User login
Private Equity in GI
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
‘Less is More’ in Myeloma
Among those that intrigue me most are the pioneering “less is more” trials that challenged conventional practices and remain relevant today. One such trial was inspired by a patient’s dissatisfaction with high doses of dexamethasone and its side effects.
Unlike the prevailing norm of frequent high doses, this trial compared a steroid dose administered weekly (as opposed to doses given several days a week). Lo and behold, the lower steroid dosage was associated with significantly better survival rates. At 1-year follow-up, 96% of patients in the lower-dose group were alive, compared with 87% in the higher-dose group.
Another noteworthy “less-is more” trial that I love, spearheaded by an Italian team, also focused on steroid dosage. This trial investigated discontinuing dexamethasone after nine cycles, along with reducing the dose of lenalidomide, versus maintaining long-term treatment without reductions. The findings revealed comparable progression-free survival with reduced toxicity, highlighting the potential benefits of this less-is-more approach.
While these trials are inspirational, a closer examination of myeloma trial history, especially those that led to regulatory approvals, reveals a preponderance of “add-on” trials. You add a potentially effective drug to an existing backbone, and you get an improvement in an outcome such as response rate (shrinking cancer) or duration of remission or progression free survival (amount of time alive and in remission).
Such trials have led to an abundance of effective options. But these same trials have almost always been a comparison of three drugs versus two drugs, and almost never three drugs versus three. And the drugs are often given continuously, especially the “newer” added drug, without a break. As a result, we are left completely unsure of how to sequence our drugs, and whether a finite course of the new drug would be equivalent to administering that new drug forever.
This problem is not unique to myeloma. Yet it is very apparent in myeloma, because we have been lucky to have so many good drugs (or at least “potentially” good drugs) that make it to phase 3 trials.
Unfortunately, the landscape of clinical trials is heavily influenced by the pharmaceutical industry, with limited funding available from alternative sources. As a result, there is a scarcity of trials exploring “less is more” approaches, despite their potential to optimize treatment outcomes and quality of life.
Even government-funded trials run by cooperative groups require industry buy-in or are run by people who have very close contacts and conflicts of interest with industry. We need so many more of these less-is-more trials, but we have limited means to fund them.
These are the kinds of discussions I have with my patients daily. We grapple with questions about the necessity of lifelong (or any) maintenance therapy or the feasibility of treatment breaks for patients with stable disease. While we strive to provide the best care possible, the lack of definitive data often leaves us making tough decisions in the clinic.
I am grateful to those who are working tirelessly to facilitate trials that prioritize quality of life and “less is more” approaches. Your efforts are invaluable. Looking forward, I aspire to contribute to this important work.
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
Among those that intrigue me most are the pioneering “less is more” trials that challenged conventional practices and remain relevant today. One such trial was inspired by a patient’s dissatisfaction with high doses of dexamethasone and its side effects.
Unlike the prevailing norm of frequent high doses, this trial compared a steroid dose administered weekly (as opposed to doses given several days a week). Lo and behold, the lower steroid dosage was associated with significantly better survival rates. At 1-year follow-up, 96% of patients in the lower-dose group were alive, compared with 87% in the higher-dose group.
Another noteworthy “less-is more” trial that I love, spearheaded by an Italian team, also focused on steroid dosage. This trial investigated discontinuing dexamethasone after nine cycles, along with reducing the dose of lenalidomide, versus maintaining long-term treatment without reductions. The findings revealed comparable progression-free survival with reduced toxicity, highlighting the potential benefits of this less-is-more approach.
While these trials are inspirational, a closer examination of myeloma trial history, especially those that led to regulatory approvals, reveals a preponderance of “add-on” trials. You add a potentially effective drug to an existing backbone, and you get an improvement in an outcome such as response rate (shrinking cancer) or duration of remission or progression free survival (amount of time alive and in remission).
Such trials have led to an abundance of effective options. But these same trials have almost always been a comparison of three drugs versus two drugs, and almost never three drugs versus three. And the drugs are often given continuously, especially the “newer” added drug, without a break. As a result, we are left completely unsure of how to sequence our drugs, and whether a finite course of the new drug would be equivalent to administering that new drug forever.
This problem is not unique to myeloma. Yet it is very apparent in myeloma, because we have been lucky to have so many good drugs (or at least “potentially” good drugs) that make it to phase 3 trials.
Unfortunately, the landscape of clinical trials is heavily influenced by the pharmaceutical industry, with limited funding available from alternative sources. As a result, there is a scarcity of trials exploring “less is more” approaches, despite their potential to optimize treatment outcomes and quality of life.
Even government-funded trials run by cooperative groups require industry buy-in or are run by people who have very close contacts and conflicts of interest with industry. We need so many more of these less-is-more trials, but we have limited means to fund them.
These are the kinds of discussions I have with my patients daily. We grapple with questions about the necessity of lifelong (or any) maintenance therapy or the feasibility of treatment breaks for patients with stable disease. While we strive to provide the best care possible, the lack of definitive data often leaves us making tough decisions in the clinic.
I am grateful to those who are working tirelessly to facilitate trials that prioritize quality of life and “less is more” approaches. Your efforts are invaluable. Looking forward, I aspire to contribute to this important work.
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
Among those that intrigue me most are the pioneering “less is more” trials that challenged conventional practices and remain relevant today. One such trial was inspired by a patient’s dissatisfaction with high doses of dexamethasone and its side effects.
Unlike the prevailing norm of frequent high doses, this trial compared a steroid dose administered weekly (as opposed to doses given several days a week). Lo and behold, the lower steroid dosage was associated with significantly better survival rates. At 1-year follow-up, 96% of patients in the lower-dose group were alive, compared with 87% in the higher-dose group.
Another noteworthy “less-is more” trial that I love, spearheaded by an Italian team, also focused on steroid dosage. This trial investigated discontinuing dexamethasone after nine cycles, along with reducing the dose of lenalidomide, versus maintaining long-term treatment without reductions. The findings revealed comparable progression-free survival with reduced toxicity, highlighting the potential benefits of this less-is-more approach.
While these trials are inspirational, a closer examination of myeloma trial history, especially those that led to regulatory approvals, reveals a preponderance of “add-on” trials. You add a potentially effective drug to an existing backbone, and you get an improvement in an outcome such as response rate (shrinking cancer) or duration of remission or progression free survival (amount of time alive and in remission).
Such trials have led to an abundance of effective options. But these same trials have almost always been a comparison of three drugs versus two drugs, and almost never three drugs versus three. And the drugs are often given continuously, especially the “newer” added drug, without a break. As a result, we are left completely unsure of how to sequence our drugs, and whether a finite course of the new drug would be equivalent to administering that new drug forever.
This problem is not unique to myeloma. Yet it is very apparent in myeloma, because we have been lucky to have so many good drugs (or at least “potentially” good drugs) that make it to phase 3 trials.
Unfortunately, the landscape of clinical trials is heavily influenced by the pharmaceutical industry, with limited funding available from alternative sources. As a result, there is a scarcity of trials exploring “less is more” approaches, despite their potential to optimize treatment outcomes and quality of life.
Even government-funded trials run by cooperative groups require industry buy-in or are run by people who have very close contacts and conflicts of interest with industry. We need so many more of these less-is-more trials, but we have limited means to fund them.
These are the kinds of discussions I have with my patients daily. We grapple with questions about the necessity of lifelong (or any) maintenance therapy or the feasibility of treatment breaks for patients with stable disease. While we strive to provide the best care possible, the lack of definitive data often leaves us making tough decisions in the clinic.
I am grateful to those who are working tirelessly to facilitate trials that prioritize quality of life and “less is more” approaches. Your efforts are invaluable. Looking forward, I aspire to contribute to this important work.
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
It Sure Looks Like Cannabis Is Bad for the Heart, Doesn’t It?
This transcript has been edited for clarity.
If you’re an epidemiologist trying to explore whether some exposure is a risk factor for a disease, you can run into a tough problem when your exposure of interest is highly correlated with another risk factor for the disease. For decades, this stymied investigations into the link, if any, between marijuana use and cardiovascular disease because, for decades, most people who used marijuana in some way also smoked cigarettes — which is a very clear risk factor for heart disease.
But the times they are a-changing.
Thanks to the legalization of marijuana for recreational use in many states, and even broader social trends, there is now a large population of people who use marijuana but do not use cigarettes. That means we can start to determine whether marijuana use is an independent risk factor for heart disease.
And this week, we have the largest study yet to attempt to answer that question, though, as I’ll explain momentarily, the smoke hasn’t entirely cleared yet.
The centerpiece of the study we are discussing this week, “Association of Cannabis Use With Cardiovascular Outcomes Among US Adults,” which appeared in the Journal of the American Heart Association, is the Behavioral Risk Factor Surveillance System, an annual telephone survey conducted by the Centers for Disease Control and Prevention since 1984 that gathers data on all sorts of stuff that we do to ourselves: our drinking habits, our smoking habits, and, more recently, our marijuana habits.
The paper combines annual data from 2016 to 2020 representing 27 states and two US territories for a total sample size of more than 430,000 individuals. The key exposure? Marijuana use, which was coded as the number of days of marijuana use in the past 30 days. The key outcome? Coronary heart disease, collected through questions such as “Has a doctor, nurse, or other health professional ever told you that you had a heart attack?”
Right away you might detect a couple of problems here. But let me show you the results before we worry about what they mean.
