User login
COVID-19 virus reinfections rare; riskiest after age 65
When researchers analyzed test results of 4 million people in Denmark, they found that less than 1% of those who tested positive experienced reinfection.
Initial infection was associated with about 80% protection overall against getting SARS-CoV-2 again. However, among those older than 65, the protection plummeted to 47%.
“Not everybody is protected against reinfection after a first infection. Older people are at higher risk of catching it again,” co–lead author Daniela Michlmayr, PhD, said in an interview. “Our findings emphasize the importance of policies to protect the elderly and of adhering to infection control measures and restrictions, even if previously infected with COVID-19.”
Verifying the need for vaccination
“The findings also highlight the need to vaccinate people who had COVID-19 before, as natural immunity to infection – especially among the elderly 65 and older – cannot be relied upon,” added Dr. Michlmayr, a researcher in the department of bacteria, parasites, and fungi at the Staten Serums Institut, Copenhagen.
The population-based observational study was published online March 17 in The Lancet.
“The findings make sense, as patients who are immunocompromised or of advanced age may not mount an immune response that is as long-lasting,” David Hirschwerk, MD, said in an interview. “It does underscore the importance of vaccination for people of more advanced age, even if they previously were infected with COVID.
“For those who were infected last spring and have not yet been vaccinated, this helps to support the value of still pursuing the vaccine,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in Manhasset, N.Y.
Evidence on reinfection risk was limited prior to this study. “Little is known about protection against SARS-CoV-2 repeat infections, but two studies in the UK have found that immunity could last at least 5 to 6 months after infection,” the authors noted.
Along with co–lead author Christian Holm Hansen, PhD, Dr. Michlmayr and colleagues found that 2.11% of 525,339 individuals tested positive for SARS-CoV-2 during the first surge in Denmark from March to May 2020. Within this group, 0.65% tested positive during a second surge from September to December.
By the end of 2020, more than 10 million people had undergone free polymerase chain reaction testing by the Danish government or through the national TestDenmark program.
“My overall take is that it is great to have such a big dataset looking at this question,” E. John Wherry, PhD, said in an interview. The findings support “what we’ve seen in previous, smaller studies.”
Natural protection against reinfection of approximately 80% “is not as good as the vaccines, but not bad,” added Dr. Wherry, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia.
Age alters immunity?
“Our finding that older people were more likely than younger people to test positive again if they had already tested positive could be explained by natural age-related changes in the immune system of older adults, also referred to as immune senescence,” the authors noted.
The investigators found no significant differences in reinfection rates between women and men.
As with the previous research, this study also indicates that an initial bout with SARS-CoV-2 infection appears to confer protection for at least 6 months. The researchers found no significant differences between people who were followed for 3-6 months and those followed for 7 months or longer.
Variants not included
To account for possible bias among people who got tested repeatedly, Dr. Michlmayr and colleagues performed a sensitivity analysis in a subgroup. They assessed reinfection rates among people who underwent testing frequently and routinely – nurses, doctors, social workers, and health care assistants – and found that 1.2% tested positive a second time during the second surge.
A limitation of the study was the inability to correlate symptoms with risk for reinfection. Also, the researchers did not account for SARS-CoV-2 variants, noting that “during the study period, such variants were not yet established in Denmark; although into 2021 this pattern is changing.”
Asked to speculate whether the results would be different had the study accounted for variants, Dr. Hirschwerk said, “It depends upon the variant, but certainly for the B.1.351 variant, there already has been data clearly demonstrating risk of reinfection with SARS-CoV-2 despite prior infection with the original strain of virus.”
The emergence of SARS-CoV-2 variants of concern that could escape natural and vaccine-related immunity “complicates matters further,” Rosemary J. Boyton, MBBS, and Daniel M. Altmann, PhD, both of Imperial College London, wrote in an accompanying comment in The Lancet.
“Emerging variants of concern might shift immunity below a protective margin, prompting the need for updated vaccines. Interestingly, vaccine responses even after single dose are substantially enhanced in individuals with a history of infection with SARS-CoV-2,” they added.
The current study confirms that “the hope of protective immunity through natural infections might not be within our reach, and a global vaccination program with high efficacy vaccines is the enduring solution,” Dr. Boyton and Dr. Altmann noted.
Cause for alarm?
Despite evidence that reinfection is relatively rare, “many will find the data reported by Hansen and colleagues about protection through natural infection relatively alarming,” Dr. Boyton and Dr. Altmann wrote in their commentary. The 80% protection rate from reinfection in general and the 47% rate among people aged 65 and older “are more concerning figures than offered by previous studies.”
Vaccines appear to provide better quality, quantity, and durability of protection against repeated infection – measured in terms of neutralizing antibodies and T cells – compared with previous infection with SARS-CoV-2, Dr. Boyton and Dr. Altmann wrote.
More research needed
The duration of natural protection against reinfection remains an unanswered question, the researchers noted, “because too little time has elapsed since the beginning of the pandemic.”
Future prospective and longitudinal cohort studies coupled with molecular surveillance are needed to characterize antibody titers and waning of protection against repeat infections, the authors noted. Furthermore, more answers are needed regarding how some virus variants might contribute to reinfection risk.
No funding for the study has been reported. Dr. Michlmayr, Dr. Hirschwerk, Dr. Wherry, Dr. Boyton, and Dr. Altmann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When researchers analyzed test results of 4 million people in Denmark, they found that less than 1% of those who tested positive experienced reinfection.
