User login
Opening the door to gene editing?
In early August, in Portland, Ore. The scale and success of such experimentation with human embryos is unprecedented in the United States. Given the highly experimental nature of fertility clinics in the United States and abroad, many suggest that these findings open the door to designer babies. A careful read of the report, however, indicates that the door is still quite closed, perhaps cracked open just a little.
The research team used a new method of cutting the genome, called CRISPR-Cas9. CRISPR utilizes two key components that the team combined in a test tube together: a Cas9 protein that can cut the DNA and a synthetic RNA that can guide the protein to cut a 20-letter sequence in the human genome specifically. In these experiments, the Cas9-RNA protein was designed to cut a pathogenic mutation in the MYBPC3 gene, which can cause hypertrophic cardiomyopathy. The research team could not obtain human zygotes with this mutation on both copies of the genome (a rare homozygous genotype). Such zygotes would have the most severe phenotype and be the most compelling test case for CRISPR. Instead, they focused on gene editing heterozygous human zygotes that have one normal maternal copy of the MYBPC3 gene and one pathogenic paternal copy. The heterozygous zygotes were produced by the research team via in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) using sperm donated by males carrying the pathogenic mutation (Nature. 2017 Aug 2. doi: 10.1038/nature23305).
When researchers injected the Cas9-RNA protein targeting the mutation into already fertilized zygotes, they found that 67% of the resulting embryos had two normal copies of the MYBPC3 gene. Without gene editing, approximately 50% of the embryos would have two normal copies, because the male sperm donor would produce equal numbers of sperm with normal and pathogenic genotypes. Thus, editing likely corrected only about 17% of the embryos that would have otherwise had one pathogenic paternal mutation. Thirty-six percent of embryos had additional mutations from imprecise gene editing. Further, some of the gene edits and additional mutations were mosaic, meaning that the resulting embryo harbored many different genotypes.
To overcome these challenges, the research team precisely controlled the timing of CRISPR injection to coincide with fertilization. With controlled timing, gene editing was restricted to only the paternal pathogenic mutation, resulting in 72% of all injected embryos having two normal copies of the gene in all cells without any mosaicism. Whole genome sequencing revealed no additional mutations above the detection limit of the assay. Finally, preimplantation development proceeded normally to the blastocyst stage, suggesting that the edited embryos have no functional deficits from the procedure.
A surprising finding was that new sequences could not be put into the embryo. The research team had coinjected a synthetic DNA template that differed from the normal maternal copy, but never saw this sequence incorporated into any embryo. Instead, the zygote utilized the maternal copy of the gene with the normal sequence as a template for repairing the DNA cut in the paternal copy produced by CRISPR. The biology behind this repair process is poorly understood and has not been previously reported with other human cell types. These observations suggest that we cannot easily “write” our genome. Instead, our vocabulary is limited to what is already within either the maternal or paternal copy of the genome. In other words, designer babies are not around the corner. While preimplantation genetic diagnosis (PGD) is still currently the safest way to avoid passing on autosomal dominant mutations, these new findings could enable correction of such mutations within IVF embryos, resulting in a larger pool of embryos for IVF clinics to work with.
Apart from these technical challenges, the National Academies has not given a green light to implant edited human embryos. Instead, the organization calls for several requirements to be met, including “broad societal consensus” on the need for this type of intervention. While it is not clear whether or how consensus could be achieved, it is clear that scientists, clinicians, and patients will need help from the rest of society for this research to have an impact clinically.
Dr. Saha is assistant professor of biomedical engineering at the Wisconsin Institute for Discovery at the University of Wisconsin, Madison. His lab works on gene editing of human cells. He has patent filings through the Wisconsin Alumni Research Foundation on gene editing inventions.
In early August, in Portland, Ore. The scale and success of such experimentation with human embryos is unprecedented in the United States. Given the highly experimental nature of fertility clinics in the United States and abroad, many suggest that these findings open the door to designer babies. A careful read of the report, however, indicates that the door is still quite closed, perhaps cracked open just a little.
The research team used a new method of cutting the genome, called CRISPR-Cas9. CRISPR utilizes two key components that the team combined in a test tube together: a Cas9 protein that can cut the DNA and a synthetic RNA that can guide the protein to cut a 20-letter sequence in the human genome specifically. In these experiments, the Cas9-RNA protein was designed to cut a pathogenic mutation in the MYBPC3 gene, which can cause hypertrophic cardiomyopathy. The research team could not obtain human zygotes with this mutation on both copies of the genome (a rare homozygous genotype). Such zygotes would have the most severe phenotype and be the most compelling test case for CRISPR. Instead, they focused on gene editing heterozygous human zygotes that have one normal maternal copy of the MYBPC3 gene and one pathogenic paternal copy. The heterozygous zygotes were produced by the research team via in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) using sperm donated by males carrying the pathogenic mutation (Nature. 2017 Aug 2. doi: 10.1038/nature23305).
When researchers injected the Cas9-RNA protein targeting the mutation into already fertilized zygotes, they found that 67% of the resulting embryos had two normal copies of the MYBPC3 gene. Without gene editing, approximately 50% of the embryos would have two normal copies, because the male sperm donor would produce equal numbers of sperm with normal and pathogenic genotypes. Thus, editing likely corrected only about 17% of the embryos that would have otherwise had one pathogenic paternal mutation. Thirty-six percent of embryos had additional mutations from imprecise gene editing. Further, some of the gene edits and additional mutations were mosaic, meaning that the resulting embryo harbored many different genotypes.
To overcome these challenges, the research team precisely controlled the timing of CRISPR injection to coincide with fertilization. With controlled timing, gene editing was restricted to only the paternal pathogenic mutation, resulting in 72% of all injected embryos having two normal copies of the gene in all cells without any mosaicism. Whole genome sequencing revealed no additional mutations above the detection limit of the assay. Finally, preimplantation development proceeded normally to the blastocyst stage, suggesting that the edited embryos have no functional deficits from the procedure.
A surprising finding was that new sequences could not be put into the embryo. The research team had coinjected a synthetic DNA template that differed from the normal maternal copy, but never saw this sequence incorporated into any embryo. Instead, the zygote utilized the maternal copy of the gene with the normal sequence as a template for repairing the DNA cut in the paternal copy produced by CRISPR. The biology behind this repair process is poorly understood and has not been previously reported with other human cell types. These observations suggest that we cannot easily “write” our genome. Instead, our vocabulary is limited to what is already within either the maternal or paternal copy of the genome. In other words, designer babies are not around the corner. While preimplantation genetic diagnosis (PGD) is still currently the safest way to avoid passing on autosomal dominant mutations, these new findings could enable correction of such mutations within IVF embryos, resulting in a larger pool of embryos for IVF clinics to work with.
Apart from these technical challenges, the National Academies has not given a green light to implant edited human embryos. Instead, the organization calls for several requirements to be met, including “broad societal consensus” on the need for this type of intervention. While it is not clear whether or how consensus could be achieved, it is clear that scientists, clinicians, and patients will need help from the rest of society for this research to have an impact clinically.
Dr. Saha is assistant professor of biomedical engineering at the Wisconsin Institute for Discovery at the University of Wisconsin, Madison. His lab works on gene editing of human cells. He has patent filings through the Wisconsin Alumni Research Foundation on gene editing inventions.
In early August, in Portland, Ore. The scale and success of such experimentation with human embryos is unprecedented in the United States. Given the highly experimental nature of fertility clinics in the United States and abroad, many suggest that these findings open the door to designer babies. A careful read of the report, however, indicates that the door is still quite closed, perhaps cracked open just a little.
The research team used a new method of cutting the genome, called CRISPR-Cas9. CRISPR utilizes two key components that the team combined in a test tube together: a Cas9 protein that can cut the DNA and a synthetic RNA that can guide the protein to cut a 20-letter sequence in the human genome specifically. In these experiments, the Cas9-RNA protein was designed to cut a pathogenic mutation in the MYBPC3 gene, which can cause hypertrophic cardiomyopathy. The research team could not obtain human zygotes with this mutation on both copies of the genome (a rare homozygous genotype). Such zygotes would have the most severe phenotype and be the most compelling test case for CRISPR. Instead, they focused on gene editing heterozygous human zygotes that have one normal maternal copy of the MYBPC3 gene and one pathogenic paternal copy. The heterozygous zygotes were produced by the research team via in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) using sperm donated by males carrying the pathogenic mutation (Nature. 2017 Aug 2. doi: 10.1038/nature23305).
When researchers injected the Cas9-RNA protein targeting the mutation into already fertilized zygotes, they found that 67% of the resulting embryos had two normal copies of the MYBPC3 gene. Without gene editing, approximately 50% of the embryos would have two normal copies, because the male sperm donor would produce equal numbers of sperm with normal and pathogenic genotypes. Thus, editing likely corrected only about 17% of the embryos that would have otherwise had one pathogenic paternal mutation. Thirty-six percent of embryos had additional mutations from imprecise gene editing. Further, some of the gene edits and additional mutations were mosaic, meaning that the resulting embryo harbored many different genotypes.
To overcome these challenges, the research team precisely controlled the timing of CRISPR injection to coincide with fertilization. With controlled timing, gene editing was restricted to only the paternal pathogenic mutation, resulting in 72% of all injected embryos having two normal copies of the gene in all cells without any mosaicism. Whole genome sequencing revealed no additional mutations above the detection limit of the assay. Finally, preimplantation development proceeded normally to the blastocyst stage, suggesting that the edited embryos have no functional deficits from the procedure.
A surprising finding was that new sequences could not be put into the embryo. The research team had coinjected a synthetic DNA template that differed from the normal maternal copy, but never saw this sequence incorporated into any embryo. Instead, the zygote utilized the maternal copy of the gene with the normal sequence as a template for repairing the DNA cut in the paternal copy produced by CRISPR. The biology behind this repair process is poorly understood and has not been previously reported with other human cell types. These observations suggest that we cannot easily “write” our genome. Instead, our vocabulary is limited to what is already within either the maternal or paternal copy of the genome. In other words, designer babies are not around the corner. While preimplantation genetic diagnosis (PGD) is still currently the safest way to avoid passing on autosomal dominant mutations, these new findings could enable correction of such mutations within IVF embryos, resulting in a larger pool of embryos for IVF clinics to work with.
Apart from these technical challenges, the National Academies has not given a green light to implant edited human embryos. Instead, the organization calls for several requirements to be met, including “broad societal consensus” on the need for this type of intervention. While it is not clear whether or how consensus could be achieved, it is clear that scientists, clinicians, and patients will need help from the rest of society for this research to have an impact clinically.
Dr. Saha is assistant professor of biomedical engineering at the Wisconsin Institute for Discovery at the University of Wisconsin, Madison. His lab works on gene editing of human cells. He has patent filings through the Wisconsin Alumni Research Foundation on gene editing inventions.
Psoriasis, psoriatic arthritis research makes headway at GRAPPA meeting
The agenda for this year’s Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) annual meeting included sessions on juvenile psoriatic arthritis (PsA), the microbiome in psoriasis and PsA, and setting up longitudinal cohort studies. There was also a workshop to define the clinical fit and feasibility of PsA outcome measures for clinical trials and updates on several GRAPPA-associated projects.
The meeting, held July 13-15 in Amsterdam, opened with the annual trainee session. This year’s trainee session attracted more than 40 abstracts, of which 6 were selected for oral presentations on a variety of topics including outcome measure assessment, imaging, the microbiome in PsA, and T-cell subsets in synovial fluid.
The jPsA session also emphasized the need for efforts to better define this disease entity and to study outcomes related to it. The current International League of Associations for Rheumatology criteria for juvenile idiopathic arthritis split jPsA into other subgroups (for example, enthesitis-associated arthritis, undifferentiated arthritis, etc).
Three investigators – Matt Stoll, MD, PhD, Devy Zisman, MD, and Elizabeth Mellins, MD – also presented studies of the epidemiology of jPsA.
Among the most intriguing sessions at the GRAPPA meeting was a series of three talks by Jose Scher, MD, Hok Bing Thio, MD, PhD, and Dirk Elewaut, PhD, that introduced the audience to the complexity of the micro-organisms that call the human host “home” and the potential role in the development of inflammatory conditions, in particular psoriasis and PsA. These talks touched on the potential uses of the microbiome of the gut, skin, and oral mucosa in predicting therapy response and modulating the immune system.
Dafna Gladman, MD, led a session on “how to set up a cohort.” In this session, speakers provided a road map for setting up a longitudinal cohort and discussed opportunities and challenges along the way. This session provided a foundation for a meeting that followed the annual meeting, the GRAPPA Research Collaborative Network meeting (held July 15-16). The RCN meeting aimed to develop a plan for a GRAPPA research network supporting collaborative research to identify biomarkers and outcomes in psoriasis and PsA. Speakers/panelists from academics and industry kicked off the meeting with a discussion of prior experiences in establishing international cohort studies. Subsequent sessions presented individual aspects of beginning a longitudinal cohort study for biomarkers, including methods for sample collection, regulatory processes, and data collected at potential sites; capturing and harmonizing clinical data for such studies; and policies for publication of RCN studies.
This year’s workshop was focused on the GRAPPA-Outcome Measures in Rheumatology (OMERACT) working group’s plan to develop a Core Outcome Measure Set, a collection of outcome measurement instruments to be used in randomized, controlled trials (RCTs) for PsA. During the plenary session, the team presented a process for the group to evaluate instruments, including assessment of match to the domain or concept of interest, feasibility, construct validity, and discrimination (including reliability and the ability to distinguish between two groups such as responders and nonresponders). Breakout groups then discussed one of the six tools being considered and voted on match to the domain of interest and feasibility for RCTs.
