User login
Labor & Delivery: An overlooked entry point for the spread of viral infection
OB hospitalists have a key role to play
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
OB hospitalists have a key role to play
OB hospitalists have a key role to play
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
Opioid use disorder up in sepsis hospitalizations
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
REPORTING FROM CCC49
Dr. Eric Howell selected as next CEO of SHM
The Society of Hospital Medicine has announced that Eric Howell, MD, MHM, will become its next CEO effective July 1, 2020. Dr. Howell will replace Laurence Wellikson, MD, MHM, who helped to found the society, and has been its first and only CEO since 2000.
“On behalf of the SHM board of directors, we welcome Dr. Howell as the incoming CEO for our organization who, with the mission-driven commitment and dedication of SHM staff, will take SHM into the future,” said Danielle Scheurer, MD, MSRC, SFHM, president-elect of SHM and chair of the CEO search committee. “With his broad knowledge of hospital medicine and extensive volunteer leadership at SHM, Dr. Howell’s experience is a natural complement to SHM’s core mission.”
Dr. Howell has a long history with SHM and has a wealth of expertise in hospital medicine. Since July 2018, he has served as chief operating officer of SHM, leading senior management’s planning and defining organizational goals to drive extensive, sustainable growth. Dr. Howell has also served as the senior physician advisor to SHM’s Center for Quality Improvement, the society’s arm that conducts quality improvement programs for hospitalist teams, since 2015. He is a past president of SHM’s board of directors and currently serves as the course director for the SHM Leadership Academies.
“Having been involved with SHM in many capacities since first joining, I am truly honored to become SHM’s CEO,” Dr. Howell said. “I always tell everyone that my goal is to make the world a better place, and I know that SHM’s staff will be able to do just that through the development and deployment of a variety of products, tools, and services to help hospitalists improve patient care.”
In addition to serving in various capacities at SHM, Dr. Howell has been a professor of medicine in the department of medicine at Johns Hopkins University, Baltimore. He has held multiple titles within the Johns Hopkins medical institutions, including chief of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, section chief of hospital medicine for Johns Hopkins Community Physicians, deputy director of hospital operations for the department of medicine at Johns Hopkins Bayview, and chief medical officer of operations at Johns Hopkins Bayview. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and oversaw nearly 200 physicians and clinical staff providing patient care in three hospitals.
Dr. Howell received his electrical engineering degree from the University of Maryland, which has proven instrumental in his mastery of managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput, and patient outcomes.
The search process was led by a CEO search committee, comprised of members of the SHM board of directors and assisted by the executive search firm Spencer Stuart. Launching a nationwide search, the firm identified candidates with the values and leadership qualities necessary to ensure the future growth of the organization.
“After a thorough search process, Dr. Eric Howell emerged as the right person to lead SHM,” said SHM board president Christopher Frost, MD, SFHM, “His experience in hospital medicine and his servant leadership style make him an ideal fit to lead SHM to even greater future success.”
In the coming weeks, the SHM board of directors will work with Dr. Howell and Dr. Wellikson on a smooth transition plan to have Dr. Howell assume the role on July 1, 2020.
The Society of Hospital Medicine has announced that Eric Howell, MD, MHM, will become its next CEO effective July 1, 2020. Dr. Howell will replace Laurence Wellikson, MD, MHM, who helped to found the society, and has been its first and only CEO since 2000.
“On behalf of the SHM board of directors, we welcome Dr. Howell as the incoming CEO for our organization who, with the mission-driven commitment and dedication of SHM staff, will take SHM into the future,” said Danielle Scheurer, MD, MSRC, SFHM, president-elect of SHM and chair of the CEO search committee. “With his broad knowledge of hospital medicine and extensive volunteer leadership at SHM, Dr. Howell’s experience is a natural complement to SHM’s core mission.”
Dr. Howell has a long history with SHM and has a wealth of expertise in hospital medicine. Since July 2018, he has served as chief operating officer of SHM, leading senior management’s planning and defining organizational goals to drive extensive, sustainable growth. Dr. Howell has also served as the senior physician advisor to SHM’s Center for Quality Improvement, the society’s arm that conducts quality improvement programs for hospitalist teams, since 2015. He is a past president of SHM’s board of directors and currently serves as the course director for the SHM Leadership Academies.
“Having been involved with SHM in many capacities since first joining, I am truly honored to become SHM’s CEO,” Dr. Howell said. “I always tell everyone that my goal is to make the world a better place, and I know that SHM’s staff will be able to do just that through the development and deployment of a variety of products, tools, and services to help hospitalists improve patient care.”
In addition to serving in various capacities at SHM, Dr. Howell has been a professor of medicine in the department of medicine at Johns Hopkins University, Baltimore. He has held multiple titles within the Johns Hopkins medical institutions, including chief of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, section chief of hospital medicine for Johns Hopkins Community Physicians, deputy director of hospital operations for the department of medicine at Johns Hopkins Bayview, and chief medical officer of operations at Johns Hopkins Bayview. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and oversaw nearly 200 physicians and clinical staff providing patient care in three hospitals.
Dr. Howell received his electrical engineering degree from the University of Maryland, which has proven instrumental in his mastery of managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput, and patient outcomes.
The search process was led by a CEO search committee, comprised of members of the SHM board of directors and assisted by the executive search firm Spencer Stuart. Launching a nationwide search, the firm identified candidates with the values and leadership qualities necessary to ensure the future growth of the organization.
“After a thorough search process, Dr. Eric Howell emerged as the right person to lead SHM,” said SHM board president Christopher Frost, MD, SFHM, “His experience in hospital medicine and his servant leadership style make him an ideal fit to lead SHM to even greater future success.”
In the coming weeks, the SHM board of directors will work with Dr. Howell and Dr. Wellikson on a smooth transition plan to have Dr. Howell assume the role on July 1, 2020.
The Society of Hospital Medicine has announced that Eric Howell, MD, MHM, will become its next CEO effective July 1, 2020. Dr. Howell will replace Laurence Wellikson, MD, MHM, who helped to found the society, and has been its first and only CEO since 2000.
“On behalf of the SHM board of directors, we welcome Dr. Howell as the incoming CEO for our organization who, with the mission-driven commitment and dedication of SHM staff, will take SHM into the future,” said Danielle Scheurer, MD, MSRC, SFHM, president-elect of SHM and chair of the CEO search committee. “With his broad knowledge of hospital medicine and extensive volunteer leadership at SHM, Dr. Howell’s experience is a natural complement to SHM’s core mission.”
Dr. Howell has a long history with SHM and has a wealth of expertise in hospital medicine. Since July 2018, he has served as chief operating officer of SHM, leading senior management’s planning and defining organizational goals to drive extensive, sustainable growth. Dr. Howell has also served as the senior physician advisor to SHM’s Center for Quality Improvement, the society’s arm that conducts quality improvement programs for hospitalist teams, since 2015. He is a past president of SHM’s board of directors and currently serves as the course director for the SHM Leadership Academies.
“Having been involved with SHM in many capacities since first joining, I am truly honored to become SHM’s CEO,” Dr. Howell said. “I always tell everyone that my goal is to make the world a better place, and I know that SHM’s staff will be able to do just that through the development and deployment of a variety of products, tools, and services to help hospitalists improve patient care.”
In addition to serving in various capacities at SHM, Dr. Howell has been a professor of medicine in the department of medicine at Johns Hopkins University, Baltimore. He has held multiple titles within the Johns Hopkins medical institutions, including chief of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, section chief of hospital medicine for Johns Hopkins Community Physicians, deputy director of hospital operations for the department of medicine at Johns Hopkins Bayview, and chief medical officer of operations at Johns Hopkins Bayview. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and oversaw nearly 200 physicians and clinical staff providing patient care in three hospitals.
Dr. Howell received his electrical engineering degree from the University of Maryland, which has proven instrumental in his mastery of managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput, and patient outcomes.
The search process was led by a CEO search committee, comprised of members of the SHM board of directors and assisted by the executive search firm Spencer Stuart. Launching a nationwide search, the firm identified candidates with the values and leadership qualities necessary to ensure the future growth of the organization.
“After a thorough search process, Dr. Eric Howell emerged as the right person to lead SHM,” said SHM board president Christopher Frost, MD, SFHM, “His experience in hospital medicine and his servant leadership style make him an ideal fit to lead SHM to even greater future success.”
In the coming weeks, the SHM board of directors will work with Dr. Howell and Dr. Wellikson on a smooth transition plan to have Dr. Howell assume the role on July 1, 2020.
The evolution of social media and visual abstracts in hospital medicine
In recent years, social media platforms like Twitter, Facebook, and Instagram have become popular gathering spots for clinicians to connect, engage, and share medical content. Medical journals, which often act as purveyors of this content, have recognized social media’s growing power and influence and have begun looking for ways to better engage their audiences.
In 2016, the Annals of Surgery was looking to better disseminate the work being published in its pages and looked to Twitter as one way of accomplishing this. At the time, most journals were only posting the title or a brief description of the published manuscript and hoping their Twitter followers would click on the article link. As journal editors were finding, if the audience was not immediately familiar with the topic or able to quickly capture the nuances of the study, there was a good chance the reader would continue to scroll past the post and never view the article.
Recognizing that social media heavily relies on visual material to garner attention, Annals turned to Andrew Ibrahim, MD, an architect turned surgeon, to help them rethink their social media strategy. Using the design training he had previously received in his career as an architect, Dr. Ibrahim created a simple visual tool that could be used to capture the often complicated and nuanced aspects of a research study. He called his creation a “visual abstract.”
