User login
Do Clinicians Understand Quality Metric Data?
Central line-associated bloodstream infections (CLABSIs) are common and serious occurrences across healthcare systems, with an attributable mortality of 12% to 25%.1,2 Given this burden,3–5 CLABSI is a focus for both high-profile public reporting and quality improvement interventions. An integral component of such interventions is audit and feedback via quality metrics. These measures are intended to allow decision makers to assess their own performance and appropriately allocate resources. Quality metrics present a substantial cost to health systems, with an estimated $15.4 billion dollars spent annually simply for reporting.6 Despite this toll, “audit and feedback” interventions have proven to be variably successful.7–9 The mechanisms that limit the effectiveness of these interventions remain
poorly understood.
One plausible explanation for limited efficacy of quality metrics is inadequate clinician numeracy—that is, “the ability to understand the quantitative aspects of clinical medicine, original research, quality improvement, and financial matters.”10 Indeed, clinicians are not consistently able to interpret probabilities and or clinical test characteristics. For example, Wegwarth et al. identified shortcomings in physician application of lead-time bias toward cancer screening.11 Additionally, studies have demonstrated systematic misinterpretations of probabilistic information in clinical settings, along with misconceptions regarding the impact of prevalence on post-test probabilities.12,13 Effective interpretation of rates may be a key—if unstated—requirement of many CLABSI quality improvement efforts.14–19 Our broader hypothesis is that clinicians who can more accurately interpret quality data, even if only from their own institution, are more likely to act on it appropriately and persistently than those who feel they must depend on a preprocessed interpretation of that same data by some other expert.
Therefore, we designed a survey to assess the numeracy of clinicians on CLABSI data presented in a prototypical feedback report. We studied 3 domains of comprehension: (1) basic numeracy: numerical tasks related to simple data; (2) risk-adjustment numeracy: numerical tasks related to risk-adjusted data; and (3) risk-adjustment interpretation: inferential tasks concerning risk-adjusted data. We hypothesized that clinician performance would vary substantially across domains, with the poorest performance in risk-
adjusted data.
METHODS
We conducted a cross-sectional survey of clinician numeracy regarding CLABSI feedback data. Respondents were also asked to provide demographic information and opinions regarding the reliability of quality metric data. Survey recruitment occurred on Twitter, a novel approach that leveraged social media to facilitate rapid recruitment of participants. The study instrument was administered using a web survey with randomized question order to preclude any possibility of order effects between questions. The study was deemed Institutional Review Board exempt by the University of Michigan: protocol HUM00106696.
Data Presentation Method
To determine the optimal mode of presenting data, we reviewed the literature on quality metric numeracy and presentation methods. Additionally, we evaluated quality metric presentation methods used by the Centers for Disease Control and Prevention (CDC), Centers for Medicare & Medicaid Services (CMS), and a tertiary academic medical center. After assessing the available literature and options, we adapted a CLABSI data presentation array from a study that had qualitatively validated the format using physician feedback (Appendix).20 We used hypothetical CLABSI data for our survey.
Survey Development
We developed a survey that included an 11-item test regarding CLABSI numeracy and data interpretation. Additional questions related to quality metric reliability and demographic information were included. No preexisting assessment tools existed for our areas of interest. Therefore, we developed a novel instrument using a broad, exploratory approach as others have employed.21
First, we defined 3 conceptual categories related to CLABSI data. Within this conceptual framework, an iterative process of development and revision was used to assemble a question bank from which the survey would be constructed. A series of think-aloud sessions were held to evaluate each prompt for precision, clarity, and accuracy in assessing the conceptual categories. Correct and incorrect answers were defined based on literature review in conjunction with input from methodological and content experts (TJI and VC) (see Appendix for answer explanations).
Within the conceptual categories related to CLABSI risk-adjustment, a key measure is the standardized infection ratio (SIR). This value is defined as the ratio of observed number of CLABSI over the expected number of CLABSIs.22 This is the primary measure to stratify hospital performance, and it was used in our assessment of risk-adjustment comprehension. In total, 54 question prompts were developed and subsequently narrowed to 11 study questions for the initial survey.
The instrument was then pretested in a cohort of 8 hospitalists and intensivists to ensure appropriate comprehension, retrieval, and judgment processes.23 Questions were revised based on feedback from this cognitive testing to constitute the final instrument. During the survey, the data table was reshown on each page directly above each question and so was always on the same screen for the respondents.
Survey Sample
We innovated by using Twitter as an online platform for recruiting participants; we used Survey Monkey to host the electronic instrument. Two authors (TJI, VC) systematically sent out solicitation tweets to their followers. These tweets clearly indicated that the recruitment was for the purpose of a research study, and participants would receive no financial reward/incentive (Appendix). A link to the survey was provided in each tweet, and the period of recruitment was 30 days. To ensure respondents were clinicians, they needed to first answer a screening question recognizing that central lines were placed in the subclavian site but not the aorta, iliac, or radial sites.
To prevent systematic or anchoring biases, the order of questions was electronically randomized for each respondent. The primary outcome was the percentage correct of attempted questions.
Statistical Analysis
Descriptive statistics were calculated for all demographic variables. The primary outcome was evaluated as a dichotomous variable for each question (correct vs. incorrect response), and as a continuous variable when assessing mean percent correct on the overall survey. Demographic and conceptual associations were assessed via t-tests, chi-square, or Fisher exact tests. Point biserial correlations were calculated to assess for associations between response to a single question and overall performance on the survey.
To evaluate the association between various respondent characteristics and responses, logistic regression analyses were performed. An ANOVA was performed to assess the association between self-reported reliability of quality metric data and the overall performance on attempted items. Analyses were conducted using STATA MP 14.0 (College Station, TX); P <0.05 was considered statistically significant.
RESULTS
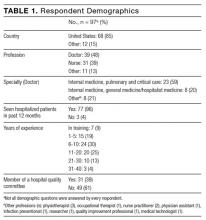
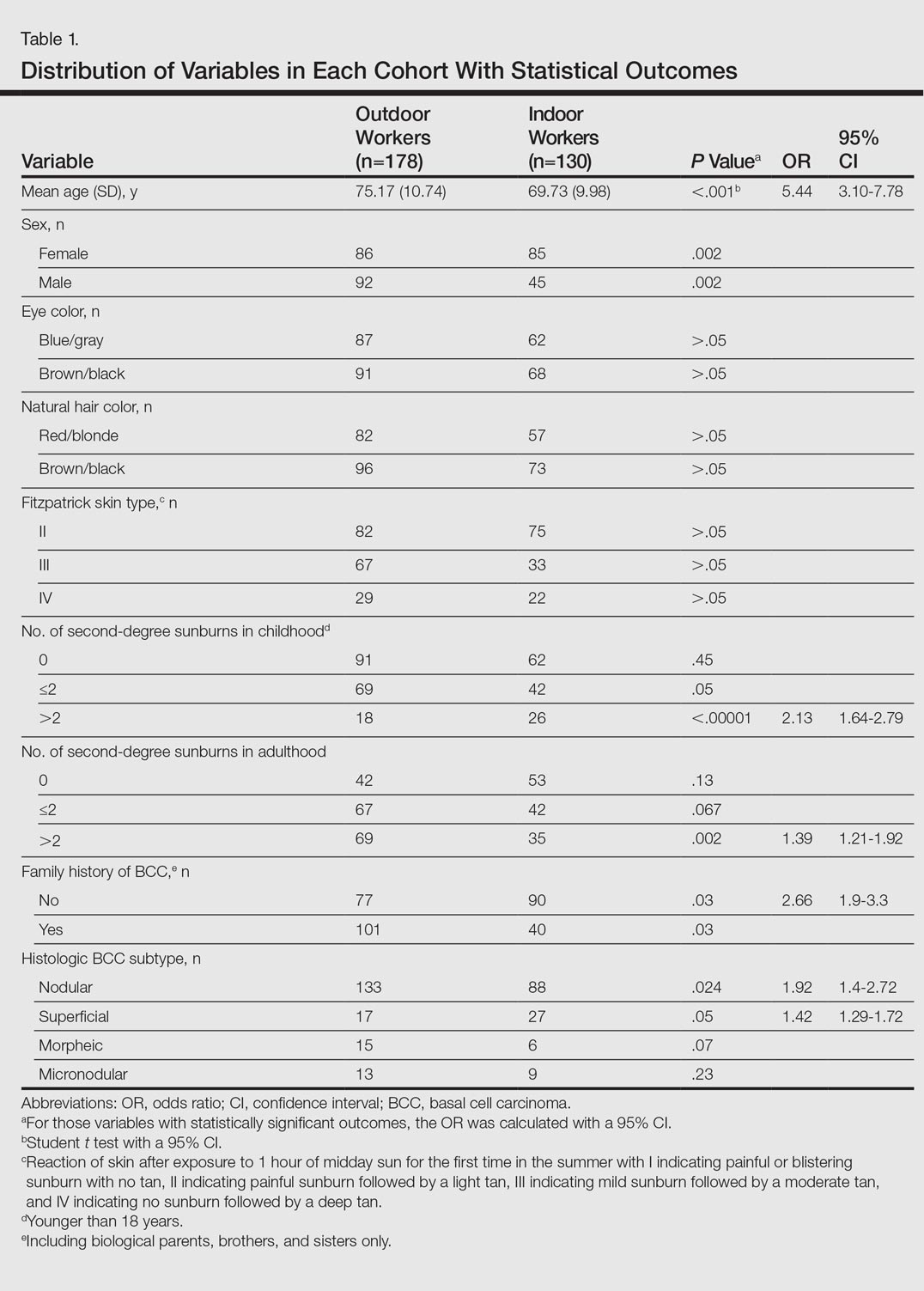
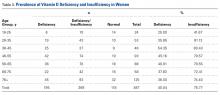
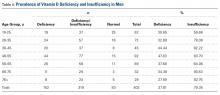
A total of 97 respondents attempted at least 1 question on the survey, and 72 respondents attempted all 11 questions, yielding 939 unique responses for analysis. Seventy respondents (87%) identified as doctors or nurses, and 44 (55%) reported having 6 to 20 years of experience; the survey cohort also came from 6 nations (Table 1). All respondents answered the CLABSI knowledge filter question correctly.
Primary Outcome
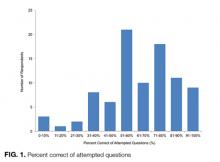
The mean percent correct of attempted questions was 61% (standard deviation 21%, interquartile range 50%-75%) (Figure 1). Of those who answered all 11 CLABSI questions, the mean percent correct was 63% (95% CI, 59%-67%). Some questions were answered correctly more often than others—ranging from 17% to 95% (Table 2). Doctors answered 68% of questions correctly (95% CI, 63%-73%), while nurses and other respondents answered 57% of questions correctly (95% CI, 52%-62%) (P = 0.003). Other demographic variables—including self-reported involvement in a quality improvement committee and being from the United States versus elsewhere—were not associated with survey performance. The point biserial correlations for each individual question with overall performance were all more than 0.2 (range 0.24–0.62) and all statistically significant at P < 0.05.
Concept-Specific Performance
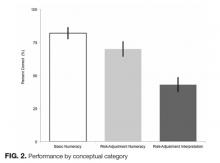
Average percent correct declined across categories as numeracy requirements increased (P < 0.05 for all pairwise comparisons). In the area of basic numeracy, respondents’ mean percent correct was 82% (95% CI, 77%-87%) of attempted. This category had 4 questions, with a performance range of 77% to 90%. For example, on the question, “Which hospital has the lowest CLABSI rate?”, 80% of respondents answered correctly. For risk-adjustment numeracy, the mean percent correct was 70% (95% CI, 64%-76%); 2 items assessed this category. For “Which is better: a higher or lower SIR?”, 95% of the cohort answered correctly. However, on “If hospital B had its number of projected infection halved, what is its SIR?”, only 46% of those who attempted the question answered correctly.
Questions featuring risk-adjustment interpretation had an average percent correct of 43% (95% CI, 37%-49%). Five questions made up this category, with a percent correct range of 17% to 75%. For example, on the question, “Which hospital’s patients are the most predisposed to developing CLABSI?”, only 32% of respondents answered this correctly. In contrast, for the question “Which hospital is most effective at preventing CLABSI?”, 51% answered correctly. Figure 2 illustrates the cohort’s performance on each conceptual category while Table 2 displays question-by-question results.
Opinions Regarding CLABSI Data Reliability
Respondents were also asked about their opinion regarding the reliability of CLABSI quality metric data. Forty-three percent of respondents stated that such data were reliable at best 50% of the time. Notably, 10% of respondents indicated that CLABSI quality metric data were rarely or never reliable. There was no association between perceived reliability of quality metric data and survey performance (P = 0.87).
DISCUSSION
This Twitter-based study found wide variation in clinician interpretation of CLABSI quality data, with low overall performance. In particular, comprehension and interpretation of risk-adjusted data were substantially worse than unadjusted data. Although doctors performed somewhat better than nurses and other respondents, those involved in quality improvement initiatives performed no better than respondents who were not. Collectively, these findings suggest clinicians may not reliably comprehend quality metric data, potentially affecting their ability to utilize audit and feedback data. These results may have important implications for policy efforts that seek to leverage quality metric data to improve patient safety.
An integral component of many contemporary quality improvement initiatives is audit and feedback through metrics.6 Unfortunately, formal audit and feedback, along with other similar methods that benchmark data, have not consistently improved outcomes.24–27 A recent meta-analysis noted that audit and feedback interventions are not becoming more efficacious over time; the study further asserted that “new trials have provided little new knowledge regarding key effect modifiers.”9 Our findings suggest that numeracy and comprehension of quality metrics may be important candidate effect modifiers not previously considered. Simply put: we hypothesize that without intrinsic comprehension of data, impetus or insight to change practice might be diminished. In other words, clinicians may be more apt to act on insights they themselves derive from the data than when they are simply told what the data “mean.”
The present study further demonstrates that clinicians do not understand risk-adjusted data as well as raw data. Risk-adjustment has long been recognized as necessary to compare outcomes among hospitals.28,29 However, risk-adjustment is complex and, by its nature, difficult to understand. Although efforts have focused on improving the statistical reliability of quality metrics, this may represent but one half of the equation. Numeracy and interpretation of the data by decision makers are potentially equally important to effecting change. Because clinicians seem to have difficulty understanding risk-adjusted data, this deficit may be of growing importance as our risk-adjustment techniques become more sophisticated.
We note that clinicians expressed concerns regarding the reliability of quality metric feedback. These findings corroborate recent research that has reported reservations from hospital leaders concerning quality data.30,31 However, as shown in the context of patients and healthcare decisions, the aversion associated with quality metrics may be related to incomplete understanding of the data.32 Whether perceptions of unreliability drive lack of understanding or, conversely, whether lack of understanding fuels perceived unreliability is an important question that requires further study.
This study has several strengths. First, we used rigorous survey development techniques to evaluate the understudied issue of quality metric numeracy. Second, our sample size was sufficient to show statistically significant differences in numeracy and comprehension of CLABSI quality metric data. Third, we leveraged social media to rapidly acquire this sample. Finally, our results provided new insights that may have important implications in the area of quality metrics.
There were also limitations to our study. First, the Twitter-derived sample precludes the calculation of a response rate and may not be representative of individuals engaged in CLABSI prevention. However, respondents were solicited from the Twitter-followers of 2 health services researchers (TJI, VC) who are actively engaged in scholarly activities pertaining to critically ill patients and hospital-acquired complications. Thus, our sample likely represents a highly motivated subset that engages in these topics on a regular basis—potentially making them more numerate than average clinicians. Second, we did not ask whether the respondents had previously seen CLABSI data specifically, so we cannot stratify by exposure to such data. Third, this study assessed only CLABSI quality metric data; generalizations regarding numeracy with other metrics should be made with caution. However, as many such data are presented in similar formats, we suspect our findings are applicable to similar audit-and-feedback initiatives.
The findings of this study serve as a stimulus for further inquiry. Research of this nature needs to be carried out in samples drawn from specific, policy-relevant populations (eg, infection control practitioners, bedside nurses, intensive care unit directors). Such studies should include longitudinal assessments of numeracy that attempt to mechanistically examine its impact on CLABSI prevention efforts and outcomes. The latter is an important issue as the link between numeracy and behavioral response, while plausible, cannot be assumed, particularly given the complexity of issues related to behavioral modification.33 Additionally, whether alternate presentations of quality data affect numeracy, interpretation, and performance is worthy of further testing; indeed, this has been shown to be the case in other forms of communication.34–37 Until data from larger samples are available, it may be prudent for quality improvement leaders to assess the comprehension of local clinicians regarding feedback and whether lack of adequate comprehension is a barrier to deploying quality improvement interventions.
Quality measurement is a cornerstone of patient safety as it seeks to assess and improve the care delivered at the bedside. Rigorous metric development is important; however, ensuring that decision makers understand complex quality metrics may be equally fundamental. Given the cost of examining quality, elucidating the mechanisms of numeracy and interpretation as decision makers engage with quality metric data is necessary, along with whether improved comprehension leads to behavior change. Such inquiry may provide an evidence-base to shape alterations in quality metric deployment that will ensure maximal efficacy in driving practice change.
Disclosures
This work was supported by VA HSR&D IIR-13-079 (TJI). Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). The views expressed here are the authors’ own and do not necessarily represent the view of the US Government or the Department of Veterans’ Affairs. The authors report no conflicts of interest.
1. Scott RD II. The direct medical costs of healthcare-associated infections in us hospitals and the benefits of prevention. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Published March 2009. Accessed November 8, 2016.
2. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 suppl 1)::S1-S34. PubMed
3. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
4. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. PubMed
5. Siempos II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
6. Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35(3):401-406. PubMed
7. Hysong SJ. Meta-analysis: audit and feedback features impact effectiveness on care quality. Med Care. 2009;47(3):356-363. PubMed
8. Ilgen DR, Fisher CD, Taylor MS. Consequences of individual feedback on behavior in organizations. J Appl Psychol. 1979;64:349-371.
9. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. PubMed
10. Rao G. Physician numeracy: essential skills for practicing evidence-based medicine. Fam Med. 2008;40(5):354-358. PubMed
11. Wegwarth O, Schwartz LM, Woloshin S, Gaissmaier W, Gigerenzer G. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Ann Intern Med. 2012;156(5):340-349. PubMed
12. Bramwell R, West H, Salmon P. Health professionals’ and service users’ interpretation of screening test results: experimental study. BMJ. 2006;333(7562):284. PubMed
13. Agoritsas T, Courvoisier DS, Combescure C, Deom M, Perneger TV. Does prevalence matter to physicians in estimating post-test probability of disease? A randomized trial. J Gen Intern Med. 2011;26(4):373-378. PubMed
14. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
15. Rinke ML, Bundy DG, Chen AR, et al. Central line maintenance bundles and CLABSIs in ambulatory oncology patients. Pediatrics. 2013;132(5):e1403-e1412. PubMed
16. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):
2725-2732. PubMed
17. Rinke ML, Chen AR, Bundy DG, et al. Implementation of a central line maintenance care bundle in hospitalized pediatric oncology patients. Pediatrics. 2012;130(4):e996-e1004. PubMed
18. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
19. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
20. Rajwan YG, Barclay PW, Lee T, Sun IF, Passaretti C, Lehmann H. Visualizing central line-associated blood stream infection (CLABSI) outcome data for decision making by health care consumers and practitioners—an evaluation study. Online J Public Health Inform. 2013;5(2):218. PubMed
21. Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making 2007;27(5):672-680. PubMed
22. HAI progress report FAQ. 2016. Available at: http://www.cdc.gov/hai/surveillance/progress-report/faq.html. Last updated March 2, 2016. Accessed November 8, 2016.
23. Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. PubMed
24. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. PubMed
25. Chatterjee P, Joynt KE. Do cardiology quality measures actually improve patient outcomes? J Am Heart Assoc. 2014;3(1):e000404. PubMed
26. Joynt KE, Blumenthal DM, Orav EJ, Resnic FS, Jha AK. Association of public reporting for percutaneous coronary intervention with utilization and outcomes among Medicare beneficiaries with acute myocardial infarction. JAMA. 2012;308(14):1460-1468. PubMed
27. Ryan AM, Nallamothu BK, Dimick JB. Medicare’s public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585-592. PubMed
28. Thomas JW. Risk adjustment for measuring health care outcomes, 3rd edition. Int J Qual Health Care. 2004;16(2):181-182.
29. Iezzoni LI. Risk Adjustment for Measuring Health Care Outcomes. Ann Arbor, Michigan: Health Administration Press; 1994.
30. Goff SL, Lagu T, Pekow PS, et al. A qualitative analysis of hospital leaders’ opinions about publicly reported measures of health care quality. Jt Comm J Qual Patient Saf. 2015;41(4):169-176. PubMed
31. Lindenauer PK, Lagu T, Ross JS, et al. Attitudes of hospital leaders toward publicly reported measures of health care quality. JAMA Intern Med. 2014;174(12):
1904-1911. PubMed
32. Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk-benefit information. Health Aff (Millwood). 2007;26(3):741-748. PubMed
33. Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Glanz K, Rimer BK, Viswanath K, eds. Health Behavior and Health Education: Theory, Research and Practice. 5th ed. San Francisco, CA: Jossey-Bass; 2015:95–124.
34. Hamstra DA, Johnson SB, Daignault S, et al. The impact of numeracy on verbatim knowledge of the longitudinal risk for prostate cancer recurrence following radiation therapy. Med Decis Making. 2015;35(1):27-36. PubMed
35. Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns. 2008;73(3):448-455. PubMed
36. Zikmund-Fisher BJ, Witteman HO, Dickson M, et al. Blocks, ovals, or people? Icon type affects risk perceptions and recall of pictographs. Med Decis Making. 2014;34(4):443-453. PubMed
37. Korfage IJ, Fuhrel-Forbis A, Ubel PA, et al. Informed choice about breast cancer prevention: randomized controlled trial of an online decision aid intervention. Breast Cancer Res. 2013;15(5):R74. PubMed
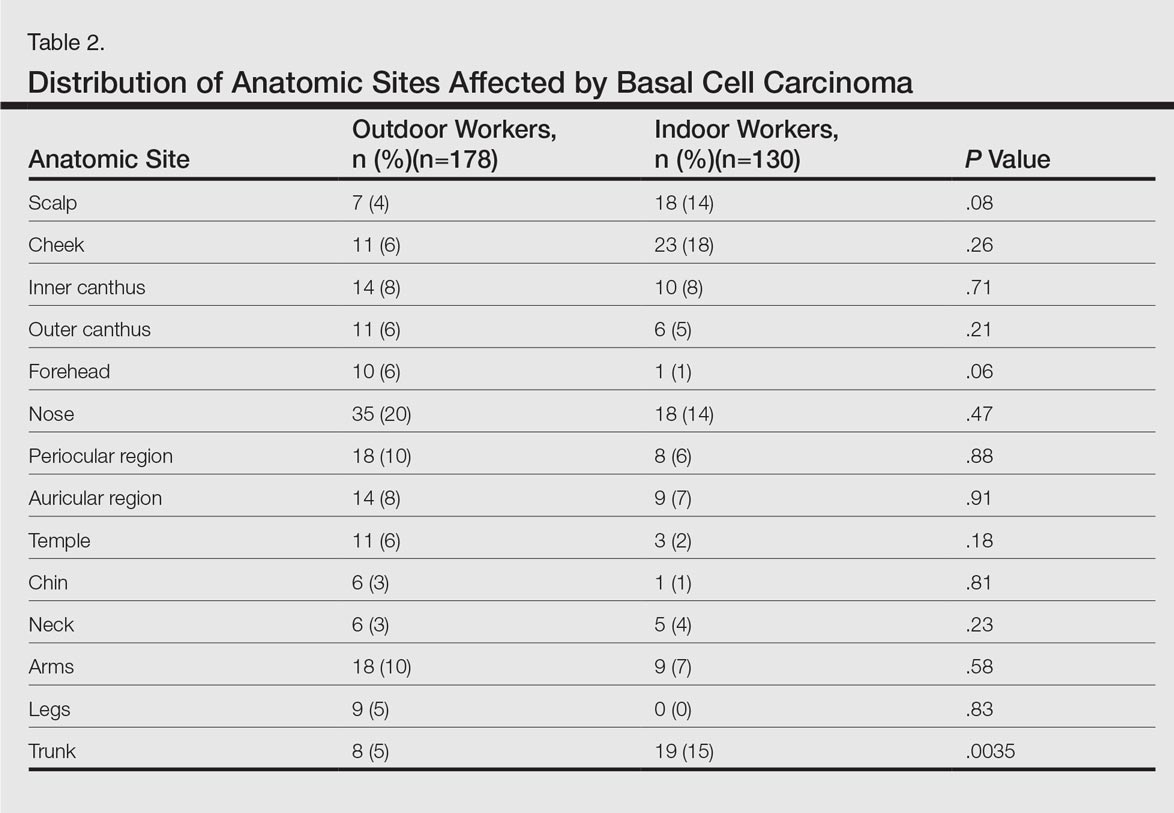
Central line-associated bloodstream infections (CLABSIs) are common and serious occurrences across healthcare systems, with an attributable mortality of 12% to 25%.1,2 Given this burden,3–5 CLABSI is a focus for both high-profile public reporting and quality improvement interventions. An integral component of such interventions is audit and feedback via quality metrics. These measures are intended to allow decision makers to assess their own performance and appropriately allocate resources. Quality metrics present a substantial cost to health systems, with an estimated $15.4 billion dollars spent annually simply for reporting.6 Despite this toll, “audit and feedback” interventions have proven to be variably successful.7–9 The mechanisms that limit the effectiveness of these interventions remain
poorly understood.
One plausible explanation for limited efficacy of quality metrics is inadequate clinician numeracy—that is, “the ability to understand the quantitative aspects of clinical medicine, original research, quality improvement, and financial matters.”10 Indeed, clinicians are not consistently able to interpret probabilities and or clinical test characteristics. For example, Wegwarth et al. identified shortcomings in physician application of lead-time bias toward cancer screening.11 Additionally, studies have demonstrated systematic misinterpretations of probabilistic information in clinical settings, along with misconceptions regarding the impact of prevalence on post-test probabilities.12,13 Effective interpretation of rates may be a key—if unstated—requirement of many CLABSI quality improvement efforts.14–19 Our broader hypothesis is that clinicians who can more accurately interpret quality data, even if only from their own institution, are more likely to act on it appropriately and persistently than those who feel they must depend on a preprocessed interpretation of that same data by some other expert.
Therefore, we designed a survey to assess the numeracy of clinicians on CLABSI data presented in a prototypical feedback report. We studied 3 domains of comprehension: (1) basic numeracy: numerical tasks related to simple data; (2) risk-adjustment numeracy: numerical tasks related to risk-adjusted data; and (3) risk-adjustment interpretation: inferential tasks concerning risk-adjusted data. We hypothesized that clinician performance would vary substantially across domains, with the poorest performance in risk-
adjusted data.
METHODS
We conducted a cross-sectional survey of clinician numeracy regarding CLABSI feedback data. Respondents were also asked to provide demographic information and opinions regarding the reliability of quality metric data. Survey recruitment occurred on Twitter, a novel approach that leveraged social media to facilitate rapid recruitment of participants. The study instrument was administered using a web survey with randomized question order to preclude any possibility of order effects between questions. The study was deemed Institutional Review Board exempt by the University of Michigan: protocol HUM00106696.
Data Presentation Method
To determine the optimal mode of presenting data, we reviewed the literature on quality metric numeracy and presentation methods. Additionally, we evaluated quality metric presentation methods used by the Centers for Disease Control and Prevention (CDC), Centers for Medicare & Medicaid Services (CMS), and a tertiary academic medical center. After assessing the available literature and options, we adapted a CLABSI data presentation array from a study that had qualitatively validated the format using physician feedback (Appendix).20 We used hypothetical CLABSI data for our survey.
Survey Development
We developed a survey that included an 11-item test regarding CLABSI numeracy and data interpretation. Additional questions related to quality metric reliability and demographic information were included. No preexisting assessment tools existed for our areas of interest. Therefore, we developed a novel instrument using a broad, exploratory approach as others have employed.21
First, we defined 3 conceptual categories related to CLABSI data. Within this conceptual framework, an iterative process of development and revision was used to assemble a question bank from which the survey would be constructed. A series of think-aloud sessions were held to evaluate each prompt for precision, clarity, and accuracy in assessing the conceptual categories. Correct and incorrect answers were defined based on literature review in conjunction with input from methodological and content experts (TJI and VC) (see Appendix for answer explanations).
Within the conceptual categories related to CLABSI risk-adjustment, a key measure is the standardized infection ratio (SIR). This value is defined as the ratio of observed number of CLABSI over the expected number of CLABSIs.22 This is the primary measure to stratify hospital performance, and it was used in our assessment of risk-adjustment comprehension. In total, 54 question prompts were developed and subsequently narrowed to 11 study questions for the initial survey.
The instrument was then pretested in a cohort of 8 hospitalists and intensivists to ensure appropriate comprehension, retrieval, and judgment processes.23 Questions were revised based on feedback from this cognitive testing to constitute the final instrument. During the survey, the data table was reshown on each page directly above each question and so was always on the same screen for the respondents.
Survey Sample
We innovated by using Twitter as an online platform for recruiting participants; we used Survey Monkey to host the electronic instrument. Two authors (TJI, VC) systematically sent out solicitation tweets to their followers. These tweets clearly indicated that the recruitment was for the purpose of a research study, and participants would receive no financial reward/incentive (Appendix). A link to the survey was provided in each tweet, and the period of recruitment was 30 days. To ensure respondents were clinicians, they needed to first answer a screening question recognizing that central lines were placed in the subclavian site but not the aorta, iliac, or radial sites.
To prevent systematic or anchoring biases, the order of questions was electronically randomized for each respondent. The primary outcome was the percentage correct of attempted questions.
Statistical Analysis
Descriptive statistics were calculated for all demographic variables. The primary outcome was evaluated as a dichotomous variable for each question (correct vs. incorrect response), and as a continuous variable when assessing mean percent correct on the overall survey. Demographic and conceptual associations were assessed via t-tests, chi-square, or Fisher exact tests. Point biserial correlations were calculated to assess for associations between response to a single question and overall performance on the survey.
To evaluate the association between various respondent characteristics and responses, logistic regression analyses were performed. An ANOVA was performed to assess the association between self-reported reliability of quality metric data and the overall performance on attempted items. Analyses were conducted using STATA MP 14.0 (College Station, TX); P <0.05 was considered statistically significant.
RESULTS
A total of 97 respondents attempted at least 1 question on the survey, and 72 respondents attempted all 11 questions, yielding 939 unique responses for analysis. Seventy respondents (87%) identified as doctors or nurses, and 44 (55%) reported having 6 to 20 years of experience; the survey cohort also came from 6 nations (Table 1). All respondents answered the CLABSI knowledge filter question correctly.
Primary Outcome
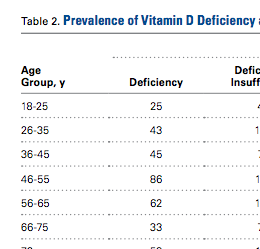
The mean percent correct of attempted questions was 61% (standard deviation 21%, interquartile range 50%-75%) (Figure 1). Of those who answered all 11 CLABSI questions, the mean percent correct was 63% (95% CI, 59%-67%). Some questions were answered correctly more often than others—ranging from 17% to 95% (Table 2). Doctors answered 68% of questions correctly (95% CI, 63%-73%), while nurses and other respondents answered 57% of questions correctly (95% CI, 52%-62%) (P = 0.003). Other demographic variables—including self-reported involvement in a quality improvement committee and being from the United States versus elsewhere—were not associated with survey performance. The point biserial correlations for each individual question with overall performance were all more than 0.2 (range 0.24–0.62) and all statistically significant at P < 0.05.
Concept-Specific Performance
Average percent correct declined across categories as numeracy requirements increased (P < 0.05 for all pairwise comparisons). In the area of basic numeracy, respondents’ mean percent correct was 82% (95% CI, 77%-87%) of attempted. This category had 4 questions, with a performance range of 77% to 90%. For example, on the question, “Which hospital has the lowest CLABSI rate?”, 80% of respondents answered correctly. For risk-adjustment numeracy, the mean percent correct was 70% (95% CI, 64%-76%); 2 items assessed this category. For “Which is better: a higher or lower SIR?”, 95% of the cohort answered correctly. However, on “If hospital B had its number of projected infection halved, what is its SIR?”, only 46% of those who attempted the question answered correctly.
Questions featuring risk-adjustment interpretation had an average percent correct of 43% (95% CI, 37%-49%). Five questions made up this category, with a percent correct range of 17% to 75%. For example, on the question, “Which hospital’s patients are the most predisposed to developing CLABSI?”, only 32% of respondents answered this correctly. In contrast, for the question “Which hospital is most effective at preventing CLABSI?”, 51% answered correctly. Figure 2 illustrates the cohort’s performance on each conceptual category while Table 2 displays question-by-question results.
Opinions Regarding CLABSI Data Reliability
Respondents were also asked about their opinion regarding the reliability of CLABSI quality metric data. Forty-three percent of respondents stated that such data were reliable at best 50% of the time. Notably, 10% of respondents indicated that CLABSI quality metric data were rarely or never reliable. There was no association between perceived reliability of quality metric data and survey performance (P = 0.87).
DISCUSSION
This Twitter-based study found wide variation in clinician interpretation of CLABSI quality data, with low overall performance. In particular, comprehension and interpretation of risk-adjusted data were substantially worse than unadjusted data. Although doctors performed somewhat better than nurses and other respondents, those involved in quality improvement initiatives performed no better than respondents who were not. Collectively, these findings suggest clinicians may not reliably comprehend quality metric data, potentially affecting their ability to utilize audit and feedback data. These results may have important implications for policy efforts that seek to leverage quality metric data to improve patient safety.
An integral component of many contemporary quality improvement initiatives is audit and feedback through metrics.6 Unfortunately, formal audit and feedback, along with other similar methods that benchmark data, have not consistently improved outcomes.24–27 A recent meta-analysis noted that audit and feedback interventions are not becoming more efficacious over time; the study further asserted that “new trials have provided little new knowledge regarding key effect modifiers.”9 Our findings suggest that numeracy and comprehension of quality metrics may be important candidate effect modifiers not previously considered. Simply put: we hypothesize that without intrinsic comprehension of data, impetus or insight to change practice might be diminished. In other words, clinicians may be more apt to act on insights they themselves derive from the data than when they are simply told what the data “mean.”
The present study further demonstrates that clinicians do not understand risk-adjusted data as well as raw data. Risk-adjustment has long been recognized as necessary to compare outcomes among hospitals.28,29 However, risk-adjustment is complex and, by its nature, difficult to understand. Although efforts have focused on improving the statistical reliability of quality metrics, this may represent but one half of the equation. Numeracy and interpretation of the data by decision makers are potentially equally important to effecting change. Because clinicians seem to have difficulty understanding risk-adjusted data, this deficit may be of growing importance as our risk-adjustment techniques become more sophisticated.
We note that clinicians expressed concerns regarding the reliability of quality metric feedback. These findings corroborate recent research that has reported reservations from hospital leaders concerning quality data.30,31 However, as shown in the context of patients and healthcare decisions, the aversion associated with quality metrics may be related to incomplete understanding of the data.32 Whether perceptions of unreliability drive lack of understanding or, conversely, whether lack of understanding fuels perceived unreliability is an important question that requires further study.
This study has several strengths. First, we used rigorous survey development techniques to evaluate the understudied issue of quality metric numeracy. Second, our sample size was sufficient to show statistically significant differences in numeracy and comprehension of CLABSI quality metric data. Third, we leveraged social media to rapidly acquire this sample. Finally, our results provided new insights that may have important implications in the area of quality metrics.
There were also limitations to our study. First, the Twitter-derived sample precludes the calculation of a response rate and may not be representative of individuals engaged in CLABSI prevention. However, respondents were solicited from the Twitter-followers of 2 health services researchers (TJI, VC) who are actively engaged in scholarly activities pertaining to critically ill patients and hospital-acquired complications. Thus, our sample likely represents a highly motivated subset that engages in these topics on a regular basis—potentially making them more numerate than average clinicians. Second, we did not ask whether the respondents had previously seen CLABSI data specifically, so we cannot stratify by exposure to such data. Third, this study assessed only CLABSI quality metric data; generalizations regarding numeracy with other metrics should be made with caution. However, as many such data are presented in similar formats, we suspect our findings are applicable to similar audit-and-feedback initiatives.
The findings of this study serve as a stimulus for further inquiry. Research of this nature needs to be carried out in samples drawn from specific, policy-relevant populations (eg, infection control practitioners, bedside nurses, intensive care unit directors). Such studies should include longitudinal assessments of numeracy that attempt to mechanistically examine its impact on CLABSI prevention efforts and outcomes. The latter is an important issue as the link between numeracy and behavioral response, while plausible, cannot be assumed, particularly given the complexity of issues related to behavioral modification.33 Additionally, whether alternate presentations of quality data affect numeracy, interpretation, and performance is worthy of further testing; indeed, this has been shown to be the case in other forms of communication.34–37 Until data from larger samples are available, it may be prudent for quality improvement leaders to assess the comprehension of local clinicians regarding feedback and whether lack of adequate comprehension is a barrier to deploying quality improvement interventions.
Quality measurement is a cornerstone of patient safety as it seeks to assess and improve the care delivered at the bedside. Rigorous metric development is important; however, ensuring that decision makers understand complex quality metrics may be equally fundamental. Given the cost of examining quality, elucidating the mechanisms of numeracy and interpretation as decision makers engage with quality metric data is necessary, along with whether improved comprehension leads to behavior change. Such inquiry may provide an evidence-base to shape alterations in quality metric deployment that will ensure maximal efficacy in driving practice change.
Disclosures
This work was supported by VA HSR&D IIR-13-079 (TJI). Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). The views expressed here are the authors’ own and do not necessarily represent the view of the US Government or the Department of Veterans’ Affairs. The authors report no conflicts of interest.
Central line-associated bloodstream infections (CLABSIs) are common and serious occurrences across healthcare systems, with an attributable mortality of 12% to 25%.1,2 Given this burden,3–5 CLABSI is a focus for both high-profile public reporting and quality improvement interventions. An integral component of such interventions is audit and feedback via quality metrics. These measures are intended to allow decision makers to assess their own performance and appropriately allocate resources. Quality metrics present a substantial cost to health systems, with an estimated $15.4 billion dollars spent annually simply for reporting.6 Despite this toll, “audit and feedback” interventions have proven to be variably successful.7–9 The mechanisms that limit the effectiveness of these interventions remain
poorly understood.
One plausible explanation for limited efficacy of quality metrics is inadequate clinician numeracy—that is, “the ability to understand the quantitative aspects of clinical medicine, original research, quality improvement, and financial matters.”10 Indeed, clinicians are not consistently able to interpret probabilities and or clinical test characteristics. For example, Wegwarth et al. identified shortcomings in physician application of lead-time bias toward cancer screening.11 Additionally, studies have demonstrated systematic misinterpretations of probabilistic information in clinical settings, along with misconceptions regarding the impact of prevalence on post-test probabilities.12,13 Effective interpretation of rates may be a key—if unstated—requirement of many CLABSI quality improvement efforts.14–19 Our broader hypothesis is that clinicians who can more accurately interpret quality data, even if only from their own institution, are more likely to act on it appropriately and persistently than those who feel they must depend on a preprocessed interpretation of that same data by some other expert.
Therefore, we designed a survey to assess the numeracy of clinicians on CLABSI data presented in a prototypical feedback report. We studied 3 domains of comprehension: (1) basic numeracy: numerical tasks related to simple data; (2) risk-adjustment numeracy: numerical tasks related to risk-adjusted data; and (3) risk-adjustment interpretation: inferential tasks concerning risk-adjusted data. We hypothesized that clinician performance would vary substantially across domains, with the poorest performance in risk-
adjusted data.
METHODS
We conducted a cross-sectional survey of clinician numeracy regarding CLABSI feedback data. Respondents were also asked to provide demographic information and opinions regarding the reliability of quality metric data. Survey recruitment occurred on Twitter, a novel approach that leveraged social media to facilitate rapid recruitment of participants. The study instrument was administered using a web survey with randomized question order to preclude any possibility of order effects between questions. The study was deemed Institutional Review Board exempt by the University of Michigan: protocol HUM00106696.
Data Presentation Method
To determine the optimal mode of presenting data, we reviewed the literature on quality metric numeracy and presentation methods. Additionally, we evaluated quality metric presentation methods used by the Centers for Disease Control and Prevention (CDC), Centers for Medicare & Medicaid Services (CMS), and a tertiary academic medical center. After assessing the available literature and options, we adapted a CLABSI data presentation array from a study that had qualitatively validated the format using physician feedback (Appendix).20 We used hypothetical CLABSI data for our survey.
Survey Development
We developed a survey that included an 11-item test regarding CLABSI numeracy and data interpretation. Additional questions related to quality metric reliability and demographic information were included. No preexisting assessment tools existed for our areas of interest. Therefore, we developed a novel instrument using a broad, exploratory approach as others have employed.21
First, we defined 3 conceptual categories related to CLABSI data. Within this conceptual framework, an iterative process of development and revision was used to assemble a question bank from which the survey would be constructed. A series of think-aloud sessions were held to evaluate each prompt for precision, clarity, and accuracy in assessing the conceptual categories. Correct and incorrect answers were defined based on literature review in conjunction with input from methodological and content experts (TJI and VC) (see Appendix for answer explanations).
Within the conceptual categories related to CLABSI risk-adjustment, a key measure is the standardized infection ratio (SIR). This value is defined as the ratio of observed number of CLABSI over the expected number of CLABSIs.22 This is the primary measure to stratify hospital performance, and it was used in our assessment of risk-adjustment comprehension. In total, 54 question prompts were developed and subsequently narrowed to 11 study questions for the initial survey.
The instrument was then pretested in a cohort of 8 hospitalists and intensivists to ensure appropriate comprehension, retrieval, and judgment processes.23 Questions were revised based on feedback from this cognitive testing to constitute the final instrument. During the survey, the data table was reshown on each page directly above each question and so was always on the same screen for the respondents.
Survey Sample
We innovated by using Twitter as an online platform for recruiting participants; we used Survey Monkey to host the electronic instrument. Two authors (TJI, VC) systematically sent out solicitation tweets to their followers. These tweets clearly indicated that the recruitment was for the purpose of a research study, and participants would receive no financial reward/incentive (Appendix). A link to the survey was provided in each tweet, and the period of recruitment was 30 days. To ensure respondents were clinicians, they needed to first answer a screening question recognizing that central lines were placed in the subclavian site but not the aorta, iliac, or radial sites.
To prevent systematic or anchoring biases, the order of questions was electronically randomized for each respondent. The primary outcome was the percentage correct of attempted questions.
Statistical Analysis
Descriptive statistics were calculated for all demographic variables. The primary outcome was evaluated as a dichotomous variable for each question (correct vs. incorrect response), and as a continuous variable when assessing mean percent correct on the overall survey. Demographic and conceptual associations were assessed via t-tests, chi-square, or Fisher exact tests. Point biserial correlations were calculated to assess for associations between response to a single question and overall performance on the survey.
To evaluate the association between various respondent characteristics and responses, logistic regression analyses were performed. An ANOVA was performed to assess the association between self-reported reliability of quality metric data and the overall performance on attempted items. Analyses were conducted using STATA MP 14.0 (College Station, TX); P <0.05 was considered statistically significant.
RESULTS
A total of 97 respondents attempted at least 1 question on the survey, and 72 respondents attempted all 11 questions, yielding 939 unique responses for analysis. Seventy respondents (87%) identified as doctors or nurses, and 44 (55%) reported having 6 to 20 years of experience; the survey cohort also came from 6 nations (Table 1). All respondents answered the CLABSI knowledge filter question correctly.
Primary Outcome
The mean percent correct of attempted questions was 61% (standard deviation 21%, interquartile range 50%-75%) (Figure 1). Of those who answered all 11 CLABSI questions, the mean percent correct was 63% (95% CI, 59%-67%). Some questions were answered correctly more often than others—ranging from 17% to 95% (Table 2). Doctors answered 68% of questions correctly (95% CI, 63%-73%), while nurses and other respondents answered 57% of questions correctly (95% CI, 52%-62%) (P = 0.003). Other demographic variables—including self-reported involvement in a quality improvement committee and being from the United States versus elsewhere—were not associated with survey performance. The point biserial correlations for each individual question with overall performance were all more than 0.2 (range 0.24–0.62) and all statistically significant at P < 0.05.
Concept-Specific Performance
Average percent correct declined across categories as numeracy requirements increased (P < 0.05 for all pairwise comparisons). In the area of basic numeracy, respondents’ mean percent correct was 82% (95% CI, 77%-87%) of attempted. This category had 4 questions, with a performance range of 77% to 90%. For example, on the question, “Which hospital has the lowest CLABSI rate?”, 80% of respondents answered correctly. For risk-adjustment numeracy, the mean percent correct was 70% (95% CI, 64%-76%); 2 items assessed this category. For “Which is better: a higher or lower SIR?”, 95% of the cohort answered correctly. However, on “If hospital B had its number of projected infection halved, what is its SIR?”, only 46% of those who attempted the question answered correctly.
