User login
Recent Evidence for Home Phototherapy Benefits May Improve Access for Patients with Psoriasis
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
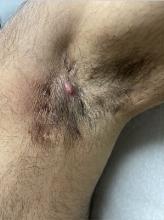
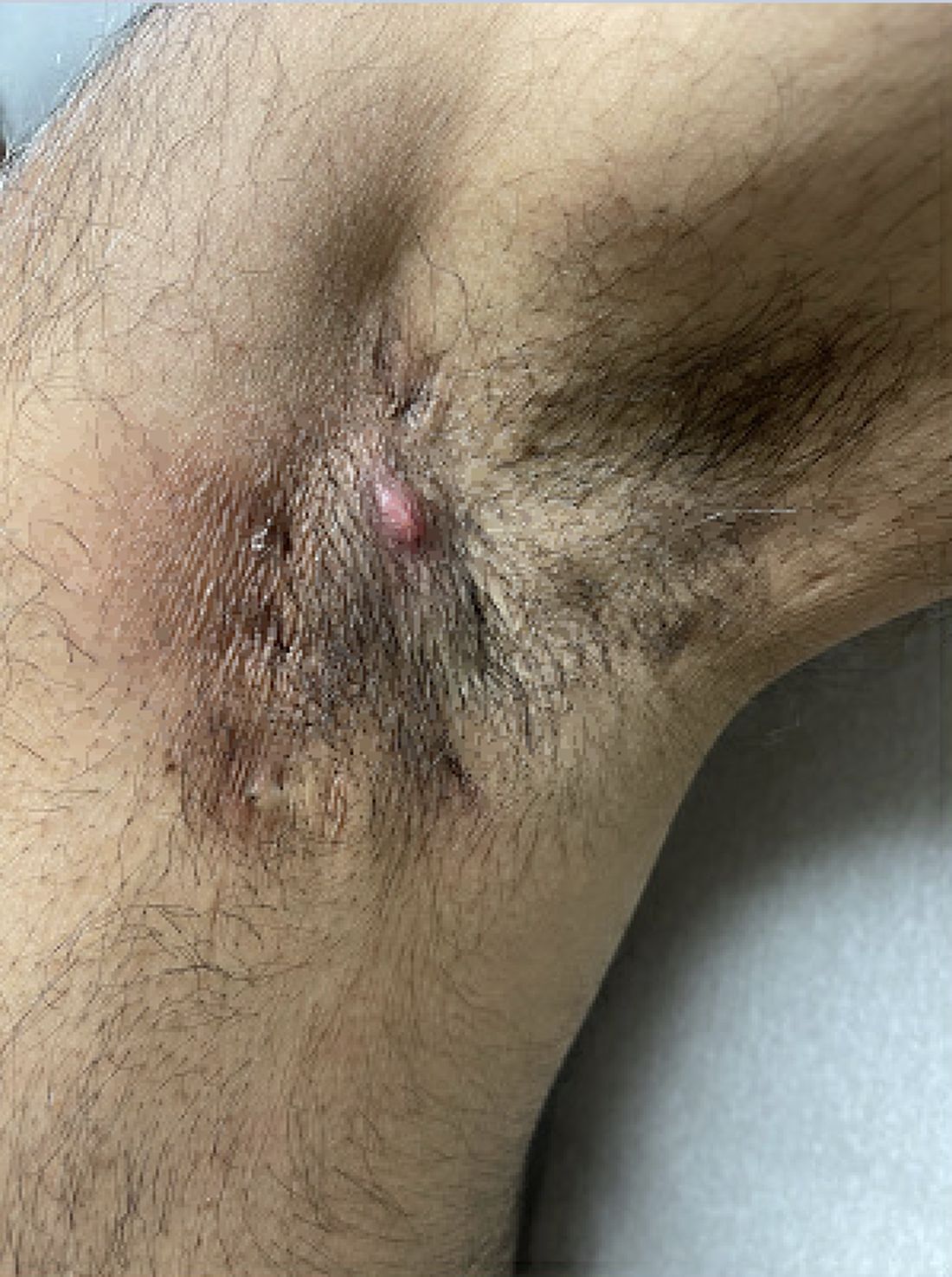
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Research Highlights From ESMO Breast Cancer
Among the topics the speakers addressed were breast cancer prevention, early breast cancer, advanced breast cancer, and supportive care.
In recent years, the way clinicians look at carcinogenesis in breast cancer has changed, and many new targets for potential early detection and prevention have emerged, said Suzette Delaloge, MD, of Gustave Roussy, Paris, France, in her presentation at the meeting.
Instant risk assessment at different time points could potentially intercept cancer among high-risk individuals, she said.
A study by Mikael Eriksson, PhD, and colleagues focused on external validation of the Profound AI tool to identify breast cancer risk in the general population. The researchers showed an area under the curve of 0.72 in their AI risk model, which has the potential to be clinically meaningful, although it must be prospectively validated, Dr. Delaloge said in her presentation.
She also reviewed two studies on the use of genes to further refine breast cancer risk among carriers. One of these, a prospective study presented in a session by Kelly-Anne Phillips, MD, of Peter MacCallum Cancer Center, Melbourne, Australia, used the CANRISK online risk assessment tool and validated increased breast cancer risk in BRCA1 and BRCA2 carriers, with AUCs of 0.79 and 0.78, respectively. The other study, which was by Maria Rezqallah Aron, MD, and colleagues examined polygenic scores as a way to refine breast cancer risk stratification among carriers of the ALM and PALB2 genes as well. These genes might be useful in identifying individuals who could benefit from early intervention, including surgery, Dr. Delaloge said.
Translational Research
“Preparing my talk, I felt like a kid in a candy store,” because of the amount of new translational research presented, including several studies of endocrine treatment–based approaches to therapy, said Marleen Kok, MD, of the Netherlands Cancer Institute, Amsterdam.
In her presentation, Dr. Kok highlighted findings from an analysis of patients in the monarchE study (a trial of high-risk patients) showing a consistent improvement in invasive disease-free survival for the subset of patients with germline BRCA1 and BRCA2 mutations who received abemaciclib plus endocrine therapy.
The value of tumor-infiltrating lymphocytes (TILs) on patients who are not receiving chemotherapy is important because of the focus on prognosis, and prospective trials are underway, she said.
A poster on the impact of chemotherapy and stromal tumor-infiltrating lymphocytes (sTILs) in stage I triple-negative breast cancer showed no association between chemotherapy and better outcomes regardless of sTILs in patients who did and did not receive chemotherapy, which has implications for potential treatment sparing in this population, Dr. Kok noted.
Artificial Intelligence (AI) was the subject of several posters at the meeting, and Dr. Kok identified a multisite European study of an automated HER2 scoring system as notable for its size and accuracy. In the study, the accuracy among pathologists was much higher with the assistance of AI, she said. Using AI for more complex analysis has shown success, she said.
Dr. Kok ended her talk with a poster that surveyed breast cancer patients about their understanding of their disease. The results showed that less than half (44%) of patients reported that their healthcare providers had given them enough information to learn about their breast cancer type, and less than one third could recall terminology about biomarkers; the study is important because it shows that clinicians need to do better in explaining these terms to patients, Dr. Kok said.
Early Breast Cancer
Right-sizing therapy, meaning identifying the right treatment for every patient, is a key element of new research in early breast cancer, said Erika Hamilton, MD, of the Sarah Cannon Research Institute, Nashville, Tenn.
She highlighted safety and treatment duration updates from the NATALEE study, which compared adjuvant ribociclib plus nonsteroidal aromatase inhibitor (NSAI) to NSAI alone for ER+/HER2- breast cancer. The current analysis presented at the meeting showed significant benefits with the addition of ribociclib and no evidence of new safety signals or adverse event exacerbations at 3 years, she said. Dose modifications had no significant impact on efficacy, she added.
The findings of no impact of dose reduction on efficacy in both the NATALEE and monarchE studies provide important information on whether dosage can be reduced in patients, which will increase the odds that patients will tolerate extended therapy with good outcomes and stay on their prescribed therapies, Dr. Hamilton emphasized.
The CARABELA study, a phase 2 trial of neoadjuvant letrozole plus abemaciclib vs adriamycin and cyclophosphamide (AC), showed clinically similar response rates but did not meet its endpoint for residual cancer burden (RCB) scores. These data add to results from other studies and show that it is too soon to universally replace neoadjuvant chemotherapy as first-line treatment for highly proliferative ER+ breast cancer, Dr. Hamilton said in her presentation.
Advanced Breast Cancer
Take-home messages about advanced breast cancer include growing evidence for the potential benefits of antibody drug conjugates (ADCs), said Eva Ciruelos, MD, of University Hospital, Madrid, Spain. The TROPION-BREAST01 study, a phase 3 randomized trial, showed significant and clinically meaningful improvement in progression-free survival in patients with previously treated, inoperable, or metastatic HR+/HER2- breast cancer who received datopotamab deruxtecan (Dato-DXd) compared with those who received chemotherapy.
Data from an additional safety analysis were presented at the meeting; although Dato-DXd, a trophoblast cell-surface antigen 2 (TROP2)–directed antibody-drug conjugate, was well-tolerated, it is important to remain aware of toxicities, notably oral mucositis, which occurred in 55.6% of the patients in the study across all grades, and ocular surface toxicity, which occurred in 40% of patients across all grades, Dr. Ciruelos emphasized.
Key research in the area of advanced triple-negative breast cancer included data from the IMPASSION 132 study. This study is “specifically centered on early relapsers,” a population often excluded from other trials, Dr. Ciruelos said. In this study, patients with advanced triple-negative breast cancer were randomized to chemotherapy with or without atezolizumab, and the study showed no benefits with atezolizumab for overall survival, progression-free survival, or overall response rate, she said. “This is something to work with, because this is a very refractory population,” Dr. Ciruelos noted.
New immunotherapy combinations are needed to improve survival in advanced breast cancer patients, Dr. Ciruelos said. At the meeting, researchers presented interim data from a subset of patients in the MORPHEUS-pan breast cancer trial, a phase 1B/2 study involving multiple treatment combinations in locally advanced/metastatic breast cancer patients.
The interim analysis included 18-week data from triple-negative breast cancer patients and compared outcomes for patients randomized to atezolizumab with or without sacituzumab govitecan (SG).
The study was small, with only 31 patients in the combination arm and 11 controls, but the results were promising, with an overall response rate of 76.7% in the combination arm vs 66.7% in the control arm, Dr. Ciruelos said.
Supportive Care
Key supportive care takeaways included data on pregnancy in young breast cancer survivors and the safety of vaginal estrogen therapy in breast cancer patients with genitourinary symptoms, said Anne May, MD, of the University Medical Center Utrecht, Utrecht, Netherlands.
A study previously published in JAMA including nearly 5000 BRCA carriers who were diagnosed with invasive breast cancer at age 40 years or younger showed no association between pregnancy after breast cancer and adverse maternal or fetal outcomes, and pregnancy had no significant impact on overall survival. The authors presented new data on the safety of assisted reproductive techniques (ART) based on the 543 pregnancies in the original study, at the meeting. Of these, 436 conceived naturally, and 107 used ART. After a median of 9.1 years, ART had no effect on disease-free survival compared to natural conception (hazard ratio [HR], 0.64). Based on these findings, fertility preservation should be offered to all women who receive a breast cancer diagnosis and are interested in future fertility, Dr. May said.
Conceiving after breast cancer treatment and follow-up should not be contraindicated for young BRCA carriers, she added.No trial data are available for the effects of vaginal estrogen therapy (VET) on disease-free survival in breast cancer survivors with genitourinary symptoms caused by declining estrogen levels, Dr. May said. However, researchers in France and Switzerland conducted an emulation of a hypothetical target trial using data from the French National social security system for more than 130,000 individuals. Although VET therapy had no impact on disease-free survival in most breast cancer survivors overall, it did have a negative impact in a subset of patients with HR-positive and HR-negative tumors who were treated with aromatase inhibitors. The study was hypothetical, but important because the results suggest that clinicians can safely propose VTE to patients who report genitourinary symptoms after treatment for early-stage breast cancer with tamoxifen, but VTE should be avoided in patients treated with aromatase inhibitors, Dr. May said.
Dr. Delaloge disclosed research support to her institution from AstraZeneca, MSD, Bristol Myers Squibb, Sanofi, Taiho, Novartis, European Commission, INCa, Banque des Territoires, and Fondation Philanthropia. She also disclosed honoraria to her institution from AstraZeneca, Gilead, Novartis, Elsan, Besins, Sanofi, Exact Sciences, and Lilly, as well as travel support from Novartis.
Dr. Kok disclosed research funding from AstraZeneca, Bristol Myers Squibb, Daichi, and Roche, and advisory board membership/speaker’s fees from Alderaan Biotechnology, BIONTECH, Domain Therapeutics, AstraZeneca, Daichi, Bristol Myers Squibb, Gilead, Medscape, MSD, and Roche.
Dr. Hamilton disclosed a consulting advisory role (to her institution) for Accutar Biotechology, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Entos, Forsum Pharma, Gilead Sciences, Greenwich LifeSciences, Jazz Pharmaceuticals, Lilly, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Roche/Genentech, Stemline Therapeutics, ands others. She also disclosed contracted research/grant support to her institution only from Abbvie, Acerta Pharma, Accutar Biotechnology , ADC Therapeutics, AKESOBIO Australia , Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond and others.
Dr. Ciruelos disclosed serving as an external advisor for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, and Lilly, as well as serving as a speaker for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, Lilly, and Pierre Fabre. She also disclosed travel grants from Roche, Pfizer, and AstraZeneca, and research grants from Seagen and Roche.
Dr. May had no financial conflicts to disclose.
Among the topics the speakers addressed were breast cancer prevention, early breast cancer, advanced breast cancer, and supportive care.
In recent years, the way clinicians look at carcinogenesis in breast cancer has changed, and many new targets for potential early detection and prevention have emerged, said Suzette Delaloge, MD, of Gustave Roussy, Paris, France, in her presentation at the meeting.
Instant risk assessment at different time points could potentially intercept cancer among high-risk individuals, she said.
A study by Mikael Eriksson, PhD, and colleagues focused on external validation of the Profound AI tool to identify breast cancer risk in the general population. The researchers showed an area under the curve of 0.72 in their AI risk model, which has the potential to be clinically meaningful, although it must be prospectively validated, Dr. Delaloge said in her presentation.
She also reviewed two studies on the use of genes to further refine breast cancer risk among carriers. One of these, a prospective study presented in a session by Kelly-Anne Phillips, MD, of Peter MacCallum Cancer Center, Melbourne, Australia, used the CANRISK online risk assessment tool and validated increased breast cancer risk in BRCA1 and BRCA2 carriers, with AUCs of 0.79 and 0.78, respectively. The other study, which was by Maria Rezqallah Aron, MD, and colleagues examined polygenic scores as a way to refine breast cancer risk stratification among carriers of the ALM and PALB2 genes as well. These genes might be useful in identifying individuals who could benefit from early intervention, including surgery, Dr. Delaloge said.
Translational Research
“Preparing my talk, I felt like a kid in a candy store,” because of the amount of new translational research presented, including several studies of endocrine treatment–based approaches to therapy, said Marleen Kok, MD, of the Netherlands Cancer Institute, Amsterdam.
In her presentation, Dr. Kok highlighted findings from an analysis of patients in the monarchE study (a trial of high-risk patients) showing a consistent improvement in invasive disease-free survival for the subset of patients with germline BRCA1 and BRCA2 mutations who received abemaciclib plus endocrine therapy.
The value of tumor-infiltrating lymphocytes (TILs) on patients who are not receiving chemotherapy is important because of the focus on prognosis, and prospective trials are underway, she said.
A poster on the impact of chemotherapy and stromal tumor-infiltrating lymphocytes (sTILs) in stage I triple-negative breast cancer showed no association between chemotherapy and better outcomes regardless of sTILs in patients who did and did not receive chemotherapy, which has implications for potential treatment sparing in this population, Dr. Kok noted.
Artificial Intelligence (AI) was the subject of several posters at the meeting, and Dr. Kok identified a multisite European study of an automated HER2 scoring system as notable for its size and accuracy. In the study, the accuracy among pathologists was much higher with the assistance of AI, she said. Using AI for more complex analysis has shown success, she said.
Dr. Kok ended her talk with a poster that surveyed breast cancer patients about their understanding of their disease. The results showed that less than half (44%) of patients reported that their healthcare providers had given them enough information to learn about their breast cancer type, and less than one third could recall terminology about biomarkers; the study is important because it shows that clinicians need to do better in explaining these terms to patients, Dr. Kok said.
Early Breast Cancer
Right-sizing therapy, meaning identifying the right treatment for every patient, is a key element of new research in early breast cancer, said Erika Hamilton, MD, of the Sarah Cannon Research Institute, Nashville, Tenn.
She highlighted safety and treatment duration updates from the NATALEE study, which compared adjuvant ribociclib plus nonsteroidal aromatase inhibitor (NSAI) to NSAI alone for ER+/HER2- breast cancer. The current analysis presented at the meeting showed significant benefits with the addition of ribociclib and no evidence of new safety signals or adverse event exacerbations at 3 years, she said. Dose modifications had no significant impact on efficacy, she added.
The findings of no impact of dose reduction on efficacy in both the NATALEE and monarchE studies provide important information on whether dosage can be reduced in patients, which will increase the odds that patients will tolerate extended therapy with good outcomes and stay on their prescribed therapies, Dr. Hamilton emphasized.
The CARABELA study, a phase 2 trial of neoadjuvant letrozole plus abemaciclib vs adriamycin and cyclophosphamide (AC), showed clinically similar response rates but did not meet its endpoint for residual cancer burden (RCB) scores. These data add to results from other studies and show that it is too soon to universally replace neoadjuvant chemotherapy as first-line treatment for highly proliferative ER+ breast cancer, Dr. Hamilton said in her presentation.
Advanced Breast Cancer
Take-home messages about advanced breast cancer include growing evidence for the potential benefits of antibody drug conjugates (ADCs), said Eva Ciruelos, MD, of University Hospital, Madrid, Spain. The TROPION-BREAST01 study, a phase 3 randomized trial, showed significant and clinically meaningful improvement in progression-free survival in patients with previously treated, inoperable, or metastatic HR+/HER2- breast cancer who received datopotamab deruxtecan (Dato-DXd) compared with those who received chemotherapy.