Initial infection was associated with about 80% protection overall against getting SARS-CoV-2 again. However, among those older than 65, the protection plummeted to 47%.
“Not everybody is protected against reinfection after a first infection. Older people are at higher risk of catching it again,” co–lead author Daniela Michlmayr, PhD, said in an interview. “Our findings emphasize the importance of policies to protect the elderly and of adhering to infection control measures and restrictions, even if previously infected with COVID-19.”
Verifying the need for vaccination
“The findings also highlight the need to vaccinate people who had COVID-19 before, as natural immunity to infection – especially among the elderly 65 and older – cannot be relied upon,” added Dr. Michlmayr, a researcher in the department of bacteria, parasites, and fungi at the Staten Serums Institut, Copenhagen.
The population-based observational study was published online March 17 in The Lancet.
“The findings make sense, as patients who are immunocompromised or of advanced age may not mount an immune response that is as long-lasting,” David Hirschwerk, MD, said in an interview. “It does underscore the importance of vaccination for people of more advanced age, even if they previously were infected with COVID.
“For those who were infected last spring and have not yet been vaccinated, this helps to support the value of still pursuing the vaccine,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in Manhasset, N.Y.
Evidence on reinfection risk was limited prior to this study. “Little is known about protection against SARS-CoV-2 repeat infections, but two studies in the UK have found that immunity could last at least 5 to 6 months after infection,” the authors noted.
Along with co–lead author Christian Holm Hansen, PhD, Dr. Michlmayr and colleagues found that 2.11% of 525,339 individuals tested positive for SARS-CoV-2 during the first surge in Denmark from March to May 2020. Within this group, 0.65% tested positive during a second surge from September to December.
By the end of 2020, more than 10 million people had undergone free polymerase chain reaction testing by the Danish government or through the national TestDenmark program.
“My overall take is that it is great to have such a big dataset looking at this question,” E. John Wherry, PhD, said in an interview. The findings support “what we’ve seen in previous, smaller studies.”
Natural protection against reinfection of approximately 80% “is not as good as the vaccines, but not bad,” added Dr. Wherry, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia.
Age alters immunity?
“Our finding that older people were more likely than younger people to test positive again if they had already tested positive could be explained by natural age-related changes in the immune system of older adults, also referred to as immune senescence,” the authors noted.
The investigators found no significant differences in reinfection rates between women and men.
As with the previous research, this study also indicates that an initial bout with SARS-CoV-2 infection appears to confer protection for at least 6 months. The researchers found no significant differences between people who were followed for 3-6 months and those followed for 7 months or longer.
Variants not included
To account for possible bias among people who got tested repeatedly, Dr. Michlmayr and colleagues performed a sensitivity analysis in a subgroup. They assessed reinfection rates among people who underwent testing frequently and routinely – nurses, doctors, social workers, and health care assistants – and found that 1.2% tested positive a second time during the second surge.
A limitation of the study was the inability to correlate symptoms with risk for reinfection. Also, the researchers did not account for SARS-CoV-2 variants, noting that “during the study period, such variants were not yet established in Denmark; although into 2021 this pattern is changing.”
Asked to speculate whether the results would be different had the study accounted for variants, Dr. Hirschwerk said, “It depends upon the variant, but certainly for the B.1.351 variant, there already has been data clearly demonstrating risk of reinfection with SARS-CoV-2 despite prior infection with the original strain of virus.”
The emergence of SARS-CoV-2 variants of concern that could escape natural and vaccine-related immunity “complicates matters further,” Rosemary J. Boyton, MBBS, and Daniel M. Altmann, PhD, both of Imperial College London, wrote in an accompanying comment in The Lancet.
“Emerging variants of concern might shift immunity below a protective margin, prompting the need for updated vaccines. Interestingly, vaccine responses even after single dose are substantially enhanced in individuals with a history of infection with SARS-CoV-2,” they added.
The current study confirms that “the hope of protective immunity through natural infections might not be within our reach, and a global vaccination program with high efficacy vaccines is the enduring solution,” Dr. Boyton and Dr. Altmann noted.
Cause for alarm?
Despite evidence that reinfection is relatively rare, “many will find the data reported by Hansen and colleagues about protection through natural infection relatively alarming,” Dr. Boyton and Dr. Altmann wrote in their commentary. The 80% protection rate from reinfection in general and the 47% rate among people aged 65 and older “are more concerning figures than offered by previous studies.”
Vaccines appear to provide better quality, quantity, and durability of protection against repeated infection – measured in terms of neutralizing antibodies and T cells – compared with previous infection with SARS-CoV-2, Dr. Boyton and Dr. Altmann wrote.
More research needed
The duration of natural protection against reinfection remains an unanswered question, the researchers noted, “because too little time has elapsed since the beginning of the pandemic.”
Future prospective and longitudinal cohort studies coupled with molecular surveillance are needed to characterize antibody titers and waning of protection against repeat infections, the authors noted. Furthermore, more answers are needed regarding how some virus variants might contribute to reinfection risk.
No funding for the study has been reported. Dr. Michlmayr, Dr. Hirschwerk, Dr. Wherry, Dr. Boyton, and Dr. Altmann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When researchers analyzed test results of 4 million people in Denmark, they found that less than 1% of those who tested positive experienced reinfection.
Initial infection was associated with about 80% protection overall against getting SARS-CoV-2 again. However, among those older than 65, the protection plummeted to 47%.