In a skin session, ongoing efforts to standardize and simplify the measurement of skin psoriasis in both the clinic and RCTs were presented. While the Psoriasis Area and Severity Index (PASI) is the current standard for measuring psoriasis severity and response to therapy in RCTs, the PASI is challenging for use in clinical practice. Joseph Merola, MD, presented data to support the use of the psoriasis Physician Global Assessment (PGA) x body surface area (BSA), a simpler measure than the PASI, as a potential substitute for the PASI. Updates from the International Dermatology Outcome Measures board included reporting of the psoriasis core domain set and a summary of the PsA symptoms working group’s efforts to identify patient-reported outcomes to identify and measure PsA among patients enrolled in psoriasis RCTs. April Armstrong, MD, discussed the National Psoriasis Foundation’s efforts to develop treat-to-target goals for psoriasis. Finally, Laura Coates, MBChB, PhD, presented a report on her efforts to examine a variety of psoriasis cut points for minimal disease activity and very low disease activity outcomes.
Dr. Ogdie is director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia, and is a member of the GRAPPA Steering Committee.
The agenda for this year’s Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) annual meeting included sessions on juvenile psoriatic arthritis (PsA), the microbiome in psoriasis and PsA, and setting up longitudinal cohort studies. There was also a workshop to define the clinical fit and feasibility of PsA outcome measures for clinical trials and updates on several GRAPPA-associated projects.
The meeting, held July 13-15 in Amsterdam, opened with the annual trainee session. This year’s trainee session attracted more than 40 abstracts, of which 6 were selected for oral presentations on a variety of topics including outcome measure assessment, imaging, the microbiome in PsA, and T-cell subsets in synovial fluid.
The jPsA session also emphasized the need for efforts to better define this disease entity and to study outcomes related to it. The current International League of Associations for Rheumatology criteria for juvenile idiopathic arthritis split jPsA into other subgroups (for example, enthesitis-associated arthritis, undifferentiated arthritis, etc).
Three investigators – Matt Stoll, MD, PhD, Devy Zisman, MD, and Elizabeth Mellins, MD – also presented studies of the epidemiology of jPsA.
Among the most intriguing sessions at the GRAPPA meeting was a series of three talks by Jose Scher, MD, Hok Bing Thio, MD, PhD, and Dirk Elewaut, PhD, that introduced the audience to the complexity of the micro-organisms that call the human host “home” and the potential role in the development of inflammatory conditions, in particular psoriasis and PsA. These talks touched on the potential uses of the microbiome of the gut, skin, and oral mucosa in predicting therapy response and modulating the immune system.
Dafna Gladman, MD, led a session on “how to set up a cohort.” In this session, speakers provided a road map for setting up a longitudinal cohort and discussed opportunities and challenges along the way. This session provided a foundation for a meeting that followed the annual meeting, the GRAPPA Research Collaborative Network meeting (held July 15-16). The RCN meeting aimed to develop a plan for a GRAPPA research network supporting collaborative research to identify biomarkers and outcomes in psoriasis and PsA. Speakers/panelists from academics and industry kicked off the meeting with a discussion of prior experiences in establishing international cohort studies. Subsequent sessions presented individual aspects of beginning a longitudinal cohort study for biomarkers, including methods for sample collection, regulatory processes, and data collected at potential sites; capturing and harmonizing clinical data for such studies; and policies for publication of RCN studies.
This year’s workshop was focused on the GRAPPA-Outcome Measures in Rheumatology (OMERACT) working group’s plan to develop a Core Outcome Measure Set, a collection of outcome measurement instruments to be used in randomized, controlled trials (RCTs) for PsA. During the plenary session, the team presented a process for the group to evaluate instruments, including assessment of match to the domain or concept of interest, feasibility, construct validity, and discrimination (including reliability and the ability to distinguish between two groups such as responders and nonresponders). Breakout groups then discussed one of the six tools being considered and voted on match to the domain of interest and feasibility for RCTs.
In a skin session, ongoing efforts to standardize and simplify the measurement of skin psoriasis in both the clinic and RCTs were presented. While the Psoriasis Area and Severity Index (PASI) is the current standard for measuring psoriasis severity and response to therapy in RCTs, the PASI is challenging for use in clinical practice. Joseph Merola, MD, presented data to support the use of the psoriasis Physician Global Assessment (PGA) x body surface area (BSA), a simpler measure than the PASI, as a potential substitute for the PASI. Updates from the International Dermatology Outcome Measures board included reporting of the psoriasis core domain set and a summary of the PsA symptoms working group’s efforts to identify patient-reported outcomes to identify and measure PsA among patients enrolled in psoriasis RCTs. April Armstrong, MD, discussed the National Psoriasis Foundation’s efforts to develop treat-to-target goals for psoriasis. Finally, Laura Coates, MBChB, PhD, presented a report on her efforts to examine a variety of psoriasis cut points for minimal disease activity and very low disease activity outcomes.
Dr. Ogdie is director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia, and is a member of the GRAPPA Steering Committee.
The agenda for this year’s Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) annual meeting included sessions on juvenile psoriatic arthritis (PsA), the microbiome in psoriasis and PsA, and setting up longitudinal cohort studies. There was also a workshop to define the clinical fit and feasibility of PsA outcome measures for clinical trials and updates on several GRAPPA-associated projects.
The meeting, held July 13-15 in Amsterdam, opened with the annual trainee session. This year’s trainee session attracted more than 40 abstracts, of which 6 were selected for oral presentations on a variety of topics including outcome measure assessment, imaging, the microbiome in PsA, and T-cell subsets in synovial fluid.
The jPsA session also emphasized the need for efforts to better define this disease entity and to study outcomes related to it. The current International League of Associations for Rheumatology criteria for juvenile idiopathic arthritis split jPsA into other subgroups (for example, enthesitis-associated arthritis, undifferentiated arthritis, etc).
Three investigators – Matt Stoll, MD, PhD, Devy Zisman, MD, and Elizabeth Mellins, MD – also presented studies of the epidemiology of jPsA.
Among the most intriguing sessions at the GRAPPA meeting was a series of three talks by Jose Scher, MD, Hok Bing Thio, MD, PhD, and Dirk Elewaut, PhD, that introduced the audience to the complexity of the micro-organisms that call the human host “home” and the potential role in the development of inflammatory conditions, in particular psoriasis and PsA. These talks touched on the potential uses of the microbiome of the gut, skin, and oral mucosa in predicting therapy response and modulating the immune system.
Dafna Gladman, MD, led a session on “how to set up a cohort.” In this session, speakers provided a road map for setting up a longitudinal cohort and discussed opportunities and challenges along the way. This session provided a foundation for a meeting that followed the annual meeting, the GRAPPA Research Collaborative Network meeting (held July 15-16). The RCN meeting aimed to develop a plan for a GRAPPA research network supporting collaborative research to identify biomarkers and outcomes in psoriasis and PsA. Speakers/panelists from academics and industry kicked off the meeting with a discussion of prior experiences in establishing international cohort studies. Subsequent sessions presented individual aspects of beginning a longitudinal cohort study for biomarkers, including methods for sample collection, regulatory processes, and data collected at potential sites; capturing and harmonizing clinical data for such studies; and policies for publication of RCN studies.
This year’s workshop was focused on the GRAPPA-Outcome Measures in Rheumatology (OMERACT) working group’s plan to develop a Core Outcome Measure Set, a collection of outcome measurement instruments to be used in randomized, controlled trials (RCTs) for PsA. During the plenary session, the team presented a process for the group to evaluate instruments, including assessment of match to the domain or concept of interest, feasibility, construct validity, and discrimination (including reliability and the ability to distinguish between two groups such as responders and nonresponders). Breakout groups then discussed one of the six tools being considered and voted on match to the domain of interest and feasibility for RCTs.
In a skin session, ongoing efforts to standardize and simplify the measurement of skin psoriasis in both the clinic and RCTs were presented. While the Psoriasis Area and Severity Index (PASI) is the current standard for measuring psoriasis severity and response to therapy in RCTs, the PASI is challenging for use in clinical practice. Joseph Merola, MD, presented data to support the use of the psoriasis Physician Global Assessment (PGA) x body surface area (BSA), a simpler measure than the PASI, as a potential substitute for the PASI. Updates from the International Dermatology Outcome Measures board included reporting of the psoriasis core domain set and a summary of the PsA symptoms working group’s efforts to identify patient-reported outcomes to identify and measure PsA among patients enrolled in psoriasis RCTs. April Armstrong, MD, discussed the National Psoriasis Foundation’s efforts to develop treat-to-target goals for psoriasis. Finally, Laura Coates, MBChB, PhD, presented a report on her efforts to examine a variety of psoriasis cut points for minimal disease activity and very low disease activity outcomes.
Dr. Ogdie is director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia, and is a member of the GRAPPA Steering Committee.
Affordable Care: Back to the Future?
In the days before this issue of Emergency Medicine (EM) went to press, the United States Senate tried unsuccessfully, first to repeal and replace the Affordable Care Act (ACA), then to repeal key provisions of ACA without a replacement bill. Despite having a majority in both the Senate and House of Representatives as well as a Republican President, after 7 years of vowing to repeal “Obama Care,” Republicans have still not been able to fulfill that vow.
When ACA was signed into law in March 2010 (See “Springing Forward,” April 2010 EM), we wrote “though the new law will undoubtedly be challenged, tested, modified, refined, used—and probably abused—it will not be repealed. As was the case with Medicare and Medicaid previously, this will change everything in subtle and not-so-subtle ways.” (For a discussion of how the healthcare industry has managed to co-opt and abuse ACA, see the recently published book An American Sickness by Elisabeth Rosenthal, who was an emergency physician [EP] in our department before becoming a senior science and healthcare reporter for the New York Times.)
But the failure of ACA to deliver on many of its promises, its uncertain financial future, and the lack of improvements to ACA since 2010, directly or indirectly affects every American. Predictably, for those in need of care who cannot find a physician to accept their insurance or schedule a timely appointment, the ED remains the safety net for obtaining care.
After the constitutionality of ACA was upheld by the Supreme Court in June 2012 (See “Our National Pastime,” July 2012 EM), we noted that “ACA contains no provisions for increasing the number of healthcare providers [and] if 24 million more Americans now have access to affordable health insurance, but there are no new providers, who will they go to for care?” Seven years after passage of ACA, the answer to this question has been provided by published studies confirming that even more insured Americans are now seeking care in EDs than before “affordable care” became available. At the same time, urgent care centers, freestanding EDs, and “convenient care” centers, have sprung up and proliferated throughout the country, while in many states, nurse practitioners, physician assistants, and now emergency medical technicians and paramedics have sought and received authorization to evaluate and treat patients independent of physician supervision and oversight. Telemedicine or “telehealth” is the latest attempt to stretch the available supply of physicians to manage patients remotely, in the hope of obviating the need for an ED visit.
But none of these measures completely addresses a basic weakness of ACA: There are not enough physicians, including EPs, in this country to care for everyone entitled to healthcare; at the same time, there is a generation of highly qualified, highly motivated young men and women seeking entrance to medical school who will never get the opportunity to become fine physicians because there are not enough places for them. The solution to these problems seems obvious and the funds needed to finance it would be well spent, though the benefits of increasing the number of medical school places would not be realized for 4 to 8 years after they are made available.
In the meantime, we leave you with the solution President George W. Bush offered to a Cleveland audience on July 10, 2007 (See “Dream On,” March 2008 EM): “people have access to healthcare in America. After all, you just go to an emergency room.”
In the days before this issue of Emergency Medicine (EM) went to press, the United States Senate tried unsuccessfully, first to repeal and replace the Affordable Care Act (ACA), then to repeal key provisions of ACA without a replacement bill. Despite having a majority in both the Senate and House of Representatives as well as a Republican President, after 7 years of vowing to repeal “Obama Care,” Republicans have still not been able to fulfill that vow.
When ACA was signed into law in March 2010 (See “Springing Forward,” April 2010 EM), we wrote “though the new law will undoubtedly be challenged, tested, modified, refined, used—and probably abused—it will not be repealed. As was the case with Medicare and Medicaid previously, this will change everything in subtle and not-so-subtle ways.” (For a discussion of how the healthcare industry has managed to co-opt and abuse ACA, see the recently published book An American Sickness by Elisabeth Rosenthal, who was an emergency physician [EP] in our department before becoming a senior science and healthcare reporter for the New York Times.)
But the failure of ACA to deliver on many of its promises, its uncertain financial future, and the lack of improvements to ACA since 2010, directly or indirectly affects every American. Predictably, for those in need of care who cannot find a physician to accept their insurance or schedule a timely appointment, the ED remains the safety net for obtaining care.
After the constitutionality of ACA was upheld by the Supreme Court in June 2012 (See “Our National Pastime,” July 2012 EM), we noted that “ACA contains no provisions for increasing the number of healthcare providers [and] if 24 million more Americans now have access to affordable health insurance, but there are no new providers, who will they go to for care?” Seven years after passage of ACA, the answer to this question has been provided by published studies confirming that even more insured Americans are now seeking care in EDs than before “affordable care” became available. At the same time, urgent care centers, freestanding EDs, and “convenient care” centers, have sprung up and proliferated throughout the country, while in many states, nurse practitioners, physician assistants, and now emergency medical technicians and paramedics have sought and received authorization to evaluate and treat patients independent of physician supervision and oversight. Telemedicine or “telehealth” is the latest attempt to stretch the available supply of physicians to manage patients remotely, in the hope of obviating the need for an ED visit.
But none of these measures completely addresses a basic weakness of ACA: There are not enough physicians, including EPs, in this country to care for everyone entitled to healthcare; at the same time, there is a generation of highly qualified, highly motivated young men and women seeking entrance to medical school who will never get the opportunity to become fine physicians because there are not enough places for them. The solution to these problems seems obvious and the funds needed to finance it would be well spent, though the benefits of increasing the number of medical school places would not be realized for 4 to 8 years after they are made available.
In the meantime, we leave you with the solution President George W. Bush offered to a Cleveland audience on July 10, 2007 (See “Dream On,” March 2008 EM): “people have access to healthcare in America. After all, you just go to an emergency room.”