Questions featuring risk-adjustment interpretation had an average percent correct of 43% (95% CI, 37%-49%). Five questions made up this category, with a percent correct range of 17% to 75%. For example, on the question, “Which hospital’s patients are the most predisposed to developing CLABSI?”, only 32% of respondents answered this correctly. In contrast, for the question “Which hospital is most effective at preventing CLABSI?”, 51% answered correctly. Figure 2 illustrates the cohort’s performance on each conceptual category while Table 2 displays question-by-question results.
Opinions Regarding CLABSI Data Reliability
Respondents were also asked about their opinion regarding the reliability of CLABSI quality metric data. Forty-three percent of respondents stated that such data were reliable at best 50% of the time. Notably, 10% of respondents indicated that CLABSI quality metric data were rarely or never reliable. There was no association between perceived reliability of quality metric data and survey performance (P = 0.87).
DISCUSSION
This Twitter-based study found wide variation in clinician interpretation of CLABSI quality data, with low overall performance. In particular, comprehension and interpretation of risk-adjusted data were substantially worse than unadjusted data. Although doctors performed somewhat better than nurses and other respondents, those involved in quality improvement initiatives performed no better than respondents who were not. Collectively, these findings suggest clinicians may not reliably comprehend quality metric data, potentially affecting their ability to utilize audit and feedback data. These results may have important implications for policy efforts that seek to leverage quality metric data to improve patient safety.
An integral component of many contemporary quality improvement initiatives is audit and feedback through metrics.6 Unfortunately, formal audit and feedback, along with other similar methods that benchmark data, have not consistently improved outcomes.24–27 A recent meta-analysis noted that audit and feedback interventions are not becoming more efficacious over time; the study further asserted that “new trials have provided little new knowledge regarding key effect modifiers.”9 Our findings suggest that numeracy and comprehension of quality metrics may be important candidate effect modifiers not previously considered. Simply put: we hypothesize that without intrinsic comprehension of data, impetus or insight to change practice might be diminished. In other words, clinicians may be more apt to act on insights they themselves derive from the data than when they are simply told what the data “mean.”
The present study further demonstrates that clinicians do not understand risk-adjusted data as well as raw data. Risk-adjustment has long been recognized as necessary to compare outcomes among hospitals.28,29 However, risk-adjustment is complex and, by its nature, difficult to understand. Although efforts have focused on improving the statistical reliability of quality metrics, this may represent but one half of the equation. Numeracy and interpretation of the data by decision makers are potentially equally important to effecting change. Because clinicians seem to have difficulty understanding risk-adjusted data, this deficit may be of growing importance as our risk-adjustment techniques become more sophisticated.
We note that clinicians expressed concerns regarding the reliability of quality metric feedback. These findings corroborate recent research that has reported reservations from hospital leaders concerning quality data.30,31 However, as shown in the context of patients and healthcare decisions, the aversion associated with quality metrics may be related to incomplete understanding of the data.32 Whether perceptions of unreliability drive lack of understanding or, conversely, whether lack of understanding fuels perceived unreliability is an important question that requires further study.
This study has several strengths. First, we used rigorous survey development techniques to evaluate the understudied issue of quality metric numeracy. Second, our sample size was sufficient to show statistically significant differences in numeracy and comprehension of CLABSI quality metric data. Third, we leveraged social media to rapidly acquire this sample. Finally, our results provided new insights that may have important implications in the area of quality metrics.
There were also limitations to our study. First, the Twitter-derived sample precludes the calculation of a response rate and may not be representative of individuals engaged in CLABSI prevention. However, respondents were solicited from the Twitter-followers of 2 health services researchers (TJI, VC) who are actively engaged in scholarly activities pertaining to critically ill patients and hospital-acquired complications. Thus, our sample likely represents a highly motivated subset that engages in these topics on a regular basis—potentially making them more numerate than average clinicians. Second, we did not ask whether the respondents had previously seen CLABSI data specifically, so we cannot stratify by exposure to such data. Third, this study assessed only CLABSI quality metric data; generalizations regarding numeracy with other metrics should be made with caution. However, as many such data are presented in similar formats, we suspect our findings are applicable to similar audit-and-feedback initiatives.
The findings of this study serve as a stimulus for further inquiry. Research of this nature needs to be carried out in samples drawn from specific, policy-relevant populations (eg, infection control practitioners, bedside nurses, intensive care unit directors). Such studies should include longitudinal assessments of numeracy that attempt to mechanistically examine its impact on CLABSI prevention efforts and outcomes. The latter is an important issue as the link between numeracy and behavioral response, while plausible, cannot be assumed, particularly given the complexity of issues related to behavioral modification.33 Additionally, whether alternate presentations of quality data affect numeracy, interpretation, and performance is worthy of further testing; indeed, this has been shown to be the case in other forms of communication.34–37 Until data from larger samples are available, it may be prudent for quality improvement leaders to assess the comprehension of local clinicians regarding feedback and whether lack of adequate comprehension is a barrier to deploying quality improvement interventions.
Quality measurement is a cornerstone of patient safety as it seeks to assess and improve the care delivered at the bedside. Rigorous metric development is important; however, ensuring that decision makers understand complex quality metrics may be equally fundamental. Given the cost of examining quality, elucidating the mechanisms of numeracy and interpretation as decision makers engage with quality metric data is necessary, along with whether improved comprehension leads to behavior change. Such inquiry may provide an evidence-base to shape alterations in quality metric deployment that will ensure maximal efficacy in driving practice change.
Disclosures
This work was supported by VA HSR&D IIR-13-079 (TJI). Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). The views expressed here are the authors’ own and do not necessarily represent the view of the US Government or the Department of Veterans’ Affairs. The authors report no conflicts of interest.
1. Scott RD II. The direct medical costs of healthcare-associated infections in us hospitals and the benefits of prevention. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Published March 2009. Accessed November 8, 2016.
2. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 suppl 1)::S1-S34. PubMed
3. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
4. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. PubMed
5. Siempos II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
6. Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35(3):401-406. PubMed
7. Hysong SJ. Meta-analysis: audit and feedback features impact effectiveness on care quality. Med Care. 2009;47(3):356-363. PubMed
8. Ilgen DR, Fisher CD, Taylor MS. Consequences of individual feedback on behavior in organizations. J Appl Psychol. 1979;64:349-371.
9. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. PubMed
10. Rao G. Physician numeracy: essential skills for practicing evidence-based medicine. Fam Med. 2008;40(5):354-358. PubMed
11. Wegwarth O, Schwartz LM, Woloshin S, Gaissmaier W, Gigerenzer G. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Ann Intern Med. 2012;156(5):340-349. PubMed
12. Bramwell R, West H, Salmon P. Health professionals’ and service users’ interpretation of screening test results: experimental study. BMJ. 2006;333(7562):284. PubMed
13. Agoritsas T, Courvoisier DS, Combescure C, Deom M, Perneger TV. Does prevalence matter to physicians in estimating post-test probability of disease? A randomized trial. J Gen Intern Med. 2011;26(4):373-378. PubMed
14. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
15. Rinke ML, Bundy DG, Chen AR, et al. Central line maintenance bundles and CLABSIs in ambulatory oncology patients. Pediatrics. 2013;132(5):e1403-e1412. PubMed
16. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):
2725-2732. PubMed
17. Rinke ML, Chen AR, Bundy DG, et al. Implementation of a central line maintenance care bundle in hospitalized pediatric oncology patients. Pediatrics. 2012;130(4):e996-e1004. PubMed
18. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
19. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
20. Rajwan YG, Barclay PW, Lee T, Sun IF, Passaretti C, Lehmann H. Visualizing central line-associated blood stream infection (CLABSI) outcome data for decision making by health care consumers and practitioners—an evaluation study. Online J Public Health Inform. 2013;5(2):218. PubMed
21. Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making 2007;27(5):672-680. PubMed
22. HAI progress report FAQ. 2016. Available at: http://www.cdc.gov/hai/surveillance/progress-report/faq.html. Last updated March 2, 2016. Accessed November 8, 2016.
23. Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. PubMed
24. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. PubMed
25. Chatterjee P, Joynt KE. Do cardiology quality measures actually improve patient outcomes? J Am Heart Assoc. 2014;3(1):e000404. PubMed
26. Joynt KE, Blumenthal DM, Orav EJ, Resnic FS, Jha AK. Association of public reporting for percutaneous coronary intervention with utilization and outcomes among Medicare beneficiaries with acute myocardial infarction. JAMA. 2012;308(14):1460-1468. PubMed
27. Ryan AM, Nallamothu BK, Dimick JB. Medicare’s public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585-592. PubMed
28. Thomas JW. Risk adjustment for measuring health care outcomes, 3rd edition. Int J Qual Health Care. 2004;16(2):181-182.
29. Iezzoni LI. Risk Adjustment for Measuring Health Care Outcomes. Ann Arbor, Michigan: Health Administration Press; 1994.
30. Goff SL, Lagu T, Pekow PS, et al. A qualitative analysis of hospital leaders’ opinions about publicly reported measures of health care quality. Jt Comm J Qual Patient Saf. 2015;41(4):169-176. PubMed
31. Lindenauer PK, Lagu T, Ross JS, et al. Attitudes of hospital leaders toward publicly reported measures of health care quality. JAMA Intern Med. 2014;174(12):
1904-1911. PubMed
32. Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk-benefit information. Health Aff (Millwood). 2007;26(3):741-748. PubMed
33. Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Glanz K, Rimer BK, Viswanath K, eds. Health Behavior and Health Education: Theory, Research and Practice. 5th ed. San Francisco, CA: Jossey-Bass; 2015:95–124.
34. Hamstra DA, Johnson SB, Daignault S, et al. The impact of numeracy on verbatim knowledge of the longitudinal risk for prostate cancer recurrence following radiation therapy. Med Decis Making. 2015;35(1):27-36. PubMed
35. Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns. 2008;73(3):448-455. PubMed
36. Zikmund-Fisher BJ, Witteman HO, Dickson M, et al. Blocks, ovals, or people? Icon type affects risk perceptions and recall of pictographs. Med Decis Making. 2014;34(4):443-453. PubMed
37. Korfage IJ, Fuhrel-Forbis A, Ubel PA, et al. Informed choice about breast cancer prevention: randomized controlled trial of an online decision aid intervention. Breast Cancer Res. 2013;15(5):R74. PubMed
1. Scott RD II. The direct medical costs of healthcare-associated infections in us hospitals and the benefits of prevention. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Published March 2009. Accessed November 8, 2016.
2. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 suppl 1)::S1-S34. PubMed
3. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
4. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. PubMed
5. Siempos II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
6. Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35(3):401-406. PubMed
7. Hysong SJ. Meta-analysis: audit and feedback features impact effectiveness on care quality. Med Care. 2009;47(3):356-363. PubMed
8. Ilgen DR, Fisher CD, Taylor MS. Consequences of individual feedback on behavior in organizations. J Appl Psychol. 1979;64:349-371.
9. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. PubMed
10. Rao G. Physician numeracy: essential skills for practicing evidence-based medicine. Fam Med. 2008;40(5):354-358. PubMed
11. Wegwarth O, Schwartz LM, Woloshin S, Gaissmaier W, Gigerenzer G. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Ann Intern Med. 2012;156(5):340-349. PubMed
12. Bramwell R, West H, Salmon P. Health professionals’ and service users’ interpretation of screening test results: experimental study. BMJ. 2006;333(7562):284. PubMed
13. Agoritsas T, Courvoisier DS, Combescure C, Deom M, Perneger TV. Does prevalence matter to physicians in estimating post-test probability of disease? A randomized trial. J Gen Intern Med. 2011;26(4):373-378. PubMed
14. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
15. Rinke ML, Bundy DG, Chen AR, et al. Central line maintenance bundles and CLABSIs in ambulatory oncology patients. Pediatrics. 2013;132(5):e1403-e1412. PubMed
16. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):
2725-2732. PubMed
17. Rinke ML, Chen AR, Bundy DG, et al. Implementation of a central line maintenance care bundle in hospitalized pediatric oncology patients. Pediatrics. 2012;130(4):e996-e1004. PubMed
18. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
19. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
20. Rajwan YG, Barclay PW, Lee T, Sun IF, Passaretti C, Lehmann H. Visualizing central line-associated blood stream infection (CLABSI) outcome data for decision making by health care consumers and practitioners—an evaluation study. Online J Public Health Inform. 2013;5(2):218. PubMed
21. Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making 2007;27(5):672-680. PubMed
22. HAI progress report FAQ. 2016. Available at: http://www.cdc.gov/hai/surveillance/progress-report/faq.html. Last updated March 2, 2016. Accessed November 8, 2016.
23. Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. PubMed
24. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. PubMed
25. Chatterjee P, Joynt KE. Do cardiology quality measures actually improve patient outcomes? J Am Heart Assoc. 2014;3(1):e000404. PubMed
26. Joynt KE, Blumenthal DM, Orav EJ, Resnic FS, Jha AK. Association of public reporting for percutaneous coronary intervention with utilization and outcomes among Medicare beneficiaries with acute myocardial infarction. JAMA. 2012;308(14):1460-1468. PubMed
27. Ryan AM, Nallamothu BK, Dimick JB. Medicare’s public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585-592. PubMed
28. Thomas JW. Risk adjustment for measuring health care outcomes, 3rd edition. Int J Qual Health Care. 2004;16(2):181-182.
29. Iezzoni LI. Risk Adjustment for Measuring Health Care Outcomes. Ann Arbor, Michigan: Health Administration Press; 1994.
30. Goff SL, Lagu T, Pekow PS, et al. A qualitative analysis of hospital leaders’ opinions about publicly reported measures of health care quality. Jt Comm J Qual Patient Saf. 2015;41(4):169-176. PubMed
31. Lindenauer PK, Lagu T, Ross JS, et al. Attitudes of hospital leaders toward publicly reported measures of health care quality. JAMA Intern Med. 2014;174(12):
1904-1911. PubMed
32. Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk-benefit information. Health Aff (Millwood). 2007;26(3):741-748. PubMed
33. Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Glanz K, Rimer BK, Viswanath K, eds. Health Behavior and Health Education: Theory, Research and Practice. 5th ed. San Francisco, CA: Jossey-Bass; 2015:95–124.
34. Hamstra DA, Johnson SB, Daignault S, et al. The impact of numeracy on verbatim knowledge of the longitudinal risk for prostate cancer recurrence following radiation therapy. Med Decis Making. 2015;35(1):27-36. PubMed
35. Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns. 2008;73(3):448-455. PubMed
36. Zikmund-Fisher BJ, Witteman HO, Dickson M, et al. Blocks, ovals, or people? Icon type affects risk perceptions and recall of pictographs. Med Decis Making. 2014;34(4):443-453. PubMed
37. Korfage IJ, Fuhrel-Forbis A, Ubel PA, et al. Informed choice about breast cancer prevention: randomized controlled trial of an online decision aid intervention. Breast Cancer Res. 2013;15(5):R74. PubMed
© 2017 Society of Hospital Medicine
Interhospital Transfer Handover Tool
The transfer of inpatients between hospitals for specialized services is common, affecting nearly 10% of all Medicare admissions1 and 4.5% of all critical care hospitalizations.2 At tertiary referral centers, 49% of medical intensive care unit (ICU) admissions are transferred from another hospital.3
Transfer patients have longer length of stay (LOS) than patients admitted directly from the emergency department or clinic. Among patients initially admitted to an ICU, transfer patients spend 1 day to 2.2 more days in the ICU and an additional 2 days to 4 more days total at the receiving hospital.4,5 Furthermore, transfer patients have higher mortality than nontransferred patients by 4% to 8%.3-5 Even after adjustment for case mix and comorbid disease, interhospital transfer is an independent predictor of both ICU death and LOS.6,7 As a result, interhospital transfer has been associated with a $9600 increase (on average) in hospital costs.4
Despite evidence detailing patient handovers as a key time when poor communication can lead to delays in care and significant patient risk, 8-10 most studies have focused on hospital discharge or change of shift, and scant effort has been dedicated to improving the interhospital handover. The process of interhospital transfer is often prolonged and discontinuous,11 commonly including delays of more than 24 hours between initiation and completion. This frequently precludes direct physician-to-physician contact at the time of transfer, and physicians rely on the discharge/transfer summary.12 Yet discharge summaries are frequently absent or incomplete,13 and often lack information for high-risk treatments such as systemic anticoagulation.14 The traditional reliance on discharge summaries for handover communication requires interpretation of unstandardized documentation and increases the risk for miscommunication, delays, and error.
To improve communication, we developed a 1-page handover tool for all inbound adult interhospital transfers to our academic medical center. We sought to determine whether implementation of this standardized handover tool improved the timeliness of initial care, LOS, and mortality among interhospital transfer patients.
METHODS
Study Design, Setting, Population
We conducted a retrospective cohort study of patients transferred into Vanderbilt University Hospital (VUH), an adult 626-bed quaternary care academic medical center in Nashville, Tennessee. The Vanderbilt University Institutional Review Board approved this study.
Population
We selected for inclusion all patients age 18 or older who were transferred into VUH between July 1, 2009 and December 31, 2010. We excluded patients whose transfer was routed outside the main VUH Patient Flow Center as well as patients who did not arrive alive at VUH. We also excluded patients transferred to the emergency department and patients admitted to obstetrics, burn, or trauma services, because these admitting services did not initially use the handover tool. Patients were followed for the duration of their hospitalization at VUH.
Intervention
The 1-page handover tool was developed with multidisciplinary physician input from house staff; medical directors from intensive care, neurology, and surgery; and the chief of staff. The tool was structured on the SBAR model (Situation, Background, Assessment, and Recommendation).15 Fields on the handover tool were limited to those deemed critical for immediate patient care and designed for 1 tool to be used for both ICU and non-ICU transfers. Fields included primary diagnosis; allergies; use and last dose of anticoagulants, vasopressors, sedative/paralytics, and antibiotics; isolation needs; indwelling devices; recent operations/procedures; code status; emergency contact information; problem list; active medication list; vital signs; pertinent exam; imaging; lab findings; and overall reason for transfer.
The handover tool was completed by the physician at the transferring hospital, faxed to VUH, and immediately scanned into the electronic record, allowing the receiving physicians to review information before patient arrival. Use of the tool was piloted first with 2 referring hospitals in April 2010 and universally recommended but not compulsory for all adult patients routed through the main VUH Patient Flow Center beginning July 1, 2010. Immediately before full implementation, the chief of staff sent letters to leadership of the 40 highest volume referral hospitals, highlighting the institutional goal of improving hand-off communication, framing completion of the tool as a step in the transfer acceptance process, and providing contact information for questions, feedback, or concerns. To ensure the tool was a standard part of the transfer process, the VUH Patient Flow Center maintained the responsibility of faxing the form to the outside facility and monitoring its receipt. The tool was processed in the same manner as other faxed patient records and treated as a part of the formal medical record to meet all standards for the Health Insurance Portability and Accountability Act (HIPAA) and medicolegal compliance. The medical center also has a separate cardiac transfer center where the handover tool was not implemented owing to its specialized workflow.
Data Source
The VUH Patient Flow Center maintains a database of all patients for whom transfer to VUH is requested, including information on the requesting hospital and the duration of transfer process. Outcome data and patient characteristics were extracted from the Enterprise Data Warehouse. Data related to comorbid illness were extracted from the Perioperative Data Warehouse, an IRB-approved data registry.
Measures
We evaluated 3 outcomes. First, we defined 2 measures of the timeliness of initial care, the time from arrival at VUH until entry of an admission order, and the time from arrival until entry of the first antibiotic order. Only antibiotics ordered within the first 36 hours of admission were included. Second, we evaluated the total LOS after transfer to VUH and the ICU LOS for patients transferred into an ICU setting. Finally, we examined in-hospital mortality at VUH. These metrics were chosen for their broad applicability across patient groups and feasibility of data capture. Length of stay and mortality also represent final common pathways for avoidance of complications. Specific patient safety indicators and complications were not abstracted due to their low frequency and burden of data collection. Due to system changes in our cost accounting systems, we were not able to obtain cost data pre- and postimplementation that provided meaningful comparisons.
Patient covariates included age, gender, payer, and Elixhauser comorbidity index as modified by van Walraven,16 calculated based on the admission of interest and the previous 365 days. We also examined admission characteristics including location (ICU vs. non-ICU), admitting service (medicine, surgery, neurology, or gynecology), and shift of arrival (day, 7:00 am to 6:00 pm; or night, 6:00 pm to 7:00 pm). Finally, we examined duration of the transfer process (ie, time between transfer request and arrival at VUH) and the volume of the transferring hospital (high was defined as 3 or more transfers to VUH per year).
Statistical analysis
Patient characteristics before and after implementation of the handover tool were compared using Pearson’s chi-square test and Fisher exact test for categorical variables and using Student t test and the Wilcoxon rank sum test for continuous variables. The outcome variables of time to admission order entry, time to antibiotic order entry, LOS, ICU LOS, and in-hospital mortality were compared between the before- and after-intervention time periods, using the Wilcoxon rank sum test for continuous outcomes and Pearson’s chi-square test for in-hospital mortality.
To account for temporal trends, the effect of the handover tool on time-to-admission order entry, hospital LOS, and mortality was measured using an interrupted time-series design with segmented linear regression analysis.17 The study period was divided into 2-week intervals, with 26 time periods in the pre-intervention period and 13 time periods in the postintervention period. Expected rates for the postintervention time periods were projected from the pre-intervention data using a linear regression model. To assess the observed effect of the intervention, rates from the postintervention periods were compared with these projected rates, assuming continuation of the trend. Restricted cubic spline models were also fit for time-to-admission order and hospital LOS; however, the F-statistics for these models were not significant, suggesting the linear regression provided a more appropriate model.
To further account for potential confounding of outcomes by comorbid disease and other patient factors, multivariate linear regression models assessed change in timeliness and LOS with implementation of the intervention. A multivariate logistic regression model was used to assess change in mortality with intervention implementation. All models adjusted for age, gender, payer, comorbid illness, admitting team, shift of arrival (day vs. night), transfer duration, volume of transferring hospital, and ICU status. Outcomes were further adjusted for calendar month to account for temporal trends in house staff efficiency. Because the cardiac transfer center did not adopt the use of the transfer tool, we evaluated adjusted in-hospital mortality for these patients as a concurrent control group.
All statistical testing was 2-sided at a significance level of 0.05. All analyses were conducted using STATA 12.1 statistical software (StataCorp LP, College Station, Texas).
RESULTS
Of 10,325 patients for whom transfer to VUH was requested during the study period, 1715 met inclusion criteria, including 798 patients (46.5%) initially admitted to an ICU setting. Specific patient exclusions are detailed in the Supplemental Figure; the majority of exclusions were due to patients being transferred directly to the emergency department setting. Table 1 summarizes patient characteristics before and after implementation of the handover tool. The median age was 57 years, with 48.6% male patients. Accepting services included medicine (56%), surgery (34%), neurology (9%), and gynecology (1%). The median duration of transfer was 8 hours, and the majority (93%) of patients came from higher volume transferring hospitals. Most (65%) of patients were admitted during night shift. The median modified Elixhauser comorbidity index was 11 (range of possible scores, -19 to 89). A slightly higher proportion of patients admitted postimplementation of the handover tool came from higher volume transferring hospitals; otherwise, there were no significant differences between the pre- and postintervention groups.
Vanderbilt University Hospital received transfers from more than 350 unique facilities in more than 25 U.S. states during the overall study period. During the postintervention period, adherence to the handover process was excellent, with more than 85% of patients having a completed handover tool available in their medical record at the time of transfer. The remaining 15% had either incomplete forms or no form.
Timeliness of Initial Care
There was no change in either the median time-to-admission order entry after implementation (47 vs. 45 minutes, unadjusted P = 0.36) or time to antibiotic order entry (199 vs. 202 minutes; unadjusted P = 0.81; Table 2).
In the time-series analysis, the pre-intervention period did not have a significant temporal trend in median time-to-admission order entry (ß-coefficient = -0.27; 95% confidence interval [CI] -0.85 to 0.31; R2 = 0.04; P = 0.34; Figure 1A). The postintervention period showed a trend toward a reduction in median time-to-admission order entry (ß-coefficient = -1.39; 95% CI -2.92 to 0.15; R2 = 0.27; P = 0.07). There was no significant difference between the actual time-to-admission order entry in the postintervention period when compared to the projected rates from the pre-intervention period (P = 0.18).
After multivariate adjustment, the postintervention time period was not associated with any significant change in the median time-to-admission order entry (P = 0.94, R2 = 0.09) nor time-to-antibiotic order entry (P = 0.91; R2 = 0.08; Table 2).
Length of Stay
Hospital LOS demonstrated a nonstatistically significant decline after implementation of the handover tool from 6.47 days to 5.81 days (unadjusted P = 0.18; Table 2). There was no significant change in ICU LOS postintervention (4.34 days to 4.55 days; P = 0.38).
In time series analysis, hospital LOS did not have a significant temporal trend in either the pre-intervention period (ß-coefficient = 0.00094; 95% CI, -0.07 to 0.07; R2 = 0.00; P = 0.98) or the postintervention period (ß-coefficient = 0.09; 95% CI, -0.07 to 0.25; R2 = 0.13; P = 0.23; Figure 1B). Similarly, there was no significant difference between the actual and projected hospital LOS after implementation of the handover tool (P = 0.31).
After multivariate adjustment, the postintervention time period was associated with a trend toward reduction in overall LOS (P = 0.06; R2 = 0.07) but no significant change in ICU LOS (P = 0.99; R2 = 0.09).
Mortality
In-hospital mortality declined significantly from 12.0% in the pre-intervention period to 8.9% in the postintervention period (P = 0.04; Table 2). In time-series analysis, mortality did not have a significant trend in the pre-intervention period (ß-coefficient = 0.00017, 95% CI, -0.0020 to 0.0024; P = 0.878) and had a trend toward reduction in the postintervention period (ß-coefficient = -0.0032; 95% CI, -0.0091 to 0.0027; P = 0.255; Figure 1C) but did not reach statistical significance due to relatively small numbers of deaths in each individual time period.
After multivariate adjustment, the postintervention period was associated with overall lower odds of mortality among transfer patients when compared with the pre-intervention period (adjusted OR 0.68; 95% CI, 0.47 – 0.99; R2 = 0.21; P = 0.04; Figure 2). Among the concurrent control group of patients routed through the cardiac transfer center, there was no significant change in mortality between the pre- and postintervention periods (adjusted OR 1.31; 95% CI, 0.88 – 1.93; R2 = 0.28; P = 0.18).
DISCUSSION
We developed a simple 1-page handover tool for interhospital transfer patients and aimed to improve timeliness, efficiency, and outcomes of care at the receiving hospital. Implementation of the handover tool was feasible and well accepted by transferring physicians despite a geographically large and diverse transfer network. Although implementation did not substantially improve measures of the timeliness of initial care among transfer patients, we noted a nonsignificant trend toward reduced LOS postintervention.
We observed a substantial and statistically significant reduction in mortality among transfer patients after implementation of the handover tool that persisted after controlling for time trends, comorbid illness, and several other patient factors. This effect was not seen in a concurrent control group of cardiac transfer patients for whom the handover tool was not implemented. Standardizing communication regarding high-risk clinical care processes may be responsible for the observed mortality reduction, similar to improvements seen in other small pilot studies.18 We acknowledge that the magnitude of the improvement in mortality is more than might be expected from the handover tool alone and could be due to chance.
In this initial evaluation, it was not feasible to determine whether information provided in the handover tool helped avert specific complications that could affect mortality, such as complications related to the use of ventilators, high-risk medications, or indwelling devices. Assessment of additional patient safety indices such as code events, unplanned ICU transfers, and medication errors could also help clarify the effect of the handover tool on patient-safety outcomes, and future work should include these metrics as well. Alternately, the improvement in mortality may result from other unmeasured processes that occurred concurrently and verification of this finding should be completed in other settings.
CONCLUSION
More work is needed to determine suitable process and outcome measures for interhospital transfers. Most literature has focused on cost and LOS at the exclusion of more proximal measures of initial care.3-7 The Institute of Medicine has identified timeliness as 1 of the 6 aims for care delivery redesign,19 yet standardized timeliness outcomes do not exist across broad inpatient populations. We chose to monitor the time-to-admission order entry and time-to-antibiotic order entry as 2 indicators of timeliness that would be applicable to a variety of patients. The lack of change in these selected measures should prompt examination for other measures of efficiency, including those that affect nontransferred patients. It is possible that nontransferred patients cared for by the same physician also benefit from fewer delays or disruptions and experience increased efficiency of care if transfer patient communication is improved. Further work is necessary to understand whether other measures of timely initial patient care may be more suitable.
The use of a time-series design to account for temporal trends adds substantial rigor to this study, since the majority of these patients were cared for by house staff whose experience and efficiency vary throughout the academic year. Furthermore, subsequent multivariate analysis demonstrated consistent findings after adjustment for comorbid illness and several other hospital and patient-level confounders.
This study has several limitations. The primary limitation is its nonrandomized design. Patient characteristics were stable across multiple variables before and after implementation, but it is possible that another confounding factor was responsible for observed improvements. Likewise, we collected data for only 6 rather than 12 months during the postintervention time period, which limited our sample size and statistical power. This was chosen because a significant restructuring of resident duty hours occurred in spring 2011 that had the potential to affect all measures studied.20,21 Finally, we did not collect data on accuracy of the information provided in the handover tool or end-user utilization and were unable to account for effects of these.
Since implementation in 2010, this process for interhospital transfers at VUH remains the same, although the volume of incoming transfers has significantly increased. Electronic handover tools with similar structure and content have since been adopted for patients being transferred to the emergency department or directly admitted from clinic. As VUH moves in the coming years from a locally developed electronic medical record to a national vendor, there will be an opportunity to transform this tool into an electronic template that will easily share data between institutions and further enhance communication.
Interhospital transfer patients represent a high-risk population whose unique handover needs have not been adequately measured or addressed. Our investigation demonstrated that a standardized handover aid can be implemented across a broad transfer network and may contribute to reductions in LOS and mortality. Further study is warranted to confirm these findings and assess the effect on other clinical outcomes.
Disclosures
This material is based upon work supported by the Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program, and was made possible by the use of the facilities at VA Tennessee Valley Healthcare System, Nashville, Tennessee. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. Additionally, this publication was supported in part by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
1. Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39:1449-1465. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47:787-793. PubMed
3. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31:1981-1986. PubMed
4. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35:1470-1476. PubMed
5. Flabouris A, Hart GK, George C. Outcomes of patients admitted to tertiary intensive care units after interhospital transfer: comparison with patients admitted from emergency departments. Crit Care Resusc. 2008;10:97-105. PubMed
6. Combes A, Luyt CE, Trouillet JL, Chastre J, Gibert C. Adverse effect on a referral intensive care unit’s performance of accepting patients transferred from another intensive care unit. Crit Care Med. 2005;33:705-710. PubMed
7. Rosenberg AL, Hofer TP, Strachan C, Watts CM, Hayward RA. Accepting critically ill transfer patients: adverse effect on a referral center’s outcome and benchmark measures. A Intern Med. 2003;138:882-890. PubMed
8. Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. Consequences of inadequate sign-out for patient care. Arch Intern Med. 2008;168:1755-1760. PubMed
9. Starmer AJ, Sectish TC, Simon DW, et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310:2262-2270. PubMed
10. Arora VM, Manjarrez E, Dressler DD, Basaviah P, Halasyamani L, Kripalani S. Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4:433-440. PubMed
11. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49:592-598. PubMed
12. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11:413-417. PubMed
13. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007;297:831-841. PubMed
14. Gandara E, Moniz TT, Ungar J, et al. Deficits in discharge documentation in patients transferred to rehabilitation facilities on anticoagulation: results of a systemwide evaluation. Jt Comm J Qual Patient Saf. 2008;34:460-463. PubMed
15. Haig KM, Sutton S, Whittington J. SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32:167-175. PubMed
16. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626-633. PubMed
17. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299-309. PubMed
18. Malpass HC, Enfield KB, Keim-Malpass J, Verghese GM. The interhospital medical intensive care unit transfer instrument facilitates early implementation of critical therapies and is associated with fewer emergent procedures upon arrival. J Intensive Care Med. 2015;30:351-357. PubMed
19. National Academy of Sciences. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. March 2005:1–360. Washington, DC. PubMed
20. Theobald CN, Stover DG, Choma NN, et al. The effect of reducing maximum shift lengths to 16 hours on internal medicine interns’ educational opportunities. Acad Med. 2013;88:512-518. PubMed
21. Choma NN, Vasilevskis EE, Sponsler KC, Hathaway J, Kripalani S. Effect of the ACGME 16-hour rule on efficiency and quality of care: duty hours 2.0. JAMA Intern Med. 2013;173:819-821. PubMed
The transfer of inpatients between hospitals for specialized services is common, affecting nearly 10% of all Medicare admissions1 and 4.5% of all critical care hospitalizations.2 At tertiary referral centers, 49% of medical intensive care unit (ICU) admissions are transferred from another hospital.3
Transfer patients have longer length of stay (LOS) than patients admitted directly from the emergency department or clinic. Among patients initially admitted to an ICU, transfer patients spend 1 day to 2.2 more days in the ICU and an additional 2 days to 4 more days total at the receiving hospital.4,5 Furthermore, transfer patients have higher mortality than nontransferred patients by 4% to 8%.3-5 Even after adjustment for case mix and comorbid disease, interhospital transfer is an independent predictor of both ICU death and LOS.6,7 As a result, interhospital transfer has been associated with a $9600 increase (on average) in hospital costs.4
Despite evidence detailing patient handovers as a key time when poor communication can lead to delays in care and significant patient risk, 8-10 most studies have focused on hospital discharge or change of shift, and scant effort has been dedicated to improving the interhospital handover. The process of interhospital transfer is often prolonged and discontinuous,11 commonly including delays of more than 24 hours between initiation and completion. This frequently precludes direct physician-to-physician contact at the time of transfer, and physicians rely on the discharge/transfer summary.12 Yet discharge summaries are frequently absent or incomplete,13 and often lack information for high-risk treatments such as systemic anticoagulation.14 The traditional reliance on discharge summaries for handover communication requires interpretation of unstandardized documentation and increases the risk for miscommunication, delays, and error.
To improve communication, we developed a 1-page handover tool for all inbound adult interhospital transfers to our academic medical center. We sought to determine whether implementation of this standardized handover tool improved the timeliness of initial care, LOS, and mortality among interhospital transfer patients.
METHODS
Study Design, Setting, Population
We conducted a retrospective cohort study of patients transferred into Vanderbilt University Hospital (VUH), an adult 626-bed quaternary care academic medical center in Nashville, Tennessee. The Vanderbilt University Institutional Review Board approved this study.
Population
We selected for inclusion all patients age 18 or older who were transferred into VUH between July 1, 2009 and December 31, 2010. We excluded patients whose transfer was routed outside the main VUH Patient Flow Center as well as patients who did not arrive alive at VUH. We also excluded patients transferred to the emergency department and patients admitted to obstetrics, burn, or trauma services, because these admitting services did not initially use the handover tool. Patients were followed for the duration of their hospitalization at VUH.
Intervention
The 1-page handover tool was developed with multidisciplinary physician input from house staff; medical directors from intensive care, neurology, and surgery; and the chief of staff. The tool was structured on the SBAR model (Situation, Background, Assessment, and Recommendation).15 Fields on the handover tool were limited to those deemed critical for immediate patient care and designed for 1 tool to be used for both ICU and non-ICU transfers. Fields included primary diagnosis; allergies; use and last dose of anticoagulants, vasopressors, sedative/paralytics, and antibiotics; isolation needs; indwelling devices; recent operations/procedures; code status; emergency contact information; problem list; active medication list; vital signs; pertinent exam; imaging; lab findings; and overall reason for transfer.
The handover tool was completed by the physician at the transferring hospital, faxed to VUH, and immediately scanned into the electronic record, allowing the receiving physicians to review information before patient arrival. Use of the tool was piloted first with 2 referring hospitals in April 2010 and universally recommended but not compulsory for all adult patients routed through the main VUH Patient Flow Center beginning July 1, 2010. Immediately before full implementation, the chief of staff sent letters to leadership of the 40 highest volume referral hospitals, highlighting the institutional goal of improving hand-off communication, framing completion of the tool as a step in the transfer acceptance process, and providing contact information for questions, feedback, or concerns. To ensure the tool was a standard part of the transfer process, the VUH Patient Flow Center maintained the responsibility of faxing the form to the outside facility and monitoring its receipt. The tool was processed in the same manner as other faxed patient records and treated as a part of the formal medical record to meet all standards for the Health Insurance Portability and Accountability Act (HIPAA) and medicolegal compliance. The medical center also has a separate cardiac transfer center where the handover tool was not implemented owing to its specialized workflow.
Data Source
The VUH Patient Flow Center maintains a database of all patients for whom transfer to VUH is requested, including information on the requesting hospital and the duration of transfer process. Outcome data and patient characteristics were extracted from the Enterprise Data Warehouse. Data related to comorbid illness were extracted from the Perioperative Data Warehouse, an IRB-approved data registry.
Measures
We evaluated 3 outcomes. First, we defined 2 measures of the timeliness of initial care, the time from arrival at VUH until entry of an admission order, and the time from arrival until entry of the first antibiotic order. Only antibiotics ordered within the first 36 hours of admission were included. Second, we evaluated the total LOS after transfer to VUH and the ICU LOS for patients transferred into an ICU setting. Finally, we examined in-hospital mortality at VUH. These metrics were chosen for their broad applicability across patient groups and feasibility of data capture. Length of stay and mortality also represent final common pathways for avoidance of complications. Specific patient safety indicators and complications were not abstracted due to their low frequency and burden of data collection. Due to system changes in our cost accounting systems, we were not able to obtain cost data pre- and postimplementation that provided meaningful comparisons.
Patient covariates included age, gender, payer, and Elixhauser comorbidity index as modified by van Walraven,16 calculated based on the admission of interest and the previous 365 days. We also examined admission characteristics including location (ICU vs. non-ICU), admitting service (medicine, surgery, neurology, or gynecology), and shift of arrival (day, 7:00 am to 6:00 pm; or night, 6:00 pm to 7:00 pm). Finally, we examined duration of the transfer process (ie, time between transfer request and arrival at VUH) and the volume of the transferring hospital (high was defined as 3 or more transfers to VUH per year).
Statistical analysis
Patient characteristics before and after implementation of the handover tool were compared using Pearson’s chi-square test and Fisher exact test for categorical variables and using Student t test and the Wilcoxon rank sum test for continuous variables. The outcome variables of time to admission order entry, time to antibiotic order entry, LOS, ICU LOS, and in-hospital mortality were compared between the before- and after-intervention time periods, using the Wilcoxon rank sum test for continuous outcomes and Pearson’s chi-square test for in-hospital mortality.
To account for temporal trends, the effect of the handover tool on time-to-admission order entry, hospital LOS, and mortality was measured using an interrupted time-series design with segmented linear regression analysis.17 The study period was divided into 2-week intervals, with 26 time periods in the pre-intervention period and 13 time periods in the postintervention period. Expected rates for the postintervention time periods were projected from the pre-intervention data using a linear regression model. To assess the observed effect of the intervention, rates from the postintervention periods were compared with these projected rates, assuming continuation of the trend. Restricted cubic spline models were also fit for time-to-admission order and hospital LOS; however, the F-statistics for these models were not significant, suggesting the linear regression provided a more appropriate model.
To further account for potential confounding of outcomes by comorbid disease and other patient factors, multivariate linear regression models assessed change in timeliness and LOS with implementation of the intervention. A multivariate logistic regression model was used to assess change in mortality with intervention implementation. All models adjusted for age, gender, payer, comorbid illness, admitting team, shift of arrival (day vs. night), transfer duration, volume of transferring hospital, and ICU status. Outcomes were further adjusted for calendar month to account for temporal trends in house staff efficiency. Because the cardiac transfer center did not adopt the use of the transfer tool, we evaluated adjusted in-hospital mortality for these patients as a concurrent control group.
All statistical testing was 2-sided at a significance level of 0.05. All analyses were conducted using STATA 12.1 statistical software (StataCorp LP, College Station, Texas).
RESULTS
Of 10,325 patients for whom transfer to VUH was requested during the study period, 1715 met inclusion criteria, including 798 patients (46.5%) initially admitted to an ICU setting. Specific patient exclusions are detailed in the Supplemental Figure; the majority of exclusions were due to patients being transferred directly to the emergency department setting. Table 1 summarizes patient characteristics before and after implementation of the handover tool. The median age was 57 years, with 48.6% male patients. Accepting services included medicine (56%), surgery (34%), neurology (9%), and gynecology (1%). The median duration of transfer was 8 hours, and the majority (93%) of patients came from higher volume transferring hospitals. Most (65%) of patients were admitted during night shift. The median modified Elixhauser comorbidity index was 11 (range of possible scores, -19 to 89). A slightly higher proportion of patients admitted postimplementation of the handover tool came from higher volume transferring hospitals; otherwise, there were no significant differences between the pre- and postintervention groups.
Vanderbilt University Hospital received transfers from more than 350 unique facilities in more than 25 U.S. states during the overall study period. During the postintervention period, adherence to the handover process was excellent, with more than 85% of patients having a completed handover tool available in their medical record at the time of transfer. The remaining 15% had either incomplete forms or no form.
Timeliness of Initial Care
There was no change in either the median time-to-admission order entry after implementation (47 vs. 45 minutes, unadjusted P = 0.36) or time to antibiotic order entry (199 vs. 202 minutes; unadjusted P = 0.81; Table 2).
In the time-series analysis, the pre-intervention period did not have a significant temporal trend in median time-to-admission order entry (ß-coefficient = -0.27; 95% confidence interval [CI] -0.85 to 0.31; R2 = 0.04; P = 0.34; Figure 1A). The postintervention period showed a trend toward a reduction in median time-to-admission order entry (ß-coefficient = -1.39; 95% CI -2.92 to 0.15; R2 = 0.27; P = 0.07). There was no significant difference between the actual time-to-admission order entry in the postintervention period when compared to the projected rates from the pre-intervention period (P = 0.18).
After multivariate adjustment, the postintervention time period was not associated with any significant change in the median time-to-admission order entry (P = 0.94, R2 = 0.09) nor time-to-antibiotic order entry (P = 0.91; R2 = 0.08; Table 2).
Length of Stay
Hospital LOS demonstrated a nonstatistically significant decline after implementation of the handover tool from 6.47 days to 5.81 days (unadjusted P = 0.18; Table 2). There was no significant change in ICU LOS postintervention (4.34 days to 4.55 days; P = 0.38).
In time series analysis, hospital LOS did not have a significant temporal trend in either the pre-intervention period (ß-coefficient = 0.00094; 95% CI, -0.07 to 0.07; R2 = 0.00; P = 0.98) or the postintervention period (ß-coefficient = 0.09; 95% CI, -0.07 to 0.25; R2 = 0.13; P = 0.23; Figure 1B). Similarly, there was no significant difference between the actual and projected hospital LOS after implementation of the handover tool (P = 0.31).
After multivariate adjustment, the postintervention time period was associated with a trend toward reduction in overall LOS (P = 0.06; R2 = 0.07) but no significant change in ICU LOS (P = 0.99; R2 = 0.09).
Mortality
In-hospital mortality declined significantly from 12.0% in the pre-intervention period to 8.9% in the postintervention period (P = 0.04; Table 2). In time-series analysis, mortality did not have a significant trend in the pre-intervention period (ß-coefficient = 0.00017, 95% CI, -0.0020 to 0.0024; P = 0.878) and had a trend toward reduction in the postintervention period (ß-coefficient = -0.0032; 95% CI, -0.0091 to 0.0027; P = 0.255; Figure 1C) but did not reach statistical significance due to relatively small numbers of deaths in each individual time period.
After multivariate adjustment, the postintervention period was associated with overall lower odds of mortality among transfer patients when compared with the pre-intervention period (adjusted OR 0.68; 95% CI, 0.47 – 0.99; R2 = 0.21; P = 0.04; Figure 2). Among the concurrent control group of patients routed through the cardiac transfer center, there was no significant change in mortality between the pre- and postintervention periods (adjusted OR 1.31; 95% CI, 0.88 – 1.93; R2 = 0.28; P = 0.18).
DISCUSSION
We developed a simple 1-page handover tool for interhospital transfer patients and aimed to improve timeliness, efficiency, and outcomes of care at the receiving hospital. Implementation of the handover tool was feasible and well accepted by transferring physicians despite a geographically large and diverse transfer network. Although implementation did not substantially improve measures of the timeliness of initial care among transfer patients, we noted a nonsignificant trend toward reduced LOS postintervention.
We observed a substantial and statistically significant reduction in mortality among transfer patients after implementation of the handover tool that persisted after controlling for time trends, comorbid illness, and several other patient factors. This effect was not seen in a concurrent control group of cardiac transfer patients for whom the handover tool was not implemented. Standardizing communication regarding high-risk clinical care processes may be responsible for the observed mortality reduction, similar to improvements seen in other small pilot studies.18 We acknowledge that the magnitude of the improvement in mortality is more than might be expected from the handover tool alone and could be due to chance.
