User login
Distress and Factors Associated with Suicidal Ideation in Veterans Living with Cancer (FULL)
It was estimated that physicians would diagnose a form of invasive cancer > 1.7 million times in 2019. As the second most common cause of death in the US, > 600,000 people were projected to die from cancer in 2019.1 Many individuals with cancer endure distress, which the National Comprehensive Cancer Network (NCCN) defines as a “multifactorial unpleasant experience of a psychological (ie, cognitive, behavioral, emotional), social, spiritual, and/or physical nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”2,3 Distress in people living with cancer has been attributed to various psychosocial concerns, such as family problems, whichinclude dealing with partners and children; emotional problems, such as depression and anxiety; and physical symptoms, such as pain and fatigue.4-9 Certain factors associated with distress may increase a patient’s risk for suicide.4
Veterans are at particularly high risk for suicide.10 In 2014, veterans accounted for 18% of completed suicides in the US but only were 8.5% of the total population that same year.10 Yet, little research has been done on the relationship between distress and suicide in veterans living with cancer. Aboumrad and colleagues found that 45% of veterans with cancer who completed suicide reported family issues and 41% endorsed chronic pain.11 This study recommended continued efforts to assess and treat distress to lessen risk of suicide in veterans living with cancer; however, to date, only 1 study has specifically evaluated distress and problems endorsed among veterans living with cancer.7
Suicide prevention is of the highest priority to the US Department of Veterans Affairs (VA).12 Consistent with the VA mission to end veteran suicide, the current study aimed to better understand the relationship between distress and suicide within a sample of veterans living with cancer. Findings would additionally be used to tailor clinical assessments and interventions for veterans living with cancer.
This study had 3 primary goals. First, we sought to understand demographic and clinical factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. Second, the study investigated the most commonly endorsed problems by veterans living with cancer. Finally, we examined which problems were related to suicidal ideation (SI). It was hypothesized that veterans who reported severe distress would be significantly more likely to endorse SI when compared with veterans who reported mild or moderate distress. Based on existing literature, it was further hypothesized that family, emotional, and physical problems would be significantly associated with SI.7,11
Methods
The current study was conducted at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida. Inclusion criteria included veterans who were diagnosed with cancer, attended an outpatient psychology-oncology evaluation, and completed mental health screening measures provided during their evaluation. Exclusion criteria included veterans who: were seen in response to an inpatient consult, were seen solely for a stem cell transplant evaluation, or did not complete the screening measures.
Measures
A veteran’s demographic (eg, age, sex, ethnicity) and clinical (eg, cancer type, stage of disease, recurrence, cancer treatments received) information was abstracted from their VA medical records. Marital status was assessed during a clinical interview and documented as part of the standardized suicide risk assessment.
The Distress Thermometer (DT) is a subjective measure developed by the NCCN.2 The DT provides a visual representation of a thermometer and asks patients to rate their level of distress over the past week with 0 indicating no distress and 10 indicating extreme distress.
The measurement additionally lists 39 problems nested within 5 domains: practical, family, emotional, spiritual/religious, and physical. Patients may endorse listed items under each problem domain by indicating yes or no. Endorsement of various items are intended to provide more detailed information about sources of distress. Due to the predominantly male and mostly older population included in this study the ability to have children measure was removed from the family problem domain.
SI was assessed in 2 ways. First, by patients’ self-report through item-9 of the Patient Health Questionnaire-9 (PHQ-9).14 Item-9 asks “over the last 2 weeks, how often have you been bothered by thoughts that you would be better off dead or of hurting yourself in some way?” Responses range from 0 (not at all) to 3 (nearly every day).14 Responses > 0 were considered a positive screen for SI.
Procedure
Participants were a sample of veterans who were referred for psychology-oncology services. The NCCN DT and Problems List were administered prior to the start of clinical interviews, which followed a checklist and included standardized assessments of SI and history of a suicide attempt(s). A licensed clinical psychologist or a postdoctoral resident conducted these assessments under the supervision of a licensed psychologist. Data gathered during the clinical interview and from the DT and problems list were documented in health records, which were retrospectively reviewed for relevant information (eg, cancer diagnosis, SI). Therefore, informed consent was waived. This study was approved by the JAHVH Institutional Review Board.
Analysis
Data were analyzed using SPSS Version 25. Data analysis proceeded in 3 steps. First, descriptive statistics included the demographic and clinical factors present in the current sample. Difference between those with and without suicidal ideation were compared using F-statistic for continuous variables and χ2 analyses for categorical variables. Second, to examine relationships between each DT problem domain and SI, χ2 analyses were conducted. Third, DT problem domains that had a significant relationship with SI were entered in a logistic regression. This analysis determined which items, if any, from a DT problem domain predicted SI. In the logistic regression model, history of suicide attempts was entered into the first block, as history of suicide attempts is a well-established risk factor for subsequent suicidal ideation. In the second block, other variables that were significantly related to suicidal ideation in the second step of analyses were included. Before interpreting the results of the logistic regression, model fit was tested using the Hosmer-Lemeshow test. Significance of each individual predictor variable in the model is reported using the Wald χ2 statistic; each Wald statistic is compared with a χ2 distribution with 1 degree of freedom (df). Results of logistic regression models also provide information about the effect of each predictor variable in the regression equation (beta weight), odds a veteran who endorsed each predictor variable in the model would also endorse SI (as indicated by the odds ratio), and an estimate of the amount of variance accounted for by each predictor variable (using Nagelkerke’s pseudo R2, which ranges in value from 0 to 1 with higher values indicating more variance explained). For all analyses, P value of .05 (2-tailed) was used for statistical significance.
Results
The sample consisted of 174 veterans (Table 1). The majority (77.6%) were male with a mean age of nearly 62 years (range, 29-87). Most identified as white (74.1%) with half reporting they were either married or living with a partner.
Prostate cancer (19.0%) was the most common type of cancer among study participants followed by head and neck (18.4%), lymphoma/leukemia (11.5%), lung (11.5%), and breast (10.9%); 31.6% had metastatic disease and 14.9% had recurrent disease. Chemotherapy (42.5%) was the most common treatment modality, followed by surgery (38.5%) and radiation (31.6%). The sample was distributed among the 3 distress DT categories: mild (18.4%), moderate (42.5%), and severe (39.1%).
Problems Endorsed
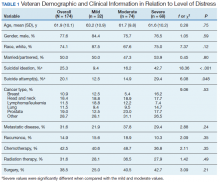
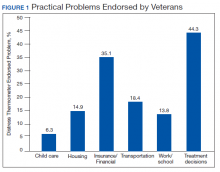
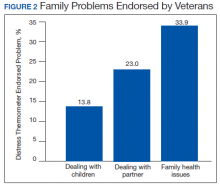
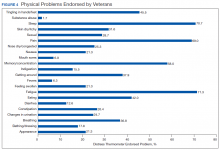
Treatment decisions (44.3%) and insurance/financial concerns (35.1%) were the most frequently endorsed practical problems (Figure 1). Family health issues (33.9%) and dealing with partner (23.0%) were the most frequently endorsed family problems (Figure 2). Worry (73.0%) and depression (69.5%) were the most frequent emotional problems; of note, all emotional problems were endorsed by at least 50% of veterans (Figure 3). Fatigue (71.3%), sleep (70.7%), and pain (69%), were the most frequently endorsed physical problems (Figure 4). Spiritual/religious problems were endorsed by 15% of veterans.
Suicidal Ideation
Overall, 25.3% of veterans endorsed SI. About 20% of veterans reported a history of ≥ 1 suicide attempts in their lifetime. A significant relationship among distress categories and SI was found (χ2 = 18.36, P < .001). Veterans with severe distress were more likely to endorse SI (42.7%) when compared with veterans with mild (9.4%) or moderate (16.2%) distress.
Similarly, a significant relationship among distress categories and a history of a suicide attempt(s) was found (χ2 = 6.08, P = .048). Veterans with severe distress were more likely to have attempted suicide (29.4%) when compared with veterans with mild (12.5%) or moderate (14.9%) distress.
χ2 analyses were conducted to examine the relationships between DT problem domains and SI. A significant relationship was found between family problems and SI (
Logistic regression analyses determined whether items representative of the family problems domain were predictive of SI. Suicide attempt(s) were entered in the first step of the model to evaluate risk factors for SI over this already established risk factor. The assumptions of logistic regression were met.
The Hosmer-Lemeshow test (χ2 = 3.66, df = 5, P = .56) demonstrated that the model fit was good. The group of predictors used in the model differentiate between people who were experiencing SI and those who were not experiencing SI at the time of evaluation. A history of a suicide attempt(s) predicted SI, as expected (Wald = 6.821, df = 1, P = .01). The odds that a veteran with a history of a suicide attempt(s) would endorse SI at the time of the evaluation was nearly 3 times greater than that of veterans without a history of a suicide attempt(s). Over and above suicide attempts, problems dealing with partner (Wald = 15.142; df = 1, P < .001) was a second significant predictor of current SI. The odds that a veteran who endorsed problems dealing with partner would also endorse SI was > 5 times higher than that of veterans who did not endorse problems dealing with partner. This finding represents a significant risk factor for SI, over and above a history of a suicide attempt(s). The other items from the family problems domains were not significant (P > .05) (Table 3).
Discussion
This study aimed to understand factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. As hypothesized, veterans who endorsed severe distress were significantly more likely to endorse SI. They also were more likely to have a history of a suicide attempt(s) when compared with those with mild or moderate distress.
A second aim of this study was to understand the most commonly endorsed problems. Consistent with prior literature, treatment decisions were the most commonly endorsed practical problem; worry and depression were the most common emotional problems; and fatigue, sleep, and pain were the most common physical problems.7
A finding unique to the current study is that family health issues and dealing with partner were specified as the most common family problems. However, a study by Smith and colleagues did not provide information about the rank of most frequently reported problems within this domain.7
The third aim was to understand which problems were related to SI. It was hypothesized that family, emotional, and physical problems would be related to SI. However, results indicated that only family problems (specifically, problems dealing with a partner) were significantly associated with SI among veterans living with cancer.
Contrary to expectations, emotional and physical problems were not found to have a significant relationship with SI. This is likely because veterans endorsed items nested within these problem domains with similar frequency. The lack of significant findings does not suggest that emotional and physical problems are not significant predictors of SI for veterans living with cancer, but that no specific emotional or physical symptom stood out as a predictor of suicidal ideation above the others.
The finding of a significant relationship between family problems (specifically, problems dealing with a partner) and SI in this study is consistent with findings of Aboumrad and colleagues in a study that examined root-cause analyses of completed suicides by veterans living with cancer.11 They found that nearly half the sample endorsed family problems prior to their death, and a small but notable percentage of veterans who completed suicide reported divorce as a stressor prior to their death.
This finding may be explained by Thomas Joiner's interpersonal-psychological theory of suicidal behavior (IPT), which suggests that completed suicide may result from a thwarted sense of belonging, perceived burdensomeness, and acquired capability for suicide.16 Problems dealing with a partner may impact a veteran’s sense of belonging or social connectedness. Problems dealing with a partner also may be attributed to perceived burdens due to limitations imposed by living with cancer and/or undergoing treatment. In both circumstances, the veteran’s social support system may be negatively impacted, and perceived social support is a well-established protective factor against suicide.17
Partner distress is a second consideration. It is likely that veterans’ partners experienced their own distress in response to the veteran’s cancer diagnosis and/or treatment. The partner’s cause, severity, and expression of distress may contribute to problems for the couple.
Finally, the latter point of the IPT refers to acquired capability, or the ability to inflict deadly harm to oneself.18 A military sample was found to have more acquired capability for suicide when compared with a college undergraduate sample.19 A history of a suicide attempt(s) and male gender have been found to significantly predict acquired capability to complete suicide.18 Furthermore, because veterans living with cancer often are in pain, fear of pain associated with suicide may be reduced and, therefore, acquired capability increased. This suggests that male veterans living with cancer who are in pain, have a history of a suicide attempt(s), and current problems with their partner may be an extremely vulnerable population at-risk for suicide. Results from the current study emphasize the importance of veterans having access to mental health and crisis resources for problems dealing with their partner. Partner problems may foreshadow a potentially lethal type of distress.
Strengths
This study’s aims are consistent with the VA’s mission to end veteran suicide and contributes to literature in several important ways.12 First, veterans living with cancer are an understudied population. The current study addresses a gap in existing literature by researching veterans living with cancer and aims to better understand the relationship between cancer-related distress and SI. Second, to the best of the authors’ knowledge, this study is the first to find that problems dealing with a partner significantly increases a veteran’s risk for SI above a history of a suicide attempt(s). Risk assessments now may be more comprehensive through inclusion of this distress factor.
It is recommended that future research use IPT to further investigate the relationship between problems dealing with a partner and SI.16 Future research may do so by including specific measures to assess for the tenants of the theory, including measurements of burdensomeness and belongingness. An expanded knowledge base about what makes problems dealing with a partner a significant suicide risk factor (eg, increased conflict, lack of support, etc.) would better enable clinicians to intervene effectively. Effective intervention may lessen suicidal behaviors or deaths from suicides within the Veteran population.
Limitations
One limitation is the focus on patients who accepted a mental health referral. This study design may limit the generalizability of results to veterans who would not accept mental health treatment. The homogenous sample of veterans is a second limitation. Most participants were male, white, and had a mean age of 62 years. These demographics are representative of the veterans that most typically utilize VA services; however, more research is needed on veterans living with cancer who are female and of diverse racial and ethnic backgrounds. There are likely differences in problems endorsed and factors associated with SI based on age, race, sex, and other socioeconomic factors. A third limitation is the cross-sectional, retrospective nature of this study. Future studies are advised to assess for distress at multiple time points. This is consistent with NCCN Standards of Care for Distress Management.2 Longitudinal data would enable more findings about distress and SI throughout the course of cancer diagnosis and treatment, therefore enhancing clinical implications and informing future research.
Conclusion
This is among the first of studies to investigate distress and factors associated with SI in veterans living with cancer who were referred for psychology services. The prevalence of distress caused by psychosocial factors (including treatment decisions, worry, and depression) highlights the importance of including mental health services as part of comprehensive cancer treatment.
Distress due to treatment decisions may be attributed to a litany of factors such as a veteran’s consideration of adverse effects, effectiveness of treatments, changes to quality of life or functioning, and inclusion of alternative or complimentary treatments. These types of decisions often are reported to be difficult conversations to have with family members or loved ones, who are likely experiencing distress of their own. The role of a mental health provider to assist veterans in exploring their treatment decisions and the implications of such decisions appears important to lessening distress.
Early intervention for emotional symptoms would likely benefit veterans’ management of distress and may lessen suicide risk as depression is known to place veterans at-risk for SI.20 This underscores the importance of timely distress assessment to prevent mild emotional distress from progressing to potentially severe or life-threatening emotional distress. For veterans with a psychiatric history, timely assessment and intervention is essential because psychiatric history is an established suicide risk factor that may be exacerbated by cancer-related distress.12
Furthermore, management of intolerable physical symptoms may lessen risk for suicide.4 Under medical guidance, fatigue may be improved using exercise.21 Behavioral intervention is commonly used as first-line treatment for sleep problems.22 While pain may be lessened through medication or nonpharmacological interventions.23
Considering the numerous ways that distress may present itself (eg, practical, emotional, or physical) and increase risk for SI, it is essential that all veterans living with cancer are assessed for distress and SI, regardless of their presentation. Although veterans may not outwardly express distress, this does not indicate the absence of either distress or risk for suicide. For example, a veteran may be distressed due to financial concerns, transportation issues, and the health of his/her partner or spouse. This veteran may not exhibit visible symptoms of distress, as would be expected when the source of distress is emotional (eg, depression, anxiety). However, this veteran is equally vulnerable to impairing distress and SI as someone who exhibits emotional distress. Distress assessments should be further developed to capture both the visible and less apparent sources of distress, while also serving the imperative function of screening for suicide. Other researchers also have noted the necessity of this development.24 Currently, the NCCN DT and Problems List does not include any assessment of SI or behavior.
Finally, this study identified a potentially critical factor to include in distress assessment: problems dealing with a partner. Problems dealing with a partner have been noted as a source of distress in existing literature, but this is the first study to find problems dealing with a partner to be a predictor of SI in veterans living with cancer.4-6
Because partners often attend appointments with veterans, it is not surprising that problems dealing with their partner are not disclosed more readily. It is recommended that clinicians ask veterans about potential problems with their partner when they are alone. Directly gathering information about such problems while assessing for distress may assist health care workers in providing the most effective, accurate type of intervention in a timely manner, and potentially mitigate risk for suicide.
As recommended by the NCCN and numerous researchers, findings from the current study underscore the importance of accurate, timely assessment of distress.2,4,8 This study makes several important recommendations about how distress assessment may be strengthened and further developed, specifically for the veteran population. This study also expands the current knowledge base of what is known about veterans living with cancer, and has begun to fill a gap in the existing literature. Consistent with the VA mission to end veteran suicide, results suggest that veterans living with cancer should be regularly screened for distress, asked about distress related to their partner, and assessed for SI. Continued efforts to enhance assessment of and response to distress may lessen suicide risk in veterans with cancer.11
Acknowledgements
This study is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
2. Riba MB, Donovan, KA, Andersen, B. National Comprehensive Cancer Network clinical practice guidelines in oncology. Distress management (Version 3.2019). J Natl Compr Can Net, 2019;17(10):1229-1249.
3. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Pianta dosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28.
4. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8(1):4-12.
5. Bulli F, Miccinesi G, Maruelli A, Katz M, Paci E. The measure of psychological distress in cancer patients: the use of distress thermometer in the oncological rehabilitation center of Florence. Support Care Cancer. 2009;17(7):771–779.
6. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
7. Smith J, Berman S, Dimick J, et al. Distress Screening and Management in an Outpatient VA Cancer Clinic: A Pilot Project Involving Ambulatory Patients Across the Disease Trajectory. Fed Pract. 2017;34(Suppl 1):43S–50S.
8. Carlson LE, Waller A, Groff SL, Bultz BD. Screening for distress, the sixth vital sign, in lung cancer patients: effects on pain, fatigue, and common problems--secondary outcomes of a randomized controlled trial. Psychooncology. 2013;22(8):1880-1888.
9. Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003;12(7):694-708.
10. US Department of Veterans Affairs Office of Suicide Prevention. Suicide among veterans and other Americans 2001-2014. https://www.mentalhealth.va.gov/docs/2016suicidedatareport.pdf. Published August 3, 2016. Accessed April 13, 2020.
11. Aboumrad M, Shiner B, Riblet N, Mills, PD, Watts BV. Factors contributing to cancer-related suicide: a study of root-cause-analysis reports. Psychooncology. 2018;27(9):2237-2244.
12. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. National Strategy for Preventing Veteran Suicide 2018–2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf Published 2018. Accessed April 13, 2020.
13. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
14. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613.
15. Martin A, Rief W, Klaiberg A, Braehler E. Validity of the brief patient health questionnaire mood scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28(1):71-77.
16. Joiner TE. Why People Die by Suicide. Cambridge, MA: Harvard University Press, 2005.
17. Kleiman EM, Riskind JH, Schaefer KE. Social support and positive events as suicide resiliency factors: examination of synergistic buffering effects. Arch Suicide Res. 2014;18(2):144-155.
18. Van Orden KA, Witte TK, Gordon KH, Bender TW, Joiner TE Jr. Suicidal desire and the capability for suicide: tests of the interpersonal-psychological theory of suicidal behavior among adults. J Consult Clin Psychol. 2008;76(1):72–83.
19. Bryan CJ, Morrow CE, Anestis MD, Joiner TE. A preliminary test of the interpersonal -psychological theory of suicidal behavior in a military sample. Personal Individual Differ. 2010;48(3):347-350.
20. Miller SN, Monahan CJ, Phillips KM, Agliata D, Gironda RJ. Mental health utilization among veterans at risk for suicide: Data from a post-deployment clinic [published online ahead of print, 2018 Oct 8]. Psychol Serv. 2018;10.1037/ser0000311.
21. Galvão DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899-909.
22. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
23. Ngamkham S, Holden JE, Smith EL. A systematic review: Mindfulness intervention for cancer-related pain. Asia Pac J Oncol Nurs. 2019;6(2):161-169.
24. Granek L, Nakash O, Ben-David M, Shapira S, Ariad S. Oncologists’, nurses’, and social workers’ strategies and barriers to identifying suicide risk in cancer patients. Psychooncology. 2018;27(1):148-154.
It was estimated that physicians would diagnose a form of invasive cancer > 1.7 million times in 2019. As the second most common cause of death in the US, > 600,000 people were projected to die from cancer in 2019.1 Many individuals with cancer endure distress, which the National Comprehensive Cancer Network (NCCN) defines as a “multifactorial unpleasant experience of a psychological (ie, cognitive, behavioral, emotional), social, spiritual, and/or physical nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”2,3 Distress in people living with cancer has been attributed to various psychosocial concerns, such as family problems, whichinclude dealing with partners and children; emotional problems, such as depression and anxiety; and physical symptoms, such as pain and fatigue.4-9 Certain factors associated with distress may increase a patient’s risk for suicide.4
Veterans are at particularly high risk for suicide.10 In 2014, veterans accounted for 18% of completed suicides in the US but only were 8.5% of the total population that same year.10 Yet, little research has been done on the relationship between distress and suicide in veterans living with cancer. Aboumrad and colleagues found that 45% of veterans with cancer who completed suicide reported family issues and 41% endorsed chronic pain.11 This study recommended continued efforts to assess and treat distress to lessen risk of suicide in veterans living with cancer; however, to date, only 1 study has specifically evaluated distress and problems endorsed among veterans living with cancer.7
Suicide prevention is of the highest priority to the US Department of Veterans Affairs (VA).12 Consistent with the VA mission to end veteran suicide, the current study aimed to better understand the relationship between distress and suicide within a sample of veterans living with cancer. Findings would additionally be used to tailor clinical assessments and interventions for veterans living with cancer.
This study had 3 primary goals. First, we sought to understand demographic and clinical factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. Second, the study investigated the most commonly endorsed problems by veterans living with cancer. Finally, we examined which problems were related to suicidal ideation (SI). It was hypothesized that veterans who reported severe distress would be significantly more likely to endorse SI when compared with veterans who reported mild or moderate distress. Based on existing literature, it was further hypothesized that family, emotional, and physical problems would be significantly associated with SI.7,11
Methods
The current study was conducted at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida. Inclusion criteria included veterans who were diagnosed with cancer, attended an outpatient psychology-oncology evaluation, and completed mental health screening measures provided during their evaluation. Exclusion criteria included veterans who: were seen in response to an inpatient consult, were seen solely for a stem cell transplant evaluation, or did not complete the screening measures.
Measures
A veteran’s demographic (eg, age, sex, ethnicity) and clinical (eg, cancer type, stage of disease, recurrence, cancer treatments received) information was abstracted from their VA medical records. Marital status was assessed during a clinical interview and documented as part of the standardized suicide risk assessment.
The Distress Thermometer (DT) is a subjective measure developed by the NCCN.2 The DT provides a visual representation of a thermometer and asks patients to rate their level of distress over the past week with 0 indicating no distress and 10 indicating extreme distress.
