User login
How a cheap liver drug may be the key to preventing COVID
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.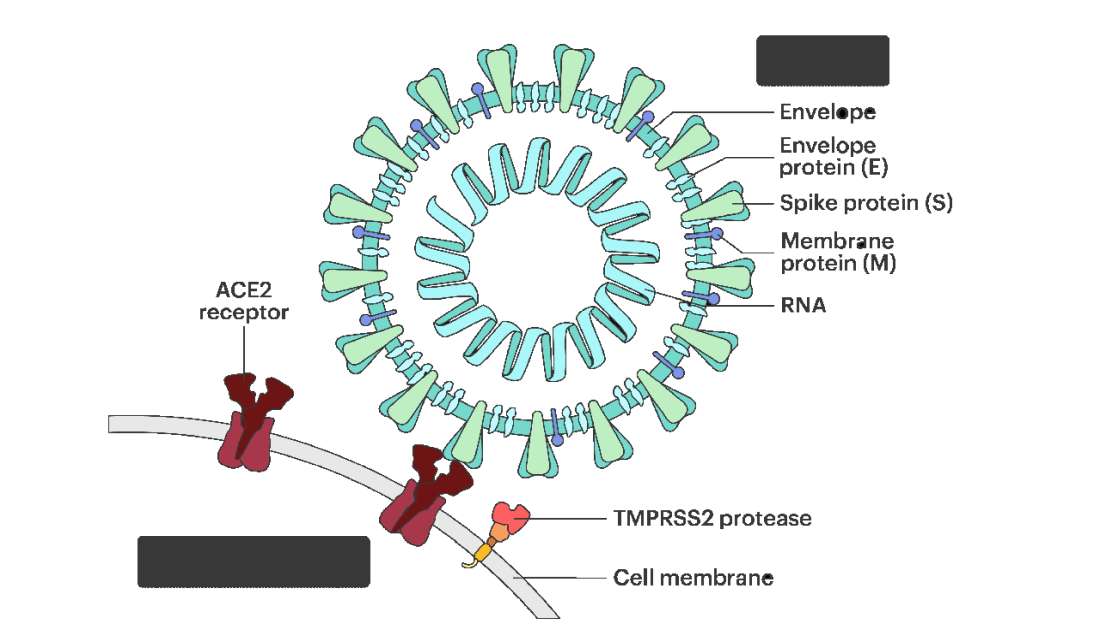
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.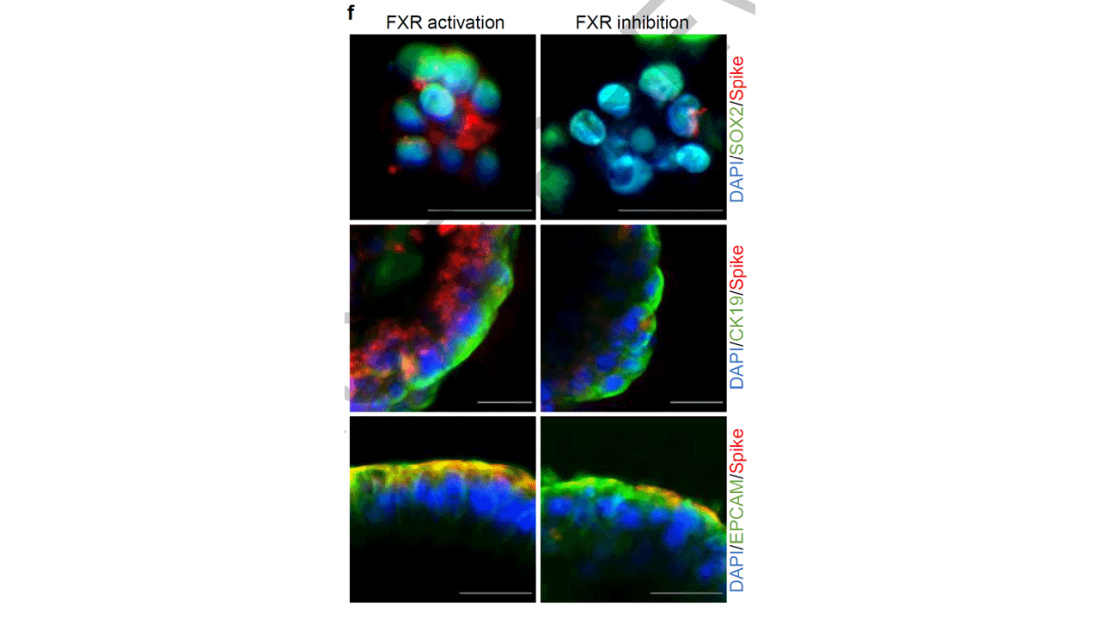
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies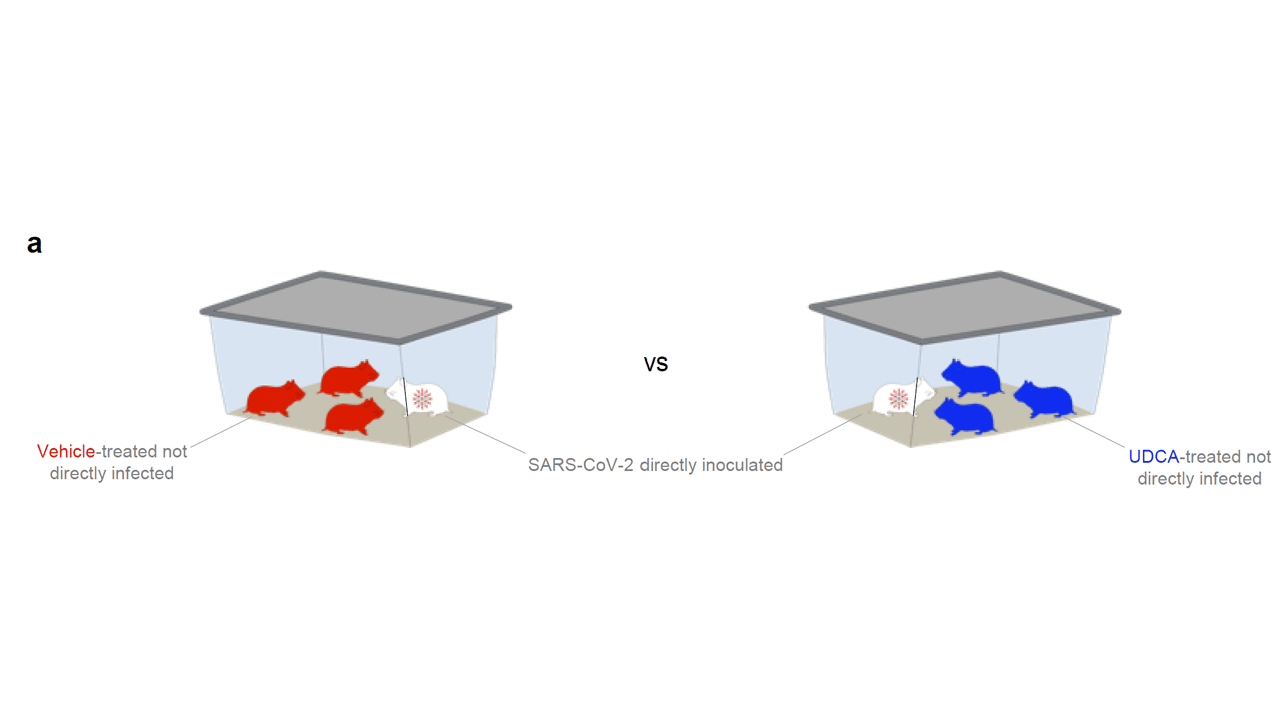
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.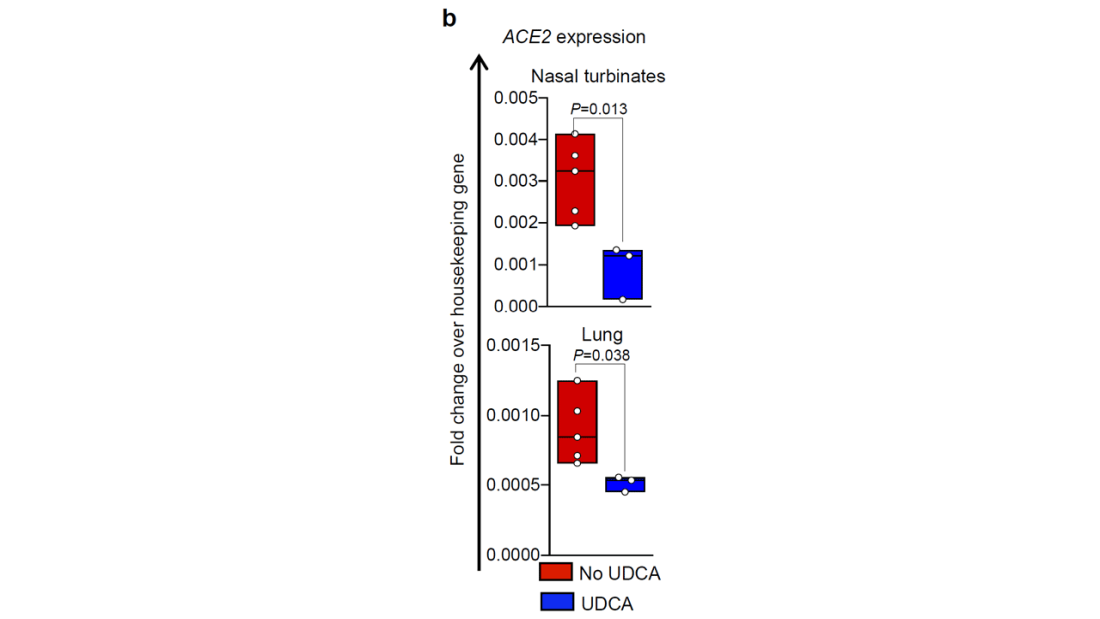
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.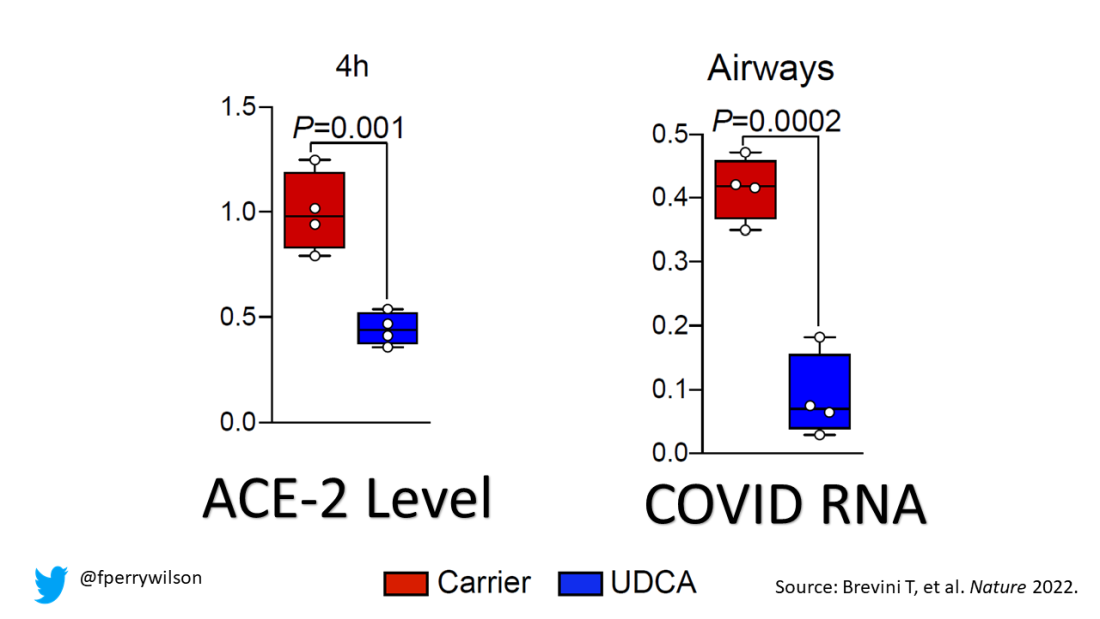
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.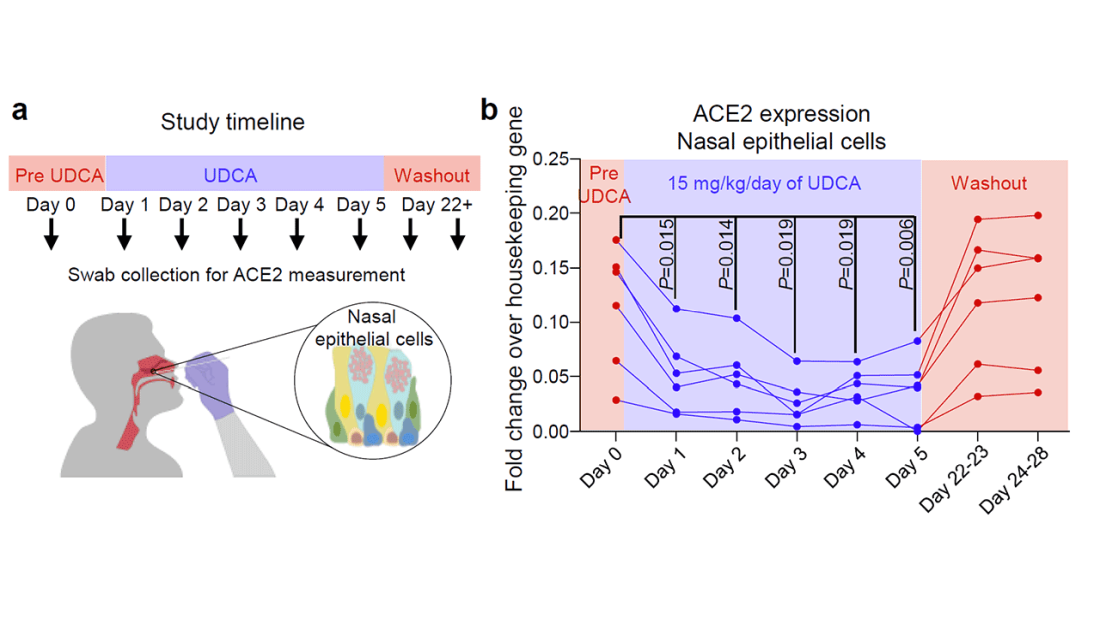
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Migraine in children and teens: managing the pain
By the time Mira Halker started high school, hardly a day passed that she wasn’t either getting a migraine attack or recovering from one. She missed volleyball team practice. She missed classes. She missed social events. And few people understood. After all, she looked healthy.
“A lot of times, people think I’m faking it,” said Mira, now 16, who lives in Phoenix. Friends called her flaky; her volleyball coaches questioned her dedication to the team. “I’m like, ‘I’m not trying to get out of this. This is not what this is about,’ ” she said.
Her mother, Rashmi B. Halker Singh, MD, is a neurologist at Mayo Clinic who happens to specialize in migraine. Even so, finding a solution was not easy. Neither ibuprofen nor triptans, nor various preventive measures such as a daily prescription for topiramate controlled the pain and associated symptoms. Mira was barely making it through her school day and had to quit volleyball. Then, in the spring of 10th grade, Mira told her mother that she couldn’t go to prom because the loud noises and lights could give her a migraine attack.
Mother and daughter decided it was time to get even more aggressive. “There are these key moments in life that you can’t get back,” Dr. Singh said. “Migraine steals so much from you.”
Diagnosis
One of the challenges Mira’s physicians faced was deciding which medications and other therapies to prescribe to a teenager. Drug companies have been releasing a steady stream of new treatments for migraine headaches, and researchers promise more are on the way soon. Here’s what works for children, what hasn’t yet been approved for use with minors, and how to diagnose migraines in the first place, from experts at some of the nation’s leading pediatric headache centers.
Migraine affects about 10% of children, according to the American Migraine Foundation. The headaches can strike children as early as age 3 or 4 years, said Robert Little, MD, a pediatric neurologist at Phoenix Children’s Hospital.
Before puberty, boys report more migraine attacks than girls, according to the American Academy of Pediatrics. But that reverses in adolescence: By age 17, as many as 8% of boys and 23% of girls have had migraine. To diagnose migraine, Juliana H. VanderPluym, MD, associate professor of neurology at Mayo Clinic in Phoenix, said she uses the criteria published in the latest edition of the International Classification of Headache Disorders (ICHD): A patient must have had at least five attacks in their life; and in children and adolescents, the attacks must last no less than 2 hours.
In addition, the headaches should exhibit at least two out of four features:
1. Occur more on one side of the head than the other (although Dr. VanderPluym said in children and adolescents headaches often are bilateral).
2. Be of moderate to severe intensity.
3. Have a pounding or throbbing quality.
4. Grow worse with activity or cause an avoidance of activity.
If the attacks meet those criteria, clinicians should check to see if they meet at least one out of the two following:
1. Are sensitive to light and sounds.
2. Are associated with nausea and/or vomiting.