You can see the rates of the major cardiovascular outcomes here, stratified by daily use of marijuana, nondaily use, and no use. Broadly speaking, the risk was highest for daily users, lowest for occasional users, and in the middle for non-users.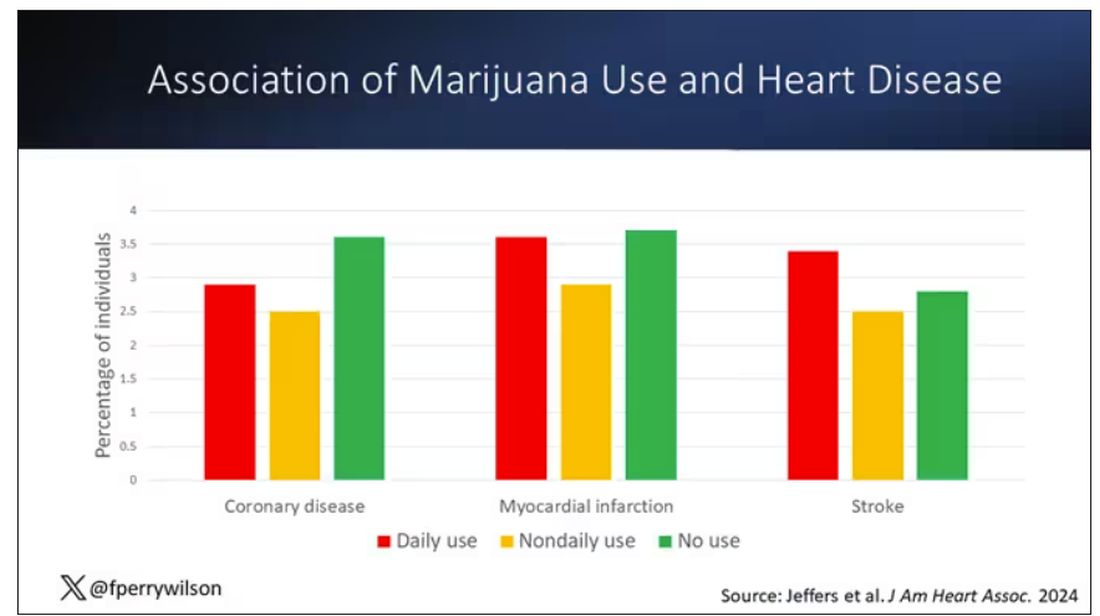
Of course, non-users and users are different in lots of other ways; non-users were quite a bit older, for example. Adjusting for all those factors showed that, independent of age, smoking status, the presence of diabetes, and so on, there was an independently increased risk for cardiovascular outcomes in people who used marijuana.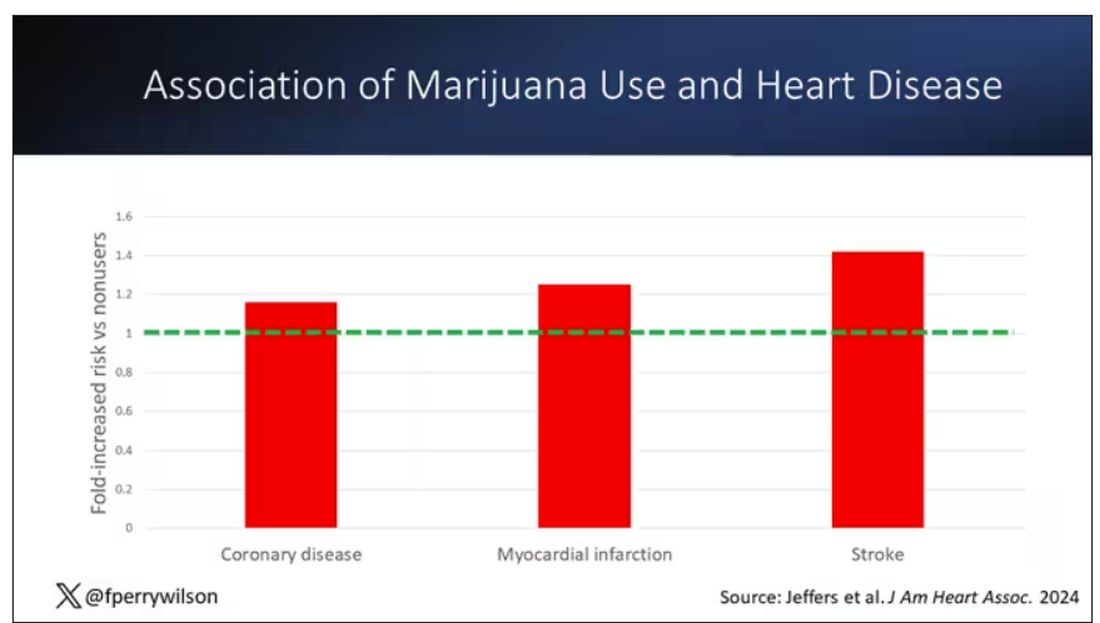
Importantly, 60% of people in this study were never smokers, and the results in that group looked pretty similar to the results overall.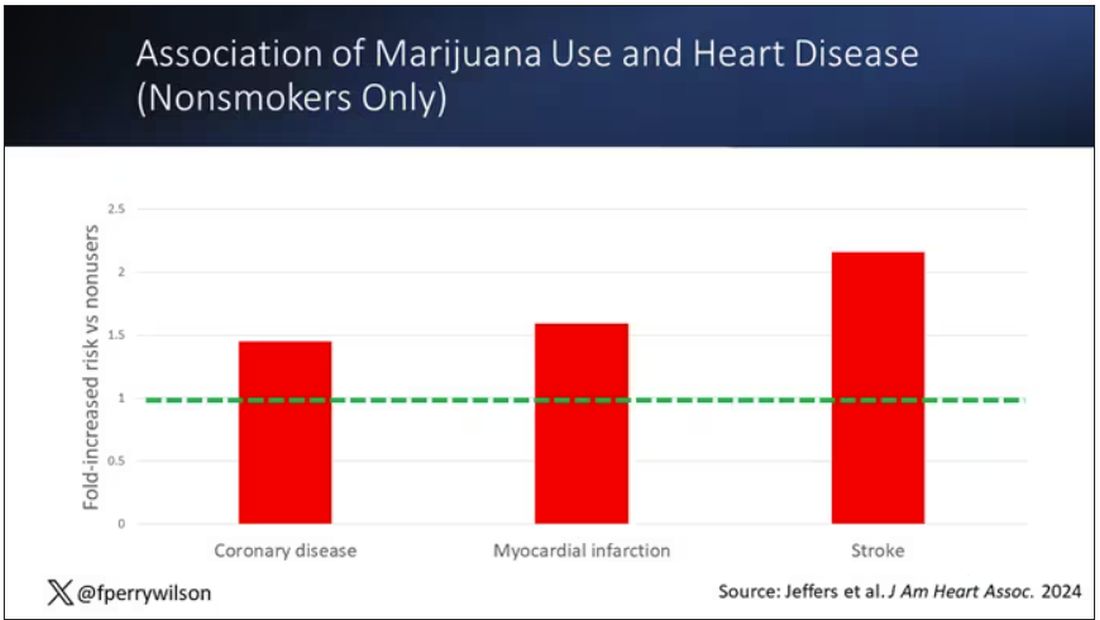
But I said there were a couple of problems, so let’s dig into those a bit.
First, like most survey studies, this one requires honest and accurate reporting from its subjects. There was no verification of heart disease using electronic health records or of marijuana usage based on biosamples. Broadly, miscategorization of exposure and outcomes in surveys tends to bias the results toward the null hypothesis, toward concluding that there is no link between exposure and outcome, so perhaps this is okay.
The bigger problem is the fact that this is a cross-sectional design. If you really wanted to know whether marijuana led to heart disease, you’d do a longitudinal study following users and non-users for some number of decades and see who developed heart disease and who didn’t. (For the pedants out there, I suppose you’d actually want to randomize people to use marijuana or not and then see who had a heart attack, but the IRB keeps rejecting my protocol when I submit it.)
Here, though, we literally can’t tell whether people who use marijuana have more heart attacks or whether people who have heart attacks use more marijuana. The authors argue that there are no data that show that people are more likely to use marijuana after a heart attack or stroke, but at the time the survey was conducted, they had already had their heart attack or stroke.
The authors also imply that they found a dose-response relationship between marijuana use and these cardiovascular outcomes. This is an important statement because dose response is one factor that we use to determine whether a risk factor may actually be causative as opposed to just correlative.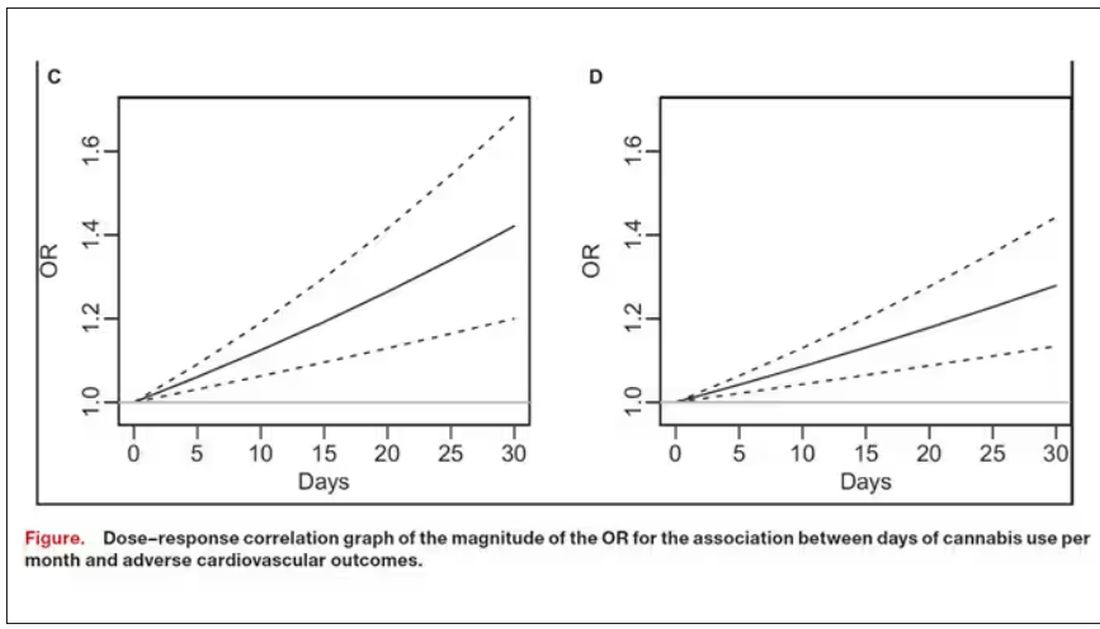
But I take issue with the dose-response language here. The model used to make these graphs classifies marijuana use as a single continuous variable ranging from 0 (no days of use in the past 30 days) to 1 (30 days of use in the past 30 days). The model is thus constrained to monotonically increase or decrease with respect to the outcome. To prove a dose response, you have to give the model the option to find something that isn’t a dose response — for example, by classifying marijuana use into discrete, independent categories rather than a single continuous number.
Am I arguing here that marijuana use is good for you? Of course not. Nor am I even arguing that it has no effect on the cardiovascular system. There are endocannabinoid receptors all over your vasculature. But a cross-sectional survey study, while a good start, is not quite the right way to answer the question. So, while the jury is still out, it’s high time for more research.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re an epidemiologist trying to explore whether some exposure is a risk factor for a disease, you can run into a tough problem when your exposure of interest is highly correlated with another risk factor for the disease. For decades, this stymied investigations into the link, if any, between marijuana use and cardiovascular disease because, for decades, most people who used marijuana in some way also smoked cigarettes — which is a very clear risk factor for heart disease.
But the times they are a-changing.
Thanks to the legalization of marijuana for recreational use in many states, and even broader social trends, there is now a large population of people who use marijuana but do not use cigarettes. That means we can start to determine whether marijuana use is an independent risk factor for heart disease.
And this week, we have the largest study yet to attempt to answer that question, though, as I’ll explain momentarily, the smoke hasn’t entirely cleared yet.
The centerpiece of the study we are discussing this week, “Association of Cannabis Use With Cardiovascular Outcomes Among US Adults,” which appeared in the Journal of the American Heart Association, is the Behavioral Risk Factor Surveillance System, an annual telephone survey conducted by the Centers for Disease Control and Prevention since 1984 that gathers data on all sorts of stuff that we do to ourselves: our drinking habits, our smoking habits, and, more recently, our marijuana habits.
The paper combines annual data from 2016 to 2020 representing 27 states and two US territories for a total sample size of more than 430,000 individuals. The key exposure? Marijuana use, which was coded as the number of days of marijuana use in the past 30 days. The key outcome? Coronary heart disease, collected through questions such as “Has a doctor, nurse, or other health professional ever told you that you had a heart attack?”
Right away you might detect a couple of problems here. But let me show you the results before we worry about what they mean.
You can see the rates of the major cardiovascular outcomes here, stratified by daily use of marijuana, nondaily use, and no use. Broadly speaking, the risk was highest for daily users, lowest for occasional users, and in the middle for non-users.
Of course, non-users and users are different in lots of other ways; non-users were quite a bit older, for example. Adjusting for all those factors showed that, independent of age, smoking status, the presence of diabetes, and so on, there was an independently increased risk for cardiovascular outcomes in people who used marijuana.
Importantly, 60% of people in this study were never smokers, and the results in that group looked pretty similar to the results overall.
But I said there were a couple of problems, so let’s dig into those a bit.
First, like most survey studies, this one requires honest and accurate reporting from its subjects. There was no verification of heart disease using electronic health records or of marijuana usage based on biosamples. Broadly, miscategorization of exposure and outcomes in surveys tends to bias the results toward the null hypothesis, toward concluding that there is no link between exposure and outcome, so perhaps this is okay.
The bigger problem is the fact that this is a cross-sectional design. If you really wanted to know whether marijuana led to heart disease, you’d do a longitudinal study following users and non-users for some number of decades and see who developed heart disease and who didn’t. (For the pedants out there, I suppose you’d actually want to randomize people to use marijuana or not and then see who had a heart attack, but the IRB keeps rejecting my protocol when I submit it.)
Here, though, we literally can’t tell whether people who use marijuana have more heart attacks or whether people who have heart attacks use more marijuana. The authors argue that there are no data that show that people are more likely to use marijuana after a heart attack or stroke, but at the time the survey was conducted, they had already had their heart attack or stroke.
The authors also imply that they found a dose-response relationship between marijuana use and these cardiovascular outcomes. This is an important statement because dose response is one factor that we use to determine whether a risk factor may actually be causative as opposed to just correlative.
But I take issue with the dose-response language here. The model used to make these graphs classifies marijuana use as a single continuous variable ranging from 0 (no days of use in the past 30 days) to 1 (30 days of use in the past 30 days). The model is thus constrained to monotonically increase or decrease with respect to the outcome. To prove a dose response, you have to give the model the option to find something that isn’t a dose response — for example, by classifying marijuana use into discrete, independent categories rather than a single continuous number.
Am I arguing here that marijuana use is good for you? Of course not. Nor am I even arguing that it has no effect on the cardiovascular system. There are endocannabinoid receptors all over your vasculature. But a cross-sectional survey study, while a good start, is not quite the right way to answer the question. So, while the jury is still out, it’s high time for more research.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re an epidemiologist trying to explore whether some exposure is a risk factor for a disease, you can run into a tough problem when your exposure of interest is highly correlated with another risk factor for the disease. For decades, this stymied investigations into the link, if any, between marijuana use and cardiovascular disease because, for decades, most people who used marijuana in some way also smoked cigarettes — which is a very clear risk factor for heart disease.
But the times they are a-changing.
