User login
Annual PSA screening important for Black men
, new data suggest.
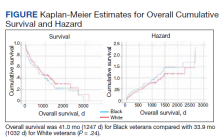
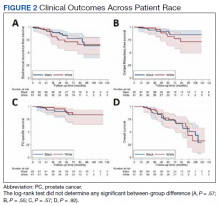
The data come from a review of 45,834 veterans (aged 55-69 years) who had been diagnosed with prostate cancer. About one-third of these men self-identified as non-Hispanic Black, and the rest were White.
During the study period (2004-2017), 2,465 men (5.4%) died of the disease.
The review found that annual prostate-specific antigen (PSA) screening significantly reduced the risk of dying from prostate cancer among Black men but not White men.
The study was published online in JAMA Oncology.
“These results may be biologically plausible because a shorter screening interval may be valuable for detecting aggressive disease, which is more common in Black men,” say investigators, led by University of California, San Diego, radiation oncology resident Michael Sherer, MD.
“Given that Black men are younger at diagnosis and have worse prostate cancer survival compared with White men,” more intensive screening recommendations “may benefit Black patients,” they write.
The study “conclusions are reasonable,” said Christopher Wallis, MD, PhD, a urologic oncologist at Mount Sinai Hospital in Toronto, when asked for comment.
Annual screening may well have “a greater potential to benefit” Black men, he said. “While we would ideally see randomized data supporting this, those data are unlikely to ever be forthcoming. Thus, this study provides a strong rationale to support the recommendations from many guideline panels (including those from the American Urological Association) that Black men, in the context of shared decision-making, may benefit more from PSA-based prostate cancer screening than the population at large,” he added.
Overall, the findings could help inform screening discussions with Black men, the investigators comments. In its most recent guidance, the U.S. Preventive Services Task Force recommends shared decision-making regarding PSA screening for men aged 55-69 years.
Similar screening frequency
For their study, the team reviewed Veterans Health Administration data to assess PSA screening patterns – which they categorized as no screening, less than annual screening, or annual screening – in the 5 years leading up to diagnosis.
They then correlated screening behaviors with the subsequent risk of dying from prostate cancer.
Overall, the reduction in risk of prostate cancer–specific mortality (PCSM) associated with screening was similar among Black men (subdistribution hazard ratio, 0.56; P = .001) and White men (sHR, 0.58; P = .001).
However, on multivariable regression, annual screening, in comparison with some screening, was associated with a significant reduction in the risk of dying from prostate cancer only among Black men (sHR, 0.65; P = .02), not among White men (sHR, 0.91; P = .35).
The cumulative incidence of PCSM among Black men was 4.7% with annual screening but 7.3% with only some screening.
Among White men, the cumulative incidence of PCSM with annual screening was 5.9% vs. 6.9% with less than annual screening.
Screening frequency was similar between Black men and White men. Black men were younger on average (61.8 vs. 63.1 years) and had slightly higher PSA levels at diagnosis but were not more likely to have regional or metastatic disease.
No funding was reported for this study. The investigators have disclosed no relevant financial relationships. Dr. Wallis has received personal fees from Janssen Canada.
A version of this article first appeared on Medscape.com.
, new data suggest.
The data come from a review of 45,834 veterans (aged 55-69 years) who had been diagnosed with prostate cancer. About one-third of these men self-identified as non-Hispanic Black, and the rest were White.
During the study period (2004-2017), 2,465 men (5.4%) died of the disease.
The review found that annual prostate-specific antigen (PSA) screening significantly reduced the risk of dying from prostate cancer among Black men but not White men.
The study was published online in JAMA Oncology.
“These results may be biologically plausible because a shorter screening interval may be valuable for detecting aggressive disease, which is more common in Black men,” say investigators, led by University of California, San Diego, radiation oncology resident Michael Sherer, MD.
“Given that Black men are younger at diagnosis and have worse prostate cancer survival compared with White men,” more intensive screening recommendations “may benefit Black patients,” they write.
The study “conclusions are reasonable,” said Christopher Wallis, MD, PhD, a urologic oncologist at Mount Sinai Hospital in Toronto, when asked for comment.
Annual screening may well have “a greater potential to benefit” Black men, he said. “While we would ideally see randomized data supporting this, those data are unlikely to ever be forthcoming. Thus, this study provides a strong rationale to support the recommendations from many guideline panels (including those from the American Urological Association) that Black men, in the context of shared decision-making, may benefit more from PSA-based prostate cancer screening than the population at large,” he added.
Overall, the findings could help inform screening discussions with Black men, the investigators comments. In its most recent guidance, the U.S. Preventive Services Task Force recommends shared decision-making regarding PSA screening for men aged 55-69 years.
Similar screening frequency
For their study, the team reviewed Veterans Health Administration data to assess PSA screening patterns – which they categorized as no screening, less than annual screening, or annual screening – in the 5 years leading up to diagnosis.
They then correlated screening behaviors with the subsequent risk of dying from prostate cancer.
Overall, the reduction in risk of prostate cancer–specific mortality (PCSM) associated with screening was similar among Black men (subdistribution hazard ratio, 0.56; P = .001) and White men (sHR, 0.58; P = .001).