The measurement additionally lists 39 problems nested within 5 domains: practical, family, emotional, spiritual/religious, and physical. Patients may endorse listed items under each problem domain by indicating yes or no. Endorsement of various items are intended to provide more detailed information about sources of distress. Due to the predominantly male and mostly older population included in this study the ability to have children measure was removed from the family problem domain.
SI was assessed in 2 ways. First, by patients’ self-report through item-9 of the Patient Health Questionnaire-9 (PHQ-9).14 Item-9 asks “over the last 2 weeks, how often have you been bothered by thoughts that you would be better off dead or of hurting yourself in some way?” Responses range from 0 (not at all) to 3 (nearly every day).14 Responses > 0 were considered a positive screen for SI.
Procedure
Participants were a sample of veterans who were referred for psychology-oncology services. The NCCN DT and Problems List were administered prior to the start of clinical interviews, which followed a checklist and included standardized assessments of SI and history of a suicide attempt(s). A licensed clinical psychologist or a postdoctoral resident conducted these assessments under the supervision of a licensed psychologist. Data gathered during the clinical interview and from the DT and problems list were documented in health records, which were retrospectively reviewed for relevant information (eg, cancer diagnosis, SI). Therefore, informed consent was waived. This study was approved by the JAHVH Institutional Review Board.
Analysis
Data were analyzed using SPSS Version 25. Data analysis proceeded in 3 steps. First, descriptive statistics included the demographic and clinical factors present in the current sample. Difference between those with and without suicidal ideation were compared using F-statistic for continuous variables and χ2 analyses for categorical variables. Second, to examine relationships between each DT problem domain and SI, χ2 analyses were conducted. Third, DT problem domains that had a significant relationship with SI were entered in a logistic regression. This analysis determined which items, if any, from a DT problem domain predicted SI. In the logistic regression model, history of suicide attempts was entered into the first block, as history of suicide attempts is a well-established risk factor for subsequent suicidal ideation. In the second block, other variables that were significantly related to suicidal ideation in the second step of analyses were included. Before interpreting the results of the logistic regression, model fit was tested using the Hosmer-Lemeshow test. Significance of each individual predictor variable in the model is reported using the Wald χ2 statistic; each Wald statistic is compared with a χ2 distribution with 1 degree of freedom (df). Results of logistic regression models also provide information about the effect of each predictor variable in the regression equation (beta weight), odds a veteran who endorsed each predictor variable in the model would also endorse SI (as indicated by the odds ratio), and an estimate of the amount of variance accounted for by each predictor variable (using Nagelkerke’s pseudo R2, which ranges in value from 0 to 1 with higher values indicating more variance explained). For all analyses, P value of .05 (2-tailed) was used for statistical significance.
Results
The sample consisted of 174 veterans (Table 1). The majority (77.6%) were male with a mean age of nearly 62 years (range, 29-87). Most identified as white (74.1%) with half reporting they were either married or living with a partner.
Prostate cancer (19.0%) was the most common type of cancer among study participants followed by head and neck (18.4%), lymphoma/leukemia (11.5%), lung (11.5%), and breast (10.9%); 31.6% had metastatic disease and 14.9% had recurrent disease. Chemotherapy (42.5%) was the most common treatment modality, followed by surgery (38.5%) and radiation (31.6%). The sample was distributed among the 3 distress DT categories: mild (18.4%), moderate (42.5%), and severe (39.1%).
Problems Endorsed
Treatment decisions (44.3%) and insurance/financial concerns (35.1%) were the most frequently endorsed practical problems (Figure 1). Family health issues (33.9%) and dealing with partner (23.0%) were the most frequently endorsed family problems (Figure 2). Worry (73.0%) and depression (69.5%) were the most frequent emotional problems; of note, all emotional problems were endorsed by at least 50% of veterans (Figure 3). Fatigue (71.3%), sleep (70.7%), and pain (69%), were the most frequently endorsed physical problems (Figure 4). Spiritual/religious problems were endorsed by 15% of veterans.
Suicidal Ideation
Overall, 25.3% of veterans endorsed SI. About 20% of veterans reported a history of ≥ 1 suicide attempts in their lifetime. A significant relationship among distress categories and SI was found (χ2 = 18.36, P < .001). Veterans with severe distress were more likely to endorse SI (42.7%) when compared with veterans with mild (9.4%) or moderate (16.2%) distress.
Similarly, a significant relationship among distress categories and a history of a suicide attempt(s) was found (χ2 = 6.08, P = .048). Veterans with severe distress were more likely to have attempted suicide (29.4%) when compared with veterans with mild (12.5%) or moderate (14.9%) distress.
χ2 analyses were conducted to examine the relationships between DT problem domains and SI. A significant relationship was found between family problems and SI (
Logistic regression analyses determined whether items representative of the family problems domain were predictive of SI. Suicide attempt(s) were entered in the first step of the model to evaluate risk factors for SI over this already established risk factor. The assumptions of logistic regression were met.
The Hosmer-Lemeshow test (χ2 = 3.66, df = 5, P = .56) demonstrated that the model fit was good. The group of predictors used in the model differentiate between people who were experiencing SI and those who were not experiencing SI at the time of evaluation. A history of a suicide attempt(s) predicted SI, as expected (Wald = 6.821, df = 1, P = .01). The odds that a veteran with a history of a suicide attempt(s) would endorse SI at the time of the evaluation was nearly 3 times greater than that of veterans without a history of a suicide attempt(s). Over and above suicide attempts, problems dealing with partner (Wald = 15.142; df = 1, P < .001) was a second significant predictor of current SI. The odds that a veteran who endorsed problems dealing with partner would also endorse SI was > 5 times higher than that of veterans who did not endorse problems dealing with partner. This finding represents a significant risk factor for SI, over and above a history of a suicide attempt(s). The other items from the family problems domains were not significant (P > .05) (Table 3).
Discussion
This study aimed to understand factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. As hypothesized, veterans who endorsed severe distress were significantly more likely to endorse SI. They also were more likely to have a history of a suicide attempt(s) when compared with those with mild or moderate distress.
A second aim of this study was to understand the most commonly endorsed problems. Consistent with prior literature, treatment decisions were the most commonly endorsed practical problem; worry and depression were the most common emotional problems; and fatigue, sleep, and pain were the most common physical problems.7
A finding unique to the current study is that family health issues and dealing with partner were specified as the most common family problems. However, a study by Smith and colleagues did not provide information about the rank of most frequently reported problems within this domain.7
The third aim was to understand which problems were related to SI. It was hypothesized that family, emotional, and physical problems would be related to SI. However, results indicated that only family problems (specifically, problems dealing with a partner) were significantly associated with SI among veterans living with cancer.
Contrary to expectations, emotional and physical problems were not found to have a significant relationship with SI. This is likely because veterans endorsed items nested within these problem domains with similar frequency. The lack of significant findings does not suggest that emotional and physical problems are not significant predictors of SI for veterans living with cancer, but that no specific emotional or physical symptom stood out as a predictor of suicidal ideation above the others.
The finding of a significant relationship between family problems (specifically, problems dealing with a partner) and SI in this study is consistent with findings of Aboumrad and colleagues in a study that examined root-cause analyses of completed suicides by veterans living with cancer.11 They found that nearly half the sample endorsed family problems prior to their death, and a small but notable percentage of veterans who completed suicide reported divorce as a stressor prior to their death.
This finding may be explained by Thomas Joiner's interpersonal-psychological theory of suicidal behavior (IPT), which suggests that completed suicide may result from a thwarted sense of belonging, perceived burdensomeness, and acquired capability for suicide.16 Problems dealing with a partner may impact a veteran’s sense of belonging or social connectedness. Problems dealing with a partner also may be attributed to perceived burdens due to limitations imposed by living with cancer and/or undergoing treatment. In both circumstances, the veteran’s social support system may be negatively impacted, and perceived social support is a well-established protective factor against suicide.17
Partner distress is a second consideration. It is likely that veterans’ partners experienced their own distress in response to the veteran’s cancer diagnosis and/or treatment. The partner’s cause, severity, and expression of distress may contribute to problems for the couple.
Finally, the latter point of the IPT refers to acquired capability, or the ability to inflict deadly harm to oneself.18 A military sample was found to have more acquired capability for suicide when compared with a college undergraduate sample.19 A history of a suicide attempt(s) and male gender have been found to significantly predict acquired capability to complete suicide.18 Furthermore, because veterans living with cancer often are in pain, fear of pain associated with suicide may be reduced and, therefore, acquired capability increased. This suggests that male veterans living with cancer who are in pain, have a history of a suicide attempt(s), and current problems with their partner may be an extremely vulnerable population at-risk for suicide. Results from the current study emphasize the importance of veterans having access to mental health and crisis resources for problems dealing with their partner. Partner problems may foreshadow a potentially lethal type of distress.
Strengths
This study’s aims are consistent with the VA’s mission to end veteran suicide and contributes to literature in several important ways.12 First, veterans living with cancer are an understudied population. The current study addresses a gap in existing literature by researching veterans living with cancer and aims to better understand the relationship between cancer-related distress and SI. Second, to the best of the authors’ knowledge, this study is the first to find that problems dealing with a partner significantly increases a veteran’s risk for SI above a history of a suicide attempt(s). Risk assessments now may be more comprehensive through inclusion of this distress factor.
It is recommended that future research use IPT to further investigate the relationship between problems dealing with a partner and SI.16 Future research may do so by including specific measures to assess for the tenants of the theory, including measurements of burdensomeness and belongingness. An expanded knowledge base about what makes problems dealing with a partner a significant suicide risk factor (eg, increased conflict, lack of support, etc.) would better enable clinicians to intervene effectively. Effective intervention may lessen suicidal behaviors or deaths from suicides within the Veteran population.
Limitations
One limitation is the focus on patients who accepted a mental health referral. This study design may limit the generalizability of results to veterans who would not accept mental health treatment. The homogenous sample of veterans is a second limitation. Most participants were male, white, and had a mean age of 62 years. These demographics are representative of the veterans that most typically utilize VA services; however, more research is needed on veterans living with cancer who are female and of diverse racial and ethnic backgrounds. There are likely differences in problems endorsed and factors associated with SI based on age, race, sex, and other socioeconomic factors. A third limitation is the cross-sectional, retrospective nature of this study. Future studies are advised to assess for distress at multiple time points. This is consistent with NCCN Standards of Care for Distress Management.2 Longitudinal data would enable more findings about distress and SI throughout the course of cancer diagnosis and treatment, therefore enhancing clinical implications and informing future research.
Conclusion
This is among the first of studies to investigate distress and factors associated with SI in veterans living with cancer who were referred for psychology services. The prevalence of distress caused by psychosocial factors (including treatment decisions, worry, and depression) highlights the importance of including mental health services as part of comprehensive cancer treatment.
Distress due to treatment decisions may be attributed to a litany of factors such as a veteran’s consideration of adverse effects, effectiveness of treatments, changes to quality of life or functioning, and inclusion of alternative or complimentary treatments. These types of decisions often are reported to be difficult conversations to have with family members or loved ones, who are likely experiencing distress of their own. The role of a mental health provider to assist veterans in exploring their treatment decisions and the implications of such decisions appears important to lessening distress.
Early intervention for emotional symptoms would likely benefit veterans’ management of distress and may lessen suicide risk as depression is known to place veterans at-risk for SI.20 This underscores the importance of timely distress assessment to prevent mild emotional distress from progressing to potentially severe or life-threatening emotional distress. For veterans with a psychiatric history, timely assessment and intervention is essential because psychiatric history is an established suicide risk factor that may be exacerbated by cancer-related distress.12
Furthermore, management of intolerable physical symptoms may lessen risk for suicide.4 Under medical guidance, fatigue may be improved using exercise.21 Behavioral intervention is commonly used as first-line treatment for sleep problems.22 While pain may be lessened through medication or nonpharmacological interventions.23
Considering the numerous ways that distress may present itself (eg, practical, emotional, or physical) and increase risk for SI, it is essential that all veterans living with cancer are assessed for distress and SI, regardless of their presentation. Although veterans may not outwardly express distress, this does not indicate the absence of either distress or risk for suicide. For example, a veteran may be distressed due to financial concerns, transportation issues, and the health of his/her partner or spouse. This veteran may not exhibit visible symptoms of distress, as would be expected when the source of distress is emotional (eg, depression, anxiety). However, this veteran is equally vulnerable to impairing distress and SI as someone who exhibits emotional distress. Distress assessments should be further developed to capture both the visible and less apparent sources of distress, while also serving the imperative function of screening for suicide. Other researchers also have noted the necessity of this development.24 Currently, the NCCN DT and Problems List does not include any assessment of SI or behavior.
Finally, this study identified a potentially critical factor to include in distress assessment: problems dealing with a partner. Problems dealing with a partner have been noted as a source of distress in existing literature, but this is the first study to find problems dealing with a partner to be a predictor of SI in veterans living with cancer.4-6
Because partners often attend appointments with veterans, it is not surprising that problems dealing with their partner are not disclosed more readily. It is recommended that clinicians ask veterans about potential problems with their partner when they are alone. Directly gathering information about such problems while assessing for distress may assist health care workers in providing the most effective, accurate type of intervention in a timely manner, and potentially mitigate risk for suicide.
As recommended by the NCCN and numerous researchers, findings from the current study underscore the importance of accurate, timely assessment of distress.2,4,8 This study makes several important recommendations about how distress assessment may be strengthened and further developed, specifically for the veteran population. This study also expands the current knowledge base of what is known about veterans living with cancer, and has begun to fill a gap in the existing literature. Consistent with the VA mission to end veteran suicide, results suggest that veterans living with cancer should be regularly screened for distress, asked about distress related to their partner, and assessed for SI. Continued efforts to enhance assessment of and response to distress may lessen suicide risk in veterans with cancer.11
Acknowledgements
This study is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
It was estimated that physicians would diagnose a form of invasive cancer > 1.7 million times in 2019. As the second most common cause of death in the US, > 600,000 people were projected to die from cancer in 2019.1 Many individuals with cancer endure distress, which the National Comprehensive Cancer Network (NCCN) defines as a “multifactorial unpleasant experience of a psychological (ie, cognitive, behavioral, emotional), social, spiritual, and/or physical nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”2,3 Distress in people living with cancer has been attributed to various psychosocial concerns, such as family problems, whichinclude dealing with partners and children; emotional problems, such as depression and anxiety; and physical symptoms, such as pain and fatigue.4-9 Certain factors associated with distress may increase a patient’s risk for suicide.4
Veterans are at particularly high risk for suicide.10 In 2014, veterans accounted for 18% of completed suicides in the US but only were 8.5% of the total population that same year.10 Yet, little research has been done on the relationship between distress and suicide in veterans living with cancer. Aboumrad and colleagues found that 45% of veterans with cancer who completed suicide reported family issues and 41% endorsed chronic pain.11 This study recommended continued efforts to assess and treat distress to lessen risk of suicide in veterans living with cancer; however, to date, only 1 study has specifically evaluated distress and problems endorsed among veterans living with cancer.7
Suicide prevention is of the highest priority to the US Department of Veterans Affairs (VA).12 Consistent with the VA mission to end veteran suicide, the current study aimed to better understand the relationship between distress and suicide within a sample of veterans living with cancer. Findings would additionally be used to tailor clinical assessments and interventions for veterans living with cancer.
This study had 3 primary goals. First, we sought to understand demographic and clinical factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. Second, the study investigated the most commonly endorsed problems by veterans living with cancer. Finally, we examined which problems were related to suicidal ideation (SI). It was hypothesized that veterans who reported severe distress would be significantly more likely to endorse SI when compared with veterans who reported mild or moderate distress. Based on existing literature, it was further hypothesized that family, emotional, and physical problems would be significantly associated with SI.7,11
Methods
The current study was conducted at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida. Inclusion criteria included veterans who were diagnosed with cancer, attended an outpatient psychology-oncology evaluation, and completed mental health screening measures provided during their evaluation. Exclusion criteria included veterans who: were seen in response to an inpatient consult, were seen solely for a stem cell transplant evaluation, or did not complete the screening measures.
Measures
A veteran’s demographic (eg, age, sex, ethnicity) and clinical (eg, cancer type, stage of disease, recurrence, cancer treatments received) information was abstracted from their VA medical records. Marital status was assessed during a clinical interview and documented as part of the standardized suicide risk assessment.
The Distress Thermometer (DT) is a subjective measure developed by the NCCN.2 The DT provides a visual representation of a thermometer and asks patients to rate their level of distress over the past week with 0 indicating no distress and 10 indicating extreme distress.
The measurement additionally lists 39 problems nested within 5 domains: practical, family, emotional, spiritual/religious, and physical. Patients may endorse listed items under each problem domain by indicating yes or no. Endorsement of various items are intended to provide more detailed information about sources of distress. Due to the predominantly male and mostly older population included in this study the ability to have children measure was removed from the family problem domain.
SI was assessed in 2 ways. First, by patients’ self-report through item-9 of the Patient Health Questionnaire-9 (PHQ-9).14 Item-9 asks “over the last 2 weeks, how often have you been bothered by thoughts that you would be better off dead or of hurting yourself in some way?” Responses range from 0 (not at all) to 3 (nearly every day).14 Responses > 0 were considered a positive screen for SI.
Procedure
Participants were a sample of veterans who were referred for psychology-oncology services. The NCCN DT and Problems List were administered prior to the start of clinical interviews, which followed a checklist and included standardized assessments of SI and history of a suicide attempt(s). A licensed clinical psychologist or a postdoctoral resident conducted these assessments under the supervision of a licensed psychologist. Data gathered during the clinical interview and from the DT and problems list were documented in health records, which were retrospectively reviewed for relevant information (eg, cancer diagnosis, SI). Therefore, informed consent was waived. This study was approved by the JAHVH Institutional Review Board.
Analysis
Data were analyzed using SPSS Version 25. Data analysis proceeded in 3 steps. First, descriptive statistics included the demographic and clinical factors present in the current sample. Difference between those with and without suicidal ideation were compared using F-statistic for continuous variables and χ2 analyses for categorical variables. Second, to examine relationships between each DT problem domain and SI, χ2 analyses were conducted. Third, DT problem domains that had a significant relationship with SI were entered in a logistic regression. This analysis determined which items, if any, from a DT problem domain predicted SI. In the logistic regression model, history of suicide attempts was entered into the first block, as history of suicide attempts is a well-established risk factor for subsequent suicidal ideation. In the second block, other variables that were significantly related to suicidal ideation in the second step of analyses were included. Before interpreting the results of the logistic regression, model fit was tested using the Hosmer-Lemeshow test. Significance of each individual predictor variable in the model is reported using the Wald χ2 statistic; each Wald statistic is compared with a χ2 distribution with 1 degree of freedom (df). Results of logistic regression models also provide information about the effect of each predictor variable in the regression equation (beta weight), odds a veteran who endorsed each predictor variable in the model would also endorse SI (as indicated by the odds ratio), and an estimate of the amount of variance accounted for by each predictor variable (using Nagelkerke’s pseudo R2, which ranges in value from 0 to 1 with higher values indicating more variance explained). For all analyses, P value of .05 (2-tailed) was used for statistical significance.
Results
The sample consisted of 174 veterans (Table 1). The majority (77.6%) were male with a mean age of nearly 62 years (range, 29-87). Most identified as white (74.1%) with half reporting they were either married or living with a partner.
Prostate cancer (19.0%) was the most common type of cancer among study participants followed by head and neck (18.4%), lymphoma/leukemia (11.5%), lung (11.5%), and breast (10.9%); 31.6% had metastatic disease and 14.9% had recurrent disease. Chemotherapy (42.5%) was the most common treatment modality, followed by surgery (38.5%) and radiation (31.6%). The sample was distributed among the 3 distress DT categories: mild (18.4%), moderate (42.5%), and severe (39.1%).
Problems Endorsed
Treatment decisions (44.3%) and insurance/financial concerns (35.1%) were the most frequently endorsed practical problems (Figure 1). Family health issues (33.9%) and dealing with partner (23.0%) were the most frequently endorsed family problems (Figure 2). Worry (73.0%) and depression (69.5%) were the most frequent emotional problems; of note, all emotional problems were endorsed by at least 50% of veterans (Figure 3). Fatigue (71.3%), sleep (70.7%), and pain (69%), were the most frequently endorsed physical problems (Figure 4). Spiritual/religious problems were endorsed by 15% of veterans.
Suicidal Ideation
Overall, 25.3% of veterans endorsed SI. About 20% of veterans reported a history of ≥ 1 suicide attempts in their lifetime. A significant relationship among distress categories and SI was found (χ2 = 18.36, P < .001). Veterans with severe distress were more likely to endorse SI (42.7%) when compared with veterans with mild (9.4%) or moderate (16.2%) distress.
Similarly, a significant relationship among distress categories and a history of a suicide attempt(s) was found (χ2 = 6.08, P = .048). Veterans with severe distress were more likely to have attempted suicide (29.4%) when compared with veterans with mild (12.5%) or moderate (14.9%) distress.
χ2 analyses were conducted to examine the relationships between DT problem domains and SI. A significant relationship was found between family problems and SI (
Logistic regression analyses determined whether items representative of the family problems domain were predictive of SI. Suicide attempt(s) were entered in the first step of the model to evaluate risk factors for SI over this already established risk factor. The assumptions of logistic regression were met.
The Hosmer-Lemeshow test (χ2 = 3.66, df = 5, P = .56) demonstrated that the model fit was good. The group of predictors used in the model differentiate between people who were experiencing SI and those who were not experiencing SI at the time of evaluation. A history of a suicide attempt(s) predicted SI, as expected (Wald = 6.821, df = 1, P = .01). The odds that a veteran with a history of a suicide attempt(s) would endorse SI at the time of the evaluation was nearly 3 times greater than that of veterans without a history of a suicide attempt(s). Over and above suicide attempts, problems dealing with partner (Wald = 15.142; df = 1, P < .001) was a second significant predictor of current SI. The odds that a veteran who endorsed problems dealing with partner would also endorse SI was > 5 times higher than that of veterans who did not endorse problems dealing with partner. This finding represents a significant risk factor for SI, over and above a history of a suicide attempt(s). The other items from the family problems domains were not significant (P > .05) (Table 3).
Discussion
This study aimed to understand factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. As hypothesized, veterans who endorsed severe distress were significantly more likely to endorse SI. They also were more likely to have a history of a suicide attempt(s) when compared with those with mild or moderate distress.
A second aim of this study was to understand the most commonly endorsed problems. Consistent with prior literature, treatment decisions were the most commonly endorsed practical problem; worry and depression were the most common emotional problems; and fatigue, sleep, and pain were the most common physical problems.7
A finding unique to the current study is that family health issues and dealing with partner were specified as the most common family problems. However, a study by Smith and colleagues did not provide information about the rank of most frequently reported problems within this domain.7
The third aim was to understand which problems were related to SI. It was hypothesized that family, emotional, and physical problems would be related to SI. However, results indicated that only family problems (specifically, problems dealing with a partner) were significantly associated with SI among veterans living with cancer.
Contrary to expectations, emotional and physical problems were not found to have a significant relationship with SI. This is likely because veterans endorsed items nested within these problem domains with similar frequency. The lack of significant findings does not suggest that emotional and physical problems are not significant predictors of SI for veterans living with cancer, but that no specific emotional or physical symptom stood out as a predictor of suicidal ideation above the others.
