User login
USPSTF: Screen all pregnant women for syphilis
The U.S. Preventive Services Task Force issued a draft recommendation that all pregnant women be screened for syphilis infection.
The recommendation, released Feb. 6, follows an evidence review of studies conducted since the task force’s most recent recommendation in 2009, which also called for universal screening of pregnant women.
“Despite consistent recommendations and legal mandates, screening for syphilis in pregnancy continues to be suboptimal in certain populations,” the evidence review noted. The rate of congenital syphilis in the United States nearly doubled from 2012 to 2016.
“Because the early stages of syphilis often don’t cause any symptoms, screening helps identify the infection in pregnant women who may not realize they have the disease,” task force member Chien-Wen Tseng, MD, of the University of Hawaii, said in a statement.
Treatment is most effective early in pregnancy, and can reduce the chances of congenital syphilis. The draft recommendation calls for pregnant women to be tested at the first prenatal visit or at delivery, if the woman has not received prenatal care.
Comments can be submitted until March 5 at www.uspreventiveservicestaskforce.org/tfcomment.htm.
[email protected]
The U.S. Preventive Services Task Force issued a draft recommendation that all pregnant women be screened for syphilis infection.
The recommendation, released Feb. 6, follows an evidence review of studies conducted since the task force’s most recent recommendation in 2009, which also called for universal screening of pregnant women.
“Despite consistent recommendations and legal mandates, screening for syphilis in pregnancy continues to be suboptimal in certain populations,” the evidence review noted. The rate of congenital syphilis in the United States nearly doubled from 2012 to 2016.
“Because the early stages of syphilis often don’t cause any symptoms, screening helps identify the infection in pregnant women who may not realize they have the disease,” task force member Chien-Wen Tseng, MD, of the University of Hawaii, said in a statement.
Treatment is most effective early in pregnancy, and can reduce the chances of congenital syphilis. The draft recommendation calls for pregnant women to be tested at the first prenatal visit or at delivery, if the woman has not received prenatal care.
Comments can be submitted until March 5 at www.uspreventiveservicestaskforce.org/tfcomment.htm.
[email protected]
The U.S. Preventive Services Task Force issued a draft recommendation that all pregnant women be screened for syphilis infection.
The recommendation, released Feb. 6, follows an evidence review of studies conducted since the task force’s most recent recommendation in 2009, which also called for universal screening of pregnant women.
“Despite consistent recommendations and legal mandates, screening for syphilis in pregnancy continues to be suboptimal in certain populations,” the evidence review noted. The rate of congenital syphilis in the United States nearly doubled from 2012 to 2016.
“Because the early stages of syphilis often don’t cause any symptoms, screening helps identify the infection in pregnant women who may not realize they have the disease,” task force member Chien-Wen Tseng, MD, of the University of Hawaii, said in a statement.
Treatment is most effective early in pregnancy, and can reduce the chances of congenital syphilis. The draft recommendation calls for pregnant women to be tested at the first prenatal visit or at delivery, if the woman has not received prenatal care.
Comments can be submitted until March 5 at www.uspreventiveservicestaskforce.org/tfcomment.htm.
[email protected]
ASCO expands recommendations on bone-modifying agents in myeloma
Bisphosphonates should be prescribed for any patient receiving treatment for active multiple myeloma, regardless of whether or not there is evidence of lytic bone destruction or spinal compression fracture, according to updated guidelines from the American Society of Clinical Oncology.
Previous guidelines from the society, last updated in 2007, recommended the use of intravenous bisphosphonates for patients with myeloma with evidence of bone disease, according to the expert panel that drafted the update.
“Fewer adverse events related to renal toxicity have been noted with denosumab, compared with zoledronic acid,” and “this may be preferred in patients with compromised renal function,” wrote the expert panel, led by cochairs Kenneth C. Anderson, MD, of Dana-Farber Cancer Institute, Boston, and Robert A. Kyle, MD, of Mayo Clinic, Rochester, Minn.
ASCO guidelines on bisphosphonates in myeloma were first drafted in 2002 and then updated in 2007. The new recommendations on bone-modifying therapy in myeloma are based on review of an additional 35 publications. The new guidelines are “consistent with the previous recommendations” while updating indications for therapy and information on denosumab, according to the expert panel.
Evidence that myeloma patients without lytic bone disease will benefit from intravenous bisphosphonates comes from the randomized MRC IX trial, in which patients who received zoledronic acid had reduced skeletal-related events at relapse and improved progression-free survival.
Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor, was noninferior to zoledronic acid for prevention of skeletal-related events in a randomized phase 3 clinical trial; however, it is “more expensive than zoledronic acid or pamidronate and must be considered in treatment decisions,” the guidelines authors wrote.
The total price in the United States for a 1-year treatment cycle of denosumab is just under $26,000, according to data included in the ASCO guideline. By comparison, the 1-year treatment cycle price for the bisphosphonates ranges from $214 to $697, depending on the regimen.
When intravenous bisphosphonate therapy is warranted, the guideline-recommended schedule is infusion of zoledronic acid 4 mg over at least 15 minutes, or pamidronate 90 mg over 2 hours, every 3-4 weeks.
The guidelines also address osteonecrosis of the jaw (ONJ), a major complication observed not only with the potent bisphosphonates pamidronate and zoledronic acid, but also with denosumab.
The panel said they were in agreement with revised labels from the Food and Drug Administration for zoledronic acid and pamidronate, among other papers or statements addressing ONJ and noted that patients need a comprehensive dental exam and preventive dentistry as appropriate before starting bone-modifying therapy.
“The risk of ONJ has prompted the use of less-frequent dosing of zoledronic acid, which may be an option for patients,” they said in their report.
Guideline authors reported ties to Amgen, Celgene, Millennium Pharmaceuticals, Gilead Sciences, Bristol-Myers Squibb, Novartis, Pfizer, and others.
SOURCE: Anderson K et al. J Clin Oncol. 2018 Jan 17:JCO2017766402. doi: 10.1200/JCO.2017.76.6402.
Bisphosphonates should be prescribed for any patient receiving treatment for active multiple myeloma, regardless of whether or not there is evidence of lytic bone destruction or spinal compression fracture, according to updated guidelines from the American Society of Clinical Oncology.
Previous guidelines from the society, last updated in 2007, recommended the use of intravenous bisphosphonates for patients with myeloma with evidence of bone disease, according to the expert panel that drafted the update.
“Fewer adverse events related to renal toxicity have been noted with denosumab, compared with zoledronic acid,” and “this may be preferred in patients with compromised renal function,” wrote the expert panel, led by cochairs Kenneth C. Anderson, MD, of Dana-Farber Cancer Institute, Boston, and Robert A. Kyle, MD, of Mayo Clinic, Rochester, Minn.
ASCO guidelines on bisphosphonates in myeloma were first drafted in 2002 and then updated in 2007. The new recommendations on bone-modifying therapy in myeloma are based on review of an additional 35 publications. The new guidelines are “consistent with the previous recommendations” while updating indications for therapy and information on denosumab, according to the expert panel.
Evidence that myeloma patients without lytic bone disease will benefit from intravenous bisphosphonates comes from the randomized MRC IX trial, in which patients who received zoledronic acid had reduced skeletal-related events at relapse and improved progression-free survival.
Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor, was noninferior to zoledronic acid for prevention of skeletal-related events in a randomized phase 3 clinical trial; however, it is “more expensive than zoledronic acid or pamidronate and must be considered in treatment decisions,” the guidelines authors wrote.
The total price in the United States for a 1-year treatment cycle of denosumab is just under $26,000, according to data included in the ASCO guideline. By comparison, the 1-year treatment cycle price for the bisphosphonates ranges from $214 to $697, depending on the regimen.
When intravenous bisphosphonate therapy is warranted, the guideline-recommended schedule is infusion of zoledronic acid 4 mg over at least 15 minutes, or pamidronate 90 mg over 2 hours, every 3-4 weeks.
The guidelines also address osteonecrosis of the jaw (ONJ), a major complication observed not only with the potent bisphosphonates pamidronate and zoledronic acid, but also with denosumab.
The panel said they were in agreement with revised labels from the Food and Drug Administration for zoledronic acid and pamidronate, among other papers or statements addressing ONJ and noted that patients need a comprehensive dental exam and preventive dentistry as appropriate before starting bone-modifying therapy.
“The risk of ONJ has prompted the use of less-frequent dosing of zoledronic acid, which may be an option for patients,” they said in their report.
Guideline authors reported ties to Amgen, Celgene, Millennium Pharmaceuticals, Gilead Sciences, Bristol-Myers Squibb, Novartis, Pfizer, and others.
SOURCE: Anderson K et al. J Clin Oncol. 2018 Jan 17:JCO2017766402. doi: 10.1200/JCO.2017.76.6402.
Bisphosphonates should be prescribed for any patient receiving treatment for active multiple myeloma, regardless of whether or not there is evidence of lytic bone destruction or spinal compression fracture, according to updated guidelines from the American Society of Clinical Oncology.
Previous guidelines from the society, last updated in 2007, recommended the use of intravenous bisphosphonates for patients with myeloma with evidence of bone disease, according to the expert panel that drafted the update.
“Fewer adverse events related to renal toxicity have been noted with denosumab, compared with zoledronic acid,” and “this may be preferred in patients with compromised renal function,” wrote the expert panel, led by cochairs Kenneth C. Anderson, MD, of Dana-Farber Cancer Institute, Boston, and Robert A. Kyle, MD, of Mayo Clinic, Rochester, Minn.
ASCO guidelines on bisphosphonates in myeloma were first drafted in 2002 and then updated in 2007. The new recommendations on bone-modifying therapy in myeloma are based on review of an additional 35 publications. The new guidelines are “consistent with the previous recommendations” while updating indications for therapy and information on denosumab, according to the expert panel.
Evidence that myeloma patients without lytic bone disease will benefit from intravenous bisphosphonates comes from the randomized MRC IX trial, in which patients who received zoledronic acid had reduced skeletal-related events at relapse and improved progression-free survival.
Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor, was noninferior to zoledronic acid for prevention of skeletal-related events in a randomized phase 3 clinical trial; however, it is “more expensive than zoledronic acid or pamidronate and must be considered in treatment decisions,” the guidelines authors wrote.
The total price in the United States for a 1-year treatment cycle of denosumab is just under $26,000, according to data included in the ASCO guideline. By comparison, the 1-year treatment cycle price for the bisphosphonates ranges from $214 to $697, depending on the regimen.
When intravenous bisphosphonate therapy is warranted, the guideline-recommended schedule is infusion of zoledronic acid 4 mg over at least 15 minutes, or pamidronate 90 mg over 2 hours, every 3-4 weeks.
The guidelines also address osteonecrosis of the jaw (ONJ), a major complication observed not only with the potent bisphosphonates pamidronate and zoledronic acid, but also with denosumab.
The panel said they were in agreement with revised labels from the Food and Drug Administration for zoledronic acid and pamidronate, among other papers or statements addressing ONJ and noted that patients need a comprehensive dental exam and preventive dentistry as appropriate before starting bone-modifying therapy.
“The risk of ONJ has prompted the use of less-frequent dosing of zoledronic acid, which may be an option for patients,” they said in their report.
Guideline authors reported ties to Amgen, Celgene, Millennium Pharmaceuticals, Gilead Sciences, Bristol-Myers Squibb, Novartis, Pfizer, and others.
SOURCE: Anderson K et al. J Clin Oncol. 2018 Jan 17:JCO2017766402. doi: 10.1200/JCO.2017.76.6402.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
nPEP for HIV: Updated CDC guidelines available for primary care physicians
In 2016, the Centers for Disease Control and Prevention provided health care providers with updated recommendations for nonoccupational postexposure prophylaxis (nPEP) with antiretroviral drugs to prevent transmission of HIV following sexual interaction, injection-drug use, or other nonoccupational exposures.1 The new recommendations include the use of more effective and more tolerable drug regimens that employ antiretroviral medications that were approved since the previous guidelines came out in 2005; they also provide updated guidance on exposure assessment, baseline and follow-up HIV testing, and longer-term prevention measures, such as pre-exposure prophylaxis (PrEP).
Screening for HIV infection has been expanding broadly in all health care settings over the past decade, so primary care physicians play an increasingly vital role in preventing HIV infection. Today, primary care physicians are also often the most likely “go-to” health care provider when patients think they may have been exposed to HIV. Clinically, this is an emergency situation, so time is of the essence: Treatment with three powerful antiretrovirals must be initiated within a few hours of – but no later than 72 hours after – an isolated exposure to blood, genital secretions, or other potentially infectious body fluids that may contain HIV.
The key issue for primary care physicians, especially those who have never prescribed PEP before, is advance planning. What you do up front, in terms of organizing materials and training staff, is worth the effort because there is so much at stake – for your patients and for society. The good news is that once you have an established nPEP protocol in place, it stays in place. When a patient asks for help, the protocol kicks in automatically.
Getting ready for nPEP
Prepare your staff:
- Educate your whole staff about the urgency of seeing potential nPEP patients immediately.
- Choose the staff person in your office who will submit requests for PEP medications to the pharmacy and/or pharmaceutical companies; your financial reimbursement staff person is likely a good candidate for this job.
- Learn about patient assistance programs (for uninsured or underinsured patients) and crime victims compensation programs (reimbursement or emergency awards for victims of violent crimes, including rape, for various out-of-pocket expenses including medical expenses).
Keep paperwork and materials on hand:
- Have information and forms for patient assistance programs for pharmaceutical companies supplying the drugs. Pharmaceutical companies are aware of the urgency for nPEP medications and are ready to respond immediately. They may mail the medication so it arrives the next day or, more likely, fax a voucher or other information for the patient to present to a local pharmacist who will fill the prescription.
- Have information on your state’s crime victims compensation program available.
- Consider keeping nPEP Starter Packs (with an initial 3-7 days’ worth of medication) readily available in your office.
Rapid evaluation of patients seeking care after potential exposure to HIV
Effective delivery of nPEP requires prompt initial evaluation of patients and assessment of HIV transmission risk. Take a methodical, step-by-step history of the exposure to address the following basic questions:
- Date and time of exposure? nPEP should be initiated as soon as possible after HIV exposure; it is unlikely to be effective if not initiated within 72 hours or less.
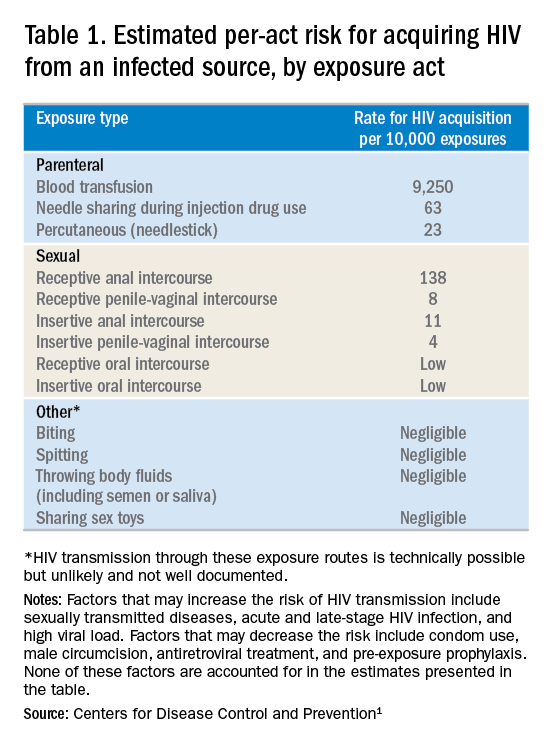
- Frequency of exposure? Type/route of exposure? nPEP is generally reserved for isolated or infrequent exposures that present a substantial risk for HIV acquisition (see Table 1 on HIV acquisition risk below).
