User login
PACT ICU Model: Interprofessional Case Conferences for High-Risk/High-Need Patients
Physician, nurse practitioner trainees, medical center faculty, and clinic staff develop proactive, team-based, interprofessional care plans to address unmet chronic care needs for high-risk patients.
This article is part of a series that illustrates strategies intended to redesign primary care education at the Veterans Health Administration (VHA), using interprofessional workplace learning. All have been implemented in the VA Centers of Excellence in Primary Care Education (CoEPCE). These models embody visionary transformation of clinical and educational environments that have potential for replication and dissemination throughout VA and other primary care clinical educational environments. For an introduction to the series see Klink K. Transforming primary care clinical learning environments to optimize education, outcomes, and satisfaction. Fed Pract. 2018;35(9):8-10.
Background
In 2011, 5 US Department of Veterans Affairs (VA) medical centers (VAMCs) were selected by the Office of Academic Affiliations (OAA) to establish CoEPCE. Part of the VA New Models of Care initiative, the 5 Centers of Excellence (CoE) in Boise, Idaho; Cleveland, Ohio; San Francisco, California; Seattle, Washington; and West Haven, Connecticut, are utilizing VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurse residents and undergraduate nursing students, and other professions of health trainees (eg, pharmacy, social work, psychology, physician assistants [PAs]) for primary care practice in the 21st century.
The Boise CoE developed and implemented a practice-based learning model. Nurse practitioner (NP) students and residents, physician residents, pharmacy residents, psychology interns, and psychology postdoctoral fellows participate in a comprehensive curriculum and practice together for 1 to 3 years. The goal is to produce providers who are able to lead and practice health care in patient-centered primary care and rural care environments. All core curricula are interprofessionally coauthored and cotaught.1
Methods
In 2015, OAA evaluators reviewed background documents and conducted open-ended interviews with 10 CoE staff, participating trainees, VA faculty, VA facility leadership, and affiliate faculty. In response to questions focused on their experiences, informants described lessons learned, challenges encountered, and benefits for participants, veterans, and the VA. Using a qualitative and quantitative approach, this case study draws on those interviews, surveys of PACT ICU (patient aligned care team interprofessional care update) participants, and analysis of presented patients to examine PACT ICU outcomes.
Interprofessional Education and Care
A key CoEPCE aim is to create more clinical opportunities for CoE trainees from a variety of professions to work as a team in ways that anticipate and address the care needs of veterans. This emphasis on workplace learning is needed since most current health care professional education programs lack settings where trainees from different professions can learn and work together with their clinic partners to provide care for patients. With the emphasis on patient-centered medical homes (PCMH) and team-based care in the Affordable Care Act, there is an imperative to develop new training models that address this gap in the preparation of future health professionals. Along with this imperative, clinicians are increasingly required to optimize the health of complex patients who consequently require a multidisciplinary approach to care, particularly high-risk, high-needs patients inappropriately using services, such as frequent emergency department (ED) use.
Addressing Complex Needs
In 2010, the Boise VA Medical Center (VAMC) phased in patient aligned care teams (PACTs), the VA-mandated version of PCMH that consist of a physician or NP primary care provider (PCP), a registered nurse (RN) care manager, a licensed vocational nurse (LVN), and a medical support assistant (MSA).
The PACT ICU also serves as a venue in which trainees and supervisors from different professions use a patient-centered framework to collaborate on these specific patient cases. The PACT ICU is easily applied to a range of health conditions, such as diabetes mellitus (DM), mental and behavioral health, lack of social support, and delivery system issues, such as ED use. The goals of PACT ICU are to improve the quality and satisfaction of patient care for high-risk patients; encourage appropriate use of health care resources by prioritizing continuity with the PACT team; and enhance interprofessional PACT team function, decreasing PCP and staff stress.
Planning and Implementation
In January 2013, Boise VAMC and the Caldwell, Idaho community-based outpatient clinic (CBOC) implemented PACT ICU. Other nonteaching clinics followed later in the year. Planning and executing PACT ICU took about 10 hours of CoE staff time and required no change in Boise VAMC policy. Program leadership approval was necessary for participation of CoE residents and postdocs. Service-line leadership support was required to protect clinic staff time (nurse care manager, social workers, chaplaincy, and ethics service). At the Caldwell CBOC, the section chief approved the program, and it took about 1 month to initiate a similar version of PACT ICU.
Curriculum
PACT ICU is a workplace clinical activity with roots in the case conference model, specifically the EFECT model (Elicit the narrative of illness, Facilitate a group meeting, Evidence-based gap analysis, Care plan, and Track changes).3 PACT ICU emphasizes a patient-centered approach to developing care plans. Staff review the 5 highest risk patients who are identified by the VA Care Assessment Need (CAN) registry. The CAN is an analytic tool that is available throughout VA and estimates patients’ risk of mortality or hospitalization in the following 90 days. Physician and NP residents choose 1 of the 5 patients to discuss in PACT ICU, while the remaining 4 serve as case-control comparisons to examine long-term patient outcomes. All trainees, faculty, and staff are provided patient data that can be discussed on a secure website.
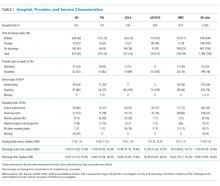
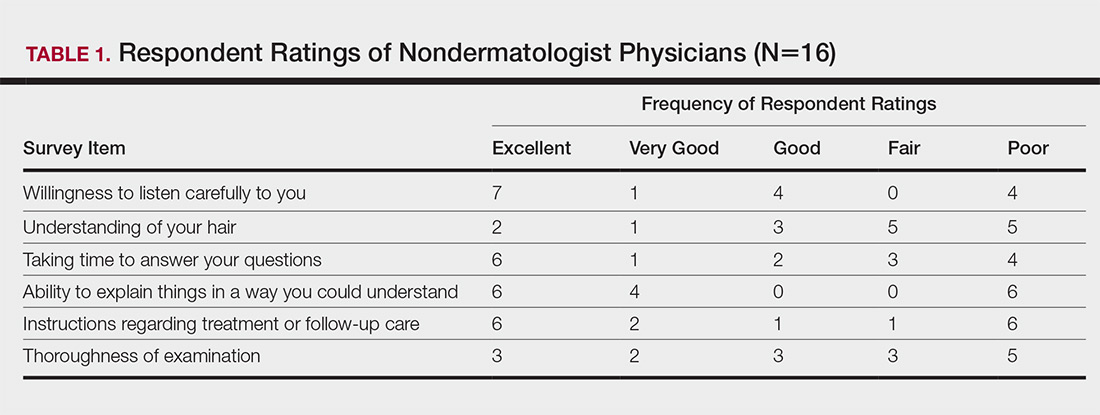
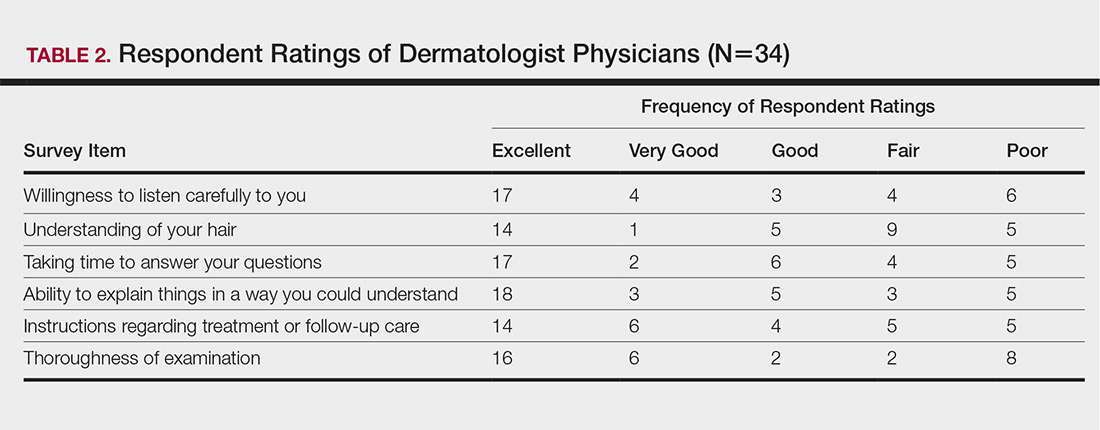
The PACT ICU combines didactic teaching with workplace learning. For example, the patient’s medical issues may lead to a formal presentation about a topic, such as secondary stroke medication prophylaxis. The workplace learning occurs as the trainees observe and participate in the decision making toward a treatment plan. Interprofessional interactions are role-modeled by clinical faculty and staff during these discussions, and the result impact the patients care. PACT ICU embodies the core domains that shape the CoEPCE curriculum: Interprofessional collaboration (IPC), performance improvement (PI), sustained relationships (SR), and shared decision making (SDM) (Table 1).
There have been some changes to the PACT ICU model over time. Initially, conferences took place on a weekly basis, to build momentum among the team and to normalize processes. After about 2 years, this decreased to every other week to reduce the time burden. Additionally, the CoE has strengthened the “tracking changes” component of the EFECT model—trainees now present a 5-minute update on the last patient they presented at the prior PACT ICU case conference. Most recently, psychology postdoctoral candidates have instituted preconference calls with patients to further improve the teams understanding of the patients’ perspective and narrative.
Related: Improving Team-Based Care Coordination Delivery and Documentation in the Health Record
Resources
The CoE faculty participate in an education program concerning facilitation of interprofessional meetings. All faculty are expected to role model collaborative behavior and mentor trainees on the cases they present.
The PACT ICU requires a room large enough to accommodate at least 12 people. One staff member is required to review patient cases prior to the case conferences (usually about 1 hour of preparation per case conference). Another staff person creates and shares a spreadsheet stored with VA-approved information security with data fields to include the site, PACT ICU date, patient identifier, the CAN score, and a checkbox for whether the patient was selected or part of a control group. Logistic support is required for reserving the room and sending information to presenters. A clinic-based RN with training in interprofessional care case management uses an online schedule to facilitate selection and review of patients. The RN care managers can use a secure management tool to track patient care and outreach.
The RN care manager also needs to be available to attend the PACT ICU case conferences. The Boise CoE built a website to share and standardize resources, such as a presenter schedule, PACT ICU worksheet, and provider questionnaire. (Contact Boise CoE staff for access.) For the initial evaluation of impact, PACT ICU utilized staff data support in the form of a data manager and biostatistician to identify, collect, and analyze data. While optional, this was helpful in refining the approach and demonstrating the impact of the project. Other resource-related requirements for exporting PACT ICU include:
- Staff members, usually RN care managers who coordinate meetings with participants and identify appropriate patients using a registry, such as CAN;
- Meeting facilitators who enforce use of the EFECT model and interprofessional participation to ensure that the interprofessional care plan is carried out by the presenting provider; and
- Interprofessional trainees and faculty who participate in PACT ICU and complete surveys after the first conference.
Monitoring and Assessment
The CoE staff have analyzed the evaluation of PACT ICU with participant self-evaluation, consultation referral patterns, and utilization data, combination of ED and episodic care visits along with hospitalizations).4 Pharmacy faculty are exploring the use polypharmacy registries, and psychology will use registries of poor psychosocial function.
Partnerships
Beyond support and engagement from VA CoEPCE and affiliate faculty, PACT ICU has greatly benefited from partnerships with VA facility department and CBOC leadership. The CoEPCE codirector and faculty are in facility committees, such as the PACT Strategic Planning Committee.
Academic affiliates are integral partners who assist with NP student and resident recruitment as well as participate in the planning and refinement of CoEPCE components. PACT ICU supports their mandate to encourage interprofessional teamwork. Faculty members from Gonzaga University (NP affiliate) were involved in the initial discussion on PACT ICU and consider it a “learning laboratory” to work through challenging problems. Gonzaga CoEPCE NP trainees are asked to talk about their PACT ICU experience—its strengths, weaknesses, and challenges—to other Gonzaga students who don’t have exposure to the team experience.
Challenges and Solutions
The demand for direct patient care puts pressure on indirect patient care approaches like PACT ICU, which is a time-intensive process with high impact on only a small number of patients. The argument for deploying strategies such as PACT ICU is that managing chronic conditions and encouraging appropriate use of services will improve outcomes for the highest risk patients and save important system resources in the long-run. However, in the short-term, a strong case must be made for the diversion of resources from usual clinic flow, particularly securing recurring blocks of provider time and clinic staff members. In addition, issues about team communication and understanding of appropriate team-based care can overflow to complex patients not presented in the PACT ICU conference.
Providing a facilitated interprofessional venue to discuss how to appropriately coordinate care improves the participation and perceived value of different team members. This approach has led to improved engagement of the team for patients discussed in the PACT ICU, as well as in general care within the participating clinic. With recent changes, the VA does see a workload benefit, and participants get encounter credit through “Non face-to-face prolonged service” codes (CPT 99358/99359), and other possibilities exist related to clinical team conference codes (CPT 99367-8) and complex chronic care management codes (CPT 99487-89). More information on documentation, scheduling and encountering/billing can be found at boisevacoe.org under Products. Other challenges include logistic challenges of finding appropriate patients and distributing sensitive patient information among the team. Additionally, PACT ICU has to wrestle with staffing shortages and episodic participation by some professions that are chronically understaffed. We have addressed many of these problems by receiving buy-in from both leadership and participants. Leadership have allowed time for participation in clinic staff schedules, and each participant has committed to recruiting a substitute in case of a schedule conflict.
Factors for Success
The commitment from the Boise VAMC facility, primary care clinic leadership and affiliated training programs to support staff and trainee participation also has been critical. Additionally, VA facility leadership commitment to ongoing improvements to PACT implementation was a key facilitating factor. Colocation of trainees and clinic staff on the academic PACT team facilitates communication between PACT ICU case conferences, while also supporting team dynamics and sustained relationships with patients. Many of these patients can and will typically seek care using the interdisciplinary trainees, and trainees were motivated to proactively coordinate warm handoffs and other models of transfer of care. PACT ICU has been successfully replicated and sustained at 4 of the 5 CoEPCE sites. The Caldwell CBOC PACT ICU has been up and running for 2 years, and 2 other nonacademic clinics have piloted PACT ICU managed care conferences thus far. Experience regarding the implementation at other academic sites has been published.5
Accomplishments and Benefits
There is evidence that PACT ICU is achieving its goals of improving trainee learning and patient outcomes. Trainees are using team skills to provide patient-centered care; trainees are strengthening their overall clinical skills by learning how to improve their responses to high-risk patients. There is also evidence of an increase in interprofessional warm handoffs within the clinic, in which “a clinician directly introduces a patient to another clinician at the time of the patient’s visit, and often a brief encounter between the patient and the health care professional occurs.”4,6
Unlike a traditional didactic with classroom case conferences on interprofessional collaboration, PACT ICU is an opportunity for health care professionals to both learn and work together providing care in a clinic. Moreover, colocation of diverse trainee and faculty professions during the case conferences better prepares trainees to work with other professions and supports all participants to work and communicate as a team.
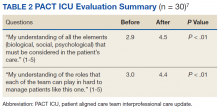
CoE staff have assessed educational outcomes before and after attendance in PACT ICU. On average, trainees (n = 30) said they found the PACT ICU case conferences to be “very helpful” in developing treatment plans.
Interprofessional Collaboration
Team building and colocating trainees, faculty, and clinic staff from different professions are a primary focus of PACT ICU. The case conferences are designed to break down silos and foster a team approach to care. Trainees learn how the team works and the ways other professionals can help them take care of the patient. For example, trainees learn early about the contributions and expertise that the pharmacist and psychologist offer in terms of their scope of practice and referral opportunities. Additionally, the RN care manager increases the integration with the PACT clinical team by sharing pertinent information on individual patients. Based on recent trainee survey findings, the CoE has observed a positive change in the team dynamic and trainee ability to interface between professions. PACT ICU participants were more likely to make referrals to other members within the PACT team, such as a warm handoff during a clinic appointment, while they were less likely to seek a consult outside the team.7
Clinical Performance
The PACT ICU is an opportunity for a trainee to increase clinical expertise. It provides exposure to a variety of patientsand their care needs and serves as an opportunity to present a high-risk, challenging patient to colleagues of various professions. As of June 2018, 96 physician resident and NP residents have presented complex patient cases.
In addition, a structured forum for discussing patients and their care options strengthens team clinical performance, which supports people to work to the full scope of their practice. Trainees learn and apply team skills, such as communication and the warm handoff.
An interprofessional care plan that is delineated during the meeting supports the trainee and is carried out with help from consultants as needed. These consultants often facilitate plans for a covisit or warm handoff at the next clinic visit, a call from the RN care manager, a virtual clinic appointment, or other nontraditional visits. The clinic staff can get information from PCPs about patient’s plan of care, and PCPs get a more complete picture of a patient’s situation (eg, history, communications, and life-style factors). In addition, surveys of PACT ICU participants suggest the curriculum’s effectiveness at encouraging use of PACT principles within the clinic team and improving appropriate referrals to other members of the PACT team, such as pharmacy and behavioral health.
Patients presented at PACT ICU can be particularly challenging, so there may be a psychological benefit to working with a team to develop a new care plan. The PCPs who feel they are overwhelmed and have exhausted every option step back, get input, and look at the patient in a new light.
Related: Interprofessional Education in Patient Aligned Care Team Primary Care-Mental Health Integration
CoEPCE Function
The PACT ICU is flexible and has been adapted to different ambulatory care settings. Currently, PACT ICU case conferences take place at Boise VAMC, the Caldwell CBOCs, and more recently at a smaller CBOC in Burns, Oregon. The PACT ICU structure is slightly different in the clinic settings since the VA primary care clinic has different resources to draw upon, such as hospital and specialty services. The Caldwell CBOC was unable to protect time for PCPs, so it holds a monthly PACT ICU case conference. In addition to continuing expansion in other nonacademic PACT clinics and collaboration with other CoEPCE sites, work is underway to disseminate generalizable principles for interprofessional education, as well as exporting the model for implementation in non-VA settings.
Primary Care Services
The PACT ICU has the potential to create efficiencies in busy clinic settings. It strengthens communication between PCPs and is an opportunity to touch base on the patient, delegate care, and keep track of high-risk patients who might otherwise receive attention only when having an acute problem. Nurses gain a deeper understanding of the patients presented at PACT ICU.
PACT ICU leverages and builds on existing PACT resources in an achievable and sustainable manner benefiting primary care. CoE trainees, who are part of the Silver Team, tap in to the information that team nurses gain from checking in with these high-risk patients biweekly. Moreover, the integration with the Silver Team improves continuity, which helps enhance a patient’s level of trust. The relationship strengthened between primary care and behavioral health at the Caldwell CBOC, providing improved patient access and increased professional sharing.
Patient Outcomes
The PACT ICU provides a forum for input beyond that of the PCP. This feature results in a more robust treatment plan than might be developed by individual PCPs who might not have time to consider options that are outside their scope of practice. Formulating an enriched care plan, informed by multiple professions, has the potential to improve utilization and provide better care.
The Boise VAMC PACT ICU has presented 219 patients as of June 2018. While clinical outcomes data are difficult to collect, the CoE has data on utilization differences on all patients presented at the PACT ICU case conferences. This includes 4 control patients from the same PCP, with similarly high risk based on CAN scores at the time of selection. A single control patient is selected based on gender, closest age, and CAN score; this serves as a comparator for subsequent utilization analysis.
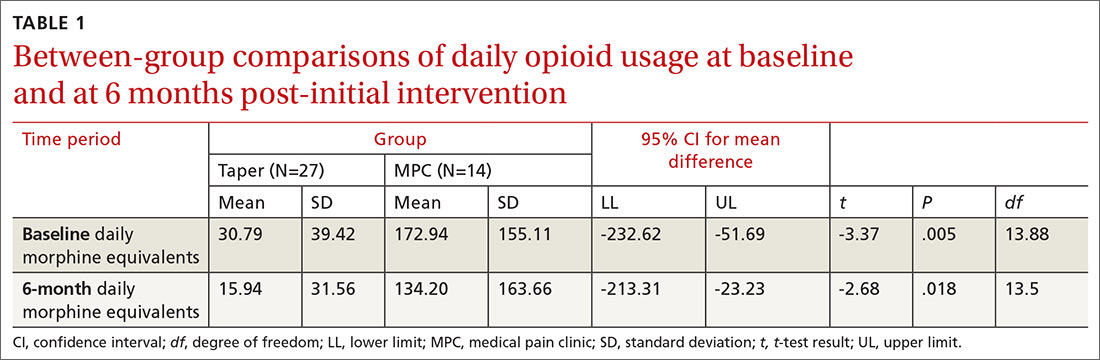
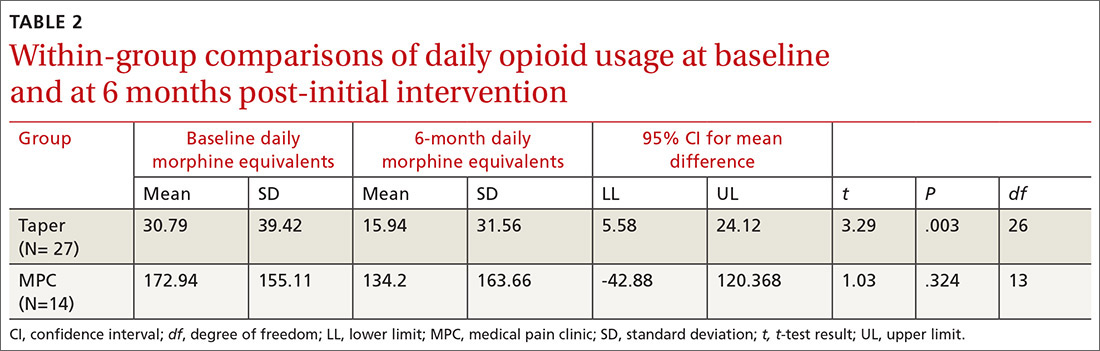
Data from the first 2 years of this study demonstrate that compared with the high-risk control group, there was an increase in contacts with PACT team members, including behavioral health, clinical pharmacists, and nurse care management, persisting up to 6 months following the PACT ICU presentation.4 However, PACT ICU participation did not increase the number of visits with the PCP, indicating better engagement with the entire team. Participation was associated with significantly decreased hospitalizations and a trend toward decreased ED visits. These findings persisted when compared with controls in the PCP’s panel with similar CAN scores, making “regression to the mean” often seen in these studies much less likely.
Analysis of patients early in the project suggests the possibility of improved glycemic control in patients with DM and improved blood pressure control in hypertensive patients presented at the PACT ICU compared with that of non-PACT ICU patients.8 Another potential benefit includes better team-based coordination. Because the patient now has a team focusing on care, this new dynamic results in improving outreach, identifying patients who could receive care by a telephone, and better preparing team members to establish rapport when the patient calls or comes in for a visit.
The Future
In stage 2 of the CoEPCE program, a multi-site trial of PACT ICU was completed to better understand which elements are critical to success, with the goal of facilitating broader exportability.5 The trial focused on 3 intertwined elements: structure, delivery, and evaluation. Using local implementation and the multisite trial, the most effective practices have been documented as part of an implementation kit, available at boisevacoe.org. The goal of the implementation kit is to facilitate step-by-step implementation of PACT ICU to other settings beyond the multisite study. Since the open-ended structure of PACT ICU enables accommodating different professions and specialties beyond the model’s Boise VAMC participants, it could be easily adapted to potentially support a variety of implementations elsewhere (Appendix).
Another opportunity for expansion is increased patient involvement. Currently, PACT ICU patients have the opportunity to review and ask questions about their multidisciplinary care plans before starting.
1. Rugen KW, Watts S, Janson S, et al. Veteran Affairs centers of excellence in primary care education: transforming nurse practitioner education. Nurs Outlook. 2014;62(2):78-88.
2. Billett S. Learning through practice: beyond informal and towards a framework for learning through practice. UNESCO-UNEVOC. https://unevoc.unesco.org/fileadmin/up/2013_epub_revisiting_global_trends_in_tvet_chapter4.pdf. Published 2013. Accessed August 30, 2018.
3. Bitton A, Pereira AG, Smith CS, Babbott SF, Bowen JL. The EFECT framework for interprofessional education in the patient centered medical home. Healthc (Amst). 2013;1(3-4):63-68.
4. Weppner WG, Davis K, Tivis R, et al. Impact of a complex chronic care patient case conference on quality and utilization. Transl Behav Med. 2018;8(3):366-374.
5. King IC, Strewler A, Wipf JE. Translating innovation: exploring dissemination of a unique case conference. J Interprof Educ Pract. 2017;6(1):55-60.
6. Cohen DJ, Balasubramanian BA, Davis M, et al. Understanding care integration from the ground up: five organizing constructs that shape integrated practices. J Am Board Fam Med. 2015;28(suppl 1):S7-S20.
7. Weppner WG, Davis K, Sordahl J, et al. Interprofessional care conferences for high risk primary care patients. Acad Med. 2016;91(6):798-802.
8. Buu J, Fisher A, Weppner W, Mason B. Impact of patient aligned care team interprofessional care updates (ICU) on metabolic parameters. Fed Pract. 2016;33(2):44-48.
Physician, nurse practitioner trainees, medical center faculty, and clinic staff develop proactive, team-based, interprofessional care plans to address unmet chronic care needs for high-risk patients.
Physician, nurse practitioner trainees, medical center faculty, and clinic staff develop proactive, team-based, interprofessional care plans to address unmet chronic care needs for high-risk patients.
This article is part of a series that illustrates strategies intended to redesign primary care education at the Veterans Health Administration (VHA), using interprofessional workplace learning. All have been implemented in the VA Centers of Excellence in Primary Care Education (CoEPCE). These models embody visionary transformation of clinical and educational environments that have potential for replication and dissemination throughout VA and other primary care clinical educational environments. For an introduction to the series see Klink K. Transforming primary care clinical learning environments to optimize education, outcomes, and satisfaction. Fed Pract. 2018;35(9):8-10.
Background
In 2011, 5 US Department of Veterans Affairs (VA) medical centers (VAMCs) were selected by the Office of Academic Affiliations (OAA) to establish CoEPCE. Part of the VA New Models of Care initiative, the 5 Centers of Excellence (CoE) in Boise, Idaho; Cleveland, Ohio; San Francisco, California; Seattle, Washington; and West Haven, Connecticut, are utilizing VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurse residents and undergraduate nursing students, and other professions of health trainees (eg, pharmacy, social work, psychology, physician assistants [PAs]) for primary care practice in the 21st century.
The Boise CoE developed and implemented a practice-based learning model. Nurse practitioner (NP) students and residents, physician residents, pharmacy residents, psychology interns, and psychology postdoctoral fellows participate in a comprehensive curriculum and practice together for 1 to 3 years. The goal is to produce providers who are able to lead and practice health care in patient-centered primary care and rural care environments. All core curricula are interprofessionally coauthored and cotaught.1
Methods
In 2015, OAA evaluators reviewed background documents and conducted open-ended interviews with 10 CoE staff, participating trainees, VA faculty, VA facility leadership, and affiliate faculty. In response to questions focused on their experiences, informants described lessons learned, challenges encountered, and benefits for participants, veterans, and the VA. Using a qualitative and quantitative approach, this case study draws on those interviews, surveys of PACT ICU (patient aligned care team interprofessional care update) participants, and analysis of presented patients to examine PACT ICU outcomes.
Interprofessional Education and Care
A key CoEPCE aim is to create more clinical opportunities for CoE trainees from a variety of professions to work as a team in ways that anticipate and address the care needs of veterans. This emphasis on workplace learning is needed since most current health care professional education programs lack settings where trainees from different professions can learn and work together with their clinic partners to provide care for patients. With the emphasis on patient-centered medical homes (PCMH) and team-based care in the Affordable Care Act, there is an imperative to develop new training models that address this gap in the preparation of future health professionals. Along with this imperative, clinicians are increasingly required to optimize the health of complex patients who consequently require a multidisciplinary approach to care, particularly high-risk, high-needs patients inappropriately using services, such as frequent emergency department (ED) use.
Addressing Complex Needs
In 2010, the Boise VA Medical Center (VAMC) phased in patient aligned care teams (PACTs), the VA-mandated version of PCMH that consist of a physician or NP primary care provider (PCP), a registered nurse (RN) care manager, a licensed vocational nurse (LVN), and a medical support assistant (MSA).
The PACT ICU also serves as a venue in which trainees and supervisors from different professions use a patient-centered framework to collaborate on these specific patient cases. The PACT ICU is easily applied to a range of health conditions, such as diabetes mellitus (DM), mental and behavioral health, lack of social support, and delivery system issues, such as ED use. The goals of PACT ICU are to improve the quality and satisfaction of patient care for high-risk patients; encourage appropriate use of health care resources by prioritizing continuity with the PACT team; and enhance interprofessional PACT team function, decreasing PCP and staff stress.
Planning and Implementation
In January 2013, Boise VAMC and the Caldwell, Idaho community-based outpatient clinic (CBOC) implemented PACT ICU. Other nonteaching clinics followed later in the year. Planning and executing PACT ICU took about 10 hours of CoE staff time and required no change in Boise VAMC policy. Program leadership approval was necessary for participation of CoE residents and postdocs. Service-line leadership support was required to protect clinic staff time (nurse care manager, social workers, chaplaincy, and ethics service). At the Caldwell CBOC, the section chief approved the program, and it took about 1 month to initiate a similar version of PACT ICU.
Curriculum
PACT ICU is a workplace clinical activity with roots in the case conference model, specifically the EFECT model (Elicit the narrative of illness, Facilitate a group meeting, Evidence-based gap analysis, Care plan, and Track changes).3 PACT ICU emphasizes a patient-centered approach to developing care plans. Staff review the 5 highest risk patients who are identified by the VA Care Assessment Need (CAN) registry. The CAN is an analytic tool that is available throughout VA and estimates patients’ risk of mortality or hospitalization in the following 90 days. Physician and NP residents choose 1 of the 5 patients to discuss in PACT ICU, while the remaining 4 serve as case-control comparisons to examine long-term patient outcomes. All trainees, faculty, and staff are provided patient data that can be discussed on a secure website.
The PACT ICU combines didactic teaching with workplace learning. For example, the patient’s medical issues may lead to a formal presentation about a topic, such as secondary stroke medication prophylaxis. The workplace learning occurs as the trainees observe and participate in the decision making toward a treatment plan. Interprofessional interactions are role-modeled by clinical faculty and staff during these discussions, and the result impact the patients care. PACT ICU embodies the core domains that shape the CoEPCE curriculum: Interprofessional collaboration (IPC), performance improvement (PI), sustained relationships (SR), and shared decision making (SDM) (Table 1).
There have been some changes to the PACT ICU model over time. Initially, conferences took place on a weekly basis, to build momentum among the team and to normalize processes. After about 2 years, this decreased to every other week to reduce the time burden. Additionally, the CoE has strengthened the “tracking changes” component of the EFECT model—trainees now present a 5-minute update on the last patient they presented at the prior PACT ICU case conference. Most recently, psychology postdoctoral candidates have instituted preconference calls with patients to further improve the teams understanding of the patients’ perspective and narrative.
Related: Improving Team-Based Care Coordination Delivery and Documentation in the Health Record
Resources
The CoE faculty participate in an education program concerning facilitation of interprofessional meetings. All faculty are expected to role model collaborative behavior and mentor trainees on the cases they present.
The PACT ICU requires a room large enough to accommodate at least 12 people. One staff member is required to review patient cases prior to the case conferences (usually about 1 hour of preparation per case conference). Another staff person creates and shares a spreadsheet stored with VA-approved information security with data fields to include the site, PACT ICU date, patient identifier, the CAN score, and a checkbox for whether the patient was selected or part of a control group. Logistic support is required for reserving the room and sending information to presenters. A clinic-based RN with training in interprofessional care case management uses an online schedule to facilitate selection and review of patients. The RN care managers can use a secure management tool to track patient care and outreach.
The RN care manager also needs to be available to attend the PACT ICU case conferences. The Boise CoE built a website to share and standardize resources, such as a presenter schedule, PACT ICU worksheet, and provider questionnaire. (Contact Boise CoE staff for access.) For the initial evaluation of impact, PACT ICU utilized staff data support in the form of a data manager and biostatistician to identify, collect, and analyze data. While optional, this was helpful in refining the approach and demonstrating the impact of the project. Other resource-related requirements for exporting PACT ICU include:
- Staff members, usually RN care managers who coordinate meetings with participants and identify appropriate patients using a registry, such as CAN;
- Meeting facilitators who enforce use of the EFECT model and interprofessional participation to ensure that the interprofessional care plan is carried out by the presenting provider; and
- Interprofessional trainees and faculty who participate in PACT ICU and complete surveys after the first conference.
Monitoring and Assessment
The CoE staff have analyzed the evaluation of PACT ICU with participant self-evaluation, consultation referral patterns, and utilization data, combination of ED and episodic care visits along with hospitalizations).4 Pharmacy faculty are exploring the use polypharmacy registries, and psychology will use registries of poor psychosocial function.
Partnerships
Beyond support and engagement from VA CoEPCE and affiliate faculty, PACT ICU has greatly benefited from partnerships with VA facility department and CBOC leadership. The CoEPCE codirector and faculty are in facility committees, such as the PACT Strategic Planning Committee.
Academic affiliates are integral partners who assist with NP student and resident recruitment as well as participate in the planning and refinement of CoEPCE components. PACT ICU supports their mandate to encourage interprofessional teamwork. Faculty members from Gonzaga University (NP affiliate) were involved in the initial discussion on PACT ICU and consider it a “learning laboratory” to work through challenging problems. Gonzaga CoEPCE NP trainees are asked to talk about their PACT ICU experience—its strengths, weaknesses, and challenges—to other Gonzaga students who don’t have exposure to the team experience.
Challenges and Solutions
The demand for direct patient care puts pressure on indirect patient care approaches like PACT ICU, which is a time-intensive process with high impact on only a small number of patients. The argument for deploying strategies such as PACT ICU is that managing chronic conditions and encouraging appropriate use of services will improve outcomes for the highest risk patients and save important system resources in the long-run. However, in the short-term, a strong case must be made for the diversion of resources from usual clinic flow, particularly securing recurring blocks of provider time and clinic staff members. In addition, issues about team communication and understanding of appropriate team-based care can overflow to complex patients not presented in the PACT ICU conference.
Providing a facilitated interprofessional venue to discuss how to appropriately coordinate care improves the participation and perceived value of different team members. This approach has led to improved engagement of the team for patients discussed in the PACT ICU, as well as in general care within the participating clinic. With recent changes, the VA does see a workload benefit, and participants get encounter credit through “Non face-to-face prolonged service” codes (CPT 99358/99359), and other possibilities exist related to clinical team conference codes (CPT 99367-8) and complex chronic care management codes (CPT 99487-89). More information on documentation, scheduling and encountering/billing can be found at boisevacoe.org under Products. Other challenges include logistic challenges of finding appropriate patients and distributing sensitive patient information among the team. Additionally, PACT ICU has to wrestle with staffing shortages and episodic participation by some professions that are chronically understaffed. We have addressed many of these problems by receiving buy-in from both leadership and participants. Leadership have allowed time for participation in clinic staff schedules, and each participant has committed to recruiting a substitute in case of a schedule conflict.
Factors for Success
The commitment from the Boise VAMC facility, primary care clinic leadership and affiliated training programs to support staff and trainee participation also has been critical. Additionally, VA facility leadership commitment to ongoing improvements to PACT implementation was a key facilitating factor. Colocation of trainees and clinic staff on the academic PACT team facilitates communication between PACT ICU case conferences, while also supporting team dynamics and sustained relationships with patients. Many of these patients can and will typically seek care using the interdisciplinary trainees, and trainees were motivated to proactively coordinate warm handoffs and other models of transfer of care. PACT ICU has been successfully replicated and sustained at 4 of the 5 CoEPCE sites. The Caldwell CBOC PACT ICU has been up and running for 2 years, and 2 other nonacademic clinics have piloted PACT ICU managed care conferences thus far. Experience regarding the implementation at other academic sites has been published.5
Accomplishments and Benefits
There is evidence that PACT ICU is achieving its goals of improving trainee learning and patient outcomes. Trainees are using team skills to provide patient-centered care; trainees are strengthening their overall clinical skills by learning how to improve their responses to high-risk patients. There is also evidence of an increase in interprofessional warm handoffs within the clinic, in which “a clinician directly introduces a patient to another clinician at the time of the patient’s visit, and often a brief encounter between the patient and the health care professional occurs.”4,6
Unlike a traditional didactic with classroom case conferences on interprofessional collaboration, PACT ICU is an opportunity for health care professionals to both learn and work together providing care in a clinic. Moreover, colocation of diverse trainee and faculty professions during the case conferences better prepares trainees to work with other professions and supports all participants to work and communicate as a team.
CoE staff have assessed educational outcomes before and after attendance in PACT ICU. On average, trainees (n = 30) said they found the PACT ICU case conferences to be “very helpful” in developing treatment plans.
Interprofessional Collaboration
Team building and colocating trainees, faculty, and clinic staff from different professions are a primary focus of PACT ICU. The case conferences are designed to break down silos and foster a team approach to care. Trainees learn how the team works and the ways other professionals can help them take care of the patient. For example, trainees learn early about the contributions and expertise that the pharmacist and psychologist offer in terms of their scope of practice and referral opportunities. Additionally, the RN care manager increases the integration with the PACT clinical team by sharing pertinent information on individual patients. Based on recent trainee survey findings, the CoE has observed a positive change in the team dynamic and trainee ability to interface between professions. PACT ICU participants were more likely to make referrals to other members within the PACT team, such as a warm handoff during a clinic appointment, while they were less likely to seek a consult outside the team.7
Clinical Performance
The PACT ICU is an opportunity for a trainee to increase clinical expertise. It provides exposure to a variety of patientsand their care needs and serves as an opportunity to present a high-risk, challenging patient to colleagues of various professions. As of June 2018, 96 physician resident and NP residents have presented complex patient cases.
In addition, a structured forum for discussing patients and their care options strengthens team clinical performance, which supports people to work to the full scope of their practice. Trainees learn and apply team skills, such as communication and the warm handoff.
An interprofessional care plan that is delineated during the meeting supports the trainee and is carried out with help from consultants as needed. These consultants often facilitate plans for a covisit or warm handoff at the next clinic visit, a call from the RN care manager, a virtual clinic appointment, or other nontraditional visits. The clinic staff can get information from PCPs about patient’s plan of care, and PCPs get a more complete picture of a patient’s situation (eg, history, communications, and life-style factors). In addition, surveys of PACT ICU participants suggest the curriculum’s effectiveness at encouraging use of PACT principles within the clinic team and improving appropriate referrals to other members of the PACT team, such as pharmacy and behavioral health.
Patients presented at PACT ICU can be particularly challenging, so there may be a psychological benefit to working with a team to develop a new care plan. The PCPs who feel they are overwhelmed and have exhausted every option step back, get input, and look at the patient in a new light.
Related: Interprofessional Education in Patient Aligned Care Team Primary Care-Mental Health Integration
CoEPCE Function
The PACT ICU is flexible and has been adapted to different ambulatory care settings. Currently, PACT ICU case conferences take place at Boise VAMC, the Caldwell CBOCs, and more recently at a smaller CBOC in Burns, Oregon. The PACT ICU structure is slightly different in the clinic settings since the VA primary care clinic has different resources to draw upon, such as hospital and specialty services. The Caldwell CBOC was unable to protect time for PCPs, so it holds a monthly PACT ICU case conference. In addition to continuing expansion in other nonacademic PACT clinics and collaboration with other CoEPCE sites, work is underway to disseminate generalizable principles for interprofessional education, as well as exporting the model for implementation in non-VA settings.
Primary Care Services
The PACT ICU has the potential to create efficiencies in busy clinic settings. It strengthens communication between PCPs and is an opportunity to touch base on the patient, delegate care, and keep track of high-risk patients who might otherwise receive attention only when having an acute problem. Nurses gain a deeper understanding of the patients presented at PACT ICU.
PACT ICU leverages and builds on existing PACT resources in an achievable and sustainable manner benefiting primary care. CoE trainees, who are part of the Silver Team, tap in to the information that team nurses gain from checking in with these high-risk patients biweekly. Moreover, the integration with the Silver Team improves continuity, which helps enhance a patient’s level of trust. The relationship strengthened between primary care and behavioral health at the Caldwell CBOC, providing improved patient access and increased professional sharing.
Patient Outcomes
The PACT ICU provides a forum for input beyond that of the PCP. This feature results in a more robust treatment plan than might be developed by individual PCPs who might not have time to consider options that are outside their scope of practice. Formulating an enriched care plan, informed by multiple professions, has the potential to improve utilization and provide better care.
The Boise VAMC PACT ICU has presented 219 patients as of June 2018. While clinical outcomes data are difficult to collect, the CoE has data on utilization differences on all patients presented at the PACT ICU case conferences. This includes 4 control patients from the same PCP, with similarly high risk based on CAN scores at the time of selection. A single control patient is selected based on gender, closest age, and CAN score; this serves as a comparator for subsequent utilization analysis.
Data from the first 2 years of this study demonstrate that compared with the high-risk control group, there was an increase in contacts with PACT team members, including behavioral health, clinical pharmacists, and nurse care management, persisting up to 6 months following the PACT ICU presentation.4 However, PACT ICU participation did not increase the number of visits with the PCP, indicating better engagement with the entire team. Participation was associated with significantly decreased hospitalizations and a trend toward decreased ED visits. These findings persisted when compared with controls in the PCP’s panel with similar CAN scores, making “regression to the mean” often seen in these studies much less likely.
Analysis of patients early in the project suggests the possibility of improved glycemic control in patients with DM and improved blood pressure control in hypertensive patients presented at the PACT ICU compared with that of non-PACT ICU patients.8 Another potential benefit includes better team-based coordination. Because the patient now has a team focusing on care, this new dynamic results in improving outreach, identifying patients who could receive care by a telephone, and better preparing team members to establish rapport when the patient calls or comes in for a visit.
The Future
In stage 2 of the CoEPCE program, a multi-site trial of PACT ICU was completed to better understand which elements are critical to success, with the goal of facilitating broader exportability.5 The trial focused on 3 intertwined elements: structure, delivery, and evaluation. Using local implementation and the multisite trial, the most effective practices have been documented as part of an implementation kit, available at boisevacoe.org. The goal of the implementation kit is to facilitate step-by-step implementation of PACT ICU to other settings beyond the multisite study. Since the open-ended structure of PACT ICU enables accommodating different professions and specialties beyond the model’s Boise VAMC participants, it could be easily adapted to potentially support a variety of implementations elsewhere (Appendix).
Another opportunity for expansion is increased patient involvement. Currently, PACT ICU patients have the opportunity to review and ask questions about their multidisciplinary care plans before starting.
This article is part of a series that illustrates strategies intended to redesign primary care education at the Veterans Health Administration (VHA), using interprofessional workplace learning. All have been implemented in the VA Centers of Excellence in Primary Care Education (CoEPCE). These models embody visionary transformation of clinical and educational environments that have potential for replication and dissemination throughout VA and other primary care clinical educational environments. For an introduction to the series see Klink K. Transforming primary care clinical learning environments to optimize education, outcomes, and satisfaction. Fed Pract. 2018;35(9):8-10.
Background
In 2011, 5 US Department of Veterans Affairs (VA) medical centers (VAMCs) were selected by the Office of Academic Affiliations (OAA) to establish CoEPCE. Part of the VA New Models of Care initiative, the 5 Centers of Excellence (CoE) in Boise, Idaho; Cleveland, Ohio; San Francisco, California; Seattle, Washington; and West Haven, Connecticut, are utilizing VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurse residents and undergraduate nursing students, and other professions of health trainees (eg, pharmacy, social work, psychology, physician assistants [PAs]) for primary care practice in the 21st century.
The Boise CoE developed and implemented a practice-based learning model. Nurse practitioner (NP) students and residents, physician residents, pharmacy residents, psychology interns, and psychology postdoctoral fellows participate in a comprehensive curriculum and practice together for 1 to 3 years. The goal is to produce providers who are able to lead and practice health care in patient-centered primary care and rural care environments. All core curricula are interprofessionally coauthored and cotaught.1
Methods
In 2015, OAA evaluators reviewed background documents and conducted open-ended interviews with 10 CoE staff, participating trainees, VA faculty, VA facility leadership, and affiliate faculty. In response to questions focused on their experiences, informants described lessons learned, challenges encountered, and benefits for participants, veterans, and the VA. Using a qualitative and quantitative approach, this case study draws on those interviews, surveys of PACT ICU (patient aligned care team interprofessional care update) participants, and analysis of presented patients to examine PACT ICU outcomes.
Interprofessional Education and Care
A key CoEPCE aim is to create more clinical opportunities for CoE trainees from a variety of professions to work as a team in ways that anticipate and address the care needs of veterans. This emphasis on workplace learning is needed since most current health care professional education programs lack settings where trainees from different professions can learn and work together with their clinic partners to provide care for patients. With the emphasis on patient-centered medical homes (PCMH) and team-based care in the Affordable Care Act, there is an imperative to develop new training models that address this gap in the preparation of future health professionals. Along with this imperative, clinicians are increasingly required to optimize the health of complex patients who consequently require a multidisciplinary approach to care, particularly high-risk, high-needs patients inappropriately using services, such as frequent emergency department (ED) use.
Addressing Complex Needs
In 2010, the Boise VA Medical Center (VAMC) phased in patient aligned care teams (PACTs), the VA-mandated version of PCMH that consist of a physician or NP primary care provider (PCP), a registered nurse (RN) care manager, a licensed vocational nurse (LVN), and a medical support assistant (MSA).
The PACT ICU also serves as a venue in which trainees and supervisors from different professions use a patient-centered framework to collaborate on these specific patient cases. The PACT ICU is easily applied to a range of health conditions, such as diabetes mellitus (DM), mental and behavioral health, lack of social support, and delivery system issues, such as ED use. The goals of PACT ICU are to improve the quality and satisfaction of patient care for high-risk patients; encourage appropriate use of health care resources by prioritizing continuity with the PACT team; and enhance interprofessional PACT team function, decreasing PCP and staff stress.
Planning and Implementation
In January 2013, Boise VAMC and the Caldwell, Idaho community-based outpatient clinic (CBOC) implemented PACT ICU. Other nonteaching clinics followed later in the year. Planning and executing PACT ICU took about 10 hours of CoE staff time and required no change in Boise VAMC policy. Program leadership approval was necessary for participation of CoE residents and postdocs. Service-line leadership support was required to protect clinic staff time (nurse care manager, social workers, chaplaincy, and ethics service). At the Caldwell CBOC, the section chief approved the program, and it took about 1 month to initiate a similar version of PACT ICU.
Curriculum
PACT ICU is a workplace clinical activity with roots in the case conference model, specifically the EFECT model (Elicit the narrative of illness, Facilitate a group meeting, Evidence-based gap analysis, Care plan, and Track changes).3 PACT ICU emphasizes a patient-centered approach to developing care plans. Staff review the 5 highest risk patients who are identified by the VA Care Assessment Need (CAN) registry. The CAN is an analytic tool that is available throughout VA and estimates patients’ risk of mortality or hospitalization in the following 90 days. Physician and NP residents choose 1 of the 5 patients to discuss in PACT ICU, while the remaining 4 serve as case-control comparisons to examine long-term patient outcomes. All trainees, faculty, and staff are provided patient data that can be discussed on a secure website.
The PACT ICU combines didactic teaching with workplace learning. For example, the patient’s medical issues may lead to a formal presentation about a topic, such as secondary stroke medication prophylaxis. The workplace learning occurs as the trainees observe and participate in the decision making toward a treatment plan. Interprofessional interactions are role-modeled by clinical faculty and staff during these discussions, and the result impact the patients care. PACT ICU embodies the core domains that shape the CoEPCE curriculum: Interprofessional collaboration (IPC), performance improvement (PI), sustained relationships (SR), and shared decision making (SDM) (Table 1).
There have been some changes to the PACT ICU model over time. Initially, conferences took place on a weekly basis, to build momentum among the team and to normalize processes. After about 2 years, this decreased to every other week to reduce the time burden. Additionally, the CoE has strengthened the “tracking changes” component of the EFECT model—trainees now present a 5-minute update on the last patient they presented at the prior PACT ICU case conference. Most recently, psychology postdoctoral candidates have instituted preconference calls with patients to further improve the teams understanding of the patients’ perspective and narrative.
Related: Improving Team-Based Care Coordination Delivery and Documentation in the Health Record
Resources
The CoE faculty participate in an education program concerning facilitation of interprofessional meetings. All faculty are expected to role model collaborative behavior and mentor trainees on the cases they present.
The PACT ICU requires a room large enough to accommodate at least 12 people. One staff member is required to review patient cases prior to the case conferences (usually about 1 hour of preparation per case conference). Another staff person creates and shares a spreadsheet stored with VA-approved information security with data fields to include the site, PACT ICU date, patient identifier, the CAN score, and a checkbox for whether the patient was selected or part of a control group. Logistic support is required for reserving the room and sending information to presenters. A clinic-based RN with training in interprofessional care case management uses an online schedule to facilitate selection and review of patients. The RN care managers can use a secure management tool to track patient care and outreach.
The RN care manager also needs to be available to attend the PACT ICU case conferences. The Boise CoE built a website to share and standardize resources, such as a presenter schedule, PACT ICU worksheet, and provider questionnaire. (Contact Boise CoE staff for access.) For the initial evaluation of impact, PACT ICU utilized staff data support in the form of a data manager and biostatistician to identify, collect, and analyze data. While optional, this was helpful in refining the approach and demonstrating the impact of the project. Other resource-related requirements for exporting PACT ICU include:
- Staff members, usually RN care managers who coordinate meetings with participants and identify appropriate patients using a registry, such as CAN;
- Meeting facilitators who enforce use of the EFECT model and interprofessional participation to ensure that the interprofessional care plan is carried out by the presenting provider; and
- Interprofessional trainees and faculty who participate in PACT ICU and complete surveys after the first conference.
Monitoring and Assessment
The CoE staff have analyzed the evaluation of PACT ICU with participant self-evaluation, consultation referral patterns, and utilization data, combination of ED and episodic care visits along with hospitalizations).4 Pharmacy faculty are exploring the use polypharmacy registries, and psychology will use registries of poor psychosocial function.
Partnerships
Beyond support and engagement from VA CoEPCE and affiliate faculty, PACT ICU has greatly benefited from partnerships with VA facility department and CBOC leadership. The CoEPCE codirector and faculty are in facility committees, such as the PACT Strategic Planning Committee.
Academic affiliates are integral partners who assist with NP student and resident recruitment as well as participate in the planning and refinement of CoEPCE components. PACT ICU supports their mandate to encourage interprofessional teamwork. Faculty members from Gonzaga University (NP affiliate) were involved in the initial discussion on PACT ICU and consider it a “learning laboratory” to work through challenging problems. Gonzaga CoEPCE NP trainees are asked to talk about their PACT ICU experience—its strengths, weaknesses, and challenges—to other Gonzaga students who don’t have exposure to the team experience.
Challenges and Solutions
The demand for direct patient care puts pressure on indirect patient care approaches like PACT ICU, which is a time-intensive process with high impact on only a small number of patients. The argument for deploying strategies such as PACT ICU is that managing chronic conditions and encouraging appropriate use of services will improve outcomes for the highest risk patients and save important system resources in the long-run. However, in the short-term, a strong case must be made for the diversion of resources from usual clinic flow, particularly securing recurring blocks of provider time and clinic staff members. In addition, issues about team communication and understanding of appropriate team-based care can overflow to complex patients not presented in the PACT ICU conference.
Providing a facilitated interprofessional venue to discuss how to appropriately coordinate care improves the participation and perceived value of different team members. This approach has led to improved engagement of the team for patients discussed in the PACT ICU, as well as in general care within the participating clinic. With recent changes, the VA does see a workload benefit, and participants get encounter credit through “Non face-to-face prolonged service” codes (CPT 99358/99359), and other possibilities exist related to clinical team conference codes (CPT 99367-8) and complex chronic care management codes (CPT 99487-89). More information on documentation, scheduling and encountering/billing can be found at boisevacoe.org under Products. Other challenges include logistic challenges of finding appropriate patients and distributing sensitive patient information among the team. Additionally, PACT ICU has to wrestle with staffing shortages and episodic participation by some professions that are chronically understaffed. We have addressed many of these problems by receiving buy-in from both leadership and participants. Leadership have allowed time for participation in clinic staff schedules, and each participant has committed to recruiting a substitute in case of a schedule conflict.
Factors for Success
The commitment from the Boise VAMC facility, primary care clinic leadership and affiliated training programs to support staff and trainee participation also has been critical. Additionally, VA facility leadership commitment to ongoing improvements to PACT implementation was a key facilitating factor. Colocation of trainees and clinic staff on the academic PACT team facilitates communication between PACT ICU case conferences, while also supporting team dynamics and sustained relationships with patients. Many of these patients can and will typically seek care using the interdisciplinary trainees, and trainees were motivated to proactively coordinate warm handoffs and other models of transfer of care. PACT ICU has been successfully replicated and sustained at 4 of the 5 CoEPCE sites. The Caldwell CBOC PACT ICU has been up and running for 2 years, and 2 other nonacademic clinics have piloted PACT ICU managed care conferences thus far. Experience regarding the implementation at other academic sites has been published.5
Accomplishments and Benefits
There is evidence that PACT ICU is achieving its goals of improving trainee learning and patient outcomes. Trainees are using team skills to provide patient-centered care; trainees are strengthening their overall clinical skills by learning how to improve their responses to high-risk patients. There is also evidence of an increase in interprofessional warm handoffs within the clinic, in which “a clinician directly introduces a patient to another clinician at the time of the patient’s visit, and often a brief encounter between the patient and the health care professional occurs.”4,6
Unlike a traditional didactic with classroom case conferences on interprofessional collaboration, PACT ICU is an opportunity for health care professionals to both learn and work together providing care in a clinic. Moreover, colocation of diverse trainee and faculty professions during the case conferences better prepares trainees to work with other professions and supports all participants to work and communicate as a team.
CoE staff have assessed educational outcomes before and after attendance in PACT ICU. On average, trainees (n = 30) said they found the PACT ICU case conferences to be “very helpful” in developing treatment plans.
Interprofessional Collaboration
Team building and colocating trainees, faculty, and clinic staff from different professions are a primary focus of PACT ICU. The case conferences are designed to break down silos and foster a team approach to care. Trainees learn how the team works and the ways other professionals can help them take care of the patient. For example, trainees learn early about the contributions and expertise that the pharmacist and psychologist offer in terms of their scope of practice and referral opportunities. Additionally, the RN care manager increases the integration with the PACT clinical team by sharing pertinent information on individual patients. Based on recent trainee survey findings, the CoE has observed a positive change in the team dynamic and trainee ability to interface between professions. PACT ICU participants were more likely to make referrals to other members within the PACT team, such as a warm handoff during a clinic appointment, while they were less likely to seek a consult outside the team.7
Clinical Performance
The PACT ICU is an opportunity for a trainee to increase clinical expertise. It provides exposure to a variety of patientsand their care needs and serves as an opportunity to present a high-risk, challenging patient to colleagues of various professions. As of June 2018, 96 physician resident and NP residents have presented complex patient cases.
In addition, a structured forum for discussing patients and their care options strengthens team clinical performance, which supports people to work to the full scope of their practice. Trainees learn and apply team skills, such as communication and the warm handoff.
An interprofessional care plan that is delineated during the meeting supports the trainee and is carried out with help from consultants as needed. These consultants often facilitate plans for a covisit or warm handoff at the next clinic visit, a call from the RN care manager, a virtual clinic appointment, or other nontraditional visits. The clinic staff can get information from PCPs about patient’s plan of care, and PCPs get a more complete picture of a patient’s situation (eg, history, communications, and life-style factors). In addition, surveys of PACT ICU participants suggest the curriculum’s effectiveness at encouraging use of PACT principles within the clinic team and improving appropriate referrals to other members of the PACT team, such as pharmacy and behavioral health.
Patients presented at PACT ICU can be particularly challenging, so there may be a psychological benefit to working with a team to develop a new care plan. The PCPs who feel they are overwhelmed and have exhausted every option step back, get input, and look at the patient in a new light.
Related: Interprofessional Education in Patient Aligned Care Team Primary Care-Mental Health Integration
CoEPCE Function
The PACT ICU is flexible and has been adapted to different ambulatory care settings. Currently, PACT ICU case conferences take place at Boise VAMC, the Caldwell CBOCs, and more recently at a smaller CBOC in Burns, Oregon. The PACT ICU structure is slightly different in the clinic settings since the VA primary care clinic has different resources to draw upon, such as hospital and specialty services. The Caldwell CBOC was unable to protect time for PCPs, so it holds a monthly PACT ICU case conference. In addition to continuing expansion in other nonacademic PACT clinics and collaboration with other CoEPCE sites, work is underway to disseminate generalizable principles for interprofessional education, as well as exporting the model for implementation in non-VA settings.
Primary Care Services
The PACT ICU has the potential to create efficiencies in busy clinic settings. It strengthens communication between PCPs and is an opportunity to touch base on the patient, delegate care, and keep track of high-risk patients who might otherwise receive attention only when having an acute problem. Nurses gain a deeper understanding of the patients presented at PACT ICU.
PACT ICU leverages and builds on existing PACT resources in an achievable and sustainable manner benefiting primary care. CoE trainees, who are part of the Silver Team, tap in to the information that team nurses gain from checking in with these high-risk patients biweekly. Moreover, the integration with the Silver Team improves continuity, which helps enhance a patient’s level of trust. The relationship strengthened between primary care and behavioral health at the Caldwell CBOC, providing improved patient access and increased professional sharing.
Patient Outcomes
The PACT ICU provides a forum for input beyond that of the PCP. This feature results in a more robust treatment plan than might be developed by individual PCPs who might not have time to consider options that are outside their scope of practice. Formulating an enriched care plan, informed by multiple professions, has the potential to improve utilization and provide better care.
The Boise VAMC PACT ICU has presented 219 patients as of June 2018. While clinical outcomes data are difficult to collect, the CoE has data on utilization differences on all patients presented at the PACT ICU case conferences. This includes 4 control patients from the same PCP, with similarly high risk based on CAN scores at the time of selection. A single control patient is selected based on gender, closest age, and CAN score; this serves as a comparator for subsequent utilization analysis.
Data from the first 2 years of this study demonstrate that compared with the high-risk control group, there was an increase in contacts with PACT team members, including behavioral health, clinical pharmacists, and nurse care management, persisting up to 6 months following the PACT ICU presentation.4 However, PACT ICU participation did not increase the number of visits with the PCP, indicating better engagement with the entire team. Participation was associated with significantly decreased hospitalizations and a trend toward decreased ED visits. These findings persisted when compared with controls in the PCP’s panel with similar CAN scores, making “regression to the mean” often seen in these studies much less likely.
Analysis of patients early in the project suggests the possibility of improved glycemic control in patients with DM and improved blood pressure control in hypertensive patients presented at the PACT ICU compared with that of non-PACT ICU patients.8 Another potential benefit includes better team-based coordination. Because the patient now has a team focusing on care, this new dynamic results in improving outreach, identifying patients who could receive care by a telephone, and better preparing team members to establish rapport when the patient calls or comes in for a visit.
The Future
In stage 2 of the CoEPCE program, a multi-site trial of PACT ICU was completed to better understand which elements are critical to success, with the goal of facilitating broader exportability.5 The trial focused on 3 intertwined elements: structure, delivery, and evaluation. Using local implementation and the multisite trial, the most effective practices have been documented as part of an implementation kit, available at boisevacoe.org. The goal of the implementation kit is to facilitate step-by-step implementation of PACT ICU to other settings beyond the multisite study. Since the open-ended structure of PACT ICU enables accommodating different professions and specialties beyond the model’s Boise VAMC participants, it could be easily adapted to potentially support a variety of implementations elsewhere (Appendix).
Another opportunity for expansion is increased patient involvement. Currently, PACT ICU patients have the opportunity to review and ask questions about their multidisciplinary care plans before starting.