A clinician should consider whether the headaches are not better accounted for by another diagnosis, according to the ICHD criteria. But, Dr. VanderPluym warned that does not necessarily mean running a slew of tests.
“In the absence of red flag features, it is more than likely going to be migraine headache,” she said. That’s especially true if a child has a family history of migraine, as the condition is often passed down from parent to child.
Ultimately, the diagnosis is fairly simple and can be made in a minute or less, said Jack Gladstein, MD, a pediatrician at the University of Maryland whose research focuses on the clinical care of children and adolescents with headache.
“Migraine is acute,” Dr. Gladstein said. “It’s really bad. And it’s recurrent.”
First line of treatment
Whatever a patient takes to treat a migraine, they should hit it early and hard, Dr. Gladstein said.
“The first thing you say, as a primary care physician, is treat your migraine at first twinge, whatever you use. Don’t wait, don’t wish it away,” he said. “The longer you wait, the less chance anything will work.”
The second piece of advice, Dr. Gladstein said, is that whatever drug a patient is taking, they should be on the highest feasible dose. “Work as fast as you can to treat them. You want the brain to reset as quickly as you can,” he said.
Patients should begin with over-the-counter pain relievers, Dr. Little said. If those prove insufficient, they can try a triptan. Rizatriptan is the only such agent that the Food and Drug Administration has approved for children aged 6-17 years. Other drugs in the class – sumatriptan/naproxen, almotriptan, and zolmitriptan – are approved for children 12 and older.
Another migraine therapy recently approved for children aged 12 and older is the use of neurostimulators. “It’s helpful to be aware of them,” Dr. VanderPluym said.
However, if neurostimulators and acute medications prove insufficient, clinicians should warn patients not to up their doses of triptans. Rebound headaches can occur if patients take triptans more than twice a week, or a maximum 10 days per month.
Another possibility is to add a preventive therapy. One mild, first option is nutraceuticals, like riboflavin (vitamin B2) or magnesium, said Anisa F. Kelley, MD, a neurologist and associate director of the headache program at the Ann and Robert H. Lurie Children’s Hospital of Chicago.
“We don’t have definitive evidence, but they’re probably doing more benefit than they are harm,” Dr. Kelley said of these therapies. “In patients who have anywhere from 4 to 8 migraine days a month, where you’re in that in-between period where you don’t necessarily need a [prescription] prophylactic, I will often start with a nutraceutical,” Dr. Kelley said.
For those patients who don’t respond to nutraceuticals, or who need more support, clinicians can prescribe amitriptyline or topiramate. Dr. VanderPluym said.
A 2017 study found such prophylactics to be no more effective than placebo in pediatric migraine patients, but experts caution the results should not be considered definitive.
For one thing, the study enrolled a highly selective group of participants, with milder forms of migraine who may have improved anyway, Dr. VanderPluym said. All participants also received lifestyle counseling.
Every time participants came in for a follow-up, they were asked questions such as how much water were they drinking and how much sleep were they getting, Dr. Kelley noted. The takeaway, she said: “Pediatric and adolescent migraine [management] is very, very much reliant on lifestyle factors.”
Lifestyle triggers
Clinicians should counsel their migraine patients about lifestyle changes, experts said. Getting adequate sleep, staying hydrated, and managing stress can help reduce the intensity and frequency of attacks.
Migraine patients should also be mindful of their screen time, Dr. Kelley added.