- HIV status of exposure source? If the source is positive, is the source person on HIV treatment with antiretroviral therapy? If unknown, is the source person an injecting drug user or a man who has sex with men (MSM)?
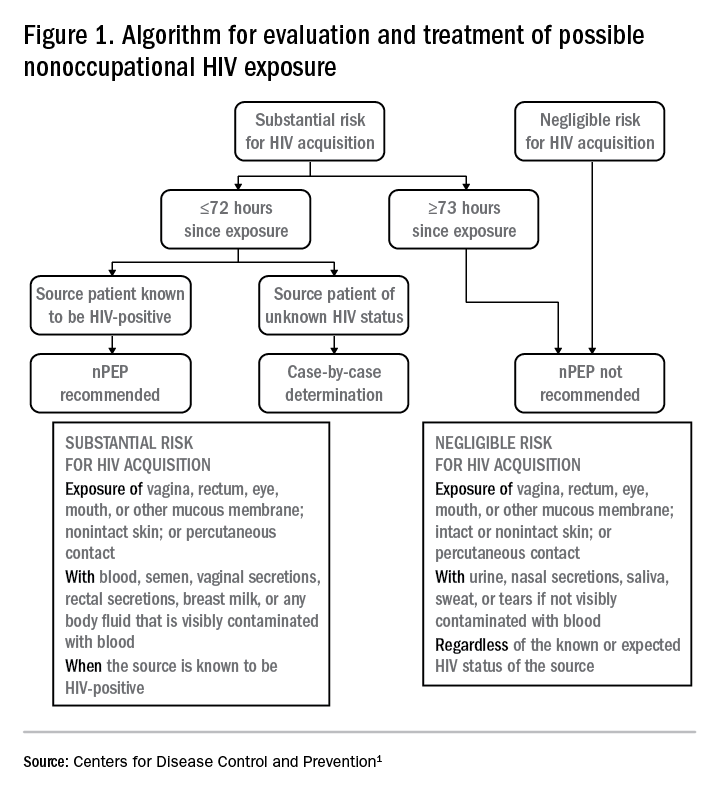
Based on the initial evaluation, is nPEP recommended?
Answers to the questions asked during the initial evaluation of the patient will determine whether nPEP is indicated. Along with its updated recommendations, the CDC provided an algorithm to help guide evaluation and treatment.
Preferred HIV test
Administer an HIV test to all patients considered for nPEP, preferably the rapid combined antigen and antibody test (Ag/Ab), or just the antibody test if the Ag/Ab test is not available. nPEP is indicated only for persons without HIV infections. However, if results are not available during the initial evaluation, assume the patient is not infected. If indicated and started, nPEP can be discontinued if tests later shown the patient already has an HIV infection.
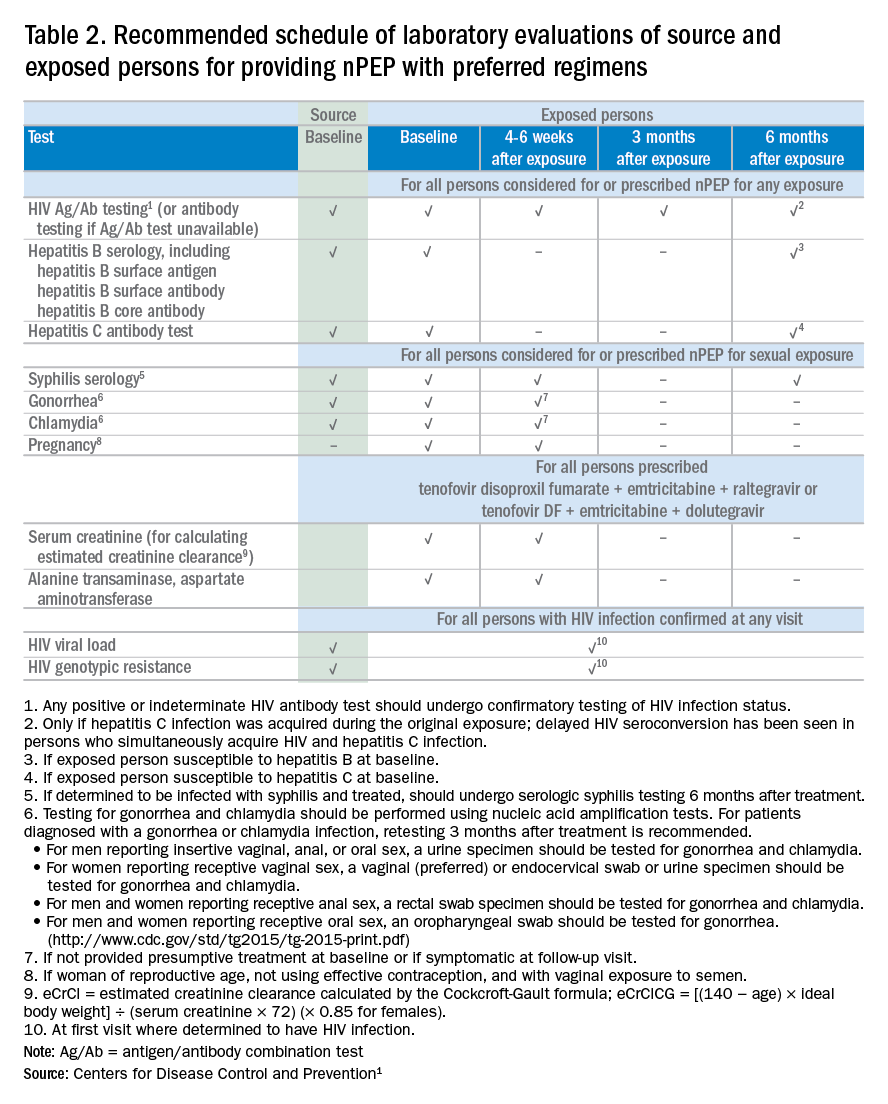
Laboratory testing
If nPEP is indicated, conduct laboratory testing. Lab testing is required to document the patient’s HIV status (and that of the source person, when available), identify and manage other conditions potentially resulting from exposure, identify conditions that may affect the nPEP medication regimen, and monitor safety or toxicities to the prescribed regimen.
nPEP treatment regimen for otherwise healthy adults and adolescents
In the absence of randomized clinical trials, data from a case/control study demonstrating an 81% reduction of HIV transmission after use of occupational PEP among hospital workers remains the strongest evidence for the benefit of nPEP.1,2 For patients offered nPEP, recommended treatment includes prescribing either of the following regimens for 28 days:
- Preferred regimen: tenofovir disoproxil fumarate (TDF) (300 mg) with emtricitabine (FTC) (200 mg) once daily plus either raltegravir (RAL) 400 mg twice daily or dolutegravir (DTG) 50 mg daily.
- Alternative regimen: TDF (300 mg) with FTC (200 mg) once daily plus darunavir (DRV) (800 mg) and ritonavir (RTV) (100 mg) once daily.
Additional considerations and nPEP treatment regimens for children, patients with decreased renal function, and pregnant women are included in the CDC guidelines.
Crucial Information for Patients on nPEP
Emphasize the importance of proper dosing and adherence.
Review the patient information for each drug in the regimen, specifically the black boxes, warnings, and side effects, and counsel your patients accordingly.
Transitioning from nPEP to PrEP or from PrEP to nPEP
If you have a patient who engages in behavior that places them at risk for frequent, recurrent exposures to HIV, consider transitioning them to PrEP (pre-exposure prophylaxis) following their 28-day course of nPEP.3 PrEP is a two-drug regimen taken daily on an ongoing basis.
Additionally, for patients who are already on PrEP but who have not taken their medications within a week before the possible exposure, consider initiating nPEP for 28 days and then reintroducing PrEP if their HIV status is negative and the problems with adherence can be addressed moving forward.
Raising Awareness About nPEP
Many people never expect to be exposed to HIV and may not know about the availability of PEP in an emergency situation. You can help raise awareness by making educational materials available in your waiting rooms and exam rooms. Brochures and other HIV/AIDS educational materials for patients are available from the CDC Act Against AIDS campaign.
Summary
Dr. Dominguez is a Captain, U.S. Public Health Service, epidemiology branch, division of HIV/AIDS prevention, CDC.
Additional resources
- The CDC recommends that everyone between the ages of 13 and 64 get tested for HIV at least once as part of routine health care. As part of its Act Against AIDS initiative, the CDC developed the HIV Screening Standard Care program, which provides free tools and resources to help clinicians and nurses incorporate routine HIV screening into primary care settings.
- HIV guidelines and recommendations .
- Postexposure prophylaxis (PEP)
- Pre-Exposure prophylaxis (PrEP)
References
1. Centers for Disease Control and Prevention. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV. United States, 2016. Accessed March 6, 2017.
2. Cardo DM et al. New Engl J Med. 1997;337(21):1485-90.
3. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States–2014: a clinical practice guideline. Accessed March 6, 2017.
In 2016, the Centers for Disease Control and Prevention provided health care providers with updated recommendations for nonoccupational postexposure prophylaxis (nPEP) with antiretroviral drugs to prevent transmission of HIV following sexual interaction, injection-drug use, or other nonoccupational exposures.1 The new recommendations include the use of more effective and more tolerable drug regimens that employ antiretroviral medications that were approved since the previous guidelines came out in 2005; they also provide updated guidance on exposure assessment, baseline and follow-up HIV testing, and longer-term prevention measures, such as pre-exposure prophylaxis (PrEP).
Screening for HIV infection has been expanding broadly in all health care settings over the past decade, so primary care physicians play an increasingly vital role in preventing HIV infection. Today, primary care physicians are also often the most likely “go-to” health care provider when patients think they may have been exposed to HIV. Clinically, this is an emergency situation, so time is of the essence: Treatment with three powerful antiretrovirals must be initiated within a few hours of – but no later than 72 hours after – an isolated exposure to blood, genital secretions, or other potentially infectious body fluids that may contain HIV.
The key issue for primary care physicians, especially those who have never prescribed PEP before, is advance planning. What you do up front, in terms of organizing materials and training staff, is worth the effort because there is so much at stake – for your patients and for society. The good news is that once you have an established nPEP protocol in place, it stays in place. When a patient asks for help, the protocol kicks in automatically.
Getting ready for nPEP
Prepare your staff:
- Educate your whole staff about the urgency of seeing potential nPEP patients immediately.
- Choose the staff person in your office who will submit requests for PEP medications to the pharmacy and/or pharmaceutical companies; your financial reimbursement staff person is likely a good candidate for this job.
- Learn about patient assistance programs (for uninsured or underinsured patients) and crime victims compensation programs (reimbursement or emergency awards for victims of violent crimes, including rape, for various out-of-pocket expenses including medical expenses).
Keep paperwork and materials on hand:
- Have information and forms for patient assistance programs for pharmaceutical companies supplying the drugs. Pharmaceutical companies are aware of the urgency for nPEP medications and are ready to respond immediately. They may mail the medication so it arrives the next day or, more likely, fax a voucher or other information for the patient to present to a local pharmacist who will fill the prescription.
- Have information on your state’s crime victims compensation program available.
- Consider keeping nPEP Starter Packs (with an initial 3-7 days’ worth of medication) readily available in your office.
Rapid evaluation of patients seeking care after potential exposure to HIV
Effective delivery of nPEP requires prompt initial evaluation of patients and assessment of HIV transmission risk. Take a methodical, step-by-step history of the exposure to address the following basic questions:
- Date and time of exposure? nPEP should be initiated as soon as possible after HIV exposure; it is unlikely to be effective if not initiated within 72 hours or less.
- Frequency of exposure? Type/route of exposure? nPEP is generally reserved for isolated or infrequent exposures that present a substantial risk for HIV acquisition (see Table 1 on HIV acquisition risk below).
- HIV status of exposure source? If the source is positive, is the source person on HIV treatment with antiretroviral therapy? If unknown, is the source person an injecting drug user or a man who has sex with men (MSM)?

Based on the initial evaluation, is nPEP recommended?
Answers to the questions asked during the initial evaluation of the patient will determine whether nPEP is indicated. Along with its updated recommendations, the CDC provided an algorithm to help guide evaluation and treatment.

Preferred HIV test
Administer an HIV test to all patients considered for nPEP, preferably the rapid combined antigen and antibody test (Ag/Ab), or just the antibody test if the Ag/Ab test is not available. nPEP is indicated only for persons without HIV infections. However, if results are not available during the initial evaluation, assume the patient is not infected. If indicated and started, nPEP can be discontinued if tests later shown the patient already has an HIV infection.
Laboratory testing
If nPEP is indicated, conduct laboratory testing. Lab testing is required to document the patient’s HIV status (and that of the source person, when available), identify and manage other conditions potentially resulting from exposure, identify conditions that may affect the nPEP medication regimen, and monitor safety or toxicities to the prescribed regimen.

nPEP treatment regimen for otherwise healthy adults and adolescents
In the absence of randomized clinical trials, data from a case/control study demonstrating an 81% reduction of HIV transmission after use of occupational PEP among hospital workers remains the strongest evidence for the benefit of nPEP.1,2 For patients offered nPEP, recommended treatment includes prescribing either of the following regimens for 28 days:
- Preferred regimen: tenofovir disoproxil fumarate (TDF) (300 mg) with emtricitabine (FTC) (200 mg) once daily plus either raltegravir (RAL) 400 mg twice daily or dolutegravir (DTG) 50 mg daily.
- Alternative regimen: TDF (300 mg) with FTC (200 mg) once daily plus darunavir (DRV) (800 mg) and ritonavir (RTV) (100 mg) once daily.
Additional considerations and nPEP treatment regimens for children, patients with decreased renal function, and pregnant women are included in the CDC guidelines.
Crucial Information for Patients on nPEP
Emphasize the importance of proper dosing and adherence.
Review the patient information for each drug in the regimen, specifically the black boxes, warnings, and side effects, and counsel your patients accordingly.
Transitioning from nPEP to PrEP or from PrEP to nPEP
If you have a patient who engages in behavior that places them at risk for frequent, recurrent exposures to HIV, consider transitioning them to PrEP (pre-exposure prophylaxis) following their 28-day course of nPEP.3 PrEP is a two-drug regimen taken daily on an ongoing basis.
Additionally, for patients who are already on PrEP but who have not taken their medications within a week before the possible exposure, consider initiating nPEP for 28 days and then reintroducing PrEP if their HIV status is negative and the problems with adherence can be addressed moving forward.
