User login
New data on COVID-19’s cognitive fallout
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
Fact or fiction? Intravascular contrast and acute kidney injury
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
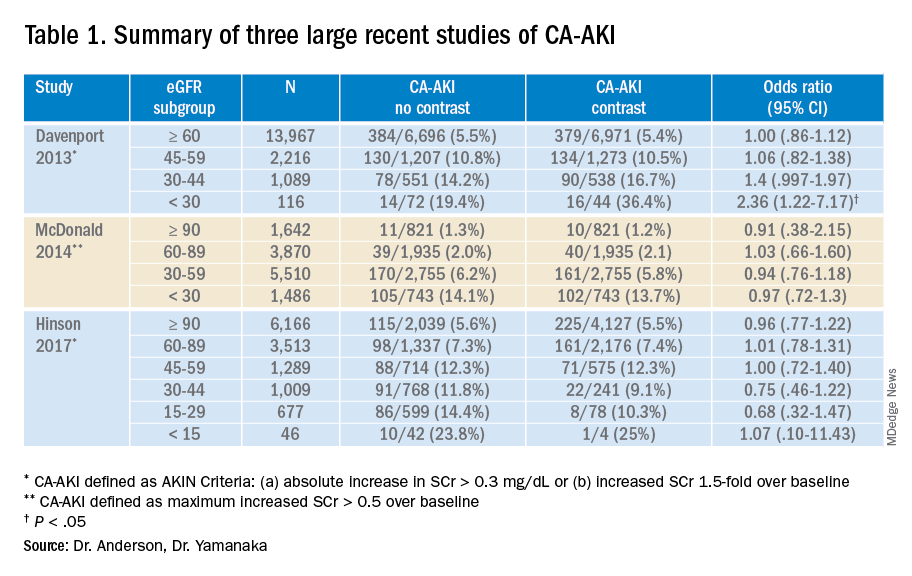
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.
Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Withholding contrast may be the greater risk
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Safety-net burden linked with poorer inpatient cirrhosis outcomes
Patients with cirrhosis treated at hospitals with the highest safety-net burden, defined by their proportion of Medicaid or uninsured patients, had a 5% higher mortality rate than patients who were treated at hospitals with the lowest burden, according to a study of over 300,000 patients.
The study, which was published in the Journal of Clinical Gastroenterology, analyzed inpatient data from the National Inpatient Sample (NIS) database focusing on a 4-year time span between 2012 and 2016. The hospitals were categorized by safety-net burden, which was defined as having either a high, medium, or low number of uninsured patients or patients with Medicaid.
This is the first-known study to evaluate the impact of a hospital’s safety-net burden on hospitalization outcomes in cirrhosis patients, wrote authors Robert J. Wong, MD, MS, of Stanford (Calif.) University and Grishma Hirode, MAS, of the University of Toronto. Previous studies have shown that safety-net hospitals, especially those with a high safety-net burden, have poorer patient outcomes. These hospitals also serve a patient population that is at high risk for chronic liver disease and cirrhosis.
The new analysis included 322,944 individual hospitalizations of patients with cirrhosis. Of these, 57.8% were male, 63.7% were White, 9.9% were Black, and 15.6% were Hispanic. In terms of safety-net burden, 107,446 hospitalizations were at high-burden hospitals, 103,508 were at medium-burden hospitals, and 111,990 hospitalizations were at low-burden hospitals.
Overall, cirrhosis-related hospitalizations in hospitals with the highest burden were found to have significantly greater odds of in-hospital mortality than the lowest tertile hospitals (odds ratio, 1.05, P = .044). The patients were also younger (mean age, 56.7 years vs. 59.8 years in low-burden hospitals). They also had a higher proportion of male patients, minority patients, Hispanic patients, and patients with Medicaid or no insurance.
The odds of hospitalization in the highest tertile hospitals were found to be significantly higher, compared with the middle and lowest tertiles for Blacks and Hispanics, compared with Whites (OR 1.26 and OR 1.63, respectively). Black patients (OR, 1.26; 95%CI, 1.17-1.35; P < .001) and Hispanic patients (OR, 1.63; 95% CI, 1.50-1.78; P< .001) were more likely to be admitted for care at high-burden hospitals (26% to 54%). In-hospital mortality rates among all hospitalizations were 5.95% and the rate did not significantly differ by hospital burden status.
“Despite adjusting for safety-net burden, our study continued to demonstrate ethnic disparities in in-hospital mortality among cirrhosis-related hospitalizations,” the researchers wrote. Overall, the odds of in-hospital mortality were 27% higher in Black patients as compared with White patients.
However, significantly lower mortality was observed in Hispanic patients as compared with White patients (4.9% vs. 6.0%, P < .001), but why this occurred was not entirely clear. “Hispanic patients may be more likely to have NASH [nonalcoholic steatohepatitis]-related cirrhosis, which generally has a slower disease progression, compared with [hepatitis C virus] or alcoholic cirrhosis. As such, it is likely that NASH-cirrhosis Hispanic patients had less severe disease at presentation,” the researchers wrote.
Study design has limitations, but shows concerning trends
The study findings were limited by several factors including the inability to show causality based on the observational study design and cross-sectional nature of the database, the researchers said. The NIS database records individual hospitalizations, not individual patient data which means that it may include repeat hospitalizations from the same patient. In addition, the study was limited by a lack of data on outpatient cirrhosis outcomes and non–liver-related comorbidities.
However, the finding that ethnic minorities with cirrhosis were significantly more likely to be hospitalized in high safety-net hospitals than White patients is concerning, and more research is needed, they said.
“These observations highlight that, while disparities in resources and health care delivery inherent to safety-net health systems may partly explain and provide opportunities to improve cirrhosis hospitalization care, they alone do not explain all of the ethnic disparities in cirrhosis outcomes observed,” they concluded.
The current study was important to conduct at this time because rates of cirrhosis are on the rise, Michael Volk, MD, of Loma Linda (Calif.) University Health, said in an interview. “Millions of patients receive care in safety-net hospitals across the country.”
Dr. Volk said that he was not surprised by the overall outcomes. “Unfortunately, I expected that patient outcomes would be worse at safety-net hospitals than wealthier hospitals. However, I was surprised that Blacks had higher in-hospital mortality than Whites, even after adjusting for the hospital.”
Dr. Volk echoed the study’s stated limitation of the lack of data to address disparities.
“Additional research is needed to determine whether the higher in-hospital mortality among Blacks is related to biological differences such as differential rates of disease progression, or social differences such as access to outpatient care,” he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Volk had no relevant financial conflicts to disclose.
Patients with cirrhosis treated at hospitals with the highest safety-net burden, defined by their proportion of Medicaid or uninsured patients, had a 5% higher mortality rate than patients who were treated at hospitals with the lowest burden, according to a study of over 300,000 patients.