“I’ve had lots and lots of patients who find excessive screen time will trigger or worsen migraine,” she said.
As for other potential triggers of attacks, the evidence is mixed.
“There’s clearly an association with disrupted sleep and migraine, and that has been very well established,” Dr. Little said. “And there is some modest amount of evidence that regular exercise can be helpful.” But for reported food triggers, he said, there have been very inconclusive results.
Commonly reported triggers include MSG, red wine, chocolate, and aged cheese. When Dr. Little’s patients keep headache diaries, tracking their meals alongside when they got migraine attacks, they often discover individualized triggers – strawberries, for instance, in one case, he said.
Scientists believe migraines result from the inappropriate activation of the trigeminal ganglion. “The question is, what causes it to get triggered? And how does it get triggered?” Dr. Gladstein said. “And that’s where there’s a lot of difference of opinion and no conclusive evidence.” Clinicians also should make sure that something else – usually depression, anxiety, insomnia, and dizziness – is not hindering effective migraine management. “If someone has terrible insomnia, until you treat the insomnia, the headaches aren’t going to get better,” he said.
As for Mira, her migraine attacks did not significantly improve, despite trying triptans, prophylactics, lifestyle changes, and shots to block nerve pain. When the headaches threatened Mira’s chance to go to her prom, her neurologist suggested trying something different. The physician persuaded the family’s insurance to cover a calcitonin gene-related peptide antagonist, an injectable monoclonal antibody treatment for migraine that the FDA has currently approved only for use in adults.
The difference for Mira has been extraordinary.
“I can do so much more than I was able to do,” said Mira, who attended the dance migraine free. “I feel liberated.”
It’s only migraine
One of the greatest challenges in diagnosing migraine can be reassuring the patient, the parents, even clinicians themselves that migraine really is the cause of all this pain and discomfort, experts said.
“A lot of migraine treatment actually comes down to migraine education,” Dr. VanderPluym said.
Patients and their parents often wonder how they can be sure that this pain is not resulting from something more dangerous than migraine, Dr. Little said. In these cases, he cites practice guidelines published by the American Academy of Neurology.
“The gist of those guidelines is that most pediatric patients do not need further workup,” he said. “But I think that there’s always a fear that you’re missing something because we don’t have a test that we can do” for migraine.
Some warning signs that further tests might be warranted, Dr. Kelley said, include:
- Headaches that wake a patient up in the middle of the night.
- Headaches that start first thing in the morning, especially those that include vomiting.
- A headache pattern that suddenly gets much worse.
- Certain symptoms that accompany the headache, such as tingling, numbness or double vision.
Although all of these signs can still stem from migraines – tingling or numbness, for instance, can be signs of migraine aura – running additional tests can rule out more serious concerns, she said.
By the time Mira Halker started high school, hardly a day passed that she wasn’t either getting a migraine attack or recovering from one. She missed volleyball team practice. She missed classes. She missed social events. And few people understood. After all, she looked healthy.
“A lot of times, people think I’m faking it,” said Mira, now 16, who lives in Phoenix. Friends called her flaky; her volleyball coaches questioned her dedication to the team. “I’m like, ‘I’m not trying to get out of this. This is not what this is about,’ ” she said.
Her mother, Rashmi B. Halker Singh, MD, is a neurologist at Mayo Clinic who happens to specialize in migraine. Even so, finding a solution was not easy. Neither ibuprofen nor triptans, nor various preventive measures such as a daily prescription for topiramate controlled the pain and associated symptoms. Mira was barely making it through her school day and had to quit volleyball. Then, in the spring of 10th grade, Mira told her mother that she couldn’t go to prom because the loud noises and lights could give her a migraine attack.
Mother and daughter decided it was time to get even more aggressive. “There are these key moments in life that you can’t get back,” Dr. Singh said. “Migraine steals so much from you.”
Diagnosis
One of the challenges Mira’s physicians faced was deciding which medications and other therapies to prescribe to a teenager. Drug companies have been releasing a steady stream of new treatments for migraine headaches, and researchers promise more are on the way soon. Here’s what works for children, what hasn’t yet been approved for use with minors, and how to diagnose migraines in the first place, from experts at some of the nation’s leading pediatric headache centers.
Migraine affects about 10% of children, according to the American Migraine Foundation. The headaches can strike children as early as age 3 or 4 years, said Robert Little, MD, a pediatric neurologist at Phoenix Children’s Hospital.
Before puberty, boys report more migraine attacks than girls, according to the American Academy of Pediatrics. But that reverses in adolescence: By age 17, as many as 8% of boys and 23% of girls have had migraine. To diagnose migraine, Juliana H. VanderPluym, MD, associate professor of neurology at Mayo Clinic in Phoenix, said she uses the criteria published in the latest edition of the International Classification of Headache Disorders (ICHD): A patient must have had at least five attacks in their life; and in children and adolescents, the attacks must last no less than 2 hours.
In addition, the headaches should exhibit at least two out of four features:
1. Occur more on one side of the head than the other (although Dr. VanderPluym said in children and adolescents headaches often are bilateral).
2. Be of moderate to severe intensity.
3. Have a pounding or throbbing quality.
4. Grow worse with activity or cause an avoidance of activity.
If the attacks meet those criteria, clinicians should check to see if they meet at least one out of the two following:
1. Are sensitive to light and sounds.
2. Are associated with nausea and/or vomiting.
A clinician should consider whether the headaches are not better accounted for by another diagnosis, according to the ICHD criteria. But, Dr. VanderPluym warned that does not necessarily mean running a slew of tests.
“In the absence of red flag features, it is more than likely going to be migraine headache,” she said. That’s especially true if a child has a family history of migraine, as the condition is often passed down from parent to child.
Ultimately, the diagnosis is fairly simple and can be made in a minute or less, said Jack Gladstein, MD, a pediatrician at the University of Maryland whose research focuses on the clinical care of children and adolescents with headache.
“Migraine is acute,” Dr. Gladstein said. “It’s really bad. And it’s recurrent.”
First line of treatment
Whatever a patient takes to treat a migraine, they should hit it early and hard, Dr. Gladstein said.
“The first thing you say, as a primary care physician, is treat your migraine at first twinge, whatever you use. Don’t wait, don’t wish it away,” he said. “The longer you wait, the less chance anything will work.”
The second piece of advice, Dr. Gladstein said, is that whatever drug a patient is taking, they should be on the highest feasible dose. “Work as fast as you can to treat them. You want the brain to reset as quickly as you can,” he said.
Patients should begin with over-the-counter pain relievers, Dr. Little said. If those prove insufficient, they can try a triptan. Rizatriptan is the only such agent that the Food and Drug Administration has approved for children aged 6-17 years. Other drugs in the class – sumatriptan/naproxen, almotriptan, and zolmitriptan – are approved for children 12 and older.
Another migraine therapy recently approved for children aged 12 and older is the use of neurostimulators. “It’s helpful to be aware of them,” Dr. VanderPluym said.
However, if neurostimulators and acute medications prove insufficient, clinicians should warn patients not to up their doses of triptans. Rebound headaches can occur if patients take triptans more than twice a week, or a maximum 10 days per month.
Another possibility is to add a preventive therapy. One mild, first option is nutraceuticals, like riboflavin (vitamin B2) or magnesium, said Anisa F. Kelley, MD, a neurologist and associate director of the headache program at the Ann and Robert H. Lurie Children’s Hospital of Chicago.
“We don’t have definitive evidence, but they’re probably doing more benefit than they are harm,” Dr. Kelley said of these therapies. “In patients who have anywhere from 4 to 8 migraine days a month, where you’re in that in-between period where you don’t necessarily need a [prescription] prophylactic, I will often start with a nutraceutical,” Dr. Kelley said.
For those patients who don’t respond to nutraceuticals, or who need more support, clinicians can prescribe amitriptyline or topiramate. Dr. VanderPluym said.
A 2017 study found such prophylactics to be no more effective than placebo in pediatric migraine patients, but experts caution the results should not be considered definitive.
For one thing, the study enrolled a highly selective group of participants, with milder forms of migraine who may have improved anyway, Dr. VanderPluym said. All participants also received lifestyle counseling.
Every time participants came in for a follow-up, they were asked questions such as how much water were they drinking and how much sleep were they getting, Dr. Kelley noted. The takeaway, she said: “Pediatric and adolescent migraine [management] is very, very much reliant on lifestyle factors.”
Lifestyle triggers
Clinicians should counsel their migraine patients about lifestyle changes, experts said. Getting adequate sleep, staying hydrated, and managing stress can help reduce the intensity and frequency of attacks.
Migraine patients should also be mindful of their screen time, Dr. Kelley added.
“I’ve had lots and lots of patients who find excessive screen time will trigger or worsen migraine,” she said.
As for other potential triggers of attacks, the evidence is mixed.
“There’s clearly an association with disrupted sleep and migraine, and that has been very well established,” Dr. Little said. “And there is some modest amount of evidence that regular exercise can be helpful.” But for reported food triggers, he said, there have been very inconclusive results.
Commonly reported triggers include MSG, red wine, chocolate, and aged cheese. When Dr. Little’s patients keep headache diaries, tracking their meals alongside when they got migraine attacks, they often discover individualized triggers – strawberries, for instance, in one case, he said.
Scientists believe migraines result from the inappropriate activation of the trigeminal ganglion. “The question is, what causes it to get triggered? And how does it get triggered?” Dr. Gladstein said. “And that’s where there’s a lot of difference of opinion and no conclusive evidence.” Clinicians also should make sure that something else – usually depression, anxiety, insomnia, and dizziness – is not hindering effective migraine management. “If someone has terrible insomnia, until you treat the insomnia, the headaches aren’t going to get better,” he said.
As for Mira, her migraine attacks did not significantly improve, despite trying triptans, prophylactics, lifestyle changes, and shots to block nerve pain. When the headaches threatened Mira’s chance to go to her prom, her neurologist suggested trying something different. The physician persuaded the family’s insurance to cover a calcitonin gene-related peptide antagonist, an injectable monoclonal antibody treatment for migraine that the FDA has currently approved only for use in adults.
The difference for Mira has been extraordinary.
“I can do so much more than I was able to do,” said Mira, who attended the dance migraine free. “I feel liberated.”
