User login
Be the Change You Wish to See
In a recent letter to the editors, we were queried as to the ratio of NP authors to PA authors represented in this journal and whether Clinician Reviews was changing its focus to PAs. Let me assure you, my NP colleagues, the journal always has been and always will be written for both professions. However, the query did give me pause—and the opportunity to encourage (as I have in the past) all NPs to pick up their pen (or keyboard) and start writing.
I often hear my NP colleagues diminish the work they do, the care they provide, the advocacy they undertake on behalf of patients and their families. Yet the stories of clinical problems they solve, access barriers they mitigate, or professional advances they have fostered are fascinating—and we want to read about them!
When I marvel at the accomplishments, and ask if the NP in question would write about his/her experience, the response is frequently “Oh, I can’t write” or “I could never author an article,” or (as most of us could say) “I’m too busy.”
I fully understand the hesitance of many with regard to penning an article (or being a lead for one of our clinical departments); many of us have been out of school for a bit and are so used to dictating clinical notes that we cringe at the thought of “trying” to write. I once had an NP tell me that she was not sure whether she could compose a full sentence anymore. Trust me, I could relate to her angst. I am reasonably confident that some of you are saying to yourself, “Yeah, sure.” Well, dear readers, it’s true.
When I was approached to undertake the position as NP Editor-in-Chief, I had much trepidation. I, too, lacked confidence in my writing ability and faith that anyone cared to read about what I thought or what I considered important. However, with the support of my colleagues and mentors, I was quickly disabused of those self-doubts.
We write every day—in one fashion or another. Think about it. Those of you with children: I am sure you have coached, restructured sentences, researched subject matter, and edited at least one book report. And what about e-mail? Surely, you compose at least one professional e-mail per day to a colleague or a patient. Is it ready for peer review, or does it meet the standard required for publication? Who knows? But it is writing. Moreover, the passion with which you communicate the information is why people read what you have to “say.”
It is said that a journey of a thousand miles begins with a single step (Lao Tsu, Chinese philosopher, 571-531 BC). So, too, does an article begin with one word or a single thought. If you are a regular reader of my editorials, you know that many of them (including this one!) start with a comment from a colleague, or something I read or heard in the news, or an occurrence that raised my ire. And so the seed of a column (limited to 1,000 words, by the way) is planted. Each of you has the seed of an article in you; I know you do.
This year, we celebrate the 50th anniversary of the NP profession, which was established because Loretta Ford and Henry Silva saw the need for better access to care for children. From the seed that they planted, the profession has grown and brought with it a monumental change in health care. (They likely weren’t thinking that far ahead at the time, but rather striving to address what they saw as an immediate and vital need.)
In 1985, a group of NPs saw the need for an organization dedicated to mitigating barriers to practice. A handful of people initiated a change—better representation for NPs—and a little entity named AANP began. As we know, the organization has flourished—all because a small group of people saw an opportunity to improve the status quo and stepped in to make a change. (To read more about the origins of AANP, “From the CR-chive”.)
My point is perhaps best summed up by a quote I read in an interview in the NY Times Book Review in the late 1960s. It stuck with me, to the extent that it became my mantra: “You can’t sit on the outside and complain if you don’t like something—go in and change it.”
Those of you willing to rise to this challenge will find that every publication posts its “guidelines for authors” on its website (and sometimes in the print edition). Take a few extra minutes when you are reading the journal or visiting the site to review those guidelines. Check out the types of articles or other submissions the editors accept and consider what you might contribute, based on your particular experiences, knowledge, and interest.
Do you have a particular specialty area or a “pet” disease state or condition that you want to share your expertise on? Have you learned something you wish you’d known sooner? Have you maneuvered through or around a particular barrier to practice or access? Others might benefit from knowing the keys to your accomplishment. We are quick to bemoan our plights; instead, share your success!
Start by jotting down a few words—the rest will follow. The great thing about writing is that you can do it anywhere, anytime. (For example, this editorial was written in an airport!) And please note: If you have the idea and the passion—and the wherewithal to start the writing process—there are dedicated editorial staff who will work with you to polish your submission for publication. Writing begins as a solitary pursuit, but you are not alone through the process.
So here is my challenge to you: Be an agent of change to increase the number of NP-authored columns in this publication. Have faith in your ability to write. Take it one word at a time; a few words form a sentence, a few sentences a paragraph—and before you know it, you’ve written an entire column or article.
Write from your head, your heart, or your outrage—but write! You can start by sending your thoughts on this editorial to [email protected].
In a recent letter to the editors, we were queried as to the ratio of NP authors to PA authors represented in this journal and whether Clinician Reviews was changing its focus to PAs. Let me assure you, my NP colleagues, the journal always has been and always will be written for both professions. However, the query did give me pause—and the opportunity to encourage (as I have in the past) all NPs to pick up their pen (or keyboard) and start writing.
I often hear my NP colleagues diminish the work they do, the care they provide, the advocacy they undertake on behalf of patients and their families. Yet the stories of clinical problems they solve, access barriers they mitigate, or professional advances they have fostered are fascinating—and we want to read about them!
When I marvel at the accomplishments, and ask if the NP in question would write about his/her experience, the response is frequently “Oh, I can’t write” or “I could never author an article,” or (as most of us could say) “I’m too busy.”
I fully understand the hesitance of many with regard to penning an article (or being a lead for one of our clinical departments); many of us have been out of school for a bit and are so used to dictating clinical notes that we cringe at the thought of “trying” to write. I once had an NP tell me that she was not sure whether she could compose a full sentence anymore. Trust me, I could relate to her angst. I am reasonably confident that some of you are saying to yourself, “Yeah, sure.” Well, dear readers, it’s true.
When I was approached to undertake the position as NP Editor-in-Chief, I had much trepidation. I, too, lacked confidence in my writing ability and faith that anyone cared to read about what I thought or what I considered important. However, with the support of my colleagues and mentors, I was quickly disabused of those self-doubts.
We write every day—in one fashion or another. Think about it. Those of you with children: I am sure you have coached, restructured sentences, researched subject matter, and edited at least one book report. And what about e-mail? Surely, you compose at least one professional e-mail per day to a colleague or a patient. Is it ready for peer review, or does it meet the standard required for publication? Who knows? But it is writing. Moreover, the passion with which you communicate the information is why people read what you have to “say.”
It is said that a journey of a thousand miles begins with a single step (Lao Tsu, Chinese philosopher, 571-531 BC). So, too, does an article begin with one word or a single thought. If you are a regular reader of my editorials, you know that many of them (including this one!) start with a comment from a colleague, or something I read or heard in the news, or an occurrence that raised my ire. And so the seed of a column (limited to 1,000 words, by the way) is planted. Each of you has the seed of an article in you; I know you do.
This year, we celebrate the 50th anniversary of the NP profession, which was established because Loretta Ford and Henry Silva saw the need for better access to care for children. From the seed that they planted, the profession has grown and brought with it a monumental change in health care. (They likely weren’t thinking that far ahead at the time, but rather striving to address what they saw as an immediate and vital need.)
In 1985, a group of NPs saw the need for an organization dedicated to mitigating barriers to practice. A handful of people initiated a change—better representation for NPs—and a little entity named AANP began. As we know, the organization has flourished—all because a small group of people saw an opportunity to improve the status quo and stepped in to make a change. (To read more about the origins of AANP, “From the CR-chive”.)
My point is perhaps best summed up by a quote I read in an interview in the NY Times Book Review in the late 1960s. It stuck with me, to the extent that it became my mantra: “You can’t sit on the outside and complain if you don’t like something—go in and change it.”
Those of you willing to rise to this challenge will find that every publication posts its “guidelines for authors” on its website (and sometimes in the print edition). Take a few extra minutes when you are reading the journal or visiting the site to review those guidelines. Check out the types of articles or other submissions the editors accept and consider what you might contribute, based on your particular experiences, knowledge, and interest.
Do you have a particular specialty area or a “pet” disease state or condition that you want to share your expertise on? Have you learned something you wish you’d known sooner? Have you maneuvered through or around a particular barrier to practice or access? Others might benefit from knowing the keys to your accomplishment. We are quick to bemoan our plights; instead, share your success!
Start by jotting down a few words—the rest will follow. The great thing about writing is that you can do it anywhere, anytime. (For example, this editorial was written in an airport!) And please note: If you have the idea and the passion—and the wherewithal to start the writing process—there are dedicated editorial staff who will work with you to polish your submission for publication. Writing begins as a solitary pursuit, but you are not alone through the process.
So here is my challenge to you: Be an agent of change to increase the number of NP-authored columns in this publication. Have faith in your ability to write. Take it one word at a time; a few words form a sentence, a few sentences a paragraph—and before you know it, you’ve written an entire column or article.
Write from your head, your heart, or your outrage—but write! You can start by sending your thoughts on this editorial to [email protected].
In a recent letter to the editors, we were queried as to the ratio of NP authors to PA authors represented in this journal and whether Clinician Reviews was changing its focus to PAs. Let me assure you, my NP colleagues, the journal always has been and always will be written for both professions. However, the query did give me pause—and the opportunity to encourage (as I have in the past) all NPs to pick up their pen (or keyboard) and start writing.
I often hear my NP colleagues diminish the work they do, the care they provide, the advocacy they undertake on behalf of patients and their families. Yet the stories of clinical problems they solve, access barriers they mitigate, or professional advances they have fostered are fascinating—and we want to read about them!
When I marvel at the accomplishments, and ask if the NP in question would write about his/her experience, the response is frequently “Oh, I can’t write” or “I could never author an article,” or (as most of us could say) “I’m too busy.”
I fully understand the hesitance of many with regard to penning an article (or being a lead for one of our clinical departments); many of us have been out of school for a bit and are so used to dictating clinical notes that we cringe at the thought of “trying” to write. I once had an NP tell me that she was not sure whether she could compose a full sentence anymore. Trust me, I could relate to her angst. I am reasonably confident that some of you are saying to yourself, “Yeah, sure.” Well, dear readers, it’s true.
When I was approached to undertake the position as NP Editor-in-Chief, I had much trepidation. I, too, lacked confidence in my writing ability and faith that anyone cared to read about what I thought or what I considered important. However, with the support of my colleagues and mentors, I was quickly disabused of those self-doubts.
We write every day—in one fashion or another. Think about it. Those of you with children: I am sure you have coached, restructured sentences, researched subject matter, and edited at least one book report. And what about e-mail? Surely, you compose at least one professional e-mail per day to a colleague or a patient. Is it ready for peer review, or does it meet the standard required for publication? Who knows? But it is writing. Moreover, the passion with which you communicate the information is why people read what you have to “say.”
It is said that a journey of a thousand miles begins with a single step (Lao Tsu, Chinese philosopher, 571-531 BC). So, too, does an article begin with one word or a single thought. If you are a regular reader of my editorials, you know that many of them (including this one!) start with a comment from a colleague, or something I read or heard in the news, or an occurrence that raised my ire. And so the seed of a column (limited to 1,000 words, by the way) is planted. Each of you has the seed of an article in you; I know you do.
This year, we celebrate the 50th anniversary of the NP profession, which was established because Loretta Ford and Henry Silva saw the need for better access to care for children. From the seed that they planted, the profession has grown and brought with it a monumental change in health care. (They likely weren’t thinking that far ahead at the time, but rather striving to address what they saw as an immediate and vital need.)
In 1985, a group of NPs saw the need for an organization dedicated to mitigating barriers to practice. A handful of people initiated a change—better representation for NPs—and a little entity named AANP began. As we know, the organization has flourished—all because a small group of people saw an opportunity to improve the status quo and stepped in to make a change. (To read more about the origins of AANP, “From the CR-chive”.)
My point is perhaps best summed up by a quote I read in an interview in the NY Times Book Review in the late 1960s. It stuck with me, to the extent that it became my mantra: “You can’t sit on the outside and complain if you don’t like something—go in and change it.”
Those of you willing to rise to this challenge will find that every publication posts its “guidelines for authors” on its website (and sometimes in the print edition). Take a few extra minutes when you are reading the journal or visiting the site to review those guidelines. Check out the types of articles or other submissions the editors accept and consider what you might contribute, based on your particular experiences, knowledge, and interest.
Do you have a particular specialty area or a “pet” disease state or condition that you want to share your expertise on? Have you learned something you wish you’d known sooner? Have you maneuvered through or around a particular barrier to practice or access? Others might benefit from knowing the keys to your accomplishment. We are quick to bemoan our plights; instead, share your success!
Start by jotting down a few words—the rest will follow. The great thing about writing is that you can do it anywhere, anytime. (For example, this editorial was written in an airport!) And please note: If you have the idea and the passion—and the wherewithal to start the writing process—there are dedicated editorial staff who will work with you to polish your submission for publication. Writing begins as a solitary pursuit, but you are not alone through the process.
So here is my challenge to you: Be an agent of change to increase the number of NP-authored columns in this publication. Have faith in your ability to write. Take it one word at a time; a few words form a sentence, a few sentences a paragraph—and before you know it, you’ve written an entire column or article.
Write from your head, your heart, or your outrage—but write! You can start by sending your thoughts on this editorial to [email protected].
A National Disgrace--Drug Shortages Continue
[Updating an editorial that first appeared in the August 2012 issue.]
Until recently, shortages of critically needed medications in this country were rare and unthinkable. But now, the unthinkable has become commonplace. In August 2012, I wrote about the seemingly endless supply problems and proposed some possible remedies, but in the 3 years since, not much has changed.
Cold War era images of empty Soviet Russia supermarket shelves still remain vivid reminders of a failed system of government. So, who would have predicted that in the 21st century, the United States of America, winner of the Cold War, would have hospital pharmacy shelves bereft of essential medications, including many of the sterile, injectable, crashcart meds we rely on during resuscitations? According to a November 2011 U.S. Government Accountability Office report (GAO-12-116), there were 1,190 drug shortages between 2001-2011 with the number increasing annually since 2006. Drug shortages have not escaped physician or public attention, and a lot of finger pointing among government agencies, manufacturers, and distributors, have made most of us feel like a parent trying to break up a loud argument between siblings: “I don’t care who started it, just stop it NOW!”
One of the most critical types of shortages involves sterile, injectable, generic meds (such as epinephrine), accounting for more than half the shortages since 2009. During resuscitations, physicians and paramedics rely on the immediate availability of premixed, ready-to-use unit doses packaged in sealed, prefilled syringes. All such preparations must be sterile and most are generic, necessitating short expiration dates and tightly controlled inventories to maintain sterility of properly produced preparations, while contamination during the manufacturing process itself results in nationwide recalls and production halts. Because these generic medications have no patent protection and do not command the higher prices of newer, brand-name drugs, there is little incentive for manufacturers to make costly changes in production methods aimed at reducing future shortages. As noted in an August 2010 NEJM article on a shortage of propofol (2010;363[9]:806-807), most such drugs are manufactured by only two or three companies Should a manufacturer suspend or discontinue production, widespread shortages are felt immediately. Although the FDA has the authority to regulate pharmaceutical production to ensure safety, it cannot mandate continued production.
Relying on information from the Utah Drug Information Service, the GAO (http://www.gao.gov/products/GAO-14-194) found that the total number of shortages increased each year since 2007, even in years (2012, 2013) when the rate of new shortages decreased, because ongoing shortages were slow to resolve. Disturbingly, current shortages include such basics as saline, dextrose, nitroglycerin, anti-emetics, and epinephrine packaged for use in resuscitations, all affecting large numbers of patients. Manufacturer-provided information to the Utah Drug Information Service (http://www.ashp.org/DocLibrary/Policy/DrugShortages/Drug-Shortages-Statistics.pdf) regarding the reasons for shortages in 2014 indicate “manufacturing problems” were responsible for 25%, “supply/demand” for 17%, “business decisions/ discontinue” for 9%, with 47% listed as “unknown.”
What can be done to avoid shortages? First, a short list of “never event” drug shortages should be identified. For established medications and formulations too essential to be allowed to fail, the federal government should consider relaxing or delaying requirements for new, costly changes in their manufacture, or should pay for the mandated changes. The government should also consider offering incentives for pharmaceutical companies to continue producing critically-needed, low-profit generics that require costly manufacturing changes, perhaps by extending patent protection for one of the company’s more profitable products. As a last resort, the government should consider manufacturing the medication itself.
In the almost 15 years since an Annals of Emergency Medicine article appeared on “the challenge of drug shortages for emergency medicine” (2002;40[8]:598-602),—enough time for three Soviet-style “5 year plans”—new shortages and their deleterious effects on patient care have continued.
[Updating an editorial that first appeared in the August 2012 issue.]
Until recently, shortages of critically needed medications in this country were rare and unthinkable. But now, the unthinkable has become commonplace. In August 2012, I wrote about the seemingly endless supply problems and proposed some possible remedies, but in the 3 years since, not much has changed.
Cold War era images of empty Soviet Russia supermarket shelves still remain vivid reminders of a failed system of government. So, who would have predicted that in the 21st century, the United States of America, winner of the Cold War, would have hospital pharmacy shelves bereft of essential medications, including many of the sterile, injectable, crashcart meds we rely on during resuscitations? According to a November 2011 U.S. Government Accountability Office report (GAO-12-116), there were 1,190 drug shortages between 2001-2011 with the number increasing annually since 2006. Drug shortages have not escaped physician or public attention, and a lot of finger pointing among government agencies, manufacturers, and distributors, have made most of us feel like a parent trying to break up a loud argument between siblings: “I don’t care who started it, just stop it NOW!”
One of the most critical types of shortages involves sterile, injectable, generic meds (such as epinephrine), accounting for more than half the shortages since 2009. During resuscitations, physicians and paramedics rely on the immediate availability of premixed, ready-to-use unit doses packaged in sealed, prefilled syringes. All such preparations must be sterile and most are generic, necessitating short expiration dates and tightly controlled inventories to maintain sterility of properly produced preparations, while contamination during the manufacturing process itself results in nationwide recalls and production halts. Because these generic medications have no patent protection and do not command the higher prices of newer, brand-name drugs, there is little incentive for manufacturers to make costly changes in production methods aimed at reducing future shortages. As noted in an August 2010 NEJM article on a shortage of propofol (2010;363[9]:806-807), most such drugs are manufactured by only two or three companies Should a manufacturer suspend or discontinue production, widespread shortages are felt immediately. Although the FDA has the authority to regulate pharmaceutical production to ensure safety, it cannot mandate continued production.
Relying on information from the Utah Drug Information Service, the GAO (http://www.gao.gov/products/GAO-14-194) found that the total number of shortages increased each year since 2007, even in years (2012, 2013) when the rate of new shortages decreased, because ongoing shortages were slow to resolve. Disturbingly, current shortages include such basics as saline, dextrose, nitroglycerin, anti-emetics, and epinephrine packaged for use in resuscitations, all affecting large numbers of patients. Manufacturer-provided information to the Utah Drug Information Service (http://www.ashp.org/DocLibrary/Policy/DrugShortages/Drug-Shortages-Statistics.pdf) regarding the reasons for shortages in 2014 indicate “manufacturing problems” were responsible for 25%, “supply/demand” for 17%, “business decisions/ discontinue” for 9%, with 47% listed as “unknown.”
What can be done to avoid shortages? First, a short list of “never event” drug shortages should be identified. For established medications and formulations too essential to be allowed to fail, the federal government should consider relaxing or delaying requirements for new, costly changes in their manufacture, or should pay for the mandated changes. The government should also consider offering incentives for pharmaceutical companies to continue producing critically-needed, low-profit generics that require costly manufacturing changes, perhaps by extending patent protection for one of the company’s more profitable products. As a last resort, the government should consider manufacturing the medication itself.
In the almost 15 years since an Annals of Emergency Medicine article appeared on “the challenge of drug shortages for emergency medicine” (2002;40[8]:598-602),—enough time for three Soviet-style “5 year plans”—new shortages and their deleterious effects on patient care have continued.
[Updating an editorial that first appeared in the August 2012 issue.]
Until recently, shortages of critically needed medications in this country were rare and unthinkable. But now, the unthinkable has become commonplace. In August 2012, I wrote about the seemingly endless supply problems and proposed some possible remedies, but in the 3 years since, not much has changed.
Cold War era images of empty Soviet Russia supermarket shelves still remain vivid reminders of a failed system of government. So, who would have predicted that in the 21st century, the United States of America, winner of the Cold War, would have hospital pharmacy shelves bereft of essential medications, including many of the sterile, injectable, crashcart meds we rely on during resuscitations? According to a November 2011 U.S. Government Accountability Office report (GAO-12-116), there were 1,190 drug shortages between 2001-2011 with the number increasing annually since 2006. Drug shortages have not escaped physician or public attention, and a lot of finger pointing among government agencies, manufacturers, and distributors, have made most of us feel like a parent trying to break up a loud argument between siblings: “I don’t care who started it, just stop it NOW!”
One of the most critical types of shortages involves sterile, injectable, generic meds (such as epinephrine), accounting for more than half the shortages since 2009. During resuscitations, physicians and paramedics rely on the immediate availability of premixed, ready-to-use unit doses packaged in sealed, prefilled syringes. All such preparations must be sterile and most are generic, necessitating short expiration dates and tightly controlled inventories to maintain sterility of properly produced preparations, while contamination during the manufacturing process itself results in nationwide recalls and production halts. Because these generic medications have no patent protection and do not command the higher prices of newer, brand-name drugs, there is little incentive for manufacturers to make costly changes in production methods aimed at reducing future shortages. As noted in an August 2010 NEJM article on a shortage of propofol (2010;363[9]:806-807), most such drugs are manufactured by only two or three companies Should a manufacturer suspend or discontinue production, widespread shortages are felt immediately. Although the FDA has the authority to regulate pharmaceutical production to ensure safety, it cannot mandate continued production.
Relying on information from the Utah Drug Information Service, the GAO (http://www.gao.gov/products/GAO-14-194) found that the total number of shortages increased each year since 2007, even in years (2012, 2013) when the rate of new shortages decreased, because ongoing shortages were slow to resolve. Disturbingly, current shortages include such basics as saline, dextrose, nitroglycerin, anti-emetics, and epinephrine packaged for use in resuscitations, all affecting large numbers of patients. Manufacturer-provided information to the Utah Drug Information Service (http://www.ashp.org/DocLibrary/Policy/DrugShortages/Drug-Shortages-Statistics.pdf) regarding the reasons for shortages in 2014 indicate “manufacturing problems” were responsible for 25%, “supply/demand” for 17%, “business decisions/ discontinue” for 9%, with 47% listed as “unknown.”
What can be done to avoid shortages? First, a short list of “never event” drug shortages should be identified. For established medications and formulations too essential to be allowed to fail, the federal government should consider relaxing or delaying requirements for new, costly changes in their manufacture, or should pay for the mandated changes. The government should also consider offering incentives for pharmaceutical companies to continue producing critically-needed, low-profit generics that require costly manufacturing changes, perhaps by extending patent protection for one of the company’s more profitable products. As a last resort, the government should consider manufacturing the medication itself.
In the almost 15 years since an Annals of Emergency Medicine article appeared on “the challenge of drug shortages for emergency medicine” (2002;40[8]:598-602),—enough time for three Soviet-style “5 year plans”—new shortages and their deleterious effects on patient care have continued.
Dr. Nathaniel Savio Beers joins Pediatric News board
We are pleased to welcome Dr. Nathaniel Savio Beers to the Pediatric News Editorial Advisory Board.
Dr. Beers is currently chief operating officer for the District of Columbia Public Schools and a general and developmental behavioral pediatrician at Children’s National Health System in Washington. At DCPS, he was previously the Chief of Specialized Instruction from 2011 to 2015. Previously, he was director of the Children’s Health Center, the largest provider of primary care in Washington, deputy director for the Community Health Administration in Washington, and Title V director for the D.C. Health Department.
He has served on the Washington Mayor’s Advisory Committee on Child Welfare, the Mayor’s Early Childhood Advisory Council, and the Children with Special Health Care Needs Advisory Board. Dr. Beers has served as president of the D.C. Chapter of the American Academy of Pediatrics, chair of the National AAP Committee on Membership, and chair of the AAP Section on Residents.
He is newly elected to the Council on School Health for the AAP. Dr. Beers graduated from George Washington University medical school and completed his pediatric residency at Children’s National. Dr. Beers was the Anne E. Dyson Child Advocacy Fellow at Children’s Hospital of Boston and Chief Fellow of General Pediatrics. He received a Master’s Degree of Public Administration at the Harvard Kennedy School, Boston.
We are pleased to welcome Dr. Nathaniel Savio Beers to the Pediatric News Editorial Advisory Board.
Dr. Beers is currently chief operating officer for the District of Columbia Public Schools and a general and developmental behavioral pediatrician at Children’s National Health System in Washington. At DCPS, he was previously the Chief of Specialized Instruction from 2011 to 2015. Previously, he was director of the Children’s Health Center, the largest provider of primary care in Washington, deputy director for the Community Health Administration in Washington, and Title V director for the D.C. Health Department.
He has served on the Washington Mayor’s Advisory Committee on Child Welfare, the Mayor’s Early Childhood Advisory Council, and the Children with Special Health Care Needs Advisory Board. Dr. Beers has served as president of the D.C. Chapter of the American Academy of Pediatrics, chair of the National AAP Committee on Membership, and chair of the AAP Section on Residents.
He is newly elected to the Council on School Health for the AAP. Dr. Beers graduated from George Washington University medical school and completed his pediatric residency at Children’s National. Dr. Beers was the Anne E. Dyson Child Advocacy Fellow at Children’s Hospital of Boston and Chief Fellow of General Pediatrics. He received a Master’s Degree of Public Administration at the Harvard Kennedy School, Boston.
We are pleased to welcome Dr. Nathaniel Savio Beers to the Pediatric News Editorial Advisory Board.
Dr. Beers is currently chief operating officer for the District of Columbia Public Schools and a general and developmental behavioral pediatrician at Children’s National Health System in Washington. At DCPS, he was previously the Chief of Specialized Instruction from 2011 to 2015. Previously, he was director of the Children’s Health Center, the largest provider of primary care in Washington, deputy director for the Community Health Administration in Washington, and Title V director for the D.C. Health Department.
He has served on the Washington Mayor’s Advisory Committee on Child Welfare, the Mayor’s Early Childhood Advisory Council, and the Children with Special Health Care Needs Advisory Board. Dr. Beers has served as president of the D.C. Chapter of the American Academy of Pediatrics, chair of the National AAP Committee on Membership, and chair of the AAP Section on Residents.
He is newly elected to the Council on School Health for the AAP. Dr. Beers graduated from George Washington University medical school and completed his pediatric residency at Children’s National. Dr. Beers was the Anne E. Dyson Child Advocacy Fellow at Children’s Hospital of Boston and Chief Fellow of General Pediatrics. He received a Master’s Degree of Public Administration at the Harvard Kennedy School, Boston.
The importance of UA in diagnosing UTIs in infants under 2 months
A 28-day-old uncircumcised male infant presents to the emergency department with fever of 38.9° C, decreased feeding, and irritability. The physical examination is normal with the exception of the irritability and your assessment of mild dehydration. The infant undergoes a sepsis work-up. The CBC is remarkable for a WBC of 16,500/mm3 with 44% neutrophils, 52% lymphocytes, and 4% monocytes. Platelet count is normal. Cerebrospinal fluid (CSF) shows no white or red blood cells with normal glucose and protein. The urinalysis (UA) has a positive 1+ leukocyte esterase (LE) with 10 WBC per high-power field (HPF), but negative nitrite and 1+ bacteria microscopically. The child is admitted to the hospital for empiric antibiotics pending blood, urine, and CSF cultures. What are the chances that a urinary tract infection (UTI) is the origin of the febrile presentation?
UTIs are currently the most common serious bacterial infection (SBI) in < 2-year-old febrile children without an apparent source of fever (Pediatrics 2011;128:595-610). Since 2000, the prevalence of UTIs in all febrile infants and young children without an apparent source is unchanged, being approximately 5%. The rate of UTIs in fever-without-apparent-source presentations at < 90 days of age is higher, ranging from 6%-15% in different studies.
Meanwhile bacteremia, sepsis, meningitis, and other previously common SBIs, mostly caused by Haemophilus influenzae type b (Hib) or pneumococcus, have decreased. We recognize these reductions as effects of universal implementation of Hib (mid-1990s) and pneumococcal (2000 and 2010) conjugate vaccines.
Given the case above, other pertinent facts are that uncircumcised males have more UTIs in the first months of life (J. Pediatr. 1996;128:23-7) and approximately 5% of young infants with UTIs also are concurrently bacteremic (Pediatrics 1999;104:79-86;J. Pediatr. 1994;124:513-9)
The elephant in the room is the fact that we also need to be cognizant of asymptomatic bacteriuria (AB). AB is colonization of the lower urinary tract without infection. Patients with AB may meet culture criteria for UTI (whether we consider > 50,000 or > 100,000 colony-forming units/mL), but there is no evidence of true infection, that is no inflammation or mucosal injury. So children with AB are not at risk for renal injury or later renal damage and do not require antibiotic treatment.
But when AB patients develop fever, for example with an enterovirus infection, their urine cultures (together with the fever) can do a good imitation of a UTI, unless we focus on the UA results. It not only remains critical to detect true UTIs in infants < 90 days old, such as the one in our case above, but also to distinguish UTI from AB.
The 1999 American Academy of Pediatrics’ UTI guidelines (Pediatrics 1999;103:843-52) included UA results as suggestive of UTI. They stated that a positive LE or nitrite test or > 5 WBC/HPF in a spun urine, or bacteria visualized in unspun gram-stained specimen suggest, but cannot be diagnostic of a UTI. Recommendation five in the guidelines states that UTI diagnosis required 100,000 CFU/mL in culture of sterilely obtained catheterized urine as the threshold criterion (strength of evidence: strong). However, AB was not fully considered because, in part, data defining AB was incomplete in 1999.
The 1999 guidelines also stated, “The urinalysis … can be valuable in selecting individuals for prompt initiation of treatment while waiting for the results of the urine culture.” So, UA was considered adjunctive. UA’s main function was to allow empiric therapy of sufficiently ill children, given positive results for LE, nitrites, or microscopic visualization of > 5 WBC/HPF or bacteria in the spun urine.
In the 2011 AAP guidelines for UTI, things have changed (Pediatrics 2011;128:595-610). The third action statement tells us that both the UA and culture taken together are necessary for UTI diagnosis. To paraphrase: The diagnosis of UTI requires urinalysis results suggesting infection (pyuria or microscopic bacteriuria) plus > 50,000 CFU/mL of a uropathogen in urine from catheterization or suprapubic aspiration. But remember that these guidelines do not apply specifically to the youngest of infants, that is < 2 months old.
Both of these criteria were changes from the 1999 UTI guidelines. Previously pyuria or microscopic bacteriuria were not considered necessary to diagnose UTI, and >100,000 CFU/mL rather than > 50,000 CFU/mL of a single pathogen species was the critical diagnostic result for catheterized urine. For suprapubic aspiration urine samples, > 10,000 CFU/HPF were considered adequate for UTI diagnoses in 1999.
Now, a recent study of children < 90 days of age (including those < 2 months of age) reports that pyuria (> 3 WBC/HPF) plus > 50,000 CFU/mL are the keys to diagnosing UTI (Pediatrics 2015;135:965-71). One caveat is that the study population was febrile infants < 90 days old with concurrent bacteremia (bacteremic UTI). Bacteremic UTI was studied to reduce as much as possible the chance that AB patients might be inadvertently included in the study. One other conclusion of this new study is that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
These data in an overall younger population than that covered by the 2011 guidelines adds evidence that pyuria (but not microscopic bacteriuria) is critical to diagnosing UTI. Pyuria plus positive culture has been a combination for the pediatric infectious diseases practitioner’s toolkit for decades. Likewise, it seems to me that primary care pediatric clinicians also often decide whether to undertake the expense of culture based on UA results. For example, a completely normal UA may obviate need for culture except in selected unusual cases.
Requiring UA evidence of inflammation to diagnose UTI (per the 2011 guidelines and the recommendations of the authors of the recent 2015 study) makes sense because most UTIs in otherwise healthy children are caused by gram-negative organisms (> 90% from Escherichia coli) (J. Pediatr. 1994;124:513-9). Why are UA results so important?
A positive nitrite test strongly suggests UTI because nitrites in the urine indicate viable gram-negative organisms also are present in the urine. Nitrates in the urine are converted to nitrites by metabolic activity of gram-negative pathogens. For WBCs or LE in the urine, their presence indicates inflammation in the urinary tract, Consider that lipopolysaccharide (LPS), also known as gram-negative endotoxin, is a major component of the cell membrane of > 90% of uropathogens like E. coli. Moreover, LPS elicits about the strongest innate immune response via toll-like receptor 4 (TLR4) from monocytes/macrophages, inducing a large pro-inflammatory and chemotactic response – interleukin-6, interleukin-8, tetrahydrofuran-alpha. Remember that LPS is also a major cause of fever and of shock during gram-negative sepsis.
So a UTI diagnosis based on a “positive” culture without evidence of metabolic products of gram negatives (nitrites) or without inflammation (no pyuria or negative LE) should be questioned. The combination of > 50,000 CFU/mL with no detectable LE or < 3-5 WBC/HPF in a febrile child is most likely evidence for AB in a child with the fever caused by some non-UTI process.
In contrast, selected SBIs may occur when the culture is “positive” without inflammation or nitrites. The first of three examples is a renal parenchymal abscess, where bacteria enter the urine sporadically in only small numbers, and do not actually infect the urinary tract mucosa. The scenario of no inflammation but “positive” culture also may occur when a large bacteremic load causes results in organisms filtering through the kidney into the urine, again without urinary mucosal infection, such as Staphylococcus aureus, group A streptococcus, or group B streptococcus bacteremia/sepsis. The third scenario with a “positive” culture and no pyuria can be with organisms that have blunted abilities to induce inflammation, such as enterococcus. Enterococcal cell components have weak inflammatory and chemotactic capability. So a urinary mucosal infection in the collecting system or bladder may occur without much if any pyuria. In fact, the patients from the recent study with insufficient evidence of pyuria/inflammation were those who had either gram-positive organisms or considerably less than 50,000 CFU/mL of gram-negative organisms.
The sensitivity and specificity of the LE or pyuria was higher in the recent study (Pediatrics 2015;135:965-71) than any prior study. The authors comment that they had not expected such a high sensitivity of 97.6% (94.5-99.2) for LE in confirmed bacteremic UTI, nor did they expect the high specificity of 93.9% (87.9-97.5). The presence of microscopic pyuria defined as > 3 WBC/HPF was nearly as sensitive, 96%, and specific, 91.3%. Disappointingly, positive nitrite testing was only 39.5% sensitive, but it was 100% specific. This likely reflects the short time that urine resides in the bladder of infants < 90 days of age, so there is insufficient time for the pathogens to metabolically convert the nitrates to nitrites.
So how would the UA help with our example case? There is microscopic bacteriuria, pyuria, and positive LE, but negative nitrites. Using the suggestions of the authors of the recent report (Pediatrics 2015;135:965-71) and those of another report on the utility of UA results (Acta Paediatr. 2010;99:581-4), the UA in our case indicates that we should be highly suspicious of a UTI in this child < 2 months old for whom the 2011 guidelines do not directly apply. But remember that these impressive sensitivity and specificity values relate to bacteremic UTI. Whether they apply to nonbacteremic UTI is not known. Likewise, the authors caution that their study design did not allow calculation of positive or negative predictive values – aspects that would clarify things even further.
So we still cannot be more than highly suspicious. Without a positive predictive value, we do not know the odds of this case having a UTI with mathematical precision. The authors do point out that only one of their subjects had a completely normal UA and actually had a bacteremic UTI. If you guessed that it was a gram-positive pathogen, you win the prize. So it seems reasonable to predict that a normal UA has a high specificity for not being a UTI (87.8%), but a positive UA remains only highly suggestive. It is still not clear if a negative UA statistically justifies not submitting the culture of the sterilely obtained urine because we still don’t have a negative predictive value.
Bottom line: The 2011 UTI guidelines provide good advice on diagnosing UTIs.
1. We have more data that evidence of inflammation is essential for diagnosing gram-negative UTIs.
2. We also have more evidence that 50,000 CFU/mL is a good threshold for diagnosing UTIs.
3. It appears that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
4. And we now have more evidence that these criteria also apply to infants < 2 months of age.
To close the loop on our case, the child’s CSF and blood cultures were negative, but the urine culture revealed > 100,000 CFU/mL of E. coli susceptible to second- and third-generation cephalosporins, ciprofloxacin, and nitrofurantoin, but resistant to trimethoprim-sulfamethoxazole.
Have a great summer and watch for UTIs in your young patients < 90 days old and fever without apparent focus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. E-mail Dr. Harrison at [email protected].
A 28-day-old uncircumcised male infant presents to the emergency department with fever of 38.9° C, decreased feeding, and irritability. The physical examination is normal with the exception of the irritability and your assessment of mild dehydration. The infant undergoes a sepsis work-up. The CBC is remarkable for a WBC of 16,500/mm3 with 44% neutrophils, 52% lymphocytes, and 4% monocytes. Platelet count is normal. Cerebrospinal fluid (CSF) shows no white or red blood cells with normal glucose and protein. The urinalysis (UA) has a positive 1+ leukocyte esterase (LE) with 10 WBC per high-power field (HPF), but negative nitrite and 1+ bacteria microscopically. The child is admitted to the hospital for empiric antibiotics pending blood, urine, and CSF cultures. What are the chances that a urinary tract infection (UTI) is the origin of the febrile presentation?
UTIs are currently the most common serious bacterial infection (SBI) in < 2-year-old febrile children without an apparent source of fever (Pediatrics 2011;128:595-610). Since 2000, the prevalence of UTIs in all febrile infants and young children without an apparent source is unchanged, being approximately 5%. The rate of UTIs in fever-without-apparent-source presentations at < 90 days of age is higher, ranging from 6%-15% in different studies.
Meanwhile bacteremia, sepsis, meningitis, and other previously common SBIs, mostly caused by Haemophilus influenzae type b (Hib) or pneumococcus, have decreased. We recognize these reductions as effects of universal implementation of Hib (mid-1990s) and pneumococcal (2000 and 2010) conjugate vaccines.
Given the case above, other pertinent facts are that uncircumcised males have more UTIs in the first months of life (J. Pediatr. 1996;128:23-7) and approximately 5% of young infants with UTIs also are concurrently bacteremic (Pediatrics 1999;104:79-86;J. Pediatr. 1994;124:513-9)
The elephant in the room is the fact that we also need to be cognizant of asymptomatic bacteriuria (AB). AB is colonization of the lower urinary tract without infection. Patients with AB may meet culture criteria for UTI (whether we consider > 50,000 or > 100,000 colony-forming units/mL), but there is no evidence of true infection, that is no inflammation or mucosal injury. So children with AB are not at risk for renal injury or later renal damage and do not require antibiotic treatment.
But when AB patients develop fever, for example with an enterovirus infection, their urine cultures (together with the fever) can do a good imitation of a UTI, unless we focus on the UA results. It not only remains critical to detect true UTIs in infants < 90 days old, such as the one in our case above, but also to distinguish UTI from AB.
The 1999 American Academy of Pediatrics’ UTI guidelines (Pediatrics 1999;103:843-52) included UA results as suggestive of UTI. They stated that a positive LE or nitrite test or > 5 WBC/HPF in a spun urine, or bacteria visualized in unspun gram-stained specimen suggest, but cannot be diagnostic of a UTI. Recommendation five in the guidelines states that UTI diagnosis required 100,000 CFU/mL in culture of sterilely obtained catheterized urine as the threshold criterion (strength of evidence: strong). However, AB was not fully considered because, in part, data defining AB was incomplete in 1999.
The 1999 guidelines also stated, “The urinalysis … can be valuable in selecting individuals for prompt initiation of treatment while waiting for the results of the urine culture.” So, UA was considered adjunctive. UA’s main function was to allow empiric therapy of sufficiently ill children, given positive results for LE, nitrites, or microscopic visualization of > 5 WBC/HPF or bacteria in the spun urine.
In the 2011 AAP guidelines for UTI, things have changed (Pediatrics 2011;128:595-610). The third action statement tells us that both the UA and culture taken together are necessary for UTI diagnosis. To paraphrase: The diagnosis of UTI requires urinalysis results suggesting infection (pyuria or microscopic bacteriuria) plus > 50,000 CFU/mL of a uropathogen in urine from catheterization or suprapubic aspiration. But remember that these guidelines do not apply specifically to the youngest of infants, that is < 2 months old.
Both of these criteria were changes from the 1999 UTI guidelines. Previously pyuria or microscopic bacteriuria were not considered necessary to diagnose UTI, and >100,000 CFU/mL rather than > 50,000 CFU/mL of a single pathogen species was the critical diagnostic result for catheterized urine. For suprapubic aspiration urine samples, > 10,000 CFU/HPF were considered adequate for UTI diagnoses in 1999.
Now, a recent study of children < 90 days of age (including those < 2 months of age) reports that pyuria (> 3 WBC/HPF) plus > 50,000 CFU/mL are the keys to diagnosing UTI (Pediatrics 2015;135:965-71). One caveat is that the study population was febrile infants < 90 days old with concurrent bacteremia (bacteremic UTI). Bacteremic UTI was studied to reduce as much as possible the chance that AB patients might be inadvertently included in the study. One other conclusion of this new study is that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
These data in an overall younger population than that covered by the 2011 guidelines adds evidence that pyuria (but not microscopic bacteriuria) is critical to diagnosing UTI. Pyuria plus positive culture has been a combination for the pediatric infectious diseases practitioner’s toolkit for decades. Likewise, it seems to me that primary care pediatric clinicians also often decide whether to undertake the expense of culture based on UA results. For example, a completely normal UA may obviate need for culture except in selected unusual cases.
Requiring UA evidence of inflammation to diagnose UTI (per the 2011 guidelines and the recommendations of the authors of the recent 2015 study) makes sense because most UTIs in otherwise healthy children are caused by gram-negative organisms (> 90% from Escherichia coli) (J. Pediatr. 1994;124:513-9). Why are UA results so important?
A positive nitrite test strongly suggests UTI because nitrites in the urine indicate viable gram-negative organisms also are present in the urine. Nitrates in the urine are converted to nitrites by metabolic activity of gram-negative pathogens. For WBCs or LE in the urine, their presence indicates inflammation in the urinary tract, Consider that lipopolysaccharide (LPS), also known as gram-negative endotoxin, is a major component of the cell membrane of > 90% of uropathogens like E. coli. Moreover, LPS elicits about the strongest innate immune response via toll-like receptor 4 (TLR4) from monocytes/macrophages, inducing a large pro-inflammatory and chemotactic response – interleukin-6, interleukin-8, tetrahydrofuran-alpha. Remember that LPS is also a major cause of fever and of shock during gram-negative sepsis.
So a UTI diagnosis based on a “positive” culture without evidence of metabolic products of gram negatives (nitrites) or without inflammation (no pyuria or negative LE) should be questioned. The combination of > 50,000 CFU/mL with no detectable LE or < 3-5 WBC/HPF in a febrile child is most likely evidence for AB in a child with the fever caused by some non-UTI process.
In contrast, selected SBIs may occur when the culture is “positive” without inflammation or nitrites. The first of three examples is a renal parenchymal abscess, where bacteria enter the urine sporadically in only small numbers, and do not actually infect the urinary tract mucosa. The scenario of no inflammation but “positive” culture also may occur when a large bacteremic load causes results in organisms filtering through the kidney into the urine, again without urinary mucosal infection, such as Staphylococcus aureus, group A streptococcus, or group B streptococcus bacteremia/sepsis. The third scenario with a “positive” culture and no pyuria can be with organisms that have blunted abilities to induce inflammation, such as enterococcus. Enterococcal cell components have weak inflammatory and chemotactic capability. So a urinary mucosal infection in the collecting system or bladder may occur without much if any pyuria. In fact, the patients from the recent study with insufficient evidence of pyuria/inflammation were those who had either gram-positive organisms or considerably less than 50,000 CFU/mL of gram-negative organisms.
The sensitivity and specificity of the LE or pyuria was higher in the recent study (Pediatrics 2015;135:965-71) than any prior study. The authors comment that they had not expected such a high sensitivity of 97.6% (94.5-99.2) for LE in confirmed bacteremic UTI, nor did they expect the high specificity of 93.9% (87.9-97.5). The presence of microscopic pyuria defined as > 3 WBC/HPF was nearly as sensitive, 96%, and specific, 91.3%. Disappointingly, positive nitrite testing was only 39.5% sensitive, but it was 100% specific. This likely reflects the short time that urine resides in the bladder of infants < 90 days of age, so there is insufficient time for the pathogens to metabolically convert the nitrates to nitrites.
So how would the UA help with our example case? There is microscopic bacteriuria, pyuria, and positive LE, but negative nitrites. Using the suggestions of the authors of the recent report (Pediatrics 2015;135:965-71) and those of another report on the utility of UA results (Acta Paediatr. 2010;99:581-4), the UA in our case indicates that we should be highly suspicious of a UTI in this child < 2 months old for whom the 2011 guidelines do not directly apply. But remember that these impressive sensitivity and specificity values relate to bacteremic UTI. Whether they apply to nonbacteremic UTI is not known. Likewise, the authors caution that their study design did not allow calculation of positive or negative predictive values – aspects that would clarify things even further.
So we still cannot be more than highly suspicious. Without a positive predictive value, we do not know the odds of this case having a UTI with mathematical precision. The authors do point out that only one of their subjects had a completely normal UA and actually had a bacteremic UTI. If you guessed that it was a gram-positive pathogen, you win the prize. So it seems reasonable to predict that a normal UA has a high specificity for not being a UTI (87.8%), but a positive UA remains only highly suggestive. It is still not clear if a negative UA statistically justifies not submitting the culture of the sterilely obtained urine because we still don’t have a negative predictive value.
Bottom line: The 2011 UTI guidelines provide good advice on diagnosing UTIs.
1. We have more data that evidence of inflammation is essential for diagnosing gram-negative UTIs.
2. We also have more evidence that 50,000 CFU/mL is a good threshold for diagnosing UTIs.
3. It appears that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
4. And we now have more evidence that these criteria also apply to infants < 2 months of age.
To close the loop on our case, the child’s CSF and blood cultures were negative, but the urine culture revealed > 100,000 CFU/mL of E. coli susceptible to second- and third-generation cephalosporins, ciprofloxacin, and nitrofurantoin, but resistant to trimethoprim-sulfamethoxazole.
Have a great summer and watch for UTIs in your young patients < 90 days old and fever without apparent focus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. E-mail Dr. Harrison at [email protected].
A 28-day-old uncircumcised male infant presents to the emergency department with fever of 38.9° C, decreased feeding, and irritability. The physical examination is normal with the exception of the irritability and your assessment of mild dehydration. The infant undergoes a sepsis work-up. The CBC is remarkable for a WBC of 16,500/mm3 with 44% neutrophils, 52% lymphocytes, and 4% monocytes. Platelet count is normal. Cerebrospinal fluid (CSF) shows no white or red blood cells with normal glucose and protein. The urinalysis (UA) has a positive 1+ leukocyte esterase (LE) with 10 WBC per high-power field (HPF), but negative nitrite and 1+ bacteria microscopically. The child is admitted to the hospital for empiric antibiotics pending blood, urine, and CSF cultures. What are the chances that a urinary tract infection (UTI) is the origin of the febrile presentation?
UTIs are currently the most common serious bacterial infection (SBI) in < 2-year-old febrile children without an apparent source of fever (Pediatrics 2011;128:595-610). Since 2000, the prevalence of UTIs in all febrile infants and young children without an apparent source is unchanged, being approximately 5%. The rate of UTIs in fever-without-apparent-source presentations at < 90 days of age is higher, ranging from 6%-15% in different studies.
Meanwhile bacteremia, sepsis, meningitis, and other previously common SBIs, mostly caused by Haemophilus influenzae type b (Hib) or pneumococcus, have decreased. We recognize these reductions as effects of universal implementation of Hib (mid-1990s) and pneumococcal (2000 and 2010) conjugate vaccines.
Given the case above, other pertinent facts are that uncircumcised males have more UTIs in the first months of life (J. Pediatr. 1996;128:23-7) and approximately 5% of young infants with UTIs also are concurrently bacteremic (Pediatrics 1999;104:79-86;J. Pediatr. 1994;124:513-9)
The elephant in the room is the fact that we also need to be cognizant of asymptomatic bacteriuria (AB). AB is colonization of the lower urinary tract without infection. Patients with AB may meet culture criteria for UTI (whether we consider > 50,000 or > 100,000 colony-forming units/mL), but there is no evidence of true infection, that is no inflammation or mucosal injury. So children with AB are not at risk for renal injury or later renal damage and do not require antibiotic treatment.
But when AB patients develop fever, for example with an enterovirus infection, their urine cultures (together with the fever) can do a good imitation of a UTI, unless we focus on the UA results. It not only remains critical to detect true UTIs in infants < 90 days old, such as the one in our case above, but also to distinguish UTI from AB.
The 1999 American Academy of Pediatrics’ UTI guidelines (Pediatrics 1999;103:843-52) included UA results as suggestive of UTI. They stated that a positive LE or nitrite test or > 5 WBC/HPF in a spun urine, or bacteria visualized in unspun gram-stained specimen suggest, but cannot be diagnostic of a UTI. Recommendation five in the guidelines states that UTI diagnosis required 100,000 CFU/mL in culture of sterilely obtained catheterized urine as the threshold criterion (strength of evidence: strong). However, AB was not fully considered because, in part, data defining AB was incomplete in 1999.
The 1999 guidelines also stated, “The urinalysis … can be valuable in selecting individuals for prompt initiation of treatment while waiting for the results of the urine culture.” So, UA was considered adjunctive. UA’s main function was to allow empiric therapy of sufficiently ill children, given positive results for LE, nitrites, or microscopic visualization of > 5 WBC/HPF or bacteria in the spun urine.
In the 2011 AAP guidelines for UTI, things have changed (Pediatrics 2011;128:595-610). The third action statement tells us that both the UA and culture taken together are necessary for UTI diagnosis. To paraphrase: The diagnosis of UTI requires urinalysis results suggesting infection (pyuria or microscopic bacteriuria) plus > 50,000 CFU/mL of a uropathogen in urine from catheterization or suprapubic aspiration. But remember that these guidelines do not apply specifically to the youngest of infants, that is < 2 months old.
Both of these criteria were changes from the 1999 UTI guidelines. Previously pyuria or microscopic bacteriuria were not considered necessary to diagnose UTI, and >100,000 CFU/mL rather than > 50,000 CFU/mL of a single pathogen species was the critical diagnostic result for catheterized urine. For suprapubic aspiration urine samples, > 10,000 CFU/HPF were considered adequate for UTI diagnoses in 1999.
Now, a recent study of children < 90 days of age (including those < 2 months of age) reports that pyuria (> 3 WBC/HPF) plus > 50,000 CFU/mL are the keys to diagnosing UTI (Pediatrics 2015;135:965-71). One caveat is that the study population was febrile infants < 90 days old with concurrent bacteremia (bacteremic UTI). Bacteremic UTI was studied to reduce as much as possible the chance that AB patients might be inadvertently included in the study. One other conclusion of this new study is that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
These data in an overall younger population than that covered by the 2011 guidelines adds evidence that pyuria (but not microscopic bacteriuria) is critical to diagnosing UTI. Pyuria plus positive culture has been a combination for the pediatric infectious diseases practitioner’s toolkit for decades. Likewise, it seems to me that primary care pediatric clinicians also often decide whether to undertake the expense of culture based on UA results. For example, a completely normal UA may obviate need for culture except in selected unusual cases.
Requiring UA evidence of inflammation to diagnose UTI (per the 2011 guidelines and the recommendations of the authors of the recent 2015 study) makes sense because most UTIs in otherwise healthy children are caused by gram-negative organisms (> 90% from Escherichia coli) (J. Pediatr. 1994;124:513-9). Why are UA results so important?
A positive nitrite test strongly suggests UTI because nitrites in the urine indicate viable gram-negative organisms also are present in the urine. Nitrates in the urine are converted to nitrites by metabolic activity of gram-negative pathogens. For WBCs or LE in the urine, their presence indicates inflammation in the urinary tract, Consider that lipopolysaccharide (LPS), also known as gram-negative endotoxin, is a major component of the cell membrane of > 90% of uropathogens like E. coli. Moreover, LPS elicits about the strongest innate immune response via toll-like receptor 4 (TLR4) from monocytes/macrophages, inducing a large pro-inflammatory and chemotactic response – interleukin-6, interleukin-8, tetrahydrofuran-alpha. Remember that LPS is also a major cause of fever and of shock during gram-negative sepsis.
So a UTI diagnosis based on a “positive” culture without evidence of metabolic products of gram negatives (nitrites) or without inflammation (no pyuria or negative LE) should be questioned. The combination of > 50,000 CFU/mL with no detectable LE or < 3-5 WBC/HPF in a febrile child is most likely evidence for AB in a child with the fever caused by some non-UTI process.
In contrast, selected SBIs may occur when the culture is “positive” without inflammation or nitrites. The first of three examples is a renal parenchymal abscess, where bacteria enter the urine sporadically in only small numbers, and do not actually infect the urinary tract mucosa. The scenario of no inflammation but “positive” culture also may occur when a large bacteremic load causes results in organisms filtering through the kidney into the urine, again without urinary mucosal infection, such as Staphylococcus aureus, group A streptococcus, or group B streptococcus bacteremia/sepsis. The third scenario with a “positive” culture and no pyuria can be with organisms that have blunted abilities to induce inflammation, such as enterococcus. Enterococcal cell components have weak inflammatory and chemotactic capability. So a urinary mucosal infection in the collecting system or bladder may occur without much if any pyuria. In fact, the patients from the recent study with insufficient evidence of pyuria/inflammation were those who had either gram-positive organisms or considerably less than 50,000 CFU/mL of gram-negative organisms.
The sensitivity and specificity of the LE or pyuria was higher in the recent study (Pediatrics 2015;135:965-71) than any prior study. The authors comment that they had not expected such a high sensitivity of 97.6% (94.5-99.2) for LE in confirmed bacteremic UTI, nor did they expect the high specificity of 93.9% (87.9-97.5). The presence of microscopic pyuria defined as > 3 WBC/HPF was nearly as sensitive, 96%, and specific, 91.3%. Disappointingly, positive nitrite testing was only 39.5% sensitive, but it was 100% specific. This likely reflects the short time that urine resides in the bladder of infants < 90 days of age, so there is insufficient time for the pathogens to metabolically convert the nitrates to nitrites.
So how would the UA help with our example case? There is microscopic bacteriuria, pyuria, and positive LE, but negative nitrites. Using the suggestions of the authors of the recent report (Pediatrics 2015;135:965-71) and those of another report on the utility of UA results (Acta Paediatr. 2010;99:581-4), the UA in our case indicates that we should be highly suspicious of a UTI in this child < 2 months old for whom the 2011 guidelines do not directly apply. But remember that these impressive sensitivity and specificity values relate to bacteremic UTI. Whether they apply to nonbacteremic UTI is not known. Likewise, the authors caution that their study design did not allow calculation of positive or negative predictive values – aspects that would clarify things even further.
So we still cannot be more than highly suspicious. Without a positive predictive value, we do not know the odds of this case having a UTI with mathematical precision. The authors do point out that only one of their subjects had a completely normal UA and actually had a bacteremic UTI. If you guessed that it was a gram-positive pathogen, you win the prize. So it seems reasonable to predict that a normal UA has a high specificity for not being a UTI (87.8%), but a positive UA remains only highly suggestive. It is still not clear if a negative UA statistically justifies not submitting the culture of the sterilely obtained urine because we still don’t have a negative predictive value.
Bottom line: The 2011 UTI guidelines provide good advice on diagnosing UTIs.
1. We have more data that evidence of inflammation is essential for diagnosing gram-negative UTIs.
2. We also have more evidence that 50,000 CFU/mL is a good threshold for diagnosing UTIs.
3. It appears that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
4. And we now have more evidence that these criteria also apply to infants < 2 months of age.
To close the loop on our case, the child’s CSF and blood cultures were negative, but the urine culture revealed > 100,000 CFU/mL of E. coli susceptible to second- and third-generation cephalosporins, ciprofloxacin, and nitrofurantoin, but resistant to trimethoprim-sulfamethoxazole.
Have a great summer and watch for UTIs in your young patients < 90 days old and fever without apparent focus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. E-mail Dr. Harrison at [email protected].
Commentary: Molecular marker testing for diagnosis and prognosis – Where are we really?
An emerging market of molecular markers for thyroid cancer diagnostic and prognostic evaluation challenges current medical and surgical management; however, here is a strong word of caution; Unless the markers have been tested in the context of clinical and surgical management their true efficacy has not been accurately assessed.
We must always ask two questions before ordering these tests that can cost more than $3,000: 1) Does the use of a molecular test add any additional information that would otherwise change our recommendations; in other words, is the clinical information already known enough for appropriate clinical decision making? 2) What is the basis upon which recommendations for its use have been made; in other words, how were the studies conducted and interpreted? Are they valid?
By way of background, the gene expression clarifier (GEC) Afirma is marketed as a diagnostic tool for the differential diagnosis of indeterminate thyroid cytology (atypical cells of undetermined significance or follicular neoplasm, Bethesda categories III and IV, respectively).
Most importantly, it has been tested and is marketed as a negative predictor only. It is also only useful if used in the appropriate setting and if the test result is negative; a positive Afirma test can tell us only that the nodule is indeterminate, something, by definition, already determined by fine needle aspiration (FNA). Furthermore, any study examining its efficacy must be conducted within the context of clinical scenarios. For example, for a patient with an indeterminate lesion and a symptomatic multinodular goiter, neither a negative nor positive Afirma result would have any impact on surgical decision making and yet would have cost the patient more than $3,000. The latter patient simply needs a thyroidectomy. A patient who has a nodule that is approaching 4 cm or is increasing in size or a patient who has undergone several FNAs, ultrasounds, and endocrinology consultations similarly would likely choose surgery regardless of the GEC test result.
The only scenario in which Afirma testing is useful is in a patient with otherwise no indications for surgery except an indeterminate FNA; for instance for a patient with an asymptomatic < 4cm, stable, single nodule. In this scenario only a negative result could be helpful and would obviate the need for surgical intervention. One also must ask what is the likelihood of the patient undergoing repeat FNA, having repeat indeterminate cytology and repeat Afirma testing over the ensuing years and at what point should we consider these costs prohibitive compared with the patient undergoing a thyroidectomy instead?
Similar concerns exist with regard to somatic mutation panels, the most commonly marketed and used being Asuragen. The mutation panel consists of Ras and BRAF mutation and RET/PTC and PAX8/PPAR-gamma chromosomal rearrangement analyses. Importantly, the expanding literature on molecular markers for thyroid cancer, however, has lost track of a vast body of literature that reports Ras mutations present in up to 30% of benign thyroid nodules; RET/PTC in 24% and PAX8/PPAR-gamma in 14%, dramatically challenging the validity of the panel purported to accurately identify thyroid malignancy. We know that BRAF mutation is exclusively found in papillary thyroid cancer, but generally a BRAF-positive tumor is one in which a cytological diagnosis is already clear; it is either suspicious or definitively malignant on FNA. One might suggest that these aforementioned markers might indicate a harbinger of malignancy or identify a precancerous lesion; however, there is no study that supports this theory. Furthermore, it is important to note that even among pathology experts the diagnoses of follicular adenoma, follicular cancer, and follicular variant of papillary thyroid cancer have significant and documented inter- and intra- observer variability, thus challenging the gold standard upon which these markers have been tested.
With regard to BRAF, Ras, RET/PTC and PAX8/PPAR-gamma as prognostic markers there are many considerations, the least of which includes the differential diagnosis of follicular lesions addressed above and the known presence of these abnormalities in benign tumors. The majority of studies to date have conducted only univariate analyses without consideration of the clinical variables already available to us to make needed clinical decisions. In order to examine the usefulness of any marker panel a multivariable study is required, incorporating the available clinical variables such as size, lymph node metastases, and extra-thyroidal extension. For instance, the majority of studies examining the presence of lymph node metastases in BRAF-negative vs. BRAF-positive tumors were almost uniformly conducted on patients in whom routine lymph node dissection was not performed, thereby skewing the results. Only patients with suspicious nodes underwent a lymph node dissection; thus for those patients who had no suspicious nodes there was no information about their nodal status. Furthermore, several studies that included patients who underwent routine lymph node dissections and, importantly, included patients who underwent prophylactic dissections for whom a marker would be most useful, did not find that BRAF mutation was associated with aggressive features, including the presence of lymph node metastases. The same can be stated for a panel that includes Ras, RET/PTC, & PAX8/PPAR-gamma. “Follicular variant of papillary thyroid cancer and follicular cancer” will more likely harbor Ras and PAX8/PPAR-gamma mutations and will, by definition, include some tumors that would be considered benign by other pathologists.
There is an emerging market for molecular markers for thyroid cancer diagnosis and prognosis, but many of the studies driving this market are lacking in scientific rigor with regard to design and interpretation of results. Furthermore, the use of the marker panel requires scrutiny of its true impact on clinical management as well as its cost-effectiveness. GEC provides a negative predictor of malignancy and should be used to obviate surgery only; if the patient requires surgery its use provides no added information. BRAF is specific for thyroid cancer diagnosis, but is often not required as FNA evaluation in BRAF-positive tumors is usually already suspicious or malignant. Finally, molecular marker panels for thyroid cancer prognosis may be promising, but scrutiny for true accuracy and true clinical impact is necessary in order to make logical and useful recommendations for our patients.
Dr. Zeiger is professor of surgery, oncology, cellular, and molecular medicine, associate vice chair for faculty development, and associate dean for postdoctoral affairs at Johns Hopkins University in Baltimore. Dr. Zeiger consults for Interpace Diagnostics.
An emerging market of molecular markers for thyroid cancer diagnostic and prognostic evaluation challenges current medical and surgical management; however, here is a strong word of caution; Unless the markers have been tested in the context of clinical and surgical management their true efficacy has not been accurately assessed.
We must always ask two questions before ordering these tests that can cost more than $3,000: 1) Does the use of a molecular test add any additional information that would otherwise change our recommendations; in other words, is the clinical information already known enough for appropriate clinical decision making? 2) What is the basis upon which recommendations for its use have been made; in other words, how were the studies conducted and interpreted? Are they valid?
By way of background, the gene expression clarifier (GEC) Afirma is marketed as a diagnostic tool for the differential diagnosis of indeterminate thyroid cytology (atypical cells of undetermined significance or follicular neoplasm, Bethesda categories III and IV, respectively).
Most importantly, it has been tested and is marketed as a negative predictor only. It is also only useful if used in the appropriate setting and if the test result is negative; a positive Afirma test can tell us only that the nodule is indeterminate, something, by definition, already determined by fine needle aspiration (FNA). Furthermore, any study examining its efficacy must be conducted within the context of clinical scenarios. For example, for a patient with an indeterminate lesion and a symptomatic multinodular goiter, neither a negative nor positive Afirma result would have any impact on surgical decision making and yet would have cost the patient more than $3,000. The latter patient simply needs a thyroidectomy. A patient who has a nodule that is approaching 4 cm or is increasing in size or a patient who has undergone several FNAs, ultrasounds, and endocrinology consultations similarly would likely choose surgery regardless of the GEC test result.
The only scenario in which Afirma testing is useful is in a patient with otherwise no indications for surgery except an indeterminate FNA; for instance for a patient with an asymptomatic < 4cm, stable, single nodule. In this scenario only a negative result could be helpful and would obviate the need for surgical intervention. One also must ask what is the likelihood of the patient undergoing repeat FNA, having repeat indeterminate cytology and repeat Afirma testing over the ensuing years and at what point should we consider these costs prohibitive compared with the patient undergoing a thyroidectomy instead?
Similar concerns exist with regard to somatic mutation panels, the most commonly marketed and used being Asuragen. The mutation panel consists of Ras and BRAF mutation and RET/PTC and PAX8/PPAR-gamma chromosomal rearrangement analyses. Importantly, the expanding literature on molecular markers for thyroid cancer, however, has lost track of a vast body of literature that reports Ras mutations present in up to 30% of benign thyroid nodules; RET/PTC in 24% and PAX8/PPAR-gamma in 14%, dramatically challenging the validity of the panel purported to accurately identify thyroid malignancy. We know that BRAF mutation is exclusively found in papillary thyroid cancer, but generally a BRAF-positive tumor is one in which a cytological diagnosis is already clear; it is either suspicious or definitively malignant on FNA. One might suggest that these aforementioned markers might indicate a harbinger of malignancy or identify a precancerous lesion; however, there is no study that supports this theory. Furthermore, it is important to note that even among pathology experts the diagnoses of follicular adenoma, follicular cancer, and follicular variant of papillary thyroid cancer have significant and documented inter- and intra- observer variability, thus challenging the gold standard upon which these markers have been tested.
With regard to BRAF, Ras, RET/PTC and PAX8/PPAR-gamma as prognostic markers there are many considerations, the least of which includes the differential diagnosis of follicular lesions addressed above and the known presence of these abnormalities in benign tumors. The majority of studies to date have conducted only univariate analyses without consideration of the clinical variables already available to us to make needed clinical decisions. In order to examine the usefulness of any marker panel a multivariable study is required, incorporating the available clinical variables such as size, lymph node metastases, and extra-thyroidal extension. For instance, the majority of studies examining the presence of lymph node metastases in BRAF-negative vs. BRAF-positive tumors were almost uniformly conducted on patients in whom routine lymph node dissection was not performed, thereby skewing the results. Only patients with suspicious nodes underwent a lymph node dissection; thus for those patients who had no suspicious nodes there was no information about their nodal status. Furthermore, several studies that included patients who underwent routine lymph node dissections and, importantly, included patients who underwent prophylactic dissections for whom a marker would be most useful, did not find that BRAF mutation was associated with aggressive features, including the presence of lymph node metastases. The same can be stated for a panel that includes Ras, RET/PTC, & PAX8/PPAR-gamma. “Follicular variant of papillary thyroid cancer and follicular cancer” will more likely harbor Ras and PAX8/PPAR-gamma mutations and will, by definition, include some tumors that would be considered benign by other pathologists.
There is an emerging market for molecular markers for thyroid cancer diagnosis and prognosis, but many of the studies driving this market are lacking in scientific rigor with regard to design and interpretation of results. Furthermore, the use of the marker panel requires scrutiny of its true impact on clinical management as well as its cost-effectiveness. GEC provides a negative predictor of malignancy and should be used to obviate surgery only; if the patient requires surgery its use provides no added information. BRAF is specific for thyroid cancer diagnosis, but is often not required as FNA evaluation in BRAF-positive tumors is usually already suspicious or malignant. Finally, molecular marker panels for thyroid cancer prognosis may be promising, but scrutiny for true accuracy and true clinical impact is necessary in order to make logical and useful recommendations for our patients.
Dr. Zeiger is professor of surgery, oncology, cellular, and molecular medicine, associate vice chair for faculty development, and associate dean for postdoctoral affairs at Johns Hopkins University in Baltimore. Dr. Zeiger consults for Interpace Diagnostics.
An emerging market of molecular markers for thyroid cancer diagnostic and prognostic evaluation challenges current medical and surgical management; however, here is a strong word of caution; Unless the markers have been tested in the context of clinical and surgical management their true efficacy has not been accurately assessed.
We must always ask two questions before ordering these tests that can cost more than $3,000: 1) Does the use of a molecular test add any additional information that would otherwise change our recommendations; in other words, is the clinical information already known enough for appropriate clinical decision making? 2) What is the basis upon which recommendations for its use have been made; in other words, how were the studies conducted and interpreted? Are they valid?
By way of background, the gene expression clarifier (GEC) Afirma is marketed as a diagnostic tool for the differential diagnosis of indeterminate thyroid cytology (atypical cells of undetermined significance or follicular neoplasm, Bethesda categories III and IV, respectively).
Most importantly, it has been tested and is marketed as a negative predictor only. It is also only useful if used in the appropriate setting and if the test result is negative; a positive Afirma test can tell us only that the nodule is indeterminate, something, by definition, already determined by fine needle aspiration (FNA). Furthermore, any study examining its efficacy must be conducted within the context of clinical scenarios. For example, for a patient with an indeterminate lesion and a symptomatic multinodular goiter, neither a negative nor positive Afirma result would have any impact on surgical decision making and yet would have cost the patient more than $3,000. The latter patient simply needs a thyroidectomy. A patient who has a nodule that is approaching 4 cm or is increasing in size or a patient who has undergone several FNAs, ultrasounds, and endocrinology consultations similarly would likely choose surgery regardless of the GEC test result.
The only scenario in which Afirma testing is useful is in a patient with otherwise no indications for surgery except an indeterminate FNA; for instance for a patient with an asymptomatic < 4cm, stable, single nodule. In this scenario only a negative result could be helpful and would obviate the need for surgical intervention. One also must ask what is the likelihood of the patient undergoing repeat FNA, having repeat indeterminate cytology and repeat Afirma testing over the ensuing years and at what point should we consider these costs prohibitive compared with the patient undergoing a thyroidectomy instead?
Similar concerns exist with regard to somatic mutation panels, the most commonly marketed and used being Asuragen. The mutation panel consists of Ras and BRAF mutation and RET/PTC and PAX8/PPAR-gamma chromosomal rearrangement analyses. Importantly, the expanding literature on molecular markers for thyroid cancer, however, has lost track of a vast body of literature that reports Ras mutations present in up to 30% of benign thyroid nodules; RET/PTC in 24% and PAX8/PPAR-gamma in 14%, dramatically challenging the validity of the panel purported to accurately identify thyroid malignancy. We know that BRAF mutation is exclusively found in papillary thyroid cancer, but generally a BRAF-positive tumor is one in which a cytological diagnosis is already clear; it is either suspicious or definitively malignant on FNA. One might suggest that these aforementioned markers might indicate a harbinger of malignancy or identify a precancerous lesion; however, there is no study that supports this theory. Furthermore, it is important to note that even among pathology experts the diagnoses of follicular adenoma, follicular cancer, and follicular variant of papillary thyroid cancer have significant and documented inter- and intra- observer variability, thus challenging the gold standard upon which these markers have been tested.
With regard to BRAF, Ras, RET/PTC and PAX8/PPAR-gamma as prognostic markers there are many considerations, the least of which includes the differential diagnosis of follicular lesions addressed above and the known presence of these abnormalities in benign tumors. The majority of studies to date have conducted only univariate analyses without consideration of the clinical variables already available to us to make needed clinical decisions. In order to examine the usefulness of any marker panel a multivariable study is required, incorporating the available clinical variables such as size, lymph node metastases, and extra-thyroidal extension. For instance, the majority of studies examining the presence of lymph node metastases in BRAF-negative vs. BRAF-positive tumors were almost uniformly conducted on patients in whom routine lymph node dissection was not performed, thereby skewing the results. Only patients with suspicious nodes underwent a lymph node dissection; thus for those patients who had no suspicious nodes there was no information about their nodal status. Furthermore, several studies that included patients who underwent routine lymph node dissections and, importantly, included patients who underwent prophylactic dissections for whom a marker would be most useful, did not find that BRAF mutation was associated with aggressive features, including the presence of lymph node metastases. The same can be stated for a panel that includes Ras, RET/PTC, & PAX8/PPAR-gamma. “Follicular variant of papillary thyroid cancer and follicular cancer” will more likely harbor Ras and PAX8/PPAR-gamma mutations and will, by definition, include some tumors that would be considered benign by other pathologists.
There is an emerging market for molecular markers for thyroid cancer diagnosis and prognosis, but many of the studies driving this market are lacking in scientific rigor with regard to design and interpretation of results. Furthermore, the use of the marker panel requires scrutiny of its true impact on clinical management as well as its cost-effectiveness. GEC provides a negative predictor of malignancy and should be used to obviate surgery only; if the patient requires surgery its use provides no added information. BRAF is specific for thyroid cancer diagnosis, but is often not required as FNA evaluation in BRAF-positive tumors is usually already suspicious or malignant. Finally, molecular marker panels for thyroid cancer prognosis may be promising, but scrutiny for true accuracy and true clinical impact is necessary in order to make logical and useful recommendations for our patients.
Dr. Zeiger is professor of surgery, oncology, cellular, and molecular medicine, associate vice chair for faculty development, and associate dean for postdoctoral affairs at Johns Hopkins University in Baltimore. Dr. Zeiger consults for Interpace Diagnostics.
Commentary: Molecular marker testing for diagnosis and prognosis – Where are we really?
An emerging market of molecular markers for thyroid cancer diagnostic and prognostic evaluation challenges current medical and surgical management; however, here is a strong word of caution; Unless the markers have been tested in the context of clinical and surgical management their true efficacy has not been accurately assessed.
We must always ask two questions before ordering these tests that can cost more than $3,000: 1) Does the use of a molecular test add any additional information that would otherwise change our recommendations; in other words, is the clinical information already known enough for appropriate clinical decision making? 2) What is the basis upon which recommendations for its use have been made; in other words, how were the studies conducted and interpreted? Are they valid?
By way of background, the gene expression clarifier (GEC) Afirma is marketed as a diagnostic tool for the differential diagnosis of indeterminate thyroid cytology (atypical cells of undetermined significance or follicular neoplasm, Bethesda categories III and IV, respectively).
Most importantly, it has been tested and is marketed as a negative predictor only. It is also only useful if used in the appropriate setting and if the test result is negative; a positive Afirma test can tell us only that the nodule is indeterminate, something, by definition, already determined by fine needle aspiration (FNA). Furthermore, any study examining its efficacy must be conducted within the context of clinical scenarios. For example, for a patient with an indeterminate lesion and a symptomatic multinodular goiter, neither a negative nor positive Afirma result would have any impact on surgical decision making and yet would have cost the patient more than $3,000. The latter patient simply needs a thyroidectomy. A patient who has a nodule that is approaching 4 cm or is increasing in size or a patient who has undergone several FNAs, ultrasounds, and endocrinology consultations similarly would likely choose surgery regardless of the GEC test result.
The only scenario in which Afirma testing is useful is in a patient with otherwise no indications for surgery except an indeterminate FNA; for instance for a patient with an asymptomatic < 4cm, stable, single nodule. In this scenario only a negative result could be helpful and would obviate the need for surgical intervention. One also must ask what is the likelihood of the patient undergoing repeat FNA, having repeat indeterminate cytology and repeat Afirma testing over the ensuing years and at what point should we consider these costs prohibitive compared with the patient undergoing a thyroidectomy instead?
Similar concerns exist with regard to somatic mutation panels, the most commonly marketed and used being Asuragen. The mutation panel consists of Ras and BRAF mutation and RET/PTC and PAX8/PPAR-gamma chromosomal rearrangement analyses. Importantly, the expanding literature on molecular markers for thyroid cancer, however, has lost track of a vast body of literature that reports Ras mutations present in up to 30% of benign thyroid nodules; RET/PTC in 24% and PAX8/PPAR-gamma in 14%, dramatically challenging the validity of the panel purported to accurately identify thyroid malignancy. We know that BRAF mutation is exclusively found in papillary thyroid cancer, but generally a BRAF-positive tumor is one in which a cytological diagnosis is already clear; it is either suspicious or definitively malignant on FNA. One might suggest that these aforementioned markers might indicate a harbinger of malignancy or identify a precancerous lesion; however, there is no study that supports this theory. Furthermore, it is important to note that even among pathology experts the diagnoses of follicular adenoma, follicular cancer, and follicular variant of papillary thyroid cancer have significant and documented inter- and intra- observer variability, thus challenging the gold standard upon which these markers have been tested.
With regard to BRAF, Ras, RET/PTC and PAX8/PPAR-gamma as prognostic markers there are many considerations, the least of which includes the differential diagnosis of follicular lesions addressed above and the known presence of these abnormalities in benign tumors. The majority of studies to date have conducted only univariate analyses without consideration of the clinical variables already available to us to make needed clinical decisions. In order to examine the usefulness of any marker panel a multivariable study is required, incorporating the available clinical variables such as size, lymph node metastases, and extra-thyroidal extension. For instance, the majority of studies examining the presence of lymph node metastases in BRAF-negative vs. BRAF-positive tumors were almost uniformly conducted on patients in whom routine lymph node dissection was not performed, thereby skewing the results. Only patients with suspicious nodes underwent a lymph node dissection; thus for those patients who had no suspicious nodes there was no information about their nodal status. Furthermore, several studies that included patients who underwent routine lymph node dissections and, importantly, included patients who underwent prophylactic dissections for whom a marker would be most useful, did not find that BRAF mutation was associated with aggressive features, including the presence of lymph node metastases. The same can be stated for a panel that includes Ras, RET/PTC, & PAX8/PPAR-gamma. “Follicular variant of papillary thyroid cancer and follicular cancer” will more likely harbor Ras and PAX8/PPAR-gamma mutations and will, by definition, include some tumors that would be considered benign by other pathologists.
There is an emerging market for molecular markers for thyroid cancer diagnosis and prognosis, but many of the studies driving this market are lacking in scientific rigor with regard to design and interpretation of results. Furthermore, the use of the marker panel requires scrutiny of its true impact on clinical management as well as its cost-effectiveness. GEC provides a negative predictor of malignancy and should be used to obviate surgery only; if the patient requires surgery its use provides no added information. BRAF is specific for thyroid cancer diagnosis, but is often not required as FNA evaluation in BRAF-positive tumors is usually already suspicious or malignant. Finally, molecular marker panels for thyroid cancer prognosis may be promising, but scrutiny for true accuracy and true clinical impact is necessary in order to make logical and useful recommendations for our patients.
Dr. Zeiger is professor of surgery, oncology, cellular, and molecular medicine, associate vice chair for faculty development, and associate dean for postdoctoral affairs at Johns Hopkins University in Baltimore. Dr. Zeiger consults for Interpace Diagnostics.
An emerging market of molecular markers for thyroid cancer diagnostic and prognostic evaluation challenges current medical and surgical management; however, here is a strong word of caution; Unless the markers have been tested in the context of clinical and surgical management their true efficacy has not been accurately assessed.
We must always ask two questions before ordering these tests that can cost more than $3,000: 1) Does the use of a molecular test add any additional information that would otherwise change our recommendations; in other words, is the clinical information already known enough for appropriate clinical decision making? 2) What is the basis upon which recommendations for its use have been made; in other words, how were the studies conducted and interpreted? Are they valid?
By way of background, the gene expression clarifier (GEC) Afirma is marketed as a diagnostic tool for the differential diagnosis of indeterminate thyroid cytology (atypical cells of undetermined significance or follicular neoplasm, Bethesda categories III and IV, respectively).
Most importantly, it has been tested and is marketed as a negative predictor only. It is also only useful if used in the appropriate setting and if the test result is negative; a positive Afirma test can tell us only that the nodule is indeterminate, something, by definition, already determined by fine needle aspiration (FNA). Furthermore, any study examining its efficacy must be conducted within the context of clinical scenarios. For example, for a patient with an indeterminate lesion and a symptomatic multinodular goiter, neither a negative nor positive Afirma result would have any impact on surgical decision making and yet would have cost the patient more than $3,000. The latter patient simply needs a thyroidectomy. A patient who has a nodule that is approaching 4 cm or is increasing in size or a patient who has undergone several FNAs, ultrasounds, and endocrinology consultations similarly would likely choose surgery regardless of the GEC test result.
The only scenario in which Afirma testing is useful is in a patient with otherwise no indications for surgery except an indeterminate FNA; for instance for a patient with an asymptomatic < 4cm, stable, single nodule. In this scenario only a negative result could be helpful and would obviate the need for surgical intervention. One also must ask what is the likelihood of the patient undergoing repeat FNA, having repeat indeterminate cytology and repeat Afirma testing over the ensuing years and at what point should we consider these costs prohibitive compared with the patient undergoing a thyroidectomy instead?
Similar concerns exist with regard to somatic mutation panels, the most commonly marketed and used being Asuragen. The mutation panel consists of Ras and BRAF mutation and RET/PTC and PAX8/PPAR-gamma chromosomal rearrangement analyses. Importantly, the expanding literature on molecular markers for thyroid cancer, however, has lost track of a vast body of literature that reports Ras mutations present in up to 30% of benign thyroid nodules; RET/PTC in 24% and PAX8/PPAR-gamma in 14%, dramatically challenging the validity of the panel purported to accurately identify thyroid malignancy. We know that BRAF mutation is exclusively found in papillary thyroid cancer, but generally a BRAF-positive tumor is one in which a cytological diagnosis is already clear; it is either suspicious or definitively malignant on FNA. One might suggest that these aforementioned markers might indicate a harbinger of malignancy or identify a precancerous lesion; however, there is no study that supports this theory. Furthermore, it is important to note that even among pathology experts the diagnoses of follicular adenoma, follicular cancer, and follicular variant of papillary thyroid cancer have significant and documented inter- and intra- observer variability, thus challenging the gold standard upon which these markers have been tested.
With regard to BRAF, Ras, RET/PTC and PAX8/PPAR-gamma as prognostic markers there are many considerations, the least of which includes the differential diagnosis of follicular lesions addressed above and the known presence of these abnormalities in benign tumors. The majority of studies to date have conducted only univariate analyses without consideration of the clinical variables already available to us to make needed clinical decisions. In order to examine the usefulness of any marker panel a multivariable study is required, incorporating the available clinical variables such as size, lymph node metastases, and extra-thyroidal extension. For instance, the majority of studies examining the presence of lymph node metastases in BRAF-negative vs. BRAF-positive tumors were almost uniformly conducted on patients in whom routine lymph node dissection was not performed, thereby skewing the results. Only patients with suspicious nodes underwent a lymph node dissection; thus for those patients who had no suspicious nodes there was no information about their nodal status. Furthermore, several studies that included patients who underwent routine lymph node dissections and, importantly, included patients who underwent prophylactic dissections for whom a marker would be most useful, did not find that BRAF mutation was associated with aggressive features, including the presence of lymph node metastases. The same can be stated for a panel that includes Ras, RET/PTC, & PAX8/PPAR-gamma. “Follicular variant of papillary thyroid cancer and follicular cancer” will more likely harbor Ras and PAX8/PPAR-gamma mutations and will, by definition, include some tumors that would be considered benign by other pathologists.
There is an emerging market for molecular markers for thyroid cancer diagnosis and prognosis, but many of the studies driving this market are lacking in scientific rigor with regard to design and interpretation of results. Furthermore, the use of the marker panel requires scrutiny of its true impact on clinical management as well as its cost-effectiveness. GEC provides a negative predictor of malignancy and should be used to obviate surgery only; if the patient requires surgery its use provides no added information. BRAF is specific for thyroid cancer diagnosis, but is often not required as FNA evaluation in BRAF-positive tumors is usually already suspicious or malignant. Finally, molecular marker panels for thyroid cancer prognosis may be promising, but scrutiny for true accuracy and true clinical impact is necessary in order to make logical and useful recommendations for our patients.
Dr. Zeiger is professor of surgery, oncology, cellular, and molecular medicine, associate vice chair for faculty development, and associate dean for postdoctoral affairs at Johns Hopkins University in Baltimore. Dr. Zeiger consults for Interpace Diagnostics.
An emerging market of molecular markers for thyroid cancer diagnostic and prognostic evaluation challenges current medical and surgical management; however, here is a strong word of caution; Unless the markers have been tested in the context of clinical and surgical management their true efficacy has not been accurately assessed.
We must always ask two questions before ordering these tests that can cost more than $3,000: 1) Does the use of a molecular test add any additional information that would otherwise change our recommendations; in other words, is the clinical information already known enough for appropriate clinical decision making? 2) What is the basis upon which recommendations for its use have been made; in other words, how were the studies conducted and interpreted? Are they valid?
By way of background, the gene expression clarifier (GEC) Afirma is marketed as a diagnostic tool for the differential diagnosis of indeterminate thyroid cytology (atypical cells of undetermined significance or follicular neoplasm, Bethesda categories III and IV, respectively).
Most importantly, it has been tested and is marketed as a negative predictor only. It is also only useful if used in the appropriate setting and if the test result is negative; a positive Afirma test can tell us only that the nodule is indeterminate, something, by definition, already determined by fine needle aspiration (FNA). Furthermore, any study examining its efficacy must be conducted within the context of clinical scenarios. For example, for a patient with an indeterminate lesion and a symptomatic multinodular goiter, neither a negative nor positive Afirma result would have any impact on surgical decision making and yet would have cost the patient more than $3,000. The latter patient simply needs a thyroidectomy. A patient who has a nodule that is approaching 4 cm or is increasing in size or a patient who has undergone several FNAs, ultrasounds, and endocrinology consultations similarly would likely choose surgery regardless of the GEC test result.
The only scenario in which Afirma testing is useful is in a patient with otherwise no indications for surgery except an indeterminate FNA; for instance for a patient with an asymptomatic < 4cm, stable, single nodule. In this scenario only a negative result could be helpful and would obviate the need for surgical intervention. One also must ask what is the likelihood of the patient undergoing repeat FNA, having repeat indeterminate cytology and repeat Afirma testing over the ensuing years and at what point should we consider these costs prohibitive compared with the patient undergoing a thyroidectomy instead?
Similar concerns exist with regard to somatic mutation panels, the most commonly marketed and used being Asuragen. The mutation panel consists of Ras and BRAF mutation and RET/PTC and PAX8/PPAR-gamma chromosomal rearrangement analyses. Importantly, the expanding literature on molecular markers for thyroid cancer, however, has lost track of a vast body of literature that reports Ras mutations present in up to 30% of benign thyroid nodules; RET/PTC in 24% and PAX8/PPAR-gamma in 14%, dramatically challenging the validity of the panel purported to accurately identify thyroid malignancy. We know that BRAF mutation is exclusively found in papillary thyroid cancer, but generally a BRAF-positive tumor is one in which a cytological diagnosis is already clear; it is either suspicious or definitively malignant on FNA. One might suggest that these aforementioned markers might indicate a harbinger of malignancy or identify a precancerous lesion; however, there is no study that supports this theory. Furthermore, it is important to note that even among pathology experts the diagnoses of follicular adenoma, follicular cancer, and follicular variant of papillary thyroid cancer have significant and documented inter- and intra- observer variability, thus challenging the gold standard upon which these markers have been tested.
With regard to BRAF, Ras, RET/PTC and PAX8/PPAR-gamma as prognostic markers there are many considerations, the least of which includes the differential diagnosis of follicular lesions addressed above and the known presence of these abnormalities in benign tumors. The majority of studies to date have conducted only univariate analyses without consideration of the clinical variables already available to us to make needed clinical decisions. In order to examine the usefulness of any marker panel a multivariable study is required, incorporating the available clinical variables such as size, lymph node metastases, and extra-thyroidal extension. For instance, the majority of studies examining the presence of lymph node metastases in BRAF-negative vs. BRAF-positive tumors were almost uniformly conducted on patients in whom routine lymph node dissection was not performed, thereby skewing the results. Only patients with suspicious nodes underwent a lymph node dissection; thus for those patients who had no suspicious nodes there was no information about their nodal status. Furthermore, several studies that included patients who underwent routine lymph node dissections and, importantly, included patients who underwent prophylactic dissections for whom a marker would be most useful, did not find that BRAF mutation was associated with aggressive features, including the presence of lymph node metastases. The same can be stated for a panel that includes Ras, RET/PTC, & PAX8/PPAR-gamma. “Follicular variant of papillary thyroid cancer and follicular cancer” will more likely harbor Ras and PAX8/PPAR-gamma mutations and will, by definition, include some tumors that would be considered benign by other pathologists.
There is an emerging market for molecular markers for thyroid cancer diagnosis and prognosis, but many of the studies driving this market are lacking in scientific rigor with regard to design and interpretation of results. Furthermore, the use of the marker panel requires scrutiny of its true impact on clinical management as well as its cost-effectiveness. GEC provides a negative predictor of malignancy and should be used to obviate surgery only; if the patient requires surgery its use provides no added information. BRAF is specific for thyroid cancer diagnosis, but is often not required as FNA evaluation in BRAF-positive tumors is usually already suspicious or malignant. Finally, molecular marker panels for thyroid cancer prognosis may be promising, but scrutiny for true accuracy and true clinical impact is necessary in order to make logical and useful recommendations for our patients.
Dr. Zeiger is professor of surgery, oncology, cellular, and molecular medicine, associate vice chair for faculty development, and associate dean for postdoctoral affairs at Johns Hopkins University in Baltimore. Dr. Zeiger consults for Interpace Diagnostics.
Documenting quantity of care rather than quality
Pneumonia shot? Check. Flu shot? Got it.
What do asking about these two immunizations mean in a neurology history? Absolutely nothing, for most cases.
But that doesn’t stop me and other neurologists from asking about them. Why? Because they’re part of the Physician Quality Reporting System (PQRS) measures, of course! None of us want to be penalized by Medicare for failing to document them. Along with medications (Measure 130: drug, dose, and route of administration), counseling for women of childbearing potential with epilepsy (Measure 268), tobacco status (Measure 226), and blood pressure at visit (Measure 317).
My colleagues in orthopedics tell me they’re now documenting a female patient’s most recent mammogram for the same reasons. While it’s unlikely to affect why they need a new knee, it’s what they have to do to avoid penalties.
How much time does this take? About 30-60 seconds per Medicare patient in my practice. That’s not a huge amount of time, but when you see about 1,000 Medicare visits per year, that adds up to 8-16 hours spent on extraneous documentation.
Do I do it to improve patient care? No. Checking off boxes that have no relation to the case at hand makes no difference at all. I doubt you’ll find a practicing physician who believes otherwise. It simply comes down to playing by the rules, no matter how irrelevant they are.
That’s part of the problem in health care today. In documentation, quantity has replaced quality as a measure of care. The concise, pointed, summary has been eclipsed by long notes that document a large amount of unimportant data. Attaching the PQRS data (a 2-page form in my office) to the bill I submit, and noting it in my chart, only wastes time and paper.
Obviously, documenting blood pressure, tobacco status, and medications are important ... but I’ve always done those. I don’t know any neurologist who doesn’t check those things regularly, since they can directly affect our care.
But the rest is just fluff, which, sadly, seems to be more important these days than actually doing something to help the patient, at least in the eyes of Medicare.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Pneumonia shot? Check. Flu shot? Got it.
What do asking about these two immunizations mean in a neurology history? Absolutely nothing, for most cases.
But that doesn’t stop me and other neurologists from asking about them. Why? Because they’re part of the Physician Quality Reporting System (PQRS) measures, of course! None of us want to be penalized by Medicare for failing to document them. Along with medications (Measure 130: drug, dose, and route of administration), counseling for women of childbearing potential with epilepsy (Measure 268), tobacco status (Measure 226), and blood pressure at visit (Measure 317).
My colleagues in orthopedics tell me they’re now documenting a female patient’s most recent mammogram for the same reasons. While it’s unlikely to affect why they need a new knee, it’s what they have to do to avoid penalties.
How much time does this take? About 30-60 seconds per Medicare patient in my practice. That’s not a huge amount of time, but when you see about 1,000 Medicare visits per year, that adds up to 8-16 hours spent on extraneous documentation.
Do I do it to improve patient care? No. Checking off boxes that have no relation to the case at hand makes no difference at all. I doubt you’ll find a practicing physician who believes otherwise. It simply comes down to playing by the rules, no matter how irrelevant they are.
That’s part of the problem in health care today. In documentation, quantity has replaced quality as a measure of care. The concise, pointed, summary has been eclipsed by long notes that document a large amount of unimportant data. Attaching the PQRS data (a 2-page form in my office) to the bill I submit, and noting it in my chart, only wastes time and paper.
Obviously, documenting blood pressure, tobacco status, and medications are important ... but I’ve always done those. I don’t know any neurologist who doesn’t check those things regularly, since they can directly affect our care.
But the rest is just fluff, which, sadly, seems to be more important these days than actually doing something to help the patient, at least in the eyes of Medicare.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Pneumonia shot? Check. Flu shot? Got it.
What do asking about these two immunizations mean in a neurology history? Absolutely nothing, for most cases.
But that doesn’t stop me and other neurologists from asking about them. Why? Because they’re part of the Physician Quality Reporting System (PQRS) measures, of course! None of us want to be penalized by Medicare for failing to document them. Along with medications (Measure 130: drug, dose, and route of administration), counseling for women of childbearing potential with epilepsy (Measure 268), tobacco status (Measure 226), and blood pressure at visit (Measure 317).
My colleagues in orthopedics tell me they’re now documenting a female patient’s most recent mammogram for the same reasons. While it’s unlikely to affect why they need a new knee, it’s what they have to do to avoid penalties.
How much time does this take? About 30-60 seconds per Medicare patient in my practice. That’s not a huge amount of time, but when you see about 1,000 Medicare visits per year, that adds up to 8-16 hours spent on extraneous documentation.
Do I do it to improve patient care? No. Checking off boxes that have no relation to the case at hand makes no difference at all. I doubt you’ll find a practicing physician who believes otherwise. It simply comes down to playing by the rules, no matter how irrelevant they are.
That’s part of the problem in health care today. In documentation, quantity has replaced quality as a measure of care. The concise, pointed, summary has been eclipsed by long notes that document a large amount of unimportant data. Attaching the PQRS data (a 2-page form in my office) to the bill I submit, and noting it in my chart, only wastes time and paper.
Obviously, documenting blood pressure, tobacco status, and medications are important ... but I’ve always done those. I don’t know any neurologist who doesn’t check those things regularly, since they can directly affect our care.
But the rest is just fluff, which, sadly, seems to be more important these days than actually doing something to help the patient, at least in the eyes of Medicare.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A new day for discharges?
“Decrease readmissions, and decrease them stat!” This mantra, or some, perhaps more subtle version thereof, is echoed over and over at hospitals across the country, and for good reason. Not only do readmissions have the potential to cost hospital systems millions of dollars through Medicare payment reductions, they also signal a more important, though less vocalized concern. If our patients keep returning to the hospital, are we really providing them with 100% of the resources they need?
On the surface, it may seem like there is little we can do for that two-pack-per-day smoker with end-stage chronic obstructive pulmonary disease who keeps getting readmitted with an exacerbation. And, while in reality, we may never get him to stop smoking and start taking his mediations as prescribed, perhaps we can help decrease the frequency of readmissions from three to four per year to two to three. While seemingly small, this decrease is actually quite dramatic, correlating to a 25%-50% reduction in the use of hospital services, not to mention the profound impact that fewer days spent in the hospital will have on his quality of life.
It is remarkable how much change occurs in the health care system over time. One year a drug may be touted as a huge breakthrough in treatment, and the next it may be taken off the market because of previously unrecognized, potentially fatal side effects. And just as the field of medicine is ever changing, so are all the fields that support it.
For example, the Agency for Healthcare Research and Qualify (AHRQ) has developed the Re-Engineered Discharge (RED) tool kit, which has been highly successful in reducing hospital readmissions. Originally developed by a group of AHRQ-funded researchers in Boston, RED provides evidence-based tools that help hospitals re-engineer their discharge process. One success story – within 3 months of implementing RED, the Valley Baptist Medical Center in Harlingen, Tex., decreased readmissions from 26% to 15%.
The RED model focuses on comprehensive discharge planning, educating patients about their discharge, and postdischarge follow-up care. It uses dedicated discharge advocates to help patients reconcile their medications and schedule much-needed follow-up appointments.
Other models exist as well. For instance, some hospitals have a palliative care team that focuses not only on keeping patients comfortable while in the hospital, but also on helping them access community services after discharge and make necessary appointments, geared at optimizing their health and ultimately decreasing the need for excessive hospitalizations.
As every health care dollar spent will be scrutinized more and more over time, innovative programs to help us rethink our long-established routines will likely play a major role in catapulting us from where we are to where we want to be.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
“Decrease readmissions, and decrease them stat!” This mantra, or some, perhaps more subtle version thereof, is echoed over and over at hospitals across the country, and for good reason. Not only do readmissions have the potential to cost hospital systems millions of dollars through Medicare payment reductions, they also signal a more important, though less vocalized concern. If our patients keep returning to the hospital, are we really providing them with 100% of the resources they need?
On the surface, it may seem like there is little we can do for that two-pack-per-day smoker with end-stage chronic obstructive pulmonary disease who keeps getting readmitted with an exacerbation. And, while in reality, we may never get him to stop smoking and start taking his mediations as prescribed, perhaps we can help decrease the frequency of readmissions from three to four per year to two to three. While seemingly small, this decrease is actually quite dramatic, correlating to a 25%-50% reduction in the use of hospital services, not to mention the profound impact that fewer days spent in the hospital will have on his quality of life.
It is remarkable how much change occurs in the health care system over time. One year a drug may be touted as a huge breakthrough in treatment, and the next it may be taken off the market because of previously unrecognized, potentially fatal side effects. And just as the field of medicine is ever changing, so are all the fields that support it.
For example, the Agency for Healthcare Research and Qualify (AHRQ) has developed the Re-Engineered Discharge (RED) tool kit, which has been highly successful in reducing hospital readmissions. Originally developed by a group of AHRQ-funded researchers in Boston, RED provides evidence-based tools that help hospitals re-engineer their discharge process. One success story – within 3 months of implementing RED, the Valley Baptist Medical Center in Harlingen, Tex., decreased readmissions from 26% to 15%.
The RED model focuses on comprehensive discharge planning, educating patients about their discharge, and postdischarge follow-up care. It uses dedicated discharge advocates to help patients reconcile their medications and schedule much-needed follow-up appointments.
Other models exist as well. For instance, some hospitals have a palliative care team that focuses not only on keeping patients comfortable while in the hospital, but also on helping them access community services after discharge and make necessary appointments, geared at optimizing their health and ultimately decreasing the need for excessive hospitalizations.
As every health care dollar spent will be scrutinized more and more over time, innovative programs to help us rethink our long-established routines will likely play a major role in catapulting us from where we are to where we want to be.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
“Decrease readmissions, and decrease them stat!” This mantra, or some, perhaps more subtle version thereof, is echoed over and over at hospitals across the country, and for good reason. Not only do readmissions have the potential to cost hospital systems millions of dollars through Medicare payment reductions, they also signal a more important, though less vocalized concern. If our patients keep returning to the hospital, are we really providing them with 100% of the resources they need?
On the surface, it may seem like there is little we can do for that two-pack-per-day smoker with end-stage chronic obstructive pulmonary disease who keeps getting readmitted with an exacerbation. And, while in reality, we may never get him to stop smoking and start taking his mediations as prescribed, perhaps we can help decrease the frequency of readmissions from three to four per year to two to three. While seemingly small, this decrease is actually quite dramatic, correlating to a 25%-50% reduction in the use of hospital services, not to mention the profound impact that fewer days spent in the hospital will have on his quality of life.
It is remarkable how much change occurs in the health care system over time. One year a drug may be touted as a huge breakthrough in treatment, and the next it may be taken off the market because of previously unrecognized, potentially fatal side effects. And just as the field of medicine is ever changing, so are all the fields that support it.
For example, the Agency for Healthcare Research and Qualify (AHRQ) has developed the Re-Engineered Discharge (RED) tool kit, which has been highly successful in reducing hospital readmissions. Originally developed by a group of AHRQ-funded researchers in Boston, RED provides evidence-based tools that help hospitals re-engineer their discharge process. One success story – within 3 months of implementing RED, the Valley Baptist Medical Center in Harlingen, Tex., decreased readmissions from 26% to 15%.
The RED model focuses on comprehensive discharge planning, educating patients about their discharge, and postdischarge follow-up care. It uses dedicated discharge advocates to help patients reconcile their medications and schedule much-needed follow-up appointments.
Other models exist as well. For instance, some hospitals have a palliative care team that focuses not only on keeping patients comfortable while in the hospital, but also on helping them access community services after discharge and make necessary appointments, geared at optimizing their health and ultimately decreasing the need for excessive hospitalizations.
As every health care dollar spent will be scrutinized more and more over time, innovative programs to help us rethink our long-established routines will likely play a major role in catapulting us from where we are to where we want to be.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
An Update on Acute Care Surgery, Part 2
In continuing with the series, An Update in Acute Care Surgery, the following section highlights the evolution of the training process for the Acute Care Surgery fellowship.
Grace S. Rozycki, MD, FACS
The Willis D. Gatch Professor of Surgery
Associate Chair, Department of Surgery, Indiana University
Chief of Surgery, IUH-Methodist Hospital, Indianapolis
Acute Care Surgery: The Training Paradigm
BY CLAY COTHREN BURLEW, MD, FACS, AND GREGORY J. JURKOVICH, MD., FACS
The acute care surgery fellowships are designed to follow core training in general surgery (J. Trauma 2007;62:553-6). Currently, this means the acute care surgery fellowship follows the completion of an Accreditation Council for Graduate Medical Education general surgery residency program and is in alignment with the core competencies of the general surgery residency. The 2-year curriculum was defined by the AAST, and incorporates the requirements of an ACGME-approved surgical critical care fellowship.
Although there are mandatory components of this fellowship, a certain amount of latitude and creativity are encouraged so to capitalize on the strength of the individual program as well as to meet the individual needs of the fellow.
The basic principles of the training paradigm include the followi
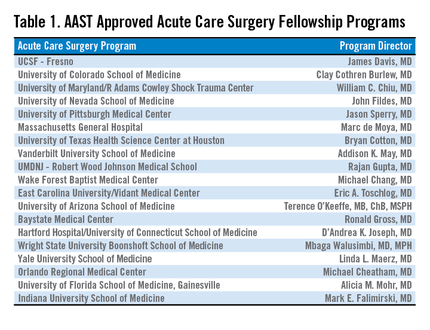
1. The program is 2 years in length.
2. The acute care surgery fellowship programs must have the ACGME approved surgical critical care residency.
3.The fellowship must include specific surgical technical training in hepatobiliary disorders, thoracic surgery, and vascular surgery.
4. Trainees should participate in acute care surgery call for at least 12 months and 52 nights of acute care surgery call (trauma and emergency general surgery).
5. Flexibility in the rotations should be used to optimize the fellow’s training.
6. The rationale for out-of-system rotations and the structure of the 24-month training should be used to optimize the fellow’s training.
7. Participation in elective surgery, both to supplement general surgery training and experience and to serve in a supervisory role to residents, is an essential component of this fellowship training.
8. An academic environment is necessary and the fellows should be trained to teach others and conduct research in acute care surgery.
The rationale for the rotations of Thoracic Surgery, Transplant/Hepatobiliary/ Pancreatic, and Vascular (including vascular interventional) is twofold: 1) Many complex operative cases in these areas are infrequently encountered in modern trauma centers; and, 2) experts in these areas can provide mentorship and operative expertise and teaching for the fellow who obtains a focused, quality operative experience in these areas. Further, these rotations have specific competency related goals so that the fellow has specific requirements to meet. The AAST is currently revising its method of confirming this training expertise by examining specific components of operative technique and exposure as well as length of time on specific rotations (see below).
Program Application and Approval
The required background and expectations for the acute care surgery fellowship include the following: 1) Fellows must have successfully completed the core training requirements of an RRC-approved residency in General Surgery; and 2) the acute care surgery fellowship programs must provide the necessary education to qualify the fellow as an acute care surgical specialist in clinical, education, and research areas. Each program must have support from its parent institution, including administrative personnel, the chairman of the department of surgery, division chief, and participating acute care surgery faculty. The program should have all of the necessary resources to fulfill the training requirements and create an environment of inquiry and scholarship while allowing for progressive responsibility throughout the training period.
The process of becoming an approved acute care surgery fellowship program can be divided into the following steps:
1. The Program Director completes the Program Information Form (PIF) form (downloaded from the AAST website, http://www.aast.org).
2. The PIF is reviewed by three members of the AAST Acute Care Surgery Committee to determine whether it is complete and if it meets the essential requirements. If the initial review is successful, then a site visit of the program is scheduled.
3. The site visit is conducted by two members of AAST Acute Care Surgery Committee. The site visit consists of an evening business dinner meeting with the following personnel: the program director for the acute care surgery fellowship, the program director for the general surgery residency program, select administrators, and division chiefs. Current fellows in the program are also invited to participate. The following day, the site visitors tour the institution, and conduct one-on-one interviews with the personnel who were present at the site visit dinner. A chart review is conducted to assess the operative case load and the involvement of the faculty, residents, and fellows in the care of the patients. At the conclusion of the day, a summation interview is conducted with the acute care surgery fellowship program director.
4. Following the site visit, a written assessment of the program is performed, which covers an overview/program description, strengths/weaknesses, major deficiencies, and a summary with recommendations.
5. If no major deficiencies are noted, the senior site visitor presents the highlights of the program to the members of the AAST Acute Care Surgery Committee and, if approved subsequently to the AAST Board of Managers for final approval.
Acute Care Surgery Committee and Curriculum
BY CLAY COTHREN BURLEW, MD
With the development of a fellowship-based training paradigm in acute care surgery, the AAST also developed an oversight committee. This committee, aptly named the Acute Care Surgery Committee, is comprised of 25 appointed AAST members. The committee represents a varied group of stakeholders including members of the senior leadership of the AAST and fellowship program directors. The Acute Care Surgery Committee’s role has evolved over the past decade. Initially the members of the Committee formulated and implemented the training paradigm for the fellowship, including the requirements enumerated above. Following the certification of several successful training fellowships in 2008-2009, the Committee encouraged additional institutions to initiate training fellows by assisting with educational development. This led to the expansion of acute care surgery training in 19 accredited programs. (see Table).
Throughout this decade of growth, the Acute Care Surgery committee has also been dedicated to the oversight and continual evaluation of the training process. Two specific measures were implemented in this regard.
First, each fellowship graduate must take not only the American Board of Surgery examination for certification in Surgical Critical Care, but also the examination in acute care surgery written by the AAST. The Acute Care Surgery Committee formed a subcommittee that has spent innumerable hours researching and writing test questions for this examination. That subcommittee is now reviewing each of the questions from the originally produced examination and reformatting the test. New questions are being created by the subcommittee, and the examination will become electronically administered.
Second, acute care surgery fellows must track their operative experience through the AAST-supported on-line case log system. Although fellows have been provided with a list of essential and desired operative cases, the case log system permits specific delineation of each fellow’s experience. Using the case log system, the Acute Care Surgery Committee was able to analyze the fellows’ experience, identify gaps in operative training, and refine the curriculum as indicated. The Committee has performed two such analyses and, based on the findings of those reports, modification of the curriculum is now underway. (J. Trauma Acute Care Surg. 2014;76:329-39.; J. Trauma Acute Care Surg. 2015;78:259-64).
One of the current initiatives of the Acute Care Surgery Committee is the revision of the acute care surgery fellowship curriculum. In revising the curriculum, several key points were considered. One observation, derived from the case log review, was that the original case list captured only a portion of the operative experience of acute care surgery fellows. Additionally, it was apparent that operative trauma cases alone do not provide adequate exposure to some of the more complex cases thought to be essential components of this specialty. Therefore, the Committee determined that incorporating specific surgical approaches or anatomic exposures performed during elective and urgent cases are valuable experiences for the fellow. The totality of training in advanced operative techniques over the breadth of anatomic locations remains the unique feature of our specialty.(J. Trauma Acute Care Surg. 2015; 78:192-196).
Based upon these observations, the new curriculum is now organized by anatomic subsections (Head/Neck, Thoracic, Abdominal, Vascular), as well as organ-based management. Each section of the curriculum now lists specific case numbers required for surgical approaches or exposures, and also addresses organ-based management. For example, within the thoracic section of the curriculum, required case numbers now exist for thoracotomy, thoracoscopy, sternotomy, and pericardotomy (exposures) as well as lung, diaphragm, heart, esophagus, and intrathoracic great vessels. In each anatomic subsection, simulation may be used to satisfy a requirement.
Opportunities to accomplish these requirements may be through the American College of Surgeons Advanced Trauma Operative Management or the Advanced Surgical Skills for Exposure in Trauma courses. Organ harvest exposures may also be used for less common surgical exposures. Fellows can choose both the exposure and the organ-based procedure code for each operative case performed when logging their cases.(J. Trauma Acute Care Surg. 2014;76:329-39). Identification of a minimum number of operative cases needed in specific body regions reflects the defined case volumes in general surgery as required by the Accreditation Council for Graduate Medical Education.
The Acute Care Surgery Committee considered that the implementation of the required case volumes would serve two purposes: First, the list would provide guidance to the fellows as to the types of cases they should actively identify and in which they should participate; and, second, it would provide guidance to program directors and subspecialty colleagues as to the cases deemed important for fellowship training. Ongoing review of the fellows’ case logs with implementation of the new curriculum will remain a focus of the Acute Care Surgery Committee as it transitions to a new case log system soon.
An earlier version of the graphic misstated the name of Clay Cothren Burlew.
In continuing with the series, An Update in Acute Care Surgery, the following section highlights the evolution of the training process for the Acute Care Surgery fellowship.
Grace S. Rozycki, MD, FACS
The Willis D. Gatch Professor of Surgery
Associate Chair, Department of Surgery, Indiana University
Chief of Surgery, IUH-Methodist Hospital, Indianapolis
Acute Care Surgery: The Training Paradigm
BY CLAY COTHREN BURLEW, MD, FACS, AND GREGORY J. JURKOVICH, MD., FACS
The acute care surgery fellowships are designed to follow core training in general surgery (J. Trauma 2007;62:553-6). Currently, this means the acute care surgery fellowship follows the completion of an Accreditation Council for Graduate Medical Education general surgery residency program and is in alignment with the core competencies of the general surgery residency. The 2-year curriculum was defined by the AAST, and incorporates the requirements of an ACGME-approved surgical critical care fellowship.
Although there are mandatory components of this fellowship, a certain amount of latitude and creativity are encouraged so to capitalize on the strength of the individual program as well as to meet the individual needs of the fellow.
The basic principles of the training paradigm include the followi

1. The program is 2 years in length.
2. The acute care surgery fellowship programs must have the ACGME approved surgical critical care residency.
3.The fellowship must include specific surgical technical training in hepatobiliary disorders, thoracic surgery, and vascular surgery.
4. Trainees should participate in acute care surgery call for at least 12 months and 52 nights of acute care surgery call (trauma and emergency general surgery).
5. Flexibility in the rotations should be used to optimize the fellow’s training.
6. The rationale for out-of-system rotations and the structure of the 24-month training should be used to optimize the fellow’s training.
7. Participation in elective surgery, both to supplement general surgery training and experience and to serve in a supervisory role to residents, is an essential component of this fellowship training.
8. An academic environment is necessary and the fellows should be trained to teach others and conduct research in acute care surgery.
The rationale for the rotations of Thoracic Surgery, Transplant/Hepatobiliary/ Pancreatic, and Vascular (including vascular interventional) is twofold: 1) Many complex operative cases in these areas are infrequently encountered in modern trauma centers; and, 2) experts in these areas can provide mentorship and operative expertise and teaching for the fellow who obtains a focused, quality operative experience in these areas. Further, these rotations have specific competency related goals so that the fellow has specific requirements to meet. The AAST is currently revising its method of confirming this training expertise by examining specific components of operative technique and exposure as well as length of time on specific rotations (see below).
Program Application and Approval
The required background and expectations for the acute care surgery fellowship include the following: 1) Fellows must have successfully completed the core training requirements of an RRC-approved residency in General Surgery; and 2) the acute care surgery fellowship programs must provide the necessary education to qualify the fellow as an acute care surgical specialist in clinical, education, and research areas. Each program must have support from its parent institution, including administrative personnel, the chairman of the department of surgery, division chief, and participating acute care surgery faculty. The program should have all of the necessary resources to fulfill the training requirements and create an environment of inquiry and scholarship while allowing for progressive responsibility throughout the training period.
The process of becoming an approved acute care surgery fellowship program can be divided into the following steps:
1. The Program Director completes the Program Information Form (PIF) form (downloaded from the AAST website, http://www.aast.org).
2. The PIF is reviewed by three members of the AAST Acute Care Surgery Committee to determine whether it is complete and if it meets the essential requirements. If the initial review is successful, then a site visit of the program is scheduled.
3. The site visit is conducted by two members of AAST Acute Care Surgery Committee. The site visit consists of an evening business dinner meeting with the following personnel: the program director for the acute care surgery fellowship, the program director for the general surgery residency program, select administrators, and division chiefs. Current fellows in the program are also invited to participate. The following day, the site visitors tour the institution, and conduct one-on-one interviews with the personnel who were present at the site visit dinner. A chart review is conducted to assess the operative case load and the involvement of the faculty, residents, and fellows in the care of the patients. At the conclusion of the day, a summation interview is conducted with the acute care surgery fellowship program director.
4. Following the site visit, a written assessment of the program is performed, which covers an overview/program description, strengths/weaknesses, major deficiencies, and a summary with recommendations.
5. If no major deficiencies are noted, the senior site visitor presents the highlights of the program to the members of the AAST Acute Care Surgery Committee and, if approved subsequently to the AAST Board of Managers for final approval.
Acute Care Surgery Committee and Curriculum
BY CLAY COTHREN BURLEW, MD
With the development of a fellowship-based training paradigm in acute care surgery, the AAST also developed an oversight committee. This committee, aptly named the Acute Care Surgery Committee, is comprised of 25 appointed AAST members. The committee represents a varied group of stakeholders including members of the senior leadership of the AAST and fellowship program directors. The Acute Care Surgery Committee’s role has evolved over the past decade. Initially the members of the Committee formulated and implemented the training paradigm for the fellowship, including the requirements enumerated above. Following the certification of several successful training fellowships in 2008-2009, the Committee encouraged additional institutions to initiate training fellows by assisting with educational development. This led to the expansion of acute care surgery training in 19 accredited programs. (see Table).
Throughout this decade of growth, the Acute Care Surgery committee has also been dedicated to the oversight and continual evaluation of the training process. Two specific measures were implemented in this regard.
First, each fellowship graduate must take not only the American Board of Surgery examination for certification in Surgical Critical Care, but also the examination in acute care surgery written by the AAST. The Acute Care Surgery Committee formed a subcommittee that has spent innumerable hours researching and writing test questions for this examination. That subcommittee is now reviewing each of the questions from the originally produced examination and reformatting the test. New questions are being created by the subcommittee, and the examination will become electronically administered.
Second, acute care surgery fellows must track their operative experience through the AAST-supported on-line case log system. Although fellows have been provided with a list of essential and desired operative cases, the case log system permits specific delineation of each fellow’s experience. Using the case log system, the Acute Care Surgery Committee was able to analyze the fellows’ experience, identify gaps in operative training, and refine the curriculum as indicated. The Committee has performed two such analyses and, based on the findings of those reports, modification of the curriculum is now underway. (J. Trauma Acute Care Surg. 2014;76:329-39.; J. Trauma Acute Care Surg. 2015;78:259-64).
One of the current initiatives of the Acute Care Surgery Committee is the revision of the acute care surgery fellowship curriculum. In revising the curriculum, several key points were considered. One observation, derived from the case log review, was that the original case list captured only a portion of the operative experience of acute care surgery fellows. Additionally, it was apparent that operative trauma cases alone do not provide adequate exposure to some of the more complex cases thought to be essential components of this specialty. Therefore, the Committee determined that incorporating specific surgical approaches or anatomic exposures performed during elective and urgent cases are valuable experiences for the fellow. The totality of training in advanced operative techniques over the breadth of anatomic locations remains the unique feature of our specialty.(J. Trauma Acute Care Surg. 2015; 78:192-196).
Based upon these observations, the new curriculum is now organized by anatomic subsections (Head/Neck, Thoracic, Abdominal, Vascular), as well as organ-based management. Each section of the curriculum now lists specific case numbers required for surgical approaches or exposures, and also addresses organ-based management. For example, within the thoracic section of the curriculum, required case numbers now exist for thoracotomy, thoracoscopy, sternotomy, and pericardotomy (exposures) as well as lung, diaphragm, heart, esophagus, and intrathoracic great vessels. In each anatomic subsection, simulation may be used to satisfy a requirement.
Opportunities to accomplish these requirements may be through the American College of Surgeons Advanced Trauma Operative Management or the Advanced Surgical Skills for Exposure in Trauma courses. Organ harvest exposures may also be used for less common surgical exposures. Fellows can choose both the exposure and the organ-based procedure code for each operative case performed when logging their cases.(J. Trauma Acute Care Surg. 2014;76:329-39). Identification of a minimum number of operative cases needed in specific body regions reflects the defined case volumes in general surgery as required by the Accreditation Council for Graduate Medical Education.
The Acute Care Surgery Committee considered that the implementation of the required case volumes would serve two purposes: First, the list would provide guidance to the fellows as to the types of cases they should actively identify and in which they should participate; and, second, it would provide guidance to program directors and subspecialty colleagues as to the cases deemed important for fellowship training. Ongoing review of the fellows’ case logs with implementation of the new curriculum will remain a focus of the Acute Care Surgery Committee as it transitions to a new case log system soon.
An earlier version of the graphic misstated the name of Clay Cothren Burlew.
In continuing with the series, An Update in Acute Care Surgery, the following section highlights the evolution of the training process for the Acute Care Surgery fellowship.
Grace S. Rozycki, MD, FACS
The Willis D. Gatch Professor of Surgery
Associate Chair, Department of Surgery, Indiana University
Chief of Surgery, IUH-Methodist Hospital, Indianapolis
Acute Care Surgery: The Training Paradigm
BY CLAY COTHREN BURLEW, MD, FACS, AND GREGORY J. JURKOVICH, MD., FACS
The acute care surgery fellowships are designed to follow core training in general surgery (J. Trauma 2007;62:553-6). Currently, this means the acute care surgery fellowship follows the completion of an Accreditation Council for Graduate Medical Education general surgery residency program and is in alignment with the core competencies of the general surgery residency. The 2-year curriculum was defined by the AAST, and incorporates the requirements of an ACGME-approved surgical critical care fellowship.
Although there are mandatory components of this fellowship, a certain amount of latitude and creativity are encouraged so to capitalize on the strength of the individual program as well as to meet the individual needs of the fellow.
The basic principles of the training paradigm include the followi

1. The program is 2 years in length.
2. The acute care surgery fellowship programs must have the ACGME approved surgical critical care residency.
3.The fellowship must include specific surgical technical training in hepatobiliary disorders, thoracic surgery, and vascular surgery.
4. Trainees should participate in acute care surgery call for at least 12 months and 52 nights of acute care surgery call (trauma and emergency general surgery).
5. Flexibility in the rotations should be used to optimize the fellow’s training.
6. The rationale for out-of-system rotations and the structure of the 24-month training should be used to optimize the fellow’s training.
7. Participation in elective surgery, both to supplement general surgery training and experience and to serve in a supervisory role to residents, is an essential component of this fellowship training.
8. An academic environment is necessary and the fellows should be trained to teach others and conduct research in acute care surgery.
The rationale for the rotations of Thoracic Surgery, Transplant/Hepatobiliary/ Pancreatic, and Vascular (including vascular interventional) is twofold: 1) Many complex operative cases in these areas are infrequently encountered in modern trauma centers; and, 2) experts in these areas can provide mentorship and operative expertise and teaching for the fellow who obtains a focused, quality operative experience in these areas. Further, these rotations have specific competency related goals so that the fellow has specific requirements to meet. The AAST is currently revising its method of confirming this training expertise by examining specific components of operative technique and exposure as well as length of time on specific rotations (see below).
Program Application and Approval
The required background and expectations for the acute care surgery fellowship include the following: 1) Fellows must have successfully completed the core training requirements of an RRC-approved residency in General Surgery; and 2) the acute care surgery fellowship programs must provide the necessary education to qualify the fellow as an acute care surgical specialist in clinical, education, and research areas. Each program must have support from its parent institution, including administrative personnel, the chairman of the department of surgery, division chief, and participating acute care surgery faculty. The program should have all of the necessary resources to fulfill the training requirements and create an environment of inquiry and scholarship while allowing for progressive responsibility throughout the training period.
The process of becoming an approved acute care surgery fellowship program can be divided into the following steps:
1. The Program Director completes the Program Information Form (PIF) form (downloaded from the AAST website, http://www.aast.org).
2. The PIF is reviewed by three members of the AAST Acute Care Surgery Committee to determine whether it is complete and if it meets the essential requirements. If the initial review is successful, then a site visit of the program is scheduled.
3. The site visit is conducted by two members of AAST Acute Care Surgery Committee. The site visit consists of an evening business dinner meeting with the following personnel: the program director for the acute care surgery fellowship, the program director for the general surgery residency program, select administrators, and division chiefs. Current fellows in the program are also invited to participate. The following day, the site visitors tour the institution, and conduct one-on-one interviews with the personnel who were present at the site visit dinner. A chart review is conducted to assess the operative case load and the involvement of the faculty, residents, and fellows in the care of the patients. At the conclusion of the day, a summation interview is conducted with the acute care surgery fellowship program director.
4. Following the site visit, a written assessment of the program is performed, which covers an overview/program description, strengths/weaknesses, major deficiencies, and a summary with recommendations.
5. If no major deficiencies are noted, the senior site visitor presents the highlights of the program to the members of the AAST Acute Care Surgery Committee and, if approved subsequently to the AAST Board of Managers for final approval.
Acute Care Surgery Committee and Curriculum
BY CLAY COTHREN BURLEW, MD
With the development of a fellowship-based training paradigm in acute care surgery, the AAST also developed an oversight committee. This committee, aptly named the Acute Care Surgery Committee, is comprised of 25 appointed AAST members. The committee represents a varied group of stakeholders including members of the senior leadership of the AAST and fellowship program directors. The Acute Care Surgery Committee’s role has evolved over the past decade. Initially the members of the Committee formulated and implemented the training paradigm for the fellowship, including the requirements enumerated above. Following the certification of several successful training fellowships in 2008-2009, the Committee encouraged additional institutions to initiate training fellows by assisting with educational development. This led to the expansion of acute care surgery training in 19 accredited programs. (see Table).
Throughout this decade of growth, the Acute Care Surgery committee has also been dedicated to the oversight and continual evaluation of the training process. Two specific measures were implemented in this regard.
First, each fellowship graduate must take not only the American Board of Surgery examination for certification in Surgical Critical Care, but also the examination in acute care surgery written by the AAST. The Acute Care Surgery Committee formed a subcommittee that has spent innumerable hours researching and writing test questions for this examination. That subcommittee is now reviewing each of the questions from the originally produced examination and reformatting the test. New questions are being created by the subcommittee, and the examination will become electronically administered.
Second, acute care surgery fellows must track their operative experience through the AAST-supported on-line case log system. Although fellows have been provided with a list of essential and desired operative cases, the case log system permits specific delineation of each fellow’s experience. Using the case log system, the Acute Care Surgery Committee was able to analyze the fellows’ experience, identify gaps in operative training, and refine the curriculum as indicated. The Committee has performed two such analyses and, based on the findings of those reports, modification of the curriculum is now underway. (J. Trauma Acute Care Surg. 2014;76:329-39.; J. Trauma Acute Care Surg. 2015;78:259-64).
One of the current initiatives of the Acute Care Surgery Committee is the revision of the acute care surgery fellowship curriculum. In revising the curriculum, several key points were considered. One observation, derived from the case log review, was that the original case list captured only a portion of the operative experience of acute care surgery fellows. Additionally, it was apparent that operative trauma cases alone do not provide adequate exposure to some of the more complex cases thought to be essential components of this specialty. Therefore, the Committee determined that incorporating specific surgical approaches or anatomic exposures performed during elective and urgent cases are valuable experiences for the fellow. The totality of training in advanced operative techniques over the breadth of anatomic locations remains the unique feature of our specialty.(J. Trauma Acute Care Surg. 2015; 78:192-196).
Based upon these observations, the new curriculum is now organized by anatomic subsections (Head/Neck, Thoracic, Abdominal, Vascular), as well as organ-based management. Each section of the curriculum now lists specific case numbers required for surgical approaches or exposures, and also addresses organ-based management. For example, within the thoracic section of the curriculum, required case numbers now exist for thoracotomy, thoracoscopy, sternotomy, and pericardotomy (exposures) as well as lung, diaphragm, heart, esophagus, and intrathoracic great vessels. In each anatomic subsection, simulation may be used to satisfy a requirement.
Opportunities to accomplish these requirements may be through the American College of Surgeons Advanced Trauma Operative Management or the Advanced Surgical Skills for Exposure in Trauma courses. Organ harvest exposures may also be used for less common surgical exposures. Fellows can choose both the exposure and the organ-based procedure code for each operative case performed when logging their cases.(J. Trauma Acute Care Surg. 2014;76:329-39). Identification of a minimum number of operative cases needed in specific body regions reflects the defined case volumes in general surgery as required by the Accreditation Council for Graduate Medical Education.
The Acute Care Surgery Committee considered that the implementation of the required case volumes would serve two purposes: First, the list would provide guidance to the fellows as to the types of cases they should actively identify and in which they should participate; and, second, it would provide guidance to program directors and subspecialty colleagues as to the cases deemed important for fellowship training. Ongoing review of the fellows’ case logs with implementation of the new curriculum will remain a focus of the Acute Care Surgery Committee as it transitions to a new case log system soon.
An earlier version of the graphic misstated the name of Clay Cothren Burlew.
In Response to "Online Entry-Level Education: The Jury Is Still Out!"
Dear Dr. Danielsen,
Thank you for addressing this latest controversy in PA/NP issues in the June 2015 Clinician Reviews. I appreciate your candor and your objectiveness in presenting this "hot topic."
I am a currently retired Certified Family Nurse Practitioner after more than 20 years in practice; I graduated from my RN program 40 years ago this month! During my career, I was a clinical instructor in an RN program, as well as a hospital nurse for most of my first two decades of nursing.
After obtaining my MSN/NP education, I preceptored NP students in a master’s prepared program for 20 years. The difference in students who were enrolled in the accelerated programs and those who were in traditional RN to BSN to MSN/NP programs were astonishing to me. They had little to no experience in how to relate to patients or how to take a history, and they certainly were not comfortable with patients.
Another concern is the growing number of "for profit" private schools now offering NP/PA programs, where enrollment seems to be at the price of well-qualified students. Those who cannot meet the requirements of a traditional university setting are now flooding these programs. Just look at the pass rates on exams and the attrition rates along with the high cost they charge.
I am concerned about the move toward e-learning programs. Anybody can memorize pathophysiology, statistics, and biochemistry, but not everyone can learn how to interact with patients and develop critical-thinking and problem-solving skills without direct supervision and experience.
Adequate clinical experience is imperative, and I suggest an internship for NPs and PAs fresh out of school. If we are going to advance our respect in the medical world with our physician colleagues, and continue to provide excellent care in this new era of medicine, then we need to make sure we do not jeopardize the hard work we have all done to bring the current high standards to PA and NP programs.
Janet Evans Emery, RN, MSN, CFNP
Irvine, California
Dear Dr. Danielsen,
Thank you for addressing this latest controversy in PA/NP issues in the June 2015 Clinician Reviews. I appreciate your candor and your objectiveness in presenting this "hot topic."
I am a currently retired Certified Family Nurse Practitioner after more than 20 years in practice; I graduated from my RN program 40 years ago this month! During my career, I was a clinical instructor in an RN program, as well as a hospital nurse for most of my first two decades of nursing.
After obtaining my MSN/NP education, I preceptored NP students in a master’s prepared program for 20 years. The difference in students who were enrolled in the accelerated programs and those who were in traditional RN to BSN to MSN/NP programs were astonishing to me. They had little to no experience in how to relate to patients or how to take a history, and they certainly were not comfortable with patients.
Another concern is the growing number of "for profit" private schools now offering NP/PA programs, where enrollment seems to be at the price of well-qualified students. Those who cannot meet the requirements of a traditional university setting are now flooding these programs. Just look at the pass rates on exams and the attrition rates along with the high cost they charge.
I am concerned about the move toward e-learning programs. Anybody can memorize pathophysiology, statistics, and biochemistry, but not everyone can learn how to interact with patients and develop critical-thinking and problem-solving skills without direct supervision and experience.
Adequate clinical experience is imperative, and I suggest an internship for NPs and PAs fresh out of school. If we are going to advance our respect in the medical world with our physician colleagues, and continue to provide excellent care in this new era of medicine, then we need to make sure we do not jeopardize the hard work we have all done to bring the current high standards to PA and NP programs.
Janet Evans Emery, RN, MSN, CFNP
Irvine, California
Dear Dr. Danielsen,
Thank you for addressing this latest controversy in PA/NP issues in the June 2015 Clinician Reviews. I appreciate your candor and your objectiveness in presenting this "hot topic."
I am a currently retired Certified Family Nurse Practitioner after more than 20 years in practice; I graduated from my RN program 40 years ago this month! During my career, I was a clinical instructor in an RN program, as well as a hospital nurse for most of my first two decades of nursing.
After obtaining my MSN/NP education, I preceptored NP students in a master’s prepared program for 20 years. The difference in students who were enrolled in the accelerated programs and those who were in traditional RN to BSN to MSN/NP programs were astonishing to me. They had little to no experience in how to relate to patients or how to take a history, and they certainly were not comfortable with patients.
Another concern is the growing number of "for profit" private schools now offering NP/PA programs, where enrollment seems to be at the price of well-qualified students. Those who cannot meet the requirements of a traditional university setting are now flooding these programs. Just look at the pass rates on exams and the attrition rates along with the high cost they charge.
I am concerned about the move toward e-learning programs. Anybody can memorize pathophysiology, statistics, and biochemistry, but not everyone can learn how to interact with patients and develop critical-thinking and problem-solving skills without direct supervision and experience.
Adequate clinical experience is imperative, and I suggest an internship for NPs and PAs fresh out of school. If we are going to advance our respect in the medical world with our physician colleagues, and continue to provide excellent care in this new era of medicine, then we need to make sure we do not jeopardize the hard work we have all done to bring the current high standards to PA and NP programs.
Janet Evans Emery, RN, MSN, CFNP
Irvine, California