“Not everybody is protected against reinfection after a first infection. Older people are at higher risk of catching it again,” co–lead author Daniela Michlmayr, PhD, said in an interview. “Our findings emphasize the importance of policies to protect the elderly and of adhering to infection control measures and restrictions, even if previously infected with COVID-19.”
Verifying the need for vaccination
“The findings also highlight the need to vaccinate people who had COVID-19 before, as natural immunity to infection – especially among the elderly 65 and older – cannot be relied upon,” added Dr. Michlmayr, a researcher in the department of bacteria, parasites, and fungi at the Staten Serums Institut, Copenhagen.
The population-based observational study was published online March 17 in The Lancet.
“The findings make sense, as patients who are immunocompromised or of advanced age may not mount an immune response that is as long-lasting,” David Hirschwerk, MD, said in an interview. “It does underscore the importance of vaccination for people of more advanced age, even if they previously were infected with COVID.
“For those who were infected last spring and have not yet been vaccinated, this helps to support the value of still pursuing the vaccine,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in Manhasset, N.Y.
Evidence on reinfection risk was limited prior to this study. “Little is known about protection against SARS-CoV-2 repeat infections, but two studies in the UK have found that immunity could last at least 5 to 6 months after infection,” the authors noted.
Along with co–lead author Christian Holm Hansen, PhD, Dr. Michlmayr and colleagues found that 2.11% of 525,339 individuals tested positive for SARS-CoV-2 during the first surge in Denmark from March to May 2020. Within this group, 0.65% tested positive during a second surge from September to December.
By the end of 2020, more than 10 million people had undergone free polymerase chain reaction testing by the Danish government or through the national TestDenmark program.
“My overall take is that it is great to have such a big dataset looking at this question,” E. John Wherry, PhD, said in an interview. The findings support “what we’ve seen in previous, smaller studies.”
Natural protection against reinfection of approximately 80% “is not as good as the vaccines, but not bad,” added Dr. Wherry, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia.
Age alters immunity?
“Our finding that older people were more likely than younger people to test positive again if they had already tested positive could be explained by natural age-related changes in the immune system of older adults, also referred to as immune senescence,” the authors noted.
The investigators found no significant differences in reinfection rates between women and men.
As with the previous research, this study also indicates that an initial bout with SARS-CoV-2 infection appears to confer protection for at least 6 months. The researchers found no significant differences between people who were followed for 3-6 months and those followed for 7 months or longer.
Variants not included
To account for possible bias among people who got tested repeatedly, Dr. Michlmayr and colleagues performed a sensitivity analysis in a subgroup. They assessed reinfection rates among people who underwent testing frequently and routinely – nurses, doctors, social workers, and health care assistants – and found that 1.2% tested positive a second time during the second surge.
A limitation of the study was the inability to correlate symptoms with risk for reinfection. Also, the researchers did not account for SARS-CoV-2 variants, noting that “during the study period, such variants were not yet established in Denmark; although into 2021 this pattern is changing.”
Asked to speculate whether the results would be different had the study accounted for variants, Dr. Hirschwerk said, “It depends upon the variant, but certainly for the B.1.351 variant, there already has been data clearly demonstrating risk of reinfection with SARS-CoV-2 despite prior infection with the original strain of virus.”
The emergence of SARS-CoV-2 variants of concern that could escape natural and vaccine-related immunity “complicates matters further,” Rosemary J. Boyton, MBBS, and Daniel M. Altmann, PhD, both of Imperial College London, wrote in an accompanying comment in The Lancet.
“Emerging variants of concern might shift immunity below a protective margin, prompting the need for updated vaccines. Interestingly, vaccine responses even after single dose are substantially enhanced in individuals with a history of infection with SARS-CoV-2,” they added.
The current study confirms that “the hope of protective immunity through natural infections might not be within our reach, and a global vaccination program with high efficacy vaccines is the enduring solution,” Dr. Boyton and Dr. Altmann noted.
Cause for alarm?
Despite evidence that reinfection is relatively rare, “many will find the data reported by Hansen and colleagues about protection through natural infection relatively alarming,” Dr. Boyton and Dr. Altmann wrote in their commentary. The 80% protection rate from reinfection in general and the 47% rate among people aged 65 and older “are more concerning figures than offered by previous studies.”
Vaccines appear to provide better quality, quantity, and durability of protection against repeated infection – measured in terms of neutralizing antibodies and T cells – compared with previous infection with SARS-CoV-2, Dr. Boyton and Dr. Altmann wrote.
More research needed
The duration of natural protection against reinfection remains an unanswered question, the researchers noted, “because too little time has elapsed since the beginning of the pandemic.”
Future prospective and longitudinal cohort studies coupled with molecular surveillance are needed to characterize antibody titers and waning of protection against repeat infections, the authors noted. Furthermore, more answers are needed regarding how some virus variants might contribute to reinfection risk.
No funding for the study has been reported. Dr. Michlmayr, Dr. Hirschwerk, Dr. Wherry, Dr. Boyton, and Dr. Altmann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Colchicine before PCI for acute MI fails to improve major outcomes
In a placebo-controlled randomized trial, a preprocedural dose of colchicine administered immediately before percutaneous coronary intervention (PCI) for an acute ST-segment elevated myocardial infarction (STEMI) did not reduce the no-reflow phenomenon or improve outcomes.