Thanks to the legalization of marijuana for recreational use in many states, and even broader social trends, there is now a large population of people who use marijuana but do not use cigarettes. That means we can start to determine whether marijuana use is an independent risk factor for heart disease.
And this week, we have the largest study yet to attempt to answer that question, though, as I’ll explain momentarily, the smoke hasn’t entirely cleared yet.
The centerpiece of the study we are discussing this week, “Association of Cannabis Use With Cardiovascular Outcomes Among US Adults,” which appeared in the Journal of the American Heart Association, is the Behavioral Risk Factor Surveillance System, an annual telephone survey conducted by the Centers for Disease Control and Prevention since 1984 that gathers data on all sorts of stuff that we do to ourselves: our drinking habits, our smoking habits, and, more recently, our marijuana habits.
The paper combines annual data from 2016 to 2020 representing 27 states and two US territories for a total sample size of more than 430,000 individuals. The key exposure? Marijuana use, which was coded as the number of days of marijuana use in the past 30 days. The key outcome? Coronary heart disease, collected through questions such as “Has a doctor, nurse, or other health professional ever told you that you had a heart attack?”
Right away you might detect a couple of problems here. But let me show you the results before we worry about what they mean.
You can see the rates of the major cardiovascular outcomes here, stratified by daily use of marijuana, nondaily use, and no use. Broadly speaking, the risk was highest for daily users, lowest for occasional users, and in the middle for non-users.
Of course, non-users and users are different in lots of other ways; non-users were quite a bit older, for example. Adjusting for all those factors showed that, independent of age, smoking status, the presence of diabetes, and so on, there was an independently increased risk for cardiovascular outcomes in people who used marijuana.
Importantly, 60% of people in this study were never smokers, and the results in that group looked pretty similar to the results overall.
But I said there were a couple of problems, so let’s dig into those a bit.
First, like most survey studies, this one requires honest and accurate reporting from its subjects. There was no verification of heart disease using electronic health records or of marijuana usage based on biosamples. Broadly, miscategorization of exposure and outcomes in surveys tends to bias the results toward the null hypothesis, toward concluding that there is no link between exposure and outcome, so perhaps this is okay.
The bigger problem is the fact that this is a cross-sectional design. If you really wanted to know whether marijuana led to heart disease, you’d do a longitudinal study following users and non-users for some number of decades and see who developed heart disease and who didn’t. (For the pedants out there, I suppose you’d actually want to randomize people to use marijuana or not and then see who had a heart attack, but the IRB keeps rejecting my protocol when I submit it.)
Here, though, we literally can’t tell whether people who use marijuana have more heart attacks or whether people who have heart attacks use more marijuana. The authors argue that there are no data that show that people are more likely to use marijuana after a heart attack or stroke, but at the time the survey was conducted, they had already had their heart attack or stroke.
The authors also imply that they found a dose-response relationship between marijuana use and these cardiovascular outcomes. This is an important statement because dose response is one factor that we use to determine whether a risk factor may actually be causative as opposed to just correlative.
But I take issue with the dose-response language here. The model used to make these graphs classifies marijuana use as a single continuous variable ranging from 0 (no days of use in the past 30 days) to 1 (30 days of use in the past 30 days). The model is thus constrained to monotonically increase or decrease with respect to the outcome. To prove a dose response, you have to give the model the option to find something that isn’t a dose response — for example, by classifying marijuana use into discrete, independent categories rather than a single continuous number.
Am I arguing here that marijuana use is good for you? Of course not. Nor am I even arguing that it has no effect on the cardiovascular system. There are endocannabinoid receptors all over your vasculature. But a cross-sectional survey study, while a good start, is not quite the right way to answer the question. So, while the jury is still out, it’s high time for more research.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Oxaliplatin in Older Adults With Resected Colorectal Cancer: Is There a Benefit?
This transcript has been edited for clarity.
Our group, the QUASAR Group, made a very significant contribution in terms of trials and knowledge in this field. One of the things that we still need to discuss and think about in our wider community is the impact of adjuvant chemotherapy in older patients.
Colorectal cancer is a disease of the elderly, with the median age of presentation around 72. We know that, at presentation, more than 50% of patients are aged 65 or over and one third of patients are 75 or over. It’s a disease predominantly of the elderly. Are we justified in giving combination chemotherapy with oxaliplatin to high-risk resected colorectal cancer patients?
There’s a very nice report of a meta-analysis by Dottorini and colleagues that came out recently in the Journal of Clinical Oncology. It’s an excellent group. They did their meta-analytical work according to a strict, rational protocol. Their statistical analyses were on point, and they collected data from all the relevant trials.
According to the results of their study, it could be concluded that the addition of oxaliplatin to adjuvant therapy for resected high-risk colorectal cancer in older patients — patients older than 70 — doesn’t result in any statistically significant gain in terms of preventing recurrences or saving lives.
When we did our QUASAR trials, initially we were looking at control vs fluoropyrimidine chemotherapy. Although there was an overall impact on survival of the whole trial group (the 5000 patients in our study), when we looked by decile, there was a significant diminution of benefit even to fluoropyrimidine therapy in our trial in patients aged 70 or above. I think this careful meta-analysis must make us question the use of oxaliplatin in elderly patients.
What could the explanation be? Why could the well-known and described benefits of oxaliplatin, particularly for stage III disease, attenuate in older people? It may be to do with reduction in dose intensity. Older people have more side effects; therefore, the chemotherapy isn’t completed as planned. Although, increasingly these days, we tend only to be giving 3 months of treatment.
Is there something biologically in terms of the biology or the somatic mutational landscape of the tumor in older people? I don’t think so. Certainly, in terms of their capacity, in terms of stem cell reserve to be as resistant to the side effects of chemotherapy as younger people, we know that does attenuate with age.
Food for thought: The majority of patients I see in the clinic for the adjuvant treatment are elderly. The majority are coming these days with high-risk stage II or stage III disease. There is a real question mark about whether we should be using oxaliplatin at all.
Clearly, one would say that we need more trials of chemotherapy in older folk to see if the addition of drugs like oxaliplatin to a fluoropyrimidine backbone really does make a difference. I’ve said many times before that we, the medical community recommending adjuvant treatment, need to have better risk stratifiers. We need to have better prognostic markers. We need to have better indices that would allow us to perhaps consider these combination treatments in a more focused group of patients who may have a higher risk for recurrence.
Have a look at the paper and see what you think. I think it’s well done. It’s certainly given me pause for thought about the treatment that we will offer our elderly patients.
Have a think about it. Let me know if there are any comments that you’d like to make. For the time being, over and out.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom. He disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our group, the QUASAR Group, made a very significant contribution in terms of trials and knowledge in this field. One of the things that we still need to discuss and think about in our wider community is the impact of adjuvant chemotherapy in older patients.
Colorectal cancer is a disease of the elderly, with the median age of presentation around 72. We know that, at presentation, more than 50% of patients are aged 65 or over and one third of patients are 75 or over. It’s a disease predominantly of the elderly. Are we justified in giving combination chemotherapy with oxaliplatin to high-risk resected colorectal cancer patients?
There’s a very nice report of a meta-analysis by Dottorini and colleagues that came out recently in the Journal of Clinical Oncology. It’s an excellent group. They did their meta-analytical work according to a strict, rational protocol. Their statistical analyses were on point, and they collected data from all the relevant trials.
According to the results of their study, it could be concluded that the addition of oxaliplatin to adjuvant therapy for resected high-risk colorectal cancer in older patients — patients older than 70 — doesn’t result in any statistically significant gain in terms of preventing recurrences or saving lives.
When we did our QUASAR trials, initially we were looking at control vs fluoropyrimidine chemotherapy. Although there was an overall impact on survival of the whole trial group (the 5000 patients in our study), when we looked by decile, there was a significant diminution of benefit even to fluoropyrimidine therapy in our trial in patients aged 70 or above. I think this careful meta-analysis must make us question the use of oxaliplatin in elderly patients.
What could the explanation be? Why could the well-known and described benefits of oxaliplatin, particularly for stage III disease, attenuate in older people? It may be to do with reduction in dose intensity. Older people have more side effects; therefore, the chemotherapy isn’t completed as planned. Although, increasingly these days, we tend only to be giving 3 months of treatment.
Is there something biologically in terms of the biology or the somatic mutational landscape of the tumor in older people? I don’t think so. Certainly, in terms of their capacity, in terms of stem cell reserve to be as resistant to the side effects of chemotherapy as younger people, we know that does attenuate with age.
Food for thought: The majority of patients I see in the clinic for the adjuvant treatment are elderly. The majority are coming these days with high-risk stage II or stage III disease. There is a real question mark about whether we should be using oxaliplatin at all.
Clearly, one would say that we need more trials of chemotherapy in older folk to see if the addition of drugs like oxaliplatin to a fluoropyrimidine backbone really does make a difference. I’ve said many times before that we, the medical community recommending adjuvant treatment, need to have better risk stratifiers. We need to have better prognostic markers. We need to have better indices that would allow us to perhaps consider these combination treatments in a more focused group of patients who may have a higher risk for recurrence.
Have a look at the paper and see what you think. I think it’s well done. It’s certainly given me pause for thought about the treatment that we will offer our elderly patients.
Have a think about it. Let me know if there are any comments that you’d like to make. For the time being, over and out.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom. He disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our group, the QUASAR Group, made a very significant contribution in terms of trials and knowledge in this field. One of the things that we still need to discuss and think about in our wider community is the impact of adjuvant chemotherapy in older patients.
Colorectal cancer is a disease of the elderly, with the median age of presentation around 72. We know that, at presentation, more than 50% of patients are aged 65 or over and one third of patients are 75 or over. It’s a disease predominantly of the elderly. Are we justified in giving combination chemotherapy with oxaliplatin to high-risk resected colorectal cancer patients?
There’s a very nice report of a meta-analysis by Dottorini and colleagues that came out recently in the Journal of Clinical Oncology. It’s an excellent group. They did their meta-analytical work according to a strict, rational protocol. Their statistical analyses were on point, and they collected data from all the relevant trials.
According to the results of their study, it could be concluded that the addition of oxaliplatin to adjuvant therapy for resected high-risk colorectal cancer in older patients — patients older than 70 — doesn’t result in any statistically significant gain in terms of preventing recurrences or saving lives.
When we did our QUASAR trials, initially we were looking at control vs fluoropyrimidine chemotherapy. Although there was an overall impact on survival of the whole trial group (the 5000 patients in our study), when we looked by decile, there was a significant diminution of benefit even to fluoropyrimidine therapy in our trial in patients aged 70 or above. I think this careful meta-analysis must make us question the use of oxaliplatin in elderly patients.
What could the explanation be? Why could the well-known and described benefits of oxaliplatin, particularly for stage III disease, attenuate in older people? It may be to do with reduction in dose intensity. Older people have more side effects; therefore, the chemotherapy isn’t completed as planned. Although, increasingly these days, we tend only to be giving 3 months of treatment.
Is there something biologically in terms of the biology or the somatic mutational landscape of the tumor in older people? I don’t think so. Certainly, in terms of their capacity, in terms of stem cell reserve to be as resistant to the side effects of chemotherapy as younger people, we know that does attenuate with age.
Food for thought: The majority of patients I see in the clinic for the adjuvant treatment are elderly. The majority are coming these days with high-risk stage II or stage III disease. There is a real question mark about whether we should be using oxaliplatin at all.
Clearly, one would say that we need more trials of chemotherapy in older folk to see if the addition of drugs like oxaliplatin to a fluoropyrimidine backbone really does make a difference. I’ve said many times before that we, the medical community recommending adjuvant treatment, need to have better risk stratifiers. We need to have better prognostic markers. We need to have better indices that would allow us to perhaps consider these combination treatments in a more focused group of patients who may have a higher risk for recurrence.
Have a look at the paper and see what you think. I think it’s well done. It’s certainly given me pause for thought about the treatment that we will offer our elderly patients.
Have a think about it. Let me know if there are any comments that you’d like to make. For the time being, over and out.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom. He disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
Poor Quality of Cancer Content on Social Media
This transcript has been edited for clarity.
I’m delighted to talk about a very interesting topic in this commentary. This is an area that we generally don’t discuss, but it’s one that’s obviously very topical, which includes the question of social media.
The paper I’m referring to is entitled, “More Than a Song and Dance”: Exploration of Patient Perspectives and Educational Quality of Gynecologic Cancer Content on TikTok. The paper was published in Gynecologic Oncology in 2023.
They had a total of 466.7 million views. They looked at 430 of the 500 top posts that were eligible, looked at 11 central themes, did an objective analysis of educational content based on published strategy for looking at this.