Raising Awareness About nPEP
Many people never expect to be exposed to HIV and may not know about the availability of PEP in an emergency situation. You can help raise awareness by making educational materials available in your waiting rooms and exam rooms. Brochures and other HIV/AIDS educational materials for patients are available from the CDC Act Against AIDS campaign.
Summary
Dr. Dominguez is a Captain, U.S. Public Health Service, epidemiology branch, division of HIV/AIDS prevention, CDC.
Additional resources
- The CDC recommends that everyone between the ages of 13 and 64 get tested for HIV at least once as part of routine health care. As part of its Act Against AIDS initiative, the CDC developed the HIV Screening Standard Care program, which provides free tools and resources to help clinicians and nurses incorporate routine HIV screening into primary care settings.
- HIV guidelines and recommendations .
- Postexposure prophylaxis (PEP)
- Pre-Exposure prophylaxis (PrEP)
References
1. Centers for Disease Control and Prevention. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV. United States, 2016. Accessed March 6, 2017.
2. Cardo DM et al. New Engl J Med. 1997;337(21):1485-90.
3. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States–2014: a clinical practice guideline. Accessed March 6, 2017.
In 2016, the Centers for Disease Control and Prevention provided health care providers with updated recommendations for nonoccupational postexposure prophylaxis (nPEP) with antiretroviral drugs to prevent transmission of HIV following sexual interaction, injection-drug use, or other nonoccupational exposures.1 The new recommendations include the use of more effective and more tolerable drug regimens that employ antiretroviral medications that were approved since the previous guidelines came out in 2005; they also provide updated guidance on exposure assessment, baseline and follow-up HIV testing, and longer-term prevention measures, such as pre-exposure prophylaxis (PrEP).
Screening for HIV infection has been expanding broadly in all health care settings over the past decade, so primary care physicians play an increasingly vital role in preventing HIV infection. Today, primary care physicians are also often the most likely “go-to” health care provider when patients think they may have been exposed to HIV. Clinically, this is an emergency situation, so time is of the essence: Treatment with three powerful antiretrovirals must be initiated within a few hours of – but no later than 72 hours after – an isolated exposure to blood, genital secretions, or other potentially infectious body fluids that may contain HIV.
The key issue for primary care physicians, especially those who have never prescribed PEP before, is advance planning. What you do up front, in terms of organizing materials and training staff, is worth the effort because there is so much at stake – for your patients and for society. The good news is that once you have an established nPEP protocol in place, it stays in place. When a patient asks for help, the protocol kicks in automatically.
Getting ready for nPEP
Prepare your staff:
- Educate your whole staff about the urgency of seeing potential nPEP patients immediately.
- Choose the staff person in your office who will submit requests for PEP medications to the pharmacy and/or pharmaceutical companies; your financial reimbursement staff person is likely a good candidate for this job.
- Learn about patient assistance programs (for uninsured or underinsured patients) and crime victims compensation programs (reimbursement or emergency awards for victims of violent crimes, including rape, for various out-of-pocket expenses including medical expenses).
Keep paperwork and materials on hand:
- Have information and forms for patient assistance programs for pharmaceutical companies supplying the drugs. Pharmaceutical companies are aware of the urgency for nPEP medications and are ready to respond immediately. They may mail the medication so it arrives the next day or, more likely, fax a voucher or other information for the patient to present to a local pharmacist who will fill the prescription.
- Have information on your state’s crime victims compensation program available.
- Consider keeping nPEP Starter Packs (with an initial 3-7 days’ worth of medication) readily available in your office.
Rapid evaluation of patients seeking care after potential exposure to HIV
Effective delivery of nPEP requires prompt initial evaluation of patients and assessment of HIV transmission risk. Take a methodical, step-by-step history of the exposure to address the following basic questions:
- Date and time of exposure? nPEP should be initiated as soon as possible after HIV exposure; it is unlikely to be effective if not initiated within 72 hours or less.
- Frequency of exposure? Type/route of exposure? nPEP is generally reserved for isolated or infrequent exposures that present a substantial risk for HIV acquisition (see Table 1 on HIV acquisition risk below).
- HIV status of exposure source? If the source is positive, is the source person on HIV treatment with antiretroviral therapy? If unknown, is the source person an injecting drug user or a man who has sex with men (MSM)?

Based on the initial evaluation, is nPEP recommended?
Answers to the questions asked during the initial evaluation of the patient will determine whether nPEP is indicated. Along with its updated recommendations, the CDC provided an algorithm to help guide evaluation and treatment.

Preferred HIV test
Administer an HIV test to all patients considered for nPEP, preferably the rapid combined antigen and antibody test (Ag/Ab), or just the antibody test if the Ag/Ab test is not available. nPEP is indicated only for persons without HIV infections. However, if results are not available during the initial evaluation, assume the patient is not infected. If indicated and started, nPEP can be discontinued if tests later shown the patient already has an HIV infection.
Laboratory testing
If nPEP is indicated, conduct laboratory testing. Lab testing is required to document the patient’s HIV status (and that of the source person, when available), identify and manage other conditions potentially resulting from exposure, identify conditions that may affect the nPEP medication regimen, and monitor safety or toxicities to the prescribed regimen.

nPEP treatment regimen for otherwise healthy adults and adolescents
In the absence of randomized clinical trials, data from a case/control study demonstrating an 81% reduction of HIV transmission after use of occupational PEP among hospital workers remains the strongest evidence for the benefit of nPEP.1,2 For patients offered nPEP, recommended treatment includes prescribing either of the following regimens for 28 days:
- Preferred regimen: tenofovir disoproxil fumarate (TDF) (300 mg) with emtricitabine (FTC) (200 mg) once daily plus either raltegravir (RAL) 400 mg twice daily or dolutegravir (DTG) 50 mg daily.
- Alternative regimen: TDF (300 mg) with FTC (200 mg) once daily plus darunavir (DRV) (800 mg) and ritonavir (RTV) (100 mg) once daily.
Additional considerations and nPEP treatment regimens for children, patients with decreased renal function, and pregnant women are included in the CDC guidelines.
Crucial Information for Patients on nPEP
Emphasize the importance of proper dosing and adherence.
Review the patient information for each drug in the regimen, specifically the black boxes, warnings, and side effects, and counsel your patients accordingly.
Transitioning from nPEP to PrEP or from PrEP to nPEP
If you have a patient who engages in behavior that places them at risk for frequent, recurrent exposures to HIV, consider transitioning them to PrEP (pre-exposure prophylaxis) following their 28-day course of nPEP.3 PrEP is a two-drug regimen taken daily on an ongoing basis.
Additionally, for patients who are already on PrEP but who have not taken their medications within a week before the possible exposure, consider initiating nPEP for 28 days and then reintroducing PrEP if their HIV status is negative and the problems with adherence can be addressed moving forward.
Raising Awareness About nPEP
Many people never expect to be exposed to HIV and may not know about the availability of PEP in an emergency situation. You can help raise awareness by making educational materials available in your waiting rooms and exam rooms. Brochures and other HIV/AIDS educational materials for patients are available from the CDC Act Against AIDS campaign.
Summary
Dr. Dominguez is a Captain, U.S. Public Health Service, epidemiology branch, division of HIV/AIDS prevention, CDC.
Additional resources
- The CDC recommends that everyone between the ages of 13 and 64 get tested for HIV at least once as part of routine health care. As part of its Act Against AIDS initiative, the CDC developed the HIV Screening Standard Care program, which provides free tools and resources to help clinicians and nurses incorporate routine HIV screening into primary care settings.
- HIV guidelines and recommendations .
- Postexposure prophylaxis (PEP)
- Pre-Exposure prophylaxis (PrEP)
References
1. Centers for Disease Control and Prevention. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV. United States, 2016. Accessed March 6, 2017.
2. Cardo DM et al. New Engl J Med. 1997;337(21):1485-90.
3. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States–2014: a clinical practice guideline. Accessed March 6, 2017.
AHA: Heart health helps optimize breast cancer outcomes
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
FROM CIRCULATION
VIDEO: New stroke guideline embraces imaging-guided thrombectomy
LOS ANGELES – When a panel organized by the American Heart Association’s Stroke Council recently revised the group’s guideline for early management of acute ischemic stroke, they were clear on the overarching change they had to make: Incorporate recent evidence collected in two trials that established brain imaging as the way to identify patients eligible for clot removal treatment by thrombectomy, a change in practice that has made this outcome-altering intervention available to more patients.
“The major take-home message [of the new guideline] is the extension of the time window for treating acute ischemic stroke,” said William J. Powers, MD, chair of the guideline group (Stroke. 2018 Jan 24. doi: 10.1161/STR.0000000000000158).
Based on recently reported results from the DAWN (N Engl J Med. 2018;378[1]:11-21) and DEFUSE 3 (N Engl J Med. 2018 Jan 24. doi: 10.1056/NEJMoa1713973) trials “we know that there are patients out to 24 hours from their stroke onset who may benefit” from thrombectomy. “This is a major, major change in how we view care for patients with stroke,” Dr. Powers said in a video interview. “Now there’s much more time. Ideally, we’ll see smaller hospitals develop the ability to do the imaging” that makes it possible to select acute ischemic stroke patients eligible for thrombectomy despite a delay of up to 24 hours from their stroke onset to the time of thrombectomy, said Dr. Powers, professor and chair of neurology at the University of North Carolina, Chapel Hill.
The big priority for the stroke community now that this major change in patient selection was incorporated into a U.S. practice guideline will be acting quickly to implement the steps needed to make this change happen, Dr. Powers and others said.
The new guideline will mean “changes in process and systems of care,” agreed Jeffrey L. Saver, MD, professor of neurology and director of the stroke unit at the University of California, Los Angeles. The imaging called for “will be practical at some primary stroke centers but not others,” he said, although most hospitals certified to provide stroke care as primary stroke centers or acute stroke–ready hospitals have a CT scanner that could provide the basic imaging needed to assess many patients. (CT angiography and perfusion CT are more informative for determining thrombectomy eligibility.) But interpretation of the brain images to distinguish patients eligible for thrombectomy from those who aren’t will likely happen at comprehensive stroke centers that perform thrombectomy or by experts using remote image reading.
Dr. Saver expects that the new guideline will translate most quickly into changes in the imaging and transfer protocols that the Joint Commission may now require from hospitals certified as primary stroke centers or acute stroke-ready hospitals, changes that could be in place sometime later in 2018, he predicted. These are steps “that would really help drive system change.”
Dr. Powers and Dr. Furie had no disclosures. Dr. Saver has received research support and personal fees from Medtronic-Abbott and Neuravia.
LOS ANGELES – When a panel organized by the American Heart Association’s Stroke Council recently revised the group’s guideline for early management of acute ischemic stroke, they were clear on the overarching change they had to make: Incorporate recent evidence collected in two trials that established brain imaging as the way to identify patients eligible for clot removal treatment by thrombectomy, a change in practice that has made this outcome-altering intervention available to more patients.
“The major take-home message [of the new guideline] is the extension of the time window for treating acute ischemic stroke,” said William J. Powers, MD, chair of the guideline group (Stroke. 2018 Jan 24. doi: 10.1161/STR.0000000000000158).
Based on recently reported results from the DAWN (N Engl J Med. 2018;378[1]:11-21) and DEFUSE 3 (N Engl J Med. 2018 Jan 24. doi: 10.1056/NEJMoa1713973) trials “we know that there are patients out to 24 hours from their stroke onset who may benefit” from thrombectomy. “This is a major, major change in how we view care for patients with stroke,” Dr. Powers said in a video interview. “Now there’s much more time. Ideally, we’ll see smaller hospitals develop the ability to do the imaging” that makes it possible to select acute ischemic stroke patients eligible for thrombectomy despite a delay of up to 24 hours from their stroke onset to the time of thrombectomy, said Dr. Powers, professor and chair of neurology at the University of North Carolina, Chapel Hill.
The big priority for the stroke community now that this major change in patient selection was incorporated into a U.S. practice guideline will be acting quickly to implement the steps needed to make this change happen, Dr. Powers and others said.
The new guideline will mean “changes in process and systems of care,” agreed Jeffrey L. Saver, MD, professor of neurology and director of the stroke unit at the University of California, Los Angeles. The imaging called for “will be practical at some primary stroke centers but not others,” he said, although most hospitals certified to provide stroke care as primary stroke centers or acute stroke–ready hospitals have a CT scanner that could provide the basic imaging needed to assess many patients. (CT angiography and perfusion CT are more informative for determining thrombectomy eligibility.) But interpretation of the brain images to distinguish patients eligible for thrombectomy from those who aren’t will likely happen at comprehensive stroke centers that perform thrombectomy or by experts using remote image reading.
Dr. Saver expects that the new guideline will translate most quickly into changes in the imaging and transfer protocols that the Joint Commission may now require from hospitals certified as primary stroke centers or acute stroke-ready hospitals, changes that could be in place sometime later in 2018, he predicted. These are steps “that would really help drive system change.”
Dr. Powers and Dr. Furie had no disclosures. Dr. Saver has received research support and personal fees from Medtronic-Abbott and Neuravia.
LOS ANGELES – When a panel organized by the American Heart Association’s Stroke Council recently revised the group’s guideline for early management of acute ischemic stroke, they were clear on the overarching change they had to make: Incorporate recent evidence collected in two trials that established brain imaging as the way to identify patients eligible for clot removal treatment by thrombectomy, a change in practice that has made this outcome-altering intervention available to more patients.
“The major take-home message [of the new guideline] is the extension of the time window for treating acute ischemic stroke,” said William J. Powers, MD, chair of the guideline group (Stroke. 2018 Jan 24. doi: 10.1161/STR.0000000000000158).
Based on recently reported results from the DAWN (N Engl J Med. 2018;378[1]:11-21) and DEFUSE 3 (N Engl J Med. 2018 Jan 24. doi: 10.1056/NEJMoa1713973) trials “we know that there are patients out to 24 hours from their stroke onset who may benefit” from thrombectomy. “This is a major, major change in how we view care for patients with stroke,” Dr. Powers said in a video interview. “Now there’s much more time. Ideally, we’ll see smaller hospitals develop the ability to do the imaging” that makes it possible to select acute ischemic stroke patients eligible for thrombectomy despite a delay of up to 24 hours from their stroke onset to the time of thrombectomy, said Dr. Powers, professor and chair of neurology at the University of North Carolina, Chapel Hill.
The big priority for the stroke community now that this major change in patient selection was incorporated into a U.S. practice guideline will be acting quickly to implement the steps needed to make this change happen, Dr. Powers and others said.
The new guideline will mean “changes in process and systems of care,” agreed Jeffrey L. Saver, MD, professor of neurology and director of the stroke unit at the University of California, Los Angeles. The imaging called for “will be practical at some primary stroke centers but not others,” he said, although most hospitals certified to provide stroke care as primary stroke centers or acute stroke–ready hospitals have a CT scanner that could provide the basic imaging needed to assess many patients. (CT angiography and perfusion CT are more informative for determining thrombectomy eligibility.) But interpretation of the brain images to distinguish patients eligible for thrombectomy from those who aren’t will likely happen at comprehensive stroke centers that perform thrombectomy or by experts using remote image reading.