In this initial evaluation, it was not feasible to determine whether information provided in the handover tool helped avert specific complications that could affect mortality, such as complications related to the use of ventilators, high-risk medications, or indwelling devices. Assessment of additional patient safety indices such as code events, unplanned ICU transfers, and medication errors could also help clarify the effect of the handover tool on patient-safety outcomes, and future work should include these metrics as well. Alternately, the improvement in mortality may result from other unmeasured processes that occurred concurrently and verification of this finding should be completed in other settings.
CONCLUSION
More work is needed to determine suitable process and outcome measures for interhospital transfers. Most literature has focused on cost and LOS at the exclusion of more proximal measures of initial care.3-7 The Institute of Medicine has identified timeliness as 1 of the 6 aims for care delivery redesign,19 yet standardized timeliness outcomes do not exist across broad inpatient populations. We chose to monitor the time-to-admission order entry and time-to-antibiotic order entry as 2 indicators of timeliness that would be applicable to a variety of patients. The lack of change in these selected measures should prompt examination for other measures of efficiency, including those that affect nontransferred patients. It is possible that nontransferred patients cared for by the same physician also benefit from fewer delays or disruptions and experience increased efficiency of care if transfer patient communication is improved. Further work is necessary to understand whether other measures of timely initial patient care may be more suitable.
The use of a time-series design to account for temporal trends adds substantial rigor to this study, since the majority of these patients were cared for by house staff whose experience and efficiency vary throughout the academic year. Furthermore, subsequent multivariate analysis demonstrated consistent findings after adjustment for comorbid illness and several other hospital and patient-level confounders.
This study has several limitations. The primary limitation is its nonrandomized design. Patient characteristics were stable across multiple variables before and after implementation, but it is possible that another confounding factor was responsible for observed improvements. Likewise, we collected data for only 6 rather than 12 months during the postintervention time period, which limited our sample size and statistical power. This was chosen because a significant restructuring of resident duty hours occurred in spring 2011 that had the potential to affect all measures studied.20,21 Finally, we did not collect data on accuracy of the information provided in the handover tool or end-user utilization and were unable to account for effects of these.
Since implementation in 2010, this process for interhospital transfers at VUH remains the same, although the volume of incoming transfers has significantly increased. Electronic handover tools with similar structure and content have since been adopted for patients being transferred to the emergency department or directly admitted from clinic. As VUH moves in the coming years from a locally developed electronic medical record to a national vendor, there will be an opportunity to transform this tool into an electronic template that will easily share data between institutions and further enhance communication.
Interhospital transfer patients represent a high-risk population whose unique handover needs have not been adequately measured or addressed. Our investigation demonstrated that a standardized handover aid can be implemented across a broad transfer network and may contribute to reductions in LOS and mortality. Further study is warranted to confirm these findings and assess the effect on other clinical outcomes.
Disclosures
This material is based upon work supported by the Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program, and was made possible by the use of the facilities at VA Tennessee Valley Healthcare System, Nashville, Tennessee. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. Additionally, this publication was supported in part by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
The transfer of inpatients between hospitals for specialized services is common, affecting nearly 10% of all Medicare admissions1 and 4.5% of all critical care hospitalizations.2 At tertiary referral centers, 49% of medical intensive care unit (ICU) admissions are transferred from another hospital.3
Transfer patients have longer length of stay (LOS) than patients admitted directly from the emergency department or clinic. Among patients initially admitted to an ICU, transfer patients spend 1 day to 2.2 more days in the ICU and an additional 2 days to 4 more days total at the receiving hospital.4,5 Furthermore, transfer patients have higher mortality than nontransferred patients by 4% to 8%.3-5 Even after adjustment for case mix and comorbid disease, interhospital transfer is an independent predictor of both ICU death and LOS.6,7 As a result, interhospital transfer has been associated with a $9600 increase (on average) in hospital costs.4
Despite evidence detailing patient handovers as a key time when poor communication can lead to delays in care and significant patient risk, 8-10 most studies have focused on hospital discharge or change of shift, and scant effort has been dedicated to improving the interhospital handover. The process of interhospital transfer is often prolonged and discontinuous,11 commonly including delays of more than 24 hours between initiation and completion. This frequently precludes direct physician-to-physician contact at the time of transfer, and physicians rely on the discharge/transfer summary.12 Yet discharge summaries are frequently absent or incomplete,13 and often lack information for high-risk treatments such as systemic anticoagulation.14 The traditional reliance on discharge summaries for handover communication requires interpretation of unstandardized documentation and increases the risk for miscommunication, delays, and error.
To improve communication, we developed a 1-page handover tool for all inbound adult interhospital transfers to our academic medical center. We sought to determine whether implementation of this standardized handover tool improved the timeliness of initial care, LOS, and mortality among interhospital transfer patients.
METHODS
Study Design, Setting, Population
We conducted a retrospective cohort study of patients transferred into Vanderbilt University Hospital (VUH), an adult 626-bed quaternary care academic medical center in Nashville, Tennessee. The Vanderbilt University Institutional Review Board approved this study.
Population
We selected for inclusion all patients age 18 or older who were transferred into VUH between July 1, 2009 and December 31, 2010. We excluded patients whose transfer was routed outside the main VUH Patient Flow Center as well as patients who did not arrive alive at VUH. We also excluded patients transferred to the emergency department and patients admitted to obstetrics, burn, or trauma services, because these admitting services did not initially use the handover tool. Patients were followed for the duration of their hospitalization at VUH.
Intervention
The 1-page handover tool was developed with multidisciplinary physician input from house staff; medical directors from intensive care, neurology, and surgery; and the chief of staff. The tool was structured on the SBAR model (Situation, Background, Assessment, and Recommendation).15 Fields on the handover tool were limited to those deemed critical for immediate patient care and designed for 1 tool to be used for both ICU and non-ICU transfers. Fields included primary diagnosis; allergies; use and last dose of anticoagulants, vasopressors, sedative/paralytics, and antibiotics; isolation needs; indwelling devices; recent operations/procedures; code status; emergency contact information; problem list; active medication list; vital signs; pertinent exam; imaging; lab findings; and overall reason for transfer.
The handover tool was completed by the physician at the transferring hospital, faxed to VUH, and immediately scanned into the electronic record, allowing the receiving physicians to review information before patient arrival. Use of the tool was piloted first with 2 referring hospitals in April 2010 and universally recommended but not compulsory for all adult patients routed through the main VUH Patient Flow Center beginning July 1, 2010. Immediately before full implementation, the chief of staff sent letters to leadership of the 40 highest volume referral hospitals, highlighting the institutional goal of improving hand-off communication, framing completion of the tool as a step in the transfer acceptance process, and providing contact information for questions, feedback, or concerns. To ensure the tool was a standard part of the transfer process, the VUH Patient Flow Center maintained the responsibility of faxing the form to the outside facility and monitoring its receipt. The tool was processed in the same manner as other faxed patient records and treated as a part of the formal medical record to meet all standards for the Health Insurance Portability and Accountability Act (HIPAA) and medicolegal compliance. The medical center also has a separate cardiac transfer center where the handover tool was not implemented owing to its specialized workflow.
Data Source
The VUH Patient Flow Center maintains a database of all patients for whom transfer to VUH is requested, including information on the requesting hospital and the duration of transfer process. Outcome data and patient characteristics were extracted from the Enterprise Data Warehouse. Data related to comorbid illness were extracted from the Perioperative Data Warehouse, an IRB-approved data registry.
Measures
We evaluated 3 outcomes. First, we defined 2 measures of the timeliness of initial care, the time from arrival at VUH until entry of an admission order, and the time from arrival until entry of the first antibiotic order. Only antibiotics ordered within the first 36 hours of admission were included. Second, we evaluated the total LOS after transfer to VUH and the ICU LOS for patients transferred into an ICU setting. Finally, we examined in-hospital mortality at VUH. These metrics were chosen for their broad applicability across patient groups and feasibility of data capture. Length of stay and mortality also represent final common pathways for avoidance of complications. Specific patient safety indicators and complications were not abstracted due to their low frequency and burden of data collection. Due to system changes in our cost accounting systems, we were not able to obtain cost data pre- and postimplementation that provided meaningful comparisons.
Patient covariates included age, gender, payer, and Elixhauser comorbidity index as modified by van Walraven,16 calculated based on the admission of interest and the previous 365 days. We also examined admission characteristics including location (ICU vs. non-ICU), admitting service (medicine, surgery, neurology, or gynecology), and shift of arrival (day, 7:00 am to 6:00 pm; or night, 6:00 pm to 7:00 pm). Finally, we examined duration of the transfer process (ie, time between transfer request and arrival at VUH) and the volume of the transferring hospital (high was defined as 3 or more transfers to VUH per year).
Statistical analysis
Patient characteristics before and after implementation of the handover tool were compared using Pearson’s chi-square test and Fisher exact test for categorical variables and using Student t test and the Wilcoxon rank sum test for continuous variables. The outcome variables of time to admission order entry, time to antibiotic order entry, LOS, ICU LOS, and in-hospital mortality were compared between the before- and after-intervention time periods, using the Wilcoxon rank sum test for continuous outcomes and Pearson’s chi-square test for in-hospital mortality.
To account for temporal trends, the effect of the handover tool on time-to-admission order entry, hospital LOS, and mortality was measured using an interrupted time-series design with segmented linear regression analysis.17 The study period was divided into 2-week intervals, with 26 time periods in the pre-intervention period and 13 time periods in the postintervention period. Expected rates for the postintervention time periods were projected from the pre-intervention data using a linear regression model. To assess the observed effect of the intervention, rates from the postintervention periods were compared with these projected rates, assuming continuation of the trend. Restricted cubic spline models were also fit for time-to-admission order and hospital LOS; however, the F-statistics for these models were not significant, suggesting the linear regression provided a more appropriate model.
To further account for potential confounding of outcomes by comorbid disease and other patient factors, multivariate linear regression models assessed change in timeliness and LOS with implementation of the intervention. A multivariate logistic regression model was used to assess change in mortality with intervention implementation. All models adjusted for age, gender, payer, comorbid illness, admitting team, shift of arrival (day vs. night), transfer duration, volume of transferring hospital, and ICU status. Outcomes were further adjusted for calendar month to account for temporal trends in house staff efficiency. Because the cardiac transfer center did not adopt the use of the transfer tool, we evaluated adjusted in-hospital mortality for these patients as a concurrent control group.
All statistical testing was 2-sided at a significance level of 0.05. All analyses were conducted using STATA 12.1 statistical software (StataCorp LP, College Station, Texas).
RESULTS
Of 10,325 patients for whom transfer to VUH was requested during the study period, 1715 met inclusion criteria, including 798 patients (46.5%) initially admitted to an ICU setting. Specific patient exclusions are detailed in the Supplemental Figure; the majority of exclusions were due to patients being transferred directly to the emergency department setting. Table 1 summarizes patient characteristics before and after implementation of the handover tool. The median age was 57 years, with 48.6% male patients. Accepting services included medicine (56%), surgery (34%), neurology (9%), and gynecology (1%). The median duration of transfer was 8 hours, and the majority (93%) of patients came from higher volume transferring hospitals. Most (65%) of patients were admitted during night shift. The median modified Elixhauser comorbidity index was 11 (range of possible scores, -19 to 89). A slightly higher proportion of patients admitted postimplementation of the handover tool came from higher volume transferring hospitals; otherwise, there were no significant differences between the pre- and postintervention groups.
Vanderbilt University Hospital received transfers from more than 350 unique facilities in more than 25 U.S. states during the overall study period. During the postintervention period, adherence to the handover process was excellent, with more than 85% of patients having a completed handover tool available in their medical record at the time of transfer. The remaining 15% had either incomplete forms or no form.
Timeliness of Initial Care
There was no change in either the median time-to-admission order entry after implementation (47 vs. 45 minutes, unadjusted P = 0.36) or time to antibiotic order entry (199 vs. 202 minutes; unadjusted P = 0.81; Table 2).
In the time-series analysis, the pre-intervention period did not have a significant temporal trend in median time-to-admission order entry (ß-coefficient = -0.27; 95% confidence interval [CI] -0.85 to 0.31; R2 = 0.04; P = 0.34; Figure 1A). The postintervention period showed a trend toward a reduction in median time-to-admission order entry (ß-coefficient = -1.39; 95% CI -2.92 to 0.15; R2 = 0.27; P = 0.07). There was no significant difference between the actual time-to-admission order entry in the postintervention period when compared to the projected rates from the pre-intervention period (P = 0.18).
After multivariate adjustment, the postintervention time period was not associated with any significant change in the median time-to-admission order entry (P = 0.94, R2 = 0.09) nor time-to-antibiotic order entry (P = 0.91; R2 = 0.08; Table 2).
Length of Stay
Hospital LOS demonstrated a nonstatistically significant decline after implementation of the handover tool from 6.47 days to 5.81 days (unadjusted P = 0.18; Table 2). There was no significant change in ICU LOS postintervention (4.34 days to 4.55 days; P = 0.38).
In time series analysis, hospital LOS did not have a significant temporal trend in either the pre-intervention period (ß-coefficient = 0.00094; 95% CI, -0.07 to 0.07; R2 = 0.00; P = 0.98) or the postintervention period (ß-coefficient = 0.09; 95% CI, -0.07 to 0.25; R2 = 0.13; P = 0.23; Figure 1B). Similarly, there was no significant difference between the actual and projected hospital LOS after implementation of the handover tool (P = 0.31).
After multivariate adjustment, the postintervention time period was associated with a trend toward reduction in overall LOS (P = 0.06; R2 = 0.07) but no significant change in ICU LOS (P = 0.99; R2 = 0.09).
Mortality
In-hospital mortality declined significantly from 12.0% in the pre-intervention period to 8.9% in the postintervention period (P = 0.04; Table 2). In time-series analysis, mortality did not have a significant trend in the pre-intervention period (ß-coefficient = 0.00017, 95% CI, -0.0020 to 0.0024; P = 0.878) and had a trend toward reduction in the postintervention period (ß-coefficient = -0.0032; 95% CI, -0.0091 to 0.0027; P = 0.255; Figure 1C) but did not reach statistical significance due to relatively small numbers of deaths in each individual time period.
After multivariate adjustment, the postintervention period was associated with overall lower odds of mortality among transfer patients when compared with the pre-intervention period (adjusted OR 0.68; 95% CI, 0.47 – 0.99; R2 = 0.21; P = 0.04; Figure 2). Among the concurrent control group of patients routed through the cardiac transfer center, there was no significant change in mortality between the pre- and postintervention periods (adjusted OR 1.31; 95% CI, 0.88 – 1.93; R2 = 0.28; P = 0.18).
DISCUSSION
We developed a simple 1-page handover tool for interhospital transfer patients and aimed to improve timeliness, efficiency, and outcomes of care at the receiving hospital. Implementation of the handover tool was feasible and well accepted by transferring physicians despite a geographically large and diverse transfer network. Although implementation did not substantially improve measures of the timeliness of initial care among transfer patients, we noted a nonsignificant trend toward reduced LOS postintervention.
We observed a substantial and statistically significant reduction in mortality among transfer patients after implementation of the handover tool that persisted after controlling for time trends, comorbid illness, and several other patient factors. This effect was not seen in a concurrent control group of cardiac transfer patients for whom the handover tool was not implemented. Standardizing communication regarding high-risk clinical care processes may be responsible for the observed mortality reduction, similar to improvements seen in other small pilot studies.18 We acknowledge that the magnitude of the improvement in mortality is more than might be expected from the handover tool alone and could be due to chance.
In this initial evaluation, it was not feasible to determine whether information provided in the handover tool helped avert specific complications that could affect mortality, such as complications related to the use of ventilators, high-risk medications, or indwelling devices. Assessment of additional patient safety indices such as code events, unplanned ICU transfers, and medication errors could also help clarify the effect of the handover tool on patient-safety outcomes, and future work should include these metrics as well. Alternately, the improvement in mortality may result from other unmeasured processes that occurred concurrently and verification of this finding should be completed in other settings.
CONCLUSION
More work is needed to determine suitable process and outcome measures for interhospital transfers. Most literature has focused on cost and LOS at the exclusion of more proximal measures of initial care.3-7 The Institute of Medicine has identified timeliness as 1 of the 6 aims for care delivery redesign,19 yet standardized timeliness outcomes do not exist across broad inpatient populations. We chose to monitor the time-to-admission order entry and time-to-antibiotic order entry as 2 indicators of timeliness that would be applicable to a variety of patients. The lack of change in these selected measures should prompt examination for other measures of efficiency, including those that affect nontransferred patients. It is possible that nontransferred patients cared for by the same physician also benefit from fewer delays or disruptions and experience increased efficiency of care if transfer patient communication is improved. Further work is necessary to understand whether other measures of timely initial patient care may be more suitable.
The use of a time-series design to account for temporal trends adds substantial rigor to this study, since the majority of these patients were cared for by house staff whose experience and efficiency vary throughout the academic year. Furthermore, subsequent multivariate analysis demonstrated consistent findings after adjustment for comorbid illness and several other hospital and patient-level confounders.
This study has several limitations. The primary limitation is its nonrandomized design. Patient characteristics were stable across multiple variables before and after implementation, but it is possible that another confounding factor was responsible for observed improvements. Likewise, we collected data for only 6 rather than 12 months during the postintervention time period, which limited our sample size and statistical power. This was chosen because a significant restructuring of resident duty hours occurred in spring 2011 that had the potential to affect all measures studied.20,21 Finally, we did not collect data on accuracy of the information provided in the handover tool or end-user utilization and were unable to account for effects of these.
Since implementation in 2010, this process for interhospital transfers at VUH remains the same, although the volume of incoming transfers has significantly increased. Electronic handover tools with similar structure and content have since been adopted for patients being transferred to the emergency department or directly admitted from clinic. As VUH moves in the coming years from a locally developed electronic medical record to a national vendor, there will be an opportunity to transform this tool into an electronic template that will easily share data between institutions and further enhance communication.
Interhospital transfer patients represent a high-risk population whose unique handover needs have not been adequately measured or addressed. Our investigation demonstrated that a standardized handover aid can be implemented across a broad transfer network and may contribute to reductions in LOS and mortality. Further study is warranted to confirm these findings and assess the effect on other clinical outcomes.
Disclosures
This material is based upon work supported by the Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program, and was made possible by the use of the facilities at VA Tennessee Valley Healthcare System, Nashville, Tennessee. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. Additionally, this publication was supported in part by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
1. Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39:1449-1465. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47:787-793. PubMed
3. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31:1981-1986. PubMed
4. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35:1470-1476. PubMed
5. Flabouris A, Hart GK, George C. Outcomes of patients admitted to tertiary intensive care units after interhospital transfer: comparison with patients admitted from emergency departments. Crit Care Resusc. 2008;10:97-105. PubMed
6. Combes A, Luyt CE, Trouillet JL, Chastre J, Gibert C. Adverse effect on a referral intensive care unit’s performance of accepting patients transferred from another intensive care unit. Crit Care Med. 2005;33:705-710. PubMed
7. Rosenberg AL, Hofer TP, Strachan C, Watts CM, Hayward RA. Accepting critically ill transfer patients: adverse effect on a referral center’s outcome and benchmark measures. A Intern Med. 2003;138:882-890. PubMed
8. Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. Consequences of inadequate sign-out for patient care. Arch Intern Med. 2008;168:1755-1760. PubMed
9. Starmer AJ, Sectish TC, Simon DW, et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310:2262-2270. PubMed
10. Arora VM, Manjarrez E, Dressler DD, Basaviah P, Halasyamani L, Kripalani S. Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4:433-440. PubMed
11. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49:592-598. PubMed
12. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11:413-417. PubMed
13. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007;297:831-841. PubMed
14. Gandara E, Moniz TT, Ungar J, et al. Deficits in discharge documentation in patients transferred to rehabilitation facilities on anticoagulation: results of a systemwide evaluation. Jt Comm J Qual Patient Saf. 2008;34:460-463. PubMed
15. Haig KM, Sutton S, Whittington J. SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32:167-175. PubMed
16. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626-633. PubMed
17. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299-309. PubMed
18. Malpass HC, Enfield KB, Keim-Malpass J, Verghese GM. The interhospital medical intensive care unit transfer instrument facilitates early implementation of critical therapies and is associated with fewer emergent procedures upon arrival. J Intensive Care Med. 2015;30:351-357. PubMed
19. National Academy of Sciences. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. March 2005:1–360. Washington, DC. PubMed
20. Theobald CN, Stover DG, Choma NN, et al. The effect of reducing maximum shift lengths to 16 hours on internal medicine interns’ educational opportunities. Acad Med. 2013;88:512-518. PubMed
21. Choma NN, Vasilevskis EE, Sponsler KC, Hathaway J, Kripalani S. Effect of the ACGME 16-hour rule on efficiency and quality of care: duty hours 2.0. JAMA Intern Med. 2013;173:819-821. PubMed
1. Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39:1449-1465. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47:787-793. PubMed
3. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31:1981-1986. PubMed
4. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35:1470-1476. PubMed
5. Flabouris A, Hart GK, George C. Outcomes of patients admitted to tertiary intensive care units after interhospital transfer: comparison with patients admitted from emergency departments. Crit Care Resusc. 2008;10:97-105. PubMed
6. Combes A, Luyt CE, Trouillet JL, Chastre J, Gibert C. Adverse effect on a referral intensive care unit’s performance of accepting patients transferred from another intensive care unit. Crit Care Med. 2005;33:705-710. PubMed
7. Rosenberg AL, Hofer TP, Strachan C, Watts CM, Hayward RA. Accepting critically ill transfer patients: adverse effect on a referral center’s outcome and benchmark measures. A Intern Med. 2003;138:882-890. PubMed
8. Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. Consequences of inadequate sign-out for patient care. Arch Intern Med. 2008;168:1755-1760. PubMed
9. Starmer AJ, Sectish TC, Simon DW, et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310:2262-2270. PubMed
10. Arora VM, Manjarrez E, Dressler DD, Basaviah P, Halasyamani L, Kripalani S. Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4:433-440. PubMed
11. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49:592-598. PubMed
12. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11:413-417. PubMed
13. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007;297:831-841. PubMed
14. Gandara E, Moniz TT, Ungar J, et al. Deficits in discharge documentation in patients transferred to rehabilitation facilities on anticoagulation: results of a systemwide evaluation. Jt Comm J Qual Patient Saf. 2008;34:460-463. PubMed
15. Haig KM, Sutton S, Whittington J. SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32:167-175. PubMed
16. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626-633. PubMed
17. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299-309. PubMed
18. Malpass HC, Enfield KB, Keim-Malpass J, Verghese GM. The interhospital medical intensive care unit transfer instrument facilitates early implementation of critical therapies and is associated with fewer emergent procedures upon arrival. J Intensive Care Med. 2015;30:351-357. PubMed
19. National Academy of Sciences. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. March 2005:1–360. Washington, DC. PubMed
20. Theobald CN, Stover DG, Choma NN, et al. The effect of reducing maximum shift lengths to 16 hours on internal medicine interns’ educational opportunities. Acad Med. 2013;88:512-518. PubMed
21. Choma NN, Vasilevskis EE, Sponsler KC, Hathaway J, Kripalani S. Effect of the ACGME 16-hour rule on efficiency and quality of care: duty hours 2.0. JAMA Intern Med. 2013;173:819-821. PubMed
© 2017 Society of Hospital Medicine
Hospital Handoffs and Readmissions in Children
Although much has been written about pediatric discharge and readmissions1-5 over the past several years, surprisingly little is known about which care practices are most effective at preventing postdischarge utilization.5 Major collaborations across the U.S. are currently focused on improving pediatric discharge processes,6-8 although the impact that these efforts will have on readmissions remains to be seen.
Research on handoffs between hospitals and primary care has mixed associations with postdischarge utilization. Although some studies observe positive relationships between specific activities and reduced postdischarge utilization,1 others suggest no relationship9-12 or, paradoxically, more utilization.13,14 Brittan et al15 found that outpatient visits were associated with more readmissions when occurring less than 4 days after discharge, but fewer readmissions when occurring 4 days to 29 days after discharge. Most studies, however, investigate single or limited sets of care activities, such as having an outpatient visit,15 timeliness of that visit,16 or receipt of a discharge summary.11 Inclusion of a more comprehensive set of hospital- to primary-care communication practices may better unravel this complex relationship between discharge care and postdischarge outcomes for children.
The purpose of this study was to characterize a set of traditional discharge handoff practices between hospital and primary care providers (PCPs) and to explore their relationships to readmissions. We hypothesized that handoff practices would be associated with fewer unplanned readmissions.
METHODS
Study Design, Setting, Participants
This project was part of a prospective cohort study with 2 aims: to investigate relationships between medical home experience and postdischarge utilization,17 and to identify relationships between common discharge communication practices and postdischarge utilization. This manuscript is focused on the second aim. Randomly selected pediatric patients and their caregivers were enrolled from any medical or surgical service during an acute hospitalization lasting more than 24 hours from October 1, 2012 to January 1, 2014, at a 100-bed tertiary children’s hospital. Patients who transferred to another facility, died, were older than 18 years or in neonatal care (ie, newborn nursery or neonatal intensive care unit) were excluded since their discharge experiences would be significantly distinct from the population of interest. Patients were enrolled once in the study.
Outcome
The study’s primary outcome was 30-day unplanned readmissions, defined as a hospitalization occurring within 30 days of the index (ie, study enrollment) hospitalization, identified through caregiver report or administrative sources.17 Although the study site is a single hospital system, readmissions could have occurred to any hospital reported by caregivers, (ie, readmissions could have occurred within or outside our health system). Readmissions for chemotherapy, radiation, dialysis, rehabilitation, or labor and delivery were excluded. If caregivers reported an admission as planned or chart review of the index discharge summary noted that a rehospitalization was scheduled in the subsequent 30 days, the readmission was labeled “planned” and excluded.
Discharge Handoff Communication
Transitional care is a set of actions designed to ensure continuity and coordination of healthcare during transfer from 1 location or level of care to another.18,19 The study team, comprised of a division chief of general pediatrics, a division chief of hospital medicine, 2 departmental vice-chairs, and the medical director for quality at the study site, identified 11 common handoff activities and reporting sources. These consensus-based activities were expected by the study team to improve continuity and coordination during hospital-to-home transfer, and included:
- verifying PCP identity during the hospitalization (caregiver report);
- notifying the PCP of admission, discharge, and providing updates during the hospitalization (PCP report);
- PCP follow-up appointment set prior to discharge (caregiver report);
- documenting planned PCP and subspecialty follow-up in the discharge summary (chart review);
- completing the discharge summary within 48 hours (chart review);
- providing a verbal or written handoff to the PCP prior to follow-up (PCP report); and
- having a PCP follow-up visit within 30 days of discharge (caregiver report).
We also asked PCPs whether they thought the follow-up interval was appropriate and whether phone follow-up with the patient would have been as appropriate as a face-to-face visit.
Covariates
Patient demographics that might confound the relationship between handoff practices and readmissions based on pediatric research20,21 were included. Medical complexity was accounted for by length-of-index stay, the number of hospitalizations and emergency department (ED) visits in past 12 months, complex chronic conditions,22,23 and seeing 3 or more subspecialists.24,25 Variables from related work included PCP scope (general pediatrics or subspecialist) and presence of a usual source for well and sick care.17
The Care Transitions Measure-3 (CTM-3), originally developed to assess the patient-centeredness of hospital transition,26,27 can discriminate adult patients at risk for readmission.26 We adapted the original CTM-3 to be answered by caregiver respondents after pilot testing with 5 caregivers not enrolled in the study: 1) “The hospital staff took my preferences and those of my family into account in deciding what my child’s health care needs would be when I left the hospital;” 2) “When I left the hospital, I had a good understanding of the things I was responsible for in managing my child’s health;” and 3) “When I left the hospital, I clearly understood the purpose for giving each of my child’s medications.” We analyzed the adapted CTM-3 on a transformed 0-100 scale as designed,26 initially hypothesizing that the CTM-3 would mediate the relationship between handoff practices and readmissions.
We assessed caregiver confidence to avoid a readmission, based on a strong independent association with readmissions described in Coller et al.17 Using questions developed for this study, caregivers were asked to rate “How confident are you that [child’s name] will stay out of the hospital for the next 30 days?” with instructions to refer to unplanned hospital visits only. Responses were reported on a 4-point Likert scale (1 = very confident, 4 = not very confident). Responses were dichotomized into very confident (ie, “1”) or not very confident (ie, “2-4”).
Enrollment and Data Collection
Computer-generated random numbers were assigned to patients admitted the previous day, and families were enrolled sequentially until the daily enrollment target was reached. Data were obtained from 3 sources: medical record, caregiver report, and PCP report. Trained research assistants systematically extracted chart review data documenting the transitions practices above, while a hospital information technology analyst extracted claims and demographic data to complement what was reported by parents and PCPs. After study conclusion, these medical record data were merged with caregiver and PCP-reported data.
Trained bilingual research assistants collected caregiver- and PCP-reported data using structured questionnaires in English or Spanish, according to preference. Timing of data collection differed by data source; caregiver-reported data were collected immediately after discharge and at 30 days postdischarge; PCP-reported data were collected at 30 days postdischarge.
Caregiver-reported data were collected through 2 separate phone calls following index discharge: immediately after discharge (caregiver confidence and CTM-3 measures) and at 30 days (readmission measures). Caregiver confidence questions were asked after (rather than immediately before) discharge to avoid biasing clinical care and revisit risk, consistent with previous work.28
PCP-reported data were collected using structured questionnaires with the PCP who was identified by the family during study enrollment. PCP-reported data were collected by telephone or fax 30 days after discharge, with up to 5 telephone attempts and 3 fax attempts. At the beginning of the questionnaire, PCPs were asked if they agreed with the designation, although they were asked to complete the questionnaire regardless.
Analyses
Descriptive statistics compared differences in handoff practices and 30-day unplanned readmissions. Exploratory factor analysis assessed whether certain handoff practices were sufficiently correlated to allow grouping of items and construction of scales. Relationships between handoff practices and readmissions were examined using bivariate, followed by multivariate, logistic regression adjusting for the covariates described. Collinearity was tested before constructing final models. Because no relationship was observed between CTM-3 and readmissions, additional mediation analyses were not pursued. All analyses were completed using STATA (SE version 14.0, StataCorp LP, College Station, Texas). This study was approved by the Institutional Review Boards at UCLA (study site) and University of Wisconsin (lead author site).
RESULTS
This study enrolled 701 of 816 eligible participants (85.9%) between October 2012 and January 2014. More than 99% of administrative data and 97% of caregiver questionnaires were complete. Of 685 patients with a reported PCP, we obtained responses from 577 PCPs (84.2%). Patient characteristics and outcomes were not significantly different for patients with and without a responding PCP; however, patients of nonresponding PCPs were more often publicly insured (64.5% vs. 48.2% for responding PCPs, P = 0.004) or seen by a subspecialist as opposed to a generalist (28.1% vs. 13.8% for responding PCPs, P = 0.001).
The overall population characteristics are summarized in Table 1: 27.4% of the cohort was younger 2 years, 49.2% were Hispanic, and the majority (51.1%) had public insurance. The average length of the index hospitalization for the overall population was 4.8 days (standard deviation = 9.6), and 53.5% had at least 1 complex chronic condition. Eighty-four percent of the cohort reported using a generalist (vs. subspecialist) for primary care.
Discharge Handoff Communication
Practices varied widely (Figure 1a). Verbal handoffs between hospital-based and PCPs were least common (10.7%), whereas discharge summary completion within 48 hours was most common (84.9%). Of variables measuring direct communication with PCPs, only notification of admission occurred at least half the time (50.8%).
Exploratory factor analysis identified 5 well-correlated items (Cronbach α = 0.77), which were combined and labeled the Hospital and Primary Care Provider Communication scale (Figure 1b). Items included PCP notification of admission, discharge, and receipt of updates during hospitalization, as well as receipt of verbal and written handoffs prior to follow-up. While these 5 items were analyzed only in this scale, other practices were analyzed as independent variables. In this assessment, 42.1% of patients had a scale score of 0 (no items performed), while 5% had all 5 items completed
Readmissions
The 30-day unplanned readmission rate to any hospital was 12.4%. Demographic characteristics were similar in patients with and without an unplanned readmission (Table 1); however, patients with a readmission were more often younger (P = 0.03) and used a subspecialist for primary care (P = 0.03). Fewer than 60% of those with an unplanned readmission had a usual source of sick and well care compared with 77.5% of those without a readmission (P < 0.001). The length of index stay was nearly 4 days longer for those with an unplanned readmission (9.3 days vs. 4.4 days, P < 0.001). These patients also had more hospitalizations or ED visits in the past year (P = 0.002 and P = 0.04, respectively) and saw more subspecialists (P < 0.001).
Frequencies of communication practices between those with and without an unplanned readmission are illustrated in Table 2. Nearly three-quarters of caregivers whose children were readmitted reported having follow-up appointments scheduled before discharge, compared to 48.9% without a readmission (P < 0.001). In 71% of discharges followed by a readmission, caregivers were not very confident about avoiding readmission, vs. 44.8% of discharges with no readmission (P < 0.001).
Readmissions were largely unrelated to handoff practices in multivariate analyses (Table 3). Having a follow-up visit scheduled prior to discharge was the only activity with a statistically significant association; however, it was actually associated with more than double the odds of readmission (adjusted odds ratio 2.20, 95% confidence interval 1.08-4.46).
DISCUSSION
The complex nature of hospital discharge care has led to general optimism that improved handoff processes might reduce readmissions for pediatric patients. Although the current literature linking transition practices to readmissions in pediatrics has mixed results,1,4,5 most studies are fragmented—investigating a single or small number of transitional care activities, such as outpatient follow-up visits, postdischarge caregiver phone calls, or PCP receipt of discharge summaries. Despite finding limited relationships with readmissions, a strength of our study was its inclusion of a more comprehensive set of traditional communication practices that the study team anticipates many primary care and hospital medicine providers would expect to be carried out for most, if not all, patients during the hospital-to-home transition.
Although our study was developed earlier, the variables in our analyses align with each domain of the conceptual model for readmission risk proposed by the Seamless Transitions and Re(admissions) Network (STARNet).6 This model identifies 7 elements believed to directly impact readmission risk in children: hospital and ED utilization, underlying diseases, ability to care for diseases, access to outpatient care, discharge processes, and discharge readiness. For example, our study included ED and hospital visits in the past year, complex chronic conditions, number of subspecialists, caregiver confidence, having a usual source of care, insurance status, and the 11 consensus-based handoff practices identified by our study team. Therefore, although the included handoff practices we included were a limited set, our models provide a relatively comprehensive analysis of readmission risk, confirming caregiver confidence, usual source of care, and hospitalizations to be associated with unplanned readmissions.
With the exception of having scheduled follow-up appointments before discharge – which was associated with more rather than fewer readmissions—the included care practices were not associated with readmissions. We suspect that these findings likely represent selection bias, with hospital providers taking additional steps in communicating with outpatient providers when they are most concerned about a patient’s vulnerability at discharge, eg, due to severity of illness, sociodemographics, health literacy, access to care, or other factors. Such selection bias could have 2 potential effects: (1) creating associations between the performance of certain handoff practices and higher readmission risk (eg, hospital providers are more likely to set follow-up appointments with the sickest patients who are also most likely to be readmitted, or (2) negating weakly effective communication practices that have small effect sizes. The currently mixed literature suggests that if associations between these handoff practices and postdischarge outcomes exist, they are often opposite to our expectation and likely driven by selection bias. If there are real effects that are hidden by this selection bias, they may be weak or inconsistent.
Recent qualitative research highlights the needs and preferences of caregivers of children with chronic or complex conditions to promote their sense of self-efficacy at discharge.29 Such needs include support from within and beyond the health system, comprehensive discharge education, and written instructions, ultimately leading to confidence and comfort in executing the home-management plan. Consistent with our work,17 a strong independent relationship between caregiver confidence and postdischarge outcomes remained even after accounting for these conventional handoff activities.
Transitions research in pediatrics has started only recently to move beyond traditional handoff communication between hospital and outpatient providers. Over the last several years, more ambitious conceptualizations of hospital discharge care have evolved2 and include constructs such as family-centeredness,4,28,29 discharge readiness,30 and social determinants of health.31 Interventions targeting these constructs are largely missing from the literature and are greatly needed. If transitions are to have an effect on downstream utilization, their focus likely needs to evolve to address such areas.
Finally, our study underscores the need to identify relevant outcomes of improved transitional care. Although the preventability of postdischarge utilization continues to be debated, most would agree that this should not detract from the importance of high-quality transitional care. The STARNet collaborative provides some examples of outcomes potentially impacted through improved transitional care,6 although the authors note that reliability, validity, and feasibility of the measures are not well understood. High-quality transitional care presumably would lead to improvements in patient and family experience and perhaps safer care. Although caregiver experience measured by an adapted CTM-3 was neither a mediator nor a predictor of postdischarge utilization for children in our study, use of more rigorously developed tools for pediatric patients32 may provide a better assessment of caregiver experience. Finally, given the well-described risks of poor communication between hospital and outpatient providers,33-35 safety events may be a better outcome of high-quality transitional care than readmissions. Investment in transitional care initiatives would be well justified if the positive patient, provider, and health system impacts can be better demonstrated through improved outcomes.
Future readmissions research should aim to accomplish several goals. Because observational studies will continue to be challenged by the selection biases described above, more rigorously designed and controlled experimental pediatric studies are needed. Family, social, and primary care characteristics should continue to be incorporated into pediatric readmission analyses given their increasingly recognized critical role. These variables, some of which could be modifiable, might represent potential targets for innovative readmission reduction interventions. Recently published conceptual models6,29,36 provide a useful starting framework.
Limitations
Because of the observational study design, we cannot draw conclusions about causal relationships between handoff practices and the measured outcomes. The tertiary care single-center nature of the study limits generalizability. Response biases are possible given that we often could not verify accuracy of PCP and caregiver responses. As noted above, we suspect that handoff practices were driven by important selection bias, not all of which could be controlled by the measured patient and clinical characteristics. The handoff practices included in this study were a limited set primarily focused on communication between hospital providers and PCPs. Therefore, the study does not rule out the possibility that other aspects of transitional care may reduce readmissions. Subsequent work investigating innovative interventions may find reductions in readmissions and other important outcomes. Additionally, not all practices have standardized definitions, eg, what 1 PCP considers a verbal handoff may be different from that of another provider. Although we assessed whether communication occurred, we were not able to assess the content or quality of communication, which may have important implications for its effectiveness.37,38
CONCLUSION
Improvements in handoffs between hospital and PCPs may have an important impact on postdischarge outcomes, but it is not clear that unplanned 30-day readmissions is among them. Efforts to reduce postdischarge utilization, if possible, likely need to focus on broader constructs such as caregiver self-efficacy, discharge readiness, and social determinants of health.
Disclosures
This study was supported by a grant from the Lucile Packard Foundation for Children’s Health, Palo Alto, California, as well as grant R40MC25677 Maternal and Child Health Research Program, Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services. The authors report no financial conflicts of interest.
1. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251-260. PubMed
2. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168:955-962; quiz 965-956. PubMed
3. Snow V, Beck D, Budnitz T, et al, American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement. American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24:971-976. PubMed
4. Desai AD, Popalisky J, Simon TD, Mangione-Smith RM. The effectiveness of family-centered transition processes from hospital settings to home: a review of the literature. Hosp Pediatr. 2015;5:219-231. PubMed
5. Berry JG, Gay JC. Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5:602-604. PubMed
6. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135:164-175. PubMed
7. Value in inpatient pediatrics network projects. American Academy of Pediatrics. Available at: https://www.aap.org/en-us/professional-resources/quality-improvement/Quality-Improvement-Innovation-Networks/Value-in-Inpatient-Pediatrics-Network/Pages/Value-in-Inpatient-Pediatrics-Network.aspx. Accessed May 18, 2015.
8. Ohio Children’s Hospitals. Solutions for patient safety. Available at: http://www.solutionsforpatientsafety.org/about-us/our-goals/. Accessed May 18, 2015.
9. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24:381-386. PubMed
10. Oduyebo I, Lehmann CU, Pollack CE, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173:624-629. PubMed
11. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17:186-192. PubMed
12. Kashiwagi DT, Burton MC, Kirkland LL, Cha S, Varkey P. Do timely outpatient follow-up visits decrease hospital readmission rates? Am J Med Qual. 2012;27:11-15. PubMed
13. Coller RJ, Klitzner TS, Lerner CF, Chung PJ. Predictors of 30-day readmission and association with primary care follow-up plans. J Pediatr. 2013;163:1027-1033. PubMed
14. Feudtner C, Pati S, Goodman DM, et al. State-level child health system performance and the likelihood of readmission to children’s hospitals. J Pediatr. 2010;157:98-102. PubMed
15. Brittan MS, Sills MR, Fox D, et al. Outpatient follow-up visits and readmission in medically complex children enrolled in Medicaid. J Pediatr. 2015;166:998-1005. PubMed
16. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397. PubMed
17. Coller RJ, Klitzner TS, Saenz AA, Lerner CF, Nelson BB, Chung PJ. The medical home and hospital readmissions. Pediatrics. 2015;136:e1550-e1560. PubMed
18. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141:533-536. PubMed
19. Coleman EA, Boult C; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51:556-557. PubMed
20. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305:682-690. PubMed
21. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123:286-293. PubMed
22. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106:205-209. PubMed
23. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. PubMed
24. Berry JG, Agrawal R, Kuo DZ, et al. Characteristics of hospitalizations for patients who use a structured clinical care program for children with medical complexity. J Pediatr. 2011;159:284-290. PubMed
25. Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH. A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med. 2011;165:1020-1026. PubMed
26. Parry C, Mahoney E, Chalmers SA, Coleman EA. Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46:317-322. PubMed
27. Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient’s perspective: the care transitions measure. Med Care. 2005;43:246-255. PubMed
28. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25:573-581. PubMed
29. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16:136-144. PubMed
30. Weiss ME, Bobay KL, Bahr SJ, Costa L, Hughes RG, Holland DE. A model for hospital discharge preparation: from case management to care transition. J Nurs Adm. 2015;45:606-614. PubMed
31. Sills MR, Hall M, Colvin JD, et al. Association of social determinants with children’s hospitals’ preventable readmissions performance. JAMA Pediatr. 2016;170:350-358. PubMed
32. Toomey SL, Zaslavsky AM, Elliott MN, et al. The development of a pediatric inpatient experience of care measure: child HCAHPS. Pediatrics. 2015;136:360-369. PubMed
33. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831-841. PubMed
34. Harlan G, Srivastava R, Harrison L, McBride G, Maloney C. Pediatric hospitalists and primary care providers: a communication needs assessment. J Hosp Med. 2009;4:187-193. PubMed
35. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170:345-349. PubMed
36. Nakamura MM, Toomey SL, Zaslavsky AM, et al. Measuring pediatric hospital readmission rates to drive quality improvement. Acad Pediatr. 2014;14:S39-S46. PubMed
37. Smith K. Effective communication with primary care providers. Pediatr Clin North Am. 2014;61671-679. PubMed
38. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15:61-68. PubMed
Although much has been written about pediatric discharge and readmissions1-5 over the past several years, surprisingly little is known about which care practices are most effective at preventing postdischarge utilization.5 Major collaborations across the U.S. are currently focused on improving pediatric discharge processes,6-8 although the impact that these efforts will have on readmissions remains to be seen.
Research on handoffs between hospitals and primary care has mixed associations with postdischarge utilization. Although some studies observe positive relationships between specific activities and reduced postdischarge utilization,1 others suggest no relationship9-12 or, paradoxically, more utilization.13,14 Brittan et al15 found that outpatient visits were associated with more readmissions when occurring less than 4 days after discharge, but fewer readmissions when occurring 4 days to 29 days after discharge. Most studies, however, investigate single or limited sets of care activities, such as having an outpatient visit,15 timeliness of that visit,16 or receipt of a discharge summary.11 Inclusion of a more comprehensive set of hospital- to primary-care communication practices may better unravel this complex relationship between discharge care and postdischarge outcomes for children.
The purpose of this study was to characterize a set of traditional discharge handoff practices between hospital and primary care providers (PCPs) and to explore their relationships to readmissions. We hypothesized that handoff practices would be associated with fewer unplanned readmissions.
METHODS
Study Design, Setting, Participants
This project was part of a prospective cohort study with 2 aims: to investigate relationships between medical home experience and postdischarge utilization,17 and to identify relationships between common discharge communication practices and postdischarge utilization. This manuscript is focused on the second aim. Randomly selected pediatric patients and their caregivers were enrolled from any medical or surgical service during an acute hospitalization lasting more than 24 hours from October 1, 2012 to January 1, 2014, at a 100-bed tertiary children’s hospital. Patients who transferred to another facility, died, were older than 18 years or in neonatal care (ie, newborn nursery or neonatal intensive care unit) were excluded since their discharge experiences would be significantly distinct from the population of interest. Patients were enrolled once in the study.