But what is a visual abstract? Simply, they are visual representations of the key findings of a published manuscript; or put another way, a “movie trailer” to the full manuscript. While they can take many different forms and designs, they often consist of three key components: (1) a simple, easy to understand title, (2) a primary focus on outcomes, and (3) the use of visual cues or images to help the reader absorb and remember the take home message. This simplified delivery of complex information allows the producer to efficiently share complex findings in a format that allows for rapid visualization and interpretation.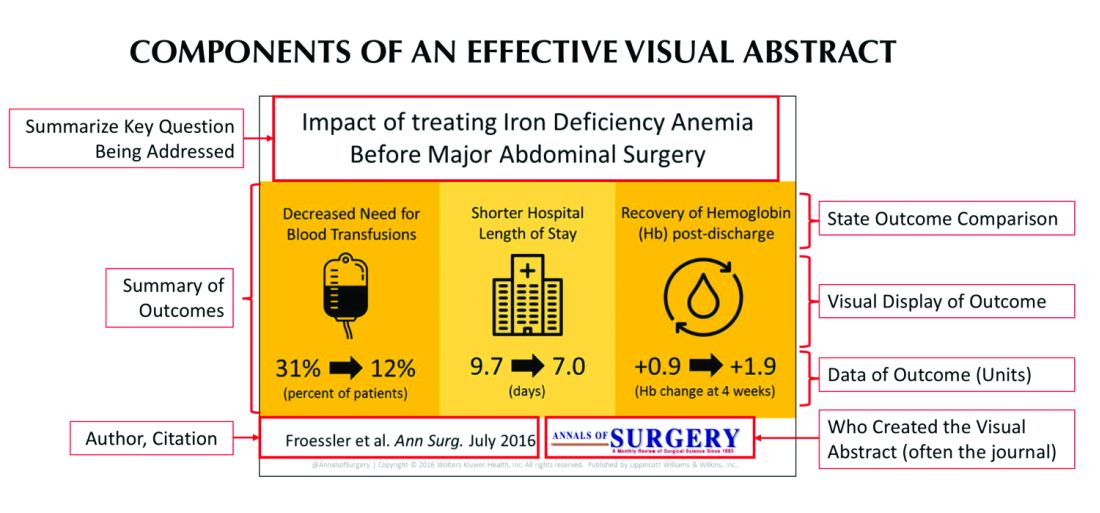
Since its inception, several studies have examined the influence visual abstracts have on disseminating research. One study conducted by Dr. Ibrahim and his colleagues found that articles tweeted with a visual abstract had an almost eightfold increase in the number of Twitter impressions (a measure of social media dissemination) and a threefold increase in article visits, compared with those manuscripts tweeted with the article title only.1 These results reflect what behavioral scientists have long understood: Humans process visual data better than any other type of data.2 For instance, according to research compiled by 3M, the company behind popular sticky notes, visual data is processed 60,000 times faster than text and has been shown to improve learning by 400%.3 Likewise, digital marketers have found that pages with videos and images draw on average 94% more views than their text-only counterparts.4
This knowledge, along with the substantial difference in engagement and dissemination characteristics from Dr. Ibrahim’s study, was far beyond what anyone might have expected and started a trend in medicine that continues to grow today. Medical journals across all practices and disciplines, including several leading journals, such as the New England Journal of Medicine, the Journal of the American Medical Association, and the Journal of Hospital Medicine (JHM), are utilizing this new tool to help disseminate their work in social media.
Visual abstracts have expanded beyond the social media sphere and are now frequently used in Grand Rounds presentations and as teaching tools among medical educators. JHM was one of the first journals to adopt the use of visual abstracts and has since published more than 150 in total. Given the growing popularity and expanded use of visual abstracts, JHM recently began archiving them on the journal’s website to allow clinicians to use the material in their own creative ways.
Visual abstracts are just one piece of the growing enterprise in social media for JHM. Recognizing the growing utilization of social media among physicians, JHM has taken a leading role in the use of online journal clubs. Since 2014, JHM has run a monthly Twitter-based journal club that discusses recently published articles and hospital medicine–based topics, called #JHMChat.5 This forum has allowed hospitalists from across the country, and around the world, to connect, network, and engage around topics important to the field of hospital medicine. The journal frequently reaches beyond hospital medicine borders and partners with other specialties and interest groups to gain perspective and insights into shared topic areas. To date, #JHMChat has one of the most robust online communities and continues to attract new followers each month.
As social media use continues to expand among clinicians, engagement tools like visual abstracts and Twitter chats will certainly continue to grow. Given that more clinicians are scrolling through websites than flipping through journal pages, medical journals like JHM will continually look for novel ways to engage their audiences and create communities among their followers. While a former architect who now practices as a surgeon led the way with visual abstracts, it remains to be seen who will create the next tool used to capture our attention on the ever-evolving sphere of social media.
Dr. Wray is a hospitalist at the University of California, San Francisco, and the San Francisco Veterans Affairs Medical Center. He also serves as a digital media and associate editor for the Journal of Hospital Medicine.
References
1. Ibrahim AM et al. Visual abstracts to disseminate research on social media: A prospective, case-control crossover study. Ann Surg. 2017;266(6):e46.
2. Tufte ER. The Visual Display of Quantitative Information. Second edition. Cheshire, Conn. Graphics Press, 2001. https://search.library.wisc.edu/catalog/999913808702121.
3. Polishing Your Presentation. http://web.archive.org/web/20001014041642/http://www.3m.com:80/meetingnetwork/files/meetingguide_pres.pdf. Accessed May 28, 2017.
4. 7 reasons you need visual content in your marketing strategy. https://medium.com/@nikos_iliopoulos/7-reasons-you-need-visual-content-in-your-marketing-strategy-bc77ca5521ac. Accessed May 28, 2017.
5. Wray CM et al. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med. 2018. doi: 10.12788/jhm.2987.
In recent years, social media platforms like Twitter, Facebook, and Instagram have become popular gathering spots for clinicians to connect, engage, and share medical content. Medical journals, which often act as purveyors of this content, have recognized social media’s growing power and influence and have begun looking for ways to better engage their audiences.
In 2016, the Annals of Surgery was looking to better disseminate the work being published in its pages and looked to Twitter as one way of accomplishing this. At the time, most journals were only posting the title or a brief description of the published manuscript and hoping their Twitter followers would click on the article link. As journal editors were finding, if the audience was not immediately familiar with the topic or able to quickly capture the nuances of the study, there was a good chance the reader would continue to scroll past the post and never view the article.
Recognizing that social media heavily relies on visual material to garner attention, Annals turned to Andrew Ibrahim, MD, an architect turned surgeon, to help them rethink their social media strategy. Using the design training he had previously received in his career as an architect, Dr. Ibrahim created a simple visual tool that could be used to capture the often complicated and nuanced aspects of a research study. He called his creation a “visual abstract.”
But what is a visual abstract? Simply, they are visual representations of the key findings of a published manuscript; or put another way, a “movie trailer” to the full manuscript. While they can take many different forms and designs, they often consist of three key components: (1) a simple, easy to understand title, (2) a primary focus on outcomes, and (3) the use of visual cues or images to help the reader absorb and remember the take home message. This simplified delivery of complex information allows the producer to efficiently share complex findings in a format that allows for rapid visualization and interpretation.
Since its inception, several studies have examined the influence visual abstracts have on disseminating research. One study conducted by Dr. Ibrahim and his colleagues found that articles tweeted with a visual abstract had an almost eightfold increase in the number of Twitter impressions (a measure of social media dissemination) and a threefold increase in article visits, compared with those manuscripts tweeted with the article title only.1 These results reflect what behavioral scientists have long understood: Humans process visual data better than any other type of data.2 For instance, according to research compiled by 3M, the company behind popular sticky notes, visual data is processed 60,000 times faster than text and has been shown to improve learning by 400%.3 Likewise, digital marketers have found that pages with videos and images draw on average 94% more views than their text-only counterparts.4
This knowledge, along with the substantial difference in engagement and dissemination characteristics from Dr. Ibrahim’s study, was far beyond what anyone might have expected and started a trend in medicine that continues to grow today. Medical journals across all practices and disciplines, including several leading journals, such as the New England Journal of Medicine, the Journal of the American Medical Association, and the Journal of Hospital Medicine (JHM), are utilizing this new tool to help disseminate their work in social media.
Visual abstracts have expanded beyond the social media sphere and are now frequently used in Grand Rounds presentations and as teaching tools among medical educators. JHM was one of the first journals to adopt the use of visual abstracts and has since published more than 150 in total. Given the growing popularity and expanded use of visual abstracts, JHM recently began archiving them on the journal’s website to allow clinicians to use the material in their own creative ways.
Visual abstracts are just one piece of the growing enterprise in social media for JHM. Recognizing the growing utilization of social media among physicians, JHM has taken a leading role in the use of online journal clubs. Since 2014, JHM has run a monthly Twitter-based journal club that discusses recently published articles and hospital medicine–based topics, called #JHMChat.5 This forum has allowed hospitalists from across the country, and around the world, to connect, network, and engage around topics important to the field of hospital medicine. The journal frequently reaches beyond hospital medicine borders and partners with other specialties and interest groups to gain perspective and insights into shared topic areas. To date, #JHMChat has one of the most robust online communities and continues to attract new followers each month.
As social media use continues to expand among clinicians, engagement tools like visual abstracts and Twitter chats will certainly continue to grow. Given that more clinicians are scrolling through websites than flipping through journal pages, medical journals like JHM will continually look for novel ways to engage their audiences and create communities among their followers. While a former architect who now practices as a surgeon led the way with visual abstracts, it remains to be seen who will create the next tool used to capture our attention on the ever-evolving sphere of social media.
Dr. Wray is a hospitalist at the University of California, San Francisco, and the San Francisco Veterans Affairs Medical Center. He also serves as a digital media and associate editor for the Journal of Hospital Medicine.
References
1. Ibrahim AM et al. Visual abstracts to disseminate research on social media: A prospective, case-control crossover study. Ann Surg. 2017;266(6):e46.
2. Tufte ER. The Visual Display of Quantitative Information. Second edition. Cheshire, Conn. Graphics Press, 2001. https://search.library.wisc.edu/catalog/999913808702121.
3. Polishing Your Presentation. http://web.archive.org/web/20001014041642/http://www.3m.com:80/meetingnetwork/files/meetingguide_pres.pdf. Accessed May 28, 2017.
4. 7 reasons you need visual content in your marketing strategy. https://medium.com/@nikos_iliopoulos/7-reasons-you-need-visual-content-in-your-marketing-strategy-bc77ca5521ac. Accessed May 28, 2017.
5. Wray CM et al. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med. 2018. doi: 10.12788/jhm.2987.
In recent years, social media platforms like Twitter, Facebook, and Instagram have become popular gathering spots for clinicians to connect, engage, and share medical content. Medical journals, which often act as purveyors of this content, have recognized social media’s growing power and influence and have begun looking for ways to better engage their audiences.
In 2016, the Annals of Surgery was looking to better disseminate the work being published in its pages and looked to Twitter as one way of accomplishing this. At the time, most journals were only posting the title or a brief description of the published manuscript and hoping their Twitter followers would click on the article link. As journal editors were finding, if the audience was not immediately familiar with the topic or able to quickly capture the nuances of the study, there was a good chance the reader would continue to scroll past the post and never view the article.
Recognizing that social media heavily relies on visual material to garner attention, Annals turned to Andrew Ibrahim, MD, an architect turned surgeon, to help them rethink their social media strategy. Using the design training he had previously received in his career as an architect, Dr. Ibrahim created a simple visual tool that could be used to capture the often complicated and nuanced aspects of a research study. He called his creation a “visual abstract.”
But what is a visual abstract? Simply, they are visual representations of the key findings of a published manuscript; or put another way, a “movie trailer” to the full manuscript. While they can take many different forms and designs, they often consist of three key components: (1) a simple, easy to understand title, (2) a primary focus on outcomes, and (3) the use of visual cues or images to help the reader absorb and remember the take home message. This simplified delivery of complex information allows the producer to efficiently share complex findings in a format that allows for rapid visualization and interpretation.