Dr. Saver expects that the new guideline will translate most quickly into changes in the imaging and transfer protocols that the Joint Commission may now require from hospitals certified as primary stroke centers or acute stroke-ready hospitals, changes that could be in place sometime later in 2018, he predicted. These are steps “that would really help drive system change.”
Dr. Powers and Dr. Furie had no disclosures. Dr. Saver has received research support and personal fees from Medtronic-Abbott and Neuravia.
EXPERT ANALYSIS FROM ISC 2018
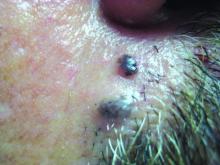
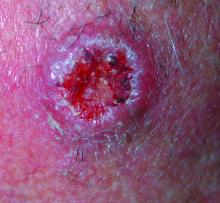
AAD guidelines favor surgery for nonmelanoma skin cancers
, according to new practice guidelines issued by the American Academy of Dermatology.
Nonsurgical approaches such as cryotherapy, photodynamic therapy, and radiation may be considered for low-risk cancers if surgery is contraindicated, but these methods have lower cure rates, according to the guidelines. Christopher K. Bichakjian, MD, professor of dermatology, University of Michigan, Ann Arbor, and Murad Alam, MD, professor of dermatology, Northwestern University, Chicago, cochaired the work groups that developed the guidelines.
The guidelines for BCC and cSCC, published online in two separate papers (J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.006; J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.007), also discuss biopsy techniques, tumor staging, and prevention of recurrence of nonmelanoma skin cancers.
The most suitable stratification for localized BCC and cSCC is the framework provided by the National Comprehensive Cancer Network, the authors said in the guidelines.
For suspected BCC and cSCC, recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy. Biopsy technique is “contingent on the clinical characteristics of the suspected tumor, including morphology, expected histologic subtype and depth, natural history, and anatomic location; patient-specific factors, such as bleeding and wound healing diatheses; and patient preference and physician judgment,” the guidelines state. If the initial biopsy proves insufficient for diagnosis, a repeat biopsy may be considered.
For surgical treatment of BCC, curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm clinical margins and histologic margin assessment is recommended for low-risk primary BCC. For high-risk BCC, Mohs micrographic surgery is recommended, the authors said.
Surgical options for cSCC also include curettage and electrodessication and standard excision for low-risk disease, and Mohs micrographic surgery for high-risk cSCC. In both BCC and cSCC, standard excision may be considered for high-risk tumors in some cases, but “strong caution is advised when selecting a treatment modality” for high-risk tumors “without complete margin assessment,” the guidelines state.
Nonsurgical therapies are generally not recommended as first-line treatment, especially in cSCC because of possible recurrence and metastasis. In cases where nonsurgical therapies are preferred, options may include cryosurgery, topical therapy, photodynamic therapy, radiation, or laser therapy, “with the understanding that the cure rate may be lower,” the authors wrote.
Patients with diagnosed nonmelanoma skin cancer should continue to undergo screening for new primary skin cancers (including BCC, cSCC, and melanoma) at least once per year, the guideline states. They should also be counseled on sun protection, tanning bed avoidance, and regular use of broad-spectrum sunscreen.
Although the new guidelines mainly “reaffirm common knowledge and current practice,” they offer a reminder of “alternative therapeutic or preventive options when insufficient evidence is available to support new therapies or previously dogmatic practice patterns,” the authors said.
These are the first guidelines of care for BCC and cSCC published by the AAD. Commonly used guidelines for the management of BCC and cSCC are published by the National Comprehensive Cancer Network, which are frequently referenced throughout the new AAD guidelines, Dr. Bichakjian said in an interview. While the aim of the cancer network is to develop multidisciplinary guidelines, reflected by the composition of the panel members, “AAD guidelines of care are established primarily by dermatologists for dermatologists,” he pointed out. “However, the work group recognizes that a variety of health care providers outside of dermatology take care of patients with BCC and cSCC, and acknowledges the importance of multidisciplinary care,” he added. “With these considerations in mind, reviewers from specialties outside of dermatology, including plastic surgery, otolaryngology/head and neck surgery, medical oncology, radiation oncology, and family medicine, were invited to critically review the current guidelines.”
The guidelines do not cover the management of actinic keratosis and cSCC in situ, he said. “The work group acknowledges the importance of appropriate management of premalignant and in situ lesions in the prevention of their potential progression to cSCC. However, additional data to provide comprehensive evidence-based recommendations were deemed too extensive to include in the current guidelines and will need to addressed separately.”
In an interview, David J. Leffell, MD, who was not an author of the guidelines, said that the new guidelines do an effective job of “highlighting where valid outcomes data exist and areas where they do not” for a wide range of therapies. They also “attempt to standardize approaches to diagnosis and care of nonmelanoma skin cancer and in general are consistent with established practice patterns,” he added. “Those contemporary approaches have developed in largely empirical fashion over many decades, but bear clarification and reinforcement,” said Dr. Leffell, professor of dermatology and surgery and chief of the section of dermatologic surgery and cutaneous oncology at Yale University, New Haven, Conn.
The guidelines “thoroughly summarize evidence-based recommendations for the entire spectrum of disease management,” Daniel D. Bennett, MD, of the department of dermatology at the University of Wisconsin – Madison, said in an interview. “While surgery remains the mainstay of treatment for BCC and cutaneous SCC, these guidelines include excellent reviews of nonsurgical management options,” he said.
Dr. Bichakjian, who is also chief of the division of cutaneous surgery and oncology at the University of Michigan, had no relevant financial disclosures to report. Dr. Alam, who is also chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern, disclosed relationships with Amway, OptMed, and 3M. Dr. Bennett and Dr. Leffell had no relevant disclosures.
, according to new practice guidelines issued by the American Academy of Dermatology.
Nonsurgical approaches such as cryotherapy, photodynamic therapy, and radiation may be considered for low-risk cancers if surgery is contraindicated, but these methods have lower cure rates, according to the guidelines. Christopher K. Bichakjian, MD, professor of dermatology, University of Michigan, Ann Arbor, and Murad Alam, MD, professor of dermatology, Northwestern University, Chicago, cochaired the work groups that developed the guidelines.
The guidelines for BCC and cSCC, published online in two separate papers (J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.006; J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.007), also discuss biopsy techniques, tumor staging, and prevention of recurrence of nonmelanoma skin cancers.
The most suitable stratification for localized BCC and cSCC is the framework provided by the National Comprehensive Cancer Network, the authors said in the guidelines.
For suspected BCC and cSCC, recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy. Biopsy technique is “contingent on the clinical characteristics of the suspected tumor, including morphology, expected histologic subtype and depth, natural history, and anatomic location; patient-specific factors, such as bleeding and wound healing diatheses; and patient preference and physician judgment,” the guidelines state. If the initial biopsy proves insufficient for diagnosis, a repeat biopsy may be considered.
For surgical treatment of BCC, curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm clinical margins and histologic margin assessment is recommended for low-risk primary BCC. For high-risk BCC, Mohs micrographic surgery is recommended, the authors said.
Surgical options for cSCC also include curettage and electrodessication and standard excision for low-risk disease, and Mohs micrographic surgery for high-risk cSCC. In both BCC and cSCC, standard excision may be considered for high-risk tumors in some cases, but “strong caution is advised when selecting a treatment modality” for high-risk tumors “without complete margin assessment,” the guidelines state.
Nonsurgical therapies are generally not recommended as first-line treatment, especially in cSCC because of possible recurrence and metastasis. In cases where nonsurgical therapies are preferred, options may include cryosurgery, topical therapy, photodynamic therapy, radiation, or laser therapy, “with the understanding that the cure rate may be lower,” the authors wrote.
Patients with diagnosed nonmelanoma skin cancer should continue to undergo screening for new primary skin cancers (including BCC, cSCC, and melanoma) at least once per year, the guideline states. They should also be counseled on sun protection, tanning bed avoidance, and regular use of broad-spectrum sunscreen.
Although the new guidelines mainly “reaffirm common knowledge and current practice,” they offer a reminder of “alternative therapeutic or preventive options when insufficient evidence is available to support new therapies or previously dogmatic practice patterns,” the authors said.
These are the first guidelines of care for BCC and cSCC published by the AAD. Commonly used guidelines for the management of BCC and cSCC are published by the National Comprehensive Cancer Network, which are frequently referenced throughout the new AAD guidelines, Dr. Bichakjian said in an interview. While the aim of the cancer network is to develop multidisciplinary guidelines, reflected by the composition of the panel members, “AAD guidelines of care are established primarily by dermatologists for dermatologists,” he pointed out. “However, the work group recognizes that a variety of health care providers outside of dermatology take care of patients with BCC and cSCC, and acknowledges the importance of multidisciplinary care,” he added. “With these considerations in mind, reviewers from specialties outside of dermatology, including plastic surgery, otolaryngology/head and neck surgery, medical oncology, radiation oncology, and family medicine, were invited to critically review the current guidelines.”
The guidelines do not cover the management of actinic keratosis and cSCC in situ, he said. “The work group acknowledges the importance of appropriate management of premalignant and in situ lesions in the prevention of their potential progression to cSCC. However, additional data to provide comprehensive evidence-based recommendations were deemed too extensive to include in the current guidelines and will need to addressed separately.”
In an interview, David J. Leffell, MD, who was not an author of the guidelines, said that the new guidelines do an effective job of “highlighting where valid outcomes data exist and areas where they do not” for a wide range of therapies. They also “attempt to standardize approaches to diagnosis and care of nonmelanoma skin cancer and in general are consistent with established practice patterns,” he added. “Those contemporary approaches have developed in largely empirical fashion over many decades, but bear clarification and reinforcement,” said Dr. Leffell, professor of dermatology and surgery and chief of the section of dermatologic surgery and cutaneous oncology at Yale University, New Haven, Conn.
The guidelines “thoroughly summarize evidence-based recommendations for the entire spectrum of disease management,” Daniel D. Bennett, MD, of the department of dermatology at the University of Wisconsin – Madison, said in an interview. “While surgery remains the mainstay of treatment for BCC and cutaneous SCC, these guidelines include excellent reviews of nonsurgical management options,” he said.
Dr. Bichakjian, who is also chief of the division of cutaneous surgery and oncology at the University of Michigan, had no relevant financial disclosures to report. Dr. Alam, who is also chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern, disclosed relationships with Amway, OptMed, and 3M. Dr. Bennett and Dr. Leffell had no relevant disclosures.
, according to new practice guidelines issued by the American Academy of Dermatology.
Nonsurgical approaches such as cryotherapy, photodynamic therapy, and radiation may be considered for low-risk cancers if surgery is contraindicated, but these methods have lower cure rates, according to the guidelines. Christopher K. Bichakjian, MD, professor of dermatology, University of Michigan, Ann Arbor, and Murad Alam, MD, professor of dermatology, Northwestern University, Chicago, cochaired the work groups that developed the guidelines.
The guidelines for BCC and cSCC, published online in two separate papers (J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.006; J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.007), also discuss biopsy techniques, tumor staging, and prevention of recurrence of nonmelanoma skin cancers.
The most suitable stratification for localized BCC and cSCC is the framework provided by the National Comprehensive Cancer Network, the authors said in the guidelines.
For suspected BCC and cSCC, recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy. Biopsy technique is “contingent on the clinical characteristics of the suspected tumor, including morphology, expected histologic subtype and depth, natural history, and anatomic location; patient-specific factors, such as bleeding and wound healing diatheses; and patient preference and physician judgment,” the guidelines state. If the initial biopsy proves insufficient for diagnosis, a repeat biopsy may be considered.
For surgical treatment of BCC, curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm clinical margins and histologic margin assessment is recommended for low-risk primary BCC. For high-risk BCC, Mohs micrographic surgery is recommended, the authors said.
Surgical options for cSCC also include curettage and electrodessication and standard excision for low-risk disease, and Mohs micrographic surgery for high-risk cSCC. In both BCC and cSCC, standard excision may be considered for high-risk tumors in some cases, but “strong caution is advised when selecting a treatment modality” for high-risk tumors “without complete margin assessment,” the guidelines state.
Nonsurgical therapies are generally not recommended as first-line treatment, especially in cSCC because of possible recurrence and metastasis. In cases where nonsurgical therapies are preferred, options may include cryosurgery, topical therapy, photodynamic therapy, radiation, or laser therapy, “with the understanding that the cure rate may be lower,” the authors wrote.
Patients with diagnosed nonmelanoma skin cancer should continue to undergo screening for new primary skin cancers (including BCC, cSCC, and melanoma) at least once per year, the guideline states. They should also be counseled on sun protection, tanning bed avoidance, and regular use of broad-spectrum sunscreen.
Although the new guidelines mainly “reaffirm common knowledge and current practice,” they offer a reminder of “alternative therapeutic or preventive options when insufficient evidence is available to support new therapies or previously dogmatic practice patterns,” the authors said.
These are the first guidelines of care for BCC and cSCC published by the AAD. Commonly used guidelines for the management of BCC and cSCC are published by the National Comprehensive Cancer Network, which are frequently referenced throughout the new AAD guidelines, Dr. Bichakjian said in an interview. While the aim of the cancer network is to develop multidisciplinary guidelines, reflected by the composition of the panel members, “AAD guidelines of care are established primarily by dermatologists for dermatologists,” he pointed out. “However, the work group recognizes that a variety of health care providers outside of dermatology take care of patients with BCC and cSCC, and acknowledges the importance of multidisciplinary care,” he added. “With these considerations in mind, reviewers from specialties outside of dermatology, including plastic surgery, otolaryngology/head and neck surgery, medical oncology, radiation oncology, and family medicine, were invited to critically review the current guidelines.”
The guidelines do not cover the management of actinic keratosis and cSCC in situ, he said. “The work group acknowledges the importance of appropriate management of premalignant and in situ lesions in the prevention of their potential progression to cSCC. However, additional data to provide comprehensive evidence-based recommendations were deemed too extensive to include in the current guidelines and will need to addressed separately.”
In an interview, David J. Leffell, MD, who was not an author of the guidelines, said that the new guidelines do an effective job of “highlighting where valid outcomes data exist and areas where they do not” for a wide range of therapies. They also “attempt to standardize approaches to diagnosis and care of nonmelanoma skin cancer and in general are consistent with established practice patterns,” he added. “Those contemporary approaches have developed in largely empirical fashion over many decades, but bear clarification and reinforcement,” said Dr. Leffell, professor of dermatology and surgery and chief of the section of dermatologic surgery and cutaneous oncology at Yale University, New Haven, Conn.
The guidelines “thoroughly summarize evidence-based recommendations for the entire spectrum of disease management,” Daniel D. Bennett, MD, of the department of dermatology at the University of Wisconsin – Madison, said in an interview. “While surgery remains the mainstay of treatment for BCC and cutaneous SCC, these guidelines include excellent reviews of nonsurgical management options,” he said.
