User login
Association Between Proton Pump Inhibitor Exposure and Clostridium difficile Infection in Elderly, Hospitalized Patients
Clostridium difficile infection (CDI) is the result of a Gram-positive bacterium, whose exotoxins are commonly associated with infectious, watery diarrhea.1Clostridium difficile infection is associated with a significant cost burden, and over the past several years, the incidence and severity of CDI have been on the rise.2,3
There are several known risk factors for CDI. The most well-elucidated risk factor is the use of antibiotics, especially fluoroquinolones, clindamycin, broad-spectrum penicillins, and broad-spectrum cephalosporins.4,5 Other risk factors include advancing age, immunosuppression, a high burden of comorbidities, hospitalization, and antineoplastic agent use.6-8 Over the past decade, gastric acid suppression has come under increased scrutiny as a possible risk factor for CDI; specifically, exposure to proton pump inhibitors (PPIs) and histamine 2 receptor antagonists (H2RAs).8-14 With the reported overuse of PPIs, the importance of understanding safety risks associated with these agents is becoming increasingly necessary.15
In 2012, the FDA issued a public safety announcement reporting a possible association between CDI and patients undergoing treatment with PPIs.16 A large meta-analysis by Janarthanan and colleagues in 2012 evaluated 23 studies with nearly 300,000 patients, showing a 1.6-fold increase in CDI in patients exposed to a PPI.8 Another large meta-analysis noted that 39 studies showed a statistically significant association between PPI use and the risk of developing CDI (odds ratio [OR] 1.74) compared with nonusers.17 A recent study by McDonald and colleagues demonstrated patients with continuous PPI use had an elevated risk of CDI recurrence compared with patients not on continuous PPI therapy.18 These large studies did not focus analysis on elderly, hospitalized patients with significant comorbidities. There are several proposed mechanisms for the reported association between PPI use and CDI. The most widely accepted mechanism is that gastric acid suppression disrupts normal gastrointestinal flora and allows for bacterial overgrowth.19-21There are few studies that have evaluated the association between PPI use and CDI in elderly, hospitalized patients. Studies conducted in a similar patient population have demonstrated no association between PPI use and CDI.22,23 Shah and colleagues reported that treatment with gastric acid antisecretory agents does not increase the risk of developing CDI among elderly, hospitalized patients who also had severe disability.23 Lowe and colleagues demonstrated no association between PPI therapy and hospitalization for elderly outpatients with CDI.22 A study was needed to determine the association between PPI use and CDI in hospitalized, elderly patients with a high burden of comorbidities.
Related: Cleaning Up? Microfiber May Be Better
Objectives
The primary objective of this study was to determine whether there is an association between PPI exposure and CDI in elderly, hospitalized patients. The secondary objective was to determine the risk factors for the development of CDI in elderly, hospitalized patients.
Methods
Approval for this study was obtained from the Emory University Institutional Review Board and the VA Research and Development Committee. The study was a single-center, retrospective, medical record review of patients with a CDI polymerase chain reaction (PCR) assay, conducted at the Atlanta VAMC between August 20, 2011, and August 20, 2013.
Two reports for the study period were generated from TheraDoc (Premier Inc., Salt Lake City, UT) medical record software: all patients with a positive CDI PCR assay and all patients with a negative CDI PCR assay. All adult inpatients aged ≥ 18 years with a positive CDI PCR assay and diarrhea were included. Patients with CDI were randomly matched 1:1, based on age, with a control patient from a large sample of eligible CDI negative assays. Any duplicate positive CDI PCR assays were deleted, and only the first positive test was analyzed. Confirmation that PCR assay with liquid stool was being performed per manufacturer recommendations was obtained from microbiology laboratory staff.
Patient-specific data were collected from the VA Computerized Patient Record System (CPRS). Potential covariates for analyses were selected based on previous literature regarding possible associations between PPI and CDI. Data were collected on patient age, gender, PPI exposure, PPI agent, PPI dose, concomitant medications, high-risk antibiotic use, comorbidities (including diabetes, chronic renal failure, liver disease, anemia, coagulopathy, myocardial infarction, chronic heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, hypertension, hypothyroidism, and any alcohol or drug abuse), length of hospital stay, bed location, and first vs recurrent CDI. Proton pump inhibitor exposure was defined as use of any PPI during hospitalization or within 2 months prior to hospitalization. High-risk antibiotics were defined as fluoroquinolones, broad-spectrum penicillins, broad-spectrum cephalosporins, and clindamycin.
Statistical Analysis
Two-sided Wilcoxon rank sum and chi-square tests were used to compare the selected variables between CDI cases and non-CDI controls. A multivariate logistic regression model was fitted to the data using CDI as the outcome and PPI use as the main exposure of interest. The large number of covariates of interest relative to the sample size suggests conditional maximum likelihood methods of estimation.24
Separate models were run using each case-control pair as a separate stratum in the model (125 pairs) as well as pooling similar-age strata to reduce the 125 pairs to 46 pooled sets. However, when comparing the Akaike information criterion (AIC; an objective measure to determine relative quality of multivariate models where a lower AIC value is preferred) between these individual and pooled strata models, the model that controlled for 125 individual case-control strata was overwhelmingly suggested as the better model (AIC, 175 vs 255, respectively).25 Analyses were conducted with SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
A total of 128 patients were positive for CDI during the 2-year study period. Three of these patients were excluded from the study due to outpatient status. The remaining 125 patients were matched 1:1 with patients negative for CDI to yield a total study population of 250 patients.
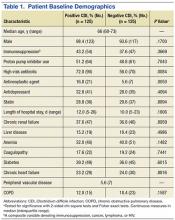
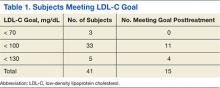
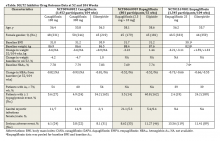
Baseline demographics are shown in Table 1. The majority of patients included were males with a median age of 66 years. Nearly half of all patients in both groups had chronic renal failure, diabetes, or anemia. Comorbidities were numerous but were not significantly different between the positive and negative CDI groups. No significant difference in immunosuppression or PPI use was detected between the 2 groups. However, there were significantly more patients taking a high-risk antibiotic or an antineoplastic agent in the positive CDI group compared with the negative CDI group. The average length of hospital stay averaged 10 to 12 days and did not statistically differ between the 2 groups.
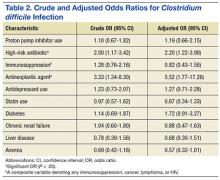
Crude ORs (cORs) and adjusted ORs (aORs) were calculated for the primary and secondary outcome measures (Table 2). There was not a statistically significant association between PPI use and CDI (cOR 1.10, 95% confidence interval [CI] 0.67-1.82; aOR 1.19, 95% CI 0.66-2.15). Other known risk factors were also evaluated for association. A statistically significant association did not exist between CDI and immunosuppression, antidepressant use, statin use, diabetes, chronic renal failure, liver disease, or anemia. However, the statistical analysis did suggest an association between CDI and high-risk antibiotic use (aOR 2.20, 95% CI 1.22-3.99) and antineoplastic agent use (aOR 5.52, 95% CI 1.77-17.26).
A sensitivity analysis was conducted to determine whether there were differing associations with CDI by PPI dose or specific agent. In both sensitivity analyses, there were no statistically significant differences in CDI between patients who took once-daily vs twice-daily PPI dosing or those who took pantoprazole vs omeprazole.
Discussion
The objective of this study was to evaluate the association between PPI use and CDI in an aging, hospitalized population. When adjusted for known risk factors, there was no association between CDI and patients exposed to PPI therapy.
Previous studies evaluating PPI use and CDI have shown conflicting results. Large meta-analyses have shown an increase in CDI in patients exposed to a PPI, whereas other studies have shown no association. In the studies that did not link PPI use and CDI, patients were elderly, hospitalized, and had other CDI risk factors. The patients in this study were hospitalized, with a median age of 66 years. They were significantly immunosuppressed and had a very high burden of comorbidities. A possible explanation for the lack of association between PPI use and CDI is that, in patients with several existing risk factors for CDI, adding a PPI confers no additional effect on CDI risk.
Well-known risk factors, including high-risk antibiotic use and antineoplastic chemotherapy use, were confirmed by this study. Other known risk factors, including immunosuppression and diabetes, were not observed to have an association with CDI in this study. This is perhaps for the same reason that PPI exposure did not show a significant association. In a study published in 2010, Howell and colleagues showed that the risk of CDI increased as acid suppression increased in a dose-dependent fashion.9 There was no association between PPI dose and PPI agent on the primary outcome measure.
About half of all patients in the current study were exposed to PPI therapy, which was a surprisingly high number. Although this study did not evaluate appropriate use of PPI therapy, it exposes the high rate of PPI use at the study site. It is known that PPI use has associated risks, and it is important that physicians continue to be vigilant in their prescribing habits.
Related: The Importance of an Antimicrobial Stewardship Program
Limitations and Future Directions
Several limitations of this study should be noted. A relatively narrow patient population was examined, which limits the generalizability of these findings. However, health care providers treating older, hospitalized patients with a high burden of comorbidities may find the results meaningful. This study was retrospective and included a relatively small sample size, which may limit the ability to detect a statistically significant difference.
Data were not collected on the duration of PPI therapy. A longer duration of therapy has been shown in previous studies to be significantly associated with CDI.26 It is unclear in this patient population whether there would have been an association between PPI duration of treatment and CDI.
Outpatient PPI exposure was determined using CPRS refill history. Patients were considered to have PPI exposure if they filled ≥ 1 prescription for a PPI within 2 months of hospitalization. Using this methodology to determine PPI exposure may not have identified patients who took over-the-counter PPIs or did not report filling a prescription for a PPI from an outside pharmacy, which would have resulted in an underestimation of PPI use in this sample. Furthermore, it is difficult to determine adherence to a prescribed regimen for outpatients.
Pantoprazole and omeprazole are the formulary PPIs at the study site. Conducting research at an institution with a formulary prevents evaluation of other PPIs, including esomeprazole, rabeprazole, dexlansoprazole, and lansoprazole. This is not seen as a significant limitation, as there have not been significant differences in the PPI agent and CDI widely reported in the literature.
Data on H2RA exposure were not collected. Any possible effect of H2RA exposure and CDI cannot be accounted for in this study. It is not likely that H2RA exposure would be associated with an increased risk of CDI in this patient population, as several studies have shown less of an association between CDI and H2RA compared with CDI and PPI use.
Further investigation to evaluate the association between CDI and PPI exposure in an elderly, hospitalized population is needed. Larger studies in these patients that evaluate duration of PPI therapy would be beneficial.
Related: Antidepressants Plus NSAIDs and the Risk of Intracranial Hemorrhage
Conclusion
In an elderly, hospitalized patient population with a high comorbidity burden, this study did not detect a statistically significant association between PPI exposure and CDI. Despite this, providers should continue to consider discontinuation of unnecessary PPI therapy.
Acknowledgements
The authors wish to thank Mehran Salles, PhD, PharmD, for her assistance. Study findings were presented at the 2014 Southeastern Residency Conference in Athens, Georgia, on May 1, 2014.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
2. Clostridium difficile infection. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_infect.html. Updated February 25, 2015. Accessed October 5, 2015.
3. Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of Clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29(9):823-828.
4. Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5. Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(1):44-50.
6. Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17(1):109-113.
7. Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40(1):1-15.
8. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001-1010.
9. Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784-790.
10. Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103(9):2308-2313.
11. Dalton BR, Lye-Maccannell T, Henderson EA, Maccannell DR, Louie TJ. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemicity, non-outbreak hospital setting. Aliment Pharmacol Ther. 2009;29(6):626-634.
12. Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
13. Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170(9):772-778.
14. Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment Pharmacol Ther. 2006;24(4):613-619.
15. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122.
16. U.S. Food and Drug Administration. FDA drug safety communication: Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm290510.htm. Updated February 15, 2013. Accessed October 5, 2015.
17. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019.
18. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;175(5):784-791.
19. Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10(4):557-561.
20. Theisen J, Nehra D, Citron D, et al. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. J Gastrointest Surg. 2000;4(1):50-54.
21. Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23(1):3-10.
22. Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43(10):1272-1276.
23. Shah S, Lewis A, Leopold D, Dunstan F, Woodhouse K. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000;93(3):175-181.
24. Kleinbaum DG, Klein M. Logistic Regression: A Self-Learning Text. 3rd ed. New York, NY: Springer; 2010.
25. Akaike H. A new look at the statistical model identification. IEEE Transact Autom Contr. 1974;19(6):716-723.
26. Barletta JF, El-Ibiary SY, Davis LE, Nguyen B, Raney CR. Proton pump inhibitors and the risk for hospital-acquired Clostridium difficile infection. Mayo Clin Proc. 2013;88(10):1085-1090.
Clostridium difficile infection (CDI) is the result of a Gram-positive bacterium, whose exotoxins are commonly associated with infectious, watery diarrhea.1Clostridium difficile infection is associated with a significant cost burden, and over the past several years, the incidence and severity of CDI have been on the rise.2,3
There are several known risk factors for CDI. The most well-elucidated risk factor is the use of antibiotics, especially fluoroquinolones, clindamycin, broad-spectrum penicillins, and broad-spectrum cephalosporins.4,5 Other risk factors include advancing age, immunosuppression, a high burden of comorbidities, hospitalization, and antineoplastic agent use.6-8 Over the past decade, gastric acid suppression has come under increased scrutiny as a possible risk factor for CDI; specifically, exposure to proton pump inhibitors (PPIs) and histamine 2 receptor antagonists (H2RAs).8-14 With the reported overuse of PPIs, the importance of understanding safety risks associated with these agents is becoming increasingly necessary.15
In 2012, the FDA issued a public safety announcement reporting a possible association between CDI and patients undergoing treatment with PPIs.16 A large meta-analysis by Janarthanan and colleagues in 2012 evaluated 23 studies with nearly 300,000 patients, showing a 1.6-fold increase in CDI in patients exposed to a PPI.8 Another large meta-analysis noted that 39 studies showed a statistically significant association between PPI use and the risk of developing CDI (odds ratio [OR] 1.74) compared with nonusers.17 A recent study by McDonald and colleagues demonstrated patients with continuous PPI use had an elevated risk of CDI recurrence compared with patients not on continuous PPI therapy.18 These large studies did not focus analysis on elderly, hospitalized patients with significant comorbidities. There are several proposed mechanisms for the reported association between PPI use and CDI. The most widely accepted mechanism is that gastric acid suppression disrupts normal gastrointestinal flora and allows for bacterial overgrowth.19-21There are few studies that have evaluated the association between PPI use and CDI in elderly, hospitalized patients. Studies conducted in a similar patient population have demonstrated no association between PPI use and CDI.22,23 Shah and colleagues reported that treatment with gastric acid antisecretory agents does not increase the risk of developing CDI among elderly, hospitalized patients who also had severe disability.23 Lowe and colleagues demonstrated no association between PPI therapy and hospitalization for elderly outpatients with CDI.22 A study was needed to determine the association between PPI use and CDI in hospitalized, elderly patients with a high burden of comorbidities.
Related: Cleaning Up? Microfiber May Be Better
Objectives
The primary objective of this study was to determine whether there is an association between PPI exposure and CDI in elderly, hospitalized patients. The secondary objective was to determine the risk factors for the development of CDI in elderly, hospitalized patients.
Methods
Approval for this study was obtained from the Emory University Institutional Review Board and the VA Research and Development Committee. The study was a single-center, retrospective, medical record review of patients with a CDI polymerase chain reaction (PCR) assay, conducted at the Atlanta VAMC between August 20, 2011, and August 20, 2013.
Two reports for the study period were generated from TheraDoc (Premier Inc., Salt Lake City, UT) medical record software: all patients with a positive CDI PCR assay and all patients with a negative CDI PCR assay. All adult inpatients aged ≥ 18 years with a positive CDI PCR assay and diarrhea were included. Patients with CDI were randomly matched 1:1, based on age, with a control patient from a large sample of eligible CDI negative assays. Any duplicate positive CDI PCR assays were deleted, and only the first positive test was analyzed. Confirmation that PCR assay with liquid stool was being performed per manufacturer recommendations was obtained from microbiology laboratory staff.
Patient-specific data were collected from the VA Computerized Patient Record System (CPRS). Potential covariates for analyses were selected based on previous literature regarding possible associations between PPI and CDI. Data were collected on patient age, gender, PPI exposure, PPI agent, PPI dose, concomitant medications, high-risk antibiotic use, comorbidities (including diabetes, chronic renal failure, liver disease, anemia, coagulopathy, myocardial infarction, chronic heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, hypertension, hypothyroidism, and any alcohol or drug abuse), length of hospital stay, bed location, and first vs recurrent CDI. Proton pump inhibitor exposure was defined as use of any PPI during hospitalization or within 2 months prior to hospitalization. High-risk antibiotics were defined as fluoroquinolones, broad-spectrum penicillins, broad-spectrum cephalosporins, and clindamycin.
Statistical Analysis
Two-sided Wilcoxon rank sum and chi-square tests were used to compare the selected variables between CDI cases and non-CDI controls. A multivariate logistic regression model was fitted to the data using CDI as the outcome and PPI use as the main exposure of interest. The large number of covariates of interest relative to the sample size suggests conditional maximum likelihood methods of estimation.24
Separate models were run using each case-control pair as a separate stratum in the model (125 pairs) as well as pooling similar-age strata to reduce the 125 pairs to 46 pooled sets. However, when comparing the Akaike information criterion (AIC; an objective measure to determine relative quality of multivariate models where a lower AIC value is preferred) between these individual and pooled strata models, the model that controlled for 125 individual case-control strata was overwhelmingly suggested as the better model (AIC, 175 vs 255, respectively).25 Analyses were conducted with SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
A total of 128 patients were positive for CDI during the 2-year study period. Three of these patients were excluded from the study due to outpatient status. The remaining 125 patients were matched 1:1 with patients negative for CDI to yield a total study population of 250 patients.
Baseline demographics are shown in Table 1. The majority of patients included were males with a median age of 66 years. Nearly half of all patients in both groups had chronic renal failure, diabetes, or anemia. Comorbidities were numerous but were not significantly different between the positive and negative CDI groups. No significant difference in immunosuppression or PPI use was detected between the 2 groups. However, there were significantly more patients taking a high-risk antibiotic or an antineoplastic agent in the positive CDI group compared with the negative CDI group. The average length of hospital stay averaged 10 to 12 days and did not statistically differ between the 2 groups.
Crude ORs (cORs) and adjusted ORs (aORs) were calculated for the primary and secondary outcome measures (Table 2). There was not a statistically significant association between PPI use and CDI (cOR 1.10, 95% confidence interval [CI] 0.67-1.82; aOR 1.19, 95% CI 0.66-2.15). Other known risk factors were also evaluated for association. A statistically significant association did not exist between CDI and immunosuppression, antidepressant use, statin use, diabetes, chronic renal failure, liver disease, or anemia. However, the statistical analysis did suggest an association between CDI and high-risk antibiotic use (aOR 2.20, 95% CI 1.22-3.99) and antineoplastic agent use (aOR 5.52, 95% CI 1.77-17.26).
A sensitivity analysis was conducted to determine whether there were differing associations with CDI by PPI dose or specific agent. In both sensitivity analyses, there were no statistically significant differences in CDI between patients who took once-daily vs twice-daily PPI dosing or those who took pantoprazole vs omeprazole.
Discussion
The objective of this study was to evaluate the association between PPI use and CDI in an aging, hospitalized population. When adjusted for known risk factors, there was no association between CDI and patients exposed to PPI therapy.
Previous studies evaluating PPI use and CDI have shown conflicting results. Large meta-analyses have shown an increase in CDI in patients exposed to a PPI, whereas other studies have shown no association. In the studies that did not link PPI use and CDI, patients were elderly, hospitalized, and had other CDI risk factors. The patients in this study were hospitalized, with a median age of 66 years. They were significantly immunosuppressed and had a very high burden of comorbidities. A possible explanation for the lack of association between PPI use and CDI is that, in patients with several existing risk factors for CDI, adding a PPI confers no additional effect on CDI risk.
Well-known risk factors, including high-risk antibiotic use and antineoplastic chemotherapy use, were confirmed by this study. Other known risk factors, including immunosuppression and diabetes, were not observed to have an association with CDI in this study. This is perhaps for the same reason that PPI exposure did not show a significant association. In a study published in 2010, Howell and colleagues showed that the risk of CDI increased as acid suppression increased in a dose-dependent fashion.9 There was no association between PPI dose and PPI agent on the primary outcome measure.
About half of all patients in the current study were exposed to PPI therapy, which was a surprisingly high number. Although this study did not evaluate appropriate use of PPI therapy, it exposes the high rate of PPI use at the study site. It is known that PPI use has associated risks, and it is important that physicians continue to be vigilant in their prescribing habits.
Related: The Importance of an Antimicrobial Stewardship Program
Limitations and Future Directions
Several limitations of this study should be noted. A relatively narrow patient population was examined, which limits the generalizability of these findings. However, health care providers treating older, hospitalized patients with a high burden of comorbidities may find the results meaningful. This study was retrospective and included a relatively small sample size, which may limit the ability to detect a statistically significant difference.
Data were not collected on the duration of PPI therapy. A longer duration of therapy has been shown in previous studies to be significantly associated with CDI.26 It is unclear in this patient population whether there would have been an association between PPI duration of treatment and CDI.
Outpatient PPI exposure was determined using CPRS refill history. Patients were considered to have PPI exposure if they filled ≥ 1 prescription for a PPI within 2 months of hospitalization. Using this methodology to determine PPI exposure may not have identified patients who took over-the-counter PPIs or did not report filling a prescription for a PPI from an outside pharmacy, which would have resulted in an underestimation of PPI use in this sample. Furthermore, it is difficult to determine adherence to a prescribed regimen for outpatients.
Pantoprazole and omeprazole are the formulary PPIs at the study site. Conducting research at an institution with a formulary prevents evaluation of other PPIs, including esomeprazole, rabeprazole, dexlansoprazole, and lansoprazole. This is not seen as a significant limitation, as there have not been significant differences in the PPI agent and CDI widely reported in the literature.
Data on H2RA exposure were not collected. Any possible effect of H2RA exposure and CDI cannot be accounted for in this study. It is not likely that H2RA exposure would be associated with an increased risk of CDI in this patient population, as several studies have shown less of an association between CDI and H2RA compared with CDI and PPI use.
Further investigation to evaluate the association between CDI and PPI exposure in an elderly, hospitalized population is needed. Larger studies in these patients that evaluate duration of PPI therapy would be beneficial.
Related: Antidepressants Plus NSAIDs and the Risk of Intracranial Hemorrhage
Conclusion
In an elderly, hospitalized patient population with a high comorbidity burden, this study did not detect a statistically significant association between PPI exposure and CDI. Despite this, providers should continue to consider discontinuation of unnecessary PPI therapy.
Acknowledgements
The authors wish to thank Mehran Salles, PhD, PharmD, for her assistance. Study findings were presented at the 2014 Southeastern Residency Conference in Athens, Georgia, on May 1, 2014.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Clostridium difficile infection (CDI) is the result of a Gram-positive bacterium, whose exotoxins are commonly associated with infectious, watery diarrhea.1Clostridium difficile infection is associated with a significant cost burden, and over the past several years, the incidence and severity of CDI have been on the rise.2,3
There are several known risk factors for CDI. The most well-elucidated risk factor is the use of antibiotics, especially fluoroquinolones, clindamycin, broad-spectrum penicillins, and broad-spectrum cephalosporins.4,5 Other risk factors include advancing age, immunosuppression, a high burden of comorbidities, hospitalization, and antineoplastic agent use.6-8 Over the past decade, gastric acid suppression has come under increased scrutiny as a possible risk factor for CDI; specifically, exposure to proton pump inhibitors (PPIs) and histamine 2 receptor antagonists (H2RAs).8-14 With the reported overuse of PPIs, the importance of understanding safety risks associated with these agents is becoming increasingly necessary.15
In 2012, the FDA issued a public safety announcement reporting a possible association between CDI and patients undergoing treatment with PPIs.16 A large meta-analysis by Janarthanan and colleagues in 2012 evaluated 23 studies with nearly 300,000 patients, showing a 1.6-fold increase in CDI in patients exposed to a PPI.8 Another large meta-analysis noted that 39 studies showed a statistically significant association between PPI use and the risk of developing CDI (odds ratio [OR] 1.74) compared with nonusers.17 A recent study by McDonald and colleagues demonstrated patients with continuous PPI use had an elevated risk of CDI recurrence compared with patients not on continuous PPI therapy.18 These large studies did not focus analysis on elderly, hospitalized patients with significant comorbidities. There are several proposed mechanisms for the reported association between PPI use and CDI. The most widely accepted mechanism is that gastric acid suppression disrupts normal gastrointestinal flora and allows for bacterial overgrowth.19-21There are few studies that have evaluated the association between PPI use and CDI in elderly, hospitalized patients. Studies conducted in a similar patient population have demonstrated no association between PPI use and CDI.22,23 Shah and colleagues reported that treatment with gastric acid antisecretory agents does not increase the risk of developing CDI among elderly, hospitalized patients who also had severe disability.23 Lowe and colleagues demonstrated no association between PPI therapy and hospitalization for elderly outpatients with CDI.22 A study was needed to determine the association between PPI use and CDI in hospitalized, elderly patients with a high burden of comorbidities.
Related: Cleaning Up? Microfiber May Be Better
Objectives
The primary objective of this study was to determine whether there is an association between PPI exposure and CDI in elderly, hospitalized patients. The secondary objective was to determine the risk factors for the development of CDI in elderly, hospitalized patients.
Methods
Approval for this study was obtained from the Emory University Institutional Review Board and the VA Research and Development Committee. The study was a single-center, retrospective, medical record review of patients with a CDI polymerase chain reaction (PCR) assay, conducted at the Atlanta VAMC between August 20, 2011, and August 20, 2013.
Two reports for the study period were generated from TheraDoc (Premier Inc., Salt Lake City, UT) medical record software: all patients with a positive CDI PCR assay and all patients with a negative CDI PCR assay. All adult inpatients aged ≥ 18 years with a positive CDI PCR assay and diarrhea were included. Patients with CDI were randomly matched 1:1, based on age, with a control patient from a large sample of eligible CDI negative assays. Any duplicate positive CDI PCR assays were deleted, and only the first positive test was analyzed. Confirmation that PCR assay with liquid stool was being performed per manufacturer recommendations was obtained from microbiology laboratory staff.
Patient-specific data were collected from the VA Computerized Patient Record System (CPRS). Potential covariates for analyses were selected based on previous literature regarding possible associations between PPI and CDI. Data were collected on patient age, gender, PPI exposure, PPI agent, PPI dose, concomitant medications, high-risk antibiotic use, comorbidities (including diabetes, chronic renal failure, liver disease, anemia, coagulopathy, myocardial infarction, chronic heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, hypertension, hypothyroidism, and any alcohol or drug abuse), length of hospital stay, bed location, and first vs recurrent CDI. Proton pump inhibitor exposure was defined as use of any PPI during hospitalization or within 2 months prior to hospitalization. High-risk antibiotics were defined as fluoroquinolones, broad-spectrum penicillins, broad-spectrum cephalosporins, and clindamycin.
Statistical Analysis
Two-sided Wilcoxon rank sum and chi-square tests were used to compare the selected variables between CDI cases and non-CDI controls. A multivariate logistic regression model was fitted to the data using CDI as the outcome and PPI use as the main exposure of interest. The large number of covariates of interest relative to the sample size suggests conditional maximum likelihood methods of estimation.24
Separate models were run using each case-control pair as a separate stratum in the model (125 pairs) as well as pooling similar-age strata to reduce the 125 pairs to 46 pooled sets. However, when comparing the Akaike information criterion (AIC; an objective measure to determine relative quality of multivariate models where a lower AIC value is preferred) between these individual and pooled strata models, the model that controlled for 125 individual case-control strata was overwhelmingly suggested as the better model (AIC, 175 vs 255, respectively).25 Analyses were conducted with SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
A total of 128 patients were positive for CDI during the 2-year study period. Three of these patients were excluded from the study due to outpatient status. The remaining 125 patients were matched 1:1 with patients negative for CDI to yield a total study population of 250 patients.
Baseline demographics are shown in Table 1. The majority of patients included were males with a median age of 66 years. Nearly half of all patients in both groups had chronic renal failure, diabetes, or anemia. Comorbidities were numerous but were not significantly different between the positive and negative CDI groups. No significant difference in immunosuppression or PPI use was detected between the 2 groups. However, there were significantly more patients taking a high-risk antibiotic or an antineoplastic agent in the positive CDI group compared with the negative CDI group. The average length of hospital stay averaged 10 to 12 days and did not statistically differ between the 2 groups.
Crude ORs (cORs) and adjusted ORs (aORs) were calculated for the primary and secondary outcome measures (Table 2). There was not a statistically significant association between PPI use and CDI (cOR 1.10, 95% confidence interval [CI] 0.67-1.82; aOR 1.19, 95% CI 0.66-2.15). Other known risk factors were also evaluated for association. A statistically significant association did not exist between CDI and immunosuppression, antidepressant use, statin use, diabetes, chronic renal failure, liver disease, or anemia. However, the statistical analysis did suggest an association between CDI and high-risk antibiotic use (aOR 2.20, 95% CI 1.22-3.99) and antineoplastic agent use (aOR 5.52, 95% CI 1.77-17.26).
A sensitivity analysis was conducted to determine whether there were differing associations with CDI by PPI dose or specific agent. In both sensitivity analyses, there were no statistically significant differences in CDI between patients who took once-daily vs twice-daily PPI dosing or those who took pantoprazole vs omeprazole.
Discussion
The objective of this study was to evaluate the association between PPI use and CDI in an aging, hospitalized population. When adjusted for known risk factors, there was no association between CDI and patients exposed to PPI therapy.
Previous studies evaluating PPI use and CDI have shown conflicting results. Large meta-analyses have shown an increase in CDI in patients exposed to a PPI, whereas other studies have shown no association. In the studies that did not link PPI use and CDI, patients were elderly, hospitalized, and had other CDI risk factors. The patients in this study were hospitalized, with a median age of 66 years. They were significantly immunosuppressed and had a very high burden of comorbidities. A possible explanation for the lack of association between PPI use and CDI is that, in patients with several existing risk factors for CDI, adding a PPI confers no additional effect on CDI risk.
Well-known risk factors, including high-risk antibiotic use and antineoplastic chemotherapy use, were confirmed by this study. Other known risk factors, including immunosuppression and diabetes, were not observed to have an association with CDI in this study. This is perhaps for the same reason that PPI exposure did not show a significant association. In a study published in 2010, Howell and colleagues showed that the risk of CDI increased as acid suppression increased in a dose-dependent fashion.9 There was no association between PPI dose and PPI agent on the primary outcome measure.
About half of all patients in the current study were exposed to PPI therapy, which was a surprisingly high number. Although this study did not evaluate appropriate use of PPI therapy, it exposes the high rate of PPI use at the study site. It is known that PPI use has associated risks, and it is important that physicians continue to be vigilant in their prescribing habits.
Related: The Importance of an Antimicrobial Stewardship Program
Limitations and Future Directions
Several limitations of this study should be noted. A relatively narrow patient population was examined, which limits the generalizability of these findings. However, health care providers treating older, hospitalized patients with a high burden of comorbidities may find the results meaningful. This study was retrospective and included a relatively small sample size, which may limit the ability to detect a statistically significant difference.
Data were not collected on the duration of PPI therapy. A longer duration of therapy has been shown in previous studies to be significantly associated with CDI.26 It is unclear in this patient population whether there would have been an association between PPI duration of treatment and CDI.
Outpatient PPI exposure was determined using CPRS refill history. Patients were considered to have PPI exposure if they filled ≥ 1 prescription for a PPI within 2 months of hospitalization. Using this methodology to determine PPI exposure may not have identified patients who took over-the-counter PPIs or did not report filling a prescription for a PPI from an outside pharmacy, which would have resulted in an underestimation of PPI use in this sample. Furthermore, it is difficult to determine adherence to a prescribed regimen for outpatients.
Pantoprazole and omeprazole are the formulary PPIs at the study site. Conducting research at an institution with a formulary prevents evaluation of other PPIs, including esomeprazole, rabeprazole, dexlansoprazole, and lansoprazole. This is not seen as a significant limitation, as there have not been significant differences in the PPI agent and CDI widely reported in the literature.
Data on H2RA exposure were not collected. Any possible effect of H2RA exposure and CDI cannot be accounted for in this study. It is not likely that H2RA exposure would be associated with an increased risk of CDI in this patient population, as several studies have shown less of an association between CDI and H2RA compared with CDI and PPI use.
Further investigation to evaluate the association between CDI and PPI exposure in an elderly, hospitalized population is needed. Larger studies in these patients that evaluate duration of PPI therapy would be beneficial.
Related: Antidepressants Plus NSAIDs and the Risk of Intracranial Hemorrhage
Conclusion
In an elderly, hospitalized patient population with a high comorbidity burden, this study did not detect a statistically significant association between PPI exposure and CDI. Despite this, providers should continue to consider discontinuation of unnecessary PPI therapy.
Acknowledgements
The authors wish to thank Mehran Salles, PhD, PharmD, for her assistance. Study findings were presented at the 2014 Southeastern Residency Conference in Athens, Georgia, on May 1, 2014.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
2. Clostridium difficile infection. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_infect.html. Updated February 25, 2015. Accessed October 5, 2015.
3. Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of Clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29(9):823-828.
4. Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5. Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(1):44-50.
6. Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17(1):109-113.
7. Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40(1):1-15.
8. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001-1010.
9. Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784-790.
10. Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103(9):2308-2313.
11. Dalton BR, Lye-Maccannell T, Henderson EA, Maccannell DR, Louie TJ. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemicity, non-outbreak hospital setting. Aliment Pharmacol Ther. 2009;29(6):626-634.
12. Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
13. Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170(9):772-778.
14. Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment Pharmacol Ther. 2006;24(4):613-619.
15. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122.
16. U.S. Food and Drug Administration. FDA drug safety communication: Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm290510.htm. Updated February 15, 2013. Accessed October 5, 2015.
17. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019.
18. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;175(5):784-791.
19. Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10(4):557-561.
20. Theisen J, Nehra D, Citron D, et al. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. J Gastrointest Surg. 2000;4(1):50-54.
21. Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23(1):3-10.
22. Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43(10):1272-1276.
23. Shah S, Lewis A, Leopold D, Dunstan F, Woodhouse K. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000;93(3):175-181.
24. Kleinbaum DG, Klein M. Logistic Regression: A Self-Learning Text. 3rd ed. New York, NY: Springer; 2010.
25. Akaike H. A new look at the statistical model identification. IEEE Transact Autom Contr. 1974;19(6):716-723.
26. Barletta JF, El-Ibiary SY, Davis LE, Nguyen B, Raney CR. Proton pump inhibitors and the risk for hospital-acquired Clostridium difficile infection. Mayo Clin Proc. 2013;88(10):1085-1090.
1. Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
2. Clostridium difficile infection. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_infect.html. Updated February 25, 2015. Accessed October 5, 2015.
3. Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of Clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29(9):823-828.
4. Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5. Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(1):44-50.
6. Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17(1):109-113.
7. Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40(1):1-15.
8. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001-1010.
9. Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784-790.
10. Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103(9):2308-2313.
11. Dalton BR, Lye-Maccannell T, Henderson EA, Maccannell DR, Louie TJ. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemicity, non-outbreak hospital setting. Aliment Pharmacol Ther. 2009;29(6):626-634.
12. Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
13. Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170(9):772-778.
14. Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment Pharmacol Ther. 2006;24(4):613-619.
15. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122.
16. U.S. Food and Drug Administration. FDA drug safety communication: Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm290510.htm. Updated February 15, 2013. Accessed October 5, 2015.
17. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019.
18. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;175(5):784-791.
19. Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10(4):557-561.
20. Theisen J, Nehra D, Citron D, et al. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. J Gastrointest Surg. 2000;4(1):50-54.
21. Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23(1):3-10.
22. Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43(10):1272-1276.
23. Shah S, Lewis A, Leopold D, Dunstan F, Woodhouse K. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000;93(3):175-181.
24. Kleinbaum DG, Klein M. Logistic Regression: A Self-Learning Text. 3rd ed. New York, NY: Springer; 2010.
25. Akaike H. A new look at the statistical model identification. IEEE Transact Autom Contr. 1974;19(6):716-723.
26. Barletta JF, El-Ibiary SY, Davis LE, Nguyen B, Raney CR. Proton pump inhibitors and the risk for hospital-acquired Clostridium difficile infection. Mayo Clin Proc. 2013;88(10):1085-1090.
Evaluating Sorafenib in Veterans With Advanced Hepatocellular Carcinoma
In 2015, more than 35,660 new cases of liver cancer and 24,550 liver cancer-related deaths are expected to occur in the U.S. About 80% of these cases will consist of hepatocellular carcinoma, (HCC).1 The incidence of HCC varies throughout the world: Incidence is as low as 5 in 100,000 individuals in North America and ranges up to > 20 in 100,000 individuals in sub-Saharan Africa and Eastern Asia.2 Nearly half of all cases of HCC are associated with hepatitis B virus (HBV), and another 25% are associated with hepatitis C virus (HCV). Other risk factors for developing HCC include alcoholic liver disease, nonalcoholic steatohepatitis, flatoxin-contaminated food, diabetes, and obesity.3
Therapeutic options for advanced HCC are limited. The FDA approved sorafenib in 2008 for the treatment of unresectable HCC.4 According to the American Association for the Study of Liver Diseases (AASLD) and the Barcelona Clinic Liver Cancer (BCLC) staging system, patients with Stage C liver cancer may undergo a trial of sorafenib.4 National Comprehensive Cancer Network (NCCN) clinical guidelines for hepatobiliary cancers reserve sorafenib for patients with inoperable tumors, metastatic disease, or extensive liver tumor burden.5 Sorafenib is shown to inhibit multiple intracellular and cell surface kinases. Several of these kinases are thought to be involved in tumor cell signaling, angiogenesis, and apoptosis.4 In the Sorafenib HCC Assessment Randomized Protocol (SHARP) trial, median overall survival (OS) was 10.7 months in the sorafenib group and 7.9 months in the placebo group.6 The predicted survival rates at 1 year were 44% in the sorafenib group and 33% in the placebo group.6The economic impact of oral chemotherapy on health care cannot be discounted. At about $50,000 to $100,000 per quality- adjusted life-year, the incremental cost-effectiveness ratio (ICER) of sorafenib over placebo was $62,473 per quality-adjusted life-year in 2007.7The purpose of this retrospective chart review was to evaluate sorafenib for efficacy and safety in a veteran population. Veterans have poorer health and more medical conditions compared with nonveterans.8 Furthermore, in the VHA, about 170,000 veterans have HCV.9 The rate of progression from HCV to HCC is about 3% to 5% annually. More than half of those diagnosed with HCC are late stage, and unfortunately, the 5-year OS rate for patients with liver cancer is 9% and 4% for those patients who are diagnosed at regional and distant stages of the disease.1 As the practice of oncology grows, it is necessary for pharmacists to be involved in the selection of chemotherapeutic agents in order to provide optimal pharmaceutical care.10
Related: VIDEO: NAFLD increasingly causing U.S. hepatocellular carcinoma
Methods
A retrospective chart review was conducted to identify patients who were prescribed sorafenib from November 1, 2007, to September 30, 2011, at the VA Greater Los Angeles Healthcare System (VAGLAHS). Inclusion criteria included patients who had a diagnosis of advanced HCC, who were initiated and managed by a VAGLAHS provider and who were eligible for a 1-year safety evaluation period. The study was approved by the VAGLAHS institutional review board.
Baseline demographic, clinical, laboratory, and medication data were collected. Demographic, clinical, laboratory, and medication data were obtained from CPRS (Computerized Patient Record System) and VistA (Veterans Health Information Systems and Technology Architecture). Data were collected on secured servers and saved on encrypted files. The master list was destroyed once the records control schedule was finalized. No identifiers were collected on the data collection sheet.
Standard practice at VAGLAHS is to monitor European Cooperative Oncology Group Performance Status (ECOG-PS), Child-Pugh class, and alpha-fetoprotein (AFP) at initiation and every 3 months and to obtain laboratory data at initiation and every month before each medication refill. Patients were seen in the Oncology Clinic periodically at the provider’s discretion. The time of drug discontinuation and the reason for drug discontinuation were recorded. Time of death at any point was collected to measure OS.
It was determined that a total sample size of 42 patients would be insufficient to achieve 80% power to demonstrate any hypothesized effects. However, the Fisher exact test was used to calculate P values for simple comparison. Patient demographics and clinical characteristics were reported as total numbers and frequencies when applicable. Survival rate was measured from the time of sorafenib initiation to 1 year after therapy initiation. Overall survival was measured from the time of sorafenib initiation to time of death. Duration of therapy was measured from the time of sorafenib initiation to time of discontinuation, either by provider or by patient.
Results
There were 83 patients who were prescribed sorafenib between November 1, 2007, and September 30, 2011. Of the 83 patients, 27 patients were ineligible for a 1-year follow-up period, 9 patients were diagnosed with non-HCC, 3 were initiated or managed by providers outside the institution, and 2 were not started on therapy. In all, 42 patients met inclusion criteria and had received at least 1 dose of sorafenib. The primary etiologies for HCC were history of alcohol abuse, HCV, and HBV. The primary risk factors were obesity, smoking, and diabetes. Many patients presented with multiple etiologies and risk factors. Ten patients (23.8%) had moderate-to-severe hepatic impairment (Child-Pugh class B or C). Baseline characteristics of these patients are listed in Table 1.
Efficacy
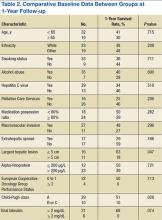
The median OS was 5.9 months and ranged from 21 days to 60 months. There were 17 patients who survived at the 1-year follow-up, including 1 patient who survived 363 days after treatment initiation, yielding an OS rate of 40.5%. Table 2 presents 1-year survival rates with respect to select baseline data. Baseline factors found to be negligible were age, smoking, alcohol abuse, obesity, presence of HCV, medication possession ratio (MPR), prior treatment, macrovascular invasion, and AFP. Neither initial dose regimen, final dose regimen achieved, or average dose correlated with the survival rate at the 1-year follow-up.
Factors possibly associated with a higher probability of survival were baseline ECOG-PS score and baseline Child-Pugh class (Table 2). Patients with an ECOG-PS score of 0 or 1 had a higher survival rate at 1 year than did patients with an ECOG-PS score of ≥ 2 (50% vs 0%, respectively; P = .113). Patients with Child-Pugh class B or C had a lower survival rate at 1 year than did patients with Child-Pugh class A (51% vs 10%, respectively; P = .028). Other indicators were size of largest hepatic lesion ≤ 5 cm, total bilirubin ≤ 2 mg/dL, concurrent treatment, almost exclusively embolization, and treatment after sorafenib discontinuation, such as another oral chemotherapeutic agent or embolization.
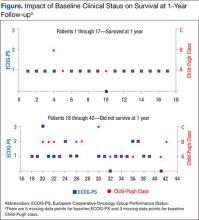
The 17 patients who survived at 1 year were reviewed to see if they shared characteristics that indicated a higher probability of survival. The figure shows the baseline ECOG-PS score and the Child-Pugh class the patients who did and did not survive at the 1-year follow-up. In the first group, all patient possessed an ECOG-PS score of 0 or 1, and only 1 patient presented with Child-Pugh class B or C. In contrast, in the group who did not survive at the 1-year follow-up, there were 4 patients with ECOG-PS scores of > 1 and 9 patients who presented with Child-Pugh class B or C. The mean AFP level of this group was < 200 µg/mL, and only 4 patients were followed by Palliative Care Services. The average normalized MPR of this group was 71.9% compared with 85.3% for those who did not survive at the 1-year follow-up.
In patients who experienced at least 1 adverse event (AE), 16 survived, whereas only 1 who did not experience an AE survived (45.7% vs 14.3%, respectively; P = .210). Thirteen patients who experienced ≥ 3 AEs survived at 1 year; and only 3 patients who reported < 3 AEs survived at 1 year (61.9% vs 14.0%, respectively; P = .011). However, when the number of AEs was normalized to duration of treatment per patient, the median frequency of AEs for all patients was 0.61 AEs per month treated. The difference in survival rates grew smaller and less significant between patients who had a frequency of AEs lower than the median compared with those with a higher ratio (52.4% vs 28.6%, respectively; P = .208). Patients affected by AEs in the first 30 days and 90 days of treatment had a survival rate at the 1-year follow-up of 42.4% and 30.2%, respectively. Patients who experienced dermatologic AEs had a higher survival rate than those who did not have dermatologic AEs (60.0% vs 29.6%, respectively; P = .099). This correlation was not found with 2 other classes of AEs, gastrointestinal (50.0% vs 27.8%; P = .208) or neurologic (64.0% vs 41.2%; P = .209).
The median overall time to discontinuation was 3.4 months. The main reasons cited for discontinuing sorafenib at 1 year included symptomatic progression (52.4%), radiographic progression (23.8%), severe AEs (16.7%), and mild-to-moderate AEs (11.9%). There was overlap, as 15 patients discontinued treatment for multiple reasons. For the 22 patients who discontinued medication due to symptomatic progression at 1 year, the median time to discontinuation was 3.8 months. For the 10 patients who discontinued medication due to radiographic progression at 1 year, median time to discontinuation was 5.6 months. Seven patients (16.7%) were still on therapy at 1 year.The study considered the impact of potential dose adjustments on survival rate and safety. The authors compared patients’ prescribed dose with the recommended dose based on the package insert and monthly laboratory values if recorded. The prescribed dose was recorded as appropriate dose, below dose, above dose, or indeterminate due to the lack of current laboratory values. Patients who survived at the 1-year follow-up had a composition of 26%, 21%, 10%, and 43%, respectively. These results were similar to those of patients who did not survive at the 1-year follow-up, 29%, 12%, 30%, and 29%, respectively.
Based on medication refill history and VA acquisition cost, the total prescription drug cost of treating 42 patients with sorafenib was $388,370.40. The total number of days survived for these patients was 16,607 days, which equates to $8,535.87 per year lived.
Safety
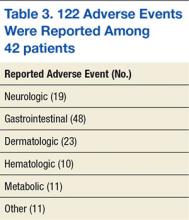
Of the 42 patients, 35 patients experienced ≥ 1 AE for a total of 122 AEs reported. The median number of AEs per patient was 2.5. The median time to the first AE was 21 days and ranged from 3 to 244 days. In the first 30 days of treatment, 23 patients (54.7%) reported 47 AEs (39.5%). In the first 90 days of treatment, 33 patients (78.6%) reported 88 AEs (73.9%). Common AEs in both instances were diarrhea, fatigue, erythematous plantar-palmar rash, and nausea (Table 3).
The predominant classes of AEs were GI (39.3%), dermatologic (18.9%), and neurologic (15.6%). Erythematous palmar-plantar rash, also known as hand-foot syndrome, has been noted as a potential dose-limiting sorafenib AE if the rash is recurrent or severe. One patient experienced recurrent grade-2 rashes, and sorafenib was immediately discontinued after an attempt to lower the dose. There were 8 patients who reported serious AEs, and 5 were hospitalized. One patient continued therapy despite GI hemorrhage. The other 4 patients discontinued therapy on hospitalization and were seen for intracranial hemorrhage, GI perforation, acute renal failure, and acute liver failure. In the first 3 cases, sorafenib could not be ruled out as the primary cause of death. None of these patients presented with comorbidities, such as hypertension, which predisposed them to AEs.
Overall, 38 patients ended therapy at the recommended regimen of 400 mg twice daily, and the average total daily dose was 619 mg, just below 80% of the recommended daily dose. Reasons for not achieving 400 mg twice daily included slow titration, AEs, and dose adjustments for compromised renal and hepatic function such as dialysis. Patients who had an ECOG-PS score of 0 or 1 or Child-Pugh class A reported ≥ 3 AEs, but when normalized to duration of treatment, no difference was observed. No correlations were found for average dose, creatinine clearance, aspartate aminotransferase, platelets, total bilirubin, or weight and number or frequency of AEs.
In regard to potential dose adjustments, the doses (400 mg twice daily, 600 mg daily [400 mg + 200 mg in 2 doses], 200 mg twice daily, and 200 mg daily) did not correlate well with AEs. Patients who had < 3 AEs presented with the breakdown 23%, 16%, 22%, and 38%, similar to patients who had ≥ 3 AEs—30%, 19%, 14%, and 37%. Likewise, patients who had a frequency of AEs lower than the median presented with the breakdown 22%, 22%, 15%, and 40% compared with patients who had more AEs than the median—37%, 9%, 23%, and 31%.
Related: Hepatocellular Carcinoma: To Biopsy or Not?
Discussion
Sorafenib is the only oral oncology medication approved by the FDA for treatment of unresectable HCC.3 Prior to sorafenib, the AASLD recommendation was supportive care for patients presenting with BCLC-Stage C liver cancer. However, guidelines changed when SHARP showed that sorafenib provided a survival benefit with a tolerable AE profile. The survival benefit of sorafenib has been replicated in a few large, multicenter trials. In Asia, Cheng and colleagues saw improved median OS of 6.5 months for sorafenib compared with 4.2 months with placebo, and in Italy, Iavarone and colleagues showed a median OS of 10.5 months without a placebo comparator.11,12
In the veteran population for this study, the OS rate of 40.5% was similar to the rate reported in the SHARP study, although the patients’ median OS fell short of the time described in SHARP and other trials. The medical complexities involved in treating veterans may explain this difference. The veteran population is heterogeneous with diverse ethnic backgrounds, several comorbidities, and varying degrees of organ dysfunction. The authors compared survival rates of different subgroups to test the hypothesis that the probability of survival while on therapy should not depend on demographics or medical history. However, in this study, patients with minimal impact from HCC, such as mild hepatic impairment and high-functional status, demonstrated higher survival rates at 1-year follow-up than did those without significant compromise.Although the high prevalence of HCV and alcohol abuse in the veteran population has resulted in a high incidence of hepatic dysfunction, this study suggests that these factors are independent of survival if liver function or integrity has not been compromised.9
Some researchers have hypothesized that clinical toxicities from tyrosine kinase inhibitors may correlate with survival.13 The authors noticed that the presentation of dermatologic AEs may reflect improved survival. In this study, patients who experienced ≥ 1 AE and ≥ 3 AEs had survival rates at the 1-year follow-up of 45.7% and 61.9%, respectively. Moreover, patients affected by AEs in the first 90 days of treatment had a survival rate at the 1-year follow-up of 42.4%.
Caution is advised when drawing conclusions from the number of AEs or when they appear, because this may falsely favor correlation. Patients who survive longer have additional time to report an AE. Therefore, the authors also looked at the ratio of AEs over time per patient to consider the number of AEs per duration of treatment and saw that there was little difference in survival rate in this regard. When considering patients affected by AEs only in the first 30 days of treatment, the survival rate at the 1-year follow-up fell to 30.2%.
A more likely factor for the survival of the 17 patients who were alive at the 1-year follow-up was their overall health relative to the rest of the study group. Overall health may indicate survival independent of sorafenib. The group of 17 who survived at the 1-year follow-up reflected a population that was different from the rest of the study population. The subset was generally healthier with better ECOG-PS scores and Child-Pugh classes, was not followed by Palliative Care Services, and had a mean AFP level under the threshold for diagnosis of HCC in patients who present with hepatic lesions and elevated AFP.14 This subset’s MPR, a surrogate marker for adherence, was less than the accepted threshold in clinical practice for oral medications.15Evaluating the patient’s dose regimen was expected to reveal a relationship between dosing and clinical outcomes, such as low survival rates with low doses or more AEs with high doses. However, the authors were not able to establish this link. In fact, the median time to discontinuation of 3.4 months for the study group, or duration of treatment, was much shorter than the median OS of 5.9 months.
These findings were consistent with Cabibbo and colleagues, who conducted a meta-analysis of survival rates for untreated patients and found that impaired performance status and Child-Pugh class B or C were independently associated with shorter survival.16 The SHARP study and Cheng and colleagues also attempted to exclude patients who were not Child-Pugh class A in their studies, which suggests a negligible correlation between sorafenib and survival time and a close relationship between baseline clinical status and survival.
The authors determined that prior treatment, including locoregional therapy, was not a factor in predicting survival. This observation is confirmed by the results of a phase 3 study that looked at sorafenib as adjuvant treatment for patients who had no detectable disease after surgical resection or local ablation.17 The trial did not meet its primary endpoint of improved recurrence-free survival. However, the authors observed in this study that 4 patients who underwent resection of the liver before sorafenib had a mean OS of 2.9 years. One patient, who was alive at the time of the study conclusion, received only 22 days of sorafenib treatment and survived for 4.9 years after sorafenib discontinuation. Patients who received concurrent or postsorafenib treatment had higher survival rates.
The cost of treatment in this study was found to be $8,535.87 per year lived. Although formal quality of life assessments were not captured, medication was discontinued at the first sign of disease progression or AE as determined by the provider or patient. When the cost of treatment was adjusted to account for median OS time and VA drug acquisition costs, estimated at average wholesale price minus 40%, the cost of treatment was within the threshold of $50,000-$100,000 per quality-adjusted life-year.7,18Of the 42 patients in this study, 28.6% discontinued therapy due to AEs, compared with 32% observed in the SHARP study. Common GI, dermatologic, and CNS AEs were comparable between the 2 studies. Serious AEs included intracranial hemorrhage, GI hemorrhage, GI perforation, acute liver failure, and acute renal failure; 3 of these events led to death. About 12% of patients experienced bleeding, regardless of severity, compared with the 18% seen in SHARP, despite no prior history of hemorrhage or GI perforation.5 The authors did not find any clinical factors at baseline that predisposed patients to AEs. It was also difficult to distinguish between drug-related AEs and general disease progression.
Although the authors did not find a relationship between dose or dose adjustments and the number or frequency of AEs, there were serious adverse outcomes in this study that were also rare complications observed in SHARP. The decision to start sorafenib should not be taken lightly.
Related: Diagnostic Dilemma of Hepatocellular Carcinoma Presenting as Hepatic Angiomyolipoma
Limitations
This retrospective review had several limitations. In SHARP and other large, multicenter trials, patients were continued on therapy until they experienced both symptomatic and radiographic progression. In this study, patients were discontinued at the first sign of progression, either symptomatic or radiographic or both. Had all patients remained on therapy until symptomatic and radiographic signs of progression were observed, there could have been a better correlation between duration of treatment and OS, symptomatic progression, or radiographic progression. The authors acknowledge, however, that there is diminishing benefit of administering chemotherapy when there are known and potentially serious AEs.
The data for this study were limited due to a small sample size, and it was not powered to evaluate for statistically significant characteristics between the patients who survived at the 1-year follow-up and the patients who did not survive at the 1-year follow-up. This information would be useful to identify potential prognostic factors and guide providers in sorafenib management. Finally, a long-term safety profile could not be established, as patients were evaluated for a 1-year period.
Ultimately, HCC is a multifactorial disease, and it is difficult to account for all potential confounding factors. Additional research, including studies comparing sunitinib or a control group to sorafenib, may provide further insight.
Conclusions
In light of these results, the authors believe that sorafenib may be considered for veterans with unresectable HCC and who are contraindicated for alternative treatments. One-year survival rates were similar to those seen in previous studies. However, there was no clear association between the duration of treatment and OS, and although the medication was well tolerated, there were also serious AEs. It is prudent to continually assess the need for therapy throughout the treatment period.
Pharmacists have a critical role in care for oncology patients, from the integration of certified clinical pharmacist practitioners into hematology-oncology clinics to patient monitoring through oral oncology pharmacy programs.19,20 These programs have been shown to improve patient outcomes and decrease overall health care use and may benefit the veteran population.
In this study, a veteran population achieved a survival rate at the 1-year follow-up similar to that found in SHARP: 40.5% vs 44%. However, OS was markedly shorter: 5.9 months vs 10.7 months. Patients with minimal impact from HCC, such as mild hepatic impairment and high functional status, demonstrated higher survival rates at the 1-year follow-up than did those with significant compromise. Thirty-five patients experienced ≥1 AE, most observed within the first 90 days of treatment, and for 3 patients, sorafenib could not be ruled out as the cause of death.
Sorafenib remains a viable therapeutic option for veterans with advanced HCC. However, it is uncertain how much benefit sorafenib affords to the veteran population, especially with the associated risks.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
2. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.
3. Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(suppl 4):14-22.
4. Nexavar [package insert]. Emeryville, CA: Bayer HealthCare Pharmaceuticals, Inc; 2009.
5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. Version 2. 2015. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed October 13, 2015.
6. Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
7. Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25(11):1739-1746.
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
9. U.S. Department of Veterans Affairs, Veterans Health Administration. National Viral Hepatitis Program. VHA Directive 1300.01. U.S. Department of Veterans Affairs Website. http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=1586. Updated February 22, 2013. Accessed October 13, 2015.
10. Patterson CJ. Best practices in specialty pharmacy management. J Manag Care Pharm. 2013;19(1):42-48.
11. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34.
12. Iavarone M, Cabibbo G, Piscaglia F, et al; SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54(6):2055-2063.
13. Di Fiore F, Rigal O, Ménager C, Michel P, Pfister C. Severe clinical toxicities are correlated with survival in patients with advanced renal cell carcinoma treated with sunitinib and sorafenib. Br J Cancer. 2011;105(12):1811-1813.
14. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
15. Blandford L, Dans PE, Ober JD, Wheelock C. Analyzing variations in medication compliance related to individual drug, drug class, and prescribing physician. J Managed Care Pharm. 1999;5(1):47-51.
16. Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51(4):1274-1283.
17. Bayer HealthCare. Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma (STORM). ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT00692770. Updated May 28, 2015. Accessed October 21, 2015.