The study, which was published in the Journal of Clinical Gastroenterology, analyzed inpatient data from the National Inpatient Sample (NIS) database focusing on a 4-year time span between 2012 and 2016. The hospitals were categorized by safety-net burden, which was defined as having either a high, medium, or low number of uninsured patients or patients with Medicaid.
This is the first-known study to evaluate the impact of a hospital’s safety-net burden on hospitalization outcomes in cirrhosis patients, wrote authors Robert J. Wong, MD, MS, of Stanford (Calif.) University and Grishma Hirode, MAS, of the University of Toronto. Previous studies have shown that safety-net hospitals, especially those with a high safety-net burden, have poorer patient outcomes. These hospitals also serve a patient population that is at high risk for chronic liver disease and cirrhosis.
The new analysis included 322,944 individual hospitalizations of patients with cirrhosis. Of these, 57.8% were male, 63.7% were White, 9.9% were Black, and 15.6% were Hispanic. In terms of safety-net burden, 107,446 hospitalizations were at high-burden hospitals, 103,508 were at medium-burden hospitals, and 111,990 hospitalizations were at low-burden hospitals.
Overall, cirrhosis-related hospitalizations in hospitals with the highest burden were found to have significantly greater odds of in-hospital mortality than the lowest tertile hospitals (odds ratio, 1.05, P = .044). The patients were also younger (mean age, 56.7 years vs. 59.8 years in low-burden hospitals). They also had a higher proportion of male patients, minority patients, Hispanic patients, and patients with Medicaid or no insurance.
The odds of hospitalization in the highest tertile hospitals were found to be significantly higher, compared with the middle and lowest tertiles for Blacks and Hispanics, compared with Whites (OR 1.26 and OR 1.63, respectively). Black patients (OR, 1.26; 95%CI, 1.17-1.35; P < .001) and Hispanic patients (OR, 1.63; 95% CI, 1.50-1.78; P< .001) were more likely to be admitted for care at high-burden hospitals (26% to 54%). In-hospital mortality rates among all hospitalizations were 5.95% and the rate did not significantly differ by hospital burden status.
“Despite adjusting for safety-net burden, our study continued to demonstrate ethnic disparities in in-hospital mortality among cirrhosis-related hospitalizations,” the researchers wrote. Overall, the odds of in-hospital mortality were 27% higher in Black patients as compared with White patients.
However, significantly lower mortality was observed in Hispanic patients as compared with White patients (4.9% vs. 6.0%, P < .001), but why this occurred was not entirely clear. “Hispanic patients may be more likely to have NASH [nonalcoholic steatohepatitis]-related cirrhosis, which generally has a slower disease progression, compared with [hepatitis C virus] or alcoholic cirrhosis. As such, it is likely that NASH-cirrhosis Hispanic patients had less severe disease at presentation,” the researchers wrote.
Study design has limitations, but shows concerning trends
The study findings were limited by several factors including the inability to show causality based on the observational study design and cross-sectional nature of the database, the researchers said. The NIS database records individual hospitalizations, not individual patient data which means that it may include repeat hospitalizations from the same patient. In addition, the study was limited by a lack of data on outpatient cirrhosis outcomes and non–liver-related comorbidities.
However, the finding that ethnic minorities with cirrhosis were significantly more likely to be hospitalized in high safety-net hospitals than White patients is concerning, and more research is needed, they said.
“These observations highlight that, while disparities in resources and health care delivery inherent to safety-net health systems may partly explain and provide opportunities to improve cirrhosis hospitalization care, they alone do not explain all of the ethnic disparities in cirrhosis outcomes observed,” they concluded.
The current study was important to conduct at this time because rates of cirrhosis are on the rise, Michael Volk, MD, of Loma Linda (Calif.) University Health, said in an interview. “Millions of patients receive care in safety-net hospitals across the country.”
Dr. Volk said that he was not surprised by the overall outcomes. “Unfortunately, I expected that patient outcomes would be worse at safety-net hospitals than wealthier hospitals. However, I was surprised that Blacks had higher in-hospital mortality than Whites, even after adjusting for the hospital.”
Dr. Volk echoed the study’s stated limitation of the lack of data to address disparities.
“Additional research is needed to determine whether the higher in-hospital mortality among Blacks is related to biological differences such as differential rates of disease progression, or social differences such as access to outpatient care,” he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Volk had no relevant financial conflicts to disclose.
Patients with cirrhosis treated at hospitals with the highest safety-net burden, defined by their proportion of Medicaid or uninsured patients, had a 5% higher mortality rate than patients who were treated at hospitals with the lowest burden, according to a study of over 300,000 patients.
The study, which was published in the Journal of Clinical Gastroenterology, analyzed inpatient data from the National Inpatient Sample (NIS) database focusing on a 4-year time span between 2012 and 2016. The hospitals were categorized by safety-net burden, which was defined as having either a high, medium, or low number of uninsured patients or patients with Medicaid.
This is the first-known study to evaluate the impact of a hospital’s safety-net burden on hospitalization outcomes in cirrhosis patients, wrote authors Robert J. Wong, MD, MS, of Stanford (Calif.) University and Grishma Hirode, MAS, of the University of Toronto. Previous studies have shown that safety-net hospitals, especially those with a high safety-net burden, have poorer patient outcomes. These hospitals also serve a patient population that is at high risk for chronic liver disease and cirrhosis.
The new analysis included 322,944 individual hospitalizations of patients with cirrhosis. Of these, 57.8% were male, 63.7% were White, 9.9% were Black, and 15.6% were Hispanic. In terms of safety-net burden, 107,446 hospitalizations were at high-burden hospitals, 103,508 were at medium-burden hospitals, and 111,990 hospitalizations were at low-burden hospitals.
Overall, cirrhosis-related hospitalizations in hospitals with the highest burden were found to have significantly greater odds of in-hospital mortality than the lowest tertile hospitals (odds ratio, 1.05, P = .044). The patients were also younger (mean age, 56.7 years vs. 59.8 years in low-burden hospitals). They also had a higher proportion of male patients, minority patients, Hispanic patients, and patients with Medicaid or no insurance.
The odds of hospitalization in the highest tertile hospitals were found to be significantly higher, compared with the middle and lowest tertiles for Blacks and Hispanics, compared with Whites (OR 1.26 and OR 1.63, respectively). Black patients (OR, 1.26; 95%CI, 1.17-1.35; P < .001) and Hispanic patients (OR, 1.63; 95% CI, 1.50-1.78; P< .001) were more likely to be admitted for care at high-burden hospitals (26% to 54%). In-hospital mortality rates among all hospitalizations were 5.95% and the rate did not significantly differ by hospital burden status.
