User login
Effects of Lumbar Fusion and Dual-Mobility Liners on Dislocation Rates Following Total Hip Arthroplasty in a Veteran Population
Effects of Lumbar Fusion and Dual-Mobility Liners on Dislocation Rates Following Total Hip Arthroplasty in a Veteran Population
Total hip arthroplasty (THA) is among the most common elective orthopedic procedures performed annually in the United States, with an estimated 635,000 to 909,000 THAs expected each year by 2030.1 Consequently, complication rates and revision surgeries related to THA have been increasing, along with the financial burden on the health care system.2-4 Optimizing outcomes for patients undergoing THA and identifying risk factors for treatment failure have become areas of focus.
Over the last decade, there has been a renewed interest in the effect of previous lumbar spine fusion (LSF) surgery on THA outcomes. Studies have explored the rates of complications, postoperative mobility, and THA implant impingement.5-8 However, the outcome receiving the most attention in recent literature is the rate and effect of dislocation in patients with lumbar fusion surgery. Large Medicare database analyses have discovered an association with increased rates of dislocations in patients with lumbar fusion surgeries compared with those without.9,10 Prosthetic hip dislocation is an expensive complication of THA and is projected to have greater impact through 2035 due to a growing number of THA procedures.11 Identifying risk factors associated with hip dislocation is paramount to mitigating its effect on patients who have undergone THA.
Recent research has found increased rates of THA dislocation and revision surgery in patients with LSF, with some studies showing previous LSF as the strongest independent predictor.6-16 However, controversy surrounds this relationship, including the sequence of procedures (LSF before or after THA), the time between procedures, and involvement of the sacrum in LSF. One study found that patients had a 106% increased risk of dislocation when LSF was performed before THA compared with patients who underwent LSF 5 years after undergoing THA, while another study showed no significant difference in dislocations pre- vs post-LSF.16,17 An additional study showed no significant difference in the rate of dislocation in patients without sacral involvement in the LSF, while also showing significantly higher rates of dislocation in LSF with sacral involvement.12 The researchers also found a trend toward more dislocations in longer lumbosacral fusions. Recent studies have also examined dislocation rates with lumbar fusion in patients treated with dual-mobility liners.18-20 The consensus from these studies is that dual-mobility liners significantly decrease the rate of dislocation in primary THAs with lumbar fusion.
The present study sought to determine the rates of hip dislocations in a US Department of Veterans Affairs (VA) hospital setting. To the authors’ knowledge, no retrospective study focusing on THAs in the veteran population has been performed. This study benefits from controlling for various surgeon techniques and surgical preferences when compared to large Medicare database studies because the orthopedic surgeon (ABK) only performed the posterior approach for all patients during the study period.
The primary objective of this study was to determine whether the rates of hip dislocation would, in fact, be higher in patients with lumbar fusion surgery, as recent database studies suggest. Secondary objectives included determining whether patient characteristics, comorbidities, number of levels fused, or inclusion of the sacrum in the fusion construct influenced dislocation rates. Furthermore, VA Dayton Healthcare System (VADHS) began routine use of dual-mobility liners for lumbar fusion patients in 2018, allowing for examination of these patients.
Methods
The Wright State University and VADHS Institutional Review Board approved this study design. A retrospective review of all primary THAs at VADHS was performed to investigate the relationship between previous lumbar spine fusion and the incidence of THA revision. Manual chart review was performed for patients who underwent primary THA between January 2003, and December 2022. One surgeon performed all surgeries using only the posterior approach. Patients were not excluded if they had bilateral procedures and all eligible hips were included. Patients with a concomitant diagnosis of fracture of the femoral head or femoral neck at the time of surgery were excluded. Additionally, only patients with ≥ 12 months of follow-up data were included.
The primary outcome was dislocation within 12 months of THA; the primary independent variable was LSF prior to THA. Covariates included patient demographics (age, sex, body mass index [BMI]) and Charlson Comorbidity Index (CCI) score, with additional data collected on the number of levels fused, sacral spine involvement, revision rates, and use of dual-mobility liners. Year of surgery was also included in analyses to account for any changes that may have occurred during the study period.
Statistical Analysis
Statistical analyses were performed in SAS 9.4. Patients were grouped into 2 cohorts, depending on whether they had received LSF prior to THA. Analyses were adjusted for repeated measures to account for the small percentage of patients with bilateral procedures.
Univariate comparisons between cohorts for covariates, as well as rates of dislocation and revision, were performed using the independent samples t test for continuous variables and the Fisher exact test for dichotomous categorical variables. Significant comorbidities, as well as age, sex, BMI, liner type, LSF cohort, and surgery year, were included in a logistic regression model to determine what effect, if any, they had on the likelihood of dislocation. Variables were removed using a backward stepwise approach, starting with the nonsignificant variable effect with the lowest χ2 value, and continuing until reaching a final model where all remaining variable effects were significant. For the variables retained in the final model, odds ratios (ORs) with 95% CIs were derived, with dislocation designated as the event. Individual comorbidity subcomponents of the CCI were also analyzed for their effects on dislocation using backward stepwise logistic regression. A secondary analysis among patients with LSF tested for the influence of the number of vertebral levels fused, the presence or absence of sacral involvement in the fusion, and the use of dual-mobility liners on the likelihood of hip dislocation.
Results
The LSF cohort included 39 patients with THA and prior LSF, 3 of whom had bilateral procedures, for a total of 42 hips. The non-LSF cohort included 813 patients with THA, 112 of whom had bilateral procedures, for a total of 925 hips. The LSF and non-LSF cohorts did not differ significantly in age, sex, BMI, CCI, or revision rates (Table). The LSF cohort included a significantly higher percentage of hips receiving dual-mobility liners than did the non-LSF cohort (23.8% vs 0.6%; P < .001) and had more than twice the rate of dislocation (4 of 42 hips [9.5%] vs 35 of 925 hips [3.8%]), although this difference was not statistically significant (P = .08).
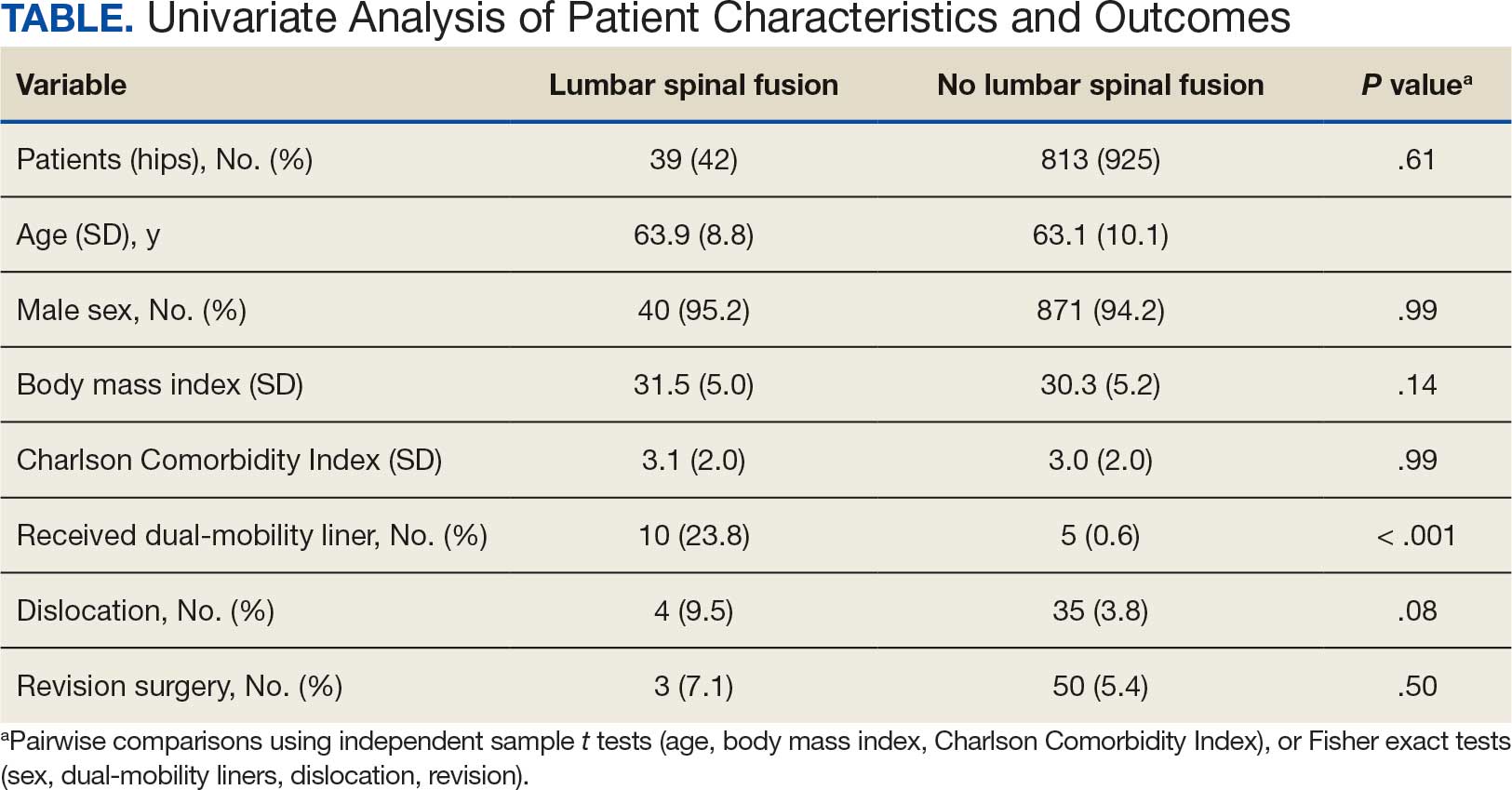
The final logistic regression model with dislocation as the outcome was statistically significant (χ2, 17.47; P < .001) and retained 2 significant predictor variables: LSF cohort (χ2, 4.63; P = .03), and sex (χ2, 18.27; P < .001). Females were more likely than males to experience dislocation (OR, 5.84; 95% CI, 2.60-13.13; P < .001) as were patients who had LSF prior to THA (OR, 3.42; 95% CI, 1.12-10.47; P = .03) (Figure). None of the CCI subcomponent comorbidities significantly affected the probability of dislocation (myocardial infarction, P = .46; congestive heart failure, P = .47; peripheral vascular disease, P = .97; stroke, P = .51; dementia, P = .99; chronic obstructive pulmonary disease, P = .95; connective tissue disease, P = .25; peptic ulcer, P = .41; liver disease, P = .30; diabetes, P = .06; hemiplegia, P = .99; chronic kidney disease, P = .82; solid tumor, P = .90; leukemia, P = .99; lymphoma, P = .99; AIDS, P = .99). Within the LSF cohort, neither the number of levels fused (P = .83) nor sacral involvement (P = .42), significantly affected the probability of hip dislocation. None of the patients in either cohort who received dual-mobility liners subsequently dislocated their hips, nor did any of them require revision surgery.
Discussion
Spinopelvic biomechanics have been an area of increasing interest and research. Spinal fusion has been shown to alter the mobility of the pelvis and has been associated with decreased stability of THA implants.21 For example, in the setting of a fused spine, the lack of compensatory changes in pelvic tilt or acetabular anteversion when adjusting to a seated or standing position may predispose patients to impingement because the acetabular component is not properly positioned. Dual-mobility constructs mitigate this risk by providing an additional articulation, which increases jump distance and range of motion prior to impingement, thereby enhancing stability.
The use of dual-mobility liners in patients with LSF has also been examined.18-20 These studies demonstrate a reduced risk of postoperative THA dislocation in patients with previous LSF. The rate of postoperative complications and revisions for LSF patients with dual-mobility liners was also found to be similar to that of THAs without dual-mobility in patients without prior LSF. This study focused on a veteran population to demonstrate the efficacy of dual-mobility liners in patients with LSF. The results indicate that LSF prior to THA and female sex were predictors for prosthetic hip dislocations in the 12-month postoperative period in this patient population, which aligns with the current literature.
The dislocation rate in the LSF-THA group (9.5%) was higher than the dislocation rate in the control group (3.8%). Although not statistically significant in the univariate analysis, LSF was shown to be a significant risk factor after controlling for patient sex. Other studies have found the dislocation rate to be 3% to 7%, which is lower than the dislocation rate observed in this study.8,10,16
The reasons for this higher rate of dislocation are not entirely clear. A veteran population has poorer overall health than the general population, which may contribute to the higher than previously reported dislocation rates.22 These results can be applied to the management of veterans seeking THA.
There have been conflicting reports regarding the impact a patient’s sex has on THA outcomes in the general population.23-26 This study found that female patients had higher rates of dislocation within 1 year of THA than male patients. This difference, which could be due to differences in baseline anatomic hip morphology between the sexes; females tend to have smaller femoral head sizes and less offset compared with males.27,28 However, this finding could have been confounded by the small number of female veterans in the study cohort.
A type 2 diabetes mellitus (T2DM) diagnosis, which is a component of CCI, trended toward increased risk of prosthetic hip dislocation. Multiple studies have also discussed the increased risk of postoperative infections and revisions following THA in patients with T2DM.29-31 One study found T2DM to be an independent risk factor for immediate in-hospital postoperative complications following hip arthroplasty.32
Another factor that may influence postoperative dislocation risk is surgical approach. The posterior approach has historically been associated with higher rates of instability when compared to anterior or lateral THA.33 Researchers have also looked at the role that surgical approach plays in patients with prior LSF. Huebschmann et al confirmed that not only is LSF a significant risk factor for dislocation following THA, but anterior and laterally based surgical approaches may mitigate this risk.34
Limitations
As a retrospective cohort study, the reliability of the data hinges on complete documentation. Documentation of all encounters for dislocations was obtained from the VA Computerized Patient Record System, which may have led to some dislocation events being missed. However, as long as there was adequate postoperative follow-up, it was assumed all events outside the VA were included. Another limitation of this study was that male patients greatly outnumbered female patients, and this fact could limit the generalizability of findings to the population as a whole.
Conclusions
This study in a veteran population found that prior LSF and female sex were significant predictors for postoperative dislocation within 1 year of THA surgery. Additionally, the use of a dual-mobility liner was found to be protective against postoperative dislocation events. These data allow clinicians to better counsel veterans on the risk factors associated with postoperative dislocation and strategies to mitigate this risk.
- Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455-1460. doi:10.2106/JBJS.17.01617
- Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128-133. doi:10.2106/JBJS.H.00155
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:144-151. doi:10.2106/JBJS.G.00587
- Kurtz SM, Ong KL, Schmier J, et al. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24:195-203. doi:10.1016/j.arth.2007.11.015
- Yamato Y, Furuhashi H, Hasegawa T, et al. Simulation of implant impingement after spinal corrective fusion surgery in patients with previous total hip arthroplasty: a retrospective case series. Spine (Phila Pa 1976). 2021;46:512-519. doi:10.1097/BRS.0000000000003836
- Mudrick CA, Melvin JS, Springer BD. Late posterior hip instability after lumbar spinopelvic fusion. Arthroplast Today. 2015;1:25-29. doi:10.1016/j.artd.2015.05.002
- Diebo BG, Beyer GA, Grieco PW, et al. Complications in patients undergoing spinal fusion after THA. Clin Orthop Relat Res. 2018;476:412-417.doi:10.1007/s11999.0000000000000009 8.
- Sing DC, Barry JJ, Aguilar TU, et al. Prior lumbar spinal arthrodesis increases risk of prosthetic-related complication in total hip arthroplasty. J Arthroplasty. 2016;31:227-232.e1. doi:10.1016/j.arth.2016.02.069
- King CA, Landy DC, Martell JM, et al. Time to dislocation analysis of lumbar spine fusion following total hip arthroplasty: breaking up a happy home. J Arthroplasty. 2018;33:3768-3772. doi:10.1016/j.arth.2018.08.029
- Buckland AJ, Puvanesarajah V, Vigdorchik J, et al. Dislocation of a primary total hip arthroplasty is more common in patients with a lumbar spinal fusion. Bone Joint J. 2017;99-B:585-591.doi:10.1302/0301-620X.99B5.BJJ-2016-0657.R1
- Pirruccio K, Premkumar A, Sheth NP. The burden of prosthetic hip dislocations in the United States is projected to significantly increase by 2035. Hip Int. 2021;31:714-721. doi:10.1177/1120700020923619
- Salib CG, Reina N, Perry KI, et al. Lumbar fusion involving the sacrum increases dislocation risk in primary total hip arthroplasty. Bone Joint J. 2019;101-B:198-206. doi:10.1302/0301-620X.101B2.BJJ-2018-0754.R1
- An VVG, Phan K, Sivakumar BS, et al. Prior lumbar spinal fusion is associated with an increased risk of dislocation and revision in total hip arthroplasty: a meta-analysis. J Arthroplasty. 2018;33:297-300. doi:10.1016/j.arth.2017.08.040
- Klemt C, Padmanabha A, Tirumala V, et al. Lumbar spine fusion before revision total hip arthroplasty is associated with increased dislocation rates. J Am Acad Orthop Surg. 2021;29:e860-e868. doi:10.5435/JAAOS-D-20-00824
- Gausden EB, Parhar HS, Popper JE, et al. Risk factors for early dislocation following primary elective total hip arthroplasty. J Arthroplasty. 2018;33:1567-1571. doi:10.1016/j.arth.2017.12.034
- Malkani AL, Himschoot KJ, Ong KL, et al. Does timing of primary total hip arthroplasty prior to or after lumbar spine fusion have an effect on dislocation and revision rates?. J Arthroplasty. 2019;34:907-911. doi:10.1016/j.arth.2019.01.009
- Parilla FW, Shah RR, Gordon AC, et al. Does it matter: total hip arthroplasty or lumbar spinal fusion first? Preoperative sagittal spinopelvic measurements guide patient-specific surgical strategies in patients requiring both. J Arthroplasty. 2019;34:2652-2662. doi:10.1016/j.arth.2019.05.053
- Chalmers BP, Syku M, Sculco TP, et al. Dual-mobility constructs in primary total hip arthroplasty in high-risk patients with spinal fusions: our institutional experience. Arthroplast Today. 2020;6:749-754. doi:10.1016/j.artd.2020.07.024
- Nessler JM, Malkani AL, Sachdeva S, et al. Use of dual mobility cups in patients undergoing primary total hip arthroplasty with prior lumbar spine fusion. Int Orthop. 2020;44:857-862. doi:10.1007/s00264-020-04507-y
- Nessler JM, Malkani AL, Yep PJ, et al. Dislocation rates of primary total hip arthroplasty in patients with prior lumbar spine fusion and lumbar degenerative disk disease with and without utilization of dual mobility cups: an American Joint Replacement Registry study. J Am Acad Orthop Surg. 2023;31:e271-e277. doi:10.5435/JAAOS-D-22-00767
- Phan D, Bederman SS, Schwarzkopf R. The influence of sagittal spinal deformity on anteversion of the acetabular component in total hip arthroplasty. Bone Joint J. 2015;97-B:1017-1023. doi:10.1302/0301-620X.97B8.35700
- Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252-3257. doi:10.1001/archinte.160.21.325223.
- Basques BA, Bell JA, Fillingham YA, et al. Gender differences for hip and knee arthroplasty: complications and healthcare utilization. J Arthroplasty. 2019;34:1593-1597.e1. doi:10.1016/j.arth.2019.03.064
- Kim YH, Choi Y, Kim JS. Influence of patient-, design-, and surgery-related factors on rate of dislocation after primary cementless total hip arthroplasty. J Arthroplasty. 2009;24:1258-1263. doi:10.1016/j.arth.2009.03.017
- Chen A, Paxton L, Zheng X, et al. Association of sex with risk of 2-year revision among patients undergoing total hip arthroplasty. JAMA Netw Open. 2021;4:e2110687. doi:10.1001/jamanetworkopen.2021.10687
- Inacio MCS, Ake CF, Paxton EW, et al. Sex and risk of hip implant failure: assessing total hip arthroplasty outcomes in the United States. JAMA Intern Med. 2013;173:435-441. doi:10.1001/jamainternmed.2013.3271
- Karlson EW, Daltroy LH, Liang MH, et al. Gender differences in patient preferences may underlie differential utilization of elective surgery. Am J Med. 1997;102:524-530. doi:10.1016/s0002-9343(97)00050-8
- Kostamo T, Bourne RB, Whittaker JP, et al. No difference in gender-specific hip replacement outcomes. Clin Orthop Relat Res. 2009;467:135-140. doi:10.1007/s11999-008-0466-2
- Papagelopoulos PJ, Idusuyi OB, Wallrichs SL, et al. Long term outcome and survivorship analysis of primary total knee arthroplasty in patients with diabetes mellitus. Clin Orthop Relat Res. 1996;(330):124-132. doi:10.1097/00003086-199609000-00015
- Fitzgerald RH Jr, Nolan DR, Ilstrup DM, et al. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59:847-855.