18. Academy of Managed Care Pharmacy. AMCP Guide to Pharmaceutical Payment Methods, 2009 Update (Version 2.0). J Manag Care Pharm. 2009;15(suppl 6-a):S3-S57.
19. Valgus JM, Faso A, Gregory KM, et al. Integration of a clinical pharmacist into the hematology-oncology clinics at an academic medical center. Am J Health Syst Pharm. 2011;68(7):613-619.
20. Tschida SJ, Aslam S, Lal LS, et al. Outcomes of a specialty pharmacy program for oral oncology medications. Am J Pharm Benefits. 2012;4(4):165-174.
In 2015, more than 35,660 new cases of liver cancer and 24,550 liver cancer-related deaths are expected to occur in the U.S. About 80% of these cases will consist of hepatocellular carcinoma, (HCC).1 The incidence of HCC varies throughout the world: Incidence is as low as 5 in 100,000 individuals in North America and ranges up to > 20 in 100,000 individuals in sub-Saharan Africa and Eastern Asia.2 Nearly half of all cases of HCC are associated with hepatitis B virus (HBV), and another 25% are associated with hepatitis C virus (HCV). Other risk factors for developing HCC include alcoholic liver disease, nonalcoholic steatohepatitis, flatoxin-contaminated food, diabetes, and obesity.3
Therapeutic options for advanced HCC are limited. The FDA approved sorafenib in 2008 for the treatment of unresectable HCC.4 According to the American Association for the Study of Liver Diseases (AASLD) and the Barcelona Clinic Liver Cancer (BCLC) staging system, patients with Stage C liver cancer may undergo a trial of sorafenib.4 National Comprehensive Cancer Network (NCCN) clinical guidelines for hepatobiliary cancers reserve sorafenib for patients with inoperable tumors, metastatic disease, or extensive liver tumor burden.5 Sorafenib is shown to inhibit multiple intracellular and cell surface kinases. Several of these kinases are thought to be involved in tumor cell signaling, angiogenesis, and apoptosis.4 In the Sorafenib HCC Assessment Randomized Protocol (SHARP) trial, median overall survival (OS) was 10.7 months in the sorafenib group and 7.9 months in the placebo group.6 The predicted survival rates at 1 year were 44% in the sorafenib group and 33% in the placebo group.6The economic impact of oral chemotherapy on health care cannot be discounted. At about $50,000 to $100,000 per quality- adjusted life-year, the incremental cost-effectiveness ratio (ICER) of sorafenib over placebo was $62,473 per quality-adjusted life-year in 2007.7The purpose of this retrospective chart review was to evaluate sorafenib for efficacy and safety in a veteran population. Veterans have poorer health and more medical conditions compared with nonveterans.8 Furthermore, in the VHA, about 170,000 veterans have HCV.9 The rate of progression from HCV to HCC is about 3% to 5% annually. More than half of those diagnosed with HCC are late stage, and unfortunately, the 5-year OS rate for patients with liver cancer is 9% and 4% for those patients who are diagnosed at regional and distant stages of the disease.1 As the practice of oncology grows, it is necessary for pharmacists to be involved in the selection of chemotherapeutic agents in order to provide optimal pharmaceutical care.10
Related: VIDEO: NAFLD increasingly causing U.S. hepatocellular carcinoma
Methods
A retrospective chart review was conducted to identify patients who were prescribed sorafenib from November 1, 2007, to September 30, 2011, at the VA Greater Los Angeles Healthcare System (VAGLAHS). Inclusion criteria included patients who had a diagnosis of advanced HCC, who were initiated and managed by a VAGLAHS provider and who were eligible for a 1-year safety evaluation period. The study was approved by the VAGLAHS institutional review board.
Baseline demographic, clinical, laboratory, and medication data were collected. Demographic, clinical, laboratory, and medication data were obtained from CPRS (Computerized Patient Record System) and VistA (Veterans Health Information Systems and Technology Architecture). Data were collected on secured servers and saved on encrypted files. The master list was destroyed once the records control schedule was finalized. No identifiers were collected on the data collection sheet.
Standard practice at VAGLAHS is to monitor European Cooperative Oncology Group Performance Status (ECOG-PS), Child-Pugh class, and alpha-fetoprotein (AFP) at initiation and every 3 months and to obtain laboratory data at initiation and every month before each medication refill. Patients were seen in the Oncology Clinic periodically at the provider’s discretion. The time of drug discontinuation and the reason for drug discontinuation were recorded. Time of death at any point was collected to measure OS.
It was determined that a total sample size of 42 patients would be insufficient to achieve 80% power to demonstrate any hypothesized effects. However, the Fisher exact test was used to calculate P values for simple comparison. Patient demographics and clinical characteristics were reported as total numbers and frequencies when applicable. Survival rate was measured from the time of sorafenib initiation to 1 year after therapy initiation. Overall survival was measured from the time of sorafenib initiation to time of death. Duration of therapy was measured from the time of sorafenib initiation to time of discontinuation, either by provider or by patient.
Results
There were 83 patients who were prescribed sorafenib between November 1, 2007, and September 30, 2011. Of the 83 patients, 27 patients were ineligible for a 1-year follow-up period, 9 patients were diagnosed with non-HCC, 3 were initiated or managed by providers outside the institution, and 2 were not started on therapy. In all, 42 patients met inclusion criteria and had received at least 1 dose of sorafenib. The primary etiologies for HCC were history of alcohol abuse, HCV, and HBV. The primary risk factors were obesity, smoking, and diabetes. Many patients presented with multiple etiologies and risk factors. Ten patients (23.8%) had moderate-to-severe hepatic impairment (Child-Pugh class B or C). Baseline characteristics of these patients are listed in Table 1.
Efficacy
The median OS was 5.9 months and ranged from 21 days to 60 months. There were 17 patients who survived at the 1-year follow-up, including 1 patient who survived 363 days after treatment initiation, yielding an OS rate of 40.5%. Table 2 presents 1-year survival rates with respect to select baseline data. Baseline factors found to be negligible were age, smoking, alcohol abuse, obesity, presence of HCV, medication possession ratio (MPR), prior treatment, macrovascular invasion, and AFP. Neither initial dose regimen, final dose regimen achieved, or average dose correlated with the survival rate at the 1-year follow-up.
Factors possibly associated with a higher probability of survival were baseline ECOG-PS score and baseline Child-Pugh class (Table 2). Patients with an ECOG-PS score of 0 or 1 had a higher survival rate at 1 year than did patients with an ECOG-PS score of ≥ 2 (50% vs 0%, respectively; P = .113). Patients with Child-Pugh class B or C had a lower survival rate at 1 year than did patients with Child-Pugh class A (51% vs 10%, respectively; P = .028). Other indicators were size of largest hepatic lesion ≤ 5 cm, total bilirubin ≤ 2 mg/dL, concurrent treatment, almost exclusively embolization, and treatment after sorafenib discontinuation, such as another oral chemotherapeutic agent or embolization.
The 17 patients who survived at 1 year were reviewed to see if they shared characteristics that indicated a higher probability of survival. The figure shows the baseline ECOG-PS score and the Child-Pugh class the patients who did and did not survive at the 1-year follow-up. In the first group, all patient possessed an ECOG-PS score of 0 or 1, and only 1 patient presented with Child-Pugh class B or C. In contrast, in the group who did not survive at the 1-year follow-up, there were 4 patients with ECOG-PS scores of > 1 and 9 patients who presented with Child-Pugh class B or C. The mean AFP level of this group was < 200 µg/mL, and only 4 patients were followed by Palliative Care Services. The average normalized MPR of this group was 71.9% compared with 85.3% for those who did not survive at the 1-year follow-up.
In patients who experienced at least 1 adverse event (AE), 16 survived, whereas only 1 who did not experience an AE survived (45.7% vs 14.3%, respectively; P = .210). Thirteen patients who experienced ≥ 3 AEs survived at 1 year; and only 3 patients who reported < 3 AEs survived at 1 year (61.9% vs 14.0%, respectively; P = .011). However, when the number of AEs was normalized to duration of treatment per patient, the median frequency of AEs for all patients was 0.61 AEs per month treated. The difference in survival rates grew smaller and less significant between patients who had a frequency of AEs lower than the median compared with those with a higher ratio (52.4% vs 28.6%, respectively; P = .208). Patients affected by AEs in the first 30 days and 90 days of treatment had a survival rate at the 1-year follow-up of 42.4% and 30.2%, respectively. Patients who experienced dermatologic AEs had a higher survival rate than those who did not have dermatologic AEs (60.0% vs 29.6%, respectively; P = .099). This correlation was not found with 2 other classes of AEs, gastrointestinal (50.0% vs 27.8%; P = .208) or neurologic (64.0% vs 41.2%; P = .209).
The median overall time to discontinuation was 3.4 months. The main reasons cited for discontinuing sorafenib at 1 year included symptomatic progression (52.4%), radiographic progression (23.8%), severe AEs (16.7%), and mild-to-moderate AEs (11.9%). There was overlap, as 15 patients discontinued treatment for multiple reasons. For the 22 patients who discontinued medication due to symptomatic progression at 1 year, the median time to discontinuation was 3.8 months. For the 10 patients who discontinued medication due to radiographic progression at 1 year, median time to discontinuation was 5.6 months. Seven patients (16.7%) were still on therapy at 1 year.The study considered the impact of potential dose adjustments on survival rate and safety. The authors compared patients’ prescribed dose with the recommended dose based on the package insert and monthly laboratory values if recorded. The prescribed dose was recorded as appropriate dose, below dose, above dose, or indeterminate due to the lack of current laboratory values. Patients who survived at the 1-year follow-up had a composition of 26%, 21%, 10%, and 43%, respectively. These results were similar to those of patients who did not survive at the 1-year follow-up, 29%, 12%, 30%, and 29%, respectively.
Based on medication refill history and VA acquisition cost, the total prescription drug cost of treating 42 patients with sorafenib was $388,370.40. The total number of days survived for these patients was 16,607 days, which equates to $8,535.87 per year lived.
Safety
Of the 42 patients, 35 patients experienced ≥ 1 AE for a total of 122 AEs reported. The median number of AEs per patient was 2.5. The median time to the first AE was 21 days and ranged from 3 to 244 days. In the first 30 days of treatment, 23 patients (54.7%) reported 47 AEs (39.5%). In the first 90 days of treatment, 33 patients (78.6%) reported 88 AEs (73.9%). Common AEs in both instances were diarrhea, fatigue, erythematous plantar-palmar rash, and nausea (Table 3).
The predominant classes of AEs were GI (39.3%), dermatologic (18.9%), and neurologic (15.6%). Erythematous palmar-plantar rash, also known as hand-foot syndrome, has been noted as a potential dose-limiting sorafenib AE if the rash is recurrent or severe. One patient experienced recurrent grade-2 rashes, and sorafenib was immediately discontinued after an attempt to lower the dose. There were 8 patients who reported serious AEs, and 5 were hospitalized. One patient continued therapy despite GI hemorrhage. The other 4 patients discontinued therapy on hospitalization and were seen for intracranial hemorrhage, GI perforation, acute renal failure, and acute liver failure. In the first 3 cases, sorafenib could not be ruled out as the primary cause of death. None of these patients presented with comorbidities, such as hypertension, which predisposed them to AEs.
Overall, 38 patients ended therapy at the recommended regimen of 400 mg twice daily, and the average total daily dose was 619 mg, just below 80% of the recommended daily dose. Reasons for not achieving 400 mg twice daily included slow titration, AEs, and dose adjustments for compromised renal and hepatic function such as dialysis. Patients who had an ECOG-PS score of 0 or 1 or Child-Pugh class A reported ≥ 3 AEs, but when normalized to duration of treatment, no difference was observed. No correlations were found for average dose, creatinine clearance, aspartate aminotransferase, platelets, total bilirubin, or weight and number or frequency of AEs.
In regard to potential dose adjustments, the doses (400 mg twice daily, 600 mg daily [400 mg + 200 mg in 2 doses], 200 mg twice daily, and 200 mg daily) did not correlate well with AEs. Patients who had < 3 AEs presented with the breakdown 23%, 16%, 22%, and 38%, similar to patients who had ≥ 3 AEs—30%, 19%, 14%, and 37%. Likewise, patients who had a frequency of AEs lower than the median presented with the breakdown 22%, 22%, 15%, and 40% compared with patients who had more AEs than the median—37%, 9%, 23%, and 31%.
Related: Hepatocellular Carcinoma: To Biopsy or Not?
Discussion
Sorafenib is the only oral oncology medication approved by the FDA for treatment of unresectable HCC.3 Prior to sorafenib, the AASLD recommendation was supportive care for patients presenting with BCLC-Stage C liver cancer. However, guidelines changed when SHARP showed that sorafenib provided a survival benefit with a tolerable AE profile. The survival benefit of sorafenib has been replicated in a few large, multicenter trials. In Asia, Cheng and colleagues saw improved median OS of 6.5 months for sorafenib compared with 4.2 months with placebo, and in Italy, Iavarone and colleagues showed a median OS of 10.5 months without a placebo comparator.11,12
In the veteran population for this study, the OS rate of 40.5% was similar to the rate reported in the SHARP study, although the patients’ median OS fell short of the time described in SHARP and other trials. The medical complexities involved in treating veterans may explain this difference. The veteran population is heterogeneous with diverse ethnic backgrounds, several comorbidities, and varying degrees of organ dysfunction. The authors compared survival rates of different subgroups to test the hypothesis that the probability of survival while on therapy should not depend on demographics or medical history. However, in this study, patients with minimal impact from HCC, such as mild hepatic impairment and high-functional status, demonstrated higher survival rates at 1-year follow-up than did those without significant compromise.Although the high prevalence of HCV and alcohol abuse in the veteran population has resulted in a high incidence of hepatic dysfunction, this study suggests that these factors are independent of survival if liver function or integrity has not been compromised.9
Some researchers have hypothesized that clinical toxicities from tyrosine kinase inhibitors may correlate with survival.13 The authors noticed that the presentation of dermatologic AEs may reflect improved survival. In this study, patients who experienced ≥ 1 AE and ≥ 3 AEs had survival rates at the 1-year follow-up of 45.7% and 61.9%, respectively. Moreover, patients affected by AEs in the first 90 days of treatment had a survival rate at the 1-year follow-up of 42.4%.
Caution is advised when drawing conclusions from the number of AEs or when they appear, because this may falsely favor correlation. Patients who survive longer have additional time to report an AE. Therefore, the authors also looked at the ratio of AEs over time per patient to consider the number of AEs per duration of treatment and saw that there was little difference in survival rate in this regard. When considering patients affected by AEs only in the first 30 days of treatment, the survival rate at the 1-year follow-up fell to 30.2%.
A more likely factor for the survival of the 17 patients who were alive at the 1-year follow-up was their overall health relative to the rest of the study group. Overall health may indicate survival independent of sorafenib. The group of 17 who survived at the 1-year follow-up reflected a population that was different from the rest of the study population. The subset was generally healthier with better ECOG-PS scores and Child-Pugh classes, was not followed by Palliative Care Services, and had a mean AFP level under the threshold for diagnosis of HCC in patients who present with hepatic lesions and elevated AFP.14 This subset’s MPR, a surrogate marker for adherence, was less than the accepted threshold in clinical practice for oral medications.15Evaluating the patient’s dose regimen was expected to reveal a relationship between dosing and clinical outcomes, such as low survival rates with low doses or more AEs with high doses. However, the authors were not able to establish this link. In fact, the median time to discontinuation of 3.4 months for the study group, or duration of treatment, was much shorter than the median OS of 5.9 months.
These findings were consistent with Cabibbo and colleagues, who conducted a meta-analysis of survival rates for untreated patients and found that impaired performance status and Child-Pugh class B or C were independently associated with shorter survival.16 The SHARP study and Cheng and colleagues also attempted to exclude patients who were not Child-Pugh class A in their studies, which suggests a negligible correlation between sorafenib and survival time and a close relationship between baseline clinical status and survival.
The authors determined that prior treatment, including locoregional therapy, was not a factor in predicting survival. This observation is confirmed by the results of a phase 3 study that looked at sorafenib as adjuvant treatment for patients who had no detectable disease after surgical resection or local ablation.17 The trial did not meet its primary endpoint of improved recurrence-free survival. However, the authors observed in this study that 4 patients who underwent resection of the liver before sorafenib had a mean OS of 2.9 years. One patient, who was alive at the time of the study conclusion, received only 22 days of sorafenib treatment and survived for 4.9 years after sorafenib discontinuation. Patients who received concurrent or postsorafenib treatment had higher survival rates.
The cost of treatment in this study was found to be $8,535.87 per year lived. Although formal quality of life assessments were not captured, medication was discontinued at the first sign of disease progression or AE as determined by the provider or patient. When the cost of treatment was adjusted to account for median OS time and VA drug acquisition costs, estimated at average wholesale price minus 40%, the cost of treatment was within the threshold of $50,000-$100,000 per quality-adjusted life-year.7,18Of the 42 patients in this study, 28.6% discontinued therapy due to AEs, compared with 32% observed in the SHARP study. Common GI, dermatologic, and CNS AEs were comparable between the 2 studies. Serious AEs included intracranial hemorrhage, GI hemorrhage, GI perforation, acute liver failure, and acute renal failure; 3 of these events led to death. About 12% of patients experienced bleeding, regardless of severity, compared with the 18% seen in SHARP, despite no prior history of hemorrhage or GI perforation.5 The authors did not find any clinical factors at baseline that predisposed patients to AEs. It was also difficult to distinguish between drug-related AEs and general disease progression.
Although the authors did not find a relationship between dose or dose adjustments and the number or frequency of AEs, there were serious adverse outcomes in this study that were also rare complications observed in SHARP. The decision to start sorafenib should not be taken lightly.
Related: Diagnostic Dilemma of Hepatocellular Carcinoma Presenting as Hepatic Angiomyolipoma
Limitations
This retrospective review had several limitations. In SHARP and other large, multicenter trials, patients were continued on therapy until they experienced both symptomatic and radiographic progression. In this study, patients were discontinued at the first sign of progression, either symptomatic or radiographic or both. Had all patients remained on therapy until symptomatic and radiographic signs of progression were observed, there could have been a better correlation between duration of treatment and OS, symptomatic progression, or radiographic progression. The authors acknowledge, however, that there is diminishing benefit of administering chemotherapy when there are known and potentially serious AEs.
The data for this study were limited due to a small sample size, and it was not powered to evaluate for statistically significant characteristics between the patients who survived at the 1-year follow-up and the patients who did not survive at the 1-year follow-up. This information would be useful to identify potential prognostic factors and guide providers in sorafenib management. Finally, a long-term safety profile could not be established, as patients were evaluated for a 1-year period.
Ultimately, HCC is a multifactorial disease, and it is difficult to account for all potential confounding factors. Additional research, including studies comparing sunitinib or a control group to sorafenib, may provide further insight.
Conclusions
In light of these results, the authors believe that sorafenib may be considered for veterans with unresectable HCC and who are contraindicated for alternative treatments. One-year survival rates were similar to those seen in previous studies. However, there was no clear association between the duration of treatment and OS, and although the medication was well tolerated, there were also serious AEs. It is prudent to continually assess the need for therapy throughout the treatment period.
Pharmacists have a critical role in care for oncology patients, from the integration of certified clinical pharmacist practitioners into hematology-oncology clinics to patient monitoring through oral oncology pharmacy programs.19,20 These programs have been shown to improve patient outcomes and decrease overall health care use and may benefit the veteran population.
In this study, a veteran population achieved a survival rate at the 1-year follow-up similar to that found in SHARP: 40.5% vs 44%. However, OS was markedly shorter: 5.9 months vs 10.7 months. Patients with minimal impact from HCC, such as mild hepatic impairment and high functional status, demonstrated higher survival rates at the 1-year follow-up than did those with significant compromise. Thirty-five patients experienced ≥1 AE, most observed within the first 90 days of treatment, and for 3 patients, sorafenib could not be ruled out as the cause of death.
Sorafenib remains a viable therapeutic option for veterans with advanced HCC. However, it is uncertain how much benefit sorafenib affords to the veteran population, especially with the associated risks.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
In 2015, more than 35,660 new cases of liver cancer and 24,550 liver cancer-related deaths are expected to occur in the U.S. About 80% of these cases will consist of hepatocellular carcinoma, (HCC).1 The incidence of HCC varies throughout the world: Incidence is as low as 5 in 100,000 individuals in North America and ranges up to > 20 in 100,000 individuals in sub-Saharan Africa and Eastern Asia.2 Nearly half of all cases of HCC are associated with hepatitis B virus (HBV), and another 25% are associated with hepatitis C virus (HCV). Other risk factors for developing HCC include alcoholic liver disease, nonalcoholic steatohepatitis, flatoxin-contaminated food, diabetes, and obesity.3
Therapeutic options for advanced HCC are limited. The FDA approved sorafenib in 2008 for the treatment of unresectable HCC.4 According to the American Association for the Study of Liver Diseases (AASLD) and the Barcelona Clinic Liver Cancer (BCLC) staging system, patients with Stage C liver cancer may undergo a trial of sorafenib.4 National Comprehensive Cancer Network (NCCN) clinical guidelines for hepatobiliary cancers reserve sorafenib for patients with inoperable tumors, metastatic disease, or extensive liver tumor burden.5 Sorafenib is shown to inhibit multiple intracellular and cell surface kinases. Several of these kinases are thought to be involved in tumor cell signaling, angiogenesis, and apoptosis.4 In the Sorafenib HCC Assessment Randomized Protocol (SHARP) trial, median overall survival (OS) was 10.7 months in the sorafenib group and 7.9 months in the placebo group.6 The predicted survival rates at 1 year were 44% in the sorafenib group and 33% in the placebo group.6The economic impact of oral chemotherapy on health care cannot be discounted. At about $50,000 to $100,000 per quality- adjusted life-year, the incremental cost-effectiveness ratio (ICER) of sorafenib over placebo was $62,473 per quality-adjusted life-year in 2007.7The purpose of this retrospective chart review was to evaluate sorafenib for efficacy and safety in a veteran population. Veterans have poorer health and more medical conditions compared with nonveterans.8 Furthermore, in the VHA, about 170,000 veterans have HCV.9 The rate of progression from HCV to HCC is about 3% to 5% annually. More than half of those diagnosed with HCC are late stage, and unfortunately, the 5-year OS rate for patients with liver cancer is 9% and 4% for those patients who are diagnosed at regional and distant stages of the disease.1 As the practice of oncology grows, it is necessary for pharmacists to be involved in the selection of chemotherapeutic agents in order to provide optimal pharmaceutical care.10
Related: VIDEO: NAFLD increasingly causing U.S. hepatocellular carcinoma
Methods
A retrospective chart review was conducted to identify patients who were prescribed sorafenib from November 1, 2007, to September 30, 2011, at the VA Greater Los Angeles Healthcare System (VAGLAHS). Inclusion criteria included patients who had a diagnosis of advanced HCC, who were initiated and managed by a VAGLAHS provider and who were eligible for a 1-year safety evaluation period. The study was approved by the VAGLAHS institutional review board.
Baseline demographic, clinical, laboratory, and medication data were collected. Demographic, clinical, laboratory, and medication data were obtained from CPRS (Computerized Patient Record System) and VistA (Veterans Health Information Systems and Technology Architecture). Data were collected on secured servers and saved on encrypted files. The master list was destroyed once the records control schedule was finalized. No identifiers were collected on the data collection sheet.
Standard practice at VAGLAHS is to monitor European Cooperative Oncology Group Performance Status (ECOG-PS), Child-Pugh class, and alpha-fetoprotein (AFP) at initiation and every 3 months and to obtain laboratory data at initiation and every month before each medication refill. Patients were seen in the Oncology Clinic periodically at the provider’s discretion. The time of drug discontinuation and the reason for drug discontinuation were recorded. Time of death at any point was collected to measure OS.
It was determined that a total sample size of 42 patients would be insufficient to achieve 80% power to demonstrate any hypothesized effects. However, the Fisher exact test was used to calculate P values for simple comparison. Patient demographics and clinical characteristics were reported as total numbers and frequencies when applicable. Survival rate was measured from the time of sorafenib initiation to 1 year after therapy initiation. Overall survival was measured from the time of sorafenib initiation to time of death. Duration of therapy was measured from the time of sorafenib initiation to time of discontinuation, either by provider or by patient.
Results
There were 83 patients who were prescribed sorafenib between November 1, 2007, and September 30, 2011. Of the 83 patients, 27 patients were ineligible for a 1-year follow-up period, 9 patients were diagnosed with non-HCC, 3 were initiated or managed by providers outside the institution, and 2 were not started on therapy. In all, 42 patients met inclusion criteria and had received at least 1 dose of sorafenib. The primary etiologies for HCC were history of alcohol abuse, HCV, and HBV. The primary risk factors were obesity, smoking, and diabetes. Many patients presented with multiple etiologies and risk factors. Ten patients (23.8%) had moderate-to-severe hepatic impairment (Child-Pugh class B or C). Baseline characteristics of these patients are listed in Table 1.
Efficacy
The median OS was 5.9 months and ranged from 21 days to 60 months. There were 17 patients who survived at the 1-year follow-up, including 1 patient who survived 363 days after treatment initiation, yielding an OS rate of 40.5%. Table 2 presents 1-year survival rates with respect to select baseline data. Baseline factors found to be negligible were age, smoking, alcohol abuse, obesity, presence of HCV, medication possession ratio (MPR), prior treatment, macrovascular invasion, and AFP. Neither initial dose regimen, final dose regimen achieved, or average dose correlated with the survival rate at the 1-year follow-up.
Factors possibly associated with a higher probability of survival were baseline ECOG-PS score and baseline Child-Pugh class (Table 2). Patients with an ECOG-PS score of 0 or 1 had a higher survival rate at 1 year than did patients with an ECOG-PS score of ≥ 2 (50% vs 0%, respectively; P = .113). Patients with Child-Pugh class B or C had a lower survival rate at 1 year than did patients with Child-Pugh class A (51% vs 10%, respectively; P = .028). Other indicators were size of largest hepatic lesion ≤ 5 cm, total bilirubin ≤ 2 mg/dL, concurrent treatment, almost exclusively embolization, and treatment after sorafenib discontinuation, such as another oral chemotherapeutic agent or embolization.
The 17 patients who survived at 1 year were reviewed to see if they shared characteristics that indicated a higher probability of survival. The figure shows the baseline ECOG-PS score and the Child-Pugh class the patients who did and did not survive at the 1-year follow-up. In the first group, all patient possessed an ECOG-PS score of 0 or 1, and only 1 patient presented with Child-Pugh class B or C. In contrast, in the group who did not survive at the 1-year follow-up, there were 4 patients with ECOG-PS scores of > 1 and 9 patients who presented with Child-Pugh class B or C. The mean AFP level of this group was < 200 µg/mL, and only 4 patients were followed by Palliative Care Services. The average normalized MPR of this group was 71.9% compared with 85.3% for those who did not survive at the 1-year follow-up.
In patients who experienced at least 1 adverse event (AE), 16 survived, whereas only 1 who did not experience an AE survived (45.7% vs 14.3%, respectively; P = .210). Thirteen patients who experienced ≥ 3 AEs survived at 1 year; and only 3 patients who reported < 3 AEs survived at 1 year (61.9% vs 14.0%, respectively; P = .011). However, when the number of AEs was normalized to duration of treatment per patient, the median frequency of AEs for all patients was 0.61 AEs per month treated. The difference in survival rates grew smaller and less significant between patients who had a frequency of AEs lower than the median compared with those with a higher ratio (52.4% vs 28.6%, respectively; P = .208). Patients affected by AEs in the first 30 days and 90 days of treatment had a survival rate at the 1-year follow-up of 42.4% and 30.2%, respectively. Patients who experienced dermatologic AEs had a higher survival rate than those who did not have dermatologic AEs (60.0% vs 29.6%, respectively; P = .099). This correlation was not found with 2 other classes of AEs, gastrointestinal (50.0% vs 27.8%; P = .208) or neurologic (64.0% vs 41.2%; P = .209).
The median overall time to discontinuation was 3.4 months. The main reasons cited for discontinuing sorafenib at 1 year included symptomatic progression (52.4%), radiographic progression (23.8%), severe AEs (16.7%), and mild-to-moderate AEs (11.9%). There was overlap, as 15 patients discontinued treatment for multiple reasons. For the 22 patients who discontinued medication due to symptomatic progression at 1 year, the median time to discontinuation was 3.8 months. For the 10 patients who discontinued medication due to radiographic progression at 1 year, median time to discontinuation was 5.6 months. Seven patients (16.7%) were still on therapy at 1 year.The study considered the impact of potential dose adjustments on survival rate and safety. The authors compared patients’ prescribed dose with the recommended dose based on the package insert and monthly laboratory values if recorded. The prescribed dose was recorded as appropriate dose, below dose, above dose, or indeterminate due to the lack of current laboratory values. Patients who survived at the 1-year follow-up had a composition of 26%, 21%, 10%, and 43%, respectively. These results were similar to those of patients who did not survive at the 1-year follow-up, 29%, 12%, 30%, and 29%, respectively.
Based on medication refill history and VA acquisition cost, the total prescription drug cost of treating 42 patients with sorafenib was $388,370.40. The total number of days survived for these patients was 16,607 days, which equates to $8,535.87 per year lived.
Safety
Of the 42 patients, 35 patients experienced ≥ 1 AE for a total of 122 AEs reported. The median number of AEs per patient was 2.5. The median time to the first AE was 21 days and ranged from 3 to 244 days. In the first 30 days of treatment, 23 patients (54.7%) reported 47 AEs (39.5%). In the first 90 days of treatment, 33 patients (78.6%) reported 88 AEs (73.9%). Common AEs in both instances were diarrhea, fatigue, erythematous plantar-palmar rash, and nausea (Table 3).
The predominant classes of AEs were GI (39.3%), dermatologic (18.9%), and neurologic (15.6%). Erythematous palmar-plantar rash, also known as hand-foot syndrome, has been noted as a potential dose-limiting sorafenib AE if the rash is recurrent or severe. One patient experienced recurrent grade-2 rashes, and sorafenib was immediately discontinued after an attempt to lower the dose. There were 8 patients who reported serious AEs, and 5 were hospitalized. One patient continued therapy despite GI hemorrhage. The other 4 patients discontinued therapy on hospitalization and were seen for intracranial hemorrhage, GI perforation, acute renal failure, and acute liver failure. In the first 3 cases, sorafenib could not be ruled out as the primary cause of death. None of these patients presented with comorbidities, such as hypertension, which predisposed them to AEs.
Overall, 38 patients ended therapy at the recommended regimen of 400 mg twice daily, and the average total daily dose was 619 mg, just below 80% of the recommended daily dose. Reasons for not achieving 400 mg twice daily included slow titration, AEs, and dose adjustments for compromised renal and hepatic function such as dialysis. Patients who had an ECOG-PS score of 0 or 1 or Child-Pugh class A reported ≥ 3 AEs, but when normalized to duration of treatment, no difference was observed. No correlations were found for average dose, creatinine clearance, aspartate aminotransferase, platelets, total bilirubin, or weight and number or frequency of AEs.
In regard to potential dose adjustments, the doses (400 mg twice daily, 600 mg daily [400 mg + 200 mg in 2 doses], 200 mg twice daily, and 200 mg daily) did not correlate well with AEs. Patients who had < 3 AEs presented with the breakdown 23%, 16%, 22%, and 38%, similar to patients who had ≥ 3 AEs—30%, 19%, 14%, and 37%. Likewise, patients who had a frequency of AEs lower than the median presented with the breakdown 22%, 22%, 15%, and 40% compared with patients who had more AEs than the median—37%, 9%, 23%, and 31%.
Related: Hepatocellular Carcinoma: To Biopsy or Not?
Discussion
Sorafenib is the only oral oncology medication approved by the FDA for treatment of unresectable HCC.3 Prior to sorafenib, the AASLD recommendation was supportive care for patients presenting with BCLC-Stage C liver cancer. However, guidelines changed when SHARP showed that sorafenib provided a survival benefit with a tolerable AE profile. The survival benefit of sorafenib has been replicated in a few large, multicenter trials. In Asia, Cheng and colleagues saw improved median OS of 6.5 months for sorafenib compared with 4.2 months with placebo, and in Italy, Iavarone and colleagues showed a median OS of 10.5 months without a placebo comparator.11,12
In the veteran population for this study, the OS rate of 40.5% was similar to the rate reported in the SHARP study, although the patients’ median OS fell short of the time described in SHARP and other trials. The medical complexities involved in treating veterans may explain this difference. The veteran population is heterogeneous with diverse ethnic backgrounds, several comorbidities, and varying degrees of organ dysfunction. The authors compared survival rates of different subgroups to test the hypothesis that the probability of survival while on therapy should not depend on demographics or medical history. However, in this study, patients with minimal impact from HCC, such as mild hepatic impairment and high-functional status, demonstrated higher survival rates at 1-year follow-up than did those without significant compromise.Although the high prevalence of HCV and alcohol abuse in the veteran population has resulted in a high incidence of hepatic dysfunction, this study suggests that these factors are independent of survival if liver function or integrity has not been compromised.9
Some researchers have hypothesized that clinical toxicities from tyrosine kinase inhibitors may correlate with survival.13 The authors noticed that the presentation of dermatologic AEs may reflect improved survival. In this study, patients who experienced ≥ 1 AE and ≥ 3 AEs had survival rates at the 1-year follow-up of 45.7% and 61.9%, respectively. Moreover, patients affected by AEs in the first 90 days of treatment had a survival rate at the 1-year follow-up of 42.4%.
Caution is advised when drawing conclusions from the number of AEs or when they appear, because this may falsely favor correlation. Patients who survive longer have additional time to report an AE. Therefore, the authors also looked at the ratio of AEs over time per patient to consider the number of AEs per duration of treatment and saw that there was little difference in survival rate in this regard. When considering patients affected by AEs only in the first 30 days of treatment, the survival rate at the 1-year follow-up fell to 30.2%.
A more likely factor for the survival of the 17 patients who were alive at the 1-year follow-up was their overall health relative to the rest of the study group. Overall health may indicate survival independent of sorafenib. The group of 17 who survived at the 1-year follow-up reflected a population that was different from the rest of the study population. The subset was generally healthier with better ECOG-PS scores and Child-Pugh classes, was not followed by Palliative Care Services, and had a mean AFP level under the threshold for diagnosis of HCC in patients who present with hepatic lesions and elevated AFP.14 This subset’s MPR, a surrogate marker for adherence, was less than the accepted threshold in clinical practice for oral medications.15Evaluating the patient’s dose regimen was expected to reveal a relationship between dosing and clinical outcomes, such as low survival rates with low doses or more AEs with high doses. However, the authors were not able to establish this link. In fact, the median time to discontinuation of 3.4 months for the study group, or duration of treatment, was much shorter than the median OS of 5.9 months.
These findings were consistent with Cabibbo and colleagues, who conducted a meta-analysis of survival rates for untreated patients and found that impaired performance status and Child-Pugh class B or C were independently associated with shorter survival.16 The SHARP study and Cheng and colleagues also attempted to exclude patients who were not Child-Pugh class A in their studies, which suggests a negligible correlation between sorafenib and survival time and a close relationship between baseline clinical status and survival.
The authors determined that prior treatment, including locoregional therapy, was not a factor in predicting survival. This observation is confirmed by the results of a phase 3 study that looked at sorafenib as adjuvant treatment for patients who had no detectable disease after surgical resection or local ablation.17 The trial did not meet its primary endpoint of improved recurrence-free survival. However, the authors observed in this study that 4 patients who underwent resection of the liver before sorafenib had a mean OS of 2.9 years. One patient, who was alive at the time of the study conclusion, received only 22 days of sorafenib treatment and survived for 4.9 years after sorafenib discontinuation. Patients who received concurrent or postsorafenib treatment had higher survival rates.
The cost of treatment in this study was found to be $8,535.87 per year lived. Although formal quality of life assessments were not captured, medication was discontinued at the first sign of disease progression or AE as determined by the provider or patient. When the cost of treatment was adjusted to account for median OS time and VA drug acquisition costs, estimated at average wholesale price minus 40%, the cost of treatment was within the threshold of $50,000-$100,000 per quality-adjusted life-year.7,18Of the 42 patients in this study, 28.6% discontinued therapy due to AEs, compared with 32% observed in the SHARP study. Common GI, dermatologic, and CNS AEs were comparable between the 2 studies. Serious AEs included intracranial hemorrhage, GI hemorrhage, GI perforation, acute liver failure, and acute renal failure; 3 of these events led to death. About 12% of patients experienced bleeding, regardless of severity, compared with the 18% seen in SHARP, despite no prior history of hemorrhage or GI perforation.5 The authors did not find any clinical factors at baseline that predisposed patients to AEs. It was also difficult to distinguish between drug-related AEs and general disease progression.
Although the authors did not find a relationship between dose or dose adjustments and the number or frequency of AEs, there were serious adverse outcomes in this study that were also rare complications observed in SHARP. The decision to start sorafenib should not be taken lightly.
Related: Diagnostic Dilemma of Hepatocellular Carcinoma Presenting as Hepatic Angiomyolipoma
Limitations
This retrospective review had several limitations. In SHARP and other large, multicenter trials, patients were continued on therapy until they experienced both symptomatic and radiographic progression. In this study, patients were discontinued at the first sign of progression, either symptomatic or radiographic or both. Had all patients remained on therapy until symptomatic and radiographic signs of progression were observed, there could have been a better correlation between duration of treatment and OS, symptomatic progression, or radiographic progression. The authors acknowledge, however, that there is diminishing benefit of administering chemotherapy when there are known and potentially serious AEs.
The data for this study were limited due to a small sample size, and it was not powered to evaluate for statistically significant characteristics between the patients who survived at the 1-year follow-up and the patients who did not survive at the 1-year follow-up. This information would be useful to identify potential prognostic factors and guide providers in sorafenib management. Finally, a long-term safety profile could not be established, as patients were evaluated for a 1-year period.
Ultimately, HCC is a multifactorial disease, and it is difficult to account for all potential confounding factors. Additional research, including studies comparing sunitinib or a control group to sorafenib, may provide further insight.
Conclusions
In light of these results, the authors believe that sorafenib may be considered for veterans with unresectable HCC and who are contraindicated for alternative treatments. One-year survival rates were similar to those seen in previous studies. However, there was no clear association between the duration of treatment and OS, and although the medication was well tolerated, there were also serious AEs. It is prudent to continually assess the need for therapy throughout the treatment period.
Pharmacists have a critical role in care for oncology patients, from the integration of certified clinical pharmacist practitioners into hematology-oncology clinics to patient monitoring through oral oncology pharmacy programs.19,20 These programs have been shown to improve patient outcomes and decrease overall health care use and may benefit the veteran population.
In this study, a veteran population achieved a survival rate at the 1-year follow-up similar to that found in SHARP: 40.5% vs 44%. However, OS was markedly shorter: 5.9 months vs 10.7 months. Patients with minimal impact from HCC, such as mild hepatic impairment and high functional status, demonstrated higher survival rates at the 1-year follow-up than did those with significant compromise. Thirty-five patients experienced ≥1 AE, most observed within the first 90 days of treatment, and for 3 patients, sorafenib could not be ruled out as the cause of death.
Sorafenib remains a viable therapeutic option for veterans with advanced HCC. However, it is uncertain how much benefit sorafenib affords to the veteran population, especially with the associated risks.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
2. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.
3. Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(suppl 4):14-22.
4. Nexavar [package insert]. Emeryville, CA: Bayer HealthCare Pharmaceuticals, Inc; 2009.
5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. Version 2. 2015. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed October 13, 2015.
6. Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
7. Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25(11):1739-1746.
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
9. U.S. Department of Veterans Affairs, Veterans Health Administration. National Viral Hepatitis Program. VHA Directive 1300.01. U.S. Department of Veterans Affairs Website. http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=1586. Updated February 22, 2013. Accessed October 13, 2015.
10. Patterson CJ. Best practices in specialty pharmacy management. J Manag Care Pharm. 2013;19(1):42-48.
11. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34.
12. Iavarone M, Cabibbo G, Piscaglia F, et al; SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54(6):2055-2063.
13. Di Fiore F, Rigal O, Ménager C, Michel P, Pfister C. Severe clinical toxicities are correlated with survival in patients with advanced renal cell carcinoma treated with sunitinib and sorafenib. Br J Cancer. 2011;105(12):1811-1813.
14. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
15. Blandford L, Dans PE, Ober JD, Wheelock C. Analyzing variations in medication compliance related to individual drug, drug class, and prescribing physician. J Managed Care Pharm. 1999;5(1):47-51.
16. Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51(4):1274-1283.
17. Bayer HealthCare. Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma (STORM). ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT00692770. Updated May 28, 2015. Accessed October 21, 2015.
18. Academy of Managed Care Pharmacy. AMCP Guide to Pharmaceutical Payment Methods, 2009 Update (Version 2.0). J Manag Care Pharm. 2009;15(suppl 6-a):S3-S57.
19. Valgus JM, Faso A, Gregory KM, et al. Integration of a clinical pharmacist into the hematology-oncology clinics at an academic medical center. Am J Health Syst Pharm. 2011;68(7):613-619.
20. Tschida SJ, Aslam S, Lal LS, et al. Outcomes of a specialty pharmacy program for oral oncology medications. Am J Pharm Benefits. 2012;4(4):165-174.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
2. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.
3. Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(suppl 4):14-22.
4. Nexavar [package insert]. Emeryville, CA: Bayer HealthCare Pharmaceuticals, Inc; 2009.
5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. Version 2. 2015. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed October 13, 2015.
6. Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
7. Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25(11):1739-1746.
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
9. U.S. Department of Veterans Affairs, Veterans Health Administration. National Viral Hepatitis Program. VHA Directive 1300.01. U.S. Department of Veterans Affairs Website. http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=1586. Updated February 22, 2013. Accessed October 13, 2015.
10. Patterson CJ. Best practices in specialty pharmacy management. J Manag Care Pharm. 2013;19(1):42-48.
11. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34.
12. Iavarone M, Cabibbo G, Piscaglia F, et al; SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54(6):2055-2063.
13. Di Fiore F, Rigal O, Ménager C, Michel P, Pfister C. Severe clinical toxicities are correlated with survival in patients with advanced renal cell carcinoma treated with sunitinib and sorafenib. Br J Cancer. 2011;105(12):1811-1813.
14. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
15. Blandford L, Dans PE, Ober JD, Wheelock C. Analyzing variations in medication compliance related to individual drug, drug class, and prescribing physician. J Managed Care Pharm. 1999;5(1):47-51.
16. Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51(4):1274-1283.
17. Bayer HealthCare. Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma (STORM). ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT00692770. Updated May 28, 2015. Accessed October 21, 2015.
18. Academy of Managed Care Pharmacy. AMCP Guide to Pharmaceutical Payment Methods, 2009 Update (Version 2.0). J Manag Care Pharm. 2009;15(suppl 6-a):S3-S57.
19. Valgus JM, Faso A, Gregory KM, et al. Integration of a clinical pharmacist into the hematology-oncology clinics at an academic medical center. Am J Health Syst Pharm. 2011;68(7):613-619.
20. Tschida SJ, Aslam S, Lal LS, et al. Outcomes of a specialty pharmacy program for oral oncology medications. Am J Pharm Benefits. 2012;4(4):165-174.
Idiopathic Intracranial Hypertension in Pregnancy
A 27-year-old white woman presented to the clinic with headaches and decreased vision through her reading glasses while performing near tasks. Her medical history was significant for herpes simplex, hyperlipidemia, and migraine headaches with aura. Her migraines began following an earlier motor vehicle accident, and her most recent magnetic resonance imaging (MRI) showed no abnormalities. Her current medications included prophylactic acyclovir for herpes and acetaminophen and caffeine tablets as needed for headache. She reported no other trauma or surgery and no known allergies. The patient’s best-corrected Snellen visual acuities in both eyes were 20/20 (distance) and 20/30 (near).
Preliminary testing, including pupils, extraocular motilities, confrontation fields, and color vision, were all within normal limits. Her slit-lamp examination was unremarkable. A dilated fundus examination revealed crowded, elevated discs without vessel obscuration, hemorrhage, hyperemia, or drusen (Figure 1). The fundus examination was otherwise unremarkable. Optical coherence tomography of the optic nerves showed increased nerve fiber layer thickness in both eyes (Figure 2). Her blood pressure (BP) at this visit was 106/77 mm/Hg.
The diagnosis based on these findings was bilateral optic nerve elevation with long-standing migraine headaches. The plan was for the patient to return to the clinic for repeat visual field testing and B-scan ultrasonography to rule out buried optic nerve head drusen.
Two months later, the patient presented to the clinic 19 weeks pregnant and reported that her headaches had increased in frequency, but she had no diplopia. All preliminary testing, including visual acuities, pupil reaction, color vision, and slit-lamp examination remained normal. Fundus examination showed the patient’s nerves were unchanged in appearance from the initial presentation. Visual fields revealed an enlarged blind spot in the right eye and paracentral defects in the left eye. The B-scan testing was negative for optic nerve drusen. Due to the increased frequency of headaches, pregnancy, and suspicious optic nerves, an urgent consult was placed to neurology.
At the neurology appointment 1 month later, the patient was diagnosed with migraine headache syndrome and idiopathic intracranial hypertension (IIH). The neurologist believed her headaches might have been resulting from analgesic rebound. He suggested that the patient discontinue or decrease use of oral butalbital, acetaminophen and caffeine tablets, and other forms of caffeine. It was decided that divalproxen sodium and verapamil were not feasible due to pregnancy. The neurologist started her on oral acetazolamide 500 mg twice daily.
The patient returned to her obstetrician 1 month later for a routine follow-up; the headaches had worsened and were now accompanied by nausea and vomiting twice daily on average. Her medications still included acetaminophen and caffeine tablets, although it had been recommended she discontinue them, prochlorperazine, and acetazolamide. Due to the worsening of her symptoms and visual fields (eFigure 1), the obstetrician recommended that the patient deliver by cesarean section at 38 to 39 weeks.
(eFigure 1.Visual Fields at Follow-up 1 and 2)
Right eye
Left Eye
Following an uncomplicated cesarean delivery at 38 weeks, the patient returned to the clinic for visual field testing. Humphrey visual fields were full in the right eye and showed some scattered central depressions in the left. Both eyes were significantly improved from previous fields (eFigure 2) . The patient had discontinued acetazolamide and reported minor tension headaches she believed were due to lack of sleep but stated that she was no longer having migraines. There was no papilledema noted on fundus examination, and Snellen distance visual acuity measured 20/20 in both eyes. An MRI had been performed after delivery and was negative for intracranial hemorrhage, mass, or hydrocephalus).
(eFigure 2. Visual Fields Postpartum)
Right eye
Left eye
Three months later, the patient returned for her yearly comprehensive examination. At that visit, she reported a decrease in frequency of the migraine headaches. Optical coherence tomography was performed and showed a significant decrease in optic nerve head swelling.
Related: Diabetes on the Rise Among Other Pregnancy Problems
Clinical Picture
Idiopathic intracranial hypertension presents clinically with signs and symptoms of increased intracranial pressure (ICP). Headache is the most common symptom, usually presenting as daily and pulsatile.1 Nausea may be associated with the headache, although vomiting is rare, and the headache may awaken the patient. The headache may remain after resolution of elevated ICP (Table).2
Papilledema is the most common sign of IIH.1,2 Visual loss associated with papilledema is generally mild at first but progressive. Transient blur lasts usually 30 seconds and may be monocular or binocular.1 The cause is thought to be related to transient ischemia of the optic nerve.1 Vision loss is typically reversible with resolution of optic nerve swelling, but 25% of patients may develop optic atrophy, which results in permanent vision loss.2 Common patterns of visual abnormalities include enlargement of the physiologic blind spot, inferonasal and arcuate defects, and eventually severe peripheral constriction.1,2 It is imperative that all patients with IIH have visual field testing performed.
About one-third of patients with IIH experience diplopia. This binocular, horizontal diplopia is caused by a sixth nerve palsy in 10% to 20% of patients.1 Cranial nerves II, VI, and VII make a 90-degree bend and seem to be prone to damage at the site of the bend.1
Pulse-synchronous tinnitus is common in IIH as well.2,3 This generally occurs unilaterally and may be eliminated by jugular compression or the head turning to the ipsilateral side.1,3 The sound is caused by the transmission of an increase in the vascular pulse due to high pressure on the cerebrospinal fluid (CSF).1,3
Idiopathic intracranial hypertension most typically presents in obese women of childbearing age.1-3 An increasing degree of obesity is generally associated with an increased risk of vision loss.1,2 Men seem to have worse acuity and visual fields at presentation than do women.2 Men are less likely to report headaches than are women and, therefore, have double the likelihood of severe vision loss.2 Hence, closer monitoring and more aggressive intervention is recommended for men due to their lesser tendency for headaches.2 Black patients also demonstrate more aggressive disease and, therefore, require closer monitoring and early aggressive intervention.1,2
Papilledema is the most common sign of IIH and may be caused by several processes. In this case, most were ruled out given the patient’s normal visual acuities, pupillary reaction, color vision testing, BP measurement, and B-scan imaging. The patient’s systemic history was negative for thyroid-related disease, diabetes, hypertension, autoimmune disease, or infection. She had no family history of vision loss or hereditary ocular conditions. The most recent MRI was negative for any long-standing space-occupying lesion or hydrocephalus.
Pathophysiology
Several mechanisms leading to increased ICP have been proposed. These include increased brain water content, excess CSF production, reduced CSF absorption, and increased cerebral venous pressure.2,3 There is also a suspicion of the role of sex hormones in IIH due to its high predilection for females.2
The role of vitamin A metabolism has also been studied in IIH.1 Retinol levels are elevated in the CSF of patients with IIH. Patients may ingest an abnormally large amount of vitamin A, metabolize it abnormally, or be sensitive to its effects.2,4 The function of adipose tissue as an actively secreting endocrine tissue may play a role in IIH due to its release of adipose tissue-derived retinol binding protein.2 Other adipose-produced cytokines include leptin, which has been implicated in IIH due to its elevated levels found in the CSF of patients with IIH.2
Stenosis of the cerebral sinuses is another proposed mechanism of IIH.1-3 Cerebrospinal fluid exits the cranium into the venous sinuses via the arachnoid villi.2 An obstruction in these sinuses may impair CSF outflow and result in intracranial hypertension. Microthrombosis caused by hypercoaguable disorders may result in increased cerebral venous pressure and impaired CSF absorption as well.2,4
Some medications have been found in association with IIH. These include tetracycline, cyclosporine, lithium, nalidixic acid, nitrofurantoin, oral contraceptives, levonorgestrel, danaxol, and tamoxifen.1-4 Tetracycline seems to have the strongest association with IIH and should be discontinued in those patients where the association is very likely to be the causative factor.2 The link to oral contraceptives may occur simply due to their association with young women most at risk for IIH.1-3
Related:Young Man With Headache, Confusion, and Hearing Loss
Management
The goals of treatment with IIH are to preserve vision and relieve symptoms, particularly headache. The general recommendation is that pregnant women with IIH should be managed and treated the same as any other patient with IIH. However, imaging and some drug contraindications exist between these 2 groups.
The diagnostic test for IIH is a lumbar puncture, which is also the most effective treatment.1-3,5 Lumbar puncture should be performed in the relaxed lateral decubitus position without sedation.1-3 The opening pressure should be measured and is the most clinically significant diagnostic tool for diagnosis of IIH. Opening pressures of > 250 mm H2O are diagnostic of IIH.1-3,5
Weight loss is an essential part of treatment in obese patients with IIH.1-3 A low-calorie, low-salt diet with mild fluid restriction seems to reverse the symptoms of IIH. A 5% to 10% reduction in body weight may reduce symptoms and signs of IIH.2
Carbonic anhydrase inhibitors (CAIs), such as acetalzolamide, have a multifactorial role in IIH.4 They are usually prescribed in 1 to 2 grams over several doses and function by decreasing CSF production.1 Carbonic anhydrase inhibitors also are known to change the taste of foods and may, therefore, aid in weight loss.1,2 Patients prescribed CAIs commonly experience a tingling in their fingers, toes, and perioral region, an indication that the medication is working.1,2 A rare but serious adverse effect (AE) is aplastic anemia, which generally occurs in the first 6 months of treatment in elderly patients.1 The use of CAIs in pregnancy is controversial, and although rare complications are reported, it is considered a class C drug.5
In patients with rapidly progressive vision loss but with minimal headache, optic nerve sheath fenestration (ONSF) is the surgical treatment of choice.2,3,6 In this procedure, a window or series of slits are created behind the globe in the optic nerve sheath.1 About 50% of patients achieve adequate headache control with ONSF, especially for frontal headaches.1,2
For patients with vision loss, papilledema, and headache that do not respond to medical therapy, a CSF diversion procedure is the preferred treatment. Cerebrospinal fluid diversion with ventriculoperitoneal or lumboperitoneal shunts may prevent progressive loss of vision.1,4,6 However, variable response rates and shunt failure requiring subsequent revisions are common and may occur in as many as half of patients undergoing these procedures.1
Increased intracranial venous pressure due to stenosis of the venous sinuses has been thought to be a possible cause of IIH. Stenting of the transverse venous sinus stenosis has been shown to reduce cerebral venous pressure, reduce ICP, and improve symptoms in patients with IIH.1-3 It is unclear whether elevations in ICP cause transverse sinus stenosis or whether transverse sinus stenosis causes increased ICP.2 Regardless, stents have a high rate of complications, including subdural hemorrhage, venous sinus perforation, in-stent thrombosis, and recurrent stenosis proximal to the stent.2
Steroids have been used to treat IIH in the past, although their mechanism of action remains unclear.2 There may be recurrence of papilledema if they are tapered too quickly. Due to their association with long-term AEs, including weight gain, they should be avoided.2
Management in Pregnancy
Several studies agree that vision loss occurs in the same frequency in pregnant and nonpregnant patients with IIH.4,7 Idiopathic intracranial hypertension can occur in any trimester in pregnancy. It has been found that patients have the same spontaneous abortion rate and visual outcomes as the general population.6-8 It has also been concluded that treatment should be the same in both patient populations with slight variability in the use of acetazolamide.4,6,7
The use of dilating drops during pregnancy is controversial. Although there have been no teratogenic effects reported with use of topical anesthetics and dilating drops, all drugs should be avoided during the first trimester.7-10 Guidelines have been established by the American Congress of Obstetricians and Gynecologists for X-ray examination and exposure during pregnancy. It has been determined that exposure from a single diagnostic X-ray procedure does not result in harmful fetal effects.11 Magnetic resonance imaging is not associated with any known adverse fetal effects and is a better imaging option during pregnancy, because it is not associated with the use of ionizing radiation.11
The use of CAIs in the first trimester is controversial.4,7 Some believe it should be avoided because it is a Pregnancy Category C drug. However, a single case of sacrococcygeal teratoma has been reported in humans; therefore, some believe this is not a strong basis for withholding the medication in patients with the potential risk for severe vision loss.4,7 In this case, a consult to the patient’s obstetrician was made, and the use of acetazolamide had no effect on the health of the baby.
In pregnant women with IIH with progressive vision loss, failed treatment, or nonadherence, surgery may be necessary. Optic nerve sheath fenestration is preferred due to lower morbidity and mortality compared with shunting procedures.1,2,4,6 The growing fetus may be affected by the peritoneal end of the shunt.4
Related: 49-Year-Old Woman With a Broken Heart
Conclusions
Vision loss associated with IIH can be severe and permanent if left untreated. The best treatments and often the most effective involve weight loss and lumbar puncture. Acetazolamide has been a proven effective treatment in some patients, but some debate exists over the safety of its use during pregnancy. This patient did not have any AEs from its use; however, it did not prove valuable in her treatment. Studies often disagree on the use of acetazolamide in pregnancy; however, all agree that proper patient counseling on potential AEs and management by an obstetrician are important. With proper management, pregnant women with IIH have had outcomes similar to those of the general population.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617.
2. Bruce BB, Biousee V, Newman NJ. Update on idiopathic intracranial hypertension. Am J Ophthalmol. 2011;152(2):163-169.
3. Fields JD, Javendani PP, Falardeau J, et al. Dural venous sinus angioplasty and stenting for the treatment of idiopathic intracranial hypertension. J Neurointerv Surg. 2013;5(1):62-68.
4. Evans RW, Lee AG. Idiopathic intracranial hypertension in pregnancy. Headache. 2010;50(9):1513-1515.
5. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59(10):1492-1495.
6. Martínez-Varea A, Diago-Almela VJ, Abad-Carrascosa A, Perales-Marín A. Progressive visual loss in a pregnant woman with idiopathic intracranial hypertension. Eur J Obstet Gynecol Reprod Biol. 2012;163(1):117-122.
7. Falardeau J, Lobb B, Golden S, Maxfield SD, Tanne E. The use of acetazolamide during pregnancy in intracranial hypertension patients. J Neuroophthalmol. 2013;33(1):9-12.
8. Dinn RB, Harris A, Marcus PS. Ocular changes in pregnancy. Obstet Gynecol Surg. 2003;58(2):137-144.
9. Shultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol. 2005;16(5):308-314.
10. Chung CY, Kwok AKH, Chung KL. Use of ophthalmic medications during pregnancy. Hong Kong Med J. 2004;10(3):191-195.
11. American Congress of Obstetricians and Gynecologists. Committee Opinion. Guidelines for diagnostic imaging during pregnancy. American Congress of Obstetricians and Gynecologists Website. http://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co299.pdf. Published 2004. Accessed October 9, 2015.
A 27-year-old white woman presented to the clinic with headaches and decreased vision through her reading glasses while performing near tasks. Her medical history was significant for herpes simplex, hyperlipidemia, and migraine headaches with aura. Her migraines began following an earlier motor vehicle accident, and her most recent magnetic resonance imaging (MRI) showed no abnormalities. Her current medications included prophylactic acyclovir for herpes and acetaminophen and caffeine tablets as needed for headache. She reported no other trauma or surgery and no known allergies. The patient’s best-corrected Snellen visual acuities in both eyes were 20/20 (distance) and 20/30 (near).
