User login
How Well Does the Braden Nutrition Subscale Agree With the VA Nutrition Classification Scheme Related to Pressure Ulcer Risk?
A pressure ulcer (PrU) is a localized injury to the skin and/or deep tissues that is due to pressure, friction, or shearing forces. Pressure ulcers are strongly associated with serious comorbidities, particularly inadequate nutrition and immobility.1,2 Pressure ulcers increase hospital costs significantly. In the U.S., PrU care is about $11 billion annually and a cost of between $2,000 and $21,410 per individual PrU.3-5
The impact of nosocomial PrUs remains a key health and economic concern of acute care facilities worldwide. In the U.S., about 2.5 million inpatients annually develop some degree of a PrU during their hospital stay. The reported incidence rates range from 0.4% to 38%.3,6 Each year about 60,000 people die of complications of a PrU.3,6,7 Inadequate nutrition is a critical factor that contributes to the incidence of PrUs.8-12 Consequences of inadequate nutrition have included alterations in skin integrity resulting in PrUs, longer hospital stays, increased costs of care, and higher rates of mortality.9 As a patient’s nutritional status becomes compromised, the likelihood of developing a PrU increases, especially if an individual is immobilized.7,9-11,13
Braden Scale History
The Braden Scale for Predicting Pressure Sore Risk was developed by Barbara Braden, PhD, RN, and Nancy Bergstrom, PhD, RN, in 1987.
The scale is composed of 6 factors: sensory perception, moisture, activity, mobility, friction and shear, and nutrition.14 Each factor is scored on a scale of 1 to 4 points (friction and shear are scored on a point scale of only 1 to 3) for a total possible score of 6 to 23 points (the lower the score, the greater the assumed PrU risk).
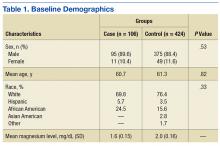
The Braden nutrition subscale relies heavily on recording observed or patient self-reported eating habits. It is typically documented by nurses who assess the daily intake of meals: recording a score of 4 if the patient’s meal intake is excellent (eats most of every meal), 3 if the patient’s intake is adequate (eats more than half of most meals), 2 if the patient’s intake is probably inadequate (rarely eats a complete meal), and 1 if a patient’s intake is very poor (never eats a complete meal) (Table 1).14
Historically, the Braden scale is reported to have good reliability when used by registered nurses as a risk prediction tool.14,16 A recent review also reported high interrater reliability of the Braden scale total score among nurses, nursing assistants, and graduate assistants.17 However, other studies suggest certain subscales (such as sensory and nutrition) may have very low interrater reliability among nurses and poor PrU predictability.18,19 To date, there are no known studies evaluating the agreement of the Braden nutrition subscale primarily used by nurses and the VA Nutrition Classification Scheme (VANCS) used by dietitians.
The VA standard of care recommends that PrU risk assessments are documented for all hospitalized veterans within 24 hours of admission, daily, with transfers or discharges, and when there is a status change in the patient. In addition, nutritional assessments by dietitians (using the VANCS) are encouraged within 24 hours of acute care hospitalization.20
The VANCS performed by dietitians consists of 4 classifications: no nutritional compromise, mild nutritional compromise, moderate nutritional compromise, and severenutritional compromise. These classifications are based on well-documented “comprehensive approaches to defining nutritional status that uses multiple parameters” including nutrition history, weight (body mass index and weight loss), diagnoses, diet (and diet orders), brief physical assessment, and preliminary laboratory data (serum albumin/pre-albumin and total lymphocyte count).20,21
The predictive ability of a risk assessment tool is critical to its clinical effectiveness in determining a clinical outcome.17 The Braden scale has been used for more than 30 years in various settings without any significant change to the scale or subscales. In a 2012 study, 4 medical factors were found to be more predictive of PrUs than the Braden scale total score in a sample of 213 acutely ill adult veterans.8 By performing a retrospective study using logistic regression predictive models, severe nutritional compromise (as identified by a dietitian), pneumonia, candidiasis, and surgery were identified as stronger predictors of PrU risk than was the Braden total score.8
With malnutrition as one of the most significant predictive factors in PrU risk, it is critical to determine whether discrepancies exist between the Braden nutrition subscale used primarily by nurses and the VANCS used by dietitians. Hence, the overall purpose of this study was to determine the level of agreement between the Braden nutrition subscale scores documented by nurses and the VANCS used by dietitians and examine the relationship of these assessments with PrU development.
Methods
The parent study was approved by the University of Florida Institutional Review Board before data collection. This secondary analysis of the parent study examined data already collected by Cowan and colleagues, which demonstrated the significance of nutritional compromise in PrU risk.
The de-identified data subset consisted of general demographics, hospital length of stay, specific diagnoses, Braden scores, PrU status, and registered dietician nutritional classification data from 213 acutely ill veterans admitted to North Florida/South Georgia Veterans Health System (NF/SGVHS) in Florida for more than 3 days between January and July 2008.8 The sample consisted of 100 veterans with nosocomial PrUs and 113 veterans without PrUs during their admission.
Scoring
Using the de-identified dataset, the variables of interest (VANCS, Braden nutrition subscale score, and the presence/absence of PrU) were coded. The VANCS was given a corresponding score ranging from 1 to 4 (1, severe nutritional compromise; 2, moderate nutritional compromise; 3, mild nutritional compromise; and 4, no nutritional compromise). The Braden nutrition subscale ranged from 1 to 4 (1 very poor nutrition; 2, probably inadequate nutrition; 3, adequate nutrition; and 4, excellent nutrition). PrU development was coded as 0, no PrU development and 1, PrU development. All nutritional assessments had been recorded in the electronic health record before any PrU reported in the parent study.
Statistical Analysis
After coding the variables of interest, the data were transferred into SAS v 9.4 (Cary, NC). The data collected compared VANCS and Braden nutrition subscale results. In addition, the authors examined the agreement between the score assigned to the VANCS and Braden nutrition subscale results with a weighted
Additionally, the authors computed sensitivity and specificity of the Braden nutrition subscale using the VANCS as the gold standard. The severe and moderately compromised categories of the VANCS combined to form the high-risk category, and the mild-to-no compromise categories were combined to form the low-risk category. The Braden nutrition subscale was similarly dichotomized with the very poor and probably inadequate intake forming the high-risk category and the adequate and excellent intake forming the low-risk category. Sensitivity and specificity of the Braden were then calculated.
Results
Nursing assessments using the Braden nutrition subscale were completed on 213 patients whose mean age (SD) was 71.0 (10.6) years. The VANCS documented by dietitians was completed on 205 patients. For 7 patients, a nutrition assessment was documented only by the Braden nutrition subscale and not the VANCS. Most of the patients were male (97%, n = 206), and white (81.4%, n = 171). The weighted
Landis and colleagues suggest that a
Figure 2 shows the percentage of patients who developed a PrU during hospitalization among different measures of Braden nutrition subscale vs VANCS. In Figure 2, nutritional categories 1, 2, and 3 correspond to very poor intake (Braden)/severe compromise (VANCS), probably inadequate intake (Braden)/moderate compromise (VANCS), and adequate intake (Braden)/mild compromise (VANCS), respectively. There were 3 patients who had a no compromise VANCS; none of these had a PrU, so their data are not represented in Figure 2.
Discussion
Findings from this study indicate that the VANCS documented by dietitians is superior in assessing nutritional risk and predicting the development of PrUs in acutely ill hospitalized veterans compared with the Braden nutrition subscale. This study also shows that the Braden nutrition subscale did not accurately predict PrU development in acutely ill veterans. This finding concurs with the Serpa and Santos study in which the Braden nutrition subscale scores were not predictive for PrU development in hospitalized patients.
One possible explanation for the findings in this study is that the nutrition subscale of the Braden tool asks the assessing clinician to evaluate the amount of food intake the patient is currently taking in for their usual meals. This assessment is highly subjective and speculative and does not account for recent intake fluctuations or weight loss. By comparison, the VANCS is more comprehensive in its ability to assess nutritional compromise based on multiple factors, such as recent weight loss, laboratory indices, body habitus, dentition, and swallowing ability.20 The National Pressure Ulcer Advisory Panel suggests that following an acute care admission, a patient receive a consult from a dietitian if the health care provider suspects that the patient may be nutritionally compromised.1 The study findings demonstrate the utility of the VANCS as predictive of PrU risk.
Unfortunately, the authors have learned that the VANCS may be phased out soon, and many VA facilities are no longer using it. Findings from this study and other recent scientific literature suggest that all inpatients may benefit from nutritional assessments by dietitians. When performed, dietitian assessments provide the basis for more accurate nursing assessment of nutritional risk and targeted interventions. Nursing professionals should be encouraged to review the dietitian assessment and consultation notes and to incorporate this information into a more comprehensive PrU prevention and treatment plan.
Interestingly, in spite of those assessed to have severe nutritional compromise by dietitian assessment (n = 39), very few of these patients (n = 4) had an ICD-9 diagnosis related to malnutrition (ICD-9 codes, 262, 273.8, 269.9, 263.9) entered in their chart for that hospitalization. This observation suggests that 88% of patients with severe nutritional compromise were not appropriately coded at discharge. Improper coding has implications for researchers using ICD-9 diagnosis codes at discharge for accurate analysis of risk factors as well as for health care providers who may look at coded diagnoses information in the charts when considering comorbid conditions for health management.
This study highlights the importance of nutritional status as a risk factor for PrU development. Reasons suggested for nutritional status seeming to be the most significant correlate to PrUs in the acute care setting include the following: decreased protein alters oncotic pressure, making tissue prone to edema; decreases in subcutaneous fat reduce protection from pressure effects; nutritional compromise alters cellular transport of nutrients and waste and makes tissue cells more vulnerable to deformation and physical stresses; and lactate (a by-product of anaerobic glycolysis) or any other metabolic by-product of malnutrition could cause biochemical stress, and tissue cells can die faster as a result of the increased plasma membrane permeability.7,24-26
Limitations
This study was limited to 1 sample of veterans hospitalized in the 2 acute care facilities of NF/SGVHS and the use of a retrospective chart review. As a result, further research is necessary to establish generalizability to other acute care settings and high-risk populations. In spite of these limitations, this and other studies highlight the need for revision of the Braden scale, specifically the nutritional subscale, to lessen the ambiguity seen between dietitian and nursing assessments while also increasing the accuracy in determining a patient’s nutrition risk of PrU development during hospitalization.
Conclusion
These findings provide evidence that dietitians’ documentation of the VANCS related to nutritional compromise are superior to current nutritional risk assessments using the Braden nutrition subscale in predicting PrU risk.
Acknowledgments
The authors acknowledge that this work was supported by the resources of the North Florida/South Georgia Veterans Health System in Gainesville, Florida, and in part by a Small Project Award from the VA Office of Nursing Services.
1. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. http://www.npuap.org/resources/educational-and-clinical -resources/prevention-and-treatment-of-pressure -ulcers-clinical-practice-guideline. Updated 2014. Accessed November 7, 2016.
2. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: quick reference guide. http://www .npuap.org/wp-content/uploads/2014/08/Updated -10-16-14-Quick-Reference-Guide-DIGITAL-NPUAP-EPUAP-PPPIA-16Oct2014.pdf. Updated October 16, 2014. Accessed October 21, 2016.
3. Sullivan N. Preventing in-facility pressure ulcers. In: Agency for Healthcare Research and Quality. Making Health Care Safer II. An Updated Critical Analysis of the Evidence for Patient Safety Practices. Evidence Reports/Technology Assessments. http://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/evidence-based-reports/services/quality/ptsafetyII-full.pdf:212-232. Published March 2013. Accessed October 21, 2016.
4. Russo CA, Steiner C, Spector W. Hospitalizations related to pressure ulcers among adults 18 years and older, 2006. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. http://www.ncbi .nlm.nih.gov/books/NBK54557. Published December 2008. Accessed October 21, 2016.
5. Spetz J, Brown DS, Aydin C, Donaldson N. The value of reducing hospital-acquired pressure ulcer prevalence: an illustrative analysis. J Nurs Adm. 2013;43(4):235-241.
6. Whittington KT, Briones R. National prevalence and incidence study: 6-year sequential acute care data. Adv Skin Wound Care. 2004;17(9):490-494.
7. Dorner B, Posthauer ME, Thomas D; National Pressure Ulcer Advisory Panel. The role of nutrition in pressure ulcer prevention and treatment: National Pressure Ulcer Advisory Panel white paper. http://www.npuap.org/wp-content/uploads/2012/03/Nutrition-White-Paper-Website-Version.pdf. Published 2009. Accessed November 7, 2016.
8. Cowan LJ, Stechmiller JK, Rowe M, Kairalla JA. Enhancing Braden pressure ulcer risk assessment in acutely ill adult veterans. Wound Repair Regen. 2012;20(2):137-148.
9. Correia MI, Hegazi RA, Higashiguchi T, et al. Evidence-based recommendations for addressing malnutrition in health care: an updated strategy from the feedM.E. Global Study Group. J Am Med Dir Assoc. 2014;15(8):544-550.
10. Malafarina V, Úriz-Otano F, Fernández-Catalán C, Tejedo-Flors D. Nutritional status and pressure ulcers. Risk assessment and estimation in older adults. J Am Geriatr Soc. 2014;62(6):1209-1210.
11. Posthauer ME, Banks M, Dorner B, Schols JM. The role of nutrition for pressure ulcer management: national pressure ulcer advisory panel, European pressure ulcer advisory panel, and pan pacific pressure injury alliance white paper. Adv Skin Wound Care. 2015;28(4):175-188.
12. Brito PA, de Vasconcelos Generoso S, Correia MI. Prevalence of pressure ulcers in hospitals in Brazil and association with nutritional status—a multicenter, cross-sectional study. Nutrition. 2013;29(4):646-649.
13. Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud. 2013;50(7):974-1003.
14. Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for predicting pressure sore risk. Nurs Res. 1987;36(4):205-210.
15. Ayello EA, Braden B. How and why to do pressure ulcer risk assessment. Adv Skin Wound Care. 2002;15(3):125-131.
16. Wang LH, Chen HL, Yan HY, et al. Inter-rater reliability of three most commonly used pressure ulcer risk assessment scales in clinical practice. Int Wound J. 2015;12(5):590-594.
17. Wilchesky M, Lungu O. Predictive and concurrent validity of the Braden scale in long-term care: a meta-analysis. Wound Repair Regen. 2015;23(1):44-56.
18. Kottner J, Dassen T. An interrater reliability study of the Braden scale in two nursing homes. Int J Nurs Stud. 2008;45(10):1501-1511.
19. Yatabe MS, Taguchi F, Ishida I, et al. Mini nutritional assessment as a useful method of predicting the development of pressure ulcers in elderly inpatients. J Am Geriatr Soc. 2013;61(10):1698-1704.
20. Hiller L, Lowery JC, Davis JA, Shore CJ, Striplin DT. Nutritional status classification in the Department of Veterans Affairs. J Am Diet Assoc. 2001;101(7):786-792.
21. U.S. Department of Veterans Affairs. VHA Handbook 1109.02. Clinical nutrition management. http://www.va.gov/vhapublications/ViewPublica tion.asp?pub_ID=2493. Published February 2012. Accessed October 21, 2016.
22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
23. Serpa LF, Santos VL. Validity of the Braden Nutrition Subscale in predicting pressure ulcer development. J Wound Ostomy Continence Nurs. 2014;41(5):436-443.
24. Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974-984.
25. Cooper KL. Evidence-based prevention of pressure ulcers in the intensive care unit. Crit Care Nurse. 2013;33(6):57-66.
26. Leopold E, Gefen A. Changes in permeability of the plasma membrane of myoblasts to fluorescent dyes with different molecular masses under sustained uniaxial stretching. Med Eng Phys. 2013;35(5):601-607.
A pressure ulcer (PrU) is a localized injury to the skin and/or deep tissues that is due to pressure, friction, or shearing forces. Pressure ulcers are strongly associated with serious comorbidities, particularly inadequate nutrition and immobility.1,2 Pressure ulcers increase hospital costs significantly. In the U.S., PrU care is about $11 billion annually and a cost of between $2,000 and $21,410 per individual PrU.3-5
The impact of nosocomial PrUs remains a key health and economic concern of acute care facilities worldwide. In the U.S., about 2.5 million inpatients annually develop some degree of a PrU during their hospital stay. The reported incidence rates range from 0.4% to 38%.3,6 Each year about 60,000 people die of complications of a PrU.3,6,7 Inadequate nutrition is a critical factor that contributes to the incidence of PrUs.8-12 Consequences of inadequate nutrition have included alterations in skin integrity resulting in PrUs, longer hospital stays, increased costs of care, and higher rates of mortality.9 As a patient’s nutritional status becomes compromised, the likelihood of developing a PrU increases, especially if an individual is immobilized.7,9-11,13
Braden Scale History
The Braden Scale for Predicting Pressure Sore Risk was developed by Barbara Braden, PhD, RN, and Nancy Bergstrom, PhD, RN, in 1987.
The scale is composed of 6 factors: sensory perception, moisture, activity, mobility, friction and shear, and nutrition.14 Each factor is scored on a scale of 1 to 4 points (friction and shear are scored on a point scale of only 1 to 3) for a total possible score of 6 to 23 points (the lower the score, the greater the assumed PrU risk).
The Braden nutrition subscale relies heavily on recording observed or patient self-reported eating habits. It is typically documented by nurses who assess the daily intake of meals: recording a score of 4 if the patient’s meal intake is excellent (eats most of every meal), 3 if the patient’s intake is adequate (eats more than half of most meals), 2 if the patient’s intake is probably inadequate (rarely eats a complete meal), and 1 if a patient’s intake is very poor (never eats a complete meal) (Table 1).14
Historically, the Braden scale is reported to have good reliability when used by registered nurses as a risk prediction tool.14,16 A recent review also reported high interrater reliability of the Braden scale total score among nurses, nursing assistants, and graduate assistants.17 However, other studies suggest certain subscales (such as sensory and nutrition) may have very low interrater reliability among nurses and poor PrU predictability.18,19 To date, there are no known studies evaluating the agreement of the Braden nutrition subscale primarily used by nurses and the VA Nutrition Classification Scheme (VANCS) used by dietitians.
The VA standard of care recommends that PrU risk assessments are documented for all hospitalized veterans within 24 hours of admission, daily, with transfers or discharges, and when there is a status change in the patient. In addition, nutritional assessments by dietitians (using the VANCS) are encouraged within 24 hours of acute care hospitalization.20
The VANCS performed by dietitians consists of 4 classifications: no nutritional compromise, mild nutritional compromise, moderate nutritional compromise, and severenutritional compromise. These classifications are based on well-documented “comprehensive approaches to defining nutritional status that uses multiple parameters” including nutrition history, weight (body mass index and weight loss), diagnoses, diet (and diet orders), brief physical assessment, and preliminary laboratory data (serum albumin/pre-albumin and total lymphocyte count).20,21
The predictive ability of a risk assessment tool is critical to its clinical effectiveness in determining a clinical outcome.17 The Braden scale has been used for more than 30 years in various settings without any significant change to the scale or subscales. In a 2012 study, 4 medical factors were found to be more predictive of PrUs than the Braden scale total score in a sample of 213 acutely ill adult veterans.8 By performing a retrospective study using logistic regression predictive models, severe nutritional compromise (as identified by a dietitian), pneumonia, candidiasis, and surgery were identified as stronger predictors of PrU risk than was the Braden total score.8
With malnutrition as one of the most significant predictive factors in PrU risk, it is critical to determine whether discrepancies exist between the Braden nutrition subscale used primarily by nurses and the VANCS used by dietitians. Hence, the overall purpose of this study was to determine the level of agreement between the Braden nutrition subscale scores documented by nurses and the VANCS used by dietitians and examine the relationship of these assessments with PrU development.
Methods
The parent study was approved by the University of Florida Institutional Review Board before data collection. This secondary analysis of the parent study examined data already collected by Cowan and colleagues, which demonstrated the significance of nutritional compromise in PrU risk.
The de-identified data subset consisted of general demographics, hospital length of stay, specific diagnoses, Braden scores, PrU status, and registered dietician nutritional classification data from 213 acutely ill veterans admitted to North Florida/South Georgia Veterans Health System (NF/SGVHS) in Florida for more than 3 days between January and July 2008.8 The sample consisted of 100 veterans with nosocomial PrUs and 113 veterans without PrUs during their admission.
Scoring
Using the de-identified dataset, the variables of interest (VANCS, Braden nutrition subscale score, and the presence/absence of PrU) were coded. The VANCS was given a corresponding score ranging from 1 to 4 (1, severe nutritional compromise; 2, moderate nutritional compromise; 3, mild nutritional compromise; and 4, no nutritional compromise). The Braden nutrition subscale ranged from 1 to 4 (1 very poor nutrition; 2, probably inadequate nutrition; 3, adequate nutrition; and 4, excellent nutrition). PrU development was coded as 0, no PrU development and 1, PrU development. All nutritional assessments had been recorded in the electronic health record before any PrU reported in the parent study.
Statistical Analysis
After coding the variables of interest, the data were transferred into SAS v 9.4 (Cary, NC). The data collected compared VANCS and Braden nutrition subscale results. In addition, the authors examined the agreement between the score assigned to the VANCS and Braden nutrition subscale results with a weighted
Additionally, the authors computed sensitivity and specificity of the Braden nutrition subscale using the VANCS as the gold standard. The severe and moderately compromised categories of the VANCS combined to form the high-risk category, and the mild-to-no compromise categories were combined to form the low-risk category. The Braden nutrition subscale was similarly dichotomized with the very poor and probably inadequate intake forming the high-risk category and the adequate and excellent intake forming the low-risk category. Sensitivity and specificity of the Braden were then calculated.
Results
Nursing assessments using the Braden nutrition subscale were completed on 213 patients whose mean age (SD) was 71.0 (10.6) years. The VANCS documented by dietitians was completed on 205 patients. For 7 patients, a nutrition assessment was documented only by the Braden nutrition subscale and not the VANCS. Most of the patients were male (97%, n = 206), and white (81.4%, n = 171). The weighted
Landis and colleagues suggest that a
Figure 2 shows the percentage of patients who developed a PrU during hospitalization among different measures of Braden nutrition subscale vs VANCS. In Figure 2, nutritional categories 1, 2, and 3 correspond to very poor intake (Braden)/severe compromise (VANCS), probably inadequate intake (Braden)/moderate compromise (VANCS), and adequate intake (Braden)/mild compromise (VANCS), respectively. There were 3 patients who had a no compromise VANCS; none of these had a PrU, so their data are not represented in Figure 2.
Discussion
Findings from this study indicate that the VANCS documented by dietitians is superior in assessing nutritional risk and predicting the development of PrUs in acutely ill hospitalized veterans compared with the Braden nutrition subscale. This study also shows that the Braden nutrition subscale did not accurately predict PrU development in acutely ill veterans. This finding concurs with the Serpa and Santos study in which the Braden nutrition subscale scores were not predictive for PrU development in hospitalized patients.
One possible explanation for the findings in this study is that the nutrition subscale of the Braden tool asks the assessing clinician to evaluate the amount of food intake the patient is currently taking in for their usual meals. This assessment is highly subjective and speculative and does not account for recent intake fluctuations or weight loss. By comparison, the VANCS is more comprehensive in its ability to assess nutritional compromise based on multiple factors, such as recent weight loss, laboratory indices, body habitus, dentition, and swallowing ability.20 The National Pressure Ulcer Advisory Panel suggests that following an acute care admission, a patient receive a consult from a dietitian if the health care provider suspects that the patient may be nutritionally compromised.1 The study findings demonstrate the utility of the VANCS as predictive of PrU risk.
Unfortunately, the authors have learned that the VANCS may be phased out soon, and many VA facilities are no longer using it. Findings from this study and other recent scientific literature suggest that all inpatients may benefit from nutritional assessments by dietitians. When performed, dietitian assessments provide the basis for more accurate nursing assessment of nutritional risk and targeted interventions. Nursing professionals should be encouraged to review the dietitian assessment and consultation notes and to incorporate this information into a more comprehensive PrU prevention and treatment plan.
Interestingly, in spite of those assessed to have severe nutritional compromise by dietitian assessment (n = 39), very few of these patients (n = 4) had an ICD-9 diagnosis related to malnutrition (ICD-9 codes, 262, 273.8, 269.9, 263.9) entered in their chart for that hospitalization. This observation suggests that 88% of patients with severe nutritional compromise were not appropriately coded at discharge. Improper coding has implications for researchers using ICD-9 diagnosis codes at discharge for accurate analysis of risk factors as well as for health care providers who may look at coded diagnoses information in the charts when considering comorbid conditions for health management.
This study highlights the importance of nutritional status as a risk factor for PrU development. Reasons suggested for nutritional status seeming to be the most significant correlate to PrUs in the acute care setting include the following: decreased protein alters oncotic pressure, making tissue prone to edema; decreases in subcutaneous fat reduce protection from pressure effects; nutritional compromise alters cellular transport of nutrients and waste and makes tissue cells more vulnerable to deformation and physical stresses; and lactate (a by-product of anaerobic glycolysis) or any other metabolic by-product of malnutrition could cause biochemical stress, and tissue cells can die faster as a result of the increased plasma membrane permeability.7,24-26
Limitations
This study was limited to 1 sample of veterans hospitalized in the 2 acute care facilities of NF/SGVHS and the use of a retrospective chart review. As a result, further research is necessary to establish generalizability to other acute care settings and high-risk populations. In spite of these limitations, this and other studies highlight the need for revision of the Braden scale, specifically the nutritional subscale, to lessen the ambiguity seen between dietitian and nursing assessments while also increasing the accuracy in determining a patient’s nutrition risk of PrU development during hospitalization.
Conclusion
These findings provide evidence that dietitians’ documentation of the VANCS related to nutritional compromise are superior to current nutritional risk assessments using the Braden nutrition subscale in predicting PrU risk.
Acknowledgments
The authors acknowledge that this work was supported by the resources of the North Florida/South Georgia Veterans Health System in Gainesville, Florida, and in part by a Small Project Award from the VA Office of Nursing Services.
A pressure ulcer (PrU) is a localized injury to the skin and/or deep tissues that is due to pressure, friction, or shearing forces. Pressure ulcers are strongly associated with serious comorbidities, particularly inadequate nutrition and immobility.1,2 Pressure ulcers increase hospital costs significantly. In the U.S., PrU care is about $11 billion annually and a cost of between $2,000 and $21,410 per individual PrU.3-5
The impact of nosocomial PrUs remains a key health and economic concern of acute care facilities worldwide. In the U.S., about 2.5 million inpatients annually develop some degree of a PrU during their hospital stay. The reported incidence rates range from 0.4% to 38%.3,6 Each year about 60,000 people die of complications of a PrU.3,6,7 Inadequate nutrition is a critical factor that contributes to the incidence of PrUs.8-12 Consequences of inadequate nutrition have included alterations in skin integrity resulting in PrUs, longer hospital stays, increased costs of care, and higher rates of mortality.9 As a patient’s nutritional status becomes compromised, the likelihood of developing a PrU increases, especially if an individual is immobilized.7,9-11,13
Braden Scale History
The Braden Scale for Predicting Pressure Sore Risk was developed by Barbara Braden, PhD, RN, and Nancy Bergstrom, PhD, RN, in 1987.
The scale is composed of 6 factors: sensory perception, moisture, activity, mobility, friction and shear, and nutrition.14 Each factor is scored on a scale of 1 to 4 points (friction and shear are scored on a point scale of only 1 to 3) for a total possible score of 6 to 23 points (the lower the score, the greater the assumed PrU risk).
The Braden nutrition subscale relies heavily on recording observed or patient self-reported eating habits. It is typically documented by nurses who assess the daily intake of meals: recording a score of 4 if the patient’s meal intake is excellent (eats most of every meal), 3 if the patient’s intake is adequate (eats more than half of most meals), 2 if the patient’s intake is probably inadequate (rarely eats a complete meal), and 1 if a patient’s intake is very poor (never eats a complete meal) (Table 1).14
Historically, the Braden scale is reported to have good reliability when used by registered nurses as a risk prediction tool.14,16 A recent review also reported high interrater reliability of the Braden scale total score among nurses, nursing assistants, and graduate assistants.17 However, other studies suggest certain subscales (such as sensory and nutrition) may have very low interrater reliability among nurses and poor PrU predictability.18,19 To date, there are no known studies evaluating the agreement of the Braden nutrition subscale primarily used by nurses and the VA Nutrition Classification Scheme (VANCS) used by dietitians.
The VA standard of care recommends that PrU risk assessments are documented for all hospitalized veterans within 24 hours of admission, daily, with transfers or discharges, and when there is a status change in the patient. In addition, nutritional assessments by dietitians (using the VANCS) are encouraged within 24 hours of acute care hospitalization.20
The VANCS performed by dietitians consists of 4 classifications: no nutritional compromise, mild nutritional compromise, moderate nutritional compromise, and severenutritional compromise. These classifications are based on well-documented “comprehensive approaches to defining nutritional status that uses multiple parameters” including nutrition history, weight (body mass index and weight loss), diagnoses, diet (and diet orders), brief physical assessment, and preliminary laboratory data (serum albumin/pre-albumin and total lymphocyte count).20,21
The predictive ability of a risk assessment tool is critical to its clinical effectiveness in determining a clinical outcome.17 The Braden scale has been used for more than 30 years in various settings without any significant change to the scale or subscales. In a 2012 study, 4 medical factors were found to be more predictive of PrUs than the Braden scale total score in a sample of 213 acutely ill adult veterans.8 By performing a retrospective study using logistic regression predictive models, severe nutritional compromise (as identified by a dietitian), pneumonia, candidiasis, and surgery were identified as stronger predictors of PrU risk than was the Braden total score.8
With malnutrition as one of the most significant predictive factors in PrU risk, it is critical to determine whether discrepancies exist between the Braden nutrition subscale used primarily by nurses and the VANCS used by dietitians. Hence, the overall purpose of this study was to determine the level of agreement between the Braden nutrition subscale scores documented by nurses and the VANCS used by dietitians and examine the relationship of these assessments with PrU development.
Methods
The parent study was approved by the University of Florida Institutional Review Board before data collection. This secondary analysis of the parent study examined data already collected by Cowan and colleagues, which demonstrated the significance of nutritional compromise in PrU risk.
The de-identified data subset consisted of general demographics, hospital length of stay, specific diagnoses, Braden scores, PrU status, and registered dietician nutritional classification data from 213 acutely ill veterans admitted to North Florida/South Georgia Veterans Health System (NF/SGVHS) in Florida for more than 3 days between January and July 2008.8 The sample consisted of 100 veterans with nosocomial PrUs and 113 veterans without PrUs during their admission.
Scoring
Using the de-identified dataset, the variables of interest (VANCS, Braden nutrition subscale score, and the presence/absence of PrU) were coded. The VANCS was given a corresponding score ranging from 1 to 4 (1, severe nutritional compromise; 2, moderate nutritional compromise; 3, mild nutritional compromise; and 4, no nutritional compromise). The Braden nutrition subscale ranged from 1 to 4 (1 very poor nutrition; 2, probably inadequate nutrition; 3, adequate nutrition; and 4, excellent nutrition). PrU development was coded as 0, no PrU development and 1, PrU development. All nutritional assessments had been recorded in the electronic health record before any PrU reported in the parent study.
Statistical Analysis
After coding the variables of interest, the data were transferred into SAS v 9.4 (Cary, NC). The data collected compared VANCS and Braden nutrition subscale results. In addition, the authors examined the agreement between the score assigned to the VANCS and Braden nutrition subscale results with a weighted
Additionally, the authors computed sensitivity and specificity of the Braden nutrition subscale using the VANCS as the gold standard. The severe and moderately compromised categories of the VANCS combined to form the high-risk category, and the mild-to-no compromise categories were combined to form the low-risk category. The Braden nutrition subscale was similarly dichotomized with the very poor and probably inadequate intake forming the high-risk category and the adequate and excellent intake forming the low-risk category. Sensitivity and specificity of the Braden were then calculated.
Results
Nursing assessments using the Braden nutrition subscale were completed on 213 patients whose mean age (SD) was 71.0 (10.6) years. The VANCS documented by dietitians was completed on 205 patients. For 7 patients, a nutrition assessment was documented only by the Braden nutrition subscale and not the VANCS. Most of the patients were male (97%, n = 206), and white (81.4%, n = 171). The weighted
Landis and colleagues suggest that a
Figure 2 shows the percentage of patients who developed a PrU during hospitalization among different measures of Braden nutrition subscale vs VANCS. In Figure 2, nutritional categories 1, 2, and 3 correspond to very poor intake (Braden)/severe compromise (VANCS), probably inadequate intake (Braden)/moderate compromise (VANCS), and adequate intake (Braden)/mild compromise (VANCS), respectively. There were 3 patients who had a no compromise VANCS; none of these had a PrU, so their data are not represented in Figure 2.
Discussion
Findings from this study indicate that the VANCS documented by dietitians is superior in assessing nutritional risk and predicting the development of PrUs in acutely ill hospitalized veterans compared with the Braden nutrition subscale. This study also shows that the Braden nutrition subscale did not accurately predict PrU development in acutely ill veterans. This finding concurs with the Serpa and Santos study in which the Braden nutrition subscale scores were not predictive for PrU development in hospitalized patients.
One possible explanation for the findings in this study is that the nutrition subscale of the Braden tool asks the assessing clinician to evaluate the amount of food intake the patient is currently taking in for their usual meals. This assessment is highly subjective and speculative and does not account for recent intake fluctuations or weight loss. By comparison, the VANCS is more comprehensive in its ability to assess nutritional compromise based on multiple factors, such as recent weight loss, laboratory indices, body habitus, dentition, and swallowing ability.20 The National Pressure Ulcer Advisory Panel suggests that following an acute care admission, a patient receive a consult from a dietitian if the health care provider suspects that the patient may be nutritionally compromised.1 The study findings demonstrate the utility of the VANCS as predictive of PrU risk.
Unfortunately, the authors have learned that the VANCS may be phased out soon, and many VA facilities are no longer using it. Findings from this study and other recent scientific literature suggest that all inpatients may benefit from nutritional assessments by dietitians. When performed, dietitian assessments provide the basis for more accurate nursing assessment of nutritional risk and targeted interventions. Nursing professionals should be encouraged to review the dietitian assessment and consultation notes and to incorporate this information into a more comprehensive PrU prevention and treatment plan.
Interestingly, in spite of those assessed to have severe nutritional compromise by dietitian assessment (n = 39), very few of these patients (n = 4) had an ICD-9 diagnosis related to malnutrition (ICD-9 codes, 262, 273.8, 269.9, 263.9) entered in their chart for that hospitalization. This observation suggests that 88% of patients with severe nutritional compromise were not appropriately coded at discharge. Improper coding has implications for researchers using ICD-9 diagnosis codes at discharge for accurate analysis of risk factors as well as for health care providers who may look at coded diagnoses information in the charts when considering comorbid conditions for health management.