- Blom AW, Brown J, Taylor AH, et al. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688-691. doi:10.1302/0301-620x.86b5.14887
- Jain NB, Guller U, Pietrobon R, et al. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;435:232-238. doi:10.1097/01.blo.0000156479.97488.a2
- Docter S, Philpott HT, Godkin L, et al. Comparison of intra and post-operative complication rates among surgical approaches in Total Hip Arthroplasty: A systematic review and meta-analysis. J Orthop. 2020;20:310-325. doi:10.1016/j.jor.2020.05.008
- Huebschmann NA, Lawrence KW, Robin JX, et al. Does surgical approach affect dislocation rate after total hip arthroplasty in patients who have prior lumbar spinal fusion? A retrospective analysis of 16,223 cases. J Arthroplasty. 2024;39:S306-S313. doi:10.1016/j.arth.2024.03.068
Total hip arthroplasty (THA) is among the most common elective orthopedic procedures performed annually in the United States, with an estimated 635,000 to 909,000 THAs expected each year by 2030.1 Consequently, complication rates and revision surgeries related to THA have been increasing, along with the financial burden on the health care system.2-4 Optimizing outcomes for patients undergoing THA and identifying risk factors for treatment failure have become areas of focus.
Over the last decade, there has been a renewed interest in the effect of previous lumbar spine fusion (LSF) surgery on THA outcomes. Studies have explored the rates of complications, postoperative mobility, and THA implant impingement.5-8 However, the outcome receiving the most attention in recent literature is the rate and effect of dislocation in patients with lumbar fusion surgery. Large Medicare database analyses have discovered an association with increased rates of dislocations in patients with lumbar fusion surgeries compared with those without.9,10 Prosthetic hip dislocation is an expensive complication of THA and is projected to have greater impact through 2035 due to a growing number of THA procedures.11 Identifying risk factors associated with hip dislocation is paramount to mitigating its effect on patients who have undergone THA.
Recent research has found increased rates of THA dislocation and revision surgery in patients with LSF, with some studies showing previous LSF as the strongest independent predictor.6-16 However, controversy surrounds this relationship, including the sequence of procedures (LSF before or after THA), the time between procedures, and involvement of the sacrum in LSF. One study found that patients had a 106% increased risk of dislocation when LSF was performed before THA compared with patients who underwent LSF 5 years after undergoing THA, while another study showed no significant difference in dislocations pre- vs post-LSF.16,17 An additional study showed no significant difference in the rate of dislocation in patients without sacral involvement in the LSF, while also showing significantly higher rates of dislocation in LSF with sacral involvement.12 The researchers also found a trend toward more dislocations in longer lumbosacral fusions. Recent studies have also examined dislocation rates with lumbar fusion in patients treated with dual-mobility liners.18-20 The consensus from these studies is that dual-mobility liners significantly decrease the rate of dislocation in primary THAs with lumbar fusion.
The present study sought to determine the rates of hip dislocations in a US Department of Veterans Affairs (VA) hospital setting. To the authors’ knowledge, no retrospective study focusing on THAs in the veteran population has been performed. This study benefits from controlling for various surgeon techniques and surgical preferences when compared to large Medicare database studies because the orthopedic surgeon (ABK) only performed the posterior approach for all patients during the study period.
The primary objective of this study was to determine whether the rates of hip dislocation would, in fact, be higher in patients with lumbar fusion surgery, as recent database studies suggest. Secondary objectives included determining whether patient characteristics, comorbidities, number of levels fused, or inclusion of the sacrum in the fusion construct influenced dislocation rates. Furthermore, VA Dayton Healthcare System (VADHS) began routine use of dual-mobility liners for lumbar fusion patients in 2018, allowing for examination of these patients.
Methods
The Wright State University and VADHS Institutional Review Board approved this study design. A retrospective review of all primary THAs at VADHS was performed to investigate the relationship between previous lumbar spine fusion and the incidence of THA revision. Manual chart review was performed for patients who underwent primary THA between January 2003, and December 2022. One surgeon performed all surgeries using only the posterior approach. Patients were not excluded if they had bilateral procedures and all eligible hips were included. Patients with a concomitant diagnosis of fracture of the femoral head or femoral neck at the time of surgery were excluded. Additionally, only patients with ≥ 12 months of follow-up data were included.
The primary outcome was dislocation within 12 months of THA; the primary independent variable was LSF prior to THA. Covariates included patient demographics (age, sex, body mass index [BMI]) and Charlson Comorbidity Index (CCI) score, with additional data collected on the number of levels fused, sacral spine involvement, revision rates, and use of dual-mobility liners. Year of surgery was also included in analyses to account for any changes that may have occurred during the study period.
Statistical Analysis
Statistical analyses were performed in SAS 9.4. Patients were grouped into 2 cohorts, depending on whether they had received LSF prior to THA. Analyses were adjusted for repeated measures to account for the small percentage of patients with bilateral procedures.
Univariate comparisons between cohorts for covariates, as well as rates of dislocation and revision, were performed using the independent samples t test for continuous variables and the Fisher exact test for dichotomous categorical variables. Significant comorbidities, as well as age, sex, BMI, liner type, LSF cohort, and surgery year, were included in a logistic regression model to determine what effect, if any, they had on the likelihood of dislocation. Variables were removed using a backward stepwise approach, starting with the nonsignificant variable effect with the lowest χ2 value, and continuing until reaching a final model where all remaining variable effects were significant. For the variables retained in the final model, odds ratios (ORs) with 95% CIs were derived, with dislocation designated as the event. Individual comorbidity subcomponents of the CCI were also analyzed for their effects on dislocation using backward stepwise logistic regression. A secondary analysis among patients with LSF tested for the influence of the number of vertebral levels fused, the presence or absence of sacral involvement in the fusion, and the use of dual-mobility liners on the likelihood of hip dislocation.
Results
The LSF cohort included 39 patients with THA and prior LSF, 3 of whom had bilateral procedures, for a total of 42 hips. The non-LSF cohort included 813 patients with THA, 112 of whom had bilateral procedures, for a total of 925 hips. The LSF and non-LSF cohorts did not differ significantly in age, sex, BMI, CCI, or revision rates (Table). The LSF cohort included a significantly higher percentage of hips receiving dual-mobility liners than did the non-LSF cohort (23.8% vs 0.6%; P < .001) and had more than twice the rate of dislocation (4 of 42 hips [9.5%] vs 35 of 925 hips [3.8%]), although this difference was not statistically significant (P = .08).

The final logistic regression model with dislocation as the outcome was statistically significant (χ2, 17.47; P < .001) and retained 2 significant predictor variables: LSF cohort (χ2, 4.63; P = .03), and sex (χ2, 18.27; P < .001). Females were more likely than males to experience dislocation (OR, 5.84; 95% CI, 2.60-13.13; P < .001) as were patients who had LSF prior to THA (OR, 3.42; 95% CI, 1.12-10.47; P = .03) (Figure). None of the CCI subcomponent comorbidities significantly affected the probability of dislocation (myocardial infarction, P = .46; congestive heart failure, P = .47; peripheral vascular disease, P = .97; stroke, P = .51; dementia, P = .99; chronic obstructive pulmonary disease, P = .95; connective tissue disease, P = .25; peptic ulcer, P = .41; liver disease, P = .30; diabetes, P = .06; hemiplegia, P = .99; chronic kidney disease, P = .82; solid tumor, P = .90; leukemia, P = .99; lymphoma, P = .99; AIDS, P = .99). Within the LSF cohort, neither the number of levels fused (P = .83) nor sacral involvement (P = .42), significantly affected the probability of hip dislocation. None of the patients in either cohort who received dual-mobility liners subsequently dislocated their hips, nor did any of them require revision surgery.

Discussion
Spinopelvic biomechanics have been an area of increasing interest and research. Spinal fusion has been shown to alter the mobility of the pelvis and has been associated with decreased stability of THA implants.21 For example, in the setting of a fused spine, the lack of compensatory changes in pelvic tilt or acetabular anteversion when adjusting to a seated or standing position may predispose patients to impingement because the acetabular component is not properly positioned. Dual-mobility constructs mitigate this risk by providing an additional articulation, which increases jump distance and range of motion prior to impingement, thereby enhancing stability.
The use of dual-mobility liners in patients with LSF has also been examined.18-20 These studies demonstrate a reduced risk of postoperative THA dislocation in patients with previous LSF. The rate of postoperative complications and revisions for LSF patients with dual-mobility liners was also found to be similar to that of THAs without dual-mobility in patients without prior LSF. This study focused on a veteran population to demonstrate the efficacy of dual-mobility liners in patients with LSF. The results indicate that LSF prior to THA and female sex were predictors for prosthetic hip dislocations in the 12-month postoperative period in this patient population, which aligns with the current literature.
The dislocation rate in the LSF-THA group (9.5%) was higher than the dislocation rate in the control group (3.8%). Although not statistically significant in the univariate analysis, LSF was shown to be a significant risk factor after controlling for patient sex. Other studies have found the dislocation rate to be 3% to 7%, which is lower than the dislocation rate observed in this study.8,10,16
The reasons for this higher rate of dislocation are not entirely clear. A veteran population has poorer overall health than the general population, which may contribute to the higher than previously reported dislocation rates.22 These results can be applied to the management of veterans seeking THA.
There have been conflicting reports regarding the impact a patient’s sex has on THA outcomes in the general population.23-26 This study found that female patients had higher rates of dislocation within 1 year of THA than male patients. This difference, which could be due to differences in baseline anatomic hip morphology between the sexes; females tend to have smaller femoral head sizes and less offset compared with males.27,28 However, this finding could have been confounded by the small number of female veterans in the study cohort.
A type 2 diabetes mellitus (T2DM) diagnosis, which is a component of CCI, trended toward increased risk of prosthetic hip dislocation. Multiple studies have also discussed the increased risk of postoperative infections and revisions following THA in patients with T2DM.29-31 One study found T2DM to be an independent risk factor for immediate in-hospital postoperative complications following hip arthroplasty.32
Another factor that may influence postoperative dislocation risk is surgical approach. The posterior approach has historically been associated with higher rates of instability when compared to anterior or lateral THA.33 Researchers have also looked at the role that surgical approach plays in patients with prior LSF. Huebschmann et al confirmed that not only is LSF a significant risk factor for dislocation following THA, but anterior and laterally based surgical approaches may mitigate this risk.34
Limitations
As a retrospective cohort study, the reliability of the data hinges on complete documentation. Documentation of all encounters for dislocations was obtained from the VA Computerized Patient Record System, which may have led to some dislocation events being missed. However, as long as there was adequate postoperative follow-up, it was assumed all events outside the VA were included. Another limitation of this study was that male patients greatly outnumbered female patients, and this fact could limit the generalizability of findings to the population as a whole.
Conclusions
This study in a veteran population found that prior LSF and female sex were significant predictors for postoperative dislocation within 1 year of THA surgery. Additionally, the use of a dual-mobility liner was found to be protective against postoperative dislocation events. These data allow clinicians to better counsel veterans on the risk factors associated with postoperative dislocation and strategies to mitigate this risk.
Total hip arthroplasty (THA) is among the most common elective orthopedic procedures performed annually in the United States, with an estimated 635,000 to 909,000 THAs expected each year by 2030.1 Consequently, complication rates and revision surgeries related to THA have been increasing, along with the financial burden on the health care system.2-4 Optimizing outcomes for patients undergoing THA and identifying risk factors for treatment failure have become areas of focus.
Over the last decade, there has been a renewed interest in the effect of previous lumbar spine fusion (LSF) surgery on THA outcomes. Studies have explored the rates of complications, postoperative mobility, and THA implant impingement.5-8 However, the outcome receiving the most attention in recent literature is the rate and effect of dislocation in patients with lumbar fusion surgery. Large Medicare database analyses have discovered an association with increased rates of dislocations in patients with lumbar fusion surgeries compared with those without.9,10 Prosthetic hip dislocation is an expensive complication of THA and is projected to have greater impact through 2035 due to a growing number of THA procedures.11 Identifying risk factors associated with hip dislocation is paramount to mitigating its effect on patients who have undergone THA.
Recent research has found increased rates of THA dislocation and revision surgery in patients with LSF, with some studies showing previous LSF as the strongest independent predictor.6-16 However, controversy surrounds this relationship, including the sequence of procedures (LSF before or after THA), the time between procedures, and involvement of the sacrum in LSF. One study found that patients had a 106% increased risk of dislocation when LSF was performed before THA compared with patients who underwent LSF 5 years after undergoing THA, while another study showed no significant difference in dislocations pre- vs post-LSF.16,17 An additional study showed no significant difference in the rate of dislocation in patients without sacral involvement in the LSF, while also showing significantly higher rates of dislocation in LSF with sacral involvement.12 The researchers also found a trend toward more dislocations in longer lumbosacral fusions. Recent studies have also examined dislocation rates with lumbar fusion in patients treated with dual-mobility liners.18-20 The consensus from these studies is that dual-mobility liners significantly decrease the rate of dislocation in primary THAs with lumbar fusion.
The present study sought to determine the rates of hip dislocations in a US Department of Veterans Affairs (VA) hospital setting. To the authors’ knowledge, no retrospective study focusing on THAs in the veteran population has been performed. This study benefits from controlling for various surgeon techniques and surgical preferences when compared to large Medicare database studies because the orthopedic surgeon (ABK) only performed the posterior approach for all patients during the study period.
The primary objective of this study was to determine whether the rates of hip dislocation would, in fact, be higher in patients with lumbar fusion surgery, as recent database studies suggest. Secondary objectives included determining whether patient characteristics, comorbidities, number of levels fused, or inclusion of the sacrum in the fusion construct influenced dislocation rates. Furthermore, VA Dayton Healthcare System (VADHS) began routine use of dual-mobility liners for lumbar fusion patients in 2018, allowing for examination of these patients.
Methods
The Wright State University and VADHS Institutional Review Board approved this study design. A retrospective review of all primary THAs at VADHS was performed to investigate the relationship between previous lumbar spine fusion and the incidence of THA revision. Manual chart review was performed for patients who underwent primary THA between January 2003, and December 2022. One surgeon performed all surgeries using only the posterior approach. Patients were not excluded if they had bilateral procedures and all eligible hips were included. Patients with a concomitant diagnosis of fracture of the femoral head or femoral neck at the time of surgery were excluded. Additionally, only patients with ≥ 12 months of follow-up data were included.
The primary outcome was dislocation within 12 months of THA; the primary independent variable was LSF prior to THA. Covariates included patient demographics (age, sex, body mass index [BMI]) and Charlson Comorbidity Index (CCI) score, with additional data collected on the number of levels fused, sacral spine involvement, revision rates, and use of dual-mobility liners. Year of surgery was also included in analyses to account for any changes that may have occurred during the study period.
Statistical Analysis
Statistical analyses were performed in SAS 9.4. Patients were grouped into 2 cohorts, depending on whether they had received LSF prior to THA. Analyses were adjusted for repeated measures to account for the small percentage of patients with bilateral procedures.
Univariate comparisons between cohorts for covariates, as well as rates of dislocation and revision, were performed using the independent samples t test for continuous variables and the Fisher exact test for dichotomous categorical variables. Significant comorbidities, as well as age, sex, BMI, liner type, LSF cohort, and surgery year, were included in a logistic regression model to determine what effect, if any, they had on the likelihood of dislocation. Variables were removed using a backward stepwise approach, starting with the nonsignificant variable effect with the lowest χ2 value, and continuing until reaching a final model where all remaining variable effects were significant. For the variables retained in the final model, odds ratios (ORs) with 95% CIs were derived, with dislocation designated as the event. Individual comorbidity subcomponents of the CCI were also analyzed for their effects on dislocation using backward stepwise logistic regression. A secondary analysis among patients with LSF tested for the influence of the number of vertebral levels fused, the presence or absence of sacral involvement in the fusion, and the use of dual-mobility liners on the likelihood of hip dislocation.
Results
The LSF cohort included 39 patients with THA and prior LSF, 3 of whom had bilateral procedures, for a total of 42 hips. The non-LSF cohort included 813 patients with THA, 112 of whom had bilateral procedures, for a total of 925 hips. The LSF and non-LSF cohorts did not differ significantly in age, sex, BMI, CCI, or revision rates (Table). The LSF cohort included a significantly higher percentage of hips receiving dual-mobility liners than did the non-LSF cohort (23.8% vs 0.6%; P < .001) and had more than twice the rate of dislocation (4 of 42 hips [9.5%] vs 35 of 925 hips [3.8%]), although this difference was not statistically significant (P = .08).

The final logistic regression model with dislocation as the outcome was statistically significant (χ2, 17.47; P < .001) and retained 2 significant predictor variables: LSF cohort (χ2, 4.63; P = .03), and sex (χ2, 18.27; P < .001). Females were more likely than males to experience dislocation (OR, 5.84; 95% CI, 2.60-13.13; P < .001) as were patients who had LSF prior to THA (OR, 3.42; 95% CI, 1.12-10.47; P = .03) (Figure). None of the CCI subcomponent comorbidities significantly affected the probability of dislocation (myocardial infarction, P = .46; congestive heart failure, P = .47; peripheral vascular disease, P = .97; stroke, P = .51; dementia, P = .99; chronic obstructive pulmonary disease, P = .95; connective tissue disease, P = .25; peptic ulcer, P = .41; liver disease, P = .30; diabetes, P = .06; hemiplegia, P = .99; chronic kidney disease, P = .82; solid tumor, P = .90; leukemia, P = .99; lymphoma, P = .99; AIDS, P = .99). Within the LSF cohort, neither the number of levels fused (P = .83) nor sacral involvement (P = .42), significantly affected the probability of hip dislocation. None of the patients in either cohort who received dual-mobility liners subsequently dislocated their hips, nor did any of them require revision surgery.

Discussion
Spinopelvic biomechanics have been an area of increasing interest and research. Spinal fusion has been shown to alter the mobility of the pelvis and has been associated with decreased stability of THA implants.21 For example, in the setting of a fused spine, the lack of compensatory changes in pelvic tilt or acetabular anteversion when adjusting to a seated or standing position may predispose patients to impingement because the acetabular component is not properly positioned. Dual-mobility constructs mitigate this risk by providing an additional articulation, which increases jump distance and range of motion prior to impingement, thereby enhancing stability.
The use of dual-mobility liners in patients with LSF has also been examined.18-20 These studies demonstrate a reduced risk of postoperative THA dislocation in patients with previous LSF. The rate of postoperative complications and revisions for LSF patients with dual-mobility liners was also found to be similar to that of THAs without dual-mobility in patients without prior LSF. This study focused on a veteran population to demonstrate the efficacy of dual-mobility liners in patients with LSF. The results indicate that LSF prior to THA and female sex were predictors for prosthetic hip dislocations in the 12-month postoperative period in this patient population, which aligns with the current literature.
The dislocation rate in the LSF-THA group (9.5%) was higher than the dislocation rate in the control group (3.8%). Although not statistically significant in the univariate analysis, LSF was shown to be a significant risk factor after controlling for patient sex. Other studies have found the dislocation rate to be 3% to 7%, which is lower than the dislocation rate observed in this study.8,10,16
The reasons for this higher rate of dislocation are not entirely clear. A veteran population has poorer overall health than the general population, which may contribute to the higher than previously reported dislocation rates.22 These results can be applied to the management of veterans seeking THA.
There have been conflicting reports regarding the impact a patient’s sex has on THA outcomes in the general population.23-26 This study found that female patients had higher rates of dislocation within 1 year of THA than male patients. This difference, which could be due to differences in baseline anatomic hip morphology between the sexes; females tend to have smaller femoral head sizes and less offset compared with males.27,28 However, this finding could have been confounded by the small number of female veterans in the study cohort.
A type 2 diabetes mellitus (T2DM) diagnosis, which is a component of CCI, trended toward increased risk of prosthetic hip dislocation. Multiple studies have also discussed the increased risk of postoperative infections and revisions following THA in patients with T2DM.29-31 One study found T2DM to be an independent risk factor for immediate in-hospital postoperative complications following hip arthroplasty.32
Another factor that may influence postoperative dislocation risk is surgical approach. The posterior approach has historically been associated with higher rates of instability when compared to anterior or lateral THA.33 Researchers have also looked at the role that surgical approach plays in patients with prior LSF. Huebschmann et al confirmed that not only is LSF a significant risk factor for dislocation following THA, but anterior and laterally based surgical approaches may mitigate this risk.34
Limitations
As a retrospective cohort study, the reliability of the data hinges on complete documentation. Documentation of all encounters for dislocations was obtained from the VA Computerized Patient Record System, which may have led to some dislocation events being missed. However, as long as there was adequate postoperative follow-up, it was assumed all events outside the VA were included. Another limitation of this study was that male patients greatly outnumbered female patients, and this fact could limit the generalizability of findings to the population as a whole.
Conclusions
This study in a veteran population found that prior LSF and female sex were significant predictors for postoperative dislocation within 1 year of THA surgery. Additionally, the use of a dual-mobility liner was found to be protective against postoperative dislocation events. These data allow clinicians to better counsel veterans on the risk factors associated with postoperative dislocation and strategies to mitigate this risk.
- Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455-1460. doi:10.2106/JBJS.17.01617
- Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128-133. doi:10.2106/JBJS.H.00155
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:144-151. doi:10.2106/JBJS.G.00587
- Kurtz SM, Ong KL, Schmier J, et al. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24:195-203. doi:10.1016/j.arth.2007.11.015
- Yamato Y, Furuhashi H, Hasegawa T, et al. Simulation of implant impingement after spinal corrective fusion surgery in patients with previous total hip arthroplasty: a retrospective case series. Spine (Phila Pa 1976). 2021;46:512-519. doi:10.1097/BRS.0000000000003836
- Mudrick CA, Melvin JS, Springer BD. Late posterior hip instability after lumbar spinopelvic fusion. Arthroplast Today. 2015;1:25-29. doi:10.1016/j.artd.2015.05.002
- Diebo BG, Beyer GA, Grieco PW, et al. Complications in patients undergoing spinal fusion after THA. Clin Orthop Relat Res. 2018;476:412-417.doi:10.1007/s11999.0000000000000009 8.