Data from an additional safety analysis were presented at the meeting; although Dato-DXd, a trophoblast cell-surface antigen 2 (TROP2)–directed antibody-drug conjugate, was well-tolerated, it is important to remain aware of toxicities, notably oral mucositis, which occurred in 55.6% of the patients in the study across all grades, and ocular surface toxicity, which occurred in 40% of patients across all grades, Dr. Ciruelos emphasized.
Key research in the area of advanced triple-negative breast cancer included data from the IMPASSION 132 study. This study is “specifically centered on early relapsers,” a population often excluded from other trials, Dr. Ciruelos said. In this study, patients with advanced triple-negative breast cancer were randomized to chemotherapy with or without atezolizumab, and the study showed no benefits with atezolizumab for overall survival, progression-free survival, or overall response rate, she said. “This is something to work with, because this is a very refractory population,” Dr. Ciruelos noted.
New immunotherapy combinations are needed to improve survival in advanced breast cancer patients, Dr. Ciruelos said. At the meeting, researchers presented interim data from a subset of patients in the MORPHEUS-pan breast cancer trial, a phase 1B/2 study involving multiple treatment combinations in locally advanced/metastatic breast cancer patients.
The interim analysis included 18-week data from triple-negative breast cancer patients and compared outcomes for patients randomized to atezolizumab with or without sacituzumab govitecan (SG).
The study was small, with only 31 patients in the combination arm and 11 controls, but the results were promising, with an overall response rate of 76.7% in the combination arm vs 66.7% in the control arm, Dr. Ciruelos said.
Supportive Care
Key supportive care takeaways included data on pregnancy in young breast cancer survivors and the safety of vaginal estrogen therapy in breast cancer patients with genitourinary symptoms, said Anne May, MD, of the University Medical Center Utrecht, Utrecht, Netherlands.
A study previously published in JAMA including nearly 5000 BRCA carriers who were diagnosed with invasive breast cancer at age 40 years or younger showed no association between pregnancy after breast cancer and adverse maternal or fetal outcomes, and pregnancy had no significant impact on overall survival. The authors presented new data on the safety of assisted reproductive techniques (ART) based on the 543 pregnancies in the original study, at the meeting. Of these, 436 conceived naturally, and 107 used ART. After a median of 9.1 years, ART had no effect on disease-free survival compared to natural conception (hazard ratio [HR], 0.64). Based on these findings, fertility preservation should be offered to all women who receive a breast cancer diagnosis and are interested in future fertility, Dr. May said.
Conceiving after breast cancer treatment and follow-up should not be contraindicated for young BRCA carriers, she added.No trial data are available for the effects of vaginal estrogen therapy (VET) on disease-free survival in breast cancer survivors with genitourinary symptoms caused by declining estrogen levels, Dr. May said. However, researchers in France and Switzerland conducted an emulation of a hypothetical target trial using data from the French National social security system for more than 130,000 individuals. Although VET therapy had no impact on disease-free survival in most breast cancer survivors overall, it did have a negative impact in a subset of patients with HR-positive and HR-negative tumors who were treated with aromatase inhibitors. The study was hypothetical, but important because the results suggest that clinicians can safely propose VTE to patients who report genitourinary symptoms after treatment for early-stage breast cancer with tamoxifen, but VTE should be avoided in patients treated with aromatase inhibitors, Dr. May said.
Dr. Delaloge disclosed research support to her institution from AstraZeneca, MSD, Bristol Myers Squibb, Sanofi, Taiho, Novartis, European Commission, INCa, Banque des Territoires, and Fondation Philanthropia. She also disclosed honoraria to her institution from AstraZeneca, Gilead, Novartis, Elsan, Besins, Sanofi, Exact Sciences, and Lilly, as well as travel support from Novartis.
Dr. Kok disclosed research funding from AstraZeneca, Bristol Myers Squibb, Daichi, and Roche, and advisory board membership/speaker’s fees from Alderaan Biotechnology, BIONTECH, Domain Therapeutics, AstraZeneca, Daichi, Bristol Myers Squibb, Gilead, Medscape, MSD, and Roche.
Dr. Hamilton disclosed a consulting advisory role (to her institution) for Accutar Biotechology, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Entos, Forsum Pharma, Gilead Sciences, Greenwich LifeSciences, Jazz Pharmaceuticals, Lilly, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Roche/Genentech, Stemline Therapeutics, ands others. She also disclosed contracted research/grant support to her institution only from Abbvie, Acerta Pharma, Accutar Biotechnology , ADC Therapeutics, AKESOBIO Australia , Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond and others.
Dr. Ciruelos disclosed serving as an external advisor for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, and Lilly, as well as serving as a speaker for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, Lilly, and Pierre Fabre. She also disclosed travel grants from Roche, Pfizer, and AstraZeneca, and research grants from Seagen and Roche.
Dr. May had no financial conflicts to disclose.
Among the topics the speakers addressed were breast cancer prevention, early breast cancer, advanced breast cancer, and supportive care.
In recent years, the way clinicians look at carcinogenesis in breast cancer has changed, and many new targets for potential early detection and prevention have emerged, said Suzette Delaloge, MD, of Gustave Roussy, Paris, France, in her presentation at the meeting.
Instant risk assessment at different time points could potentially intercept cancer among high-risk individuals, she said.
A study by Mikael Eriksson, PhD, and colleagues focused on external validation of the Profound AI tool to identify breast cancer risk in the general population. The researchers showed an area under the curve of 0.72 in their AI risk model, which has the potential to be clinically meaningful, although it must be prospectively validated, Dr. Delaloge said in her presentation.
She also reviewed two studies on the use of genes to further refine breast cancer risk among carriers. One of these, a prospective study presented in a session by Kelly-Anne Phillips, MD, of Peter MacCallum Cancer Center, Melbourne, Australia, used the CANRISK online risk assessment tool and validated increased breast cancer risk in BRCA1 and BRCA2 carriers, with AUCs of 0.79 and 0.78, respectively. The other study, which was by Maria Rezqallah Aron, MD, and colleagues examined polygenic scores as a way to refine breast cancer risk stratification among carriers of the ALM and PALB2 genes as well. These genes might be useful in identifying individuals who could benefit from early intervention, including surgery, Dr. Delaloge said.
Translational Research
“Preparing my talk, I felt like a kid in a candy store,” because of the amount of new translational research presented, including several studies of endocrine treatment–based approaches to therapy, said Marleen Kok, MD, of the Netherlands Cancer Institute, Amsterdam.
In her presentation, Dr. Kok highlighted findings from an analysis of patients in the monarchE study (a trial of high-risk patients) showing a consistent improvement in invasive disease-free survival for the subset of patients with germline BRCA1 and BRCA2 mutations who received abemaciclib plus endocrine therapy.
The value of tumor-infiltrating lymphocytes (TILs) on patients who are not receiving chemotherapy is important because of the focus on prognosis, and prospective trials are underway, she said.
A poster on the impact of chemotherapy and stromal tumor-infiltrating lymphocytes (sTILs) in stage I triple-negative breast cancer showed no association between chemotherapy and better outcomes regardless of sTILs in patients who did and did not receive chemotherapy, which has implications for potential treatment sparing in this population, Dr. Kok noted.
Artificial Intelligence (AI) was the subject of several posters at the meeting, and Dr. Kok identified a multisite European study of an automated HER2 scoring system as notable for its size and accuracy. In the study, the accuracy among pathologists was much higher with the assistance of AI, she said. Using AI for more complex analysis has shown success, she said.
Dr. Kok ended her talk with a poster that surveyed breast cancer patients about their understanding of their disease. The results showed that less than half (44%) of patients reported that their healthcare providers had given them enough information to learn about their breast cancer type, and less than one third could recall terminology about biomarkers; the study is important because it shows that clinicians need to do better in explaining these terms to patients, Dr. Kok said.
Early Breast Cancer
Right-sizing therapy, meaning identifying the right treatment for every patient, is a key element of new research in early breast cancer, said Erika Hamilton, MD, of the Sarah Cannon Research Institute, Nashville, Tenn.
She highlighted safety and treatment duration updates from the NATALEE study, which compared adjuvant ribociclib plus nonsteroidal aromatase inhibitor (NSAI) to NSAI alone for ER+/HER2- breast cancer. The current analysis presented at the meeting showed significant benefits with the addition of ribociclib and no evidence of new safety signals or adverse event exacerbations at 3 years, she said. Dose modifications had no significant impact on efficacy, she added.
The findings of no impact of dose reduction on efficacy in both the NATALEE and monarchE studies provide important information on whether dosage can be reduced in patients, which will increase the odds that patients will tolerate extended therapy with good outcomes and stay on their prescribed therapies, Dr. Hamilton emphasized.
The CARABELA study, a phase 2 trial of neoadjuvant letrozole plus abemaciclib vs adriamycin and cyclophosphamide (AC), showed clinically similar response rates but did not meet its endpoint for residual cancer burden (RCB) scores. These data add to results from other studies and show that it is too soon to universally replace neoadjuvant chemotherapy as first-line treatment for highly proliferative ER+ breast cancer, Dr. Hamilton said in her presentation.
Advanced Breast Cancer
Take-home messages about advanced breast cancer include growing evidence for the potential benefits of antibody drug conjugates (ADCs), said Eva Ciruelos, MD, of University Hospital, Madrid, Spain. The TROPION-BREAST01 study, a phase 3 randomized trial, showed significant and clinically meaningful improvement in progression-free survival in patients with previously treated, inoperable, or metastatic HR+/HER2- breast cancer who received datopotamab deruxtecan (Dato-DXd) compared with those who received chemotherapy.
Data from an additional safety analysis were presented at the meeting; although Dato-DXd, a trophoblast cell-surface antigen 2 (TROP2)–directed antibody-drug conjugate, was well-tolerated, it is important to remain aware of toxicities, notably oral mucositis, which occurred in 55.6% of the patients in the study across all grades, and ocular surface toxicity, which occurred in 40% of patients across all grades, Dr. Ciruelos emphasized.
Key research in the area of advanced triple-negative breast cancer included data from the IMPASSION 132 study. This study is “specifically centered on early relapsers,” a population often excluded from other trials, Dr. Ciruelos said. In this study, patients with advanced triple-negative breast cancer were randomized to chemotherapy with or without atezolizumab, and the study showed no benefits with atezolizumab for overall survival, progression-free survival, or overall response rate, she said. “This is something to work with, because this is a very refractory population,” Dr. Ciruelos noted.
New immunotherapy combinations are needed to improve survival in advanced breast cancer patients, Dr. Ciruelos said. At the meeting, researchers presented interim data from a subset of patients in the MORPHEUS-pan breast cancer trial, a phase 1B/2 study involving multiple treatment combinations in locally advanced/metastatic breast cancer patients.
The interim analysis included 18-week data from triple-negative breast cancer patients and compared outcomes for patients randomized to atezolizumab with or without sacituzumab govitecan (SG).
The study was small, with only 31 patients in the combination arm and 11 controls, but the results were promising, with an overall response rate of 76.7% in the combination arm vs 66.7% in the control arm, Dr. Ciruelos said.
Supportive Care
Key supportive care takeaways included data on pregnancy in young breast cancer survivors and the safety of vaginal estrogen therapy in breast cancer patients with genitourinary symptoms, said Anne May, MD, of the University Medical Center Utrecht, Utrecht, Netherlands.
A study previously published in JAMA including nearly 5000 BRCA carriers who were diagnosed with invasive breast cancer at age 40 years or younger showed no association between pregnancy after breast cancer and adverse maternal or fetal outcomes, and pregnancy had no significant impact on overall survival. The authors presented new data on the safety of assisted reproductive techniques (ART) based on the 543 pregnancies in the original study, at the meeting. Of these, 436 conceived naturally, and 107 used ART. After a median of 9.1 years, ART had no effect on disease-free survival compared to natural conception (hazard ratio [HR], 0.64). Based on these findings, fertility preservation should be offered to all women who receive a breast cancer diagnosis and are interested in future fertility, Dr. May said.
Conceiving after breast cancer treatment and follow-up should not be contraindicated for young BRCA carriers, she added.No trial data are available for the effects of vaginal estrogen therapy (VET) on disease-free survival in breast cancer survivors with genitourinary symptoms caused by declining estrogen levels, Dr. May said. However, researchers in France and Switzerland conducted an emulation of a hypothetical target trial using data from the French National social security system for more than 130,000 individuals. Although VET therapy had no impact on disease-free survival in most breast cancer survivors overall, it did have a negative impact in a subset of patients with HR-positive and HR-negative tumors who were treated with aromatase inhibitors. The study was hypothetical, but important because the results suggest that clinicians can safely propose VTE to patients who report genitourinary symptoms after treatment for early-stage breast cancer with tamoxifen, but VTE should be avoided in patients treated with aromatase inhibitors, Dr. May said.
Dr. Delaloge disclosed research support to her institution from AstraZeneca, MSD, Bristol Myers Squibb, Sanofi, Taiho, Novartis, European Commission, INCa, Banque des Territoires, and Fondation Philanthropia. She also disclosed honoraria to her institution from AstraZeneca, Gilead, Novartis, Elsan, Besins, Sanofi, Exact Sciences, and Lilly, as well as travel support from Novartis.
Dr. Kok disclosed research funding from AstraZeneca, Bristol Myers Squibb, Daichi, and Roche, and advisory board membership/speaker’s fees from Alderaan Biotechnology, BIONTECH, Domain Therapeutics, AstraZeneca, Daichi, Bristol Myers Squibb, Gilead, Medscape, MSD, and Roche.
Dr. Hamilton disclosed a consulting advisory role (to her institution) for Accutar Biotechology, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Entos, Forsum Pharma, Gilead Sciences, Greenwich LifeSciences, Jazz Pharmaceuticals, Lilly, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Roche/Genentech, Stemline Therapeutics, ands others. She also disclosed contracted research/grant support to her institution only from Abbvie, Acerta Pharma, Accutar Biotechnology , ADC Therapeutics, AKESOBIO Australia , Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond and others.
Dr. Ciruelos disclosed serving as an external advisor for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, and Lilly, as well as serving as a speaker for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, Lilly, and Pierre Fabre. She also disclosed travel grants from Roche, Pfizer, and AstraZeneca, and research grants from Seagen and Roche.
Dr. May had no financial conflicts to disclose.
FROM ESMO BREAST CANCER 2024
Does More Systemic Treatment for Advanced Cancer Improve Survival?
This conclusion of a new study published online May 16 in JAMA Oncology may help reassure oncologists that giving systemic anticancer therapy (SACT) at the most advanced stages of cancer will not improve the patient’s life, the authors wrote. It also may encourage them to instead focus more on honest communication with patients about their choices, Maureen E. Canavan, PhD, at the Cancer and Outcomes, Public Policy and Effectiveness Research (COPPER) Center at the Yale School of Medicine in New Haven, Connecticut, and colleagues, wrote in their paper.
How Was the Study Conducted?
Researchers used Flatiron Health, a nationwide electronic health records database of academic and community practices throughout the United State. They identified 78,446 adults with advanced or metastatic stages of one of six common cancers (breast, colorectal, urothelial, non–small cell lung cancer [NSCLC], pancreatic and renal cell carcinoma) who were treated at healthcare practices from 2015 to 2019. They then stratified practices into quintiles based on how often the practices treated patients with any systemic therapy, including chemotherapy and immunotherapy, in their last 14 days of life. They compared whether patients in practices with greater use of systemic treatment at very advanced stages had longer overall survival.
What Were the Main Findings?
“We saw that there were absolutely no survival differences between the practices that used more systemic therapy for very advanced cancer than the practices that use less,” said senior author Kerin Adelson, MD, chief quality and value officer at MD Anderson Cancer Center in Houston, Texas. In some cancers, those in the lowest quintile (those with the lowest rates of systemic end-of-life care) lived fewer years compared with those in the highest quintiles. In other cancers, those in the lowest quintiles lived more years than those in the highest quintiles.
“What’s important is that none of those differences, after you control for other factors, was statistically significant,” Dr. Adelson said. “That was the same in every cancer type we looked at.”
An example is seen in advanced urothelial cancer. Those in the first quintile (lowest rates of systemic care at end of life) had an SACT rate range of 4.0-9.1. The SACT rate range in the highest quintile was 19.8-42.6. But the median overall survival (OS) rate for those in the lowest quintile was 12.7 months, not statistically different from the median OS in the highest quintile (11 months.)
How Does This Study Add to the Literature?
The American Society of Clinical Oncology (ASCO) and the National Quality Forum (NQF) developed a cancer quality metric to reduce SACT at the end of life. The NQF 0210 is a ratio of patients who get systemic treatment within 14 days of death over all patients who die of cancer. The quality metric has been widely adopted and used in value-based care reporting.
But the metric has been criticized because it focuses only on people who died and not people who lived longer because they benefited from the systemic therapy, the authors wrote.
Dr. Canavan’s team focused on all patients treated in the practice, not just those who died, Dr. Adelson said. This may put that criticism to rest, Dr. Adelson said.
“I personally believed the ASCO and NQF metric was appropriate and the criticisms were off base,” said Otis Brawley, MD, associate director of community outreach and engagement at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University School of Medicine in Baltimore. “Canavan’s study is evidence suggesting the metrics were appropriate.”
This study included not just chemotherapy, as some other studies have, but targeted therapies and immunotherapies as well. Dr. Adelson said some think that the newer drugs might change the prognosis at end of life. But this study shows “even those drugs are not helping patients to survive with very advanced cancer,” she said.
Could This Change Practice?
The authors noted that end-of life SACT has been linked with more acute care use, delays in conversations about care goals, late enrollment in hospice, higher costs, and potentially shorter and poorer quality life.
Dr. Adelson said she’s hoping that the knowledge that there’s no survival benefit for use of SACT for patients with advanced solid tumors who are nearing the end of life will lead instead to more conversations about prognosis with patients and transitions to palliative care.