The finding of a significant relationship between family problems (specifically, problems dealing with a partner) and SI in this study is consistent with findings of Aboumrad and colleagues in a study that examined root-cause analyses of completed suicides by veterans living with cancer.11 They found that nearly half the sample endorsed family problems prior to their death, and a small but notable percentage of veterans who completed suicide reported divorce as a stressor prior to their death.
This finding may be explained by Thomas Joiner's interpersonal-psychological theory of suicidal behavior (IPT), which suggests that completed suicide may result from a thwarted sense of belonging, perceived burdensomeness, and acquired capability for suicide.16 Problems dealing with a partner may impact a veteran’s sense of belonging or social connectedness. Problems dealing with a partner also may be attributed to perceived burdens due to limitations imposed by living with cancer and/or undergoing treatment. In both circumstances, the veteran’s social support system may be negatively impacted, and perceived social support is a well-established protective factor against suicide.17
Partner distress is a second consideration. It is likely that veterans’ partners experienced their own distress in response to the veteran’s cancer diagnosis and/or treatment. The partner’s cause, severity, and expression of distress may contribute to problems for the couple.
Finally, the latter point of the IPT refers to acquired capability, or the ability to inflict deadly harm to oneself.18 A military sample was found to have more acquired capability for suicide when compared with a college undergraduate sample.19 A history of a suicide attempt(s) and male gender have been found to significantly predict acquired capability to complete suicide.18 Furthermore, because veterans living with cancer often are in pain, fear of pain associated with suicide may be reduced and, therefore, acquired capability increased. This suggests that male veterans living with cancer who are in pain, have a history of a suicide attempt(s), and current problems with their partner may be an extremely vulnerable population at-risk for suicide. Results from the current study emphasize the importance of veterans having access to mental health and crisis resources for problems dealing with their partner. Partner problems may foreshadow a potentially lethal type of distress.
Strengths
This study’s aims are consistent with the VA’s mission to end veteran suicide and contributes to literature in several important ways.12 First, veterans living with cancer are an understudied population. The current study addresses a gap in existing literature by researching veterans living with cancer and aims to better understand the relationship between cancer-related distress and SI. Second, to the best of the authors’ knowledge, this study is the first to find that problems dealing with a partner significantly increases a veteran’s risk for SI above a history of a suicide attempt(s). Risk assessments now may be more comprehensive through inclusion of this distress factor.
It is recommended that future research use IPT to further investigate the relationship between problems dealing with a partner and SI.16 Future research may do so by including specific measures to assess for the tenants of the theory, including measurements of burdensomeness and belongingness. An expanded knowledge base about what makes problems dealing with a partner a significant suicide risk factor (eg, increased conflict, lack of support, etc.) would better enable clinicians to intervene effectively. Effective intervention may lessen suicidal behaviors or deaths from suicides within the Veteran population.
Limitations
One limitation is the focus on patients who accepted a mental health referral. This study design may limit the generalizability of results to veterans who would not accept mental health treatment. The homogenous sample of veterans is a second limitation. Most participants were male, white, and had a mean age of 62 years. These demographics are representative of the veterans that most typically utilize VA services; however, more research is needed on veterans living with cancer who are female and of diverse racial and ethnic backgrounds. There are likely differences in problems endorsed and factors associated with SI based on age, race, sex, and other socioeconomic factors. A third limitation is the cross-sectional, retrospective nature of this study. Future studies are advised to assess for distress at multiple time points. This is consistent with NCCN Standards of Care for Distress Management.2 Longitudinal data would enable more findings about distress and SI throughout the course of cancer diagnosis and treatment, therefore enhancing clinical implications and informing future research.
Conclusion
This is among the first of studies to investigate distress and factors associated with SI in veterans living with cancer who were referred for psychology services. The prevalence of distress caused by psychosocial factors (including treatment decisions, worry, and depression) highlights the importance of including mental health services as part of comprehensive cancer treatment.
Distress due to treatment decisions may be attributed to a litany of factors such as a veteran’s consideration of adverse effects, effectiveness of treatments, changes to quality of life or functioning, and inclusion of alternative or complimentary treatments. These types of decisions often are reported to be difficult conversations to have with family members or loved ones, who are likely experiencing distress of their own. The role of a mental health provider to assist veterans in exploring their treatment decisions and the implications of such decisions appears important to lessening distress.
Early intervention for emotional symptoms would likely benefit veterans’ management of distress and may lessen suicide risk as depression is known to place veterans at-risk for SI.20 This underscores the importance of timely distress assessment to prevent mild emotional distress from progressing to potentially severe or life-threatening emotional distress. For veterans with a psychiatric history, timely assessment and intervention is essential because psychiatric history is an established suicide risk factor that may be exacerbated by cancer-related distress.12
Furthermore, management of intolerable physical symptoms may lessen risk for suicide.4 Under medical guidance, fatigue may be improved using exercise.21 Behavioral intervention is commonly used as first-line treatment for sleep problems.22 While pain may be lessened through medication or nonpharmacological interventions.23
Considering the numerous ways that distress may present itself (eg, practical, emotional, or physical) and increase risk for SI, it is essential that all veterans living with cancer are assessed for distress and SI, regardless of their presentation. Although veterans may not outwardly express distress, this does not indicate the absence of either distress or risk for suicide. For example, a veteran may be distressed due to financial concerns, transportation issues, and the health of his/her partner or spouse. This veteran may not exhibit visible symptoms of distress, as would be expected when the source of distress is emotional (eg, depression, anxiety). However, this veteran is equally vulnerable to impairing distress and SI as someone who exhibits emotional distress. Distress assessments should be further developed to capture both the visible and less apparent sources of distress, while also serving the imperative function of screening for suicide. Other researchers also have noted the necessity of this development.24 Currently, the NCCN DT and Problems List does not include any assessment of SI or behavior.
Finally, this study identified a potentially critical factor to include in distress assessment: problems dealing with a partner. Problems dealing with a partner have been noted as a source of distress in existing literature, but this is the first study to find problems dealing with a partner to be a predictor of SI in veterans living with cancer.4-6
Because partners often attend appointments with veterans, it is not surprising that problems dealing with their partner are not disclosed more readily. It is recommended that clinicians ask veterans about potential problems with their partner when they are alone. Directly gathering information about such problems while assessing for distress may assist health care workers in providing the most effective, accurate type of intervention in a timely manner, and potentially mitigate risk for suicide.
As recommended by the NCCN and numerous researchers, findings from the current study underscore the importance of accurate, timely assessment of distress.2,4,8 This study makes several important recommendations about how distress assessment may be strengthened and further developed, specifically for the veteran population. This study also expands the current knowledge base of what is known about veterans living with cancer, and has begun to fill a gap in the existing literature. Consistent with the VA mission to end veteran suicide, results suggest that veterans living with cancer should be regularly screened for distress, asked about distress related to their partner, and assessed for SI. Continued efforts to enhance assessment of and response to distress may lessen suicide risk in veterans with cancer.11
Acknowledgements
This study is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
2. Riba MB, Donovan, KA, Andersen, B. National Comprehensive Cancer Network clinical practice guidelines in oncology. Distress management (Version 3.2019). J Natl Compr Can Net, 2019;17(10):1229-1249.
3. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Pianta dosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28.
4. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8(1):4-12.
5. Bulli F, Miccinesi G, Maruelli A, Katz M, Paci E. The measure of psychological distress in cancer patients: the use of distress thermometer in the oncological rehabilitation center of Florence. Support Care Cancer. 2009;17(7):771–779.
6. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
7. Smith J, Berman S, Dimick J, et al. Distress Screening and Management in an Outpatient VA Cancer Clinic: A Pilot Project Involving Ambulatory Patients Across the Disease Trajectory. Fed Pract. 2017;34(Suppl 1):43S–50S.
8. Carlson LE, Waller A, Groff SL, Bultz BD. Screening for distress, the sixth vital sign, in lung cancer patients: effects on pain, fatigue, and common problems--secondary outcomes of a randomized controlled trial. Psychooncology. 2013;22(8):1880-1888.
9. Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003;12(7):694-708.
10. US Department of Veterans Affairs Office of Suicide Prevention. Suicide among veterans and other Americans 2001-2014. https://www.mentalhealth.va.gov/docs/2016suicidedatareport.pdf. Published August 3, 2016. Accessed April 13, 2020.
11. Aboumrad M, Shiner B, Riblet N, Mills, PD, Watts BV. Factors contributing to cancer-related suicide: a study of root-cause-analysis reports. Psychooncology. 2018;27(9):2237-2244.
12. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. National Strategy for Preventing Veteran Suicide 2018–2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf Published 2018. Accessed April 13, 2020.
13. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
14. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613.
15. Martin A, Rief W, Klaiberg A, Braehler E. Validity of the brief patient health questionnaire mood scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28(1):71-77.
16. Joiner TE. Why People Die by Suicide. Cambridge, MA: Harvard University Press, 2005.
17. Kleiman EM, Riskind JH, Schaefer KE. Social support and positive events as suicide resiliency factors: examination of synergistic buffering effects. Arch Suicide Res. 2014;18(2):144-155.
18. Van Orden KA, Witte TK, Gordon KH, Bender TW, Joiner TE Jr. Suicidal desire and the capability for suicide: tests of the interpersonal-psychological theory of suicidal behavior among adults. J Consult Clin Psychol. 2008;76(1):72–83.
19. Bryan CJ, Morrow CE, Anestis MD, Joiner TE. A preliminary test of the interpersonal -psychological theory of suicidal behavior in a military sample. Personal Individual Differ. 2010;48(3):347-350.
20. Miller SN, Monahan CJ, Phillips KM, Agliata D, Gironda RJ. Mental health utilization among veterans at risk for suicide: Data from a post-deployment clinic [published online ahead of print, 2018 Oct 8]. Psychol Serv. 2018;10.1037/ser0000311.
21. Galvão DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899-909.
22. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
23. Ngamkham S, Holden JE, Smith EL. A systematic review: Mindfulness intervention for cancer-related pain. Asia Pac J Oncol Nurs. 2019;6(2):161-169.
24. Granek L, Nakash O, Ben-David M, Shapira S, Ariad S. Oncologists’, nurses’, and social workers’ strategies and barriers to identifying suicide risk in cancer patients. Psychooncology. 2018;27(1):148-154.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
2. Riba MB, Donovan, KA, Andersen, B. National Comprehensive Cancer Network clinical practice guidelines in oncology. Distress management (Version 3.2019). J Natl Compr Can Net, 2019;17(10):1229-1249.
3. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Pianta dosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28.
4. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8(1):4-12.
5. Bulli F, Miccinesi G, Maruelli A, Katz M, Paci E. The measure of psychological distress in cancer patients: the use of distress thermometer in the oncological rehabilitation center of Florence. Support Care Cancer. 2009;17(7):771–779.
6. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
7. Smith J, Berman S, Dimick J, et al. Distress Screening and Management in an Outpatient VA Cancer Clinic: A Pilot Project Involving Ambulatory Patients Across the Disease Trajectory. Fed Pract. 2017;34(Suppl 1):43S–50S.
8. Carlson LE, Waller A, Groff SL, Bultz BD. Screening for distress, the sixth vital sign, in lung cancer patients: effects on pain, fatigue, and common problems--secondary outcomes of a randomized controlled trial. Psychooncology. 2013;22(8):1880-1888.
9. Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003;12(7):694-708.
10. US Department of Veterans Affairs Office of Suicide Prevention. Suicide among veterans and other Americans 2001-2014. https://www.mentalhealth.va.gov/docs/2016suicidedatareport.pdf. Published August 3, 2016. Accessed April 13, 2020.
11. Aboumrad M, Shiner B, Riblet N, Mills, PD, Watts BV. Factors contributing to cancer-related suicide: a study of root-cause-analysis reports. Psychooncology. 2018;27(9):2237-2244.
12. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. National Strategy for Preventing Veteran Suicide 2018–2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf Published 2018. Accessed April 13, 2020.
13. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
14. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613.
15. Martin A, Rief W, Klaiberg A, Braehler E. Validity of the brief patient health questionnaire mood scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28(1):71-77.
16. Joiner TE. Why People Die by Suicide. Cambridge, MA: Harvard University Press, 2005.
17. Kleiman EM, Riskind JH, Schaefer KE. Social support and positive events as suicide resiliency factors: examination of synergistic buffering effects. Arch Suicide Res. 2014;18(2):144-155.
18. Van Orden KA, Witte TK, Gordon KH, Bender TW, Joiner TE Jr. Suicidal desire and the capability for suicide: tests of the interpersonal-psychological theory of suicidal behavior among adults. J Consult Clin Psychol. 2008;76(1):72–83.
19. Bryan CJ, Morrow CE, Anestis MD, Joiner TE. A preliminary test of the interpersonal -psychological theory of suicidal behavior in a military sample. Personal Individual Differ. 2010;48(3):347-350.
20. Miller SN, Monahan CJ, Phillips KM, Agliata D, Gironda RJ. Mental health utilization among veterans at risk for suicide: Data from a post-deployment clinic [published online ahead of print, 2018 Oct 8]. Psychol Serv. 2018;10.1037/ser0000311.
21. Galvão DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899-909.
22. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
23. Ngamkham S, Holden JE, Smith EL. A systematic review: Mindfulness intervention for cancer-related pain. Asia Pac J Oncol Nurs. 2019;6(2):161-169.
24. Granek L, Nakash O, Ben-David M, Shapira S, Ariad S. Oncologists’, nurses’, and social workers’ strategies and barriers to identifying suicide risk in cancer patients. Psychooncology. 2018;27(1):148-154.
Atrial Fibrillation and Bleeding in Patients With Chronic Lymphocytic Leukemia Treated with Ibrutinib in the Veterans Health Administration (FULL)
Chronic lymphocytic leukemia (CLL) is the most common leukemia diagnosed in developed countries, with an estimated 21,040 new diagnoses of CLL expected in the US in 2020. 1-3 CLL is an indolent cancer characterized by the accumulation of B-lymphocytes in the blood, marrow, and lymphoid tissues. 4 It has a heterogeneous clinical course; the majority of patients are observed or receive delayed treatment following diagnosis, while a minority of patients require immediate treatment. After first-line treatment, some patients experience prolonged remissions while others require retreatment within 1 or 2 years. Fortunately, advances in cancer biology and therapeutics in the last decade have increased the number of treatment options available for patients with CLL.
Until recently, most CLL treatments relied on a chemotherapy or a chemoimmunotherapy backbone; however, the last few years have seen novel therapies introduced, such as small molecule inhibitors to target molecular pathways that promote the normal development, expansion, and survival of B-cells.5 One such therapy is ibrutinib, a targeted Bruton tyrosine kinase inhibitor that received accelerated approval by the US Food and Drug Administration (FDA) in February 2014 for patients with CLL who received at least 1 prior therapy. The FDA later expanded this approval to include use of ibrutinib in patients with CLL with relapsed or refractory disease, with or without chromosome 17p deletion. In 2016, based on data from the RESONATE-17 study, the FDA approved ibrutinib for first-line therapy in patients with CLL.6
Ibrutinib’s efficacy, ease of administration and dosing (all doses are oral and fixed, rather than based on weight or body surface area), and relatively favorable safety profile have resulted in a rapid growth in its adoption.7 Since its adverse event (AE) profile is generally more tolerable than that of a typical chemoimmunotherapy, its use in older patients with CLL and patients with significant comorbidities is particularly appealing.8
However, the results of some clinical trials suggest an association between treatment with ibrutinib and an increased risk of bleeding-related events of any grade (44%) and major bleeding events (4%).7,8 The incidence of major bleeding events was reported to be higher (9%) in one clinical trial and at 5-year follow-up, although this trial did not exclude patients receiving concomitant oral anticoagulation with warfarin.6,9
Heterogeneity in clinical trials’ definitions of major bleeding confounded the ability to calculate bleeding risk in patients treated with ibrutinib in a systematic review and meta-analysis that called for more data.10 Additionally, patients with factors that might increase the risk of major bleeding with ibrutinib treatment were likely underrepresented in clinical trials, given the carefully selected nature of clinical trial subjects. These factors include renal or hepatic disease, gastrointestinal disease, and use of a number of concomitant medications such as antiplatelets or anticoagulant medications. Accounting for use of the latter is particularly important because patients who develop atrial fibrillation (Afib), one of the recognized AEs of treatment with ibrutinib, often are treated with anticoagulant medications in order to decrease the risk of stroke or other thromboembolic complications.
A single-site observational study of patients treated with ibrutinib reported a high utilization rate of antiplatelet medications (70%), anticoagulant medications (17%), or both (13%) with a concomitant major bleeding rate of 18% of patients.11 Prevalence of bleeding events seemed to be highly affected by the presence of concomitant medications: 78% of patients treated with ibrutinib while concurrently receiving both antiplatelet and anticoagulant medications developed a major bleeding event, while none of the patients who were not receiving antiplatelets, anticoagulants, or medications that interact with cytochrome P450 (an enzyme that metabolized chemotherapeutic agents used to treat cancer) experienced a major bleeding event.11
The prevalence of major bleeding events, comorbidities, and utilization of medications that could increase the risk of major bleeding in patients with CLL on ibrutinib in the Veterans Health Administration (VHA) is not known. The VHA is the largest integrated health care system in the US. To address these knowledge gaps, a retrospective observational study was conducted using data on demographics, comorbidities that could affect bleeding, use of anticoagulant and antiplatelet medications, and bleeding events in patients with CLL who were treated in the first year of ibrutinib availability from the VHA.
The first year of ibrutinib availability was chosen for this study since we anticipated that many health care providers would be unfamiliar with ibrutinib during that time given its novelty, and therefore more likely to codispense ibrutinib with medications that could increase the risk of a bleeding event. Since Afib is both an AE associated with ibrutinib treatment and a condition that often is treated with anticoagulants, the prevalence of Afib in this population was also included. For context, the incidence of bleeding and Afib and use of anticoagulant and antiplatelet medications during treatment in a cohort of patients with CLL treated with bendamustine + rituximab (BR) also was reported.
Methods
The VHA maintains the centralized US Department of Veterans Affairs Cancer Registry System (VACRS), with electronic medical record data and other sources captured in its Corporate Data Warehouse (CDW). The VHA CDW is a national repository comprising data from several VHA clinical and administrative systems. The CDW includes patient identifiers; demographics; vital status; lab information; administrative information (such as diagnostic International Statistical Classification of Diseases and Related Health Problems [ICD-9] codes); medication dispensation tables (such as outpatient fill); IV package information; and notes from radiology, pathology, outpatient and inpatient admission, discharge, and daily progress.
Registrars abstract all cancer cases within the VHA system (or diagnosed outside the VHA, if patients subsequently receive treatment in the VHA). It is estimated that VACRS captures 3% of cancer cases in the US.12 Like most registries, VACRS captures data such as diagnosis, age, gender, race, and vital status.
The study received approval from the University of Utah Institutional Review Board and used individual patient-level historical administrative, cancer registry, and electronic health care record data. Patients diagnosed and treated for CLL at the VHA from 2010 to 2014 were identified through the VACRS and CDW; patients with a prior malignancy were excluded. Patients who received ibrutinib or BR based on pharmacy dispensation information were selected. Patients were followed until December 31, 2016 or death; patients with documentation of another cancer or lack of utilization of the VHA hematology or oncology services (defined as absence of any hematology and/or oncology clinic visits for ≥ 18 months) were omitted from the final analysis (Figure).
Previous and concomitant utilization of antiplatelet (aspirin, clopidogrel) or anticoagulant (dalteparin, enoxaparin, fondaparinux, heparin, rivaroxaban, and warfarin) medications was extracted 6 months before and after the first dispensation of ibrutinib or BR using pharmacy dispensation records.
Study Definitions
Prevalence of comorbidities that could increase bleeding risk was determined using administrative ICD-9-CM codes. Liver disease was identified by presence of cirrhosis, hepatitis C virus, or alcoholic liver disease using administrative codes validated by Kramer and colleagues, who reported positive and negative predictive values of 90% and 87% for cirrhosis, 93% and 92% for hepatitis C virus, and 71% and 98% for alcoholic liver disease.13 Similarly, end-stage liver disease was identified using a validated coding algorithm developed by Goldberg and colleagues, with a positive predictive value of 89.3%.14 The presence of controlled or uncontrolled diabetes mellitus (DM) was identified using the procedure described by Guzman and colleagues.15 Quan’s algorithm was used to calculate Charlson Comorbidity Index (CCI) based on ICD-9-CM codes for inpatient and outpatient visits within a 6-month lookback period prior to treatment initiation.16
A major bleeding event was defined as a hospitalization with an ICD-9-CM code suggestive of major bleeding as the primary reason, as defined by Lane and colleagues in their study of major bleeding related to warfarin in a cohort of patients treated within the VHA.17 Incidence rates of major bleeding events were identified during the first 6 months of treatment. Incidence of Afib—defined as an inpatient or outpatient encounter with the 427.31 ICD-9-CM code—also was examined within the first 6 months after starting treatment. The period of 6 months was chosen because bendamustine must be discontinued after 6 months.
Study Analysis
Descriptive statistics were used to examine patient demographics, disease characteristics, and treatment history from initial CLL diagnosis through end of study observation period. Categorical variables were summarized using frequencies and accompanying proportions, while a mean and standard deviation were used to summarize continuous variables. For the means of continuous variables and of categorical data, 95% CIs were used. Proportions and accompanying 95% CIs characterized treatment patterns, including line of therapy, comorbidities, and bleeding events. Treatment duration was described using mean and accompanying 95% CI. Statistical tests were not conducted for comparisons among treatment groups. Patients were censored at the end of follow-up, defined as the earliest of the following scenarios: (1) end of study observation period (December 31, 2016); (2) development of a secondary cancer; or (3) last day of contact given absence of care within the VHA for ≥ 18 months (with care defined as oncology and/or oncology/hematology visit with an associated note). Analysis was performed using R 3.4.0.
Results
Between 2010 and 2014, 2,796 patients were diagnosed and received care for CLL within the VHA. Overall, all 172 patients who were treated with ibrutinib during our inclusion period were selected. These patients were treated between January 1, 2014 and December 31, 2016, following ibrutinib’s approval in early 2014. An additional 291 patients were selected who received BR (Table). Reflecting the predominantly male population of the VHA, 282 (97%) BR patients and 167 (97%) ibrutinib patients were male. The median age at diagnosis was 67 years for BR patients and 69 years for ibrutinib patients. About 76% of patients who received ibrutinib and 82% of patients who received BR were non-Hispanic white; 17% and 14% were African American, respectively.
Less than 10% of patients receiving either ibrutinib or BR had liver disease per criteria used by Kramer and colleagues, or end-stage liver disease using criteria developed by Goldberg and colleagues.12,13 About 5% of patients had a history of previous bleeding in the 6-month period prior to initiating either therapy. Mean CCI (excluding malignancy) score was 1.5 (range, 0-11) for the ibrutinib group, and 2.1 (range, 0-9) for the BR group. About 16% of the ibrutinib group had controlled DM and fewer than 10% had uncontrolled DM, while 4% of patients in the BR group met the criteria for controlled DM and another 4% met the criteria for uncontrolled DM.
There was very low utilization of anticoagulant or antiplatelet medication prior to initiation of ibrutinib (2.9% and 2.3%, respectively) or BR (< 1% each). In the first 6 months after treatment initiation, about 8% of patients in both ibrutinib and BR cohorts received anticoagulant medication while antiplatelet utilization was < 5% in either group.