However, on multivariable regression, annual screening, in comparison with some screening, was associated with a significant reduction in the risk of dying from prostate cancer only among Black men (sHR, 0.65; P = .02), not among White men (sHR, 0.91; P = .35).
The cumulative incidence of PCSM among Black men was 4.7% with annual screening but 7.3% with only some screening.
Among White men, the cumulative incidence of PCSM with annual screening was 5.9% vs. 6.9% with less than annual screening.
Screening frequency was similar between Black men and White men. Black men were younger on average (61.8 vs. 63.1 years) and had slightly higher PSA levels at diagnosis but were not more likely to have regional or metastatic disease.
No funding was reported for this study. The investigators have disclosed no relevant financial relationships. Dr. Wallis has received personal fees from Janssen Canada.
A version of this article first appeared on Medscape.com.
, new data suggest.
The data come from a review of 45,834 veterans (aged 55-69 years) who had been diagnosed with prostate cancer. About one-third of these men self-identified as non-Hispanic Black, and the rest were White.
During the study period (2004-2017), 2,465 men (5.4%) died of the disease.
The review found that annual prostate-specific antigen (PSA) screening significantly reduced the risk of dying from prostate cancer among Black men but not White men.
The study was published online in JAMA Oncology.
“These results may be biologically plausible because a shorter screening interval may be valuable for detecting aggressive disease, which is more common in Black men,” say investigators, led by University of California, San Diego, radiation oncology resident Michael Sherer, MD.
“Given that Black men are younger at diagnosis and have worse prostate cancer survival compared with White men,” more intensive screening recommendations “may benefit Black patients,” they write.
The study “conclusions are reasonable,” said Christopher Wallis, MD, PhD, a urologic oncologist at Mount Sinai Hospital in Toronto, when asked for comment.
Annual screening may well have “a greater potential to benefit” Black men, he said. “While we would ideally see randomized data supporting this, those data are unlikely to ever be forthcoming. Thus, this study provides a strong rationale to support the recommendations from many guideline panels (including those from the American Urological Association) that Black men, in the context of shared decision-making, may benefit more from PSA-based prostate cancer screening than the population at large,” he added.
Overall, the findings could help inform screening discussions with Black men, the investigators comments. In its most recent guidance, the U.S. Preventive Services Task Force recommends shared decision-making regarding PSA screening for men aged 55-69 years.
Similar screening frequency
For their study, the team reviewed Veterans Health Administration data to assess PSA screening patterns – which they categorized as no screening, less than annual screening, or annual screening – in the 5 years leading up to diagnosis.
They then correlated screening behaviors with the subsequent risk of dying from prostate cancer.
Overall, the reduction in risk of prostate cancer–specific mortality (PCSM) associated with screening was similar among Black men (subdistribution hazard ratio, 0.56; P = .001) and White men (sHR, 0.58; P = .001).
However, on multivariable regression, annual screening, in comparison with some screening, was associated with a significant reduction in the risk of dying from prostate cancer only among Black men (sHR, 0.65; P = .02), not among White men (sHR, 0.91; P = .35).
The cumulative incidence of PCSM among Black men was 4.7% with annual screening but 7.3% with only some screening.
Among White men, the cumulative incidence of PCSM with annual screening was 5.9% vs. 6.9% with less than annual screening.
Screening frequency was similar between Black men and White men. Black men were younger on average (61.8 vs. 63.1 years) and had slightly higher PSA levels at diagnosis but were not more likely to have regional or metastatic disease.
No funding was reported for this study. The investigators have disclosed no relevant financial relationships. Dr. Wallis has received personal fees from Janssen Canada.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
‘Obesity paradox’ in AFib challenged as mortality climbs with BMI
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.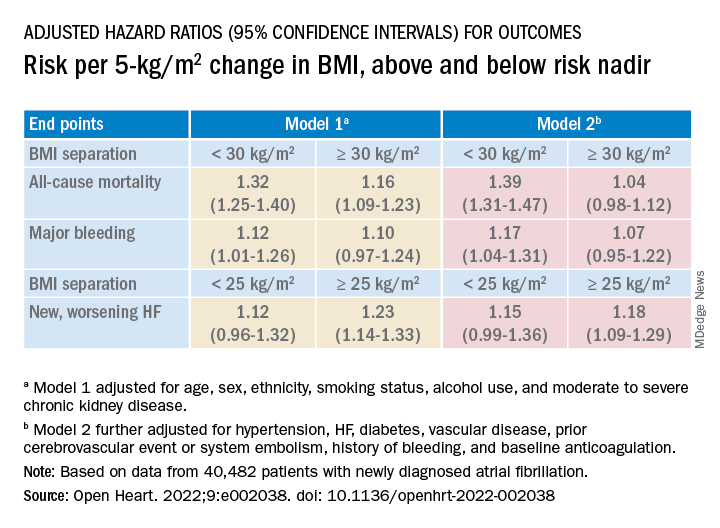
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
FROM OPEN HEART
Safety Profile of Mutant EGFR-TK Inhibitors in Advanced Non–Small Cell Lung Cancer: A Meta-analysis
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
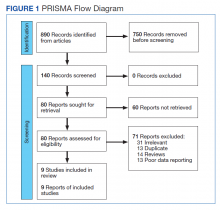
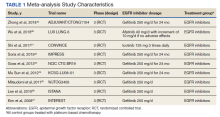
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
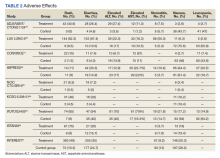
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
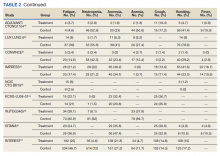
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Obesity drug shortage triggers frustrations, workarounds
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The Effect of Race on Outcomes in Veterans With Hepatocellular Carcinoma at a Single Center
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
Hepatocellular carcinoma (HCC) is the sixth most common and third most deadly malignancy worldwide, carrying a mean survival rate without treatment of 6 to 20 months depending on stage.1 Fifty-seven percent of patients with liver cancer are diagnosed with regional or distant metastatic disease that carries 5-year relative survival rates of 10.7% and 3.1%, respectively.2 HCC arises most commonly from liver cirrhosis due to chronic hepatocyte injury, which may be mediated by viral hepatitis, alcoholism, and metabolic disease. Other less common causes include autoimmune disease, exposure to environmental hazards, and certain genetic diseases, such as α-1 antitrypsin deficiency and Wilson disease.