Since its inception, several studies have examined the influence visual abstracts have on disseminating research. One study conducted by Dr. Ibrahim and his colleagues found that articles tweeted with a visual abstract had an almost eightfold increase in the number of Twitter impressions (a measure of social media dissemination) and a threefold increase in article visits, compared with those manuscripts tweeted with the article title only.1 These results reflect what behavioral scientists have long understood: Humans process visual data better than any other type of data.2 For instance, according to research compiled by 3M, the company behind popular sticky notes, visual data is processed 60,000 times faster than text and has been shown to improve learning by 400%.3 Likewise, digital marketers have found that pages with videos and images draw on average 94% more views than their text-only counterparts.4
This knowledge, along with the substantial difference in engagement and dissemination characteristics from Dr. Ibrahim’s study, was far beyond what anyone might have expected and started a trend in medicine that continues to grow today. Medical journals across all practices and disciplines, including several leading journals, such as the New England Journal of Medicine, the Journal of the American Medical Association, and the Journal of Hospital Medicine (JHM), are utilizing this new tool to help disseminate their work in social media.
Visual abstracts have expanded beyond the social media sphere and are now frequently used in Grand Rounds presentations and as teaching tools among medical educators. JHM was one of the first journals to adopt the use of visual abstracts and has since published more than 150 in total. Given the growing popularity and expanded use of visual abstracts, JHM recently began archiving them on the journal’s website to allow clinicians to use the material in their own creative ways.
Visual abstracts are just one piece of the growing enterprise in social media for JHM. Recognizing the growing utilization of social media among physicians, JHM has taken a leading role in the use of online journal clubs. Since 2014, JHM has run a monthly Twitter-based journal club that discusses recently published articles and hospital medicine–based topics, called #JHMChat.5 This forum has allowed hospitalists from across the country, and around the world, to connect, network, and engage around topics important to the field of hospital medicine. The journal frequently reaches beyond hospital medicine borders and partners with other specialties and interest groups to gain perspective and insights into shared topic areas. To date, #JHMChat has one of the most robust online communities and continues to attract new followers each month.
As social media use continues to expand among clinicians, engagement tools like visual abstracts and Twitter chats will certainly continue to grow. Given that more clinicians are scrolling through websites than flipping through journal pages, medical journals like JHM will continually look for novel ways to engage their audiences and create communities among their followers. While a former architect who now practices as a surgeon led the way with visual abstracts, it remains to be seen who will create the next tool used to capture our attention on the ever-evolving sphere of social media.
Dr. Wray is a hospitalist at the University of California, San Francisco, and the San Francisco Veterans Affairs Medical Center. He also serves as a digital media and associate editor for the Journal of Hospital Medicine.
References
1. Ibrahim AM et al. Visual abstracts to disseminate research on social media: A prospective, case-control crossover study. Ann Surg. 2017;266(6):e46.
2. Tufte ER. The Visual Display of Quantitative Information. Second edition. Cheshire, Conn. Graphics Press, 2001. https://search.library.wisc.edu/catalog/999913808702121.
3. Polishing Your Presentation. http://web.archive.org/web/20001014041642/http://www.3m.com:80/meetingnetwork/files/meetingguide_pres.pdf. Accessed May 28, 2017.
4. 7 reasons you need visual content in your marketing strategy. https://medium.com/@nikos_iliopoulos/7-reasons-you-need-visual-content-in-your-marketing-strategy-bc77ca5521ac. Accessed May 28, 2017.
5. Wray CM et al. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med. 2018. doi: 10.12788/jhm.2987.
When is a troponin elevation an acute myocardial infarction?
Misdiagnosis can have ‘downstream repercussions’
Hospitalists encounter troponin elevations daily, but we have to use clinical judgment to determine if the troponin elevation represents either a myocardial infarction (MI), or a non-MI troponin elevation (i.e. a , nonischemic myocardial injury).
It is important to remember that an MI specifically refers to myocardial injury due to acute myocardial ischemia to the myocardium. This lack of blood supply can be due to an acute absolute or relative deficiency in coronary artery blood flow. However, there are also many mechanisms of myocardial injury unrelated to reduced coronary artery blood flow, and these should be more appropriately termed non-MI troponin elevations.
Historically, when an ischemic mechanism of myocardial injury was suspected, providers would categorize troponin elevations into ST-elevation MI (STEMI) versus non-ST-elevation MI (NSTEMI) based on the electrocardiogram (ECG). We would further classify the NSTEMI into type 1 or type 2, depending on the mechanism of injury. The term “NSTEMI” served as a “catch-all” term to describe both type 1 NSTEMIs and type 2 MIs, but that classification system is no longer valid.
As of Oct. 1, 2017, ICD-10 and the Centers for Medicare & Medicaid Services have a new ICD-10 diagnosis code for type 2 MI (I21.A1), distinct from NSTEMI (I21.4) based on updated definitions from the American College of Cardiology, American Heart Association, European Society of Cardiology, and World Heart Federation. The term “NSTEMI” should be used only when referring to a type 1 MI not when referring to a type 2 MI.1
Classification of MI types
The Fourth Universal Definition of MI published in August 2018 further updated the definitions of MI (summarized in Figure 1).2 This review focuses on type 1 and type 2 MIs, which are the most common types encountered by hospitalists. Types 3-5 MI (grouped under a common ICD-10 diagnosis code for “Other MI Types,” or I21.A9) would rarely be diagnosed by hospitalists.
Figure 1: Classification of MI
MI Type | Classification |
1 | STEMI (acute coronary artery thrombosis) |
2 | Supply/demand mismatch (heterogeneous underlying causes) |
3 | Sudden cardiac death with ECG evidence of acute myocardial ischemia before cardiac troponins could be drawn |
4 | MI due to percutaneous coronary intervention (PCI) |
5 | MI due to coronary artery bypass grafting (CABG) |
The diagnosis of a type 1 MIs (STEMI and NSTEMI) is supported by the presence of an acute coronary thrombus or plaque rupture/erosion on coronary angiography or a strong suspicion for these when angiography is unavailable or contraindicated. Type 1 MI (also referred to as spontaneous MI) is generally a primary reason (or “principal” diagnosis) for a patient’s presentation to a hospital.3 Please note that a very high or rising troponin level alone is not diagnostic for a type 1 or type 2 NSTEMI. The lab has to be taken in the context of the patient’s presentation and other supporting findings.
In contrast to a type 1 MI (STEMI and NSTEMI), at type 2 MI results from an imbalance between myocardial oxygen supply and demand unrelated to acute coronary artery thrombosis or plaque rupture. A type 2 MI is a relative (as opposed to an absolute) deficiency in coronary artery blood flow triggered by an abrupt increase in myocardial oxygen demand, drop in myocardial blood supply, or both. In type 2 MI, myocardial injury occurs secondary to an underlying process, and therefore requires correct documentation of the underlying cause as well.
Common examples of underlying causes of type 2 MI include acute blood loss anemia (e.g. GI bleed), acute hypoxia (e.g. COPD exacerbation), shock states (cardiogenic, hypovolemic, hemorrhagic, or septic), coronary vasospasm (e.g. spontaneous), and bradyarrhythmias. Patients with type 2 MI often have a history of fixed obstructive coronary disease, which when coupled with the acute trigger facilitates the type 2 MI; however, underlying CAD is not always present.
Diagnosing a type 2 MI requires evidence of acute myocardial ischemia (Figure 2) with an elevated troponin but must also have at least one of the following:2
- Symptoms of acute myocardial ischemia such as typical chest pain.
- New ischemic ECG changes.
- Development of pathological Q waves.
- Imaging evidence of new loss of viable myocardium, significant reversible perfusion defect on nuclear imaging, or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.
Distinguishing a type 1 NSTEMI from a type 2 MI depends mainly on the clinical context and clinical judgment. A patient whose presenting symptoms include acute chest discomfort, acute ST-T wave changes, and a rise in troponin would be suspected of having a type 1 NSTEMI. However, in a patient presenting with other or vague complaints where an elevated troponin was found amongst a battery of tests, a type 2 MI may be favored, particularly if there is evidence of an underlying trigger for a supply-demand mismatch. In challenging cases, cardiology consultation can help determine the MI type and/or the next diagnostic and treatment considerations.
When there is only elevated troponin levels (or even a rise and fall in troponin) without new symptoms or ECG/imaging evidence of myocardial ischemia, it is most appropriate to document a non-MI troponin elevation due to a nonischemic mechanism of myocardial injury.
Non-MI troponin elevation (nonischemic myocardial injury)
The number of conditions known to cause myocardial injury through mechanisms other than myocardial ischemia (see Figure 2) is growing, especially in the current era of high-sensitivity troponin assays.4
Common examples of underlying causes of non-MI troponin elevation include:
- Acute (on chronic) systolic or diastolic heart failure: Usually due to acute ventricular wall stretch/strain. Troponin elevations tend to be mild, with more indolent (or even flat) troponin trajectories.
- Pericarditis and myocarditis: Due to direct injury from myocardial inflammation.
- Cardiopulmonary resuscitation (CPR): Due to physical injury to the heart from mechanical chest compressions and from electrical shocks of external defibrillation.
- Stress-induced (takotsubo) cardiomyopathy: Stress-induced release of neurohormonal factors and catecholamines that cause direct myocyte injury and transient dilatation of the ventricle.
- Acute pulmonary embolism: Result of acute right ventricular wall stretch/strain, not from myocardial ischemia.
- Sepsis without shock: Direct toxicity of circulating cytokines to cardiac myocytes. In the absence of evidence of shock and symptoms/signs of myocardial ischemia, do not document type 2 MI.
- Renal failure (acute kidney injury or chronic kidney disease): Multiple etiologies, but at least partially related to reduced renal clearance of troponin. In general, renal failure in the absence of symptoms/signs of ischemia is best classified as a non-MI troponin elevation. ESRD patients who present with volume overload due to missed dialysis also typically have a non-MI troponin elevation.
- Stroke/intracranial hemorrhage: Mechanisms of myocardial injury and troponin elevation are incompletely understood, but may include catecholamine surges that injure the heart.
Some underlying conditions can cause a type 2 MI or a non-MI troponin elevation depending on the clinical context. For example, hypertensive emergency, severe aortic valve stenosis, hypertrophic cardiomyopathy, and tachyarrhythmias (including atrial fibrillation with rapid ventricular response) may cause increased myocardial oxygen demand, and in patients with underlying CAD, could precipitate a type 2 MI.
However, these same conditions could cause a non-MI troponin elevation in patients without CAD and could also cause myocardial injury and troponin release by causing acute left ventricular stretch/strain. Distinguishing the diagnose of type 2 MI vs. non-MI troponin elevation depends on documenting whether there are ancillary ischemic symptoms, ECG findings, imaging, and/or cath findings of acute myocardial ischemia.
Case examples
1. A 60-year-old male presents with fever, cough, shortness of breath, and an infiltrate on CXR and is diagnosed with sepsis secondary to pneumonia. His initial troponin of 0.07 (normal < 0.05) rises to 0.11, peaks at 0.23, then subsequently trends down.