In the days before this issue of Emergency Medicine (EM) went to press, the United States Senate tried unsuccessfully, first to repeal and replace the Affordable Care Act (ACA), then to repeal key provisions of ACA without a replacement bill. Despite having a majority in both the Senate and House of Representatives as well as a Republican President, after 7 years of vowing to repeal “Obama Care,” Republicans have still not been able to fulfill that vow.
When ACA was signed into law in March 2010 (See “Springing Forward,” April 2010 EM), we wrote “though the new law will undoubtedly be challenged, tested, modified, refined, used—and probably abused—it will not be repealed. As was the case with Medicare and Medicaid previously, this will change everything in subtle and not-so-subtle ways.” (For a discussion of how the healthcare industry has managed to co-opt and abuse ACA, see the recently published book An American Sickness by Elisabeth Rosenthal, who was an emergency physician [EP] in our department before becoming a senior science and healthcare reporter for the New York Times.)
But the failure of ACA to deliver on many of its promises, its uncertain financial future, and the lack of improvements to ACA since 2010, directly or indirectly affects every American. Predictably, for those in need of care who cannot find a physician to accept their insurance or schedule a timely appointment, the ED remains the safety net for obtaining care.
After the constitutionality of ACA was upheld by the Supreme Court in June 2012 (See “Our National Pastime,” July 2012 EM), we noted that “ACA contains no provisions for increasing the number of healthcare providers [and] if 24 million more Americans now have access to affordable health insurance, but there are no new providers, who will they go to for care?” Seven years after passage of ACA, the answer to this question has been provided by published studies confirming that even more insured Americans are now seeking care in EDs than before “affordable care” became available. At the same time, urgent care centers, freestanding EDs, and “convenient care” centers, have sprung up and proliferated throughout the country, while in many states, nurse practitioners, physician assistants, and now emergency medical technicians and paramedics have sought and received authorization to evaluate and treat patients independent of physician supervision and oversight. Telemedicine or “telehealth” is the latest attempt to stretch the available supply of physicians to manage patients remotely, in the hope of obviating the need for an ED visit.
But none of these measures completely addresses a basic weakness of ACA: There are not enough physicians, including EPs, in this country to care for everyone entitled to healthcare; at the same time, there is a generation of highly qualified, highly motivated young men and women seeking entrance to medical school who will never get the opportunity to become fine physicians because there are not enough places for them. The solution to these problems seems obvious and the funds needed to finance it would be well spent, though the benefits of increasing the number of medical school places would not be realized for 4 to 8 years after they are made available.
In the meantime, we leave you with the solution President George W. Bush offered to a Cleveland audience on July 10, 2007 (See “Dream On,” March 2008 EM): “people have access to healthcare in America. After all, you just go to an emergency room.”
Federal medical tort reform: Has its time come?
Question: Congressional proposals on medical tort reform can be expected to include the following, except:
A. A no-fault system akin to automobile no-fault insurance.
B. A cap on noneconomic damages.
C. “Safe-harbor” immunity against medical negligence.
D. Health courts in place of the judge/jury system to adjudicate claims.
E. Promotion of laws that encourage apologies and error disclosures.
Answer: A. Under the current Republican administration, one can expect legislative efforts at federal tort reform, especially given that Thomas E. Price, MD, the new secretary of the Department of Health & Human Services, is an orthopedic surgeon who has spoken passionately about defensive medicine, damage caps, health tribunals, and practice guidelines. As a former House representative for Georgia, Dr. Price has introduced several tort reform bills, so it is likely that any omnibus federal law will incorporate some of his proposals.1
Over the decades, many states have gone ahead in enacting their own statutes while awaiting federal action. Iowa is the latest example. It recently passed legislation that included a noneconomic damages cap of $250,000, stronger expert witness standards, a certificate of merit in all medical liability lawsuits, and an expansion of its “candor” protections.2 Additional reforms in other states include pretrial screening panels; arbitration; structured periodic payments in lieu of lump sum payments; penalties for frivolous suits; shortened statutes of limitations; making the loser bear all litigation costs; abolishing the collateral source rule, as well as joint and several liability; and limiting attorney contingency fees.
The best-known reform is a cap on noneconomic losses, such as pain and suffering, that doesn’t abridge compensation for economic losses, i.e., medical expenses and lost wages. This provides some predictability because noneconomic damages are difficult to quantify, and jury sympathy may result in unrealistically high payments.
Interestingly, Dr. Price himself has not pushed for a federal cap on noneconomic damages, but other Republican bills have proposed a cap of $250,000. Many states, such as California, Kansas, and Texas, have seen their cap statutes withstand constitutional challenge. However, other jurisdictions, notably Georgia, Illinois, and Missouri, have ruled them unconstitutional.
California’s law, popularly known as MICRA (Medical Injury Compensation Reform Act), came under renewed attack in 2015 with a wrongful death suit from hemorrhagic complications related to Coumadin (warfarin) use following heart surgery.3 The plaintiff’s constitutional challenges included violation of equal protection, due process, and the right to a jury trial, but these were essentially all grounded on an entitlement to recover additional noneconomic damages sufficient to cover attorney fees. The trial court had reduced her $1 million noneconomic damages to $250,000, as required under MICRA. A California court of appeal rejected her claim as being “contrary to many well-established legal principles.”
On the other hand, Florida’s Supreme Court recently held in a closely divided decision of 4-3 that the state’s caps were unconstitutional.4 The law limited noneconomic damages in malpractice cases to either $500,000 or $1 million if the injuries were catastrophic. The court ruled that the caps were arbitrary and unfairly hurt the most severely injured. It was unconvinced that they would reduce malpractice insurance rates; at any rate, there was no present crisis to justify the caps. The decision came 3 years after the court had struck down caps in a case of wrongful death.5
Three relative newcomers to the legal landscape – health courts, apology laws, and safe harbors – appear to be taking center stage in any forthcoming federal reform measures.
Health courts
Under this proposal, so-called health panels and tribunals would now adjudicate malpractice claims. Such health courts would dispense with the jury; further, regular judges would be replaced with specialized judges who would make binding determinations. In one version, a panel of medical experts would initially screen the complaint, followed by an administrative health care tribunal that would feature judges with medical expertise. These tribunals would issue binding rulings, but either party could appeal to a state court for a reversal.
In countries such as Scandinavia and New Zealand, these administrative compensation approaches are coupled to a no-fault system and appear to work well. However, unlike auto no-fault and workers’ compensation, the notion of medical no-fault has never caught on in the United States.
As currently construed, health courts evince dramatic departures from traditional rules of civil procedure. For one, the panels may render decisions before discovery has occurred, which would substantially limit a patient’s ability to learn the facts of what had happened to cause the injury. The panel may rely on a standard of “gross negligence” instead of “ordinary negligence,” requiring evidence not merely of substandard care but of recklessness. This would be a heavier burden on the victim, and could be expected to generate stiff opposition from the plaintiff’s bar. In addition, evidentiary rules may be modified, requiring that an appeal show with clear and convincing proof that the tribunal had erred.
Apology law
Disclosure of medical errors to the injured patient is believed to serve as an ethical and effective way of thwarting potential malpractice claims. Many states have enacted so-called apology laws that disallow statements of sympathy from being admitted into evidence. In some cases, these laws may assist the physician.
For example, the Ohio Supreme Court ruled that a surgeon’s comments and alleged admission of guilt (“I take full responsibility for this” regarding accidentally sectioning the common bile duct) were properly shielded from discovery by the state’s apology statute.6 Apology laws vary from state to state, and some do not shield admissions regarding causation of error or fault.
However, it is unclear if apology laws work. A recent study from Vanderbilt University reported that, for physicians who do not regularly perform surgery, apology laws actually increased the probability of facing a lawsuit.7 And for surgeons, apology laws do not have a substantial effect on the probability of facing a claim or the average payment made to resolve a claim.
Safe harbors
A proposal released by U.S. House Speaker Paul Ryan (R-Wis.) in June 2016 made reference to “safe harbors” from liability for those adhering to clinical practice guidelines. The Institute of Medicine defines practice guidelines as “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.”
There are thousands of guidelines that have been developed by medical organizations and governmental agencies, as well as by insurance carriers, managed care organizations, and others. They purport to define the best evidence-based medicine, and if they were arrived at by the consensus of an authoritative body of experts, courts will tend to view them as reflective, though not necessarily dispositive, of customary medical standards.
Theoretically, adherence to guidelines could reduce the practice of defensive medicine and improve the quality of care. However, the available evidence does not indicate that guideline-based safe harbors will prove very effective in reducing malpractice claims: They are inapplicable in 85% of cases, and they have been estimated to eliminate defendants’ payments in less than 1% of claims.
Whether any form of tort reform emerges from the current Congress is as much about politics as it is about justice. It comes at an inopportune time, given the impasse over the health care debate. Still, on June 29, 2017, the U.S. House passed a medical liability reform bill with a vote of 218-210 along party lines that would cap noneconomic damages at $250,000, shorten the statute of limitations to 3 years after the date of injury, and abolish joint and several liability.8 The outlook in the U.S. Senate, however, is anything but certain.
References
1. N Engl J Med. 2017 May 11;376(19):1806-8.
2. “Sweeping new tort reforms will protect Iowa physicians” AMA Wire. June 1, 2017.
3. Chan v. Curran, 237 CA 4th 601 (2015).
4. N. Broward Hospital District v. Kalitan, (Florida Supreme Court, decided June 8, 2017).
5. Estate of Michelle Evette McCall v. U.S., 2014 Fla LEXIS 933 (No. SC 11-1148; March 13, 2014).
6. Estate of Johnson v. Randall Smith, Inc., 135 Ohio St.3d 440 (2013).
7. “Sorry is Never Enough: The Effect of State Apology Laws on Medical Malpractice Liability Risk” SSRN. 2016 Dec 10.
8. Protecting Access to Care Act of 2017 (H.R. 1215).
Question: Congressional proposals on medical tort reform can be expected to include the following, except:
A. A no-fault system akin to automobile no-fault insurance.
B. A cap on noneconomic damages.
C. “Safe-harbor” immunity against medical negligence.
D. Health courts in place of the judge/jury system to adjudicate claims.
E. Promotion of laws that encourage apologies and error disclosures.
Answer: A. Under the current Republican administration, one can expect legislative efforts at federal tort reform, especially given that Thomas E. Price, MD, the new secretary of the Department of Health & Human Services, is an orthopedic surgeon who has spoken passionately about defensive medicine, damage caps, health tribunals, and practice guidelines. As a former House representative for Georgia, Dr. Price has introduced several tort reform bills, so it is likely that any omnibus federal law will incorporate some of his proposals.1
Over the decades, many states have gone ahead in enacting their own statutes while awaiting federal action. Iowa is the latest example. It recently passed legislation that included a noneconomic damages cap of $250,000, stronger expert witness standards, a certificate of merit in all medical liability lawsuits, and an expansion of its “candor” protections.2 Additional reforms in other states include pretrial screening panels; arbitration; structured periodic payments in lieu of lump sum payments; penalties for frivolous suits; shortened statutes of limitations; making the loser bear all litigation costs; abolishing the collateral source rule, as well as joint and several liability; and limiting attorney contingency fees.
The best-known reform is a cap on noneconomic losses, such as pain and suffering, that doesn’t abridge compensation for economic losses, i.e., medical expenses and lost wages. This provides some predictability because noneconomic damages are difficult to quantify, and jury sympathy may result in unrealistically high payments.
Interestingly, Dr. Price himself has not pushed for a federal cap on noneconomic damages, but other Republican bills have proposed a cap of $250,000. Many states, such as California, Kansas, and Texas, have seen their cap statutes withstand constitutional challenge. However, other jurisdictions, notably Georgia, Illinois, and Missouri, have ruled them unconstitutional.
California’s law, popularly known as MICRA (Medical Injury Compensation Reform Act), came under renewed attack in 2015 with a wrongful death suit from hemorrhagic complications related to Coumadin (warfarin) use following heart surgery.3 The plaintiff’s constitutional challenges included violation of equal protection, due process, and the right to a jury trial, but these were essentially all grounded on an entitlement to recover additional noneconomic damages sufficient to cover attorney fees. The trial court had reduced her $1 million noneconomic damages to $250,000, as required under MICRA. A California court of appeal rejected her claim as being “contrary to many well-established legal principles.”
On the other hand, Florida’s Supreme Court recently held in a closely divided decision of 4-3 that the state’s caps were unconstitutional.4 The law limited noneconomic damages in malpractice cases to either $500,000 or $1 million if the injuries were catastrophic. The court ruled that the caps were arbitrary and unfairly hurt the most severely injured. It was unconvinced that they would reduce malpractice insurance rates; at any rate, there was no present crisis to justify the caps. The decision came 3 years after the court had struck down caps in a case of wrongful death.5
Three relative newcomers to the legal landscape – health courts, apology laws, and safe harbors – appear to be taking center stage in any forthcoming federal reform measures.
Health courts
Under this proposal, so-called health panels and tribunals would now adjudicate malpractice claims. Such health courts would dispense with the jury; further, regular judges would be replaced with specialized judges who would make binding determinations. In one version, a panel of medical experts would initially screen the complaint, followed by an administrative health care tribunal that would feature judges with medical expertise. These tribunals would issue binding rulings, but either party could appeal to a state court for a reversal.
In countries such as Scandinavia and New Zealand, these administrative compensation approaches are coupled to a no-fault system and appear to work well. However, unlike auto no-fault and workers’ compensation, the notion of medical no-fault has never caught on in the United States.
As currently construed, health courts evince dramatic departures from traditional rules of civil procedure. For one, the panels may render decisions before discovery has occurred, which would substantially limit a patient’s ability to learn the facts of what had happened to cause the injury. The panel may rely on a standard of “gross negligence” instead of “ordinary negligence,” requiring evidence not merely of substandard care but of recklessness. This would be a heavier burden on the victim, and could be expected to generate stiff opposition from the plaintiff’s bar. In addition, evidentiary rules may be modified, requiring that an appeal show with clear and convincing proof that the tribunal had erred.