Preliminary testing, including pupils, extraocular motilities, confrontation fields, and color vision, were all within normal limits. Her slit-lamp examination was unremarkable. A dilated fundus examination revealed crowded, elevated discs without vessel obscuration, hemorrhage, hyperemia, or drusen (Figure 1). The fundus examination was otherwise unremarkable. Optical coherence tomography of the optic nerves showed increased nerve fiber layer thickness in both eyes (Figure 2). Her blood pressure (BP) at this visit was 106/77 mm/Hg.
The diagnosis based on these findings was bilateral optic nerve elevation with long-standing migraine headaches. The plan was for the patient to return to the clinic for repeat visual field testing and B-scan ultrasonography to rule out buried optic nerve head drusen.
Two months later, the patient presented to the clinic 19 weeks pregnant and reported that her headaches had increased in frequency, but she had no diplopia. All preliminary testing, including visual acuities, pupil reaction, color vision, and slit-lamp examination remained normal. Fundus examination showed the patient’s nerves were unchanged in appearance from the initial presentation. Visual fields revealed an enlarged blind spot in the right eye and paracentral defects in the left eye. The B-scan testing was negative for optic nerve drusen. Due to the increased frequency of headaches, pregnancy, and suspicious optic nerves, an urgent consult was placed to neurology.
At the neurology appointment 1 month later, the patient was diagnosed with migraine headache syndrome and idiopathic intracranial hypertension (IIH). The neurologist believed her headaches might have been resulting from analgesic rebound. He suggested that the patient discontinue or decrease use of oral butalbital, acetaminophen and caffeine tablets, and other forms of caffeine. It was decided that divalproxen sodium and verapamil were not feasible due to pregnancy. The neurologist started her on oral acetazolamide 500 mg twice daily.
The patient returned to her obstetrician 1 month later for a routine follow-up; the headaches had worsened and were now accompanied by nausea and vomiting twice daily on average. Her medications still included acetaminophen and caffeine tablets, although it had been recommended she discontinue them, prochlorperazine, and acetazolamide. Due to the worsening of her symptoms and visual fields (eFigure 1), the obstetrician recommended that the patient deliver by cesarean section at 38 to 39 weeks.
(eFigure 1.Visual Fields at Follow-up 1 and 2)
Right eye
Left Eye
Following an uncomplicated cesarean delivery at 38 weeks, the patient returned to the clinic for visual field testing. Humphrey visual fields were full in the right eye and showed some scattered central depressions in the left. Both eyes were significantly improved from previous fields (eFigure 2) . The patient had discontinued acetazolamide and reported minor tension headaches she believed were due to lack of sleep but stated that she was no longer having migraines. There was no papilledema noted on fundus examination, and Snellen distance visual acuity measured 20/20 in both eyes. An MRI had been performed after delivery and was negative for intracranial hemorrhage, mass, or hydrocephalus).
(eFigure 2. Visual Fields Postpartum)
Right eye
Left eye
Three months later, the patient returned for her yearly comprehensive examination. At that visit, she reported a decrease in frequency of the migraine headaches. Optical coherence tomography was performed and showed a significant decrease in optic nerve head swelling.
Related: Diabetes on the Rise Among Other Pregnancy Problems
Clinical Picture
Idiopathic intracranial hypertension presents clinically with signs and symptoms of increased intracranial pressure (ICP). Headache is the most common symptom, usually presenting as daily and pulsatile.1 Nausea may be associated with the headache, although vomiting is rare, and the headache may awaken the patient. The headache may remain after resolution of elevated ICP (Table).2
Papilledema is the most common sign of IIH.1,2 Visual loss associated with papilledema is generally mild at first but progressive. Transient blur lasts usually 30 seconds and may be monocular or binocular.1 The cause is thought to be related to transient ischemia of the optic nerve.1 Vision loss is typically reversible with resolution of optic nerve swelling, but 25% of patients may develop optic atrophy, which results in permanent vision loss.2 Common patterns of visual abnormalities include enlargement of the physiologic blind spot, inferonasal and arcuate defects, and eventually severe peripheral constriction.1,2 It is imperative that all patients with IIH have visual field testing performed.
About one-third of patients with IIH experience diplopia. This binocular, horizontal diplopia is caused by a sixth nerve palsy in 10% to 20% of patients.1 Cranial nerves II, VI, and VII make a 90-degree bend and seem to be prone to damage at the site of the bend.1
Pulse-synchronous tinnitus is common in IIH as well.2,3 This generally occurs unilaterally and may be eliminated by jugular compression or the head turning to the ipsilateral side.1,3 The sound is caused by the transmission of an increase in the vascular pulse due to high pressure on the cerebrospinal fluid (CSF).1,3
Idiopathic intracranial hypertension most typically presents in obese women of childbearing age.1-3 An increasing degree of obesity is generally associated with an increased risk of vision loss.1,2 Men seem to have worse acuity and visual fields at presentation than do women.2 Men are less likely to report headaches than are women and, therefore, have double the likelihood of severe vision loss.2 Hence, closer monitoring and more aggressive intervention is recommended for men due to their lesser tendency for headaches.2 Black patients also demonstrate more aggressive disease and, therefore, require closer monitoring and early aggressive intervention.1,2
Papilledema is the most common sign of IIH and may be caused by several processes. In this case, most were ruled out given the patient’s normal visual acuities, pupillary reaction, color vision testing, BP measurement, and B-scan imaging. The patient’s systemic history was negative for thyroid-related disease, diabetes, hypertension, autoimmune disease, or infection. She had no family history of vision loss or hereditary ocular conditions. The most recent MRI was negative for any long-standing space-occupying lesion or hydrocephalus.
Pathophysiology
Several mechanisms leading to increased ICP have been proposed. These include increased brain water content, excess CSF production, reduced CSF absorption, and increased cerebral venous pressure.2,3 There is also a suspicion of the role of sex hormones in IIH due to its high predilection for females.2
The role of vitamin A metabolism has also been studied in IIH.1 Retinol levels are elevated in the CSF of patients with IIH. Patients may ingest an abnormally large amount of vitamin A, metabolize it abnormally, or be sensitive to its effects.2,4 The function of adipose tissue as an actively secreting endocrine tissue may play a role in IIH due to its release of adipose tissue-derived retinol binding protein.2 Other adipose-produced cytokines include leptin, which has been implicated in IIH due to its elevated levels found in the CSF of patients with IIH.2
Stenosis of the cerebral sinuses is another proposed mechanism of IIH.1-3 Cerebrospinal fluid exits the cranium into the venous sinuses via the arachnoid villi.2 An obstruction in these sinuses may impair CSF outflow and result in intracranial hypertension. Microthrombosis caused by hypercoaguable disorders may result in increased cerebral venous pressure and impaired CSF absorption as well.2,4
Some medications have been found in association with IIH. These include tetracycline, cyclosporine, lithium, nalidixic acid, nitrofurantoin, oral contraceptives, levonorgestrel, danaxol, and tamoxifen.1-4 Tetracycline seems to have the strongest association with IIH and should be discontinued in those patients where the association is very likely to be the causative factor.2 The link to oral contraceptives may occur simply due to their association with young women most at risk for IIH.1-3
Related:Young Man With Headache, Confusion, and Hearing Loss
Management
The goals of treatment with IIH are to preserve vision and relieve symptoms, particularly headache. The general recommendation is that pregnant women with IIH should be managed and treated the same as any other patient with IIH. However, imaging and some drug contraindications exist between these 2 groups.
The diagnostic test for IIH is a lumbar puncture, which is also the most effective treatment.1-3,5 Lumbar puncture should be performed in the relaxed lateral decubitus position without sedation.1-3 The opening pressure should be measured and is the most clinically significant diagnostic tool for diagnosis of IIH. Opening pressures of > 250 mm H2O are diagnostic of IIH.1-3,5
Weight loss is an essential part of treatment in obese patients with IIH.1-3 A low-calorie, low-salt diet with mild fluid restriction seems to reverse the symptoms of IIH. A 5% to 10% reduction in body weight may reduce symptoms and signs of IIH.2
Carbonic anhydrase inhibitors (CAIs), such as acetalzolamide, have a multifactorial role in IIH.4 They are usually prescribed in 1 to 2 grams over several doses and function by decreasing CSF production.1 Carbonic anhydrase inhibitors also are known to change the taste of foods and may, therefore, aid in weight loss.1,2 Patients prescribed CAIs commonly experience a tingling in their fingers, toes, and perioral region, an indication that the medication is working.1,2 A rare but serious adverse effect (AE) is aplastic anemia, which generally occurs in the first 6 months of treatment in elderly patients.1 The use of CAIs in pregnancy is controversial, and although rare complications are reported, it is considered a class C drug.5
In patients with rapidly progressive vision loss but with minimal headache, optic nerve sheath fenestration (ONSF) is the surgical treatment of choice.2,3,6 In this procedure, a window or series of slits are created behind the globe in the optic nerve sheath.1 About 50% of patients achieve adequate headache control with ONSF, especially for frontal headaches.1,2
For patients with vision loss, papilledema, and headache that do not respond to medical therapy, a CSF diversion procedure is the preferred treatment. Cerebrospinal fluid diversion with ventriculoperitoneal or lumboperitoneal shunts may prevent progressive loss of vision.1,4,6 However, variable response rates and shunt failure requiring subsequent revisions are common and may occur in as many as half of patients undergoing these procedures.1
Increased intracranial venous pressure due to stenosis of the venous sinuses has been thought to be a possible cause of IIH. Stenting of the transverse venous sinus stenosis has been shown to reduce cerebral venous pressure, reduce ICP, and improve symptoms in patients with IIH.1-3 It is unclear whether elevations in ICP cause transverse sinus stenosis or whether transverse sinus stenosis causes increased ICP.2 Regardless, stents have a high rate of complications, including subdural hemorrhage, venous sinus perforation, in-stent thrombosis, and recurrent stenosis proximal to the stent.2
Steroids have been used to treat IIH in the past, although their mechanism of action remains unclear.2 There may be recurrence of papilledema if they are tapered too quickly. Due to their association with long-term AEs, including weight gain, they should be avoided.2
Management in Pregnancy
Several studies agree that vision loss occurs in the same frequency in pregnant and nonpregnant patients with IIH.4,7 Idiopathic intracranial hypertension can occur in any trimester in pregnancy. It has been found that patients have the same spontaneous abortion rate and visual outcomes as the general population.6-8 It has also been concluded that treatment should be the same in both patient populations with slight variability in the use of acetazolamide.4,6,7
The use of dilating drops during pregnancy is controversial. Although there have been no teratogenic effects reported with use of topical anesthetics and dilating drops, all drugs should be avoided during the first trimester.7-10 Guidelines have been established by the American Congress of Obstetricians and Gynecologists for X-ray examination and exposure during pregnancy. It has been determined that exposure from a single diagnostic X-ray procedure does not result in harmful fetal effects.11 Magnetic resonance imaging is not associated with any known adverse fetal effects and is a better imaging option during pregnancy, because it is not associated with the use of ionizing radiation.11
The use of CAIs in the first trimester is controversial.4,7 Some believe it should be avoided because it is a Pregnancy Category C drug. However, a single case of sacrococcygeal teratoma has been reported in humans; therefore, some believe this is not a strong basis for withholding the medication in patients with the potential risk for severe vision loss.4,7 In this case, a consult to the patient’s obstetrician was made, and the use of acetazolamide had no effect on the health of the baby.
In pregnant women with IIH with progressive vision loss, failed treatment, or nonadherence, surgery may be necessary. Optic nerve sheath fenestration is preferred due to lower morbidity and mortality compared with shunting procedures.1,2,4,6 The growing fetus may be affected by the peritoneal end of the shunt.4
Related: 49-Year-Old Woman With a Broken Heart
Conclusions
Vision loss associated with IIH can be severe and permanent if left untreated. The best treatments and often the most effective involve weight loss and lumbar puncture. Acetazolamide has been a proven effective treatment in some patients, but some debate exists over the safety of its use during pregnancy. This patient did not have any AEs from its use; however, it did not prove valuable in her treatment. Studies often disagree on the use of acetazolamide in pregnancy; however, all agree that proper patient counseling on potential AEs and management by an obstetrician are important. With proper management, pregnant women with IIH have had outcomes similar to those of the general population.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
A 27-year-old white woman presented to the clinic with headaches and decreased vision through her reading glasses while performing near tasks. Her medical history was significant for herpes simplex, hyperlipidemia, and migraine headaches with aura. Her migraines began following an earlier motor vehicle accident, and her most recent magnetic resonance imaging (MRI) showed no abnormalities. Her current medications included prophylactic acyclovir for herpes and acetaminophen and caffeine tablets as needed for headache. She reported no other trauma or surgery and no known allergies. The patient’s best-corrected Snellen visual acuities in both eyes were 20/20 (distance) and 20/30 (near).
Preliminary testing, including pupils, extraocular motilities, confrontation fields, and color vision, were all within normal limits. Her slit-lamp examination was unremarkable. A dilated fundus examination revealed crowded, elevated discs without vessel obscuration, hemorrhage, hyperemia, or drusen (Figure 1). The fundus examination was otherwise unremarkable. Optical coherence tomography of the optic nerves showed increased nerve fiber layer thickness in both eyes (Figure 2). Her blood pressure (BP) at this visit was 106/77 mm/Hg.
The diagnosis based on these findings was bilateral optic nerve elevation with long-standing migraine headaches. The plan was for the patient to return to the clinic for repeat visual field testing and B-scan ultrasonography to rule out buried optic nerve head drusen.
Two months later, the patient presented to the clinic 19 weeks pregnant and reported that her headaches had increased in frequency, but she had no diplopia. All preliminary testing, including visual acuities, pupil reaction, color vision, and slit-lamp examination remained normal. Fundus examination showed the patient’s nerves were unchanged in appearance from the initial presentation. Visual fields revealed an enlarged blind spot in the right eye and paracentral defects in the left eye. The B-scan testing was negative for optic nerve drusen. Due to the increased frequency of headaches, pregnancy, and suspicious optic nerves, an urgent consult was placed to neurology.
At the neurology appointment 1 month later, the patient was diagnosed with migraine headache syndrome and idiopathic intracranial hypertension (IIH). The neurologist believed her headaches might have been resulting from analgesic rebound. He suggested that the patient discontinue or decrease use of oral butalbital, acetaminophen and caffeine tablets, and other forms of caffeine. It was decided that divalproxen sodium and verapamil were not feasible due to pregnancy. The neurologist started her on oral acetazolamide 500 mg twice daily.
The patient returned to her obstetrician 1 month later for a routine follow-up; the headaches had worsened and were now accompanied by nausea and vomiting twice daily on average. Her medications still included acetaminophen and caffeine tablets, although it had been recommended she discontinue them, prochlorperazine, and acetazolamide. Due to the worsening of her symptoms and visual fields (eFigure 1), the obstetrician recommended that the patient deliver by cesarean section at 38 to 39 weeks.
(eFigure 1.Visual Fields at Follow-up 1 and 2)
Right eye
Left Eye
Following an uncomplicated cesarean delivery at 38 weeks, the patient returned to the clinic for visual field testing. Humphrey visual fields were full in the right eye and showed some scattered central depressions in the left. Both eyes were significantly improved from previous fields (eFigure 2) . The patient had discontinued acetazolamide and reported minor tension headaches she believed were due to lack of sleep but stated that she was no longer having migraines. There was no papilledema noted on fundus examination, and Snellen distance visual acuity measured 20/20 in both eyes. An MRI had been performed after delivery and was negative for intracranial hemorrhage, mass, or hydrocephalus).
(eFigure 2. Visual Fields Postpartum)
Right eye
Left eye
Three months later, the patient returned for her yearly comprehensive examination. At that visit, she reported a decrease in frequency of the migraine headaches. Optical coherence tomography was performed and showed a significant decrease in optic nerve head swelling.
Related: Diabetes on the Rise Among Other Pregnancy Problems
Clinical Picture
Idiopathic intracranial hypertension presents clinically with signs and symptoms of increased intracranial pressure (ICP). Headache is the most common symptom, usually presenting as daily and pulsatile.1 Nausea may be associated with the headache, although vomiting is rare, and the headache may awaken the patient. The headache may remain after resolution of elevated ICP (Table).2
Papilledema is the most common sign of IIH.1,2 Visual loss associated with papilledema is generally mild at first but progressive. Transient blur lasts usually 30 seconds and may be monocular or binocular.1 The cause is thought to be related to transient ischemia of the optic nerve.1 Vision loss is typically reversible with resolution of optic nerve swelling, but 25% of patients may develop optic atrophy, which results in permanent vision loss.2 Common patterns of visual abnormalities include enlargement of the physiologic blind spot, inferonasal and arcuate defects, and eventually severe peripheral constriction.1,2 It is imperative that all patients with IIH have visual field testing performed.
About one-third of patients with IIH experience diplopia. This binocular, horizontal diplopia is caused by a sixth nerve palsy in 10% to 20% of patients.1 Cranial nerves II, VI, and VII make a 90-degree bend and seem to be prone to damage at the site of the bend.1
Pulse-synchronous tinnitus is common in IIH as well.2,3 This generally occurs unilaterally and may be eliminated by jugular compression or the head turning to the ipsilateral side.1,3 The sound is caused by the transmission of an increase in the vascular pulse due to high pressure on the cerebrospinal fluid (CSF).1,3
Idiopathic intracranial hypertension most typically presents in obese women of childbearing age.1-3 An increasing degree of obesity is generally associated with an increased risk of vision loss.1,2 Men seem to have worse acuity and visual fields at presentation than do women.2 Men are less likely to report headaches than are women and, therefore, have double the likelihood of severe vision loss.2 Hence, closer monitoring and more aggressive intervention is recommended for men due to their lesser tendency for headaches.2 Black patients also demonstrate more aggressive disease and, therefore, require closer monitoring and early aggressive intervention.1,2
Papilledema is the most common sign of IIH and may be caused by several processes. In this case, most were ruled out given the patient’s normal visual acuities, pupillary reaction, color vision testing, BP measurement, and B-scan imaging. The patient’s systemic history was negative for thyroid-related disease, diabetes, hypertension, autoimmune disease, or infection. She had no family history of vision loss or hereditary ocular conditions. The most recent MRI was negative for any long-standing space-occupying lesion or hydrocephalus.
Pathophysiology
Several mechanisms leading to increased ICP have been proposed. These include increased brain water content, excess CSF production, reduced CSF absorption, and increased cerebral venous pressure.2,3 There is also a suspicion of the role of sex hormones in IIH due to its high predilection for females.2
The role of vitamin A metabolism has also been studied in IIH.1 Retinol levels are elevated in the CSF of patients with IIH. Patients may ingest an abnormally large amount of vitamin A, metabolize it abnormally, or be sensitive to its effects.2,4 The function of adipose tissue as an actively secreting endocrine tissue may play a role in IIH due to its release of adipose tissue-derived retinol binding protein.2 Other adipose-produced cytokines include leptin, which has been implicated in IIH due to its elevated levels found in the CSF of patients with IIH.2
Stenosis of the cerebral sinuses is another proposed mechanism of IIH.1-3 Cerebrospinal fluid exits the cranium into the venous sinuses via the arachnoid villi.2 An obstruction in these sinuses may impair CSF outflow and result in intracranial hypertension. Microthrombosis caused by hypercoaguable disorders may result in increased cerebral venous pressure and impaired CSF absorption as well.2,4
Some medications have been found in association with IIH. These include tetracycline, cyclosporine, lithium, nalidixic acid, nitrofurantoin, oral contraceptives, levonorgestrel, danaxol, and tamoxifen.1-4 Tetracycline seems to have the strongest association with IIH and should be discontinued in those patients where the association is very likely to be the causative factor.2 The link to oral contraceptives may occur simply due to their association with young women most at risk for IIH.1-3
Related:Young Man With Headache, Confusion, and Hearing Loss
Management
The goals of treatment with IIH are to preserve vision and relieve symptoms, particularly headache. The general recommendation is that pregnant women with IIH should be managed and treated the same as any other patient with IIH. However, imaging and some drug contraindications exist between these 2 groups.
The diagnostic test for IIH is a lumbar puncture, which is also the most effective treatment.1-3,5 Lumbar puncture should be performed in the relaxed lateral decubitus position without sedation.1-3 The opening pressure should be measured and is the most clinically significant diagnostic tool for diagnosis of IIH. Opening pressures of > 250 mm H2O are diagnostic of IIH.1-3,5
Weight loss is an essential part of treatment in obese patients with IIH.1-3 A low-calorie, low-salt diet with mild fluid restriction seems to reverse the symptoms of IIH. A 5% to 10% reduction in body weight may reduce symptoms and signs of IIH.2
Carbonic anhydrase inhibitors (CAIs), such as acetalzolamide, have a multifactorial role in IIH.4 They are usually prescribed in 1 to 2 grams over several doses and function by decreasing CSF production.1 Carbonic anhydrase inhibitors also are known to change the taste of foods and may, therefore, aid in weight loss.1,2 Patients prescribed CAIs commonly experience a tingling in their fingers, toes, and perioral region, an indication that the medication is working.1,2 A rare but serious adverse effect (AE) is aplastic anemia, which generally occurs in the first 6 months of treatment in elderly patients.1 The use of CAIs in pregnancy is controversial, and although rare complications are reported, it is considered a class C drug.5
In patients with rapidly progressive vision loss but with minimal headache, optic nerve sheath fenestration (ONSF) is the surgical treatment of choice.2,3,6 In this procedure, a window or series of slits are created behind the globe in the optic nerve sheath.1 About 50% of patients achieve adequate headache control with ONSF, especially for frontal headaches.1,2
For patients with vision loss, papilledema, and headache that do not respond to medical therapy, a CSF diversion procedure is the preferred treatment. Cerebrospinal fluid diversion with ventriculoperitoneal or lumboperitoneal shunts may prevent progressive loss of vision.1,4,6 However, variable response rates and shunt failure requiring subsequent revisions are common and may occur in as many as half of patients undergoing these procedures.1
Increased intracranial venous pressure due to stenosis of the venous sinuses has been thought to be a possible cause of IIH. Stenting of the transverse venous sinus stenosis has been shown to reduce cerebral venous pressure, reduce ICP, and improve symptoms in patients with IIH.1-3 It is unclear whether elevations in ICP cause transverse sinus stenosis or whether transverse sinus stenosis causes increased ICP.2 Regardless, stents have a high rate of complications, including subdural hemorrhage, venous sinus perforation, in-stent thrombosis, and recurrent stenosis proximal to the stent.2
Steroids have been used to treat IIH in the past, although their mechanism of action remains unclear.2 There may be recurrence of papilledema if they are tapered too quickly. Due to their association with long-term AEs, including weight gain, they should be avoided.2
Management in Pregnancy
Several studies agree that vision loss occurs in the same frequency in pregnant and nonpregnant patients with IIH.4,7 Idiopathic intracranial hypertension can occur in any trimester in pregnancy. It has been found that patients have the same spontaneous abortion rate and visual outcomes as the general population.6-8 It has also been concluded that treatment should be the same in both patient populations with slight variability in the use of acetazolamide.4,6,7
The use of dilating drops during pregnancy is controversial. Although there have been no teratogenic effects reported with use of topical anesthetics and dilating drops, all drugs should be avoided during the first trimester.7-10 Guidelines have been established by the American Congress of Obstetricians and Gynecologists for X-ray examination and exposure during pregnancy. It has been determined that exposure from a single diagnostic X-ray procedure does not result in harmful fetal effects.11 Magnetic resonance imaging is not associated with any known adverse fetal effects and is a better imaging option during pregnancy, because it is not associated with the use of ionizing radiation.11
The use of CAIs in the first trimester is controversial.4,7 Some believe it should be avoided because it is a Pregnancy Category C drug. However, a single case of sacrococcygeal teratoma has been reported in humans; therefore, some believe this is not a strong basis for withholding the medication in patients with the potential risk for severe vision loss.4,7 In this case, a consult to the patient’s obstetrician was made, and the use of acetazolamide had no effect on the health of the baby.
In pregnant women with IIH with progressive vision loss, failed treatment, or nonadherence, surgery may be necessary. Optic nerve sheath fenestration is preferred due to lower morbidity and mortality compared with shunting procedures.1,2,4,6 The growing fetus may be affected by the peritoneal end of the shunt.4
Related: 49-Year-Old Woman With a Broken Heart
Conclusions
Vision loss associated with IIH can be severe and permanent if left untreated. The best treatments and often the most effective involve weight loss and lumbar puncture. Acetazolamide has been a proven effective treatment in some patients, but some debate exists over the safety of its use during pregnancy. This patient did not have any AEs from its use; however, it did not prove valuable in her treatment. Studies often disagree on the use of acetazolamide in pregnancy; however, all agree that proper patient counseling on potential AEs and management by an obstetrician are important. With proper management, pregnant women with IIH have had outcomes similar to those of the general population.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617.
2. Bruce BB, Biousee V, Newman NJ. Update on idiopathic intracranial hypertension. Am J Ophthalmol. 2011;152(2):163-169.
3. Fields JD, Javendani PP, Falardeau J, et al. Dural venous sinus angioplasty and stenting for the treatment of idiopathic intracranial hypertension. J Neurointerv Surg. 2013;5(1):62-68.
4. Evans RW, Lee AG. Idiopathic intracranial hypertension in pregnancy. Headache. 2010;50(9):1513-1515.
5. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59(10):1492-1495.
6. Martínez-Varea A, Diago-Almela VJ, Abad-Carrascosa A, Perales-Marín A. Progressive visual loss in a pregnant woman with idiopathic intracranial hypertension. Eur J Obstet Gynecol Reprod Biol. 2012;163(1):117-122.
7. Falardeau J, Lobb B, Golden S, Maxfield SD, Tanne E. The use of acetazolamide during pregnancy in intracranial hypertension patients. J Neuroophthalmol. 2013;33(1):9-12.
8. Dinn RB, Harris A, Marcus PS. Ocular changes in pregnancy. Obstet Gynecol Surg. 2003;58(2):137-144.
9. Shultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol. 2005;16(5):308-314.
10. Chung CY, Kwok AKH, Chung KL. Use of ophthalmic medications during pregnancy. Hong Kong Med J. 2004;10(3):191-195.
11. American Congress of Obstetricians and Gynecologists. Committee Opinion. Guidelines for diagnostic imaging during pregnancy. American Congress of Obstetricians and Gynecologists Website. http://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co299.pdf. Published 2004. Accessed October 9, 2015.
1. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617.
2. Bruce BB, Biousee V, Newman NJ. Update on idiopathic intracranial hypertension. Am J Ophthalmol. 2011;152(2):163-169.
3. Fields JD, Javendani PP, Falardeau J, et al. Dural venous sinus angioplasty and stenting for the treatment of idiopathic intracranial hypertension. J Neurointerv Surg. 2013;5(1):62-68.
4. Evans RW, Lee AG. Idiopathic intracranial hypertension in pregnancy. Headache. 2010;50(9):1513-1515.
5. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59(10):1492-1495.
6. Martínez-Varea A, Diago-Almela VJ, Abad-Carrascosa A, Perales-Marín A. Progressive visual loss in a pregnant woman with idiopathic intracranial hypertension. Eur J Obstet Gynecol Reprod Biol. 2012;163(1):117-122.
7. Falardeau J, Lobb B, Golden S, Maxfield SD, Tanne E. The use of acetazolamide during pregnancy in intracranial hypertension patients. J Neuroophthalmol. 2013;33(1):9-12.
8. Dinn RB, Harris A, Marcus PS. Ocular changes in pregnancy. Obstet Gynecol Surg. 2003;58(2):137-144.
9. Shultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol. 2005;16(5):308-314.
10. Chung CY, Kwok AKH, Chung KL. Use of ophthalmic medications during pregnancy. Hong Kong Med J. 2004;10(3):191-195.
11. American Congress of Obstetricians and Gynecologists. Committee Opinion. Guidelines for diagnostic imaging during pregnancy. American Congress of Obstetricians and Gynecologists Website. http://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co299.pdf. Published 2004. Accessed October 9, 2015.
Efficacy of Patient Aligned Care Team Pharmacist Services in Reaching Diabetes and Hyperlipidemia Treatment Goals
According to the CDC, diabetes mellitus (DM) and hyperlipidemia have been distinguished as major contributors to death and disability among adults within the U.S. Although these diseases may often escape a directly malignant etiology, the complications of these metabolic disorders are correlated with long-term disability. Uncontrolled diabetes contributes to 5 major complications in U.S. adults, including myocardial infarction, cerebral vascular accident, lower extremity amputation, renal failure, and hyperglycemic crisis. Hyperlipidemia is another major risk factor listed for advancing heart disease and ischemic stroke. Medical and preventive care are effective means for declining complication rates, but these chronic diseases continue to increase in frequency.1,2
The prevalence of DM and hyperlipidemia among U.S. veterans is uniquely higher than that of the general population. About 9.3% of the U.S. population has been diagnosed with diabetes compared with almost 25% of veterans receiving care through the VHA.3,4 According to the 2012 National Ambulatory Medical Care Survey, 15.2% of patients receiving nonfederal care had a hyperlipidemia diagnosis compared with > 20% of the U.S. veteran population.5,6
Patient-Centered Care
A key initiative of the VHA Office of Patient Care Services in providing coordinated health care is the patient aligned care team (PACT). The PACT model seeks to provide communicative patient-centered care and involves primary care providers (PCPs) as well as other clinical and nonclinical affiliates.7 These team members often include a PCP, a registered and licensed practical nurse, a dietitian, a social worker, clerical support, and a clinical pharmacy specialist (CPS). Each professional uses his or her unique specialty to provide evidence-based care to the veteran. Clinical pharmacy specialist integration into the PACT model is one way to provide greater continuity of care for patients and more comprehensive treatment of chronic diseases. Given the need for regular medication titration, these patients may require a greater allocation of time and resources than PCPs can feasibly give. For this reason, CPSs were integrated into PACTs to allow for focused management of chronic conditions.
Most PACT CPSs at the VA Illiana Health Care System (VAIHCS) have advanced residency training and/or board certification, making them proficient in patient communication, drug knowledge, pharmacology, and therapeutics. Within the VHA, CPSs practice as midlevel providers with a scope of practice. This scope grants them the ability to clinically assess drug therapy, order and evaluate laboratory data, prescribe pertinent medications to treat the disease within the scope, and order consults with other professionals of the PACT team.8
Research Studies
Several studies have revealed that pharmacist-driven outpatient interventions for patients with dyslipidemia have significantly reduced low-density lipoprotein cholesterol (LDL-C).9-14 Mazzolini and colleagues found that VHA pharmacist intervention produced a mean LDL-C reduction of 24.5 mg/dL and increased the percentage of patients reaching their LDL-C goal from 36.8% to 64.3%.9 Similarly, at another VHA facility, telephone interventions with patients were also effective in reducing veterans’ LDL-C levels. Fabbio and colleagues found a mean LDL-C reduction of 44.3 mg/dL when performing retrospective chart reviews of pharmacist interventions.10 Other pharmacist-driven LDL-C outcomes were also positive compared with that of usual care by PCPs, showing mean LDL-C reductions of 10.7 mg/dL and 10.4 mg/dL.11,12 All these studies showed positive impacts on outcomes for patients with dyslipidemia. Additionally, these types of interventions have been shown to maintain both patient and PCP satisfaction.15
Clinical pharmacist interventions in the primary care setting have shown positive impacts in DM control with hemoglobin A1c (A1c) reductions by as much as 1.3% to 3.4%.16-19 The highest A1c reductions were evident when pharmacists had the ability to prescribe medications or work in a collaborative practice model with PCPs.16-18 Independent practice and the ability to prescribe medications have been shown to have more impact than recommendations to physicians alone. Recommendation letters from pharmacists did not produce a significant reduction of A1c in one physician group compared with another physician group not receiving DM management recommendations.20Given the increased prevalence of chronic diseases in the veteran population and the literature to support the value of CPSs as provider extenders, the focus of this analysis was to determine the potential benefit of CPS services to the PACT.
The primary objectives of this analysis were to determine the true impact of PACT CPSs on LDL-C and A1c in the veterans enrolled in VAIHCS Disease State Management (DSM) clinics. If positive impacts were revealed, this study would support expansion of CPS services to include additional staff and the management of additional diseases.
Related: Experiences of Veterans With Diabetes From Shared Medical Appointments
Methods
This analysis was a retrospective chart review approved by the VA Illiana Publication and Presentation Committee as a quality improvement (QI) project. Data were collected through the VistA electronic medical record. Subject data were analyzed in a multicenter fashion. A total of 5 sites within VAIHCS were included for review. The study subjects acted as their own controls and were distributed proportionally by volume of DSM visits at each VAIHCS location.
The primary objectives of this QI analysis were to determine the efficacy of PACT CPSs in reducing LDL-C and/or A1c levels in veterans enrolled in VAIHCS DSM clinics. The primary endpoints of this study were change from baseline LDL-C to first LDL-C drawn between 6 and 9 months and change from baseline A1c to first A1c drawn between 9 and 12 months after enrollment in DSM clinics.
The secondary objectives of this QI analysis were to determine the efficacy of PACT CPSs in improving high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), and total cholesterol (TC) levels in veterans enrolled in DSM clinics. The secondary hyperlipidemia endpoints were the change from baseline HDL-C, TG, and TC to first blood work results and percentage of patients who achieved National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) LDL-C goal between 6 and 9 months after clinic enrollment.21 The secondary DM endpoint was the percentage of patients who achieved the recommended American Diabetes Association A1c goal between 9 and 12 months after enrollment. Mean percentage reduction of primary and relevant secondary endpoints were determined for each study subject.
Subjects selected for inclusion within this analysis were U.S. veterans aged 18 to 75 years who were enrolled in DSM clinics for hyperlipidemia or type 2 DM (T2DM) between September 1, 2011, and September 1, 2013. These subjects did not meet VA performance measures for hyperlipidemia or T2DM at baseline. The key focus of these measures was to include disease prevention and management of diagnosed disease by clinical practice guideline standards. To be included in the analysis, subjects were required to attend DSM clinic appointments for a minimum of 3 months for hyperlipidemia or 6 months for T2DM.
Subjects were excluded from this study if they were nonadherent to clinic visits (defined as missing > 50% of their appointments), were discharged from the clinic due to nonadherence to drug therapy and/or lifestyle interventions, met LDL-C or A1c goals prior to the laboratory collection interval, or had a baseline LDL-C of < 110 mg/dL or baseline A1c of < 8%. Subjects were also excluded if they failed to receive any antihyperlipidemic or antidiabetic agents through the course of their enrollment. Statistics were derived by averaging the percentage change of laboratory parameters per subject. The time frame used was from baseline to the time of primary and secondary endpoint collection. Due to the QI nature of this analysis, power was not targeted for attainment. A randomized sample of 49 subjects was pulled from the population for complete analysis, which was determined by using a random number generator and analyzing corresponding alphabetized patient charts.
Related:Diabetes Patient-Centered Medical Home Approach
Results
Two hundred ninety-five charts were reviewed to yield 49 subjects eligible for the analysis (Figure 1). One subject was eligible for both hyperlipidemia and T2DM. The primary reasons for exclusion were consults for DSM services not related to T2DM or hyperlipidemia (49.4%) and inadequate time of enrollment (30.2%). Less than 10% of exclusions were due to baseline LDL-C < 110 mg/dL or A1c < 8%, unavailable blood work within the collection interval, nonadherence to clinic visits or medications, or other reasons.
Hyperlipidemia
Means and ranges for LDL-C, TG, and TC were all significantly reduced from baseline (Figure 2). The primary endpoint for hyperlipidemia included a 25.1% reduction in mean LDL-C (95% CI, 0.173-0.327). Secondary endpoints included a 12.9% reduction in mean TG from baseline (95% CI, 0.017-0.241) and a 22.5% reduction in mean TC from baseline (95% CI, 0.174-0.276). A 2.1% increase in mean HDL-C was considered nonsignificant (95% CI, -0.082 to -0.042). The percentage of subjects meeting LDL-C goal between 6 and 9 months after enrollment was 36.7% (Table 1).
Twenty-six subjects (63.4%) did not reach their LDL-C goal between 6 and 9 months after clinic enrollment. Of these subjects, an additional analysis was performed to determine potential contributing factors. Eleven of these subjects received moderate- to high-intensity statin therapy, 2 received low-intensity statin therapy, and 3 (without documented statin intolerance) received no statin therapy. Seven subjects had statin intolerance documented in their charts at baseline or during treatment in DSM clinics. Three subjects had documented nonadherence. Subjects receiving no statin therapy due to intolerance or other reasons were prescribed fibrates, cholestyramine, psyllium, or therapeutic lifestyle changes.
Diabetes
Mean A1c and A1c range resulted in a significant reduction from baseline (Figure 3). The primary endpoint for T2DM included a 3.1% reduction in mean A1c (95% CI, 1.45-5.52). The percentage meeting A1c goal between 9 and 12 months after enrollment was 44.4% (Table 2).
Discussion
The results of this analysis suggest a positive impact of CPSs on the care of veterans within VAIHCS, consistent with previous literature. The strengths of this study include a true measure of pharmacist intervention via an extended length of enrollment and regular CPS follow-up visits. Additionally, this was a multicenter design across numerous sites within VAIHCS. The variety of sites showed the impact of differing prescribing practice or consulting habits among CPSs and their associated PACT providers. Subjects were analyzed only if they received a prescription for antihyperlipidemic or antidiabetic medications. This exclusion allowed the analysis to focus on CPS medication adjustment skills.
Related: The Clinical Impact of Electronic Consultation in Diabetes Care
Limitations
This analysis is limited by its retrospective design and the reliance on chart reviews to collect data. As a retrospective analysis, a direct causality between CPS intervention and change in endpoints cannot be determined. Retrospective chart reviews are also subject to both bias and influence from confounding variables due to inability to establish blinding. One confounding variable not assessed was the impact of ancillary PACT members on subject outcomes. Therapeutic lifestyle changes implemented by registered dietitians could have confounded A1c and lipid profile improvements throughout the course of the analysis.
A specific limitation for hyperlipidemia included an early exclusion for meeting LDL-C goal before 3 months. After the completion of several chart reviews, it was determined that many of these patients required rapid or minimal medication adjustment to meet their therapeutic goals. The major limitation for T2DM included a small sample size. This limitation was partially due to the establishment of hyperlipidemia services before T2DM services within VAIHCS DSM clinics. Due to earlier establishment, hyperlipidemia management was better recognized, and consults for this disease were more prevalent. Sample size was also limited for T2DM due to the nature of the chart review and the original data attainment. The review of both diseases was limited due to some subjects not acquiring laboratory values within the predefined collection periods. In some cases, useful data outside the collection interval could not be used.
Although CPSs produced significant reductions in LDL-C, TG, and TC, their ability to provide more impactful results was likely limited due to enrollment for statin intolerance. Some studies indicated the incidence of statin intolerance to be about 5% to 10% of the general population.22 However, in this analysis, 17.1% of patients who did not meet LDL-C goal had some history of or current statin intolerance. Despite this high degree of intolerance, CPS management was still able to effectively improve lipid profiles but to a less significant degree.
A final point to consider is the design of the analysis before the release of the American College of Cardiology/American Heart Association (ACC/AHA) 2013 cholesterol guidelines.23 Target LDL-C reduction is no longer considered the most appropriate management technique for reducing the risk of atherosclerotic cardiovascular disease (ASCVD). However, the hyperlipidemia endpoints in this analysis were directly related to NCEP-ATP III recommendations. The current guidelines focus on the intensity of statin therapy for patients with ASCVD or elevated risk for ASCVD. With the release of this new guideline, a poststudy analysis was completed to apply the new information to previous practice in VAIHCS DSM clinics. Many subjects were already meeting their statin intensity goal without further intervention. In fact, 46.3% of subjects were meeting their goal at the time of primary endpoint collection. Between the release of the new clinical guideline and February 2014, another 14.6% of subjects had changed therapy and were meeting their statin-intensity goal, with or without pharmacist intervention. Another 17.1% of patients had statin intolerance that may have limited their ability to reach their statin-intensity goal. The remaining 22% of subjects (without statin intolerance) did not have any adjustments in hyperlipidemia profiles since the release of the updated guideline; these patients were scheduled to be contacted as a result of this analysis. Further review of patients meeting LDL-C goal at primary endpoint collection would also be beneficial to ensure appropriate management per current ACC/AHA 2013 guidelines.
Conclusion
Pharmacists were able to produce significant improvements in LDL-C and A1c profiles despite the confounding factors mentioned previously. With further analysis, VAIHCS may demonstrate efficacy in other CPS services and have greater potential to expand its services.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
This quality improvement analysis was performed to improve patient care at the VAIHCS, Danville, IL. It was reviewed by the VHA education department, privacy officer, information security officer, and VAIHCS leadership and was determined to meet guidelines for nonresearch, which is exempt from IRB review. As a quality improvement project, these data are not generalizable.
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Centers for Disease Control and Prevention. Diabetes report card, 2014. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2014. www .cdc.gov/diabetes/pdfs/library/diabetesreport card2014.pdf. Accessed August 25, 2015.
2. Fryar CD, Chen T-C, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999-2010. National Center for Health Statistics Data Brief, No. 103. National Center for Health Statistics, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services Website. http://www.cdc.gov /nchs/data/databriefs/db103.htm. Updated August 3, 2012. Accessed August 10, 2015.
3. American Diabetes Association. Statistics about diabetes. American Diabetes Association Website. http://www.diabetes.org/diabetes-basics/statistics. Updated May 18, 2015. Accessed August 10, 2015.
4. U.S. Department of Veterans Affairs. Close to 25% of VA patients have diabetes. U.S. Department of Veterans Affairs Website. http://www.va.gov/health /NewsFeatures/20111115a.asp. Updated April 17, 2015. Accessed August 11, 2015.
5. Centers for Disease Control and Prevention. National ambulatory medical care survey: 2012 summary tables. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs /data/ahcd/namcs_summary/2012_namcs_web _tables.pdf. Accessed August 25, 2015.
6. Utilization of Veterans Affairs Medical Care Services by United States Veterans. New York, NY: Pfizer Inc; 2003.
7. U.S. Department of Veterans Affairs. Primary care services. U.S. Department of Veterans Affairs Website. http://www.va.gov/primarycare/pcmh. Updated May 13, 2015. Accessed August 11, 2015.
8. U.S. Department of Veterans Affairs. Clinical Pharmacy Services. VHA Handbook 1108.11. http://www.va.gov/vhapublications/ViewPublication .asp?pub_ID=3120. Accessed August 25, 2015.
9. Mazzolini TA, Irons BK, Schell EC, Seifert CF. Lipid levels and use of lipid-lowering drugs for patients in pharmacist-managed lipid clinics versus usual care in 2 VA medical centers. J Manag Care Pharm. 2005;11(9):763-771.
10. Fabbio KL, Bradley M, Chrymko M. Evaluation of a pharmacist-managed telephone lipid clinic at a Veterans Affairs Medical Center. Ann Pharmacother. 2010;44(1):50-56.
11. Charrois TL, Zolezzi M, Koshman SL, et al. A systematic review of the evidence for pharmacist care of patients with dyslipidemia. Pharmacother. 2012;32(3):222-233.
12. Smith MC, Boldt AS, Walston CM, Zillich AJ. Effectiveness of a pharmacy care management program for veterans with dyslipidemia. Pharmacother. 2013;33(7):736-743.
13. Till LT, Voris JC, Horst JB. Assessment of clinical pharmacist management of lipid-lowering therapy in a primary care setting. J Manag Care Pharm. 2003;9(3):269-273.
14. Machado M, Nassor N, Bajcar JM, Guzzo GC, Einarson TR. Sensitivity of patient outcomes to pharmacist interventions. Part III: systematic review and meta-analysis in hyperlipidemia management. Ann Pharmacother. 2008;42(9):1195-1207.
15. Collins C, Kramer A, O’Day ME, Low MB. Evaluation of patient and provider satisfaction with a pharmacist-managed lipid clinic in a Veterans Affairs medical center. Am J Health Syst Pharm. 2006;63(18):1723-1727.
16. American Association of Diabetes Educators. The scope and standards for the practice of diabetes education by pharmacists. American Association of Diabetes Educators Website. http://www .diabeteseducator.org/docs/default-source/legacy -docs/_resources/pdf/PharmDScopeStandards.pdf. Updated 2011. Accessed August 11, 2015.
17. Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacother. 2008;28(4):421-436.
18. Armor BL, Britton ML, Dennis VC, Letassy NA. A review of pharmacist contributions to diabetes care in the United States. J Pharm Pract. 2010;23(3):250-264.
19. Jarab AS, Alqudah SG, Mukattash TL, Shattat G, Al-Qirim T. Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan. J Manag Care Pharm. 2012;18(7):516-526.
20. Kirwin JL, Cunningham RJ, Sequist TD. Pharmacist recommendations to improve the quality of diabetes care: a randomized controlled trial. J Manag Care Pharm. 2010;16(2):104-113.
21. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
22. Kennedy SP, Barnas GP, Schmidt MJ, Glisczinski MS, Paniagua AC. Efficacy and tolerability of once-weekly rosuvastatin in patients with previous statin intolerance. J Clin Lipidol. 2011;5(4):308-315.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
According to the CDC, diabetes mellitus (DM) and hyperlipidemia have been distinguished as major contributors to death and disability among adults within the U.S. Although these diseases may often escape a directly malignant etiology, the complications of these metabolic disorders are correlated with long-term disability. Uncontrolled diabetes contributes to 5 major complications in U.S. adults, including myocardial infarction, cerebral vascular accident, lower extremity amputation, renal failure, and hyperglycemic crisis. Hyperlipidemia is another major risk factor listed for advancing heart disease and ischemic stroke. Medical and preventive care are effective means for declining complication rates, but these chronic diseases continue to increase in frequency.1,2
The prevalence of DM and hyperlipidemia among U.S. veterans is uniquely higher than that of the general population. About 9.3% of the U.S. population has been diagnosed with diabetes compared with almost 25% of veterans receiving care through the VHA.3,4 According to the 2012 National Ambulatory Medical Care Survey, 15.2% of patients receiving nonfederal care had a hyperlipidemia diagnosis compared with > 20% of the U.S. veteran population.5,6
Patient-Centered Care
A key initiative of the VHA Office of Patient Care Services in providing coordinated health care is the patient aligned care team (PACT). The PACT model seeks to provide communicative patient-centered care and involves primary care providers (PCPs) as well as other clinical and nonclinical affiliates.7 These team members often include a PCP, a registered and licensed practical nurse, a dietitian, a social worker, clerical support, and a clinical pharmacy specialist (CPS). Each professional uses his or her unique specialty to provide evidence-based care to the veteran. Clinical pharmacy specialist integration into the PACT model is one way to provide greater continuity of care for patients and more comprehensive treatment of chronic diseases. Given the need for regular medication titration, these patients may require a greater allocation of time and resources than PCPs can feasibly give. For this reason, CPSs were integrated into PACTs to allow for focused management of chronic conditions.
Most PACT CPSs at the VA Illiana Health Care System (VAIHCS) have advanced residency training and/or board certification, making them proficient in patient communication, drug knowledge, pharmacology, and therapeutics. Within the VHA, CPSs practice as midlevel providers with a scope of practice. This scope grants them the ability to clinically assess drug therapy, order and evaluate laboratory data, prescribe pertinent medications to treat the disease within the scope, and order consults with other professionals of the PACT team.8
Research Studies
Several studies have revealed that pharmacist-driven outpatient interventions for patients with dyslipidemia have significantly reduced low-density lipoprotein cholesterol (LDL-C).9-14 Mazzolini and colleagues found that VHA pharmacist intervention produced a mean LDL-C reduction of 24.5 mg/dL and increased the percentage of patients reaching their LDL-C goal from 36.8% to 64.3%.9 Similarly, at another VHA facility, telephone interventions with patients were also effective in reducing veterans’ LDL-C levels. Fabbio and colleagues found a mean LDL-C reduction of 44.3 mg/dL when performing retrospective chart reviews of pharmacist interventions.10 Other pharmacist-driven LDL-C outcomes were also positive compared with that of usual care by PCPs, showing mean LDL-C reductions of 10.7 mg/dL and 10.4 mg/dL.11,12 All these studies showed positive impacts on outcomes for patients with dyslipidemia. Additionally, these types of interventions have been shown to maintain both patient and PCP satisfaction.15
Clinical pharmacist interventions in the primary care setting have shown positive impacts in DM control with hemoglobin A1c (A1c) reductions by as much as 1.3% to 3.4%.16-19 The highest A1c reductions were evident when pharmacists had the ability to prescribe medications or work in a collaborative practice model with PCPs.16-18 Independent practice and the ability to prescribe medications have been shown to have more impact than recommendations to physicians alone. Recommendation letters from pharmacists did not produce a significant reduction of A1c in one physician group compared with another physician group not receiving DM management recommendations.20Given the increased prevalence of chronic diseases in the veteran population and the literature to support the value of CPSs as provider extenders, the focus of this analysis was to determine the potential benefit of CPS services to the PACT.
The primary objectives of this analysis were to determine the true impact of PACT CPSs on LDL-C and A1c in the veterans enrolled in VAIHCS Disease State Management (DSM) clinics. If positive impacts were revealed, this study would support expansion of CPS services to include additional staff and the management of additional diseases.
Related: Experiences of Veterans With Diabetes From Shared Medical Appointments
Methods
This analysis was a retrospective chart review approved by the VA Illiana Publication and Presentation Committee as a quality improvement (QI) project. Data were collected through the VistA electronic medical record. Subject data were analyzed in a multicenter fashion. A total of 5 sites within VAIHCS were included for review. The study subjects acted as their own controls and were distributed proportionally by volume of DSM visits at each VAIHCS location.
The primary objectives of this QI analysis were to determine the efficacy of PACT CPSs in reducing LDL-C and/or A1c levels in veterans enrolled in VAIHCS DSM clinics. The primary endpoints of this study were change from baseline LDL-C to first LDL-C drawn between 6 and 9 months and change from baseline A1c to first A1c drawn between 9 and 12 months after enrollment in DSM clinics.
The secondary objectives of this QI analysis were to determine the efficacy of PACT CPSs in improving high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), and total cholesterol (TC) levels in veterans enrolled in DSM clinics. The secondary hyperlipidemia endpoints were the change from baseline HDL-C, TG, and TC to first blood work results and percentage of patients who achieved National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) LDL-C goal between 6 and 9 months after clinic enrollment.21 The secondary DM endpoint was the percentage of patients who achieved the recommended American Diabetes Association A1c goal between 9 and 12 months after enrollment. Mean percentage reduction of primary and relevant secondary endpoints were determined for each study subject.
Subjects selected for inclusion within this analysis were U.S. veterans aged 18 to 75 years who were enrolled in DSM clinics for hyperlipidemia or type 2 DM (T2DM) between September 1, 2011, and September 1, 2013. These subjects did not meet VA performance measures for hyperlipidemia or T2DM at baseline. The key focus of these measures was to include disease prevention and management of diagnosed disease by clinical practice guideline standards. To be included in the analysis, subjects were required to attend DSM clinic appointments for a minimum of 3 months for hyperlipidemia or 6 months for T2DM.
Subjects were excluded from this study if they were nonadherent to clinic visits (defined as missing > 50% of their appointments), were discharged from the clinic due to nonadherence to drug therapy and/or lifestyle interventions, met LDL-C or A1c goals prior to the laboratory collection interval, or had a baseline LDL-C of < 110 mg/dL or baseline A1c of < 8%. Subjects were also excluded if they failed to receive any antihyperlipidemic or antidiabetic agents through the course of their enrollment. Statistics were derived by averaging the percentage change of laboratory parameters per subject. The time frame used was from baseline to the time of primary and secondary endpoint collection. Due to the QI nature of this analysis, power was not targeted for attainment. A randomized sample of 49 subjects was pulled from the population for complete analysis, which was determined by using a random number generator and analyzing corresponding alphabetized patient charts.
Related:Diabetes Patient-Centered Medical Home Approach
Results
Two hundred ninety-five charts were reviewed to yield 49 subjects eligible for the analysis (Figure 1). One subject was eligible for both hyperlipidemia and T2DM. The primary reasons for exclusion were consults for DSM services not related to T2DM or hyperlipidemia (49.4%) and inadequate time of enrollment (30.2%). Less than 10% of exclusions were due to baseline LDL-C < 110 mg/dL or A1c < 8%, unavailable blood work within the collection interval, nonadherence to clinic visits or medications, or other reasons.
Hyperlipidemia
Means and ranges for LDL-C, TG, and TC were all significantly reduced from baseline (Figure 2). The primary endpoint for hyperlipidemia included a 25.1% reduction in mean LDL-C (95% CI, 0.173-0.327). Secondary endpoints included a 12.9% reduction in mean TG from baseline (95% CI, 0.017-0.241) and a 22.5% reduction in mean TC from baseline (95% CI, 0.174-0.276). A 2.1% increase in mean HDL-C was considered nonsignificant (95% CI, -0.082 to -0.042). The percentage of subjects meeting LDL-C goal between 6 and 9 months after enrollment was 36.7% (Table 1).
Twenty-six subjects (63.4%) did not reach their LDL-C goal between 6 and 9 months after clinic enrollment. Of these subjects, an additional analysis was performed to determine potential contributing factors. Eleven of these subjects received moderate- to high-intensity statin therapy, 2 received low-intensity statin therapy, and 3 (without documented statin intolerance) received no statin therapy. Seven subjects had statin intolerance documented in their charts at baseline or during treatment in DSM clinics. Three subjects had documented nonadherence. Subjects receiving no statin therapy due to intolerance or other reasons were prescribed fibrates, cholestyramine, psyllium, or therapeutic lifestyle changes.
Diabetes
Mean A1c and A1c range resulted in a significant reduction from baseline (Figure 3). The primary endpoint for T2DM included a 3.1% reduction in mean A1c (95% CI, 1.45-5.52). The percentage meeting A1c goal between 9 and 12 months after enrollment was 44.4% (Table 2).
Discussion
The results of this analysis suggest a positive impact of CPSs on the care of veterans within VAIHCS, consistent with previous literature. The strengths of this study include a true measure of pharmacist intervention via an extended length of enrollment and regular CPS follow-up visits. Additionally, this was a multicenter design across numerous sites within VAIHCS. The variety of sites showed the impact of differing prescribing practice or consulting habits among CPSs and their associated PACT providers. Subjects were analyzed only if they received a prescription for antihyperlipidemic or antidiabetic medications. This exclusion allowed the analysis to focus on CPS medication adjustment skills.
Related: The Clinical Impact of Electronic Consultation in Diabetes Care
Limitations
This analysis is limited by its retrospective design and the reliance on chart reviews to collect data. As a retrospective analysis, a direct causality between CPS intervention and change in endpoints cannot be determined. Retrospective chart reviews are also subject to both bias and influence from confounding variables due to inability to establish blinding. One confounding variable not assessed was the impact of ancillary PACT members on subject outcomes. Therapeutic lifestyle changes implemented by registered dietitians could have confounded A1c and lipid profile improvements throughout the course of the analysis.
A specific limitation for hyperlipidemia included an early exclusion for meeting LDL-C goal before 3 months. After the completion of several chart reviews, it was determined that many of these patients required rapid or minimal medication adjustment to meet their therapeutic goals. The major limitation for T2DM included a small sample size. This limitation was partially due to the establishment of hyperlipidemia services before T2DM services within VAIHCS DSM clinics. Due to earlier establishment, hyperlipidemia management was better recognized, and consults for this disease were more prevalent. Sample size was also limited for T2DM due to the nature of the chart review and the original data attainment. The review of both diseases was limited due to some subjects not acquiring laboratory values within the predefined collection periods. In some cases, useful data outside the collection interval could not be used.
Although CPSs produced significant reductions in LDL-C, TG, and TC, their ability to provide more impactful results was likely limited due to enrollment for statin intolerance. Some studies indicated the incidence of statin intolerance to be about 5% to 10% of the general population.22 However, in this analysis, 17.1% of patients who did not meet LDL-C goal had some history of or current statin intolerance. Despite this high degree of intolerance, CPS management was still able to effectively improve lipid profiles but to a less significant degree.
A final point to consider is the design of the analysis before the release of the American College of Cardiology/American Heart Association (ACC/AHA) 2013 cholesterol guidelines.23 Target LDL-C reduction is no longer considered the most appropriate management technique for reducing the risk of atherosclerotic cardiovascular disease (ASCVD). However, the hyperlipidemia endpoints in this analysis were directly related to NCEP-ATP III recommendations. The current guidelines focus on the intensity of statin therapy for patients with ASCVD or elevated risk for ASCVD. With the release of this new guideline, a poststudy analysis was completed to apply the new information to previous practice in VAIHCS DSM clinics. Many subjects were already meeting their statin intensity goal without further intervention. In fact, 46.3% of subjects were meeting their goal at the time of primary endpoint collection. Between the release of the new clinical guideline and February 2014, another 14.6% of subjects had changed therapy and were meeting their statin-intensity goal, with or without pharmacist intervention. Another 17.1% of patients had statin intolerance that may have limited their ability to reach their statin-intensity goal. The remaining 22% of subjects (without statin intolerance) did not have any adjustments in hyperlipidemia profiles since the release of the updated guideline; these patients were scheduled to be contacted as a result of this analysis. Further review of patients meeting LDL-C goal at primary endpoint collection would also be beneficial to ensure appropriate management per current ACC/AHA 2013 guidelines.