Dr. Bichakjian, who is also chief of the division of cutaneous surgery and oncology at the University of Michigan, had no relevant financial disclosures to report. Dr. Alam, who is also chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern, disclosed relationships with Amway, OptMed, and 3M. Dr. Bennett and Dr. Leffell had no relevant disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
APA guideline backs naltrexone, acamprosate for alcohol use disorder
Naltrexone or acamprosate should be offered as first-line pharmacologic therapy to patients with moderate to severe alcohol use disorder (AUD) who do not respond to nonpharmacologic therapy alone, according to a practice guideline published by the American Psychiatric Association.
if naltrexone and acamprosate are not effective or well tolerated, the report said.
The APA guideline recommends against the use of antidepressants and benzodiazepines for patients with alcohol use disorder, except for situations where a co-occurring disorder requires treatment. In addition, the guideline recommends against the use of acamprosate in patients with renal impairment, and specifies that naltrexone should not be used by patients with acute hepatitis, hepatic failure, or opioid dependence.
“Naltrexone and acamprosate have the best available evidence as pharmacotherapy for patients with AUD,” wrote Victor I. Reus, MD, and his coauthors in the APA Guideline Writing Group, which formed the guideline using a systematic review of current literature in accordance with Institute of Medicine (now called the National Academy of Medicine) and Agency for Healthcare Research and Quality standards.
Naltrexone, an opioid receptor antagonist, is effective in treating both AUD and opioid use disorder. Studies have shown that it may decrease cravings, and is associated with fewer drinking days and a reduced likelihood of return to drinking, the authors reported. In patients with a history of renal impairment, serum creatinine should be measured, and results should be reviewed before initiating treatment with acamprosate – a synthetic amino acid.
Disulfiram breaks down acetaldehyde, an ethanol byproduct, and should be used only to treat patients with a goal of abstinence. It is not recommended as a first-line therapy because of the side effects of concurrent alcohol use, including tachycardia, flushing, headache, nausea, and vomiting, reported Dr. Reus of the psychiatry department at the University of California, San Francisco, and his coauthors.
“Patients should be fully informed of the physiological consequences of consuming alcohol while taking disulfiram and should agree to taking the medication,” the authors wrote. “They should be instructed to abstain from drinking alcohol for at least 12 hours before taking a dose of the medication and be advised that reactions with alcohol can occur up to 14 days after taking disulfiram.”
Lastly, topiramate and gabapentin may be used in patients for whom naltrexone and acamprosate are ineffective, though topiramate may have side effects of concern to the patient, including cognitive dysfunction and numbness, tingling, paresthesias, dizziness, taste abnormalities, and decreased appetite or weight loss, the report said.
Although the APA guideline acknowledges the importance of psychiatric evaluation and nonpharmacologic treatments such as cognitive-behavioral therapy and 12-step programs, it does not provide recommendations on those treatment options.
Further research on alcohol use disorder should include study of quantitative measures for longitudinal monitoring, co-occurring medical and psychiatric conditions, and the effectiveness of naltrexone versus combination therapy for patients with both AUD and opioid use disorder, the authors said.
“The overall goal of this guideline is to enhance the treatment of AUD for millions of affected individuals, thereby reducing the significant psychosocial and public health consequences of this important psychiatric condition,” the report concluded. An executive summary of the guideline was published in the American Journal of Psychiatry.
The guideline authors disclosed no conflicts of interest with their work on the guideline.
SOURCE: Reus VI et al. Am J Psychiatry. 2018;175:86-90.
Naltrexone or acamprosate should be offered as first-line pharmacologic therapy to patients with moderate to severe alcohol use disorder (AUD) who do not respond to nonpharmacologic therapy alone, according to a practice guideline published by the American Psychiatric Association.
if naltrexone and acamprosate are not effective or well tolerated, the report said.
The APA guideline recommends against the use of antidepressants and benzodiazepines for patients with alcohol use disorder, except for situations where a co-occurring disorder requires treatment. In addition, the guideline recommends against the use of acamprosate in patients with renal impairment, and specifies that naltrexone should not be used by patients with acute hepatitis, hepatic failure, or opioid dependence.
“Naltrexone and acamprosate have the best available evidence as pharmacotherapy for patients with AUD,” wrote Victor I. Reus, MD, and his coauthors in the APA Guideline Writing Group, which formed the guideline using a systematic review of current literature in accordance with Institute of Medicine (now called the National Academy of Medicine) and Agency for Healthcare Research and Quality standards.
Naltrexone, an opioid receptor antagonist, is effective in treating both AUD and opioid use disorder. Studies have shown that it may decrease cravings, and is associated with fewer drinking days and a reduced likelihood of return to drinking, the authors reported. In patients with a history of renal impairment, serum creatinine should be measured, and results should be reviewed before initiating treatment with acamprosate – a synthetic amino acid.
Disulfiram breaks down acetaldehyde, an ethanol byproduct, and should be used only to treat patients with a goal of abstinence. It is not recommended as a first-line therapy because of the side effects of concurrent alcohol use, including tachycardia, flushing, headache, nausea, and vomiting, reported Dr. Reus of the psychiatry department at the University of California, San Francisco, and his coauthors.
“Patients should be fully informed of the physiological consequences of consuming alcohol while taking disulfiram and should agree to taking the medication,” the authors wrote. “They should be instructed to abstain from drinking alcohol for at least 12 hours before taking a dose of the medication and be advised that reactions with alcohol can occur up to 14 days after taking disulfiram.”
Lastly, topiramate and gabapentin may be used in patients for whom naltrexone and acamprosate are ineffective, though topiramate may have side effects of concern to the patient, including cognitive dysfunction and numbness, tingling, paresthesias, dizziness, taste abnormalities, and decreased appetite or weight loss, the report said.
Although the APA guideline acknowledges the importance of psychiatric evaluation and nonpharmacologic treatments such as cognitive-behavioral therapy and 12-step programs, it does not provide recommendations on those treatment options.
Further research on alcohol use disorder should include study of quantitative measures for longitudinal monitoring, co-occurring medical and psychiatric conditions, and the effectiveness of naltrexone versus combination therapy for patients with both AUD and opioid use disorder, the authors said.
“The overall goal of this guideline is to enhance the treatment of AUD for millions of affected individuals, thereby reducing the significant psychosocial and public health consequences of this important psychiatric condition,” the report concluded. An executive summary of the guideline was published in the American Journal of Psychiatry.
The guideline authors disclosed no conflicts of interest with their work on the guideline.
SOURCE: Reus VI et al. Am J Psychiatry. 2018;175:86-90.
Naltrexone or acamprosate should be offered as first-line pharmacologic therapy to patients with moderate to severe alcohol use disorder (AUD) who do not respond to nonpharmacologic therapy alone, according to a practice guideline published by the American Psychiatric Association.
if naltrexone and acamprosate are not effective or well tolerated, the report said.
The APA guideline recommends against the use of antidepressants and benzodiazepines for patients with alcohol use disorder, except for situations where a co-occurring disorder requires treatment. In addition, the guideline recommends against the use of acamprosate in patients with renal impairment, and specifies that naltrexone should not be used by patients with acute hepatitis, hepatic failure, or opioid dependence.
“Naltrexone and acamprosate have the best available evidence as pharmacotherapy for patients with AUD,” wrote Victor I. Reus, MD, and his coauthors in the APA Guideline Writing Group, which formed the guideline using a systematic review of current literature in accordance with Institute of Medicine (now called the National Academy of Medicine) and Agency for Healthcare Research and Quality standards.
Naltrexone, an opioid receptor antagonist, is effective in treating both AUD and opioid use disorder. Studies have shown that it may decrease cravings, and is associated with fewer drinking days and a reduced likelihood of return to drinking, the authors reported. In patients with a history of renal impairment, serum creatinine should be measured, and results should be reviewed before initiating treatment with acamprosate – a synthetic amino acid.
Disulfiram breaks down acetaldehyde, an ethanol byproduct, and should be used only to treat patients with a goal of abstinence. It is not recommended as a first-line therapy because of the side effects of concurrent alcohol use, including tachycardia, flushing, headache, nausea, and vomiting, reported Dr. Reus of the psychiatry department at the University of California, San Francisco, and his coauthors.
“Patients should be fully informed of the physiological consequences of consuming alcohol while taking disulfiram and should agree to taking the medication,” the authors wrote. “They should be instructed to abstain from drinking alcohol for at least 12 hours before taking a dose of the medication and be advised that reactions with alcohol can occur up to 14 days after taking disulfiram.”
Lastly, topiramate and gabapentin may be used in patients for whom naltrexone and acamprosate are ineffective, though topiramate may have side effects of concern to the patient, including cognitive dysfunction and numbness, tingling, paresthesias, dizziness, taste abnormalities, and decreased appetite or weight loss, the report said.
Although the APA guideline acknowledges the importance of psychiatric evaluation and nonpharmacologic treatments such as cognitive-behavioral therapy and 12-step programs, it does not provide recommendations on those treatment options.
Further research on alcohol use disorder should include study of quantitative measures for longitudinal monitoring, co-occurring medical and psychiatric conditions, and the effectiveness of naltrexone versus combination therapy for patients with both AUD and opioid use disorder, the authors said.
“The overall goal of this guideline is to enhance the treatment of AUD for millions of affected individuals, thereby reducing the significant psychosocial and public health consequences of this important psychiatric condition,” the report concluded. An executive summary of the guideline was published in the American Journal of Psychiatry.
The guideline authors disclosed no conflicts of interest with their work on the guideline.
SOURCE: Reus VI et al. Am J Psychiatry. 2018;175:86-90.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
Bright Futures 4th Edition gets a clinical refresher
CHICAGO – Bracing his audience for a whirlwind tour of the many updates to the fourth edition of Bright Futures, Joseph F. Hagan Jr., MD, said that it’s still completely possible to fit Bright Futures visits into a clinic day.
“I practice primary care pediatrics,” said Dr. Hagan, a pediatrician in private practice and clinical professor of pediatrics at the University of Vermont, both in Burlington. “I said to my Bright Futures colleagues, if I didn’t think I could do this in 18 minutes, I wouldn’t ask you to do it.”
The Bright Futures framework, described by Dr. Hagan as the health prevention and disease prevention component of the medical home for children and youth, emerges in the Fourth Edition with a significant evidence-based refresher. The changes and updates are built within the existing framework and encompass surveillance and screening recommendations as well as anticipatory guidance. All content, including family handouts, has been updated, said Dr. Hagan, a coeditor of the Fourth Edition of Bright Futures. He spoke at the annual meeting of the American Academy of Pediatrics.
New clinical content
“What’s new? Maternal depression screening is new,” said Dr. Hagan, noting that the recommendation has long been under discussion. Now, supported by a 2016 United States Preventative Task Force (USPSTF) recommendation that carries a grade B level of evidence, all mothers should be screened for depression at the 1-, 2-, 4-, and 6-month Bright Futures visits.
However, he said, know your local regulations. “State mandates to do more might overrule this.” And conversely, “Just because we’re doing it universally until 6 months doesn’t mean you couldn’t selectively screen later if you have concerns.”
Safe sleep is another area with new clinical focus, he said. The new recommendation for the child to sleep in the parent’s room for “at least 6 months” draws on data from European studies showing lower mortality for children who share a room with parents during this period.
Clinicians should continue to recommend that parents not sleep with their infants in couches, chairs, or beds. As before, parents should be told not to have loose blankets, stuffed toys, or crib bumpers in their babies’ cribs. Another key message, said Dr. Hagan, is that “There is no such thing as safe ‘breast-sleeping.’ ”
Parents should be reminded not to swaddle at nap – or bedtime. The risk is that even a 2-month-old infant may be capable of wriggling over from back to front, and a swaddled infant whose hands are trapped may not be able to move to protect her airway once prone. “Swaddle for comfort, swaddle for crying, swaddle for nursing, but don’t swaddle for sleep” is the message, said Dr. Hagan.
For breast-fed babies, iron supplementation should begin at the 4-month visit. The notion is to prevent progression from iron deficiency to frank anemia, said Dr. Hagan. “We know that we screen for iron deficiency anemia … but we also know that before you’re iron deficient anemic, you’re iron deficient,” he said, and iron’s also critical to brain development. For convenience, switching from vitamin D alone to a multivitamin drop with iron at 4 months is a practical choice.
New dental health recommendations bring prevention to the pediatrician’s office. “Fluoride varnish? Do it!” said Dr. Hagan. Although the USPSTF made a 2014 grade B recommendation that primary care clinicians apply fluoride varnish to primary teeth as soon as they erupt, “It’s new to the Bright Futures periodicity schedule,” he said; parents can be assured that fluoride varnish does not cause fluorosis.
The good news for clinicians, he noted. “Once it hits the periodicity schedule, now, it’s a billable service that must be paid” under Affordable Care Act regulations, said Dr. Hagan. “Don’t let your insurer say, ‘That’s part of what you’re already being paid for.’ ” He recommends avoiding the pressure to bundle this important service. Use the discrete CPT code 99188, “Application of a fluoride varnish by a physician or other qualified health care professional.”
Although Bright Futures has updated recommendations for dyslipidemia blood screening, the USPSTF found insufficient evidence to back lipid screening for those younger than 20 years of age, citing an inability to assess the balance of benefits and harms for universal, rather than risk-based, screening. However, said Dr. Hagan, the American Academy of Pediatrics (AAP), and the National Heart, Lung, and Blood Institute (NHLBI) were looking at this issue at about the same time, and they “did a really good job of showing their work,” to show that if family history alone guided screening in the pediatric population, it “just wasn’t getting done.” And AAP and NHLBI did demonstrate evidence sufficient to support this recommendation.
Accordingly, Bright Futures recommends one screening between ages 9 and 11 years and an additional screening between ages 17 and 21. These windows are designed to bracket puberty, said Dr. Hagan, because values can be skewed during that period. “It’s billable, it’s not bundle-able, and I’d recommend that you do it,” he said.
Developmental surveillance and screening
What’s new with developmental surveillance and screening? “Well, we could argue that the milestones are something to think about, because the milestones are the cornerstone of developmental surveillance,” said Dr. Hagan. “You’re in the room with the child. You’re trained, you’re experienced, you’re smart, your gestalt tells you if their development is good or bad.”
As important as surveillance is, though, he said, it is “nowhere near as important as screening.” Surveillance happens at every well-child visit, but there’s no substitute for formal developmental screening. For the Fourth Edition guidance and toolkit, gross motor milestones have been adjusted to reflect what’s really being seen as more parents adopt the Back to Sleep recommendations as well.
A standardized developmental screening tool is used at the 9-, 18-, and 30-month visits, and when parents or caregivers express concern about development. Autism-specific screening happens at 18 and 24 months.
“Remember this, if you remember nothing else: If the screening is positive, and you believe there’s a problem, you’re going to refer,” not just to the appropriate specialist but also for early intervention services, so time isn’t lost as the child is waiting for further evaluation and a formal diagnosis, said Dr. Hagan. This coordinated effort appropriately places the responsibility for early identification of developmental delays and disorders at the doorstep of the child’s medical home.
The federally-coordinated Birth to 5: Watch Me Thrive! effort has aggregated research-based screening tools, users’ guides targeted at a variety of audiences, and resources to help caregivers, said Dr. Hagan.
Four commonly-used tools to consider using during the visit are the Parents’ Evaluation of Developmental Status, the Ages and Stages Questionnaire, the Child Health and Development Interactive System, and the Survey of Wellbeing of Young Children. Of these, said Dr. Hagan, the latter is the only tool that’s in the public domain. However, he said, they are “all really good.”