- Sing DC, Barry JJ, Aguilar TU, et al. Prior lumbar spinal arthrodesis increases risk of prosthetic-related complication in total hip arthroplasty. J Arthroplasty. 2016;31:227-232.e1. doi:10.1016/j.arth.2016.02.069
- King CA, Landy DC, Martell JM, et al. Time to dislocation analysis of lumbar spine fusion following total hip arthroplasty: breaking up a happy home. J Arthroplasty. 2018;33:3768-3772. doi:10.1016/j.arth.2018.08.029
- Buckland AJ, Puvanesarajah V, Vigdorchik J, et al. Dislocation of a primary total hip arthroplasty is more common in patients with a lumbar spinal fusion. Bone Joint J. 2017;99-B:585-591.doi:10.1302/0301-620X.99B5.BJJ-2016-0657.R1
- Pirruccio K, Premkumar A, Sheth NP. The burden of prosthetic hip dislocations in the United States is projected to significantly increase by 2035. Hip Int. 2021;31:714-721. doi:10.1177/1120700020923619
- Salib CG, Reina N, Perry KI, et al. Lumbar fusion involving the sacrum increases dislocation risk in primary total hip arthroplasty. Bone Joint J. 2019;101-B:198-206. doi:10.1302/0301-620X.101B2.BJJ-2018-0754.R1
- An VVG, Phan K, Sivakumar BS, et al. Prior lumbar spinal fusion is associated with an increased risk of dislocation and revision in total hip arthroplasty: a meta-analysis. J Arthroplasty. 2018;33:297-300. doi:10.1016/j.arth.2017.08.040
- Klemt C, Padmanabha A, Tirumala V, et al. Lumbar spine fusion before revision total hip arthroplasty is associated with increased dislocation rates. J Am Acad Orthop Surg. 2021;29:e860-e868. doi:10.5435/JAAOS-D-20-00824
- Gausden EB, Parhar HS, Popper JE, et al. Risk factors for early dislocation following primary elective total hip arthroplasty. J Arthroplasty. 2018;33:1567-1571. doi:10.1016/j.arth.2017.12.034
- Malkani AL, Himschoot KJ, Ong KL, et al. Does timing of primary total hip arthroplasty prior to or after lumbar spine fusion have an effect on dislocation and revision rates?. J Arthroplasty. 2019;34:907-911. doi:10.1016/j.arth.2019.01.009
- Parilla FW, Shah RR, Gordon AC, et al. Does it matter: total hip arthroplasty or lumbar spinal fusion first? Preoperative sagittal spinopelvic measurements guide patient-specific surgical strategies in patients requiring both. J Arthroplasty. 2019;34:2652-2662. doi:10.1016/j.arth.2019.05.053
- Chalmers BP, Syku M, Sculco TP, et al. Dual-mobility constructs in primary total hip arthroplasty in high-risk patients with spinal fusions: our institutional experience. Arthroplast Today. 2020;6:749-754. doi:10.1016/j.artd.2020.07.024
- Nessler JM, Malkani AL, Sachdeva S, et al. Use of dual mobility cups in patients undergoing primary total hip arthroplasty with prior lumbar spine fusion. Int Orthop. 2020;44:857-862. doi:10.1007/s00264-020-04507-y
- Nessler JM, Malkani AL, Yep PJ, et al. Dislocation rates of primary total hip arthroplasty in patients with prior lumbar spine fusion and lumbar degenerative disk disease with and without utilization of dual mobility cups: an American Joint Replacement Registry study. J Am Acad Orthop Surg. 2023;31:e271-e277. doi:10.5435/JAAOS-D-22-00767
- Phan D, Bederman SS, Schwarzkopf R. The influence of sagittal spinal deformity on anteversion of the acetabular component in total hip arthroplasty. Bone Joint J. 2015;97-B:1017-1023. doi:10.1302/0301-620X.97B8.35700
- Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252-3257. doi:10.1001/archinte.160.21.325223.
- Basques BA, Bell JA, Fillingham YA, et al. Gender differences for hip and knee arthroplasty: complications and healthcare utilization. J Arthroplasty. 2019;34:1593-1597.e1. doi:10.1016/j.arth.2019.03.064
- Kim YH, Choi Y, Kim JS. Influence of patient-, design-, and surgery-related factors on rate of dislocation after primary cementless total hip arthroplasty. J Arthroplasty. 2009;24:1258-1263. doi:10.1016/j.arth.2009.03.017
- Chen A, Paxton L, Zheng X, et al. Association of sex with risk of 2-year revision among patients undergoing total hip arthroplasty. JAMA Netw Open. 2021;4:e2110687. doi:10.1001/jamanetworkopen.2021.10687
- Inacio MCS, Ake CF, Paxton EW, et al. Sex and risk of hip implant failure: assessing total hip arthroplasty outcomes in the United States. JAMA Intern Med. 2013;173:435-441. doi:10.1001/jamainternmed.2013.3271
- Karlson EW, Daltroy LH, Liang MH, et al. Gender differences in patient preferences may underlie differential utilization of elective surgery. Am J Med. 1997;102:524-530. doi:10.1016/s0002-9343(97)00050-8
- Kostamo T, Bourne RB, Whittaker JP, et al. No difference in gender-specific hip replacement outcomes. Clin Orthop Relat Res. 2009;467:135-140. doi:10.1007/s11999-008-0466-2
- Papagelopoulos PJ, Idusuyi OB, Wallrichs SL, et al. Long term outcome and survivorship analysis of primary total knee arthroplasty in patients with diabetes mellitus. Clin Orthop Relat Res. 1996;(330):124-132. doi:10.1097/00003086-199609000-00015
- Fitzgerald RH Jr, Nolan DR, Ilstrup DM, et al. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59:847-855.
- Blom AW, Brown J, Taylor AH, et al. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688-691. doi:10.1302/0301-620x.86b5.14887
- Jain NB, Guller U, Pietrobon R, et al. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;435:232-238. doi:10.1097/01.blo.0000156479.97488.a2
- Docter S, Philpott HT, Godkin L, et al. Comparison of intra and post-operative complication rates among surgical approaches in Total Hip Arthroplasty: A systematic review and meta-analysis. J Orthop. 2020;20:310-325. doi:10.1016/j.jor.2020.05.008
- Huebschmann NA, Lawrence KW, Robin JX, et al. Does surgical approach affect dislocation rate after total hip arthroplasty in patients who have prior lumbar spinal fusion? A retrospective analysis of 16,223 cases. J Arthroplasty. 2024;39:S306-S313. doi:10.1016/j.arth.2024.03.068
- Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455-1460. doi:10.2106/JBJS.17.01617
- Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128-133. doi:10.2106/JBJS.H.00155
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:144-151. doi:10.2106/JBJS.G.00587
- Kurtz SM, Ong KL, Schmier J, et al. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24:195-203. doi:10.1016/j.arth.2007.11.015
- Yamato Y, Furuhashi H, Hasegawa T, et al. Simulation of implant impingement after spinal corrective fusion surgery in patients with previous total hip arthroplasty: a retrospective case series. Spine (Phila Pa 1976). 2021;46:512-519. doi:10.1097/BRS.0000000000003836
- Mudrick CA, Melvin JS, Springer BD. Late posterior hip instability after lumbar spinopelvic fusion. Arthroplast Today. 2015;1:25-29. doi:10.1016/j.artd.2015.05.002
- Diebo BG, Beyer GA, Grieco PW, et al. Complications in patients undergoing spinal fusion after THA. Clin Orthop Relat Res. 2018;476:412-417.doi:10.1007/s11999.0000000000000009 8.
- Sing DC, Barry JJ, Aguilar TU, et al. Prior lumbar spinal arthrodesis increases risk of prosthetic-related complication in total hip arthroplasty. J Arthroplasty. 2016;31:227-232.e1. doi:10.1016/j.arth.2016.02.069
- King CA, Landy DC, Martell JM, et al. Time to dislocation analysis of lumbar spine fusion following total hip arthroplasty: breaking up a happy home. J Arthroplasty. 2018;33:3768-3772. doi:10.1016/j.arth.2018.08.029
- Buckland AJ, Puvanesarajah V, Vigdorchik J, et al. Dislocation of a primary total hip arthroplasty is more common in patients with a lumbar spinal fusion. Bone Joint J. 2017;99-B:585-591.doi:10.1302/0301-620X.99B5.BJJ-2016-0657.R1
- Pirruccio K, Premkumar A, Sheth NP. The burden of prosthetic hip dislocations in the United States is projected to significantly increase by 2035. Hip Int. 2021;31:714-721. doi:10.1177/1120700020923619
- Salib CG, Reina N, Perry KI, et al. Lumbar fusion involving the sacrum increases dislocation risk in primary total hip arthroplasty. Bone Joint J. 2019;101-B:198-206. doi:10.1302/0301-620X.101B2.BJJ-2018-0754.R1
- An VVG, Phan K, Sivakumar BS, et al. Prior lumbar spinal fusion is associated with an increased risk of dislocation and revision in total hip arthroplasty: a meta-analysis. J Arthroplasty. 2018;33:297-300. doi:10.1016/j.arth.2017.08.040
- Klemt C, Padmanabha A, Tirumala V, et al. Lumbar spine fusion before revision total hip arthroplasty is associated with increased dislocation rates. J Am Acad Orthop Surg. 2021;29:e860-e868. doi:10.5435/JAAOS-D-20-00824
- Gausden EB, Parhar HS, Popper JE, et al. Risk factors for early dislocation following primary elective total hip arthroplasty. J Arthroplasty. 2018;33:1567-1571. doi:10.1016/j.arth.2017.12.034
- Malkani AL, Himschoot KJ, Ong KL, et al. Does timing of primary total hip arthroplasty prior to or after lumbar spine fusion have an effect on dislocation and revision rates?. J Arthroplasty. 2019;34:907-911. doi:10.1016/j.arth.2019.01.009
- Parilla FW, Shah RR, Gordon AC, et al. Does it matter: total hip arthroplasty or lumbar spinal fusion first? Preoperative sagittal spinopelvic measurements guide patient-specific surgical strategies in patients requiring both. J Arthroplasty. 2019;34:2652-2662. doi:10.1016/j.arth.2019.05.053
- Chalmers BP, Syku M, Sculco TP, et al. Dual-mobility constructs in primary total hip arthroplasty in high-risk patients with spinal fusions: our institutional experience. Arthroplast Today. 2020;6:749-754. doi:10.1016/j.artd.2020.07.024
- Nessler JM, Malkani AL, Sachdeva S, et al. Use of dual mobility cups in patients undergoing primary total hip arthroplasty with prior lumbar spine fusion. Int Orthop. 2020;44:857-862. doi:10.1007/s00264-020-04507-y
- Nessler JM, Malkani AL, Yep PJ, et al. Dislocation rates of primary total hip arthroplasty in patients with prior lumbar spine fusion and lumbar degenerative disk disease with and without utilization of dual mobility cups: an American Joint Replacement Registry study. J Am Acad Orthop Surg. 2023;31:e271-e277. doi:10.5435/JAAOS-D-22-00767
- Phan D, Bederman SS, Schwarzkopf R. The influence of sagittal spinal deformity on anteversion of the acetabular component in total hip arthroplasty. Bone Joint J. 2015;97-B:1017-1023. doi:10.1302/0301-620X.97B8.35700
- Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252-3257. doi:10.1001/archinte.160.21.325223.
- Basques BA, Bell JA, Fillingham YA, et al. Gender differences for hip and knee arthroplasty: complications and healthcare utilization. J Arthroplasty. 2019;34:1593-1597.e1. doi:10.1016/j.arth.2019.03.064
- Kim YH, Choi Y, Kim JS. Influence of patient-, design-, and surgery-related factors on rate of dislocation after primary cementless total hip arthroplasty. J Arthroplasty. 2009;24:1258-1263. doi:10.1016/j.arth.2009.03.017
- Chen A, Paxton L, Zheng X, et al. Association of sex with risk of 2-year revision among patients undergoing total hip arthroplasty. JAMA Netw Open. 2021;4:e2110687. doi:10.1001/jamanetworkopen.2021.10687
- Inacio MCS, Ake CF, Paxton EW, et al. Sex and risk of hip implant failure: assessing total hip arthroplasty outcomes in the United States. JAMA Intern Med. 2013;173:435-441. doi:10.1001/jamainternmed.2013.3271
- Karlson EW, Daltroy LH, Liang MH, et al. Gender differences in patient preferences may underlie differential utilization of elective surgery. Am J Med. 1997;102:524-530. doi:10.1016/s0002-9343(97)00050-8
- Kostamo T, Bourne RB, Whittaker JP, et al. No difference in gender-specific hip replacement outcomes. Clin Orthop Relat Res. 2009;467:135-140. doi:10.1007/s11999-008-0466-2
- Papagelopoulos PJ, Idusuyi OB, Wallrichs SL, et al. Long term outcome and survivorship analysis of primary total knee arthroplasty in patients with diabetes mellitus. Clin Orthop Relat Res. 1996;(330):124-132. doi:10.1097/00003086-199609000-00015
- Fitzgerald RH Jr, Nolan DR, Ilstrup DM, et al. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59:847-855.
- Blom AW, Brown J, Taylor AH, et al. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688-691. doi:10.1302/0301-620x.86b5.14887
- Jain NB, Guller U, Pietrobon R, et al. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;435:232-238. doi:10.1097/01.blo.0000156479.97488.a2
- Docter S, Philpott HT, Godkin L, et al. Comparison of intra and post-operative complication rates among surgical approaches in Total Hip Arthroplasty: A systematic review and meta-analysis. J Orthop. 2020;20:310-325. doi:10.1016/j.jor.2020.05.008
- Huebschmann NA, Lawrence KW, Robin JX, et al. Does surgical approach affect dislocation rate after total hip arthroplasty in patients who have prior lumbar spinal fusion? A retrospective analysis of 16,223 cases. J Arthroplasty. 2024;39:S306-S313. doi:10.1016/j.arth.2024.03.068
Effects of Lumbar Fusion and Dual-Mobility Liners on Dislocation Rates Following Total Hip Arthroplasty in a Veteran Population
Effects of Lumbar Fusion and Dual-Mobility Liners on Dislocation Rates Following Total Hip Arthroplasty in a Veteran Population
Evaluation of Pharmacist-Driven Inhaled Corticosteroid De-escalation in Veterans
Evaluation of Pharmacist-Driven Inhaled Corticosteroid De-escalation in Veterans
Systemic glucocorticoids play an important role in the treatment of chronic obstructive pulmonary disease (COPD) exacerbations. They are recommended to shorten recovery time and increase forced expiratory volume in 1 second (FEV1) during exacerbations.1 However, the role of the chronic use of inhaled corticosteroids (ICSs) in the treatment of COPD is less clear.
When added to inhaled β-2 agonists and muscarinic antagonists, ICSs can decrease the risk of exacerbations.1 However, not all patients with COPD benefit from ICS therapy. The degree of benefit an ICS can provide has been shown to correlate with eosinophil count—a marker of inflammation. The expected benefit of using an ICS increases as the eosinophil count increases.1 Maximum benefit can be observed with eosinophil counts ≥ 300 cells/µL, and minimal benefit is observed with eosinophil counts < 100 cells/µL. Adverse effects (AEs) of ICSs include a hoarse voice, oral candidiasis, and an increased risk of pneumonia.1 Given the risk of AEs, it is important to limit ICS use in patients who are unlikely to reap any benefits.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines suggest the use of ICSs in patients who experience exacerbations while using long-acting β agonist (LABA) plus long-acting muscarinic antagonist (LAMA) therapy and have an eosinophil count ≥ 100 cells/µL. Switching from LABA or LAMA monotherapy to triple therapy with LAMA/LABA/ICS may be considered if patients have continued exacerbations and an eosinophil count ≥ 300 cells/µL. De-escalation of ICS therapy should be considered if patients do not meet these criteria or if patients experience ICS AEs, such as pneumonia. The patients most likely to have increased exacerbations or decreased FEV1 with ICS withdrawal are those with eosinophil counts ≥ 300 cells/µL.1,2
Several studies have explored the effects of ICS de-escalation in real-world clinical settings. A systematic review of 11 studies indicated that de-escalation of ICS in COPD does not result in increased exacerbations.3 A prospective study by Rossi et al found that in a 6-month period, 141 of 482 patients on ICS therapy (29%) had an exacerbation. In the opposing arm of the study, 88 of 334 patients (26%) with deprescribed ICS experienced an exacerbation. The difference between these 2 groups was not statistically significant.4 The researchers concluded that in real-world practice, ICS withdrawal can be safe in patients at low risk of exacerbation.
About 25% of veterans (1.25 million) have been diagnosed with COPD.5 To address this, the US Department of Veterans Affairs (VA) and US Department of Defense published updated COPD guidelines in 2021 that specify criteria for de-escalation of ICS.6 Guidelines, however, may not be reflected in common clinical practice for several years following publication. The VA Academic Detailing Service (ADS) provides tools to help clinicians identify patients who may benefit from changes in treatment plans. A recent ADS focus was the implementation of a COPD dashboard, which identifies patients with COPD who are candidates for ICS de-escalation based on comorbid diagnoses, exacerbation history, and eosinophil count. VA pharmacists have an expanded role in the management of primary care disease states and are therefore well-positioned to increase adherence to guideline-directed therapy. The objective of this quality improvement project was to determine the impact of pharmacist-driven de-escalation on ICS usage in veterans with COPD.
Methods
This project was conducted in an outpatient clinic at the Robley Rex VA Medical Center beginning September 21, 2023, with a progress note in the Computerized Patient Record System (CPRS). Eligible patients were selected using the COPD Dashboard provided by ADS. The COPD Dashboard defined patients with COPD as those with ≥ 2 outpatient COPD diagnoses in the past 2 years, 1 inpatient discharge COPD diagnosis in the past year, or COPD listed as an active problem. COPD diagnoses were identified using International Statistical Classification of Disease, Tenth Revision (ICD-10) codes
Candidates identified for ICS de-escalation by the dashboard were excluded if they had a history of COPD exacerbation in the previous 2 years. The dashboard identified COPD exacerbations via ICD-10 codes for COPD or acute respiratory failure for inpatient discharges, emergency department (ED) visits, urgent care visits, and community care consults with 1 of the following terms: emergency, inpatient, hospital, urgent, ED (self). The COPD dashboard excluded patients with a diagnosis of asthma.
After patients were selected, they were screened for additional exclusion criteria. Patients were excluded if a pulmonary care practitioner managed their COPD; if identified via an active pulmonary consult in CPRS; if a non-VA clinician prescribed their ICS; or if they were being treated with roflumilast, theophylline, or chronic azithromycin. Individuals taking these 3 drugs were excluded due to potential severe and/or refractory COPD. Patients also were excluded if they: (1) had prior ICS de-escalation failure (defined as a COPD exacerbation following ICS de-escalation that resulted in ICS resumption); (2) had a COPD exacerbation requiring systemic corticosteroids or antibiotics in the previous year; (3) had active lung cancer; (4) did not have any eosinophil levels in CPRS within the previous 2 years; or (5) had any eosinophil levels ≥ 300 cells/µL in the previous year.
Each patient who met the inclusion criteria and was not excluded received a focused medication review by a pharmacist who created a templated progress note, with patient-specific recommendations, that was entered in the CPRS (eAppendix). The recommendations were also attached as an addendum to the patient’s last primary care visit note, and the primary care practitioner (PCP) was alerted via CPRS to consider ICS de-escalation and non-ICS alternatives. Tapering of ICS therapy was offered as an option to de-escalate if abrupt discontinuation was deemed inappropriate. PCPs were also prompted to consider referral to a primary care clinical pharmacy specialist for management and follow-up of ICS de-escalation.
The primary outcome was the number of patients with de-escalated ICS at 3 and 6 months following the recommendation. Secondary outcomes included the number of: patients who were no longer prescribed an ICS or who had a non-ICS alternative initiated at a pharmacist’s recommendation; patients who were referred to a primary care clinical pharmacy specialist for ICS de-escalation; COPD exacerbations requiring systemic steroids or antibiotics, or requiring an ED visit, inpatient admission, or urgent-care clinic visit; and cases of pneumonia or oral candidiasis. Primary and secondary outcomes were evaluated via chart review in CPRS. For secondary outcomes of pneumonia and COPD exacerbation, identification was made by documented diagnosis in CPRS. For continuous data such as age, the mean was calculated.
Results
Pharmacist ICS de-escalation recommendations were made between September 21, 2023, and November 19, 2023, for 106 patients. The mean age was 72 years and 99 (93%) patients were male (Table 1). Forty-one (39%) of the patients used tobacco at the time of the study. FEV1 was available for 69 patients with a mean of 63% (GOLD grade 2).1 Based on FEV1 values, 16 patients had mild COPD (GOLD grade 1), 37 patients had moderate COPD (GOLD grade 2), 14 patients had severe COPD (GOLD grade 3), and 2 patients had very severe COPD (GOLD grade 4).1 Thirty-four patients received LABA + LAMA + ICS, 65 received LABA + ICS, 2 received LAMA + ICS, and 5 received ICS monotherapy. The most common dose of ICS was a moderate dose (Table 2). Only 2 patients had an ICS AE in the previous year.