“Palliative care has actually been shown to improve quality of life and, in some studies, even survival,” she said.
“I doubt it will change practice, but it should,” Dr. Brawley said. “The study suggests that doctors and patients have too much hope for chemotherapy as patients’ disease progresses. In the US especially, there is a tendency to believe we have better therapies than we truly do and we have difficulty accepting that the patient is dying. Many patients get third- and fourth-line chemotherapy that is highly likely to increase suffering without realistic hope of prolonging life and especially no hope of prolonging life with good quality.”
Dr. Adelson disclosed ties with AbbVie, Quantum Health, Gilead, ParetoHealth, and Carrum Health. Various coauthors disclosed ties with Roche, AbbVie, Johnson & Johnson, Genentech, the National Comprehensive Cancer Network, and AstraZeneca. The study was funded by Flatiron Health, an independent member of the Roche group. Dr. Brawley reports no relevant financial disclosures.
This conclusion of a new study published online May 16 in JAMA Oncology may help reassure oncologists that giving systemic anticancer therapy (SACT) at the most advanced stages of cancer will not improve the patient’s life, the authors wrote. It also may encourage them to instead focus more on honest communication with patients about their choices, Maureen E. Canavan, PhD, at the Cancer and Outcomes, Public Policy and Effectiveness Research (COPPER) Center at the Yale School of Medicine in New Haven, Connecticut, and colleagues, wrote in their paper.
How Was the Study Conducted?
Researchers used Flatiron Health, a nationwide electronic health records database of academic and community practices throughout the United State. They identified 78,446 adults with advanced or metastatic stages of one of six common cancers (breast, colorectal, urothelial, non–small cell lung cancer [NSCLC], pancreatic and renal cell carcinoma) who were treated at healthcare practices from 2015 to 2019. They then stratified practices into quintiles based on how often the practices treated patients with any systemic therapy, including chemotherapy and immunotherapy, in their last 14 days of life. They compared whether patients in practices with greater use of systemic treatment at very advanced stages had longer overall survival.
What Were the Main Findings?
“We saw that there were absolutely no survival differences between the practices that used more systemic therapy for very advanced cancer than the practices that use less,” said senior author Kerin Adelson, MD, chief quality and value officer at MD Anderson Cancer Center in Houston, Texas. In some cancers, those in the lowest quintile (those with the lowest rates of systemic end-of-life care) lived fewer years compared with those in the highest quintiles. In other cancers, those in the lowest quintiles lived more years than those in the highest quintiles.
“What’s important is that none of those differences, after you control for other factors, was statistically significant,” Dr. Adelson said. “That was the same in every cancer type we looked at.”
An example is seen in advanced urothelial cancer. Those in the first quintile (lowest rates of systemic care at end of life) had an SACT rate range of 4.0-9.1. The SACT rate range in the highest quintile was 19.8-42.6. But the median overall survival (OS) rate for those in the lowest quintile was 12.7 months, not statistically different from the median OS in the highest quintile (11 months.)
How Does This Study Add to the Literature?
The American Society of Clinical Oncology (ASCO) and the National Quality Forum (NQF) developed a cancer quality metric to reduce SACT at the end of life. The NQF 0210 is a ratio of patients who get systemic treatment within 14 days of death over all patients who die of cancer. The quality metric has been widely adopted and used in value-based care reporting.
But the metric has been criticized because it focuses only on people who died and not people who lived longer because they benefited from the systemic therapy, the authors wrote.
Dr. Canavan’s team focused on all patients treated in the practice, not just those who died, Dr. Adelson said. This may put that criticism to rest, Dr. Adelson said.
“I personally believed the ASCO and NQF metric was appropriate and the criticisms were off base,” said Otis Brawley, MD, associate director of community outreach and engagement at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University School of Medicine in Baltimore. “Canavan’s study is evidence suggesting the metrics were appropriate.”
This study included not just chemotherapy, as some other studies have, but targeted therapies and immunotherapies as well. Dr. Adelson said some think that the newer drugs might change the prognosis at end of life. But this study shows “even those drugs are not helping patients to survive with very advanced cancer,” she said.
Could This Change Practice?
The authors noted that end-of life SACT has been linked with more acute care use, delays in conversations about care goals, late enrollment in hospice, higher costs, and potentially shorter and poorer quality life.
Dr. Adelson said she’s hoping that the knowledge that there’s no survival benefit for use of SACT for patients with advanced solid tumors who are nearing the end of life will lead instead to more conversations about prognosis with patients and transitions to palliative care.
“Palliative care has actually been shown to improve quality of life and, in some studies, even survival,” she said.
“I doubt it will change practice, but it should,” Dr. Brawley said. “The study suggests that doctors and patients have too much hope for chemotherapy as patients’ disease progresses. In the US especially, there is a tendency to believe we have better therapies than we truly do and we have difficulty accepting that the patient is dying. Many patients get third- and fourth-line chemotherapy that is highly likely to increase suffering without realistic hope of prolonging life and especially no hope of prolonging life with good quality.”
Dr. Adelson disclosed ties with AbbVie, Quantum Health, Gilead, ParetoHealth, and Carrum Health. Various coauthors disclosed ties with Roche, AbbVie, Johnson & Johnson, Genentech, the National Comprehensive Cancer Network, and AstraZeneca. The study was funded by Flatiron Health, an independent member of the Roche group. Dr. Brawley reports no relevant financial disclosures.
This conclusion of a new study published online May 16 in JAMA Oncology may help reassure oncologists that giving systemic anticancer therapy (SACT) at the most advanced stages of cancer will not improve the patient’s life, the authors wrote. It also may encourage them to instead focus more on honest communication with patients about their choices, Maureen E. Canavan, PhD, at the Cancer and Outcomes, Public Policy and Effectiveness Research (COPPER) Center at the Yale School of Medicine in New Haven, Connecticut, and colleagues, wrote in their paper.
How Was the Study Conducted?
Researchers used Flatiron Health, a nationwide electronic health records database of academic and community practices throughout the United State. They identified 78,446 adults with advanced or metastatic stages of one of six common cancers (breast, colorectal, urothelial, non–small cell lung cancer [NSCLC], pancreatic and renal cell carcinoma) who were treated at healthcare practices from 2015 to 2019. They then stratified practices into quintiles based on how often the practices treated patients with any systemic therapy, including chemotherapy and immunotherapy, in their last 14 days of life. They compared whether patients in practices with greater use of systemic treatment at very advanced stages had longer overall survival.
What Were the Main Findings?
“We saw that there were absolutely no survival differences between the practices that used more systemic therapy for very advanced cancer than the practices that use less,” said senior author Kerin Adelson, MD, chief quality and value officer at MD Anderson Cancer Center in Houston, Texas. In some cancers, those in the lowest quintile (those with the lowest rates of systemic end-of-life care) lived fewer years compared with those in the highest quintiles. In other cancers, those in the lowest quintiles lived more years than those in the highest quintiles.
“What’s important is that none of those differences, after you control for other factors, was statistically significant,” Dr. Adelson said. “That was the same in every cancer type we looked at.”
An example is seen in advanced urothelial cancer. Those in the first quintile (lowest rates of systemic care at end of life) had an SACT rate range of 4.0-9.1. The SACT rate range in the highest quintile was 19.8-42.6. But the median overall survival (OS) rate for those in the lowest quintile was 12.7 months, not statistically different from the median OS in the highest quintile (11 months.)
How Does This Study Add to the Literature?
The American Society of Clinical Oncology (ASCO) and the National Quality Forum (NQF) developed a cancer quality metric to reduce SACT at the end of life. The NQF 0210 is a ratio of patients who get systemic treatment within 14 days of death over all patients who die of cancer. The quality metric has been widely adopted and used in value-based care reporting.
But the metric has been criticized because it focuses only on people who died and not people who lived longer because they benefited from the systemic therapy, the authors wrote.
Dr. Canavan’s team focused on all patients treated in the practice, not just those who died, Dr. Adelson said. This may put that criticism to rest, Dr. Adelson said.
“I personally believed the ASCO and NQF metric was appropriate and the criticisms were off base,” said Otis Brawley, MD, associate director of community outreach and engagement at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University School of Medicine in Baltimore. “Canavan’s study is evidence suggesting the metrics were appropriate.”
This study included not just chemotherapy, as some other studies have, but targeted therapies and immunotherapies as well. Dr. Adelson said some think that the newer drugs might change the prognosis at end of life. But this study shows “even those drugs are not helping patients to survive with very advanced cancer,” she said.
Could This Change Practice?
The authors noted that end-of life SACT has been linked with more acute care use, delays in conversations about care goals, late enrollment in hospice, higher costs, and potentially shorter and poorer quality life.
Dr. Adelson said she’s hoping that the knowledge that there’s no survival benefit for use of SACT for patients with advanced solid tumors who are nearing the end of life will lead instead to more conversations about prognosis with patients and transitions to palliative care.
“Palliative care has actually been shown to improve quality of life and, in some studies, even survival,” she said.
“I doubt it will change practice, but it should,” Dr. Brawley said. “The study suggests that doctors and patients have too much hope for chemotherapy as patients’ disease progresses. In the US especially, there is a tendency to believe we have better therapies than we truly do and we have difficulty accepting that the patient is dying. Many patients get third- and fourth-line chemotherapy that is highly likely to increase suffering without realistic hope of prolonging life and especially no hope of prolonging life with good quality.”
Dr. Adelson disclosed ties with AbbVie, Quantum Health, Gilead, ParetoHealth, and Carrum Health. Various coauthors disclosed ties with Roche, AbbVie, Johnson & Johnson, Genentech, the National Comprehensive Cancer Network, and AstraZeneca. The study was funded by Flatiron Health, an independent member of the Roche group. Dr. Brawley reports no relevant financial disclosures.
FROM JAMA ONCOLOGY
Liposomal Irinotecan for Pancreatic Cancer: Is It Worth It?
In February, the US Food and Drug Administration (FDA) approved irinotecan liposome (Onivyde) as part of a new regimen for first-line metastatic pancreatic adenocarcinoma called NALIRIFOX.
The main difference between NALIRIFOX and a standard go-to regimen for the indication, modified FOLFIRINOX, is that liposomal irinotecan — irinotecan encased in a lipid nanoparticle — is used instead of free irinotecan.
Trial data suggested a better overall response rate, a slight progression-free survival advantage, and potentially fewer adverse events with the liposomal formulation.
The substitution, however, raises the cost of treatment substantially. According to one estimate, a single cycle of FOLFIRINOX costs about $500 at a body surface area of 2 m2, while the equivalent single cycle of NALIRIFOX costs $7800 — over 15-fold more expensive.
While some oncologists have called the NALIRIFOX regimen a potential new standard first-line treatment for metastatic pancreatic adenocarcinoma, others have expressed serious doubts about whether the potential benefits are worth the extra cost.
“I can’t really see a single scenario where I would recommend NALIRIFOX over FOLFIRINOX” Ignacio Garrido-Laguna, MD, PhD, a gastrointestinal oncologist and pancreatic cancer researcher at the University of Utah, Salt Lake City, told this news organization. “Most of us in the academic setting have the same take on this.”
No Head-to-Head Comparison
Instead, the 770-patient NAPOLI 3 trial compared NALIRIFOX — which also includes oxaliplatin, fluorouracil, and leucovorin — with a two-drug regimen, nab-paclitaxel and gemcitabine. In the trial, overall survival and other outcomes were moderately better with NALIRIFOX.
Oncologists have said that the true value of the trial is that it conclusively demonstrates that a four-drug regimen is superior to a two-drug regimen for patients who can tolerate the more intensive therapy.
Eileen M. O’Reilly, MD, the senior investigator on NAPOLI 3, made this point when she presented the phase 3 results at the 2023 ASCO annual meeting.
The trial “answers the question of four drugs versus two” for first-line metastatic pancreatic cancer but “does not address the question of NALIRIFOX versus FOLFIRINOX,” said Dr. O’Reilly, a pancreatic and hepatobiliary oncologist and researcher at Memorial Sloan Kettering Cancer Center in New York City.
Comparing them directly in the study “probably wouldn’t have been in the interest of the sponsor,” said Dr. O’Reilly.
With no head-to-head comparison, oncologists have been comparing NAPOLI 3 results with those from PRODIGE 4, the 2011 trial that won FOLFIRINOX its place as a first-line regimen.
When comparing the trials, median overall survival was exactly the same for the two regimens — 11.1 months. FOLFIRINOX was associated with a slightly higher 1-year survival rate — 48.4% with FOLFIRINOX vs 45.6% with NALIRIFOX.
However, Dr. O’Reilly and her colleagues also highlighted comparisons between the two trials that favored NAPOLI 3.
NAPOLI 3 had no age limit, while PRODIGE subjects were no older than 75 years. Median progression-free survival was 1 month longer among patients receiving NALIRIFOX — 7.4 months vs 6.4 months in PRODIGE — and overall response rates were higher as well — 41.8% in NAPOLI 3 vs 31.6%. Patients receiving NALIRIFOX also had lower rates of grade 3/4 neutropenia (23.8% vs 45.7%, respectively) and peripheral sensory neuropathy (3.5% vs 9.0%, respectively).
The authors explained that the lower rate of neuropathy could be because NALIRIFOX uses a lower dose of oxaliplatin (FOLFIRINOX), at 60 mg/m2 instead of 85 mg/m2.
Is It Worth It?
During a presentation of the phase 3 findings last year, study author Zev A. Wainberg, MD, of the University of California, Los Angeles, said the NALIRIFOX regimen can be considered the new reference regimen for first-line treatment of metastatic pancreatic adenocarcinoma.
The study discussant, Laura Goff, MD, MSCI, of Vanderbilt-Ingram Cancer Center, Nashville, Tennessee, agreed that the results support the NALIRIFOX regimen as “the new standard for fit patients.”
However, other oncologists remain skeptical about the benefits of the new regimen over FOLFIRINOX for patients with metastatic pancreatic adenocarcinoma.
In a recent editorial, Dr. Garrido-Laguna and University of Utah gastrointestinal oncologist Christopher Nevala-Plagemann, MD, compared the evidence for both regimens.
The experts pointed out that overall response rates were assessed by investigators in NAPOLI 3 and not by an independent review committee, as in PRODIGE 4, and might have been overestimated.
Although the lack of an age limit was touted as a benefit of NAPOLI 3, Dr. Garrido-Laguna and Dr. Nevala-Plagemann doubt whether enough patients over 75 years old participated to draw any meaningful conclusions about using NALIRIFOX in older, frailer patients. If anything, patients in PRODIGE 4 might have been less fit because, among other things, the trial allowed patients with serum albumins < 3 g/dL.
On the adverse event front, the authors highlighted the higher incidences of grade 3 or worse diarrhea with NALIRIFOX (20% vs 12.7%) and questioned if there truly is less neutropenia with NALIRIFOX because high-risk patients in NAPOLI 3 were treated with granulocyte colony-stimulating factor to prevent it. The pair also questioned whether the differences in neuropathy rates between the two trials were big enough to be clinically meaningful.
Insights from a recent meta-analysis may further clarify some of the lingering questions about the efficacy of NALIRIFOX vs FOLFIRINOX.
In the analysis, the team found no meaningful difference in overall and progression-free survival between the two regimens. Differences in rates of peripheral neuropathy and diarrhea were not statistically significant, but NALIRIFOX did carry a statistically significant advantage in lower rates of febrile neutropenia, thrombocytopenia, and vomiting.
The team concluded that “NALIRIFOX and FOLFIRINOX may provide equal efficacy as first-line treatment of metastatic pancreatic cancer, but with different toxicity profiles,” and called for careful patient selection when choosing between the two regimens as well as consideration of financial toxicity.
Dr. Garrido-Laguna had a different take. With the current data, NALIRIFOX does not seem to “add anything substantially different to what we already” have with FOLFIRINOX, he told this news organization. Given that, “we can’t really justify NALIRIFOX over FOLFIRINOX without more of a head-to-head comparison.”
The higher cost of NALIRIFOX, in particular, remains a major drawback.
“We think it would be an economic disservice to our healthcare systems if we used NALIRIFOX instead of FOLFIRINOX for these patients on the basis of [NAPOLI 3] data,” Bishal Gyawali, MD, PhD, and Christopher Booth, MD, gastrointestinal oncologists at Queen’s University in Kingston, Ontario, Canada, said in a recent essay.
Dr. Garrido-Laguna and Dr. Nevala-Plagemann reiterated this concern.
Overall, “NALIRIFOX does not seem to raise the bar but rather exposes patients and healthcare systems to financial toxicities,” Dr. Garrido-Laguna and Dr. Nevala-Plagemann wrote in their review.
NAPOLI 3 was funded by Ipsen and PRODIGE 4 was funded by the government of France. No funding source was reported for the meta-analysis. NAPOLI 3 investigators included Ipsen employees. Dr. O’Reilly disclosed grants or contracts from Ipsen and many other companies. Dr. Garrido-Laguna reported institutional research funding from Bristol Myers Squibb, Novartis, Pfizer, and other companies, but not Ipsen. Dr. Nevala-Plagemann is an advisor for Seagen and reported institutional research funding from Theriva. Dr. Gyawali is a consultant for Vivio Health; Dr. Booth had no disclosures. Two meta-analysis authors reported grants or personal fees from Ipsen as well as ties to other companies.
A version of this article appeared on Medscape.com.
In February, the US Food and Drug Administration (FDA) approved irinotecan liposome (Onivyde) as part of a new regimen for first-line metastatic pancreatic adenocarcinoma called NALIRIFOX.
The main difference between NALIRIFOX and a standard go-to regimen for the indication, modified FOLFIRINOX, is that liposomal irinotecan — irinotecan encased in a lipid nanoparticle — is used instead of free irinotecan.
Trial data suggested a better overall response rate, a slight progression-free survival advantage, and potentially fewer adverse events with the liposomal formulation.
The substitution, however, raises the cost of treatment substantially. According to one estimate, a single cycle of FOLFIRINOX costs about $500 at a body surface area of 2 m2, while the equivalent single cycle of NALIRIFOX costs $7800 — over 15-fold more expensive.
While some oncologists have called the NALIRIFOX regimen a potential new standard first-line treatment for metastatic pancreatic adenocarcinoma, others have expressed serious doubts about whether the potential benefits are worth the extra cost.