No-reflow, in which insufficient myocardial perfusion is present even though the coronary artery appears patent, was the primary outcome, and the proportion of patients experiencing this event was exactly the same (14.4%) in the colchicine and placebo groups, reported Yaser Jenab, MD, at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
The hypothesis that colchicine would offer benefit in this setting was largely based on the Colchicine Cardiovascular Outcomes Trial (COLCOT). In that study, colchicine was associated with a 23% reduction in risk for major adverse cardiovascular events (MACE) relative to placebo when administered within 30 days after a myocardial infarction (hazard ratio, 0.77; P = .02).
The benefit in that trial was attributed to an anti-inflammatory effect, according to Dr. Jenab, associate professor of cardiology at Tehran (Iran) Heart Center. In particular as it relates to vascular disease, he cited experimental studies associating colchicine with a reduction in neutrophil activation and adherence to vascular endothelium.
The rationale for a preprocedural approach to colchicine was supplied by a subsequent time-to-treatment COLCOT analysis. In this study, MACE risk reduction for colchicine climbed to 48% (HR 0.52) for those treated within 3 days of the MI but largely disappeared (HR 0.96) if treatment was started at least 8 days post MI.
PodCAST-PCI trial
In the preprocedural study, called the PodCAST-PCI trial, 321 acute STEMI patients were randomized. Patients received a 1-mg dose of oral colchicine or placebo at the time PCI was scheduled. Another dose of colchicine (0.5 mg) or placebo was administered 1 hour after the procedure.
Of secondary outcomes, which included MACE at 1 month and 1 year, ST-segment resolution at 1 month, and change in inflammatory markers at 1 month, none were significant. Few even trended for significance.
For MACE, which included cardiac death, stroke, nonfatal MI, new hospitalization due to heart failure, or target vessel revascularization, the rates were lower in the colchicine group at 1 month (4.3% vs. 7.5%) and 1 year (9.3% vs. 11.2%), but neither approached significance.
For ST-segment resolution, the proportions were generally comparable among the colchicine and placebo groups, respectively, for the proportion below 50% (18.6% vs. 23.1%), between 50% and 70% (16.8% vs. 15.6%), and above 70% (64.6% vs. 61.3%).
The average troponin levels were nonsignificantly lower at 6 hours (1,847 vs. 2,883 ng/mL) in the colchicine group but higher at 48 hours (1,197 vs. 1,147 ng/mL). The average C-reactive protein (CRP) levels at 48 hours were nonsignificantly lower on colchicine (176.5 vs. 244.5 mg/L).
There were no significant differences in postprocedural perfusion, as measured with TIMI blood flow, or in the rate of stent thrombosis, which occurred in roughly 3% of each group of patients.
The small sample size was one limitation of this study, Dr. Jenab acknowledged. For this and other reasons, he cautioned that these data are not definitive and do not preclude a benefit on clinical outcomes in a study with a larger size, a different design, or different dosing.
Timing might be the issue
However, even if colchicine has a potential benefit in this setting, timing might be a major obstacle, according to Binata Shah, MD, associate director of research for the Cardiac Catheterization Laboratory at New York University.
“We have learned from our rheumatology colleagues that peak plasma levels of colchicine are not achieved for at least 1 hour after the full loading dose,” Dr. Shah said. “With us moving so quickly in a primary PCI setting, it is hard to imagine that colchicine would have had time to really kick in and exert its anti-inflammatory effect.”
Indeed, the problem might be worse than reaching the peak plasma level.
“Even though peak plasma levels occur as early as 1 hour after a full loading dose, we see that it takes about 24 hours to really see the effects translate downstream into more systemic inflammatory markers such as CRP and interleukin-6,” she added. If lowering these signals of inflammation is predictive of benefit, than this might be the biggest obstacle to benefit from colchicine in an urgent treatment setting.
Dr. Jenab and Dr. Shah reported no potential conflicts of interest.
In a placebo-controlled randomized trial, a preprocedural dose of colchicine administered immediately before percutaneous coronary intervention (PCI) for an acute ST-segment elevated myocardial infarction (STEMI) did not reduce the no-reflow phenomenon or improve outcomes.
No-reflow, in which insufficient myocardial perfusion is present even though the coronary artery appears patent, was the primary outcome, and the proportion of patients experiencing this event was exactly the same (14.4%) in the colchicine and placebo groups, reported Yaser Jenab, MD, at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
The hypothesis that colchicine would offer benefit in this setting was largely based on the Colchicine Cardiovascular Outcomes Trial (COLCOT). In that study, colchicine was associated with a 23% reduction in risk for major adverse cardiovascular events (MACE) relative to placebo when administered within 30 days after a myocardial infarction (hazard ratio, 0.77; P = .02).
The benefit in that trial was attributed to an anti-inflammatory effect, according to Dr. Jenab, associate professor of cardiology at Tehran (Iran) Heart Center. In particular as it relates to vascular disease, he cited experimental studies associating colchicine with a reduction in neutrophil activation and adherence to vascular endothelium.
The rationale for a preprocedural approach to colchicine was supplied by a subsequent time-to-treatment COLCOT analysis. In this study, MACE risk reduction for colchicine climbed to 48% (HR 0.52) for those treated within 3 days of the MI but largely disappeared (HR 0.96) if treatment was started at least 8 days post MI.
PodCAST-PCI trial
In the preprocedural study, called the PodCAST-PCI trial, 321 acute STEMI patients were randomized. Patients received a 1-mg dose of oral colchicine or placebo at the time PCI was scheduled. Another dose of colchicine (0.5 mg) or placebo was administered 1 hour after the procedure.
Of secondary outcomes, which included MACE at 1 month and 1 year, ST-segment resolution at 1 month, and change in inflammatory markers at 1 month, none were significant. Few even trended for significance.