“Despite adjusting for safety-net burden, our study continued to demonstrate ethnic disparities in in-hospital mortality among cirrhosis-related hospitalizations,” the researchers wrote. Overall, the odds of in-hospital mortality were 27% higher in Black patients as compared with White patients.
However, significantly lower mortality was observed in Hispanic patients as compared with White patients (4.9% vs. 6.0%, P < .001), but why this occurred was not entirely clear. “Hispanic patients may be more likely to have NASH [nonalcoholic steatohepatitis]-related cirrhosis, which generally has a slower disease progression, compared with [hepatitis C virus] or alcoholic cirrhosis. As such, it is likely that NASH-cirrhosis Hispanic patients had less severe disease at presentation,” the researchers wrote.
Study design has limitations, but shows concerning trends
The study findings were limited by several factors including the inability to show causality based on the observational study design and cross-sectional nature of the database, the researchers said. The NIS database records individual hospitalizations, not individual patient data which means that it may include repeat hospitalizations from the same patient. In addition, the study was limited by a lack of data on outpatient cirrhosis outcomes and non–liver-related comorbidities.
However, the finding that ethnic minorities with cirrhosis were significantly more likely to be hospitalized in high safety-net hospitals than White patients is concerning, and more research is needed, they said.
“These observations highlight that, while disparities in resources and health care delivery inherent to safety-net health systems may partly explain and provide opportunities to improve cirrhosis hospitalization care, they alone do not explain all of the ethnic disparities in cirrhosis outcomes observed,” they concluded.
The current study was important to conduct at this time because rates of cirrhosis are on the rise, Michael Volk, MD, of Loma Linda (Calif.) University Health, said in an interview. “Millions of patients receive care in safety-net hospitals across the country.”
Dr. Volk said that he was not surprised by the overall outcomes. “Unfortunately, I expected that patient outcomes would be worse at safety-net hospitals than wealthier hospitals. However, I was surprised that Blacks had higher in-hospital mortality than Whites, even after adjusting for the hospital.”
Dr. Volk echoed the study’s stated limitation of the lack of data to address disparities.
“Additional research is needed to determine whether the higher in-hospital mortality among Blacks is related to biological differences such as differential rates of disease progression, or social differences such as access to outpatient care,” he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Volk had no relevant financial conflicts to disclose.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Prophylactic anticoagulation tied to lower death rate in COVID
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S., international MIS-C studies yield disparate results
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Reversal agents curb DOAC-related bleeding but deaths still high
Agents that reverse the effect of direct oral anticoagulants (DOACs) are highly effective in patients with severe bleeding, but mortality rates remain high despite their use, a meta-analysis shows.
Effective hemostasis was achieved in 78.5% of patients treated with a reversal agent, whereas failure to achieve hemostasis was associated with more than a threefold higher relative risk for death (relative risk, 3.63; 95% confidence interval, 2.56-5.16).
“This has implications in practice because it emphasizes the need for achieving effective hemostasis, if not with only one agent, trying other agents or treatment modalities, because it is a strong predictor of survival,” lead author Antonio Gómez-Outes, MD, PhD, said in an interview.
The bad news, he said, is that the mortality rate was still significant, at 17.7%, and approximately half of patients with DOAC-related severe intracranial bleeding survived with long-term moderate/severe disability.
“The lesson is to prevent these bleeding events because once they appear, even if you give an antidote, the outcome is poor, particularly for intracranial bleeding,” said Dr. Gómez-Outes, division of pharmacology and clinical drug evaluation, Spanish Agency for Medicines and Medical Devices, Madrid.
To put this in context, mortality rates were close to 50% after intracranial bleeding a decade ago when there were no antidotes or reversal agents, he observed. “So to some extent, patient care has improved, and the outcome has improved, but there is a long road to improve regarding disability.”
More than 100,000 DOAC-related major bleeding cases occur each year in the United States and European Union, Dr. Gómez-Outes said, and about half are severe enough to require hospitalization and potentially the use of a reversal agent. These include idarucizumab (Praxbind) for dabigatran reversal and prothombin complex concentrates (4CCC) or andexanet alpha (Andexxa) for reversal of direct factor Xa inhibitors like rivaroxaban, apixaban, and edoxaban.
As reported in the June 22 issue of the Journal of the American College of Cardiology, the meta-analysis comprised 4,735 patients (mean age, 77 years; 57% male) with severe DOAC-related bleeding who received 4PCC (n = 2,688), idarucizumab (n = 1,111), or andexanet (n = 936) in 60 studies between January 2010 and December 2020.
Atrial fibrillation (AFib) was the most common reason for use of a DOAC (82%), followed by venous thromboembolism (14%). Rivaroxaban was used in 36%, apixaban in 32%, dabigatran in 31%, and edoxaban in 1%.
The index bleeding event was intracranial hemorrhage (ICH) in 55%. Anticoagulation was restarted in 57% of patients an average of 11 days after admission.
Mortality rates were 20.2% in patients with ICH and 15.4% in those with extracranial bleeding. There were no differences in death rates by reversal agent used, type of study, risk for bias, or study sponsorship in meta-regression analysis.
Rebleeding occurred in 13.2% of patients; 82.0% of these events were described as an ICH, and 78.0% occurred after anticoagulation was restarted.
The overall rate of thromboembolism was 4.6%. The risk was particularly high with andexanet, at 10.7%, and relatively low with idarucizumab (3.8%) and 4PCC (4.3%), the authors note.
“Our meta-analysis suggests specific reversal with andexanet is not superior to unspecific reversal with 4PCC, and that’s good news because many centers, in many countries, have no access to specific antidotes that are more costly,” Dr. Gómez-Outes said. “4PCC is an effective and relatively safe drug, so it’s still a good option for these patients.”
Labeling for andexanet includes a warning for thromboembolic events, but in the absence of direct comparisons, the findings should be interpreted with caution, he added. Further insights are expected from an ongoing randomized trial of andexanet and standard of care in 900 patients who present with acute ICH less than 15 hours after taking an oral factor Xa inhibitor. The preliminary completion date is set for 2023.
“The meta-analysis raises awareness about the rates of mortality and thromboembolism after reversal agent administration, although understanding the implications of these data is challenging,” Christopher Granger, MD, and Sean P. Pokomey, MD, MBA, Duke University Medical Center, Durham, N.C., say in an accompanying editorial.
The fact that failure to achieve hemostasis was associated with death is expected and might be related to the way hemostasis was defined, rather than the actual failure of the hemostatic treatments, they suggest. “The prothrombotic effects of each agent, including andexanet, need to be better understood, as clinicians work toward including reversal agents into algorithms for bleeding management.”