“I can’t really see a single scenario where I would recommend NALIRIFOX over FOLFIRINOX” Ignacio Garrido-Laguna, MD, PhD, a gastrointestinal oncologist and pancreatic cancer researcher at the University of Utah, Salt Lake City, told this news organization. “Most of us in the academic setting have the same take on this.”
No Head-to-Head Comparison
Instead, the 770-patient NAPOLI 3 trial compared NALIRIFOX — which also includes oxaliplatin, fluorouracil, and leucovorin — with a two-drug regimen, nab-paclitaxel and gemcitabine. In the trial, overall survival and other outcomes were moderately better with NALIRIFOX.
Oncologists have said that the true value of the trial is that it conclusively demonstrates that a four-drug regimen is superior to a two-drug regimen for patients who can tolerate the more intensive therapy.
Eileen M. O’Reilly, MD, the senior investigator on NAPOLI 3, made this point when she presented the phase 3 results at the 2023 ASCO annual meeting.
The trial “answers the question of four drugs versus two” for first-line metastatic pancreatic cancer but “does not address the question of NALIRIFOX versus FOLFIRINOX,” said Dr. O’Reilly, a pancreatic and hepatobiliary oncologist and researcher at Memorial Sloan Kettering Cancer Center in New York City.
Comparing them directly in the study “probably wouldn’t have been in the interest of the sponsor,” said Dr. O’Reilly.
With no head-to-head comparison, oncologists have been comparing NAPOLI 3 results with those from PRODIGE 4, the 2011 trial that won FOLFIRINOX its place as a first-line regimen.
When comparing the trials, median overall survival was exactly the same for the two regimens — 11.1 months. FOLFIRINOX was associated with a slightly higher 1-year survival rate — 48.4% with FOLFIRINOX vs 45.6% with NALIRIFOX.
However, Dr. O’Reilly and her colleagues also highlighted comparisons between the two trials that favored NAPOLI 3.
NAPOLI 3 had no age limit, while PRODIGE subjects were no older than 75 years. Median progression-free survival was 1 month longer among patients receiving NALIRIFOX — 7.4 months vs 6.4 months in PRODIGE — and overall response rates were higher as well — 41.8% in NAPOLI 3 vs 31.6%. Patients receiving NALIRIFOX also had lower rates of grade 3/4 neutropenia (23.8% vs 45.7%, respectively) and peripheral sensory neuropathy (3.5% vs 9.0%, respectively).
The authors explained that the lower rate of neuropathy could be because NALIRIFOX uses a lower dose of oxaliplatin (FOLFIRINOX), at 60 mg/m2 instead of 85 mg/m2.
Is It Worth It?
During a presentation of the phase 3 findings last year, study author Zev A. Wainberg, MD, of the University of California, Los Angeles, said the NALIRIFOX regimen can be considered the new reference regimen for first-line treatment of metastatic pancreatic adenocarcinoma.
The study discussant, Laura Goff, MD, MSCI, of Vanderbilt-Ingram Cancer Center, Nashville, Tennessee, agreed that the results support the NALIRIFOX regimen as “the new standard for fit patients.”
However, other oncologists remain skeptical about the benefits of the new regimen over FOLFIRINOX for patients with metastatic pancreatic adenocarcinoma.
In a recent editorial, Dr. Garrido-Laguna and University of Utah gastrointestinal oncologist Christopher Nevala-Plagemann, MD, compared the evidence for both regimens.
The experts pointed out that overall response rates were assessed by investigators in NAPOLI 3 and not by an independent review committee, as in PRODIGE 4, and might have been overestimated.
Although the lack of an age limit was touted as a benefit of NAPOLI 3, Dr. Garrido-Laguna and Dr. Nevala-Plagemann doubt whether enough patients over 75 years old participated to draw any meaningful conclusions about using NALIRIFOX in older, frailer patients. If anything, patients in PRODIGE 4 might have been less fit because, among other things, the trial allowed patients with serum albumins < 3 g/dL.
On the adverse event front, the authors highlighted the higher incidences of grade 3 or worse diarrhea with NALIRIFOX (20% vs 12.7%) and questioned if there truly is less neutropenia with NALIRIFOX because high-risk patients in NAPOLI 3 were treated with granulocyte colony-stimulating factor to prevent it. The pair also questioned whether the differences in neuropathy rates between the two trials were big enough to be clinically meaningful.
Insights from a recent meta-analysis may further clarify some of the lingering questions about the efficacy of NALIRIFOX vs FOLFIRINOX.
In the analysis, the team found no meaningful difference in overall and progression-free survival between the two regimens. Differences in rates of peripheral neuropathy and diarrhea were not statistically significant, but NALIRIFOX did carry a statistically significant advantage in lower rates of febrile neutropenia, thrombocytopenia, and vomiting.
The team concluded that “NALIRIFOX and FOLFIRINOX may provide equal efficacy as first-line treatment of metastatic pancreatic cancer, but with different toxicity profiles,” and called for careful patient selection when choosing between the two regimens as well as consideration of financial toxicity.
Dr. Garrido-Laguna had a different take. With the current data, NALIRIFOX does not seem to “add anything substantially different to what we already” have with FOLFIRINOX, he told this news organization. Given that, “we can’t really justify NALIRIFOX over FOLFIRINOX without more of a head-to-head comparison.”
The higher cost of NALIRIFOX, in particular, remains a major drawback.
“We think it would be an economic disservice to our healthcare systems if we used NALIRIFOX instead of FOLFIRINOX for these patients on the basis of [NAPOLI 3] data,” Bishal Gyawali, MD, PhD, and Christopher Booth, MD, gastrointestinal oncologists at Queen’s University in Kingston, Ontario, Canada, said in a recent essay.
Dr. Garrido-Laguna and Dr. Nevala-Plagemann reiterated this concern.
Overall, “NALIRIFOX does not seem to raise the bar but rather exposes patients and healthcare systems to financial toxicities,” Dr. Garrido-Laguna and Dr. Nevala-Plagemann wrote in their review.
NAPOLI 3 was funded by Ipsen and PRODIGE 4 was funded by the government of France. No funding source was reported for the meta-analysis. NAPOLI 3 investigators included Ipsen employees. Dr. O’Reilly disclosed grants or contracts from Ipsen and many other companies. Dr. Garrido-Laguna reported institutional research funding from Bristol Myers Squibb, Novartis, Pfizer, and other companies, but not Ipsen. Dr. Nevala-Plagemann is an advisor for Seagen and reported institutional research funding from Theriva. Dr. Gyawali is a consultant for Vivio Health; Dr. Booth had no disclosures. Two meta-analysis authors reported grants or personal fees from Ipsen as well as ties to other companies.
A version of this article appeared on Medscape.com.
In February, the US Food and Drug Administration (FDA) approved irinotecan liposome (Onivyde) as part of a new regimen for first-line metastatic pancreatic adenocarcinoma called NALIRIFOX.
The main difference between NALIRIFOX and a standard go-to regimen for the indication, modified FOLFIRINOX, is that liposomal irinotecan — irinotecan encased in a lipid nanoparticle — is used instead of free irinotecan.
Trial data suggested a better overall response rate, a slight progression-free survival advantage, and potentially fewer adverse events with the liposomal formulation.
The substitution, however, raises the cost of treatment substantially. According to one estimate, a single cycle of FOLFIRINOX costs about $500 at a body surface area of 2 m2, while the equivalent single cycle of NALIRIFOX costs $7800 — over 15-fold more expensive.
While some oncologists have called the NALIRIFOX regimen a potential new standard first-line treatment for metastatic pancreatic adenocarcinoma, others have expressed serious doubts about whether the potential benefits are worth the extra cost.
“I can’t really see a single scenario where I would recommend NALIRIFOX over FOLFIRINOX” Ignacio Garrido-Laguna, MD, PhD, a gastrointestinal oncologist and pancreatic cancer researcher at the University of Utah, Salt Lake City, told this news organization. “Most of us in the academic setting have the same take on this.”
No Head-to-Head Comparison
Instead, the 770-patient NAPOLI 3 trial compared NALIRIFOX — which also includes oxaliplatin, fluorouracil, and leucovorin — with a two-drug regimen, nab-paclitaxel and gemcitabine. In the trial, overall survival and other outcomes were moderately better with NALIRIFOX.
Oncologists have said that the true value of the trial is that it conclusively demonstrates that a four-drug regimen is superior to a two-drug regimen for patients who can tolerate the more intensive therapy.
Eileen M. O’Reilly, MD, the senior investigator on NAPOLI 3, made this point when she presented the phase 3 results at the 2023 ASCO annual meeting.
The trial “answers the question of four drugs versus two” for first-line metastatic pancreatic cancer but “does not address the question of NALIRIFOX versus FOLFIRINOX,” said Dr. O’Reilly, a pancreatic and hepatobiliary oncologist and researcher at Memorial Sloan Kettering Cancer Center in New York City.
Comparing them directly in the study “probably wouldn’t have been in the interest of the sponsor,” said Dr. O’Reilly.
With no head-to-head comparison, oncologists have been comparing NAPOLI 3 results with those from PRODIGE 4, the 2011 trial that won FOLFIRINOX its place as a first-line regimen.
When comparing the trials, median overall survival was exactly the same for the two regimens — 11.1 months. FOLFIRINOX was associated with a slightly higher 1-year survival rate — 48.4% with FOLFIRINOX vs 45.6% with NALIRIFOX.
However, Dr. O’Reilly and her colleagues also highlighted comparisons between the two trials that favored NAPOLI 3.
NAPOLI 3 had no age limit, while PRODIGE subjects were no older than 75 years. Median progression-free survival was 1 month longer among patients receiving NALIRIFOX — 7.4 months vs 6.4 months in PRODIGE — and overall response rates were higher as well — 41.8% in NAPOLI 3 vs 31.6%. Patients receiving NALIRIFOX also had lower rates of grade 3/4 neutropenia (23.8% vs 45.7%, respectively) and peripheral sensory neuropathy (3.5% vs 9.0%, respectively).
The authors explained that the lower rate of neuropathy could be because NALIRIFOX uses a lower dose of oxaliplatin (FOLFIRINOX), at 60 mg/m2 instead of 85 mg/m2.
Is It Worth It?
During a presentation of the phase 3 findings last year, study author Zev A. Wainberg, MD, of the University of California, Los Angeles, said the NALIRIFOX regimen can be considered the new reference regimen for first-line treatment of metastatic pancreatic adenocarcinoma.
The study discussant, Laura Goff, MD, MSCI, of Vanderbilt-Ingram Cancer Center, Nashville, Tennessee, agreed that the results support the NALIRIFOX regimen as “the new standard for fit patients.”
However, other oncologists remain skeptical about the benefits of the new regimen over FOLFIRINOX for patients with metastatic pancreatic adenocarcinoma.
In a recent editorial, Dr. Garrido-Laguna and University of Utah gastrointestinal oncologist Christopher Nevala-Plagemann, MD, compared the evidence for both regimens.
The experts pointed out that overall response rates were assessed by investigators in NAPOLI 3 and not by an independent review committee, as in PRODIGE 4, and might have been overestimated.
Although the lack of an age limit was touted as a benefit of NAPOLI 3, Dr. Garrido-Laguna and Dr. Nevala-Plagemann doubt whether enough patients over 75 years old participated to draw any meaningful conclusions about using NALIRIFOX in older, frailer patients. If anything, patients in PRODIGE 4 might have been less fit because, among other things, the trial allowed patients with serum albumins < 3 g/dL.
On the adverse event front, the authors highlighted the higher incidences of grade 3 or worse diarrhea with NALIRIFOX (20% vs 12.7%) and questioned if there truly is less neutropenia with NALIRIFOX because high-risk patients in NAPOLI 3 were treated with granulocyte colony-stimulating factor to prevent it. The pair also questioned whether the differences in neuropathy rates between the two trials were big enough to be clinically meaningful.
Insights from a recent meta-analysis may further clarify some of the lingering questions about the efficacy of NALIRIFOX vs FOLFIRINOX.
In the analysis, the team found no meaningful difference in overall and progression-free survival between the two regimens. Differences in rates of peripheral neuropathy and diarrhea were not statistically significant, but NALIRIFOX did carry a statistically significant advantage in lower rates of febrile neutropenia, thrombocytopenia, and vomiting.
The team concluded that “NALIRIFOX and FOLFIRINOX may provide equal efficacy as first-line treatment of metastatic pancreatic cancer, but with different toxicity profiles,” and called for careful patient selection when choosing between the two regimens as well as consideration of financial toxicity.
Dr. Garrido-Laguna had a different take. With the current data, NALIRIFOX does not seem to “add anything substantially different to what we already” have with FOLFIRINOX, he told this news organization. Given that, “we can’t really justify NALIRIFOX over FOLFIRINOX without more of a head-to-head comparison.”
The higher cost of NALIRIFOX, in particular, remains a major drawback.
“We think it would be an economic disservice to our healthcare systems if we used NALIRIFOX instead of FOLFIRINOX for these patients on the basis of [NAPOLI 3] data,” Bishal Gyawali, MD, PhD, and Christopher Booth, MD, gastrointestinal oncologists at Queen’s University in Kingston, Ontario, Canada, said in a recent essay.
Dr. Garrido-Laguna and Dr. Nevala-Plagemann reiterated this concern.
Overall, “NALIRIFOX does not seem to raise the bar but rather exposes patients and healthcare systems to financial toxicities,” Dr. Garrido-Laguna and Dr. Nevala-Plagemann wrote in their review.
NAPOLI 3 was funded by Ipsen and PRODIGE 4 was funded by the government of France. No funding source was reported for the meta-analysis. NAPOLI 3 investigators included Ipsen employees. Dr. O’Reilly disclosed grants or contracts from Ipsen and many other companies. Dr. Garrido-Laguna reported institutional research funding from Bristol Myers Squibb, Novartis, Pfizer, and other companies, but not Ipsen. Dr. Nevala-Plagemann is an advisor for Seagen and reported institutional research funding from Theriva. Dr. Gyawali is a consultant for Vivio Health; Dr. Booth had no disclosures. Two meta-analysis authors reported grants or personal fees from Ipsen as well as ties to other companies.
A version of this article appeared on Medscape.com.
Urine Tests Could Be ‘Enormous Step’ in Diagnosing Cancer
Emerging science suggests that the body’s “liquid gold” could be particularly useful for liquid biopsies, offering a convenient, pain-free, and cost-effective way to spot otherwise hard-to-detect cancers.
“The search for cancer biomarkers that can be detected in urine could provide an enormous step forward to decrease cancer patient mortality,” said Kenneth R. Shroyer, MD, PhD, a pathologist at Stony Brook University, Stony Brook, New York, who studies cancer biomarkers.
Physicians have long known that urine can reveal a lot about our health — that’s why urinalysis has been part of medicine for 6000 years. Urine tests can detect diabetes, pregnancy, drug use, and urinary or kidney conditions.
But other conditions leave clues in urine, too, and cancer may be one of the most promising. “Urine testing could detect biomarkers of early-stage cancers, not only from local but also distant sites,” Dr. Shroyer said. It could also help flag recurrence in cancer survivors who have undergone treatment.
Granted, cancer biomarkers in urine are not nearly as widely studied as those in the blood, Dr. Shroyer noted. But a new wave of urine tests suggests research is gaining pace.
“The recent availability of high-throughput screening technologies has enabled researchers to investigate cancer from a top-down, comprehensive approach,” said Pak Kin Wong, PhD, professor of mechanical engineering, biomedical engineering, and surgery at The Pennsylvania State University. “We are starting to understand the rich information that can be obtained from urine.”
Urine is mostly water (about 95%) and urea, a metabolic byproduct that imparts that signature yellow color (about 2%). The other 3% is a mix of waste products, minerals, and other compounds the kidneys removed from the blood. Even in trace amounts, these substances say a lot.
Among them are “exfoliated cancer cells, cell-free DNA, hormones, and the urine microbiota — the collection of microbes in our urinary tract system,” Dr. Wong said.
“It is highly promising to be one of the major biological fluids used for screening, diagnosis, prognosis, and monitoring treatment efficiency in the era of precision medicine,” Dr. Wong said.
How Urine Testing Could Reveal Cancer
Still, as exciting as the prospect is, there’s a lot to consider in the hunt for cancer biomarkers in urine. These biomarkers must be able to pass through the renal nephrons (filtering units), remain stable in urine, and have high-level sensitivity, Dr. Shroyer said. They should also have high specificity for cancer vs benign conditions and be expressed at early stages, before the primary tumor has spread.
“At this stage, few circulating biomarkers have been found that are both sensitive and specific for early-stage disease,” said Dr. Shroyer.
But there are a few promising examples under investigation in humans:
Prostate cancer. Researchers at the University of Michigan have developed a urine test that detects high-grade prostate cancer more accurately than existing tests, including PHI, SelectMDx, 4Kscore, EPI, MPS, and IsoPSA.
The MyProstateScore 2.0 (MPS2) test, which looks for 18 genes associated with high-grade tumors, could reduce unnecessary biopsies in men with elevated prostate-specific antigen levels, according to a paper published in JAMA Oncology.
It makes sense. The prostate gland secretes fluid that becomes part of the semen, traces of which enter urine. After a digital rectal exam, even more prostate fluid enters the urine. If a patient has prostate cancer, genetic material from the cancer cells will infiltrate the urine.
In the MPS2 test, researchers used polymerase chain reaction (PCR) testing in urine. “The technology used for COVID PCR is essentially the same as the PCR used to detect transcripts associated with high-grade prostate cancer in urine,” said study author Arul Chinnaiyan, MD, PhD, director of the Michigan Center for Translational Pathology at the University of Michigan, Ann Arbor. “In the case of the MPS2 test, we are doing PCR on 18 genes simultaneously on urine samples.”
A statistical model uses levels of that genetic material to predict the risk for high-grade disease, helping doctors decide what to do next. At 95% sensitivity, the MPS2 model could eliminate 35%-45% of unnecessary biopsies, compared with 15%-30% for the other tests, and reduce repeat biopsies by 46%-51%, compared with 9%-21% for the other tests.