It’s only migraine
One of the greatest challenges in diagnosing migraine can be reassuring the patient, the parents, even clinicians themselves that migraine really is the cause of all this pain and discomfort, experts said.
“A lot of migraine treatment actually comes down to migraine education,” Dr. VanderPluym said.
Patients and their parents often wonder how they can be sure that this pain is not resulting from something more dangerous than migraine, Dr. Little said. In these cases, he cites practice guidelines published by the American Academy of Neurology.
“The gist of those guidelines is that most pediatric patients do not need further workup,” he said. “But I think that there’s always a fear that you’re missing something because we don’t have a test that we can do” for migraine.
Some warning signs that further tests might be warranted, Dr. Kelley said, include:
- Headaches that wake a patient up in the middle of the night.
- Headaches that start first thing in the morning, especially those that include vomiting.
- A headache pattern that suddenly gets much worse.
- Certain symptoms that accompany the headache, such as tingling, numbness or double vision.
Although all of these signs can still stem from migraines – tingling or numbness, for instance, can be signs of migraine aura – running additional tests can rule out more serious concerns, she said.
By the time Mira Halker started high school, hardly a day passed that she wasn’t either getting a migraine attack or recovering from one. She missed volleyball team practice. She missed classes. She missed social events. And few people understood. After all, she looked healthy.
“A lot of times, people think I’m faking it,” said Mira, now 16, who lives in Phoenix. Friends called her flaky; her volleyball coaches questioned her dedication to the team. “I’m like, ‘I’m not trying to get out of this. This is not what this is about,’ ” she said.
Her mother, Rashmi B. Halker Singh, MD, is a neurologist at Mayo Clinic who happens to specialize in migraine. Even so, finding a solution was not easy. Neither ibuprofen nor triptans, nor various preventive measures such as a daily prescription for topiramate controlled the pain and associated symptoms. Mira was barely making it through her school day and had to quit volleyball. Then, in the spring of 10th grade, Mira told her mother that she couldn’t go to prom because the loud noises and lights could give her a migraine attack.
Mother and daughter decided it was time to get even more aggressive. “There are these key moments in life that you can’t get back,” Dr. Singh said. “Migraine steals so much from you.”
Diagnosis
One of the challenges Mira’s physicians faced was deciding which medications and other therapies to prescribe to a teenager. Drug companies have been releasing a steady stream of new treatments for migraine headaches, and researchers promise more are on the way soon. Here’s what works for children, what hasn’t yet been approved for use with minors, and how to diagnose migraines in the first place, from experts at some of the nation’s leading pediatric headache centers.
Migraine affects about 10% of children, according to the American Migraine Foundation. The headaches can strike children as early as age 3 or 4 years, said Robert Little, MD, a pediatric neurologist at Phoenix Children’s Hospital.
Before puberty, boys report more migraine attacks than girls, according to the American Academy of Pediatrics. But that reverses in adolescence: By age 17, as many as 8% of boys and 23% of girls have had migraine. To diagnose migraine, Juliana H. VanderPluym, MD, associate professor of neurology at Mayo Clinic in Phoenix, said she uses the criteria published in the latest edition of the International Classification of Headache Disorders (ICHD): A patient must have had at least five attacks in their life; and in children and adolescents, the attacks must last no less than 2 hours.
In addition, the headaches should exhibit at least two out of four features:
1. Occur more on one side of the head than the other (although Dr. VanderPluym said in children and adolescents headaches often are bilateral).
2. Be of moderate to severe intensity.
3. Have a pounding or throbbing quality.
4. Grow worse with activity or cause an avoidance of activity.
If the attacks meet those criteria, clinicians should check to see if they meet at least one out of the two following:
1. Are sensitive to light and sounds.
2. Are associated with nausea and/or vomiting.
A clinician should consider whether the headaches are not better accounted for by another diagnosis, according to the ICHD criteria. But, Dr. VanderPluym warned that does not necessarily mean running a slew of tests.
“In the absence of red flag features, it is more than likely going to be migraine headache,” she said. That’s especially true if a child has a family history of migraine, as the condition is often passed down from parent to child.
Ultimately, the diagnosis is fairly simple and can be made in a minute or less, said Jack Gladstein, MD, a pediatrician at the University of Maryland whose research focuses on the clinical care of children and adolescents with headache.
“Migraine is acute,” Dr. Gladstein said. “It’s really bad. And it’s recurrent.”
First line of treatment
Whatever a patient takes to treat a migraine, they should hit it early and hard, Dr. Gladstein said.
“The first thing you say, as a primary care physician, is treat your migraine at first twinge, whatever you use. Don’t wait, don’t wish it away,” he said. “The longer you wait, the less chance anything will work.”
The second piece of advice, Dr. Gladstein said, is that whatever drug a patient is taking, they should be on the highest feasible dose. “Work as fast as you can to treat them. You want the brain to reset as quickly as you can,” he said.
Patients should begin with over-the-counter pain relievers, Dr. Little said. If those prove insufficient, they can try a triptan. Rizatriptan is the only such agent that the Food and Drug Administration has approved for children aged 6-17 years. Other drugs in the class – sumatriptan/naproxen, almotriptan, and zolmitriptan – are approved for children 12 and older.
Another migraine therapy recently approved for children aged 12 and older is the use of neurostimulators. “It’s helpful to be aware of them,” Dr. VanderPluym said.
However, if neurostimulators and acute medications prove insufficient, clinicians should warn patients not to up their doses of triptans. Rebound headaches can occur if patients take triptans more than twice a week, or a maximum 10 days per month.
Another possibility is to add a preventive therapy. One mild, first option is nutraceuticals, like riboflavin (vitamin B2) or magnesium, said Anisa F. Kelley, MD, a neurologist and associate director of the headache program at the Ann and Robert H. Lurie Children’s Hospital of Chicago.
“We don’t have definitive evidence, but they’re probably doing more benefit than they are harm,” Dr. Kelley said of these therapies. “In patients who have anywhere from 4 to 8 migraine days a month, where you’re in that in-between period where you don’t necessarily need a [prescription] prophylactic, I will often start with a nutraceutical,” Dr. Kelley said.
For those patients who don’t respond to nutraceuticals, or who need more support, clinicians can prescribe amitriptyline or topiramate. Dr. VanderPluym said.
A 2017 study found such prophylactics to be no more effective than placebo in pediatric migraine patients, but experts caution the results should not be considered definitive.
For one thing, the study enrolled a highly selective group of participants, with milder forms of migraine who may have improved anyway, Dr. VanderPluym said. All participants also received lifestyle counseling.
Every time participants came in for a follow-up, they were asked questions such as how much water were they drinking and how much sleep were they getting, Dr. Kelley noted. The takeaway, she said: “Pediatric and adolescent migraine [management] is very, very much reliant on lifestyle factors.”
Lifestyle triggers
Clinicians should counsel their migraine patients about lifestyle changes, experts said. Getting adequate sleep, staying hydrated, and managing stress can help reduce the intensity and frequency of attacks.
Migraine patients should also be mindful of their screen time, Dr. Kelley added.
“I’ve had lots and lots of patients who find excessive screen time will trigger or worsen migraine,” she said.
As for other potential triggers of attacks, the evidence is mixed.
“There’s clearly an association with disrupted sleep and migraine, and that has been very well established,” Dr. Little said. “And there is some modest amount of evidence that regular exercise can be helpful.” But for reported food triggers, he said, there have been very inconclusive results.
Commonly reported triggers include MSG, red wine, chocolate, and aged cheese. When Dr. Little’s patients keep headache diaries, tracking their meals alongside when they got migraine attacks, they often discover individualized triggers – strawberries, for instance, in one case, he said.
Scientists believe migraines result from the inappropriate activation of the trigeminal ganglion. “The question is, what causes it to get triggered? And how does it get triggered?” Dr. Gladstein said. “And that’s where there’s a lot of difference of opinion and no conclusive evidence.” Clinicians also should make sure that something else – usually depression, anxiety, insomnia, and dizziness – is not hindering effective migraine management. “If someone has terrible insomnia, until you treat the insomnia, the headaches aren’t going to get better,” he said.
As for Mira, her migraine attacks did not significantly improve, despite trying triptans, prophylactics, lifestyle changes, and shots to block nerve pain. When the headaches threatened Mira’s chance to go to her prom, her neurologist suggested trying something different. The physician persuaded the family’s insurance to cover a calcitonin gene-related peptide antagonist, an injectable monoclonal antibody treatment for migraine that the FDA has currently approved only for use in adults.
The difference for Mira has been extraordinary.
“I can do so much more than I was able to do,” said Mira, who attended the dance migraine free. “I feel liberated.”
It’s only migraine
One of the greatest challenges in diagnosing migraine can be reassuring the patient, the parents, even clinicians themselves that migraine really is the cause of all this pain and discomfort, experts said.
“A lot of migraine treatment actually comes down to migraine education,” Dr. VanderPluym said.
Patients and their parents often wonder how they can be sure that this pain is not resulting from something more dangerous than migraine, Dr. Little said. In these cases, he cites practice guidelines published by the American Academy of Neurology.
“The gist of those guidelines is that most pediatric patients do not need further workup,” he said. “But I think that there’s always a fear that you’re missing something because we don’t have a test that we can do” for migraine.
Some warning signs that further tests might be warranted, Dr. Kelley said, include:
- Headaches that wake a patient up in the middle of the night.
- Headaches that start first thing in the morning, especially those that include vomiting.
- A headache pattern that suddenly gets much worse.
- Certain symptoms that accompany the headache, such as tingling, numbness or double vision.
Although all of these signs can still stem from migraines – tingling or numbness, for instance, can be signs of migraine aura – running additional tests can rule out more serious concerns, she said.
New and Improved Devices Add More Therapeutic Options for Treatment of Migraine
Since the mid-2010s, the US Food and Drug Administration (FDA) has approved or cleared no fewer than 10 migraine treatments in the form of orals, injectables, nasal sprays, and devices. The medical achievements of the last decade in the field of migraine have been nothing less than stunning for physicians and their patients, whether they relied on off-label medications or those sanctioned by the FDA to treat patients living with migraine.