For MACE, which included cardiac death, stroke, nonfatal MI, new hospitalization due to heart failure, or target vessel revascularization, the rates were lower in the colchicine group at 1 month (4.3% vs. 7.5%) and 1 year (9.3% vs. 11.2%), but neither approached significance.
For ST-segment resolution, the proportions were generally comparable among the colchicine and placebo groups, respectively, for the proportion below 50% (18.6% vs. 23.1%), between 50% and 70% (16.8% vs. 15.6%), and above 70% (64.6% vs. 61.3%).
The average troponin levels were nonsignificantly lower at 6 hours (1,847 vs. 2,883 ng/mL) in the colchicine group but higher at 48 hours (1,197 vs. 1,147 ng/mL). The average C-reactive protein (CRP) levels at 48 hours were nonsignificantly lower on colchicine (176.5 vs. 244.5 mg/L).
There were no significant differences in postprocedural perfusion, as measured with TIMI blood flow, or in the rate of stent thrombosis, which occurred in roughly 3% of each group of patients.
The small sample size was one limitation of this study, Dr. Jenab acknowledged. For this and other reasons, he cautioned that these data are not definitive and do not preclude a benefit on clinical outcomes in a study with a larger size, a different design, or different dosing.
Timing might be the issue
However, even if colchicine has a potential benefit in this setting, timing might be a major obstacle, according to Binata Shah, MD, associate director of research for the Cardiac Catheterization Laboratory at New York University.
“We have learned from our rheumatology colleagues that peak plasma levels of colchicine are not achieved for at least 1 hour after the full loading dose,” Dr. Shah said. “With us moving so quickly in a primary PCI setting, it is hard to imagine that colchicine would have had time to really kick in and exert its anti-inflammatory effect.”
Indeed, the problem might be worse than reaching the peak plasma level.
“Even though peak plasma levels occur as early as 1 hour after a full loading dose, we see that it takes about 24 hours to really see the effects translate downstream into more systemic inflammatory markers such as CRP and interleukin-6,” she added. If lowering these signals of inflammation is predictive of benefit, than this might be the biggest obstacle to benefit from colchicine in an urgent treatment setting.
Dr. Jenab and Dr. Shah reported no potential conflicts of interest.
In a placebo-controlled randomized trial, a preprocedural dose of colchicine administered immediately before percutaneous coronary intervention (PCI) for an acute ST-segment elevated myocardial infarction (STEMI) did not reduce the no-reflow phenomenon or improve outcomes.
No-reflow, in which insufficient myocardial perfusion is present even though the coronary artery appears patent, was the primary outcome, and the proportion of patients experiencing this event was exactly the same (14.4%) in the colchicine and placebo groups, reported Yaser Jenab, MD, at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
The hypothesis that colchicine would offer benefit in this setting was largely based on the Colchicine Cardiovascular Outcomes Trial (COLCOT). In that study, colchicine was associated with a 23% reduction in risk for major adverse cardiovascular events (MACE) relative to placebo when administered within 30 days after a myocardial infarction (hazard ratio, 0.77; P = .02).
The benefit in that trial was attributed to an anti-inflammatory effect, according to Dr. Jenab, associate professor of cardiology at Tehran (Iran) Heart Center. In particular as it relates to vascular disease, he cited experimental studies associating colchicine with a reduction in neutrophil activation and adherence to vascular endothelium.
The rationale for a preprocedural approach to colchicine was supplied by a subsequent time-to-treatment COLCOT analysis. In this study, MACE risk reduction for colchicine climbed to 48% (HR 0.52) for those treated within 3 days of the MI but largely disappeared (HR 0.96) if treatment was started at least 8 days post MI.
PodCAST-PCI trial
In the preprocedural study, called the PodCAST-PCI trial, 321 acute STEMI patients were randomized. Patients received a 1-mg dose of oral colchicine or placebo at the time PCI was scheduled. Another dose of colchicine (0.5 mg) or placebo was administered 1 hour after the procedure.
Of secondary outcomes, which included MACE at 1 month and 1 year, ST-segment resolution at 1 month, and change in inflammatory markers at 1 month, none were significant. Few even trended for significance.
For MACE, which included cardiac death, stroke, nonfatal MI, new hospitalization due to heart failure, or target vessel revascularization, the rates were lower in the colchicine group at 1 month (4.3% vs. 7.5%) and 1 year (9.3% vs. 11.2%), but neither approached significance.
For ST-segment resolution, the proportions were generally comparable among the colchicine and placebo groups, respectively, for the proportion below 50% (18.6% vs. 23.1%), between 50% and 70% (16.8% vs. 15.6%), and above 70% (64.6% vs. 61.3%).
The average troponin levels were nonsignificantly lower at 6 hours (1,847 vs. 2,883 ng/mL) in the colchicine group but higher at 48 hours (1,197 vs. 1,147 ng/mL). The average C-reactive protein (CRP) levels at 48 hours were nonsignificantly lower on colchicine (176.5 vs. 244.5 mg/L).
There were no significant differences in postprocedural perfusion, as measured with TIMI blood flow, or in the rate of stent thrombosis, which occurred in roughly 3% of each group of patients.
The small sample size was one limitation of this study, Dr. Jenab acknowledged. For this and other reasons, he cautioned that these data are not definitive and do not preclude a benefit on clinical outcomes in a study with a larger size, a different design, or different dosing.