Outcome
The study’s primary outcome was 30-day unplanned readmissions, defined as a hospitalization occurring within 30 days of the index (ie, study enrollment) hospitalization, identified through caregiver report or administrative sources.17 Although the study site is a single hospital system, readmissions could have occurred to any hospital reported by caregivers, (ie, readmissions could have occurred within or outside our health system). Readmissions for chemotherapy, radiation, dialysis, rehabilitation, or labor and delivery were excluded. If caregivers reported an admission as planned or chart review of the index discharge summary noted that a rehospitalization was scheduled in the subsequent 30 days, the readmission was labeled “planned” and excluded.
Discharge Handoff Communication
Transitional care is a set of actions designed to ensure continuity and coordination of healthcare during transfer from 1 location or level of care to another.18,19 The study team, comprised of a division chief of general pediatrics, a division chief of hospital medicine, 2 departmental vice-chairs, and the medical director for quality at the study site, identified 11 common handoff activities and reporting sources. These consensus-based activities were expected by the study team to improve continuity and coordination during hospital-to-home transfer, and included:
- verifying PCP identity during the hospitalization (caregiver report);
- notifying the PCP of admission, discharge, and providing updates during the hospitalization (PCP report);
- PCP follow-up appointment set prior to discharge (caregiver report);
- documenting planned PCP and subspecialty follow-up in the discharge summary (chart review);
- completing the discharge summary within 48 hours (chart review);
- providing a verbal or written handoff to the PCP prior to follow-up (PCP report); and
- having a PCP follow-up visit within 30 days of discharge (caregiver report).
We also asked PCPs whether they thought the follow-up interval was appropriate and whether phone follow-up with the patient would have been as appropriate as a face-to-face visit.
Covariates
Patient demographics that might confound the relationship between handoff practices and readmissions based on pediatric research20,21 were included. Medical complexity was accounted for by length-of-index stay, the number of hospitalizations and emergency department (ED) visits in past 12 months, complex chronic conditions,22,23 and seeing 3 or more subspecialists.24,25 Variables from related work included PCP scope (general pediatrics or subspecialist) and presence of a usual source for well and sick care.17
The Care Transitions Measure-3 (CTM-3), originally developed to assess the patient-centeredness of hospital transition,26,27 can discriminate adult patients at risk for readmission.26 We adapted the original CTM-3 to be answered by caregiver respondents after pilot testing with 5 caregivers not enrolled in the study: 1) “The hospital staff took my preferences and those of my family into account in deciding what my child’s health care needs would be when I left the hospital;” 2) “When I left the hospital, I had a good understanding of the things I was responsible for in managing my child’s health;” and 3) “When I left the hospital, I clearly understood the purpose for giving each of my child’s medications.” We analyzed the adapted CTM-3 on a transformed 0-100 scale as designed,26 initially hypothesizing that the CTM-3 would mediate the relationship between handoff practices and readmissions.
We assessed caregiver confidence to avoid a readmission, based on a strong independent association with readmissions described in Coller et al.17 Using questions developed for this study, caregivers were asked to rate “How confident are you that [child’s name] will stay out of the hospital for the next 30 days?” with instructions to refer to unplanned hospital visits only. Responses were reported on a 4-point Likert scale (1 = very confident, 4 = not very confident). Responses were dichotomized into very confident (ie, “1”) or not very confident (ie, “2-4”).
Enrollment and Data Collection
Computer-generated random numbers were assigned to patients admitted the previous day, and families were enrolled sequentially until the daily enrollment target was reached. Data were obtained from 3 sources: medical record, caregiver report, and PCP report. Trained research assistants systematically extracted chart review data documenting the transitions practices above, while a hospital information technology analyst extracted claims and demographic data to complement what was reported by parents and PCPs. After study conclusion, these medical record data were merged with caregiver and PCP-reported data.
Trained bilingual research assistants collected caregiver- and PCP-reported data using structured questionnaires in English or Spanish, according to preference. Timing of data collection differed by data source; caregiver-reported data were collected immediately after discharge and at 30 days postdischarge; PCP-reported data were collected at 30 days postdischarge.
Caregiver-reported data were collected through 2 separate phone calls following index discharge: immediately after discharge (caregiver confidence and CTM-3 measures) and at 30 days (readmission measures). Caregiver confidence questions were asked after (rather than immediately before) discharge to avoid biasing clinical care and revisit risk, consistent with previous work.28
PCP-reported data were collected using structured questionnaires with the PCP who was identified by the family during study enrollment. PCP-reported data were collected by telephone or fax 30 days after discharge, with up to 5 telephone attempts and 3 fax attempts. At the beginning of the questionnaire, PCPs were asked if they agreed with the designation, although they were asked to complete the questionnaire regardless.
Analyses
Descriptive statistics compared differences in handoff practices and 30-day unplanned readmissions. Exploratory factor analysis assessed whether certain handoff practices were sufficiently correlated to allow grouping of items and construction of scales. Relationships between handoff practices and readmissions were examined using bivariate, followed by multivariate, logistic regression adjusting for the covariates described. Collinearity was tested before constructing final models. Because no relationship was observed between CTM-3 and readmissions, additional mediation analyses were not pursued. All analyses were completed using STATA (SE version 14.0, StataCorp LP, College Station, Texas). This study was approved by the Institutional Review Boards at UCLA (study site) and University of Wisconsin (lead author site).
RESULTS
This study enrolled 701 of 816 eligible participants (85.9%) between October 2012 and January 2014. More than 99% of administrative data and 97% of caregiver questionnaires were complete. Of 685 patients with a reported PCP, we obtained responses from 577 PCPs (84.2%). Patient characteristics and outcomes were not significantly different for patients with and without a responding PCP; however, patients of nonresponding PCPs were more often publicly insured (64.5% vs. 48.2% for responding PCPs, P = 0.004) or seen by a subspecialist as opposed to a generalist (28.1% vs. 13.8% for responding PCPs, P = 0.001).
The overall population characteristics are summarized in Table 1: 27.4% of the cohort was younger 2 years, 49.2% were Hispanic, and the majority (51.1%) had public insurance. The average length of the index hospitalization for the overall population was 4.8 days (standard deviation = 9.6), and 53.5% had at least 1 complex chronic condition. Eighty-four percent of the cohort reported using a generalist (vs. subspecialist) for primary care.
Discharge Handoff Communication
Practices varied widely (Figure 1a). Verbal handoffs between hospital-based and PCPs were least common (10.7%), whereas discharge summary completion within 48 hours was most common (84.9%). Of variables measuring direct communication with PCPs, only notification of admission occurred at least half the time (50.8%).
Exploratory factor analysis identified 5 well-correlated items (Cronbach α = 0.77), which were combined and labeled the Hospital and Primary Care Provider Communication scale (Figure 1b). Items included PCP notification of admission, discharge, and receipt of updates during hospitalization, as well as receipt of verbal and written handoffs prior to follow-up. While these 5 items were analyzed only in this scale, other practices were analyzed as independent variables. In this assessment, 42.1% of patients had a scale score of 0 (no items performed), while 5% had all 5 items completed
Readmissions
The 30-day unplanned readmission rate to any hospital was 12.4%. Demographic characteristics were similar in patients with and without an unplanned readmission (Table 1); however, patients with a readmission were more often younger (P = 0.03) and used a subspecialist for primary care (P = 0.03). Fewer than 60% of those with an unplanned readmission had a usual source of sick and well care compared with 77.5% of those without a readmission (P < 0.001). The length of index stay was nearly 4 days longer for those with an unplanned readmission (9.3 days vs. 4.4 days, P < 0.001). These patients also had more hospitalizations or ED visits in the past year (P = 0.002 and P = 0.04, respectively) and saw more subspecialists (P < 0.001).
Frequencies of communication practices between those with and without an unplanned readmission are illustrated in Table 2. Nearly three-quarters of caregivers whose children were readmitted reported having follow-up appointments scheduled before discharge, compared to 48.9% without a readmission (P < 0.001). In 71% of discharges followed by a readmission, caregivers were not very confident about avoiding readmission, vs. 44.8% of discharges with no readmission (P < 0.001).
Readmissions were largely unrelated to handoff practices in multivariate analyses (Table 3). Having a follow-up visit scheduled prior to discharge was the only activity with a statistically significant association; however, it was actually associated with more than double the odds of readmission (adjusted odds ratio 2.20, 95% confidence interval 1.08-4.46).
DISCUSSION
The complex nature of hospital discharge care has led to general optimism that improved handoff processes might reduce readmissions for pediatric patients. Although the current literature linking transition practices to readmissions in pediatrics has mixed results,1,4,5 most studies are fragmented—investigating a single or small number of transitional care activities, such as outpatient follow-up visits, postdischarge caregiver phone calls, or PCP receipt of discharge summaries. Despite finding limited relationships with readmissions, a strength of our study was its inclusion of a more comprehensive set of traditional communication practices that the study team anticipates many primary care and hospital medicine providers would expect to be carried out for most, if not all, patients during the hospital-to-home transition.
Although our study was developed earlier, the variables in our analyses align with each domain of the conceptual model for readmission risk proposed by the Seamless Transitions and Re(admissions) Network (STARNet).6 This model identifies 7 elements believed to directly impact readmission risk in children: hospital and ED utilization, underlying diseases, ability to care for diseases, access to outpatient care, discharge processes, and discharge readiness. For example, our study included ED and hospital visits in the past year, complex chronic conditions, number of subspecialists, caregiver confidence, having a usual source of care, insurance status, and the 11 consensus-based handoff practices identified by our study team. Therefore, although the included handoff practices we included were a limited set, our models provide a relatively comprehensive analysis of readmission risk, confirming caregiver confidence, usual source of care, and hospitalizations to be associated with unplanned readmissions.
With the exception of having scheduled follow-up appointments before discharge – which was associated with more rather than fewer readmissions—the included care practices were not associated with readmissions. We suspect that these findings likely represent selection bias, with hospital providers taking additional steps in communicating with outpatient providers when they are most concerned about a patient’s vulnerability at discharge, eg, due to severity of illness, sociodemographics, health literacy, access to care, or other factors. Such selection bias could have 2 potential effects: (1) creating associations between the performance of certain handoff practices and higher readmission risk (eg, hospital providers are more likely to set follow-up appointments with the sickest patients who are also most likely to be readmitted, or (2) negating weakly effective communication practices that have small effect sizes. The currently mixed literature suggests that if associations between these handoff practices and postdischarge outcomes exist, they are often opposite to our expectation and likely driven by selection bias. If there are real effects that are hidden by this selection bias, they may be weak or inconsistent.
Recent qualitative research highlights the needs and preferences of caregivers of children with chronic or complex conditions to promote their sense of self-efficacy at discharge.29 Such needs include support from within and beyond the health system, comprehensive discharge education, and written instructions, ultimately leading to confidence and comfort in executing the home-management plan. Consistent with our work,17 a strong independent relationship between caregiver confidence and postdischarge outcomes remained even after accounting for these conventional handoff activities.
Transitions research in pediatrics has started only recently to move beyond traditional handoff communication between hospital and outpatient providers. Over the last several years, more ambitious conceptualizations of hospital discharge care have evolved2 and include constructs such as family-centeredness,4,28,29 discharge readiness,30 and social determinants of health.31 Interventions targeting these constructs are largely missing from the literature and are greatly needed. If transitions are to have an effect on downstream utilization, their focus likely needs to evolve to address such areas.
Finally, our study underscores the need to identify relevant outcomes of improved transitional care. Although the preventability of postdischarge utilization continues to be debated, most would agree that this should not detract from the importance of high-quality transitional care. The STARNet collaborative provides some examples of outcomes potentially impacted through improved transitional care,6 although the authors note that reliability, validity, and feasibility of the measures are not well understood. High-quality transitional care presumably would lead to improvements in patient and family experience and perhaps safer care. Although caregiver experience measured by an adapted CTM-3 was neither a mediator nor a predictor of postdischarge utilization for children in our study, use of more rigorously developed tools for pediatric patients32 may provide a better assessment of caregiver experience. Finally, given the well-described risks of poor communication between hospital and outpatient providers,33-35 safety events may be a better outcome of high-quality transitional care than readmissions. Investment in transitional care initiatives would be well justified if the positive patient, provider, and health system impacts can be better demonstrated through improved outcomes.
Future readmissions research should aim to accomplish several goals. Because observational studies will continue to be challenged by the selection biases described above, more rigorously designed and controlled experimental pediatric studies are needed. Family, social, and primary care characteristics should continue to be incorporated into pediatric readmission analyses given their increasingly recognized critical role. These variables, some of which could be modifiable, might represent potential targets for innovative readmission reduction interventions. Recently published conceptual models6,29,36 provide a useful starting framework.
Limitations
Because of the observational study design, we cannot draw conclusions about causal relationships between handoff practices and the measured outcomes. The tertiary care single-center nature of the study limits generalizability. Response biases are possible given that we often could not verify accuracy of PCP and caregiver responses. As noted above, we suspect that handoff practices were driven by important selection bias, not all of which could be controlled by the measured patient and clinical characteristics. The handoff practices included in this study were a limited set primarily focused on communication between hospital providers and PCPs. Therefore, the study does not rule out the possibility that other aspects of transitional care may reduce readmissions. Subsequent work investigating innovative interventions may find reductions in readmissions and other important outcomes. Additionally, not all practices have standardized definitions, eg, what 1 PCP considers a verbal handoff may be different from that of another provider. Although we assessed whether communication occurred, we were not able to assess the content or quality of communication, which may have important implications for its effectiveness.37,38
CONCLUSION
Improvements in handoffs between hospital and PCPs may have an important impact on postdischarge outcomes, but it is not clear that unplanned 30-day readmissions is among them. Efforts to reduce postdischarge utilization, if possible, likely need to focus on broader constructs such as caregiver self-efficacy, discharge readiness, and social determinants of health.
Disclosures
This study was supported by a grant from the Lucile Packard Foundation for Children’s Health, Palo Alto, California, as well as grant R40MC25677 Maternal and Child Health Research Program, Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services. The authors report no financial conflicts of interest.
Although much has been written about pediatric discharge and readmissions1-5 over the past several years, surprisingly little is known about which care practices are most effective at preventing postdischarge utilization.5 Major collaborations across the U.S. are currently focused on improving pediatric discharge processes,6-8 although the impact that these efforts will have on readmissions remains to be seen.
Research on handoffs between hospitals and primary care has mixed associations with postdischarge utilization. Although some studies observe positive relationships between specific activities and reduced postdischarge utilization,1 others suggest no relationship9-12 or, paradoxically, more utilization.13,14 Brittan et al15 found that outpatient visits were associated with more readmissions when occurring less than 4 days after discharge, but fewer readmissions when occurring 4 days to 29 days after discharge. Most studies, however, investigate single or limited sets of care activities, such as having an outpatient visit,15 timeliness of that visit,16 or receipt of a discharge summary.11 Inclusion of a more comprehensive set of hospital- to primary-care communication practices may better unravel this complex relationship between discharge care and postdischarge outcomes for children.
The purpose of this study was to characterize a set of traditional discharge handoff practices between hospital and primary care providers (PCPs) and to explore their relationships to readmissions. We hypothesized that handoff practices would be associated with fewer unplanned readmissions.
METHODS
Study Design, Setting, Participants
This project was part of a prospective cohort study with 2 aims: to investigate relationships between medical home experience and postdischarge utilization,17 and to identify relationships between common discharge communication practices and postdischarge utilization. This manuscript is focused on the second aim. Randomly selected pediatric patients and their caregivers were enrolled from any medical or surgical service during an acute hospitalization lasting more than 24 hours from October 1, 2012 to January 1, 2014, at a 100-bed tertiary children’s hospital. Patients who transferred to another facility, died, were older than 18 years or in neonatal care (ie, newborn nursery or neonatal intensive care unit) were excluded since their discharge experiences would be significantly distinct from the population of interest. Patients were enrolled once in the study.
Outcome
The study’s primary outcome was 30-day unplanned readmissions, defined as a hospitalization occurring within 30 days of the index (ie, study enrollment) hospitalization, identified through caregiver report or administrative sources.17 Although the study site is a single hospital system, readmissions could have occurred to any hospital reported by caregivers, (ie, readmissions could have occurred within or outside our health system). Readmissions for chemotherapy, radiation, dialysis, rehabilitation, or labor and delivery were excluded. If caregivers reported an admission as planned or chart review of the index discharge summary noted that a rehospitalization was scheduled in the subsequent 30 days, the readmission was labeled “planned” and excluded.
Discharge Handoff Communication
Transitional care is a set of actions designed to ensure continuity and coordination of healthcare during transfer from 1 location or level of care to another.18,19 The study team, comprised of a division chief of general pediatrics, a division chief of hospital medicine, 2 departmental vice-chairs, and the medical director for quality at the study site, identified 11 common handoff activities and reporting sources. These consensus-based activities were expected by the study team to improve continuity and coordination during hospital-to-home transfer, and included:
- verifying PCP identity during the hospitalization (caregiver report);
- notifying the PCP of admission, discharge, and providing updates during the hospitalization (PCP report);
- PCP follow-up appointment set prior to discharge (caregiver report);
- documenting planned PCP and subspecialty follow-up in the discharge summary (chart review);
- completing the discharge summary within 48 hours (chart review);
- providing a verbal or written handoff to the PCP prior to follow-up (PCP report); and
- having a PCP follow-up visit within 30 days of discharge (caregiver report).
We also asked PCPs whether they thought the follow-up interval was appropriate and whether phone follow-up with the patient would have been as appropriate as a face-to-face visit.
Covariates
Patient demographics that might confound the relationship between handoff practices and readmissions based on pediatric research20,21 were included. Medical complexity was accounted for by length-of-index stay, the number of hospitalizations and emergency department (ED) visits in past 12 months, complex chronic conditions,22,23 and seeing 3 or more subspecialists.24,25 Variables from related work included PCP scope (general pediatrics or subspecialist) and presence of a usual source for well and sick care.17
The Care Transitions Measure-3 (CTM-3), originally developed to assess the patient-centeredness of hospital transition,26,27 can discriminate adult patients at risk for readmission.26 We adapted the original CTM-3 to be answered by caregiver respondents after pilot testing with 5 caregivers not enrolled in the study: 1) “The hospital staff took my preferences and those of my family into account in deciding what my child’s health care needs would be when I left the hospital;” 2) “When I left the hospital, I had a good understanding of the things I was responsible for in managing my child’s health;” and 3) “When I left the hospital, I clearly understood the purpose for giving each of my child’s medications.” We analyzed the adapted CTM-3 on a transformed 0-100 scale as designed,26 initially hypothesizing that the CTM-3 would mediate the relationship between handoff practices and readmissions.
We assessed caregiver confidence to avoid a readmission, based on a strong independent association with readmissions described in Coller et al.17 Using questions developed for this study, caregivers were asked to rate “How confident are you that [child’s name] will stay out of the hospital for the next 30 days?” with instructions to refer to unplanned hospital visits only. Responses were reported on a 4-point Likert scale (1 = very confident, 4 = not very confident). Responses were dichotomized into very confident (ie, “1”) or not very confident (ie, “2-4”).
Enrollment and Data Collection
Computer-generated random numbers were assigned to patients admitted the previous day, and families were enrolled sequentially until the daily enrollment target was reached. Data were obtained from 3 sources: medical record, caregiver report, and PCP report. Trained research assistants systematically extracted chart review data documenting the transitions practices above, while a hospital information technology analyst extracted claims and demographic data to complement what was reported by parents and PCPs. After study conclusion, these medical record data were merged with caregiver and PCP-reported data.
Trained bilingual research assistants collected caregiver- and PCP-reported data using structured questionnaires in English or Spanish, according to preference. Timing of data collection differed by data source; caregiver-reported data were collected immediately after discharge and at 30 days postdischarge; PCP-reported data were collected at 30 days postdischarge.
Caregiver-reported data were collected through 2 separate phone calls following index discharge: immediately after discharge (caregiver confidence and CTM-3 measures) and at 30 days (readmission measures). Caregiver confidence questions were asked after (rather than immediately before) discharge to avoid biasing clinical care and revisit risk, consistent with previous work.28
PCP-reported data were collected using structured questionnaires with the PCP who was identified by the family during study enrollment. PCP-reported data were collected by telephone or fax 30 days after discharge, with up to 5 telephone attempts and 3 fax attempts. At the beginning of the questionnaire, PCPs were asked if they agreed with the designation, although they were asked to complete the questionnaire regardless.
Analyses
Descriptive statistics compared differences in handoff practices and 30-day unplanned readmissions. Exploratory factor analysis assessed whether certain handoff practices were sufficiently correlated to allow grouping of items and construction of scales. Relationships between handoff practices and readmissions were examined using bivariate, followed by multivariate, logistic regression adjusting for the covariates described. Collinearity was tested before constructing final models. Because no relationship was observed between CTM-3 and readmissions, additional mediation analyses were not pursued. All analyses were completed using STATA (SE version 14.0, StataCorp LP, College Station, Texas). This study was approved by the Institutional Review Boards at UCLA (study site) and University of Wisconsin (lead author site).
RESULTS
This study enrolled 701 of 816 eligible participants (85.9%) between October 2012 and January 2014. More than 99% of administrative data and 97% of caregiver questionnaires were complete. Of 685 patients with a reported PCP, we obtained responses from 577 PCPs (84.2%). Patient characteristics and outcomes were not significantly different for patients with and without a responding PCP; however, patients of nonresponding PCPs were more often publicly insured (64.5% vs. 48.2% for responding PCPs, P = 0.004) or seen by a subspecialist as opposed to a generalist (28.1% vs. 13.8% for responding PCPs, P = 0.001).
The overall population characteristics are summarized in Table 1: 27.4% of the cohort was younger 2 years, 49.2% were Hispanic, and the majority (51.1%) had public insurance. The average length of the index hospitalization for the overall population was 4.8 days (standard deviation = 9.6), and 53.5% had at least 1 complex chronic condition. Eighty-four percent of the cohort reported using a generalist (vs. subspecialist) for primary care.
Discharge Handoff Communication
Practices varied widely (Figure 1a). Verbal handoffs between hospital-based and PCPs were least common (10.7%), whereas discharge summary completion within 48 hours was most common (84.9%). Of variables measuring direct communication with PCPs, only notification of admission occurred at least half the time (50.8%).
Exploratory factor analysis identified 5 well-correlated items (Cronbach α = 0.77), which were combined and labeled the Hospital and Primary Care Provider Communication scale (Figure 1b). Items included PCP notification of admission, discharge, and receipt of updates during hospitalization, as well as receipt of verbal and written handoffs prior to follow-up. While these 5 items were analyzed only in this scale, other practices were analyzed as independent variables. In this assessment, 42.1% of patients had a scale score of 0 (no items performed), while 5% had all 5 items completed
Readmissions
The 30-day unplanned readmission rate to any hospital was 12.4%. Demographic characteristics were similar in patients with and without an unplanned readmission (Table 1); however, patients with a readmission were more often younger (P = 0.03) and used a subspecialist for primary care (P = 0.03). Fewer than 60% of those with an unplanned readmission had a usual source of sick and well care compared with 77.5% of those without a readmission (P < 0.001). The length of index stay was nearly 4 days longer for those with an unplanned readmission (9.3 days vs. 4.4 days, P < 0.001). These patients also had more hospitalizations or ED visits in the past year (P = 0.002 and P = 0.04, respectively) and saw more subspecialists (P < 0.001).
Frequencies of communication practices between those with and without an unplanned readmission are illustrated in Table 2. Nearly three-quarters of caregivers whose children were readmitted reported having follow-up appointments scheduled before discharge, compared to 48.9% without a readmission (P < 0.001). In 71% of discharges followed by a readmission, caregivers were not very confident about avoiding readmission, vs. 44.8% of discharges with no readmission (P < 0.001).
Readmissions were largely unrelated to handoff practices in multivariate analyses (Table 3). Having a follow-up visit scheduled prior to discharge was the only activity with a statistically significant association; however, it was actually associated with more than double the odds of readmission (adjusted odds ratio 2.20, 95% confidence interval 1.08-4.46).
DISCUSSION
The complex nature of hospital discharge care has led to general optimism that improved handoff processes might reduce readmissions for pediatric patients. Although the current literature linking transition practices to readmissions in pediatrics has mixed results,1,4,5 most studies are fragmented—investigating a single or small number of transitional care activities, such as outpatient follow-up visits, postdischarge caregiver phone calls, or PCP receipt of discharge summaries. Despite finding limited relationships with readmissions, a strength of our study was its inclusion of a more comprehensive set of traditional communication practices that the study team anticipates many primary care and hospital medicine providers would expect to be carried out for most, if not all, patients during the hospital-to-home transition.
Although our study was developed earlier, the variables in our analyses align with each domain of the conceptual model for readmission risk proposed by the Seamless Transitions and Re(admissions) Network (STARNet).6 This model identifies 7 elements believed to directly impact readmission risk in children: hospital and ED utilization, underlying diseases, ability to care for diseases, access to outpatient care, discharge processes, and discharge readiness. For example, our study included ED and hospital visits in the past year, complex chronic conditions, number of subspecialists, caregiver confidence, having a usual source of care, insurance status, and the 11 consensus-based handoff practices identified by our study team. Therefore, although the included handoff practices we included were a limited set, our models provide a relatively comprehensive analysis of readmission risk, confirming caregiver confidence, usual source of care, and hospitalizations to be associated with unplanned readmissions.
With the exception of having scheduled follow-up appointments before discharge – which was associated with more rather than fewer readmissions—the included care practices were not associated with readmissions. We suspect that these findings likely represent selection bias, with hospital providers taking additional steps in communicating with outpatient providers when they are most concerned about a patient’s vulnerability at discharge, eg, due to severity of illness, sociodemographics, health literacy, access to care, or other factors. Such selection bias could have 2 potential effects: (1) creating associations between the performance of certain handoff practices and higher readmission risk (eg, hospital providers are more likely to set follow-up appointments with the sickest patients who are also most likely to be readmitted, or (2) negating weakly effective communication practices that have small effect sizes. The currently mixed literature suggests that if associations between these handoff practices and postdischarge outcomes exist, they are often opposite to our expectation and likely driven by selection bias. If there are real effects that are hidden by this selection bias, they may be weak or inconsistent.
Recent qualitative research highlights the needs and preferences of caregivers of children with chronic or complex conditions to promote their sense of self-efficacy at discharge.29 Such needs include support from within and beyond the health system, comprehensive discharge education, and written instructions, ultimately leading to confidence and comfort in executing the home-management plan. Consistent with our work,17 a strong independent relationship between caregiver confidence and postdischarge outcomes remained even after accounting for these conventional handoff activities.
Transitions research in pediatrics has started only recently to move beyond traditional handoff communication between hospital and outpatient providers. Over the last several years, more ambitious conceptualizations of hospital discharge care have evolved2 and include constructs such as family-centeredness,4,28,29 discharge readiness,30 and social determinants of health.31 Interventions targeting these constructs are largely missing from the literature and are greatly needed. If transitions are to have an effect on downstream utilization, their focus likely needs to evolve to address such areas.
Finally, our study underscores the need to identify relevant outcomes of improved transitional care. Although the preventability of postdischarge utilization continues to be debated, most would agree that this should not detract from the importance of high-quality transitional care. The STARNet collaborative provides some examples of outcomes potentially impacted through improved transitional care,6 although the authors note that reliability, validity, and feasibility of the measures are not well understood. High-quality transitional care presumably would lead to improvements in patient and family experience and perhaps safer care. Although caregiver experience measured by an adapted CTM-3 was neither a mediator nor a predictor of postdischarge utilization for children in our study, use of more rigorously developed tools for pediatric patients32 may provide a better assessment of caregiver experience. Finally, given the well-described risks of poor communication between hospital and outpatient providers,33-35 safety events may be a better outcome of high-quality transitional care than readmissions. Investment in transitional care initiatives would be well justified if the positive patient, provider, and health system impacts can be better demonstrated through improved outcomes.
Future readmissions research should aim to accomplish several goals. Because observational studies will continue to be challenged by the selection biases described above, more rigorously designed and controlled experimental pediatric studies are needed. Family, social, and primary care characteristics should continue to be incorporated into pediatric readmission analyses given their increasingly recognized critical role. These variables, some of which could be modifiable, might represent potential targets for innovative readmission reduction interventions. Recently published conceptual models6,29,36 provide a useful starting framework.
Limitations
Because of the observational study design, we cannot draw conclusions about causal relationships between handoff practices and the measured outcomes. The tertiary care single-center nature of the study limits generalizability. Response biases are possible given that we often could not verify accuracy of PCP and caregiver responses. As noted above, we suspect that handoff practices were driven by important selection bias, not all of which could be controlled by the measured patient and clinical characteristics. The handoff practices included in this study were a limited set primarily focused on communication between hospital providers and PCPs. Therefore, the study does not rule out the possibility that other aspects of transitional care may reduce readmissions. Subsequent work investigating innovative interventions may find reductions in readmissions and other important outcomes. Additionally, not all practices have standardized definitions, eg, what 1 PCP considers a verbal handoff may be different from that of another provider. Although we assessed whether communication occurred, we were not able to assess the content or quality of communication, which may have important implications for its effectiveness.37,38
CONCLUSION
Improvements in handoffs between hospital and PCPs may have an important impact on postdischarge outcomes, but it is not clear that unplanned 30-day readmissions is among them. Efforts to reduce postdischarge utilization, if possible, likely need to focus on broader constructs such as caregiver self-efficacy, discharge readiness, and social determinants of health.
Disclosures
This study was supported by a grant from the Lucile Packard Foundation for Children’s Health, Palo Alto, California, as well as grant R40MC25677 Maternal and Child Health Research Program, Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services. The authors report no financial conflicts of interest.
1. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251-260. PubMed
2. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168:955-962; quiz 965-956. PubMed
3. Snow V, Beck D, Budnitz T, et al, American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement. American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24:971-976. PubMed
4. Desai AD, Popalisky J, Simon TD, Mangione-Smith RM. The effectiveness of family-centered transition processes from hospital settings to home: a review of the literature. Hosp Pediatr. 2015;5:219-231. PubMed
5. Berry JG, Gay JC. Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5:602-604. PubMed
6. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135:164-175. PubMed
7. Value in inpatient pediatrics network projects. American Academy of Pediatrics. Available at: https://www.aap.org/en-us/professional-resources/quality-improvement/Quality-Improvement-Innovation-Networks/Value-in-Inpatient-Pediatrics-Network/Pages/Value-in-Inpatient-Pediatrics-Network.aspx. Accessed May 18, 2015.
8. Ohio Children’s Hospitals. Solutions for patient safety. Available at: http://www.solutionsforpatientsafety.org/about-us/our-goals/. Accessed May 18, 2015.
9. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24:381-386. PubMed
10. Oduyebo I, Lehmann CU, Pollack CE, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173:624-629. PubMed
11. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17:186-192. PubMed
12. Kashiwagi DT, Burton MC, Kirkland LL, Cha S, Varkey P. Do timely outpatient follow-up visits decrease hospital readmission rates? Am J Med Qual. 2012;27:11-15. PubMed
13. Coller RJ, Klitzner TS, Lerner CF, Chung PJ. Predictors of 30-day readmission and association with primary care follow-up plans. J Pediatr. 2013;163:1027-1033. PubMed
14. Feudtner C, Pati S, Goodman DM, et al. State-level child health system performance and the likelihood of readmission to children’s hospitals. J Pediatr. 2010;157:98-102. PubMed
15. Brittan MS, Sills MR, Fox D, et al. Outpatient follow-up visits and readmission in medically complex children enrolled in Medicaid. J Pediatr. 2015;166:998-1005. PubMed
16. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397. PubMed
17. Coller RJ, Klitzner TS, Saenz AA, Lerner CF, Nelson BB, Chung PJ. The medical home and hospital readmissions. Pediatrics. 2015;136:e1550-e1560. PubMed
18. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141:533-536. PubMed
19. Coleman EA, Boult C; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51:556-557. PubMed
20. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305:682-690. PubMed
21. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123:286-293. PubMed
22. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106:205-209. PubMed
23. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. PubMed
24. Berry JG, Agrawal R, Kuo DZ, et al. Characteristics of hospitalizations for patients who use a structured clinical care program for children with medical complexity. J Pediatr. 2011;159:284-290. PubMed
25. Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH. A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med. 2011;165:1020-1026. PubMed
26. Parry C, Mahoney E, Chalmers SA, Coleman EA. Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46:317-322. PubMed
27. Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient’s perspective: the care transitions measure. Med Care. 2005;43:246-255. PubMed
28. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25:573-581. PubMed
29. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16:136-144. PubMed
30. Weiss ME, Bobay KL, Bahr SJ, Costa L, Hughes RG, Holland DE. A model for hospital discharge preparation: from case management to care transition. J Nurs Adm. 2015;45:606-614. PubMed
31. Sills MR, Hall M, Colvin JD, et al. Association of social determinants with children’s hospitals’ preventable readmissions performance. JAMA Pediatr. 2016;170:350-358. PubMed
32. Toomey SL, Zaslavsky AM, Elliott MN, et al. The development of a pediatric inpatient experience of care measure: child HCAHPS. Pediatrics. 2015;136:360-369. PubMed
33. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831-841. PubMed
34. Harlan G, Srivastava R, Harrison L, McBride G, Maloney C. Pediatric hospitalists and primary care providers: a communication needs assessment. J Hosp Med. 2009;4:187-193. PubMed
35. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170:345-349. PubMed
36. Nakamura MM, Toomey SL, Zaslavsky AM, et al. Measuring pediatric hospital readmission rates to drive quality improvement. Acad Pediatr. 2014;14:S39-S46. PubMed
37. Smith K. Effective communication with primary care providers. Pediatr Clin North Am. 2014;61671-679. PubMed
38. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15:61-68. PubMed
1. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251-260. PubMed
2. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168:955-962; quiz 965-956. PubMed
3. Snow V, Beck D, Budnitz T, et al, American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement. American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24:971-976. PubMed
4. Desai AD, Popalisky J, Simon TD, Mangione-Smith RM. The effectiveness of family-centered transition processes from hospital settings to home: a review of the literature. Hosp Pediatr. 2015;5:219-231. PubMed
5. Berry JG, Gay JC. Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5:602-604. PubMed
6. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135:164-175. PubMed
7. Value in inpatient pediatrics network projects. American Academy of Pediatrics. Available at: https://www.aap.org/en-us/professional-resources/quality-improvement/Quality-Improvement-Innovation-Networks/Value-in-Inpatient-Pediatrics-Network/Pages/Value-in-Inpatient-Pediatrics-Network.aspx. Accessed May 18, 2015.
8. Ohio Children’s Hospitals. Solutions for patient safety. Available at: http://www.solutionsforpatientsafety.org/about-us/our-goals/. Accessed May 18, 2015.
9. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24:381-386. PubMed
10. Oduyebo I, Lehmann CU, Pollack CE, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173:624-629. PubMed
11. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17:186-192. PubMed
12. Kashiwagi DT, Burton MC, Kirkland LL, Cha S, Varkey P. Do timely outpatient follow-up visits decrease hospital readmission rates? Am J Med Qual. 2012;27:11-15. PubMed
13. Coller RJ, Klitzner TS, Lerner CF, Chung PJ. Predictors of 30-day readmission and association with primary care follow-up plans. J Pediatr. 2013;163:1027-1033. PubMed
14. Feudtner C, Pati S, Goodman DM, et al. State-level child health system performance and the likelihood of readmission to children’s hospitals. J Pediatr. 2010;157:98-102. PubMed
15. Brittan MS, Sills MR, Fox D, et al. Outpatient follow-up visits and readmission in medically complex children enrolled in Medicaid. J Pediatr. 2015;166:998-1005. PubMed
16. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397. PubMed
17. Coller RJ, Klitzner TS, Saenz AA, Lerner CF, Nelson BB, Chung PJ. The medical home and hospital readmissions. Pediatrics. 2015;136:e1550-e1560. PubMed
18. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141:533-536. PubMed
19. Coleman EA, Boult C; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51:556-557. PubMed
20. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305:682-690. PubMed
21. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123:286-293. PubMed
22. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106:205-209. PubMed
23. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. PubMed
24. Berry JG, Agrawal R, Kuo DZ, et al. Characteristics of hospitalizations for patients who use a structured clinical care program for children with medical complexity. J Pediatr. 2011;159:284-290. PubMed
25. Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH. A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med. 2011;165:1020-1026. PubMed
26. Parry C, Mahoney E, Chalmers SA, Coleman EA. Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46:317-322. PubMed
27. Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient’s perspective: the care transitions measure. Med Care. 2005;43:246-255. PubMed
28. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25:573-581. PubMed
29. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16:136-144. PubMed
30. Weiss ME, Bobay KL, Bahr SJ, Costa L, Hughes RG, Holland DE. A model for hospital discharge preparation: from case management to care transition. J Nurs Adm. 2015;45:606-614. PubMed
31. Sills MR, Hall M, Colvin JD, et al. Association of social determinants with children’s hospitals’ preventable readmissions performance. JAMA Pediatr. 2016;170:350-358. PubMed
32. Toomey SL, Zaslavsky AM, Elliott MN, et al. The development of a pediatric inpatient experience of care measure: child HCAHPS. Pediatrics. 2015;136:360-369. PubMed
33. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831-841. PubMed
34. Harlan G, Srivastava R, Harrison L, McBride G, Maloney C. Pediatric hospitalists and primary care providers: a communication needs assessment. J Hosp Med. 2009;4:187-193. PubMed
35. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170:345-349. PubMed
36. Nakamura MM, Toomey SL, Zaslavsky AM, et al. Measuring pediatric hospital readmission rates to drive quality improvement. Acad Pediatr. 2014;14:S39-S46. PubMed
37. Smith K. Effective communication with primary care providers. Pediatr Clin North Am. 2014;61671-679. PubMed
38. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15:61-68. PubMed
© 2017 Society of Hospital Medicine
Basal Cell Carcinoma Arising in Outdoor Workers Versus Indoor Workers: A Retrospective Study
Basal cell carcinoma (BCC) is the most prevalent malignancy in white individuals and its incidence is rapidly increasing. Despite its low mortality rate, BCC can cause severe morbidity and remains a serious health problem with a high economic burden for health care systems. The incidence of BCC is higher in individuals who have red or blonde hair, light eye color, and/or Fitzpatrick skin types I and II. The risk for developing BCC also increases with age, and men are more frequently affected than women.1,2 Although several factors have been implicated in the etiology of this condition, such as exposure to ionizing radiation, trauma, chemical carcinogenesis, immunosuppression, predisposing syndromes, and host factors (eg, traits that affect susceptibility to disease),3-5 exposure to UV radiation is considered to be a major risk factor, with most BCCs presenting in sun-exposed areas of the body (eg, face, neck). Prolongate suberythrodermal UV doses, which do not burn the skin but cause erythema in the histological level, can lead to formation of pyrimidine dimers in the dermal and epidermal tissues and cause DNA mutation with potential carcinogenic effects. Due to a large number of outdoor occupations, it is likely that outdoor workers (OWs) with a history of UV exposure may develop BCCs with different features than those seen in indoor workers (IWs). However, there has been debate about the relevance of occupational UV exposure as a risk factor for BCC development.6,7 The aim of this study was to compare the clinical and histological features of BCCs in OWs versus IWs at a referral hospital in southern Spain.
Methods
Using the electronic pathology records at a referral hospital in southern Spain, we identified medical records between May 1, 2010, and May 1, 2011, of specimens containing the term skin in the specimen box and basal cell carcinoma in the diagnosis box. We excluded patients with a history of or concomitant squamous cell carcinoma. Reexcision of incompletely excised lesions; punch, shave or incisional biopsies; and palliative excisions also were excluded. The specimens were reviewed and classified according to the differentiation pattern of BCC (ie, nodular, superficial, morpheic, micronodular). Basal cell carcinomas with mixed features were classified according to the most predominant subtype.
We also gathered information regarding the patients’ work history (ie, any job held during their lifetime with a minimum duration of 6 months). Patients were asked about the type of work and start/end dates. In patients who performed OW, we evaluated hours per day and months as well as the type of clothing worn (eg, head covering, socks/stockings during work in the summer months).
Each patient was classified as an OW or IW based on his/her stated occupation. The OWs included those who performed all or most of their work (≥6 hours per day for at least 6 months) outdoors in direct sunlight. Most patients in this group included farmers and fishermen. Indoor workers were those who performed most of their work in an indoor environment (eg, shop, factory, office, hospital, library, bank, school, laboratory). Most patients in this group included mechanics and shop assistants. A small group of individuals could not be classified as OWs or IWs and therefore were excluded from the study. Individuals with a history of exposure to ionizing radiation, chemical carcinogenesis, immunosuppression, or predisposing syndromes also were excluded.
We included variables that could be considered independent risk factors for BCC, including age, sex, eye color, natural hair color, Fitzpatrick skin type, history of sunburns, and family history. All data were collected via a personal interview performed by a single dermatologist (H.H-E.) during the follow-up with the patients conducted after obtaining all medical records and contacting eligible patients; none of the patients were lost on follow-up.
The study was approved by the hospital’s ethics committee and written consent was obtained from all recruited patients for analyzing the data acquired and accessing the relevant diagnostic documents (eg, pathology reports).
The cohorts were compared by a χ2 test and Student t test, which were performed using the SPSS software version 15. Statistical significance was determined using α=.05, and all tests were 2-sided.
Results
A total of 308 patients were included in the study, comprising 178 (58%) OWs and 130 (42%) IWs. Table 1 summarizes the characteristics of each cohort with the statistical outcomes.
The mean age (SD) of the OWs was significantly higher than the IWs (75.17 [10.74] vs 69.73 [9.98] years; P<.001). The sex distribution among the 2 cohorts was significantly different (P=.002); the OW group featured a slightly higher proportion of men than women (92 [52%] vs 86 [48%]), whereas women were clearly more prevalent in the IW group than men (85 [65%] vs 45 [35%]).
No significant differences regarding eye color (blue/gray vs brown/black) between the 2 cohorts were found (P>.05). In the same way, the 2 cohorts did not show differences in the natural hair color (red/blonde vs brown/black)(P>.05).
Fitzpatrick skin type II was the most common between both cohorts (82 [46%] OWs and 75 [58%] IWs), but no statistical differences regarding the proportions of each skin type were found (P>.05).
History of sunburns (>2 episodes) was significantly different between the 2 cohorts. The incidence of second-degree sunburns in childhood was higher in IWs (P<.00001), while the incidence of second-degree sunburns in adulthood was higher in OWs (P=.002).
Most OWs had a positive family history of BCC (101 [57%]), while the majority of IWs had a negative family history of BCC (90 [69%]). This difference was statistically significant (P=.03).
Table 2 shows the distribution of anatomic sites of BCCs in OWs and IWs. The nose was the most frequently affected area in OWs (35 cases [20%]), while the cheek was the most common location (23 [18%]) in IWs. Comparison of the frequency of BCC incidence for each anatomic location revealed that only the rate for truncal BCC was significantly different; IWs had a higher incidence of truncal BCCs than OWs (P=.0035). Although the differences between groups were not statistically significant, there was a trend toward a higher incidence of BCCs on the forehead in OW (P=.06).
In both cohorts, the most prevalent histologic subtype was nodular BCC (133 [75%] OWs and 88 [68%] IWs), followed by superficial BCC (17 [10%] OWs and 27 [21%] IWs). The incidence rate of nodular BCCs was statistically different between the 2 cohorts, with OWs showing a higher incidence compared to IWs (P=.024). Regarding the superficial subtype, the opposite was observed: IWs had significantly increased risk compared to OWs (P=.05). There was a trend toward a higher incidence of morpheic BCCs in OWs than IWs, but the difference was not statistically significant (P=.07).
Comment
Skin cancer due to occupational UV exposure is more common than is generally recognized,6,7 but occupational UV exposure as a risk factor for BCC is still an ongoing debate. In this study, we analyzed the different clinical and histological features of BCC in OWs versus IWs.
The geographic area where this study was performed is characterized by a subtropical Mediterranean climate with irregular rainfall; a short, cool to mild winter; and long, dry, hot summers. Summer temperatures usually are hot and regularly exceed 35°C (95°F). UV index (UVI) is a measure of the amount of skin-damaging UV radiation expected to reach the earth’s surface when the sun is highest in the sky (around midday) and ranges from 1 (low risk) to 10 (maximum risk). In southern Spain, the mean UVI is approximately 6 and can reach up to 9 or sometimes 10 in the summer months. Although Fitzpatrick skin types II and III are most common, the elevated UVI indicates that the general population in southern Spain is at a high risk for developing skin cancer.
In our study the mean age of IWs was lower than OWs, which suggests that IWs may develop BCC at a younger age than OWs. This finding is consistent with studies showing that cumulative occupational UV exposure has been associated with development of BCCs in older age groups, while acute intermittent recreational sun exposure, particularly sustained in childhood and adolescence, is linked with BCC in younger patients.6
The role of sex as a risk factor for BCC remains unclear. Some reports show that BCC is more common in men than in women.8-10 In our study, sex distribution was statistically significant (P=.002); there were more women in the IW cohort and more men in the OW cohort. These differences may be explained by cultural and lifestyle patterns, as women who are IWs tend to have office jobs in urban settings and wear modern fashion clothes at work and for recreation. In rural settings, women have agricultural jobs and tend to wear more traditional clothes that offer sun protection.