That said, the newer orals and injectables cannot help everyone living with migraine. The small molecule calcitonin gene-related peptide (CGRP) receptor antagonists (gepants) and the monoclonal antibodies that target the CGRP ligand or receptor, while well received by patients and physicians alike, have drawbacks for some patients, including lack of efficacy, slow response rate, and adverse events that prevent some patients from taking them. The gepants, which are oral medications—as opposed to the CGRP monoclonal antibody injectables—can occasionally cause enough nausea, drowsiness, and constipation for patients to choose to discontinue their use.
Certain patients have other reasons to shun orals and injectables. Some cannot swallow pills while others fear or do not tolerate injections. Insurance companies limit the quantity of acute care medications, so some patients cannot treat every migraine attack. Then there are those who have failed so many therapies in the past that they will not try the latest one. Consequently, some lie in bed, vomiting until the pain is gone, and some take too many over-the-counter or migraine-specific products, which make migraine symptoms worse if they develop medication overuse headache. And lastly, there are patients who have never walked through a physician’s door to secure a migraine diagnosis and get appropriate treatment.
Non interventional medical devices cleared by the FDA now allow physicians to offer relief to patients with migraine. They work either through various types of electrical neuromodulation to nerves outside the brain or they apply magnetic stimulation to the back of the brain itself to reach pain-associated pathways. A 2019 report on pain management from the US Department of Health and Human Services noted that some randomized control trials (RCTs) and other studies “have demonstrated that noninvasive vagal nerve stimulation can be effective in ameliorating pain in various types of cluster headaches and migraines.”
At least 3 devices, 1 designed to stimulate both the occipital and trigeminal nerves (eCOT-NS, Relivion, Neurolief Ltd), 1 that stimulates the vagus nerve noninvasively (nVNS, gammaCORE, electroCore), and 1 that stimulates peripheral nerves in the upper arm (remote electrical neuromodulation [REN], Nerivio, Theranica Bio-Electronics Ltd), are FDA cleared to treat episodic and chronic migraine. nVNS is also cleared to treat migraine, episodic cluster headache acutely, and chronic cluster acutely in connection with medication.
Real-world studies on all migraine treatments, especially the devices, are flooding PubMed. As for a physician’s observation, we will get to that shortly.
The Devices
Nerivio
Theranica Bio-Electronics Ltd makes a REN called Nerivio, which was FDA cleared in January 2021 to treat episodic migraine acutely in adults and adolescents. Studies have shown its effectiveness for chronic migraine patients who are treated acutely, and it has also helped patients with menstrual migraine. The patient wears the device on the upper arm. Sensory fibers, once stimulated in the arm, send an impulse to the brainstem to affect the serotonin- and norepinephrine-modulated descending inhibitory pathway to disrupt incoming pain messaging. Theranica has applied to the FDA for clearance to treat patients with chronic migraine, as well as for prevention.
Relivion
Neurolief Ltd created the external combined occipital and trigeminal nerve stimulation device (eCOT-NS), which stimulates both the occipital and trigeminal nerves. It has multiple output electrodes, which are placed on the forehead to stimulate the trigeminal supraorbital and supratrochlear nerve branches bilaterally, and over the occipital nerves in the back of the head. It is worn like a tiara as it must be in good contact with the forehead and the back of the head simultaneously. It is FDA cleared to treat acute migraine.
gammaCORE
gammaCORE is a nVNS device that is FDA cleared for acute and preventive treatment of migraine in adolescents and adults, and acute and preventive treatment of episodic cluster headache in adults. It is also cleared to treat chronic cluster headache acutely along with medication. The patient applies gel to the device’s 2 electrical contacts and then locates the vagus nerve on the side of the neck and applies the electrodes to the area that will be treated. Patients can adjust the stimulation’s intensity so that they can barely feel the stimulation; it has not been reported to be painful. nVNS is also an FDA cleared treatment for paroxysmal hemicrania and hemicrania continua.
SAVI Dual
The s-TMS (SAVI Dual, formerly called the Spring TMS and the sTMS mini), made by eNeura, is a single-pulse, transcranial magnetic stimulation applied to the back of the head to stimulate the occipital lobes in the brain. It was FDA cleared for acute and preventive care of migraine in adolescents over 12 years and for adults in February 2019. The patient holds a handheld magnetic device against their occiput, and when the tool is discharged, a brief magnetic pulse interrupts the pattern of neuronal firing (probably cortical spreading depression) that can trigger migraine and the visual aura associated with migraine in one-third of patients.
Cefaly
The e-TNS (Cefaly) works by external trigeminal nerve stimulation of the supraorbital and trochlear nerves bilaterally in the forehead. It gradually and automatically increases in intensity and can be controlled by the patient. It is FDA cleared for acute and preventive treatment of migraine, and, unlike the other devices, it is sold over the counter without a prescription. According to the company website, there are 3 devices: 1 is for acute treatment, 1 is for preventive treatment, and 1 device has 2 settings for both acute and preventive treatment.
The Studies
While most of the published studies on devices are company-sponsored, these device makers have underwritten numerous, sometimes very well-designed, studies on their products. A review by VanderPluym et al described those studies and their various risks of bias.
There are at least 10 studies on REN published so far. These include 2 randomized, sham-controlled trials looking at pain freedom and pain relief at 2 hours after stimulation begins. Another study detailed treatment reports from many patients in which 66.5% experienced pain relief at 2 hours post treatment initiation in half of their treatments. A subgroup of 16% of those patients were prescribed REN by their primary care physicians. Of that group, 77.8% experienced pain relief in half their treatments. That figure was very close to another study that found that 23 of 31 (74.2%) of the study patients treated virtually by non headache providers found relief in 50% of their headaches. REN comes with an education and behavioral medicine app that is used during treatment. A study done by the company shows that when a patient uses the relaxation app along with the standard stimulation, they do considerably better than with stimulation alone.
The eCOT-NS has also been tested in an RCT. At 2 hours, the responder rate was twice as high as in the sham group (66.7% vs 32%). Overall headache relief at 2 hours was higher in the responder group (76% vs 31.6%). In a study collecting real-world data on the efficacy of eCOT-NS in the preventive treatment of migraine (abstract data were presented at the American Headache Society meeting in June 2022), there was a 65.3% reduction in monthly migraine days (MMD) from baseline through 6 months. Treatment reduced MMD by 10.0 (from 15.3 to 5.3—a 76.8% reduction), and reduced acute medication use days (12.5 at baseline to 2.9) at 6 months.
Users of nVNS discussed their experiences with the device, which is the size of a large bar of soap, in a patient registry. They reported 192 attacks, with a mean pain score starting at 2.7 and dropping to 1.3 after 30 minutes. The pain levels of 70% of the attacks dropped to either mild or nonexistent. In a multicenter study on nNVS, 48 patients and 44 sham patients with episodic and chronic cluster headache showed no significant difference in the primary endpoint of pain freedom at 15 minutes between the nVNS and sham. There was also no difference in the chronic cluster headache group. But the episodic cluster subgroup showed a difference; nVNS was superior to sham, 48% to 6% (P
The e-TNS device is cleared for treating adults with migraine, acutely and preventively. It received initial clearance in 2017; in 2020, Cefaly Technology received clearance from the FDA to sell its products over the counter. The device, which resembles a large diamond that affixes to the forehead, has received differing reviews between various patient reports (found online at major retailer sites) and study results. In a blinded, intent-to-treat study involving 538 patients, 25.5% of the verum group reported they were pain-free at 2 hours; 18.3% in the sham group reported the same. Additionally, 56.4% of the subjects in the verum group reported they were free of the most bothersome migraine symptoms, as opposed to 42.3% of the sham group.
Adverse Events
The adverse events observed with these devices were, overall, relatively mild, and disappeared once the device was shut off. A few nVNS users said they experienced discomfort at the application site. With REN, 59 of 12,368 patients reported device-related issues; the vast majority were considered mild and consisted mostly of a sensation of warmth under the device. Of the 259 e-TNS users, 8.5% reported minor and reversible occurrences, such as treatment-related discomfort, paresthesia, and burning.
Patients in the Clinic
A few observations from the clinic regarding these devices:
Some devices are easier to use than others. I know this, because at a recent demonstration session in a course for physicians on headache treatment, I agreed to be the person on whom the device was demonstrated. The physician applying the device had difficulty aligning the device’s sensors with the appropriate nerves. Making sure your patients use these devices correctly is essential, and you or your staff should demonstrate their use to the patient. No doubt, this could be time-consuming in some cases, and patients who are reading the device’s instructions while in pain will likely get frustrated if they cannot get the device to work.
Some patients who have failed every medication class can occasionally find partial relief with these devices. One longtime patient of mine came to me severely disabled from chronic migraine and medication overuse headache but was somewhat better with 2 preventive medications. Triptans worked acutely, but she developed nearly every side effect imaginable. I was able to reverse her medication overuse headache, but the gepants, although they worked somewhat, took too long to take effect. We agreed the next step would be to use REN for each migraine attack, combined with acute care medication if necessary. (She uses REN alone for a milder headache and adds a gepant with naproxen if necessary.) She has found using the relaxation module on the REN app increases her chances of eliminating the migraine. She is not pain free all the time, but she appreciates the pain-free intervals.
One chronic cluster patient has relied on subcutaneous sumatriptan and breathing 100% oxygen at 12 liters per minute through a mask over his nose and mouth for acute relief from his headaches. His headache pain can climb from a 3 to a 10 in a matter of minutes. It starts behind and a bit above the right eye where he feels a tremendous pressure building up. He says that at times it feels like a screwdriver has been thrust into his eye and is being turned. Along with the pain, the eye becomes red, the pupil constricts, and the eyelid droops. He also has dripping from the right nostril, which stuffs up when the pain abates. The pain lasts for 1 to 2 hours, then returns 3 to 5 times a day for 5 days a week, on average. The pain never goes away for more than 3 weeks in a year’s time, hence the reason for his chronic cluster headache diagnosis. He is now using nVNS as soon as he feels the pain coming on. If the device does not provide sufficient relief, he uses oxygen or takes the sumatriptan injection.
Some patients who get cluster headaches think of suicide if the pain cannot be stopped; but in my experience, most can become pain free, or at least realize some partial relief from a variety of treatments (sometimes given at the same time).