What they found, unfortunately but not surprisingly, overall was that the educational quality and reliability were quite poor. They also noticed considerable differences in disparities based on racial background and really emphasized in their analysis not only how common it is for individuals to look at this content on TikTok but also concerns about what it is that the public, patients, and their families are actually seeing.
This, of course, specifically relates to gynecologic cancers, but almost certainly relates to other cancers as well. Clearly, this is a topic that needs to be discussed widely. It’s very complex and very controversial, but when you think about the information that might be provided to our patients and their families going to social media, it’s important that we understand what they’re seeing, what they’re hearing, what they’re viewing, and the impact this might have on their care and outcomes.
I encourage you to read this very interesting paper if you have an interest in this topic. Again, it was recently published in Gynecologic Oncology. I thank you for your attention.
Dr. Markman is professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California; president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m delighted to talk about a very interesting topic in this commentary. This is an area that we generally don’t discuss, but it’s one that’s obviously very topical, which includes the question of social media.
The paper I’m referring to is entitled, “More Than a Song and Dance”: Exploration of Patient Perspectives and Educational Quality of Gynecologic Cancer Content on TikTok. The paper was published in Gynecologic Oncology in 2023.
They had a total of 466.7 million views. They looked at 430 of the 500 top posts that were eligible, looked at 11 central themes, did an objective analysis of educational content based on published strategy for looking at this.
What they found, unfortunately but not surprisingly, overall was that the educational quality and reliability were quite poor. They also noticed considerable differences in disparities based on racial background and really emphasized in their analysis not only how common it is for individuals to look at this content on TikTok but also concerns about what it is that the public, patients, and their families are actually seeing.
This, of course, specifically relates to gynecologic cancers, but almost certainly relates to other cancers as well. Clearly, this is a topic that needs to be discussed widely. It’s very complex and very controversial, but when you think about the information that might be provided to our patients and their families going to social media, it’s important that we understand what they’re seeing, what they’re hearing, what they’re viewing, and the impact this might have on their care and outcomes.
I encourage you to read this very interesting paper if you have an interest in this topic. Again, it was recently published in Gynecologic Oncology. I thank you for your attention.
Dr. Markman is professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California; president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m delighted to talk about a very interesting topic in this commentary. This is an area that we generally don’t discuss, but it’s one that’s obviously very topical, which includes the question of social media.
The paper I’m referring to is entitled, “More Than a Song and Dance”: Exploration of Patient Perspectives and Educational Quality of Gynecologic Cancer Content on TikTok. The paper was published in Gynecologic Oncology in 2023.
They had a total of 466.7 million views. They looked at 430 of the 500 top posts that were eligible, looked at 11 central themes, did an objective analysis of educational content based on published strategy for looking at this.
What they found, unfortunately but not surprisingly, overall was that the educational quality and reliability were quite poor. They also noticed considerable differences in disparities based on racial background and really emphasized in their analysis not only how common it is for individuals to look at this content on TikTok but also concerns about what it is that the public, patients, and their families are actually seeing.
This, of course, specifically relates to gynecologic cancers, but almost certainly relates to other cancers as well. Clearly, this is a topic that needs to be discussed widely. It’s very complex and very controversial, but when you think about the information that might be provided to our patients and their families going to social media, it’s important that we understand what they’re seeing, what they’re hearing, what they’re viewing, and the impact this might have on their care and outcomes.
I encourage you to read this very interesting paper if you have an interest in this topic. Again, it was recently published in Gynecologic Oncology. I thank you for your attention.
Dr. Markman is professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California; president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
Bent but Not Broken: The Truth About Penile Curvature
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. This is Sex Matters, and I’m here today with my friend and colleague, Dr. Matt Ziegelmann, who is the sexual medicine expert at the Mayo Clinic and who does all things men’s health, including penile curvature, testosterone, and sexual function.
Matthew J. Ziegelmann, MD: Penile curvature (often due to Peyronie’s disease) is actually incredibly common; as many as 10% of men have this condition. We need to normalize it and let men know that this is something we see often, and we have treatments for it. One of the biggest concerns these men have is cancer, but I have yet to see this as an indicator of cancer.
Penile curvature has a significant impact on affected men — their relationships, psychological well-being, sexual functioning, and overall health. We can provide treatment if they are interested. A small subset of men are born with natural penile curvature, which is different from Peyronie’s disease. Natural curvature can still affect their mental health, and we have treatment options.
Rubin: What happens when a patient is referred to urology? We want to tell our patients what to expect. What’s in our toolbox to help patients with penile curvature?
Dr. Ziegelmann: Many patient resources are available. For example, the Sexual Medicine Society of North America (SMSNA) has a patient-facing website on sexual health with lots of information about Peyronie’s disease and other aspects of sexual health.
Patients who are bothered by their penile curvature can be referred to us to find out about treatment options, or even just to get reassurance. It might be as simple as a conversation and a physical exam — that’s all we need to make the diagnosis. We can provide reassurance and get an idea of how bothered they are by this condition without doing anything invasive.
If they are considering definitive treatment, we would need to do more invasive testing. Sometimes we have the patient bring in photos of their erection to help establish the change they see in the shape of their penis.
Dr. Rubin: What about the patient who asks, “Doc, did I do this to myself? Did I break my penis? What do I do?”
Dr. Ziegelmann: That’s a common question — “How the heck did this happen?” No, you didn’t do this to yourself. We still have much to understand about why this happens to some men. Our approach is to acknowledge what we do and don’t know, and partner with the patient to discuss treatment.
Dr. Rubin: It’s very important to support your patient’s mental health, because this can be really devastating. So, what are the treatment options from conservative to invasive? What do you recommend for patients?
Dr. Ziegelmann: It can be as simple as observation if the patient is just seeking reassurance that it’s not cancer. This is a benign condition, but for men who are more bothered by their curvature, we’ll talk about options. We have oral medications that help improved the rigidity of the penis. Many men are also suffering from inadequate functioning. We can use devices called traction or vacuum to stretch the area of the penis that’s curved. We can use injections of medications, including FDA-approved agents that are injected into the penis in the outpatient setting. For men who are either later in the treatment protocol or want to resolve the problem right away, we can offer surgical intervention. We have a host of options, and a very individualized approach and a shared decision-making model. It’s not a one-size-fits-all problem.
Dr. Rubin: The real takeaway is that there is a lot of hope for this condition. Many doctors care deeply about these issues and are ready to partner with specialists to figure out the right treatment strategy. The SMSNA is a great place to find a provider like Dr Ziegelmann or myself, or any of our incredible colleagues throughout North America and the world. Thank you for joining us today.
Dr. Rubin is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. This is Sex Matters, and I’m here today with my friend and colleague, Dr. Matt Ziegelmann, who is the sexual medicine expert at the Mayo Clinic and who does all things men’s health, including penile curvature, testosterone, and sexual function.
Matthew J. Ziegelmann, MD: Penile curvature (often due to Peyronie’s disease) is actually incredibly common; as many as 10% of men have this condition. We need to normalize it and let men know that this is something we see often, and we have treatments for it. One of the biggest concerns these men have is cancer, but I have yet to see this as an indicator of cancer.
Penile curvature has a significant impact on affected men — their relationships, psychological well-being, sexual functioning, and overall health. We can provide treatment if they are interested. A small subset of men are born with natural penile curvature, which is different from Peyronie’s disease. Natural curvature can still affect their mental health, and we have treatment options.
Rubin: What happens when a patient is referred to urology? We want to tell our patients what to expect. What’s in our toolbox to help patients with penile curvature?
Dr. Ziegelmann: Many patient resources are available. For example, the Sexual Medicine Society of North America (SMSNA) has a patient-facing website on sexual health with lots of information about Peyronie’s disease and other aspects of sexual health.
Patients who are bothered by their penile curvature can be referred to us to find out about treatment options, or even just to get reassurance. It might be as simple as a conversation and a physical exam — that’s all we need to make the diagnosis. We can provide reassurance and get an idea of how bothered they are by this condition without doing anything invasive.
If they are considering definitive treatment, we would need to do more invasive testing. Sometimes we have the patient bring in photos of their erection to help establish the change they see in the shape of their penis.
Dr. Rubin: What about the patient who asks, “Doc, did I do this to myself? Did I break my penis? What do I do?”
Dr. Ziegelmann: That’s a common question — “How the heck did this happen?” No, you didn’t do this to yourself. We still have much to understand about why this happens to some men. Our approach is to acknowledge what we do and don’t know, and partner with the patient to discuss treatment.
Dr. Rubin: It’s very important to support your patient’s mental health, because this can be really devastating. So, what are the treatment options from conservative to invasive? What do you recommend for patients?
Dr. Ziegelmann: It can be as simple as observation if the patient is just seeking reassurance that it’s not cancer. This is a benign condition, but for men who are more bothered by their curvature, we’ll talk about options. We have oral medications that help improved the rigidity of the penis. Many men are also suffering from inadequate functioning. We can use devices called traction or vacuum to stretch the area of the penis that’s curved. We can use injections of medications, including FDA-approved agents that are injected into the penis in the outpatient setting. For men who are either later in the treatment protocol or want to resolve the problem right away, we can offer surgical intervention. We have a host of options, and a very individualized approach and a shared decision-making model. It’s not a one-size-fits-all problem.
Dr. Rubin: The real takeaway is that there is a lot of hope for this condition. Many doctors care deeply about these issues and are ready to partner with specialists to figure out the right treatment strategy. The SMSNA is a great place to find a provider like Dr Ziegelmann or myself, or any of our incredible colleagues throughout North America and the world. Thank you for joining us today.
Dr. Rubin is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. This is Sex Matters, and I’m here today with my friend and colleague, Dr. Matt Ziegelmann, who is the sexual medicine expert at the Mayo Clinic and who does all things men’s health, including penile curvature, testosterone, and sexual function.
Matthew J. Ziegelmann, MD: Penile curvature (often due to Peyronie’s disease) is actually incredibly common; as many as 10% of men have this condition. We need to normalize it and let men know that this is something we see often, and we have treatments for it. One of the biggest concerns these men have is cancer, but I have yet to see this as an indicator of cancer.
Penile curvature has a significant impact on affected men — their relationships, psychological well-being, sexual functioning, and overall health. We can provide treatment if they are interested. A small subset of men are born with natural penile curvature, which is different from Peyronie’s disease. Natural curvature can still affect their mental health, and we have treatment options.
Rubin: What happens when a patient is referred to urology? We want to tell our patients what to expect. What’s in our toolbox to help patients with penile curvature?
Dr. Ziegelmann: Many patient resources are available. For example, the Sexual Medicine Society of North America (SMSNA) has a patient-facing website on sexual health with lots of information about Peyronie’s disease and other aspects of sexual health.
Patients who are bothered by their penile curvature can be referred to us to find out about treatment options, or even just to get reassurance. It might be as simple as a conversation and a physical exam — that’s all we need to make the diagnosis. We can provide reassurance and get an idea of how bothered they are by this condition without doing anything invasive.
If they are considering definitive treatment, we would need to do more invasive testing. Sometimes we have the patient bring in photos of their erection to help establish the change they see in the shape of their penis.
Dr. Rubin: What about the patient who asks, “Doc, did I do this to myself? Did I break my penis? What do I do?”
Dr. Ziegelmann: That’s a common question — “How the heck did this happen?” No, you didn’t do this to yourself. We still have much to understand about why this happens to some men. Our approach is to acknowledge what we do and don’t know, and partner with the patient to discuss treatment.
Dr. Rubin: It’s very important to support your patient’s mental health, because this can be really devastating. So, what are the treatment options from conservative to invasive? What do you recommend for patients?
Dr. Ziegelmann: It can be as simple as observation if the patient is just seeking reassurance that it’s not cancer. This is a benign condition, but for men who are more bothered by their curvature, we’ll talk about options. We have oral medications that help improved the rigidity of the penis. Many men are also suffering from inadequate functioning. We can use devices called traction or vacuum to stretch the area of the penis that’s curved. We can use injections of medications, including FDA-approved agents that are injected into the penis in the outpatient setting. For men who are either later in the treatment protocol or want to resolve the problem right away, we can offer surgical intervention. We have a host of options, and a very individualized approach and a shared decision-making model. It’s not a one-size-fits-all problem.
Dr. Rubin: The real takeaway is that there is a lot of hope for this condition. Many doctors care deeply about these issues and are ready to partner with specialists to figure out the right treatment strategy. The SMSNA is a great place to find a provider like Dr Ziegelmann or myself, or any of our incredible colleagues throughout North America and the world. Thank you for joining us today.