Positive family history has been suggested to be a constitutional risk factor for BCC development.8,11,12 In our study, we observed that positive family history was more common in OWs, while most IWs had a negative family history. These differences were significant (P=.03), and OWs had a 2.6-fold increased likelihood of having a positive family history of BCC compared to IWs. Cultural and lifestyle patterns may partially explain this finding. In rural settings, workers tend to have the same job as their parents as a traditional way of life and therefore have similar patterns of UV exposure; in urban settings, individuals may have different jobs than their parents and therefore the pattern of UV exposure may be different. However, a genetic predisposition for developing BCC cannot be excluded. In addition, we have to consider that the information on family history of BCC in the patients was self-reported and not validated, which may limit the results.
The difference in history of second-degree sunburn in childhood was significantly higher in IWs than in OWs (P<.00001). The OW group had a significant rate of sunburns in adulthood (P=.002). The relationship between UV radiation and BCC is complex, and the patterns of sun exposure and their occurrence in different periods of lifetime (ie, childhood vs adulthood) remain controversial.13 The overall history of severe sunburns seems to be more important than simply the tendency to burn or tan,14,15 and a history of sunburns in childhood and adolescence has been associated with early-onset BCC.6 Our findings were consistent in that the age of onset of BCCs was lower in IWs who had a history of sunburns in childhood. Basal cell carcinomas developed at older ages in OWs who had a higher incidence of sunburns in adulthood. However, we have to consider that the retrospective nature of the data collection on sunburns in childhood and adulthood was potentially limited, as the information was based on the patients’ memory. Additionally, other non-UV risk factors for BCC, such as ionizing radiation exposure, were not analyzed.
The majority of BCCs developed in sun-exposed areas of the head and neck in both cohorts, and only 35 (20%) and 28 (22%) BCCs were located on the trunk, arms, or legs in OWs and IWs, respectively. In our study, the rate of BCCs on the trunk was significantly lower in OWs than in IWs (P=.0035). Basal cell carcinomas on the trunk have been suggested to be linked to genetic susceptibility16,17 and reduced DNA repair capacity18 rather than sun exposure. Our findings support this hypothesis and suggest that occupational sun exposure has no direct relation with truncal BCC. This outcome is consistent with the result of a case-control study conducted by Pelucchi et al19 (N=1040). The authors concluded that occupational UV exposure was not associated with truncal BCC development but with head/neck BCC, indicating that there may be different etiological mechanisms between truncal and head/neck BCC.19 In the largest BCC case series published in the literature with 13,457 specimens, the authors stated that tumors on the trunk may represent a particular variant of BCC, in which the theory of chronic versus intermittent UV exposure cannot be simply extrapolated as it is for the rest of BCC sites. Other factors such as genetic predisposition could be involved in the development of truncal BCC.20 Similarly, Ramos et al21 suggested that nonmelanoma skin cancers in sun-protected anatomic sites may occur in individuals with impairment in the DNA repair process.
The classification of histological subtypes of BCC helps to predict tumor behavior,22 which can impact the prognosis. In our study, nodular BCC was the most common subtype in both cohorts, followed by superficial BCC. The nodular subtype was increased in OWs compared to IWs, while the superficial subtype was most common in IWs. Bastiaens et al23 and McCormack et al24 have suggested that the most frequent subtypes of BCC (nodular and superficial) may represent different tumors with distinct causal factors. According to these authors, nodular subtypes are associated with cumulative UV exposure, while superficial subtypes are associated with more intense and intermittent UV exposure. The results of the current study support this hypothesis, as the OW cohort with cumulative UV exposure showed more incidence of nodular BCC than IWs, while the patients with intense and intermittent sun exposure (the IWs) showed more risk of superficial BCC.
The importance of occupational UV exposure in OWs as a risk factor for BCC is still an ongoing discussion. Our data show that occupational UV exposure may be considered an etiological factor for BCC according to histological subtype and anatomic site. Our study is limited by the retrospective nature of the data collection regarding occupation and childhood sunburns, which were based on the patients’ memory and therefore potentially biased. Data regarding family history of BCC also was self-reported and not validated. Another limiting factor was that other non-UV risk factors for BCC, such as ionizing radiation exposure, were not considered. The limited sample size also may have impacted the study results. Among the strengths of the study are the complete response rate, the similar catchment area of OWs and IWs, the common hospital setting of the 2 cohorts, and the similar attention to medical history. All patients were obtained from the practice of a single referral dermatologist and are felt to be representative of our working area. The use of a single dermatologist reduces provider-associated variability.
Conclusion
According to the results of this study, OWs are more likely to develop nodular BCCs with no increased risk for superficial BCCs. The age of onset in OWs is older than in IWs. Some anatomical sites such as the trunk are more commonly affected in IWs. Truncal BCCs may have etiological factors other than UV exposure, such as a genetic predisposition. This study is useful to occupational safety representatives and physicians to stimulate the implementation of prevention strategies for this easily preventable malignancy and may encourage further research.
- de Vries E, van de Poll-Franse LV, Louwman WJ, et al. Predictions of skin cancer incidence in the Netherlands up to 2015. Br J Dermatol. 2005;152:481-488.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(suppl 61):1-6.
- Netscher DT, Spira M. Basal cell carcinoma: an overview of tumor biology and treatment. Plast Reconstr Surg. 2004;113:e74-e94.
- Miller SJ. Etiology and pathogenesis of basal cell carcinoma. Clin Dermatol. 1995;13:527-536.
- Dessinioti C, Tzannis K, Sypsa V, et al. Epidemiologic risk factors of basal cell carcinoma development and age at onset in a Southern European population from Greece. Exp Dermatol. 2011;20:622-626.
- Bauer A, Diepgen TL, Schmitt J. Is occupational solar UV-irradiation a relevant risk factor for basal cell carcinoma? a systematic review and meta-analysis of the epidemiologic literature. Br J Dermatol. 2011;165:612-625.
- Tran H, Chen K, Shumack S. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2003;149(suppl 66):50-52.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Stern RS. The mysteries of geographic variability in nonmelanoma skin cancer incidence. Arch Dermatol. 1999;135:843-844.
- Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. An Bras Dermatol. 2011;86:292-305.
- Wong CS, Strange RC, Lear JT. Basal cell carcinoma. Br Med J. 2003;327:794-798.
- Dessinioti C, Antoniou C, Katsambas AD, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Van Dam RM, Huang Z, Rimm EB, et al. Risk factors for basal cell carcinoma of the skin in men: results from the health professionals follow-up study. Am J Epidemiol. 1999;150:459-468.
- Hunter DJ, Colditz GA, Stampfer MJ, et al. Risk factors for basal cell carcinoma in a prospective cohort of women. Ann Epidemiol. 1990;1:13-23.
- Ramachandran S, Fryer AA, Smith A, et al. Cutaneous basal cell carcinomas: distinct host factors are associated with the development of tumors on the trunk and on the head and neck. Cancer. 2001;92:354-358.
- Ramachandran S, Lear JT, Ramsay H, et al. Presentation with multiple cutaneous basal cell carcinomas: association of glutathione S-transferase and cytochrome P450 genotypes with clinical phenotype. Cancer Epidemiol Biomarkers Prev. 1999;8:61-67.
- Wei Q, Matanoski GM, Farmer ER, et al. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci USA. 1993;90:1614-1618.
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study [published online ahead of print Oct 19, 2006]. J Invest Dermatol. 2007;127:935-944.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Ramos J, Villa J, Ruiz A, et al. UV dose determines key characteristics of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:2006-2011.
- Rippey JJ. Why classify basal cell carcinomas? Histopathology. 1998;32:393-398.
- Bastiaens MT, Hoefnagel JJ, Bruijn JA, et al. Differences in age, site distribution and sex between nodular and superficial basal cell carcinomas indicate different type of tumors. J Invest Dermatol. 1998;110:880-884.
- McCormack CJ, Kelly JW, Dorevitch AP. Differences in age and body site distribution of histological subtypes of basal cell carcinoma. a possible indicator of different causes. Arch Dermatol. 1997;133:593-596.
Basal cell carcinoma (BCC) is the most prevalent malignancy in white individuals and its incidence is rapidly increasing. Despite its low mortality rate, BCC can cause severe morbidity and remains a serious health problem with a high economic burden for health care systems. The incidence of BCC is higher in individuals who have red or blonde hair, light eye color, and/or Fitzpatrick skin types I and II. The risk for developing BCC also increases with age, and men are more frequently affected than women.1,2 Although several factors have been implicated in the etiology of this condition, such as exposure to ionizing radiation, trauma, chemical carcinogenesis, immunosuppression, predisposing syndromes, and host factors (eg, traits that affect susceptibility to disease),3-5 exposure to UV radiation is considered to be a major risk factor, with most BCCs presenting in sun-exposed areas of the body (eg, face, neck). Prolongate suberythrodermal UV doses, which do not burn the skin but cause erythema in the histological level, can lead to formation of pyrimidine dimers in the dermal and epidermal tissues and cause DNA mutation with potential carcinogenic effects. Due to a large number of outdoor occupations, it is likely that outdoor workers (OWs) with a history of UV exposure may develop BCCs with different features than those seen in indoor workers (IWs). However, there has been debate about the relevance of occupational UV exposure as a risk factor for BCC development.6,7 The aim of this study was to compare the clinical and histological features of BCCs in OWs versus IWs at a referral hospital in southern Spain.
Methods
Using the electronic pathology records at a referral hospital in southern Spain, we identified medical records between May 1, 2010, and May 1, 2011, of specimens containing the term skin in the specimen box and basal cell carcinoma in the diagnosis box. We excluded patients with a history of or concomitant squamous cell carcinoma. Reexcision of incompletely excised lesions; punch, shave or incisional biopsies; and palliative excisions also were excluded. The specimens were reviewed and classified according to the differentiation pattern of BCC (ie, nodular, superficial, morpheic, micronodular). Basal cell carcinomas with mixed features were classified according to the most predominant subtype.
We also gathered information regarding the patients’ work history (ie, any job held during their lifetime with a minimum duration of 6 months). Patients were asked about the type of work and start/end dates. In patients who performed OW, we evaluated hours per day and months as well as the type of clothing worn (eg, head covering, socks/stockings during work in the summer months).
Each patient was classified as an OW or IW based on his/her stated occupation. The OWs included those who performed all or most of their work (≥6 hours per day for at least 6 months) outdoors in direct sunlight. Most patients in this group included farmers and fishermen. Indoor workers were those who performed most of their work in an indoor environment (eg, shop, factory, office, hospital, library, bank, school, laboratory). Most patients in this group included mechanics and shop assistants. A small group of individuals could not be classified as OWs or IWs and therefore were excluded from the study. Individuals with a history of exposure to ionizing radiation, chemical carcinogenesis, immunosuppression, or predisposing syndromes also were excluded.
We included variables that could be considered independent risk factors for BCC, including age, sex, eye color, natural hair color, Fitzpatrick skin type, history of sunburns, and family history. All data were collected via a personal interview performed by a single dermatologist (H.H-E.) during the follow-up with the patients conducted after obtaining all medical records and contacting eligible patients; none of the patients were lost on follow-up.
The study was approved by the hospital’s ethics committee and written consent was obtained from all recruited patients for analyzing the data acquired and accessing the relevant diagnostic documents (eg, pathology reports).
The cohorts were compared by a χ2 test and Student t test, which were performed using the SPSS software version 15. Statistical significance was determined using α=.05, and all tests were 2-sided.
Results
A total of 308 patients were included in the study, comprising 178 (58%) OWs and 130 (42%) IWs. Table 1 summarizes the characteristics of each cohort with the statistical outcomes.
The mean age (SD) of the OWs was significantly higher than the IWs (75.17 [10.74] vs 69.73 [9.98] years; P<.001). The sex distribution among the 2 cohorts was significantly different (P=.002); the OW group featured a slightly higher proportion of men than women (92 [52%] vs 86 [48%]), whereas women were clearly more prevalent in the IW group than men (85 [65%] vs 45 [35%]).
No significant differences regarding eye color (blue/gray vs brown/black) between the 2 cohorts were found (P>.05). In the same way, the 2 cohorts did not show differences in the natural hair color (red/blonde vs brown/black)(P>.05).
Fitzpatrick skin type II was the most common between both cohorts (82 [46%] OWs and 75 [58%] IWs), but no statistical differences regarding the proportions of each skin type were found (P>.05).
History of sunburns (>2 episodes) was significantly different between the 2 cohorts. The incidence of second-degree sunburns in childhood was higher in IWs (P<.00001), while the incidence of second-degree sunburns in adulthood was higher in OWs (P=.002).
Most OWs had a positive family history of BCC (101 [57%]), while the majority of IWs had a negative family history of BCC (90 [69%]). This difference was statistically significant (P=.03).
Table 2 shows the distribution of anatomic sites of BCCs in OWs and IWs. The nose was the most frequently affected area in OWs (35 cases [20%]), while the cheek was the most common location (23 [18%]) in IWs. Comparison of the frequency of BCC incidence for each anatomic location revealed that only the rate for truncal BCC was significantly different; IWs had a higher incidence of truncal BCCs than OWs (P=.0035). Although the differences between groups were not statistically significant, there was a trend toward a higher incidence of BCCs on the forehead in OW (P=.06).
In both cohorts, the most prevalent histologic subtype was nodular BCC (133 [75%] OWs and 88 [68%] IWs), followed by superficial BCC (17 [10%] OWs and 27 [21%] IWs). The incidence rate of nodular BCCs was statistically different between the 2 cohorts, with OWs showing a higher incidence compared to IWs (P=.024). Regarding the superficial subtype, the opposite was observed: IWs had significantly increased risk compared to OWs (P=.05). There was a trend toward a higher incidence of morpheic BCCs in OWs than IWs, but the difference was not statistically significant (P=.07).
Comment
Skin cancer due to occupational UV exposure is more common than is generally recognized,6,7 but occupational UV exposure as a risk factor for BCC is still an ongoing debate. In this study, we analyzed the different clinical and histological features of BCC in OWs versus IWs.
The geographic area where this study was performed is characterized by a subtropical Mediterranean climate with irregular rainfall; a short, cool to mild winter; and long, dry, hot summers. Summer temperatures usually are hot and regularly exceed 35°C (95°F). UV index (UVI) is a measure of the amount of skin-damaging UV radiation expected to reach the earth’s surface when the sun is highest in the sky (around midday) and ranges from 1 (low risk) to 10 (maximum risk). In southern Spain, the mean UVI is approximately 6 and can reach up to 9 or sometimes 10 in the summer months. Although Fitzpatrick skin types II and III are most common, the elevated UVI indicates that the general population in southern Spain is at a high risk for developing skin cancer.
In our study the mean age of IWs was lower than OWs, which suggests that IWs may develop BCC at a younger age than OWs. This finding is consistent with studies showing that cumulative occupational UV exposure has been associated with development of BCCs in older age groups, while acute intermittent recreational sun exposure, particularly sustained in childhood and adolescence, is linked with BCC in younger patients.6
The role of sex as a risk factor for BCC remains unclear. Some reports show that BCC is more common in men than in women.8-10 In our study, sex distribution was statistically significant (P=.002); there were more women in the IW cohort and more men in the OW cohort. These differences may be explained by cultural and lifestyle patterns, as women who are IWs tend to have office jobs in urban settings and wear modern fashion clothes at work and for recreation. In rural settings, women have agricultural jobs and tend to wear more traditional clothes that offer sun protection.
Positive family history has been suggested to be a constitutional risk factor for BCC development.8,11,12 In our study, we observed that positive family history was more common in OWs, while most IWs had a negative family history. These differences were significant (P=.03), and OWs had a 2.6-fold increased likelihood of having a positive family history of BCC compared to IWs. Cultural and lifestyle patterns may partially explain this finding. In rural settings, workers tend to have the same job as their parents as a traditional way of life and therefore have similar patterns of UV exposure; in urban settings, individuals may have different jobs than their parents and therefore the pattern of UV exposure may be different. However, a genetic predisposition for developing BCC cannot be excluded. In addition, we have to consider that the information on family history of BCC in the patients was self-reported and not validated, which may limit the results.
The difference in history of second-degree sunburn in childhood was significantly higher in IWs than in OWs (P<.00001). The OW group had a significant rate of sunburns in adulthood (P=.002). The relationship between UV radiation and BCC is complex, and the patterns of sun exposure and their occurrence in different periods of lifetime (ie, childhood vs adulthood) remain controversial.13 The overall history of severe sunburns seems to be more important than simply the tendency to burn or tan,14,15 and a history of sunburns in childhood and adolescence has been associated with early-onset BCC.6 Our findings were consistent in that the age of onset of BCCs was lower in IWs who had a history of sunburns in childhood. Basal cell carcinomas developed at older ages in OWs who had a higher incidence of sunburns in adulthood. However, we have to consider that the retrospective nature of the data collection on sunburns in childhood and adulthood was potentially limited, as the information was based on the patients’ memory. Additionally, other non-UV risk factors for BCC, such as ionizing radiation exposure, were not analyzed.
The majority of BCCs developed in sun-exposed areas of the head and neck in both cohorts, and only 35 (20%) and 28 (22%) BCCs were located on the trunk, arms, or legs in OWs and IWs, respectively. In our study, the rate of BCCs on the trunk was significantly lower in OWs than in IWs (P=.0035). Basal cell carcinomas on the trunk have been suggested to be linked to genetic susceptibility16,17 and reduced DNA repair capacity18 rather than sun exposure. Our findings support this hypothesis and suggest that occupational sun exposure has no direct relation with truncal BCC. This outcome is consistent with the result of a case-control study conducted by Pelucchi et al19 (N=1040). The authors concluded that occupational UV exposure was not associated with truncal BCC development but with head/neck BCC, indicating that there may be different etiological mechanisms between truncal and head/neck BCC.19 In the largest BCC case series published in the literature with 13,457 specimens, the authors stated that tumors on the trunk may represent a particular variant of BCC, in which the theory of chronic versus intermittent UV exposure cannot be simply extrapolated as it is for the rest of BCC sites. Other factors such as genetic predisposition could be involved in the development of truncal BCC.20 Similarly, Ramos et al21 suggested that nonmelanoma skin cancers in sun-protected anatomic sites may occur in individuals with impairment in the DNA repair process.
The classification of histological subtypes of BCC helps to predict tumor behavior,22 which can impact the prognosis. In our study, nodular BCC was the most common subtype in both cohorts, followed by superficial BCC. The nodular subtype was increased in OWs compared to IWs, while the superficial subtype was most common in IWs. Bastiaens et al23 and McCormack et al24 have suggested that the most frequent subtypes of BCC (nodular and superficial) may represent different tumors with distinct causal factors. According to these authors, nodular subtypes are associated with cumulative UV exposure, while superficial subtypes are associated with more intense and intermittent UV exposure. The results of the current study support this hypothesis, as the OW cohort with cumulative UV exposure showed more incidence of nodular BCC than IWs, while the patients with intense and intermittent sun exposure (the IWs) showed more risk of superficial BCC.
The importance of occupational UV exposure in OWs as a risk factor for BCC is still an ongoing discussion. Our data show that occupational UV exposure may be considered an etiological factor for BCC according to histological subtype and anatomic site. Our study is limited by the retrospective nature of the data collection regarding occupation and childhood sunburns, which were based on the patients’ memory and therefore potentially biased. Data regarding family history of BCC also was self-reported and not validated. Another limiting factor was that other non-UV risk factors for BCC, such as ionizing radiation exposure, were not considered. The limited sample size also may have impacted the study results. Among the strengths of the study are the complete response rate, the similar catchment area of OWs and IWs, the common hospital setting of the 2 cohorts, and the similar attention to medical history. All patients were obtained from the practice of a single referral dermatologist and are felt to be representative of our working area. The use of a single dermatologist reduces provider-associated variability.
Conclusion
According to the results of this study, OWs are more likely to develop nodular BCCs with no increased risk for superficial BCCs. The age of onset in OWs is older than in IWs. Some anatomical sites such as the trunk are more commonly affected in IWs. Truncal BCCs may have etiological factors other than UV exposure, such as a genetic predisposition. This study is useful to occupational safety representatives and physicians to stimulate the implementation of prevention strategies for this easily preventable malignancy and may encourage further research.
Basal cell carcinoma (BCC) is the most prevalent malignancy in white individuals and its incidence is rapidly increasing. Despite its low mortality rate, BCC can cause severe morbidity and remains a serious health problem with a high economic burden for health care systems. The incidence of BCC is higher in individuals who have red or blonde hair, light eye color, and/or Fitzpatrick skin types I and II. The risk for developing BCC also increases with age, and men are more frequently affected than women.1,2 Although several factors have been implicated in the etiology of this condition, such as exposure to ionizing radiation, trauma, chemical carcinogenesis, immunosuppression, predisposing syndromes, and host factors (eg, traits that affect susceptibility to disease),3-5 exposure to UV radiation is considered to be a major risk factor, with most BCCs presenting in sun-exposed areas of the body (eg, face, neck). Prolongate suberythrodermal UV doses, which do not burn the skin but cause erythema in the histological level, can lead to formation of pyrimidine dimers in the dermal and epidermal tissues and cause DNA mutation with potential carcinogenic effects. Due to a large number of outdoor occupations, it is likely that outdoor workers (OWs) with a history of UV exposure may develop BCCs with different features than those seen in indoor workers (IWs). However, there has been debate about the relevance of occupational UV exposure as a risk factor for BCC development.6,7 The aim of this study was to compare the clinical and histological features of BCCs in OWs versus IWs at a referral hospital in southern Spain.
Methods
Using the electronic pathology records at a referral hospital in southern Spain, we identified medical records between May 1, 2010, and May 1, 2011, of specimens containing the term skin in the specimen box and basal cell carcinoma in the diagnosis box. We excluded patients with a history of or concomitant squamous cell carcinoma. Reexcision of incompletely excised lesions; punch, shave or incisional biopsies; and palliative excisions also were excluded. The specimens were reviewed and classified according to the differentiation pattern of BCC (ie, nodular, superficial, morpheic, micronodular). Basal cell carcinomas with mixed features were classified according to the most predominant subtype.
We also gathered information regarding the patients’ work history (ie, any job held during their lifetime with a minimum duration of 6 months). Patients were asked about the type of work and start/end dates. In patients who performed OW, we evaluated hours per day and months as well as the type of clothing worn (eg, head covering, socks/stockings during work in the summer months).
Each patient was classified as an OW or IW based on his/her stated occupation. The OWs included those who performed all or most of their work (≥6 hours per day for at least 6 months) outdoors in direct sunlight. Most patients in this group included farmers and fishermen. Indoor workers were those who performed most of their work in an indoor environment (eg, shop, factory, office, hospital, library, bank, school, laboratory). Most patients in this group included mechanics and shop assistants. A small group of individuals could not be classified as OWs or IWs and therefore were excluded from the study. Individuals with a history of exposure to ionizing radiation, chemical carcinogenesis, immunosuppression, or predisposing syndromes also were excluded.
We included variables that could be considered independent risk factors for BCC, including age, sex, eye color, natural hair color, Fitzpatrick skin type, history of sunburns, and family history. All data were collected via a personal interview performed by a single dermatologist (H.H-E.) during the follow-up with the patients conducted after obtaining all medical records and contacting eligible patients; none of the patients were lost on follow-up.
The study was approved by the hospital’s ethics committee and written consent was obtained from all recruited patients for analyzing the data acquired and accessing the relevant diagnostic documents (eg, pathology reports).
The cohorts were compared by a χ2 test and Student t test, which were performed using the SPSS software version 15. Statistical significance was determined using α=.05, and all tests were 2-sided.
Results
A total of 308 patients were included in the study, comprising 178 (58%) OWs and 130 (42%) IWs. Table 1 summarizes the characteristics of each cohort with the statistical outcomes.
The mean age (SD) of the OWs was significantly higher than the IWs (75.17 [10.74] vs 69.73 [9.98] years; P<.001). The sex distribution among the 2 cohorts was significantly different (P=.002); the OW group featured a slightly higher proportion of men than women (92 [52%] vs 86 [48%]), whereas women were clearly more prevalent in the IW group than men (85 [65%] vs 45 [35%]).
No significant differences regarding eye color (blue/gray vs brown/black) between the 2 cohorts were found (P>.05). In the same way, the 2 cohorts did not show differences in the natural hair color (red/blonde vs brown/black)(P>.05).
Fitzpatrick skin type II was the most common between both cohorts (82 [46%] OWs and 75 [58%] IWs), but no statistical differences regarding the proportions of each skin type were found (P>.05).
History of sunburns (>2 episodes) was significantly different between the 2 cohorts. The incidence of second-degree sunburns in childhood was higher in IWs (P<.00001), while the incidence of second-degree sunburns in adulthood was higher in OWs (P=.002).
Most OWs had a positive family history of BCC (101 [57%]), while the majority of IWs had a negative family history of BCC (90 [69%]). This difference was statistically significant (P=.03).
Table 2 shows the distribution of anatomic sites of BCCs in OWs and IWs. The nose was the most frequently affected area in OWs (35 cases [20%]), while the cheek was the most common location (23 [18%]) in IWs. Comparison of the frequency of BCC incidence for each anatomic location revealed that only the rate for truncal BCC was significantly different; IWs had a higher incidence of truncal BCCs than OWs (P=.0035). Although the differences between groups were not statistically significant, there was a trend toward a higher incidence of BCCs on the forehead in OW (P=.06).
In both cohorts, the most prevalent histologic subtype was nodular BCC (133 [75%] OWs and 88 [68%] IWs), followed by superficial BCC (17 [10%] OWs and 27 [21%] IWs). The incidence rate of nodular BCCs was statistically different between the 2 cohorts, with OWs showing a higher incidence compared to IWs (P=.024). Regarding the superficial subtype, the opposite was observed: IWs had significantly increased risk compared to OWs (P=.05). There was a trend toward a higher incidence of morpheic BCCs in OWs than IWs, but the difference was not statistically significant (P=.07).
Comment
Skin cancer due to occupational UV exposure is more common than is generally recognized,6,7 but occupational UV exposure as a risk factor for BCC is still an ongoing debate. In this study, we analyzed the different clinical and histological features of BCC in OWs versus IWs.
The geographic area where this study was performed is characterized by a subtropical Mediterranean climate with irregular rainfall; a short, cool to mild winter; and long, dry, hot summers. Summer temperatures usually are hot and regularly exceed 35°C (95°F). UV index (UVI) is a measure of the amount of skin-damaging UV radiation expected to reach the earth’s surface when the sun is highest in the sky (around midday) and ranges from 1 (low risk) to 10 (maximum risk). In southern Spain, the mean UVI is approximately 6 and can reach up to 9 or sometimes 10 in the summer months. Although Fitzpatrick skin types II and III are most common, the elevated UVI indicates that the general population in southern Spain is at a high risk for developing skin cancer.
In our study the mean age of IWs was lower than OWs, which suggests that IWs may develop BCC at a younger age than OWs. This finding is consistent with studies showing that cumulative occupational UV exposure has been associated with development of BCCs in older age groups, while acute intermittent recreational sun exposure, particularly sustained in childhood and adolescence, is linked with BCC in younger patients.6
The role of sex as a risk factor for BCC remains unclear. Some reports show that BCC is more common in men than in women.8-10 In our study, sex distribution was statistically significant (P=.002); there were more women in the IW cohort and more men in the OW cohort. These differences may be explained by cultural and lifestyle patterns, as women who are IWs tend to have office jobs in urban settings and wear modern fashion clothes at work and for recreation. In rural settings, women have agricultural jobs and tend to wear more traditional clothes that offer sun protection.
Positive family history has been suggested to be a constitutional risk factor for BCC development.8,11,12 In our study, we observed that positive family history was more common in OWs, while most IWs had a negative family history. These differences were significant (P=.03), and OWs had a 2.6-fold increased likelihood of having a positive family history of BCC compared to IWs. Cultural and lifestyle patterns may partially explain this finding. In rural settings, workers tend to have the same job as their parents as a traditional way of life and therefore have similar patterns of UV exposure; in urban settings, individuals may have different jobs than their parents and therefore the pattern of UV exposure may be different. However, a genetic predisposition for developing BCC cannot be excluded. In addition, we have to consider that the information on family history of BCC in the patients was self-reported and not validated, which may limit the results.
The difference in history of second-degree sunburn in childhood was significantly higher in IWs than in OWs (P<.00001). The OW group had a significant rate of sunburns in adulthood (P=.002). The relationship between UV radiation and BCC is complex, and the patterns of sun exposure and their occurrence in different periods of lifetime (ie, childhood vs adulthood) remain controversial.13 The overall history of severe sunburns seems to be more important than simply the tendency to burn or tan,14,15 and a history of sunburns in childhood and adolescence has been associated with early-onset BCC.6 Our findings were consistent in that the age of onset of BCCs was lower in IWs who had a history of sunburns in childhood. Basal cell carcinomas developed at older ages in OWs who had a higher incidence of sunburns in adulthood. However, we have to consider that the retrospective nature of the data collection on sunburns in childhood and adulthood was potentially limited, as the information was based on the patients’ memory. Additionally, other non-UV risk factors for BCC, such as ionizing radiation exposure, were not analyzed.
The majority of BCCs developed in sun-exposed areas of the head and neck in both cohorts, and only 35 (20%) and 28 (22%) BCCs were located on the trunk, arms, or legs in OWs and IWs, respectively. In our study, the rate of BCCs on the trunk was significantly lower in OWs than in IWs (P=.0035). Basal cell carcinomas on the trunk have been suggested to be linked to genetic susceptibility16,17 and reduced DNA repair capacity18 rather than sun exposure. Our findings support this hypothesis and suggest that occupational sun exposure has no direct relation with truncal BCC. This outcome is consistent with the result of a case-control study conducted by Pelucchi et al19 (N=1040). The authors concluded that occupational UV exposure was not associated with truncal BCC development but with head/neck BCC, indicating that there may be different etiological mechanisms between truncal and head/neck BCC.19 In the largest BCC case series published in the literature with 13,457 specimens, the authors stated that tumors on the trunk may represent a particular variant of BCC, in which the theory of chronic versus intermittent UV exposure cannot be simply extrapolated as it is for the rest of BCC sites. Other factors such as genetic predisposition could be involved in the development of truncal BCC.20 Similarly, Ramos et al21 suggested that nonmelanoma skin cancers in sun-protected anatomic sites may occur in individuals with impairment in the DNA repair process.
The classification of histological subtypes of BCC helps to predict tumor behavior,22 which can impact the prognosis. In our study, nodular BCC was the most common subtype in both cohorts, followed by superficial BCC. The nodular subtype was increased in OWs compared to IWs, while the superficial subtype was most common in IWs. Bastiaens et al23 and McCormack et al24 have suggested that the most frequent subtypes of BCC (nodular and superficial) may represent different tumors with distinct causal factors. According to these authors, nodular subtypes are associated with cumulative UV exposure, while superficial subtypes are associated with more intense and intermittent UV exposure. The results of the current study support this hypothesis, as the OW cohort with cumulative UV exposure showed more incidence of nodular BCC than IWs, while the patients with intense and intermittent sun exposure (the IWs) showed more risk of superficial BCC.
The importance of occupational UV exposure in OWs as a risk factor for BCC is still an ongoing discussion. Our data show that occupational UV exposure may be considered an etiological factor for BCC according to histological subtype and anatomic site. Our study is limited by the retrospective nature of the data collection regarding occupation and childhood sunburns, which were based on the patients’ memory and therefore potentially biased. Data regarding family history of BCC also was self-reported and not validated. Another limiting factor was that other non-UV risk factors for BCC, such as ionizing radiation exposure, were not considered. The limited sample size also may have impacted the study results. Among the strengths of the study are the complete response rate, the similar catchment area of OWs and IWs, the common hospital setting of the 2 cohorts, and the similar attention to medical history. All patients were obtained from the practice of a single referral dermatologist and are felt to be representative of our working area. The use of a single dermatologist reduces provider-associated variability.
Conclusion
According to the results of this study, OWs are more likely to develop nodular BCCs with no increased risk for superficial BCCs. The age of onset in OWs is older than in IWs. Some anatomical sites such as the trunk are more commonly affected in IWs. Truncal BCCs may have etiological factors other than UV exposure, such as a genetic predisposition. This study is useful to occupational safety representatives and physicians to stimulate the implementation of prevention strategies for this easily preventable malignancy and may encourage further research.
- de Vries E, van de Poll-Franse LV, Louwman WJ, et al. Predictions of skin cancer incidence in the Netherlands up to 2015. Br J Dermatol. 2005;152:481-488.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(suppl 61):1-6.
- Netscher DT, Spira M. Basal cell carcinoma: an overview of tumor biology and treatment. Plast Reconstr Surg. 2004;113:e74-e94.
- Miller SJ. Etiology and pathogenesis of basal cell carcinoma. Clin Dermatol. 1995;13:527-536.
- Dessinioti C, Tzannis K, Sypsa V, et al. Epidemiologic risk factors of basal cell carcinoma development and age at onset in a Southern European population from Greece. Exp Dermatol. 2011;20:622-626.
- Bauer A, Diepgen TL, Schmitt J. Is occupational solar UV-irradiation a relevant risk factor for basal cell carcinoma? a systematic review and meta-analysis of the epidemiologic literature. Br J Dermatol. 2011;165:612-625.
- Tran H, Chen K, Shumack S. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2003;149(suppl 66):50-52.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Stern RS. The mysteries of geographic variability in nonmelanoma skin cancer incidence. Arch Dermatol. 1999;135:843-844.
- Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. An Bras Dermatol. 2011;86:292-305.
- Wong CS, Strange RC, Lear JT. Basal cell carcinoma. Br Med J. 2003;327:794-798.
- Dessinioti C, Antoniou C, Katsambas AD, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Van Dam RM, Huang Z, Rimm EB, et al. Risk factors for basal cell carcinoma of the skin in men: results from the health professionals follow-up study. Am J Epidemiol. 1999;150:459-468.
- Hunter DJ, Colditz GA, Stampfer MJ, et al. Risk factors for basal cell carcinoma in a prospective cohort of women. Ann Epidemiol. 1990;1:13-23.
- Ramachandran S, Fryer AA, Smith A, et al. Cutaneous basal cell carcinomas: distinct host factors are associated with the development of tumors on the trunk and on the head and neck. Cancer. 2001;92:354-358.
- Ramachandran S, Lear JT, Ramsay H, et al. Presentation with multiple cutaneous basal cell carcinomas: association of glutathione S-transferase and cytochrome P450 genotypes with clinical phenotype. Cancer Epidemiol Biomarkers Prev. 1999;8:61-67.
- Wei Q, Matanoski GM, Farmer ER, et al. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci USA. 1993;90:1614-1618.
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study [published online ahead of print Oct 19, 2006]. J Invest Dermatol. 2007;127:935-944.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Ramos J, Villa J, Ruiz A, et al. UV dose determines key characteristics of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:2006-2011.
- Rippey JJ. Why classify basal cell carcinomas? Histopathology. 1998;32:393-398.
- Bastiaens MT, Hoefnagel JJ, Bruijn JA, et al. Differences in age, site distribution and sex between nodular and superficial basal cell carcinomas indicate different type of tumors. J Invest Dermatol. 1998;110:880-884.
- McCormack CJ, Kelly JW, Dorevitch AP. Differences in age and body site distribution of histological subtypes of basal cell carcinoma. a possible indicator of different causes. Arch Dermatol. 1997;133:593-596.
- de Vries E, van de Poll-Franse LV, Louwman WJ, et al. Predictions of skin cancer incidence in the Netherlands up to 2015. Br J Dermatol. 2005;152:481-488.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(suppl 61):1-6.
- Netscher DT, Spira M. Basal cell carcinoma: an overview of tumor biology and treatment. Plast Reconstr Surg. 2004;113:e74-e94.
- Miller SJ. Etiology and pathogenesis of basal cell carcinoma. Clin Dermatol. 1995;13:527-536.
- Dessinioti C, Tzannis K, Sypsa V, et al. Epidemiologic risk factors of basal cell carcinoma development and age at onset in a Southern European population from Greece. Exp Dermatol. 2011;20:622-626.
- Bauer A, Diepgen TL, Schmitt J. Is occupational solar UV-irradiation a relevant risk factor for basal cell carcinoma? a systematic review and meta-analysis of the epidemiologic literature. Br J Dermatol. 2011;165:612-625.
- Tran H, Chen K, Shumack S. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2003;149(suppl 66):50-52.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Stern RS. The mysteries of geographic variability in nonmelanoma skin cancer incidence. Arch Dermatol. 1999;135:843-844.
- Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. An Bras Dermatol. 2011;86:292-305.
- Wong CS, Strange RC, Lear JT. Basal cell carcinoma. Br Med J. 2003;327:794-798.
- Dessinioti C, Antoniou C, Katsambas AD, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Van Dam RM, Huang Z, Rimm EB, et al. Risk factors for basal cell carcinoma of the skin in men: results from the health professionals follow-up study. Am J Epidemiol. 1999;150:459-468.
- Hunter DJ, Colditz GA, Stampfer MJ, et al. Risk factors for basal cell carcinoma in a prospective cohort of women. Ann Epidemiol. 1990;1:13-23.
- Ramachandran S, Fryer AA, Smith A, et al. Cutaneous basal cell carcinomas: distinct host factors are associated with the development of tumors on the trunk and on the head and neck. Cancer. 2001;92:354-358.
- Ramachandran S, Lear JT, Ramsay H, et al. Presentation with multiple cutaneous basal cell carcinomas: association of glutathione S-transferase and cytochrome P450 genotypes with clinical phenotype. Cancer Epidemiol Biomarkers Prev. 1999;8:61-67.
- Wei Q, Matanoski GM, Farmer ER, et al. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci USA. 1993;90:1614-1618.
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study [published online ahead of print Oct 19, 2006]. J Invest Dermatol. 2007;127:935-944.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Ramos J, Villa J, Ruiz A, et al. UV dose determines key characteristics of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:2006-2011.
- Rippey JJ. Why classify basal cell carcinomas? Histopathology. 1998;32:393-398.
- Bastiaens MT, Hoefnagel JJ, Bruijn JA, et al. Differences in age, site distribution and sex between nodular and superficial basal cell carcinomas indicate different type of tumors. J Invest Dermatol. 1998;110:880-884.
- McCormack CJ, Kelly JW, Dorevitch AP. Differences in age and body site distribution of histological subtypes of basal cell carcinoma. a possible indicator of different causes. Arch Dermatol. 1997;133:593-596.
Practice Points
- Basal cell carcinoma (BCC) is the most common cancer in white individuals with rapidly increasing incidence rates and a high economic burden.
- Despite a large number of epidemiologic studies and the known importance of UV exposure in BCC carcinogenesis, there are no clear conclusions regarding the role of chronic and acute sun exposure related to BCC subtypes.
- It is reasonable to assume that outdoor workers with a history of UV exposure may develop BCCs with different features than those observed in indoor workers.
How in-office and ambulatory BP monitoring compare: A systematic review and meta-analysis
ABSTRACT
Purpose We performed a literature review and meta-analysis to ascertain the validity of office blood pressure (BP) measurement in a primary care setting, using ambulatory blood pressure measurement (ABPM) as a benchmark in the monitoring of hypertensive patients receiving treatment.
Methods We conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting. We compared the mean office BP with readings obtained by ABPM. We summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LR), with a 95% confidence interval (CI).
Results Only 12 studies met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements. Five were suitable for calculating sensitivity, specificity, and likelihood ratios, and 4 contained sufficient extractable data for meta-analysis. Compared with ABPM (thresholds of 140/90 mm Hg for office BP; 130/80 mmHg for ABPM) in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 81.9% (95% CI, 74.8%-87%) and specificity of 41.1% (95% CI, 35.1%-48.4%). Positive LR was 1.35 (95% CI, 1.32-1.38), and the negative LR was 0.44 (95% CI, 0.37-0.53).
Conclusion Likelihood ratios show that isolated BP measurement in the office does not confirm or rule out the presence of poor BP control. Likelihood of underestimating or overestimating BP control is high when relying on in-office BP measurement alone.
A growing body of evidence supports more frequent use of ambulatory blood pressure monitoring (ABPM) to confirm a diagnosis of hypertension1 and to monitor blood pressure (BP) response to treatment.2 The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure has long accepted ABPM for diagnosis of hypertension,3 and many clinicians consider ABPM the reference standard for diagnosing true hypertension and for accurately assessing associated cardiovascular risk in adults, regardless of office BP readings.4 The US Preventive Services Task Force (USPSTF) recommends obtaining BP measurements outside the clinical setting to confirm a diagnosis of hypertension before starting treatment.5 The USPSTF also asserts that elevated 24-hour ambulatory systolic BP is consistently and significantly associated with stroke and other cardiovascular events independent of office BP readings and has greater predictive value than office monitoring.5 The USPSTF concludes that ABPM, because of its large evidence base, is the best confirmatory test for hypertension.6 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7
The challenge. Despite the considerable support for ABPM, this method of BP measurement is still not sufficiently integrated into primary care. And some guidelines, such as those of
But ABPM’s advantages are numerous. Ambulatory monitors, which can record BP for 24 hours, are typically programmed to take readings every 15 to 30 minutes, providing estimates of mean daytime and nighttime BP and revealing an individual’s circadian pattern of BP.8-10 Ambulatory BP values usually considered the uppermost limit of normal are 135/85 mm Hg (day), 120/70 mm Hg (night), and 130/80 mm Hg (24 hour).8
Office BP monitoring, usually performed manually by medical staff, has 2 main drawbacks: the well-known white-coat effect experienced by many patients, and the relatively small number of possible measurements. A more reliable in-office BP estimation of BP would require repeated measurements at each of several visits.
By comparing ABPM and office measurements, 4 clinical findings are possible: isolated clinic or office (white-coat) hypertension (ICH); isolated ambulatory (masked) hypertension (IAH); consistent normotension; or sustained hypertension. With ICH, BP is high in the office and normal with ABPM. With IAH, BP is normal in the office and high with ABPM. With consistent normotension and sustained hypertension, BP readings with both types of measurement agree.8,9
In patients being treated for hypertension, ICH leads to an overestimation of uncontrolled BP and may result in overtreatment. The cardiovascular risk, although controversial, is usually lower than in patients diagnosed with sustained hypertension.11 IAH leads to an underestimation of uncontrolled BP and may result in undertreatment; its associated cardiovascular risk is similar to that of sustained hypertension.12
Our research objective. We recently published a study conducted with 137 hypertensive patients in a primary care center.13 Our conclusion was that in-office measurement of BP had insufficient clinical validity to be recommended as a sole method of monitoring BP control. In accurately classifying BP as controlled or uncontrolled, clinic measurement agreed with 24h-ABPM in just 64.2% of cases.13
In our present study, we performed a literature review and meta-analysis to ascertain the validity of office BP measurement in a primary care setting, using ABPM as a benchmark in the monitoring of hypertensive patients receiving treatment.
METHODS
Most published studies comparing conventional office BP measurement with ABPM have been conducted with patients not taking antihypertensive medication. We excluded these studies and conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting.
We searched Medline (from 1950 onward) and the Cochrane Database of Systematic Reviews. For the Medline search, we combined keywords for office BP, hypertension, and ambulatory BP with keywords for outpatient setting and primary care, using the following syntax: (((“clinic blood pressure” OR “office blood pressure” OR “casual blood pressure”))) AND (“hypertension” AND ((((“24-h ambulatory blood pressure”) OR “24 h ambulatory blood pressure”) OR “24 hour ambulatory blood pressure”) OR “blood pressure monitoring, ambulatory”[Mesh]) AND ((((((“outpatient setting”) OR “primary care”) OR “family care”) OR “family physician”) OR “family practice”) OR “general practice”)). We chose studies published in English and reviewed the titles and abstracts of identified articles.
With the aim of identifying additional candidate studies, we reviewed the reference lists of eligible primary studies, narrative reviews, and systematic reviews. The studies were generally of good quality and used appropriate statistical methods. Only primary studies qualified for meta-analysis.
Inclusion and exclusion criteria
Acceptable studies had to be conducted in a primary care setting with patients being treated for hypertension, and had to provide data comparing office BP measurement with ABPM. We excluded studies in which participants were treated in the hospital, were untreated, or had not been diagnosed with hypertension.
The quality of the studies included in the meta-analysis was judged by 2 independent observers according to the following criteria: the clear classification and initial comparison of both measurements; explicit and defined diagnostic criteria; compliance with the inclusion/exclusion criteria; and clear and precise definition of outcome variables.
Data extraction
We extracted the following data from each included study: study population, number of patients included, age, gender distribution, number of measurements (ambulatory and office BP), equipment validation, mean office and ambulatory BP, and the period of ambulatory BP measurement. We included adult patients of all ages, and we compared the mean office BP with those obtained by ABPM in hypertensive patients.