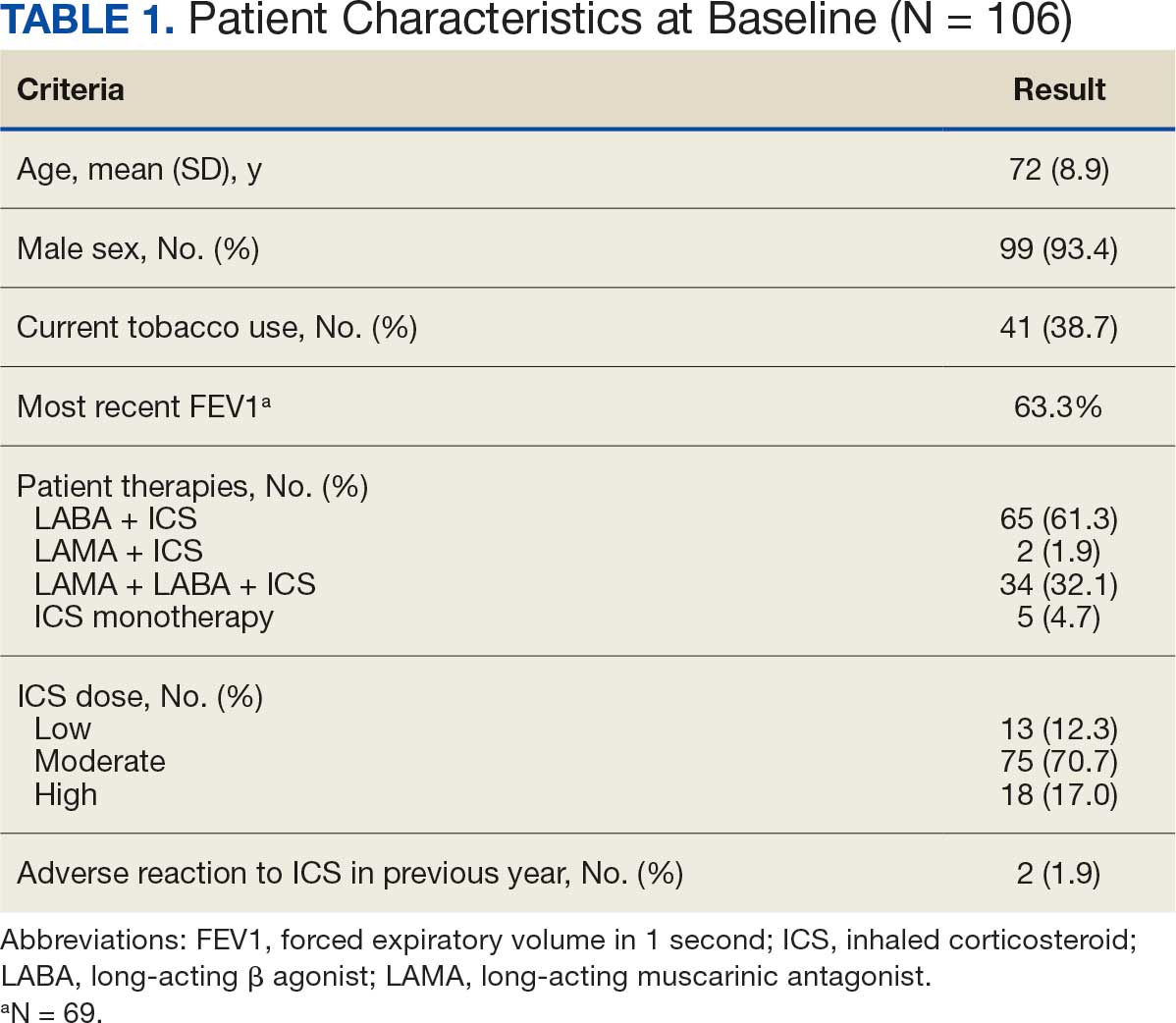
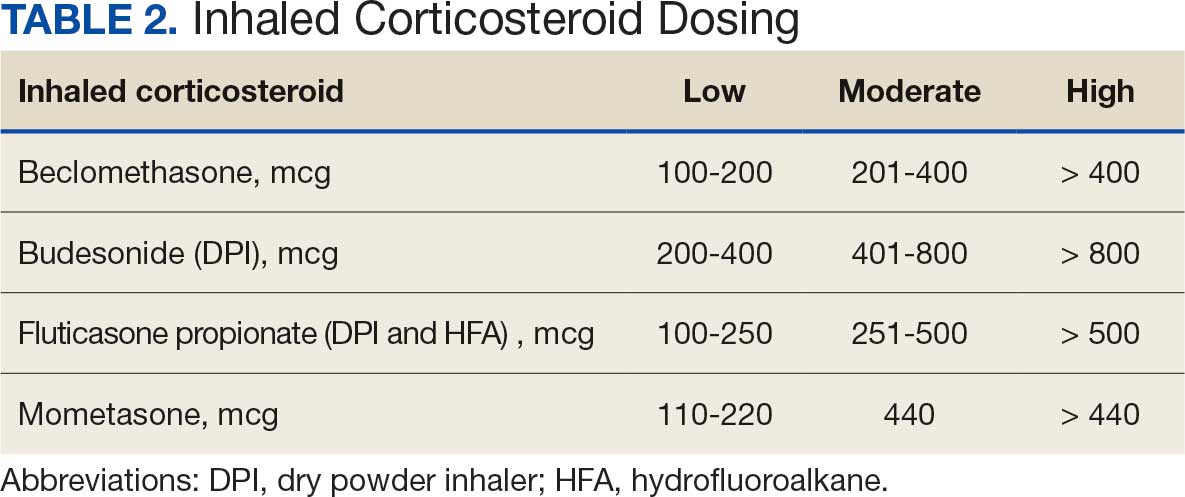
ICS de-escalation recommendations resulted in ICS de-escalation in 50 (47.2%) and 62 (58.5%) patients at 3 and 6 months, respectively. The 6-month ICS de-escalation rate by ICS dose at baseline was 72.2% (high dose), 60.0% (moderate), and 30.8% (low). De-escalation at 6 months by GOLD grade at baseline was 56.3% (9 of 16 patients, GOLD 1), 64.9% (24 of 37 patients, GOLD 2), 50% (7 of 14 patients, GOLD 3), and 50% (1 of 2 patients, GOLD 4). Six months after the ICS de-escalation recommendation appeared in the CPRS, the percentage of patients on LABA + ICS therapy dropped from 65 patients (61.3%) at baseline to 25 patients (23.6%).
Secondary outcomes were assessed at 3 and 6 months following the recommendation. Most patients with de-escalated ICS had their ICS discontinued and a non-ICS alternative initiated per pharmacist recommendations. At 6 months, 39 patients (36.8%) patients were referred to a patient aligned care team (PACT) pharmacist for de-escalation. Of the 39 patients referred to pharmacists, 69.2% (27 patients) were de-escalated; this compared to 52.2% (35 patients) who were not referred to pharmacists (Table 3).
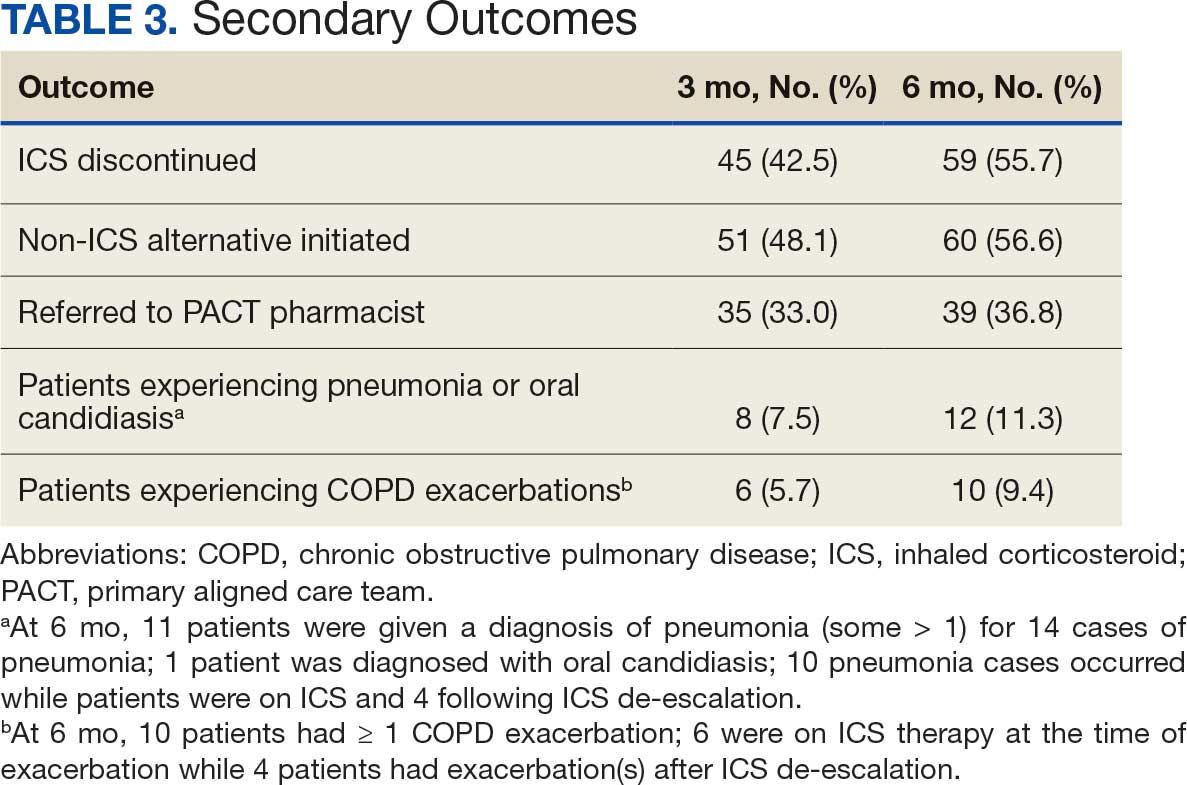
ICS use increases the risk of pneumonia.1 At 6 months, 11 patients were diagnosed with pneumonia; 3 patients were diagnosed with pneumonia twice, resulting in a total of 14 cases. Ten cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation. One patient had a documented case of oral candidiasis that occurred while on ICS therapy; no patients with discontinued ICS were diagnosed with oral candidiasis. In addition, 10 patients had COPD exacerbations; however no patients had exacerbations both before and after de-escalation. Six patients were on ICS therapy when they experienced an exacerbation, and 4 patients had an exacerbation after ICS de-escalation.
Discussion
More than half of patients receiving the pharmacist intervention achieved the primary outcome of ICS de-escalation at 6 months. Furthermore, a larger percentage of patients referred to pharmacists for the management of ICS de-escalation successfully achieved de-escalation compared to those who were not referred. These outcomes reflect the important role pharmacists can play in identifying appropriate candidates for ICS de-escalation and assisting in the management of ICS de-escalation. Patients referred to pharmacists also received other services such as smoking cessation pharmacotherapy and counseling on inhaler technique and adherence. These interventions can support improved COPD clinical outcomes.
The purpose of de-escalating ICS therapy is to reduce the risk of AEs such as pneumonia and oral candidiasis.1 The secondary outcomes of this study support previous evidence that patients who have de-escalated ICS therapy may have reduced risk of AEs compared to those who remain on ICS therapy.3 Specifically, of the 14 cases of pneumonia that occurred during the study, 10 cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation.
ICS de-escalation may increase risk of increased COPD exacerbations.1 However, the secondary outcomes of this study do not indicate that those with de-escalated ICS had more COPD exacerbations compared to those who continued on ICS. Pharmacists’ recommendations were more effective for patients with less severe COPD based on baseline FEV1.
The previous GOLD Guidelines for COPD suggested LABA + ICS therapy as an option for patients with a high symptom and exacerbation burden (previously known as GOLD Group D). Guidelines no longer recommend LABA + ICS therapy due to the superiority of triple inhaled therapy for exacerbations and the superiority of LAMA + LABA therapy for dyspnea.7 A majority of identified patients in this project were on LABA + ICS therapy alone at baseline. The ICS de-escalation recommendation resulted in a 61.5% reduction in patients on LABA + ICS therapy at 6 months. By decreasing the number of patients on LABA + ICS without LAMA, recommendations increased the number of patients on guideline-directed therapy.
Limitations
This study lacked a control group, and the rate of ICS de-escalation in patients who did not receive a pharmacist recommendation was not assessed. Therefore, it could not be determined whether the pharmacist recommendation is more effective than no recommendation. Another limitation was our inability to access records from non-VA health care facilities. This may have resulted in missed COPD exacerbations, pneumonia, and oral candidiasis prior to or following the pharmacist recommendation.
In addition, the method used to notify PCPs of the pharmacist recommendation was a CPRS alert. Clinicians often receive multiple daily alerts and may not always pay close attention to them due to alert fatigue. Early in the study, some PCPs were unknowingly omitted from the alert of the pharmacist recommendation for 10 patients due to human error. For 8 of these 10 patients, the PCP was notified of the recommendations during the 3-month follow-up period. However, 2 patients had COPD exacerbations during the 3-month follow-up period. In these cases, the PCP was not alerted to de-escalate ICS. The data for these patients were collected at 3 and 6 months in the same manner as all other patients. Also, 7 of 35 patients who were referred to a pharmacist for ICS de-escalation did not have a scheduled appointment. These patients were considered to be lost to follow-up and this may have resulted in an underestimation of the ability of pharmacists to successfully de-escalate ICS in patients with COPD.
Other studies have evaluated the efficacy of a pharmacy-driven ICS de-escalation.8,9 Hegland et al reported ICS de-escalation for 22% of 141 eligible ambulatory patients with COPD on triple inhaled therapy following pharmacist appointments.8 A study by Hahn et al resulted in 63.8% of 58 patients with COPD being maintained off ICS following a pharmacist de-escalation initiative.9 However, these studies relied upon more time-consuming de-escalation interventions, including at least 1 phone, video, or in-person patient visit.8,9
This project used a single chart review and templated progress note to recommend ICS de-escalation and achieved similar or improved de-escalation rates compared to previous studies.8,9 Previous studies were conducted prior to the updated 2023 GOLD guidelines for COPD which no longer recommend LABA + ICS therapy. This project addressed ICS de-escalation in patients on LABA + ICS therapy in addition to those on triple inhaled therapy. Additionally, previous studies did not address rates of moderate to severe COPD exacerbation and adverse events to ICS following the pharmacist intervention.8,9
This study included COPD exacerbations and cases of pneumonia or oral candidiasis as secondary outcomes to assess the safety and efficacy of the ICS de-escalation. It appeared there were similar or lower rates of COPD exacerbations, pneumonia, and oral candidiasis in those with de-escalated ICS therapy in this study. However, these secondary outcomes are exploratory and would need to be confirmed by larger studies powered to address these outcomes.
CONCLUSIONS
Pharmacist-driven ICS de-escalation may be an effective method for reducing ICS usage in veterans as seen in this study. Additional controlled studies are required to evaluate the efficacy and safety of pharmacist-driven ICS de-escalation.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2024 Report). Accessed October 14, 2025. https://goldcopd.org/2024-gold-report/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2025 Report). Accessed November 14, 2025. https://goldcopd.org/2025-gold-report/
- Rogliani P, Ritondo BL, Gabriele M, et al. Optimizing de-escalation of inhaled corticosteroids in COPD: a systematic review of real-world findings. Expert Rev Clin Pharmacol. 2020;13(9):977-990. doi:10.1080/17512433.2020.1817739
- Rossi A, Guerriero M, Corrado A; OPTIMO/AIPO Study Group. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15(1):77. doi:10.1186/1465-9921-15-77
- Anderson E, Wiener RS, Resnick K, et al. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/ajmc.2020.42394
- US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Chronic Obstructive Pulmonary Disease. 2021. Accessed October 14, 2025. https://www.healthquality.va.gov/guidelines/CD/copd/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). Accessed October 14, 2025. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
- Hegland AJ, Bolduc J, Jones L, Kunisaki KM, Melzer AC. Pharmacist-driven deprescribing of inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2021;18(4):730-733. doi:10.1513/AnnalsATS.202007-871RL
- Hahn NM, Nagy MW. Implementation of a targeted inhaled corticosteroid de-escalation process in patients with chronic obstructive pulmonary disease in the primary care setting. Innov Pharm. 2022;13(1):10.24926/iip.v13i1.4349. doi:10.24926/iip.v13i1.4349
Systemic glucocorticoids play an important role in the treatment of chronic obstructive pulmonary disease (COPD) exacerbations. They are recommended to shorten recovery time and increase forced expiratory volume in 1 second (FEV1) during exacerbations.1 However, the role of the chronic use of inhaled corticosteroids (ICSs) in the treatment of COPD is less clear.
When added to inhaled β-2 agonists and muscarinic antagonists, ICSs can decrease the risk of exacerbations.1 However, not all patients with COPD benefit from ICS therapy. The degree of benefit an ICS can provide has been shown to correlate with eosinophil count—a marker of inflammation. The expected benefit of using an ICS increases as the eosinophil count increases.1 Maximum benefit can be observed with eosinophil counts ≥ 300 cells/µL, and minimal benefit is observed with eosinophil counts < 100 cells/µL. Adverse effects (AEs) of ICSs include a hoarse voice, oral candidiasis, and an increased risk of pneumonia.1 Given the risk of AEs, it is important to limit ICS use in patients who are unlikely to reap any benefits.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines suggest the use of ICSs in patients who experience exacerbations while using long-acting β agonist (LABA) plus long-acting muscarinic antagonist (LAMA) therapy and have an eosinophil count ≥ 100 cells/µL. Switching from LABA or LAMA monotherapy to triple therapy with LAMA/LABA/ICS may be considered if patients have continued exacerbations and an eosinophil count ≥ 300 cells/µL. De-escalation of ICS therapy should be considered if patients do not meet these criteria or if patients experience ICS AEs, such as pneumonia. The patients most likely to have increased exacerbations or decreased FEV1 with ICS withdrawal are those with eosinophil counts ≥ 300 cells/µL.1,2
Several studies have explored the effects of ICS de-escalation in real-world clinical settings. A systematic review of 11 studies indicated that de-escalation of ICS in COPD does not result in increased exacerbations.3 A prospective study by Rossi et al found that in a 6-month period, 141 of 482 patients on ICS therapy (29%) had an exacerbation. In the opposing arm of the study, 88 of 334 patients (26%) with deprescribed ICS experienced an exacerbation. The difference between these 2 groups was not statistically significant.4 The researchers concluded that in real-world practice, ICS withdrawal can be safe in patients at low risk of exacerbation.
About 25% of veterans (1.25 million) have been diagnosed with COPD.5 To address this, the US Department of Veterans Affairs (VA) and US Department of Defense published updated COPD guidelines in 2021 that specify criteria for de-escalation of ICS.6 Guidelines, however, may not be reflected in common clinical practice for several years following publication. The VA Academic Detailing Service (ADS) provides tools to help clinicians identify patients who may benefit from changes in treatment plans. A recent ADS focus was the implementation of a COPD dashboard, which identifies patients with COPD who are candidates for ICS de-escalation based on comorbid diagnoses, exacerbation history, and eosinophil count. VA pharmacists have an expanded role in the management of primary care disease states and are therefore well-positioned to increase adherence to guideline-directed therapy. The objective of this quality improvement project was to determine the impact of pharmacist-driven de-escalation on ICS usage in veterans with COPD.
Methods
This project was conducted in an outpatient clinic at the Robley Rex VA Medical Center beginning September 21, 2023, with a progress note in the Computerized Patient Record System (CPRS). Eligible patients were selected using the COPD Dashboard provided by ADS. The COPD Dashboard defined patients with COPD as those with ≥ 2 outpatient COPD diagnoses in the past 2 years, 1 inpatient discharge COPD diagnosis in the past year, or COPD listed as an active problem. COPD diagnoses were identified using International Statistical Classification of Disease, Tenth Revision (ICD-10) codes
Candidates identified for ICS de-escalation by the dashboard were excluded if they had a history of COPD exacerbation in the previous 2 years. The dashboard identified COPD exacerbations via ICD-10 codes for COPD or acute respiratory failure for inpatient discharges, emergency department (ED) visits, urgent care visits, and community care consults with 1 of the following terms: emergency, inpatient, hospital, urgent, ED (self). The COPD dashboard excluded patients with a diagnosis of asthma.
After patients were selected, they were screened for additional exclusion criteria. Patients were excluded if a pulmonary care practitioner managed their COPD; if identified via an active pulmonary consult in CPRS; if a non-VA clinician prescribed their ICS; or if they were being treated with roflumilast, theophylline, or chronic azithromycin. Individuals taking these 3 drugs were excluded due to potential severe and/or refractory COPD. Patients also were excluded if they: (1) had prior ICS de-escalation failure (defined as a COPD exacerbation following ICS de-escalation that resulted in ICS resumption); (2) had a COPD exacerbation requiring systemic corticosteroids or antibiotics in the previous year; (3) had active lung cancer; (4) did not have any eosinophil levels in CPRS within the previous 2 years; or (5) had any eosinophil levels ≥ 300 cells/µL in the previous year.