Head and neck cancer. In a paper published in JCI Insight, researchers described a test that finds ultra-short fragments of DNA in urine to enable early detection of head and neck cancers caused by human papillomavirus.
“Our data show that a relatively small volume of urine (30-60 mL) gives overall detection results comparable to a tube of blood,” said study author Muneesh Tewari, MD, PhD, professor of hematology and oncology at the University of Michigan .
A larger volume of urine could potentially “make cancer detection even more sensitive than blood,” Dr. Tewari said, “allowing cancers to be detected at the earliest stages when they are more curable.”
The team used a technique called droplet digital PCR to detect DNA fragments that are “ultra-short” (less than 50 base pairs long) and usually missed by conventional PCR testing. This transrenal cell-free tumor DNA, which travels from the tumor into the bloodstream, is broken down small enough to pass through the kidneys and into the urine. But the fragments are still long enough to carry information about the tumor’s genetic signature.
This test could spot cancer before a tumor grows big enough — about a centimeter wide and carrying a billion cells — to spot on a CT scan or other imaging test. “When we are instead detecting fragments of DNA released from a tumor,” said Dr. Tewari, “our testing methods are very sensitive and can detect DNA in urine that came from just 5-10 cells in a tumor that died and released their DNA into the blood, which then made its way into the urine.”
Pancreatic cancer. Pancreatic ductal adenocarcinoma is one of the deadliest cancers, largely because it is diagnosed so late. A urine panel now in clinical trials could help doctors diagnose the cancer before it has spread so more people can have the tumor surgically removed, improving prognosis.
Using enzyme-linked immunosorbent assay test, a common lab method that detects antibodies and other proteins, the team measured expression levels for three genes (LYVE1, REG1B, and TFF1) in urine samples collected from people up to 5 years before they were diagnosed with pancreatic cancer. The researchers combined this result with patients’ urinary creatinine levels, a common component of existing urinalysis, and their age to develop a risk score.
This score performed similarly to an existing blood test, CA19-9, in predicting patients’ risk for pancreatic cancer up to 1 year before diagnosis. When combined with CA19-9, the urinary panel helped spot cancer up to 2 years before diagnosis.
According to a paper in the International Journal of Cancer, “the urine panel and affiliated PancRISK are currently being validated in a prospective clinical study (UroPanc).” If all goes well, they could be implemented in clinical practice in a few years as a “noninvasive stratification tool” to identify patients for further testing, speeding up diagnosis, and saving lives.
Limitations and Promises
Each cancer type is different, and more research is needed to map out which substances in urine predict which cancers and to develop tests for mass adoption. “There are medical and technological hurdles to the large-scale implementation of urine analysis for complex diseases such as cancer,” said Dr. Wong.
One possibility: Scientists and clinicians could collaborate and use artificial intelligence techniques to combine urine test results with other data.
“It is likely that future diagnostics may combine urine with other biological samples such as feces and saliva, among others,” said Dr. Wong. “This is especially true when novel data science and machine learning techniques can integrate comprehensive data from patients that span genetic, proteomic, metabolic, microbiomic, and even behavioral data to evaluate a patient’s condition.”
One thing that excites Dr. Tewari about urine-based cancer testing: “We think it could be especially impactful for patients living in rural areas or other areas with less access to healthcare services,” he said.
A version of this article appeared on Medscape.com.
Emerging science suggests that the body’s “liquid gold” could be particularly useful for liquid biopsies, offering a convenient, pain-free, and cost-effective way to spot otherwise hard-to-detect cancers.
“The search for cancer biomarkers that can be detected in urine could provide an enormous step forward to decrease cancer patient mortality,” said Kenneth R. Shroyer, MD, PhD, a pathologist at Stony Brook University, Stony Brook, New York, who studies cancer biomarkers.
Physicians have long known that urine can reveal a lot about our health — that’s why urinalysis has been part of medicine for 6000 years. Urine tests can detect diabetes, pregnancy, drug use, and urinary or kidney conditions.
But other conditions leave clues in urine, too, and cancer may be one of the most promising. “Urine testing could detect biomarkers of early-stage cancers, not only from local but also distant sites,” Dr. Shroyer said. It could also help flag recurrence in cancer survivors who have undergone treatment.
Granted, cancer biomarkers in urine are not nearly as widely studied as those in the blood, Dr. Shroyer noted. But a new wave of urine tests suggests research is gaining pace.
“The recent availability of high-throughput screening technologies has enabled researchers to investigate cancer from a top-down, comprehensive approach,” said Pak Kin Wong, PhD, professor of mechanical engineering, biomedical engineering, and surgery at The Pennsylvania State University. “We are starting to understand the rich information that can be obtained from urine.”
Urine is mostly water (about 95%) and urea, a metabolic byproduct that imparts that signature yellow color (about 2%). The other 3% is a mix of waste products, minerals, and other compounds the kidneys removed from the blood. Even in trace amounts, these substances say a lot.
Among them are “exfoliated cancer cells, cell-free DNA, hormones, and the urine microbiota — the collection of microbes in our urinary tract system,” Dr. Wong said.
“It is highly promising to be one of the major biological fluids used for screening, diagnosis, prognosis, and monitoring treatment efficiency in the era of precision medicine,” Dr. Wong said.
How Urine Testing Could Reveal Cancer
Still, as exciting as the prospect is, there’s a lot to consider in the hunt for cancer biomarkers in urine. These biomarkers must be able to pass through the renal nephrons (filtering units), remain stable in urine, and have high-level sensitivity, Dr. Shroyer said. They should also have high specificity for cancer vs benign conditions and be expressed at early stages, before the primary tumor has spread.
“At this stage, few circulating biomarkers have been found that are both sensitive and specific for early-stage disease,” said Dr. Shroyer.
But there are a few promising examples under investigation in humans:
Prostate cancer. Researchers at the University of Michigan have developed a urine test that detects high-grade prostate cancer more accurately than existing tests, including PHI, SelectMDx, 4Kscore, EPI, MPS, and IsoPSA.
The MyProstateScore 2.0 (MPS2) test, which looks for 18 genes associated with high-grade tumors, could reduce unnecessary biopsies in men with elevated prostate-specific antigen levels, according to a paper published in JAMA Oncology.
It makes sense. The prostate gland secretes fluid that becomes part of the semen, traces of which enter urine. After a digital rectal exam, even more prostate fluid enters the urine. If a patient has prostate cancer, genetic material from the cancer cells will infiltrate the urine.
In the MPS2 test, researchers used polymerase chain reaction (PCR) testing in urine. “The technology used for COVID PCR is essentially the same as the PCR used to detect transcripts associated with high-grade prostate cancer in urine,” said study author Arul Chinnaiyan, MD, PhD, director of the Michigan Center for Translational Pathology at the University of Michigan, Ann Arbor. “In the case of the MPS2 test, we are doing PCR on 18 genes simultaneously on urine samples.”
A statistical model uses levels of that genetic material to predict the risk for high-grade disease, helping doctors decide what to do next. At 95% sensitivity, the MPS2 model could eliminate 35%-45% of unnecessary biopsies, compared with 15%-30% for the other tests, and reduce repeat biopsies by 46%-51%, compared with 9%-21% for the other tests.
Head and neck cancer. In a paper published in JCI Insight, researchers described a test that finds ultra-short fragments of DNA in urine to enable early detection of head and neck cancers caused by human papillomavirus.
“Our data show that a relatively small volume of urine (30-60 mL) gives overall detection results comparable to a tube of blood,” said study author Muneesh Tewari, MD, PhD, professor of hematology and oncology at the University of Michigan .
A larger volume of urine could potentially “make cancer detection even more sensitive than blood,” Dr. Tewari said, “allowing cancers to be detected at the earliest stages when they are more curable.”
The team used a technique called droplet digital PCR to detect DNA fragments that are “ultra-short” (less than 50 base pairs long) and usually missed by conventional PCR testing. This transrenal cell-free tumor DNA, which travels from the tumor into the bloodstream, is broken down small enough to pass through the kidneys and into the urine. But the fragments are still long enough to carry information about the tumor’s genetic signature.
This test could spot cancer before a tumor grows big enough — about a centimeter wide and carrying a billion cells — to spot on a CT scan or other imaging test. “When we are instead detecting fragments of DNA released from a tumor,” said Dr. Tewari, “our testing methods are very sensitive and can detect DNA in urine that came from just 5-10 cells in a tumor that died and released their DNA into the blood, which then made its way into the urine.”
Pancreatic cancer. Pancreatic ductal adenocarcinoma is one of the deadliest cancers, largely because it is diagnosed so late. A urine panel now in clinical trials could help doctors diagnose the cancer before it has spread so more people can have the tumor surgically removed, improving prognosis.
Using enzyme-linked immunosorbent assay test, a common lab method that detects antibodies and other proteins, the team measured expression levels for three genes (LYVE1, REG1B, and TFF1) in urine samples collected from people up to 5 years before they were diagnosed with pancreatic cancer. The researchers combined this result with patients’ urinary creatinine levels, a common component of existing urinalysis, and their age to develop a risk score.
This score performed similarly to an existing blood test, CA19-9, in predicting patients’ risk for pancreatic cancer up to 1 year before diagnosis. When combined with CA19-9, the urinary panel helped spot cancer up to 2 years before diagnosis.
According to a paper in the International Journal of Cancer, “the urine panel and affiliated PancRISK are currently being validated in a prospective clinical study (UroPanc).” If all goes well, they could be implemented in clinical practice in a few years as a “noninvasive stratification tool” to identify patients for further testing, speeding up diagnosis, and saving lives.
Limitations and Promises
Each cancer type is different, and more research is needed to map out which substances in urine predict which cancers and to develop tests for mass adoption. “There are medical and technological hurdles to the large-scale implementation of urine analysis for complex diseases such as cancer,” said Dr. Wong.
One possibility: Scientists and clinicians could collaborate and use artificial intelligence techniques to combine urine test results with other data.
“It is likely that future diagnostics may combine urine with other biological samples such as feces and saliva, among others,” said Dr. Wong. “This is especially true when novel data science and machine learning techniques can integrate comprehensive data from patients that span genetic, proteomic, metabolic, microbiomic, and even behavioral data to evaluate a patient’s condition.”
One thing that excites Dr. Tewari about urine-based cancer testing: “We think it could be especially impactful for patients living in rural areas or other areas with less access to healthcare services,” he said.
A version of this article appeared on Medscape.com.
Emerging science suggests that the body’s “liquid gold” could be particularly useful for liquid biopsies, offering a convenient, pain-free, and cost-effective way to spot otherwise hard-to-detect cancers.
“The search for cancer biomarkers that can be detected in urine could provide an enormous step forward to decrease cancer patient mortality,” said Kenneth R. Shroyer, MD, PhD, a pathologist at Stony Brook University, Stony Brook, New York, who studies cancer biomarkers.
Physicians have long known that urine can reveal a lot about our health — that’s why urinalysis has been part of medicine for 6000 years. Urine tests can detect diabetes, pregnancy, drug use, and urinary or kidney conditions.
But other conditions leave clues in urine, too, and cancer may be one of the most promising. “Urine testing could detect biomarkers of early-stage cancers, not only from local but also distant sites,” Dr. Shroyer said. It could also help flag recurrence in cancer survivors who have undergone treatment.
Granted, cancer biomarkers in urine are not nearly as widely studied as those in the blood, Dr. Shroyer noted. But a new wave of urine tests suggests research is gaining pace.
“The recent availability of high-throughput screening technologies has enabled researchers to investigate cancer from a top-down, comprehensive approach,” said Pak Kin Wong, PhD, professor of mechanical engineering, biomedical engineering, and surgery at The Pennsylvania State University. “We are starting to understand the rich information that can be obtained from urine.”
Urine is mostly water (about 95%) and urea, a metabolic byproduct that imparts that signature yellow color (about 2%). The other 3% is a mix of waste products, minerals, and other compounds the kidneys removed from the blood. Even in trace amounts, these substances say a lot.
Among them are “exfoliated cancer cells, cell-free DNA, hormones, and the urine microbiota — the collection of microbes in our urinary tract system,” Dr. Wong said.
“It is highly promising to be one of the major biological fluids used for screening, diagnosis, prognosis, and monitoring treatment efficiency in the era of precision medicine,” Dr. Wong said.
How Urine Testing Could Reveal Cancer
Still, as exciting as the prospect is, there’s a lot to consider in the hunt for cancer biomarkers in urine. These biomarkers must be able to pass through the renal nephrons (filtering units), remain stable in urine, and have high-level sensitivity, Dr. Shroyer said. They should also have high specificity for cancer vs benign conditions and be expressed at early stages, before the primary tumor has spread.
“At this stage, few circulating biomarkers have been found that are both sensitive and specific for early-stage disease,” said Dr. Shroyer.
But there are a few promising examples under investigation in humans:
Prostate cancer. Researchers at the University of Michigan have developed a urine test that detects high-grade prostate cancer more accurately than existing tests, including PHI, SelectMDx, 4Kscore, EPI, MPS, and IsoPSA.
The MyProstateScore 2.0 (MPS2) test, which looks for 18 genes associated with high-grade tumors, could reduce unnecessary biopsies in men with elevated prostate-specific antigen levels, according to a paper published in JAMA Oncology.
It makes sense. The prostate gland secretes fluid that becomes part of the semen, traces of which enter urine. After a digital rectal exam, even more prostate fluid enters the urine. If a patient has prostate cancer, genetic material from the cancer cells will infiltrate the urine.
In the MPS2 test, researchers used polymerase chain reaction (PCR) testing in urine. “The technology used for COVID PCR is essentially the same as the PCR used to detect transcripts associated with high-grade prostate cancer in urine,” said study author Arul Chinnaiyan, MD, PhD, director of the Michigan Center for Translational Pathology at the University of Michigan, Ann Arbor. “In the case of the MPS2 test, we are doing PCR on 18 genes simultaneously on urine samples.”
A statistical model uses levels of that genetic material to predict the risk for high-grade disease, helping doctors decide what to do next. At 95% sensitivity, the MPS2 model could eliminate 35%-45% of unnecessary biopsies, compared with 15%-30% for the other tests, and reduce repeat biopsies by 46%-51%, compared with 9%-21% for the other tests.
Head and neck cancer. In a paper published in JCI Insight, researchers described a test that finds ultra-short fragments of DNA in urine to enable early detection of head and neck cancers caused by human papillomavirus.
“Our data show that a relatively small volume of urine (30-60 mL) gives overall detection results comparable to a tube of blood,” said study author Muneesh Tewari, MD, PhD, professor of hematology and oncology at the University of Michigan .
A larger volume of urine could potentially “make cancer detection even more sensitive than blood,” Dr. Tewari said, “allowing cancers to be detected at the earliest stages when they are more curable.”
The team used a technique called droplet digital PCR to detect DNA fragments that are “ultra-short” (less than 50 base pairs long) and usually missed by conventional PCR testing. This transrenal cell-free tumor DNA, which travels from the tumor into the bloodstream, is broken down small enough to pass through the kidneys and into the urine. But the fragments are still long enough to carry information about the tumor’s genetic signature.
This test could spot cancer before a tumor grows big enough — about a centimeter wide and carrying a billion cells — to spot on a CT scan or other imaging test. “When we are instead detecting fragments of DNA released from a tumor,” said Dr. Tewari, “our testing methods are very sensitive and can detect DNA in urine that came from just 5-10 cells in a tumor that died and released their DNA into the blood, which then made its way into the urine.”
Pancreatic cancer. Pancreatic ductal adenocarcinoma is one of the deadliest cancers, largely because it is diagnosed so late. A urine panel now in clinical trials could help doctors diagnose the cancer before it has spread so more people can have the tumor surgically removed, improving prognosis.
Using enzyme-linked immunosorbent assay test, a common lab method that detects antibodies and other proteins, the team measured expression levels for three genes (LYVE1, REG1B, and TFF1) in urine samples collected from people up to 5 years before they were diagnosed with pancreatic cancer. The researchers combined this result with patients’ urinary creatinine levels, a common component of existing urinalysis, and their age to develop a risk score.
This score performed similarly to an existing blood test, CA19-9, in predicting patients’ risk for pancreatic cancer up to 1 year before diagnosis. When combined with CA19-9, the urinary panel helped spot cancer up to 2 years before diagnosis.
According to a paper in the International Journal of Cancer, “the urine panel and affiliated PancRISK are currently being validated in a prospective clinical study (UroPanc).” If all goes well, they could be implemented in clinical practice in a few years as a “noninvasive stratification tool” to identify patients for further testing, speeding up diagnosis, and saving lives.
Limitations and Promises
Each cancer type is different, and more research is needed to map out which substances in urine predict which cancers and to develop tests for mass adoption. “There are medical and technological hurdles to the large-scale implementation of urine analysis for complex diseases such as cancer,” said Dr. Wong.
One possibility: Scientists and clinicians could collaborate and use artificial intelligence techniques to combine urine test results with other data.
“It is likely that future diagnostics may combine urine with other biological samples such as feces and saliva, among others,” said Dr. Wong. “This is especially true when novel data science and machine learning techniques can integrate comprehensive data from patients that span genetic, proteomic, metabolic, microbiomic, and even behavioral data to evaluate a patient’s condition.”
One thing that excites Dr. Tewari about urine-based cancer testing: “We think it could be especially impactful for patients living in rural areas or other areas with less access to healthcare services,” he said.
A version of this article appeared on Medscape.com.
Treatments for Early HS Range From Topical Therapies to Laser Hair Removal
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
Diagnosing Mild Hidradenitis Suppurativa: Early Stage Can Mimic Other Diseases
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
The Lure of Specialty Medicine Pulls Nurse Practitioners From Primary Care
For many patients, seeing a nurse practitioner has become a routine part of primary care, in which these “NPs” often perform the same tasks that patients have relied on doctors for.
But NPs in specialty care? That’s not routine, at least not yet. Increasingly, though, nurse practitioners and physician assistants are joining cardiology, dermatology, and other specialty practices, broadening their skills and increasing their income.
This development worries some people who track the health workforce, because current trends suggest primary care, which has counted on nurse practitioners to backstop physician shortages, soon might not be able to rely on them to the same extent.