Doctors often do not think of devices as options, and some doctors think devices do not work even though they have no experience with using them. Devices can give good relief on their own, and when a severe headache needs stronger treatment, medications added to a device usually work better than either treatment alone.
Since the mid-2010s, the US Food and Drug Administration (FDA) has approved or cleared no fewer than 10 migraine treatments in the form of orals, injectables, nasal sprays, and devices. The medical achievements of the last decade in the field of migraine have been nothing less than stunning for physicians and their patients, whether they relied on off-label medications or those sanctioned by the FDA to treat patients living with migraine.
That said, the newer orals and injectables cannot help everyone living with migraine. The small molecule calcitonin gene-related peptide (CGRP) receptor antagonists (gepants) and the monoclonal antibodies that target the CGRP ligand or receptor, while well received by patients and physicians alike, have drawbacks for some patients, including lack of efficacy, slow response rate, and adverse events that prevent some patients from taking them. The gepants, which are oral medications—as opposed to the CGRP monoclonal antibody injectables—can occasionally cause enough nausea, drowsiness, and constipation for patients to choose to discontinue their use.
Certain patients have other reasons to shun orals and injectables. Some cannot swallow pills while others fear or do not tolerate injections. Insurance companies limit the quantity of acute care medications, so some patients cannot treat every migraine attack. Then there are those who have failed so many therapies in the past that they will not try the latest one. Consequently, some lie in bed, vomiting until the pain is gone, and some take too many over-the-counter or migraine-specific products, which make migraine symptoms worse if they develop medication overuse headache. And lastly, there are patients who have never walked through a physician’s door to secure a migraine diagnosis and get appropriate treatment.
Non interventional medical devices cleared by the FDA now allow physicians to offer relief to patients with migraine. They work either through various types of electrical neuromodulation to nerves outside the brain or they apply magnetic stimulation to the back of the brain itself to reach pain-associated pathways. A 2019 report on pain management from the US Department of Health and Human Services noted that some randomized control trials (RCTs) and other studies “have demonstrated that noninvasive vagal nerve stimulation can be effective in ameliorating pain in various types of cluster headaches and migraines.”
At least 3 devices, 1 designed to stimulate both the occipital and trigeminal nerves (eCOT-NS, Relivion, Neurolief Ltd), 1 that stimulates the vagus nerve noninvasively (nVNS, gammaCORE, electroCore), and 1 that stimulates peripheral nerves in the upper arm (remote electrical neuromodulation [REN], Nerivio, Theranica Bio-Electronics Ltd), are FDA cleared to treat episodic and chronic migraine. nVNS is also cleared to treat migraine, episodic cluster headache acutely, and chronic cluster acutely in connection with medication.
Real-world studies on all migraine treatments, especially the devices, are flooding PubMed. As for a physician’s observation, we will get to that shortly.
The Devices
Nerivio
Theranica Bio-Electronics Ltd makes a REN called Nerivio, which was FDA cleared in January 2021 to treat episodic migraine acutely in adults and adolescents. Studies have shown its effectiveness for chronic migraine patients who are treated acutely, and it has also helped patients with menstrual migraine. The patient wears the device on the upper arm. Sensory fibers, once stimulated in the arm, send an impulse to the brainstem to affect the serotonin- and norepinephrine-modulated descending inhibitory pathway to disrupt incoming pain messaging. Theranica has applied to the FDA for clearance to treat patients with chronic migraine, as well as for prevention.
Relivion
Neurolief Ltd created the external combined occipital and trigeminal nerve stimulation device (eCOT-NS), which stimulates both the occipital and trigeminal nerves. It has multiple output electrodes, which are placed on the forehead to stimulate the trigeminal supraorbital and supratrochlear nerve branches bilaterally, and over the occipital nerves in the back of the head. It is worn like a tiara as it must be in good contact with the forehead and the back of the head simultaneously. It is FDA cleared to treat acute migraine.
gammaCORE
gammaCORE is a nVNS device that is FDA cleared for acute and preventive treatment of migraine in adolescents and adults, and acute and preventive treatment of episodic cluster headache in adults. It is also cleared to treat chronic cluster headache acutely along with medication. The patient applies gel to the device’s 2 electrical contacts and then locates the vagus nerve on the side of the neck and applies the electrodes to the area that will be treated. Patients can adjust the stimulation’s intensity so that they can barely feel the stimulation; it has not been reported to be painful. nVNS is also an FDA cleared treatment for paroxysmal hemicrania and hemicrania continua.
SAVI Dual
The s-TMS (SAVI Dual, formerly called the Spring TMS and the sTMS mini), made by eNeura, is a single-pulse, transcranial magnetic stimulation applied to the back of the head to stimulate the occipital lobes in the brain. It was FDA cleared for acute and preventive care of migraine in adolescents over 12 years and for adults in February 2019. The patient holds a handheld magnetic device against their occiput, and when the tool is discharged, a brief magnetic pulse interrupts the pattern of neuronal firing (probably cortical spreading depression) that can trigger migraine and the visual aura associated with migraine in one-third of patients.
Cefaly
The e-TNS (Cefaly) works by external trigeminal nerve stimulation of the supraorbital and trochlear nerves bilaterally in the forehead. It gradually and automatically increases in intensity and can be controlled by the patient. It is FDA cleared for acute and preventive treatment of migraine, and, unlike the other devices, it is sold over the counter without a prescription. According to the company website, there are 3 devices: 1 is for acute treatment, 1 is for preventive treatment, and 1 device has 2 settings for both acute and preventive treatment.
The Studies
While most of the published studies on devices are company-sponsored, these device makers have underwritten numerous, sometimes very well-designed, studies on their products. A review by VanderPluym et al described those studies and their various risks of bias.
There are at least 10 studies on REN published so far. These include 2 randomized, sham-controlled trials looking at pain freedom and pain relief at 2 hours after stimulation begins. Another study detailed treatment reports from many patients in which 66.5% experienced pain relief at 2 hours post treatment initiation in half of their treatments. A subgroup of 16% of those patients were prescribed REN by their primary care physicians. Of that group, 77.8% experienced pain relief in half their treatments. That figure was very close to another study that found that 23 of 31 (74.2%) of the study patients treated virtually by non headache providers found relief in 50% of their headaches. REN comes with an education and behavioral medicine app that is used during treatment. A study done by the company shows that when a patient uses the relaxation app along with the standard stimulation, they do considerably better than with stimulation alone.
The eCOT-NS has also been tested in an RCT. At 2 hours, the responder rate was twice as high as in the sham group (66.7% vs 32%). Overall headache relief at 2 hours was higher in the responder group (76% vs 31.6%). In a study collecting real-world data on the efficacy of eCOT-NS in the preventive treatment of migraine (abstract data were presented at the American Headache Society meeting in June 2022), there was a 65.3% reduction in monthly migraine days (MMD) from baseline through 6 months. Treatment reduced MMD by 10.0 (from 15.3 to 5.3—a 76.8% reduction), and reduced acute medication use days (12.5 at baseline to 2.9) at 6 months.
Users of nVNS discussed their experiences with the device, which is the size of a large bar of soap, in a patient registry. They reported 192 attacks, with a mean pain score starting at 2.7 and dropping to 1.3 after 30 minutes. The pain levels of 70% of the attacks dropped to either mild or nonexistent. In a multicenter study on nNVS, 48 patients and 44 sham patients with episodic and chronic cluster headache showed no significant difference in the primary endpoint of pain freedom at 15 minutes between the nVNS and sham. There was also no difference in the chronic cluster headache group. But the episodic cluster subgroup showed a difference; nVNS was superior to sham, 48% to 6% (P
The e-TNS device is cleared for treating adults with migraine, acutely and preventively. It received initial clearance in 2017; in 2020, Cefaly Technology received clearance from the FDA to sell its products over the counter. The device, which resembles a large diamond that affixes to the forehead, has received differing reviews between various patient reports (found online at major retailer sites) and study results. In a blinded, intent-to-treat study involving 538 patients, 25.5% of the verum group reported they were pain-free at 2 hours; 18.3% in the sham group reported the same. Additionally, 56.4% of the subjects in the verum group reported they were free of the most bothersome migraine symptoms, as opposed to 42.3% of the sham group.
Adverse Events
The adverse events observed with these devices were, overall, relatively mild, and disappeared once the device was shut off. A few nVNS users said they experienced discomfort at the application site. With REN, 59 of 12,368 patients reported device-related issues; the vast majority were considered mild and consisted mostly of a sensation of warmth under the device. Of the 259 e-TNS users, 8.5% reported minor and reversible occurrences, such as treatment-related discomfort, paresthesia, and burning.
Patients in the Clinic
A few observations from the clinic regarding these devices:
Some devices are easier to use than others. I know this, because at a recent demonstration session in a course for physicians on headache treatment, I agreed to be the person on whom the device was demonstrated. The physician applying the device had difficulty aligning the device’s sensors with the appropriate nerves. Making sure your patients use these devices correctly is essential, and you or your staff should demonstrate their use to the patient. No doubt, this could be time-consuming in some cases, and patients who are reading the device’s instructions while in pain will likely get frustrated if they cannot get the device to work.
Some patients who have failed every medication class can occasionally find partial relief with these devices. One longtime patient of mine came to me severely disabled from chronic migraine and medication overuse headache but was somewhat better with 2 preventive medications. Triptans worked acutely, but she developed nearly every side effect imaginable. I was able to reverse her medication overuse headache, but the gepants, although they worked somewhat, took too long to take effect. We agreed the next step would be to use REN for each migraine attack, combined with acute care medication if necessary. (She uses REN alone for a milder headache and adds a gepant with naproxen if necessary.) She has found using the relaxation module on the REN app increases her chances of eliminating the migraine. She is not pain free all the time, but she appreciates the pain-free intervals.
One chronic cluster patient has relied on subcutaneous sumatriptan and breathing 100% oxygen at 12 liters per minute through a mask over his nose and mouth for acute relief from his headaches. His headache pain can climb from a 3 to a 10 in a matter of minutes. It starts behind and a bit above the right eye where he feels a tremendous pressure building up. He says that at times it feels like a screwdriver has been thrust into his eye and is being turned. Along with the pain, the eye becomes red, the pupil constricts, and the eyelid droops. He also has dripping from the right nostril, which stuffs up when the pain abates. The pain lasts for 1 to 2 hours, then returns 3 to 5 times a day for 5 days a week, on average. The pain never goes away for more than 3 weeks in a year’s time, hence the reason for his chronic cluster headache diagnosis. He is now using nVNS as soon as he feels the pain coming on. If the device does not provide sufficient relief, he uses oxygen or takes the sumatriptan injection.