Each patient who met the inclusion criteria and was not excluded received a focused medication review by a pharmacist who created a templated progress note, with patient-specific recommendations, that was entered in the CPRS (eAppendix). The recommendations were also attached as an addendum to the patient’s last primary care visit note, and the primary care practitioner (PCP) was alerted via CPRS to consider ICS de-escalation and non-ICS alternatives. Tapering of ICS therapy was offered as an option to de-escalate if abrupt discontinuation was deemed inappropriate. PCPs were also prompted to consider referral to a primary care clinical pharmacy specialist for management and follow-up of ICS de-escalation.
The primary outcome was the number of patients with de-escalated ICS at 3 and 6 months following the recommendation. Secondary outcomes included the number of: patients who were no longer prescribed an ICS or who had a non-ICS alternative initiated at a pharmacist’s recommendation; patients who were referred to a primary care clinical pharmacy specialist for ICS de-escalation; COPD exacerbations requiring systemic steroids or antibiotics, or requiring an ED visit, inpatient admission, or urgent-care clinic visit; and cases of pneumonia or oral candidiasis. Primary and secondary outcomes were evaluated via chart review in CPRS. For secondary outcomes of pneumonia and COPD exacerbation, identification was made by documented diagnosis in CPRS. For continuous data such as age, the mean was calculated.
Results
Pharmacist ICS de-escalation recommendations were made between September 21, 2023, and November 19, 2023, for 106 patients. The mean age was 72 years and 99 (93%) patients were male (Table 1). Forty-one (39%) of the patients used tobacco at the time of the study. FEV1 was available for 69 patients with a mean of 63% (GOLD grade 2).1 Based on FEV1 values, 16 patients had mild COPD (GOLD grade 1), 37 patients had moderate COPD (GOLD grade 2), 14 patients had severe COPD (GOLD grade 3), and 2 patients had very severe COPD (GOLD grade 4).1 Thirty-four patients received LABA + LAMA + ICS, 65 received LABA + ICS, 2 received LAMA + ICS, and 5 received ICS monotherapy. The most common dose of ICS was a moderate dose (Table 2). Only 2 patients had an ICS AE in the previous year.


ICS de-escalation recommendations resulted in ICS de-escalation in 50 (47.2%) and 62 (58.5%) patients at 3 and 6 months, respectively. The 6-month ICS de-escalation rate by ICS dose at baseline was 72.2% (high dose), 60.0% (moderate), and 30.8% (low). De-escalation at 6 months by GOLD grade at baseline was 56.3% (9 of 16 patients, GOLD 1), 64.9% (24 of 37 patients, GOLD 2), 50% (7 of 14 patients, GOLD 3), and 50% (1 of 2 patients, GOLD 4). Six months after the ICS de-escalation recommendation appeared in the CPRS, the percentage of patients on LABA + ICS therapy dropped from 65 patients (61.3%) at baseline to 25 patients (23.6%).
Secondary outcomes were assessed at 3 and 6 months following the recommendation. Most patients with de-escalated ICS had their ICS discontinued and a non-ICS alternative initiated per pharmacist recommendations. At 6 months, 39 patients (36.8%) patients were referred to a patient aligned care team (PACT) pharmacist for de-escalation. Of the 39 patients referred to pharmacists, 69.2% (27 patients) were de-escalated; this compared to 52.2% (35 patients) who were not referred to pharmacists (Table 3).

ICS use increases the risk of pneumonia.1 At 6 months, 11 patients were diagnosed with pneumonia; 3 patients were diagnosed with pneumonia twice, resulting in a total of 14 cases. Ten cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation. One patient had a documented case of oral candidiasis that occurred while on ICS therapy; no patients with discontinued ICS were diagnosed with oral candidiasis. In addition, 10 patients had COPD exacerbations; however no patients had exacerbations both before and after de-escalation. Six patients were on ICS therapy when they experienced an exacerbation, and 4 patients had an exacerbation after ICS de-escalation.
Discussion
More than half of patients receiving the pharmacist intervention achieved the primary outcome of ICS de-escalation at 6 months. Furthermore, a larger percentage of patients referred to pharmacists for the management of ICS de-escalation successfully achieved de-escalation compared to those who were not referred. These outcomes reflect the important role pharmacists can play in identifying appropriate candidates for ICS de-escalation and assisting in the management of ICS de-escalation. Patients referred to pharmacists also received other services such as smoking cessation pharmacotherapy and counseling on inhaler technique and adherence. These interventions can support improved COPD clinical outcomes.
The purpose of de-escalating ICS therapy is to reduce the risk of AEs such as pneumonia and oral candidiasis.1 The secondary outcomes of this study support previous evidence that patients who have de-escalated ICS therapy may have reduced risk of AEs compared to those who remain on ICS therapy.3 Specifically, of the 14 cases of pneumonia that occurred during the study, 10 cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation.
ICS de-escalation may increase risk of increased COPD exacerbations.1 However, the secondary outcomes of this study do not indicate that those with de-escalated ICS had more COPD exacerbations compared to those who continued on ICS. Pharmacists’ recommendations were more effective for patients with less severe COPD based on baseline FEV1.
The previous GOLD Guidelines for COPD suggested LABA + ICS therapy as an option for patients with a high symptom and exacerbation burden (previously known as GOLD Group D). Guidelines no longer recommend LABA + ICS therapy due to the superiority of triple inhaled therapy for exacerbations and the superiority of LAMA + LABA therapy for dyspnea.7 A majority of identified patients in this project were on LABA + ICS therapy alone at baseline. The ICS de-escalation recommendation resulted in a 61.5% reduction in patients on LABA + ICS therapy at 6 months. By decreasing the number of patients on LABA + ICS without LAMA, recommendations increased the number of patients on guideline-directed therapy.
Limitations
This study lacked a control group, and the rate of ICS de-escalation in patients who did not receive a pharmacist recommendation was not assessed. Therefore, it could not be determined whether the pharmacist recommendation is more effective than no recommendation. Another limitation was our inability to access records from non-VA health care facilities. This may have resulted in missed COPD exacerbations, pneumonia, and oral candidiasis prior to or following the pharmacist recommendation.
In addition, the method used to notify PCPs of the pharmacist recommendation was a CPRS alert. Clinicians often receive multiple daily alerts and may not always pay close attention to them due to alert fatigue. Early in the study, some PCPs were unknowingly omitted from the alert of the pharmacist recommendation for 10 patients due to human error. For 8 of these 10 patients, the PCP was notified of the recommendations during the 3-month follow-up period. However, 2 patients had COPD exacerbations during the 3-month follow-up period. In these cases, the PCP was not alerted to de-escalate ICS. The data for these patients were collected at 3 and 6 months in the same manner as all other patients. Also, 7 of 35 patients who were referred to a pharmacist for ICS de-escalation did not have a scheduled appointment. These patients were considered to be lost to follow-up and this may have resulted in an underestimation of the ability of pharmacists to successfully de-escalate ICS in patients with COPD.
Other studies have evaluated the efficacy of a pharmacy-driven ICS de-escalation.8,9 Hegland et al reported ICS de-escalation for 22% of 141 eligible ambulatory patients with COPD on triple inhaled therapy following pharmacist appointments.8 A study by Hahn et al resulted in 63.8% of 58 patients with COPD being maintained off ICS following a pharmacist de-escalation initiative.9 However, these studies relied upon more time-consuming de-escalation interventions, including at least 1 phone, video, or in-person patient visit.8,9
This project used a single chart review and templated progress note to recommend ICS de-escalation and achieved similar or improved de-escalation rates compared to previous studies.8,9 Previous studies were conducted prior to the updated 2023 GOLD guidelines for COPD which no longer recommend LABA + ICS therapy. This project addressed ICS de-escalation in patients on LABA + ICS therapy in addition to those on triple inhaled therapy. Additionally, previous studies did not address rates of moderate to severe COPD exacerbation and adverse events to ICS following the pharmacist intervention.8,9
This study included COPD exacerbations and cases of pneumonia or oral candidiasis as secondary outcomes to assess the safety and efficacy of the ICS de-escalation. It appeared there were similar or lower rates of COPD exacerbations, pneumonia, and oral candidiasis in those with de-escalated ICS therapy in this study. However, these secondary outcomes are exploratory and would need to be confirmed by larger studies powered to address these outcomes.
CONCLUSIONS
Pharmacist-driven ICS de-escalation may be an effective method for reducing ICS usage in veterans as seen in this study. Additional controlled studies are required to evaluate the efficacy and safety of pharmacist-driven ICS de-escalation.

Systemic glucocorticoids play an important role in the treatment of chronic obstructive pulmonary disease (COPD) exacerbations. They are recommended to shorten recovery time and increase forced expiratory volume in 1 second (FEV1) during exacerbations.1 However, the role of the chronic use of inhaled corticosteroids (ICSs) in the treatment of COPD is less clear.
When added to inhaled β-2 agonists and muscarinic antagonists, ICSs can decrease the risk of exacerbations.1 However, not all patients with COPD benefit from ICS therapy. The degree of benefit an ICS can provide has been shown to correlate with eosinophil count—a marker of inflammation. The expected benefit of using an ICS increases as the eosinophil count increases.1 Maximum benefit can be observed with eosinophil counts ≥ 300 cells/µL, and minimal benefit is observed with eosinophil counts < 100 cells/µL. Adverse effects (AEs) of ICSs include a hoarse voice, oral candidiasis, and an increased risk of pneumonia.1 Given the risk of AEs, it is important to limit ICS use in patients who are unlikely to reap any benefits.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines suggest the use of ICSs in patients who experience exacerbations while using long-acting β agonist (LABA) plus long-acting muscarinic antagonist (LAMA) therapy and have an eosinophil count ≥ 100 cells/µL. Switching from LABA or LAMA monotherapy to triple therapy with LAMA/LABA/ICS may be considered if patients have continued exacerbations and an eosinophil count ≥ 300 cells/µL. De-escalation of ICS therapy should be considered if patients do not meet these criteria or if patients experience ICS AEs, such as pneumonia. The patients most likely to have increased exacerbations or decreased FEV1 with ICS withdrawal are those with eosinophil counts ≥ 300 cells/µL.1,2
Several studies have explored the effects of ICS de-escalation in real-world clinical settings. A systematic review of 11 studies indicated that de-escalation of ICS in COPD does not result in increased exacerbations.3 A prospective study by Rossi et al found that in a 6-month period, 141 of 482 patients on ICS therapy (29%) had an exacerbation. In the opposing arm of the study, 88 of 334 patients (26%) with deprescribed ICS experienced an exacerbation. The difference between these 2 groups was not statistically significant.4 The researchers concluded that in real-world practice, ICS withdrawal can be safe in patients at low risk of exacerbation.
About 25% of veterans (1.25 million) have been diagnosed with COPD.5 To address this, the US Department of Veterans Affairs (VA) and US Department of Defense published updated COPD guidelines in 2021 that specify criteria for de-escalation of ICS.6 Guidelines, however, may not be reflected in common clinical practice for several years following publication. The VA Academic Detailing Service (ADS) provides tools to help clinicians identify patients who may benefit from changes in treatment plans. A recent ADS focus was the implementation of a COPD dashboard, which identifies patients with COPD who are candidates for ICS de-escalation based on comorbid diagnoses, exacerbation history, and eosinophil count. VA pharmacists have an expanded role in the management of primary care disease states and are therefore well-positioned to increase adherence to guideline-directed therapy. The objective of this quality improvement project was to determine the impact of pharmacist-driven de-escalation on ICS usage in veterans with COPD.
Methods
This project was conducted in an outpatient clinic at the Robley Rex VA Medical Center beginning September 21, 2023, with a progress note in the Computerized Patient Record System (CPRS). Eligible patients were selected using the COPD Dashboard provided by ADS. The COPD Dashboard defined patients with COPD as those with ≥ 2 outpatient COPD diagnoses in the past 2 years, 1 inpatient discharge COPD diagnosis in the past year, or COPD listed as an active problem. COPD diagnoses were identified using International Statistical Classification of Disease, Tenth Revision (ICD-10) codes
Candidates identified for ICS de-escalation by the dashboard were excluded if they had a history of COPD exacerbation in the previous 2 years. The dashboard identified COPD exacerbations via ICD-10 codes for COPD or acute respiratory failure for inpatient discharges, emergency department (ED) visits, urgent care visits, and community care consults with 1 of the following terms: emergency, inpatient, hospital, urgent, ED (self). The COPD dashboard excluded patients with a diagnosis of asthma.
After patients were selected, they were screened for additional exclusion criteria. Patients were excluded if a pulmonary care practitioner managed their COPD; if identified via an active pulmonary consult in CPRS; if a non-VA clinician prescribed their ICS; or if they were being treated with roflumilast, theophylline, or chronic azithromycin. Individuals taking these 3 drugs were excluded due to potential severe and/or refractory COPD. Patients also were excluded if they: (1) had prior ICS de-escalation failure (defined as a COPD exacerbation following ICS de-escalation that resulted in ICS resumption); (2) had a COPD exacerbation requiring systemic corticosteroids or antibiotics in the previous year; (3) had active lung cancer; (4) did not have any eosinophil levels in CPRS within the previous 2 years; or (5) had any eosinophil levels ≥ 300 cells/µL in the previous year.
Each patient who met the inclusion criteria and was not excluded received a focused medication review by a pharmacist who created a templated progress note, with patient-specific recommendations, that was entered in the CPRS (eAppendix). The recommendations were also attached as an addendum to the patient’s last primary care visit note, and the primary care practitioner (PCP) was alerted via CPRS to consider ICS de-escalation and non-ICS alternatives. Tapering of ICS therapy was offered as an option to de-escalate if abrupt discontinuation was deemed inappropriate. PCPs were also prompted to consider referral to a primary care clinical pharmacy specialist for management and follow-up of ICS de-escalation.
The primary outcome was the number of patients with de-escalated ICS at 3 and 6 months following the recommendation. Secondary outcomes included the number of: patients who were no longer prescribed an ICS or who had a non-ICS alternative initiated at a pharmacist’s recommendation; patients who were referred to a primary care clinical pharmacy specialist for ICS de-escalation; COPD exacerbations requiring systemic steroids or antibiotics, or requiring an ED visit, inpatient admission, or urgent-care clinic visit; and cases of pneumonia or oral candidiasis. Primary and secondary outcomes were evaluated via chart review in CPRS. For secondary outcomes of pneumonia and COPD exacerbation, identification was made by documented diagnosis in CPRS. For continuous data such as age, the mean was calculated.
Results
Pharmacist ICS de-escalation recommendations were made between September 21, 2023, and November 19, 2023, for 106 patients. The mean age was 72 years and 99 (93%) patients were male (Table 1). Forty-one (39%) of the patients used tobacco at the time of the study. FEV1 was available for 69 patients with a mean of 63% (GOLD grade 2).1 Based on FEV1 values, 16 patients had mild COPD (GOLD grade 1), 37 patients had moderate COPD (GOLD grade 2), 14 patients had severe COPD (GOLD grade 3), and 2 patients had very severe COPD (GOLD grade 4).1 Thirty-four patients received LABA + LAMA + ICS, 65 received LABA + ICS, 2 received LAMA + ICS, and 5 received ICS monotherapy. The most common dose of ICS was a moderate dose (Table 2). Only 2 patients had an ICS AE in the previous year.


ICS de-escalation recommendations resulted in ICS de-escalation in 50 (47.2%) and 62 (58.5%) patients at 3 and 6 months, respectively. The 6-month ICS de-escalation rate by ICS dose at baseline was 72.2% (high dose), 60.0% (moderate), and 30.8% (low). De-escalation at 6 months by GOLD grade at baseline was 56.3% (9 of 16 patients, GOLD 1), 64.9% (24 of 37 patients, GOLD 2), 50% (7 of 14 patients, GOLD 3), and 50% (1 of 2 patients, GOLD 4). Six months after the ICS de-escalation recommendation appeared in the CPRS, the percentage of patients on LABA + ICS therapy dropped from 65 patients (61.3%) at baseline to 25 patients (23.6%).
Secondary outcomes were assessed at 3 and 6 months following the recommendation. Most patients with de-escalated ICS had their ICS discontinued and a non-ICS alternative initiated per pharmacist recommendations. At 6 months, 39 patients (36.8%) patients were referred to a patient aligned care team (PACT) pharmacist for de-escalation. Of the 39 patients referred to pharmacists, 69.2% (27 patients) were de-escalated; this compared to 52.2% (35 patients) who were not referred to pharmacists (Table 3).

ICS use increases the risk of pneumonia.1 At 6 months, 11 patients were diagnosed with pneumonia; 3 patients were diagnosed with pneumonia twice, resulting in a total of 14 cases. Ten cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation. One patient had a documented case of oral candidiasis that occurred while on ICS therapy; no patients with discontinued ICS were diagnosed with oral candidiasis. In addition, 10 patients had COPD exacerbations; however no patients had exacerbations both before and after de-escalation. Six patients were on ICS therapy when they experienced an exacerbation, and 4 patients had an exacerbation after ICS de-escalation.
Discussion
More than half of patients receiving the pharmacist intervention achieved the primary outcome of ICS de-escalation at 6 months. Furthermore, a larger percentage of patients referred to pharmacists for the management of ICS de-escalation successfully achieved de-escalation compared to those who were not referred. These outcomes reflect the important role pharmacists can play in identifying appropriate candidates for ICS de-escalation and assisting in the management of ICS de-escalation. Patients referred to pharmacists also received other services such as smoking cessation pharmacotherapy and counseling on inhaler technique and adherence. These interventions can support improved COPD clinical outcomes.
The purpose of de-escalating ICS therapy is to reduce the risk of AEs such as pneumonia and oral candidiasis.1 The secondary outcomes of this study support previous evidence that patients who have de-escalated ICS therapy may have reduced risk of AEs compared to those who remain on ICS therapy.3 Specifically, of the 14 cases of pneumonia that occurred during the study, 10 cases occurred while patients were on ICS and 4 cases occurred following ICS de-escalation.
ICS de-escalation may increase risk of increased COPD exacerbations.1 However, the secondary outcomes of this study do not indicate that those with de-escalated ICS had more COPD exacerbations compared to those who continued on ICS. Pharmacists’ recommendations were more effective for patients with less severe COPD based on baseline FEV1.
The previous GOLD Guidelines for COPD suggested LABA + ICS therapy as an option for patients with a high symptom and exacerbation burden (previously known as GOLD Group D). Guidelines no longer recommend LABA + ICS therapy due to the superiority of triple inhaled therapy for exacerbations and the superiority of LAMA + LABA therapy for dyspnea.7 A majority of identified patients in this project were on LABA + ICS therapy alone at baseline. The ICS de-escalation recommendation resulted in a 61.5% reduction in patients on LABA + ICS therapy at 6 months. By decreasing the number of patients on LABA + ICS without LAMA, recommendations increased the number of patients on guideline-directed therapy.
Limitations
This study lacked a control group, and the rate of ICS de-escalation in patients who did not receive a pharmacist recommendation was not assessed. Therefore, it could not be determined whether the pharmacist recommendation is more effective than no recommendation. Another limitation was our inability to access records from non-VA health care facilities. This may have resulted in missed COPD exacerbations, pneumonia, and oral candidiasis prior to or following the pharmacist recommendation.
In addition, the method used to notify PCPs of the pharmacist recommendation was a CPRS alert. Clinicians often receive multiple daily alerts and may not always pay close attention to them due to alert fatigue. Early in the study, some PCPs were unknowingly omitted from the alert of the pharmacist recommendation for 10 patients due to human error. For 8 of these 10 patients, the PCP was notified of the recommendations during the 3-month follow-up period. However, 2 patients had COPD exacerbations during the 3-month follow-up period. In these cases, the PCP was not alerted to de-escalate ICS. The data for these patients were collected at 3 and 6 months in the same manner as all other patients. Also, 7 of 35 patients who were referred to a pharmacist for ICS de-escalation did not have a scheduled appointment. These patients were considered to be lost to follow-up and this may have resulted in an underestimation of the ability of pharmacists to successfully de-escalate ICS in patients with COPD.
Other studies have evaluated the efficacy of a pharmacy-driven ICS de-escalation.8,9 Hegland et al reported ICS de-escalation for 22% of 141 eligible ambulatory patients with COPD on triple inhaled therapy following pharmacist appointments.8 A study by Hahn et al resulted in 63.8% of 58 patients with COPD being maintained off ICS following a pharmacist de-escalation initiative.9 However, these studies relied upon more time-consuming de-escalation interventions, including at least 1 phone, video, or in-person patient visit.8,9
This project used a single chart review and templated progress note to recommend ICS de-escalation and achieved similar or improved de-escalation rates compared to previous studies.8,9 Previous studies were conducted prior to the updated 2023 GOLD guidelines for COPD which no longer recommend LABA + ICS therapy. This project addressed ICS de-escalation in patients on LABA + ICS therapy in addition to those on triple inhaled therapy. Additionally, previous studies did not address rates of moderate to severe COPD exacerbation and adverse events to ICS following the pharmacist intervention.8,9
This study included COPD exacerbations and cases of pneumonia or oral candidiasis as secondary outcomes to assess the safety and efficacy of the ICS de-escalation. It appeared there were similar or lower rates of COPD exacerbations, pneumonia, and oral candidiasis in those with de-escalated ICS therapy in this study. However, these secondary outcomes are exploratory and would need to be confirmed by larger studies powered to address these outcomes.