“They’re succumbing to the same challenges that we have with physicians,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute at the Association of American Medical Colleges. The rates NPs can command in a specialty practice “are quite a bit higher” than practice salaries in primary care, he said.
When nurse practitioner programs began to proliferate in the 1970s, “at first it looked great, producing all these nurse practitioners that go to work with primary care physicians,” said Yalda Jabbarpour, MD, director of the American Academy of Family Physicians’ Robert Graham Center for Policy Studies. “But now only 30% are going into primary care.”
Dr. Jabbarpour was referring to the 2024 primary care scorecard by the Milbank Memorial Fund, which found that from 2016 to 2021 the proportion of nurse practitioners who worked in primary care practices hovered between 32% and 34%, even though their numbers grew rapidly. The proportion of physician assistants, also known as physician associates, in primary care ranged from 27% to 30%, the study found.
Both nurse practitioners and physician assistants are advanced practice clinicians who, in addition to graduate degrees, must complete distinct education, training, and certification steps. NPs can practice without a doctor’s supervision in more than two dozen states, while PAs have similar independence in only a handful of states.
About 88% of nurse practitioners are certified in an area of primary care, according to the American Association of Nurse Practitioners. But it is difficult to track exactly how many work in primary care or in specialty practices. Unlike physicians, they’re generally not required to be endorsed by a national standard-setting body to practice in specialties like oncology or cardiology, for example. The AANP declined to answer questions about its annual workforce survey or the extent to which primary care NPs are moving toward specialties.
Though data tracking the change is sparse, specialty practices are adding these advanced practice clinicians at almost the same rate as primary care practices, according to frequently cited research published in 2018.
The clearest evidence of the shift: From 2008 to 2016, there was a 22% increase in the number of specialty practices that employed nurse practitioners and physician assistants, according to that study. The increase in the number of primary care practices that employed these professionals was 24%.
Once more, the most recent projections by the Association of American Medical Colleges predict a dearth of at least 20,200 primary care physicians by 2036. There will also be a shortfall of non-primary care specialists, including a deficiency of at least 10,100 surgical physicians and up to 25,000 physicians in other specialties.
When it comes to the actual work performed, the lines between primary and specialty care are often blurred, said Candice Chen, MD, MPH, associate professor of health policy and management at George Washington University.
“You might be a nurse practitioner working in a gastroenterology clinic or cardiology clinic, but the scope of what you do is starting to overlap with primary care,” she said.
Nurse practitioners’ salaries vary widely by location, type of facility, and experience. Still, according to data from health care recruiter AMN Healthcare Physician Solutions, formerly known as Merritt Hawkins, the total annual average starting compensation, including signing bonus, for nurse practitioners and physician assistants in specialty practice was $172,544 in the year that ended March 31, slightly higher than the $166,544 for those in primary care.
According to forecasts from the federal Bureau of Labor Statistics, nurse practitioner jobs will increase faster than jobs in almost any other occupation in the decade leading up to 2032, growing by 123,600 jobs or 45%. (Wind turbine service technician is the only other occupation projected to grow as fast.) The growth rate for physician assistants is also much faster than average, at 27%. There are more than twice as many nurse practitioners as physician assistants, however: 323,900 versus 148,000, in 2022.
To Dr. Grover of the AAMC, numbers like this signal that there will probably be enough NPs, PAs, and physicians to meet primary care needs. At the same time, “expect more NPs and PAs to also flow out into other specialties,” he said.
When Pamela Ograbisz started working as a registered nurse 27 years ago, she worked in a cardiothoracic intensive care unit. After she became a family nurse practitioner a few years later, she found a job with a similar specialty practice, which trained her to take on a bigger role, first running their outpatient clinic, then working on the floor, and later in the intensive care unit.
If nurse practitioners want to specialize, often “the doctors mentor them just like they would with a physician residency,” said Ms. Ograbisz, now vice president of clinical operations at temporary placement recruiter LocumTenens.com.
If physician assistants want to specialize, they also can do so through mentoring, or they can receive “certificates of added qualifications” in 10 specialties to demonstrate their expertise. Most employers don’t “encourage or require” these certificates, however, said Jennifer Orozco, DMSc, PA-C, DFAAPA, chief medical officer at the American Academy of Physician Associates.
There are a number of training programs for family nurse practitioners who want to develop skills in other areas.
Raina Hoebelheinrich, 40, a family nurse practitioner at a regional medical center in Yankton, South Dakota, recently enrolled in a three-semester post-master’s endocrinology training program at Mount Marty University. She lives on a farm in nearby northeastern Nebraska with her husband and five sons.
Ms. Hoebelheinrich’s new skills could be helpful in her current hospital job, in which she sees a lot of patients with acute diabetes, or in a clinic setting like the one in Sioux Falls, South Dakota, where she is doing her clinical endocrinology training.
Lack of access to endocrinology care in rural areas is a real problem, and many people may travel hundreds of miles to see a specialist.
“There aren’t a lot of options,” she said.
A version of this article first appeared on Medscape.com.
For many patients, seeing a nurse practitioner has become a routine part of primary care, in which these “NPs” often perform the same tasks that patients have relied on doctors for.
But NPs in specialty care? That’s not routine, at least not yet. Increasingly, though, nurse practitioners and physician assistants are joining cardiology, dermatology, and other specialty practices, broadening their skills and increasing their income.
This development worries some people who track the health workforce, because current trends suggest primary care, which has counted on nurse practitioners to backstop physician shortages, soon might not be able to rely on them to the same extent.
“They’re succumbing to the same challenges that we have with physicians,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute at the Association of American Medical Colleges. The rates NPs can command in a specialty practice “are quite a bit higher” than practice salaries in primary care, he said.
When nurse practitioner programs began to proliferate in the 1970s, “at first it looked great, producing all these nurse practitioners that go to work with primary care physicians,” said Yalda Jabbarpour, MD, director of the American Academy of Family Physicians’ Robert Graham Center for Policy Studies. “But now only 30% are going into primary care.”
Dr. Jabbarpour was referring to the 2024 primary care scorecard by the Milbank Memorial Fund, which found that from 2016 to 2021 the proportion of nurse practitioners who worked in primary care practices hovered between 32% and 34%, even though their numbers grew rapidly. The proportion of physician assistants, also known as physician associates, in primary care ranged from 27% to 30%, the study found.
Both nurse practitioners and physician assistants are advanced practice clinicians who, in addition to graduate degrees, must complete distinct education, training, and certification steps. NPs can practice without a doctor’s supervision in more than two dozen states, while PAs have similar independence in only a handful of states.
About 88% of nurse practitioners are certified in an area of primary care, according to the American Association of Nurse Practitioners. But it is difficult to track exactly how many work in primary care or in specialty practices. Unlike physicians, they’re generally not required to be endorsed by a national standard-setting body to practice in specialties like oncology or cardiology, for example. The AANP declined to answer questions about its annual workforce survey or the extent to which primary care NPs are moving toward specialties.
Though data tracking the change is sparse, specialty practices are adding these advanced practice clinicians at almost the same rate as primary care practices, according to frequently cited research published in 2018.
The clearest evidence of the shift: From 2008 to 2016, there was a 22% increase in the number of specialty practices that employed nurse practitioners and physician assistants, according to that study. The increase in the number of primary care practices that employed these professionals was 24%.
Once more, the most recent projections by the Association of American Medical Colleges predict a dearth of at least 20,200 primary care physicians by 2036. There will also be a shortfall of non-primary care specialists, including a deficiency of at least 10,100 surgical physicians and up to 25,000 physicians in other specialties.
When it comes to the actual work performed, the lines between primary and specialty care are often blurred, said Candice Chen, MD, MPH, associate professor of health policy and management at George Washington University.
“You might be a nurse practitioner working in a gastroenterology clinic or cardiology clinic, but the scope of what you do is starting to overlap with primary care,” she said.
Nurse practitioners’ salaries vary widely by location, type of facility, and experience. Still, according to data from health care recruiter AMN Healthcare Physician Solutions, formerly known as Merritt Hawkins, the total annual average starting compensation, including signing bonus, for nurse practitioners and physician assistants in specialty practice was $172,544 in the year that ended March 31, slightly higher than the $166,544 for those in primary care.
According to forecasts from the federal Bureau of Labor Statistics, nurse practitioner jobs will increase faster than jobs in almost any other occupation in the decade leading up to 2032, growing by 123,600 jobs or 45%. (Wind turbine service technician is the only other occupation projected to grow as fast.) The growth rate for physician assistants is also much faster than average, at 27%. There are more than twice as many nurse practitioners as physician assistants, however: 323,900 versus 148,000, in 2022.
To Dr. Grover of the AAMC, numbers like this signal that there will probably be enough NPs, PAs, and physicians to meet primary care needs. At the same time, “expect more NPs and PAs to also flow out into other specialties,” he said.
When Pamela Ograbisz started working as a registered nurse 27 years ago, she worked in a cardiothoracic intensive care unit. After she became a family nurse practitioner a few years later, she found a job with a similar specialty practice, which trained her to take on a bigger role, first running their outpatient clinic, then working on the floor, and later in the intensive care unit.
If nurse practitioners want to specialize, often “the doctors mentor them just like they would with a physician residency,” said Ms. Ograbisz, now vice president of clinical operations at temporary placement recruiter LocumTenens.com.
If physician assistants want to specialize, they also can do so through mentoring, or they can receive “certificates of added qualifications” in 10 specialties to demonstrate their expertise. Most employers don’t “encourage or require” these certificates, however, said Jennifer Orozco, DMSc, PA-C, DFAAPA, chief medical officer at the American Academy of Physician Associates.
There are a number of training programs for family nurse practitioners who want to develop skills in other areas.
Raina Hoebelheinrich, 40, a family nurse practitioner at a regional medical center in Yankton, South Dakota, recently enrolled in a three-semester post-master’s endocrinology training program at Mount Marty University. She lives on a farm in nearby northeastern Nebraska with her husband and five sons.
Ms. Hoebelheinrich’s new skills could be helpful in her current hospital job, in which she sees a lot of patients with acute diabetes, or in a clinic setting like the one in Sioux Falls, South Dakota, where she is doing her clinical endocrinology training.
Lack of access to endocrinology care in rural areas is a real problem, and many people may travel hundreds of miles to see a specialist.
“There aren’t a lot of options,” she said.
A version of this article first appeared on Medscape.com.
For many patients, seeing a nurse practitioner has become a routine part of primary care, in which these “NPs” often perform the same tasks that patients have relied on doctors for.
But NPs in specialty care? That’s not routine, at least not yet. Increasingly, though, nurse practitioners and physician assistants are joining cardiology, dermatology, and other specialty practices, broadening their skills and increasing their income.
This development worries some people who track the health workforce, because current trends suggest primary care, which has counted on nurse practitioners to backstop physician shortages, soon might not be able to rely on them to the same extent.
“They’re succumbing to the same challenges that we have with physicians,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute at the Association of American Medical Colleges. The rates NPs can command in a specialty practice “are quite a bit higher” than practice salaries in primary care, he said.
When nurse practitioner programs began to proliferate in the 1970s, “at first it looked great, producing all these nurse practitioners that go to work with primary care physicians,” said Yalda Jabbarpour, MD, director of the American Academy of Family Physicians’ Robert Graham Center for Policy Studies. “But now only 30% are going into primary care.”
Dr. Jabbarpour was referring to the 2024 primary care scorecard by the Milbank Memorial Fund, which found that from 2016 to 2021 the proportion of nurse practitioners who worked in primary care practices hovered between 32% and 34%, even though their numbers grew rapidly. The proportion of physician assistants, also known as physician associates, in primary care ranged from 27% to 30%, the study found.
Both nurse practitioners and physician assistants are advanced practice clinicians who, in addition to graduate degrees, must complete distinct education, training, and certification steps. NPs can practice without a doctor’s supervision in more than two dozen states, while PAs have similar independence in only a handful of states.
About 88% of nurse practitioners are certified in an area of primary care, according to the American Association of Nurse Practitioners. But it is difficult to track exactly how many work in primary care or in specialty practices. Unlike physicians, they’re generally not required to be endorsed by a national standard-setting body to practice in specialties like oncology or cardiology, for example. The AANP declined to answer questions about its annual workforce survey or the extent to which primary care NPs are moving toward specialties.
Though data tracking the change is sparse, specialty practices are adding these advanced practice clinicians at almost the same rate as primary care practices, according to frequently cited research published in 2018.
The clearest evidence of the shift: From 2008 to 2016, there was a 22% increase in the number of specialty practices that employed nurse practitioners and physician assistants, according to that study. The increase in the number of primary care practices that employed these professionals was 24%.
Once more, the most recent projections by the Association of American Medical Colleges predict a dearth of at least 20,200 primary care physicians by 2036. There will also be a shortfall of non-primary care specialists, including a deficiency of at least 10,100 surgical physicians and up to 25,000 physicians in other specialties.
When it comes to the actual work performed, the lines between primary and specialty care are often blurred, said Candice Chen, MD, MPH, associate professor of health policy and management at George Washington University.
“You might be a nurse practitioner working in a gastroenterology clinic or cardiology clinic, but the scope of what you do is starting to overlap with primary care,” she said.
Nurse practitioners’ salaries vary widely by location, type of facility, and experience. Still, according to data from health care recruiter AMN Healthcare Physician Solutions, formerly known as Merritt Hawkins, the total annual average starting compensation, including signing bonus, for nurse practitioners and physician assistants in specialty practice was $172,544 in the year that ended March 31, slightly higher than the $166,544 for those in primary care.
According to forecasts from the federal Bureau of Labor Statistics, nurse practitioner jobs will increase faster than jobs in almost any other occupation in the decade leading up to 2032, growing by 123,600 jobs or 45%. (Wind turbine service technician is the only other occupation projected to grow as fast.) The growth rate for physician assistants is also much faster than average, at 27%. There are more than twice as many nurse practitioners as physician assistants, however: 323,900 versus 148,000, in 2022.
To Dr. Grover of the AAMC, numbers like this signal that there will probably be enough NPs, PAs, and physicians to meet primary care needs. At the same time, “expect more NPs and PAs to also flow out into other specialties,” he said.
When Pamela Ograbisz started working as a registered nurse 27 years ago, she worked in a cardiothoracic intensive care unit. After she became a family nurse practitioner a few years later, she found a job with a similar specialty practice, which trained her to take on a bigger role, first running their outpatient clinic, then working on the floor, and later in the intensive care unit.
If nurse practitioners want to specialize, often “the doctors mentor them just like they would with a physician residency,” said Ms. Ograbisz, now vice president of clinical operations at temporary placement recruiter LocumTenens.com.
If physician assistants want to specialize, they also can do so through mentoring, or they can receive “certificates of added qualifications” in 10 specialties to demonstrate their expertise. Most employers don’t “encourage or require” these certificates, however, said Jennifer Orozco, DMSc, PA-C, DFAAPA, chief medical officer at the American Academy of Physician Associates.
There are a number of training programs for family nurse practitioners who want to develop skills in other areas.
Raina Hoebelheinrich, 40, a family nurse practitioner at a regional medical center in Yankton, South Dakota, recently enrolled in a three-semester post-master’s endocrinology training program at Mount Marty University. She lives on a farm in nearby northeastern Nebraska with her husband and five sons.
Ms. Hoebelheinrich’s new skills could be helpful in her current hospital job, in which she sees a lot of patients with acute diabetes, or in a clinic setting like the one in Sioux Falls, South Dakota, where she is doing her clinical endocrinology training.
Lack of access to endocrinology care in rural areas is a real problem, and many people may travel hundreds of miles to see a specialist.
“There aren’t a lot of options,” she said.
A version of this article first appeared on Medscape.com.
Statin Use May Extend Life for Early Breast Cancer Patients
Previous research examining the association between cholesterol and breast cancer metabolism suggests that cholesterol-lowering medications such as statins may improve outcomes in breast cancer patients, Sixten Harborg, a medical student and PhD student at Aarhus University, Denmark, said in a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
In addition, cardiovascular-related death is the second most common cause of death for breast cancer survivors, and given the survival rates in early breast cancer, there is a demand for cardioprotective initiatives and maintenance of cardioprotective drugs after diagnosis, he said in an interview.
What Is Known About Statins and Breast Cancer?
Statins are the most common drugs used to lower cholesterol and may deprive tumor cells of the cholesterol needed for cell membrane synthesis, Mr. Harborg said in his presentation.
Data from a randomized trial published in the Journal of Clinical Oncology in 2017 showed significantly improved disease-free survival, breast cancer–free interval, and distant recurrence–free interval in early stage breast cancer patients randomized to cholesterol-lowering medication vs. those who did not receive cholesterol-lowering medication.
The 2017 study prompted the creation of the MASTER study, a randomized, multicenter, double-blind, placebo-controlled trial comparing standard adjuvant therapy plus placebo to standard adjuvant therapy plus atorvastatin in patients with early breast cancer (NCT04601116), Mr. Harborg said. The MASTER trial is currently recruiting patients in Denmark.
How Was the Current Study Designed?
To provide preliminary analysis, Mr. Harborg and colleagues used an emulation trial design based on electronic health care data from 110,160 females with a diagnosis of stage I, II, or III breast cancer who were part of the Danish Breast Cancer Group, a national clinical registry in Denmark, between 2000 and 2020.
As defined in the European Journal of Epidemiology in 2017, target trial emulation involves application of randomized trial designs to observational data with the goal of improving the quality of observational epidemiology when a comparator trial is not yet available.
The researchers created a cohort of patients based on electronic health care data to simulate a target trial of the use of atorvastatin after breast cancer diagnosis. Patients were randomized to one of two treatment strategies: starting to use statins within 36 months of diagnosis, or not using statins. The primary outcome was death from breast cancer. The follow-up for the MASTER study starts with inclusion and ends with death, emigration from Denmark, end of clinical follow-up, or 10 years of follow-up (whichever comes first); the follow-up was the same in the current study.
The researchers calculated hazard ratios (HR) of breast cancer mortality in statin users vs. non–statin users and used a technique known as inverse-probability of censoring-weighting (IPCW) to estimate the effects of statin use based on prognostic factors.
What Did the Results Show?
The results favored statin use for improved survival in early breast cancer patients, Mr. Harborg said. Overall, the hazard ratio for breast cancer mortality was 0.96 in statin users compared with non–statin users, and was similar in both a Cox regression analysis (HR 0.81), and in a 10-year landmark analysis (HR 0.86).
The difference in mortality between statin and non–statin users was even stronger in patients who were receiving adjuvant chemotherapy (HR 0.94, 0.64, and 0.76 on the IPCW, Cox, and landmark analyses, respectively).
The results were in line with previous reports of statins’ effect on breast cancer survival, Mr. Harborg said in an interview.
“We believe the results encourage the continuous effort of the currently enrolling MASTER trial,” he said.
The results also suggest that deprescribing statins at the time of breast cancer diagnosis is not recommended, and that statin treatment can safely be prescribed to breast cancer patients with increased cardiovascular disease risk and/or dyslipidemia, Mr. Harborg said in the interview.
What Is the Takeaway Message for Clinical Practice?
“The clinical takeaway from our study is that statin use is associated with reduced risk of dying from breast cancer, but that it is not possible to determine the true effect of statins on breast cancer survival without a randomized, placebo-controlled trial,” Mr. Harborg told this publication. “Statins are inexpensive and well-tolerated drugs and may have a beneficial effect in terms of survival for breast cancer patients. However, with the current level of evidence [because the MASTER study is ongoing], we still cannot recommend that oncologists prescribe statins to prevent mortality from breast cancer,” he said.
What Are the Next Steps for Research?
The findings were limited by the study design, and real-world data are needed, Dr. Harborg said. Other limitations include the presence of residual bias, and the use of data based on prescription codes, but these were not considered to have an effect on the main conclusion of the study, Mr. Harborg said in the interview.
However, the results suggest that the addition of statins may improve outcomes for early breast cancer patients, especially when used with chemotherapy, and support the value of the ongoing MASTER study, he concluded.
Ultimately, the MASTER study will provide a more definitive answer to the question of whether statins should be added to the adjuvant treatment regimen of breast cancer to improve breast cancer outcomes, he said.
What Do Clinicians Think of the Study?
The current study is timely and highlights the need for phase 3 trials to examine the potential of statin use for breast cancer outcomes, Malinda T. West, MD, a medical oncologist and breast oncologist at the University of Wisconsin Carbone Cancer Center, Madison, said in an interview.
Questions for future research include whether statins can be used in combination with adjuvant abemaciclib if indicated, or how to best sequence these agents, said Dr. West, who was not involved in the study. Other questions raised by the current study include whether other cholesterol-lowering agents have a potential adjuvant benefit in reducing breast cancer recurrent and/or mortality, and whether the addition of statins would benefit subgroups such as HER2+ and triple negative breast cancer, she said.
“I was not surprised to see another study reporting benefit with statins and reduced risk of breast cancer recurrence and/or mortality, but I think the larger question is defining the subgroups who benefit the most, and identifying predictors for benefit or resistance,” Dr. West said in an interview.
Previous studies have shown that cholesterol elevation, specifically LDL levels, can be linked to increased tumor growth in breast cancer, so the lower mortality risk associated with lipid-lowering therapies in the current study was consistent, Peyton L. Reves, MD, a hematology/oncology fellow, also at the University of Wisconsin, said in an interview. In practice, data from the current study and previous research could be especially useful for patients with elevated LDL levels, said Dr. Reves, who was not involved in the study.
“These results could impact clinical practice in many ways, including leading to routine cholesterol monitoring in breast cancer patients on adjuvant therapy as well as the addition of lipid-lowering therapy with statins in these patients,” Dr. Reves said.
The findings showing particular benefit for patients on adjuvant chemotherapy highlight the need for more research on this specific population and the effect of statins on overall breast cancer mortality, to explore the extent to which the results of the current study were driven by the benefit seen in patients receiving adjuvant chemotherapy, Dr. Reves said.
The study was supported by Director Michael Hermann Nielsen’s Memorial Grant, Manufacturer Einar Willumsen’s Memorial Grant, Astrid Thaysen’s Grant for Medical Basic Research, Eva and Henry Fraenkel’s Memorial Fund, and the Novo Nordisk Foundation.
The researchers had no financial conflicts to disclose. Dr. West and Dr. Reves had no financial conflicts to disclose.
Previous research examining the association between cholesterol and breast cancer metabolism suggests that cholesterol-lowering medications such as statins may improve outcomes in breast cancer patients, Sixten Harborg, a medical student and PhD student at Aarhus University, Denmark, said in a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
In addition, cardiovascular-related death is the second most common cause of death for breast cancer survivors, and given the survival rates in early breast cancer, there is a demand for cardioprotective initiatives and maintenance of cardioprotective drugs after diagnosis, he said in an interview.
What Is Known About Statins and Breast Cancer?
Statins are the most common drugs used to lower cholesterol and may deprive tumor cells of the cholesterol needed for cell membrane synthesis, Mr. Harborg said in his presentation.
Data from a randomized trial published in the Journal of Clinical Oncology in 2017 showed significantly improved disease-free survival, breast cancer–free interval, and distant recurrence–free interval in early stage breast cancer patients randomized to cholesterol-lowering medication vs. those who did not receive cholesterol-lowering medication.
The 2017 study prompted the creation of the MASTER study, a randomized, multicenter, double-blind, placebo-controlled trial comparing standard adjuvant therapy plus placebo to standard adjuvant therapy plus atorvastatin in patients with early breast cancer (NCT04601116), Mr. Harborg said. The MASTER trial is currently recruiting patients in Denmark.
How Was the Current Study Designed?
To provide preliminary analysis, Mr. Harborg and colleagues used an emulation trial design based on electronic health care data from 110,160 females with a diagnosis of stage I, II, or III breast cancer who were part of the Danish Breast Cancer Group, a national clinical registry in Denmark, between 2000 and 2020.
As defined in the European Journal of Epidemiology in 2017, target trial emulation involves application of randomized trial designs to observational data with the goal of improving the quality of observational epidemiology when a comparator trial is not yet available.
The researchers created a cohort of patients based on electronic health care data to simulate a target trial of the use of atorvastatin after breast cancer diagnosis. Patients were randomized to one of two treatment strategies: starting to use statins within 36 months of diagnosis, or not using statins. The primary outcome was death from breast cancer. The follow-up for the MASTER study starts with inclusion and ends with death, emigration from Denmark, end of clinical follow-up, or 10 years of follow-up (whichever comes first); the follow-up was the same in the current study.
The researchers calculated hazard ratios (HR) of breast cancer mortality in statin users vs. non–statin users and used a technique known as inverse-probability of censoring-weighting (IPCW) to estimate the effects of statin use based on prognostic factors.
What Did the Results Show?
The results favored statin use for improved survival in early breast cancer patients, Mr. Harborg said. Overall, the hazard ratio for breast cancer mortality was 0.96 in statin users compared with non–statin users, and was similar in both a Cox regression analysis (HR 0.81), and in a 10-year landmark analysis (HR 0.86).
The difference in mortality between statin and non–statin users was even stronger in patients who were receiving adjuvant chemotherapy (HR 0.94, 0.64, and 0.76 on the IPCW, Cox, and landmark analyses, respectively).
The results were in line with previous reports of statins’ effect on breast cancer survival, Mr. Harborg said in an interview.
“We believe the results encourage the continuous effort of the currently enrolling MASTER trial,” he said.
The results also suggest that deprescribing statins at the time of breast cancer diagnosis is not recommended, and that statin treatment can safely be prescribed to breast cancer patients with increased cardiovascular disease risk and/or dyslipidemia, Mr. Harborg said in the interview.
What Is the Takeaway Message for Clinical Practice?
“The clinical takeaway from our study is that statin use is associated with reduced risk of dying from breast cancer, but that it is not possible to determine the true effect of statins on breast cancer survival without a randomized, placebo-controlled trial,” Mr. Harborg told this publication. “Statins are inexpensive and well-tolerated drugs and may have a beneficial effect in terms of survival for breast cancer patients. However, with the current level of evidence [because the MASTER study is ongoing], we still cannot recommend that oncologists prescribe statins to prevent mortality from breast cancer,” he said.
What Are the Next Steps for Research?
The findings were limited by the study design, and real-world data are needed, Dr. Harborg said. Other limitations include the presence of residual bias, and the use of data based on prescription codes, but these were not considered to have an effect on the main conclusion of the study, Mr. Harborg said in the interview.
However, the results suggest that the addition of statins may improve outcomes for early breast cancer patients, especially when used with chemotherapy, and support the value of the ongoing MASTER study, he concluded.
Ultimately, the MASTER study will provide a more definitive answer to the question of whether statins should be added to the adjuvant treatment regimen of breast cancer to improve breast cancer outcomes, he said.
What Do Clinicians Think of the Study?
The current study is timely and highlights the need for phase 3 trials to examine the potential of statin use for breast cancer outcomes, Malinda T. West, MD, a medical oncologist and breast oncologist at the University of Wisconsin Carbone Cancer Center, Madison, said in an interview.
Questions for future research include whether statins can be used in combination with adjuvant abemaciclib if indicated, or how to best sequence these agents, said Dr. West, who was not involved in the study. Other questions raised by the current study include whether other cholesterol-lowering agents have a potential adjuvant benefit in reducing breast cancer recurrent and/or mortality, and whether the addition of statins would benefit subgroups such as HER2+ and triple negative breast cancer, she said.
“I was not surprised to see another study reporting benefit with statins and reduced risk of breast cancer recurrence and/or mortality, but I think the larger question is defining the subgroups who benefit the most, and identifying predictors for benefit or resistance,” Dr. West said in an interview.
Previous studies have shown that cholesterol elevation, specifically LDL levels, can be linked to increased tumor growth in breast cancer, so the lower mortality risk associated with lipid-lowering therapies in the current study was consistent, Peyton L. Reves, MD, a hematology/oncology fellow, also at the University of Wisconsin, said in an interview. In practice, data from the current study and previous research could be especially useful for patients with elevated LDL levels, said Dr. Reves, who was not involved in the study.
“These results could impact clinical practice in many ways, including leading to routine cholesterol monitoring in breast cancer patients on adjuvant therapy as well as the addition of lipid-lowering therapy with statins in these patients,” Dr. Reves said.
The findings showing particular benefit for patients on adjuvant chemotherapy highlight the need for more research on this specific population and the effect of statins on overall breast cancer mortality, to explore the extent to which the results of the current study were driven by the benefit seen in patients receiving adjuvant chemotherapy, Dr. Reves said.
The study was supported by Director Michael Hermann Nielsen’s Memorial Grant, Manufacturer Einar Willumsen’s Memorial Grant, Astrid Thaysen’s Grant for Medical Basic Research, Eva and Henry Fraenkel’s Memorial Fund, and the Novo Nordisk Foundation.
The researchers had no financial conflicts to disclose. Dr. West and Dr. Reves had no financial conflicts to disclose.
Previous research examining the association between cholesterol and breast cancer metabolism suggests that cholesterol-lowering medications such as statins may improve outcomes in breast cancer patients, Sixten Harborg, a medical student and PhD student at Aarhus University, Denmark, said in a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
In addition, cardiovascular-related death is the second most common cause of death for breast cancer survivors, and given the survival rates in early breast cancer, there is a demand for cardioprotective initiatives and maintenance of cardioprotective drugs after diagnosis, he said in an interview.
What Is Known About Statins and Breast Cancer?
Statins are the most common drugs used to lower cholesterol and may deprive tumor cells of the cholesterol needed for cell membrane synthesis, Mr. Harborg said in his presentation.
Data from a randomized trial published in the Journal of Clinical Oncology in 2017 showed significantly improved disease-free survival, breast cancer–free interval, and distant recurrence–free interval in early stage breast cancer patients randomized to cholesterol-lowering medication vs. those who did not receive cholesterol-lowering medication.
The 2017 study prompted the creation of the MASTER study, a randomized, multicenter, double-blind, placebo-controlled trial comparing standard adjuvant therapy plus placebo to standard adjuvant therapy plus atorvastatin in patients with early breast cancer (NCT04601116), Mr. Harborg said. The MASTER trial is currently recruiting patients in Denmark.
How Was the Current Study Designed?
To provide preliminary analysis, Mr. Harborg and colleagues used an emulation trial design based on electronic health care data from 110,160 females with a diagnosis of stage I, II, or III breast cancer who were part of the Danish Breast Cancer Group, a national clinical registry in Denmark, between 2000 and 2020.
As defined in the European Journal of Epidemiology in 2017, target trial emulation involves application of randomized trial designs to observational data with the goal of improving the quality of observational epidemiology when a comparator trial is not yet available.
The researchers created a cohort of patients based on electronic health care data to simulate a target trial of the use of atorvastatin after breast cancer diagnosis. Patients were randomized to one of two treatment strategies: starting to use statins within 36 months of diagnosis, or not using statins. The primary outcome was death from breast cancer. The follow-up for the MASTER study starts with inclusion and ends with death, emigration from Denmark, end of clinical follow-up, or 10 years of follow-up (whichever comes first); the follow-up was the same in the current study.
The researchers calculated hazard ratios (HR) of breast cancer mortality in statin users vs. non–statin users and used a technique known as inverse-probability of censoring-weighting (IPCW) to estimate the effects of statin use based on prognostic factors.
What Did the Results Show?
The results favored statin use for improved survival in early breast cancer patients, Mr. Harborg said. Overall, the hazard ratio for breast cancer mortality was 0.96 in statin users compared with non–statin users, and was similar in both a Cox regression analysis (HR 0.81), and in a 10-year landmark analysis (HR 0.86).
The difference in mortality between statin and non–statin users was even stronger in patients who were receiving adjuvant chemotherapy (HR 0.94, 0.64, and 0.76 on the IPCW, Cox, and landmark analyses, respectively).
The results were in line with previous reports of statins’ effect on breast cancer survival, Mr. Harborg said in an interview.
“We believe the results encourage the continuous effort of the currently enrolling MASTER trial,” he said.
The results also suggest that deprescribing statins at the time of breast cancer diagnosis is not recommended, and that statin treatment can safely be prescribed to breast cancer patients with increased cardiovascular disease risk and/or dyslipidemia, Mr. Harborg said in the interview.
What Is the Takeaway Message for Clinical Practice?
“The clinical takeaway from our study is that statin use is associated with reduced risk of dying from breast cancer, but that it is not possible to determine the true effect of statins on breast cancer survival without a randomized, placebo-controlled trial,” Mr. Harborg told this publication. “Statins are inexpensive and well-tolerated drugs and may have a beneficial effect in terms of survival for breast cancer patients. However, with the current level of evidence [because the MASTER study is ongoing], we still cannot recommend that oncologists prescribe statins to prevent mortality from breast cancer,” he said.
What Are the Next Steps for Research?
The findings were limited by the study design, and real-world data are needed, Dr. Harborg said. Other limitations include the presence of residual bias, and the use of data based on prescription codes, but these were not considered to have an effect on the main conclusion of the study, Mr. Harborg said in the interview.
However, the results suggest that the addition of statins may improve outcomes for early breast cancer patients, especially when used with chemotherapy, and support the value of the ongoing MASTER study, he concluded.
Ultimately, the MASTER study will provide a more definitive answer to the question of whether statins should be added to the adjuvant treatment regimen of breast cancer to improve breast cancer outcomes, he said.
What Do Clinicians Think of the Study?
The current study is timely and highlights the need for phase 3 trials to examine the potential of statin use for breast cancer outcomes, Malinda T. West, MD, a medical oncologist and breast oncologist at the University of Wisconsin Carbone Cancer Center, Madison, said in an interview.
Questions for future research include whether statins can be used in combination with adjuvant abemaciclib if indicated, or how to best sequence these agents, said Dr. West, who was not involved in the study. Other questions raised by the current study include whether other cholesterol-lowering agents have a potential adjuvant benefit in reducing breast cancer recurrent and/or mortality, and whether the addition of statins would benefit subgroups such as HER2+ and triple negative breast cancer, she said.
“I was not surprised to see another study reporting benefit with statins and reduced risk of breast cancer recurrence and/or mortality, but I think the larger question is defining the subgroups who benefit the most, and identifying predictors for benefit or resistance,” Dr. West said in an interview.
Previous studies have shown that cholesterol elevation, specifically LDL levels, can be linked to increased tumor growth in breast cancer, so the lower mortality risk associated with lipid-lowering therapies in the current study was consistent, Peyton L. Reves, MD, a hematology/oncology fellow, also at the University of Wisconsin, said in an interview. In practice, data from the current study and previous research could be especially useful for patients with elevated LDL levels, said Dr. Reves, who was not involved in the study.
“These results could impact clinical practice in many ways, including leading to routine cholesterol monitoring in breast cancer patients on adjuvant therapy as well as the addition of lipid-lowering therapy with statins in these patients,” Dr. Reves said.
The findings showing particular benefit for patients on adjuvant chemotherapy highlight the need for more research on this specific population and the effect of statins on overall breast cancer mortality, to explore the extent to which the results of the current study were driven by the benefit seen in patients receiving adjuvant chemotherapy, Dr. Reves said.
The study was supported by Director Michael Hermann Nielsen’s Memorial Grant, Manufacturer Einar Willumsen’s Memorial Grant, Astrid Thaysen’s Grant for Medical Basic Research, Eva and Henry Fraenkel’s Memorial Fund, and the Novo Nordisk Foundation.
The researchers had no financial conflicts to disclose. Dr. West and Dr. Reves had no financial conflicts to disclose.
FROM ESMO BREAST CANCER 2024
Follow-Up Outcomes Data Often Missing for FDA Drug Approvals Based on Surrogate Markers
Over the past few decades, the US Food and Drug Administration (FDA) has increasingly relied on surrogate measures such as blood tests instead of clinical outcomes for medication approvals. But critics say the agency lacks consistent standards to ensure the surrogate aligns with clinical outcomes that matter to patients — things like improvements in symptoms and gains in function.