Effective hemostasis was defined in the studies through various methods as: “Excellent/good” using the Sarode and ANNEXA-4 scales; “yes” in the International Society on Thrombosis and Hemostasis Scale; and with other scales and through clinical judgment.
Although the size of the meta-analysis dwarfs previous reviews, the editorialists and authors point out that 47 of the 60 studies were retrospective, only two had control groups, and 45 had a high risk for bias.
In general, there was also poor reporting of key clinical data, such as postbleeding anticoagulation management, and a limitation of the mortality analysis is that it was based in selected patients with effective hemostasis assessed within 48 hours, which may not capture early deaths, the authors note.
“The morbidity and mortality from ischemic strokes as a result of undertreatment of stroke prevention in patients with AFib continue to dwarf the bleeding related mortality among patients with AFib and on DOACs, and thus the number one priority is to treat nearly all patients with AFib with a DOAC,” Dr. Granger and Dr. Pokomey conclude. “The availability of reversal agents for DOACs should provide reassurance, with another tool in our armamentarium, to providers to prescribe OACs for stroke prevention.”
No funding/grant support was received to conduct the study. Coauthor Ramón Lecumberri has received personal fees from Boehringer Ingelheim and Bristol Myers Squibb outside the submitted work. All other authors report no relevant financial relationships. Dr. Granger has received research and consulting fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Bayer, Janssen, Boston Scientific, Apple, AstraZeneca, Novartis, AbbVie, Biomed, CeleCor, GSK, Novartis, Medtronic, Merck, Novo Nordisk, Philips, Rho, and the U.S. Food and Drug Administration. Dr. Pokomey has received modest consulting support from Bristol Myers Squibb, Pfizer, Boston Scientific, Medtronic, Janssen, and Zoll; modest research support from Gilead, Boston Scientific, Bristol Myers Squibb, Pfizer, and Janssen; and significant research support from the FDA.
A version of this article first appeared on Medscape.com.
Agents that reverse the effect of direct oral anticoagulants (DOACs) are highly effective in patients with severe bleeding, but mortality rates remain high despite their use, a meta-analysis shows.
Effective hemostasis was achieved in 78.5% of patients treated with a reversal agent, whereas failure to achieve hemostasis was associated with more than a threefold higher relative risk for death (relative risk, 3.63; 95% confidence interval, 2.56-5.16).
“This has implications in practice because it emphasizes the need for achieving effective hemostasis, if not with only one agent, trying other agents or treatment modalities, because it is a strong predictor of survival,” lead author Antonio Gómez-Outes, MD, PhD, said in an interview.
The bad news, he said, is that the mortality rate was still significant, at 17.7%, and approximately half of patients with DOAC-related severe intracranial bleeding survived with long-term moderate/severe disability.
“The lesson is to prevent these bleeding events because once they appear, even if you give an antidote, the outcome is poor, particularly for intracranial bleeding,” said Dr. Gómez-Outes, division of pharmacology and clinical drug evaluation, Spanish Agency for Medicines and Medical Devices, Madrid.
To put this in context, mortality rates were close to 50% after intracranial bleeding a decade ago when there were no antidotes or reversal agents, he observed. “So to some extent, patient care has improved, and the outcome has improved, but there is a long road to improve regarding disability.”
More than 100,000 DOAC-related major bleeding cases occur each year in the United States and European Union, Dr. Gómez-Outes said, and about half are severe enough to require hospitalization and potentially the use of a reversal agent. These include idarucizumab (Praxbind) for dabigatran reversal and prothombin complex concentrates (4CCC) or andexanet alpha (Andexxa) for reversal of direct factor Xa inhibitors like rivaroxaban, apixaban, and edoxaban.
As reported in the June 22 issue of the Journal of the American College of Cardiology, the meta-analysis comprised 4,735 patients (mean age, 77 years; 57% male) with severe DOAC-related bleeding who received 4PCC (n = 2,688), idarucizumab (n = 1,111), or andexanet (n = 936) in 60 studies between January 2010 and December 2020.
Atrial fibrillation (AFib) was the most common reason for use of a DOAC (82%), followed by venous thromboembolism (14%). Rivaroxaban was used in 36%, apixaban in 32%, dabigatran in 31%, and edoxaban in 1%.
The index bleeding event was intracranial hemorrhage (ICH) in 55%. Anticoagulation was restarted in 57% of patients an average of 11 days after admission.
Mortality rates were 20.2% in patients with ICH and 15.4% in those with extracranial bleeding. There were no differences in death rates by reversal agent used, type of study, risk for bias, or study sponsorship in meta-regression analysis.
Rebleeding occurred in 13.2% of patients; 82.0% of these events were described as an ICH, and 78.0% occurred after anticoagulation was restarted.
The overall rate of thromboembolism was 4.6%. The risk was particularly high with andexanet, at 10.7%, and relatively low with idarucizumab (3.8%) and 4PCC (4.3%), the authors note.
“Our meta-analysis suggests specific reversal with andexanet is not superior to unspecific reversal with 4PCC, and that’s good news because many centers, in many countries, have no access to specific antidotes that are more costly,” Dr. Gómez-Outes said. “4PCC is an effective and relatively safe drug, so it’s still a good option for these patients.”
Labeling for andexanet includes a warning for thromboembolic events, but in the absence of direct comparisons, the findings should be interpreted with caution, he added. Further insights are expected from an ongoing randomized trial of andexanet and standard of care in 900 patients who present with acute ICH less than 15 hours after taking an oral factor Xa inhibitor. The preliminary completion date is set for 2023.
“The meta-analysis raises awareness about the rates of mortality and thromboembolism after reversal agent administration, although understanding the implications of these data is challenging,” Christopher Granger, MD, and Sean P. Pokomey, MD, MBA, Duke University Medical Center, Durham, N.C., say in an accompanying editorial.
The fact that failure to achieve hemostasis was associated with death is expected and might be related to the way hemostasis was defined, rather than the actual failure of the hemostatic treatments, they suggest. “The prothrombotic effects of each agent, including andexanet, need to be better understood, as clinicians work toward including reversal agents into algorithms for bleeding management.”
Effective hemostasis was defined in the studies through various methods as: “Excellent/good” using the Sarode and ANNEXA-4 scales; “yes” in the International Society on Thrombosis and Hemostasis Scale; and with other scales and through clinical judgment.
Although the size of the meta-analysis dwarfs previous reviews, the editorialists and authors point out that 47 of the 60 studies were retrospective, only two had control groups, and 45 had a high risk for bias.
In general, there was also poor reporting of key clinical data, such as postbleeding anticoagulation management, and a limitation of the mortality analysis is that it was based in selected patients with effective hemostasis assessed within 48 hours, which may not capture early deaths, the authors note.