In the BR group, 8 patients (2.7%) experienced a major bleeding event, while 14 patients (8.1%) in the ibrutinib group experienced a bleeding event (P = .008). While these numbers were too low to perform a formal statistical analysis of the association between clinical covariates and bleeding in either group, there did not seem to be an association between bleeding and liver disease or DM. Of patients who experienced a bleeding event, about 1 in 4 patients had had a prior bleeding event in both the ibrutinib and the BR groups. Interestingly, while none of the patients who experienced a bleeding event while receiving BR were taking concomitant anticoagulant medication, 3 of the 14 patients who experienced a bleeding event in the ibrutinib group showed evidence of anticoagulant utilization. Finally, the incidence of Afib (defined as patients with no evidence of Afib in the 6 months prior to treatment but with evidence of Afib in the 6 months following treatment initiation) was 4% in the BR group, and about 8% in the ibrutinib group (P = .003).
Discussion
To the authors’ knowledge, this study is the first to examine the real-world incidence of bleeding and Afib in veterans who received ibrutinib for CLL in the first year of its availability. The study found minimal use of anticoagulants and/or antiplatelet agents prior to receiving first-line ibrutinib or BR, and very low use of these agents in the first 6 months following the initiation of first-line treatment. This finding suggests a high awareness among VA providers of potential adverse effects (AEs) of ibrutinib and chemotherapy, and a careful selection of patients that lack risk factors for AEs.
In patients treated with first-line ibrutinib when compared with patients treated with first-line BR, moderate increases in bleeding (2.7% vs 8.1%, P = .008) and Afib (10.5% vs 3%, P = .003) also were observed. These results are concordant with previous findings examining the use of ibrutinib in patients with CLL.18-20
Limitations
The results of this study should be interpreted with caution, as some limitations must be considered. The study was conducted in the early days of ibrutinib adoption. Since then, more patients have been treated with ibrutinib and for longer durations. As clinicians gain more familiarity and with ibrutinib, and as additional novel therapeutics emerge, it is possible that the initial awareness about risks for possible AEs may diminish; patients with high comorbidity burdens and concomitant medications would be especially vulnerable in cases of reduced physician vigilance.
Another limitation of this study stems from the potential for dual system use among patients treated in the VHA. Concurrent or alternating use of multiple health care systems (use of VHA and private-sector facilities) may present gaps in the reconstruction of patient histories, resulting in missing data as patients transition between commercial, the Centers for Medicare and Medicaid Services, and VHA care. As a result, the results presented here do not reflect instances where a patient experienced a bleeding event treated outside the VA.
Problems with missing data also may occur due to incomplete extraction from the electronic health record; these issues were addressed by leveraging an understanding of the multiple data marts within the CDW environment to harmonize missing and/or erroneous information through use of other data marts when possible. Lastly, this research represents a population-level study of the VHA, thus all findings are directly relevant to the VHA. The generalizability of the findings outside the VHA would depend on the characteristics of the external population.
Conclusion
Real-world evidence from a nationwide cohort of veteran patients with CLL treated with ibrutinib suggest that, while there is an association of increased bleeding-related events and Afib, the risk is comparable to those reported in previous studies.18-20 These findings suggest that patients in real-world clinical care settings with higher levels of comorbidities may be at a slight increased risk for bleeding events and Afib.
1. Scarfò L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169-182.
2. Devereux S, Cuthill K. Chronic lymphocytic leukaemia. Medicine (Baltimore). 2017;45(5):292-296.
3. American Cancer Society. Cancer facts & figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed April 24, 2020.
4. Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096.
5. Owen C, Assouline S, Kuruvilla J, Uchida C, Bellingham C, Sehn L. Novel therapies for chronic lymphocytic leukemia: a Canadian perspective. Clin Lymphoma Myeloma Leuk. 2015;15(11):627-634.e5.
6. O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418.
7. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437.
8. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
9. O’Brien S, Furman R, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919.
10. Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772-778.
11. Kunk PR, Mock J, Devitt ME, Palkimas S, et al. Major bleeding with ibrutinib: more than expected. Blood. 2016;128(22):3229.
12. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
13. Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274-282.
14. Goldberg D, Lewis JD, Halpern SD, Weiner M, Lo Re V 3rd. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21(7):765-769.
15. Guzman JZ, Iatridis JC, Skovrlj B, et al. Outcomes and complications of diabetes mellitus on patients undergoing degenerative lumbar spine surgery. Spine (Phila Pa 1976). 2014;39(19):1596-1604.
16. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
17. Lane MA, Zeringue A, McDonald JR. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127(7):657–663.e2.
18. Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138-140.
19. Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571-1578.
20. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796-1805.
Chronic lymphocytic leukemia (CLL) is the most common leukemia diagnosed in developed countries, with an estimated 21,040 new diagnoses of CLL expected in the US in 2020. 1-3 CLL is an indolent cancer characterized by the accumulation of B-lymphocytes in the blood, marrow, and lymphoid tissues. 4 It has a heterogeneous clinical course; the majority of patients are observed or receive delayed treatment following diagnosis, while a minority of patients require immediate treatment. After first-line treatment, some patients experience prolonged remissions while others require retreatment within 1 or 2 years. Fortunately, advances in cancer biology and therapeutics in the last decade have increased the number of treatment options available for patients with CLL.
Until recently, most CLL treatments relied on a chemotherapy or a chemoimmunotherapy backbone; however, the last few years have seen novel therapies introduced, such as small molecule inhibitors to target molecular pathways that promote the normal development, expansion, and survival of B-cells.5 One such therapy is ibrutinib, a targeted Bruton tyrosine kinase inhibitor that received accelerated approval by the US Food and Drug Administration (FDA) in February 2014 for patients with CLL who received at least 1 prior therapy. The FDA later expanded this approval to include use of ibrutinib in patients with CLL with relapsed or refractory disease, with or without chromosome 17p deletion. In 2016, based on data from the RESONATE-17 study, the FDA approved ibrutinib for first-line therapy in patients with CLL.6
Ibrutinib’s efficacy, ease of administration and dosing (all doses are oral and fixed, rather than based on weight or body surface area), and relatively favorable safety profile have resulted in a rapid growth in its adoption.7 Since its adverse event (AE) profile is generally more tolerable than that of a typical chemoimmunotherapy, its use in older patients with CLL and patients with significant comorbidities is particularly appealing.8
However, the results of some clinical trials suggest an association between treatment with ibrutinib and an increased risk of bleeding-related events of any grade (44%) and major bleeding events (4%).7,8 The incidence of major bleeding events was reported to be higher (9%) in one clinical trial and at 5-year follow-up, although this trial did not exclude patients receiving concomitant oral anticoagulation with warfarin.6,9
Heterogeneity in clinical trials’ definitions of major bleeding confounded the ability to calculate bleeding risk in patients treated with ibrutinib in a systematic review and meta-analysis that called for more data.10 Additionally, patients with factors that might increase the risk of major bleeding with ibrutinib treatment were likely underrepresented in clinical trials, given the carefully selected nature of clinical trial subjects. These factors include renal or hepatic disease, gastrointestinal disease, and use of a number of concomitant medications such as antiplatelets or anticoagulant medications. Accounting for use of the latter is particularly important because patients who develop atrial fibrillation (Afib), one of the recognized AEs of treatment with ibrutinib, often are treated with anticoagulant medications in order to decrease the risk of stroke or other thromboembolic complications.
A single-site observational study of patients treated with ibrutinib reported a high utilization rate of antiplatelet medications (70%), anticoagulant medications (17%), or both (13%) with a concomitant major bleeding rate of 18% of patients.11 Prevalence of bleeding events seemed to be highly affected by the presence of concomitant medications: 78% of patients treated with ibrutinib while concurrently receiving both antiplatelet and anticoagulant medications developed a major bleeding event, while none of the patients who were not receiving antiplatelets, anticoagulants, or medications that interact with cytochrome P450 (an enzyme that metabolized chemotherapeutic agents used to treat cancer) experienced a major bleeding event.11
The prevalence of major bleeding events, comorbidities, and utilization of medications that could increase the risk of major bleeding in patients with CLL on ibrutinib in the Veterans Health Administration (VHA) is not known. The VHA is the largest integrated health care system in the US. To address these knowledge gaps, a retrospective observational study was conducted using data on demographics, comorbidities that could affect bleeding, use of anticoagulant and antiplatelet medications, and bleeding events in patients with CLL who were treated in the first year of ibrutinib availability from the VHA.
The first year of ibrutinib availability was chosen for this study since we anticipated that many health care providers would be unfamiliar with ibrutinib during that time given its novelty, and therefore more likely to codispense ibrutinib with medications that could increase the risk of a bleeding event. Since Afib is both an AE associated with ibrutinib treatment and a condition that often is treated with anticoagulants, the prevalence of Afib in this population was also included. For context, the incidence of bleeding and Afib and use of anticoagulant and antiplatelet medications during treatment in a cohort of patients with CLL treated with bendamustine + rituximab (BR) also was reported.
Methods
The VHA maintains the centralized US Department of Veterans Affairs Cancer Registry System (VACRS), with electronic medical record data and other sources captured in its Corporate Data Warehouse (CDW). The VHA CDW is a national repository comprising data from several VHA clinical and administrative systems. The CDW includes patient identifiers; demographics; vital status; lab information; administrative information (such as diagnostic International Statistical Classification of Diseases and Related Health Problems [ICD-9] codes); medication dispensation tables (such as outpatient fill); IV package information; and notes from radiology, pathology, outpatient and inpatient admission, discharge, and daily progress.
Registrars abstract all cancer cases within the VHA system (or diagnosed outside the VHA, if patients subsequently receive treatment in the VHA). It is estimated that VACRS captures 3% of cancer cases in the US.12 Like most registries, VACRS captures data such as diagnosis, age, gender, race, and vital status.
The study received approval from the University of Utah Institutional Review Board and used individual patient-level historical administrative, cancer registry, and electronic health care record data. Patients diagnosed and treated for CLL at the VHA from 2010 to 2014 were identified through the VACRS and CDW; patients with a prior malignancy were excluded. Patients who received ibrutinib or BR based on pharmacy dispensation information were selected. Patients were followed until December 31, 2016 or death; patients with documentation of another cancer or lack of utilization of the VHA hematology or oncology services (defined as absence of any hematology and/or oncology clinic visits for ≥ 18 months) were omitted from the final analysis (Figure).
Previous and concomitant utilization of antiplatelet (aspirin, clopidogrel) or anticoagulant (dalteparin, enoxaparin, fondaparinux, heparin, rivaroxaban, and warfarin) medications was extracted 6 months before and after the first dispensation of ibrutinib or BR using pharmacy dispensation records.
Study Definitions
Prevalence of comorbidities that could increase bleeding risk was determined using administrative ICD-9-CM codes. Liver disease was identified by presence of cirrhosis, hepatitis C virus, or alcoholic liver disease using administrative codes validated by Kramer and colleagues, who reported positive and negative predictive values of 90% and 87% for cirrhosis, 93% and 92% for hepatitis C virus, and 71% and 98% for alcoholic liver disease.13 Similarly, end-stage liver disease was identified using a validated coding algorithm developed by Goldberg and colleagues, with a positive predictive value of 89.3%.14 The presence of controlled or uncontrolled diabetes mellitus (DM) was identified using the procedure described by Guzman and colleagues.15 Quan’s algorithm was used to calculate Charlson Comorbidity Index (CCI) based on ICD-9-CM codes for inpatient and outpatient visits within a 6-month lookback period prior to treatment initiation.16
A major bleeding event was defined as a hospitalization with an ICD-9-CM code suggestive of major bleeding as the primary reason, as defined by Lane and colleagues in their study of major bleeding related to warfarin in a cohort of patients treated within the VHA.17 Incidence rates of major bleeding events were identified during the first 6 months of treatment. Incidence of Afib—defined as an inpatient or outpatient encounter with the 427.31 ICD-9-CM code—also was examined within the first 6 months after starting treatment. The period of 6 months was chosen because bendamustine must be discontinued after 6 months.
Study Analysis
Descriptive statistics were used to examine patient demographics, disease characteristics, and treatment history from initial CLL diagnosis through end of study observation period. Categorical variables were summarized using frequencies and accompanying proportions, while a mean and standard deviation were used to summarize continuous variables. For the means of continuous variables and of categorical data, 95% CIs were used. Proportions and accompanying 95% CIs characterized treatment patterns, including line of therapy, comorbidities, and bleeding events. Treatment duration was described using mean and accompanying 95% CI. Statistical tests were not conducted for comparisons among treatment groups. Patients were censored at the end of follow-up, defined as the earliest of the following scenarios: (1) end of study observation period (December 31, 2016); (2) development of a secondary cancer; or (3) last day of contact given absence of care within the VHA for ≥ 18 months (with care defined as oncology and/or oncology/hematology visit with an associated note). Analysis was performed using R 3.4.0.
Results
Between 2010 and 2014, 2,796 patients were diagnosed and received care for CLL within the VHA. Overall, all 172 patients who were treated with ibrutinib during our inclusion period were selected. These patients were treated between January 1, 2014 and December 31, 2016, following ibrutinib’s approval in early 2014. An additional 291 patients were selected who received BR (Table). Reflecting the predominantly male population of the VHA, 282 (97%) BR patients and 167 (97%) ibrutinib patients were male. The median age at diagnosis was 67 years for BR patients and 69 years for ibrutinib patients. About 76% of patients who received ibrutinib and 82% of patients who received BR were non-Hispanic white; 17% and 14% were African American, respectively.
Less than 10% of patients receiving either ibrutinib or BR had liver disease per criteria used by Kramer and colleagues, or end-stage liver disease using criteria developed by Goldberg and colleagues.12,13 About 5% of patients had a history of previous bleeding in the 6-month period prior to initiating either therapy. Mean CCI (excluding malignancy) score was 1.5 (range, 0-11) for the ibrutinib group, and 2.1 (range, 0-9) for the BR group. About 16% of the ibrutinib group had controlled DM and fewer than 10% had uncontrolled DM, while 4% of patients in the BR group met the criteria for controlled DM and another 4% met the criteria for uncontrolled DM.
There was very low utilization of anticoagulant or antiplatelet medication prior to initiation of ibrutinib (2.9% and 2.3%, respectively) or BR (< 1% each). In the first 6 months after treatment initiation, about 8% of patients in both ibrutinib and BR cohorts received anticoagulant medication while antiplatelet utilization was < 5% in either group.
In the BR group, 8 patients (2.7%) experienced a major bleeding event, while 14 patients (8.1%) in the ibrutinib group experienced a bleeding event (P = .008). While these numbers were too low to perform a formal statistical analysis of the association between clinical covariates and bleeding in either group, there did not seem to be an association between bleeding and liver disease or DM. Of patients who experienced a bleeding event, about 1 in 4 patients had had a prior bleeding event in both the ibrutinib and the BR groups. Interestingly, while none of the patients who experienced a bleeding event while receiving BR were taking concomitant anticoagulant medication, 3 of the 14 patients who experienced a bleeding event in the ibrutinib group showed evidence of anticoagulant utilization. Finally, the incidence of Afib (defined as patients with no evidence of Afib in the 6 months prior to treatment but with evidence of Afib in the 6 months following treatment initiation) was 4% in the BR group, and about 8% in the ibrutinib group (P = .003).
Discussion
To the authors’ knowledge, this study is the first to examine the real-world incidence of bleeding and Afib in veterans who received ibrutinib for CLL in the first year of its availability. The study found minimal use of anticoagulants and/or antiplatelet agents prior to receiving first-line ibrutinib or BR, and very low use of these agents in the first 6 months following the initiation of first-line treatment. This finding suggests a high awareness among VA providers of potential adverse effects (AEs) of ibrutinib and chemotherapy, and a careful selection of patients that lack risk factors for AEs.
In patients treated with first-line ibrutinib when compared with patients treated with first-line BR, moderate increases in bleeding (2.7% vs 8.1%, P = .008) and Afib (10.5% vs 3%, P = .003) also were observed. These results are concordant with previous findings examining the use of ibrutinib in patients with CLL.18-20
Limitations
The results of this study should be interpreted with caution, as some limitations must be considered. The study was conducted in the early days of ibrutinib adoption. Since then, more patients have been treated with ibrutinib and for longer durations. As clinicians gain more familiarity and with ibrutinib, and as additional novel therapeutics emerge, it is possible that the initial awareness about risks for possible AEs may diminish; patients with high comorbidity burdens and concomitant medications would be especially vulnerable in cases of reduced physician vigilance.
Another limitation of this study stems from the potential for dual system use among patients treated in the VHA. Concurrent or alternating use of multiple health care systems (use of VHA and private-sector facilities) may present gaps in the reconstruction of patient histories, resulting in missing data as patients transition between commercial, the Centers for Medicare and Medicaid Services, and VHA care. As a result, the results presented here do not reflect instances where a patient experienced a bleeding event treated outside the VA.
Problems with missing data also may occur due to incomplete extraction from the electronic health record; these issues were addressed by leveraging an understanding of the multiple data marts within the CDW environment to harmonize missing and/or erroneous information through use of other data marts when possible. Lastly, this research represents a population-level study of the VHA, thus all findings are directly relevant to the VHA. The generalizability of the findings outside the VHA would depend on the characteristics of the external population.
Conclusion
Real-world evidence from a nationwide cohort of veteran patients with CLL treated with ibrutinib suggest that, while there is an association of increased bleeding-related events and Afib, the risk is comparable to those reported in previous studies.18-20 These findings suggest that patients in real-world clinical care settings with higher levels of comorbidities may be at a slight increased risk for bleeding events and Afib.
Chronic lymphocytic leukemia (CLL) is the most common leukemia diagnosed in developed countries, with an estimated 21,040 new diagnoses of CLL expected in the US in 2020. 1-3 CLL is an indolent cancer characterized by the accumulation of B-lymphocytes in the blood, marrow, and lymphoid tissues. 4 It has a heterogeneous clinical course; the majority of patients are observed or receive delayed treatment following diagnosis, while a minority of patients require immediate treatment. After first-line treatment, some patients experience prolonged remissions while others require retreatment within 1 or 2 years. Fortunately, advances in cancer biology and therapeutics in the last decade have increased the number of treatment options available for patients with CLL.
Until recently, most CLL treatments relied on a chemotherapy or a chemoimmunotherapy backbone; however, the last few years have seen novel therapies introduced, such as small molecule inhibitors to target molecular pathways that promote the normal development, expansion, and survival of B-cells.5 One such therapy is ibrutinib, a targeted Bruton tyrosine kinase inhibitor that received accelerated approval by the US Food and Drug Administration (FDA) in February 2014 for patients with CLL who received at least 1 prior therapy. The FDA later expanded this approval to include use of ibrutinib in patients with CLL with relapsed or refractory disease, with or without chromosome 17p deletion. In 2016, based on data from the RESONATE-17 study, the FDA approved ibrutinib for first-line therapy in patients with CLL.6
Ibrutinib’s efficacy, ease of administration and dosing (all doses are oral and fixed, rather than based on weight or body surface area), and relatively favorable safety profile have resulted in a rapid growth in its adoption.7 Since its adverse event (AE) profile is generally more tolerable than that of a typical chemoimmunotherapy, its use in older patients with CLL and patients with significant comorbidities is particularly appealing.8
However, the results of some clinical trials suggest an association between treatment with ibrutinib and an increased risk of bleeding-related events of any grade (44%) and major bleeding events (4%).7,8 The incidence of major bleeding events was reported to be higher (9%) in one clinical trial and at 5-year follow-up, although this trial did not exclude patients receiving concomitant oral anticoagulation with warfarin.6,9
Heterogeneity in clinical trials’ definitions of major bleeding confounded the ability to calculate bleeding risk in patients treated with ibrutinib in a systematic review and meta-analysis that called for more data.10 Additionally, patients with factors that might increase the risk of major bleeding with ibrutinib treatment were likely underrepresented in clinical trials, given the carefully selected nature of clinical trial subjects. These factors include renal or hepatic disease, gastrointestinal disease, and use of a number of concomitant medications such as antiplatelets or anticoagulant medications. Accounting for use of the latter is particularly important because patients who develop atrial fibrillation (Afib), one of the recognized AEs of treatment with ibrutinib, often are treated with anticoagulant medications in order to decrease the risk of stroke or other thromboembolic complications.
A single-site observational study of patients treated with ibrutinib reported a high utilization rate of antiplatelet medications (70%), anticoagulant medications (17%), or both (13%) with a concomitant major bleeding rate of 18% of patients.11 Prevalence of bleeding events seemed to be highly affected by the presence of concomitant medications: 78% of patients treated with ibrutinib while concurrently receiving both antiplatelet and anticoagulant medications developed a major bleeding event, while none of the patients who were not receiving antiplatelets, anticoagulants, or medications that interact with cytochrome P450 (an enzyme that metabolized chemotherapeutic agents used to treat cancer) experienced a major bleeding event.11
The prevalence of major bleeding events, comorbidities, and utilization of medications that could increase the risk of major bleeding in patients with CLL on ibrutinib in the Veterans Health Administration (VHA) is not known. The VHA is the largest integrated health care system in the US. To address these knowledge gaps, a retrospective observational study was conducted using data on demographics, comorbidities that could affect bleeding, use of anticoagulant and antiplatelet medications, and bleeding events in patients with CLL who were treated in the first year of ibrutinib availability from the VHA.
The first year of ibrutinib availability was chosen for this study since we anticipated that many health care providers would be unfamiliar with ibrutinib during that time given its novelty, and therefore more likely to codispense ibrutinib with medications that could increase the risk of a bleeding event. Since Afib is both an AE associated with ibrutinib treatment and a condition that often is treated with anticoagulants, the prevalence of Afib in this population was also included. For context, the incidence of bleeding and Afib and use of anticoagulant and antiplatelet medications during treatment in a cohort of patients with CLL treated with bendamustine + rituximab (BR) also was reported.
Methods
The VHA maintains the centralized US Department of Veterans Affairs Cancer Registry System (VACRS), with electronic medical record data and other sources captured in its Corporate Data Warehouse (CDW). The VHA CDW is a national repository comprising data from several VHA clinical and administrative systems. The CDW includes patient identifiers; demographics; vital status; lab information; administrative information (such as diagnostic International Statistical Classification of Diseases and Related Health Problems [ICD-9] codes); medication dispensation tables (such as outpatient fill); IV package information; and notes from radiology, pathology, outpatient and inpatient admission, discharge, and daily progress.
Registrars abstract all cancer cases within the VHA system (or diagnosed outside the VHA, if patients subsequently receive treatment in the VHA). It is estimated that VACRS captures 3% of cancer cases in the US.12 Like most registries, VACRS captures data such as diagnosis, age, gender, race, and vital status.
The study received approval from the University of Utah Institutional Review Board and used individual patient-level historical administrative, cancer registry, and electronic health care record data. Patients diagnosed and treated for CLL at the VHA from 2010 to 2014 were identified through the VACRS and CDW; patients with a prior malignancy were excluded. Patients who received ibrutinib or BR based on pharmacy dispensation information were selected. Patients were followed until December 31, 2016 or death; patients with documentation of another cancer or lack of utilization of the VHA hematology or oncology services (defined as absence of any hematology and/or oncology clinic visits for ≥ 18 months) were omitted from the final analysis (Figure).