This study highlights the importance of nutritional status as a risk factor for PrU development. Reasons suggested for nutritional status seeming to be the most significant correlate to PrUs in the acute care setting include the following: decreased protein alters oncotic pressure, making tissue prone to edema; decreases in subcutaneous fat reduce protection from pressure effects; nutritional compromise alters cellular transport of nutrients and waste and makes tissue cells more vulnerable to deformation and physical stresses; and lactate (a by-product of anaerobic glycolysis) or any other metabolic by-product of malnutrition could cause biochemical stress, and tissue cells can die faster as a result of the increased plasma membrane permeability.7,24-26
Limitations
This study was limited to 1 sample of veterans hospitalized in the 2 acute care facilities of NF/SGVHS and the use of a retrospective chart review. As a result, further research is necessary to establish generalizability to other acute care settings and high-risk populations. In spite of these limitations, this and other studies highlight the need for revision of the Braden scale, specifically the nutritional subscale, to lessen the ambiguity seen between dietitian and nursing assessments while also increasing the accuracy in determining a patient’s nutrition risk of PrU development during hospitalization.
Conclusion
These findings provide evidence that dietitians’ documentation of the VANCS related to nutritional compromise are superior to current nutritional risk assessments using the Braden nutrition subscale in predicting PrU risk.
Acknowledgments
The authors acknowledge that this work was supported by the resources of the North Florida/South Georgia Veterans Health System in Gainesville, Florida, and in part by a Small Project Award from the VA Office of Nursing Services.
1. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. http://www.npuap.org/resources/educational-and-clinical -resources/prevention-and-treatment-of-pressure -ulcers-clinical-practice-guideline. Updated 2014. Accessed November 7, 2016.
2. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: quick reference guide. http://www .npuap.org/wp-content/uploads/2014/08/Updated -10-16-14-Quick-Reference-Guide-DIGITAL-NPUAP-EPUAP-PPPIA-16Oct2014.pdf. Updated October 16, 2014. Accessed October 21, 2016.
3. Sullivan N. Preventing in-facility pressure ulcers. In: Agency for Healthcare Research and Quality. Making Health Care Safer II. An Updated Critical Analysis of the Evidence for Patient Safety Practices. Evidence Reports/Technology Assessments. http://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/evidence-based-reports/services/quality/ptsafetyII-full.pdf:212-232. Published March 2013. Accessed October 21, 2016.
4. Russo CA, Steiner C, Spector W. Hospitalizations related to pressure ulcers among adults 18 years and older, 2006. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. http://www.ncbi .nlm.nih.gov/books/NBK54557. Published December 2008. Accessed October 21, 2016.
5. Spetz J, Brown DS, Aydin C, Donaldson N. The value of reducing hospital-acquired pressure ulcer prevalence: an illustrative analysis. J Nurs Adm. 2013;43(4):235-241.
6. Whittington KT, Briones R. National prevalence and incidence study: 6-year sequential acute care data. Adv Skin Wound Care. 2004;17(9):490-494.
7. Dorner B, Posthauer ME, Thomas D; National Pressure Ulcer Advisory Panel. The role of nutrition in pressure ulcer prevention and treatment: National Pressure Ulcer Advisory Panel white paper. http://www.npuap.org/wp-content/uploads/2012/03/Nutrition-White-Paper-Website-Version.pdf. Published 2009. Accessed November 7, 2016.
8. Cowan LJ, Stechmiller JK, Rowe M, Kairalla JA. Enhancing Braden pressure ulcer risk assessment in acutely ill adult veterans. Wound Repair Regen. 2012;20(2):137-148.
9. Correia MI, Hegazi RA, Higashiguchi T, et al. Evidence-based recommendations for addressing malnutrition in health care: an updated strategy from the feedM.E. Global Study Group. J Am Med Dir Assoc. 2014;15(8):544-550.
10. Malafarina V, Úriz-Otano F, Fernández-Catalán C, Tejedo-Flors D. Nutritional status and pressure ulcers. Risk assessment and estimation in older adults. J Am Geriatr Soc. 2014;62(6):1209-1210.
11. Posthauer ME, Banks M, Dorner B, Schols JM. The role of nutrition for pressure ulcer management: national pressure ulcer advisory panel, European pressure ulcer advisory panel, and pan pacific pressure injury alliance white paper. Adv Skin Wound Care. 2015;28(4):175-188.
12. Brito PA, de Vasconcelos Generoso S, Correia MI. Prevalence of pressure ulcers in hospitals in Brazil and association with nutritional status—a multicenter, cross-sectional study. Nutrition. 2013;29(4):646-649.
13. Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud. 2013;50(7):974-1003.
14. Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for predicting pressure sore risk. Nurs Res. 1987;36(4):205-210.
15. Ayello EA, Braden B. How and why to do pressure ulcer risk assessment. Adv Skin Wound Care. 2002;15(3):125-131.
16. Wang LH, Chen HL, Yan HY, et al. Inter-rater reliability of three most commonly used pressure ulcer risk assessment scales in clinical practice. Int Wound J. 2015;12(5):590-594.
17. Wilchesky M, Lungu O. Predictive and concurrent validity of the Braden scale in long-term care: a meta-analysis. Wound Repair Regen. 2015;23(1):44-56.
18. Kottner J, Dassen T. An interrater reliability study of the Braden scale in two nursing homes. Int J Nurs Stud. 2008;45(10):1501-1511.
19. Yatabe MS, Taguchi F, Ishida I, et al. Mini nutritional assessment as a useful method of predicting the development of pressure ulcers in elderly inpatients. J Am Geriatr Soc. 2013;61(10):1698-1704.
20. Hiller L, Lowery JC, Davis JA, Shore CJ, Striplin DT. Nutritional status classification in the Department of Veterans Affairs. J Am Diet Assoc. 2001;101(7):786-792.
21. U.S. Department of Veterans Affairs. VHA Handbook 1109.02. Clinical nutrition management. http://www.va.gov/vhapublications/ViewPublica tion.asp?pub_ID=2493. Published February 2012. Accessed October 21, 2016.
22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
23. Serpa LF, Santos VL. Validity of the Braden Nutrition Subscale in predicting pressure ulcer development. J Wound Ostomy Continence Nurs. 2014;41(5):436-443.
24. Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974-984.
25. Cooper KL. Evidence-based prevention of pressure ulcers in the intensive care unit. Crit Care Nurse. 2013;33(6):57-66.
26. Leopold E, Gefen A. Changes in permeability of the plasma membrane of myoblasts to fluorescent dyes with different molecular masses under sustained uniaxial stretching. Med Eng Phys. 2013;35(5):601-607.
1. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. http://www.npuap.org/resources/educational-and-clinical -resources/prevention-and-treatment-of-pressure -ulcers-clinical-practice-guideline. Updated 2014. Accessed November 7, 2016.
2. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: quick reference guide. http://www .npuap.org/wp-content/uploads/2014/08/Updated -10-16-14-Quick-Reference-Guide-DIGITAL-NPUAP-EPUAP-PPPIA-16Oct2014.pdf. Updated October 16, 2014. Accessed October 21, 2016.
3. Sullivan N. Preventing in-facility pressure ulcers. In: Agency for Healthcare Research and Quality. Making Health Care Safer II. An Updated Critical Analysis of the Evidence for Patient Safety Practices. Evidence Reports/Technology Assessments. http://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/evidence-based-reports/services/quality/ptsafetyII-full.pdf:212-232. Published March 2013. Accessed October 21, 2016.
4. Russo CA, Steiner C, Spector W. Hospitalizations related to pressure ulcers among adults 18 years and older, 2006. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. http://www.ncbi .nlm.nih.gov/books/NBK54557. Published December 2008. Accessed October 21, 2016.
5. Spetz J, Brown DS, Aydin C, Donaldson N. The value of reducing hospital-acquired pressure ulcer prevalence: an illustrative analysis. J Nurs Adm. 2013;43(4):235-241.
6. Whittington KT, Briones R. National prevalence and incidence study: 6-year sequential acute care data. Adv Skin Wound Care. 2004;17(9):490-494.
7. Dorner B, Posthauer ME, Thomas D; National Pressure Ulcer Advisory Panel. The role of nutrition in pressure ulcer prevention and treatment: National Pressure Ulcer Advisory Panel white paper. http://www.npuap.org/wp-content/uploads/2012/03/Nutrition-White-Paper-Website-Version.pdf. Published 2009. Accessed November 7, 2016.
8. Cowan LJ, Stechmiller JK, Rowe M, Kairalla JA. Enhancing Braden pressure ulcer risk assessment in acutely ill adult veterans. Wound Repair Regen. 2012;20(2):137-148.
9. Correia MI, Hegazi RA, Higashiguchi T, et al. Evidence-based recommendations for addressing malnutrition in health care: an updated strategy from the feedM.E. Global Study Group. J Am Med Dir Assoc. 2014;15(8):544-550.
10. Malafarina V, Úriz-Otano F, Fernández-Catalán C, Tejedo-Flors D. Nutritional status and pressure ulcers. Risk assessment and estimation in older adults. J Am Geriatr Soc. 2014;62(6):1209-1210.
11. Posthauer ME, Banks M, Dorner B, Schols JM. The role of nutrition for pressure ulcer management: national pressure ulcer advisory panel, European pressure ulcer advisory panel, and pan pacific pressure injury alliance white paper. Adv Skin Wound Care. 2015;28(4):175-188.
12. Brito PA, de Vasconcelos Generoso S, Correia MI. Prevalence of pressure ulcers in hospitals in Brazil and association with nutritional status—a multicenter, cross-sectional study. Nutrition. 2013;29(4):646-649.
13. Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud. 2013;50(7):974-1003.
14. Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for predicting pressure sore risk. Nurs Res. 1987;36(4):205-210.
15. Ayello EA, Braden B. How and why to do pressure ulcer risk assessment. Adv Skin Wound Care. 2002;15(3):125-131.
16. Wang LH, Chen HL, Yan HY, et al. Inter-rater reliability of three most commonly used pressure ulcer risk assessment scales in clinical practice. Int Wound J. 2015;12(5):590-594.
17. Wilchesky M, Lungu O. Predictive and concurrent validity of the Braden scale in long-term care: a meta-analysis. Wound Repair Regen. 2015;23(1):44-56.
18. Kottner J, Dassen T. An interrater reliability study of the Braden scale in two nursing homes. Int J Nurs Stud. 2008;45(10):1501-1511.
19. Yatabe MS, Taguchi F, Ishida I, et al. Mini nutritional assessment as a useful method of predicting the development of pressure ulcers in elderly inpatients. J Am Geriatr Soc. 2013;61(10):1698-1704.
20. Hiller L, Lowery JC, Davis JA, Shore CJ, Striplin DT. Nutritional status classification in the Department of Veterans Affairs. J Am Diet Assoc. 2001;101(7):786-792.
21. U.S. Department of Veterans Affairs. VHA Handbook 1109.02. Clinical nutrition management. http://www.va.gov/vhapublications/ViewPublica tion.asp?pub_ID=2493. Published February 2012. Accessed October 21, 2016.
22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
23. Serpa LF, Santos VL. Validity of the Braden Nutrition Subscale in predicting pressure ulcer development. J Wound Ostomy Continence Nurs. 2014;41(5):436-443.
24. Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974-984.
25. Cooper KL. Evidence-based prevention of pressure ulcers in the intensive care unit. Crit Care Nurse. 2013;33(6):57-66.
26. Leopold E, Gefen A. Changes in permeability of the plasma membrane of myoblasts to fluorescent dyes with different molecular masses under sustained uniaxial stretching. Med Eng Phys. 2013;35(5):601-607.
Laxative Use with Patient-Controlled Analgesia in the Hospital and Associated Outcomes
From the Division of General Internal Medicine (Dr. Lenz), Division of Biomedical Statistics and Informatics (Mr. Schroeder), and the Division of Hospital Internal Medicine (Ms. Lawson and Dr. Yu), Mayo Clinic, Rochester, MN.
Abstract
- Objective: To describe prophylactic laxative effectiveness and prescribing patterns in patients initiated on intravenous (IV) opioid analgesia.
- Design: Retrospective cohort study.
- Setting and participants: All patients who were on IV narcotics with a patient-controlled pump while admitted to a general medicine service at the Mayo Clinic in Rochester in 2011 and 2012 were identified. Patients were excluded if constipation or diarrhea were diagnosed prior to IV opioid analgesia initiation.
- Measurements: Prophylactic laxatives were defined as laxatives prescribed within 24 hours of IV opioid analgesia initiation to be given even in the absence of constipation. Constipation was recorded when diagnosed during the hospitalization. Severe constipation was defined as constipation resulting in an abdominal CT or X-ray; abdominal distension, pain, or bloating; or if an enema was performed during the hospitalization.
- Results: Of 283 patients, 101 (36%) received prophylactic laxatives and 182 (64%) did not. Constipation occurred in 61 (34%) not on prophylactic laxatives and in 49 (49%) on prophylactic laxatives (P = 0.015). Severe constipation occurred in 23 (13%) not on prophylactic laxatives and in 33 (33%) on prophylactic laxatives (P < 0.001).
- Conclusion: A large percentage of patients are not receiving prophylactic laxatives when receiving IV opioid analgesia in the hospital. Current laxative strategies are not effectively preventing constipation in patients when prescribed.
Key words: constipation; opioids; hospital medicine; patient-controlled analgesia; laxatives.
Opioid-induced constipation (OIC) is defined as a change, when initiating opioid therapy, from baseline bowel habits and defecation patterns that is characterized by any of the following: reduced bowel frequency; development or worsening of straining; a sense of incomplete evacuation; or a patient’s perception of distress related to bowel habits [1]. It is an important side effect to consider when initiating narcotic analgesia. It has been estimated that approximately 3% to 4% of the population is on chronic narcotic pain relievers in the outpatient setting [2,3], and 37% to 81% of these patients will experience constipation [3–9]. Because of the high incidence of constipation, the prophylactic prescription of laxatives with initiation of opioid pain relievers is frequently recommended [10–15]. Furthermore, it has been shown that among patients admitted to the hospital with cancer, there is a lower incidence of constipation amongst those who have received prophylactic laxatives [16]. However, there is no evidence in the literature that prophylactic laxatives improve outcomes in patients on opioid analgesia in the general medicine inpatient setting. Furthermore, studies have illustrated that recommendations for prophylactic laxative use are not reliably followed [3,9].
While the incidence of OIC is well described in the outpatient setting [17,18], there are few studies looking at the incidence of OIC in the hospital setting. It has been shown, however, that occurrence during even a brief hospitalization is possible [4,6]. Acute constipation while hospitalized can theoretically lead to longer hospitalizations, increased pain, and decreased quality of life [6,7,19]. Recent research has focused heavily on the use of novel agents such as peripherally acting mu-opioid receptor antagonists in the treatment of OIC [20–23]. However, the expense of these agents makes them less than ideal in the prophylactic setting. This study will assess the effectiveness and prescribing patterns of prophylactic laxatives in the inpatient general medicine setting over a 2-year period at our institution in patients initiated on patient-controlled analgesia with hydromorphone, morphine, or fentanyl.
Methods
This study was approved by the institutional review board at the Mayo Clinic Rochester. All patients who were initiated on intravenous analgesia with an electronic patient-controlled opioid pump (PCA) while admitted to a general medicine service in 2011 and 2012 were identified. Patients who received PCA therapy were identified through a pharmacy database. Only patients older than 18 years of age were included in the study. PCA therapy was selected for our analysis because PCA therapy is not regularly administered on an outpatient basis. All of these patients, therefore, had a change in their narcotic regimen on admission to the hospital. Patients were excluded from the study if they were on a PCA for less than 24 hours; had a PCA initiated on a service other than a general medicine service; were on a scheduled laxative regimen prior to admission; or carried a diagnosis of bowel obstruction, chronic diarrhea, constipation, or intestinal discontinuity (eg, those with previous diversions or ostomies).
A retrospective review of each patient’s chart was conducted with the assistance of a team of nurse abstractors. Basic demographic data were recorded for each patient. Date of hospital admission and discharge; scheduled laxatives ordered and administered (any dose of sennosides, polyethylene glycol, docusate, bisacodyl, lactulose, or magnesium citrate); abdominal X-rays and abdominal CT scans performed for constipation; and any administration of enemas were recorded. Fiber supplements were not considered laxatives. If a patient was documented to have constipation during their hospitalization this was recorded. Patients were classified as having severe constipation if an abdominal CT or x-ray was performed for the indication of constipation; if abdominal distension, pain, or bloating were documented due to constipation; or if an enema was performed during the hospitalization.
For analysis purposes, patients who started receiving scheduled laxatives (as opposed to laxatives “as needed”)on or before the day of PCA initiation were classified as receiving prophylactic laxatives. Baseline patient characteristics and outcomes were compared using the chi-square test for nominal variables and the rank sum test for continuous variables. In all cases, 2-tailed tests were performed with P values ≤ 0.05 considered statistically significant. A nominal logistic regression model was utilized to assess for independent association of risk factors with the outcome of constipation.
Results
Discussion
Patients initiated on opioid therapy were not prescribed prophylactic laxatives in 64% of our cohort in the inpatient setting. When prescribed, current laxative strategies did not effectively prevent constipation with 49% experiencing OIC. Our data serves as a strong reminder of the magnitude of the problem of OIC in the inpatient setting.
The strength of our paper lies in its role as a magnitude assessment. This retrospective review reveals for that among a diverse group of patients hospitalized within a large academic institution, OIC remains prevalent. Furthermore, the high incidence of severe constipation indicates the potential for increased health care costs and patient discomfort secondary to OIC emphasizing the importance of prevention of OIC. Recent guidelines have made a push toward prophylactic laxative utilization earlier. Specifically, the European Palliative Research Collaborative offers a “strong recommendation to routinely prescribe laxatives for the management or prophylaxis of opioid-induced constipation” [10]. Additionally, the American Society of Interventional Pain Physicians suggests that “a physician should consider the initiation of a bowel regimen even before the development of constipation and definitely after the development of constipation” [11]. Our manuscript serves as a reminder that OIC remains a very prevalent problem and that prophylactic laxatives are still being underutilized.
This is a retrospective study and thus has inherent limitations. Specifically, we are limited to those cases of constipation that were documented in the medical record. The presentation of constipation is varied between patients. This variation in presentation of OIC is inherent to the disease process as is demonstrated in the broad definition for OIC [1]. The cases of constipation that we are reporting clearly were bothersome enough to warrant documentation in the medical record, and while there may have been cases that escaped documentation, we can be confident that the cases of OIC we are reporting are true cases of OIC. The numbers we report can therefore be taken to represent a minimum number of cases of constipation occurring in our study population.
It has been suggested that OIC prevalence varies with type of opioid and duration of opioid therapy [24]. We did not compare dose, type, or duration of opioid therapy in this study. This could certainly account for the seemingly higher rate of constipation within the group treated with prophylactic laxatives as compared with those not treated with prophylactic laxatives. Physicians likely have a higher propensity to prescribe prophylactic laxatives to patients receiving high doses of opioids who are in turn at higher risk for OIC. We cannot say whether differences in efficacy exist between prophylactic laxative regimens or which opioids (dose and duration) cause the most constipation based upon our data. Future studies incorporating dose, duration, and opioid type along with the variables we collected in this study could potentially construct successful logistic regression models with predictive power to identify those at highest risk of OIC.
Our rate of OIC is consistent with previously published figures [3–9]. However, we demonstrate for the first time that prophylactic laxatives are prescribed infrequently and unsuccessfully in the inpatient setting. This is consistent with prescribing rates in the outpatient setting [9,25]. Furthermore, we observed a higher rate of constipation in those treated with prophylactic laxatives compared to those that did not receive prophylactic laxatives. Pottegard et al similarly demonstrated an increased rate of constipation in those utilizing laxative therapy [25]. This is likely secondary to providers recommending prophylactic laxatives to those patients most likely to develop constipation. Despite being able to recognize high-risk patients, providers are unable to prevent OIC as little is known regarding optimal laxative strategies. Previous studies comparing treatment regimens for the relief of constipation in the palliative care population have been largely inconclusive [26]. There have been no studies to date comparing different prophylactic laxatives in the inpatient setting.
Future directions for research in this area would ideally take the form of randomized controlled trials investigating efficacy of different prophylactic laxatives in the inpatient setting. These trials would ideally include well-defined patient groups receiving specific narcotics for specific reasons. These studies would be best if powered to assess the effect of narcotic dosage and duration of therapy as well. Alternatively, larger retrospective chart reviews could be performed including narcotic dosage, type, and duration of therapy with a planned logistic regression model attempting to account for likely independent variables.
Conclusion
Our study demonstrates for the first time that prophylactic laxatives are not being prescribed frequently to patients on opioid analgesia in the inpatient general medicine setting. Additionally, while providers seem to be identifying patients at higher risk of constipation, they are still unable to prevent constipation in a high percentage of patients. Further research into this area would be beneficial to prevent this uncomfortable, costly, and preventable complication of opioid analgesia.
Corresponding author: Roger Yu, MD, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, [email protected].
Funding/support: This research was supported by the Mayo Clinic Return to Work program nurses for data abstraction.
Financial disclosures: None.
1. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016.
2. Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18:1166–75.
3. Choung RS, Locke GR 3rd, Zinsmeister AR, et al. Opioid bowel dysfunction and narcotic bowel syndrome: a population-based study. Am J Gastroenterol 2009;104:1199–204.
4. Droney J, Ross J, Gretton S, et al. Constipation in cancer patients on morphine. Support Care Cancer 2008;16:453–9.
5. Sykes NP. The relationship between opioid use and laxative use in terminally ill cancer patients. Palliat Med 1998;12:375–82.
6. Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009;10:35–42.
7. Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther 2008;27:1224–32.
8. Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005:7:R1046–51.
9. Bouvy ML, Buurma H, Egberts TC. Laxative prescribing in relation to opioid use and the influence of pharmacy-based intervention. J Clin Pharm Ther 2002;27:107–10.
10. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012:13:e58–68.
11. Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2--guidance. Pain Physician 2012;15(3 Suppl):S67–116.
12. Cameron JC. Constipation related to narcotic therapy. A protocol for nurses and patients. Cancer Nurs 1992;15:372–7.
13. Levy MH. Pharmacologic treatment of cancer pain. N Engl J Med 1996;335:1124–32.
14. Swegle JM, Logemann D. Management of common opioid-induced adverse effects. Am Fam Physician 2006;74:1347–54.
15. Donnelly S, Davis MP, Walsh D, Naughton M. Morphine in cancer pain management: a practical guide. Support Care Cancer 2002;10:13–35.
16. Ishihara M, Ikesue H Matsunaga H, et al. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin J Pain 2012;28:373–81.
17. Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004;112:372–80.
18. Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 2010; 22:424–30, e96.
19. Brock C, Olesen SS, Olesen AE, et al. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs 2012;72:1847–65.
20. Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol 2011;106:835–42.
21. Candy B, Jones L, Goodman ML, et al. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev 2011(1):CD003448.
22. Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterol 2013;108:1566–74.
23. Jansen JP, Lorch D, Langan J, et al. A randomized, placebo-controlled phase 3 trial (Study SB-767905/012) of alvimopan for opioid-induced bowel dysfunction in patients with non-cancer pain. J Pain 2011;12:185–93.
24. Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 2014;26:1386–95.
25. Pottegard A, Knudsen TB, van Heesch K, et al. Information on risk of constipation for Danish users of opioids, and their laxative use. Int J Clin Pharm 2014;36:291–4.
26. Candy B, Jones L, Larkin PJ, et al. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015(5):CD003448.
From the Division of General Internal Medicine (Dr. Lenz), Division of Biomedical Statistics and Informatics (Mr. Schroeder), and the Division of Hospital Internal Medicine (Ms. Lawson and Dr. Yu), Mayo Clinic, Rochester, MN.
Abstract
- Objective: To describe prophylactic laxative effectiveness and prescribing patterns in patients initiated on intravenous (IV) opioid analgesia.
- Design: Retrospective cohort study.
- Setting and participants: All patients who were on IV narcotics with a patient-controlled pump while admitted to a general medicine service at the Mayo Clinic in Rochester in 2011 and 2012 were identified. Patients were excluded if constipation or diarrhea were diagnosed prior to IV opioid analgesia initiation.
- Measurements: Prophylactic laxatives were defined as laxatives prescribed within 24 hours of IV opioid analgesia initiation to be given even in the absence of constipation. Constipation was recorded when diagnosed during the hospitalization. Severe constipation was defined as constipation resulting in an abdominal CT or X-ray; abdominal distension, pain, or bloating; or if an enema was performed during the hospitalization.
- Results: Of 283 patients, 101 (36%) received prophylactic laxatives and 182 (64%) did not. Constipation occurred in 61 (34%) not on prophylactic laxatives and in 49 (49%) on prophylactic laxatives (P = 0.015). Severe constipation occurred in 23 (13%) not on prophylactic laxatives and in 33 (33%) on prophylactic laxatives (P < 0.001).
- Conclusion: A large percentage of patients are not receiving prophylactic laxatives when receiving IV opioid analgesia in the hospital. Current laxative strategies are not effectively preventing constipation in patients when prescribed.
Key words: constipation; opioids; hospital medicine; patient-controlled analgesia; laxatives.
Opioid-induced constipation (OIC) is defined as a change, when initiating opioid therapy, from baseline bowel habits and defecation patterns that is characterized by any of the following: reduced bowel frequency; development or worsening of straining; a sense of incomplete evacuation; or a patient’s perception of distress related to bowel habits [1]. It is an important side effect to consider when initiating narcotic analgesia. It has been estimated that approximately 3% to 4% of the population is on chronic narcotic pain relievers in the outpatient setting [2,3], and 37% to 81% of these patients will experience constipation [3–9]. Because of the high incidence of constipation, the prophylactic prescription of laxatives with initiation of opioid pain relievers is frequently recommended [10–15]. Furthermore, it has been shown that among patients admitted to the hospital with cancer, there is a lower incidence of constipation amongst those who have received prophylactic laxatives [16]. However, there is no evidence in the literature that prophylactic laxatives improve outcomes in patients on opioid analgesia in the general medicine inpatient setting. Furthermore, studies have illustrated that recommendations for prophylactic laxative use are not reliably followed [3,9].
While the incidence of OIC is well described in the outpatient setting [17,18], there are few studies looking at the incidence of OIC in the hospital setting. It has been shown, however, that occurrence during even a brief hospitalization is possible [4,6]. Acute constipation while hospitalized can theoretically lead to longer hospitalizations, increased pain, and decreased quality of life [6,7,19]. Recent research has focused heavily on the use of novel agents such as peripherally acting mu-opioid receptor antagonists in the treatment of OIC [20–23]. However, the expense of these agents makes them less than ideal in the prophylactic setting. This study will assess the effectiveness and prescribing patterns of prophylactic laxatives in the inpatient general medicine setting over a 2-year period at our institution in patients initiated on patient-controlled analgesia with hydromorphone, morphine, or fentanyl.
Methods
This study was approved by the institutional review board at the Mayo Clinic Rochester. All patients who were initiated on intravenous analgesia with an electronic patient-controlled opioid pump (PCA) while admitted to a general medicine service in 2011 and 2012 were identified. Patients who received PCA therapy were identified through a pharmacy database. Only patients older than 18 years of age were included in the study. PCA therapy was selected for our analysis because PCA therapy is not regularly administered on an outpatient basis. All of these patients, therefore, had a change in their narcotic regimen on admission to the hospital. Patients were excluded from the study if they were on a PCA for less than 24 hours; had a PCA initiated on a service other than a general medicine service; were on a scheduled laxative regimen prior to admission; or carried a diagnosis of bowel obstruction, chronic diarrhea, constipation, or intestinal discontinuity (eg, those with previous diversions or ostomies).
A retrospective review of each patient’s chart was conducted with the assistance of a team of nurse abstractors. Basic demographic data were recorded for each patient. Date of hospital admission and discharge; scheduled laxatives ordered and administered (any dose of sennosides, polyethylene glycol, docusate, bisacodyl, lactulose, or magnesium citrate); abdominal X-rays and abdominal CT scans performed for constipation; and any administration of enemas were recorded. Fiber supplements were not considered laxatives. If a patient was documented to have constipation during their hospitalization this was recorded. Patients were classified as having severe constipation if an abdominal CT or x-ray was performed for the indication of constipation; if abdominal distension, pain, or bloating were documented due to constipation; or if an enema was performed during the hospitalization.
For analysis purposes, patients who started receiving scheduled laxatives (as opposed to laxatives “as needed”)on or before the day of PCA initiation were classified as receiving prophylactic laxatives. Baseline patient characteristics and outcomes were compared using the chi-square test for nominal variables and the rank sum test for continuous variables. In all cases, 2-tailed tests were performed with P values ≤ 0.05 considered statistically significant. A nominal logistic regression model was utilized to assess for independent association of risk factors with the outcome of constipation.
Results
Discussion
Patients initiated on opioid therapy were not prescribed prophylactic laxatives in 64% of our cohort in the inpatient setting. When prescribed, current laxative strategies did not effectively prevent constipation with 49% experiencing OIC. Our data serves as a strong reminder of the magnitude of the problem of OIC in the inpatient setting.
The strength of our paper lies in its role as a magnitude assessment. This retrospective review reveals for that among a diverse group of patients hospitalized within a large academic institution, OIC remains prevalent. Furthermore, the high incidence of severe constipation indicates the potential for increased health care costs and patient discomfort secondary to OIC emphasizing the importance of prevention of OIC. Recent guidelines have made a push toward prophylactic laxative utilization earlier. Specifically, the European Palliative Research Collaborative offers a “strong recommendation to routinely prescribe laxatives for the management or prophylaxis of opioid-induced constipation” [10]. Additionally, the American Society of Interventional Pain Physicians suggests that “a physician should consider the initiation of a bowel regimen even before the development of constipation and definitely after the development of constipation” [11]. Our manuscript serves as a reminder that OIC remains a very prevalent problem and that prophylactic laxatives are still being underutilized.
This is a retrospective study and thus has inherent limitations. Specifically, we are limited to those cases of constipation that were documented in the medical record. The presentation of constipation is varied between patients. This variation in presentation of OIC is inherent to the disease process as is demonstrated in the broad definition for OIC [1]. The cases of constipation that we are reporting clearly were bothersome enough to warrant documentation in the medical record, and while there may have been cases that escaped documentation, we can be confident that the cases of OIC we are reporting are true cases of OIC. The numbers we report can therefore be taken to represent a minimum number of cases of constipation occurring in our study population.
It has been suggested that OIC prevalence varies with type of opioid and duration of opioid therapy [24]. We did not compare dose, type, or duration of opioid therapy in this study. This could certainly account for the seemingly higher rate of constipation within the group treated with prophylactic laxatives as compared with those not treated with prophylactic laxatives. Physicians likely have a higher propensity to prescribe prophylactic laxatives to patients receiving high doses of opioids who are in turn at higher risk for OIC. We cannot say whether differences in efficacy exist between prophylactic laxative regimens or which opioids (dose and duration) cause the most constipation based upon our data. Future studies incorporating dose, duration, and opioid type along with the variables we collected in this study could potentially construct successful logistic regression models with predictive power to identify those at highest risk of OIC.
Our rate of OIC is consistent with previously published figures [3–9]. However, we demonstrate for the first time that prophylactic laxatives are prescribed infrequently and unsuccessfully in the inpatient setting. This is consistent with prescribing rates in the outpatient setting [9,25]. Furthermore, we observed a higher rate of constipation in those treated with prophylactic laxatives compared to those that did not receive prophylactic laxatives. Pottegard et al similarly demonstrated an increased rate of constipation in those utilizing laxative therapy [25]. This is likely secondary to providers recommending prophylactic laxatives to those patients most likely to develop constipation. Despite being able to recognize high-risk patients, providers are unable to prevent OIC as little is known regarding optimal laxative strategies. Previous studies comparing treatment regimens for the relief of constipation in the palliative care population have been largely inconclusive [26]. There have been no studies to date comparing different prophylactic laxatives in the inpatient setting.
Future directions for research in this area would ideally take the form of randomized controlled trials investigating efficacy of different prophylactic laxatives in the inpatient setting. These trials would ideally include well-defined patient groups receiving specific narcotics for specific reasons. These studies would be best if powered to assess the effect of narcotic dosage and duration of therapy as well. Alternatively, larger retrospective chart reviews could be performed including narcotic dosage, type, and duration of therapy with a planned logistic regression model attempting to account for likely independent variables.
Conclusion
Our study demonstrates for the first time that prophylactic laxatives are not being prescribed frequently to patients on opioid analgesia in the inpatient general medicine setting. Additionally, while providers seem to be identifying patients at higher risk of constipation, they are still unable to prevent constipation in a high percentage of patients. Further research into this area would be beneficial to prevent this uncomfortable, costly, and preventable complication of opioid analgesia.
Corresponding author: Roger Yu, MD, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, [email protected].
Funding/support: This research was supported by the Mayo Clinic Return to Work program nurses for data abstraction.
Financial disclosures: None.
From the Division of General Internal Medicine (Dr. Lenz), Division of Biomedical Statistics and Informatics (Mr. Schroeder), and the Division of Hospital Internal Medicine (Ms. Lawson and Dr. Yu), Mayo Clinic, Rochester, MN.
Abstract
- Objective: To describe prophylactic laxative effectiveness and prescribing patterns in patients initiated on intravenous (IV) opioid analgesia.
- Design: Retrospective cohort study.
- Setting and participants: All patients who were on IV narcotics with a patient-controlled pump while admitted to a general medicine service at the Mayo Clinic in Rochester in 2011 and 2012 were identified. Patients were excluded if constipation or diarrhea were diagnosed prior to IV opioid analgesia initiation.
- Measurements: Prophylactic laxatives were defined as laxatives prescribed within 24 hours of IV opioid analgesia initiation to be given even in the absence of constipation. Constipation was recorded when diagnosed during the hospitalization. Severe constipation was defined as constipation resulting in an abdominal CT or X-ray; abdominal distension, pain, or bloating; or if an enema was performed during the hospitalization.
- Results: Of 283 patients, 101 (36%) received prophylactic laxatives and 182 (64%) did not. Constipation occurred in 61 (34%) not on prophylactic laxatives and in 49 (49%) on prophylactic laxatives (P = 0.015). Severe constipation occurred in 23 (13%) not on prophylactic laxatives and in 33 (33%) on prophylactic laxatives (P < 0.001).
- Conclusion: A large percentage of patients are not receiving prophylactic laxatives when receiving IV opioid analgesia in the hospital. Current laxative strategies are not effectively preventing constipation in patients when prescribed.
