User login
The Paradox of Achalasia Symptoms
In contrast to most diseases, as achalasia progresses, the symptoms improve. Specifically, reduction of symptoms of dysphagia lulls the gastroenterologist into thinking their patients are doing well.
This improvement in dysphagia is likely due to two mechanisms. The first is that as the esophagus dilates, there is a greater capacity for food accumulation before sensation occurs. Whether this is completely a volume issue or whether there is a contribution from increased esophageal body distensibility is unclear. Similarly, as achalasia results from inflammation and destruction of the motor neurons of the myenteric plexus, sensory neurons are also damaged. As a result, the patient’s ability to sense food retention lessens. To some degree, this explains the phenomenon of patients presenting with megaesophagus; after years of initially diminishing or stable symptoms managed with patient accommodation, patients present with end-stage disease manifested by a food-impacted esophagus, nocturnal aspiration, and weight loss.
This aspect of the natural history of achalasia has led esophagologists to follow patients with achalasia after treatment at regular intervals with objective examinations such as timed esophagography to mitigate against this worsening yet symptomatically stable course.
Dr. Katzka is based in the Division of Digestive and Liver Diseases, Columbia University Medical Center, New York. He receives research support from Medtronic and is an associate editor for GI & Hepatology News. Previously published in Gastro Hep Advances. 2024 Jan 19. doi: 10.1016/j.gastha.2024.01.006.
In contrast to most diseases, as achalasia progresses, the symptoms improve. Specifically, reduction of symptoms of dysphagia lulls the gastroenterologist into thinking their patients are doing well.
This improvement in dysphagia is likely due to two mechanisms. The first is that as the esophagus dilates, there is a greater capacity for food accumulation before sensation occurs. Whether this is completely a volume issue or whether there is a contribution from increased esophageal body distensibility is unclear. Similarly, as achalasia results from inflammation and destruction of the motor neurons of the myenteric plexus, sensory neurons are also damaged. As a result, the patient’s ability to sense food retention lessens. To some degree, this explains the phenomenon of patients presenting with megaesophagus; after years of initially diminishing or stable symptoms managed with patient accommodation, patients present with end-stage disease manifested by a food-impacted esophagus, nocturnal aspiration, and weight loss.
This aspect of the natural history of achalasia has led esophagologists to follow patients with achalasia after treatment at regular intervals with objective examinations such as timed esophagography to mitigate against this worsening yet symptomatically stable course.
Dr. Katzka is based in the Division of Digestive and Liver Diseases, Columbia University Medical Center, New York. He receives research support from Medtronic and is an associate editor for GI & Hepatology News. Previously published in Gastro Hep Advances. 2024 Jan 19. doi: 10.1016/j.gastha.2024.01.006.
In contrast to most diseases, as achalasia progresses, the symptoms improve. Specifically, reduction of symptoms of dysphagia lulls the gastroenterologist into thinking their patients are doing well.
This improvement in dysphagia is likely due to two mechanisms. The first is that as the esophagus dilates, there is a greater capacity for food accumulation before sensation occurs. Whether this is completely a volume issue or whether there is a contribution from increased esophageal body distensibility is unclear. Similarly, as achalasia results from inflammation and destruction of the motor neurons of the myenteric plexus, sensory neurons are also damaged. As a result, the patient’s ability to sense food retention lessens. To some degree, this explains the phenomenon of patients presenting with megaesophagus; after years of initially diminishing or stable symptoms managed with patient accommodation, patients present with end-stage disease manifested by a food-impacted esophagus, nocturnal aspiration, and weight loss.
This aspect of the natural history of achalasia has led esophagologists to follow patients with achalasia after treatment at regular intervals with objective examinations such as timed esophagography to mitigate against this worsening yet symptomatically stable course.
Dr. Katzka is based in the Division of Digestive and Liver Diseases, Columbia University Medical Center, New York. He receives research support from Medtronic and is an associate editor for GI & Hepatology News. Previously published in Gastro Hep Advances. 2024 Jan 19. doi: 10.1016/j.gastha.2024.01.006.
Residents Unionizing: What Are the Benefits, the Downsides?
This transcript has been edited for clarity.
Hospital administrators and some department heads have been vocal about the potential for unions to affect both the attending-resident relationship and the ability for residents to directly discuss concerns and educational plans.
Sometimes, there are institution-specific issues as well. One example was at Loma Linda. They argued that unionization would go against their religious principles. They filed a lawsuit. That didn’t go through, and the residents won a few months later.
I know there’s always that one senior, older doctor who says, “Back in our day, we just worked, and we never complained.”
Look at the current situation that residents are facing now, with housing and rent prices and increasing costs of childcare. Sprinkle in some inflation, poor hospital staffing, increasing workload, and add in the fact that the average first-year resident salary in 2023 was around $64,000.
Now, if you look back to 2012, the average salary was around $55,000. If you adjust that for inflation, it would be around $75,000 today, which is more than what the average resident is getting paid.
Then, there are hospital administrators who say that the hospital does not have the money to meet these demands; meanwhile, hospital graduate medical education (GME) offices receive about $150,000 of Medicare funds per resident.
Obviously, there are additional costs when it comes to training and supporting residents. In general, unionizing freaks out the people handling all the cash.
There’s also the threat of a strike, which no hospital wants on their public record. A recent highly publicized event happened at New York’s Elmhurst Hospital, when 160 residents went on strike for 3 days until a deal was made.
Critics of unionizing also cite a particular study in JAMA, which included a survey of 5700 general surgery residents at 285 programs. It found that while unions helped with vacation time and housing stipends, the unions were not associated with improved burnout rates, suicidality, job satisfaction, duty hour violations, mistreatment, educational environment, or salary.
Now, granted, this isn’t the strongest study. It only sampled one group of residents, so I wouldn’t generalize these findings, but it’s still commonly cited by anti-union advocates.
Another potential downside, which is purely anecdotal because I can’t find any data to support this, is potential retaliation against residents or harm to the attending-resident relationship.
I’m an attending. I don’t really understand this one. I don’t exactly own stock in my hospital, nor am I making millions of dollars by siphoning GME money. I’m just trying to focus on educating and supporting my residents the best I can.
Dr. Patel is Clinical Instructor, Department of Pediatrics, Columbia University College of Physicians and Surgeons; Pediatric Hospitalist, Morgan Stanley Children’s Hospital of NewYork–Presbyterian, and Benioff Children’s Hospital, University of California San Francisco. He disclosed ties with Medumo Inc.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hospital administrators and some department heads have been vocal about the potential for unions to affect both the attending-resident relationship and the ability for residents to directly discuss concerns and educational plans.
Sometimes, there are institution-specific issues as well. One example was at Loma Linda. They argued that unionization would go against their religious principles. They filed a lawsuit. That didn’t go through, and the residents won a few months later.
I know there’s always that one senior, older doctor who says, “Back in our day, we just worked, and we never complained.”
Look at the current situation that residents are facing now, with housing and rent prices and increasing costs of childcare. Sprinkle in some inflation, poor hospital staffing, increasing workload, and add in the fact that the average first-year resident salary in 2023 was around $64,000.
Now, if you look back to 2012, the average salary was around $55,000. If you adjust that for inflation, it would be around $75,000 today, which is more than what the average resident is getting paid.
Then, there are hospital administrators who say that the hospital does not have the money to meet these demands; meanwhile, hospital graduate medical education (GME) offices receive about $150,000 of Medicare funds per resident.
Obviously, there are additional costs when it comes to training and supporting residents. In general, unionizing freaks out the people handling all the cash.
There’s also the threat of a strike, which no hospital wants on their public record. A recent highly publicized event happened at New York’s Elmhurst Hospital, when 160 residents went on strike for 3 days until a deal was made.
Critics of unionizing also cite a particular study in JAMA, which included a survey of 5700 general surgery residents at 285 programs. It found that while unions helped with vacation time and housing stipends, the unions were not associated with improved burnout rates, suicidality, job satisfaction, duty hour violations, mistreatment, educational environment, or salary.
Now, granted, this isn’t the strongest study. It only sampled one group of residents, so I wouldn’t generalize these findings, but it’s still commonly cited by anti-union advocates.
Another potential downside, which is purely anecdotal because I can’t find any data to support this, is potential retaliation against residents or harm to the attending-resident relationship.
I’m an attending. I don’t really understand this one. I don’t exactly own stock in my hospital, nor am I making millions of dollars by siphoning GME money. I’m just trying to focus on educating and supporting my residents the best I can.
Dr. Patel is Clinical Instructor, Department of Pediatrics, Columbia University College of Physicians and Surgeons; Pediatric Hospitalist, Morgan Stanley Children’s Hospital of NewYork–Presbyterian, and Benioff Children’s Hospital, University of California San Francisco. He disclosed ties with Medumo Inc.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hospital administrators and some department heads have been vocal about the potential for unions to affect both the attending-resident relationship and the ability for residents to directly discuss concerns and educational plans.
Sometimes, there are institution-specific issues as well. One example was at Loma Linda. They argued that unionization would go against their religious principles. They filed a lawsuit. That didn’t go through, and the residents won a few months later.
I know there’s always that one senior, older doctor who says, “Back in our day, we just worked, and we never complained.”
Look at the current situation that residents are facing now, with housing and rent prices and increasing costs of childcare. Sprinkle in some inflation, poor hospital staffing, increasing workload, and add in the fact that the average first-year resident salary in 2023 was around $64,000.
Now, if you look back to 2012, the average salary was around $55,000. If you adjust that for inflation, it would be around $75,000 today, which is more than what the average resident is getting paid.
Then, there are hospital administrators who say that the hospital does not have the money to meet these demands; meanwhile, hospital graduate medical education (GME) offices receive about $150,000 of Medicare funds per resident.
Obviously, there are additional costs when it comes to training and supporting residents. In general, unionizing freaks out the people handling all the cash.
There’s also the threat of a strike, which no hospital wants on their public record. A recent highly publicized event happened at New York’s Elmhurst Hospital, when 160 residents went on strike for 3 days until a deal was made.
Critics of unionizing also cite a particular study in JAMA, which included a survey of 5700 general surgery residents at 285 programs. It found that while unions helped with vacation time and housing stipends, the unions were not associated with improved burnout rates, suicidality, job satisfaction, duty hour violations, mistreatment, educational environment, or salary.
Now, granted, this isn’t the strongest study. It only sampled one group of residents, so I wouldn’t generalize these findings, but it’s still commonly cited by anti-union advocates.
Another potential downside, which is purely anecdotal because I can’t find any data to support this, is potential retaliation against residents or harm to the attending-resident relationship.
I’m an attending. I don’t really understand this one. I don’t exactly own stock in my hospital, nor am I making millions of dollars by siphoning GME money. I’m just trying to focus on educating and supporting my residents the best I can.
Dr. Patel is Clinical Instructor, Department of Pediatrics, Columbia University College of Physicians and Surgeons; Pediatric Hospitalist, Morgan Stanley Children’s Hospital of NewYork–Presbyterian, and Benioff Children’s Hospital, University of California San Francisco. He disclosed ties with Medumo Inc.
A version of this article first appeared on Medscape.com.
Myasthenia Gravis: 5 Things to Know
Myasthenia gravis (MG) is a rare autoimmune neurologic disorder that occurs when the transmission between nerves and muscles is disrupted. It is caused by autoantibodies against acetylcholine receptors (AChRs), which results in muscle weakness that is often fatigable and affects various muscles in the body, including those that move the eyes, eyelids, and limbs. Ocular MG affects only the muscles that move the eyes and eyelids, whereas generalized MG (gMG) affects muscles throughout the body. When MG occurs with a thymoma, it is called thymoma-associated MG and is considered a paraneoplastic disease. In severe cases of MG, patients can experience a myasthenic crisis (MC), during which respiratory muscles weaken and necessitate mechanical ventilation. Diagnosis of MG is based on clinical examination, and laboratory tests are used to confirm the diagnosis. Treatment options include cholinesterase enzyme inhibitors and immunosuppressive agents, which aim to either reduce symptoms or cause nonspecific immunosuppression, respectively, but do not target the pathogenetic autoantibodies that characterize the disease.
1. The most common age at onset of gMG is the second and third decades in women and the seventh and eighth decades in men.
MG has an annual incidence of approximately four to 30 new cases per million population. Prevalence rates range from 150 to 200 cases per million population, and they have steadily increased over the past 50 years. This increase in prevalence is probably the result of better disease recognition, aging of the population, and an increased life span in patients.
MG can occur at any age; however, onset is more common in females in the second and third decades and is more common in males in the seventh to eighth decades. Before age 40 years, the female-to-male ratio is 3:1, and after age 50 years, the female-to-male ratio is 3:2.
2. gMG commonly weakens muscles responsible for eye movement, facial expressions, and functions such as chewing, swallowing, and speaking.
gMG typically manifests as muscle weakness that worsens with repeated use. Patients often report that their function is best in the morning, with more pronounced weakness at the end of the day. Permanent muscle damage is rare, however, and maximal muscle strength is often good.
Extraocular muscles are more commonly affected, as twitch fibers in these muscles develop tension faster, have a higher frequency of synaptic firing than limb muscles, and have fewer AChRs, making them more susceptible to fatigue. Patients present asymmetrically; intermittent drooping of the upper eyelid (ptosis) and double vision (diplopia) are the most common symptoms.
Muscles innervated by the cranial nerves (bulbar muscles) are involved in 60% of patients with gMG and can lead to fatigable chewing, reduced facial expression, speech difficulties (dysarthria), and weakness of swallowing (dysphagia). Up to 15% of patients initially present with bulbar muscle involvement, including dysarthria and painless dysphagia.
3. Emotional stress can trigger an MC.
MC is a complication of MG characterized by worsening muscle weakness that results in respiratory failure and necessitates mechanical ventilation.
MC is often the result of respiratory muscle weakness but can also be due to bulbar weakness with upper airway collapse. MC can occur in 15%-20% of patients within the first 2-3 years of the disease; however, it can also be the first presentation of MG in 18%-28% of cases.
MC can be triggered by multiple causes, including emotional or physical stress. The most common precipitant is infection; other precipitants include surgery, pregnancy, perimenstrual state, certain medications, tapering of immune-modulating medications, exposure to temperature extremes, pain, and sleep deprivation. Approximately one third to one half of patients with MC may have no obvious cause.
4. High levels of anti-AChR antibodies strongly indicate MG, but normal levels do not rule it out.
All patients with a clinical history suggestive of MG should be tested for antibodies for confirmation. Most patients have anti-AChR antibodies (~85%), and those without have anti–muscle-specific kinase (MuSK antibodies) (6%) and anti–lipoprotein receptor-related protein 4 (LRP4) antibodies (2%).
The sensitivity of anti-AChR antibodies varies depending on whether the antibody is binding, modulating, or blocking the AChR. Binding antibody is the most common, and when combined with blocking antibodies, has a high sensitivity (99.6%) and is typically tested first. Higher AChR antibody titers are more specific for the diagnosis of MG than are low titers, but they do not correlate with disease severity.
For patients who do not have anti-AChR antibodies but do have clinical features of MG, anti-MuSK antibodies and anti-LRP4 antibodies are measured to increase diagnostic sensitivity. For symptomatic patients who do not have any autoantibodies (seronegative), electrodiagnostic testing that shows evidence of impaired signal transmission at the neuromuscular junction is used to confirm the diagnosis of MG.
5. Studies suggest that over 75% of seropositive MG patients show distinct thymus abnormalities.
More than 75% of patients with AChR antibody–positive MG have abnormalities in their thymus, and up to 40% of patients with a thymoma have MG. Among those with thymic pathology, thymic hyperplasia is the most common type (85%), but other thymic tumors (mainly thymoma) can be present in up to 15% of cases. Thymomas are typically noninvasive and cortical, but in some rare cases, invasive thymic carcinoma can occur.