“The morbidity and mortality from ischemic strokes as a result of undertreatment of stroke prevention in patients with AFib continue to dwarf the bleeding related mortality among patients with AFib and on DOACs, and thus the number one priority is to treat nearly all patients with AFib with a DOAC,” Dr. Granger and Dr. Pokomey conclude. “The availability of reversal agents for DOACs should provide reassurance, with another tool in our armamentarium, to providers to prescribe OACs for stroke prevention.”
No funding/grant support was received to conduct the study. Coauthor Ramón Lecumberri has received personal fees from Boehringer Ingelheim and Bristol Myers Squibb outside the submitted work. All other authors report no relevant financial relationships. Dr. Granger has received research and consulting fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Bayer, Janssen, Boston Scientific, Apple, AstraZeneca, Novartis, AbbVie, Biomed, CeleCor, GSK, Novartis, Medtronic, Merck, Novo Nordisk, Philips, Rho, and the U.S. Food and Drug Administration. Dr. Pokomey has received modest consulting support from Bristol Myers Squibb, Pfizer, Boston Scientific, Medtronic, Janssen, and Zoll; modest research support from Gilead, Boston Scientific, Bristol Myers Squibb, Pfizer, and Janssen; and significant research support from the FDA.
A version of this article first appeared on Medscape.com.
Agents that reverse the effect of direct oral anticoagulants (DOACs) are highly effective in patients with severe bleeding, but mortality rates remain high despite their use, a meta-analysis shows.
Effective hemostasis was achieved in 78.5% of patients treated with a reversal agent, whereas failure to achieve hemostasis was associated with more than a threefold higher relative risk for death (relative risk, 3.63; 95% confidence interval, 2.56-5.16).
“This has implications in practice because it emphasizes the need for achieving effective hemostasis, if not with only one agent, trying other agents or treatment modalities, because it is a strong predictor of survival,” lead author Antonio Gómez-Outes, MD, PhD, said in an interview.
The bad news, he said, is that the mortality rate was still significant, at 17.7%, and approximately half of patients with DOAC-related severe intracranial bleeding survived with long-term moderate/severe disability.
“The lesson is to prevent these bleeding events because once they appear, even if you give an antidote, the outcome is poor, particularly for intracranial bleeding,” said Dr. Gómez-Outes, division of pharmacology and clinical drug evaluation, Spanish Agency for Medicines and Medical Devices, Madrid.
To put this in context, mortality rates were close to 50% after intracranial bleeding a decade ago when there were no antidotes or reversal agents, he observed. “So to some extent, patient care has improved, and the outcome has improved, but there is a long road to improve regarding disability.”
More than 100,000 DOAC-related major bleeding cases occur each year in the United States and European Union, Dr. Gómez-Outes said, and about half are severe enough to require hospitalization and potentially the use of a reversal agent. These include idarucizumab (Praxbind) for dabigatran reversal and prothombin complex concentrates (4CCC) or andexanet alpha (Andexxa) for reversal of direct factor Xa inhibitors like rivaroxaban, apixaban, and edoxaban.
As reported in the June 22 issue of the Journal of the American College of Cardiology, the meta-analysis comprised 4,735 patients (mean age, 77 years; 57% male) with severe DOAC-related bleeding who received 4PCC (n = 2,688), idarucizumab (n = 1,111), or andexanet (n = 936) in 60 studies between January 2010 and December 2020.
Atrial fibrillation (AFib) was the most common reason for use of a DOAC (82%), followed by venous thromboembolism (14%). Rivaroxaban was used in 36%, apixaban in 32%, dabigatran in 31%, and edoxaban in 1%.
The index bleeding event was intracranial hemorrhage (ICH) in 55%. Anticoagulation was restarted in 57% of patients an average of 11 days after admission.
Mortality rates were 20.2% in patients with ICH and 15.4% in those with extracranial bleeding. There were no differences in death rates by reversal agent used, type of study, risk for bias, or study sponsorship in meta-regression analysis.
Rebleeding occurred in 13.2% of patients; 82.0% of these events were described as an ICH, and 78.0% occurred after anticoagulation was restarted.
The overall rate of thromboembolism was 4.6%. The risk was particularly high with andexanet, at 10.7%, and relatively low with idarucizumab (3.8%) and 4PCC (4.3%), the authors note.
“Our meta-analysis suggests specific reversal with andexanet is not superior to unspecific reversal with 4PCC, and that’s good news because many centers, in many countries, have no access to specific antidotes that are more costly,” Dr. Gómez-Outes said. “4PCC is an effective and relatively safe drug, so it’s still a good option for these patients.”
Labeling for andexanet includes a warning for thromboembolic events, but in the absence of direct comparisons, the findings should be interpreted with caution, he added. Further insights are expected from an ongoing randomized trial of andexanet and standard of care in 900 patients who present with acute ICH less than 15 hours after taking an oral factor Xa inhibitor. The preliminary completion date is set for 2023.
“The meta-analysis raises awareness about the rates of mortality and thromboembolism after reversal agent administration, although understanding the implications of these data is challenging,” Christopher Granger, MD, and Sean P. Pokomey, MD, MBA, Duke University Medical Center, Durham, N.C., say in an accompanying editorial.
The fact that failure to achieve hemostasis was associated with death is expected and might be related to the way hemostasis was defined, rather than the actual failure of the hemostatic treatments, they suggest. “The prothrombotic effects of each agent, including andexanet, need to be better understood, as clinicians work toward including reversal agents into algorithms for bleeding management.”
Effective hemostasis was defined in the studies through various methods as: “Excellent/good” using the Sarode and ANNEXA-4 scales; “yes” in the International Society on Thrombosis and Hemostasis Scale; and with other scales and through clinical judgment.
Although the size of the meta-analysis dwarfs previous reviews, the editorialists and authors point out that 47 of the 60 studies were retrospective, only two had control groups, and 45 had a high risk for bias.
In general, there was also poor reporting of key clinical data, such as postbleeding anticoagulation management, and a limitation of the mortality analysis is that it was based in selected patients with effective hemostasis assessed within 48 hours, which may not capture early deaths, the authors note.
“The morbidity and mortality from ischemic strokes as a result of undertreatment of stroke prevention in patients with AFib continue to dwarf the bleeding related mortality among patients with AFib and on DOACs, and thus the number one priority is to treat nearly all patients with AFib with a DOAC,” Dr. Granger and Dr. Pokomey conclude. “The availability of reversal agents for DOACs should provide reassurance, with another tool in our armamentarium, to providers to prescribe OACs for stroke prevention.”