Consider having parents fill out screening questionnaires in the waiting room before the visit, said Dr. Hagan. “I always tell my colleagues, ‘Have them start the visit without you, if you want to get it done in 18 minutes.’ ”
Two questionnaires per visit are available in the Bright Futures toolkit. One questionnaire asks developmental surveillance and risk assessment questions for selective screening. The second questionnaire asks prescreening questions to help with the anticipatory guidance part of the visit, he said. Having families do these ahead of time, said Dr. Hagan, “allows you to become more focused.”
Paying attention to practicalities can make all this go more smoothly, and maximize reimbursement as well. In his own practice, Dr. Hagan said, screening tools and questionnaires are integrated into the EHR system, so that appropriate paperwork prints automatically ahead of the visit.
It’s also worth reviewing billing practices to make sure that CPT code 96110 is used when administering screening with a standardized instrument and completing scoring and documentation. According to the Bright Futures periodicity schedule, this may be done at the 9-, 18-, and 30-month visits for developmental screening, as well as at 18 and 24 months for autism-specific screening.
Promoting lifelong health
Since the initial Bright Futures guidelines were published in the late 1990s, said Dr. Hagan, the focus has always been on seeing the child as part of the family, who, in turn, are part of the community, forming a framework that addresses the social components of child health. “If you’re not looking at the whole picture, you’re not promoting health,” he said. “It’s no big surprise that we now have a specific, called-out focus on promoting lifelong health.”
In the Fourth Edition, the theme of promoting lifelong health for families and communities is woven throughout, with social determinants of health being a specific visit priority. For example, questions about food insecurity have been drawn from the published literature and are included. Also, said Dr. Hagan, there’s specific anticipatory guidance content that’s clearly marked as addressing social determinants of health.
The fundamental importance of socioeconomic status as a social determinant of health was brought home by the Robert Wood Johnson Foundation’s Commission to Build a Healthier America, which demonstrated that, “Your ZIP code is more important to your health than your genetic code,” said Dr. Hagan. “So your work in health supervision is important, and you have been leaders in this effort.”
Research guides Bright Futures updates
The fourth edition of Bright Futures builds on health promotion themes to support the mental and physical health of children and adolescents, and has a robust framework of evidence underpinning the guidelines, said Dr. Hagan.
The goal is for clinicians to “use evidence to decide upon content of their own health supervision visits,” he explained.
The chapter of the Bright Futures guidelines that addresses the evidence and rationale for the guidelines has been expanded to better answer two questions, said Dr. Hagan: “What evidence grounds our recommendations?” and “What rationale did we use when evidence was insufficient or lacking?”
When possible, the editors of the guidelines used evidence-based sources such as recommendations from the USPSTF, the Centers for Disease Control Community Guide, and the Cochrane Collaboration.
There were many more evidence-based recommendations available to those working on the 4th edition than there had been when writing the previous edition, when, said Dr. Hagan, the USPSTF had exactly two recommendations for those under the age of 21 years. The current expanded number of USPSTF pediatric recommendations was due in part to the attention the AAP was able to bring regarding the need for evidence-based recommendations in pediatrics, he said.
When guidelines were not available, the editors also turned to high quality studies from peer reviewed publications. When such high quality evidence was lacking in a particular area, the guidelines make clear what rationale was used to formulate a given recommendation, and that some recommendations should be interpreted with a degree of caution.
And, said Dr. Hagan, even science-based guidelines will change as more data accumulates. “Don’t forget about peanuts!” he said. “It was really logical 15 years ago when we said don’t give peanut products until 1 year of age. And about 2 years ago, we found out that it really didn’t work.”
Although there are specific updates to clinical content, there also were changes made in broader strokes throughout the 4th edition. One of these shifts embeds social determinants of health in many visits. This adjustment acknowledges the growing body of knowledge that “strengths and protective factors make a difference, and risk factors make a difference” in pediatric outcomes.
A greater focus on lifelong physical and mental health is included under the general rubric of promoting lifelong health for families and communities. More emphasis is placed on promoting health for children and youth who have special health care needs as well.
Nuts-and-bolts changes in the updated 4th edition include updates for milestones of development and accompanying developmental surveillance questions, new clinical content and guidance for implementation that have been added based on strong evidence, and a variety of updates for adolescent screenings in particular.
The full 4th edition Bright Futures toolkit will be available for use in 2018.
Dr. Hagan was a coeditor of the Fourth Edition of Bright Futures.
*This article was updated on December 21, 2017
CHICAGO – Bracing his audience for a whirlwind tour of the many updates to the fourth edition of Bright Futures, Joseph F. Hagan Jr., MD, said that it’s still completely possible to fit Bright Futures visits into a clinic day.
“I practice primary care pediatrics,” said Dr. Hagan, a pediatrician in private practice and clinical professor of pediatrics at the University of Vermont, both in Burlington. “I said to my Bright Futures colleagues, if I didn’t think I could do this in 18 minutes, I wouldn’t ask you to do it.”
The Bright Futures framework, described by Dr. Hagan as the health prevention and disease prevention component of the medical home for children and youth, emerges in the Fourth Edition with a significant evidence-based refresher. The changes and updates are built within the existing framework and encompass surveillance and screening recommendations as well as anticipatory guidance. All content, including family handouts, has been updated, said Dr. Hagan, a coeditor of the Fourth Edition of Bright Futures. He spoke at the annual meeting of the American Academy of Pediatrics.
New clinical content
“What’s new? Maternal depression screening is new,” said Dr. Hagan, noting that the recommendation has long been under discussion. Now, supported by a 2016 United States Preventative Task Force (USPSTF) recommendation that carries a grade B level of evidence, all mothers should be screened for depression at the 1-, 2-, 4-, and 6-month Bright Futures visits.
However, he said, know your local regulations. “State mandates to do more might overrule this.” And conversely, “Just because we’re doing it universally until 6 months doesn’t mean you couldn’t selectively screen later if you have concerns.”
Safe sleep is another area with new clinical focus, he said. The new recommendation for the child to sleep in the parent’s room for “at least 6 months” draws on data from European studies showing lower mortality for children who share a room with parents during this period.
Clinicians should continue to recommend that parents not sleep with their infants in couches, chairs, or beds. As before, parents should be told not to have loose blankets, stuffed toys, or crib bumpers in their babies’ cribs. Another key message, said Dr. Hagan, is that “There is no such thing as safe ‘breast-sleeping.’ ”
Parents should be reminded not to swaddle at nap – or bedtime. The risk is that even a 2-month-old infant may be capable of wriggling over from back to front, and a swaddled infant whose hands are trapped may not be able to move to protect her airway once prone. “Swaddle for comfort, swaddle for crying, swaddle for nursing, but don’t swaddle for sleep” is the message, said Dr. Hagan.
For breast-fed babies, iron supplementation should begin at the 4-month visit. The notion is to prevent progression from iron deficiency to frank anemia, said Dr. Hagan. “We know that we screen for iron deficiency anemia … but we also know that before you’re iron deficient anemic, you’re iron deficient,” he said, and iron’s also critical to brain development. For convenience, switching from vitamin D alone to a multivitamin drop with iron at 4 months is a practical choice.
New dental health recommendations bring prevention to the pediatrician’s office. “Fluoride varnish? Do it!” said Dr. Hagan. Although the USPSTF made a 2014 grade B recommendation that primary care clinicians apply fluoride varnish to primary teeth as soon as they erupt, “It’s new to the Bright Futures periodicity schedule,” he said; parents can be assured that fluoride varnish does not cause fluorosis.
The good news for clinicians, he noted. “Once it hits the periodicity schedule, now, it’s a billable service that must be paid” under Affordable Care Act regulations, said Dr. Hagan. “Don’t let your insurer say, ‘That’s part of what you’re already being paid for.’ ” He recommends avoiding the pressure to bundle this important service. Use the discrete CPT code 99188, “Application of a fluoride varnish by a physician or other qualified health care professional.”
Although Bright Futures has updated recommendations for dyslipidemia blood screening, the USPSTF found insufficient evidence to back lipid screening for those younger than 20 years of age, citing an inability to assess the balance of benefits and harms for universal, rather than risk-based, screening. However, said Dr. Hagan, the American Academy of Pediatrics (AAP), and the National Heart, Lung, and Blood Institute (NHLBI) were looking at this issue at about the same time, and they “did a really good job of showing their work,” to show that if family history alone guided screening in the pediatric population, it “just wasn’t getting done.” And AAP and NHLBI did demonstrate evidence sufficient to support this recommendation.
Accordingly, Bright Futures recommends one screening between ages 9 and 11 years and an additional screening between ages 17 and 21. These windows are designed to bracket puberty, said Dr. Hagan, because values can be skewed during that period. “It’s billable, it’s not bundle-able, and I’d recommend that you do it,” he said.
Developmental surveillance and screening
What’s new with developmental surveillance and screening? “Well, we could argue that the milestones are something to think about, because the milestones are the cornerstone of developmental surveillance,” said Dr. Hagan. “You’re in the room with the child. You’re trained, you’re experienced, you’re smart, your gestalt tells you if their development is good or bad.”
As important as surveillance is, though, he said, it is “nowhere near as important as screening.” Surveillance happens at every well-child visit, but there’s no substitute for formal developmental screening. For the Fourth Edition guidance and toolkit, gross motor milestones have been adjusted to reflect what’s really being seen as more parents adopt the Back to Sleep recommendations as well.
A standardized developmental screening tool is used at the 9-, 18-, and 30-month visits, and when parents or caregivers express concern about development. Autism-specific screening happens at 18 and 24 months.
“Remember this, if you remember nothing else: If the screening is positive, and you believe there’s a problem, you’re going to refer,” not just to the appropriate specialist but also for early intervention services, so time isn’t lost as the child is waiting for further evaluation and a formal diagnosis, said Dr. Hagan. This coordinated effort appropriately places the responsibility for early identification of developmental delays and disorders at the doorstep of the child’s medical home.
The federally-coordinated Birth to 5: Watch Me Thrive! effort has aggregated research-based screening tools, users’ guides targeted at a variety of audiences, and resources to help caregivers, said Dr. Hagan.
Four commonly-used tools to consider using during the visit are the Parents’ Evaluation of Developmental Status, the Ages and Stages Questionnaire, the Child Health and Development Interactive System, and the Survey of Wellbeing of Young Children. Of these, said Dr. Hagan, the latter is the only tool that’s in the public domain. However, he said, they are “all really good.”
Consider having parents fill out screening questionnaires in the waiting room before the visit, said Dr. Hagan. “I always tell my colleagues, ‘Have them start the visit without you, if you want to get it done in 18 minutes.’ ”
Two questionnaires per visit are available in the Bright Futures toolkit. One questionnaire asks developmental surveillance and risk assessment questions for selective screening. The second questionnaire asks prescreening questions to help with the anticipatory guidance part of the visit, he said. Having families do these ahead of time, said Dr. Hagan, “allows you to become more focused.”
Paying attention to practicalities can make all this go more smoothly, and maximize reimbursement as well. In his own practice, Dr. Hagan said, screening tools and questionnaires are integrated into the EHR system, so that appropriate paperwork prints automatically ahead of the visit.
It’s also worth reviewing billing practices to make sure that CPT code 96110 is used when administering screening with a standardized instrument and completing scoring and documentation. According to the Bright Futures periodicity schedule, this may be done at the 9-, 18-, and 30-month visits for developmental screening, as well as at 18 and 24 months for autism-specific screening.
Promoting lifelong health
Since the initial Bright Futures guidelines were published in the late 1990s, said Dr. Hagan, the focus has always been on seeing the child as part of the family, who, in turn, are part of the community, forming a framework that addresses the social components of child health. “If you’re not looking at the whole picture, you’re not promoting health,” he said. “It’s no big surprise that we now have a specific, called-out focus on promoting lifelong health.”
In the Fourth Edition, the theme of promoting lifelong health for families and communities is woven throughout, with social determinants of health being a specific visit priority. For example, questions about food insecurity have been drawn from the published literature and are included. Also, said Dr. Hagan, there’s specific anticipatory guidance content that’s clearly marked as addressing social determinants of health.
The fundamental importance of socioeconomic status as a social determinant of health was brought home by the Robert Wood Johnson Foundation’s Commission to Build a Healthier America, which demonstrated that, “Your ZIP code is more important to your health than your genetic code,” said Dr. Hagan. “So your work in health supervision is important, and you have been leaders in this effort.”
Research guides Bright Futures updates
The fourth edition of Bright Futures builds on health promotion themes to support the mental and physical health of children and adolescents, and has a robust framework of evidence underpinning the guidelines, said Dr. Hagan.
The goal is for clinicians to “use evidence to decide upon content of their own health supervision visits,” he explained.
The chapter of the Bright Futures guidelines that addresses the evidence and rationale for the guidelines has been expanded to better answer two questions, said Dr. Hagan: “What evidence grounds our recommendations?” and “What rationale did we use when evidence was insufficient or lacking?”
When possible, the editors of the guidelines used evidence-based sources such as recommendations from the USPSTF, the Centers for Disease Control Community Guide, and the Cochrane Collaboration.
There were many more evidence-based recommendations available to those working on the 4th edition than there had been when writing the previous edition, when, said Dr. Hagan, the USPSTF had exactly two recommendations for those under the age of 21 years. The current expanded number of USPSTF pediatric recommendations was due in part to the attention the AAP was able to bring regarding the need for evidence-based recommendations in pediatrics, he said.
When guidelines were not available, the editors also turned to high quality studies from peer reviewed publications. When such high quality evidence was lacking in a particular area, the guidelines make clear what rationale was used to formulate a given recommendation, and that some recommendations should be interpreted with a degree of caution.
And, said Dr. Hagan, even science-based guidelines will change as more data accumulates. “Don’t forget about peanuts!” he said. “It was really logical 15 years ago when we said don’t give peanut products until 1 year of age. And about 2 years ago, we found out that it really didn’t work.”
Although there are specific updates to clinical content, there also were changes made in broader strokes throughout the 4th edition. One of these shifts embeds social determinants of health in many visits. This adjustment acknowledges the growing body of knowledge that “strengths and protective factors make a difference, and risk factors make a difference” in pediatric outcomes.
A greater focus on lifelong physical and mental health is included under the general rubric of promoting lifelong health for families and communities. More emphasis is placed on promoting health for children and youth who have special health care needs as well.
Nuts-and-bolts changes in the updated 4th edition include updates for milestones of development and accompanying developmental surveillance questions, new clinical content and guidance for implementation that have been added based on strong evidence, and a variety of updates for adolescent screenings in particular.
The full 4th edition Bright Futures toolkit will be available for use in 2018.
Dr. Hagan was a coeditor of the Fourth Edition of Bright Futures.
*This article was updated on December 21, 2017
CHICAGO – Bracing his audience for a whirlwind tour of the many updates to the fourth edition of Bright Futures, Joseph F. Hagan Jr., MD, said that it’s still completely possible to fit Bright Futures visits into a clinic day.