Given this overlap in presentation, it is recommended that patients with seronegative and seropositive MG undergo chest CT or MRI for evaluation of their anterior mediastinal anatomy and to detect the presence of a thymoma. For patients with MG and a thymoma, as well as selected (nonthymomatous) patients with seropositive or seronegative MG, therapeutic thymectomy is recommended.
Myasthenia gravis (MG) is a rare autoimmune neurologic disorder that occurs when the transmission between nerves and muscles is disrupted. It is caused by autoantibodies against acetylcholine receptors (AChRs), which results in muscle weakness that is often fatigable and affects various muscles in the body, including those that move the eyes, eyelids, and limbs. Ocular MG affects only the muscles that move the eyes and eyelids, whereas generalized MG (gMG) affects muscles throughout the body. When MG occurs with a thymoma, it is called thymoma-associated MG and is considered a paraneoplastic disease. In severe cases of MG, patients can experience a myasthenic crisis (MC), during which respiratory muscles weaken and necessitate mechanical ventilation. Diagnosis of MG is based on clinical examination, and laboratory tests are used to confirm the diagnosis. Treatment options include cholinesterase enzyme inhibitors and immunosuppressive agents, which aim to either reduce symptoms or cause nonspecific immunosuppression, respectively, but do not target the pathogenetic autoantibodies that characterize the disease.
1. The most common age at onset of gMG is the second and third decades in women and the seventh and eighth decades in men.
MG has an annual incidence of approximately four to 30 new cases per million population. Prevalence rates range from 150 to 200 cases per million population, and they have steadily increased over the past 50 years. This increase in prevalence is probably the result of better disease recognition, aging of the population, and an increased life span in patients.
MG can occur at any age; however, onset is more common in females in the second and third decades and is more common in males in the seventh to eighth decades. Before age 40 years, the female-to-male ratio is 3:1, and after age 50 years, the female-to-male ratio is 3:2.
2. gMG commonly weakens muscles responsible for eye movement, facial expressions, and functions such as chewing, swallowing, and speaking.
gMG typically manifests as muscle weakness that worsens with repeated use. Patients often report that their function is best in the morning, with more pronounced weakness at the end of the day. Permanent muscle damage is rare, however, and maximal muscle strength is often good.
Extraocular muscles are more commonly affected, as twitch fibers in these muscles develop tension faster, have a higher frequency of synaptic firing than limb muscles, and have fewer AChRs, making them more susceptible to fatigue. Patients present asymmetrically; intermittent drooping of the upper eyelid (ptosis) and double vision (diplopia) are the most common symptoms.
Muscles innervated by the cranial nerves (bulbar muscles) are involved in 60% of patients with gMG and can lead to fatigable chewing, reduced facial expression, speech difficulties (dysarthria), and weakness of swallowing (dysphagia). Up to 15% of patients initially present with bulbar muscle involvement, including dysarthria and painless dysphagia.
3. Emotional stress can trigger an MC.
MC is a complication of MG characterized by worsening muscle weakness that results in respiratory failure and necessitates mechanical ventilation.
MC is often the result of respiratory muscle weakness but can also be due to bulbar weakness with upper airway collapse. MC can occur in 15%-20% of patients within the first 2-3 years of the disease; however, it can also be the first presentation of MG in 18%-28% of cases.
MC can be triggered by multiple causes, including emotional or physical stress. The most common precipitant is infection; other precipitants include surgery, pregnancy, perimenstrual state, certain medications, tapering of immune-modulating medications, exposure to temperature extremes, pain, and sleep deprivation. Approximately one third to one half of patients with MC may have no obvious cause.
4. High levels of anti-AChR antibodies strongly indicate MG, but normal levels do not rule it out.
All patients with a clinical history suggestive of MG should be tested for antibodies for confirmation. Most patients have anti-AChR antibodies (~85%), and those without have anti–muscle-specific kinase (MuSK antibodies) (6%) and anti–lipoprotein receptor-related protein 4 (LRP4) antibodies (2%).
The sensitivity of anti-AChR antibodies varies depending on whether the antibody is binding, modulating, or blocking the AChR. Binding antibody is the most common, and when combined with blocking antibodies, has a high sensitivity (99.6%) and is typically tested first. Higher AChR antibody titers are more specific for the diagnosis of MG than are low titers, but they do not correlate with disease severity.
For patients who do not have anti-AChR antibodies but do have clinical features of MG, anti-MuSK antibodies and anti-LRP4 antibodies are measured to increase diagnostic sensitivity. For symptomatic patients who do not have any autoantibodies (seronegative), electrodiagnostic testing that shows evidence of impaired signal transmission at the neuromuscular junction is used to confirm the diagnosis of MG.
5. Studies suggest that over 75% of seropositive MG patients show distinct thymus abnormalities.
More than 75% of patients with AChR antibody–positive MG have abnormalities in their thymus, and up to 40% of patients with a thymoma have MG. Among those with thymic pathology, thymic hyperplasia is the most common type (85%), but other thymic tumors (mainly thymoma) can be present in up to 15% of cases. Thymomas are typically noninvasive and cortical, but in some rare cases, invasive thymic carcinoma can occur.
Given this overlap in presentation, it is recommended that patients with seronegative and seropositive MG undergo chest CT or MRI for evaluation of their anterior mediastinal anatomy and to detect the presence of a thymoma. For patients with MG and a thymoma, as well as selected (nonthymomatous) patients with seropositive or seronegative MG, therapeutic thymectomy is recommended.
Myasthenia gravis (MG) is a rare autoimmune neurologic disorder that occurs when the transmission between nerves and muscles is disrupted. It is caused by autoantibodies against acetylcholine receptors (AChRs), which results in muscle weakness that is often fatigable and affects various muscles in the body, including those that move the eyes, eyelids, and limbs. Ocular MG affects only the muscles that move the eyes and eyelids, whereas generalized MG (gMG) affects muscles throughout the body. When MG occurs with a thymoma, it is called thymoma-associated MG and is considered a paraneoplastic disease. In severe cases of MG, patients can experience a myasthenic crisis (MC), during which respiratory muscles weaken and necessitate mechanical ventilation. Diagnosis of MG is based on clinical examination, and laboratory tests are used to confirm the diagnosis. Treatment options include cholinesterase enzyme inhibitors and immunosuppressive agents, which aim to either reduce symptoms or cause nonspecific immunosuppression, respectively, but do not target the pathogenetic autoantibodies that characterize the disease.
1. The most common age at onset of gMG is the second and third decades in women and the seventh and eighth decades in men.
MG has an annual incidence of approximately four to 30 new cases per million population. Prevalence rates range from 150 to 200 cases per million population, and they have steadily increased over the past 50 years. This increase in prevalence is probably the result of better disease recognition, aging of the population, and an increased life span in patients.
MG can occur at any age; however, onset is more common in females in the second and third decades and is more common in males in the seventh to eighth decades. Before age 40 years, the female-to-male ratio is 3:1, and after age 50 years, the female-to-male ratio is 3:2.
2. gMG commonly weakens muscles responsible for eye movement, facial expressions, and functions such as chewing, swallowing, and speaking.
gMG typically manifests as muscle weakness that worsens with repeated use. Patients often report that their function is best in the morning, with more pronounced weakness at the end of the day. Permanent muscle damage is rare, however, and maximal muscle strength is often good.