No funding/grant support was received to conduct the study. Coauthor Ramón Lecumberri has received personal fees from Boehringer Ingelheim and Bristol Myers Squibb outside the submitted work. All other authors report no relevant financial relationships. Dr. Granger has received research and consulting fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Bayer, Janssen, Boston Scientific, Apple, AstraZeneca, Novartis, AbbVie, Biomed, CeleCor, GSK, Novartis, Medtronic, Merck, Novo Nordisk, Philips, Rho, and the U.S. Food and Drug Administration. Dr. Pokomey has received modest consulting support from Bristol Myers Squibb, Pfizer, Boston Scientific, Medtronic, Janssen, and Zoll; modest research support from Gilead, Boston Scientific, Bristol Myers Squibb, Pfizer, and Janssen; and significant research support from the FDA.
A version of this article first appeared on Medscape.com.
AHA: Don’t delay COVID shot while CDC reviews myocarditis cases
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
‘COVID toes’ chilblain-like lesions not related to COVID-19
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
As new cases fall, U.S. passes 4 million children with COVID-19
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Simple risk assessment predicts post-PCI ischemic events
A patient’s risk for ischemic events, but not bleeding, after percutaneous coronary intervention (PCI) can be predicted simply based on whether they have one or more guideline-based standardized risk criteria, a large-scale real-world analysis suggests.
Haoyu Wang, MD, and colleagues showed that having at least one high-risk feature, as outlined in the 2018 European Society of Cardiology and European Association for Cardiothoracic Surgery (ESC/EACTS) Guidelines on Myocardial Revascularization, was associated with an increased risk for target vessel failure by 48% and for a patient-oriented composite outcome by 44%.
Moreover, they showed that implantation of at least three stents and the presence of diabetes and diffuse multivessel disease were the only high-risk features from the guidelines that were independent predictors of the two outcomes.
The study of more than 10,000 PCI patients also showed that determining whether patients were at high bleeding risk (HBR) did not modify their ischemic risk.
This, said Dr. Wang, from the National Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, underscores the importance of applying the high ischemic risk (HIR) criteria from the ESC/EACTS guidelines when tailoring dual antiplatelet therapy (DAPT).
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress on June 2, and published online in the Journal of Atherosclerosis and Thrombosis.
Dr. Wang told theheart.org | Medscape Cardiology that they conducted the study to determine which – HIR or HBR – is “most important to balance when treating patients undergoing PCI and then having dual antiplatelet therapy.”
The results showed that when patients have both a HIR and HBR, it is the ESC/EACTS guideline HIR criteria that have “a higher impact” than the bleeding risk, and that this can be “used to guide our choice of the duration of dual anti-platelet therapy.”
“Maybe we can extend, or use more potent, P2Y12 inhibitors” in those situations, he said.
S. Lale Tokgözoglu, MD, PhD, professor of cardiology, Hacettepe University, Ankara, Turkey, who was not involved in the study, said the HIR assessment “performed well,” adding that the HBR score might have been expected to attenuate its “prognostic advantage.”
She told this news organization that the results “are interesting since previous observations have suggested that Asian patients may be more prone to medication side effects and bleeding.”
These findings emphasize the importance of assessing HIR in daily PCI practice and confirm that it “performs well in different populations in real life,” added Dr. Tokgözoglu, a former president of the EAS.
The ESC/EACTS guidelines aimed to standardize the definition of HIR, Dr. Wang said during the presentation.
They set out 10 high-risk features for ischemic events for patients undergoing revascularization, which included patient medical history, comorbid conditions, and the characteristics of the PCI procedure.
Although the goals of the criteria are to inform decision-making and stimulate research, Dr. Wang said that their “prevalence and prognostic association with clinical outcomes are yet to be established in real-world PCI practice.”
Alongside, the Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE-DAPT) score was developed to predict out-of-hospital bleeding in patients receiving DAPT after stent implantation.
Although a PRECISE-DAPT score of at least 25 constitutes a patient at high bleeding risk, Dr. Wang pointed out that such patients are typically also at risk for ischemic events after PCI, and it is “unclear” whether being at HBR modifies this risk.
To investigate further, they used the prospective, real-world Fuwai PCI registry to collate an all-comer patient population with unselected use of drug-eluting stents at the National Center for Cardiovascular Diseases at Fuwai Hospital.
They excluded individuals who were treated with balloon angioplasty alone, bioresorbable scaffolds, or bare metal stents, leaving a total population of 10,167 patients who were treated in 2013.
In that cohort, 5,149 patients (50.6%) met at least one risk criterion from the ESC/EACTS guidelines (HIR patients) and 5,018 (49.4%) met none of the risk criteria (non-HIR patients).
The most common criteria were implantation of at least three stents (23.5%); total stent length greater than 60 mm (20.2%); diffuse multivessel disease, especially in diabetic patients (18.5%); and a history of ST-segment elevation myocardial infarction (13.9%).
HIR patients were significantly older than non-HIR patients (average age, 58.86 vs. 57.77 years; P < .001), were more likely to have diabetes mellitus (42.6% vs. 16.9%; P < .001); and were more likely to have already had a myocardial infarction (32.2% vs. 5.2%; P < .001).
HIR patients also had higher average PRECISE-ADAPT scores than those without HIR (11.22 vs. 9.94; P < .001), and were conversely less likely to have the left anterior descending artery as the target vessel than non-HIR patients (86.0% vs. 94.6%; P < .001).
Cox regression analysis taking into account a range of patient and clinical factors revealed that HIR patients were significantly more likely than their non-HIR counterparts to experience target vessel failure (hazard ratio, 1.48; 95% confidence interval, 1.25-1.74; P < .001).
They were also significantly more likely to have a patient-oriented composite outcome, defined as all-cause death, any myocardial infarction, or any revascularization (HR, 1.44; 95% CI, 1.28-1.63; P < .001).
There was also a significantly higher risk for cardiac death in HIR than in non-HIR patients (HR, 1.95; 95% CI, 1.16-3.29; P = .012).
However, there was no significant association between HIR status and clinically relevant bleeding (HR, 0.84; 95% CI, 0.66-1.06; P = .143).
When the researchers looked at individual ischemic risk features, they found that, on fully adjusted analyses, only two were independent predictors of target vessel failure and the patient-oriented composite outcome.
Having at least three stents implanted was significantly associated with target vessel failure (HR, 1.36; 95% CI, 1.02-1.80; P = .038), and borderline significantly associated with the patient oriented composite outcome (HR, 1.23; 95% CI, 1.00-1.53; P = .056).
Diffuse multivessel disease, especially in diabetic patients, was significantly associated with both target vessel failure (HR, 1.24; 95% CI, 1.02-1.51; P = .035) and with the patient-oriented composite outcome (HR, 1.20; 95% CI, 1.04-1.39; P = .012).