While some may be tempted to diagnose a type 2 MI, remember that sepsis can cause direct myocardial cell injury via direct cell toxicity. Unless this patient had at least one additional criteria (anginal chest pain, new ischemic ECG changes, or imaging evidence of new loss of viable myocardium, which does not recover with treatment of sepsis), this was most likely myocardial injury via direct cell toxicity, and should be documented as a non-MI troponin elevation due to sepsis without shock.
If there were ischemic ECG changes and the patient had chest pain, one would have to use clinical suspicion to differentiate between a type 1 NSTEMI and a type 2 MI. If there is a high clinical suspicion for an acute plaque rupture/thrombus, one would call it an NSTEMI and would have to document treatment as such (e.g. start heparin drip). Again, cardiology consultation can be helpful in cases where it may be hard to decide how to manage. Many times, the true mechanism is not determined until the patient is taken to the cath lab and if no acute plaque rupture is seen, then it was likely a type 2 MI.
2. A 70-year-old male with chronic systolic heart failure, noncompliant with medications, presents with 3 days of dyspnea on exertion and lower extremity edema. He had no chest discomfort. Exam shows bibasilar crackles and hepatojugular reflux. ECG shows no ischemic changes. Serial troponin values over 48 hours were: 0.48, 0.58, 0.51. A transthoracic echocardiogram reveals an LVEF of 40% with poor movement in the apex, similar to his prior echo.
This patient had no overt evidence of ischemia (no chest pain, ischemic ECG, or imaging changes) so the troponin elevation was most likely a non-MI troponin elevation secondary to acute on chronic systolic heart failure (in which the mechanism of troponin elevation is left ventricular chamber stretch from volume overload, and not demand ischemia). Generally, it is uncommon for a heart failure exacerbation to cause a type 2 MI.
Why is it so important to get this diagnosis right?
Misdiagnosing an MI when the patient does not have one can have multiple downstream repercussions. Because it stays on their medical record, it impacts their ability to get insurance and their premium costs. We expose patients to additional medications (e.g. dual antiplatelet therapy, statins), which can have adverse effects. As a result, it is very important to classify the etiology of the troponin elevation and treat accordingly.
Finally, when we incorrectly label a patient as having an MI, this can impact billing and reimbursement, DRG denials, insurance premiums, and quality metrics for both the hospital and the physicians. Hospitals’ 30-day readmission rates for AMI will suffer and quality metrics can be significantly impacted. We must be diligent and as precise as possible with our diagnoses and documentation to ensure the maximum benefit for our patients and our health care system.
Dr. Nave is assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta. Dr. Goyal is associate professor of medicine (cardiology), at Emory University, and chief quality officer, Emory Heart and Vascular Center, Emory Healthcare. He is also codirector of nuclear cardiology at Emory University Hospital.
Key points
- A diagnosis of a type 1 MI is supported by evidence or strong suspicion of acute coronary artery thrombus or plaque rupture/erosion.
- A very high troponin level alone is not diagnostic for a type 1 or type 2 MI. It has to be contextualized with the patient’s presentation and other supporting findings.
- Type 2 MI is a mismatch between myocardial oxygen supply and demand unrelated to acute coronary thrombosis or plaque rupture triggered by an abrupt increase in myocardial oxygen demand, drop in myocardial blood supply, or both. Type 2 MI should be documented along with its underlying cause.
- To diagnose an MI (either type 1 or type 2 MI), in addition to the troponin elevation, the patient must have symptoms of acute ischemia, ischemic ECG findings, and/or imaging suggestive of new ischemia.
- An elevated troponin level without new symptoms or ECG/imaging evidence of myocardial ischemia should be documented as a non-MI troponin elevation secondary to an underlying cause.
References
1. Goyal A, Gluckman TJ, Tcheng JE. What’s in a name? The new ICD-10 (10th revision of the international statistical classification of diseases and related health problems) codes and type 2 myocardial infarction. Circulation. 2017;136:1180-2.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;Aug 25:[Epub ahead of print].
3. Goyal, et al. Translating the Fourth Universal Definition of Myocardial Infarction into Clinical Documentation: Ten Pearls For Frontline Clinicians. Cardiology Magazine. Nov 2018.
4. Roongsritong C, Warraich I, Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: incidence and clinical significance. Chest. 2004;125:1877-84.
Misdiagnosis can have ‘downstream repercussions’
Misdiagnosis can have ‘downstream repercussions’
Hospitalists encounter troponin elevations daily, but we have to use clinical judgment to determine if the troponin elevation represents either a myocardial infarction (MI), or a non-MI troponin elevation (i.e. a , nonischemic myocardial injury).
It is important to remember that an MI specifically refers to myocardial injury due to acute myocardial ischemia to the myocardium. This lack of blood supply can be due to an acute absolute or relative deficiency in coronary artery blood flow. However, there are also many mechanisms of myocardial injury unrelated to reduced coronary artery blood flow, and these should be more appropriately termed non-MI troponin elevations.
Historically, when an ischemic mechanism of myocardial injury was suspected, providers would categorize troponin elevations into ST-elevation MI (STEMI) versus non-ST-elevation MI (NSTEMI) based on the electrocardiogram (ECG). We would further classify the NSTEMI into type 1 or type 2, depending on the mechanism of injury. The term “NSTEMI” served as a “catch-all” term to describe both type 1 NSTEMIs and type 2 MIs, but that classification system is no longer valid.
As of Oct. 1, 2017, ICD-10 and the Centers for Medicare & Medicaid Services have a new ICD-10 diagnosis code for type 2 MI (I21.A1), distinct from NSTEMI (I21.4) based on updated definitions from the American College of Cardiology, American Heart Association, European Society of Cardiology, and World Heart Federation. The term “NSTEMI” should be used only when referring to a type 1 MI not when referring to a type 2 MI.1
Classification of MI types
The Fourth Universal Definition of MI published in August 2018 further updated the definitions of MI (summarized in Figure 1).2 This review focuses on type 1 and type 2 MIs, which are the most common types encountered by hospitalists. Types 3-5 MI (grouped under a common ICD-10 diagnosis code for “Other MI Types,” or I21.A9) would rarely be diagnosed by hospitalists.
Figure 1: Classification of MI
MI Type | Classification |
1 | STEMI (acute coronary artery thrombosis) |
2 | Supply/demand mismatch (heterogeneous underlying causes) |
3 | Sudden cardiac death with ECG evidence of acute myocardial ischemia before cardiac troponins could be drawn |
4 | MI due to percutaneous coronary intervention (PCI) |
5 | MI due to coronary artery bypass grafting (CABG) |
The diagnosis of a type 1 MIs (STEMI and NSTEMI) is supported by the presence of an acute coronary thrombus or plaque rupture/erosion on coronary angiography or a strong suspicion for these when angiography is unavailable or contraindicated. Type 1 MI (also referred to as spontaneous MI) is generally a primary reason (or “principal” diagnosis) for a patient’s presentation to a hospital.3 Please note that a very high or rising troponin level alone is not diagnostic for a type 1 or type 2 NSTEMI. The lab has to be taken in the context of the patient’s presentation and other supporting findings.
In contrast to a type 1 MI (STEMI and NSTEMI), at type 2 MI results from an imbalance between myocardial oxygen supply and demand unrelated to acute coronary artery thrombosis or plaque rupture. A type 2 MI is a relative (as opposed to an absolute) deficiency in coronary artery blood flow triggered by an abrupt increase in myocardial oxygen demand, drop in myocardial blood supply, or both. In type 2 MI, myocardial injury occurs secondary to an underlying process, and therefore requires correct documentation of the underlying cause as well.
Common examples of underlying causes of type 2 MI include acute blood loss anemia (e.g. GI bleed), acute hypoxia (e.g. COPD exacerbation), shock states (cardiogenic, hypovolemic, hemorrhagic, or septic), coronary vasospasm (e.g. spontaneous), and bradyarrhythmias. Patients with type 2 MI often have a history of fixed obstructive coronary disease, which when coupled with the acute trigger facilitates the type 2 MI; however, underlying CAD is not always present.
Diagnosing a type 2 MI requires evidence of acute myocardial ischemia (Figure 2) with an elevated troponin but must also have at least one of the following:2
- Symptoms of acute myocardial ischemia such as typical chest pain.
- New ischemic ECG changes.
- Development of pathological Q waves.
- Imaging evidence of new loss of viable myocardium, significant reversible perfusion defect on nuclear imaging, or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.
Distinguishing a type 1 NSTEMI from a type 2 MI depends mainly on the clinical context and clinical judgment. A patient whose presenting symptoms include acute chest discomfort, acute ST-T wave changes, and a rise in troponin would be suspected of having a type 1 NSTEMI. However, in a patient presenting with other or vague complaints where an elevated troponin was found amongst a battery of tests, a type 2 MI may be favored, particularly if there is evidence of an underlying trigger for a supply-demand mismatch. In challenging cases, cardiology consultation can help determine the MI type and/or the next diagnostic and treatment considerations.
When there is only elevated troponin levels (or even a rise and fall in troponin) without new symptoms or ECG/imaging evidence of myocardial ischemia, it is most appropriate to document a non-MI troponin elevation due to a nonischemic mechanism of myocardial injury.
Non-MI troponin elevation (nonischemic myocardial injury)
The number of conditions known to cause myocardial injury through mechanisms other than myocardial ischemia (see Figure 2) is growing, especially in the current era of high-sensitivity troponin assays.4
Common examples of underlying causes of non-MI troponin elevation include:
- Acute (on chronic) systolic or diastolic heart failure: Usually due to acute ventricular wall stretch/strain. Troponin elevations tend to be mild, with more indolent (or even flat) troponin trajectories.
- Pericarditis and myocarditis: Due to direct injury from myocardial inflammation.
- Cardiopulmonary resuscitation (CPR): Due to physical injury to the heart from mechanical chest compressions and from electrical shocks of external defibrillation.
- Stress-induced (takotsubo) cardiomyopathy: Stress-induced release of neurohormonal factors and catecholamines that cause direct myocyte injury and transient dilatation of the ventricle.
- Acute pulmonary embolism: Result of acute right ventricular wall stretch/strain, not from myocardial ischemia.
- Sepsis without shock: Direct toxicity of circulating cytokines to cardiac myocytes. In the absence of evidence of shock and symptoms/signs of myocardial ischemia, do not document type 2 MI.
- Renal failure (acute kidney injury or chronic kidney disease): Multiple etiologies, but at least partially related to reduced renal clearance of troponin. In general, renal failure in the absence of symptoms/signs of ischemia is best classified as a non-MI troponin elevation. ESRD patients who present with volume overload due to missed dialysis also typically have a non-MI troponin elevation.
- Stroke/intracranial hemorrhage: Mechanisms of myocardial injury and troponin elevation are incompletely understood, but may include catecholamine surges that injure the heart.
Some underlying conditions can cause a type 2 MI or a non-MI troponin elevation depending on the clinical context. For example, hypertensive emergency, severe aortic valve stenosis, hypertrophic cardiomyopathy, and tachyarrhythmias (including atrial fibrillation with rapid ventricular response) may cause increased myocardial oxygen demand, and in patients with underlying CAD, could precipitate a type 2 MI.