Previous and concomitant utilization of antiplatelet (aspirin, clopidogrel) or anticoagulant (dalteparin, enoxaparin, fondaparinux, heparin, rivaroxaban, and warfarin) medications was extracted 6 months before and after the first dispensation of ibrutinib or BR using pharmacy dispensation records.
Study Definitions
Prevalence of comorbidities that could increase bleeding risk was determined using administrative ICD-9-CM codes. Liver disease was identified by presence of cirrhosis, hepatitis C virus, or alcoholic liver disease using administrative codes validated by Kramer and colleagues, who reported positive and negative predictive values of 90% and 87% for cirrhosis, 93% and 92% for hepatitis C virus, and 71% and 98% for alcoholic liver disease.13 Similarly, end-stage liver disease was identified using a validated coding algorithm developed by Goldberg and colleagues, with a positive predictive value of 89.3%.14 The presence of controlled or uncontrolled diabetes mellitus (DM) was identified using the procedure described by Guzman and colleagues.15 Quan’s algorithm was used to calculate Charlson Comorbidity Index (CCI) based on ICD-9-CM codes for inpatient and outpatient visits within a 6-month lookback period prior to treatment initiation.16
A major bleeding event was defined as a hospitalization with an ICD-9-CM code suggestive of major bleeding as the primary reason, as defined by Lane and colleagues in their study of major bleeding related to warfarin in a cohort of patients treated within the VHA.17 Incidence rates of major bleeding events were identified during the first 6 months of treatment. Incidence of Afib—defined as an inpatient or outpatient encounter with the 427.31 ICD-9-CM code—also was examined within the first 6 months after starting treatment. The period of 6 months was chosen because bendamustine must be discontinued after 6 months.
Study Analysis
Descriptive statistics were used to examine patient demographics, disease characteristics, and treatment history from initial CLL diagnosis through end of study observation period. Categorical variables were summarized using frequencies and accompanying proportions, while a mean and standard deviation were used to summarize continuous variables. For the means of continuous variables and of categorical data, 95% CIs were used. Proportions and accompanying 95% CIs characterized treatment patterns, including line of therapy, comorbidities, and bleeding events. Treatment duration was described using mean and accompanying 95% CI. Statistical tests were not conducted for comparisons among treatment groups. Patients were censored at the end of follow-up, defined as the earliest of the following scenarios: (1) end of study observation period (December 31, 2016); (2) development of a secondary cancer; or (3) last day of contact given absence of care within the VHA for ≥ 18 months (with care defined as oncology and/or oncology/hematology visit with an associated note). Analysis was performed using R 3.4.0.
Results
Between 2010 and 2014, 2,796 patients were diagnosed and received care for CLL within the VHA. Overall, all 172 patients who were treated with ibrutinib during our inclusion period were selected. These patients were treated between January 1, 2014 and December 31, 2016, following ibrutinib’s approval in early 2014. An additional 291 patients were selected who received BR (Table). Reflecting the predominantly male population of the VHA, 282 (97%) BR patients and 167 (97%) ibrutinib patients were male. The median age at diagnosis was 67 years for BR patients and 69 years for ibrutinib patients. About 76% of patients who received ibrutinib and 82% of patients who received BR were non-Hispanic white; 17% and 14% were African American, respectively.
Less than 10% of patients receiving either ibrutinib or BR had liver disease per criteria used by Kramer and colleagues, or end-stage liver disease using criteria developed by Goldberg and colleagues.12,13 About 5% of patients had a history of previous bleeding in the 6-month period prior to initiating either therapy. Mean CCI (excluding malignancy) score was 1.5 (range, 0-11) for the ibrutinib group, and 2.1 (range, 0-9) for the BR group. About 16% of the ibrutinib group had controlled DM and fewer than 10% had uncontrolled DM, while 4% of patients in the BR group met the criteria for controlled DM and another 4% met the criteria for uncontrolled DM.
There was very low utilization of anticoagulant or antiplatelet medication prior to initiation of ibrutinib (2.9% and 2.3%, respectively) or BR (< 1% each). In the first 6 months after treatment initiation, about 8% of patients in both ibrutinib and BR cohorts received anticoagulant medication while antiplatelet utilization was < 5% in either group.
In the BR group, 8 patients (2.7%) experienced a major bleeding event, while 14 patients (8.1%) in the ibrutinib group experienced a bleeding event (P = .008). While these numbers were too low to perform a formal statistical analysis of the association between clinical covariates and bleeding in either group, there did not seem to be an association between bleeding and liver disease or DM. Of patients who experienced a bleeding event, about 1 in 4 patients had had a prior bleeding event in both the ibrutinib and the BR groups. Interestingly, while none of the patients who experienced a bleeding event while receiving BR were taking concomitant anticoagulant medication, 3 of the 14 patients who experienced a bleeding event in the ibrutinib group showed evidence of anticoagulant utilization. Finally, the incidence of Afib (defined as patients with no evidence of Afib in the 6 months prior to treatment but with evidence of Afib in the 6 months following treatment initiation) was 4% in the BR group, and about 8% in the ibrutinib group (P = .003).
Discussion
To the authors’ knowledge, this study is the first to examine the real-world incidence of bleeding and Afib in veterans who received ibrutinib for CLL in the first year of its availability. The study found minimal use of anticoagulants and/or antiplatelet agents prior to receiving first-line ibrutinib or BR, and very low use of these agents in the first 6 months following the initiation of first-line treatment. This finding suggests a high awareness among VA providers of potential adverse effects (AEs) of ibrutinib and chemotherapy, and a careful selection of patients that lack risk factors for AEs.
In patients treated with first-line ibrutinib when compared with patients treated with first-line BR, moderate increases in bleeding (2.7% vs 8.1%, P = .008) and Afib (10.5% vs 3%, P = .003) also were observed. These results are concordant with previous findings examining the use of ibrutinib in patients with CLL.18-20
Limitations
The results of this study should be interpreted with caution, as some limitations must be considered. The study was conducted in the early days of ibrutinib adoption. Since then, more patients have been treated with ibrutinib and for longer durations. As clinicians gain more familiarity and with ibrutinib, and as additional novel therapeutics emerge, it is possible that the initial awareness about risks for possible AEs may diminish; patients with high comorbidity burdens and concomitant medications would be especially vulnerable in cases of reduced physician vigilance.
Another limitation of this study stems from the potential for dual system use among patients treated in the VHA. Concurrent or alternating use of multiple health care systems (use of VHA and private-sector facilities) may present gaps in the reconstruction of patient histories, resulting in missing data as patients transition between commercial, the Centers for Medicare and Medicaid Services, and VHA care. As a result, the results presented here do not reflect instances where a patient experienced a bleeding event treated outside the VA.
Problems with missing data also may occur due to incomplete extraction from the electronic health record; these issues were addressed by leveraging an understanding of the multiple data marts within the CDW environment to harmonize missing and/or erroneous information through use of other data marts when possible. Lastly, this research represents a population-level study of the VHA, thus all findings are directly relevant to the VHA. The generalizability of the findings outside the VHA would depend on the characteristics of the external population.
Conclusion
Real-world evidence from a nationwide cohort of veteran patients with CLL treated with ibrutinib suggest that, while there is an association of increased bleeding-related events and Afib, the risk is comparable to those reported in previous studies.18-20 These findings suggest that patients in real-world clinical care settings with higher levels of comorbidities may be at a slight increased risk for bleeding events and Afib.
1. Scarfò L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169-182.
2. Devereux S, Cuthill K. Chronic lymphocytic leukaemia. Medicine (Baltimore). 2017;45(5):292-296.
3. American Cancer Society. Cancer facts & figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed April 24, 2020.
4. Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096.
5. Owen C, Assouline S, Kuruvilla J, Uchida C, Bellingham C, Sehn L. Novel therapies for chronic lymphocytic leukemia: a Canadian perspective. Clin Lymphoma Myeloma Leuk. 2015;15(11):627-634.e5.
6. O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418.
7. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437.
8. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
9. O’Brien S, Furman R, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919.
10. Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772-778.
11. Kunk PR, Mock J, Devitt ME, Palkimas S, et al. Major bleeding with ibrutinib: more than expected. Blood. 2016;128(22):3229.
12. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
13. Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274-282.
14. Goldberg D, Lewis JD, Halpern SD, Weiner M, Lo Re V 3rd. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21(7):765-769.
15. Guzman JZ, Iatridis JC, Skovrlj B, et al. Outcomes and complications of diabetes mellitus on patients undergoing degenerative lumbar spine surgery. Spine (Phila Pa 1976). 2014;39(19):1596-1604.
16. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
17. Lane MA, Zeringue A, McDonald JR. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127(7):657–663.e2.
18. Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138-140.
19. Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571-1578.
20. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796-1805.
1. Scarfò L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169-182.
2. Devereux S, Cuthill K. Chronic lymphocytic leukaemia. Medicine (Baltimore). 2017;45(5):292-296.
3. American Cancer Society. Cancer facts & figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed April 24, 2020.
4. Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096.
5. Owen C, Assouline S, Kuruvilla J, Uchida C, Bellingham C, Sehn L. Novel therapies for chronic lymphocytic leukemia: a Canadian perspective. Clin Lymphoma Myeloma Leuk. 2015;15(11):627-634.e5.
6. O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418.
7. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437.
8. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
9. O’Brien S, Furman R, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919.
10. Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772-778.
11. Kunk PR, Mock J, Devitt ME, Palkimas S, et al. Major bleeding with ibrutinib: more than expected. Blood. 2016;128(22):3229.
12. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
13. Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274-282.
14. Goldberg D, Lewis JD, Halpern SD, Weiner M, Lo Re V 3rd. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21(7):765-769.
15. Guzman JZ, Iatridis JC, Skovrlj B, et al. Outcomes and complications of diabetes mellitus on patients undergoing degenerative lumbar spine surgery. Spine (Phila Pa 1976). 2014;39(19):1596-1604.
16. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
17. Lane MA, Zeringue A, McDonald JR. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127(7):657–663.e2.
18. Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138-140.
19. Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571-1578.
20. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796-1805.
Radiotherapeutic Care of Patients With Stage IV Lung Cancer with Thoracic Symptoms in the Veterans Health Administration (FULL)
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
Neurologic drug prices jump 50% in five years
, new research shows. Results of the retrospective study also showed that most of the increased costs for these agents were due to rising costs for neuroimmunology drugs, mainly for those used to treat multiple sclerosis (MS).
“The same brand name medication in 2017 cost approximately 50% more than in 2013,” said Adam de Havenon, MD, assistant professor of neurology, University of Utah, Salt Lake City.
“An analogy would be if you bought an iPhone 5 in 2013 for $500, and then in 2017, you were asked to pay $750 for the exact same iPhone 5,” Dr. de Havenon added.
The study findings were published online March 10 in the journal Neurology.
$26 billion in payments
Both neurologists and patients are concerned about the high cost of prescription drugs for neurologic diseases, and Medicare Part D data indicate that these drugs are the most expensive component of neurologic care, the researchers noted. In addition, out-of-pocket costs have increased significantly for patients with neurologic disease such as Parkinson’s disease, epilepsy, and MS.
To understand trends in payments for neurologic drugs, Dr. de Havenon and colleagues analyzed Medicare Part D claims filed from 2013 to 2017. The payments include costs paid by Medicare, the patient, government subsidies, and other third-party payers.
In addition to examining more current Medicare Part D data than previous studies, the current analysis examined all medications prescribed by neurologists that consistently remained branded or generic during the 5-year study period, said Dr. de Havenon. This approach resulted in a large number of claims and a large total cost.
To calculate the percentage change in annual payment claims, the researchers used 2013 prices as a reference point. They identified drugs named in 2013 claims and classified them as generic, brand-name only, or brand-name with generic equivalent. Researchers also divided the drugs by neurologic subspecialty.
The analysis included 520 drugs, all of which were available in each year of the study period. Of these drugs, 322 were generic, 61 were brand-name only, and 137 were brand-name with a generic equivalent. There were 90.7 million total claims.
Results showed total payments amounted to $26.65 billion. Yearly total payments increased from $4.05 billion in 2013 to $6.09 billion in 2017, representing a 50.4% increase, even after adjusting for inflation. Total claims increased by 7.6% – from 17.1 million in 2013 to 18.4 million in 2017.
From 2013 to 2017, claim payments increased by 0.6% for generic drugs, 42.4% for brand-name only drugs, and 45% for brand-name drugs with generic equivalents. The proportion of claims increased from 81.9% to 88% for generic drugs and from 4.9% to 6.2% for brand-name only drugs.
However, the proportion of claims for brand-name drugs with generic equivalents decreased from 13.3% to 5.8%.
Treatment barrier
Neuroimmunologic drugs, most of which were prescribed for MS, had exceptional cost, the researchers noted. These drugs accounted for more than 50% of payments but only 4.3% of claims. Claim payment for these drugs increased by 46.9% during the study period, from $3,337 to $4,902.
When neuroimmunologic drugs were removed from the analysis there was still significant increase in claim payments for brand-name only drugs (50.4%) and brand-name drugs with generic equivalents (45.6%).
Although neuroimmunologic medicines, including monoclonal antibodies, are more expensive to produce, this factor alone does not explain their exceptional cost, said Dr. de Havenon. “The high cost of brand-name drugs in this speciality is likely because the market bears it,” he added. “In other words, MS is a disabling disease and the medications work, so historically the Centers for Medicare & Medicaid Services have been willing to tolerate the high cost of these primarily brand-name medications.”
Several countries have controlled drug costs by negotiating with pharmaceutical companies and through legislation, Dr. de Havenon noted.
“My intent with this article was to raise awareness on the topic, which I struggle with frequently as a clinician. I know I want my patients to have a medication, but the cost prevents it,” he said.
‘Unfettered’ price-setting
Commenting on the findings, Robert J. Fox, MD, vice chair for research at the Neurological Institute of the Cleveland Clinic, said the study “brings into clear light” what neurologists, particularly those who treat MS, have long suspected but did not really know. These neurologists “are typically distanced from the payment aspects of the medications they prescribe,” said Dr. Fox, who was not involved with the research.
Although a particular strength of the study was its comprehensiveness, the researchers excluded infusion claims – which account for a large portion of total patient care costs for many disorders, he noted.
Drugs for MS historically have been expensive, ostensibly because of their high cost of development. In addition, the large and continued price increase that occurs long after these drugs have been approved remains unexplained, said Dr. Fox.
He noted that the study findings might not directly affect clinical practice because neurologists will continue prescribing medications they think are best for their patients. “Instead, I think this is a lesson to lawmakers about the massive error in the Medicare Modernization Act of 2003, where the federal government was prohibited from negotiating drug prices. If the seller is unfettered in setting a price, then no one should be surprised when the price rises,” Dr. Fox said.
Because many new drugs and new generic formulations for treating MS have become available during the past year, “repeating these types of economic studies for the period 2020-2025 will help us understand if generic competition – as well as new laws if they are passed – alter price,” he concluded.
The study was funded by the American Academy of Neurology, which publishes Neurology. Dr. de Havenon has received clinical research funding from AMAG Pharmaceuticals and Regeneron Pharmaceuticals. Dr. Fox receives consulting fees from many pharmaceutical companies involved in the development of therapies for MS.
A version of this article first appeared on Medscape.com.
, new research shows. Results of the retrospective study also showed that most of the increased costs for these agents were due to rising costs for neuroimmunology drugs, mainly for those used to treat multiple sclerosis (MS).
“The same brand name medication in 2017 cost approximately 50% more than in 2013,” said Adam de Havenon, MD, assistant professor of neurology, University of Utah, Salt Lake City.
“An analogy would be if you bought an iPhone 5 in 2013 for $500, and then in 2017, you were asked to pay $750 for the exact same iPhone 5,” Dr. de Havenon added.
The study findings were published online March 10 in the journal Neurology.
$26 billion in payments
Both neurologists and patients are concerned about the high cost of prescription drugs for neurologic diseases, and Medicare Part D data indicate that these drugs are the most expensive component of neurologic care, the researchers noted. In addition, out-of-pocket costs have increased significantly for patients with neurologic disease such as Parkinson’s disease, epilepsy, and MS.
To understand trends in payments for neurologic drugs, Dr. de Havenon and colleagues analyzed Medicare Part D claims filed from 2013 to 2017. The payments include costs paid by Medicare, the patient, government subsidies, and other third-party payers.
In addition to examining more current Medicare Part D data than previous studies, the current analysis examined all medications prescribed by neurologists that consistently remained branded or generic during the 5-year study period, said Dr. de Havenon. This approach resulted in a large number of claims and a large total cost.
To calculate the percentage change in annual payment claims, the researchers used 2013 prices as a reference point. They identified drugs named in 2013 claims and classified them as generic, brand-name only, or brand-name with generic equivalent. Researchers also divided the drugs by neurologic subspecialty.
The analysis included 520 drugs, all of which were available in each year of the study period. Of these drugs, 322 were generic, 61 were brand-name only, and 137 were brand-name with a generic equivalent. There were 90.7 million total claims.
Results showed total payments amounted to $26.65 billion. Yearly total payments increased from $4.05 billion in 2013 to $6.09 billion in 2017, representing a 50.4% increase, even after adjusting for inflation. Total claims increased by 7.6% – from 17.1 million in 2013 to 18.4 million in 2017.
From 2013 to 2017, claim payments increased by 0.6% for generic drugs, 42.4% for brand-name only drugs, and 45% for brand-name drugs with generic equivalents. The proportion of claims increased from 81.9% to 88% for generic drugs and from 4.9% to 6.2% for brand-name only drugs.
However, the proportion of claims for brand-name drugs with generic equivalents decreased from 13.3% to 5.8%.
Treatment barrier
Neuroimmunologic drugs, most of which were prescribed for MS, had exceptional cost, the researchers noted. These drugs accounted for more than 50% of payments but only 4.3% of claims. Claim payment for these drugs increased by 46.9% during the study period, from $3,337 to $4,902.
When neuroimmunologic drugs were removed from the analysis there was still significant increase in claim payments for brand-name only drugs (50.4%) and brand-name drugs with generic equivalents (45.6%).
Although neuroimmunologic medicines, including monoclonal antibodies, are more expensive to produce, this factor alone does not explain their exceptional cost, said Dr. de Havenon. “The high cost of brand-name drugs in this speciality is likely because the market bears it,” he added. “In other words, MS is a disabling disease and the medications work, so historically the Centers for Medicare & Medicaid Services have been willing to tolerate the high cost of these primarily brand-name medications.”
Several countries have controlled drug costs by negotiating with pharmaceutical companies and through legislation, Dr. de Havenon noted.
“My intent with this article was to raise awareness on the topic, which I struggle with frequently as a clinician. I know I want my patients to have a medication, but the cost prevents it,” he said.
‘Unfettered’ price-setting
Commenting on the findings, Robert J. Fox, MD, vice chair for research at the Neurological Institute of the Cleveland Clinic, said the study “brings into clear light” what neurologists, particularly those who treat MS, have long suspected but did not really know. These neurologists “are typically distanced from the payment aspects of the medications they prescribe,” said Dr. Fox, who was not involved with the research.
Although a particular strength of the study was its comprehensiveness, the researchers excluded infusion claims – which account for a large portion of total patient care costs for many disorders, he noted.
Drugs for MS historically have been expensive, ostensibly because of their high cost of development. In addition, the large and continued price increase that occurs long after these drugs have been approved remains unexplained, said Dr. Fox.
He noted that the study findings might not directly affect clinical practice because neurologists will continue prescribing medications they think are best for their patients. “Instead, I think this is a lesson to lawmakers about the massive error in the Medicare Modernization Act of 2003, where the federal government was prohibited from negotiating drug prices. If the seller is unfettered in setting a price, then no one should be surprised when the price rises,” Dr. Fox said.
Because many new drugs and new generic formulations for treating MS have become available during the past year, “repeating these types of economic studies for the period 2020-2025 will help us understand if generic competition – as well as new laws if they are passed – alter price,” he concluded.
The study was funded by the American Academy of Neurology, which publishes Neurology. Dr. de Havenon has received clinical research funding from AMAG Pharmaceuticals and Regeneron Pharmaceuticals. Dr. Fox receives consulting fees from many pharmaceutical companies involved in the development of therapies for MS.
A version of this article first appeared on Medscape.com.
, new research shows. Results of the retrospective study also showed that most of the increased costs for these agents were due to rising costs for neuroimmunology drugs, mainly for those used to treat multiple sclerosis (MS).
“The same brand name medication in 2017 cost approximately 50% more than in 2013,” said Adam de Havenon, MD, assistant professor of neurology, University of Utah, Salt Lake City.
“An analogy would be if you bought an iPhone 5 in 2013 for $500, and then in 2017, you were asked to pay $750 for the exact same iPhone 5,” Dr. de Havenon added.
The study findings were published online March 10 in the journal Neurology.
$26 billion in payments
Both neurologists and patients are concerned about the high cost of prescription drugs for neurologic diseases, and Medicare Part D data indicate that these drugs are the most expensive component of neurologic care, the researchers noted. In addition, out-of-pocket costs have increased significantly for patients with neurologic disease such as Parkinson’s disease, epilepsy, and MS.
To understand trends in payments for neurologic drugs, Dr. de Havenon and colleagues analyzed Medicare Part D claims filed from 2013 to 2017. The payments include costs paid by Medicare, the patient, government subsidies, and other third-party payers.
In addition to examining more current Medicare Part D data than previous studies, the current analysis examined all medications prescribed by neurologists that consistently remained branded or generic during the 5-year study period, said Dr. de Havenon. This approach resulted in a large number of claims and a large total cost.
To calculate the percentage change in annual payment claims, the researchers used 2013 prices as a reference point. They identified drugs named in 2013 claims and classified them as generic, brand-name only, or brand-name with generic equivalent. Researchers also divided the drugs by neurologic subspecialty.
The analysis included 520 drugs, all of which were available in each year of the study period. Of these drugs, 322 were generic, 61 were brand-name only, and 137 were brand-name with a generic equivalent. There were 90.7 million total claims.
Results showed total payments amounted to $26.65 billion. Yearly total payments increased from $4.05 billion in 2013 to $6.09 billion in 2017, representing a 50.4% increase, even after adjusting for inflation. Total claims increased by 7.6% – from 17.1 million in 2013 to 18.4 million in 2017.
From 2013 to 2017, claim payments increased by 0.6% for generic drugs, 42.4% for brand-name only drugs, and 45% for brand-name drugs with generic equivalents. The proportion of claims increased from 81.9% to 88% for generic drugs and from 4.9% to 6.2% for brand-name only drugs.
However, the proportion of claims for brand-name drugs with generic equivalents decreased from 13.3% to 5.8%.
Treatment barrier
Neuroimmunologic drugs, most of which were prescribed for MS, had exceptional cost, the researchers noted. These drugs accounted for more than 50% of payments but only 4.3% of claims. Claim payment for these drugs increased by 46.9% during the study period, from $3,337 to $4,902.
When neuroimmunologic drugs were removed from the analysis there was still significant increase in claim payments for brand-name only drugs (50.4%) and brand-name drugs with generic equivalents (45.6%).
Although neuroimmunologic medicines, including monoclonal antibodies, are more expensive to produce, this factor alone does not explain their exceptional cost, said Dr. de Havenon. “The high cost of brand-name drugs in this speciality is likely because the market bears it,” he added. “In other words, MS is a disabling disease and the medications work, so historically the Centers for Medicare & Medicaid Services have been willing to tolerate the high cost of these primarily brand-name medications.”