Timing might be the issue
However, even if colchicine has a potential benefit in this setting, timing might be a major obstacle, according to Binata Shah, MD, associate director of research for the Cardiac Catheterization Laboratory at New York University.
“We have learned from our rheumatology colleagues that peak plasma levels of colchicine are not achieved for at least 1 hour after the full loading dose,” Dr. Shah said. “With us moving so quickly in a primary PCI setting, it is hard to imagine that colchicine would have had time to really kick in and exert its anti-inflammatory effect.”
Indeed, the problem might be worse than reaching the peak plasma level.
“Even though peak plasma levels occur as early as 1 hour after a full loading dose, we see that it takes about 24 hours to really see the effects translate downstream into more systemic inflammatory markers such as CRP and interleukin-6,” she added. If lowering these signals of inflammation is predictive of benefit, than this might be the biggest obstacle to benefit from colchicine in an urgent treatment setting.
Dr. Jenab and Dr. Shah reported no potential conflicts of interest.
FROM CRT 2021
Decline in child COVID-19 cases picks up after 2-week slowdown
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.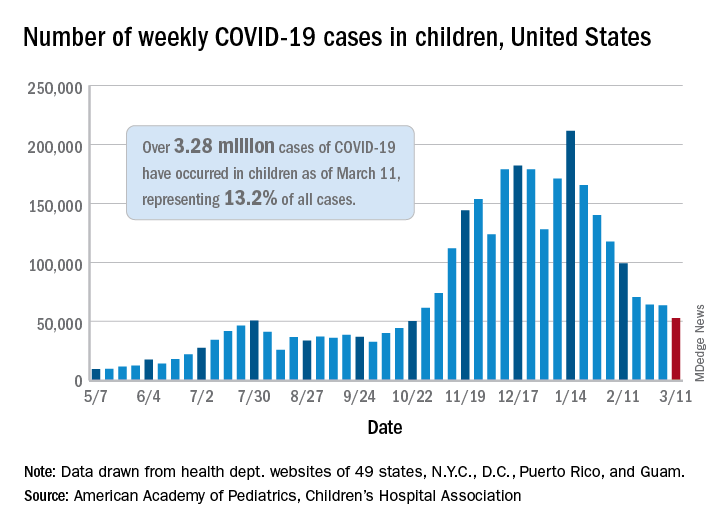
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
‘Major update’ of BP guidance for kidney disease; treat to 120 mm Hg
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
First pill for COVID-19 could be ready by year’s end
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
Don’t discontinue osteoporosis meds for COVID-19 vaccines, expert guidance says
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
Inpatient sodium imbalances linked to adverse COVID-19 outcomes
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Delay surgery by 7 weeks after COVID-19 diagnosis, study shows
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Palliative care and hospital medicine partnerships in the pandemic
Patients dying without their loved ones, families forced to remotely decide goals of care without the physical presence or human connection of the care team, overworked staff physically isolated from their critically ill patients, and at-risk community members with uncertain and undocumented goals for care are among the universal challenges confronted by hospitals and hospitalists during this COVID-19 pandemic. Partnerships among hospital medicine (HM) and palliative care (PC) teams at Dell Medical School/Dell Seton Medical Center thrive on mutually shared core values of patient centered care – compassion, empathy, and humanity.
A key PC-HM collaboration was adapting our multidisciplinary huddle to focus on communication effectiveness and efficiency in the medical intensive care unit (MICU). Expanded interprofessional and cross-specialty collaboration promoted streamlined, succinct, and standardized communication with patients’ families while their loved ones were critically ill with COVID-19. The PC team attended daily MICU multidisciplinary huddles, attentive to both the medical and psychosocial updates for each patient. During huddles, residents or HM providers were asked to end their presentation with a clinical status “headline” and solicited feedback from the multidisciplinary team before messaging to the family. The PC team then communicated with families a succinct and cohesive medical update and continuously explored goals of care. This allowed the HM team, often overwhelmed with admissions, co-managing intensive care patients, and facilitating safe discharges, to focus on urgent issues while PC provided continuity and personalized support for patients and families. PC’s ability to synthesize and summarize clinical information from multiple teams and then provide cohesive updates in patient-friendly language modeled important communication skills for learners and simultaneously benefited HM providers.
Our chaplains, too, were central to facilitating timely, proactive conversations and documentation of Medical Power of Attorney (MPOA) for patients with COVID-19 admitted to our hospital. HM prioritized early admission conversations with patients to counsel them on severity of illness, prognosis based on risk factors, to elucidate wishes for intubation or resuscitation, and to capture their desired medical decision maker. HM was notified of all COVID and PUI admissions, allowing us to speak with even critically ill patients in the ER or ICU prior to intubation in order to quickly and accurately capture patients’ wishes for treatment and delegate decision makers. Our chaplains supported and supplemented these efforts by diligently and dutifully soliciting, hearing, and documenting patient MPOA delegates, with over 50% MPOA completion by 24 hours of hospitalization.
Another early PC-HM project, “Meet My Loved One,” was adapted from the University of Alabama at Birmingham Palliative and Comfort Unit. The absence of families visiting the ICU and sharing pictures, stories, anecdotes of our patients left a deeply felt, dehumanizing void in the halls and rooms of our hospital. To fill this space with life and humanity, furloughed medical students on their “transition of care” electives contacted family members of their “continuity” patients focusing primarily on those patients expected to have prolonged ICU or hospital stays and solicited personal, humanizing information about our patients. Questions included: “What is your loved one’s preferred name or nickname?” and “What are three things we should know to take better care of your loved one?” With family permission, we posted this information on the door outside the patient’s room. Nursing staff, in particular, appreciated getting to know their patients more personally and families appreciated the staff’s desire to know their loved one as an individual.