However, these same conditions could cause a non-MI troponin elevation in patients without CAD and could also cause myocardial injury and troponin release by causing acute left ventricular stretch/strain. Distinguishing the diagnose of type 2 MI vs. non-MI troponin elevation depends on documenting whether there are ancillary ischemic symptoms, ECG findings, imaging, and/or cath findings of acute myocardial ischemia.
Case examples
1. A 60-year-old male presents with fever, cough, shortness of breath, and an infiltrate on CXR and is diagnosed with sepsis secondary to pneumonia. His initial troponin of 0.07 (normal < 0.05) rises to 0.11, peaks at 0.23, then subsequently trends down.
While some may be tempted to diagnose a type 2 MI, remember that sepsis can cause direct myocardial cell injury via direct cell toxicity. Unless this patient had at least one additional criteria (anginal chest pain, new ischemic ECG changes, or imaging evidence of new loss of viable myocardium, which does not recover with treatment of sepsis), this was most likely myocardial injury via direct cell toxicity, and should be documented as a non-MI troponin elevation due to sepsis without shock.
If there were ischemic ECG changes and the patient had chest pain, one would have to use clinical suspicion to differentiate between a type 1 NSTEMI and a type 2 MI. If there is a high clinical suspicion for an acute plaque rupture/thrombus, one would call it an NSTEMI and would have to document treatment as such (e.g. start heparin drip). Again, cardiology consultation can be helpful in cases where it may be hard to decide how to manage. Many times, the true mechanism is not determined until the patient is taken to the cath lab and if no acute plaque rupture is seen, then it was likely a type 2 MI.
2. A 70-year-old male with chronic systolic heart failure, noncompliant with medications, presents with 3 days of dyspnea on exertion and lower extremity edema. He had no chest discomfort. Exam shows bibasilar crackles and hepatojugular reflux. ECG shows no ischemic changes. Serial troponin values over 48 hours were: 0.48, 0.58, 0.51. A transthoracic echocardiogram reveals an LVEF of 40% with poor movement in the apex, similar to his prior echo.
This patient had no overt evidence of ischemia (no chest pain, ischemic ECG, or imaging changes) so the troponin elevation was most likely a non-MI troponin elevation secondary to acute on chronic systolic heart failure (in which the mechanism of troponin elevation is left ventricular chamber stretch from volume overload, and not demand ischemia). Generally, it is uncommon for a heart failure exacerbation to cause a type 2 MI.
Why is it so important to get this diagnosis right?
Misdiagnosing an MI when the patient does not have one can have multiple downstream repercussions. Because it stays on their medical record, it impacts their ability to get insurance and their premium costs. We expose patients to additional medications (e.g. dual antiplatelet therapy, statins), which can have adverse effects. As a result, it is very important to classify the etiology of the troponin elevation and treat accordingly.
Finally, when we incorrectly label a patient as having an MI, this can impact billing and reimbursement, DRG denials, insurance premiums, and quality metrics for both the hospital and the physicians. Hospitals’ 30-day readmission rates for AMI will suffer and quality metrics can be significantly impacted. We must be diligent and as precise as possible with our diagnoses and documentation to ensure the maximum benefit for our patients and our health care system.
Dr. Nave is assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta. Dr. Goyal is associate professor of medicine (cardiology), at Emory University, and chief quality officer, Emory Heart and Vascular Center, Emory Healthcare. He is also codirector of nuclear cardiology at Emory University Hospital.
Key points
- A diagnosis of a type 1 MI is supported by evidence or strong suspicion of acute coronary artery thrombus or plaque rupture/erosion.
- A very high troponin level alone is not diagnostic for a type 1 or type 2 MI. It has to be contextualized with the patient’s presentation and other supporting findings.
- Type 2 MI is a mismatch between myocardial oxygen supply and demand unrelated to acute coronary thrombosis or plaque rupture triggered by an abrupt increase in myocardial oxygen demand, drop in myocardial blood supply, or both. Type 2 MI should be documented along with its underlying cause.
- To diagnose an MI (either type 1 or type 2 MI), in addition to the troponin elevation, the patient must have symptoms of acute ischemia, ischemic ECG findings, and/or imaging suggestive of new ischemia.
- An elevated troponin level without new symptoms or ECG/imaging evidence of myocardial ischemia should be documented as a non-MI troponin elevation secondary to an underlying cause.
References
1. Goyal A, Gluckman TJ, Tcheng JE. What’s in a name? The new ICD-10 (10th revision of the international statistical classification of diseases and related health problems) codes and type 2 myocardial infarction. Circulation. 2017;136:1180-2.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;Aug 25:[Epub ahead of print].
3. Goyal, et al. Translating the Fourth Universal Definition of Myocardial Infarction into Clinical Documentation: Ten Pearls For Frontline Clinicians. Cardiology Magazine. Nov 2018.
4. Roongsritong C, Warraich I, Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: incidence and clinical significance. Chest. 2004;125:1877-84.
Hospitalists encounter troponin elevations daily, but we have to use clinical judgment to determine if the troponin elevation represents either a myocardial infarction (MI), or a non-MI troponin elevation (i.e. a , nonischemic myocardial injury).
It is important to remember that an MI specifically refers to myocardial injury due to acute myocardial ischemia to the myocardium. This lack of blood supply can be due to an acute absolute or relative deficiency in coronary artery blood flow. However, there are also many mechanisms of myocardial injury unrelated to reduced coronary artery blood flow, and these should be more appropriately termed non-MI troponin elevations.
Historically, when an ischemic mechanism of myocardial injury was suspected, providers would categorize troponin elevations into ST-elevation MI (STEMI) versus non-ST-elevation MI (NSTEMI) based on the electrocardiogram (ECG). We would further classify the NSTEMI into type 1 or type 2, depending on the mechanism of injury. The term “NSTEMI” served as a “catch-all” term to describe both type 1 NSTEMIs and type 2 MIs, but that classification system is no longer valid.
As of Oct. 1, 2017, ICD-10 and the Centers for Medicare & Medicaid Services have a new ICD-10 diagnosis code for type 2 MI (I21.A1), distinct from NSTEMI (I21.4) based on updated definitions from the American College of Cardiology, American Heart Association, European Society of Cardiology, and World Heart Federation. The term “NSTEMI” should be used only when referring to a type 1 MI not when referring to a type 2 MI.1
Classification of MI types
The Fourth Universal Definition of MI published in August 2018 further updated the definitions of MI (summarized in Figure 1).2 This review focuses on type 1 and type 2 MIs, which are the most common types encountered by hospitalists. Types 3-5 MI (grouped under a common ICD-10 diagnosis code for “Other MI Types,” or I21.A9) would rarely be diagnosed by hospitalists.
Figure 1: Classification of MI
MI Type | Classification |
1 | STEMI (acute coronary artery thrombosis) |
2 | Supply/demand mismatch (heterogeneous underlying causes) |
3 | Sudden cardiac death with ECG evidence of acute myocardial ischemia before cardiac troponins could be drawn |
4 | MI due to percutaneous coronary intervention (PCI) |
5 | MI due to coronary artery bypass grafting (CABG) |
The diagnosis of a type 1 MIs (STEMI and NSTEMI) is supported by the presence of an acute coronary thrombus or plaque rupture/erosion on coronary angiography or a strong suspicion for these when angiography is unavailable or contraindicated. Type 1 MI (also referred to as spontaneous MI) is generally a primary reason (or “principal” diagnosis) for a patient’s presentation to a hospital.3 Please note that a very high or rising troponin level alone is not diagnostic for a type 1 or type 2 NSTEMI. The lab has to be taken in the context of the patient’s presentation and other supporting findings.
In contrast to a type 1 MI (STEMI and NSTEMI), at type 2 MI results from an imbalance between myocardial oxygen supply and demand unrelated to acute coronary artery thrombosis or plaque rupture. A type 2 MI is a relative (as opposed to an absolute) deficiency in coronary artery blood flow triggered by an abrupt increase in myocardial oxygen demand, drop in myocardial blood supply, or both. In type 2 MI, myocardial injury occurs secondary to an underlying process, and therefore requires correct documentation of the underlying cause as well.
Common examples of underlying causes of type 2 MI include acute blood loss anemia (e.g. GI bleed), acute hypoxia (e.g. COPD exacerbation), shock states (cardiogenic, hypovolemic, hemorrhagic, or septic), coronary vasospasm (e.g. spontaneous), and bradyarrhythmias. Patients with type 2 MI often have a history of fixed obstructive coronary disease, which when coupled with the acute trigger facilitates the type 2 MI; however, underlying CAD is not always present.
Diagnosing a type 2 MI requires evidence of acute myocardial ischemia (Figure 2) with an elevated troponin but must also have at least one of the following:2
- Symptoms of acute myocardial ischemia such as typical chest pain.
- New ischemic ECG changes.
- Development of pathological Q waves.
- Imaging evidence of new loss of viable myocardium, significant reversible perfusion defect on nuclear imaging, or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.
Distinguishing a type 1 NSTEMI from a type 2 MI depends mainly on the clinical context and clinical judgment. A patient whose presenting symptoms include acute chest discomfort, acute ST-T wave changes, and a rise in troponin would be suspected of having a type 1 NSTEMI. However, in a patient presenting with other or vague complaints where an elevated troponin was found amongst a battery of tests, a type 2 MI may be favored, particularly if there is evidence of an underlying trigger for a supply-demand mismatch. In challenging cases, cardiology consultation can help determine the MI type and/or the next diagnostic and treatment considerations.
When there is only elevated troponin levels (or even a rise and fall in troponin) without new symptoms or ECG/imaging evidence of myocardial ischemia, it is most appropriate to document a non-MI troponin elevation due to a nonischemic mechanism of myocardial injury.
Non-MI troponin elevation (nonischemic myocardial injury)
The number of conditions known to cause myocardial injury through mechanisms other than myocardial ischemia (see Figure 2) is growing, especially in the current era of high-sensitivity troponin assays.4
Common examples of underlying causes of non-MI troponin elevation include:
- Acute (on chronic) systolic or diastolic heart failure: Usually due to acute ventricular wall stretch/strain. Troponin elevations tend to be mild, with more indolent (or even flat) troponin trajectories.
- Pericarditis and myocarditis: Due to direct injury from myocardial inflammation.
- Cardiopulmonary resuscitation (CPR): Due to physical injury to the heart from mechanical chest compressions and from electrical shocks of external defibrillation.
- Stress-induced (takotsubo) cardiomyopathy: Stress-induced release of neurohormonal factors and catecholamines that cause direct myocyte injury and transient dilatation of the ventricle.
- Acute pulmonary embolism: Result of acute right ventricular wall stretch/strain, not from myocardial ischemia.
- Sepsis without shock: Direct toxicity of circulating cytokines to cardiac myocytes. In the absence of evidence of shock and symptoms/signs of myocardial ischemia, do not document type 2 MI.