Multiple staging systems for HCC exist that incorporate some variation of the following features: size and invasion of the tumor, distant metastases, and liver function. Stage-directed treatments for HCC include ablation, embolization, resection, transplant, and systemic therapy, such as tyrosine kinase inhibitors, immunotherapies, and monoclonal antibodies. In addition to tumor/node/metastasis (TNM) staging, α-fetoprotein (AFP) is a diagnostic marker with prognostic value in HCC with higher levels correlating to higher tumor burden and a worse prognosis. With treatment, the 5-year survival rate for early stage HCC ranges from 60% to 80% but decreases significantly with higher stages.1 HCC screening in at-risk populations has accounted for > 40% of diagnoses since the practice became widely adopted, and earlier recognition has led to an improvement in survival even when adjusting for lead time bias.3
Systemic therapy for advanced disease continues to improve. Sorafenib remained the standard first-line systemic therapy since it was introduced in 2008.4 First-line therapy improved with immunotherapies. The phase 3 IMBrave150 trial comparing atezolizumab plus bevacizumab to sorafenib showed a median overall survival (OS) > 19 months with 7.7% of patients achieving a complete response.5 HIMALAYA, another phase 3 trial set for publication later this year, also reported promising results when a priming dose of the CTLA-4 inhibitor tremelimumab followed by durvalumab was compared with sorafenib.6
There has been a rise in incidence of HCC in the United States across all races and ethnicities, though Black, Hispanic, and Asian patients remain disproportionately affected. Subsequently, identifying causative biologic, socioeconomic, and cultural factors, as well as implicit bias in health care continues to be a topic of great interest.7-9 Using Surveillance, Epidemiology, and End Results (SEER) data, a number of large studies have found that Black patients with HCC were more likely to present with an advanced stage, less likely to receive curative intent treatment, and had significantly reduced survival compared with that of White patients.1,7-9 An analysis of 1117 patients by Rich and colleagues noted a 34% increased risk of death for Black patients with HCC compared with that of White patients, and other studies have shown about a 50% reduction in rate of liver transplantation for Black patients.10-12 Our study aimed to investigate potential disparities in incidence, etiology, AFP level at diagnosis, and outcomes of HCC in Black and White veterans managed at the Memphis Veterans Affairs Medical Center (VAMC) in Tennessee.
Methods
A single center retrospective chart review was conducted at the Memphis VAMC using the Computerized Patient Record System (CPRS) and the International Statistical Classification of Diseases, Tenth Revision (ICD-10) code C22.0 for HCC. Initial results were manually refined by prespecified criteria. Patients were included if they were diagnosed with HCC and received HCC treatment at the Memphis VAMC. Patients were excluded if HCC was not diagnosed histologically or clinically by imaging characteristics and AFP level, if the patient’s primary treatment was not provided at the Memphis VAMC, if they were lost to follow-up, or if race was not specified as either Black or White.
The following patient variables were examined: age, sex, comorbidities (alcohol or substance use disorder, cirrhosis, HIV), tumor stage, AFP, method of diagnosis, first-line treatments, systemic treatment, surgical options offered, and mortality. Staging was based on the American Joint Committee on Cancer TNM staging for HCC.13 Surgical options were recorded as resection or transplant. Patients who were offered treatment but lost to follow-up were excluded from the analysis.
Data Analysis
Our primary endpoint was identifying differences in OS among Memphis VAMC patients with HCC related to race. Kaplan-Meier analysis was used to investigate differences in OS and cumulative hazard ratio (HR) for death. Cox regression multivariate analysis further evaluated discrepancies among investigated patient variables, including age, race, alcohol, tobacco, or illicit drug use, HIV coinfection, and cirrhosis. Treatment factors were further defined by first-line treatment, systemic therapy, surgical resection, and transplant. χ2 analysis was used to investigate differences in treatment modalities.
Results
We identified 227 veterans, 95 Black and 132 White, between 2009 and 2021 meeting criteria for primary HCC treated at the Memphis VAMC. This study did not show a significant difference in OS between White and Black veterans (P = .24). Kaplan-Meier assessment showed OS was 1247 days (41 months) for Black veterans compared with 1032 days (34 months) for White veterans (Figure; Table 1).