“I practice primary care pediatrics,” said Dr. Hagan, a pediatrician in private practice and clinical professor of pediatrics at the University of Vermont, both in Burlington. “I said to my Bright Futures colleagues, if I didn’t think I could do this in 18 minutes, I wouldn’t ask you to do it.”
The Bright Futures framework, described by Dr. Hagan as the health prevention and disease prevention component of the medical home for children and youth, emerges in the Fourth Edition with a significant evidence-based refresher. The changes and updates are built within the existing framework and encompass surveillance and screening recommendations as well as anticipatory guidance. All content, including family handouts, has been updated, said Dr. Hagan, a coeditor of the Fourth Edition of Bright Futures. He spoke at the annual meeting of the American Academy of Pediatrics.
New clinical content
“What’s new? Maternal depression screening is new,” said Dr. Hagan, noting that the recommendation has long been under discussion. Now, supported by a 2016 United States Preventative Task Force (USPSTF) recommendation that carries a grade B level of evidence, all mothers should be screened for depression at the 1-, 2-, 4-, and 6-month Bright Futures visits.
However, he said, know your local regulations. “State mandates to do more might overrule this.” And conversely, “Just because we’re doing it universally until 6 months doesn’t mean you couldn’t selectively screen later if you have concerns.”
Safe sleep is another area with new clinical focus, he said. The new recommendation for the child to sleep in the parent’s room for “at least 6 months” draws on data from European studies showing lower mortality for children who share a room with parents during this period.
Clinicians should continue to recommend that parents not sleep with their infants in couches, chairs, or beds. As before, parents should be told not to have loose blankets, stuffed toys, or crib bumpers in their babies’ cribs. Another key message, said Dr. Hagan, is that “There is no such thing as safe ‘breast-sleeping.’ ”
Parents should be reminded not to swaddle at nap – or bedtime. The risk is that even a 2-month-old infant may be capable of wriggling over from back to front, and a swaddled infant whose hands are trapped may not be able to move to protect her airway once prone. “Swaddle for comfort, swaddle for crying, swaddle for nursing, but don’t swaddle for sleep” is the message, said Dr. Hagan.
For breast-fed babies, iron supplementation should begin at the 4-month visit. The notion is to prevent progression from iron deficiency to frank anemia, said Dr. Hagan. “We know that we screen for iron deficiency anemia … but we also know that before you’re iron deficient anemic, you’re iron deficient,” he said, and iron’s also critical to brain development. For convenience, switching from vitamin D alone to a multivitamin drop with iron at 4 months is a practical choice.
New dental health recommendations bring prevention to the pediatrician’s office. “Fluoride varnish? Do it!” said Dr. Hagan. Although the USPSTF made a 2014 grade B recommendation that primary care clinicians apply fluoride varnish to primary teeth as soon as they erupt, “It’s new to the Bright Futures periodicity schedule,” he said; parents can be assured that fluoride varnish does not cause fluorosis.
The good news for clinicians, he noted. “Once it hits the periodicity schedule, now, it’s a billable service that must be paid” under Affordable Care Act regulations, said Dr. Hagan. “Don’t let your insurer say, ‘That’s part of what you’re already being paid for.’ ” He recommends avoiding the pressure to bundle this important service. Use the discrete CPT code 99188, “Application of a fluoride varnish by a physician or other qualified health care professional.”
Although Bright Futures has updated recommendations for dyslipidemia blood screening, the USPSTF found insufficient evidence to back lipid screening for those younger than 20 years of age, citing an inability to assess the balance of benefits and harms for universal, rather than risk-based, screening. However, said Dr. Hagan, the American Academy of Pediatrics (AAP), and the National Heart, Lung, and Blood Institute (NHLBI) were looking at this issue at about the same time, and they “did a really good job of showing their work,” to show that if family history alone guided screening in the pediatric population, it “just wasn’t getting done.” And AAP and NHLBI did demonstrate evidence sufficient to support this recommendation.
Accordingly, Bright Futures recommends one screening between ages 9 and 11 years and an additional screening between ages 17 and 21. These windows are designed to bracket puberty, said Dr. Hagan, because values can be skewed during that period. “It’s billable, it’s not bundle-able, and I’d recommend that you do it,” he said.
Developmental surveillance and screening
What’s new with developmental surveillance and screening? “Well, we could argue that the milestones are something to think about, because the milestones are the cornerstone of developmental surveillance,” said Dr. Hagan. “You’re in the room with the child. You’re trained, you’re experienced, you’re smart, your gestalt tells you if their development is good or bad.”
As important as surveillance is, though, he said, it is “nowhere near as important as screening.” Surveillance happens at every well-child visit, but there’s no substitute for formal developmental screening. For the Fourth Edition guidance and toolkit, gross motor milestones have been adjusted to reflect what’s really being seen as more parents adopt the Back to Sleep recommendations as well.
A standardized developmental screening tool is used at the 9-, 18-, and 30-month visits, and when parents or caregivers express concern about development. Autism-specific screening happens at 18 and 24 months.
“Remember this, if you remember nothing else: If the screening is positive, and you believe there’s a problem, you’re going to refer,” not just to the appropriate specialist but also for early intervention services, so time isn’t lost as the child is waiting for further evaluation and a formal diagnosis, said Dr. Hagan. This coordinated effort appropriately places the responsibility for early identification of developmental delays and disorders at the doorstep of the child’s medical home.
The federally-coordinated Birth to 5: Watch Me Thrive! effort has aggregated research-based screening tools, users’ guides targeted at a variety of audiences, and resources to help caregivers, said Dr. Hagan.
Four commonly-used tools to consider using during the visit are the Parents’ Evaluation of Developmental Status, the Ages and Stages Questionnaire, the Child Health and Development Interactive System, and the Survey of Wellbeing of Young Children. Of these, said Dr. Hagan, the latter is the only tool that’s in the public domain. However, he said, they are “all really good.”
Consider having parents fill out screening questionnaires in the waiting room before the visit, said Dr. Hagan. “I always tell my colleagues, ‘Have them start the visit without you, if you want to get it done in 18 minutes.’ ”
Two questionnaires per visit are available in the Bright Futures toolkit. One questionnaire asks developmental surveillance and risk assessment questions for selective screening. The second questionnaire asks prescreening questions to help with the anticipatory guidance part of the visit, he said. Having families do these ahead of time, said Dr. Hagan, “allows you to become more focused.”
Paying attention to practicalities can make all this go more smoothly, and maximize reimbursement as well. In his own practice, Dr. Hagan said, screening tools and questionnaires are integrated into the EHR system, so that appropriate paperwork prints automatically ahead of the visit.
It’s also worth reviewing billing practices to make sure that CPT code 96110 is used when administering screening with a standardized instrument and completing scoring and documentation. According to the Bright Futures periodicity schedule, this may be done at the 9-, 18-, and 30-month visits for developmental screening, as well as at 18 and 24 months for autism-specific screening.
Promoting lifelong health
Since the initial Bright Futures guidelines were published in the late 1990s, said Dr. Hagan, the focus has always been on seeing the child as part of the family, who, in turn, are part of the community, forming a framework that addresses the social components of child health. “If you’re not looking at the whole picture, you’re not promoting health,” he said. “It’s no big surprise that we now have a specific, called-out focus on promoting lifelong health.”
In the Fourth Edition, the theme of promoting lifelong health for families and communities is woven throughout, with social determinants of health being a specific visit priority. For example, questions about food insecurity have been drawn from the published literature and are included. Also, said Dr. Hagan, there’s specific anticipatory guidance content that’s clearly marked as addressing social determinants of health.
The fundamental importance of socioeconomic status as a social determinant of health was brought home by the Robert Wood Johnson Foundation’s Commission to Build a Healthier America, which demonstrated that, “Your ZIP code is more important to your health than your genetic code,” said Dr. Hagan. “So your work in health supervision is important, and you have been leaders in this effort.”
Research guides Bright Futures updates
The fourth edition of Bright Futures builds on health promotion themes to support the mental and physical health of children and adolescents, and has a robust framework of evidence underpinning the guidelines, said Dr. Hagan.
The goal is for clinicians to “use evidence to decide upon content of their own health supervision visits,” he explained.
The chapter of the Bright Futures guidelines that addresses the evidence and rationale for the guidelines has been expanded to better answer two questions, said Dr. Hagan: “What evidence grounds our recommendations?” and “What rationale did we use when evidence was insufficient or lacking?”
When possible, the editors of the guidelines used evidence-based sources such as recommendations from the USPSTF, the Centers for Disease Control Community Guide, and the Cochrane Collaboration.
There were many more evidence-based recommendations available to those working on the 4th edition than there had been when writing the previous edition, when, said Dr. Hagan, the USPSTF had exactly two recommendations for those under the age of 21 years. The current expanded number of USPSTF pediatric recommendations was due in part to the attention the AAP was able to bring regarding the need for evidence-based recommendations in pediatrics, he said.
When guidelines were not available, the editors also turned to high quality studies from peer reviewed publications. When such high quality evidence was lacking in a particular area, the guidelines make clear what rationale was used to formulate a given recommendation, and that some recommendations should be interpreted with a degree of caution.
And, said Dr. Hagan, even science-based guidelines will change as more data accumulates. “Don’t forget about peanuts!” he said. “It was really logical 15 years ago when we said don’t give peanut products until 1 year of age. And about 2 years ago, we found out that it really didn’t work.”
Although there are specific updates to clinical content, there also were changes made in broader strokes throughout the 4th edition. One of these shifts embeds social determinants of health in many visits. This adjustment acknowledges the growing body of knowledge that “strengths and protective factors make a difference, and risk factors make a difference” in pediatric outcomes.
A greater focus on lifelong physical and mental health is included under the general rubric of promoting lifelong health for families and communities. More emphasis is placed on promoting health for children and youth who have special health care needs as well.
Nuts-and-bolts changes in the updated 4th edition include updates for milestones of development and accompanying developmental surveillance questions, new clinical content and guidance for implementation that have been added based on strong evidence, and a variety of updates for adolescent screenings in particular.
The full 4th edition Bright Futures toolkit will be available for use in 2018.
Dr. Hagan was a coeditor of the Fourth Edition of Bright Futures.
*This article was updated on December 21, 2017
EXPERT ANALYSIS FROM AAP 2017
ADA guidelines embrace heart health
Recent studies that confirm the cardiovascular benefit of some antihyperglycemic agents are shaping the newest therapeutic recommendations for patients with type 2 diabetes and comorbid atherosclerotic cardiovascular disease (ASCVD).
Treatment for these patients – as all with diabetes – should start with lifestyle modifications and metformin. But in its new position statement, the American Diabetes Association now recommends that clinicians consider adding agents proved to reduce major cardiovascular events and cardiovascular death – such as the sodium glucose cotransporter-2 (SGLT2) inhibitor empagliflozin or the glucagon-like peptide 1 (GLP-1) agonist liraglutide – to the regimens of patients with diabetes and ASCVD (Diabetes Care 2018;41(Suppl. 1):S86-S104. doi: 10.2337/dc18-S009).
The medications are indicated if, after being treated with lifestyle and metformin therapy, the patient isn’t meeting hemoglobin A1c goals, said Rita R. Kalyani, MD, who led the ADA’s 12-member writing committee. But clinicians may also consider adding these agents for cardiovascular benefit alone, even when glucose control is adequate on a regimen of lifestyle modification and metformin, with dose adjustments as appropriate, she said in an interview.
“A1c remains the main target of sequencing antihyperglycemic therapies, if it’s not reached after 3 months,” said Dr. Kalyani of Johns Hopkins University, Baltimore. “But, it could also be that the provider, after consulting with the patient, feels it’s appropriate to add one of these agents solely for cardioprotective benefit in patients with ASCVD.”
The recommendation to incorporate agents with cardiovascular benefit is related directly to data from two trials, LEADER and EMPA-REG, which support this recommendation. All of these cardiovascular outcome trials included a majority of patients who were already on metformin. “We developed these evidence-based recommendations based on these trials and to appropriately reflect the populations studied,” said Dr. Kalyani.
The 2018 update contains a number of new recommendations; more will be added as new data emerge, since the ADA intends it to be a continuously refreshed “living document.” This makes it especially clinically useful, Paul S. Jellinger, MD, said in an interview. A member of the writing committee of the American Association of Clinical Endocrinologists’ diabetes management guidelines, Dr. Jellinger feels ADA’s previous versions have not been as targeted as this new one and, he hopes, its subsequent iterations.
“This is a nice enhancement of previously published guidelines for diabetes therapy,” said Dr. Jellinger, professor of clinical medicine at the University of Miami. “For the first time, ADA is providing some guidance in terms of which agents to use. It’s definitely more prescriptive than it was in the past, when, unlike the AACE Diabetes Guidelines, it was a palette of choice for clinicians, but with very little guidance about which agent to pick. The guidance for patients with cardiovascular disease in particular is big news because these antihyperglycemic agents showed such a significant cardiovascular benefit in the trials.”
While the document gives a detailed algorithm of advancing therapy in patients with ASCVD, it doesn’t specify a preference for a specific drug class after metformin therapy in patients without ASCVD. Instead, it provides a detailed table listing the drug-specific effects and patient factors to consider when selecting from different classes of antihyperglycemic agents ( SGLT2 inhibitors, GLP-1 agonists, DPP-4 inhibitors, thiazolidinones, sulfonylureas, and insulins). The table notes the drugs’ general efficacy in diabetes, and their impact on hypoglycemia, weight gain, and cardiovascular and renal health. The table also includes the Food and Drug Administration black box warnings that are on some of these medications.
Another helpful feature is a cost comparison of antidiabetic agents, Dr. Kalyani noted. “Last year we added comprehensive cost tables for all the different insulins and noninsulins, and this year we added a second data set of cost information, to assist the provider when prescribing these agents.”
The pricing information is a very important addition to this guideline, and one that clinicians will appreciate, said Richard Hellman, MD, clinical professor of medicine at the University of Missouri–Kansas City.
“In this document, ADA is urging providers of care to ask about whether the cost of their diabetes care is more than they can deal with. They present tables which compare the costs of the current blood glucose lowering agents used in the U.S., and it is plain to see that many patients, without insurance coverage, will find some of the medications unaffordable,” said Dr. Hellman, a past president of AACE. “They also provide data that show half of all patients with diabetes have financial problems,” and he suspects that medication costs are an important component of their financial insecurity.
The document also notes data from the 2017 National Health and Nutrition Examination Survey, which found that 10% of people with diabetes have severe food insecurity and 20% have mild food insecurity (Diabetes Educ. 2017;43:260-71. doi: 10.1177/0145721717699890).
“Another thing the document points out is that two-thirds of the patients who don’t take all their medications due to cost don’t tell their doctor,” Dr. Hellman said. “The ADA is making the point that providers have a responsibility to ask if a patient is not taking certain medications because of the cost. We have so many better tools to manage this disease, but so many of these tools are unaffordable.”
While the treatment algorithm for patients with ASCVD will likely be embraced, another new recommendation may stir the pot a bit, Dr. Hellman noted. The section on cardiovascular disease and risk management sticks to a definition of hypertension as 140/90 mm Hg or higher – a striking diversion from the new 130/80 mm Hg limit set this fall by both the American Heart Association and the American College of Cardiology.