Conclusion
Pharmacists were able to produce significant improvements in LDL-C and A1c profiles despite the confounding factors mentioned previously. With further analysis, VAIHCS may demonstrate efficacy in other CPS services and have greater potential to expand its services.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
This quality improvement analysis was performed to improve patient care at the VAIHCS, Danville, IL. It was reviewed by the VHA education department, privacy officer, information security officer, and VAIHCS leadership and was determined to meet guidelines for nonresearch, which is exempt from IRB review. As a quality improvement project, these data are not generalizable.
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
According to the CDC, diabetes mellitus (DM) and hyperlipidemia have been distinguished as major contributors to death and disability among adults within the U.S. Although these diseases may often escape a directly malignant etiology, the complications of these metabolic disorders are correlated with long-term disability. Uncontrolled diabetes contributes to 5 major complications in U.S. adults, including myocardial infarction, cerebral vascular accident, lower extremity amputation, renal failure, and hyperglycemic crisis. Hyperlipidemia is another major risk factor listed for advancing heart disease and ischemic stroke. Medical and preventive care are effective means for declining complication rates, but these chronic diseases continue to increase in frequency.1,2
The prevalence of DM and hyperlipidemia among U.S. veterans is uniquely higher than that of the general population. About 9.3% of the U.S. population has been diagnosed with diabetes compared with almost 25% of veterans receiving care through the VHA.3,4 According to the 2012 National Ambulatory Medical Care Survey, 15.2% of patients receiving nonfederal care had a hyperlipidemia diagnosis compared with > 20% of the U.S. veteran population.5,6
Patient-Centered Care
A key initiative of the VHA Office of Patient Care Services in providing coordinated health care is the patient aligned care team (PACT). The PACT model seeks to provide communicative patient-centered care and involves primary care providers (PCPs) as well as other clinical and nonclinical affiliates.7 These team members often include a PCP, a registered and licensed practical nurse, a dietitian, a social worker, clerical support, and a clinical pharmacy specialist (CPS). Each professional uses his or her unique specialty to provide evidence-based care to the veteran. Clinical pharmacy specialist integration into the PACT model is one way to provide greater continuity of care for patients and more comprehensive treatment of chronic diseases. Given the need for regular medication titration, these patients may require a greater allocation of time and resources than PCPs can feasibly give. For this reason, CPSs were integrated into PACTs to allow for focused management of chronic conditions.
Most PACT CPSs at the VA Illiana Health Care System (VAIHCS) have advanced residency training and/or board certification, making them proficient in patient communication, drug knowledge, pharmacology, and therapeutics. Within the VHA, CPSs practice as midlevel providers with a scope of practice. This scope grants them the ability to clinically assess drug therapy, order and evaluate laboratory data, prescribe pertinent medications to treat the disease within the scope, and order consults with other professionals of the PACT team.8
Research Studies
Several studies have revealed that pharmacist-driven outpatient interventions for patients with dyslipidemia have significantly reduced low-density lipoprotein cholesterol (LDL-C).9-14 Mazzolini and colleagues found that VHA pharmacist intervention produced a mean LDL-C reduction of 24.5 mg/dL and increased the percentage of patients reaching their LDL-C goal from 36.8% to 64.3%.9 Similarly, at another VHA facility, telephone interventions with patients were also effective in reducing veterans’ LDL-C levels. Fabbio and colleagues found a mean LDL-C reduction of 44.3 mg/dL when performing retrospective chart reviews of pharmacist interventions.10 Other pharmacist-driven LDL-C outcomes were also positive compared with that of usual care by PCPs, showing mean LDL-C reductions of 10.7 mg/dL and 10.4 mg/dL.11,12 All these studies showed positive impacts on outcomes for patients with dyslipidemia. Additionally, these types of interventions have been shown to maintain both patient and PCP satisfaction.15
Clinical pharmacist interventions in the primary care setting have shown positive impacts in DM control with hemoglobin A1c (A1c) reductions by as much as 1.3% to 3.4%.16-19 The highest A1c reductions were evident when pharmacists had the ability to prescribe medications or work in a collaborative practice model with PCPs.16-18 Independent practice and the ability to prescribe medications have been shown to have more impact than recommendations to physicians alone. Recommendation letters from pharmacists did not produce a significant reduction of A1c in one physician group compared with another physician group not receiving DM management recommendations.20Given the increased prevalence of chronic diseases in the veteran population and the literature to support the value of CPSs as provider extenders, the focus of this analysis was to determine the potential benefit of CPS services to the PACT.
The primary objectives of this analysis were to determine the true impact of PACT CPSs on LDL-C and A1c in the veterans enrolled in VAIHCS Disease State Management (DSM) clinics. If positive impacts were revealed, this study would support expansion of CPS services to include additional staff and the management of additional diseases.
Related: Experiences of Veterans With Diabetes From Shared Medical Appointments
Methods
This analysis was a retrospective chart review approved by the VA Illiana Publication and Presentation Committee as a quality improvement (QI) project. Data were collected through the VistA electronic medical record. Subject data were analyzed in a multicenter fashion. A total of 5 sites within VAIHCS were included for review. The study subjects acted as their own controls and were distributed proportionally by volume of DSM visits at each VAIHCS location.
The primary objectives of this QI analysis were to determine the efficacy of PACT CPSs in reducing LDL-C and/or A1c levels in veterans enrolled in VAIHCS DSM clinics. The primary endpoints of this study were change from baseline LDL-C to first LDL-C drawn between 6 and 9 months and change from baseline A1c to first A1c drawn between 9 and 12 months after enrollment in DSM clinics.
The secondary objectives of this QI analysis were to determine the efficacy of PACT CPSs in improving high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), and total cholesterol (TC) levels in veterans enrolled in DSM clinics. The secondary hyperlipidemia endpoints were the change from baseline HDL-C, TG, and TC to first blood work results and percentage of patients who achieved National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) LDL-C goal between 6 and 9 months after clinic enrollment.21 The secondary DM endpoint was the percentage of patients who achieved the recommended American Diabetes Association A1c goal between 9 and 12 months after enrollment. Mean percentage reduction of primary and relevant secondary endpoints were determined for each study subject.
Subjects selected for inclusion within this analysis were U.S. veterans aged 18 to 75 years who were enrolled in DSM clinics for hyperlipidemia or type 2 DM (T2DM) between September 1, 2011, and September 1, 2013. These subjects did not meet VA performance measures for hyperlipidemia or T2DM at baseline. The key focus of these measures was to include disease prevention and management of diagnosed disease by clinical practice guideline standards. To be included in the analysis, subjects were required to attend DSM clinic appointments for a minimum of 3 months for hyperlipidemia or 6 months for T2DM.
Subjects were excluded from this study if they were nonadherent to clinic visits (defined as missing > 50% of their appointments), were discharged from the clinic due to nonadherence to drug therapy and/or lifestyle interventions, met LDL-C or A1c goals prior to the laboratory collection interval, or had a baseline LDL-C of < 110 mg/dL or baseline A1c of < 8%. Subjects were also excluded if they failed to receive any antihyperlipidemic or antidiabetic agents through the course of their enrollment. Statistics were derived by averaging the percentage change of laboratory parameters per subject. The time frame used was from baseline to the time of primary and secondary endpoint collection. Due to the QI nature of this analysis, power was not targeted for attainment. A randomized sample of 49 subjects was pulled from the population for complete analysis, which was determined by using a random number generator and analyzing corresponding alphabetized patient charts.
Related:Diabetes Patient-Centered Medical Home Approach
Results
Two hundred ninety-five charts were reviewed to yield 49 subjects eligible for the analysis (Figure 1). One subject was eligible for both hyperlipidemia and T2DM. The primary reasons for exclusion were consults for DSM services not related to T2DM or hyperlipidemia (49.4%) and inadequate time of enrollment (30.2%). Less than 10% of exclusions were due to baseline LDL-C < 110 mg/dL or A1c < 8%, unavailable blood work within the collection interval, nonadherence to clinic visits or medications, or other reasons.
Hyperlipidemia
Means and ranges for LDL-C, TG, and TC were all significantly reduced from baseline (Figure 2). The primary endpoint for hyperlipidemia included a 25.1% reduction in mean LDL-C (95% CI, 0.173-0.327). Secondary endpoints included a 12.9% reduction in mean TG from baseline (95% CI, 0.017-0.241) and a 22.5% reduction in mean TC from baseline (95% CI, 0.174-0.276). A 2.1% increase in mean HDL-C was considered nonsignificant (95% CI, -0.082 to -0.042). The percentage of subjects meeting LDL-C goal between 6 and 9 months after enrollment was 36.7% (Table 1).
Twenty-six subjects (63.4%) did not reach their LDL-C goal between 6 and 9 months after clinic enrollment. Of these subjects, an additional analysis was performed to determine potential contributing factors. Eleven of these subjects received moderate- to high-intensity statin therapy, 2 received low-intensity statin therapy, and 3 (without documented statin intolerance) received no statin therapy. Seven subjects had statin intolerance documented in their charts at baseline or during treatment in DSM clinics. Three subjects had documented nonadherence. Subjects receiving no statin therapy due to intolerance or other reasons were prescribed fibrates, cholestyramine, psyllium, or therapeutic lifestyle changes.
Diabetes
Mean A1c and A1c range resulted in a significant reduction from baseline (Figure 3). The primary endpoint for T2DM included a 3.1% reduction in mean A1c (95% CI, 1.45-5.52). The percentage meeting A1c goal between 9 and 12 months after enrollment was 44.4% (Table 2).
Discussion
The results of this analysis suggest a positive impact of CPSs on the care of veterans within VAIHCS, consistent with previous literature. The strengths of this study include a true measure of pharmacist intervention via an extended length of enrollment and regular CPS follow-up visits. Additionally, this was a multicenter design across numerous sites within VAIHCS. The variety of sites showed the impact of differing prescribing practice or consulting habits among CPSs and their associated PACT providers. Subjects were analyzed only if they received a prescription for antihyperlipidemic or antidiabetic medications. This exclusion allowed the analysis to focus on CPS medication adjustment skills.
Related: The Clinical Impact of Electronic Consultation in Diabetes Care
Limitations
This analysis is limited by its retrospective design and the reliance on chart reviews to collect data. As a retrospective analysis, a direct causality between CPS intervention and change in endpoints cannot be determined. Retrospective chart reviews are also subject to both bias and influence from confounding variables due to inability to establish blinding. One confounding variable not assessed was the impact of ancillary PACT members on subject outcomes. Therapeutic lifestyle changes implemented by registered dietitians could have confounded A1c and lipid profile improvements throughout the course of the analysis.
A specific limitation for hyperlipidemia included an early exclusion for meeting LDL-C goal before 3 months. After the completion of several chart reviews, it was determined that many of these patients required rapid or minimal medication adjustment to meet their therapeutic goals. The major limitation for T2DM included a small sample size. This limitation was partially due to the establishment of hyperlipidemia services before T2DM services within VAIHCS DSM clinics. Due to earlier establishment, hyperlipidemia management was better recognized, and consults for this disease were more prevalent. Sample size was also limited for T2DM due to the nature of the chart review and the original data attainment. The review of both diseases was limited due to some subjects not acquiring laboratory values within the predefined collection periods. In some cases, useful data outside the collection interval could not be used.
Although CPSs produced significant reductions in LDL-C, TG, and TC, their ability to provide more impactful results was likely limited due to enrollment for statin intolerance. Some studies indicated the incidence of statin intolerance to be about 5% to 10% of the general population.22 However, in this analysis, 17.1% of patients who did not meet LDL-C goal had some history of or current statin intolerance. Despite this high degree of intolerance, CPS management was still able to effectively improve lipid profiles but to a less significant degree.
A final point to consider is the design of the analysis before the release of the American College of Cardiology/American Heart Association (ACC/AHA) 2013 cholesterol guidelines.23 Target LDL-C reduction is no longer considered the most appropriate management technique for reducing the risk of atherosclerotic cardiovascular disease (ASCVD). However, the hyperlipidemia endpoints in this analysis were directly related to NCEP-ATP III recommendations. The current guidelines focus on the intensity of statin therapy for patients with ASCVD or elevated risk for ASCVD. With the release of this new guideline, a poststudy analysis was completed to apply the new information to previous practice in VAIHCS DSM clinics. Many subjects were already meeting their statin intensity goal without further intervention. In fact, 46.3% of subjects were meeting their goal at the time of primary endpoint collection. Between the release of the new clinical guideline and February 2014, another 14.6% of subjects had changed therapy and were meeting their statin-intensity goal, with or without pharmacist intervention. Another 17.1% of patients had statin intolerance that may have limited their ability to reach their statin-intensity goal. The remaining 22% of subjects (without statin intolerance) did not have any adjustments in hyperlipidemia profiles since the release of the updated guideline; these patients were scheduled to be contacted as a result of this analysis. Further review of patients meeting LDL-C goal at primary endpoint collection would also be beneficial to ensure appropriate management per current ACC/AHA 2013 guidelines.
Conclusion
Pharmacists were able to produce significant improvements in LDL-C and A1c profiles despite the confounding factors mentioned previously. With further analysis, VAIHCS may demonstrate efficacy in other CPS services and have greater potential to expand its services.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
This quality improvement analysis was performed to improve patient care at the VAIHCS, Danville, IL. It was reviewed by the VHA education department, privacy officer, information security officer, and VAIHCS leadership and was determined to meet guidelines for nonresearch, which is exempt from IRB review. As a quality improvement project, these data are not generalizable.
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Centers for Disease Control and Prevention. Diabetes report card, 2014. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2014. www .cdc.gov/diabetes/pdfs/library/diabetesreport card2014.pdf. Accessed August 25, 2015.
2. Fryar CD, Chen T-C, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999-2010. National Center for Health Statistics Data Brief, No. 103. National Center for Health Statistics, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services Website. http://www.cdc.gov /nchs/data/databriefs/db103.htm. Updated August 3, 2012. Accessed August 10, 2015.
3. American Diabetes Association. Statistics about diabetes. American Diabetes Association Website. http://www.diabetes.org/diabetes-basics/statistics. Updated May 18, 2015. Accessed August 10, 2015.
4. U.S. Department of Veterans Affairs. Close to 25% of VA patients have diabetes. U.S. Department of Veterans Affairs Website. http://www.va.gov/health /NewsFeatures/20111115a.asp. Updated April 17, 2015. Accessed August 11, 2015.
5. Centers for Disease Control and Prevention. National ambulatory medical care survey: 2012 summary tables. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs /data/ahcd/namcs_summary/2012_namcs_web _tables.pdf. Accessed August 25, 2015.
6. Utilization of Veterans Affairs Medical Care Services by United States Veterans. New York, NY: Pfizer Inc; 2003.
7. U.S. Department of Veterans Affairs. Primary care services. U.S. Department of Veterans Affairs Website. http://www.va.gov/primarycare/pcmh. Updated May 13, 2015. Accessed August 11, 2015.
8. U.S. Department of Veterans Affairs. Clinical Pharmacy Services. VHA Handbook 1108.11. http://www.va.gov/vhapublications/ViewPublication .asp?pub_ID=3120. Accessed August 25, 2015.
9. Mazzolini TA, Irons BK, Schell EC, Seifert CF. Lipid levels and use of lipid-lowering drugs for patients in pharmacist-managed lipid clinics versus usual care in 2 VA medical centers. J Manag Care Pharm. 2005;11(9):763-771.
10. Fabbio KL, Bradley M, Chrymko M. Evaluation of a pharmacist-managed telephone lipid clinic at a Veterans Affairs Medical Center. Ann Pharmacother. 2010;44(1):50-56.
11. Charrois TL, Zolezzi M, Koshman SL, et al. A systematic review of the evidence for pharmacist care of patients with dyslipidemia. Pharmacother. 2012;32(3):222-233.
12. Smith MC, Boldt AS, Walston CM, Zillich AJ. Effectiveness of a pharmacy care management program for veterans with dyslipidemia. Pharmacother. 2013;33(7):736-743.
13. Till LT, Voris JC, Horst JB. Assessment of clinical pharmacist management of lipid-lowering therapy in a primary care setting. J Manag Care Pharm. 2003;9(3):269-273.
14. Machado M, Nassor N, Bajcar JM, Guzzo GC, Einarson TR. Sensitivity of patient outcomes to pharmacist interventions. Part III: systematic review and meta-analysis in hyperlipidemia management. Ann Pharmacother. 2008;42(9):1195-1207.
15. Collins C, Kramer A, O’Day ME, Low MB. Evaluation of patient and provider satisfaction with a pharmacist-managed lipid clinic in a Veterans Affairs medical center. Am J Health Syst Pharm. 2006;63(18):1723-1727.
16. American Association of Diabetes Educators. The scope and standards for the practice of diabetes education by pharmacists. American Association of Diabetes Educators Website. http://www .diabeteseducator.org/docs/default-source/legacy -docs/_resources/pdf/PharmDScopeStandards.pdf. Updated 2011. Accessed August 11, 2015.
17. Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacother. 2008;28(4):421-436.
18. Armor BL, Britton ML, Dennis VC, Letassy NA. A review of pharmacist contributions to diabetes care in the United States. J Pharm Pract. 2010;23(3):250-264.
19. Jarab AS, Alqudah SG, Mukattash TL, Shattat G, Al-Qirim T. Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan. J Manag Care Pharm. 2012;18(7):516-526.
20. Kirwin JL, Cunningham RJ, Sequist TD. Pharmacist recommendations to improve the quality of diabetes care: a randomized controlled trial. J Manag Care Pharm. 2010;16(2):104-113.
21. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
22. Kennedy SP, Barnas GP, Schmidt MJ, Glisczinski MS, Paniagua AC. Efficacy and tolerability of once-weekly rosuvastatin in patients with previous statin intolerance. J Clin Lipidol. 2011;5(4):308-315.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
1. Centers for Disease Control and Prevention. Diabetes report card, 2014. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2014. www .cdc.gov/diabetes/pdfs/library/diabetesreport card2014.pdf. Accessed August 25, 2015.
2. Fryar CD, Chen T-C, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999-2010. National Center for Health Statistics Data Brief, No. 103. National Center for Health Statistics, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services Website. http://www.cdc.gov /nchs/data/databriefs/db103.htm. Updated August 3, 2012. Accessed August 10, 2015.
3. American Diabetes Association. Statistics about diabetes. American Diabetes Association Website. http://www.diabetes.org/diabetes-basics/statistics. Updated May 18, 2015. Accessed August 10, 2015.
4. U.S. Department of Veterans Affairs. Close to 25% of VA patients have diabetes. U.S. Department of Veterans Affairs Website. http://www.va.gov/health /NewsFeatures/20111115a.asp. Updated April 17, 2015. Accessed August 11, 2015.
5. Centers for Disease Control and Prevention. National ambulatory medical care survey: 2012 summary tables. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs /data/ahcd/namcs_summary/2012_namcs_web _tables.pdf. Accessed August 25, 2015.
6. Utilization of Veterans Affairs Medical Care Services by United States Veterans. New York, NY: Pfizer Inc; 2003.
7. U.S. Department of Veterans Affairs. Primary care services. U.S. Department of Veterans Affairs Website. http://www.va.gov/primarycare/pcmh. Updated May 13, 2015. Accessed August 11, 2015.
8. U.S. Department of Veterans Affairs. Clinical Pharmacy Services. VHA Handbook 1108.11. http://www.va.gov/vhapublications/ViewPublication .asp?pub_ID=3120. Accessed August 25, 2015.
9. Mazzolini TA, Irons BK, Schell EC, Seifert CF. Lipid levels and use of lipid-lowering drugs for patients in pharmacist-managed lipid clinics versus usual care in 2 VA medical centers. J Manag Care Pharm. 2005;11(9):763-771.
10. Fabbio KL, Bradley M, Chrymko M. Evaluation of a pharmacist-managed telephone lipid clinic at a Veterans Affairs Medical Center. Ann Pharmacother. 2010;44(1):50-56.
11. Charrois TL, Zolezzi M, Koshman SL, et al. A systematic review of the evidence for pharmacist care of patients with dyslipidemia. Pharmacother. 2012;32(3):222-233.
12. Smith MC, Boldt AS, Walston CM, Zillich AJ. Effectiveness of a pharmacy care management program for veterans with dyslipidemia. Pharmacother. 2013;33(7):736-743.
13. Till LT, Voris JC, Horst JB. Assessment of clinical pharmacist management of lipid-lowering therapy in a primary care setting. J Manag Care Pharm. 2003;9(3):269-273.
14. Machado M, Nassor N, Bajcar JM, Guzzo GC, Einarson TR. Sensitivity of patient outcomes to pharmacist interventions. Part III: systematic review and meta-analysis in hyperlipidemia management. Ann Pharmacother. 2008;42(9):1195-1207.
15. Collins C, Kramer A, O’Day ME, Low MB. Evaluation of patient and provider satisfaction with a pharmacist-managed lipid clinic in a Veterans Affairs medical center. Am J Health Syst Pharm. 2006;63(18):1723-1727.
16. American Association of Diabetes Educators. The scope and standards for the practice of diabetes education by pharmacists. American Association of Diabetes Educators Website. http://www .diabeteseducator.org/docs/default-source/legacy -docs/_resources/pdf/PharmDScopeStandards.pdf. Updated 2011. Accessed August 11, 2015.
17. Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacother. 2008;28(4):421-436.
18. Armor BL, Britton ML, Dennis VC, Letassy NA. A review of pharmacist contributions to diabetes care in the United States. J Pharm Pract. 2010;23(3):250-264.
19. Jarab AS, Alqudah SG, Mukattash TL, Shattat G, Al-Qirim T. Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan. J Manag Care Pharm. 2012;18(7):516-526.
20. Kirwin JL, Cunningham RJ, Sequist TD. Pharmacist recommendations to improve the quality of diabetes care: a randomized controlled trial. J Manag Care Pharm. 2010;16(2):104-113.
21. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
22. Kennedy SP, Barnas GP, Schmidt MJ, Glisczinski MS, Paniagua AC. Efficacy and tolerability of once-weekly rosuvastatin in patients with previous statin intolerance. J Clin Lipidol. 2011;5(4):308-315.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
Barriers to the Prevention and Treatment of Geriatric Diabetes
Diabetes mellitus (DM) is a chronic disease that is commonly reported in older adults in primary care. Many adults aged > 65 years with DM have other chronic diseases that make management of their care more complex. Overseeing DM care in older adults while comanaging other chronic diseases is a challenge to health care providers (HCPs). The terms older adults and geriatric define persons aged ≥ 65 years.
Diabetes mellitus is growing at a rapid rate, and older adults are at higher risk. In 2012, about 29.1 million people in the U.S. (9.3%) were diagnosed with DM. Of that number, 11.2 million were aged ≥ 65 years. Additionally, 86 million adults had prediabetes when fasting blood glucose and A1c levels were reviewed. Also in 2012, more than 400,000 new cases (11.5 per 1,000 people) were diagnosed in the aged ≥ 65 years group.1-3 This age group is anticipated to double in 25 years, and the incidence of DM is projected to increase 3.2-fold.4 By 2050, 26.7 million older adults—55% of the older adult population—will have DM. As a result, HCPs are faced with treating escalating numbers of older adults with DM as the population ages.4
In 2012, the total cost of DM for the U.S. population was $245 billion: The direct cost of medical care was $176 billion, and the indirect costs in productivity, absenteeism, unemployment, disability, and premature death was nearly $69 billion.2 This is a significant burden in terms of health care costs, productivity, disability, sick days, early retirement, and premature death. Diabetes mellitus increases atherosclerosis and thus accelerates the risk for heart disease, stroke, kidney disease, blindness, and limb amputations.2
Managing DM concurrently with multiple chronic comorbid conditions is challenging. Patients are asked to bring blood glucose under tight control, perform regular blood glucose testing, take antiglycemic medications, watch their diet, lose weight, and exercise regularly—all while managing other chronic diseases. Many older patients are overwhelmed by the demands of self-management recommended by their HCPs. Similarly, HCPs are frustrated with their older patients, who are unable to adequately meet targeted goals for DM management and thereby reduce the associated risks for complications.
The purpose of this article is to discuss the common barriers to DM management, the experiences of patients and HCPs regarding those barriers, and the management strategies for overcoming barriers in treating older adults with DM.
What Are The Barriers?
The experiences of both patients and HCPs matter when working to overcome DM barriers. If no one understands the problem, no one can fix it. What concerns do patients and HCPs have? Do they really value each other’s perspectives? To overcome barriers, can HCPs and patients develop mutually agreed on goals that are reasonable and practical to implement within the framework of a partnership?
Patient Experiences
Continuity of care and access. Some older adults are seen by multiple HCPs during health care visits, and as a result, they receive mixed messages on what is expected of them.3 Patients feel they have a greater sense of security and confidence when they have a therapeutic relationship with a trusted HCP; they feel more connected and confident about their health care system.5
Lack of education. Many patients say they need more DM education, guidance, and support.6 They report that HCPs tell them how to control DM to avoid complications but say they need more education on how DM affects their lives and concrete suggestions on how to change their lifestyles. Researchers say patients need to feel empowered so they can take a leadership role in managing medications, diet, exercise, preventive foot and eye care, and stress.7 In contrast, an empowerment approach identifies patients’ inherent capacity to self-direct and motivate themselves to develop a self-managed plan based on their personal goals and priorities.8 Patients want to be part of the solution.
Communication and language. A significant challenge for elderly patients is loss of hearing and/or vision, which results in difficulty communicating with their HCP.9 The loss of hearing or vision decreases the ability to adequately collaborate with HCPs and hampers an older adult’s ability to take the lead on self-management. As the U.S. population becomes more diverse, language also poses a significant barrier to care. A language barrier inherently affects health literacy about the disease as well as patients’ perceived trust in HCPs to manage their disease.3
Medication regimen. There are numerous barriers to taking medications. Polypharmacy is a common cause of more drug interactions and adverse effects, which are the most common reason for stopping medications.3,10 Cost of medications and difficulty keeping track of multiple medications are also a deterrent to self-management and adherence. Polypharmacy is seen as detrimental to quality of life (QOL).3 Some patients are also resistant to the initiation and titration of insulin.11
Lack of resources. Many patients cite a lack of resources to facilitate DM care. Common barriers include delays in being scheduled for medical appointments, lack of transportation to appointments, difficulty paying out-of-pocket copays, high cost of medications, and cost of DM supplies (eg, glucometers, test strips, insulin pens and/or pumps). The lack of access to community green spaces or gyms to increase physical activity is also a common barrier.12
Health Care Provider Experiences
Lack of motivation. Health care providers’ experiences and motivations can also present barriers to care. Some HCPs believe that evidence-based guidelines are simply theoretical frameworks; they disagree with using these guidelines as a basis to initiate statin therapy or antiglycemic medications, which reduce cardiovascular complications.13 Many also feel justified in taking a more lax approach when treating older adults due to a lack of time.13
Lack of education about DM management. Health care providers often feel less prepared to provide DM care and believe additional education in DM care is needed. Many lack formal postgraduate DM education or professional development, and 19.6% have no postgraduate DM education or training.14 Some are uncomfortable managing insulin because of a lack of knowledge of insulin therapy and its effect on cardiovascular risk. This results in patients remaining too long on oral DM medications and delaying the necessary initiation of insulin.13
Lack of resources. Some HCPs do not have qualified staff, such as dieticians and diabetes nurse educators, to support DM care. Fearing a loss of control over individual patient care, some HCPs also find it difficult to collaborate with multidisciplinary diabetes care team members, such as psychologists and diabetes educators.13 A lack of awareness of community programs hampers HCPs’ ability to get patients connected to resources that help them make lifestyle changes.14
Lack of involvement or empowerment. Health care providers often think patients do not act with a sense of empowerment in DM management. Health care providers commonly perceive patients as lacking the motivation to change and say that as many as 30% of patients are uncooperative, regardless of proposed changes.13 Many are convinced that patients are unwilling to make even small lifestyle adjustments, such as getting physically active and losing weight. Health care providers say patients do not ask questions about DM selfmanagement during visits and often do not verbalize how HCPs can best support their needs.15 They say that patients are so entrenched in their habits, they even refuse DM education.13
Management Strategies
Several strategies can be deployed to overcome barriers in DM care. Of utmost importance is the need to provide patient-centered care with age-specific characteristics of older adults (Table). To foster mutual collaboration in DM care, HCPs need to ask patients about their health care goals. Patients often view their health through a functional and social perspective rather than from a biomedical perspective. In one study, 71% of patients said their most common health goal was to be independent with activities of daily living, which was more important than the specific details of DM care.16 Preventing DM complications was among their secondary health care goals.
A DM care plan for older adults should be individualized with careful consideration given to medical history, functional capacity, home care environment, and life expectancy. Many older adults have health problems, such as impaired vision, cognitive impairment, depression, and peripheral sensory neuropathy. They may have osteoarthritis of the knees, osteoporosis of the hip and spine, or urinary incontinence; all these conditions increase the risk for falls. Many older adults are on multiple medications, which can increase falls by causing dizziness, dehydration, or hypotension.17 Polypharmacy can negatively impact one or more comorbid conditions and QOL.18
The clinical guidelines for DM management are based on studies conducted in younger populations. However, the 2015 guidelines from the American Diabetes Association (ADA) have been tailored to consider level of health, frailty, cognition, comorbidities, and life expectancy of older adults. The 2015 ADA recommendations provide a framework to guide treatment goals in older adults. A reasonable goal for healthy older adults with few chronic diseases, intact cognition, high functional status, and an anticipated longer remaining life expectancy is an A1c of < 7.5%.19 For older adults with comorbidities of intermediate complexity, such as mild cognitive impairment, an A1c treatment goal of < 8.0% is suggested. An A1c goal of < 8.5% is recommended for older adults in poor health, such as those with end-stage chronic disease, significant cognitive impairment, or those in long-term care. Health care providers may choose to further individualize A1c treatment goals to < 7% if patients are healthy and if treatment burdens are not severe or excessive.19
Multidisciplinary Collaboration
Chronic illness in older adults can be complex to manage due to competing comorbidities and polypharmacy. A diabetes care team consisting of a dietician, social worker, pharmacist, and certified diabetes educator is well suited to effectively manage DM in older adults. Up to 10 hours of DM education with a registered dietician or certified diabetes educator is covered under Medicare in a 12-month period if at least one of the following criteria are met: new diagnosis of DM with A1c > 8.5%, recent initiation of medication, or a high risk for complications.20
Motivational Interviewing
Many HCPs are frustrated that they are unable to persuade patients to adhere to their DM care recommendations. Health care providers often use strategies such as badgering or blaming patients for being nonadherent or scare tactics about the negative consequences of the disease.21 This approach is often ineffective and results in patients becoming more resistant to change.
Motivational interviewing using open-ended questions is an evidenced-based counseling technique that has been shown to elicit sustained behavioral changes. Motivational interviewing increases intrinsic motivation within patients and establishes a goal of incorporating patient-centered values into care by examining ambivalence and passivity in a nonjudgmental way.22 Motivational interviewing facilitates empowerment by using a decision-making process based on each individual’s unique physical, emotional, and environmental circumstances. With guidance from HCPs, patients are able to set the ground rules for DM management by defining a plan that works best for them. For example, a patient may consider a meal plan with stricter caloric intake vs one with a higher calorie count but with more frequent insulin injections or blood glucose monitoring. This strategy puts patients at the center of decision making about medications, diet, and exercise. It also allows them to implement an individualized plan that they believe will work best for them based on their own perceived goals, priorities, and stressors. This approach is shown to work effectively in DM care.8,23
Medication Regimen and Glucose Monitoring
Hypoglycemia is a major concern when managing DM in older adults.20 Hypoglycemia can be triggered by polypharmacy, cognitive impairment, renal insufficiency, sedatives, alcohol intake, malnutrition, and the use of sulfonylureas or insulin. Medications should be considered within the context of other geriatric problems such as falls, depression, urinary incontinence, and pain.20 A simplified approach based on the patient’s functional and cognitive abilities is a good starting point.20 Unless contraindicated, medication initiation could begin with a biguanide.1 Sulfonylureas should generally be avoided in older adults due to the high risk of hypoglycemia.1 Older adults with frequent hypoglycemia should be referred to an endocrinologist or diabetes educator for further management.24
Insulin therapy is recommended if oral therapy alone is insufficient or fails.20 Insulin can be prescribed with adequate DM education and blood glucose monitoring. When prescribing insulin, HCPs should consider older patients’ physical dexterity, visual acuity, cognitive function, financial circumstances, and family support to determine whether insulin therapy is a realistic option that patients can appropriately manage.12,20
Many older adults are resistant to starting insulin and are often reluctant to titrate insulin doses between clinic visits as prescribed by HCPs.12 Older adults on insulin need reassurance and education from a diabetes educator or HCP to gain confidence in adjusting insulin.12 A simple approach to starting insulin can be to start with an evening dose of long-acting insulin.20 Short-acting agents can be added later as needed to control postprandial hyperglycemia.20 Prefilled insulin flex pens also provide a quick and easy way to administer insulin in precise doses.20
Barriers to self-monitoring of blood glucose include pain from finger sticks, inconvenience of testing, and the expense of test strips.25 The newer glucometers and test strips use smaller amounts of blood from other body parts such as the upper arm, calf, or thigh, and these glucometers are ideally suited for older adults.20
Lifestyle Modifications
Lifestyle interventions, including weight loss and exercise, are the mainstay in glycemic control of diabetes. The Diabetes Prevention Program study found a group of patients on lifestyle intervention alone (weight loss goal of 7% and ≥ 150 minutes of weekly exercise) had a 58% lower prevalence of DM compared with a group of patients taking metformin, which had a 31% lower prevalence.26 When older adults were compared with younger persons, lifestyle interventions were more effective than was taking metformin. High-intensity resistance training with moderate weights and repetition lowered glycemic index and caused a 3-fold reduction in A1c in older patients.27,28
Case Management
Older adults may have difficulty getting in touch with HCPs through traditional automated telephone systems. Many have difficulty transmitting glucose monitoring log sheets to HCPs for medication adjustments, which can result in delayed interventions. Telephone visits initiated by a competent case manager can serve as a primary point of contact between HCPs and older adults to optimize treatment and effectively get patients to targeted goals.
Telemedicine is an important tool for monitoring older adults in their home. The technology includes installing a home telemedicine unit, which supports videoconferencing, exchanging messages with case managers, uploading blood glucose readings, and accessing DM educational materials. A study on medication adherence in older diabetic patients found increased adherence through telemonitoring.29 Telemedicine can quickly identify new or persistent barriers between clinic visits so interventions can be made.
A case manager can also facilitate family and social support to address issues such as infrequent glucose monitoring, infrequent medical appointments, caregiver stress, lack of transportation, and financial difficulties, all of which can adversely affect DM care for older adults. The use of a network of family and friends is a good tool for DM management. One study found that when family or friends attended clinic visits, patients were more motivated to understand, follow HCP advice, and find resolutions to difficult issues in DM care.30
Conclusion
Diabetes is a chronic illness with a high burden for older adults. It is important to understand the experiences of patients and HCPs that influence common diabetes barriers. In older adults, barriers should be evaluated in an age-specific context to devise practical interventions to overcome them. Individualizing therapies and empowering older adults prepares them to live confidently while maintaining a sense of control over their lives. A patientcentered collaboration between HCPs and older adults that incorporates a multidisciplinary team approach to resolve problems can improve patient outcomes.
Additional research is needed to identify methods that are most suitable and applicable to older adults. If new evidenced-based research can eliminate diabetes barriers and improve diabetes care in older adults, the consequential burden of diabetes is more likely to decline.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Geriatrics Society Expert Panel on Care of Older Adults With Diabetes Mellitus; Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 Update. J Am Geriatr Soc. 2013;61(11):2020-2026.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
3. Williams A, Manias E. Exploring motivation and confidence in taking prescribed medicines in coexisting diseases: a qualitative study. J Clin Nurs. 2014;23(3-4):471-481.
4. Narayan KMV, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent
increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care.
2006;29(9):2114-2116.
5. von Bültzingslöwen I, Eliasson G, Sarvimäki A, Mattsson B, Hjortdahl P. Patients’ views on interpersonal continuity in primary care: a sense of security based on four core foundations. Fam Pract. 2006;23(2):210-219.
6. Koenigsberg MR, Bartlett D, Cramer JS. Facilitating treatment adherence with lifestyle changes in diabetes. Am Fam Physician. 2004;69(2):309-316.
7. Mensing C, Boucher J, Cypress M, et al. National standards for diabetes self-management education. Task Force to Review and Revise the National Standards for Diabetes Self-Management Education Programs. Diabetes Care. 2000;23(5):682-689.
8. Funnell MM, Weiss MA. Empowering patients with diabetes. Nursing. 2009;39(3):34-37.
9. Hammouda EI. Overcoming barriers to diabetes control in geriatrics. Int J Clin Pract. 2011;65(4):420-424.
10. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650-2664.
11. Brod M, Kongsø JH, Lessard S, Christensen TL. Psychological insulin resistance: patient beliefs and implications for diabetes management. Qual Life Res. 2009;18(1):23-32.
12. Munshi MN, Segal AR, Suhl E, et al. Assessment of barriers to improve diabetes management in older adults: a randomized controlled study. Diabetes Care. 2013;36(3):543-549.
13. Goderis G, Borgermans L, Mathieu C, et al. Barriers and facilitators to evidence based care of type 2 diabetes patients: experiences of general practitioners participating to a quality improvement program. Implement Sci. 2009;4:41.
14. Raaijmakers LGM, Hamers FJM, Martens MK, Bagchus C, de Vries NK, Kremers SPJ. Perceived facilitators and barriers in diabetes care: a qualitative study among health care professionals in the Netherlands. BMC Fam Pract. 2013;14(1):114.
15. Holt RIG, Nicolucci A, Kovacs Burns K, et al; DAWN2 Study Group. Diabetes Attitudes, Wishes and Needs second study (DAWN2™): cross-national comparisons on barriers and resources for optimal care—healthcare professional perspective. Diabet Med. 2013;30(7):789-798.
16. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53(2):306-311.
17. Haas LB. Special considerations for older adults with diabetes residing in skilled nursing facilities. Diabetes Spectrum. 2014;27(1):37-43.
18. Mason NA, Bakus JL. Strategies for reducing polypharmacy and other medicationrelated problems in chronic kidney disease. Semin Dial. 2010;23(1):55-61.
19. American Diabetes Association. Older adults. Sec.10. In Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S67-S69.
20. Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med. 2008;75(1):70-78.
21. Gabbay RA, Durdock K. Strategies to increase adherence through diabetes technology. J Diabetes Sci Technol. 2010;4(3):661-665.
22. Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: The Guilford Press; 2008.
23. West D, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081-1087.
24. Graber AL, Elasy TA, Quinn D, Wolff K, Brown A. Improving glycemic control in adults with diabetes mellitus: shared responsibility in primary care practices. South Med J. 2002;95(7):684-690.
25. Zgibor JC, Simmons D. Barriers to blood glucose monitoring in a multiethnic community. Diabetes Care. 2002;25(10):1772-1777.
26. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
27. Witham MD, Avenell A. Interventions to achieve long-term weight loss in obese older people: a systematic review and meta-analysis. Age Ageing. 2010;39(2):176-184.
28. Dunstan DW, Daly RM, Owen N, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729-1736.
29. Brath H, Morak J, Kästenbauer T, et al. Mobile health (mHealth) based medication adherence measurement–a pilot trial using electronic blisters in diabetes patients. Br J Clin Pharmacol. 2013;76(suppl 1):47-55.
30. Rosland AM, Piette JD, Choi H, Heisler M. Family and friend participation in primary care visits of patients with diabetes or heart failure: patient and physician determinants and experiences. Med Care. 2011;49(1):37-45.
Diabetes mellitus (DM) is a chronic disease that is commonly reported in older adults in primary care. Many adults aged > 65 years with DM have other chronic diseases that make management of their care more complex. Overseeing DM care in older adults while comanaging other chronic diseases is a challenge to health care providers (HCPs). The terms older adults and geriatric define persons aged ≥ 65 years.
Diabetes mellitus is growing at a rapid rate, and older adults are at higher risk. In 2012, about 29.1 million people in the U.S. (9.3%) were diagnosed with DM. Of that number, 11.2 million were aged ≥ 65 years. Additionally, 86 million adults had prediabetes when fasting blood glucose and A1c levels were reviewed. Also in 2012, more than 400,000 new cases (11.5 per 1,000 people) were diagnosed in the aged ≥ 65 years group.1-3 This age group is anticipated to double in 25 years, and the incidence of DM is projected to increase 3.2-fold.4 By 2050, 26.7 million older adults—55% of the older adult population—will have DM. As a result, HCPs are faced with treating escalating numbers of older adults with DM as the population ages.4
In 2012, the total cost of DM for the U.S. population was $245 billion: The direct cost of medical care was $176 billion, and the indirect costs in productivity, absenteeism, unemployment, disability, and premature death was nearly $69 billion.2 This is a significant burden in terms of health care costs, productivity, disability, sick days, early retirement, and premature death. Diabetes mellitus increases atherosclerosis and thus accelerates the risk for heart disease, stroke, kidney disease, blindness, and limb amputations.2
Managing DM concurrently with multiple chronic comorbid conditions is challenging. Patients are asked to bring blood glucose under tight control, perform regular blood glucose testing, take antiglycemic medications, watch their diet, lose weight, and exercise regularly—all while managing other chronic diseases. Many older patients are overwhelmed by the demands of self-management recommended by their HCPs. Similarly, HCPs are frustrated with their older patients, who are unable to adequately meet targeted goals for DM management and thereby reduce the associated risks for complications.
The purpose of this article is to discuss the common barriers to DM management, the experiences of patients and HCPs regarding those barriers, and the management strategies for overcoming barriers in treating older adults with DM.
What Are The Barriers?
The experiences of both patients and HCPs matter when working to overcome DM barriers. If no one understands the problem, no one can fix it. What concerns do patients and HCPs have? Do they really value each other’s perspectives? To overcome barriers, can HCPs and patients develop mutually agreed on goals that are reasonable and practical to implement within the framework of a partnership?
Patient Experiences
Continuity of care and access. Some older adults are seen by multiple HCPs during health care visits, and as a result, they receive mixed messages on what is expected of them.3 Patients feel they have a greater sense of security and confidence when they have a therapeutic relationship with a trusted HCP; they feel more connected and confident about their health care system.5
Lack of education. Many patients say they need more DM education, guidance, and support.6 They report that HCPs tell them how to control DM to avoid complications but say they need more education on how DM affects their lives and concrete suggestions on how to change their lifestyles. Researchers say patients need to feel empowered so they can take a leadership role in managing medications, diet, exercise, preventive foot and eye care, and stress.7 In contrast, an empowerment approach identifies patients’ inherent capacity to self-direct and motivate themselves to develop a self-managed plan based on their personal goals and priorities.8 Patients want to be part of the solution.
Communication and language. A significant challenge for elderly patients is loss of hearing and/or vision, which results in difficulty communicating with their HCP.9 The loss of hearing or vision decreases the ability to adequately collaborate with HCPs and hampers an older adult’s ability to take the lead on self-management. As the U.S. population becomes more diverse, language also poses a significant barrier to care. A language barrier inherently affects health literacy about the disease as well as patients’ perceived trust in HCPs to manage their disease.3
Medication regimen. There are numerous barriers to taking medications. Polypharmacy is a common cause of more drug interactions and adverse effects, which are the most common reason for stopping medications.3,10 Cost of medications and difficulty keeping track of multiple medications are also a deterrent to self-management and adherence. Polypharmacy is seen as detrimental to quality of life (QOL).3 Some patients are also resistant to the initiation and titration of insulin.11
Lack of resources. Many patients cite a lack of resources to facilitate DM care. Common barriers include delays in being scheduled for medical appointments, lack of transportation to appointments, difficulty paying out-of-pocket copays, high cost of medications, and cost of DM supplies (eg, glucometers, test strips, insulin pens and/or pumps). The lack of access to community green spaces or gyms to increase physical activity is also a common barrier.12
Health Care Provider Experiences
Lack of motivation. Health care providers’ experiences and motivations can also present barriers to care. Some HCPs believe that evidence-based guidelines are simply theoretical frameworks; they disagree with using these guidelines as a basis to initiate statin therapy or antiglycemic medications, which reduce cardiovascular complications.13 Many also feel justified in taking a more lax approach when treating older adults due to a lack of time.13
Lack of education about DM management. Health care providers often feel less prepared to provide DM care and believe additional education in DM care is needed. Many lack formal postgraduate DM education or professional development, and 19.6% have no postgraduate DM education or training.14 Some are uncomfortable managing insulin because of a lack of knowledge of insulin therapy and its effect on cardiovascular risk. This results in patients remaining too long on oral DM medications and delaying the necessary initiation of insulin.13
Lack of resources. Some HCPs do not have qualified staff, such as dieticians and diabetes nurse educators, to support DM care. Fearing a loss of control over individual patient care, some HCPs also find it difficult to collaborate with multidisciplinary diabetes care team members, such as psychologists and diabetes educators.13 A lack of awareness of community programs hampers HCPs’ ability to get patients connected to resources that help them make lifestyle changes.14
Lack of involvement or empowerment. Health care providers often think patients do not act with a sense of empowerment in DM management. Health care providers commonly perceive patients as lacking the motivation to change and say that as many as 30% of patients are uncooperative, regardless of proposed changes.13 Many are convinced that patients are unwilling to make even small lifestyle adjustments, such as getting physically active and losing weight. Health care providers say patients do not ask questions about DM selfmanagement during visits and often do not verbalize how HCPs can best support their needs.15 They say that patients are so entrenched in their habits, they even refuse DM education.13
Management Strategies
Several strategies can be deployed to overcome barriers in DM care. Of utmost importance is the need to provide patient-centered care with age-specific characteristics of older adults (Table). To foster mutual collaboration in DM care, HCPs need to ask patients about their health care goals. Patients often view their health through a functional and social perspective rather than from a biomedical perspective. In one study, 71% of patients said their most common health goal was to be independent with activities of daily living, which was more important than the specific details of DM care.16 Preventing DM complications was among their secondary health care goals.
A DM care plan for older adults should be individualized with careful consideration given to medical history, functional capacity, home care environment, and life expectancy. Many older adults have health problems, such as impaired vision, cognitive impairment, depression, and peripheral sensory neuropathy. They may have osteoarthritis of the knees, osteoporosis of the hip and spine, or urinary incontinence; all these conditions increase the risk for falls. Many older adults are on multiple medications, which can increase falls by causing dizziness, dehydration, or hypotension.17 Polypharmacy can negatively impact one or more comorbid conditions and QOL.18
The clinical guidelines for DM management are based on studies conducted in younger populations. However, the 2015 guidelines from the American Diabetes Association (ADA) have been tailored to consider level of health, frailty, cognition, comorbidities, and life expectancy of older adults. The 2015 ADA recommendations provide a framework to guide treatment goals in older adults. A reasonable goal for healthy older adults with few chronic diseases, intact cognition, high functional status, and an anticipated longer remaining life expectancy is an A1c of < 7.5%.19 For older adults with comorbidities of intermediate complexity, such as mild cognitive impairment, an A1c treatment goal of < 8.0% is suggested. An A1c goal of < 8.5% is recommended for older adults in poor health, such as those with end-stage chronic disease, significant cognitive impairment, or those in long-term care. Health care providers may choose to further individualize A1c treatment goals to < 7% if patients are healthy and if treatment burdens are not severe or excessive.19
Multidisciplinary Collaboration
Chronic illness in older adults can be complex to manage due to competing comorbidities and polypharmacy. A diabetes care team consisting of a dietician, social worker, pharmacist, and certified diabetes educator is well suited to effectively manage DM in older adults. Up to 10 hours of DM education with a registered dietician or certified diabetes educator is covered under Medicare in a 12-month period if at least one of the following criteria are met: new diagnosis of DM with A1c > 8.5%, recent initiation of medication, or a high risk for complications.20
Motivational Interviewing
Many HCPs are frustrated that they are unable to persuade patients to adhere to their DM care recommendations. Health care providers often use strategies such as badgering or blaming patients for being nonadherent or scare tactics about the negative consequences of the disease.21 This approach is often ineffective and results in patients becoming more resistant to change.
Motivational interviewing using open-ended questions is an evidenced-based counseling technique that has been shown to elicit sustained behavioral changes. Motivational interviewing increases intrinsic motivation within patients and establishes a goal of incorporating patient-centered values into care by examining ambivalence and passivity in a nonjudgmental way.22 Motivational interviewing facilitates empowerment by using a decision-making process based on each individual’s unique physical, emotional, and environmental circumstances. With guidance from HCPs, patients are able to set the ground rules for DM management by defining a plan that works best for them. For example, a patient may consider a meal plan with stricter caloric intake vs one with a higher calorie count but with more frequent insulin injections or blood glucose monitoring. This strategy puts patients at the center of decision making about medications, diet, and exercise. It also allows them to implement an individualized plan that they believe will work best for them based on their own perceived goals, priorities, and stressors. This approach is shown to work effectively in DM care.8,23
Medication Regimen and Glucose Monitoring
Hypoglycemia is a major concern when managing DM in older adults.20 Hypoglycemia can be triggered by polypharmacy, cognitive impairment, renal insufficiency, sedatives, alcohol intake, malnutrition, and the use of sulfonylureas or insulin. Medications should be considered within the context of other geriatric problems such as falls, depression, urinary incontinence, and pain.20 A simplified approach based on the patient’s functional and cognitive abilities is a good starting point.20 Unless contraindicated, medication initiation could begin with a biguanide.1 Sulfonylureas should generally be avoided in older adults due to the high risk of hypoglycemia.1 Older adults with frequent hypoglycemia should be referred to an endocrinologist or diabetes educator for further management.24
Insulin therapy is recommended if oral therapy alone is insufficient or fails.20 Insulin can be prescribed with adequate DM education and blood glucose monitoring. When prescribing insulin, HCPs should consider older patients’ physical dexterity, visual acuity, cognitive function, financial circumstances, and family support to determine whether insulin therapy is a realistic option that patients can appropriately manage.12,20
Many older adults are resistant to starting insulin and are often reluctant to titrate insulin doses between clinic visits as prescribed by HCPs.12 Older adults on insulin need reassurance and education from a diabetes educator or HCP to gain confidence in adjusting insulin.12 A simple approach to starting insulin can be to start with an evening dose of long-acting insulin.20 Short-acting agents can be added later as needed to control postprandial hyperglycemia.20 Prefilled insulin flex pens also provide a quick and easy way to administer insulin in precise doses.20
Barriers to self-monitoring of blood glucose include pain from finger sticks, inconvenience of testing, and the expense of test strips.25 The newer glucometers and test strips use smaller amounts of blood from other body parts such as the upper arm, calf, or thigh, and these glucometers are ideally suited for older adults.20
Lifestyle Modifications
Lifestyle interventions, including weight loss and exercise, are the mainstay in glycemic control of diabetes. The Diabetes Prevention Program study found a group of patients on lifestyle intervention alone (weight loss goal of 7% and ≥ 150 minutes of weekly exercise) had a 58% lower prevalence of DM compared with a group of patients taking metformin, which had a 31% lower prevalence.26 When older adults were compared with younger persons, lifestyle interventions were more effective than was taking metformin. High-intensity resistance training with moderate weights and repetition lowered glycemic index and caused a 3-fold reduction in A1c in older patients.27,28
Case Management
Older adults may have difficulty getting in touch with HCPs through traditional automated telephone systems. Many have difficulty transmitting glucose monitoring log sheets to HCPs for medication adjustments, which can result in delayed interventions. Telephone visits initiated by a competent case manager can serve as a primary point of contact between HCPs and older adults to optimize treatment and effectively get patients to targeted goals.
Telemedicine is an important tool for monitoring older adults in their home. The technology includes installing a home telemedicine unit, which supports videoconferencing, exchanging messages with case managers, uploading blood glucose readings, and accessing DM educational materials. A study on medication adherence in older diabetic patients found increased adherence through telemonitoring.29 Telemedicine can quickly identify new or persistent barriers between clinic visits so interventions can be made.
A case manager can also facilitate family and social support to address issues such as infrequent glucose monitoring, infrequent medical appointments, caregiver stress, lack of transportation, and financial difficulties, all of which can adversely affect DM care for older adults. The use of a network of family and friends is a good tool for DM management. One study found that when family or friends attended clinic visits, patients were more motivated to understand, follow HCP advice, and find resolutions to difficult issues in DM care.30
Conclusion
Diabetes is a chronic illness with a high burden for older adults. It is important to understand the experiences of patients and HCPs that influence common diabetes barriers. In older adults, barriers should be evaluated in an age-specific context to devise practical interventions to overcome them. Individualizing therapies and empowering older adults prepares them to live confidently while maintaining a sense of control over their lives. A patientcentered collaboration between HCPs and older adults that incorporates a multidisciplinary team approach to resolve problems can improve patient outcomes.
Additional research is needed to identify methods that are most suitable and applicable to older adults. If new evidenced-based research can eliminate diabetes barriers and improve diabetes care in older adults, the consequential burden of diabetes is more likely to decline.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Diabetes mellitus (DM) is a chronic disease that is commonly reported in older adults in primary care. Many adults aged > 65 years with DM have other chronic diseases that make management of their care more complex. Overseeing DM care in older adults while comanaging other chronic diseases is a challenge to health care providers (HCPs). The terms older adults and geriatric define persons aged ≥ 65 years.
Diabetes mellitus is growing at a rapid rate, and older adults are at higher risk. In 2012, about 29.1 million people in the U.S. (9.3%) were diagnosed with DM. Of that number, 11.2 million were aged ≥ 65 years. Additionally, 86 million adults had prediabetes when fasting blood glucose and A1c levels were reviewed. Also in 2012, more than 400,000 new cases (11.5 per 1,000 people) were diagnosed in the aged ≥ 65 years group.1-3 This age group is anticipated to double in 25 years, and the incidence of DM is projected to increase 3.2-fold.4 By 2050, 26.7 million older adults—55% of the older adult population—will have DM. As a result, HCPs are faced with treating escalating numbers of older adults with DM as the population ages.4
In 2012, the total cost of DM for the U.S. population was $245 billion: The direct cost of medical care was $176 billion, and the indirect costs in productivity, absenteeism, unemployment, disability, and premature death was nearly $69 billion.2 This is a significant burden in terms of health care costs, productivity, disability, sick days, early retirement, and premature death. Diabetes mellitus increases atherosclerosis and thus accelerates the risk for heart disease, stroke, kidney disease, blindness, and limb amputations.2
Managing DM concurrently with multiple chronic comorbid conditions is challenging. Patients are asked to bring blood glucose under tight control, perform regular blood glucose testing, take antiglycemic medications, watch their diet, lose weight, and exercise regularly—all while managing other chronic diseases. Many older patients are overwhelmed by the demands of self-management recommended by their HCPs. Similarly, HCPs are frustrated with their older patients, who are unable to adequately meet targeted goals for DM management and thereby reduce the associated risks for complications.
The purpose of this article is to discuss the common barriers to DM management, the experiences of patients and HCPs regarding those barriers, and the management strategies for overcoming barriers in treating older adults with DM.
What Are The Barriers?
The experiences of both patients and HCPs matter when working to overcome DM barriers. If no one understands the problem, no one can fix it. What concerns do patients and HCPs have? Do they really value each other’s perspectives? To overcome barriers, can HCPs and patients develop mutually agreed on goals that are reasonable and practical to implement within the framework of a partnership?
Patient Experiences
Continuity of care and access. Some older adults are seen by multiple HCPs during health care visits, and as a result, they receive mixed messages on what is expected of them.3 Patients feel they have a greater sense of security and confidence when they have a therapeutic relationship with a trusted HCP; they feel more connected and confident about their health care system.5
Lack of education. Many patients say they need more DM education, guidance, and support.6 They report that HCPs tell them how to control DM to avoid complications but say they need more education on how DM affects their lives and concrete suggestions on how to change their lifestyles. Researchers say patients need to feel empowered so they can take a leadership role in managing medications, diet, exercise, preventive foot and eye care, and stress.7 In contrast, an empowerment approach identifies patients’ inherent capacity to self-direct and motivate themselves to develop a self-managed plan based on their personal goals and priorities.8 Patients want to be part of the solution.
Communication and language. A significant challenge for elderly patients is loss of hearing and/or vision, which results in difficulty communicating with their HCP.9 The loss of hearing or vision decreases the ability to adequately collaborate with HCPs and hampers an older adult’s ability to take the lead on self-management. As the U.S. population becomes more diverse, language also poses a significant barrier to care. A language barrier inherently affects health literacy about the disease as well as patients’ perceived trust in HCPs to manage their disease.3
Medication regimen. There are numerous barriers to taking medications. Polypharmacy is a common cause of more drug interactions and adverse effects, which are the most common reason for stopping medications.3,10 Cost of medications and difficulty keeping track of multiple medications are also a deterrent to self-management and adherence. Polypharmacy is seen as detrimental to quality of life (QOL).3 Some patients are also resistant to the initiation and titration of insulin.11
Lack of resources. Many patients cite a lack of resources to facilitate DM care. Common barriers include delays in being scheduled for medical appointments, lack of transportation to appointments, difficulty paying out-of-pocket copays, high cost of medications, and cost of DM supplies (eg, glucometers, test strips, insulin pens and/or pumps). The lack of access to community green spaces or gyms to increase physical activity is also a common barrier.12
Health Care Provider Experiences
Lack of motivation. Health care providers’ experiences and motivations can also present barriers to care. Some HCPs believe that evidence-based guidelines are simply theoretical frameworks; they disagree with using these guidelines as a basis to initiate statin therapy or antiglycemic medications, which reduce cardiovascular complications.13 Many also feel justified in taking a more lax approach when treating older adults due to a lack of time.13
Lack of education about DM management. Health care providers often feel less prepared to provide DM care and believe additional education in DM care is needed. Many lack formal postgraduate DM education or professional development, and 19.6% have no postgraduate DM education or training.14 Some are uncomfortable managing insulin because of a lack of knowledge of insulin therapy and its effect on cardiovascular risk. This results in patients remaining too long on oral DM medications and delaying the necessary initiation of insulin.13
Lack of resources. Some HCPs do not have qualified staff, such as dieticians and diabetes nurse educators, to support DM care. Fearing a loss of control over individual patient care, some HCPs also find it difficult to collaborate with multidisciplinary diabetes care team members, such as psychologists and diabetes educators.13 A lack of awareness of community programs hampers HCPs’ ability to get patients connected to resources that help them make lifestyle changes.14
Lack of involvement or empowerment. Health care providers often think patients do not act with a sense of empowerment in DM management. Health care providers commonly perceive patients as lacking the motivation to change and say that as many as 30% of patients are uncooperative, regardless of proposed changes.13 Many are convinced that patients are unwilling to make even small lifestyle adjustments, such as getting physically active and losing weight. Health care providers say patients do not ask questions about DM selfmanagement during visits and often do not verbalize how HCPs can best support their needs.15 They say that patients are so entrenched in their habits, they even refuse DM education.13
Management Strategies
Several strategies can be deployed to overcome barriers in DM care. Of utmost importance is the need to provide patient-centered care with age-specific characteristics of older adults (Table). To foster mutual collaboration in DM care, HCPs need to ask patients about their health care goals. Patients often view their health through a functional and social perspective rather than from a biomedical perspective. In one study, 71% of patients said their most common health goal was to be independent with activities of daily living, which was more important than the specific details of DM care.16 Preventing DM complications was among their secondary health care goals.