Several countries have controlled drug costs by negotiating with pharmaceutical companies and through legislation, Dr. de Havenon noted.
“My intent with this article was to raise awareness on the topic, which I struggle with frequently as a clinician. I know I want my patients to have a medication, but the cost prevents it,” he said.
‘Unfettered’ price-setting
Commenting on the findings, Robert J. Fox, MD, vice chair for research at the Neurological Institute of the Cleveland Clinic, said the study “brings into clear light” what neurologists, particularly those who treat MS, have long suspected but did not really know. These neurologists “are typically distanced from the payment aspects of the medications they prescribe,” said Dr. Fox, who was not involved with the research.
Although a particular strength of the study was its comprehensiveness, the researchers excluded infusion claims – which account for a large portion of total patient care costs for many disorders, he noted.
Drugs for MS historically have been expensive, ostensibly because of their high cost of development. In addition, the large and continued price increase that occurs long after these drugs have been approved remains unexplained, said Dr. Fox.
He noted that the study findings might not directly affect clinical practice because neurologists will continue prescribing medications they think are best for their patients. “Instead, I think this is a lesson to lawmakers about the massive error in the Medicare Modernization Act of 2003, where the federal government was prohibited from negotiating drug prices. If the seller is unfettered in setting a price, then no one should be surprised when the price rises,” Dr. Fox said.
Because many new drugs and new generic formulations for treating MS have become available during the past year, “repeating these types of economic studies for the period 2020-2025 will help us understand if generic competition – as well as new laws if they are passed – alter price,” he concluded.
The study was funded by the American Academy of Neurology, which publishes Neurology. Dr. de Havenon has received clinical research funding from AMAG Pharmaceuticals and Regeneron Pharmaceuticals. Dr. Fox receives consulting fees from many pharmaceutical companies involved in the development of therapies for MS.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Acute care of migraine and cluster headaches: Mainstay treatments and emerging strategies
Acute migraine headache attacks
A recent review in the Journal of Neuro-Ophthalmology by Konstantinos Spingos and colleagues (including me as the senior author) details typical and new treatments for migraine. We all know about the longstanding options, including the 7 triptans and ergots, as well as over-the-counter analgesics, which can be combined with caffeine, nonsteroidal anti-inflammatory drugs; and many use the 2 categories of medication that I no longer use for migraine, butalbital-containing medications, and opioids.
Now 2 gepants are available—small molecule calcitonin gene-related peptide (CGRP) receptor antagonists. These medications are thought to be a useful alternative for those in whom triptans do not work or are relatively contraindicated due to coronary and cerebrovascular problems and other cardiac risk factors like obesity, smoking, lack of exercise, high cholesterol, and diabetes. Ubrogepant was approved by the FDA in 2019, and rimegepant soon followed in 2020.
- Ubrogepant: In the ACHIEVE trials, approximately 1 in 5 participants who received the 50 mg dose were pain-free at 2 hours. Moreover, nearly 40% of individuals who received it said their worst migraine symptom was resolved at 2 hours. Pain relief at 2 hours was 59%
- Rimegepant: Like ubrogepant, about 20% of trial participants who received the 75 mg melt tablet dose of rimegepant were pain-free at 2 hours. Thirty-seven percent reported that their worst migraine symptom was gone at 2 hours. Patients began to return to normal functioning in 15 minutes.
In addition to gepants, there is 1 ditan approved, which stimulates 5-HT1F receptors. Lasmiditan is the first medication in this class to be FDA-approved. It, too, is considered an alternative in patients in whom triptans are ineffective or when patients should not take a vasoconstrictor. In the most recent phase 3 study, the percentage of individuals who received lasmiditan and were pain-free at 2 hours were 28% (50 mg), 31% (100 mg) and 39% (200 mg). Relief from the migraine sufferers’ most bothersome symptom at 2 hours occurred in 41%, 44%, and 49% of patients, respectively. Lasmiditan is a Class V controlled substance. It has 18% dizziness in clinical trials. After administration, patients should not drive for 8 hours, and it should only be used once in a 24-hour period.
Non-pharmaceutical treatment options for acute migraine include nerve stimulation using electrical and magnetic stimulation devices, and behavioral approaches such as biofeedback training and mindfulness. The Nerivio device for the upper arm is controlled by a smart phone app and seems to work as well as a triptan in some patients with almost no adverse events. Just approved in February is the Relivion device which is worn like a tiara on the head and stimulates the frontal branches of the trigeminal nerve as well as the 2 occipital nerves in the back of the head.
Acute care of cluster headache attacks
In 2011, Ashkenazi and Schwedt published a comprehensive table in Headache outlining the treatment options for acute cluster headache. More recently, a review in CNS Drugs by Brandt and colleagues presented the choices with level 1 evidence for efficacy. They include:
- Sumatriptan, 6 mg subcutaneous injection, or 20 mg nasal spray
- Zolmitriptan, 5 or 10 mg nasal spray
- Oxygen, 100%, 7 to 12 liters per minute via a mask over the nose and mouth
The authors recommend subcutaneous sumatriptan 6 mg and/or high-flow oxygen at 9- to 12- liters per minute for 15 minutes. Subcutaneous sumatriptan, they note, has been shown to achieve pain relief within 15 minutes in 75% of patients who receive it. Moreover, one-third report pain freedom. Oxygen’s efficacy has long been established, and relief comes with no adverse events. As for mask type, though no significant differences have been observed in studies, patients appear to express a preference for the demand valve oxygen type, which allows a high flow rate and is dependent on the user’s breathing rate.
Lidocaine intranasally has been found to be effective when triptans or oxygen do not work, according to a review in The Lancet Neurology by Hoffman and May. The medication is dripped or sprayed into the ipsilateral nostril at a concentration of between 4% and 10%. Pain relief is typically achieved within 10 minutes. This review also reports efficacy with percutaneous vagus nerve stimulation with the gammaCore device and neurostimulation of the sphenopalatine ganglion, though the mechanisms of these approaches are poorly understood.
Evolving therapies for acute cluster headache include the aforementioned CGRP receptor-antagonists. Additionally, intranasal ketamine hydrochloride is under investigation in an open-label, proof-of-concept study; and a zolmitriptan patch is being evaluated in a double-blind, placebo-controlled trial.
Attacks of migraine occur in 12% of the adult population, 3 times more in women than men and are painful and debilitating. Cluster attacks are even more painful and occur in about 0.1% of the population, somewhat more in men. Both types of headache have a variety of effective treatment as detailed above.
Acute migraine headache attacks
A recent review in the Journal of Neuro-Ophthalmology by Konstantinos Spingos and colleagues (including me as the senior author) details typical and new treatments for migraine. We all know about the longstanding options, including the 7 triptans and ergots, as well as over-the-counter analgesics, which can be combined with caffeine, nonsteroidal anti-inflammatory drugs; and many use the 2 categories of medication that I no longer use for migraine, butalbital-containing medications, and opioids.
Now 2 gepants are available—small molecule calcitonin gene-related peptide (CGRP) receptor antagonists. These medications are thought to be a useful alternative for those in whom triptans do not work or are relatively contraindicated due to coronary and cerebrovascular problems and other cardiac risk factors like obesity, smoking, lack of exercise, high cholesterol, and diabetes. Ubrogepant was approved by the FDA in 2019, and rimegepant soon followed in 2020.
- Ubrogepant: In the ACHIEVE trials, approximately 1 in 5 participants who received the 50 mg dose were pain-free at 2 hours. Moreover, nearly 40% of individuals who received it said their worst migraine symptom was resolved at 2 hours. Pain relief at 2 hours was 59%
- Rimegepant: Like ubrogepant, about 20% of trial participants who received the 75 mg melt tablet dose of rimegepant were pain-free at 2 hours. Thirty-seven percent reported that their worst migraine symptom was gone at 2 hours. Patients began to return to normal functioning in 15 minutes.
In addition to gepants, there is 1 ditan approved, which stimulates 5-HT1F receptors. Lasmiditan is the first medication in this class to be FDA-approved. It, too, is considered an alternative in patients in whom triptans are ineffective or when patients should not take a vasoconstrictor. In the most recent phase 3 study, the percentage of individuals who received lasmiditan and were pain-free at 2 hours were 28% (50 mg), 31% (100 mg) and 39% (200 mg). Relief from the migraine sufferers’ most bothersome symptom at 2 hours occurred in 41%, 44%, and 49% of patients, respectively. Lasmiditan is a Class V controlled substance. It has 18% dizziness in clinical trials. After administration, patients should not drive for 8 hours, and it should only be used once in a 24-hour period.
Non-pharmaceutical treatment options for acute migraine include nerve stimulation using electrical and magnetic stimulation devices, and behavioral approaches such as biofeedback training and mindfulness. The Nerivio device for the upper arm is controlled by a smart phone app and seems to work as well as a triptan in some patients with almost no adverse events. Just approved in February is the Relivion device which is worn like a tiara on the head and stimulates the frontal branches of the trigeminal nerve as well as the 2 occipital nerves in the back of the head.
Acute care of cluster headache attacks
In 2011, Ashkenazi and Schwedt published a comprehensive table in Headache outlining the treatment options for acute cluster headache. More recently, a review in CNS Drugs by Brandt and colleagues presented the choices with level 1 evidence for efficacy. They include:
- Sumatriptan, 6 mg subcutaneous injection, or 20 mg nasal spray
- Zolmitriptan, 5 or 10 mg nasal spray
- Oxygen, 100%, 7 to 12 liters per minute via a mask over the nose and mouth
The authors recommend subcutaneous sumatriptan 6 mg and/or high-flow oxygen at 9- to 12- liters per minute for 15 minutes. Subcutaneous sumatriptan, they note, has been shown to achieve pain relief within 15 minutes in 75% of patients who receive it. Moreover, one-third report pain freedom. Oxygen’s efficacy has long been established, and relief comes with no adverse events. As for mask type, though no significant differences have been observed in studies, patients appear to express a preference for the demand valve oxygen type, which allows a high flow rate and is dependent on the user’s breathing rate.
Lidocaine intranasally has been found to be effective when triptans or oxygen do not work, according to a review in The Lancet Neurology by Hoffman and May. The medication is dripped or sprayed into the ipsilateral nostril at a concentration of between 4% and 10%. Pain relief is typically achieved within 10 minutes. This review also reports efficacy with percutaneous vagus nerve stimulation with the gammaCore device and neurostimulation of the sphenopalatine ganglion, though the mechanisms of these approaches are poorly understood.
Evolving therapies for acute cluster headache include the aforementioned CGRP receptor-antagonists. Additionally, intranasal ketamine hydrochloride is under investigation in an open-label, proof-of-concept study; and a zolmitriptan patch is being evaluated in a double-blind, placebo-controlled trial.
Attacks of migraine occur in 12% of the adult population, 3 times more in women than men and are painful and debilitating. Cluster attacks are even more painful and occur in about 0.1% of the population, somewhat more in men. Both types of headache have a variety of effective treatment as detailed above.
Acute migraine headache attacks
A recent review in the Journal of Neuro-Ophthalmology by Konstantinos Spingos and colleagues (including me as the senior author) details typical and new treatments for migraine. We all know about the longstanding options, including the 7 triptans and ergots, as well as over-the-counter analgesics, which can be combined with caffeine, nonsteroidal anti-inflammatory drugs; and many use the 2 categories of medication that I no longer use for migraine, butalbital-containing medications, and opioids.
Now 2 gepants are available—small molecule calcitonin gene-related peptide (CGRP) receptor antagonists. These medications are thought to be a useful alternative for those in whom triptans do not work or are relatively contraindicated due to coronary and cerebrovascular problems and other cardiac risk factors like obesity, smoking, lack of exercise, high cholesterol, and diabetes. Ubrogepant was approved by the FDA in 2019, and rimegepant soon followed in 2020.
- Ubrogepant: In the ACHIEVE trials, approximately 1 in 5 participants who received the 50 mg dose were pain-free at 2 hours. Moreover, nearly 40% of individuals who received it said their worst migraine symptom was resolved at 2 hours. Pain relief at 2 hours was 59%
- Rimegepant: Like ubrogepant, about 20% of trial participants who received the 75 mg melt tablet dose of rimegepant were pain-free at 2 hours. Thirty-seven percent reported that their worst migraine symptom was gone at 2 hours. Patients began to return to normal functioning in 15 minutes.
In addition to gepants, there is 1 ditan approved, which stimulates 5-HT1F receptors. Lasmiditan is the first medication in this class to be FDA-approved. It, too, is considered an alternative in patients in whom triptans are ineffective or when patients should not take a vasoconstrictor. In the most recent phase 3 study, the percentage of individuals who received lasmiditan and were pain-free at 2 hours were 28% (50 mg), 31% (100 mg) and 39% (200 mg). Relief from the migraine sufferers’ most bothersome symptom at 2 hours occurred in 41%, 44%, and 49% of patients, respectively. Lasmiditan is a Class V controlled substance. It has 18% dizziness in clinical trials. After administration, patients should not drive for 8 hours, and it should only be used once in a 24-hour period.
Non-pharmaceutical treatment options for acute migraine include nerve stimulation using electrical and magnetic stimulation devices, and behavioral approaches such as biofeedback training and mindfulness. The Nerivio device for the upper arm is controlled by a smart phone app and seems to work as well as a triptan in some patients with almost no adverse events. Just approved in February is the Relivion device which is worn like a tiara on the head and stimulates the frontal branches of the trigeminal nerve as well as the 2 occipital nerves in the back of the head.
Acute care of cluster headache attacks
In 2011, Ashkenazi and Schwedt published a comprehensive table in Headache outlining the treatment options for acute cluster headache. More recently, a review in CNS Drugs by Brandt and colleagues presented the choices with level 1 evidence for efficacy. They include:
- Sumatriptan, 6 mg subcutaneous injection, or 20 mg nasal spray
- Zolmitriptan, 5 or 10 mg nasal spray
- Oxygen, 100%, 7 to 12 liters per minute via a mask over the nose and mouth
The authors recommend subcutaneous sumatriptan 6 mg and/or high-flow oxygen at 9- to 12- liters per minute for 15 minutes. Subcutaneous sumatriptan, they note, has been shown to achieve pain relief within 15 minutes in 75% of patients who receive it. Moreover, one-third report pain freedom. Oxygen’s efficacy has long been established, and relief comes with no adverse events. As for mask type, though no significant differences have been observed in studies, patients appear to express a preference for the demand valve oxygen type, which allows a high flow rate and is dependent on the user’s breathing rate.
Lidocaine intranasally has been found to be effective when triptans or oxygen do not work, according to a review in The Lancet Neurology by Hoffman and May. The medication is dripped or sprayed into the ipsilateral nostril at a concentration of between 4% and 10%. Pain relief is typically achieved within 10 minutes. This review also reports efficacy with percutaneous vagus nerve stimulation with the gammaCore device and neurostimulation of the sphenopalatine ganglion, though the mechanisms of these approaches are poorly understood.
Evolving therapies for acute cluster headache include the aforementioned CGRP receptor-antagonists. Additionally, intranasal ketamine hydrochloride is under investigation in an open-label, proof-of-concept study; and a zolmitriptan patch is being evaluated in a double-blind, placebo-controlled trial.
Attacks of migraine occur in 12% of the adult population, 3 times more in women than men and are painful and debilitating. Cluster attacks are even more painful and occur in about 0.1% of the population, somewhat more in men. Both types of headache have a variety of effective treatment as detailed above.
Headache and COVID-19: Key questions answered
Although coronavirus 19 disease (COVID-19), caused by severe acute respiratory coronavirus 2, is characterized by symptoms that primarily impact the respiratory system, many patients experience neurological manifestations, with headache among leading complaints. Moreover, headache symptoms, including migraine-like headache, can last long after patients recover from COVID-19.
Last November, in an interview with 60 Minutes, Sadie Nagamootoo described her experience. “There are days when I do nothing and cannot get out of bed. The migraines are 10 times worse than a flu headache.”
To help individuals like Nagamootoo and others who experience headache as a result of COVID-19, it is important to understand the data that are emerging and how to incorporate them into practice. Following are answers to important questions that can guide front-line neurologists and other clinicians who are practicing during the pandemic.
Why is headache a symptom of COVID-19? It should come as no surprise that patients with COVID-19 can experience headache. Peng reminds us, in a November 2020 editorial in Cephalalgia, that headache is a common symptom in individuals with acute respiratory disease, representing a physiological response to acute infection. Headache is often the primary reason patients seek treatment.
How is headache associated with COVID-19? It is too early to know with certainty the mechanisms underlying COVID-19 headache, but a possible explanation—according to Uygun and colleagues, writing in the The Journal of Headache and Pain—is that the virus directly invades trigeminal nerve endings in the nasal and oral cavities.
How does headache tend to present in COVID-19? Patricia Pozo-Rosich, MD, PhD, presented on this topic at the American Headache Society’s 2020 Virtual Annual Scientific Meeting in June. In a recent interview with Neurology Reviews, Dr. Pozo-Rosich, head of the Headache & Craniofacial Pain Unit at Vall d’Hebron University Hospital, Barcelona, Spain, noted that “headache seems to have 2 different presentations: 1) migraine-like characteristics that are severe, disabling, and usually start before other COVID symptoms and 2) tension-type headache characteristics, which usually start together with the rest of COVID symptoms.”
Are there symptoms that tend to occur more frequently in patients with COVID-19 and headache? Caronna and colleagues recently published an analysis in Cephalalgia of 130 individuals with COVID-19, showing that loss of smell and/or taste occurred in more than half of patients with headache, compared with fewer than 20% of those without headache. This finding is notable because it has been frequently reported in case reports of patients with COVID-19 and headache.
What does the presence of headache indicate about COVID-19 prognosis? The good news for individuals with COVID-19 who experience headache is that the duration of their COVID-19 illness might very well be shorter. In the Caronna study, COVID-19 duration in individuals with headache was, on average, 1 week shorter (24 days) than in those without headache symptoms (31 days). “We don’t know why,” said Dr. Pozo-Rosich, who is one of the study’s authors. She hypothesizes that it is because of a balance between neuroinflammation and systemic inflammation. “Having an extraordinary initial reaction at the nasal cavity might protect us from having greater systemic inflammation.”
What is the cause of headache from COVID-19? Bolay and colleagues reported in Headache in Spring 2020 that patients developed new-onset, moderate-to-severe, bilateral pulsating or pressing headache toward the frontal area and forehead during the viral phase of disease. The virus activates peripheral trigeminal nerve endings directly or through vasculopathy and/or increased circulating pro-inflammatory cytokines.
What else is important to be aware of regarding headache evolution in individuals with COVID-19? The bad news for many of these individuals is that, although their COVID-19 illness might dissipate more quickly, headaches could linger. Moreover, many will be experiencing chronic headache for the first time in their life. Caronna reported that that one third of follow-up patients who reported headache were experiencing persistent disabling headache daily after 6 weeks, and more than half had no history of recurrent headache.
What is the recommended treatment for headache associated with COVID-19? Dr. Pozo-Rosich recommends starting with a nonsteroidal anti-inflammatory medication. Eventually, steroids might be indicated, “especially if the disease progresses.”
It is important for neurologists to be aware of new-onset headache associated with anosmia early in the disease. Test for the virus in such a patient; hopefully, their course will be shorter, milder, and non-respiratory.
Although coronavirus 19 disease (COVID-19), caused by severe acute respiratory coronavirus 2, is characterized by symptoms that primarily impact the respiratory system, many patients experience neurological manifestations, with headache among leading complaints. Moreover, headache symptoms, including migraine-like headache, can last long after patients recover from COVID-19.
Last November, in an interview with 60 Minutes, Sadie Nagamootoo described her experience. “There are days when I do nothing and cannot get out of bed. The migraines are 10 times worse than a flu headache.”
To help individuals like Nagamootoo and others who experience headache as a result of COVID-19, it is important to understand the data that are emerging and how to incorporate them into practice. Following are answers to important questions that can guide front-line neurologists and other clinicians who are practicing during the pandemic.
Why is headache a symptom of COVID-19? It should come as no surprise that patients with COVID-19 can experience headache. Peng reminds us, in a November 2020 editorial in Cephalalgia, that headache is a common symptom in individuals with acute respiratory disease, representing a physiological response to acute infection. Headache is often the primary reason patients seek treatment.
How is headache associated with COVID-19? It is too early to know with certainty the mechanisms underlying COVID-19 headache, but a possible explanation—according to Uygun and colleagues, writing in the The Journal of Headache and Pain—is that the virus directly invades trigeminal nerve endings in the nasal and oral cavities.
How does headache tend to present in COVID-19? Patricia Pozo-Rosich, MD, PhD, presented on this topic at the American Headache Society’s 2020 Virtual Annual Scientific Meeting in June. In a recent interview with Neurology Reviews, Dr. Pozo-Rosich, head of the Headache & Craniofacial Pain Unit at Vall d’Hebron University Hospital, Barcelona, Spain, noted that “headache seems to have 2 different presentations: 1) migraine-like characteristics that are severe, disabling, and usually start before other COVID symptoms and 2) tension-type headache characteristics, which usually start together with the rest of COVID symptoms.”
Are there symptoms that tend to occur more frequently in patients with COVID-19 and headache? Caronna and colleagues recently published an analysis in Cephalalgia of 130 individuals with COVID-19, showing that loss of smell and/or taste occurred in more than half of patients with headache, compared with fewer than 20% of those without headache. This finding is notable because it has been frequently reported in case reports of patients with COVID-19 and headache.
What does the presence of headache indicate about COVID-19 prognosis? The good news for individuals with COVID-19 who experience headache is that the duration of their COVID-19 illness might very well be shorter. In the Caronna study, COVID-19 duration in individuals with headache was, on average, 1 week shorter (24 days) than in those without headache symptoms (31 days). “We don’t know why,” said Dr. Pozo-Rosich, who is one of the study’s authors. She hypothesizes that it is because of a balance between neuroinflammation and systemic inflammation. “Having an extraordinary initial reaction at the nasal cavity might protect us from having greater systemic inflammation.”
What is the cause of headache from COVID-19? Bolay and colleagues reported in Headache in Spring 2020 that patients developed new-onset, moderate-to-severe, bilateral pulsating or pressing headache toward the frontal area and forehead during the viral phase of disease. The virus activates peripheral trigeminal nerve endings directly or through vasculopathy and/or increased circulating pro-inflammatory cytokines.
What else is important to be aware of regarding headache evolution in individuals with COVID-19? The bad news for many of these individuals is that, although their COVID-19 illness might dissipate more quickly, headaches could linger. Moreover, many will be experiencing chronic headache for the first time in their life. Caronna reported that that one third of follow-up patients who reported headache were experiencing persistent disabling headache daily after 6 weeks, and more than half had no history of recurrent headache.
What is the recommended treatment for headache associated with COVID-19? Dr. Pozo-Rosich recommends starting with a nonsteroidal anti-inflammatory medication. Eventually, steroids might be indicated, “especially if the disease progresses.”
It is important for neurologists to be aware of new-onset headache associated with anosmia early in the disease. Test for the virus in such a patient; hopefully, their course will be shorter, milder, and non-respiratory.
Although coronavirus 19 disease (COVID-19), caused by severe acute respiratory coronavirus 2, is characterized by symptoms that primarily impact the respiratory system, many patients experience neurological manifestations, with headache among leading complaints. Moreover, headache symptoms, including migraine-like headache, can last long after patients recover from COVID-19.