1. Rugen KW, Watts S, Janson S, et al. Veteran Affairs centers of excellence in primary care education: transforming nurse practitioner education. Nurs Outlook. 2014;62(2):78-88.
2. Billett S. Learning through practice: beyond informal and towards a framework for learning through practice. UNESCO-UNEVOC. https://unevoc.unesco.org/fileadmin/up/2013_epub_revisiting_global_trends_in_tvet_chapter4.pdf. Published 2013. Accessed August 30, 2018.
3. Bitton A, Pereira AG, Smith CS, Babbott SF, Bowen JL. The EFECT framework for interprofessional education in the patient centered medical home. Healthc (Amst). 2013;1(3-4):63-68.
4. Weppner WG, Davis K, Tivis R, et al. Impact of a complex chronic care patient case conference on quality and utilization. Transl Behav Med. 2018;8(3):366-374.
5. King IC, Strewler A, Wipf JE. Translating innovation: exploring dissemination of a unique case conference. J Interprof Educ Pract. 2017;6(1):55-60.
6. Cohen DJ, Balasubramanian BA, Davis M, et al. Understanding care integration from the ground up: five organizing constructs that shape integrated practices. J Am Board Fam Med. 2015;28(suppl 1):S7-S20.
7. Weppner WG, Davis K, Sordahl J, et al. Interprofessional care conferences for high risk primary care patients. Acad Med. 2016;91(6):798-802.
8. Buu J, Fisher A, Weppner W, Mason B. Impact of patient aligned care team interprofessional care updates (ICU) on metabolic parameters. Fed Pract. 2016;33(2):44-48.
1. Rugen KW, Watts S, Janson S, et al. Veteran Affairs centers of excellence in primary care education: transforming nurse practitioner education. Nurs Outlook. 2014;62(2):78-88.
2. Billett S. Learning through practice: beyond informal and towards a framework for learning through practice. UNESCO-UNEVOC. https://unevoc.unesco.org/fileadmin/up/2013_epub_revisiting_global_trends_in_tvet_chapter4.pdf. Published 2013. Accessed August 30, 2018.
3. Bitton A, Pereira AG, Smith CS, Babbott SF, Bowen JL. The EFECT framework for interprofessional education in the patient centered medical home. Healthc (Amst). 2013;1(3-4):63-68.
4. Weppner WG, Davis K, Tivis R, et al. Impact of a complex chronic care patient case conference on quality and utilization. Transl Behav Med. 2018;8(3):366-374.
5. King IC, Strewler A, Wipf JE. Translating innovation: exploring dissemination of a unique case conference. J Interprof Educ Pract. 2017;6(1):55-60.
6. Cohen DJ, Balasubramanian BA, Davis M, et al. Understanding care integration from the ground up: five organizing constructs that shape integrated practices. J Am Board Fam Med. 2015;28(suppl 1):S7-S20.
7. Weppner WG, Davis K, Sordahl J, et al. Interprofessional care conferences for high risk primary care patients. Acad Med. 2016;91(6):798-802.
8. Buu J, Fisher A, Weppner W, Mason B. Impact of patient aligned care team interprofessional care updates (ICU) on metabolic parameters. Fed Pract. 2016;33(2):44-48.
Role of Point-of-Care Ultrasonography in the Evaluation and Management of Kidney Disease
Imaging at the nephrology point of care provides an important and continuously expanding tool to improve diagnostic accuracy in concert with history and physical examination.
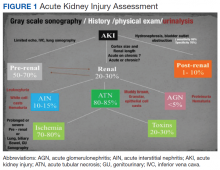
The evaluation of acute kidney injury (AKI) often starts with the classic prerenal, renal, and postrenal causalities, delineating a practical workable approach in its differential diagnosis. Accordingly, the history, physical examination, urinalysis, and kidney-bladder sonography are standard resources in the initial approach to renal disease assessment. Ultrasonography has a well-established role as an important adjuvant for postrenal diagnosis of renal failure. Nevertheless, most of the causes of AKI are prerenal and renal.
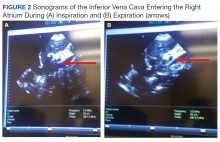
Some etiologies of kidney injury are sequelae of systemic diseases in which sonography can be diagnostically analogous to the history and physical examination. Furthermore, ultrasonography may be informative in various clinical scenarios, for example, patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD). In this narrative review, the contribution of point-of-care (POC) sonography to the evaluation and management of AKI, CKD, and associated diseases are explored beyond the traditional sonogram uses for kidney biopsy, central catheter placement, and/or screening of hydronephrosis.
Two important elements made possible the incorporation of POC sonography into nephrology practice.1,2 First, the development of handheld reliable and portable ultrasound devices and, second, the derived capacity of POC sonography to obtain objective signs of physiologic and/or pathophysiologic phenomena. The latter clinical application is realized through the incorporation of POC protocols into the modified focused assessment with sonography for trauma (FAST) examination in conjunction with limited echocardiography and lung sonography (Figure 1).
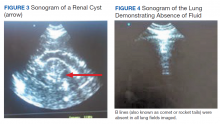
These protocols have allowed the evaluation of extracellular volume, which is important to measure for the diagnosis and management of renal diseases. For example, the evaluation of lung water by POC ultrasonography for patients with ESRD is emerging as a promising tool. In a study of patients with ESRD undergoing hemodialysis, POC ultrasonography detected moderate-to-severe lung congestion in 45% of patients, most of whom (71%) were asymptomatic. Two years of follow-up of patients was associated with 3 to 4 times greater risk of heart attack and death, respectively, compared with individuals without congestion on sonography.4-6 Thus, ultrasound assessment of lung water in patients with ESRD may prove to be an essential tool to assure an adequate ultrafiltration and improve patient outcomes.
Related: Nephrogenic Systemic Fibrosis in a Patient With Multiple Inflammatory Disorders
Acute Kidney Injury
Prerenal
The physical examination provides evaluation of effective arterial circulatory flow (EACF) and is clinically useful in the evaluation of prerenal azotemia. The utility is more obvious in the extremes of EACF. However, in the case of blood volume losses of > 10% or the physiologic equivalent, heart rate, blood pressure, skin turgor, urinary output, and capillary refill may be within normal limits. Obvious changes in these parameters during the physical examination are considered relatively late manifestations.7-10 Therefore, prerenal failure is frequently diagnosed retrospectively after correction of the EACF through use of crystalloids, blood products, vasopressors, inotropic agents, discontinuation of antihypertensive agents, or treatment of its prerenal causes. Certain sonographic maneuvers, performed at the bedside during acute renal injury, may be useful in many patients to evaluate a multitude of prerenal causes of AKI.
Sonographic inferior vena cava (IVC) luminal diameter and inspiratory collapsibility together serve as a surrogate marker of preload venous return and right side heart function. Such imaging results have been shown to be more accurate than jugular venous distension on physical examination but only modestly helpful as a surrogate for central venous pressure (CVP), with more accuracy in the lower values of the CVP.11 However, this procedure can be repeated often after volume resuscitation to achieve a 1.5- to 2.5-cm diameter dimension of the IVC and < 25% inspiratory collapsibility as a goal.
An IVC with a diameter > 2.5 cm in the context of a suspected prerenal AKI is more likely the consequence of heart failure (HF) rather than hypovolemia. The caveat to this finding is that pulmonary hypertension may induce false-positive results.12,13 Hepatic vein dilation is another sign of HF and/or pulmonary hypertension. Furthermore, sonographic images of the left ventricle either from the parasternal long axis or subxiphoid approach can identify supranormal left ventricular ejection fraction (LVEF) or hyperdynamic heart as an important clue of the absolute or relative decrease of EACF.14 Conversely, a decrease in EACF in patients with low LVEF can be assessed qualitatively at the bedside in patients with systolic HF. Supporting evidence of prerenal azotemia as the result of HF can be suggested by the presence of pleural effusions and bilateral comet/rockets tails or B lines in lung sonography.15
The easily recognizable hypoechoic ascitic fluid in the presence of small, hyperechoic gross changes in the echocardiographic texture of liver may indicate a hepatorenal component as the cause of prerenal failure. A small increase of > 20% in the diameter of the portal vein with deep inspiration indicates portal hypertension, with a sensitivity of 80% and a specificity of 100%.15,16 Other clinical scenarios leading to AKI in association with systemic hypotension may be identified quickly with the aid of POC sonography. These scenarios include cardiac tamponade, tension pneumothorax, right ventricular dysfunction (as a surrogate of pulmonary embolism), or an acute coronary event.16,17 Alternatively, identifying the presence of severe left ventricular hypertrophy through POC ultrasonography in a patient with AKI and normal or low normal blood pressures may alert clinicians to the diagnosis of normotensive renal failure in individuals with previously unrecognized severe hypertension. In this clinical context, keeping mean arterial pressures higher than usual with vasopressors may improve renal function while decreasing dialysis utilization.18-21
Likewise, in clinical scenarios of shock with AKI, POC ultrasonography has proven to be an indispensable tool. For example, rapid exploration of the biliary tree demonstrating anterior gallbladder wall thickening, a stone or sludge, common bile duct dilation, or perigallbladder inflammation suggests acute cholecystitis and/or cholangitis as the cause. The presence of dyspnea in association with hypotension and unilateral signs of a higher proportion of comet tails and/or a lung consolidation suggests pneumonia. Rapid differentiation between acute respiratory distress syndrome (ARDS) and pulmonary edema from HF is possible with ultrasonography. When pleural line abnormalities are seen, ARDS is a common cause.
POC ultrasonography will be key in management of ARDS, as ultrasound results will help avoid the use of excessive diuretics, which can result in renal hypoperfusion and AKI.22 In trauma patients, the ultrasound examination will identify free fluid (bleeding) as the source of the prerenal failure, along with its cause (aortic dissection, hepatic hemorrhage, splenic hemorrhage, ectopic pregnancy, etc).23 Sonographic free air observed in the abdomen can provide the clue of a perforated viscus.24 The sonographic image of an inflamed pancreas can suggest pancreatitis as the cause of the systemic hypotension. Ultimately, intravascular losses in the hypoechoic edematous bowel wall in obstruction, ileus, pseudomembranous, or infectious or autoimmune enterocolitis can lead to significant decreases in the EACF and cause prerenal injury.
Related: Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
Intrinsic Renal Disease
In intrinsic AKI, acute tubular necrosis (ATN), glomerulonephritis, and interstitial nephritis are the typical causes. Although no signs are specific to each of the potential causes, a poor corticomedullary differentiation, kidney size < 9 cm, and cortex size < 1 cm help to distinguish CKD from AKI, especially if no previous serum creatinine values are available. The early diagnosis of ATN continues to be clinically relevant in the management of acute renal failure. Despite not being a practical tool for POC sonography currently, the use of bedside Doppler repetitive renal vasculature measures of resistive index predict occurrence and severity of ATN in the critical care setting and are an independent risk factor for poor survival in arterial hypertension and HF.25-30
Other POC sonographic evaluations of intrinsic AKI have been helpful in the following clinical scenarios. The presence of an ultrasonographic sign of sinusitis in the context of nephritic sediment and a rapid decline of renal function suggest antineutrophil cytoplasmic antibody (ANCA)-related vasculitis. Likewise, in younger adults, nephritic sediment and bilateral sonographic lung interstitial fluid in the absence of infection and a normal POC echocardiogram without significant edema elsewhere suggest glomerulonephritis in the category of pulmonary lung syndrome caused by antiglomerular basement membrane antibodies.
In the elderly, a similar systemic presentation suggests an ANCA vasculitis. Pleural effusion, synovitis, proteinuria, and/or hematuria will suggest lupus nephritis. Another important cause of acute renal failure in the critical care setting is intra-abdominal compartment syndrome. Here, bladder pressure measurement protocols are the standard of care. A human model evaluated the predictive value of intra-abdominal compartment syndrome pressures using the IVC square surface. In this study, a normal surface area of the IVC of > 1 cm2/m2 excluded the presence of intra-abdominal hypertension 87.5% of the time. However, the sensitivity of detection of the intra-abdominal hypertension was only 67.5% when the surface area of the IVC was < 1 cm2/m2.31
CKD and Associated Diseases
The diagnostic validity of ultrasonography is well established in adult-onset polycystic kidney disease. Bedside visualization of a parathyroid adenoma may be an important clue for a patient with CKD, echogenic kidneys, or nephrolithiasis with or without hypercalcemia to diagnose primary hyperparathyroidism. The sonographic diagnosis of abnormal parathyroid gland compared with parathyroid surgical exploration had a sensitivity, specificity, and positive predictive value of 74%, 96%, and 90%, respectively.32 In the clinical presentation of severe hypertension with headaches, ultrasonography at bedside can provide valuable diagnostic and risk assessment information of endocranial hypertension from measuring the optic nerve sheath. Sensitivity and specificity of papilledema was 90% and 79%, respectively, when 3.3 mm was the cutoff of the nerve sheath with a 30-degrees sign.33 The carotid artery intima media thickness measured on sonography correlates with the future development of atherogenesis, left ventricular hypertrophy, cognition deficits, CKD, and cardiovascular disease in asymptomatic patients. An intima media thickness of > 1.1 mm has been associated with a higher cardiovascular mortality.
Early initiation of antihypertensive medications and/or statins has been suggested to lower risk in these asymptomatic patients.34 The size and contour (smooth or irregular) of kidneys may provide clues to reflux nephropathy, dysplastic kidneys, radiation nephritis, or chronic pyelonephritis. The presence of nephrotic syndrome and abnormal free light chains ratio with a bedside echocardiogram showing the typical refractile myocardial walls with a peculiar speckled pattern is strongly suggestive of amyloidosis.35 Conditions associated with chronic hypercalcemia, medullary sponge kidney, milk alkali syndrome, sarcoidosis, and distal renal tubular acidosis are causes of nephrocalcinosis. Some degree of CKD is a constant feature in nephrocalcinosis. The initial imaging of choice in nephrocalcinosis and specially the medullary type is ultrasonography preferable to X-ray and perhaps to computed tomography.36
End-Stage Renal Disease
In a patient undergoing peritoneal dialysis with exit-site infection, the presence of > 1 mm radiolucent rim around the subcutaneous catheter after antibiotics has a bad prognosis and prompts catheter removal. This sonographic sign has a positive and negative predictive value for a tunneled infection of 84.6% and 94.1%, respectively.37,38 A risk factor for peritonitis in peritoneal dialysis is air in the peritoneum, which can be seen in one-third of patients. These individuals have 2.4 times more risk of peritonitis compared with patients without pneumoperitoneum. The sensitivity and specificity of sonographic detection of pneumoperitoneum is 94% and 100%, respectively, using the scissor technique.39 Proper training in performing home peritoneal dialysis decreases the incidence of pneumoperitoneum. Although not formally assessed, patient education and change in procedure techniques may decrease the incidence of pneumoperitoneum and peritonitis. The use of prelaparoscopic ultrasonography before insertion of the peritoneal dialysis catheter has detected intra-abdominal adhesions (visceral slide sign) with a sensitivity of 90% to 92%.40
History and physical examination are frequently helpful in the diagnosis of malfunctioning arteriovenous fistulas (AVF) for inflow or outflow disturbances, with sensitivity ranging from 70% to 100% and specificity ranging from 71% to 93% compared with angiography. Frequently, POC limited ultrasound can be helpful for a problematic AVF, either for cannulation or diagnosis. The congruence of duplex sonography with arteriogram is 85% to 96%. Various etiologies of a dysfunctional AVF (pseudo- or true aneurysm, poor development, stenosis, thrombi, or accessory veins) can be observed in the dialysis unit through limited sonography.41-44
After placement of a hemodialysis catheter using real-time ultrasonography, pneumohemothorax can be diagnosed reliably and rapidly. Catheter misplacement outside of the right atrium was detected by thoracic echocardiogram with a sensitivity of 96%, a specificity of 83%, and a positive predictive value of 98%.45,46 Ultimately, ultrasonography may replace chest X-ray in most cases after central vein dialysis catheter placement in the acute care setting.
Postrenal Failure
The sensitivity of ultrasonography to detect dilation to hydronephrosis of the pelvicaliceal system is well established. Sonography is the diagnostic examination of choice in pregnancy and the initial screening test for the nonpregnant patient. Computed tomography is the preferred imaging study in nephroureterolithiasis; however, due to ionizing radiation and cost, ultrasonography is gaining popularity for initial and/or follow-up evaluations. The ureteral jet is a relatively unexplored color and Doppler sonographic methodology that can provide insight into pelvicalyceal peristalsis, potentially yielding evidence of functional obstruction.47-51 Postvoid bladder residual volumes and bladder wall hypertrophy may provide important clues as to the cause(s) of the obstructive uropathy.
Telenephrology
In our institution, sonography is used in the evaluation of IVC, lungs, and kidneys via telemedicine. The probe is handled by trained nurses at the distant site.
Cardiac Arrest in ESRD
Patients with ESRD may have sudden cardiac arrest as a result of several etiologies. During the advance cardiac life support algorithm, there is a brief period of evaluation of the electrical rhythm in which echocardiography can be helpful with the diagnosis immediately after the 2 initial minutes of cardiopulmonary resuscitation. An enlarged right ventricular cavity (> 2/3 of the left ventricle) is a sonographic sign of a pulmonary embolism.
Bedside sonography has the potential to alter the current guidelines of advance cardiac life support management. For example, if the bedside echo shows a significant pericardial effusion, a pericardiocentesis could be performed faster as it would be diagnosed faster. In addition, at times the heart may appear to be beating rapidly but there is a small amount of fluid (blood) within the cardiac chambers. This may be from an extreme case of dehydration for which rapid administration of IV fluids may help manage. Therefore, a quick bedside point of care echocardiography may reveal a cardiac anomaly that may be able to be restored in a efficient manner.
Related: General Applications of Ultrasound in Rheumatology Practice
Conclusion
Ultrasonography at the POC provides an important and continuously expanding tool to improve nephrological diagnostic accuracy in concert with history and physical examination. Extracellular fluid evaluation is paramount in all kidney disease conditions. Recent clinical studies in lung ultrasonography suggest that the learning curve for the medical provider is quicker than with other organs. Because POC sonography in association with limited bedside echocardiography may reveal discriminatory signs of pneumonia and differentiate between cardiogenic vs noncardiogenic pulmonary edema, such imaging may be important cost-effective strategies in the management of dyspnea and in the categorization/etiology of AKI. Therefore, incorporation of POC sonography into clinical practice will require that medical schools, residency programs, and nephrology fellowship programs design teaching strategies within their respective curricula. Research studies with outcomes regarding diagnosis, morbidity, and mortality are necessary in these areas.
1. Remer EM, Papanicolaou N, Casalino DD, et al. ACR Appropriateness Criteria® on renal failure. Am J Med. 2014;127(11):1041-1048.e1.
2. Tublin M, Thurston W, Wilson SR. The kidney and urinary tract. In: Rumack C, Wilson S, Charboneau JW, Levine D, eds. Diagnostic Ultrasound. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:317-391.
3. Bahner D, Blaivas M, Cohen HL, et al; American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med. 2008;27(2):313-318.
4. Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging. 2010;3(6):586-594.
5. Enia G, Torino C, Panuccio V, et al; Lung Comets Cohort Working Group. Asymptomatic pulmonary congestion and physical functioning in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(8):1343-1348.
6. Zoccali C, Torino C, Tripepi R, et al; Lung US in CKD Working Group. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol. 2013;24(4):639-646.
7. Fortes MB, Owen JA, Raymond-Barker P, et al. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J Am Med Dir Assoc. 2015;16(3):221-228.
8. Jauregui J, Nelson D, Choo E, et al. The BUDDY (Bedside Ultrasound to Detect Dehydration in Youth) study. Crit Ultrasound J. 2014;6(1):15.
9. McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
10. Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
11. Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
12. Stawicki SP, Adkins EJ, Eiferman DS, et al. Prospective evaluation of intravascular volume status in critically ill patients: does inferior vena cava collapsibility correlate with central venous pressure? J Trauma Acute Care Surg. 2014;76(4):956-963.
13. Thanakitcharu P, Charoenwut M, Siriwiwatanakul N. Inferior vena cava diameter and collapsibility index: a practical non-invasive evaluation of intravascular fluid volume in critically-ill patients. J Med Assoc Thai. 2013;96(suppl 3):S14-S22.
14. Gustafsson M, Alehagen U, Johansson P. Pocket-sized ultrasound examination of fluid imbalance in patients with heart failure: a pilot and feasibility study of heart failure nurses without prior experience of ultrasonography. Eur J Cardiovasc Nurs. 2015;14(4):294-302.
15. Peguero A, Lamarche J, Courville C, Taha M, Antar-Shultz M. Ultrasonography to evaluate pulmonary edema resolution with blood pressure control in a hemodialysis patient. Abstract 263 presented at: 2016 Spring Clinical National Kidney Foundation Meeting; April 27-May 1, 2016; Boston, MA.
16. Bolondi L, Mazziotti A, Arienti V, et al. Ultrasonographic study of portal venous system in portal hypertension and after portosystemic shunt operations. Surgery. 1984;95(3):261-269.
17. Al-Nakshabandi NA. The role of ultrasonography in portal hypertension. Saudi J Gastroenterol. 2006;12(3):111-117.
18. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357(8):797-805.
19. Messerli FH. Clinical determinants and consequences of left ventricular hypertrophy. Am J Med. 1983;75(3A):51-56.
20. Chen SC, Su HM, Hung CC, et al. Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(12):2750-2758.
21. Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(7):496-507.
22. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16.
23. ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693.
24. Hefny AF, Abu-Zidan FM. Sonographic diagnosis of intraperitoneal free air. J Emerg Trauma Shock. 2011;4(4):511-513.
25. Meola M, Petrucci I. Ultrasound and color Doppler in nephrology. Acute kidney injury [in Italian]. G Ital Nefrol. 2012;29(5):599-615.
26. Corradi F, Brusasco C, Vezzani A, et al. Hemorrhagic shock in polytrauma patients: early detection with renal Doppler resistive index measurements. Radiology. 2011;260(1):112-118.
27. Viazzi F, Leoncini G, Derchi LE, Pontremoli R. Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. J Hypertens. 2014;32(1):149-153.
28. Marty P, Szatjnic S, Ferre F, et al. Doppler renal resistive index for early detection of acute kidney injury after major orthopaedic surgery : a prospective observational study. Eur J Anaesthesiol. 2015;32(1):37-43.
29. Kastelan S, Ljubicic N, Kastelan Z, Ostojic R, Uravic M. The role of duplex-doppler ultrasonography in the diagnosis of renal dysfunction and hepatorenal syndrome in patients with liver cirrhosis. Hepatogastroenterology. 2004;51(59):1408-1412.
30. Capotondo L, Nicolai GA, Garosi G. The role of color Doppler in acute kidney injury. Arch Ital Urol Androl. 2010;82(4):275-279.
31. Cavaliere F, Cina A, Biasucci D, et al. Sonographic assessment of abdominal vein dimensional and hemodynamic changes induced in human volunteers by a model of abdominal hypertension. Crit Care Med. 2011;39(2):344-348.
32. Tublin ME, Pryma DA, Yim JH, et al. Localization of parathyroid adenomas by sonography and technetium tc 99m sestamibi single-photon emission computed tomography before minimally invasive parathyroidectomy: are both studies really needed? J Ultrasound Med. 2009;28(2):183-190.
33. Carter SB, Pistilli M, Livingston KG, et al. The role of orbital ultrasonography in distinguishing papilledema from pseudopapilledema. Eye (Lond). 2014;28(12):1425-1430.
34. Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50-e103.
35. Huang Y, Zhan J, Wei X, et al. Clinical characteristics of 42 patients with cardiac amyloidosis. [Article in Chinese] Zhonghua Nei Ke Za Zhi. 2014;53(7):546-549.
36. Boyce AM, Shawker TH, Hill SC, et al. Ultrasound is superior to computed tomography for assessment of medullary nephrocalcinosis in hypoparathyroidism. J Clin Endocrinol Metab. 2013;98(3):989-994.
37. Kwan TH, Tong MK, Siu YP, Leung KT, Luk SH, Cheung YK. Ultrasonography in the management of exit site infections in peritoneal dialysis patients. Nephrology (Carlton). 2004;9(6):348-352.
38. Karahan OI, Taskapan H, Yikilmaz A, Oymak O, Utas C. Ultrasound evaluation of peritoneal catheter tunnel in catheter related infections in CAPD. Int Urol Nephrol. 2005;37(2):363-366.
39. Karahan OI, Kurt A, Yikilmaz A, Kahriman G. New method for the detection of intraperitoneal free air by sonography: scissors maneuver. J Clin Ultrasound. 2004;32(8):381-385.
40. Okamoto T, Ikenoue T, Matsui K, et al. Free air on CT and the risk of peritonitis in peritoneal dialysis patients: a retrospective study. Ren Fail. 2014;36(10):1492-1496.
41. Arshad FH, Sutijono D, Moore CL. Emergency ultrasound diagnosis of a pseudoaneurysm associated with an arteriovenous fistula. Acad Emerg Med. 2010;17(6):e43-e45.
42. Teodorescu V, Gustavson S, Schanzer H. Duplex ultrasound evaluation of hemodialysis access: a detailed protocol. Int J Nephrol. 2012;2012:508956.
43. Coentrão L, Turmel-Rodrigues L. Monitoring dialysis arteriovenous fistulae: it’s in our hands. J Vasc Access. 2013;14(3):209-215.
44. Chandra AP, Dimascio D, Gruenewald S, Nankivell B, Allen RD, Swinnen J. Colour duplex ultrasound accurately identifies focal stenoses in dysfunctional autogenous arteriovenous fistulae. Nephrology (Carlton). 2010;15(3):300-306.
45. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937.
46. Vezzani A, Brusasco C, Palermo S, Launo C, Mergoni M, Corradi F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538.
47. Celik S, Altay C, Bozkurt O, et al. Association between ureteral jet dynamics and nonobstructive kidney stones: a prospective-controlled study. Urology. 2014;84(5):1016-1020.
48. Tullus K. Does the ureteric jet Doppler waveform have a role in detecting vesicoureteric reflux? Pediatr Nephrol. 2013;28(9):1719-1721.
49. Jandaghi AB, Falahatkar S, Alizadeh A, et al. Assessment of ureterovesical jet dynamics in obstructed ureter by urinary stone with color Doppler and duplex Doppler examinations. Urolithiasis. 2013;41(2):159-163.
50. Pepe P, Motta L, Pennisi M, Aragona F. Functional evaluation of the urinary tract by color-Doppler ultrasonography (CDU) in 100 patients with renal colic. Eur J Radiol. 2005;53(1):131-135.
51. Leung VY, Metreweli C. Ureteric jet in renal transplantation patient. Ultrasound Med Biol. 2002;28(7):885-888.
Imaging at the nephrology point of care provides an important and continuously expanding tool to improve diagnostic accuracy in concert with history and physical examination.
Imaging at the nephrology point of care provides an important and continuously expanding tool to improve diagnostic accuracy in concert with history and physical examination.
The evaluation of acute kidney injury (AKI) often starts with the classic prerenal, renal, and postrenal causalities, delineating a practical workable approach in its differential diagnosis. Accordingly, the history, physical examination, urinalysis, and kidney-bladder sonography are standard resources in the initial approach to renal disease assessment. Ultrasonography has a well-established role as an important adjuvant for postrenal diagnosis of renal failure. Nevertheless, most of the causes of AKI are prerenal and renal.
Some etiologies of kidney injury are sequelae of systemic diseases in which sonography can be diagnostically analogous to the history and physical examination. Furthermore, ultrasonography may be informative in various clinical scenarios, for example, patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD). In this narrative review, the contribution of point-of-care (POC) sonography to the evaluation and management of AKI, CKD, and associated diseases are explored beyond the traditional sonogram uses for kidney biopsy, central catheter placement, and/or screening of hydronephrosis.
Two important elements made possible the incorporation of POC sonography into nephrology practice.1,2 First, the development of handheld reliable and portable ultrasound devices and, second, the derived capacity of POC sonography to obtain objective signs of physiologic and/or pathophysiologic phenomena. The latter clinical application is realized through the incorporation of POC protocols into the modified focused assessment with sonography for trauma (FAST) examination in conjunction with limited echocardiography and lung sonography (Figure 1).
These protocols have allowed the evaluation of extracellular volume, which is important to measure for the diagnosis and management of renal diseases. For example, the evaluation of lung water by POC ultrasonography for patients with ESRD is emerging as a promising tool. In a study of patients with ESRD undergoing hemodialysis, POC ultrasonography detected moderate-to-severe lung congestion in 45% of patients, most of whom (71%) were asymptomatic. Two years of follow-up of patients was associated with 3 to 4 times greater risk of heart attack and death, respectively, compared with individuals without congestion on sonography.4-6 Thus, ultrasound assessment of lung water in patients with ESRD may prove to be an essential tool to assure an adequate ultrafiltration and improve patient outcomes.
Related: Nephrogenic Systemic Fibrosis in a Patient With Multiple Inflammatory Disorders
Acute Kidney Injury
Prerenal
The physical examination provides evaluation of effective arterial circulatory flow (EACF) and is clinically useful in the evaluation of prerenal azotemia. The utility is more obvious in the extremes of EACF. However, in the case of blood volume losses of > 10% or the physiologic equivalent, heart rate, blood pressure, skin turgor, urinary output, and capillary refill may be within normal limits. Obvious changes in these parameters during the physical examination are considered relatively late manifestations.7-10 Therefore, prerenal failure is frequently diagnosed retrospectively after correction of the EACF through use of crystalloids, blood products, vasopressors, inotropic agents, discontinuation of antihypertensive agents, or treatment of its prerenal causes. Certain sonographic maneuvers, performed at the bedside during acute renal injury, may be useful in many patients to evaluate a multitude of prerenal causes of AKI.
Sonographic inferior vena cava (IVC) luminal diameter and inspiratory collapsibility together serve as a surrogate marker of preload venous return and right side heart function. Such imaging results have been shown to be more accurate than jugular venous distension on physical examination but only modestly helpful as a surrogate for central venous pressure (CVP), with more accuracy in the lower values of the CVP.11 However, this procedure can be repeated often after volume resuscitation to achieve a 1.5- to 2.5-cm diameter dimension of the IVC and < 25% inspiratory collapsibility as a goal.
An IVC with a diameter > 2.5 cm in the context of a suspected prerenal AKI is more likely the consequence of heart failure (HF) rather than hypovolemia. The caveat to this finding is that pulmonary hypertension may induce false-positive results.12,13 Hepatic vein dilation is another sign of HF and/or pulmonary hypertension. Furthermore, sonographic images of the left ventricle either from the parasternal long axis or subxiphoid approach can identify supranormal left ventricular ejection fraction (LVEF) or hyperdynamic heart as an important clue of the absolute or relative decrease of EACF.14 Conversely, a decrease in EACF in patients with low LVEF can be assessed qualitatively at the bedside in patients with systolic HF. Supporting evidence of prerenal azotemia as the result of HF can be suggested by the presence of pleural effusions and bilateral comet/rockets tails or B lines in lung sonography.15
The easily recognizable hypoechoic ascitic fluid in the presence of small, hyperechoic gross changes in the echocardiographic texture of liver may indicate a hepatorenal component as the cause of prerenal failure. A small increase of > 20% in the diameter of the portal vein with deep inspiration indicates portal hypertension, with a sensitivity of 80% and a specificity of 100%.15,16 Other clinical scenarios leading to AKI in association with systemic hypotension may be identified quickly with the aid of POC sonography. These scenarios include cardiac tamponade, tension pneumothorax, right ventricular dysfunction (as a surrogate of pulmonary embolism), or an acute coronary event.16,17 Alternatively, identifying the presence of severe left ventricular hypertrophy through POC ultrasonography in a patient with AKI and normal or low normal blood pressures may alert clinicians to the diagnosis of normotensive renal failure in individuals with previously unrecognized severe hypertension. In this clinical context, keeping mean arterial pressures higher than usual with vasopressors may improve renal function while decreasing dialysis utilization.18-21
Likewise, in clinical scenarios of shock with AKI, POC ultrasonography has proven to be an indispensable tool. For example, rapid exploration of the biliary tree demonstrating anterior gallbladder wall thickening, a stone or sludge, common bile duct dilation, or perigallbladder inflammation suggests acute cholecystitis and/or cholangitis as the cause. The presence of dyspnea in association with hypotension and unilateral signs of a higher proportion of comet tails and/or a lung consolidation suggests pneumonia. Rapid differentiation between acute respiratory distress syndrome (ARDS) and pulmonary edema from HF is possible with ultrasonography. When pleural line abnormalities are seen, ARDS is a common cause.
POC ultrasonography will be key in management of ARDS, as ultrasound results will help avoid the use of excessive diuretics, which can result in renal hypoperfusion and AKI.22 In trauma patients, the ultrasound examination will identify free fluid (bleeding) as the source of the prerenal failure, along with its cause (aortic dissection, hepatic hemorrhage, splenic hemorrhage, ectopic pregnancy, etc).23 Sonographic free air observed in the abdomen can provide the clue of a perforated viscus.24 The sonographic image of an inflamed pancreas can suggest pancreatitis as the cause of the systemic hypotension. Ultimately, intravascular losses in the hypoechoic edematous bowel wall in obstruction, ileus, pseudomembranous, or infectious or autoimmune enterocolitis can lead to significant decreases in the EACF and cause prerenal injury.
Related: Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
Intrinsic Renal Disease
In intrinsic AKI, acute tubular necrosis (ATN), glomerulonephritis, and interstitial nephritis are the typical causes. Although no signs are specific to each of the potential causes, a poor corticomedullary differentiation, kidney size < 9 cm, and cortex size < 1 cm help to distinguish CKD from AKI, especially if no previous serum creatinine values are available. The early diagnosis of ATN continues to be clinically relevant in the management of acute renal failure. Despite not being a practical tool for POC sonography currently, the use of bedside Doppler repetitive renal vasculature measures of resistive index predict occurrence and severity of ATN in the critical care setting and are an independent risk factor for poor survival in arterial hypertension and HF.25-30
Other POC sonographic evaluations of intrinsic AKI have been helpful in the following clinical scenarios. The presence of an ultrasonographic sign of sinusitis in the context of nephritic sediment and a rapid decline of renal function suggest antineutrophil cytoplasmic antibody (ANCA)-related vasculitis. Likewise, in younger adults, nephritic sediment and bilateral sonographic lung interstitial fluid in the absence of infection and a normal POC echocardiogram without significant edema elsewhere suggest glomerulonephritis in the category of pulmonary lung syndrome caused by antiglomerular basement membrane antibodies.
In the elderly, a similar systemic presentation suggests an ANCA vasculitis. Pleural effusion, synovitis, proteinuria, and/or hematuria will suggest lupus nephritis. Another important cause of acute renal failure in the critical care setting is intra-abdominal compartment syndrome. Here, bladder pressure measurement protocols are the standard of care. A human model evaluated the predictive value of intra-abdominal compartment syndrome pressures using the IVC square surface. In this study, a normal surface area of the IVC of > 1 cm2/m2 excluded the presence of intra-abdominal hypertension 87.5% of the time. However, the sensitivity of detection of the intra-abdominal hypertension was only 67.5% when the surface area of the IVC was < 1 cm2/m2.31
CKD and Associated Diseases
The diagnostic validity of ultrasonography is well established in adult-onset polycystic kidney disease. Bedside visualization of a parathyroid adenoma may be an important clue for a patient with CKD, echogenic kidneys, or nephrolithiasis with or without hypercalcemia to diagnose primary hyperparathyroidism. The sonographic diagnosis of abnormal parathyroid gland compared with parathyroid surgical exploration had a sensitivity, specificity, and positive predictive value of 74%, 96%, and 90%, respectively.32 In the clinical presentation of severe hypertension with headaches, ultrasonography at bedside can provide valuable diagnostic and risk assessment information of endocranial hypertension from measuring the optic nerve sheath. Sensitivity and specificity of papilledema was 90% and 79%, respectively, when 3.3 mm was the cutoff of the nerve sheath with a 30-degrees sign.33 The carotid artery intima media thickness measured on sonography correlates with the future development of atherogenesis, left ventricular hypertrophy, cognition deficits, CKD, and cardiovascular disease in asymptomatic patients. An intima media thickness of > 1.1 mm has been associated with a higher cardiovascular mortality.
Early initiation of antihypertensive medications and/or statins has been suggested to lower risk in these asymptomatic patients.34 The size and contour (smooth or irregular) of kidneys may provide clues to reflux nephropathy, dysplastic kidneys, radiation nephritis, or chronic pyelonephritis. The presence of nephrotic syndrome and abnormal free light chains ratio with a bedside echocardiogram showing the typical refractile myocardial walls with a peculiar speckled pattern is strongly suggestive of amyloidosis.35 Conditions associated with chronic hypercalcemia, medullary sponge kidney, milk alkali syndrome, sarcoidosis, and distal renal tubular acidosis are causes of nephrocalcinosis. Some degree of CKD is a constant feature in nephrocalcinosis. The initial imaging of choice in nephrocalcinosis and specially the medullary type is ultrasonography preferable to X-ray and perhaps to computed tomography.36
End-Stage Renal Disease
In a patient undergoing peritoneal dialysis with exit-site infection, the presence of > 1 mm radiolucent rim around the subcutaneous catheter after antibiotics has a bad prognosis and prompts catheter removal. This sonographic sign has a positive and negative predictive value for a tunneled infection of 84.6% and 94.1%, respectively.37,38 A risk factor for peritonitis in peritoneal dialysis is air in the peritoneum, which can be seen in one-third of patients. These individuals have 2.4 times more risk of peritonitis compared with patients without pneumoperitoneum. The sensitivity and specificity of sonographic detection of pneumoperitoneum is 94% and 100%, respectively, using the scissor technique.39 Proper training in performing home peritoneal dialysis decreases the incidence of pneumoperitoneum. Although not formally assessed, patient education and change in procedure techniques may decrease the incidence of pneumoperitoneum and peritonitis. The use of prelaparoscopic ultrasonography before insertion of the peritoneal dialysis catheter has detected intra-abdominal adhesions (visceral slide sign) with a sensitivity of 90% to 92%.40
History and physical examination are frequently helpful in the diagnosis of malfunctioning arteriovenous fistulas (AVF) for inflow or outflow disturbances, with sensitivity ranging from 70% to 100% and specificity ranging from 71% to 93% compared with angiography. Frequently, POC limited ultrasound can be helpful for a problematic AVF, either for cannulation or diagnosis. The congruence of duplex sonography with arteriogram is 85% to 96%. Various etiologies of a dysfunctional AVF (pseudo- or true aneurysm, poor development, stenosis, thrombi, or accessory veins) can be observed in the dialysis unit through limited sonography.41-44
After placement of a hemodialysis catheter using real-time ultrasonography, pneumohemothorax can be diagnosed reliably and rapidly. Catheter misplacement outside of the right atrium was detected by thoracic echocardiogram with a sensitivity of 96%, a specificity of 83%, and a positive predictive value of 98%.45,46 Ultimately, ultrasonography may replace chest X-ray in most cases after central vein dialysis catheter placement in the acute care setting.
Postrenal Failure
The sensitivity of ultrasonography to detect dilation to hydronephrosis of the pelvicaliceal system is well established. Sonography is the diagnostic examination of choice in pregnancy and the initial screening test for the nonpregnant patient. Computed tomography is the preferred imaging study in nephroureterolithiasis; however, due to ionizing radiation and cost, ultrasonography is gaining popularity for initial and/or follow-up evaluations. The ureteral jet is a relatively unexplored color and Doppler sonographic methodology that can provide insight into pelvicalyceal peristalsis, potentially yielding evidence of functional obstruction.47-51 Postvoid bladder residual volumes and bladder wall hypertrophy may provide important clues as to the cause(s) of the obstructive uropathy.
Telenephrology
In our institution, sonography is used in the evaluation of IVC, lungs, and kidneys via telemedicine. The probe is handled by trained nurses at the distant site.
Cardiac Arrest in ESRD
Patients with ESRD may have sudden cardiac arrest as a result of several etiologies. During the advance cardiac life support algorithm, there is a brief period of evaluation of the electrical rhythm in which echocardiography can be helpful with the diagnosis immediately after the 2 initial minutes of cardiopulmonary resuscitation. An enlarged right ventricular cavity (> 2/3 of the left ventricle) is a sonographic sign of a pulmonary embolism.
Bedside sonography has the potential to alter the current guidelines of advance cardiac life support management. For example, if the bedside echo shows a significant pericardial effusion, a pericardiocentesis could be performed faster as it would be diagnosed faster. In addition, at times the heart may appear to be beating rapidly but there is a small amount of fluid (blood) within the cardiac chambers. This may be from an extreme case of dehydration for which rapid administration of IV fluids may help manage. Therefore, a quick bedside point of care echocardiography may reveal a cardiac anomaly that may be able to be restored in a efficient manner.
Related: General Applications of Ultrasound in Rheumatology Practice
Conclusion
Ultrasonography at the POC provides an important and continuously expanding tool to improve nephrological diagnostic accuracy in concert with history and physical examination. Extracellular fluid evaluation is paramount in all kidney disease conditions. Recent clinical studies in lung ultrasonography suggest that the learning curve for the medical provider is quicker than with other organs. Because POC sonography in association with limited bedside echocardiography may reveal discriminatory signs of pneumonia and differentiate between cardiogenic vs noncardiogenic pulmonary edema, such imaging may be important cost-effective strategies in the management of dyspnea and in the categorization/etiology of AKI. Therefore, incorporation of POC sonography into clinical practice will require that medical schools, residency programs, and nephrology fellowship programs design teaching strategies within their respective curricula. Research studies with outcomes regarding diagnosis, morbidity, and mortality are necessary in these areas.
The evaluation of acute kidney injury (AKI) often starts with the classic prerenal, renal, and postrenal causalities, delineating a practical workable approach in its differential diagnosis. Accordingly, the history, physical examination, urinalysis, and kidney-bladder sonography are standard resources in the initial approach to renal disease assessment. Ultrasonography has a well-established role as an important adjuvant for postrenal diagnosis of renal failure. Nevertheless, most of the causes of AKI are prerenal and renal.
Some etiologies of kidney injury are sequelae of systemic diseases in which sonography can be diagnostically analogous to the history and physical examination. Furthermore, ultrasonography may be informative in various clinical scenarios, for example, patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD). In this narrative review, the contribution of point-of-care (POC) sonography to the evaluation and management of AKI, CKD, and associated diseases are explored beyond the traditional sonogram uses for kidney biopsy, central catheter placement, and/or screening of hydronephrosis.
Two important elements made possible the incorporation of POC sonography into nephrology practice.1,2 First, the development of handheld reliable and portable ultrasound devices and, second, the derived capacity of POC sonography to obtain objective signs of physiologic and/or pathophysiologic phenomena. The latter clinical application is realized through the incorporation of POC protocols into the modified focused assessment with sonography for trauma (FAST) examination in conjunction with limited echocardiography and lung sonography (Figure 1).
These protocols have allowed the evaluation of extracellular volume, which is important to measure for the diagnosis and management of renal diseases. For example, the evaluation of lung water by POC ultrasonography for patients with ESRD is emerging as a promising tool. In a study of patients with ESRD undergoing hemodialysis, POC ultrasonography detected moderate-to-severe lung congestion in 45% of patients, most of whom (71%) were asymptomatic. Two years of follow-up of patients was associated with 3 to 4 times greater risk of heart attack and death, respectively, compared with individuals without congestion on sonography.4-6 Thus, ultrasound assessment of lung water in patients with ESRD may prove to be an essential tool to assure an adequate ultrafiltration and improve patient outcomes.
Related: Nephrogenic Systemic Fibrosis in a Patient With Multiple Inflammatory Disorders
Acute Kidney Injury
Prerenal
The physical examination provides evaluation of effective arterial circulatory flow (EACF) and is clinically useful in the evaluation of prerenal azotemia. The utility is more obvious in the extremes of EACF. However, in the case of blood volume losses of > 10% or the physiologic equivalent, heart rate, blood pressure, skin turgor, urinary output, and capillary refill may be within normal limits. Obvious changes in these parameters during the physical examination are considered relatively late manifestations.7-10 Therefore, prerenal failure is frequently diagnosed retrospectively after correction of the EACF through use of crystalloids, blood products, vasopressors, inotropic agents, discontinuation of antihypertensive agents, or treatment of its prerenal causes. Certain sonographic maneuvers, performed at the bedside during acute renal injury, may be useful in many patients to evaluate a multitude of prerenal causes of AKI.
Sonographic inferior vena cava (IVC) luminal diameter and inspiratory collapsibility together serve as a surrogate marker of preload venous return and right side heart function. Such imaging results have been shown to be more accurate than jugular venous distension on physical examination but only modestly helpful as a surrogate for central venous pressure (CVP), with more accuracy in the lower values of the CVP.11 However, this procedure can be repeated often after volume resuscitation to achieve a 1.5- to 2.5-cm diameter dimension of the IVC and < 25% inspiratory collapsibility as a goal.
An IVC with a diameter > 2.5 cm in the context of a suspected prerenal AKI is more likely the consequence of heart failure (HF) rather than hypovolemia. The caveat to this finding is that pulmonary hypertension may induce false-positive results.12,13 Hepatic vein dilation is another sign of HF and/or pulmonary hypertension. Furthermore, sonographic images of the left ventricle either from the parasternal long axis or subxiphoid approach can identify supranormal left ventricular ejection fraction (LVEF) or hyperdynamic heart as an important clue of the absolute or relative decrease of EACF.14 Conversely, a decrease in EACF in patients with low LVEF can be assessed qualitatively at the bedside in patients with systolic HF. Supporting evidence of prerenal azotemia as the result of HF can be suggested by the presence of pleural effusions and bilateral comet/rockets tails or B lines in lung sonography.15
The easily recognizable hypoechoic ascitic fluid in the presence of small, hyperechoic gross changes in the echocardiographic texture of liver may indicate a hepatorenal component as the cause of prerenal failure. A small increase of > 20% in the diameter of the portal vein with deep inspiration indicates portal hypertension, with a sensitivity of 80% and a specificity of 100%.15,16 Other clinical scenarios leading to AKI in association with systemic hypotension may be identified quickly with the aid of POC sonography. These scenarios include cardiac tamponade, tension pneumothorax, right ventricular dysfunction (as a surrogate of pulmonary embolism), or an acute coronary event.16,17 Alternatively, identifying the presence of severe left ventricular hypertrophy through POC ultrasonography in a patient with AKI and normal or low normal blood pressures may alert clinicians to the diagnosis of normotensive renal failure in individuals with previously unrecognized severe hypertension. In this clinical context, keeping mean arterial pressures higher than usual with vasopressors may improve renal function while decreasing dialysis utilization.18-21
Likewise, in clinical scenarios of shock with AKI, POC ultrasonography has proven to be an indispensable tool. For example, rapid exploration of the biliary tree demonstrating anterior gallbladder wall thickening, a stone or sludge, common bile duct dilation, or perigallbladder inflammation suggests acute cholecystitis and/or cholangitis as the cause. The presence of dyspnea in association with hypotension and unilateral signs of a higher proportion of comet tails and/or a lung consolidation suggests pneumonia. Rapid differentiation between acute respiratory distress syndrome (ARDS) and pulmonary edema from HF is possible with ultrasonography. When pleural line abnormalities are seen, ARDS is a common cause.
POC ultrasonography will be key in management of ARDS, as ultrasound results will help avoid the use of excessive diuretics, which can result in renal hypoperfusion and AKI.22 In trauma patients, the ultrasound examination will identify free fluid (bleeding) as the source of the prerenal failure, along with its cause (aortic dissection, hepatic hemorrhage, splenic hemorrhage, ectopic pregnancy, etc).23 Sonographic free air observed in the abdomen can provide the clue of a perforated viscus.24 The sonographic image of an inflamed pancreas can suggest pancreatitis as the cause of the systemic hypotension. Ultimately, intravascular losses in the hypoechoic edematous bowel wall in obstruction, ileus, pseudomembranous, or infectious or autoimmune enterocolitis can lead to significant decreases in the EACF and cause prerenal injury.
Related: Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
Intrinsic Renal Disease
In intrinsic AKI, acute tubular necrosis (ATN), glomerulonephritis, and interstitial nephritis are the typical causes. Although no signs are specific to each of the potential causes, a poor corticomedullary differentiation, kidney size < 9 cm, and cortex size < 1 cm help to distinguish CKD from AKI, especially if no previous serum creatinine values are available. The early diagnosis of ATN continues to be clinically relevant in the management of acute renal failure. Despite not being a practical tool for POC sonography currently, the use of bedside Doppler repetitive renal vasculature measures of resistive index predict occurrence and severity of ATN in the critical care setting and are an independent risk factor for poor survival in arterial hypertension and HF.25-30
Other POC sonographic evaluations of intrinsic AKI have been helpful in the following clinical scenarios. The presence of an ultrasonographic sign of sinusitis in the context of nephritic sediment and a rapid decline of renal function suggest antineutrophil cytoplasmic antibody (ANCA)-related vasculitis. Likewise, in younger adults, nephritic sediment and bilateral sonographic lung interstitial fluid in the absence of infection and a normal POC echocardiogram without significant edema elsewhere suggest glomerulonephritis in the category of pulmonary lung syndrome caused by antiglomerular basement membrane antibodies.
In the elderly, a similar systemic presentation suggests an ANCA vasculitis. Pleural effusion, synovitis, proteinuria, and/or hematuria will suggest lupus nephritis. Another important cause of acute renal failure in the critical care setting is intra-abdominal compartment syndrome. Here, bladder pressure measurement protocols are the standard of care. A human model evaluated the predictive value of intra-abdominal compartment syndrome pressures using the IVC square surface. In this study, a normal surface area of the IVC of > 1 cm2/m2 excluded the presence of intra-abdominal hypertension 87.5% of the time. However, the sensitivity of detection of the intra-abdominal hypertension was only 67.5% when the surface area of the IVC was < 1 cm2/m2.31
CKD and Associated Diseases
The diagnostic validity of ultrasonography is well established in adult-onset polycystic kidney disease. Bedside visualization of a parathyroid adenoma may be an important clue for a patient with CKD, echogenic kidneys, or nephrolithiasis with or without hypercalcemia to diagnose primary hyperparathyroidism. The sonographic diagnosis of abnormal parathyroid gland compared with parathyroid surgical exploration had a sensitivity, specificity, and positive predictive value of 74%, 96%, and 90%, respectively.32 In the clinical presentation of severe hypertension with headaches, ultrasonography at bedside can provide valuable diagnostic and risk assessment information of endocranial hypertension from measuring the optic nerve sheath. Sensitivity and specificity of papilledema was 90% and 79%, respectively, when 3.3 mm was the cutoff of the nerve sheath with a 30-degrees sign.33 The carotid artery intima media thickness measured on sonography correlates with the future development of atherogenesis, left ventricular hypertrophy, cognition deficits, CKD, and cardiovascular disease in asymptomatic patients. An intima media thickness of > 1.1 mm has been associated with a higher cardiovascular mortality.
Early initiation of antihypertensive medications and/or statins has been suggested to lower risk in these asymptomatic patients.34 The size and contour (smooth or irregular) of kidneys may provide clues to reflux nephropathy, dysplastic kidneys, radiation nephritis, or chronic pyelonephritis. The presence of nephrotic syndrome and abnormal free light chains ratio with a bedside echocardiogram showing the typical refractile myocardial walls with a peculiar speckled pattern is strongly suggestive of amyloidosis.35 Conditions associated with chronic hypercalcemia, medullary sponge kidney, milk alkali syndrome, sarcoidosis, and distal renal tubular acidosis are causes of nephrocalcinosis. Some degree of CKD is a constant feature in nephrocalcinosis. The initial imaging of choice in nephrocalcinosis and specially the medullary type is ultrasonography preferable to X-ray and perhaps to computed tomography.36
End-Stage Renal Disease
In a patient undergoing peritoneal dialysis with exit-site infection, the presence of > 1 mm radiolucent rim around the subcutaneous catheter after antibiotics has a bad prognosis and prompts catheter removal. This sonographic sign has a positive and negative predictive value for a tunneled infection of 84.6% and 94.1%, respectively.37,38 A risk factor for peritonitis in peritoneal dialysis is air in the peritoneum, which can be seen in one-third of patients. These individuals have 2.4 times more risk of peritonitis compared with patients without pneumoperitoneum. The sensitivity and specificity of sonographic detection of pneumoperitoneum is 94% and 100%, respectively, using the scissor technique.39 Proper training in performing home peritoneal dialysis decreases the incidence of pneumoperitoneum. Although not formally assessed, patient education and change in procedure techniques may decrease the incidence of pneumoperitoneum and peritonitis. The use of prelaparoscopic ultrasonography before insertion of the peritoneal dialysis catheter has detected intra-abdominal adhesions (visceral slide sign) with a sensitivity of 90% to 92%.40
History and physical examination are frequently helpful in the diagnosis of malfunctioning arteriovenous fistulas (AVF) for inflow or outflow disturbances, with sensitivity ranging from 70% to 100% and specificity ranging from 71% to 93% compared with angiography. Frequently, POC limited ultrasound can be helpful for a problematic AVF, either for cannulation or diagnosis. The congruence of duplex sonography with arteriogram is 85% to 96%. Various etiologies of a dysfunctional AVF (pseudo- or true aneurysm, poor development, stenosis, thrombi, or accessory veins) can be observed in the dialysis unit through limited sonography.41-44
After placement of a hemodialysis catheter using real-time ultrasonography, pneumohemothorax can be diagnosed reliably and rapidly. Catheter misplacement outside of the right atrium was detected by thoracic echocardiogram with a sensitivity of 96%, a specificity of 83%, and a positive predictive value of 98%.45,46 Ultimately, ultrasonography may replace chest X-ray in most cases after central vein dialysis catheter placement in the acute care setting.
Postrenal Failure
The sensitivity of ultrasonography to detect dilation to hydronephrosis of the pelvicaliceal system is well established. Sonography is the diagnostic examination of choice in pregnancy and the initial screening test for the nonpregnant patient. Computed tomography is the preferred imaging study in nephroureterolithiasis; however, due to ionizing radiation and cost, ultrasonography is gaining popularity for initial and/or follow-up evaluations. The ureteral jet is a relatively unexplored color and Doppler sonographic methodology that can provide insight into pelvicalyceal peristalsis, potentially yielding evidence of functional obstruction.47-51 Postvoid bladder residual volumes and bladder wall hypertrophy may provide important clues as to the cause(s) of the obstructive uropathy.
Telenephrology
In our institution, sonography is used in the evaluation of IVC, lungs, and kidneys via telemedicine. The probe is handled by trained nurses at the distant site.
Cardiac Arrest in ESRD
Patients with ESRD may have sudden cardiac arrest as a result of several etiologies. During the advance cardiac life support algorithm, there is a brief period of evaluation of the electrical rhythm in which echocardiography can be helpful with the diagnosis immediately after the 2 initial minutes of cardiopulmonary resuscitation. An enlarged right ventricular cavity (> 2/3 of the left ventricle) is a sonographic sign of a pulmonary embolism.
Bedside sonography has the potential to alter the current guidelines of advance cardiac life support management. For example, if the bedside echo shows a significant pericardial effusion, a pericardiocentesis could be performed faster as it would be diagnosed faster. In addition, at times the heart may appear to be beating rapidly but there is a small amount of fluid (blood) within the cardiac chambers. This may be from an extreme case of dehydration for which rapid administration of IV fluids may help manage. Therefore, a quick bedside point of care echocardiography may reveal a cardiac anomaly that may be able to be restored in a efficient manner.
Related: General Applications of Ultrasound in Rheumatology Practice
Conclusion
Ultrasonography at the POC provides an important and continuously expanding tool to improve nephrological diagnostic accuracy in concert with history and physical examination. Extracellular fluid evaluation is paramount in all kidney disease conditions. Recent clinical studies in lung ultrasonography suggest that the learning curve for the medical provider is quicker than with other organs. Because POC sonography in association with limited bedside echocardiography may reveal discriminatory signs of pneumonia and differentiate between cardiogenic vs noncardiogenic pulmonary edema, such imaging may be important cost-effective strategies in the management of dyspnea and in the categorization/etiology of AKI. Therefore, incorporation of POC sonography into clinical practice will require that medical schools, residency programs, and nephrology fellowship programs design teaching strategies within their respective curricula. Research studies with outcomes regarding diagnosis, morbidity, and mortality are necessary in these areas.
1. Remer EM, Papanicolaou N, Casalino DD, et al. ACR Appropriateness Criteria® on renal failure. Am J Med. 2014;127(11):1041-1048.e1.
2. Tublin M, Thurston W, Wilson SR. The kidney and urinary tract. In: Rumack C, Wilson S, Charboneau JW, Levine D, eds. Diagnostic Ultrasound. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:317-391.
3. Bahner D, Blaivas M, Cohen HL, et al; American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med. 2008;27(2):313-318.
4. Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging. 2010;3(6):586-594.
5. Enia G, Torino C, Panuccio V, et al; Lung Comets Cohort Working Group. Asymptomatic pulmonary congestion and physical functioning in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(8):1343-1348.
6. Zoccali C, Torino C, Tripepi R, et al; Lung US in CKD Working Group. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol. 2013;24(4):639-646.
7. Fortes MB, Owen JA, Raymond-Barker P, et al. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J Am Med Dir Assoc. 2015;16(3):221-228.
8. Jauregui J, Nelson D, Choo E, et al. The BUDDY (Bedside Ultrasound to Detect Dehydration in Youth) study. Crit Ultrasound J. 2014;6(1):15.
9. McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
10. Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
11. Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
12. Stawicki SP, Adkins EJ, Eiferman DS, et al. Prospective evaluation of intravascular volume status in critically ill patients: does inferior vena cava collapsibility correlate with central venous pressure? J Trauma Acute Care Surg. 2014;76(4):956-963.
13. Thanakitcharu P, Charoenwut M, Siriwiwatanakul N. Inferior vena cava diameter and collapsibility index: a practical non-invasive evaluation of intravascular fluid volume in critically-ill patients. J Med Assoc Thai. 2013;96(suppl 3):S14-S22.
14. Gustafsson M, Alehagen U, Johansson P. Pocket-sized ultrasound examination of fluid imbalance in patients with heart failure: a pilot and feasibility study of heart failure nurses without prior experience of ultrasonography. Eur J Cardiovasc Nurs. 2015;14(4):294-302.
15. Peguero A, Lamarche J, Courville C, Taha M, Antar-Shultz M. Ultrasonography to evaluate pulmonary edema resolution with blood pressure control in a hemodialysis patient. Abstract 263 presented at: 2016 Spring Clinical National Kidney Foundation Meeting; April 27-May 1, 2016; Boston, MA.
16. Bolondi L, Mazziotti A, Arienti V, et al. Ultrasonographic study of portal venous system in portal hypertension and after portosystemic shunt operations. Surgery. 1984;95(3):261-269.
17. Al-Nakshabandi NA. The role of ultrasonography in portal hypertension. Saudi J Gastroenterol. 2006;12(3):111-117.
18. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357(8):797-805.
19. Messerli FH. Clinical determinants and consequences of left ventricular hypertrophy. Am J Med. 1983;75(3A):51-56.
20. Chen SC, Su HM, Hung CC, et al. Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(12):2750-2758.
21. Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(7):496-507.
22. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16.
23. ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693.
24. Hefny AF, Abu-Zidan FM. Sonographic diagnosis of intraperitoneal free air. J Emerg Trauma Shock. 2011;4(4):511-513.
25. Meola M, Petrucci I. Ultrasound and color Doppler in nephrology. Acute kidney injury [in Italian]. G Ital Nefrol. 2012;29(5):599-615.
26. Corradi F, Brusasco C, Vezzani A, et al. Hemorrhagic shock in polytrauma patients: early detection with renal Doppler resistive index measurements. Radiology. 2011;260(1):112-118.
27. Viazzi F, Leoncini G, Derchi LE, Pontremoli R. Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. J Hypertens. 2014;32(1):149-153.
28. Marty P, Szatjnic S, Ferre F, et al. Doppler renal resistive index for early detection of acute kidney injury after major orthopaedic surgery : a prospective observational study. Eur J Anaesthesiol. 2015;32(1):37-43.
29. Kastelan S, Ljubicic N, Kastelan Z, Ostojic R, Uravic M. The role of duplex-doppler ultrasonography in the diagnosis of renal dysfunction and hepatorenal syndrome in patients with liver cirrhosis. Hepatogastroenterology. 2004;51(59):1408-1412.
30. Capotondo L, Nicolai GA, Garosi G. The role of color Doppler in acute kidney injury. Arch Ital Urol Androl. 2010;82(4):275-279.
31. Cavaliere F, Cina A, Biasucci D, et al. Sonographic assessment of abdominal vein dimensional and hemodynamic changes induced in human volunteers by a model of abdominal hypertension. Crit Care Med. 2011;39(2):344-348.
32. Tublin ME, Pryma DA, Yim JH, et al. Localization of parathyroid adenomas by sonography and technetium tc 99m sestamibi single-photon emission computed tomography before minimally invasive parathyroidectomy: are both studies really needed? J Ultrasound Med. 2009;28(2):183-190.
33. Carter SB, Pistilli M, Livingston KG, et al. The role of orbital ultrasonography in distinguishing papilledema from pseudopapilledema. Eye (Lond). 2014;28(12):1425-1430.
34. Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50-e103.
35. Huang Y, Zhan J, Wei X, et al. Clinical characteristics of 42 patients with cardiac amyloidosis. [Article in Chinese] Zhonghua Nei Ke Za Zhi. 2014;53(7):546-549.
36. Boyce AM, Shawker TH, Hill SC, et al. Ultrasound is superior to computed tomography for assessment of medullary nephrocalcinosis in hypoparathyroidism. J Clin Endocrinol Metab. 2013;98(3):989-994.
37. Kwan TH, Tong MK, Siu YP, Leung KT, Luk SH, Cheung YK. Ultrasonography in the management of exit site infections in peritoneal dialysis patients. Nephrology (Carlton). 2004;9(6):348-352.
38. Karahan OI, Taskapan H, Yikilmaz A, Oymak O, Utas C. Ultrasound evaluation of peritoneal catheter tunnel in catheter related infections in CAPD. Int Urol Nephrol. 2005;37(2):363-366.
39. Karahan OI, Kurt A, Yikilmaz A, Kahriman G. New method for the detection of intraperitoneal free air by sonography: scissors maneuver. J Clin Ultrasound. 2004;32(8):381-385.
40. Okamoto T, Ikenoue T, Matsui K, et al. Free air on CT and the risk of peritonitis in peritoneal dialysis patients: a retrospective study. Ren Fail. 2014;36(10):1492-1496.
41. Arshad FH, Sutijono D, Moore CL. Emergency ultrasound diagnosis of a pseudoaneurysm associated with an arteriovenous fistula. Acad Emerg Med. 2010;17(6):e43-e45.
42. Teodorescu V, Gustavson S, Schanzer H. Duplex ultrasound evaluation of hemodialysis access: a detailed protocol. Int J Nephrol. 2012;2012:508956.
43. Coentrão L, Turmel-Rodrigues L. Monitoring dialysis arteriovenous fistulae: it’s in our hands. J Vasc Access. 2013;14(3):209-215.
44. Chandra AP, Dimascio D, Gruenewald S, Nankivell B, Allen RD, Swinnen J. Colour duplex ultrasound accurately identifies focal stenoses in dysfunctional autogenous arteriovenous fistulae. Nephrology (Carlton). 2010;15(3):300-306.
45. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937.
46. Vezzani A, Brusasco C, Palermo S, Launo C, Mergoni M, Corradi F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538.
47. Celik S, Altay C, Bozkurt O, et al. Association between ureteral jet dynamics and nonobstructive kidney stones: a prospective-controlled study. Urology. 2014;84(5):1016-1020.
48. Tullus K. Does the ureteric jet Doppler waveform have a role in detecting vesicoureteric reflux? Pediatr Nephrol. 2013;28(9):1719-1721.
49. Jandaghi AB, Falahatkar S, Alizadeh A, et al. Assessment of ureterovesical jet dynamics in obstructed ureter by urinary stone with color Doppler and duplex Doppler examinations. Urolithiasis. 2013;41(2):159-163.
50. Pepe P, Motta L, Pennisi M, Aragona F. Functional evaluation of the urinary tract by color-Doppler ultrasonography (CDU) in 100 patients with renal colic. Eur J Radiol. 2005;53(1):131-135.
51. Leung VY, Metreweli C. Ureteric jet in renal transplantation patient. Ultrasound Med Biol. 2002;28(7):885-888.
1. Remer EM, Papanicolaou N, Casalino DD, et al. ACR Appropriateness Criteria® on renal failure. Am J Med. 2014;127(11):1041-1048.e1.
2. Tublin M, Thurston W, Wilson SR. The kidney and urinary tract. In: Rumack C, Wilson S, Charboneau JW, Levine D, eds. Diagnostic Ultrasound. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:317-391.
3. Bahner D, Blaivas M, Cohen HL, et al; American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med. 2008;27(2):313-318.
4. Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging. 2010;3(6):586-594.
5. Enia G, Torino C, Panuccio V, et al; Lung Comets Cohort Working Group. Asymptomatic pulmonary congestion and physical functioning in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(8):1343-1348.
6. Zoccali C, Torino C, Tripepi R, et al; Lung US in CKD Working Group. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol. 2013;24(4):639-646.
7. Fortes MB, Owen JA, Raymond-Barker P, et al. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J Am Med Dir Assoc. 2015;16(3):221-228.
8. Jauregui J, Nelson D, Choo E, et al. The BUDDY (Bedside Ultrasound to Detect Dehydration in Youth) study. Crit Ultrasound J. 2014;6(1):15.
9. McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
10. Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
11. Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
12. Stawicki SP, Adkins EJ, Eiferman DS, et al. Prospective evaluation of intravascular volume status in critically ill patients: does inferior vena cava collapsibility correlate with central venous pressure? J Trauma Acute Care Surg. 2014;76(4):956-963.
13. Thanakitcharu P, Charoenwut M, Siriwiwatanakul N. Inferior vena cava diameter and collapsibility index: a practical non-invasive evaluation of intravascular fluid volume in critically-ill patients. J Med Assoc Thai. 2013;96(suppl 3):S14-S22.
14. Gustafsson M, Alehagen U, Johansson P. Pocket-sized ultrasound examination of fluid imbalance in patients with heart failure: a pilot and feasibility study of heart failure nurses without prior experience of ultrasonography. Eur J Cardiovasc Nurs. 2015;14(4):294-302.
15. Peguero A, Lamarche J, Courville C, Taha M, Antar-Shultz M. Ultrasonography to evaluate pulmonary edema resolution with blood pressure control in a hemodialysis patient. Abstract 263 presented at: 2016 Spring Clinical National Kidney Foundation Meeting; April 27-May 1, 2016; Boston, MA.
16. Bolondi L, Mazziotti A, Arienti V, et al. Ultrasonographic study of portal venous system in portal hypertension and after portosystemic shunt operations. Surgery. 1984;95(3):261-269.
17. Al-Nakshabandi NA. The role of ultrasonography in portal hypertension. Saudi J Gastroenterol. 2006;12(3):111-117.
18. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357(8):797-805.
19. Messerli FH. Clinical determinants and consequences of left ventricular hypertrophy. Am J Med. 1983;75(3A):51-56.
20. Chen SC, Su HM, Hung CC, et al. Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(12):2750-2758.
21. Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(7):496-507.
22. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16.
23. ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693.
24. Hefny AF, Abu-Zidan FM. Sonographic diagnosis of intraperitoneal free air. J Emerg Trauma Shock. 2011;4(4):511-513.
25. Meola M, Petrucci I. Ultrasound and color Doppler in nephrology. Acute kidney injury [in Italian]. G Ital Nefrol. 2012;29(5):599-615.
26. Corradi F, Brusasco C, Vezzani A, et al. Hemorrhagic shock in polytrauma patients: early detection with renal Doppler resistive index measurements. Radiology. 2011;260(1):112-118.
27. Viazzi F, Leoncini G, Derchi LE, Pontremoli R. Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. J Hypertens. 2014;32(1):149-153.
28. Marty P, Szatjnic S, Ferre F, et al. Doppler renal resistive index for early detection of acute kidney injury after major orthopaedic surgery : a prospective observational study. Eur J Anaesthesiol. 2015;32(1):37-43.
29. Kastelan S, Ljubicic N, Kastelan Z, Ostojic R, Uravic M. The role of duplex-doppler ultrasonography in the diagnosis of renal dysfunction and hepatorenal syndrome in patients with liver cirrhosis. Hepatogastroenterology. 2004;51(59):1408-1412.
30. Capotondo L, Nicolai GA, Garosi G. The role of color Doppler in acute kidney injury. Arch Ital Urol Androl. 2010;82(4):275-279.
31. Cavaliere F, Cina A, Biasucci D, et al. Sonographic assessment of abdominal vein dimensional and hemodynamic changes induced in human volunteers by a model of abdominal hypertension. Crit Care Med. 2011;39(2):344-348.
32. Tublin ME, Pryma DA, Yim JH, et al. Localization of parathyroid adenomas by sonography and technetium tc 99m sestamibi single-photon emission computed tomography before minimally invasive parathyroidectomy: are both studies really needed? J Ultrasound Med. 2009;28(2):183-190.
33. Carter SB, Pistilli M, Livingston KG, et al. The role of orbital ultrasonography in distinguishing papilledema from pseudopapilledema. Eye (Lond). 2014;28(12):1425-1430.
34. Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50-e103.
35. Huang Y, Zhan J, Wei X, et al. Clinical characteristics of 42 patients with cardiac amyloidosis. [Article in Chinese] Zhonghua Nei Ke Za Zhi. 2014;53(7):546-549.
36. Boyce AM, Shawker TH, Hill SC, et al. Ultrasound is superior to computed tomography for assessment of medullary nephrocalcinosis in hypoparathyroidism. J Clin Endocrinol Metab. 2013;98(3):989-994.
37. Kwan TH, Tong MK, Siu YP, Leung KT, Luk SH, Cheung YK. Ultrasonography in the management of exit site infections in peritoneal dialysis patients. Nephrology (Carlton). 2004;9(6):348-352.
38. Karahan OI, Taskapan H, Yikilmaz A, Oymak O, Utas C. Ultrasound evaluation of peritoneal catheter tunnel in catheter related infections in CAPD. Int Urol Nephrol. 2005;37(2):363-366.
39. Karahan OI, Kurt A, Yikilmaz A, Kahriman G. New method for the detection of intraperitoneal free air by sonography: scissors maneuver. J Clin Ultrasound. 2004;32(8):381-385.
40. Okamoto T, Ikenoue T, Matsui K, et al. Free air on CT and the risk of peritonitis in peritoneal dialysis patients: a retrospective study. Ren Fail. 2014;36(10):1492-1496.
41. Arshad FH, Sutijono D, Moore CL. Emergency ultrasound diagnosis of a pseudoaneurysm associated with an arteriovenous fistula. Acad Emerg Med. 2010;17(6):e43-e45.
42. Teodorescu V, Gustavson S, Schanzer H. Duplex ultrasound evaluation of hemodialysis access: a detailed protocol. Int J Nephrol. 2012;2012:508956.
43. Coentrão L, Turmel-Rodrigues L. Monitoring dialysis arteriovenous fistulae: it’s in our hands. J Vasc Access. 2013;14(3):209-215.
44. Chandra AP, Dimascio D, Gruenewald S, Nankivell B, Allen RD, Swinnen J. Colour duplex ultrasound accurately identifies focal stenoses in dysfunctional autogenous arteriovenous fistulae. Nephrology (Carlton). 2010;15(3):300-306.
45. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937.
46. Vezzani A, Brusasco C, Palermo S, Launo C, Mergoni M, Corradi F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538.
47. Celik S, Altay C, Bozkurt O, et al. Association between ureteral jet dynamics and nonobstructive kidney stones: a prospective-controlled study. Urology. 2014;84(5):1016-1020.
48. Tullus K. Does the ureteric jet Doppler waveform have a role in detecting vesicoureteric reflux? Pediatr Nephrol. 2013;28(9):1719-1721.
49. Jandaghi AB, Falahatkar S, Alizadeh A, et al. Assessment of ureterovesical jet dynamics in obstructed ureter by urinary stone with color Doppler and duplex Doppler examinations. Urolithiasis. 2013;41(2):159-163.
50. Pepe P, Motta L, Pennisi M, Aragona F. Functional evaluation of the urinary tract by color-Doppler ultrasonography (CDU) in 100 patients with renal colic. Eur J Radiol. 2005;53(1):131-135.
51. Leung VY, Metreweli C. Ureteric jet in renal transplantation patient. Ultrasound Med Biol. 2002;28(7):885-888.
Anesthesia Care Practice Models in the Veterans Health Administration
Although the VHA primarily relies on teams for anesthesia care, unsupervised certified registered nurse anesthetists also are used to meet veterans’ surgical care needs.
Anesthesia care is provided by physician anesthesiologists, certified registered nurse anesthetists (CRNAs), anesthesiology residents, and anesthesiologist assistants. These providers may practice alone (anesthesiologists or CRNAs) or in various combinations of supervised roles and teams. Previous studies reveal mixed findings regarding whether patient outcomes differ by anesthesia practice models.1-7However, little is known about the prevalence of various anesthesia models in the US.
Background
In recent years, anesthesiology has undergone substantial expansion in its scope of services provided, the settings in which it is provided, and the diversity of its workforce.8As the field continues to evolve, especially within the context of value-based health care reform, it is imperative to evaluate how anesthesia care models are used in health systems and how these models may optimize care delivery.
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing surgical care in 110 inpatient medical centers and 27 ambulatory surgery centers. Despite national integration, anesthesia practices vary widely among facilities. The question of which model of anesthesia care is associated with the best outcomes and offers the most value is widely debated.1,5,7,9 As an important first step in understanding anesthesia care delivery, a baseline assessment of the practice patterns of anesthesia providers is necessary and may benefit future studies of the impact of these care models on outcomes. Thus, the aim of this work was to understand and describe the previously unassessed landscape of anesthesia care delivery within the VHA.
Methods
As part of a larger evaluation of anesthesia care delivery in the VHA, an observational assessment of anesthesia provider practice patterns was conducted using retrospective surgical data. This project complies with VHA policy pertaining to nonresearch operational activities and did not require institutional review board approval and adheres to the EQUATOR Network guidelines described in Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).10
Data were obtained from the VHA Managerial Cost Accounting National Data Extract for Surgery package for all surgical procedures (n = 726,706) between October 1, 2013 and March 31, 2015. There were 420 facilities represented in these surgical data. The VHA facility records were used to specifically identify inpatient and ambulatory surgery facilities for inclusion. Additionally, to ensure facilities were valid surgical sites with sufficient surgical volume, those with 100 or fewer cases during the period were excluded. In total, 288 facilities with 9,434 surgical cases (representing 1% of cases) were excluded. These excluded facilities included nursing homes (38%), domiciliaries (26%), outpatient clinics (11%), rehabilitation programs (9%), other nonsurgical facilities (8%), and medical centers (8%). The majority (80%) of excluded medical centers had 30 or fewer surgical cases.
In 6 instances, data from subfacilities were combined with their organizationally affiliated main facilities. The final sample included 125 facilities. The VHA assigns a complexity level designation to facilities, defined as follows: 1a (most complex), 1b, 1c, 2, and 3 (least complex).11 Facilities with 1a designation perform the most complex surgical cases, such as cardiovascular surgery or neurosurgery and have more staff and resource support, whereas levels 2 and 3 facilities perform fewer and less complex cases.
Surgical records were excluded when the primary Current Procedural Terminology (CPT) code was missing (n = 85,748, or 12% of cases). This resulted in 631,524 remaining cases. The surgical CPT codes were mapped to anesthesia CPT codes to obtain the associated base unit (BU) values via a published crosswalk by the American Society of Anesthesiologists (ASA).12 A higher number of associated BUs indicates a more complex procedure. For example, procedures such as biopsies, arthroscopies, and laparoscopies receive 3 to 4 BUs, whereas a venous thrombectomy of the leg and a transurethral resection of the prostate are both 5 BUs, a total knee arthroplasty is 7 BUs, a craniotomy is 10 BUs, and a coronary artery bypass receives 18 BUs. Surgical case complexity was defined as low (3 or 4 BUs), medium (5 BUs), and high (≥ 6 BUs). Although the VHA has an existing case complexity assignment process based on CPT codes, it defines complexity differently for inpatient facilities and ambulatory surgery centers. Thus, the BU-defined complexity permitted a standardized complexity categorization across all facilities. Categorization of BUs similar to this has previously been used in the literature as a proxy for case complexity.13,14
Patient-level information included the ASA physical status classification, a measure of overall health status determined by an anesthesia provider preoperatively.15 These classifications included ASA I (healthy), ASA II (mild systemic disease), ASA III (severe systemic disease), ASA IV (severe systemic disease that is a constant threat to life), and ASA V (moribund patient who is not expected to survive without surgery). The last classification, ASA VI: brain-dead with planned organ donation, was excluded. The “E” subcategory denoting “emergency” was subsumed within the corresponding ASA category (eg, ASA V-E was combined with ASA V).
Provider data identified the principal and supervising (if present) anesthetists involved in the case. The provision of anesthesia care was categorized into 3 models: Model 1—a physician anesthesiologist supervising a CRNA; Model 2—a physician anesthesiologist practicing independently or supervising an anesthesiology resident; and Model 3—a CRNA without supervision. Surgical cases were excluded when there was no anesthesia provider (n = 95,795, or 15% of remaining cases), or a nonanesthesia provider (n = 51,647, or 8% of remaining cases) on record. The final sample was 484,082 surgical cases conducted at 125 facilities.
Related: Improving Care and Reducing Length of Stay in Patients Undergoing Total Knee Replacement
Statistical Analysis
The percentage of surgical cases in each anesthesia care model was calculated overall and by the following characteristics: surgical case complexity, ASA classification, and facility complexity. The anesthesia model was determined for each case and summed at the facility level, yielding a total number of cases attributed to each model for each facility, thus identifying the predominant anesthesia model for each facility. The facilities were geographically displayed by their predominant anesthesia model and total number of surgical cases during the period. Because the aim was to present a descriptive representation of anesthesia care models, rather than infer significance, statistical testing was not included.
Results
A total of 484,082 surgical cases met inclusion criteria (Table). These cases were from 109 inpatient facilities and 16 ambulatory surgery facilities.
The percentage of cases in Model 1 was similar across the levels of surgical case complexity. However, a higher proportion of highly complex cases had a physician anesthesiologist (Model 2, 38.8%) than a CRNA (Model 3, 6.4%) as the primary anesthesia provider. Patients in each ASA classification were most likely to receive anesthesia care via Model 1. As ASA level increased, fewer patients had their anesthesia managed by a CRNA without supervision (Model 3: 18.4% of ASA 1 patients vs 8.3% of ASA 4 patients).
Facility complexity demonstrated notable differences in the proportions of surgical cases within each model. More than half of surgical cases in the largest, most complex facilities used Model 1 (64.9%, 58.2%, and 57.7% of cases in 1a, 1b, and 1c facilities, respectively). In comparison, Model 3 was found almost exclusively among surgical cases in smaller facilities with lower complexity (52% and 74% of cases in level 2 and 3 facilities, respectively).
The Figure displays the 125 facilities by their predominant model of anesthesia care. The diameter of the dots is relative to the facility’s total number of surgical cases. For each facility, the predominant model accounted for about half or more of cases but was not necessarily the only model of care used at a particular facility.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Discussion
Anesthesia care in more than half of surgical cases in VHA facilities was delivered by physician anesthesiologists supervising CRNAs. This model of anesthesia care was the dominant model in 54% of the facilities included in the sample. Consistent with a study of non-VHA facilities, this assessment found that the type of facility may influence the model of anesthesia care, with smaller, less complex facilities more often using a CRNA without supervision model.4 In these data, it was noted that among the 28 facilities that predominantly used Model 3, half had 12% or fewer cases that indicated a physician anesthesiologist model of care, and 6 had no cases with physician anesthesiologist involvement. These findings may reflect the limited scope of surgical services offered at lower complexity facilities and/or the reduced availability and/or utilization of physician anesthesiologists in these facilities.
Limitations
We recognize limitations in our assessment of anesthesia care. The documented presence or absence of a supervising anesthesia provider on the surgical record may not adequately characterize the model of anesthesia care in use at a facility, thus limiting an understanding of care delivery relationships among anesthesia providers. In addition, the patterns of anesthesia care delivery are likely influenced by factors not accounted for in this assessment, including the labor market share and economic forces.16,17 The veteran population tends to be older, male, and with substantial chronic disease burden, thus may have differing surgical needs and experiences than that of the general public.18,19 The surgical services offered in VHA facilities as well as the policies and practice environment surrounding anesthesia care also may vary from those found in nongovernmental facilities. However, as the largest health care system in the US, the VHA provides a diverse and robust surgical program. Many VHA facilities are large teaching hospitals with academic affiliations that would parallel some in the public sector. For example, studies have demonstrated similar surgical outcomes for patients in VHA vs non-VHA facilities.20 Therefore, the findings regarding anesthesia care models in VHA are likely relevant to non-VHA surgical sites.
Related: Improving Team-Based Care Coordination Delivery and Documentation in the Health Record
Conclusion
This preliminary assessment of the different models of anesthesia care demonstrates that although primarily relying on teams of anesthesiologists and CRNAs, the VA also uses unsupervised CRNAs to meet veterans’ surgical care needs. Although CRNA practice without supervision represented only 12% of surgical cases in our data, we identified 28 facilities (22%) that predominantly used CRNAs without supervision. Thus, CRNAs with and without supervision deliver a substantial portion of anesthesia care in the VA. The prevalence of CRNAs in documented VA surgical records and among surgical facilities nationwide highlights the importance of further examining their supervised and unsupervised roles in anesthesia care delivery.21 As the practice of anesthesiology continues to evolve, it is imperative that research efforts further investigate ways anesthesia care models may optimize care delivery, benefit anesthesia providers, and improve health outcomes for patients.
1. Dulisse B, Cromwell J. No harm found when nurse anesthetists work without supervision by physicians. Health Aff (Millwood). 2010;29(8):1469-1475
2. Simonson DC, Ahern MM, Hendryx MS. Anesthesia staffing and anesthetic complications during cesarean delivery: a retrospective analysis. Nurs Res. 2007;56(1):9-17.
3. Smith AF, Kane M, Milne R. Comparative effectiveness and safety of physician and nurse anaesthetists: a narrative systematic review. Br J Anaesth. 2004;93(4):540-545.
4. Needleman J, Minnick AF. Anesthesia provider model, hospital resources, and maternal outcomes. Health Serv Res. 2009;44(2, pt 1):464-482.
5. Lewis SR, Nicholson A, Smith AF, Alderson P. Physician anaesthetists versus non-physician providers of anaesthesia for surgical patients. Cochrane Database Syst Rev. 2014(7):CD010357.
6. Silber JH, Kennedy SK, Even-Shoshan O, et al. Anesthesiologist direction and patient outcomes. Anesthesiology. 2000;93(1):152-163.
7. Negrusa B, Hogan PF, Warner JT, Schroeder CH, Pang B. Scope of practice laws and anesthesia complications: no measurable impact of certified registered nurse anesthetist expanded scope of practice on anesthesia-related complications. Med Care. 2016;54(10):913-920.
8. Prielipp RC, Cohen NH. The future of anesthesiology: implications of the changing healthcare environment. Curr Opin Anaesthesiol. 2016;29(2):198-205.
9. Memtsoudis SG, Ma Y, Swamidoss CP, Edwards AM, Mazumdar M, Liguori GA. Factors influencing unexpected disposition after orthopedic ambulatory surgery. J Clin Anesth. 2012;24(2):89-95.
10. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epid. 2008;61:344-349.
11. US Department of Veterans Affairs, Veterans Health Administration, Office of Productivity Efficiency & Staffing. Facility Complexity Levels. http://opes.vssc.med.va.gov/FacilityComplexityLevels/Pages/default.aspx. [Nonpublic document; source not verified.
13. Mathis MR, Sathishkumar S, Kheterpal S, et al. Complications, risk factors, and staffing patterns for noncardiac surgery in patients with left ventricular assist devices. Anesthesiology. 2017;126(3):450-460.
14. Chen Y, Gabriel RA, Kodali BS, Urman RD. Effect of anesthesia staffing ratio on first-case surgical start time. J Med Syst. 2016;40(5):115.
15. American Society of Anesthesiologists. Standards, guidelines and related resources. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system. Published October 15, 2014. Accessed November 5, 2018.
16. Kalist DE, Molinari NA, Spurr SJ. Cooperation and conflict between very similar occupations: the case of anesthesia. Health Econ Policy Law. 2011;6(2):237-264.
17. Daugherty L, Fonseca R, Kumar KB, Michaud PC. An analysis of the labor markets for anesthesiology. Rand Health Q. 2011;1(3):18.
18. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
19. Yoon J, Scott JY, Phibbs CS, Wagner TH. Recent trends in Veterans Affairs chronic condition spending. Popul Health Manag. 2011;14(6):293-298.
20. Shekelle PG, Asch S, Glassman P, Matula S, Trivedi A, Miake-Lye I. Comparison of Quality of Care in VA and Non-VA Settings: A Systematic Review. VA Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2010.
21. Baird M, Daugherty L, Kumar KB, Arifkhanova A. Regional and gender differences and trends in the anesthesiologist workforce. Anesthesiology. 2015;123(5):997-1012.
Although the VHA primarily relies on teams for anesthesia care, unsupervised certified registered nurse anesthetists also are used to meet veterans’ surgical care needs.
Although the VHA primarily relies on teams for anesthesia care, unsupervised certified registered nurse anesthetists also are used to meet veterans’ surgical care needs.
Anesthesia care is provided by physician anesthesiologists, certified registered nurse anesthetists (CRNAs), anesthesiology residents, and anesthesiologist assistants. These providers may practice alone (anesthesiologists or CRNAs) or in various combinations of supervised roles and teams. Previous studies reveal mixed findings regarding whether patient outcomes differ by anesthesia practice models.1-7However, little is known about the prevalence of various anesthesia models in the US.
Background
In recent years, anesthesiology has undergone substantial expansion in its scope of services provided, the settings in which it is provided, and the diversity of its workforce.8As the field continues to evolve, especially within the context of value-based health care reform, it is imperative to evaluate how anesthesia care models are used in health systems and how these models may optimize care delivery.
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing surgical care in 110 inpatient medical centers and 27 ambulatory surgery centers. Despite national integration, anesthesia practices vary widely among facilities. The question of which model of anesthesia care is associated with the best outcomes and offers the most value is widely debated.1,5,7,9 As an important first step in understanding anesthesia care delivery, a baseline assessment of the practice patterns of anesthesia providers is necessary and may benefit future studies of the impact of these care models on outcomes. Thus, the aim of this work was to understand and describe the previously unassessed landscape of anesthesia care delivery within the VHA.
Methods
As part of a larger evaluation of anesthesia care delivery in the VHA, an observational assessment of anesthesia provider practice patterns was conducted using retrospective surgical data. This project complies with VHA policy pertaining to nonresearch operational activities and did not require institutional review board approval and adheres to the EQUATOR Network guidelines described in Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).10
Data were obtained from the VHA Managerial Cost Accounting National Data Extract for Surgery package for all surgical procedures (n = 726,706) between October 1, 2013 and March 31, 2015. There were 420 facilities represented in these surgical data. The VHA facility records were used to specifically identify inpatient and ambulatory surgery facilities for inclusion. Additionally, to ensure facilities were valid surgical sites with sufficient surgical volume, those with 100 or fewer cases during the period were excluded. In total, 288 facilities with 9,434 surgical cases (representing 1% of cases) were excluded. These excluded facilities included nursing homes (38%), domiciliaries (26%), outpatient clinics (11%), rehabilitation programs (9%), other nonsurgical facilities (8%), and medical centers (8%). The majority (80%) of excluded medical centers had 30 or fewer surgical cases.
In 6 instances, data from subfacilities were combined with their organizationally affiliated main facilities. The final sample included 125 facilities. The VHA assigns a complexity level designation to facilities, defined as follows: 1a (most complex), 1b, 1c, 2, and 3 (least complex).11 Facilities with 1a designation perform the most complex surgical cases, such as cardiovascular surgery or neurosurgery and have more staff and resource support, whereas levels 2 and 3 facilities perform fewer and less complex cases.
Surgical records were excluded when the primary Current Procedural Terminology (CPT) code was missing (n = 85,748, or 12% of cases). This resulted in 631,524 remaining cases. The surgical CPT codes were mapped to anesthesia CPT codes to obtain the associated base unit (BU) values via a published crosswalk by the American Society of Anesthesiologists (ASA).12 A higher number of associated BUs indicates a more complex procedure. For example, procedures such as biopsies, arthroscopies, and laparoscopies receive 3 to 4 BUs, whereas a venous thrombectomy of the leg and a transurethral resection of the prostate are both 5 BUs, a total knee arthroplasty is 7 BUs, a craniotomy is 10 BUs, and a coronary artery bypass receives 18 BUs. Surgical case complexity was defined as low (3 or 4 BUs), medium (5 BUs), and high (≥ 6 BUs). Although the VHA has an existing case complexity assignment process based on CPT codes, it defines complexity differently for inpatient facilities and ambulatory surgery centers. Thus, the BU-defined complexity permitted a standardized complexity categorization across all facilities. Categorization of BUs similar to this has previously been used in the literature as a proxy for case complexity.13,14
Patient-level information included the ASA physical status classification, a measure of overall health status determined by an anesthesia provider preoperatively.15 These classifications included ASA I (healthy), ASA II (mild systemic disease), ASA III (severe systemic disease), ASA IV (severe systemic disease that is a constant threat to life), and ASA V (moribund patient who is not expected to survive without surgery). The last classification, ASA VI: brain-dead with planned organ donation, was excluded. The “E” subcategory denoting “emergency” was subsumed within the corresponding ASA category (eg, ASA V-E was combined with ASA V).
Provider data identified the principal and supervising (if present) anesthetists involved in the case. The provision of anesthesia care was categorized into 3 models: Model 1—a physician anesthesiologist supervising a CRNA; Model 2—a physician anesthesiologist practicing independently or supervising an anesthesiology resident; and Model 3—a CRNA without supervision. Surgical cases were excluded when there was no anesthesia provider (n = 95,795, or 15% of remaining cases), or a nonanesthesia provider (n = 51,647, or 8% of remaining cases) on record. The final sample was 484,082 surgical cases conducted at 125 facilities.
Related: Improving Care and Reducing Length of Stay in Patients Undergoing Total Knee Replacement
Statistical Analysis
The percentage of surgical cases in each anesthesia care model was calculated overall and by the following characteristics: surgical case complexity, ASA classification, and facility complexity. The anesthesia model was determined for each case and summed at the facility level, yielding a total number of cases attributed to each model for each facility, thus identifying the predominant anesthesia model for each facility. The facilities were geographically displayed by their predominant anesthesia model and total number of surgical cases during the period. Because the aim was to present a descriptive representation of anesthesia care models, rather than infer significance, statistical testing was not included.
Results
A total of 484,082 surgical cases met inclusion criteria (Table). These cases were from 109 inpatient facilities and 16 ambulatory surgery facilities.
The percentage of cases in Model 1 was similar across the levels of surgical case complexity. However, a higher proportion of highly complex cases had a physician anesthesiologist (Model 2, 38.8%) than a CRNA (Model 3, 6.4%) as the primary anesthesia provider. Patients in each ASA classification were most likely to receive anesthesia care via Model 1. As ASA level increased, fewer patients had their anesthesia managed by a CRNA without supervision (Model 3: 18.4% of ASA 1 patients vs 8.3% of ASA 4 patients).
Facility complexity demonstrated notable differences in the proportions of surgical cases within each model. More than half of surgical cases in the largest, most complex facilities used Model 1 (64.9%, 58.2%, and 57.7% of cases in 1a, 1b, and 1c facilities, respectively). In comparison, Model 3 was found almost exclusively among surgical cases in smaller facilities with lower complexity (52% and 74% of cases in level 2 and 3 facilities, respectively).
The Figure displays the 125 facilities by their predominant model of anesthesia care. The diameter of the dots is relative to the facility’s total number of surgical cases. For each facility, the predominant model accounted for about half or more of cases but was not necessarily the only model of care used at a particular facility.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Discussion
Anesthesia care in more than half of surgical cases in VHA facilities was delivered by physician anesthesiologists supervising CRNAs. This model of anesthesia care was the dominant model in 54% of the facilities included in the sample. Consistent with a study of non-VHA facilities, this assessment found that the type of facility may influence the model of anesthesia care, with smaller, less complex facilities more often using a CRNA without supervision model.4 In these data, it was noted that among the 28 facilities that predominantly used Model 3, half had 12% or fewer cases that indicated a physician anesthesiologist model of care, and 6 had no cases with physician anesthesiologist involvement. These findings may reflect the limited scope of surgical services offered at lower complexity facilities and/or the reduced availability and/or utilization of physician anesthesiologists in these facilities.
Limitations
We recognize limitations in our assessment of anesthesia care. The documented presence or absence of a supervising anesthesia provider on the surgical record may not adequately characterize the model of anesthesia care in use at a facility, thus limiting an understanding of care delivery relationships among anesthesia providers. In addition, the patterns of anesthesia care delivery are likely influenced by factors not accounted for in this assessment, including the labor market share and economic forces.16,17 The veteran population tends to be older, male, and with substantial chronic disease burden, thus may have differing surgical needs and experiences than that of the general public.18,19 The surgical services offered in VHA facilities as well as the policies and practice environment surrounding anesthesia care also may vary from those found in nongovernmental facilities. However, as the largest health care system in the US, the VHA provides a diverse and robust surgical program. Many VHA facilities are large teaching hospitals with academic affiliations that would parallel some in the public sector. For example, studies have demonstrated similar surgical outcomes for patients in VHA vs non-VHA facilities.20 Therefore, the findings regarding anesthesia care models in VHA are likely relevant to non-VHA surgical sites.
Related: Improving Team-Based Care Coordination Delivery and Documentation in the Health Record
Conclusion
This preliminary assessment of the different models of anesthesia care demonstrates that although primarily relying on teams of anesthesiologists and CRNAs, the VA also uses unsupervised CRNAs to meet veterans’ surgical care needs. Although CRNA practice without supervision represented only 12% of surgical cases in our data, we identified 28 facilities (22%) that predominantly used CRNAs without supervision. Thus, CRNAs with and without supervision deliver a substantial portion of anesthesia care in the VA. The prevalence of CRNAs in documented VA surgical records and among surgical facilities nationwide highlights the importance of further examining their supervised and unsupervised roles in anesthesia care delivery.21 As the practice of anesthesiology continues to evolve, it is imperative that research efforts further investigate ways anesthesia care models may optimize care delivery, benefit anesthesia providers, and improve health outcomes for patients.
Anesthesia care is provided by physician anesthesiologists, certified registered nurse anesthetists (CRNAs), anesthesiology residents, and anesthesiologist assistants. These providers may practice alone (anesthesiologists or CRNAs) or in various combinations of supervised roles and teams. Previous studies reveal mixed findings regarding whether patient outcomes differ by anesthesia practice models.1-7However, little is known about the prevalence of various anesthesia models in the US.
Background
In recent years, anesthesiology has undergone substantial expansion in its scope of services provided, the settings in which it is provided, and the diversity of its workforce.8As the field continues to evolve, especially within the context of value-based health care reform, it is imperative to evaluate how anesthesia care models are used in health systems and how these models may optimize care delivery.
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing surgical care in 110 inpatient medical centers and 27 ambulatory surgery centers. Despite national integration, anesthesia practices vary widely among facilities. The question of which model of anesthesia care is associated with the best outcomes and offers the most value is widely debated.1,5,7,9 As an important first step in understanding anesthesia care delivery, a baseline assessment of the practice patterns of anesthesia providers is necessary and may benefit future studies of the impact of these care models on outcomes. Thus, the aim of this work was to understand and describe the previously unassessed landscape of anesthesia care delivery within the VHA.
Methods
As part of a larger evaluation of anesthesia care delivery in the VHA, an observational assessment of anesthesia provider practice patterns was conducted using retrospective surgical data. This project complies with VHA policy pertaining to nonresearch operational activities and did not require institutional review board approval and adheres to the EQUATOR Network guidelines described in Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).10
Data were obtained from the VHA Managerial Cost Accounting National Data Extract for Surgery package for all surgical procedures (n = 726,706) between October 1, 2013 and March 31, 2015. There were 420 facilities represented in these surgical data. The VHA facility records were used to specifically identify inpatient and ambulatory surgery facilities for inclusion. Additionally, to ensure facilities were valid surgical sites with sufficient surgical volume, those with 100 or fewer cases during the period were excluded. In total, 288 facilities with 9,434 surgical cases (representing 1% of cases) were excluded. These excluded facilities included nursing homes (38%), domiciliaries (26%), outpatient clinics (11%), rehabilitation programs (9%), other nonsurgical facilities (8%), and medical centers (8%). The majority (80%) of excluded medical centers had 30 or fewer surgical cases.
In 6 instances, data from subfacilities were combined with their organizationally affiliated main facilities. The final sample included 125 facilities. The VHA assigns a complexity level designation to facilities, defined as follows: 1a (most complex), 1b, 1c, 2, and 3 (least complex).11 Facilities with 1a designation perform the most complex surgical cases, such as cardiovascular surgery or neurosurgery and have more staff and resource support, whereas levels 2 and 3 facilities perform fewer and less complex cases.
Surgical records were excluded when the primary Current Procedural Terminology (CPT) code was missing (n = 85,748, or 12% of cases). This resulted in 631,524 remaining cases. The surgical CPT codes were mapped to anesthesia CPT codes to obtain the associated base unit (BU) values via a published crosswalk by the American Society of Anesthesiologists (ASA).12 A higher number of associated BUs indicates a more complex procedure. For example, procedures such as biopsies, arthroscopies, and laparoscopies receive 3 to 4 BUs, whereas a venous thrombectomy of the leg and a transurethral resection of the prostate are both 5 BUs, a total knee arthroplasty is 7 BUs, a craniotomy is 10 BUs, and a coronary artery bypass receives 18 BUs. Surgical case complexity was defined as low (3 or 4 BUs), medium (5 BUs), and high (≥ 6 BUs). Although the VHA has an existing case complexity assignment process based on CPT codes, it defines complexity differently for inpatient facilities and ambulatory surgery centers. Thus, the BU-defined complexity permitted a standardized complexity categorization across all facilities. Categorization of BUs similar to this has previously been used in the literature as a proxy for case complexity.13,14
Patient-level information included the ASA physical status classification, a measure of overall health status determined by an anesthesia provider preoperatively.15 These classifications included ASA I (healthy), ASA II (mild systemic disease), ASA III (severe systemic disease), ASA IV (severe systemic disease that is a constant threat to life), and ASA V (moribund patient who is not expected to survive without surgery). The last classification, ASA VI: brain-dead with planned organ donation, was excluded. The “E” subcategory denoting “emergency” was subsumed within the corresponding ASA category (eg, ASA V-E was combined with ASA V).
Provider data identified the principal and supervising (if present) anesthetists involved in the case. The provision of anesthesia care was categorized into 3 models: Model 1—a physician anesthesiologist supervising a CRNA; Model 2—a physician anesthesiologist practicing independently or supervising an anesthesiology resident; and Model 3—a CRNA without supervision. Surgical cases were excluded when there was no anesthesia provider (n = 95,795, or 15% of remaining cases), or a nonanesthesia provider (n = 51,647, or 8% of remaining cases) on record. The final sample was 484,082 surgical cases conducted at 125 facilities.
Related: Improving Care and Reducing Length of Stay in Patients Undergoing Total Knee Replacement
Statistical Analysis
The percentage of surgical cases in each anesthesia care model was calculated overall and by the following characteristics: surgical case complexity, ASA classification, and facility complexity. The anesthesia model was determined for each case and summed at the facility level, yielding a total number of cases attributed to each model for each facility, thus identifying the predominant anesthesia model for each facility. The facilities were geographically displayed by their predominant anesthesia model and total number of surgical cases during the period. Because the aim was to present a descriptive representation of anesthesia care models, rather than infer significance, statistical testing was not included.
Results
A total of 484,082 surgical cases met inclusion criteria (Table). These cases were from 109 inpatient facilities and 16 ambulatory surgery facilities.
The percentage of cases in Model 1 was similar across the levels of surgical case complexity. However, a higher proportion of highly complex cases had a physician anesthesiologist (Model 2, 38.8%) than a CRNA (Model 3, 6.4%) as the primary anesthesia provider. Patients in each ASA classification were most likely to receive anesthesia care via Model 1. As ASA level increased, fewer patients had their anesthesia managed by a CRNA without supervision (Model 3: 18.4% of ASA 1 patients vs 8.3% of ASA 4 patients).
Facility complexity demonstrated notable differences in the proportions of surgical cases within each model. More than half of surgical cases in the largest, most complex facilities used Model 1 (64.9%, 58.2%, and 57.7% of cases in 1a, 1b, and 1c facilities, respectively). In comparison, Model 3 was found almost exclusively among surgical cases in smaller facilities with lower complexity (52% and 74% of cases in level 2 and 3 facilities, respectively).
The Figure displays the 125 facilities by their predominant model of anesthesia care. The diameter of the dots is relative to the facility’s total number of surgical cases. For each facility, the predominant model accounted for about half or more of cases but was not necessarily the only model of care used at a particular facility.
Related: Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic
Discussion
Anesthesia care in more than half of surgical cases in VHA facilities was delivered by physician anesthesiologists supervising CRNAs. This model of anesthesia care was the dominant model in 54% of the facilities included in the sample. Consistent with a study of non-VHA facilities, this assessment found that the type of facility may influence the model of anesthesia care, with smaller, less complex facilities more often using a CRNA without supervision model.4 In these data, it was noted that among the 28 facilities that predominantly used Model 3, half had 12% or fewer cases that indicated a physician anesthesiologist model of care, and 6 had no cases with physician anesthesiologist involvement. These findings may reflect the limited scope of surgical services offered at lower complexity facilities and/or the reduced availability and/or utilization of physician anesthesiologists in these facilities.
Limitations
We recognize limitations in our assessment of anesthesia care. The documented presence or absence of a supervising anesthesia provider on the surgical record may not adequately characterize the model of anesthesia care in use at a facility, thus limiting an understanding of care delivery relationships among anesthesia providers. In addition, the patterns of anesthesia care delivery are likely influenced by factors not accounted for in this assessment, including the labor market share and economic forces.16,17 The veteran population tends to be older, male, and with substantial chronic disease burden, thus may have differing surgical needs and experiences than that of the general public.18,19 The surgical services offered in VHA facilities as well as the policies and practice environment surrounding anesthesia care also may vary from those found in nongovernmental facilities. However, as the largest health care system in the US, the VHA provides a diverse and robust surgical program. Many VHA facilities are large teaching hospitals with academic affiliations that would parallel some in the public sector. For example, studies have demonstrated similar surgical outcomes for patients in VHA vs non-VHA facilities.20 Therefore, the findings regarding anesthesia care models in VHA are likely relevant to non-VHA surgical sites.
Related: Improving Team-Based Care Coordination Delivery and Documentation in the Health Record
Conclusion
This preliminary assessment of the different models of anesthesia care demonstrates that although primarily relying on teams of anesthesiologists and CRNAs, the VA also uses unsupervised CRNAs to meet veterans’ surgical care needs. Although CRNA practice without supervision represented only 12% of surgical cases in our data, we identified 28 facilities (22%) that predominantly used CRNAs without supervision. Thus, CRNAs with and without supervision deliver a substantial portion of anesthesia care in the VA. The prevalence of CRNAs in documented VA surgical records and among surgical facilities nationwide highlights the importance of further examining their supervised and unsupervised roles in anesthesia care delivery.21 As the practice of anesthesiology continues to evolve, it is imperative that research efforts further investigate ways anesthesia care models may optimize care delivery, benefit anesthesia providers, and improve health outcomes for patients.
1. Dulisse B, Cromwell J. No harm found when nurse anesthetists work without supervision by physicians. Health Aff (Millwood). 2010;29(8):1469-1475
2. Simonson DC, Ahern MM, Hendryx MS. Anesthesia staffing and anesthetic complications during cesarean delivery: a retrospective analysis. Nurs Res. 2007;56(1):9-17.
3. Smith AF, Kane M, Milne R. Comparative effectiveness and safety of physician and nurse anaesthetists: a narrative systematic review. Br J Anaesth. 2004;93(4):540-545.
4. Needleman J, Minnick AF. Anesthesia provider model, hospital resources, and maternal outcomes. Health Serv Res. 2009;44(2, pt 1):464-482.
5. Lewis SR, Nicholson A, Smith AF, Alderson P. Physician anaesthetists versus non-physician providers of anaesthesia for surgical patients. Cochrane Database Syst Rev. 2014(7):CD010357.
6. Silber JH, Kennedy SK, Even-Shoshan O, et al. Anesthesiologist direction and patient outcomes. Anesthesiology. 2000;93(1):152-163.
7. Negrusa B, Hogan PF, Warner JT, Schroeder CH, Pang B. Scope of practice laws and anesthesia complications: no measurable impact of certified registered nurse anesthetist expanded scope of practice on anesthesia-related complications. Med Care. 2016;54(10):913-920.
8. Prielipp RC, Cohen NH. The future of anesthesiology: implications of the changing healthcare environment. Curr Opin Anaesthesiol. 2016;29(2):198-205.
9. Memtsoudis SG, Ma Y, Swamidoss CP, Edwards AM, Mazumdar M, Liguori GA. Factors influencing unexpected disposition after orthopedic ambulatory surgery. J Clin Anesth. 2012;24(2):89-95.
10. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epid. 2008;61:344-349.
11. US Department of Veterans Affairs, Veterans Health Administration, Office of Productivity Efficiency & Staffing. Facility Complexity Levels. http://opes.vssc.med.va.gov/FacilityComplexityLevels/Pages/default.aspx. [Nonpublic document; source not verified.
13. Mathis MR, Sathishkumar S, Kheterpal S, et al. Complications, risk factors, and staffing patterns for noncardiac surgery in patients with left ventricular assist devices. Anesthesiology. 2017;126(3):450-460.
14. Chen Y, Gabriel RA, Kodali BS, Urman RD. Effect of anesthesia staffing ratio on first-case surgical start time. J Med Syst. 2016;40(5):115.
15. American Society of Anesthesiologists. Standards, guidelines and related resources. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system. Published October 15, 2014. Accessed November 5, 2018.
16. Kalist DE, Molinari NA, Spurr SJ. Cooperation and conflict between very similar occupations: the case of anesthesia. Health Econ Policy Law. 2011;6(2):237-264.
17. Daugherty L, Fonseca R, Kumar KB, Michaud PC. An analysis of the labor markets for anesthesiology. Rand Health Q. 2011;1(3):18.
18. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
19. Yoon J, Scott JY, Phibbs CS, Wagner TH. Recent trends in Veterans Affairs chronic condition spending. Popul Health Manag. 2011;14(6):293-298.
20. Shekelle PG, Asch S, Glassman P, Matula S, Trivedi A, Miake-Lye I. Comparison of Quality of Care in VA and Non-VA Settings: A Systematic Review. VA Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2010.
21. Baird M, Daugherty L, Kumar KB, Arifkhanova A. Regional and gender differences and trends in the anesthesiologist workforce. Anesthesiology. 2015;123(5):997-1012.
1. Dulisse B, Cromwell J. No harm found when nurse anesthetists work without supervision by physicians. Health Aff (Millwood). 2010;29(8):1469-1475
2. Simonson DC, Ahern MM, Hendryx MS. Anesthesia staffing and anesthetic complications during cesarean delivery: a retrospective analysis. Nurs Res. 2007;56(1):9-17.
3. Smith AF, Kane M, Milne R. Comparative effectiveness and safety of physician and nurse anaesthetists: a narrative systematic review. Br J Anaesth. 2004;93(4):540-545.
4. Needleman J, Minnick AF. Anesthesia provider model, hospital resources, and maternal outcomes. Health Serv Res. 2009;44(2, pt 1):464-482.
5. Lewis SR, Nicholson A, Smith AF, Alderson P. Physician anaesthetists versus non-physician providers of anaesthesia for surgical patients. Cochrane Database Syst Rev. 2014(7):CD010357.
6. Silber JH, Kennedy SK, Even-Shoshan O, et al. Anesthesiologist direction and patient outcomes. Anesthesiology. 2000;93(1):152-163.
7. Negrusa B, Hogan PF, Warner JT, Schroeder CH, Pang B. Scope of practice laws and anesthesia complications: no measurable impact of certified registered nurse anesthetist expanded scope of practice on anesthesia-related complications. Med Care. 2016;54(10):913-920.
8. Prielipp RC, Cohen NH. The future of anesthesiology: implications of the changing healthcare environment. Curr Opin Anaesthesiol. 2016;29(2):198-205.
9. Memtsoudis SG, Ma Y, Swamidoss CP, Edwards AM, Mazumdar M, Liguori GA. Factors influencing unexpected disposition after orthopedic ambulatory surgery. J Clin Anesth. 2012;24(2):89-95.
10. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epid. 2008;61:344-349.
11. US Department of Veterans Affairs, Veterans Health Administration, Office of Productivity Efficiency & Staffing. Facility Complexity Levels. http://opes.vssc.med.va.gov/FacilityComplexityLevels/Pages/default.aspx. [Nonpublic document; source not verified.
13. Mathis MR, Sathishkumar S, Kheterpal S, et al. Complications, risk factors, and staffing patterns for noncardiac surgery in patients with left ventricular assist devices. Anesthesiology. 2017;126(3):450-460.
14. Chen Y, Gabriel RA, Kodali BS, Urman RD. Effect of anesthesia staffing ratio on first-case surgical start time. J Med Syst. 2016;40(5):115.
15. American Society of Anesthesiologists. Standards, guidelines and related resources. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system. Published October 15, 2014. Accessed November 5, 2018.
16. Kalist DE, Molinari NA, Spurr SJ. Cooperation and conflict between very similar occupations: the case of anesthesia. Health Econ Policy Law. 2011;6(2):237-264.
17. Daugherty L, Fonseca R, Kumar KB, Michaud PC. An analysis of the labor markets for anesthesiology. Rand Health Q. 2011;1(3):18.
18. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
19. Yoon J, Scott JY, Phibbs CS, Wagner TH. Recent trends in Veterans Affairs chronic condition spending. Popul Health Manag. 2011;14(6):293-298.
20. Shekelle PG, Asch S, Glassman P, Matula S, Trivedi A, Miake-Lye I. Comparison of Quality of Care in VA and Non-VA Settings: A Systematic Review. VA Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2010.
21. Baird M, Daugherty L, Kumar KB, Arifkhanova A. Regional and gender differences and trends in the anesthesiologist workforce. Anesthesiology. 2015;123(5):997-1012.
Dialing back opioids for chronic pain one conversation at a time
ABSTRACT
Purpose Our study examined the efficacy of a primary-care intervention in reducing opioid use among patients who have chronic non-cancer pain (CNCP). We also recorded the intervention’s effect on patients’ decisions to leave (or stay) with the primary-care practice.
Methods A family physician (FP) identified 41 patients in his practice who had CNCP of at least 6 month’s duration and were using opioids. The intervention with each patient involved an initial discussion of ethical principles, evidence-based practice, and current published guidelines. Following the discussion, patients self-selected to participate with their FP in a continuing tapering program or to accept referral to a pain center for management of their opioid medications. Tapering ranged from a 10% reduction per week to a more rapid 25% to 50% reduction every few days. Twenty-seven patients continued tapering with their FP, and 6 months later were retrospectively placed in the Taper Group. Fourteen patients chose not to pursue the tapering option and were referred to a single-modality medical pain clinic (MPC). All patients had the option of staying with the FP for other medical care.
Results At baseline and again at 6 months post-initial intervention, the MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group. The Taper Group at 6 months was taking significantly lower average daily narcotic doses in morphine equivalents than at baseline. No significant baseline-to-6 month differences were found in the MPC Group. Contrary to many physicians’ fear of losing patients following candid discussions about opioid use, 40 of the 41 patients continued with the FP for other health needs.
Conclusions FPs can frankly discuss opioid use with their patients based on ethical principles and evidence-based recommendations and employ a tapering protocol consistent with current opioid treatment guidelines without jeopardizing the patient-physician relationship.
[polldaddy:10180698]
Opioid prescriptions for chronic noncancer pain (CNCP) have increased significantly over the past 25 years in the United States.1 Despite methodologic concerns surrounding research on opioid harms, prescription opioid misuse among CNCP patients is estimated to be 21% to 29% and prescription addiction 8% to 12%.2 Tragically, with the overall increase in opioid use for CNCP, substance-related hospital admissions and deaths due to opioid overdose have also risen.3
Increased opioid use began in 1985 when the World Health Organization expanded its ethical mandate for pain relief in dying patients to include relief from all cancer pain.3 Opioid use then accelerated following Portenoy and Foley’s 1986 article4 and the 1997 consensus statement by the American Academy of Pain Medicine (AAPM) and the American Pain Society (APS),5 with both organizations arguing that opioids have a role in the treatment of CNCP. Increased use of opioids for CNCP continued throughout the 1990s and 2000s, as many states passed legislation removing sanctions on prescribing long-term and high-dose opioid therapy, and pharmaceutical companies aggressively marketed sustained-release opioids.3
A balanced approach to opioids. While acknowledging the serious public health problems of drug abuse, addiction, and diversion of opioids from licit to illicit uses, clinical research and regulation leaders have called for a balanced approach that recognizes the legitimate medical need for opioids for CNCP. In 2009 the APS, in partnership with the AAPM, published evidence-based guidelines on chronic opioid therapy (COT) for adults with CNCP.6 In developing these guidelines, a multidisciplinary panel of experts conducted systematic reviews of available evidence and made recommendations on formulating COT for individuals, initiating and titrating therapy, regularly monitoring patients, and managing opioid-related adverse effects. Additional recommendations addressed the use of therapies focusing on psychosocial factors. The APS-AAPM guidelines received the highest rating in a systematic review critically appraising 13 guidelines that address the use of opioids for CNCP.7
Continue to: When opioid use is prolonged...
When opioid use is prolonged. Most primary care physicians are aware of the risks of prolonged opioid use, and many have successfully tapered or discontinued opioid medications for patients in acute or pre-chronic stages of pain.8 However, many physicians face the challenge of patients who have used COT for a longer time. The APS-AAPM guidelines may help primary care physicians at any stage of treating CNCP patients.
METHODS
Purpose and design. This retrospective study, which reviewed pretest-posttest findings between and within study groups, received an exempt status from Creighton University’s institutional review board. We designed the study to determine the efficacy of an intervention protocol to reduce opioid use by patients with CNCP who had been in a family physician (FP)'s panel for quite some time. Furthermore, because a common fear among primary care providers is that raising concerns with patients about their opioid use may cause those patients to leave their panel,9 our study also recorded how many patients stayed with their FP after initiation of the opioid management protocol.
Subjects. This study tracked 41 patients with CNCP in 1 FP’s panel. Inclusion criteria for participation was: 1) presence of CNCP for at least 6 months, 2) current use of opioid medication for CNCP, 3) age of at least 16 years, and 4) ability to read and write English. Two exclusion criteria were the presence of a surgically correctable condition or an organic brain syndrome or psychosis.
Clinical intervention. The FP identified eligible patients in his practice that were taking opioids for CNCP and initiated a discussion with each of them emphasizing his desire to follow the ethical principles of beneficence, nonmaleficence, respect for autonomy, and justice.10 The FP also presented his reasons for wanting the patient to stop using opioid medication. They included his beliefs that:
1) COT was not safe for the patient based on a growing body of published evidence of harm and death from COT3;
2) long-term use of opioids could lead to misuse, abuse, or addiction2;
3) prolonged opioid use paradoxically increases pain sensitivity that does not resolve
4) the patient’s current pain medications were not in line with published guidelines for use of opioids for CNCP.6
Initially, 45 patients were eligible for the study, but 4 declined participation before the intervention discussion and were immediately referred to a single-modality medical pain clinic (MPC). These patients were not included in subsequent analyses. Of the remaining 41 patients, all had a discussion with the MD about ethical principles, practice guidelines, and the importance of opioid tapering. After the discussion, patients decided whether to continue with the plan to taper their opioid therapy or to not taper their therapy and so receive a referral to an MPC.
Continue to: The 27 patients who chose to work with...
The 27 patients who chose to work with their FP started an individually tailored opioid-tapering program and were retrospectively placed in the Taper Group 6 months later. Tapering ranged from a slow 10% reduction in dosage per week to a more rapid 25% to 50% reduction every few days. Although evidence to guide specific recommendations on the rate of reduction is lacking, a slower rate may reduce unpleasant symptoms of opioid withdrawal.6 Following the patient-FP discussion, the 14 patients who chose not to pursue the tapering option were referred to an MPC for pain management, but could opt to remain with the FP for all other medical care. At 6 months post-discussion, we retrospectively assigned these 14 patients to the MPC Group.
Measures. We obtained demographic and medical information, including age, gender, race, marital status, and medication level in morphine equivalents, from the electronic health record. Medication level in morphine equivalents was recorded at the beginning of the intervention and again 6 months later. All analyses were conducted using SPSS Version 24 (IBM Corp, Armonk, NY) with P<.05 used to indicate statistical significance.
RESULTS
Between-group differences. The Taper and MPC groups did not differ significantly on demographic variables, with mean ages, respectively, at 57 and 51 years, sex 56% and 50% female, race 74% and 79% white, and marital status 48% and 50% married.
We found significant differences between the Taper and MPC groups on total daily dose in morphine equivalents at baseline and at 6 months following initial intervention. The Levene’s test for equality of variances was statistically significant, indicating unequal variances between the groups. In our SPSS analyses, we therefore used the option “equal variances not assumed.” TABLE 1 lists resultant means, standard deviations, individual sample t-test scores, and confidence intervals. The MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group both at baseline and at 6 months following initial intervention.
Within-group differences. Paired sample t tests indicated significant differences between baseline and 6-month average daily narcotic doses in morphine equivalents for the Taper Group. No significant difference was found between baseline and 6-month daily morphine equivalents for the MPC group. These results indicated that patients who continued opioid tapering with the FP significantly reduced their daily morphine equivalents over the 6 months of the study. Patients in the MPC Group reduced morphine equivalents over the 6 months, but the reduction was not statistically significant. Paired sample t test results are presented in TABLE 2.
Continue to: Patient retention
Patient retention. All but one of the 41 patients in the Tapering and MPC groups continued with the FP for the remainder of their health care needs. Contrary to some physicians’ fears, the patients in this study maintained continuity with their FP.
DISCUSSION
Results of this study indicate that an intervention consisting of a physician-patient discussion of ethical principles and evidence-based practice, followed by individualized opioid tapering per published guidelines, led to a significant reduction in opioid use in patients with CNCP. The Taper Group, which completed the intervention, exhibited significant morphine reductions between baseline and 6-month follow-up. This did not hold true for the MPC Group.
The MPC Group, despite participating in the discussion with the FP, chose not to complete the tapering program and was referred to a single-modality MPC where opioids were managed rather than tapered. While the MPC group reduced daily opioid dose levels, the reduction was not statistically significant. A possible reason for no difference within the MPC Group may be that they had greater dependence on opioids, as their baseline average daily dose was much higher than that in the Taper Group (173 mg vs 31 mg, respectively). Although we did not assess anxiety directly, we speculate that the MPC Group was more anxious about opioid reduction than the Taper Group, and that this anxiety potentially led 4 patients to opt out of the initial FP discussion and 14 patients to self-select out of the tapering program following the discussion.
The FP intervention was successful for the Taper Group. For MPC patients, an enhanced intervention including behavior health strategies13 might have reduced anxiety and increased motivation14 to continue tapering. Based on moderate-quality evidence, APS-AAPM guidelines strongly recommend that CNCP be viewed as a complex biopsychosocial condition. Therefore, clinicians who prescribe opioids should routinely integrate psychotherapeutic interventions, functional restoration, interdisciplinary therapy, and other adjunctive nonopioid therapies.6
Opioid tapering within multidisciplinary rehabilitation programs is possible without significant worsening of pain, mood, and function.15 Recently, an outpatient opioid-tapering support intervention showed promise for efficacy in reducing prescription opioid doses without resultant increases in pain intensity or pain interference.16
Continue to: The tapering protocol in our study...
The tapering protocol in our study and the inclusion of behavioral health co-interventions are also recommended by the 2016 guidelines published by the Center for Disease Control and Prevention.17 More information on the similarities and differences among the various guidelines is available online.18,19
Caveats with our study. Patients’ entry into the Taper or MPC groups occurred through self-selection rather than random assignment. Thus, caution is recommended in interpreting findings of the FP intervention. And, we did not measure patients’ levels of pain, so differences between groups may have been possible. In addition, the number of patients per group was relatively small, which may have accounted for the lack of significance in the MPC Group findings. Conversely, significant reductions in opioid use in the small tapering sample suggests a relatively robust intervention, despite a lack of random assignment to treatment conditions.
These findings suggest that FPs can have a frank conversation about opioid use with their patients based on ethical principles and evidence-based practice, and employ a tapering protocol consistent with current opioid treatment guidelines. Furthermore, this approach appears not to jeopardize the patient-physician relationship.
CORRESPONDENCE
Thomas P. Guck, PhD, Creighton University School of Medicine, 2412 Cuming Street, Omaha, NE 68131; [email protected].
1. Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9-ES38.
2. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576.
3. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154:S94-S100.
4. Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25:171-186.
5. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6-8.
6. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
7. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
8. Hwang CS, Turner LW, Kruszewski SP, et al. Primary care physicians’ knowledge and attitudes regarding prescription opioid abuse and diversion. Clin J Pain. 2016;279-284.
9. Top 15 challenges facing physicians in 2015. Medical Economics. http://www.medicaleconomics.com/medical-economics/news/top-15-challenges-facing-physicians-2015?page=0,12. Accessed October 18, 2018.
10. Kotalik J. Controlling pain and reducing misuse of opioids: ethical considerations. Can Fam Physician. 2012;58:381-385.
11. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570-587.
12. Wachholtz A, Gonzalez G. Co-morbid pain and opioid addiction: long term effect of opioid maintenance on acute pain. Drug Alcohol Depend. 2014;145:143-149.
13. Hunter CL, Goodie JL, Oordt MS, Dobmeyer AC. Integrated Behavioral Health in Primary Care. 2nd ed. Washington DC: American Psychological Association; 2017.
14. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
15. Townsend CO, Kerkvliet JL, Bruce BK, et al. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Pain. 2008;140:177-189.
16. Sullivan MD, Turner JA, DiLodovico C, et al. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. 2017;18:308-318.
17. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
18. Barth KS, Guille C, McCauley J, et al. Targeting practitioners: a review of guidelines, training, and policy in pain management. Drug Alcohol Depend. 2017;173:S22-S30.
19. CDC. Common Elements in Guidelines for Prescribing Opioids for Chronic Pain. Injury Prevention & Control: Prescription Drug Overdose 2016. http://www.cdc.gov/drugoverdose/prescribing/common-elements.html. Accessed October 18, 2018.
ABSTRACT
Purpose Our study examined the efficacy of a primary-care intervention in reducing opioid use among patients who have chronic non-cancer pain (CNCP). We also recorded the intervention’s effect on patients’ decisions to leave (or stay) with the primary-care practice.
Methods A family physician (FP) identified 41 patients in his practice who had CNCP of at least 6 month’s duration and were using opioids. The intervention with each patient involved an initial discussion of ethical principles, evidence-based practice, and current published guidelines. Following the discussion, patients self-selected to participate with their FP in a continuing tapering program or to accept referral to a pain center for management of their opioid medications. Tapering ranged from a 10% reduction per week to a more rapid 25% to 50% reduction every few days. Twenty-seven patients continued tapering with their FP, and 6 months later were retrospectively placed in the Taper Group. Fourteen patients chose not to pursue the tapering option and were referred to a single-modality medical pain clinic (MPC). All patients had the option of staying with the FP for other medical care.
Results At baseline and again at 6 months post-initial intervention, the MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group. The Taper Group at 6 months was taking significantly lower average daily narcotic doses in morphine equivalents than at baseline. No significant baseline-to-6 month differences were found in the MPC Group. Contrary to many physicians’ fear of losing patients following candid discussions about opioid use, 40 of the 41 patients continued with the FP for other health needs.
Conclusions FPs can frankly discuss opioid use with their patients based on ethical principles and evidence-based recommendations and employ a tapering protocol consistent with current opioid treatment guidelines without jeopardizing the patient-physician relationship.
[polldaddy:10180698]
Opioid prescriptions for chronic noncancer pain (CNCP) have increased significantly over the past 25 years in the United States.1 Despite methodologic concerns surrounding research on opioid harms, prescription opioid misuse among CNCP patients is estimated to be 21% to 29% and prescription addiction 8% to 12%.2 Tragically, with the overall increase in opioid use for CNCP, substance-related hospital admissions and deaths due to opioid overdose have also risen.3
Increased opioid use began in 1985 when the World Health Organization expanded its ethical mandate for pain relief in dying patients to include relief from all cancer pain.3 Opioid use then accelerated following Portenoy and Foley’s 1986 article4 and the 1997 consensus statement by the American Academy of Pain Medicine (AAPM) and the American Pain Society (APS),5 with both organizations arguing that opioids have a role in the treatment of CNCP. Increased use of opioids for CNCP continued throughout the 1990s and 2000s, as many states passed legislation removing sanctions on prescribing long-term and high-dose opioid therapy, and pharmaceutical companies aggressively marketed sustained-release opioids.3
A balanced approach to opioids. While acknowledging the serious public health problems of drug abuse, addiction, and diversion of opioids from licit to illicit uses, clinical research and regulation leaders have called for a balanced approach that recognizes the legitimate medical need for opioids for CNCP. In 2009 the APS, in partnership with the AAPM, published evidence-based guidelines on chronic opioid therapy (COT) for adults with CNCP.6 In developing these guidelines, a multidisciplinary panel of experts conducted systematic reviews of available evidence and made recommendations on formulating COT for individuals, initiating and titrating therapy, regularly monitoring patients, and managing opioid-related adverse effects. Additional recommendations addressed the use of therapies focusing on psychosocial factors. The APS-AAPM guidelines received the highest rating in a systematic review critically appraising 13 guidelines that address the use of opioids for CNCP.7
Continue to: When opioid use is prolonged...
When opioid use is prolonged. Most primary care physicians are aware of the risks of prolonged opioid use, and many have successfully tapered or discontinued opioid medications for patients in acute or pre-chronic stages of pain.8 However, many physicians face the challenge of patients who have used COT for a longer time. The APS-AAPM guidelines may help primary care physicians at any stage of treating CNCP patients.
METHODS
Purpose and design. This retrospective study, which reviewed pretest-posttest findings between and within study groups, received an exempt status from Creighton University’s institutional review board. We designed the study to determine the efficacy of an intervention protocol to reduce opioid use by patients with CNCP who had been in a family physician (FP)'s panel for quite some time. Furthermore, because a common fear among primary care providers is that raising concerns with patients about their opioid use may cause those patients to leave their panel,9 our study also recorded how many patients stayed with their FP after initiation of the opioid management protocol.
Subjects. This study tracked 41 patients with CNCP in 1 FP’s panel. Inclusion criteria for participation was: 1) presence of CNCP for at least 6 months, 2) current use of opioid medication for CNCP, 3) age of at least 16 years, and 4) ability to read and write English. Two exclusion criteria were the presence of a surgically correctable condition or an organic brain syndrome or psychosis.
Clinical intervention. The FP identified eligible patients in his practice that were taking opioids for CNCP and initiated a discussion with each of them emphasizing his desire to follow the ethical principles of beneficence, nonmaleficence, respect for autonomy, and justice.10 The FP also presented his reasons for wanting the patient to stop using opioid medication. They included his beliefs that:
1) COT was not safe for the patient based on a growing body of published evidence of harm and death from COT3;
2) long-term use of opioids could lead to misuse, abuse, or addiction2;
3) prolonged opioid use paradoxically increases pain sensitivity that does not resolve
4) the patient’s current pain medications were not in line with published guidelines for use of opioids for CNCP.6
Initially, 45 patients were eligible for the study, but 4 declined participation before the intervention discussion and were immediately referred to a single-modality medical pain clinic (MPC). These patients were not included in subsequent analyses. Of the remaining 41 patients, all had a discussion with the MD about ethical principles, practice guidelines, and the importance of opioid tapering. After the discussion, patients decided whether to continue with the plan to taper their opioid therapy or to not taper their therapy and so receive a referral to an MPC.
Continue to: The 27 patients who chose to work with...
The 27 patients who chose to work with their FP started an individually tailored opioid-tapering program and were retrospectively placed in the Taper Group 6 months later. Tapering ranged from a slow 10% reduction in dosage per week to a more rapid 25% to 50% reduction every few days. Although evidence to guide specific recommendations on the rate of reduction is lacking, a slower rate may reduce unpleasant symptoms of opioid withdrawal.6 Following the patient-FP discussion, the 14 patients who chose not to pursue the tapering option were referred to an MPC for pain management, but could opt to remain with the FP for all other medical care. At 6 months post-discussion, we retrospectively assigned these 14 patients to the MPC Group.
Measures. We obtained demographic and medical information, including age, gender, race, marital status, and medication level in morphine equivalents, from the electronic health record. Medication level in morphine equivalents was recorded at the beginning of the intervention and again 6 months later. All analyses were conducted using SPSS Version 24 (IBM Corp, Armonk, NY) with P<.05 used to indicate statistical significance.
RESULTS
Between-group differences. The Taper and MPC groups did not differ significantly on demographic variables, with mean ages, respectively, at 57 and 51 years, sex 56% and 50% female, race 74% and 79% white, and marital status 48% and 50% married.
We found significant differences between the Taper and MPC groups on total daily dose in morphine equivalents at baseline and at 6 months following initial intervention. The Levene’s test for equality of variances was statistically significant, indicating unequal variances between the groups. In our SPSS analyses, we therefore used the option “equal variances not assumed.” TABLE 1 lists resultant means, standard deviations, individual sample t-test scores, and confidence intervals. The MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group both at baseline and at 6 months following initial intervention.
Within-group differences. Paired sample t tests indicated significant differences between baseline and 6-month average daily narcotic doses in morphine equivalents for the Taper Group. No significant difference was found between baseline and 6-month daily morphine equivalents for the MPC group. These results indicated that patients who continued opioid tapering with the FP significantly reduced their daily morphine equivalents over the 6 months of the study. Patients in the MPC Group reduced morphine equivalents over the 6 months, but the reduction was not statistically significant. Paired sample t test results are presented in TABLE 2.
Continue to: Patient retention
Patient retention. All but one of the 41 patients in the Tapering and MPC groups continued with the FP for the remainder of their health care needs. Contrary to some physicians’ fears, the patients in this study maintained continuity with their FP.
DISCUSSION
Results of this study indicate that an intervention consisting of a physician-patient discussion of ethical principles and evidence-based practice, followed by individualized opioid tapering per published guidelines, led to a significant reduction in opioid use in patients with CNCP. The Taper Group, which completed the intervention, exhibited significant morphine reductions between baseline and 6-month follow-up. This did not hold true for the MPC Group.
The MPC Group, despite participating in the discussion with the FP, chose not to complete the tapering program and was referred to a single-modality MPC where opioids were managed rather than tapered. While the MPC group reduced daily opioid dose levels, the reduction was not statistically significant. A possible reason for no difference within the MPC Group may be that they had greater dependence on opioids, as their baseline average daily dose was much higher than that in the Taper Group (173 mg vs 31 mg, respectively). Although we did not assess anxiety directly, we speculate that the MPC Group was more anxious about opioid reduction than the Taper Group, and that this anxiety potentially led 4 patients to opt out of the initial FP discussion and 14 patients to self-select out of the tapering program following the discussion.
The FP intervention was successful for the Taper Group. For MPC patients, an enhanced intervention including behavior health strategies13 might have reduced anxiety and increased motivation14 to continue tapering. Based on moderate-quality evidence, APS-AAPM guidelines strongly recommend that CNCP be viewed as a complex biopsychosocial condition. Therefore, clinicians who prescribe opioids should routinely integrate psychotherapeutic interventions, functional restoration, interdisciplinary therapy, and other adjunctive nonopioid therapies.6
Opioid tapering within multidisciplinary rehabilitation programs is possible without significant worsening of pain, mood, and function.15 Recently, an outpatient opioid-tapering support intervention showed promise for efficacy in reducing prescription opioid doses without resultant increases in pain intensity or pain interference.16
Continue to: The tapering protocol in our study...
The tapering protocol in our study and the inclusion of behavioral health co-interventions are also recommended by the 2016 guidelines published by the Center for Disease Control and Prevention.17 More information on the similarities and differences among the various guidelines is available online.18,19
Caveats with our study. Patients’ entry into the Taper or MPC groups occurred through self-selection rather than random assignment. Thus, caution is recommended in interpreting findings of the FP intervention. And, we did not measure patients’ levels of pain, so differences between groups may have been possible. In addition, the number of patients per group was relatively small, which may have accounted for the lack of significance in the MPC Group findings. Conversely, significant reductions in opioid use in the small tapering sample suggests a relatively robust intervention, despite a lack of random assignment to treatment conditions.
These findings suggest that FPs can have a frank conversation about opioid use with their patients based on ethical principles and evidence-based practice, and employ a tapering protocol consistent with current opioid treatment guidelines. Furthermore, this approach appears not to jeopardize the patient-physician relationship.
CORRESPONDENCE
Thomas P. Guck, PhD, Creighton University School of Medicine, 2412 Cuming Street, Omaha, NE 68131; [email protected].
ABSTRACT
Purpose Our study examined the efficacy of a primary-care intervention in reducing opioid use among patients who have chronic non-cancer pain (CNCP). We also recorded the intervention’s effect on patients’ decisions to leave (or stay) with the primary-care practice.
Methods A family physician (FP) identified 41 patients in his practice who had CNCP of at least 6 month’s duration and were using opioids. The intervention with each patient involved an initial discussion of ethical principles, evidence-based practice, and current published guidelines. Following the discussion, patients self-selected to participate with their FP in a continuing tapering program or to accept referral to a pain center for management of their opioid medications. Tapering ranged from a 10% reduction per week to a more rapid 25% to 50% reduction every few days. Twenty-seven patients continued tapering with their FP, and 6 months later were retrospectively placed in the Taper Group. Fourteen patients chose not to pursue the tapering option and were referred to a single-modality medical pain clinic (MPC). All patients had the option of staying with the FP for other medical care.
Results At baseline and again at 6 months post-initial intervention, the MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group. The Taper Group at 6 months was taking significantly lower average daily narcotic doses in morphine equivalents than at baseline. No significant baseline-to-6 month differences were found in the MPC Group. Contrary to many physicians’ fear of losing patients following candid discussions about opioid use, 40 of the 41 patients continued with the FP for other health needs.
Conclusions FPs can frankly discuss opioid use with their patients based on ethical principles and evidence-based recommendations and employ a tapering protocol consistent with current opioid treatment guidelines without jeopardizing the patient-physician relationship.
[polldaddy:10180698]
Opioid prescriptions for chronic noncancer pain (CNCP) have increased significantly over the past 25 years in the United States.1 Despite methodologic concerns surrounding research on opioid harms, prescription opioid misuse among CNCP patients is estimated to be 21% to 29% and prescription addiction 8% to 12%.2 Tragically, with the overall increase in opioid use for CNCP, substance-related hospital admissions and deaths due to opioid overdose have also risen.3
Increased opioid use began in 1985 when the World Health Organization expanded its ethical mandate for pain relief in dying patients to include relief from all cancer pain.3 Opioid use then accelerated following Portenoy and Foley’s 1986 article4 and the 1997 consensus statement by the American Academy of Pain Medicine (AAPM) and the American Pain Society (APS),5 with both organizations arguing that opioids have a role in the treatment of CNCP. Increased use of opioids for CNCP continued throughout the 1990s and 2000s, as many states passed legislation removing sanctions on prescribing long-term and high-dose opioid therapy, and pharmaceutical companies aggressively marketed sustained-release opioids.3
A balanced approach to opioids. While acknowledging the serious public health problems of drug abuse, addiction, and diversion of opioids from licit to illicit uses, clinical research and regulation leaders have called for a balanced approach that recognizes the legitimate medical need for opioids for CNCP. In 2009 the APS, in partnership with the AAPM, published evidence-based guidelines on chronic opioid therapy (COT) for adults with CNCP.6 In developing these guidelines, a multidisciplinary panel of experts conducted systematic reviews of available evidence and made recommendations on formulating COT for individuals, initiating and titrating therapy, regularly monitoring patients, and managing opioid-related adverse effects. Additional recommendations addressed the use of therapies focusing on psychosocial factors. The APS-AAPM guidelines received the highest rating in a systematic review critically appraising 13 guidelines that address the use of opioids for CNCP.7
Continue to: When opioid use is prolonged...
When opioid use is prolonged. Most primary care physicians are aware of the risks of prolonged opioid use, and many have successfully tapered or discontinued opioid medications for patients in acute or pre-chronic stages of pain.8 However, many physicians face the challenge of patients who have used COT for a longer time. The APS-AAPM guidelines may help primary care physicians at any stage of treating CNCP patients.
METHODS
Purpose and design. This retrospective study, which reviewed pretest-posttest findings between and within study groups, received an exempt status from Creighton University’s institutional review board. We designed the study to determine the efficacy of an intervention protocol to reduce opioid use by patients with CNCP who had been in a family physician (FP)'s panel for quite some time. Furthermore, because a common fear among primary care providers is that raising concerns with patients about their opioid use may cause those patients to leave their panel,9 our study also recorded how many patients stayed with their FP after initiation of the opioid management protocol.
Subjects. This study tracked 41 patients with CNCP in 1 FP’s panel. Inclusion criteria for participation was: 1) presence of CNCP for at least 6 months, 2) current use of opioid medication for CNCP, 3) age of at least 16 years, and 4) ability to read and write English. Two exclusion criteria were the presence of a surgically correctable condition or an organic brain syndrome or psychosis.
Clinical intervention. The FP identified eligible patients in his practice that were taking opioids for CNCP and initiated a discussion with each of them emphasizing his desire to follow the ethical principles of beneficence, nonmaleficence, respect for autonomy, and justice.10 The FP also presented his reasons for wanting the patient to stop using opioid medication. They included his beliefs that:
1) COT was not safe for the patient based on a growing body of published evidence of harm and death from COT3;
2) long-term use of opioids could lead to misuse, abuse, or addiction2;
3) prolonged opioid use paradoxically increases pain sensitivity that does not resolve
4) the patient’s current pain medications were not in line with published guidelines for use of opioids for CNCP.6
Initially, 45 patients were eligible for the study, but 4 declined participation before the intervention discussion and were immediately referred to a single-modality medical pain clinic (MPC). These patients were not included in subsequent analyses. Of the remaining 41 patients, all had a discussion with the MD about ethical principles, practice guidelines, and the importance of opioid tapering. After the discussion, patients decided whether to continue with the plan to taper their opioid therapy or to not taper their therapy and so receive a referral to an MPC.
Continue to: The 27 patients who chose to work with...
The 27 patients who chose to work with their FP started an individually tailored opioid-tapering program and were retrospectively placed in the Taper Group 6 months later. Tapering ranged from a slow 10% reduction in dosage per week to a more rapid 25% to 50% reduction every few days. Although evidence to guide specific recommendations on the rate of reduction is lacking, a slower rate may reduce unpleasant symptoms of opioid withdrawal.6 Following the patient-FP discussion, the 14 patients who chose not to pursue the tapering option were referred to an MPC for pain management, but could opt to remain with the FP for all other medical care. At 6 months post-discussion, we retrospectively assigned these 14 patients to the MPC Group.
Measures. We obtained demographic and medical information, including age, gender, race, marital status, and medication level in morphine equivalents, from the electronic health record. Medication level in morphine equivalents was recorded at the beginning of the intervention and again 6 months later. All analyses were conducted using SPSS Version 24 (IBM Corp, Armonk, NY) with P<.05 used to indicate statistical significance.
RESULTS
Between-group differences. The Taper and MPC groups did not differ significantly on demographic variables, with mean ages, respectively, at 57 and 51 years, sex 56% and 50% female, race 74% and 79% white, and marital status 48% and 50% married.
We found significant differences between the Taper and MPC groups on total daily dose in morphine equivalents at baseline and at 6 months following initial intervention. The Levene’s test for equality of variances was statistically significant, indicating unequal variances between the groups. In our SPSS analyses, we therefore used the option “equal variances not assumed.” TABLE 1 lists resultant means, standard deviations, individual sample t-test scores, and confidence intervals. The MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group both at baseline and at 6 months following initial intervention.
Within-group differences. Paired sample t tests indicated significant differences between baseline and 6-month average daily narcotic doses in morphine equivalents for the Taper Group. No significant difference was found between baseline and 6-month daily morphine equivalents for the MPC group. These results indicated that patients who continued opioid tapering with the FP significantly reduced their daily morphine equivalents over the 6 months of the study. Patients in the MPC Group reduced morphine equivalents over the 6 months, but the reduction was not statistically significant. Paired sample t test results are presented in TABLE 2.
Continue to: Patient retention
Patient retention. All but one of the 41 patients in the Tapering and MPC groups continued with the FP for the remainder of their health care needs. Contrary to some physicians’ fears, the patients in this study maintained continuity with their FP.
DISCUSSION
Results of this study indicate that an intervention consisting of a physician-patient discussion of ethical principles and evidence-based practice, followed by individualized opioid tapering per published guidelines, led to a significant reduction in opioid use in patients with CNCP. The Taper Group, which completed the intervention, exhibited significant morphine reductions between baseline and 6-month follow-up. This did not hold true for the MPC Group.
The MPC Group, despite participating in the discussion with the FP, chose not to complete the tapering program and was referred to a single-modality MPC where opioids were managed rather than tapered. While the MPC group reduced daily opioid dose levels, the reduction was not statistically significant. A possible reason for no difference within the MPC Group may be that they had greater dependence on opioids, as their baseline average daily dose was much higher than that in the Taper Group (173 mg vs 31 mg, respectively). Although we did not assess anxiety directly, we speculate that the MPC Group was more anxious about opioid reduction than the Taper Group, and that this anxiety potentially led 4 patients to opt out of the initial FP discussion and 14 patients to self-select out of the tapering program following the discussion.
The FP intervention was successful for the Taper Group. For MPC patients, an enhanced intervention including behavior health strategies13 might have reduced anxiety and increased motivation14 to continue tapering. Based on moderate-quality evidence, APS-AAPM guidelines strongly recommend that CNCP be viewed as a complex biopsychosocial condition. Therefore, clinicians who prescribe opioids should routinely integrate psychotherapeutic interventions, functional restoration, interdisciplinary therapy, and other adjunctive nonopioid therapies.6
Opioid tapering within multidisciplinary rehabilitation programs is possible without significant worsening of pain, mood, and function.15 Recently, an outpatient opioid-tapering support intervention showed promise for efficacy in reducing prescription opioid doses without resultant increases in pain intensity or pain interference.16
Continue to: The tapering protocol in our study...
The tapering protocol in our study and the inclusion of behavioral health co-interventions are also recommended by the 2016 guidelines published by the Center for Disease Control and Prevention.17 More information on the similarities and differences among the various guidelines is available online.18,19
Caveats with our study. Patients’ entry into the Taper or MPC groups occurred through self-selection rather than random assignment. Thus, caution is recommended in interpreting findings of the FP intervention. And, we did not measure patients’ levels of pain, so differences between groups may have been possible. In addition, the number of patients per group was relatively small, which may have accounted for the lack of significance in the MPC Group findings. Conversely, significant reductions in opioid use in the small tapering sample suggests a relatively robust intervention, despite a lack of random assignment to treatment conditions.
These findings suggest that FPs can have a frank conversation about opioid use with their patients based on ethical principles and evidence-based practice, and employ a tapering protocol consistent with current opioid treatment guidelines. Furthermore, this approach appears not to jeopardize the patient-physician relationship.
CORRESPONDENCE
Thomas P. Guck, PhD, Creighton University School of Medicine, 2412 Cuming Street, Omaha, NE 68131; [email protected].
1. Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9-ES38.
2. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576.
3. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154:S94-S100.
4. Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25:171-186.
5. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6-8.
6. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
7. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
8. Hwang CS, Turner LW, Kruszewski SP, et al. Primary care physicians’ knowledge and attitudes regarding prescription opioid abuse and diversion. Clin J Pain. 2016;279-284.
9. Top 15 challenges facing physicians in 2015. Medical Economics. http://www.medicaleconomics.com/medical-economics/news/top-15-challenges-facing-physicians-2015?page=0,12. Accessed October 18, 2018.
10. Kotalik J. Controlling pain and reducing misuse of opioids: ethical considerations. Can Fam Physician. 2012;58:381-385.
11. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570-587.
12. Wachholtz A, Gonzalez G. Co-morbid pain and opioid addiction: long term effect of opioid maintenance on acute pain. Drug Alcohol Depend. 2014;145:143-149.
13. Hunter CL, Goodie JL, Oordt MS, Dobmeyer AC. Integrated Behavioral Health in Primary Care. 2nd ed. Washington DC: American Psychological Association; 2017.
14. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
15. Townsend CO, Kerkvliet JL, Bruce BK, et al. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Pain. 2008;140:177-189.
16. Sullivan MD, Turner JA, DiLodovico C, et al. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. 2017;18:308-318.
17. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
18. Barth KS, Guille C, McCauley J, et al. Targeting practitioners: a review of guidelines, training, and policy in pain management. Drug Alcohol Depend. 2017;173:S22-S30.
19. CDC. Common Elements in Guidelines for Prescribing Opioids for Chronic Pain. Injury Prevention & Control: Prescription Drug Overdose 2016. http://www.cdc.gov/drugoverdose/prescribing/common-elements.html. Accessed October 18, 2018.
1. Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9-ES38.
2. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576.
3. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154:S94-S100.
4. Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25:171-186.
5. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6-8.
6. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
7. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
8. Hwang CS, Turner LW, Kruszewski SP, et al. Primary care physicians’ knowledge and attitudes regarding prescription opioid abuse and diversion. Clin J Pain. 2016;279-284.
9. Top 15 challenges facing physicians in 2015. Medical Economics. http://www.medicaleconomics.com/medical-economics/news/top-15-challenges-facing-physicians-2015?page=0,12. Accessed October 18, 2018.
10. Kotalik J. Controlling pain and reducing misuse of opioids: ethical considerations. Can Fam Physician. 2012;58:381-385.
11. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570-587.
12. Wachholtz A, Gonzalez G. Co-morbid pain and opioid addiction: long term effect of opioid maintenance on acute pain. Drug Alcohol Depend. 2014;145:143-149.
13. Hunter CL, Goodie JL, Oordt MS, Dobmeyer AC. Integrated Behavioral Health in Primary Care. 2nd ed. Washington DC: American Psychological Association; 2017.
14. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
15. Townsend CO, Kerkvliet JL, Bruce BK, et al. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Pain. 2008;140:177-189.
16. Sullivan MD, Turner JA, DiLodovico C, et al. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. 2017;18:308-318.
17. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
18. Barth KS, Guille C, McCauley J, et al. Targeting practitioners: a review of guidelines, training, and policy in pain management. Drug Alcohol Depend. 2017;173:S22-S30.
19. CDC. Common Elements in Guidelines for Prescribing Opioids for Chronic Pain. Injury Prevention & Control: Prescription Drug Overdose 2016. http://www.cdc.gov/drugoverdose/prescribing/common-elements.html. Accessed October 18, 2018.
Barriers to Early Hospital Discharge: A Cross-Sectional Study at Five Academic Hospitals
Hospital discharges frequently occur in the afternoon or evening hours.1-5 Late discharges can adversely affect patient flow throughout the hospital,3,6-9 which, in turn, can result in delays in care,10-16 more medication errors,17 increased mortality,18-20 longer lengths of stay,20-22 higher costs,23 and lower patient satisfaction.24
Various interventions have been employed in the attempts to find ways of moving discharge times to earlier in the day, including preparing the discharge paperwork and medications the previous night,25 using checklists,1,25 team huddles,2 providing real-time feedback to unit staff,1 and employing multidisciplinary teamwork.1,2,6,25,26
The purpose of this study was to identify and determine the relative frequency of barriers to writing discharge orders in the hopes of identifying issues that might be addressed by targeted interventions. We also assessed the effects of daily team census, patients being on teaching versus nonteaching services, and how daily rounds were structured at the time that the discharge orders were written.
METHODS
Study Design, Setting, and Participants
We conducted a prospective, cross-sectional survey of house-staff and attending physicians on general medicine teaching and nonteaching services from November 13, 2014, through May 31, 2016. The study was conducted at the following five hospitals: Denver Health Medical Center (DHMC) and Presbyterian/Saint Luke’s Medical Center (PSL) in Denver, Colorado; Ronald Reagan University (UCLA) and Los Angeles County/University of Southern California Medical Center (LAC+USC) in Los Angeles, California; and Harborview Medical Center (HMC) in Seattle, Washington. The study was approved by the Colorado Multi-Institutional Review Board as well as by the review boards of the other participating sites.
Data Collection
The results of the focus groups composed of attending physicians at DHMC were used to develop our initial data collection template. Additional sites joining the study provided feedback, leading to modifications (Appendix 1).
Physicians were surveyed at three different time points on study days that were selected according to the convenience of the investigators. The sampling occurred only on weekdays and was done based on the investigators’ availability. Investigators would attempt to survey as many teams as they were able to but, secondary to feasibility, not all teams could be surveyed on study days. The specific time points varied as a function of physician workflows but were standardized as much as possible to occur in the early morning, around noon, and midafternoon on weekdays. Physicians were contacted either in person or by telephone for verbal consent prior to administering the first survey. All general medicine teams were eligible. For teaching teams, the order of contact was resident, intern, and then attending based on which physician was available at the time of the survey and on which member of the team was thought to know the patients the best. For the nonteaching services, the attending physicians were contacted.
During the initial survey, the investigators assessed the provider role (ie, attending or housestaff), whether the service was a teaching or a nonteaching service, and the starting patient census on that service primarily based on interviewing the provider of record for the team and looking at team census lists. Physicians were asked about their rounding style (ie, sickest patients first, patients likely to be discharged first, room-by-room, most recently admitted patients first, patients on the team the longest, or other) and then to identify all patients they thought would be definite discharges sometime during the day of the survey. Definite discharges were defined as patients whom the provider thought were either currently ready for discharge or who had only minor barriers that, if unresolved, would not prevent same-day discharge. They were asked if the discharge order had been entered and, if not, what was preventing them from doing so, if the discharge could in their opinion have occurred the day prior and, if so, why this did not occur. We also obtained the date and time of the admission and discharge orders, the actual discharge time, as well as the length of stay either through chart review (majority of sites) or from data warehouses (Denver Health and Presbyterian St. Lukes had length of stay data retrieved from their data warehouse).
Physicians were also asked to identify all patients whom they thought might possibly be discharged that day. Possible discharges were defined as patients with barriers to discharge that, if unresolved, would prevent same-day discharge. For each of these, the physicians were asked to list whatever issues needed to be resolved prior to placing the discharge order (Appendix 1).
The second survey was administered late morning on the same day, typically between 11
The third survey was administered midafternoon, typically around 3 PM similar to the first two surveys, with the exception that the third survey did not attempt to identify new definite or possible discharges.
Sample Size
We stopped collecting data after obtaining a convenience sample of 5% of total discharges at each study site or on the study end date, which was May 31, 2016, whichever came first.
Data Analysis
Data were collected and managed using a secure, web-based application electronic data capture tool (REDCap), hosted at Denver Health. REDCap (Research Electronic Data Capture, Nashville, Tennessee) is designed to support data collection for research studies.27 Data were then analyzed using SAS Enterprise Guide 5.1 (SAS Institute, Inc., Cary, North Carolina). All data entered into REDCap were reviewed by the principal investigator to ensure that data were not missing, and when there were missing data, a query was sent to verify if the data were retrievable. If retrievable, then the data would be entered. The volume of missing data that remained is described in our results.
Continuous variables were described using means and standard deviations (SD) or medians and interquartile ranges (IQR) based on tests of normality. Differences in the time that the discharge orders were placed in the electronic medical record according to morning patient census, teaching versus nonteaching service, and rounding style were compared using the Wilcoxon rank sum test. Linear regression was used to evaluate the effect of patient census on discharge order time. P < .05 was considered as significant.
RESULTS
We conducted 1,584 patient evaluations through surveys of 254 physicians over 156 days. Given surveys coincided with the existing work we had full participation (ie, 100% participation) and no dropout during the study days. Median (IQR) survey time points were 8:30
The characteristics of the five hospitals participating in the study, the patients’ final discharge status, the types of physicians surveyed, the services on which they were working, the rounding styles employed, and the median starting daily census are summarized in Table 1. The majority of the physicians surveyed were housestaff working on teaching services, and only a small minority structured rounds such that patients ready for discharge were seen first.
Over the course of the three surveys, 949 patients were identified as being definite discharges at any time point, and the large majority of these (863, 91%) were discharged on the day of the survey. The median (IQR) time that the discharge orders were written was 11:50
During the initial morning survey, 314 patients were identified as being definite discharges for that day (representing approximately 6% of the total number of patients being cared for, or 33% of the patients identified as definite discharges throughout the day). Of these, the physicians thought that 44 (<1% of the total number of patients being cared for on the services) could have been discharged on the previous day. The most frequent reasons cited for why these patients were not discharged on the previous day were “Patient did not want to leave” (n = 15, 34%), “Too late in the day” (n = 10, 23%), and “No ride” (n = 9, 20%). The remaining 10 patients (23%) had a variety of reasons related to system or social issues (ie, shelter not available, miscommunication).
At the morning time point, the most common barriers to discharge identified were that the physicians had not finished rounding on their team of patients and that the housestaff needed to staff their patients with their attending. At noon, caring for other patients and tending to the discharge processes were most commonly cited, and in the afternoon, the most common barriers were that the physicians were in the process of completing the discharge paperwork for those patients or were discharging other patients (Table 2). When comparing barriers on teaching to nonteaching teams, a higher proportion of teaching teams were still rounding on all patients and were working on discharge paperwork at the second survey. Barriers cited by sites were similar; however, the frequency at which the barriers were mentioned varied (data not shown).
The physicians identified 1,237 patients at any time point as being possible discharges during the day of the survey and these had a mean (±SD) of 1.3 (±0.5) barriers cited for why these patients were possible rather than definite discharges. The most common were that clinical improvement was needed, one or more pending issues related to their care needed to be resolved, and/or awaiting pending test results. The need to see clinical improvement generally decreased throughout the day as did the need to staff patients with an attending physician, but barriers related to consultant recommendations or completing procedures increased (Table 3). Of the 1,237 patients ever identified as possible discharges, 594 (48%) became a definite discharge by the third call and 444 (36%) became a no discharge as their final status. As with definite discharges, barriers cited by sites were similar; however, the frequency at which the barriers were mentioned varied.
Among the 949 and 1,237 patients who were ever identified as definite or possible discharges, respectively, at any time point during the study day, 28 (3%) and 444 (36%), respectively, had their discharge status changed to no discharge, most commonly because their clinical condition either worsened or expected improvements did not occur or that barriers pertaining to social work, physical therapy, or occupational therapy were not resolved.
The median time that the discharge orders were entered into the electronic medical record was 43 minutes earlier if patients were on teams with a lower versus a higher starting census (P = .0003), 48 minutes earlier if they were seen by physicians whose rounding style was to see patients first who potentially could be discharged (P = .0026), and 58 minutes earlier if they were on nonteaching versus teaching services (P < .0001; Table 4). For every one-person increase in census, the discharge order time increased by 6 minutes (β = 5.6, SE = 1.6, P = .0003).
DISCUSSION
The important findings of this study are that (1) the large majority of issues thought to delay discharging patients identified as definite discharges were related to physicians caring for other patients on their team, (2) although 91% of patients ever identified as being definite discharges were discharged on the day of the survey, only 48% of those identified as possible discharges became definite discharges by the afternoon time point, largely because the anticipated clinical improvement did not occur or care being provided by ancillary services had not been completed, and (3) discharge orders on patients identified as definite discharges were written on average 50 minutes earlier by physicians on teams with a smaller starting patient census, on nonteaching services, or when the rounding style was to see patients ready for discharges first.
Previous research has reported that physician-perceived barriers to discharge were extrinsic to providers and even extrinsic to the hospital setting (eg, awaiting subacute nursing placement and transportation).28,29 However, many of the barriers that we identified were related directly to the providers’ workload and rounding styles and whether the patients were on teaching versus nonteaching services. We also found that delays in the ability of hospital services to complete care also contributed to delayed discharges.
Our observational data suggest that delays resulting from caring for other patients might be reduced by changing rounding styles such that patients ready for discharge are seen first and are discharged prior to seeing other patients on the team, as previously reported by Beck et al.30 Intuitively, this would seem to be a straightforward way of freeing up beds earlier in the day, but such a change will, of necessity, lead to delaying care for other patients, which, in turn, could increase their length of stays. Durvasula et al. suggested that discharges could be moved to earlier in the day by completing orders and paperwork the day prior to discharge.25 Such an approach might be effective on an Obstetrical or elective Orthopedic service on which patients predictably are hospitalized for a fixed number of days (or even hours) but may be less relevant to patients on internal medicine services where lengths of stay are less predictable. Interventions to improve discharge times have resulted in earlier discharge times in some studies,2,4 but the overall length of stay either did not decrease25 or increased31 in others. Werthheimer et al.1 did find earlier discharge times, but other interventions also occurred during the study period (eg, extending social work services to include weekends).1,32
We found that discharge times were approximately 50 minutes earlier on teams with a smaller starting census, on nonteaching compared with teaching services, or when the attending’s rounding style was to see patients ready for discharges first. Although 50 minutes may seem like a small change in discharge time, Khanna et al.33 found that when discharges occur even 1 hour earlier, hospital overcrowding is reduced. To have a lower team census would require having more teams and more providers to staff these teams, raising cost-effectiveness concerns. Moving to more nonteaching services could represent a conflict with respect to one of the missions of teaching hospitals and raises a cost-benefit issue as several teaching hospitals receive substantial funding in support of their teaching activities and housestaff would have to be replaced with more expensive providers.
Delays attributable to ancillary services indicate imbalances between demand and availability of these services. Inappropriate demand and inefficiencies could be reduced by systems redesign, but in at least some instances, additional resources will be needed to add staff, increase space, or add additional equipment.
Our study has several limitations. First, we surveyed only physicians working in university-affiliated hospitals, and three of these were public safety-net hospitals. Accordingly, our results may not be generalizable to different patient populations. Second, we surveyed only physicians, and Minichiello et al.29 found that barriers to discharge perceived by physicians were different from those of other staff. Third, our data were observational and were collected only on weekdays. Fourth, we did not differentiate interns from residents, and thus, potentially the level of training could have affected these results. Similarly, the decision for a “possible” and a “definite” discharge is likely dependent on the knowledge base of the participant, such that less experienced participants may have had differing perspectives than someone with more experience. Fifth, the sites did vary based on the infrastructure and support but also had several similarities. All sites had social work and case management involved in care, although at some sites, they were assigned according to team and at others according to geographic location. Similarly, rounding times varied. Most of the services surveyed did not utilize advanced practice providers (the exception was the nonteaching services at Denver Health, and their presence was variable). These differences in staffing models could also have affected these results.
Our study also has a number of strengths. First, we assessed the barriers at five different hospitals. Second, we collected real-time data related to specific barriers at multiple time points throughout the day, allowing us to assess the dynamic nature of identifying patients as being ready or nearly ready for discharge. Third, we assessed the perceptions of barriers to discharge from physicians working on teaching as well as nonteaching services and from physicians utilizing a variety of rounding styles. Fourth, we had a very high participation rate (100%), probably due to the fact that our study was strategically aligned with participants’ daily work activities.
In conclusion, we found two distinct categories of issues that physicians perceived as most commonly delaying writing discharge orders on their patients. The first pertained to patients thought to definitely be ready for discharge and was related to the physicians having to care for other patients on their team. The second pertained to patients identified as possibly ready for discharge and was related to the need for care to be completed by a variety of ancillary services. Addressing each of these barriers would require different interventions and a need to weigh the potential improvements that could be achieved against the increased costs and/or delays in care for other patients that may result.
Disclosures
The authors report no conflicts of interest relevant to this work.
1. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
2. Kane M, Weinacker A, Arthofer R, et al. A multidisciplinary initiative to increase inpatient discharges before noon. J Nurs Adm. 2016;46(12):630-635. doi: 10.1097/NNA.0000000000000418. PubMed
3. Khanna S, Sier D, Boyle J, Zeitz K. Discharge timeliness and its impact on hospital crowding and emergency department flow performance. Emerg Med Australas. 2016;28(2):164-170. doi: 10.1111/1742-6723.12543. PubMed
4. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag (Frederick). 2007;26:142-146. doi: 10.1097/01.HCM.0000268617.33491.60. PubMed
5. Khanna S, Boyle J, Good N, Lind J. Impact of admission and discharge peak times on hospital overcrowding. Stud Health Technol Inform. 2011;168:82-88. doi: 10.3233/978-1-60750-791-8-82. PubMed
6. McGowan JE, Truwit JD, Cipriano P, et al. Operating room efficiency and hospital capacity: factors affecting operating room use during maximum hospital census. J Am Coll Surg. 2007;204(5):865-871; discussion 71-72. doi: 10.1016/j.jamcollsurg.2007.01.052 PubMed
7. Khanna S, Boyle J, Good N, Lind J. Early discharge and its effect on ED length of stay and access block. Stud Health Technol Inform. 2012;178:92-98. doi: 10.3233/978-1-61499-078-9-92 PubMed
8. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi: 10.1016/j.jemermed.2010.06.028. PubMed
9. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi: 10.1002/jhm.2412. PubMed
10. Sikka R, Mehta S, Kaucky C, Kulstad EB. ED crowding is associated with an increased time to pneumonia treatment. Am J Emerg Med. 2010;28(7):809-812. doi: 10.1016/j.ajem.2009.06.023. PubMed
11. Coil CJ, Flood JD, Belyeu BM, Young P, Kaji AH, Lewis RJ. The effect of emergency department boarding on order completion. Ann Emerg Med. 2016;67:730-736 e2. doi: 10.1016/j.annemergmed.2015.09.018. PubMed
12. Gaieski DF, Agarwal AK, Mikkelsen ME, et al. The impact of ED crowding on early interventions and mortality in patients with severe sepsis. Am J Emerg Med. 2017;35:953-960. doi: 10.1016/j.ajem.2017.01.061. PubMed
13. Pines JM, Localio AR, Hollander JE, et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007;50(5):510-516. doi: 10.1016/j.annemergmed.2007.07.021. PubMed
14. Hwang U, Richardson L, Livote E, Harris B, Spencer N, Sean Morrison R. Emergency department crowding and decreased quality of pain care. Acad Emerg Med. 2008;15:1248-1255. doi: 10.1111/j.1553-2712.2008.00267.x. PubMed
15. Mills AM, Shofer FS, Chen EH, Hollander JE, Pines JM. The association between emergency department crowding and analgesia administration in acute abdominal pain patients. Acad Emerg Med. 2009;16:603-608. doi: 10.1111/j.1553-2712.2009.00441.x. PubMed
16. Pines JM, Shofer FS, Isserman JA, Abbuhl SB, Mills AM. The effect of emergency department crowding on analgesia in patients with back pain in two hospitals. Acad Emerg Med. 2010;17(3):276-283. doi: 10.1111/j.1553-2712.2009.00676.x. PubMed
17. Kulstad EB, Sikka R, Sweis RT, Kelley KM, Rzechula KH. ED overcrowding is associated with an increased frequency of medication errors. Am J Emerg Med. 2010;28:304-309. doi: 10.1016/j.ajem.2008.12.014. PubMed
18. Richardson DB. Increase in patient mortality at 10 days associated with emergency department overcrowding. Med J Aust. 2006;184(5):213-216. PubMed
19. Hoot NR, Aronsky D. Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med. 2008;52(2):126-136. doi: 10.1016/j.annemergmed.2008.03.014. PubMed
20. Singer AJ, Thode HC, Jr., Viccellio P, Pines JM. The association between length of emergency department boarding and mortality. Acad Emerg Med. 2011;18(12):1324-1329. doi: 10.1111/j.1553-2712.2011.01236.x. PubMed
21. White BA, Biddinger PD, Chang Y, Grabowski B, Carignan S, Brown DF. Boarding inpatients in the emergency department increases discharged patient length of stay. J Emerg Med. 2013;44(1):230-235. doi: 10.1016/j.jemermed.2012.05.007. PubMed
22. Forster AJ, Stiell I, Wells G, Lee AJ, van Walraven C. The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127-133. doi: 10.1197/aemj.10.2.127. PubMed
23. Foley M, Kifaieh N, Mallon WK. Financial impact of emergency department crowding. West J Emerg Med. 2011;12(2):192-197. PubMed
24. Pines JM, Iyer S, Disbot M, Hollander JE, Shofer FS, Datner EM. The effect of emergency department crowding on patient satisfaction for admitted patients. Acad Emerg Med. 2008;15(9):825-831. doi: 10.1111/j.1553-2712.2008.00200.x. PubMed
25. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24:45-51. doi: 10.1097/QMH.0000000000000049. PubMed
26. Cho HJ, Desai N, Florendo A, et al. E-DIP: Early Discharge Project. A Model for Throughput and Early Discharge for 1-Day Admissions. BMJ Qual Improv Rep. 2016;5(1): pii: u210035.w4128. doi: 10.1136/bmjquality.u210035.w4128. PubMed
27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010. PubMed
28. Patel H, Fang MC, Mourad M, et al. Hospitalist and internal medicine leaders’ perspectives of early discharge challenges at academic medical centers. J Hosp Med. 2018;13(6):388-391. doi: 10.12788/jhm.2885. PubMed
29. Minichiello TM, Auerbach AD, Wachter RM. Caregiver perceptions of the reasons for delayed hospital discharge. Eff Clin Pract. 2001;4(6):250-255. PubMed
30. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract (1995). 2016;44(5):252-259. doi: 10.1080/21548331.2016.1254559. PubMed
31. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. doi: 10.1002/jhm.2529. PubMed
32. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. doi: 10.1016/j.amjmed.2014.12.011. PubMed 33. Khanna S, Boyle J, Good N, Lind J. Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Australas. 2012;24(5):510-517. doi: 10.1111/j.1742-6723.2012.01587.x. PubMed
33. Khanna S, Boyle J, Good N, Lind J. Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Australas. 2012;24(5):510-517. doi: 10.1111/j.1742-6723.2012.01587.x. PubMed
Hospital discharges frequently occur in the afternoon or evening hours.1-5 Late discharges can adversely affect patient flow throughout the hospital,3,6-9 which, in turn, can result in delays in care,10-16 more medication errors,17 increased mortality,18-20 longer lengths of stay,20-22 higher costs,23 and lower patient satisfaction.24
Various interventions have been employed in the attempts to find ways of moving discharge times to earlier in the day, including preparing the discharge paperwork and medications the previous night,25 using checklists,1,25 team huddles,2 providing real-time feedback to unit staff,1 and employing multidisciplinary teamwork.1,2,6,25,26
The purpose of this study was to identify and determine the relative frequency of barriers to writing discharge orders in the hopes of identifying issues that might be addressed by targeted interventions. We also assessed the effects of daily team census, patients being on teaching versus nonteaching services, and how daily rounds were structured at the time that the discharge orders were written.
METHODS
Study Design, Setting, and Participants
We conducted a prospective, cross-sectional survey of house-staff and attending physicians on general medicine teaching and nonteaching services from November 13, 2014, through May 31, 2016. The study was conducted at the following five hospitals: Denver Health Medical Center (DHMC) and Presbyterian/Saint Luke’s Medical Center (PSL) in Denver, Colorado; Ronald Reagan University (UCLA) and Los Angeles County/University of Southern California Medical Center (LAC+USC) in Los Angeles, California; and Harborview Medical Center (HMC) in Seattle, Washington. The study was approved by the Colorado Multi-Institutional Review Board as well as by the review boards of the other participating sites.
Data Collection
The results of the focus groups composed of attending physicians at DHMC were used to develop our initial data collection template. Additional sites joining the study provided feedback, leading to modifications (Appendix 1).
Physicians were surveyed at three different time points on study days that were selected according to the convenience of the investigators. The sampling occurred only on weekdays and was done based on the investigators’ availability. Investigators would attempt to survey as many teams as they were able to but, secondary to feasibility, not all teams could be surveyed on study days. The specific time points varied as a function of physician workflows but were standardized as much as possible to occur in the early morning, around noon, and midafternoon on weekdays. Physicians were contacted either in person or by telephone for verbal consent prior to administering the first survey. All general medicine teams were eligible. For teaching teams, the order of contact was resident, intern, and then attending based on which physician was available at the time of the survey and on which member of the team was thought to know the patients the best. For the nonteaching services, the attending physicians were contacted.
During the initial survey, the investigators assessed the provider role (ie, attending or housestaff), whether the service was a teaching or a nonteaching service, and the starting patient census on that service primarily based on interviewing the provider of record for the team and looking at team census lists. Physicians were asked about their rounding style (ie, sickest patients first, patients likely to be discharged first, room-by-room, most recently admitted patients first, patients on the team the longest, or other) and then to identify all patients they thought would be definite discharges sometime during the day of the survey. Definite discharges were defined as patients whom the provider thought were either currently ready for discharge or who had only minor barriers that, if unresolved, would not prevent same-day discharge. They were asked if the discharge order had been entered and, if not, what was preventing them from doing so, if the discharge could in their opinion have occurred the day prior and, if so, why this did not occur. We also obtained the date and time of the admission and discharge orders, the actual discharge time, as well as the length of stay either through chart review (majority of sites) or from data warehouses (Denver Health and Presbyterian St. Lukes had length of stay data retrieved from their data warehouse).
Physicians were also asked to identify all patients whom they thought might possibly be discharged that day. Possible discharges were defined as patients with barriers to discharge that, if unresolved, would prevent same-day discharge. For each of these, the physicians were asked to list whatever issues needed to be resolved prior to placing the discharge order (Appendix 1).
The second survey was administered late morning on the same day, typically between 11
The third survey was administered midafternoon, typically around 3 PM similar to the first two surveys, with the exception that the third survey did not attempt to identify new definite or possible discharges.
Sample Size
We stopped collecting data after obtaining a convenience sample of 5% of total discharges at each study site or on the study end date, which was May 31, 2016, whichever came first.
Data Analysis
Data were collected and managed using a secure, web-based application electronic data capture tool (REDCap), hosted at Denver Health. REDCap (Research Electronic Data Capture, Nashville, Tennessee) is designed to support data collection for research studies.27 Data were then analyzed using SAS Enterprise Guide 5.1 (SAS Institute, Inc., Cary, North Carolina). All data entered into REDCap were reviewed by the principal investigator to ensure that data were not missing, and when there were missing data, a query was sent to verify if the data were retrievable. If retrievable, then the data would be entered. The volume of missing data that remained is described in our results.
Continuous variables were described using means and standard deviations (SD) or medians and interquartile ranges (IQR) based on tests of normality. Differences in the time that the discharge orders were placed in the electronic medical record according to morning patient census, teaching versus nonteaching service, and rounding style were compared using the Wilcoxon rank sum test. Linear regression was used to evaluate the effect of patient census on discharge order time. P < .05 was considered as significant.
RESULTS
We conducted 1,584 patient evaluations through surveys of 254 physicians over 156 days. Given surveys coincided with the existing work we had full participation (ie, 100% participation) and no dropout during the study days. Median (IQR) survey time points were 8:30
The characteristics of the five hospitals participating in the study, the patients’ final discharge status, the types of physicians surveyed, the services on which they were working, the rounding styles employed, and the median starting daily census are summarized in Table 1. The majority of the physicians surveyed were housestaff working on teaching services, and only a small minority structured rounds such that patients ready for discharge were seen first.
Over the course of the three surveys, 949 patients were identified as being definite discharges at any time point, and the large majority of these (863, 91%) were discharged on the day of the survey. The median (IQR) time that the discharge orders were written was 11:50
During the initial morning survey, 314 patients were identified as being definite discharges for that day (representing approximately 6% of the total number of patients being cared for, or 33% of the patients identified as definite discharges throughout the day). Of these, the physicians thought that 44 (<1% of the total number of patients being cared for on the services) could have been discharged on the previous day. The most frequent reasons cited for why these patients were not discharged on the previous day were “Patient did not want to leave” (n = 15, 34%), “Too late in the day” (n = 10, 23%), and “No ride” (n = 9, 20%). The remaining 10 patients (23%) had a variety of reasons related to system or social issues (ie, shelter not available, miscommunication).
At the morning time point, the most common barriers to discharge identified were that the physicians had not finished rounding on their team of patients and that the housestaff needed to staff their patients with their attending. At noon, caring for other patients and tending to the discharge processes were most commonly cited, and in the afternoon, the most common barriers were that the physicians were in the process of completing the discharge paperwork for those patients or were discharging other patients (Table 2). When comparing barriers on teaching to nonteaching teams, a higher proportion of teaching teams were still rounding on all patients and were working on discharge paperwork at the second survey. Barriers cited by sites were similar; however, the frequency at which the barriers were mentioned varied (data not shown).
The physicians identified 1,237 patients at any time point as being possible discharges during the day of the survey and these had a mean (±SD) of 1.3 (±0.5) barriers cited for why these patients were possible rather than definite discharges. The most common were that clinical improvement was needed, one or more pending issues related to their care needed to be resolved, and/or awaiting pending test results. The need to see clinical improvement generally decreased throughout the day as did the need to staff patients with an attending physician, but barriers related to consultant recommendations or completing procedures increased (Table 3). Of the 1,237 patients ever identified as possible discharges, 594 (48%) became a definite discharge by the third call and 444 (36%) became a no discharge as their final status. As with definite discharges, barriers cited by sites were similar; however, the frequency at which the barriers were mentioned varied.
Among the 949 and 1,237 patients who were ever identified as definite or possible discharges, respectively, at any time point during the study day, 28 (3%) and 444 (36%), respectively, had their discharge status changed to no discharge, most commonly because their clinical condition either worsened or expected improvements did not occur or that barriers pertaining to social work, physical therapy, or occupational therapy were not resolved.
The median time that the discharge orders were entered into the electronic medical record was 43 minutes earlier if patients were on teams with a lower versus a higher starting census (P = .0003), 48 minutes earlier if they were seen by physicians whose rounding style was to see patients first who potentially could be discharged (P = .0026), and 58 minutes earlier if they were on nonteaching versus teaching services (P < .0001; Table 4). For every one-person increase in census, the discharge order time increased by 6 minutes (β = 5.6, SE = 1.6, P = .0003).
DISCUSSION
The important findings of this study are that (1) the large majority of issues thought to delay discharging patients identified as definite discharges were related to physicians caring for other patients on their team, (2) although 91% of patients ever identified as being definite discharges were discharged on the day of the survey, only 48% of those identified as possible discharges became definite discharges by the afternoon time point, largely because the anticipated clinical improvement did not occur or care being provided by ancillary services had not been completed, and (3) discharge orders on patients identified as definite discharges were written on average 50 minutes earlier by physicians on teams with a smaller starting patient census, on nonteaching services, or when the rounding style was to see patients ready for discharges first.
Previous research has reported that physician-perceived barriers to discharge were extrinsic to providers and even extrinsic to the hospital setting (eg, awaiting subacute nursing placement and transportation).28,29 However, many of the barriers that we identified were related directly to the providers’ workload and rounding styles and whether the patients were on teaching versus nonteaching services. We also found that delays in the ability of hospital services to complete care also contributed to delayed discharges.
Our observational data suggest that delays resulting from caring for other patients might be reduced by changing rounding styles such that patients ready for discharge are seen first and are discharged prior to seeing other patients on the team, as previously reported by Beck et al.30 Intuitively, this would seem to be a straightforward way of freeing up beds earlier in the day, but such a change will, of necessity, lead to delaying care for other patients, which, in turn, could increase their length of stays. Durvasula et al. suggested that discharges could be moved to earlier in the day by completing orders and paperwork the day prior to discharge.25 Such an approach might be effective on an Obstetrical or elective Orthopedic service on which patients predictably are hospitalized for a fixed number of days (or even hours) but may be less relevant to patients on internal medicine services where lengths of stay are less predictable. Interventions to improve discharge times have resulted in earlier discharge times in some studies,2,4 but the overall length of stay either did not decrease25 or increased31 in others. Werthheimer et al.1 did find earlier discharge times, but other interventions also occurred during the study period (eg, extending social work services to include weekends).1,32
We found that discharge times were approximately 50 minutes earlier on teams with a smaller starting census, on nonteaching compared with teaching services, or when the attending’s rounding style was to see patients ready for discharges first. Although 50 minutes may seem like a small change in discharge time, Khanna et al.33 found that when discharges occur even 1 hour earlier, hospital overcrowding is reduced. To have a lower team census would require having more teams and more providers to staff these teams, raising cost-effectiveness concerns. Moving to more nonteaching services could represent a conflict with respect to one of the missions of teaching hospitals and raises a cost-benefit issue as several teaching hospitals receive substantial funding in support of their teaching activities and housestaff would have to be replaced with more expensive providers.
Delays attributable to ancillary services indicate imbalances between demand and availability of these services. Inappropriate demand and inefficiencies could be reduced by systems redesign, but in at least some instances, additional resources will be needed to add staff, increase space, or add additional equipment.
Our study has several limitations. First, we surveyed only physicians working in university-affiliated hospitals, and three of these were public safety-net hospitals. Accordingly, our results may not be generalizable to different patient populations. Second, we surveyed only physicians, and Minichiello et al.29 found that barriers to discharge perceived by physicians were different from those of other staff. Third, our data were observational and were collected only on weekdays. Fourth, we did not differentiate interns from residents, and thus, potentially the level of training could have affected these results. Similarly, the decision for a “possible” and a “definite” discharge is likely dependent on the knowledge base of the participant, such that less experienced participants may have had differing perspectives than someone with more experience. Fifth, the sites did vary based on the infrastructure and support but also had several similarities. All sites had social work and case management involved in care, although at some sites, they were assigned according to team and at others according to geographic location. Similarly, rounding times varied. Most of the services surveyed did not utilize advanced practice providers (the exception was the nonteaching services at Denver Health, and their presence was variable). These differences in staffing models could also have affected these results.
Our study also has a number of strengths. First, we assessed the barriers at five different hospitals. Second, we collected real-time data related to specific barriers at multiple time points throughout the day, allowing us to assess the dynamic nature of identifying patients as being ready or nearly ready for discharge. Third, we assessed the perceptions of barriers to discharge from physicians working on teaching as well as nonteaching services and from physicians utilizing a variety of rounding styles. Fourth, we had a very high participation rate (100%), probably due to the fact that our study was strategically aligned with participants’ daily work activities.
In conclusion, we found two distinct categories of issues that physicians perceived as most commonly delaying writing discharge orders on their patients. The first pertained to patients thought to definitely be ready for discharge and was related to the physicians having to care for other patients on their team. The second pertained to patients identified as possibly ready for discharge and was related to the need for care to be completed by a variety of ancillary services. Addressing each of these barriers would require different interventions and a need to weigh the potential improvements that could be achieved against the increased costs and/or delays in care for other patients that may result.
Disclosures
The authors report no conflicts of interest relevant to this work.
Hospital discharges frequently occur in the afternoon or evening hours.1-5 Late discharges can adversely affect patient flow throughout the hospital,3,6-9 which, in turn, can result in delays in care,10-16 more medication errors,17 increased mortality,18-20 longer lengths of stay,20-22 higher costs,23 and lower patient satisfaction.24
Various interventions have been employed in the attempts to find ways of moving discharge times to earlier in the day, including preparing the discharge paperwork and medications the previous night,25 using checklists,1,25 team huddles,2 providing real-time feedback to unit staff,1 and employing multidisciplinary teamwork.1,2,6,25,26
The purpose of this study was to identify and determine the relative frequency of barriers to writing discharge orders in the hopes of identifying issues that might be addressed by targeted interventions. We also assessed the effects of daily team census, patients being on teaching versus nonteaching services, and how daily rounds were structured at the time that the discharge orders were written.
METHODS
Study Design, Setting, and Participants
We conducted a prospective, cross-sectional survey of house-staff and attending physicians on general medicine teaching and nonteaching services from November 13, 2014, through May 31, 2016. The study was conducted at the following five hospitals: Denver Health Medical Center (DHMC) and Presbyterian/Saint Luke’s Medical Center (PSL) in Denver, Colorado; Ronald Reagan University (UCLA) and Los Angeles County/University of Southern California Medical Center (LAC+USC) in Los Angeles, California; and Harborview Medical Center (HMC) in Seattle, Washington. The study was approved by the Colorado Multi-Institutional Review Board as well as by the review boards of the other participating sites.
Data Collection
The results of the focus groups composed of attending physicians at DHMC were used to develop our initial data collection template. Additional sites joining the study provided feedback, leading to modifications (Appendix 1).
Physicians were surveyed at three different time points on study days that were selected according to the convenience of the investigators. The sampling occurred only on weekdays and was done based on the investigators’ availability. Investigators would attempt to survey as many teams as they were able to but, secondary to feasibility, not all teams could be surveyed on study days. The specific time points varied as a function of physician workflows but were standardized as much as possible to occur in the early morning, around noon, and midafternoon on weekdays. Physicians were contacted either in person or by telephone for verbal consent prior to administering the first survey. All general medicine teams were eligible. For teaching teams, the order of contact was resident, intern, and then attending based on which physician was available at the time of the survey and on which member of the team was thought to know the patients the best. For the nonteaching services, the attending physicians were contacted.
During the initial survey, the investigators assessed the provider role (ie, attending or housestaff), whether the service was a teaching or a nonteaching service, and the starting patient census on that service primarily based on interviewing the provider of record for the team and looking at team census lists. Physicians were asked about their rounding style (ie, sickest patients first, patients likely to be discharged first, room-by-room, most recently admitted patients first, patients on the team the longest, or other) and then to identify all patients they thought would be definite discharges sometime during the day of the survey. Definite discharges were defined as patients whom the provider thought were either currently ready for discharge or who had only minor barriers that, if unresolved, would not prevent same-day discharge. They were asked if the discharge order had been entered and, if not, what was preventing them from doing so, if the discharge could in their opinion have occurred the day prior and, if so, why this did not occur. We also obtained the date and time of the admission and discharge orders, the actual discharge time, as well as the length of stay either through chart review (majority of sites) or from data warehouses (Denver Health and Presbyterian St. Lukes had length of stay data retrieved from their data warehouse).
Physicians were also asked to identify all patients whom they thought might possibly be discharged that day. Possible discharges were defined as patients with barriers to discharge that, if unresolved, would prevent same-day discharge. For each of these, the physicians were asked to list whatever issues needed to be resolved prior to placing the discharge order (Appendix 1).
The second survey was administered late morning on the same day, typically between 11
The third survey was administered midafternoon, typically around 3 PM similar to the first two surveys, with the exception that the third survey did not attempt to identify new definite or possible discharges.
Sample Size
We stopped collecting data after obtaining a convenience sample of 5% of total discharges at each study site or on the study end date, which was May 31, 2016, whichever came first.
Data Analysis
Data were collected and managed using a secure, web-based application electronic data capture tool (REDCap), hosted at Denver Health. REDCap (Research Electronic Data Capture, Nashville, Tennessee) is designed to support data collection for research studies.27 Data were then analyzed using SAS Enterprise Guide 5.1 (SAS Institute, Inc., Cary, North Carolina). All data entered into REDCap were reviewed by the principal investigator to ensure that data were not missing, and when there were missing data, a query was sent to verify if the data were retrievable. If retrievable, then the data would be entered. The volume of missing data that remained is described in our results.
Continuous variables were described using means and standard deviations (SD) or medians and interquartile ranges (IQR) based on tests of normality. Differences in the time that the discharge orders were placed in the electronic medical record according to morning patient census, teaching versus nonteaching service, and rounding style were compared using the Wilcoxon rank sum test. Linear regression was used to evaluate the effect of patient census on discharge order time. P < .05 was considered as significant.
RESULTS
We conducted 1,584 patient evaluations through surveys of 254 physicians over 156 days. Given surveys coincided with the existing work we had full participation (ie, 100% participation) and no dropout during the study days. Median (IQR) survey time points were 8:30
The characteristics of the five hospitals participating in the study, the patients’ final discharge status, the types of physicians surveyed, the services on which they were working, the rounding styles employed, and the median starting daily census are summarized in Table 1. The majority of the physicians surveyed were housestaff working on teaching services, and only a small minority structured rounds such that patients ready for discharge were seen first.
Over the course of the three surveys, 949 patients were identified as being definite discharges at any time point, and the large majority of these (863, 91%) were discharged on the day of the survey. The median (IQR) time that the discharge orders were written was 11:50
During the initial morning survey, 314 patients were identified as being definite discharges for that day (representing approximately 6% of the total number of patients being cared for, or 33% of the patients identified as definite discharges throughout the day). Of these, the physicians thought that 44 (<1% of the total number of patients being cared for on the services) could have been discharged on the previous day. The most frequent reasons cited for why these patients were not discharged on the previous day were “Patient did not want to leave” (n = 15, 34%), “Too late in the day” (n = 10, 23%), and “No ride” (n = 9, 20%). The remaining 10 patients (23%) had a variety of reasons related to system or social issues (ie, shelter not available, miscommunication).
At the morning time point, the most common barriers to discharge identified were that the physicians had not finished rounding on their team of patients and that the housestaff needed to staff their patients with their attending. At noon, caring for other patients and tending to the discharge processes were most commonly cited, and in the afternoon, the most common barriers were that the physicians were in the process of completing the discharge paperwork for those patients or were discharging other patients (Table 2). When comparing barriers on teaching to nonteaching teams, a higher proportion of teaching teams were still rounding on all patients and were working on discharge paperwork at the second survey. Barriers cited by sites were similar; however, the frequency at which the barriers were mentioned varied (data not shown).
The physicians identified 1,237 patients at any time point as being possible discharges during the day of the survey and these had a mean (±SD) of 1.3 (±0.5) barriers cited for why these patients were possible rather than definite discharges. The most common were that clinical improvement was needed, one or more pending issues related to their care needed to be resolved, and/or awaiting pending test results. The need to see clinical improvement generally decreased throughout the day as did the need to staff patients with an attending physician, but barriers related to consultant recommendations or completing procedures increased (Table 3). Of the 1,237 patients ever identified as possible discharges, 594 (48%) became a definite discharge by the third call and 444 (36%) became a no discharge as their final status. As with definite discharges, barriers cited by sites were similar; however, the frequency at which the barriers were mentioned varied.
Among the 949 and 1,237 patients who were ever identified as definite or possible discharges, respectively, at any time point during the study day, 28 (3%) and 444 (36%), respectively, had their discharge status changed to no discharge, most commonly because their clinical condition either worsened or expected improvements did not occur or that barriers pertaining to social work, physical therapy, or occupational therapy were not resolved.
The median time that the discharge orders were entered into the electronic medical record was 43 minutes earlier if patients were on teams with a lower versus a higher starting census (P = .0003), 48 minutes earlier if they were seen by physicians whose rounding style was to see patients first who potentially could be discharged (P = .0026), and 58 minutes earlier if they were on nonteaching versus teaching services (P < .0001; Table 4). For every one-person increase in census, the discharge order time increased by 6 minutes (β = 5.6, SE = 1.6, P = .0003).
DISCUSSION
The important findings of this study are that (1) the large majority of issues thought to delay discharging patients identified as definite discharges were related to physicians caring for other patients on their team, (2) although 91% of patients ever identified as being definite discharges were discharged on the day of the survey, only 48% of those identified as possible discharges became definite discharges by the afternoon time point, largely because the anticipated clinical improvement did not occur or care being provided by ancillary services had not been completed, and (3) discharge orders on patients identified as definite discharges were written on average 50 minutes earlier by physicians on teams with a smaller starting patient census, on nonteaching services, or when the rounding style was to see patients ready for discharges first.
Previous research has reported that physician-perceived barriers to discharge were extrinsic to providers and even extrinsic to the hospital setting (eg, awaiting subacute nursing placement and transportation).28,29 However, many of the barriers that we identified were related directly to the providers’ workload and rounding styles and whether the patients were on teaching versus nonteaching services. We also found that delays in the ability of hospital services to complete care also contributed to delayed discharges.
Our observational data suggest that delays resulting from caring for other patients might be reduced by changing rounding styles such that patients ready for discharge are seen first and are discharged prior to seeing other patients on the team, as previously reported by Beck et al.30 Intuitively, this would seem to be a straightforward way of freeing up beds earlier in the day, but such a change will, of necessity, lead to delaying care for other patients, which, in turn, could increase their length of stays. Durvasula et al. suggested that discharges could be moved to earlier in the day by completing orders and paperwork the day prior to discharge.25 Such an approach might be effective on an Obstetrical or elective Orthopedic service on which patients predictably are hospitalized for a fixed number of days (or even hours) but may be less relevant to patients on internal medicine services where lengths of stay are less predictable. Interventions to improve discharge times have resulted in earlier discharge times in some studies,2,4 but the overall length of stay either did not decrease25 or increased31 in others. Werthheimer et al.1 did find earlier discharge times, but other interventions also occurred during the study period (eg, extending social work services to include weekends).1,32
We found that discharge times were approximately 50 minutes earlier on teams with a smaller starting census, on nonteaching compared with teaching services, or when the attending’s rounding style was to see patients ready for discharges first. Although 50 minutes may seem like a small change in discharge time, Khanna et al.33 found that when discharges occur even 1 hour earlier, hospital overcrowding is reduced. To have a lower team census would require having more teams and more providers to staff these teams, raising cost-effectiveness concerns. Moving to more nonteaching services could represent a conflict with respect to one of the missions of teaching hospitals and raises a cost-benefit issue as several teaching hospitals receive substantial funding in support of their teaching activities and housestaff would have to be replaced with more expensive providers.
Delays attributable to ancillary services indicate imbalances between demand and availability of these services. Inappropriate demand and inefficiencies could be reduced by systems redesign, but in at least some instances, additional resources will be needed to add staff, increase space, or add additional equipment.
Our study has several limitations. First, we surveyed only physicians working in university-affiliated hospitals, and three of these were public safety-net hospitals. Accordingly, our results may not be generalizable to different patient populations. Second, we surveyed only physicians, and Minichiello et al.29 found that barriers to discharge perceived by physicians were different from those of other staff. Third, our data were observational and were collected only on weekdays. Fourth, we did not differentiate interns from residents, and thus, potentially the level of training could have affected these results. Similarly, the decision for a “possible” and a “definite” discharge is likely dependent on the knowledge base of the participant, such that less experienced participants may have had differing perspectives than someone with more experience. Fifth, the sites did vary based on the infrastructure and support but also had several similarities. All sites had social work and case management involved in care, although at some sites, they were assigned according to team and at others according to geographic location. Similarly, rounding times varied. Most of the services surveyed did not utilize advanced practice providers (the exception was the nonteaching services at Denver Health, and their presence was variable). These differences in staffing models could also have affected these results.
Our study also has a number of strengths. First, we assessed the barriers at five different hospitals. Second, we collected real-time data related to specific barriers at multiple time points throughout the day, allowing us to assess the dynamic nature of identifying patients as being ready or nearly ready for discharge. Third, we assessed the perceptions of barriers to discharge from physicians working on teaching as well as nonteaching services and from physicians utilizing a variety of rounding styles. Fourth, we had a very high participation rate (100%), probably due to the fact that our study was strategically aligned with participants’ daily work activities.
In conclusion, we found two distinct categories of issues that physicians perceived as most commonly delaying writing discharge orders on their patients. The first pertained to patients thought to definitely be ready for discharge and was related to the physicians having to care for other patients on their team. The second pertained to patients identified as possibly ready for discharge and was related to the need for care to be completed by a variety of ancillary services. Addressing each of these barriers would require different interventions and a need to weigh the potential improvements that could be achieved against the increased costs and/or delays in care for other patients that may result.
Disclosures
The authors report no conflicts of interest relevant to this work.
1. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
2. Kane M, Weinacker A, Arthofer R, et al. A multidisciplinary initiative to increase inpatient discharges before noon. J Nurs Adm. 2016;46(12):630-635. doi: 10.1097/NNA.0000000000000418. PubMed
3. Khanna S, Sier D, Boyle J, Zeitz K. Discharge timeliness and its impact on hospital crowding and emergency department flow performance. Emerg Med Australas. 2016;28(2):164-170. doi: 10.1111/1742-6723.12543. PubMed
4. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag (Frederick). 2007;26:142-146. doi: 10.1097/01.HCM.0000268617.33491.60. PubMed
5. Khanna S, Boyle J, Good N, Lind J. Impact of admission and discharge peak times on hospital overcrowding. Stud Health Technol Inform. 2011;168:82-88. doi: 10.3233/978-1-60750-791-8-82. PubMed
6. McGowan JE, Truwit JD, Cipriano P, et al. Operating room efficiency and hospital capacity: factors affecting operating room use during maximum hospital census. J Am Coll Surg. 2007;204(5):865-871; discussion 71-72. doi: 10.1016/j.jamcollsurg.2007.01.052 PubMed
7. Khanna S, Boyle J, Good N, Lind J. Early discharge and its effect on ED length of stay and access block. Stud Health Technol Inform. 2012;178:92-98. doi: 10.3233/978-1-61499-078-9-92 PubMed
8. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi: 10.1016/j.jemermed.2010.06.028. PubMed
9. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi: 10.1002/jhm.2412. PubMed
10. Sikka R, Mehta S, Kaucky C, Kulstad EB. ED crowding is associated with an increased time to pneumonia treatment. Am J Emerg Med. 2010;28(7):809-812. doi: 10.1016/j.ajem.2009.06.023. PubMed
11. Coil CJ, Flood JD, Belyeu BM, Young P, Kaji AH, Lewis RJ. The effect of emergency department boarding on order completion. Ann Emerg Med. 2016;67:730-736 e2. doi: 10.1016/j.annemergmed.2015.09.018. PubMed
12. Gaieski DF, Agarwal AK, Mikkelsen ME, et al. The impact of ED crowding on early interventions and mortality in patients with severe sepsis. Am J Emerg Med. 2017;35:953-960. doi: 10.1016/j.ajem.2017.01.061. PubMed
13. Pines JM, Localio AR, Hollander JE, et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007;50(5):510-516. doi: 10.1016/j.annemergmed.2007.07.021. PubMed
14. Hwang U, Richardson L, Livote E, Harris B, Spencer N, Sean Morrison R. Emergency department crowding and decreased quality of pain care. Acad Emerg Med. 2008;15:1248-1255. doi: 10.1111/j.1553-2712.2008.00267.x. PubMed
15. Mills AM, Shofer FS, Chen EH, Hollander JE, Pines JM. The association between emergency department crowding and analgesia administration in acute abdominal pain patients. Acad Emerg Med. 2009;16:603-608. doi: 10.1111/j.1553-2712.2009.00441.x. PubMed
16. Pines JM, Shofer FS, Isserman JA, Abbuhl SB, Mills AM. The effect of emergency department crowding on analgesia in patients with back pain in two hospitals. Acad Emerg Med. 2010;17(3):276-283. doi: 10.1111/j.1553-2712.2009.00676.x. PubMed
17. Kulstad EB, Sikka R, Sweis RT, Kelley KM, Rzechula KH. ED overcrowding is associated with an increased frequency of medication errors. Am J Emerg Med. 2010;28:304-309. doi: 10.1016/j.ajem.2008.12.014. PubMed
18. Richardson DB. Increase in patient mortality at 10 days associated with emergency department overcrowding. Med J Aust. 2006;184(5):213-216. PubMed
19. Hoot NR, Aronsky D. Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med. 2008;52(2):126-136. doi: 10.1016/j.annemergmed.2008.03.014. PubMed
20. Singer AJ, Thode HC, Jr., Viccellio P, Pines JM. The association between length of emergency department boarding and mortality. Acad Emerg Med. 2011;18(12):1324-1329. doi: 10.1111/j.1553-2712.2011.01236.x. PubMed
21. White BA, Biddinger PD, Chang Y, Grabowski B, Carignan S, Brown DF. Boarding inpatients in the emergency department increases discharged patient length of stay. J Emerg Med. 2013;44(1):230-235. doi: 10.1016/j.jemermed.2012.05.007. PubMed
22. Forster AJ, Stiell I, Wells G, Lee AJ, van Walraven C. The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127-133. doi: 10.1197/aemj.10.2.127. PubMed
23. Foley M, Kifaieh N, Mallon WK. Financial impact of emergency department crowding. West J Emerg Med. 2011;12(2):192-197. PubMed
24. Pines JM, Iyer S, Disbot M, Hollander JE, Shofer FS, Datner EM. The effect of emergency department crowding on patient satisfaction for admitted patients. Acad Emerg Med. 2008;15(9):825-831. doi: 10.1111/j.1553-2712.2008.00200.x. PubMed
25. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24:45-51. doi: 10.1097/QMH.0000000000000049. PubMed
26. Cho HJ, Desai N, Florendo A, et al. E-DIP: Early Discharge Project. A Model for Throughput and Early Discharge for 1-Day Admissions. BMJ Qual Improv Rep. 2016;5(1): pii: u210035.w4128. doi: 10.1136/bmjquality.u210035.w4128. PubMed
27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010. PubMed
28. Patel H, Fang MC, Mourad M, et al. Hospitalist and internal medicine leaders’ perspectives of early discharge challenges at academic medical centers. J Hosp Med. 2018;13(6):388-391. doi: 10.12788/jhm.2885. PubMed
29. Minichiello TM, Auerbach AD, Wachter RM. Caregiver perceptions of the reasons for delayed hospital discharge. Eff Clin Pract. 2001;4(6):250-255. PubMed
30. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract (1995). 2016;44(5):252-259. doi: 10.1080/21548331.2016.1254559. PubMed
31. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. doi: 10.1002/jhm.2529. PubMed
32. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. doi: 10.1016/j.amjmed.2014.12.011. PubMed 33. Khanna S, Boyle J, Good N, Lind J. Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Australas. 2012;24(5):510-517. doi: 10.1111/j.1742-6723.2012.01587.x. PubMed
33. Khanna S, Boyle J, Good N, Lind J. Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Australas. 2012;24(5):510-517. doi: 10.1111/j.1742-6723.2012.01587.x. PubMed
1. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
2. Kane M, Weinacker A, Arthofer R, et al. A multidisciplinary initiative to increase inpatient discharges before noon. J Nurs Adm. 2016;46(12):630-635. doi: 10.1097/NNA.0000000000000418. PubMed
3. Khanna S, Sier D, Boyle J, Zeitz K. Discharge timeliness and its impact on hospital crowding and emergency department flow performance. Emerg Med Australas. 2016;28(2):164-170. doi: 10.1111/1742-6723.12543. PubMed
4. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag (Frederick). 2007;26:142-146. doi: 10.1097/01.HCM.0000268617.33491.60. PubMed
5. Khanna S, Boyle J, Good N, Lind J. Impact of admission and discharge peak times on hospital overcrowding. Stud Health Technol Inform. 2011;168:82-88. doi: 10.3233/978-1-60750-791-8-82. PubMed
6. McGowan JE, Truwit JD, Cipriano P, et al. Operating room efficiency and hospital capacity: factors affecting operating room use during maximum hospital census. J Am Coll Surg. 2007;204(5):865-871; discussion 71-72. doi: 10.1016/j.jamcollsurg.2007.01.052 PubMed
7. Khanna S, Boyle J, Good N, Lind J. Early discharge and its effect on ED length of stay and access block. Stud Health Technol Inform. 2012;178:92-98. doi: 10.3233/978-1-61499-078-9-92 PubMed
8. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi: 10.1016/j.jemermed.2010.06.028. PubMed
9. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi: 10.1002/jhm.2412. PubMed
10. Sikka R, Mehta S, Kaucky C, Kulstad EB. ED crowding is associated with an increased time to pneumonia treatment. Am J Emerg Med. 2010;28(7):809-812. doi: 10.1016/j.ajem.2009.06.023. PubMed
11. Coil CJ, Flood JD, Belyeu BM, Young P, Kaji AH, Lewis RJ. The effect of emergency department boarding on order completion. Ann Emerg Med. 2016;67:730-736 e2. doi: 10.1016/j.annemergmed.2015.09.018. PubMed
12. Gaieski DF, Agarwal AK, Mikkelsen ME, et al. The impact of ED crowding on early interventions and mortality in patients with severe sepsis. Am J Emerg Med. 2017;35:953-960. doi: 10.1016/j.ajem.2017.01.061. PubMed
13. Pines JM, Localio AR, Hollander JE, et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007;50(5):510-516. doi: 10.1016/j.annemergmed.2007.07.021. PubMed
14. Hwang U, Richardson L, Livote E, Harris B, Spencer N, Sean Morrison R. Emergency department crowding and decreased quality of pain care. Acad Emerg Med. 2008;15:1248-1255. doi: 10.1111/j.1553-2712.2008.00267.x. PubMed
15. Mills AM, Shofer FS, Chen EH, Hollander JE, Pines JM. The association between emergency department crowding and analgesia administration in acute abdominal pain patients. Acad Emerg Med. 2009;16:603-608. doi: 10.1111/j.1553-2712.2009.00441.x. PubMed
16. Pines JM, Shofer FS, Isserman JA, Abbuhl SB, Mills AM. The effect of emergency department crowding on analgesia in patients with back pain in two hospitals. Acad Emerg Med. 2010;17(3):276-283. doi: 10.1111/j.1553-2712.2009.00676.x. PubMed
17. Kulstad EB, Sikka R, Sweis RT, Kelley KM, Rzechula KH. ED overcrowding is associated with an increased frequency of medication errors. Am J Emerg Med. 2010;28:304-309. doi: 10.1016/j.ajem.2008.12.014. PubMed
18. Richardson DB. Increase in patient mortality at 10 days associated with emergency department overcrowding. Med J Aust. 2006;184(5):213-216. PubMed
19. Hoot NR, Aronsky D. Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med. 2008;52(2):126-136. doi: 10.1016/j.annemergmed.2008.03.014. PubMed
20. Singer AJ, Thode HC, Jr., Viccellio P, Pines JM. The association between length of emergency department boarding and mortality. Acad Emerg Med. 2011;18(12):1324-1329. doi: 10.1111/j.1553-2712.2011.01236.x. PubMed
21. White BA, Biddinger PD, Chang Y, Grabowski B, Carignan S, Brown DF. Boarding inpatients in the emergency department increases discharged patient length of stay. J Emerg Med. 2013;44(1):230-235. doi: 10.1016/j.jemermed.2012.05.007. PubMed
22. Forster AJ, Stiell I, Wells G, Lee AJ, van Walraven C. The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127-133. doi: 10.1197/aemj.10.2.127. PubMed
23. Foley M, Kifaieh N, Mallon WK. Financial impact of emergency department crowding. West J Emerg Med. 2011;12(2):192-197. PubMed
24. Pines JM, Iyer S, Disbot M, Hollander JE, Shofer FS, Datner EM. The effect of emergency department crowding on patient satisfaction for admitted patients. Acad Emerg Med. 2008;15(9):825-831. doi: 10.1111/j.1553-2712.2008.00200.x. PubMed
25. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24:45-51. doi: 10.1097/QMH.0000000000000049. PubMed
26. Cho HJ, Desai N, Florendo A, et al. E-DIP: Early Discharge Project. A Model for Throughput and Early Discharge for 1-Day Admissions. BMJ Qual Improv Rep. 2016;5(1): pii: u210035.w4128. doi: 10.1136/bmjquality.u210035.w4128. PubMed
27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010. PubMed
28. Patel H, Fang MC, Mourad M, et al. Hospitalist and internal medicine leaders’ perspectives of early discharge challenges at academic medical centers. J Hosp Med. 2018;13(6):388-391. doi: 10.12788/jhm.2885. PubMed
29. Minichiello TM, Auerbach AD, Wachter RM. Caregiver perceptions of the reasons for delayed hospital discharge. Eff Clin Pract. 2001;4(6):250-255. PubMed
30. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract (1995). 2016;44(5):252-259. doi: 10.1080/21548331.2016.1254559. PubMed
31. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. doi: 10.1002/jhm.2529. PubMed
32. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. doi: 10.1016/j.amjmed.2014.12.011. PubMed 33. Khanna S, Boyle J, Good N, Lind J. Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Australas. 2012;24(5):510-517. doi: 10.1111/j.1742-6723.2012.01587.x. PubMed
33. Khanna S, Boyle J, Good N, Lind J. Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Australas. 2012;24(5):510-517. doi: 10.1111/j.1742-6723.2012.01587.x. PubMed
Predictors of Clinically Significant Echocardiography Findings in Older Adults with Syncope: A Secondary Analysis
Syncope, defined as a transient loss of consciousness and postural tone followed by complete, spontaneous return to neurological baseline, accounts for over 1 million (or approximately 1%) of all emergency department (ED) visits per year in the United States (US).12 Given the breadth of etiologies for syncope, including certain life-threatening conditions, extensive diagnostic evaluation and hospitalization for this complaint is common.3-7 The estimated costs of syncope-related hospitalizations are over $2.4 billion annually in the US.8
The 2011 American College of Cardiology Foundation appropriate use criteria for echocardiography state that syncope is an appropriate indication for transthoracic echocardiography (TTE) even when there are no other symptoms or signs of cardiovascular disease.9 This broad recommendation may be appropriate since a finding of severe valvular disease would generally merit consultation with a cardiothoracic surgeon to assess the potential for surgical intervention.10 However, routine use of echocardiogram in all syncope patients could result in increased healthcare costs, patient discomfort, and incidental findings of unclear significance, while rarely changing diagnosis or management.11,12
In an attempt to reduce potentially unnecessary TTE testing, several studies have tried to identify patients at very low risk of structural heart disease.13-17 These investigations suggest that TTE is not indicated in syncope patients with a normal ECG and a normal cardiac exam. However, this literature is limited by retrospective study design and/or small sample sizes. The 2017 American Heart Association/American College of Cardiology/Heart Rhythm Society syncope guidelines recommend TTE for a patient in whom structural heart disease is suspected, but they are not explicit about how to make this determination. 18 Thus, it is still unclear which syncope patients require TTE since a standardized approach to assessing risk of clinically significant findings on TTE has not yet been rigorously developed.
The objective of this study was to develop a risk-stratification tool to identify older adults at very low risk of having a major, clinically significant finding on rest TTE after presenting to the ED with syncope or near-syncope. Using clinical, ECG, and cardiac biomarker data, we created the ROMEO (Risk Of Major Echocardiography findings in Older adults with syncope) score to help optimize resource utilization for syncope.
METHODS
Study Design and Setting
We conducted a large, multicenter, prospective, observational cohort study of older adults who presented to an ED with syncope or near-syncope (ClinicalTrials.gov identifier: NCT01802398). The study was approved by the institutional review boards at all sites and written informed consent was obtained from all participating subjects. The study was conducted at 11 academic EDs across the US (See Appendix Table 1).
Study Population
Patient inclusion criteria for eligibility were age ≥60 years with a complaint of syncope or near-syncope. Syncope was defined as transient loss of consciousness, associated with postural loss of tone, with immediate, spontaneous, and complete recovery. Near syncope was defined as the sensation of imminent loss of consciousness. Patients were excluded if their symptoms were thought to be due to intoxication, seizure, stroke, head trauma, or hypoglycemia. Additional exclusion criteria were the need for medical intervention to restore consciousness (eg, defibrillation), new or worsening confusion, and inability to obtain informed consent from the patient or a legally authorized representative.
This analysis included only patients who received a TTE during the index visit (either in the ED, observation unit, or while admitted to the hospital). This dataset was also used for other analyses addressing questions relevant to the ED management of syncope.
Measurements
All patients underwent a standardized history, physical examination, laboratory, and 12-lead ECG testing. Trained research assistants (RA) directly queried patients about symptoms associated with the syncopal episode. Data on the patient’s past medical history, medications, and physical examination findings were collected prospectively from treating providers.
Research staff obtained blood samples for testing at a core laboratory (University of Rochester, Rochester, NY). Two assays were performed using the Roche Elecsys platform: N-terminal pro B-type natriuretic peptide (NT-proBNP) and the 5th generation high-sensitivity cardiac troponin T (hs-TnT). NT-proBNP was classified as abnormal above a cutoff of 125 pg/mL. Hs-TnT was classified as abnormal above the 99th percentile for a reference population (14 pg/mL). Although hs-TnT was not approved by the U.S. Food and Drug Administration (FDA) at the time of the study, we anticipated that this assay would receive approval and be integrated into future standard of care (FDA approval was granted in January 2017). Rest TTEs were ordered at the discretion of the treating providers.
Outcome Measures
The primary outcome for this secondary analysis was a major, clinically significant finding on TTE.13,14,16,19 These included severe aortic stenosis (<1 cm2), severe mitral stenosis, severe aortic/mitral regurgitation, reduced ejection fraction (defined either quantitatively as less than 45% or qualitatively as “severe left ventricular dysfunction”), hypertrophic cardiomyopathy with outflow tract obstruction, severe pulmonary hypertension, right ventricular dysfunction/strain, large pericardial effusion, atrial myxoma, or regional wall motion abnormalities.
All echocardiogram reports were independently reviewed by two research physicians. Discrepant reviews were resolved by the research physicians and two of the study investigators (BS, CB). Of note, all the TTEs obtained were formal echocardiographic studies, not bedside ultrasonography performed by the emergency physician.
Candidate Predictors
Potential candidate predictors were identified through a prior expert panel process.20,21 Candidate predictors included age, sex, abnormal heart sounds, exertional syncope, shortness of breath, chest pain, near-syncope, family history of sudden cardiac death, high (>180 mm Hg) or low (<90 mm Hg) systolic blood pressure, abnormal ECG, elevated hs-TnT, elevated NT-proBNP, and history of the following: hypertension, cardiac dysrhythmia, renal failure, diabetes, congestive heart failure (CHF), and coronary artery disease (CAD).
The first obtained ECG was abstracted by one of five research study physicians blinded to all clinical data. Research study physicians demonstrated high interrater reliability (kappa > 0.80) in distinguishing normal from abnormal ECGs in a training set of 50 ECGs. Abnormal ECG interpretations included nonsinus rhythms (including paced rhythms), multiple premature ventricular complexes, sinus bradycardias (≤40 bpm), ventricular hypertrophies, short PR segment intervals (<100 milliseconds [ms]), axis deviations, first degree blocks (>200 ms), complete bundle branch blocks, Brugada patterns, Wolff-Parkinson-White patterns, abnormal QRS duration (>120 ms) or abnormal QTc prolongations (>450 ms), and Q/ST/T segment abnormalities suggestive of acute or chronic ischemia.
Statistical Analysis
We calculated descriptive statistics for each predictor variable, stratified by the presence or absence of TTE findings. Chi-square and t-tests were used to test associations between categorical or continuous variables and TTE findings using a significance level of 0.05 and 2-sided hypothesis testing. To identify a robust set of predictors of the primary outcome, we used multivariate logistic regression with the LASSO (Least Absolute Shrinkage and Selection Operator) to fit a parsimonious model.22 The LASSO selects variables and shrinks the associated coefficients to avoid overfitting.23-25 We then used a bootstrap to generate confidence intervals for coefficient estimates. Cases with missing echocardiography reports were excluded from the analysis. Bootstrap results were summarized as the percentage of bootstrap iterations in which each variable’s coefficient was 1) chosen and negative, 2) shrunk to zero, or 3) chosen and positive.
We assessed different weighting schemes to generate a risk score from significant variables identified by regression modeling. These included weighting by regression coefficients rounded to the nearest integer and simple summation of the presence or absence of each variable.
Based on these results, a predictive score was developed to risk stratify patients on their probability of major, clinically significant findings on TTE. The sensitivity and specificity of a score of zero to predict findings on TTE was calculated. For confidence intervals, we used Wilson’s method for binomial confidence intervals.26 The receiver operating characteristic (ROC) curve and its associated area under the curve (AUC) were calculated, and a confidence interval for the AUC was obtained through bootstrap resampling with 2,000 iterations. As part of our sensitivity analyses, we also calculated the ROC curve and AUC after excluding the patients with a known history of CHF and significant finding on TTE. Data analyses were performed in R.27 Two sensitivity analyses were performed: 1) we used multiple imputation to impute 1,000 complete data sets and then used the same LASSO methodology as with the complete data to assess whether incorporating missing data changed the results; and 2) we simulated a conventional troponin assay by raising the positive threshold for hs-TnT to >30 pg/mL (corresponding to the limit of detection for conventional troponin).28
RESULTS
Characteristics of Study Subjects
Patient screening occurred from April 2013 to September 2016. There were 6,930 patients who met eligibility criteria, of whom 3,686 (53%) consented and enrolled in the study (See Figure 1). Of these, 995 (27%) received TTE. The mean age of patients receiving TTE was 74 years; 55% were male. Characteristics of patients obtaining and not obtaining TTE are presented in Appendix Table 2. Patients who received TTE were more likely to be older, have abnormal heart sounds, abnormal EKGs, elevated hs-TnT, elevated NT-proBNP, and have a history of CHF. Of the 995 subjects receiving TTE, 215 (21.6%) had a major, clinically significant finding.
Main Results
Univariate analysis identified 14 variables significantly associated with major findings on TTE. These included male gender, shortness of breath, abnormal heart sounds, history of renal failure, diabetes, CHF, CAD, abnormal ECG, and elevated cardiac biomarkers, among others (See Table 1). The most common major finding on TTE was regional wall motion abnormality, followed by reduced left ventricular ejection fraction (See Table 2). Of the 995 patients who received TTE, 20 (2%) were discharged directly from the ED, 444 (45%) were observed, and 531 (53%) were admitted. On average, patients who received TTE had a longer length of stay than did those that did not (3.4 days vs 1.9 days).
LASSO multivariable logistic regression produced five predictors associated with major findings on TTE: 1) history of CHF, 2) history of CAD, 3) abnormal ECG, 4) hs-TnT above 14 pg/mL, and 5) NT-proBNP above 125 pg/mL (See Table 3).
These five high-risk clinical variables retained their importance after multivariate analysis and form the ROMEO score.
The sensitivity and specificity of a ROMEO score of zero for excluding major findings on TTE was 99.5% (95% CI: 97.4%-99.9%) and 15.4% (95% CI: 13.0%-18.1%), respectively. Patients with a ROMEO score of 0 were at very low risk of having a major finding on TTE: 0.8% (95% CI: 0.02%-4.5%; Appendix Table 3). Only one out of 121 patients with none of the ROMEO criteria was found to have a major finding on TTE (regional wall motion abnormality). Patients with a score of 1 or more were at moderate-to-high risk of having a major finding (7.3% to 55.6%).
There was a linear relationship between the ROMEO score and probability of major findings on TTE (See Appendix Figure 1). The AUC was 0.77 (95% CI = 0.72-0.79) indicating good accuracy of the combination of the five high-risk clinical variables to predict major findings on TTE (See Appendix Figure 2). After excluding the 72 patients with known CHF and significant findings on TTE, the AUC was similar: 0.73 (95% CI: 0.69-0.77). There were 139 patients with at least one missing variable (14%) (See Appendix Table 4). A multiple imputation sensitivity analysis identified the same five high-risk clinical variables in 85% of imputations.
There were 253 patients with high-sensitivity troponin levels between 15 and 30 pg/mL (inclusive). Using a higher hs-TnT threshold (>30 pg/mL) to simulate a conventional troponin assay again identified the same five high-risk variables along with shortness of breath as a potential sixth variable though with an odds ratio approaching unity (See Appendix Table 5). The ROMEO score would have missed two additional patients with major findings if the troponin cutoff were raised to 30 pg/mL from 14 pg/mL, ie, it would have identified 212/215 (98.6%) of the major findings rather than 214/215 (99.5%).
DISCUSSION
Older adults with syncope often present to the ED and undergo a variety of diagnostic tests, including TTE, and a significant proportion are admitted to the hospital.2 There is currently no standardized, evidence-based approach to guide TTE ordering for these patients. Using a large, prospective dataset of syncope patients, we sought to develop a risk-stratification tool to help clinicians identify which syncope patients would be at very low risk for clinically significant findings on TTE. We found that in the absence of these five high-risk clinical variables, the rate of significant findings on TTE in our sample was less than 1%. All five high-risk variables included in the tool remained predictive in our sensitivity analyses, speaking to the robustness of our model.
Other retrospective, and smaller prospective, studies have identified a combination of low-risk criteria including: a normal ECG alone,15 a normal physical exam and normal ECG,14,17 a negative cardiac history and normal ECG.16 Han et al. performed a chart review of 241 patients presenting to the ED with syncope and identified three risk factors for abnormal TTE findings using multiple logistic regression: age, abnormal ECG, and BNP greater than 100 pg/mL.13 While these studies’ results are generally consistent with ours, the retrospective nature and small sample size of these studies limit the generalizability of these results. Thus, using a large, multicenter prospective dataset, we derived a clinical decision instrument (the ROMEO score) to determine which older adults with syncope are at very low risk for major, clinically significant findings on TTE.
Our results add to the recent American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines on the management of syncope which recommend TTE in “selected patients presenting with syncope if structural heart disease is suspected.”18 Our risk-stratification tool offers a simple, standardized approach to determine specifically when to defer TTE testing.
Our findings can guide clinicians in deciding when to obtain TTE for ED syncope patients in the following way: Older adults presenting with syncope or near-syncope to the ED who have none of the ROMEO criteria are at extremely low risk for clinically significant findings on TTE and thus need not undergo such testing solely because of the syncopal event. Patients who have only one or more high-risk clinical variables are at higher risk (7.3%-56%) of significant TTE findings. In this subset, other factors, (eg, physician gestalt, recent previous echocardiography, patient preference, availability of echocardiography) can help guide TTE ordering. Patients with a greater number of high-risk variables may benefit from a more urgent echocardiographic evaluation.
Although on average, patients undergoing TTE had a longer length of stay than those that did not, this finding does not necessarily imply that ordering a TTE was the cause of the increased length of stay. It is possible that this positive association was due to greater underlying medical complexity or acuity of illness that resulted in a greater likelihood of admission/observation, and in turn, a greater length of stay.
Prior to implementation, our results should be externally validated in other clinical settings. In the interim, this risk-stratification tool may be used by clinicians, in conjunction with clinical judgement, to help guide the appropriate use of TTE in older adults presenting with syncope.
Our study has certain limitations. As we only enrolled patients 60 years and older, our findings may not necessarily be valid in younger populations of syncope patients. However, structural heart disease is less common in younger patients and is generally more of a concern for clinicians when evaluating syncope patients in the older age range.29 In our study, 47% of eligible patients declined to participate and thus sampling bias may have occurred. TTEs were ordered at the discretion of treating providers, which was likely subject to physician, institutional, and regional variation; the prevalence of major TTE findings may be lower in the overall cohort than in patients who received TTE. Prior TTE reports were not available; therefore, we were not able to determine if these major findings were previously known. Importantly, we did not perform an internal or external validation of the ROMEO score due to time and resource constraints. Thus, this study represents a derivation of the score solely and would require external validation prior to clinical implementation. Also, to calculate the ROMEO score, both an hs-TnT and NT-proBNP level must be obtained. Thus, the cost savings of any potential reduction in TTE ordering may be partially offset by the costs of increased laboratory testing. Lastly, hs-TnT assays are not currently widely available in hospitals in the United States; earlier generation cardiac troponin assays may not be a perfect substitute for hs-TnT assays. Our sensitivity analysis using an elevated threshold for hs-TnT attempted to mitigate this limitation and resulted in similar findings.
In summary, this risk-stratification tool, using five simple criteria, could help clinicians determine which older adult syncope patients can safely forgo TTE. If validated, this tool could help optimize resource utilization, and increase the value of healthcare for patients presenting with syncope.
Acknowledgments
The authors would like to thank the research assistants at all 11 sites who enrolled patients and collected data for this study.
Disclosures
Dr. Adler has received research funding from Roche. Dr. Bastani has received research funding from Radiometer and Portola and has been a consultant for Portola. Dr. Baugh has received advisory board and speaker’s fees from Roche, research funding from Janssen and Boehringer Ingelheim, and consulting and advisory board fees from Janssen. Dr. Casterino has received funding from Astra Zeneca. Dr. Clark has received research funding from Radiometer, Ortho Clinical Trials, Janssen, Pfizer, NIH, Portola, Biocryst, Glaxo Smith Klein, Hospital Quality Foundation, and Abbott. She is a consultant for Portola, Janssen, and Hospital Quality Foundation. Dr. Diercks is a consultant for Siemens, Janssen, and Roche has received institutional research support from Novartis, Ortho Scientific, and Roche. Dr. Hollander has received research funding from Alere, Siemens, Roche, Portola, and Trinity. Dr. Hollander has also received royalties from UpToDate. Dr. Nishijima has received an honorarium from Pfizer. Dr. Storrow is a consultant for Siemens and Quidel, has received speaking fees from MCM Education, and is on the Data and Safety Monitoring Board for Trevena. Dr. Sun is a consultant for Medtronic. The other authors report no relevant conflicts of interest.
Funding
This project was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01 HL111033. Dr. Probst is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL132052-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Roche Diagnostics supplied the high-sensitivity troponin-T assays. The sponsoring organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, or review of the manuscript.
1. Sun BC, Emond JA, Camargo CA, Jr. Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992-2000. Acad Emerg Med. 2004;11(10):1029-1034. doi: 10.1197/j.aem.2004.05.032. PubMed
2. Probst MA, Kanzaria HK, Gbedemah M, Richardson LD, Sun BC. National trends in resource utilization associated with ED visits for syncope. Am J Emerg Med. 2015;33(8):998-1001. doi: 10.1016/j.ajem.2015.04.030. PubMed
3. Kapoor WN, Karpf M, Maher Y, Miller RA, Levey GS. Syncope of unknown origin. The need for a more cost-effective approach to its diagnosis evaluation. JAMA. 1982;247(19):2687-2691. doi: 10.1001/jama.247.19.2687. PubMed
4. Pires LA, Ganji JR, Jarandila R, Steele R. Diagnostic patterns and temporal trends in the evaluation of adult patients hospitalized with syncope. Arch Intern Med. 2001;161(15):1889-1895. doi: 10.1001/archinte.161.15.1889. PubMed
5. Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43(2):224-232. doi: 10.1016/S0196064403008230. PubMed
6. Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 1: Value of history, physical examination, and electrocardiography. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med. 1997;126(12):989-996. doi: 10.7326/0003-4819-126-12-199706150-00012. PubMed
7. Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med. 1997;127(1):76-86. doi: 10.7326/0003-4819-127-1-199707010-00014. PubMed
8. Sun BC, Emond JA, Camargo CA, Jr. Direct medical costs of syncope-related hospitalizations in the United States. Am J Cardiol. 2005;95(5):668-671. doi: 10.1016/j.amjcard.2004.11.013. PubMed
9. American College of Cardiology Foundation. Appropriate Use Criteria Task F, American Society of Echocardiography, American Heart Association, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011;57(9):1126-1166. doi: 10.1016/j.echo.2010.12.008.
10. Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85(5):483-500. doi: 10.4065/mcp.2009.0706. PubMed
11. Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14):1299-1305. doi: 10.1001/archinternmed.2009.204. PubMed
12. Madeira CL, Craig MJ, Donohoe A, Stephens JR. Things we do for no reason: echocardiogram in unselected patients with syncope. J Hosp Med. 2017;12(12):984–988. doi: http://dx.doi.org/10.12788/jhm.2864. PubMed
13. Han SK, Yeom SR, Lee SH, et al. Transthoracic echocardiogram in syncope patients with normal initial evaluation. Am J Emerg Med. 2017;35(2):281-284. doi: 10.1016/j.ajem.2016.10.078. PubMed
14. Chang NL, Shah P, Bajaj S, Virk H, Bikkina M, Shamoon F. Diagnostic yield of echocardiography in syncope patients with normal ECG. Cardiol Res Pract. 2016;2016:1251637. doi: http://dx.doi.org/10.1155/2016/1251637. PubMed
15. Anderson KL, Limkakeng A, Damuth E, Chandra A. Cardiac evaluation for structural abnormalities may not be required in patients presenting with syncope and a normal ECG result in an observation unit setting. Ann Emerg Med. 2012;60(4):478–84.e1. doi: 10.1016/j.annemergmed.2012.04.023. PubMed
16. Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart. 2002;88(4):363-367. doi: 10.1136/heart.88.4.363. PubMed
17. Recchia D, Barzilai B. Echocardiography in the evaluation of patients with syncope. J Gen Intern Med. 1995;10(12):649-655. doi: 10.1007/BF02602755. PubMed
18. Shen WK, Sheldon RS, Benditt DG, et al. ACC/AHA/HRS guideline for the evaluation and management of patients With syncope: executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;2017(70(5)):620-663. PubMed
19. Chiu DT, Shapiro NI, Sun BC, Mottley JL, Grossman SA. Are echocardiography, telemetry, ambulatory electrocardiography monitoring, and cardiac enzymes in emergency department patients presenting with syncope useful tests? A preliminary investigation. J Emerg Med. 2014;47(1):113-118. doi: 10.1016/j.jemermed.2014.01.018. PubMed
20. Sun BC, Costantino G, Barbic F, et al. Priorities for emergency department syncope research. Ann Emerg Med. 2014;64(6):649–55.e2. doi: 10.1016/j.annemergmed.2014.04.014. PubMed
21. Sun BC, Derose SF, Liang LJ, et al. Predictors of 30-day serious events in older patients with syncope. Ann Emerg Med. 2009;54(6):769–778.e1-5. doi: 10.1016/j.annemergmed.2009.07.027. PubMed
22. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc. 1996;58(1):267-288.
23. Friedman J, Hastie T, Tibshirani R. He Elements of Statistical Learning;Vol 1. New York, NY: Springer-Verlag; 2001. PubMed
24. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1-22. doi: 10.18637/jss.v033.i01. PubMed
25. James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning;Vol 112. New York, NY: Springer-Verlag; 2013.
26. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927 ;22(158):209-212. doi: 10.1080/01621459.1927.10502953. PubMed
27. R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
28. Chew DP, Zeitz C, Worthley M, et al. Randomized comparison of high-sensitivity troponin reporting in undifferentiated chest pain assessment. Circ Cardiovasc Qual Outcomes. 2016;9(5):542-553. doi: 10.1161/CIRCOUTCOMES.115.002488. PubMed
29. Chen RS, Bivens MJ, Grossman SA. Diagnosis and management of valvular heart disease in emergency medicine. Emerg Med Clin North Am. 2011;29(4):801–10, vii. doi: 10.1016/j.emc.2011.08.001. PubMed
Syncope, defined as a transient loss of consciousness and postural tone followed by complete, spontaneous return to neurological baseline, accounts for over 1 million (or approximately 1%) of all emergency department (ED) visits per year in the United States (US).12 Given the breadth of etiologies for syncope, including certain life-threatening conditions, extensive diagnostic evaluation and hospitalization for this complaint is common.3-7 The estimated costs of syncope-related hospitalizations are over $2.4 billion annually in the US.8
The 2011 American College of Cardiology Foundation appropriate use criteria for echocardiography state that syncope is an appropriate indication for transthoracic echocardiography (TTE) even when there are no other symptoms or signs of cardiovascular disease.9 This broad recommendation may be appropriate since a finding of severe valvular disease would generally merit consultation with a cardiothoracic surgeon to assess the potential for surgical intervention.10 However, routine use of echocardiogram in all syncope patients could result in increased healthcare costs, patient discomfort, and incidental findings of unclear significance, while rarely changing diagnosis or management.11,12
In an attempt to reduce potentially unnecessary TTE testing, several studies have tried to identify patients at very low risk of structural heart disease.13-17 These investigations suggest that TTE is not indicated in syncope patients with a normal ECG and a normal cardiac exam. However, this literature is limited by retrospective study design and/or small sample sizes. The 2017 American Heart Association/American College of Cardiology/Heart Rhythm Society syncope guidelines recommend TTE for a patient in whom structural heart disease is suspected, but they are not explicit about how to make this determination. 18 Thus, it is still unclear which syncope patients require TTE since a standardized approach to assessing risk of clinically significant findings on TTE has not yet been rigorously developed.
The objective of this study was to develop a risk-stratification tool to identify older adults at very low risk of having a major, clinically significant finding on rest TTE after presenting to the ED with syncope or near-syncope. Using clinical, ECG, and cardiac biomarker data, we created the ROMEO (Risk Of Major Echocardiography findings in Older adults with syncope) score to help optimize resource utilization for syncope.
METHODS
Study Design and Setting
We conducted a large, multicenter, prospective, observational cohort study of older adults who presented to an ED with syncope or near-syncope (ClinicalTrials.gov identifier: NCT01802398). The study was approved by the institutional review boards at all sites and written informed consent was obtained from all participating subjects. The study was conducted at 11 academic EDs across the US (See Appendix Table 1).
Study Population
Patient inclusion criteria for eligibility were age ≥60 years with a complaint of syncope or near-syncope. Syncope was defined as transient loss of consciousness, associated with postural loss of tone, with immediate, spontaneous, and complete recovery. Near syncope was defined as the sensation of imminent loss of consciousness. Patients were excluded if their symptoms were thought to be due to intoxication, seizure, stroke, head trauma, or hypoglycemia. Additional exclusion criteria were the need for medical intervention to restore consciousness (eg, defibrillation), new or worsening confusion, and inability to obtain informed consent from the patient or a legally authorized representative.
This analysis included only patients who received a TTE during the index visit (either in the ED, observation unit, or while admitted to the hospital). This dataset was also used for other analyses addressing questions relevant to the ED management of syncope.
Measurements
All patients underwent a standardized history, physical examination, laboratory, and 12-lead ECG testing. Trained research assistants (RA) directly queried patients about symptoms associated with the syncopal episode. Data on the patient’s past medical history, medications, and physical examination findings were collected prospectively from treating providers.
Research staff obtained blood samples for testing at a core laboratory (University of Rochester, Rochester, NY). Two assays were performed using the Roche Elecsys platform: N-terminal pro B-type natriuretic peptide (NT-proBNP) and the 5th generation high-sensitivity cardiac troponin T (hs-TnT). NT-proBNP was classified as abnormal above a cutoff of 125 pg/mL. Hs-TnT was classified as abnormal above the 99th percentile for a reference population (14 pg/mL). Although hs-TnT was not approved by the U.S. Food and Drug Administration (FDA) at the time of the study, we anticipated that this assay would receive approval and be integrated into future standard of care (FDA approval was granted in January 2017). Rest TTEs were ordered at the discretion of the treating providers.
Outcome Measures
The primary outcome for this secondary analysis was a major, clinically significant finding on TTE.13,14,16,19 These included severe aortic stenosis (<1 cm2), severe mitral stenosis, severe aortic/mitral regurgitation, reduced ejection fraction (defined either quantitatively as less than 45% or qualitatively as “severe left ventricular dysfunction”), hypertrophic cardiomyopathy with outflow tract obstruction, severe pulmonary hypertension, right ventricular dysfunction/strain, large pericardial effusion, atrial myxoma, or regional wall motion abnormalities.
All echocardiogram reports were independently reviewed by two research physicians. Discrepant reviews were resolved by the research physicians and two of the study investigators (BS, CB). Of note, all the TTEs obtained were formal echocardiographic studies, not bedside ultrasonography performed by the emergency physician.
Candidate Predictors
Potential candidate predictors were identified through a prior expert panel process.20,21 Candidate predictors included age, sex, abnormal heart sounds, exertional syncope, shortness of breath, chest pain, near-syncope, family history of sudden cardiac death, high (>180 mm Hg) or low (<90 mm Hg) systolic blood pressure, abnormal ECG, elevated hs-TnT, elevated NT-proBNP, and history of the following: hypertension, cardiac dysrhythmia, renal failure, diabetes, congestive heart failure (CHF), and coronary artery disease (CAD).
The first obtained ECG was abstracted by one of five research study physicians blinded to all clinical data. Research study physicians demonstrated high interrater reliability (kappa > 0.80) in distinguishing normal from abnormal ECGs in a training set of 50 ECGs. Abnormal ECG interpretations included nonsinus rhythms (including paced rhythms), multiple premature ventricular complexes, sinus bradycardias (≤40 bpm), ventricular hypertrophies, short PR segment intervals (<100 milliseconds [ms]), axis deviations, first degree blocks (>200 ms), complete bundle branch blocks, Brugada patterns, Wolff-Parkinson-White patterns, abnormal QRS duration (>120 ms) or abnormal QTc prolongations (>450 ms), and Q/ST/T segment abnormalities suggestive of acute or chronic ischemia.
Statistical Analysis
We calculated descriptive statistics for each predictor variable, stratified by the presence or absence of TTE findings. Chi-square and t-tests were used to test associations between categorical or continuous variables and TTE findings using a significance level of 0.05 and 2-sided hypothesis testing. To identify a robust set of predictors of the primary outcome, we used multivariate logistic regression with the LASSO (Least Absolute Shrinkage and Selection Operator) to fit a parsimonious model.22 The LASSO selects variables and shrinks the associated coefficients to avoid overfitting.23-25 We then used a bootstrap to generate confidence intervals for coefficient estimates. Cases with missing echocardiography reports were excluded from the analysis. Bootstrap results were summarized as the percentage of bootstrap iterations in which each variable’s coefficient was 1) chosen and negative, 2) shrunk to zero, or 3) chosen and positive.
We assessed different weighting schemes to generate a risk score from significant variables identified by regression modeling. These included weighting by regression coefficients rounded to the nearest integer and simple summation of the presence or absence of each variable.
Based on these results, a predictive score was developed to risk stratify patients on their probability of major, clinically significant findings on TTE. The sensitivity and specificity of a score of zero to predict findings on TTE was calculated. For confidence intervals, we used Wilson’s method for binomial confidence intervals.26 The receiver operating characteristic (ROC) curve and its associated area under the curve (AUC) were calculated, and a confidence interval for the AUC was obtained through bootstrap resampling with 2,000 iterations. As part of our sensitivity analyses, we also calculated the ROC curve and AUC after excluding the patients with a known history of CHF and significant finding on TTE. Data analyses were performed in R.27 Two sensitivity analyses were performed: 1) we used multiple imputation to impute 1,000 complete data sets and then used the same LASSO methodology as with the complete data to assess whether incorporating missing data changed the results; and 2) we simulated a conventional troponin assay by raising the positive threshold for hs-TnT to >30 pg/mL (corresponding to the limit of detection for conventional troponin).28
RESULTS
Characteristics of Study Subjects
Patient screening occurred from April 2013 to September 2016. There were 6,930 patients who met eligibility criteria, of whom 3,686 (53%) consented and enrolled in the study (See Figure 1). Of these, 995 (27%) received TTE. The mean age of patients receiving TTE was 74 years; 55% were male. Characteristics of patients obtaining and not obtaining TTE are presented in Appendix Table 2. Patients who received TTE were more likely to be older, have abnormal heart sounds, abnormal EKGs, elevated hs-TnT, elevated NT-proBNP, and have a history of CHF. Of the 995 subjects receiving TTE, 215 (21.6%) had a major, clinically significant finding.
Main Results
Univariate analysis identified 14 variables significantly associated with major findings on TTE. These included male gender, shortness of breath, abnormal heart sounds, history of renal failure, diabetes, CHF, CAD, abnormal ECG, and elevated cardiac biomarkers, among others (See Table 1). The most common major finding on TTE was regional wall motion abnormality, followed by reduced left ventricular ejection fraction (See Table 2). Of the 995 patients who received TTE, 20 (2%) were discharged directly from the ED, 444 (45%) were observed, and 531 (53%) were admitted. On average, patients who received TTE had a longer length of stay than did those that did not (3.4 days vs 1.9 days).
LASSO multivariable logistic regression produced five predictors associated with major findings on TTE: 1) history of CHF, 2) history of CAD, 3) abnormal ECG, 4) hs-TnT above 14 pg/mL, and 5) NT-proBNP above 125 pg/mL (See Table 3).
These five high-risk clinical variables retained their importance after multivariate analysis and form the ROMEO score.
The sensitivity and specificity of a ROMEO score of zero for excluding major findings on TTE was 99.5% (95% CI: 97.4%-99.9%) and 15.4% (95% CI: 13.0%-18.1%), respectively. Patients with a ROMEO score of 0 were at very low risk of having a major finding on TTE: 0.8% (95% CI: 0.02%-4.5%; Appendix Table 3). Only one out of 121 patients with none of the ROMEO criteria was found to have a major finding on TTE (regional wall motion abnormality). Patients with a score of 1 or more were at moderate-to-high risk of having a major finding (7.3% to 55.6%).
There was a linear relationship between the ROMEO score and probability of major findings on TTE (See Appendix Figure 1). The AUC was 0.77 (95% CI = 0.72-0.79) indicating good accuracy of the combination of the five high-risk clinical variables to predict major findings on TTE (See Appendix Figure 2). After excluding the 72 patients with known CHF and significant findings on TTE, the AUC was similar: 0.73 (95% CI: 0.69-0.77). There were 139 patients with at least one missing variable (14%) (See Appendix Table 4). A multiple imputation sensitivity analysis identified the same five high-risk clinical variables in 85% of imputations.
There were 253 patients with high-sensitivity troponin levels between 15 and 30 pg/mL (inclusive). Using a higher hs-TnT threshold (>30 pg/mL) to simulate a conventional troponin assay again identified the same five high-risk variables along with shortness of breath as a potential sixth variable though with an odds ratio approaching unity (See Appendix Table 5). The ROMEO score would have missed two additional patients with major findings if the troponin cutoff were raised to 30 pg/mL from 14 pg/mL, ie, it would have identified 212/215 (98.6%) of the major findings rather than 214/215 (99.5%).
DISCUSSION
Older adults with syncope often present to the ED and undergo a variety of diagnostic tests, including TTE, and a significant proportion are admitted to the hospital.2 There is currently no standardized, evidence-based approach to guide TTE ordering for these patients. Using a large, prospective dataset of syncope patients, we sought to develop a risk-stratification tool to help clinicians identify which syncope patients would be at very low risk for clinically significant findings on TTE. We found that in the absence of these five high-risk clinical variables, the rate of significant findings on TTE in our sample was less than 1%. All five high-risk variables included in the tool remained predictive in our sensitivity analyses, speaking to the robustness of our model.
Other retrospective, and smaller prospective, studies have identified a combination of low-risk criteria including: a normal ECG alone,15 a normal physical exam and normal ECG,14,17 a negative cardiac history and normal ECG.16 Han et al. performed a chart review of 241 patients presenting to the ED with syncope and identified three risk factors for abnormal TTE findings using multiple logistic regression: age, abnormal ECG, and BNP greater than 100 pg/mL.13 While these studies’ results are generally consistent with ours, the retrospective nature and small sample size of these studies limit the generalizability of these results. Thus, using a large, multicenter prospective dataset, we derived a clinical decision instrument (the ROMEO score) to determine which older adults with syncope are at very low risk for major, clinically significant findings on TTE.
Our results add to the recent American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines on the management of syncope which recommend TTE in “selected patients presenting with syncope if structural heart disease is suspected.”18 Our risk-stratification tool offers a simple, standardized approach to determine specifically when to defer TTE testing.
Our findings can guide clinicians in deciding when to obtain TTE for ED syncope patients in the following way: Older adults presenting with syncope or near-syncope to the ED who have none of the ROMEO criteria are at extremely low risk for clinically significant findings on TTE and thus need not undergo such testing solely because of the syncopal event. Patients who have only one or more high-risk clinical variables are at higher risk (7.3%-56%) of significant TTE findings. In this subset, other factors, (eg, physician gestalt, recent previous echocardiography, patient preference, availability of echocardiography) can help guide TTE ordering. Patients with a greater number of high-risk variables may benefit from a more urgent echocardiographic evaluation.
Although on average, patients undergoing TTE had a longer length of stay than those that did not, this finding does not necessarily imply that ordering a TTE was the cause of the increased length of stay. It is possible that this positive association was due to greater underlying medical complexity or acuity of illness that resulted in a greater likelihood of admission/observation, and in turn, a greater length of stay.
Prior to implementation, our results should be externally validated in other clinical settings. In the interim, this risk-stratification tool may be used by clinicians, in conjunction with clinical judgement, to help guide the appropriate use of TTE in older adults presenting with syncope.
Our study has certain limitations. As we only enrolled patients 60 years and older, our findings may not necessarily be valid in younger populations of syncope patients. However, structural heart disease is less common in younger patients and is generally more of a concern for clinicians when evaluating syncope patients in the older age range.29 In our study, 47% of eligible patients declined to participate and thus sampling bias may have occurred. TTEs were ordered at the discretion of treating providers, which was likely subject to physician, institutional, and regional variation; the prevalence of major TTE findings may be lower in the overall cohort than in patients who received TTE. Prior TTE reports were not available; therefore, we were not able to determine if these major findings were previously known. Importantly, we did not perform an internal or external validation of the ROMEO score due to time and resource constraints. Thus, this study represents a derivation of the score solely and would require external validation prior to clinical implementation. Also, to calculate the ROMEO score, both an hs-TnT and NT-proBNP level must be obtained. Thus, the cost savings of any potential reduction in TTE ordering may be partially offset by the costs of increased laboratory testing. Lastly, hs-TnT assays are not currently widely available in hospitals in the United States; earlier generation cardiac troponin assays may not be a perfect substitute for hs-TnT assays. Our sensitivity analysis using an elevated threshold for hs-TnT attempted to mitigate this limitation and resulted in similar findings.
In summary, this risk-stratification tool, using five simple criteria, could help clinicians determine which older adult syncope patients can safely forgo TTE. If validated, this tool could help optimize resource utilization, and increase the value of healthcare for patients presenting with syncope.
Acknowledgments
The authors would like to thank the research assistants at all 11 sites who enrolled patients and collected data for this study.
Disclosures
Dr. Adler has received research funding from Roche. Dr. Bastani has received research funding from Radiometer and Portola and has been a consultant for Portola. Dr. Baugh has received advisory board and speaker’s fees from Roche, research funding from Janssen and Boehringer Ingelheim, and consulting and advisory board fees from Janssen. Dr. Casterino has received funding from Astra Zeneca. Dr. Clark has received research funding from Radiometer, Ortho Clinical Trials, Janssen, Pfizer, NIH, Portola, Biocryst, Glaxo Smith Klein, Hospital Quality Foundation, and Abbott. She is a consultant for Portola, Janssen, and Hospital Quality Foundation. Dr. Diercks is a consultant for Siemens, Janssen, and Roche has received institutional research support from Novartis, Ortho Scientific, and Roche. Dr. Hollander has received research funding from Alere, Siemens, Roche, Portola, and Trinity. Dr. Hollander has also received royalties from UpToDate. Dr. Nishijima has received an honorarium from Pfizer. Dr. Storrow is a consultant for Siemens and Quidel, has received speaking fees from MCM Education, and is on the Data and Safety Monitoring Board for Trevena. Dr. Sun is a consultant for Medtronic. The other authors report no relevant conflicts of interest.
Funding
This project was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01 HL111033. Dr. Probst is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL132052-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Roche Diagnostics supplied the high-sensitivity troponin-T assays. The sponsoring organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, or review of the manuscript.
Syncope, defined as a transient loss of consciousness and postural tone followed by complete, spontaneous return to neurological baseline, accounts for over 1 million (or approximately 1%) of all emergency department (ED) visits per year in the United States (US).12 Given the breadth of etiologies for syncope, including certain life-threatening conditions, extensive diagnostic evaluation and hospitalization for this complaint is common.3-7 The estimated costs of syncope-related hospitalizations are over $2.4 billion annually in the US.8
The 2011 American College of Cardiology Foundation appropriate use criteria for echocardiography state that syncope is an appropriate indication for transthoracic echocardiography (TTE) even when there are no other symptoms or signs of cardiovascular disease.9 This broad recommendation may be appropriate since a finding of severe valvular disease would generally merit consultation with a cardiothoracic surgeon to assess the potential for surgical intervention.10 However, routine use of echocardiogram in all syncope patients could result in increased healthcare costs, patient discomfort, and incidental findings of unclear significance, while rarely changing diagnosis or management.11,12
In an attempt to reduce potentially unnecessary TTE testing, several studies have tried to identify patients at very low risk of structural heart disease.13-17 These investigations suggest that TTE is not indicated in syncope patients with a normal ECG and a normal cardiac exam. However, this literature is limited by retrospective study design and/or small sample sizes. The 2017 American Heart Association/American College of Cardiology/Heart Rhythm Society syncope guidelines recommend TTE for a patient in whom structural heart disease is suspected, but they are not explicit about how to make this determination. 18 Thus, it is still unclear which syncope patients require TTE since a standardized approach to assessing risk of clinically significant findings on TTE has not yet been rigorously developed.
The objective of this study was to develop a risk-stratification tool to identify older adults at very low risk of having a major, clinically significant finding on rest TTE after presenting to the ED with syncope or near-syncope. Using clinical, ECG, and cardiac biomarker data, we created the ROMEO (Risk Of Major Echocardiography findings in Older adults with syncope) score to help optimize resource utilization for syncope.
METHODS
Study Design and Setting
We conducted a large, multicenter, prospective, observational cohort study of older adults who presented to an ED with syncope or near-syncope (ClinicalTrials.gov identifier: NCT01802398). The study was approved by the institutional review boards at all sites and written informed consent was obtained from all participating subjects. The study was conducted at 11 academic EDs across the US (See Appendix Table 1).
Study Population
Patient inclusion criteria for eligibility were age ≥60 years with a complaint of syncope or near-syncope. Syncope was defined as transient loss of consciousness, associated with postural loss of tone, with immediate, spontaneous, and complete recovery. Near syncope was defined as the sensation of imminent loss of consciousness. Patients were excluded if their symptoms were thought to be due to intoxication, seizure, stroke, head trauma, or hypoglycemia. Additional exclusion criteria were the need for medical intervention to restore consciousness (eg, defibrillation), new or worsening confusion, and inability to obtain informed consent from the patient or a legally authorized representative.
This analysis included only patients who received a TTE during the index visit (either in the ED, observation unit, or while admitted to the hospital). This dataset was also used for other analyses addressing questions relevant to the ED management of syncope.
Measurements
All patients underwent a standardized history, physical examination, laboratory, and 12-lead ECG testing. Trained research assistants (RA) directly queried patients about symptoms associated with the syncopal episode. Data on the patient’s past medical history, medications, and physical examination findings were collected prospectively from treating providers.
Research staff obtained blood samples for testing at a core laboratory (University of Rochester, Rochester, NY). Two assays were performed using the Roche Elecsys platform: N-terminal pro B-type natriuretic peptide (NT-proBNP) and the 5th generation high-sensitivity cardiac troponin T (hs-TnT). NT-proBNP was classified as abnormal above a cutoff of 125 pg/mL. Hs-TnT was classified as abnormal above the 99th percentile for a reference population (14 pg/mL). Although hs-TnT was not approved by the U.S. Food and Drug Administration (FDA) at the time of the study, we anticipated that this assay would receive approval and be integrated into future standard of care (FDA approval was granted in January 2017). Rest TTEs were ordered at the discretion of the treating providers.
Outcome Measures
The primary outcome for this secondary analysis was a major, clinically significant finding on TTE.13,14,16,19 These included severe aortic stenosis (<1 cm2), severe mitral stenosis, severe aortic/mitral regurgitation, reduced ejection fraction (defined either quantitatively as less than 45% or qualitatively as “severe left ventricular dysfunction”), hypertrophic cardiomyopathy with outflow tract obstruction, severe pulmonary hypertension, right ventricular dysfunction/strain, large pericardial effusion, atrial myxoma, or regional wall motion abnormalities.
All echocardiogram reports were independently reviewed by two research physicians. Discrepant reviews were resolved by the research physicians and two of the study investigators (BS, CB). Of note, all the TTEs obtained were formal echocardiographic studies, not bedside ultrasonography performed by the emergency physician.
Candidate Predictors
Potential candidate predictors were identified through a prior expert panel process.20,21 Candidate predictors included age, sex, abnormal heart sounds, exertional syncope, shortness of breath, chest pain, near-syncope, family history of sudden cardiac death, high (>180 mm Hg) or low (<90 mm Hg) systolic blood pressure, abnormal ECG, elevated hs-TnT, elevated NT-proBNP, and history of the following: hypertension, cardiac dysrhythmia, renal failure, diabetes, congestive heart failure (CHF), and coronary artery disease (CAD).
The first obtained ECG was abstracted by one of five research study physicians blinded to all clinical data. Research study physicians demonstrated high interrater reliability (kappa > 0.80) in distinguishing normal from abnormal ECGs in a training set of 50 ECGs. Abnormal ECG interpretations included nonsinus rhythms (including paced rhythms), multiple premature ventricular complexes, sinus bradycardias (≤40 bpm), ventricular hypertrophies, short PR segment intervals (<100 milliseconds [ms]), axis deviations, first degree blocks (>200 ms), complete bundle branch blocks, Brugada patterns, Wolff-Parkinson-White patterns, abnormal QRS duration (>120 ms) or abnormal QTc prolongations (>450 ms), and Q/ST/T segment abnormalities suggestive of acute or chronic ischemia.
Statistical Analysis
We calculated descriptive statistics for each predictor variable, stratified by the presence or absence of TTE findings. Chi-square and t-tests were used to test associations between categorical or continuous variables and TTE findings using a significance level of 0.05 and 2-sided hypothesis testing. To identify a robust set of predictors of the primary outcome, we used multivariate logistic regression with the LASSO (Least Absolute Shrinkage and Selection Operator) to fit a parsimonious model.22 The LASSO selects variables and shrinks the associated coefficients to avoid overfitting.23-25 We then used a bootstrap to generate confidence intervals for coefficient estimates. Cases with missing echocardiography reports were excluded from the analysis. Bootstrap results were summarized as the percentage of bootstrap iterations in which each variable’s coefficient was 1) chosen and negative, 2) shrunk to zero, or 3) chosen and positive.
We assessed different weighting schemes to generate a risk score from significant variables identified by regression modeling. These included weighting by regression coefficients rounded to the nearest integer and simple summation of the presence or absence of each variable.
Based on these results, a predictive score was developed to risk stratify patients on their probability of major, clinically significant findings on TTE. The sensitivity and specificity of a score of zero to predict findings on TTE was calculated. For confidence intervals, we used Wilson’s method for binomial confidence intervals.26 The receiver operating characteristic (ROC) curve and its associated area under the curve (AUC) were calculated, and a confidence interval for the AUC was obtained through bootstrap resampling with 2,000 iterations. As part of our sensitivity analyses, we also calculated the ROC curve and AUC after excluding the patients with a known history of CHF and significant finding on TTE. Data analyses were performed in R.27 Two sensitivity analyses were performed: 1) we used multiple imputation to impute 1,000 complete data sets and then used the same LASSO methodology as with the complete data to assess whether incorporating missing data changed the results; and 2) we simulated a conventional troponin assay by raising the positive threshold for hs-TnT to >30 pg/mL (corresponding to the limit of detection for conventional troponin).28
RESULTS
Characteristics of Study Subjects
Patient screening occurred from April 2013 to September 2016. There were 6,930 patients who met eligibility criteria, of whom 3,686 (53%) consented and enrolled in the study (See Figure 1). Of these, 995 (27%) received TTE. The mean age of patients receiving TTE was 74 years; 55% were male. Characteristics of patients obtaining and not obtaining TTE are presented in Appendix Table 2. Patients who received TTE were more likely to be older, have abnormal heart sounds, abnormal EKGs, elevated hs-TnT, elevated NT-proBNP, and have a history of CHF. Of the 995 subjects receiving TTE, 215 (21.6%) had a major, clinically significant finding.
Main Results
Univariate analysis identified 14 variables significantly associated with major findings on TTE. These included male gender, shortness of breath, abnormal heart sounds, history of renal failure, diabetes, CHF, CAD, abnormal ECG, and elevated cardiac biomarkers, among others (See Table 1). The most common major finding on TTE was regional wall motion abnormality, followed by reduced left ventricular ejection fraction (See Table 2). Of the 995 patients who received TTE, 20 (2%) were discharged directly from the ED, 444 (45%) were observed, and 531 (53%) were admitted. On average, patients who received TTE had a longer length of stay than did those that did not (3.4 days vs 1.9 days).
LASSO multivariable logistic regression produced five predictors associated with major findings on TTE: 1) history of CHF, 2) history of CAD, 3) abnormal ECG, 4) hs-TnT above 14 pg/mL, and 5) NT-proBNP above 125 pg/mL (See Table 3).
These five high-risk clinical variables retained their importance after multivariate analysis and form the ROMEO score.
The sensitivity and specificity of a ROMEO score of zero for excluding major findings on TTE was 99.5% (95% CI: 97.4%-99.9%) and 15.4% (95% CI: 13.0%-18.1%), respectively. Patients with a ROMEO score of 0 were at very low risk of having a major finding on TTE: 0.8% (95% CI: 0.02%-4.5%; Appendix Table 3). Only one out of 121 patients with none of the ROMEO criteria was found to have a major finding on TTE (regional wall motion abnormality). Patients with a score of 1 or more were at moderate-to-high risk of having a major finding (7.3% to 55.6%).
There was a linear relationship between the ROMEO score and probability of major findings on TTE (See Appendix Figure 1). The AUC was 0.77 (95% CI = 0.72-0.79) indicating good accuracy of the combination of the five high-risk clinical variables to predict major findings on TTE (See Appendix Figure 2). After excluding the 72 patients with known CHF and significant findings on TTE, the AUC was similar: 0.73 (95% CI: 0.69-0.77). There were 139 patients with at least one missing variable (14%) (See Appendix Table 4). A multiple imputation sensitivity analysis identified the same five high-risk clinical variables in 85% of imputations.
There were 253 patients with high-sensitivity troponin levels between 15 and 30 pg/mL (inclusive). Using a higher hs-TnT threshold (>30 pg/mL) to simulate a conventional troponin assay again identified the same five high-risk variables along with shortness of breath as a potential sixth variable though with an odds ratio approaching unity (See Appendix Table 5). The ROMEO score would have missed two additional patients with major findings if the troponin cutoff were raised to 30 pg/mL from 14 pg/mL, ie, it would have identified 212/215 (98.6%) of the major findings rather than 214/215 (99.5%).
DISCUSSION
Older adults with syncope often present to the ED and undergo a variety of diagnostic tests, including TTE, and a significant proportion are admitted to the hospital.2 There is currently no standardized, evidence-based approach to guide TTE ordering for these patients. Using a large, prospective dataset of syncope patients, we sought to develop a risk-stratification tool to help clinicians identify which syncope patients would be at very low risk for clinically significant findings on TTE. We found that in the absence of these five high-risk clinical variables, the rate of significant findings on TTE in our sample was less than 1%. All five high-risk variables included in the tool remained predictive in our sensitivity analyses, speaking to the robustness of our model.
Other retrospective, and smaller prospective, studies have identified a combination of low-risk criteria including: a normal ECG alone,15 a normal physical exam and normal ECG,14,17 a negative cardiac history and normal ECG.16 Han et al. performed a chart review of 241 patients presenting to the ED with syncope and identified three risk factors for abnormal TTE findings using multiple logistic regression: age, abnormal ECG, and BNP greater than 100 pg/mL.13 While these studies’ results are generally consistent with ours, the retrospective nature and small sample size of these studies limit the generalizability of these results. Thus, using a large, multicenter prospective dataset, we derived a clinical decision instrument (the ROMEO score) to determine which older adults with syncope are at very low risk for major, clinically significant findings on TTE.
Our results add to the recent American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines on the management of syncope which recommend TTE in “selected patients presenting with syncope if structural heart disease is suspected.”18 Our risk-stratification tool offers a simple, standardized approach to determine specifically when to defer TTE testing.
Our findings can guide clinicians in deciding when to obtain TTE for ED syncope patients in the following way: Older adults presenting with syncope or near-syncope to the ED who have none of the ROMEO criteria are at extremely low risk for clinically significant findings on TTE and thus need not undergo such testing solely because of the syncopal event. Patients who have only one or more high-risk clinical variables are at higher risk (7.3%-56%) of significant TTE findings. In this subset, other factors, (eg, physician gestalt, recent previous echocardiography, patient preference, availability of echocardiography) can help guide TTE ordering. Patients with a greater number of high-risk variables may benefit from a more urgent echocardiographic evaluation.
Although on average, patients undergoing TTE had a longer length of stay than those that did not, this finding does not necessarily imply that ordering a TTE was the cause of the increased length of stay. It is possible that this positive association was due to greater underlying medical complexity or acuity of illness that resulted in a greater likelihood of admission/observation, and in turn, a greater length of stay.
Prior to implementation, our results should be externally validated in other clinical settings. In the interim, this risk-stratification tool may be used by clinicians, in conjunction with clinical judgement, to help guide the appropriate use of TTE in older adults presenting with syncope.
Our study has certain limitations. As we only enrolled patients 60 years and older, our findings may not necessarily be valid in younger populations of syncope patients. However, structural heart disease is less common in younger patients and is generally more of a concern for clinicians when evaluating syncope patients in the older age range.29 In our study, 47% of eligible patients declined to participate and thus sampling bias may have occurred. TTEs were ordered at the discretion of treating providers, which was likely subject to physician, institutional, and regional variation; the prevalence of major TTE findings may be lower in the overall cohort than in patients who received TTE. Prior TTE reports were not available; therefore, we were not able to determine if these major findings were previously known. Importantly, we did not perform an internal or external validation of the ROMEO score due to time and resource constraints. Thus, this study represents a derivation of the score solely and would require external validation prior to clinical implementation. Also, to calculate the ROMEO score, both an hs-TnT and NT-proBNP level must be obtained. Thus, the cost savings of any potential reduction in TTE ordering may be partially offset by the costs of increased laboratory testing. Lastly, hs-TnT assays are not currently widely available in hospitals in the United States; earlier generation cardiac troponin assays may not be a perfect substitute for hs-TnT assays. Our sensitivity analysis using an elevated threshold for hs-TnT attempted to mitigate this limitation and resulted in similar findings.
In summary, this risk-stratification tool, using five simple criteria, could help clinicians determine which older adult syncope patients can safely forgo TTE. If validated, this tool could help optimize resource utilization, and increase the value of healthcare for patients presenting with syncope.
Acknowledgments
The authors would like to thank the research assistants at all 11 sites who enrolled patients and collected data for this study.
Disclosures
Dr. Adler has received research funding from Roche. Dr. Bastani has received research funding from Radiometer and Portola and has been a consultant for Portola. Dr. Baugh has received advisory board and speaker’s fees from Roche, research funding from Janssen and Boehringer Ingelheim, and consulting and advisory board fees from Janssen. Dr. Casterino has received funding from Astra Zeneca. Dr. Clark has received research funding from Radiometer, Ortho Clinical Trials, Janssen, Pfizer, NIH, Portola, Biocryst, Glaxo Smith Klein, Hospital Quality Foundation, and Abbott. She is a consultant for Portola, Janssen, and Hospital Quality Foundation. Dr. Diercks is a consultant for Siemens, Janssen, and Roche has received institutional research support from Novartis, Ortho Scientific, and Roche. Dr. Hollander has received research funding from Alere, Siemens, Roche, Portola, and Trinity. Dr. Hollander has also received royalties from UpToDate. Dr. Nishijima has received an honorarium from Pfizer. Dr. Storrow is a consultant for Siemens and Quidel, has received speaking fees from MCM Education, and is on the Data and Safety Monitoring Board for Trevena. Dr. Sun is a consultant for Medtronic. The other authors report no relevant conflicts of interest.
Funding
This project was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01 HL111033. Dr. Probst is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL132052-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Roche Diagnostics supplied the high-sensitivity troponin-T assays. The sponsoring organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, or review of the manuscript.
1. Sun BC, Emond JA, Camargo CA, Jr. Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992-2000. Acad Emerg Med. 2004;11(10):1029-1034. doi: 10.1197/j.aem.2004.05.032. PubMed
2. Probst MA, Kanzaria HK, Gbedemah M, Richardson LD, Sun BC. National trends in resource utilization associated with ED visits for syncope. Am J Emerg Med. 2015;33(8):998-1001. doi: 10.1016/j.ajem.2015.04.030. PubMed
3. Kapoor WN, Karpf M, Maher Y, Miller RA, Levey GS. Syncope of unknown origin. The need for a more cost-effective approach to its diagnosis evaluation. JAMA. 1982;247(19):2687-2691. doi: 10.1001/jama.247.19.2687. PubMed
4. Pires LA, Ganji JR, Jarandila R, Steele R. Diagnostic patterns and temporal trends in the evaluation of adult patients hospitalized with syncope. Arch Intern Med. 2001;161(15):1889-1895. doi: 10.1001/archinte.161.15.1889. PubMed
5. Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43(2):224-232. doi: 10.1016/S0196064403008230. PubMed
6. Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 1: Value of history, physical examination, and electrocardiography. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med. 1997;126(12):989-996. doi: 10.7326/0003-4819-126-12-199706150-00012. PubMed
7. Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med. 1997;127(1):76-86. doi: 10.7326/0003-4819-127-1-199707010-00014. PubMed
8. Sun BC, Emond JA, Camargo CA, Jr. Direct medical costs of syncope-related hospitalizations in the United States. Am J Cardiol. 2005;95(5):668-671. doi: 10.1016/j.amjcard.2004.11.013. PubMed
9. American College of Cardiology Foundation. Appropriate Use Criteria Task F, American Society of Echocardiography, American Heart Association, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011;57(9):1126-1166. doi: 10.1016/j.echo.2010.12.008.
10. Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85(5):483-500. doi: 10.4065/mcp.2009.0706. PubMed
11. Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14):1299-1305. doi: 10.1001/archinternmed.2009.204. PubMed
12. Madeira CL, Craig MJ, Donohoe A, Stephens JR. Things we do for no reason: echocardiogram in unselected patients with syncope. J Hosp Med. 2017;12(12):984–988. doi: http://dx.doi.org/10.12788/jhm.2864. PubMed
13. Han SK, Yeom SR, Lee SH, et al. Transthoracic echocardiogram in syncope patients with normal initial evaluation. Am J Emerg Med. 2017;35(2):281-284. doi: 10.1016/j.ajem.2016.10.078. PubMed
14. Chang NL, Shah P, Bajaj S, Virk H, Bikkina M, Shamoon F. Diagnostic yield of echocardiography in syncope patients with normal ECG. Cardiol Res Pract. 2016;2016:1251637. doi: http://dx.doi.org/10.1155/2016/1251637. PubMed
15. Anderson KL, Limkakeng A, Damuth E, Chandra A. Cardiac evaluation for structural abnormalities may not be required in patients presenting with syncope and a normal ECG result in an observation unit setting. Ann Emerg Med. 2012;60(4):478–84.e1. doi: 10.1016/j.annemergmed.2012.04.023. PubMed
16. Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart. 2002;88(4):363-367. doi: 10.1136/heart.88.4.363. PubMed
17. Recchia D, Barzilai B. Echocardiography in the evaluation of patients with syncope. J Gen Intern Med. 1995;10(12):649-655. doi: 10.1007/BF02602755. PubMed
18. Shen WK, Sheldon RS, Benditt DG, et al. ACC/AHA/HRS guideline for the evaluation and management of patients With syncope: executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;2017(70(5)):620-663. PubMed
19. Chiu DT, Shapiro NI, Sun BC, Mottley JL, Grossman SA. Are echocardiography, telemetry, ambulatory electrocardiography monitoring, and cardiac enzymes in emergency department patients presenting with syncope useful tests? A preliminary investigation. J Emerg Med. 2014;47(1):113-118. doi: 10.1016/j.jemermed.2014.01.018. PubMed
20. Sun BC, Costantino G, Barbic F, et al. Priorities for emergency department syncope research. Ann Emerg Med. 2014;64(6):649–55.e2. doi: 10.1016/j.annemergmed.2014.04.014. PubMed
21. Sun BC, Derose SF, Liang LJ, et al. Predictors of 30-day serious events in older patients with syncope. Ann Emerg Med. 2009;54(6):769–778.e1-5. doi: 10.1016/j.annemergmed.2009.07.027. PubMed
22. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc. 1996;58(1):267-288.
23. Friedman J, Hastie T, Tibshirani R. He Elements of Statistical Learning;Vol 1. New York, NY: Springer-Verlag; 2001. PubMed
24. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1-22. doi: 10.18637/jss.v033.i01. PubMed
25. James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning;Vol 112. New York, NY: Springer-Verlag; 2013.
26. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927 ;22(158):209-212. doi: 10.1080/01621459.1927.10502953. PubMed
27. R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
28. Chew DP, Zeitz C, Worthley M, et al. Randomized comparison of high-sensitivity troponin reporting in undifferentiated chest pain assessment. Circ Cardiovasc Qual Outcomes. 2016;9(5):542-553. doi: 10.1161/CIRCOUTCOMES.115.002488. PubMed
29. Chen RS, Bivens MJ, Grossman SA. Diagnosis and management of valvular heart disease in emergency medicine. Emerg Med Clin North Am. 2011;29(4):801–10, vii. doi: 10.1016/j.emc.2011.08.001. PubMed
1. Sun BC, Emond JA, Camargo CA, Jr. Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992-2000. Acad Emerg Med. 2004;11(10):1029-1034. doi: 10.1197/j.aem.2004.05.032. PubMed
2. Probst MA, Kanzaria HK, Gbedemah M, Richardson LD, Sun BC. National trends in resource utilization associated with ED visits for syncope. Am J Emerg Med. 2015;33(8):998-1001. doi: 10.1016/j.ajem.2015.04.030. PubMed
3. Kapoor WN, Karpf M, Maher Y, Miller RA, Levey GS. Syncope of unknown origin. The need for a more cost-effective approach to its diagnosis evaluation. JAMA. 1982;247(19):2687-2691. doi: 10.1001/jama.247.19.2687. PubMed
4. Pires LA, Ganji JR, Jarandila R, Steele R. Diagnostic patterns and temporal trends in the evaluation of adult patients hospitalized with syncope. Arch Intern Med. 2001;161(15):1889-1895. doi: 10.1001/archinte.161.15.1889. PubMed
5. Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43(2):224-232. doi: 10.1016/S0196064403008230. PubMed
6. Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 1: Value of history, physical examination, and electrocardiography. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med. 1997;126(12):989-996. doi: 10.7326/0003-4819-126-12-199706150-00012. PubMed
7. Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med. 1997;127(1):76-86. doi: 10.7326/0003-4819-127-1-199707010-00014. PubMed
8. Sun BC, Emond JA, Camargo CA, Jr. Direct medical costs of syncope-related hospitalizations in the United States. Am J Cardiol. 2005;95(5):668-671. doi: 10.1016/j.amjcard.2004.11.013. PubMed
9. American College of Cardiology Foundation. Appropriate Use Criteria Task F, American Society of Echocardiography, American Heart Association, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011;57(9):1126-1166. doi: 10.1016/j.echo.2010.12.008.
10. Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85(5):483-500. doi: 10.4065/mcp.2009.0706. PubMed
11. Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14):1299-1305. doi: 10.1001/archinternmed.2009.204. PubMed
12. Madeira CL, Craig MJ, Donohoe A, Stephens JR. Things we do for no reason: echocardiogram in unselected patients with syncope. J Hosp Med. 2017;12(12):984–988. doi: http://dx.doi.org/10.12788/jhm.2864. PubMed
13. Han SK, Yeom SR, Lee SH, et al. Transthoracic echocardiogram in syncope patients with normal initial evaluation. Am J Emerg Med. 2017;35(2):281-284. doi: 10.1016/j.ajem.2016.10.078. PubMed
14. Chang NL, Shah P, Bajaj S, Virk H, Bikkina M, Shamoon F. Diagnostic yield of echocardiography in syncope patients with normal ECG. Cardiol Res Pract. 2016;2016:1251637. doi: http://dx.doi.org/10.1155/2016/1251637. PubMed
15. Anderson KL, Limkakeng A, Damuth E, Chandra A. Cardiac evaluation for structural abnormalities may not be required in patients presenting with syncope and a normal ECG result in an observation unit setting. Ann Emerg Med. 2012;60(4):478–84.e1. doi: 10.1016/j.annemergmed.2012.04.023. PubMed
16. Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart. 2002;88(4):363-367. doi: 10.1136/heart.88.4.363. PubMed
17. Recchia D, Barzilai B. Echocardiography in the evaluation of patients with syncope. J Gen Intern Med. 1995;10(12):649-655. doi: 10.1007/BF02602755. PubMed
18. Shen WK, Sheldon RS, Benditt DG, et al. ACC/AHA/HRS guideline for the evaluation and management of patients With syncope: executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;2017(70(5)):620-663. PubMed
19. Chiu DT, Shapiro NI, Sun BC, Mottley JL, Grossman SA. Are echocardiography, telemetry, ambulatory electrocardiography monitoring, and cardiac enzymes in emergency department patients presenting with syncope useful tests? A preliminary investigation. J Emerg Med. 2014;47(1):113-118. doi: 10.1016/j.jemermed.2014.01.018. PubMed
20. Sun BC, Costantino G, Barbic F, et al. Priorities for emergency department syncope research. Ann Emerg Med. 2014;64(6):649–55.e2. doi: 10.1016/j.annemergmed.2014.04.014. PubMed
21. Sun BC, Derose SF, Liang LJ, et al. Predictors of 30-day serious events in older patients with syncope. Ann Emerg Med. 2009;54(6):769–778.e1-5. doi: 10.1016/j.annemergmed.2009.07.027. PubMed
22. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc. 1996;58(1):267-288.
23. Friedman J, Hastie T, Tibshirani R. He Elements of Statistical Learning;Vol 1. New York, NY: Springer-Verlag; 2001. PubMed
24. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1-22. doi: 10.18637/jss.v033.i01. PubMed
25. James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning;Vol 112. New York, NY: Springer-Verlag; 2013.
26. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927 ;22(158):209-212. doi: 10.1080/01621459.1927.10502953. PubMed
27. R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
28. Chew DP, Zeitz C, Worthley M, et al. Randomized comparison of high-sensitivity troponin reporting in undifferentiated chest pain assessment. Circ Cardiovasc Qual Outcomes. 2016;9(5):542-553. doi: 10.1161/CIRCOUTCOMES.115.002488. PubMed
29. Chen RS, Bivens MJ, Grossman SA. Diagnosis and management of valvular heart disease in emergency medicine. Emerg Med Clin North Am. 2011;29(4):801–10, vii. doi: 10.1016/j.emc.2011.08.001. PubMed
© 2018 Society of Hospital Medicine
Electronic Order Volume as a Meaningful Component in Estimating Patient Complexity and Resident Physician Workload
Resident physician workload has traditionally been measured by patient census.1,2 However, census and other volume-based metrics such as daily admissions may not accurately reflect workload due to variation in patient complexity. Relative value units (RVUs) are another commonly used marker of workload, but the validity of this metric relies on accurate coding, usually done by the attending physician, and is less directly related to resident physician workload. Because much of hospital-based medicine is mediated through the electronic health record (EHR), which can capture differences in patient complexity,3 electronic records could be harnessed to more comprehensively describe residents’ work. Current government estimates indicate that several hundred companies offer certified EHRs, thanks in large part to the Health Information Technology for Economic and Clinical Health (HITECH) Act of 2009, which aimed to promote adoption and meaningful use of health information technology.4, 5 These systems can collect important data about the usage and operating patterns of physicians, which may provide an insight into workload.6-8
Accurately measuring workload is important because of the direct link that has been drawn between physician workload and quality metrics. In a study of attending hospitalists, higher workload, as measured by patient census and RVUs, was associated with longer lengths of stay and higher costs of hospitalization.9 Another study among medical residents found that as daily admissions increased, length of stay, cost, and inpatient mortality appeared to rise.10 Although these studies used only volume-based workload metrics, the implication that high workload may negatively impact patient care hints at a possible trade-off between the two that should inform discussions of physician productivity.
In the current study, we examine whether data obtained from the EHR, particularly electronic order volume, could provide valuable information, in addition to patient volume, about resident physician workload. We first tested the feasibility and validity of using electronic order volume as an important component of clinical workload by examining the relationship between electronic order volume and well-established factors that are likely to increase the workload of residents, including patient level of care and severity of illness. Then, using order volume as a marker for workload, we sought to describe whether higher order volumes were associated with two discharge-related quality metrics, completion of a high-quality after-visit summary and timely discharge summary, postulating that quality metrics may suffer when residents are busier.
METHODS
Study Design and Setting
We performed a single-center retrospective cohort study of patients admitted to the internal medicine service at the University of California, San Francisco (UCSF) Medical Center between May 1, 2015 and July 31, 2016. UCSF is a 600-bed academic medical center, and the inpatient internal medicine teaching service manages an average daily census of 80-90 patients. Medicine teams care for patients on the general acute-care wards, the step-down units (for patients with higher acuity of care), and also patients in the intensive care unit (ICU). ICU patients are comanaged by general medicine teams and intensive care teams; internal medicine teams enter all electronic orders for ICU patients, except for orders for respiratory care or sedating medications. The inpatient internal medicine teaching service comprises eight teams each supervised by an attending physician, a senior resident (in the second or third year of residency training), two interns, and a third- and/or fourth-year medical student. Residents place all clinical orders and complete all clinical documentation through the EHR (Epic Systems, Verona, Wisconsin).11 Typically, the bulk of the orders and documentation, including discharge documentation, is performed by interns; however, the degree of senior resident involvement in these tasks is variable and team-dependent. In addition to the eight resident teams, there are also four attending hospitalist-only internal medicine teams, who manage a service of ~30-40 patients.
Study Population
Our study population comprised all hospitalized adults admitted to the eight resident-run teams on the internal medicine teaching service. Patients cared for by hospitalist-only teams were not included in this analysis. Because the focus of our study was on hospitalizations, individual patients may have been included multiple times over the course of the study. Hospitalizations were excluded if they did not have complete Medicare Severity-Diagnosis Related Group (MS-DRG) data,12 since this was used as our severity of illness marker. This occurred either because patients were not discharged by the end of the study period or because they had a length of stay of less than one day, because this metric was not assigned to these short-stay (observation) patients.
Data Collection
All electronic orders placed during the study period were obtained by extracting data from Epic’s Clarity database. Our EHR allows for the use of order sets; each order in these sets was counted individually, so that an order set with several orders would not be identified as one order. We identified the time and date that the order was placed, the ordering physician, the identity of the patient for which the order was placed, and the location of the patient when the order was placed, to determine the level of care (ICU, step-down, or general medicine unit). To track the composite volume of orders placed by resident teams, we matched each ordering physician to his or her corresponding resident team using our physician scheduling database, Amion (Spiral Software). We obtained team census by tabulating the total number of patients that a single resident team placed orders on over the course of a given calendar day. From billing data, we identified the MS-DRG weight that was assigned at the end of each hospitalization. Finally, we collected data on adherence to two discharge-related quality metrics to determine whether increased order volume was associated with decreased rates of adherence to these metrics. Using departmental patient-level quality improvement data, we determined whether each metric was met on discharge at the patient level. We also extracted patient-level demographic data, including age, sex, and insurance status, from this departmental quality improvement database.
Discharge Quality Outcome Metrics
We hypothesized that as the total daily electronic orders of a resident team increased, the rate of completion of two discharge-related quality metrics would decline due to the greater time constraints placed on the teams. The first metric we used was the completion of a high-quality after-visit summary (AVS), which has been described by the Centers for Medicare and Medicaid Services as part of its Meaningful Use Initiative.13 It was selected by the residents in our program as a particularly high-priority quality metric. Our institution specifically defines a “high-quality” AVS as including the following three components: a principal hospital problem, patient instructions, and follow-up information. The second discharge-related quality metric was the completion of a timely discharge summary, another measure recognized as a critical component in high-quality care.14 To be considered timely, the discharge summary had to be filed no later than 24 hours after the discharge order was entered into the EHR. This metric was more recently tracked by the internal medicine department and was not selected by the residents as a high-priority metric.
Statistical Analysis
To examine how the order volume per day changed throughout each sequential day of hospital admission, mean orders per hospital day with 95% CIs were plotted. We performed an aggregate analysis of all orders placed for each patient per day across three different levels of care (ICU, step-down, and general medicine). For each day of the study period, we summed all orders for all patients according to their location and divided by the number of total patients in each location to identify the average number of orders written for an ICU, step-down, and general medicine patient that day. We then calculated the mean daily orders for an ICU, step-down, and general medicine patient over the entire study period. We used ANOVA to test for statistically significant differences between the mean daily orders between these locations.
To examine the relationship between severity of illness and order volume, we performed an unadjusted patient-level analysis of orders per patient in the first three days of each hospitalization and stratified the data by the MS-DRG payment weight, which we divided into four quartiles. For each quartile, we calculated the mean number of orders placed in the first three days of admission and used ANOVA to test for statistically significant differences. We restricted the orders to the first three days of hospitalization instead of calculating mean orders per day of hospitalization because we postulated that the majority of orders were entered in these first few days and that with increasing length of stay (which we expected to occur with higher MS-DRG weight), the order volume becomes highly variable, which would tend to skew the mean orders per day.
We used multivariable logistic regression to determine whether the volume of electronic orders on the day of a given patient’s discharge, and also on the day before a given patient’s discharge, was a significant predictor of receiving a high-quality AVS. We adjusted for team census on the day of discharge, MS-DRG weight, age, sex, and insurance status. We then conducted a separate analysis of the association between electronic order volume and likelihood of completing a timely discharge summary among patients where discharge summary data were available. Logistic regression for each case was performed independently, so that team orders on the day prior to a patient’s discharge were not included in the model for the relationship between team orders on the day of a patient’s discharge and the discharge-related quality metric of interest, and vice versa, since including both in the model would be potentially disruptive given that orders on the day before and day of a patient’s discharge are likely correlated.
We also performed a subanalysis in which we restricted orders to only those placed during the daytime hours (7
IRB Approval
The study was approved by the UCSF Institutional Review Board and was granted a waiver of informed consent.
RESULTS
Population
We identified 7,296 eligible hospitalizations during the study period. After removing hospitalizations according to our exclusion criteria (Figure 1), there were 5,032 hospitalizations that were used in the analysis for which a total of 929,153 orders were written. The vast majority of patients received at least one order per day; fewer than 1% of encounter-days had zero associated orders. The top 10 discharge diagnoses identified in the cohort are listed in Appendix Table 1. A breakdown of orders by order type, across the entire cohort, is displayed in Appendix Table 2. The mean number of orders per patient per day of hospitalization is plotted in the Appendix Figure, which indicates that the number of orders is highest on the day of admission, decreases significantly after the first few days, and becomes increasingly variable with longer lengths of stay.
Patient Level of Care and Severity of Illness Metrics
Patients at a higher level of care had, on average, more orders entered per day. The mean order frequency was 40 orders per day for an ICU patient (standard deviation [SD] 13, range 13-134), 24 for a step-down patient (SD 6, range 11-48), and 19 for a general medicine unit patient (SD 3, range 10-31). The difference in mean daily orders was statistically significant (P < .001, Figure 2a).
Orders also correlated with increasing severity of illness. Patients in the lowest quartile of MS-DRG weight received, on average, 98 orders in the first three days of hospitalization (SD 35, range 2-349), those in the second quartile received 105 orders (SD 38, range 10-380), those in the third quartile received 132 orders (SD 51, range 17-436), and those in the fourth and highest quartile received 149 orders (SD 59, range 32-482). Comparisons between each of these severity of illness categories were significant (P < .001, Figure 2b).
Discharge-Related Quality Metrics
The median number of orders per internal medicine team per day was 343 (IQR 261- 446). Of the 5,032 total discharged patients, 3,657 (73%) received a high-quality AVS on discharge. After controlling for team census, severity of illness, and demographic factors, there was no statistically significant association between total orders on the day of discharge and odds of receiving a high-quality AVS (OR 1.01; 95% CI 0.96-1.06), or between team orders placed the day prior to discharge and odds of receiving a high-quality AVS (OR 0.99; 95% CI 0.95-1.04; Table 1). When we restricted our analysis to orders placed during daytime hours (7
There were 3,835 patients for whom data on timing of discharge summary were available. Of these, 3,455 (91.2%) had a discharge summary completed within 24 hours. After controlling for team census, severity of illness, and demographic factors, there was no statistically significant association between total orders placed by the team on a patient’s day of discharge and odds of receiving a timely discharge summary (OR 0.96; 95% CI 0.88-1.05). However, patients were 12% less likely to receive a timely discharge summary for every 100 extra orders the team placed on the day prior to discharge (OR 0.88, 95% CI 0.82-0.95). Patients who received a timely discharge summary were cared for by teams who placed a median of 345 orders the day prior to their discharge, whereas those that did not receive a timely discharge summary were cared for by teams who placed a significantly higher number of orders (375) on the day prior to discharge (Table 2). When we restricted our analysis to only daytime orders, there were no significant changes in the findings (OR 1.00; 95% CI 0.88-1.14 for orders on the day of discharge; OR 0.84; 95% CI 0.75-0.95 for orders on the day prior to discharge).
DISCUSSION
We found that electronic order volume may be a marker for patient complexity, which encompasses both level of care and severity of illness, and could be a marker of resident physician workload that harnesses readily available data from an EHR. Recent time-motion studies of internal medicine residents indicate that the majority of trainees’ time is spent on computers, engaged in indirect patient care activities such as reading electronic charts, entering electronic orders, and writing computerized notes.15-18 Capturing these tasks through metrics such as electronic order volume, as we did in this study, can provide valuable insights into resident physician workflow.
We found that ICU patients received more than twice as many orders per day than did general acute care-level patients. Furthermore, we found that patients whose hospitalizations fell into the highest MS-DRG weight quartile received approximately 50% more orders during the first three days of admission compared to that of patients whose hospitalizations fell into the lowest quartile. This strong association indicates that electronic order volume could provide meaningful additional information, in concert with other factors such as census, to describe resident physician workload.
We did not find that our workload measure was significantly associated with high-quality AVS completion. There are several possible explanations for this finding. First, adherence to this quality metric may be independent of workload, possibly because it is highly prioritized by residents at our institution. Second, adherence may only be impacted at levels of workload greater than what was experienced by the residents in our study. Finally, electronic order volume may not encompass enough of total workload to be reliably representative of resident work. However, the tight correlation between electronic order volume with severity of illness and level of care, in conjunction with the finding that patients were less likely to receive a timely discharge summary when workload was high on the day prior to a patient’s discharge, suggests that electronic order volume does indeed encompass a meaningful component of workload, and that with higher workload, adherence to some quality metrics may decline. We found that patients who received a timely discharge summary were discharged by teams who entered 30 fewer orders on the day before discharge compared with patients who did not receive a timely discharge summary. In addition to being statistically significant, it is also likely that this difference is clinically significant, although a determination of clinical significance is outside the scope of this study. Further exploration into the relationship between order volume and other quality metrics that are perhaps more sensitive to workload would be interesting.
The primary strength of our study is in how it demonstrates that EHRs can be harnessed to provide additional insights into clinical workload in a quantifiable and automated manner. Although there are a wide range of EHRs currently in use across the country, the capability to track electronic orders is common and could therefore be used broadly across institutions, with tailoring and standardization specific to each site. This technique is similar to that used by prior investigators who characterized the workload of pediatric residents by orders entered and notes written in the electronic medical record.19 However, our study is unique, in that we explored the relationship between electronic order volume and patient-level severity metrics as well as discharge-related quality metrics.
Our study is limited by several factors. When conceptualizing resident workload, several other elements that contribute to a sense of “busyness” may be independent of electronic orders and were not measured in our study.20 These include communication factors (such as language discordance, discussion with consulting services, and difficult end-of-life discussions), environmental factors (such as geographic localization), resident physician team factors (such as competing clinical or educational responsibilities), timing (in terms of day of week as well as time of year, since residents in July likely feel “busier” than residents in May), and ultimate discharge destination for patients (those going to a skilled nursing facility may require discharge documentation more urgently). Additionally, we chose to focus on the workload of resident teams, as represented by team orders, as opposed to individual work, which may be more directly correlated to our outcomes of interest, completion of a high-quality AVS, and timely discharge summary, which are usually performed by individuals.
Furthermore, we did not measure the relationship between our objective measure of workload and clinical endpoints. Instead, we chose to focus on process measures because they are less likely to be confounded by clinical factors independent of physician workload.21 Future studies should also consider obtaining direct resident-level measures of “busyness” or burnout, or other resident-centered endpoints, such as whether residents left the hospital at times consistent with duty hour regulations or whether they were able to attend educational conferences.
These limitations pose opportunities for further efforts to more comprehensively characterize clinical workload. Additional research is needed to understand and quantify the impact of patient, physician, and environmental factors that are not reflected by electronic order volume. Furthermore, an exploration of other electronic surrogates for clinical workload, such as paging volume and other EHR-derived data points, could also prove valuable in further describing the clinical workload. Future studies should also examine whether there is a relationship between these novel markers of workload and further outcomes, including both process measures and clinical endpoints.
CONCLUSIONS
Electronic order volume may provide valuable additional information for estimating the workload of resident physicians caring for hospitalized patients. Further investigation to determine whether the statistically significant differences identified in this study are clinically significant, how the technique used in this work may be applied to different EHRs, an examination of other EHR-derived metrics that may represent workload, and an exploration of additional patient-centered outcomes may be warranted.
Disclosures
Rajkomar reports personal fees from Google LLC, outside the submitted work. Dr. Khanna reports that during the conduct of the study, his salary, and the development of CareWeb (a communication platform that includes a smartphone-based paging application in use in several inpatient clinical units at University of California, San Francisco [UCSF] Medical Center) were supported by funding from the Center for Digital Health Innovation at UCSF. The CareWeb software has been licensed by Voalte.
Disclaimer
The views expressed in the submitted article are of the authors and not an official position of the institution.
1. Lurie JD, Wachter RM. Hospitalist staffing requirements. Eff Clin Pract. 1999;2(3):126-30. PubMed
2. Wachter RM. Hospitalist workload: The search for the magic number. JAMA Intern Med. 2014;174(5):794-795. doi: 10.1001/jamainternmed.2014.18. PubMed
3. Adler-Milstein J, DesRoches CM, Kralovec P, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015;34(12):2174-2180. doi: 10.1377/hlthaff.2015.0992. PubMed
4. The Office of the National Coordinator for Health Information Technology, Health IT Dashboard. [cited 2018 April 4]. https://dashboard.healthit.gov/quickstats/quickstats.php Accessed June 28, 2018.
5. Index for Excerpts from the American Recovery and Reinvestment Act of 2009. Health Information Technology (HITECH) Act 2009. p. 112-164.
6. van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13(2):138-147. doi: 10.1197/jamia.M1809. PubMed
7. Ancker JS, Kern LM1, Edwards A, et al. How is the electronic health record being used? Use of EHR data to assess physician-level variability in technology use. J Am Med Inform Assoc. 2014;21(6):1001-1008. doi: 10.1136/amiajnl-2013-002627. PubMed
8. Hendey GW, Barth BE, Soliz T. Overnight and postcall errors in medication orders. Acad Emerg Med. 2005;12(7):629-634. doi: 10.1197/j.aem.2005.02.009. PubMed
9. Elliott DJ, Young RS2, Brice J3, Aguiar R4, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. doi: 10.1001/jamainternmed.2014.300. PubMed
10. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi: 10.1001/archinte.167.1.47. PubMed
11. Epic Systems. [cited 2017 March 28]; Available from: http://www.epic.com/. Accessed June 28, 2018.
12. MS-DRG Classifications and software. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software.html. Accessed June 28, 2018.
13. Hummel J, Evans P. Providing Clinical Summaries to Patients after Each Office Visit: A Technical Guide. [cited 2017 March 27]. https://www.healthit.gov/sites/default/files/measure-tools/avs-tech-guide.pdf. Accessed June 28, 2018.
14. Haycock M, Stuttaford L, Ruscombe-King O, Barker Z, Callaghan K, Davis T. Improving the percentage of electronic discharge summaries completed within 24 hours of discharge. BMJ Qual Improv Rep. 2014;3(1) pii: u205963.w2604. doi: 10.1136/bmjquality.u205963.w2604. PubMed
15. Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042-1047. doi: 10.1007/s11606-013-2376-6. PubMed
16. Wenger N, Méan M, Castioni J, Marques-Vidal P, Waeber G, Garnier A. Allocation of internal medicine resident time in a Swiss hospital: a time and motion study of day and evening shifts. Ann Intern Med. 2017;166(8):579-586. doi: 10.7326/M16-2238. PubMed
17. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. doi: 10.1097/ACM.0000000000001148. PubMed
18. Fletcher KE, Visotcky AM, Slagle JM, Tarima S, Weinger MB, Schapira MM. The composition of intern work while on call. J Gen Intern Med. 2012;27(11):1432-1437. doi: 10.1007/s11606-012-2120-7. PubMed
19. Was A, Blankenburg R, Park KT. Pediatric resident workload intensity and variability. Pediatrics 2016;138(1):e20154371. doi: 10.1542/peds.2015-4371. PubMed
20. Michtalik HJ, Pronovost PJ, Marsteller JA, Spetz J, Brotman DJ. Developing a model for attending physician workload and outcomes. JAMA Intern Med. 2013;173(11):1026-1028. doi: 10.1001/jamainternmed.2013.405. PubMed
21. Mant J. Process versus outcome indicators in the assessment of quality of health care. Int J Qual Health Care. 2001;13(6):475-480. doi: 10.1093/intqhc/13.6.475. PubMed
Resident physician workload has traditionally been measured by patient census.1,2 However, census and other volume-based metrics such as daily admissions may not accurately reflect workload due to variation in patient complexity. Relative value units (RVUs) are another commonly used marker of workload, but the validity of this metric relies on accurate coding, usually done by the attending physician, and is less directly related to resident physician workload. Because much of hospital-based medicine is mediated through the electronic health record (EHR), which can capture differences in patient complexity,3 electronic records could be harnessed to more comprehensively describe residents’ work. Current government estimates indicate that several hundred companies offer certified EHRs, thanks in large part to the Health Information Technology for Economic and Clinical Health (HITECH) Act of 2009, which aimed to promote adoption and meaningful use of health information technology.4, 5 These systems can collect important data about the usage and operating patterns of physicians, which may provide an insight into workload.6-8
Accurately measuring workload is important because of the direct link that has been drawn between physician workload and quality metrics. In a study of attending hospitalists, higher workload, as measured by patient census and RVUs, was associated with longer lengths of stay and higher costs of hospitalization.9 Another study among medical residents found that as daily admissions increased, length of stay, cost, and inpatient mortality appeared to rise.10 Although these studies used only volume-based workload metrics, the implication that high workload may negatively impact patient care hints at a possible trade-off between the two that should inform discussions of physician productivity.
In the current study, we examine whether data obtained from the EHR, particularly electronic order volume, could provide valuable information, in addition to patient volume, about resident physician workload. We first tested the feasibility and validity of using electronic order volume as an important component of clinical workload by examining the relationship between electronic order volume and well-established factors that are likely to increase the workload of residents, including patient level of care and severity of illness. Then, using order volume as a marker for workload, we sought to describe whether higher order volumes were associated with two discharge-related quality metrics, completion of a high-quality after-visit summary and timely discharge summary, postulating that quality metrics may suffer when residents are busier.
METHODS
Study Design and Setting
We performed a single-center retrospective cohort study of patients admitted to the internal medicine service at the University of California, San Francisco (UCSF) Medical Center between May 1, 2015 and July 31, 2016. UCSF is a 600-bed academic medical center, and the inpatient internal medicine teaching service manages an average daily census of 80-90 patients. Medicine teams care for patients on the general acute-care wards, the step-down units (for patients with higher acuity of care), and also patients in the intensive care unit (ICU). ICU patients are comanaged by general medicine teams and intensive care teams; internal medicine teams enter all electronic orders for ICU patients, except for orders for respiratory care or sedating medications. The inpatient internal medicine teaching service comprises eight teams each supervised by an attending physician, a senior resident (in the second or third year of residency training), two interns, and a third- and/or fourth-year medical student. Residents place all clinical orders and complete all clinical documentation through the EHR (Epic Systems, Verona, Wisconsin).11 Typically, the bulk of the orders and documentation, including discharge documentation, is performed by interns; however, the degree of senior resident involvement in these tasks is variable and team-dependent. In addition to the eight resident teams, there are also four attending hospitalist-only internal medicine teams, who manage a service of ~30-40 patients.
Study Population
Our study population comprised all hospitalized adults admitted to the eight resident-run teams on the internal medicine teaching service. Patients cared for by hospitalist-only teams were not included in this analysis. Because the focus of our study was on hospitalizations, individual patients may have been included multiple times over the course of the study. Hospitalizations were excluded if they did not have complete Medicare Severity-Diagnosis Related Group (MS-DRG) data,12 since this was used as our severity of illness marker. This occurred either because patients were not discharged by the end of the study period or because they had a length of stay of less than one day, because this metric was not assigned to these short-stay (observation) patients.
Data Collection
All electronic orders placed during the study period were obtained by extracting data from Epic’s Clarity database. Our EHR allows for the use of order sets; each order in these sets was counted individually, so that an order set with several orders would not be identified as one order. We identified the time and date that the order was placed, the ordering physician, the identity of the patient for which the order was placed, and the location of the patient when the order was placed, to determine the level of care (ICU, step-down, or general medicine unit). To track the composite volume of orders placed by resident teams, we matched each ordering physician to his or her corresponding resident team using our physician scheduling database, Amion (Spiral Software). We obtained team census by tabulating the total number of patients that a single resident team placed orders on over the course of a given calendar day. From billing data, we identified the MS-DRG weight that was assigned at the end of each hospitalization. Finally, we collected data on adherence to two discharge-related quality metrics to determine whether increased order volume was associated with decreased rates of adherence to these metrics. Using departmental patient-level quality improvement data, we determined whether each metric was met on discharge at the patient level. We also extracted patient-level demographic data, including age, sex, and insurance status, from this departmental quality improvement database.
Discharge Quality Outcome Metrics
We hypothesized that as the total daily electronic orders of a resident team increased, the rate of completion of two discharge-related quality metrics would decline due to the greater time constraints placed on the teams. The first metric we used was the completion of a high-quality after-visit summary (AVS), which has been described by the Centers for Medicare and Medicaid Services as part of its Meaningful Use Initiative.13 It was selected by the residents in our program as a particularly high-priority quality metric. Our institution specifically defines a “high-quality” AVS as including the following three components: a principal hospital problem, patient instructions, and follow-up information. The second discharge-related quality metric was the completion of a timely discharge summary, another measure recognized as a critical component in high-quality care.14 To be considered timely, the discharge summary had to be filed no later than 24 hours after the discharge order was entered into the EHR. This metric was more recently tracked by the internal medicine department and was not selected by the residents as a high-priority metric.
Statistical Analysis
To examine how the order volume per day changed throughout each sequential day of hospital admission, mean orders per hospital day with 95% CIs were plotted. We performed an aggregate analysis of all orders placed for each patient per day across three different levels of care (ICU, step-down, and general medicine). For each day of the study period, we summed all orders for all patients according to their location and divided by the number of total patients in each location to identify the average number of orders written for an ICU, step-down, and general medicine patient that day. We then calculated the mean daily orders for an ICU, step-down, and general medicine patient over the entire study period. We used ANOVA to test for statistically significant differences between the mean daily orders between these locations.
To examine the relationship between severity of illness and order volume, we performed an unadjusted patient-level analysis of orders per patient in the first three days of each hospitalization and stratified the data by the MS-DRG payment weight, which we divided into four quartiles. For each quartile, we calculated the mean number of orders placed in the first three days of admission and used ANOVA to test for statistically significant differences. We restricted the orders to the first three days of hospitalization instead of calculating mean orders per day of hospitalization because we postulated that the majority of orders were entered in these first few days and that with increasing length of stay (which we expected to occur with higher MS-DRG weight), the order volume becomes highly variable, which would tend to skew the mean orders per day.
We used multivariable logistic regression to determine whether the volume of electronic orders on the day of a given patient’s discharge, and also on the day before a given patient’s discharge, was a significant predictor of receiving a high-quality AVS. We adjusted for team census on the day of discharge, MS-DRG weight, age, sex, and insurance status. We then conducted a separate analysis of the association between electronic order volume and likelihood of completing a timely discharge summary among patients where discharge summary data were available. Logistic regression for each case was performed independently, so that team orders on the day prior to a patient’s discharge were not included in the model for the relationship between team orders on the day of a patient’s discharge and the discharge-related quality metric of interest, and vice versa, since including both in the model would be potentially disruptive given that orders on the day before and day of a patient’s discharge are likely correlated.
We also performed a subanalysis in which we restricted orders to only those placed during the daytime hours (7
IRB Approval
The study was approved by the UCSF Institutional Review Board and was granted a waiver of informed consent.
RESULTS
Population
We identified 7,296 eligible hospitalizations during the study period. After removing hospitalizations according to our exclusion criteria (Figure 1), there were 5,032 hospitalizations that were used in the analysis for which a total of 929,153 orders were written. The vast majority of patients received at least one order per day; fewer than 1% of encounter-days had zero associated orders. The top 10 discharge diagnoses identified in the cohort are listed in Appendix Table 1. A breakdown of orders by order type, across the entire cohort, is displayed in Appendix Table 2. The mean number of orders per patient per day of hospitalization is plotted in the Appendix Figure, which indicates that the number of orders is highest on the day of admission, decreases significantly after the first few days, and becomes increasingly variable with longer lengths of stay.
Patient Level of Care and Severity of Illness Metrics
Patients at a higher level of care had, on average, more orders entered per day. The mean order frequency was 40 orders per day for an ICU patient (standard deviation [SD] 13, range 13-134), 24 for a step-down patient (SD 6, range 11-48), and 19 for a general medicine unit patient (SD 3, range 10-31). The difference in mean daily orders was statistically significant (P < .001, Figure 2a).
Orders also correlated with increasing severity of illness. Patients in the lowest quartile of MS-DRG weight received, on average, 98 orders in the first three days of hospitalization (SD 35, range 2-349), those in the second quartile received 105 orders (SD 38, range 10-380), those in the third quartile received 132 orders (SD 51, range 17-436), and those in the fourth and highest quartile received 149 orders (SD 59, range 32-482). Comparisons between each of these severity of illness categories were significant (P < .001, Figure 2b).
Discharge-Related Quality Metrics
The median number of orders per internal medicine team per day was 343 (IQR 261- 446). Of the 5,032 total discharged patients, 3,657 (73%) received a high-quality AVS on discharge. After controlling for team census, severity of illness, and demographic factors, there was no statistically significant association between total orders on the day of discharge and odds of receiving a high-quality AVS (OR 1.01; 95% CI 0.96-1.06), or between team orders placed the day prior to discharge and odds of receiving a high-quality AVS (OR 0.99; 95% CI 0.95-1.04; Table 1). When we restricted our analysis to orders placed during daytime hours (7
There were 3,835 patients for whom data on timing of discharge summary were available. Of these, 3,455 (91.2%) had a discharge summary completed within 24 hours. After controlling for team census, severity of illness, and demographic factors, there was no statistically significant association between total orders placed by the team on a patient’s day of discharge and odds of receiving a timely discharge summary (OR 0.96; 95% CI 0.88-1.05). However, patients were 12% less likely to receive a timely discharge summary for every 100 extra orders the team placed on the day prior to discharge (OR 0.88, 95% CI 0.82-0.95). Patients who received a timely discharge summary were cared for by teams who placed a median of 345 orders the day prior to their discharge, whereas those that did not receive a timely discharge summary were cared for by teams who placed a significantly higher number of orders (375) on the day prior to discharge (Table 2). When we restricted our analysis to only daytime orders, there were no significant changes in the findings (OR 1.00; 95% CI 0.88-1.14 for orders on the day of discharge; OR 0.84; 95% CI 0.75-0.95 for orders on the day prior to discharge).
DISCUSSION
We found that electronic order volume may be a marker for patient complexity, which encompasses both level of care and severity of illness, and could be a marker of resident physician workload that harnesses readily available data from an EHR. Recent time-motion studies of internal medicine residents indicate that the majority of trainees’ time is spent on computers, engaged in indirect patient care activities such as reading electronic charts, entering electronic orders, and writing computerized notes.15-18 Capturing these tasks through metrics such as electronic order volume, as we did in this study, can provide valuable insights into resident physician workflow.
We found that ICU patients received more than twice as many orders per day than did general acute care-level patients. Furthermore, we found that patients whose hospitalizations fell into the highest MS-DRG weight quartile received approximately 50% more orders during the first three days of admission compared to that of patients whose hospitalizations fell into the lowest quartile. This strong association indicates that electronic order volume could provide meaningful additional information, in concert with other factors such as census, to describe resident physician workload.
We did not find that our workload measure was significantly associated with high-quality AVS completion. There are several possible explanations for this finding. First, adherence to this quality metric may be independent of workload, possibly because it is highly prioritized by residents at our institution. Second, adherence may only be impacted at levels of workload greater than what was experienced by the residents in our study. Finally, electronic order volume may not encompass enough of total workload to be reliably representative of resident work. However, the tight correlation between electronic order volume with severity of illness and level of care, in conjunction with the finding that patients were less likely to receive a timely discharge summary when workload was high on the day prior to a patient’s discharge, suggests that electronic order volume does indeed encompass a meaningful component of workload, and that with higher workload, adherence to some quality metrics may decline. We found that patients who received a timely discharge summary were discharged by teams who entered 30 fewer orders on the day before discharge compared with patients who did not receive a timely discharge summary. In addition to being statistically significant, it is also likely that this difference is clinically significant, although a determination of clinical significance is outside the scope of this study. Further exploration into the relationship between order volume and other quality metrics that are perhaps more sensitive to workload would be interesting.
The primary strength of our study is in how it demonstrates that EHRs can be harnessed to provide additional insights into clinical workload in a quantifiable and automated manner. Although there are a wide range of EHRs currently in use across the country, the capability to track electronic orders is common and could therefore be used broadly across institutions, with tailoring and standardization specific to each site. This technique is similar to that used by prior investigators who characterized the workload of pediatric residents by orders entered and notes written in the electronic medical record.19 However, our study is unique, in that we explored the relationship between electronic order volume and patient-level severity metrics as well as discharge-related quality metrics.
Our study is limited by several factors. When conceptualizing resident workload, several other elements that contribute to a sense of “busyness” may be independent of electronic orders and were not measured in our study.20 These include communication factors (such as language discordance, discussion with consulting services, and difficult end-of-life discussions), environmental factors (such as geographic localization), resident physician team factors (such as competing clinical or educational responsibilities), timing (in terms of day of week as well as time of year, since residents in July likely feel “busier” than residents in May), and ultimate discharge destination for patients (those going to a skilled nursing facility may require discharge documentation more urgently). Additionally, we chose to focus on the workload of resident teams, as represented by team orders, as opposed to individual work, which may be more directly correlated to our outcomes of interest, completion of a high-quality AVS, and timely discharge summary, which are usually performed by individuals.
Furthermore, we did not measure the relationship between our objective measure of workload and clinical endpoints. Instead, we chose to focus on process measures because they are less likely to be confounded by clinical factors independent of physician workload.21 Future studies should also consider obtaining direct resident-level measures of “busyness” or burnout, or other resident-centered endpoints, such as whether residents left the hospital at times consistent with duty hour regulations or whether they were able to attend educational conferences.
These limitations pose opportunities for further efforts to more comprehensively characterize clinical workload. Additional research is needed to understand and quantify the impact of patient, physician, and environmental factors that are not reflected by electronic order volume. Furthermore, an exploration of other electronic surrogates for clinical workload, such as paging volume and other EHR-derived data points, could also prove valuable in further describing the clinical workload. Future studies should also examine whether there is a relationship between these novel markers of workload and further outcomes, including both process measures and clinical endpoints.
CONCLUSIONS
Electronic order volume may provide valuable additional information for estimating the workload of resident physicians caring for hospitalized patients. Further investigation to determine whether the statistically significant differences identified in this study are clinically significant, how the technique used in this work may be applied to different EHRs, an examination of other EHR-derived metrics that may represent workload, and an exploration of additional patient-centered outcomes may be warranted.
Disclosures
Rajkomar reports personal fees from Google LLC, outside the submitted work. Dr. Khanna reports that during the conduct of the study, his salary, and the development of CareWeb (a communication platform that includes a smartphone-based paging application in use in several inpatient clinical units at University of California, San Francisco [UCSF] Medical Center) were supported by funding from the Center for Digital Health Innovation at UCSF. The CareWeb software has been licensed by Voalte.
Disclaimer
The views expressed in the submitted article are of the authors and not an official position of the institution.
Resident physician workload has traditionally been measured by patient census.1,2 However, census and other volume-based metrics such as daily admissions may not accurately reflect workload due to variation in patient complexity. Relative value units (RVUs) are another commonly used marker of workload, but the validity of this metric relies on accurate coding, usually done by the attending physician, and is less directly related to resident physician workload. Because much of hospital-based medicine is mediated through the electronic health record (EHR), which can capture differences in patient complexity,3 electronic records could be harnessed to more comprehensively describe residents’ work. Current government estimates indicate that several hundred companies offer certified EHRs, thanks in large part to the Health Information Technology for Economic and Clinical Health (HITECH) Act of 2009, which aimed to promote adoption and meaningful use of health information technology.4, 5 These systems can collect important data about the usage and operating patterns of physicians, which may provide an insight into workload.6-8
Accurately measuring workload is important because of the direct link that has been drawn between physician workload and quality metrics. In a study of attending hospitalists, higher workload, as measured by patient census and RVUs, was associated with longer lengths of stay and higher costs of hospitalization.9 Another study among medical residents found that as daily admissions increased, length of stay, cost, and inpatient mortality appeared to rise.10 Although these studies used only volume-based workload metrics, the implication that high workload may negatively impact patient care hints at a possible trade-off between the two that should inform discussions of physician productivity.
In the current study, we examine whether data obtained from the EHR, particularly electronic order volume, could provide valuable information, in addition to patient volume, about resident physician workload. We first tested the feasibility and validity of using electronic order volume as an important component of clinical workload by examining the relationship between electronic order volume and well-established factors that are likely to increase the workload of residents, including patient level of care and severity of illness. Then, using order volume as a marker for workload, we sought to describe whether higher order volumes were associated with two discharge-related quality metrics, completion of a high-quality after-visit summary and timely discharge summary, postulating that quality metrics may suffer when residents are busier.
METHODS
Study Design and Setting
We performed a single-center retrospective cohort study of patients admitted to the internal medicine service at the University of California, San Francisco (UCSF) Medical Center between May 1, 2015 and July 31, 2016. UCSF is a 600-bed academic medical center, and the inpatient internal medicine teaching service manages an average daily census of 80-90 patients. Medicine teams care for patients on the general acute-care wards, the step-down units (for patients with higher acuity of care), and also patients in the intensive care unit (ICU). ICU patients are comanaged by general medicine teams and intensive care teams; internal medicine teams enter all electronic orders for ICU patients, except for orders for respiratory care or sedating medications. The inpatient internal medicine teaching service comprises eight teams each supervised by an attending physician, a senior resident (in the second or third year of residency training), two interns, and a third- and/or fourth-year medical student. Residents place all clinical orders and complete all clinical documentation through the EHR (Epic Systems, Verona, Wisconsin).11 Typically, the bulk of the orders and documentation, including discharge documentation, is performed by interns; however, the degree of senior resident involvement in these tasks is variable and team-dependent. In addition to the eight resident teams, there are also four attending hospitalist-only internal medicine teams, who manage a service of ~30-40 patients.
Study Population
Our study population comprised all hospitalized adults admitted to the eight resident-run teams on the internal medicine teaching service. Patients cared for by hospitalist-only teams were not included in this analysis. Because the focus of our study was on hospitalizations, individual patients may have been included multiple times over the course of the study. Hospitalizations were excluded if they did not have complete Medicare Severity-Diagnosis Related Group (MS-DRG) data,12 since this was used as our severity of illness marker. This occurred either because patients were not discharged by the end of the study period or because they had a length of stay of less than one day, because this metric was not assigned to these short-stay (observation) patients.
Data Collection
All electronic orders placed during the study period were obtained by extracting data from Epic’s Clarity database. Our EHR allows for the use of order sets; each order in these sets was counted individually, so that an order set with several orders would not be identified as one order. We identified the time and date that the order was placed, the ordering physician, the identity of the patient for which the order was placed, and the location of the patient when the order was placed, to determine the level of care (ICU, step-down, or general medicine unit). To track the composite volume of orders placed by resident teams, we matched each ordering physician to his or her corresponding resident team using our physician scheduling database, Amion (Spiral Software). We obtained team census by tabulating the total number of patients that a single resident team placed orders on over the course of a given calendar day. From billing data, we identified the MS-DRG weight that was assigned at the end of each hospitalization. Finally, we collected data on adherence to two discharge-related quality metrics to determine whether increased order volume was associated with decreased rates of adherence to these metrics. Using departmental patient-level quality improvement data, we determined whether each metric was met on discharge at the patient level. We also extracted patient-level demographic data, including age, sex, and insurance status, from this departmental quality improvement database.
Discharge Quality Outcome Metrics
We hypothesized that as the total daily electronic orders of a resident team increased, the rate of completion of two discharge-related quality metrics would decline due to the greater time constraints placed on the teams. The first metric we used was the completion of a high-quality after-visit summary (AVS), which has been described by the Centers for Medicare and Medicaid Services as part of its Meaningful Use Initiative.13 It was selected by the residents in our program as a particularly high-priority quality metric. Our institution specifically defines a “high-quality” AVS as including the following three components: a principal hospital problem, patient instructions, and follow-up information. The second discharge-related quality metric was the completion of a timely discharge summary, another measure recognized as a critical component in high-quality care.14 To be considered timely, the discharge summary had to be filed no later than 24 hours after the discharge order was entered into the EHR. This metric was more recently tracked by the internal medicine department and was not selected by the residents as a high-priority metric.
Statistical Analysis
To examine how the order volume per day changed throughout each sequential day of hospital admission, mean orders per hospital day with 95% CIs were plotted. We performed an aggregate analysis of all orders placed for each patient per day across three different levels of care (ICU, step-down, and general medicine). For each day of the study period, we summed all orders for all patients according to their location and divided by the number of total patients in each location to identify the average number of orders written for an ICU, step-down, and general medicine patient that day. We then calculated the mean daily orders for an ICU, step-down, and general medicine patient over the entire study period. We used ANOVA to test for statistically significant differences between the mean daily orders between these locations.
To examine the relationship between severity of illness and order volume, we performed an unadjusted patient-level analysis of orders per patient in the first three days of each hospitalization and stratified the data by the MS-DRG payment weight, which we divided into four quartiles. For each quartile, we calculated the mean number of orders placed in the first three days of admission and used ANOVA to test for statistically significant differences. We restricted the orders to the first three days of hospitalization instead of calculating mean orders per day of hospitalization because we postulated that the majority of orders were entered in these first few days and that with increasing length of stay (which we expected to occur with higher MS-DRG weight), the order volume becomes highly variable, which would tend to skew the mean orders per day.
We used multivariable logistic regression to determine whether the volume of electronic orders on the day of a given patient’s discharge, and also on the day before a given patient’s discharge, was a significant predictor of receiving a high-quality AVS. We adjusted for team census on the day of discharge, MS-DRG weight, age, sex, and insurance status. We then conducted a separate analysis of the association between electronic order volume and likelihood of completing a timely discharge summary among patients where discharge summary data were available. Logistic regression for each case was performed independently, so that team orders on the day prior to a patient’s discharge were not included in the model for the relationship between team orders on the day of a patient’s discharge and the discharge-related quality metric of interest, and vice versa, since including both in the model would be potentially disruptive given that orders on the day before and day of a patient’s discharge are likely correlated.
We also performed a subanalysis in which we restricted orders to only those placed during the daytime hours (7
IRB Approval
The study was approved by the UCSF Institutional Review Board and was granted a waiver of informed consent.
RESULTS
Population
We identified 7,296 eligible hospitalizations during the study period. After removing hospitalizations according to our exclusion criteria (Figure 1), there were 5,032 hospitalizations that were used in the analysis for which a total of 929,153 orders were written. The vast majority of patients received at least one order per day; fewer than 1% of encounter-days had zero associated orders. The top 10 discharge diagnoses identified in the cohort are listed in Appendix Table 1. A breakdown of orders by order type, across the entire cohort, is displayed in Appendix Table 2. The mean number of orders per patient per day of hospitalization is plotted in the Appendix Figure, which indicates that the number of orders is highest on the day of admission, decreases significantly after the first few days, and becomes increasingly variable with longer lengths of stay.
Patient Level of Care and Severity of Illness Metrics
Patients at a higher level of care had, on average, more orders entered per day. The mean order frequency was 40 orders per day for an ICU patient (standard deviation [SD] 13, range 13-134), 24 for a step-down patient (SD 6, range 11-48), and 19 for a general medicine unit patient (SD 3, range 10-31). The difference in mean daily orders was statistically significant (P < .001, Figure 2a).
Orders also correlated with increasing severity of illness. Patients in the lowest quartile of MS-DRG weight received, on average, 98 orders in the first three days of hospitalization (SD 35, range 2-349), those in the second quartile received 105 orders (SD 38, range 10-380), those in the third quartile received 132 orders (SD 51, range 17-436), and those in the fourth and highest quartile received 149 orders (SD 59, range 32-482). Comparisons between each of these severity of illness categories were significant (P < .001, Figure 2b).
Discharge-Related Quality Metrics
The median number of orders per internal medicine team per day was 343 (IQR 261- 446). Of the 5,032 total discharged patients, 3,657 (73%) received a high-quality AVS on discharge. After controlling for team census, severity of illness, and demographic factors, there was no statistically significant association between total orders on the day of discharge and odds of receiving a high-quality AVS (OR 1.01; 95% CI 0.96-1.06), or between team orders placed the day prior to discharge and odds of receiving a high-quality AVS (OR 0.99; 95% CI 0.95-1.04; Table 1). When we restricted our analysis to orders placed during daytime hours (7
There were 3,835 patients for whom data on timing of discharge summary were available. Of these, 3,455 (91.2%) had a discharge summary completed within 24 hours. After controlling for team census, severity of illness, and demographic factors, there was no statistically significant association between total orders placed by the team on a patient’s day of discharge and odds of receiving a timely discharge summary (OR 0.96; 95% CI 0.88-1.05). However, patients were 12% less likely to receive a timely discharge summary for every 100 extra orders the team placed on the day prior to discharge (OR 0.88, 95% CI 0.82-0.95). Patients who received a timely discharge summary were cared for by teams who placed a median of 345 orders the day prior to their discharge, whereas those that did not receive a timely discharge summary were cared for by teams who placed a significantly higher number of orders (375) on the day prior to discharge (Table 2). When we restricted our analysis to only daytime orders, there were no significant changes in the findings (OR 1.00; 95% CI 0.88-1.14 for orders on the day of discharge; OR 0.84; 95% CI 0.75-0.95 for orders on the day prior to discharge).
DISCUSSION
We found that electronic order volume may be a marker for patient complexity, which encompasses both level of care and severity of illness, and could be a marker of resident physician workload that harnesses readily available data from an EHR. Recent time-motion studies of internal medicine residents indicate that the majority of trainees’ time is spent on computers, engaged in indirect patient care activities such as reading electronic charts, entering electronic orders, and writing computerized notes.15-18 Capturing these tasks through metrics such as electronic order volume, as we did in this study, can provide valuable insights into resident physician workflow.
We found that ICU patients received more than twice as many orders per day than did general acute care-level patients. Furthermore, we found that patients whose hospitalizations fell into the highest MS-DRG weight quartile received approximately 50% more orders during the first three days of admission compared to that of patients whose hospitalizations fell into the lowest quartile. This strong association indicates that electronic order volume could provide meaningful additional information, in concert with other factors such as census, to describe resident physician workload.
We did not find that our workload measure was significantly associated with high-quality AVS completion. There are several possible explanations for this finding. First, adherence to this quality metric may be independent of workload, possibly because it is highly prioritized by residents at our institution. Second, adherence may only be impacted at levels of workload greater than what was experienced by the residents in our study. Finally, electronic order volume may not encompass enough of total workload to be reliably representative of resident work. However, the tight correlation between electronic order volume with severity of illness and level of care, in conjunction with the finding that patients were less likely to receive a timely discharge summary when workload was high on the day prior to a patient’s discharge, suggests that electronic order volume does indeed encompass a meaningful component of workload, and that with higher workload, adherence to some quality metrics may decline. We found that patients who received a timely discharge summary were discharged by teams who entered 30 fewer orders on the day before discharge compared with patients who did not receive a timely discharge summary. In addition to being statistically significant, it is also likely that this difference is clinically significant, although a determination of clinical significance is outside the scope of this study. Further exploration into the relationship between order volume and other quality metrics that are perhaps more sensitive to workload would be interesting.
The primary strength of our study is in how it demonstrates that EHRs can be harnessed to provide additional insights into clinical workload in a quantifiable and automated manner. Although there are a wide range of EHRs currently in use across the country, the capability to track electronic orders is common and could therefore be used broadly across institutions, with tailoring and standardization specific to each site. This technique is similar to that used by prior investigators who characterized the workload of pediatric residents by orders entered and notes written in the electronic medical record.19 However, our study is unique, in that we explored the relationship between electronic order volume and patient-level severity metrics as well as discharge-related quality metrics.
Our study is limited by several factors. When conceptualizing resident workload, several other elements that contribute to a sense of “busyness” may be independent of electronic orders and were not measured in our study.20 These include communication factors (such as language discordance, discussion with consulting services, and difficult end-of-life discussions), environmental factors (such as geographic localization), resident physician team factors (such as competing clinical or educational responsibilities), timing (in terms of day of week as well as time of year, since residents in July likely feel “busier” than residents in May), and ultimate discharge destination for patients (those going to a skilled nursing facility may require discharge documentation more urgently). Additionally, we chose to focus on the workload of resident teams, as represented by team orders, as opposed to individual work, which may be more directly correlated to our outcomes of interest, completion of a high-quality AVS, and timely discharge summary, which are usually performed by individuals.
Furthermore, we did not measure the relationship between our objective measure of workload and clinical endpoints. Instead, we chose to focus on process measures because they are less likely to be confounded by clinical factors independent of physician workload.21 Future studies should also consider obtaining direct resident-level measures of “busyness” or burnout, or other resident-centered endpoints, such as whether residents left the hospital at times consistent with duty hour regulations or whether they were able to attend educational conferences.
These limitations pose opportunities for further efforts to more comprehensively characterize clinical workload. Additional research is needed to understand and quantify the impact of patient, physician, and environmental factors that are not reflected by electronic order volume. Furthermore, an exploration of other electronic surrogates for clinical workload, such as paging volume and other EHR-derived data points, could also prove valuable in further describing the clinical workload. Future studies should also examine whether there is a relationship between these novel markers of workload and further outcomes, including both process measures and clinical endpoints.
CONCLUSIONS
Electronic order volume may provide valuable additional information for estimating the workload of resident physicians caring for hospitalized patients. Further investigation to determine whether the statistically significant differences identified in this study are clinically significant, how the technique used in this work may be applied to different EHRs, an examination of other EHR-derived metrics that may represent workload, and an exploration of additional patient-centered outcomes may be warranted.
Disclosures
Rajkomar reports personal fees from Google LLC, outside the submitted work. Dr. Khanna reports that during the conduct of the study, his salary, and the development of CareWeb (a communication platform that includes a smartphone-based paging application in use in several inpatient clinical units at University of California, San Francisco [UCSF] Medical Center) were supported by funding from the Center for Digital Health Innovation at UCSF. The CareWeb software has been licensed by Voalte.
Disclaimer
The views expressed in the submitted article are of the authors and not an official position of the institution.
1. Lurie JD, Wachter RM. Hospitalist staffing requirements. Eff Clin Pract. 1999;2(3):126-30. PubMed
2. Wachter RM. Hospitalist workload: The search for the magic number. JAMA Intern Med. 2014;174(5):794-795. doi: 10.1001/jamainternmed.2014.18. PubMed
3. Adler-Milstein J, DesRoches CM, Kralovec P, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015;34(12):2174-2180. doi: 10.1377/hlthaff.2015.0992. PubMed
4. The Office of the National Coordinator for Health Information Technology, Health IT Dashboard. [cited 2018 April 4]. https://dashboard.healthit.gov/quickstats/quickstats.php Accessed June 28, 2018.
5. Index for Excerpts from the American Recovery and Reinvestment Act of 2009. Health Information Technology (HITECH) Act 2009. p. 112-164.
6. van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13(2):138-147. doi: 10.1197/jamia.M1809. PubMed
7. Ancker JS, Kern LM1, Edwards A, et al. How is the electronic health record being used? Use of EHR data to assess physician-level variability in technology use. J Am Med Inform Assoc. 2014;21(6):1001-1008. doi: 10.1136/amiajnl-2013-002627. PubMed
8. Hendey GW, Barth BE, Soliz T. Overnight and postcall errors in medication orders. Acad Emerg Med. 2005;12(7):629-634. doi: 10.1197/j.aem.2005.02.009. PubMed
9. Elliott DJ, Young RS2, Brice J3, Aguiar R4, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. doi: 10.1001/jamainternmed.2014.300. PubMed
10. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi: 10.1001/archinte.167.1.47. PubMed
11. Epic Systems. [cited 2017 March 28]; Available from: http://www.epic.com/. Accessed June 28, 2018.
12. MS-DRG Classifications and software. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software.html. Accessed June 28, 2018.
13. Hummel J, Evans P. Providing Clinical Summaries to Patients after Each Office Visit: A Technical Guide. [cited 2017 March 27]. https://www.healthit.gov/sites/default/files/measure-tools/avs-tech-guide.pdf. Accessed June 28, 2018.
14. Haycock M, Stuttaford L, Ruscombe-King O, Barker Z, Callaghan K, Davis T. Improving the percentage of electronic discharge summaries completed within 24 hours of discharge. BMJ Qual Improv Rep. 2014;3(1) pii: u205963.w2604. doi: 10.1136/bmjquality.u205963.w2604. PubMed
15. Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042-1047. doi: 10.1007/s11606-013-2376-6. PubMed
16. Wenger N, Méan M, Castioni J, Marques-Vidal P, Waeber G, Garnier A. Allocation of internal medicine resident time in a Swiss hospital: a time and motion study of day and evening shifts. Ann Intern Med. 2017;166(8):579-586. doi: 10.7326/M16-2238. PubMed
17. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. doi: 10.1097/ACM.0000000000001148. PubMed
18. Fletcher KE, Visotcky AM, Slagle JM, Tarima S, Weinger MB, Schapira MM. The composition of intern work while on call. J Gen Intern Med. 2012;27(11):1432-1437. doi: 10.1007/s11606-012-2120-7. PubMed
19. Was A, Blankenburg R, Park KT. Pediatric resident workload intensity and variability. Pediatrics 2016;138(1):e20154371. doi: 10.1542/peds.2015-4371. PubMed
20. Michtalik HJ, Pronovost PJ, Marsteller JA, Spetz J, Brotman DJ. Developing a model for attending physician workload and outcomes. JAMA Intern Med. 2013;173(11):1026-1028. doi: 10.1001/jamainternmed.2013.405. PubMed
21. Mant J. Process versus outcome indicators in the assessment of quality of health care. Int J Qual Health Care. 2001;13(6):475-480. doi: 10.1093/intqhc/13.6.475. PubMed
1. Lurie JD, Wachter RM. Hospitalist staffing requirements. Eff Clin Pract. 1999;2(3):126-30. PubMed
2. Wachter RM. Hospitalist workload: The search for the magic number. JAMA Intern Med. 2014;174(5):794-795. doi: 10.1001/jamainternmed.2014.18. PubMed
3. Adler-Milstein J, DesRoches CM, Kralovec P, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015;34(12):2174-2180. doi: 10.1377/hlthaff.2015.0992. PubMed
4. The Office of the National Coordinator for Health Information Technology, Health IT Dashboard. [cited 2018 April 4]. https://dashboard.healthit.gov/quickstats/quickstats.php Accessed June 28, 2018.
5. Index for Excerpts from the American Recovery and Reinvestment Act of 2009. Health Information Technology (HITECH) Act 2009. p. 112-164.
6. van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13(2):138-147. doi: 10.1197/jamia.M1809. PubMed
7. Ancker JS, Kern LM1, Edwards A, et al. How is the electronic health record being used? Use of EHR data to assess physician-level variability in technology use. J Am Med Inform Assoc. 2014;21(6):1001-1008. doi: 10.1136/amiajnl-2013-002627. PubMed
8. Hendey GW, Barth BE, Soliz T. Overnight and postcall errors in medication orders. Acad Emerg Med. 2005;12(7):629-634. doi: 10.1197/j.aem.2005.02.009. PubMed
9. Elliott DJ, Young RS2, Brice J3, Aguiar R4, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. doi: 10.1001/jamainternmed.2014.300. PubMed
10. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi: 10.1001/archinte.167.1.47. PubMed
11. Epic Systems. [cited 2017 March 28]; Available from: http://www.epic.com/. Accessed June 28, 2018.
12. MS-DRG Classifications and software. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software.html. Accessed June 28, 2018.
13. Hummel J, Evans P. Providing Clinical Summaries to Patients after Each Office Visit: A Technical Guide. [cited 2017 March 27]. https://www.healthit.gov/sites/default/files/measure-tools/avs-tech-guide.pdf. Accessed June 28, 2018.
14. Haycock M, Stuttaford L, Ruscombe-King O, Barker Z, Callaghan K, Davis T. Improving the percentage of electronic discharge summaries completed within 24 hours of discharge. BMJ Qual Improv Rep. 2014;3(1) pii: u205963.w2604. doi: 10.1136/bmjquality.u205963.w2604. PubMed
15. Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042-1047. doi: 10.1007/s11606-013-2376-6. PubMed
16. Wenger N, Méan M, Castioni J, Marques-Vidal P, Waeber G, Garnier A. Allocation of internal medicine resident time in a Swiss hospital: a time and motion study of day and evening shifts. Ann Intern Med. 2017;166(8):579-586. doi: 10.7326/M16-2238. PubMed
17. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. doi: 10.1097/ACM.0000000000001148. PubMed
18. Fletcher KE, Visotcky AM, Slagle JM, Tarima S, Weinger MB, Schapira MM. The composition of intern work while on call. J Gen Intern Med. 2012;27(11):1432-1437. doi: 10.1007/s11606-012-2120-7. PubMed
19. Was A, Blankenburg R, Park KT. Pediatric resident workload intensity and variability. Pediatrics 2016;138(1):e20154371. doi: 10.1542/peds.2015-4371. PubMed
20. Michtalik HJ, Pronovost PJ, Marsteller JA, Spetz J, Brotman DJ. Developing a model for attending physician workload and outcomes. JAMA Intern Med. 2013;173(11):1026-1028. doi: 10.1001/jamainternmed.2013.405. PubMed
21. Mant J. Process versus outcome indicators in the assessment of quality of health care. Int J Qual Health Care. 2001;13(6):475-480. doi: 10.1093/intqhc/13.6.475. PubMed
Health Care Barriers and Quality of Life in Central Centrifugal Cicatricial Alopecia Patients
The etiology of central centrifugal cicatricial alopecia (CCCA), a clinical and histological pattern of hair loss on the central scalp, has been well studied. This disease is chronic and progressive, with extensive follicular destruction and eventual burnout.1,2 Central centrifugal cicatricial alopecia is most commonly seen in patients of African descent and has been shown to be 1 of the 5 most common dermatologic diagnoses in black patients.3,4 The top 5 dermatologic diagnoses within this population include acne vulgaris (28.4%), dyschromia (19.9%), eczema (9.1%), alopecia (8.3%), and seborrheic dermatitis (6.7%).4 The incidence rate of CCCA is estimated to be 5.6%.3,5 Most patients are women, with onset between the second and fourth decades of life.6
Central centrifugal cicatricial alopecia treatment efficacy is inversely correlated with disease duration. The primary goal of treatment is to prevent progression. Efforts are made to stimulate regrowth in areas that are not permanently scarred. When patients present with a substantial amount of scarring hair loss, dermatologists often are limited in their ability to achieve a cosmetically acceptable pattern of growth. Generally, hair is connected to a sense of self-worth in black women, and any type of hair loss has been shown to lead to frustration and decreased self-esteem.7 A 1994 study showed that 75% (44/58) of women with androgenetic alopecia had decreased self-esteem and 50% (29/58) had social challenges.8
The purpose of this pilot study was to determine the personal, historical, logistical, or environmental factors that preclude women from obtaining medical care for CCCA and to investigate how CCCA affects quality of life (QOL) and psychological well-being.
Methods
The investigators designed a survey study of adult, English-speaking, black women diagnosed with CCCA at the Northwestern University Department of Dermatology (Chicago, Illinois) between 2011 and 2017. Patients were selected from the electronic data warehouse compiled by the Department of Dermatology and were included if they fulfilled the following criteria: evaluated in the dermatology department between September 1, 2011, and September 30, 2017, by any faculty physician; diagnosed with CCCA; and aged 18 years or older. Patients were excluded if they did not speak English, as interpreters were not available. All patients who fulfilled the inclusion criteria provided signed informed consent prior to participation. All surveys were disseminated in the office or via telephone from fall 2016 to spring 2017 and took 10 to 15 minutes to complete. The research was approved by the authors’ institutional review board (IRB ID STU00203449).
Survey Instrument
The
Data Analysis
Analyses were completed using data analysis software JMP Pro 13 from SAS and a Microsoft Excel spreadsheet. Continuous data were presented as mean, SD, median, minimum, and maximum. Categorical data were presented as counts and percentages. Nine QOL items were aggregated into a self-esteem category (questions 30–38).
Cronbach α, a statistical measure of internal consistency and how closely related items are in a group, was used to evaluate internal consistency reliability; values of 0.70 or greater indicate acceptable reliability.
Results
Of 501 individuals contacted, 34 completed the survey (7% completion rate). Nonrespondents included 7 who refused to participate and 460 who could not be contacted. All respondents self-identified as black women. Median age at time of survey administration was 46 years (range, 28–79 years); median age at CCCA diagnosis was 42 years (range, 15–73 years). Respondents did not significantly differ in age from nonrespondents (P=.46). The majority of respondents had an associate’s degree, bachelor’s degree, or advanced degree of education (master of arts, doctor of medicine, doctor of jurisprudence, doctor of philosophy); however, 8 women reported completing some college, 1 reported completing high school, and 1 reported no schooling. Three respondents had no health insurance.
Initial Hair Loss Discovery
The majority of respondents (22/34 [65%]) were first to notice their hair loss, while 5 (15%) reported hairstylists as the initial observers. Twelve respondents (35%) initially went to a physician to learn why they were losing hair; 6 (18%) instead utilized hairstylists or the Internet. Fifteen women (44%) waited more than 1 month up to 6 months after noticing hair loss before seeing a physician instead of going immediately within a 4-week period, and 16 (47%) waited 1 year or more.
Nondermatologist Consultation
Almost half (16/34 [47%]) of the women went to a nondermatologist physician regarding their hair loss; of them, half (8/16 [50%]) reported their physician did not examine the scalp, 3 (19%) reported their physician offered a biopsy, and none of them reported that their physician diagnosed them with CCCA. The median patient rating of their nondermatologist physician interactions was good (3 on a 5-point scale). Table 1 and Figure 1 show responses to individual items.
Dermatologist Consultation
All 34 respondents presented to a dermatologist. The majority of respondents (22/34 [65%]) saw either 1 or 2 dermatologists for their hair loss. Three (9%) reported their dermatologist did not examine their scalp. Twelve respondents (35%) reported their dermatologist did not offer a biopsy. Twenty-one respondents (62%) reported a CCCA diagnosis from the first dermatologist they saw. Twenty-three respondents (68%) were diagnosed by dermatologists with expertise in hair disorders. Sixteen (47%) were diagnosed by dermatologists within a skin-of-color center. Fourteen (41%) initial dermatology consultations were race concordant.
The median patient rating of their dermatologist interactions was excellent (5 on a 5-point scale). Table 2 and Figure 2 show responses to individual items. Respondents saw an average of 3 different providers, both dermatologists and otherwise.
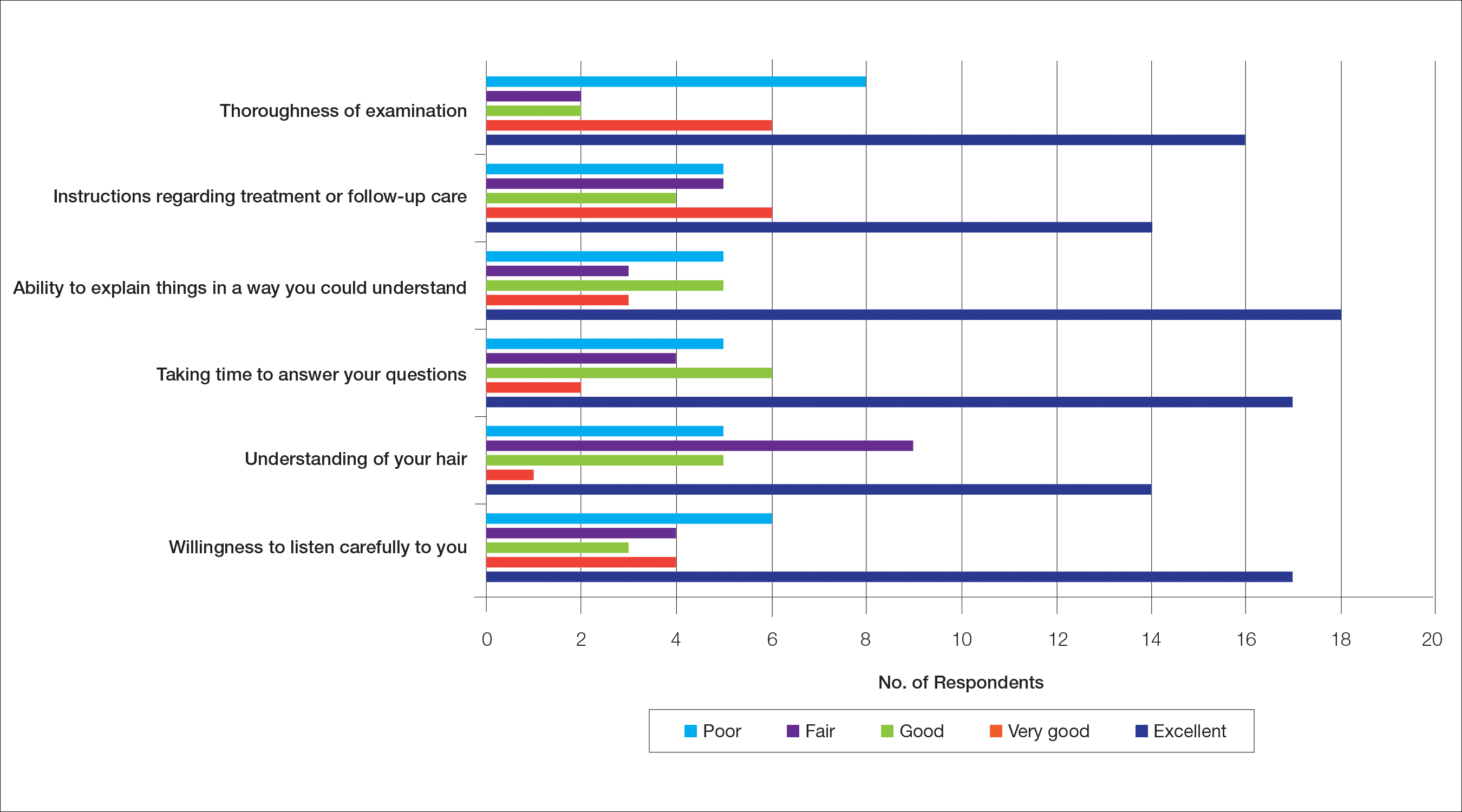
Waiting to See a Dermatologist
Nearly all respondents (31/34 [91%]) recommended that other women with hair loss immediately go see a dermatologist.
Barriers to Care
The top 5 factors reported as most important when initially seeking care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Table 3 shows frequency counts for these freely reported factors.
Quality of Life
The median score on 9 aggregated self-esteem items was 4 on a 5-point scale, representing an agree response to statements such as “I feel embarrassed, self-conscious, or frustrated about my hair loss” (28/34 [82%]) and “My hair loss bothers me” (28/34 [82%])(Table 4). Cronbach α for self-esteem survey items was 0.7826.
For the nonaggregated items, many respondents strongly disagreed with statements pertaining to activities of daily living, including “I take care of where I sit or stand at social gatherings due to my hair loss” (18/34 [53%]), “My hair loss makes it difficult for me to go to the grocery store” (29/34 [85%]), “My hair loss makes it difficult for me to attend faith-based activities” (30/34 [88%]), “My hair loss makes it difficult for me to exercise” (23/34 [68%]), “My hair loss makes it difficult for me to go to work and/or school” (24/34 [71%]), “My hair loss makes it difficult for me to go out with a significant other” (24/34 [71%]), “My hair loss makes it difficult for me to spend time with family” (27/34 [79%]), and “My hair loss makes it difficult for me to go to a hairstylist” (16/34 [47%]).
Comment
The majority of respondents were first to discover their hair loss. Harbingers of CCCA hair loss include paresthesia, tenderness, and itch,6 symptoms that are hard to ignore. Unfortunately, many patients notice hair thinning years after the scarring process has begun and a notable amount of hair has already been lost.6,9
Fifteen percent of respondents learned about their hair loss from their hairstylist. Women of African descent often maintain hairstyles that require frequent interactions with a hair care professional.7,10 As a result, hairstylists are at the forefront of early alopecia detection and are a valued resource in the black community. Open dialogue between dermatologists and hair care professionals could funnel women with hair loss into treatment before extensive damage.
Fifteen women (44%) recalled a waiting period of several months before seeking medical assistance, and 16 (47%) reported waiting 1 year or more. However, 91% of respondents indicated that women with hair loss should immediately see a physician for evaluation, thus patient experiences underscore the importance of early treatment. In our experience, many patients wait years before presenting to a physician. Some work with their hairstylists first to address the issue, while others do not realize how notable the loss has become. Some have a negative experience with one provider or are told there is nothing that can be done and then wait many years to see a second provider. Proper education of patients, physicians, and hairstylists is important in the identification and prompt treatment of this condition.
It is perhaps to be expected that patients rated interactions with dermatologists as excellent and very good more frequently than interactions with nondermatologists, which may be due to an absence of thorough hair evaluation with nondermatologists. Respondents reported that only half of nondermatologist providers actually examined their scalp during an initial encounter. However, both physician groups had the lowest frequencies of excellent and very good ratings on “understanding of your hair” (Tables 1 and 2). Patients with hair loss seek immediate answers, and often it is the specialist that can give them a firm diagnosis as opposed to a primary care provider. The fact that dermatologists and nondermatologists alike scored poorly on patient-perceived understanding of CCCA indicates an area for improvement within patient-physician interactions and physician knowledge.
The top 5 factors important to respondents when obtaining medical care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Patients with CCCA seeing dermatologists may discern a lack of experience with ethnic hair that leads patients to doubt their physicians’ ability to provide adequate care and decreased shared decision-making.11,12 These patient perceptions are not unfounded; a 2008 study showed that dermatology residents are not uniformly trained in diseases pertaining to patients with skin of color.13 Thus, incorporation of education on skin of color in dermatology training programs is critical.
Finally, hair loss patients often have concerns regarding how medical therapeutics could adversely affect personal hair care regimens, including washing and hairstyling practices. Current research demonstrates that patients consider treatment effectiveness and ability to be integrated into daily routines after establishing medical care.14 The present study shows that some CCCA patients contemplate how well a therapy will work before seeking medical care, demonstrating that patients continue to have these concerns after establishing medical care. Consideration of treatment effectiveness is important for both patients and providers, as there is minimal evidence behind current CCCA management practices. The ability for treatments to be easily integrated into daily hair care habits is important to maintain patient compliance.
Participants’ median self-esteem scores indicate the effect of CCCA on morale and self-perception. Items scrutinizing this construct had acceptable internal consistency reliability. It is interesting to note that activities of daily living were not impacted by hair loss. Examination of self-esteem is important in the alopecia population because the effect of hair loss on mental status is well documented.15-17 Low self-esteem has been reported as a prospective risk factor for clinical depression.18-20 In black patients, clinical depression rates surpass those of Hispanics and non-Hispanic white individuals.21 Dermatologists must consider the psychological status of all patients, particularly populations at risk for severe disease.
Limitations of this study include the small (34 participants) and mostly highly educated sample size, limited survey validity, and potential patient bias. Because many patients changed their address and/or telephone number in the time between CCCA diagnosis and the present study, we were left with a small pilot study, which minimizes the impact of our findings. Furthermore, our survey was created by a single expert’s opinion and modeling from preexisting alopecia questionnaires16; full validity procedures analyzing face, content, and criterion validity were not undertaken. Finally, the majority of respondents were patients of one of the study’s authors (S.S.L.P.), which could influence survey responses. The fact that some providers were hair experts and some were race concordant with their patients also could potentially affect the responses received, which was not analyzed in the present study. Future studies with more respondents from multiple providers would help clarify our preliminary findings.
Conclusion
Analysis of barriers to care and QOL in patients with skin of color is an essential addition to dermatologic discourse. Alopecia is particularly important to investigate, as prior research has found it to be one of the top 5 diagnoses made in patients with skin of color.3,4 Alopecia has been shown to negatively affect QOL.15,22,23 This study, although limited by small sample size, suggests CCCA also is a contributor to self-esteem challenges, similar to other forms of hair loss. Patient-physician interactions and personal hairstyling practices are prominent barriers to care for CCCA patients, demonstrating the need for quality education on skin of color and cultural competency in dermatology residencies across the country.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181.
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Gathers RC, Mahan MG. African american women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Van Der Donk J, Hunfeld JA, Passchier J, et al. Quality of life and maladjustment associated with hair loss in women with alopecia androgenetica. Social Sci Med. 1994;38:159-163.
- Sperling LC, Sau P. The follicular degeneration syndrome in black patients. ‘hot comb alopecia’ revisited and revised. Arch Dermatol. 1992;128:68-74.
- Gathers RC, Jankowski M, Eide M, et al. Hair grooming practices and central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2009;60:574-578.
- Harvey VM, Ozoemena U, Paul J, et al. Patient-provider communication, concordance, and ratings of care in dermatology: results of a cross-sectional study. Dermatol Online J. 2016;22. pii: 13030/qt06j6p7gh.
- Laveist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43:296-306.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Suchonwanit P, Hector CE, Bin Saif GA, et al. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:E338-E343.
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15:137-139.
- Fabbrocini G, Panariello L, De Vita V, et al. Quality of life in alopecia areata: a disease-specific questionnaire. J Eur Acad Dermatol Venereol. 2013;27:E276-E281.
- Ramos PM, Miot HA. Female pattern hair loss: a clinical and pathophysiological review. An Bras Dermatol. 2015;90:529-543.
- Sowislo JF, Orth U. Does low self-esteem predict depression and anxiety? a meta-analysis of longitudinal studies. Psychol Bull. 2013;139:213-240.
- Steiger AE, Allemand M, Robins RW, et al. Low and decreasing self-esteem during adolescence predict adult depression two decades later. J Pers Soc Psychol. 2014;106:325-338.
- Wegener I, Geiser F, Alfter S, et al. Changes of explicitly and implicitly measured self-esteem in the treatment of major depression: evidence for implicit self-esteem compensation. Compr Psychiatry. 2015;58:57-67.
- Pratt LAB, Brody DJ. Depression in the U.S. Household Population, 2009-2012. Hyattsville, MD: National Center for Health Statistics; 2014. NCHS Data Brief, No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed November 19, 2018.
- Schmidt S, Fischer TW, Chren MM, et al. Strategies of coping and quality of life in women with alopecia. Br J Dermatol. 2001;144:1038-1043.
- Hunt N, McHale S. The psychological impact of alopecia. Br Med J. 2005;331:951-953.
The etiology of central centrifugal cicatricial alopecia (CCCA), a clinical and histological pattern of hair loss on the central scalp, has been well studied. This disease is chronic and progressive, with extensive follicular destruction and eventual burnout.1,2 Central centrifugal cicatricial alopecia is most commonly seen in patients of African descent and has been shown to be 1 of the 5 most common dermatologic diagnoses in black patients.3,4 The top 5 dermatologic diagnoses within this population include acne vulgaris (28.4%), dyschromia (19.9%), eczema (9.1%), alopecia (8.3%), and seborrheic dermatitis (6.7%).4 The incidence rate of CCCA is estimated to be 5.6%.3,5 Most patients are women, with onset between the second and fourth decades of life.6
Central centrifugal cicatricial alopecia treatment efficacy is inversely correlated with disease duration. The primary goal of treatment is to prevent progression. Efforts are made to stimulate regrowth in areas that are not permanently scarred. When patients present with a substantial amount of scarring hair loss, dermatologists often are limited in their ability to achieve a cosmetically acceptable pattern of growth. Generally, hair is connected to a sense of self-worth in black women, and any type of hair loss has been shown to lead to frustration and decreased self-esteem.7 A 1994 study showed that 75% (44/58) of women with androgenetic alopecia had decreased self-esteem and 50% (29/58) had social challenges.8
The purpose of this pilot study was to determine the personal, historical, logistical, or environmental factors that preclude women from obtaining medical care for CCCA and to investigate how CCCA affects quality of life (QOL) and psychological well-being.
Methods
The investigators designed a survey study of adult, English-speaking, black women diagnosed with CCCA at the Northwestern University Department of Dermatology (Chicago, Illinois) between 2011 and 2017. Patients were selected from the electronic data warehouse compiled by the Department of Dermatology and were included if they fulfilled the following criteria: evaluated in the dermatology department between September 1, 2011, and September 30, 2017, by any faculty physician; diagnosed with CCCA; and aged 18 years or older. Patients were excluded if they did not speak English, as interpreters were not available. All patients who fulfilled the inclusion criteria provided signed informed consent prior to participation. All surveys were disseminated in the office or via telephone from fall 2016 to spring 2017 and took 10 to 15 minutes to complete. The research was approved by the authors’ institutional review board (IRB ID STU00203449).
Survey Instrument
The
Data Analysis
Analyses were completed using data analysis software JMP Pro 13 from SAS and a Microsoft Excel spreadsheet. Continuous data were presented as mean, SD, median, minimum, and maximum. Categorical data were presented as counts and percentages. Nine QOL items were aggregated into a self-esteem category (questions 30–38).
Cronbach α, a statistical measure of internal consistency and how closely related items are in a group, was used to evaluate internal consistency reliability; values of 0.70 or greater indicate acceptable reliability.
Results
Of 501 individuals contacted, 34 completed the survey (7% completion rate). Nonrespondents included 7 who refused to participate and 460 who could not be contacted. All respondents self-identified as black women. Median age at time of survey administration was 46 years (range, 28–79 years); median age at CCCA diagnosis was 42 years (range, 15–73 years). Respondents did not significantly differ in age from nonrespondents (P=.46). The majority of respondents had an associate’s degree, bachelor’s degree, or advanced degree of education (master of arts, doctor of medicine, doctor of jurisprudence, doctor of philosophy); however, 8 women reported completing some college, 1 reported completing high school, and 1 reported no schooling. Three respondents had no health insurance.
Initial Hair Loss Discovery
The majority of respondents (22/34 [65%]) were first to notice their hair loss, while 5 (15%) reported hairstylists as the initial observers. Twelve respondents (35%) initially went to a physician to learn why they were losing hair; 6 (18%) instead utilized hairstylists or the Internet. Fifteen women (44%) waited more than 1 month up to 6 months after noticing hair loss before seeing a physician instead of going immediately within a 4-week period, and 16 (47%) waited 1 year or more.
Nondermatologist Consultation
Almost half (16/34 [47%]) of the women went to a nondermatologist physician regarding their hair loss; of them, half (8/16 [50%]) reported their physician did not examine the scalp, 3 (19%) reported their physician offered a biopsy, and none of them reported that their physician diagnosed them with CCCA. The median patient rating of their nondermatologist physician interactions was good (3 on a 5-point scale). Table 1 and Figure 1 show responses to individual items.

Dermatologist Consultation
All 34 respondents presented to a dermatologist. The majority of respondents (22/34 [65%]) saw either 1 or 2 dermatologists for their hair loss. Three (9%) reported their dermatologist did not examine their scalp. Twelve respondents (35%) reported their dermatologist did not offer a biopsy. Twenty-one respondents (62%) reported a CCCA diagnosis from the first dermatologist they saw. Twenty-three respondents (68%) were diagnosed by dermatologists with expertise in hair disorders. Sixteen (47%) were diagnosed by dermatologists within a skin-of-color center. Fourteen (41%) initial dermatology consultations were race concordant.
The median patient rating of their dermatologist interactions was excellent (5 on a 5-point scale). Table 2 and Figure 2 show responses to individual items. Respondents saw an average of 3 different providers, both dermatologists and otherwise.

Waiting to See a Dermatologist
Nearly all respondents (31/34 [91%]) recommended that other women with hair loss immediately go see a dermatologist.
Barriers to Care
The top 5 factors reported as most important when initially seeking care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Table 3 shows frequency counts for these freely reported factors.
Quality of Life
The median score on 9 aggregated self-esteem items was 4 on a 5-point scale, representing an agree response to statements such as “I feel embarrassed, self-conscious, or frustrated about my hair loss” (28/34 [82%]) and “My hair loss bothers me” (28/34 [82%])(Table 4). Cronbach α for self-esteem survey items was 0.7826.
For the nonaggregated items, many respondents strongly disagreed with statements pertaining to activities of daily living, including “I take care of where I sit or stand at social gatherings due to my hair loss” (18/34 [53%]), “My hair loss makes it difficult for me to go to the grocery store” (29/34 [85%]), “My hair loss makes it difficult for me to attend faith-based activities” (30/34 [88%]), “My hair loss makes it difficult for me to exercise” (23/34 [68%]), “My hair loss makes it difficult for me to go to work and/or school” (24/34 [71%]), “My hair loss makes it difficult for me to go out with a significant other” (24/34 [71%]), “My hair loss makes it difficult for me to spend time with family” (27/34 [79%]), and “My hair loss makes it difficult for me to go to a hairstylist” (16/34 [47%]).
Comment
The majority of respondents were first to discover their hair loss. Harbingers of CCCA hair loss include paresthesia, tenderness, and itch,6 symptoms that are hard to ignore. Unfortunately, many patients notice hair thinning years after the scarring process has begun and a notable amount of hair has already been lost.6,9
Fifteen percent of respondents learned about their hair loss from their hairstylist. Women of African descent often maintain hairstyles that require frequent interactions with a hair care professional.7,10 As a result, hairstylists are at the forefront of early alopecia detection and are a valued resource in the black community. Open dialogue between dermatologists and hair care professionals could funnel women with hair loss into treatment before extensive damage.
Fifteen women (44%) recalled a waiting period of several months before seeking medical assistance, and 16 (47%) reported waiting 1 year or more. However, 91% of respondents indicated that women with hair loss should immediately see a physician for evaluation, thus patient experiences underscore the importance of early treatment. In our experience, many patients wait years before presenting to a physician. Some work with their hairstylists first to address the issue, while others do not realize how notable the loss has become. Some have a negative experience with one provider or are told there is nothing that can be done and then wait many years to see a second provider. Proper education of patients, physicians, and hairstylists is important in the identification and prompt treatment of this condition.
It is perhaps to be expected that patients rated interactions with dermatologists as excellent and very good more frequently than interactions with nondermatologists, which may be due to an absence of thorough hair evaluation with nondermatologists. Respondents reported that only half of nondermatologist providers actually examined their scalp during an initial encounter. However, both physician groups had the lowest frequencies of excellent and very good ratings on “understanding of your hair” (Tables 1 and 2). Patients with hair loss seek immediate answers, and often it is the specialist that can give them a firm diagnosis as opposed to a primary care provider. The fact that dermatologists and nondermatologists alike scored poorly on patient-perceived understanding of CCCA indicates an area for improvement within patient-physician interactions and physician knowledge.
The top 5 factors important to respondents when obtaining medical care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Patients with CCCA seeing dermatologists may discern a lack of experience with ethnic hair that leads patients to doubt their physicians’ ability to provide adequate care and decreased shared decision-making.11,12 These patient perceptions are not unfounded; a 2008 study showed that dermatology residents are not uniformly trained in diseases pertaining to patients with skin of color.13 Thus, incorporation of education on skin of color in dermatology training programs is critical.
Finally, hair loss patients often have concerns regarding how medical therapeutics could adversely affect personal hair care regimens, including washing and hairstyling practices. Current research demonstrates that patients consider treatment effectiveness and ability to be integrated into daily routines after establishing medical care.14 The present study shows that some CCCA patients contemplate how well a therapy will work before seeking medical care, demonstrating that patients continue to have these concerns after establishing medical care. Consideration of treatment effectiveness is important for both patients and providers, as there is minimal evidence behind current CCCA management practices. The ability for treatments to be easily integrated into daily hair care habits is important to maintain patient compliance.
Participants’ median self-esteem scores indicate the effect of CCCA on morale and self-perception. Items scrutinizing this construct had acceptable internal consistency reliability. It is interesting to note that activities of daily living were not impacted by hair loss. Examination of self-esteem is important in the alopecia population because the effect of hair loss on mental status is well documented.15-17 Low self-esteem has been reported as a prospective risk factor for clinical depression.18-20 In black patients, clinical depression rates surpass those of Hispanics and non-Hispanic white individuals.21 Dermatologists must consider the psychological status of all patients, particularly populations at risk for severe disease.
Limitations of this study include the small (34 participants) and mostly highly educated sample size, limited survey validity, and potential patient bias. Because many patients changed their address and/or telephone number in the time between CCCA diagnosis and the present study, we were left with a small pilot study, which minimizes the impact of our findings. Furthermore, our survey was created by a single expert’s opinion and modeling from preexisting alopecia questionnaires16; full validity procedures analyzing face, content, and criterion validity were not undertaken. Finally, the majority of respondents were patients of one of the study’s authors (S.S.L.P.), which could influence survey responses. The fact that some providers were hair experts and some were race concordant with their patients also could potentially affect the responses received, which was not analyzed in the present study. Future studies with more respondents from multiple providers would help clarify our preliminary findings.
Conclusion
Analysis of barriers to care and QOL in patients with skin of color is an essential addition to dermatologic discourse. Alopecia is particularly important to investigate, as prior research has found it to be one of the top 5 diagnoses made in patients with skin of color.3,4 Alopecia has been shown to negatively affect QOL.15,22,23 This study, although limited by small sample size, suggests CCCA also is a contributor to self-esteem challenges, similar to other forms of hair loss. Patient-physician interactions and personal hairstyling practices are prominent barriers to care for CCCA patients, demonstrating the need for quality education on skin of color and cultural competency in dermatology residencies across the country.
The etiology of central centrifugal cicatricial alopecia (CCCA), a clinical and histological pattern of hair loss on the central scalp, has been well studied. This disease is chronic and progressive, with extensive follicular destruction and eventual burnout.1,2 Central centrifugal cicatricial alopecia is most commonly seen in patients of African descent and has been shown to be 1 of the 5 most common dermatologic diagnoses in black patients.3,4 The top 5 dermatologic diagnoses within this population include acne vulgaris (28.4%), dyschromia (19.9%), eczema (9.1%), alopecia (8.3%), and seborrheic dermatitis (6.7%).4 The incidence rate of CCCA is estimated to be 5.6%.3,5 Most patients are women, with onset between the second and fourth decades of life.6
Central centrifugal cicatricial alopecia treatment efficacy is inversely correlated with disease duration. The primary goal of treatment is to prevent progression. Efforts are made to stimulate regrowth in areas that are not permanently scarred. When patients present with a substantial amount of scarring hair loss, dermatologists often are limited in their ability to achieve a cosmetically acceptable pattern of growth. Generally, hair is connected to a sense of self-worth in black women, and any type of hair loss has been shown to lead to frustration and decreased self-esteem.7 A 1994 study showed that 75% (44/58) of women with androgenetic alopecia had decreased self-esteem and 50% (29/58) had social challenges.8
The purpose of this pilot study was to determine the personal, historical, logistical, or environmental factors that preclude women from obtaining medical care for CCCA and to investigate how CCCA affects quality of life (QOL) and psychological well-being.
Methods
The investigators designed a survey study of adult, English-speaking, black women diagnosed with CCCA at the Northwestern University Department of Dermatology (Chicago, Illinois) between 2011 and 2017. Patients were selected from the electronic data warehouse compiled by the Department of Dermatology and were included if they fulfilled the following criteria: evaluated in the dermatology department between September 1, 2011, and September 30, 2017, by any faculty physician; diagnosed with CCCA; and aged 18 years or older. Patients were excluded if they did not speak English, as interpreters were not available. All patients who fulfilled the inclusion criteria provided signed informed consent prior to participation. All surveys were disseminated in the office or via telephone from fall 2016 to spring 2017 and took 10 to 15 minutes to complete. The research was approved by the authors’ institutional review board (IRB ID STU00203449).
Survey Instrument
The
Data Analysis
Analyses were completed using data analysis software JMP Pro 13 from SAS and a Microsoft Excel spreadsheet. Continuous data were presented as mean, SD, median, minimum, and maximum. Categorical data were presented as counts and percentages. Nine QOL items were aggregated into a self-esteem category (questions 30–38).
Cronbach α, a statistical measure of internal consistency and how closely related items are in a group, was used to evaluate internal consistency reliability; values of 0.70 or greater indicate acceptable reliability.
Results
Of 501 individuals contacted, 34 completed the survey (7% completion rate). Nonrespondents included 7 who refused to participate and 460 who could not be contacted. All respondents self-identified as black women. Median age at time of survey administration was 46 years (range, 28–79 years); median age at CCCA diagnosis was 42 years (range, 15–73 years). Respondents did not significantly differ in age from nonrespondents (P=.46). The majority of respondents had an associate’s degree, bachelor’s degree, or advanced degree of education (master of arts, doctor of medicine, doctor of jurisprudence, doctor of philosophy); however, 8 women reported completing some college, 1 reported completing high school, and 1 reported no schooling. Three respondents had no health insurance.
Initial Hair Loss Discovery
The majority of respondents (22/34 [65%]) were first to notice their hair loss, while 5 (15%) reported hairstylists as the initial observers. Twelve respondents (35%) initially went to a physician to learn why they were losing hair; 6 (18%) instead utilized hairstylists or the Internet. Fifteen women (44%) waited more than 1 month up to 6 months after noticing hair loss before seeing a physician instead of going immediately within a 4-week period, and 16 (47%) waited 1 year or more.
Nondermatologist Consultation
Almost half (16/34 [47%]) of the women went to a nondermatologist physician regarding their hair loss; of them, half (8/16 [50%]) reported their physician did not examine the scalp, 3 (19%) reported their physician offered a biopsy, and none of them reported that their physician diagnosed them with CCCA. The median patient rating of their nondermatologist physician interactions was good (3 on a 5-point scale). Table 1 and Figure 1 show responses to individual items.

Dermatologist Consultation
All 34 respondents presented to a dermatologist. The majority of respondents (22/34 [65%]) saw either 1 or 2 dermatologists for their hair loss. Three (9%) reported their dermatologist did not examine their scalp. Twelve respondents (35%) reported their dermatologist did not offer a biopsy. Twenty-one respondents (62%) reported a CCCA diagnosis from the first dermatologist they saw. Twenty-three respondents (68%) were diagnosed by dermatologists with expertise in hair disorders. Sixteen (47%) were diagnosed by dermatologists within a skin-of-color center. Fourteen (41%) initial dermatology consultations were race concordant.
The median patient rating of their dermatologist interactions was excellent (5 on a 5-point scale). Table 2 and Figure 2 show responses to individual items. Respondents saw an average of 3 different providers, both dermatologists and otherwise.

Waiting to See a Dermatologist
Nearly all respondents (31/34 [91%]) recommended that other women with hair loss immediately go see a dermatologist.
Barriers to Care
The top 5 factors reported as most important when initially seeking care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Table 3 shows frequency counts for these freely reported factors.
Quality of Life
The median score on 9 aggregated self-esteem items was 4 on a 5-point scale, representing an agree response to statements such as “I feel embarrassed, self-conscious, or frustrated about my hair loss” (28/34 [82%]) and “My hair loss bothers me” (28/34 [82%])(Table 4). Cronbach α for self-esteem survey items was 0.7826.
For the nonaggregated items, many respondents strongly disagreed with statements pertaining to activities of daily living, including “I take care of where I sit or stand at social gatherings due to my hair loss” (18/34 [53%]), “My hair loss makes it difficult for me to go to the grocery store” (29/34 [85%]), “My hair loss makes it difficult for me to attend faith-based activities” (30/34 [88%]), “My hair loss makes it difficult for me to exercise” (23/34 [68%]), “My hair loss makes it difficult for me to go to work and/or school” (24/34 [71%]), “My hair loss makes it difficult for me to go out with a significant other” (24/34 [71%]), “My hair loss makes it difficult for me to spend time with family” (27/34 [79%]), and “My hair loss makes it difficult for me to go to a hairstylist” (16/34 [47%]).
Comment
The majority of respondents were first to discover their hair loss. Harbingers of CCCA hair loss include paresthesia, tenderness, and itch,6 symptoms that are hard to ignore. Unfortunately, many patients notice hair thinning years after the scarring process has begun and a notable amount of hair has already been lost.6,9
Fifteen percent of respondents learned about their hair loss from their hairstylist. Women of African descent often maintain hairstyles that require frequent interactions with a hair care professional.7,10 As a result, hairstylists are at the forefront of early alopecia detection and are a valued resource in the black community. Open dialogue between dermatologists and hair care professionals could funnel women with hair loss into treatment before extensive damage.
Fifteen women (44%) recalled a waiting period of several months before seeking medical assistance, and 16 (47%) reported waiting 1 year or more. However, 91% of respondents indicated that women with hair loss should immediately see a physician for evaluation, thus patient experiences underscore the importance of early treatment. In our experience, many patients wait years before presenting to a physician. Some work with their hairstylists first to address the issue, while others do not realize how notable the loss has become. Some have a negative experience with one provider or are told there is nothing that can be done and then wait many years to see a second provider. Proper education of patients, physicians, and hairstylists is important in the identification and prompt treatment of this condition.
It is perhaps to be expected that patients rated interactions with dermatologists as excellent and very good more frequently than interactions with nondermatologists, which may be due to an absence of thorough hair evaluation with nondermatologists. Respondents reported that only half of nondermatologist providers actually examined their scalp during an initial encounter. However, both physician groups had the lowest frequencies of excellent and very good ratings on “understanding of your hair” (Tables 1 and 2). Patients with hair loss seek immediate answers, and often it is the specialist that can give them a firm diagnosis as opposed to a primary care provider. The fact that dermatologists and nondermatologists alike scored poorly on patient-perceived understanding of CCCA indicates an area for improvement within patient-physician interactions and physician knowledge.
The top 5 factors important to respondents when obtaining medical care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Patients with CCCA seeing dermatologists may discern a lack of experience with ethnic hair that leads patients to doubt their physicians’ ability to provide adequate care and decreased shared decision-making.11,12 These patient perceptions are not unfounded; a 2008 study showed that dermatology residents are not uniformly trained in diseases pertaining to patients with skin of color.13 Thus, incorporation of education on skin of color in dermatology training programs is critical.
Finally, hair loss patients often have concerns regarding how medical therapeutics could adversely affect personal hair care regimens, including washing and hairstyling practices. Current research demonstrates that patients consider treatment effectiveness and ability to be integrated into daily routines after establishing medical care.14 The present study shows that some CCCA patients contemplate how well a therapy will work before seeking medical care, demonstrating that patients continue to have these concerns after establishing medical care. Consideration of treatment effectiveness is important for both patients and providers, as there is minimal evidence behind current CCCA management practices. The ability for treatments to be easily integrated into daily hair care habits is important to maintain patient compliance.
Participants’ median self-esteem scores indicate the effect of CCCA on morale and self-perception. Items scrutinizing this construct had acceptable internal consistency reliability. It is interesting to note that activities of daily living were not impacted by hair loss. Examination of self-esteem is important in the alopecia population because the effect of hair loss on mental status is well documented.15-17 Low self-esteem has been reported as a prospective risk factor for clinical depression.18-20 In black patients, clinical depression rates surpass those of Hispanics and non-Hispanic white individuals.21 Dermatologists must consider the psychological status of all patients, particularly populations at risk for severe disease.
Limitations of this study include the small (34 participants) and mostly highly educated sample size, limited survey validity, and potential patient bias. Because many patients changed their address and/or telephone number in the time between CCCA diagnosis and the present study, we were left with a small pilot study, which minimizes the impact of our findings. Furthermore, our survey was created by a single expert’s opinion and modeling from preexisting alopecia questionnaires16; full validity procedures analyzing face, content, and criterion validity were not undertaken. Finally, the majority of respondents were patients of one of the study’s authors (S.S.L.P.), which could influence survey responses. The fact that some providers were hair experts and some were race concordant with their patients also could potentially affect the responses received, which was not analyzed in the present study. Future studies with more respondents from multiple providers would help clarify our preliminary findings.
Conclusion
Analysis of barriers to care and QOL in patients with skin of color is an essential addition to dermatologic discourse. Alopecia is particularly important to investigate, as prior research has found it to be one of the top 5 diagnoses made in patients with skin of color.3,4 Alopecia has been shown to negatively affect QOL.15,22,23 This study, although limited by small sample size, suggests CCCA also is a contributor to self-esteem challenges, similar to other forms of hair loss. Patient-physician interactions and personal hairstyling practices are prominent barriers to care for CCCA patients, demonstrating the need for quality education on skin of color and cultural competency in dermatology residencies across the country.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181.
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Gathers RC, Mahan MG. African american women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Van Der Donk J, Hunfeld JA, Passchier J, et al. Quality of life and maladjustment associated with hair loss in women with alopecia androgenetica. Social Sci Med. 1994;38:159-163.
- Sperling LC, Sau P. The follicular degeneration syndrome in black patients. ‘hot comb alopecia’ revisited and revised. Arch Dermatol. 1992;128:68-74.
- Gathers RC, Jankowski M, Eide M, et al. Hair grooming practices and central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2009;60:574-578.
- Harvey VM, Ozoemena U, Paul J, et al. Patient-provider communication, concordance, and ratings of care in dermatology: results of a cross-sectional study. Dermatol Online J. 2016;22. pii: 13030/qt06j6p7gh.
- Laveist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43:296-306.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Suchonwanit P, Hector CE, Bin Saif GA, et al. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:E338-E343.
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15:137-139.
- Fabbrocini G, Panariello L, De Vita V, et al. Quality of life in alopecia areata: a disease-specific questionnaire. J Eur Acad Dermatol Venereol. 2013;27:E276-E281.
- Ramos PM, Miot HA. Female pattern hair loss: a clinical and pathophysiological review. An Bras Dermatol. 2015;90:529-543.
- Sowislo JF, Orth U. Does low self-esteem predict depression and anxiety? a meta-analysis of longitudinal studies. Psychol Bull. 2013;139:213-240.
- Steiger AE, Allemand M, Robins RW, et al. Low and decreasing self-esteem during adolescence predict adult depression two decades later. J Pers Soc Psychol. 2014;106:325-338.
- Wegener I, Geiser F, Alfter S, et al. Changes of explicitly and implicitly measured self-esteem in the treatment of major depression: evidence for implicit self-esteem compensation. Compr Psychiatry. 2015;58:57-67.
- Pratt LAB, Brody DJ. Depression in the U.S. Household Population, 2009-2012. Hyattsville, MD: National Center for Health Statistics; 2014. NCHS Data Brief, No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed November 19, 2018.
- Schmidt S, Fischer TW, Chren MM, et al. Strategies of coping and quality of life in women with alopecia. Br J Dermatol. 2001;144:1038-1043.
- Hunt N, McHale S. The psychological impact of alopecia. Br Med J. 2005;331:951-953.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181.
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Gathers RC, Mahan MG. African american women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Van Der Donk J, Hunfeld JA, Passchier J, et al. Quality of life and maladjustment associated with hair loss in women with alopecia androgenetica. Social Sci Med. 1994;38:159-163.
- Sperling LC, Sau P. The follicular degeneration syndrome in black patients. ‘hot comb alopecia’ revisited and revised. Arch Dermatol. 1992;128:68-74.
- Gathers RC, Jankowski M, Eide M, et al. Hair grooming practices and central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2009;60:574-578.
- Harvey VM, Ozoemena U, Paul J, et al. Patient-provider communication, concordance, and ratings of care in dermatology: results of a cross-sectional study. Dermatol Online J. 2016;22. pii: 13030/qt06j6p7gh.
- Laveist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43:296-306.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Suchonwanit P, Hector CE, Bin Saif GA, et al. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:E338-E343.
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15:137-139.
- Fabbrocini G, Panariello L, De Vita V, et al. Quality of life in alopecia areata: a disease-specific questionnaire. J Eur Acad Dermatol Venereol. 2013;27:E276-E281.
- Ramos PM, Miot HA. Female pattern hair loss: a clinical and pathophysiological review. An Bras Dermatol. 2015;90:529-543.
- Sowislo JF, Orth U. Does low self-esteem predict depression and anxiety? a meta-analysis of longitudinal studies. Psychol Bull. 2013;139:213-240.
- Steiger AE, Allemand M, Robins RW, et al. Low and decreasing self-esteem during adolescence predict adult depression two decades later. J Pers Soc Psychol. 2014;106:325-338.
- Wegener I, Geiser F, Alfter S, et al. Changes of explicitly and implicitly measured self-esteem in the treatment of major depression: evidence for implicit self-esteem compensation. Compr Psychiatry. 2015;58:57-67.
- Pratt LAB, Brody DJ. Depression in the U.S. Household Population, 2009-2012. Hyattsville, MD: National Center for Health Statistics; 2014. NCHS Data Brief, No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed November 19, 2018.
- Schmidt S, Fischer TW, Chren MM, et al. Strategies of coping and quality of life in women with alopecia. Br J Dermatol. 2001;144:1038-1043.
- Hunt N, McHale S. The psychological impact of alopecia. Br Med J. 2005;331:951-953.
Practice Points
- Central centrifugal cicatricial alopecia (CCCA) presents a unique set of challenges for both patients and providers.
- Lack of physician experience with black hair/CCCA and the potential impact of care on personal hairstyling practices are 2 barriers to care for many patients with this disease.
- There is a need for improved patient-provider communication strategies, quality education on hair in skin of color patients, and cultural competency training in dermatology residencies across the country.
Safety and Efficacy of Percutaneous Injection of Lipogems Micro-Fractured Adipose Tissue for Osteoarthritic Knees
ABSTRACT
The aim of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with refractory knee osteoarthritis (OA). A total of 17 subjects (26 knees) with a median age of 72 years (range: 54-78 years) and a history of knee OA (Kellgren–Lawrence, grade of 3 or 4) underwent treatment with ultrasound-guided injection of micro-fractured adipose tissue. Micro-fractured fat was obtained using a minimal manipulation technique in a closed system (Lipogems), without the addition of enzymes or any other additives. The study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months following this procedure.
When compared with baseline, significant improvements were noted in the mean values of NPRS, FXN, and LEAS at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved at 6 weeks and 12 months. In particular, the average KSS score improved from 74 to 82, the FXN score improved from 65 to 76, and the LEAS score improved from 36 to 47. These values were significantly greater than the previously published minimal clinically important difference described for KSS and FXN in patients who underwent total knee arthroplasty for primary OA. No serious adverse events were reported. The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option for patients with refractory, severe (grade 3 or 4) knee OA.
This study demonstrated significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
Continue to: Knee OA is...
Knee OA is a chronic disease that affects all races, genders, and ages, but it is most prevalent in obese and elderly people. Worldwide, arthritis is considered to be the fourth leading cause of disability.1 In developing and developed countries, knee OA may cause a significant decline in the quality of life for individuals >65 years due to joint pain and disability.1 Nonoperative treatment can be successful in patients with mild to moderate arthritis with pain.
Current treatment options for knee OA, including physical therapy and anti-inflammatory drugs, aim to remedy the symptoms, but they do little to treat the underlying causes of knee OA pain. When a patient presents with advanced arthritis of the knee as confirmed by radiographic findings (classified as Kellgren–Lawrence grade of 3 or 4), the standard approach has been a total knee arthroplasty (TKA) after the patient has failed conservative treatment. The annual rate of total knee replacement in the United States has doubled since 2000, especially in those 45 – 65 years.2 The total number of procedures performed each year now exceeds 640,000, at a total annual cost of about $10.2 billion.2 Multiple studies show that TKA has favorable outcomes in pain relief and functional improvement in patients >60 years when evaluated at a follow-up of 10 years after surgery.2
However, some patients are hesitant to proceed with surgery due to fear of surgical pain and procedural complications. The known complications include deep vein thrombosis, pulmonary embolism, nerve injury, and infection. In addition, up to 20% of patients continue to complain of pain following a total knee replacement.3 Finally, in the young population (<50 years), there are concerns related to the potential need of revision knee surgery in the future.3
Alternative treatments for knee OA have recently emerged, including the use of platelet-rich plasma (PRP). A recent meta-analysis that included 10 randomized controlled trials with a total of 1069 patients demonstrated that, compared with hyaluronic acid and saline, intra-articular PRP injection may have more benefits in pain relief and functional improvement in patients with symptomatic knee OA at 1-year post-injection.4 Another smaller study examined patients who had experienced mild knee OA (Kellgren–Lawrence grade <3) for an average of 14 months. Each patient underwent magnetic resonance imaging for the evaluation of joint damage and then received a single PRP injection. The patients were assessed at regular intervals, with improvement in pain lasting up to 12 months.5
Additional orthobiologic options include the use of bone marrow and adipose-derived stem cell (ASC) injections for a variety of knee conditions, including knee OA. Mesenchymal stem cells (MSCs) are multipotent cells that have been used for the treatment of OA in clinical trials because of their regeneration potential and anti-inflammatory effects.6 Bone marrow stem cells (BMSCs) were first used to repair cartilage damage in humans in 1998. However, BMSCs had particular challenges, including low stem cell yield, pain, and possible morbidities during bone marrow aspiration. An alternative is ASCs, which may be more suitable clinically because of the high stem cell yield from lipoaspirates, faster cell proliferation, and less discomfort and morbidities during the harvesting procedure.7 In addition, these adult stem cells can contribute to the chondrogenic, osteogenic, adipogenic, myogenic, and neurogenic lineages.8 One study demonstrated that the contents of cartilage glycosaminoglycans significantly increased in specific areas of a knee joint treated with ASCs.9,10 This increased glycosaminoglycan content in hyaline cartilage may explain the observed visual analog score (VAS) improvement and clinical results. Other studies suggest that the chondrogenic action of ASCs may depend more on regenerative signaling by activated perivascular cells and signaling of trophic and paracrine mediators, such as vascular endothelial growth factor.9,10 Finally, the mechanism of action may include providing volume, support, cushioning, and filling of soft tissue defects.11
The Lipogems method and device, approved by the U.S. Food and Drug Administration, is used to harvest ASCs, cleanse, and micro-fracture adipose tissue while maintaining the perivascular niche that contains pericytes. The purpose of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with severe refractory knee OA.
Continue to: This report details...
STUDY PRESENTATION
This report details the outcome of an IRB-approved study of 17 subjects with 26 symptomatic knees with a history of knee OA (Kellgren–Lawrence grade of 3 or 4) diagnosed by a radiograph. Patient demographics are described in the Table.
TABLE. Patient Demographics | |
Male n (%) | 10 (58.8) |
Age, mean ± SD (range) | 68.27 ± 7.43 |
BMI, mean ± SD (range) | 28.98 ± 4.50 |
Kellgren–Lawrence grade 3 (n) | 7 |
Kellgren–Lawrence grade 4 (n) | 19 |
Abbreviation: BMI, body mass index.
The study patients were evaluated by an orthopedic surgeon, Mitchell Sheinkop, who commonly performs total joint replacement in his practice and considers potential patients as candidates for TKA. These patients presented with a Kellgren-Lawrence grade of 3 or 4 knee OA, and all had significant pain that was refractory to conservative treatment, which included medications, physical therapy, and injections. The study patients were offered the Lipogems procedure as an alternative to TKA. Following this procedure, the study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months. The 1989 KSS12 was used for this study. Adverse reactions were also monitored throughout the study period.
METHODS
After obtaining informed consent, the subjects were taken into the operating room, moved to the procedure table, and placed in the prone position for aspiration. After scrubbing with Betadine and draping, 1 mL of lidocaine was used to anesthetize the skin, and a pre-prepared preparation of lidocaine, epinephrine, and sterile saline was infused into the subcutaneous tissue. The micro-fragmented adipose tissue was obtained with minimal manipulation using Lipogems, a closed system using mild mechanical forces and reduction filters. The system processes the lipoaspirate without the addition of enzymes or any other additives. The final product consists of adipose tissue clusters with preserved vascular stromal niche of approximately 500 microns. The lipoaspirate was processed in the same room via a closed system. During the processing, the subject’s puncture wounds were dressed. The knee injection site was prepped with a Betadine swab and DuraPrep. Then, Lipogems was injected intra-articularly under ultrasound guidance.
After the completion of the injection, manual range of motion was administered to the treated joint. The subject was then transferred to the recovery room where vital signs were monitored. Post-procedure instructions were reviewed with the patient by the study staff. The subject was instructed to use an assistive device and avoid weight-bearing for 48 hours and maintain the activities of daily living to a minimum on the day of the procedure. Non-weight-bearing for 48 hours was recommended for reducing discomfort to avoid the use of opioids. Nonsteroidal anti-inflammatory drugs, alcohol, and marijuana must be avoided for 4 weeks after the procedure. Pretreatment and post-treatment outcomes were collected using the NPRS, the 100-point KSS with its FXN, and the LEAS at 6 weeks, 6 months, and 12 months after this procedure. The 1989 KSS12 was used for this study since the same scale was used for previous TKA procedures by our authors, allowing for future comparisons of results.
STATISTICAL ANALYSIS
Mean and standard deviation were used to estimate central tendency and variability. Outcome measures were analyzed using the t test, with the pairwise t test was used for paired and subsequent measurements of the same patient or a knee. All analyses were performed with significance set at P <.05. The minimal clinically important difference (MCID) in patients who underwent TKA for primary OA was between 5.3 and 5.9 for KSS, while the MCID for FXN was between 6.1 and 6.4.13 These values were referenced for our analysis.
Continue to: No significant adverse...
RESULTS
No significant adverse events were reported in the subjects of this study. Common minor adverse events included pain and swelling, which generally resolved in 48 to 72 hours after the procedure.
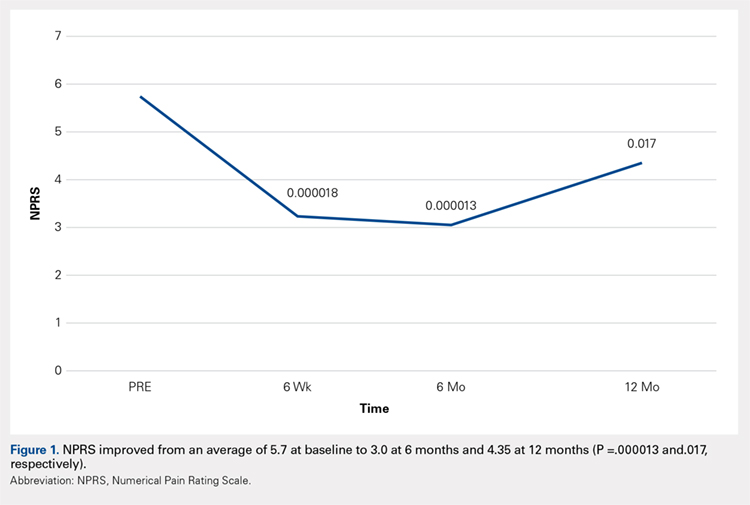
Compared with baseline, significant improvements were noted in the mean values of NPRS (Figure 1) at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved from baseline at 6 weeks and 12 months (Figure 2). Significant improvements were also noted in the mean values of FXN (Figure 3) and the mean LEAS significantly improved from baseline at 6 weeks and 6 months (Figure 4).
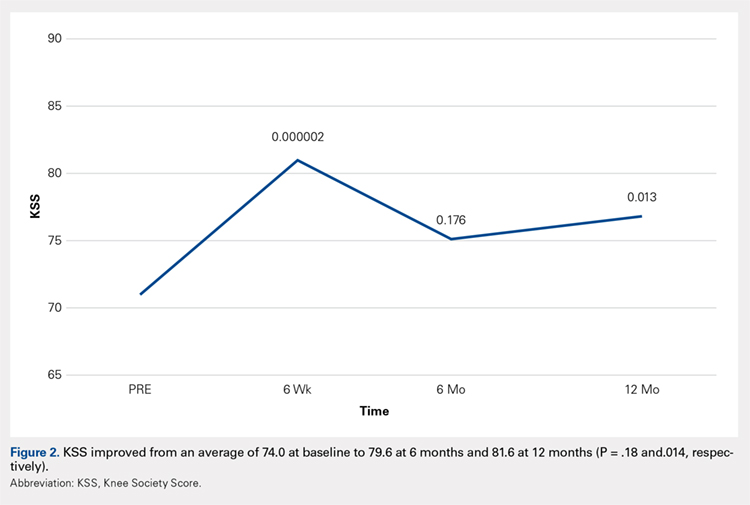
DISCUSSION
Knee OA is a disabling condition that affects a substantial proportion of the aging population. The current treatment methods do little to address the degenerative environment of the joint, which includes cytokines such as IL-1 and IL-2. Orthobiologic agents have been used recently to address these issues, which include PRP and MSCs from various sources, including bone marrow and adipose tissue.
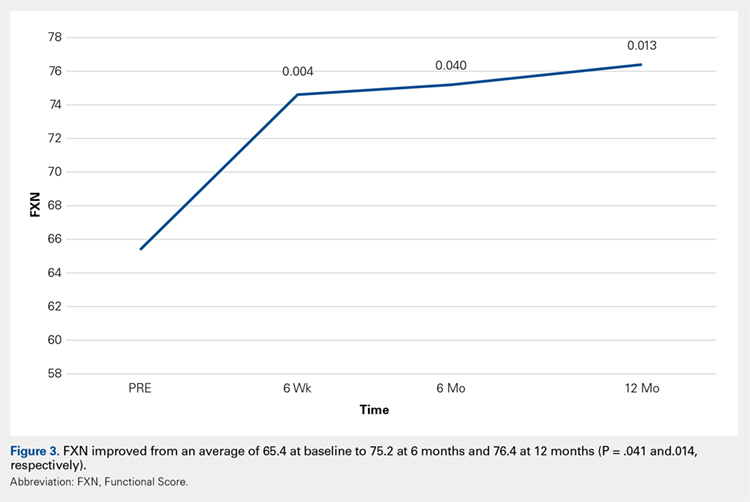
A recent meta-analysis conducted by Cui and colleagues14 evaluated 18 studies of MSC treatment for knee OA with a total of 565 participants (226 males and 339 females). The duration from the onset of knee pain to registration in each study ranged from 3 months to ≥7 years. The follow-up period was 3 months -24 months. The majority of studies recruited patients with knee OA with a severity grade of 1-4 on the K-L scale; K-L grades 1 and 2 and grades 3 and 4 were defined as early OA and advanced OA, respectively. The results suggested that MSC treatment significantly improved pain and functional status, relative to the baseline evaluations in knee OA, and the beneficial effect was maintained for 2 years after treatment. Furthermore, the treatment effectiveness was not reduced over time.14
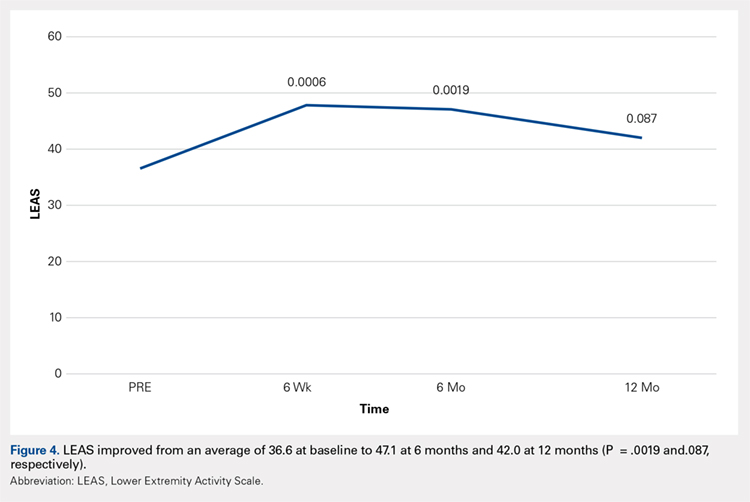
Included in the abovementioned meta-analysis were 2 papers by Koh and colleagues in 2012 and 2013 on the use of AMSCs for the treatment of OA. 15,16 The first study included 18 patients whose adipose tissue was harvested from the inner side of the infrapatellar fat pad via a skin incision after arthroscopic debridement. The cells were centrifuged and injected into the patient’s knee the same day. The results showed a significant reduction of pain and an increased quality of life for all patients, and a positive correlation was found between the number of cells injected and pain improvements. The authors concluded that AMSCs were a valid cell source for treating cartilage damage.15
In their second study, Koh and colleagues reported their results of treating 30 elderly patients with OA (≥65 years), who had failed conventional treatment, using intra-articular injections of AMSCs.16 This patient population is important since OA most commonly occurs in the elderly population. Patients underwent arthroscopic lavage and cartilage evaluation before receiving an injection of AMSCs delivered in PRP. The authors demonstrated that AMSC therapy for elderly patients with mild to moderate OA was an effective treatment resulting in reduction of pain and regeneration of cartilage.16
In another study, Adriani and colleagues17 performed autologous percutaneous fat injection from January 2012 to March 2015 for the treatment of knee OA. Their 30 patients (12 males and 18 females; mean age of 63.3 years; mean body mass index of 25.1) had stable or progressive knee OA for at least 12 months, no other injection treatments during the previous 12 months, and no prior knee surgeries. The patients were evaluated at baseline and 1 week and at 1, 3, 6, and 12 months after treatment using the NPRS and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as outcome measures. The average VAS was 7.7 at baseline and improved to 4.3 at 3-month follow-up; however, a slight deterioration (VAS 5.0) was noted at 1 year. Total WOMAC score was 89.9 at baseline, 68.6 at 3 months, and 73.2 at 12-month follow-up.17
Continue to: The results of...
The results of this study demonstrated significant improvements in pain, quality of life, and function at 12 months after ultrasound-guided injection of ASCs in patients with severe knee OA. Significant improvement that was noted at 6 weeks was maintained through 12 months after the treatment. Improvement was noted in all scales, including the NPRS, the KSS, and the FXN beginning at 3 months and continuing through 12 months. The LEAS was statistically significant through 6 months after the treatment but not significant at 12 months. No serious adverse events were recorded.
In a study by Lee and colleagues,13 the MCID was described for KSS and FXN in patients who underwent TKA for primary OA. This is the minimal change in a scoring measure that is perceived by the patient to be beneficial or harmful. The MCID for KSS was noted to be between 5.3 and 5.9, while the MCID for FXN was between 6.1 and 6.4.13 In our study, the KSS score improved from an average of 74.0 at baseline to 79.6 at 6 months and 81.6 at 12 months (a difference of 5.6 and 7.6; P = .18 and.014, respectively). The FXN improved from an average of 65.4 at baseline to 75.2 at 6 months and 76.4 at 12 months (a difference of 9.9 and 11; P = .041 and.014, respectively). Therefore, a clinically important difference of KSS and FXN scores was noted at both 6 and 12 months.
The technique used in this study provides autologous, minimally manipulated, fat graft performed in a short time (60-90 minutes), without expansion and/or enzymatic treatment. In addition, the harvesting and the injection of stem cells on the same day is a simple, office-based procedure, and compliant with the U. S. Food and Drug Administration regulations.18 The cost of the procedure averages $3500.
A study limitation is that it is a case series with relatively small numbers and not a randomized controlled study. Therefore, a placebo effect may play a role in our results. Further study with a larger number of patients and randomized controlled studies would be beneficial to support the findings of this study.
CONCLUSION
The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option in patients with refractory severe (grade 3 or 4) knee OA. This study showed significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
- Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One. 2017;12(4):e0175449.
- Jauregui JJ, Cherian JJ, Pierce TP, Beaver WB, Issa K, Mont MA. Long-Term Survivorship and Clinical Outcomes Following Total Knee Arthroplasty. J Arthroplasty. 2015;30(12):2164-2166.
- Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
- Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy.33(3):659-670.e651.
- Halpern B CS, Rodeo SA, Hayter C, Bogner E, Potter HG, Nguyen J. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013 May;23.
- Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307-1316.
- Tang Y, Pan ZY, Zou Y, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017.
- Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. Journal of cellular biochemistry. 2006;99(5):1285-1297.
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16(5):203-209.
- Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. A J Physiol Renal Physiol. 2007;292(5):F1626-1635.
- Mestak O, Sukop A, Hsueh YS, et al. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J Surg Oncol. 2014;12:178.
- Insall JN DL, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989 Nov;(248):13-4.
- Lee WC, Kwan YH, Chong HC, Yeo SJ. The minimal clinically important difference for Knee Society Clinical Rating System after total knee arthroplasty for primary osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy. 2016.
- Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp Ther Med. 2016;12(5):3390-3400.
- Koh Y-GC, Yun-Jin. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902-907.
- Koh Y-GC, Yun-Jin. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
- Adriani E. MM, et al. Percutaneous Fat Transfer to Treat Knee Osteoarthritis Symptoms: Preliminary Results. Joints. 2017.
- Bianchi F, Maioli M, Leonardi E, et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces From Human Lipoaspirates. Cell Transplantation. 2013;22(11):2063-2077
ABSTRACT
The aim of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with refractory knee osteoarthritis (OA). A total of 17 subjects (26 knees) with a median age of 72 years (range: 54-78 years) and a history of knee OA (Kellgren–Lawrence, grade of 3 or 4) underwent treatment with ultrasound-guided injection of micro-fractured adipose tissue. Micro-fractured fat was obtained using a minimal manipulation technique in a closed system (Lipogems), without the addition of enzymes or any other additives. The study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months following this procedure.
When compared with baseline, significant improvements were noted in the mean values of NPRS, FXN, and LEAS at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved at 6 weeks and 12 months. In particular, the average KSS score improved from 74 to 82, the FXN score improved from 65 to 76, and the LEAS score improved from 36 to 47. These values were significantly greater than the previously published minimal clinically important difference described for KSS and FXN in patients who underwent total knee arthroplasty for primary OA. No serious adverse events were reported. The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option for patients with refractory, severe (grade 3 or 4) knee OA.
This study demonstrated significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
Continue to: Knee OA is...
Knee OA is a chronic disease that affects all races, genders, and ages, but it is most prevalent in obese and elderly people. Worldwide, arthritis is considered to be the fourth leading cause of disability.1 In developing and developed countries, knee OA may cause a significant decline in the quality of life for individuals >65 years due to joint pain and disability.1 Nonoperative treatment can be successful in patients with mild to moderate arthritis with pain.
Current treatment options for knee OA, including physical therapy and anti-inflammatory drugs, aim to remedy the symptoms, but they do little to treat the underlying causes of knee OA pain. When a patient presents with advanced arthritis of the knee as confirmed by radiographic findings (classified as Kellgren–Lawrence grade of 3 or 4), the standard approach has been a total knee arthroplasty (TKA) after the patient has failed conservative treatment. The annual rate of total knee replacement in the United States has doubled since 2000, especially in those 45 – 65 years.2 The total number of procedures performed each year now exceeds 640,000, at a total annual cost of about $10.2 billion.2 Multiple studies show that TKA has favorable outcomes in pain relief and functional improvement in patients >60 years when evaluated at a follow-up of 10 years after surgery.2
However, some patients are hesitant to proceed with surgery due to fear of surgical pain and procedural complications. The known complications include deep vein thrombosis, pulmonary embolism, nerve injury, and infection. In addition, up to 20% of patients continue to complain of pain following a total knee replacement.3 Finally, in the young population (<50 years), there are concerns related to the potential need of revision knee surgery in the future.3
Alternative treatments for knee OA have recently emerged, including the use of platelet-rich plasma (PRP). A recent meta-analysis that included 10 randomized controlled trials with a total of 1069 patients demonstrated that, compared with hyaluronic acid and saline, intra-articular PRP injection may have more benefits in pain relief and functional improvement in patients with symptomatic knee OA at 1-year post-injection.4 Another smaller study examined patients who had experienced mild knee OA (Kellgren–Lawrence grade <3) for an average of 14 months. Each patient underwent magnetic resonance imaging for the evaluation of joint damage and then received a single PRP injection. The patients were assessed at regular intervals, with improvement in pain lasting up to 12 months.5
Additional orthobiologic options include the use of bone marrow and adipose-derived stem cell (ASC) injections for a variety of knee conditions, including knee OA. Mesenchymal stem cells (MSCs) are multipotent cells that have been used for the treatment of OA in clinical trials because of their regeneration potential and anti-inflammatory effects.6 Bone marrow stem cells (BMSCs) were first used to repair cartilage damage in humans in 1998. However, BMSCs had particular challenges, including low stem cell yield, pain, and possible morbidities during bone marrow aspiration. An alternative is ASCs, which may be more suitable clinically because of the high stem cell yield from lipoaspirates, faster cell proliferation, and less discomfort and morbidities during the harvesting procedure.7 In addition, these adult stem cells can contribute to the chondrogenic, osteogenic, adipogenic, myogenic, and neurogenic lineages.8 One study demonstrated that the contents of cartilage glycosaminoglycans significantly increased in specific areas of a knee joint treated with ASCs.9,10 This increased glycosaminoglycan content in hyaline cartilage may explain the observed visual analog score (VAS) improvement and clinical results. Other studies suggest that the chondrogenic action of ASCs may depend more on regenerative signaling by activated perivascular cells and signaling of trophic and paracrine mediators, such as vascular endothelial growth factor.9,10 Finally, the mechanism of action may include providing volume, support, cushioning, and filling of soft tissue defects.11
The Lipogems method and device, approved by the U.S. Food and Drug Administration, is used to harvest ASCs, cleanse, and micro-fracture adipose tissue while maintaining the perivascular niche that contains pericytes. The purpose of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with severe refractory knee OA.
Continue to: This report details...
STUDY PRESENTATION
This report details the outcome of an IRB-approved study of 17 subjects with 26 symptomatic knees with a history of knee OA (Kellgren–Lawrence grade of 3 or 4) diagnosed by a radiograph. Patient demographics are described in the Table.
TABLE. Patient Demographics | |
Male n (%) | 10 (58.8) |
Age, mean ± SD (range) | 68.27 ± 7.43 |
BMI, mean ± SD (range) | 28.98 ± 4.50 |
Kellgren–Lawrence grade 3 (n) | 7 |
Kellgren–Lawrence grade 4 (n) | 19 |
Abbreviation: BMI, body mass index.
The study patients were evaluated by an orthopedic surgeon, Mitchell Sheinkop, who commonly performs total joint replacement in his practice and considers potential patients as candidates for TKA. These patients presented with a Kellgren-Lawrence grade of 3 or 4 knee OA, and all had significant pain that was refractory to conservative treatment, which included medications, physical therapy, and injections. The study patients were offered the Lipogems procedure as an alternative to TKA. Following this procedure, the study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months. The 1989 KSS12 was used for this study. Adverse reactions were also monitored throughout the study period.
METHODS
After obtaining informed consent, the subjects were taken into the operating room, moved to the procedure table, and placed in the prone position for aspiration. After scrubbing with Betadine and draping, 1 mL of lidocaine was used to anesthetize the skin, and a pre-prepared preparation of lidocaine, epinephrine, and sterile saline was infused into the subcutaneous tissue. The micro-fragmented adipose tissue was obtained with minimal manipulation using Lipogems, a closed system using mild mechanical forces and reduction filters. The system processes the lipoaspirate without the addition of enzymes or any other additives. The final product consists of adipose tissue clusters with preserved vascular stromal niche of approximately 500 microns. The lipoaspirate was processed in the same room via a closed system. During the processing, the subject’s puncture wounds were dressed. The knee injection site was prepped with a Betadine swab and DuraPrep. Then, Lipogems was injected intra-articularly under ultrasound guidance.
After the completion of the injection, manual range of motion was administered to the treated joint. The subject was then transferred to the recovery room where vital signs were monitored. Post-procedure instructions were reviewed with the patient by the study staff. The subject was instructed to use an assistive device and avoid weight-bearing for 48 hours and maintain the activities of daily living to a minimum on the day of the procedure. Non-weight-bearing for 48 hours was recommended for reducing discomfort to avoid the use of opioids. Nonsteroidal anti-inflammatory drugs, alcohol, and marijuana must be avoided for 4 weeks after the procedure. Pretreatment and post-treatment outcomes were collected using the NPRS, the 100-point KSS with its FXN, and the LEAS at 6 weeks, 6 months, and 12 months after this procedure. The 1989 KSS12 was used for this study since the same scale was used for previous TKA procedures by our authors, allowing for future comparisons of results.
STATISTICAL ANALYSIS
Mean and standard deviation were used to estimate central tendency and variability. Outcome measures were analyzed using the t test, with the pairwise t test was used for paired and subsequent measurements of the same patient or a knee. All analyses were performed with significance set at P <.05. The minimal clinically important difference (MCID) in patients who underwent TKA for primary OA was between 5.3 and 5.9 for KSS, while the MCID for FXN was between 6.1 and 6.4.13 These values were referenced for our analysis.
Continue to: No significant adverse...
RESULTS
No significant adverse events were reported in the subjects of this study. Common minor adverse events included pain and swelling, which generally resolved in 48 to 72 hours after the procedure.

Compared with baseline, significant improvements were noted in the mean values of NPRS (Figure 1) at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved from baseline at 6 weeks and 12 months (Figure 2). Significant improvements were also noted in the mean values of FXN (Figure 3) and the mean LEAS significantly improved from baseline at 6 weeks and 6 months (Figure 4).

DISCUSSION
Knee OA is a disabling condition that affects a substantial proportion of the aging population. The current treatment methods do little to address the degenerative environment of the joint, which includes cytokines such as IL-1 and IL-2. Orthobiologic agents have been used recently to address these issues, which include PRP and MSCs from various sources, including bone marrow and adipose tissue.

A recent meta-analysis conducted by Cui and colleagues14 evaluated 18 studies of MSC treatment for knee OA with a total of 565 participants (226 males and 339 females). The duration from the onset of knee pain to registration in each study ranged from 3 months to ≥7 years. The follow-up period was 3 months -24 months. The majority of studies recruited patients with knee OA with a severity grade of 1-4 on the K-L scale; K-L grades 1 and 2 and grades 3 and 4 were defined as early OA and advanced OA, respectively. The results suggested that MSC treatment significantly improved pain and functional status, relative to the baseline evaluations in knee OA, and the beneficial effect was maintained for 2 years after treatment. Furthermore, the treatment effectiveness was not reduced over time.14

Included in the abovementioned meta-analysis were 2 papers by Koh and colleagues in 2012 and 2013 on the use of AMSCs for the treatment of OA. 15,16 The first study included 18 patients whose adipose tissue was harvested from the inner side of the infrapatellar fat pad via a skin incision after arthroscopic debridement. The cells were centrifuged and injected into the patient’s knee the same day. The results showed a significant reduction of pain and an increased quality of life for all patients, and a positive correlation was found between the number of cells injected and pain improvements. The authors concluded that AMSCs were a valid cell source for treating cartilage damage.15
In their second study, Koh and colleagues reported their results of treating 30 elderly patients with OA (≥65 years), who had failed conventional treatment, using intra-articular injections of AMSCs.16 This patient population is important since OA most commonly occurs in the elderly population. Patients underwent arthroscopic lavage and cartilage evaluation before receiving an injection of AMSCs delivered in PRP. The authors demonstrated that AMSC therapy for elderly patients with mild to moderate OA was an effective treatment resulting in reduction of pain and regeneration of cartilage.16
In another study, Adriani and colleagues17 performed autologous percutaneous fat injection from January 2012 to March 2015 for the treatment of knee OA. Their 30 patients (12 males and 18 females; mean age of 63.3 years; mean body mass index of 25.1) had stable or progressive knee OA for at least 12 months, no other injection treatments during the previous 12 months, and no prior knee surgeries. The patients were evaluated at baseline and 1 week and at 1, 3, 6, and 12 months after treatment using the NPRS and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as outcome measures. The average VAS was 7.7 at baseline and improved to 4.3 at 3-month follow-up; however, a slight deterioration (VAS 5.0) was noted at 1 year. Total WOMAC score was 89.9 at baseline, 68.6 at 3 months, and 73.2 at 12-month follow-up.17
Continue to: The results of...
The results of this study demonstrated significant improvements in pain, quality of life, and function at 12 months after ultrasound-guided injection of ASCs in patients with severe knee OA. Significant improvement that was noted at 6 weeks was maintained through 12 months after the treatment. Improvement was noted in all scales, including the NPRS, the KSS, and the FXN beginning at 3 months and continuing through 12 months. The LEAS was statistically significant through 6 months after the treatment but not significant at 12 months. No serious adverse events were recorded.
In a study by Lee and colleagues,13 the MCID was described for KSS and FXN in patients who underwent TKA for primary OA. This is the minimal change in a scoring measure that is perceived by the patient to be beneficial or harmful. The MCID for KSS was noted to be between 5.3 and 5.9, while the MCID for FXN was between 6.1 and 6.4.13 In our study, the KSS score improved from an average of 74.0 at baseline to 79.6 at 6 months and 81.6 at 12 months (a difference of 5.6 and 7.6; P = .18 and.014, respectively). The FXN improved from an average of 65.4 at baseline to 75.2 at 6 months and 76.4 at 12 months (a difference of 9.9 and 11; P = .041 and.014, respectively). Therefore, a clinically important difference of KSS and FXN scores was noted at both 6 and 12 months.
The technique used in this study provides autologous, minimally manipulated, fat graft performed in a short time (60-90 minutes), without expansion and/or enzymatic treatment. In addition, the harvesting and the injection of stem cells on the same day is a simple, office-based procedure, and compliant with the U. S. Food and Drug Administration regulations.18 The cost of the procedure averages $3500.
A study limitation is that it is a case series with relatively small numbers and not a randomized controlled study. Therefore, a placebo effect may play a role in our results. Further study with a larger number of patients and randomized controlled studies would be beneficial to support the findings of this study.
CONCLUSION
The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option in patients with refractory severe (grade 3 or 4) knee OA. This study showed significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
ABSTRACT
The aim of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with refractory knee osteoarthritis (OA). A total of 17 subjects (26 knees) with a median age of 72 years (range: 54-78 years) and a history of knee OA (Kellgren–Lawrence, grade of 3 or 4) underwent treatment with ultrasound-guided injection of micro-fractured adipose tissue. Micro-fractured fat was obtained using a minimal manipulation technique in a closed system (Lipogems), without the addition of enzymes or any other additives. The study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months following this procedure.
When compared with baseline, significant improvements were noted in the mean values of NPRS, FXN, and LEAS at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved at 6 weeks and 12 months. In particular, the average KSS score improved from 74 to 82, the FXN score improved from 65 to 76, and the LEAS score improved from 36 to 47. These values were significantly greater than the previously published minimal clinically important difference described for KSS and FXN in patients who underwent total knee arthroplasty for primary OA. No serious adverse events were reported. The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option for patients with refractory, severe (grade 3 or 4) knee OA.
This study demonstrated significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
Continue to: Knee OA is...
Knee OA is a chronic disease that affects all races, genders, and ages, but it is most prevalent in obese and elderly people. Worldwide, arthritis is considered to be the fourth leading cause of disability.1 In developing and developed countries, knee OA may cause a significant decline in the quality of life for individuals >65 years due to joint pain and disability.1 Nonoperative treatment can be successful in patients with mild to moderate arthritis with pain.
Current treatment options for knee OA, including physical therapy and anti-inflammatory drugs, aim to remedy the symptoms, but they do little to treat the underlying causes of knee OA pain. When a patient presents with advanced arthritis of the knee as confirmed by radiographic findings (classified as Kellgren–Lawrence grade of 3 or 4), the standard approach has been a total knee arthroplasty (TKA) after the patient has failed conservative treatment. The annual rate of total knee replacement in the United States has doubled since 2000, especially in those 45 – 65 years.2 The total number of procedures performed each year now exceeds 640,000, at a total annual cost of about $10.2 billion.2 Multiple studies show that TKA has favorable outcomes in pain relief and functional improvement in patients >60 years when evaluated at a follow-up of 10 years after surgery.2
However, some patients are hesitant to proceed with surgery due to fear of surgical pain and procedural complications. The known complications include deep vein thrombosis, pulmonary embolism, nerve injury, and infection. In addition, up to 20% of patients continue to complain of pain following a total knee replacement.3 Finally, in the young population (<50 years), there are concerns related to the potential need of revision knee surgery in the future.3
Alternative treatments for knee OA have recently emerged, including the use of platelet-rich plasma (PRP). A recent meta-analysis that included 10 randomized controlled trials with a total of 1069 patients demonstrated that, compared with hyaluronic acid and saline, intra-articular PRP injection may have more benefits in pain relief and functional improvement in patients with symptomatic knee OA at 1-year post-injection.4 Another smaller study examined patients who had experienced mild knee OA (Kellgren–Lawrence grade <3) for an average of 14 months. Each patient underwent magnetic resonance imaging for the evaluation of joint damage and then received a single PRP injection. The patients were assessed at regular intervals, with improvement in pain lasting up to 12 months.5
Additional orthobiologic options include the use of bone marrow and adipose-derived stem cell (ASC) injections for a variety of knee conditions, including knee OA. Mesenchymal stem cells (MSCs) are multipotent cells that have been used for the treatment of OA in clinical trials because of their regeneration potential and anti-inflammatory effects.6 Bone marrow stem cells (BMSCs) were first used to repair cartilage damage in humans in 1998. However, BMSCs had particular challenges, including low stem cell yield, pain, and possible morbidities during bone marrow aspiration. An alternative is ASCs, which may be more suitable clinically because of the high stem cell yield from lipoaspirates, faster cell proliferation, and less discomfort and morbidities during the harvesting procedure.7 In addition, these adult stem cells can contribute to the chondrogenic, osteogenic, adipogenic, myogenic, and neurogenic lineages.8 One study demonstrated that the contents of cartilage glycosaminoglycans significantly increased in specific areas of a knee joint treated with ASCs.9,10 This increased glycosaminoglycan content in hyaline cartilage may explain the observed visual analog score (VAS) improvement and clinical results. Other studies suggest that the chondrogenic action of ASCs may depend more on regenerative signaling by activated perivascular cells and signaling of trophic and paracrine mediators, such as vascular endothelial growth factor.9,10 Finally, the mechanism of action may include providing volume, support, cushioning, and filling of soft tissue defects.11
The Lipogems method and device, approved by the U.S. Food and Drug Administration, is used to harvest ASCs, cleanse, and micro-fracture adipose tissue while maintaining the perivascular niche that contains pericytes. The purpose of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with severe refractory knee OA.
Continue to: This report details...
STUDY PRESENTATION
This report details the outcome of an IRB-approved study of 17 subjects with 26 symptomatic knees with a history of knee OA (Kellgren–Lawrence grade of 3 or 4) diagnosed by a radiograph. Patient demographics are described in the Table.
TABLE. Patient Demographics | |
Male n (%) | 10 (58.8) |
Age, mean ± SD (range) | 68.27 ± 7.43 |
BMI, mean ± SD (range) | 28.98 ± 4.50 |
Kellgren–Lawrence grade 3 (n) | 7 |
Kellgren–Lawrence grade 4 (n) | 19 |
Abbreviation: BMI, body mass index.
The study patients were evaluated by an orthopedic surgeon, Mitchell Sheinkop, who commonly performs total joint replacement in his practice and considers potential patients as candidates for TKA. These patients presented with a Kellgren-Lawrence grade of 3 or 4 knee OA, and all had significant pain that was refractory to conservative treatment, which included medications, physical therapy, and injections. The study patients were offered the Lipogems procedure as an alternative to TKA. Following this procedure, the study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months. The 1989 KSS12 was used for this study. Adverse reactions were also monitored throughout the study period.
METHODS
After obtaining informed consent, the subjects were taken into the operating room, moved to the procedure table, and placed in the prone position for aspiration. After scrubbing with Betadine and draping, 1 mL of lidocaine was used to anesthetize the skin, and a pre-prepared preparation of lidocaine, epinephrine, and sterile saline was infused into the subcutaneous tissue. The micro-fragmented adipose tissue was obtained with minimal manipulation using Lipogems, a closed system using mild mechanical forces and reduction filters. The system processes the lipoaspirate without the addition of enzymes or any other additives. The final product consists of adipose tissue clusters with preserved vascular stromal niche of approximately 500 microns. The lipoaspirate was processed in the same room via a closed system. During the processing, the subject’s puncture wounds were dressed. The knee injection site was prepped with a Betadine swab and DuraPrep. Then, Lipogems was injected intra-articularly under ultrasound guidance.
After the completion of the injection, manual range of motion was administered to the treated joint. The subject was then transferred to the recovery room where vital signs were monitored. Post-procedure instructions were reviewed with the patient by the study staff. The subject was instructed to use an assistive device and avoid weight-bearing for 48 hours and maintain the activities of daily living to a minimum on the day of the procedure. Non-weight-bearing for 48 hours was recommended for reducing discomfort to avoid the use of opioids. Nonsteroidal anti-inflammatory drugs, alcohol, and marijuana must be avoided for 4 weeks after the procedure. Pretreatment and post-treatment outcomes were collected using the NPRS, the 100-point KSS with its FXN, and the LEAS at 6 weeks, 6 months, and 12 months after this procedure. The 1989 KSS12 was used for this study since the same scale was used for previous TKA procedures by our authors, allowing for future comparisons of results.
STATISTICAL ANALYSIS
Mean and standard deviation were used to estimate central tendency and variability. Outcome measures were analyzed using the t test, with the pairwise t test was used for paired and subsequent measurements of the same patient or a knee. All analyses were performed with significance set at P <.05. The minimal clinically important difference (MCID) in patients who underwent TKA for primary OA was between 5.3 and 5.9 for KSS, while the MCID for FXN was between 6.1 and 6.4.13 These values were referenced for our analysis.
Continue to: No significant adverse...
RESULTS
No significant adverse events were reported in the subjects of this study. Common minor adverse events included pain and swelling, which generally resolved in 48 to 72 hours after the procedure.

Compared with baseline, significant improvements were noted in the mean values of NPRS (Figure 1) at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved from baseline at 6 weeks and 12 months (Figure 2). Significant improvements were also noted in the mean values of FXN (Figure 3) and the mean LEAS significantly improved from baseline at 6 weeks and 6 months (Figure 4).

DISCUSSION
Knee OA is a disabling condition that affects a substantial proportion of the aging population. The current treatment methods do little to address the degenerative environment of the joint, which includes cytokines such as IL-1 and IL-2. Orthobiologic agents have been used recently to address these issues, which include PRP and MSCs from various sources, including bone marrow and adipose tissue.

A recent meta-analysis conducted by Cui and colleagues14 evaluated 18 studies of MSC treatment for knee OA with a total of 565 participants (226 males and 339 females). The duration from the onset of knee pain to registration in each study ranged from 3 months to ≥7 years. The follow-up period was 3 months -24 months. The majority of studies recruited patients with knee OA with a severity grade of 1-4 on the K-L scale; K-L grades 1 and 2 and grades 3 and 4 were defined as early OA and advanced OA, respectively. The results suggested that MSC treatment significantly improved pain and functional status, relative to the baseline evaluations in knee OA, and the beneficial effect was maintained for 2 years after treatment. Furthermore, the treatment effectiveness was not reduced over time.14

Included in the abovementioned meta-analysis were 2 papers by Koh and colleagues in 2012 and 2013 on the use of AMSCs for the treatment of OA. 15,16 The first study included 18 patients whose adipose tissue was harvested from the inner side of the infrapatellar fat pad via a skin incision after arthroscopic debridement. The cells were centrifuged and injected into the patient’s knee the same day. The results showed a significant reduction of pain and an increased quality of life for all patients, and a positive correlation was found between the number of cells injected and pain improvements. The authors concluded that AMSCs were a valid cell source for treating cartilage damage.15
In their second study, Koh and colleagues reported their results of treating 30 elderly patients with OA (≥65 years), who had failed conventional treatment, using intra-articular injections of AMSCs.16 This patient population is important since OA most commonly occurs in the elderly population. Patients underwent arthroscopic lavage and cartilage evaluation before receiving an injection of AMSCs delivered in PRP. The authors demonstrated that AMSC therapy for elderly patients with mild to moderate OA was an effective treatment resulting in reduction of pain and regeneration of cartilage.16
In another study, Adriani and colleagues17 performed autologous percutaneous fat injection from January 2012 to March 2015 for the treatment of knee OA. Their 30 patients (12 males and 18 females; mean age of 63.3 years; mean body mass index of 25.1) had stable or progressive knee OA for at least 12 months, no other injection treatments during the previous 12 months, and no prior knee surgeries. The patients were evaluated at baseline and 1 week and at 1, 3, 6, and 12 months after treatment using the NPRS and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as outcome measures. The average VAS was 7.7 at baseline and improved to 4.3 at 3-month follow-up; however, a slight deterioration (VAS 5.0) was noted at 1 year. Total WOMAC score was 89.9 at baseline, 68.6 at 3 months, and 73.2 at 12-month follow-up.17
Continue to: The results of...
The results of this study demonstrated significant improvements in pain, quality of life, and function at 12 months after ultrasound-guided injection of ASCs in patients with severe knee OA. Significant improvement that was noted at 6 weeks was maintained through 12 months after the treatment. Improvement was noted in all scales, including the NPRS, the KSS, and the FXN beginning at 3 months and continuing through 12 months. The LEAS was statistically significant through 6 months after the treatment but not significant at 12 months. No serious adverse events were recorded.
In a study by Lee and colleagues,13 the MCID was described for KSS and FXN in patients who underwent TKA for primary OA. This is the minimal change in a scoring measure that is perceived by the patient to be beneficial or harmful. The MCID for KSS was noted to be between 5.3 and 5.9, while the MCID for FXN was between 6.1 and 6.4.13 In our study, the KSS score improved from an average of 74.0 at baseline to 79.6 at 6 months and 81.6 at 12 months (a difference of 5.6 and 7.6; P = .18 and.014, respectively). The FXN improved from an average of 65.4 at baseline to 75.2 at 6 months and 76.4 at 12 months (a difference of 9.9 and 11; P = .041 and.014, respectively). Therefore, a clinically important difference of KSS and FXN scores was noted at both 6 and 12 months.
The technique used in this study provides autologous, minimally manipulated, fat graft performed in a short time (60-90 minutes), without expansion and/or enzymatic treatment. In addition, the harvesting and the injection of stem cells on the same day is a simple, office-based procedure, and compliant with the U. S. Food and Drug Administration regulations.18 The cost of the procedure averages $3500.
A study limitation is that it is a case series with relatively small numbers and not a randomized controlled study. Therefore, a placebo effect may play a role in our results. Further study with a larger number of patients and randomized controlled studies would be beneficial to support the findings of this study.
CONCLUSION
The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option in patients with refractory severe (grade 3 or 4) knee OA. This study showed significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
- Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One. 2017;12(4):e0175449.
- Jauregui JJ, Cherian JJ, Pierce TP, Beaver WB, Issa K, Mont MA. Long-Term Survivorship and Clinical Outcomes Following Total Knee Arthroplasty. J Arthroplasty. 2015;30(12):2164-2166.
- Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
- Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy.33(3):659-670.e651.
- Halpern B CS, Rodeo SA, Hayter C, Bogner E, Potter HG, Nguyen J. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013 May;23.
- Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307-1316.
- Tang Y, Pan ZY, Zou Y, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017.
- Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. Journal of cellular biochemistry. 2006;99(5):1285-1297.
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16(5):203-209.
- Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. A J Physiol Renal Physiol. 2007;292(5):F1626-1635.
- Mestak O, Sukop A, Hsueh YS, et al. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J Surg Oncol. 2014;12:178.
- Insall JN DL, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989 Nov;(248):13-4.
- Lee WC, Kwan YH, Chong HC, Yeo SJ. The minimal clinically important difference for Knee Society Clinical Rating System after total knee arthroplasty for primary osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy. 2016.
- Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp Ther Med. 2016;12(5):3390-3400.
- Koh Y-GC, Yun-Jin. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902-907.
- Koh Y-GC, Yun-Jin. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
- Adriani E. MM, et al. Percutaneous Fat Transfer to Treat Knee Osteoarthritis Symptoms: Preliminary Results. Joints. 2017.
- Bianchi F, Maioli M, Leonardi E, et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces From Human Lipoaspirates. Cell Transplantation. 2013;22(11):2063-2077
- Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One. 2017;12(4):e0175449.
- Jauregui JJ, Cherian JJ, Pierce TP, Beaver WB, Issa K, Mont MA. Long-Term Survivorship and Clinical Outcomes Following Total Knee Arthroplasty. J Arthroplasty. 2015;30(12):2164-2166.
- Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
- Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy.33(3):659-670.e651.
- Halpern B CS, Rodeo SA, Hayter C, Bogner E, Potter HG, Nguyen J. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013 May;23.
- Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307-1316.
- Tang Y, Pan ZY, Zou Y, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017.
- Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. Journal of cellular biochemistry. 2006;99(5):1285-1297.
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16(5):203-209.
- Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. A J Physiol Renal Physiol. 2007;292(5):F1626-1635.
- Mestak O, Sukop A, Hsueh YS, et al. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J Surg Oncol. 2014;12:178.
- Insall JN DL, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989 Nov;(248):13-4.
- Lee WC, Kwan YH, Chong HC, Yeo SJ. The minimal clinically important difference for Knee Society Clinical Rating System after total knee arthroplasty for primary osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy. 2016.
- Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp Ther Med. 2016;12(5):3390-3400.
- Koh Y-GC, Yun-Jin. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902-907.
- Koh Y-GC, Yun-Jin. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
- Adriani E. MM, et al. Percutaneous Fat Transfer to Treat Knee Osteoarthritis Symptoms: Preliminary Results. Joints. 2017.
- Bianchi F, Maioli M, Leonardi E, et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces From Human Lipoaspirates. Cell Transplantation. 2013;22(11):2063-2077
TAKE-HOME POINTS
- Severe knee osteoarthritis causes pain and limits functions in a substantial proportion of the aging population.
- Total knee arthroplasty is often recommended in this group of patients when conservative management has failed.
- Many patients in this group continue to seek a nonsurgical option for this process.
- Autologous, micro-fractured, minimally manipulated adipose tissue is easy to harvest, and injection into a knee joint resulted in significant improvement in pain and function for at least 12 months in this study population.
- This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population.