Some patients who get cluster headaches think of suicide if the pain cannot be stopped; but in my experience, most can become pain free, or at least realize some partial relief from a variety of treatments (sometimes given at the same time).
Doctors often do not think of devices as options, and some doctors think devices do not work even though they have no experience with using them. Devices can give good relief on their own, and when a severe headache needs stronger treatment, medications added to a device usually work better than either treatment alone.
Since the mid-2010s, the US Food and Drug Administration (FDA) has approved or cleared no fewer than 10 migraine treatments in the form of orals, injectables, nasal sprays, and devices. The medical achievements of the last decade in the field of migraine have been nothing less than stunning for physicians and their patients, whether they relied on off-label medications or those sanctioned by the FDA to treat patients living with migraine.
That said, the newer orals and injectables cannot help everyone living with migraine. The small molecule calcitonin gene-related peptide (CGRP) receptor antagonists (gepants) and the monoclonal antibodies that target the CGRP ligand or receptor, while well received by patients and physicians alike, have drawbacks for some patients, including lack of efficacy, slow response rate, and adverse events that prevent some patients from taking them. The gepants, which are oral medications—as opposed to the CGRP monoclonal antibody injectables—can occasionally cause enough nausea, drowsiness, and constipation for patients to choose to discontinue their use.
Certain patients have other reasons to shun orals and injectables. Some cannot swallow pills while others fear or do not tolerate injections. Insurance companies limit the quantity of acute care medications, so some patients cannot treat every migraine attack. Then there are those who have failed so many therapies in the past that they will not try the latest one. Consequently, some lie in bed, vomiting until the pain is gone, and some take too many over-the-counter or migraine-specific products, which make migraine symptoms worse if they develop medication overuse headache. And lastly, there are patients who have never walked through a physician’s door to secure a migraine diagnosis and get appropriate treatment.
Non interventional medical devices cleared by the FDA now allow physicians to offer relief to patients with migraine. They work either through various types of electrical neuromodulation to nerves outside the brain or they apply magnetic stimulation to the back of the brain itself to reach pain-associated pathways. A 2019 report on pain management from the US Department of Health and Human Services noted that some randomized control trials (RCTs) and other studies “have demonstrated that noninvasive vagal nerve stimulation can be effective in ameliorating pain in various types of cluster headaches and migraines.”
At least 3 devices, 1 designed to stimulate both the occipital and trigeminal nerves (eCOT-NS, Relivion, Neurolief Ltd), 1 that stimulates the vagus nerve noninvasively (nVNS, gammaCORE, electroCore), and 1 that stimulates peripheral nerves in the upper arm (remote electrical neuromodulation [REN], Nerivio, Theranica Bio-Electronics Ltd), are FDA cleared to treat episodic and chronic migraine. nVNS is also cleared to treat migraine, episodic cluster headache acutely, and chronic cluster acutely in connection with medication.
Real-world studies on all migraine treatments, especially the devices, are flooding PubMed. As for a physician’s observation, we will get to that shortly.
The Devices
Nerivio
Theranica Bio-Electronics Ltd makes a REN called Nerivio, which was FDA cleared in January 2021 to treat episodic migraine acutely in adults and adolescents. Studies have shown its effectiveness for chronic migraine patients who are treated acutely, and it has also helped patients with menstrual migraine. The patient wears the device on the upper arm. Sensory fibers, once stimulated in the arm, send an impulse to the brainstem to affect the serotonin- and norepinephrine-modulated descending inhibitory pathway to disrupt incoming pain messaging. Theranica has applied to the FDA for clearance to treat patients with chronic migraine, as well as for prevention.
Relivion
Neurolief Ltd created the external combined occipital and trigeminal nerve stimulation device (eCOT-NS), which stimulates both the occipital and trigeminal nerves. It has multiple output electrodes, which are placed on the forehead to stimulate the trigeminal supraorbital and supratrochlear nerve branches bilaterally, and over the occipital nerves in the back of the head. It is worn like a tiara as it must be in good contact with the forehead and the back of the head simultaneously. It is FDA cleared to treat acute migraine.
gammaCORE
gammaCORE is a nVNS device that is FDA cleared for acute and preventive treatment of migraine in adolescents and adults, and acute and preventive treatment of episodic cluster headache in adults. It is also cleared to treat chronic cluster headache acutely along with medication. The patient applies gel to the device’s 2 electrical contacts and then locates the vagus nerve on the side of the neck and applies the electrodes to the area that will be treated. Patients can adjust the stimulation’s intensity so that they can barely feel the stimulation; it has not been reported to be painful. nVNS is also an FDA cleared treatment for paroxysmal hemicrania and hemicrania continua.
SAVI Dual
The s-TMS (SAVI Dual, formerly called the Spring TMS and the sTMS mini), made by eNeura, is a single-pulse, transcranial magnetic stimulation applied to the back of the head to stimulate the occipital lobes in the brain. It was FDA cleared for acute and preventive care of migraine in adolescents over 12 years and for adults in February 2019. The patient holds a handheld magnetic device against their occiput, and when the tool is discharged, a brief magnetic pulse interrupts the pattern of neuronal firing (probably cortical spreading depression) that can trigger migraine and the visual aura associated with migraine in one-third of patients.
Cefaly
The e-TNS (Cefaly) works by external trigeminal nerve stimulation of the supraorbital and trochlear nerves bilaterally in the forehead. It gradually and automatically increases in intensity and can be controlled by the patient. It is FDA cleared for acute and preventive treatment of migraine, and, unlike the other devices, it is sold over the counter without a prescription. According to the company website, there are 3 devices: 1 is for acute treatment, 1 is for preventive treatment, and 1 device has 2 settings for both acute and preventive treatment.
The Studies
While most of the published studies on devices are company-sponsored, these device makers have underwritten numerous, sometimes very well-designed, studies on their products. A review by VanderPluym et al described those studies and their various risks of bias.
There are at least 10 studies on REN published so far. These include 2 randomized, sham-controlled trials looking at pain freedom and pain relief at 2 hours after stimulation begins. Another study detailed treatment reports from many patients in which 66.5% experienced pain relief at 2 hours post treatment initiation in half of their treatments. A subgroup of 16% of those patients were prescribed REN by their primary care physicians. Of that group, 77.8% experienced pain relief in half their treatments. That figure was very close to another study that found that 23 of 31 (74.2%) of the study patients treated virtually by non headache providers found relief in 50% of their headaches. REN comes with an education and behavioral medicine app that is used during treatment. A study done by the company shows that when a patient uses the relaxation app along with the standard stimulation, they do considerably better than with stimulation alone.
The eCOT-NS has also been tested in an RCT. At 2 hours, the responder rate was twice as high as in the sham group (66.7% vs 32%). Overall headache relief at 2 hours was higher in the responder group (76% vs 31.6%). In a study collecting real-world data on the efficacy of eCOT-NS in the preventive treatment of migraine (abstract data were presented at the American Headache Society meeting in June 2022), there was a 65.3% reduction in monthly migraine days (MMD) from baseline through 6 months. Treatment reduced MMD by 10.0 (from 15.3 to 5.3—a 76.8% reduction), and reduced acute medication use days (12.5 at baseline to 2.9) at 6 months.
Users of nVNS discussed their experiences with the device, which is the size of a large bar of soap, in a patient registry. They reported 192 attacks, with a mean pain score starting at 2.7 and dropping to 1.3 after 30 minutes. The pain levels of 70% of the attacks dropped to either mild or nonexistent. In a multicenter study on nNVS, 48 patients and 44 sham patients with episodic and chronic cluster headache showed no significant difference in the primary endpoint of pain freedom at 15 minutes between the nVNS and sham. There was also no difference in the chronic cluster headache group. But the episodic cluster subgroup showed a difference; nVNS was superior to sham, 48% to 6% (P
The e-TNS device is cleared for treating adults with migraine, acutely and preventively. It received initial clearance in 2017; in 2020, Cefaly Technology received clearance from the FDA to sell its products over the counter. The device, which resembles a large diamond that affixes to the forehead, has received differing reviews between various patient reports (found online at major retailer sites) and study results. In a blinded, intent-to-treat study involving 538 patients, 25.5% of the verum group reported they were pain-free at 2 hours; 18.3% in the sham group reported the same. Additionally, 56.4% of the subjects in the verum group reported they were free of the most bothersome migraine symptoms, as opposed to 42.3% of the sham group.
Adverse Events
The adverse events observed with these devices were, overall, relatively mild, and disappeared once the device was shut off. A few nVNS users said they experienced discomfort at the application site. With REN, 59 of 12,368 patients reported device-related issues; the vast majority were considered mild and consisted mostly of a sensation of warmth under the device. Of the 259 e-TNS users, 8.5% reported minor and reversible occurrences, such as treatment-related discomfort, paresthesia, and burning.
Patients in the Clinic
A few observations from the clinic regarding these devices:
Some devices are easier to use than others. I know this, because at a recent demonstration session in a course for physicians on headache treatment, I agreed to be the person on whom the device was demonstrated. The physician applying the device had difficulty aligning the device’s sensors with the appropriate nerves. Making sure your patients use these devices correctly is essential, and you or your staff should demonstrate their use to the patient. No doubt, this could be time-consuming in some cases, and patients who are reading the device’s instructions while in pain will likely get frustrated if they cannot get the device to work.
Some patients who have failed every medication class can occasionally find partial relief with these devices. One longtime patient of mine came to me severely disabled from chronic migraine and medication overuse headache but was somewhat better with 2 preventive medications. Triptans worked acutely, but she developed nearly every side effect imaginable. I was able to reverse her medication overuse headache, but the gepants, although they worked somewhat, took too long to take effect. We agreed the next step would be to use REN for each migraine attack, combined with acute care medication if necessary. (She uses REN alone for a milder headache and adds a gepant with naproxen if necessary.) She has found using the relaxation module on the REN app increases her chances of eliminating the migraine. She is not pain free all the time, but she appreciates the pain-free intervals.