It is also important to acknowledge setbacks. Early efforts to engage technology proved more foe than friend. We continue to struggle with using our iPads for video visits. Most of our families prefer “WhatsApp” for video communication, which is not compatible with operating systems on early versions of the iPad, which were generously and widely donated by local school systems. Desperate to allow families to connect, many providers resorted to using personal devices to facilitate video visits and family meetings. And we discovered that many video visits caused more not less family angst, especially for critically ill patients. Families often required preparation and coaching on what to expect and how to interact with intubated, sedated, proned, and paralyzed loved ones.
Our hospital medicine and palliative care teams have an established strong partnership. The COVID-19 pandemic created novel communication challenges but our shared mission toward patient-centered care allowed us to effectively collaborate to bring the patients goals of care to the forefront aligning patients, families, physicians, nurses, and staff during the COVID-19 surge.
Dr. Johnston is associate professor at Dell Medical School at The University of Texas in Austin. She practices hospital medicine and inpatient palliative care at Dell Seton Medical Center. Dr. Cooremans is a resident physician at Dell Medical School. Dr. Salib is the internal medicine clerkship director and an associate professor at Dell Medical School. Dr. Nieto is an assistant professor and associate chief of the Division of Hospital Medicine at Dell Medical School. Dr. Patel is an assistant professor at Dell Medical School. This article is part of a series written by members of the Division of Hospital Medicine at Dell Medical School, exploring lessons learned from the coronavirus pandemic and outlining an approach for creating COVID-19 Centers of Excellence. The article first appeared in The Hospital Leader, the official blog of SHM.
Patients dying without their loved ones, families forced to remotely decide goals of care without the physical presence or human connection of the care team, overworked staff physically isolated from their critically ill patients, and at-risk community members with uncertain and undocumented goals for care are among the universal challenges confronted by hospitals and hospitalists during this COVID-19 pandemic. Partnerships among hospital medicine (HM) and palliative care (PC) teams at Dell Medical School/Dell Seton Medical Center thrive on mutually shared core values of patient centered care – compassion, empathy, and humanity.
A key PC-HM collaboration was adapting our multidisciplinary huddle to focus on communication effectiveness and efficiency in the medical intensive care unit (MICU). Expanded interprofessional and cross-specialty collaboration promoted streamlined, succinct, and standardized communication with patients’ families while their loved ones were critically ill with COVID-19. The PC team attended daily MICU multidisciplinary huddles, attentive to both the medical and psychosocial updates for each patient. During huddles, residents or HM providers were asked to end their presentation with a clinical status “headline” and solicited feedback from the multidisciplinary team before messaging to the family. The PC team then communicated with families a succinct and cohesive medical update and continuously explored goals of care. This allowed the HM team, often overwhelmed with admissions, co-managing intensive care patients, and facilitating safe discharges, to focus on urgent issues while PC provided continuity and personalized support for patients and families. PC’s ability to synthesize and summarize clinical information from multiple teams and then provide cohesive updates in patient-friendly language modeled important communication skills for learners and simultaneously benefited HM providers.
Our chaplains, too, were central to facilitating timely, proactive conversations and documentation of Medical Power of Attorney (MPOA) for patients with COVID-19 admitted to our hospital. HM prioritized early admission conversations with patients to counsel them on severity of illness, prognosis based on risk factors, to elucidate wishes for intubation or resuscitation, and to capture their desired medical decision maker. HM was notified of all COVID and PUI admissions, allowing us to speak with even critically ill patients in the ER or ICU prior to intubation in order to quickly and accurately capture patients’ wishes for treatment and delegate decision makers. Our chaplains supported and supplemented these efforts by diligently and dutifully soliciting, hearing, and documenting patient MPOA delegates, with over 50% MPOA completion by 24 hours of hospitalization.
Another early PC-HM project, “Meet My Loved One,” was adapted from the University of Alabama at Birmingham Palliative and Comfort Unit. The absence of families visiting the ICU and sharing pictures, stories, anecdotes of our patients left a deeply felt, dehumanizing void in the halls and rooms of our hospital. To fill this space with life and humanity, furloughed medical students on their “transition of care” electives contacted family members of their “continuity” patients focusing primarily on those patients expected to have prolonged ICU or hospital stays and solicited personal, humanizing information about our patients. Questions included: “What is your loved one’s preferred name or nickname?” and “What are three things we should know to take better care of your loved one?” With family permission, we posted this information on the door outside the patient’s room. Nursing staff, in particular, appreciated getting to know their patients more personally and families appreciated the staff’s desire to know their loved one as an individual.
It is also important to acknowledge setbacks. Early efforts to engage technology proved more foe than friend. We continue to struggle with using our iPads for video visits. Most of our families prefer “WhatsApp” for video communication, which is not compatible with operating systems on early versions of the iPad, which were generously and widely donated by local school systems. Desperate to allow families to connect, many providers resorted to using personal devices to facilitate video visits and family meetings. And we discovered that many video visits caused more not less family angst, especially for critically ill patients. Families often required preparation and coaching on what to expect and how to interact with intubated, sedated, proned, and paralyzed loved ones.