CONCLUSIONS
Pharmacist-driven ICS de-escalation may be an effective method for reducing ICS usage in veterans as seen in this study. Additional controlled studies are required to evaluate the efficacy and safety of pharmacist-driven ICS de-escalation.

- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2024 Report). Accessed October 14, 2025. https://goldcopd.org/2024-gold-report/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2025 Report). Accessed November 14, 2025. https://goldcopd.org/2025-gold-report/
- Rogliani P, Ritondo BL, Gabriele M, et al. Optimizing de-escalation of inhaled corticosteroids in COPD: a systematic review of real-world findings. Expert Rev Clin Pharmacol. 2020;13(9):977-990. doi:10.1080/17512433.2020.1817739
- Rossi A, Guerriero M, Corrado A; OPTIMO/AIPO Study Group. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15(1):77. doi:10.1186/1465-9921-15-77
- Anderson E, Wiener RS, Resnick K, et al. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/ajmc.2020.42394
- US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Chronic Obstructive Pulmonary Disease. 2021. Accessed October 14, 2025. https://www.healthquality.va.gov/guidelines/CD/copd/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). Accessed October 14, 2025. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
- Hegland AJ, Bolduc J, Jones L, Kunisaki KM, Melzer AC. Pharmacist-driven deprescribing of inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2021;18(4):730-733. doi:10.1513/AnnalsATS.202007-871RL
- Hahn NM, Nagy MW. Implementation of a targeted inhaled corticosteroid de-escalation process in patients with chronic obstructive pulmonary disease in the primary care setting. Innov Pharm. 2022;13(1):10.24926/iip.v13i1.4349. doi:10.24926/iip.v13i1.4349
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2024 Report). Accessed October 14, 2025. https://goldcopd.org/2024-gold-report/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2025 Report). Accessed November 14, 2025. https://goldcopd.org/2025-gold-report/
- Rogliani P, Ritondo BL, Gabriele M, et al. Optimizing de-escalation of inhaled corticosteroids in COPD: a systematic review of real-world findings. Expert Rev Clin Pharmacol. 2020;13(9):977-990. doi:10.1080/17512433.2020.1817739
- Rossi A, Guerriero M, Corrado A; OPTIMO/AIPO Study Group. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15(1):77. doi:10.1186/1465-9921-15-77
- Anderson E, Wiener RS, Resnick K, et al. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/ajmc.2020.42394
- US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Chronic Obstructive Pulmonary Disease. 2021. Accessed October 14, 2025. https://www.healthquality.va.gov/guidelines/CD/copd/
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). Accessed October 14, 2025. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
- Hegland AJ, Bolduc J, Jones L, Kunisaki KM, Melzer AC. Pharmacist-driven deprescribing of inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2021;18(4):730-733. doi:10.1513/AnnalsATS.202007-871RL
- Hahn NM, Nagy MW. Implementation of a targeted inhaled corticosteroid de-escalation process in patients with chronic obstructive pulmonary disease in the primary care setting. Innov Pharm. 2022;13(1):10.24926/iip.v13i1.4349. doi:10.24926/iip.v13i1.4349
Evaluation of Pharmacist-Driven Inhaled Corticosteroid De-escalation in Veterans
Evaluation of Pharmacist-Driven Inhaled Corticosteroid De-escalation in Veterans
Development and Implementation of an Anti-Human Trafficking Education for Veterans and Clinicians
Background
Veterans may have a greater risk of experiencing human trafficking (HT) than the general population because of social aspects of health, including housing insecurity, justice involvement, food insecurity, and adverse childhood events.1-4 Since 2023, the U.S. Department of Veterans Affairs (VA) has explored veterans’ experiences of HT through the Anti-Human Trafficking (AHT) Pilot Project. This quality improvement project evaluated: 1) development of clinician AHT training materials to enhance identification and response to Veterans experiencing HT, and 2) educational resources aimed at raising awareness tailored to veterans and clinicians.
Methods
South Central Mental Illness Research, Education and Clinical Center (SCMIRECC) facilitated two focus group discussions with AHT coordinators implementing the pilot at six sites. Based on discussions and leadership input, SCMIRECC developed a training curriculum, with bi-weekly readings culminating in a two-hour workshop. Training evaluation followed Kirkpatrick’s model using questions adapted from the Provider Responses, Treatment, and Care for Trafficked People (PROTECT) Survey.5,6 Veteran-facing materials, including a brochure and whiteboard video, were reviewed by two Veteran Consumer Advisory Boards (CAB). The brochures, whiteboard video, and awareness modules were developed and revised based on feedback from focus group discussions. VA Central Office cleared all materials.
Results
Coordinators were satisfied with the training (mean, 4.20). After the training, none of the coordinators (n = 6) felt unprepared to assist Veterans (pre-training mean, 2.25; post-training mean, 1.40), and confidence in documentation improved (pre-training mean, 3.00; post-training mean, 3.40). Veteran CAB members recommended simplified language and veteran-centered messaging. The coordinators found the brochures and training useful. Recommendations included adding more representation to brochure covers, advanced training, a list of commonly asked questions, and a simplified screening tool. Barriers included delays in material development due to language guidance under recent executive orders.
Conclusions
The AHT training improved coordinators’ preparedness and confidence in supporting Veterans with trafficking experiences. Feedback emphasized the value of concise, Veteran-centered materials and a practical HT screening tool. These findings support the continued implementation of AHT education across VA settings to enhance identification and response for Veterans at risk of HT.
- US Department of Veterans Affairs, Veterans Health Administration. Annual Report 2023 Veterans Health Administration Homeless Programs Office.
- Tsai J, Kasprow WJ, Rosenheck RA. Alcohol and drug use disorders among homeless veterans: prevalence and association with supported housing outcomes. Addict Behav. 2014;39(2):455-460. doi:10.1016/j.addbeh.2013.02.002
- Wang EA, McGinnis KA, Goulet J, et al. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268. doi:10.1177/003335491513000313
- Blosnich JR, Garfin DR, Maguen S, et al. Differences in childhood adversity, suicidal ideation, and suicide attempt among veterans and nonveterans. Am Psychol. 2021;76(2):284-299. doi:10.1037/amp0000755
- Kirkpatrick D. Great ideas revisited. Training & Development. 1996;50(1):54-60.
- Ross C, Dimitrova S, Howard LM, Dewey M, Zimmerman C, Oram S. Human trafficking and health: a cross-sectional survey of NHS professionals' contact with victims of human trafficking. BMJ Open. 2015;5(8):e008682. Published 2015 Aug 20. doi:10.1136/bmjopen-2015-008682
Background
Veterans may have a greater risk of experiencing human trafficking (HT) than the general population because of social aspects of health, including housing insecurity, justice involvement, food insecurity, and adverse childhood events.1-4 Since 2023, the U.S. Department of Veterans Affairs (VA) has explored veterans’ experiences of HT through the Anti-Human Trafficking (AHT) Pilot Project. This quality improvement project evaluated: 1) development of clinician AHT training materials to enhance identification and response to Veterans experiencing HT, and 2) educational resources aimed at raising awareness tailored to veterans and clinicians.
Methods
South Central Mental Illness Research, Education and Clinical Center (SCMIRECC) facilitated two focus group discussions with AHT coordinators implementing the pilot at six sites. Based on discussions and leadership input, SCMIRECC developed a training curriculum, with bi-weekly readings culminating in a two-hour workshop. Training evaluation followed Kirkpatrick’s model using questions adapted from the Provider Responses, Treatment, and Care for Trafficked People (PROTECT) Survey.5,6 Veteran-facing materials, including a brochure and whiteboard video, were reviewed by two Veteran Consumer Advisory Boards (CAB). The brochures, whiteboard video, and awareness modules were developed and revised based on feedback from focus group discussions. VA Central Office cleared all materials.
Results
Coordinators were satisfied with the training (mean, 4.20). After the training, none of the coordinators (n = 6) felt unprepared to assist Veterans (pre-training mean, 2.25; post-training mean, 1.40), and confidence in documentation improved (pre-training mean, 3.00; post-training mean, 3.40). Veteran CAB members recommended simplified language and veteran-centered messaging. The coordinators found the brochures and training useful. Recommendations included adding more representation to brochure covers, advanced training, a list of commonly asked questions, and a simplified screening tool. Barriers included delays in material development due to language guidance under recent executive orders.
Conclusions
The AHT training improved coordinators’ preparedness and confidence in supporting Veterans with trafficking experiences. Feedback emphasized the value of concise, Veteran-centered materials and a practical HT screening tool. These findings support the continued implementation of AHT education across VA settings to enhance identification and response for Veterans at risk of HT.
Background
Veterans may have a greater risk of experiencing human trafficking (HT) than the general population because of social aspects of health, including housing insecurity, justice involvement, food insecurity, and adverse childhood events.1-4 Since 2023, the U.S. Department of Veterans Affairs (VA) has explored veterans’ experiences of HT through the Anti-Human Trafficking (AHT) Pilot Project. This quality improvement project evaluated: 1) development of clinician AHT training materials to enhance identification and response to Veterans experiencing HT, and 2) educational resources aimed at raising awareness tailored to veterans and clinicians.
Methods
South Central Mental Illness Research, Education and Clinical Center (SCMIRECC) facilitated two focus group discussions with AHT coordinators implementing the pilot at six sites. Based on discussions and leadership input, SCMIRECC developed a training curriculum, with bi-weekly readings culminating in a two-hour workshop. Training evaluation followed Kirkpatrick’s model using questions adapted from the Provider Responses, Treatment, and Care for Trafficked People (PROTECT) Survey.5,6 Veteran-facing materials, including a brochure and whiteboard video, were reviewed by two Veteran Consumer Advisory Boards (CAB). The brochures, whiteboard video, and awareness modules were developed and revised based on feedback from focus group discussions. VA Central Office cleared all materials.
Results
Coordinators were satisfied with the training (mean, 4.20). After the training, none of the coordinators (n = 6) felt unprepared to assist Veterans (pre-training mean, 2.25; post-training mean, 1.40), and confidence in documentation improved (pre-training mean, 3.00; post-training mean, 3.40). Veteran CAB members recommended simplified language and veteran-centered messaging. The coordinators found the brochures and training useful. Recommendations included adding more representation to brochure covers, advanced training, a list of commonly asked questions, and a simplified screening tool. Barriers included delays in material development due to language guidance under recent executive orders.
Conclusions
The AHT training improved coordinators’ preparedness and confidence in supporting Veterans with trafficking experiences. Feedback emphasized the value of concise, Veteran-centered materials and a practical HT screening tool. These findings support the continued implementation of AHT education across VA settings to enhance identification and response for Veterans at risk of HT.
- US Department of Veterans Affairs, Veterans Health Administration. Annual Report 2023 Veterans Health Administration Homeless Programs Office.
- Tsai J, Kasprow WJ, Rosenheck RA. Alcohol and drug use disorders among homeless veterans: prevalence and association with supported housing outcomes. Addict Behav. 2014;39(2):455-460. doi:10.1016/j.addbeh.2013.02.002
- Wang EA, McGinnis KA, Goulet J, et al. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268. doi:10.1177/003335491513000313
- Blosnich JR, Garfin DR, Maguen S, et al. Differences in childhood adversity, suicidal ideation, and suicide attempt among veterans and nonveterans. Am Psychol. 2021;76(2):284-299. doi:10.1037/amp0000755
- Kirkpatrick D. Great ideas revisited. Training & Development. 1996;50(1):54-60.
- Ross C, Dimitrova S, Howard LM, Dewey M, Zimmerman C, Oram S. Human trafficking and health: a cross-sectional survey of NHS professionals' contact with victims of human trafficking. BMJ Open. 2015;5(8):e008682. Published 2015 Aug 20. doi:10.1136/bmjopen-2015-008682
- US Department of Veterans Affairs, Veterans Health Administration. Annual Report 2023 Veterans Health Administration Homeless Programs Office.
- Tsai J, Kasprow WJ, Rosenheck RA. Alcohol and drug use disorders among homeless veterans: prevalence and association with supported housing outcomes. Addict Behav. 2014;39(2):455-460. doi:10.1016/j.addbeh.2013.02.002
- Wang EA, McGinnis KA, Goulet J, et al. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268. doi:10.1177/003335491513000313
- Blosnich JR, Garfin DR, Maguen S, et al. Differences in childhood adversity, suicidal ideation, and suicide attempt among veterans and nonveterans. Am Psychol. 2021;76(2):284-299. doi:10.1037/amp0000755
- Kirkpatrick D. Great ideas revisited. Training & Development. 1996;50(1):54-60.
- Ross C, Dimitrova S, Howard LM, Dewey M, Zimmerman C, Oram S. Human trafficking and health: a cross-sectional survey of NHS professionals' contact with victims of human trafficking. BMJ Open. 2015;5(8):e008682. Published 2015 Aug 20. doi:10.1136/bmjopen-2015-008682
Weekends Off on Clinical Rotations? Examining Clinical Opportunity Trends on Weekdays vs Weekends During Internal Medicine Clerkship Rotations in Veterans Health Administration Inpatient Wards
Background
The Accreditation Council for Graduate Medical Education (ACGME) mandates an 80-hour weekly work limit for residents.1 In contrast, decisions regarding undergraduate medical education (UME) are strongly influenced locally, with individual institutions setting academic policy for students. These differences in oversight reflect fundamental differences in residents’ and students’ roles in patient care, power, and responsibility. Considering rotation schedules, internal medicine (IM) clerkship directors have discussed the relative value of weekend vs weekday duty during inpatient rotations, a scheduling topic of interest to students as well, though these conversations are limited by a lack of knowledge regarding admission patterns. Addressing this information gap would inform policy decisions.
The Veterans Health Administration (VHA) is uniquely positioned to address questions about UME clinical experiences nationwide: annually, over 118,000 students representing 97% of US medical schools train at VHA facilities.2,3 We aim to compare the number and variety of patient encounter opportunities presenting during inpatient VHA IM rotations on weekdays versus weekends to inform policy decisions for UME rotation schedules.
Innovation
The VHA Corporate Data Warehouse will be queried for all admissions, diagnoses, and length of stay on inpatient IM services at the 420 VHA hospitals affiliated with US medical schools from 2016-2026. We will aggregate case data for day of week, floor, hospital, and Veteran Integrated Service Network (VISN), and determine number of admissions by weekday (Monday-Friday) and weekend (Saturday-Sunday). Weekday vs. weekend admission data will be compared using generalized mixed effects models for clustered longitudinal data. Heterogeneity across hospitals and VISNs will be explored to examine unique regional trends.
Results
We have drafted strategies to query and curate relevant datasets, developed a preliminary analysis plan, and await data deployment from VHA data stewards.
Conclusions
We believe this will be the first VHA-wide evaluation of patient encounter trends on IM services to examine potential training experiences for medical students. This will increase understanding of the critical role VHA has in developing the nations’ healthcare workforce, and how patterns of opportunities for clinical education may be distributed over time, informing decisions about rotation schedules to maximize students’ abilities to interact with, learn from, and serve our nation’s veterans
- Dimitris KD, Taylor BC, Fankhauser RA. Resident work-week regulations: historical review and modern perspectives. J Surg Educ. 2008;65(4):290-296. doi:10.1016/j.jsurg.2008.05.011
- Health professions education statistics. Veterans Health Administration. Accessed March 19, 2025. https://www.va.gov/oaa/docs/OAACurrentStats.pdf
- Medical education at VA: It’s all about the Veterans. VA News. Updated August 16, 2021. Accessed March 19, 2025. https://news.va.gov/93370/medical-education-at-va-its-all-about-the-veterans/
Background
The Accreditation Council for Graduate Medical Education (ACGME) mandates an 80-hour weekly work limit for residents.1 In contrast, decisions regarding undergraduate medical education (UME) are strongly influenced locally, with individual institutions setting academic policy for students. These differences in oversight reflect fundamental differences in residents’ and students’ roles in patient care, power, and responsibility. Considering rotation schedules, internal medicine (IM) clerkship directors have discussed the relative value of weekend vs weekday duty during inpatient rotations, a scheduling topic of interest to students as well, though these conversations are limited by a lack of knowledge regarding admission patterns. Addressing this information gap would inform policy decisions.
The Veterans Health Administration (VHA) is uniquely positioned to address questions about UME clinical experiences nationwide: annually, over 118,000 students representing 97% of US medical schools train at VHA facilities.2,3 We aim to compare the number and variety of patient encounter opportunities presenting during inpatient VHA IM rotations on weekdays versus weekends to inform policy decisions for UME rotation schedules.
Innovation
The VHA Corporate Data Warehouse will be queried for all admissions, diagnoses, and length of stay on inpatient IM services at the 420 VHA hospitals affiliated with US medical schools from 2016-2026. We will aggregate case data for day of week, floor, hospital, and Veteran Integrated Service Network (VISN), and determine number of admissions by weekday (Monday-Friday) and weekend (Saturday-Sunday). Weekday vs. weekend admission data will be compared using generalized mixed effects models for clustered longitudinal data. Heterogeneity across hospitals and VISNs will be explored to examine unique regional trends.
Results
We have drafted strategies to query and curate relevant datasets, developed a preliminary analysis plan, and await data deployment from VHA data stewards.
Conclusions
We believe this will be the first VHA-wide evaluation of patient encounter trends on IM services to examine potential training experiences for medical students. This will increase understanding of the critical role VHA has in developing the nations’ healthcare workforce, and how patterns of opportunities for clinical education may be distributed over time, informing decisions about rotation schedules to maximize students’ abilities to interact with, learn from, and serve our nation’s veterans
Background
The Accreditation Council for Graduate Medical Education (ACGME) mandates an 80-hour weekly work limit for residents.1 In contrast, decisions regarding undergraduate medical education (UME) are strongly influenced locally, with individual institutions setting academic policy for students. These differences in oversight reflect fundamental differences in residents’ and students’ roles in patient care, power, and responsibility. Considering rotation schedules, internal medicine (IM) clerkship directors have discussed the relative value of weekend vs weekday duty during inpatient rotations, a scheduling topic of interest to students as well, though these conversations are limited by a lack of knowledge regarding admission patterns. Addressing this information gap would inform policy decisions.
The Veterans Health Administration (VHA) is uniquely positioned to address questions about UME clinical experiences nationwide: annually, over 118,000 students representing 97% of US medical schools train at VHA facilities.2,3 We aim to compare the number and variety of patient encounter opportunities presenting during inpatient VHA IM rotations on weekdays versus weekends to inform policy decisions for UME rotation schedules.
Innovation
The VHA Corporate Data Warehouse will be queried for all admissions, diagnoses, and length of stay on inpatient IM services at the 420 VHA hospitals affiliated with US medical schools from 2016-2026. We will aggregate case data for day of week, floor, hospital, and Veteran Integrated Service Network (VISN), and determine number of admissions by weekday (Monday-Friday) and weekend (Saturday-Sunday). Weekday vs. weekend admission data will be compared using generalized mixed effects models for clustered longitudinal data. Heterogeneity across hospitals and VISNs will be explored to examine unique regional trends.
Results
We have drafted strategies to query and curate relevant datasets, developed a preliminary analysis plan, and await data deployment from VHA data stewards.
Conclusions
We believe this will be the first VHA-wide evaluation of patient encounter trends on IM services to examine potential training experiences for medical students. This will increase understanding of the critical role VHA has in developing the nations’ healthcare workforce, and how patterns of opportunities for clinical education may be distributed over time, informing decisions about rotation schedules to maximize students’ abilities to interact with, learn from, and serve our nation’s veterans
- Dimitris KD, Taylor BC, Fankhauser RA. Resident work-week regulations: historical review and modern perspectives. J Surg Educ. 2008;65(4):290-296. doi:10.1016/j.jsurg.2008.05.011
- Health professions education statistics. Veterans Health Administration. Accessed March 19, 2025. https://www.va.gov/oaa/docs/OAACurrentStats.pdf
- Medical education at VA: It’s all about the Veterans. VA News. Updated August 16, 2021. Accessed March 19, 2025. https://news.va.gov/93370/medical-education-at-va-its-all-about-the-veterans/
- Dimitris KD, Taylor BC, Fankhauser RA. Resident work-week regulations: historical review and modern perspectives. J Surg Educ. 2008;65(4):290-296. doi:10.1016/j.jsurg.2008.05.011
- Health professions education statistics. Veterans Health Administration. Accessed March 19, 2025. https://www.va.gov/oaa/docs/OAACurrentStats.pdf
- Medical education at VA: It’s all about the Veterans. VA News. Updated August 16, 2021. Accessed March 19, 2025. https://news.va.gov/93370/medical-education-at-va-its-all-about-the-veterans/
Developing a Multi-Disciplinary Integrative Health Elective at the San Francisco VA
Background
Integrative health (IH) combines conventional and complementary medicine in a coordinated, evidence-based approach to treat the whole person. Nearly 40% of American adults have used complementary health approaches,1 yet IH exposure in medical training is limited. In 2022, the San Francisco VA Health Care Center launched a multidisciplinary clinical IH elective for University of California San Francisco (UCSF) internal medicine and SFVA nurse practitioner residents. Based on findings from a general and targeted needs assessment, including faculty and learner feedback, we found that the elective was well-received, but relied on one-on-one patient-based teaching. This structure created variable learning experiences and high faculty burden. Our project aims to formalize and evaluate the IH elective curriculum to better address the needs of both faculty and learners.