Key words: constipation; opioids; hospital medicine; patient-controlled analgesia; laxatives.
Opioid-induced constipation (OIC) is defined as a change, when initiating opioid therapy, from baseline bowel habits and defecation patterns that is characterized by any of the following: reduced bowel frequency; development or worsening of straining; a sense of incomplete evacuation; or a patient’s perception of distress related to bowel habits [1]. It is an important side effect to consider when initiating narcotic analgesia. It has been estimated that approximately 3% to 4% of the population is on chronic narcotic pain relievers in the outpatient setting [2,3], and 37% to 81% of these patients will experience constipation [3–9]. Because of the high incidence of constipation, the prophylactic prescription of laxatives with initiation of opioid pain relievers is frequently recommended [10–15]. Furthermore, it has been shown that among patients admitted to the hospital with cancer, there is a lower incidence of constipation amongst those who have received prophylactic laxatives [16]. However, there is no evidence in the literature that prophylactic laxatives improve outcomes in patients on opioid analgesia in the general medicine inpatient setting. Furthermore, studies have illustrated that recommendations for prophylactic laxative use are not reliably followed [3,9].
While the incidence of OIC is well described in the outpatient setting [17,18], there are few studies looking at the incidence of OIC in the hospital setting. It has been shown, however, that occurrence during even a brief hospitalization is possible [4,6]. Acute constipation while hospitalized can theoretically lead to longer hospitalizations, increased pain, and decreased quality of life [6,7,19]. Recent research has focused heavily on the use of novel agents such as peripherally acting mu-opioid receptor antagonists in the treatment of OIC [20–23]. However, the expense of these agents makes them less than ideal in the prophylactic setting. This study will assess the effectiveness and prescribing patterns of prophylactic laxatives in the inpatient general medicine setting over a 2-year period at our institution in patients initiated on patient-controlled analgesia with hydromorphone, morphine, or fentanyl.
Methods
This study was approved by the institutional review board at the Mayo Clinic Rochester. All patients who were initiated on intravenous analgesia with an electronic patient-controlled opioid pump (PCA) while admitted to a general medicine service in 2011 and 2012 were identified. Patients who received PCA therapy were identified through a pharmacy database. Only patients older than 18 years of age were included in the study. PCA therapy was selected for our analysis because PCA therapy is not regularly administered on an outpatient basis. All of these patients, therefore, had a change in their narcotic regimen on admission to the hospital. Patients were excluded from the study if they were on a PCA for less than 24 hours; had a PCA initiated on a service other than a general medicine service; were on a scheduled laxative regimen prior to admission; or carried a diagnosis of bowel obstruction, chronic diarrhea, constipation, or intestinal discontinuity (eg, those with previous diversions or ostomies).
A retrospective review of each patient’s chart was conducted with the assistance of a team of nurse abstractors. Basic demographic data were recorded for each patient. Date of hospital admission and discharge; scheduled laxatives ordered and administered (any dose of sennosides, polyethylene glycol, docusate, bisacodyl, lactulose, or magnesium citrate); abdominal X-rays and abdominal CT scans performed for constipation; and any administration of enemas were recorded. Fiber supplements were not considered laxatives. If a patient was documented to have constipation during their hospitalization this was recorded. Patients were classified as having severe constipation if an abdominal CT or x-ray was performed for the indication of constipation; if abdominal distension, pain, or bloating were documented due to constipation; or if an enema was performed during the hospitalization.
For analysis purposes, patients who started receiving scheduled laxatives (as opposed to laxatives “as needed”)on or before the day of PCA initiation were classified as receiving prophylactic laxatives. Baseline patient characteristics and outcomes were compared using the chi-square test for nominal variables and the rank sum test for continuous variables. In all cases, 2-tailed tests were performed with P values ≤ 0.05 considered statistically significant. A nominal logistic regression model was utilized to assess for independent association of risk factors with the outcome of constipation.
Results
Discussion
Patients initiated on opioid therapy were not prescribed prophylactic laxatives in 64% of our cohort in the inpatient setting. When prescribed, current laxative strategies did not effectively prevent constipation with 49% experiencing OIC. Our data serves as a strong reminder of the magnitude of the problem of OIC in the inpatient setting.
The strength of our paper lies in its role as a magnitude assessment. This retrospective review reveals for that among a diverse group of patients hospitalized within a large academic institution, OIC remains prevalent. Furthermore, the high incidence of severe constipation indicates the potential for increased health care costs and patient discomfort secondary to OIC emphasizing the importance of prevention of OIC. Recent guidelines have made a push toward prophylactic laxative utilization earlier. Specifically, the European Palliative Research Collaborative offers a “strong recommendation to routinely prescribe laxatives for the management or prophylaxis of opioid-induced constipation” [10]. Additionally, the American Society of Interventional Pain Physicians suggests that “a physician should consider the initiation of a bowel regimen even before the development of constipation and definitely after the development of constipation” [11]. Our manuscript serves as a reminder that OIC remains a very prevalent problem and that prophylactic laxatives are still being underutilized.
This is a retrospective study and thus has inherent limitations. Specifically, we are limited to those cases of constipation that were documented in the medical record. The presentation of constipation is varied between patients. This variation in presentation of OIC is inherent to the disease process as is demonstrated in the broad definition for OIC [1]. The cases of constipation that we are reporting clearly were bothersome enough to warrant documentation in the medical record, and while there may have been cases that escaped documentation, we can be confident that the cases of OIC we are reporting are true cases of OIC. The numbers we report can therefore be taken to represent a minimum number of cases of constipation occurring in our study population.
It has been suggested that OIC prevalence varies with type of opioid and duration of opioid therapy [24]. We did not compare dose, type, or duration of opioid therapy in this study. This could certainly account for the seemingly higher rate of constipation within the group treated with prophylactic laxatives as compared with those not treated with prophylactic laxatives. Physicians likely have a higher propensity to prescribe prophylactic laxatives to patients receiving high doses of opioids who are in turn at higher risk for OIC. We cannot say whether differences in efficacy exist between prophylactic laxative regimens or which opioids (dose and duration) cause the most constipation based upon our data. Future studies incorporating dose, duration, and opioid type along with the variables we collected in this study could potentially construct successful logistic regression models with predictive power to identify those at highest risk of OIC.
Our rate of OIC is consistent with previously published figures [3–9]. However, we demonstrate for the first time that prophylactic laxatives are prescribed infrequently and unsuccessfully in the inpatient setting. This is consistent with prescribing rates in the outpatient setting [9,25]. Furthermore, we observed a higher rate of constipation in those treated with prophylactic laxatives compared to those that did not receive prophylactic laxatives. Pottegard et al similarly demonstrated an increased rate of constipation in those utilizing laxative therapy [25]. This is likely secondary to providers recommending prophylactic laxatives to those patients most likely to develop constipation. Despite being able to recognize high-risk patients, providers are unable to prevent OIC as little is known regarding optimal laxative strategies. Previous studies comparing treatment regimens for the relief of constipation in the palliative care population have been largely inconclusive [26]. There have been no studies to date comparing different prophylactic laxatives in the inpatient setting.
Future directions for research in this area would ideally take the form of randomized controlled trials investigating efficacy of different prophylactic laxatives in the inpatient setting. These trials would ideally include well-defined patient groups receiving specific narcotics for specific reasons. These studies would be best if powered to assess the effect of narcotic dosage and duration of therapy as well. Alternatively, larger retrospective chart reviews could be performed including narcotic dosage, type, and duration of therapy with a planned logistic regression model attempting to account for likely independent variables.
Conclusion
Our study demonstrates for the first time that prophylactic laxatives are not being prescribed frequently to patients on opioid analgesia in the inpatient general medicine setting. Additionally, while providers seem to be identifying patients at higher risk of constipation, they are still unable to prevent constipation in a high percentage of patients. Further research into this area would be beneficial to prevent this uncomfortable, costly, and preventable complication of opioid analgesia.
Corresponding author: Roger Yu, MD, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, [email protected].
Funding/support: This research was supported by the Mayo Clinic Return to Work program nurses for data abstraction.
Financial disclosures: None.
1. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016.
2. Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18:1166–75.
3. Choung RS, Locke GR 3rd, Zinsmeister AR, et al. Opioid bowel dysfunction and narcotic bowel syndrome: a population-based study. Am J Gastroenterol 2009;104:1199–204.
4. Droney J, Ross J, Gretton S, et al. Constipation in cancer patients on morphine. Support Care Cancer 2008;16:453–9.
5. Sykes NP. The relationship between opioid use and laxative use in terminally ill cancer patients. Palliat Med 1998;12:375–82.
6. Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009;10:35–42.
7. Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther 2008;27:1224–32.
8. Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005:7:R1046–51.
9. Bouvy ML, Buurma H, Egberts TC. Laxative prescribing in relation to opioid use and the influence of pharmacy-based intervention. J Clin Pharm Ther 2002;27:107–10.
10. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012:13:e58–68.
11. Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2--guidance. Pain Physician 2012;15(3 Suppl):S67–116.
12. Cameron JC. Constipation related to narcotic therapy. A protocol for nurses and patients. Cancer Nurs 1992;15:372–7.
13. Levy MH. Pharmacologic treatment of cancer pain. N Engl J Med 1996;335:1124–32.
14. Swegle JM, Logemann D. Management of common opioid-induced adverse effects. Am Fam Physician 2006;74:1347–54.
15. Donnelly S, Davis MP, Walsh D, Naughton M. Morphine in cancer pain management: a practical guide. Support Care Cancer 2002;10:13–35.
16. Ishihara M, Ikesue H Matsunaga H, et al. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin J Pain 2012;28:373–81.
17. Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004;112:372–80.
18. Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 2010; 22:424–30, e96.
19. Brock C, Olesen SS, Olesen AE, et al. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs 2012;72:1847–65.
20. Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol 2011;106:835–42.
21. Candy B, Jones L, Goodman ML, et al. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev 2011(1):CD003448.
22. Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterol 2013;108:1566–74.
23. Jansen JP, Lorch D, Langan J, et al. A randomized, placebo-controlled phase 3 trial (Study SB-767905/012) of alvimopan for opioid-induced bowel dysfunction in patients with non-cancer pain. J Pain 2011;12:185–93.
24. Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 2014;26:1386–95.
25. Pottegard A, Knudsen TB, van Heesch K, et al. Information on risk of constipation for Danish users of opioids, and their laxative use. Int J Clin Pharm 2014;36:291–4.
26. Candy B, Jones L, Larkin PJ, et al. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015(5):CD003448.
1. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016.
2. Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18:1166–75.
3. Choung RS, Locke GR 3rd, Zinsmeister AR, et al. Opioid bowel dysfunction and narcotic bowel syndrome: a population-based study. Am J Gastroenterol 2009;104:1199–204.
4. Droney J, Ross J, Gretton S, et al. Constipation in cancer patients on morphine. Support Care Cancer 2008;16:453–9.
5. Sykes NP. The relationship between opioid use and laxative use in terminally ill cancer patients. Palliat Med 1998;12:375–82.
6. Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009;10:35–42.
7. Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther 2008;27:1224–32.
8. Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005:7:R1046–51.
9. Bouvy ML, Buurma H, Egberts TC. Laxative prescribing in relation to opioid use and the influence of pharmacy-based intervention. J Clin Pharm Ther 2002;27:107–10.
10. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012:13:e58–68.
11. Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2--guidance. Pain Physician 2012;15(3 Suppl):S67–116.
12. Cameron JC. Constipation related to narcotic therapy. A protocol for nurses and patients. Cancer Nurs 1992;15:372–7.
13. Levy MH. Pharmacologic treatment of cancer pain. N Engl J Med 1996;335:1124–32.
14. Swegle JM, Logemann D. Management of common opioid-induced adverse effects. Am Fam Physician 2006;74:1347–54.
15. Donnelly S, Davis MP, Walsh D, Naughton M. Morphine in cancer pain management: a practical guide. Support Care Cancer 2002;10:13–35.
16. Ishihara M, Ikesue H Matsunaga H, et al. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin J Pain 2012;28:373–81.
17. Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004;112:372–80.
18. Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 2010; 22:424–30, e96.
19. Brock C, Olesen SS, Olesen AE, et al. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs 2012;72:1847–65.
20. Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol 2011;106:835–42.
21. Candy B, Jones L, Goodman ML, et al. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev 2011(1):CD003448.
22. Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterol 2013;108:1566–74.
23. Jansen JP, Lorch D, Langan J, et al. A randomized, placebo-controlled phase 3 trial (Study SB-767905/012) of alvimopan for opioid-induced bowel dysfunction in patients with non-cancer pain. J Pain 2011;12:185–93.
24. Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 2014;26:1386–95.
25. Pottegard A, Knudsen TB, van Heesch K, et al. Information on risk of constipation for Danish users of opioids, and their laxative use. Int J Clin Pharm 2014;36:291–4.
26. Candy B, Jones L, Larkin PJ, et al. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015(5):CD003448.
Patient-Reported Outcome Measures: How Do Digital Tablets Stack Up to Paper Forms? A Randomized, Controlled Study
Over the past several decades, patient-reported outcomes (PROs) have become increasingly important in assessing the quality and effectiveness of medical and surgical care.1,2 The benefit lies in the ability of PROs to characterize the impact of medical interventions on symptoms, function, and other outcomes from the patient’s perspective. Consequently, clinical practices can improve patients’ objective findings (from radiographic and clinical examinations) as well as their preferences in a social-psychological context.2,3 As a patient’s satisfaction with a surgical intervention may not correlate with the surgeon’s objective assessment of outcome, PROs offer unique insight into the patient’s perceptions of well-being.4
Health-related quality-of-life assessments can be made with either general-health or disease-specific instruments. These instruments traditionally are administered with pen and paper—a data collection method with several limitations, chief being the need to manually transfer the data into an electronic medical record, a research database, or both. In addition, administering surveys on paper risks potential disqualification of partially or incorrectly completed surveys. With pen and paper, it is difficult to mandate that every question be answered accurately.
Currently, there is a potential role for electronic medical records and digital tablet devices in survey administration and data collection and storage. Theoretical advantages include direct input of survey data into databases (eliminating manual data entry and associated entry errors), improved accuracy and completion rates, and long-term storage not dependent on paper charts.5To our knowledge, there have been no prospective studies of different orthopedic outcomes collection methods. Some studies have evaluated use of touch-based tablets in data collection. Dy and colleagues6 considered administration of the DASH (Disabilities of the Arm, Shoulder, and Hand) survey on an iPad tablet (Apple Computers) and retrospectively compared the tablet and paper completion rates. The tablet group’s rate (98%) was significantly higher than the paper group’s rate (76%). Aktas and colleagues7 reported a high completion rate for a tablet survey of palliative care outcomes (they did not compare modalities). A handful of other studies have found higher intraclass correlation and validation for digital data collection than for paper collection.7-14 The comparability of the data collected digitally vs on paper was the nidus for our decision to prospectively evaluate the ease and reliability of digital data collection.
We conducted a prospective, randomized study to compare the performance of tablet and paper versions of several general-health and musculoskeletal disease–specific questionnaires. We hypothesized the tablet and paper surveys would have similar completion rates and times.
Methods
This study was approved by our Institutional Review Board. Participants were recruited during their clinic visit to 3 subspecialty orthopedic services (upper extremity, spine, arthroplasty). The questionnaires included basic demographics questions and questions about tablet use (comfort level with computers, measured on a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree), and ownership of a tablet or smartphone). Also included were European Quality of Life–5 Dimensions (EQ-5D, General Health), a disease questionnaire specific to 1 of the 3 subspecialty services, and a satisfaction survey. Patients were asked to complete the Oswestry Disability Index (ODI) for low-back pain, the Neck Disability Index (NDI) for neck pain, the Hip Disability and Osteoarthritis Outcomes Score (HOOS) for hip pain, the Knee Injury and Osteoarthritis Outcomes Score (KOOS) for knee pain, or the QuickDASH survey for upper extremity complaints (subspecialty-specific). After recruitment, a computer-generated randomization technique was used to randomly assign patients to either a paper or an electronic (iPad) data collection group.15 We included all surveys for which patients had sufficient completion time (no clinic staff interruptions) and excluded surveys marked incomplete (because of interruptions for clinic workflow efficiency). For direct input from tablets and for data storage, we used the Research Electronic Data Capture (REDCap) system hosted at our institution.16 Our staff registered patients as REDCap participants, assigned them to their disease-specific study arms, and gave them tablets to use to complete the surveys.
Patients who were randomly assigned to take the surveys on paper were given a packet that included the demographics survey, the EQ-5D, a disease-specific survey, and a satisfaction survey. Their responses were then manually entered by the investigators into the REDCap system.
Patients who were randomly assigned to take the surveys on tablets used the REDCap survey feature, which allowed them to directly input their responses into the database (Figure).
Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction with survey length (Likert scale, 1-5), ease of completion (Likert scale, 1-5), ability to comprehend questions (Likert scale, 1-5), and preference for the other survey modality (Appendix).
We used SPSS statistical software (IBM) to analyze our data, t test to compare continuous variables, χ2 test to compare categorical variables, and linear regression to test the relationship between number of questions and completion rate. Statistical significance was set at P < .05.
Results
Of the 510 patients enrolled in the study, 483 completed the initial demographics questionnaire and were included in the analysis. Patients were excluded if they were unable to complete the initial demographics questionnaire because of clinic workflow (eg, immediate need to be seen by physician, need to transfer to radiology for imaging and not being able to revisit the survey). Mean age was 56 years (range, 14-93 years), and 51% of the respondents were female. Fifty percent owned tablets, 70% owned smartphones, and mean (SD) self-rating of computer skills was 3.13 (1.16) (Likert scale, 1-5). There were no significant demographic differences between the tablet and paper groups (Table 1).
For each disease-specific questionnaire, the instrument’s published instructions for calculating scores were followed; these scores were then compared in order to further characterize the groups. There were significant differences in scores on the EQ-5D descriptive questions, a pain visual analog scale (VAS), and the NDI. Mean EQ-5D score was 0.664 for the tablet group and 0.699 for the paper group (P = .041), mean pain VAS score was 62.5 for the tablet group and 71.6 for the paper group (P < .001), and mean NDI score was 42.8 for the tablet group and 32.4 for the paper group (P = .033).
The overall completion rate for all questionnaires was 84.4%. The KOOS completion rate was 83.3% for the tablet group and 54.5% for the paper group (P = .023). Although it was not statistically significant, there was a trend toward higher rates of completing all disease-specific questionnaires in the tablet group relative to the paper group.
Satisfaction regarding the surveys and their modalities was similar between the groups.
Discussion
Electronic data entry has many advantages over traditional paper-based data collection and can be used with PRO surveys to measure response to treatment. Our study evaluated whether completion rates differed between surveys administered on digital tablets and those administered on traditional paper forms in a clinic setting. We selected general-health and disease-specific instruments commonly used to collect PROs from orthopedic patients. Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction, and survey preferences.
In this study, our tablet and paper groups had similar overall survey completion rates, which suggests digital tablet-based data collection is noninferior to traditional pen-and-paper data collection with respect to patient response rate in the clinical setting. It is worth emphasizing that the tablet surveys were made to resemble and function as much as possible like the paper surveys. For example, patients were allowed to select multiple answers as well as advance without answering a question. Paper surveys were mimicked so we could study inherent differences in patient responsiveness without adding digital features to prevent patients from selecting multiple answers or skipping questions. We postulate that adding these digital features could have introduced a significant difference in patient responsiveness.
Time for survey completion was not significantly different between the tablet and paper groups, demonstrating that data can be digitally collected and the aforementioned advantages realized without significant delay or clinic workflow disruption. In the future, patients may be able to complete their forms digitally, on their own devices, before arriving for their clinic visits—resulting in improved clinic workflow and data collection efficiency.
Scores computed for the health-related quality-of-life questionnaires were not significantly different between the tablet and paper groups, except for EQ-5D and NDI. Although statistically significant, the 0.035 difference between the groups’ EQ-5D scores (0.664, 0.699) is not clinically significant. (Pickard and colleagues17 established that 0.06 is the clinically significant difference between EQ-5D scores in the United States.) If there were any clinical difference in the present study, our paper group patients appeared to be in better health than our tablet group patients.
Patients’ motivation to complete surveys often plays a large role in meaningful rates of completion. On our subjective satisfaction survey, a larger percentage of patients reported they would prefer to use a tablet for future surveys (Table 4). This finding may be driven by the novelty or ease of using a popular device. Nevertheless, we think it is worthwhile to heed patient preferences, as they may point to more successful data collection and compliance.
Several other studies have compared electronic and paper data capture.6,7,9-14,18-22 Dy and colleagues6 reported on administering the DASH survey on an iPad tablet using REDCap in an outpatient setting. They found that the percentage of surveys that could be scored (<3 questions left unanswered) was significantly higher for their tablet group (98%) than their paper group (76%). The larger difference in survey completion rates in their study (vs ours) may be attributable to their use of DASH, which has more survey items (compared with QuickDASH, the instrument we used) and thus may be more sensitive to detecting differences, at the risk of increasing the burden on survey takers.23 Aktas and colleagues7 conducted a similar but smaller study of completion rates, completion times, and overall practicality of using digital tablets to collect PROs in a palliative care clinic (they did not compare tablet and paper modalities). Marsh and colleagues,12 who studied the agreement between data collected on electronic and paper versions of the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index and the SF-12 (12-item Short Form Health Survey, Version 2) after total hip and total knee arthroplasty, found a high intraclass correlation coefficient between the 2 methods. Griffiths-Jones and colleagues11 also found a high degree of agreement between patient data collected on digital and paper surveys. In a similar study, Fanning and McAuley10 compared digital tablet and paper survey administration in an older population and found a higher percentage of preference for tablets, with ease of use and anxiety during survey completion correlating with preference. These findings mirror ours, even with our inclusion of patients in a broader age range.
Strengths of our study included its overall cohort size and the variety of measurement instruments used. In addition, we measured time for survey completion to assess the practicality of tablet-based data collection and refrained from using digital features that could have artificially improved the completion rate for this survey modality.
Our study had a few limitations. First, we recruited unequal numbers of patients from the different subspecialties—a result of each subspecialty having a different number of attending physicians and a different patient volume. Given randomization and use of similar patients across the study arms, however, this likely did not present any significant bias. Second, each patient completed a tablet survey or a paper survey but not both, and therefore we could not compare a patient’s performance on the 2 modalities. However, the burden of completing the same survey more than once likely would have lowered our participation rate and introduced additional biases we wanted to avoid. Third, despite our attempt to mimic the look of a paper survey, the tablet’s user interface presented several potential difficulties. For example, its small text and small answer buttons may have been limiting for patients with poor vision. These design features emphasize the importance of having a user interface that can be adapted to the individual, regardless of handicap. Indeed, adaptability is a potential strength of digital interfaces. For adaptability, an interface designer can use large, scalable text and add audio prompts and other features.
Our findings can be useful in evaluating patient responsiveness to surveys administered on digital tablets in an outpatient clinic setting. In this prospective, randomized study, we found that, for survey completion, use of a tablet device did not require more time than use of a paper form. In addition, the administration modalities had similar completion and error rates for a variety of orthopedic outcomes surveys. We did not activate digital features that would have given unfair advantage to the digital data collection modality. We also found a strong preference for use of technology in PRO data collection, and this may help improve collection rates. Last, though optimizing the flow of patients in our clinic was not a strict research metric, we prioritized making sure patients were not spending any more time completing these surveys than in the past. Given the potential benefits of digital surveys—immediate and accurate transfer of collected data into multiple databases, including the patient’s electronic medical record—our experience supports continuing validation of these instruments for potential wider use.
Am J Orthop. 2016;45(7):E451-E457. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Higginson IJ, Carr AJ. Measuring quality of life: using quality of life measures in the clinical setting. BMJ. 2001;322(7297):1297-1300.
3. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102-109.
4. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622-629.
5. Paudel D, Ahmed M, Pradhan A, Lal Dangol R. Successful use of tablet personal computers and wireless technologies for the 2011 Nepal Demographic and Health Survey. Glob Heal Sci Pract. 2013;1(2):277-284.
6. Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A. The use of a tablet computer to complete the DASH questionnaire. J Hand Surg Am. 2012;37(12):2589-2594.
7. Aktas A, Hullihen B, Shrotriya S, Thomas S, Walsh D, Estfan B. Connected health: cancer symptom and quality-of-life assessment using a tablet computer: a pilot study. Am J Hosp Palliat Care. 2015;32(2):189-197.
8. Basnov M, Kongsved SM, Bech P, Hjollund NH. Reliability of Short Form-36 in an internet- and a pen-and-paper version. Inform Health Soc Care. 2009;34(1):53-58.
9. Bellamy N, Wilson C, Hendrikz J, et al; EDC Study Group. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J Clin Epidemiol. 2011;64(2):182-190.
10. Fanning J, McAuley E. A comparison of tablet computer and paper-based questionnaires in healthy aging research. JMIR Res Protoc. 2014;3(3):e38.
11. Griffiths-Jones W, Norton MR, Fern ED, Williams DH. The equivalence of remote electronic and paper patient reported outcome (PRO) collection. J Arthroplasty. 2014;29(11):2136-2139.
12. Marsh JD, Bryant DM, Macdonald SJ, Naudie DD. Patients respond similarly to paper and electronic versions of the WOMAC and SF-12 following total joint arthroplasty. J Arthroplasty. 2014;29(4):670-673.
13. Olajos-Clow J, Minard J, Szpiro K, et al. Validation of an electronic version of the Mini Asthma Quality of Life Questionnaire. Respir Med. 2010;104(5):658-667.
14. Shervin N, Dorrwachter J, Bragdon CR, Shervin D, Zurakowski D, Malchau H. Comparison of paper and computer-based questionnaire modes for measuring health outcomes in patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2011;93(3):285-293.
15. Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8-11.
16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
17. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
18. Abdel Messih M, Naylor JM, Descallar J, Manickam A, Mittal R, Harris IA. Mail versus telephone administration of the Oxford Knee and Hip Scores. J Arthroplasty. 2014;29(3):491-494.
19. Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of internet versus paper-and-pencil versions. J Med Internet Res. 2007;9(3):e25.
20. Theiler R, Bischoff-Ferrari HA, Good M, Bellamy N. Responsiveness of the electronic touch screen WOMAC 3.1 OA Index in a short term clinical trial with rofecoxib. Osteoarthritis Cartilage. 2004;12(11):912-916.
21. Ryan JM, Corry JR, Attewell R, Smithson MJ. A comparison of an electronic version of the SF-36 General Health Questionnaire to the standard paper version. Qual Life Res. 2002;11(1):19-26.
22. Wilson AS, Kitas GD, Carruthers DM, et al. Computerized information-gathering in specialist rheumatology clinics: an initial evaluation of an electronic version of the Short Form 36. Rheumatology. 2002;41(3):268-273.
23. Angst F, Goldhahn J, Drerup S, Flury M, Schwyzer HK, Simmen BR. How sharp is the short QuickDASH? A refined content and validity analysis of the Short Form of the Disabilities of the Shoulder, Arm and Hand questionnaire in the strata of symptoms and function and specific joint conditions. Qual Life Res. 2009;18(8):1043-1051.
Over the past several decades, patient-reported outcomes (PROs) have become increasingly important in assessing the quality and effectiveness of medical and surgical care.1,2 The benefit lies in the ability of PROs to characterize the impact of medical interventions on symptoms, function, and other outcomes from the patient’s perspective. Consequently, clinical practices can improve patients’ objective findings (from radiographic and clinical examinations) as well as their preferences in a social-psychological context.2,3 As a patient’s satisfaction with a surgical intervention may not correlate with the surgeon’s objective assessment of outcome, PROs offer unique insight into the patient’s perceptions of well-being.4
Health-related quality-of-life assessments can be made with either general-health or disease-specific instruments. These instruments traditionally are administered with pen and paper—a data collection method with several limitations, chief being the need to manually transfer the data into an electronic medical record, a research database, or both. In addition, administering surveys on paper risks potential disqualification of partially or incorrectly completed surveys. With pen and paper, it is difficult to mandate that every question be answered accurately.
Currently, there is a potential role for electronic medical records and digital tablet devices in survey administration and data collection and storage. Theoretical advantages include direct input of survey data into databases (eliminating manual data entry and associated entry errors), improved accuracy and completion rates, and long-term storage not dependent on paper charts.5To our knowledge, there have been no prospective studies of different orthopedic outcomes collection methods. Some studies have evaluated use of touch-based tablets in data collection. Dy and colleagues6 considered administration of the DASH (Disabilities of the Arm, Shoulder, and Hand) survey on an iPad tablet (Apple Computers) and retrospectively compared the tablet and paper completion rates. The tablet group’s rate (98%) was significantly higher than the paper group’s rate (76%). Aktas and colleagues7 reported a high completion rate for a tablet survey of palliative care outcomes (they did not compare modalities). A handful of other studies have found higher intraclass correlation and validation for digital data collection than for paper collection.7-14 The comparability of the data collected digitally vs on paper was the nidus for our decision to prospectively evaluate the ease and reliability of digital data collection.
We conducted a prospective, randomized study to compare the performance of tablet and paper versions of several general-health and musculoskeletal disease–specific questionnaires. We hypothesized the tablet and paper surveys would have similar completion rates and times.
Methods
This study was approved by our Institutional Review Board. Participants were recruited during their clinic visit to 3 subspecialty orthopedic services (upper extremity, spine, arthroplasty). The questionnaires included basic demographics questions and questions about tablet use (comfort level with computers, measured on a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree), and ownership of a tablet or smartphone). Also included were European Quality of Life–5 Dimensions (EQ-5D, General Health), a disease questionnaire specific to 1 of the 3 subspecialty services, and a satisfaction survey. Patients were asked to complete the Oswestry Disability Index (ODI) for low-back pain, the Neck Disability Index (NDI) for neck pain, the Hip Disability and Osteoarthritis Outcomes Score (HOOS) for hip pain, the Knee Injury and Osteoarthritis Outcomes Score (KOOS) for knee pain, or the QuickDASH survey for upper extremity complaints (subspecialty-specific). After recruitment, a computer-generated randomization technique was used to randomly assign patients to either a paper or an electronic (iPad) data collection group.15 We included all surveys for which patients had sufficient completion time (no clinic staff interruptions) and excluded surveys marked incomplete (because of interruptions for clinic workflow efficiency). For direct input from tablets and for data storage, we used the Research Electronic Data Capture (REDCap) system hosted at our institution.16 Our staff registered patients as REDCap participants, assigned them to their disease-specific study arms, and gave them tablets to use to complete the surveys.
Patients who were randomly assigned to take the surveys on paper were given a packet that included the demographics survey, the EQ-5D, a disease-specific survey, and a satisfaction survey. Their responses were then manually entered by the investigators into the REDCap system.
Patients who were randomly assigned to take the surveys on tablets used the REDCap survey feature, which allowed them to directly input their responses into the database (Figure).
Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction with survey length (Likert scale, 1-5), ease of completion (Likert scale, 1-5), ability to comprehend questions (Likert scale, 1-5), and preference for the other survey modality (Appendix).
We used SPSS statistical software (IBM) to analyze our data, t test to compare continuous variables, χ2 test to compare categorical variables, and linear regression to test the relationship between number of questions and completion rate. Statistical significance was set at P < .05.
Results
Of the 510 patients enrolled in the study, 483 completed the initial demographics questionnaire and were included in the analysis. Patients were excluded if they were unable to complete the initial demographics questionnaire because of clinic workflow (eg, immediate need to be seen by physician, need to transfer to radiology for imaging and not being able to revisit the survey). Mean age was 56 years (range, 14-93 years), and 51% of the respondents were female. Fifty percent owned tablets, 70% owned smartphones, and mean (SD) self-rating of computer skills was 3.13 (1.16) (Likert scale, 1-5). There were no significant demographic differences between the tablet and paper groups (Table 1).
For each disease-specific questionnaire, the instrument’s published instructions for calculating scores were followed; these scores were then compared in order to further characterize the groups. There were significant differences in scores on the EQ-5D descriptive questions, a pain visual analog scale (VAS), and the NDI. Mean EQ-5D score was 0.664 for the tablet group and 0.699 for the paper group (P = .041), mean pain VAS score was 62.5 for the tablet group and 71.6 for the paper group (P < .001), and mean NDI score was 42.8 for the tablet group and 32.4 for the paper group (P = .033).
The overall completion rate for all questionnaires was 84.4%. The KOOS completion rate was 83.3% for the tablet group and 54.5% for the paper group (P = .023). Although it was not statistically significant, there was a trend toward higher rates of completing all disease-specific questionnaires in the tablet group relative to the paper group.
Satisfaction regarding the surveys and their modalities was similar between the groups.
Discussion
Electronic data entry has many advantages over traditional paper-based data collection and can be used with PRO surveys to measure response to treatment. Our study evaluated whether completion rates differed between surveys administered on digital tablets and those administered on traditional paper forms in a clinic setting. We selected general-health and disease-specific instruments commonly used to collect PROs from orthopedic patients. Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction, and survey preferences.
In this study, our tablet and paper groups had similar overall survey completion rates, which suggests digital tablet-based data collection is noninferior to traditional pen-and-paper data collection with respect to patient response rate in the clinical setting. It is worth emphasizing that the tablet surveys were made to resemble and function as much as possible like the paper surveys. For example, patients were allowed to select multiple answers as well as advance without answering a question. Paper surveys were mimicked so we could study inherent differences in patient responsiveness without adding digital features to prevent patients from selecting multiple answers or skipping questions. We postulate that adding these digital features could have introduced a significant difference in patient responsiveness.
Time for survey completion was not significantly different between the tablet and paper groups, demonstrating that data can be digitally collected and the aforementioned advantages realized without significant delay or clinic workflow disruption. In the future, patients may be able to complete their forms digitally, on their own devices, before arriving for their clinic visits—resulting in improved clinic workflow and data collection efficiency.
Scores computed for the health-related quality-of-life questionnaires were not significantly different between the tablet and paper groups, except for EQ-5D and NDI. Although statistically significant, the 0.035 difference between the groups’ EQ-5D scores (0.664, 0.699) is not clinically significant. (Pickard and colleagues17 established that 0.06 is the clinically significant difference between EQ-5D scores in the United States.) If there were any clinical difference in the present study, our paper group patients appeared to be in better health than our tablet group patients.
Patients’ motivation to complete surveys often plays a large role in meaningful rates of completion. On our subjective satisfaction survey, a larger percentage of patients reported they would prefer to use a tablet for future surveys (Table 4). This finding may be driven by the novelty or ease of using a popular device. Nevertheless, we think it is worthwhile to heed patient preferences, as they may point to more successful data collection and compliance.
Several other studies have compared electronic and paper data capture.6,7,9-14,18-22 Dy and colleagues6 reported on administering the DASH survey on an iPad tablet using REDCap in an outpatient setting. They found that the percentage of surveys that could be scored (<3 questions left unanswered) was significantly higher for their tablet group (98%) than their paper group (76%). The larger difference in survey completion rates in their study (vs ours) may be attributable to their use of DASH, which has more survey items (compared with QuickDASH, the instrument we used) and thus may be more sensitive to detecting differences, at the risk of increasing the burden on survey takers.23 Aktas and colleagues7 conducted a similar but smaller study of completion rates, completion times, and overall practicality of using digital tablets to collect PROs in a palliative care clinic (they did not compare tablet and paper modalities). Marsh and colleagues,12 who studied the agreement between data collected on electronic and paper versions of the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index and the SF-12 (12-item Short Form Health Survey, Version 2) after total hip and total knee arthroplasty, found a high intraclass correlation coefficient between the 2 methods. Griffiths-Jones and colleagues11 also found a high degree of agreement between patient data collected on digital and paper surveys. In a similar study, Fanning and McAuley10 compared digital tablet and paper survey administration in an older population and found a higher percentage of preference for tablets, with ease of use and anxiety during survey completion correlating with preference. These findings mirror ours, even with our inclusion of patients in a broader age range.
Strengths of our study included its overall cohort size and the variety of measurement instruments used. In addition, we measured time for survey completion to assess the practicality of tablet-based data collection and refrained from using digital features that could have artificially improved the completion rate for this survey modality.
Our study had a few limitations. First, we recruited unequal numbers of patients from the different subspecialties—a result of each subspecialty having a different number of attending physicians and a different patient volume. Given randomization and use of similar patients across the study arms, however, this likely did not present any significant bias. Second, each patient completed a tablet survey or a paper survey but not both, and therefore we could not compare a patient’s performance on the 2 modalities. However, the burden of completing the same survey more than once likely would have lowered our participation rate and introduced additional biases we wanted to avoid. Third, despite our attempt to mimic the look of a paper survey, the tablet’s user interface presented several potential difficulties. For example, its small text and small answer buttons may have been limiting for patients with poor vision. These design features emphasize the importance of having a user interface that can be adapted to the individual, regardless of handicap. Indeed, adaptability is a potential strength of digital interfaces. For adaptability, an interface designer can use large, scalable text and add audio prompts and other features.
Our findings can be useful in evaluating patient responsiveness to surveys administered on digital tablets in an outpatient clinic setting. In this prospective, randomized study, we found that, for survey completion, use of a tablet device did not require more time than use of a paper form. In addition, the administration modalities had similar completion and error rates for a variety of orthopedic outcomes surveys. We did not activate digital features that would have given unfair advantage to the digital data collection modality. We also found a strong preference for use of technology in PRO data collection, and this may help improve collection rates. Last, though optimizing the flow of patients in our clinic was not a strict research metric, we prioritized making sure patients were not spending any more time completing these surveys than in the past. Given the potential benefits of digital surveys—immediate and accurate transfer of collected data into multiple databases, including the patient’s electronic medical record—our experience supports continuing validation of these instruments for potential wider use.
Am J Orthop. 2016;45(7):E451-E457. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Over the past several decades, patient-reported outcomes (PROs) have become increasingly important in assessing the quality and effectiveness of medical and surgical care.1,2 The benefit lies in the ability of PROs to characterize the impact of medical interventions on symptoms, function, and other outcomes from the patient’s perspective. Consequently, clinical practices can improve patients’ objective findings (from radiographic and clinical examinations) as well as their preferences in a social-psychological context.2,3 As a patient’s satisfaction with a surgical intervention may not correlate with the surgeon’s objective assessment of outcome, PROs offer unique insight into the patient’s perceptions of well-being.4
Health-related quality-of-life assessments can be made with either general-health or disease-specific instruments. These instruments traditionally are administered with pen and paper—a data collection method with several limitations, chief being the need to manually transfer the data into an electronic medical record, a research database, or both. In addition, administering surveys on paper risks potential disqualification of partially or incorrectly completed surveys. With pen and paper, it is difficult to mandate that every question be answered accurately.
Currently, there is a potential role for electronic medical records and digital tablet devices in survey administration and data collection and storage. Theoretical advantages include direct input of survey data into databases (eliminating manual data entry and associated entry errors), improved accuracy and completion rates, and long-term storage not dependent on paper charts.5To our knowledge, there have been no prospective studies of different orthopedic outcomes collection methods. Some studies have evaluated use of touch-based tablets in data collection. Dy and colleagues6 considered administration of the DASH (Disabilities of the Arm, Shoulder, and Hand) survey on an iPad tablet (Apple Computers) and retrospectively compared the tablet and paper completion rates. The tablet group’s rate (98%) was significantly higher than the paper group’s rate (76%). Aktas and colleagues7 reported a high completion rate for a tablet survey of palliative care outcomes (they did not compare modalities). A handful of other studies have found higher intraclass correlation and validation for digital data collection than for paper collection.7-14 The comparability of the data collected digitally vs on paper was the nidus for our decision to prospectively evaluate the ease and reliability of digital data collection.
We conducted a prospective, randomized study to compare the performance of tablet and paper versions of several general-health and musculoskeletal disease–specific questionnaires. We hypothesized the tablet and paper surveys would have similar completion rates and times.
Methods
This study was approved by our Institutional Review Board. Participants were recruited during their clinic visit to 3 subspecialty orthopedic services (upper extremity, spine, arthroplasty). The questionnaires included basic demographics questions and questions about tablet use (comfort level with computers, measured on a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree), and ownership of a tablet or smartphone). Also included were European Quality of Life–5 Dimensions (EQ-5D, General Health), a disease questionnaire specific to 1 of the 3 subspecialty services, and a satisfaction survey. Patients were asked to complete the Oswestry Disability Index (ODI) for low-back pain, the Neck Disability Index (NDI) for neck pain, the Hip Disability and Osteoarthritis Outcomes Score (HOOS) for hip pain, the Knee Injury and Osteoarthritis Outcomes Score (KOOS) for knee pain, or the QuickDASH survey for upper extremity complaints (subspecialty-specific). After recruitment, a computer-generated randomization technique was used to randomly assign patients to either a paper or an electronic (iPad) data collection group.15 We included all surveys for which patients had sufficient completion time (no clinic staff interruptions) and excluded surveys marked incomplete (because of interruptions for clinic workflow efficiency). For direct input from tablets and for data storage, we used the Research Electronic Data Capture (REDCap) system hosted at our institution.16 Our staff registered patients as REDCap participants, assigned them to their disease-specific study arms, and gave them tablets to use to complete the surveys.
Patients who were randomly assigned to take the surveys on paper were given a packet that included the demographics survey, the EQ-5D, a disease-specific survey, and a satisfaction survey. Their responses were then manually entered by the investigators into the REDCap system.
Patients who were randomly assigned to take the surveys on tablets used the REDCap survey feature, which allowed them to directly input their responses into the database (Figure).
Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction with survey length (Likert scale, 1-5), ease of completion (Likert scale, 1-5), ability to comprehend questions (Likert scale, 1-5), and preference for the other survey modality (Appendix).
We used SPSS statistical software (IBM) to analyze our data, t test to compare continuous variables, χ2 test to compare categorical variables, and linear regression to test the relationship between number of questions and completion rate. Statistical significance was set at P < .05.
Results
Of the 510 patients enrolled in the study, 483 completed the initial demographics questionnaire and were included in the analysis. Patients were excluded if they were unable to complete the initial demographics questionnaire because of clinic workflow (eg, immediate need to be seen by physician, need to transfer to radiology for imaging and not being able to revisit the survey). Mean age was 56 years (range, 14-93 years), and 51% of the respondents were female. Fifty percent owned tablets, 70% owned smartphones, and mean (SD) self-rating of computer skills was 3.13 (1.16) (Likert scale, 1-5). There were no significant demographic differences between the tablet and paper groups (Table 1).
For each disease-specific questionnaire, the instrument’s published instructions for calculating scores were followed; these scores were then compared in order to further characterize the groups. There were significant differences in scores on the EQ-5D descriptive questions, a pain visual analog scale (VAS), and the NDI. Mean EQ-5D score was 0.664 for the tablet group and 0.699 for the paper group (P = .041), mean pain VAS score was 62.5 for the tablet group and 71.6 for the paper group (P < .001), and mean NDI score was 42.8 for the tablet group and 32.4 for the paper group (P = .033).
The overall completion rate for all questionnaires was 84.4%. The KOOS completion rate was 83.3% for the tablet group and 54.5% for the paper group (P = .023). Although it was not statistically significant, there was a trend toward higher rates of completing all disease-specific questionnaires in the tablet group relative to the paper group.
Satisfaction regarding the surveys and their modalities was similar between the groups.
Discussion
Electronic data entry has many advantages over traditional paper-based data collection and can be used with PRO surveys to measure response to treatment. Our study evaluated whether completion rates differed between surveys administered on digital tablets and those administered on traditional paper forms in a clinic setting. We selected general-health and disease-specific instruments commonly used to collect PROs from orthopedic patients. Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction, and survey preferences.
In this study, our tablet and paper groups had similar overall survey completion rates, which suggests digital tablet-based data collection is noninferior to traditional pen-and-paper data collection with respect to patient response rate in the clinical setting. It is worth emphasizing that the tablet surveys were made to resemble and function as much as possible like the paper surveys. For example, patients were allowed to select multiple answers as well as advance without answering a question. Paper surveys were mimicked so we could study inherent differences in patient responsiveness without adding digital features to prevent patients from selecting multiple answers or skipping questions. We postulate that adding these digital features could have introduced a significant difference in patient responsiveness.
Time for survey completion was not significantly different between the tablet and paper groups, demonstrating that data can be digitally collected and the aforementioned advantages realized without significant delay or clinic workflow disruption. In the future, patients may be able to complete their forms digitally, on their own devices, before arriving for their clinic visits—resulting in improved clinic workflow and data collection efficiency.
Scores computed for the health-related quality-of-life questionnaires were not significantly different between the tablet and paper groups, except for EQ-5D and NDI. Although statistically significant, the 0.035 difference between the groups’ EQ-5D scores (0.664, 0.699) is not clinically significant. (Pickard and colleagues17 established that 0.06 is the clinically significant difference between EQ-5D scores in the United States.) If there were any clinical difference in the present study, our paper group patients appeared to be in better health than our tablet group patients.
Patients’ motivation to complete surveys often plays a large role in meaningful rates of completion. On our subjective satisfaction survey, a larger percentage of patients reported they would prefer to use a tablet for future surveys (Table 4). This finding may be driven by the novelty or ease of using a popular device. Nevertheless, we think it is worthwhile to heed patient preferences, as they may point to more successful data collection and compliance.
Several other studies have compared electronic and paper data capture.6,7,9-14,18-22 Dy and colleagues6 reported on administering the DASH survey on an iPad tablet using REDCap in an outpatient setting. They found that the percentage of surveys that could be scored (<3 questions left unanswered) was significantly higher for their tablet group (98%) than their paper group (76%). The larger difference in survey completion rates in their study (vs ours) may be attributable to their use of DASH, which has more survey items (compared with QuickDASH, the instrument we used) and thus may be more sensitive to detecting differences, at the risk of increasing the burden on survey takers.23 Aktas and colleagues7 conducted a similar but smaller study of completion rates, completion times, and overall practicality of using digital tablets to collect PROs in a palliative care clinic (they did not compare tablet and paper modalities). Marsh and colleagues,12 who studied the agreement between data collected on electronic and paper versions of the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index and the SF-12 (12-item Short Form Health Survey, Version 2) after total hip and total knee arthroplasty, found a high intraclass correlation coefficient between the 2 methods. Griffiths-Jones and colleagues11 also found a high degree of agreement between patient data collected on digital and paper surveys. In a similar study, Fanning and McAuley10 compared digital tablet and paper survey administration in an older population and found a higher percentage of preference for tablets, with ease of use and anxiety during survey completion correlating with preference. These findings mirror ours, even with our inclusion of patients in a broader age range.
Strengths of our study included its overall cohort size and the variety of measurement instruments used. In addition, we measured time for survey completion to assess the practicality of tablet-based data collection and refrained from using digital features that could have artificially improved the completion rate for this survey modality.
Our study had a few limitations. First, we recruited unequal numbers of patients from the different subspecialties—a result of each subspecialty having a different number of attending physicians and a different patient volume. Given randomization and use of similar patients across the study arms, however, this likely did not present any significant bias. Second, each patient completed a tablet survey or a paper survey but not both, and therefore we could not compare a patient’s performance on the 2 modalities. However, the burden of completing the same survey more than once likely would have lowered our participation rate and introduced additional biases we wanted to avoid. Third, despite our attempt to mimic the look of a paper survey, the tablet’s user interface presented several potential difficulties. For example, its small text and small answer buttons may have been limiting for patients with poor vision. These design features emphasize the importance of having a user interface that can be adapted to the individual, regardless of handicap. Indeed, adaptability is a potential strength of digital interfaces. For adaptability, an interface designer can use large, scalable text and add audio prompts and other features.
Our findings can be useful in evaluating patient responsiveness to surveys administered on digital tablets in an outpatient clinic setting. In this prospective, randomized study, we found that, for survey completion, use of a tablet device did not require more time than use of a paper form. In addition, the administration modalities had similar completion and error rates for a variety of orthopedic outcomes surveys. We did not activate digital features that would have given unfair advantage to the digital data collection modality. We also found a strong preference for use of technology in PRO data collection, and this may help improve collection rates. Last, though optimizing the flow of patients in our clinic was not a strict research metric, we prioritized making sure patients were not spending any more time completing these surveys than in the past. Given the potential benefits of digital surveys—immediate and accurate transfer of collected data into multiple databases, including the patient’s electronic medical record—our experience supports continuing validation of these instruments for potential wider use.
Am J Orthop. 2016;45(7):E451-E457. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Higginson IJ, Carr AJ. Measuring quality of life: using quality of life measures in the clinical setting. BMJ. 2001;322(7297):1297-1300.
3. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102-109.
4. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622-629.
5. Paudel D, Ahmed M, Pradhan A, Lal Dangol R. Successful use of tablet personal computers and wireless technologies for the 2011 Nepal Demographic and Health Survey. Glob Heal Sci Pract. 2013;1(2):277-284.
6. Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A. The use of a tablet computer to complete the DASH questionnaire. J Hand Surg Am. 2012;37(12):2589-2594.
7. Aktas A, Hullihen B, Shrotriya S, Thomas S, Walsh D, Estfan B. Connected health: cancer symptom and quality-of-life assessment using a tablet computer: a pilot study. Am J Hosp Palliat Care. 2015;32(2):189-197.
8. Basnov M, Kongsved SM, Bech P, Hjollund NH. Reliability of Short Form-36 in an internet- and a pen-and-paper version. Inform Health Soc Care. 2009;34(1):53-58.
9. Bellamy N, Wilson C, Hendrikz J, et al; EDC Study Group. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J Clin Epidemiol. 2011;64(2):182-190.
10. Fanning J, McAuley E. A comparison of tablet computer and paper-based questionnaires in healthy aging research. JMIR Res Protoc. 2014;3(3):e38.
11. Griffiths-Jones W, Norton MR, Fern ED, Williams DH. The equivalence of remote electronic and paper patient reported outcome (PRO) collection. J Arthroplasty. 2014;29(11):2136-2139.
12. Marsh JD, Bryant DM, Macdonald SJ, Naudie DD. Patients respond similarly to paper and electronic versions of the WOMAC and SF-12 following total joint arthroplasty. J Arthroplasty. 2014;29(4):670-673.
13. Olajos-Clow J, Minard J, Szpiro K, et al. Validation of an electronic version of the Mini Asthma Quality of Life Questionnaire. Respir Med. 2010;104(5):658-667.
14. Shervin N, Dorrwachter J, Bragdon CR, Shervin D, Zurakowski D, Malchau H. Comparison of paper and computer-based questionnaire modes for measuring health outcomes in patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2011;93(3):285-293.
15. Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8-11.
16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
17. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
18. Abdel Messih M, Naylor JM, Descallar J, Manickam A, Mittal R, Harris IA. Mail versus telephone administration of the Oxford Knee and Hip Scores. J Arthroplasty. 2014;29(3):491-494.
19. Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of internet versus paper-and-pencil versions. J Med Internet Res. 2007;9(3):e25.
20. Theiler R, Bischoff-Ferrari HA, Good M, Bellamy N. Responsiveness of the electronic touch screen WOMAC 3.1 OA Index in a short term clinical trial with rofecoxib. Osteoarthritis Cartilage. 2004;12(11):912-916.
21. Ryan JM, Corry JR, Attewell R, Smithson MJ. A comparison of an electronic version of the SF-36 General Health Questionnaire to the standard paper version. Qual Life Res. 2002;11(1):19-26.
22. Wilson AS, Kitas GD, Carruthers DM, et al. Computerized information-gathering in specialist rheumatology clinics: an initial evaluation of an electronic version of the Short Form 36. Rheumatology. 2002;41(3):268-273.
23. Angst F, Goldhahn J, Drerup S, Flury M, Schwyzer HK, Simmen BR. How sharp is the short QuickDASH? A refined content and validity analysis of the Short Form of the Disabilities of the Shoulder, Arm and Hand questionnaire in the strata of symptoms and function and specific joint conditions. Qual Life Res. 2009;18(8):1043-1051.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Higginson IJ, Carr AJ. Measuring quality of life: using quality of life measures in the clinical setting. BMJ. 2001;322(7297):1297-1300.
3. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102-109.
4. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622-629.
5. Paudel D, Ahmed M, Pradhan A, Lal Dangol R. Successful use of tablet personal computers and wireless technologies for the 2011 Nepal Demographic and Health Survey. Glob Heal Sci Pract. 2013;1(2):277-284.
6. Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A. The use of a tablet computer to complete the DASH questionnaire. J Hand Surg Am. 2012;37(12):2589-2594.
7. Aktas A, Hullihen B, Shrotriya S, Thomas S, Walsh D, Estfan B. Connected health: cancer symptom and quality-of-life assessment using a tablet computer: a pilot study. Am J Hosp Palliat Care. 2015;32(2):189-197.
8. Basnov M, Kongsved SM, Bech P, Hjollund NH. Reliability of Short Form-36 in an internet- and a pen-and-paper version. Inform Health Soc Care. 2009;34(1):53-58.
9. Bellamy N, Wilson C, Hendrikz J, et al; EDC Study Group. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J Clin Epidemiol. 2011;64(2):182-190.
10. Fanning J, McAuley E. A comparison of tablet computer and paper-based questionnaires in healthy aging research. JMIR Res Protoc. 2014;3(3):e38.
11. Griffiths-Jones W, Norton MR, Fern ED, Williams DH. The equivalence of remote electronic and paper patient reported outcome (PRO) collection. J Arthroplasty. 2014;29(11):2136-2139.
12. Marsh JD, Bryant DM, Macdonald SJ, Naudie DD. Patients respond similarly to paper and electronic versions of the WOMAC and SF-12 following total joint arthroplasty. J Arthroplasty. 2014;29(4):670-673.
13. Olajos-Clow J, Minard J, Szpiro K, et al. Validation of an electronic version of the Mini Asthma Quality of Life Questionnaire. Respir Med. 2010;104(5):658-667.
14. Shervin N, Dorrwachter J, Bragdon CR, Shervin D, Zurakowski D, Malchau H. Comparison of paper and computer-based questionnaire modes for measuring health outcomes in patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2011;93(3):285-293.
15. Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8-11.
16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
17. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
18. Abdel Messih M, Naylor JM, Descallar J, Manickam A, Mittal R, Harris IA. Mail versus telephone administration of the Oxford Knee and Hip Scores. J Arthroplasty. 2014;29(3):491-494.
19. Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of internet versus paper-and-pencil versions. J Med Internet Res. 2007;9(3):e25.
20. Theiler R, Bischoff-Ferrari HA, Good M, Bellamy N. Responsiveness of the electronic touch screen WOMAC 3.1 OA Index in a short term clinical trial with rofecoxib. Osteoarthritis Cartilage. 2004;12(11):912-916.
21. Ryan JM, Corry JR, Attewell R, Smithson MJ. A comparison of an electronic version of the SF-36 General Health Questionnaire to the standard paper version. Qual Life Res. 2002;11(1):19-26.
22. Wilson AS, Kitas GD, Carruthers DM, et al. Computerized information-gathering in specialist rheumatology clinics: an initial evaluation of an electronic version of the Short Form 36. Rheumatology. 2002;41(3):268-273.
23. Angst F, Goldhahn J, Drerup S, Flury M, Schwyzer HK, Simmen BR. How sharp is the short QuickDASH? A refined content and validity analysis of the Short Form of the Disabilities of the Shoulder, Arm and Hand questionnaire in the strata of symptoms and function and specific joint conditions. Qual Life Res. 2009;18(8):1043-1051.
Can a Total Knee Arthroplasty Perioperative Surgical Home Close the Gap Between Primary and Revision TKA Outcomes?
Total knee arthroplasty (TKA) is an efficacious procedure for end-stage knee arthritis. Although TKA is cost-effective and has a high rate of success,1-6 TKAs fail and may require revision surgery. Failure mechanisms include periprosthetic fracture, aseptic loosening, wear, osteolysis, instability, and infection.7-9 In these cases, revision arthroplasty may be needed in order to restore function.
There has been a steady increase in the number of primary and revision TKAs performed in the United States.8,10,11 Revision rates are 4% at 5 years after index TKA and 8.9% at 9 years.12 However, surgical techniques and improved implants have led to improved outcomes after primary TKA, as evidenced by the reduction in revisions performed for polyethylene wear and osteolysis.13 Given the continuing need for revision TKAs (despite technical improvements13), evidence-based standard protocols that improve outcomes after revision TKA are necessary.
The Total Joint Replacement Perioperative Surgical Home (TJR-PSH) implemented and used by surgeons and anesthesiologists at our institution has shown that an evidence-based perioperative protocol can provide consistent and improved outcomes in primary TKA.14-16
Garson and colleagues14 and Chaurasia and colleagues15 found that patients who underwent primary TKA in a TJA-PSH had a predicted short length of stay (LOS): <3 days. About half were discharged to a location other than home, and 1.1% were readmitted within the first 30 days after surgery. There were no major complications and no mortalities. Conversely, as shown in different nationwide database analysis,17,18 mean LOS after primary unilateral TKA was 5.3 days, 8.2% of patients had procedure-related complications, 30-day readmission rate was 4.2%, and the in-hospital mortality rate was 0.3%. As with TJA-PSH, about half the patients were discharged to a place other than home.
We conducted a study to test the effect of the TJA-PSH clinical pathway on revision TKA patients. Early perioperative outcomes, such as LOS, readmission rate, and reoperation rate, are invaluable tools in measuring TKA outcomes and correlate with the dedicated orthopedic complication grading system proposed by the Knee Society.14,15,17,19 We hypothesized that the TJR-PSH clinical pathway would close the perioperative morbidity gap between primary and revision TKAs and yield equivalent perioperative outcomes.
Materials and Methods
In this study, which received Institutional Review Board approval, we performed a prospective cross-sectional analysis comparing the perioperative outcomes of patients who underwent primary TKA with those of patients who underwent revision TKA. Medical records and our institution’s data registry were queried for LOS, discharge disposition, readmission rates, and reoperation rates.
The study included all primary and revision TKAs performed at our institution since the inception of TJA-PSH. Unicompartmental knee arthroplasties and exchanges of a single component (patella, tibia, or femur) were excluded. We identified a total of 285 consecutive primary or revision TKAs, all performed by a single surgeon. Three cases lacked complete data and were excluded, leaving 282 cases: 235 primary and 50 revision TKAs (no simultaneous bilateral TKAs). The demographic data we collected included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, calculated Charlson Comorbidity Index (CCI), LOS, and discharge disposition.
The same established perioperative surgical home clinical pathway was used to care for all patients, whether they underwent primary or revision TKA. The primary outcomes studied were LOS, discharge disposition (subacute nursing facility or home), 30-day orthopedic readmission, and return to operating room. All reoperations on the same knee were analyzed.
Statistical Analysis
Primary and revision TKAs were compared on LOS (with an independent-sample t test) and discharge disposition, 30-day readmissions, and reoperations (χ2 Fisher exact test). Multivariate regression analysis was performed with each primary outcome, using age, sex, BMI, ASA score, and CCI as covariates. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 16.0 (SPSS Inc.) and Microsoft Excel 2011 (Microsoft).
Results
Mean (SD) age was 66 (13.2) years for primary TKA patients and 62 (12.8) years for revision TKA patients. The cohort had more women (62.5%) than men (37.5%). There was no statistical difference in patient demographics with respect to age (P = .169) or BMI (P = .701) between the 2 groups. There was an even age distribution within each group and between the groups (Table).
There was no statistically significant difference in LOS between the groups. Mean (SD) LOS was 2.55 (1.25) days for primary TKA and 2.92 (1.24) days for revision TKA (P = .061; 95% confidence interval [CI], 0.017-0.749). Regression analysis showed a correlation between ASA score and LOS for primary TKAs but not revision TKAs. For every unit increase in ASA score, there was a 0.39-day increase in LOS for primary TKA (P = .46; 95% CI, 0.006-0.781). There was no correlation between ASA score and LOS for revision TKA when controlling for covariates (P = .124). Eighty (34%) of the 235 primary TKA patients and 21 (41%) of the 50 revision TKA patients were discharged to a subacute nursing facility; the difference was not significant (P = .123). No patient was discharged to an acute inpatient rehabilitation unit. In addition, there was no significant difference in 30-day readmission rates between primary and revision TKA (P = .081). One primary TKA patient (0.4%) and 2 revision TKA patients (4%) were readmitted within 30 days after surgery (P = .081). The primary TKA readmission was for severe spasticity and a history of cerebral palsy leading to a quadriceps avulsion fracture from the superior pole of the patella. One revision TKA readmission was for acute periprosthetic joint infection, and the other for periprosthetic fracture around a press-fit distal femoral replacement stem. There was no significant difference in number of 30-day reoperations between the groups (P = .993). None of the primary TKAs and 2 (4%) of the revision TKAs underwent reoperation. Of the revision TKA patients who returned to the operating room within 30 days after surgery, one was treated for an acute periprosthetic joint infection, the other for a femoral periprosthetic fracture.
Discussion
Advances in multidisciplinary co-management of TKA patients and their clinical effects are highlighted in the TJR-PSH.14 TJR-PSH allows the health team and the patient to prepare for surgery with an understanding of probable outcomes and to optimize the patient’s medical and educational standing to better meet expectations and increase satisfaction.
Previous studies have focused on the etiologies of revision TKA7,8 and on understanding the factors that may predict increased risk for a poor outcome after primary TKA and indicate a possible need for revision.8,12 The present study focused on practical clinical processes that could potentially constitute a standardized perioperative protocol for revision TKA. An organized TJR-PSH may allow the health team to educate patients that LOS, rehabilitation and acute recovery, risk of acute (30-day) complications, and risk of readmission and return to the operating room within the first 30 days after surgery are similar for revision and primary TKAs, as long as proper preoperative optimization and education occur within the TJR-PSH.
Studies have found correlations between revision TKA and significantly increased LOS and postoperative complications.20,21 In contrast, we found no significant difference in LOS between our primary and revision TKA groups. LOS was 2.6 days for primary TKA and 2.9 days for revision TKA—a significant improvement in care and cost for revision TKA patients. That the reduced mean LOS for revision TKA is similar to the mean LOS for primary TKA also implies a reduction in the higher cost of care in revision TKA.20 In addition to obtaining similar LOS for primary and revision TKA, TJR-PSH achieved an overall reduction in LOS.17,22Our results also showed no difference in discharge disposition between primary and revision TKA in our protocol. Discharge disposition also did not correlate with age, sex, BMI, ASA score, or CCI. In TJR-PSH, discharge planning starts before admission and is patient-oriented for optimal recovery. About 66% of primary TKA patients and 58% of revision TKA patients in our cohort were discharged home—implying we are able to send a majority of our postoperative patients home after a shorter hospital stay, while obtaining the same good outcomes. Discharging fewer revision TKA patients to extended-care facilities also indicates a possible reduction in the cost of postoperative care, bringing it in line with the cost in primary TKA. Early individualized discharge planning in TJA-PSH accounts for the similar outcomes in primary and revision TKAs.
There was no significant difference in 30-day readmission rates between our primary and revision TKA patients. An important component of the TJR-PSH pathway is the individualized postdischarge recovery plan, which helps with optimal recovery and reduces readmission rates. Our cohort’s 30-day readmission rate was 0.4% for primary TKA and 4% for revision TKA (P = .081). Thirty-day readmission is a good indicator of postoperative complications and recovery from surgery. We have previously reported on primary TKA outcomes.14,15,,18,22,23 In a study using an NSQIP (National Surgical Quality Improvement Program) database, 11,814 primary TKAs had a 30-day readmission rate of 4.2%.18 In an outcomes study of 17,994 patients who underwent primary TKA in a single fiscal year, the 30-day readmission rate was 5.9%.9 In addition, in a single-institution cohort study of 1032 primary TKA patients, Schairer and colleagues23 found a 30-day unplanned readmission rate of 3.4%. Compared with primary TKA, revision TKA traditionally has had a higher postoperative complication rate.20,21 There is also concern that shorter hospital stays may indicate that significant complications of revision TKAs are being missed. In this study, however, we established that the equal outcomes obtained in the perioperative period carry over to the 30-day postoperative period in our revision TKA group. Good postoperative follow-up and planning are important factors in readmission reduction. Readmissions also have significant overall cost implications.24There was no statistical difference in 30-day reoperation rates between our primary and revision TKA patients. The primary TKA patients had no 30-day reoperations. Previous studies have found reoperation rates ranging from 1.8% to 4.7%.25,26 Revision TKA patients are up to 6 times more likely than primary TKA patients to require reoperation.20 Our study found no significant difference in outcomes between primary and revision TKAs.
Comparison of the outcomes of primary TKA and revision TKA in TJR-PSH showed no difference in acute recovery from surgery. LOS and discharge disposition, 30-day readmission rate, and 30-day return to the operating room were the same for primary and revision TKAs. The morbidity gap between primary and revision TKA patients has been closed in our research cohort. This outcome is important, as indications for primary TKA continue to expand and more primary TKAs are performed in younger patients.18,23 The implication is that, in the future, more knees will need to be revised as patients outlive their prostheses.
Our study had some limitations. First, it involved a small sample of patients, operated on by a single surgeon in a well-organized TJR-PSH at a large academic center. This population might not represent the US patient population, but that should not have adversely affected data analysis, because patients were compared with a similar population. Second, the data might be incomplete because some patients with complications might have sought care at other medical facilities, and we might not have been aware of these cases. Third, we focused on objective clinical outcomes in order to measure the success of TKAs. We did not include any subjective, patient-reported data, such as rehabilitation advances and functioning levels. Fourth, multiple parameters can be used to address complication outcomes, but we used LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate because current payers and institutions often consider these variables when assessing quality of care. These parameters can be influenced by factors such as inpatient physical therapy goals, facility discharge practices, individual social support structure, and hospital pay-for-performance model. The implication is that different facilities have different outcomes in terms of LOS, discharge disposition, readmissions, and reoperations. However, we expect proportionate similarities in these parameters as patient perioperative outcomes become more complicated. Nevertheless, a multicenter study would be able to answer questions raised by this limitation. Fifth, our statistical analysis might have been affected by decreased power of some of the outcome variables.
TJR-PSH has succeeded in closing the perioperative morbidity and outcomes gap between primary and revision TKAs. Outcome parameters used to measure the success of TJR-PSH are standard measures of the immediate postoperative recovery and short-term outcomes of TKA patients. These measures are linked to complication rates and overall outcomes in many TKA studies.14,15,17,19 Also important is that hospital costs can be drastically cut by reducing LOS, readmissions, and reoperations. Presence of any complication of primary or revision TKA raises the cost up to 34%. This increase can go as high as 64% in the 90 days after surgery.27
Conclusion
The major challenge of the changing medical landscape is to integrate quality care and a continually improving healthcare system with the goal of cost-effective delivery of healthcare. Surgical care costs can be significantly increased by evitable hospital stays, complications that lead to readmissions, and unplanned returns to the operating room after index surgery. The new perioperative surgical home created for TJA has helped drastically reduce LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate in revision TKA. This study demonstrates similar outcomes in our revision TKA patients relative to their primary TKA counterparts.
Am J Orthop. 2016;45(7):E458-E464. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Berger RA, Rosenberg AG, Barden RM, Sheinkop MB, Jacobs JJ, Galante JO. Long-term followup of the Miller-Galante total knee replacement. Clin Orthop Relat Res. 2001;(388):58-67.
2. Rissanen P, Aro S, Slatis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty. 1995;10(2):169-175.
3. March LM, Cross MJ, Lapsley H, et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients’ quality of life before and after surgery with age-related population norms. Med J Aust. 1999;171(5):235-238.
4. Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576-1584.
5. Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745-1752.
6. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
7. Mulhall KJ, Ghomrawi HM, Scully S, Callaghan JJ, Saleh KJ. Current etiologies and modes of failure in total knee arthroplasty revision. Clin Orthop Relat Res. 2006;(446):45-50.
8. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
9. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
10. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
11 Maloney WJ. National joint replacement registries: has the time come? J Bone Joint Surg Am. 2001;83(10):1582-1585.
12. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
13. Dalury DF, Pomeroy DL, Gorab RS, Adams MJ. Why are total knee arthroplasties being revised? J Arthroplasty. 2013;28(8 suppl):120-121.
14. Garson L, Schwarzkopf R, Vakharia S, et al. Implementation of a total joint replacement-focused perioperative surgical home: a management case report. Anesth Analg. 2014;118(5):1081-1089.
15. Chaurasia A, Garson L, Kain ZL, Schwarzkopf R. Outcomes of a joint replacement surgical home model clinical pathway. Biomed Res Int. 2014;2014:296302.
16. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130.
17. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627.
18. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
19. Harris DY, McAngus JK, Kuo YF, Lindsey RW. Correlations between a dedicated orthopaedic complications grading system and early adverse outcomes in joint arthroplasty. Clin Orthop Relat Res. 2015;473(4):1524-1531.
20. Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin Orthop Relat Res. 2010;468(11):3070-3076.
21. Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87(3):570-576.
22. Singh JA, Kwoh CK, Richardson D, Chen W, Ibrahim SA. Sex and surgical outcomes and mortality after primary total knee arthroplasty: a risk-adjusted analysis. Arthritis Care Res. 2013;65(7):1095-1102.
23. Schairer WW, Vail TP, Bozic KJ. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014;472(1):181-187.
24. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
25. Zmistowski B, Restrepo C, Kahl LK, Parvizi J, Sharkey PF. Incidence and reasons for nonrevision reoperation after total knee arthroplasty. Clin Orthop Relat Res 2011;469(1):138-145.26. Bottle A, Aylin P, Loeffler M. Return to theatre for elective hip and knee replacements: what is the relative importance of patient factors, surgeon and hospital? Bone Joint J Br. 2014;96(12):1663-1668.
27. Maradit Kremers H, Visscher SL, Moriarty JP, et al. Determinants of direct medical costs in primary and revision total knee arthroplasty. Clin Orthop Relat Res. 2013;471(1):206-214.
Total knee arthroplasty (TKA) is an efficacious procedure for end-stage knee arthritis. Although TKA is cost-effective and has a high rate of success,1-6 TKAs fail and may require revision surgery. Failure mechanisms include periprosthetic fracture, aseptic loosening, wear, osteolysis, instability, and infection.7-9 In these cases, revision arthroplasty may be needed in order to restore function.
There has been a steady increase in the number of primary and revision TKAs performed in the United States.8,10,11 Revision rates are 4% at 5 years after index TKA and 8.9% at 9 years.12 However, surgical techniques and improved implants have led to improved outcomes after primary TKA, as evidenced by the reduction in revisions performed for polyethylene wear and osteolysis.13 Given the continuing need for revision TKAs (despite technical improvements13), evidence-based standard protocols that improve outcomes after revision TKA are necessary.
The Total Joint Replacement Perioperative Surgical Home (TJR-PSH) implemented and used by surgeons and anesthesiologists at our institution has shown that an evidence-based perioperative protocol can provide consistent and improved outcomes in primary TKA.14-16
Garson and colleagues14 and Chaurasia and colleagues15 found that patients who underwent primary TKA in a TJA-PSH had a predicted short length of stay (LOS): <3 days. About half were discharged to a location other than home, and 1.1% were readmitted within the first 30 days after surgery. There were no major complications and no mortalities. Conversely, as shown in different nationwide database analysis,17,18 mean LOS after primary unilateral TKA was 5.3 days, 8.2% of patients had procedure-related complications, 30-day readmission rate was 4.2%, and the in-hospital mortality rate was 0.3%. As with TJA-PSH, about half the patients were discharged to a place other than home.
We conducted a study to test the effect of the TJA-PSH clinical pathway on revision TKA patients. Early perioperative outcomes, such as LOS, readmission rate, and reoperation rate, are invaluable tools in measuring TKA outcomes and correlate with the dedicated orthopedic complication grading system proposed by the Knee Society.14,15,17,19 We hypothesized that the TJR-PSH clinical pathway would close the perioperative morbidity gap between primary and revision TKAs and yield equivalent perioperative outcomes.
Materials and Methods
In this study, which received Institutional Review Board approval, we performed a prospective cross-sectional analysis comparing the perioperative outcomes of patients who underwent primary TKA with those of patients who underwent revision TKA. Medical records and our institution’s data registry were queried for LOS, discharge disposition, readmission rates, and reoperation rates.
The study included all primary and revision TKAs performed at our institution since the inception of TJA-PSH. Unicompartmental knee arthroplasties and exchanges of a single component (patella, tibia, or femur) were excluded. We identified a total of 285 consecutive primary or revision TKAs, all performed by a single surgeon. Three cases lacked complete data and were excluded, leaving 282 cases: 235 primary and 50 revision TKAs (no simultaneous bilateral TKAs). The demographic data we collected included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, calculated Charlson Comorbidity Index (CCI), LOS, and discharge disposition.
The same established perioperative surgical home clinical pathway was used to care for all patients, whether they underwent primary or revision TKA. The primary outcomes studied were LOS, discharge disposition (subacute nursing facility or home), 30-day orthopedic readmission, and return to operating room. All reoperations on the same knee were analyzed.
Statistical Analysis
Primary and revision TKAs were compared on LOS (with an independent-sample t test) and discharge disposition, 30-day readmissions, and reoperations (χ2 Fisher exact test). Multivariate regression analysis was performed with each primary outcome, using age, sex, BMI, ASA score, and CCI as covariates. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 16.0 (SPSS Inc.) and Microsoft Excel 2011 (Microsoft).
Results
Mean (SD) age was 66 (13.2) years for primary TKA patients and 62 (12.8) years for revision TKA patients. The cohort had more women (62.5%) than men (37.5%). There was no statistical difference in patient demographics with respect to age (P = .169) or BMI (P = .701) between the 2 groups. There was an even age distribution within each group and between the groups (Table).
There was no statistically significant difference in LOS between the groups. Mean (SD) LOS was 2.55 (1.25) days for primary TKA and 2.92 (1.24) days for revision TKA (P = .061; 95% confidence interval [CI], 0.017-0.749). Regression analysis showed a correlation between ASA score and LOS for primary TKAs but not revision TKAs. For every unit increase in ASA score, there was a 0.39-day increase in LOS for primary TKA (P = .46; 95% CI, 0.006-0.781). There was no correlation between ASA score and LOS for revision TKA when controlling for covariates (P = .124). Eighty (34%) of the 235 primary TKA patients and 21 (41%) of the 50 revision TKA patients were discharged to a subacute nursing facility; the difference was not significant (P = .123). No patient was discharged to an acute inpatient rehabilitation unit. In addition, there was no significant difference in 30-day readmission rates between primary and revision TKA (P = .081). One primary TKA patient (0.4%) and 2 revision TKA patients (4%) were readmitted within 30 days after surgery (P = .081). The primary TKA readmission was for severe spasticity and a history of cerebral palsy leading to a quadriceps avulsion fracture from the superior pole of the patella. One revision TKA readmission was for acute periprosthetic joint infection, and the other for periprosthetic fracture around a press-fit distal femoral replacement stem. There was no significant difference in number of 30-day reoperations between the groups (P = .993). None of the primary TKAs and 2 (4%) of the revision TKAs underwent reoperation. Of the revision TKA patients who returned to the operating room within 30 days after surgery, one was treated for an acute periprosthetic joint infection, the other for a femoral periprosthetic fracture.
Discussion
Advances in multidisciplinary co-management of TKA patients and their clinical effects are highlighted in the TJR-PSH.14 TJR-PSH allows the health team and the patient to prepare for surgery with an understanding of probable outcomes and to optimize the patient’s medical and educational standing to better meet expectations and increase satisfaction.
Previous studies have focused on the etiologies of revision TKA7,8 and on understanding the factors that may predict increased risk for a poor outcome after primary TKA and indicate a possible need for revision.8,12 The present study focused on practical clinical processes that could potentially constitute a standardized perioperative protocol for revision TKA. An organized TJR-PSH may allow the health team to educate patients that LOS, rehabilitation and acute recovery, risk of acute (30-day) complications, and risk of readmission and return to the operating room within the first 30 days after surgery are similar for revision and primary TKAs, as long as proper preoperative optimization and education occur within the TJR-PSH.
Studies have found correlations between revision TKA and significantly increased LOS and postoperative complications.20,21 In contrast, we found no significant difference in LOS between our primary and revision TKA groups. LOS was 2.6 days for primary TKA and 2.9 days for revision TKA—a significant improvement in care and cost for revision TKA patients. That the reduced mean LOS for revision TKA is similar to the mean LOS for primary TKA also implies a reduction in the higher cost of care in revision TKA.20 In addition to obtaining similar LOS for primary and revision TKA, TJR-PSH achieved an overall reduction in LOS.17,22Our results also showed no difference in discharge disposition between primary and revision TKA in our protocol. Discharge disposition also did not correlate with age, sex, BMI, ASA score, or CCI. In TJR-PSH, discharge planning starts before admission and is patient-oriented for optimal recovery. About 66% of primary TKA patients and 58% of revision TKA patients in our cohort were discharged home—implying we are able to send a majority of our postoperative patients home after a shorter hospital stay, while obtaining the same good outcomes. Discharging fewer revision TKA patients to extended-care facilities also indicates a possible reduction in the cost of postoperative care, bringing it in line with the cost in primary TKA. Early individualized discharge planning in TJA-PSH accounts for the similar outcomes in primary and revision TKAs.
There was no significant difference in 30-day readmission rates between our primary and revision TKA patients. An important component of the TJR-PSH pathway is the individualized postdischarge recovery plan, which helps with optimal recovery and reduces readmission rates. Our cohort’s 30-day readmission rate was 0.4% for primary TKA and 4% for revision TKA (P = .081). Thirty-day readmission is a good indicator of postoperative complications and recovery from surgery. We have previously reported on primary TKA outcomes.14,15,,18,22,23 In a study using an NSQIP (National Surgical Quality Improvement Program) database, 11,814 primary TKAs had a 30-day readmission rate of 4.2%.18 In an outcomes study of 17,994 patients who underwent primary TKA in a single fiscal year, the 30-day readmission rate was 5.9%.9 In addition, in a single-institution cohort study of 1032 primary TKA patients, Schairer and colleagues23 found a 30-day unplanned readmission rate of 3.4%. Compared with primary TKA, revision TKA traditionally has had a higher postoperative complication rate.20,21 There is also concern that shorter hospital stays may indicate that significant complications of revision TKAs are being missed. In this study, however, we established that the equal outcomes obtained in the perioperative period carry over to the 30-day postoperative period in our revision TKA group. Good postoperative follow-up and planning are important factors in readmission reduction. Readmissions also have significant overall cost implications.24There was no statistical difference in 30-day reoperation rates between our primary and revision TKA patients. The primary TKA patients had no 30-day reoperations. Previous studies have found reoperation rates ranging from 1.8% to 4.7%.25,26 Revision TKA patients are up to 6 times more likely than primary TKA patients to require reoperation.20 Our study found no significant difference in outcomes between primary and revision TKAs.
Comparison of the outcomes of primary TKA and revision TKA in TJR-PSH showed no difference in acute recovery from surgery. LOS and discharge disposition, 30-day readmission rate, and 30-day return to the operating room were the same for primary and revision TKAs. The morbidity gap between primary and revision TKA patients has been closed in our research cohort. This outcome is important, as indications for primary TKA continue to expand and more primary TKAs are performed in younger patients.18,23 The implication is that, in the future, more knees will need to be revised as patients outlive their prostheses.
Our study had some limitations. First, it involved a small sample of patients, operated on by a single surgeon in a well-organized TJR-PSH at a large academic center. This population might not represent the US patient population, but that should not have adversely affected data analysis, because patients were compared with a similar population. Second, the data might be incomplete because some patients with complications might have sought care at other medical facilities, and we might not have been aware of these cases. Third, we focused on objective clinical outcomes in order to measure the success of TKAs. We did not include any subjective, patient-reported data, such as rehabilitation advances and functioning levels. Fourth, multiple parameters can be used to address complication outcomes, but we used LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate because current payers and institutions often consider these variables when assessing quality of care. These parameters can be influenced by factors such as inpatient physical therapy goals, facility discharge practices, individual social support structure, and hospital pay-for-performance model. The implication is that different facilities have different outcomes in terms of LOS, discharge disposition, readmissions, and reoperations. However, we expect proportionate similarities in these parameters as patient perioperative outcomes become more complicated. Nevertheless, a multicenter study would be able to answer questions raised by this limitation. Fifth, our statistical analysis might have been affected by decreased power of some of the outcome variables.
TJR-PSH has succeeded in closing the perioperative morbidity and outcomes gap between primary and revision TKAs. Outcome parameters used to measure the success of TJR-PSH are standard measures of the immediate postoperative recovery and short-term outcomes of TKA patients. These measures are linked to complication rates and overall outcomes in many TKA studies.14,15,17,19 Also important is that hospital costs can be drastically cut by reducing LOS, readmissions, and reoperations. Presence of any complication of primary or revision TKA raises the cost up to 34%. This increase can go as high as 64% in the 90 days after surgery.27
Conclusion
The major challenge of the changing medical landscape is to integrate quality care and a continually improving healthcare system with the goal of cost-effective delivery of healthcare. Surgical care costs can be significantly increased by evitable hospital stays, complications that lead to readmissions, and unplanned returns to the operating room after index surgery. The new perioperative surgical home created for TJA has helped drastically reduce LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate in revision TKA. This study demonstrates similar outcomes in our revision TKA patients relative to their primary TKA counterparts.
Am J Orthop. 2016;45(7):E458-E464. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total knee arthroplasty (TKA) is an efficacious procedure for end-stage knee arthritis. Although TKA is cost-effective and has a high rate of success,1-6 TKAs fail and may require revision surgery. Failure mechanisms include periprosthetic fracture, aseptic loosening, wear, osteolysis, instability, and infection.7-9 In these cases, revision arthroplasty may be needed in order to restore function.
There has been a steady increase in the number of primary and revision TKAs performed in the United States.8,10,11 Revision rates are 4% at 5 years after index TKA and 8.9% at 9 years.12 However, surgical techniques and improved implants have led to improved outcomes after primary TKA, as evidenced by the reduction in revisions performed for polyethylene wear and osteolysis.13 Given the continuing need for revision TKAs (despite technical improvements13), evidence-based standard protocols that improve outcomes after revision TKA are necessary.
The Total Joint Replacement Perioperative Surgical Home (TJR-PSH) implemented and used by surgeons and anesthesiologists at our institution has shown that an evidence-based perioperative protocol can provide consistent and improved outcomes in primary TKA.14-16
Garson and colleagues14 and Chaurasia and colleagues15 found that patients who underwent primary TKA in a TJA-PSH had a predicted short length of stay (LOS): <3 days. About half were discharged to a location other than home, and 1.1% were readmitted within the first 30 days after surgery. There were no major complications and no mortalities. Conversely, as shown in different nationwide database analysis,17,18 mean LOS after primary unilateral TKA was 5.3 days, 8.2% of patients had procedure-related complications, 30-day readmission rate was 4.2%, and the in-hospital mortality rate was 0.3%. As with TJA-PSH, about half the patients were discharged to a place other than home.
We conducted a study to test the effect of the TJA-PSH clinical pathway on revision TKA patients. Early perioperative outcomes, such as LOS, readmission rate, and reoperation rate, are invaluable tools in measuring TKA outcomes and correlate with the dedicated orthopedic complication grading system proposed by the Knee Society.14,15,17,19 We hypothesized that the TJR-PSH clinical pathway would close the perioperative morbidity gap between primary and revision TKAs and yield equivalent perioperative outcomes.
Materials and Methods
In this study, which received Institutional Review Board approval, we performed a prospective cross-sectional analysis comparing the perioperative outcomes of patients who underwent primary TKA with those of patients who underwent revision TKA. Medical records and our institution’s data registry were queried for LOS, discharge disposition, readmission rates, and reoperation rates.
The study included all primary and revision TKAs performed at our institution since the inception of TJA-PSH. Unicompartmental knee arthroplasties and exchanges of a single component (patella, tibia, or femur) were excluded. We identified a total of 285 consecutive primary or revision TKAs, all performed by a single surgeon. Three cases lacked complete data and were excluded, leaving 282 cases: 235 primary and 50 revision TKAs (no simultaneous bilateral TKAs). The demographic data we collected included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, calculated Charlson Comorbidity Index (CCI), LOS, and discharge disposition.
The same established perioperative surgical home clinical pathway was used to care for all patients, whether they underwent primary or revision TKA. The primary outcomes studied were LOS, discharge disposition (subacute nursing facility or home), 30-day orthopedic readmission, and return to operating room. All reoperations on the same knee were analyzed.
Statistical Analysis
Primary and revision TKAs were compared on LOS (with an independent-sample t test) and discharge disposition, 30-day readmissions, and reoperations (χ2 Fisher exact test). Multivariate regression analysis was performed with each primary outcome, using age, sex, BMI, ASA score, and CCI as covariates. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 16.0 (SPSS Inc.) and Microsoft Excel 2011 (Microsoft).
Results
Mean (SD) age was 66 (13.2) years for primary TKA patients and 62 (12.8) years for revision TKA patients. The cohort had more women (62.5%) than men (37.5%). There was no statistical difference in patient demographics with respect to age (P = .169) or BMI (P = .701) between the 2 groups. There was an even age distribution within each group and between the groups (Table).
There was no statistically significant difference in LOS between the groups. Mean (SD) LOS was 2.55 (1.25) days for primary TKA and 2.92 (1.24) days for revision TKA (P = .061; 95% confidence interval [CI], 0.017-0.749). Regression analysis showed a correlation between ASA score and LOS for primary TKAs but not revision TKAs. For every unit increase in ASA score, there was a 0.39-day increase in LOS for primary TKA (P = .46; 95% CI, 0.006-0.781). There was no correlation between ASA score and LOS for revision TKA when controlling for covariates (P = .124). Eighty (34%) of the 235 primary TKA patients and 21 (41%) of the 50 revision TKA patients were discharged to a subacute nursing facility; the difference was not significant (P = .123). No patient was discharged to an acute inpatient rehabilitation unit. In addition, there was no significant difference in 30-day readmission rates between primary and revision TKA (P = .081). One primary TKA patient (0.4%) and 2 revision TKA patients (4%) were readmitted within 30 days after surgery (P = .081). The primary TKA readmission was for severe spasticity and a history of cerebral palsy leading to a quadriceps avulsion fracture from the superior pole of the patella. One revision TKA readmission was for acute periprosthetic joint infection, and the other for periprosthetic fracture around a press-fit distal femoral replacement stem. There was no significant difference in number of 30-day reoperations between the groups (P = .993). None of the primary TKAs and 2 (4%) of the revision TKAs underwent reoperation. Of the revision TKA patients who returned to the operating room within 30 days after surgery, one was treated for an acute periprosthetic joint infection, the other for a femoral periprosthetic fracture.
Discussion
Advances in multidisciplinary co-management of TKA patients and their clinical effects are highlighted in the TJR-PSH.14 TJR-PSH allows the health team and the patient to prepare for surgery with an understanding of probable outcomes and to optimize the patient’s medical and educational standing to better meet expectations and increase satisfaction.
Previous studies have focused on the etiologies of revision TKA7,8 and on understanding the factors that may predict increased risk for a poor outcome after primary TKA and indicate a possible need for revision.8,12 The present study focused on practical clinical processes that could potentially constitute a standardized perioperative protocol for revision TKA. An organized TJR-PSH may allow the health team to educate patients that LOS, rehabilitation and acute recovery, risk of acute (30-day) complications, and risk of readmission and return to the operating room within the first 30 days after surgery are similar for revision and primary TKAs, as long as proper preoperative optimization and education occur within the TJR-PSH.
Studies have found correlations between revision TKA and significantly increased LOS and postoperative complications.20,21 In contrast, we found no significant difference in LOS between our primary and revision TKA groups. LOS was 2.6 days for primary TKA and 2.9 days for revision TKA—a significant improvement in care and cost for revision TKA patients. That the reduced mean LOS for revision TKA is similar to the mean LOS for primary TKA also implies a reduction in the higher cost of care in revision TKA.20 In addition to obtaining similar LOS for primary and revision TKA, TJR-PSH achieved an overall reduction in LOS.17,22Our results also showed no difference in discharge disposition between primary and revision TKA in our protocol. Discharge disposition also did not correlate with age, sex, BMI, ASA score, or CCI. In TJR-PSH, discharge planning starts before admission and is patient-oriented for optimal recovery. About 66% of primary TKA patients and 58% of revision TKA patients in our cohort were discharged home—implying we are able to send a majority of our postoperative patients home after a shorter hospital stay, while obtaining the same good outcomes. Discharging fewer revision TKA patients to extended-care facilities also indicates a possible reduction in the cost of postoperative care, bringing it in line with the cost in primary TKA. Early individualized discharge planning in TJA-PSH accounts for the similar outcomes in primary and revision TKAs.
There was no significant difference in 30-day readmission rates between our primary and revision TKA patients. An important component of the TJR-PSH pathway is the individualized postdischarge recovery plan, which helps with optimal recovery and reduces readmission rates. Our cohort’s 30-day readmission rate was 0.4% for primary TKA and 4% for revision TKA (P = .081). Thirty-day readmission is a good indicator of postoperative complications and recovery from surgery. We have previously reported on primary TKA outcomes.14,15,,18,22,23 In a study using an NSQIP (National Surgical Quality Improvement Program) database, 11,814 primary TKAs had a 30-day readmission rate of 4.2%.18 In an outcomes study of 17,994 patients who underwent primary TKA in a single fiscal year, the 30-day readmission rate was 5.9%.9 In addition, in a single-institution cohort study of 1032 primary TKA patients, Schairer and colleagues23 found a 30-day unplanned readmission rate of 3.4%. Compared with primary TKA, revision TKA traditionally has had a higher postoperative complication rate.20,21 There is also concern that shorter hospital stays may indicate that significant complications of revision TKAs are being missed. In this study, however, we established that the equal outcomes obtained in the perioperative period carry over to the 30-day postoperative period in our revision TKA group. Good postoperative follow-up and planning are important factors in readmission reduction. Readmissions also have significant overall cost implications.24There was no statistical difference in 30-day reoperation rates between our primary and revision TKA patients. The primary TKA patients had no 30-day reoperations. Previous studies have found reoperation rates ranging from 1.8% to 4.7%.25,26 Revision TKA patients are up to 6 times more likely than primary TKA patients to require reoperation.20 Our study found no significant difference in outcomes between primary and revision TKAs.
Comparison of the outcomes of primary TKA and revision TKA in TJR-PSH showed no difference in acute recovery from surgery. LOS and discharge disposition, 30-day readmission rate, and 30-day return to the operating room were the same for primary and revision TKAs. The morbidity gap between primary and revision TKA patients has been closed in our research cohort. This outcome is important, as indications for primary TKA continue to expand and more primary TKAs are performed in younger patients.18,23 The implication is that, in the future, more knees will need to be revised as patients outlive their prostheses.
Our study had some limitations. First, it involved a small sample of patients, operated on by a single surgeon in a well-organized TJR-PSH at a large academic center. This population might not represent the US patient population, but that should not have adversely affected data analysis, because patients were compared with a similar population. Second, the data might be incomplete because some patients with complications might have sought care at other medical facilities, and we might not have been aware of these cases. Third, we focused on objective clinical outcomes in order to measure the success of TKAs. We did not include any subjective, patient-reported data, such as rehabilitation advances and functioning levels. Fourth, multiple parameters can be used to address complication outcomes, but we used LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate because current payers and institutions often consider these variables when assessing quality of care. These parameters can be influenced by factors such as inpatient physical therapy goals, facility discharge practices, individual social support structure, and hospital pay-for-performance model. The implication is that different facilities have different outcomes in terms of LOS, discharge disposition, readmissions, and reoperations. However, we expect proportionate similarities in these parameters as patient perioperative outcomes become more complicated. Nevertheless, a multicenter study would be able to answer questions raised by this limitation. Fifth, our statistical analysis might have been affected by decreased power of some of the outcome variables.
TJR-PSH has succeeded in closing the perioperative morbidity and outcomes gap between primary and revision TKAs. Outcome parameters used to measure the success of TJR-PSH are standard measures of the immediate postoperative recovery and short-term outcomes of TKA patients. These measures are linked to complication rates and overall outcomes in many TKA studies.14,15,17,19 Also important is that hospital costs can be drastically cut by reducing LOS, readmissions, and reoperations. Presence of any complication of primary or revision TKA raises the cost up to 34%. This increase can go as high as 64% in the 90 days after surgery.27
Conclusion
The major challenge of the changing medical landscape is to integrate quality care and a continually improving healthcare system with the goal of cost-effective delivery of healthcare. Surgical care costs can be significantly increased by evitable hospital stays, complications that lead to readmissions, and unplanned returns to the operating room after index surgery. The new perioperative surgical home created for TJA has helped drastically reduce LOS, discharge disposition, 30-day readmission rate, and 30-day reoperation rate in revision TKA. This study demonstrates similar outcomes in our revision TKA patients relative to their primary TKA counterparts.
Am J Orthop. 2016;45(7):E458-E464. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Berger RA, Rosenberg AG, Barden RM, Sheinkop MB, Jacobs JJ, Galante JO. Long-term followup of the Miller-Galante total knee replacement. Clin Orthop Relat Res. 2001;(388):58-67.
2. Rissanen P, Aro S, Slatis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty. 1995;10(2):169-175.
3. March LM, Cross MJ, Lapsley H, et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients’ quality of life before and after surgery with age-related population norms. Med J Aust. 1999;171(5):235-238.
4. Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576-1584.
5. Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745-1752.
6. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
7. Mulhall KJ, Ghomrawi HM, Scully S, Callaghan JJ, Saleh KJ. Current etiologies and modes of failure in total knee arthroplasty revision. Clin Orthop Relat Res. 2006;(446):45-50.
8. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
9. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
10. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
11 Maloney WJ. National joint replacement registries: has the time come? J Bone Joint Surg Am. 2001;83(10):1582-1585.
12. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
13. Dalury DF, Pomeroy DL, Gorab RS, Adams MJ. Why are total knee arthroplasties being revised? J Arthroplasty. 2013;28(8 suppl):120-121.
14. Garson L, Schwarzkopf R, Vakharia S, et al. Implementation of a total joint replacement-focused perioperative surgical home: a management case report. Anesth Analg. 2014;118(5):1081-1089.
15. Chaurasia A, Garson L, Kain ZL, Schwarzkopf R. Outcomes of a joint replacement surgical home model clinical pathway. Biomed Res Int. 2014;2014:296302.
16. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130.
17. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627.
18. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
19. Harris DY, McAngus JK, Kuo YF, Lindsey RW. Correlations between a dedicated orthopaedic complications grading system and early adverse outcomes in joint arthroplasty. Clin Orthop Relat Res. 2015;473(4):1524-1531.
20. Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin Orthop Relat Res. 2010;468(11):3070-3076.
21. Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87(3):570-576.
22. Singh JA, Kwoh CK, Richardson D, Chen W, Ibrahim SA. Sex and surgical outcomes and mortality after primary total knee arthroplasty: a risk-adjusted analysis. Arthritis Care Res. 2013;65(7):1095-1102.
23. Schairer WW, Vail TP, Bozic KJ. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014;472(1):181-187.
24. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
25. Zmistowski B, Restrepo C, Kahl LK, Parvizi J, Sharkey PF. Incidence and reasons for nonrevision reoperation after total knee arthroplasty. Clin Orthop Relat Res 2011;469(1):138-145.26. Bottle A, Aylin P, Loeffler M. Return to theatre for elective hip and knee replacements: what is the relative importance of patient factors, surgeon and hospital? Bone Joint J Br. 2014;96(12):1663-1668.
27. Maradit Kremers H, Visscher SL, Moriarty JP, et al. Determinants of direct medical costs in primary and revision total knee arthroplasty. Clin Orthop Relat Res. 2013;471(1):206-214.
1. Berger RA, Rosenberg AG, Barden RM, Sheinkop MB, Jacobs JJ, Galante JO. Long-term followup of the Miller-Galante total knee replacement. Clin Orthop Relat Res. 2001;(388):58-67.
2. Rissanen P, Aro S, Slatis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty. 1995;10(2):169-175.
3. March LM, Cross MJ, Lapsley H, et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients’ quality of life before and after surgery with age-related population norms. Med J Aust. 1999;171(5):235-238.
4. Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576-1584.
5. Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745-1752.
6. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
7. Mulhall KJ, Ghomrawi HM, Scully S, Callaghan JJ, Saleh KJ. Current etiologies and modes of failure in total knee arthroplasty revision. Clin Orthop Relat Res. 2006;(446):45-50.
8. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
9. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
10. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
11 Maloney WJ. National joint replacement registries: has the time come? J Bone Joint Surg Am. 2001;83(10):1582-1585.
12. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
13. Dalury DF, Pomeroy DL, Gorab RS, Adams MJ. Why are total knee arthroplasties being revised? J Arthroplasty. 2013;28(8 suppl):120-121.
14. Garson L, Schwarzkopf R, Vakharia S, et al. Implementation of a total joint replacement-focused perioperative surgical home: a management case report. Anesth Analg. 2014;118(5):1081-1089.
15. Chaurasia A, Garson L, Kain ZL, Schwarzkopf R. Outcomes of a joint replacement surgical home model clinical pathway. Biomed Res Int. 2014;2014:296302.
16. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130.
17. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627.
18. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
19. Harris DY, McAngus JK, Kuo YF, Lindsey RW. Correlations between a dedicated orthopaedic complications grading system and early adverse outcomes in joint arthroplasty. Clin Orthop Relat Res. 2015;473(4):1524-1531.
20. Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin Orthop Relat Res. 2010;468(11):3070-3076.
21. Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87(3):570-576.
22. Singh JA, Kwoh CK, Richardson D, Chen W, Ibrahim SA. Sex and surgical outcomes and mortality after primary total knee arthroplasty: a risk-adjusted analysis. Arthritis Care Res. 2013;65(7):1095-1102.
23. Schairer WW, Vail TP, Bozic KJ. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014;472(1):181-187.
24. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
25. Zmistowski B, Restrepo C, Kahl LK, Parvizi J, Sharkey PF. Incidence and reasons for nonrevision reoperation after total knee arthroplasty. Clin Orthop Relat Res 2011;469(1):138-145.26. Bottle A, Aylin P, Loeffler M. Return to theatre for elective hip and knee replacements: what is the relative importance of patient factors, surgeon and hospital? Bone Joint J Br. 2014;96(12):1663-1668.
27. Maradit Kremers H, Visscher SL, Moriarty JP, et al. Determinants of direct medical costs in primary and revision total knee arthroplasty. Clin Orthop Relat Res. 2013;471(1):206-214.
A look at the burden of opioid management in primary care
ABSTRACT
Purpose Pain management with opioids in primary care is challenging. The objective of this study was to identify the number of opioid-related tasks in our clinics and determine whether opioid-related tasks occur more often in a residency setting.
Methods This was a retrospective observational review of an electronic health record (EHR) system to evaluate tasks related to the use of opioids and other controlled substances. Tasks are created in the EHR when patients call the clinic; the task-box system is a means of communication within the EHR. The study setting was 2 university-based family medicine clinics. Clinic 1 has faculty and resident providers in an urban area. Clinic 2 has only faculty providers in a suburban area. We reviewed all tasks recorded in November 2010.
Results A total of 3193 patients were seen at the clinics. In addition, 1028 call-related tasks were created, 220 of which (21.4%) were opioid-related. More than half of the tasks were about chronic (ongoing) patient issues. More than one‑third of the tasks required follow-up phone calls. Multiple logistic regression analysis showed more opioid-related tasks in the residency setting (Clinic 1) compared with the nonresidency setting (Clinic 2), (23.1% vs 16.7%; P<.001). However, multiple logistic regression analysis did not show any correlations between opioid-related tasks and who addressed the tasks or the day tasks were created.
Conclusions Primary care physicians prescribe significant amounts of opioids. Due to the nature of opioid use and abuse, a well-planned protocol customized to the practice or institution is required to streamline this process and decrease the number of unnecessary phone calls and follow-ups.
Pain management with opioids in primary care is challenging,1,2 and many physicians find it unsatisfying and burdensome.3 More than 60 million patient visits for chronic pain occur annually in the United States, consuming large amounts of time and resources.4 Contributing to the challenge is the need to ensure patient safety and satisfaction, as well as staff satisfaction with pain management.5-8 Opioid-related death is a major cause of iatrogenic mortality in the United States:9,10 From 1999 to 2006, fatal opioid-involved intoxications more than tripled from 4000 to 13,800.7
At issue for many providers, as well as patients and staff, is dissatisfaction with current systems in place for managing chronic non-cancer pain with opioids.2,3,8,11 In developing this study, we decided to focus on the systems aspect of care with 2 primary outcome measures in mind. Specifically, we sought to identify the tasks related to managing opioids and other controlled substances in 2 primary care clinics in a university-based family medicine program and to determine what proportion of all routine tasks in these 2 clinics could be attributed to opioid-related issues. With our secondary outcome measures, we sought to compare the number of opioid-related tasks in the residency setting with those in a nonresidency setting, and to identify factors that might be associated with an increase in the number of opioid-related tasks.
METHODS
Setting and design
We conducted a retrospective observational pilot study reviewing our electronic health record (EHR) system (Allscripts TouchWorks) at 2 of our outpatient family medicine clinics at the University of Colorado. When patients call the clinics, or when patient-care-related concerns need to be addressed, an electronic task message is created and sent to the appropriate task box for staff or provider response. The task box system is how staff and providers communicate within the EHR. Each provider has a personal task box, and there are other task boxes in the system (eg, triage, medication refill) for urgent and non-urgent patient care issues.
For example, when a patient calls to request a refill, a medical assistant (MA), care team assistant (CTA), or nurse will create a task for the medication refill box. If the task is urgent, it is marked with a red asterisk and a triage provider will address the task that same day. Non-urgent triage tasks will be addressed by the patient’s primary care provider within 2 to 3 days. Depending on the issue at hand, the task may or may not require phone calls to the patient, pharmacy, or insurance company.
Clinic 1, in urban Denver, has 13 physicians (many of them part-time clinical faculty), one nurse practitioner (NP), one physician assistant (PA), and 18 family medicine residents. Clinic 2, in a suburb of Denver, has 5 physicians (only one is part-time) and one nurse practitioner. Clinic 1 is divided into 3 pods, and each has the same number of attending physicians, residents, and MAs, and either a PA or NP.
We reviewed, one by one, all tasks created from November 1 to 30, 2010. One of the study’s investigators categorized each task according to the following descriptors: who created the task, who addressed the task, what day of the week the task was created, urgency of the task, whether the task required a follow-up phone call, and whether the task was related to opioid/controlled-substance issues. The task was categorized as acute if the issue was related to a condition that had been present for fewer than 3 weeks. Chronic tasks were created for conditions present for ≥3 weeks. At the time the study was completed, our EHR had no portal through which we could communicate with patients.
ANALYSIS
We conducted statistical analyses with the IBM SPSS, version 22.0 (SPSS, Inc, Chicago, Illinois). We used descriptive statistics to examine the frequency and percentage for all variables. We used a chi-squared (χ2) test to assess the differences between the 2 clinics, and used a binary multiple logistic regression model to determine possible factors related to opioid-related tasks. P values <.05 were considered statistically significant. The Colorado Multiple Institutional Review Board approved this study.
RESULTS
Clinics 1 and 2, respectively, saw 2007 and 1186 patients during the study period (TABLE 1). The additional 1028 tasks generated by phone calls were almost equally distributed among the 3 pods of Clinic 1 (290, 202, and 260) and Clinic 2 (276). For data analysis, we compared Clinic 1 with Clinic 2 and also compared the 3 pods of Clinic 1 individually with Clinic 2. Both approaches produced similar results.
Most tasks (54% for Clinic 1 and 99% for Clinic 2) were created by MAs and CTAs. At Clinic 1, tasks were also created by residents (17%), PA/NPs (8%), attending physicians (7%), and others/clinical nurses (14%). Tasks at Clinic 1 were addressed by attending physicians (49%), residents (25%), PA/NPs (25%), and others (1%). At Clinic 2, tasks were addressed by attending physicians (75%) and PA/NPs (25%). Approximately half of the tasks (51%) in both clinics were created during weekdays, compared with the day after weekends/holidays (28%), the day before weekends/holidays (17%), and during weekends/holidays (4%). Chronic patient issues, acute patient issues, and other issues accounted for 54%, 29%, and 17% of tasks, respectively. Follow-up phone calls to patients, pharmacies, or others occurred in 37% of tasks. Two hundred twenty tasks (21%) in the clinics combined were related to opioids and controlled substances.
Multiple logistic regression analysis of data from both clinics (TABLE 2) showed more opioid-related tasks in Clinic 1 compared with Clinic 2 (P<.001), and that these tasks were more often related to chronic issues than to acute issues (P<.001). Tasks created by MAs, CTAs, clinical nurses, and others were more likely to be opioid-related compared with the tasks created by attending physicians, residents, NPs, or a PA (25% vs 15%; P<.05). Compared with non-opioid-related tasks, opioid-related tasks required more follow-up phone calls (P<.001). Follow-up phone calls to pharmacies occurred more often with opioid-related tasks than with non-opioid tasks (11% vs 5%), while follow-up phone calls to patients occurred more often for non-opioid related tasks than opioid-related tasks (28% vs 18%). No correlations with task creation were found for who addressed the opioid-related task or the day the task was created.
DISCUSSION
This study demonstrated that our process of handling patient issues related to opioids accounts for a large proportion of all tasks. Dealing with tasks is time consuming, not only for attending physicians and residents but also for clinic nurses and staff. Almost a quarter of clinic tasks were opioid related. As has been shown in previous studies,5-8 chronic pain management with opioids is an unsatisfying task for staff and care providers at our clinics. We also found that tasks created by non-providers were more likely to be opioid-related than were tasks created by providers. This is most likely due to the fact that non-providers cannot write prescriptions and they have to ask providers for further reviews.
Khalid et al found that, compared with attending physicians, residents had more patients on chronic opioids who displayed concerning behaviors, including early refills and refills from multiple providers.13 The higher number of part-time providers at Clinic 1 in our study may have also caused insufficient continuity of care at that site. Nevertheless, this model of practice is used in many academic primary care institutions.4 Another possible reason for the difference could be a lack of resident training on current guidelines for managing opiates for chronic pain.3,13,14 Again, this was a pilot study and we drew no solid conclusion about the reasons for differences between these 2 clinics.
It is obvious, however, that we spend a significant amount of time and resources dealing with chronic pain management. Our institution created an opioid/controlled-substance patient registry about 3 years ago. The data for 2014 showed that 22.8% and 18% of patients seen at least once at Clinic 1 and Clinic 2, respectively, were prescribed opioids/controlled substances (TABLE 3).
Possible solutions to reduce tasks related to opioid management. For both small and large practices, one way to reduce the number of tasks related to opioid management and, therefore, the time allocated to completing those tasks, would be to have a clear protocol to follow.3,4,8,11,14,15 The protocol may include the creation of an opioid/controlled-substance registry and the development and implementation of clinical decision support programs.
We also recommend the dissemination of tools for clinical management at the point of care. These can include a controlled-substance risk assessment tool for aberrant behaviors, a controlled-substance informed consent form, a functional and quality-of-life assessment, electronic clinical-note templates in the EHR, urine drug screening, and routine use of existing state pharmacy prescription drug monitoring programs. Also essential would be the provision of routine educational programs for clinicians regarding chronic pain management based on existing evidence and guidelines. (See “Opioids for chronic pain: The CDC’s 12 recommendations.”) It has been demonstrated that an EHR opioid dashboard or an EHR-based protocol improved adherence to guidelines for prescribing opiates.16
This study has several limitations. First, this was a small pilot study completed over a short period of time, although we believe the findings are likely representative of the prescribing practices in the 2 clinics we evaluated. Second, it was a retrospective study, which was appropriate for evaluating our questions. Third, we were unable to account for other factors that could potentially confound the results, including, but not limited to, the amount of time allocated to each task, and the total number of patients at each clinic who were on opioids for management of chronic pain during the study period. However, due to our recent addition of an opioid/controlled-substance patient registry, we were able to add information for the year 2014 (TABLE 3). Multi-center large scale studies are required to evaluate this further.
ACKNOWLEDGEMENTS
We thank Dr. Corey Lyon for his editorial assistance.
CORRESPONDENCE
Morteza Khodaee, MD, AFW Family Medicine Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected].
1. Smith BH, Torrance N. Management of chronic pain in primary care. Curr Opin Support Palliat Care. 2011;5:137-142.
2. Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307:1377-1378.
3. Leverence RR, Williams RL, Potter M, et al; PRIME Net Clinicians. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
4. Watkins A, Wasmann S, Dodson L, et al. An evaluation of the care provided to patients prescribed controlled substances for chronic nonmalignant pain at an academic family medicine center. Fam Med. 2004;36:487-489.
5. Brown J, Setnik B, Lee K, et al. Assessment, stratification, and monitoring of the risk for prescription opioid misuse and abuse in the primary care setting. J Opioid Manag. 2011;7:467-483.
6. Duensing L, Eksterowicz N, Macario A, et al. Patient and physician perceptions of treatment of moderate-to-severe chronic pain with oral opioids. Curr Med Res Opin. 2010;26:1579-1585.
7. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12:S26-S35.
8. Wenghofer EF, Wilson L, Kahan M, et al. Survey of Ontario primary care physicians’ experiences with opioid prescribing. Can Fam Physician. 2011;57:324-332.
9. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
10. Hartrick CT, Gatchel RJ, Conroy S. Identification and management of pain medication abuse and misuse: current state and future directions. Expert Rev Neurother. 2012;12:601-610.
11. Wiedemer NL, Harden PS, Arndt IO, et al. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573-584.
12. Colburn JL, Jasinski DR, Rastegar DA. Long-term opioid therapy, aberrant behaviors, and substance misuse: comparison of patients treated by resident and attending physicians in a general medical clinic. J Opioid Manag. 2012;8:153-160.
13. Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16:480-487.
14. Canada RE, DiRocco D, Day S. A better approach to opioid prescribing in primary care. J Fam Pract. 2014;63:E1-E8.
15. Clark LG, Upshur CC. Family medicine physicians’ views of how to improve chronic pain management. J Am Board Fam Med. 2007;20:479-482.
16. Anderson D, Zlateva I, Khatri K, et al. Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015;31:573-579.
ABSTRACT
Purpose Pain management with opioids in primary care is challenging. The objective of this study was to identify the number of opioid-related tasks in our clinics and determine whether opioid-related tasks occur more often in a residency setting.
Methods This was a retrospective observational review of an electronic health record (EHR) system to evaluate tasks related to the use of opioids and other controlled substances. Tasks are created in the EHR when patients call the clinic; the task-box system is a means of communication within the EHR. The study setting was 2 university-based family medicine clinics. Clinic 1 has faculty and resident providers in an urban area. Clinic 2 has only faculty providers in a suburban area. We reviewed all tasks recorded in November 2010.
Results A total of 3193 patients were seen at the clinics. In addition, 1028 call-related tasks were created, 220 of which (21.4%) were opioid-related. More than half of the tasks were about chronic (ongoing) patient issues. More than one‑third of the tasks required follow-up phone calls. Multiple logistic regression analysis showed more opioid-related tasks in the residency setting (Clinic 1) compared with the nonresidency setting (Clinic 2), (23.1% vs 16.7%; P<.001). However, multiple logistic regression analysis did not show any correlations between opioid-related tasks and who addressed the tasks or the day tasks were created.
Conclusions Primary care physicians prescribe significant amounts of opioids. Due to the nature of opioid use and abuse, a well-planned protocol customized to the practice or institution is required to streamline this process and decrease the number of unnecessary phone calls and follow-ups.
Pain management with opioids in primary care is challenging,1,2 and many physicians find it unsatisfying and burdensome.3 More than 60 million patient visits for chronic pain occur annually in the United States, consuming large amounts of time and resources.4 Contributing to the challenge is the need to ensure patient safety and satisfaction, as well as staff satisfaction with pain management.5-8 Opioid-related death is a major cause of iatrogenic mortality in the United States:9,10 From 1999 to 2006, fatal opioid-involved intoxications more than tripled from 4000 to 13,800.7
At issue for many providers, as well as patients and staff, is dissatisfaction with current systems in place for managing chronic non-cancer pain with opioids.2,3,8,11 In developing this study, we decided to focus on the systems aspect of care with 2 primary outcome measures in mind. Specifically, we sought to identify the tasks related to managing opioids and other controlled substances in 2 primary care clinics in a university-based family medicine program and to determine what proportion of all routine tasks in these 2 clinics could be attributed to opioid-related issues. With our secondary outcome measures, we sought to compare the number of opioid-related tasks in the residency setting with those in a nonresidency setting, and to identify factors that might be associated with an increase in the number of opioid-related tasks.
METHODS
Setting and design
We conducted a retrospective observational pilot study reviewing our electronic health record (EHR) system (Allscripts TouchWorks) at 2 of our outpatient family medicine clinics at the University of Colorado. When patients call the clinics, or when patient-care-related concerns need to be addressed, an electronic task message is created and sent to the appropriate task box for staff or provider response. The task box system is how staff and providers communicate within the EHR. Each provider has a personal task box, and there are other task boxes in the system (eg, triage, medication refill) for urgent and non-urgent patient care issues.
For example, when a patient calls to request a refill, a medical assistant (MA), care team assistant (CTA), or nurse will create a task for the medication refill box. If the task is urgent, it is marked with a red asterisk and a triage provider will address the task that same day. Non-urgent triage tasks will be addressed by the patient’s primary care provider within 2 to 3 days. Depending on the issue at hand, the task may or may not require phone calls to the patient, pharmacy, or insurance company.
Clinic 1, in urban Denver, has 13 physicians (many of them part-time clinical faculty), one nurse practitioner (NP), one physician assistant (PA), and 18 family medicine residents. Clinic 2, in a suburb of Denver, has 5 physicians (only one is part-time) and one nurse practitioner. Clinic 1 is divided into 3 pods, and each has the same number of attending physicians, residents, and MAs, and either a PA or NP.
We reviewed, one by one, all tasks created from November 1 to 30, 2010. One of the study’s investigators categorized each task according to the following descriptors: who created the task, who addressed the task, what day of the week the task was created, urgency of the task, whether the task required a follow-up phone call, and whether the task was related to opioid/controlled-substance issues. The task was categorized as acute if the issue was related to a condition that had been present for fewer than 3 weeks. Chronic tasks were created for conditions present for ≥3 weeks. At the time the study was completed, our EHR had no portal through which we could communicate with patients.
ANALYSIS
We conducted statistical analyses with the IBM SPSS, version 22.0 (SPSS, Inc, Chicago, Illinois). We used descriptive statistics to examine the frequency and percentage for all variables. We used a chi-squared (χ2) test to assess the differences between the 2 clinics, and used a binary multiple logistic regression model to determine possible factors related to opioid-related tasks. P values <.05 were considered statistically significant. The Colorado Multiple Institutional Review Board approved this study.
RESULTS
Clinics 1 and 2, respectively, saw 2007 and 1186 patients during the study period (TABLE 1). The additional 1028 tasks generated by phone calls were almost equally distributed among the 3 pods of Clinic 1 (290, 202, and 260) and Clinic 2 (276). For data analysis, we compared Clinic 1 with Clinic 2 and also compared the 3 pods of Clinic 1 individually with Clinic 2. Both approaches produced similar results.
Most tasks (54% for Clinic 1 and 99% for Clinic 2) were created by MAs and CTAs. At Clinic 1, tasks were also created by residents (17%), PA/NPs (8%), attending physicians (7%), and others/clinical nurses (14%). Tasks at Clinic 1 were addressed by attending physicians (49%), residents (25%), PA/NPs (25%), and others (1%). At Clinic 2, tasks were addressed by attending physicians (75%) and PA/NPs (25%). Approximately half of the tasks (51%) in both clinics were created during weekdays, compared with the day after weekends/holidays (28%), the day before weekends/holidays (17%), and during weekends/holidays (4%). Chronic patient issues, acute patient issues, and other issues accounted for 54%, 29%, and 17% of tasks, respectively. Follow-up phone calls to patients, pharmacies, or others occurred in 37% of tasks. Two hundred twenty tasks (21%) in the clinics combined were related to opioids and controlled substances.
Multiple logistic regression analysis of data from both clinics (TABLE 2) showed more opioid-related tasks in Clinic 1 compared with Clinic 2 (P<.001), and that these tasks were more often related to chronic issues than to acute issues (P<.001). Tasks created by MAs, CTAs, clinical nurses, and others were more likely to be opioid-related compared with the tasks created by attending physicians, residents, NPs, or a PA (25% vs 15%; P<.05). Compared with non-opioid-related tasks, opioid-related tasks required more follow-up phone calls (P<.001). Follow-up phone calls to pharmacies occurred more often with opioid-related tasks than with non-opioid tasks (11% vs 5%), while follow-up phone calls to patients occurred more often for non-opioid related tasks than opioid-related tasks (28% vs 18%). No correlations with task creation were found for who addressed the opioid-related task or the day the task was created.
DISCUSSION
This study demonstrated that our process of handling patient issues related to opioids accounts for a large proportion of all tasks. Dealing with tasks is time consuming, not only for attending physicians and residents but also for clinic nurses and staff. Almost a quarter of clinic tasks were opioid related. As has been shown in previous studies,5-8 chronic pain management with opioids is an unsatisfying task for staff and care providers at our clinics. We also found that tasks created by non-providers were more likely to be opioid-related than were tasks created by providers. This is most likely due to the fact that non-providers cannot write prescriptions and they have to ask providers for further reviews.
Khalid et al found that, compared with attending physicians, residents had more patients on chronic opioids who displayed concerning behaviors, including early refills and refills from multiple providers.13 The higher number of part-time providers at Clinic 1 in our study may have also caused insufficient continuity of care at that site. Nevertheless, this model of practice is used in many academic primary care institutions.4 Another possible reason for the difference could be a lack of resident training on current guidelines for managing opiates for chronic pain.3,13,14 Again, this was a pilot study and we drew no solid conclusion about the reasons for differences between these 2 clinics.
It is obvious, however, that we spend a significant amount of time and resources dealing with chronic pain management. Our institution created an opioid/controlled-substance patient registry about 3 years ago. The data for 2014 showed that 22.8% and 18% of patients seen at least once at Clinic 1 and Clinic 2, respectively, were prescribed opioids/controlled substances (TABLE 3).
Possible solutions to reduce tasks related to opioid management. For both small and large practices, one way to reduce the number of tasks related to opioid management and, therefore, the time allocated to completing those tasks, would be to have a clear protocol to follow.3,4,8,11,14,15 The protocol may include the creation of an opioid/controlled-substance registry and the development and implementation of clinical decision support programs.
We also recommend the dissemination of tools for clinical management at the point of care. These can include a controlled-substance risk assessment tool for aberrant behaviors, a controlled-substance informed consent form, a functional and quality-of-life assessment, electronic clinical-note templates in the EHR, urine drug screening, and routine use of existing state pharmacy prescription drug monitoring programs. Also essential would be the provision of routine educational programs for clinicians regarding chronic pain management based on existing evidence and guidelines. (See “Opioids for chronic pain: The CDC’s 12 recommendations.”) It has been demonstrated that an EHR opioid dashboard or an EHR-based protocol improved adherence to guidelines for prescribing opiates.16
This study has several limitations. First, this was a small pilot study completed over a short period of time, although we believe the findings are likely representative of the prescribing practices in the 2 clinics we evaluated. Second, it was a retrospective study, which was appropriate for evaluating our questions. Third, we were unable to account for other factors that could potentially confound the results, including, but not limited to, the amount of time allocated to each task, and the total number of patients at each clinic who were on opioids for management of chronic pain during the study period. However, due to our recent addition of an opioid/controlled-substance patient registry, we were able to add information for the year 2014 (TABLE 3). Multi-center large scale studies are required to evaluate this further.
ACKNOWLEDGEMENTS
We thank Dr. Corey Lyon for his editorial assistance.
CORRESPONDENCE
Morteza Khodaee, MD, AFW Family Medicine Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected].
ABSTRACT
Purpose Pain management with opioids in primary care is challenging. The objective of this study was to identify the number of opioid-related tasks in our clinics and determine whether opioid-related tasks occur more often in a residency setting.
Methods This was a retrospective observational review of an electronic health record (EHR) system to evaluate tasks related to the use of opioids and other controlled substances. Tasks are created in the EHR when patients call the clinic; the task-box system is a means of communication within the EHR. The study setting was 2 university-based family medicine clinics. Clinic 1 has faculty and resident providers in an urban area. Clinic 2 has only faculty providers in a suburban area. We reviewed all tasks recorded in November 2010.
Results A total of 3193 patients were seen at the clinics. In addition, 1028 call-related tasks were created, 220 of which (21.4%) were opioid-related. More than half of the tasks were about chronic (ongoing) patient issues. More than one‑third of the tasks required follow-up phone calls. Multiple logistic regression analysis showed more opioid-related tasks in the residency setting (Clinic 1) compared with the nonresidency setting (Clinic 2), (23.1% vs 16.7%; P<.001). However, multiple logistic regression analysis did not show any correlations between opioid-related tasks and who addressed the tasks or the day tasks were created.
Conclusions Primary care physicians prescribe significant amounts of opioids. Due to the nature of opioid use and abuse, a well-planned protocol customized to the practice or institution is required to streamline this process and decrease the number of unnecessary phone calls and follow-ups.
Pain management with opioids in primary care is challenging,1,2 and many physicians find it unsatisfying and burdensome.3 More than 60 million patient visits for chronic pain occur annually in the United States, consuming large amounts of time and resources.4 Contributing to the challenge is the need to ensure patient safety and satisfaction, as well as staff satisfaction with pain management.5-8 Opioid-related death is a major cause of iatrogenic mortality in the United States:9,10 From 1999 to 2006, fatal opioid-involved intoxications more than tripled from 4000 to 13,800.7
At issue for many providers, as well as patients and staff, is dissatisfaction with current systems in place for managing chronic non-cancer pain with opioids.2,3,8,11 In developing this study, we decided to focus on the systems aspect of care with 2 primary outcome measures in mind. Specifically, we sought to identify the tasks related to managing opioids and other controlled substances in 2 primary care clinics in a university-based family medicine program and to determine what proportion of all routine tasks in these 2 clinics could be attributed to opioid-related issues. With our secondary outcome measures, we sought to compare the number of opioid-related tasks in the residency setting with those in a nonresidency setting, and to identify factors that might be associated with an increase in the number of opioid-related tasks.
METHODS
Setting and design
We conducted a retrospective observational pilot study reviewing our electronic health record (EHR) system (Allscripts TouchWorks) at 2 of our outpatient family medicine clinics at the University of Colorado. When patients call the clinics, or when patient-care-related concerns need to be addressed, an electronic task message is created and sent to the appropriate task box for staff or provider response. The task box system is how staff and providers communicate within the EHR. Each provider has a personal task box, and there are other task boxes in the system (eg, triage, medication refill) for urgent and non-urgent patient care issues.
For example, when a patient calls to request a refill, a medical assistant (MA), care team assistant (CTA), or nurse will create a task for the medication refill box. If the task is urgent, it is marked with a red asterisk and a triage provider will address the task that same day. Non-urgent triage tasks will be addressed by the patient’s primary care provider within 2 to 3 days. Depending on the issue at hand, the task may or may not require phone calls to the patient, pharmacy, or insurance company.
Clinic 1, in urban Denver, has 13 physicians (many of them part-time clinical faculty), one nurse practitioner (NP), one physician assistant (PA), and 18 family medicine residents. Clinic 2, in a suburb of Denver, has 5 physicians (only one is part-time) and one nurse practitioner. Clinic 1 is divided into 3 pods, and each has the same number of attending physicians, residents, and MAs, and either a PA or NP.
We reviewed, one by one, all tasks created from November 1 to 30, 2010. One of the study’s investigators categorized each task according to the following descriptors: who created the task, who addressed the task, what day of the week the task was created, urgency of the task, whether the task required a follow-up phone call, and whether the task was related to opioid/controlled-substance issues. The task was categorized as acute if the issue was related to a condition that had been present for fewer than 3 weeks. Chronic tasks were created for conditions present for ≥3 weeks. At the time the study was completed, our EHR had no portal through which we could communicate with patients.
ANALYSIS
We conducted statistical analyses with the IBM SPSS, version 22.0 (SPSS, Inc, Chicago, Illinois). We used descriptive statistics to examine the frequency and percentage for all variables. We used a chi-squared (χ2) test to assess the differences between the 2 clinics, and used a binary multiple logistic regression model to determine possible factors related to opioid-related tasks. P values <.05 were considered statistically significant. The Colorado Multiple Institutional Review Board approved this study.
RESULTS
Clinics 1 and 2, respectively, saw 2007 and 1186 patients during the study period (TABLE 1). The additional 1028 tasks generated by phone calls were almost equally distributed among the 3 pods of Clinic 1 (290, 202, and 260) and Clinic 2 (276). For data analysis, we compared Clinic 1 with Clinic 2 and also compared the 3 pods of Clinic 1 individually with Clinic 2. Both approaches produced similar results.
Most tasks (54% for Clinic 1 and 99% for Clinic 2) were created by MAs and CTAs. At Clinic 1, tasks were also created by residents (17%), PA/NPs (8%), attending physicians (7%), and others/clinical nurses (14%). Tasks at Clinic 1 were addressed by attending physicians (49%), residents (25%), PA/NPs (25%), and others (1%). At Clinic 2, tasks were addressed by attending physicians (75%) and PA/NPs (25%). Approximately half of the tasks (51%) in both clinics were created during weekdays, compared with the day after weekends/holidays (28%), the day before weekends/holidays (17%), and during weekends/holidays (4%). Chronic patient issues, acute patient issues, and other issues accounted for 54%, 29%, and 17% of tasks, respectively. Follow-up phone calls to patients, pharmacies, or others occurred in 37% of tasks. Two hundred twenty tasks (21%) in the clinics combined were related to opioids and controlled substances.
Multiple logistic regression analysis of data from both clinics (TABLE 2) showed more opioid-related tasks in Clinic 1 compared with Clinic 2 (P<.001), and that these tasks were more often related to chronic issues than to acute issues (P<.001). Tasks created by MAs, CTAs, clinical nurses, and others were more likely to be opioid-related compared with the tasks created by attending physicians, residents, NPs, or a PA (25% vs 15%; P<.05). Compared with non-opioid-related tasks, opioid-related tasks required more follow-up phone calls (P<.001). Follow-up phone calls to pharmacies occurred more often with opioid-related tasks than with non-opioid tasks (11% vs 5%), while follow-up phone calls to patients occurred more often for non-opioid related tasks than opioid-related tasks (28% vs 18%). No correlations with task creation were found for who addressed the opioid-related task or the day the task was created.
DISCUSSION
This study demonstrated that our process of handling patient issues related to opioids accounts for a large proportion of all tasks. Dealing with tasks is time consuming, not only for attending physicians and residents but also for clinic nurses and staff. Almost a quarter of clinic tasks were opioid related. As has been shown in previous studies,5-8 chronic pain management with opioids is an unsatisfying task for staff and care providers at our clinics. We also found that tasks created by non-providers were more likely to be opioid-related than were tasks created by providers. This is most likely due to the fact that non-providers cannot write prescriptions and they have to ask providers for further reviews.
Khalid et al found that, compared with attending physicians, residents had more patients on chronic opioids who displayed concerning behaviors, including early refills and refills from multiple providers.13 The higher number of part-time providers at Clinic 1 in our study may have also caused insufficient continuity of care at that site. Nevertheless, this model of practice is used in many academic primary care institutions.4 Another possible reason for the difference could be a lack of resident training on current guidelines for managing opiates for chronic pain.3,13,14 Again, this was a pilot study and we drew no solid conclusion about the reasons for differences between these 2 clinics.
It is obvious, however, that we spend a significant amount of time and resources dealing with chronic pain management. Our institution created an opioid/controlled-substance patient registry about 3 years ago. The data for 2014 showed that 22.8% and 18% of patients seen at least once at Clinic 1 and Clinic 2, respectively, were prescribed opioids/controlled substances (TABLE 3).
Possible solutions to reduce tasks related to opioid management. For both small and large practices, one way to reduce the number of tasks related to opioid management and, therefore, the time allocated to completing those tasks, would be to have a clear protocol to follow.3,4,8,11,14,15 The protocol may include the creation of an opioid/controlled-substance registry and the development and implementation of clinical decision support programs.
We also recommend the dissemination of tools for clinical management at the point of care. These can include a controlled-substance risk assessment tool for aberrant behaviors, a controlled-substance informed consent form, a functional and quality-of-life assessment, electronic clinical-note templates in the EHR, urine drug screening, and routine use of existing state pharmacy prescription drug monitoring programs. Also essential would be the provision of routine educational programs for clinicians regarding chronic pain management based on existing evidence and guidelines. (See “Opioids for chronic pain: The CDC’s 12 recommendations.”) It has been demonstrated that an EHR opioid dashboard or an EHR-based protocol improved adherence to guidelines for prescribing opiates.16
This study has several limitations. First, this was a small pilot study completed over a short period of time, although we believe the findings are likely representative of the prescribing practices in the 2 clinics we evaluated. Second, it was a retrospective study, which was appropriate for evaluating our questions. Third, we were unable to account for other factors that could potentially confound the results, including, but not limited to, the amount of time allocated to each task, and the total number of patients at each clinic who were on opioids for management of chronic pain during the study period. However, due to our recent addition of an opioid/controlled-substance patient registry, we were able to add information for the year 2014 (TABLE 3). Multi-center large scale studies are required to evaluate this further.
ACKNOWLEDGEMENTS
We thank Dr. Corey Lyon for his editorial assistance.
CORRESPONDENCE
Morteza Khodaee, MD, AFW Family Medicine Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected].
1. Smith BH, Torrance N. Management of chronic pain in primary care. Curr Opin Support Palliat Care. 2011;5:137-142.
2. Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307:1377-1378.
3. Leverence RR, Williams RL, Potter M, et al; PRIME Net Clinicians. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
4. Watkins A, Wasmann S, Dodson L, et al. An evaluation of the care provided to patients prescribed controlled substances for chronic nonmalignant pain at an academic family medicine center. Fam Med. 2004;36:487-489.
5. Brown J, Setnik B, Lee K, et al. Assessment, stratification, and monitoring of the risk for prescription opioid misuse and abuse in the primary care setting. J Opioid Manag. 2011;7:467-483.
6. Duensing L, Eksterowicz N, Macario A, et al. Patient and physician perceptions of treatment of moderate-to-severe chronic pain with oral opioids. Curr Med Res Opin. 2010;26:1579-1585.
7. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12:S26-S35.
8. Wenghofer EF, Wilson L, Kahan M, et al. Survey of Ontario primary care physicians’ experiences with opioid prescribing. Can Fam Physician. 2011;57:324-332.
9. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
10. Hartrick CT, Gatchel RJ, Conroy S. Identification and management of pain medication abuse and misuse: current state and future directions. Expert Rev Neurother. 2012;12:601-610.
11. Wiedemer NL, Harden PS, Arndt IO, et al. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573-584.
12. Colburn JL, Jasinski DR, Rastegar DA. Long-term opioid therapy, aberrant behaviors, and substance misuse: comparison of patients treated by resident and attending physicians in a general medical clinic. J Opioid Manag. 2012;8:153-160.
13. Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16:480-487.
14. Canada RE, DiRocco D, Day S. A better approach to opioid prescribing in primary care. J Fam Pract. 2014;63:E1-E8.
15. Clark LG, Upshur CC. Family medicine physicians’ views of how to improve chronic pain management. J Am Board Fam Med. 2007;20:479-482.
16. Anderson D, Zlateva I, Khatri K, et al. Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015;31:573-579.
1. Smith BH, Torrance N. Management of chronic pain in primary care. Curr Opin Support Palliat Care. 2011;5:137-142.
2. Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307:1377-1378.
3. Leverence RR, Williams RL, Potter M, et al; PRIME Net Clinicians. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
4. Watkins A, Wasmann S, Dodson L, et al. An evaluation of the care provided to patients prescribed controlled substances for chronic nonmalignant pain at an academic family medicine center. Fam Med. 2004;36:487-489.
5. Brown J, Setnik B, Lee K, et al. Assessment, stratification, and monitoring of the risk for prescription opioid misuse and abuse in the primary care setting. J Opioid Manag. 2011;7:467-483.
6. Duensing L, Eksterowicz N, Macario A, et al. Patient and physician perceptions of treatment of moderate-to-severe chronic pain with oral opioids. Curr Med Res Opin. 2010;26:1579-1585.
7. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12:S26-S35.
8. Wenghofer EF, Wilson L, Kahan M, et al. Survey of Ontario primary care physicians’ experiences with opioid prescribing. Can Fam Physician. 2011;57:324-332.
9. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
10. Hartrick CT, Gatchel RJ, Conroy S. Identification and management of pain medication abuse and misuse: current state and future directions. Expert Rev Neurother. 2012;12:601-610.
11. Wiedemer NL, Harden PS, Arndt IO, et al. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573-584.
12. Colburn JL, Jasinski DR, Rastegar DA. Long-term opioid therapy, aberrant behaviors, and substance misuse: comparison of patients treated by resident and attending physicians in a general medical clinic. J Opioid Manag. 2012;8:153-160.
13. Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16:480-487.
14. Canada RE, DiRocco D, Day S. A better approach to opioid prescribing in primary care. J Fam Pract. 2014;63:E1-E8.
15. Clark LG, Upshur CC. Family medicine physicians’ views of how to improve chronic pain management. J Am Board Fam Med. 2007;20:479-482.
16. Anderson D, Zlateva I, Khatri K, et al. Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015;31:573-579.
Vitiligo Patients Experience Barriers in Accessing Care
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
Practice Points
- Patients with vitiligo may experience difficulty receiving the care prescribed to them.
- It is best to identify barriers such as work schedule or distance before recommending a treatment plan.
Proton Pump Inhibitor-Associated Hypomagnesemia: A Retrospective Case-Control Study
In the U.S., proton pump inhibitors (PPIs) are one of the best-selling drug classes—more than $9 billion were spent on PPIs in 2012.1 These medications, available both by prescription and over-the-counter (OTC), are used to treat a variety of gastrointestinal conditions, including heartburn, gastroesophageal reflux disease, and peptic ulcer disease.1
Proton pump inhibitors are generally recognized as safe and effective. In 2011, however, the FDA reviewed Adverse Event Reporting System (AERS) reports, medical literature, and periodic safety updates and issued a safety communication outlining the risk for hypomagnesemia with prolonged PPI use.2 The FDA focused on 53 cases: 30 AERS cases, 15 in the literature, and 8 reported both through AERS and in the literature. The majority involved PPI use that continued for 1 year or longer, but in some cases hypomagnesemia developed after only 3 months. Labeling for prescription PPIs was updated with information about the hypomagnesemia risk, but labeling for the OTC drugs was not affected, as the FDA stated there is little risk with OTC use, and the label already indicated that use should be limited to 14 days at a time and up to 3 courses within 1 year.
Magnesium is an important intracellular cation that plays a role in multiple cellular activities. Low levels of magnesium can lead to a wide variety of adverse events (AEs), including vomiting, diarrhea, cramps, convulsions, bradycardia, and even death.3,4 The mechanism of PPI-associated hypomagnesemia is yet to be established but could be related to, as has been proposed, altered intestinal absorption of magnesium with long-term PPI use.4
Results from investigations of PPI-associated hypomagnesemia have been inconclusive. In a study of PPI-associated AEs reported to the FDA, Luk and colleagues estimated that 1% of patients who experienced an AE reported hypomagnesemia and concluded that all PPIs are associated with hypomagnesemia, but the risk varies. Of the 6 PPIs that have been FDA approved, esomeprazole was associated with the lowest risk, pantoprazole with the most. Results also suggested that the risk was higher for elderly and male patients.
In another study of prior PPI use and its effects on magnesium levels among 11,490 intensive care unit admissions, Danziger and colleagues found that the association of PPI use and hypomagnesemia was limited to patients who concomitantly received a diuretic, and use of a histamine 2 receptor antagonist was not associated with hypomagnesemia.3 A third cross-sectional study of 402 adults with hypomagnesemia on hospital admission found no association between outpatient PPI regimens and hypomagnesemia.5 Other studies designed to investigate PPI-associated hypomagnesemia were limited by short-term PPI use, small samples, concurrent diseases, and confounding variables (eg, history of alcoholism).6,7
Need for Present Study
The evidence needed to establish the incidence of PPI-associated hypomagnesemia is limited. Hypomagnesemia can lead to serious AEs, as just outlined, and is a common indication for hospitalization.8 The hypomagnesemia rate is about 12% in hospitalized patients and sharply higher (60%-65%) in those who are critically ill. Proton pump inhibitor-associated hypomagnesemia is preventable, and monitoring parameters can be recommended to patients undergoing long-term therapy.
Ajumobi and colleagues found that 13,713 (23.4%) of 58,605 patients treated at a VA center over a 12-month period were receiving a PPI.9 Gawron and colleagues found that many veterans had been prescribed a PPI and were receiving high total daily doses for the treatment of gastroesophageal reflux disease.10 The majority of patients received a 90-day or longer supply and showed minimal evidence of step-down therapy or cessation of PPI therapy.
In the present study, the authors investigated the rate of PPI-associated hypomagnesemia in a veteran population at a facility where the majority of PPIs were by prescription, not OTC. The Captain James A. Lovell Federal Health Care Center (FHCC) is a combined DoD and VA facility where veterans and active military members and their dependents receive medical care and prescription drugs.
This study’s primary objective was to determine the rate of PPI-induced hypomagnesemia. The secondary objective was to identify any clinical factors (eg, PPI dose and therapy duration, concomitant use of a diuretic) that might further increase the risk of hypomagnesemia.
Methods
After the study protocol was approved by the Lovell FHCC institutional review board, the authors retrospectively compared patients with a low magnesium level (case group) with patients with a normal magnesium level (control group). In each group, the authors identified patients who underwent PPI therapy and those who did not (Figure).
Study inclusion criteria were low magnesium level (< 1.8 mg/dL) within the past 5 years for veterans in the case group and normal magnesium level (1.8-2.4 mg/dL) within the past 5 years for veterans in the control group. Exclusion criteria were nonveterans and no prior magnesium level for a veteran.
Patients were assigned in a ratio of 1 (case group) to 4 (control group) and were added only after confirmation that multiple magnesium levels had been recorded (January 2008-January 2013).
Patients who met the inclusion criteria were enrolled in the study. Patient’s Computerized Patient Record System charts were reviewed for demographics (sex, age, race); magnesium level; active order for PPI during same period magnesium level was drawn; PPI name, dose, and therapy duration; and concomitant use of a diuretic (yes or no) and, if yes, type of diuretic.
To assess a significance criterion (α) of 0.05 and a power of 80% 1,375 patients in a 1:4 ratio (275 cases, 1,100 controls) were required in order to detect a difference in rates of hypomagnesemia between patients who received a PPI and those who did not. Primary outcome data are reported as percentages and calculated odds ratios (ORs). Significance of ORs was determined with 95% confidence intervals (CIs). Secondary outcomes were PPI dose and therapy duration and concomitant use of a diuretic. Descriptive statistics were used for secondary outcomes.
Results
Five hundred thirty charts (106 cases, 424 controls) were included and reviewed. Table 1 lists the baseline demographics. There were no statistically significant differences in age, sex, or race between the case and control groups. Mean (SD) magnesium level was 1.6 (0.15)
The authors assessed for other clinical factors that might concurrently or
Discussion
One of the most widely prescribed classes of medications, PPIs are often regarded as safe and effective and therefore continued as long-term therapy. Results of this study showed an association of PPI use and hypomagnesemia—thereby adding to the literature. Results for the secondary objective suggest that the association does not necessarily depend on PPI dose, but, given that a statistical analysis of the difference between the case and control groups was not conducted, the statistical significance is unknown.
Although the hypomagnesemia rate remains undetermined, the results of this NNH study suggest a rate higher than previously proposed. Other investigators have estimated the rate of PPI-associated hypomagnesemia at 1%, which does not correlate well with the NNH often calculated in this study. For 2 possible reasons, the poor correlation may be attributable to underreporting of hypomagnesemia: Magnesium levels are not commonly checked with a basic metabolic panel, and many patients who are mildly hypomagnesemic remain asymptomatic.
Future research directions include determining whether the risk for hypomagnesemia is related to patient status (eg, inpatient vs outpatient) and performing statistical analyses on the secondary objective to determine the clinical significance of potential risk factors. Other research directions might involve assessing PPI discontinuation rates in a hypomagnesemic population and assessing outcomes such as hospitalizations and AEs (eg, seizure, tetany, arrhythmia).
Limitations
This study had several limitations. First was the overall design. Study results described only a potential association of PPI use and hypomagnesemia, not definitive cause and effect. Results also depended on an assumed, previously reported rate of PPI-associated hypomagnesemia and a rate of exposure to PPIs, as these data were taken into account in the overall study design. In addition, patient adherence to prescribed therapy and accuracy of medication history were assumed from the medication and dispensing history, as not all medications obtained outside the Lovell FHCC were accurately documented. There also was an external validity limitation in that older men make up the typical FHCC patient population. Last, as inherent to all studies that use objective measures, there was the potential for laboratory magnesium level reporting errors.
Conclusion
The study results identified an association of PPI use and hypomagnesemia in a VA patient population of older men. More studies need to be conducted with non-VA patient populations to further assess the incidence of PPI-associated hypomagnesemia.
1. Consumers Union. Consumer Reports Best Buy Drugs: Using the proton pump inhibitors to treat heartburn and stomach acid reflux, comparing effectiveness, safety, and price. http://www.consumer reports.org/health/resources/pdf/best-buy-drugs/PPIsUpdate-FINAL.pdf. Updated July 2013. Accessed November 4, 2016.
2. U.S. Food and Drug Administration. FDA drug safety communication: low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs). http://www.fda.gov/Drugs /DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed November 4, 2016.
3. Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692-699.
4. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
5. Koulouridis I, Alfayez M, Tighiouart H, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62(4):730-737.
6. Mackay JD, Bladon PT. Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM. 2010;103(6):387-395.
7. Faulhaber GA, Ascoli BA, Lubina A, et al. Serum magnesium and proton-pump inhibitors use: a cross-sectional study. Rev Assoc Med Bras (1992). 2013;59(3):276-279.
8. Yu ASL. Causes of hypomagnesemia. UpToDate. http://www.uptodate.com/contents/causes-of-hypomagnesemia. Updated February 4, 2016. Accessed November 4, 2016.
9. Ajumobi AB, Vuong R, Ahaneku H. Analysis of nonformulary use of PPIs and excess drug cost in a Veterans Affairs population. J Manag Care Pharm. 2012;18(1):63-67.
10. Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28(7):930-937.
In the U.S., proton pump inhibitors (PPIs) are one of the best-selling drug classes—more than $9 billion were spent on PPIs in 2012.1 These medications, available both by prescription and over-the-counter (OTC), are used to treat a variety of gastrointestinal conditions, including heartburn, gastroesophageal reflux disease, and peptic ulcer disease.1
Proton pump inhibitors are generally recognized as safe and effective. In 2011, however, the FDA reviewed Adverse Event Reporting System (AERS) reports, medical literature, and periodic safety updates and issued a safety communication outlining the risk for hypomagnesemia with prolonged PPI use.2 The FDA focused on 53 cases: 30 AERS cases, 15 in the literature, and 8 reported both through AERS and in the literature. The majority involved PPI use that continued for 1 year or longer, but in some cases hypomagnesemia developed after only 3 months. Labeling for prescription PPIs was updated with information about the hypomagnesemia risk, but labeling for the OTC drugs was not affected, as the FDA stated there is little risk with OTC use, and the label already indicated that use should be limited to 14 days at a time and up to 3 courses within 1 year.
Magnesium is an important intracellular cation that plays a role in multiple cellular activities. Low levels of magnesium can lead to a wide variety of adverse events (AEs), including vomiting, diarrhea, cramps, convulsions, bradycardia, and even death.3,4 The mechanism of PPI-associated hypomagnesemia is yet to be established but could be related to, as has been proposed, altered intestinal absorption of magnesium with long-term PPI use.4
Results from investigations of PPI-associated hypomagnesemia have been inconclusive. In a study of PPI-associated AEs reported to the FDA, Luk and colleagues estimated that 1% of patients who experienced an AE reported hypomagnesemia and concluded that all PPIs are associated with hypomagnesemia, but the risk varies. Of the 6 PPIs that have been FDA approved, esomeprazole was associated with the lowest risk, pantoprazole with the most. Results also suggested that the risk was higher for elderly and male patients.
In another study of prior PPI use and its effects on magnesium levels among 11,490 intensive care unit admissions, Danziger and colleagues found that the association of PPI use and hypomagnesemia was limited to patients who concomitantly received a diuretic, and use of a histamine 2 receptor antagonist was not associated with hypomagnesemia.3 A third cross-sectional study of 402 adults with hypomagnesemia on hospital admission found no association between outpatient PPI regimens and hypomagnesemia.5 Other studies designed to investigate PPI-associated hypomagnesemia were limited by short-term PPI use, small samples, concurrent diseases, and confounding variables (eg, history of alcoholism).6,7
Need for Present Study
The evidence needed to establish the incidence of PPI-associated hypomagnesemia is limited. Hypomagnesemia can lead to serious AEs, as just outlined, and is a common indication for hospitalization.8 The hypomagnesemia rate is about 12% in hospitalized patients and sharply higher (60%-65%) in those who are critically ill. Proton pump inhibitor-associated hypomagnesemia is preventable, and monitoring parameters can be recommended to patients undergoing long-term therapy.
Ajumobi and colleagues found that 13,713 (23.4%) of 58,605 patients treated at a VA center over a 12-month period were receiving a PPI.9 Gawron and colleagues found that many veterans had been prescribed a PPI and were receiving high total daily doses for the treatment of gastroesophageal reflux disease.10 The majority of patients received a 90-day or longer supply and showed minimal evidence of step-down therapy or cessation of PPI therapy.
In the present study, the authors investigated the rate of PPI-associated hypomagnesemia in a veteran population at a facility where the majority of PPIs were by prescription, not OTC. The Captain James A. Lovell Federal Health Care Center (FHCC) is a combined DoD and VA facility where veterans and active military members and their dependents receive medical care and prescription drugs.
This study’s primary objective was to determine the rate of PPI-induced hypomagnesemia. The secondary objective was to identify any clinical factors (eg, PPI dose and therapy duration, concomitant use of a diuretic) that might further increase the risk of hypomagnesemia.
Methods
After the study protocol was approved by the Lovell FHCC institutional review board, the authors retrospectively compared patients with a low magnesium level (case group) with patients with a normal magnesium level (control group). In each group, the authors identified patients who underwent PPI therapy and those who did not (Figure).
Study inclusion criteria were low magnesium level (< 1.8 mg/dL) within the past 5 years for veterans in the case group and normal magnesium level (1.8-2.4 mg/dL) within the past 5 years for veterans in the control group. Exclusion criteria were nonveterans and no prior magnesium level for a veteran.
Patients were assigned in a ratio of 1 (case group) to 4 (control group) and were added only after confirmation that multiple magnesium levels had been recorded (January 2008-January 2013).
Patients who met the inclusion criteria were enrolled in the study. Patient’s Computerized Patient Record System charts were reviewed for demographics (sex, age, race); magnesium level; active order for PPI during same period magnesium level was drawn; PPI name, dose, and therapy duration; and concomitant use of a diuretic (yes or no) and, if yes, type of diuretic.
To assess a significance criterion (α) of 0.05 and a power of 80% 1,375 patients in a 1:4 ratio (275 cases, 1,100 controls) were required in order to detect a difference in rates of hypomagnesemia between patients who received a PPI and those who did not. Primary outcome data are reported as percentages and calculated odds ratios (ORs). Significance of ORs was determined with 95% confidence intervals (CIs). Secondary outcomes were PPI dose and therapy duration and concomitant use of a diuretic. Descriptive statistics were used for secondary outcomes.
Results
Five hundred thirty charts (106 cases, 424 controls) were included and reviewed. Table 1 lists the baseline demographics. There were no statistically significant differences in age, sex, or race between the case and control groups. Mean (SD) magnesium level was 1.6 (0.15)
The authors assessed for other clinical factors that might concurrently or
Discussion
One of the most widely prescribed classes of medications, PPIs are often regarded as safe and effective and therefore continued as long-term therapy. Results of this study showed an association of PPI use and hypomagnesemia—thereby adding to the literature. Results for the secondary objective suggest that the association does not necessarily depend on PPI dose, but, given that a statistical analysis of the difference between the case and control groups was not conducted, the statistical significance is unknown.
Although the hypomagnesemia rate remains undetermined, the results of this NNH study suggest a rate higher than previously proposed. Other investigators have estimated the rate of PPI-associated hypomagnesemia at 1%, which does not correlate well with the NNH often calculated in this study. For 2 possible reasons, the poor correlation may be attributable to underreporting of hypomagnesemia: Magnesium levels are not commonly checked with a basic metabolic panel, and many patients who are mildly hypomagnesemic remain asymptomatic.
Future research directions include determining whether the risk for hypomagnesemia is related to patient status (eg, inpatient vs outpatient) and performing statistical analyses on the secondary objective to determine the clinical significance of potential risk factors. Other research directions might involve assessing PPI discontinuation rates in a hypomagnesemic population and assessing outcomes such as hospitalizations and AEs (eg, seizure, tetany, arrhythmia).
Limitations
This study had several limitations. First was the overall design. Study results described only a potential association of PPI use and hypomagnesemia, not definitive cause and effect. Results also depended on an assumed, previously reported rate of PPI-associated hypomagnesemia and a rate of exposure to PPIs, as these data were taken into account in the overall study design. In addition, patient adherence to prescribed therapy and accuracy of medication history were assumed from the medication and dispensing history, as not all medications obtained outside the Lovell FHCC were accurately documented. There also was an external validity limitation in that older men make up the typical FHCC patient population. Last, as inherent to all studies that use objective measures, there was the potential for laboratory magnesium level reporting errors.
Conclusion
The study results identified an association of PPI use and hypomagnesemia in a VA patient population of older men. More studies need to be conducted with non-VA patient populations to further assess the incidence of PPI-associated hypomagnesemia.
In the U.S., proton pump inhibitors (PPIs) are one of the best-selling drug classes—more than $9 billion were spent on PPIs in 2012.1 These medications, available both by prescription and over-the-counter (OTC), are used to treat a variety of gastrointestinal conditions, including heartburn, gastroesophageal reflux disease, and peptic ulcer disease.1
Proton pump inhibitors are generally recognized as safe and effective. In 2011, however, the FDA reviewed Adverse Event Reporting System (AERS) reports, medical literature, and periodic safety updates and issued a safety communication outlining the risk for hypomagnesemia with prolonged PPI use.2 The FDA focused on 53 cases: 30 AERS cases, 15 in the literature, and 8 reported both through AERS and in the literature. The majority involved PPI use that continued for 1 year or longer, but in some cases hypomagnesemia developed after only 3 months. Labeling for prescription PPIs was updated with information about the hypomagnesemia risk, but labeling for the OTC drugs was not affected, as the FDA stated there is little risk with OTC use, and the label already indicated that use should be limited to 14 days at a time and up to 3 courses within 1 year.
Magnesium is an important intracellular cation that plays a role in multiple cellular activities. Low levels of magnesium can lead to a wide variety of adverse events (AEs), including vomiting, diarrhea, cramps, convulsions, bradycardia, and even death.3,4 The mechanism of PPI-associated hypomagnesemia is yet to be established but could be related to, as has been proposed, altered intestinal absorption of magnesium with long-term PPI use.4
Results from investigations of PPI-associated hypomagnesemia have been inconclusive. In a study of PPI-associated AEs reported to the FDA, Luk and colleagues estimated that 1% of patients who experienced an AE reported hypomagnesemia and concluded that all PPIs are associated with hypomagnesemia, but the risk varies. Of the 6 PPIs that have been FDA approved, esomeprazole was associated with the lowest risk, pantoprazole with the most. Results also suggested that the risk was higher for elderly and male patients.
In another study of prior PPI use and its effects on magnesium levels among 11,490 intensive care unit admissions, Danziger and colleagues found that the association of PPI use and hypomagnesemia was limited to patients who concomitantly received a diuretic, and use of a histamine 2 receptor antagonist was not associated with hypomagnesemia.3 A third cross-sectional study of 402 adults with hypomagnesemia on hospital admission found no association between outpatient PPI regimens and hypomagnesemia.5 Other studies designed to investigate PPI-associated hypomagnesemia were limited by short-term PPI use, small samples, concurrent diseases, and confounding variables (eg, history of alcoholism).6,7
Need for Present Study
The evidence needed to establish the incidence of PPI-associated hypomagnesemia is limited. Hypomagnesemia can lead to serious AEs, as just outlined, and is a common indication for hospitalization.8 The hypomagnesemia rate is about 12% in hospitalized patients and sharply higher (60%-65%) in those who are critically ill. Proton pump inhibitor-associated hypomagnesemia is preventable, and monitoring parameters can be recommended to patients undergoing long-term therapy.
Ajumobi and colleagues found that 13,713 (23.4%) of 58,605 patients treated at a VA center over a 12-month period were receiving a PPI.9 Gawron and colleagues found that many veterans had been prescribed a PPI and were receiving high total daily doses for the treatment of gastroesophageal reflux disease.10 The majority of patients received a 90-day or longer supply and showed minimal evidence of step-down therapy or cessation of PPI therapy.
In the present study, the authors investigated the rate of PPI-associated hypomagnesemia in a veteran population at a facility where the majority of PPIs were by prescription, not OTC. The Captain James A. Lovell Federal Health Care Center (FHCC) is a combined DoD and VA facility where veterans and active military members and their dependents receive medical care and prescription drugs.
This study’s primary objective was to determine the rate of PPI-induced hypomagnesemia. The secondary objective was to identify any clinical factors (eg, PPI dose and therapy duration, concomitant use of a diuretic) that might further increase the risk of hypomagnesemia.
Methods
After the study protocol was approved by the Lovell FHCC institutional review board, the authors retrospectively compared patients with a low magnesium level (case group) with patients with a normal magnesium level (control group). In each group, the authors identified patients who underwent PPI therapy and those who did not (Figure).
Study inclusion criteria were low magnesium level (< 1.8 mg/dL) within the past 5 years for veterans in the case group and normal magnesium level (1.8-2.4 mg/dL) within the past 5 years for veterans in the control group. Exclusion criteria were nonveterans and no prior magnesium level for a veteran.
Patients were assigned in a ratio of 1 (case group) to 4 (control group) and were added only after confirmation that multiple magnesium levels had been recorded (January 2008-January 2013).
Patients who met the inclusion criteria were enrolled in the study. Patient’s Computerized Patient Record System charts were reviewed for demographics (sex, age, race); magnesium level; active order for PPI during same period magnesium level was drawn; PPI name, dose, and therapy duration; and concomitant use of a diuretic (yes or no) and, if yes, type of diuretic.
To assess a significance criterion (α) of 0.05 and a power of 80% 1,375 patients in a 1:4 ratio (275 cases, 1,100 controls) were required in order to detect a difference in rates of hypomagnesemia between patients who received a PPI and those who did not. Primary outcome data are reported as percentages and calculated odds ratios (ORs). Significance of ORs was determined with 95% confidence intervals (CIs). Secondary outcomes were PPI dose and therapy duration and concomitant use of a diuretic. Descriptive statistics were used for secondary outcomes.
Results
Five hundred thirty charts (106 cases, 424 controls) were included and reviewed. Table 1 lists the baseline demographics. There were no statistically significant differences in age, sex, or race between the case and control groups. Mean (SD) magnesium level was 1.6 (0.15)
The authors assessed for other clinical factors that might concurrently or
Discussion
One of the most widely prescribed classes of medications, PPIs are often regarded as safe and effective and therefore continued as long-term therapy. Results of this study showed an association of PPI use and hypomagnesemia—thereby adding to the literature. Results for the secondary objective suggest that the association does not necessarily depend on PPI dose, but, given that a statistical analysis of the difference between the case and control groups was not conducted, the statistical significance is unknown.
Although the hypomagnesemia rate remains undetermined, the results of this NNH study suggest a rate higher than previously proposed. Other investigators have estimated the rate of PPI-associated hypomagnesemia at 1%, which does not correlate well with the NNH often calculated in this study. For 2 possible reasons, the poor correlation may be attributable to underreporting of hypomagnesemia: Magnesium levels are not commonly checked with a basic metabolic panel, and many patients who are mildly hypomagnesemic remain asymptomatic.
Future research directions include determining whether the risk for hypomagnesemia is related to patient status (eg, inpatient vs outpatient) and performing statistical analyses on the secondary objective to determine the clinical significance of potential risk factors. Other research directions might involve assessing PPI discontinuation rates in a hypomagnesemic population and assessing outcomes such as hospitalizations and AEs (eg, seizure, tetany, arrhythmia).
Limitations
This study had several limitations. First was the overall design. Study results described only a potential association of PPI use and hypomagnesemia, not definitive cause and effect. Results also depended on an assumed, previously reported rate of PPI-associated hypomagnesemia and a rate of exposure to PPIs, as these data were taken into account in the overall study design. In addition, patient adherence to prescribed therapy and accuracy of medication history were assumed from the medication and dispensing history, as not all medications obtained outside the Lovell FHCC were accurately documented. There also was an external validity limitation in that older men make up the typical FHCC patient population. Last, as inherent to all studies that use objective measures, there was the potential for laboratory magnesium level reporting errors.
Conclusion
The study results identified an association of PPI use and hypomagnesemia in a VA patient population of older men. More studies need to be conducted with non-VA patient populations to further assess the incidence of PPI-associated hypomagnesemia.
1. Consumers Union. Consumer Reports Best Buy Drugs: Using the proton pump inhibitors to treat heartburn and stomach acid reflux, comparing effectiveness, safety, and price. http://www.consumer reports.org/health/resources/pdf/best-buy-drugs/PPIsUpdate-FINAL.pdf. Updated July 2013. Accessed November 4, 2016.
2. U.S. Food and Drug Administration. FDA drug safety communication: low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs). http://www.fda.gov/Drugs /DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed November 4, 2016.
3. Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692-699.
4. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
5. Koulouridis I, Alfayez M, Tighiouart H, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62(4):730-737.
6. Mackay JD, Bladon PT. Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM. 2010;103(6):387-395.
7. Faulhaber GA, Ascoli BA, Lubina A, et al. Serum magnesium and proton-pump inhibitors use: a cross-sectional study. Rev Assoc Med Bras (1992). 2013;59(3):276-279.
8. Yu ASL. Causes of hypomagnesemia. UpToDate. http://www.uptodate.com/contents/causes-of-hypomagnesemia. Updated February 4, 2016. Accessed November 4, 2016.
9. Ajumobi AB, Vuong R, Ahaneku H. Analysis of nonformulary use of PPIs and excess drug cost in a Veterans Affairs population. J Manag Care Pharm. 2012;18(1):63-67.
10. Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28(7):930-937.
1. Consumers Union. Consumer Reports Best Buy Drugs: Using the proton pump inhibitors to treat heartburn and stomach acid reflux, comparing effectiveness, safety, and price. http://www.consumer reports.org/health/resources/pdf/best-buy-drugs/PPIsUpdate-FINAL.pdf. Updated July 2013. Accessed November 4, 2016.
2. U.S. Food and Drug Administration. FDA drug safety communication: low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs). http://www.fda.gov/Drugs /DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed November 4, 2016.
3. Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692-699.
4. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
5. Koulouridis I, Alfayez M, Tighiouart H, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62(4):730-737.
6. Mackay JD, Bladon PT. Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM. 2010;103(6):387-395.
7. Faulhaber GA, Ascoli BA, Lubina A, et al. Serum magnesium and proton-pump inhibitors use: a cross-sectional study. Rev Assoc Med Bras (1992). 2013;59(3):276-279.
8. Yu ASL. Causes of hypomagnesemia. UpToDate. http://www.uptodate.com/contents/causes-of-hypomagnesemia. Updated February 4, 2016. Accessed November 4, 2016.
9. Ajumobi AB, Vuong R, Ahaneku H. Analysis of nonformulary use of PPIs and excess drug cost in a Veterans Affairs population. J Manag Care Pharm. 2012;18(1):63-67.
10. Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28(7):930-937.
A Noninvasive Mechanical Treatment to Reduce the Visible Appearance of Cellulite
Cellulite is a cosmetic problem, not a disease process. It affects 85% to 90% of all women worldwide and was described nearly 100 years ago.1 Causes may be genetic, hormonal, or vascular in nature and may be related to the septa configuration in the subdermal tissue. Fibrosis at the dermal-subcutaneous junction as well as decreased vascular and lymphatic circulation also may be causative factors.
Cellulite has a multifactorial etiology. Khan et al2 noted that there are specific classic patterns of cellulite that affect women exclusively. White women tend to have somewhat higher rates of cellulite than Asian women. The authors also stated that lifestyle factors such as high carbohydrate diets may lead to an increase in total body fat content, which enhances the appearance of cellulite.2
The subdermal anatomy affects the appearance of cellulite. Utilizing in vivo magnetic resonance imaging, Querleux et al3 showed that women with visible cellulite have dermal septa that are thinner and generally more perpendicular to the skin’s surface than women without cellulite. In women without cellulite, the orientation of the septa is more angled into a crisscross pattern. In women with a high percentage of perpendicular septa, the perpendicular septa allow for fat herniation with dimpling of the skin compared to the crisscross septa pattern.2 Other investigators have discussed the reduction of blood flow in specific areas of the body in women, particularly in cellulite-prone areas such as the buttocks and thighs, as another causative factor.2,4,5 Rossi and Vergnanini6 showed that the blood flow was 35% lower in affected cellulite regions than in nonaffected regions without cellulite, which can cause congestion of blood and lymphatic flow and increased subdermal pressure, thus increasing the appearance of cellulite.
Although there is some controversy regarding the effects of weight loss on the appearance of cellulite,2,7 it appears that the subdermal septa and morphology have more of an effect on the appearance of cellulite.2,3,8
Rossi and Vergnanini6 proposed a 4-grade system for evaluating the appearance of cellulite (grade I, no cellulite; grade II, skin that is smooth and without any pronounced dimpling upon standing or lying down but may show some dimpling upon pinching and strong muscle contraction; grade III, cellulite is present in upright positions but not when the patient is in a supine position; grade IV, cellulite can be seen when the patient is standing and in a supine position). Both grades III and IV can be exacerbated by maximal voluntary contraction and strong pinching of the skin because these actions cause the subcutaneous fat to move toward the surface of the skin between the septa. This grading system aligns with categories I through III described by Mirrashed et al.9
There are many cellulite treatments available but few actually create a reduction in the visible appearance of cellulite. A number of these treatments were reviewed by Khan et al,10 including massage; a noninvasive suction-assisted massage technique; and topical agents such as xanthine, retinols, and other botanicals.4,11-14 Liposuction has not been shown to be effective in the treatment of cellulite and in fact may increase the appearance of cellulite.9,15 Mesotherapy, a modality that entails injecting substances into the subcutaneous fat layer, is another treatment of cellulite. Two of the most common agents purported to dissolve fat include phosphatidylcholine and sodium deoxycholate. The efficacy and safety of mesotherapy remains controversial and unproven. A July 2008 position statement from the American Society of Plastic Surgeons stated that “low levels of validity and quality of the literature does not allow [American Society of Plastic Surgeons] to support a recommendation for the use of mesotherapy/injection lipolysis for fat reduction.”16 Other modalities such as noninvasive dual-wavelength laser/suction devices; low-energy diode laser, contact cooling, suction, and massage devices; and infrared, bipolar radiofrequency, and suction with mechanical massage devices are available and show some small improvements in the visible appearance of cellulite, but no rating scales were used in any of these studies.17,18 DiBernardo19 utilized a 1440-nm pulsed laser to treat cellulite. It is an invasive treatment that works by breaking down some of the connective tissue septa responsible for the majority and greater severity of the dermal dimpling seen in cellulite, increasing the thickness of the dermis as well as its elasticity, reducing subcutaneous fat, and improving circulation and reducing general lymphatic congestion.19 The system showed promise but was an invasive treatment, and one session could cost $5000 to $7000 for bilateral areas and another $2500 for each additional area.20 Burns21 expressed that the short-term results showed promise in reducing the appearance of cellulite. Noninvasive ultrasound22,23 as well as extracorporeal shock wave therapy24,25 also has shown some improvement in the firmness of collagen but generally not in the appearance of cellulite.
We sought to evaluate the efficacy and safety of a noninvasive mechanical treatment of cellulite.
Methods
This study was conducted in accordance with the guidelines set forth by the US Department of Health and Human Services’ Policy for Protection of Human Research Subjects and the World Medical Association’s Declaration of Helsinki. Participants were recruited through local area medical facilities in southeastern Michigan. Written informed consent was obtained from all participants prior to beginning the study.
Patients with grades II to IV cellulite, according to the Rossi and Vergnanini6 grading system, were allowed to participate. All participants in the study were asked not to make lifestyle changes (eg, exercise habits, diet) or use any other treatments for cellulite that might be available to them during the study period. Exclusion criteria included history of deep vein thrombosis, cancer diagnosed within the last year, pregnancy, hemophilia, severe lymphedema, presence of a pacemaker, epilepsy, seizure disorder, or current use of anticoagulants. History of partial or total joint replacements, acute hernia, nonunited fractures, advanced arthritis, or detached retina also excluded participation in the study.
Participants completed an 8-week, twice-weekly treatment protocol with a noninvasive mechanical device performed in clinic. The device consisted of a 10.16-cm belt with a layer of nonslip material wrapped around the belt. The belt was attached to a mechanical oscillator. We adjusted the stroke length to approximately 2 cm and moved the dermis at that length at approximately 1000 strokes per minute.
Each participant was treated for a total treatment time of 18 to 24 minutes. The total treatment area included the top of the iliac crest to just above the top of the popliteal space. The width of the belt (10.16 cm) was equal to 1 individual treatment area. Each individual treatment area was treated for 2 minutes. First the buttocks and bilateral thighs were treated, followed by the right lateral thigh and the left lateral thigh. The belt was moved progressively down the total treatment area until all individual treatment areas were addressed. The average participant had 3 to 4 bilateral thigh and buttocks treatment areas and 3 to 4 lateral treatment areas on both the left and right sides of the body.
Digital photographs were taken with standardized lighting for all participants. Photographs were taken before the first treatment on the lateral and posterior aspects of the participant and were taken again at the end of the treatment program immediately before the last treatment. Participants were asked to contract the gluteal musculature for all photographs.
Two board-certified plastic surgeons were asked to rate the before/after photographs in a blinded manner. They graded each photograph on a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite). These data were analyzed using a Wilcoxon signed rank test. These data were compared to the participants self-evaluation of the appearance of cellulite in the photographs from the initial and final treatments using a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite).
The circumference of the widest part of the gluteal area was measured before and after treatment (+/–0.5 cm). The data were analyzed using a paired t test.
Results
The study included 43 participants (age range, 21–67 years; mean age, 37.6 years; weight range, 51–97 kg; mean weight, 64.95 kg) who resided in the Midwestern United States, were interested in reducing their cellulite, and were willing to commit to treatment 2 times weekly for the duration of the 8-week study. Fourteen percent (6/43) of participants were smokers. Participant self-assessments were divided into 3 categories based on the Rossi and Vergnanini6 grading system: category II, n=7; category III, n=12; and category IV, n=24. Although all the categories in our analysis showed statistically significant improvements, we found that there was more improvement in category II participants versus category III, and then again more improvement in category III versus category IV. The data for each treatment were analyzed separately using a paired t test, as we were not interested in comparing categories, only the effect of the treatment. We were testing to see if the difference was greater than 0, and the paired t values were statistically significant in all cases (category II, P=.003; category III, P=.001; category IV, P=.002)(Figure 1).
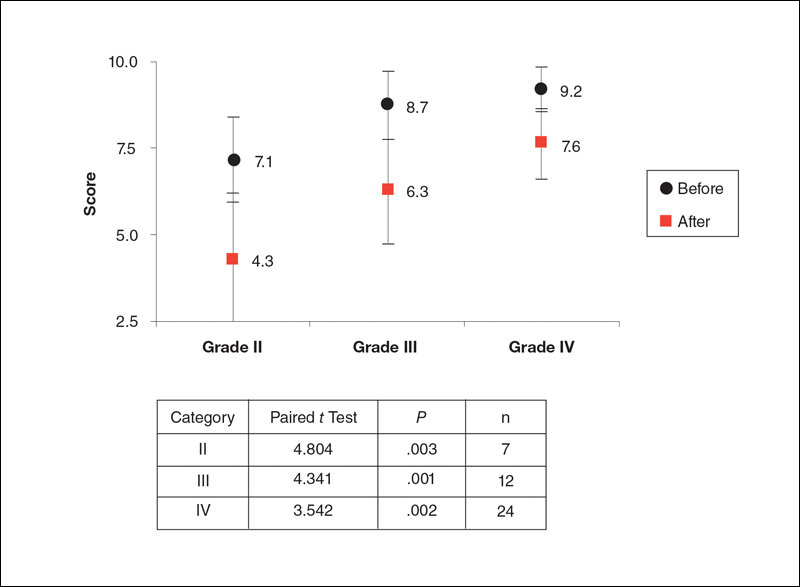
Using a correlation analysis, we found that age, body weight, or body mass index were not significantly correlated with the difference between the before and after physician rating. The difference between before and after treatment also was independent of whether or not the participant exercised or had an adverse reaction to the belt. Adverse reactions to the belt were characterized by redness and/or minor raising of the skin immediately following the treatment. These reactions all dissipated within 12 hours. It also appeared that the rating scales correlated well with the participants self-perception of their cellulite and the improvements seen in the photographs (Figures 2 and 3).
The mean circumference of the widest part of the gluteal area before treatment was 100.2 cm and the standard deviation was 8.14 cm. The mean circumference after treatment was 98.3 cm and the standard deviation was 8.02 (t=–2.81; P<.05). Many of the women commented that they felt more “toned,” which probably accounted for the slight difference in circumference rather than weight loss.
Of the 2 blinded board-certified plastic surgeons, one physician rated all participants in category III as significantly improved (P<.05) and rated the other categories as marginally insignificantly improved; the second physician rated all categories as marginally insignificantly improved.
Comment
Although there are a large number of treatment protocols that have been introduced and studied for the reduction of the appearance of cellulite,4,9,11-18 many have not shown promising long-term results. Some treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.22-25 One of the only treatments that has shown some promise is an expensive invasive treatment.20
The system used in this study was shown to be safe in all study participants. No significant adverse reactions were noted, and each participant successfully completed the protocol. Figures 2 and 3 show the strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population, which was supported by statistical analysis, particularly the participant self-reported ratings. The participants and the blinded physicians were not in agreement on the improvement of cellulite. Although the participants knew the changes that occurred to their bodies, the physicians only had photographs from which to make their decisions. The participants clearly observed noticeable differences to their bodies, while the physicians either saw no change or some improvement.
The physicians were asked to evaluate only the cellulite, but the process we employed changed more than the cellulite. The first step in the process was a toning of the legs and buttocks, which was readily observable by the patients but was outside the scope of the physicians’ assessment. After the body toning, the cellulite began to improve. It is possible that the participants were responding to the entire process, which clearly was positive, while the physicians were responding only to the cellulite end point.
Our treatment regimen accomplished reduction of the visible appearance of cellulite by breaking down connective tissue septa as well as increasing the thickness of the dermis and its elasticity. It also helped reduce subcutaneous fat, improve circulation, and reduce general lymphatic congestion. The parallel motions of the unit could be adjusted, but we kept them at a mid-level range of motion. The motion at this frequency would have a tendency to not only heat the epidermis and dermal layer that we were attempting to affect but would also help accomplish breaking down the septa and improving the elasticity of the dermis. Also, the rapid motion over a period of time of pulling the dermis parallel to the subdermal tissue and fascia most likely helped improve the circulation and lymphatic flow in treated areas as well as possibly broke down the subcutaneous fat. All of these factors appear to have led to an improvement in the appearance of cellulite in our study participants.
A maintenance-type program, if continued, would likely demonstrate improved results by further breaking down the septa and improving the other factors that reduce the appearance of cellulite. We believe that the participants would eventually be able to discontinue the use of the unit or reduce its use substantially once the desired results were obtained.
When utilizing the device, the participants were in a standing posture and leaning into the belt with a moderate force, which seemed to secondarily improve the tone of the gluteal and thigh musculature that was being treated. It may be that the oscillatory motion and the standing posture caused the muscles to isometrically co-contract, adding a secondary exerciselike effect.26-29
Proving our suggested mechanisms of action would require tissue biopsies and/or magnetic resonance imaging studies that were beyond the scope of this study. However, regardless of the mechanism of action, we do believe that this treatment has been shown to be effective, convenient, and most importantly safe.
Conclusion
The unique device that was utilized in our study is a safe and cost-effective method of reducing the appearance of cellulite for home use and would allow for a noninvasive, low-risk procedure.
- Scherwitz C, Braun-Falco O. So-called cellulite. J Dermatol Surg Oncol. 1978;4:230-234.
- Khan MH, Victor F, Rao B, et al. Treatment of cellulite: part I. pathophysiology. J Am Acad Dermatol. 2010;62:361-370, quiz 371-372.
- Querleux B, Cornillon C, Jolivet O, et al. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118-124.
- Rawlings A. Cellulite and its treatment. Int J Cos Sci. 2006;28:175-190.
- Rosenbaum M, Prieto V, Hellmer J, et al. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg. 1998;101:1934-1939.
- Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14:251-262.
- Smalls LK, Hicks M, Passeretti D, et al. Effect of weight loss on cellulite: gynoid lypodystrophy. Plast Reconstr Surg. 2006;118:510-516.
- Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221-229.
- Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161-168.
- Khan M, Victor F, Rao B, et al. Treatment of cellulite, part II. advances and controversies. J Am Acad Dermatol. 2010;62:373-384.
- Collis N, Elliot L, Sharp C, et al. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg. 1999;104:1110-1114.
- Adcock D, Paulsen S, Jabour K, et al. Analysis of the effects of deep mechanical massage in the porcine model. Plast Reconstr Surg. 2000;108:233-240.
- Güleç AT. Treatment of cellulite with LPG endermologie. Int J Dermatol. 2009;48:265-270.
- Piérard-Franchimont C, Piérard GE, Henry F, et al. A randomized, placebo-controlled trial of tropical retinol in the treatment of cellulite. Am J Clin Dermatol. 2000;1:369-374.
- Coleman WP. Liposuction. In: Coleman WP, Hanke CW, Alt TH, eds. Cosmetic Surgery of the Skin: Principles and Practice. Philadelphia, PA: BC Decker; 1991:213-238.
- ASPS guiding principles for mesotherapy/injection lipolysis. American Society of Plastic Surgeons website. http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/guiding-principles/ASPS-Guiding-Principles-for-Mesotherapy-Injection-Lipolysis-7-08.pdf. Published July 2008. Accessed February 17, 2016.
- Kulick MI. Evaluation of a noninvasive, dual-wavelength laser-suction and massage device for the regional treatment of cellulite. Plast Reconstr Surg. 2010;125:1788-1796.
- Nootheti PK, Magpantay A, Yosowitz G, et al. A single center, randomized, comparative, prospective clinical study to determine the efficacy of the VelaSmooth system versus the TriActive system for the treatment of cellulite. Lasers Surg Med. 2006;38:908-912.
- DiBernardo BE. Treatment of cellulite using a 1440-nm pulsed laser with one-year follow up. Aesthet Surg J. 2011;31:328-341.
- Johannes L. New laser aims to zap cellulite at the source. Wall Street Journal. July 3, 2012. http://www.wsj.com/articles/SB10001424052702303649504577496981754619546. Accessed November 21, 2016.
- Burns AJ. Commentary on: treatment of cellulite using a 1440-nm pulsed laser with one-year follow up: preliminary report. Aesthet Surg J. 2011;31:342-343.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety efficacy of the contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Brown SA, Greenbaum L, Shtukmaster S, et al. Characterization of nonthermal focused ultrasound for noninvasive selective fat cell disruption (lysis): technical and preclinical assessment. Plast Reconstr Surg. 2009;124:92-101.
- Angehrn F, Kuhn C, Voss A. Can cellulite be treated with low energy extracorporeal shock wave therapy? Clin Interv Aging. 2007;2:623-630.
- Christ C, Brenke R, Sattler G, et al. Improvement in skin elasticity in the treatment of cellulite and connective tissue weakness by means of extracorporeal pulse activation therapy. Aesthet Surg J. 2008;28:538-544.
- Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183-187.
- Luo J, McNamara B, Moran K. The use of vibration training to enhance muscle strength and power. Sports Med. 2005;35:23-41.
- Annino G, Padua E, Castagna C, et al. Effect of whole body vibration training on lower limb performance in selected high-level ballet students. J Strength Cond Res. 2007;21:1072-1076.
- Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study [published online December 22, 2003]. J Bone Miner Res. 2004;19:352-359.
Cellulite is a cosmetic problem, not a disease process. It affects 85% to 90% of all women worldwide and was described nearly 100 years ago.1 Causes may be genetic, hormonal, or vascular in nature and may be related to the septa configuration in the subdermal tissue. Fibrosis at the dermal-subcutaneous junction as well as decreased vascular and lymphatic circulation also may be causative factors.
Cellulite has a multifactorial etiology. Khan et al2 noted that there are specific classic patterns of cellulite that affect women exclusively. White women tend to have somewhat higher rates of cellulite than Asian women. The authors also stated that lifestyle factors such as high carbohydrate diets may lead to an increase in total body fat content, which enhances the appearance of cellulite.2
The subdermal anatomy affects the appearance of cellulite. Utilizing in vivo magnetic resonance imaging, Querleux et al3 showed that women with visible cellulite have dermal septa that are thinner and generally more perpendicular to the skin’s surface than women without cellulite. In women without cellulite, the orientation of the septa is more angled into a crisscross pattern. In women with a high percentage of perpendicular septa, the perpendicular septa allow for fat herniation with dimpling of the skin compared to the crisscross septa pattern.2 Other investigators have discussed the reduction of blood flow in specific areas of the body in women, particularly in cellulite-prone areas such as the buttocks and thighs, as another causative factor.2,4,5 Rossi and Vergnanini6 showed that the blood flow was 35% lower in affected cellulite regions than in nonaffected regions without cellulite, which can cause congestion of blood and lymphatic flow and increased subdermal pressure, thus increasing the appearance of cellulite.
Although there is some controversy regarding the effects of weight loss on the appearance of cellulite,2,7 it appears that the subdermal septa and morphology have more of an effect on the appearance of cellulite.2,3,8
Rossi and Vergnanini6 proposed a 4-grade system for evaluating the appearance of cellulite (grade I, no cellulite; grade II, skin that is smooth and without any pronounced dimpling upon standing or lying down but may show some dimpling upon pinching and strong muscle contraction; grade III, cellulite is present in upright positions but not when the patient is in a supine position; grade IV, cellulite can be seen when the patient is standing and in a supine position). Both grades III and IV can be exacerbated by maximal voluntary contraction and strong pinching of the skin because these actions cause the subcutaneous fat to move toward the surface of the skin between the septa. This grading system aligns with categories I through III described by Mirrashed et al.9
There are many cellulite treatments available but few actually create a reduction in the visible appearance of cellulite. A number of these treatments were reviewed by Khan et al,10 including massage; a noninvasive suction-assisted massage technique; and topical agents such as xanthine, retinols, and other botanicals.4,11-14 Liposuction has not been shown to be effective in the treatment of cellulite and in fact may increase the appearance of cellulite.9,15 Mesotherapy, a modality that entails injecting substances into the subcutaneous fat layer, is another treatment of cellulite. Two of the most common agents purported to dissolve fat include phosphatidylcholine and sodium deoxycholate. The efficacy and safety of mesotherapy remains controversial and unproven. A July 2008 position statement from the American Society of Plastic Surgeons stated that “low levels of validity and quality of the literature does not allow [American Society of Plastic Surgeons] to support a recommendation for the use of mesotherapy/injection lipolysis for fat reduction.”16 Other modalities such as noninvasive dual-wavelength laser/suction devices; low-energy diode laser, contact cooling, suction, and massage devices; and infrared, bipolar radiofrequency, and suction with mechanical massage devices are available and show some small improvements in the visible appearance of cellulite, but no rating scales were used in any of these studies.17,18 DiBernardo19 utilized a 1440-nm pulsed laser to treat cellulite. It is an invasive treatment that works by breaking down some of the connective tissue septa responsible for the majority and greater severity of the dermal dimpling seen in cellulite, increasing the thickness of the dermis as well as its elasticity, reducing subcutaneous fat, and improving circulation and reducing general lymphatic congestion.19 The system showed promise but was an invasive treatment, and one session could cost $5000 to $7000 for bilateral areas and another $2500 for each additional area.20 Burns21 expressed that the short-term results showed promise in reducing the appearance of cellulite. Noninvasive ultrasound22,23 as well as extracorporeal shock wave therapy24,25 also has shown some improvement in the firmness of collagen but generally not in the appearance of cellulite.
We sought to evaluate the efficacy and safety of a noninvasive mechanical treatment of cellulite.
Methods
This study was conducted in accordance with the guidelines set forth by the US Department of Health and Human Services’ Policy for Protection of Human Research Subjects and the World Medical Association’s Declaration of Helsinki. Participants were recruited through local area medical facilities in southeastern Michigan. Written informed consent was obtained from all participants prior to beginning the study.
Patients with grades II to IV cellulite, according to the Rossi and Vergnanini6 grading system, were allowed to participate. All participants in the study were asked not to make lifestyle changes (eg, exercise habits, diet) or use any other treatments for cellulite that might be available to them during the study period. Exclusion criteria included history of deep vein thrombosis, cancer diagnosed within the last year, pregnancy, hemophilia, severe lymphedema, presence of a pacemaker, epilepsy, seizure disorder, or current use of anticoagulants. History of partial or total joint replacements, acute hernia, nonunited fractures, advanced arthritis, or detached retina also excluded participation in the study.
Participants completed an 8-week, twice-weekly treatment protocol with a noninvasive mechanical device performed in clinic. The device consisted of a 10.16-cm belt with a layer of nonslip material wrapped around the belt. The belt was attached to a mechanical oscillator. We adjusted the stroke length to approximately 2 cm and moved the dermis at that length at approximately 1000 strokes per minute.
Each participant was treated for a total treatment time of 18 to 24 minutes. The total treatment area included the top of the iliac crest to just above the top of the popliteal space. The width of the belt (10.16 cm) was equal to 1 individual treatment area. Each individual treatment area was treated for 2 minutes. First the buttocks and bilateral thighs were treated, followed by the right lateral thigh and the left lateral thigh. The belt was moved progressively down the total treatment area until all individual treatment areas were addressed. The average participant had 3 to 4 bilateral thigh and buttocks treatment areas and 3 to 4 lateral treatment areas on both the left and right sides of the body.
Digital photographs were taken with standardized lighting for all participants. Photographs were taken before the first treatment on the lateral and posterior aspects of the participant and were taken again at the end of the treatment program immediately before the last treatment. Participants were asked to contract the gluteal musculature for all photographs.
Two board-certified plastic surgeons were asked to rate the before/after photographs in a blinded manner. They graded each photograph on a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite). These data were analyzed using a Wilcoxon signed rank test. These data were compared to the participants self-evaluation of the appearance of cellulite in the photographs from the initial and final treatments using a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite).
The circumference of the widest part of the gluteal area was measured before and after treatment (+/–0.5 cm). The data were analyzed using a paired t test.
Results
The study included 43 participants (age range, 21–67 years; mean age, 37.6 years; weight range, 51–97 kg; mean weight, 64.95 kg) who resided in the Midwestern United States, were interested in reducing their cellulite, and were willing to commit to treatment 2 times weekly for the duration of the 8-week study. Fourteen percent (6/43) of participants were smokers. Participant self-assessments were divided into 3 categories based on the Rossi and Vergnanini6 grading system: category II, n=7; category III, n=12; and category IV, n=24. Although all the categories in our analysis showed statistically significant improvements, we found that there was more improvement in category II participants versus category III, and then again more improvement in category III versus category IV. The data for each treatment were analyzed separately using a paired t test, as we were not interested in comparing categories, only the effect of the treatment. We were testing to see if the difference was greater than 0, and the paired t values were statistically significant in all cases (category II, P=.003; category III, P=.001; category IV, P=.002)(Figure 1).

Using a correlation analysis, we found that age, body weight, or body mass index were not significantly correlated with the difference between the before and after physician rating. The difference between before and after treatment also was independent of whether or not the participant exercised or had an adverse reaction to the belt. Adverse reactions to the belt were characterized by redness and/or minor raising of the skin immediately following the treatment. These reactions all dissipated within 12 hours. It also appeared that the rating scales correlated well with the participants self-perception of their cellulite and the improvements seen in the photographs (Figures 2 and 3).


The mean circumference of the widest part of the gluteal area before treatment was 100.2 cm and the standard deviation was 8.14 cm. The mean circumference after treatment was 98.3 cm and the standard deviation was 8.02 (t=–2.81; P<.05). Many of the women commented that they felt more “toned,” which probably accounted for the slight difference in circumference rather than weight loss.
Of the 2 blinded board-certified plastic surgeons, one physician rated all participants in category III as significantly improved (P<.05) and rated the other categories as marginally insignificantly improved; the second physician rated all categories as marginally insignificantly improved.
Comment
Although there are a large number of treatment protocols that have been introduced and studied for the reduction of the appearance of cellulite,4,9,11-18 many have not shown promising long-term results. Some treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.22-25 One of the only treatments that has shown some promise is an expensive invasive treatment.20
The system used in this study was shown to be safe in all study participants. No significant adverse reactions were noted, and each participant successfully completed the protocol. Figures 2 and 3 show the strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population, which was supported by statistical analysis, particularly the participant self-reported ratings. The participants and the blinded physicians were not in agreement on the improvement of cellulite. Although the participants knew the changes that occurred to their bodies, the physicians only had photographs from which to make their decisions. The participants clearly observed noticeable differences to their bodies, while the physicians either saw no change or some improvement.
The physicians were asked to evaluate only the cellulite, but the process we employed changed more than the cellulite. The first step in the process was a toning of the legs and buttocks, which was readily observable by the patients but was outside the scope of the physicians’ assessment. After the body toning, the cellulite began to improve. It is possible that the participants were responding to the entire process, which clearly was positive, while the physicians were responding only to the cellulite end point.
Our treatment regimen accomplished reduction of the visible appearance of cellulite by breaking down connective tissue septa as well as increasing the thickness of the dermis and its elasticity. It also helped reduce subcutaneous fat, improve circulation, and reduce general lymphatic congestion. The parallel motions of the unit could be adjusted, but we kept them at a mid-level range of motion. The motion at this frequency would have a tendency to not only heat the epidermis and dermal layer that we were attempting to affect but would also help accomplish breaking down the septa and improving the elasticity of the dermis. Also, the rapid motion over a period of time of pulling the dermis parallel to the subdermal tissue and fascia most likely helped improve the circulation and lymphatic flow in treated areas as well as possibly broke down the subcutaneous fat. All of these factors appear to have led to an improvement in the appearance of cellulite in our study participants.
A maintenance-type program, if continued, would likely demonstrate improved results by further breaking down the septa and improving the other factors that reduce the appearance of cellulite. We believe that the participants would eventually be able to discontinue the use of the unit or reduce its use substantially once the desired results were obtained.
When utilizing the device, the participants were in a standing posture and leaning into the belt with a moderate force, which seemed to secondarily improve the tone of the gluteal and thigh musculature that was being treated. It may be that the oscillatory motion and the standing posture caused the muscles to isometrically co-contract, adding a secondary exerciselike effect.26-29
Proving our suggested mechanisms of action would require tissue biopsies and/or magnetic resonance imaging studies that were beyond the scope of this study. However, regardless of the mechanism of action, we do believe that this treatment has been shown to be effective, convenient, and most importantly safe.
Conclusion
The unique device that was utilized in our study is a safe and cost-effective method of reducing the appearance of cellulite for home use and would allow for a noninvasive, low-risk procedure.
Cellulite is a cosmetic problem, not a disease process. It affects 85% to 90% of all women worldwide and was described nearly 100 years ago.1 Causes may be genetic, hormonal, or vascular in nature and may be related to the septa configuration in the subdermal tissue. Fibrosis at the dermal-subcutaneous junction as well as decreased vascular and lymphatic circulation also may be causative factors.
Cellulite has a multifactorial etiology. Khan et al2 noted that there are specific classic patterns of cellulite that affect women exclusively. White women tend to have somewhat higher rates of cellulite than Asian women. The authors also stated that lifestyle factors such as high carbohydrate diets may lead to an increase in total body fat content, which enhances the appearance of cellulite.2
The subdermal anatomy affects the appearance of cellulite. Utilizing in vivo magnetic resonance imaging, Querleux et al3 showed that women with visible cellulite have dermal septa that are thinner and generally more perpendicular to the skin’s surface than women without cellulite. In women without cellulite, the orientation of the septa is more angled into a crisscross pattern. In women with a high percentage of perpendicular septa, the perpendicular septa allow for fat herniation with dimpling of the skin compared to the crisscross septa pattern.2 Other investigators have discussed the reduction of blood flow in specific areas of the body in women, particularly in cellulite-prone areas such as the buttocks and thighs, as another causative factor.2,4,5 Rossi and Vergnanini6 showed that the blood flow was 35% lower in affected cellulite regions than in nonaffected regions without cellulite, which can cause congestion of blood and lymphatic flow and increased subdermal pressure, thus increasing the appearance of cellulite.
Although there is some controversy regarding the effects of weight loss on the appearance of cellulite,2,7 it appears that the subdermal septa and morphology have more of an effect on the appearance of cellulite.2,3,8
Rossi and Vergnanini6 proposed a 4-grade system for evaluating the appearance of cellulite (grade I, no cellulite; grade II, skin that is smooth and without any pronounced dimpling upon standing or lying down but may show some dimpling upon pinching and strong muscle contraction; grade III, cellulite is present in upright positions but not when the patient is in a supine position; grade IV, cellulite can be seen when the patient is standing and in a supine position). Both grades III and IV can be exacerbated by maximal voluntary contraction and strong pinching of the skin because these actions cause the subcutaneous fat to move toward the surface of the skin between the septa. This grading system aligns with categories I through III described by Mirrashed et al.9
There are many cellulite treatments available but few actually create a reduction in the visible appearance of cellulite. A number of these treatments were reviewed by Khan et al,10 including massage; a noninvasive suction-assisted massage technique; and topical agents such as xanthine, retinols, and other botanicals.4,11-14 Liposuction has not been shown to be effective in the treatment of cellulite and in fact may increase the appearance of cellulite.9,15 Mesotherapy, a modality that entails injecting substances into the subcutaneous fat layer, is another treatment of cellulite. Two of the most common agents purported to dissolve fat include phosphatidylcholine and sodium deoxycholate. The efficacy and safety of mesotherapy remains controversial and unproven. A July 2008 position statement from the American Society of Plastic Surgeons stated that “low levels of validity and quality of the literature does not allow [American Society of Plastic Surgeons] to support a recommendation for the use of mesotherapy/injection lipolysis for fat reduction.”16 Other modalities such as noninvasive dual-wavelength laser/suction devices; low-energy diode laser, contact cooling, suction, and massage devices; and infrared, bipolar radiofrequency, and suction with mechanical massage devices are available and show some small improvements in the visible appearance of cellulite, but no rating scales were used in any of these studies.17,18 DiBernardo19 utilized a 1440-nm pulsed laser to treat cellulite. It is an invasive treatment that works by breaking down some of the connective tissue septa responsible for the majority and greater severity of the dermal dimpling seen in cellulite, increasing the thickness of the dermis as well as its elasticity, reducing subcutaneous fat, and improving circulation and reducing general lymphatic congestion.19 The system showed promise but was an invasive treatment, and one session could cost $5000 to $7000 for bilateral areas and another $2500 for each additional area.20 Burns21 expressed that the short-term results showed promise in reducing the appearance of cellulite. Noninvasive ultrasound22,23 as well as extracorporeal shock wave therapy24,25 also has shown some improvement in the firmness of collagen but generally not in the appearance of cellulite.
We sought to evaluate the efficacy and safety of a noninvasive mechanical treatment of cellulite.
Methods
This study was conducted in accordance with the guidelines set forth by the US Department of Health and Human Services’ Policy for Protection of Human Research Subjects and the World Medical Association’s Declaration of Helsinki. Participants were recruited through local area medical facilities in southeastern Michigan. Written informed consent was obtained from all participants prior to beginning the study.
Patients with grades II to IV cellulite, according to the Rossi and Vergnanini6 grading system, were allowed to participate. All participants in the study were asked not to make lifestyle changes (eg, exercise habits, diet) or use any other treatments for cellulite that might be available to them during the study period. Exclusion criteria included history of deep vein thrombosis, cancer diagnosed within the last year, pregnancy, hemophilia, severe lymphedema, presence of a pacemaker, epilepsy, seizure disorder, or current use of anticoagulants. History of partial or total joint replacements, acute hernia, nonunited fractures, advanced arthritis, or detached retina also excluded participation in the study.
Participants completed an 8-week, twice-weekly treatment protocol with a noninvasive mechanical device performed in clinic. The device consisted of a 10.16-cm belt with a layer of nonslip material wrapped around the belt. The belt was attached to a mechanical oscillator. We adjusted the stroke length to approximately 2 cm and moved the dermis at that length at approximately 1000 strokes per minute.
Each participant was treated for a total treatment time of 18 to 24 minutes. The total treatment area included the top of the iliac crest to just above the top of the popliteal space. The width of the belt (10.16 cm) was equal to 1 individual treatment area. Each individual treatment area was treated for 2 minutes. First the buttocks and bilateral thighs were treated, followed by the right lateral thigh and the left lateral thigh. The belt was moved progressively down the total treatment area until all individual treatment areas were addressed. The average participant had 3 to 4 bilateral thigh and buttocks treatment areas and 3 to 4 lateral treatment areas on both the left and right sides of the body.
Digital photographs were taken with standardized lighting for all participants. Photographs were taken before the first treatment on the lateral and posterior aspects of the participant and were taken again at the end of the treatment program immediately before the last treatment. Participants were asked to contract the gluteal musculature for all photographs.
Two board-certified plastic surgeons were asked to rate the before/after photographs in a blinded manner. They graded each photograph on a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite). These data were analyzed using a Wilcoxon signed rank test. These data were compared to the participants self-evaluation of the appearance of cellulite in the photographs from the initial and final treatments using a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite).
The circumference of the widest part of the gluteal area was measured before and after treatment (+/–0.5 cm). The data were analyzed using a paired t test.
Results
The study included 43 participants (age range, 21–67 years; mean age, 37.6 years; weight range, 51–97 kg; mean weight, 64.95 kg) who resided in the Midwestern United States, were interested in reducing their cellulite, and were willing to commit to treatment 2 times weekly for the duration of the 8-week study. Fourteen percent (6/43) of participants were smokers. Participant self-assessments were divided into 3 categories based on the Rossi and Vergnanini6 grading system: category II, n=7; category III, n=12; and category IV, n=24. Although all the categories in our analysis showed statistically significant improvements, we found that there was more improvement in category II participants versus category III, and then again more improvement in category III versus category IV. The data for each treatment were analyzed separately using a paired t test, as we were not interested in comparing categories, only the effect of the treatment. We were testing to see if the difference was greater than 0, and the paired t values were statistically significant in all cases (category II, P=.003; category III, P=.001; category IV, P=.002)(Figure 1).

Using a correlation analysis, we found that age, body weight, or body mass index were not significantly correlated with the difference between the before and after physician rating. The difference between before and after treatment also was independent of whether or not the participant exercised or had an adverse reaction to the belt. Adverse reactions to the belt were characterized by redness and/or minor raising of the skin immediately following the treatment. These reactions all dissipated within 12 hours. It also appeared that the rating scales correlated well with the participants self-perception of their cellulite and the improvements seen in the photographs (Figures 2 and 3).


The mean circumference of the widest part of the gluteal area before treatment was 100.2 cm and the standard deviation was 8.14 cm. The mean circumference after treatment was 98.3 cm and the standard deviation was 8.02 (t=–2.81; P<.05). Many of the women commented that they felt more “toned,” which probably accounted for the slight difference in circumference rather than weight loss.
Of the 2 blinded board-certified plastic surgeons, one physician rated all participants in category III as significantly improved (P<.05) and rated the other categories as marginally insignificantly improved; the second physician rated all categories as marginally insignificantly improved.
Comment
Although there are a large number of treatment protocols that have been introduced and studied for the reduction of the appearance of cellulite,4,9,11-18 many have not shown promising long-term results. Some treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.22-25 One of the only treatments that has shown some promise is an expensive invasive treatment.20
The system used in this study was shown to be safe in all study participants. No significant adverse reactions were noted, and each participant successfully completed the protocol. Figures 2 and 3 show the strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population, which was supported by statistical analysis, particularly the participant self-reported ratings. The participants and the blinded physicians were not in agreement on the improvement of cellulite. Although the participants knew the changes that occurred to their bodies, the physicians only had photographs from which to make their decisions. The participants clearly observed noticeable differences to their bodies, while the physicians either saw no change or some improvement.
The physicians were asked to evaluate only the cellulite, but the process we employed changed more than the cellulite. The first step in the process was a toning of the legs and buttocks, which was readily observable by the patients but was outside the scope of the physicians’ assessment. After the body toning, the cellulite began to improve. It is possible that the participants were responding to the entire process, which clearly was positive, while the physicians were responding only to the cellulite end point.
Our treatment regimen accomplished reduction of the visible appearance of cellulite by breaking down connective tissue septa as well as increasing the thickness of the dermis and its elasticity. It also helped reduce subcutaneous fat, improve circulation, and reduce general lymphatic congestion. The parallel motions of the unit could be adjusted, but we kept them at a mid-level range of motion. The motion at this frequency would have a tendency to not only heat the epidermis and dermal layer that we were attempting to affect but would also help accomplish breaking down the septa and improving the elasticity of the dermis. Also, the rapid motion over a period of time of pulling the dermis parallel to the subdermal tissue and fascia most likely helped improve the circulation and lymphatic flow in treated areas as well as possibly broke down the subcutaneous fat. All of these factors appear to have led to an improvement in the appearance of cellulite in our study participants.
A maintenance-type program, if continued, would likely demonstrate improved results by further breaking down the septa and improving the other factors that reduce the appearance of cellulite. We believe that the participants would eventually be able to discontinue the use of the unit or reduce its use substantially once the desired results were obtained.
When utilizing the device, the participants were in a standing posture and leaning into the belt with a moderate force, which seemed to secondarily improve the tone of the gluteal and thigh musculature that was being treated. It may be that the oscillatory motion and the standing posture caused the muscles to isometrically co-contract, adding a secondary exerciselike effect.26-29
Proving our suggested mechanisms of action would require tissue biopsies and/or magnetic resonance imaging studies that were beyond the scope of this study. However, regardless of the mechanism of action, we do believe that this treatment has been shown to be effective, convenient, and most importantly safe.
Conclusion
The unique device that was utilized in our study is a safe and cost-effective method of reducing the appearance of cellulite for home use and would allow for a noninvasive, low-risk procedure.
- Scherwitz C, Braun-Falco O. So-called cellulite. J Dermatol Surg Oncol. 1978;4:230-234.
- Khan MH, Victor F, Rao B, et al. Treatment of cellulite: part I. pathophysiology. J Am Acad Dermatol. 2010;62:361-370, quiz 371-372.
- Querleux B, Cornillon C, Jolivet O, et al. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118-124.
- Rawlings A. Cellulite and its treatment. Int J Cos Sci. 2006;28:175-190.
- Rosenbaum M, Prieto V, Hellmer J, et al. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg. 1998;101:1934-1939.
- Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14:251-262.
- Smalls LK, Hicks M, Passeretti D, et al. Effect of weight loss on cellulite: gynoid lypodystrophy. Plast Reconstr Surg. 2006;118:510-516.
- Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221-229.
- Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161-168.
- Khan M, Victor F, Rao B, et al. Treatment of cellulite, part II. advances and controversies. J Am Acad Dermatol. 2010;62:373-384.
- Collis N, Elliot L, Sharp C, et al. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg. 1999;104:1110-1114.
- Adcock D, Paulsen S, Jabour K, et al. Analysis of the effects of deep mechanical massage in the porcine model. Plast Reconstr Surg. 2000;108:233-240.
- Güleç AT. Treatment of cellulite with LPG endermologie. Int J Dermatol. 2009;48:265-270.
- Piérard-Franchimont C, Piérard GE, Henry F, et al. A randomized, placebo-controlled trial of tropical retinol in the treatment of cellulite. Am J Clin Dermatol. 2000;1:369-374.
- Coleman WP. Liposuction. In: Coleman WP, Hanke CW, Alt TH, eds. Cosmetic Surgery of the Skin: Principles and Practice. Philadelphia, PA: BC Decker; 1991:213-238.
- ASPS guiding principles for mesotherapy/injection lipolysis. American Society of Plastic Surgeons website. http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/guiding-principles/ASPS-Guiding-Principles-for-Mesotherapy-Injection-Lipolysis-7-08.pdf. Published July 2008. Accessed February 17, 2016.
- Kulick MI. Evaluation of a noninvasive, dual-wavelength laser-suction and massage device for the regional treatment of cellulite. Plast Reconstr Surg. 2010;125:1788-1796.
- Nootheti PK, Magpantay A, Yosowitz G, et al. A single center, randomized, comparative, prospective clinical study to determine the efficacy of the VelaSmooth system versus the TriActive system for the treatment of cellulite. Lasers Surg Med. 2006;38:908-912.
- DiBernardo BE. Treatment of cellulite using a 1440-nm pulsed laser with one-year follow up. Aesthet Surg J. 2011;31:328-341.
- Johannes L. New laser aims to zap cellulite at the source. Wall Street Journal. July 3, 2012. http://www.wsj.com/articles/SB10001424052702303649504577496981754619546. Accessed November 21, 2016.
- Burns AJ. Commentary on: treatment of cellulite using a 1440-nm pulsed laser with one-year follow up: preliminary report. Aesthet Surg J. 2011;31:342-343.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety efficacy of the contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Brown SA, Greenbaum L, Shtukmaster S, et al. Characterization of nonthermal focused ultrasound for noninvasive selective fat cell disruption (lysis): technical and preclinical assessment. Plast Reconstr Surg. 2009;124:92-101.
- Angehrn F, Kuhn C, Voss A. Can cellulite be treated with low energy extracorporeal shock wave therapy? Clin Interv Aging. 2007;2:623-630.
- Christ C, Brenke R, Sattler G, et al. Improvement in skin elasticity in the treatment of cellulite and connective tissue weakness by means of extracorporeal pulse activation therapy. Aesthet Surg J. 2008;28:538-544.
- Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183-187.
- Luo J, McNamara B, Moran K. The use of vibration training to enhance muscle strength and power. Sports Med. 2005;35:23-41.
- Annino G, Padua E, Castagna C, et al. Effect of whole body vibration training on lower limb performance in selected high-level ballet students. J Strength Cond Res. 2007;21:1072-1076.
- Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study [published online December 22, 2003]. J Bone Miner Res. 2004;19:352-359.
- Scherwitz C, Braun-Falco O. So-called cellulite. J Dermatol Surg Oncol. 1978;4:230-234.
- Khan MH, Victor F, Rao B, et al. Treatment of cellulite: part I. pathophysiology. J Am Acad Dermatol. 2010;62:361-370, quiz 371-372.
- Querleux B, Cornillon C, Jolivet O, et al. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118-124.
- Rawlings A. Cellulite and its treatment. Int J Cos Sci. 2006;28:175-190.
- Rosenbaum M, Prieto V, Hellmer J, et al. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg. 1998;101:1934-1939.
- Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14:251-262.
- Smalls LK, Hicks M, Passeretti D, et al. Effect of weight loss on cellulite: gynoid lypodystrophy. Plast Reconstr Surg. 2006;118:510-516.
- Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221-229.
- Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161-168.
- Khan M, Victor F, Rao B, et al. Treatment of cellulite, part II. advances and controversies. J Am Acad Dermatol. 2010;62:373-384.
- Collis N, Elliot L, Sharp C, et al. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg. 1999;104:1110-1114.
- Adcock D, Paulsen S, Jabour K, et al. Analysis of the effects of deep mechanical massage in the porcine model. Plast Reconstr Surg. 2000;108:233-240.
- Güleç AT. Treatment of cellulite with LPG endermologie. Int J Dermatol. 2009;48:265-270.
- Piérard-Franchimont C, Piérard GE, Henry F, et al. A randomized, placebo-controlled trial of tropical retinol in the treatment of cellulite. Am J Clin Dermatol. 2000;1:369-374.
- Coleman WP. Liposuction. In: Coleman WP, Hanke CW, Alt TH, eds. Cosmetic Surgery of the Skin: Principles and Practice. Philadelphia, PA: BC Decker; 1991:213-238.
- ASPS guiding principles for mesotherapy/injection lipolysis. American Society of Plastic Surgeons website. http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/guiding-principles/ASPS-Guiding-Principles-for-Mesotherapy-Injection-Lipolysis-7-08.pdf. Published July 2008. Accessed February 17, 2016.
- Kulick MI. Evaluation of a noninvasive, dual-wavelength laser-suction and massage device for the regional treatment of cellulite. Plast Reconstr Surg. 2010;125:1788-1796.
- Nootheti PK, Magpantay A, Yosowitz G, et al. A single center, randomized, comparative, prospective clinical study to determine the efficacy of the VelaSmooth system versus the TriActive system for the treatment of cellulite. Lasers Surg Med. 2006;38:908-912.
- DiBernardo BE. Treatment of cellulite using a 1440-nm pulsed laser with one-year follow up. Aesthet Surg J. 2011;31:328-341.
- Johannes L. New laser aims to zap cellulite at the source. Wall Street Journal. July 3, 2012. http://www.wsj.com/articles/SB10001424052702303649504577496981754619546. Accessed November 21, 2016.
- Burns AJ. Commentary on: treatment of cellulite using a 1440-nm pulsed laser with one-year follow up: preliminary report. Aesthet Surg J. 2011;31:342-343.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety efficacy of the contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Brown SA, Greenbaum L, Shtukmaster S, et al. Characterization of nonthermal focused ultrasound for noninvasive selective fat cell disruption (lysis): technical and preclinical assessment. Plast Reconstr Surg. 2009;124:92-101.
- Angehrn F, Kuhn C, Voss A. Can cellulite be treated with low energy extracorporeal shock wave therapy? Clin Interv Aging. 2007;2:623-630.
- Christ C, Brenke R, Sattler G, et al. Improvement in skin elasticity in the treatment of cellulite and connective tissue weakness by means of extracorporeal pulse activation therapy. Aesthet Surg J. 2008;28:538-544.
- Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183-187.
- Luo J, McNamara B, Moran K. The use of vibration training to enhance muscle strength and power. Sports Med. 2005;35:23-41.
- Annino G, Padua E, Castagna C, et al. Effect of whole body vibration training on lower limb performance in selected high-level ballet students. J Strength Cond Res. 2007;21:1072-1076.
- Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study [published online December 22, 2003]. J Bone Miner Res. 2004;19:352-359.
Practice Points
- Several cellulite treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.
- The noninvasive mechanical treatment for women with cellulite evaluated in this study showed a strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population.
Comparing Cost, Efficacy, and Safety of Intravenous and Topical Tranexamic Acid in Total Hip and Knee Arthroplasty
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.