Sometimes those decisions backfire. Consider: In July 2021, the FDA approved aducanumab for the treatment of Alzheimer’s disease, bucking the advice of an advisory panel for the agency that questioned the effectiveness of the medication. Regulators relied on data from the drugmaker, Biogen, showing the monoclonal antibody could reduce levels of amyloid beta plaques in blood — a surrogate marker officials hoped would translate to clinical benefit.
The FDA’s decision triggered significant controversy, and Biogen in January announced it is pulling it from the market this year, citing disappointing sales.
Although the case of aducanumab might seem extreme, given the stakes — Alzheimer’s remains a disease without an effective treatment — it’s far from unusual.
“When we prescribe a drug, there is an underlying assumption that the FDA has done its due diligence to confirm the drug is safe and of benefit,” said Reshma Ramachandran, MD, MPP, MHS, a researcher at Yale School of Medicine, New Haven, Connecticut, and a coauthor of a recent review of surrogate outcomes. “In fact, we found either no evidence or low-quality evidence.” Such markers are associated with clinical outcomes. “We just don’t know if they work meaningfully to treat the patient’s condition. The results were pretty shocking for us,” she said.
The FDA in 2018 released an Adult Surrogate Endpoint Table listing markers that can be used as substitutes for clinical outcomes to more quickly test, review, and approve new therapies. The analysis found the majority of these endpoints lacked subsequent confirmations, defined as published meta-analyses of clinical studies to validate the association between the marker and a clinical outcome important to patients.
In a paper published in JAMA, Dr. Ramachandran and her colleagues looked at 37 surrogate endpoints for nearly 3 dozen nononcologic diseases in the table.
Approval with surrogate markers implies responsibility for postapproval or validation studies — not just lab measures or imaging findings but mortality, morbidity, or improved quality of life, said Joshua D. Wallach, PhD, MS, assistant professor in the department of epidemiology at the Emory Rollins School of Public Health in Atlanta and lead author of the JAMA review.
Dr. Wallach said surrogate markers are easier to measure and do not require large and long trials. But the FDA has not provided clear rules for what makes a surrogate marker valid in clinical trials.
“They’ve said that at a minimum, it requires meta-analytical evidence from studies that have looked at the correlation or the association between the surrogate and the clinical outcome,” Dr. Wallach said. “Our understanding was that if that’s a minimum expectation, we should be able to find those studies in the literature. And the reality is that we were unable to find evidence from those types of studies supporting the association between the surrogate and the clinical outcome.”
Physicians generally do not receive training about the FDA approval process and the difference between biomarkers, surrogate markers, and clinical endpoints, Dr. Ramachandran said. “Our study shows that things are much more uncertain than we thought when it comes to the prescribing of new drugs,” she said.
Surrogate Markers on the Rise
Dr. Wallach’s group looked for published meta-analyses compiling randomized controlled trials reporting surrogate endpoints for more than 3 dozen chronic nononcologic conditions, including type 2 diabetes, Alzheimer’s, kidney disease, HIV, gout, and lupus. They found no meta-analyses at all for 59% of the surrogate markers, while for those that were studied, few reported high-strength evidence of an association with clinical outcomes.
The findings echo previous research. In a 2020 study in JAMA Network Open, researchers tallied primary endpoints for all FDA approvals of new drugs and therapies during three 3-year periods: 1995-1997, 2005-2007, and 2015-2017. The proportion of products whose approvals were based on the use of clinical endpoints decreased from 43.8% in 1995-1997 to 28.4% in 2005-2007 to 23.3% in 2015-2017. The share based on surrogate endpoints rose from 43.3% to roughly 60% over the same interval.
A 2017 study in the Journal of Health Economics found the use of “imperfect” surrogate endpoints helped support the approval of an average of 16 new drugs per year between 2010 and 2014 compared with six per year from 1998 to 2008.
Similar concerns about weak associations between surrogate markers and drugs used to treat cancer have been documented before, including in a 2020 study published in eClinicalMedicine. The researchers found the surrogate endpoints in the FDA table either were not tested or were tested but proven to be weak surrogates.
“And yet the FDA considered these as good enough not only for accelerated approval but also for regular approval,” said Bishal Gyawali, MD, PhD, associate professor in the department of oncology at Queen’s University, Kingston, Ontario, Canada, who led the group.
The use of surrogate endpoints is also increasing in Europe, said Huseyin Naci, MHS, PhD, associate professor of health policy at the London School of Economics and Political Science in England. He cited a cohort study of 298 randomized clinical trials (RCTs) in JAMA Oncology suggesting “contemporary oncology RCTs now largely measure putative surrogate endpoints.” Dr. Wallach called the FDA’s surrogate table “a great first step toward transparency. But a key column is missing from that table, telling us what is the basis for which the FDA allows drug companies to use the recognized surrogate markers. What is the evidence they are considering?”
If the agency allows companies the flexibility to validate surrogate endpoints, postmarketing studies designed to confirm the clinical utility of those endpoints should follow.
“We obviously want physicians to be guided by evidence when they’re selecting treatments, and they need to be able to interpret the clinical benefits of the drug that they’re prescribing,” he said. “This is really about having the research consumer, patients, and physicians, as well as industry, understand why certain markers are considered and not considered.”
Dr. Wallach reported receiving grants from the FDA (through the Yale University — Mayo Clinic Center of Excellence in Regulatory Science and Innovation), National Institute on Alcohol Abuse and Alcoholism (1K01AA028258), and Johnson & Johnson (through the Yale University Open Data Access Project); and consulting fees from Hagens Berman Sobol Shapiro LLP and Dugan Law Firm APLC outside the submitted work. Dr. Ramachandran reported receiving grants from the Stavros Niarchos Foundation and FDA; receiving consulting fees from ReAct Action on Antibiotic Resistance strategy policy program outside the submitted work; and serving in an unpaid capacity as chair of the FDA task force for the nonprofit organization Doctors for America and in an unpaid capacity as board president for Universities Allied for Essential Medicines North America.
A version of this article appeared on Medscape.com.
Over the past few decades, the US Food and Drug Administration (FDA) has increasingly relied on surrogate measures such as blood tests instead of clinical outcomes for medication approvals. But critics say the agency lacks consistent standards to ensure the surrogate aligns with clinical outcomes that matter to patients — things like improvements in symptoms and gains in function.
Sometimes those decisions backfire. Consider: In July 2021, the FDA approved aducanumab for the treatment of Alzheimer’s disease, bucking the advice of an advisory panel for the agency that questioned the effectiveness of the medication. Regulators relied on data from the drugmaker, Biogen, showing the monoclonal antibody could reduce levels of amyloid beta plaques in blood — a surrogate marker officials hoped would translate to clinical benefit.
The FDA’s decision triggered significant controversy, and Biogen in January announced it is pulling it from the market this year, citing disappointing sales.
Although the case of aducanumab might seem extreme, given the stakes — Alzheimer’s remains a disease without an effective treatment — it’s far from unusual.
“When we prescribe a drug, there is an underlying assumption that the FDA has done its due diligence to confirm the drug is safe and of benefit,” said Reshma Ramachandran, MD, MPP, MHS, a researcher at Yale School of Medicine, New Haven, Connecticut, and a coauthor of a recent review of surrogate outcomes. “In fact, we found either no evidence or low-quality evidence.” Such markers are associated with clinical outcomes. “We just don’t know if they work meaningfully to treat the patient’s condition. The results were pretty shocking for us,” she said.
The FDA in 2018 released an Adult Surrogate Endpoint Table listing markers that can be used as substitutes for clinical outcomes to more quickly test, review, and approve new therapies. The analysis found the majority of these endpoints lacked subsequent confirmations, defined as published meta-analyses of clinical studies to validate the association between the marker and a clinical outcome important to patients.
In a paper published in JAMA, Dr. Ramachandran and her colleagues looked at 37 surrogate endpoints for nearly 3 dozen nononcologic diseases in the table.
Approval with surrogate markers implies responsibility for postapproval or validation studies — not just lab measures or imaging findings but mortality, morbidity, or improved quality of life, said Joshua D. Wallach, PhD, MS, assistant professor in the department of epidemiology at the Emory Rollins School of Public Health in Atlanta and lead author of the JAMA review.
Dr. Wallach said surrogate markers are easier to measure and do not require large and long trials. But the FDA has not provided clear rules for what makes a surrogate marker valid in clinical trials.
“They’ve said that at a minimum, it requires meta-analytical evidence from studies that have looked at the correlation or the association between the surrogate and the clinical outcome,” Dr. Wallach said. “Our understanding was that if that’s a minimum expectation, we should be able to find those studies in the literature. And the reality is that we were unable to find evidence from those types of studies supporting the association between the surrogate and the clinical outcome.”
Physicians generally do not receive training about the FDA approval process and the difference between biomarkers, surrogate markers, and clinical endpoints, Dr. Ramachandran said. “Our study shows that things are much more uncertain than we thought when it comes to the prescribing of new drugs,” she said.
Surrogate Markers on the Rise
Dr. Wallach’s group looked for published meta-analyses compiling randomized controlled trials reporting surrogate endpoints for more than 3 dozen chronic nononcologic conditions, including type 2 diabetes, Alzheimer’s, kidney disease, HIV, gout, and lupus. They found no meta-analyses at all for 59% of the surrogate markers, while for those that were studied, few reported high-strength evidence of an association with clinical outcomes.
The findings echo previous research. In a 2020 study in JAMA Network Open, researchers tallied primary endpoints for all FDA approvals of new drugs and therapies during three 3-year periods: 1995-1997, 2005-2007, and 2015-2017. The proportion of products whose approvals were based on the use of clinical endpoints decreased from 43.8% in 1995-1997 to 28.4% in 2005-2007 to 23.3% in 2015-2017. The share based on surrogate endpoints rose from 43.3% to roughly 60% over the same interval.
A 2017 study in the Journal of Health Economics found the use of “imperfect” surrogate endpoints helped support the approval of an average of 16 new drugs per year between 2010 and 2014 compared with six per year from 1998 to 2008.
Similar concerns about weak associations between surrogate markers and drugs used to treat cancer have been documented before, including in a 2020 study published in eClinicalMedicine. The researchers found the surrogate endpoints in the FDA table either were not tested or were tested but proven to be weak surrogates.
“And yet the FDA considered these as good enough not only for accelerated approval but also for regular approval,” said Bishal Gyawali, MD, PhD, associate professor in the department of oncology at Queen’s University, Kingston, Ontario, Canada, who led the group.
The use of surrogate endpoints is also increasing in Europe, said Huseyin Naci, MHS, PhD, associate professor of health policy at the London School of Economics and Political Science in England. He cited a cohort study of 298 randomized clinical trials (RCTs) in JAMA Oncology suggesting “contemporary oncology RCTs now largely measure putative surrogate endpoints.” Dr. Wallach called the FDA’s surrogate table “a great first step toward transparency. But a key column is missing from that table, telling us what is the basis for which the FDA allows drug companies to use the recognized surrogate markers. What is the evidence they are considering?”
If the agency allows companies the flexibility to validate surrogate endpoints, postmarketing studies designed to confirm the clinical utility of those endpoints should follow.
“We obviously want physicians to be guided by evidence when they’re selecting treatments, and they need to be able to interpret the clinical benefits of the drug that they’re prescribing,” he said. “This is really about having the research consumer, patients, and physicians, as well as industry, understand why certain markers are considered and not considered.”
Dr. Wallach reported receiving grants from the FDA (through the Yale University — Mayo Clinic Center of Excellence in Regulatory Science and Innovation), National Institute on Alcohol Abuse and Alcoholism (1K01AA028258), and Johnson & Johnson (through the Yale University Open Data Access Project); and consulting fees from Hagens Berman Sobol Shapiro LLP and Dugan Law Firm APLC outside the submitted work. Dr. Ramachandran reported receiving grants from the Stavros Niarchos Foundation and FDA; receiving consulting fees from ReAct Action on Antibiotic Resistance strategy policy program outside the submitted work; and serving in an unpaid capacity as chair of the FDA task force for the nonprofit organization Doctors for America and in an unpaid capacity as board president for Universities Allied for Essential Medicines North America.
A version of this article appeared on Medscape.com.
Over the past few decades, the US Food and Drug Administration (FDA) has increasingly relied on surrogate measures such as blood tests instead of clinical outcomes for medication approvals. But critics say the agency lacks consistent standards to ensure the surrogate aligns with clinical outcomes that matter to patients — things like improvements in symptoms and gains in function.
Sometimes those decisions backfire. Consider: In July 2021, the FDA approved aducanumab for the treatment of Alzheimer’s disease, bucking the advice of an advisory panel for the agency that questioned the effectiveness of the medication. Regulators relied on data from the drugmaker, Biogen, showing the monoclonal antibody could reduce levels of amyloid beta plaques in blood — a surrogate marker officials hoped would translate to clinical benefit.
The FDA’s decision triggered significant controversy, and Biogen in January announced it is pulling it from the market this year, citing disappointing sales.
Although the case of aducanumab might seem extreme, given the stakes — Alzheimer’s remains a disease without an effective treatment — it’s far from unusual.
“When we prescribe a drug, there is an underlying assumption that the FDA has done its due diligence to confirm the drug is safe and of benefit,” said Reshma Ramachandran, MD, MPP, MHS, a researcher at Yale School of Medicine, New Haven, Connecticut, and a coauthor of a recent review of surrogate outcomes. “In fact, we found either no evidence or low-quality evidence.” Such markers are associated with clinical outcomes. “We just don’t know if they work meaningfully to treat the patient’s condition. The results were pretty shocking for us,” she said.
The FDA in 2018 released an Adult Surrogate Endpoint Table listing markers that can be used as substitutes for clinical outcomes to more quickly test, review, and approve new therapies. The analysis found the majority of these endpoints lacked subsequent confirmations, defined as published meta-analyses of clinical studies to validate the association between the marker and a clinical outcome important to patients.
In a paper published in JAMA, Dr. Ramachandran and her colleagues looked at 37 surrogate endpoints for nearly 3 dozen nononcologic diseases in the table.
Approval with surrogate markers implies responsibility for postapproval or validation studies — not just lab measures or imaging findings but mortality, morbidity, or improved quality of life, said Joshua D. Wallach, PhD, MS, assistant professor in the department of epidemiology at the Emory Rollins School of Public Health in Atlanta and lead author of the JAMA review.
Dr. Wallach said surrogate markers are easier to measure and do not require large and long trials. But the FDA has not provided clear rules for what makes a surrogate marker valid in clinical trials.
“They’ve said that at a minimum, it requires meta-analytical evidence from studies that have looked at the correlation or the association between the surrogate and the clinical outcome,” Dr. Wallach said. “Our understanding was that if that’s a minimum expectation, we should be able to find those studies in the literature. And the reality is that we were unable to find evidence from those types of studies supporting the association between the surrogate and the clinical outcome.”
Physicians generally do not receive training about the FDA approval process and the difference between biomarkers, surrogate markers, and clinical endpoints, Dr. Ramachandran said. “Our study shows that things are much more uncertain than we thought when it comes to the prescribing of new drugs,” she said.
Surrogate Markers on the Rise
Dr. Wallach’s group looked for published meta-analyses compiling randomized controlled trials reporting surrogate endpoints for more than 3 dozen chronic nononcologic conditions, including type 2 diabetes, Alzheimer’s, kidney disease, HIV, gout, and lupus. They found no meta-analyses at all for 59% of the surrogate markers, while for those that were studied, few reported high-strength evidence of an association with clinical outcomes.
The findings echo previous research. In a 2020 study in JAMA Network Open, researchers tallied primary endpoints for all FDA approvals of new drugs and therapies during three 3-year periods: 1995-1997, 2005-2007, and 2015-2017. The proportion of products whose approvals were based on the use of clinical endpoints decreased from 43.8% in 1995-1997 to 28.4% in 2005-2007 to 23.3% in 2015-2017. The share based on surrogate endpoints rose from 43.3% to roughly 60% over the same interval.
A 2017 study in the Journal of Health Economics found the use of “imperfect” surrogate endpoints helped support the approval of an average of 16 new drugs per year between 2010 and 2014 compared with six per year from 1998 to 2008.
Similar concerns about weak associations between surrogate markers and drugs used to treat cancer have been documented before, including in a 2020 study published in eClinicalMedicine. The researchers found the surrogate endpoints in the FDA table either were not tested or were tested but proven to be weak surrogates.
“And yet the FDA considered these as good enough not only for accelerated approval but also for regular approval,” said Bishal Gyawali, MD, PhD, associate professor in the department of oncology at Queen’s University, Kingston, Ontario, Canada, who led the group.
The use of surrogate endpoints is also increasing in Europe, said Huseyin Naci, MHS, PhD, associate professor of health policy at the London School of Economics and Political Science in England. He cited a cohort study of 298 randomized clinical trials (RCTs) in JAMA Oncology suggesting “contemporary oncology RCTs now largely measure putative surrogate endpoints.” Dr. Wallach called the FDA’s surrogate table “a great first step toward transparency. But a key column is missing from that table, telling us what is the basis for which the FDA allows drug companies to use the recognized surrogate markers. What is the evidence they are considering?”
If the agency allows companies the flexibility to validate surrogate endpoints, postmarketing studies designed to confirm the clinical utility of those endpoints should follow.
“We obviously want physicians to be guided by evidence when they’re selecting treatments, and they need to be able to interpret the clinical benefits of the drug that they’re prescribing,” he said. “This is really about having the research consumer, patients, and physicians, as well as industry, understand why certain markers are considered and not considered.”
Dr. Wallach reported receiving grants from the FDA (through the Yale University — Mayo Clinic Center of Excellence in Regulatory Science and Innovation), National Institute on Alcohol Abuse and Alcoholism (1K01AA028258), and Johnson & Johnson (through the Yale University Open Data Access Project); and consulting fees from Hagens Berman Sobol Shapiro LLP and Dugan Law Firm APLC outside the submitted work. Dr. Ramachandran reported receiving grants from the Stavros Niarchos Foundation and FDA; receiving consulting fees from ReAct Action on Antibiotic Resistance strategy policy program outside the submitted work; and serving in an unpaid capacity as chair of the FDA task force for the nonprofit organization Doctors for America and in an unpaid capacity as board president for Universities Allied for Essential Medicines North America.
A version of this article appeared on Medscape.com.
FROM JAMA