Last November, in an interview with 60 Minutes, Sadie Nagamootoo described her experience. “There are days when I do nothing and cannot get out of bed. The migraines are 10 times worse than a flu headache.”
To help individuals like Nagamootoo and others who experience headache as a result of COVID-19, it is important to understand the data that are emerging and how to incorporate them into practice. Following are answers to important questions that can guide front-line neurologists and other clinicians who are practicing during the pandemic.
Why is headache a symptom of COVID-19? It should come as no surprise that patients with COVID-19 can experience headache. Peng reminds us, in a November 2020 editorial in Cephalalgia, that headache is a common symptom in individuals with acute respiratory disease, representing a physiological response to acute infection. Headache is often the primary reason patients seek treatment.
How is headache associated with COVID-19? It is too early to know with certainty the mechanisms underlying COVID-19 headache, but a possible explanation—according to Uygun and colleagues, writing in the The Journal of Headache and Pain—is that the virus directly invades trigeminal nerve endings in the nasal and oral cavities.
How does headache tend to present in COVID-19? Patricia Pozo-Rosich, MD, PhD, presented on this topic at the American Headache Society’s 2020 Virtual Annual Scientific Meeting in June. In a recent interview with Neurology Reviews, Dr. Pozo-Rosich, head of the Headache & Craniofacial Pain Unit at Vall d’Hebron University Hospital, Barcelona, Spain, noted that “headache seems to have 2 different presentations: 1) migraine-like characteristics that are severe, disabling, and usually start before other COVID symptoms and 2) tension-type headache characteristics, which usually start together with the rest of COVID symptoms.”
Are there symptoms that tend to occur more frequently in patients with COVID-19 and headache? Caronna and colleagues recently published an analysis in Cephalalgia of 130 individuals with COVID-19, showing that loss of smell and/or taste occurred in more than half of patients with headache, compared with fewer than 20% of those without headache. This finding is notable because it has been frequently reported in case reports of patients with COVID-19 and headache.
What does the presence of headache indicate about COVID-19 prognosis? The good news for individuals with COVID-19 who experience headache is that the duration of their COVID-19 illness might very well be shorter. In the Caronna study, COVID-19 duration in individuals with headache was, on average, 1 week shorter (24 days) than in those without headache symptoms (31 days). “We don’t know why,” said Dr. Pozo-Rosich, who is one of the study’s authors. She hypothesizes that it is because of a balance between neuroinflammation and systemic inflammation. “Having an extraordinary initial reaction at the nasal cavity might protect us from having greater systemic inflammation.”
What is the cause of headache from COVID-19? Bolay and colleagues reported in Headache in Spring 2020 that patients developed new-onset, moderate-to-severe, bilateral pulsating or pressing headache toward the frontal area and forehead during the viral phase of disease. The virus activates peripheral trigeminal nerve endings directly or through vasculopathy and/or increased circulating pro-inflammatory cytokines.
What else is important to be aware of regarding headache evolution in individuals with COVID-19? The bad news for many of these individuals is that, although their COVID-19 illness might dissipate more quickly, headaches could linger. Moreover, many will be experiencing chronic headache for the first time in their life. Caronna reported that that one third of follow-up patients who reported headache were experiencing persistent disabling headache daily after 6 weeks, and more than half had no history of recurrent headache.
What is the recommended treatment for headache associated with COVID-19? Dr. Pozo-Rosich recommends starting with a nonsteroidal anti-inflammatory medication. Eventually, steroids might be indicated, “especially if the disease progresses.”
It is important for neurologists to be aware of new-onset headache associated with anosmia early in the disease. Test for the virus in such a patient; hopefully, their course will be shorter, milder, and non-respiratory.
MS bears no effect on certain pregnancy complications, stillbirth, or congenital deformation
, according to a new study published online Feb. 3 in Neurology Clinical Practice. While pregnancy and childbirth are not regarded as conditions that engender high-risk pregnancy in the MS population, previous studies evaluating the effects of MS on pregnancy and parturition have yet to fully elucidate some outcomes for pregnant women and their babies in multiple sclerosis.
“Women with multiple sclerosis may be understandably concerned about the risk of pregnancy,” said Melinda Magyari, MD, PhD, a consultant at the University of Copenhagen. “While previous research has shown there is no higher risk of birth defect for babies born to women with MS, we wanted to find out if women with MS are at risk for a variety of pregnancy complications.”
MS is regarded as a progressive, neurological disease mediated by the immune system that demands careful consideration of numerous situations and life changes including family planning. The MS population is overwhelmingly female, as women account for three out of every four cases of MS. The majority of these women range from 20 to 40 years of age at the time of being diagnosed with MS. Despite the unknown risks of pregnancy-related complications and various perinatal complications in this patient population, women who have MS are not discouraged from conceiving.
Assessing pregnancy outcomes
This nationwide, population-based, cross-sectional study evaluated the pregnancies of 2,930 women with MS between Jan. 1, 1997, and Dec. 31, 2016, registered in the Danish Multiple Sclerosis Registry. The researchers compared pregnancy-related and prenatal outcomes to a 5% random sample of 56,958 randomly-selected pregnant women from Denmark’s general population who did not have MS. They found no differences in the risks associated with several pregnancy-related complications (e.g., preeclampsia, gestational diabetes, or placental complications), emergency Cesarean section (C-section), instrumental delivery, stillbirth, preterm birth, or congenital malformation. Apgar scores were low in both groups. A composite of various biometrics in newborns such as reflexes, muscle tone, and heart rate immediately following birth, the Apgar score is used to help assess the neonatal health, with a value of less than 7 considered low. Here, preterm birth is defined as delivery occurring before 37 weeks of gestation, and stillbirth describes a fetus born dead after 22 weeks of gestation.
Women in the MS cohort were more likely to have elective C-sections (odds ratio, 2.89 [95% confidence interval, 1.65-2.16]), induced labor (OR, 1.15 [95%CI, 1.01-1.31]) and have babies with low birth weight based on their gestational age (OR, 1.29 [95% CI, 1.04-1.60]). Nearly 30% of babies born in the cohort (n = 851) were born to mothers who had received disease-modifying therapy (DMT). Neonates exposed to DMT weighed an average of 116 g less than babies born to mothers who had not received DMT (3,378 g vs. 3,494 g) with a slightly lower gestational age (39 weeks as opposed to 40 weeks). However, babies born to mothers with MS were less likely to show signs of asphyxia (OR, 0.87 [95% CI, 0.78-0.97]) than the comparison cohort.
“We found overall, their pregnancies were just as healthy as those of the moms without MS,” Dr. Magyari said.
Comprehensive data
Denmark’s health care system has two key features that make it an attractive setting in which to conduct such a study – the first being its universal health care. The second advantage is that the country enacted several health registries in the 1970s and 1980s that enable the collection of more comprehensive data. For example, the Danish National Patient Register is a population-based registry that spans the entire nation, facilitating epidemiological research with what the study’s authors describe as “high generalizability.” Providing additional insights regarding the patient story helps add context to pregnancy and outcomes. Among the data collected on the women studied were demographics, contact information, and abortions, both spontaneous and medically induced. The country uses other databases and registries to capture additional data. For example, the Register of Legally Induced Abortions provides data regarding the context of medically induced abortions. In contrast, the Danish Medical Birth Registry provides context regarding specified variables regarding women’s pregnancies, delivery, and perinatal outcomes. Finally, the population’s education register offers information regarding patients’ educational history.
A key strength of this study is that the long duration of follow-up data from the Danish Medical Birth Registry, along with its comprehensive data collection, eliminates recall bias. Universal access to health care also improves the generalizability of data. A limitation of the study is its lack of data on maternal smoking and its effects on low gestational weights. The study also has some data gaps, including body mass index information missing from a large portion of the cohort. Finally, the sample size of newborns born to mothers who had received DMT therapy within the last 6 months of gestation was too underpowered to stratify based on first on first-line or second-line treatment.
Dr. Magyari served on scientific advisory boards for Biogen, Sanofi, Teva, Roche, Novartis, and Merck. She has also received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi, Genzyme, and has received research support and support for congress participation from Biogen, Genzyme, Teva, Roche, Merck, and Novartis. Coauthors disclosed various fees received from Merck, Novartis, Biogen, Roche, Sanofi Genzyme, and Teva.
, according to a new study published online Feb. 3 in Neurology Clinical Practice. While pregnancy and childbirth are not regarded as conditions that engender high-risk pregnancy in the MS population, previous studies evaluating the effects of MS on pregnancy and parturition have yet to fully elucidate some outcomes for pregnant women and their babies in multiple sclerosis.
“Women with multiple sclerosis may be understandably concerned about the risk of pregnancy,” said Melinda Magyari, MD, PhD, a consultant at the University of Copenhagen. “While previous research has shown there is no higher risk of birth defect for babies born to women with MS, we wanted to find out if women with MS are at risk for a variety of pregnancy complications.”
MS is regarded as a progressive, neurological disease mediated by the immune system that demands careful consideration of numerous situations and life changes including family planning. The MS population is overwhelmingly female, as women account for three out of every four cases of MS. The majority of these women range from 20 to 40 years of age at the time of being diagnosed with MS. Despite the unknown risks of pregnancy-related complications and various perinatal complications in this patient population, women who have MS are not discouraged from conceiving.
Assessing pregnancy outcomes
This nationwide, population-based, cross-sectional study evaluated the pregnancies of 2,930 women with MS between Jan. 1, 1997, and Dec. 31, 2016, registered in the Danish Multiple Sclerosis Registry. The researchers compared pregnancy-related and prenatal outcomes to a 5% random sample of 56,958 randomly-selected pregnant women from Denmark’s general population who did not have MS. They found no differences in the risks associated with several pregnancy-related complications (e.g., preeclampsia, gestational diabetes, or placental complications), emergency Cesarean section (C-section), instrumental delivery, stillbirth, preterm birth, or congenital malformation. Apgar scores were low in both groups. A composite of various biometrics in newborns such as reflexes, muscle tone, and heart rate immediately following birth, the Apgar score is used to help assess the neonatal health, with a value of less than 7 considered low. Here, preterm birth is defined as delivery occurring before 37 weeks of gestation, and stillbirth describes a fetus born dead after 22 weeks of gestation.
Women in the MS cohort were more likely to have elective C-sections (odds ratio, 2.89 [95% confidence interval, 1.65-2.16]), induced labor (OR, 1.15 [95%CI, 1.01-1.31]) and have babies with low birth weight based on their gestational age (OR, 1.29 [95% CI, 1.04-1.60]). Nearly 30% of babies born in the cohort (n = 851) were born to mothers who had received disease-modifying therapy (DMT). Neonates exposed to DMT weighed an average of 116 g less than babies born to mothers who had not received DMT (3,378 g vs. 3,494 g) with a slightly lower gestational age (39 weeks as opposed to 40 weeks). However, babies born to mothers with MS were less likely to show signs of asphyxia (OR, 0.87 [95% CI, 0.78-0.97]) than the comparison cohort.
“We found overall, their pregnancies were just as healthy as those of the moms without MS,” Dr. Magyari said.
Comprehensive data
Denmark’s health care system has two key features that make it an attractive setting in which to conduct such a study – the first being its universal health care. The second advantage is that the country enacted several health registries in the 1970s and 1980s that enable the collection of more comprehensive data. For example, the Danish National Patient Register is a population-based registry that spans the entire nation, facilitating epidemiological research with what the study’s authors describe as “high generalizability.” Providing additional insights regarding the patient story helps add context to pregnancy and outcomes. Among the data collected on the women studied were demographics, contact information, and abortions, both spontaneous and medically induced. The country uses other databases and registries to capture additional data. For example, the Register of Legally Induced Abortions provides data regarding the context of medically induced abortions. In contrast, the Danish Medical Birth Registry provides context regarding specified variables regarding women’s pregnancies, delivery, and perinatal outcomes. Finally, the population’s education register offers information regarding patients’ educational history.
A key strength of this study is that the long duration of follow-up data from the Danish Medical Birth Registry, along with its comprehensive data collection, eliminates recall bias. Universal access to health care also improves the generalizability of data. A limitation of the study is its lack of data on maternal smoking and its effects on low gestational weights. The study also has some data gaps, including body mass index information missing from a large portion of the cohort. Finally, the sample size of newborns born to mothers who had received DMT therapy within the last 6 months of gestation was too underpowered to stratify based on first on first-line or second-line treatment.
Dr. Magyari served on scientific advisory boards for Biogen, Sanofi, Teva, Roche, Novartis, and Merck. She has also received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi, Genzyme, and has received research support and support for congress participation from Biogen, Genzyme, Teva, Roche, Merck, and Novartis. Coauthors disclosed various fees received from Merck, Novartis, Biogen, Roche, Sanofi Genzyme, and Teva.
, according to a new study published online Feb. 3 in Neurology Clinical Practice. While pregnancy and childbirth are not regarded as conditions that engender high-risk pregnancy in the MS population, previous studies evaluating the effects of MS on pregnancy and parturition have yet to fully elucidate some outcomes for pregnant women and their babies in multiple sclerosis.
“Women with multiple sclerosis may be understandably concerned about the risk of pregnancy,” said Melinda Magyari, MD, PhD, a consultant at the University of Copenhagen. “While previous research has shown there is no higher risk of birth defect for babies born to women with MS, we wanted to find out if women with MS are at risk for a variety of pregnancy complications.”
MS is regarded as a progressive, neurological disease mediated by the immune system that demands careful consideration of numerous situations and life changes including family planning. The MS population is overwhelmingly female, as women account for three out of every four cases of MS. The majority of these women range from 20 to 40 years of age at the time of being diagnosed with MS. Despite the unknown risks of pregnancy-related complications and various perinatal complications in this patient population, women who have MS are not discouraged from conceiving.
Assessing pregnancy outcomes
This nationwide, population-based, cross-sectional study evaluated the pregnancies of 2,930 women with MS between Jan. 1, 1997, and Dec. 31, 2016, registered in the Danish Multiple Sclerosis Registry. The researchers compared pregnancy-related and prenatal outcomes to a 5% random sample of 56,958 randomly-selected pregnant women from Denmark’s general population who did not have MS. They found no differences in the risks associated with several pregnancy-related complications (e.g., preeclampsia, gestational diabetes, or placental complications), emergency Cesarean section (C-section), instrumental delivery, stillbirth, preterm birth, or congenital malformation. Apgar scores were low in both groups. A composite of various biometrics in newborns such as reflexes, muscle tone, and heart rate immediately following birth, the Apgar score is used to help assess the neonatal health, with a value of less than 7 considered low. Here, preterm birth is defined as delivery occurring before 37 weeks of gestation, and stillbirth describes a fetus born dead after 22 weeks of gestation.
Women in the MS cohort were more likely to have elective C-sections (odds ratio, 2.89 [95% confidence interval, 1.65-2.16]), induced labor (OR, 1.15 [95%CI, 1.01-1.31]) and have babies with low birth weight based on their gestational age (OR, 1.29 [95% CI, 1.04-1.60]). Nearly 30% of babies born in the cohort (n = 851) were born to mothers who had received disease-modifying therapy (DMT). Neonates exposed to DMT weighed an average of 116 g less than babies born to mothers who had not received DMT (3,378 g vs. 3,494 g) with a slightly lower gestational age (39 weeks as opposed to 40 weeks). However, babies born to mothers with MS were less likely to show signs of asphyxia (OR, 0.87 [95% CI, 0.78-0.97]) than the comparison cohort.
“We found overall, their pregnancies were just as healthy as those of the moms without MS,” Dr. Magyari said.
Comprehensive data
Denmark’s health care system has two key features that make it an attractive setting in which to conduct such a study – the first being its universal health care. The second advantage is that the country enacted several health registries in the 1970s and 1980s that enable the collection of more comprehensive data. For example, the Danish National Patient Register is a population-based registry that spans the entire nation, facilitating epidemiological research with what the study’s authors describe as “high generalizability.” Providing additional insights regarding the patient story helps add context to pregnancy and outcomes. Among the data collected on the women studied were demographics, contact information, and abortions, both spontaneous and medically induced. The country uses other databases and registries to capture additional data. For example, the Register of Legally Induced Abortions provides data regarding the context of medically induced abortions. In contrast, the Danish Medical Birth Registry provides context regarding specified variables regarding women’s pregnancies, delivery, and perinatal outcomes. Finally, the population’s education register offers information regarding patients’ educational history.
A key strength of this study is that the long duration of follow-up data from the Danish Medical Birth Registry, along with its comprehensive data collection, eliminates recall bias. Universal access to health care also improves the generalizability of data. A limitation of the study is its lack of data on maternal smoking and its effects on low gestational weights. The study also has some data gaps, including body mass index information missing from a large portion of the cohort. Finally, the sample size of newborns born to mothers who had received DMT therapy within the last 6 months of gestation was too underpowered to stratify based on first on first-line or second-line treatment.
Dr. Magyari served on scientific advisory boards for Biogen, Sanofi, Teva, Roche, Novartis, and Merck. She has also received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi, Genzyme, and has received research support and support for congress participation from Biogen, Genzyme, Teva, Roche, Merck, and Novartis. Coauthors disclosed various fees received from Merck, Novartis, Biogen, Roche, Sanofi Genzyme, and Teva.
FROM NEUROLOGY CLINICAL PRACTICE
FDA approves intramuscular administration for peginterferon beta-1a in MS
“The new IM administration offers people living with relapsing MS the well-characterized efficacy and safety of Plegridy with the potential for significantly reduced injection site reactions,” Biogen said in a news release announcing the FDA action.
Plegridy is a pegylated version of interferon beta-1a, which prolongs the circulation time of the molecule in the body by increasing its size. The process extends the drug’s half-life, allowing for a less-frequent dosing schedule.
Peginterferon beta-1a administered subcutaneously was first approved by the FDA in 2014 based on data showing it significantly reduces MS relapses, disability progression, and brain lesions.
The FDA approved IM administration for peginterferon beta-1a based on data evaluating bioequivalence and adverse reactions associated with IM administration compared with subcutaneous (SC) administration in healthy volunteers.
Bioequivalence of the IM and SC dosing regimens was confirmed and volunteers receiving the drug through IM administration experienced fewer injection site reactions relative to those receiving SC administration (14.4% vs. 32.1%), the company said.
The overall safety profiles of IM and SC administration were generally similar, with no new safety signals.
The European Commission allowed marketing authorization for IM administration of peginterferon beta-1a in December 2020.
A version of this article first appeared on Medscape.com.
“The new IM administration offers people living with relapsing MS the well-characterized efficacy and safety of Plegridy with the potential for significantly reduced injection site reactions,” Biogen said in a news release announcing the FDA action.
Plegridy is a pegylated version of interferon beta-1a, which prolongs the circulation time of the molecule in the body by increasing its size. The process extends the drug’s half-life, allowing for a less-frequent dosing schedule.
Peginterferon beta-1a administered subcutaneously was first approved by the FDA in 2014 based on data showing it significantly reduces MS relapses, disability progression, and brain lesions.
The FDA approved IM administration for peginterferon beta-1a based on data evaluating bioequivalence and adverse reactions associated with IM administration compared with subcutaneous (SC) administration in healthy volunteers.
Bioequivalence of the IM and SC dosing regimens was confirmed and volunteers receiving the drug through IM administration experienced fewer injection site reactions relative to those receiving SC administration (14.4% vs. 32.1%), the company said.
The overall safety profiles of IM and SC administration were generally similar, with no new safety signals.
The European Commission allowed marketing authorization for IM administration of peginterferon beta-1a in December 2020.
A version of this article first appeared on Medscape.com.
“The new IM administration offers people living with relapsing MS the well-characterized efficacy and safety of Plegridy with the potential for significantly reduced injection site reactions,” Biogen said in a news release announcing the FDA action.
Plegridy is a pegylated version of interferon beta-1a, which prolongs the circulation time of the molecule in the body by increasing its size. The process extends the drug’s half-life, allowing for a less-frequent dosing schedule.
Peginterferon beta-1a administered subcutaneously was first approved by the FDA in 2014 based on data showing it significantly reduces MS relapses, disability progression, and brain lesions.
The FDA approved IM administration for peginterferon beta-1a based on data evaluating bioequivalence and adverse reactions associated with IM administration compared with subcutaneous (SC) administration in healthy volunteers.
Bioequivalence of the IM and SC dosing regimens was confirmed and volunteers receiving the drug through IM administration experienced fewer injection site reactions relative to those receiving SC administration (14.4% vs. 32.1%), the company said.
The overall safety profiles of IM and SC administration were generally similar, with no new safety signals.
The European Commission allowed marketing authorization for IM administration of peginterferon beta-1a in December 2020.
A version of this article first appeared on Medscape.com.
Is the EDSS an adequate outcome measure in secondary progressive MS trials?
Clinical trials enrolling patients with progressive multiple sclerosis (MS) commonly use the Expanded Disability Status Scale (EDSS), an instrument that looks at impairment across several different functional domains, as a primary outcome measure. But results from
For their research, published in the Jan. 5 issue of Neurology, Marcus W. Koch, MD, PhD, of the department of neurosciences at Hotchkiss Brain Institute at the University of Calgary (Alta.) and colleagues looked at data from the placebo arms of two randomized trials that collectively enrolled nearly 700 patients with secondary progressive MS (SPMS). The trials were similar in terms of baseline patient characteristics and level of disability.
Comparing three outcome measures
The investigators compared disability progression and improvement across each of the three instruments and their combinations. Because improvement is understood to occur only rarely in untreated secondary progressive MS, most improvement picked up in the placebo arm of a trial is assumed to be noise from random variation or measurement error.
Dr. Koch and colleagues found that the EDSS showed higher rates of improvement than the other tests. The EDSS also showed the smallest differences between progression and improvement among the three instruments, with improvement rate over time increasing in parallel with disability progression rates. With the other two tests, improvement rates remained low – at 10% or less – while disability was seen steadily increasing over time.
The results, the investigators wrote in their analysis, suggest that the timed 25-foot walk and 9-hole peg test are the more reliable outcome measures. The reason “may simply lie in the fact that both the timed 25-foot walk and 9-hole peg test are objective and quantitative interval-scaled measures while the EDSS is a graded categorical measure.” As primary outcome measures in clinical trials, “the lower noise of the timed 25-foot walk and 9-hole peg test may make them preferable over the EDSS,” Dr. Koch and colleagues concluded. The investigators noted that a 2019 analysis of different MS disability scales across more than 13,000 patients in 14 trials did not find such stark differences – but that the patients in the pooled trials had less disability at baseline (median EDSS score of 2.5, compared with 6.0 for the two trials in Dr. Koch and colleagues’ study). This suggests, the investigators wrote, “that the timed 25-foot walk and 9-hole peg test may be more useful outcomes in patients with a progressive disease course and with greater baseline disability.”
‘Considerable implications’ for the design of future clinical trials
In an accompanying editorial, Tomas Kalincik, MD, PhD, of the University of Melbourne, along with colleagues in Italy and Britain, praised Dr. Koch and colleagues’ study as having “considerable implications for the design of future clinical trials because detecting a treatment effect on an outcome that is subject to large measurement error is difficult.” Most trials in progressive MS use change in EDSS score as their primary or key secondary outcomes. “However, as the authors elegantly show, other, more reliable clinical outcomes are needed. As we are revisiting our biological hypotheses for treatment of progressive MS, perhaps the time has come that we should also revisit the instruments that we use to examine their efficacy.”