Additionally, no significant difference was found between veterans for age or stage at diagnosis when stratified by race. The mean age of diagnosis for both groups was 65 years (P = .09). The mean TNM staging was 1.7 for White veterans vs 1.8 for Black veterans (P = .57). There was a significant increase in the AFP level at diagnosis for Black veterans (P = .001) (Table 2).
The most common initial treatment for both groups was transarterial chemoembolization and radiofrequency ablation with 68% of White and 64% of Black veterans receiving this therapy. There was no significant difference between who received systemic therapy.
However, we found significant differences by race for some forms of treatment. In our analysis, significant differences existed between those who did not receive any form of treatment as well as who received surgical resection and transplant. Among Black veterans, 11.6% received no treatment vs 6.1% for White veterans (P = .001). Only 2.1% of Black veterans underwent surgical resection vs 8.3% of White veterans (P = .046). Similarly, 13 (9.8%) White veterans vs 3 (3.2%) Black veterans received orthotopic liver transplantation (P = .052) in our cohort (eAppendix available at doi:10.12788/fp.0304). We found no differences in patient characteristics affecting OS, including alcohol use, tobacco use, illicit drug use, HIV coinfection, or liver cirrhosis (Table 3).
Discussion
In this retrospective analysis, Black veterans with HCC did not experience a statistically significant decrease in OS compared with that of White veterans despite some differences in therapy offered. Other studies have found that surgery was less frequently recommended to Black patients across multiple cancer types, and in most cases this carried a negative impact on OS.8,10,11,14,15 A number of other studies have demonstrated a greater percentage of Black patients receiving no treatment, although these studies are often based on SEER data, which captures only cancer-directed surgery and no other methods of treatment. Inequities in patient factors like insurance and socioeconomic status as well as willingness to receive certain treatments are often cited as major influences in health care disparities, but systemic and clinician factors like hospital volume, clinician expertise, specialist availability, and implicit racial bias all affect outcomes.16 One benefit of our study was that CPRS provided a centralized recording of all treatments received. Interestingly, the treatment discrepancy in our study was not attributable to a statistically significant difference in tumor stage at presentation. There should be no misconception that US Department of Veterans Affairs patients are less affected by socioeconomic inequities, though still this suggests clinician and systemic factors were significant drivers behind our findings.
This study did not intend to determine differences in incidence of HCC by race, although many studies have shown an age-adjusted incidence of HCC among Black and Hispanic patients up to twice that of White patients.1,8-10 Notably, the rate of orthotopic liver transplantation in this study was low regardless of race compared with that of other larger studies of patients with HCC.12,15 Discrepancies in HCC care among White and Black patients have been suggested to stem from a variety of influences, including access to early diagnosis and treatment of hepatitis C virus, comorbid conditions, as well as complex socioeconomic factors. It also has been shown that oncologists’ implicit racial bias has a negative impact on patients’ perceived quality of communication, their confidence in the recommended treatment, and the understood difficulty of the treatment by the patient and should be considered as a contributor to health disparities.17,18
Studies evaluating survival in HCC using SEER data generally stratify disease by localized, regional, or distant metastasis. For our study, TNM staging provided a more accurate assessment of the disease and reduced the chances that broader staging definitions could obscure differences in treatment choices. Future studies could be improved by stratifying patients by variables impacting treatment choice, such as Child-Pugh score or Barcelona Clinic Liver Cancer staging. Our study demonstrated a statistically significant difference in AFP level between White and Black veterans. This has been observed in prior studies as well, and while no specific cause has been identified, it suggests differences in tumor biologic features across different races. In addition, we found that an elevated AFP level at the time of diagnosis (defined as > 400) correlates with a worsened OS (HR, 1.36; P = .01).
Limitations
This study has several limitations, notably the number of veterans eligible for analysis at a single institution. A larger cohort would be needed to evaluate for statistically significant differences in outcomes by race. Additionally, our study did not account for therapy that was offered to but not pursued by the patient, and this would be useful to determine whether patient or practitioner factors were the more significant influence on the type of therapy received.
Conclusions
This study demonstrated a statistically significant difference in the rate of resection and liver transplantation between White and Black veterans at a single institution, although no difference in OS was observed. This discrepancy was not explained by differences in tumor staging. Additional, larger studies will be useful in clarifying the biologic, cultural, and socioeconomic drivers in HCC treatment and mortality.
Acknowledgments
The authors thank Lorri Reaves, Memphis Veterans Affairs Medical Center, Department of Hepatology.
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi:10.1200/JCO.2008.20.7753
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Accessed July 8, 2022. https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf#page=8
3. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.e1. doi:10.1016/j.amjmed.2017.01.021
4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
6. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
7. Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018;55(5)(suppl 1):S40-S48. doi:10.1016/j.amepre.2018.05.030
8. Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol. 2016;50(5):423-430. doi:10.1097/MCG.0000000000000448
9. Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525-531. doi:10.1016/j.amjmed.2008.03.005
10. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551-559.e1. doi:10.1016/j.cgh.2018.05.039
11. Peters NA, Javed AA, He J, Wolfgang CL, Weiss MJ. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253-260. doi:10.1016/j.jss.2016.11.042
12. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528-535. doi:10.1002/lt.23820
13. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909-922. doi:10.1097/01.sla.0000254368.65878.da.
14. Harrison LE, Reichman T, Koneru B, et al. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139(9):992-996. doi:10.1001/archsurg.139.9.992
15. Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98(12):1934-1939.
16. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-92.e12. doi:10.1016/j.jamcollsurg.2012.11.014
17. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. doi:10.2105/AJPH.2011.300558
18. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874-2880. doi:10.1200/JCO.2015.66.3658
Short walks after meals can cut diabetes risk
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Long COVID’s grip will likely tighten as infections continue
COVID-19 is far from done in the United States, with more than 111,000 new cases being recorded a day in the second week of August, according to Johns Hopkins University, and 625 deaths being reported every day. , a condition that already has affected between 7.7 million and 23 million Americans, according to U.S. government estimates.
“It is evident that long COVID is real, that it already impacts a substantial number of people, and that this number may continue to grow as new infections occur,” the U.S. Department of Health and Human Services (HHS) said in a research action plan released Aug. 4.
“We are heading towards a big problem on our hands,” says Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs Hospital in St. Louis. “It’s like if we are falling in a plane, hurtling towards the ground. It doesn’t matter at what speed we are falling; what matters is that we are all falling, and falling fast. It’s a real problem. We needed to bring attention to this, yesterday,” he said.
Bryan Lau, PhD, professor of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and co-lead of a long COVID study there, says whether it’s 5% of the 92 million officially recorded U.S. COVID-19 cases, or 30% – on the higher end of estimates – that means anywhere between 4.5 million and 27 million Americans will have the effects of long COVID.
Other experts put the estimates even higher.
“If we conservatively assume 100 million working-age adults have been infected, that implies 10 to 33 million may have long COVID,” Alice Burns, PhD, associate director for the Kaiser Family Foundation’s Program on Medicaid and the Uninsured, wrote in an analysis.
And even the Centers for Disease Control and Prevention says only a fraction of cases have been recorded.
That, in turn, means tens of millions of people who struggle to work, to get to school, and to take care of their families – and who will be making demands on an already stressed U.S. health care system.
The HHS said in its Aug. 4 report that long COVID could keep 1 million people a day out of work, with a loss of $50 billion in annual pay.
Dr. Lau said health workers and policymakers are woefully unprepared.
“If you have a family unit, and the mom or dad can’t work, or has trouble taking their child to activities, where does the question of support come into play? Where is there potential for food issues, or housing issues?” he asked. “I see the potential for the burden to be extremely large in that capacity.”
Dr. Lau said he has yet to see any strong estimates of how many cases of long COVID might develop. Because a person has to get COVID-19 to ultimately get long COVID, the two are linked. In other words, as COVID-19 cases rise, so will cases of long COVID, and vice versa.
Evidence from the Kaiser Family Foundation analysis suggests a significant impact on employment: Surveys showed more than half of adults with long COVID who worked before becoming infected are either out of work or working fewer hours. Conditions associated with long COVID – such as fatigue, malaise, or problems concentrating – limit people’s ability to work, even if they have jobs that allow for accommodations.
Two surveys of people with long COVID who had worked before becoming infected showed that between 22% and 27% of them were out of work after getting long COVID. In comparison, among all working-age adults in 2019, only 7% were out of work. Given the sheer number of working-age adults with long COVID, the effects on employment may be profound and are likely to involve more people over time. One study estimates that long COVID already accounts for 15% of unfilled jobs.
The most severe symptoms of long COVID include brain fog and heart complications, known to persist for weeks for months after a COVID-19 infection.
A study from the University of Norway published in Open Forum Infectious Diseases found 53% of people tested had at least one symptom of thinking problems 13 months after infection with COVID-19. According to the HHS’ latest report on long COVID, people with thinking problems, heart conditions, mobility issues, and other symptoms are going to need a considerable amount of care. Many will need lengthy periods of rehabilitation.
Dr. Al-Aly worries that long COVID has already severely affected the labor force and the job market, all while burdening the country’s health care system.
“While there are variations in how individuals respond and cope with long COVID, the unifying thread is that with the level of disability it causes, more people will be struggling to keep up with the demands of the workforce and more people will be out on disability than ever before,” he said.
Studies from Johns Hopkins and the University of Washington estimate that 5%-30% of people could get long COVID in the future. Projections beyond that are hazy.
“So far, all the studies we have done on long COVID have been reactionary. Much of the activism around long COVID has been patient led. We are seeing more and more people with lasting symptoms. We need our research to catch up,” Dr. Lau said.
Theo Vos, MD, PhD, professor of health sciences at University of Washington, Seattle, said the main reasons for the huge range of predictions are the variety of methods used, as well as differences in sample size. Also, much long COVID data is self-reported, making it difficult for epidemiologists to track.
“With self-reported data, you can’t plug people into a machine and say this is what they have or this is what they don’t have. At the population level, the only thing you can do is ask questions. There is no systematic way to define long COVID,” he said.
Dr. Vos’s most recent study, which is being peer-reviewed and revised, found that most people with long COVID have symptoms similar to those seen in other autoimmune diseases. But sometimes the immune system can overreact, causing the more severe symptoms, such as brain fog and heart problems, associated with long COVID.
One reason that researchers struggle to come up with numbers, said Dr. Al-Aly, is the rapid rise of new variants. These variants appear to sometimes cause less severe disease than previous ones, but it’s not clear whether that means different risks for long COVID.