A DM care plan for older adults should be individualized with careful consideration given to medical history, functional capacity, home care environment, and life expectancy. Many older adults have health problems, such as impaired vision, cognitive impairment, depression, and peripheral sensory neuropathy. They may have osteoarthritis of the knees, osteoporosis of the hip and spine, or urinary incontinence; all these conditions increase the risk for falls. Many older adults are on multiple medications, which can increase falls by causing dizziness, dehydration, or hypotension.17 Polypharmacy can negatively impact one or more comorbid conditions and QOL.18
The clinical guidelines for DM management are based on studies conducted in younger populations. However, the 2015 guidelines from the American Diabetes Association (ADA) have been tailored to consider level of health, frailty, cognition, comorbidities, and life expectancy of older adults. The 2015 ADA recommendations provide a framework to guide treatment goals in older adults. A reasonable goal for healthy older adults with few chronic diseases, intact cognition, high functional status, and an anticipated longer remaining life expectancy is an A1c of < 7.5%.19 For older adults with comorbidities of intermediate complexity, such as mild cognitive impairment, an A1c treatment goal of < 8.0% is suggested. An A1c goal of < 8.5% is recommended for older adults in poor health, such as those with end-stage chronic disease, significant cognitive impairment, or those in long-term care. Health care providers may choose to further individualize A1c treatment goals to < 7% if patients are healthy and if treatment burdens are not severe or excessive.19
Multidisciplinary Collaboration
Chronic illness in older adults can be complex to manage due to competing comorbidities and polypharmacy. A diabetes care team consisting of a dietician, social worker, pharmacist, and certified diabetes educator is well suited to effectively manage DM in older adults. Up to 10 hours of DM education with a registered dietician or certified diabetes educator is covered under Medicare in a 12-month period if at least one of the following criteria are met: new diagnosis of DM with A1c > 8.5%, recent initiation of medication, or a high risk for complications.20
Motivational Interviewing
Many HCPs are frustrated that they are unable to persuade patients to adhere to their DM care recommendations. Health care providers often use strategies such as badgering or blaming patients for being nonadherent or scare tactics about the negative consequences of the disease.21 This approach is often ineffective and results in patients becoming more resistant to change.
Motivational interviewing using open-ended questions is an evidenced-based counseling technique that has been shown to elicit sustained behavioral changes. Motivational interviewing increases intrinsic motivation within patients and establishes a goal of incorporating patient-centered values into care by examining ambivalence and passivity in a nonjudgmental way.22 Motivational interviewing facilitates empowerment by using a decision-making process based on each individual’s unique physical, emotional, and environmental circumstances. With guidance from HCPs, patients are able to set the ground rules for DM management by defining a plan that works best for them. For example, a patient may consider a meal plan with stricter caloric intake vs one with a higher calorie count but with more frequent insulin injections or blood glucose monitoring. This strategy puts patients at the center of decision making about medications, diet, and exercise. It also allows them to implement an individualized plan that they believe will work best for them based on their own perceived goals, priorities, and stressors. This approach is shown to work effectively in DM care.8,23
Medication Regimen and Glucose Monitoring
Hypoglycemia is a major concern when managing DM in older adults.20 Hypoglycemia can be triggered by polypharmacy, cognitive impairment, renal insufficiency, sedatives, alcohol intake, malnutrition, and the use of sulfonylureas or insulin. Medications should be considered within the context of other geriatric problems such as falls, depression, urinary incontinence, and pain.20 A simplified approach based on the patient’s functional and cognitive abilities is a good starting point.20 Unless contraindicated, medication initiation could begin with a biguanide.1 Sulfonylureas should generally be avoided in older adults due to the high risk of hypoglycemia.1 Older adults with frequent hypoglycemia should be referred to an endocrinologist or diabetes educator for further management.24
Insulin therapy is recommended if oral therapy alone is insufficient or fails.20 Insulin can be prescribed with adequate DM education and blood glucose monitoring. When prescribing insulin, HCPs should consider older patients’ physical dexterity, visual acuity, cognitive function, financial circumstances, and family support to determine whether insulin therapy is a realistic option that patients can appropriately manage.12,20
Many older adults are resistant to starting insulin and are often reluctant to titrate insulin doses between clinic visits as prescribed by HCPs.12 Older adults on insulin need reassurance and education from a diabetes educator or HCP to gain confidence in adjusting insulin.12 A simple approach to starting insulin can be to start with an evening dose of long-acting insulin.20 Short-acting agents can be added later as needed to control postprandial hyperglycemia.20 Prefilled insulin flex pens also provide a quick and easy way to administer insulin in precise doses.20
Barriers to self-monitoring of blood glucose include pain from finger sticks, inconvenience of testing, and the expense of test strips.25 The newer glucometers and test strips use smaller amounts of blood from other body parts such as the upper arm, calf, or thigh, and these glucometers are ideally suited for older adults.20
Lifestyle Modifications
Lifestyle interventions, including weight loss and exercise, are the mainstay in glycemic control of diabetes. The Diabetes Prevention Program study found a group of patients on lifestyle intervention alone (weight loss goal of 7% and ≥ 150 minutes of weekly exercise) had a 58% lower prevalence of DM compared with a group of patients taking metformin, which had a 31% lower prevalence.26 When older adults were compared with younger persons, lifestyle interventions were more effective than was taking metformin. High-intensity resistance training with moderate weights and repetition lowered glycemic index and caused a 3-fold reduction in A1c in older patients.27,28
Case Management
Older adults may have difficulty getting in touch with HCPs through traditional automated telephone systems. Many have difficulty transmitting glucose monitoring log sheets to HCPs for medication adjustments, which can result in delayed interventions. Telephone visits initiated by a competent case manager can serve as a primary point of contact between HCPs and older adults to optimize treatment and effectively get patients to targeted goals.
Telemedicine is an important tool for monitoring older adults in their home. The technology includes installing a home telemedicine unit, which supports videoconferencing, exchanging messages with case managers, uploading blood glucose readings, and accessing DM educational materials. A study on medication adherence in older diabetic patients found increased adherence through telemonitoring.29 Telemedicine can quickly identify new or persistent barriers between clinic visits so interventions can be made.
A case manager can also facilitate family and social support to address issues such as infrequent glucose monitoring, infrequent medical appointments, caregiver stress, lack of transportation, and financial difficulties, all of which can adversely affect DM care for older adults. The use of a network of family and friends is a good tool for DM management. One study found that when family or friends attended clinic visits, patients were more motivated to understand, follow HCP advice, and find resolutions to difficult issues in DM care.30
Conclusion
Diabetes is a chronic illness with a high burden for older adults. It is important to understand the experiences of patients and HCPs that influence common diabetes barriers. In older adults, barriers should be evaluated in an age-specific context to devise practical interventions to overcome them. Individualizing therapies and empowering older adults prepares them to live confidently while maintaining a sense of control over their lives. A patientcentered collaboration between HCPs and older adults that incorporates a multidisciplinary team approach to resolve problems can improve patient outcomes.
Additional research is needed to identify methods that are most suitable and applicable to older adults. If new evidenced-based research can eliminate diabetes barriers and improve diabetes care in older adults, the consequential burden of diabetes is more likely to decline.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Geriatrics Society Expert Panel on Care of Older Adults With Diabetes Mellitus; Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 Update. J Am Geriatr Soc. 2013;61(11):2020-2026.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
3. Williams A, Manias E. Exploring motivation and confidence in taking prescribed medicines in coexisting diseases: a qualitative study. J Clin Nurs. 2014;23(3-4):471-481.
4. Narayan KMV, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent
increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care.
2006;29(9):2114-2116.
5. von Bültzingslöwen I, Eliasson G, Sarvimäki A, Mattsson B, Hjortdahl P. Patients’ views on interpersonal continuity in primary care: a sense of security based on four core foundations. Fam Pract. 2006;23(2):210-219.
6. Koenigsberg MR, Bartlett D, Cramer JS. Facilitating treatment adherence with lifestyle changes in diabetes. Am Fam Physician. 2004;69(2):309-316.
7. Mensing C, Boucher J, Cypress M, et al. National standards for diabetes self-management education. Task Force to Review and Revise the National Standards for Diabetes Self-Management Education Programs. Diabetes Care. 2000;23(5):682-689.
8. Funnell MM, Weiss MA. Empowering patients with diabetes. Nursing. 2009;39(3):34-37.
9. Hammouda EI. Overcoming barriers to diabetes control in geriatrics. Int J Clin Pract. 2011;65(4):420-424.
10. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650-2664.
11. Brod M, Kongsø JH, Lessard S, Christensen TL. Psychological insulin resistance: patient beliefs and implications for diabetes management. Qual Life Res. 2009;18(1):23-32.
12. Munshi MN, Segal AR, Suhl E, et al. Assessment of barriers to improve diabetes management in older adults: a randomized controlled study. Diabetes Care. 2013;36(3):543-549.
13. Goderis G, Borgermans L, Mathieu C, et al. Barriers and facilitators to evidence based care of type 2 diabetes patients: experiences of general practitioners participating to a quality improvement program. Implement Sci. 2009;4:41.
14. Raaijmakers LGM, Hamers FJM, Martens MK, Bagchus C, de Vries NK, Kremers SPJ. Perceived facilitators and barriers in diabetes care: a qualitative study among health care professionals in the Netherlands. BMC Fam Pract. 2013;14(1):114.
15. Holt RIG, Nicolucci A, Kovacs Burns K, et al; DAWN2 Study Group. Diabetes Attitudes, Wishes and Needs second study (DAWN2™): cross-national comparisons on barriers and resources for optimal care—healthcare professional perspective. Diabet Med. 2013;30(7):789-798.
16. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53(2):306-311.
17. Haas LB. Special considerations for older adults with diabetes residing in skilled nursing facilities. Diabetes Spectrum. 2014;27(1):37-43.
18. Mason NA, Bakus JL. Strategies for reducing polypharmacy and other medicationrelated problems in chronic kidney disease. Semin Dial. 2010;23(1):55-61.
19. American Diabetes Association. Older adults. Sec.10. In Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S67-S69.
20. Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med. 2008;75(1):70-78.
21. Gabbay RA, Durdock K. Strategies to increase adherence through diabetes technology. J Diabetes Sci Technol. 2010;4(3):661-665.
22. Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: The Guilford Press; 2008.
23. West D, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081-1087.
24. Graber AL, Elasy TA, Quinn D, Wolff K, Brown A. Improving glycemic control in adults with diabetes mellitus: shared responsibility in primary care practices. South Med J. 2002;95(7):684-690.
25. Zgibor JC, Simmons D. Barriers to blood glucose monitoring in a multiethnic community. Diabetes Care. 2002;25(10):1772-1777.
26. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
27. Witham MD, Avenell A. Interventions to achieve long-term weight loss in obese older people: a systematic review and meta-analysis. Age Ageing. 2010;39(2):176-184.
28. Dunstan DW, Daly RM, Owen N, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729-1736.
29. Brath H, Morak J, Kästenbauer T, et al. Mobile health (mHealth) based medication adherence measurement–a pilot trial using electronic blisters in diabetes patients. Br J Clin Pharmacol. 2013;76(suppl 1):47-55.
30. Rosland AM, Piette JD, Choi H, Heisler M. Family and friend participation in primary care visits of patients with diabetes or heart failure: patient and physician determinants and experiences. Med Care. 2011;49(1):37-45.
1. American Geriatrics Society Expert Panel on Care of Older Adults With Diabetes Mellitus; Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 Update. J Am Geriatr Soc. 2013;61(11):2020-2026.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
3. Williams A, Manias E. Exploring motivation and confidence in taking prescribed medicines in coexisting diseases: a qualitative study. J Clin Nurs. 2014;23(3-4):471-481.
4. Narayan KMV, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent
increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care.
2006;29(9):2114-2116.
5. von Bültzingslöwen I, Eliasson G, Sarvimäki A, Mattsson B, Hjortdahl P. Patients’ views on interpersonal continuity in primary care: a sense of security based on four core foundations. Fam Pract. 2006;23(2):210-219.
6. Koenigsberg MR, Bartlett D, Cramer JS. Facilitating treatment adherence with lifestyle changes in diabetes. Am Fam Physician. 2004;69(2):309-316.
7. Mensing C, Boucher J, Cypress M, et al. National standards for diabetes self-management education. Task Force to Review and Revise the National Standards for Diabetes Self-Management Education Programs. Diabetes Care. 2000;23(5):682-689.
8. Funnell MM, Weiss MA. Empowering patients with diabetes. Nursing. 2009;39(3):34-37.
9. Hammouda EI. Overcoming barriers to diabetes control in geriatrics. Int J Clin Pract. 2011;65(4):420-424.
10. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650-2664.
11. Brod M, Kongsø JH, Lessard S, Christensen TL. Psychological insulin resistance: patient beliefs and implications for diabetes management. Qual Life Res. 2009;18(1):23-32.
12. Munshi MN, Segal AR, Suhl E, et al. Assessment of barriers to improve diabetes management in older adults: a randomized controlled study. Diabetes Care. 2013;36(3):543-549.
13. Goderis G, Borgermans L, Mathieu C, et al. Barriers and facilitators to evidence based care of type 2 diabetes patients: experiences of general practitioners participating to a quality improvement program. Implement Sci. 2009;4:41.
14. Raaijmakers LGM, Hamers FJM, Martens MK, Bagchus C, de Vries NK, Kremers SPJ. Perceived facilitators and barriers in diabetes care: a qualitative study among health care professionals in the Netherlands. BMC Fam Pract. 2013;14(1):114.
15. Holt RIG, Nicolucci A, Kovacs Burns K, et al; DAWN2 Study Group. Diabetes Attitudes, Wishes and Needs second study (DAWN2™): cross-national comparisons on barriers and resources for optimal care—healthcare professional perspective. Diabet Med. 2013;30(7):789-798.
16. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53(2):306-311.
17. Haas LB. Special considerations for older adults with diabetes residing in skilled nursing facilities. Diabetes Spectrum. 2014;27(1):37-43.
18. Mason NA, Bakus JL. Strategies for reducing polypharmacy and other medicationrelated problems in chronic kidney disease. Semin Dial. 2010;23(1):55-61.
19. American Diabetes Association. Older adults. Sec.10. In Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S67-S69.
20. Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med. 2008;75(1):70-78.
21. Gabbay RA, Durdock K. Strategies to increase adherence through diabetes technology. J Diabetes Sci Technol. 2010;4(3):661-665.
22. Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: The Guilford Press; 2008.
23. West D, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081-1087.
24. Graber AL, Elasy TA, Quinn D, Wolff K, Brown A. Improving glycemic control in adults with diabetes mellitus: shared responsibility in primary care practices. South Med J. 2002;95(7):684-690.
25. Zgibor JC, Simmons D. Barriers to blood glucose monitoring in a multiethnic community. Diabetes Care. 2002;25(10):1772-1777.
26. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
27. Witham MD, Avenell A. Interventions to achieve long-term weight loss in obese older people: a systematic review and meta-analysis. Age Ageing. 2010;39(2):176-184.
28. Dunstan DW, Daly RM, Owen N, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729-1736.
29. Brath H, Morak J, Kästenbauer T, et al. Mobile health (mHealth) based medication adherence measurement–a pilot trial using electronic blisters in diabetes patients. Br J Clin Pharmacol. 2013;76(suppl 1):47-55.
30. Rosland AM, Piette JD, Choi H, Heisler M. Family and friend participation in primary care visits of patients with diabetes or heart failure: patient and physician determinants and experiences. Med Care. 2011;49(1):37-45.
SGLT2 Inhibitors for Type 2 Diabetes Mellitus Treatment
Over the past 2 decades, the treatment of type 2 diabetes mellitus (T2DM) has been an evolving science. With therapeutic advances, the prevalence of catastrophic complications such as amputations, renal failure requiring dialysis, and blindness due to retinopathy have significantly declined. Developed drugs have successfully met treatment goals; however, they are often associated with a higher risk of hypoglycemia and weight gain. Now that better glucose control is possible, the science of diabetes care continues to evolve. Newly developed drugs should control glucose without significant hypoglycemia and also promote weight reduction. The sodium-glucose transport protein 2 (SGLT2) inhibitor drug class has these characteristics, and the novel mechanism of action complements older medications used to treat T2DM.
Phlorizin is a plant-based compound originally discovered in 1935 when it was derived from the bark of apple trees.1 It is a naturally occurring botanical glucoside and is fairly nonselective between SGLT1 and SGLT2. Due to its poor bioavailability and its degradation in the gastrointestinal tract, it was not an ideal drug candidate in humans.
Canagliflozin
Canagliflozin is a SGLT2 inhibitor and a low-potency SGLT1 inhibitor. It was the first SGLT2 inhibitor approved by the FDA (March 2013) to be used with diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 100 mg once daily for patients who have an estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73m2 and can be increased to 300 mg once daily. It is also available in a fixed-dose combination with metformin. Canagliflozin SGLT-2 inhibition leads to increased glycosuria and osmotic diuresis that lowers plasma glucose concentrations. Lower blood pressure (BP) is likely an effect of the osmotic diuresis. Increased urinary excretion of glucose also leads to a loss of calories and weight loss. It was studied alone and in combination with metformin, sulfonylurea, pioglitazone, and insulin therapy.
The pharmacokinetics of canagliflozin is similar in healthy subjects and patients with T2DM. Peak plasma concentrations (Cmax) and area under the cover (AUC) of canagliflozin increased in a dose-proportional manner from 50 mg to 300 mg. Following single-dose oral administration of 100 mg and 300 mg of canagliflozin, time to Cmax (Tmax) of canagliflozin occurs within 1 to 2 hours postdose. The apparent terminal half-life (t1/2) was 10.6 hours and 13.1 hours for the 100 mg and 300 mg doses, respectively. Steady state was reached after 4 to 5 days of once-daily dosing with canagliflozin 100 mg to 300 mg. Glucuronidation is the major metabolic pathway. There is balanced renal and biliary excretion of metabolites, and there are no active metabolites.
Following oral doses of canagliflozin in patients with T2DM, dose-dependent decreases were seen in the renal threshold for glucose (RTG). From a starting value of about 240 mg/dL, the 300-mg dose suppressed the mean (RTG) to about 70 to 90 mg/dL in T2DM in phase 1 studies. The reduction in RTG led to increase in urinary excretion of glucose of about 100 g/d.
In addition to renal SGLT2 inhibition leading to increased urinary glucose excretion (UGE), canagliflozin has been shown to lower postprandial glucose excursion (PPGE) and insulin concentrations by delaying intestinal glucose absorption.2 A study was done in 20 healthy subjects who received either placebo or canagliflozin 300 mg 20 minutes before a 600 kcal mixed-meal tolerance test. Compared with placebo, canagliflozin reduced PPGE and insulin excursions (0-2 h) AUC by 35% and 43%, respectively (P < .001 for both). This may present a difference between canagliflozin and the other SGLT2 inhibitors.
Because of the potential differences due to canagliflozin’s inhibition of intestinal SGLT1, the pharmacodynamic differences between canagliflozin and dapagliflozin were studied.3 The randomized, double-blind, crossover study consisted of 54 subjects. The subjects received the maximum approved doses of canagliflozin 300 mg or dapagliflozin 10 mg a day. Each group was treated with the study drug for 2 days, and then a 600 kcal mixed-meal tolerance test was performed. The results of the PPGE 0- to 2-hour AUC analysis showed 3.66 mmol*h/L with canagliflozin 300 mg and 4.08 mmol*h/L with dapagliflozin 10 mg. There was a difference of 0.42 (P = .0122), which was a 10.3% reduction in AUC PPGE by canagliflozin compared with dapagliflozin.
Canagliflozin has been studied in patients with T2DM and stage 3 nephropathy. Data were pooled from 4 randomized, placebo-controlled, phase 3 studies in which subjects had baseline eGFR > 30 to < 60 mL/min/1.73 m2.4 In the setting of decreased eGFR associated with stage 3 chronic kidney disease, subjects treated with canagliflozin 100 mg and canagliflozin 300 mg had placebo-subtracted reductions in hemoglobin A1c (A1c) of -0.38% and -0.47%, respectively, and placebosubtracted reduction in weight of -1.6% and -1.9%, respectively. Decreases in eGFR were seen at week 6 but trended toward baseline over time with a mean change in eGFR of 0.7, -1.7, -2.2 mL/min/1.73 m2 for placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively.
Clinical Efficacy Trials
Canagliflozin was studied as add-on therapy to metformin and compared with glimepiride (a sulfonylurea).5 The randomized, double-blind study included 1,450 subjects for a core study period of 52 weeks followed by a 52-week extension. Eligible subjects were aged > 18 and < 80 years, A1c > 7% and < 9.5%, and were receiving metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. The study groups were canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, and baseline A1c measurements were 7.78%, 7.79%, and 7.83%, respectively. The glimepiride was titrated up if > 50% of fasting blood glucose measurements were > 108 mg/dL with no hypoglycemic events in the previous 2 weeks.
Over 104 weeks, canagliflozin 100 mg and 300 mg and glimepiride reduced A1c from mean baseline values by -0.65%, -0.74%, and -0.55%, respectively, and the proportions of patients achieving A1c < 7% at week 104 was 42.5%, 50.2%, and 43.9%, respectively. Weight fell over 104 weeks with canagliflozin 100 mg (-4.1%, -3.6 kg) and canagliflozin 300 mg (-4.2%, -3.6 kg). In contrast, glimepiride showed weight increase (0.9%, 0.8 kg). Documented hypoglycemia episodes were lower in canagliflozin 100 mg and 300 mg than with glimepiride (6.8%, 8.2%, and 40.9%, respectively).
A study was undertaken to compare canagliflozin with the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin in patients with T2DM on background therapy of metformin.6 This randomized, double-blind trial studied subjects aged > 18 and < 80 years with inadequate glucose control A1c > 7% and < 10.5%. Subjects received metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. At week 52, canagliflozin showed noninferiority to sitagliptin, with both drugs lowering A1c by 0.73%. Canagliflozin 300 mg showed superiority to sitagliptin with -0.88% change in A1c. Both canagliflozin 100 mg and 300 mg were superior to sitagliptin 100 mg in weight reduction: -3.8%, -4.2%, and -1.3%, respectively. Genital mycotic infections were higher in the canagliflozin groups. Rates for mycotic infections for sitagliptin 100 mg, canagliflozin 100 mg, and canagliflozin 300 mg were 1.2%, 5.2%, and 2.4% in men, respectively, and 2.6%, 11.3%, and 9.9% in women, respectively.
Canagliflozin was also studied in combination with insulin to determine efficacy and safety in this setting.7 Subjects were randomized to receive placebo, canagliflozin 100 mg, or canagliflozin 300 mg. Subjects had a mean baseline A1c of 8.3%. The median daily insulin dose was 60 IU, and most individuals were using basal/bolus regimens. The primary endpoint was 18 weeks of therapy, and A1c was lowered 0.62% (P < .001) with canagliflozin 100 mg and 0.73% with canagliflozin 300 mg compared with placebo. Weight decreased 1.9% (P < .001) with canagliflozin 100 mg and 2.4% (P < .001) with canagliflozin 300 mg compared with placebo.
Adverse Effects and Precautions
Canagliflozin adverse effects (AEs) were generally low. In the aforementioned 104-week study comparing canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, AEs leading to discontinuation were low at 6.2%, 9.5%, and 7.3%, respectively. Serious AEs were lower in the canagliflozin 100 mg and 300 mg groups compared with glimepiride at 9.7%, 9.7%, 14.3%, respectively.5
Limitations for use of canagliflozin are T1DM and diabetic ketoacidosis (DKA). In mild renal impairment, there is no dose adjustment in patients with eGFR > 60 mL/min/1.73 m2. In moderate renal impairment (eGFR 45-60 mL/min/1.73 m2), dose is limited to 100 mg once daily. It is recommended not to initiate canagliflozin if eGFR is < 45 mL/min/1.73 m2. Canagliflozin is contraindicated if eGFR is < 30 mL/min/1.73 m2.8
Dapagliflozin
Dapagliflozin is a highly selective SGLT2 inhibitor. It is a 1,400-fold greater inhibitor of SGLT2 vs SGLT1 and was approved in January 2014. It is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 5 mg once daily, taken in the morning, with or without food, and the dosage can be increased to 10 mg once daily in patients who require additional glycemic control. Dapagliflozin should not be initiated if eGFR is < 60 mL/min/1.73 m2, and it should be discontinued if eGFR is persistently < 60 mL/min/1.73 m2.
By inhibiting SGLT2, dapagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose, thereby increasing UGE. Increased glucose secretion also leads to weight reduction. Dapagliflozin has been studied alone and in combination with glipizide, glimepiride, pioglitazone, and a DDP-4 inhibitor and as an add-on to insulin with and without other oral antidiabetic drugs. It is also available in a fixed-dose combination with metformin.
Following oral administration of dapagliflozin, the Tmax is usually attained within 2 hours under a fasting state. The Cmax and AUC values increase the dose proportionally with increase in dapagliflozin dose in the therapeutic dose range. The absolute oral bioavailability of dapagliflozin following the administration of a 10-mg dose is 78%. The mean plasma t1⁄2 for dapagliflozin is about 12.9 hours following a single oral dose of 10 mg. In patients with normal renal function, the renal glucose excretion was 85 g per day at maximal dose. In humans, 75% of the dose of dapagliflozin is primarily metabolized through the uridine diphosphate glucuronosyltransferase 1A9 pathway. Dapagliflozin and related metabolites are primarily eliminated via the renal pathway.9
Clinical Efficacy Trials
Dapagliflozin was studied as add-on therapy to metformin with glipizide (a sulfonylurea) as the comparator.10 This 52-week double-blind, multicenter, active-controlled, noninferiority trial randomized 801 patients. Eligible subjects were aged > 18 years with A1c > 6.5% and < 10% and were receiving metformin or metformin and 1 other oral antidiabetic drug up to half-maximal dose for at least 8 weeks. During an 18-week titration period, all patients started dapagliflozin 2.5 mg/d and glipizide 5 mg/d. At 21-day intervals, patients were titrated up to the next dosage level if fasting plasma glucose was > 110 mg/dL. Dapagliflozin could be titrated to 5 mg and then 10 mg per protocol. Glipizide could be titrated to 10 mg or 20 mg per protocol.
The A1c change with dapagliflozin was noninferior to glipizide at week 52. The A1c adjusted mean change from baseline was -0.52 for both dapagliflozin and glipizide. The secondary endpoint was weight change. The glipizide group had a +1.44 kg weight gain, whereas the dapagliflozin group had a -3.22 kg weight loss. The number of patients with ≥ 1 episode of hypoglycemia, either symptomatic or with no symptoms, with blood glucose ≤ 63 mg/dL was assessed. The dapagliflozin group had a 3.5% rate compared with the glipizide group, which had a 40.8% rate of patients with > 1 hypoglycemic episode.
A study examined the efficacy and safety of dapagliflozin in combination with and also vs a DPP-4 inhibitor with metformin background therapy. The study looked at add-ons of saxagliptin plus dapagliflozin vs saxagliptin or dapagliflozin added alone.11 This was a randomized, double-blind, 24-week study with patients aged > 18 years with inadequate glucose control A1c > 8.0% and < 12.0%. Patients had to be on a stable dose of metformin > 1,500 mg/d for at least 8 weeks. Patients were randomized 1:1:1 to receive either saxagliptin 5 mg/d and dapagliflozin 10 mg/d plus metformin, saxagliptin 5 mg/d and placebo plus metformin, or dapagliflozin 10 mg/d and placebo plus metformin.
The patients had a mean age of 54 years and a mean duration of T2DM of 7.6 years. The mean baseline A1c was 8.94%. The addition of saxagliptin plus dapagliflozin to metformin resulted in significantly greater A1c reduction compared with saxagliptin plus metformin or dapagliflozin plus metformin from baseline A1c levels: -1.47%, -0.88%, -1.2%, respectively.11
Dapagliflozin was also studied in combination with insulin to determine the safety and efficacy in this setting.12 This double-blind, placebo-controlled, parallel-group trial had an initial study period of 24 weeks followed by an extension period totaling 104 weeks. At 48 weeks, patients on dapagliflozin 5 mg were switched to 10 mg. Outcomes over 104 weeks were changed from baseline A1c, insulin dose, and body weight. Up-titration of insulin was permitted if at least 3 self-monitored blood glucose readings from the 7 days prior to the study visit were > 240 mg/dL up to week 12; > 220 mg/dL between weeks 12 and 24; > 178 mg/dL or if A1c was > 8% between weeks 24 and 48. Between weeks 52 and 65, insulin titrated up was allowed if A1c was > 7.5% and between weeks 78 and 104 if A1c was > 7%. Insulin could be titrated down if ≥ 2 self-monitored blood glucose readings were < 68 mg/dL.
The study group had a T2DM diagnosis for 13.6 years and a mean duration of insulin therapy for about 6 years. The mean daily insulin dose was 77.1 IU. The mean baseline A1c was 8.5%. At 104 weeks, the differences from placebo in A1c adjusted mean change from baseline were -4.0% (P = .0002) and -0.4% (P = .0007) in the dapagliflozin 5 mg (switched to 10 mg at week 48) and 10 mg groups, respectively. Insulin requirements increased progressively in the placebo group +18.3 IU/d at 104 weeks. Insulin requirements stayed stable over 104 weeks in the dapagliflozin groups. Body weight increased in the placebo group, whereas it decreased in the dapagliflozin groups. At 104 weeks, the weight changes from baseline in placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg were -0.99 kg, -1.03 kg (P < .001), and -1.5 kg (P < .0001), respectively. The frequency of ≥ 1 minor or major episodes of hypoglycemia was fairly balanced across placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg at 61.9%, 61.3%, and 60.7%, respectively.
Adverse Effects and Precautions
Dapagliflozin was generally well tolerated. In the trial of dapagliflozin compared with glipizide in the setting of metformin background therapy, AEs led to discontinuation rates of 9.1% vs 5.9%, respectively. Serious AEs were lower in the dapagliflozin group compared with glipizide at 8.6% vs 11.3%, respectively.10
Limitations for use of dapagliflozin are the treatment of T1DM or the treatment of DKA. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. No dose adjustment is needed in patients with mild renal impairment (eGFR of ≥ 60 mL/ min/1.73 m2), and dapagliflozin should be discontinued when eGFR is persistently < 60 mL/min/1.73 m2. In the clinical trials, there was an imbalance in the number of bladder cancer reported with dapagliflozin compared with placebo. Across 22 clinical studies, newly diagnosed cases of bladder cancer were reported in 10 of 6,045 patients (0.17%) treated with dapagliflozin and 1 of 3,512 patients (0.03%) treated with placebo or a comparator.9 There were too few cases to determine whether the emergence of these events is related to dapagliflozin. Product labeling states dapagliflozin should not be used in patients with active bladder cancer and should be used with caution in those with a prior history of bladder cancer.
Empaglifilozin
Empagliflozin is the most recently FDA-approved SGLT2 inhibitor (August 2014) for improved glycemic control in T2DM. It has the highest SGLT2 selectivity: > 2,500-fold selectivity for SGLT2 over SGLT1.13 Empagliflozin regulates blood glucose levels by increased UGE, independent of endogenous insulin secretion. It is associated with modest reductions in body weight, visceral adiposity, and systolic BP.
Empagliflozin is available in 10-mg and 25-mg tablets, with a recommended initial dose of 10 mg daily.13 Dosing adjustments are not required for geriatric patients or for those patients with hepatic impairment.14 The use of empagliflozin is contraindicated in patients with eGFR < 45 mL/min/1.73 m2 or for those whose eGFR declines to < 45 mL/min/1.73 m2 during therapy.15,16 Empagliflozin has a pregnancy risk category C. Drug transference during lactation is unknown; therefore, empagliflozin during breast-feeding is not recommended. It is also available in a fixed-dose combination with metformin.
Empagliflozin has been studied alone and in combination therapy with other oral antidiabetic drugs as well as insulin therapy. Metformin, pioglitazone, sitagliptin, and linagliptin have been studied in combination with empagliflozin with sustained glycemic improvement without significantly increased risk of hypoglycemia.17-21 Empagliflozin/linagliptin combination was recently approved after phase 3 trials demonstrated 62% of patients achieved an A1c value < 7% on the 25/5-mg dose at 24 weeks.21 Empagliflozin, coadministered with multiple daily injections of insulin (MDI), has been shown to safely improve glycemic control and reduce total daily insulin requirements without an increased risk of hypoglycemia.22 Currently, it is not approved for use in patients with T1DM, but phase 3 trials are ongoing.
Pharmacokinetics of empagliflozin among healthy volunteers paralleled those of people with T2DM with rapid absorption. The plasma glucose lowering effect of empagliflozin was evident after the first dose and became more pronounced with treatment duration. The AUC and Cmax were dose-proportional over a range of empagliflozin doses in a single rising dose study, with maximum UGE of 90.8 g in healthy volunteers reached at the 400-mg dose.23 The Tmax was 1.5 to 2.1 hours after dosing, comparable to dapagliflozin.24 Steady state with once-daily dosing is reached by day 5 with t1/2 range of 10 to 19 hours.24-27 Plasma levels of empagliflozin declined in a biphasic pattern, with a rapid distribution phase and a slower elimination phase. Total urine volume did not differ significantly in the empagliflozin group compared with placebo.
Healthy subjects treated with placebo or empagliflozin had comparable plasma glucose concentrations. Patients with T2DM demonstrated a decrease in mean daily glucose of -37.0 mg/dL for the 10-mg dose compared with -13.5 mg/dL for placebo. Doses up to 10 mg were found to inhibit renal tubular reabsorption up to 40%, and higher doses inhibit up to 60% of filtered glucose.22-26 Empagliflozin has been shown to have similar efficacy independent of food.27 The pharmacodynamic response declines with increasing renal impairment in SGLT2 inhibitors. Empagliflozin has been associated with a decline from UGE of 97.6 g in normal renal function to 18.2 g in severe renal impairment.
Phase 1 studies of healthy male volunteers initially elucidated empagliflozin’s therapeutic potential of dosedependent increases in UGE and associated reduction in A1c with daily administration, respectively.24,28 Single rising dose studies further defined the linear pharmacokinetics and excellent tolerability of empagliflozin.23 Heise and colleagues demonstrated in 2 separate studies with multiple oral doses (2.5 mg, 10 mg, 25 mg, and 100 mg) of empagliflozin in people with T2DM similar pharmacokinetics and efficacy in UGE, tolerability, and reduction in plasma glucose.24
Ferrannini and colleagues, in a 12-week phase 2 study, demonstrated a statistically significant reduction in A1c of 0.5% and 0.6% with 10-mg and 25-mg doses, respectively, as well as a universal body weight decline by 2 kg.29
Clinical Efficacy Trials
A phase 3 trial of 1,549 randomized people with T2DM (aged > 18 years with baseline A1c of 7%-10%), comparing empagliflozin and glimepiride as an add-on to metformin, demonstrated noninferiority at 52 weeks and statistically significant superiority for the empagliflozin group at 104 weeks.30 The adjusted mean difference in change from baseline in A1c with empagliflozin vs glimepiride at week 104 was -0.11% (95% confidence interval, -0.19 to -0.02; P = .0153 for superiority). Despite 39% of both groups achieving a A1c < 7% at week 52, the empagliflozin group had significantly less hypoglycemia (2% vs 24%). In the body composition substudy, empagliflozin demonstrated a significant reduction of 3 kg compared with an increase of slightly over 1 kg in the glimepiride group at week 104. The majority of the empagliflozin-associated weight loss was found to be a reduction in fat mass.
An international phase 2B randomized, controlled, open-label extension study of 659 people with T2DM (aged > 18 and < 79 years with a body mass index (BMI) < 40 kg/m2 and baseline A1c of 7%-10%) described the long-term safety and efficacy of empagliflozin monotherapy in combination with metformin as compared with sitagliptin monotherapy in combination with metformin. At week 90, the changes from baseline A1c were -0.34%/ -0.47% in the empagliflozin 10 mg/25 mg monotherapy group, -0.34%/0.63% in the empagliflozin 10 mg/25 mg/ metformin combination, -0.56% with metformin monotherapy, and -0.40% with sitagliptin/metformin combination.31 These data provided evidence that empagliflozin had sustained weight loss effects (-2.2 kg to -4.0 kg).
Empagliflozin monotherapy was studied with sitagliptin as an active comparator in a phase 3 trial of 899 people with T2DM (aged > 18 years with baseline A1c of 7%-10%), with the primary endpoint of change in baseline in A1c at week 24. Both the 10-mg and 25-mg dose sof empagliflozin were associated with greater reductions in A1c from baseline (-0.66%, -0.77%) at week 24 than that of sitagliptin (-1.04%). Additionally, both doses of empagliflozin were associated with greater reductions in bodyweight (-2.26 kg and -2.48 kg) as compared with sitagliptin 100 mg (+0.18 kg).20
Empagliflozin’s efficacy and safety in combination with MDI insulin as well as add-on to basal insulin was tested in 2 separate studies.23,32 People with T2DM (mean age 58.8 years, A1c 8.2%) on basal insulin were randomized to empagliflozin (10 mg or 25 mg) or placebo for 78 weeks with a constant basal insulin dose for the first 18 weeks and titration allowed thereafter. Empagliflozin was found to significantly reduce A1c at both 18 and 78 weeks (-0.48% and -0.64% for 10-mg/25-mg doses, respectively, vs -0.02% for placebo) and insulin dose at week 78 vs placebo (-8.8 IU on empagliflozin 10 mg and -11.2 IU on empagliflozin 25 mg).32 Similar rates of hypoglycemia were reported (36.1% of patients on empagliflozin and 35.3% on placebo)
Furthermore, empagliflozin was studied in obese people with uncontrolled, insulin-dependent T2DM (A1c 8.3%, BMI 34.8 kg/m2) on MDI insulin (average 92 IU/d) over a 52-week study.22 Patients were randomized to once-daily empagliflozin 10 mg, empagliflozin 25 mg, or placebo. The study demonstrated improved glycemic control (A1c reduction of -1.27% and -1.18% on empagliflozin 10 mg and 25 mg doses, respectively, compared with -0.81% on placebo) with lower insulin doses (-9 to -11 IU/d) and weight loss (-1.95 kg and -2.04 kg on empagliflozin 10-mg and 25-mg doses, respectively, compared with 0.44 kg on placebo.) There was no increased risk of hypoglycemia noted.
Adverse Effects and Precautions
Empagliflozin is generally well tolerated with low occurrence of AEs. Adverse effects reported in the pooled empagliflozin phase 3 studies were mild to moderate. Serious AEs reported were higher in the placebo group as compared with those of the empagliflozin subjects but did not result in study discontinuation.33
Drug-drug studies of empagliflozin co-administered with other commonly prescribed medication in T2DM showed very limited, if any, interaction. Empagliflozin had no effect on the pharmacokinetics of warfarin or on its anticoagulant activity; therefore, these 2 drugs were deemed safe to be co-administered. No AEs were reported when combining empagliflozin with the loop diuretic hydrochlorothiazide.33 Due to the mode of action of SGLT2 inhibitors, osmotic diuresis may result in a modest reduction in BP.34
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA.35 The FDA Adverse Event Reporting System identified 20 cases of DKA in patients treated with SGLT2 inhibitors from March 2013 to June 6, 2014. Diabetic ketoacidosis is typically accompanied by levels of ketone bodies > 3,000 μmol/L and develops almost exclusively in states of absolute insulin deficiency. The highest level of ketone bodies observed in patients receiving 25 mg of empagliflozin was 1,449 μmol/L compared with a mean of 1,300 μmol/L in a nondiabetic overnight fast.36 Therefore, it is unlikely that the modest empagliflozin-induced ketosis would increase the risk of developing DKA in the absence of absolute insulin deficiency or extreme ketogenic diet.37 Physiologic explanation at the present time is not clear.
Clinical Application
The SGLT2 inhibitors are the latest of 14 classes of drugs approved to treat T2DM. This class offers many beneficial characteristics besides blood sugar lowering. The drugs lower systolic BP, induce weight loss through diuresis of glucose (calories), and carry low risks for hypoglycemia. The American Association of Clinical Endocrinologists recently published their 2015 diabetes management algorithm.38 In this algorithm, they recognized metformin as first-line therapy along with diet and exercise intensification for the treatment of T2DM.
The glucagon-like peptide-1 (GLP-1) analogs and SGLT2 inhibitors are recognized as plausible second-line drugs. They go together well with metformin, providing powerful additional A1c-lowering effects while inducing weight loss without hypoglycemia. Because all GLP-1 analogs are currently available only in injectable forms, the SGLT2 inhibitors offer the additional advantage of being available in pill forms.
Conclusions
All 3 of the current SGLT2 inhibitors are effective tools for treating T2DM. The 3 drugs share many similar traits, and efficacy is generally similar. Empagliflozin has the highest SGLT2 selectivity, > 2,500-fold selectivity for SGLT2 over SGLT1. However, canagliflozin has mild SGLT1 activity, which may offer additional benefits with regards to attenuating PPGE excursions by delaying intestinal glucose absorption (Table).
The SGLT2 inhibitor class had been shown to be effective when used as monotherapy as well as in combination with other oral antidiabetic medications. All 3 SGLT2 inhibitors have also been shown to be effective in combination with insulin and have similar efficacy in these clinical settings (eTable).
All 3 SGLT2 inhibitors are generally well tolerated. There is less hypoglycemia compared with sulfonylureas. However, when the SGLT2 drugs are combined with drugs that can cause hypoglycemia, such as sulfonylureas and insulin, patients must be monitored for hypoglycemia, and titrating down the sulfonylurea or insulin may be necessary. Genital mycotic infections and urinary tract infections in men and woman are common AEs with SGLT2 drugs. Patients must be advised of these possible AEs, and treatment should be prompt if these AEs occur. Because of the mild osmotic diuresis, patients should be reminded to keep well hydrated. SGLT2s have a very mild effect on increasing low-density lipoprotein cholesterol (LDL-C), so care should be taken to ensure that patients’ LDL-C stays at goal.
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA. Although these reports are rare, clinicians should be vigilant. The FDA has suggested that patients should pay close attention for any signs of DKA and seek medical attention immediately if they experience difficulty breathing, nausea, vomiting, abdominal pain, confusion, and unusual fatigue or sleepiness.35
There are subtle differences in the eGFR thresholds for the use of the 3 drugs. It should be kept in mind that with all 3 drugs, the efficacy decreases as the eGFR decreases. It is recommended not to initiate canagliflozin if the patient’s eGFR is < 45 mL/min/1.73 m2. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. Empagliflozin should not be initiated in patients with an eGFR < 45 mL/min/1.73 m2.
The SGLT2 inhibitors are useful tools to lower blood glucose levels in people with T2DM. They can be used as monotherapy or in combination. They also cause weight reduction. Thus, their unique mechanism of action is complementary to the other oral antidiabetic medications and insulin, so a wide variety of patients can benefit from this class.
Author disclosures
Dr. Nguyen is affiliated with the Astra Zeneca Speakers Bureau and Janssen Pharmaceutical Speakers Bureau. Dr. Plodkowski is a Janssen Pharmaceutical consultant. The remaining authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
2. Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36(8):2154-2161.
3. Sha S, Polidori D, Farrell, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab. 2015;17(2):188-197.
4. Yamout H, Perkovic V, Davies M, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol. 2014;40(1):64-74.
5. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38(3):355-364.
6. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582-2592.
7. Neal B, Perkovic V, de Zeeuw D, et al; CANVAS Trial Collaborative Group. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38(3):403-411.
8. INVOKANA [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2013.
9. FARXIGA [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015.
10. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34(9):2015-2022.
11. Rosenstock J, Hansen L, Zee P, et al. Dual Add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376-383.
12. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S; Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16(2):124-136.
13. Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(7):613-621.
14. Macha S, Rose P, Mattheus M, et al. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16(2):118-123.
15. Barnett A, Mithal A, Manassie J, et al; EMPA-REG RENAL trial investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, doubleblind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369-384.
16. Macha S, Mattheus M, Halabi A, Pinnetti S, Woerle HJ, Broedl UC. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes Metab. 2014;16(3):215-222.
17. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG MET Trial Investigators.
Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650-1699.
18. Ridderstråle M, Svaerd R, Zeller C, Kim G, Woerle HJ, Broedi UC; EMPA-REG H2H-SU trial investigators. Rational, design and baseline characteristics of a 4-year (208-week) phase III trial of empagliflozin, an SGLT2 inhibitor, versus glimepiride as add-on to metformin in patients with type 2 diabetes mellitus with insufficient glycemic control. Cardiovasc Diabetol. 2013;12:129.
19. Kovacs CS, Seshiah V, Swallow R, et al; EMPA-REG PIO trial investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16(2):147-158.
20. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
21. Friedrich C, Metzmann K, Rose P, Mattheus M, Pinnetti S, Woerle HJ. A randomized, open-label, crossover study to evaluate the pharmacokinetics of empagliflozin and linagliptin after coadministration in healthy male volunteers. Clin Ther. 2013;35(1):A33-A42.
22. Rosenstock J, Jelaska A, Frappin G, et al; EMPA-REG MDI Trial Investigators. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(3):1815-1823.
23. Seman L, Macha S, Nehmiz G, et al. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Drug Dev. 2013;2(2):152-161.
24. Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. 2013;4(2):331-345.
25. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12(2):90-100.
26. Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53(3):213-225.
27. Macha S, Jungnik A, Hohl K, Hobson D, Salsali A, Woerle HJ. Effect of food on the pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, and assessment of dose proportionality in healthy volunteers. Int J Clinical Pharmacol Therapy. 2013;51(11):873-879.
28. Sarashina A, Koiwai K, Seman LJ, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab Pharmacokinet. 2013;28(3):213-219.
29. Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(8):721-728.
30. Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC; EMPAREG H2H-SU trial investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691-700.
31. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36(12):4015-4021.
32. Rosenstock J, Jelaska A, Kim G, et al. Empagliflozin as add-on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin-treated (T2DM) [Abstract 1102-P]. Diabetes. 2013;62(suppl 1):A285.
33. JARDIANCE [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals,
Inc.; 2015.
34. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11):3396-3404.
35. FDA Drug Safety Communication: FDA Warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Updated May 19, 2015. Accessed September 23, 2015.
36. Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11.
37. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients
with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
38. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21(4):438-447.
Over the past 2 decades, the treatment of type 2 diabetes mellitus (T2DM) has been an evolving science. With therapeutic advances, the prevalence of catastrophic complications such as amputations, renal failure requiring dialysis, and blindness due to retinopathy have significantly declined. Developed drugs have successfully met treatment goals; however, they are often associated with a higher risk of hypoglycemia and weight gain. Now that better glucose control is possible, the science of diabetes care continues to evolve. Newly developed drugs should control glucose without significant hypoglycemia and also promote weight reduction. The sodium-glucose transport protein 2 (SGLT2) inhibitor drug class has these characteristics, and the novel mechanism of action complements older medications used to treat T2DM.
Phlorizin is a plant-based compound originally discovered in 1935 when it was derived from the bark of apple trees.1 It is a naturally occurring botanical glucoside and is fairly nonselective between SGLT1 and SGLT2. Due to its poor bioavailability and its degradation in the gastrointestinal tract, it was not an ideal drug candidate in humans.
Canagliflozin
Canagliflozin is a SGLT2 inhibitor and a low-potency SGLT1 inhibitor. It was the first SGLT2 inhibitor approved by the FDA (March 2013) to be used with diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 100 mg once daily for patients who have an estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73m2 and can be increased to 300 mg once daily. It is also available in a fixed-dose combination with metformin. Canagliflozin SGLT-2 inhibition leads to increased glycosuria and osmotic diuresis that lowers plasma glucose concentrations. Lower blood pressure (BP) is likely an effect of the osmotic diuresis. Increased urinary excretion of glucose also leads to a loss of calories and weight loss. It was studied alone and in combination with metformin, sulfonylurea, pioglitazone, and insulin therapy.
The pharmacokinetics of canagliflozin is similar in healthy subjects and patients with T2DM. Peak plasma concentrations (Cmax) and area under the cover (AUC) of canagliflozin increased in a dose-proportional manner from 50 mg to 300 mg. Following single-dose oral administration of 100 mg and 300 mg of canagliflozin, time to Cmax (Tmax) of canagliflozin occurs within 1 to 2 hours postdose. The apparent terminal half-life (t1/2) was 10.6 hours and 13.1 hours for the 100 mg and 300 mg doses, respectively. Steady state was reached after 4 to 5 days of once-daily dosing with canagliflozin 100 mg to 300 mg. Glucuronidation is the major metabolic pathway. There is balanced renal and biliary excretion of metabolites, and there are no active metabolites.
Following oral doses of canagliflozin in patients with T2DM, dose-dependent decreases were seen in the renal threshold for glucose (RTG). From a starting value of about 240 mg/dL, the 300-mg dose suppressed the mean (RTG) to about 70 to 90 mg/dL in T2DM in phase 1 studies. The reduction in RTG led to increase in urinary excretion of glucose of about 100 g/d.
In addition to renal SGLT2 inhibition leading to increased urinary glucose excretion (UGE), canagliflozin has been shown to lower postprandial glucose excursion (PPGE) and insulin concentrations by delaying intestinal glucose absorption.2 A study was done in 20 healthy subjects who received either placebo or canagliflozin 300 mg 20 minutes before a 600 kcal mixed-meal tolerance test. Compared with placebo, canagliflozin reduced PPGE and insulin excursions (0-2 h) AUC by 35% and 43%, respectively (P < .001 for both). This may present a difference between canagliflozin and the other SGLT2 inhibitors.
Because of the potential differences due to canagliflozin’s inhibition of intestinal SGLT1, the pharmacodynamic differences between canagliflozin and dapagliflozin were studied.3 The randomized, double-blind, crossover study consisted of 54 subjects. The subjects received the maximum approved doses of canagliflozin 300 mg or dapagliflozin 10 mg a day. Each group was treated with the study drug for 2 days, and then a 600 kcal mixed-meal tolerance test was performed. The results of the PPGE 0- to 2-hour AUC analysis showed 3.66 mmol*h/L with canagliflozin 300 mg and 4.08 mmol*h/L with dapagliflozin 10 mg. There was a difference of 0.42 (P = .0122), which was a 10.3% reduction in AUC PPGE by canagliflozin compared with dapagliflozin.
Canagliflozin has been studied in patients with T2DM and stage 3 nephropathy. Data were pooled from 4 randomized, placebo-controlled, phase 3 studies in which subjects had baseline eGFR > 30 to < 60 mL/min/1.73 m2.4 In the setting of decreased eGFR associated with stage 3 chronic kidney disease, subjects treated with canagliflozin 100 mg and canagliflozin 300 mg had placebo-subtracted reductions in hemoglobin A1c (A1c) of -0.38% and -0.47%, respectively, and placebosubtracted reduction in weight of -1.6% and -1.9%, respectively. Decreases in eGFR were seen at week 6 but trended toward baseline over time with a mean change in eGFR of 0.7, -1.7, -2.2 mL/min/1.73 m2 for placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively.
Clinical Efficacy Trials
Canagliflozin was studied as add-on therapy to metformin and compared with glimepiride (a sulfonylurea).5 The randomized, double-blind study included 1,450 subjects for a core study period of 52 weeks followed by a 52-week extension. Eligible subjects were aged > 18 and < 80 years, A1c > 7% and < 9.5%, and were receiving metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. The study groups were canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, and baseline A1c measurements were 7.78%, 7.79%, and 7.83%, respectively. The glimepiride was titrated up if > 50% of fasting blood glucose measurements were > 108 mg/dL with no hypoglycemic events in the previous 2 weeks.
Over 104 weeks, canagliflozin 100 mg and 300 mg and glimepiride reduced A1c from mean baseline values by -0.65%, -0.74%, and -0.55%, respectively, and the proportions of patients achieving A1c < 7% at week 104 was 42.5%, 50.2%, and 43.9%, respectively. Weight fell over 104 weeks with canagliflozin 100 mg (-4.1%, -3.6 kg) and canagliflozin 300 mg (-4.2%, -3.6 kg). In contrast, glimepiride showed weight increase (0.9%, 0.8 kg). Documented hypoglycemia episodes were lower in canagliflozin 100 mg and 300 mg than with glimepiride (6.8%, 8.2%, and 40.9%, respectively).
A study was undertaken to compare canagliflozin with the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin in patients with T2DM on background therapy of metformin.6 This randomized, double-blind trial studied subjects aged > 18 and < 80 years with inadequate glucose control A1c > 7% and < 10.5%. Subjects received metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. At week 52, canagliflozin showed noninferiority to sitagliptin, with both drugs lowering A1c by 0.73%. Canagliflozin 300 mg showed superiority to sitagliptin with -0.88% change in A1c. Both canagliflozin 100 mg and 300 mg were superior to sitagliptin 100 mg in weight reduction: -3.8%, -4.2%, and -1.3%, respectively. Genital mycotic infections were higher in the canagliflozin groups. Rates for mycotic infections for sitagliptin 100 mg, canagliflozin 100 mg, and canagliflozin 300 mg were 1.2%, 5.2%, and 2.4% in men, respectively, and 2.6%, 11.3%, and 9.9% in women, respectively.
Canagliflozin was also studied in combination with insulin to determine efficacy and safety in this setting.7 Subjects were randomized to receive placebo, canagliflozin 100 mg, or canagliflozin 300 mg. Subjects had a mean baseline A1c of 8.3%. The median daily insulin dose was 60 IU, and most individuals were using basal/bolus regimens. The primary endpoint was 18 weeks of therapy, and A1c was lowered 0.62% (P < .001) with canagliflozin 100 mg and 0.73% with canagliflozin 300 mg compared with placebo. Weight decreased 1.9% (P < .001) with canagliflozin 100 mg and 2.4% (P < .001) with canagliflozin 300 mg compared with placebo.
Adverse Effects and Precautions
Canagliflozin adverse effects (AEs) were generally low. In the aforementioned 104-week study comparing canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, AEs leading to discontinuation were low at 6.2%, 9.5%, and 7.3%, respectively. Serious AEs were lower in the canagliflozin 100 mg and 300 mg groups compared with glimepiride at 9.7%, 9.7%, 14.3%, respectively.5
Limitations for use of canagliflozin are T1DM and diabetic ketoacidosis (DKA). In mild renal impairment, there is no dose adjustment in patients with eGFR > 60 mL/min/1.73 m2. In moderate renal impairment (eGFR 45-60 mL/min/1.73 m2), dose is limited to 100 mg once daily. It is recommended not to initiate canagliflozin if eGFR is < 45 mL/min/1.73 m2. Canagliflozin is contraindicated if eGFR is < 30 mL/min/1.73 m2.8
Dapagliflozin
Dapagliflozin is a highly selective SGLT2 inhibitor. It is a 1,400-fold greater inhibitor of SGLT2 vs SGLT1 and was approved in January 2014. It is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 5 mg once daily, taken in the morning, with or without food, and the dosage can be increased to 10 mg once daily in patients who require additional glycemic control. Dapagliflozin should not be initiated if eGFR is < 60 mL/min/1.73 m2, and it should be discontinued if eGFR is persistently < 60 mL/min/1.73 m2.
By inhibiting SGLT2, dapagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose, thereby increasing UGE. Increased glucose secretion also leads to weight reduction. Dapagliflozin has been studied alone and in combination with glipizide, glimepiride, pioglitazone, and a DDP-4 inhibitor and as an add-on to insulin with and without other oral antidiabetic drugs. It is also available in a fixed-dose combination with metformin.
Following oral administration of dapagliflozin, the Tmax is usually attained within 2 hours under a fasting state. The Cmax and AUC values increase the dose proportionally with increase in dapagliflozin dose in the therapeutic dose range. The absolute oral bioavailability of dapagliflozin following the administration of a 10-mg dose is 78%. The mean plasma t1⁄2 for dapagliflozin is about 12.9 hours following a single oral dose of 10 mg. In patients with normal renal function, the renal glucose excretion was 85 g per day at maximal dose. In humans, 75% of the dose of dapagliflozin is primarily metabolized through the uridine diphosphate glucuronosyltransferase 1A9 pathway. Dapagliflozin and related metabolites are primarily eliminated via the renal pathway.9
Clinical Efficacy Trials
Dapagliflozin was studied as add-on therapy to metformin with glipizide (a sulfonylurea) as the comparator.10 This 52-week double-blind, multicenter, active-controlled, noninferiority trial randomized 801 patients. Eligible subjects were aged > 18 years with A1c > 6.5% and < 10% and were receiving metformin or metformin and 1 other oral antidiabetic drug up to half-maximal dose for at least 8 weeks. During an 18-week titration period, all patients started dapagliflozin 2.5 mg/d and glipizide 5 mg/d. At 21-day intervals, patients were titrated up to the next dosage level if fasting plasma glucose was > 110 mg/dL. Dapagliflozin could be titrated to 5 mg and then 10 mg per protocol. Glipizide could be titrated to 10 mg or 20 mg per protocol.