Dr. Rubin is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
CRC: Troubling Mortality Rates for a Preventable Cancer
This transcript has been edited for clarity.
The American Cancer Society has just published its cancer statistics for 2024. This is an annual report, the latest version of which has some alarming news for gastroenterologists. Usually, we think of being “number one” as a positive thing, but that’s not the case this year when it comes to the projections for colorectal cancer.
But first, let’s discuss the report’s overall findings. That decline over the past four decades is due to reductions in smoking, earlier detection, and improved screening and treatments for localized or metastatic disease. But these gains are now threatened by some offsets that we’re seeing, with increasing rates of six of the top 10 cancers in the past several years.
Increasing Rates of Gastrointestinal Cancers
The incidence rate of pancreas cancer has increased from 0.6% to 1% annually.
Pancreas cancer has a 5-year relative survival rate of 13%, which ranks as one of the three worst rates for cancers. This cancer represents a real screening challenge for us, as it typically presents asymptomatically.
Women have experienced a 2%-3% annual increase in incidence rates for liver cancer.
I suspect that this is due to cases of fibrotic liver disease resulting from viral hepatitis and metabolic liver diseases with nonalcoholic fatty liver and advanced fibrosis (F3 and F4). These cases may be carried over from before, thereby contributing to the increasing incremental cancer risk.
We can’t overlook the need for risk reduction here and should focus on applying regular screening efforts in our female patients. However, it’s also true that we require better liver cancer screening tests to accomplish that goal.
In Those Under 50, CRC the Leading Cause of Cancer Death in Men, Second in Women
I really want to focus on the news around colorectal cancer.
To put this in perspective, in the late 1990s, colorectal cancer was the fourth leading cause of death in men and women. The current report extrapolated 2024 projections using the Surveillance, Epidemiology, and End Results (SEER) database ending in 2020, which was necessary given the incremental time it takes to develop cancers. The SEER database suggests that in 2024, colorectal cancer in those younger than 50 years of age will become the number-one leading cause of cancer death in men and number-two in women. The increasing incidence of colorectal cancer in younger people is probably the result of a number of epidemiologic and other reasons.
The current report offers evidence of racial disparities in cancer mortality rates in general, which are twofold higher in Black people compared with White people, particularly for gastric cancer. There is also an evident disparity in Native Americans, who have higher rates of gastric and liver cancer. This is a reminder of the increasing need for equity to address racial disparities across these populations.
But returning to colon cancer, it’s a marked change to go from being the fourth-leading cause of cancer death in those younger than 50 years of age to being number one for men and number two for women.
Being “number one” is supposed to make you famous. This “number one,” however, should in fact be infamous. It’s a travesty, because colorectal cancer is a potentially preventable disease.
As we move into March, which happens to be Colorectal Cancer Awareness Month, hopefully this fires up some of the conversations you have with your younger at-risk population, who may be reticent or resistant to colorectal cancer screening.
We have to do better at getting this message out to that population at large. “Number one” is not where we want to be for this potentially preventable problem.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
The American Cancer Society has just published its cancer statistics for 2024. This is an annual report, the latest version of which has some alarming news for gastroenterologists. Usually, we think of being “number one” as a positive thing, but that’s not the case this year when it comes to the projections for colorectal cancer.
But first, let’s discuss the report’s overall findings. That decline over the past four decades is due to reductions in smoking, earlier detection, and improved screening and treatments for localized or metastatic disease. But these gains are now threatened by some offsets that we’re seeing, with increasing rates of six of the top 10 cancers in the past several years.
Increasing Rates of Gastrointestinal Cancers
The incidence rate of pancreas cancer has increased from 0.6% to 1% annually.
Pancreas cancer has a 5-year relative survival rate of 13%, which ranks as one of the three worst rates for cancers. This cancer represents a real screening challenge for us, as it typically presents asymptomatically.
Women have experienced a 2%-3% annual increase in incidence rates for liver cancer.
I suspect that this is due to cases of fibrotic liver disease resulting from viral hepatitis and metabolic liver diseases with nonalcoholic fatty liver and advanced fibrosis (F3 and F4). These cases may be carried over from before, thereby contributing to the increasing incremental cancer risk.
We can’t overlook the need for risk reduction here and should focus on applying regular screening efforts in our female patients. However, it’s also true that we require better liver cancer screening tests to accomplish that goal.
In Those Under 50, CRC the Leading Cause of Cancer Death in Men, Second in Women
I really want to focus on the news around colorectal cancer.
To put this in perspective, in the late 1990s, colorectal cancer was the fourth leading cause of death in men and women. The current report extrapolated 2024 projections using the Surveillance, Epidemiology, and End Results (SEER) database ending in 2020, which was necessary given the incremental time it takes to develop cancers. The SEER database suggests that in 2024, colorectal cancer in those younger than 50 years of age will become the number-one leading cause of cancer death in men and number-two in women. The increasing incidence of colorectal cancer in younger people is probably the result of a number of epidemiologic and other reasons.
The current report offers evidence of racial disparities in cancer mortality rates in general, which are twofold higher in Black people compared with White people, particularly for gastric cancer. There is also an evident disparity in Native Americans, who have higher rates of gastric and liver cancer. This is a reminder of the increasing need for equity to address racial disparities across these populations.
But returning to colon cancer, it’s a marked change to go from being the fourth-leading cause of cancer death in those younger than 50 years of age to being number one for men and number two for women.
Being “number one” is supposed to make you famous. This “number one,” however, should in fact be infamous. It’s a travesty, because colorectal cancer is a potentially preventable disease.
As we move into March, which happens to be Colorectal Cancer Awareness Month, hopefully this fires up some of the conversations you have with your younger at-risk population, who may be reticent or resistant to colorectal cancer screening.
We have to do better at getting this message out to that population at large. “Number one” is not where we want to be for this potentially preventable problem.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
The American Cancer Society has just published its cancer statistics for 2024. This is an annual report, the latest version of which has some alarming news for gastroenterologists. Usually, we think of being “number one” as a positive thing, but that’s not the case this year when it comes to the projections for colorectal cancer.
But first, let’s discuss the report’s overall findings. That decline over the past four decades is due to reductions in smoking, earlier detection, and improved screening and treatments for localized or metastatic disease. But these gains are now threatened by some offsets that we’re seeing, with increasing rates of six of the top 10 cancers in the past several years.
Increasing Rates of Gastrointestinal Cancers
The incidence rate of pancreas cancer has increased from 0.6% to 1% annually.
Pancreas cancer has a 5-year relative survival rate of 13%, which ranks as one of the three worst rates for cancers. This cancer represents a real screening challenge for us, as it typically presents asymptomatically.
Women have experienced a 2%-3% annual increase in incidence rates for liver cancer.
I suspect that this is due to cases of fibrotic liver disease resulting from viral hepatitis and metabolic liver diseases with nonalcoholic fatty liver and advanced fibrosis (F3 and F4). These cases may be carried over from before, thereby contributing to the increasing incremental cancer risk.
We can’t overlook the need for risk reduction here and should focus on applying regular screening efforts in our female patients. However, it’s also true that we require better liver cancer screening tests to accomplish that goal.
In Those Under 50, CRC the Leading Cause of Cancer Death in Men, Second in Women
I really want to focus on the news around colorectal cancer.
To put this in perspective, in the late 1990s, colorectal cancer was the fourth leading cause of death in men and women. The current report extrapolated 2024 projections using the Surveillance, Epidemiology, and End Results (SEER) database ending in 2020, which was necessary given the incremental time it takes to develop cancers. The SEER database suggests that in 2024, colorectal cancer in those younger than 50 years of age will become the number-one leading cause of cancer death in men and number-two in women. The increasing incidence of colorectal cancer in younger people is probably the result of a number of epidemiologic and other reasons.
The current report offers evidence of racial disparities in cancer mortality rates in general, which are twofold higher in Black people compared with White people, particularly for gastric cancer. There is also an evident disparity in Native Americans, who have higher rates of gastric and liver cancer. This is a reminder of the increasing need for equity to address racial disparities across these populations.
But returning to colon cancer, it’s a marked change to go from being the fourth-leading cause of cancer death in those younger than 50 years of age to being number one for men and number two for women.
Being “number one” is supposed to make you famous. This “number one,” however, should in fact be infamous. It’s a travesty, because colorectal cancer is a potentially preventable disease.
As we move into March, which happens to be Colorectal Cancer Awareness Month, hopefully this fires up some of the conversations you have with your younger at-risk population, who may be reticent or resistant to colorectal cancer screening.
We have to do better at getting this message out to that population at large. “Number one” is not where we want to be for this potentially preventable problem.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Unleashing Our Immune Response to Quash Cancer
This article was originally published on February 10 in Eric Topol’s substack “Ground Truths.”
It’s astounding how devious cancer cells and tumor tissue can be. This week in Science we learned how certain lung cancer cells can function like “Catch Me If You Can” — changing their driver mutation and cell identity to escape targeted therapy. This histologic transformation, as seen in an experimental model, is just one of so many cancer tricks that we are learning about.
Recently, as shown by single-cell sequencing, cancer cells can steal the mitochondria from T cells, a double whammy that turbocharges cancer cells with the hijacked fuel supply and, at the same time, dismantles the immune response.
Last week, we saw how tumor cells can release a virus-like protein that unleashes a vicious autoimmune response.
And then there’s the finding that cancer cell spread predominantly is occurring while we sleep.
As I previously reviewed, the ability for cancer cells to hijack neurons and neural circuits is now well established, no less their ability to reprogram neurons to become adrenergic and stimulate tumor progression, and interfere with the immune response. Stay tuned on that for a new Ground Truths podcast with Prof Michelle Monje, a leader in cancer neuroscience, which will post soon.
Add advancing age’s immunosenescence as yet another challenge to the long and growing list of formidable ways that cancer cells, and the tumor microenvironment, evade our immune response.
An Ever-Expanding Armamentarium
Immune Checkpoint Inhibitors
The field of immunotherapies took off with the immune checkpoint inhibitors, first approved by the FDA in 2011, that take the brakes off of T cells, with the programmed death-1 (PD-1), PD-ligand1, and anti-CTLA-4 monoclonal antibodies.
But we’re clearly learning they are not enough to prevail over cancer with common recurrences, only short term success in most patients, with some notable exceptions. Adding other immune response strategies, such as a vaccine, or antibody-drug conjugates, or engineered T cells, are showing improved chances for success.
Therapeutic Cancer Vaccines
There are many therapeutic cancer vaccines in the works, as reviewed in depth here.
Here’s a list of ongoing clinical trials of cancer vaccines. You’ll note most of these are on top of a checkpoint inhibitor and use personalized neoantigens (cancer cell surface proteins) derived from sequencing (whole-exome or whole genome, RNA-sequencing and HLA-profiling) the patient’s tumor.
An example of positive findings is with the combination of an mRNA-nanoparticle vaccine with up to 34 personalized neoantigens and pembrolizumab (Keytruda) vs pembrolizumab alone in advanced melanoma after resection, with improved outcomes at 3-year follow-up, cutting death or relapse rate in half.
Antibody-Drug Conjugates (ADC)
There is considerable excitement about antibody-drug conjugates (ADC) whereby a linker is used to attach a chemotherapy agent to the checkpoint inhibitor antibody, specifically targeting the cancer cell and facilitating entry of the chemotherapy into the cell. Akin to these are bispecific antibodies (BiTEs, binding to a tumor antigen and T cell receptor simultaneously), both of these conjugates acting as “biologic” or “guided” missiles.
A very good example of the potency of an ADC was seen in a “HER2-low” breast cancer randomized trial. The absence or very low expression or amplification of the HER2 receptor is common in breast cancer and successful treatment has been elusive. A randomized trial of an ADC (trastuzumab deruxtecan) compared to physician’s choice therapy demonstrated a marked success for progression-free survival in HER2-low patients, which was characterized as “unheard-of success” by media coverage.
This strategy is being used to target some of the most difficult cancer driver mutations such as TP53 and KRAS.
Oncolytic Viruses
Modifying viruses to infect the tumor and make it more visible to the immune system, potentiating anti-tumor responses, known as oncolytic viruses, have been proposed as a way to rev up the immune response for a long time but without positive Phase 3 clinical trials.
After decades of failure, a recent trial in refractory bladder cancer showed marked success, along with others, summarized here, now providing very encouraging results. It looks like oncolytic viruses are on a comeback path.
Engineering T Cells (Chimeric Antigen Receptor [CAR-T])
As I recently reviewed, there are over 500 ongoing clinical trials to build on the success of the first CAR-T approval for leukemia 7 years ago. I won’t go through that all again here, but to reiterate most of the success to date has been in “liquid” blood (leukemia and lymphoma) cancer tumors. This week in Nature is the discovery of a T cell cancer mutation, a gene fusion CARD11-PIK3R3, from a T cell lymphoma that can potentially be used to augment CAR-T efficacy. It has pronounced and prolonged effects in the experimental model. Instead of 1 million cells needed for treatment, even 20,000 were enough to melt the tumor. This is a noteworthy discovery since CAR-T work to date has largely not exploited such naturally occurring mutations, while instead concentrating on those seen in the patient’s set of key tumor mutations.
As currently conceived, CAR-T, and what is being referred to more broadly as adoptive cell therapies, involves removing T cells from the patient’s body and engineering their activation, then reintroducing them back to the patient. This is laborious, technically difficult, and very expensive. Recently, the idea of achieving all of this via an injection of virus that specifically infects T cells and inserts the genes needed, was advanced by two biotech companies with preclinical results, one in non-human primates.
Gearing up to meet the challenge of solid tumor CAR-T intervention, there’s more work using CRISPR genome editing of T cell receptors. A.I. is increasingly being exploited to process the data from sequencing and identify optimal neoantigens.
Instead of just CAR-T, we’re seeing the emergence of CAR-macrophage and CAR-natural killer (NK) cells strategies, and rapidly expanding potential combinations of all the strategies I’ve mentioned. No less, there’s been maturation of on-off suicide switches programmed in, to limit cytokine release and promote safety of these interventions. Overall, major side effects of immunotherapies are not only cytokine release syndromes, but also include interstitial pneumonitis and neurotoxicity.
Summary
Given the multitude of ways cancer cells and tumor tissue can evade our immune response, durably successful treatment remains a daunting challenge. But the ingenuity of so many different approaches to unleash our immune response, and their combinations, provides considerable hope that we’ll increasingly meet the challenge in the years ahead. We have clearly learned that combining different immunotherapy strategies will be essential for many patients with the most resilient solid tumors.
Of concern, as noted by a recent editorial in The Lancet, entitled “Cancer Research Equity: Innovations For The Many, Not The Few,” is that these individualized, sophisticated strategies are not scalable; they will have limited reach and benefit. The movement towards “off the shelf” CAR-T and inexpensive, orally active checkpoint inhibitors may help mitigate this issue.
Notwithstanding this important concern, we’re seeing an array of diverse and potent immunotherapy strategies that are providing highly encouraging results, engendering more excitement than we’ve seen in this space for some time. These should propel substantial improvements in outcomes for patients in the years ahead. It can’t happen soon enough.
Thanks for reading this edition of Ground Truths. If you found it informative, please share it with your colleagues.
Dr. Topol has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Dexcom; Illumina; Molecular Stethoscope; Quest Diagnostics; Blue Cross Blue Shield Association. Received research grant from National Institutes of Health.
A version of this article appeared on Medscape.com.
This article was originally published on February 10 in Eric Topol’s substack “Ground Truths.”
It’s astounding how devious cancer cells and tumor tissue can be. This week in Science we learned how certain lung cancer cells can function like “Catch Me If You Can” — changing their driver mutation and cell identity to escape targeted therapy. This histologic transformation, as seen in an experimental model, is just one of so many cancer tricks that we are learning about.
Recently, as shown by single-cell sequencing, cancer cells can steal the mitochondria from T cells, a double whammy that turbocharges cancer cells with the hijacked fuel supply and, at the same time, dismantles the immune response.
Last week, we saw how tumor cells can release a virus-like protein that unleashes a vicious autoimmune response.
And then there’s the finding that cancer cell spread predominantly is occurring while we sleep.
As I previously reviewed, the ability for cancer cells to hijack neurons and neural circuits is now well established, no less their ability to reprogram neurons to become adrenergic and stimulate tumor progression, and interfere with the immune response. Stay tuned on that for a new Ground Truths podcast with Prof Michelle Monje, a leader in cancer neuroscience, which will post soon.
Add advancing age’s immunosenescence as yet another challenge to the long and growing list of formidable ways that cancer cells, and the tumor microenvironment, evade our immune response.
An Ever-Expanding Armamentarium
Immune Checkpoint Inhibitors
The field of immunotherapies took off with the immune checkpoint inhibitors, first approved by the FDA in 2011, that take the brakes off of T cells, with the programmed death-1 (PD-1), PD-ligand1, and anti-CTLA-4 monoclonal antibodies.
But we’re clearly learning they are not enough to prevail over cancer with common recurrences, only short term success in most patients, with some notable exceptions. Adding other immune response strategies, such as a vaccine, or antibody-drug conjugates, or engineered T cells, are showing improved chances for success.
Therapeutic Cancer Vaccines
There are many therapeutic cancer vaccines in the works, as reviewed in depth here.
Here’s a list of ongoing clinical trials of cancer vaccines. You’ll note most of these are on top of a checkpoint inhibitor and use personalized neoantigens (cancer cell surface proteins) derived from sequencing (whole-exome or whole genome, RNA-sequencing and HLA-profiling) the patient’s tumor.
An example of positive findings is with the combination of an mRNA-nanoparticle vaccine with up to 34 personalized neoantigens and pembrolizumab (Keytruda) vs pembrolizumab alone in advanced melanoma after resection, with improved outcomes at 3-year follow-up, cutting death or relapse rate in half.
Antibody-Drug Conjugates (ADC)
There is considerable excitement about antibody-drug conjugates (ADC) whereby a linker is used to attach a chemotherapy agent to the checkpoint inhibitor antibody, specifically targeting the cancer cell and facilitating entry of the chemotherapy into the cell. Akin to these are bispecific antibodies (BiTEs, binding to a tumor antigen and T cell receptor simultaneously), both of these conjugates acting as “biologic” or “guided” missiles.
A very good example of the potency of an ADC was seen in a “HER2-low” breast cancer randomized trial. The absence or very low expression or amplification of the HER2 receptor is common in breast cancer and successful treatment has been elusive. A randomized trial of an ADC (trastuzumab deruxtecan) compared to physician’s choice therapy demonstrated a marked success for progression-free survival in HER2-low patients, which was characterized as “unheard-of success” by media coverage.
This strategy is being used to target some of the most difficult cancer driver mutations such as TP53 and KRAS.
Oncolytic Viruses
Modifying viruses to infect the tumor and make it more visible to the immune system, potentiating anti-tumor responses, known as oncolytic viruses, have been proposed as a way to rev up the immune response for a long time but without positive Phase 3 clinical trials.
After decades of failure, a recent trial in refractory bladder cancer showed marked success, along with others, summarized here, now providing very encouraging results. It looks like oncolytic viruses are on a comeback path.
Engineering T Cells (Chimeric Antigen Receptor [CAR-T])
As I recently reviewed, there are over 500 ongoing clinical trials to build on the success of the first CAR-T approval for leukemia 7 years ago. I won’t go through that all again here, but to reiterate most of the success to date has been in “liquid” blood (leukemia and lymphoma) cancer tumors. This week in Nature is the discovery of a T cell cancer mutation, a gene fusion CARD11-PIK3R3, from a T cell lymphoma that can potentially be used to augment CAR-T efficacy. It has pronounced and prolonged effects in the experimental model. Instead of 1 million cells needed for treatment, even 20,000 were enough to melt the tumor. This is a noteworthy discovery since CAR-T work to date has largely not exploited such naturally occurring mutations, while instead concentrating on those seen in the patient’s set of key tumor mutations.
As currently conceived, CAR-T, and what is being referred to more broadly as adoptive cell therapies, involves removing T cells from the patient’s body and engineering their activation, then reintroducing them back to the patient. This is laborious, technically difficult, and very expensive. Recently, the idea of achieving all of this via an injection of virus that specifically infects T cells and inserts the genes needed, was advanced by two biotech companies with preclinical results, one in non-human primates.
Gearing up to meet the challenge of solid tumor CAR-T intervention, there’s more work using CRISPR genome editing of T cell receptors. A.I. is increasingly being exploited to process the data from sequencing and identify optimal neoantigens.
Instead of just CAR-T, we’re seeing the emergence of CAR-macrophage and CAR-natural killer (NK) cells strategies, and rapidly expanding potential combinations of all the strategies I’ve mentioned. No less, there’s been maturation of on-off suicide switches programmed in, to limit cytokine release and promote safety of these interventions. Overall, major side effects of immunotherapies are not only cytokine release syndromes, but also include interstitial pneumonitis and neurotoxicity.
Summary
Given the multitude of ways cancer cells and tumor tissue can evade our immune response, durably successful treatment remains a daunting challenge. But the ingenuity of so many different approaches to unleash our immune response, and their combinations, provides considerable hope that we’ll increasingly meet the challenge in the years ahead. We have clearly learned that combining different immunotherapy strategies will be essential for many patients with the most resilient solid tumors.
Of concern, as noted by a recent editorial in The Lancet, entitled “Cancer Research Equity: Innovations For The Many, Not The Few,” is that these individualized, sophisticated strategies are not scalable; they will have limited reach and benefit. The movement towards “off the shelf” CAR-T and inexpensive, orally active checkpoint inhibitors may help mitigate this issue.
Notwithstanding this important concern, we’re seeing an array of diverse and potent immunotherapy strategies that are providing highly encouraging results, engendering more excitement than we’ve seen in this space for some time. These should propel substantial improvements in outcomes for patients in the years ahead. It can’t happen soon enough.
Thanks for reading this edition of Ground Truths. If you found it informative, please share it with your colleagues.
Dr. Topol has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Dexcom; Illumina; Molecular Stethoscope; Quest Diagnostics; Blue Cross Blue Shield Association. Received research grant from National Institutes of Health.
A version of this article appeared on Medscape.com.
This article was originally published on February 10 in Eric Topol’s substack “Ground Truths.”
It’s astounding how devious cancer cells and tumor tissue can be. This week in Science we learned how certain lung cancer cells can function like “Catch Me If You Can” — changing their driver mutation and cell identity to escape targeted therapy. This histologic transformation, as seen in an experimental model, is just one of so many cancer tricks that we are learning about.
Recently, as shown by single-cell sequencing, cancer cells can steal the mitochondria from T cells, a double whammy that turbocharges cancer cells with the hijacked fuel supply and, at the same time, dismantles the immune response.
Last week, we saw how tumor cells can release a virus-like protein that unleashes a vicious autoimmune response.
And then there’s the finding that cancer cell spread predominantly is occurring while we sleep.
As I previously reviewed, the ability for cancer cells to hijack neurons and neural circuits is now well established, no less their ability to reprogram neurons to become adrenergic and stimulate tumor progression, and interfere with the immune response. Stay tuned on that for a new Ground Truths podcast with Prof Michelle Monje, a leader in cancer neuroscience, which will post soon.
Add advancing age’s immunosenescence as yet another challenge to the long and growing list of formidable ways that cancer cells, and the tumor microenvironment, evade our immune response.
An Ever-Expanding Armamentarium
Immune Checkpoint Inhibitors
The field of immunotherapies took off with the immune checkpoint inhibitors, first approved by the FDA in 2011, that take the brakes off of T cells, with the programmed death-1 (PD-1), PD-ligand1, and anti-CTLA-4 monoclonal antibodies.
But we’re clearly learning they are not enough to prevail over cancer with common recurrences, only short term success in most patients, with some notable exceptions. Adding other immune response strategies, such as a vaccine, or antibody-drug conjugates, or engineered T cells, are showing improved chances for success.
Therapeutic Cancer Vaccines
There are many therapeutic cancer vaccines in the works, as reviewed in depth here.
Here’s a list of ongoing clinical trials of cancer vaccines. You’ll note most of these are on top of a checkpoint inhibitor and use personalized neoantigens (cancer cell surface proteins) derived from sequencing (whole-exome or whole genome, RNA-sequencing and HLA-profiling) the patient’s tumor.
An example of positive findings is with the combination of an mRNA-nanoparticle vaccine with up to 34 personalized neoantigens and pembrolizumab (Keytruda) vs pembrolizumab alone in advanced melanoma after resection, with improved outcomes at 3-year follow-up, cutting death or relapse rate in half.
Antibody-Drug Conjugates (ADC)
There is considerable excitement about antibody-drug conjugates (ADC) whereby a linker is used to attach a chemotherapy agent to the checkpoint inhibitor antibody, specifically targeting the cancer cell and facilitating entry of the chemotherapy into the cell. Akin to these are bispecific antibodies (BiTEs, binding to a tumor antigen and T cell receptor simultaneously), both of these conjugates acting as “biologic” or “guided” missiles.
A very good example of the potency of an ADC was seen in a “HER2-low” breast cancer randomized trial. The absence or very low expression or amplification of the HER2 receptor is common in breast cancer and successful treatment has been elusive. A randomized trial of an ADC (trastuzumab deruxtecan) compared to physician’s choice therapy demonstrated a marked success for progression-free survival in HER2-low patients, which was characterized as “unheard-of success” by media coverage.
This strategy is being used to target some of the most difficult cancer driver mutations such as TP53 and KRAS.
Oncolytic Viruses
Modifying viruses to infect the tumor and make it more visible to the immune system, potentiating anti-tumor responses, known as oncolytic viruses, have been proposed as a way to rev up the immune response for a long time but without positive Phase 3 clinical trials.
After decades of failure, a recent trial in refractory bladder cancer showed marked success, along with others, summarized here, now providing very encouraging results. It looks like oncolytic viruses are on a comeback path.
Engineering T Cells (Chimeric Antigen Receptor [CAR-T])
As I recently reviewed, there are over 500 ongoing clinical trials to build on the success of the first CAR-T approval for leukemia 7 years ago. I won’t go through that all again here, but to reiterate most of the success to date has been in “liquid” blood (leukemia and lymphoma) cancer tumors. This week in Nature is the discovery of a T cell cancer mutation, a gene fusion CARD11-PIK3R3, from a T cell lymphoma that can potentially be used to augment CAR-T efficacy. It has pronounced and prolonged effects in the experimental model. Instead of 1 million cells needed for treatment, even 20,000 were enough to melt the tumor. This is a noteworthy discovery since CAR-T work to date has largely not exploited such naturally occurring mutations, while instead concentrating on those seen in the patient’s set of key tumor mutations.
As currently conceived, CAR-T, and what is being referred to more broadly as adoptive cell therapies, involves removing T cells from the patient’s body and engineering their activation, then reintroducing them back to the patient. This is laborious, technically difficult, and very expensive. Recently, the idea of achieving all of this via an injection of virus that specifically infects T cells and inserts the genes needed, was advanced by two biotech companies with preclinical results, one in non-human primates.
Gearing up to meet the challenge of solid tumor CAR-T intervention, there’s more work using CRISPR genome editing of T cell receptors. A.I. is increasingly being exploited to process the data from sequencing and identify optimal neoantigens.
Instead of just CAR-T, we’re seeing the emergence of CAR-macrophage and CAR-natural killer (NK) cells strategies, and rapidly expanding potential combinations of all the strategies I’ve mentioned. No less, there’s been maturation of on-off suicide switches programmed in, to limit cytokine release and promote safety of these interventions. Overall, major side effects of immunotherapies are not only cytokine release syndromes, but also include interstitial pneumonitis and neurotoxicity.
Summary
Given the multitude of ways cancer cells and tumor tissue can evade our immune response, durably successful treatment remains a daunting challenge. But the ingenuity of so many different approaches to unleash our immune response, and their combinations, provides considerable hope that we’ll increasingly meet the challenge in the years ahead. We have clearly learned that combining different immunotherapy strategies will be essential for many patients with the most resilient solid tumors.
Of concern, as noted by a recent editorial in The Lancet, entitled “Cancer Research Equity: Innovations For The Many, Not The Few,” is that these individualized, sophisticated strategies are not scalable; they will have limited reach and benefit. The movement towards “off the shelf” CAR-T and inexpensive, orally active checkpoint inhibitors may help mitigate this issue.
Notwithstanding this important concern, we’re seeing an array of diverse and potent immunotherapy strategies that are providing highly encouraging results, engendering more excitement than we’ve seen in this space for some time. These should propel substantial improvements in outcomes for patients in the years ahead. It can’t happen soon enough.
Thanks for reading this edition of Ground Truths. If you found it informative, please share it with your colleagues.
Dr. Topol has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Dexcom; Illumina; Molecular Stethoscope; Quest Diagnostics; Blue Cross Blue Shield Association. Received research grant from National Institutes of Health.
A version of this article appeared on Medscape.com.
Kimchi: Not Magically Protective Against Weight Gain
How much of societal diet-related scientific illiteracy can be blamed on the publication decisions of medical journals around food studies?
That was the question I pondered when reading “Association between kimchi consumption and obesity based on BMI and abdominal obesity in Korean adults: a cross-sectional analysis of the Health Examinees study,” recently published in BMJ Open. Although I will get to the study particulars momentarily, that it’s 2024 and journals are still publishing cross-sectional studies of the impact of a single food’s subjectively reported consumption on health outcomes is mind boggling.
You might wonder why I wasn’t mind boggled by the authors rather than the journal — but the authors’ interest in publishing a study on kimchi’s supposed impact on obesity is an easy thing to explain, in that the study was funded by the World Institute of Kimchi, where two of its four authors are employed.
You might also wonder why I wasn’t mind boggled by media running with this story — but the media’s job is to capture eyeballs, and who doesn’t love a good magic food story, doubly so for one involving obesity and one with a study backing it up?
Back to this World Institute of Kimchi project looking at kimchi intake on obesity rates. No doubt if I worked for the World Institute of Kimchi, I would want kimchi to be shown to be somehow magically protective against weight gain. So how might I go about exploring that?
Well, I could look to the data from the Health Examinees (HEXA) Study. The HEXA study was a cross-sectional look at South Koreans; included in their data collection was a 106-item food frequency questionnaire (FFQ).
That questionnaire looked at 106 food items — yep, you guessed it, explicitly including kimchi. Not included in this FFQ, though, were prepared foods, meaning that it was unable to measure seasonings, spices, or cooking oils. Also perhaps problematic is that no doubt most of us consume more than 106 total food items in our diets. Perhaps this is why the validation study of HEXA’s food item–based FFQ found that it had “relatively low validity” when compared against 12-day food diaries and why its creators themselves report it to be in their study’s conclusion only “reasonably acceptable” to apply to a population. But yes, kimchi!
So for the sake of this exercise, though, let’s assume that instead of only a reasonably acceptable FFQ with low validity, the FFQ was fantastic and its data robust. How great then is kimchi at preventing obesity? Certainly, the media report it’s pretty darn good. Here’s a smattering from the literal dozens of headlines of stories covering this paper:
Eating kimchi every day could help stave off weight gain, new study says — NBC News
Eating kimchi every day may prevent weight gain, research suggests — Sky News
Want to avoid piling on the pounds? Try kimchi for breakfast — The Telegraph
But when we turn to the paper itself, suddenly things aren’t so clear.
According to the paper, men who reported eating two to three servings of kimchi per day were found to have lower rates of obesity, whereas men who reported eating three to five servings of kimchi per day were not. But these are overlapping groups! Also found was that men consuming more than five servings of kimchi per day have higher rates of obesity. When taken together, these findings do not demonstrate a statistically significant trend of kimchi intake on obesity in men. Whereas in women, things are worse in that the more kimchi reportedly consumed, the more obesity, in a trend that did (just) reach statistical significance.
So even if we pretend the FFQs were robust enough to make conclusions about a single food’s impact on obesity, and we pretend there was a well-described, plausible mechanistic reason to believe same (there isn’t), and we pretend that this particular FFQ had better than “relatively low validity,” there is no conclusion here to be drawn about kimchi’s impact on obesity.
What we can conclude is that when it comes to publishing papers purporting to find the impact of single foods on obesity, journals will still happily publish them and their publication will lead to hyperbolic headlines and stories, which in turn reinforce the scientifically illiterate notion that the highly complex multifactorial problem of obesity boils down to simple food choices, which in turn keeps weight loss grifters everywhere in business while fueling societal weight bias.
Dr. Freedhoff is Associate Professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He disclosed ties with Bariatric Medical Institute, Constant Health, and Novo Nordisk.
A version of this article appeared on Medscape.com.
How much of societal diet-related scientific illiteracy can be blamed on the publication decisions of medical journals around food studies?
That was the question I pondered when reading “Association between kimchi consumption and obesity based on BMI and abdominal obesity in Korean adults: a cross-sectional analysis of the Health Examinees study,” recently published in BMJ Open. Although I will get to the study particulars momentarily, that it’s 2024 and journals are still publishing cross-sectional studies of the impact of a single food’s subjectively reported consumption on health outcomes is mind boggling.
You might wonder why I wasn’t mind boggled by the authors rather than the journal — but the authors’ interest in publishing a study on kimchi’s supposed impact on obesity is an easy thing to explain, in that the study was funded by the World Institute of Kimchi, where two of its four authors are employed.
You might also wonder why I wasn’t mind boggled by media running with this story — but the media’s job is to capture eyeballs, and who doesn’t love a good magic food story, doubly so for one involving obesity and one with a study backing it up?
Back to this World Institute of Kimchi project looking at kimchi intake on obesity rates. No doubt if I worked for the World Institute of Kimchi, I would want kimchi to be shown to be somehow magically protective against weight gain. So how might I go about exploring that?
Well, I could look to the data from the Health Examinees (HEXA) Study. The HEXA study was a cross-sectional look at South Koreans; included in their data collection was a 106-item food frequency questionnaire (FFQ).
That questionnaire looked at 106 food items — yep, you guessed it, explicitly including kimchi. Not included in this FFQ, though, were prepared foods, meaning that it was unable to measure seasonings, spices, or cooking oils. Also perhaps problematic is that no doubt most of us consume more than 106 total food items in our diets. Perhaps this is why the validation study of HEXA’s food item–based FFQ found that it had “relatively low validity” when compared against 12-day food diaries and why its creators themselves report it to be in their study’s conclusion only “reasonably acceptable” to apply to a population. But yes, kimchi!
So for the sake of this exercise, though, let’s assume that instead of only a reasonably acceptable FFQ with low validity, the FFQ was fantastic and its data robust. How great then is kimchi at preventing obesity? Certainly, the media report it’s pretty darn good. Here’s a smattering from the literal dozens of headlines of stories covering this paper:
Eating kimchi every day could help stave off weight gain, new study says — NBC News
Eating kimchi every day may prevent weight gain, research suggests — Sky News
Want to avoid piling on the pounds? Try kimchi for breakfast — The Telegraph
But when we turn to the paper itself, suddenly things aren’t so clear.
According to the paper, men who reported eating two to three servings of kimchi per day were found to have lower rates of obesity, whereas men who reported eating three to five servings of kimchi per day were not. But these are overlapping groups! Also found was that men consuming more than five servings of kimchi per day have higher rates of obesity. When taken together, these findings do not demonstrate a statistically significant trend of kimchi intake on obesity in men. Whereas in women, things are worse in that the more kimchi reportedly consumed, the more obesity, in a trend that did (just) reach statistical significance.
So even if we pretend the FFQs were robust enough to make conclusions about a single food’s impact on obesity, and we pretend there was a well-described, plausible mechanistic reason to believe same (there isn’t), and we pretend that this particular FFQ had better than “relatively low validity,” there is no conclusion here to be drawn about kimchi’s impact on obesity.
What we can conclude is that when it comes to publishing papers purporting to find the impact of single foods on obesity, journals will still happily publish them and their publication will lead to hyperbolic headlines and stories, which in turn reinforce the scientifically illiterate notion that the highly complex multifactorial problem of obesity boils down to simple food choices, which in turn keeps weight loss grifters everywhere in business while fueling societal weight bias.
Dr. Freedhoff is Associate Professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He disclosed ties with Bariatric Medical Institute, Constant Health, and Novo Nordisk.
A version of this article appeared on Medscape.com.
How much of societal diet-related scientific illiteracy can be blamed on the publication decisions of medical journals around food studies?
That was the question I pondered when reading “Association between kimchi consumption and obesity based on BMI and abdominal obesity in Korean adults: a cross-sectional analysis of the Health Examinees study,” recently published in BMJ Open. Although I will get to the study particulars momentarily, that it’s 2024 and journals are still publishing cross-sectional studies of the impact of a single food’s subjectively reported consumption on health outcomes is mind boggling.
You might wonder why I wasn’t mind boggled by the authors rather than the journal — but the authors’ interest in publishing a study on kimchi’s supposed impact on obesity is an easy thing to explain, in that the study was funded by the World Institute of Kimchi, where two of its four authors are employed.
You might also wonder why I wasn’t mind boggled by media running with this story — but the media’s job is to capture eyeballs, and who doesn’t love a good magic food story, doubly so for one involving obesity and one with a study backing it up?
Back to this World Institute of Kimchi project looking at kimchi intake on obesity rates. No doubt if I worked for the World Institute of Kimchi, I would want kimchi to be shown to be somehow magically protective against weight gain. So how might I go about exploring that?
Well, I could look to the data from the Health Examinees (HEXA) Study. The HEXA study was a cross-sectional look at South Koreans; included in their data collection was a 106-item food frequency questionnaire (FFQ).
That questionnaire looked at 106 food items — yep, you guessed it, explicitly including kimchi. Not included in this FFQ, though, were prepared foods, meaning that it was unable to measure seasonings, spices, or cooking oils. Also perhaps problematic is that no doubt most of us consume more than 106 total food items in our diets. Perhaps this is why the validation study of HEXA’s food item–based FFQ found that it had “relatively low validity” when compared against 12-day food diaries and why its creators themselves report it to be in their study’s conclusion only “reasonably acceptable” to apply to a population. But yes, kimchi!
So for the sake of this exercise, though, let’s assume that instead of only a reasonably acceptable FFQ with low validity, the FFQ was fantastic and its data robust. How great then is kimchi at preventing obesity? Certainly, the media report it’s pretty darn good. Here’s a smattering from the literal dozens of headlines of stories covering this paper:
Eating kimchi every day could help stave off weight gain, new study says — NBC News
Eating kimchi every day may prevent weight gain, research suggests — Sky News
Want to avoid piling on the pounds? Try kimchi for breakfast — The Telegraph
But when we turn to the paper itself, suddenly things aren’t so clear.
According to the paper, men who reported eating two to three servings of kimchi per day were found to have lower rates of obesity, whereas men who reported eating three to five servings of kimchi per day were not. But these are overlapping groups! Also found was that men consuming more than five servings of kimchi per day have higher rates of obesity. When taken together, these findings do not demonstrate a statistically significant trend of kimchi intake on obesity in men. Whereas in women, things are worse in that the more kimchi reportedly consumed, the more obesity, in a trend that did (just) reach statistical significance.
So even if we pretend the FFQs were robust enough to make conclusions about a single food’s impact on obesity, and we pretend there was a well-described, plausible mechanistic reason to believe same (there isn’t), and we pretend that this particular FFQ had better than “relatively low validity,” there is no conclusion here to be drawn about kimchi’s impact on obesity.
What we can conclude is that when it comes to publishing papers purporting to find the impact of single foods on obesity, journals will still happily publish them and their publication will lead to hyperbolic headlines and stories, which in turn reinforce the scientifically illiterate notion that the highly complex multifactorial problem of obesity boils down to simple food choices, which in turn keeps weight loss grifters everywhere in business while fueling societal weight bias.
Dr. Freedhoff is Associate Professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He disclosed ties with Bariatric Medical Institute, Constant Health, and Novo Nordisk.
A version of this article appeared on Medscape.com.
Beware the Letter of Intent
I recently received an email from a distraught physician. Several months previously, He could continue running his office any way he wished, set his own hours and fees, and keep his employees. All his overhead expenses would disappear. His income would remain the same, maybe even increase. He signed it eagerly.
When he received the actual sale and employment contracts, none of the details promised in the LOI were included; but he figured that since they were spelled out in the LOI, which both he and the buyer had signed, he was covered. His attorney — a family friend with no experience in medical practice transactions — approved the documents.
The deal seemed too good to be true, and it was. The day after the sale closed, all his employees received termination notices. The group offered to rehire some of them, but at lower salaries and reduced benefits. (Most declined.) The new staffers he received were inadequately trained and unfamiliar with his standard office procedures. Patients complained that fees had increased substantially. His own compensation was contingent on meeting strict billing and performance goals. Malpractice premiums remained his responsibility. His office hours were lengthened to include evenings and Saturday mornings.
When he complained to the group that none of the things promised in the LOI had been delivered, he was informed that the LOI was not legally binding. In fact, the sale and employment contracts both clearly specified that they “replaced any previous written or oral agreements between the parties.”
There are some valuable lessons to be learned here. First, whether you are a young physician seeking a new job with a hospital or large practice, or an older one looking to sell an established practice, retain an attorney experienced in medical transactions early, before you sign anything, binding or not. Second, recognize that any promises made in an LOI must be spelled out in the employment and/or sale contract as well.
You might ask, if the terms in an LOI are not binding, why bother with one at all? For one thing, you want to make sure that you and your potential employer or buyer are on the same page with respect to major terms before you get down to details in the employment agreement and/or the medical practice sale agreement. For another, in most states certain LOI provisions are legally binding. For example, the document will most likely provide that each party is responsible for its own attorneys’ fees and for maintaining confidentiality during the negotiations, and that you will not negotiate with any other parties for some specified period of time. In most cases, such provisions are binding whether you go on to sign a formal contract or not.
When you receive an LOI, go through it carefully and identify areas of concern. The offering party will likely be in a rush to sign you up; but once you sign, you won’t be able to negotiate with anyone else for a while, which weakens your negotiating position. Regardless of what is said about time being “of the essence,” proceed slowly and with caution.
Bear in mind that employers and buyers never begin with their best offer. Unless you have been through this before, it is unlikely that you will know your value as an employee or the value of your practice, or what exactly you are entitled to ask for. Rather than signing something you don’t completely understand, explain to the offering party that you need time to consider and evaluate their offer.
This is the time to hire a competent medical attorney to do some due diligence on the offering party and review their offer, and to educate yourself about practice value and compensation benchmarks in your area. You and your counsel should assemble a list of things that you want changed in the LOI, then present them to the other side. They should be amenable to negotiation. If they are not (as was the case in the example presented earlier), you should reconsider whether you really want to be associated with that particular buyer or employer.
Once you have signed the LOI, experts say speed then works to your advantage. “Time kills all deals,” as one lawyer put it. “The longer it takes to close the transaction, the more that can go wrong.” The prospective employer or buyer could uncover information about you or your practice that decreases their perception of value, or economic conditions might change.
While speed is now important, and most of the core issues should already have been resolved in the LOI, don’t be afraid to ask for everything you want, whether it’s a better sale price, higher compensation, a favorable call schedule, more vacation time, or anything else. You won’t know what you can get if you don’t ask for it.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I recently received an email from a distraught physician. Several months previously, He could continue running his office any way he wished, set his own hours and fees, and keep his employees. All his overhead expenses would disappear. His income would remain the same, maybe even increase. He signed it eagerly.
When he received the actual sale and employment contracts, none of the details promised in the LOI were included; but he figured that since they were spelled out in the LOI, which both he and the buyer had signed, he was covered. His attorney — a family friend with no experience in medical practice transactions — approved the documents.
The deal seemed too good to be true, and it was. The day after the sale closed, all his employees received termination notices. The group offered to rehire some of them, but at lower salaries and reduced benefits. (Most declined.) The new staffers he received were inadequately trained and unfamiliar with his standard office procedures. Patients complained that fees had increased substantially. His own compensation was contingent on meeting strict billing and performance goals. Malpractice premiums remained his responsibility. His office hours were lengthened to include evenings and Saturday mornings.
When he complained to the group that none of the things promised in the LOI had been delivered, he was informed that the LOI was not legally binding. In fact, the sale and employment contracts both clearly specified that they “replaced any previous written or oral agreements between the parties.”
There are some valuable lessons to be learned here. First, whether you are a young physician seeking a new job with a hospital or large practice, or an older one looking to sell an established practice, retain an attorney experienced in medical transactions early, before you sign anything, binding or not. Second, recognize that any promises made in an LOI must be spelled out in the employment and/or sale contract as well.
You might ask, if the terms in an LOI are not binding, why bother with one at all? For one thing, you want to make sure that you and your potential employer or buyer are on the same page with respect to major terms before you get down to details in the employment agreement and/or the medical practice sale agreement. For another, in most states certain LOI provisions are legally binding. For example, the document will most likely provide that each party is responsible for its own attorneys’ fees and for maintaining confidentiality during the negotiations, and that you will not negotiate with any other parties for some specified period of time. In most cases, such provisions are binding whether you go on to sign a formal contract or not.
When you receive an LOI, go through it carefully and identify areas of concern. The offering party will likely be in a rush to sign you up; but once you sign, you won’t be able to negotiate with anyone else for a while, which weakens your negotiating position. Regardless of what is said about time being “of the essence,” proceed slowly and with caution.
Bear in mind that employers and buyers never begin with their best offer. Unless you have been through this before, it is unlikely that you will know your value as an employee or the value of your practice, or what exactly you are entitled to ask for. Rather than signing something you don’t completely understand, explain to the offering party that you need time to consider and evaluate their offer.
This is the time to hire a competent medical attorney to do some due diligence on the offering party and review their offer, and to educate yourself about practice value and compensation benchmarks in your area. You and your counsel should assemble a list of things that you want changed in the LOI, then present them to the other side. They should be amenable to negotiation. If they are not (as was the case in the example presented earlier), you should reconsider whether you really want to be associated with that particular buyer or employer.
Once you have signed the LOI, experts say speed then works to your advantage. “Time kills all deals,” as one lawyer put it. “The longer it takes to close the transaction, the more that can go wrong.” The prospective employer or buyer could uncover information about you or your practice that decreases their perception of value, or economic conditions might change.
While speed is now important, and most of the core issues should already have been resolved in the LOI, don’t be afraid to ask for everything you want, whether it’s a better sale price, higher compensation, a favorable call schedule, more vacation time, or anything else. You won’t know what you can get if you don’t ask for it.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I recently received an email from a distraught physician. Several months previously, He could continue running his office any way he wished, set his own hours and fees, and keep his employees. All his overhead expenses would disappear. His income would remain the same, maybe even increase. He signed it eagerly.
When he received the actual sale and employment contracts, none of the details promised in the LOI were included; but he figured that since they were spelled out in the LOI, which both he and the buyer had signed, he was covered. His attorney — a family friend with no experience in medical practice transactions — approved the documents.
The deal seemed too good to be true, and it was. The day after the sale closed, all his employees received termination notices. The group offered to rehire some of them, but at lower salaries and reduced benefits. (Most declined.) The new staffers he received were inadequately trained and unfamiliar with his standard office procedures. Patients complained that fees had increased substantially. His own compensation was contingent on meeting strict billing and performance goals. Malpractice premiums remained his responsibility. His office hours were lengthened to include evenings and Saturday mornings.
When he complained to the group that none of the things promised in the LOI had been delivered, he was informed that the LOI was not legally binding. In fact, the sale and employment contracts both clearly specified that they “replaced any previous written or oral agreements between the parties.”
There are some valuable lessons to be learned here. First, whether you are a young physician seeking a new job with a hospital or large practice, or an older one looking to sell an established practice, retain an attorney experienced in medical transactions early, before you sign anything, binding or not. Second, recognize that any promises made in an LOI must be spelled out in the employment and/or sale contract as well.
You might ask, if the terms in an LOI are not binding, why bother with one at all? For one thing, you want to make sure that you and your potential employer or buyer are on the same page with respect to major terms before you get down to details in the employment agreement and/or the medical practice sale agreement. For another, in most states certain LOI provisions are legally binding. For example, the document will most likely provide that each party is responsible for its own attorneys’ fees and for maintaining confidentiality during the negotiations, and that you will not negotiate with any other parties for some specified period of time. In most cases, such provisions are binding whether you go on to sign a formal contract or not.
When you receive an LOI, go through it carefully and identify areas of concern. The offering party will likely be in a rush to sign you up; but once you sign, you won’t be able to negotiate with anyone else for a while, which weakens your negotiating position. Regardless of what is said about time being “of the essence,” proceed slowly and with caution.
Bear in mind that employers and buyers never begin with their best offer. Unless you have been through this before, it is unlikely that you will know your value as an employee or the value of your practice, or what exactly you are entitled to ask for. Rather than signing something you don’t completely understand, explain to the offering party that you need time to consider and evaluate their offer.
This is the time to hire a competent medical attorney to do some due diligence on the offering party and review their offer, and to educate yourself about practice value and compensation benchmarks in your area. You and your counsel should assemble a list of things that you want changed in the LOI, then present them to the other side. They should be amenable to negotiation. If they are not (as was the case in the example presented earlier), you should reconsider whether you really want to be associated with that particular buyer or employer.
Once you have signed the LOI, experts say speed then works to your advantage. “Time kills all deals,” as one lawyer put it. “The longer it takes to close the transaction, the more that can go wrong.” The prospective employer or buyer could uncover information about you or your practice that decreases their perception of value, or economic conditions might change.
While speed is now important, and most of the core issues should already have been resolved in the LOI, don’t be afraid to ask for everything you want, whether it’s a better sale price, higher compensation, a favorable call schedule, more vacation time, or anything else. You won’t know what you can get if you don’t ask for it.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].