“There’s a wide diversity in severity. Someone can have long COVID and be fully functional, while others are not functional at all. We still have a long way to go before we figure out why,” Dr. Lau said.
A version of this article first appeared on WebMD.com.
COVID-19 is far from done in the United States, with more than 111,000 new cases being recorded a day in the second week of August, according to Johns Hopkins University, and 625 deaths being reported every day. , a condition that already has affected between 7.7 million and 23 million Americans, according to U.S. government estimates.
“It is evident that long COVID is real, that it already impacts a substantial number of people, and that this number may continue to grow as new infections occur,” the U.S. Department of Health and Human Services (HHS) said in a research action plan released Aug. 4.
“We are heading towards a big problem on our hands,” says Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs Hospital in St. Louis. “It’s like if we are falling in a plane, hurtling towards the ground. It doesn’t matter at what speed we are falling; what matters is that we are all falling, and falling fast. It’s a real problem. We needed to bring attention to this, yesterday,” he said.
Bryan Lau, PhD, professor of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and co-lead of a long COVID study there, says whether it’s 5% of the 92 million officially recorded U.S. COVID-19 cases, or 30% – on the higher end of estimates – that means anywhere between 4.5 million and 27 million Americans will have the effects of long COVID.
Other experts put the estimates even higher.
“If we conservatively assume 100 million working-age adults have been infected, that implies 10 to 33 million may have long COVID,” Alice Burns, PhD, associate director for the Kaiser Family Foundation’s Program on Medicaid and the Uninsured, wrote in an analysis.
And even the Centers for Disease Control and Prevention says only a fraction of cases have been recorded.
That, in turn, means tens of millions of people who struggle to work, to get to school, and to take care of their families – and who will be making demands on an already stressed U.S. health care system.
The HHS said in its Aug. 4 report that long COVID could keep 1 million people a day out of work, with a loss of $50 billion in annual pay.
Dr. Lau said health workers and policymakers are woefully unprepared.
“If you have a family unit, and the mom or dad can’t work, or has trouble taking their child to activities, where does the question of support come into play? Where is there potential for food issues, or housing issues?” he asked. “I see the potential for the burden to be extremely large in that capacity.”
Dr. Lau said he has yet to see any strong estimates of how many cases of long COVID might develop. Because a person has to get COVID-19 to ultimately get long COVID, the two are linked. In other words, as COVID-19 cases rise, so will cases of long COVID, and vice versa.
Evidence from the Kaiser Family Foundation analysis suggests a significant impact on employment: Surveys showed more than half of adults with long COVID who worked before becoming infected are either out of work or working fewer hours. Conditions associated with long COVID – such as fatigue, malaise, or problems concentrating – limit people’s ability to work, even if they have jobs that allow for accommodations.
Two surveys of people with long COVID who had worked before becoming infected showed that between 22% and 27% of them were out of work after getting long COVID. In comparison, among all working-age adults in 2019, only 7% were out of work. Given the sheer number of working-age adults with long COVID, the effects on employment may be profound and are likely to involve more people over time. One study estimates that long COVID already accounts for 15% of unfilled jobs.
The most severe symptoms of long COVID include brain fog and heart complications, known to persist for weeks for months after a COVID-19 infection.
A study from the University of Norway published in Open Forum Infectious Diseases found 53% of people tested had at least one symptom of thinking problems 13 months after infection with COVID-19. According to the HHS’ latest report on long COVID, people with thinking problems, heart conditions, mobility issues, and other symptoms are going to need a considerable amount of care. Many will need lengthy periods of rehabilitation.
Dr. Al-Aly worries that long COVID has already severely affected the labor force and the job market, all while burdening the country’s health care system.
“While there are variations in how individuals respond and cope with long COVID, the unifying thread is that with the level of disability it causes, more people will be struggling to keep up with the demands of the workforce and more people will be out on disability than ever before,” he said.
Studies from Johns Hopkins and the University of Washington estimate that 5%-30% of people could get long COVID in the future. Projections beyond that are hazy.
“So far, all the studies we have done on long COVID have been reactionary. Much of the activism around long COVID has been patient led. We are seeing more and more people with lasting symptoms. We need our research to catch up,” Dr. Lau said.
Theo Vos, MD, PhD, professor of health sciences at University of Washington, Seattle, said the main reasons for the huge range of predictions are the variety of methods used, as well as differences in sample size. Also, much long COVID data is self-reported, making it difficult for epidemiologists to track.
“With self-reported data, you can’t plug people into a machine and say this is what they have or this is what they don’t have. At the population level, the only thing you can do is ask questions. There is no systematic way to define long COVID,” he said.
Dr. Vos’s most recent study, which is being peer-reviewed and revised, found that most people with long COVID have symptoms similar to those seen in other autoimmune diseases. But sometimes the immune system can overreact, causing the more severe symptoms, such as brain fog and heart problems, associated with long COVID.
One reason that researchers struggle to come up with numbers, said Dr. Al-Aly, is the rapid rise of new variants. These variants appear to sometimes cause less severe disease than previous ones, but it’s not clear whether that means different risks for long COVID.
“There’s a wide diversity in severity. Someone can have long COVID and be fully functional, while others are not functional at all. We still have a long way to go before we figure out why,” Dr. Lau said.