Extraocular muscles are more commonly affected, as twitch fibers in these muscles develop tension faster, have a higher frequency of synaptic firing than limb muscles, and have fewer AChRs, making them more susceptible to fatigue. Patients present asymmetrically; intermittent drooping of the upper eyelid (ptosis) and double vision (diplopia) are the most common symptoms.
Muscles innervated by the cranial nerves (bulbar muscles) are involved in 60% of patients with gMG and can lead to fatigable chewing, reduced facial expression, speech difficulties (dysarthria), and weakness of swallowing (dysphagia). Up to 15% of patients initially present with bulbar muscle involvement, including dysarthria and painless dysphagia.
3. Emotional stress can trigger an MC.
MC is a complication of MG characterized by worsening muscle weakness that results in respiratory failure and necessitates mechanical ventilation.
MC is often the result of respiratory muscle weakness but can also be due to bulbar weakness with upper airway collapse. MC can occur in 15%-20% of patients within the first 2-3 years of the disease; however, it can also be the first presentation of MG in 18%-28% of cases.
MC can be triggered by multiple causes, including emotional or physical stress. The most common precipitant is infection; other precipitants include surgery, pregnancy, perimenstrual state, certain medications, tapering of immune-modulating medications, exposure to temperature extremes, pain, and sleep deprivation. Approximately one third to one half of patients with MC may have no obvious cause.
4. High levels of anti-AChR antibodies strongly indicate MG, but normal levels do not rule it out.
All patients with a clinical history suggestive of MG should be tested for antibodies for confirmation. Most patients have anti-AChR antibodies (~85%), and those without have anti–muscle-specific kinase (MuSK antibodies) (6%) and anti–lipoprotein receptor-related protein 4 (LRP4) antibodies (2%).
The sensitivity of anti-AChR antibodies varies depending on whether the antibody is binding, modulating, or blocking the AChR. Binding antibody is the most common, and when combined with blocking antibodies, has a high sensitivity (99.6%) and is typically tested first. Higher AChR antibody titers are more specific for the diagnosis of MG than are low titers, but they do not correlate with disease severity.
For patients who do not have anti-AChR antibodies but do have clinical features of MG, anti-MuSK antibodies and anti-LRP4 antibodies are measured to increase diagnostic sensitivity. For symptomatic patients who do not have any autoantibodies (seronegative), electrodiagnostic testing that shows evidence of impaired signal transmission at the neuromuscular junction is used to confirm the diagnosis of MG.
5. Studies suggest that over 75% of seropositive MG patients show distinct thymus abnormalities.
More than 75% of patients with AChR antibody–positive MG have abnormalities in their thymus, and up to 40% of patients with a thymoma have MG. Among those with thymic pathology, thymic hyperplasia is the most common type (85%), but other thymic tumors (mainly thymoma) can be present in up to 15% of cases. Thymomas are typically noninvasive and cortical, but in some rare cases, invasive thymic carcinoma can occur.
Given this overlap in presentation, it is recommended that patients with seronegative and seropositive MG undergo chest CT or MRI for evaluation of their anterior mediastinal anatomy and to detect the presence of a thymoma. For patients with MG and a thymoma, as well as selected (nonthymomatous) patients with seropositive or seronegative MG, therapeutic thymectomy is recommended.
COVID-19 Is a Very Weird Virus
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.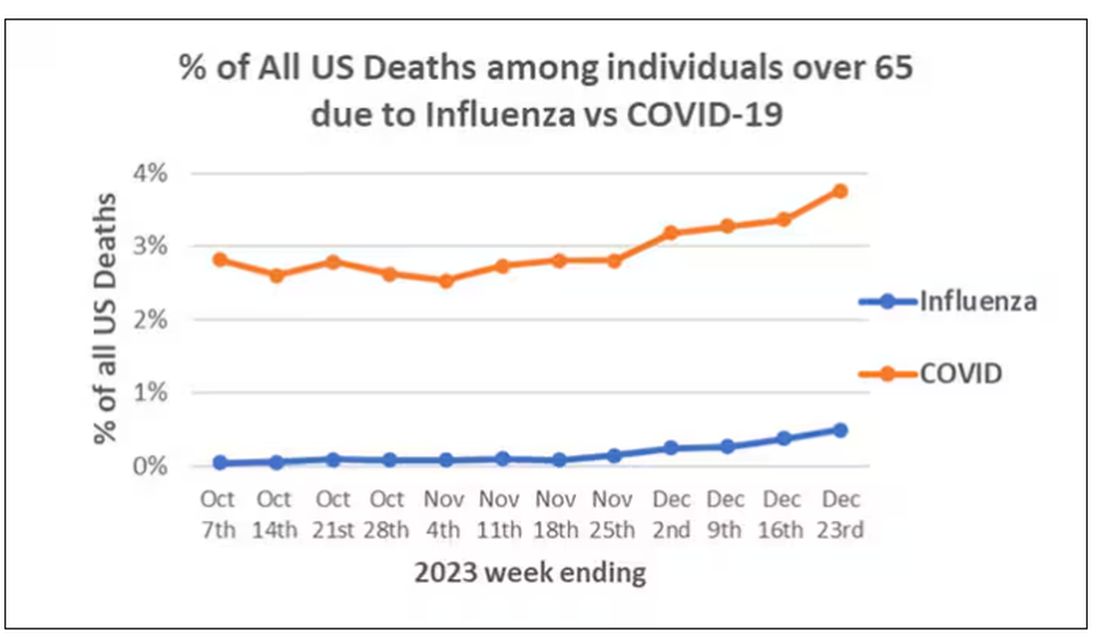
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)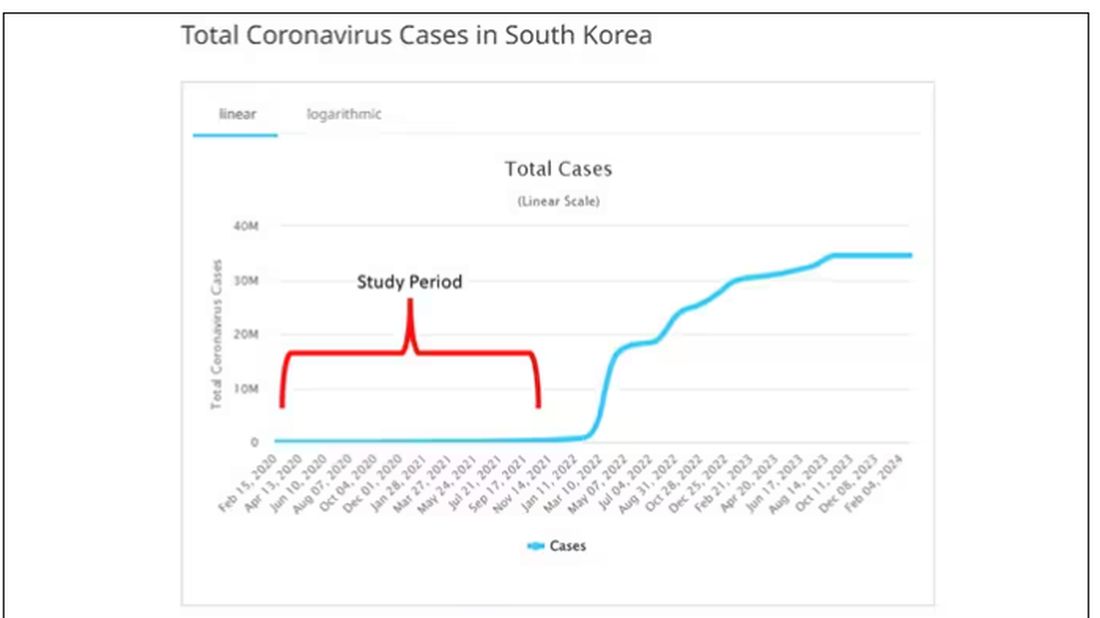
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.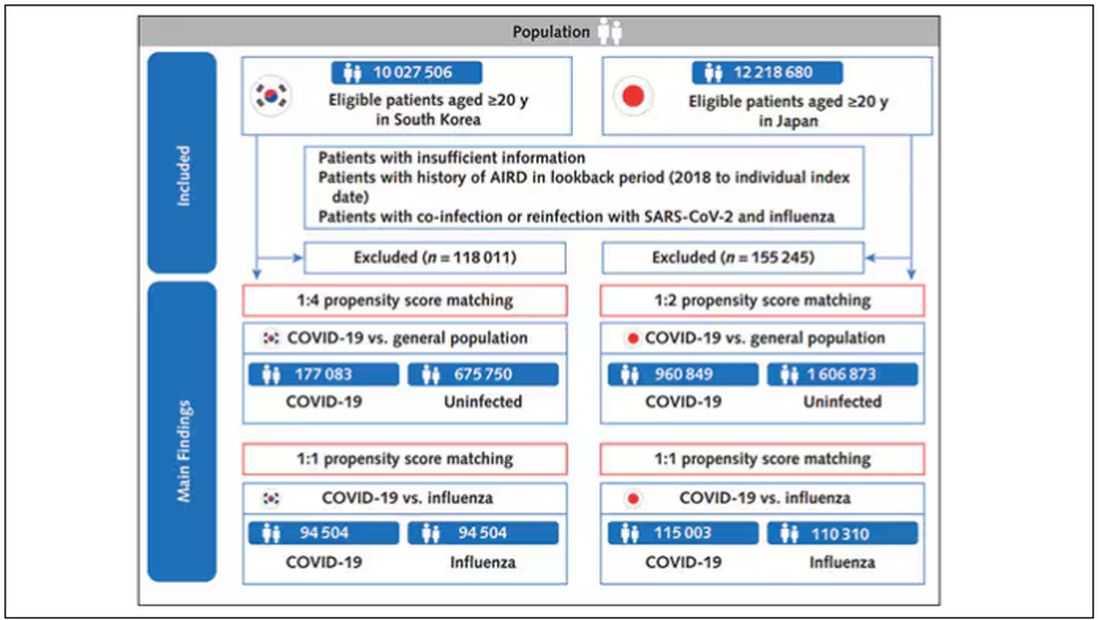
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).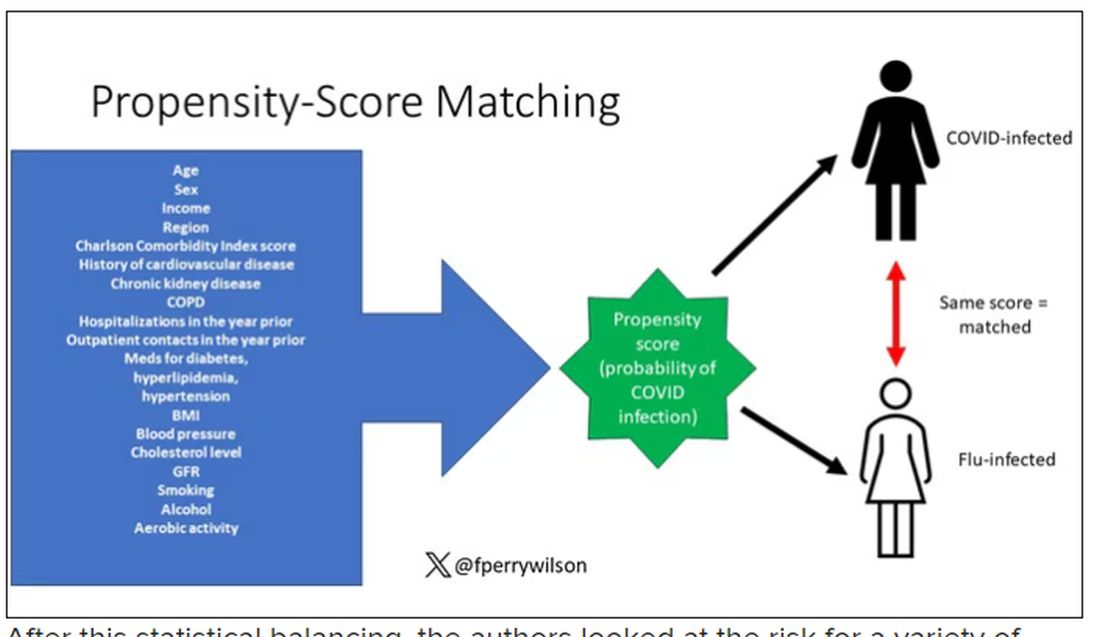
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.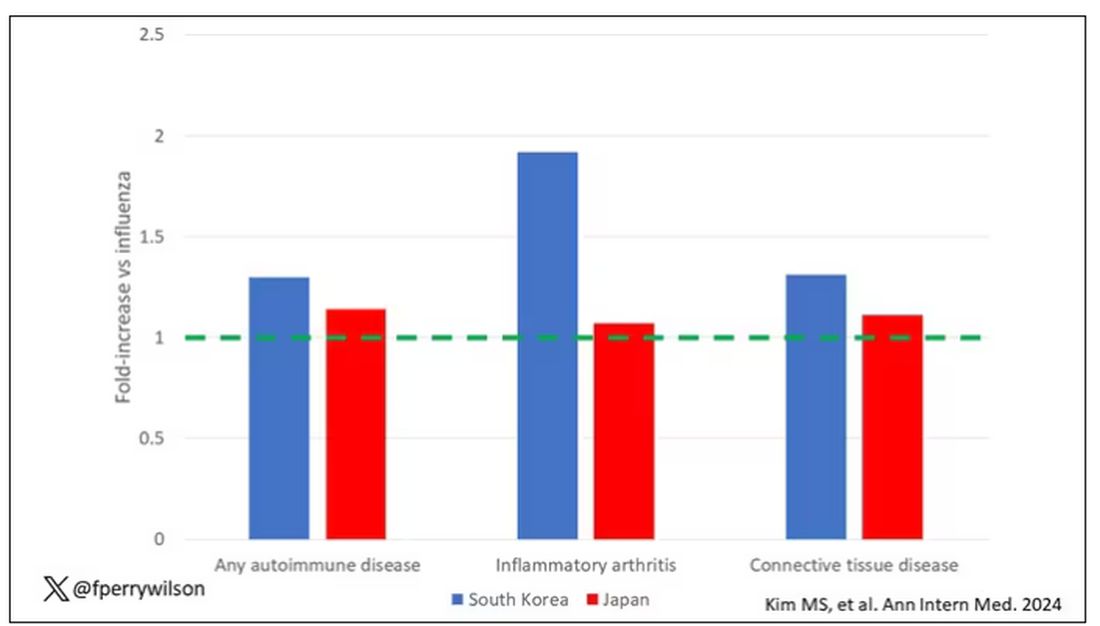
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.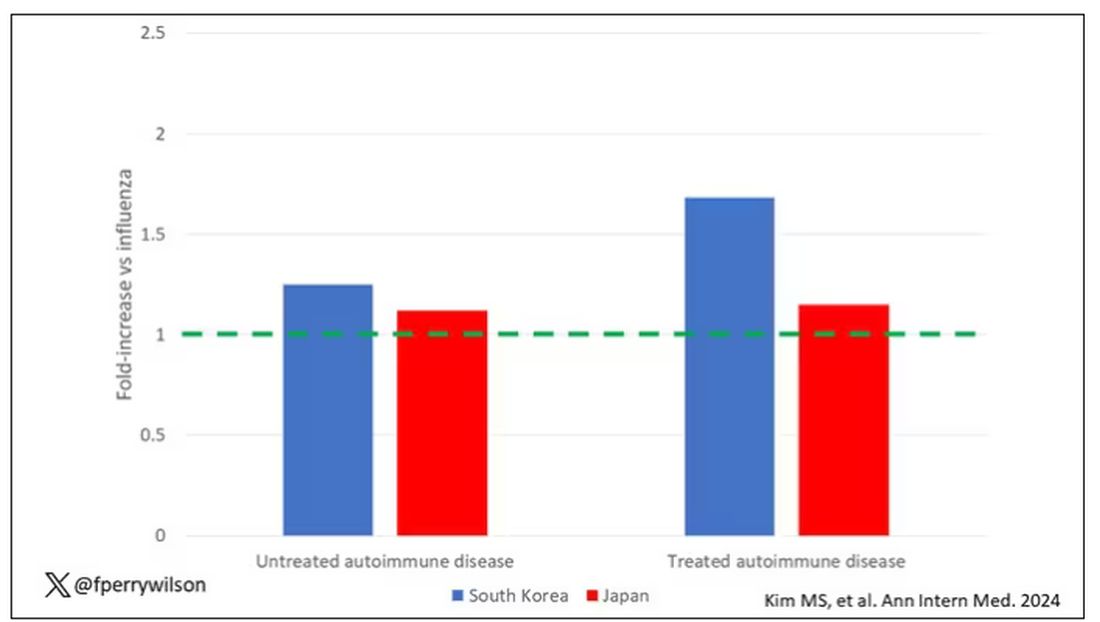
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.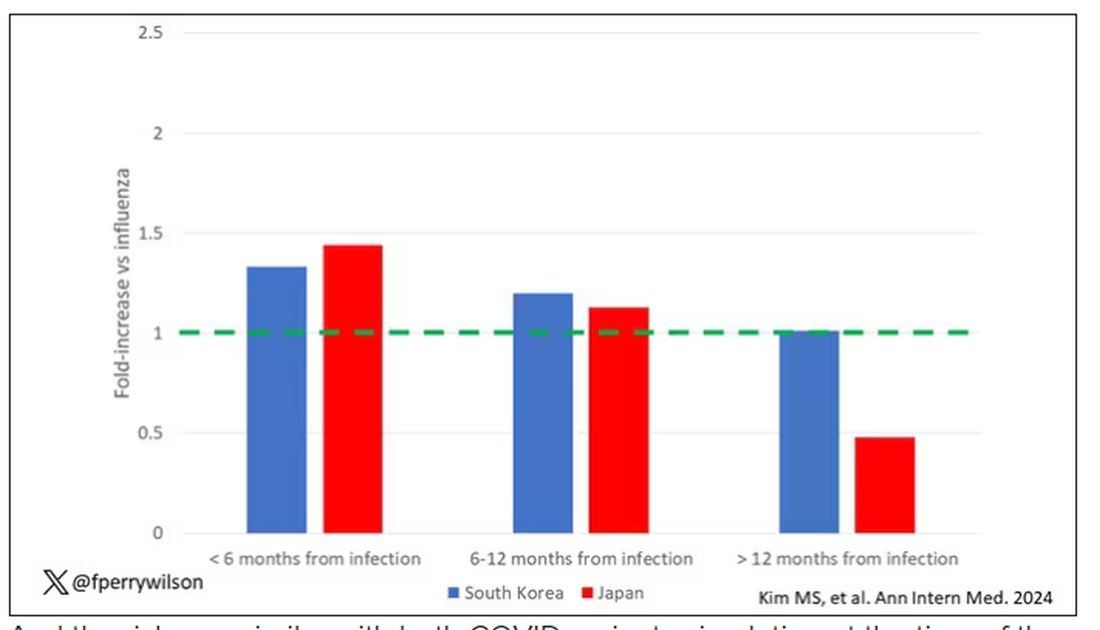
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?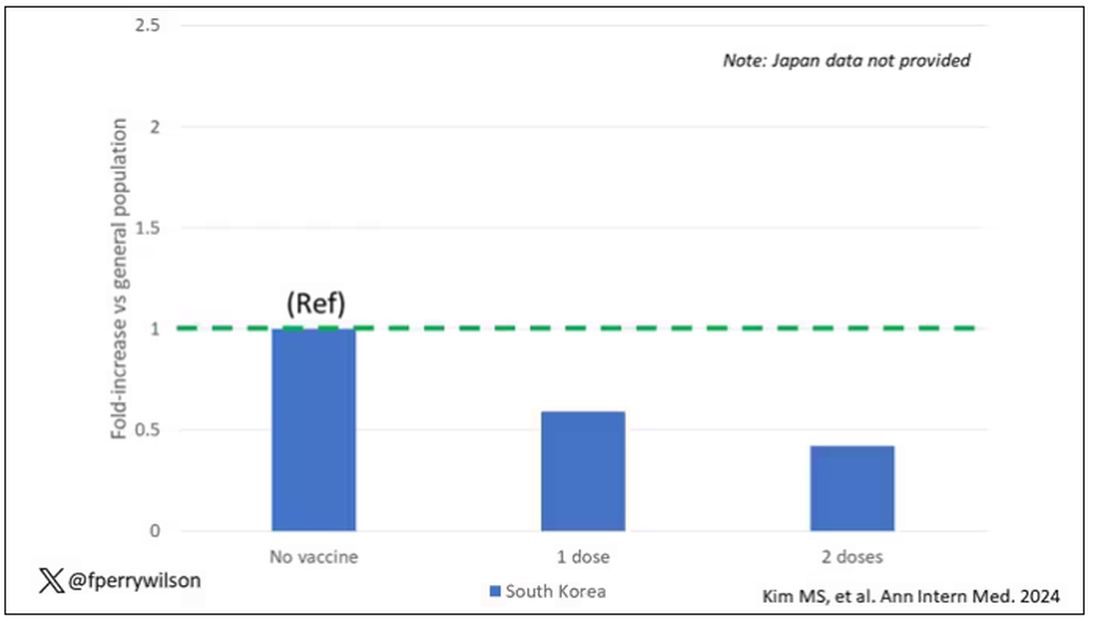
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
What’s Changed in Asthma Treatment? Quite a Bit
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
Bartonella henselae Infection May Occasionally Distract Immune Control of Latent Human Herpesviruses
To the Editor:
We read with interest the September 2023 Cutis article by Swink et al,1 “Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl.” The authors documented the possibility of Bartonella henselae infection as another causative agent for pityriasis rosea (PR) even though the association of PR with human herpesvirus (HHV) 6 and HHV-7 infection is based on several consistent observations and not on occasional findings. The association of PR with endogenous systemic reactivation of HHV-6 and HHV-7 has been identified with different investigations and laboratory techniques. Using polymerase chain reaction, real-time calibrated quantitative polymerase chain reaction, in situ hybridization, immunohistochemistry, and electron microscopy, HHV-6 and HHV-7 have been detected in plasma (a marker of active viral replication), peripheral blood mononuclear cells, and skin lesions from patients with PR.2 In addition, HHV-6 and HHV-7 messenger RNA expression and their specific antigens have been detected in PR skin lesions and herpesvirus virions in various stages of morphogenesis as well as in the supernatant of co-cultured peripheral blood mononuclear cells of patients with PR.2,3 Lastly, the increased levels of several particular cytokines and chemokinesin the sera of patients with PR support a viral role in its pathogenesis.4
Bartonella henselae is a gram-negative intracellular facultative bacterium that is commonly implicated in causing zoonotic infections worldwide. The incidence of cat-scratch disease (CSD) was reported to be 6.4 cases per 100,000 population in adults and 9.4 cases per 100,000 population in children aged 5 to 9 years globally.5 Approximately 24,000 cases of CSD are reported in the United States every year.6 Therefore, considering these data, if B henselae was a causative agent for PR, the eruption would be observed frequently in many patients with CSD, which is not the case. On the contrary, it is possible that B henselae infection may have reactivated HHV-6 and/or HHV-7 infection. It is well established that B henselae causes a robust cell-mediated immune response by activating natural killer and helper T cells (TH1) and enhancement of cytotoxic T lymphocytes.7 It could be assumed that by strongly stimulating the immune response and polarizing it to a specific antigen cell response, B henselae infection may temporarily distract the T cell-mediated control of the latent infections, such as HHV-6 and HHV-7, which may reactivate and cause PR.
It is important to point out that a case of concomitant B henselae and Epstein-Barr virus infection has been described.8 Even in that case, the B henselae infection may have reactivated Epstein-Barr virus as well as HHV-6 and HHV-7 in the case described by Swink et al.1 Epstein-Barr virus reactivation has been detected in one case8 through serologic testing—IgM, IgG, Epstein-Barr virus nuclear antigen IgG, and heterophile antibodies—as there were no dermatologic manifestations that may be related to Epstein-Barr virus reactivation from latency.9
In conclusion, a viral or bacterial infection such as Epstein-Barr virus or B henselae may have a transactivating function allowing another (latent) virus such as HHV-6 or HHV-7 to reactivate. Indeed, it has been described that SARS-CoV-2 may act as a transactivator agent triggering HHV-6/HHV-7 reactivation, thereby indirectly causing PR clinical manifestation.10
- Swink SM, Rhodes LP, Levin J. Cat scratch disease presenting with concurrent pityriasis rosea in a 10-year-old girl. Cutis. 2023;112:E24-E26. doi:10.12788/cutis.0861
- Broccolo F, Drago F, Careddu AM, et al. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus-6 and -7. J Invest Dermatol. 2005;124:1234-1240.