“This difference in recommendations is very important and will be controversial,” Dr. Hellman said, adding that he agrees with this clinical point.
Again, this recommendation is grounded in clinical trials, which suggest that people with diabetes don’t benefit from overly strict blood pressure control. The new AHA/ACC recommendations largely drew on data from the SPRINT trial, which was conducted in an entirely nondiabetic population. “These gave a clear signal that a lower BP target is beneficial to that group,” Dr. Hellman said.
But large well-designed randomized controlled trials of intensive blood pressure lowering in people with diabetes, such as ACCORD-BP, did not demonstrate that intensive blood-pressure lowering targeting a systolic less than120 mm Hg had a significant benefit on the composite primary cardiovascular endpoint. And while the ADVANCE BP trial found that the composite endpoint was improved with intensive blood pressure lowering, the blood pressure level achieved in the intervention group was 136/73 mm Hg.
“This recommendation is based on current evidence for people with diabetes,” Dr. Kalyani said. “We maintain our definition of hypertension as 140/90 mm Hg or higher based on the results of large clinical trials specifically in people with diabetes but emphasize that intensification of antihypertensive therapy to target lower blood pressures (less than 130/80 mm Hg) may be beneficial for high-risk patients with diabetes such as those with cardiovascular disease. We are constantly assessing the evidence and will continue to review the results of new studies for potential incorporation into recommendations in the future.”
Dr. Kalyani and Dr. Hellman had no financial disclosures. Dr. Jellinger has been a speaker for several pharmaceutical companies.
SOURCE: Kalyani R et al. Diabetes Care 2018;41(Suppl. 1):S86-S104 doi: 10.2337/dc18-S009
This article was updated 12/21/17.
Recent studies that confirm the cardiovascular benefit of some antihyperglycemic agents are shaping the newest therapeutic recommendations for patients with type 2 diabetes and comorbid atherosclerotic cardiovascular disease (ASCVD).
Treatment for these patients – as all with diabetes – should start with lifestyle modifications and metformin. But in its new position statement, the American Diabetes Association now recommends that clinicians consider adding agents proved to reduce major cardiovascular events and cardiovascular death – such as the sodium glucose cotransporter-2 (SGLT2) inhibitor empagliflozin or the glucagon-like peptide 1 (GLP-1) agonist liraglutide – to the regimens of patients with diabetes and ASCVD (Diabetes Care 2018;41(Suppl. 1):S86-S104. doi: 10.2337/dc18-S009).
The medications are indicated if, after being treated with lifestyle and metformin therapy, the patient isn’t meeting hemoglobin A1c goals, said Rita R. Kalyani, MD, who led the ADA’s 12-member writing committee. But clinicians may also consider adding these agents for cardiovascular benefit alone, even when glucose control is adequate on a regimen of lifestyle modification and metformin, with dose adjustments as appropriate, she said in an interview.
“A1c remains the main target of sequencing antihyperglycemic therapies, if it’s not reached after 3 months,” said Dr. Kalyani of Johns Hopkins University, Baltimore. “But, it could also be that the provider, after consulting with the patient, feels it’s appropriate to add one of these agents solely for cardioprotective benefit in patients with ASCVD.”
The recommendation to incorporate agents with cardiovascular benefit is related directly to data from two trials, LEADER and EMPA-REG, which support this recommendation. All of these cardiovascular outcome trials included a majority of patients who were already on metformin. “We developed these evidence-based recommendations based on these trials and to appropriately reflect the populations studied,” said Dr. Kalyani.
The 2018 update contains a number of new recommendations; more will be added as new data emerge, since the ADA intends it to be a continuously refreshed “living document.” This makes it especially clinically useful, Paul S. Jellinger, MD, said in an interview. A member of the writing committee of the American Association of Clinical Endocrinologists’ diabetes management guidelines, Dr. Jellinger feels ADA’s previous versions have not been as targeted as this new one and, he hopes, its subsequent iterations.
“This is a nice enhancement of previously published guidelines for diabetes therapy,” said Dr. Jellinger, professor of clinical medicine at the University of Miami. “For the first time, ADA is providing some guidance in terms of which agents to use. It’s definitely more prescriptive than it was in the past, when, unlike the AACE Diabetes Guidelines, it was a palette of choice for clinicians, but with very little guidance about which agent to pick. The guidance for patients with cardiovascular disease in particular is big news because these antihyperglycemic agents showed such a significant cardiovascular benefit in the trials.”
While the document gives a detailed algorithm of advancing therapy in patients with ASCVD, it doesn’t specify a preference for a specific drug class after metformin therapy in patients without ASCVD. Instead, it provides a detailed table listing the drug-specific effects and patient factors to consider when selecting from different classes of antihyperglycemic agents ( SGLT2 inhibitors, GLP-1 agonists, DPP-4 inhibitors, thiazolidinones, sulfonylureas, and insulins). The table notes the drugs’ general efficacy in diabetes, and their impact on hypoglycemia, weight gain, and cardiovascular and renal health. The table also includes the Food and Drug Administration black box warnings that are on some of these medications.
Another helpful feature is a cost comparison of antidiabetic agents, Dr. Kalyani noted. “Last year we added comprehensive cost tables for all the different insulins and noninsulins, and this year we added a second data set of cost information, to assist the provider when prescribing these agents.”
The pricing information is a very important addition to this guideline, and one that clinicians will appreciate, said Richard Hellman, MD, clinical professor of medicine at the University of Missouri–Kansas City.
“In this document, ADA is urging providers of care to ask about whether the cost of their diabetes care is more than they can deal with. They present tables which compare the costs of the current blood glucose lowering agents used in the U.S., and it is plain to see that many patients, without insurance coverage, will find some of the medications unaffordable,” said Dr. Hellman, a past president of AACE. “They also provide data that show half of all patients with diabetes have financial problems,” and he suspects that medication costs are an important component of their financial insecurity.
The document also notes data from the 2017 National Health and Nutrition Examination Survey, which found that 10% of people with diabetes have severe food insecurity and 20% have mild food insecurity (Diabetes Educ. 2017;43:260-71. doi: 10.1177/0145721717699890).
“Another thing the document points out is that two-thirds of the patients who don’t take all their medications due to cost don’t tell their doctor,” Dr. Hellman said. “The ADA is making the point that providers have a responsibility to ask if a patient is not taking certain medications because of the cost. We have so many better tools to manage this disease, but so many of these tools are unaffordable.”
While the treatment algorithm for patients with ASCVD will likely be embraced, another new recommendation may stir the pot a bit, Dr. Hellman noted. The section on cardiovascular disease and risk management sticks to a definition of hypertension as 140/90 mm Hg or higher – a striking diversion from the new 130/80 mm Hg limit set this fall by both the American Heart Association and the American College of Cardiology.
“This difference in recommendations is very important and will be controversial,” Dr. Hellman said, adding that he agrees with this clinical point.
Again, this recommendation is grounded in clinical trials, which suggest that people with diabetes don’t benefit from overly strict blood pressure control. The new AHA/ACC recommendations largely drew on data from the SPRINT trial, which was conducted in an entirely nondiabetic population. “These gave a clear signal that a lower BP target is beneficial to that group,” Dr. Hellman said.
But large well-designed randomized controlled trials of intensive blood pressure lowering in people with diabetes, such as ACCORD-BP, did not demonstrate that intensive blood-pressure lowering targeting a systolic less than120 mm Hg had a significant benefit on the composite primary cardiovascular endpoint. And while the ADVANCE BP trial found that the composite endpoint was improved with intensive blood pressure lowering, the blood pressure level achieved in the intervention group was 136/73 mm Hg.
“This recommendation is based on current evidence for people with diabetes,” Dr. Kalyani said. “We maintain our definition of hypertension as 140/90 mm Hg or higher based on the results of large clinical trials specifically in people with diabetes but emphasize that intensification of antihypertensive therapy to target lower blood pressures (less than 130/80 mm Hg) may be beneficial for high-risk patients with diabetes such as those with cardiovascular disease. We are constantly assessing the evidence and will continue to review the results of new studies for potential incorporation into recommendations in the future.”
Dr. Kalyani and Dr. Hellman had no financial disclosures. Dr. Jellinger has been a speaker for several pharmaceutical companies.
SOURCE: Kalyani R et al. Diabetes Care 2018;41(Suppl. 1):S86-S104 doi: 10.2337/dc18-S009
This article was updated 12/21/17.
Recent studies that confirm the cardiovascular benefit of some antihyperglycemic agents are shaping the newest therapeutic recommendations for patients with type 2 diabetes and comorbid atherosclerotic cardiovascular disease (ASCVD).
Treatment for these patients – as all with diabetes – should start with lifestyle modifications and metformin. But in its new position statement, the American Diabetes Association now recommends that clinicians consider adding agents proved to reduce major cardiovascular events and cardiovascular death – such as the sodium glucose cotransporter-2 (SGLT2) inhibitor empagliflozin or the glucagon-like peptide 1 (GLP-1) agonist liraglutide – to the regimens of patients with diabetes and ASCVD (Diabetes Care 2018;41(Suppl. 1):S86-S104. doi: 10.2337/dc18-S009).
The medications are indicated if, after being treated with lifestyle and metformin therapy, the patient isn’t meeting hemoglobin A1c goals, said Rita R. Kalyani, MD, who led the ADA’s 12-member writing committee. But clinicians may also consider adding these agents for cardiovascular benefit alone, even when glucose control is adequate on a regimen of lifestyle modification and metformin, with dose adjustments as appropriate, she said in an interview.
“A1c remains the main target of sequencing antihyperglycemic therapies, if it’s not reached after 3 months,” said Dr. Kalyani of Johns Hopkins University, Baltimore. “But, it could also be that the provider, after consulting with the patient, feels it’s appropriate to add one of these agents solely for cardioprotective benefit in patients with ASCVD.”
The recommendation to incorporate agents with cardiovascular benefit is related directly to data from two trials, LEADER and EMPA-REG, which support this recommendation. All of these cardiovascular outcome trials included a majority of patients who were already on metformin. “We developed these evidence-based recommendations based on these trials and to appropriately reflect the populations studied,” said Dr. Kalyani.
The 2018 update contains a number of new recommendations; more will be added as new data emerge, since the ADA intends it to be a continuously refreshed “living document.” This makes it especially clinically useful, Paul S. Jellinger, MD, said in an interview. A member of the writing committee of the American Association of Clinical Endocrinologists’ diabetes management guidelines, Dr. Jellinger feels ADA’s previous versions have not been as targeted as this new one and, he hopes, its subsequent iterations.
“This is a nice enhancement of previously published guidelines for diabetes therapy,” said Dr. Jellinger, professor of clinical medicine at the University of Miami. “For the first time, ADA is providing some guidance in terms of which agents to use. It’s definitely more prescriptive than it was in the past, when, unlike the AACE Diabetes Guidelines, it was a palette of choice for clinicians, but with very little guidance about which agent to pick. The guidance for patients with cardiovascular disease in particular is big news because these antihyperglycemic agents showed such a significant cardiovascular benefit in the trials.”
While the document gives a detailed algorithm of advancing therapy in patients with ASCVD, it doesn’t specify a preference for a specific drug class after metformin therapy in patients without ASCVD. Instead, it provides a detailed table listing the drug-specific effects and patient factors to consider when selecting from different classes of antihyperglycemic agents ( SGLT2 inhibitors, GLP-1 agonists, DPP-4 inhibitors, thiazolidinones, sulfonylureas, and insulins). The table notes the drugs’ general efficacy in diabetes, and their impact on hypoglycemia, weight gain, and cardiovascular and renal health. The table also includes the Food and Drug Administration black box warnings that are on some of these medications.
Another helpful feature is a cost comparison of antidiabetic agents, Dr. Kalyani noted. “Last year we added comprehensive cost tables for all the different insulins and noninsulins, and this year we added a second data set of cost information, to assist the provider when prescribing these agents.”
The pricing information is a very important addition to this guideline, and one that clinicians will appreciate, said Richard Hellman, MD, clinical professor of medicine at the University of Missouri–Kansas City.
“In this document, ADA is urging providers of care to ask about whether the cost of their diabetes care is more than they can deal with. They present tables which compare the costs of the current blood glucose lowering agents used in the U.S., and it is plain to see that many patients, without insurance coverage, will find some of the medications unaffordable,” said Dr. Hellman, a past president of AACE. “They also provide data that show half of all patients with diabetes have financial problems,” and he suspects that medication costs are an important component of their financial insecurity.
The document also notes data from the 2017 National Health and Nutrition Examination Survey, which found that 10% of people with diabetes have severe food insecurity and 20% have mild food insecurity (Diabetes Educ. 2017;43:260-71. doi: 10.1177/0145721717699890).
“Another thing the document points out is that two-thirds of the patients who don’t take all their medications due to cost don’t tell their doctor,” Dr. Hellman said. “The ADA is making the point that providers have a responsibility to ask if a patient is not taking certain medications because of the cost. We have so many better tools to manage this disease, but so many of these tools are unaffordable.”
While the treatment algorithm for patients with ASCVD will likely be embraced, another new recommendation may stir the pot a bit, Dr. Hellman noted. The section on cardiovascular disease and risk management sticks to a definition of hypertension as 140/90 mm Hg or higher – a striking diversion from the new 130/80 mm Hg limit set this fall by both the American Heart Association and the American College of Cardiology.
“This difference in recommendations is very important and will be controversial,” Dr. Hellman said, adding that he agrees with this clinical point.
Again, this recommendation is grounded in clinical trials, which suggest that people with diabetes don’t benefit from overly strict blood pressure control. The new AHA/ACC recommendations largely drew on data from the SPRINT trial, which was conducted in an entirely nondiabetic population. “These gave a clear signal that a lower BP target is beneficial to that group,” Dr. Hellman said.
But large well-designed randomized controlled trials of intensive blood pressure lowering in people with diabetes, such as ACCORD-BP, did not demonstrate that intensive blood-pressure lowering targeting a systolic less than120 mm Hg had a significant benefit on the composite primary cardiovascular endpoint. And while the ADVANCE BP trial found that the composite endpoint was improved with intensive blood pressure lowering, the blood pressure level achieved in the intervention group was 136/73 mm Hg.
“This recommendation is based on current evidence for people with diabetes,” Dr. Kalyani said. “We maintain our definition of hypertension as 140/90 mm Hg or higher based on the results of large clinical trials specifically in people with diabetes but emphasize that intensification of antihypertensive therapy to target lower blood pressures (less than 130/80 mm Hg) may be beneficial for high-risk patients with diabetes such as those with cardiovascular disease. We are constantly assessing the evidence and will continue to review the results of new studies for potential incorporation into recommendations in the future.”
Dr. Kalyani and Dr. Hellman had no financial disclosures. Dr. Jellinger has been a speaker for several pharmaceutical companies.
SOURCE: Kalyani R et al. Diabetes Care 2018;41(Suppl. 1):S86-S104 doi: 10.2337/dc18-S009
This article was updated 12/21/17.
EXPERT ANALYSIS FROM DIABETES CARE
Testing for latent tuberculosis infection
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.