STATISTICAL ANALYSIS
For each study, we summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LRs), with the 95% confidence interval (CI), if available. If these rates were not directly reported in the original papers, we used the published data to calculate them.
We used the R v2.15.1 software with the “mada” package for meta-analysis.14 Although a bivariate approach is preferred for the meta-analysis of diagnostic accuracy, it cannot be recommended if the number of primary studies to pool is too small,14 as happened in our case. Therefore, we used a univariate approach and pooled summary statistics for positive LR, negative LR, and the diagnostic odds ratio (DOR) with their 95% confidence intervals. We used the DerSimonian-Laird method to perform a random-effect meta-analysis. To explore heterogeneity between the studies, we used the Cochran’s Q heterogeneity test, I2 index, and Galbraith and L’Abbé plots.
RESULTS
Our search identified 237 studies, only 12 of which met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements (TABLES 1 AND 2).15-26 Of these 12 studies, 5 were suitable for calculating sensitivity, specificity, and LR (TABLE 3),16,18,22,24,26 and 4 contained sufficient extractable data for meta-analysis. The study by Little et al18 was not included in the meta-analysis, as the number of true-positive, true-negative, false-positive, and false-negative results could not be deduced from published data.
The studies differed in sample size (40-31,530), patient ages (mean, 55-72.8 years), sex (percentage of men, 31%-52.9%), and number of measurements for office BP (1-9) and ABPM (32-96) (TABLE 1),15-26 as well as in daytime and nighttime periods for ABPM and BP thresholds, and in differences between the mean office and ambulatory BPs (TABLE 2).15-26
In general, the mean office BP measurements were higher than those obtained with ABPM in any period—from 5/0 mm Hg to 27.4/10.1 mm Hg in the day, and from 7.9/6.3 mm Hg to 31.2/13.7 mm Hg over 24 hours (TABLE 2).15-26
Compared with ABPM in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 55.7% to 91.2% and a specificity of 25.8% to 61.8% (depending on whether the measure was carried out by the doctor or nurse18); positive LR ranged from 1.2 to 1.4, and negative LR from 0.3 to 0.72 (TABLE 3).16,18,22,24,26
For meta-analysis, we pooled studies with the same thresholds (140/90 mm Hg for office BP; 130/80 mm Hg for ABPM), with diagnostic accuracy of office BP expressed as pooled positive and negative LR, and as pooled DOR. The meta-analysis revealed that the pooled positive LR was 1.35 (95% CI, 1.32-1.38), and the pooled negative LR was 0.44 (95% CI, 0.37-0.53). The pooled DOR was 3.47 (95% CI, 3.02-3.98). Sensitivity was 81.9% (95% CI, 74.8%-87%) and specificity was 41.1% (95% CI, 35.1%-48.4%).
One study16 had a slightly different ambulatory diagnostic threshold (133/78 mm Hg), so we excluded it from a second meta-analysis. Results after the exclusion did not change significantly: positive LR was 1.39 (95% CI, 1.34-1.45); negative LR was 0.38 (95% CI, 0.33-0.44); and DOR was 3.77 (95% CI, 3.31-4.43).
In conclusion, the use of office-based BP readings in the outpatient clinic does not correlate well with ABPM. Therefore, caution must be used when making management decisions based solely on in-office readings of BP.
DISCUSSION
The European Society of Hypertension still regards office BP measurement as the gold standard in screening for, diagnosing, and managing hypertension. As previously mentioned, though, office measurements are usually handled by medical staff and can be compromised by the white-coat effect and a small number of measurements. The USPSTF now considers ABPM the reference standard in primary care to diagnose hypertension in adults, to corroborate or contradict office-based determinations of elevated BP (whether based on single or repeated-interval measurements), and to avoid overtreatment of individuals displaying elevated office BP yet proven normotensive by ABPM.4,7 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7 Therefore, evidence supports ABPM as the reference standard for confirming elevated office BP screening results to avoid misdiagnosis and overtreatment of individuals with isolated clinic hypertension.7
How office measurements stack up against ABPM
Checking the validity of decisions in clinical practice is extremely important for patient management. One of the tools used for decision-making is an estimate of the LR. We used the LR to assess the value of office BP measurement in determining controlled or uncontrolled BP. A high LR (eg, >10) indicates that the office BP can be used to rule in the disease (uncontrolled BP) with a high probability, while a low LR (eg, <0.1) could rule it out. An LR of around one indicates that the office BP measurement cannot rule the diagnosis of uncontrolled BP in or out.27 In our meta-analysis, the positive LR is 1.35 and negative LR is 0.44. Therefore, in treated hypertensive patients, an indication of uncontrolled BP as measured in the clinic does not confirm a diagnosis of uncontrolled BP (as judged by the reference standard of ABPM). On the other hand, the negative LR means that normal office BP does not rule out uncontrolled BP, which may be detected with ABPM. Consequently, the measurement of BP in the office does not change the degree of (un)certainty of adequate control of BP. This knowledge is important, to avoid overtreatment of white coat hypertension and undertreatment of masked cases.
As previously mentioned, we reported similar results in a study designed to determine the validity of office BP measurement in a primary care setting compared with ABPM.13 In that paper, the level of agreement between both methods was poor, indicating that clinic measurements could not be recommended as a single method of BP control in hypertensive patients.
The use of ABPM in diagnosing hypertension is likely to increase as a consequence of some guideline updates.2 Our study emphasizes the importance of their use in the control of hypertensive patients.
Another published meta-analysis1 investigated the validity of office BP for the diagnosis of hypertension in untreated patients, with diagnostic thresholds for arterial hypertension set at 140/90 mm Hg for office measurement, and 135/85 mm Hg for ABPM. In that paper, the sensitivity of office BP was 74.6% (95% CI, 60.7-84.8) and the specificity was 74.6% (95% CI, 47.9-90.4).
In our present study carried out with hypertensive patients receiving treatment, we obtained a slightly higher sensitivity value of 81.9% (within the CI of this meta-analysis) and a lower specificity of 41.1%. Therefore, the discordance between office BP and ABPM seems to be similar for the diagnosis of hypertension and the classification of hypertension as being well or poorly controlled. This confirms the low validity of the office BP, both for diagnosis and monitoring of hypertensive patients.
Strengths of our study. The study focused on (treated) hypertensive patients in a primary care setting, where hypertension is most often managed. It confirms that ABPM is indispensable to a good clinical practice.
Limitations of our study are those inherent to meta-analyses. The main weakness of our study is the paucity of data available regarding the utility of ABPM for monitoring BP control with treatment in a primary care setting. Other limitations are the variability in BP thresholds used, the number of measurements performed, and the ambulatory BP devices used. These differences could contribute to the observed heterogeneity.
Application of our results must take into account that we included only those studies performed in a primary care setting with treated hypertensive patients.
Moreover, this study was not designed to evaluate the consequences of over- and undertreatment of blood pressure, nor to address the accuracy of automated blood pressure machines or newer health and fitness devices.
Implications for practice, policy, or future research. Alternative monitoring methods are home BP self-measurement and automated 30-minute clinic BP measurement.28 However, ABPM provides us with unique information about the BP pattern (dipping or non-dipping), BP variability, and mean nighttime BP. This paper establishes that the measurement of BP in the office is not an accurate method to monitor BP control. ABPM should be incorporated in usual clinical practice in primary care. Although the consequences of ambulatory monitoring are not the focus of this study, we acknowledge that the decision to incorporate ABPM in clinical practice depends on the availability of ambulatory devices, proper training of health care workers, and a cost-effectiveness analysis of its use.
CORRESPONDENCE
Sergio Reino-González, MD, PhD, Adormideras Primary Health Center, Poligono de Adormideras s/n. 15002 A Coruña, Spain; [email protected].
1. Hodgkinson J, Mant J, Martin U, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621.
2. National Institute for Health and Clinical Excellence. Hypertension in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/CG127. Accessed November 15, 2016.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
4. Hermida RC, Smolensky MH, Ayala DE, et al. Ambulatory Blood Pressure Monitoring (ABPM) as the reference standard for diagnosis of hypertension and assessment of vascular risk in adults. Chronobiol Int. 2015;32:1329-1342.
5. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
6. Piper MA, Evans CV
7. American Academy of Family Physicians. Hypertension. Available at: www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 10, 2016.
8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2013;23:3-16.
9. Marin R, de la Sierra A, Armario P, et al. 2005 Spanish guidelines in diagnosis and treatment of arterial hypertension. Medicina Clínica. 2005;125:24-34.
10. Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55-61.
11. Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation. 2001;104:1385-1392.
12. Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21:969-975.
13. Reino-González S, Pita-Fernández S, Cibiriain-Sola M, et al. Validity of clinic blood pressure compared to ambulatory monitoring in hypertensive patients in a primary care setting. Blood Press. 2015;24:111-118.
14. Doebler P, Holling H. Meta-analysis of diagnostic accuracy with mada. Available at: https://cran.r-project.org/web/packages/mada/vignettes/mada.pdf. Accessed October 5, 2015.
15. Myers MG, Oh PI, Reeves RA, et al. Prevalence of white coat effect in treated hypertensive patients in the community. Am J Hypertens. 1995;8:591-597.
16. Imai Y, Tsuji I, Nagai K, et al. Ambulatory blood pressure monitoring in evaluating the prevalence of hypertension in adults in Ohasama, a rural Japanese community. Hypertens Res. 1996;19:207-212.
17. Taylor RS, Stockman J, Kernick D, et al. Ambulatory blood pressure monitoring for hypertension in general practice. J R Soc Med. 1998;91:301-304.
18. Little P, Barnett J, Barnsley L, et al. Comparison of agreement between different measures of blood pressure in primary care and daytime ambulatory blood pressure. BMJ. 2002;325:254.
19. Bur A, Herkner H, Vlcek M, et al. Classification of blood pressure levels by ambulatory blood pressure in hypertension. Hypertension. 2002;40:817-822.
20. Lindbaek M, Sandvik E, Liodden K, et al. Predictors for the white coat effect in general practice patients with suspected and treated hypertension. Br J Gen Pract. 2003;53:790-793.
21. Martínez MA, Sancho T, García P, et al. Home blood pressure in poorly controlled hypertension: relationship with ambulatory blood pressure and organ damage. Blood Press Monit. 2006;11:207-213.
22. Sierra BC, de la Sierra IA, Sobrino J, et al. Monitorización ambulatoria de la presión arterial (MAPA): características clínicas de 31.530 pacientes. Medicina Clínica. 2007;129:1-5.
23. Gómez MA, García L, Sánchez Á, et al. Agreement and disagreement between different methods of measuring blood pressure. Hipertensión (Madr). 2008;25:231-239.
24. Banegas JR, Segura J, De la Sierra A, et al. Gender differences in office and ambulatory control of hypertension. Am J Med. 2008;121:1078-1084.
25. Zaninelli A, Parati G, Cricelli C, et al. Office and 24-h ambulatory blood pressure control by treatment in general practice: the ‘Monitoraggio della pressione ARteriosa nella medicina TErritoriale’ study. J Hypertens. 2010;28:910-917.
26. Llisterri JL, Morillas P, Pallarés V, et al. Differences in the degree of control of arterial hypertension according to the measurement procedure of blood pressure in patients ≥ 65 years. FAPRES study. Rev Clin Esp. 2011;211:76-84.
27. Straus SE, Richardson WS, Glasziou P, et al. Evidence-Based Medicine: How to practice and teach it. 4th ed. Edinburgh, Scotland: Churchill Livingstone; 2010.
28. Van der Wel MC, Buunk IE, van Weel C, et al. A novel approach to office blood pressure measurement: 30-minute office blood pressure vs daytime ambulatory blood pressure. Ann Fam Med. 2011;9:128-135.
ABSTRACT
Purpose We performed a literature review and meta-analysis to ascertain the validity of office blood pressure (BP) measurement in a primary care setting, using ambulatory blood pressure measurement (ABPM) as a benchmark in the monitoring of hypertensive patients receiving treatment.
Methods We conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting. We compared the mean office BP with readings obtained by ABPM. We summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LR), with a 95% confidence interval (CI).
Results Only 12 studies met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements. Five were suitable for calculating sensitivity, specificity, and likelihood ratios, and 4 contained sufficient extractable data for meta-analysis. Compared with ABPM (thresholds of 140/90 mm Hg for office BP; 130/80 mmHg for ABPM) in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 81.9% (95% CI, 74.8%-87%) and specificity of 41.1% (95% CI, 35.1%-48.4%). Positive LR was 1.35 (95% CI, 1.32-1.38), and the negative LR was 0.44 (95% CI, 0.37-0.53).
Conclusion Likelihood ratios show that isolated BP measurement in the office does not confirm or rule out the presence of poor BP control. Likelihood of underestimating or overestimating BP control is high when relying on in-office BP measurement alone.
A growing body of evidence supports more frequent use of ambulatory blood pressure monitoring (ABPM) to confirm a diagnosis of hypertension1 and to monitor blood pressure (BP) response to treatment.2 The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure has long accepted ABPM for diagnosis of hypertension,3 and many clinicians consider ABPM the reference standard for diagnosing true hypertension and for accurately assessing associated cardiovascular risk in adults, regardless of office BP readings.4 The US Preventive Services Task Force (USPSTF) recommends obtaining BP measurements outside the clinical setting to confirm a diagnosis of hypertension before starting treatment.5 The USPSTF also asserts that elevated 24-hour ambulatory systolic BP is consistently and significantly associated with stroke and other cardiovascular events independent of office BP readings and has greater predictive value than office monitoring.5 The USPSTF concludes that ABPM, because of its large evidence base, is the best confirmatory test for hypertension.6 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7
The challenge. Despite the considerable support for ABPM, this method of BP measurement is still not sufficiently integrated into primary care. And some guidelines, such as those of
But ABPM’s advantages are numerous. Ambulatory monitors, which can record BP for 24 hours, are typically programmed to take readings every 15 to 30 minutes, providing estimates of mean daytime and nighttime BP and revealing an individual’s circadian pattern of BP.8-10 Ambulatory BP values usually considered the uppermost limit of normal are 135/85 mm Hg (day), 120/70 mm Hg (night), and 130/80 mm Hg (24 hour).8
Office BP monitoring, usually performed manually by medical staff, has 2 main drawbacks: the well-known white-coat effect experienced by many patients, and the relatively small number of possible measurements. A more reliable in-office BP estimation of BP would require repeated measurements at each of several visits.
By comparing ABPM and office measurements, 4 clinical findings are possible: isolated clinic or office (white-coat) hypertension (ICH); isolated ambulatory (masked) hypertension (IAH); consistent normotension; or sustained hypertension. With ICH, BP is high in the office and normal with ABPM. With IAH, BP is normal in the office and high with ABPM. With consistent normotension and sustained hypertension, BP readings with both types of measurement agree.8,9
In patients being treated for hypertension, ICH leads to an overestimation of uncontrolled BP and may result in overtreatment. The cardiovascular risk, although controversial, is usually lower than in patients diagnosed with sustained hypertension.11 IAH leads to an underestimation of uncontrolled BP and may result in undertreatment; its associated cardiovascular risk is similar to that of sustained hypertension.12
Our research objective. We recently published a study conducted with 137 hypertensive patients in a primary care center.13 Our conclusion was that in-office measurement of BP had insufficient clinical validity to be recommended as a sole method of monitoring BP control. In accurately classifying BP as controlled or uncontrolled, clinic measurement agreed with 24h-ABPM in just 64.2% of cases.13
In our present study, we performed a literature review and meta-analysis to ascertain the validity of office BP measurement in a primary care setting, using ABPM as a benchmark in the monitoring of hypertensive patients receiving treatment.
METHODS
Most published studies comparing conventional office BP measurement with ABPM have been conducted with patients not taking antihypertensive medication. We excluded these studies and conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting.
We searched Medline (from 1950 onward) and the Cochrane Database of Systematic Reviews. For the Medline search, we combined keywords for office BP, hypertension, and ambulatory BP with keywords for outpatient setting and primary care, using the following syntax: (((“clinic blood pressure” OR “office blood pressure” OR “casual blood pressure”))) AND (“hypertension” AND ((((“24-h ambulatory blood pressure”) OR “24 h ambulatory blood pressure”) OR “24 hour ambulatory blood pressure”) OR “blood pressure monitoring, ambulatory”[Mesh]) AND ((((((“outpatient setting”) OR “primary care”) OR “family care”) OR “family physician”) OR “family practice”) OR “general practice”)). We chose studies published in English and reviewed the titles and abstracts of identified articles.
With the aim of identifying additional candidate studies, we reviewed the reference lists of eligible primary studies, narrative reviews, and systematic reviews. The studies were generally of good quality and used appropriate statistical methods. Only primary studies qualified for meta-analysis.
Inclusion and exclusion criteria
Acceptable studies had to be conducted in a primary care setting with patients being treated for hypertension, and had to provide data comparing office BP measurement with ABPM. We excluded studies in which participants were treated in the hospital, were untreated, or had not been diagnosed with hypertension.
The quality of the studies included in the meta-analysis was judged by 2 independent observers according to the following criteria: the clear classification and initial comparison of both measurements; explicit and defined diagnostic criteria; compliance with the inclusion/exclusion criteria; and clear and precise definition of outcome variables.
Data extraction
We extracted the following data from each included study: study population, number of patients included, age, gender distribution, number of measurements (ambulatory and office BP), equipment validation, mean office and ambulatory BP, and the period of ambulatory BP measurement. We included adult patients of all ages, and we compared the mean office BP with those obtained by ABPM in hypertensive patients.
STATISTICAL ANALYSIS
For each study, we summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LRs), with the 95% confidence interval (CI), if available. If these rates were not directly reported in the original papers, we used the published data to calculate them.
We used the R v2.15.1 software with the “mada” package for meta-analysis.14 Although a bivariate approach is preferred for the meta-analysis of diagnostic accuracy, it cannot be recommended if the number of primary studies to pool is too small,14 as happened in our case. Therefore, we used a univariate approach and pooled summary statistics for positive LR, negative LR, and the diagnostic odds ratio (DOR) with their 95% confidence intervals. We used the DerSimonian-Laird method to perform a random-effect meta-analysis. To explore heterogeneity between the studies, we used the Cochran’s Q heterogeneity test, I2 index, and Galbraith and L’Abbé plots.
RESULTS
Our search identified 237 studies, only 12 of which met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements (TABLES 1 AND 2).15-26 Of these 12 studies, 5 were suitable for calculating sensitivity, specificity, and LR (TABLE 3),16,18,22,24,26 and 4 contained sufficient extractable data for meta-analysis. The study by Little et al18 was not included in the meta-analysis, as the number of true-positive, true-negative, false-positive, and false-negative results could not be deduced from published data.
The studies differed in sample size (40-31,530), patient ages (mean, 55-72.8 years), sex (percentage of men, 31%-52.9%), and number of measurements for office BP (1-9) and ABPM (32-96) (TABLE 1),15-26 as well as in daytime and nighttime periods for ABPM and BP thresholds, and in differences between the mean office and ambulatory BPs (TABLE 2).15-26
In general, the mean office BP measurements were higher than those obtained with ABPM in any period—from 5/0 mm Hg to 27.4/10.1 mm Hg in the day, and from 7.9/6.3 mm Hg to 31.2/13.7 mm Hg over 24 hours (TABLE 2).15-26
Compared with ABPM in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 55.7% to 91.2% and a specificity of 25.8% to 61.8% (depending on whether the measure was carried out by the doctor or nurse18); positive LR ranged from 1.2 to 1.4, and negative LR from 0.3 to 0.72 (TABLE 3).16,18,22,24,26
For meta-analysis, we pooled studies with the same thresholds (140/90 mm Hg for office BP; 130/80 mm Hg for ABPM), with diagnostic accuracy of office BP expressed as pooled positive and negative LR, and as pooled DOR. The meta-analysis revealed that the pooled positive LR was 1.35 (95% CI, 1.32-1.38), and the pooled negative LR was 0.44 (95% CI, 0.37-0.53). The pooled DOR was 3.47 (95% CI, 3.02-3.98). Sensitivity was 81.9% (95% CI, 74.8%-87%) and specificity was 41.1% (95% CI, 35.1%-48.4%).
One study16 had a slightly different ambulatory diagnostic threshold (133/78 mm Hg), so we excluded it from a second meta-analysis. Results after the exclusion did not change significantly: positive LR was 1.39 (95% CI, 1.34-1.45); negative LR was 0.38 (95% CI, 0.33-0.44); and DOR was 3.77 (95% CI, 3.31-4.43).
In conclusion, the use of office-based BP readings in the outpatient clinic does not correlate well with ABPM. Therefore, caution must be used when making management decisions based solely on in-office readings of BP.
DISCUSSION
The European Society of Hypertension still regards office BP measurement as the gold standard in screening for, diagnosing, and managing hypertension. As previously mentioned, though, office measurements are usually handled by medical staff and can be compromised by the white-coat effect and a small number of measurements. The USPSTF now considers ABPM the reference standard in primary care to diagnose hypertension in adults, to corroborate or contradict office-based determinations of elevated BP (whether based on single or repeated-interval measurements), and to avoid overtreatment of individuals displaying elevated office BP yet proven normotensive by ABPM.4,7 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7 Therefore, evidence supports ABPM as the reference standard for confirming elevated office BP screening results to avoid misdiagnosis and overtreatment of individuals with isolated clinic hypertension.7
How office measurements stack up against ABPM
Checking the validity of decisions in clinical practice is extremely important for patient management. One of the tools used for decision-making is an estimate of the LR. We used the LR to assess the value of office BP measurement in determining controlled or uncontrolled BP. A high LR (eg, >10) indicates that the office BP can be used to rule in the disease (uncontrolled BP) with a high probability, while a low LR (eg, <0.1) could rule it out. An LR of around one indicates that the office BP measurement cannot rule the diagnosis of uncontrolled BP in or out.27 In our meta-analysis, the positive LR is 1.35 and negative LR is 0.44. Therefore, in treated hypertensive patients, an indication of uncontrolled BP as measured in the clinic does not confirm a diagnosis of uncontrolled BP (as judged by the reference standard of ABPM). On the other hand, the negative LR means that normal office BP does not rule out uncontrolled BP, which may be detected with ABPM. Consequently, the measurement of BP in the office does not change the degree of (un)certainty of adequate control of BP. This knowledge is important, to avoid overtreatment of white coat hypertension and undertreatment of masked cases.
As previously mentioned, we reported similar results in a study designed to determine the validity of office BP measurement in a primary care setting compared with ABPM.13 In that paper, the level of agreement between both methods was poor, indicating that clinic measurements could not be recommended as a single method of BP control in hypertensive patients.
The use of ABPM in diagnosing hypertension is likely to increase as a consequence of some guideline updates.2 Our study emphasizes the importance of their use in the control of hypertensive patients.
Another published meta-analysis1 investigated the validity of office BP for the diagnosis of hypertension in untreated patients, with diagnostic thresholds for arterial hypertension set at 140/90 mm Hg for office measurement, and 135/85 mm Hg for ABPM. In that paper, the sensitivity of office BP was 74.6% (95% CI, 60.7-84.8) and the specificity was 74.6% (95% CI, 47.9-90.4).
In our present study carried out with hypertensive patients receiving treatment, we obtained a slightly higher sensitivity value of 81.9% (within the CI of this meta-analysis) and a lower specificity of 41.1%. Therefore, the discordance between office BP and ABPM seems to be similar for the diagnosis of hypertension and the classification of hypertension as being well or poorly controlled. This confirms the low validity of the office BP, both for diagnosis and monitoring of hypertensive patients.
Strengths of our study. The study focused on (treated) hypertensive patients in a primary care setting, where hypertension is most often managed. It confirms that ABPM is indispensable to a good clinical practice.
Limitations of our study are those inherent to meta-analyses. The main weakness of our study is the paucity of data available regarding the utility of ABPM for monitoring BP control with treatment in a primary care setting. Other limitations are the variability in BP thresholds used, the number of measurements performed, and the ambulatory BP devices used. These differences could contribute to the observed heterogeneity.
Application of our results must take into account that we included only those studies performed in a primary care setting with treated hypertensive patients.
Moreover, this study was not designed to evaluate the consequences of over- and undertreatment of blood pressure, nor to address the accuracy of automated blood pressure machines or newer health and fitness devices.
Implications for practice, policy, or future research. Alternative monitoring methods are home BP self-measurement and automated 30-minute clinic BP measurement.28 However, ABPM provides us with unique information about the BP pattern (dipping or non-dipping), BP variability, and mean nighttime BP. This paper establishes that the measurement of BP in the office is not an accurate method to monitor BP control. ABPM should be incorporated in usual clinical practice in primary care. Although the consequences of ambulatory monitoring are not the focus of this study, we acknowledge that the decision to incorporate ABPM in clinical practice depends on the availability of ambulatory devices, proper training of health care workers, and a cost-effectiveness analysis of its use.
CORRESPONDENCE
Sergio Reino-González, MD, PhD, Adormideras Primary Health Center, Poligono de Adormideras s/n. 15002 A Coruña, Spain; [email protected].
ABSTRACT
Purpose We performed a literature review and meta-analysis to ascertain the validity of office blood pressure (BP) measurement in a primary care setting, using ambulatory blood pressure measurement (ABPM) as a benchmark in the monitoring of hypertensive patients receiving treatment.
Methods We conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting. We compared the mean office BP with readings obtained by ABPM. We summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LR), with a 95% confidence interval (CI).
Results Only 12 studies met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements. Five were suitable for calculating sensitivity, specificity, and likelihood ratios, and 4 contained sufficient extractable data for meta-analysis. Compared with ABPM (thresholds of 140/90 mm Hg for office BP; 130/80 mmHg for ABPM) in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 81.9% (95% CI, 74.8%-87%) and specificity of 41.1% (95% CI, 35.1%-48.4%). Positive LR was 1.35 (95% CI, 1.32-1.38), and the negative LR was 0.44 (95% CI, 0.37-0.53).
Conclusion Likelihood ratios show that isolated BP measurement in the office does not confirm or rule out the presence of poor BP control. Likelihood of underestimating or overestimating BP control is high when relying on in-office BP measurement alone.
A growing body of evidence supports more frequent use of ambulatory blood pressure monitoring (ABPM) to confirm a diagnosis of hypertension1 and to monitor blood pressure (BP) response to treatment.2 The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure has long accepted ABPM for diagnosis of hypertension,3 and many clinicians consider ABPM the reference standard for diagnosing true hypertension and for accurately assessing associated cardiovascular risk in adults, regardless of office BP readings.4 The US Preventive Services Task Force (USPSTF) recommends obtaining BP measurements outside the clinical setting to confirm a diagnosis of hypertension before starting treatment.5 The USPSTF also asserts that elevated 24-hour ambulatory systolic BP is consistently and significantly associated with stroke and other cardiovascular events independent of office BP readings and has greater predictive value than office monitoring.5 The USPSTF concludes that ABPM, because of its large evidence base, is the best confirmatory test for hypertension.6 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7
The challenge. Despite the considerable support for ABPM, this method of BP measurement is still not sufficiently integrated into primary care. And some guidelines, such as those of
But ABPM’s advantages are numerous. Ambulatory monitors, which can record BP for 24 hours, are typically programmed to take readings every 15 to 30 minutes, providing estimates of mean daytime and nighttime BP and revealing an individual’s circadian pattern of BP.8-10 Ambulatory BP values usually considered the uppermost limit of normal are 135/85 mm Hg (day), 120/70 mm Hg (night), and 130/80 mm Hg (24 hour).8
Office BP monitoring, usually performed manually by medical staff, has 2 main drawbacks: the well-known white-coat effect experienced by many patients, and the relatively small number of possible measurements. A more reliable in-office BP estimation of BP would require repeated measurements at each of several visits.
By comparing ABPM and office measurements, 4 clinical findings are possible: isolated clinic or office (white-coat) hypertension (ICH); isolated ambulatory (masked) hypertension (IAH); consistent normotension; or sustained hypertension. With ICH, BP is high in the office and normal with ABPM. With IAH, BP is normal in the office and high with ABPM. With consistent normotension and sustained hypertension, BP readings with both types of measurement agree.8,9
In patients being treated for hypertension, ICH leads to an overestimation of uncontrolled BP and may result in overtreatment. The cardiovascular risk, although controversial, is usually lower than in patients diagnosed with sustained hypertension.11 IAH leads to an underestimation of uncontrolled BP and may result in undertreatment; its associated cardiovascular risk is similar to that of sustained hypertension.12
Our research objective. We recently published a study conducted with 137 hypertensive patients in a primary care center.13 Our conclusion was that in-office measurement of BP had insufficient clinical validity to be recommended as a sole method of monitoring BP control. In accurately classifying BP as controlled or uncontrolled, clinic measurement agreed with 24h-ABPM in just 64.2% of cases.13
In our present study, we performed a literature review and meta-analysis to ascertain the validity of office BP measurement in a primary care setting, using ABPM as a benchmark in the monitoring of hypertensive patients receiving treatment.
METHODS
Most published studies comparing conventional office BP measurement with ABPM have been conducted with patients not taking antihypertensive medication. We excluded these studies and conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting.
We searched Medline (from 1950 onward) and the Cochrane Database of Systematic Reviews. For the Medline search, we combined keywords for office BP, hypertension, and ambulatory BP with keywords for outpatient setting and primary care, using the following syntax: (((“clinic blood pressure” OR “office blood pressure” OR “casual blood pressure”))) AND (“hypertension” AND ((((“24-h ambulatory blood pressure”) OR “24 h ambulatory blood pressure”) OR “24 hour ambulatory blood pressure”) OR “blood pressure monitoring, ambulatory”[Mesh]) AND ((((((“outpatient setting”) OR “primary care”) OR “family care”) OR “family physician”) OR “family practice”) OR “general practice”)). We chose studies published in English and reviewed the titles and abstracts of identified articles.
With the aim of identifying additional candidate studies, we reviewed the reference lists of eligible primary studies, narrative reviews, and systematic reviews. The studies were generally of good quality and used appropriate statistical methods. Only primary studies qualified for meta-analysis.
Inclusion and exclusion criteria
Acceptable studies had to be conducted in a primary care setting with patients being treated for hypertension, and had to provide data comparing office BP measurement with ABPM. We excluded studies in which participants were treated in the hospital, were untreated, or had not been diagnosed with hypertension.
The quality of the studies included in the meta-analysis was judged by 2 independent observers according to the following criteria: the clear classification and initial comparison of both measurements; explicit and defined diagnostic criteria; compliance with the inclusion/exclusion criteria; and clear and precise definition of outcome variables.
Data extraction
We extracted the following data from each included study: study population, number of patients included, age, gender distribution, number of measurements (ambulatory and office BP), equipment validation, mean office and ambulatory BP, and the period of ambulatory BP measurement. We included adult patients of all ages, and we compared the mean office BP with those obtained by ABPM in hypertensive patients.
STATISTICAL ANALYSIS
For each study, we summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LRs), with the 95% confidence interval (CI), if available. If these rates were not directly reported in the original papers, we used the published data to calculate them.
We used the R v2.15.1 software with the “mada” package for meta-analysis.14 Although a bivariate approach is preferred for the meta-analysis of diagnostic accuracy, it cannot be recommended if the number of primary studies to pool is too small,14 as happened in our case. Therefore, we used a univariate approach and pooled summary statistics for positive LR, negative LR, and the diagnostic odds ratio (DOR) with their 95% confidence intervals. We used the DerSimonian-Laird method to perform a random-effect meta-analysis. To explore heterogeneity between the studies, we used the Cochran’s Q heterogeneity test, I2 index, and Galbraith and L’Abbé plots.
RESULTS
Our search identified 237 studies, only 12 of which met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements (TABLES 1 AND 2).15-26 Of these 12 studies, 5 were suitable for calculating sensitivity, specificity, and LR (TABLE 3),16,18,22,24,26 and 4 contained sufficient extractable data for meta-analysis. The study by Little et al18 was not included in the meta-analysis, as the number of true-positive, true-negative, false-positive, and false-negative results could not be deduced from published data.
The studies differed in sample size (40-31,530), patient ages (mean, 55-72.8 years), sex (percentage of men, 31%-52.9%), and number of measurements for office BP (1-9) and ABPM (32-96) (TABLE 1),15-26 as well as in daytime and nighttime periods for ABPM and BP thresholds, and in differences between the mean office and ambulatory BPs (TABLE 2).15-26
In general, the mean office BP measurements were higher than those obtained with ABPM in any period—from 5/0 mm Hg to 27.4/10.1 mm Hg in the day, and from 7.9/6.3 mm Hg to 31.2/13.7 mm Hg over 24 hours (TABLE 2).15-26
Compared with ABPM in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 55.7% to 91.2% and a specificity of 25.8% to 61.8% (depending on whether the measure was carried out by the doctor or nurse18); positive LR ranged from 1.2 to 1.4, and negative LR from 0.3 to 0.72 (TABLE 3).16,18,22,24,26
For meta-analysis, we pooled studies with the same thresholds (140/90 mm Hg for office BP; 130/80 mm Hg for ABPM), with diagnostic accuracy of office BP expressed as pooled positive and negative LR, and as pooled DOR. The meta-analysis revealed that the pooled positive LR was 1.35 (95% CI, 1.32-1.38), and the pooled negative LR was 0.44 (95% CI, 0.37-0.53). The pooled DOR was 3.47 (95% CI, 3.02-3.98). Sensitivity was 81.9% (95% CI, 74.8%-87%) and specificity was 41.1% (95% CI, 35.1%-48.4%).
One study16 had a slightly different ambulatory diagnostic threshold (133/78 mm Hg), so we excluded it from a second meta-analysis. Results after the exclusion did not change significantly: positive LR was 1.39 (95% CI, 1.34-1.45); negative LR was 0.38 (95% CI, 0.33-0.44); and DOR was 3.77 (95% CI, 3.31-4.43).
In conclusion, the use of office-based BP readings in the outpatient clinic does not correlate well with ABPM. Therefore, caution must be used when making management decisions based solely on in-office readings of BP.
DISCUSSION
The European Society of Hypertension still regards office BP measurement as the gold standard in screening for, diagnosing, and managing hypertension. As previously mentioned, though, office measurements are usually handled by medical staff and can be compromised by the white-coat effect and a small number of measurements. The USPSTF now considers ABPM the reference standard in primary care to diagnose hypertension in adults, to corroborate or contradict office-based determinations of elevated BP (whether based on single or repeated-interval measurements), and to avoid overtreatment of individuals displaying elevated office BP yet proven normotensive by ABPM.4,7 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7 Therefore, evidence supports ABPM as the reference standard for confirming elevated office BP screening results to avoid misdiagnosis and overtreatment of individuals with isolated clinic hypertension.7
How office measurements stack up against ABPM
Checking the validity of decisions in clinical practice is extremely important for patient management. One of the tools used for decision-making is an estimate of the LR. We used the LR to assess the value of office BP measurement in determining controlled or uncontrolled BP. A high LR (eg, >10) indicates that the office BP can be used to rule in the disease (uncontrolled BP) with a high probability, while a low LR (eg, <0.1) could rule it out. An LR of around one indicates that the office BP measurement cannot rule the diagnosis of uncontrolled BP in or out.27 In our meta-analysis, the positive LR is 1.35 and negative LR is 0.44. Therefore, in treated hypertensive patients, an indication of uncontrolled BP as measured in the clinic does not confirm a diagnosis of uncontrolled BP (as judged by the reference standard of ABPM). On the other hand, the negative LR means that normal office BP does not rule out uncontrolled BP, which may be detected with ABPM. Consequently, the measurement of BP in the office does not change the degree of (un)certainty of adequate control of BP. This knowledge is important, to avoid overtreatment of white coat hypertension and undertreatment of masked cases.
As previously mentioned, we reported similar results in a study designed to determine the validity of office BP measurement in a primary care setting compared with ABPM.13 In that paper, the level of agreement between both methods was poor, indicating that clinic measurements could not be recommended as a single method of BP control in hypertensive patients.
The use of ABPM in diagnosing hypertension is likely to increase as a consequence of some guideline updates.2 Our study emphasizes the importance of their use in the control of hypertensive patients.
Another published meta-analysis1 investigated the validity of office BP for the diagnosis of hypertension in untreated patients, with diagnostic thresholds for arterial hypertension set at 140/90 mm Hg for office measurement, and 135/85 mm Hg for ABPM. In that paper, the sensitivity of office BP was 74.6% (95% CI, 60.7-84.8) and the specificity was 74.6% (95% CI, 47.9-90.4).
In our present study carried out with hypertensive patients receiving treatment, we obtained a slightly higher sensitivity value of 81.9% (within the CI of this meta-analysis) and a lower specificity of 41.1%. Therefore, the discordance between office BP and ABPM seems to be similar for the diagnosis of hypertension and the classification of hypertension as being well or poorly controlled. This confirms the low validity of the office BP, both for diagnosis and monitoring of hypertensive patients.
Strengths of our study. The study focused on (treated) hypertensive patients in a primary care setting, where hypertension is most often managed. It confirms that ABPM is indispensable to a good clinical practice.
Limitations of our study are those inherent to meta-analyses. The main weakness of our study is the paucity of data available regarding the utility of ABPM for monitoring BP control with treatment in a primary care setting. Other limitations are the variability in BP thresholds used, the number of measurements performed, and the ambulatory BP devices used. These differences could contribute to the observed heterogeneity.
Application of our results must take into account that we included only those studies performed in a primary care setting with treated hypertensive patients.
Moreover, this study was not designed to evaluate the consequences of over- and undertreatment of blood pressure, nor to address the accuracy of automated blood pressure machines or newer health and fitness devices.
Implications for practice, policy, or future research. Alternative monitoring methods are home BP self-measurement and automated 30-minute clinic BP measurement.28 However, ABPM provides us with unique information about the BP pattern (dipping or non-dipping), BP variability, and mean nighttime BP. This paper establishes that the measurement of BP in the office is not an accurate method to monitor BP control. ABPM should be incorporated in usual clinical practice in primary care. Although the consequences of ambulatory monitoring are not the focus of this study, we acknowledge that the decision to incorporate ABPM in clinical practice depends on the availability of ambulatory devices, proper training of health care workers, and a cost-effectiveness analysis of its use.
CORRESPONDENCE
Sergio Reino-González, MD, PhD, Adormideras Primary Health Center, Poligono de Adormideras s/n. 15002 A Coruña, Spain; [email protected].
1. Hodgkinson J, Mant J, Martin U, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621.
2. National Institute for Health and Clinical Excellence. Hypertension in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/CG127. Accessed November 15, 2016.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
4. Hermida RC, Smolensky MH, Ayala DE, et al. Ambulatory Blood Pressure Monitoring (ABPM) as the reference standard for diagnosis of hypertension and assessment of vascular risk in adults. Chronobiol Int. 2015;32:1329-1342.
5. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
6. Piper MA, Evans CV
7. American Academy of Family Physicians. Hypertension. Available at: www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 10, 2016.
8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2013;23:3-16.
9. Marin R, de la Sierra A, Armario P, et al. 2005 Spanish guidelines in diagnosis and treatment of arterial hypertension. Medicina Clínica. 2005;125:24-34.
10. Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55-61.
11. Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation. 2001;104:1385-1392.
12. Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21:969-975.
13. Reino-González S, Pita-Fernández S, Cibiriain-Sola M, et al. Validity of clinic blood pressure compared to ambulatory monitoring in hypertensive patients in a primary care setting. Blood Press. 2015;24:111-118.
14. Doebler P, Holling H. Meta-analysis of diagnostic accuracy with mada. Available at: https://cran.r-project.org/web/packages/mada/vignettes/mada.pdf. Accessed October 5, 2015.
15. Myers MG, Oh PI, Reeves RA, et al. Prevalence of white coat effect in treated hypertensive patients in the community. Am J Hypertens. 1995;8:591-597.
16. Imai Y, Tsuji I, Nagai K, et al. Ambulatory blood pressure monitoring in evaluating the prevalence of hypertension in adults in Ohasama, a rural Japanese community. Hypertens Res. 1996;19:207-212.
17. Taylor RS, Stockman J, Kernick D, et al. Ambulatory blood pressure monitoring for hypertension in general practice. J R Soc Med. 1998;91:301-304.
18. Little P, Barnett J, Barnsley L, et al. Comparison of agreement between different measures of blood pressure in primary care and daytime ambulatory blood pressure. BMJ. 2002;325:254.
19. Bur A, Herkner H, Vlcek M, et al. Classification of blood pressure levels by ambulatory blood pressure in hypertension. Hypertension. 2002;40:817-822.
20. Lindbaek M, Sandvik E, Liodden K, et al. Predictors for the white coat effect in general practice patients with suspected and treated hypertension. Br J Gen Pract. 2003;53:790-793.
21. Martínez MA, Sancho T, García P, et al. Home blood pressure in poorly controlled hypertension: relationship with ambulatory blood pressure and organ damage. Blood Press Monit. 2006;11:207-213.
22. Sierra BC, de la Sierra IA, Sobrino J, et al. Monitorización ambulatoria de la presión arterial (MAPA): características clínicas de 31.530 pacientes. Medicina Clínica. 2007;129:1-5.
23. Gómez MA, García L, Sánchez Á, et al. Agreement and disagreement between different methods of measuring blood pressure. Hipertensión (Madr). 2008;25:231-239.
24. Banegas JR, Segura J, De la Sierra A, et al. Gender differences in office and ambulatory control of hypertension. Am J Med. 2008;121:1078-1084.
25. Zaninelli A, Parati G, Cricelli C, et al. Office and 24-h ambulatory blood pressure control by treatment in general practice: the ‘Monitoraggio della pressione ARteriosa nella medicina TErritoriale’ study. J Hypertens. 2010;28:910-917.
26. Llisterri JL, Morillas P, Pallarés V, et al. Differences in the degree of control of arterial hypertension according to the measurement procedure of blood pressure in patients ≥ 65 years. FAPRES study. Rev Clin Esp. 2011;211:76-84.
27. Straus SE, Richardson WS, Glasziou P, et al. Evidence-Based Medicine: How to practice and teach it. 4th ed. Edinburgh, Scotland: Churchill Livingstone; 2010.
28. Van der Wel MC, Buunk IE, van Weel C, et al. A novel approach to office blood pressure measurement: 30-minute office blood pressure vs daytime ambulatory blood pressure. Ann Fam Med. 2011;9:128-135.
1. Hodgkinson J, Mant J, Martin U, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621.
2. National Institute for Health and Clinical Excellence. Hypertension in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/CG127. Accessed November 15, 2016.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
4. Hermida RC, Smolensky MH, Ayala DE, et al. Ambulatory Blood Pressure Monitoring (ABPM) as the reference standard for diagnosis of hypertension and assessment of vascular risk in adults. Chronobiol Int. 2015;32:1329-1342.
5. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
6. Piper MA, Evans CV
7. American Academy of Family Physicians. Hypertension. Available at: www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 10, 2016.
8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2013;23:3-16.
9. Marin R, de la Sierra A, Armario P, et al. 2005 Spanish guidelines in diagnosis and treatment of arterial hypertension. Medicina Clínica. 2005;125:24-34.
10. Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55-61.
11. Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation. 2001;104:1385-1392.
12. Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21:969-975.
13. Reino-González S, Pita-Fernández S, Cibiriain-Sola M, et al. Validity of clinic blood pressure compared to ambulatory monitoring in hypertensive patients in a primary care setting. Blood Press. 2015;24:111-118.
14. Doebler P, Holling H. Meta-analysis of diagnostic accuracy with mada. Available at: https://cran.r-project.org/web/packages/mada/vignettes/mada.pdf. Accessed October 5, 2015.
15. Myers MG, Oh PI, Reeves RA, et al. Prevalence of white coat effect in treated hypertensive patients in the community. Am J Hypertens. 1995;8:591-597.
16. Imai Y, Tsuji I, Nagai K, et al. Ambulatory blood pressure monitoring in evaluating the prevalence of hypertension in adults in Ohasama, a rural Japanese community. Hypertens Res. 1996;19:207-212.
17. Taylor RS, Stockman J, Kernick D, et al. Ambulatory blood pressure monitoring for hypertension in general practice. J R Soc Med. 1998;91:301-304.
18. Little P, Barnett J, Barnsley L, et al. Comparison of agreement between different measures of blood pressure in primary care and daytime ambulatory blood pressure. BMJ. 2002;325:254.
19. Bur A, Herkner H, Vlcek M, et al. Classification of blood pressure levels by ambulatory blood pressure in hypertension. Hypertension. 2002;40:817-822.
20. Lindbaek M, Sandvik E, Liodden K, et al. Predictors for the white coat effect in general practice patients with suspected and treated hypertension. Br J Gen Pract. 2003;53:790-793.
21. Martínez MA, Sancho T, García P, et al. Home blood pressure in poorly controlled hypertension: relationship with ambulatory blood pressure and organ damage. Blood Press Monit. 2006;11:207-213.
22. Sierra BC, de la Sierra IA, Sobrino J, et al. Monitorización ambulatoria de la presión arterial (MAPA): características clínicas de 31.530 pacientes. Medicina Clínica. 2007;129:1-5.
23. Gómez MA, García L, Sánchez Á, et al. Agreement and disagreement between different methods of measuring blood pressure. Hipertensión (Madr). 2008;25:231-239.
24. Banegas JR, Segura J, De la Sierra A, et al. Gender differences in office and ambulatory control of hypertension. Am J Med. 2008;121:1078-1084.
25. Zaninelli A, Parati G, Cricelli C, et al. Office and 24-h ambulatory blood pressure control by treatment in general practice: the ‘Monitoraggio della pressione ARteriosa nella medicina TErritoriale’ study. J Hypertens. 2010;28:910-917.
26. Llisterri JL, Morillas P, Pallarés V, et al. Differences in the degree of control of arterial hypertension according to the measurement procedure of blood pressure in patients ≥ 65 years. FAPRES study. Rev Clin Esp. 2011;211:76-84.
27. Straus SE, Richardson WS, Glasziou P, et al. Evidence-Based Medicine: How to practice and teach it. 4th ed. Edinburgh, Scotland: Churchill Livingstone; 2010.
28. Van der Wel MC, Buunk IE, van Weel C, et al. A novel approach to office blood pressure measurement: 30-minute office blood pressure vs daytime ambulatory blood pressure. Ann Fam Med. 2011;9:128-135.
Biomechanics of Polyhydroxyalkanoate Mesh–Augmented Single-Row Rotator Cuff Repairs
Healing after rotator cuff repair (RCR) can be challenging, especially in cases of large and massive tears, revision repairs, and tendons with poor tissue quality.1-3 Poor tissue quality is associated with increased risk for recurrent tears, independent of age and tear size.3 Various techniques have been used to improve tendon fixation strength in these difficult situations, including augmented suture configurations (eg, massive cuff stitches, rip-stop stitches) and tissue grafts (eg, acellular dermal matrix).4-9 Clinical studies have found improved healing rates for larger tears and revision repairs using acellular dermal matrix grafts.6,10 Synthetic patches are another option for RCR augmentation, but limited clinical data and biomechanical evidence support use of synthetic grafts as an augment for RCRs.11-13
Polyhydroxyalkanoates (PHAs) are a class of biodegradable polymers that have been used as orthopedic devices, tissue scaffolds, patches, and other applications with increasing frequency over the past decade.14 In the laboratory, these implanted materials have been shown to support cell migration and growth.15 The PHA family of polymers typically degrades by hydrolytic and bacterial depolymerase mechanisms over 52-plus weeks in vivo.14PHA grafts have been studied in the setting of RCR. An expanded polytetrafluoroethylene scaffold was shown to improve repair mechanics when used as a bursal side graft in an in vitro ovine model.11 The graft increased tendon footprint contact pressure and failure loads by almost 180 N. In clinical studies, poly-L-lactic acid augmentations have been used to reinforce massive RCRs. Lenart and colleagues16 found that 38% of 16 patients with such tears had an intact rotator cuff at 1.2-year follow-up, and improvement in clinical scores. Proctor13 reported on use of a poly-L-lactic acid retrograde patch for reinforcement of massive tears with both single- and double-row repairs in 18 patients. The cohort had more favorable rates of intact cuffs at 12 months (83%) and 42 months (78%), and ASES (American Shoulder and Elbow Surgeons) scores improved from 25 before surgery to 82 at latest follow-up after surgery.
RCR augmentation traditionally has been performed with an open or mini-open technique.6 Recently, several authors have reported on arthroscopic techniques for augmentation with either acellular dermal matrix or synthetic grafts.13,17,18 Most techniques have involved “bridging” with a graft or patch used to stress-shield a single-row repair.8,9,13 This bridging typically involves placing several sutures medial to where the anchor repair stitches pass through the tendon. An alternative is to pass the repair stitches through both the tendon and the graft.17-19 The overall volume of tissue incorporated into the repair stitches (rotator cuff plus graft) is increased with the augmented technique relative to the bridging technique. Both can be technically challenging, but the augmented technique may be easier to perform arthroscopically.9,19 Regardless, these techniques are complicated and require a higher level of arthroscopic skills compared with those required in arthroscopic RCR without a graft. Simplifying arthroscopic graft augmentation likely will increase its utility because, even for skilled surgeons, adding a graft can increase operative time by 20 to 30 minutes. Simplification will also extend use of the technique to surgeons with less experience and proficiency with arthroscopic repair.
We developed a simple method for augmenting single-row RCR with a strip of bioresorbable soft-tissue scaffold. We also conducted a study to evaluate the initial biomechanical properties of single-row RCR in cadaveric shoulder specimens augmented with PHA mesh (BioFiber; Tornier) graft as compared with single-row RCR without augmentation. Both cyclic gap formation and ultimate failure loads and displacement were quantified. We hypothesized that the augmented RCRs would have decreased gap formation and increased ultimate failure loads compared with nonaugmented RCRs. This study was exempt from having to obtain Institutional Review B
Methods
Eight pairs of fresh-frozen cadaver humeri (6 male, 2 female; mean [SD] age, 61 [9] years) were dissected of all soft tissue (except rotator cuff) by Dr. Tashjian, a board-certified, fellowship-trained orthopedic surgeon. There were no qualitative differences in tendon condition between tendons within a pair. The supraspinatus muscle and tendon were separated from the other rotator cuff muscles. The infraspinatus, subscapularis, and teres minor were removed from the humerus. Last, the supraspinatus was resected at its insertion. Humeral pairs were then randomized into augmented and nonaugmented RCRs within each pair.
In the nonaugmented group, the supraspinatus was reattached to its insertion in a single-row RCR with 2 triple-loaded suture anchors (5.5-mm Insite FT Ti, No. 2 Force Fiber suture; Tornier) and 6 simple stitches (Figure 1A). Anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity at about 45° to the bone surface.
In the contralateral shoulders, augmented RCRs were performed. Specimens were prepared exactly as they were for the nonaugmented RCRs, including anchor placement and suture passage. Before knot tying, RCRs were augmented with 2 strips of 13-mm × 23-mm PHA mesh (BioFiber) (Figure 1B). One strip was used to augment the 3 sutures of each anchor, overlying the residual tendon, to reinforce the tendon–knot interface. After each suture was passed through the supraspinatus tendon from the intra-articular surface, the stitch was passed through the strip of PHA mesh. Stitches were separated by 5 mm in each mesh strip. All 6 sutures were then tied with a Revo knot between the free end of each suture leg and the leg that passed through the tendon and mesh.
Each humerus was transected at the midshaft and potted and mounted in an Instron 1331 load frame with Model 8800 controller (Instron). A cryoclamp was used to grasp the supraspinatus muscle belly above the musculotendinous junction (Figure 2).
Three rows of 2-mm fiducial markers were affixed to the bone, tendon, and muscle belly with cyanoacrylate for tracking with a digital video system (DMAS Version 6.5; Spicatek) (Figure 3).21
A 0.1-MPa pre-stress (applied force/tendon cross-sectional area) was applied to each construct to determine the starting position for the deformation profile. Each repair underwent 1000 cycles of uniaxial load-controlled displacement between 0.1 and 1.0 MPa of effective stress at 1 Hz. Effective stress was determined as the ratio of applied force to cross-sectional area of the tendon at harvest to normalize the applied loads between tendons of varying size. During cyclic testing, gapping of more than 5 mm was defined as construct failure.22 After cyclic loading, each construct was loaded to failure at 1.0 mm/s. Ultimate failure load was defined as the highest load achieved at the maximum displacement before rapid decline in load supported by the construct.
Statistical Analysis
Paired t tests were used to compare the matched pairs of constructs. For all tests, significance was set at P ≤ .05. Post hoc power was calculated for significant results using G*Power Version 3.1.6.23 All data are presented as means (SDs).
Results
After 1000 cycles of displacement, mean (SD) gapping was 3.8 (0.9) mm for the nonaugmented repairs and 3.9 (1.1) mm for the PHA mesh–augmented repairs (P = .879) (Figure 4).
For the nonaugmented repairs, mean (SD) failure displacement was 6.3 (1.7) mm, and mean (SD) ultimate failure load was 472.1 (120.3) N. For the PHA-augmented repairs, failure displacement was 5.5 (1.9) mm, and ultimate failure load was 571.2 (173.0) N. There was no difference in failure displacement (P = .393), but there was a difference in ultimate failure load (P = .042; power = 0.57). During failure testing, mean (SD) tissue deformation was higher (P = .012; power = 0.83) for the PHA-augmented repairs, 1.2 (0.7) mm, than for the nonaugmented repairs, 0.8 (0.5) mm. Failures, which were consistent within pairs, were caused by tissue failure, with sutures pulling through the tissue (4 pairs) or single anchor pullout before ultimate tissue failure (4 pairs). Of the 4 failures with anchor pullout, 3 had anterior anchor pullout, and 1 had posterior anchor pullout. In all specimens with anchor pullout, the second anchor remained stable, and ultimate failure occurred with tissue tearing at the suture interface. There were no significant differences in any metrics between specimens that failed with intact anchors and specimens with single anchor pullout (P ≥ .122). Therefore, both groups were pooled for the failure analysis.
Discussion
RCR augmentation with a synthetic graft is a viable option for improving fixation strength of supraspinatus repairs, as shown in otherwise healthy tendon in the present study. Our hypothesis that there would be decreased gap formation with graft augmentation was not supported, whereas the hypothesis of increased failure loads with graft augmentation was supported. These findings may also be applicable in cases of large tears, revisions, and tendons with poor tissue quality. Simplification of graft application techniques will allow quick and easy arthroscopic augmentation.
Studies of RCRs for large or massive tears have reported retear rates of 25% to 79%.24-26 Latissimus dorsi tendon transfers also show promise in posterosuperior RCRs, with failure rates near 10%.27,28 Although use of PHA patches in RCR augmentation is relatively new, short-term and midterm failure rates are in the range of 20% to 60% in the few small cohorts currently being studied.13,16 It is possible that these rates may improve as indications, surgical experience, and techniques for use of PHA patches are further refined. Regardless, with PHA currently being used in practice, it is important to quantify the biomechanics of the augmentation as a baseline for its performance in reinforcing the tendon–suture interface.
We determined that the initial fixation strength of single-row repairs was higher with the addition of PHA synthetic grafts using a very simple technique. Single-row triple-loaded anchor repairs already provide high initial mechanical strength, and our results are similar to those of another study of this technique.29 Despite the already high mechanical strength of a triple-loaded anchor repair, PHA mesh increased ultimate strength by about 100 N (~25%). Of note, tissue elongation during failure was higher (P = .012; power = 0.83) in the PHA-augmented group (1.2 mm) than in the nonaugmented group (0.8 mm). This was not surprising—failure loads were almost 100 N higher in the PHA-augmented group than in the nonaugmented group. Consequently, much higher forces were placed on the muscle belly, likely resulting in additional elongation of the intact tissue medial to the repair construct.
The ultimate failure loads in our study compare favorably with the biomechanical strength of augmented repairs reported by others.8,9,18 Barber and colleagues18 evaluated an augmented single-row repair with 2 double-loaded suture anchors and an acellular dermal matrix graft. The ultimate failure load of the augmented repairs was 325 N. In contrast, Omae and colleagues8 tested a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft. Ultimate failure load of the augmented repairs was 560 N, similar to our finding. Last, Shea and colleagues9 evaluated a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft, with ultimate failure load of 429 N. The techniques in all 3 studies can be performed arthroscopically but are challenging and require multiple extra sutures and anchors that need management and tying. Our technique provides similar initial fixation strength, has no requirement for extra sutures or anchors, and is very simple to perform.
The supraspinatus tendon is estimated to fail between 800 N and 1000 N.30,31 Biomechanical shoulder simulators use supraspinatus forces in the range of 20 N to 200 N for scapular plane abduction.32-36 Therefore, the single-row repair failures in our study fell between functional and full-thickness failure loads. Studies on the mechanics of degenerated human supraspinatus tendon are limited, but there is evidence the mechanical properties of these tissues are inferior to those of healthy tendon.37 A 100-N increase in failure loads with PHA augmentation may prove highly significant in reinforcing the suture–tendon interface in degenerated tendons.
Adding the mesh did not have any effect on gapping at the repair site after cyclic loading. This finding suggests that construct gapping under cyclic loading is not a function of a reinforced knot–tendon interface but is instead caused by microtearing and cinching of the suture constructs in relation to the underlying bone. Tissue elongation likely was not a strong contributor to overall cyclic gapping, as elongation did not differ between the nonaugmented and augmented repairs (0.5 mm vs 0.7 mm; P = .276) and was small relative to the nearly 4 mm of construct gapping. Gapping may be affected by healing and integration of the mesh into the repaired tendon over time, but this effect could not be captured in the present study. Patients are initially immobilized and passive shoulder motion gradually introduced, in stark contrast to the immediate loading protocol in the present study. Regardless, the 25% increase in overall strength may be clinically important, especially in cases of difficult repair or poor tissue quality.
Our technique simplifies arthroscopic augmentation—stitches are passed through the rotator cuff in simple fashion. Before being tied, the limbs that were passed through the rotator cuff are removed through a cannula and then passed through the synthetic graft.
Study Limitations
This study had several limitations. First, it was a cadaveric biomechanical study that evaluated only time-zero biomechanical properties. Loads were normalized to tendon size, specimens were randomized between sides, and paired specimens were used to minimize the effects of tendon and bone quality on outcome metrics. In addition, donor tendons were representative of otherwise healthy tissue. Chronic tears and associated resorption/atrophy could have affected the magnitude of forces and gapping detected in this study. Theoretically, over time the tendon tissue will adhere to and grow into the mesh, which could minimize potential differences. Studies are needed to determine the effects of healing on long-term repair strength in affected patients. Last, all constructs were performed in open fashion to improve repeatability of construct placement and provide accessibility for Instron testing. Our technique did not directly replicate the arthroscopic approach, but, unlike other augmentation techniques, it is so simple that transition to all-arthroscopic augmentation is realistic.
Patch augmentation increases the cost of materials and operative time and should be considered a limitation of its utility. We do not recommend augmentation in all RCRs, as it likely is cost-ineffective. Instead, we recommend augmentation in cases of poor tissue quality, which could lead to healing failure, revision surgery, and higher overall patient costs beyond the cost of adding augmentation. Similarly, we recommend augmentation for revision cases in which tendon healing has failed and tissue quality is poor. The goal is to prevent another failure.
Conclusion
PHA graft augmentation of single-row triple-loaded anchor repairs of the supraspinatus tendon improves the overall ultimate load to failure by 25%. There was no difference in gap formation after cyclic loading between augmented and nonaugmented repairs. This technique for arthroscopic augmentation can be used to improve initial biomechanical repair strength in tears at risk for failure.
Am J Orthop. 2016;45(7):E527-E533. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
2. Keener JD, Wei AS, Kim HM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am. 2010;92(3):590-598.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Barber FA, Herbert MA, Schroeder FA, Aziz-Jacobo J, Mays MM, Rapley JH. Biomechanical advantages of triple-loaded suture anchors compared with double-row rotator cuff repairs. Arthroscopy. 2010;26(3):316-323.
5. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
6. Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872-879.
7. Ma CB, MacGillivray JD, Clabeaux J, Lee S, Otis JC. Biomechanical evaluation of arthroscopic rotator cuff stitches. J Bone Joint Surg Am. 2004;86(6):1211-1216.
8. Omae H, Steinmann SP, Zhao C, et al. Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clin Biomech. 2012;27(8):789-792.
9. Shea KP, Obopilwe E, Sperling JW, Iannotti JP. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg. 2012;21(8):1072-1079.
10. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
11. Beimers L, Lam PH, Murrell GA. The biomechanical effects of polytetrafluoroethylene suture augmentations in lateral-row rotator cuff repairs in an ovine model. J Shoulder Elbow Surg. 2014;23(10):1545-1552.
12. McCarron JA, Milks RA, Chen X, Iannotti JP, Derwin KA. Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg. 2010;19(5):688-696.
13. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23(10):1508-1513.
14. Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7(8):2249-2258.
15. Ellis G, Cano P, Jadraque M, et al. Laser microperforated biodegradable microbial polyhydroxyalkanoate substrates for tissue repair strategies: an infrared microspectroscopy study. Anal Bioanal Chem. 2011;399(7):2379-2388.
16. Lenart BA, Martens KA, Kearns KA, Gillespie RJ, Zoga AC, Williams GR. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg. 2015;24(6):915-921.
17. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
18. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
19. Gilot GJ, Attia AK, Alvarez AM. Arthroscopic repair of rotator cuff tears using extracellular matrix graft. Arthrosc Tech. 2014;3(4):e487-e489.
20. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
21. Kullar RS, Reagan JM, Kolz CW, Burks RT, Henninger HB. Suture placement near the musculotendinous junction in the supraspinatus: implications for rotator cuff repair. Am J Sports Med. 2015;43(1):57-62.
22. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athanasiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
23. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
24. Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24(9):1493-1505.
25. Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy. 2015;31(11):2274-2281.
26. Henry P, Wasserstein D, Park S, et al. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31(12):2472-2480.
27. El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015;97(6):462-469.
28. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
29. Coons DA, Barber FA, Herbert MA. Triple-loaded single-anchor stitch configurations: an analysis of cyclically loaded suture–tendon interface security. Arthroscopy. 2006;22(11):1154-1158.
30. Itoi E, Berglund LJ, Grabowski JJ, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13(4):578-584.
31. Matsuhashi T, Hooke AW, Zhao KD, et al. Tensile properties of a morphologically split supraspinatus tendon. Clin Anat. 2014;27(5):702-706.
32. Apreleva M, Parsons IM 4th, Warner JJ, Fu FH, Woo SL. Experimental investigation of reaction forces at the glenohumeral joint during active abduction. J Shoulder Elbow Surg. 2000;9(5):409-417.
33. Giles JW, Ferreira LM, Athwal GS, Johnson JA. Development and performance evaluation of a multi-PID muscle loading driven in vitro active-motion shoulder simulator and application to assessing reverse total shoulder arthroplasty. J Biomech Eng. 2014;136(12):121007.
34. Hansen ML, Otis JC, Johnson JS, Cordasco FA, Craig EV, Warren RF. Biomechanics of massive rotator cuff tears: implications for treatment. J Bone Joint Surg Am. 2008;90(2):316-325.
35. Henninger HB, Barg A, Anderson AE, Bachus KN, Tashjian RZ, Burks RT. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(4):483-490.
36. Mihata T, Gates J, McGarry MH, Lee J, Kinoshita M, Lee TQ. Effect of rotator cuff muscle imbalance on forceful internal impingement and peel-back of the superior labrum: a cadaveric study. Am J Sports Med. 2009;37(11):2222-2227.
37. Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone–tendon complex. J Orthop Res. 1997;15(5):719-726.
Healing after rotator cuff repair (RCR) can be challenging, especially in cases of large and massive tears, revision repairs, and tendons with poor tissue quality.1-3 Poor tissue quality is associated with increased risk for recurrent tears, independent of age and tear size.3 Various techniques have been used to improve tendon fixation strength in these difficult situations, including augmented suture configurations (eg, massive cuff stitches, rip-stop stitches) and tissue grafts (eg, acellular dermal matrix).4-9 Clinical studies have found improved healing rates for larger tears and revision repairs using acellular dermal matrix grafts.6,10 Synthetic patches are another option for RCR augmentation, but limited clinical data and biomechanical evidence support use of synthetic grafts as an augment for RCRs.11-13
Polyhydroxyalkanoates (PHAs) are a class of biodegradable polymers that have been used as orthopedic devices, tissue scaffolds, patches, and other applications with increasing frequency over the past decade.14 In the laboratory, these implanted materials have been shown to support cell migration and growth.15 The PHA family of polymers typically degrades by hydrolytic and bacterial depolymerase mechanisms over 52-plus weeks in vivo.14PHA grafts have been studied in the setting of RCR. An expanded polytetrafluoroethylene scaffold was shown to improve repair mechanics when used as a bursal side graft in an in vitro ovine model.11 The graft increased tendon footprint contact pressure and failure loads by almost 180 N. In clinical studies, poly-L-lactic acid augmentations have been used to reinforce massive RCRs. Lenart and colleagues16 found that 38% of 16 patients with such tears had an intact rotator cuff at 1.2-year follow-up, and improvement in clinical scores. Proctor13 reported on use of a poly-L-lactic acid retrograde patch for reinforcement of massive tears with both single- and double-row repairs in 18 patients. The cohort had more favorable rates of intact cuffs at 12 months (83%) and 42 months (78%), and ASES (American Shoulder and Elbow Surgeons) scores improved from 25 before surgery to 82 at latest follow-up after surgery.
RCR augmentation traditionally has been performed with an open or mini-open technique.6 Recently, several authors have reported on arthroscopic techniques for augmentation with either acellular dermal matrix or synthetic grafts.13,17,18 Most techniques have involved “bridging” with a graft or patch used to stress-shield a single-row repair.8,9,13 This bridging typically involves placing several sutures medial to where the anchor repair stitches pass through the tendon. An alternative is to pass the repair stitches through both the tendon and the graft.17-19 The overall volume of tissue incorporated into the repair stitches (rotator cuff plus graft) is increased with the augmented technique relative to the bridging technique. Both can be technically challenging, but the augmented technique may be easier to perform arthroscopically.9,19 Regardless, these techniques are complicated and require a higher level of arthroscopic skills compared with those required in arthroscopic RCR without a graft. Simplifying arthroscopic graft augmentation likely will increase its utility because, even for skilled surgeons, adding a graft can increase operative time by 20 to 30 minutes. Simplification will also extend use of the technique to surgeons with less experience and proficiency with arthroscopic repair.
We developed a simple method for augmenting single-row RCR with a strip of bioresorbable soft-tissue scaffold. We also conducted a study to evaluate the initial biomechanical properties of single-row RCR in cadaveric shoulder specimens augmented with PHA mesh (BioFiber; Tornier) graft as compared with single-row RCR without augmentation. Both cyclic gap formation and ultimate failure loads and displacement were quantified. We hypothesized that the augmented RCRs would have decreased gap formation and increased ultimate failure loads compared with nonaugmented RCRs. This study was exempt from having to obtain Institutional Review B
Methods
Eight pairs of fresh-frozen cadaver humeri (6 male, 2 female; mean [SD] age, 61 [9] years) were dissected of all soft tissue (except rotator cuff) by Dr. Tashjian, a board-certified, fellowship-trained orthopedic surgeon. There were no qualitative differences in tendon condition between tendons within a pair. The supraspinatus muscle and tendon were separated from the other rotator cuff muscles. The infraspinatus, subscapularis, and teres minor were removed from the humerus. Last, the supraspinatus was resected at its insertion. Humeral pairs were then randomized into augmented and nonaugmented RCRs within each pair.
In the nonaugmented group, the supraspinatus was reattached to its insertion in a single-row RCR with 2 triple-loaded suture anchors (5.5-mm Insite FT Ti, No. 2 Force Fiber suture; Tornier) and 6 simple stitches (Figure 1A). Anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity at about 45° to the bone surface.
In the contralateral shoulders, augmented RCRs were performed. Specimens were prepared exactly as they were for the nonaugmented RCRs, including anchor placement and suture passage. Before knot tying, RCRs were augmented with 2 strips of 13-mm × 23-mm PHA mesh (BioFiber) (Figure 1B). One strip was used to augment the 3 sutures of each anchor, overlying the residual tendon, to reinforce the tendon–knot interface. After each suture was passed through the supraspinatus tendon from the intra-articular surface, the stitch was passed through the strip of PHA mesh. Stitches were separated by 5 mm in each mesh strip. All 6 sutures were then tied with a Revo knot between the free end of each suture leg and the leg that passed through the tendon and mesh.
Each humerus was transected at the midshaft and potted and mounted in an Instron 1331 load frame with Model 8800 controller (Instron). A cryoclamp was used to grasp the supraspinatus muscle belly above the musculotendinous junction (Figure 2).
Three rows of 2-mm fiducial markers were affixed to the bone, tendon, and muscle belly with cyanoacrylate for tracking with a digital video system (DMAS Version 6.5; Spicatek) (Figure 3).21
A 0.1-MPa pre-stress (applied force/tendon cross-sectional area) was applied to each construct to determine the starting position for the deformation profile. Each repair underwent 1000 cycles of uniaxial load-controlled displacement between 0.1 and 1.0 MPa of effective stress at 1 Hz. Effective stress was determined as the ratio of applied force to cross-sectional area of the tendon at harvest to normalize the applied loads between tendons of varying size. During cyclic testing, gapping of more than 5 mm was defined as construct failure.22 After cyclic loading, each construct was loaded to failure at 1.0 mm/s. Ultimate failure load was defined as the highest load achieved at the maximum displacement before rapid decline in load supported by the construct.
Statistical Analysis
Paired t tests were used to compare the matched pairs of constructs. For all tests, significance was set at P ≤ .05. Post hoc power was calculated for significant results using G*Power Version 3.1.6.23 All data are presented as means (SDs).
Results
After 1000 cycles of displacement, mean (SD) gapping was 3.8 (0.9) mm for the nonaugmented repairs and 3.9 (1.1) mm for the PHA mesh–augmented repairs (P = .879) (Figure 4).
For the nonaugmented repairs, mean (SD) failure displacement was 6.3 (1.7) mm, and mean (SD) ultimate failure load was 472.1 (120.3) N. For the PHA-augmented repairs, failure displacement was 5.5 (1.9) mm, and ultimate failure load was 571.2 (173.0) N. There was no difference in failure displacement (P = .393), but there was a difference in ultimate failure load (P = .042; power = 0.57). During failure testing, mean (SD) tissue deformation was higher (P = .012; power = 0.83) for the PHA-augmented repairs, 1.2 (0.7) mm, than for the nonaugmented repairs, 0.8 (0.5) mm. Failures, which were consistent within pairs, were caused by tissue failure, with sutures pulling through the tissue (4 pairs) or single anchor pullout before ultimate tissue failure (4 pairs). Of the 4 failures with anchor pullout, 3 had anterior anchor pullout, and 1 had posterior anchor pullout. In all specimens with anchor pullout, the second anchor remained stable, and ultimate failure occurred with tissue tearing at the suture interface. There were no significant differences in any metrics between specimens that failed with intact anchors and specimens with single anchor pullout (P ≥ .122). Therefore, both groups were pooled for the failure analysis.
Discussion
RCR augmentation with a synthetic graft is a viable option for improving fixation strength of supraspinatus repairs, as shown in otherwise healthy tendon in the present study. Our hypothesis that there would be decreased gap formation with graft augmentation was not supported, whereas the hypothesis of increased failure loads with graft augmentation was supported. These findings may also be applicable in cases of large tears, revisions, and tendons with poor tissue quality. Simplification of graft application techniques will allow quick and easy arthroscopic augmentation.
Studies of RCRs for large or massive tears have reported retear rates of 25% to 79%.24-26 Latissimus dorsi tendon transfers also show promise in posterosuperior RCRs, with failure rates near 10%.27,28 Although use of PHA patches in RCR augmentation is relatively new, short-term and midterm failure rates are in the range of 20% to 60% in the few small cohorts currently being studied.13,16 It is possible that these rates may improve as indications, surgical experience, and techniques for use of PHA patches are further refined. Regardless, with PHA currently being used in practice, it is important to quantify the biomechanics of the augmentation as a baseline for its performance in reinforcing the tendon–suture interface.
We determined that the initial fixation strength of single-row repairs was higher with the addition of PHA synthetic grafts using a very simple technique. Single-row triple-loaded anchor repairs already provide high initial mechanical strength, and our results are similar to those of another study of this technique.29 Despite the already high mechanical strength of a triple-loaded anchor repair, PHA mesh increased ultimate strength by about 100 N (~25%). Of note, tissue elongation during failure was higher (P = .012; power = 0.83) in the PHA-augmented group (1.2 mm) than in the nonaugmented group (0.8 mm). This was not surprising—failure loads were almost 100 N higher in the PHA-augmented group than in the nonaugmented group. Consequently, much higher forces were placed on the muscle belly, likely resulting in additional elongation of the intact tissue medial to the repair construct.
The ultimate failure loads in our study compare favorably with the biomechanical strength of augmented repairs reported by others.8,9,18 Barber and colleagues18 evaluated an augmented single-row repair with 2 double-loaded suture anchors and an acellular dermal matrix graft. The ultimate failure load of the augmented repairs was 325 N. In contrast, Omae and colleagues8 tested a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft. Ultimate failure load of the augmented repairs was 560 N, similar to our finding. Last, Shea and colleagues9 evaluated a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft, with ultimate failure load of 429 N. The techniques in all 3 studies can be performed arthroscopically but are challenging and require multiple extra sutures and anchors that need management and tying. Our technique provides similar initial fixation strength, has no requirement for extra sutures or anchors, and is very simple to perform.
The supraspinatus tendon is estimated to fail between 800 N and 1000 N.30,31 Biomechanical shoulder simulators use supraspinatus forces in the range of 20 N to 200 N for scapular plane abduction.32-36 Therefore, the single-row repair failures in our study fell between functional and full-thickness failure loads. Studies on the mechanics of degenerated human supraspinatus tendon are limited, but there is evidence the mechanical properties of these tissues are inferior to those of healthy tendon.37 A 100-N increase in failure loads with PHA augmentation may prove highly significant in reinforcing the suture–tendon interface in degenerated tendons.
Adding the mesh did not have any effect on gapping at the repair site after cyclic loading. This finding suggests that construct gapping under cyclic loading is not a function of a reinforced knot–tendon interface but is instead caused by microtearing and cinching of the suture constructs in relation to the underlying bone. Tissue elongation likely was not a strong contributor to overall cyclic gapping, as elongation did not differ between the nonaugmented and augmented repairs (0.5 mm vs 0.7 mm; P = .276) and was small relative to the nearly 4 mm of construct gapping. Gapping may be affected by healing and integration of the mesh into the repaired tendon over time, but this effect could not be captured in the present study. Patients are initially immobilized and passive shoulder motion gradually introduced, in stark contrast to the immediate loading protocol in the present study. Regardless, the 25% increase in overall strength may be clinically important, especially in cases of difficult repair or poor tissue quality.
Our technique simplifies arthroscopic augmentation—stitches are passed through the rotator cuff in simple fashion. Before being tied, the limbs that were passed through the rotator cuff are removed through a cannula and then passed through the synthetic graft.
Study Limitations
This study had several limitations. First, it was a cadaveric biomechanical study that evaluated only time-zero biomechanical properties. Loads were normalized to tendon size, specimens were randomized between sides, and paired specimens were used to minimize the effects of tendon and bone quality on outcome metrics. In addition, donor tendons were representative of otherwise healthy tissue. Chronic tears and associated resorption/atrophy could have affected the magnitude of forces and gapping detected in this study. Theoretically, over time the tendon tissue will adhere to and grow into the mesh, which could minimize potential differences. Studies are needed to determine the effects of healing on long-term repair strength in affected patients. Last, all constructs were performed in open fashion to improve repeatability of construct placement and provide accessibility for Instron testing. Our technique did not directly replicate the arthroscopic approach, but, unlike other augmentation techniques, it is so simple that transition to all-arthroscopic augmentation is realistic.
Patch augmentation increases the cost of materials and operative time and should be considered a limitation of its utility. We do not recommend augmentation in all RCRs, as it likely is cost-ineffective. Instead, we recommend augmentation in cases of poor tissue quality, which could lead to healing failure, revision surgery, and higher overall patient costs beyond the cost of adding augmentation. Similarly, we recommend augmentation for revision cases in which tendon healing has failed and tissue quality is poor. The goal is to prevent another failure.
Conclusion
PHA graft augmentation of single-row triple-loaded anchor repairs of the supraspinatus tendon improves the overall ultimate load to failure by 25%. There was no difference in gap formation after cyclic loading between augmented and nonaugmented repairs. This technique for arthroscopic augmentation can be used to improve initial biomechanical repair strength in tears at risk for failure.
Am J Orthop. 2016;45(7):E527-E533. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Healing after rotator cuff repair (RCR) can be challenging, especially in cases of large and massive tears, revision repairs, and tendons with poor tissue quality.1-3 Poor tissue quality is associated with increased risk for recurrent tears, independent of age and tear size.3 Various techniques have been used to improve tendon fixation strength in these difficult situations, including augmented suture configurations (eg, massive cuff stitches, rip-stop stitches) and tissue grafts (eg, acellular dermal matrix).4-9 Clinical studies have found improved healing rates for larger tears and revision repairs using acellular dermal matrix grafts.6,10 Synthetic patches are another option for RCR augmentation, but limited clinical data and biomechanical evidence support use of synthetic grafts as an augment for RCRs.11-13
Polyhydroxyalkanoates (PHAs) are a class of biodegradable polymers that have been used as orthopedic devices, tissue scaffolds, patches, and other applications with increasing frequency over the past decade.14 In the laboratory, these implanted materials have been shown to support cell migration and growth.15 The PHA family of polymers typically degrades by hydrolytic and bacterial depolymerase mechanisms over 52-plus weeks in vivo.14PHA grafts have been studied in the setting of RCR. An expanded polytetrafluoroethylene scaffold was shown to improve repair mechanics when used as a bursal side graft in an in vitro ovine model.11 The graft increased tendon footprint contact pressure and failure loads by almost 180 N. In clinical studies, poly-L-lactic acid augmentations have been used to reinforce massive RCRs. Lenart and colleagues16 found that 38% of 16 patients with such tears had an intact rotator cuff at 1.2-year follow-up, and improvement in clinical scores. Proctor13 reported on use of a poly-L-lactic acid retrograde patch for reinforcement of massive tears with both single- and double-row repairs in 18 patients. The cohort had more favorable rates of intact cuffs at 12 months (83%) and 42 months (78%), and ASES (American Shoulder and Elbow Surgeons) scores improved from 25 before surgery to 82 at latest follow-up after surgery.
RCR augmentation traditionally has been performed with an open or mini-open technique.6 Recently, several authors have reported on arthroscopic techniques for augmentation with either acellular dermal matrix or synthetic grafts.13,17,18 Most techniques have involved “bridging” with a graft or patch used to stress-shield a single-row repair.8,9,13 This bridging typically involves placing several sutures medial to where the anchor repair stitches pass through the tendon. An alternative is to pass the repair stitches through both the tendon and the graft.17-19 The overall volume of tissue incorporated into the repair stitches (rotator cuff plus graft) is increased with the augmented technique relative to the bridging technique. Both can be technically challenging, but the augmented technique may be easier to perform arthroscopically.9,19 Regardless, these techniques are complicated and require a higher level of arthroscopic skills compared with those required in arthroscopic RCR without a graft. Simplifying arthroscopic graft augmentation likely will increase its utility because, even for skilled surgeons, adding a graft can increase operative time by 20 to 30 minutes. Simplification will also extend use of the technique to surgeons with less experience and proficiency with arthroscopic repair.
We developed a simple method for augmenting single-row RCR with a strip of bioresorbable soft-tissue scaffold. We also conducted a study to evaluate the initial biomechanical properties of single-row RCR in cadaveric shoulder specimens augmented with PHA mesh (BioFiber; Tornier) graft as compared with single-row RCR without augmentation. Both cyclic gap formation and ultimate failure loads and displacement were quantified. We hypothesized that the augmented RCRs would have decreased gap formation and increased ultimate failure loads compared with nonaugmented RCRs. This study was exempt from having to obtain Institutional Review B
Methods
Eight pairs of fresh-frozen cadaver humeri (6 male, 2 female; mean [SD] age, 61 [9] years) were dissected of all soft tissue (except rotator cuff) by Dr. Tashjian, a board-certified, fellowship-trained orthopedic surgeon. There were no qualitative differences in tendon condition between tendons within a pair. The supraspinatus muscle and tendon were separated from the other rotator cuff muscles. The infraspinatus, subscapularis, and teres minor were removed from the humerus. Last, the supraspinatus was resected at its insertion. Humeral pairs were then randomized into augmented and nonaugmented RCRs within each pair.
In the nonaugmented group, the supraspinatus was reattached to its insertion in a single-row RCR with 2 triple-loaded suture anchors (5.5-mm Insite FT Ti, No. 2 Force Fiber suture; Tornier) and 6 simple stitches (Figure 1A). Anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity at about 45° to the bone surface.
In the contralateral shoulders, augmented RCRs were performed. Specimens were prepared exactly as they were for the nonaugmented RCRs, including anchor placement and suture passage. Before knot tying, RCRs were augmented with 2 strips of 13-mm × 23-mm PHA mesh (BioFiber) (Figure 1B). One strip was used to augment the 3 sutures of each anchor, overlying the residual tendon, to reinforce the tendon–knot interface. After each suture was passed through the supraspinatus tendon from the intra-articular surface, the stitch was passed through the strip of PHA mesh. Stitches were separated by 5 mm in each mesh strip. All 6 sutures were then tied with a Revo knot between the free end of each suture leg and the leg that passed through the tendon and mesh.
Each humerus was transected at the midshaft and potted and mounted in an Instron 1331 load frame with Model 8800 controller (Instron). A cryoclamp was used to grasp the supraspinatus muscle belly above the musculotendinous junction (Figure 2).
Three rows of 2-mm fiducial markers were affixed to the bone, tendon, and muscle belly with cyanoacrylate for tracking with a digital video system (DMAS Version 6.5; Spicatek) (Figure 3).21
A 0.1-MPa pre-stress (applied force/tendon cross-sectional area) was applied to each construct to determine the starting position for the deformation profile. Each repair underwent 1000 cycles of uniaxial load-controlled displacement between 0.1 and 1.0 MPa of effective stress at 1 Hz. Effective stress was determined as the ratio of applied force to cross-sectional area of the tendon at harvest to normalize the applied loads between tendons of varying size. During cyclic testing, gapping of more than 5 mm was defined as construct failure.22 After cyclic loading, each construct was loaded to failure at 1.0 mm/s. Ultimate failure load was defined as the highest load achieved at the maximum displacement before rapid decline in load supported by the construct.
Statistical Analysis
Paired t tests were used to compare the matched pairs of constructs. For all tests, significance was set at P ≤ .05. Post hoc power was calculated for significant results using G*Power Version 3.1.6.23 All data are presented as means (SDs).
Results
After 1000 cycles of displacement, mean (SD) gapping was 3.8 (0.9) mm for the nonaugmented repairs and 3.9 (1.1) mm for the PHA mesh–augmented repairs (P = .879) (Figure 4).
For the nonaugmented repairs, mean (SD) failure displacement was 6.3 (1.7) mm, and mean (SD) ultimate failure load was 472.1 (120.3) N. For the PHA-augmented repairs, failure displacement was 5.5 (1.9) mm, and ultimate failure load was 571.2 (173.0) N. There was no difference in failure displacement (P = .393), but there was a difference in ultimate failure load (P = .042; power = 0.57). During failure testing, mean (SD) tissue deformation was higher (P = .012; power = 0.83) for the PHA-augmented repairs, 1.2 (0.7) mm, than for the nonaugmented repairs, 0.8 (0.5) mm. Failures, which were consistent within pairs, were caused by tissue failure, with sutures pulling through the tissue (4 pairs) or single anchor pullout before ultimate tissue failure (4 pairs). Of the 4 failures with anchor pullout, 3 had anterior anchor pullout, and 1 had posterior anchor pullout. In all specimens with anchor pullout, the second anchor remained stable, and ultimate failure occurred with tissue tearing at the suture interface. There were no significant differences in any metrics between specimens that failed with intact anchors and specimens with single anchor pullout (P ≥ .122). Therefore, both groups were pooled for the failure analysis.
Discussion
RCR augmentation with a synthetic graft is a viable option for improving fixation strength of supraspinatus repairs, as shown in otherwise healthy tendon in the present study. Our hypothesis that there would be decreased gap formation with graft augmentation was not supported, whereas the hypothesis of increased failure loads with graft augmentation was supported. These findings may also be applicable in cases of large tears, revisions, and tendons with poor tissue quality. Simplification of graft application techniques will allow quick and easy arthroscopic augmentation.
Studies of RCRs for large or massive tears have reported retear rates of 25% to 79%.24-26 Latissimus dorsi tendon transfers also show promise in posterosuperior RCRs, with failure rates near 10%.27,28 Although use of PHA patches in RCR augmentation is relatively new, short-term and midterm failure rates are in the range of 20% to 60% in the few small cohorts currently being studied.13,16 It is possible that these rates may improve as indications, surgical experience, and techniques for use of PHA patches are further refined. Regardless, with PHA currently being used in practice, it is important to quantify the biomechanics of the augmentation as a baseline for its performance in reinforcing the tendon–suture interface.
We determined that the initial fixation strength of single-row repairs was higher with the addition of PHA synthetic grafts using a very simple technique. Single-row triple-loaded anchor repairs already provide high initial mechanical strength, and our results are similar to those of another study of this technique.29 Despite the already high mechanical strength of a triple-loaded anchor repair, PHA mesh increased ultimate strength by about 100 N (~25%). Of note, tissue elongation during failure was higher (P = .012; power = 0.83) in the PHA-augmented group (1.2 mm) than in the nonaugmented group (0.8 mm). This was not surprising—failure loads were almost 100 N higher in the PHA-augmented group than in the nonaugmented group. Consequently, much higher forces were placed on the muscle belly, likely resulting in additional elongation of the intact tissue medial to the repair construct.
The ultimate failure loads in our study compare favorably with the biomechanical strength of augmented repairs reported by others.8,9,18 Barber and colleagues18 evaluated an augmented single-row repair with 2 double-loaded suture anchors and an acellular dermal matrix graft. The ultimate failure load of the augmented repairs was 325 N. In contrast, Omae and colleagues8 tested a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft. Ultimate failure load of the augmented repairs was 560 N, similar to our finding. Last, Shea and colleagues9 evaluated a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft, with ultimate failure load of 429 N. The techniques in all 3 studies can be performed arthroscopically but are challenging and require multiple extra sutures and anchors that need management and tying. Our technique provides similar initial fixation strength, has no requirement for extra sutures or anchors, and is very simple to perform.
The supraspinatus tendon is estimated to fail between 800 N and 1000 N.30,31 Biomechanical shoulder simulators use supraspinatus forces in the range of 20 N to 200 N for scapular plane abduction.32-36 Therefore, the single-row repair failures in our study fell between functional and full-thickness failure loads. Studies on the mechanics of degenerated human supraspinatus tendon are limited, but there is evidence the mechanical properties of these tissues are inferior to those of healthy tendon.37 A 100-N increase in failure loads with PHA augmentation may prove highly significant in reinforcing the suture–tendon interface in degenerated tendons.
Adding the mesh did not have any effect on gapping at the repair site after cyclic loading. This finding suggests that construct gapping under cyclic loading is not a function of a reinforced knot–tendon interface but is instead caused by microtearing and cinching of the suture constructs in relation to the underlying bone. Tissue elongation likely was not a strong contributor to overall cyclic gapping, as elongation did not differ between the nonaugmented and augmented repairs (0.5 mm vs 0.7 mm; P = .276) and was small relative to the nearly 4 mm of construct gapping. Gapping may be affected by healing and integration of the mesh into the repaired tendon over time, but this effect could not be captured in the present study. Patients are initially immobilized and passive shoulder motion gradually introduced, in stark contrast to the immediate loading protocol in the present study. Regardless, the 25% increase in overall strength may be clinically important, especially in cases of difficult repair or poor tissue quality.
Our technique simplifies arthroscopic augmentation—stitches are passed through the rotator cuff in simple fashion. Before being tied, the limbs that were passed through the rotator cuff are removed through a cannula and then passed through the synthetic graft.
Study Limitations
This study had several limitations. First, it was a cadaveric biomechanical study that evaluated only time-zero biomechanical properties. Loads were normalized to tendon size, specimens were randomized between sides, and paired specimens were used to minimize the effects of tendon and bone quality on outcome metrics. In addition, donor tendons were representative of otherwise healthy tissue. Chronic tears and associated resorption/atrophy could have affected the magnitude of forces and gapping detected in this study. Theoretically, over time the tendon tissue will adhere to and grow into the mesh, which could minimize potential differences. Studies are needed to determine the effects of healing on long-term repair strength in affected patients. Last, all constructs were performed in open fashion to improve repeatability of construct placement and provide accessibility for Instron testing. Our technique did not directly replicate the arthroscopic approach, but, unlike other augmentation techniques, it is so simple that transition to all-arthroscopic augmentation is realistic.
Patch augmentation increases the cost of materials and operative time and should be considered a limitation of its utility. We do not recommend augmentation in all RCRs, as it likely is cost-ineffective. Instead, we recommend augmentation in cases of poor tissue quality, which could lead to healing failure, revision surgery, and higher overall patient costs beyond the cost of adding augmentation. Similarly, we recommend augmentation for revision cases in which tendon healing has failed and tissue quality is poor. The goal is to prevent another failure.
Conclusion
PHA graft augmentation of single-row triple-loaded anchor repairs of the supraspinatus tendon improves the overall ultimate load to failure by 25%. There was no difference in gap formation after cyclic loading between augmented and nonaugmented repairs. This technique for arthroscopic augmentation can be used to improve initial biomechanical repair strength in tears at risk for failure.
Am J Orthop. 2016;45(7):E527-E533. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
2. Keener JD, Wei AS, Kim HM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am. 2010;92(3):590-598.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Barber FA, Herbert MA, Schroeder FA, Aziz-Jacobo J, Mays MM, Rapley JH. Biomechanical advantages of triple-loaded suture anchors compared with double-row rotator cuff repairs. Arthroscopy. 2010;26(3):316-323.
5. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
6. Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872-879.
7. Ma CB, MacGillivray JD, Clabeaux J, Lee S, Otis JC. Biomechanical evaluation of arthroscopic rotator cuff stitches. J Bone Joint Surg Am. 2004;86(6):1211-1216.
8. Omae H, Steinmann SP, Zhao C, et al. Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clin Biomech. 2012;27(8):789-792.
9. Shea KP, Obopilwe E, Sperling JW, Iannotti JP. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg. 2012;21(8):1072-1079.
10. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
11. Beimers L, Lam PH, Murrell GA. The biomechanical effects of polytetrafluoroethylene suture augmentations in lateral-row rotator cuff repairs in an ovine model. J Shoulder Elbow Surg. 2014;23(10):1545-1552.
12. McCarron JA, Milks RA, Chen X, Iannotti JP, Derwin KA. Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg. 2010;19(5):688-696.
13. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23(10):1508-1513.
14. Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7(8):2249-2258.
15. Ellis G, Cano P, Jadraque M, et al. Laser microperforated biodegradable microbial polyhydroxyalkanoate substrates for tissue repair strategies: an infrared microspectroscopy study. Anal Bioanal Chem. 2011;399(7):2379-2388.
16. Lenart BA, Martens KA, Kearns KA, Gillespie RJ, Zoga AC, Williams GR. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg. 2015;24(6):915-921.
17. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
18. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
19. Gilot GJ, Attia AK, Alvarez AM. Arthroscopic repair of rotator cuff tears using extracellular matrix graft. Arthrosc Tech. 2014;3(4):e487-e489.
20. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
21. Kullar RS, Reagan JM, Kolz CW, Burks RT, Henninger HB. Suture placement near the musculotendinous junction in the supraspinatus: implications for rotator cuff repair. Am J Sports Med. 2015;43(1):57-62.
22. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athanasiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
23. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
24. Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24(9):1493-1505.
25. Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy. 2015;31(11):2274-2281.
26. Henry P, Wasserstein D, Park S, et al. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31(12):2472-2480.
27. El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015;97(6):462-469.
28. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
29. Coons DA, Barber FA, Herbert MA. Triple-loaded single-anchor stitch configurations: an analysis of cyclically loaded suture–tendon interface security. Arthroscopy. 2006;22(11):1154-1158.
30. Itoi E, Berglund LJ, Grabowski JJ, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13(4):578-584.
31. Matsuhashi T, Hooke AW, Zhao KD, et al. Tensile properties of a morphologically split supraspinatus tendon. Clin Anat. 2014;27(5):702-706.
32. Apreleva M, Parsons IM 4th, Warner JJ, Fu FH, Woo SL. Experimental investigation of reaction forces at the glenohumeral joint during active abduction. J Shoulder Elbow Surg. 2000;9(5):409-417.
33. Giles JW, Ferreira LM, Athwal GS, Johnson JA. Development and performance evaluation of a multi-PID muscle loading driven in vitro active-motion shoulder simulator and application to assessing reverse total shoulder arthroplasty. J Biomech Eng. 2014;136(12):121007.
34. Hansen ML, Otis JC, Johnson JS, Cordasco FA, Craig EV, Warren RF. Biomechanics of massive rotator cuff tears: implications for treatment. J Bone Joint Surg Am. 2008;90(2):316-325.
35. Henninger HB, Barg A, Anderson AE, Bachus KN, Tashjian RZ, Burks RT. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(4):483-490.
36. Mihata T, Gates J, McGarry MH, Lee J, Kinoshita M, Lee TQ. Effect of rotator cuff muscle imbalance on forceful internal impingement and peel-back of the superior labrum: a cadaveric study. Am J Sports Med. 2009;37(11):2222-2227.
37. Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone–tendon complex. J Orthop Res. 1997;15(5):719-726.
1. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
2. Keener JD, Wei AS, Kim HM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am. 2010;92(3):590-598.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Barber FA, Herbert MA, Schroeder FA, Aziz-Jacobo J, Mays MM, Rapley JH. Biomechanical advantages of triple-loaded suture anchors compared with double-row rotator cuff repairs. Arthroscopy. 2010;26(3):316-323.
5. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
6. Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872-879.
7. Ma CB, MacGillivray JD, Clabeaux J, Lee S, Otis JC. Biomechanical evaluation of arthroscopic rotator cuff stitches. J Bone Joint Surg Am. 2004;86(6):1211-1216.
8. Omae H, Steinmann SP, Zhao C, et al. Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clin Biomech. 2012;27(8):789-792.
9. Shea KP, Obopilwe E, Sperling JW, Iannotti JP. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg. 2012;21(8):1072-1079.
10. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
11. Beimers L, Lam PH, Murrell GA. The biomechanical effects of polytetrafluoroethylene suture augmentations in lateral-row rotator cuff repairs in an ovine model. J Shoulder Elbow Surg. 2014;23(10):1545-1552.
12. McCarron JA, Milks RA, Chen X, Iannotti JP, Derwin KA. Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg. 2010;19(5):688-696.
13. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23(10):1508-1513.
14. Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7(8):2249-2258.
15. Ellis G, Cano P, Jadraque M, et al. Laser microperforated biodegradable microbial polyhydroxyalkanoate substrates for tissue repair strategies: an infrared microspectroscopy study. Anal Bioanal Chem. 2011;399(7):2379-2388.
16. Lenart BA, Martens KA, Kearns KA, Gillespie RJ, Zoga AC, Williams GR. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg. 2015;24(6):915-921.
17. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
18. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
19. Gilot GJ, Attia AK, Alvarez AM. Arthroscopic repair of rotator cuff tears using extracellular matrix graft. Arthrosc Tech. 2014;3(4):e487-e489.
20. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
21. Kullar RS, Reagan JM, Kolz CW, Burks RT, Henninger HB. Suture placement near the musculotendinous junction in the supraspinatus: implications for rotator cuff repair. Am J Sports Med. 2015;43(1):57-62.
22. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athanasiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
23. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
24. Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24(9):1493-1505.
25. Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy. 2015;31(11):2274-2281.
26. Henry P, Wasserstein D, Park S, et al. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31(12):2472-2480.
27. El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015;97(6):462-469.
28. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
29. Coons DA, Barber FA, Herbert MA. Triple-loaded single-anchor stitch configurations: an analysis of cyclically loaded suture–tendon interface security. Arthroscopy. 2006;22(11):1154-1158.
30. Itoi E, Berglund LJ, Grabowski JJ, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13(4):578-584.
31. Matsuhashi T, Hooke AW, Zhao KD, et al. Tensile properties of a morphologically split supraspinatus tendon. Clin Anat. 2014;27(5):702-706.
32. Apreleva M, Parsons IM 4th, Warner JJ, Fu FH, Woo SL. Experimental investigation of reaction forces at the glenohumeral joint during active abduction. J Shoulder Elbow Surg. 2000;9(5):409-417.
33. Giles JW, Ferreira LM, Athwal GS, Johnson JA. Development and performance evaluation of a multi-PID muscle loading driven in vitro active-motion shoulder simulator and application to assessing reverse total shoulder arthroplasty. J Biomech Eng. 2014;136(12):121007.
34. Hansen ML, Otis JC, Johnson JS, Cordasco FA, Craig EV, Warren RF. Biomechanics of massive rotator cuff tears: implications for treatment. J Bone Joint Surg Am. 2008;90(2):316-325.
35. Henninger HB, Barg A, Anderson AE, Bachus KN, Tashjian RZ, Burks RT. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(4):483-490.
36. Mihata T, Gates J, McGarry MH, Lee J, Kinoshita M, Lee TQ. Effect of rotator cuff muscle imbalance on forceful internal impingement and peel-back of the superior labrum: a cadaveric study. Am J Sports Med. 2009;37(11):2222-2227.
37. Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone–tendon complex. J Orthop Res. 1997;15(5):719-726.
An Update on Management of Syndesmosis Injury: A National US Database Study
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
Prevalence of Low Vitamin D Levels in Patients With Orthopedic Trauma
The role of vitamin D in general health maintenance is a topic of increasing interest and importance in the medical community. Not only has vitamin D deficiency been linked to a myriad of nonorthopedic maladies, including cancer, diabetes, and cardiovascular disease, but it has demonstrated an adverse effect on musculoskeletal health.1 Authors have found a correlation between vitamin D deficiency and muscle weakness, fragility fractures, and, most recently, fracture nonunion.1 Despite the detrimental effects of vitamin D deficiency on musculoskeletal and general health, evidence exists that vitamin D deficiency is surprisingly prevalent.2 This deficiency is known to be associated with increasing age, but recent studies have also found alarming rates of deficiency in younger populations.3,4
Although there has been some discussion regarding optimal serum levels of 25-hydroxyvitamin D, most experts have defined vitamin D deficiency as a 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.5 Hollis and Wagner5 found increased serum parathyroid hormone and bone resorption and impaired dietary absorption of calcium when 25-hydroxyvitamin D levels were under 32 ng/mL. Given these data, a 25-hydroxyvitamin D level of 21 to 32 ng/mL (52-72 nmol/L) can be considered as indicating a relative insufficiency of vitamin D, and a level of 20 ng/mL or less can be considered as indicating vitamin D deficiency.
Vitamin D plays a vital role in bone metabolism and has been implicated in increased fracture risk and in fracture healing ability. Therefore, documenting the prevalence of vitamin D deficiency in patients with trauma is the first step in raising awareness among orthopedic traumatologists and further developing a screening-and-treatment strategy for vitamin D deficiency in these patients. Steele and colleagues6 retrospectively studied 44 patients with high- and low-energy fractures and found an almost 60% prevalence of vitamin D insufficiency. If vitamin D insufficiency is this prevalent, treatment protocols for patients with fractures may require modifications that include routine screening and treatment for low vitamin D levels.
After noting a regular occurrence of hypovitaminosis D in our patient population (independent of age, sex, or medical comorbidities), we conducted a study to determine the prevalence of vitamin D deficiency in a large orthopedic trauma population.
Patients and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the charts of all patients with a fracture treated by 1 of 4 orthopedic traumatologists within a 21-month period (January 1, 2009 to September 30, 2010). Acute fracture and recorded 25-hydroxyvitamin D level were the primary criteria for study inclusion. Given the concern about vitamin D deficiency, it became common protocol to check the serum 25-hydroxyvitamin D levels of patients with acute fractures during the review period. Exclusion criteria were age under 18 years and presence of vitamin D deficiency risk factors, including renal insufficiency (creatinine level, ≥2 mg/dL), malabsorption, gastrectomy, active liver disease, acute myocardial infarction, alcoholism, anorexia nervosa, and steroid dependency.
During the period studied, 1830 patients over age 18 years were treated by 4 fellowship-trained orthopedic traumatologists. Of these patients, 889 (487 female, 402 male) met the inclusion criteria. Mean age was 53.8 years. Demographic data (age, sex, race, independent living status, comorbid medical conditions, medications) were collected from the patients’ medical records. Clinical data collected were mechanism of injury, fracture location and type, injury date, surgery date and surgical procedure performed (when applicable), and serum 25-hydroxyvitamin D levels.
Statistical Methods
Descriptive statistics (mean, median, mode) were calculated. The χ2 test was used when all cell frequencies were more than 5, and the Fisher exact probability test was used when any cell frequency was 5 or less. Prevalence of vitamin D deficiency and insufficiency was calculated in multiple patient populations. Patients were analyzed according to age and sex subgroups.
Definitions
Vitamin D deficiency was defined as a serum 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.2 As the serum test was performed independent of the investigators and with use of standard medical laboratory protocols and techniques, there should be no bias in the results. We had intended to have all patients undergo serum testing during the review period because that was our usual protocol. However, test results were available for only 889 (49%) of the 1830 patients with orthopedic trauma during the review period. Although a false-positive is theoretically possible, this series of orthopedic trauma patients is the largest in the literature and therefore should be more accurate than the previously reported small series.
Results
There were no significant (P < .05) age or sex differences in prevalence of vitamin D deficiency or insufficiency in our patient population. Overall prevalence of deficiency/insufficiency was 77.39%, and prevalence of deficiency alone was 39.03% (Table 1).
Women in the 18- to 25-year age group had a lower prevalence of deficiency (25%; P = .41) and insufficiency (41.7%; P = .16) than women in the other age groups (Table 3).
Discussion
We conducted this study to determine the prevalence of vitamin D deficiency in a large population of patients with orthopedic trauma. Results showed that vitamin D deficiency and insufficiency were prevalent in this population, which to our knowledge is the largest studied for vitamin D deficiency. In a 6-month study of 44 fractures, Steele and colleagues6 found an overall 60% rate of deficiency/insufficiency. Although their investigation is important—it was the first of its kind to evaluate patients with various fracture types, including those with high-energy causes—its numbers were small, and the period evaluated (June 1, 2006 to February 1, 2007) was short (8 months). Use of that time frame may have led to an underestimate of the prevalence of vitamin D deficiency, as vitamin D levels are higher in late summer because of increased sun exposure. Our study of 889 patients over 21 months allowed for seasonal variability of vitamin D levels. We did not notice a specific difference in patients who were treated during winter vs summer. Furthermore, our 77% prevalence of vitamin D insufficiency and 39% prevalence of vitamin D deficiency indicate how widespread low vitamin D levels are in a large Midwestern orthopedic trauma population. In the Pacific Northwest, Bee and colleagues7 studied seasonal differences in patients with surgically treated fractures and found an average difference of 3 ng/mL between winter and summer serum levels. However, the real issue, which should not be overlooked, is that the average 25-hydroxyvitamin D level was under 30 ng/mL in both cohorts (26.4 ng/mL in winter vs 29.8 ng/mL in summer). The emphasis should be that both levels were insufficient and that seasonal variance does not really change prevalence.
With use of the current definitions, it has been estimated that 1 billion people worldwide have vitamin D deficiency or insufficiency, with the elderly and certain ethnic populations at higher risk.8-10Vitamin D deficiency is a common diagnosis among elderly patients with hip fractures. According to various reports, 60% to 90% of patients treated for hip fractures are deficient or insufficient in vitamin D.8,9Hypovitaminosis D has also been noted in medical inpatients with and without risks for this deficiency.2 Surprisingly, low vitamin D levels are not isolated to the elderly. In Massachusetts, Gordon and colleagues11 found a 52% prevalence of vitamin D deficiency in Hispanic and black adolescents. Nesby-O’Dell and colleagues10 found that 42% of 15- to 49-year-old black women in the United States had vitamin D deficiency at the end of winter. Bogunovic and colleagues12 noted 5.5 times higher risk of low vitamin D levels in patients with darker skin tones. Although vitamin D deficiency has been linked to specific races, it frequently occurs in lower-risk populations as well. Sullivan and colleagues4 found a 48% prevalence of vitamin D deficiency in white preadolescent girls in Maine. Tangpricha and colleagues3 reported a 32% prevalence of vitamin D deficiency in otherwise fit healthcare providers sampled at a Boston hospital. Bogunovic and colleagues12 also showed that patients between ages 18 years and 50 years, and men, were more likely to have low vitamin D levels.
Establishing the prevalence of hypovitaminosis D in orthopedic trauma patients is needed in order to raise awareness of the disease and modify screening and treatment protocols. Brinker and O’Connor13 found vitamin D deficiency in 68% of patients with fracture nonunions, which suggests that hypovitaminosis D may partly account for difficulty in achieving fracture union. Bogunovic and colleagues12 found vitamin D insufficiency in 43% of 723 patients who underwent orthopedic surgery. Isolating the 121 patients on the trauma service revealed a 66% prevalence of low vitamin D levels. Our 77% prevalence of low vitamin D levels in 889 patients adds to the evidence that low levels are common in patients with orthopedic trauma. Understanding the importance of vitamin D deficiency can be significant in reducing the risk of complications, including delayed unions and nonunions, associated with treating orthopedic trauma cases.
Although our study indicates an alarming prevalence of insufficient vitamin D levels in our patient population, it does not provide a cause-and-effect link between low serum 25-hydroxyvitamin D levels and risk of fracture or nonunion. However, further investigations may yield clinically relevant data linking hypovitaminosis D with fracture risk. Although we did not include patients with nonunion in this study, new prospective investigations will address nonunions and subgroup analysis of race, fracture type, management type (surgical vs nonsurgical), injury date (to determine seasonal effect), and different treatment regimens.
The primary limitation of this study was its retrospective design. In addition, though we collected vitamin D data from 889 patients with acute fracture, our serum collection protocols were not standardized. Most patients who were admitted during initial orthopedic consultation in the emergency department had serum 25-hydroxyvitamin D levels drawn during their hospital stay, and patients initially treated in an ambulatory setting may not have had serum vitamin D levels drawn for up to 2 weeks after injury (the significance of this delay is unknown). Furthermore, the serum result rate for the overall orthopedic trauma population during the review period was only 49%, which could indicate selection bias. There are multiple explanations for the low rate. As with any new protocol or method, it takes time for the order to become standard practice; in the early stages, individuals can forget to ask for the test. In addition, during the review period, the serum test was also relatively new at our facility, and it was a “send-out” test, which could partly account for the lack of consistency. For example, some specimens were lost, and, in a number of other cases, excluded patients mistakenly had their 1,25-hydroxyvitamin D levels measured and were not comparable to included patients. Nevertheless, our sample of 889 patients with acute fractures remains the largest (by several hundred) reported in the literature.
From a practical standpoint, the present results were useful in updating our treatment protocols. Now we typically treat patients only prophylactically, with 50,000 units of vitamin D2 for 8 weeks and daily vitamin D3 and calcium until fracture healing. Patients are encouraged to continue daily vitamin D and calcium supplementation after fracture healing to maintain bone health. Compliance, however, remains a continued challenge and lack thereof can potentially explain the confusing effect of a supplementation protocol on the serum 25-hydroxyvitamin D level.14 The only patients who are not given prophylactic treatment are those who previously had been denied it (patients with chronic kidney disease or elevated blood calcium levels).
Vitamin D deficiency and insufficiency are prevalent in patients with orthopedic trauma. Studies are needed to further elucidate the relationship between low vitamin D levels and risk of complications. Retrospectively, without compliance monitoring, we have not seen a direct correlation with fracture complications.15 Our goal here was to increase orthopedic surgeons’ awareness of the problem and of the need to consider addressing low serum vitamin D levels. The treatment is low cost and low risk. The ultimate goal—if there is a prospective direct correlation between low serum vitamin D levels and complications—is to develop treatment strategies that can effectively lower the prevalence of low vitamin D levels.
Am J Orthop. 2016;45(7):E522-E526. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Zaidi SA, Singh G, Owojori O, et al. Vitamin D deficiency in medical inpatients: a retrospective study of implications of untreated versus treated deficiency. Nutr Metab Insights. 2016;9:65-69.
2. Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777-783.
3. Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112(8):659-662.
4. Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105(6):971-974.
5. Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352(5):515-516.
6. Steele B, Serota A, Helfet DL, Peterson M, Lyman S, Lane JM. Vitamin D deficiency: a common occurrence in both high- and low-energy fractures. HSS J. 2008;4(2):143-148.
7. Bee CR, Sheerin DV, Wuest TK, Fitzpatrick DC. Serum vitamin D levels in orthopaedic trauma patients living in the northwestern United States. J Orthop Trauma. 2013;27(5):e103-e106.
8. Bischoff-Ferrari HA, Can U, Staehelin HB, et al. Severe vitamin D deficiency in Swiss hip fracture patients. Bone. 2008;42(3):597-602.
9. Pieper CF, Colon-Emeric C, Caminis J, et al. Distribution and correlates of serum 25-hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother. 2007;5(4):335-340.
10. Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187-192.
11. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531-537.
12. Bogunovic L, Kim AD, Beamer BS, Nguyen J, Lane JM. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92(13):2300-2304.
13. Brinker MR, O’Connor DP. Outcomes of tibial nonunion in older adults following treatment using the Ilizarov method. J Orthop Trauma. 2007;21(9):634-642.
14. Robertson DS, Jenkins T, Murtha YM, et al. Effectiveness of vitamin D therapy in orthopaedic trauma patients. J Orthop Trauma. 2015;29(11):e451-e453.
15. Bodendorfer BM, Cook JL, Robertson DS, et al. Do 25-hydroxyvitamin D levels correlate with fracture complications: J Orthop Trauma. 2016;30(9):e312-e317.
The role of vitamin D in general health maintenance is a topic of increasing interest and importance in the medical community. Not only has vitamin D deficiency been linked to a myriad of nonorthopedic maladies, including cancer, diabetes, and cardiovascular disease, but it has demonstrated an adverse effect on musculoskeletal health.1 Authors have found a correlation between vitamin D deficiency and muscle weakness, fragility fractures, and, most recently, fracture nonunion.1 Despite the detrimental effects of vitamin D deficiency on musculoskeletal and general health, evidence exists that vitamin D deficiency is surprisingly prevalent.2 This deficiency is known to be associated with increasing age, but recent studies have also found alarming rates of deficiency in younger populations.3,4
Although there has been some discussion regarding optimal serum levels of 25-hydroxyvitamin D, most experts have defined vitamin D deficiency as a 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.5 Hollis and Wagner5 found increased serum parathyroid hormone and bone resorption and impaired dietary absorption of calcium when 25-hydroxyvitamin D levels were under 32 ng/mL. Given these data, a 25-hydroxyvitamin D level of 21 to 32 ng/mL (52-72 nmol/L) can be considered as indicating a relative insufficiency of vitamin D, and a level of 20 ng/mL or less can be considered as indicating vitamin D deficiency.
Vitamin D plays a vital role in bone metabolism and has been implicated in increased fracture risk and in fracture healing ability. Therefore, documenting the prevalence of vitamin D deficiency in patients with trauma is the first step in raising awareness among orthopedic traumatologists and further developing a screening-and-treatment strategy for vitamin D deficiency in these patients. Steele and colleagues6 retrospectively studied 44 patients with high- and low-energy fractures and found an almost 60% prevalence of vitamin D insufficiency. If vitamin D insufficiency is this prevalent, treatment protocols for patients with fractures may require modifications that include routine screening and treatment for low vitamin D levels.
After noting a regular occurrence of hypovitaminosis D in our patient population (independent of age, sex, or medical comorbidities), we conducted a study to determine the prevalence of vitamin D deficiency in a large orthopedic trauma population.
Patients and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the charts of all patients with a fracture treated by 1 of 4 orthopedic traumatologists within a 21-month period (January 1, 2009 to September 30, 2010). Acute fracture and recorded 25-hydroxyvitamin D level were the primary criteria for study inclusion. Given the concern about vitamin D deficiency, it became common protocol to check the serum 25-hydroxyvitamin D levels of patients with acute fractures during the review period. Exclusion criteria were age under 18 years and presence of vitamin D deficiency risk factors, including renal insufficiency (creatinine level, ≥2 mg/dL), malabsorption, gastrectomy, active liver disease, acute myocardial infarction, alcoholism, anorexia nervosa, and steroid dependency.
During the period studied, 1830 patients over age 18 years were treated by 4 fellowship-trained orthopedic traumatologists. Of these patients, 889 (487 female, 402 male) met the inclusion criteria. Mean age was 53.8 years. Demographic data (age, sex, race, independent living status, comorbid medical conditions, medications) were collected from the patients’ medical records. Clinical data collected were mechanism of injury, fracture location and type, injury date, surgery date and surgical procedure performed (when applicable), and serum 25-hydroxyvitamin D levels.
Statistical Methods
Descriptive statistics (mean, median, mode) were calculated. The χ2 test was used when all cell frequencies were more than 5, and the Fisher exact probability test was used when any cell frequency was 5 or less. Prevalence of vitamin D deficiency and insufficiency was calculated in multiple patient populations. Patients were analyzed according to age and sex subgroups.
Definitions
Vitamin D deficiency was defined as a serum 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.2 As the serum test was performed independent of the investigators and with use of standard medical laboratory protocols and techniques, there should be no bias in the results. We had intended to have all patients undergo serum testing during the review period because that was our usual protocol. However, test results were available for only 889 (49%) of the 1830 patients with orthopedic trauma during the review period. Although a false-positive is theoretically possible, this series of orthopedic trauma patients is the largest in the literature and therefore should be more accurate than the previously reported small series.
Results
There were no significant (P < .05) age or sex differences in prevalence of vitamin D deficiency or insufficiency in our patient population. Overall prevalence of deficiency/insufficiency was 77.39%, and prevalence of deficiency alone was 39.03% (Table 1).
Women in the 18- to 25-year age group had a lower prevalence of deficiency (25%; P = .41) and insufficiency (41.7%; P = .16) than women in the other age groups (Table 3).
Discussion
We conducted this study to determine the prevalence of vitamin D deficiency in a large population of patients with orthopedic trauma. Results showed that vitamin D deficiency and insufficiency were prevalent in this population, which to our knowledge is the largest studied for vitamin D deficiency. In a 6-month study of 44 fractures, Steele and colleagues6 found an overall 60% rate of deficiency/insufficiency. Although their investigation is important—it was the first of its kind to evaluate patients with various fracture types, including those with high-energy causes—its numbers were small, and the period evaluated (June 1, 2006 to February 1, 2007) was short (8 months). Use of that time frame may have led to an underestimate of the prevalence of vitamin D deficiency, as vitamin D levels are higher in late summer because of increased sun exposure. Our study of 889 patients over 21 months allowed for seasonal variability of vitamin D levels. We did not notice a specific difference in patients who were treated during winter vs summer. Furthermore, our 77% prevalence of vitamin D insufficiency and 39% prevalence of vitamin D deficiency indicate how widespread low vitamin D levels are in a large Midwestern orthopedic trauma population. In the Pacific Northwest, Bee and colleagues7 studied seasonal differences in patients with surgically treated fractures and found an average difference of 3 ng/mL between winter and summer serum levels. However, the real issue, which should not be overlooked, is that the average 25-hydroxyvitamin D level was under 30 ng/mL in both cohorts (26.4 ng/mL in winter vs 29.8 ng/mL in summer). The emphasis should be that both levels were insufficient and that seasonal variance does not really change prevalence.
With use of the current definitions, it has been estimated that 1 billion people worldwide have vitamin D deficiency or insufficiency, with the elderly and certain ethnic populations at higher risk.8-10Vitamin D deficiency is a common diagnosis among elderly patients with hip fractures. According to various reports, 60% to 90% of patients treated for hip fractures are deficient or insufficient in vitamin D.8,9Hypovitaminosis D has also been noted in medical inpatients with and without risks for this deficiency.2 Surprisingly, low vitamin D levels are not isolated to the elderly. In Massachusetts, Gordon and colleagues11 found a 52% prevalence of vitamin D deficiency in Hispanic and black adolescents. Nesby-O’Dell and colleagues10 found that 42% of 15- to 49-year-old black women in the United States had vitamin D deficiency at the end of winter. Bogunovic and colleagues12 noted 5.5 times higher risk of low vitamin D levels in patients with darker skin tones. Although vitamin D deficiency has been linked to specific races, it frequently occurs in lower-risk populations as well. Sullivan and colleagues4 found a 48% prevalence of vitamin D deficiency in white preadolescent girls in Maine. Tangpricha and colleagues3 reported a 32% prevalence of vitamin D deficiency in otherwise fit healthcare providers sampled at a Boston hospital. Bogunovic and colleagues12 also showed that patients between ages 18 years and 50 years, and men, were more likely to have low vitamin D levels.
Establishing the prevalence of hypovitaminosis D in orthopedic trauma patients is needed in order to raise awareness of the disease and modify screening and treatment protocols. Brinker and O’Connor13 found vitamin D deficiency in 68% of patients with fracture nonunions, which suggests that hypovitaminosis D may partly account for difficulty in achieving fracture union. Bogunovic and colleagues12 found vitamin D insufficiency in 43% of 723 patients who underwent orthopedic surgery. Isolating the 121 patients on the trauma service revealed a 66% prevalence of low vitamin D levels. Our 77% prevalence of low vitamin D levels in 889 patients adds to the evidence that low levels are common in patients with orthopedic trauma. Understanding the importance of vitamin D deficiency can be significant in reducing the risk of complications, including delayed unions and nonunions, associated with treating orthopedic trauma cases.
Although our study indicates an alarming prevalence of insufficient vitamin D levels in our patient population, it does not provide a cause-and-effect link between low serum 25-hydroxyvitamin D levels and risk of fracture or nonunion. However, further investigations may yield clinically relevant data linking hypovitaminosis D with fracture risk. Although we did not include patients with nonunion in this study, new prospective investigations will address nonunions and subgroup analysis of race, fracture type, management type (surgical vs nonsurgical), injury date (to determine seasonal effect), and different treatment regimens.
The primary limitation of this study was its retrospective design. In addition, though we collected vitamin D data from 889 patients with acute fracture, our serum collection protocols were not standardized. Most patients who were admitted during initial orthopedic consultation in the emergency department had serum 25-hydroxyvitamin D levels drawn during their hospital stay, and patients initially treated in an ambulatory setting may not have had serum vitamin D levels drawn for up to 2 weeks after injury (the significance of this delay is unknown). Furthermore, the serum result rate for the overall orthopedic trauma population during the review period was only 49%, which could indicate selection bias. There are multiple explanations for the low rate. As with any new protocol or method, it takes time for the order to become standard practice; in the early stages, individuals can forget to ask for the test. In addition, during the review period, the serum test was also relatively new at our facility, and it was a “send-out” test, which could partly account for the lack of consistency. For example, some specimens were lost, and, in a number of other cases, excluded patients mistakenly had their 1,25-hydroxyvitamin D levels measured and were not comparable to included patients. Nevertheless, our sample of 889 patients with acute fractures remains the largest (by several hundred) reported in the literature.
From a practical standpoint, the present results were useful in updating our treatment protocols. Now we typically treat patients only prophylactically, with 50,000 units of vitamin D2 for 8 weeks and daily vitamin D3 and calcium until fracture healing. Patients are encouraged to continue daily vitamin D and calcium supplementation after fracture healing to maintain bone health. Compliance, however, remains a continued challenge and lack thereof can potentially explain the confusing effect of a supplementation protocol on the serum 25-hydroxyvitamin D level.14 The only patients who are not given prophylactic treatment are those who previously had been denied it (patients with chronic kidney disease or elevated blood calcium levels).
Vitamin D deficiency and insufficiency are prevalent in patients with orthopedic trauma. Studies are needed to further elucidate the relationship between low vitamin D levels and risk of complications. Retrospectively, without compliance monitoring, we have not seen a direct correlation with fracture complications.15 Our goal here was to increase orthopedic surgeons’ awareness of the problem and of the need to consider addressing low serum vitamin D levels. The treatment is low cost and low risk. The ultimate goal—if there is a prospective direct correlation between low serum vitamin D levels and complications—is to develop treatment strategies that can effectively lower the prevalence of low vitamin D levels.
Am J Orthop. 2016;45(7):E522-E526. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The role of vitamin D in general health maintenance is a topic of increasing interest and importance in the medical community. Not only has vitamin D deficiency been linked to a myriad of nonorthopedic maladies, including cancer, diabetes, and cardiovascular disease, but it has demonstrated an adverse effect on musculoskeletal health.1 Authors have found a correlation between vitamin D deficiency and muscle weakness, fragility fractures, and, most recently, fracture nonunion.1 Despite the detrimental effects of vitamin D deficiency on musculoskeletal and general health, evidence exists that vitamin D deficiency is surprisingly prevalent.2 This deficiency is known to be associated with increasing age, but recent studies have also found alarming rates of deficiency in younger populations.3,4
Although there has been some discussion regarding optimal serum levels of 25-hydroxyvitamin D, most experts have defined vitamin D deficiency as a 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.5 Hollis and Wagner5 found increased serum parathyroid hormone and bone resorption and impaired dietary absorption of calcium when 25-hydroxyvitamin D levels were under 32 ng/mL. Given these data, a 25-hydroxyvitamin D level of 21 to 32 ng/mL (52-72 nmol/L) can be considered as indicating a relative insufficiency of vitamin D, and a level of 20 ng/mL or less can be considered as indicating vitamin D deficiency.
Vitamin D plays a vital role in bone metabolism and has been implicated in increased fracture risk and in fracture healing ability. Therefore, documenting the prevalence of vitamin D deficiency in patients with trauma is the first step in raising awareness among orthopedic traumatologists and further developing a screening-and-treatment strategy for vitamin D deficiency in these patients. Steele and colleagues6 retrospectively studied 44 patients with high- and low-energy fractures and found an almost 60% prevalence of vitamin D insufficiency. If vitamin D insufficiency is this prevalent, treatment protocols for patients with fractures may require modifications that include routine screening and treatment for low vitamin D levels.
After noting a regular occurrence of hypovitaminosis D in our patient population (independent of age, sex, or medical comorbidities), we conducted a study to determine the prevalence of vitamin D deficiency in a large orthopedic trauma population.
Patients and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the charts of all patients with a fracture treated by 1 of 4 orthopedic traumatologists within a 21-month period (January 1, 2009 to September 30, 2010). Acute fracture and recorded 25-hydroxyvitamin D level were the primary criteria for study inclusion. Given the concern about vitamin D deficiency, it became common protocol to check the serum 25-hydroxyvitamin D levels of patients with acute fractures during the review period. Exclusion criteria were age under 18 years and presence of vitamin D deficiency risk factors, including renal insufficiency (creatinine level, ≥2 mg/dL), malabsorption, gastrectomy, active liver disease, acute myocardial infarction, alcoholism, anorexia nervosa, and steroid dependency.
During the period studied, 1830 patients over age 18 years were treated by 4 fellowship-trained orthopedic traumatologists. Of these patients, 889 (487 female, 402 male) met the inclusion criteria. Mean age was 53.8 years. Demographic data (age, sex, race, independent living status, comorbid medical conditions, medications) were collected from the patients’ medical records. Clinical data collected were mechanism of injury, fracture location and type, injury date, surgery date and surgical procedure performed (when applicable), and serum 25-hydroxyvitamin D levels.
Statistical Methods
Descriptive statistics (mean, median, mode) were calculated. The χ2 test was used when all cell frequencies were more than 5, and the Fisher exact probability test was used when any cell frequency was 5 or less. Prevalence of vitamin D deficiency and insufficiency was calculated in multiple patient populations. Patients were analyzed according to age and sex subgroups.
Definitions
Vitamin D deficiency was defined as a serum 25-hydroxyvitamin D level of 20 ng/mL or less and insufficiency as 21 to 32 ng/mL.2 As the serum test was performed independent of the investigators and with use of standard medical laboratory protocols and techniques, there should be no bias in the results. We had intended to have all patients undergo serum testing during the review period because that was our usual protocol. However, test results were available for only 889 (49%) of the 1830 patients with orthopedic trauma during the review period. Although a false-positive is theoretically possible, this series of orthopedic trauma patients is the largest in the literature and therefore should be more accurate than the previously reported small series.
Results
There were no significant (P < .05) age or sex differences in prevalence of vitamin D deficiency or insufficiency in our patient population. Overall prevalence of deficiency/insufficiency was 77.39%, and prevalence of deficiency alone was 39.03% (Table 1).
Women in the 18- to 25-year age group had a lower prevalence of deficiency (25%; P = .41) and insufficiency (41.7%; P = .16) than women in the other age groups (Table 3).
Discussion
We conducted this study to determine the prevalence of vitamin D deficiency in a large population of patients with orthopedic trauma. Results showed that vitamin D deficiency and insufficiency were prevalent in this population, which to our knowledge is the largest studied for vitamin D deficiency. In a 6-month study of 44 fractures, Steele and colleagues6 found an overall 60% rate of deficiency/insufficiency. Although their investigation is important—it was the first of its kind to evaluate patients with various fracture types, including those with high-energy causes—its numbers were small, and the period evaluated (June 1, 2006 to February 1, 2007) was short (8 months). Use of that time frame may have led to an underestimate of the prevalence of vitamin D deficiency, as vitamin D levels are higher in late summer because of increased sun exposure. Our study of 889 patients over 21 months allowed for seasonal variability of vitamin D levels. We did not notice a specific difference in patients who were treated during winter vs summer. Furthermore, our 77% prevalence of vitamin D insufficiency and 39% prevalence of vitamin D deficiency indicate how widespread low vitamin D levels are in a large Midwestern orthopedic trauma population. In the Pacific Northwest, Bee and colleagues7 studied seasonal differences in patients with surgically treated fractures and found an average difference of 3 ng/mL between winter and summer serum levels. However, the real issue, which should not be overlooked, is that the average 25-hydroxyvitamin D level was under 30 ng/mL in both cohorts (26.4 ng/mL in winter vs 29.8 ng/mL in summer). The emphasis should be that both levels were insufficient and that seasonal variance does not really change prevalence.
With use of the current definitions, it has been estimated that 1 billion people worldwide have vitamin D deficiency or insufficiency, with the elderly and certain ethnic populations at higher risk.8-10Vitamin D deficiency is a common diagnosis among elderly patients with hip fractures. According to various reports, 60% to 90% of patients treated for hip fractures are deficient or insufficient in vitamin D.8,9Hypovitaminosis D has also been noted in medical inpatients with and without risks for this deficiency.2 Surprisingly, low vitamin D levels are not isolated to the elderly. In Massachusetts, Gordon and colleagues11 found a 52% prevalence of vitamin D deficiency in Hispanic and black adolescents. Nesby-O’Dell and colleagues10 found that 42% of 15- to 49-year-old black women in the United States had vitamin D deficiency at the end of winter. Bogunovic and colleagues12 noted 5.5 times higher risk of low vitamin D levels in patients with darker skin tones. Although vitamin D deficiency has been linked to specific races, it frequently occurs in lower-risk populations as well. Sullivan and colleagues4 found a 48% prevalence of vitamin D deficiency in white preadolescent girls in Maine. Tangpricha and colleagues3 reported a 32% prevalence of vitamin D deficiency in otherwise fit healthcare providers sampled at a Boston hospital. Bogunovic and colleagues12 also showed that patients between ages 18 years and 50 years, and men, were more likely to have low vitamin D levels.
Establishing the prevalence of hypovitaminosis D in orthopedic trauma patients is needed in order to raise awareness of the disease and modify screening and treatment protocols. Brinker and O’Connor13 found vitamin D deficiency in 68% of patients with fracture nonunions, which suggests that hypovitaminosis D may partly account for difficulty in achieving fracture union. Bogunovic and colleagues12 found vitamin D insufficiency in 43% of 723 patients who underwent orthopedic surgery. Isolating the 121 patients on the trauma service revealed a 66% prevalence of low vitamin D levels. Our 77% prevalence of low vitamin D levels in 889 patients adds to the evidence that low levels are common in patients with orthopedic trauma. Understanding the importance of vitamin D deficiency can be significant in reducing the risk of complications, including delayed unions and nonunions, associated with treating orthopedic trauma cases.
Although our study indicates an alarming prevalence of insufficient vitamin D levels in our patient population, it does not provide a cause-and-effect link between low serum 25-hydroxyvitamin D levels and risk of fracture or nonunion. However, further investigations may yield clinically relevant data linking hypovitaminosis D with fracture risk. Although we did not include patients with nonunion in this study, new prospective investigations will address nonunions and subgroup analysis of race, fracture type, management type (surgical vs nonsurgical), injury date (to determine seasonal effect), and different treatment regimens.
The primary limitation of this study was its retrospective design. In addition, though we collected vitamin D data from 889 patients with acute fracture, our serum collection protocols were not standardized. Most patients who were admitted during initial orthopedic consultation in the emergency department had serum 25-hydroxyvitamin D levels drawn during their hospital stay, and patients initially treated in an ambulatory setting may not have had serum vitamin D levels drawn for up to 2 weeks after injury (the significance of this delay is unknown). Furthermore, the serum result rate for the overall orthopedic trauma population during the review period was only 49%, which could indicate selection bias. There are multiple explanations for the low rate. As with any new protocol or method, it takes time for the order to become standard practice; in the early stages, individuals can forget to ask for the test. In addition, during the review period, the serum test was also relatively new at our facility, and it was a “send-out” test, which could partly account for the lack of consistency. For example, some specimens were lost, and, in a number of other cases, excluded patients mistakenly had their 1,25-hydroxyvitamin D levels measured and were not comparable to included patients. Nevertheless, our sample of 889 patients with acute fractures remains the largest (by several hundred) reported in the literature.
From a practical standpoint, the present results were useful in updating our treatment protocols. Now we typically treat patients only prophylactically, with 50,000 units of vitamin D2 for 8 weeks and daily vitamin D3 and calcium until fracture healing. Patients are encouraged to continue daily vitamin D and calcium supplementation after fracture healing to maintain bone health. Compliance, however, remains a continued challenge and lack thereof can potentially explain the confusing effect of a supplementation protocol on the serum 25-hydroxyvitamin D level.14 The only patients who are not given prophylactic treatment are those who previously had been denied it (patients with chronic kidney disease or elevated blood calcium levels).
Vitamin D deficiency and insufficiency are prevalent in patients with orthopedic trauma. Studies are needed to further elucidate the relationship between low vitamin D levels and risk of complications. Retrospectively, without compliance monitoring, we have not seen a direct correlation with fracture complications.15 Our goal here was to increase orthopedic surgeons’ awareness of the problem and of the need to consider addressing low serum vitamin D levels. The treatment is low cost and low risk. The ultimate goal—if there is a prospective direct correlation between low serum vitamin D levels and complications—is to develop treatment strategies that can effectively lower the prevalence of low vitamin D levels.
Am J Orthop. 2016;45(7):E522-E526. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Zaidi SA, Singh G, Owojori O, et al. Vitamin D deficiency in medical inpatients: a retrospective study of implications of untreated versus treated deficiency. Nutr Metab Insights. 2016;9:65-69.
2. Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777-783.
3. Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112(8):659-662.
4. Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105(6):971-974.
5. Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352(5):515-516.
6. Steele B, Serota A, Helfet DL, Peterson M, Lyman S, Lane JM. Vitamin D deficiency: a common occurrence in both high- and low-energy fractures. HSS J. 2008;4(2):143-148.
7. Bee CR, Sheerin DV, Wuest TK, Fitzpatrick DC. Serum vitamin D levels in orthopaedic trauma patients living in the northwestern United States. J Orthop Trauma. 2013;27(5):e103-e106.
8. Bischoff-Ferrari HA, Can U, Staehelin HB, et al. Severe vitamin D deficiency in Swiss hip fracture patients. Bone. 2008;42(3):597-602.
9. Pieper CF, Colon-Emeric C, Caminis J, et al. Distribution and correlates of serum 25-hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother. 2007;5(4):335-340.
10. Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187-192.
11. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531-537.
12. Bogunovic L, Kim AD, Beamer BS, Nguyen J, Lane JM. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92(13):2300-2304.
13. Brinker MR, O’Connor DP. Outcomes of tibial nonunion in older adults following treatment using the Ilizarov method. J Orthop Trauma. 2007;21(9):634-642.
14. Robertson DS, Jenkins T, Murtha YM, et al. Effectiveness of vitamin D therapy in orthopaedic trauma patients. J Orthop Trauma. 2015;29(11):e451-e453.
15. Bodendorfer BM, Cook JL, Robertson DS, et al. Do 25-hydroxyvitamin D levels correlate with fracture complications: J Orthop Trauma. 2016;30(9):e312-e317.
1. Zaidi SA, Singh G, Owojori O, et al. Vitamin D deficiency in medical inpatients: a retrospective study of implications of untreated versus treated deficiency. Nutr Metab Insights. 2016;9:65-69.
2. Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777-783.
3. Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112(8):659-662.
4. Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105(6):971-974.
5. Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352(5):515-516.
6. Steele B, Serota A, Helfet DL, Peterson M, Lyman S, Lane JM. Vitamin D deficiency: a common occurrence in both high- and low-energy fractures. HSS J. 2008;4(2):143-148.
7. Bee CR, Sheerin DV, Wuest TK, Fitzpatrick DC. Serum vitamin D levels in orthopaedic trauma patients living in the northwestern United States. J Orthop Trauma. 2013;27(5):e103-e106.
8. Bischoff-Ferrari HA, Can U, Staehelin HB, et al. Severe vitamin D deficiency in Swiss hip fracture patients. Bone. 2008;42(3):597-602.
9. Pieper CF, Colon-Emeric C, Caminis J, et al. Distribution and correlates of serum 25-hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother. 2007;5(4):335-340.
10. Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187-192.
11. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531-537.
12. Bogunovic L, Kim AD, Beamer BS, Nguyen J, Lane JM. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92(13):2300-2304.
13. Brinker MR, O’Connor DP. Outcomes of tibial nonunion in older adults following treatment using the Ilizarov method. J Orthop Trauma. 2007;21(9):634-642.
14. Robertson DS, Jenkins T, Murtha YM, et al. Effectiveness of vitamin D therapy in orthopaedic trauma patients. J Orthop Trauma. 2015;29(11):e451-e453.
15. Bodendorfer BM, Cook JL, Robertson DS, et al. Do 25-hydroxyvitamin D levels correlate with fracture complications: J Orthop Trauma. 2016;30(9):e312-e317.