- Renal failure (acute kidney injury or chronic kidney disease): Multiple etiologies, but at least partially related to reduced renal clearance of troponin. In general, renal failure in the absence of symptoms/signs of ischemia is best classified as a non-MI troponin elevation. ESRD patients who present with volume overload due to missed dialysis also typically have a non-MI troponin elevation.
- Stroke/intracranial hemorrhage: Mechanisms of myocardial injury and troponin elevation are incompletely understood, but may include catecholamine surges that injure the heart.
Some underlying conditions can cause a type 2 MI or a non-MI troponin elevation depending on the clinical context. For example, hypertensive emergency, severe aortic valve stenosis, hypertrophic cardiomyopathy, and tachyarrhythmias (including atrial fibrillation with rapid ventricular response) may cause increased myocardial oxygen demand, and in patients with underlying CAD, could precipitate a type 2 MI.
However, these same conditions could cause a non-MI troponin elevation in patients without CAD and could also cause myocardial injury and troponin release by causing acute left ventricular stretch/strain. Distinguishing the diagnose of type 2 MI vs. non-MI troponin elevation depends on documenting whether there are ancillary ischemic symptoms, ECG findings, imaging, and/or cath findings of acute myocardial ischemia.
Case examples
1. A 60-year-old male presents with fever, cough, shortness of breath, and an infiltrate on CXR and is diagnosed with sepsis secondary to pneumonia. His initial troponin of 0.07 (normal < 0.05) rises to 0.11, peaks at 0.23, then subsequently trends down.
While some may be tempted to diagnose a type 2 MI, remember that sepsis can cause direct myocardial cell injury via direct cell toxicity. Unless this patient had at least one additional criteria (anginal chest pain, new ischemic ECG changes, or imaging evidence of new loss of viable myocardium, which does not recover with treatment of sepsis), this was most likely myocardial injury via direct cell toxicity, and should be documented as a non-MI troponin elevation due to sepsis without shock.
If there were ischemic ECG changes and the patient had chest pain, one would have to use clinical suspicion to differentiate between a type 1 NSTEMI and a type 2 MI. If there is a high clinical suspicion for an acute plaque rupture/thrombus, one would call it an NSTEMI and would have to document treatment as such (e.g. start heparin drip). Again, cardiology consultation can be helpful in cases where it may be hard to decide how to manage. Many times, the true mechanism is not determined until the patient is taken to the cath lab and if no acute plaque rupture is seen, then it was likely a type 2 MI.
2. A 70-year-old male with chronic systolic heart failure, noncompliant with medications, presents with 3 days of dyspnea on exertion and lower extremity edema. He had no chest discomfort. Exam shows bibasilar crackles and hepatojugular reflux. ECG shows no ischemic changes. Serial troponin values over 48 hours were: 0.48, 0.58, 0.51. A transthoracic echocardiogram reveals an LVEF of 40% with poor movement in the apex, similar to his prior echo.
This patient had no overt evidence of ischemia (no chest pain, ischemic ECG, or imaging changes) so the troponin elevation was most likely a non-MI troponin elevation secondary to acute on chronic systolic heart failure (in which the mechanism of troponin elevation is left ventricular chamber stretch from volume overload, and not demand ischemia). Generally, it is uncommon for a heart failure exacerbation to cause a type 2 MI.
Why is it so important to get this diagnosis right?
Misdiagnosing an MI when the patient does not have one can have multiple downstream repercussions. Because it stays on their medical record, it impacts their ability to get insurance and their premium costs. We expose patients to additional medications (e.g. dual antiplatelet therapy, statins), which can have adverse effects. As a result, it is very important to classify the etiology of the troponin elevation and treat accordingly.
Finally, when we incorrectly label a patient as having an MI, this can impact billing and reimbursement, DRG denials, insurance premiums, and quality metrics for both the hospital and the physicians. Hospitals’ 30-day readmission rates for AMI will suffer and quality metrics can be significantly impacted. We must be diligent and as precise as possible with our diagnoses and documentation to ensure the maximum benefit for our patients and our health care system.
Dr. Nave is assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta. Dr. Goyal is associate professor of medicine (cardiology), at Emory University, and chief quality officer, Emory Heart and Vascular Center, Emory Healthcare. He is also codirector of nuclear cardiology at Emory University Hospital.
Key points
- A diagnosis of a type 1 MI is supported by evidence or strong suspicion of acute coronary artery thrombus or plaque rupture/erosion.
- A very high troponin level alone is not diagnostic for a type 1 or type 2 MI. It has to be contextualized with the patient’s presentation and other supporting findings.
- Type 2 MI is a mismatch between myocardial oxygen supply and demand unrelated to acute coronary thrombosis or plaque rupture triggered by an abrupt increase in myocardial oxygen demand, drop in myocardial blood supply, or both. Type 2 MI should be documented along with its underlying cause.
- To diagnose an MI (either type 1 or type 2 MI), in addition to the troponin elevation, the patient must have symptoms of acute ischemia, ischemic ECG findings, and/or imaging suggestive of new ischemia.
- An elevated troponin level without new symptoms or ECG/imaging evidence of myocardial ischemia should be documented as a non-MI troponin elevation secondary to an underlying cause.
References
1. Goyal A, Gluckman TJ, Tcheng JE. What’s in a name? The new ICD-10 (10th revision of the international statistical classification of diseases and related health problems) codes and type 2 myocardial infarction. Circulation. 2017;136:1180-2.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;Aug 25:[Epub ahead of print].
3. Goyal, et al. Translating the Fourth Universal Definition of Myocardial Infarction into Clinical Documentation: Ten Pearls For Frontline Clinicians. Cardiology Magazine. Nov 2018.
4. Roongsritong C, Warraich I, Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: incidence and clinical significance. Chest. 2004;125:1877-84.
Infection with 2019 novel coronavirus extends to infants
between Dec. 8, 2019, and Feb. 6, 2020, based on data from the Chinese central government and local health departments.
“As of February 6, 2020, China reported 31,211 confirmed cases of COVID-19 and 637 fatalities,” wrote Min Wei, MD, of Wuhan University, China, and colleagues. However, “few infections in children have been reported.”
In a research letter published in JAMA, the investigators reviewed data from nine infants aged 28 days to 1 year who were hospitalized with a diagnosis of COVID-19 between Dec. 8, 2019, and Feb. 6, 2020. The ages of the infants ranged from 1 month to 11 months, and seven were female. The patients included two children from Beijing, two from Hainan, and one each from the areas of Guangdong, Anhui, Shanghai, Zhejiang, and Guizhou.
All infected infants had at least one infected family member, and the infants’ infections occurred after the family members’ infections; seven infants lived in Wuhan or had family members who had visited Wuhan.
One of the infants had no symptoms but tested positive for the 2019 novel coronavirus, and two others had a diagnosis but missing information on any symptoms. Fever occurred in four patients, and mild upper respiratory tract symptoms occurred in two patients.
None of the infants died, and none reported severe complications or the need for intensive care or mechanical ventilation, the investigators said. The fact that most of the infants were female might suggest that they are more susceptible to the virus than males, although overall COVID-19 viral infections have been more common in adult men, especially those with chronic comorbidities, Dr. Wei and associates noted.
The study findings were limited by the small sample size and lack of symptom data for some patients, the researchers said. However, the results confirm that the COVID-19 virus is transmissible to infants younger than 1 year, and adult caregivers should exercise protective measures including wearing masks, washing hands before contact with infants, and routinely sterilizing toys and tableware, they emphasized.
The study was supported by the National Natural Science Foundation of China and the Fundamental Research Funds for the Central Universities. The researchers had no financial conflicts to disclose.
SOURCE: Wei M et al. JAMA. 2020 Feb 14. doi:10.1001/jama.2020.2131.
between Dec. 8, 2019, and Feb. 6, 2020, based on data from the Chinese central government and local health departments.
“As of February 6, 2020, China reported 31,211 confirmed cases of COVID-19 and 637 fatalities,” wrote Min Wei, MD, of Wuhan University, China, and colleagues. However, “few infections in children have been reported.”
In a research letter published in JAMA, the investigators reviewed data from nine infants aged 28 days to 1 year who were hospitalized with a diagnosis of COVID-19 between Dec. 8, 2019, and Feb. 6, 2020. The ages of the infants ranged from 1 month to 11 months, and seven were female. The patients included two children from Beijing, two from Hainan, and one each from the areas of Guangdong, Anhui, Shanghai, Zhejiang, and Guizhou.
All infected infants had at least one infected family member, and the infants’ infections occurred after the family members’ infections; seven infants lived in Wuhan or had family members who had visited Wuhan.
One of the infants had no symptoms but tested positive for the 2019 novel coronavirus, and two others had a diagnosis but missing information on any symptoms. Fever occurred in four patients, and mild upper respiratory tract symptoms occurred in two patients.
None of the infants died, and none reported severe complications or the need for intensive care or mechanical ventilation, the investigators said. The fact that most of the infants were female might suggest that they are more susceptible to the virus than males, although overall COVID-19 viral infections have been more common in adult men, especially those with chronic comorbidities, Dr. Wei and associates noted.
The study findings were limited by the small sample size and lack of symptom data for some patients, the researchers said. However, the results confirm that the COVID-19 virus is transmissible to infants younger than 1 year, and adult caregivers should exercise protective measures including wearing masks, washing hands before contact with infants, and routinely sterilizing toys and tableware, they emphasized.
The study was supported by the National Natural Science Foundation of China and the Fundamental Research Funds for the Central Universities. The researchers had no financial conflicts to disclose.
SOURCE: Wei M et al. JAMA. 2020 Feb 14. doi:10.1001/jama.2020.2131.
between Dec. 8, 2019, and Feb. 6, 2020, based on data from the Chinese central government and local health departments.
“As of February 6, 2020, China reported 31,211 confirmed cases of COVID-19 and 637 fatalities,” wrote Min Wei, MD, of Wuhan University, China, and colleagues. However, “few infections in children have been reported.”
In a research letter published in JAMA, the investigators reviewed data from nine infants aged 28 days to 1 year who were hospitalized with a diagnosis of COVID-19 between Dec. 8, 2019, and Feb. 6, 2020. The ages of the infants ranged from 1 month to 11 months, and seven were female. The patients included two children from Beijing, two from Hainan, and one each from the areas of Guangdong, Anhui, Shanghai, Zhejiang, and Guizhou.
All infected infants had at least one infected family member, and the infants’ infections occurred after the family members’ infections; seven infants lived in Wuhan or had family members who had visited Wuhan.
One of the infants had no symptoms but tested positive for the 2019 novel coronavirus, and two others had a diagnosis but missing information on any symptoms. Fever occurred in four patients, and mild upper respiratory tract symptoms occurred in two patients.
None of the infants died, and none reported severe complications or the need for intensive care or mechanical ventilation, the investigators said. The fact that most of the infants were female might suggest that they are more susceptible to the virus than males, although overall COVID-19 viral infections have been more common in adult men, especially those with chronic comorbidities, Dr. Wei and associates noted.
The study findings were limited by the small sample size and lack of symptom data for some patients, the researchers said. However, the results confirm that the COVID-19 virus is transmissible to infants younger than 1 year, and adult caregivers should exercise protective measures including wearing masks, washing hands before contact with infants, and routinely sterilizing toys and tableware, they emphasized.
The study was supported by the National Natural Science Foundation of China and the Fundamental Research Funds for the Central Universities. The researchers had no financial conflicts to disclose.
SOURCE: Wei M et al. JAMA. 2020 Feb 14. doi:10.1001/jama.2020.2131.
FROM JAMA
As novel coronavirus outbreak evolves, critical care providers need to be prepared
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
EXPERT ANALYSIS FROM CCC49
Resetting your compensation
Using the State of Hospital Medicine Report to bolster your proposal
In the ever-changing world of health care, one thing is for sure: If you’re not paying attention, you’re falling behind. In this column, I will discuss how you may utilize the Society of Hospital Medicine (SHM) State of Hospital Medicine Report (SoHM) to evaluate your current compensation structure and strengthen your business plan for change. For purposes of this exercise, I will focus on data referenced as Internal Medicine only, hospital-owned Hospital Medicine Groups (HMG).
Issues with retention, recruitment, or burnout may be among the first factors that lead you to reevaluate your compensation plan. The SoHM Report can help you to take a dive into feedback for these areas. Look for any indications that compensation may be affecting your turnover, inability to hire, or leaving your current team frustrated with their current pay structure. Feedback surrounding each of these factors may drive you to evaluate your comp plan but remain mindful that money is not always the answer.
You may complete your evaluation and find the data could suggest you are in fact well paid for the work you do. Even though this may be the case, the evaluation and transparency to your provider team may help flush out the real reason you are struggling with recruitment, retention, or burnout. However, if you do find you have an opportunity to improve your compensation structure, remember that you will need a compelling, data-driven case, to present to your C-suite.
Let us start by understanding how the market has changed over time using data from the 2014, 2016, and 2018 SoHM Reports. Of note, each report is based on data from the prior year. Since 2013, hospital owned HMGs have seen a 16% increase in total compensation while experiencing only a 9% increase in collections. Meanwhile RVU productivity has remained relatively stable over time. From this, we see hospitalists are earning more for similar productivity. The hospital reimbursement for professional fees has not grown at the same rate as compensation. Also, the collection per RVU has remained relatively flat over time.
It’s simple: Hospitalists are earning more and professional revenues are not making up the difference. This market change is driving hospitals to invest more money to maintain their HMGs. If your hospital hasn’t been responding to these data, you will need a strong business plan to get buy in from your hospital administration.
Now that you have evaluated the market change, it is time to put some optics on where your compensation falls in the current market. When you combine your total compensation with your total RVU productivity, you can use the SoHM Report to evaluate the current reported benchmarks for Compensation per RVU. Plotting these benchmarks against your own compensation and any proposed changes can help your administration really begin to see whether a change should be considered. Providing that clear picture in relevance to the SoHM benchmark is important, as a chart or graph can simplify your C-suite’s understanding of your proposal.
By simplifying your example using Compensation per RVU, you are making the conversation easier to follow. Your hospital leaders can clearly see the cost for every RVU generated and understand the impact. This is not to say that you should base your compensation around productivity. It is merely a way to roll in all compensation factors, whether quality related, performance based, or productivity driven, and tie them to a metric that is clear and easy for administration to understand.
Remember, when designing your new compensation plan, you can reference the SoHM Report to see how HMGs around the country are providing incentive and what percentage of compensation is based on incentive. There are sections within the report directly outlining these data points.
Now that we have reviewed market change and how to visualize change between your current and proposed future state, I will leave you with some final thoughts regarding other considerations when building your business plan:
- Focus on only physician-generated RVUs.
- Consider Length of Stay impact on productivity.
- Decide if Case Mix Index changes have impacted your staffing needs.
- Understand your E and M coding practices in reference to industry benchmarks. The SoHM Report provides benchmarks for billing practices across the country.
- Lastly, clearly identify the issues you want to address and set goals with measurable outcomes.
There is still time for your group to be part of the 2020 State of Hospital Medicine Report data by participating in the 2020 Survey. Data are being accepted through Feb. 28, 2020. Submit your data at www.hospitalmedicine.org/2020survey.
Mr. Sandroni is director of operations, hospitalists, at Rochester (N.Y.) Regional Health.
Using the State of Hospital Medicine Report to bolster your proposal
Using the State of Hospital Medicine Report to bolster your proposal
In the ever-changing world of health care, one thing is for sure: If you’re not paying attention, you’re falling behind. In this column, I will discuss how you may utilize the Society of Hospital Medicine (SHM) State of Hospital Medicine Report (SoHM) to evaluate your current compensation structure and strengthen your business plan for change. For purposes of this exercise, I will focus on data referenced as Internal Medicine only, hospital-owned Hospital Medicine Groups (HMG).
Issues with retention, recruitment, or burnout may be among the first factors that lead you to reevaluate your compensation plan. The SoHM Report can help you to take a dive into feedback for these areas. Look for any indications that compensation may be affecting your turnover, inability to hire, or leaving your current team frustrated with their current pay structure. Feedback surrounding each of these factors may drive you to evaluate your comp plan but remain mindful that money is not always the answer.
You may complete your evaluation and find the data could suggest you are in fact well paid for the work you do. Even though this may be the case, the evaluation and transparency to your provider team may help flush out the real reason you are struggling with recruitment, retention, or burnout. However, if you do find you have an opportunity to improve your compensation structure, remember that you will need a compelling, data-driven case, to present to your C-suite.
Let us start by understanding how the market has changed over time using data from the 2014, 2016, and 2018 SoHM Reports. Of note, each report is based on data from the prior year. Since 2013, hospital owned HMGs have seen a 16% increase in total compensation while experiencing only a 9% increase in collections. Meanwhile RVU productivity has remained relatively stable over time. From this, we see hospitalists are earning more for similar productivity. The hospital reimbursement for professional fees has not grown at the same rate as compensation. Also, the collection per RVU has remained relatively flat over time.
It’s simple: Hospitalists are earning more and professional revenues are not making up the difference. This market change is driving hospitals to invest more money to maintain their HMGs. If your hospital hasn’t been responding to these data, you will need a strong business plan to get buy in from your hospital administration.
Now that you have evaluated the market change, it is time to put some optics on where your compensation falls in the current market. When you combine your total compensation with your total RVU productivity, you can use the SoHM Report to evaluate the current reported benchmarks for Compensation per RVU. Plotting these benchmarks against your own compensation and any proposed changes can help your administration really begin to see whether a change should be considered. Providing that clear picture in relevance to the SoHM benchmark is important, as a chart or graph can simplify your C-suite’s understanding of your proposal.
By simplifying your example using Compensation per RVU, you are making the conversation easier to follow. Your hospital leaders can clearly see the cost for every RVU generated and understand the impact. This is not to say that you should base your compensation around productivity. It is merely a way to roll in all compensation factors, whether quality related, performance based, or productivity driven, and tie them to a metric that is clear and easy for administration to understand.
Remember, when designing your new compensation plan, you can reference the SoHM Report to see how HMGs around the country are providing incentive and what percentage of compensation is based on incentive. There are sections within the report directly outlining these data points.
Now that we have reviewed market change and how to visualize change between your current and proposed future state, I will leave you with some final thoughts regarding other considerations when building your business plan:
- Focus on only physician-generated RVUs.
- Consider Length of Stay impact on productivity.
- Decide if Case Mix Index changes have impacted your staffing needs.
- Understand your E and M coding practices in reference to industry benchmarks. The SoHM Report provides benchmarks for billing practices across the country.
- Lastly, clearly identify the issues you want to address and set goals with measurable outcomes.
There is still time for your group to be part of the 2020 State of Hospital Medicine Report data by participating in the 2020 Survey. Data are being accepted through Feb. 28, 2020. Submit your data at www.hospitalmedicine.org/2020survey.
Mr. Sandroni is director of operations, hospitalists, at Rochester (N.Y.) Regional Health.
In the ever-changing world of health care, one thing is for sure: If you’re not paying attention, you’re falling behind. In this column, I will discuss how you may utilize the Society of Hospital Medicine (SHM) State of Hospital Medicine Report (SoHM) to evaluate your current compensation structure and strengthen your business plan for change. For purposes of this exercise, I will focus on data referenced as Internal Medicine only, hospital-owned Hospital Medicine Groups (HMG).
Issues with retention, recruitment, or burnout may be among the first factors that lead you to reevaluate your compensation plan. The SoHM Report can help you to take a dive into feedback for these areas. Look for any indications that compensation may be affecting your turnover, inability to hire, or leaving your current team frustrated with their current pay structure. Feedback surrounding each of these factors may drive you to evaluate your comp plan but remain mindful that money is not always the answer.
You may complete your evaluation and find the data could suggest you are in fact well paid for the work you do. Even though this may be the case, the evaluation and transparency to your provider team may help flush out the real reason you are struggling with recruitment, retention, or burnout. However, if you do find you have an opportunity to improve your compensation structure, remember that you will need a compelling, data-driven case, to present to your C-suite.
Let us start by understanding how the market has changed over time using data from the 2014, 2016, and 2018 SoHM Reports. Of note, each report is based on data from the prior year. Since 2013, hospital owned HMGs have seen a 16% increase in total compensation while experiencing only a 9% increase in collections. Meanwhile RVU productivity has remained relatively stable over time. From this, we see hospitalists are earning more for similar productivity. The hospital reimbursement for professional fees has not grown at the same rate as compensation. Also, the collection per RVU has remained relatively flat over time.
It’s simple: Hospitalists are earning more and professional revenues are not making up the difference. This market change is driving hospitals to invest more money to maintain their HMGs. If your hospital hasn’t been responding to these data, you will need a strong business plan to get buy in from your hospital administration.
Now that you have evaluated the market change, it is time to put some optics on where your compensation falls in the current market. When you combine your total compensation with your total RVU productivity, you can use the SoHM Report to evaluate the current reported benchmarks for Compensation per RVU. Plotting these benchmarks against your own compensation and any proposed changes can help your administration really begin to see whether a change should be considered. Providing that clear picture in relevance to the SoHM benchmark is important, as a chart or graph can simplify your C-suite’s understanding of your proposal.
By simplifying your example using Compensation per RVU, you are making the conversation easier to follow. Your hospital leaders can clearly see the cost for every RVU generated and understand the impact. This is not to say that you should base your compensation around productivity. It is merely a way to roll in all compensation factors, whether quality related, performance based, or productivity driven, and tie them to a metric that is clear and easy for administration to understand.
Remember, when designing your new compensation plan, you can reference the SoHM Report to see how HMGs around the country are providing incentive and what percentage of compensation is based on incentive. There are sections within the report directly outlining these data points.
Now that we have reviewed market change and how to visualize change between your current and proposed future state, I will leave you with some final thoughts regarding other considerations when building your business plan:
- Focus on only physician-generated RVUs.