Apology law
Disclosure of medical errors to the injured patient is believed to serve as an ethical and effective way of thwarting potential malpractice claims. Many states have enacted so-called apology laws that disallow statements of sympathy from being admitted into evidence. In some cases, these laws may assist the physician.
For example, the Ohio Supreme Court ruled that a surgeon’s comments and alleged admission of guilt (“I take full responsibility for this” regarding accidentally sectioning the common bile duct) were properly shielded from discovery by the state’s apology statute.6 Apology laws vary from state to state, and some do not shield admissions regarding causation of error or fault.
However, it is unclear if apology laws work. A recent study from Vanderbilt University reported that, for physicians who do not regularly perform surgery, apology laws actually increased the probability of facing a lawsuit.7 And for surgeons, apology laws do not have a substantial effect on the probability of facing a claim or the average payment made to resolve a claim.
Safe harbors
A proposal released by U.S. House Speaker Paul Ryan (R-Wis.) in June 2016 made reference to “safe harbors” from liability for those adhering to clinical practice guidelines. The Institute of Medicine defines practice guidelines as “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.”
There are thousands of guidelines that have been developed by medical organizations and governmental agencies, as well as by insurance carriers, managed care organizations, and others. They purport to define the best evidence-based medicine, and if they were arrived at by the consensus of an authoritative body of experts, courts will tend to view them as reflective, though not necessarily dispositive, of customary medical standards.
Theoretically, adherence to guidelines could reduce the practice of defensive medicine and improve the quality of care. However, the available evidence does not indicate that guideline-based safe harbors will prove very effective in reducing malpractice claims: They are inapplicable in 85% of cases, and they have been estimated to eliminate defendants’ payments in less than 1% of claims.
Whether any form of tort reform emerges from the current Congress is as much about politics as it is about justice. It comes at an inopportune time, given the impasse over the health care debate. Still, on June 29, 2017, the U.S. House passed a medical liability reform bill with a vote of 218-210 along party lines that would cap noneconomic damages at $250,000, shorten the statute of limitations to 3 years after the date of injury, and abolish joint and several liability.8 The outlook in the U.S. Senate, however, is anything but certain.
References
1. N Engl J Med. 2017 May 11;376(19):1806-8.
2. “Sweeping new tort reforms will protect Iowa physicians” AMA Wire. June 1, 2017.
3. Chan v. Curran, 237 CA 4th 601 (2015).
4. N. Broward Hospital District v. Kalitan, (Florida Supreme Court, decided June 8, 2017).
5. Estate of Michelle Evette McCall v. U.S., 2014 Fla LEXIS 933 (No. SC 11-1148; March 13, 2014).
6. Estate of Johnson v. Randall Smith, Inc., 135 Ohio St.3d 440 (2013).
7. “Sorry is Never Enough: The Effect of State Apology Laws on Medical Malpractice Liability Risk” SSRN. 2016 Dec 10.
8. Protecting Access to Care Act of 2017 (H.R. 1215).
Question: Congressional proposals on medical tort reform can be expected to include the following, except:
A. A no-fault system akin to automobile no-fault insurance.
B. A cap on noneconomic damages.
C. “Safe-harbor” immunity against medical negligence.
D. Health courts in place of the judge/jury system to adjudicate claims.
E. Promotion of laws that encourage apologies and error disclosures.
Answer: A. Under the current Republican administration, one can expect legislative efforts at federal tort reform, especially given that Thomas E. Price, MD, the new secretary of the Department of Health & Human Services, is an orthopedic surgeon who has spoken passionately about defensive medicine, damage caps, health tribunals, and practice guidelines. As a former House representative for Georgia, Dr. Price has introduced several tort reform bills, so it is likely that any omnibus federal law will incorporate some of his proposals.1
Over the decades, many states have gone ahead in enacting their own statutes while awaiting federal action. Iowa is the latest example. It recently passed legislation that included a noneconomic damages cap of $250,000, stronger expert witness standards, a certificate of merit in all medical liability lawsuits, and an expansion of its “candor” protections.2 Additional reforms in other states include pretrial screening panels; arbitration; structured periodic payments in lieu of lump sum payments; penalties for frivolous suits; shortened statutes of limitations; making the loser bear all litigation costs; abolishing the collateral source rule, as well as joint and several liability; and limiting attorney contingency fees.
The best-known reform is a cap on noneconomic losses, such as pain and suffering, that doesn’t abridge compensation for economic losses, i.e., medical expenses and lost wages. This provides some predictability because noneconomic damages are difficult to quantify, and jury sympathy may result in unrealistically high payments.
Interestingly, Dr. Price himself has not pushed for a federal cap on noneconomic damages, but other Republican bills have proposed a cap of $250,000. Many states, such as California, Kansas, and Texas, have seen their cap statutes withstand constitutional challenge. However, other jurisdictions, notably Georgia, Illinois, and Missouri, have ruled them unconstitutional.
California’s law, popularly known as MICRA (Medical Injury Compensation Reform Act), came under renewed attack in 2015 with a wrongful death suit from hemorrhagic complications related to Coumadin (warfarin) use following heart surgery.3 The plaintiff’s constitutional challenges included violation of equal protection, due process, and the right to a jury trial, but these were essentially all grounded on an entitlement to recover additional noneconomic damages sufficient to cover attorney fees. The trial court had reduced her $1 million noneconomic damages to $250,000, as required under MICRA. A California court of appeal rejected her claim as being “contrary to many well-established legal principles.”
On the other hand, Florida’s Supreme Court recently held in a closely divided decision of 4-3 that the state’s caps were unconstitutional.4 The law limited noneconomic damages in malpractice cases to either $500,000 or $1 million if the injuries were catastrophic. The court ruled that the caps were arbitrary and unfairly hurt the most severely injured. It was unconvinced that they would reduce malpractice insurance rates; at any rate, there was no present crisis to justify the caps. The decision came 3 years after the court had struck down caps in a case of wrongful death.5
Three relative newcomers to the legal landscape – health courts, apology laws, and safe harbors – appear to be taking center stage in any forthcoming federal reform measures.
Health courts
Under this proposal, so-called health panels and tribunals would now adjudicate malpractice claims. Such health courts would dispense with the jury; further, regular judges would be replaced with specialized judges who would make binding determinations. In one version, a panel of medical experts would initially screen the complaint, followed by an administrative health care tribunal that would feature judges with medical expertise. These tribunals would issue binding rulings, but either party could appeal to a state court for a reversal.
In countries such as Scandinavia and New Zealand, these administrative compensation approaches are coupled to a no-fault system and appear to work well. However, unlike auto no-fault and workers’ compensation, the notion of medical no-fault has never caught on in the United States.
As currently construed, health courts evince dramatic departures from traditional rules of civil procedure. For one, the panels may render decisions before discovery has occurred, which would substantially limit a patient’s ability to learn the facts of what had happened to cause the injury. The panel may rely on a standard of “gross negligence” instead of “ordinary negligence,” requiring evidence not merely of substandard care but of recklessness. This would be a heavier burden on the victim, and could be expected to generate stiff opposition from the plaintiff’s bar. In addition, evidentiary rules may be modified, requiring that an appeal show with clear and convincing proof that the tribunal had erred.
Apology law
Disclosure of medical errors to the injured patient is believed to serve as an ethical and effective way of thwarting potential malpractice claims. Many states have enacted so-called apology laws that disallow statements of sympathy from being admitted into evidence. In some cases, these laws may assist the physician.
For example, the Ohio Supreme Court ruled that a surgeon’s comments and alleged admission of guilt (“I take full responsibility for this” regarding accidentally sectioning the common bile duct) were properly shielded from discovery by the state’s apology statute.6 Apology laws vary from state to state, and some do not shield admissions regarding causation of error or fault.
However, it is unclear if apology laws work. A recent study from Vanderbilt University reported that, for physicians who do not regularly perform surgery, apology laws actually increased the probability of facing a lawsuit.7 And for surgeons, apology laws do not have a substantial effect on the probability of facing a claim or the average payment made to resolve a claim.
Safe harbors
A proposal released by U.S. House Speaker Paul Ryan (R-Wis.) in June 2016 made reference to “safe harbors” from liability for those adhering to clinical practice guidelines. The Institute of Medicine defines practice guidelines as “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.”
There are thousands of guidelines that have been developed by medical organizations and governmental agencies, as well as by insurance carriers, managed care organizations, and others. They purport to define the best evidence-based medicine, and if they were arrived at by the consensus of an authoritative body of experts, courts will tend to view them as reflective, though not necessarily dispositive, of customary medical standards.
Theoretically, adherence to guidelines could reduce the practice of defensive medicine and improve the quality of care. However, the available evidence does not indicate that guideline-based safe harbors will prove very effective in reducing malpractice claims: They are inapplicable in 85% of cases, and they have been estimated to eliminate defendants’ payments in less than 1% of claims.
Whether any form of tort reform emerges from the current Congress is as much about politics as it is about justice. It comes at an inopportune time, given the impasse over the health care debate. Still, on June 29, 2017, the U.S. House passed a medical liability reform bill with a vote of 218-210 along party lines that would cap noneconomic damages at $250,000, shorten the statute of limitations to 3 years after the date of injury, and abolish joint and several liability.8 The outlook in the U.S. Senate, however, is anything but certain.
References
1. N Engl J Med. 2017 May 11;376(19):1806-8.
2. “Sweeping new tort reforms will protect Iowa physicians” AMA Wire. June 1, 2017.
3. Chan v. Curran, 237 CA 4th 601 (2015).
4. N. Broward Hospital District v. Kalitan, (Florida Supreme Court, decided June 8, 2017).
5. Estate of Michelle Evette McCall v. U.S., 2014 Fla LEXIS 933 (No. SC 11-1148; March 13, 2014).
6. Estate of Johnson v. Randall Smith, Inc., 135 Ohio St.3d 440 (2013).
7. “Sorry is Never Enough: The Effect of State Apology Laws on Medical Malpractice Liability Risk” SSRN. 2016 Dec 10.
8. Protecting Access to Care Act of 2017 (H.R. 1215).
Now boarding: How we can skip coach and bump our patients up to first class
Cruising above the earth at 37,000 feet on the way back from vacation, my mind starts wandering. The impending reality of returning to work is setting in, and I can’t help but reflect on how the experience of a weary traveler trying to get home is like that of a weary patient trying to navigate modern health care. As it turns out, there are more than a few similarities, and that is not necessarily a good thing.
The modern airline industry is often cited by experts as a model for safety, efficiency, and innovation, though just a few decades ago this wasn’t the case. Several factors (for example, catastrophic crashes; the events of September 11th, 2001; the economic downturn) forced airlines to make radical improvements in how they operated – many of which I am quite thankful for as I gaze down upon America’s heartland from my window seat. Still, there are many who would say that in spite of (and sometimes because of) these improvements, air travel is the worst it’s ever been; airport lines are longer than ever, costs have steadily increased, and customer service has become little more than a quaint idea from a bygone era.
Most people deride the frustrations of air travel yet accept them as normal. The same expectations have unfortunately been set in health care. Patients wait, though waiting only contributes to anxiety and leads them to question the quality of their care. They also expect their journey to have many layover stops, though these involve even more waiting and often unnecessary redundancy. We need to streamline the care delivery process, and this is where technology can help.
First of all, we need to address the waiting. In health care, we tend to call this “access,” an ever-present problem for patients and providers. Thankfully, some recent innovations have helped significantly. The first of these innovations is online scheduling, which allows patients to find openings and schedule visits without the need to pick up the phone. Much like the ability to book a dinner reservation online, this is becoming an expectation for health care consumers. Participating practices and health systems can also use it as a marketing advantage; it is a fantastic way to recruit new patients as they search for a new provider online (that is, seeing that a physician has immediate openings may make the decision easier).
There are several companies providing third-party online scheduling services, and many of these can interface directly with electronic health records. EHR vendors themselves also provide this functionality to existing patients through an online web portal or mobile app. Either way, if you haven’t considered it yet, you should. It’s a great way to fill last-minute schedule openings and increase your patient base, all while improving access and patient satisfaction.
Another way to improve access is through telemedicine. We’ve written about this in prior columns, but it has certainly become more prevalent and available since then. Now more insurers are reimbursing for telemedicine services, and consumers are starting to embrace it as well. Consider some advantages: it’s more convenient for patients and often less expensive for those without insurance – cash prices tend to be in the $50-$75 range. It can also be more convenient for providers, as the typical telemedicine visit lasts only about 10 minutes and can be easily fit in last-minute. Better still, telemedicine can be a way for providers to now be paid for services they might have previously provided for free by telephone. It is critical to choose patients and conditions appropriate for these “virtual visits.” Medication checks, lab follow-ups, or rash evaluations are just a few examples, but with a little bit of thought it is easy to find dozens of other opportunities to use telemedicine to improve access.
In addition to access, we need to look for ways to improve efficiency and decrease redundancy when sending patients for testing and consultations. Recently, I had the experience of visiting a specialist for a minor medical issue. In spite of the fact that the specialist was a member of the same health system as my PCP, I still spent the first 15 minutes of my visit filling out paperwork that requested information easily available from my health record. There must be a better way.
Patients are beginning to question why, in the world of ubiquitous social media and connectivity, our computerized medical records can’t communicate. This is especially true when they are seeing physicians who are part of the same health system (as in my case). Thankfully, vendors have gotten the message and have begun allowing providers to collaborate, not only with physicians using the same software, but also with those using other EHRs through Health Information Exchanges (HIEs). Unfortunately, this alone won’t be enough. We must continue to promote the notion of patient-owned medical records, as that will be the only way to ensure true patient-centered care. In a future column, we’ll explore this concept in greater detail, but for now we’ll confirm our belief that universal interoperability is reasonable and possible.
As we are getting ready to land, I reflect on the wonderful vacation I just had and the tasks ahead at home, most of which I enjoy. Patients aren’t always as lucky; they are accessing medical care because they have to, not because they want to. Their “destination” is all too often an unfortunate diagnosis, unexpected surgical procedure, or lifetime of chronic discomfort. It is therefore incumbent on us, their care providers, to use the tools at our disposal to offer them the most efficient, most comfortable, and most connected journey possible.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
Cruising above the earth at 37,000 feet on the way back from vacation, my mind starts wandering. The impending reality of returning to work is setting in, and I can’t help but reflect on how the experience of a weary traveler trying to get home is like that of a weary patient trying to navigate modern health care. As it turns out, there are more than a few similarities, and that is not necessarily a good thing.
The modern airline industry is often cited by experts as a model for safety, efficiency, and innovation, though just a few decades ago this wasn’t the case. Several factors (for example, catastrophic crashes; the events of September 11th, 2001; the economic downturn) forced airlines to make radical improvements in how they operated – many of which I am quite thankful for as I gaze down upon America’s heartland from my window seat. Still, there are many who would say that in spite of (and sometimes because of) these improvements, air travel is the worst it’s ever been; airport lines are longer than ever, costs have steadily increased, and customer service has become little more than a quaint idea from a bygone era.
Most people deride the frustrations of air travel yet accept them as normal. The same expectations have unfortunately been set in health care. Patients wait, though waiting only contributes to anxiety and leads them to question the quality of their care. They also expect their journey to have many layover stops, though these involve even more waiting and often unnecessary redundancy. We need to streamline the care delivery process, and this is where technology can help.
First of all, we need to address the waiting. In health care, we tend to call this “access,” an ever-present problem for patients and providers. Thankfully, some recent innovations have helped significantly. The first of these innovations is online scheduling, which allows patients to find openings and schedule visits without the need to pick up the phone. Much like the ability to book a dinner reservation online, this is becoming an expectation for health care consumers. Participating practices and health systems can also use it as a marketing advantage; it is a fantastic way to recruit new patients as they search for a new provider online (that is, seeing that a physician has immediate openings may make the decision easier).
There are several companies providing third-party online scheduling services, and many of these can interface directly with electronic health records. EHR vendors themselves also provide this functionality to existing patients through an online web portal or mobile app. Either way, if you haven’t considered it yet, you should. It’s a great way to fill last-minute schedule openings and increase your patient base, all while improving access and patient satisfaction.
Another way to improve access is through telemedicine. We’ve written about this in prior columns, but it has certainly become more prevalent and available since then. Now more insurers are reimbursing for telemedicine services, and consumers are starting to embrace it as well. Consider some advantages: it’s more convenient for patients and often less expensive for those without insurance – cash prices tend to be in the $50-$75 range. It can also be more convenient for providers, as the typical telemedicine visit lasts only about 10 minutes and can be easily fit in last-minute. Better still, telemedicine can be a way for providers to now be paid for services they might have previously provided for free by telephone. It is critical to choose patients and conditions appropriate for these “virtual visits.” Medication checks, lab follow-ups, or rash evaluations are just a few examples, but with a little bit of thought it is easy to find dozens of other opportunities to use telemedicine to improve access.
In addition to access, we need to look for ways to improve efficiency and decrease redundancy when sending patients for testing and consultations. Recently, I had the experience of visiting a specialist for a minor medical issue. In spite of the fact that the specialist was a member of the same health system as my PCP, I still spent the first 15 minutes of my visit filling out paperwork that requested information easily available from my health record. There must be a better way.
Patients are beginning to question why, in the world of ubiquitous social media and connectivity, our computerized medical records can’t communicate. This is especially true when they are seeing physicians who are part of the same health system (as in my case). Thankfully, vendors have gotten the message and have begun allowing providers to collaborate, not only with physicians using the same software, but also with those using other EHRs through Health Information Exchanges (HIEs). Unfortunately, this alone won’t be enough. We must continue to promote the notion of patient-owned medical records, as that will be the only way to ensure true patient-centered care. In a future column, we’ll explore this concept in greater detail, but for now we’ll confirm our belief that universal interoperability is reasonable and possible.
As we are getting ready to land, I reflect on the wonderful vacation I just had and the tasks ahead at home, most of which I enjoy. Patients aren’t always as lucky; they are accessing medical care because they have to, not because they want to. Their “destination” is all too often an unfortunate diagnosis, unexpected surgical procedure, or lifetime of chronic discomfort. It is therefore incumbent on us, their care providers, to use the tools at our disposal to offer them the most efficient, most comfortable, and most connected journey possible.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
Cruising above the earth at 37,000 feet on the way back from vacation, my mind starts wandering. The impending reality of returning to work is setting in, and I can’t help but reflect on how the experience of a weary traveler trying to get home is like that of a weary patient trying to navigate modern health care. As it turns out, there are more than a few similarities, and that is not necessarily a good thing.
The modern airline industry is often cited by experts as a model for safety, efficiency, and innovation, though just a few decades ago this wasn’t the case. Several factors (for example, catastrophic crashes; the events of September 11th, 2001; the economic downturn) forced airlines to make radical improvements in how they operated – many of which I am quite thankful for as I gaze down upon America’s heartland from my window seat. Still, there are many who would say that in spite of (and sometimes because of) these improvements, air travel is the worst it’s ever been; airport lines are longer than ever, costs have steadily increased, and customer service has become little more than a quaint idea from a bygone era.
Most people deride the frustrations of air travel yet accept them as normal. The same expectations have unfortunately been set in health care. Patients wait, though waiting only contributes to anxiety and leads them to question the quality of their care. They also expect their journey to have many layover stops, though these involve even more waiting and often unnecessary redundancy. We need to streamline the care delivery process, and this is where technology can help.
First of all, we need to address the waiting. In health care, we tend to call this “access,” an ever-present problem for patients and providers. Thankfully, some recent innovations have helped significantly. The first of these innovations is online scheduling, which allows patients to find openings and schedule visits without the need to pick up the phone. Much like the ability to book a dinner reservation online, this is becoming an expectation for health care consumers. Participating practices and health systems can also use it as a marketing advantage; it is a fantastic way to recruit new patients as they search for a new provider online (that is, seeing that a physician has immediate openings may make the decision easier).
There are several companies providing third-party online scheduling services, and many of these can interface directly with electronic health records. EHR vendors themselves also provide this functionality to existing patients through an online web portal or mobile app. Either way, if you haven’t considered it yet, you should. It’s a great way to fill last-minute schedule openings and increase your patient base, all while improving access and patient satisfaction.
Another way to improve access is through telemedicine. We’ve written about this in prior columns, but it has certainly become more prevalent and available since then. Now more insurers are reimbursing for telemedicine services, and consumers are starting to embrace it as well. Consider some advantages: it’s more convenient for patients and often less expensive for those without insurance – cash prices tend to be in the $50-$75 range. It can also be more convenient for providers, as the typical telemedicine visit lasts only about 10 minutes and can be easily fit in last-minute. Better still, telemedicine can be a way for providers to now be paid for services they might have previously provided for free by telephone. It is critical to choose patients and conditions appropriate for these “virtual visits.” Medication checks, lab follow-ups, or rash evaluations are just a few examples, but with a little bit of thought it is easy to find dozens of other opportunities to use telemedicine to improve access.
In addition to access, we need to look for ways to improve efficiency and decrease redundancy when sending patients for testing and consultations. Recently, I had the experience of visiting a specialist for a minor medical issue. In spite of the fact that the specialist was a member of the same health system as my PCP, I still spent the first 15 minutes of my visit filling out paperwork that requested information easily available from my health record. There must be a better way.
Patients are beginning to question why, in the world of ubiquitous social media and connectivity, our computerized medical records can’t communicate. This is especially true when they are seeing physicians who are part of the same health system (as in my case). Thankfully, vendors have gotten the message and have begun allowing providers to collaborate, not only with physicians using the same software, but also with those using other EHRs through Health Information Exchanges (HIEs). Unfortunately, this alone won’t be enough. We must continue to promote the notion of patient-owned medical records, as that will be the only way to ensure true patient-centered care. In a future column, we’ll explore this concept in greater detail, but for now we’ll confirm our belief that universal interoperability is reasonable and possible.
As we are getting ready to land, I reflect on the wonderful vacation I just had and the tasks ahead at home, most of which I enjoy. Patients aren’t always as lucky; they are accessing medical care because they have to, not because they want to. Their “destination” is all too often an unfortunate diagnosis, unexpected surgical procedure, or lifetime of chronic discomfort. It is therefore incumbent on us, their care providers, to use the tools at our disposal to offer them the most efficient, most comfortable, and most connected journey possible.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
Crisis in psychiatry: Top 5 problems, many solutions
Lack of access to psychiatric services has been a challenge for decades, resulting in significant delays to treatment with associated consequences in reduced quality of care, low patient satisfaction, poor patient outcomes, and higher costs.
The problem is exacerbated by a growing shortage of psychiatrists, an increased demand for psychiatric services, and inadequate payment rates. The result is a crisis that is resonating throughout the U.S. health care system.
As many know, a few months ago, the Medical Director Institute of the National Council for Behavioral Health, in partnership with the American Psychiatric Association, convened an expert panel to develop a report responding to this evolving quandary. The findings of our 60-page report, “The Psychiatric Shortage: Causes and Solutions,” suggest that psychiatry is uniquely positioned to address the issues that face our specialty.
The institute identified five areas of critical concern: workforce development, improved efficiency of service delivery, reducing burdensome regulations and confidentiality restrictions, broader implementation of innovative models, and adoption of novel reimbursement methods.
1. Workforce development
Psychiatrists come out of residency training without the skills they need to practice in today’s rapidly evolving health care environment. We need better preparation in measurement-based care, telepsychiatry, collaborative care, and other methods of efficient team collaboration with primary care.
Funding for graduate medical education and training programs must be expanded with an infusion of new funding – not only for psychiatrists – but also for psychiatric nurse practitioners and psychiatric physician assistants.
2. Improved efficiency of service delivery
Providers of psychiatric services in outpatient psychiatric programs face a cramped daily routine with increasingly briefer appointments scheduled back-to-back that limit in-depth clinical assessment, collaboration with other members of the treatment team, and consultation with primary care providers outside of the program. Such a schedule leads to lower-quality care.
Psychiatrists must have the same level of nurse and paraprofessional assistance and support provided to other medical specialties. In addition, regulations that prevent the broader use of telepsychiatry must be revised. All behavioral health providers should implement open access scheduling, a proven modality for reducing missed appointments.
3. Reducing burdensome regulations and confidentiality restrictions
Excessive documentation requirements, especially necessarily detailed, lengthy assessments and treatment must be revised and 42 CFR Part 2 must be made consistent with HIPAA requirements.
4. Broader implementation of innovative models
The shortage of psychiatrists will only worsen with the integration of primary care and behavioral health, and the shift to Accountable Care Organizations as part of health care reform (which, as of this writing, faces much uncertainty). Thanks to more efficient screening for mental health and substance use disorders now occurring in primary care, there will be growing demand for access to psychiatric services.
The collaborative care model for providing psychiatric services should be implemented throughout primary care. Behavioral health organizations must develop their own version of collaborative care that targets the limited psychiatric resource where it is most needed by using measurement-based care and collaborating more effectively with other team members.
5. Adoption of novel reimbursement methods
Inappropriately low rates limit access to care. Today, 40% of psychiatrists choose cash-only private practices, the second-highest among medical specialties after dermatologists, and 75% of provider organizations employing psychiatrists report that they lose money on their psychiatric services. At the same time, the shrinking number of inpatient psychiatric services has become a significant obstacle to improved access. Beds have been eliminated because of lower rates of reimbursement, compared with other medical-surgical procedures and difficulty in recruiting psychiatrists to staff inpatient units.
Psychiatric service rates must be reset to be consistent with the actual cost of providing care. Prospective payment models like Certified Community Behavioral Health Clinics should be expanded, and bundled payments for services like collaborative care and complex care should be covered by payers.
The Medical Director Institute recommends these solutions so that access to psychiatric services does not remain a barrier to the overall success of health care reform and service delivery improving the health of Americans. Multiple stakeholders – federal and state governments, payers, providers, provider trade associations, and advocates – must take action within their spheres of influence in the design, funding, regulation, and delivery of behavioral health care to improve access to psychiatric services. Such broad collaboration is imperative for our patients to get the care they need.
Dr. Parks is the medical director for the National Council for Behavioral Health. He practices psychiatry on an outpatient basis at Family Health Center, a federally funded community health center established to expand services to uninsured and underinsured patients in central Missouri. He also holds the position of Distinguished Research Professor of Science at the University of Missouri–St. Louis and is a clinical assistant professor of psychiatry at the University of Missouri–Columbia.
Lack of access to psychiatric services has been a challenge for decades, resulting in significant delays to treatment with associated consequences in reduced quality of care, low patient satisfaction, poor patient outcomes, and higher costs.
The problem is exacerbated by a growing shortage of psychiatrists, an increased demand for psychiatric services, and inadequate payment rates. The result is a crisis that is resonating throughout the U.S. health care system.
As many know, a few months ago, the Medical Director Institute of the National Council for Behavioral Health, in partnership with the American Psychiatric Association, convened an expert panel to develop a report responding to this evolving quandary. The findings of our 60-page report, “The Psychiatric Shortage: Causes and Solutions,” suggest that psychiatry is uniquely positioned to address the issues that face our specialty.
The institute identified five areas of critical concern: workforce development, improved efficiency of service delivery, reducing burdensome regulations and confidentiality restrictions, broader implementation of innovative models, and adoption of novel reimbursement methods.
1. Workforce development
Psychiatrists come out of residency training without the skills they need to practice in today’s rapidly evolving health care environment. We need better preparation in measurement-based care, telepsychiatry, collaborative care, and other methods of efficient team collaboration with primary care.
Funding for graduate medical education and training programs must be expanded with an infusion of new funding – not only for psychiatrists – but also for psychiatric nurse practitioners and psychiatric physician assistants.
2. Improved efficiency of service delivery
Providers of psychiatric services in outpatient psychiatric programs face a cramped daily routine with increasingly briefer appointments scheduled back-to-back that limit in-depth clinical assessment, collaboration with other members of the treatment team, and consultation with primary care providers outside of the program. Such a schedule leads to lower-quality care.
Psychiatrists must have the same level of nurse and paraprofessional assistance and support provided to other medical specialties. In addition, regulations that prevent the broader use of telepsychiatry must be revised. All behavioral health providers should implement open access scheduling, a proven modality for reducing missed appointments.
3. Reducing burdensome regulations and confidentiality restrictions
Excessive documentation requirements, especially necessarily detailed, lengthy assessments and treatment must be revised and 42 CFR Part 2 must be made consistent with HIPAA requirements.
4. Broader implementation of innovative models
The shortage of psychiatrists will only worsen with the integration of primary care and behavioral health, and the shift to Accountable Care Organizations as part of health care reform (which, as of this writing, faces much uncertainty). Thanks to more efficient screening for mental health and substance use disorders now occurring in primary care, there will be growing demand for access to psychiatric services.
The collaborative care model for providing psychiatric services should be implemented throughout primary care. Behavioral health organizations must develop their own version of collaborative care that targets the limited psychiatric resource where it is most needed by using measurement-based care and collaborating more effectively with other team members.
5. Adoption of novel reimbursement methods
Inappropriately low rates limit access to care. Today, 40% of psychiatrists choose cash-only private practices, the second-highest among medical specialties after dermatologists, and 75% of provider organizations employing psychiatrists report that they lose money on their psychiatric services. At the same time, the shrinking number of inpatient psychiatric services has become a significant obstacle to improved access. Beds have been eliminated because of lower rates of reimbursement, compared with other medical-surgical procedures and difficulty in recruiting psychiatrists to staff inpatient units.
Psychiatric service rates must be reset to be consistent with the actual cost of providing care. Prospective payment models like Certified Community Behavioral Health Clinics should be expanded, and bundled payments for services like collaborative care and complex care should be covered by payers.
The Medical Director Institute recommends these solutions so that access to psychiatric services does not remain a barrier to the overall success of health care reform and service delivery improving the health of Americans. Multiple stakeholders – federal and state governments, payers, providers, provider trade associations, and advocates – must take action within their spheres of influence in the design, funding, regulation, and delivery of behavioral health care to improve access to psychiatric services. Such broad collaboration is imperative for our patients to get the care they need.
Dr. Parks is the medical director for the National Council for Behavioral Health. He practices psychiatry on an outpatient basis at Family Health Center, a federally funded community health center established to expand services to uninsured and underinsured patients in central Missouri. He also holds the position of Distinguished Research Professor of Science at the University of Missouri–St. Louis and is a clinical assistant professor of psychiatry at the University of Missouri–Columbia.
Lack of access to psychiatric services has been a challenge for decades, resulting in significant delays to treatment with associated consequences in reduced quality of care, low patient satisfaction, poor patient outcomes, and higher costs.
The problem is exacerbated by a growing shortage of psychiatrists, an increased demand for psychiatric services, and inadequate payment rates. The result is a crisis that is resonating throughout the U.S. health care system.
As many know, a few months ago, the Medical Director Institute of the National Council for Behavioral Health, in partnership with the American Psychiatric Association, convened an expert panel to develop a report responding to this evolving quandary. The findings of our 60-page report, “The Psychiatric Shortage: Causes and Solutions,” suggest that psychiatry is uniquely positioned to address the issues that face our specialty.
The institute identified five areas of critical concern: workforce development, improved efficiency of service delivery, reducing burdensome regulations and confidentiality restrictions, broader implementation of innovative models, and adoption of novel reimbursement methods.
1. Workforce development
Psychiatrists come out of residency training without the skills they need to practice in today’s rapidly evolving health care environment. We need better preparation in measurement-based care, telepsychiatry, collaborative care, and other methods of efficient team collaboration with primary care.
Funding for graduate medical education and training programs must be expanded with an infusion of new funding – not only for psychiatrists – but also for psychiatric nurse practitioners and psychiatric physician assistants.
2. Improved efficiency of service delivery
Providers of psychiatric services in outpatient psychiatric programs face a cramped daily routine with increasingly briefer appointments scheduled back-to-back that limit in-depth clinical assessment, collaboration with other members of the treatment team, and consultation with primary care providers outside of the program. Such a schedule leads to lower-quality care.
Psychiatrists must have the same level of nurse and paraprofessional assistance and support provided to other medical specialties. In addition, regulations that prevent the broader use of telepsychiatry must be revised. All behavioral health providers should implement open access scheduling, a proven modality for reducing missed appointments.
3. Reducing burdensome regulations and confidentiality restrictions
Excessive documentation requirements, especially necessarily detailed, lengthy assessments and treatment must be revised and 42 CFR Part 2 must be made consistent with HIPAA requirements.
4. Broader implementation of innovative models
The shortage of psychiatrists will only worsen with the integration of primary care and behavioral health, and the shift to Accountable Care Organizations as part of health care reform (which, as of this writing, faces much uncertainty). Thanks to more efficient screening for mental health and substance use disorders now occurring in primary care, there will be growing demand for access to psychiatric services.
The collaborative care model for providing psychiatric services should be implemented throughout primary care. Behavioral health organizations must develop their own version of collaborative care that targets the limited psychiatric resource where it is most needed by using measurement-based care and collaborating more effectively with other team members.
5. Adoption of novel reimbursement methods
Inappropriately low rates limit access to care. Today, 40% of psychiatrists choose cash-only private practices, the second-highest among medical specialties after dermatologists, and 75% of provider organizations employing psychiatrists report that they lose money on their psychiatric services. At the same time, the shrinking number of inpatient psychiatric services has become a significant obstacle to improved access. Beds have been eliminated because of lower rates of reimbursement, compared with other medical-surgical procedures and difficulty in recruiting psychiatrists to staff inpatient units.
Psychiatric service rates must be reset to be consistent with the actual cost of providing care. Prospective payment models like Certified Community Behavioral Health Clinics should be expanded, and bundled payments for services like collaborative care and complex care should be covered by payers.
The Medical Director Institute recommends these solutions so that access to psychiatric services does not remain a barrier to the overall success of health care reform and service delivery improving the health of Americans. Multiple stakeholders – federal and state governments, payers, providers, provider trade associations, and advocates – must take action within their spheres of influence in the design, funding, regulation, and delivery of behavioral health care to improve access to psychiatric services. Such broad collaboration is imperative for our patients to get the care they need.
Dr. Parks is the medical director for the National Council for Behavioral Health. He practices psychiatry on an outpatient basis at Family Health Center, a federally funded community health center established to expand services to uninsured and underinsured patients in central Missouri. He also holds the position of Distinguished Research Professor of Science at the University of Missouri–St. Louis and is a clinical assistant professor of psychiatry at the University of Missouri–Columbia.
Diabetes’ social determinants: What they mean in our practices
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.
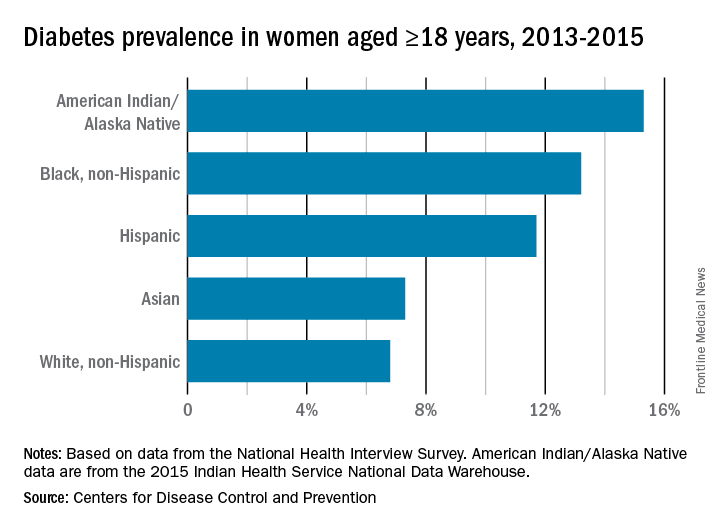
In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.

In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.

In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.
The moving target of gestational diabetes care
With the rise of obesity and diabetes, especially type 2 diabetes, in the general population, the likelihood of encountering a patient with diabetes in pregnancy also continues to increase. Women with diabetes who are pregnant require specialized medical guidance, additional monitoring, and a health care team well versed in the possible complications that can arise during pregnancy, delivery, and after birth.
Even with strict glycemic control, women with diabetes in pregnancy are much more likely to experience complications, such as preeclampsia, babies with major congenital defects, large-for-gestational-age fetuses, and children with a higher propensity for chronic diseases later in life, compared with women without diabetes.
Therefore, it has been an incredible honor for me to have taken part in the work of the Diabetes in Pregnancy Study Group of North America (DPSG-NA) for the last 20 years. The DPSG-NA meetings have served as a forum for the dissemination of data, gathered through collaboration between researchers and clinical care teams in the United States and abroad. This year, the DPSG-NA will meet in Washington, D.C., Oct. 26-28, to discuss a range of topics under the theme of managing and preventing diabetes and obesity in pregnancy.
I am delighted that one of the speakers at the DPSG-NA meeting is this month’s Master Class guest author, Lynn Yee, MD, assistant professor of obstetrics and gynecology at Northwestern University Feinberg School of Medicine, Chicago. Dr. Yee will address the need to reduce disparities in the quality and availability of care for patients with diabetes in pregnancy, an extension of the June Master Class column that discussed the critical role that ob.gyns. can play in improving health equity for all patients.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
With the rise of obesity and diabetes, especially type 2 diabetes, in the general population, the likelihood of encountering a patient with diabetes in pregnancy also continues to increase. Women with diabetes who are pregnant require specialized medical guidance, additional monitoring, and a health care team well versed in the possible complications that can arise during pregnancy, delivery, and after birth.
Even with strict glycemic control, women with diabetes in pregnancy are much more likely to experience complications, such as preeclampsia, babies with major congenital defects, large-for-gestational-age fetuses, and children with a higher propensity for chronic diseases later in life, compared with women without diabetes.
Therefore, it has been an incredible honor for me to have taken part in the work of the Diabetes in Pregnancy Study Group of North America (DPSG-NA) for the last 20 years. The DPSG-NA meetings have served as a forum for the dissemination of data, gathered through collaboration between researchers and clinical care teams in the United States and abroad. This year, the DPSG-NA will meet in Washington, D.C., Oct. 26-28, to discuss a range of topics under the theme of managing and preventing diabetes and obesity in pregnancy.
I am delighted that one of the speakers at the DPSG-NA meeting is this month’s Master Class guest author, Lynn Yee, MD, assistant professor of obstetrics and gynecology at Northwestern University Feinberg School of Medicine, Chicago. Dr. Yee will address the need to reduce disparities in the quality and availability of care for patients with diabetes in pregnancy, an extension of the June Master Class column that discussed the critical role that ob.gyns. can play in improving health equity for all patients.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
With the rise of obesity and diabetes, especially type 2 diabetes, in the general population, the likelihood of encountering a patient with diabetes in pregnancy also continues to increase. Women with diabetes who are pregnant require specialized medical guidance, additional monitoring, and a health care team well versed in the possible complications that can arise during pregnancy, delivery, and after birth.
Even with strict glycemic control, women with diabetes in pregnancy are much more likely to experience complications, such as preeclampsia, babies with major congenital defects, large-for-gestational-age fetuses, and children with a higher propensity for chronic diseases later in life, compared with women without diabetes.
Therefore, it has been an incredible honor for me to have taken part in the work of the Diabetes in Pregnancy Study Group of North America (DPSG-NA) for the last 20 years. The DPSG-NA meetings have served as a forum for the dissemination of data, gathered through collaboration between researchers and clinical care teams in the United States and abroad. This year, the DPSG-NA will meet in Washington, D.C., Oct. 26-28, to discuss a range of topics under the theme of managing and preventing diabetes and obesity in pregnancy.
I am delighted that one of the speakers at the DPSG-NA meeting is this month’s Master Class guest author, Lynn Yee, MD, assistant professor of obstetrics and gynecology at Northwestern University Feinberg School of Medicine, Chicago. Dr. Yee will address the need to reduce disparities in the quality and availability of care for patients with diabetes in pregnancy, an extension of the June Master Class column that discussed the critical role that ob.gyns. can play in improving health equity for all patients.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Safety of oral antidiabetic agents in pregnancy
The three most potent human teratogens, with the possible inclusion of some of the first antineoplastics, are isotretinoin, alcohol, and hyperglycemia.
As with all teratogens, the toxicity is dose related. For example, the risk of embryo-fetal harm from hyperglycemia increases markedly when the HbA1c is greater than 8%. Moreover, diabetes accounts for more than 90% of the harm caused by chronic diseases. Consequently, control of glucose levels in pregnancy is critical.
If these agents are used near term, there is a risk that they will cause hypoglycemia in the newborn. Changing from oral therapy to insulin is the safest course.
There are seven pharmacologic subclasses of oral antidiabetic agents: alpha-glucosidase inhibitors, biguanides, dipeptidyl peptidase-4 inhibitors, meglitinides, sulfonylureas, sodium-glucose cotransporter-2 inhibitors, and thiazolidinediones. Many of these drugs are available in combination with metformin. All of these agents are indicated as adjunct to diet and exercise for type 2 diabetes, but they also can be used for gestational diabetes. Although the human pregnancy data are very limited or nonexistent for most of these agents, none are known to cause structural defects in humans. Additional details of the exposures are available in the 11th edition of “Drugs in Pregnancy and Lactation” (2017: Wolters Kluwer).
Alpha-glucosidase inhibitors
The two agents is this subclass are acarbose (Precose) and miglitol (Glyset). The human pregnancy data with acarbose are limited, and no human pregnancy data have been found for miglitol. The animal data for both drugs suggest low risk.
Biguanides
There are substantial human pregnancy data for metformin in both type 2 and gestational diabetes. When combined with insulin, it is effective in significantly lowering the amount of insulin required to control hyperglycemia. It also may be effective when used alone. The risk of embryo-fetal harm with this drug appears to be very low or nonexistent. The animal data suggest low risk.
Dipeptidyl peptidase-4 inhibitors
There are four drugs in this subclass: alogliptin (Nesina), linagliptin (Tradjenta), saxagliptin (Onglyza), and sitagliptin (Januvia). No reports of the use of the first three drugs in human pregnancy have been found. However, the Merck Pregnancy Registries (2006-2009) described the outcomes of eight women who were exposed to sitagliptin or sitagliptin/metformin in the first trimester. The outcomes of these pregnancies were five healthy newborns, two spontaneous abortions, and one fetal death at 34 weeks’ gestation. In that case, the mother took sitagliptin and metformin separately during the first 5 weeks of gestation. The animal data for all four drugs suggest low risk.
Meglitinides
Nateglinide (Starlix) and repaglinide (Prandin) are the agents in this subclass. There is no human pregnancy data for nateglinide, but there is limited data (eight pregnancies) for repaglinide. No birth defects or other toxicity was noted in these cases. The animal data suggest low risk.
Sulfonylureas
Six drugs are included in this subclass: chlorpropamide, glimepiride (Amaryl), glipizide (Glucotrol), glyburide, tolazamide (Tolinase), and tolbutamide. These agents were among the first oral antidiabetic agents. As a result, they have the most human pregnancy data. Although birth defects were observed in newborns of mothers who had used one of these drugs, the defects were thought to be the result of uncontrolled diabetes. The animal data suggest low risk.
SGLT2 inhibitors
There are three drugs in this sodium-glucose cotransporter-2 inhibitor subclass: canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance). No reports describing the use of these drugs in human pregnancy have been located. The animal data suggest low risk.
Thiazolidinediones
Pioglitazone (Actos) and rosiglitazone (Avandia) form this subclass. There are limited human pregnancy data for both drugs. The animal data suggest moderate risk for embryo-fetal toxicity but not for structural defects.
Lactation
All of the above drugs will probably be excreted into breast milk, but the amounts are typically unknown. When they have been measured, the amounts were usually low. However, there is still a risk for hypoglycemia in a nursing infant. Combination products containing two antidiabetic agents are best avoided. The safest course is to use insulin, but, if this is not an option, then the lowest effective dose should be used. In addition, the infant’s blood glucose levels should be routinely monitored.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
The three most potent human teratogens, with the possible inclusion of some of the first antineoplastics, are isotretinoin, alcohol, and hyperglycemia.
As with all teratogens, the toxicity is dose related. For example, the risk of embryo-fetal harm from hyperglycemia increases markedly when the HbA1c is greater than 8%. Moreover, diabetes accounts for more than 90% of the harm caused by chronic diseases. Consequently, control of glucose levels in pregnancy is critical.
If these agents are used near term, there is a risk that they will cause hypoglycemia in the newborn. Changing from oral therapy to insulin is the safest course.
There are seven pharmacologic subclasses of oral antidiabetic agents: alpha-glucosidase inhibitors, biguanides, dipeptidyl peptidase-4 inhibitors, meglitinides, sulfonylureas, sodium-glucose cotransporter-2 inhibitors, and thiazolidinediones. Many of these drugs are available in combination with metformin. All of these agents are indicated as adjunct to diet and exercise for type 2 diabetes, but they also can be used for gestational diabetes. Although the human pregnancy data are very limited or nonexistent for most of these agents, none are known to cause structural defects in humans. Additional details of the exposures are available in the 11th edition of “Drugs in Pregnancy and Lactation” (2017: Wolters Kluwer).
Alpha-glucosidase inhibitors
The two agents is this subclass are acarbose (Precose) and miglitol (Glyset). The human pregnancy data with acarbose are limited, and no human pregnancy data have been found for miglitol. The animal data for both drugs suggest low risk.
Biguanides
There are substantial human pregnancy data for metformin in both type 2 and gestational diabetes. When combined with insulin, it is effective in significantly lowering the amount of insulin required to control hyperglycemia. It also may be effective when used alone. The risk of embryo-fetal harm with this drug appears to be very low or nonexistent. The animal data suggest low risk.
Dipeptidyl peptidase-4 inhibitors
There are four drugs in this subclass: alogliptin (Nesina), linagliptin (Tradjenta), saxagliptin (Onglyza), and sitagliptin (Januvia). No reports of the use of the first three drugs in human pregnancy have been found. However, the Merck Pregnancy Registries (2006-2009) described the outcomes of eight women who were exposed to sitagliptin or sitagliptin/metformin in the first trimester. The outcomes of these pregnancies were five healthy newborns, two spontaneous abortions, and one fetal death at 34 weeks’ gestation. In that case, the mother took sitagliptin and metformin separately during the first 5 weeks of gestation. The animal data for all four drugs suggest low risk.
Meglitinides
Nateglinide (Starlix) and repaglinide (Prandin) are the agents in this subclass. There is no human pregnancy data for nateglinide, but there is limited data (eight pregnancies) for repaglinide. No birth defects or other toxicity was noted in these cases. The animal data suggest low risk.
Sulfonylureas
Six drugs are included in this subclass: chlorpropamide, glimepiride (Amaryl), glipizide (Glucotrol), glyburide, tolazamide (Tolinase), and tolbutamide. These agents were among the first oral antidiabetic agents. As a result, they have the most human pregnancy data. Although birth defects were observed in newborns of mothers who had used one of these drugs, the defects were thought to be the result of uncontrolled diabetes. The animal data suggest low risk.
SGLT2 inhibitors
There are three drugs in this sodium-glucose cotransporter-2 inhibitor subclass: canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance). No reports describing the use of these drugs in human pregnancy have been located. The animal data suggest low risk.
Thiazolidinediones
Pioglitazone (Actos) and rosiglitazone (Avandia) form this subclass. There are limited human pregnancy data for both drugs. The animal data suggest moderate risk for embryo-fetal toxicity but not for structural defects.
Lactation
All of the above drugs will probably be excreted into breast milk, but the amounts are typically unknown. When they have been measured, the amounts were usually low. However, there is still a risk for hypoglycemia in a nursing infant. Combination products containing two antidiabetic agents are best avoided. The safest course is to use insulin, but, if this is not an option, then the lowest effective dose should be used. In addition, the infant’s blood glucose levels should be routinely monitored.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
The three most potent human teratogens, with the possible inclusion of some of the first antineoplastics, are isotretinoin, alcohol, and hyperglycemia.
As with all teratogens, the toxicity is dose related. For example, the risk of embryo-fetal harm from hyperglycemia increases markedly when the HbA1c is greater than 8%. Moreover, diabetes accounts for more than 90% of the harm caused by chronic diseases. Consequently, control of glucose levels in pregnancy is critical.
If these agents are used near term, there is a risk that they will cause hypoglycemia in the newborn. Changing from oral therapy to insulin is the safest course.
There are seven pharmacologic subclasses of oral antidiabetic agents: alpha-glucosidase inhibitors, biguanides, dipeptidyl peptidase-4 inhibitors, meglitinides, sulfonylureas, sodium-glucose cotransporter-2 inhibitors, and thiazolidinediones. Many of these drugs are available in combination with metformin. All of these agents are indicated as adjunct to diet and exercise for type 2 diabetes, but they also can be used for gestational diabetes. Although the human pregnancy data are very limited or nonexistent for most of these agents, none are known to cause structural defects in humans. Additional details of the exposures are available in the 11th edition of “Drugs in Pregnancy and Lactation” (2017: Wolters Kluwer).
Alpha-glucosidase inhibitors
The two agents is this subclass are acarbose (Precose) and miglitol (Glyset). The human pregnancy data with acarbose are limited, and no human pregnancy data have been found for miglitol. The animal data for both drugs suggest low risk.
Biguanides
There are substantial human pregnancy data for metformin in both type 2 and gestational diabetes. When combined with insulin, it is effective in significantly lowering the amount of insulin required to control hyperglycemia. It also may be effective when used alone. The risk of embryo-fetal harm with this drug appears to be very low or nonexistent. The animal data suggest low risk.
Dipeptidyl peptidase-4 inhibitors
There are four drugs in this subclass: alogliptin (Nesina), linagliptin (Tradjenta), saxagliptin (Onglyza), and sitagliptin (Januvia). No reports of the use of the first three drugs in human pregnancy have been found. However, the Merck Pregnancy Registries (2006-2009) described the outcomes of eight women who were exposed to sitagliptin or sitagliptin/metformin in the first trimester. The outcomes of these pregnancies were five healthy newborns, two spontaneous abortions, and one fetal death at 34 weeks’ gestation. In that case, the mother took sitagliptin and metformin separately during the first 5 weeks of gestation. The animal data for all four drugs suggest low risk.
Meglitinides
Nateglinide (Starlix) and repaglinide (Prandin) are the agents in this subclass. There is no human pregnancy data for nateglinide, but there is limited data (eight pregnancies) for repaglinide. No birth defects or other toxicity was noted in these cases. The animal data suggest low risk.
Sulfonylureas
Six drugs are included in this subclass: chlorpropamide, glimepiride (Amaryl), glipizide (Glucotrol), glyburide, tolazamide (Tolinase), and tolbutamide. These agents were among the first oral antidiabetic agents. As a result, they have the most human pregnancy data. Although birth defects were observed in newborns of mothers who had used one of these drugs, the defects were thought to be the result of uncontrolled diabetes. The animal data suggest low risk.
SGLT2 inhibitors
There are three drugs in this sodium-glucose cotransporter-2 inhibitor subclass: canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance). No reports describing the use of these drugs in human pregnancy have been located. The animal data suggest low risk.
Thiazolidinediones
Pioglitazone (Actos) and rosiglitazone (Avandia) form this subclass. There are limited human pregnancy data for both drugs. The animal data suggest moderate risk for embryo-fetal toxicity but not for structural defects.
Lactation
All of the above drugs will probably be excreted into breast milk, but the amounts are typically unknown. When they have been measured, the amounts were usually low. However, there is still a risk for hypoglycemia in a nursing infant. Combination products containing two antidiabetic agents are best avoided. The safest course is to use insulin, but, if this is not an option, then the lowest effective dose should be used. In addition, the infant’s blood glucose levels should be routinely monitored.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Optimizing HPV vaccination
Human papillomavirus (HPV) is the most common sexually transmitted infection. Exposure is widespread and most individuals clear the infection without symptoms or development of disease. However, a subset of individuals experience persistent infection, a state which can lead to carcinogenesis of lower genital tract malignancies, particularly cervical cancer.1
Vaccine coverage
Persistent infection with high-risk (oncogenic) HPV is well known to be the cause of cervical cancer. There are two HPV vaccines manufactured for the purposes of cervical cancer, anal cancer, and genital wart prevention (Cervarix and Gardasil). The Cervarix vaccine covers high-risk HPV subtypes 16 and 18 and the Gardasil vaccine prevents both low-risk HPV subtypes 6 and 11, which can cause genital warts, and high-risk HPV subtypes 16, 18, 31, 33, 45, 52 and 58, which cause cervical dysplasia and cancer.
High-risk HPV is also associated with head and neck, vulvar, vaginal, and penile cancers, though the vaccines are not approved by the Food and Drug Administration for prevention of these diseases.2
Vaccination indications
Since vaccination prevents multiple subtypes of HPV, an individual who has already been exposed will still benefit from protection from other subtypes of HPV through vaccination. HPV vaccination is not approved during pregnancy but can be initiated in the postpartum period when women are engaged in their health care and receiving other vaccinations, such as varicella or the MMR vaccine.
Recommended schedule
Until October 2016, the vaccination schedule was based on a three-dose series (0, 2, and 6 months). Currently, the CDC recommends that children aged under 15 years at the time of first dose may opt for a two-dose series (0 and 6-12 months). For those aged 15-26 years, the three-dose schedule remains the recommended course.
The benefits of two-dose schedule are convenience, cost, and increased likelihood of completion. Data presented at the 2017 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer showed that rates of cervical dysplasia were equivalent for women who completed a two-dose schedule versus a three-dose schedule.4
Efficacy
A recent meta-analysis of clinical trials of the HPV vaccines describe efficacy of 95%-97% in prevention of CIN 1-3.5 While its greatest efficacy is in its ability to prevent primary HPV infection, there still is some benefit for individuals who already were exposed to HPV prior to vaccination. As stated previously, women with a history of prior HPV vaccination have lower rates of recurrence of cervical dysplasia after treatment. Additionally, recent research has shown that women who received HPV vaccinations after a LEEP procedure for CIN 2 or 3 experience significantly lower recurrence rates, compared with women who did not receive vaccinations after LEEP (2.5% vs. 8.5%).6 This raises the possibility of a therapeutic role for HPV vaccination in women infected with HPV. Prospective studies are currently evaluating this question.
Myths
The most common side effects of the HPV vaccine are pain, redness, or swelling at the injection site. Other known side effects include fever, headache or malaise, nausea, syncope, or muscle/joint pain – similar to other vaccinations. Anaphylaxis is a rare complication.
Some parents and pediatricians report concerns that vaccination could lead to earlier sexual activity. Multiple studies have shown that girls who receive HPV vaccination are no more likely to become pregnant or get a sexually transmitted infection (proxies for intercourse) than are girls who were not vaccinated.7,8
Maximizing vaccination rates
HPV vaccination rates in the United States lag significantly behind rates in countries with national vaccine programs, such as Australia and Denmark.9 Early data from Australia already have shown a decrease in genital warts and CIN 2+ incidence within the 10 years of starting its school-based vaccine program, with approximately 73% of 12- to 15-year-olds having completed the vaccine series.2 In contrast, just 40% and 22% of 13- to 17-year-old girls and boys in the United States, respectively, had completed the vaccine series in 2014, according to the CDC.10
Vaccination gaps between girls and boys are narrowing, and more teens will be able to complete the series with the new two-dose recommendation for those younger than 15 years. However, our current rates of vaccination are significantly lower for HPV than for other routinely recommended adolescent vaccines (such as Tdap and meningococcal) and more must be done to encourage vaccination.
Studies have shown that parents are more likely to vaccinate their children if providers recommend the vaccine.11 As women’s health care providers, we do not always see children during the time period that is ideal for vaccination. However, we take care of many women who are presenting for routine gynecologic care, pregnancy, or with abnormal Pap smear screenings. These are ideal opportunities to educate and offer HPV vaccination to women in the approved age groups, as well as to encourage parents to vaccinate their children.
As with other vaccines, the recommendation should be clear and focused on the cancer prevention benefit. Using methods in which the recommendation is “announced” in a brief statement assuming parents/patients are ready to vaccinate versus open-ended conversations, has been studied as a potentially successful method to increase uptake of HPV vaccination.12 Additionally, documentation of HPV vaccination status should be built into electronic medical record templates to prompt clinicians to ask and offer HPV vaccination at visits, including postpartum visits.
Dr. Rahangdale is an associate professor of ob.gyn. at the University of North Carolina, Chapel Hill, and is director of the North Carolina Women’s Hospital Cervical Dysplasia Clinic. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Epidemiol. 2008 Jul 15;168(2):123-37.
2. Int J Cancer. 2012 Nov 1;131(9):1969-82.
3. BMJ. 2012 Mar 27;344:e1401. doi: 10.1136/bmj.e1401.
4. Gynecol Oncol. 2017 Jun. doi. org/10.1016/j.ygyno.2017.03.031.
5. Int J Prev Med. 2017 Jun 1;8:44. doi: 10.4103/ijpvm.IJPVM_413_16.
6. Gynecol Oncol. 2013 Aug;130(2):264-8.
7. Pediatrics. 2012 Nov;130(5):798-805.
8. JAMA Intern Med. 2015 Apr;175(4):617-23.
9. Clin Pediatr (Phila). 2016 Sep;55(10):904-14.
10. MMWR Morb Mortal Wkly Rep. 2015 Jul 31;64(29):784-92.
11. Vaccine. 2016 Feb 24;34(9):1187-92.
12. Pediatrics. 2017 Jan;139(1). pii:e20161764. doi: 10.1542/peds.2016-1764.
Human papillomavirus (HPV) is the most common sexually transmitted infection. Exposure is widespread and most individuals clear the infection without symptoms or development of disease. However, a subset of individuals experience persistent infection, a state which can lead to carcinogenesis of lower genital tract malignancies, particularly cervical cancer.1
Vaccine coverage
Persistent infection with high-risk (oncogenic) HPV is well known to be the cause of cervical cancer. There are two HPV vaccines manufactured for the purposes of cervical cancer, anal cancer, and genital wart prevention (Cervarix and Gardasil). The Cervarix vaccine covers high-risk HPV subtypes 16 and 18 and the Gardasil vaccine prevents both low-risk HPV subtypes 6 and 11, which can cause genital warts, and high-risk HPV subtypes 16, 18, 31, 33, 45, 52 and 58, which cause cervical dysplasia and cancer.
High-risk HPV is also associated with head and neck, vulvar, vaginal, and penile cancers, though the vaccines are not approved by the Food and Drug Administration for prevention of these diseases.2
Vaccination indications
Since vaccination prevents multiple subtypes of HPV, an individual who has already been exposed will still benefit from protection from other subtypes of HPV through vaccination. HPV vaccination is not approved during pregnancy but can be initiated in the postpartum period when women are engaged in their health care and receiving other vaccinations, such as varicella or the MMR vaccine.
Recommended schedule
Until October 2016, the vaccination schedule was based on a three-dose series (0, 2, and 6 months). Currently, the CDC recommends that children aged under 15 years at the time of first dose may opt for a two-dose series (0 and 6-12 months). For those aged 15-26 years, the three-dose schedule remains the recommended course.
The benefits of two-dose schedule are convenience, cost, and increased likelihood of completion. Data presented at the 2017 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer showed that rates of cervical dysplasia were equivalent for women who completed a two-dose schedule versus a three-dose schedule.4
Efficacy
A recent meta-analysis of clinical trials of the HPV vaccines describe efficacy of 95%-97% in prevention of CIN 1-3.5 While its greatest efficacy is in its ability to prevent primary HPV infection, there still is some benefit for individuals who already were exposed to HPV prior to vaccination. As stated previously, women with a history of prior HPV vaccination have lower rates of recurrence of cervical dysplasia after treatment. Additionally, recent research has shown that women who received HPV vaccinations after a LEEP procedure for CIN 2 or 3 experience significantly lower recurrence rates, compared with women who did not receive vaccinations after LEEP (2.5% vs. 8.5%).6 This raises the possibility of a therapeutic role for HPV vaccination in women infected with HPV. Prospective studies are currently evaluating this question.
Myths
The most common side effects of the HPV vaccine are pain, redness, or swelling at the injection site. Other known side effects include fever, headache or malaise, nausea, syncope, or muscle/joint pain – similar to other vaccinations. Anaphylaxis is a rare complication.
Some parents and pediatricians report concerns that vaccination could lead to earlier sexual activity. Multiple studies have shown that girls who receive HPV vaccination are no more likely to become pregnant or get a sexually transmitted infection (proxies for intercourse) than are girls who were not vaccinated.7,8
Maximizing vaccination rates
HPV vaccination rates in the United States lag significantly behind rates in countries with national vaccine programs, such as Australia and Denmark.9 Early data from Australia already have shown a decrease in genital warts and CIN 2+ incidence within the 10 years of starting its school-based vaccine program, with approximately 73% of 12- to 15-year-olds having completed the vaccine series.2 In contrast, just 40% and 22% of 13- to 17-year-old girls and boys in the United States, respectively, had completed the vaccine series in 2014, according to the CDC.10
Vaccination gaps between girls and boys are narrowing, and more teens will be able to complete the series with the new two-dose recommendation for those younger than 15 years. However, our current rates of vaccination are significantly lower for HPV than for other routinely recommended adolescent vaccines (such as Tdap and meningococcal) and more must be done to encourage vaccination.
Studies have shown that parents are more likely to vaccinate their children if providers recommend the vaccine.11 As women’s health care providers, we do not always see children during the time period that is ideal for vaccination. However, we take care of many women who are presenting for routine gynecologic care, pregnancy, or with abnormal Pap smear screenings. These are ideal opportunities to educate and offer HPV vaccination to women in the approved age groups, as well as to encourage parents to vaccinate their children.
As with other vaccines, the recommendation should be clear and focused on the cancer prevention benefit. Using methods in which the recommendation is “announced” in a brief statement assuming parents/patients are ready to vaccinate versus open-ended conversations, has been studied as a potentially successful method to increase uptake of HPV vaccination.12 Additionally, documentation of HPV vaccination status should be built into electronic medical record templates to prompt clinicians to ask and offer HPV vaccination at visits, including postpartum visits.
Dr. Rahangdale is an associate professor of ob.gyn. at the University of North Carolina, Chapel Hill, and is director of the North Carolina Women’s Hospital Cervical Dysplasia Clinic. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Epidemiol. 2008 Jul 15;168(2):123-37.
2. Int J Cancer. 2012 Nov 1;131(9):1969-82.
3. BMJ. 2012 Mar 27;344:e1401. doi: 10.1136/bmj.e1401.
4. Gynecol Oncol. 2017 Jun. doi. org/10.1016/j.ygyno.2017.03.031.
5. Int J Prev Med. 2017 Jun 1;8:44. doi: 10.4103/ijpvm.IJPVM_413_16.
6. Gynecol Oncol. 2013 Aug;130(2):264-8.
7. Pediatrics. 2012 Nov;130(5):798-805.
8. JAMA Intern Med. 2015 Apr;175(4):617-23.
9. Clin Pediatr (Phila). 2016 Sep;55(10):904-14.
10. MMWR Morb Mortal Wkly Rep. 2015 Jul 31;64(29):784-92.
11. Vaccine. 2016 Feb 24;34(9):1187-92.
12. Pediatrics. 2017 Jan;139(1). pii:e20161764. doi: 10.1542/peds.2016-1764.
Human papillomavirus (HPV) is the most common sexually transmitted infection. Exposure is widespread and most individuals clear the infection without symptoms or development of disease. However, a subset of individuals experience persistent infection, a state which can lead to carcinogenesis of lower genital tract malignancies, particularly cervical cancer.1
Vaccine coverage
Persistent infection with high-risk (oncogenic) HPV is well known to be the cause of cervical cancer. There are two HPV vaccines manufactured for the purposes of cervical cancer, anal cancer, and genital wart prevention (Cervarix and Gardasil). The Cervarix vaccine covers high-risk HPV subtypes 16 and 18 and the Gardasil vaccine prevents both low-risk HPV subtypes 6 and 11, which can cause genital warts, and high-risk HPV subtypes 16, 18, 31, 33, 45, 52 and 58, which cause cervical dysplasia and cancer.
High-risk HPV is also associated with head and neck, vulvar, vaginal, and penile cancers, though the vaccines are not approved by the Food and Drug Administration for prevention of these diseases.2
Vaccination indications
Since vaccination prevents multiple subtypes of HPV, an individual who has already been exposed will still benefit from protection from other subtypes of HPV through vaccination. HPV vaccination is not approved during pregnancy but can be initiated in the postpartum period when women are engaged in their health care and receiving other vaccinations, such as varicella or the MMR vaccine.
Recommended schedule
Until October 2016, the vaccination schedule was based on a three-dose series (0, 2, and 6 months). Currently, the CDC recommends that children aged under 15 years at the time of first dose may opt for a two-dose series (0 and 6-12 months). For those aged 15-26 years, the three-dose schedule remains the recommended course.
The benefits of two-dose schedule are convenience, cost, and increased likelihood of completion. Data presented at the 2017 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer showed that rates of cervical dysplasia were equivalent for women who completed a two-dose schedule versus a three-dose schedule.4
Efficacy
A recent meta-analysis of clinical trials of the HPV vaccines describe efficacy of 95%-97% in prevention of CIN 1-3.5 While its greatest efficacy is in its ability to prevent primary HPV infection, there still is some benefit for individuals who already were exposed to HPV prior to vaccination. As stated previously, women with a history of prior HPV vaccination have lower rates of recurrence of cervical dysplasia after treatment. Additionally, recent research has shown that women who received HPV vaccinations after a LEEP procedure for CIN 2 or 3 experience significantly lower recurrence rates, compared with women who did not receive vaccinations after LEEP (2.5% vs. 8.5%).6 This raises the possibility of a therapeutic role for HPV vaccination in women infected with HPV. Prospective studies are currently evaluating this question.
Myths
The most common side effects of the HPV vaccine are pain, redness, or swelling at the injection site. Other known side effects include fever, headache or malaise, nausea, syncope, or muscle/joint pain – similar to other vaccinations. Anaphylaxis is a rare complication.
Some parents and pediatricians report concerns that vaccination could lead to earlier sexual activity. Multiple studies have shown that girls who receive HPV vaccination are no more likely to become pregnant or get a sexually transmitted infection (proxies for intercourse) than are girls who were not vaccinated.7,8
Maximizing vaccination rates
HPV vaccination rates in the United States lag significantly behind rates in countries with national vaccine programs, such as Australia and Denmark.9 Early data from Australia already have shown a decrease in genital warts and CIN 2+ incidence within the 10 years of starting its school-based vaccine program, with approximately 73% of 12- to 15-year-olds having completed the vaccine series.2 In contrast, just 40% and 22% of 13- to 17-year-old girls and boys in the United States, respectively, had completed the vaccine series in 2014, according to the CDC.10
Vaccination gaps between girls and boys are narrowing, and more teens will be able to complete the series with the new two-dose recommendation for those younger than 15 years. However, our current rates of vaccination are significantly lower for HPV than for other routinely recommended adolescent vaccines (such as Tdap and meningococcal) and more must be done to encourage vaccination.
Studies have shown that parents are more likely to vaccinate their children if providers recommend the vaccine.11 As women’s health care providers, we do not always see children during the time period that is ideal for vaccination. However, we take care of many women who are presenting for routine gynecologic care, pregnancy, or with abnormal Pap smear screenings. These are ideal opportunities to educate and offer HPV vaccination to women in the approved age groups, as well as to encourage parents to vaccinate their children.
As with other vaccines, the recommendation should be clear and focused on the cancer prevention benefit. Using methods in which the recommendation is “announced” in a brief statement assuming parents/patients are ready to vaccinate versus open-ended conversations, has been studied as a potentially successful method to increase uptake of HPV vaccination.12 Additionally, documentation of HPV vaccination status should be built into electronic medical record templates to prompt clinicians to ask and offer HPV vaccination at visits, including postpartum visits.
Dr. Rahangdale is an associate professor of ob.gyn. at the University of North Carolina, Chapel Hill, and is director of the North Carolina Women’s Hospital Cervical Dysplasia Clinic. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Epidemiol. 2008 Jul 15;168(2):123-37.
2. Int J Cancer. 2012 Nov 1;131(9):1969-82.
3. BMJ. 2012 Mar 27;344:e1401. doi: 10.1136/bmj.e1401.
4. Gynecol Oncol. 2017 Jun. doi. org/10.1016/j.ygyno.2017.03.031.
5. Int J Prev Med. 2017 Jun 1;8:44. doi: 10.4103/ijpvm.IJPVM_413_16.
6. Gynecol Oncol. 2013 Aug;130(2):264-8.
7. Pediatrics. 2012 Nov;130(5):798-805.
8. JAMA Intern Med. 2015 Apr;175(4):617-23.
9. Clin Pediatr (Phila). 2016 Sep;55(10):904-14.
10. MMWR Morb Mortal Wkly Rep. 2015 Jul 31;64(29):784-92.
11. Vaccine. 2016 Feb 24;34(9):1187-92.
12. Pediatrics. 2017 Jan;139(1). pii:e20161764. doi: 10.1542/peds.2016-1764.