- Rebora A, Ciccarese G, Herzum A, et al. Pityriasis rosea and other infectious eruptions during pregnancy: possible life-threatening health conditions for the fetus. Clin Dermatol. 2020;38:105-112.
- Drago F, Ciccarese G, Broccolo F, et al. The role of cytokines, chemokines, and growth factors in the pathogenesis of pityriasis rosea. Mediators Inflamm. 2015;2015:438963. doi:10.1155/2015/438963
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Ackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707-1711.
- Resto-Ruiz S, Burgess A, Anderson BE. The role of the host immune response in pathogenesis of Bartonella henselae. DNA Cell Biol. 2003; 22:431-440.
- Aparicio-Casares H, Puente-Rico MH, Tomé-Nestal C, et al. A pediatric case of Bartonella henselae and Epstein Barr virus disease with bone and hepatosplenic involvement. Bol Med Hosp Infant Mex. 2021;78:467-473.
- Ciccarese G, Trave I, Herzum A, et al. Dermatological manifestations of Epstein-Barr virus systemic infection: a case report and literature review. Int J Dermatol. 2020;59:1202-1209.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;40:586-590.
To the Editor:
We read with interest the September 2023 Cutis article by Swink et al,1 “Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl.” The authors documented the possibility of Bartonella henselae infection as another causative agent for pityriasis rosea (PR) even though the association of PR with human herpesvirus (HHV) 6 and HHV-7 infection is based on several consistent observations and not on occasional findings. The association of PR with endogenous systemic reactivation of HHV-6 and HHV-7 has been identified with different investigations and laboratory techniques. Using polymerase chain reaction, real-time calibrated quantitative polymerase chain reaction, in situ hybridization, immunohistochemistry, and electron microscopy, HHV-6 and HHV-7 have been detected in plasma (a marker of active viral replication), peripheral blood mononuclear cells, and skin lesions from patients with PR.2 In addition, HHV-6 and HHV-7 messenger RNA expression and their specific antigens have been detected in PR skin lesions and herpesvirus virions in various stages of morphogenesis as well as in the supernatant of co-cultured peripheral blood mononuclear cells of patients with PR.2,3 Lastly, the increased levels of several particular cytokines and chemokinesin the sera of patients with PR support a viral role in its pathogenesis.4
Bartonella henselae is a gram-negative intracellular facultative bacterium that is commonly implicated in causing zoonotic infections worldwide. The incidence of cat-scratch disease (CSD) was reported to be 6.4 cases per 100,000 population in adults and 9.4 cases per 100,000 population in children aged 5 to 9 years globally.5 Approximately 24,000 cases of CSD are reported in the United States every year.6 Therefore, considering these data, if B henselae was a causative agent for PR, the eruption would be observed frequently in many patients with CSD, which is not the case. On the contrary, it is possible that B henselae infection may have reactivated HHV-6 and/or HHV-7 infection. It is well established that B henselae causes a robust cell-mediated immune response by activating natural killer and helper T cells (TH1) and enhancement of cytotoxic T lymphocytes.7 It could be assumed that by strongly stimulating the immune response and polarizing it to a specific antigen cell response, B henselae infection may temporarily distract the T cell-mediated control of the latent infections, such as HHV-6 and HHV-7, which may reactivate and cause PR.
It is important to point out that a case of concomitant B henselae and Epstein-Barr virus infection has been described.8 Even in that case, the B henselae infection may have reactivated Epstein-Barr virus as well as HHV-6 and HHV-7 in the case described by Swink et al.1 Epstein-Barr virus reactivation has been detected in one case8 through serologic testing—IgM, IgG, Epstein-Barr virus nuclear antigen IgG, and heterophile antibodies—as there were no dermatologic manifestations that may be related to Epstein-Barr virus reactivation from latency.9
In conclusion, a viral or bacterial infection such as Epstein-Barr virus or B henselae may have a transactivating function allowing another (latent) virus such as HHV-6 or HHV-7 to reactivate. Indeed, it has been described that SARS-CoV-2 may act as a transactivator agent triggering HHV-6/HHV-7 reactivation, thereby indirectly causing PR clinical manifestation.10
To the Editor:
We read with interest the September 2023 Cutis article by Swink et al,1 “Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl.” The authors documented the possibility of Bartonella henselae infection as another causative agent for pityriasis rosea (PR) even though the association of PR with human herpesvirus (HHV) 6 and HHV-7 infection is based on several consistent observations and not on occasional findings. The association of PR with endogenous systemic reactivation of HHV-6 and HHV-7 has been identified with different investigations and laboratory techniques. Using polymerase chain reaction, real-time calibrated quantitative polymerase chain reaction, in situ hybridization, immunohistochemistry, and electron microscopy, HHV-6 and HHV-7 have been detected in plasma (a marker of active viral replication), peripheral blood mononuclear cells, and skin lesions from patients with PR.2 In addition, HHV-6 and HHV-7 messenger RNA expression and their specific antigens have been detected in PR skin lesions and herpesvirus virions in various stages of morphogenesis as well as in the supernatant of co-cultured peripheral blood mononuclear cells of patients with PR.2,3 Lastly, the increased levels of several particular cytokines and chemokinesin the sera of patients with PR support a viral role in its pathogenesis.4
Bartonella henselae is a gram-negative intracellular facultative bacterium that is commonly implicated in causing zoonotic infections worldwide. The incidence of cat-scratch disease (CSD) was reported to be 6.4 cases per 100,000 population in adults and 9.4 cases per 100,000 population in children aged 5 to 9 years globally.5 Approximately 24,000 cases of CSD are reported in the United States every year.6 Therefore, considering these data, if B henselae was a causative agent for PR, the eruption would be observed frequently in many patients with CSD, which is not the case. On the contrary, it is possible that B henselae infection may have reactivated HHV-6 and/or HHV-7 infection. It is well established that B henselae causes a robust cell-mediated immune response by activating natural killer and helper T cells (TH1) and enhancement of cytotoxic T lymphocytes.7 It could be assumed that by strongly stimulating the immune response and polarizing it to a specific antigen cell response, B henselae infection may temporarily distract the T cell-mediated control of the latent infections, such as HHV-6 and HHV-7, which may reactivate and cause PR.
It is important to point out that a case of concomitant B henselae and Epstein-Barr virus infection has been described.8 Even in that case, the B henselae infection may have reactivated Epstein-Barr virus as well as HHV-6 and HHV-7 in the case described by Swink et al.1 Epstein-Barr virus reactivation has been detected in one case8 through serologic testing—IgM, IgG, Epstein-Barr virus nuclear antigen IgG, and heterophile antibodies—as there were no dermatologic manifestations that may be related to Epstein-Barr virus reactivation from latency.9
In conclusion, a viral or bacterial infection such as Epstein-Barr virus or B henselae may have a transactivating function allowing another (latent) virus such as HHV-6 or HHV-7 to reactivate. Indeed, it has been described that SARS-CoV-2 may act as a transactivator agent triggering HHV-6/HHV-7 reactivation, thereby indirectly causing PR clinical manifestation.10
- Swink SM, Rhodes LP, Levin J. Cat scratch disease presenting with concurrent pityriasis rosea in a 10-year-old girl. Cutis. 2023;112:E24-E26. doi:10.12788/cutis.0861
- Broccolo F, Drago F, Careddu AM, et al. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus-6 and -7. J Invest Dermatol. 2005;124:1234-1240.
- Rebora A, Ciccarese G, Herzum A, et al. Pityriasis rosea and other infectious eruptions during pregnancy: possible life-threatening health conditions for the fetus. Clin Dermatol. 2020;38:105-112.
- Drago F, Ciccarese G, Broccolo F, et al. The role of cytokines, chemokines, and growth factors in the pathogenesis of pityriasis rosea. Mediators Inflamm. 2015;2015:438963. doi:10.1155/2015/438963
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Ackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707-1711.
- Resto-Ruiz S, Burgess A, Anderson BE. The role of the host immune response in pathogenesis of Bartonella henselae. DNA Cell Biol. 2003; 22:431-440.
- Aparicio-Casares H, Puente-Rico MH, Tomé-Nestal C, et al. A pediatric case of Bartonella henselae and Epstein Barr virus disease with bone and hepatosplenic involvement. Bol Med Hosp Infant Mex. 2021;78:467-473.
- Ciccarese G, Trave I, Herzum A, et al. Dermatological manifestations of Epstein-Barr virus systemic infection: a case report and literature review. Int J Dermatol. 2020;59:1202-1209.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;40:586-590.
- Swink SM, Rhodes LP, Levin J. Cat scratch disease presenting with concurrent pityriasis rosea in a 10-year-old girl. Cutis. 2023;112:E24-E26. doi:10.12788/cutis.0861
- Broccolo F, Drago F, Careddu AM, et al. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus-6 and -7. J Invest Dermatol. 2005;124:1234-1240.
- Rebora A, Ciccarese G, Herzum A, et al. Pityriasis rosea and other infectious eruptions during pregnancy: possible life-threatening health conditions for the fetus. Clin Dermatol. 2020;38:105-112.
- Drago F, Ciccarese G, Broccolo F, et al. The role of cytokines, chemokines, and growth factors in the pathogenesis of pityriasis rosea. Mediators Inflamm. 2015;2015:438963. doi:10.1155/2015/438963
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Ackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707-1711.
- Resto-Ruiz S, Burgess A, Anderson BE. The role of the host immune response in pathogenesis of Bartonella henselae. DNA Cell Biol. 2003; 22:431-440.
- Aparicio-Casares H, Puente-Rico MH, Tomé-Nestal C, et al. A pediatric case of Bartonella henselae and Epstein Barr virus disease with bone and hepatosplenic involvement. Bol Med Hosp Infant Mex. 2021;78:467-473.
- Ciccarese G, Trave I, Herzum A, et al. Dermatological manifestations of Epstein-Barr virus systemic infection: a case report and literature review. Int J Dermatol. 2020;59:1202-1209.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;40:586-590.
Vulvar Lichen Sclerosus: What’s New?
Vulvar lichen sclerosus (VLS) is an underserved area in medicine and dermatology. We discuss updates in VLS, which include the following: (1) development of core outcome domains to include in all future clinical trials, with current efforts focused on determining outcome measurements for each domain; (2) increased understanding of the impact VLS has on quality-of-life (QOL) outcomes; (3) expanded disease associations; (4) clinical and histologic variants, including vestibular sclerosis and nonsclerotic VLS; and (5) updates in management of VLS.
Core Outcomes Measures
The burden of VLS is challenging to quantify, with little agreement among experts.1 Recently there has been a focus on developing scoring scales to measure disease progression and treatment response. Simpson et al2 pioneered the development of a core outcome set to be included in all future clinical trials for genital lichen sclerosus (LS)—clinical (visible) signs, symptoms, and LS-specific QOL.
Although there is no standardized method for assessing disease severity, various scales have been proposed to measure clinical findings in VLS, such as the vulvar architecture severity scale3 as well as the clinical LS score,4 which is the only validated scale to incorporate the signs and architectural changes identified by a 2018 Delphi consensus group of the International Society for the Study of Vulvovaginal Disease.5 Work is ongoing to identify and evaluate outcome measurement instruments for each of the 3 core outcome domains.
Increased Understanding of QOL Impacts
Pain, pruritus, impairment of sexual function, genitourinary complications, architectural changes, and risk for squamous cell carcinoma (SCC) all have been well established as VLS sequelae.6,7 Recent studies have focused on the QOL impact and associations with psychiatric comorbidities. A matched case-control study found that LS was significantly associated with depression and anxiety among US women (P<.001), and individuals with LS had a more than 2-fold increased odds of receiving a diagnosis of depression or anxiety.8
A review evaluating QOL outcomes in LS found that overall QOL was impaired. Female patients reported worse QOL in the work-school domain of the dermatology life quality index compared with male counterparts.9
Finally, a study exploring the experiences of patients living with VLS highlighted the secrecy and stigma of the condition,10 which serves as a call to action to improve the general population’s knowledge about vulvar anatomy and create change in societal attitudes on vulvar conditions.
Although there are several instruments assessing vulvar-specific QOL, most are for patients with vulvar cancer and focus on sexual function. In 2020, Saunderson et al11 published the 15-item vulvar quality of life index (VQLI), which has broad implications for measuring vulvar disease burden and is an important tool for standardizing vulvar disease measurements and outcomes for clinical research.12 The VQLI, though not specific to VLS, consists of 4 domains to assess vulvar QOL including symptoms, anxiety, activities of daily living, and sexuality. Studies have evaluated this scoring system in patients with VLS, with 1 study finding that VQLI correlated with clinician-rated severity scores (P=.01) and overall patient itch/discomfort score (P<.001) in VLS.13,14
Expanded Disease Associations
Lichen sclerosus has a well-known association with vulvar SCC and other autoimmune conditions, including thyroid disease and bullous pemphigoid.15-17 Recent studies also have revealed an association between LS and psoriasis.18 A case-control study from a single center found VLS was associated with elevated body mass index, statin usage, and cholecystectomy.19 Gynecologic pain syndromes, interstitial cystitis, urinary incontinence, and some gastrointestinal tract disorders including celiac disease also have been found to be increased in patients with VLS.20 Finally, the incidence of cutaneous immune-related adverse events such as LS has increased as the use of immune checkpoint therapies as anticancer treatments has expanded.21 Clinicians should be aware of these potential disease associations when caring for patients with VLS.
The incidence of VLS is higher in lower estrogen states throughout the lifespan, and a recent case-control study evaluated the cutaneous hormonal and microbial landscapes in postmenopausal patients (6 patients with VLS; 12 controls).22 Levels of the following cutaneous hormones in the groin were found to be altered in patients with VLS compared with controls: estrone (lower; P=.006), progesterone (higher; P<.0001), and testosterone (lower; P=.02). The authors found that most hormone levels normalized following treatment with a topical steroid. Additionally, bacterial microbiome alterations were seen in patients with VLS compared with controls. Thus, cutaneous sex hormone and skin microbiome alterations may be associated with VLS.22
Updates in Clinical and Histologic Variants
Less-recognized variants of VLS have been characterized in recent years. Vestibular sclerosis is a variant of VLS with unique clinical and histopathologic features; it is characterized by involvement localized to the anterior vestibule and either an absent or sparse lymphocytic infiltrate on histopathology.23,24 Nonsclerotic VLS is a variant with clinical features consistent with VLS that does not exhibit dermal sclerosis on histopathology. Thus, a diagnosis of nonsclerotic VLS requires clinicopathologic correlation. Four nonsclerotic histopathologic subtypes are proposed: lichenoid, hypertrophic lichenoid, dermal fibrosis without acanthosis, and dermal fibrosis with acanthosis.25 Longitudinal studies that correlate duration, signs, and symptoms will be important to further understand these variants.
Management Updates
First-line treatment of VLS still consists of ultrapotent topical corticosteroids with chronic maintenance therapy (usually lifetime) to decrease the risk for SCC and architectural changes.26 However, a survey across social media platforms found steroid phobia is common in patients with VLS (N=865), with approximately 40% of respondents endorsing waiting as long as they could before using topical corticosteroids and stopping as soon as possible.27 Clinicians should be aware of possible patient perceptions in the use of chronic steroids when discussing this therapy.
Randomized controlled trials utilizing fractional CO2 devices for VLS have been performed with conflicting results and no consensus regarding outcome measurement.28,29 Additionally, long-term disease outcomes following laser use have not been investigated. Although there is evidence that both ablative and nonablative devices can improve symptoms and signs, there is no evidence that they offer a cure for a chronic inflammatory skin condition. Current evidence suggests that even for patients undergoing these procedures, maintenance therapy is still essential to prevent sequelae.30 Future studies incorporating standardized outcome measures will be important for assessing the benefits of laser therapy in VLS. Finally, the reasons why topical corticosteroids may fail in an individual patient are multifaceted and should be explored thoroughly when considering laser therapy for VLS.
Studies evaluating the role of systemic therapies for refractory cases of VLS have expanded. A systematic review of systemic therapies for both genital and extragenital LS found oral corticosteroids and methotrexate were the most-reported systemic treatment regimens.31 Use of biologics in LS has been reported, with cases utilizing adalimumab for VLS and dupilumab for extragenital LS. Use of Janus kinase inhibitors including abrocitinib and baricitinib also has been reported for LS.31 A clinical trial to evaluate the safety and efficacy of topical ruxolitinib in VLS was recently completed (ClinicalTrials.govidentifier NCT05593445). Future research studies likely will focus on the safety and efficacy of targeted and steroid-sparing therapies for patients with VLS.
Final Thoughts
Vulvar lichen sclerosus increasingly is becoming recognized as a chronic genital skin condition that impacts QOL and health outcomes, with a need to develop more effective and safe evidence-based therapies. Recent literature has focused on the importance of developing and standardizing disease outcomes; identifying disease associations including the role of cutaneous hormones and microbiome alterations; characterizing histologic and clinical variants; and staying up-to-date on management, including the need for understanding patient perceptions of chronic topical steroid therapy. Each of these are important updates for clinicians to consider when caring for patients with VLS. Future studies likely will focus on elucidating disease etiology and mechanisms to gain a better understanding of VLS pathogenesis and potential targets for therapies as well as implementation of clinical trials that incorporate standardized outcome domains to test efficacy and safety of additional therapies.
- Sheinis M, Green N, Vieira-Baptista P, et al. Adult vulvar lichen sclerosus: can experts agree on the assessment of disease severity? J Low Genit Tract Dis. 2020;24:295-298. doi:10.1097/LGT.0000000000000534
- Simpson RC, Kirtschig G, Selk A, et al. Core outcome domains for lichen sclerosus: a CORALS initiative consensus statement. Br J Dermatol. 2023;188:628-635. doi:10.1093/bjd/ljac145
- Almadori A, Zenner N, Boyle D, et al. Development and validation of a clinical grading scale to assess the vulvar region: the Vulvar Architecture Severity Scale. Aesthet Surg J. 2020;40:1319-1326. doi:10.1093/asj/sjz342
- Erni B, Navarini AA, Huang D, et al. Proposition of a severity scale for lichen sclerosus: the “Clinical Lichen Sclerosus Score.” Dermatol Ther. 2021;34:E14773. doi:10.1111/dth.14773
- Sheinis M, Selk A. Development of the Adult Vulvar Lichen Sclerosus Severity Scale—a Delphi Consensus Exercise for Item Generation. J Low Genit Tract Dis. 2018;22:66-73. doi:10.1097/LGT.0000000000000361
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases. J Am Acad Dermatol. 2020;82:1287-1298. doi:10.1016/j.jaad.2019.10.077
- Wijaya M, Lee G, Fischer G. Why do some patients with vulval lichen sclerosus on long-term topical corticosteroid treatment experience ongoing poor quality of life? Australas J Dermatol. 2022;63:463-472. doi:10.1111/ajd.13926
- Fan R, Leasure AC, Maisha FI, et al. Depression and anxiety in patients with lichen sclerosus. JAMA Dermatol. 2022;158:953-954. doi:10.1001/jamadermatol.2022.1964
- Ranum A, Pearson DR. The impact of genital lichen sclerosus and lichen planus on quality of life: a review. Int J Womens Dermatol. 2022;8:E042. doi:10.1097/JW9.0000000000000042
- Arnold S, Fernando S, Rees S. Living with vulval lichen sclerosus: a qualitative interview study. Br J Dermatol. 2022;187:909-918. doi:10.1111/bjd.21777
- Saunderson RB, Harris V, Yeh R, et al. Vulvar quality of life index (VQLI)—a simple tool to measure quality of life in patients with vulvar disease. Australas J Dermatol. 2020;61:152-157. doi:10.1111/ajd.13235
- Pyle HJ, Evans JC, Vandergriff TW, et al. Vulvar lichen sclerosus clinical severity scales and histopathologic correlation: a case series. Am J Dermatopathol. 2023;45:588-592. doi:10.1097/DAD.0000000000002471
- Wijaya M, Lee G, Fischer G. Quality of life of women with untreated vulval lichen sclerosus assessed with vulval quality of life index (VQLI) [published online January 28, 2021]. Australas J Dermatol. 2021;62:177-182. doi:10.1111/ajd.13530
- Felmingham C, Chan L, Doyle LW, et al. The Vulval Disease Quality of Life Index in women with vulval lichen sclerosus correlates with clinician and symptom scores [published online November 14, 2019]. Australas J Dermatol. 2020;61:110-118. doi:10.1111/ajd.13197
- Walsh ML, Leonard N, Shawki H, et al. Lichen sclerosus and immunobullous disease. J Low Genit Tract Dis. 2012;16:468-470. doi:10.1097/LGT.0b013e31825e9b18
- Chin S, Scurry J, Bradford J, et al. Association of topical corticosteroids with reduced vulvar squamous cell carcinoma recurrence in patients with vulvar lichen sclerosus. JAMA Dermatol. 2020;156:813. doi:10.1001/jamadermatol.2020.1074
- Fan R, Leasure AC, Maisha FI, et al. Thyroid disorders associated with lichen sclerosus: a case–control study in the All of Us Research Program. Br J Dermatol. 2022;187:797-799. doi:10.1111/bjd.21702
- Fan R, Leasure AC, Little AJ, et al. Lichen sclerosus among women with psoriasis: a cross-sectional study in the All of Us research program. J Am Acad Dermatol. 2023;88:1175-1177. doi:10.1016/j.jaad.2022.12.012
- Luu Y, Cheng AL, Reisz C. Elevated body mass index, statin use, and cholecystectomy are associated with vulvar lichen sclerosus: a retrospective, case-control study. J Am Acad Dermatol. 2023;88:1376-1378. doi:10.1016/j.jaad.2023.01.023
- Söderlund JM, Hieta NK, Kurki SH, et al. Comorbidity of urogynecological and gastrointestinal disorders in female patients with lichen sclerosus. J Low Genit Tract Dis. 2023;2:156-160. doi:10.1097/LGT.0000000000000727
- Shin L, Smith J, Shiu J, et al. Association of lichen sclerosus and morphea with immune checkpoint therapy: a systematic review. Int J Womens Dermatol. 2023;9:E070. doi:10.1097/JW9.0000000000000070
- Pyle HJ, Evans JC, Artami M, et al. Assessment of the cutaneous hormone landscapes and microbiomes in vulvar lichen sclerosus [published online February 16, 2024]. J Invest Dermatol. 2024:S0022-202X(24)00111-8. doi:10.1016/j.jid.2024.01.027
- Day T, Burston K, Dennerstein G, et al. Vestibulovaginal sclerosis versus lichen sclerosus. Int J Gynecol Pathol. 2018;37:356-363. doi:10.1097/PGP.0000000000000441
- Croker BA, Scurry JP, Petry FM, et al. Vestibular sclerosis: is this a new, distinct clinicopathological entity? J Low Genit Tract Dis. 2018;22:260-263. doi:10.1097/LGT.0000000000000404
- Day T, Selim MA, Allbritton JI, et al. Nonsclerotic lichen sclerosus: definition of a concept and pathologic description. J Low Genit Tract Dis. 2023;27:358-364. doi:10.1097/LGT.0000000000000760
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061. doi:10.1001/jamadermatol.2015.0643
- Delpero E, Sriharan A, Selk A. Steroid phobia in patients with vulvar lichen sclerosus. J Low Genit Tract Dis. 2023;27:286-290. doi:10.1097/LGT.0000000000000753
- Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi:10.1097/AOG.0000000000004332
- Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi:10.1097/AOG.0000000000004409
- Li HOY, Bailey AMJ, Tan MG, Dover JS. Lasers as an adjuvant for vulvar lichen sclerosus: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;86:694-696. doi:10.1016/j.jaad.2021.02.081
- Hargis A, Ngo M, Kraus CN, et al. Systemic therapy for lichen sclerosus: a systematic review [published online November 4, 2023]. J Low Genit Tract Dis. doi:10.1097/LGT.0000000000000775
Vulvar lichen sclerosus (VLS) is an underserved area in medicine and dermatology. We discuss updates in VLS, which include the following: (1) development of core outcome domains to include in all future clinical trials, with current efforts focused on determining outcome measurements for each domain; (2) increased understanding of the impact VLS has on quality-of-life (QOL) outcomes; (3) expanded disease associations; (4) clinical and histologic variants, including vestibular sclerosis and nonsclerotic VLS; and (5) updates in management of VLS.
Core Outcomes Measures
The burden of VLS is challenging to quantify, with little agreement among experts.1 Recently there has been a focus on developing scoring scales to measure disease progression and treatment response. Simpson et al2 pioneered the development of a core outcome set to be included in all future clinical trials for genital lichen sclerosus (LS)—clinical (visible) signs, symptoms, and LS-specific QOL.
Although there is no standardized method for assessing disease severity, various scales have been proposed to measure clinical findings in VLS, such as the vulvar architecture severity scale3 as well as the clinical LS score,4 which is the only validated scale to incorporate the signs and architectural changes identified by a 2018 Delphi consensus group of the International Society for the Study of Vulvovaginal Disease.5 Work is ongoing to identify and evaluate outcome measurement instruments for each of the 3 core outcome domains.
Increased Understanding of QOL Impacts
Pain, pruritus, impairment of sexual function, genitourinary complications, architectural changes, and risk for squamous cell carcinoma (SCC) all have been well established as VLS sequelae.6,7 Recent studies have focused on the QOL impact and associations with psychiatric comorbidities. A matched case-control study found that LS was significantly associated with depression and anxiety among US women (P<.001), and individuals with LS had a more than 2-fold increased odds of receiving a diagnosis of depression or anxiety.8
A review evaluating QOL outcomes in LS found that overall QOL was impaired. Female patients reported worse QOL in the work-school domain of the dermatology life quality index compared with male counterparts.9
Finally, a study exploring the experiences of patients living with VLS highlighted the secrecy and stigma of the condition,10 which serves as a call to action to improve the general population’s knowledge about vulvar anatomy and create change in societal attitudes on vulvar conditions.
Although there are several instruments assessing vulvar-specific QOL, most are for patients with vulvar cancer and focus on sexual function. In 2020, Saunderson et al11 published the 15-item vulvar quality of life index (VQLI), which has broad implications for measuring vulvar disease burden and is an important tool for standardizing vulvar disease measurements and outcomes for clinical research.12 The VQLI, though not specific to VLS, consists of 4 domains to assess vulvar QOL including symptoms, anxiety, activities of daily living, and sexuality. Studies have evaluated this scoring system in patients with VLS, with 1 study finding that VQLI correlated with clinician-rated severity scores (P=.01) and overall patient itch/discomfort score (P<.001) in VLS.13,14
Expanded Disease Associations
Lichen sclerosus has a well-known association with vulvar SCC and other autoimmune conditions, including thyroid disease and bullous pemphigoid.15-17 Recent studies also have revealed an association between LS and psoriasis.18 A case-control study from a single center found VLS was associated with elevated body mass index, statin usage, and cholecystectomy.19 Gynecologic pain syndromes, interstitial cystitis, urinary incontinence, and some gastrointestinal tract disorders including celiac disease also have been found to be increased in patients with VLS.20 Finally, the incidence of cutaneous immune-related adverse events such as LS has increased as the use of immune checkpoint therapies as anticancer treatments has expanded.21 Clinicians should be aware of these potential disease associations when caring for patients with VLS.
The incidence of VLS is higher in lower estrogen states throughout the lifespan, and a recent case-control study evaluated the cutaneous hormonal and microbial landscapes in postmenopausal patients (6 patients with VLS; 12 controls).22 Levels of the following cutaneous hormones in the groin were found to be altered in patients with VLS compared with controls: estrone (lower; P=.006), progesterone (higher; P<.0001), and testosterone (lower; P=.02). The authors found that most hormone levels normalized following treatment with a topical steroid. Additionally, bacterial microbiome alterations were seen in patients with VLS compared with controls. Thus, cutaneous sex hormone and skin microbiome alterations may be associated with VLS.22
Updates in Clinical and Histologic Variants
Less-recognized variants of VLS have been characterized in recent years. Vestibular sclerosis is a variant of VLS with unique clinical and histopathologic features; it is characterized by involvement localized to the anterior vestibule and either an absent or sparse lymphocytic infiltrate on histopathology.23,24 Nonsclerotic VLS is a variant with clinical features consistent with VLS that does not exhibit dermal sclerosis on histopathology. Thus, a diagnosis of nonsclerotic VLS requires clinicopathologic correlation. Four nonsclerotic histopathologic subtypes are proposed: lichenoid, hypertrophic lichenoid, dermal fibrosis without acanthosis, and dermal fibrosis with acanthosis.25 Longitudinal studies that correlate duration, signs, and symptoms will be important to further understand these variants.
Management Updates
First-line treatment of VLS still consists of ultrapotent topical corticosteroids with chronic maintenance therapy (usually lifetime) to decrease the risk for SCC and architectural changes.26 However, a survey across social media platforms found steroid phobia is common in patients with VLS (N=865), with approximately 40% of respondents endorsing waiting as long as they could before using topical corticosteroids and stopping as soon as possible.27 Clinicians should be aware of possible patient perceptions in the use of chronic steroids when discussing this therapy.
Randomized controlled trials utilizing fractional CO2 devices for VLS have been performed with conflicting results and no consensus regarding outcome measurement.28,29 Additionally, long-term disease outcomes following laser use have not been investigated. Although there is evidence that both ablative and nonablative devices can improve symptoms and signs, there is no evidence that they offer a cure for a chronic inflammatory skin condition. Current evidence suggests that even for patients undergoing these procedures, maintenance therapy is still essential to prevent sequelae.30 Future studies incorporating standardized outcome measures will be important for assessing the benefits of laser therapy in VLS. Finally, the reasons why topical corticosteroids may fail in an individual patient are multifaceted and should be explored thoroughly when considering laser therapy for VLS.
Studies evaluating the role of systemic therapies for refractory cases of VLS have expanded. A systematic review of systemic therapies for both genital and extragenital LS found oral corticosteroids and methotrexate were the most-reported systemic treatment regimens.31 Use of biologics in LS has been reported, with cases utilizing adalimumab for VLS and dupilumab for extragenital LS. Use of Janus kinase inhibitors including abrocitinib and baricitinib also has been reported for LS.31 A clinical trial to evaluate the safety and efficacy of topical ruxolitinib in VLS was recently completed (ClinicalTrials.govidentifier NCT05593445). Future research studies likely will focus on the safety and efficacy of targeted and steroid-sparing therapies for patients with VLS.
Final Thoughts
Vulvar lichen sclerosus increasingly is becoming recognized as a chronic genital skin condition that impacts QOL and health outcomes, with a need to develop more effective and safe evidence-based therapies. Recent literature has focused on the importance of developing and standardizing disease outcomes; identifying disease associations including the role of cutaneous hormones and microbiome alterations; characterizing histologic and clinical variants; and staying up-to-date on management, including the need for understanding patient perceptions of chronic topical steroid therapy. Each of these are important updates for clinicians to consider when caring for patients with VLS. Future studies likely will focus on elucidating disease etiology and mechanisms to gain a better understanding of VLS pathogenesis and potential targets for therapies as well as implementation of clinical trials that incorporate standardized outcome domains to test efficacy and safety of additional therapies.
Vulvar lichen sclerosus (VLS) is an underserved area in medicine and dermatology. We discuss updates in VLS, which include the following: (1) development of core outcome domains to include in all future clinical trials, with current efforts focused on determining outcome measurements for each domain; (2) increased understanding of the impact VLS has on quality-of-life (QOL) outcomes; (3) expanded disease associations; (4) clinical and histologic variants, including vestibular sclerosis and nonsclerotic VLS; and (5) updates in management of VLS.
Core Outcomes Measures
The burden of VLS is challenging to quantify, with little agreement among experts.1 Recently there has been a focus on developing scoring scales to measure disease progression and treatment response. Simpson et al2 pioneered the development of a core outcome set to be included in all future clinical trials for genital lichen sclerosus (LS)—clinical (visible) signs, symptoms, and LS-specific QOL.
Although there is no standardized method for assessing disease severity, various scales have been proposed to measure clinical findings in VLS, such as the vulvar architecture severity scale3 as well as the clinical LS score,4 which is the only validated scale to incorporate the signs and architectural changes identified by a 2018 Delphi consensus group of the International Society for the Study of Vulvovaginal Disease.5 Work is ongoing to identify and evaluate outcome measurement instruments for each of the 3 core outcome domains.
Increased Understanding of QOL Impacts
Pain, pruritus, impairment of sexual function, genitourinary complications, architectural changes, and risk for squamous cell carcinoma (SCC) all have been well established as VLS sequelae.6,7 Recent studies have focused on the QOL impact and associations with psychiatric comorbidities. A matched case-control study found that LS was significantly associated with depression and anxiety among US women (P<.001), and individuals with LS had a more than 2-fold increased odds of receiving a diagnosis of depression or anxiety.8
A review evaluating QOL outcomes in LS found that overall QOL was impaired. Female patients reported worse QOL in the work-school domain of the dermatology life quality index compared with male counterparts.9
Finally, a study exploring the experiences of patients living with VLS highlighted the secrecy and stigma of the condition,10 which serves as a call to action to improve the general population’s knowledge about vulvar anatomy and create change in societal attitudes on vulvar conditions.
Although there are several instruments assessing vulvar-specific QOL, most are for patients with vulvar cancer and focus on sexual function. In 2020, Saunderson et al11 published the 15-item vulvar quality of life index (VQLI), which has broad implications for measuring vulvar disease burden and is an important tool for standardizing vulvar disease measurements and outcomes for clinical research.12 The VQLI, though not specific to VLS, consists of 4 domains to assess vulvar QOL including symptoms, anxiety, activities of daily living, and sexuality. Studies have evaluated this scoring system in patients with VLS, with 1 study finding that VQLI correlated with clinician-rated severity scores (P=.01) and overall patient itch/discomfort score (P<.001) in VLS.13,14
Expanded Disease Associations
Lichen sclerosus has a well-known association with vulvar SCC and other autoimmune conditions, including thyroid disease and bullous pemphigoid.15-17 Recent studies also have revealed an association between LS and psoriasis.18 A case-control study from a single center found VLS was associated with elevated body mass index, statin usage, and cholecystectomy.19 Gynecologic pain syndromes, interstitial cystitis, urinary incontinence, and some gastrointestinal tract disorders including celiac disease also have been found to be increased in patients with VLS.20 Finally, the incidence of cutaneous immune-related adverse events such as LS has increased as the use of immune checkpoint therapies as anticancer treatments has expanded.21 Clinicians should be aware of these potential disease associations when caring for patients with VLS.
The incidence of VLS is higher in lower estrogen states throughout the lifespan, and a recent case-control study evaluated the cutaneous hormonal and microbial landscapes in postmenopausal patients (6 patients with VLS; 12 controls).22 Levels of the following cutaneous hormones in the groin were found to be altered in patients with VLS compared with controls: estrone (lower; P=.006), progesterone (higher; P<.0001), and testosterone (lower; P=.02). The authors found that most hormone levels normalized following treatment with a topical steroid. Additionally, bacterial microbiome alterations were seen in patients with VLS compared with controls. Thus, cutaneous sex hormone and skin microbiome alterations may be associated with VLS.22
Updates in Clinical and Histologic Variants
Less-recognized variants of VLS have been characterized in recent years. Vestibular sclerosis is a variant of VLS with unique clinical and histopathologic features; it is characterized by involvement localized to the anterior vestibule and either an absent or sparse lymphocytic infiltrate on histopathology.23,24 Nonsclerotic VLS is a variant with clinical features consistent with VLS that does not exhibit dermal sclerosis on histopathology. Thus, a diagnosis of nonsclerotic VLS requires clinicopathologic correlation. Four nonsclerotic histopathologic subtypes are proposed: lichenoid, hypertrophic lichenoid, dermal fibrosis without acanthosis, and dermal fibrosis with acanthosis.25 Longitudinal studies that correlate duration, signs, and symptoms will be important to further understand these variants.
Management Updates
First-line treatment of VLS still consists of ultrapotent topical corticosteroids with chronic maintenance therapy (usually lifetime) to decrease the risk for SCC and architectural changes.26 However, a survey across social media platforms found steroid phobia is common in patients with VLS (N=865), with approximately 40% of respondents endorsing waiting as long as they could before using topical corticosteroids and stopping as soon as possible.27 Clinicians should be aware of possible patient perceptions in the use of chronic steroids when discussing this therapy.
Randomized controlled trials utilizing fractional CO2 devices for VLS have been performed with conflicting results and no consensus regarding outcome measurement.28,29 Additionally, long-term disease outcomes following laser use have not been investigated. Although there is evidence that both ablative and nonablative devices can improve symptoms and signs, there is no evidence that they offer a cure for a chronic inflammatory skin condition. Current evidence suggests that even for patients undergoing these procedures, maintenance therapy is still essential to prevent sequelae.30 Future studies incorporating standardized outcome measures will be important for assessing the benefits of laser therapy in VLS. Finally, the reasons why topical corticosteroids may fail in an individual patient are multifaceted and should be explored thoroughly when considering laser therapy for VLS.
Studies evaluating the role of systemic therapies for refractory cases of VLS have expanded. A systematic review of systemic therapies for both genital and extragenital LS found oral corticosteroids and methotrexate were the most-reported systemic treatment regimens.31 Use of biologics in LS has been reported, with cases utilizing adalimumab for VLS and dupilumab for extragenital LS. Use of Janus kinase inhibitors including abrocitinib and baricitinib also has been reported for LS.31 A clinical trial to evaluate the safety and efficacy of topical ruxolitinib in VLS was recently completed (ClinicalTrials.govidentifier NCT05593445). Future research studies likely will focus on the safety and efficacy of targeted and steroid-sparing therapies for patients with VLS.
Final Thoughts
Vulvar lichen sclerosus increasingly is becoming recognized as a chronic genital skin condition that impacts QOL and health outcomes, with a need to develop more effective and safe evidence-based therapies. Recent literature has focused on the importance of developing and standardizing disease outcomes; identifying disease associations including the role of cutaneous hormones and microbiome alterations; characterizing histologic and clinical variants; and staying up-to-date on management, including the need for understanding patient perceptions of chronic topical steroid therapy. Each of these are important updates for clinicians to consider when caring for patients with VLS. Future studies likely will focus on elucidating disease etiology and mechanisms to gain a better understanding of VLS pathogenesis and potential targets for therapies as well as implementation of clinical trials that incorporate standardized outcome domains to test efficacy and safety of additional therapies.
- Sheinis M, Green N, Vieira-Baptista P, et al. Adult vulvar lichen sclerosus: can experts agree on the assessment of disease severity? J Low Genit Tract Dis. 2020;24:295-298. doi:10.1097/LGT.0000000000000534
- Simpson RC, Kirtschig G, Selk A, et al. Core outcome domains for lichen sclerosus: a CORALS initiative consensus statement. Br J Dermatol. 2023;188:628-635. doi:10.1093/bjd/ljac145
- Almadori A, Zenner N, Boyle D, et al. Development and validation of a clinical grading scale to assess the vulvar region: the Vulvar Architecture Severity Scale. Aesthet Surg J. 2020;40:1319-1326. doi:10.1093/asj/sjz342
- Erni B, Navarini AA, Huang D, et al. Proposition of a severity scale for lichen sclerosus: the “Clinical Lichen Sclerosus Score.” Dermatol Ther. 2021;34:E14773. doi:10.1111/dth.14773
- Sheinis M, Selk A. Development of the Adult Vulvar Lichen Sclerosus Severity Scale—a Delphi Consensus Exercise for Item Generation. J Low Genit Tract Dis. 2018;22:66-73. doi:10.1097/LGT.0000000000000361
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases. J Am Acad Dermatol. 2020;82:1287-1298. doi:10.1016/j.jaad.2019.10.077
- Wijaya M, Lee G, Fischer G. Why do some patients with vulval lichen sclerosus on long-term topical corticosteroid treatment experience ongoing poor quality of life? Australas J Dermatol. 2022;63:463-472. doi:10.1111/ajd.13926
- Fan R, Leasure AC, Maisha FI, et al. Depression and anxiety in patients with lichen sclerosus. JAMA Dermatol. 2022;158:953-954. doi:10.1001/jamadermatol.2022.1964
- Ranum A, Pearson DR. The impact of genital lichen sclerosus and lichen planus on quality of life: a review. Int J Womens Dermatol. 2022;8:E042. doi:10.1097/JW9.0000000000000042
- Arnold S, Fernando S, Rees S. Living with vulval lichen sclerosus: a qualitative interview study. Br J Dermatol. 2022;187:909-918. doi:10.1111/bjd.21777
- Saunderson RB, Harris V, Yeh R, et al. Vulvar quality of life index (VQLI)—a simple tool to measure quality of life in patients with vulvar disease. Australas J Dermatol. 2020;61:152-157. doi:10.1111/ajd.13235
- Pyle HJ, Evans JC, Vandergriff TW, et al. Vulvar lichen sclerosus clinical severity scales and histopathologic correlation: a case series. Am J Dermatopathol. 2023;45:588-592. doi:10.1097/DAD.0000000000002471
- Wijaya M, Lee G, Fischer G. Quality of life of women with untreated vulval lichen sclerosus assessed with vulval quality of life index (VQLI) [published online January 28, 2021]. Australas J Dermatol. 2021;62:177-182. doi:10.1111/ajd.13530
- Felmingham C, Chan L, Doyle LW, et al. The Vulval Disease Quality of Life Index in women with vulval lichen sclerosus correlates with clinician and symptom scores [published online November 14, 2019]. Australas J Dermatol. 2020;61:110-118. doi:10.1111/ajd.13197
- Walsh ML, Leonard N, Shawki H, et al. Lichen sclerosus and immunobullous disease. J Low Genit Tract Dis. 2012;16:468-470. doi:10.1097/LGT.0b013e31825e9b18
- Chin S, Scurry J, Bradford J, et al. Association of topical corticosteroids with reduced vulvar squamous cell carcinoma recurrence in patients with vulvar lichen sclerosus. JAMA Dermatol. 2020;156:813. doi:10.1001/jamadermatol.2020.1074
- Fan R, Leasure AC, Maisha FI, et al. Thyroid disorders associated with lichen sclerosus: a case–control study in the All of Us Research Program. Br J Dermatol. 2022;187:797-799. doi:10.1111/bjd.21702
- Fan R, Leasure AC, Little AJ, et al. Lichen sclerosus among women with psoriasis: a cross-sectional study in the All of Us research program. J Am Acad Dermatol. 2023;88:1175-1177. doi:10.1016/j.jaad.2022.12.012
- Luu Y, Cheng AL, Reisz C. Elevated body mass index, statin use, and cholecystectomy are associated with vulvar lichen sclerosus: a retrospective, case-control study. J Am Acad Dermatol. 2023;88:1376-1378. doi:10.1016/j.jaad.2023.01.023
- Söderlund JM, Hieta NK, Kurki SH, et al. Comorbidity of urogynecological and gastrointestinal disorders in female patients with lichen sclerosus. J Low Genit Tract Dis. 2023;2:156-160. doi:10.1097/LGT.0000000000000727
- Shin L, Smith J, Shiu J, et al. Association of lichen sclerosus and morphea with immune checkpoint therapy: a systematic review. Int J Womens Dermatol. 2023;9:E070. doi:10.1097/JW9.0000000000000070
- Pyle HJ, Evans JC, Artami M, et al. Assessment of the cutaneous hormone landscapes and microbiomes in vulvar lichen sclerosus [published online February 16, 2024]. J Invest Dermatol. 2024:S0022-202X(24)00111-8. doi:10.1016/j.jid.2024.01.027
- Day T, Burston K, Dennerstein G, et al. Vestibulovaginal sclerosis versus lichen sclerosus. Int J Gynecol Pathol. 2018;37:356-363. doi:10.1097/PGP.0000000000000441
- Croker BA, Scurry JP, Petry FM, et al. Vestibular sclerosis: is this a new, distinct clinicopathological entity? J Low Genit Tract Dis. 2018;22:260-263. doi:10.1097/LGT.0000000000000404
- Day T, Selim MA, Allbritton JI, et al. Nonsclerotic lichen sclerosus: definition of a concept and pathologic description. J Low Genit Tract Dis. 2023;27:358-364. doi:10.1097/LGT.0000000000000760
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061. doi:10.1001/jamadermatol.2015.0643
- Delpero E, Sriharan A, Selk A. Steroid phobia in patients with vulvar lichen sclerosus. J Low Genit Tract Dis. 2023;27:286-290. doi:10.1097/LGT.0000000000000753
- Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi:10.1097/AOG.0000000000004332
- Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi:10.1097/AOG.0000000000004409
- Li HOY, Bailey AMJ, Tan MG, Dover JS. Lasers as an adjuvant for vulvar lichen sclerosus: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;86:694-696. doi:10.1016/j.jaad.2021.02.081
- Hargis A, Ngo M, Kraus CN, et al. Systemic therapy for lichen sclerosus: a systematic review [published online November 4, 2023]. J Low Genit Tract Dis. doi:10.1097/LGT.0000000000000775
- Sheinis M, Green N, Vieira-Baptista P, et al. Adult vulvar lichen sclerosus: can experts agree on the assessment of disease severity? J Low Genit Tract Dis. 2020;24:295-298. doi:10.1097/LGT.0000000000000534
- Simpson RC, Kirtschig G, Selk A, et al. Core outcome domains for lichen sclerosus: a CORALS initiative consensus statement. Br J Dermatol. 2023;188:628-635. doi:10.1093/bjd/ljac145
- Almadori A, Zenner N, Boyle D, et al. Development and validation of a clinical grading scale to assess the vulvar region: the Vulvar Architecture Severity Scale. Aesthet Surg J. 2020;40:1319-1326. doi:10.1093/asj/sjz342
- Erni B, Navarini AA, Huang D, et al. Proposition of a severity scale for lichen sclerosus: the “Clinical Lichen Sclerosus Score.” Dermatol Ther. 2021;34:E14773. doi:10.1111/dth.14773
- Sheinis M, Selk A. Development of the Adult Vulvar Lichen Sclerosus Severity Scale—a Delphi Consensus Exercise for Item Generation. J Low Genit Tract Dis. 2018;22:66-73. doi:10.1097/LGT.0000000000000361
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases. J Am Acad Dermatol. 2020;82:1287-1298. doi:10.1016/j.jaad.2019.10.077
- Wijaya M, Lee G, Fischer G. Why do some patients with vulval lichen sclerosus on long-term topical corticosteroid treatment experience ongoing poor quality of life? Australas J Dermatol. 2022;63:463-472. doi:10.1111/ajd.13926
- Fan R, Leasure AC, Maisha FI, et al. Depression and anxiety in patients with lichen sclerosus. JAMA Dermatol. 2022;158:953-954. doi:10.1001/jamadermatol.2022.1964
- Ranum A, Pearson DR. The impact of genital lichen sclerosus and lichen planus on quality of life: a review. Int J Womens Dermatol. 2022;8:E042. doi:10.1097/JW9.0000000000000042
- Arnold S, Fernando S, Rees S. Living with vulval lichen sclerosus: a qualitative interview study. Br J Dermatol. 2022;187:909-918. doi:10.1111/bjd.21777
- Saunderson RB, Harris V, Yeh R, et al. Vulvar quality of life index (VQLI)—a simple tool to measure quality of life in patients with vulvar disease. Australas J Dermatol. 2020;61:152-157. doi:10.1111/ajd.13235
- Pyle HJ, Evans JC, Vandergriff TW, et al. Vulvar lichen sclerosus clinical severity scales and histopathologic correlation: a case series. Am J Dermatopathol. 2023;45:588-592. doi:10.1097/DAD.0000000000002471
- Wijaya M, Lee G, Fischer G. Quality of life of women with untreated vulval lichen sclerosus assessed with vulval quality of life index (VQLI) [published online January 28, 2021]. Australas J Dermatol. 2021;62:177-182. doi:10.1111/ajd.13530
- Felmingham C, Chan L, Doyle LW, et al. The Vulval Disease Quality of Life Index in women with vulval lichen sclerosus correlates with clinician and symptom scores [published online November 14, 2019]. Australas J Dermatol. 2020;61:110-118. doi:10.1111/ajd.13197
- Walsh ML, Leonard N, Shawki H, et al. Lichen sclerosus and immunobullous disease. J Low Genit Tract Dis. 2012;16:468-470. doi:10.1097/LGT.0b013e31825e9b18
- Chin S, Scurry J, Bradford J, et al. Association of topical corticosteroids with reduced vulvar squamous cell carcinoma recurrence in patients with vulvar lichen sclerosus. JAMA Dermatol. 2020;156:813. doi:10.1001/jamadermatol.2020.1074
- Fan R, Leasure AC, Maisha FI, et al. Thyroid disorders associated with lichen sclerosus: a case–control study in the All of Us Research Program. Br J Dermatol. 2022;187:797-799. doi:10.1111/bjd.21702
- Fan R, Leasure AC, Little AJ, et al. Lichen sclerosus among women with psoriasis: a cross-sectional study in the All of Us research program. J Am Acad Dermatol. 2023;88:1175-1177. doi:10.1016/j.jaad.2022.12.012
- Luu Y, Cheng AL, Reisz C. Elevated body mass index, statin use, and cholecystectomy are associated with vulvar lichen sclerosus: a retrospective, case-control study. J Am Acad Dermatol. 2023;88:1376-1378. doi:10.1016/j.jaad.2023.01.023
- Söderlund JM, Hieta NK, Kurki SH, et al. Comorbidity of urogynecological and gastrointestinal disorders in female patients with lichen sclerosus. J Low Genit Tract Dis. 2023;2:156-160. doi:10.1097/LGT.0000000000000727
- Shin L, Smith J, Shiu J, et al. Association of lichen sclerosus and morphea with immune checkpoint therapy: a systematic review. Int J Womens Dermatol. 2023;9:E070. doi:10.1097/JW9.0000000000000070
- Pyle HJ, Evans JC, Artami M, et al. Assessment of the cutaneous hormone landscapes and microbiomes in vulvar lichen sclerosus [published online February 16, 2024]. J Invest Dermatol. 2024:S0022-202X(24)00111-8. doi:10.1016/j.jid.2024.01.027
- Day T, Burston K, Dennerstein G, et al. Vestibulovaginal sclerosis versus lichen sclerosus. Int J Gynecol Pathol. 2018;37:356-363. doi:10.1097/PGP.0000000000000441
- Croker BA, Scurry JP, Petry FM, et al. Vestibular sclerosis: is this a new, distinct clinicopathological entity? J Low Genit Tract Dis. 2018;22:260-263. doi:10.1097/LGT.0000000000000404
- Day T, Selim MA, Allbritton JI, et al. Nonsclerotic lichen sclerosus: definition of a concept and pathologic description. J Low Genit Tract Dis. 2023;27:358-364. doi:10.1097/LGT.0000000000000760
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061. doi:10.1001/jamadermatol.2015.0643
- Delpero E, Sriharan A, Selk A. Steroid phobia in patients with vulvar lichen sclerosus. J Low Genit Tract Dis. 2023;27:286-290. doi:10.1097/LGT.0000000000000753
- Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi:10.1097/AOG.0000000000004332
- Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi:10.1097/AOG.0000000000004409
- Li HOY, Bailey AMJ, Tan MG, Dover JS. Lasers as an adjuvant for vulvar lichen sclerosus: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;86:694-696. doi:10.1016/j.jaad.2021.02.081
- Hargis A, Ngo M, Kraus CN, et al. Systemic therapy for lichen sclerosus: a systematic review [published online November 4, 2023]. J Low Genit Tract Dis. doi:10.1097/LGT.0000000000000775
Fighting to Serve: Women in Military Medicine
Let the generations know that women in uniform also guaranteed their freedom.
Mary Walker, MD
Hoping to make a career in nursing, my mother, a newly graduated registered nurse, enlisted in the US Army Nurse Corps shortly after the United States entered World War II. When she married my father, a US Army doctor, in 1942, she was summarily discharged (the Army Nurse Corp changed its policy and permitted married nurses to serve later that year), while my father went on to decades of distinguished service in military medicine.1 My mother always regretted being unable to advance through the ranks of the US Army as other woman nurses did in her training class.
March is Women’s History Month. My personal narrative of discrimination against women in military medicine is a footnote in a long volume of inequitable treatment. This column will examine a few of the most famous—or rather from a justice perspective, infamous—chapters in that story to illustrate how for centuries women heroically fought for the right to serve.
A theme of the early epochs of the American military is that women were forced to come to the difficult realization that the only way to serve was to conceal their identity. In 1776, Margaret Cochran Corbin felt called as her husband did to defend the new nation. She dressed as a man and joined him at the ramparts, helping load his cannon until he was killed, and took over firing at the enemy. Even after being shot, she remained in the ranks, entering the Invalid Regiment at West Point, New York, dedicated to caring for other injured soldiers. As recognition of her exemplary service and battlefield injury Corbin became the first US woman to receive a military pension. The Veterans Affairs New York Harbor Healthcare System Manhattan campus is named in her honor.2
The hypocrisy of the military’s gender politics was nowhere more evident than in the case of Mary Walker, MD, and the Congressional Medal of Honor. Walker graduated from Syracuse Medical College in 1855. At the beginning of the Civil War, Walker’s request to enlist as a surgeon was refused on the grounds of her gender. She declined to be a nurse, and instead volunteered for the Army where she cared for the wounded in various hospitals. Her medical degree was accepted in 1863, enabling her to become a paid surgical officer in the War Department, including 4 months as a prisoner of war.
An early and avid feminist, Walker wore men’s clothing and when she was arrested on the charge of impersonating a male, declared the government had given her permission to dress as a man to facilitate her surgical work. Walker separated from the military in 1865 and President Andrew Johnson awarded her the Congressional Medal of Honor that year. After Walker’s death in 1917, the Medal of Honor was rescinded on the grounds that she had never actually been commissioned and the medal could not be awarded to a civilian. It took 60 years of lobbying before President Jimmy Carter restored her award in 1977.3 That millions of women have served in the military since the Civil War, and Walker remains the only woman among the 3517 service members to have won the nation’s highest military honor, underscores the ongoing injustice.4
February commemorated Black History Month and a second theme that emerges from the study of the history of women in military medicine is intersectionality: How race, gender, sexual orientation, and other identities overlap and interact to generate distinctive forms of discrimination. Ethicists have applied the concept of intersectionality to health care and there are a plethora of examples in military medicine.5 Despite a dire need for nurses in the first and second world wars, and a track record of their exemplary service in prior conflicts, the government repeatedly set up arbitrary obstacles barring highly-qualified Black nurses from enlisting.6 Technically allowed to join the Army Nurse Corps in 1941, Black nurses confronted bureaucratic barriers that restricted them to only caring for Black servicemen and prisoners of war, and racial quotas that resulted in 500 Black nurses vs 59,000 White nurses that served during World War II. Black nurses and their supporters in government and society persisted, and once in uniform, broke through barriers to achieve administrative and clinical excellence.7
My mother’s experience mirrors that of thousands of women whose dreams for a career in military medicine were shattered or who enlisted only to find their aspirations for advancement in the service thwarted. Medical historians remind us that due to bias, much of the book of women healer’s accomplishments remains unwritten, itself a testimony to the pervasive and enduring marginalization of women in Western society. Yet, as this brief glimpse of women in military medicine shows, there is sufficient evidence for us to appreciate their impressive contributions.8
Reflecting on this sketch of women’s struggle for acceptance in military medicine in March 2024, we may presume that the fight for equity has been continuously trending upward.8 President Joseph R. Biden appointed, and even more surprisingly, the US Congress confirmed Rachel Levine, MD, as US Department of Health and Human Services Assistant Under Secretary for Health in 2021, making Levine the highest ranking openly transgender health official in the history of the US government.9 Levine also has the distinction of being the first 4-star admiral in the Commissioned Corps of the US Public Health Service and the only transgender person to achieve this rank in any branch of the US uniformed services.10
However, research suggests that the history of women in the military is far more like an undulating curve. A 2019 study of academic military surgery found evidence of gender disparity even greater than that of the civilian sector.11 True and lasting equity in federal health care practice will require all of us to follow the inspiring examples of so many women known and unknown who fought the military establishment within for the right to heal those wounded fighting the enemy without.
1. Treadwell ME. The Women’s Army Corps. US Army Center of Military History; 1991: Chap 25. Accessed February 20, 2024. https://history.army.mil/books/wwii/Wac/ch25.htm
2. Hayes P. Meet five inspiring women veterans. Published November 10, 2022. Accessed February 20, 2024. https://news.va.gov/110571/meet-five-inspiring-women-veterans/
3. Lange K. Meet Dr. Mary Walker: the only female recipient of the Medical of Honor recipient. Published March 7, 2017. Accessed February 20, 2024. https://www.army.mil/article/183800/meet_dr_mary_walker_the_only_female_medal_of_honor_recipient
4. The National Medal of Honor Museum. Accessed February 20, 2024. https://mohmuseum.org/the-medal
5. Wilson Y, White A, Jefferson A, Danis M. Intersectionality in Clinical Medicine: The Need for a Conceptual Framework. Am J Bioeth. 2019;19(2):8-19. doi:10.1080/15265161.2018.1557275
6. National Women’s History Museum. African American Nurses in World War II. Published July 8, 2019. Accessed February 20, 2024. https://www.womenshistory.org/articles/african-american-nurses-world-war-ii
7. O’Gan P. Smithsonian National Museum of African American History and Culture. Victory at Home and Abroad: African American Army Nurses in World War II. Published May 8, 2023. Accessed February 20, 2024. https://nmaahc.si.edu/explore/stories/nurses-WWII
8. Neve M. Conclusion. In Conrad LI, Neve M, Nutton V, Porter R, and Wear A, eds. The Western Medical Tradition 800 BC to AD 1800. Cambridge University Press; 1995:477-494.
9. Stolberg SG. ‘This is politics’: Dr. Rachel Levine’s rise as transgender issues gain prominence. The New York Times. Updated May 10, 2021. Accessed February 20, 2024. https://www.nytimes.com/2021/05/08/us/politics/rachel-levine-transgender.html
10. Franklin J. Dr. Rachel Levine is sworn in as the nation’s first transgender four-star officer. October 19, 2021. Accessed February 20, 2024. https://www.npr.org/2021/10/19/1047423156/rachel-levine-first-transgender-four-star-officer
11. Herrick-Reynolds K, Brooks D, Wind G, Jackson P, Latham K. Military medicine and the academic surgery gender gap. Mil Med. 2019;184(9-10):383-387. doi:10.1093/milmed/usz083
Let the generations know that women in uniform also guaranteed their freedom.
Mary Walker, MD
Hoping to make a career in nursing, my mother, a newly graduated registered nurse, enlisted in the US Army Nurse Corps shortly after the United States entered World War II. When she married my father, a US Army doctor, in 1942, she was summarily discharged (the Army Nurse Corp changed its policy and permitted married nurses to serve later that year), while my father went on to decades of distinguished service in military medicine.1 My mother always regretted being unable to advance through the ranks of the US Army as other woman nurses did in her training class.
March is Women’s History Month. My personal narrative of discrimination against women in military medicine is a footnote in a long volume of inequitable treatment. This column will examine a few of the most famous—or rather from a justice perspective, infamous—chapters in that story to illustrate how for centuries women heroically fought for the right to serve.
A theme of the early epochs of the American military is that women were forced to come to the difficult realization that the only way to serve was to conceal their identity. In 1776, Margaret Cochran Corbin felt called as her husband did to defend the new nation. She dressed as a man and joined him at the ramparts, helping load his cannon until he was killed, and took over firing at the enemy. Even after being shot, she remained in the ranks, entering the Invalid Regiment at West Point, New York, dedicated to caring for other injured soldiers. As recognition of her exemplary service and battlefield injury Corbin became the first US woman to receive a military pension. The Veterans Affairs New York Harbor Healthcare System Manhattan campus is named in her honor.2
The hypocrisy of the military’s gender politics was nowhere more evident than in the case of Mary Walker, MD, and the Congressional Medal of Honor. Walker graduated from Syracuse Medical College in 1855. At the beginning of the Civil War, Walker’s request to enlist as a surgeon was refused on the grounds of her gender. She declined to be a nurse, and instead volunteered for the Army where she cared for the wounded in various hospitals. Her medical degree was accepted in 1863, enabling her to become a paid surgical officer in the War Department, including 4 months as a prisoner of war.
An early and avid feminist, Walker wore men’s clothing and when she was arrested on the charge of impersonating a male, declared the government had given her permission to dress as a man to facilitate her surgical work. Walker separated from the military in 1865 and President Andrew Johnson awarded her the Congressional Medal of Honor that year. After Walker’s death in 1917, the Medal of Honor was rescinded on the grounds that she had never actually been commissioned and the medal could not be awarded to a civilian. It took 60 years of lobbying before President Jimmy Carter restored her award in 1977.3 That millions of women have served in the military since the Civil War, and Walker remains the only woman among the 3517 service members to have won the nation’s highest military honor, underscores the ongoing injustice.4
February commemorated Black History Month and a second theme that emerges from the study of the history of women in military medicine is intersectionality: How race, gender, sexual orientation, and other identities overlap and interact to generate distinctive forms of discrimination. Ethicists have applied the concept of intersectionality to health care and there are a plethora of examples in military medicine.5 Despite a dire need for nurses in the first and second world wars, and a track record of their exemplary service in prior conflicts, the government repeatedly set up arbitrary obstacles barring highly-qualified Black nurses from enlisting.6 Technically allowed to join the Army Nurse Corps in 1941, Black nurses confronted bureaucratic barriers that restricted them to only caring for Black servicemen and prisoners of war, and racial quotas that resulted in 500 Black nurses vs 59,000 White nurses that served during World War II. Black nurses and their supporters in government and society persisted, and once in uniform, broke through barriers to achieve administrative and clinical excellence.7
My mother’s experience mirrors that of thousands of women whose dreams for a career in military medicine were shattered or who enlisted only to find their aspirations for advancement in the service thwarted. Medical historians remind us that due to bias, much of the book of women healer’s accomplishments remains unwritten, itself a testimony to the pervasive and enduring marginalization of women in Western society. Yet, as this brief glimpse of women in military medicine shows, there is sufficient evidence for us to appreciate their impressive contributions.8
Reflecting on this sketch of women’s struggle for acceptance in military medicine in March 2024, we may presume that the fight for equity has been continuously trending upward.8 President Joseph R. Biden appointed, and even more surprisingly, the US Congress confirmed Rachel Levine, MD, as US Department of Health and Human Services Assistant Under Secretary for Health in 2021, making Levine the highest ranking openly transgender health official in the history of the US government.9 Levine also has the distinction of being the first 4-star admiral in the Commissioned Corps of the US Public Health Service and the only transgender person to achieve this rank in any branch of the US uniformed services.10
However, research suggests that the history of women in the military is far more like an undulating curve. A 2019 study of academic military surgery found evidence of gender disparity even greater than that of the civilian sector.11 True and lasting equity in federal health care practice will require all of us to follow the inspiring examples of so many women known and unknown who fought the military establishment within for the right to heal those wounded fighting the enemy without.
Let the generations know that women in uniform also guaranteed their freedom.
Mary Walker, MD
Hoping to make a career in nursing, my mother, a newly graduated registered nurse, enlisted in the US Army Nurse Corps shortly after the United States entered World War II. When she married my father, a US Army doctor, in 1942, she was summarily discharged (the Army Nurse Corp changed its policy and permitted married nurses to serve later that year), while my father went on to decades of distinguished service in military medicine.1 My mother always regretted being unable to advance through the ranks of the US Army as other woman nurses did in her training class.
March is Women’s History Month. My personal narrative of discrimination against women in military medicine is a footnote in a long volume of inequitable treatment. This column will examine a few of the most famous—or rather from a justice perspective, infamous—chapters in that story to illustrate how for centuries women heroically fought for the right to serve.
A theme of the early epochs of the American military is that women were forced to come to the difficult realization that the only way to serve was to conceal their identity. In 1776, Margaret Cochran Corbin felt called as her husband did to defend the new nation. She dressed as a man and joined him at the ramparts, helping load his cannon until he was killed, and took over firing at the enemy. Even after being shot, she remained in the ranks, entering the Invalid Regiment at West Point, New York, dedicated to caring for other injured soldiers. As recognition of her exemplary service and battlefield injury Corbin became the first US woman to receive a military pension. The Veterans Affairs New York Harbor Healthcare System Manhattan campus is named in her honor.2
The hypocrisy of the military’s gender politics was nowhere more evident than in the case of Mary Walker, MD, and the Congressional Medal of Honor. Walker graduated from Syracuse Medical College in 1855. At the beginning of the Civil War, Walker’s request to enlist as a surgeon was refused on the grounds of her gender. She declined to be a nurse, and instead volunteered for the Army where she cared for the wounded in various hospitals. Her medical degree was accepted in 1863, enabling her to become a paid surgical officer in the War Department, including 4 months as a prisoner of war.
An early and avid feminist, Walker wore men’s clothing and when she was arrested on the charge of impersonating a male, declared the government had given her permission to dress as a man to facilitate her surgical work. Walker separated from the military in 1865 and President Andrew Johnson awarded her the Congressional Medal of Honor that year. After Walker’s death in 1917, the Medal of Honor was rescinded on the grounds that she had never actually been commissioned and the medal could not be awarded to a civilian. It took 60 years of lobbying before President Jimmy Carter restored her award in 1977.3 That millions of women have served in the military since the Civil War, and Walker remains the only woman among the 3517 service members to have won the nation’s highest military honor, underscores the ongoing injustice.4
February commemorated Black History Month and a second theme that emerges from the study of the history of women in military medicine is intersectionality: How race, gender, sexual orientation, and other identities overlap and interact to generate distinctive forms of discrimination. Ethicists have applied the concept of intersectionality to health care and there are a plethora of examples in military medicine.5 Despite a dire need for nurses in the first and second world wars, and a track record of their exemplary service in prior conflicts, the government repeatedly set up arbitrary obstacles barring highly-qualified Black nurses from enlisting.6 Technically allowed to join the Army Nurse Corps in 1941, Black nurses confronted bureaucratic barriers that restricted them to only caring for Black servicemen and prisoners of war, and racial quotas that resulted in 500 Black nurses vs 59,000 White nurses that served during World War II. Black nurses and their supporters in government and society persisted, and once in uniform, broke through barriers to achieve administrative and clinical excellence.7
My mother’s experience mirrors that of thousands of women whose dreams for a career in military medicine were shattered or who enlisted only to find their aspirations for advancement in the service thwarted. Medical historians remind us that due to bias, much of the book of women healer’s accomplishments remains unwritten, itself a testimony to the pervasive and enduring marginalization of women in Western society. Yet, as this brief glimpse of women in military medicine shows, there is sufficient evidence for us to appreciate their impressive contributions.8
Reflecting on this sketch of women’s struggle for acceptance in military medicine in March 2024, we may presume that the fight for equity has been continuously trending upward.8 President Joseph R. Biden appointed, and even more surprisingly, the US Congress confirmed Rachel Levine, MD, as US Department of Health and Human Services Assistant Under Secretary for Health in 2021, making Levine the highest ranking openly transgender health official in the history of the US government.9 Levine also has the distinction of being the first 4-star admiral in the Commissioned Corps of the US Public Health Service and the only transgender person to achieve this rank in any branch of the US uniformed services.10
However, research suggests that the history of women in the military is far more like an undulating curve. A 2019 study of academic military surgery found evidence of gender disparity even greater than that of the civilian sector.11 True and lasting equity in federal health care practice will require all of us to follow the inspiring examples of so many women known and unknown who fought the military establishment within for the right to heal those wounded fighting the enemy without.
1. Treadwell ME. The Women’s Army Corps. US Army Center of Military History; 1991: Chap 25. Accessed February 20, 2024. https://history.army.mil/books/wwii/Wac/ch25.htm
2. Hayes P. Meet five inspiring women veterans. Published November 10, 2022. Accessed February 20, 2024. https://news.va.gov/110571/meet-five-inspiring-women-veterans/
3. Lange K. Meet Dr. Mary Walker: the only female recipient of the Medical of Honor recipient. Published March 7, 2017. Accessed February 20, 2024. https://www.army.mil/article/183800/meet_dr_mary_walker_the_only_female_medal_of_honor_recipient
4. The National Medal of Honor Museum. Accessed February 20, 2024. https://mohmuseum.org/the-medal
5. Wilson Y, White A, Jefferson A, Danis M. Intersectionality in Clinical Medicine: The Need for a Conceptual Framework. Am J Bioeth. 2019;19(2):8-19. doi:10.1080/15265161.2018.1557275
6. National Women’s History Museum. African American Nurses in World War II. Published July 8, 2019. Accessed February 20, 2024. https://www.womenshistory.org/articles/african-american-nurses-world-war-ii
7. O’Gan P. Smithsonian National Museum of African American History and Culture. Victory at Home and Abroad: African American Army Nurses in World War II. Published May 8, 2023. Accessed February 20, 2024. https://nmaahc.si.edu/explore/stories/nurses-WWII
8. Neve M. Conclusion. In Conrad LI, Neve M, Nutton V, Porter R, and Wear A, eds. The Western Medical Tradition 800 BC to AD 1800. Cambridge University Press; 1995:477-494.
9. Stolberg SG. ‘This is politics’: Dr. Rachel Levine’s rise as transgender issues gain prominence. The New York Times. Updated May 10, 2021. Accessed February 20, 2024. https://www.nytimes.com/2021/05/08/us/politics/rachel-levine-transgender.html
10. Franklin J. Dr. Rachel Levine is sworn in as the nation’s first transgender four-star officer. October 19, 2021. Accessed February 20, 2024. https://www.npr.org/2021/10/19/1047423156/rachel-levine-first-transgender-four-star-officer
11. Herrick-Reynolds K, Brooks D, Wind G, Jackson P, Latham K. Military medicine and the academic surgery gender gap. Mil Med. 2019;184(9-10):383-387. doi:10.1093/milmed/usz083
1. Treadwell ME. The Women’s Army Corps. US Army Center of Military History; 1991: Chap 25. Accessed February 20, 2024. https://history.army.mil/books/wwii/Wac/ch25.htm
2. Hayes P. Meet five inspiring women veterans. Published November 10, 2022. Accessed February 20, 2024. https://news.va.gov/110571/meet-five-inspiring-women-veterans/
3. Lange K. Meet Dr. Mary Walker: the only female recipient of the Medical of Honor recipient. Published March 7, 2017. Accessed February 20, 2024. https://www.army.mil/article/183800/meet_dr_mary_walker_the_only_female_medal_of_honor_recipient
4. The National Medal of Honor Museum. Accessed February 20, 2024. https://mohmuseum.org/the-medal
5. Wilson Y, White A, Jefferson A, Danis M. Intersectionality in Clinical Medicine: The Need for a Conceptual Framework. Am J Bioeth. 2019;19(2):8-19. doi:10.1080/15265161.2018.1557275
6. National Women’s History Museum. African American Nurses in World War II. Published July 8, 2019. Accessed February 20, 2024. https://www.womenshistory.org/articles/african-american-nurses-world-war-ii
7. O’Gan P. Smithsonian National Museum of African American History and Culture. Victory at Home and Abroad: African American Army Nurses in World War II. Published May 8, 2023. Accessed February 20, 2024. https://nmaahc.si.edu/explore/stories/nurses-WWII
8. Neve M. Conclusion. In Conrad LI, Neve M, Nutton V, Porter R, and Wear A, eds. The Western Medical Tradition 800 BC to AD 1800. Cambridge University Press; 1995:477-494.
9. Stolberg SG. ‘This is politics’: Dr. Rachel Levine’s rise as transgender issues gain prominence. The New York Times. Updated May 10, 2021. Accessed February 20, 2024. https://www.nytimes.com/2021/05/08/us/politics/rachel-levine-transgender.html
10. Franklin J. Dr. Rachel Levine is sworn in as the nation’s first transgender four-star officer. October 19, 2021. Accessed February 20, 2024. https://www.npr.org/2021/10/19/1047423156/rachel-levine-first-transgender-four-star-officer
11. Herrick-Reynolds K, Brooks D, Wind G, Jackson P, Latham K. Military medicine and the academic surgery gender gap. Mil Med. 2019;184(9-10):383-387. doi:10.1093/milmed/usz083
Unexpectedly Helpful Effects of Drugs Used For Other Reasons
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
Shining a Light on Colorectal Cancer
For more than two decades, March has been designated Colorectal Cancer Awareness Month. This annual observance serves as a reminder to spread the word in our local and national communities regarding the value of colorectal cancer screening and prevention. CRC prevention through screening and surveillance is a core part of our practice as gastroenterologists and plays a critical role in improving outcomes and reducing mortality from the second leading cause of cancer deaths in the US.
While we have made great strides in increasing awareness among patients of the need for screening, overall screening rates remain well below our national target of 80% and significant disparities in screening persist. By disseminating key information about risk factors, promoting early detection through evidence-based screening, continuing to improve access to care by reducing financial and other barriers, and educating patients about available screening options that best fit their needs and preferences, we can continue to move the needle in improving overall screening rates and optimizing outcomes.
In this month’s issue of GIHN, we feature an excellent narrative review by Dr. Samir Gupta and colleagues describing the phenomenon of “birth cohort CRC,” which is thought to explain recent changes in CRC epidemiology, including rising incidence of early-onset colorectal cancer. We also highlight a timely study out of Kaiser Permanente investigating how best to communicate with patients with prior low-risk adenomas regarding updated colonoscopy intervals given recent guideline changes extending surveillance intervals from 5 to 7-10 years. This question is particularly relevant to resource-constrained healthcare settings, where proactive de-implementation of outdated surveillance intervals could improve access for other patients with more immediate need.
In our March Member Spotlight, we feature Dr. Andy Tau of Austin Gastroenterology, who shares important insights regarding his career as a GI hospitalist, a growing area of GI practice. Finally, in this month’s Perspectives column, Drs. Michael Weinstein of Capital Digestive Care and Paul Berggreen of GI Alliance provide powerful contrasting perspectives highlighting the pros and cons of private equity in GI and how to evaluate if it’s right for your practice. I found it to be a particularly fascinating read!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
For more than two decades, March has been designated Colorectal Cancer Awareness Month. This annual observance serves as a reminder to spread the word in our local and national communities regarding the value of colorectal cancer screening and prevention. CRC prevention through screening and surveillance is a core part of our practice as gastroenterologists and plays a critical role in improving outcomes and reducing mortality from the second leading cause of cancer deaths in the US.
While we have made great strides in increasing awareness among patients of the need for screening, overall screening rates remain well below our national target of 80% and significant disparities in screening persist. By disseminating key information about risk factors, promoting early detection through evidence-based screening, continuing to improve access to care by reducing financial and other barriers, and educating patients about available screening options that best fit their needs and preferences, we can continue to move the needle in improving overall screening rates and optimizing outcomes.
In this month’s issue of GIHN, we feature an excellent narrative review by Dr. Samir Gupta and colleagues describing the phenomenon of “birth cohort CRC,” which is thought to explain recent changes in CRC epidemiology, including rising incidence of early-onset colorectal cancer. We also highlight a timely study out of Kaiser Permanente investigating how best to communicate with patients with prior low-risk adenomas regarding updated colonoscopy intervals given recent guideline changes extending surveillance intervals from 5 to 7-10 years. This question is particularly relevant to resource-constrained healthcare settings, where proactive de-implementation of outdated surveillance intervals could improve access for other patients with more immediate need.
In our March Member Spotlight, we feature Dr. Andy Tau of Austin Gastroenterology, who shares important insights regarding his career as a GI hospitalist, a growing area of GI practice. Finally, in this month’s Perspectives column, Drs. Michael Weinstein of Capital Digestive Care and Paul Berggreen of GI Alliance provide powerful contrasting perspectives highlighting the pros and cons of private equity in GI and how to evaluate if it’s right for your practice. I found it to be a particularly fascinating read!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
For more than two decades, March has been designated Colorectal Cancer Awareness Month. This annual observance serves as a reminder to spread the word in our local and national communities regarding the value of colorectal cancer screening and prevention. CRC prevention through screening and surveillance is a core part of our practice as gastroenterologists and plays a critical role in improving outcomes and reducing mortality from the second leading cause of cancer deaths in the US.
While we have made great strides in increasing awareness among patients of the need for screening, overall screening rates remain well below our national target of 80% and significant disparities in screening persist. By disseminating key information about risk factors, promoting early detection through evidence-based screening, continuing to improve access to care by reducing financial and other barriers, and educating patients about available screening options that best fit their needs and preferences, we can continue to move the needle in improving overall screening rates and optimizing outcomes.
In this month’s issue of GIHN, we feature an excellent narrative review by Dr. Samir Gupta and colleagues describing the phenomenon of “birth cohort CRC,” which is thought to explain recent changes in CRC epidemiology, including rising incidence of early-onset colorectal cancer. We also highlight a timely study out of Kaiser Permanente investigating how best to communicate with patients with prior low-risk adenomas regarding updated colonoscopy intervals given recent guideline changes extending surveillance intervals from 5 to 7-10 years. This question is particularly relevant to resource-constrained healthcare settings, where proactive de-implementation of outdated surveillance intervals could improve access for other patients with more immediate need.
In our March Member Spotlight, we feature Dr. Andy Tau of Austin Gastroenterology, who shares important insights regarding his career as a GI hospitalist, a growing area of GI practice. Finally, in this month’s Perspectives column, Drs. Michael Weinstein of Capital Digestive Care and Paul Berggreen of GI Alliance provide powerful contrasting perspectives highlighting the pros and cons of private equity in GI and how to evaluate if it’s right for your practice. I found it to be a particularly fascinating read!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief





