User login
Diagnosis and treatment of uterine isthmocele
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
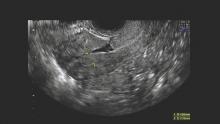
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
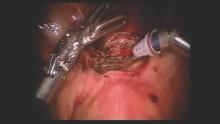
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
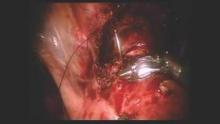
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.
Call to action: Saving 100,000 U.S. mothers in 5 years
The United States now ranks 60th in the world, and worst among developed nations, in maternal mortality. Each year more than 600 women in the United States die from pregnancy and childbirth, and more than 50,000 suffer a life-threatening complication (“severe maternal morbidity”).
The maternal mortality ratio doubled between 1987 and 2011, from 7.2 to 17.8 deaths per 100,000 live births; severe maternal morbidity doubled between 1998 and 2011, from 74 to 163 per 10,000 delivery hospitalizations. There continues to be large and persistent disparities; for example, a black woman is more than three times as likely to die from pregnancy and childbirth as a white woman, a gap we haven’t been able to close in decades.
The Maternal and Child Health Bureau is partnering with the American College of Obstetricians and Gynecologists and the Council on Patient Safety in Women’s Health Care (“the Council”) in launching a new national campaign to reduce maternal mortality and severe morbidity in the United States. The goal of the campaign is to prevent 100,000 maternal deaths or severe morbidities in the next 5 years by doing three things:
• Improving women’s health before pregnancy, with a focus on promoting preventive services including preconception, interconception, and postpartum care.
• Reducing primary cesarean deliveries, with an immediate focus on developing an evidence-based patient safety bundle to reduce low-risk, nulliparous, term, spontaneous, and vertex (NTSV) cesarean deliveries.
• Improving the quality and safety of maternity care, with a focus on implementing patient safety bundles to reduce mortality and morbidity associated with hemorrhage, preeclampsia, and thromboembolism in every birthing hospital and facility across the country in the next 3 years.
Safety bundles are small, straightforward sets of evidence-based practices that, when performed collectively and reliably, have improved patient outcomes. Several states have begun to pilot these safety bundles. In New York, ob.gyn. leaders such as Dr. Mary D’Alton are working with more than 1,000 health care providers to implement these safety bundles in 118 hospitals throughout the state.
In California, under the leadership of Dr. Elliott Main and other clinical and public health leaders, and in partnership with California Department of Public Health, the maternal mortality ratio decreased from a high of 16.9 in 2006 to 6.2 deaths per 100,000 live births in 2012.
If we are going to move the needle on maternal mortality and severe morbidity in this country, we are going to need an ob.gyn. champion in every hospital and every state. To learn more about the campaign and to find out how you can help, please visit the Council’s website at safehealthcareforeverywoman.org and click on the AIM Program.
Dr. Lu is the director of the Maternal and Child Health Bureau at the Health Resources and Services Administration, and an associate professor of obstetrics, gynecology, and public health at the University of California, Los Angeles.
The United States now ranks 60th in the world, and worst among developed nations, in maternal mortality. Each year more than 600 women in the United States die from pregnancy and childbirth, and more than 50,000 suffer a life-threatening complication (“severe maternal morbidity”).
The maternal mortality ratio doubled between 1987 and 2011, from 7.2 to 17.8 deaths per 100,000 live births; severe maternal morbidity doubled between 1998 and 2011, from 74 to 163 per 10,000 delivery hospitalizations. There continues to be large and persistent disparities; for example, a black woman is more than three times as likely to die from pregnancy and childbirth as a white woman, a gap we haven’t been able to close in decades.
The Maternal and Child Health Bureau is partnering with the American College of Obstetricians and Gynecologists and the Council on Patient Safety in Women’s Health Care (“the Council”) in launching a new national campaign to reduce maternal mortality and severe morbidity in the United States. The goal of the campaign is to prevent 100,000 maternal deaths or severe morbidities in the next 5 years by doing three things:
• Improving women’s health before pregnancy, with a focus on promoting preventive services including preconception, interconception, and postpartum care.
• Reducing primary cesarean deliveries, with an immediate focus on developing an evidence-based patient safety bundle to reduce low-risk, nulliparous, term, spontaneous, and vertex (NTSV) cesarean deliveries.
• Improving the quality and safety of maternity care, with a focus on implementing patient safety bundles to reduce mortality and morbidity associated with hemorrhage, preeclampsia, and thromboembolism in every birthing hospital and facility across the country in the next 3 years.
Safety bundles are small, straightforward sets of evidence-based practices that, when performed collectively and reliably, have improved patient outcomes. Several states have begun to pilot these safety bundles. In New York, ob.gyn. leaders such as Dr. Mary D’Alton are working with more than 1,000 health care providers to implement these safety bundles in 118 hospitals throughout the state.
In California, under the leadership of Dr. Elliott Main and other clinical and public health leaders, and in partnership with California Department of Public Health, the maternal mortality ratio decreased from a high of 16.9 in 2006 to 6.2 deaths per 100,000 live births in 2012.
If we are going to move the needle on maternal mortality and severe morbidity in this country, we are going to need an ob.gyn. champion in every hospital and every state. To learn more about the campaign and to find out how you can help, please visit the Council’s website at safehealthcareforeverywoman.org and click on the AIM Program.
Dr. Lu is the director of the Maternal and Child Health Bureau at the Health Resources and Services Administration, and an associate professor of obstetrics, gynecology, and public health at the University of California, Los Angeles.
The United States now ranks 60th in the world, and worst among developed nations, in maternal mortality. Each year more than 600 women in the United States die from pregnancy and childbirth, and more than 50,000 suffer a life-threatening complication (“severe maternal morbidity”).
The maternal mortality ratio doubled between 1987 and 2011, from 7.2 to 17.8 deaths per 100,000 live births; severe maternal morbidity doubled between 1998 and 2011, from 74 to 163 per 10,000 delivery hospitalizations. There continues to be large and persistent disparities; for example, a black woman is more than three times as likely to die from pregnancy and childbirth as a white woman, a gap we haven’t been able to close in decades.
The Maternal and Child Health Bureau is partnering with the American College of Obstetricians and Gynecologists and the Council on Patient Safety in Women’s Health Care (“the Council”) in launching a new national campaign to reduce maternal mortality and severe morbidity in the United States. The goal of the campaign is to prevent 100,000 maternal deaths or severe morbidities in the next 5 years by doing three things:
• Improving women’s health before pregnancy, with a focus on promoting preventive services including preconception, interconception, and postpartum care.
• Reducing primary cesarean deliveries, with an immediate focus on developing an evidence-based patient safety bundle to reduce low-risk, nulliparous, term, spontaneous, and vertex (NTSV) cesarean deliveries.
• Improving the quality and safety of maternity care, with a focus on implementing patient safety bundles to reduce mortality and morbidity associated with hemorrhage, preeclampsia, and thromboembolism in every birthing hospital and facility across the country in the next 3 years.
Safety bundles are small, straightforward sets of evidence-based practices that, when performed collectively and reliably, have improved patient outcomes. Several states have begun to pilot these safety bundles. In New York, ob.gyn. leaders such as Dr. Mary D’Alton are working with more than 1,000 health care providers to implement these safety bundles in 118 hospitals throughout the state.
In California, under the leadership of Dr. Elliott Main and other clinical and public health leaders, and in partnership with California Department of Public Health, the maternal mortality ratio decreased from a high of 16.9 in 2006 to 6.2 deaths per 100,000 live births in 2012.
If we are going to move the needle on maternal mortality and severe morbidity in this country, we are going to need an ob.gyn. champion in every hospital and every state. To learn more about the campaign and to find out how you can help, please visit the Council’s website at safehealthcareforeverywoman.org and click on the AIM Program.
Dr. Lu is the director of the Maternal and Child Health Bureau at the Health Resources and Services Administration, and an associate professor of obstetrics, gynecology, and public health at the University of California, Los Angeles.
Beyond myalgic encephalomyelitis/chronic fatigue syndrome – redefining an illness
According to the Institute of Medicine, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) affects 836,000 to 2.5 million Americans. ME/CFS is a disease that is characterized by profound fatigue, cognitive dysfunctions, sleep abnormalities, autonomic manifestations, pain, and other symptoms, all of which are made worse by any exertion. The Institute of Medicine (IOM) created a report developed by an expert committee to help redefine the illness and proposed new diagnostic criteria that will help medical professionals understand the illness and accurately diagnose and manage patients with this often-misunderstood disease. The IOM committee also recommended that it be renamed Systemic Exertion Intolerance Disease (SEID) to reflect the main characteristics of the disease.
Background
The true prevalence of MF/CSF is unknown because an estimated 84%-91% of affected people have not been diagnosed, and its cause is unknown; however, symptoms may be triggered by certain infections such as Epstein-Barr Virus. MF/CFS is a disease that is more common in women than men, with an average age of onset at 33 years. At some point in the course of this illness, one quarter of affected patients have been bed- or house bound. As explained by the IOM report, most patients have symptoms for years and never regain their predisease functioning level. There is no cure; however, there are interventions and therapies that are helpful in managing symptoms.
Because of the patients’ loss of functioning, high medical costs accrue that add to the overall annual economic burden of $17 billion to $24 billion.
Diagnostic criteria
The following three symptoms must be present to make the diagnosis as stated in the IOM report:
At least one of the two following manifestations also is required:
Key considerations
Diagnosing ME/CFS can be challenging. The health professional should diagnose only after a full history, physical, medical work-up, referrals to appropriate specialists to help rule out other potential disorders, and, ultimately, fulfillment of the diagnostic criteria. The severity and frequency of a patient’s symptoms over the past month and beyond should be assessed by the health professional to determine if the symptoms meet the diagnostic criteria of being chronic, moderate to severe, and frequent. An important distinguishing feature of ME/CFS is that the patient needs to have been symptomatic for 6 months, because most other causes of fatigue do not last that long. Fibromyalgia and irritable bowel syndrome are common comorbidities found in patients with ME/CFS.
Core symptoms
Fatigue and impairment. ME/CFS causes a profound fatigue that does not improve a lot by rest and is not associated with excessive exertion. This type of fatigue makes a substantial impact in decreasing a patient’s functioning and impairing the ability to return to a pre-illness state within occupational, educational, social or personal activities. The impairment secondary to fatigue must persist for at least 6 months.
Postexertional malaise. This symptom is unique to ME/CFS and was described as the primary feature. Physical or cognitive stressors that were previously tolerated now produce worsening symptoms and functioning.
Unrefreshing sleep. There was no subjective evidence of sleep disorders due to ME/CFS, but sufficient data did show that unrefreshing sleep was a complaint universally among ME/CFS patients. The IOM recommends that while polysomnography is not a requirement to diagnose ME/CFS, it is encouraged, to rule out other primary sleep disorders.
Cognitive impairment. Increased stress, effort, or time pressure all can exacerbate existing problems that a patient with ME/CFS has with thinking or executive functioning. Evidence has shown that patients with ME/CFS have slowed information processing and this may be a central aspect of these patients’ overall neurocognitive impairment.
Orthostatic intolerance. Symptoms have been shown to worsen with an upright posture according to objective measures such as orthostatic vital signs and head-up tilt testing, and symptoms improve with rest and leg elevation.
Additional symptoms
Additional common manifestations that were found present in ME/CFS are pain, immune impairment, and infection. Pain was prevalent in more severe cases and manifested as headaches, arthralgia, and myalgia, among others. The pain that these patients experience was indistinguishable from pain experienced in other disease states and healthy people. Immune impairment was evident in people with ME/CFS, in that there were data demonstrating poor NK cell cytotoxicity function. The severity of the illness correlated to the degree of immune impairment. The function of this NK cell was proposed to potentially be a biomarker for the severity of ME/CSF, although not specific to the disease. Finally, there was evidence that ME/CFS can often follow an infection with Epstein Barr Virus (EBV).
The Bottom line
ME/CFS (SEID) is a serious disease that affects many Americans and impacts their lives in cognitive, emotional, physical, and economic realms. The IOM has described a clear diagnostic algorithm for patients presenting with profound fatigue. There are tools that can be found within the report that help to assess the quality, severity, and frequency of the core symptoms. Further research is needed to determine what causes this SEID, what factors affect its course, and what therapies work for which patients.
References
IOM (Institute of Medicine). Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington, DC: The National Academies Press; 2015. http://www.iom.edu/mecfs.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Baranck Drumond is a chief resident in the family medicine residency program at Abington Memorial Hospital and is going into practice at a Federally Qualified Health Center, The Community Health Center of Cape Cod in Mashpee, Mass.
According to the Institute of Medicine, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) affects 836,000 to 2.5 million Americans. ME/CFS is a disease that is characterized by profound fatigue, cognitive dysfunctions, sleep abnormalities, autonomic manifestations, pain, and other symptoms, all of which are made worse by any exertion. The Institute of Medicine (IOM) created a report developed by an expert committee to help redefine the illness and proposed new diagnostic criteria that will help medical professionals understand the illness and accurately diagnose and manage patients with this often-misunderstood disease. The IOM committee also recommended that it be renamed Systemic Exertion Intolerance Disease (SEID) to reflect the main characteristics of the disease.
Background
The true prevalence of MF/CSF is unknown because an estimated 84%-91% of affected people have not been diagnosed, and its cause is unknown; however, symptoms may be triggered by certain infections such as Epstein-Barr Virus. MF/CFS is a disease that is more common in women than men, with an average age of onset at 33 years. At some point in the course of this illness, one quarter of affected patients have been bed- or house bound. As explained by the IOM report, most patients have symptoms for years and never regain their predisease functioning level. There is no cure; however, there are interventions and therapies that are helpful in managing symptoms.
Because of the patients’ loss of functioning, high medical costs accrue that add to the overall annual economic burden of $17 billion to $24 billion.
Diagnostic criteria
The following three symptoms must be present to make the diagnosis as stated in the IOM report:
At least one of the two following manifestations also is required:
Key considerations
Diagnosing ME/CFS can be challenging. The health professional should diagnose only after a full history, physical, medical work-up, referrals to appropriate specialists to help rule out other potential disorders, and, ultimately, fulfillment of the diagnostic criteria. The severity and frequency of a patient’s symptoms over the past month and beyond should be assessed by the health professional to determine if the symptoms meet the diagnostic criteria of being chronic, moderate to severe, and frequent. An important distinguishing feature of ME/CFS is that the patient needs to have been symptomatic for 6 months, because most other causes of fatigue do not last that long. Fibromyalgia and irritable bowel syndrome are common comorbidities found in patients with ME/CFS.
Core symptoms
Fatigue and impairment. ME/CFS causes a profound fatigue that does not improve a lot by rest and is not associated with excessive exertion. This type of fatigue makes a substantial impact in decreasing a patient’s functioning and impairing the ability to return to a pre-illness state within occupational, educational, social or personal activities. The impairment secondary to fatigue must persist for at least 6 months.
Postexertional malaise. This symptom is unique to ME/CFS and was described as the primary feature. Physical or cognitive stressors that were previously tolerated now produce worsening symptoms and functioning.
Unrefreshing sleep. There was no subjective evidence of sleep disorders due to ME/CFS, but sufficient data did show that unrefreshing sleep was a complaint universally among ME/CFS patients. The IOM recommends that while polysomnography is not a requirement to diagnose ME/CFS, it is encouraged, to rule out other primary sleep disorders.
Cognitive impairment. Increased stress, effort, or time pressure all can exacerbate existing problems that a patient with ME/CFS has with thinking or executive functioning. Evidence has shown that patients with ME/CFS have slowed information processing and this may be a central aspect of these patients’ overall neurocognitive impairment.
Orthostatic intolerance. Symptoms have been shown to worsen with an upright posture according to objective measures such as orthostatic vital signs and head-up tilt testing, and symptoms improve with rest and leg elevation.
Additional symptoms
Additional common manifestations that were found present in ME/CFS are pain, immune impairment, and infection. Pain was prevalent in more severe cases and manifested as headaches, arthralgia, and myalgia, among others. The pain that these patients experience was indistinguishable from pain experienced in other disease states and healthy people. Immune impairment was evident in people with ME/CFS, in that there were data demonstrating poor NK cell cytotoxicity function. The severity of the illness correlated to the degree of immune impairment. The function of this NK cell was proposed to potentially be a biomarker for the severity of ME/CSF, although not specific to the disease. Finally, there was evidence that ME/CFS can often follow an infection with Epstein Barr Virus (EBV).
The Bottom line
ME/CFS (SEID) is a serious disease that affects many Americans and impacts their lives in cognitive, emotional, physical, and economic realms. The IOM has described a clear diagnostic algorithm for patients presenting with profound fatigue. There are tools that can be found within the report that help to assess the quality, severity, and frequency of the core symptoms. Further research is needed to determine what causes this SEID, what factors affect its course, and what therapies work for which patients.
References
IOM (Institute of Medicine). Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington, DC: The National Academies Press; 2015. http://www.iom.edu/mecfs.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Baranck Drumond is a chief resident in the family medicine residency program at Abington Memorial Hospital and is going into practice at a Federally Qualified Health Center, The Community Health Center of Cape Cod in Mashpee, Mass.
According to the Institute of Medicine, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) affects 836,000 to 2.5 million Americans. ME/CFS is a disease that is characterized by profound fatigue, cognitive dysfunctions, sleep abnormalities, autonomic manifestations, pain, and other symptoms, all of which are made worse by any exertion. The Institute of Medicine (IOM) created a report developed by an expert committee to help redefine the illness and proposed new diagnostic criteria that will help medical professionals understand the illness and accurately diagnose and manage patients with this often-misunderstood disease. The IOM committee also recommended that it be renamed Systemic Exertion Intolerance Disease (SEID) to reflect the main characteristics of the disease.
Background
The true prevalence of MF/CSF is unknown because an estimated 84%-91% of affected people have not been diagnosed, and its cause is unknown; however, symptoms may be triggered by certain infections such as Epstein-Barr Virus. MF/CFS is a disease that is more common in women than men, with an average age of onset at 33 years. At some point in the course of this illness, one quarter of affected patients have been bed- or house bound. As explained by the IOM report, most patients have symptoms for years and never regain their predisease functioning level. There is no cure; however, there are interventions and therapies that are helpful in managing symptoms.
Because of the patients’ loss of functioning, high medical costs accrue that add to the overall annual economic burden of $17 billion to $24 billion.
Diagnostic criteria
The following three symptoms must be present to make the diagnosis as stated in the IOM report:
At least one of the two following manifestations also is required:
Key considerations
Diagnosing ME/CFS can be challenging. The health professional should diagnose only after a full history, physical, medical work-up, referrals to appropriate specialists to help rule out other potential disorders, and, ultimately, fulfillment of the diagnostic criteria. The severity and frequency of a patient’s symptoms over the past month and beyond should be assessed by the health professional to determine if the symptoms meet the diagnostic criteria of being chronic, moderate to severe, and frequent. An important distinguishing feature of ME/CFS is that the patient needs to have been symptomatic for 6 months, because most other causes of fatigue do not last that long. Fibromyalgia and irritable bowel syndrome are common comorbidities found in patients with ME/CFS.
Core symptoms
Fatigue and impairment. ME/CFS causes a profound fatigue that does not improve a lot by rest and is not associated with excessive exertion. This type of fatigue makes a substantial impact in decreasing a patient’s functioning and impairing the ability to return to a pre-illness state within occupational, educational, social or personal activities. The impairment secondary to fatigue must persist for at least 6 months.
Postexertional malaise. This symptom is unique to ME/CFS and was described as the primary feature. Physical or cognitive stressors that were previously tolerated now produce worsening symptoms and functioning.
Unrefreshing sleep. There was no subjective evidence of sleep disorders due to ME/CFS, but sufficient data did show that unrefreshing sleep was a complaint universally among ME/CFS patients. The IOM recommends that while polysomnography is not a requirement to diagnose ME/CFS, it is encouraged, to rule out other primary sleep disorders.
Cognitive impairment. Increased stress, effort, or time pressure all can exacerbate existing problems that a patient with ME/CFS has with thinking or executive functioning. Evidence has shown that patients with ME/CFS have slowed information processing and this may be a central aspect of these patients’ overall neurocognitive impairment.
Orthostatic intolerance. Symptoms have been shown to worsen with an upright posture according to objective measures such as orthostatic vital signs and head-up tilt testing, and symptoms improve with rest and leg elevation.
Additional symptoms
Additional common manifestations that were found present in ME/CFS are pain, immune impairment, and infection. Pain was prevalent in more severe cases and manifested as headaches, arthralgia, and myalgia, among others. The pain that these patients experience was indistinguishable from pain experienced in other disease states and healthy people. Immune impairment was evident in people with ME/CFS, in that there were data demonstrating poor NK cell cytotoxicity function. The severity of the illness correlated to the degree of immune impairment. The function of this NK cell was proposed to potentially be a biomarker for the severity of ME/CSF, although not specific to the disease. Finally, there was evidence that ME/CFS can often follow an infection with Epstein Barr Virus (EBV).
The Bottom line
ME/CFS (SEID) is a serious disease that affects many Americans and impacts their lives in cognitive, emotional, physical, and economic realms. The IOM has described a clear diagnostic algorithm for patients presenting with profound fatigue. There are tools that can be found within the report that help to assess the quality, severity, and frequency of the core symptoms. Further research is needed to determine what causes this SEID, what factors affect its course, and what therapies work for which patients.
References
IOM (Institute of Medicine). Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington, DC: The National Academies Press; 2015. http://www.iom.edu/mecfs.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Baranck Drumond is a chief resident in the family medicine residency program at Abington Memorial Hospital and is going into practice at a Federally Qualified Health Center, The Community Health Center of Cape Cod in Mashpee, Mass.
Food recalls highlight risk of listeriosis
Recently, after a long day at the hospital, I stopped at the grocery store to pick up something for a quick dinner. I drifted to the frozen food case in the organic food section, but pulled up short when I saw empty shelves. A paper sign announced that Amy’s Kitchen, a manufacturer of organic and natural frozen foods, had become the latest company to recall its products because of concern about Listeria monocytogenes contamination.
According to information posted on the Food and Drug Administration website, this facultative, anaerobic gram-positive bacillus has been the impetus behind 10 national recalls of food products between April 1 and May 8, 2015 alone. Implicated food products have ranged from gourmet ice cream to soybean sprouts to frozen vegetables. Unlike some other bacterial causes of food-borne illness, Listeria organisms can thrive at cold temperatures. Historically, outbreaks of disease have been linked to a variety of foods, including raw produce, contaminated ready-to-eat foods such as deli meats and prepared salads, and unpasteurized milk and milk products.
Clinical manifestations of listeriosis range from febrile gastroenteritis to bacteremia and meningitis, with severe disease seen primarily in immunocompromised individuals and adults 65 and older.
Pregnant women are especially susceptible, with incidence rates 13 times higher than in the general population. Probably as a result of food choices, Hispanic women are disproportionately affected, with rates up to 24 times higher. Maternal infection may be asymptomatic or may manifest with flulike symptoms that include fever, myalgias, headache, and backache, with or without a preceding diarrhea illness. Even mild maternal illness may result in adverse pregnancy outcomes such as fetal loss, premature labor, and severe neonatal infection.
While medical students and residents are still taught to think of Listeria infection as one of the “big three” causes of neonatal sepsis along with group B streptococcus and Escherichia coli, many pediatricians have never seen a case of this rare, but potentially devastating disease. As with group B streptococcus, both early-onset and late-onset disease occur. Sepsis is the most common presentation of disease in the first week of life, while meningitis predominates in late-onset disease. Pneumonia and myocarditis are occasionally seen. Granulomatosis infantisepticum is an uncommon manifestation of severe, disseminated Listeria infection. Granuloma can occur in nearly every organ, although involvement of the liver and skin is most common.
In 2002, investigators from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists surveyed more than 400 pregnant women from across the United States about their knowledge of the transmission, risk factors, symptoms, and prevention of listeriosis (Infect. Dis. Obstet. Gyn. 2005;13:11-15). A year later, the Minnesota Department of Health surveyed an additional 286 pregnant women from their state using the same survey instrument.
More than 80% of survey respondents had never heard of the disease, and knowledge about prevention strategies was therefore predictably limited. Only 33% of respondents in the national survey and 17% of respondents in the Minnesota survey knew, for example, that infection could be prevented by avoiding delicatessen meats and soft cheeses. Investigators concluded that “timely and appropriate education” of pregnant women about listeriosis could reduce cases of perinatal infection.
Data from the CDC suggest we have more work to do. The Listeria Initiative is an enhanced national surveillance system that collects laboratory, clinical, and food exposure data about listeriosis cases in the United States. Between 2009 and 2011, 14% of the 1,651 invasive Listeria infections reported were classified as pregnancy associated. Morbidity and mortality were significant, with 40 fetal losses and 6 neonatal deaths (MMWR 2013;62:448-52).
The CDC offers some common sense tips for preventing listeriosis and other food-borne illness. Raw fruits and vegetables should be thoroughly rinsed with tap water and dried with a clean cloth or paper towel before being eaten or cooked. Even foods that are typically peeled first should be washed, and firm produce, such as cantaloupe, should be scrubbed with a produce brush to reduce surface contamination. Uncooked meats and poultry should never come in contact with other food. Hands, knives, cutting boards, and other food preparation surfaces should be washed thoroughly after uncooked food is handled.
Pregnant women and others at increased risk for listeriosis should not eat hot dogs or deli meats unless they are cooked to steaming. Soft cheeses, including feta, brie, Camembert, queso blanco, or anything blue veined, should be avoided unless the label clearly states that the product has been made with pasteurized milk. Even then, it might not be safe. Pasteurized Mexican-style cheeses, such as queso fresco, have been linked to Listeria infections, likely as a result of contamination during the cheese-making process.
Physicians should be prepared to field calls from concerned parents who believe their children may have consumed a product potentially contaminated with Listeria. In general, someone who has eaten a recalled food product but has no symptoms doesn’t need a laboratory evaluation or treatment. Screening blood cultures is not indicated, and routine tests such as a complete blood count are unlikely to be helpful. Instead, patients should be counseled about the symptoms of listeriosis and undergo prompt evaluation if any develop within 2 months of exposure. The typical interval between exposure and the development of symptoms is 1 day to 3 weeks, but may be as long as 70 days.
Although Listeria infection may result in gastrointestinal symptoms, stool cultures are not recommended for diagnosis. According to the CDC, ingestion of food contaminated with Listeria occurs frequently because the organisms are commonly found in the environment. Although uncommon, intermittent fecal carriage and shedding have been observed in asymptomatic individuals.
Back at the grocery, I sighed and resigned myself to a grilled cheese sandwich for dinner. I turned and saw another woman in the aisle stop and read the sign on the freezer case.
“It’s a little scary,” she said with a sigh. “It seems like there is another recall every week, and I’m wondering what’s safe to eat.”
The parents of our patients may have similar questions. Although the Food and Drug Administration offers detailed guidance for food manufacturers about reducing Listeria contamination, perfect compliance wouldn’t eliminate the risk for consumers because L. monocytogenes is widespread in the environment. The organisms are found in water, soil, sewage, and decaying vegetation, and can be isolated from a variety of animals. Fresh fruits and vegetables are “healthy” choices as long as they are handled and prepared appropriately. Conversely, unpasteurized milk and milk products can never be considered safe.
That’s food for thought.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Recently, after a long day at the hospital, I stopped at the grocery store to pick up something for a quick dinner. I drifted to the frozen food case in the organic food section, but pulled up short when I saw empty shelves. A paper sign announced that Amy’s Kitchen, a manufacturer of organic and natural frozen foods, had become the latest company to recall its products because of concern about Listeria monocytogenes contamination.
According to information posted on the Food and Drug Administration website, this facultative, anaerobic gram-positive bacillus has been the impetus behind 10 national recalls of food products between April 1 and May 8, 2015 alone. Implicated food products have ranged from gourmet ice cream to soybean sprouts to frozen vegetables. Unlike some other bacterial causes of food-borne illness, Listeria organisms can thrive at cold temperatures. Historically, outbreaks of disease have been linked to a variety of foods, including raw produce, contaminated ready-to-eat foods such as deli meats and prepared salads, and unpasteurized milk and milk products.
Clinical manifestations of listeriosis range from febrile gastroenteritis to bacteremia and meningitis, with severe disease seen primarily in immunocompromised individuals and adults 65 and older.
Pregnant women are especially susceptible, with incidence rates 13 times higher than in the general population. Probably as a result of food choices, Hispanic women are disproportionately affected, with rates up to 24 times higher. Maternal infection may be asymptomatic or may manifest with flulike symptoms that include fever, myalgias, headache, and backache, with or without a preceding diarrhea illness. Even mild maternal illness may result in adverse pregnancy outcomes such as fetal loss, premature labor, and severe neonatal infection.
While medical students and residents are still taught to think of Listeria infection as one of the “big three” causes of neonatal sepsis along with group B streptococcus and Escherichia coli, many pediatricians have never seen a case of this rare, but potentially devastating disease. As with group B streptococcus, both early-onset and late-onset disease occur. Sepsis is the most common presentation of disease in the first week of life, while meningitis predominates in late-onset disease. Pneumonia and myocarditis are occasionally seen. Granulomatosis infantisepticum is an uncommon manifestation of severe, disseminated Listeria infection. Granuloma can occur in nearly every organ, although involvement of the liver and skin is most common.
In 2002, investigators from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists surveyed more than 400 pregnant women from across the United States about their knowledge of the transmission, risk factors, symptoms, and prevention of listeriosis (Infect. Dis. Obstet. Gyn. 2005;13:11-15). A year later, the Minnesota Department of Health surveyed an additional 286 pregnant women from their state using the same survey instrument.
More than 80% of survey respondents had never heard of the disease, and knowledge about prevention strategies was therefore predictably limited. Only 33% of respondents in the national survey and 17% of respondents in the Minnesota survey knew, for example, that infection could be prevented by avoiding delicatessen meats and soft cheeses. Investigators concluded that “timely and appropriate education” of pregnant women about listeriosis could reduce cases of perinatal infection.
Data from the CDC suggest we have more work to do. The Listeria Initiative is an enhanced national surveillance system that collects laboratory, clinical, and food exposure data about listeriosis cases in the United States. Between 2009 and 2011, 14% of the 1,651 invasive Listeria infections reported were classified as pregnancy associated. Morbidity and mortality were significant, with 40 fetal losses and 6 neonatal deaths (MMWR 2013;62:448-52).
The CDC offers some common sense tips for preventing listeriosis and other food-borne illness. Raw fruits and vegetables should be thoroughly rinsed with tap water and dried with a clean cloth or paper towel before being eaten or cooked. Even foods that are typically peeled first should be washed, and firm produce, such as cantaloupe, should be scrubbed with a produce brush to reduce surface contamination. Uncooked meats and poultry should never come in contact with other food. Hands, knives, cutting boards, and other food preparation surfaces should be washed thoroughly after uncooked food is handled.
Pregnant women and others at increased risk for listeriosis should not eat hot dogs or deli meats unless they are cooked to steaming. Soft cheeses, including feta, brie, Camembert, queso blanco, or anything blue veined, should be avoided unless the label clearly states that the product has been made with pasteurized milk. Even then, it might not be safe. Pasteurized Mexican-style cheeses, such as queso fresco, have been linked to Listeria infections, likely as a result of contamination during the cheese-making process.
Physicians should be prepared to field calls from concerned parents who believe their children may have consumed a product potentially contaminated with Listeria. In general, someone who has eaten a recalled food product but has no symptoms doesn’t need a laboratory evaluation or treatment. Screening blood cultures is not indicated, and routine tests such as a complete blood count are unlikely to be helpful. Instead, patients should be counseled about the symptoms of listeriosis and undergo prompt evaluation if any develop within 2 months of exposure. The typical interval between exposure and the development of symptoms is 1 day to 3 weeks, but may be as long as 70 days.
Although Listeria infection may result in gastrointestinal symptoms, stool cultures are not recommended for diagnosis. According to the CDC, ingestion of food contaminated with Listeria occurs frequently because the organisms are commonly found in the environment. Although uncommon, intermittent fecal carriage and shedding have been observed in asymptomatic individuals.
Back at the grocery, I sighed and resigned myself to a grilled cheese sandwich for dinner. I turned and saw another woman in the aisle stop and read the sign on the freezer case.
“It’s a little scary,” she said with a sigh. “It seems like there is another recall every week, and I’m wondering what’s safe to eat.”
The parents of our patients may have similar questions. Although the Food and Drug Administration offers detailed guidance for food manufacturers about reducing Listeria contamination, perfect compliance wouldn’t eliminate the risk for consumers because L. monocytogenes is widespread in the environment. The organisms are found in water, soil, sewage, and decaying vegetation, and can be isolated from a variety of animals. Fresh fruits and vegetables are “healthy” choices as long as they are handled and prepared appropriately. Conversely, unpasteurized milk and milk products can never be considered safe.
That’s food for thought.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Recently, after a long day at the hospital, I stopped at the grocery store to pick up something for a quick dinner. I drifted to the frozen food case in the organic food section, but pulled up short when I saw empty shelves. A paper sign announced that Amy’s Kitchen, a manufacturer of organic and natural frozen foods, had become the latest company to recall its products because of concern about Listeria monocytogenes contamination.
According to information posted on the Food and Drug Administration website, this facultative, anaerobic gram-positive bacillus has been the impetus behind 10 national recalls of food products between April 1 and May 8, 2015 alone. Implicated food products have ranged from gourmet ice cream to soybean sprouts to frozen vegetables. Unlike some other bacterial causes of food-borne illness, Listeria organisms can thrive at cold temperatures. Historically, outbreaks of disease have been linked to a variety of foods, including raw produce, contaminated ready-to-eat foods such as deli meats and prepared salads, and unpasteurized milk and milk products.
Clinical manifestations of listeriosis range from febrile gastroenteritis to bacteremia and meningitis, with severe disease seen primarily in immunocompromised individuals and adults 65 and older.
Pregnant women are especially susceptible, with incidence rates 13 times higher than in the general population. Probably as a result of food choices, Hispanic women are disproportionately affected, with rates up to 24 times higher. Maternal infection may be asymptomatic or may manifest with flulike symptoms that include fever, myalgias, headache, and backache, with or without a preceding diarrhea illness. Even mild maternal illness may result in adverse pregnancy outcomes such as fetal loss, premature labor, and severe neonatal infection.
While medical students and residents are still taught to think of Listeria infection as one of the “big three” causes of neonatal sepsis along with group B streptococcus and Escherichia coli, many pediatricians have never seen a case of this rare, but potentially devastating disease. As with group B streptococcus, both early-onset and late-onset disease occur. Sepsis is the most common presentation of disease in the first week of life, while meningitis predominates in late-onset disease. Pneumonia and myocarditis are occasionally seen. Granulomatosis infantisepticum is an uncommon manifestation of severe, disseminated Listeria infection. Granuloma can occur in nearly every organ, although involvement of the liver and skin is most common.
In 2002, investigators from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists surveyed more than 400 pregnant women from across the United States about their knowledge of the transmission, risk factors, symptoms, and prevention of listeriosis (Infect. Dis. Obstet. Gyn. 2005;13:11-15). A year later, the Minnesota Department of Health surveyed an additional 286 pregnant women from their state using the same survey instrument.
More than 80% of survey respondents had never heard of the disease, and knowledge about prevention strategies was therefore predictably limited. Only 33% of respondents in the national survey and 17% of respondents in the Minnesota survey knew, for example, that infection could be prevented by avoiding delicatessen meats and soft cheeses. Investigators concluded that “timely and appropriate education” of pregnant women about listeriosis could reduce cases of perinatal infection.
Data from the CDC suggest we have more work to do. The Listeria Initiative is an enhanced national surveillance system that collects laboratory, clinical, and food exposure data about listeriosis cases in the United States. Between 2009 and 2011, 14% of the 1,651 invasive Listeria infections reported were classified as pregnancy associated. Morbidity and mortality were significant, with 40 fetal losses and 6 neonatal deaths (MMWR 2013;62:448-52).
The CDC offers some common sense tips for preventing listeriosis and other food-borne illness. Raw fruits and vegetables should be thoroughly rinsed with tap water and dried with a clean cloth or paper towel before being eaten or cooked. Even foods that are typically peeled first should be washed, and firm produce, such as cantaloupe, should be scrubbed with a produce brush to reduce surface contamination. Uncooked meats and poultry should never come in contact with other food. Hands, knives, cutting boards, and other food preparation surfaces should be washed thoroughly after uncooked food is handled.
Pregnant women and others at increased risk for listeriosis should not eat hot dogs or deli meats unless they are cooked to steaming. Soft cheeses, including feta, brie, Camembert, queso blanco, or anything blue veined, should be avoided unless the label clearly states that the product has been made with pasteurized milk. Even then, it might not be safe. Pasteurized Mexican-style cheeses, such as queso fresco, have been linked to Listeria infections, likely as a result of contamination during the cheese-making process.
Physicians should be prepared to field calls from concerned parents who believe their children may have consumed a product potentially contaminated with Listeria. In general, someone who has eaten a recalled food product but has no symptoms doesn’t need a laboratory evaluation or treatment. Screening blood cultures is not indicated, and routine tests such as a complete blood count are unlikely to be helpful. Instead, patients should be counseled about the symptoms of listeriosis and undergo prompt evaluation if any develop within 2 months of exposure. The typical interval between exposure and the development of symptoms is 1 day to 3 weeks, but may be as long as 70 days.
Although Listeria infection may result in gastrointestinal symptoms, stool cultures are not recommended for diagnosis. According to the CDC, ingestion of food contaminated with Listeria occurs frequently because the organisms are commonly found in the environment. Although uncommon, intermittent fecal carriage and shedding have been observed in asymptomatic individuals.
Back at the grocery, I sighed and resigned myself to a grilled cheese sandwich for dinner. I turned and saw another woman in the aisle stop and read the sign on the freezer case.
“It’s a little scary,” she said with a sigh. “It seems like there is another recall every week, and I’m wondering what’s safe to eat.”
The parents of our patients may have similar questions. Although the Food and Drug Administration offers detailed guidance for food manufacturers about reducing Listeria contamination, perfect compliance wouldn’t eliminate the risk for consumers because L. monocytogenes is widespread in the environment. The organisms are found in water, soil, sewage, and decaying vegetation, and can be isolated from a variety of animals. Fresh fruits and vegetables are “healthy” choices as long as they are handled and prepared appropriately. Conversely, unpasteurized milk and milk products can never be considered safe.
That’s food for thought.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Are birthing centers a safe alternative for women?
Yes: Time to design seamless care
Since the first freestanding birth center opened in the mid-1970s, a demonstration model, designed by Maternity Center Association in New York, the evidence has consistently shown quality and safe care for the mother and newborn in low-risk populations, as well as a reduced cost of care.
The first National Birth Center study published in the New England Journal of Medicine in 1989, as well as the National Birth Center Study II, Outcomes of Care in Birth Centers published in the Journal of Midwifery and Women’s Health in 2013, continue to demonstrate safe outcomes (N. Engl. J. Med. 1989;321:1804-11).
The most recent Birth Center II study collected data from 2007 through 2010 on 15,574 women admitted to 79 midwifery-led freestanding birthing centers (FSBCs) in 33 states. Findings included a rate of 92.8% for normal spontaneous vaginal delivery, 6.1% cesarean delivery, and 84% of women gave birth at the FSBCs, with 12.4% transferred after admission. There were no maternal deaths (J. Midwifery. Womens. Health. 2013;58:3-14).
The most common reason for nonemergent maternal transfer to hospital was prolonged or arrested labor, request for pharmacologic pain relief, and maternal exhaustion. For emergent transfer, the primary reason was nonreassuring fetal heart rate pattern (0.5%) and postpartum hemorrhage (0.2%). Newborn outcomes include transfer of 2.6% and 14 neonatal deaths (7 occurred before admission to the center). The intrapartum fetal mortality rate for admitted mothers was 0.47/1,000 and neonatal mortality rate 0.40/1,000, which is similar to what the United Kingdom found among low-risk women in hospitals, FSBCs, and home births. The primary reason for newborn transfer was respiratory observation.
In 2011, the American College of Obstetricians and Gynecologists Committee Opinion on Planned Home Birth references that hospitals and birthing centers are the safest setting for birth. This was followed up in 2015 with the consensus document on Levels of Maternal Care by ACOG and the Society for Maternal-Fetal Medicine that acknowledges and addresses care provided at birth centers, including the role of caregivers.
The future focus of the FSBCs should include a push to mandate accreditation in all facilities, and ongoing research related to outcomes of: midwifery-led care, provider types, FSBC licensure and/or accreditation status, and types of collegial partnerships. This debate must move from a polarizing discussion of “either/or” to “both/and,” which would expand interprofessional collaborations and design seamless care for the growing number of women choosing out-of-hospital birth.
Dr. Breedlove is president of the American College of Nurse-Midwives.
No: Hospitals are still the safest choice
According to analysis of currently available data, and recognizing that there has not been a randomized controlled trial addressing the site of delivery, hospitals are safer than freestanding birthing centers.
How much safer is a point of contention and depends on several key factors: 1. whether the birthing center is accredited and staffed by certified nurse midwives; 2. how far from the nearest hospital with delivery capability the birthing center is located; and 3. the thoroughness of the selection process in identifying low-risk women who are appropriate candidates to deliver in a birthing center.
Even women correctly classified as low risk may require nonemergent cesarean delivery for failure to progress in labor. Since freestanding birthing centers do not provide cesarean delivery, such women must be transferred to a hospital. If the status of both mother and fetus is reassuring, transfer represents just an inconvenience. Overall, the need for transfer arises fairly often, especially in first pregnancies.
However, unexpected emergencies like cord prolapse can and do occur in the course of labor in low-risk women. Hospitals can respond with immediate cesarean delivery, whereas the only recourse for birthing centers is emergency transfer to a hospital.
The idea of a safety net is the No. 1 reason why women choose to deliver in hospitals and that safety net covers not only their newborns, but women themselves. A second reason is the availability of epidural anesthesia for relief of pain in labor.
In answer to the question of whether birthing centers are a safe alternative to hospitals for delivering babies, I would say it depends on women’s tolerance of risk. Adverse outcomes at freestanding birthing centers are uncommon, but some of those that occur could be prevented in a hospital setting.
One compromise may be to locate birthing centers inside hospitals and this model has been successfully implemented in a number of cities across the United States. This suggestion dates back a quarter of a century but is still meritorious today (N. Engl. J. Med. 1989;321:1824-5). Could it be a way for women to have their cake and eat it too?
Dr. Yeomans is the chair of the department of ob.gyn. at Texas Tech University in Lubbock.
Yes: Time to design seamless care
Since the first freestanding birth center opened in the mid-1970s, a demonstration model, designed by Maternity Center Association in New York, the evidence has consistently shown quality and safe care for the mother and newborn in low-risk populations, as well as a reduced cost of care.
The first National Birth Center study published in the New England Journal of Medicine in 1989, as well as the National Birth Center Study II, Outcomes of Care in Birth Centers published in the Journal of Midwifery and Women’s Health in 2013, continue to demonstrate safe outcomes (N. Engl. J. Med. 1989;321:1804-11).
The most recent Birth Center II study collected data from 2007 through 2010 on 15,574 women admitted to 79 midwifery-led freestanding birthing centers (FSBCs) in 33 states. Findings included a rate of 92.8% for normal spontaneous vaginal delivery, 6.1% cesarean delivery, and 84% of women gave birth at the FSBCs, with 12.4% transferred after admission. There were no maternal deaths (J. Midwifery. Womens. Health. 2013;58:3-14).
The most common reason for nonemergent maternal transfer to hospital was prolonged or arrested labor, request for pharmacologic pain relief, and maternal exhaustion. For emergent transfer, the primary reason was nonreassuring fetal heart rate pattern (0.5%) and postpartum hemorrhage (0.2%). Newborn outcomes include transfer of 2.6% and 14 neonatal deaths (7 occurred before admission to the center). The intrapartum fetal mortality rate for admitted mothers was 0.47/1,000 and neonatal mortality rate 0.40/1,000, which is similar to what the United Kingdom found among low-risk women in hospitals, FSBCs, and home births. The primary reason for newborn transfer was respiratory observation.
In 2011, the American College of Obstetricians and Gynecologists Committee Opinion on Planned Home Birth references that hospitals and birthing centers are the safest setting for birth. This was followed up in 2015 with the consensus document on Levels of Maternal Care by ACOG and the Society for Maternal-Fetal Medicine that acknowledges and addresses care provided at birth centers, including the role of caregivers.
The future focus of the FSBCs should include a push to mandate accreditation in all facilities, and ongoing research related to outcomes of: midwifery-led care, provider types, FSBC licensure and/or accreditation status, and types of collegial partnerships. This debate must move from a polarizing discussion of “either/or” to “both/and,” which would expand interprofessional collaborations and design seamless care for the growing number of women choosing out-of-hospital birth.
Dr. Breedlove is president of the American College of Nurse-Midwives.
No: Hospitals are still the safest choice
According to analysis of currently available data, and recognizing that there has not been a randomized controlled trial addressing the site of delivery, hospitals are safer than freestanding birthing centers.
How much safer is a point of contention and depends on several key factors: 1. whether the birthing center is accredited and staffed by certified nurse midwives; 2. how far from the nearest hospital with delivery capability the birthing center is located; and 3. the thoroughness of the selection process in identifying low-risk women who are appropriate candidates to deliver in a birthing center.
Even women correctly classified as low risk may require nonemergent cesarean delivery for failure to progress in labor. Since freestanding birthing centers do not provide cesarean delivery, such women must be transferred to a hospital. If the status of both mother and fetus is reassuring, transfer represents just an inconvenience. Overall, the need for transfer arises fairly often, especially in first pregnancies.
However, unexpected emergencies like cord prolapse can and do occur in the course of labor in low-risk women. Hospitals can respond with immediate cesarean delivery, whereas the only recourse for birthing centers is emergency transfer to a hospital.
The idea of a safety net is the No. 1 reason why women choose to deliver in hospitals and that safety net covers not only their newborns, but women themselves. A second reason is the availability of epidural anesthesia for relief of pain in labor.
In answer to the question of whether birthing centers are a safe alternative to hospitals for delivering babies, I would say it depends on women’s tolerance of risk. Adverse outcomes at freestanding birthing centers are uncommon, but some of those that occur could be prevented in a hospital setting.
One compromise may be to locate birthing centers inside hospitals and this model has been successfully implemented in a number of cities across the United States. This suggestion dates back a quarter of a century but is still meritorious today (N. Engl. J. Med. 1989;321:1824-5). Could it be a way for women to have their cake and eat it too?
Dr. Yeomans is the chair of the department of ob.gyn. at Texas Tech University in Lubbock.
Yes: Time to design seamless care
Since the first freestanding birth center opened in the mid-1970s, a demonstration model, designed by Maternity Center Association in New York, the evidence has consistently shown quality and safe care for the mother and newborn in low-risk populations, as well as a reduced cost of care.
The first National Birth Center study published in the New England Journal of Medicine in 1989, as well as the National Birth Center Study II, Outcomes of Care in Birth Centers published in the Journal of Midwifery and Women’s Health in 2013, continue to demonstrate safe outcomes (N. Engl. J. Med. 1989;321:1804-11).
The most recent Birth Center II study collected data from 2007 through 2010 on 15,574 women admitted to 79 midwifery-led freestanding birthing centers (FSBCs) in 33 states. Findings included a rate of 92.8% for normal spontaneous vaginal delivery, 6.1% cesarean delivery, and 84% of women gave birth at the FSBCs, with 12.4% transferred after admission. There were no maternal deaths (J. Midwifery. Womens. Health. 2013;58:3-14).
The most common reason for nonemergent maternal transfer to hospital was prolonged or arrested labor, request for pharmacologic pain relief, and maternal exhaustion. For emergent transfer, the primary reason was nonreassuring fetal heart rate pattern (0.5%) and postpartum hemorrhage (0.2%). Newborn outcomes include transfer of 2.6% and 14 neonatal deaths (7 occurred before admission to the center). The intrapartum fetal mortality rate for admitted mothers was 0.47/1,000 and neonatal mortality rate 0.40/1,000, which is similar to what the United Kingdom found among low-risk women in hospitals, FSBCs, and home births. The primary reason for newborn transfer was respiratory observation.
In 2011, the American College of Obstetricians and Gynecologists Committee Opinion on Planned Home Birth references that hospitals and birthing centers are the safest setting for birth. This was followed up in 2015 with the consensus document on Levels of Maternal Care by ACOG and the Society for Maternal-Fetal Medicine that acknowledges and addresses care provided at birth centers, including the role of caregivers.
The future focus of the FSBCs should include a push to mandate accreditation in all facilities, and ongoing research related to outcomes of: midwifery-led care, provider types, FSBC licensure and/or accreditation status, and types of collegial partnerships. This debate must move from a polarizing discussion of “either/or” to “both/and,” which would expand interprofessional collaborations and design seamless care for the growing number of women choosing out-of-hospital birth.
Dr. Breedlove is president of the American College of Nurse-Midwives.
No: Hospitals are still the safest choice
According to analysis of currently available data, and recognizing that there has not been a randomized controlled trial addressing the site of delivery, hospitals are safer than freestanding birthing centers.
How much safer is a point of contention and depends on several key factors: 1. whether the birthing center is accredited and staffed by certified nurse midwives; 2. how far from the nearest hospital with delivery capability the birthing center is located; and 3. the thoroughness of the selection process in identifying low-risk women who are appropriate candidates to deliver in a birthing center.
Even women correctly classified as low risk may require nonemergent cesarean delivery for failure to progress in labor. Since freestanding birthing centers do not provide cesarean delivery, such women must be transferred to a hospital. If the status of both mother and fetus is reassuring, transfer represents just an inconvenience. Overall, the need for transfer arises fairly often, especially in first pregnancies.
However, unexpected emergencies like cord prolapse can and do occur in the course of labor in low-risk women. Hospitals can respond with immediate cesarean delivery, whereas the only recourse for birthing centers is emergency transfer to a hospital.
The idea of a safety net is the No. 1 reason why women choose to deliver in hospitals and that safety net covers not only their newborns, but women themselves. A second reason is the availability of epidural anesthesia for relief of pain in labor.
In answer to the question of whether birthing centers are a safe alternative to hospitals for delivering babies, I would say it depends on women’s tolerance of risk. Adverse outcomes at freestanding birthing centers are uncommon, but some of those that occur could be prevented in a hospital setting.
One compromise may be to locate birthing centers inside hospitals and this model has been successfully implemented in a number of cities across the United States. This suggestion dates back a quarter of a century but is still meritorious today (N. Engl. J. Med. 1989;321:1824-5). Could it be a way for women to have their cake and eat it too?
Dr. Yeomans is the chair of the department of ob.gyn. at Texas Tech University in Lubbock.
Do unto others...
In his most recent bestseller, Being Mortal, Dr. Atul Gawande has again raised social awareness of the inadequacies of our health care system in assisting patients with end-of-life decisions and care. In his treatise, he laments his own lack of training in medical school and residency regarding what he has emphasized should be a key component of any physician’s education.
To some degree, there has been a greater emphasis on palliative care training in our medical schools since Dr. Gawande graduated two decades or so ago. The subject of physician-patient communication, including those difficult discussions that should occur near the end of life, is now incorporated into most medical school curricula. Additionally, palliative care has become a respected and growing subspecialty within both medicine and surgery.* Despite these improvements, still far too many patients die while receiving futile end-of-life care in our nation’s intensive care units and hospital wards rather than in the comfort of their homes surrounded by loved ones. Although referrals have increased, too few patients are afforded the opportunity to utilize hospice care and, those that do, are often referred too late in the course of their terminal disease to obtain full benefit
Why are we not doing better? Two likely contributors include a physician mindset that only cure is success and death represents failure, and unrealistic expectations of patients as to what modern medicine can accomplish. A more fixable and probably more important factor is the failure of doctors to effectively communicate during these highly stressful circumstances. As emphasized by Gawande and from my own experience, the key to negotiating a sensible path in hopeless, end-of-life situations is frequent, reasonable, and realistic consultation with our patients.
Not only are the conversations usually difficult and demanding, but the choices of whether to pursue treatment or remove life-sustaining efforts are frequently not well-defined. While these challenging clinical scenarios are often painted as black and white in the lay press, any physician or surgeon who has cared for such patients realizes that there is a delicate and precarious balance between providing hope, administering appropriate aggressive treatment, and ensuring patient comfort. In a well-intentioned attempt to leave some remnant of hope, we physicians too frequently paint an unrealistic picture for our patients.
Advance patient directives have been promoted as one means for patients to avoid futile, uncomfortable, and unnecessary care during the last stage of their lives. Though I by no means wish to discourage these often useful legal documents, they should not be entered into naively. For example, aggressive life-sustaining care for a patient with extensive metastatic lung cancer is likely inappropriate. On the other hand, short-term ventilator assistance for an elderly unconscious person recovering from an automobile accident may result in many additional years of enjoyable and productive life. Patients need to understand that all grave clinical situations are not equal and that their advance directives should be flexible enough to cover a variety of circumstances.
It has been well established that most patients and families have selective hearing and understanding. Even when the details of a major operation with a greater likelihood of a negative rather than a positive outcome are carefully and clearly presented using lay language, the potential positive outcomes frequently push the more probable adverse consequences into hidden recesses of the brain. In my experience, the more desperate the situation, the more often it is that the possibility of an unsuccessful outcome will be masked or denied by patients or their family members. Even though in my practice I carefully explained the high probability of eventual recurrence when operating on patients with pancreatic cancer, many of them were surprised and some were even quite indignant when this disappointing consequence developed. In my opinion, the most effective means to avoid such misunderstandings is to always have the patient and/or family relate their comprehension of the just-completed conversation. It is then essential to re-emphasize the important details that they suppressed and pushed to the background from your initial explanation.
In these challenging end-of-life moments, what advice should we offer? One question that should almost never be asked of the patient or his/her representative is: “Would you like everything possible done?” Especially for a family member who may take on considerable guilt by answering in the negative, the response will nearly always be “yes” no matter how unlikely a successful outcome. Rather, I believe that recommending only reasonable options, including and possibly emphasizing the choice of comfort therapy alone despite the certainty of death, is our obligation. We should be cognizant of the fact that the decision made by the patient is often highly dependent on how the alternatives are presented by his/her doctor. After clearly presenting the therapeutic options and their likely consequences, it may be helpful to relate what you would do yourself for a loved one in the same circumstances.
As in many other aspects of our lives, a useful guidepost in these situations is the Golden Rule: “Do unto others as you would have done unto yourself.” Interestingly, most probably based on our intimate exposure to numerous unnecessarily complicated and uncomfortable deaths, there is evidence that we physicians choose to die differently than our patients. In a recent essay, Dr. Ken Murray presented data from the John Hopkins Precursors Study that suggested doctors are less likely than their patients to submit themselves to futile end-of-life care. (Murray K: Doctors really do die differently. Zocalopublicsquare.org; accessed March 29, 2015). The proof he presents is from a survey of graduates of Johns Hopkins School of Medicine between 1948 and 1964. It revealed that 65% of them had written their own advance directives in comparison to 20% for the public at large. In addition, only 10% of the graduates would opt for cardiopulmonary resuscitation if they were comatose, compared with 75% of the general population.
I suspect that most surgeons, desiring a dignified death for themselves, are not surprised by these statistics. Therefore, we owe it to our patients to be as compassionate and thoughtful in managing the last stage of their lives as we have traditionally been trained to do in earlier phases when cure was a realistic expectation.
Dr. Rikkers is Editor in Chief of ACS Surgery News.
*Recognizing the importance of end-of-life issues in a surgeon’s education, in 2012 ACS Surgery News initiated a series of articles on various aspects of palliative care.
In his most recent bestseller, Being Mortal, Dr. Atul Gawande has again raised social awareness of the inadequacies of our health care system in assisting patients with end-of-life decisions and care. In his treatise, he laments his own lack of training in medical school and residency regarding what he has emphasized should be a key component of any physician’s education.
To some degree, there has been a greater emphasis on palliative care training in our medical schools since Dr. Gawande graduated two decades or so ago. The subject of physician-patient communication, including those difficult discussions that should occur near the end of life, is now incorporated into most medical school curricula. Additionally, palliative care has become a respected and growing subspecialty within both medicine and surgery.* Despite these improvements, still far too many patients die while receiving futile end-of-life care in our nation’s intensive care units and hospital wards rather than in the comfort of their homes surrounded by loved ones. Although referrals have increased, too few patients are afforded the opportunity to utilize hospice care and, those that do, are often referred too late in the course of their terminal disease to obtain full benefit
Why are we not doing better? Two likely contributors include a physician mindset that only cure is success and death represents failure, and unrealistic expectations of patients as to what modern medicine can accomplish. A more fixable and probably more important factor is the failure of doctors to effectively communicate during these highly stressful circumstances. As emphasized by Gawande and from my own experience, the key to negotiating a sensible path in hopeless, end-of-life situations is frequent, reasonable, and realistic consultation with our patients.
Not only are the conversations usually difficult and demanding, but the choices of whether to pursue treatment or remove life-sustaining efforts are frequently not well-defined. While these challenging clinical scenarios are often painted as black and white in the lay press, any physician or surgeon who has cared for such patients realizes that there is a delicate and precarious balance between providing hope, administering appropriate aggressive treatment, and ensuring patient comfort. In a well-intentioned attempt to leave some remnant of hope, we physicians too frequently paint an unrealistic picture for our patients.
Advance patient directives have been promoted as one means for patients to avoid futile, uncomfortable, and unnecessary care during the last stage of their lives. Though I by no means wish to discourage these often useful legal documents, they should not be entered into naively. For example, aggressive life-sustaining care for a patient with extensive metastatic lung cancer is likely inappropriate. On the other hand, short-term ventilator assistance for an elderly unconscious person recovering from an automobile accident may result in many additional years of enjoyable and productive life. Patients need to understand that all grave clinical situations are not equal and that their advance directives should be flexible enough to cover a variety of circumstances.
It has been well established that most patients and families have selective hearing and understanding. Even when the details of a major operation with a greater likelihood of a negative rather than a positive outcome are carefully and clearly presented using lay language, the potential positive outcomes frequently push the more probable adverse consequences into hidden recesses of the brain. In my experience, the more desperate the situation, the more often it is that the possibility of an unsuccessful outcome will be masked or denied by patients or their family members. Even though in my practice I carefully explained the high probability of eventual recurrence when operating on patients with pancreatic cancer, many of them were surprised and some were even quite indignant when this disappointing consequence developed. In my opinion, the most effective means to avoid such misunderstandings is to always have the patient and/or family relate their comprehension of the just-completed conversation. It is then essential to re-emphasize the important details that they suppressed and pushed to the background from your initial explanation.
In these challenging end-of-life moments, what advice should we offer? One question that should almost never be asked of the patient or his/her representative is: “Would you like everything possible done?” Especially for a family member who may take on considerable guilt by answering in the negative, the response will nearly always be “yes” no matter how unlikely a successful outcome. Rather, I believe that recommending only reasonable options, including and possibly emphasizing the choice of comfort therapy alone despite the certainty of death, is our obligation. We should be cognizant of the fact that the decision made by the patient is often highly dependent on how the alternatives are presented by his/her doctor. After clearly presenting the therapeutic options and their likely consequences, it may be helpful to relate what you would do yourself for a loved one in the same circumstances.
As in many other aspects of our lives, a useful guidepost in these situations is the Golden Rule: “Do unto others as you would have done unto yourself.” Interestingly, most probably based on our intimate exposure to numerous unnecessarily complicated and uncomfortable deaths, there is evidence that we physicians choose to die differently than our patients. In a recent essay, Dr. Ken Murray presented data from the John Hopkins Precursors Study that suggested doctors are less likely than their patients to submit themselves to futile end-of-life care. (Murray K: Doctors really do die differently. Zocalopublicsquare.org; accessed March 29, 2015). The proof he presents is from a survey of graduates of Johns Hopkins School of Medicine between 1948 and 1964. It revealed that 65% of them had written their own advance directives in comparison to 20% for the public at large. In addition, only 10% of the graduates would opt for cardiopulmonary resuscitation if they were comatose, compared with 75% of the general population.
I suspect that most surgeons, desiring a dignified death for themselves, are not surprised by these statistics. Therefore, we owe it to our patients to be as compassionate and thoughtful in managing the last stage of their lives as we have traditionally been trained to do in earlier phases when cure was a realistic expectation.
Dr. Rikkers is Editor in Chief of ACS Surgery News.
*Recognizing the importance of end-of-life issues in a surgeon’s education, in 2012 ACS Surgery News initiated a series of articles on various aspects of palliative care.
In his most recent bestseller, Being Mortal, Dr. Atul Gawande has again raised social awareness of the inadequacies of our health care system in assisting patients with end-of-life decisions and care. In his treatise, he laments his own lack of training in medical school and residency regarding what he has emphasized should be a key component of any physician’s education.
To some degree, there has been a greater emphasis on palliative care training in our medical schools since Dr. Gawande graduated two decades or so ago. The subject of physician-patient communication, including those difficult discussions that should occur near the end of life, is now incorporated into most medical school curricula. Additionally, palliative care has become a respected and growing subspecialty within both medicine and surgery.* Despite these improvements, still far too many patients die while receiving futile end-of-life care in our nation’s intensive care units and hospital wards rather than in the comfort of their homes surrounded by loved ones. Although referrals have increased, too few patients are afforded the opportunity to utilize hospice care and, those that do, are often referred too late in the course of their terminal disease to obtain full benefit
Why are we not doing better? Two likely contributors include a physician mindset that only cure is success and death represents failure, and unrealistic expectations of patients as to what modern medicine can accomplish. A more fixable and probably more important factor is the failure of doctors to effectively communicate during these highly stressful circumstances. As emphasized by Gawande and from my own experience, the key to negotiating a sensible path in hopeless, end-of-life situations is frequent, reasonable, and realistic consultation with our patients.
Not only are the conversations usually difficult and demanding, but the choices of whether to pursue treatment or remove life-sustaining efforts are frequently not well-defined. While these challenging clinical scenarios are often painted as black and white in the lay press, any physician or surgeon who has cared for such patients realizes that there is a delicate and precarious balance between providing hope, administering appropriate aggressive treatment, and ensuring patient comfort. In a well-intentioned attempt to leave some remnant of hope, we physicians too frequently paint an unrealistic picture for our patients.
Advance patient directives have been promoted as one means for patients to avoid futile, uncomfortable, and unnecessary care during the last stage of their lives. Though I by no means wish to discourage these often useful legal documents, they should not be entered into naively. For example, aggressive life-sustaining care for a patient with extensive metastatic lung cancer is likely inappropriate. On the other hand, short-term ventilator assistance for an elderly unconscious person recovering from an automobile accident may result in many additional years of enjoyable and productive life. Patients need to understand that all grave clinical situations are not equal and that their advance directives should be flexible enough to cover a variety of circumstances.
It has been well established that most patients and families have selective hearing and understanding. Even when the details of a major operation with a greater likelihood of a negative rather than a positive outcome are carefully and clearly presented using lay language, the potential positive outcomes frequently push the more probable adverse consequences into hidden recesses of the brain. In my experience, the more desperate the situation, the more often it is that the possibility of an unsuccessful outcome will be masked or denied by patients or their family members. Even though in my practice I carefully explained the high probability of eventual recurrence when operating on patients with pancreatic cancer, many of them were surprised and some were even quite indignant when this disappointing consequence developed. In my opinion, the most effective means to avoid such misunderstandings is to always have the patient and/or family relate their comprehension of the just-completed conversation. It is then essential to re-emphasize the important details that they suppressed and pushed to the background from your initial explanation.
In these challenging end-of-life moments, what advice should we offer? One question that should almost never be asked of the patient or his/her representative is: “Would you like everything possible done?” Especially for a family member who may take on considerable guilt by answering in the negative, the response will nearly always be “yes” no matter how unlikely a successful outcome. Rather, I believe that recommending only reasonable options, including and possibly emphasizing the choice of comfort therapy alone despite the certainty of death, is our obligation. We should be cognizant of the fact that the decision made by the patient is often highly dependent on how the alternatives are presented by his/her doctor. After clearly presenting the therapeutic options and their likely consequences, it may be helpful to relate what you would do yourself for a loved one in the same circumstances.
As in many other aspects of our lives, a useful guidepost in these situations is the Golden Rule: “Do unto others as you would have done unto yourself.” Interestingly, most probably based on our intimate exposure to numerous unnecessarily complicated and uncomfortable deaths, there is evidence that we physicians choose to die differently than our patients. In a recent essay, Dr. Ken Murray presented data from the John Hopkins Precursors Study that suggested doctors are less likely than their patients to submit themselves to futile end-of-life care. (Murray K: Doctors really do die differently. Zocalopublicsquare.org; accessed March 29, 2015). The proof he presents is from a survey of graduates of Johns Hopkins School of Medicine between 1948 and 1964. It revealed that 65% of them had written their own advance directives in comparison to 20% for the public at large. In addition, only 10% of the graduates would opt for cardiopulmonary resuscitation if they were comatose, compared with 75% of the general population.
I suspect that most surgeons, desiring a dignified death for themselves, are not surprised by these statistics. Therefore, we owe it to our patients to be as compassionate and thoughtful in managing the last stage of their lives as we have traditionally been trained to do in earlier phases when cure was a realistic expectation.
Dr. Rikkers is Editor in Chief of ACS Surgery News.
*Recognizing the importance of end-of-life issues in a surgeon’s education, in 2012 ACS Surgery News initiated a series of articles on various aspects of palliative care.
Managing open wounds in ob.gyn.
Negative pressure wound therapy is a wound management system for chronic open subcutaneous or intra-abdominal wounds. Some popular commercial systems include V.A.C. therapy (KCI, San Antonio) and the Chariker-Jeter wound-sealing kit (Smith and Nephew, London). Within ob.gyn. and gynecologic oncology, they have use in the management of postoperative superficial wound dehiscence from routine surgery and in the management of the open abdomen.
The primary benefit of negative pressure wound therapy (NPWT) is the acceleration of wound healing. Postoperative superficial wound dehiscence can occur as a result of surgical factors such as wound infection and subcutaneous seroma/hematoma or systematic factors such as poor nutrition and wound ischemia.
Acceleration of wound healing results from the design of the NPWT systems. They consist of semipermeable dressings (foam), sealed with an adhesive sheet that is connected to a portable pump. By the application of –50 to –175 mm Hg of continuous or intermittent suction, the edges of the wound are drawn together, and this deforming process promotes tissue remodeling at the cellular level. Other potential benefits of negative pressure are increased blood flow, a decrease in mediators of inflammation, and an increase in collagen organization via changes in wound biochemistry.
An alternative to NPWT would be traditional gauze dressings, which can also be applied in the case of superficial wound dehiscence. These are changed up to three times a day, however, and this can result in significant patient discomfort, caregiver difficulties, and prolonged healing of weeks to months. In contrast, NPWT dressings are changed once every 2-3 days. They are also versatile and can be fit to traditionally shaped abdominal wounds, as well as difficult to dress vulvar and groin wounds (J. Obstet. Gynaecol. Can. 2011;33:1031-7).
In a series of 27 gynecologic oncology patients in whom NPWT was employed after primary wound–healing failure, there was a 96% reduction in the size of the wounds with a median number of therapy days of 32 (range, 3-88). The majority of these patients were also managed as outpatients without complication (Gynecol. Oncol. 2004;92:586-91).
There are some contraindications to NPWT that should be considered. The major, and perhaps most common, is an ongoing wound infection.
A wound that needs to be evaluated at least daily to assess the response to antibiotic therapy or need for debridement should not be managed with NPWT until the wound is deemed stable. There should be no devitalized tissue present in the wound upon application of the NPWT. If any necrotic tissue is present, then wound debridement is warranted until only well-vascularized tissue remains.
Another contraindication is the presence of malignant tissue in the wound. Negative pressure can promote this tissue growth and lead to chronic nonhealing. Other considerations would include adhesive allergies and fragile skin due to chronic steroid use or collagen vascular disorders, as NPWT can lead to skin necrosis.
Finally, the involvement of vital organs, such as exposed bowel, is a contraindication to the NPWT systems, as constant suction can promote fistula formation or hemorrhage. However, in the setting of an open abdomen after trauma surgery, there has been the development of intra-abdominal wound management systems that may be appropriate.
Although rare in obstetrics, gynecology, and gynecologic oncology, delayed abdominal closure may be necessary. This can occur after reoperation for bowel injury, in cases where bowel wall edema and increased intra-abdominal pressure preclude closure, or in cases of massive hemorrhage (for example, ruptured ectopic pregnancy) where patient instability necessitates rapid termination of the surgical case. These wounds can be managed with temporary abdominal closure techniques such as retention sutures, a Bogota bag, or loose packing (World. J. Surg. 2015; 39: 912-25).
The negative pressure systems developed for these instances are the V.A.C. abdominal dressing (KCI), Renasys NPWT (Smith and Nephew), and ABThera open abdomen negative pressure therapy (KCI). They consist of a perforated plastic sheet with foam attachments that is placed directly in the abdomen to cover the intestine. This is then covered with an adhesive dressing that is cut to accommodate the suction attachment for the negative pressure pump. This setup is easily applied and taken down, and therefore facilitates frequent abdominal washouts until true facial closure can be achieved.
There are many benefits to NPWT for the management of superficial and deep wound dehiscence in the ob.gyn. or gynecologic oncology patient. NPWT should be considered primarily with any surgical wound healing by secondary intention.
Dr. Doll is a third-year fellow in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. The authors reported having no relevant financial disclosures.
Negative pressure wound therapy is a wound management system for chronic open subcutaneous or intra-abdominal wounds. Some popular commercial systems include V.A.C. therapy (KCI, San Antonio) and the Chariker-Jeter wound-sealing kit (Smith and Nephew, London). Within ob.gyn. and gynecologic oncology, they have use in the management of postoperative superficial wound dehiscence from routine surgery and in the management of the open abdomen.
The primary benefit of negative pressure wound therapy (NPWT) is the acceleration of wound healing. Postoperative superficial wound dehiscence can occur as a result of surgical factors such as wound infection and subcutaneous seroma/hematoma or systematic factors such as poor nutrition and wound ischemia.
Acceleration of wound healing results from the design of the NPWT systems. They consist of semipermeable dressings (foam), sealed with an adhesive sheet that is connected to a portable pump. By the application of –50 to –175 mm Hg of continuous or intermittent suction, the edges of the wound are drawn together, and this deforming process promotes tissue remodeling at the cellular level. Other potential benefits of negative pressure are increased blood flow, a decrease in mediators of inflammation, and an increase in collagen organization via changes in wound biochemistry.
An alternative to NPWT would be traditional gauze dressings, which can also be applied in the case of superficial wound dehiscence. These are changed up to three times a day, however, and this can result in significant patient discomfort, caregiver difficulties, and prolonged healing of weeks to months. In contrast, NPWT dressings are changed once every 2-3 days. They are also versatile and can be fit to traditionally shaped abdominal wounds, as well as difficult to dress vulvar and groin wounds (J. Obstet. Gynaecol. Can. 2011;33:1031-7).
In a series of 27 gynecologic oncology patients in whom NPWT was employed after primary wound–healing failure, there was a 96% reduction in the size of the wounds with a median number of therapy days of 32 (range, 3-88). The majority of these patients were also managed as outpatients without complication (Gynecol. Oncol. 2004;92:586-91).
There are some contraindications to NPWT that should be considered. The major, and perhaps most common, is an ongoing wound infection.
A wound that needs to be evaluated at least daily to assess the response to antibiotic therapy or need for debridement should not be managed with NPWT until the wound is deemed stable. There should be no devitalized tissue present in the wound upon application of the NPWT. If any necrotic tissue is present, then wound debridement is warranted until only well-vascularized tissue remains.
Another contraindication is the presence of malignant tissue in the wound. Negative pressure can promote this tissue growth and lead to chronic nonhealing. Other considerations would include adhesive allergies and fragile skin due to chronic steroid use or collagen vascular disorders, as NPWT can lead to skin necrosis.
Finally, the involvement of vital organs, such as exposed bowel, is a contraindication to the NPWT systems, as constant suction can promote fistula formation or hemorrhage. However, in the setting of an open abdomen after trauma surgery, there has been the development of intra-abdominal wound management systems that may be appropriate.
Although rare in obstetrics, gynecology, and gynecologic oncology, delayed abdominal closure may be necessary. This can occur after reoperation for bowel injury, in cases where bowel wall edema and increased intra-abdominal pressure preclude closure, or in cases of massive hemorrhage (for example, ruptured ectopic pregnancy) where patient instability necessitates rapid termination of the surgical case. These wounds can be managed with temporary abdominal closure techniques such as retention sutures, a Bogota bag, or loose packing (World. J. Surg. 2015; 39: 912-25).
The negative pressure systems developed for these instances are the V.A.C. abdominal dressing (KCI), Renasys NPWT (Smith and Nephew), and ABThera open abdomen negative pressure therapy (KCI). They consist of a perforated plastic sheet with foam attachments that is placed directly in the abdomen to cover the intestine. This is then covered with an adhesive dressing that is cut to accommodate the suction attachment for the negative pressure pump. This setup is easily applied and taken down, and therefore facilitates frequent abdominal washouts until true facial closure can be achieved.
There are many benefits to NPWT for the management of superficial and deep wound dehiscence in the ob.gyn. or gynecologic oncology patient. NPWT should be considered primarily with any surgical wound healing by secondary intention.
Dr. Doll is a third-year fellow in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. The authors reported having no relevant financial disclosures.
Negative pressure wound therapy is a wound management system for chronic open subcutaneous or intra-abdominal wounds. Some popular commercial systems include V.A.C. therapy (KCI, San Antonio) and the Chariker-Jeter wound-sealing kit (Smith and Nephew, London). Within ob.gyn. and gynecologic oncology, they have use in the management of postoperative superficial wound dehiscence from routine surgery and in the management of the open abdomen.
The primary benefit of negative pressure wound therapy (NPWT) is the acceleration of wound healing. Postoperative superficial wound dehiscence can occur as a result of surgical factors such as wound infection and subcutaneous seroma/hematoma or systematic factors such as poor nutrition and wound ischemia.
Acceleration of wound healing results from the design of the NPWT systems. They consist of semipermeable dressings (foam), sealed with an adhesive sheet that is connected to a portable pump. By the application of –50 to –175 mm Hg of continuous or intermittent suction, the edges of the wound are drawn together, and this deforming process promotes tissue remodeling at the cellular level. Other potential benefits of negative pressure are increased blood flow, a decrease in mediators of inflammation, and an increase in collagen organization via changes in wound biochemistry.
An alternative to NPWT would be traditional gauze dressings, which can also be applied in the case of superficial wound dehiscence. These are changed up to three times a day, however, and this can result in significant patient discomfort, caregiver difficulties, and prolonged healing of weeks to months. In contrast, NPWT dressings are changed once every 2-3 days. They are also versatile and can be fit to traditionally shaped abdominal wounds, as well as difficult to dress vulvar and groin wounds (J. Obstet. Gynaecol. Can. 2011;33:1031-7).
In a series of 27 gynecologic oncology patients in whom NPWT was employed after primary wound–healing failure, there was a 96% reduction in the size of the wounds with a median number of therapy days of 32 (range, 3-88). The majority of these patients were also managed as outpatients without complication (Gynecol. Oncol. 2004;92:586-91).
There are some contraindications to NPWT that should be considered. The major, and perhaps most common, is an ongoing wound infection.
A wound that needs to be evaluated at least daily to assess the response to antibiotic therapy or need for debridement should not be managed with NPWT until the wound is deemed stable. There should be no devitalized tissue present in the wound upon application of the NPWT. If any necrotic tissue is present, then wound debridement is warranted until only well-vascularized tissue remains.
Another contraindication is the presence of malignant tissue in the wound. Negative pressure can promote this tissue growth and lead to chronic nonhealing. Other considerations would include adhesive allergies and fragile skin due to chronic steroid use or collagen vascular disorders, as NPWT can lead to skin necrosis.
Finally, the involvement of vital organs, such as exposed bowel, is a contraindication to the NPWT systems, as constant suction can promote fistula formation or hemorrhage. However, in the setting of an open abdomen after trauma surgery, there has been the development of intra-abdominal wound management systems that may be appropriate.
Although rare in obstetrics, gynecology, and gynecologic oncology, delayed abdominal closure may be necessary. This can occur after reoperation for bowel injury, in cases where bowel wall edema and increased intra-abdominal pressure preclude closure, or in cases of massive hemorrhage (for example, ruptured ectopic pregnancy) where patient instability necessitates rapid termination of the surgical case. These wounds can be managed with temporary abdominal closure techniques such as retention sutures, a Bogota bag, or loose packing (World. J. Surg. 2015; 39: 912-25).
The negative pressure systems developed for these instances are the V.A.C. abdominal dressing (KCI), Renasys NPWT (Smith and Nephew), and ABThera open abdomen negative pressure therapy (KCI). They consist of a perforated plastic sheet with foam attachments that is placed directly in the abdomen to cover the intestine. This is then covered with an adhesive dressing that is cut to accommodate the suction attachment for the negative pressure pump. This setup is easily applied and taken down, and therefore facilitates frequent abdominal washouts until true facial closure can be achieved.
There are many benefits to NPWT for the management of superficial and deep wound dehiscence in the ob.gyn. or gynecologic oncology patient. NPWT should be considered primarily with any surgical wound healing by secondary intention.
Dr. Doll is a third-year fellow in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. The authors reported having no relevant financial disclosures.
Spacing out
The number of parents asking their pediatricians to stray from the recommended immunization schedule by spreading out the vaccines is increasing, and so is the number of pediatricians who are agreeing to follow these spaced-out schedules.
One of the two reasons most often given by pediatricians for agreeing to the less than optimal immunization schedules is that by showing a willingness to compromise, that physician may be helping to build a trusting relationship with these families. The other reason is a concern – let’s be honest and call it a fear – that a dissatisfied family will move its care to another physician/provider.
When we scratch the surface of these two rationales, neither seems to make much sense. The conflict over immunization spacing comes to a head at the 2-month well-child visit recommended call for six injections. If the infant has had an unremarkable neonatal course, there may not have been any situation in which the physician was forced to demonstrate her trustworthiness. As long as she has dressed professionally, showed up on time for appointments, washed her hands, and appeared genuinely interested in the child’s well-being, that’s about all she has had to do.
The physician may give the impression that she can be trusted, but real trust is usually something that must accumulate over time, in monthly – or more likely yearly – increments. Occasionally a crisis allows the physician to behave so heroically that her route to a trusting relationship is compressed to just a few hours, but fortunately these crises are rare.
Does agreeing to an unnecessary and unsubstantiated diversion from the recommended immunization schedule play a role in trust building? It may signal that the physician is willing to compromise, which in some situations may not be a bad attribute. For example, the mother who has struggled and failed at breastfeeding her 6 weeks despite everyone’s best efforts will appreciate her pediatrician’s willingness to compromise. But should compromise of scientifically validated practices really be one of the cornerstones of a physician-patient relationship?
I have never had a family request that the immunization schedule be spread out for their second child because they have seen for themselves that the process is not what they have feared. I gave all the immunizations myself, and my administration style was quick and matter-of-fact. The problem, of course, is getting hesitant parents up to and over that hurdle of the 2-month visit. Unfortunately, the evidence seems to be that education and extra time and reassurance are of little value in getting them to that point of trust.
The more difficult issue is a physician’s fear that by failing to agree to a spaced-out schedule, she will open a spigot and families will flow out of her practice to other more compromising providers. Is this just an ego thing? No one likes to feel rejected. Will the feared patient exodus seriously depress the physician’s income or will it be merely a trickle that can be ignored? Obviously, the answer varies from community to community. Do families have so many options that they will easily be able to find a provider who is eager to grow his or her practice, and is less concerned about the immunization level of the community? Or, is the pediatrician so busy that a firm adherence to the standard schedule might provide a welcome opportunity to have a more manageable panel size, and at the same time shift the patient mix toward families that don’t require the extra time in fruitless “educational” discussions?
These are questions that don’t seem to be getting asked. What are the numbers? Is the loss of patients just an irrational fear for physicians created by an irrational fear of a small segment of the population? If the physician practices in a group, could her fear of patient loss be eased if the entire group committed itself to following the standard immunization schedule? Are group members discussing this issue among themselves and with their practice managers? Or, is everyone just spacing out?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected]. Scan this QR code to read similar articles or go to pediatricnews.com.
The number of parents asking their pediatricians to stray from the recommended immunization schedule by spreading out the vaccines is increasing, and so is the number of pediatricians who are agreeing to follow these spaced-out schedules.
One of the two reasons most often given by pediatricians for agreeing to the less than optimal immunization schedules is that by showing a willingness to compromise, that physician may be helping to build a trusting relationship with these families. The other reason is a concern – let’s be honest and call it a fear – that a dissatisfied family will move its care to another physician/provider.
When we scratch the surface of these two rationales, neither seems to make much sense. The conflict over immunization spacing comes to a head at the 2-month well-child visit recommended call for six injections. If the infant has had an unremarkable neonatal course, there may not have been any situation in which the physician was forced to demonstrate her trustworthiness. As long as she has dressed professionally, showed up on time for appointments, washed her hands, and appeared genuinely interested in the child’s well-being, that’s about all she has had to do.
The physician may give the impression that she can be trusted, but real trust is usually something that must accumulate over time, in monthly – or more likely yearly – increments. Occasionally a crisis allows the physician to behave so heroically that her route to a trusting relationship is compressed to just a few hours, but fortunately these crises are rare.
Does agreeing to an unnecessary and unsubstantiated diversion from the recommended immunization schedule play a role in trust building? It may signal that the physician is willing to compromise, which in some situations may not be a bad attribute. For example, the mother who has struggled and failed at breastfeeding her 6 weeks despite everyone’s best efforts will appreciate her pediatrician’s willingness to compromise. But should compromise of scientifically validated practices really be one of the cornerstones of a physician-patient relationship?
I have never had a family request that the immunization schedule be spread out for their second child because they have seen for themselves that the process is not what they have feared. I gave all the immunizations myself, and my administration style was quick and matter-of-fact. The problem, of course, is getting hesitant parents up to and over that hurdle of the 2-month visit. Unfortunately, the evidence seems to be that education and extra time and reassurance are of little value in getting them to that point of trust.
The more difficult issue is a physician’s fear that by failing to agree to a spaced-out schedule, she will open a spigot and families will flow out of her practice to other more compromising providers. Is this just an ego thing? No one likes to feel rejected. Will the feared patient exodus seriously depress the physician’s income or will it be merely a trickle that can be ignored? Obviously, the answer varies from community to community. Do families have so many options that they will easily be able to find a provider who is eager to grow his or her practice, and is less concerned about the immunization level of the community? Or, is the pediatrician so busy that a firm adherence to the standard schedule might provide a welcome opportunity to have a more manageable panel size, and at the same time shift the patient mix toward families that don’t require the extra time in fruitless “educational” discussions?
These are questions that don’t seem to be getting asked. What are the numbers? Is the loss of patients just an irrational fear for physicians created by an irrational fear of a small segment of the population? If the physician practices in a group, could her fear of patient loss be eased if the entire group committed itself to following the standard immunization schedule? Are group members discussing this issue among themselves and with their practice managers? Or, is everyone just spacing out?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected]. Scan this QR code to read similar articles or go to pediatricnews.com.
The number of parents asking their pediatricians to stray from the recommended immunization schedule by spreading out the vaccines is increasing, and so is the number of pediatricians who are agreeing to follow these spaced-out schedules.
One of the two reasons most often given by pediatricians for agreeing to the less than optimal immunization schedules is that by showing a willingness to compromise, that physician may be helping to build a trusting relationship with these families. The other reason is a concern – let’s be honest and call it a fear – that a dissatisfied family will move its care to another physician/provider.
When we scratch the surface of these two rationales, neither seems to make much sense. The conflict over immunization spacing comes to a head at the 2-month well-child visit recommended call for six injections. If the infant has had an unremarkable neonatal course, there may not have been any situation in which the physician was forced to demonstrate her trustworthiness. As long as she has dressed professionally, showed up on time for appointments, washed her hands, and appeared genuinely interested in the child’s well-being, that’s about all she has had to do.
The physician may give the impression that she can be trusted, but real trust is usually something that must accumulate over time, in monthly – or more likely yearly – increments. Occasionally a crisis allows the physician to behave so heroically that her route to a trusting relationship is compressed to just a few hours, but fortunately these crises are rare.
Does agreeing to an unnecessary and unsubstantiated diversion from the recommended immunization schedule play a role in trust building? It may signal that the physician is willing to compromise, which in some situations may not be a bad attribute. For example, the mother who has struggled and failed at breastfeeding her 6 weeks despite everyone’s best efforts will appreciate her pediatrician’s willingness to compromise. But should compromise of scientifically validated practices really be one of the cornerstones of a physician-patient relationship?
I have never had a family request that the immunization schedule be spread out for their second child because they have seen for themselves that the process is not what they have feared. I gave all the immunizations myself, and my administration style was quick and matter-of-fact. The problem, of course, is getting hesitant parents up to and over that hurdle of the 2-month visit. Unfortunately, the evidence seems to be that education and extra time and reassurance are of little value in getting them to that point of trust.
The more difficult issue is a physician’s fear that by failing to agree to a spaced-out schedule, she will open a spigot and families will flow out of her practice to other more compromising providers. Is this just an ego thing? No one likes to feel rejected. Will the feared patient exodus seriously depress the physician’s income or will it be merely a trickle that can be ignored? Obviously, the answer varies from community to community. Do families have so many options that they will easily be able to find a provider who is eager to grow his or her practice, and is less concerned about the immunization level of the community? Or, is the pediatrician so busy that a firm adherence to the standard schedule might provide a welcome opportunity to have a more manageable panel size, and at the same time shift the patient mix toward families that don’t require the extra time in fruitless “educational” discussions?
These are questions that don’t seem to be getting asked. What are the numbers? Is the loss of patients just an irrational fear for physicians created by an irrational fear of a small segment of the population? If the physician practices in a group, could her fear of patient loss be eased if the entire group committed itself to following the standard immunization schedule? Are group members discussing this issue among themselves and with their practice managers? Or, is everyone just spacing out?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected]. Scan this QR code to read similar articles or go to pediatricnews.com.
Portulaca oleracea (purslane)
Portulaca oleracea, also known as purslane, has long been used in various traditional medicine systems to relieve pain and edema.1Portulaca oleracea is a warm-climate annual plant originally found in the Middle East, North Africa, and the Indian subcontinent and now cultivated in the Arabian peninsula; Japan, where it is an abundant garden plant from spring to fall;2,3 and throughout the world.
The use of P. oleracea, a member of the Portulacaceae family, as a vegetable as well as herbal medicine dates back several centuries.4 In modern times, purslane has been found to be rich in antioxidants, particularly omega-3 fatty acids, vitamins C and E, beta-carotene, melatonin, and glutathione, as well as several minerals.5,6,7 Currently, it is considered one of the top ten most common plants in the world, and one of the most-used medical plants according to the World Health Organization.6 It is considered a weed in the United States, but is eaten in many parts of the world.
Antioxidant activity
Using two different assays, Uddin et al. determined in 2012 that P. oleracea cultivars exhibited significant antioxidant activity through various growth stages. In addition, the researchers suggested that purslane could provide multiple minerals as well as antioxidants in the context of nutraceutical products and functional food.7 Early this year, Silva et al. studied the antioxidant activity of P. oleracea leaves, flowers, and stems from two different locations in Portugal, with assays revealing significantly greater antioxidant activity in the stems of both samples compared to the leaves and flowers. However, the phenolic extracts of all three plant sections from both samples were found to protect DNA against hydroxyl radicals. The investigators concluded that their findings, particularly related to high antioxidant activity, support the potential benefits of purslane consumption to human health.8
A 2014 analysis of 13 collected purslane accessions revealed significant mineral content (particularly potassium, followed by nitrogen, sodium, calcium, magnesium, phosphorus, iron, zinc, and manganese) and showed that antioxidant activity was more strongly associated with ornamental as opposed to common purslane, the latter of which was richer in mineral content.6
Anti-inflammatory activity
In 2000, Chan et al. found that a 10% ethanolic extract of the dried leaves and stem of a P. oleracea cultivar displayed significant anti-inflammatory and analgesic properties after topical and intraperitoneal, but not oral, administration in comparison to diclofenac sodium, a synthetic drug used as active control. They added that these activities corresponded to the reputed effects of the traditional uses of the wild species.9
Wound healing activity
Rashed et al. reported in 2003 that a crude extract of P. oleracea accelerates wound healing. They used Mus musculus JVI-1 to show that fresh homogenized crude aerial parts of the plant topically applied on excision wound surfaces reduced wound surface areas and increased tensile strength. The best documented contraction was associated with a single dose of 50 mg, followed by two doses of 25 mg each.10
Oral lichen planus treatment
In 2010, Agha-Hosseini et al. conducted a randomized double-blind placebo-controlled 3-month study to assess the effectiveness of purslane in the treatment of oral lichen planus. Thirty-seven symptomatic patients (confirmed by biopsy) were divided into a purslane treatment group (n = 20) and a placebo group (n = 17). The investigators reported that partial to complete clinical improvement was observed in 83% of the treatment group, with no response in the remaining 17%, whereas partial improvement was seen in 17% of the placebo group, 73% had no response, and the condition was aggravated in 10% of the placebo group. No adverse side effects were reported in either group, and the researchers concluded that purslane was clinically effective in treating oral lichen planus and warrants consideration as a treatment option for the disorder.5
Other activities
In 2001, Radhakrishnan et al. identified several neuropharmacological actions, particularly anti-nociceptive and muscle-relaxing activity, with a range of effects on the central and peripheral nervous system observed in animal studies.1 The betacyanins found in P. oleracea have subsequently been found to confer a protective effect against neurotoxicity, specifically, ameliorating the D-galactose-induced cognitive deficits in senescent mice.11P. oleracea also has been shown to efficiently eliminate the endocrine-disrupting chemical bisphenol A from a hydroponic solution.12
In 2012, Yan et al. showed that three newly isolated homoisoflavonoids, known as portulacanones, and the compound 2,2’-dihydroxy-4’,6’-dimethoxychalcone selectively exhibited in vitro cytotoxic activities against four human cancer cell lines.2
Conclusions
This antioxidant-rich plant is found throughout the world and has long been associated with traditional health care. Modern research into its potential dermatologic uses is ongoing, but the evidence is relatively scarce. There are indications that the antioxidant, anti-inflammatory, and wound healing activity reportedly exhibited by purslane may be harnessed for various cutaneous applications. However, much more research is necessary to determine how extensive a role purslane may play in skin care.
References
1.J. Ethnopharmacol. 2001;76:171-6
2.Phytochemistry 2012;80:37-41
3.J. Biosci. Bioeng. 2007;103:420-6
4.J. Ethnopharmacol. 2000;73:445-51
5.Phytother. Res. 2010;24:240-4
6.Biomed. Res. Int. 2014;2014:296063
7.Int. J. Mol. Sci. 2012;13:10257-67
8.Nat Prod. Commun. 2014;9:45-50
9. J. Ethnopharmacol. 2000;73:445-51
10. J. Ethnopharmacol. 2003;88:131-6
11. Phytomedicine. 2010 Jun;17:527-32
12. Biosci. Biotechnol. Biochem. 2012;76:1015-7
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Portulaca oleracea, also known as purslane, has long been used in various traditional medicine systems to relieve pain and edema.1Portulaca oleracea is a warm-climate annual plant originally found in the Middle East, North Africa, and the Indian subcontinent and now cultivated in the Arabian peninsula; Japan, where it is an abundant garden plant from spring to fall;2,3 and throughout the world.
The use of P. oleracea, a member of the Portulacaceae family, as a vegetable as well as herbal medicine dates back several centuries.4 In modern times, purslane has been found to be rich in antioxidants, particularly omega-3 fatty acids, vitamins C and E, beta-carotene, melatonin, and glutathione, as well as several minerals.5,6,7 Currently, it is considered one of the top ten most common plants in the world, and one of the most-used medical plants according to the World Health Organization.6 It is considered a weed in the United States, but is eaten in many parts of the world.
Antioxidant activity
Using two different assays, Uddin et al. determined in 2012 that P. oleracea cultivars exhibited significant antioxidant activity through various growth stages. In addition, the researchers suggested that purslane could provide multiple minerals as well as antioxidants in the context of nutraceutical products and functional food.7 Early this year, Silva et al. studied the antioxidant activity of P. oleracea leaves, flowers, and stems from two different locations in Portugal, with assays revealing significantly greater antioxidant activity in the stems of both samples compared to the leaves and flowers. However, the phenolic extracts of all three plant sections from both samples were found to protect DNA against hydroxyl radicals. The investigators concluded that their findings, particularly related to high antioxidant activity, support the potential benefits of purslane consumption to human health.8
A 2014 analysis of 13 collected purslane accessions revealed significant mineral content (particularly potassium, followed by nitrogen, sodium, calcium, magnesium, phosphorus, iron, zinc, and manganese) and showed that antioxidant activity was more strongly associated with ornamental as opposed to common purslane, the latter of which was richer in mineral content.6
Anti-inflammatory activity
In 2000, Chan et al. found that a 10% ethanolic extract of the dried leaves and stem of a P. oleracea cultivar displayed significant anti-inflammatory and analgesic properties after topical and intraperitoneal, but not oral, administration in comparison to diclofenac sodium, a synthetic drug used as active control. They added that these activities corresponded to the reputed effects of the traditional uses of the wild species.9
Wound healing activity
Rashed et al. reported in 2003 that a crude extract of P. oleracea accelerates wound healing. They used Mus musculus JVI-1 to show that fresh homogenized crude aerial parts of the plant topically applied on excision wound surfaces reduced wound surface areas and increased tensile strength. The best documented contraction was associated with a single dose of 50 mg, followed by two doses of 25 mg each.10
Oral lichen planus treatment
In 2010, Agha-Hosseini et al. conducted a randomized double-blind placebo-controlled 3-month study to assess the effectiveness of purslane in the treatment of oral lichen planus. Thirty-seven symptomatic patients (confirmed by biopsy) were divided into a purslane treatment group (n = 20) and a placebo group (n = 17). The investigators reported that partial to complete clinical improvement was observed in 83% of the treatment group, with no response in the remaining 17%, whereas partial improvement was seen in 17% of the placebo group, 73% had no response, and the condition was aggravated in 10% of the placebo group. No adverse side effects were reported in either group, and the researchers concluded that purslane was clinically effective in treating oral lichen planus and warrants consideration as a treatment option for the disorder.5
Other activities
In 2001, Radhakrishnan et al. identified several neuropharmacological actions, particularly anti-nociceptive and muscle-relaxing activity, with a range of effects on the central and peripheral nervous system observed in animal studies.1 The betacyanins found in P. oleracea have subsequently been found to confer a protective effect against neurotoxicity, specifically, ameliorating the D-galactose-induced cognitive deficits in senescent mice.11P. oleracea also has been shown to efficiently eliminate the endocrine-disrupting chemical bisphenol A from a hydroponic solution.12
In 2012, Yan et al. showed that three newly isolated homoisoflavonoids, known as portulacanones, and the compound 2,2’-dihydroxy-4’,6’-dimethoxychalcone selectively exhibited in vitro cytotoxic activities against four human cancer cell lines.2
Conclusions
This antioxidant-rich plant is found throughout the world and has long been associated with traditional health care. Modern research into its potential dermatologic uses is ongoing, but the evidence is relatively scarce. There are indications that the antioxidant, anti-inflammatory, and wound healing activity reportedly exhibited by purslane may be harnessed for various cutaneous applications. However, much more research is necessary to determine how extensive a role purslane may play in skin care.
References
1.J. Ethnopharmacol. 2001;76:171-6
2.Phytochemistry 2012;80:37-41
3.J. Biosci. Bioeng. 2007;103:420-6
4.J. Ethnopharmacol. 2000;73:445-51
5.Phytother. Res. 2010;24:240-4
6.Biomed. Res. Int. 2014;2014:296063
7.Int. J. Mol. Sci. 2012;13:10257-67
8.Nat Prod. Commun. 2014;9:45-50
9. J. Ethnopharmacol. 2000;73:445-51
10. J. Ethnopharmacol. 2003;88:131-6
11. Phytomedicine. 2010 Jun;17:527-32
12. Biosci. Biotechnol. Biochem. 2012;76:1015-7
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Portulaca oleracea, also known as purslane, has long been used in various traditional medicine systems to relieve pain and edema.1Portulaca oleracea is a warm-climate annual plant originally found in the Middle East, North Africa, and the Indian subcontinent and now cultivated in the Arabian peninsula; Japan, where it is an abundant garden plant from spring to fall;2,3 and throughout the world.
The use of P. oleracea, a member of the Portulacaceae family, as a vegetable as well as herbal medicine dates back several centuries.4 In modern times, purslane has been found to be rich in antioxidants, particularly omega-3 fatty acids, vitamins C and E, beta-carotene, melatonin, and glutathione, as well as several minerals.5,6,7 Currently, it is considered one of the top ten most common plants in the world, and one of the most-used medical plants according to the World Health Organization.6 It is considered a weed in the United States, but is eaten in many parts of the world.
Antioxidant activity
Using two different assays, Uddin et al. determined in 2012 that P. oleracea cultivars exhibited significant antioxidant activity through various growth stages. In addition, the researchers suggested that purslane could provide multiple minerals as well as antioxidants in the context of nutraceutical products and functional food.7 Early this year, Silva et al. studied the antioxidant activity of P. oleracea leaves, flowers, and stems from two different locations in Portugal, with assays revealing significantly greater antioxidant activity in the stems of both samples compared to the leaves and flowers. However, the phenolic extracts of all three plant sections from both samples were found to protect DNA against hydroxyl radicals. The investigators concluded that their findings, particularly related to high antioxidant activity, support the potential benefits of purslane consumption to human health.8
A 2014 analysis of 13 collected purslane accessions revealed significant mineral content (particularly potassium, followed by nitrogen, sodium, calcium, magnesium, phosphorus, iron, zinc, and manganese) and showed that antioxidant activity was more strongly associated with ornamental as opposed to common purslane, the latter of which was richer in mineral content.6
Anti-inflammatory activity
In 2000, Chan et al. found that a 10% ethanolic extract of the dried leaves and stem of a P. oleracea cultivar displayed significant anti-inflammatory and analgesic properties after topical and intraperitoneal, but not oral, administration in comparison to diclofenac sodium, a synthetic drug used as active control. They added that these activities corresponded to the reputed effects of the traditional uses of the wild species.9
Wound healing activity
Rashed et al. reported in 2003 that a crude extract of P. oleracea accelerates wound healing. They used Mus musculus JVI-1 to show that fresh homogenized crude aerial parts of the plant topically applied on excision wound surfaces reduced wound surface areas and increased tensile strength. The best documented contraction was associated with a single dose of 50 mg, followed by two doses of 25 mg each.10
Oral lichen planus treatment
In 2010, Agha-Hosseini et al. conducted a randomized double-blind placebo-controlled 3-month study to assess the effectiveness of purslane in the treatment of oral lichen planus. Thirty-seven symptomatic patients (confirmed by biopsy) were divided into a purslane treatment group (n = 20) and a placebo group (n = 17). The investigators reported that partial to complete clinical improvement was observed in 83% of the treatment group, with no response in the remaining 17%, whereas partial improvement was seen in 17% of the placebo group, 73% had no response, and the condition was aggravated in 10% of the placebo group. No adverse side effects were reported in either group, and the researchers concluded that purslane was clinically effective in treating oral lichen planus and warrants consideration as a treatment option for the disorder.5
Other activities
In 2001, Radhakrishnan et al. identified several neuropharmacological actions, particularly anti-nociceptive and muscle-relaxing activity, with a range of effects on the central and peripheral nervous system observed in animal studies.1 The betacyanins found in P. oleracea have subsequently been found to confer a protective effect against neurotoxicity, specifically, ameliorating the D-galactose-induced cognitive deficits in senescent mice.11P. oleracea also has been shown to efficiently eliminate the endocrine-disrupting chemical bisphenol A from a hydroponic solution.12
In 2012, Yan et al. showed that three newly isolated homoisoflavonoids, known as portulacanones, and the compound 2,2’-dihydroxy-4’,6’-dimethoxychalcone selectively exhibited in vitro cytotoxic activities against four human cancer cell lines.2
Conclusions
This antioxidant-rich plant is found throughout the world and has long been associated with traditional health care. Modern research into its potential dermatologic uses is ongoing, but the evidence is relatively scarce. There are indications that the antioxidant, anti-inflammatory, and wound healing activity reportedly exhibited by purslane may be harnessed for various cutaneous applications. However, much more research is necessary to determine how extensive a role purslane may play in skin care.
References
1.J. Ethnopharmacol. 2001;76:171-6
2.Phytochemistry 2012;80:37-41
3.J. Biosci. Bioeng. 2007;103:420-6
4.J. Ethnopharmacol. 2000;73:445-51
5.Phytother. Res. 2010;24:240-4
6.Biomed. Res. Int. 2014;2014:296063
7.Int. J. Mol. Sci. 2012;13:10257-67
8.Nat Prod. Commun. 2014;9:45-50
9. J. Ethnopharmacol. 2000;73:445-51
10. J. Ethnopharmacol. 2003;88:131-6
11. Phytomedicine. 2010 Jun;17:527-32
12. Biosci. Biotechnol. Biochem. 2012;76:1015-7
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Aesthetic Dermatology: Sun protection after aesthetic procedures
As summertime approaches, opportunities for outdoor activities increase. For many of our patients, summer inspires a desire to have aesthetic procedures in preparation for outdoor events, such as weddings and vacations. We must, however, be mindful that increased sun exposure after some aesthetic procedures can mean an increased risk of complications.
The main complication we worry about with sun exposure is, of course, hyperpigmentation. The risk is low with injectable procedures such as botulinum toxin and fillers, but sun protection is still encouraged, especially in skin types III-VI. The risk increases greatly with chemical peels and laser and light-based procedures, such as intense pulsed light, vascular lasers, pigment lasers, laser hair removal, and especially nonablative and ablative resurfacing (including nonlaser resurfacing such as dermabrasion).
Sun protection should be encouraged, even with seemingly less invasive procedures, such as electrodessication. I once had a patient with type-IV skin tell me at her first visit that, years before, she had electrodessication on her face for DPN (dermatosis papulosa nigra), a procedure she had done on several occasions without complications and great results. However, she went to a party on a boat the weekend after the procedure and developed hyperpigmentation at the procedure areas, and she still had a few dark macules several years later.
At a follow-up visit, she said the doctor told her she should not have gone out on the boat and should have worn sunscreen. Of course, she was highly upset that she wasn’t advised about sun protection at the time of the procedure. This is one of several stories I’ve heard or seen of complications and postinflammatory hyperpigmentation after an aesthetic procedure, when the patients felt that the treating physician or practitioner did not counsel them about sun exposure during the consultation or treatment visit. It seems intuitive, but I’ve made it a habit to make sun protection part of my counseling routine.
In my practice, we often give patients sunscreen to apply immediately after a procedure. Specifically encouraging the use of zinc- and/or titanium-based, broad-spectrum, noncomedogenic physical blockers that are SPF 30 or higher may help reduce the risk of potential irritation or allergy and subsequent postinflammatory pigmentary alteration from chemical blocking ingredients. We provide a postprocedure handout, and the medical assistant also will counsel about sun protection when applying it to the patient or reviewing postprocedure instructions. So the patient is counseled at least three times: By me during consultation or pre-procedure, by the medical assistant post procedure, and by written instructions.
Vigorous sun protection is encouraged for at least 1 week after any aesthetic procedure (and longer if the downtime is longer or if multiple treatments are required). Some practices also use antioxidant serums to reduce free radicals, encourage healing, and reduce the risk of hyperpigmentation after procedures. Wide-brimmed hats also are encouraged, particularly after resurfacing or photodynamic therapy (PDT). We give patients sun-protective hats when they leave our office after PDT. We counsel them to practice vigorous sun protection for at least 1 week and to avoid sitting by a window for 48 hours after the procedure so as to not reactivate the levulan.
Delaying more high-risk procedures, such as laser treatments, until after the summer months may be appropriate if sun cannot be avoided to mitigate the risk of complications. If a patient comes to the office for a laser procedure and is visibly more tan than at the time of the last treatment, I will counsel about risks, adjust the settings appropriately, or even delay the treatment altogether to a time when the tan has faded. This is particularly important for lasers and light treatments for which melanin is the target chromosphere, such as intense pulsed light and laser hair removal. Although UV exposure is more intense in the summer, in our practice in Southern California we follow these principles year-round for the safety of our patients.
Dr. Wesley and Dr. Talakoub are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
As summertime approaches, opportunities for outdoor activities increase. For many of our patients, summer inspires a desire to have aesthetic procedures in preparation for outdoor events, such as weddings and vacations. We must, however, be mindful that increased sun exposure after some aesthetic procedures can mean an increased risk of complications.
The main complication we worry about with sun exposure is, of course, hyperpigmentation. The risk is low with injectable procedures such as botulinum toxin and fillers, but sun protection is still encouraged, especially in skin types III-VI. The risk increases greatly with chemical peels and laser and light-based procedures, such as intense pulsed light, vascular lasers, pigment lasers, laser hair removal, and especially nonablative and ablative resurfacing (including nonlaser resurfacing such as dermabrasion).
Sun protection should be encouraged, even with seemingly less invasive procedures, such as electrodessication. I once had a patient with type-IV skin tell me at her first visit that, years before, she had electrodessication on her face for DPN (dermatosis papulosa nigra), a procedure she had done on several occasions without complications and great results. However, she went to a party on a boat the weekend after the procedure and developed hyperpigmentation at the procedure areas, and she still had a few dark macules several years later.
At a follow-up visit, she said the doctor told her she should not have gone out on the boat and should have worn sunscreen. Of course, she was highly upset that she wasn’t advised about sun protection at the time of the procedure. This is one of several stories I’ve heard or seen of complications and postinflammatory hyperpigmentation after an aesthetic procedure, when the patients felt that the treating physician or practitioner did not counsel them about sun exposure during the consultation or treatment visit. It seems intuitive, but I’ve made it a habit to make sun protection part of my counseling routine.
In my practice, we often give patients sunscreen to apply immediately after a procedure. Specifically encouraging the use of zinc- and/or titanium-based, broad-spectrum, noncomedogenic physical blockers that are SPF 30 or higher may help reduce the risk of potential irritation or allergy and subsequent postinflammatory pigmentary alteration from chemical blocking ingredients. We provide a postprocedure handout, and the medical assistant also will counsel about sun protection when applying it to the patient or reviewing postprocedure instructions. So the patient is counseled at least three times: By me during consultation or pre-procedure, by the medical assistant post procedure, and by written instructions.
Vigorous sun protection is encouraged for at least 1 week after any aesthetic procedure (and longer if the downtime is longer or if multiple treatments are required). Some practices also use antioxidant serums to reduce free radicals, encourage healing, and reduce the risk of hyperpigmentation after procedures. Wide-brimmed hats also are encouraged, particularly after resurfacing or photodynamic therapy (PDT). We give patients sun-protective hats when they leave our office after PDT. We counsel them to practice vigorous sun protection for at least 1 week and to avoid sitting by a window for 48 hours after the procedure so as to not reactivate the levulan.
Delaying more high-risk procedures, such as laser treatments, until after the summer months may be appropriate if sun cannot be avoided to mitigate the risk of complications. If a patient comes to the office for a laser procedure and is visibly more tan than at the time of the last treatment, I will counsel about risks, adjust the settings appropriately, or even delay the treatment altogether to a time when the tan has faded. This is particularly important for lasers and light treatments for which melanin is the target chromosphere, such as intense pulsed light and laser hair removal. Although UV exposure is more intense in the summer, in our practice in Southern California we follow these principles year-round for the safety of our patients.
Dr. Wesley and Dr. Talakoub are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
As summertime approaches, opportunities for outdoor activities increase. For many of our patients, summer inspires a desire to have aesthetic procedures in preparation for outdoor events, such as weddings and vacations. We must, however, be mindful that increased sun exposure after some aesthetic procedures can mean an increased risk of complications.
The main complication we worry about with sun exposure is, of course, hyperpigmentation. The risk is low with injectable procedures such as botulinum toxin and fillers, but sun protection is still encouraged, especially in skin types III-VI. The risk increases greatly with chemical peels and laser and light-based procedures, such as intense pulsed light, vascular lasers, pigment lasers, laser hair removal, and especially nonablative and ablative resurfacing (including nonlaser resurfacing such as dermabrasion).
Sun protection should be encouraged, even with seemingly less invasive procedures, such as electrodessication. I once had a patient with type-IV skin tell me at her first visit that, years before, she had electrodessication on her face for DPN (dermatosis papulosa nigra), a procedure she had done on several occasions without complications and great results. However, she went to a party on a boat the weekend after the procedure and developed hyperpigmentation at the procedure areas, and she still had a few dark macules several years later.
At a follow-up visit, she said the doctor told her she should not have gone out on the boat and should have worn sunscreen. Of course, she was highly upset that she wasn’t advised about sun protection at the time of the procedure. This is one of several stories I’ve heard or seen of complications and postinflammatory hyperpigmentation after an aesthetic procedure, when the patients felt that the treating physician or practitioner did not counsel them about sun exposure during the consultation or treatment visit. It seems intuitive, but I’ve made it a habit to make sun protection part of my counseling routine.
In my practice, we often give patients sunscreen to apply immediately after a procedure. Specifically encouraging the use of zinc- and/or titanium-based, broad-spectrum, noncomedogenic physical blockers that are SPF 30 or higher may help reduce the risk of potential irritation or allergy and subsequent postinflammatory pigmentary alteration from chemical blocking ingredients. We provide a postprocedure handout, and the medical assistant also will counsel about sun protection when applying it to the patient or reviewing postprocedure instructions. So the patient is counseled at least three times: By me during consultation or pre-procedure, by the medical assistant post procedure, and by written instructions.
Vigorous sun protection is encouraged for at least 1 week after any aesthetic procedure (and longer if the downtime is longer or if multiple treatments are required). Some practices also use antioxidant serums to reduce free radicals, encourage healing, and reduce the risk of hyperpigmentation after procedures. Wide-brimmed hats also are encouraged, particularly after resurfacing or photodynamic therapy (PDT). We give patients sun-protective hats when they leave our office after PDT. We counsel them to practice vigorous sun protection for at least 1 week and to avoid sitting by a window for 48 hours after the procedure so as to not reactivate the levulan.
Delaying more high-risk procedures, such as laser treatments, until after the summer months may be appropriate if sun cannot be avoided to mitigate the risk of complications. If a patient comes to the office for a laser procedure and is visibly more tan than at the time of the last treatment, I will counsel about risks, adjust the settings appropriately, or even delay the treatment altogether to a time when the tan has faded. This is particularly important for lasers and light treatments for which melanin is the target chromosphere, such as intense pulsed light and laser hair removal. Although UV exposure is more intense in the summer, in our practice in Southern California we follow these principles year-round for the safety of our patients.
Dr. Wesley and Dr. Talakoub are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.