Neither risk feature was significantly associated with clinically relevant bleeding, Dr. Wang noted.
Stratifying the patients by HBR status, the team found that rates of target vessel failure, the patient-oriented composite outcome, cardiac death, myocardial infarction, and definite/probable stent thrombosis were higher in patients with both HIR and HBR than those with neither HIR nor HBR (P < .001).
Further stratifying patients by PRECISE-ADAPT scores – 10 or less indicating very low risk, 11-17 indicating low risk, 18-24 indicating moderate risk, and at least 25 indicating high risk – showed that HIR features had a consistent effect on ischemic and bleeding outcomes, regardless of bleeding risk.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
A patient’s risk for ischemic events, but not bleeding, after percutaneous coronary intervention (PCI) can be predicted simply based on whether they have one or more guideline-based standardized risk criteria, a large-scale real-world analysis suggests.
Haoyu Wang, MD, and colleagues showed that having at least one high-risk feature, as outlined in the 2018 European Society of Cardiology and European Association for Cardiothoracic Surgery (ESC/EACTS) Guidelines on Myocardial Revascularization, was associated with an increased risk for target vessel failure by 48% and for a patient-oriented composite outcome by 44%.
Moreover, they showed that implantation of at least three stents and the presence of diabetes and diffuse multivessel disease were the only high-risk features from the guidelines that were independent predictors of the two outcomes.
The study of more than 10,000 PCI patients also showed that determining whether patients were at high bleeding risk (HBR) did not modify their ischemic risk.
This, said Dr. Wang, from the National Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, underscores the importance of applying the high ischemic risk (HIR) criteria from the ESC/EACTS guidelines when tailoring dual antiplatelet therapy (DAPT).
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress on June 2, and published online in the Journal of Atherosclerosis and Thrombosis.
Dr. Wang told theheart.org | Medscape Cardiology that they conducted the study to determine which – HIR or HBR – is “most important to balance when treating patients undergoing PCI and then having dual antiplatelet therapy.”
The results showed that when patients have both a HIR and HBR, it is the ESC/EACTS guideline HIR criteria that have “a higher impact” than the bleeding risk, and that this can be “used to guide our choice of the duration of dual anti-platelet therapy.”
“Maybe we can extend, or use more potent, P2Y12 inhibitors” in those situations, he said.
S. Lale Tokgözoglu, MD, PhD, professor of cardiology, Hacettepe University, Ankara, Turkey, who was not involved in the study, said the HIR assessment “performed well,” adding that the HBR score might have been expected to attenuate its “prognostic advantage.”
She told this news organization that the results “are interesting since previous observations have suggested that Asian patients may be more prone to medication side effects and bleeding.”
These findings emphasize the importance of assessing HIR in daily PCI practice and confirm that it “performs well in different populations in real life,” added Dr. Tokgözoglu, a former president of the EAS.
The ESC/EACTS guidelines aimed to standardize the definition of HIR, Dr. Wang said during the presentation.
They set out 10 high-risk features for ischemic events for patients undergoing revascularization, which included patient medical history, comorbid conditions, and the characteristics of the PCI procedure.
Although the goals of the criteria are to inform decision-making and stimulate research, Dr. Wang said that their “prevalence and prognostic association with clinical outcomes are yet to be established in real-world PCI practice.”
Alongside, the Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE-DAPT) score was developed to predict out-of-hospital bleeding in patients receiving DAPT after stent implantation.
Although a PRECISE-DAPT score of at least 25 constitutes a patient at high bleeding risk, Dr. Wang pointed out that such patients are typically also at risk for ischemic events after PCI, and it is “unclear” whether being at HBR modifies this risk.
To investigate further, they used the prospective, real-world Fuwai PCI registry to collate an all-comer patient population with unselected use of drug-eluting stents at the National Center for Cardiovascular Diseases at Fuwai Hospital.
They excluded individuals who were treated with balloon angioplasty alone, bioresorbable scaffolds, or bare metal stents, leaving a total population of 10,167 patients who were treated in 2013.
In that cohort, 5,149 patients (50.6%) met at least one risk criterion from the ESC/EACTS guidelines (HIR patients) and 5,018 (49.4%) met none of the risk criteria (non-HIR patients).
The most common criteria were implantation of at least three stents (23.5%); total stent length greater than 60 mm (20.2%); diffuse multivessel disease, especially in diabetic patients (18.5%); and a history of ST-segment elevation myocardial infarction (13.9%).
HIR patients were significantly older than non-HIR patients (average age, 58.86 vs. 57.77 years; P < .001), were more likely to have diabetes mellitus (42.6% vs. 16.9%; P < .001); and were more likely to have already had a myocardial infarction (32.2% vs. 5.2%; P < .001).
HIR patients also had higher average PRECISE-ADAPT scores than those without HIR (11.22 vs. 9.94; P < .001), and were conversely less likely to have the left anterior descending artery as the target vessel than non-HIR patients (86.0% vs. 94.6%; P < .001).
Cox regression analysis taking into account a range of patient and clinical factors revealed that HIR patients were significantly more likely than their non-HIR counterparts to experience target vessel failure (hazard ratio, 1.48; 95% confidence interval, 1.25-1.74; P < .001).
They were also significantly more likely to have a patient-oriented composite outcome, defined as all-cause death, any myocardial infarction, or any revascularization (HR, 1.44; 95% CI, 1.28-1.63; P < .001).
There was also a significantly higher risk for cardiac death in HIR than in non-HIR patients (HR, 1.95; 95% CI, 1.16-3.29; P = .012).
However, there was no significant association between HIR status and clinically relevant bleeding (HR, 0.84; 95% CI, 0.66-1.06; P = .143).
When the researchers looked at individual ischemic risk features, they found that, on fully adjusted analyses, only two were independent predictors of target vessel failure and the patient-oriented composite outcome.
Having at least three stents implanted was significantly associated with target vessel failure (HR, 1.36; 95% CI, 1.02-1.80; P = .038), and borderline significantly associated with the patient oriented composite outcome (HR, 1.23; 95% CI, 1.00-1.53; P = .056).
Diffuse multivessel disease, especially in diabetic patients, was significantly associated with both target vessel failure (HR, 1.24; 95% CI, 1.02-1.51; P = .035) and with the patient-oriented composite outcome (HR, 1.20; 95% CI, 1.04-1.39; P = .012).
Neither risk feature was significantly associated with clinically relevant bleeding, Dr. Wang noted.
Stratifying the patients by HBR status, the team found that rates of target vessel failure, the patient-oriented composite outcome, cardiac death, myocardial infarction, and definite/probable stent thrombosis were higher in patients with both HIR and HBR than those with neither HIR nor HBR (P < .001).