One chronic cluster patient has relied on subcutaneous sumatriptan and breathing 100% oxygen at 12 liters per minute through a mask over his nose and mouth for acute relief from his headaches. His headache pain can climb from a 3 to a 10 in a matter of minutes. It starts behind and a bit above the right eye where he feels a tremendous pressure building up. He says that at times it feels like a screwdriver has been thrust into his eye and is being turned. Along with the pain, the eye becomes red, the pupil constricts, and the eyelid droops. He also has dripping from the right nostril, which stuffs up when the pain abates. The pain lasts for 1 to 2 hours, then returns 3 to 5 times a day for 5 days a week, on average. The pain never goes away for more than 3 weeks in a year’s time, hence the reason for his chronic cluster headache diagnosis. He is now using nVNS as soon as he feels the pain coming on. If the device does not provide sufficient relief, he uses oxygen or takes the sumatriptan injection.
Some patients who get cluster headaches think of suicide if the pain cannot be stopped; but in my experience, most can become pain free, or at least realize some partial relief from a variety of treatments (sometimes given at the same time).
Doctors often do not think of devices as options, and some doctors think devices do not work even though they have no experience with using them. Devices can give good relief on their own, and when a severe headache needs stronger treatment, medications added to a device usually work better than either treatment alone.
New recommendations for hyperglycemia management
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
Love them or hate them, masks in schools work
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The body of evidence for Paxlovid therapy
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
I’m a physician battling long COVID. I can assure you it’s real
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
Ivermectin for COVID-19: Final nail in the coffin
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.
But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.

But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.

But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
The marked contrast in pandemic outcomes between Japan and the United States
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
Why people lie about COVID
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Have you ever lied about COVID-19?
Before you get upset, before the “how dare you,” I want you to think carefully.
Did you have COVID-19 (or think you did) and not mention it to someone you were going to be with? Did you tell someone you were taking more COVID precautions than you really were? Did you tell someone you were vaccinated when you weren’t? Have you avoided getting a COVID test even though you knew you should have?
Researchers appreciated the fact that public health interventions in COVID are important but are only as good as the percentage of people who actually abide by them. So, they designed a survey to ask the questions that many people don’t want to hear the answer to.
A total of 1,733 participants – 80% of those invited – responded to the survey. By design, approximately one-third of respondents (477) had already had COVID, one-third (499) were vaccinated and not yet infected, and one-third (509) were unvaccinated and not yet infected.
Of those surveyed, 41.6% admitted that they lied about COVID or didn’t adhere to COVID guidelines - a conservative estimate, if you ask me.
Breaking down some of the results, about 20% of people who previously were infected with COVID said they didn’t mention it when meeting with someone. A similar number said they didn’t tell anyone when they were entering a public place. A bit more concerning to me, roughly 20% reported not disclosing their COVID-positive status when going to a health care provider’s office.
About 10% of those who had not been vaccinated reported lying about their vaccination status. That’s actually less than the 15% of vaccinated people who lied and told someone they weren’t vaccinated.
About 17% of people lied about the need to quarantine, and many more broke quarantine rules.
The authors tried to see if certain personal characteristics predicted people who were more likely to lie about COVID-19–related issues. Turns out there was only one thing that predicted honesty: age.
Older people were more honest about their COVID status and COVID habits. Other factors – gender, education, race, political affiliation, COVID-19 conspiracy beliefs, and where you got your COVID information – did not seem to make much of a difference. Why are older people more honest? Because older people take COVID more seriously. And they should; COVID is more severe in older people.
The problem arises, of course, because people who are at lower risk for COVID complications interact with people at higher risk – and in those situations, honesty matters more.
On the other hand, isn’t lying about COVID stuff inevitable? If you know that a positive test means you can’t go to work, and not going to work means you won’t get paid, might you not be more likely to lie about the test? Or not get the test at all?
The authors explored the reasons for dishonesty and they are fairly broad, ranging from the desire for life to feel normal (more than half of people who lied) to not believing that COVID was real (a whopping 30%). Some of the reasons for lying included:
- Wanted life to feel normal (50%).
- Freedom (45%).
- It’s no one’s business (40%).
- COVID isn’t real (30%).
In the end, though, we need to realize that public health recommendations are not going to be universally followed, and people may tell us they are following them when, in fact, they are not.
What this adds is another data point to a trend we’ve seen across the course of the pandemic, a shift from collective to individual responsibility. If you can’t be sure what others are doing in regard to COVID, you need to focus on protecting yourself. Perhaps that shift was inevitable. Doesn’t mean we have to like it.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Have you ever lied about COVID-19?
Before you get upset, before the “how dare you,” I want you to think carefully.
Did you have COVID-19 (or think you did) and not mention it to someone you were going to be with? Did you tell someone you were taking more COVID precautions than you really were? Did you tell someone you were vaccinated when you weren’t? Have you avoided getting a COVID test even though you knew you should have?
Researchers appreciated the fact that public health interventions in COVID are important but are only as good as the percentage of people who actually abide by them. So, they designed a survey to ask the questions that many people don’t want to hear the answer to.
A total of 1,733 participants – 80% of those invited – responded to the survey. By design, approximately one-third of respondents (477) had already had COVID, one-third (499) were vaccinated and not yet infected, and one-third (509) were unvaccinated and not yet infected.
Of those surveyed, 41.6% admitted that they lied about COVID or didn’t adhere to COVID guidelines - a conservative estimate, if you ask me.
Breaking down some of the results, about 20% of people who previously were infected with COVID said they didn’t mention it when meeting with someone. A similar number said they didn’t tell anyone when they were entering a public place. A bit more concerning to me, roughly 20% reported not disclosing their COVID-positive status when going to a health care provider’s office.
About 10% of those who had not been vaccinated reported lying about their vaccination status. That’s actually less than the 15% of vaccinated people who lied and told someone they weren’t vaccinated.
About 17% of people lied about the need to quarantine, and many more broke quarantine rules.
The authors tried to see if certain personal characteristics predicted people who were more likely to lie about COVID-19–related issues. Turns out there was only one thing that predicted honesty: age.
Older people were more honest about their COVID status and COVID habits. Other factors – gender, education, race, political affiliation, COVID-19 conspiracy beliefs, and where you got your COVID information – did not seem to make much of a difference. Why are older people more honest? Because older people take COVID more seriously. And they should; COVID is more severe in older people.
The problem arises, of course, because people who are at lower risk for COVID complications interact with people at higher risk – and in those situations, honesty matters more.
On the other hand, isn’t lying about COVID stuff inevitable? If you know that a positive test means you can’t go to work, and not going to work means you won’t get paid, might you not be more likely to lie about the test? Or not get the test at all?
The authors explored the reasons for dishonesty and they are fairly broad, ranging from the desire for life to feel normal (more than half of people who lied) to not believing that COVID was real (a whopping 30%). Some of the reasons for lying included:
- Wanted life to feel normal (50%).
- Freedom (45%).
- It’s no one’s business (40%).
- COVID isn’t real (30%).
In the end, though, we need to realize that public health recommendations are not going to be universally followed, and people may tell us they are following them when, in fact, they are not.
What this adds is another data point to a trend we’ve seen across the course of the pandemic, a shift from collective to individual responsibility. If you can’t be sure what others are doing in regard to COVID, you need to focus on protecting yourself. Perhaps that shift was inevitable. Doesn’t mean we have to like it.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Have you ever lied about COVID-19?
Before you get upset, before the “how dare you,” I want you to think carefully.
Did you have COVID-19 (or think you did) and not mention it to someone you were going to be with? Did you tell someone you were taking more COVID precautions than you really were? Did you tell someone you were vaccinated when you weren’t? Have you avoided getting a COVID test even though you knew you should have?
Researchers appreciated the fact that public health interventions in COVID are important but are only as good as the percentage of people who actually abide by them. So, they designed a survey to ask the questions that many people don’t want to hear the answer to.
A total of 1,733 participants – 80% of those invited – responded to the survey. By design, approximately one-third of respondents (477) had already had COVID, one-third (499) were vaccinated and not yet infected, and one-third (509) were unvaccinated and not yet infected.
Of those surveyed, 41.6% admitted that they lied about COVID or didn’t adhere to COVID guidelines - a conservative estimate, if you ask me.
Breaking down some of the results, about 20% of people who previously were infected with COVID said they didn’t mention it when meeting with someone. A similar number said they didn’t tell anyone when they were entering a public place. A bit more concerning to me, roughly 20% reported not disclosing their COVID-positive status when going to a health care provider’s office.
About 10% of those who had not been vaccinated reported lying about their vaccination status. That’s actually less than the 15% of vaccinated people who lied and told someone they weren’t vaccinated.
About 17% of people lied about the need to quarantine, and many more broke quarantine rules.
The authors tried to see if certain personal characteristics predicted people who were more likely to lie about COVID-19–related issues. Turns out there was only one thing that predicted honesty: age.
Older people were more honest about their COVID status and COVID habits. Other factors – gender, education, race, political affiliation, COVID-19 conspiracy beliefs, and where you got your COVID information – did not seem to make much of a difference. Why are older people more honest? Because older people take COVID more seriously. And they should; COVID is more severe in older people.
The problem arises, of course, because people who are at lower risk for COVID complications interact with people at higher risk – and in those situations, honesty matters more.
On the other hand, isn’t lying about COVID stuff inevitable? If you know that a positive test means you can’t go to work, and not going to work means you won’t get paid, might you not be more likely to lie about the test? Or not get the test at all?
The authors explored the reasons for dishonesty and they are fairly broad, ranging from the desire for life to feel normal (more than half of people who lied) to not believing that COVID was real (a whopping 30%). Some of the reasons for lying included:
- Wanted life to feel normal (50%).
- Freedom (45%).
- It’s no one’s business (40%).
- COVID isn’t real (30%).
In the end, though, we need to realize that public health recommendations are not going to be universally followed, and people may tell us they are following them when, in fact, they are not.
What this adds is another data point to a trend we’ve seen across the course of the pandemic, a shift from collective to individual responsibility. If you can’t be sure what others are doing in regard to COVID, you need to focus on protecting yourself. Perhaps that shift was inevitable. Doesn’t mean we have to like it.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com.