Methods
We used Kern’s six-step framework for curriculum development. To reduce variability, we sought to formalize the core curricular content by: 1) reviewing existing elective components, comparing them to similar curricula nationwide, and outlining foundational knowledge based on the exam domains of the American Board of Integrative Medicine (ABOIM);2 2) creating eleven learning objectives across three themes: patient-centered care, systems-based practice, and IH-specific knowledge; 3) developing IH subspecialty experience guides to standardize clinical teaching with suggested takeaways, guided reflection, and curated resources. To reduce faculty burden, we consolidated elective resources into a centralized e-learning hub. Trainees complete a pre/post self-assessment and evaluation at the end of the elective.
Results
We identified key learning opportunities in each IH shadowing experience to enhance learners’ knowledge. We developed an IH e-Learning Hub to provide easy access to elective materials and IH clinical tools. Evaluations from the first two learners who completed the elective indicate that the learning objectives were met and that learners gained increased knowledge of lifestyle medicine, mind-body medicine, manual medicine, and botanicals/dietary supplements. Learners valued increased IH subspecialty familiarity and reported high likelihood of future practice change.
Discussion
The project is ongoing. Next steps include collecting faculty evaluations about their experience, continuing to create and refine experience guides, promoting clinical tools for learner’s future practice, and developing strategies to recruit more learners to the elective.
- Nahin RL, Rhee A, Stussman B. Use of Complementary Health Approaches Overall and for Pain Management by US Adults. JAMA. 2024;331(7):613-615. doi:10.1001/jama.2023.26775
- Integrative medicine exam description. American Board of Physician Specialties. Updated July 2021. Accessed December 12, 2025. https://www.abpsus.org/integrative-medicine-description
Background
Integrative health (IH) combines conventional and complementary medicine in a coordinated, evidence-based approach to treat the whole person. Nearly 40% of American adults have used complementary health approaches,1 yet IH exposure in medical training is limited. In 2022, the San Francisco VA Health Care Center launched a multidisciplinary clinical IH elective for University of California San Francisco (UCSF) internal medicine and SFVA nurse practitioner residents. Based on findings from a general and targeted needs assessment, including faculty and learner feedback, we found that the elective was well-received, but relied on one-on-one patient-based teaching. This structure created variable learning experiences and high faculty burden. Our project aims to formalize and evaluate the IH elective curriculum to better address the needs of both faculty and learners.
Methods
We used Kern’s six-step framework for curriculum development. To reduce variability, we sought to formalize the core curricular content by: 1) reviewing existing elective components, comparing them to similar curricula nationwide, and outlining foundational knowledge based on the exam domains of the American Board of Integrative Medicine (ABOIM);2 2) creating eleven learning objectives across three themes: patient-centered care, systems-based practice, and IH-specific knowledge; 3) developing IH subspecialty experience guides to standardize clinical teaching with suggested takeaways, guided reflection, and curated resources. To reduce faculty burden, we consolidated elective resources into a centralized e-learning hub. Trainees complete a pre/post self-assessment and evaluation at the end of the elective.
Results
We identified key learning opportunities in each IH shadowing experience to enhance learners’ knowledge. We developed an IH e-Learning Hub to provide easy access to elective materials and IH clinical tools. Evaluations from the first two learners who completed the elective indicate that the learning objectives were met and that learners gained increased knowledge of lifestyle medicine, mind-body medicine, manual medicine, and botanicals/dietary supplements. Learners valued increased IH subspecialty familiarity and reported high likelihood of future practice change.
Discussion
The project is ongoing. Next steps include collecting faculty evaluations about their experience, continuing to create and refine experience guides, promoting clinical tools for learner’s future practice, and developing strategies to recruit more learners to the elective.
Background
Integrative health (IH) combines conventional and complementary medicine in a coordinated, evidence-based approach to treat the whole person. Nearly 40% of American adults have used complementary health approaches,1 yet IH exposure in medical training is limited. In 2022, the San Francisco VA Health Care Center launched a multidisciplinary clinical IH elective for University of California San Francisco (UCSF) internal medicine and SFVA nurse practitioner residents. Based on findings from a general and targeted needs assessment, including faculty and learner feedback, we found that the elective was well-received, but relied on one-on-one patient-based teaching. This structure created variable learning experiences and high faculty burden. Our project aims to formalize and evaluate the IH elective curriculum to better address the needs of both faculty and learners.
Methods
We used Kern’s six-step framework for curriculum development. To reduce variability, we sought to formalize the core curricular content by: 1) reviewing existing elective components, comparing them to similar curricula nationwide, and outlining foundational knowledge based on the exam domains of the American Board of Integrative Medicine (ABOIM);2 2) creating eleven learning objectives across three themes: patient-centered care, systems-based practice, and IH-specific knowledge; 3) developing IH subspecialty experience guides to standardize clinical teaching with suggested takeaways, guided reflection, and curated resources. To reduce faculty burden, we consolidated elective resources into a centralized e-learning hub. Trainees complete a pre/post self-assessment and evaluation at the end of the elective.
Results
We identified key learning opportunities in each IH shadowing experience to enhance learners’ knowledge. We developed an IH e-Learning Hub to provide easy access to elective materials and IH clinical tools. Evaluations from the first two learners who completed the elective indicate that the learning objectives were met and that learners gained increased knowledge of lifestyle medicine, mind-body medicine, manual medicine, and botanicals/dietary supplements. Learners valued increased IH subspecialty familiarity and reported high likelihood of future practice change.
Discussion
The project is ongoing. Next steps include collecting faculty evaluations about their experience, continuing to create and refine experience guides, promoting clinical tools for learner’s future practice, and developing strategies to recruit more learners to the elective.
- Nahin RL, Rhee A, Stussman B. Use of Complementary Health Approaches Overall and for Pain Management by US Adults. JAMA. 2024;331(7):613-615. doi:10.1001/jama.2023.26775
- Integrative medicine exam description. American Board of Physician Specialties. Updated July 2021. Accessed December 12, 2025. https://www.abpsus.org/integrative-medicine-description
- Nahin RL, Rhee A, Stussman B. Use of Complementary Health Approaches Overall and for Pain Management by US Adults. JAMA. 2024;331(7):613-615. doi:10.1001/jama.2023.26775
- Integrative medicine exam description. American Board of Physician Specialties. Updated July 2021. Accessed December 12, 2025. https://www.abpsus.org/integrative-medicine-description
Harm Reduction Integration in an Interprofessional Primary Care Training Clinic
Background
Among people who use drugs (PWUD), harm reduction (HR) is an evidence-based low barrier approach to mitigating ongoing substance use risks and is considered a key pillar of the Department of Health and Human Service’s Overdose Prevention Strategy.1 Given the accessibility and continuity, primary care (PC) clinics are optimal sites for education about and provision of HR services.2,3
Aim
- Determining the impact of active and passive methods for HR supply.
- Recognizing the importance of clinician addiction education in the provision of HR services.
Methods
In January 2024, physician and nurse practitioner trainees in the West Haven Veterans Affairs (VA) Center of Education (CoE) in Interprofessional Primary Care received addiction care and HR strategy education. Initially, all patients presenting to the CoE completed a single-item substance use screening. Patients screening positive were offered HR supplies, including fentanyl and xylazine test strips (FTS, XTS), during the encounter (active distribution). Starting October 2024, HR kiosks were implemented in the clinic lobby, offering patients self-serve access to HR supplies (passive distribution). Test strip uptake was tracked through clinical encounter documentation and weekly kiosk inventory.
Results
Between January 2024 and June 2024, 92 FTS and 84 XTS were actively distributed. Upon implementation of the harm reduction kiosk, 253 FTS and 164 XTS were distributed between October 2024 and February 2025. In the CoE, FTS and XTS distribution increased by 275% and 195%, respectively, through passive kiosk distribution relative to active distribution during clinical encounters.
Conclusions
HR kiosk implementation resulted in significantly increased test strip uptake in the CoE, proving passive distribution to be an effective low barrier method of increasing access to HR and substance use disorder (SUD) resources. Although this model may reduce stigma and logistical barriers when presenting for a healthcare encounter, it limits the ability to track and engage patients for more intensive services. While each approach has unique advantages and disadvantages, test strip demand via both methods highlights the significant need for HR resources in PC settings. Continuing education for PC clinicians on low barrier SUD care and HR is critical to optimizing care for this population.
- Haffajee, RL, Sherry, TB, Dubenitz, JM, et al. Overdose prevention strategy. US Department of Health and Human Services (Issue Brief). Published October 27, 2021. Accessed December 11, 2025. https://aspe.hhs.gov/sites/default/files/documents/101936da95b69acb8446a4bad9179cc0/overdose-prevention-strategy.pdf
- Substance Abuse and Mental Health Services Administration. Advisory: low barrier models of care for substance use disorders. SAMHSA Publication No. PEP23-02-00-005. Published December 2023. Accessed December 11, 2025. https://library.samhsa.gov/sites/default/files/advisory-low-barrier-models-of-care-pep23-02-00-005.pdf
- Substance Abuse and Mental Health Services Administration: Harm Reduction Framework. Center for Substance Abuse Prevention, Substance Abuse and Mental Health Services Administration, 2023.
Background
Among people who use drugs (PWUD), harm reduction (HR) is an evidence-based low barrier approach to mitigating ongoing substance use risks and is considered a key pillar of the Department of Health and Human Service’s Overdose Prevention Strategy.1 Given the accessibility and continuity, primary care (PC) clinics are optimal sites for education about and provision of HR services.2,3
Aim
- Determining the impact of active and passive methods for HR supply.
- Recognizing the importance of clinician addiction education in the provision of HR services.
Methods
In January 2024, physician and nurse practitioner trainees in the West Haven Veterans Affairs (VA) Center of Education (CoE) in Interprofessional Primary Care received addiction care and HR strategy education. Initially, all patients presenting to the CoE completed a single-item substance use screening. Patients screening positive were offered HR supplies, including fentanyl and xylazine test strips (FTS, XTS), during the encounter (active distribution). Starting October 2024, HR kiosks were implemented in the clinic lobby, offering patients self-serve access to HR supplies (passive distribution). Test strip uptake was tracked through clinical encounter documentation and weekly kiosk inventory.
Results
Between January 2024 and June 2024, 92 FTS and 84 XTS were actively distributed. Upon implementation of the harm reduction kiosk, 253 FTS and 164 XTS were distributed between October 2024 and February 2025. In the CoE, FTS and XTS distribution increased by 275% and 195%, respectively, through passive kiosk distribution relative to active distribution during clinical encounters.
Conclusions
HR kiosk implementation resulted in significantly increased test strip uptake in the CoE, proving passive distribution to be an effective low barrier method of increasing access to HR and substance use disorder (SUD) resources. Although this model may reduce stigma and logistical barriers when presenting for a healthcare encounter, it limits the ability to track and engage patients for more intensive services. While each approach has unique advantages and disadvantages, test strip demand via both methods highlights the significant need for HR resources in PC settings. Continuing education for PC clinicians on low barrier SUD care and HR is critical to optimizing care for this population.
Background
Among people who use drugs (PWUD), harm reduction (HR) is an evidence-based low barrier approach to mitigating ongoing substance use risks and is considered a key pillar of the Department of Health and Human Service’s Overdose Prevention Strategy.1 Given the accessibility and continuity, primary care (PC) clinics are optimal sites for education about and provision of HR services.2,3
Aim
- Determining the impact of active and passive methods for HR supply.
- Recognizing the importance of clinician addiction education in the provision of HR services.
Methods
In January 2024, physician and nurse practitioner trainees in the West Haven Veterans Affairs (VA) Center of Education (CoE) in Interprofessional Primary Care received addiction care and HR strategy education. Initially, all patients presenting to the CoE completed a single-item substance use screening. Patients screening positive were offered HR supplies, including fentanyl and xylazine test strips (FTS, XTS), during the encounter (active distribution). Starting October 2024, HR kiosks were implemented in the clinic lobby, offering patients self-serve access to HR supplies (passive distribution). Test strip uptake was tracked through clinical encounter documentation and weekly kiosk inventory.
Results
Between January 2024 and June 2024, 92 FTS and 84 XTS were actively distributed. Upon implementation of the harm reduction kiosk, 253 FTS and 164 XTS were distributed between October 2024 and February 2025. In the CoE, FTS and XTS distribution increased by 275% and 195%, respectively, through passive kiosk distribution relative to active distribution during clinical encounters.
Conclusions
HR kiosk implementation resulted in significantly increased test strip uptake in the CoE, proving passive distribution to be an effective low barrier method of increasing access to HR and substance use disorder (SUD) resources. Although this model may reduce stigma and logistical barriers when presenting for a healthcare encounter, it limits the ability to track and engage patients for more intensive services. While each approach has unique advantages and disadvantages, test strip demand via both methods highlights the significant need for HR resources in PC settings. Continuing education for PC clinicians on low barrier SUD care and HR is critical to optimizing care for this population.
- Haffajee, RL, Sherry, TB, Dubenitz, JM, et al. Overdose prevention strategy. US Department of Health and Human Services (Issue Brief). Published October 27, 2021. Accessed December 11, 2025. https://aspe.hhs.gov/sites/default/files/documents/101936da95b69acb8446a4bad9179cc0/overdose-prevention-strategy.pdf
- Substance Abuse and Mental Health Services Administration. Advisory: low barrier models of care for substance use disorders. SAMHSA Publication No. PEP23-02-00-005. Published December 2023. Accessed December 11, 2025. https://library.samhsa.gov/sites/default/files/advisory-low-barrier-models-of-care-pep23-02-00-005.pdf
- Substance Abuse and Mental Health Services Administration: Harm Reduction Framework. Center for Substance Abuse Prevention, Substance Abuse and Mental Health Services Administration, 2023.
- Haffajee, RL, Sherry, TB, Dubenitz, JM, et al. Overdose prevention strategy. US Department of Health and Human Services (Issue Brief). Published October 27, 2021. Accessed December 11, 2025. https://aspe.hhs.gov/sites/default/files/documents/101936da95b69acb8446a4bad9179cc0/overdose-prevention-strategy.pdf
- Substance Abuse and Mental Health Services Administration. Advisory: low barrier models of care for substance use disorders. SAMHSA Publication No. PEP23-02-00-005. Published December 2023. Accessed December 11, 2025. https://library.samhsa.gov/sites/default/files/advisory-low-barrier-models-of-care-pep23-02-00-005.pdf
- Substance Abuse and Mental Health Services Administration: Harm Reduction Framework. Center for Substance Abuse Prevention, Substance Abuse and Mental Health Services Administration, 2023.
Building Trust: Enhancing Rural Women Veterans’ Healthcare Experiences Through Need-Supportive Patient-Centered Communication
Background
Rural women veterans often confront unique healthcare barriers—geographic isolation, gender-related stigma, and limited provider cultural sensitivity that undermine trust and engagement. In response, we co-designed an interprofessional communication curriculum to promote relational, patient-centered care grounded in psychological need support.
Innovation
Anchored in Self Determination Theory (SDT), this curriculum equips nurses and social workers with need-supportive communication strategies that nurture autonomy, competence, and relatedness, integrating two transformative learning methods for enhancing respectful and inclusive listening:
- Cultural humility reflections for veteran-centered care—personal narratives, storytelling, and power-awareness discussions to build lifelong reflective practices.
- Medical improv simulations—adaptive improvisational role plays for healthcare environments fostering presence, adaptability, empathy, trust-building, and real-time responsiveness.
Delivered via a multiday health professions learning lab, the training combines asynchronous workshops with in-person facilitated interactions. Core modules cover SDT foundations, need supportive dialogue, veteran-centered cultural humility, and shared decision-making practices that uplift rural women veterans’ voices. Using Kirkpatrick’s Four Level Model, we assess impact at multiple tiers:
- Reaction: Participant satisfaction and perceived training relevance.
- Learning: Pre/post assessments track SDT knowledge and communication skills gains.
- Behavior: Observe simulations and self-reported changes in communication practices.
- Results: Qualitative satisfaction metrics and care engagement trends among rural women veterans.
Results
A pilot cohort (N = 20) across two rural sites is pending implementation. pre/post surveys will assess any improved confidence in applying need supportive communication and the most effective component in building empathetic presence. Feedback measures will also indicate the significance of combined uses of medical improv and cultural humility on deepened relational capacity and trust.
Discussion
This program operationalizes SDT within healthcare communications, integrating cultural humility and improvisation learning modalities to enhance care quality for rural women veterans, ultimately strengthening provider-patient connections. Using health professions learning lab environments can foster sustained behavioral impacts. Future iterations will expand to additional rural VA sites, co-designing with the voices of women veterans through focus groups.
Background
Rural women veterans often confront unique healthcare barriers—geographic isolation, gender-related stigma, and limited provider cultural sensitivity that undermine trust and engagement. In response, we co-designed an interprofessional communication curriculum to promote relational, patient-centered care grounded in psychological need support.
Innovation
Anchored in Self Determination Theory (SDT), this curriculum equips nurses and social workers with need-supportive communication strategies that nurture autonomy, competence, and relatedness, integrating two transformative learning methods for enhancing respectful and inclusive listening:
- Cultural humility reflections for veteran-centered care—personal narratives, storytelling, and power-awareness discussions to build lifelong reflective practices.
- Medical improv simulations—adaptive improvisational role plays for healthcare environments fostering presence, adaptability, empathy, trust-building, and real-time responsiveness.
Delivered via a multiday health professions learning lab, the training combines asynchronous workshops with in-person facilitated interactions. Core modules cover SDT foundations, need supportive dialogue, veteran-centered cultural humility, and shared decision-making practices that uplift rural women veterans’ voices. Using Kirkpatrick’s Four Level Model, we assess impact at multiple tiers:
- Reaction: Participant satisfaction and perceived training relevance.
- Learning: Pre/post assessments track SDT knowledge and communication skills gains.
- Behavior: Observe simulations and self-reported changes in communication practices.
- Results: Qualitative satisfaction metrics and care engagement trends among rural women veterans.
Results
A pilot cohort (N = 20) across two rural sites is pending implementation. pre/post surveys will assess any improved confidence in applying need supportive communication and the most effective component in building empathetic presence. Feedback measures will also indicate the significance of combined uses of medical improv and cultural humility on deepened relational capacity and trust.
Discussion
This program operationalizes SDT within healthcare communications, integrating cultural humility and improvisation learning modalities to enhance care quality for rural women veterans, ultimately strengthening provider-patient connections. Using health professions learning lab environments can foster sustained behavioral impacts. Future iterations will expand to additional rural VA sites, co-designing with the voices of women veterans through focus groups.
Background
Rural women veterans often confront unique healthcare barriers—geographic isolation, gender-related stigma, and limited provider cultural sensitivity that undermine trust and engagement. In response, we co-designed an interprofessional communication curriculum to promote relational, patient-centered care grounded in psychological need support.
Innovation
Anchored in Self Determination Theory (SDT), this curriculum equips nurses and social workers with need-supportive communication strategies that nurture autonomy, competence, and relatedness, integrating two transformative learning methods for enhancing respectful and inclusive listening:
- Cultural humility reflections for veteran-centered care—personal narratives, storytelling, and power-awareness discussions to build lifelong reflective practices.
- Medical improv simulations—adaptive improvisational role plays for healthcare environments fostering presence, adaptability, empathy, trust-building, and real-time responsiveness.
Delivered via a multiday health professions learning lab, the training combines asynchronous workshops with in-person facilitated interactions. Core modules cover SDT foundations, need supportive dialogue, veteran-centered cultural humility, and shared decision-making practices that uplift rural women veterans’ voices. Using Kirkpatrick’s Four Level Model, we assess impact at multiple tiers:
- Reaction: Participant satisfaction and perceived training relevance.
- Learning: Pre/post assessments track SDT knowledge and communication skills gains.
- Behavior: Observe simulations and self-reported changes in communication practices.
- Results: Qualitative satisfaction metrics and care engagement trends among rural women veterans.
Results
A pilot cohort (N = 20) across two rural sites is pending implementation. pre/post surveys will assess any improved confidence in applying need supportive communication and the most effective component in building empathetic presence. Feedback measures will also indicate the significance of combined uses of medical improv and cultural humility on deepened relational capacity and trust.
Discussion
This program operationalizes SDT within healthcare communications, integrating cultural humility and improvisation learning modalities to enhance care quality for rural women veterans, ultimately strengthening provider-patient connections. Using health professions learning lab environments can foster sustained behavioral impacts. Future iterations will expand to additional rural VA sites, co-designing with the voices of women veterans through focus groups.
Tai Chi Modification and Supplemental Movements Quality Improvement Program
Background
The original program consisted of 12 movements that were to be split up between 3 weeks teaching 4 movements each week. Range of mobility was the main consideration for developing this HPE quality improvement project. Veterans who wanted to participate in Tai Chi were not able to engage in the activity due to the range of movement traditional Tai Chi required.