Our hospital medicine and palliative care teams have an established strong partnership. The COVID-19 pandemic created novel communication challenges but our shared mission toward patient-centered care allowed us to effectively collaborate to bring the patients goals of care to the forefront aligning patients, families, physicians, nurses, and staff during the COVID-19 surge.
Dr. Johnston is associate professor at Dell Medical School at The University of Texas in Austin. She practices hospital medicine and inpatient palliative care at Dell Seton Medical Center. Dr. Cooremans is a resident physician at Dell Medical School. Dr. Salib is the internal medicine clerkship director and an associate professor at Dell Medical School. Dr. Nieto is an assistant professor and associate chief of the Division of Hospital Medicine at Dell Medical School. Dr. Patel is an assistant professor at Dell Medical School. This article is part of a series written by members of the Division of Hospital Medicine at Dell Medical School, exploring lessons learned from the coronavirus pandemic and outlining an approach for creating COVID-19 Centers of Excellence. The article first appeared in The Hospital Leader, the official blog of SHM.
Patients dying without their loved ones, families forced to remotely decide goals of care without the physical presence or human connection of the care team, overworked staff physically isolated from their critically ill patients, and at-risk community members with uncertain and undocumented goals for care are among the universal challenges confronted by hospitals and hospitalists during this COVID-19 pandemic. Partnerships among hospital medicine (HM) and palliative care (PC) teams at Dell Medical School/Dell Seton Medical Center thrive on mutually shared core values of patient centered care – compassion, empathy, and humanity.
A key PC-HM collaboration was adapting our multidisciplinary huddle to focus on communication effectiveness and efficiency in the medical intensive care unit (MICU). Expanded interprofessional and cross-specialty collaboration promoted streamlined, succinct, and standardized communication with patients’ families while their loved ones were critically ill with COVID-19. The PC team attended daily MICU multidisciplinary huddles, attentive to both the medical and psychosocial updates for each patient. During huddles, residents or HM providers were asked to end their presentation with a clinical status “headline” and solicited feedback from the multidisciplinary team before messaging to the family. The PC team then communicated with families a succinct and cohesive medical update and continuously explored goals of care. This allowed the HM team, often overwhelmed with admissions, co-managing intensive care patients, and facilitating safe discharges, to focus on urgent issues while PC provided continuity and personalized support for patients and families. PC’s ability to synthesize and summarize clinical information from multiple teams and then provide cohesive updates in patient-friendly language modeled important communication skills for learners and simultaneously benefited HM providers.
Our chaplains, too, were central to facilitating timely, proactive conversations and documentation of Medical Power of Attorney (MPOA) for patients with COVID-19 admitted to our hospital. HM prioritized early admission conversations with patients to counsel them on severity of illness, prognosis based on risk factors, to elucidate wishes for intubation or resuscitation, and to capture their desired medical decision maker. HM was notified of all COVID and PUI admissions, allowing us to speak with even critically ill patients in the ER or ICU prior to intubation in order to quickly and accurately capture patients’ wishes for treatment and delegate decision makers. Our chaplains supported and supplemented these efforts by diligently and dutifully soliciting, hearing, and documenting patient MPOA delegates, with over 50% MPOA completion by 24 hours of hospitalization.
Another early PC-HM project, “Meet My Loved One,” was adapted from the University of Alabama at Birmingham Palliative and Comfort Unit. The absence of families visiting the ICU and sharing pictures, stories, anecdotes of our patients left a deeply felt, dehumanizing void in the halls and rooms of our hospital. To fill this space with life and humanity, furloughed medical students on their “transition of care” electives contacted family members of their “continuity” patients focusing primarily on those patients expected to have prolonged ICU or hospital stays and solicited personal, humanizing information about our patients. Questions included: “What is your loved one’s preferred name or nickname?” and “What are three things we should know to take better care of your loved one?” With family permission, we posted this information on the door outside the patient’s room. Nursing staff, in particular, appreciated getting to know their patients more personally and families appreciated the staff’s desire to know their loved one as an individual.
It is also important to acknowledge setbacks. Early efforts to engage technology proved more foe than friend. We continue to struggle with using our iPads for video visits. Most of our families prefer “WhatsApp” for video communication, which is not compatible with operating systems on early versions of the iPad, which were generously and widely donated by local school systems. Desperate to allow families to connect, many providers resorted to using personal devices to facilitate video visits and family meetings. And we discovered that many video visits caused more not less family angst, especially for critically ill patients. Families often required preparation and coaching on what to expect and how to interact with intubated, sedated, proned, and paralyzed loved ones.
Our hospital medicine and palliative care teams have an established strong partnership. The COVID-19 pandemic created novel communication challenges but our shared mission toward patient-centered care allowed us to effectively collaborate to bring the patients goals of care to the forefront aligning patients, families, physicians, nurses, and staff during the COVID-19 surge.
Dr. Johnston is associate professor at Dell Medical School at The University of Texas in Austin. She practices hospital medicine and inpatient palliative care at Dell Seton Medical Center. Dr. Cooremans is a resident physician at Dell Medical School. Dr. Salib is the internal medicine clerkship director and an associate professor at Dell Medical School. Dr. Nieto is an assistant professor and associate chief of the Division of Hospital Medicine at Dell Medical School. Dr. Patel is an assistant professor at Dell Medical School. This article is part of a series written by members of the Division of Hospital Medicine at Dell Medical School, exploring lessons learned from the coronavirus pandemic and outlining an approach for creating COVID-19 Centers of Excellence. The article first appeared in The Hospital Leader, the official blog of SHM.
CDC data strengthen link between obesity and severe COVID
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.