- Consider Length of Stay impact on productivity.
- Decide if Case Mix Index changes have impacted your staffing needs.
- Understand your E and M coding practices in reference to industry benchmarks. The SoHM Report provides benchmarks for billing practices across the country.
- Lastly, clearly identify the issues you want to address and set goals with measurable outcomes.
There is still time for your group to be part of the 2020 State of Hospital Medicine Report data by participating in the 2020 Survey. Data are being accepted through Feb. 28, 2020. Submit your data at www.hospitalmedicine.org/2020survey.
Mr. Sandroni is director of operations, hospitalists, at Rochester (N.Y.) Regional Health.
Work the program for NP/PAs, and the program will work
A ‘knowledge gap’ in best practices exists
Hospital medicine has been the fastest growing medical specialty since the term “hospitalist” was coined by Bob Wachter, MD, in the famous 1996 New England Journal of Medicine article (doi: 10.1056/NEJM199608153350713). The growth and change within this specialty is also reflected in the changing and migrating target of hospitals and hospital systems as they continue to effectively and safely move from fee-for-service to a payer model that rewards value and improvement in the health of a population – both in and outside of hospital walls.
In a short time, nurse practitioners and physician assistants have become a growing population in the hospital medicine workforce. The 2018 State of Hospital Medicine Report notes a 42% increase in 4 years, and about 75% of hospital medicine groups across the country currently incorporate NP/PAs within a hospital medicine practice. This evolution has occurred in the setting of a looming and well-documented physician shortage, a variety of cost pressures on hospitals that reflect the need for an efficient and cost-effective care delivery model, an increasing NP/PA workforce (the Department of Labor notes increases of 35% and 36% respectively by 2036), and data that indicates similar outcomes, for example, HCAHPS (the Hospital Consumer Assessment of Healthcare Providers and Systems), readmission, and morbidity and mortality in NP/PA-driven care.
This evolution, however, reveals a true knowledge gap in best practices related to integration of these providers. This is impacted by wide variability in the preparation of NPs – they may enter hospitalist practice from a variety of clinical exposures and training, for example, adult gerontology acute care, adult, or even, in some states, family NPs. For PAs, this is reflected in the variety of clinical rotations and pregraduate clinical exposure.
This variability is compounded, too, by the lack of standardization of hospital medicine practices, both with site size and patient acuity, a variety of challenges that drive the need for integration of NP/PA providers, and by-laws that define advanced practice clinical models and function.
In that perspective, it is important to define what constitutes a leading and successful advanced practice provider (APP) integration program. I would suggest:
- A structured and formalized transition-to-practice program for all new graduates and those new to hospital medicine. This program should consist of clinical volume progression, formalized didactic congruent with the Society of Hospital Medicine Core Competencies, and a process for evaluating knowledge and decision making throughout the program and upon completion.
- Development of physician competencies related to APP integration. Physicians are not prepared in their medical school training or residency to understand the differences and similarities of NP/PA providers. These competencies should be required and can best be developed through steady leadership, formalized instruction and accountability for professional teamwork.
- Allowance for NP/PA providers to work at the top of their skills and license. This means utilizing NP/PAs as providers who care for patients – not as scribes or clerical workers. The evolution of the acuity of patients provided for may evolve with the skill set and experience of NP/PAs, but it will evolve – especially if steps 1 and 2 are in place.
- Productivity expectations that reach near physician level of volume. In 2016 State of Hospital Medicine Report data, yearly billable encounters for NP/PAs were within 10% of that of physicians. I think 15% is a reasonable goal.
- Implementation and support of APP administrative leadership structure at the system/site level. This can be as simple as having APPs on the same leadership committees as physician team members, being involved in hiring and training newer physicians and NP/PAs or as broad as having all NP/PAs report to an APP leader. Having an intentional leadership structure that demonstrates and reflects inclusivity and belonging is crucial.
Consistent application of these frameworks will provide a strong infrastructure for successful NP/PA practice.
Ms. Cardin is currently the vice president of advanced practice providers at Sound Physicians and serves on SHM’s board of directors as its secretary. This article appeared initially at the Hospital Leader, the official blog of SHM.
A ‘knowledge gap’ in best practices exists
A ‘knowledge gap’ in best practices exists
Hospital medicine has been the fastest growing medical specialty since the term “hospitalist” was coined by Bob Wachter, MD, in the famous 1996 New England Journal of Medicine article (doi: 10.1056/NEJM199608153350713). The growth and change within this specialty is also reflected in the changing and migrating target of hospitals and hospital systems as they continue to effectively and safely move from fee-for-service to a payer model that rewards value and improvement in the health of a population – both in and outside of hospital walls.
In a short time, nurse practitioners and physician assistants have become a growing population in the hospital medicine workforce. The 2018 State of Hospital Medicine Report notes a 42% increase in 4 years, and about 75% of hospital medicine groups across the country currently incorporate NP/PAs within a hospital medicine practice. This evolution has occurred in the setting of a looming and well-documented physician shortage, a variety of cost pressures on hospitals that reflect the need for an efficient and cost-effective care delivery model, an increasing NP/PA workforce (the Department of Labor notes increases of 35% and 36% respectively by 2036), and data that indicates similar outcomes, for example, HCAHPS (the Hospital Consumer Assessment of Healthcare Providers and Systems), readmission, and morbidity and mortality in NP/PA-driven care.
This evolution, however, reveals a true knowledge gap in best practices related to integration of these providers. This is impacted by wide variability in the preparation of NPs – they may enter hospitalist practice from a variety of clinical exposures and training, for example, adult gerontology acute care, adult, or even, in some states, family NPs. For PAs, this is reflected in the variety of clinical rotations and pregraduate clinical exposure.
This variability is compounded, too, by the lack of standardization of hospital medicine practices, both with site size and patient acuity, a variety of challenges that drive the need for integration of NP/PA providers, and by-laws that define advanced practice clinical models and function.
In that perspective, it is important to define what constitutes a leading and successful advanced practice provider (APP) integration program. I would suggest:
- A structured and formalized transition-to-practice program for all new graduates and those new to hospital medicine. This program should consist of clinical volume progression, formalized didactic congruent with the Society of Hospital Medicine Core Competencies, and a process for evaluating knowledge and decision making throughout the program and upon completion.
- Development of physician competencies related to APP integration. Physicians are not prepared in their medical school training or residency to understand the differences and similarities of NP/PA providers. These competencies should be required and can best be developed through steady leadership, formalized instruction and accountability for professional teamwork.
- Allowance for NP/PA providers to work at the top of their skills and license. This means utilizing NP/PAs as providers who care for patients – not as scribes or clerical workers. The evolution of the acuity of patients provided for may evolve with the skill set and experience of NP/PAs, but it will evolve – especially if steps 1 and 2 are in place.
- Productivity expectations that reach near physician level of volume. In 2016 State of Hospital Medicine Report data, yearly billable encounters for NP/PAs were within 10% of that of physicians. I think 15% is a reasonable goal.
- Implementation and support of APP administrative leadership structure at the system/site level. This can be as simple as having APPs on the same leadership committees as physician team members, being involved in hiring and training newer physicians and NP/PAs or as broad as having all NP/PAs report to an APP leader. Having an intentional leadership structure that demonstrates and reflects inclusivity and belonging is crucial.
Consistent application of these frameworks will provide a strong infrastructure for successful NP/PA practice.
Ms. Cardin is currently the vice president of advanced practice providers at Sound Physicians and serves on SHM’s board of directors as its secretary. This article appeared initially at the Hospital Leader, the official blog of SHM.
Hospital medicine has been the fastest growing medical specialty since the term “hospitalist” was coined by Bob Wachter, MD, in the famous 1996 New England Journal of Medicine article (doi: 10.1056/NEJM199608153350713). The growth and change within this specialty is also reflected in the changing and migrating target of hospitals and hospital systems as they continue to effectively and safely move from fee-for-service to a payer model that rewards value and improvement in the health of a population – both in and outside of hospital walls.
In a short time, nurse practitioners and physician assistants have become a growing population in the hospital medicine workforce. The 2018 State of Hospital Medicine Report notes a 42% increase in 4 years, and about 75% of hospital medicine groups across the country currently incorporate NP/PAs within a hospital medicine practice. This evolution has occurred in the setting of a looming and well-documented physician shortage, a variety of cost pressures on hospitals that reflect the need for an efficient and cost-effective care delivery model, an increasing NP/PA workforce (the Department of Labor notes increases of 35% and 36% respectively by 2036), and data that indicates similar outcomes, for example, HCAHPS (the Hospital Consumer Assessment of Healthcare Providers and Systems), readmission, and morbidity and mortality in NP/PA-driven care.
This evolution, however, reveals a true knowledge gap in best practices related to integration of these providers. This is impacted by wide variability in the preparation of NPs – they may enter hospitalist practice from a variety of clinical exposures and training, for example, adult gerontology acute care, adult, or even, in some states, family NPs. For PAs, this is reflected in the variety of clinical rotations and pregraduate clinical exposure.
This variability is compounded, too, by the lack of standardization of hospital medicine practices, both with site size and patient acuity, a variety of challenges that drive the need for integration of NP/PA providers, and by-laws that define advanced practice clinical models and function.
In that perspective, it is important to define what constitutes a leading and successful advanced practice provider (APP) integration program. I would suggest:
- A structured and formalized transition-to-practice program for all new graduates and those new to hospital medicine. This program should consist of clinical volume progression, formalized didactic congruent with the Society of Hospital Medicine Core Competencies, and a process for evaluating knowledge and decision making throughout the program and upon completion.
- Development of physician competencies related to APP integration. Physicians are not prepared in their medical school training or residency to understand the differences and similarities of NP/PA providers. These competencies should be required and can best be developed through steady leadership, formalized instruction and accountability for professional teamwork.
- Allowance for NP/PA providers to work at the top of their skills and license. This means utilizing NP/PAs as providers who care for patients – not as scribes or clerical workers. The evolution of the acuity of patients provided for may evolve with the skill set and experience of NP/PAs, but it will evolve – especially if steps 1 and 2 are in place.
- Productivity expectations that reach near physician level of volume. In 2016 State of Hospital Medicine Report data, yearly billable encounters for NP/PAs were within 10% of that of physicians. I think 15% is a reasonable goal.
- Implementation and support of APP administrative leadership structure at the system/site level. This can be as simple as having APPs on the same leadership committees as physician team members, being involved in hiring and training newer physicians and NP/PAs or as broad as having all NP/PAs report to an APP leader. Having an intentional leadership structure that demonstrates and reflects inclusivity and belonging is crucial.
Consistent application of these frameworks will provide a strong infrastructure for successful NP/PA practice.
Ms. Cardin is currently the vice president of advanced practice providers at Sound Physicians and serves on SHM’s board of directors as its secretary. This article appeared initially at the Hospital Leader, the official blog of SHM.
What you absolutely need to know about tail coverage
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.