The A1c change with dapagliflozin was noninferior to glipizide at week 52. The A1c adjusted mean change from baseline was -0.52 for both dapagliflozin and glipizide. The secondary endpoint was weight change. The glipizide group had a +1.44 kg weight gain, whereas the dapagliflozin group had a -3.22 kg weight loss. The number of patients with ≥ 1 episode of hypoglycemia, either symptomatic or with no symptoms, with blood glucose ≤ 63 mg/dL was assessed. The dapagliflozin group had a 3.5% rate compared with the glipizide group, which had a 40.8% rate of patients with > 1 hypoglycemic episode.
A study examined the efficacy and safety of dapagliflozin in combination with and also vs a DPP-4 inhibitor with metformin background therapy. The study looked at add-ons of saxagliptin plus dapagliflozin vs saxagliptin or dapagliflozin added alone.11 This was a randomized, double-blind, 24-week study with patients aged > 18 years with inadequate glucose control A1c > 8.0% and < 12.0%. Patients had to be on a stable dose of metformin > 1,500 mg/d for at least 8 weeks. Patients were randomized 1:1:1 to receive either saxagliptin 5 mg/d and dapagliflozin 10 mg/d plus metformin, saxagliptin 5 mg/d and placebo plus metformin, or dapagliflozin 10 mg/d and placebo plus metformin.
The patients had a mean age of 54 years and a mean duration of T2DM of 7.6 years. The mean baseline A1c was 8.94%. The addition of saxagliptin plus dapagliflozin to metformin resulted in significantly greater A1c reduction compared with saxagliptin plus metformin or dapagliflozin plus metformin from baseline A1c levels: -1.47%, -0.88%, -1.2%, respectively.11
Dapagliflozin was also studied in combination with insulin to determine the safety and efficacy in this setting.12 This double-blind, placebo-controlled, parallel-group trial had an initial study period of 24 weeks followed by an extension period totaling 104 weeks. At 48 weeks, patients on dapagliflozin 5 mg were switched to 10 mg. Outcomes over 104 weeks were changed from baseline A1c, insulin dose, and body weight. Up-titration of insulin was permitted if at least 3 self-monitored blood glucose readings from the 7 days prior to the study visit were > 240 mg/dL up to week 12; > 220 mg/dL between weeks 12 and 24; > 178 mg/dL or if A1c was > 8% between weeks 24 and 48. Between weeks 52 and 65, insulin titrated up was allowed if A1c was > 7.5% and between weeks 78 and 104 if A1c was > 7%. Insulin could be titrated down if ≥ 2 self-monitored blood glucose readings were < 68 mg/dL.
The study group had a T2DM diagnosis for 13.6 years and a mean duration of insulin therapy for about 6 years. The mean daily insulin dose was 77.1 IU. The mean baseline A1c was 8.5%. At 104 weeks, the differences from placebo in A1c adjusted mean change from baseline were -4.0% (P = .0002) and -0.4% (P = .0007) in the dapagliflozin 5 mg (switched to 10 mg at week 48) and 10 mg groups, respectively. Insulin requirements increased progressively in the placebo group +18.3 IU/d at 104 weeks. Insulin requirements stayed stable over 104 weeks in the dapagliflozin groups. Body weight increased in the placebo group, whereas it decreased in the dapagliflozin groups. At 104 weeks, the weight changes from baseline in placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg were -0.99 kg, -1.03 kg (P < .001), and -1.5 kg (P < .0001), respectively. The frequency of ≥ 1 minor or major episodes of hypoglycemia was fairly balanced across placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg at 61.9%, 61.3%, and 60.7%, respectively.
Adverse Effects and Precautions
Dapagliflozin was generally well tolerated. In the trial of dapagliflozin compared with glipizide in the setting of metformin background therapy, AEs led to discontinuation rates of 9.1% vs 5.9%, respectively. Serious AEs were lower in the dapagliflozin group compared with glipizide at 8.6% vs 11.3%, respectively.10
Limitations for use of dapagliflozin are the treatment of T1DM or the treatment of DKA. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. No dose adjustment is needed in patients with mild renal impairment (eGFR of ≥ 60 mL/ min/1.73 m2), and dapagliflozin should be discontinued when eGFR is persistently < 60 mL/min/1.73 m2. In the clinical trials, there was an imbalance in the number of bladder cancer reported with dapagliflozin compared with placebo. Across 22 clinical studies, newly diagnosed cases of bladder cancer were reported in 10 of 6,045 patients (0.17%) treated with dapagliflozin and 1 of 3,512 patients (0.03%) treated with placebo or a comparator.9 There were too few cases to determine whether the emergence of these events is related to dapagliflozin. Product labeling states dapagliflozin should not be used in patients with active bladder cancer and should be used with caution in those with a prior history of bladder cancer.
Empaglifilozin
Empagliflozin is the most recently FDA-approved SGLT2 inhibitor (August 2014) for improved glycemic control in T2DM. It has the highest SGLT2 selectivity: > 2,500-fold selectivity for SGLT2 over SGLT1.13 Empagliflozin regulates blood glucose levels by increased UGE, independent of endogenous insulin secretion. It is associated with modest reductions in body weight, visceral adiposity, and systolic BP.
Empagliflozin is available in 10-mg and 25-mg tablets, with a recommended initial dose of 10 mg daily.13 Dosing adjustments are not required for geriatric patients or for those patients with hepatic impairment.14 The use of empagliflozin is contraindicated in patients with eGFR < 45 mL/min/1.73 m2 or for those whose eGFR declines to < 45 mL/min/1.73 m2 during therapy.15,16 Empagliflozin has a pregnancy risk category C. Drug transference during lactation is unknown; therefore, empagliflozin during breast-feeding is not recommended. It is also available in a fixed-dose combination with metformin.
Empagliflozin has been studied alone and in combination therapy with other oral antidiabetic drugs as well as insulin therapy. Metformin, pioglitazone, sitagliptin, and linagliptin have been studied in combination with empagliflozin with sustained glycemic improvement without significantly increased risk of hypoglycemia.17-21 Empagliflozin/linagliptin combination was recently approved after phase 3 trials demonstrated 62% of patients achieved an A1c value < 7% on the 25/5-mg dose at 24 weeks.21 Empagliflozin, coadministered with multiple daily injections of insulin (MDI), has been shown to safely improve glycemic control and reduce total daily insulin requirements without an increased risk of hypoglycemia.22 Currently, it is not approved for use in patients with T1DM, but phase 3 trials are ongoing.
Pharmacokinetics of empagliflozin among healthy volunteers paralleled those of people with T2DM with rapid absorption. The plasma glucose lowering effect of empagliflozin was evident after the first dose and became more pronounced with treatment duration. The AUC and Cmax were dose-proportional over a range of empagliflozin doses in a single rising dose study, with maximum UGE of 90.8 g in healthy volunteers reached at the 400-mg dose.23 The Tmax was 1.5 to 2.1 hours after dosing, comparable to dapagliflozin.24 Steady state with once-daily dosing is reached by day 5 with t1/2 range of 10 to 19 hours.24-27 Plasma levels of empagliflozin declined in a biphasic pattern, with a rapid distribution phase and a slower elimination phase. Total urine volume did not differ significantly in the empagliflozin group compared with placebo.
Healthy subjects treated with placebo or empagliflozin had comparable plasma glucose concentrations. Patients with T2DM demonstrated a decrease in mean daily glucose of -37.0 mg/dL for the 10-mg dose compared with -13.5 mg/dL for placebo. Doses up to 10 mg were found to inhibit renal tubular reabsorption up to 40%, and higher doses inhibit up to 60% of filtered glucose.22-26 Empagliflozin has been shown to have similar efficacy independent of food.27 The pharmacodynamic response declines with increasing renal impairment in SGLT2 inhibitors. Empagliflozin has been associated with a decline from UGE of 97.6 g in normal renal function to 18.2 g in severe renal impairment.
Phase 1 studies of healthy male volunteers initially elucidated empagliflozin’s therapeutic potential of dosedependent increases in UGE and associated reduction in A1c with daily administration, respectively.24,28 Single rising dose studies further defined the linear pharmacokinetics and excellent tolerability of empagliflozin.23 Heise and colleagues demonstrated in 2 separate studies with multiple oral doses (2.5 mg, 10 mg, 25 mg, and 100 mg) of empagliflozin in people with T2DM similar pharmacokinetics and efficacy in UGE, tolerability, and reduction in plasma glucose.24
Ferrannini and colleagues, in a 12-week phase 2 study, demonstrated a statistically significant reduction in A1c of 0.5% and 0.6% with 10-mg and 25-mg doses, respectively, as well as a universal body weight decline by 2 kg.29
Clinical Efficacy Trials
A phase 3 trial of 1,549 randomized people with T2DM (aged > 18 years with baseline A1c of 7%-10%), comparing empagliflozin and glimepiride as an add-on to metformin, demonstrated noninferiority at 52 weeks and statistically significant superiority for the empagliflozin group at 104 weeks.30 The adjusted mean difference in change from baseline in A1c with empagliflozin vs glimepiride at week 104 was -0.11% (95% confidence interval, -0.19 to -0.02; P = .0153 for superiority). Despite 39% of both groups achieving a A1c < 7% at week 52, the empagliflozin group had significantly less hypoglycemia (2% vs 24%). In the body composition substudy, empagliflozin demonstrated a significant reduction of 3 kg compared with an increase of slightly over 1 kg in the glimepiride group at week 104. The majority of the empagliflozin-associated weight loss was found to be a reduction in fat mass.
An international phase 2B randomized, controlled, open-label extension study of 659 people with T2DM (aged > 18 and < 79 years with a body mass index (BMI) < 40 kg/m2 and baseline A1c of 7%-10%) described the long-term safety and efficacy of empagliflozin monotherapy in combination with metformin as compared with sitagliptin monotherapy in combination with metformin. At week 90, the changes from baseline A1c were -0.34%/ -0.47% in the empagliflozin 10 mg/25 mg monotherapy group, -0.34%/0.63% in the empagliflozin 10 mg/25 mg/ metformin combination, -0.56% with metformin monotherapy, and -0.40% with sitagliptin/metformin combination.31 These data provided evidence that empagliflozin had sustained weight loss effects (-2.2 kg to -4.0 kg).
Empagliflozin monotherapy was studied with sitagliptin as an active comparator in a phase 3 trial of 899 people with T2DM (aged > 18 years with baseline A1c of 7%-10%), with the primary endpoint of change in baseline in A1c at week 24. Both the 10-mg and 25-mg dose sof empagliflozin were associated with greater reductions in A1c from baseline (-0.66%, -0.77%) at week 24 than that of sitagliptin (-1.04%). Additionally, both doses of empagliflozin were associated with greater reductions in bodyweight (-2.26 kg and -2.48 kg) as compared with sitagliptin 100 mg (+0.18 kg).20
Empagliflozin’s efficacy and safety in combination with MDI insulin as well as add-on to basal insulin was tested in 2 separate studies.23,32 People with T2DM (mean age 58.8 years, A1c 8.2%) on basal insulin were randomized to empagliflozin (10 mg or 25 mg) or placebo for 78 weeks with a constant basal insulin dose for the first 18 weeks and titration allowed thereafter. Empagliflozin was found to significantly reduce A1c at both 18 and 78 weeks (-0.48% and -0.64% for 10-mg/25-mg doses, respectively, vs -0.02% for placebo) and insulin dose at week 78 vs placebo (-8.8 IU on empagliflozin 10 mg and -11.2 IU on empagliflozin 25 mg).32 Similar rates of hypoglycemia were reported (36.1% of patients on empagliflozin and 35.3% on placebo)
Furthermore, empagliflozin was studied in obese people with uncontrolled, insulin-dependent T2DM (A1c 8.3%, BMI 34.8 kg/m2) on MDI insulin (average 92 IU/d) over a 52-week study.22 Patients were randomized to once-daily empagliflozin 10 mg, empagliflozin 25 mg, or placebo. The study demonstrated improved glycemic control (A1c reduction of -1.27% and -1.18% on empagliflozin 10 mg and 25 mg doses, respectively, compared with -0.81% on placebo) with lower insulin doses (-9 to -11 IU/d) and weight loss (-1.95 kg and -2.04 kg on empagliflozin 10-mg and 25-mg doses, respectively, compared with 0.44 kg on placebo.) There was no increased risk of hypoglycemia noted.
Adverse Effects and Precautions
Empagliflozin is generally well tolerated with low occurrence of AEs. Adverse effects reported in the pooled empagliflozin phase 3 studies were mild to moderate. Serious AEs reported were higher in the placebo group as compared with those of the empagliflozin subjects but did not result in study discontinuation.33
Drug-drug studies of empagliflozin co-administered with other commonly prescribed medication in T2DM showed very limited, if any, interaction. Empagliflozin had no effect on the pharmacokinetics of warfarin or on its anticoagulant activity; therefore, these 2 drugs were deemed safe to be co-administered. No AEs were reported when combining empagliflozin with the loop diuretic hydrochlorothiazide.33 Due to the mode of action of SGLT2 inhibitors, osmotic diuresis may result in a modest reduction in BP.34
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA.35 The FDA Adverse Event Reporting System identified 20 cases of DKA in patients treated with SGLT2 inhibitors from March 2013 to June 6, 2014. Diabetic ketoacidosis is typically accompanied by levels of ketone bodies > 3,000 μmol/L and develops almost exclusively in states of absolute insulin deficiency. The highest level of ketone bodies observed in patients receiving 25 mg of empagliflozin was 1,449 μmol/L compared with a mean of 1,300 μmol/L in a nondiabetic overnight fast.36 Therefore, it is unlikely that the modest empagliflozin-induced ketosis would increase the risk of developing DKA in the absence of absolute insulin deficiency or extreme ketogenic diet.37 Physiologic explanation at the present time is not clear.
Clinical Application
The SGLT2 inhibitors are the latest of 14 classes of drugs approved to treat T2DM. This class offers many beneficial characteristics besides blood sugar lowering. The drugs lower systolic BP, induce weight loss through diuresis of glucose (calories), and carry low risks for hypoglycemia. The American Association of Clinical Endocrinologists recently published their 2015 diabetes management algorithm.38 In this algorithm, they recognized metformin as first-line therapy along with diet and exercise intensification for the treatment of T2DM.
The glucagon-like peptide-1 (GLP-1) analogs and SGLT2 inhibitors are recognized as plausible second-line drugs. They go together well with metformin, providing powerful additional A1c-lowering effects while inducing weight loss without hypoglycemia. Because all GLP-1 analogs are currently available only in injectable forms, the SGLT2 inhibitors offer the additional advantage of being available in pill forms.
Conclusions
All 3 of the current SGLT2 inhibitors are effective tools for treating T2DM. The 3 drugs share many similar traits, and efficacy is generally similar. Empagliflozin has the highest SGLT2 selectivity, > 2,500-fold selectivity for SGLT2 over SGLT1. However, canagliflozin has mild SGLT1 activity, which may offer additional benefits with regards to attenuating PPGE excursions by delaying intestinal glucose absorption (Table).
The SGLT2 inhibitor class had been shown to be effective when used as monotherapy as well as in combination with other oral antidiabetic medications. All 3 SGLT2 inhibitors have also been shown to be effective in combination with insulin and have similar efficacy in these clinical settings (eTable).
All 3 SGLT2 inhibitors are generally well tolerated. There is less hypoglycemia compared with sulfonylureas. However, when the SGLT2 drugs are combined with drugs that can cause hypoglycemia, such as sulfonylureas and insulin, patients must be monitored for hypoglycemia, and titrating down the sulfonylurea or insulin may be necessary. Genital mycotic infections and urinary tract infections in men and woman are common AEs with SGLT2 drugs. Patients must be advised of these possible AEs, and treatment should be prompt if these AEs occur. Because of the mild osmotic diuresis, patients should be reminded to keep well hydrated. SGLT2s have a very mild effect on increasing low-density lipoprotein cholesterol (LDL-C), so care should be taken to ensure that patients’ LDL-C stays at goal.
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA. Although these reports are rare, clinicians should be vigilant. The FDA has suggested that patients should pay close attention for any signs of DKA and seek medical attention immediately if they experience difficulty breathing, nausea, vomiting, abdominal pain, confusion, and unusual fatigue or sleepiness.35
There are subtle differences in the eGFR thresholds for the use of the 3 drugs. It should be kept in mind that with all 3 drugs, the efficacy decreases as the eGFR decreases. It is recommended not to initiate canagliflozin if the patient’s eGFR is < 45 mL/min/1.73 m2. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. Empagliflozin should not be initiated in patients with an eGFR < 45 mL/min/1.73 m2.
The SGLT2 inhibitors are useful tools to lower blood glucose levels in people with T2DM. They can be used as monotherapy or in combination. They also cause weight reduction. Thus, their unique mechanism of action is complementary to the other oral antidiabetic medications and insulin, so a wide variety of patients can benefit from this class.
Author disclosures
Dr. Nguyen is affiliated with the Astra Zeneca Speakers Bureau and Janssen Pharmaceutical Speakers Bureau. Dr. Plodkowski is a Janssen Pharmaceutical consultant. The remaining authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Over the past 2 decades, the treatment of type 2 diabetes mellitus (T2DM) has been an evolving science. With therapeutic advances, the prevalence of catastrophic complications such as amputations, renal failure requiring dialysis, and blindness due to retinopathy have significantly declined. Developed drugs have successfully met treatment goals; however, they are often associated with a higher risk of hypoglycemia and weight gain. Now that better glucose control is possible, the science of diabetes care continues to evolve. Newly developed drugs should control glucose without significant hypoglycemia and also promote weight reduction. The sodium-glucose transport protein 2 (SGLT2) inhibitor drug class has these characteristics, and the novel mechanism of action complements older medications used to treat T2DM.
Phlorizin is a plant-based compound originally discovered in 1935 when it was derived from the bark of apple trees.1 It is a naturally occurring botanical glucoside and is fairly nonselective between SGLT1 and SGLT2. Due to its poor bioavailability and its degradation in the gastrointestinal tract, it was not an ideal drug candidate in humans.
Canagliflozin
Canagliflozin is a SGLT2 inhibitor and a low-potency SGLT1 inhibitor. It was the first SGLT2 inhibitor approved by the FDA (March 2013) to be used with diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 100 mg once daily for patients who have an estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73m2 and can be increased to 300 mg once daily. It is also available in a fixed-dose combination with metformin. Canagliflozin SGLT-2 inhibition leads to increased glycosuria and osmotic diuresis that lowers plasma glucose concentrations. Lower blood pressure (BP) is likely an effect of the osmotic diuresis. Increased urinary excretion of glucose also leads to a loss of calories and weight loss. It was studied alone and in combination with metformin, sulfonylurea, pioglitazone, and insulin therapy.
The pharmacokinetics of canagliflozin is similar in healthy subjects and patients with T2DM. Peak plasma concentrations (Cmax) and area under the cover (AUC) of canagliflozin increased in a dose-proportional manner from 50 mg to 300 mg. Following single-dose oral administration of 100 mg and 300 mg of canagliflozin, time to Cmax (Tmax) of canagliflozin occurs within 1 to 2 hours postdose. The apparent terminal half-life (t1/2) was 10.6 hours and 13.1 hours for the 100 mg and 300 mg doses, respectively. Steady state was reached after 4 to 5 days of once-daily dosing with canagliflozin 100 mg to 300 mg. Glucuronidation is the major metabolic pathway. There is balanced renal and biliary excretion of metabolites, and there are no active metabolites.
Following oral doses of canagliflozin in patients with T2DM, dose-dependent decreases were seen in the renal threshold for glucose (RTG). From a starting value of about 240 mg/dL, the 300-mg dose suppressed the mean (RTG) to about 70 to 90 mg/dL in T2DM in phase 1 studies. The reduction in RTG led to increase in urinary excretion of glucose of about 100 g/d.
In addition to renal SGLT2 inhibition leading to increased urinary glucose excretion (UGE), canagliflozin has been shown to lower postprandial glucose excursion (PPGE) and insulin concentrations by delaying intestinal glucose absorption.2 A study was done in 20 healthy subjects who received either placebo or canagliflozin 300 mg 20 minutes before a 600 kcal mixed-meal tolerance test. Compared with placebo, canagliflozin reduced PPGE and insulin excursions (0-2 h) AUC by 35% and 43%, respectively (P < .001 for both). This may present a difference between canagliflozin and the other SGLT2 inhibitors.
Because of the potential differences due to canagliflozin’s inhibition of intestinal SGLT1, the pharmacodynamic differences between canagliflozin and dapagliflozin were studied.3 The randomized, double-blind, crossover study consisted of 54 subjects. The subjects received the maximum approved doses of canagliflozin 300 mg or dapagliflozin 10 mg a day. Each group was treated with the study drug for 2 days, and then a 600 kcal mixed-meal tolerance test was performed. The results of the PPGE 0- to 2-hour AUC analysis showed 3.66 mmol*h/L with canagliflozin 300 mg and 4.08 mmol*h/L with dapagliflozin 10 mg. There was a difference of 0.42 (P = .0122), which was a 10.3% reduction in AUC PPGE by canagliflozin compared with dapagliflozin.
Canagliflozin has been studied in patients with T2DM and stage 3 nephropathy. Data were pooled from 4 randomized, placebo-controlled, phase 3 studies in which subjects had baseline eGFR > 30 to < 60 mL/min/1.73 m2.4 In the setting of decreased eGFR associated with stage 3 chronic kidney disease, subjects treated with canagliflozin 100 mg and canagliflozin 300 mg had placebo-subtracted reductions in hemoglobin A1c (A1c) of -0.38% and -0.47%, respectively, and placebosubtracted reduction in weight of -1.6% and -1.9%, respectively. Decreases in eGFR were seen at week 6 but trended toward baseline over time with a mean change in eGFR of 0.7, -1.7, -2.2 mL/min/1.73 m2 for placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively.
Clinical Efficacy Trials
Canagliflozin was studied as add-on therapy to metformin and compared with glimepiride (a sulfonylurea).5 The randomized, double-blind study included 1,450 subjects for a core study period of 52 weeks followed by a 52-week extension. Eligible subjects were aged > 18 and < 80 years, A1c > 7% and < 9.5%, and were receiving metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. The study groups were canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, and baseline A1c measurements were 7.78%, 7.79%, and 7.83%, respectively. The glimepiride was titrated up if > 50% of fasting blood glucose measurements were > 108 mg/dL with no hypoglycemic events in the previous 2 weeks.
Over 104 weeks, canagliflozin 100 mg and 300 mg and glimepiride reduced A1c from mean baseline values by -0.65%, -0.74%, and -0.55%, respectively, and the proportions of patients achieving A1c < 7% at week 104 was 42.5%, 50.2%, and 43.9%, respectively. Weight fell over 104 weeks with canagliflozin 100 mg (-4.1%, -3.6 kg) and canagliflozin 300 mg (-4.2%, -3.6 kg). In contrast, glimepiride showed weight increase (0.9%, 0.8 kg). Documented hypoglycemia episodes were lower in canagliflozin 100 mg and 300 mg than with glimepiride (6.8%, 8.2%, and 40.9%, respectively).
A study was undertaken to compare canagliflozin with the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin in patients with T2DM on background therapy of metformin.6 This randomized, double-blind trial studied subjects aged > 18 and < 80 years with inadequate glucose control A1c > 7% and < 10.5%. Subjects received metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. At week 52, canagliflozin showed noninferiority to sitagliptin, with both drugs lowering A1c by 0.73%. Canagliflozin 300 mg showed superiority to sitagliptin with -0.88% change in A1c. Both canagliflozin 100 mg and 300 mg were superior to sitagliptin 100 mg in weight reduction: -3.8%, -4.2%, and -1.3%, respectively. Genital mycotic infections were higher in the canagliflozin groups. Rates for mycotic infections for sitagliptin 100 mg, canagliflozin 100 mg, and canagliflozin 300 mg were 1.2%, 5.2%, and 2.4% in men, respectively, and 2.6%, 11.3%, and 9.9% in women, respectively.
Canagliflozin was also studied in combination with insulin to determine efficacy and safety in this setting.7 Subjects were randomized to receive placebo, canagliflozin 100 mg, or canagliflozin 300 mg. Subjects had a mean baseline A1c of 8.3%. The median daily insulin dose was 60 IU, and most individuals were using basal/bolus regimens. The primary endpoint was 18 weeks of therapy, and A1c was lowered 0.62% (P < .001) with canagliflozin 100 mg and 0.73% with canagliflozin 300 mg compared with placebo. Weight decreased 1.9% (P < .001) with canagliflozin 100 mg and 2.4% (P < .001) with canagliflozin 300 mg compared with placebo.
Adverse Effects and Precautions
Canagliflozin adverse effects (AEs) were generally low. In the aforementioned 104-week study comparing canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, AEs leading to discontinuation were low at 6.2%, 9.5%, and 7.3%, respectively. Serious AEs were lower in the canagliflozin 100 mg and 300 mg groups compared with glimepiride at 9.7%, 9.7%, 14.3%, respectively.5
Limitations for use of canagliflozin are T1DM and diabetic ketoacidosis (DKA). In mild renal impairment, there is no dose adjustment in patients with eGFR > 60 mL/min/1.73 m2. In moderate renal impairment (eGFR 45-60 mL/min/1.73 m2), dose is limited to 100 mg once daily. It is recommended not to initiate canagliflozin if eGFR is < 45 mL/min/1.73 m2. Canagliflozin is contraindicated if eGFR is < 30 mL/min/1.73 m2.8
Dapagliflozin
Dapagliflozin is a highly selective SGLT2 inhibitor. It is a 1,400-fold greater inhibitor of SGLT2 vs SGLT1 and was approved in January 2014. It is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 5 mg once daily, taken in the morning, with or without food, and the dosage can be increased to 10 mg once daily in patients who require additional glycemic control. Dapagliflozin should not be initiated if eGFR is < 60 mL/min/1.73 m2, and it should be discontinued if eGFR is persistently < 60 mL/min/1.73 m2.
By inhibiting SGLT2, dapagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose, thereby increasing UGE. Increased glucose secretion also leads to weight reduction. Dapagliflozin has been studied alone and in combination with glipizide, glimepiride, pioglitazone, and a DDP-4 inhibitor and as an add-on to insulin with and without other oral antidiabetic drugs. It is also available in a fixed-dose combination with metformin.
Following oral administration of dapagliflozin, the Tmax is usually attained within 2 hours under a fasting state. The Cmax and AUC values increase the dose proportionally with increase in dapagliflozin dose in the therapeutic dose range. The absolute oral bioavailability of dapagliflozin following the administration of a 10-mg dose is 78%. The mean plasma t1⁄2 for dapagliflozin is about 12.9 hours following a single oral dose of 10 mg. In patients with normal renal function, the renal glucose excretion was 85 g per day at maximal dose. In humans, 75% of the dose of dapagliflozin is primarily metabolized through the uridine diphosphate glucuronosyltransferase 1A9 pathway. Dapagliflozin and related metabolites are primarily eliminated via the renal pathway.9
Clinical Efficacy Trials
Dapagliflozin was studied as add-on therapy to metformin with glipizide (a sulfonylurea) as the comparator.10 This 52-week double-blind, multicenter, active-controlled, noninferiority trial randomized 801 patients. Eligible subjects were aged > 18 years with A1c > 6.5% and < 10% and were receiving metformin or metformin and 1 other oral antidiabetic drug up to half-maximal dose for at least 8 weeks. During an 18-week titration period, all patients started dapagliflozin 2.5 mg/d and glipizide 5 mg/d. At 21-day intervals, patients were titrated up to the next dosage level if fasting plasma glucose was > 110 mg/dL. Dapagliflozin could be titrated to 5 mg and then 10 mg per protocol. Glipizide could be titrated to 10 mg or 20 mg per protocol.
The A1c change with dapagliflozin was noninferior to glipizide at week 52. The A1c adjusted mean change from baseline was -0.52 for both dapagliflozin and glipizide. The secondary endpoint was weight change. The glipizide group had a +1.44 kg weight gain, whereas the dapagliflozin group had a -3.22 kg weight loss. The number of patients with ≥ 1 episode of hypoglycemia, either symptomatic or with no symptoms, with blood glucose ≤ 63 mg/dL was assessed. The dapagliflozin group had a 3.5% rate compared with the glipizide group, which had a 40.8% rate of patients with > 1 hypoglycemic episode.
A study examined the efficacy and safety of dapagliflozin in combination with and also vs a DPP-4 inhibitor with metformin background therapy. The study looked at add-ons of saxagliptin plus dapagliflozin vs saxagliptin or dapagliflozin added alone.11 This was a randomized, double-blind, 24-week study with patients aged > 18 years with inadequate glucose control A1c > 8.0% and < 12.0%. Patients had to be on a stable dose of metformin > 1,500 mg/d for at least 8 weeks. Patients were randomized 1:1:1 to receive either saxagliptin 5 mg/d and dapagliflozin 10 mg/d plus metformin, saxagliptin 5 mg/d and placebo plus metformin, or dapagliflozin 10 mg/d and placebo plus metformin.
The patients had a mean age of 54 years and a mean duration of T2DM of 7.6 years. The mean baseline A1c was 8.94%. The addition of saxagliptin plus dapagliflozin to metformin resulted in significantly greater A1c reduction compared with saxagliptin plus metformin or dapagliflozin plus metformin from baseline A1c levels: -1.47%, -0.88%, -1.2%, respectively.11
Dapagliflozin was also studied in combination with insulin to determine the safety and efficacy in this setting.12 This double-blind, placebo-controlled, parallel-group trial had an initial study period of 24 weeks followed by an extension period totaling 104 weeks. At 48 weeks, patients on dapagliflozin 5 mg were switched to 10 mg. Outcomes over 104 weeks were changed from baseline A1c, insulin dose, and body weight. Up-titration of insulin was permitted if at least 3 self-monitored blood glucose readings from the 7 days prior to the study visit were > 240 mg/dL up to week 12; > 220 mg/dL between weeks 12 and 24; > 178 mg/dL or if A1c was > 8% between weeks 24 and 48. Between weeks 52 and 65, insulin titrated up was allowed if A1c was > 7.5% and between weeks 78 and 104 if A1c was > 7%. Insulin could be titrated down if ≥ 2 self-monitored blood glucose readings were < 68 mg/dL.
The study group had a T2DM diagnosis for 13.6 years and a mean duration of insulin therapy for about 6 years. The mean daily insulin dose was 77.1 IU. The mean baseline A1c was 8.5%. At 104 weeks, the differences from placebo in A1c adjusted mean change from baseline were -4.0% (P = .0002) and -0.4% (P = .0007) in the dapagliflozin 5 mg (switched to 10 mg at week 48) and 10 mg groups, respectively. Insulin requirements increased progressively in the placebo group +18.3 IU/d at 104 weeks. Insulin requirements stayed stable over 104 weeks in the dapagliflozin groups. Body weight increased in the placebo group, whereas it decreased in the dapagliflozin groups. At 104 weeks, the weight changes from baseline in placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg were -0.99 kg, -1.03 kg (P < .001), and -1.5 kg (P < .0001), respectively. The frequency of ≥ 1 minor or major episodes of hypoglycemia was fairly balanced across placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg at 61.9%, 61.3%, and 60.7%, respectively.
Adverse Effects and Precautions
Dapagliflozin was generally well tolerated. In the trial of dapagliflozin compared with glipizide in the setting of metformin background therapy, AEs led to discontinuation rates of 9.1% vs 5.9%, respectively. Serious AEs were lower in the dapagliflozin group compared with glipizide at 8.6% vs 11.3%, respectively.10
Limitations for use of dapagliflozin are the treatment of T1DM or the treatment of DKA. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. No dose adjustment is needed in patients with mild renal impairment (eGFR of ≥ 60 mL/ min/1.73 m2), and dapagliflozin should be discontinued when eGFR is persistently < 60 mL/min/1.73 m2. In the clinical trials, there was an imbalance in the number of bladder cancer reported with dapagliflozin compared with placebo. Across 22 clinical studies, newly diagnosed cases of bladder cancer were reported in 10 of 6,045 patients (0.17%) treated with dapagliflozin and 1 of 3,512 patients (0.03%) treated with placebo or a comparator.9 There were too few cases to determine whether the emergence of these events is related to dapagliflozin. Product labeling states dapagliflozin should not be used in patients with active bladder cancer and should be used with caution in those with a prior history of bladder cancer.
Empaglifilozin
Empagliflozin is the most recently FDA-approved SGLT2 inhibitor (August 2014) for improved glycemic control in T2DM. It has the highest SGLT2 selectivity: > 2,500-fold selectivity for SGLT2 over SGLT1.13 Empagliflozin regulates blood glucose levels by increased UGE, independent of endogenous insulin secretion. It is associated with modest reductions in body weight, visceral adiposity, and systolic BP.
Empagliflozin is available in 10-mg and 25-mg tablets, with a recommended initial dose of 10 mg daily.13 Dosing adjustments are not required for geriatric patients or for those patients with hepatic impairment.14 The use of empagliflozin is contraindicated in patients with eGFR < 45 mL/min/1.73 m2 or for those whose eGFR declines to < 45 mL/min/1.73 m2 during therapy.15,16 Empagliflozin has a pregnancy risk category C. Drug transference during lactation is unknown; therefore, empagliflozin during breast-feeding is not recommended. It is also available in a fixed-dose combination with metformin.
Empagliflozin has been studied alone and in combination therapy with other oral antidiabetic drugs as well as insulin therapy. Metformin, pioglitazone, sitagliptin, and linagliptin have been studied in combination with empagliflozin with sustained glycemic improvement without significantly increased risk of hypoglycemia.17-21 Empagliflozin/linagliptin combination was recently approved after phase 3 trials demonstrated 62% of patients achieved an A1c value < 7% on the 25/5-mg dose at 24 weeks.21 Empagliflozin, coadministered with multiple daily injections of insulin (MDI), has been shown to safely improve glycemic control and reduce total daily insulin requirements without an increased risk of hypoglycemia.22 Currently, it is not approved for use in patients with T1DM, but phase 3 trials are ongoing.
Pharmacokinetics of empagliflozin among healthy volunteers paralleled those of people with T2DM with rapid absorption. The plasma glucose lowering effect of empagliflozin was evident after the first dose and became more pronounced with treatment duration. The AUC and Cmax were dose-proportional over a range of empagliflozin doses in a single rising dose study, with maximum UGE of 90.8 g in healthy volunteers reached at the 400-mg dose.23 The Tmax was 1.5 to 2.1 hours after dosing, comparable to dapagliflozin.24 Steady state with once-daily dosing is reached by day 5 with t1/2 range of 10 to 19 hours.24-27 Plasma levels of empagliflozin declined in a biphasic pattern, with a rapid distribution phase and a slower elimination phase. Total urine volume did not differ significantly in the empagliflozin group compared with placebo.
Healthy subjects treated with placebo or empagliflozin had comparable plasma glucose concentrations. Patients with T2DM demonstrated a decrease in mean daily glucose of -37.0 mg/dL for the 10-mg dose compared with -13.5 mg/dL for placebo. Doses up to 10 mg were found to inhibit renal tubular reabsorption up to 40%, and higher doses inhibit up to 60% of filtered glucose.22-26 Empagliflozin has been shown to have similar efficacy independent of food.27 The pharmacodynamic response declines with increasing renal impairment in SGLT2 inhibitors. Empagliflozin has been associated with a decline from UGE of 97.6 g in normal renal function to 18.2 g in severe renal impairment.
Phase 1 studies of healthy male volunteers initially elucidated empagliflozin’s therapeutic potential of dosedependent increases in UGE and associated reduction in A1c with daily administration, respectively.24,28 Single rising dose studies further defined the linear pharmacokinetics and excellent tolerability of empagliflozin.23 Heise and colleagues demonstrated in 2 separate studies with multiple oral doses (2.5 mg, 10 mg, 25 mg, and 100 mg) of empagliflozin in people with T2DM similar pharmacokinetics and efficacy in UGE, tolerability, and reduction in plasma glucose.24
Ferrannini and colleagues, in a 12-week phase 2 study, demonstrated a statistically significant reduction in A1c of 0.5% and 0.6% with 10-mg and 25-mg doses, respectively, as well as a universal body weight decline by 2 kg.29
Clinical Efficacy Trials
A phase 3 trial of 1,549 randomized people with T2DM (aged > 18 years with baseline A1c of 7%-10%), comparing empagliflozin and glimepiride as an add-on to metformin, demonstrated noninferiority at 52 weeks and statistically significant superiority for the empagliflozin group at 104 weeks.30 The adjusted mean difference in change from baseline in A1c with empagliflozin vs glimepiride at week 104 was -0.11% (95% confidence interval, -0.19 to -0.02; P = .0153 for superiority). Despite 39% of both groups achieving a A1c < 7% at week 52, the empagliflozin group had significantly less hypoglycemia (2% vs 24%). In the body composition substudy, empagliflozin demonstrated a significant reduction of 3 kg compared with an increase of slightly over 1 kg in the glimepiride group at week 104. The majority of the empagliflozin-associated weight loss was found to be a reduction in fat mass.
An international phase 2B randomized, controlled, open-label extension study of 659 people with T2DM (aged > 18 and < 79 years with a body mass index (BMI) < 40 kg/m2 and baseline A1c of 7%-10%) described the long-term safety and efficacy of empagliflozin monotherapy in combination with metformin as compared with sitagliptin monotherapy in combination with metformin. At week 90, the changes from baseline A1c were -0.34%/ -0.47% in the empagliflozin 10 mg/25 mg monotherapy group, -0.34%/0.63% in the empagliflozin 10 mg/25 mg/ metformin combination, -0.56% with metformin monotherapy, and -0.40% with sitagliptin/metformin combination.31 These data provided evidence that empagliflozin had sustained weight loss effects (-2.2 kg to -4.0 kg).
Empagliflozin monotherapy was studied with sitagliptin as an active comparator in a phase 3 trial of 899 people with T2DM (aged > 18 years with baseline A1c of 7%-10%), with the primary endpoint of change in baseline in A1c at week 24. Both the 10-mg and 25-mg dose sof empagliflozin were associated with greater reductions in A1c from baseline (-0.66%, -0.77%) at week 24 than that of sitagliptin (-1.04%). Additionally, both doses of empagliflozin were associated with greater reductions in bodyweight (-2.26 kg and -2.48 kg) as compared with sitagliptin 100 mg (+0.18 kg).20
Empagliflozin’s efficacy and safety in combination with MDI insulin as well as add-on to basal insulin was tested in 2 separate studies.23,32 People with T2DM (mean age 58.8 years, A1c 8.2%) on basal insulin were randomized to empagliflozin (10 mg or 25 mg) or placebo for 78 weeks with a constant basal insulin dose for the first 18 weeks and titration allowed thereafter. Empagliflozin was found to significantly reduce A1c at both 18 and 78 weeks (-0.48% and -0.64% for 10-mg/25-mg doses, respectively, vs -0.02% for placebo) and insulin dose at week 78 vs placebo (-8.8 IU on empagliflozin 10 mg and -11.2 IU on empagliflozin 25 mg).32 Similar rates of hypoglycemia were reported (36.1% of patients on empagliflozin and 35.3% on placebo)
Furthermore, empagliflozin was studied in obese people with uncontrolled, insulin-dependent T2DM (A1c 8.3%, BMI 34.8 kg/m2) on MDI insulin (average 92 IU/d) over a 52-week study.22 Patients were randomized to once-daily empagliflozin 10 mg, empagliflozin 25 mg, or placebo. The study demonstrated improved glycemic control (A1c reduction of -1.27% and -1.18% on empagliflozin 10 mg and 25 mg doses, respectively, compared with -0.81% on placebo) with lower insulin doses (-9 to -11 IU/d) and weight loss (-1.95 kg and -2.04 kg on empagliflozin 10-mg and 25-mg doses, respectively, compared with 0.44 kg on placebo.) There was no increased risk of hypoglycemia noted.
Adverse Effects and Precautions
Empagliflozin is generally well tolerated with low occurrence of AEs. Adverse effects reported in the pooled empagliflozin phase 3 studies were mild to moderate. Serious AEs reported were higher in the placebo group as compared with those of the empagliflozin subjects but did not result in study discontinuation.33
Drug-drug studies of empagliflozin co-administered with other commonly prescribed medication in T2DM showed very limited, if any, interaction. Empagliflozin had no effect on the pharmacokinetics of warfarin or on its anticoagulant activity; therefore, these 2 drugs were deemed safe to be co-administered. No AEs were reported when combining empagliflozin with the loop diuretic hydrochlorothiazide.33 Due to the mode of action of SGLT2 inhibitors, osmotic diuresis may result in a modest reduction in BP.34
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA.35 The FDA Adverse Event Reporting System identified 20 cases of DKA in patients treated with SGLT2 inhibitors from March 2013 to June 6, 2014. Diabetic ketoacidosis is typically accompanied by levels of ketone bodies > 3,000 μmol/L and develops almost exclusively in states of absolute insulin deficiency. The highest level of ketone bodies observed in patients receiving 25 mg of empagliflozin was 1,449 μmol/L compared with a mean of 1,300 μmol/L in a nondiabetic overnight fast.36 Therefore, it is unlikely that the modest empagliflozin-induced ketosis would increase the risk of developing DKA in the absence of absolute insulin deficiency or extreme ketogenic diet.37 Physiologic explanation at the present time is not clear.
Clinical Application
The SGLT2 inhibitors are the latest of 14 classes of drugs approved to treat T2DM. This class offers many beneficial characteristics besides blood sugar lowering. The drugs lower systolic BP, induce weight loss through diuresis of glucose (calories), and carry low risks for hypoglycemia. The American Association of Clinical Endocrinologists recently published their 2015 diabetes management algorithm.38 In this algorithm, they recognized metformin as first-line therapy along with diet and exercise intensification for the treatment of T2DM.
The glucagon-like peptide-1 (GLP-1) analogs and SGLT2 inhibitors are recognized as plausible second-line drugs. They go together well with metformin, providing powerful additional A1c-lowering effects while inducing weight loss without hypoglycemia. Because all GLP-1 analogs are currently available only in injectable forms, the SGLT2 inhibitors offer the additional advantage of being available in pill forms.
Conclusions
All 3 of the current SGLT2 inhibitors are effective tools for treating T2DM. The 3 drugs share many similar traits, and efficacy is generally similar. Empagliflozin has the highest SGLT2 selectivity, > 2,500-fold selectivity for SGLT2 over SGLT1. However, canagliflozin has mild SGLT1 activity, which may offer additional benefits with regards to attenuating PPGE excursions by delaying intestinal glucose absorption (Table).
The SGLT2 inhibitor class had been shown to be effective when used as monotherapy as well as in combination with other oral antidiabetic medications. All 3 SGLT2 inhibitors have also been shown to be effective in combination with insulin and have similar efficacy in these clinical settings (eTable).
All 3 SGLT2 inhibitors are generally well tolerated. There is less hypoglycemia compared with sulfonylureas. However, when the SGLT2 drugs are combined with drugs that can cause hypoglycemia, such as sulfonylureas and insulin, patients must be monitored for hypoglycemia, and titrating down the sulfonylurea or insulin may be necessary. Genital mycotic infections and urinary tract infections in men and woman are common AEs with SGLT2 drugs. Patients must be advised of these possible AEs, and treatment should be prompt if these AEs occur. Because of the mild osmotic diuresis, patients should be reminded to keep well hydrated. SGLT2s have a very mild effect on increasing low-density lipoprotein cholesterol (LDL-C), so care should be taken to ensure that patients’ LDL-C stays at goal.
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA. Although these reports are rare, clinicians should be vigilant. The FDA has suggested that patients should pay close attention for any signs of DKA and seek medical attention immediately if they experience difficulty breathing, nausea, vomiting, abdominal pain, confusion, and unusual fatigue or sleepiness.35
There are subtle differences in the eGFR thresholds for the use of the 3 drugs. It should be kept in mind that with all 3 drugs, the efficacy decreases as the eGFR decreases. It is recommended not to initiate canagliflozin if the patient’s eGFR is < 45 mL/min/1.73 m2. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. Empagliflozin should not be initiated in patients with an eGFR < 45 mL/min/1.73 m2.
The SGLT2 inhibitors are useful tools to lower blood glucose levels in people with T2DM. They can be used as monotherapy or in combination. They also cause weight reduction. Thus, their unique mechanism of action is complementary to the other oral antidiabetic medications and insulin, so a wide variety of patients can benefit from this class.
Author disclosures
Dr. Nguyen is affiliated with the Astra Zeneca Speakers Bureau and Janssen Pharmaceutical Speakers Bureau. Dr. Plodkowski is a Janssen Pharmaceutical consultant. The remaining authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
2. Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36(8):2154-2161.
3. Sha S, Polidori D, Farrell, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab. 2015;17(2):188-197.
4. Yamout H, Perkovic V, Davies M, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol. 2014;40(1):64-74.
5. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38(3):355-364.
6. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582-2592.
7. Neal B, Perkovic V, de Zeeuw D, et al; CANVAS Trial Collaborative Group. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38(3):403-411.
8. INVOKANA [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2013.
9. FARXIGA [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015.
10. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34(9):2015-2022.
11. Rosenstock J, Hansen L, Zee P, et al. Dual Add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376-383.
12. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S; Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16(2):124-136.
13. Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(7):613-621.
14. Macha S, Rose P, Mattheus M, et al. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16(2):118-123.
15. Barnett A, Mithal A, Manassie J, et al; EMPA-REG RENAL trial investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, doubleblind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369-384.
16. Macha S, Mattheus M, Halabi A, Pinnetti S, Woerle HJ, Broedl UC. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes Metab. 2014;16(3):215-222.
17. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG MET Trial Investigators.
Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650-1699.
18. Ridderstråle M, Svaerd R, Zeller C, Kim G, Woerle HJ, Broedi UC; EMPA-REG H2H-SU trial investigators. Rational, design and baseline characteristics of a 4-year (208-week) phase III trial of empagliflozin, an SGLT2 inhibitor, versus glimepiride as add-on to metformin in patients with type 2 diabetes mellitus with insufficient glycemic control. Cardiovasc Diabetol. 2013;12:129.
19. Kovacs CS, Seshiah V, Swallow R, et al; EMPA-REG PIO trial investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16(2):147-158.
20. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
21. Friedrich C, Metzmann K, Rose P, Mattheus M, Pinnetti S, Woerle HJ. A randomized, open-label, crossover study to evaluate the pharmacokinetics of empagliflozin and linagliptin after coadministration in healthy male volunteers. Clin Ther. 2013;35(1):A33-A42.
22. Rosenstock J, Jelaska A, Frappin G, et al; EMPA-REG MDI Trial Investigators. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(3):1815-1823.
23. Seman L, Macha S, Nehmiz G, et al. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Drug Dev. 2013;2(2):152-161.
24. Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. 2013;4(2):331-345.
25. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12(2):90-100.
26. Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53(3):213-225.
27. Macha S, Jungnik A, Hohl K, Hobson D, Salsali A, Woerle HJ. Effect of food on the pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, and assessment of dose proportionality in healthy volunteers. Int J Clinical Pharmacol Therapy. 2013;51(11):873-879.
28. Sarashina A, Koiwai K, Seman LJ, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab Pharmacokinet. 2013;28(3):213-219.
29. Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(8):721-728.
30. Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC; EMPAREG H2H-SU trial investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691-700.
31. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36(12):4015-4021.
32. Rosenstock J, Jelaska A, Kim G, et al. Empagliflozin as add-on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin-treated (T2DM) [Abstract 1102-P]. Diabetes. 2013;62(suppl 1):A285.
33. JARDIANCE [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals,
Inc.; 2015.
34. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11):3396-3404.
35. FDA Drug Safety Communication: FDA Warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Updated May 19, 2015. Accessed September 23, 2015.
36. Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11.
37. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients
with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
38. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21(4):438-447.
1. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
2. Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36(8):2154-2161.
3. Sha S, Polidori D, Farrell, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab. 2015;17(2):188-197.
4. Yamout H, Perkovic V, Davies M, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol. 2014;40(1):64-74.
5. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38(3):355-364.
6. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582-2592.
7. Neal B, Perkovic V, de Zeeuw D, et al; CANVAS Trial Collaborative Group. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38(3):403-411.
8. INVOKANA [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2013.
9. FARXIGA [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015.
10. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34(9):2015-2022.
11. Rosenstock J, Hansen L, Zee P, et al. Dual Add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376-383.
12. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S; Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16(2):124-136.
13. Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(7):613-621.
14. Macha S, Rose P, Mattheus M, et al. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16(2):118-123.
15. Barnett A, Mithal A, Manassie J, et al; EMPA-REG RENAL trial investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, doubleblind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369-384.
16. Macha S, Mattheus M, Halabi A, Pinnetti S, Woerle HJ, Broedl UC. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes Metab. 2014;16(3):215-222.
17. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG MET Trial Investigators.
Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650-1699.
18. Ridderstråle M, Svaerd R, Zeller C, Kim G, Woerle HJ, Broedi UC; EMPA-REG H2H-SU trial investigators. Rational, design and baseline characteristics of a 4-year (208-week) phase III trial of empagliflozin, an SGLT2 inhibitor, versus glimepiride as add-on to metformin in patients with type 2 diabetes mellitus with insufficient glycemic control. Cardiovasc Diabetol. 2013;12:129.
19. Kovacs CS, Seshiah V, Swallow R, et al; EMPA-REG PIO trial investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16(2):147-158.
20. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
21. Friedrich C, Metzmann K, Rose P, Mattheus M, Pinnetti S, Woerle HJ. A randomized, open-label, crossover study to evaluate the pharmacokinetics of empagliflozin and linagliptin after coadministration in healthy male volunteers. Clin Ther. 2013;35(1):A33-A42.
22. Rosenstock J, Jelaska A, Frappin G, et al; EMPA-REG MDI Trial Investigators. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(3):1815-1823.
23. Seman L, Macha S, Nehmiz G, et al. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Drug Dev. 2013;2(2):152-161.
24. Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. 2013;4(2):331-345.
25. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12(2):90-100.
26. Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53(3):213-225.
27. Macha S, Jungnik A, Hohl K, Hobson D, Salsali A, Woerle HJ. Effect of food on the pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, and assessment of dose proportionality in healthy volunteers. Int J Clinical Pharmacol Therapy. 2013;51(11):873-879.
28. Sarashina A, Koiwai K, Seman LJ, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab Pharmacokinet. 2013;28(3):213-219.
29. Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(8):721-728.
30. Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC; EMPAREG H2H-SU trial investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691-700.
31. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36(12):4015-4021.
32. Rosenstock J, Jelaska A, Kim G, et al. Empagliflozin as add-on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin-treated (T2DM) [Abstract 1102-P]. Diabetes. 2013;62(suppl 1):A285.
33. JARDIANCE [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals,
Inc.; 2015.
34. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11):3396-3404.
35. FDA Drug Safety Communication: FDA Warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Updated May 19, 2015. Accessed September 23, 2015.
36. Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11.
37. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients
with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
38. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21(4):438-447.
Diabetic Macular Edema: Is Your Patient Going Blind?
Diabetes mellitus (DM) affects about 347 million people worldwide, making it the new global epidemic.1 In the U.S alone, the number of adults with DM has more than tripled over the past 30 years. Now, almost 10% of the U.S. population has the disease and is at risk for serious systemic complications, including blindness.2
Diabetes is the leading cause of new cases of legal blindness in adults aged 18 to 74 years in the U.S. Diabetic retinopathy, seen as vascular changes in the retina related to DM, is found in almost half of all patients with DM.3 As the number of people with DM is expected to increase, so is the number of people affected with and blinded by diabetic retinopathy. Providers in both primary care and subspecialty settings have a critical role to play in the management and prevention of blindness in diabetic patients.
According to the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), diabetic retinopathy will affect 99% of patients with type 1 DM (T1DM) and 60% of patients with type 2 DM (T2DM) after 20 years of having diabetes.4 Diabetic retinopathy is a result of the microvascular damage that occurs from diabetes. The most common findings seen on a dilated fundus exam in nonproliferative diabetic retinopathy are microaneurysms, intraretinal hemorrhages, hard exudates, and cotton wool spots (Figure 1). Cotton wool spots represent focal areas of retinal ischemia. Nonproliferative disease progresses to proliferative retinopathy when neovascularization develops.
One of the major causes of vision loss in the setting of DM is diabetic macular edema (DME). Between 4% and 7% of people with DM currently have DME.5,6 Diabetic macular edema is a result of the break down of the bloodretinal barrier, which is an extension of the blood-brain barrier. Hyperglycemia causes a disruption of the cellular tight junctions, pericyte loss, and thickening of the basement membrane. These changes cause weakness in the walls of the retinal blood vessels, allowing microaneurysms to form. Hyperglycemia also causes upregulation of the production of inflammatory markers such as vascular endothelial growth factor (VEGF), protein kinase C, prostaglandins, and cytokines, which increase retinal vascular permeability (Figure 2).7
Risk Factors for DME
Several studies have found the prevalence of DME to be higher in black (10.4%-15.6%) and Hispanic (18%) patients vs non-Hispanic white patients (6.3%-8.4%); Asian patients have the lowest prevalence of DME (5%).5,6,8,9 The modifiable risk factors for the development of DME include hyperglycemia, duration of disease, hypertension, and dyslipidemia. Patients who have had DM for a longer period of time (> 10 years) and those with a higher hemoglobin A1c (A1c ) are more likely to have DME.4,6 The WESDR showed that for each percentage point increase in baseline A1c, there was a 28% increase in the incidence of visual impairment at the 25-year follow-up.10 An A1c < 7% is recommended in patients, though some may benefit from an A1c < 6.5%.11
Epidemiologic studies have also found hypertension and dyslipidemia to be associated with an increased risk of DME.12-15 Both diseases lead to an increased vascular permeability, compounding the microvascular damage already present from DM. In hypertension, DME and retinal hemorrhages are thought to be influenced by the increased perfusion pressure in retinal vessels.16 For every 10 mm Hg increase in blood pressure over 160 mm Hg, the risk of DME increases by 25%.17 Dyslipidemia contributes to DME by damaging endothelial cells and causing increased vascular permeability through cytokine and VEGF upregulation.18
Other risk factors, such as nephropathy, anemia, sleep apnea, and thiazolidinedione (glitazone) use, may also affect the development of DME. Patients with microalbuminuria have a lower serum protein concentration and thus, a reduced plasma colloidal osmotic pressure. This decreased osmotic pressure allows fluid to exit the retinal blood vessels and causes DME.7 Serum osmolarity may also play a role in DME. Some patients were noted to have decreased DME after receiving hemodialysis.19 Retinal vascular permeability can be increased by ischemia caused by hypoxia from anemia or sleep apnea.7 Glitazones have been associated with an increased risk of developing DME, although the cause is unclear.7
Examination
The American Academy of Ophthalmology recommends annual diabetic retinopathy screening for all patients with DM. Screening exams should start at the time of diagnosis for patients with T2DM and at 5 years after diagnosis for patients with T1DM. Currently, patients without a history of diabetic retinopathy can be screened via an ophthalmologic exam or review of color fundus photographs, which can be taken by trained personnel in the primary care or subspecialty settings.
Unfortunately, only 60% of patients with DM are screened annually. It is important to emphasize to patients the importance of a screening eye exam. Many patients do not understand that diabetic retinopathy may be present even if they are not experiencing any changes in vision. The patient should be referred to an ophthalmologist immediately if he or she reports blurry vision, wavy lines, or dark spots in the vision, especially if those symptoms are acute. The goal of a screening program is early detection: to identify those patients who are at risk for vision loss from DM and to provide close follow-up and timely treatment. Any patient with a history of diabetic retinopathy should be followed at the interval recommended by the eye care provider.11
Patient history is an important part of the screening exam, including symptoms, duration of DM, A1c, medications, medical history (hypertension, nephropathy, dyslipidemia, obesity, pregnancy), and ocular history. If there is evidence of diabetic retinopathy or DME, recommendations for better systemic control of DM or its comorbidities can be made based on the patient history. During the screening exam, the patient’s visual acuity and intraocular pressure are measured. A basic examination of the anterior segment looking for neovascularization of the iris is also completed. Iris neovascularization is a sign of proliferative diabetic retinopathy that would indicate laser treatment, also known as panretinal photocoagulation, or intravitreal injection.
The patient’s pupils are dilated, which enables the eye care provider to examine the retina. Patients often dislike this portion of the examination, because the dilation drop causes their vision to be blurry for 4 to 6 hours. However, dilation ensures that the provider has a view of the entire retina and can detect early stages of diabetic retinopathy.
If the screening is taking place via color fundus photographs, a nonmydriatic fundus camera, which does not require dilation, can be used. The purpose of the screening examination is to assess changes that can lead to vision loss. Important features that must be detected if present are macular edema, extensive microvascular changes, vitreous hemorrhage, and neovascularization of the optic nerve, retina, or iris.11 It is important to remember that the diabetic screening examination does not take the place of a complete ophthalmologic examination for other ocular disease, such as glaucoma. The patient may need to schedule additional appointments with an eye care provider if other eye problems exist.
Imaging
Clinically significant macular edema (CSME) was first defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) as macular edema that involves the center of vision, called the fovea, and can be visualized with clinical examination of the retina. The criteria for CSME are used to identify edema that is mostly likely to cause vision loss and to guide laser treatment.20 Today, adjunctive testing, such as optical coherence tomography (OCT) and fluorescein angiography (FA), aid in the earlier detection and diagnosis of DME. These imaging techniques are capable of detecting small amounts of macular edema that are vision-threatening but are not visible on exam. Center-involving DME is edema that involves the fovea; noncenter-involving DME is edema that does not involve the fovea but is found within the macula. Color and redfree fundus photographs, OCT, and FA are also used to guide treatment in patients with DME.
Color fundus photographs are helpful as a screening tool that identifies those patients who need to be seen by an eye care provider for a dilated fundus exam. Photographs are also helpful in documenting the changes in DME after treatment. Optical coherence tomography allows for high-resolution imaging of the retinal layers and objective measurement of the amount and location of DME. This information can be used to detect any change in DME between visits and evaluate the response to treatment. Further treatment decisions are frequently made based in part on the findings seen on OCT (Figure 2).
Fluorescein angiography is an imaging test that uses an IV dye called fluorescein to detect areas of retinal nonperfusion, edema, and neovascularization. Fluorescein is injected into a peripheral vein (frequently in the antecubital area or in the hand). Photographs are then taken
using special filters that allow only the wavelength corresponding to the fluorescein dye to be visible. The fluorescein can be seen filling the retinal arteries within 20 seconds of the peripheral injection. Photographs are usually taken intermittently for 15 minutes. As time passes, the dye will leak out of any blood vessels that have increased vascular permeability and highlight any microaneurysms, because the dye pools in the outpouching of the blood vessel. Leakage of the dye out of the vessels can be seen as an increase in fluorescence, or whitening, outside of the blood vessels in the photograph. This leakage leads to the accumulation of fluid in the retina, causing DME. Neovascularization is also very permeable, and areas of neovascularization, an indicator of proliferative diabetic retinopathy, are apparent on FA as spots of intense hyperfluorescence (Figure 3).
Treatment
In 1985, results from ETDRS revolutionized the treatment of DME. The study showed that by applying laser burns to leaking microaneurysms or in a grid pattern over an area of diffuse edema, severe vision loss could be reduced by 50%. In the past few years, the role of laser treatment has shifted so that it is now indicated for the treatment of noncenter-involving DME. The impetus for this change was the development of anti-VEGF therapy, which is now the first-line therapy for centerinvolving DME.
As aforementioned, VEGF causes increased vascular permeability and breakdown of the blood-retinal barrier. Patients with DME have been shown to have increased levels of VEGF in the vitreous when compared with nondiabetic controls.21 There are now 3 anti-VEGF agents that are commonly used in clinical practice for the treatment of DME: ranibizumab, aflibercept, and bevacizumab. Ranibizumab is an antibody fragment targeted against VEGF that is FDA approved for use in patients with DME. The Diabetic Retinopathy Clinical Research Network Protocol I showed that treatment with ranibizumab, paired with deferred laser treatment, results in greater visual improvement than does prompt laser treatment alone.22 Treatment with aflibercept is a recombinant fusion protein of VEGF receptors. It was shown to be superior in terms of visual improvement when compared with laser treatment.23 Bevacizumab is a full-length antibody that is more affordable than other anti-VEGF medications and is often used off label for the treatment of DME. All of the anti-VEGF therapies are intravitreal injections. After topical anesthesia, the medication is injected through the sclera into the vitreous cavity in the outpatient clinic setting.
A significant disadvantage of the anti-VEGF therapies is that many patients need monthly injections, especially in the first year of treatment, necessitating many office visits, which can decrease adherence. In some patients, the edema may not respond to anti-VEGF therapy. In these cases, steroid therapy may be helpful to suppress the inflammatory pathways that are independent of VEGF. Intravitreal triamcinolone in combination with laser treatment has been shown to be as effective as ranibizumab in a small group of patients.24 An intravitreal dexamethasone implant, which has a treatment effect lasting for 3 months, was also shown to improve visual acuity over sham treatment in patients with DME.25 Most recently, an intravitreal fluocinolone implant that lasts 3 years was approved by the FDA for treatment of DME.26 A significant benefit of the steroid implants is the long duration of treatment effect compared with that of the anti-VEGF injections. However, steroid therapy is associated with the development of cataracts and glaucoma, the rates of which are increased when treatment is prolonged. Because of these adverse effects, steroids are currently used as second-line or third-line treatment in DME. Retinal surgery may be indicated if there is vitreomacular traction that is exacerbating the DME. A vitrectomy is performed to remove the vitreous and relieve any adhesion to the surface of the retina.
Conclusion
Despite the new ophthalmic treatment modalities, it is important to remember that DME is a chronic condition that will require long-term follow-up. Many patients will not experience complete resolution of DME with a single therapy alone. Control of systemic risk factors, including blood sugar with a goal of A1c < 7%, blood pressure, and cholesterol, remains the key to a successful treatment program. Primary care physicians, endocrinologists, diabetologists, optometrists, comprehensive ophthalmologists, retina specialists, and patients must work together to create an individualized treatment regimen that will optimize the patient’s vision by preventing blindness and improving his/her quality of life for years to come.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Danaei G, Finucane MM, Lu Y, et al; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31-40.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimate of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
3. National Institutes of Health. Diabetic retinopathy: causes and risk factors. NIH Senior Health Website. http://nihseniorhealth.gov/diabeticretinopathy/causesandriskfactors/01.html. Updated February 2015. Accessed September 3, 2015.
4. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102(1):7-16.
5. Yau JW, Rogers SL, Kawasaki R, et al; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564.
6. Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334-1340.
7. Diep TM, Tsui I. Risk factors associated with diabetic macular edema. Diabetes Res Clin Pract. 2013;100(3):298-305.
8. Varma R, Choudhury F, Klein R, Chung J, Torres M, Azen SP; Los Angeles Latino Eye Study Group. Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5):752-761.e1-e3.
9. Emanuele N, Moritz T, Klein R, et al; Veterans Affairs Diabetes Trial Study Group. Ethnicity, race, and clinically significant macular edema in the Veterans Affairs Diabetes Trial (VADT). Diabetes Res Clin Pract. 2009;86(2):104-110.
10. Klein R, Lee KE, Gangnon RE, Klein BE. The 25-year incidence of visual impairment in type 1 diabetes mellitus: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2010;117(1):63-70.
11. American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern: Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2014.
12. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984;91(12):1464-1474.
13. Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116(3):497-503.
14. Chew EY. Diabetic retinopathy and lipid abnormalities. Curr Opin Ophthalmol. 1997;8(3):59-62.
15. Klein R, Sharrett AR, Klein BE, et al; ARIC Group. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology. 2002;109(7):1225-1234.
16. Haefliger IO, Meyer P, Flammer J, Lüscher TF. The vascular endothelium as a regulator of the ocular circulation: a new concept in ophthalmology? Surv Ophthalmol. 1994;39(2):123-132.
17. Lopes de Faria JM, Jalkh AE, Trempe CL, McMeel JW. Diabetic macular edema: risk factors and concomitants. Acta Ophthalmol Scand. 1999;77(2):170-175.
18. Langeler EG, Snelting-Havinga I, van Hinsbergh VW. Passage of low density lipoproteins through monolayers of human arterial endothelial cells. Effects of vasoactive substances in an in vitro model. Arteriosclerosis. 1989;9(4):550-559.
19. Theodossiadis PG, Theodoropoulou S, Neamonitou G, et al. Hemodialysisinduced alterations in macular thickness measured by optical coherence tomography in diabetic patients with end-stage renal disease. Ophthalmologica. 2012;227(2):90-94.
20. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796-1806.
21. Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116(1):73-79.
22. Diabetic Retinopathy Clinical Research Network; Elman MJ, Qin H, Aiello LP, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119(11):2312-2318.
23. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247-2254.
24. Diabetic Retinopathy Clinical Research Network; Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.
25. Boyer DS, Yoon YH, Belfort R Jr, et al; Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904-1914.
26. Campochiaro PA, Brown DM, Pearson A, et al; FAME Study Group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626-635.e2.
Diabetes mellitus (DM) affects about 347 million people worldwide, making it the new global epidemic.1 In the U.S alone, the number of adults with DM has more than tripled over the past 30 years. Now, almost 10% of the U.S. population has the disease and is at risk for serious systemic complications, including blindness.2
Diabetes is the leading cause of new cases of legal blindness in adults aged 18 to 74 years in the U.S. Diabetic retinopathy, seen as vascular changes in the retina related to DM, is found in almost half of all patients with DM.3 As the number of people with DM is expected to increase, so is the number of people affected with and blinded by diabetic retinopathy. Providers in both primary care and subspecialty settings have a critical role to play in the management and prevention of blindness in diabetic patients.
According to the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), diabetic retinopathy will affect 99% of patients with type 1 DM (T1DM) and 60% of patients with type 2 DM (T2DM) after 20 years of having diabetes.4 Diabetic retinopathy is a result of the microvascular damage that occurs from diabetes. The most common findings seen on a dilated fundus exam in nonproliferative diabetic retinopathy are microaneurysms, intraretinal hemorrhages, hard exudates, and cotton wool spots (Figure 1). Cotton wool spots represent focal areas of retinal ischemia. Nonproliferative disease progresses to proliferative retinopathy when neovascularization develops.
One of the major causes of vision loss in the setting of DM is diabetic macular edema (DME). Between 4% and 7% of people with DM currently have DME.5,6 Diabetic macular edema is a result of the break down of the bloodretinal barrier, which is an extension of the blood-brain barrier. Hyperglycemia causes a disruption of the cellular tight junctions, pericyte loss, and thickening of the basement membrane. These changes cause weakness in the walls of the retinal blood vessels, allowing microaneurysms to form. Hyperglycemia also causes upregulation of the production of inflammatory markers such as vascular endothelial growth factor (VEGF), protein kinase C, prostaglandins, and cytokines, which increase retinal vascular permeability (Figure 2).7
Risk Factors for DME
Several studies have found the prevalence of DME to be higher in black (10.4%-15.6%) and Hispanic (18%) patients vs non-Hispanic white patients (6.3%-8.4%); Asian patients have the lowest prevalence of DME (5%).5,6,8,9 The modifiable risk factors for the development of DME include hyperglycemia, duration of disease, hypertension, and dyslipidemia. Patients who have had DM for a longer period of time (> 10 years) and those with a higher hemoglobin A1c (A1c ) are more likely to have DME.4,6 The WESDR showed that for each percentage point increase in baseline A1c, there was a 28% increase in the incidence of visual impairment at the 25-year follow-up.10 An A1c < 7% is recommended in patients, though some may benefit from an A1c < 6.5%.11
Epidemiologic studies have also found hypertension and dyslipidemia to be associated with an increased risk of DME.12-15 Both diseases lead to an increased vascular permeability, compounding the microvascular damage already present from DM. In hypertension, DME and retinal hemorrhages are thought to be influenced by the increased perfusion pressure in retinal vessels.16 For every 10 mm Hg increase in blood pressure over 160 mm Hg, the risk of DME increases by 25%.17 Dyslipidemia contributes to DME by damaging endothelial cells and causing increased vascular permeability through cytokine and VEGF upregulation.18
Other risk factors, such as nephropathy, anemia, sleep apnea, and thiazolidinedione (glitazone) use, may also affect the development of DME. Patients with microalbuminuria have a lower serum protein concentration and thus, a reduced plasma colloidal osmotic pressure. This decreased osmotic pressure allows fluid to exit the retinal blood vessels and causes DME.7 Serum osmolarity may also play a role in DME. Some patients were noted to have decreased DME after receiving hemodialysis.19 Retinal vascular permeability can be increased by ischemia caused by hypoxia from anemia or sleep apnea.7 Glitazones have been associated with an increased risk of developing DME, although the cause is unclear.7
Examination
The American Academy of Ophthalmology recommends annual diabetic retinopathy screening for all patients with DM. Screening exams should start at the time of diagnosis for patients with T2DM and at 5 years after diagnosis for patients with T1DM. Currently, patients without a history of diabetic retinopathy can be screened via an ophthalmologic exam or review of color fundus photographs, which can be taken by trained personnel in the primary care or subspecialty settings.
Unfortunately, only 60% of patients with DM are screened annually. It is important to emphasize to patients the importance of a screening eye exam. Many patients do not understand that diabetic retinopathy may be present even if they are not experiencing any changes in vision. The patient should be referred to an ophthalmologist immediately if he or she reports blurry vision, wavy lines, or dark spots in the vision, especially if those symptoms are acute. The goal of a screening program is early detection: to identify those patients who are at risk for vision loss from DM and to provide close follow-up and timely treatment. Any patient with a history of diabetic retinopathy should be followed at the interval recommended by the eye care provider.11
Patient history is an important part of the screening exam, including symptoms, duration of DM, A1c, medications, medical history (hypertension, nephropathy, dyslipidemia, obesity, pregnancy), and ocular history. If there is evidence of diabetic retinopathy or DME, recommendations for better systemic control of DM or its comorbidities can be made based on the patient history. During the screening exam, the patient’s visual acuity and intraocular pressure are measured. A basic examination of the anterior segment looking for neovascularization of the iris is also completed. Iris neovascularization is a sign of proliferative diabetic retinopathy that would indicate laser treatment, also known as panretinal photocoagulation, or intravitreal injection.
The patient’s pupils are dilated, which enables the eye care provider to examine the retina. Patients often dislike this portion of the examination, because the dilation drop causes their vision to be blurry for 4 to 6 hours. However, dilation ensures that the provider has a view of the entire retina and can detect early stages of diabetic retinopathy.
If the screening is taking place via color fundus photographs, a nonmydriatic fundus camera, which does not require dilation, can be used. The purpose of the screening examination is to assess changes that can lead to vision loss. Important features that must be detected if present are macular edema, extensive microvascular changes, vitreous hemorrhage, and neovascularization of the optic nerve, retina, or iris.11 It is important to remember that the diabetic screening examination does not take the place of a complete ophthalmologic examination for other ocular disease, such as glaucoma. The patient may need to schedule additional appointments with an eye care provider if other eye problems exist.
Imaging
Clinically significant macular edema (CSME) was first defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) as macular edema that involves the center of vision, called the fovea, and can be visualized with clinical examination of the retina. The criteria for CSME are used to identify edema that is mostly likely to cause vision loss and to guide laser treatment.20 Today, adjunctive testing, such as optical coherence tomography (OCT) and fluorescein angiography (FA), aid in the earlier detection and diagnosis of DME. These imaging techniques are capable of detecting small amounts of macular edema that are vision-threatening but are not visible on exam. Center-involving DME is edema that involves the fovea; noncenter-involving DME is edema that does not involve the fovea but is found within the macula. Color and redfree fundus photographs, OCT, and FA are also used to guide treatment in patients with DME.
Color fundus photographs are helpful as a screening tool that identifies those patients who need to be seen by an eye care provider for a dilated fundus exam. Photographs are also helpful in documenting the changes in DME after treatment. Optical coherence tomography allows for high-resolution imaging of the retinal layers and objective measurement of the amount and location of DME. This information can be used to detect any change in DME between visits and evaluate the response to treatment. Further treatment decisions are frequently made based in part on the findings seen on OCT (Figure 2).
Fluorescein angiography is an imaging test that uses an IV dye called fluorescein to detect areas of retinal nonperfusion, edema, and neovascularization. Fluorescein is injected into a peripheral vein (frequently in the antecubital area or in the hand). Photographs are then taken
using special filters that allow only the wavelength corresponding to the fluorescein dye to be visible. The fluorescein can be seen filling the retinal arteries within 20 seconds of the peripheral injection. Photographs are usually taken intermittently for 15 minutes. As time passes, the dye will leak out of any blood vessels that have increased vascular permeability and highlight any microaneurysms, because the dye pools in the outpouching of the blood vessel. Leakage of the dye out of the vessels can be seen as an increase in fluorescence, or whitening, outside of the blood vessels in the photograph. This leakage leads to the accumulation of fluid in the retina, causing DME. Neovascularization is also very permeable, and areas of neovascularization, an indicator of proliferative diabetic retinopathy, are apparent on FA as spots of intense hyperfluorescence (Figure 3).
Treatment
In 1985, results from ETDRS revolutionized the treatment of DME. The study showed that by applying laser burns to leaking microaneurysms or in a grid pattern over an area of diffuse edema, severe vision loss could be reduced by 50%. In the past few years, the role of laser treatment has shifted so that it is now indicated for the treatment of noncenter-involving DME. The impetus for this change was the development of anti-VEGF therapy, which is now the first-line therapy for centerinvolving DME.
As aforementioned, VEGF causes increased vascular permeability and breakdown of the blood-retinal barrier. Patients with DME have been shown to have increased levels of VEGF in the vitreous when compared with nondiabetic controls.21 There are now 3 anti-VEGF agents that are commonly used in clinical practice for the treatment of DME: ranibizumab, aflibercept, and bevacizumab. Ranibizumab is an antibody fragment targeted against VEGF that is FDA approved for use in patients with DME. The Diabetic Retinopathy Clinical Research Network Protocol I showed that treatment with ranibizumab, paired with deferred laser treatment, results in greater visual improvement than does prompt laser treatment alone.22 Treatment with aflibercept is a recombinant fusion protein of VEGF receptors. It was shown to be superior in terms of visual improvement when compared with laser treatment.23 Bevacizumab is a full-length antibody that is more affordable than other anti-VEGF medications and is often used off label for the treatment of DME. All of the anti-VEGF therapies are intravitreal injections. After topical anesthesia, the medication is injected through the sclera into the vitreous cavity in the outpatient clinic setting.
A significant disadvantage of the anti-VEGF therapies is that many patients need monthly injections, especially in the first year of treatment, necessitating many office visits, which can decrease adherence. In some patients, the edema may not respond to anti-VEGF therapy. In these cases, steroid therapy may be helpful to suppress the inflammatory pathways that are independent of VEGF. Intravitreal triamcinolone in combination with laser treatment has been shown to be as effective as ranibizumab in a small group of patients.24 An intravitreal dexamethasone implant, which has a treatment effect lasting for 3 months, was also shown to improve visual acuity over sham treatment in patients with DME.25 Most recently, an intravitreal fluocinolone implant that lasts 3 years was approved by the FDA for treatment of DME.26 A significant benefit of the steroid implants is the long duration of treatment effect compared with that of the anti-VEGF injections. However, steroid therapy is associated with the development of cataracts and glaucoma, the rates of which are increased when treatment is prolonged. Because of these adverse effects, steroids are currently used as second-line or third-line treatment in DME. Retinal surgery may be indicated if there is vitreomacular traction that is exacerbating the DME. A vitrectomy is performed to remove the vitreous and relieve any adhesion to the surface of the retina.
Conclusion
Despite the new ophthalmic treatment modalities, it is important to remember that DME is a chronic condition that will require long-term follow-up. Many patients will not experience complete resolution of DME with a single therapy alone. Control of systemic risk factors, including blood sugar with a goal of A1c < 7%, blood pressure, and cholesterol, remains the key to a successful treatment program. Primary care physicians, endocrinologists, diabetologists, optometrists, comprehensive ophthalmologists, retina specialists, and patients must work together to create an individualized treatment regimen that will optimize the patient’s vision by preventing blindness and improving his/her quality of life for years to come.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Diabetes mellitus (DM) affects about 347 million people worldwide, making it the new global epidemic.1 In the U.S alone, the number of adults with DM has more than tripled over the past 30 years. Now, almost 10% of the U.S. population has the disease and is at risk for serious systemic complications, including blindness.2
Diabetes is the leading cause of new cases of legal blindness in adults aged 18 to 74 years in the U.S. Diabetic retinopathy, seen as vascular changes in the retina related to DM, is found in almost half of all patients with DM.3 As the number of people with DM is expected to increase, so is the number of people affected with and blinded by diabetic retinopathy. Providers in both primary care and subspecialty settings have a critical role to play in the management and prevention of blindness in diabetic patients.
According to the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), diabetic retinopathy will affect 99% of patients with type 1 DM (T1DM) and 60% of patients with type 2 DM (T2DM) after 20 years of having diabetes.4 Diabetic retinopathy is a result of the microvascular damage that occurs from diabetes. The most common findings seen on a dilated fundus exam in nonproliferative diabetic retinopathy are microaneurysms, intraretinal hemorrhages, hard exudates, and cotton wool spots (Figure 1). Cotton wool spots represent focal areas of retinal ischemia. Nonproliferative disease progresses to proliferative retinopathy when neovascularization develops.
One of the major causes of vision loss in the setting of DM is diabetic macular edema (DME). Between 4% and 7% of people with DM currently have DME.5,6 Diabetic macular edema is a result of the break down of the bloodretinal barrier, which is an extension of the blood-brain barrier. Hyperglycemia causes a disruption of the cellular tight junctions, pericyte loss, and thickening of the basement membrane. These changes cause weakness in the walls of the retinal blood vessels, allowing microaneurysms to form. Hyperglycemia also causes upregulation of the production of inflammatory markers such as vascular endothelial growth factor (VEGF), protein kinase C, prostaglandins, and cytokines, which increase retinal vascular permeability (Figure 2).7
Risk Factors for DME
Several studies have found the prevalence of DME to be higher in black (10.4%-15.6%) and Hispanic (18%) patients vs non-Hispanic white patients (6.3%-8.4%); Asian patients have the lowest prevalence of DME (5%).5,6,8,9 The modifiable risk factors for the development of DME include hyperglycemia, duration of disease, hypertension, and dyslipidemia. Patients who have had DM for a longer period of time (> 10 years) and those with a higher hemoglobin A1c (A1c ) are more likely to have DME.4,6 The WESDR showed that for each percentage point increase in baseline A1c, there was a 28% increase in the incidence of visual impairment at the 25-year follow-up.10 An A1c < 7% is recommended in patients, though some may benefit from an A1c < 6.5%.11
Epidemiologic studies have also found hypertension and dyslipidemia to be associated with an increased risk of DME.12-15 Both diseases lead to an increased vascular permeability, compounding the microvascular damage already present from DM. In hypertension, DME and retinal hemorrhages are thought to be influenced by the increased perfusion pressure in retinal vessels.16 For every 10 mm Hg increase in blood pressure over 160 mm Hg, the risk of DME increases by 25%.17 Dyslipidemia contributes to DME by damaging endothelial cells and causing increased vascular permeability through cytokine and VEGF upregulation.18
Other risk factors, such as nephropathy, anemia, sleep apnea, and thiazolidinedione (glitazone) use, may also affect the development of DME. Patients with microalbuminuria have a lower serum protein concentration and thus, a reduced plasma colloidal osmotic pressure. This decreased osmotic pressure allows fluid to exit the retinal blood vessels and causes DME.7 Serum osmolarity may also play a role in DME. Some patients were noted to have decreased DME after receiving hemodialysis.19 Retinal vascular permeability can be increased by ischemia caused by hypoxia from anemia or sleep apnea.7 Glitazones have been associated with an increased risk of developing DME, although the cause is unclear.7
Examination
The American Academy of Ophthalmology recommends annual diabetic retinopathy screening for all patients with DM. Screening exams should start at the time of diagnosis for patients with T2DM and at 5 years after diagnosis for patients with T1DM. Currently, patients without a history of diabetic retinopathy can be screened via an ophthalmologic exam or review of color fundus photographs, which can be taken by trained personnel in the primary care or subspecialty settings.
Unfortunately, only 60% of patients with DM are screened annually. It is important to emphasize to patients the importance of a screening eye exam. Many patients do not understand that diabetic retinopathy may be present even if they are not experiencing any changes in vision. The patient should be referred to an ophthalmologist immediately if he or she reports blurry vision, wavy lines, or dark spots in the vision, especially if those symptoms are acute. The goal of a screening program is early detection: to identify those patients who are at risk for vision loss from DM and to provide close follow-up and timely treatment. Any patient with a history of diabetic retinopathy should be followed at the interval recommended by the eye care provider.11
Patient history is an important part of the screening exam, including symptoms, duration of DM, A1c, medications, medical history (hypertension, nephropathy, dyslipidemia, obesity, pregnancy), and ocular history. If there is evidence of diabetic retinopathy or DME, recommendations for better systemic control of DM or its comorbidities can be made based on the patient history. During the screening exam, the patient’s visual acuity and intraocular pressure are measured. A basic examination of the anterior segment looking for neovascularization of the iris is also completed. Iris neovascularization is a sign of proliferative diabetic retinopathy that would indicate laser treatment, also known as panretinal photocoagulation, or intravitreal injection.
The patient’s pupils are dilated, which enables the eye care provider to examine the retina. Patients often dislike this portion of the examination, because the dilation drop causes their vision to be blurry for 4 to 6 hours. However, dilation ensures that the provider has a view of the entire retina and can detect early stages of diabetic retinopathy.
If the screening is taking place via color fundus photographs, a nonmydriatic fundus camera, which does not require dilation, can be used. The purpose of the screening examination is to assess changes that can lead to vision loss. Important features that must be detected if present are macular edema, extensive microvascular changes, vitreous hemorrhage, and neovascularization of the optic nerve, retina, or iris.11 It is important to remember that the diabetic screening examination does not take the place of a complete ophthalmologic examination for other ocular disease, such as glaucoma. The patient may need to schedule additional appointments with an eye care provider if other eye problems exist.
Imaging
Clinically significant macular edema (CSME) was first defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) as macular edema that involves the center of vision, called the fovea, and can be visualized with clinical examination of the retina. The criteria for CSME are used to identify edema that is mostly likely to cause vision loss and to guide laser treatment.20 Today, adjunctive testing, such as optical coherence tomography (OCT) and fluorescein angiography (FA), aid in the earlier detection and diagnosis of DME. These imaging techniques are capable of detecting small amounts of macular edema that are vision-threatening but are not visible on exam. Center-involving DME is edema that involves the fovea; noncenter-involving DME is edema that does not involve the fovea but is found within the macula. Color and redfree fundus photographs, OCT, and FA are also used to guide treatment in patients with DME.
Color fundus photographs are helpful as a screening tool that identifies those patients who need to be seen by an eye care provider for a dilated fundus exam. Photographs are also helpful in documenting the changes in DME after treatment. Optical coherence tomography allows for high-resolution imaging of the retinal layers and objective measurement of the amount and location of DME. This information can be used to detect any change in DME between visits and evaluate the response to treatment. Further treatment decisions are frequently made based in part on the findings seen on OCT (Figure 2).
Fluorescein angiography is an imaging test that uses an IV dye called fluorescein to detect areas of retinal nonperfusion, edema, and neovascularization. Fluorescein is injected into a peripheral vein (frequently in the antecubital area or in the hand). Photographs are then taken
using special filters that allow only the wavelength corresponding to the fluorescein dye to be visible. The fluorescein can be seen filling the retinal arteries within 20 seconds of the peripheral injection. Photographs are usually taken intermittently for 15 minutes. As time passes, the dye will leak out of any blood vessels that have increased vascular permeability and highlight any microaneurysms, because the dye pools in the outpouching of the blood vessel. Leakage of the dye out of the vessels can be seen as an increase in fluorescence, or whitening, outside of the blood vessels in the photograph. This leakage leads to the accumulation of fluid in the retina, causing DME. Neovascularization is also very permeable, and areas of neovascularization, an indicator of proliferative diabetic retinopathy, are apparent on FA as spots of intense hyperfluorescence (Figure 3).
Treatment
In 1985, results from ETDRS revolutionized the treatment of DME. The study showed that by applying laser burns to leaking microaneurysms or in a grid pattern over an area of diffuse edema, severe vision loss could be reduced by 50%. In the past few years, the role of laser treatment has shifted so that it is now indicated for the treatment of noncenter-involving DME. The impetus for this change was the development of anti-VEGF therapy, which is now the first-line therapy for centerinvolving DME.
As aforementioned, VEGF causes increased vascular permeability and breakdown of the blood-retinal barrier. Patients with DME have been shown to have increased levels of VEGF in the vitreous when compared with nondiabetic controls.21 There are now 3 anti-VEGF agents that are commonly used in clinical practice for the treatment of DME: ranibizumab, aflibercept, and bevacizumab. Ranibizumab is an antibody fragment targeted against VEGF that is FDA approved for use in patients with DME. The Diabetic Retinopathy Clinical Research Network Protocol I showed that treatment with ranibizumab, paired with deferred laser treatment, results in greater visual improvement than does prompt laser treatment alone.22 Treatment with aflibercept is a recombinant fusion protein of VEGF receptors. It was shown to be superior in terms of visual improvement when compared with laser treatment.23 Bevacizumab is a full-length antibody that is more affordable than other anti-VEGF medications and is often used off label for the treatment of DME. All of the anti-VEGF therapies are intravitreal injections. After topical anesthesia, the medication is injected through the sclera into the vitreous cavity in the outpatient clinic setting.
A significant disadvantage of the anti-VEGF therapies is that many patients need monthly injections, especially in the first year of treatment, necessitating many office visits, which can decrease adherence. In some patients, the edema may not respond to anti-VEGF therapy. In these cases, steroid therapy may be helpful to suppress the inflammatory pathways that are independent of VEGF. Intravitreal triamcinolone in combination with laser treatment has been shown to be as effective as ranibizumab in a small group of patients.24 An intravitreal dexamethasone implant, which has a treatment effect lasting for 3 months, was also shown to improve visual acuity over sham treatment in patients with DME.25 Most recently, an intravitreal fluocinolone implant that lasts 3 years was approved by the FDA for treatment of DME.26 A significant benefit of the steroid implants is the long duration of treatment effect compared with that of the anti-VEGF injections. However, steroid therapy is associated with the development of cataracts and glaucoma, the rates of which are increased when treatment is prolonged. Because of these adverse effects, steroids are currently used as second-line or third-line treatment in DME. Retinal surgery may be indicated if there is vitreomacular traction that is exacerbating the DME. A vitrectomy is performed to remove the vitreous and relieve any adhesion to the surface of the retina.
Conclusion
Despite the new ophthalmic treatment modalities, it is important to remember that DME is a chronic condition that will require long-term follow-up. Many patients will not experience complete resolution of DME with a single therapy alone. Control of systemic risk factors, including blood sugar with a goal of A1c < 7%, blood pressure, and cholesterol, remains the key to a successful treatment program. Primary care physicians, endocrinologists, diabetologists, optometrists, comprehensive ophthalmologists, retina specialists, and patients must work together to create an individualized treatment regimen that will optimize the patient’s vision by preventing blindness and improving his/her quality of life for years to come.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Danaei G, Finucane MM, Lu Y, et al; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31-40.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimate of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
3. National Institutes of Health. Diabetic retinopathy: causes and risk factors. NIH Senior Health Website. http://nihseniorhealth.gov/diabeticretinopathy/causesandriskfactors/01.html. Updated February 2015. Accessed September 3, 2015.
4. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102(1):7-16.
5. Yau JW, Rogers SL, Kawasaki R, et al; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564.
6. Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334-1340.
7. Diep TM, Tsui I. Risk factors associated with diabetic macular edema. Diabetes Res Clin Pract. 2013;100(3):298-305.
8. Varma R, Choudhury F, Klein R, Chung J, Torres M, Azen SP; Los Angeles Latino Eye Study Group. Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5):752-761.e1-e3.
9. Emanuele N, Moritz T, Klein R, et al; Veterans Affairs Diabetes Trial Study Group. Ethnicity, race, and clinically significant macular edema in the Veterans Affairs Diabetes Trial (VADT). Diabetes Res Clin Pract. 2009;86(2):104-110.
10. Klein R, Lee KE, Gangnon RE, Klein BE. The 25-year incidence of visual impairment in type 1 diabetes mellitus: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2010;117(1):63-70.
11. American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern: Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2014.
12. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984;91(12):1464-1474.
13. Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116(3):497-503.
14. Chew EY. Diabetic retinopathy and lipid abnormalities. Curr Opin Ophthalmol. 1997;8(3):59-62.
15. Klein R, Sharrett AR, Klein BE, et al; ARIC Group. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology. 2002;109(7):1225-1234.
16. Haefliger IO, Meyer P, Flammer J, Lüscher TF. The vascular endothelium as a regulator of the ocular circulation: a new concept in ophthalmology? Surv Ophthalmol. 1994;39(2):123-132.
17. Lopes de Faria JM, Jalkh AE, Trempe CL, McMeel JW. Diabetic macular edema: risk factors and concomitants. Acta Ophthalmol Scand. 1999;77(2):170-175.
18. Langeler EG, Snelting-Havinga I, van Hinsbergh VW. Passage of low density lipoproteins through monolayers of human arterial endothelial cells. Effects of vasoactive substances in an in vitro model. Arteriosclerosis. 1989;9(4):550-559.
19. Theodossiadis PG, Theodoropoulou S, Neamonitou G, et al. Hemodialysisinduced alterations in macular thickness measured by optical coherence tomography in diabetic patients with end-stage renal disease. Ophthalmologica. 2012;227(2):90-94.
20. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796-1806.
21. Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116(1):73-79.
22. Diabetic Retinopathy Clinical Research Network; Elman MJ, Qin H, Aiello LP, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119(11):2312-2318.
23. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247-2254.
24. Diabetic Retinopathy Clinical Research Network; Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.
25. Boyer DS, Yoon YH, Belfort R Jr, et al; Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904-1914.
26. Campochiaro PA, Brown DM, Pearson A, et al; FAME Study Group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626-635.e2.
1. Danaei G, Finucane MM, Lu Y, et al; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31-40.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimate of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
3. National Institutes of Health. Diabetic retinopathy: causes and risk factors. NIH Senior Health Website. http://nihseniorhealth.gov/diabeticretinopathy/causesandriskfactors/01.html. Updated February 2015. Accessed September 3, 2015.
4. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102(1):7-16.
5. Yau JW, Rogers SL, Kawasaki R, et al; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564.
6. Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334-1340.
7. Diep TM, Tsui I. Risk factors associated with diabetic macular edema. Diabetes Res Clin Pract. 2013;100(3):298-305.
8. Varma R, Choudhury F, Klein R, Chung J, Torres M, Azen SP; Los Angeles Latino Eye Study Group. Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5):752-761.e1-e3.
9. Emanuele N, Moritz T, Klein R, et al; Veterans Affairs Diabetes Trial Study Group. Ethnicity, race, and clinically significant macular edema in the Veterans Affairs Diabetes Trial (VADT). Diabetes Res Clin Pract. 2009;86(2):104-110.
10. Klein R, Lee KE, Gangnon RE, Klein BE. The 25-year incidence of visual impairment in type 1 diabetes mellitus: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2010;117(1):63-70.
11. American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern: Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2014.
12. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984;91(12):1464-1474.
13. Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116(3):497-503.
14. Chew EY. Diabetic retinopathy and lipid abnormalities. Curr Opin Ophthalmol. 1997;8(3):59-62.
15. Klein R, Sharrett AR, Klein BE, et al; ARIC Group. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology. 2002;109(7):1225-1234.
16. Haefliger IO, Meyer P, Flammer J, Lüscher TF. The vascular endothelium as a regulator of the ocular circulation: a new concept in ophthalmology? Surv Ophthalmol. 1994;39(2):123-132.
17. Lopes de Faria JM, Jalkh AE, Trempe CL, McMeel JW. Diabetic macular edema: risk factors and concomitants. Acta Ophthalmol Scand. 1999;77(2):170-175.
18. Langeler EG, Snelting-Havinga I, van Hinsbergh VW. Passage of low density lipoproteins through monolayers of human arterial endothelial cells. Effects of vasoactive substances in an in vitro model. Arteriosclerosis. 1989;9(4):550-559.
19. Theodossiadis PG, Theodoropoulou S, Neamonitou G, et al. Hemodialysisinduced alterations in macular thickness measured by optical coherence tomography in diabetic patients with end-stage renal disease. Ophthalmologica. 2012;227(2):90-94.
20. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796-1806.
21. Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116(1):73-79.
22. Diabetic Retinopathy Clinical Research Network; Elman MJ, Qin H, Aiello LP, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119(11):2312-2318.
23. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247-2254.
24. Diabetic Retinopathy Clinical Research Network; Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.
25. Boyer DS, Yoon YH, Belfort R Jr, et al; Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904-1914.
26. Campochiaro PA, Brown DM, Pearson A, et al; FAME Study Group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626-635.e2.
Confronting the Diabetes Epidemic
Obesity and type 2 diabetes mellitus (DM) are major clinical and public health problems in the U.S. From 1980 to 2012, the number of U.S. adults with diagnosed DM nearly quadrupled. More than 21 million Americans have DM and another 8.1 million people with DM are undiagnosed.1 Hispanics, non-Hispanic blacks, and American Indians/Alaska Natives are disproportionately impacted with rates up to twice as high as whites. The CDC estimates that 40% of the adult population will develop DM during their lifetime, and > 50% of Hispanic men and women and non-Hispanic black women are predicted to develop the disease.2
A 2015 CDC study analyzed 2011 and 2012 National Health and Nutrition Examination Survey data and estimated that the prevalence of DM for all American adults was 12% to 14%.3 Diabetes mellitus prevalence increased in every age, sex, level of education, income, and racial/ethnic group. Although the proportion of people with DM who were undiagnosed decreased, more than half of Asian Americans and nearly half of Hispanics with DM remained undiagnosed. Prediabetes, an important predicator of the risk of developing DM, also increased from about 33% to 37% to 38%.2,3 It was estimated that 49% to 53% of Americans have either DM or prediabetes.3
The increase in incidence of DM has been attributed to a rise in the prevalence of obesity, which doubled between 1980 and 2000. Current estimates place the prevalence of obesity at 35% for adults aged 20 years. The primary components of DM prevention in adults are weight loss and increased physical activity. The Diabetes Prevention Program study showed that participants in the lifestyle intervention group—those receiving intensive individual counseling and motivational support on effective diet, exercise, and behavior modification—reduced their risk of developing DM by 58%. Evidence suggests that higher intensity programs are more likely to lead to greater weight loss and reduction in new onset disease. However, even small and incremental steps in improving diet and increasing activity can produce benefits for individuals.
Recommendations issued by the U.S. Surgeon General and the Institute of Medicine (IOM) focus on not only personal behaviors, but also characteristics of the social and physical environment.4,5 The IOM recognizes 5 critical goals for preventing obesity: (1) integrating physical activity into people’s daily lives; (2) making healthful food and beverage options routinely and easily available; (3) transforming marketing and messages about nutrition and activity; (4) making schools a focal point for obesity prevention; and (5) galvanizing employers and health care professionals to support healthy lifestyles.
A multifaceted approach toward prevention and management is needed to combat DM and obesity. Changes in U.S. society have resulted in decreases in physical activity and increases in caloric intake. Communities without safe environments to walk and play, lacking affordable and healthful food options, and surrounded by advertisements for unhealthful food and beverage options will continue to struggle with obesity and DM. Broad changes are needed to support and sustain individuals and families. As health care providers, we can have profound effects in assisting patients to achieve better health by encouraging, motivating, and empowering them with the tools needed to make these changes for meaningful and permanent lifestyle changes.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
2. Centers for Disease Control and Prevention. Diabetes Report Card 2014. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2015.
3. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029.
4. U.S. Department of Health and Human Services. The Surgeon General’s Vision for a Healthy and Fit Nation, 2010. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2010.
5. Institute of Medicine. Accelerating Progress in Obesity Prevention: Solving the Weight
of the Nation. Washington, DC: National Academies Press; 2012.
Obesity and type 2 diabetes mellitus (DM) are major clinical and public health problems in the U.S. From 1980 to 2012, the number of U.S. adults with diagnosed DM nearly quadrupled. More than 21 million Americans have DM and another 8.1 million people with DM are undiagnosed.1 Hispanics, non-Hispanic blacks, and American Indians/Alaska Natives are disproportionately impacted with rates up to twice as high as whites. The CDC estimates that 40% of the adult population will develop DM during their lifetime, and > 50% of Hispanic men and women and non-Hispanic black women are predicted to develop the disease.2
A 2015 CDC study analyzed 2011 and 2012 National Health and Nutrition Examination Survey data and estimated that the prevalence of DM for all American adults was 12% to 14%.3 Diabetes mellitus prevalence increased in every age, sex, level of education, income, and racial/ethnic group. Although the proportion of people with DM who were undiagnosed decreased, more than half of Asian Americans and nearly half of Hispanics with DM remained undiagnosed. Prediabetes, an important predicator of the risk of developing DM, also increased from about 33% to 37% to 38%.2,3 It was estimated that 49% to 53% of Americans have either DM or prediabetes.3
The increase in incidence of DM has been attributed to a rise in the prevalence of obesity, which doubled between 1980 and 2000. Current estimates place the prevalence of obesity at 35% for adults aged 20 years. The primary components of DM prevention in adults are weight loss and increased physical activity. The Diabetes Prevention Program study showed that participants in the lifestyle intervention group—those receiving intensive individual counseling and motivational support on effective diet, exercise, and behavior modification—reduced their risk of developing DM by 58%. Evidence suggests that higher intensity programs are more likely to lead to greater weight loss and reduction in new onset disease. However, even small and incremental steps in improving diet and increasing activity can produce benefits for individuals.
Recommendations issued by the U.S. Surgeon General and the Institute of Medicine (IOM) focus on not only personal behaviors, but also characteristics of the social and physical environment.4,5 The IOM recognizes 5 critical goals for preventing obesity: (1) integrating physical activity into people’s daily lives; (2) making healthful food and beverage options routinely and easily available; (3) transforming marketing and messages about nutrition and activity; (4) making schools a focal point for obesity prevention; and (5) galvanizing employers and health care professionals to support healthy lifestyles.
A multifaceted approach toward prevention and management is needed to combat DM and obesity. Changes in U.S. society have resulted in decreases in physical activity and increases in caloric intake. Communities without safe environments to walk and play, lacking affordable and healthful food options, and surrounded by advertisements for unhealthful food and beverage options will continue to struggle with obesity and DM. Broad changes are needed to support and sustain individuals and families. As health care providers, we can have profound effects in assisting patients to achieve better health by encouraging, motivating, and empowering them with the tools needed to make these changes for meaningful and permanent lifestyle changes.
Click here to read the digital edition.
Obesity and type 2 diabetes mellitus (DM) are major clinical and public health problems in the U.S. From 1980 to 2012, the number of U.S. adults with diagnosed DM nearly quadrupled. More than 21 million Americans have DM and another 8.1 million people with DM are undiagnosed.1 Hispanics, non-Hispanic blacks, and American Indians/Alaska Natives are disproportionately impacted with rates up to twice as high as whites. The CDC estimates that 40% of the adult population will develop DM during their lifetime, and > 50% of Hispanic men and women and non-Hispanic black women are predicted to develop the disease.2
A 2015 CDC study analyzed 2011 and 2012 National Health and Nutrition Examination Survey data and estimated that the prevalence of DM for all American adults was 12% to 14%.3 Diabetes mellitus prevalence increased in every age, sex, level of education, income, and racial/ethnic group. Although the proportion of people with DM who were undiagnosed decreased, more than half of Asian Americans and nearly half of Hispanics with DM remained undiagnosed. Prediabetes, an important predicator of the risk of developing DM, also increased from about 33% to 37% to 38%.2,3 It was estimated that 49% to 53% of Americans have either DM or prediabetes.3
The increase in incidence of DM has been attributed to a rise in the prevalence of obesity, which doubled between 1980 and 2000. Current estimates place the prevalence of obesity at 35% for adults aged 20 years. The primary components of DM prevention in adults are weight loss and increased physical activity. The Diabetes Prevention Program study showed that participants in the lifestyle intervention group—those receiving intensive individual counseling and motivational support on effective diet, exercise, and behavior modification—reduced their risk of developing DM by 58%. Evidence suggests that higher intensity programs are more likely to lead to greater weight loss and reduction in new onset disease. However, even small and incremental steps in improving diet and increasing activity can produce benefits for individuals.
Recommendations issued by the U.S. Surgeon General and the Institute of Medicine (IOM) focus on not only personal behaviors, but also characteristics of the social and physical environment.4,5 The IOM recognizes 5 critical goals for preventing obesity: (1) integrating physical activity into people’s daily lives; (2) making healthful food and beverage options routinely and easily available; (3) transforming marketing and messages about nutrition and activity; (4) making schools a focal point for obesity prevention; and (5) galvanizing employers and health care professionals to support healthy lifestyles.
A multifaceted approach toward prevention and management is needed to combat DM and obesity. Changes in U.S. society have resulted in decreases in physical activity and increases in caloric intake. Communities without safe environments to walk and play, lacking affordable and healthful food options, and surrounded by advertisements for unhealthful food and beverage options will continue to struggle with obesity and DM. Broad changes are needed to support and sustain individuals and families. As health care providers, we can have profound effects in assisting patients to achieve better health by encouraging, motivating, and empowering them with the tools needed to make these changes for meaningful and permanent lifestyle changes.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
2. Centers for Disease Control and Prevention. Diabetes Report Card 2014. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2015.
3. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029.
4. U.S. Department of Health and Human Services. The Surgeon General’s Vision for a Healthy and Fit Nation, 2010. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2010.
5. Institute of Medicine. Accelerating Progress in Obesity Prevention: Solving the Weight
of the Nation. Washington, DC: National Academies Press; 2012.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
2. Centers for Disease Control and Prevention. Diabetes Report Card 2014. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2015.
3. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029.
4. U.S. Department of Health and Human Services. The Surgeon General’s Vision for a Healthy and Fit Nation, 2010. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2010.
5. Institute of Medicine. Accelerating Progress in Obesity Prevention: Solving the Weight
of the Nation. Washington, DC: National Academies Press; 2012.
“I Feel Dizzy, Doctor”
› Refer a patient who reports that his dizziness is accompanied by hearing loss to an otolaryngologist for evaluation. C
› Use the HINTS (Head Impulse, Nystagmus, and Test of Skew) procedure to differentiate central from peripheral vertigo. A
› Use the Dix-Hallpike procedure to diagnose benign paroxysmal positional vertigo. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With an estimated lifetime prevalence of 17% to 30%,1 dizziness is a relatively common clinical symptom, but the underlying cause can be difficult to diagnose. That’s because patients’ descriptions of dizziness are often imprecise, and this symptom is associated with a wide range of conditions. A careful history and physical examination are key to diagnosis, as is an understanding of the mechanisms of dizziness.
This article covers the range of diagnoses that should be considered when a patient presents with dizziness, and provides insight regarding features of the patient’s history that can better elucidate the specific etiology.
What do patients mean when they say, “I feel dizzy”?
“Dizziness” is a vague term, and patients who report dizziness should be asked to further describe the sensation. Patients may use the word dizziness in an attempt to describe many sensations, including faintness, giddiness, light-headedness, or unsteadiness.2 In 1972, Drachman and Hart proposed a classification system for dizziness that describes 4 categories—presyncope, vertigo, disequilibrium, and atypical (TABLE 1).3 These classifications are still commonly used today, and the discussion that follows describes potential causes of dizziness in each of these 4 categories. A stepwise approach for evaluating a patient who reports dizziness can be found in the ALGORITHM.3-6
Syncopal-related dizziness can have a cardiovascular cause
Presyncope is a feeling of impending loss of consciousness that’s sometimes accompanied by generalized muscle weakness and/or partial vision loss. Taking a careful history regarding the events surrounding the episode should distinguish this type of dizziness, and doing so is essential because most of the underlying pathogenesis involves the cardiovascular system and requires specific interventions.
Dysrhythmias can cause syncope and may or may not be accompanied by a feeling of palpitations. Diagnosis is made by electrocardiogram (EKG) followed by the use of a Holter monitor.
Vasovagal syncope is caused by a sudden slowing of the pulse that’s the result of stimulation of the vagal nerve. It can occur from direct stimulation of the nerve from palpation (or strangulation), or from an intense autonomic discharge, as when people are frightened or confronted with something upsetting (eg, the sight of blood.)
Orthostatic hypotension results from a change in body position in which either autonomic mechanisms cannot maintain venous tone, causing a sudden drop in blood pressure, or in which the heart cannot compensate by speeding up, as when a patient is taking a beta-adrenergic antagonist or has first-degree heart block. It can also result from hypovolemia.
Measuring the patient’s blood pressure in the recumbent, seated, and standing positions can verify the diagnosis if an episode occurred soon before the examination. This kind of dizziness can be treated by instructing the patient to rise slowly, or by making appropriate medication adjustments. If conservative measures fail, medications such as midodrine or droxidopa can be tried.7
Hypoglycemia, hypoxia, or hyperventilation can also precipitate syncopal symptoms. Taking a careful history to assess for the presence of seizure-related features such as tonic/clonic movements or loss of bowel and bladder control can be helpful in distinguishing this form of dizziness.
Vertigo can have a central or peripheral cause
Vertigo is dizziness that is characterized by the sensation of spinning. The presence of vertigo implies disease of the inner ear or central nervous system. The “wiring diagram” of the vestibulo-ocular reflex is fairly straightforward, but sorting out the symptoms that arise from lesions within the system can be a diagnostic challenge. Vertigo has classically been divided into causes that are central (originating in the central nervous system) or peripheral (originating in the peripheral nervous system).
The HINTS (Head Impulse, Nystagmus, and Test of Skew) protocol is a group of 3 tests that can be used to differentiate central from peripheral vertigo (TABLE 2).8,9 To perform the head impulse test, the examiner asks the patient to focus his gaze on a target and then rapidly turns the patient’s head to the side, watching the eyes for any corrective movements.10 When the eyes make a corrective saccade, the test is considered to be positive for a peripheral lesion.
Horizontal nystagmus is assessed by having the patient look in the direction of the fast phase of the nystagmus. If the nystagmus increases in intensity, then the test is considered positive for a peripheral lesion.
Vertigo can have many possible causes
Finally, the “test of skew” is performed by again having the patient fixate on the examiner’s nose. Each eye is tested by being covered, and then uncovered. If the uncovered eye has to move to refocus on the examiner’s nose, then the test is positive for a central lesion. A positive head impulse, positive horizontal nystagmus, and negative test of skew is 100% sensitive and 96% specific for a peripheral lesion.11
Benign paroxysmal positional vertigo (BPPV) is vertigo that is triggered by movement of the head. It occurs when otoconia that are normally embedded in gel in the utricle become dislodged and migrate into the 3 fluid-filled semicircular canals, where they interfere with the normal fluid movement these canals use to sense head motion, causing the inner ear to send false signals to the brain.12
Diagnosis is confirmed by performing the Dix-Hallpike maneuver to elicit nystagmus. The patient is moved from a seated to a supine position with her head turned 45 degrees to the right and held for 30 seconds. For a demonstration of the Dix-Hallpike maneuver, see https://youtu.be/8RYB2QlO1N4. The Dix-Hallpike maneuver is also the first step of a treatment for BBPV known as the Epley maneuver. (See “The Epley maneuver: A procedure for treating BPPV”.13,14)
Benign paroxysmal positional vertigo (BPPV) can be treated with the Epley maneuver. Like the Dix-Hallpike maneuver, the Epley maneuver isolates the posterior semicircular canal of the affected ear. However, it goes a step further to reposition otolithic debris away from the ampulla of the posterior canal, rolling it through the canal and depositing it in the utricle, where it will not stimulate nerve endings and produce symptoms.
For a demonstration of the Epley maneuver, see https://youtu.be/jBzID5nVQjk. A computer-controlled form of the Epley maneuver has been developed and can be as effective as the manual version of this procedure.13
In 38% of patients, BPPV spontaneously resolves. The Epley maneuver can improve this rate to 64% with a single treatment, and one additional maneuver improves the success rate to 83.3%.14 If this procedure doesn’t work the first time, there may be more sediment that didn’t have enough time to settle during the procedure. Therefore, the Epley maneuver can be repeated 3 times a day, and performed on subsequent days as needed.
Labyrinthitis—inflammation of the inner ear that can cause vertigo—is suggested by an acute, non-recurrent episode of dizziness that is often preceded by an upper respiratory infection. If the external canal is extremely painful and/or develops a vesicular rash, the patient might have herpes zoster of the geniculate ganglion (Ramsay Hunt syndrome type 2).
Vertigo can have many possible causes
Vestibular migraine and Meniere’s disease. When a patient who has a history of migraines experiences symptoms of vertigo, vestibular migraine should be suspected, and treatment should focus on migraine therapy rather than vestibular therapy.15
Symptoms of Meniere’s disease and vestibular migraine can overlap.16 The current definition of Meniere’s disease requires ≥2 definitive episodes of vertigo with hearing loss plus tinnitus and/or aural symptoms.17 Thirty percent of vertigo episodes in patients with Meniere's disease can be attributed to BPPV.18
Acoustic neuroma. In addition to vertigo, acoustic neuroma is often associated with gradual hearing loss, tinnitus, and facial numbness (from compression of cranial nerve V preoperatively) or facial weakness (from compression of cranial nerve VII postoperatively). Unilateral hearing loss should prompt evaluation with magnetic resonance imaging.
“Acoustic neuroma” is a misnomer. The lesion arises from the vestibular (not the acoustic) portion of the 8th cranial nerve, and isn’t a neuroma; it is a schwannoma.19 Although it actually arises peripherally within the vestibular canal, it typically expands centrally and compresses other nerves centrally, which can make the clinical diagnosis more challenging if one were using the classical schema of differentiating between peripheral and central causes of vertigo.
Age-related vestibular loss occurs when the aging process causes deterioration of most of the components of the vestibulo-ocular reflex, resulting in dizziness and vertigo. Usually, the cerebral override mechanisms can compensate for the degeneration.
Other causes of vertigo include cerebellar infarction (3% of patients with vertigo),20 sound-induced vertigo (Tullio phenomenon),21 obstructive sleep apnea,22 and systemic sclerosis.23 Diabetes can cause a reduction in vestibular sensitivity that is evidenced by an increased reliance on visual stimuli to resolve vestibulo-visual conflict.24
Disequilibrium
Disequilibrium is predominantly a loss of balance. Patients with disequilibrium have the feeling that they are about to fall, specifically without the sensation of spinning. They may appear to sway, and will reach out for something to support them. Disequilibrium can be a component of vertigo, or it may suggest a more specific diagnosis, such as ataxia, which is a lack of coordination when walking.
Atypical causes of dizziness
“Light-headedness” may have an element of euphoria or may be indistinguishable from the early part of a syncopal episode. Because other causes of light-headedness can be difficult to distinguish from presyncope, it is important to consider syncope in the differential diagnosis.
The differential of light-headedness can also include panic attack, early hyperventilation, and toxin exposure (such as diphenylarsinic acid,25 pregabalin,26 or paint thinner27).
CORRESPONDENCE
Shannon Paul Starr, MD, Louisiana State University Health Sciences Center, 200 W. Esplanade #412, Kenner, LA 70065; [email protected].
1. Murdin L, Schilder AG. Epidemiology of balance symptoms and disorders in the community: a systematic review. Otol Neurotol. 2015;36:387-392.
2. Stedman TL. Stedman’s medical dictionary, illustrated. 24th ed. Baltimore, Md: William & Wilkins; 1982:419.
3. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology. 1972;22:323-334.
4. Angtuaco EJ, Wippold FJ II, Cornelius RS, et al; Expert Panel on Neurologic Imaging. ACR appropriateness criteria: hearing loss and/or vertigo. 2013. American College of Radiology Web site. Available at: http://www.acr.org/~/media/914834f9cfa74e6c803e8e9c6909cd7e.pdf. Accessed September 3, 2015.
5. Dros J, Maarsingh OR, van der Windt DA, et al. Profiling dizziness in older primary care patients: an empirical study. PLoS One. 2011;6:e16481.
6. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-369.
7. Biaggioni I. New developments in the management of neurogenic orthostatic hypotension. Curr Cardiol Rep. 2014;16:542.
8. Batuecas-Caletrío Á, Yáñez-González R, Sánchez-Blanco C, et al. [Peripheral vertigo versus central vertigo. Application of the HINTS protocol]. Rev Neurol. 2014;59:349-353.
9. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504-3510.
10. Barraclough K, Bronstein A. Vertigo. BMJ. 2009;339:b3493.
11. Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. 2013;20:986-996.
12. Vestibular Disorders Association. Benign Paroxysmal Positional Vertigo. Vestibular Disorders Association Web site. Available at: http://vestibular.org/understanding-vestibular-disorders/types-vestibular-disorders/benign-paroxysmal-positional-vertigo. Accessed September 1, 2015.
13. Shan X, Peng X, Wang E. Efficacy of computer-controlled repositioning procedure for benign paroxysmal positional vertigo. Laryngoscope. 2015;125:715-719.
14. Lee JD, Shim DB, Park HJ, et al. A multicenter randomized double-blind study: comparison of the Epley, Semont, and sham maneuvers for the treatment of posterior canal benign paroxysmal positional vertigo. Audiol Neurootol. 2014;19:336-341.
15. Stolte B, Holle D, Naegel S, et al. Vestibular migraine. Cephalalgia. 2015;35:262-270.
16. Lopez-Escamez JA, Dlugaiczyk J, Jacobs J, et al. Accompanying symptoms overlap during attacks in Menière’s disease and vestibular migraine. Front Neurol. 2014;5:265.
17. Beasley NJ, Jones NS. Menière’s disease: evolution of a definition. J Laryngol Otol. 1996;110:1107-1113.
18. Taura A, Funabiki K, Ohgita H, et al. One-third of vertiginous episodes during the follow-up period are caused by benign paroxysmal positional vertigo in patients with Meniere’s disease. Acta Otolaryngol. 2014;134:1140-1145.
19. Pineda A, Feder BH. Acoustic neuroma: a misnomer. Is Surg. 1967;33:40-43.
20. Seemungal BM. Neuro-otological emergencies. Curr Opin Neurol. 2007;20:32-39.
21. Harrison RV. On the biological plausibility of Wind Turbine Syndrome. Int J Environ Health Res. 2015;25:463-468.
22. Kayabasi S, Iriz A, Cayonu M, et al. Vestibular functions were found to be impaired in patients with moderate-tosevere obstructive sleep apnea. Laryngoscope. 2015;125:1244-1248.
23. Rabelo MB, Corona AP. Auditory and vestibular dysfunctions in systemic sclerosis: literature review. Codas. 2014;26:337-342.
24. Razzak RA, Bagust J, Docherty S, et al. Augmented asymmetrical visual field dependence in asymptomatic diabetics: evidence of subclinical asymmetrical bilateral vestibular dysfunction. J Diabetes Complications. 2015;29:68-72.
25. Ogata T, Nakamura Y, Endo G, et al. [Subjective symptoms and miscarriage after drinking well water exposed to diphenylarsinic acid]. Nihon Koshu Eisei Zasshi. 2014;61:556-564.
26. Qu C, Xie Y, Qin F, et al. Neuropsychiatric symptoms accompanying thrombocytopenia following pregabalin treatment for neuralgia: a case report. Int J Clin Pharm. 2014;36:1138-1140.
27. Rahimi HR, Agin K, Shadnia S, et al. Clinical and biochemical analysis of acute paint thinner intoxication in adults: a retrospective descriptive study. Toxicol Mech Methods. 2015;25:42-47.
› Refer a patient who reports that his dizziness is accompanied by hearing loss to an otolaryngologist for evaluation. C
› Use the HINTS (Head Impulse, Nystagmus, and Test of Skew) procedure to differentiate central from peripheral vertigo. A
› Use the Dix-Hallpike procedure to diagnose benign paroxysmal positional vertigo. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With an estimated lifetime prevalence of 17% to 30%,1 dizziness is a relatively common clinical symptom, but the underlying cause can be difficult to diagnose. That’s because patients’ descriptions of dizziness are often imprecise, and this symptom is associated with a wide range of conditions. A careful history and physical examination are key to diagnosis, as is an understanding of the mechanisms of dizziness.
This article covers the range of diagnoses that should be considered when a patient presents with dizziness, and provides insight regarding features of the patient’s history that can better elucidate the specific etiology.
What do patients mean when they say, “I feel dizzy”?
“Dizziness” is a vague term, and patients who report dizziness should be asked to further describe the sensation. Patients may use the word dizziness in an attempt to describe many sensations, including faintness, giddiness, light-headedness, or unsteadiness.2 In 1972, Drachman and Hart proposed a classification system for dizziness that describes 4 categories—presyncope, vertigo, disequilibrium, and atypical (TABLE 1).3 These classifications are still commonly used today, and the discussion that follows describes potential causes of dizziness in each of these 4 categories. A stepwise approach for evaluating a patient who reports dizziness can be found in the ALGORITHM.3-6
Syncopal-related dizziness can have a cardiovascular cause
Presyncope is a feeling of impending loss of consciousness that’s sometimes accompanied by generalized muscle weakness and/or partial vision loss. Taking a careful history regarding the events surrounding the episode should distinguish this type of dizziness, and doing so is essential because most of the underlying pathogenesis involves the cardiovascular system and requires specific interventions.
Dysrhythmias can cause syncope and may or may not be accompanied by a feeling of palpitations. Diagnosis is made by electrocardiogram (EKG) followed by the use of a Holter monitor.
Vasovagal syncope is caused by a sudden slowing of the pulse that’s the result of stimulation of the vagal nerve. It can occur from direct stimulation of the nerve from palpation (or strangulation), or from an intense autonomic discharge, as when people are frightened or confronted with something upsetting (eg, the sight of blood.)
Orthostatic hypotension results from a change in body position in which either autonomic mechanisms cannot maintain venous tone, causing a sudden drop in blood pressure, or in which the heart cannot compensate by speeding up, as when a patient is taking a beta-adrenergic antagonist or has first-degree heart block. It can also result from hypovolemia.
Measuring the patient’s blood pressure in the recumbent, seated, and standing positions can verify the diagnosis if an episode occurred soon before the examination. This kind of dizziness can be treated by instructing the patient to rise slowly, or by making appropriate medication adjustments. If conservative measures fail, medications such as midodrine or droxidopa can be tried.7
Hypoglycemia, hypoxia, or hyperventilation can also precipitate syncopal symptoms. Taking a careful history to assess for the presence of seizure-related features such as tonic/clonic movements or loss of bowel and bladder control can be helpful in distinguishing this form of dizziness.
Vertigo can have a central or peripheral cause
Vertigo is dizziness that is characterized by the sensation of spinning. The presence of vertigo implies disease of the inner ear or central nervous system. The “wiring diagram” of the vestibulo-ocular reflex is fairly straightforward, but sorting out the symptoms that arise from lesions within the system can be a diagnostic challenge. Vertigo has classically been divided into causes that are central (originating in the central nervous system) or peripheral (originating in the peripheral nervous system).
The HINTS (Head Impulse, Nystagmus, and Test of Skew) protocol is a group of 3 tests that can be used to differentiate central from peripheral vertigo (TABLE 2).8,9 To perform the head impulse test, the examiner asks the patient to focus his gaze on a target and then rapidly turns the patient’s head to the side, watching the eyes for any corrective movements.10 When the eyes make a corrective saccade, the test is considered to be positive for a peripheral lesion.
Horizontal nystagmus is assessed by having the patient look in the direction of the fast phase of the nystagmus. If the nystagmus increases in intensity, then the test is considered positive for a peripheral lesion.
Vertigo can have many possible causes
Finally, the “test of skew” is performed by again having the patient fixate on the examiner’s nose. Each eye is tested by being covered, and then uncovered. If the uncovered eye has to move to refocus on the examiner’s nose, then the test is positive for a central lesion. A positive head impulse, positive horizontal nystagmus, and negative test of skew is 100% sensitive and 96% specific for a peripheral lesion.11
Benign paroxysmal positional vertigo (BPPV) is vertigo that is triggered by movement of the head. It occurs when otoconia that are normally embedded in gel in the utricle become dislodged and migrate into the 3 fluid-filled semicircular canals, where they interfere with the normal fluid movement these canals use to sense head motion, causing the inner ear to send false signals to the brain.12
Diagnosis is confirmed by performing the Dix-Hallpike maneuver to elicit nystagmus. The patient is moved from a seated to a supine position with her head turned 45 degrees to the right and held for 30 seconds. For a demonstration of the Dix-Hallpike maneuver, see https://youtu.be/8RYB2QlO1N4. The Dix-Hallpike maneuver is also the first step of a treatment for BBPV known as the Epley maneuver. (See “The Epley maneuver: A procedure for treating BPPV”.13,14)
Benign paroxysmal positional vertigo (BPPV) can be treated with the Epley maneuver. Like the Dix-Hallpike maneuver, the Epley maneuver isolates the posterior semicircular canal of the affected ear. However, it goes a step further to reposition otolithic debris away from the ampulla of the posterior canal, rolling it through the canal and depositing it in the utricle, where it will not stimulate nerve endings and produce symptoms.
For a demonstration of the Epley maneuver, see https://youtu.be/jBzID5nVQjk. A computer-controlled form of the Epley maneuver has been developed and can be as effective as the manual version of this procedure.13
In 38% of patients, BPPV spontaneously resolves. The Epley maneuver can improve this rate to 64% with a single treatment, and one additional maneuver improves the success rate to 83.3%.14 If this procedure doesn’t work the first time, there may be more sediment that didn’t have enough time to settle during the procedure. Therefore, the Epley maneuver can be repeated 3 times a day, and performed on subsequent days as needed.
Labyrinthitis—inflammation of the inner ear that can cause vertigo—is suggested by an acute, non-recurrent episode of dizziness that is often preceded by an upper respiratory infection. If the external canal is extremely painful and/or develops a vesicular rash, the patient might have herpes zoster of the geniculate ganglion (Ramsay Hunt syndrome type 2).
Vertigo can have many possible causes
Vestibular migraine and Meniere’s disease. When a patient who has a history of migraines experiences symptoms of vertigo, vestibular migraine should be suspected, and treatment should focus on migraine therapy rather than vestibular therapy.15
Symptoms of Meniere’s disease and vestibular migraine can overlap.16 The current definition of Meniere’s disease requires ≥2 definitive episodes of vertigo with hearing loss plus tinnitus and/or aural symptoms.17 Thirty percent of vertigo episodes in patients with Meniere's disease can be attributed to BPPV.18
Acoustic neuroma. In addition to vertigo, acoustic neuroma is often associated with gradual hearing loss, tinnitus, and facial numbness (from compression of cranial nerve V preoperatively) or facial weakness (from compression of cranial nerve VII postoperatively). Unilateral hearing loss should prompt evaluation with magnetic resonance imaging.
“Acoustic neuroma” is a misnomer. The lesion arises from the vestibular (not the acoustic) portion of the 8th cranial nerve, and isn’t a neuroma; it is a schwannoma.19 Although it actually arises peripherally within the vestibular canal, it typically expands centrally and compresses other nerves centrally, which can make the clinical diagnosis more challenging if one were using the classical schema of differentiating between peripheral and central causes of vertigo.
Age-related vestibular loss occurs when the aging process causes deterioration of most of the components of the vestibulo-ocular reflex, resulting in dizziness and vertigo. Usually, the cerebral override mechanisms can compensate for the degeneration.
Other causes of vertigo include cerebellar infarction (3% of patients with vertigo),20 sound-induced vertigo (Tullio phenomenon),21 obstructive sleep apnea,22 and systemic sclerosis.23 Diabetes can cause a reduction in vestibular sensitivity that is evidenced by an increased reliance on visual stimuli to resolve vestibulo-visual conflict.24
Disequilibrium
Disequilibrium is predominantly a loss of balance. Patients with disequilibrium have the feeling that they are about to fall, specifically without the sensation of spinning. They may appear to sway, and will reach out for something to support them. Disequilibrium can be a component of vertigo, or it may suggest a more specific diagnosis, such as ataxia, which is a lack of coordination when walking.
Atypical causes of dizziness
“Light-headedness” may have an element of euphoria or may be indistinguishable from the early part of a syncopal episode. Because other causes of light-headedness can be difficult to distinguish from presyncope, it is important to consider syncope in the differential diagnosis.
The differential of light-headedness can also include panic attack, early hyperventilation, and toxin exposure (such as diphenylarsinic acid,25 pregabalin,26 or paint thinner27).
CORRESPONDENCE
Shannon Paul Starr, MD, Louisiana State University Health Sciences Center, 200 W. Esplanade #412, Kenner, LA 70065; [email protected].
› Refer a patient who reports that his dizziness is accompanied by hearing loss to an otolaryngologist for evaluation. C
› Use the HINTS (Head Impulse, Nystagmus, and Test of Skew) procedure to differentiate central from peripheral vertigo. A
› Use the Dix-Hallpike procedure to diagnose benign paroxysmal positional vertigo. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With an estimated lifetime prevalence of 17% to 30%,1 dizziness is a relatively common clinical symptom, but the underlying cause can be difficult to diagnose. That’s because patients’ descriptions of dizziness are often imprecise, and this symptom is associated with a wide range of conditions. A careful history and physical examination are key to diagnosis, as is an understanding of the mechanisms of dizziness.
This article covers the range of diagnoses that should be considered when a patient presents with dizziness, and provides insight regarding features of the patient’s history that can better elucidate the specific etiology.
What do patients mean when they say, “I feel dizzy”?
“Dizziness” is a vague term, and patients who report dizziness should be asked to further describe the sensation. Patients may use the word dizziness in an attempt to describe many sensations, including faintness, giddiness, light-headedness, or unsteadiness.2 In 1972, Drachman and Hart proposed a classification system for dizziness that describes 4 categories—presyncope, vertigo, disequilibrium, and atypical (TABLE 1).3 These classifications are still commonly used today, and the discussion that follows describes potential causes of dizziness in each of these 4 categories. A stepwise approach for evaluating a patient who reports dizziness can be found in the ALGORITHM.3-6
Syncopal-related dizziness can have a cardiovascular cause
Presyncope is a feeling of impending loss of consciousness that’s sometimes accompanied by generalized muscle weakness and/or partial vision loss. Taking a careful history regarding the events surrounding the episode should distinguish this type of dizziness, and doing so is essential because most of the underlying pathogenesis involves the cardiovascular system and requires specific interventions.
Dysrhythmias can cause syncope and may or may not be accompanied by a feeling of palpitations. Diagnosis is made by electrocardiogram (EKG) followed by the use of a Holter monitor.
Vasovagal syncope is caused by a sudden slowing of the pulse that’s the result of stimulation of the vagal nerve. It can occur from direct stimulation of the nerve from palpation (or strangulation), or from an intense autonomic discharge, as when people are frightened or confronted with something upsetting (eg, the sight of blood.)
Orthostatic hypotension results from a change in body position in which either autonomic mechanisms cannot maintain venous tone, causing a sudden drop in blood pressure, or in which the heart cannot compensate by speeding up, as when a patient is taking a beta-adrenergic antagonist or has first-degree heart block. It can also result from hypovolemia.
Measuring the patient’s blood pressure in the recumbent, seated, and standing positions can verify the diagnosis if an episode occurred soon before the examination. This kind of dizziness can be treated by instructing the patient to rise slowly, or by making appropriate medication adjustments. If conservative measures fail, medications such as midodrine or droxidopa can be tried.7
Hypoglycemia, hypoxia, or hyperventilation can also precipitate syncopal symptoms. Taking a careful history to assess for the presence of seizure-related features such as tonic/clonic movements or loss of bowel and bladder control can be helpful in distinguishing this form of dizziness.
Vertigo can have a central or peripheral cause
Vertigo is dizziness that is characterized by the sensation of spinning. The presence of vertigo implies disease of the inner ear or central nervous system. The “wiring diagram” of the vestibulo-ocular reflex is fairly straightforward, but sorting out the symptoms that arise from lesions within the system can be a diagnostic challenge. Vertigo has classically been divided into causes that are central (originating in the central nervous system) or peripheral (originating in the peripheral nervous system).
The HINTS (Head Impulse, Nystagmus, and Test of Skew) protocol is a group of 3 tests that can be used to differentiate central from peripheral vertigo (TABLE 2).8,9 To perform the head impulse test, the examiner asks the patient to focus his gaze on a target and then rapidly turns the patient’s head to the side, watching the eyes for any corrective movements.10 When the eyes make a corrective saccade, the test is considered to be positive for a peripheral lesion.
Horizontal nystagmus is assessed by having the patient look in the direction of the fast phase of the nystagmus. If the nystagmus increases in intensity, then the test is considered positive for a peripheral lesion.
Vertigo can have many possible causes
Finally, the “test of skew” is performed by again having the patient fixate on the examiner’s nose. Each eye is tested by being covered, and then uncovered. If the uncovered eye has to move to refocus on the examiner’s nose, then the test is positive for a central lesion. A positive head impulse, positive horizontal nystagmus, and negative test of skew is 100% sensitive and 96% specific for a peripheral lesion.11
Benign paroxysmal positional vertigo (BPPV) is vertigo that is triggered by movement of the head. It occurs when otoconia that are normally embedded in gel in the utricle become dislodged and migrate into the 3 fluid-filled semicircular canals, where they interfere with the normal fluid movement these canals use to sense head motion, causing the inner ear to send false signals to the brain.12
Diagnosis is confirmed by performing the Dix-Hallpike maneuver to elicit nystagmus. The patient is moved from a seated to a supine position with her head turned 45 degrees to the right and held for 30 seconds. For a demonstration of the Dix-Hallpike maneuver, see https://youtu.be/8RYB2QlO1N4. The Dix-Hallpike maneuver is also the first step of a treatment for BBPV known as the Epley maneuver. (See “The Epley maneuver: A procedure for treating BPPV”.13,14)
Benign paroxysmal positional vertigo (BPPV) can be treated with the Epley maneuver. Like the Dix-Hallpike maneuver, the Epley maneuver isolates the posterior semicircular canal of the affected ear. However, it goes a step further to reposition otolithic debris away from the ampulla of the posterior canal, rolling it through the canal and depositing it in the utricle, where it will not stimulate nerve endings and produce symptoms.
For a demonstration of the Epley maneuver, see https://youtu.be/jBzID5nVQjk. A computer-controlled form of the Epley maneuver has been developed and can be as effective as the manual version of this procedure.13
In 38% of patients, BPPV spontaneously resolves. The Epley maneuver can improve this rate to 64% with a single treatment, and one additional maneuver improves the success rate to 83.3%.14 If this procedure doesn’t work the first time, there may be more sediment that didn’t have enough time to settle during the procedure. Therefore, the Epley maneuver can be repeated 3 times a day, and performed on subsequent days as needed.
Labyrinthitis—inflammation of the inner ear that can cause vertigo—is suggested by an acute, non-recurrent episode of dizziness that is often preceded by an upper respiratory infection. If the external canal is extremely painful and/or develops a vesicular rash, the patient might have herpes zoster of the geniculate ganglion (Ramsay Hunt syndrome type 2).
Vertigo can have many possible causes
Vestibular migraine and Meniere’s disease. When a patient who has a history of migraines experiences symptoms of vertigo, vestibular migraine should be suspected, and treatment should focus on migraine therapy rather than vestibular therapy.15
Symptoms of Meniere’s disease and vestibular migraine can overlap.16 The current definition of Meniere’s disease requires ≥2 definitive episodes of vertigo with hearing loss plus tinnitus and/or aural symptoms.17 Thirty percent of vertigo episodes in patients with Meniere's disease can be attributed to BPPV.18
Acoustic neuroma. In addition to vertigo, acoustic neuroma is often associated with gradual hearing loss, tinnitus, and facial numbness (from compression of cranial nerve V preoperatively) or facial weakness (from compression of cranial nerve VII postoperatively). Unilateral hearing loss should prompt evaluation with magnetic resonance imaging.
“Acoustic neuroma” is a misnomer. The lesion arises from the vestibular (not the acoustic) portion of the 8th cranial nerve, and isn’t a neuroma; it is a schwannoma.19 Although it actually arises peripherally within the vestibular canal, it typically expands centrally and compresses other nerves centrally, which can make the clinical diagnosis more challenging if one were using the classical schema of differentiating between peripheral and central causes of vertigo.
Age-related vestibular loss occurs when the aging process causes deterioration of most of the components of the vestibulo-ocular reflex, resulting in dizziness and vertigo. Usually, the cerebral override mechanisms can compensate for the degeneration.
Other causes of vertigo include cerebellar infarction (3% of patients with vertigo),20 sound-induced vertigo (Tullio phenomenon),21 obstructive sleep apnea,22 and systemic sclerosis.23 Diabetes can cause a reduction in vestibular sensitivity that is evidenced by an increased reliance on visual stimuli to resolve vestibulo-visual conflict.24
Disequilibrium
Disequilibrium is predominantly a loss of balance. Patients with disequilibrium have the feeling that they are about to fall, specifically without the sensation of spinning. They may appear to sway, and will reach out for something to support them. Disequilibrium can be a component of vertigo, or it may suggest a more specific diagnosis, such as ataxia, which is a lack of coordination when walking.
Atypical causes of dizziness
“Light-headedness” may have an element of euphoria or may be indistinguishable from the early part of a syncopal episode. Because other causes of light-headedness can be difficult to distinguish from presyncope, it is important to consider syncope in the differential diagnosis.
The differential of light-headedness can also include panic attack, early hyperventilation, and toxin exposure (such as diphenylarsinic acid,25 pregabalin,26 or paint thinner27).
CORRESPONDENCE
Shannon Paul Starr, MD, Louisiana State University Health Sciences Center, 200 W. Esplanade #412, Kenner, LA 70065; [email protected].
1. Murdin L, Schilder AG. Epidemiology of balance symptoms and disorders in the community: a systematic review. Otol Neurotol. 2015;36:387-392.
2. Stedman TL. Stedman’s medical dictionary, illustrated. 24th ed. Baltimore, Md: William & Wilkins; 1982:419.
3. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology. 1972;22:323-334.
4. Angtuaco EJ, Wippold FJ II, Cornelius RS, et al; Expert Panel on Neurologic Imaging. ACR appropriateness criteria: hearing loss and/or vertigo. 2013. American College of Radiology Web site. Available at: http://www.acr.org/~/media/914834f9cfa74e6c803e8e9c6909cd7e.pdf. Accessed September 3, 2015.
5. Dros J, Maarsingh OR, van der Windt DA, et al. Profiling dizziness in older primary care patients: an empirical study. PLoS One. 2011;6:e16481.
6. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-369.
7. Biaggioni I. New developments in the management of neurogenic orthostatic hypotension. Curr Cardiol Rep. 2014;16:542.
8. Batuecas-Caletrío Á, Yáñez-González R, Sánchez-Blanco C, et al. [Peripheral vertigo versus central vertigo. Application of the HINTS protocol]. Rev Neurol. 2014;59:349-353.
9. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504-3510.
10. Barraclough K, Bronstein A. Vertigo. BMJ. 2009;339:b3493.
11. Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. 2013;20:986-996.
12. Vestibular Disorders Association. Benign Paroxysmal Positional Vertigo. Vestibular Disorders Association Web site. Available at: http://vestibular.org/understanding-vestibular-disorders/types-vestibular-disorders/benign-paroxysmal-positional-vertigo. Accessed September 1, 2015.
13. Shan X, Peng X, Wang E. Efficacy of computer-controlled repositioning procedure for benign paroxysmal positional vertigo. Laryngoscope. 2015;125:715-719.
14. Lee JD, Shim DB, Park HJ, et al. A multicenter randomized double-blind study: comparison of the Epley, Semont, and sham maneuvers for the treatment of posterior canal benign paroxysmal positional vertigo. Audiol Neurootol. 2014;19:336-341.
15. Stolte B, Holle D, Naegel S, et al. Vestibular migraine. Cephalalgia. 2015;35:262-270.
16. Lopez-Escamez JA, Dlugaiczyk J, Jacobs J, et al. Accompanying symptoms overlap during attacks in Menière’s disease and vestibular migraine. Front Neurol. 2014;5:265.
17. Beasley NJ, Jones NS. Menière’s disease: evolution of a definition. J Laryngol Otol. 1996;110:1107-1113.
18. Taura A, Funabiki K, Ohgita H, et al. One-third of vertiginous episodes during the follow-up period are caused by benign paroxysmal positional vertigo in patients with Meniere’s disease. Acta Otolaryngol. 2014;134:1140-1145.
19. Pineda A, Feder BH. Acoustic neuroma: a misnomer. Is Surg. 1967;33:40-43.
20. Seemungal BM. Neuro-otological emergencies. Curr Opin Neurol. 2007;20:32-39.
21. Harrison RV. On the biological plausibility of Wind Turbine Syndrome. Int J Environ Health Res. 2015;25:463-468.
22. Kayabasi S, Iriz A, Cayonu M, et al. Vestibular functions were found to be impaired in patients with moderate-tosevere obstructive sleep apnea. Laryngoscope. 2015;125:1244-1248.
23. Rabelo MB, Corona AP. Auditory and vestibular dysfunctions in systemic sclerosis: literature review. Codas. 2014;26:337-342.
24. Razzak RA, Bagust J, Docherty S, et al. Augmented asymmetrical visual field dependence in asymptomatic diabetics: evidence of subclinical asymmetrical bilateral vestibular dysfunction. J Diabetes Complications. 2015;29:68-72.
25. Ogata T, Nakamura Y, Endo G, et al. [Subjective symptoms and miscarriage after drinking well water exposed to diphenylarsinic acid]. Nihon Koshu Eisei Zasshi. 2014;61:556-564.
26. Qu C, Xie Y, Qin F, et al. Neuropsychiatric symptoms accompanying thrombocytopenia following pregabalin treatment for neuralgia: a case report. Int J Clin Pharm. 2014;36:1138-1140.
27. Rahimi HR, Agin K, Shadnia S, et al. Clinical and biochemical analysis of acute paint thinner intoxication in adults: a retrospective descriptive study. Toxicol Mech Methods. 2015;25:42-47.
1. Murdin L, Schilder AG. Epidemiology of balance symptoms and disorders in the community: a systematic review. Otol Neurotol. 2015;36:387-392.
2. Stedman TL. Stedman’s medical dictionary, illustrated. 24th ed. Baltimore, Md: William & Wilkins; 1982:419.
3. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology. 1972;22:323-334.
4. Angtuaco EJ, Wippold FJ II, Cornelius RS, et al; Expert Panel on Neurologic Imaging. ACR appropriateness criteria: hearing loss and/or vertigo. 2013. American College of Radiology Web site. Available at: http://www.acr.org/~/media/914834f9cfa74e6c803e8e9c6909cd7e.pdf. Accessed September 3, 2015.
5. Dros J, Maarsingh OR, van der Windt DA, et al. Profiling dizziness in older primary care patients: an empirical study. PLoS One. 2011;6:e16481.
6. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-369.
7. Biaggioni I. New developments in the management of neurogenic orthostatic hypotension. Curr Cardiol Rep. 2014;16:542.
8. Batuecas-Caletrío Á, Yáñez-González R, Sánchez-Blanco C, et al. [Peripheral vertigo versus central vertigo. Application of the HINTS protocol]. Rev Neurol. 2014;59:349-353.
9. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504-3510.
10. Barraclough K, Bronstein A. Vertigo. BMJ. 2009;339:b3493.
11. Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. 2013;20:986-996.
12. Vestibular Disorders Association. Benign Paroxysmal Positional Vertigo. Vestibular Disorders Association Web site. Available at: http://vestibular.org/understanding-vestibular-disorders/types-vestibular-disorders/benign-paroxysmal-positional-vertigo. Accessed September 1, 2015.
13. Shan X, Peng X, Wang E. Efficacy of computer-controlled repositioning procedure for benign paroxysmal positional vertigo. Laryngoscope. 2015;125:715-719.
14. Lee JD, Shim DB, Park HJ, et al. A multicenter randomized double-blind study: comparison of the Epley, Semont, and sham maneuvers for the treatment of posterior canal benign paroxysmal positional vertigo. Audiol Neurootol. 2014;19:336-341.
15. Stolte B, Holle D, Naegel S, et al. Vestibular migraine. Cephalalgia. 2015;35:262-270.
16. Lopez-Escamez JA, Dlugaiczyk J, Jacobs J, et al. Accompanying symptoms overlap during attacks in Menière’s disease and vestibular migraine. Front Neurol. 2014;5:265.
17. Beasley NJ, Jones NS. Menière’s disease: evolution of a definition. J Laryngol Otol. 1996;110:1107-1113.
18. Taura A, Funabiki K, Ohgita H, et al. One-third of vertiginous episodes during the follow-up period are caused by benign paroxysmal positional vertigo in patients with Meniere’s disease. Acta Otolaryngol. 2014;134:1140-1145.
19. Pineda A, Feder BH. Acoustic neuroma: a misnomer. Is Surg. 1967;33:40-43.
20. Seemungal BM. Neuro-otological emergencies. Curr Opin Neurol. 2007;20:32-39.
21. Harrison RV. On the biological plausibility of Wind Turbine Syndrome. Int J Environ Health Res. 2015;25:463-468.
22. Kayabasi S, Iriz A, Cayonu M, et al. Vestibular functions were found to be impaired in patients with moderate-tosevere obstructive sleep apnea. Laryngoscope. 2015;125:1244-1248.
23. Rabelo MB, Corona AP. Auditory and vestibular dysfunctions in systemic sclerosis: literature review. Codas. 2014;26:337-342.
24. Razzak RA, Bagust J, Docherty S, et al. Augmented asymmetrical visual field dependence in asymptomatic diabetics: evidence of subclinical asymmetrical bilateral vestibular dysfunction. J Diabetes Complications. 2015;29:68-72.
25. Ogata T, Nakamura Y, Endo G, et al. [Subjective symptoms and miscarriage after drinking well water exposed to diphenylarsinic acid]. Nihon Koshu Eisei Zasshi. 2014;61:556-564.
26. Qu C, Xie Y, Qin F, et al. Neuropsychiatric symptoms accompanying thrombocytopenia following pregabalin treatment for neuralgia: a case report. Int J Clin Pharm. 2014;36:1138-1140.
27. Rahimi HR, Agin K, Shadnia S, et al. Clinical and biochemical analysis of acute paint thinner intoxication in adults: a retrospective descriptive study. Toxicol Mech Methods. 2015;25:42-47.
Latest Clinical Guidelines
IN THIS ARTICLE
• ACP: Telemedicine in Primary Care Settings
• AAP: Binge Drinking Among Adolescents
• Management of Primary Immunodeficiency
• Atopic Dermatitis in Children
• Obesity Treatment in Primary Care
• Management of Chronic Kidney Disease
ACP: TELEMEDICINE IN PRIMARY CARE SETTINGS
Daniel H, Sulmasy, LS. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med. [Epub ahead of print September 8, 2015]. doi:10.7326/M15-0498.
The American College of Physicians (ACP) has issued policy recommendations to guide the use of telemedicine in primary care settings, along with clinician considerations for those who use telemedicine and policy recommendations on the practice and reimbursement of telemedicine. ACP’s position is that telemedicine can potentially be a beneficial and important part of the future of health care delivery; however, it also stresses the importance of balancing the benefits of telemedicine against the potential risks for patients. Among the ACP position statements and recommendations are:
• ACP believes that a valid patient-provider relationship must be established for a professionally responsible telemedicine service to take place.
• ACP recommends the telehealth activities address the needs of all patients without disenfranchising financially disadvantaged populations or those with low literacy or low technologic literacy.
ACP believes that clinicians should use their professional judgment about whether the use of telemedicine is appropriate for a patient.
COMMENTARY
The issue of professional judgment about when it is sufficient to see a patient using a digital interface will ultimately determine the safety and effectiveness of telemedicine. It is a mode of health care delivery that was nonexistent just a few years ago and now has an estimated annual growth rate of 20% per year, with an expected 7 million visits per year by 2018. The potential advantages include health savings, convenience, and the potential to deliver specialized services to people who might otherwise not have access to them. In addition, the use of telemedicine as a part of case-management and patient follow-up has shown promise. In this era of ever-changing technologies, we need to embrace new modes of care with skeptical open arms and be honest about the potential benefits as well as the risks.
Continue for AAP: Binge drinking among adolescents >>
AAP: BINGE DRINKING AMONG ADOLESCENTS
Siqueira L, Smith VC; Committee on Substance Abuse. Binge drinking (clinical report). Pediatrics. 2015;136(3):e718-e726. doi: 10.1542/peds.2015-2337.
A clinical report released by the American Academy of Pediatrics (AAP) details alcohol abuse by children and adolescents in the United States and offers guidance and recommendations to combat this high-risk behavior. The report states that among youth who drink, the proportion that drinks heavily is higher than among adult drinkers.
Among those who drink, binge drinking increases from approximately 50% in those ages 12 to 14 to 72% among those ages 18 to 20. Alcohol use is also associated with the leading causes of death and serious injury in this age-group, including motor vehicle accidents, homicides, and suicides. Recommendations offered in the report include
• In the office setting, provide programs designed to deliver messages about binge-drinking prevention to parents.
• Ask adolescents about alcohol use during office visits.
• Encourage schools to adopt preventive measures, including school-based health education programs.
COMMENTARY
Binge drinking in adults is defined as consumption of five or more alcoholic drinks in a two-hour period for men and four or more drinks for women. The number of drinks that qualifies as binge drinking in teenagers is slightly less and varies by age.
Using a 30-day time period, 14% of adolescents (1 out of 7) reported binge drinking. When teenagers drink alcohol, they tend to binge drink. Of students who consume alcohol, two-thirds report binge drinking, and 10% report having drunk 10 or more drinks in a row.
It is important to address this problem with parents and youth beginning at about age 9, as the change in attitudes toward drinking appears to begin between ages 9 and 12. It is also important to remind parents, supported by good evidence, that the message they send to their children about alcohol is the most important influence on teenage and young adult decisions about drinking.1
1. Turrisi R, Mallett KA, Cleveland MJ, et al. Evaluation of timing and dosage of a parent-based intervention to minimize college students’ alcohol consumption. J Stud Alcohol Drugs. 2013;74(1):30-40.
Continue for management of primary immunodeficiency >>
MANAGEMENT OF PRIMARY IMMUNODEFICIENCY
Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. [Epub ahead of print September 11, 2015]. doi: 10.1016/j.jaci.2015.04.049.
This practice parameter is intended to provide practical guidance on the clinical recognition and diagnosis of primary immunodeficiency (PIDD), along with general principles on management of these disorders. Highlights include
• PIDD has a prevalence of 1:2,000 children.
• PIDD is subdivided into humoral or antibody deficiencies and combined immunodeficiency.
• Initial evaluation is guided by the clinical presentation, and screening tests are applied and followed by advanced tests, ensuring efficient and thorough evaluation of mechanisms of immune dysfunction that underlie the clinical presentation.
• Diagnosis and therapy should be guided overall or performed in consultation with persons and centers with knowledge and experience diagnosing and treating a broad range of immunodeficiencies.
COMMENTARY
Clinicians should be aware of PIDD in order to refer appropriate patients on to an allergist/immunologist for further evaluation. While many different types of PIDD exist, they generally present with recurrent or severe infections or infections by unusual organisms. For example, approximately a quarter of patients older than 2 with invasive pneumococcal disease have an identifiable PIDD. This is a group of disorders that, while rare, is helpful to be aware of and to refer on for further evaluation when indicated.
Continue for atopic dermatitis in children >>
ATOPIC DERMATITIS IN CHILDREN
Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. [Epub ahead of print August 3, 2015]. doi: 10.1542/peds.2014-3678.
Treatment guidelines for atopic dermatitis (AD) in children, designed specifically for use by pediatricians and other primary care providers, include basic management such as skin care, antiseptic measures, and trigger avoidance, to be used regardless of AD severity, according to a roundtable discussion to address challenges in AD management. Recommendations for the primary care provider include
• The diagnosis of eczema is a clinical one, based on a chronic or relapsing course of a pruritic dermatitis consisting of erythematous papules or patches of scaling and/or excoriated skin.
• Basic management is important and should include skin hydration with an appropriate moisturizer, use of diluted bleach baths, trigger avoidance, and acute treatment for flares.
• Treatment of acute flares is managed with topical corticosteroids, using a more potent topical steroid initially and then deescalating therapy to a less potent agent after a few days to weeks.
For patients with moderate-to-severe eczema, maintenance therapy for flare-prone areas should be applied regularly or at first sign of a flare-up. Recommended agents include tacrolimus or pimecrolimus (topical calcineurin inhibitors) or medium- or low-potency topical corticosteroids (avoiding medium-potency topical steroids on the face), depending on the severity of eczema.
COMMENTARY
Eczema affects about 12% of US children (ages 0 to 17 years), most of whom have mild disease and are well taken care of by primary care providers. This article provides clear guidance for treatment of a disease that we see quite frequently. Use of diluted bleach baths or washes is an underappreciated approach, and I suspect the clear recommendation for it, based on very good evidence, will lead to a helpful addition to many clinicians’ standard approach.
Continue for obesity treatment in primary care >>
OBESITY TREATMENT IN PRIMARY CARE
Fitzpatrick SL, Wischenka D, Appelhans BM, et al. An evidence-based guide for obesity treatment in primary care. Am J Med. [Epub ahead of print July 31, 2015]. doi: 10.1016/j.amjmed.2015.07.015.
A new evidence-based guideline from the Society of Behavioral Medicine for obesity management and treatment in primary care is based on the “5As” counseling framework (assess, advise, agree, assist, and arrange). The guide recommends building a multidisciplinary team that helps patients lose weight and maintain their weight loss by
• Addressing patients’ psychosocial issues and medical and psychiatric comorbidities associated with obesity treatment failure
• Delivering intensive counseling consisting of goal setting, self-monitoring, and problem solving
• Connecting patients with community resources to assist them in making healthy lifestyle changes.
COMMENTARY
Combating obesity is the critical health issue of the next decade. Currently, two-thirds of the adult population is either overweight or obese, and if the current trend continues, diabetes, one of the most important consequences of obesity, will develop in one out of three Americans born today. Clinicians are generally good at accomplishing the first and second of the three “As”: assessing and advising.1 The challenge for most of us in busy office practices is in assisting patients with the development of specific, concrete goals using specific, concrete behaviors, and then, when appropriate, arranging for referral to nutritionists, personal trainers, and multicomponent programs to help patients accomplish the agreed-upon goals.
1. Spring B, Ockene JK, Gidding SS, et al. Better population health through behavior change in adults. Circulation. 2013;128:2169-2176.
Continue for management of CKD >>
MANAGEMENT OF CKD
Vassalotti JA, Centor R, Turner BJ, et al; US Kidney Disease Outcomes Quality Initiative. A practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. [Epub ahead of print September 25, 2015]. doi: 10.1016/j.amjmed.2015.08.025.
This guideline was developed for the primary care provider to guide assessment and care of chronic kidney disease (CKD). Recommendations include
• Assessment of estimated glomerular filtration rate (GFR) and albuminuria should be performed for persons with diabetes and/or hypertension but is not recommended for the general population.
• Prevention of CKD progression requires blood pressure < 140/90 mm Hg, use of ACE inhibitors or angiotensin receptor blockers (ARB) for patients with albuminuria and hypertension, A1C < 7% for patients with diabetes, and correction of CKD-associated metabolic acidosis.
• Nephrotoxic drugs (eg, NSAIDs) should be avoided, and providers should be aware to use reduced doses of medications that are renally excreted, such as insulin, many antibiotics, and some statins.
The ultimate goal of CKD management is to prevent disease progression, minimize complications, and promote quality of life.
COMMENTARY
More than 10% of the US population has CKD, defined as a GFR < 60 mL/min/1.73 m2 and/or albumin-creatinine ratio > 30 mg/g. Both GFR and albuminuria independently predict progression of CKD. Control of blood pressure and A1C and use of an ACE inhibitor or an ARB are well-appreciated methods of slowing CKD progression. What is not as well appreciated is that treatment with ACE inhibitors or ARBs remains renal protective even with GFR < 30. Also important is the use of oral alkali therapy to maintain normal serum bicarbonate levels, which may slow CKD progression. When bicarbonate levels decrease to < 22 mmol/L, sodium bicarbonate (650 mg tid) should be added to raise the bicarbonate level above 22 mmol/L. For patients with severe CKD, referral to a nephrologist is appropriate.
IN THIS ARTICLE
• ACP: Telemedicine in Primary Care Settings
• AAP: Binge Drinking Among Adolescents
• Management of Primary Immunodeficiency
• Atopic Dermatitis in Children
• Obesity Treatment in Primary Care
• Management of Chronic Kidney Disease
ACP: TELEMEDICINE IN PRIMARY CARE SETTINGS
Daniel H, Sulmasy, LS. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med. [Epub ahead of print September 8, 2015]. doi:10.7326/M15-0498.
The American College of Physicians (ACP) has issued policy recommendations to guide the use of telemedicine in primary care settings, along with clinician considerations for those who use telemedicine and policy recommendations on the practice and reimbursement of telemedicine. ACP’s position is that telemedicine can potentially be a beneficial and important part of the future of health care delivery; however, it also stresses the importance of balancing the benefits of telemedicine against the potential risks for patients. Among the ACP position statements and recommendations are:
• ACP believes that a valid patient-provider relationship must be established for a professionally responsible telemedicine service to take place.
• ACP recommends the telehealth activities address the needs of all patients without disenfranchising financially disadvantaged populations or those with low literacy or low technologic literacy.
ACP believes that clinicians should use their professional judgment about whether the use of telemedicine is appropriate for a patient.
COMMENTARY
The issue of professional judgment about when it is sufficient to see a patient using a digital interface will ultimately determine the safety and effectiveness of telemedicine. It is a mode of health care delivery that was nonexistent just a few years ago and now has an estimated annual growth rate of 20% per year, with an expected 7 million visits per year by 2018. The potential advantages include health savings, convenience, and the potential to deliver specialized services to people who might otherwise not have access to them. In addition, the use of telemedicine as a part of case-management and patient follow-up has shown promise. In this era of ever-changing technologies, we need to embrace new modes of care with skeptical open arms and be honest about the potential benefits as well as the risks.
Continue for AAP: Binge drinking among adolescents >>
AAP: BINGE DRINKING AMONG ADOLESCENTS
Siqueira L, Smith VC; Committee on Substance Abuse. Binge drinking (clinical report). Pediatrics. 2015;136(3):e718-e726. doi: 10.1542/peds.2015-2337.
A clinical report released by the American Academy of Pediatrics (AAP) details alcohol abuse by children and adolescents in the United States and offers guidance and recommendations to combat this high-risk behavior. The report states that among youth who drink, the proportion that drinks heavily is higher than among adult drinkers.
Among those who drink, binge drinking increases from approximately 50% in those ages 12 to 14 to 72% among those ages 18 to 20. Alcohol use is also associated with the leading causes of death and serious injury in this age-group, including motor vehicle accidents, homicides, and suicides. Recommendations offered in the report include
• In the office setting, provide programs designed to deliver messages about binge-drinking prevention to parents.
• Ask adolescents about alcohol use during office visits.
• Encourage schools to adopt preventive measures, including school-based health education programs.
COMMENTARY
Binge drinking in adults is defined as consumption of five or more alcoholic drinks in a two-hour period for men and four or more drinks for women. The number of drinks that qualifies as binge drinking in teenagers is slightly less and varies by age.
Using a 30-day time period, 14% of adolescents (1 out of 7) reported binge drinking. When teenagers drink alcohol, they tend to binge drink. Of students who consume alcohol, two-thirds report binge drinking, and 10% report having drunk 10 or more drinks in a row.
It is important to address this problem with parents and youth beginning at about age 9, as the change in attitudes toward drinking appears to begin between ages 9 and 12. It is also important to remind parents, supported by good evidence, that the message they send to their children about alcohol is the most important influence on teenage and young adult decisions about drinking.1
1. Turrisi R, Mallett KA, Cleveland MJ, et al. Evaluation of timing and dosage of a parent-based intervention to minimize college students’ alcohol consumption. J Stud Alcohol Drugs. 2013;74(1):30-40.
Continue for management of primary immunodeficiency >>
MANAGEMENT OF PRIMARY IMMUNODEFICIENCY
Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. [Epub ahead of print September 11, 2015]. doi: 10.1016/j.jaci.2015.04.049.
This practice parameter is intended to provide practical guidance on the clinical recognition and diagnosis of primary immunodeficiency (PIDD), along with general principles on management of these disorders. Highlights include
• PIDD has a prevalence of 1:2,000 children.
• PIDD is subdivided into humoral or antibody deficiencies and combined immunodeficiency.
• Initial evaluation is guided by the clinical presentation, and screening tests are applied and followed by advanced tests, ensuring efficient and thorough evaluation of mechanisms of immune dysfunction that underlie the clinical presentation.
• Diagnosis and therapy should be guided overall or performed in consultation with persons and centers with knowledge and experience diagnosing and treating a broad range of immunodeficiencies.
COMMENTARY
Clinicians should be aware of PIDD in order to refer appropriate patients on to an allergist/immunologist for further evaluation. While many different types of PIDD exist, they generally present with recurrent or severe infections or infections by unusual organisms. For example, approximately a quarter of patients older than 2 with invasive pneumococcal disease have an identifiable PIDD. This is a group of disorders that, while rare, is helpful to be aware of and to refer on for further evaluation when indicated.
Continue for atopic dermatitis in children >>
ATOPIC DERMATITIS IN CHILDREN
Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. [Epub ahead of print August 3, 2015]. doi: 10.1542/peds.2014-3678.
Treatment guidelines for atopic dermatitis (AD) in children, designed specifically for use by pediatricians and other primary care providers, include basic management such as skin care, antiseptic measures, and trigger avoidance, to be used regardless of AD severity, according to a roundtable discussion to address challenges in AD management. Recommendations for the primary care provider include
• The diagnosis of eczema is a clinical one, based on a chronic or relapsing course of a pruritic dermatitis consisting of erythematous papules or patches of scaling and/or excoriated skin.
• Basic management is important and should include skin hydration with an appropriate moisturizer, use of diluted bleach baths, trigger avoidance, and acute treatment for flares.
• Treatment of acute flares is managed with topical corticosteroids, using a more potent topical steroid initially and then deescalating therapy to a less potent agent after a few days to weeks.
For patients with moderate-to-severe eczema, maintenance therapy for flare-prone areas should be applied regularly or at first sign of a flare-up. Recommended agents include tacrolimus or pimecrolimus (topical calcineurin inhibitors) or medium- or low-potency topical corticosteroids (avoiding medium-potency topical steroids on the face), depending on the severity of eczema.
COMMENTARY
Eczema affects about 12% of US children (ages 0 to 17 years), most of whom have mild disease and are well taken care of by primary care providers. This article provides clear guidance for treatment of a disease that we see quite frequently. Use of diluted bleach baths or washes is an underappreciated approach, and I suspect the clear recommendation for it, based on very good evidence, will lead to a helpful addition to many clinicians’ standard approach.
Continue for obesity treatment in primary care >>
OBESITY TREATMENT IN PRIMARY CARE
Fitzpatrick SL, Wischenka D, Appelhans BM, et al. An evidence-based guide for obesity treatment in primary care. Am J Med. [Epub ahead of print July 31, 2015]. doi: 10.1016/j.amjmed.2015.07.015.
A new evidence-based guideline from the Society of Behavioral Medicine for obesity management and treatment in primary care is based on the “5As” counseling framework (assess, advise, agree, assist, and arrange). The guide recommends building a multidisciplinary team that helps patients lose weight and maintain their weight loss by
• Addressing patients’ psychosocial issues and medical and psychiatric comorbidities associated with obesity treatment failure
• Delivering intensive counseling consisting of goal setting, self-monitoring, and problem solving
• Connecting patients with community resources to assist them in making healthy lifestyle changes.
COMMENTARY
Combating obesity is the critical health issue of the next decade. Currently, two-thirds of the adult population is either overweight or obese, and if the current trend continues, diabetes, one of the most important consequences of obesity, will develop in one out of three Americans born today. Clinicians are generally good at accomplishing the first and second of the three “As”: assessing and advising.1 The challenge for most of us in busy office practices is in assisting patients with the development of specific, concrete goals using specific, concrete behaviors, and then, when appropriate, arranging for referral to nutritionists, personal trainers, and multicomponent programs to help patients accomplish the agreed-upon goals.
1. Spring B, Ockene JK, Gidding SS, et al. Better population health through behavior change in adults. Circulation. 2013;128:2169-2176.
Continue for management of CKD >>
MANAGEMENT OF CKD
Vassalotti JA, Centor R, Turner BJ, et al; US Kidney Disease Outcomes Quality Initiative. A practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. [Epub ahead of print September 25, 2015]. doi: 10.1016/j.amjmed.2015.08.025.
This guideline was developed for the primary care provider to guide assessment and care of chronic kidney disease (CKD). Recommendations include
• Assessment of estimated glomerular filtration rate (GFR) and albuminuria should be performed for persons with diabetes and/or hypertension but is not recommended for the general population.
• Prevention of CKD progression requires blood pressure < 140/90 mm Hg, use of ACE inhibitors or angiotensin receptor blockers (ARB) for patients with albuminuria and hypertension, A1C < 7% for patients with diabetes, and correction of CKD-associated metabolic acidosis.
• Nephrotoxic drugs (eg, NSAIDs) should be avoided, and providers should be aware to use reduced doses of medications that are renally excreted, such as insulin, many antibiotics, and some statins.
The ultimate goal of CKD management is to prevent disease progression, minimize complications, and promote quality of life.
COMMENTARY
More than 10% of the US population has CKD, defined as a GFR < 60 mL/min/1.73 m2 and/or albumin-creatinine ratio > 30 mg/g. Both GFR and albuminuria independently predict progression of CKD. Control of blood pressure and A1C and use of an ACE inhibitor or an ARB are well-appreciated methods of slowing CKD progression. What is not as well appreciated is that treatment with ACE inhibitors or ARBs remains renal protective even with GFR < 30. Also important is the use of oral alkali therapy to maintain normal serum bicarbonate levels, which may slow CKD progression. When bicarbonate levels decrease to < 22 mmol/L, sodium bicarbonate (650 mg tid) should be added to raise the bicarbonate level above 22 mmol/L. For patients with severe CKD, referral to a nephrologist is appropriate.
IN THIS ARTICLE
• ACP: Telemedicine in Primary Care Settings
• AAP: Binge Drinking Among Adolescents
• Management of Primary Immunodeficiency
• Atopic Dermatitis in Children
• Obesity Treatment in Primary Care
• Management of Chronic Kidney Disease
ACP: TELEMEDICINE IN PRIMARY CARE SETTINGS
Daniel H, Sulmasy, LS. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med. [Epub ahead of print September 8, 2015]. doi:10.7326/M15-0498.
The American College of Physicians (ACP) has issued policy recommendations to guide the use of telemedicine in primary care settings, along with clinician considerations for those who use telemedicine and policy recommendations on the practice and reimbursement of telemedicine. ACP’s position is that telemedicine can potentially be a beneficial and important part of the future of health care delivery; however, it also stresses the importance of balancing the benefits of telemedicine against the potential risks for patients. Among the ACP position statements and recommendations are:
• ACP believes that a valid patient-provider relationship must be established for a professionally responsible telemedicine service to take place.
• ACP recommends the telehealth activities address the needs of all patients without disenfranchising financially disadvantaged populations or those with low literacy or low technologic literacy.
ACP believes that clinicians should use their professional judgment about whether the use of telemedicine is appropriate for a patient.
COMMENTARY
The issue of professional judgment about when it is sufficient to see a patient using a digital interface will ultimately determine the safety and effectiveness of telemedicine. It is a mode of health care delivery that was nonexistent just a few years ago and now has an estimated annual growth rate of 20% per year, with an expected 7 million visits per year by 2018. The potential advantages include health savings, convenience, and the potential to deliver specialized services to people who might otherwise not have access to them. In addition, the use of telemedicine as a part of case-management and patient follow-up has shown promise. In this era of ever-changing technologies, we need to embrace new modes of care with skeptical open arms and be honest about the potential benefits as well as the risks.
Continue for AAP: Binge drinking among adolescents >>
AAP: BINGE DRINKING AMONG ADOLESCENTS
Siqueira L, Smith VC; Committee on Substance Abuse. Binge drinking (clinical report). Pediatrics. 2015;136(3):e718-e726. doi: 10.1542/peds.2015-2337.
A clinical report released by the American Academy of Pediatrics (AAP) details alcohol abuse by children and adolescents in the United States and offers guidance and recommendations to combat this high-risk behavior. The report states that among youth who drink, the proportion that drinks heavily is higher than among adult drinkers.
Among those who drink, binge drinking increases from approximately 50% in those ages 12 to 14 to 72% among those ages 18 to 20. Alcohol use is also associated with the leading causes of death and serious injury in this age-group, including motor vehicle accidents, homicides, and suicides. Recommendations offered in the report include
• In the office setting, provide programs designed to deliver messages about binge-drinking prevention to parents.
• Ask adolescents about alcohol use during office visits.
• Encourage schools to adopt preventive measures, including school-based health education programs.
COMMENTARY
Binge drinking in adults is defined as consumption of five or more alcoholic drinks in a two-hour period for men and four or more drinks for women. The number of drinks that qualifies as binge drinking in teenagers is slightly less and varies by age.
Using a 30-day time period, 14% of adolescents (1 out of 7) reported binge drinking. When teenagers drink alcohol, they tend to binge drink. Of students who consume alcohol, two-thirds report binge drinking, and 10% report having drunk 10 or more drinks in a row.
It is important to address this problem with parents and youth beginning at about age 9, as the change in attitudes toward drinking appears to begin between ages 9 and 12. It is also important to remind parents, supported by good evidence, that the message they send to their children about alcohol is the most important influence on teenage and young adult decisions about drinking.1
1. Turrisi R, Mallett KA, Cleveland MJ, et al. Evaluation of timing and dosage of a parent-based intervention to minimize college students’ alcohol consumption. J Stud Alcohol Drugs. 2013;74(1):30-40.
Continue for management of primary immunodeficiency >>
MANAGEMENT OF PRIMARY IMMUNODEFICIENCY
Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. [Epub ahead of print September 11, 2015]. doi: 10.1016/j.jaci.2015.04.049.
This practice parameter is intended to provide practical guidance on the clinical recognition and diagnosis of primary immunodeficiency (PIDD), along with general principles on management of these disorders. Highlights include
• PIDD has a prevalence of 1:2,000 children.
• PIDD is subdivided into humoral or antibody deficiencies and combined immunodeficiency.
• Initial evaluation is guided by the clinical presentation, and screening tests are applied and followed by advanced tests, ensuring efficient and thorough evaluation of mechanisms of immune dysfunction that underlie the clinical presentation.
• Diagnosis and therapy should be guided overall or performed in consultation with persons and centers with knowledge and experience diagnosing and treating a broad range of immunodeficiencies.
COMMENTARY
Clinicians should be aware of PIDD in order to refer appropriate patients on to an allergist/immunologist for further evaluation. While many different types of PIDD exist, they generally present with recurrent or severe infections or infections by unusual organisms. For example, approximately a quarter of patients older than 2 with invasive pneumococcal disease have an identifiable PIDD. This is a group of disorders that, while rare, is helpful to be aware of and to refer on for further evaluation when indicated.
Continue for atopic dermatitis in children >>
ATOPIC DERMATITIS IN CHILDREN
Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. [Epub ahead of print August 3, 2015]. doi: 10.1542/peds.2014-3678.
Treatment guidelines for atopic dermatitis (AD) in children, designed specifically for use by pediatricians and other primary care providers, include basic management such as skin care, antiseptic measures, and trigger avoidance, to be used regardless of AD severity, according to a roundtable discussion to address challenges in AD management. Recommendations for the primary care provider include
• The diagnosis of eczema is a clinical one, based on a chronic or relapsing course of a pruritic dermatitis consisting of erythematous papules or patches of scaling and/or excoriated skin.
• Basic management is important and should include skin hydration with an appropriate moisturizer, use of diluted bleach baths, trigger avoidance, and acute treatment for flares.
• Treatment of acute flares is managed with topical corticosteroids, using a more potent topical steroid initially and then deescalating therapy to a less potent agent after a few days to weeks.
For patients with moderate-to-severe eczema, maintenance therapy for flare-prone areas should be applied regularly or at first sign of a flare-up. Recommended agents include tacrolimus or pimecrolimus (topical calcineurin inhibitors) or medium- or low-potency topical corticosteroids (avoiding medium-potency topical steroids on the face), depending on the severity of eczema.
COMMENTARY
Eczema affects about 12% of US children (ages 0 to 17 years), most of whom have mild disease and are well taken care of by primary care providers. This article provides clear guidance for treatment of a disease that we see quite frequently. Use of diluted bleach baths or washes is an underappreciated approach, and I suspect the clear recommendation for it, based on very good evidence, will lead to a helpful addition to many clinicians’ standard approach.
Continue for obesity treatment in primary care >>
OBESITY TREATMENT IN PRIMARY CARE
Fitzpatrick SL, Wischenka D, Appelhans BM, et al. An evidence-based guide for obesity treatment in primary care. Am J Med. [Epub ahead of print July 31, 2015]. doi: 10.1016/j.amjmed.2015.07.015.
A new evidence-based guideline from the Society of Behavioral Medicine for obesity management and treatment in primary care is based on the “5As” counseling framework (assess, advise, agree, assist, and arrange). The guide recommends building a multidisciplinary team that helps patients lose weight and maintain their weight loss by
• Addressing patients’ psychosocial issues and medical and psychiatric comorbidities associated with obesity treatment failure
• Delivering intensive counseling consisting of goal setting, self-monitoring, and problem solving
• Connecting patients with community resources to assist them in making healthy lifestyle changes.
COMMENTARY
Combating obesity is the critical health issue of the next decade. Currently, two-thirds of the adult population is either overweight or obese, and if the current trend continues, diabetes, one of the most important consequences of obesity, will develop in one out of three Americans born today. Clinicians are generally good at accomplishing the first and second of the three “As”: assessing and advising.1 The challenge for most of us in busy office practices is in assisting patients with the development of specific, concrete goals using specific, concrete behaviors, and then, when appropriate, arranging for referral to nutritionists, personal trainers, and multicomponent programs to help patients accomplish the agreed-upon goals.
1. Spring B, Ockene JK, Gidding SS, et al. Better population health through behavior change in adults. Circulation. 2013;128:2169-2176.
Continue for management of CKD >>
MANAGEMENT OF CKD
Vassalotti JA, Centor R, Turner BJ, et al; US Kidney Disease Outcomes Quality Initiative. A practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. [Epub ahead of print September 25, 2015]. doi: 10.1016/j.amjmed.2015.08.025.
This guideline was developed for the primary care provider to guide assessment and care of chronic kidney disease (CKD). Recommendations include
• Assessment of estimated glomerular filtration rate (GFR) and albuminuria should be performed for persons with diabetes and/or hypertension but is not recommended for the general population.
• Prevention of CKD progression requires blood pressure < 140/90 mm Hg, use of ACE inhibitors or angiotensin receptor blockers (ARB) for patients with albuminuria and hypertension, A1C < 7% for patients with diabetes, and correction of CKD-associated metabolic acidosis.
• Nephrotoxic drugs (eg, NSAIDs) should be avoided, and providers should be aware to use reduced doses of medications that are renally excreted, such as insulin, many antibiotics, and some statins.
The ultimate goal of CKD management is to prevent disease progression, minimize complications, and promote quality of life.
COMMENTARY
More than 10% of the US population has CKD, defined as a GFR < 60 mL/min/1.73 m2 and/or albumin-creatinine ratio > 30 mg/g. Both GFR and albuminuria independently predict progression of CKD. Control of blood pressure and A1C and use of an ACE inhibitor or an ARB are well-appreciated methods of slowing CKD progression. What is not as well appreciated is that treatment with ACE inhibitors or ARBs remains renal protective even with GFR < 30. Also important is the use of oral alkali therapy to maintain normal serum bicarbonate levels, which may slow CKD progression. When bicarbonate levels decrease to < 22 mmol/L, sodium bicarbonate (650 mg tid) should be added to raise the bicarbonate level above 22 mmol/L. For patients with severe CKD, referral to a nephrologist is appropriate.