Innovation
The HPE Quality Improvement program developed a 15-movement warm-up, 12 co-ordinational movements consistent with the original program, 18 supplemental Tai Chi movements that were not included in the original program all of which focus on movements remaining below the shoulders and can be done standing or sitting. Four advanced exercises including “hip over heel” were included to target participants balance if able and to improve their hip strength, knee tendon/ligament strength. Tai Chi loses its potential to increase balance when performed in a sitting position.1 The movements drew upon Fu style Tai Chi and the program developer was given permission from Tommy Kirchoff to use his DVD Healing Exercises. The HPE program consisted of four 30–60-minute weekly sessions of learning the movements with another 4 weekly sessions of demonstrating the movements. Instructors were given written and visual documents to learn from and were evaluated by the developer during the last 4 weeks.
.
Results
Qualitative Data: Instructors notice a difference in how they feel, and appreciate having another option to offer veterans with mobility/standing issues. Patients expressed improvement in mobility relating to bending, arm extension, arm raising, muscle strengthening, hip strengthening and rotation.
Discussion
Future research will want to look at taking measurements before and after patient implementation to determine quantitative data related to balance, strength and range of movement including grip strength, stand up and go, and one-legged stands.
- Skelton DA, Mavroeidi A. How do muscle and bone strengthening and balance activities (MBSBA) vary across the life course, and are there particular ages where MBSBA are most important?. J Frailty Sarcopenia Falls. 2018;3(2):74-84. Published 2018 Jun 1. doi:10.22540/JFSF-03-074
Background
The original program consisted of 12 movements that were to be split up between 3 weeks teaching 4 movements each week. Range of mobility was the main consideration for developing this HPE quality improvement project. Veterans who wanted to participate in Tai Chi were not able to engage in the activity due to the range of movement traditional Tai Chi required.
Innovation
The HPE Quality Improvement program developed a 15-movement warm-up, 12 co-ordinational movements consistent with the original program, 18 supplemental Tai Chi movements that were not included in the original program all of which focus on movements remaining below the shoulders and can be done standing or sitting. Four advanced exercises including “hip over heel” were included to target participants balance if able and to improve their hip strength, knee tendon/ligament strength. Tai Chi loses its potential to increase balance when performed in a sitting position.1 The movements drew upon Fu style Tai Chi and the program developer was given permission from Tommy Kirchoff to use his DVD Healing Exercises. The HPE program consisted of four 30–60-minute weekly sessions of learning the movements with another 4 weekly sessions of demonstrating the movements. Instructors were given written and visual documents to learn from and were evaluated by the developer during the last 4 weeks.
.
Results
Qualitative Data: Instructors notice a difference in how they feel, and appreciate having another option to offer veterans with mobility/standing issues. Patients expressed improvement in mobility relating to bending, arm extension, arm raising, muscle strengthening, hip strengthening and rotation.
Discussion
Future research will want to look at taking measurements before and after patient implementation to determine quantitative data related to balance, strength and range of movement including grip strength, stand up and go, and one-legged stands.
Background
The original program consisted of 12 movements that were to be split up between 3 weeks teaching 4 movements each week. Range of mobility was the main consideration for developing this HPE quality improvement project. Veterans who wanted to participate in Tai Chi were not able to engage in the activity due to the range of movement traditional Tai Chi required.
Innovation
The HPE Quality Improvement program developed a 15-movement warm-up, 12 co-ordinational movements consistent with the original program, 18 supplemental Tai Chi movements that were not included in the original program all of which focus on movements remaining below the shoulders and can be done standing or sitting. Four advanced exercises including “hip over heel” were included to target participants balance if able and to improve their hip strength, knee tendon/ligament strength. Tai Chi loses its potential to increase balance when performed in a sitting position.1 The movements drew upon Fu style Tai Chi and the program developer was given permission from Tommy Kirchoff to use his DVD Healing Exercises. The HPE program consisted of four 30–60-minute weekly sessions of learning the movements with another 4 weekly sessions of demonstrating the movements. Instructors were given written and visual documents to learn from and were evaluated by the developer during the last 4 weeks.
.
Results
Qualitative Data: Instructors notice a difference in how they feel, and appreciate having another option to offer veterans with mobility/standing issues. Patients expressed improvement in mobility relating to bending, arm extension, arm raising, muscle strengthening, hip strengthening and rotation.
Discussion
Future research will want to look at taking measurements before and after patient implementation to determine quantitative data related to balance, strength and range of movement including grip strength, stand up and go, and one-legged stands.
- Skelton DA, Mavroeidi A. How do muscle and bone strengthening and balance activities (MBSBA) vary across the life course, and are there particular ages where MBSBA are most important?. J Frailty Sarcopenia Falls. 2018;3(2):74-84. Published 2018 Jun 1. doi:10.22540/JFSF-03-074
- Skelton DA, Mavroeidi A. How do muscle and bone strengthening and balance activities (MBSBA) vary across the life course, and are there particular ages where MBSBA are most important?. J Frailty Sarcopenia Falls. 2018;3(2):74-84. Published 2018 Jun 1. doi:10.22540/JFSF-03-074
Improving Life-Sustaining Treatment Discussions and Order Quality in a Primary Care Clinic
Background
Veterans Health Administration Directive 1004.03(1) (Advance Care Planning) aims to establish a “system-wide, patient-centered and evidence-based approach to Advance Care Planning.”1 Life-sustaining treatment (LST) orders are documents of patient preference regarding interventions such as mechanical ventilation, CPR, dialysis, artificial nutrition and hydration; and are considered part of an Advance Care Plan. From a bioethics perspective, these orders promote patient autonomy by formalizing patient preferences around LSTs in the medical record, particularly for when a patient lacks capacity and/or cannot make decisions on their own.2 Through consensus building, our team defined vague, inactionable, or incorrectly written LST orders as Potentially Problematic Orders (PPO). PPOs which cause confusion at the bedside or lack clarity around preferences can pose serious risks to patient safety and autonomy by exposing patients to inappropriate initiation or withholding of LSTs. Improving the quality of LST orders and reducing the number of PPOs is a crucial element for safe and effective implementation of Directive 1004.03(1).
Aim
The aim of this quality improvement project was to reduce the number of PPOs in a VA Community-Based Outpatient Clinic (CBOC) by 75% by the end of 2025.
Methods
The Model for Improvement was used for this quality improvement project.3 One year of LST orders were audited and thematic analysis identified 7 subtypes of PPO. Some PPO subtypes included clerical errors, potentially mismatched order sets (e.g., Comfort Care order with no associated DNR order) ill-defined or vague orders, and clinically impractical orders (eg, “consents to one shock during CPR”). We defined vague, ill-defined, and impractical orders as the most ethically and clinically challenging given the possibility of confusion or error at the bedside. Initial data were collected from October 2022 to October 2023, and post-intervention data were collected from February 2024 to September 2024. Interventions included process changes (clarifying role responsibility, documentation practices, patient education), regular auditing and feedback from a supervisor, and staff education.
Results
Post-intervention analysis demonstrated that the proportion of PPO remained the same, with 25% of patient charts containing at least one PPO. However, the distribution of PPO in the most ethically and clinically problematic categories (vague, ill-defined, and impractical orders) decreased from 14.7% to <1%.
Conclusions
We successfully reduced the most ethically and clinically challenging PPOs to <1% in our initial intervention. To reduce the overall proportion of PPO, we plan enhancements in process automations, additional physical educational resources, and minor changes in audit criteria. Future projects will aim to address the remaining PPO error types and prepare this project for implementation in other CBOCs.
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1004.03(1): Advance care planning. Published December 12, 2023. Accessed December 11, 2025. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11610
- White DB, Curtis JR, Lo B, Luce JM. Decisions to limit life-sustaining treatment for critically ill patients who lack both decision-making capacity and surrogate decision-makers. Crit Care Med. 2006;34(8):2053-2059. doi:10.1097/01.CCM.0000227654.38708.C1
- Ogrinc GS, Headrick LA, Barton AJ, Dolansky MA, Madigosky WS, Miltner RS, Hall AG. Fundamentals of Health Care Improvement: A Guide to Improving Your Patients’ Care (4th edition). Joint Commission Resources and Institute for Healthcare Improvement; 2022.
Background
Veterans Health Administration Directive 1004.03(1) (Advance Care Planning) aims to establish a “system-wide, patient-centered and evidence-based approach to Advance Care Planning.”1 Life-sustaining treatment (LST) orders are documents of patient preference regarding interventions such as mechanical ventilation, CPR, dialysis, artificial nutrition and hydration; and are considered part of an Advance Care Plan. From a bioethics perspective, these orders promote patient autonomy by formalizing patient preferences around LSTs in the medical record, particularly for when a patient lacks capacity and/or cannot make decisions on their own.2 Through consensus building, our team defined vague, inactionable, or incorrectly written LST orders as Potentially Problematic Orders (PPO). PPOs which cause confusion at the bedside or lack clarity around preferences can pose serious risks to patient safety and autonomy by exposing patients to inappropriate initiation or withholding of LSTs. Improving the quality of LST orders and reducing the number of PPOs is a crucial element for safe and effective implementation of Directive 1004.03(1).
Aim
The aim of this quality improvement project was to reduce the number of PPOs in a VA Community-Based Outpatient Clinic (CBOC) by 75% by the end of 2025.
Methods
The Model for Improvement was used for this quality improvement project.3 One year of LST orders were audited and thematic analysis identified 7 subtypes of PPO. Some PPO subtypes included clerical errors, potentially mismatched order sets (e.g., Comfort Care order with no associated DNR order) ill-defined or vague orders, and clinically impractical orders (eg, “consents to one shock during CPR”). We defined vague, ill-defined, and impractical orders as the most ethically and clinically challenging given the possibility of confusion or error at the bedside. Initial data were collected from October 2022 to October 2023, and post-intervention data were collected from February 2024 to September 2024. Interventions included process changes (clarifying role responsibility, documentation practices, patient education), regular auditing and feedback from a supervisor, and staff education.
Results
Post-intervention analysis demonstrated that the proportion of PPO remained the same, with 25% of patient charts containing at least one PPO. However, the distribution of PPO in the most ethically and clinically problematic categories (vague, ill-defined, and impractical orders) decreased from 14.7% to <1%.
Conclusions
We successfully reduced the most ethically and clinically challenging PPOs to <1% in our initial intervention. To reduce the overall proportion of PPO, we plan enhancements in process automations, additional physical educational resources, and minor changes in audit criteria. Future projects will aim to address the remaining PPO error types and prepare this project for implementation in other CBOCs.
Background
Veterans Health Administration Directive 1004.03(1) (Advance Care Planning) aims to establish a “system-wide, patient-centered and evidence-based approach to Advance Care Planning.”1 Life-sustaining treatment (LST) orders are documents of patient preference regarding interventions such as mechanical ventilation, CPR, dialysis, artificial nutrition and hydration; and are considered part of an Advance Care Plan. From a bioethics perspective, these orders promote patient autonomy by formalizing patient preferences around LSTs in the medical record, particularly for when a patient lacks capacity and/or cannot make decisions on their own.2 Through consensus building, our team defined vague, inactionable, or incorrectly written LST orders as Potentially Problematic Orders (PPO). PPOs which cause confusion at the bedside or lack clarity around preferences can pose serious risks to patient safety and autonomy by exposing patients to inappropriate initiation or withholding of LSTs. Improving the quality of LST orders and reducing the number of PPOs is a crucial element for safe and effective implementation of Directive 1004.03(1).
Aim
The aim of this quality improvement project was to reduce the number of PPOs in a VA Community-Based Outpatient Clinic (CBOC) by 75% by the end of 2025.
Methods
The Model for Improvement was used for this quality improvement project.3 One year of LST orders were audited and thematic analysis identified 7 subtypes of PPO. Some PPO subtypes included clerical errors, potentially mismatched order sets (e.g., Comfort Care order with no associated DNR order) ill-defined or vague orders, and clinically impractical orders (eg, “consents to one shock during CPR”). We defined vague, ill-defined, and impractical orders as the most ethically and clinically challenging given the possibility of confusion or error at the bedside. Initial data were collected from October 2022 to October 2023, and post-intervention data were collected from February 2024 to September 2024. Interventions included process changes (clarifying role responsibility, documentation practices, patient education), regular auditing and feedback from a supervisor, and staff education.
Results
Post-intervention analysis demonstrated that the proportion of PPO remained the same, with 25% of patient charts containing at least one PPO. However, the distribution of PPO in the most ethically and clinically problematic categories (vague, ill-defined, and impractical orders) decreased from 14.7% to <1%.
Conclusions
We successfully reduced the most ethically and clinically challenging PPOs to <1% in our initial intervention. To reduce the overall proportion of PPO, we plan enhancements in process automations, additional physical educational resources, and minor changes in audit criteria. Future projects will aim to address the remaining PPO error types and prepare this project for implementation in other CBOCs.
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1004.03(1): Advance care planning. Published December 12, 2023. Accessed December 11, 2025. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11610
- White DB, Curtis JR, Lo B, Luce JM. Decisions to limit life-sustaining treatment for critically ill patients who lack both decision-making capacity and surrogate decision-makers. Crit Care Med. 2006;34(8):2053-2059. doi:10.1097/01.CCM.0000227654.38708.C1
- Ogrinc GS, Headrick LA, Barton AJ, Dolansky MA, Madigosky WS, Miltner RS, Hall AG. Fundamentals of Health Care Improvement: A Guide to Improving Your Patients’ Care (4th edition). Joint Commission Resources and Institute for Healthcare Improvement; 2022.
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1004.03(1): Advance care planning. Published December 12, 2023. Accessed December 11, 2025. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11610
- White DB, Curtis JR, Lo B, Luce JM. Decisions to limit life-sustaining treatment for critically ill patients who lack both decision-making capacity and surrogate decision-makers. Crit Care Med. 2006;34(8):2053-2059. doi:10.1097/01.CCM.0000227654.38708.C1
- Ogrinc GS, Headrick LA, Barton AJ, Dolansky MA, Madigosky WS, Miltner RS, Hall AG. Fundamentals of Health Care Improvement: A Guide to Improving Your Patients’ Care (4th edition). Joint Commission Resources and Institute for Healthcare Improvement; 2022.
A Health Educator’s Primer to Cost-Effectiveness in Health Professions Education
Background
Cost-effectiveness (CE) evaluations, for existing and anticipated programs, are common in healthcare, but are rarely used in health professions education (HPE). A systematic review of HPE literature found not only few examples of CE evaluations, but also unclear and inconsistent methodology.1 One proposed reason HPE has been slow to adopt CE evaluations is uncertainty over terminology and how to adapt this methodology to HPE.2 CE evaluations present further challenges for HPE since educational outcomes are often not easily monetized. However, given the reality of constrained budgets and limited resources, CE evaluations can be a powerful tool for educators to strengthen arguments for proposed innovations, and for scholars seeking to conduct rigorous work that sustains critical review.
Innovation
This project aims to make CE evaluations more understandable to HPE educators, using a one-page infographic and glossary. This will provide a primer, operationalizing the steps involved in CE evaluations and addressing why and when CE evaluations might be considered in HPE. To improve comprehension, this is being developed collaboratively with health professions educators and an economist. This infographic will be submitted for publication, as a resource to facilitate educators’ scholarly work and conversations with fiscal administrators.
Results
The infographic includes 1) an overview of CE evaluations, 2) information about inputs required for CE evaluations, 3) guidance on interpreting results, 4) a glossary of key terminology, and 5) considerations for why educators might consider this type of analysis. A final draft will be pilot tested with a focus group to assess interdisciplinary accessibility.
Discussion
Discussions between health professions educators and an economist on this infographic uncovered concepts that were poorly understood or defined differently across disciplines, determining specific knowledge gaps and misunderstandings. For example, facilitating conversation between educators and economists highlighted key terms that were a source of misunderstanding. These were then added to the glossary, creating a shared vocabulary. This also helped clarify the steps and information necessary for conducting CE evaluations in HPE, particularly the issue of perspective choice for the analysis (educator, patient, learner, etc.). Overall, this collaboration aimed at making CE evaluations more approachable and understandable for HPE professionals through this infographic.
- Foo J, Cook DA, Walsh K, et al. Cost evaluations in health professions education: a systematic review of methods and reporting quality. Med Educ. 2019;53(12):1196-1208. doi:10.1111/medu.13936
- Maloney S, Reeves S, Rivers G, Ilic D, Foo J, Walsh K. The Prato Statement on cost and value in professional and interprofessional education. J Interprof Care. 2017;31(1):1-4. doi:10.1080/13561820.2016.1257255
Background
Cost-effectiveness (CE) evaluations, for existing and anticipated programs, are common in healthcare, but are rarely used in health professions education (HPE). A systematic review of HPE literature found not only few examples of CE evaluations, but also unclear and inconsistent methodology.1 One proposed reason HPE has been slow to adopt CE evaluations is uncertainty over terminology and how to adapt this methodology to HPE.2 CE evaluations present further challenges for HPE since educational outcomes are often not easily monetized. However, given the reality of constrained budgets and limited resources, CE evaluations can be a powerful tool for educators to strengthen arguments for proposed innovations, and for scholars seeking to conduct rigorous work that sustains critical review.
Innovation
This project aims to make CE evaluations more understandable to HPE educators, using a one-page infographic and glossary. This will provide a primer, operationalizing the steps involved in CE evaluations and addressing why and when CE evaluations might be considered in HPE. To improve comprehension, this is being developed collaboratively with health professions educators and an economist. This infographic will be submitted for publication, as a resource to facilitate educators’ scholarly work and conversations with fiscal administrators.
Results
The infographic includes 1) an overview of CE evaluations, 2) information about inputs required for CE evaluations, 3) guidance on interpreting results, 4) a glossary of key terminology, and 5) considerations for why educators might consider this type of analysis. A final draft will be pilot tested with a focus group to assess interdisciplinary accessibility.
Discussion
Discussions between health professions educators and an economist on this infographic uncovered concepts that were poorly understood or defined differently across disciplines, determining specific knowledge gaps and misunderstandings. For example, facilitating conversation between educators and economists highlighted key terms that were a source of misunderstanding. These were then added to the glossary, creating a shared vocabulary. This also helped clarify the steps and information necessary for conducting CE evaluations in HPE, particularly the issue of perspective choice for the analysis (educator, patient, learner, etc.). Overall, this collaboration aimed at making CE evaluations more approachable and understandable for HPE professionals through this infographic.
Background
Cost-effectiveness (CE) evaluations, for existing and anticipated programs, are common in healthcare, but are rarely used in health professions education (HPE). A systematic review of HPE literature found not only few examples of CE evaluations, but also unclear and inconsistent methodology.1 One proposed reason HPE has been slow to adopt CE evaluations is uncertainty over terminology and how to adapt this methodology to HPE.2 CE evaluations present further challenges for HPE since educational outcomes are often not easily monetized. However, given the reality of constrained budgets and limited resources, CE evaluations can be a powerful tool for educators to strengthen arguments for proposed innovations, and for scholars seeking to conduct rigorous work that sustains critical review.
Innovation
This project aims to make CE evaluations more understandable to HPE educators, using a one-page infographic and glossary. This will provide a primer, operationalizing the steps involved in CE evaluations and addressing why and when CE evaluations might be considered in HPE. To improve comprehension, this is being developed collaboratively with health professions educators and an economist. This infographic will be submitted for publication, as a resource to facilitate educators’ scholarly work and conversations with fiscal administrators.
Results
The infographic includes 1) an overview of CE evaluations, 2) information about inputs required for CE evaluations, 3) guidance on interpreting results, 4) a glossary of key terminology, and 5) considerations for why educators might consider this type of analysis. A final draft will be pilot tested with a focus group to assess interdisciplinary accessibility.
Discussion
Discussions between health professions educators and an economist on this infographic uncovered concepts that were poorly understood or defined differently across disciplines, determining specific knowledge gaps and misunderstandings. For example, facilitating conversation between educators and economists highlighted key terms that were a source of misunderstanding. These were then added to the glossary, creating a shared vocabulary. This also helped clarify the steps and information necessary for conducting CE evaluations in HPE, particularly the issue of perspective choice for the analysis (educator, patient, learner, etc.). Overall, this collaboration aimed at making CE evaluations more approachable and understandable for HPE professionals through this infographic.
- Foo J, Cook DA, Walsh K, et al. Cost evaluations in health professions education: a systematic review of methods and reporting quality. Med Educ. 2019;53(12):1196-1208. doi:10.1111/medu.13936
- Maloney S, Reeves S, Rivers G, Ilic D, Foo J, Walsh K. The Prato Statement on cost and value in professional and interprofessional education. J Interprof Care. 2017;31(1):1-4. doi:10.1080/13561820.2016.1257255
- Foo J, Cook DA, Walsh K, et al. Cost evaluations in health professions education: a systematic review of methods and reporting quality. Med Educ. 2019;53(12):1196-1208. doi:10.1111/medu.13936
- Maloney S, Reeves S, Rivers G, Ilic D, Foo J, Walsh K. The Prato Statement on cost and value in professional and interprofessional education. J Interprof Care. 2017;31(1):1-4. doi:10.1080/13561820.2016.1257255