A version of this article first appeared on WebMD.com.
COVID-19 is far from done in the United States, with more than 111,000 new cases being recorded a day in the second week of August, according to Johns Hopkins University, and 625 deaths being reported every day. , a condition that already has affected between 7.7 million and 23 million Americans, according to U.S. government estimates.
“It is evident that long COVID is real, that it already impacts a substantial number of people, and that this number may continue to grow as new infections occur,” the U.S. Department of Health and Human Services (HHS) said in a research action plan released Aug. 4.
“We are heading towards a big problem on our hands,” says Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs Hospital in St. Louis. “It’s like if we are falling in a plane, hurtling towards the ground. It doesn’t matter at what speed we are falling; what matters is that we are all falling, and falling fast. It’s a real problem. We needed to bring attention to this, yesterday,” he said.
Bryan Lau, PhD, professor of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and co-lead of a long COVID study there, says whether it’s 5% of the 92 million officially recorded U.S. COVID-19 cases, or 30% – on the higher end of estimates – that means anywhere between 4.5 million and 27 million Americans will have the effects of long COVID.
Other experts put the estimates even higher.
“If we conservatively assume 100 million working-age adults have been infected, that implies 10 to 33 million may have long COVID,” Alice Burns, PhD, associate director for the Kaiser Family Foundation’s Program on Medicaid and the Uninsured, wrote in an analysis.
And even the Centers for Disease Control and Prevention says only a fraction of cases have been recorded.
That, in turn, means tens of millions of people who struggle to work, to get to school, and to take care of their families – and who will be making demands on an already stressed U.S. health care system.
The HHS said in its Aug. 4 report that long COVID could keep 1 million people a day out of work, with a loss of $50 billion in annual pay.
Dr. Lau said health workers and policymakers are woefully unprepared.
“If you have a family unit, and the mom or dad can’t work, or has trouble taking their child to activities, where does the question of support come into play? Where is there potential for food issues, or housing issues?” he asked. “I see the potential for the burden to be extremely large in that capacity.”
Dr. Lau said he has yet to see any strong estimates of how many cases of long COVID might develop. Because a person has to get COVID-19 to ultimately get long COVID, the two are linked. In other words, as COVID-19 cases rise, so will cases of long COVID, and vice versa.
Evidence from the Kaiser Family Foundation analysis suggests a significant impact on employment: Surveys showed more than half of adults with long COVID who worked before becoming infected are either out of work or working fewer hours. Conditions associated with long COVID – such as fatigue, malaise, or problems concentrating – limit people’s ability to work, even if they have jobs that allow for accommodations.
Two surveys of people with long COVID who had worked before becoming infected showed that between 22% and 27% of them were out of work after getting long COVID. In comparison, among all working-age adults in 2019, only 7% were out of work. Given the sheer number of working-age adults with long COVID, the effects on employment may be profound and are likely to involve more people over time. One study estimates that long COVID already accounts for 15% of unfilled jobs.
The most severe symptoms of long COVID include brain fog and heart complications, known to persist for weeks for months after a COVID-19 infection.
A study from the University of Norway published in Open Forum Infectious Diseases found 53% of people tested had at least one symptom of thinking problems 13 months after infection with COVID-19. According to the HHS’ latest report on long COVID, people with thinking problems, heart conditions, mobility issues, and other symptoms are going to need a considerable amount of care. Many will need lengthy periods of rehabilitation.
Dr. Al-Aly worries that long COVID has already severely affected the labor force and the job market, all while burdening the country’s health care system.
“While there are variations in how individuals respond and cope with long COVID, the unifying thread is that with the level of disability it causes, more people will be struggling to keep up with the demands of the workforce and more people will be out on disability than ever before,” he said.
Studies from Johns Hopkins and the University of Washington estimate that 5%-30% of people could get long COVID in the future. Projections beyond that are hazy.
“So far, all the studies we have done on long COVID have been reactionary. Much of the activism around long COVID has been patient led. We are seeing more and more people with lasting symptoms. We need our research to catch up,” Dr. Lau said.
Theo Vos, MD, PhD, professor of health sciences at University of Washington, Seattle, said the main reasons for the huge range of predictions are the variety of methods used, as well as differences in sample size. Also, much long COVID data is self-reported, making it difficult for epidemiologists to track.
“With self-reported data, you can’t plug people into a machine and say this is what they have or this is what they don’t have. At the population level, the only thing you can do is ask questions. There is no systematic way to define long COVID,” he said.
Dr. Vos’s most recent study, which is being peer-reviewed and revised, found that most people with long COVID have symptoms similar to those seen in other autoimmune diseases. But sometimes the immune system can overreact, causing the more severe symptoms, such as brain fog and heart problems, associated with long COVID.
One reason that researchers struggle to come up with numbers, said Dr. Al-Aly, is the rapid rise of new variants. These variants appear to sometimes cause less severe disease than previous ones, but it’s not clear whether that means different risks for long COVID.
“There’s a wide diversity in severity. Someone can have long COVID and be fully functional, while others are not functional at all. We still have a long way to go before we figure out why,” Dr. Lau said.
A version of this article first appeared on WebMD.com.
Impact of Race on Outcomes of High-Risk Patients With Prostate Cancer Treated With Moderately Hypofractionated Radiotherapy in an Equal Access Setting
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
Genetic counseling for cancer often costs patients nothing
But even among those who do, the cost is $16 or less, a cohort study shows.
“The findings highlight the relatively low financial costs of genetic counseling, a form of care with potentially substantial implications for cancer treatment,” lead author Mya Roberson, PhD, Vanderbilt University, Nashville, Tenn., and colleagues explained.
The study was published online in JAMA Health Forum.
Genetic counseling is an important feature of cancer care that can affect treatment decisions and surveillance. But coverage of genetic counseling services varies across insurance types.
To understand the costs to patients, the investigators used data from IBM Watson Health MarketScan to create a cohort of privately insured patients with breast, prostate, endometrial, ovarian, colorectal, and pancreatic cancer who had at least one genetic counseling session from 2013 to the end of 2019.
Dr. Roberson and colleagues then calculated out-of-pocket costs – including coinsurance, copayments, and deductibles – and total costs paid on claims for genetic counseling encounters. The cohort included 16,791 patients, the majority of whom had breast cancer.
Although the median insurance payment for genetic counseling encounters was $118 ($58-$211), most patients paid nothing out of pocket for these services. Among the 31% of patients with an out-of-pocket expense, the cost was $16 or less.
Compared with breast cancer patients, men with prostate cancer were 28% more likely to have out-of-pocket costs for genetic counseling, which may “reflect a lack of awareness about the medical necessity of genetic counseling,” the authors suggested.
Overall, the study highlights the value of genetic counseling in cancer care.
“Cancer genetic counseling not only promotes informed decision-making about genetic testing and cancer treatment in the era of precision medicine, but it also is a form of low-cost, high-value care,” the authors wrote.
The study was funded by a grant from the National Cancer Institute. Dr. Roberson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
But even among those who do, the cost is $16 or less, a cohort study shows.
“The findings highlight the relatively low financial costs of genetic counseling, a form of care with potentially substantial implications for cancer treatment,” lead author Mya Roberson, PhD, Vanderbilt University, Nashville, Tenn., and colleagues explained.
The study was published online in JAMA Health Forum.
Genetic counseling is an important feature of cancer care that can affect treatment decisions and surveillance. But coverage of genetic counseling services varies across insurance types.
To understand the costs to patients, the investigators used data from IBM Watson Health MarketScan to create a cohort of privately insured patients with breast, prostate, endometrial, ovarian, colorectal, and pancreatic cancer who had at least one genetic counseling session from 2013 to the end of 2019.
Dr. Roberson and colleagues then calculated out-of-pocket costs – including coinsurance, copayments, and deductibles – and total costs paid on claims for genetic counseling encounters. The cohort included 16,791 patients, the majority of whom had breast cancer.
Although the median insurance payment for genetic counseling encounters was $118 ($58-$211), most patients paid nothing out of pocket for these services. Among the 31% of patients with an out-of-pocket expense, the cost was $16 or less.
Compared with breast cancer patients, men with prostate cancer were 28% more likely to have out-of-pocket costs for genetic counseling, which may “reflect a lack of awareness about the medical necessity of genetic counseling,” the authors suggested.
Overall, the study highlights the value of genetic counseling in cancer care.
“Cancer genetic counseling not only promotes informed decision-making about genetic testing and cancer treatment in the era of precision medicine, but it also is a form of low-cost, high-value care,” the authors wrote.
The study was funded by a grant from the National Cancer Institute. Dr. Roberson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
But even among those who do, the cost is $16 or less, a cohort study shows.
“The findings highlight the relatively low financial costs of genetic counseling, a form of care with potentially substantial implications for cancer treatment,” lead author Mya Roberson, PhD, Vanderbilt University, Nashville, Tenn., and colleagues explained.
The study was published online in JAMA Health Forum.
Genetic counseling is an important feature of cancer care that can affect treatment decisions and surveillance. But coverage of genetic counseling services varies across insurance types.
To understand the costs to patients, the investigators used data from IBM Watson Health MarketScan to create a cohort of privately insured patients with breast, prostate, endometrial, ovarian, colorectal, and pancreatic cancer who had at least one genetic counseling session from 2013 to the end of 2019.
Dr. Roberson and colleagues then calculated out-of-pocket costs – including coinsurance, copayments, and deductibles – and total costs paid on claims for genetic counseling encounters. The cohort included 16,791 patients, the majority of whom had breast cancer.
Although the median insurance payment for genetic counseling encounters was $118 ($58-$211), most patients paid nothing out of pocket for these services. Among the 31% of patients with an out-of-pocket expense, the cost was $16 or less.
Compared with breast cancer patients, men with prostate cancer were 28% more likely to have out-of-pocket costs for genetic counseling, which may “reflect a lack of awareness about the medical necessity of genetic counseling,” the authors suggested.
Overall, the study highlights the value of genetic counseling in cancer care.
“Cancer genetic counseling not only promotes informed decision-making about genetic testing and cancer treatment in the era of precision medicine, but it also is a form of low-cost, high-value care,” the authors wrote.
The study was funded by a grant from the National Cancer Institute. Dr. Roberson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA HEALTH FORUM
Updates on treatment/prevention of VTE in cancer patients
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
FROM THE LANCET ONCOLOGY