Further stratifying patients by PRECISE-ADAPT scores – 10 or less indicating very low risk, 11-17 indicating low risk, 18-24 indicating moderate risk, and at least 25 indicating high risk – showed that HIR features had a consistent effect on ischemic and bleeding outcomes, regardless of bleeding risk.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
A patient’s risk for ischemic events, but not bleeding, after percutaneous coronary intervention (PCI) can be predicted simply based on whether they have one or more guideline-based standardized risk criteria, a large-scale real-world analysis suggests.
Haoyu Wang, MD, and colleagues showed that having at least one high-risk feature, as outlined in the 2018 European Society of Cardiology and European Association for Cardiothoracic Surgery (ESC/EACTS) Guidelines on Myocardial Revascularization, was associated with an increased risk for target vessel failure by 48% and for a patient-oriented composite outcome by 44%.
Moreover, they showed that implantation of at least three stents and the presence of diabetes and diffuse multivessel disease were the only high-risk features from the guidelines that were independent predictors of the two outcomes.
The study of more than 10,000 PCI patients also showed that determining whether patients were at high bleeding risk (HBR) did not modify their ischemic risk.
This, said Dr. Wang, from the National Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, underscores the importance of applying the high ischemic risk (HIR) criteria from the ESC/EACTS guidelines when tailoring dual antiplatelet therapy (DAPT).
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress on June 2, and published online in the Journal of Atherosclerosis and Thrombosis.
Dr. Wang told theheart.org | Medscape Cardiology that they conducted the study to determine which – HIR or HBR – is “most important to balance when treating patients undergoing PCI and then having dual antiplatelet therapy.”
The results showed that when patients have both a HIR and HBR, it is the ESC/EACTS guideline HIR criteria that have “a higher impact” than the bleeding risk, and that this can be “used to guide our choice of the duration of dual anti-platelet therapy.”
“Maybe we can extend, or use more potent, P2Y12 inhibitors” in those situations, he said.
S. Lale Tokgözoglu, MD, PhD, professor of cardiology, Hacettepe University, Ankara, Turkey, who was not involved in the study, said the HIR assessment “performed well,” adding that the HBR score might have been expected to attenuate its “prognostic advantage.”
She told this news organization that the results “are interesting since previous observations have suggested that Asian patients may be more prone to medication side effects and bleeding.”
These findings emphasize the importance of assessing HIR in daily PCI practice and confirm that it “performs well in different populations in real life,” added Dr. Tokgözoglu, a former president of the EAS.
The ESC/EACTS guidelines aimed to standardize the definition of HIR, Dr. Wang said during the presentation.
They set out 10 high-risk features for ischemic events for patients undergoing revascularization, which included patient medical history, comorbid conditions, and the characteristics of the PCI procedure.
Although the goals of the criteria are to inform decision-making and stimulate research, Dr. Wang said that their “prevalence and prognostic association with clinical outcomes are yet to be established in real-world PCI practice.”
Alongside, the Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE-DAPT) score was developed to predict out-of-hospital bleeding in patients receiving DAPT after stent implantation.
Although a PRECISE-DAPT score of at least 25 constitutes a patient at high bleeding risk, Dr. Wang pointed out that such patients are typically also at risk for ischemic events after PCI, and it is “unclear” whether being at HBR modifies this risk.
To investigate further, they used the prospective, real-world Fuwai PCI registry to collate an all-comer patient population with unselected use of drug-eluting stents at the National Center for Cardiovascular Diseases at Fuwai Hospital.
They excluded individuals who were treated with balloon angioplasty alone, bioresorbable scaffolds, or bare metal stents, leaving a total population of 10,167 patients who were treated in 2013.
In that cohort, 5,149 patients (50.6%) met at least one risk criterion from the ESC/EACTS guidelines (HIR patients) and 5,018 (49.4%) met none of the risk criteria (non-HIR patients).
The most common criteria were implantation of at least three stents (23.5%); total stent length greater than 60 mm (20.2%); diffuse multivessel disease, especially in diabetic patients (18.5%); and a history of ST-segment elevation myocardial infarction (13.9%).
HIR patients were significantly older than non-HIR patients (average age, 58.86 vs. 57.77 years; P < .001), were more likely to have diabetes mellitus (42.6% vs. 16.9%; P < .001); and were more likely to have already had a myocardial infarction (32.2% vs. 5.2%; P < .001).
HIR patients also had higher average PRECISE-ADAPT scores than those without HIR (11.22 vs. 9.94; P < .001), and were conversely less likely to have the left anterior descending artery as the target vessel than non-HIR patients (86.0% vs. 94.6%; P < .001).
Cox regression analysis taking into account a range of patient and clinical factors revealed that HIR patients were significantly more likely than their non-HIR counterparts to experience target vessel failure (hazard ratio, 1.48; 95% confidence interval, 1.25-1.74; P < .001).
They were also significantly more likely to have a patient-oriented composite outcome, defined as all-cause death, any myocardial infarction, or any revascularization (HR, 1.44; 95% CI, 1.28-1.63; P < .001).
There was also a significantly higher risk for cardiac death in HIR than in non-HIR patients (HR, 1.95; 95% CI, 1.16-3.29; P = .012).
However, there was no significant association between HIR status and clinically relevant bleeding (HR, 0.84; 95% CI, 0.66-1.06; P = .143).
When the researchers looked at individual ischemic risk features, they found that, on fully adjusted analyses, only two were independent predictors of target vessel failure and the patient-oriented composite outcome.
Having at least three stents implanted was significantly associated with target vessel failure (HR, 1.36; 95% CI, 1.02-1.80; P = .038), and borderline significantly associated with the patient oriented composite outcome (HR, 1.23; 95% CI, 1.00-1.53; P = .056).
Diffuse multivessel disease, especially in diabetic patients, was significantly associated with both target vessel failure (HR, 1.24; 95% CI, 1.02-1.51; P = .035) and with the patient-oriented composite outcome (HR, 1.20; 95% CI, 1.04-1.39; P = .012).
Neither risk feature was significantly associated with clinically relevant bleeding, Dr. Wang noted.
Stratifying the patients by HBR status, the team found that rates of target vessel failure, the patient-oriented composite outcome, cardiac death, myocardial infarction, and definite/probable stent thrombosis were higher in patients with both HIR and HBR than those with neither HIR nor HBR (P < .001).
Further stratifying patients by PRECISE-ADAPT scores – 10 or less indicating very low risk, 11-17 indicating low risk, 18-24 indicating moderate risk, and at least 25 indicating high risk – showed that HIR features had a consistent effect on ischemic and bleeding outcomes, regardless of bleeding risk.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.