The editorialists allowed for the possibility that something besides noise or measurement error could be responsible for the disparities seen across the instruments. “An alternative interpretation of the presented results could be that recovery of neurologic function is more common in SPMS than what we had previously thought and that EDSS is more sensitive to its detection than the other two measures,” they wrote.
Dr. Koch and colleagues’ study received no outside funding. Dr. Koch disclosed consulting fees and other financial support from several drug manufacturers, and three coauthors also disclosed financial relationships with pharmaceutical companies. All three editorial writers disclosed similar relationships.
Clinical trials enrolling patients with progressive multiple sclerosis (MS) commonly use the Expanded Disability Status Scale (EDSS), an instrument that looks at impairment across several different functional domains, as a primary outcome measure. But results from
For their research, published in the Jan. 5 issue of Neurology, Marcus W. Koch, MD, PhD, of the department of neurosciences at Hotchkiss Brain Institute at the University of Calgary (Alta.) and colleagues looked at data from the placebo arms of two randomized trials that collectively enrolled nearly 700 patients with secondary progressive MS (SPMS). The trials were similar in terms of baseline patient characteristics and level of disability.
Comparing three outcome measures
The investigators compared disability progression and improvement across each of the three instruments and their combinations. Because improvement is understood to occur only rarely in untreated secondary progressive MS, most improvement picked up in the placebo arm of a trial is assumed to be noise from random variation or measurement error.
Dr. Koch and colleagues found that the EDSS showed higher rates of improvement than the other tests. The EDSS also showed the smallest differences between progression and improvement among the three instruments, with improvement rate over time increasing in parallel with disability progression rates. With the other two tests, improvement rates remained low – at 10% or less – while disability was seen steadily increasing over time.
The results, the investigators wrote in their analysis, suggest that the timed 25-foot walk and 9-hole peg test are the more reliable outcome measures. The reason “may simply lie in the fact that both the timed 25-foot walk and 9-hole peg test are objective and quantitative interval-scaled measures while the EDSS is a graded categorical measure.” As primary outcome measures in clinical trials, “the lower noise of the timed 25-foot walk and 9-hole peg test may make them preferable over the EDSS,” Dr. Koch and colleagues concluded. The investigators noted that a 2019 analysis of different MS disability scales across more than 13,000 patients in 14 trials did not find such stark differences – but that the patients in the pooled trials had less disability at baseline (median EDSS score of 2.5, compared with 6.0 for the two trials in Dr. Koch and colleagues’ study). This suggests, the investigators wrote, “that the timed 25-foot walk and 9-hole peg test may be more useful outcomes in patients with a progressive disease course and with greater baseline disability.”
‘Considerable implications’ for the design of future clinical trials
In an accompanying editorial, Tomas Kalincik, MD, PhD, of the University of Melbourne, along with colleagues in Italy and Britain, praised Dr. Koch and colleagues’ study as having “considerable implications for the design of future clinical trials because detecting a treatment effect on an outcome that is subject to large measurement error is difficult.” Most trials in progressive MS use change in EDSS score as their primary or key secondary outcomes. “However, as the authors elegantly show, other, more reliable clinical outcomes are needed. As we are revisiting our biological hypotheses for treatment of progressive MS, perhaps the time has come that we should also revisit the instruments that we use to examine their efficacy.”
The editorialists allowed for the possibility that something besides noise or measurement error could be responsible for the disparities seen across the instruments. “An alternative interpretation of the presented results could be that recovery of neurologic function is more common in SPMS than what we had previously thought and that EDSS is more sensitive to its detection than the other two measures,” they wrote.
Dr. Koch and colleagues’ study received no outside funding. Dr. Koch disclosed consulting fees and other financial support from several drug manufacturers, and three coauthors also disclosed financial relationships with pharmaceutical companies. All three editorial writers disclosed similar relationships.
Clinical trials enrolling patients with progressive multiple sclerosis (MS) commonly use the Expanded Disability Status Scale (EDSS), an instrument that looks at impairment across several different functional domains, as a primary outcome measure. But results from
For their research, published in the Jan. 5 issue of Neurology, Marcus W. Koch, MD, PhD, of the department of neurosciences at Hotchkiss Brain Institute at the University of Calgary (Alta.) and colleagues looked at data from the placebo arms of two randomized trials that collectively enrolled nearly 700 patients with secondary progressive MS (SPMS). The trials were similar in terms of baseline patient characteristics and level of disability.
Comparing three outcome measures
The investigators compared disability progression and improvement across each of the three instruments and their combinations. Because improvement is understood to occur only rarely in untreated secondary progressive MS, most improvement picked up in the placebo arm of a trial is assumed to be noise from random variation or measurement error.
Dr. Koch and colleagues found that the EDSS showed higher rates of improvement than the other tests. The EDSS also showed the smallest differences between progression and improvement among the three instruments, with improvement rate over time increasing in parallel with disability progression rates. With the other two tests, improvement rates remained low – at 10% or less – while disability was seen steadily increasing over time.
The results, the investigators wrote in their analysis, suggest that the timed 25-foot walk and 9-hole peg test are the more reliable outcome measures. The reason “may simply lie in the fact that both the timed 25-foot walk and 9-hole peg test are objective and quantitative interval-scaled measures while the EDSS is a graded categorical measure.” As primary outcome measures in clinical trials, “the lower noise of the timed 25-foot walk and 9-hole peg test may make them preferable over the EDSS,” Dr. Koch and colleagues concluded. The investigators noted that a 2019 analysis of different MS disability scales across more than 13,000 patients in 14 trials did not find such stark differences – but that the patients in the pooled trials had less disability at baseline (median EDSS score of 2.5, compared with 6.0 for the two trials in Dr. Koch and colleagues’ study). This suggests, the investigators wrote, “that the timed 25-foot walk and 9-hole peg test may be more useful outcomes in patients with a progressive disease course and with greater baseline disability.”
‘Considerable implications’ for the design of future clinical trials
In an accompanying editorial, Tomas Kalincik, MD, PhD, of the University of Melbourne, along with colleagues in Italy and Britain, praised Dr. Koch and colleagues’ study as having “considerable implications for the design of future clinical trials because detecting a treatment effect on an outcome that is subject to large measurement error is difficult.” Most trials in progressive MS use change in EDSS score as their primary or key secondary outcomes. “However, as the authors elegantly show, other, more reliable clinical outcomes are needed. As we are revisiting our biological hypotheses for treatment of progressive MS, perhaps the time has come that we should also revisit the instruments that we use to examine their efficacy.”
The editorialists allowed for the possibility that something besides noise or measurement error could be responsible for the disparities seen across the instruments. “An alternative interpretation of the presented results could be that recovery of neurologic function is more common in SPMS than what we had previously thought and that EDSS is more sensitive to its detection than the other two measures,” they wrote.
Dr. Koch and colleagues’ study received no outside funding. Dr. Koch disclosed consulting fees and other financial support from several drug manufacturers, and three coauthors also disclosed financial relationships with pharmaceutical companies. All three editorial writers disclosed similar relationships.
FROM NEUROLOGY
Stem cell transplant shows long-term benefit in MS
The benefits of autologous hematopoietic stem cell transplant (AHSCT) for patients with multiple sclerosis (MS) persist for more than 10 years in the majority of patents, new data show. The study reports on 210 Italian patients who underwent AHSCT between 2007 and 2019. Among the entire study cohort, 79.5% of patients had not experienced worsening of disability at 5 years, and 65.5% had not experienced it at 10 years.
Patients with relapsing remitting MS had better results, with 85.5% experiencing no worsening of disability at 5 years, and 71.3% at 10 years. Among patients with progressive MS, 71.0% showed no worsening of disability at 5 years, and 57.2% at 10 years.
“This is the longest follow-up of AHSCT in MS patients so far to be reported,” said study author Matilde Inglese, MD, University of Genoa (Italy). “We have shown AHSCT to be highly effective to prevent long-term disability worsening in most treated patients.”
The study was published online Jan. 20 in Neurology.
“We suggest that AHSCT should be considered as a treatment strategy for MS not responding to conventional therapy,” the authors concluded.
The study had no control group, so a direct comparison is not possible. Nevertheless, Dr. Inglese said she believed these results are better than those that would be achieved with disease-modifying drug therapy for similar patients.
“The best patient candidates for this procedure are those with highly active multiple sclerosis who are not responsive to high-efficacy drugs, such as alemtuzumab or ocrelizumab,” Dr. Inglese commented. “Younger patients with an aggressive form of relapsing remitting MS tend to do the best, although patients with progressive forms of MS who still have active lesions on MRI also benefit.”
Renewing the immune system
The transplant procedure involves giving high-dose cyclophosphamide to stimulate mobilization of bone marrow stem cells, which are collected from peripheral blood. Patients then undergo intense immunosuppression with a cocktail of drugs to remove the autoreactive T cells, and the stem cells, which are not autoreactive, are reinfused.
“We are effectively renewing the immune system,” Dr. Inglese said. “While it is not correct to call it a cure, as we are not eliminating the etiology of the disease, it is the closest to complete suppression of the disease that we can get.”
Other results from the study show that among patients with relapsing remitting MS, rates of relapse-free survival were 78.1% at 5 years and 63.5% at 10 years.
Better results were achieved for patients who received the BEAM+ATG conditioning regimen for immunosuppression. That regimen includes carmustine, cytosine-arabinoside, etoposide, and melphalan, followed by rabbit antithymocyte globulin. Among patients with relapsing remitting disease who were treated with this protocol, rates of relapse-free survival were 86.4% at 5 years and 77.0% at 10 years.
For patients with relapsing remitting MS, the probability of achieving NEDA-3 status (no evidence of disease activity, including the absence of clinical relapses, disability worsening, and MRI inflammatory activity) was 62.2% at 5 years and 40.5% at 10 years.
Among those patients with relapsing remitting MS who received the BEAM+ATG conditioning protocol, NEDA-3 status was achieved in 67.7% at 5 years and in 54.9% at 10 years.
Three deaths occurred within 100 days following AHSCT (1.4% of the entire study population). One patient developed pulmonary thromboembolism, received fibrinolytic treatment, and died 48 hours later after intracranial hemorrhage. The second patient experienced engraftment failure and died 24 days after transplant because of an opportunistic infection. The third patient died 1 month after transplant from Wernicke-like encephalopathy. All the patients who died received the BEAM+ATG conditioning regimen. No transplant-related deaths occurred in patients who underwent transplant after 2007.
Dr. Inglese noted that the mortality rate associated with AHSCT has been greatly reduced in recent years. “We are seeing a very low mortality rate – about 0.3% – thanks to improvements in the procedure and better patient selection. This seems acceptable, given that we are treating patients with very aggressive disease who have a high risk of becoming significantly disabled relatively early in life,” she commented.
However, it is vitally important that the procedure be conducted in a specialized center with a highly experienced multidisciplinary team, she stressed.
In the Neurology article, the authors concluded: “Although patients with RRMS [relapsing remitting MS] are those who benefit the most from transplant, AHSCT has been also shown to prevent disability worsening in a large proportion of patients with active progressive MS.
“The BEAM+ATG conditioning protocol, although associated with a higher transplant mortality rate, was associated with a more pronounced suppression of clinical relapses and MRI inflammatory activity, allowing complete disease control in a higher proportion of patients,” they wrote.
Potent and durable efficacy, with caveats
Commenting on these latest findings, Jeffrey A. Cohen, MD, of the Mellen Center for Multiple Sclerosis at the Cleveland Clinic, said: “AHSCT appears to have potent and durable efficacy in MS but is associated with significant risk and cost.”
The patients who are most likely to benefit are young and have experienced the onset of disease relatively recently. They are still ambulatory with highly active MS and have experienced recent clinical relapses and/or MRI lesion activity, and such activity continues despite disease-modifying therapy, Dr. Cohen noted. He added that “AHSCT is a reasonable option for such patients who have essentially failed the available disease-modifying therapy options.”
He pointed out that the key question is where AHSCT belongs in the overall MS algorithm relative to other high-efficacy therapies. “We need to know whether it should be used more broadly rather than as a last resort.”
To address that question, several randomized trials comparing AHSCT with high-efficacy disease-modifying therapy are in progress, including the National Institutes of Health–sponsored BEAT-MS trial in the United States (for which Dr. Cohen is the lead investigator) and four European trials – NET-MS (for which Dr. Inglese is the lead investigator), STAR-MS, RAM-MS, and COAST-MS.
The current study was partially funded and supported by the Italian Multiple Sclerosis Foundation. Dr. Inglese disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The benefits of autologous hematopoietic stem cell transplant (AHSCT) for patients with multiple sclerosis (MS) persist for more than 10 years in the majority of patents, new data show. The study reports on 210 Italian patients who underwent AHSCT between 2007 and 2019. Among the entire study cohort, 79.5% of patients had not experienced worsening of disability at 5 years, and 65.5% had not experienced it at 10 years.
Patients with relapsing remitting MS had better results, with 85.5% experiencing no worsening of disability at 5 years, and 71.3% at 10 years. Among patients with progressive MS, 71.0% showed no worsening of disability at 5 years, and 57.2% at 10 years.
“This is the longest follow-up of AHSCT in MS patients so far to be reported,” said study author Matilde Inglese, MD, University of Genoa (Italy). “We have shown AHSCT to be highly effective to prevent long-term disability worsening in most treated patients.”
The study was published online Jan. 20 in Neurology.
“We suggest that AHSCT should be considered as a treatment strategy for MS not responding to conventional therapy,” the authors concluded.
The study had no control group, so a direct comparison is not possible. Nevertheless, Dr. Inglese said she believed these results are better than those that would be achieved with disease-modifying drug therapy for similar patients.
“The best patient candidates for this procedure are those with highly active multiple sclerosis who are not responsive to high-efficacy drugs, such as alemtuzumab or ocrelizumab,” Dr. Inglese commented. “Younger patients with an aggressive form of relapsing remitting MS tend to do the best, although patients with progressive forms of MS who still have active lesions on MRI also benefit.”
Renewing the immune system
The transplant procedure involves giving high-dose cyclophosphamide to stimulate mobilization of bone marrow stem cells, which are collected from peripheral blood. Patients then undergo intense immunosuppression with a cocktail of drugs to remove the autoreactive T cells, and the stem cells, which are not autoreactive, are reinfused.
“We are effectively renewing the immune system,” Dr. Inglese said. “While it is not correct to call it a cure, as we are not eliminating the etiology of the disease, it is the closest to complete suppression of the disease that we can get.”
Other results from the study show that among patients with relapsing remitting MS, rates of relapse-free survival were 78.1% at 5 years and 63.5% at 10 years.
Better results were achieved for patients who received the BEAM+ATG conditioning regimen for immunosuppression. That regimen includes carmustine, cytosine-arabinoside, etoposide, and melphalan, followed by rabbit antithymocyte globulin. Among patients with relapsing remitting disease who were treated with this protocol, rates of relapse-free survival were 86.4% at 5 years and 77.0% at 10 years.
For patients with relapsing remitting MS, the probability of achieving NEDA-3 status (no evidence of disease activity, including the absence of clinical relapses, disability worsening, and MRI inflammatory activity) was 62.2% at 5 years and 40.5% at 10 years.
Among those patients with relapsing remitting MS who received the BEAM+ATG conditioning protocol, NEDA-3 status was achieved in 67.7% at 5 years and in 54.9% at 10 years.
Three deaths occurred within 100 days following AHSCT (1.4% of the entire study population). One patient developed pulmonary thromboembolism, received fibrinolytic treatment, and died 48 hours later after intracranial hemorrhage. The second patient experienced engraftment failure and died 24 days after transplant because of an opportunistic infection. The third patient died 1 month after transplant from Wernicke-like encephalopathy. All the patients who died received the BEAM+ATG conditioning regimen. No transplant-related deaths occurred in patients who underwent transplant after 2007.
Dr. Inglese noted that the mortality rate associated with AHSCT has been greatly reduced in recent years. “We are seeing a very low mortality rate – about 0.3% – thanks to improvements in the procedure and better patient selection. This seems acceptable, given that we are treating patients with very aggressive disease who have a high risk of becoming significantly disabled relatively early in life,” she commented.
However, it is vitally important that the procedure be conducted in a specialized center with a highly experienced multidisciplinary team, she stressed.
In the Neurology article, the authors concluded: “Although patients with RRMS [relapsing remitting MS] are those who benefit the most from transplant, AHSCT has been also shown to prevent disability worsening in a large proportion of patients with active progressive MS.
“The BEAM+ATG conditioning protocol, although associated with a higher transplant mortality rate, was associated with a more pronounced suppression of clinical relapses and MRI inflammatory activity, allowing complete disease control in a higher proportion of patients,” they wrote.
Potent and durable efficacy, with caveats
Commenting on these latest findings, Jeffrey A. Cohen, MD, of the Mellen Center for Multiple Sclerosis at the Cleveland Clinic, said: “AHSCT appears to have potent and durable efficacy in MS but is associated with significant risk and cost.”
The patients who are most likely to benefit are young and have experienced the onset of disease relatively recently. They are still ambulatory with highly active MS and have experienced recent clinical relapses and/or MRI lesion activity, and such activity continues despite disease-modifying therapy, Dr. Cohen noted. He added that “AHSCT is a reasonable option for such patients who have essentially failed the available disease-modifying therapy options.”
He pointed out that the key question is where AHSCT belongs in the overall MS algorithm relative to other high-efficacy therapies. “We need to know whether it should be used more broadly rather than as a last resort.”
To address that question, several randomized trials comparing AHSCT with high-efficacy disease-modifying therapy are in progress, including the National Institutes of Health–sponsored BEAT-MS trial in the United States (for which Dr. Cohen is the lead investigator) and four European trials – NET-MS (for which Dr. Inglese is the lead investigator), STAR-MS, RAM-MS, and COAST-MS.
The current study was partially funded and supported by the Italian Multiple Sclerosis Foundation. Dr. Inglese disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The benefits of autologous hematopoietic stem cell transplant (AHSCT) for patients with multiple sclerosis (MS) persist for more than 10 years in the majority of patents, new data show. The study reports on 210 Italian patients who underwent AHSCT between 2007 and 2019. Among the entire study cohort, 79.5% of patients had not experienced worsening of disability at 5 years, and 65.5% had not experienced it at 10 years.
Patients with relapsing remitting MS had better results, with 85.5% experiencing no worsening of disability at 5 years, and 71.3% at 10 years. Among patients with progressive MS, 71.0% showed no worsening of disability at 5 years, and 57.2% at 10 years.
“This is the longest follow-up of AHSCT in MS patients so far to be reported,” said study author Matilde Inglese, MD, University of Genoa (Italy). “We have shown AHSCT to be highly effective to prevent long-term disability worsening in most treated patients.”
The study was published online Jan. 20 in Neurology.
“We suggest that AHSCT should be considered as a treatment strategy for MS not responding to conventional therapy,” the authors concluded.
The study had no control group, so a direct comparison is not possible. Nevertheless, Dr. Inglese said she believed these results are better than those that would be achieved with disease-modifying drug therapy for similar patients.
“The best patient candidates for this procedure are those with highly active multiple sclerosis who are not responsive to high-efficacy drugs, such as alemtuzumab or ocrelizumab,” Dr. Inglese commented. “Younger patients with an aggressive form of relapsing remitting MS tend to do the best, although patients with progressive forms of MS who still have active lesions on MRI also benefit.”
Renewing the immune system
The transplant procedure involves giving high-dose cyclophosphamide to stimulate mobilization of bone marrow stem cells, which are collected from peripheral blood. Patients then undergo intense immunosuppression with a cocktail of drugs to remove the autoreactive T cells, and the stem cells, which are not autoreactive, are reinfused.
“We are effectively renewing the immune system,” Dr. Inglese said. “While it is not correct to call it a cure, as we are not eliminating the etiology of the disease, it is the closest to complete suppression of the disease that we can get.”
Other results from the study show that among patients with relapsing remitting MS, rates of relapse-free survival were 78.1% at 5 years and 63.5% at 10 years.
Better results were achieved for patients who received the BEAM+ATG conditioning regimen for immunosuppression. That regimen includes carmustine, cytosine-arabinoside, etoposide, and melphalan, followed by rabbit antithymocyte globulin. Among patients with relapsing remitting disease who were treated with this protocol, rates of relapse-free survival were 86.4% at 5 years and 77.0% at 10 years.
For patients with relapsing remitting MS, the probability of achieving NEDA-3 status (no evidence of disease activity, including the absence of clinical relapses, disability worsening, and MRI inflammatory activity) was 62.2% at 5 years and 40.5% at 10 years.
Among those patients with relapsing remitting MS who received the BEAM+ATG conditioning protocol, NEDA-3 status was achieved in 67.7% at 5 years and in 54.9% at 10 years.
Three deaths occurred within 100 days following AHSCT (1.4% of the entire study population). One patient developed pulmonary thromboembolism, received fibrinolytic treatment, and died 48 hours later after intracranial hemorrhage. The second patient experienced engraftment failure and died 24 days after transplant because of an opportunistic infection. The third patient died 1 month after transplant from Wernicke-like encephalopathy. All the patients who died received the BEAM+ATG conditioning regimen. No transplant-related deaths occurred in patients who underwent transplant after 2007.
Dr. Inglese noted that the mortality rate associated with AHSCT has been greatly reduced in recent years. “We are seeing a very low mortality rate – about 0.3% – thanks to improvements in the procedure and better patient selection. This seems acceptable, given that we are treating patients with very aggressive disease who have a high risk of becoming significantly disabled relatively early in life,” she commented.
However, it is vitally important that the procedure be conducted in a specialized center with a highly experienced multidisciplinary team, she stressed.
In the Neurology article, the authors concluded: “Although patients with RRMS [relapsing remitting MS] are those who benefit the most from transplant, AHSCT has been also shown to prevent disability worsening in a large proportion of patients with active progressive MS.
“The BEAM+ATG conditioning protocol, although associated with a higher transplant mortality rate, was associated with a more pronounced suppression of clinical relapses and MRI inflammatory activity, allowing complete disease control in a higher proportion of patients,” they wrote.
Potent and durable efficacy, with caveats
Commenting on these latest findings, Jeffrey A. Cohen, MD, of the Mellen Center for Multiple Sclerosis at the Cleveland Clinic, said: “AHSCT appears to have potent and durable efficacy in MS but is associated with significant risk and cost.”
The patients who are most likely to benefit are young and have experienced the onset of disease relatively recently. They are still ambulatory with highly active MS and have experienced recent clinical relapses and/or MRI lesion activity, and such activity continues despite disease-modifying therapy, Dr. Cohen noted. He added that “AHSCT is a reasonable option for such patients who have essentially failed the available disease-modifying therapy options.”
He pointed out that the key question is where AHSCT belongs in the overall MS algorithm relative to other high-efficacy therapies. “We need to know whether it should be used more broadly rather than as a last resort.”
To address that question, several randomized trials comparing AHSCT with high-efficacy disease-modifying therapy are in progress, including the National Institutes of Health–sponsored BEAT-MS trial in the United States (for which Dr. Cohen is the lead investigator) and four European trials – NET-MS (for which Dr. Inglese is the lead investigator), STAR-MS, RAM-MS, and COAST-MS.
The current study was partially funded and supported by the Italian Multiple Sclerosis Foundation. Dr. Inglese disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY