User login
Magnetic Resonance Imaging Evaluation of the Distal Biceps Tendon
ABSTRACT
Injuries to the distal biceps occur at the tendinous insertion at the radial tuberosity. Distal biceps injuries range from tendinosis to partial tears to non-retracted and retracted complete tears. Acute and chronic complete tears result from a tendinous avulsion at the radial tuberosity. Acute tears result from a strong force exerted on an eccentric biceps contraction, leading to tendon injury.
Distal biceps tendon injuries are uncommon (1.2 per 100,000 patients in one study).1 An underlying degenerative component is involved in all distal biceps tendon tears and tendinosis.2 Partial tears can be caused by the same mechanism or by no particular inciting event.3 Magnetic resonance imaging (MRI) is the optimal imaging modality for distal tendon tears because of its excellent specificity and sensitivity in the detection of complete tears.4,5 Imaging also accurately diagnoses and characterizes partial tears and tendinosis.5 On MRI, fast spin-echo intermediate-weighted and T2-weighted or short tau inversion recovery (STIR) sequences are normally obtained to assess tendon integrity. Along with standard axial and sagittal views, the FABS (flexed elbow, abducted shoulder, supinated forearm) view is an important tool in the diagnosis of distal biceps tendon tears.6 The FABS view is obtained with the patient prone with the shoulder abducted 180° (above the head), with the elbow flexed to 90°, and the forearm supinated. This position allows a longitudinal view of along the entire length of the distal tendon.
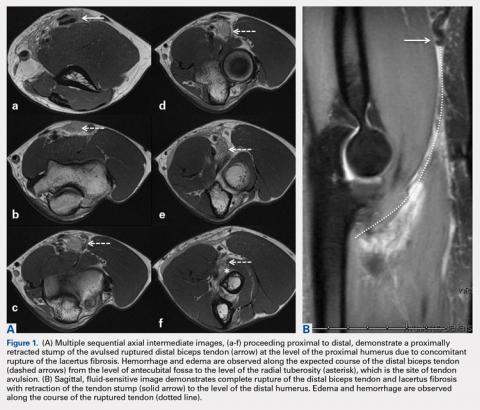
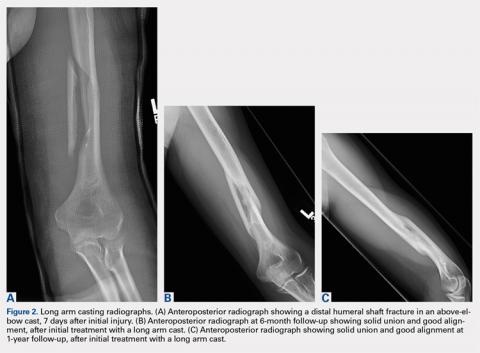
Complete distal biceps tears can usually be diagnosed by history and physical examinations. However, imaging can be helpful when intact brachialis function can compensate for a completely torn tendon. MRI is also useful in the setting of a complete tear to locate the torn tendon stump, and assess the degree of retraction for tendon retrieval7,8 and quality of the tendon stump for repair. For associated rupture of the lacertus, the degree of proximal tendon retraction can be significant (Figures 1A, 1B).
Continue to: Partial distal bicep tears...
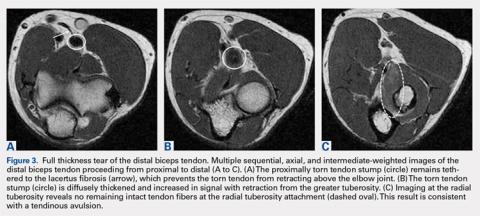
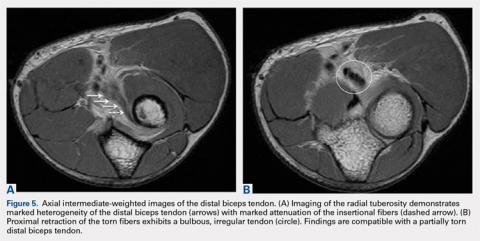
Partial distal bicep tears are characterized on MRI by focal or partial detachment of the tendon at the radial tuberosity with fluid filling the site of the tear. The degree of partial tearing can be assessed on MRI (Figures 5A, 5B).
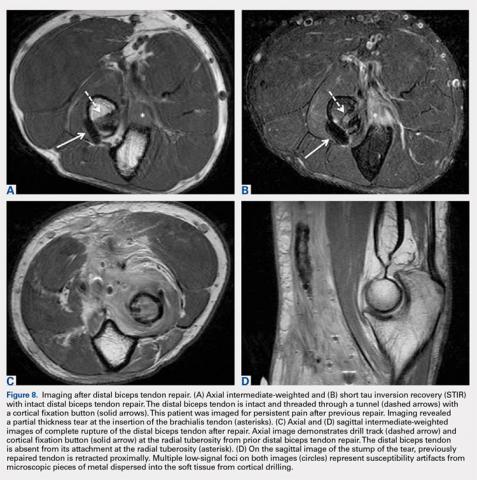
MRI is useful in assessing the distal biceps tendon in the postoperative setting to evaluate the integrity of a repaired tendon. Cortical fixation button technique for repair creates minimal susceptibility artifacts on MRI. Postoperative MRI typically demonstrates a transverse hole drilled through the proximal radius at the site of the tuberosity with a cortical fixation button flush against the posterior radial cortex (Figures 8A-8D).
1. Safran M, Graham S. Distal biceps tendon ruptures. Clin Orthop Relat Res. 2002;404:275-283.
2. Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525. doi:10.2106/00004623-199173100-00009.
3. Frazier M, Boardman M, Westland M, Imbriglia J. Surgical treatment of partial distal biceps tendon ruptures. J Hand Surg Am. 2010;35(7):1111-1114. doi:10.1016/j.jhsa.2010.04.024.
4. Festa A, Mulieri P, Newman J, Spitz D, Leslie B. Effectiveness of magnetic resonance imaging in detecting partial and complete distal biceps tendon rupture. J Hand Surg Am. 2010;35(1):77-83. doi:10.1016/j.jhsa.2009.08.016.
5. O'Driscoll S, Goncalves L, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869. doi:10.1177/0363546507305016.
6. Giuffrè B, Moss M. Optimal positioning for MRI of the distal biceps brachii tendon: flexed abducted supinated view. Am J Roentgenol. 2004;182(4):944-946. doi:10.2214/ajr.182.4.1820944.
7. Falchook F, Zlatkin M, Erbacher G, Moulton J, Bisset G. Murphy B. Rupture of the distal biceps tendon: evaluation with MR imaging. Radiology. 1994;190(3):659-663. doi:10.1148/radiology.190.3.8115606.
8. Fitzgerald S, Curry D, Erickson S, Quinn S, Friedman H. Distal biceps tendon injury: MR imaging diagnosis. Radiology. 1994;191(1):203-206. doi:10.1148/radiology.191.1.8134571.
9. Lehuec J, Zipoli B, Liquois F, Moinard M, Chauveaux D, Le Rebeller A. Distal rupture of the biceps tendon MRI evaluation and surgical repair. J Shoulder Elbow Surg. 1996;5(2):S49.
10. Dirim B, Brouha S, Pretterklieber M, et al. Terminal bifurcation of the biceps brachii muscle and tendon: anatomic considerations and clinical implications. Am J Roentgenol. 2008;191(6):W248-W255. doi:10.2214/AJR.08.1048.
11. Quach T, Jazayeri R, Sherman O, Rosen J. Distal biceps tendon injuries--current treatment options. Bull NYU Hosp Jt Dis. 2010;68(2):103-111.
ABSTRACT
Injuries to the distal biceps occur at the tendinous insertion at the radial tuberosity. Distal biceps injuries range from tendinosis to partial tears to non-retracted and retracted complete tears. Acute and chronic complete tears result from a tendinous avulsion at the radial tuberosity. Acute tears result from a strong force exerted on an eccentric biceps contraction, leading to tendon injury.
Distal biceps tendon injuries are uncommon (1.2 per 100,000 patients in one study).1 An underlying degenerative component is involved in all distal biceps tendon tears and tendinosis.2 Partial tears can be caused by the same mechanism or by no particular inciting event.3 Magnetic resonance imaging (MRI) is the optimal imaging modality for distal tendon tears because of its excellent specificity and sensitivity in the detection of complete tears.4,5 Imaging also accurately diagnoses and characterizes partial tears and tendinosis.5 On MRI, fast spin-echo intermediate-weighted and T2-weighted or short tau inversion recovery (STIR) sequences are normally obtained to assess tendon integrity. Along with standard axial and sagittal views, the FABS (flexed elbow, abducted shoulder, supinated forearm) view is an important tool in the diagnosis of distal biceps tendon tears.6 The FABS view is obtained with the patient prone with the shoulder abducted 180° (above the head), with the elbow flexed to 90°, and the forearm supinated. This position allows a longitudinal view of along the entire length of the distal tendon.
Complete distal biceps tears can usually be diagnosed by history and physical examinations. However, imaging can be helpful when intact brachialis function can compensate for a completely torn tendon. MRI is also useful in the setting of a complete tear to locate the torn tendon stump, and assess the degree of retraction for tendon retrieval7,8 and quality of the tendon stump for repair. For associated rupture of the lacertus, the degree of proximal tendon retraction can be significant (Figures 1A, 1B).
Continue to: Partial distal bicep tears...
Partial distal bicep tears are characterized on MRI by focal or partial detachment of the tendon at the radial tuberosity with fluid filling the site of the tear. The degree of partial tearing can be assessed on MRI (Figures 5A, 5B).
MRI is useful in assessing the distal biceps tendon in the postoperative setting to evaluate the integrity of a repaired tendon. Cortical fixation button technique for repair creates minimal susceptibility artifacts on MRI. Postoperative MRI typically demonstrates a transverse hole drilled through the proximal radius at the site of the tuberosity with a cortical fixation button flush against the posterior radial cortex (Figures 8A-8D).
ABSTRACT
Injuries to the distal biceps occur at the tendinous insertion at the radial tuberosity. Distal biceps injuries range from tendinosis to partial tears to non-retracted and retracted complete tears. Acute and chronic complete tears result from a tendinous avulsion at the radial tuberosity. Acute tears result from a strong force exerted on an eccentric biceps contraction, leading to tendon injury.
Distal biceps tendon injuries are uncommon (1.2 per 100,000 patients in one study).1 An underlying degenerative component is involved in all distal biceps tendon tears and tendinosis.2 Partial tears can be caused by the same mechanism or by no particular inciting event.3 Magnetic resonance imaging (MRI) is the optimal imaging modality for distal tendon tears because of its excellent specificity and sensitivity in the detection of complete tears.4,5 Imaging also accurately diagnoses and characterizes partial tears and tendinosis.5 On MRI, fast spin-echo intermediate-weighted and T2-weighted or short tau inversion recovery (STIR) sequences are normally obtained to assess tendon integrity. Along with standard axial and sagittal views, the FABS (flexed elbow, abducted shoulder, supinated forearm) view is an important tool in the diagnosis of distal biceps tendon tears.6 The FABS view is obtained with the patient prone with the shoulder abducted 180° (above the head), with the elbow flexed to 90°, and the forearm supinated. This position allows a longitudinal view of along the entire length of the distal tendon.
Complete distal biceps tears can usually be diagnosed by history and physical examinations. However, imaging can be helpful when intact brachialis function can compensate for a completely torn tendon. MRI is also useful in the setting of a complete tear to locate the torn tendon stump, and assess the degree of retraction for tendon retrieval7,8 and quality of the tendon stump for repair. For associated rupture of the lacertus, the degree of proximal tendon retraction can be significant (Figures 1A, 1B).
Continue to: Partial distal bicep tears...
Partial distal bicep tears are characterized on MRI by focal or partial detachment of the tendon at the radial tuberosity with fluid filling the site of the tear. The degree of partial tearing can be assessed on MRI (Figures 5A, 5B).
MRI is useful in assessing the distal biceps tendon in the postoperative setting to evaluate the integrity of a repaired tendon. Cortical fixation button technique for repair creates minimal susceptibility artifacts on MRI. Postoperative MRI typically demonstrates a transverse hole drilled through the proximal radius at the site of the tuberosity with a cortical fixation button flush against the posterior radial cortex (Figures 8A-8D).
1. Safran M, Graham S. Distal biceps tendon ruptures. Clin Orthop Relat Res. 2002;404:275-283.
2. Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525. doi:10.2106/00004623-199173100-00009.
3. Frazier M, Boardman M, Westland M, Imbriglia J. Surgical treatment of partial distal biceps tendon ruptures. J Hand Surg Am. 2010;35(7):1111-1114. doi:10.1016/j.jhsa.2010.04.024.
4. Festa A, Mulieri P, Newman J, Spitz D, Leslie B. Effectiveness of magnetic resonance imaging in detecting partial and complete distal biceps tendon rupture. J Hand Surg Am. 2010;35(1):77-83. doi:10.1016/j.jhsa.2009.08.016.
5. O'Driscoll S, Goncalves L, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869. doi:10.1177/0363546507305016.
6. Giuffrè B, Moss M. Optimal positioning for MRI of the distal biceps brachii tendon: flexed abducted supinated view. Am J Roentgenol. 2004;182(4):944-946. doi:10.2214/ajr.182.4.1820944.
7. Falchook F, Zlatkin M, Erbacher G, Moulton J, Bisset G. Murphy B. Rupture of the distal biceps tendon: evaluation with MR imaging. Radiology. 1994;190(3):659-663. doi:10.1148/radiology.190.3.8115606.
8. Fitzgerald S, Curry D, Erickson S, Quinn S, Friedman H. Distal biceps tendon injury: MR imaging diagnosis. Radiology. 1994;191(1):203-206. doi:10.1148/radiology.191.1.8134571.
9. Lehuec J, Zipoli B, Liquois F, Moinard M, Chauveaux D, Le Rebeller A. Distal rupture of the biceps tendon MRI evaluation and surgical repair. J Shoulder Elbow Surg. 1996;5(2):S49.
10. Dirim B, Brouha S, Pretterklieber M, et al. Terminal bifurcation of the biceps brachii muscle and tendon: anatomic considerations and clinical implications. Am J Roentgenol. 2008;191(6):W248-W255. doi:10.2214/AJR.08.1048.
11. Quach T, Jazayeri R, Sherman O, Rosen J. Distal biceps tendon injuries--current treatment options. Bull NYU Hosp Jt Dis. 2010;68(2):103-111.
1. Safran M, Graham S. Distal biceps tendon ruptures. Clin Orthop Relat Res. 2002;404:275-283.
2. Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525. doi:10.2106/00004623-199173100-00009.
3. Frazier M, Boardman M, Westland M, Imbriglia J. Surgical treatment of partial distal biceps tendon ruptures. J Hand Surg Am. 2010;35(7):1111-1114. doi:10.1016/j.jhsa.2010.04.024.
4. Festa A, Mulieri P, Newman J, Spitz D, Leslie B. Effectiveness of magnetic resonance imaging in detecting partial and complete distal biceps tendon rupture. J Hand Surg Am. 2010;35(1):77-83. doi:10.1016/j.jhsa.2009.08.016.
5. O'Driscoll S, Goncalves L, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869. doi:10.1177/0363546507305016.
6. Giuffrè B, Moss M. Optimal positioning for MRI of the distal biceps brachii tendon: flexed abducted supinated view. Am J Roentgenol. 2004;182(4):944-946. doi:10.2214/ajr.182.4.1820944.
7. Falchook F, Zlatkin M, Erbacher G, Moulton J, Bisset G. Murphy B. Rupture of the distal biceps tendon: evaluation with MR imaging. Radiology. 1994;190(3):659-663. doi:10.1148/radiology.190.3.8115606.
8. Fitzgerald S, Curry D, Erickson S, Quinn S, Friedman H. Distal biceps tendon injury: MR imaging diagnosis. Radiology. 1994;191(1):203-206. doi:10.1148/radiology.191.1.8134571.
9. Lehuec J, Zipoli B, Liquois F, Moinard M, Chauveaux D, Le Rebeller A. Distal rupture of the biceps tendon MRI evaluation and surgical repair. J Shoulder Elbow Surg. 1996;5(2):S49.
10. Dirim B, Brouha S, Pretterklieber M, et al. Terminal bifurcation of the biceps brachii muscle and tendon: anatomic considerations and clinical implications. Am J Roentgenol. 2008;191(6):W248-W255. doi:10.2214/AJR.08.1048.
11. Quach T, Jazayeri R, Sherman O, Rosen J. Distal biceps tendon injuries--current treatment options. Bull NYU Hosp Jt Dis. 2010;68(2):103-111.
TAKE-HOME POINTS
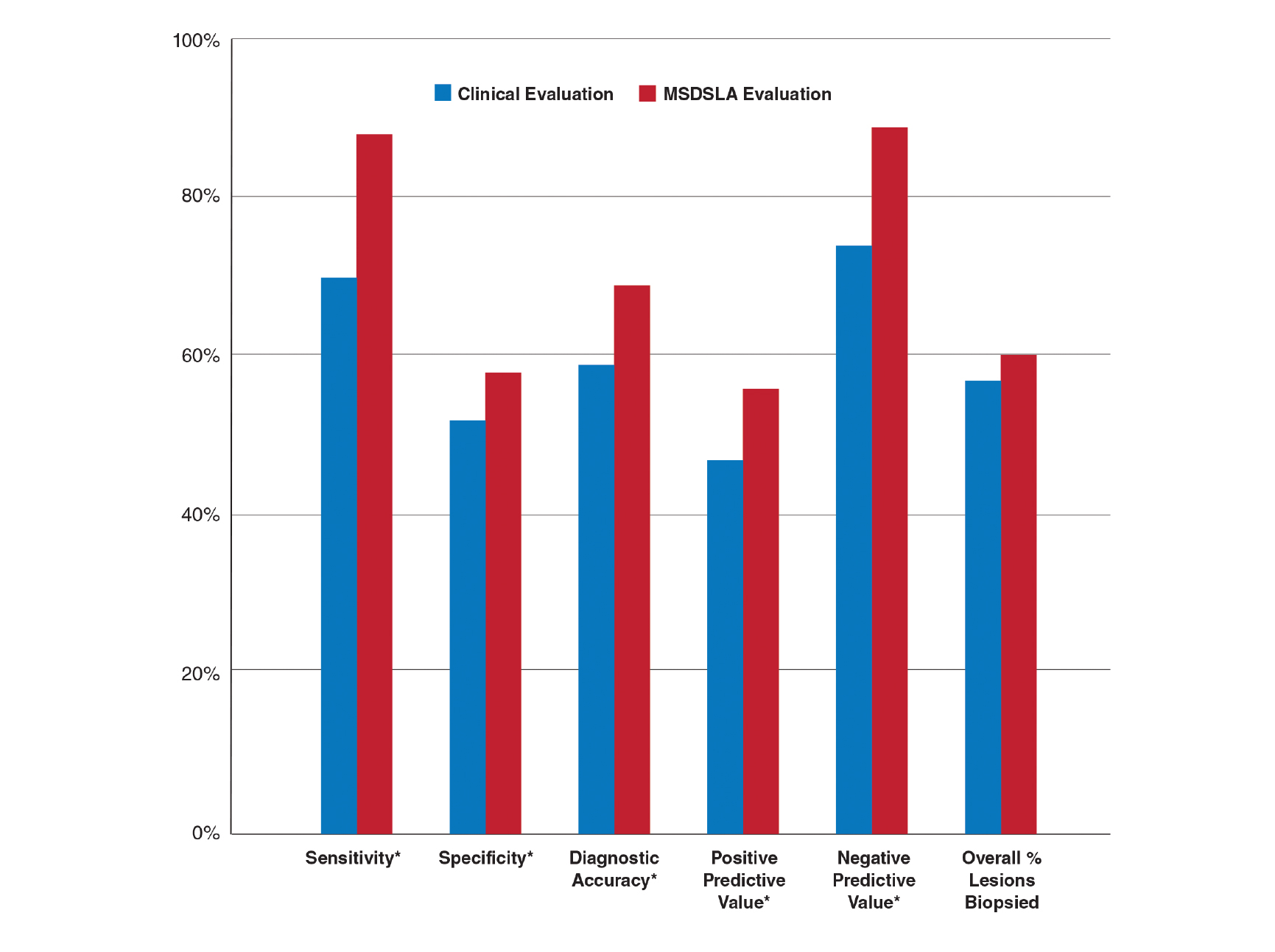
- There are a variety of injuries to the distal biceps tendon.
- Injuries vary from tendinosis to full thickness, retracted tears.
- The degree of retraction of full thickness tears depends on the integrity of the lacertus fibrosis.
- The FABS view allows for MRI of the entire length of the distal biceps tendon.
- MRI is the most useful imaging modality to determine the integrity of the postoperative biceps tendon.
Radiographic Study of Humeral Stem in Shoulder Arthroplasty After Lesser Tuberosity Osteotomy or Subscapularis Tenotomy
ABSTRACT
Lesser tuberosity osteotomy (LTO) and subscapularis tenotomy (ST) are used for takedown of the subscapularis during shoulder arthroplasty. LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis. However, humeral stem subsidence and loosening may be greater when osteotomy is performed, which may compromise functional outcomes. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique.
During the surgical approach for total shoulder arthroplasty (TSA), the subscapularis is taken down for adequate exposure to the glenohumeral joint. Various methods are available for taking down the subscapularis, including lesser tuberosity osteotomy (LTO) and a subscapularis tenotomy (ST). LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis secondary to bone-to-bone healing. One concern, however, is that humeral stem subsidence may be greater when an osteotomy is performed owing to compromise of metaphyseal cortical bone, which may compromise functional outcomes. The humeral stem design may also influence subsidence when metaphyseal bone proximally is compromised. This is a concern in both metaphyseal and diaphyseal fitting stems. Metaphyseal collars on diaphyseal fitting stems rely on adequate bone stock in the metaphysis to provide the additional support needed. Also, posterior subluxation remains a challenge in shoulder arthroplasty. The integrity of the subscapularis is important in prevention of posterior subluxation.1 To our knowledge, no study to date has directly compared differences in humeral stem subsidence, loosening, or posterior subluxation between LTO and ST techniques with any humeral stem design. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique. We also hypothesize that no difference in posterior subluxation exists between LTO and ST techniques.
MATERIALS AND METHODS
INCLUSION CRITERIA
Consecutive patients with a minimum of 12 months of radiographic follow-up were selected from 2007 to 2010 after TSA was performed by 1 of the senior authors (Dr. Miller and Dr. Voloshin). Study patients underwent primary TSA for primary osteoarthritis or rheumatoid arthritis.
EXCLUSION CRITERIA
Patients were excluded if they underwent TSA for posttraumatic glenohumeral arthritis, hemiarthroplasty, or osteonecrosis. Patients were also excluded if a rotator cuff tear was discovered intraoperatively or if they had a history of a rotator cuff repair. Additional exclusion criteria included postoperative trauma to the operative shoulder, postoperative infection, extensive documentation of chronic pain, and underlying neurologic disorder (eg, Parkinson disease, dystonia). Patients with a history of diabetes mellitus were not excluded.
SURGICAL TECHNIQUE
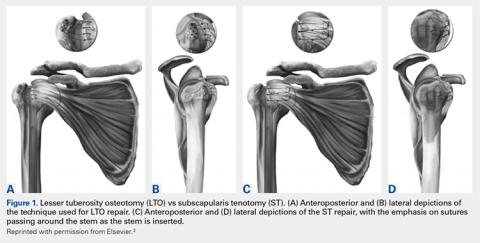
All patients underwent TSA via a deltopectoral approach in a modified beach chair position. Biceps tendons were tenodesed at the level of the pectoralis major. All patients received the same proximal collar press-fit implant (Bigliani-Flatow; Zimmer Biomet). These stems provide rotational stability in the metaphyseal segment via fins, vertical stability with the proximal collar, and distal fixation via an interference fit. All parts of the procedure were performed in similar fashion with the exception of ST vs LTO (Figures 1A-1D).
Continue to: LTO was performed as the primary...
LESSER TUBEROSITY OSTEOTOMY
LTO was performed as the primary or preferred technique of 1 surgeon. After completion of the biceps tenodesis, the lesser tuberosity is reflected off with the subscapularis intact using an osteotome. After placement of the press-fit humeral stem, the LTO is repaired using No. 5 Ethibond Excel sutures (Ethicon) passed through previously created bone tunnels in the greater tuberosity. These sutures are tied over metal buttons over the lateral cortex of the greater tuberosity. Last, the lateral corner of the rotator interval is repaired using a single No. 2 FiberWire (Arthrex).2
SUBSCAPULARIS TENOTOMY
ST is the preferred surgical technique of the second surgeon. After a biceps tenodesis, the subscapularis tendon is released from the lesser tuberosity at the margin of the bicipital groove. Through careful dissection, a single flap including the underlying capsule is created and reflected medially to the level of the coracoid. After placement of the press-fit humeral stem and humeral head, the subscapularis is repaired back in place through previous bone tunnels and with a No. 5 Ethibond Excel suture under the appropriate tension. Then, the lateral corner of the rotator interval is closed using a single No. 2 Ethibond Excel suture in a figure-of-eight fashion.2
RADIOGRAPHIC ANALYSIS
The primary variables analyzed were subsidence and loosening. Additional variables, including humeral-acromial distance (HAD) and subluxation index, were also analyzed to assess for any additional impact caused by subsidence or loosening.3 All radiographic measurements were taken from the Grashey (true anteroposterior) view, except subluxation index, which was calculated using the axillary view. All radiographic measurements were completed by 3 independent reviewers. All radiographs were completed in a consistent manner according to postoperative protocols.
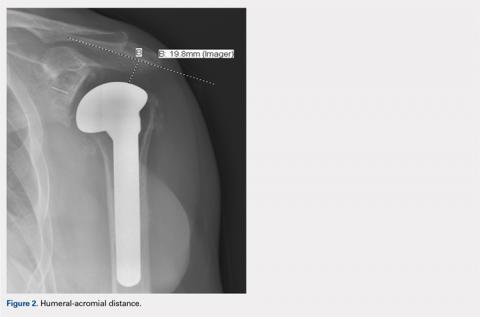
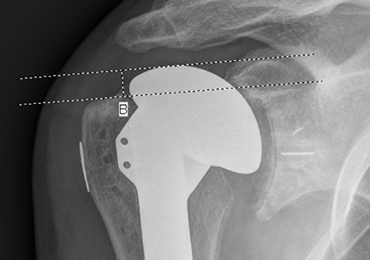
HAD was measured preoperatively, immediately postoperatively, and at final follow-up at a minimum of 1 year. The HAD was measured from the lowest point on the acromion to the humerus using a perpendicular line (Figure 2).
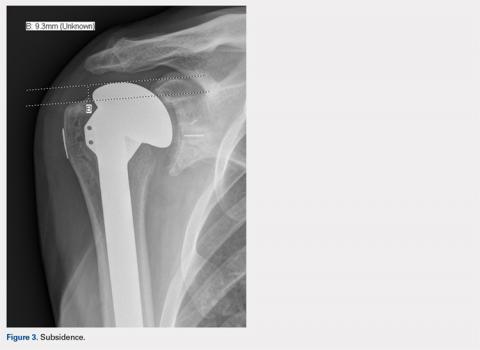
Subsidence of the prosthesis was calculated by determining the difference between immediate postoperative heights of the prosthesis in comparison to the value of the final follow-up films. To calculate the height, 2 lines were drawn, 1 line was drawn perpendicular to the top of the prosthetic head and 1 perpendicular to the top of the greater tuberosity (Figure 3).
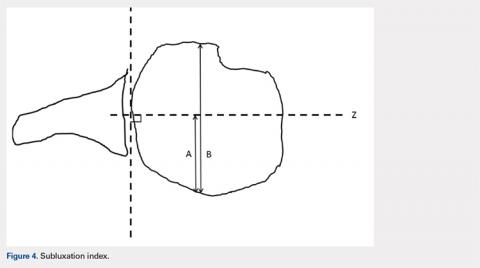
Continue to: Posterior subluxation is indicated...
Posterior subluxation is indicated by a value >65%, a centered head is between 35% and 65%, and anterior subluxation is indicated by a value <35% (Figure 4).3
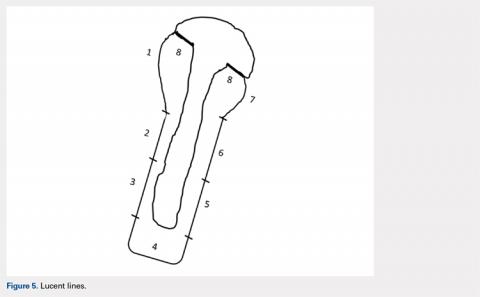
The humeral stems were evaluated for loosening by assessing for lucency on final radiographic follow-up films. These were evaluated in a zonal fashion as demonstrated by Sanchez-Sotelo and colleagues4 and in Figure 5.
FUNCTIONAL OUTCOME EVALUATION
Before clinical evaluation, each study patient completed the Western Ontario Osteoarthritis of the Shoulder (WOOS) index; the Disabilities of the Hand, Arm and Shoulder (DASH) questionnaire, and the pain and function sections of the Constant score. The functional outcomes scores were captured postoperatively from October to November 2011. The WOOS is a validated outcome measure specific to osteoarthritis of the shoulder and has been used in prior studies evaluating outcomes of TSA.5-7 Previous studies have determined that the minimal clinically important difference for the WOOS score is 15 on a normalized 0 to 100 scale (100 being the best). The DASH score is a validated outcome measure for disorders of the upper extremity but is not specific to osteoarthritis of the shoulder.8 The Constant score is a validated outcome measure for a number of shoulder disorders, including TSA.9,10
STATISTICAL ANALYSIS
Statistical analyses were completed by a trained biostatistician. A power analysis was calculated using the noninferiority test to determine if adequate data had been obtained for this study. This was calculated by using previously accepted data demonstrating a statistically significant difference for subsidence and HAD. The data from these studies were used to make assumptions regarding accepted standard deviations and noninferiority margins, as calculated from the mean values of the 2 groups analyzed in each respective study.4,11 This analysis demonstrated power of 0.97 and 0.85 for the subsidence and HAD, respectively, given the current sample sizes. Intraclass coefficients were calculated to evaluate the measurements obtained during the radiographic analysis to determine the interrater agreement. Two samples’ t tests were calculated for the variables analyzed, along with P values and means.
RESULTS
DEMOGRAPHICS
A total of 51 consecutive patients were retrospectively selected for analysis. Of these, 16 patients were excluded from the study because they had <9 months of radiographic follow-up and were unavailable for further follow-up evaluation. Of the remaining 35 patients available for analysis, 4 patients had bilateral TSA, providing 39 shoulders for evaluation. Demographic characteristics of the study cohort are reported in Table 1.
| Table 1. Demographic Characteristics | |||
| Tenotomy (n = 24) | Osteotomy (n = 15) | P-value | |
| Age | 68.2 [7.4] | 70.2 [7.1] | 0.46 |
| Follow-up | 20.6 [11.5] | 18.5 [6.25] | 0.94 |
| Females | 7 (29%) | 6 (40%) | 0.58 |
| Dominant shoulder | 14 (58%) | 8 (53%) | 0.81 |
| Primary Diagnosis | |||
| Osteoarthritis | 22 (92%) | 15 (100%) | |
| Rheumatoid arthritis | 2 (8%) | 0 (0%) |
Fifteen patients underwent LTO, and 24 underwent ST. One patient underwent a tenotomy of the right shoulder and LTO of the left shoulder. Three LTOs were performed by the surgeon who primarily performed ST, owing to potential benefits of LTO. He eventually returned to his preferred technique of ST because of surgeon preference. Three ST procedures were completed by the surgeon who typically performed LTO at the start of the series prior to establishing LTO as his preferred technique. There was no significant difference between the study populations in terms of age, follow-up, male-to-female ratio, hand dominance, and primary diagnosis of osteoarthritis vs rheumatoid arthritis.
Continue to: There was no significant difference...
RADIOGRAPHIC DATA
There was no significant difference in preoperative HAD between the LTO and ST groups (9.5 ± 2.4 mm vs 10.9 ± 2.7 mm, P = .11). The immediate postoperative HAD was statistically significant between the LTO and ST groups (11.9 ± 3.7 mm vs 15.9 ± 4.5 mm, P = .005). There was as statistically significant difference noted in the final follow-up films between the LTO and ST groups (11.8 ± 3.2 mm vs 14.5 ± 3.9 mm, P = .025) (Table 2).
Table 2. Radiographic Data | |||||
Humeral Acromial Distance | |||||
| LTO | ST | P-Value | ||
Preoperative, mm | 9.5 | [2.4] | 10.9 | [2.7] | 0.11 |
Postoperative, mm | 11.9 | [3.7] | 15.9 | [4.5] | 0.005 |
Final follow-up, mm | 11.8 | [3.2] | 14.5 | [3.9] | 0.025 |
Subsidence | |||||
| LTO | ST | P-Value | ||
Subsidence, mm | 2.8 | [3.1] | 2.5 | [3.1] | 0.72 |
Subluxation Index | |||||
| LTO | ST | P-Value | ||
Preoperative, % | 0.55 | [0.06] | 0.54 | [0.07] | 0.45 |
Postoperative, % | 0.55 | [0.09] | 0.48 | [0.05] | 0.015 |
Lucent Lines | |||||
| LTO | ST | P-Value | ||
Lines >2 mm, % | 0.00 | 0.08 | 0.51 | ||
Abbreviations: LTO, lesser tuberosity osteotomy; ST, subscapularis tenotomy.
There were no statistically significant differences found in subsidence between LTO and ST groups at final follow-up (2.8 mm ± 3.1 mm vs 2.5 mm ± 3.1 mm, P = .72) (Table 2). No statistically significant difference was noted in the subluxation index between the LTO and ST groups (0.55% ± .06% vs 0.54% ± 0.07%, P = .45), but there was a statistically significant difference noted postoperatively between the LTO and ST groups (0.55% ± 0.09% vs .48% ± 0.05%, P = .015) (Table 2).
Two stems were noted to have lucent lines >2 mm, both within the ST cohort. Each had 1 stem zone >2 mm, 1 in zone 7, and 1 in zone 4. No statistically significant difference was identified between the LTO and ST groups (0/15 vs 2/24, P = .51) (Table 2).
FUNCTIONAL OUTCOMES
Study patients were evaluated using functional outcome scores, including the Constant, WOOS, and DASH scores (Table 3).
| Table 3. Functional Data | |||||
| LTO | ST | P-Value | |||
| WOOS index | 93.3 | [5.3] | 81.5 | [20.8] | 0.013 |
| DASH score | 8.4 | [6.6] | 13.8 | [4.9] | 0.13 |
| Constant score | 83.3 | [9.1] | 81.8 | [10.1] | 0.64 |
Abbreviations: DASH, disabilities of the arm, shoulder and hand; WOOS, Western Ontario Osteoarthritis of the Shoulder.
No statistically significant differences were noted in the DASH scores (8.4 ± 6.6 vs 13.8 ± 4.9, P = .13) or Constant scores (83.3 ± 9.1 vs 81.8 ± 10.1, P = .64) between the LTO and ST cohorts. There was a statistically significant difference between the WOOS scores (93.3 ± 5.3 vs 81.5 ± 20.8, P = .013). Because separate radiographic reviews were done by 3 independent personnel at 3 different times, it was important to ensure agreement among the reviewers. This was compared using the intraclass correlation coefficients. In the statistical analysis completed, the intraclass coefficients showed the 3 reviewers agreed with each other throughout the radiographic analysis (Table 4).
| Table 4. Testing Agreement: ICC | ||||
| ICC | CI, 2.5% | CI, 97.5% | ||
| HAD | Preoperative | 0.4451 | 0.2202 | 0.6443 |
| Postoperative | 0.6997 | 0.4836 | 0.834 | |
| Final follow-up | 0.5575 | 0.3592 | 0.7218 | |
| Subsidence | 0.6863 | 0.5349 | 0.807 | |
| SI | Preoperative | 0.3087 | 0.1061 | 0.5213 |
| Final follow-up | 0.5364 | 0.299 | 0.7186 |
Abbreviations: CI, confidence interval; HAD, humeral acromial distance; ICC, intraclass correlation coefficient; SI, subluxation index.
DISCUSSION
At final follow-up, we identified no statistically significant difference between the LTO and ST patients in subsidence, lucent lines >2 mm, or functional outcomes (Constant and DASH scores) in patients who underwent TSA with the same proximal collar press-fit humeral stem. In regard to the functional outcome scores, although the WOOS score was statistically significant (P = .013) between the LTO and ST cohorts, we do not feel that this is clinically relevant because it does not reach the minimal clinically important difference threshold of 15 points.8
A statistically significant difference was noted in postoperative subluxation index but was not clinically relevant, because the values between the LTO and ST groups (0.55 vs 0.48) still showed a centered humeral head. Gerber and colleagues3 discussed using a value of 0.65 as a measure of posterior humeral head subluxation, whereas Walch and colleagues12 defined posterior humeral head subluxation as a value >0.55. On the basis of these numbers, the values obtained in this study demonstrated that the postoperative values were still centered on the glenoid, and therefore were not clinically significant.3,12
Continue to: In regard to HAD, there...
In regard to HAD, there was a statistically significant difference noted postoperatively (P = .005) and at final follow-up (P = .025) between the LTO and ST cohorts. Saupe and colleagues13 demonstrated that a HAD <7 mm was considered abnormal and reflected subacromial space narrowing. The values noted in the LTO and ST patients on postoperative and final follow-up radiographs were statistically significant (Table 2), but not clinically relevant because both were >7 mm. A potential source for the variation in HAD may be due to X-ray position and angle.
Studies have shown a concern regarding the integrity of the subscapularis after tenotomy or peel used in TSA with abnormal subscapularis function.14,15 Miller and colleagues15 reported 41 patients, nearly two-thirds, of whom described subscapularis dysfunction. Those authors’ response to the poor clinical outcomes was to remove a fleck of bone with the tendon to achieve “bone-to-bone” healing.14 Gerber and colleagues16 reported on a series of patients using LTO and repair in TSA with 75% and 89% intact subscapularis function on clinical testing.16 Studies by Qureshi and colleagues17 and Scalise and colleagues18 showed similar results after LTO. Biomechanical studies have shown mixed results. Ponce and colleagues19 showed biomechanically superior results for LTO in comparison to the various repair techniques for ST. In another study, Giuseffi and colleagues20 showed no difference in LTO vs ST during biomechanical testing. In response to the increased concern regarding subscapularis integrity, Caplan and colleagues21 reported on 45 arthroplasties in 43 patients with improved postoperative testing with intact subscapularis testing in 90% to 100% of patients. A level 1 randomized control trial conducted by Lapner and colleagues22 did not demonstrate any clear clinical advantage of LTO vs ST. Controversy still exists regarding which is the preferred technique for TSA.
Sanchez-Sotelo and colleagues4 evaluated uncemented humeral components in 72 patients who underwent TSA. They found a humeral component was at risk for loosening if a radiolucent line ≥2 mm was present in at least 3 radiographic zones. They also evaluated tilt or subsidence by measurement and whether the components were observed to have changed. Their measured values correlated with their observed values. That study provided a benchmark for evaluation of loosening and subsidence used during this study.4 Although radiographic follow-up is limited in this study, we feel that any potential subsidence secondary to use of the LTO technique would be radiographically apparent at 1 year. There were 16 patients without adequate radiographic follow-up included in the study. However, we feel that this was not a large concern, because the study was adequately powered with the patients available to determine a difference based on subsidence.
CONCLUSION
We found no difference in subsidence, lucent lines >2 mm, posterior subluxation, and the Constant and DASH functional outcome scores when we compared TSA performed by a LTO with an ST technique with proximal collar press-fit humeral stem. These data cannot be extrapolated to metaphyseal fit stems, which may exhibit different settling characteristics in the setting of the LTO technique.
This paper will be judged for the Resident Writer’s Award.
1. Blasier R, Soslowsky L, Malicky D, Palmer M. Posterior glenohumeral subluxation: Active and passive stabilization in a biomechanical model. J Bone Joint Surg Am. 1997;79-A(3):433-440.
2. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317. doi:10.1016/j.jse.2013.12.009.
3. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510. doi:10.1016/j.jse.2009.03.003.
4. Sanchez-Sotelo J, Wright TW, O'Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplasty. 2001;16(2):180-187.
5. Litchfield RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthrtitis of the shoulder: A prospective, randomized, double-blind clinical trial-A JOINTs Canada Project. J Shoulder Elbow Surg. 2013;20(4):529-536. doi:10.1016/j.jse.2011.01.041.
6. Lo IK, Griffin S, Kirkley A. The development of a disease specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778. doi:10.1053/joca.2001.0474
7. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality of life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185. doi:10.2106/JBJS.D.02198
8. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602-608. doi:10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
9. Constant CR, Gerber C, Emery RJ, Sojbjerg JO, Gohlke F, Boileau P. A review of the constant score: Modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17(2):355-361. doi:10.1016/j.jse.2007.06.022.
10. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
11. Mayerhoefer ME, Breitenseher MJ, Wurnig C, Roposch A. Shoulder impingement: Relationship of clinical symptoms and imaging criteria. Clin J Sport Med. 2009;19(2):83-89. doi:10.1097/JSM.0b013e318198e2e3.
12. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasy. 1999;14(6):756-760.
13. Saupe N, Pfirmann CW, Schmid MR, et al. Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006;187(2):376-382. doi:10.2214/AJR.05.0435.
14. Jackson J, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090. doi:10.1016/j.jse.2010.04.001.
15. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34. doi:10.1067/mse.2003.128195.
16. Gerber C, Yian EH, Pfirrmann AW, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745. doi:10.2106/JBJS.D.02788.
17. Qureshi S, Hsiao A, Klug RA, Lee E, Braman J, Flatow EL. Subscapularis function after total shoulder replacement: results with lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2008;17(1): 68-72. doi:10.1016/j.jse.2007.04.018.
18. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634. doi:10.2106/JBJS.G.01461.
19. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87 Suppl 2:1-8.
20. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095. doi:10.1016/j.jse.2011.07.008.
21. Caplan JL, Whitfield W, Nevasier RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196. doi:10.1016/j.jse.2008.10.019.
22. Lapner PLC, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of LTO to subscapularis peel in shoulder arthroplasty. J Bone Joint Surg Am. 2012;94(24):2239-2246. doi:10.2106/JBJS.K.01365.
ABSTRACT
Lesser tuberosity osteotomy (LTO) and subscapularis tenotomy (ST) are used for takedown of the subscapularis during shoulder arthroplasty. LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis. However, humeral stem subsidence and loosening may be greater when osteotomy is performed, which may compromise functional outcomes. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique.
During the surgical approach for total shoulder arthroplasty (TSA), the subscapularis is taken down for adequate exposure to the glenohumeral joint. Various methods are available for taking down the subscapularis, including lesser tuberosity osteotomy (LTO) and a subscapularis tenotomy (ST). LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis secondary to bone-to-bone healing. One concern, however, is that humeral stem subsidence may be greater when an osteotomy is performed owing to compromise of metaphyseal cortical bone, which may compromise functional outcomes. The humeral stem design may also influence subsidence when metaphyseal bone proximally is compromised. This is a concern in both metaphyseal and diaphyseal fitting stems. Metaphyseal collars on diaphyseal fitting stems rely on adequate bone stock in the metaphysis to provide the additional support needed. Also, posterior subluxation remains a challenge in shoulder arthroplasty. The integrity of the subscapularis is important in prevention of posterior subluxation.1 To our knowledge, no study to date has directly compared differences in humeral stem subsidence, loosening, or posterior subluxation between LTO and ST techniques with any humeral stem design. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique. We also hypothesize that no difference in posterior subluxation exists between LTO and ST techniques.
MATERIALS AND METHODS
INCLUSION CRITERIA
Consecutive patients with a minimum of 12 months of radiographic follow-up were selected from 2007 to 2010 after TSA was performed by 1 of the senior authors (Dr. Miller and Dr. Voloshin). Study patients underwent primary TSA for primary osteoarthritis or rheumatoid arthritis.
EXCLUSION CRITERIA
Patients were excluded if they underwent TSA for posttraumatic glenohumeral arthritis, hemiarthroplasty, or osteonecrosis. Patients were also excluded if a rotator cuff tear was discovered intraoperatively or if they had a history of a rotator cuff repair. Additional exclusion criteria included postoperative trauma to the operative shoulder, postoperative infection, extensive documentation of chronic pain, and underlying neurologic disorder (eg, Parkinson disease, dystonia). Patients with a history of diabetes mellitus were not excluded.
SURGICAL TECHNIQUE
All patients underwent TSA via a deltopectoral approach in a modified beach chair position. Biceps tendons were tenodesed at the level of the pectoralis major. All patients received the same proximal collar press-fit implant (Bigliani-Flatow; Zimmer Biomet). These stems provide rotational stability in the metaphyseal segment via fins, vertical stability with the proximal collar, and distal fixation via an interference fit. All parts of the procedure were performed in similar fashion with the exception of ST vs LTO (Figures 1A-1D).
Continue to: LTO was performed as the primary...
LESSER TUBEROSITY OSTEOTOMY
LTO was performed as the primary or preferred technique of 1 surgeon. After completion of the biceps tenodesis, the lesser tuberosity is reflected off with the subscapularis intact using an osteotome. After placement of the press-fit humeral stem, the LTO is repaired using No. 5 Ethibond Excel sutures (Ethicon) passed through previously created bone tunnels in the greater tuberosity. These sutures are tied over metal buttons over the lateral cortex of the greater tuberosity. Last, the lateral corner of the rotator interval is repaired using a single No. 2 FiberWire (Arthrex).2
SUBSCAPULARIS TENOTOMY
ST is the preferred surgical technique of the second surgeon. After a biceps tenodesis, the subscapularis tendon is released from the lesser tuberosity at the margin of the bicipital groove. Through careful dissection, a single flap including the underlying capsule is created and reflected medially to the level of the coracoid. After placement of the press-fit humeral stem and humeral head, the subscapularis is repaired back in place through previous bone tunnels and with a No. 5 Ethibond Excel suture under the appropriate tension. Then, the lateral corner of the rotator interval is closed using a single No. 2 Ethibond Excel suture in a figure-of-eight fashion.2
RADIOGRAPHIC ANALYSIS
The primary variables analyzed were subsidence and loosening. Additional variables, including humeral-acromial distance (HAD) and subluxation index, were also analyzed to assess for any additional impact caused by subsidence or loosening.3 All radiographic measurements were taken from the Grashey (true anteroposterior) view, except subluxation index, which was calculated using the axillary view. All radiographic measurements were completed by 3 independent reviewers. All radiographs were completed in a consistent manner according to postoperative protocols.
HAD was measured preoperatively, immediately postoperatively, and at final follow-up at a minimum of 1 year. The HAD was measured from the lowest point on the acromion to the humerus using a perpendicular line (Figure 2).
Subsidence of the prosthesis was calculated by determining the difference between immediate postoperative heights of the prosthesis in comparison to the value of the final follow-up films. To calculate the height, 2 lines were drawn, 1 line was drawn perpendicular to the top of the prosthetic head and 1 perpendicular to the top of the greater tuberosity (Figure 3).
Continue to: Posterior subluxation is indicated...
Posterior subluxation is indicated by a value >65%, a centered head is between 35% and 65%, and anterior subluxation is indicated by a value <35% (Figure 4).3
The humeral stems were evaluated for loosening by assessing for lucency on final radiographic follow-up films. These were evaluated in a zonal fashion as demonstrated by Sanchez-Sotelo and colleagues4 and in Figure 5.
FUNCTIONAL OUTCOME EVALUATION
Before clinical evaluation, each study patient completed the Western Ontario Osteoarthritis of the Shoulder (WOOS) index; the Disabilities of the Hand, Arm and Shoulder (DASH) questionnaire, and the pain and function sections of the Constant score. The functional outcomes scores were captured postoperatively from October to November 2011. The WOOS is a validated outcome measure specific to osteoarthritis of the shoulder and has been used in prior studies evaluating outcomes of TSA.5-7 Previous studies have determined that the minimal clinically important difference for the WOOS score is 15 on a normalized 0 to 100 scale (100 being the best). The DASH score is a validated outcome measure for disorders of the upper extremity but is not specific to osteoarthritis of the shoulder.8 The Constant score is a validated outcome measure for a number of shoulder disorders, including TSA.9,10
STATISTICAL ANALYSIS
Statistical analyses were completed by a trained biostatistician. A power analysis was calculated using the noninferiority test to determine if adequate data had been obtained for this study. This was calculated by using previously accepted data demonstrating a statistically significant difference for subsidence and HAD. The data from these studies were used to make assumptions regarding accepted standard deviations and noninferiority margins, as calculated from the mean values of the 2 groups analyzed in each respective study.4,11 This analysis demonstrated power of 0.97 and 0.85 for the subsidence and HAD, respectively, given the current sample sizes. Intraclass coefficients were calculated to evaluate the measurements obtained during the radiographic analysis to determine the interrater agreement. Two samples’ t tests were calculated for the variables analyzed, along with P values and means.
RESULTS
DEMOGRAPHICS
A total of 51 consecutive patients were retrospectively selected for analysis. Of these, 16 patients were excluded from the study because they had <9 months of radiographic follow-up and were unavailable for further follow-up evaluation. Of the remaining 35 patients available for analysis, 4 patients had bilateral TSA, providing 39 shoulders for evaluation. Demographic characteristics of the study cohort are reported in Table 1.
| Table 1. Demographic Characteristics | |||
| Tenotomy (n = 24) | Osteotomy (n = 15) | P-value | |
| Age | 68.2 [7.4] | 70.2 [7.1] | 0.46 |
| Follow-up | 20.6 [11.5] | 18.5 [6.25] | 0.94 |
| Females | 7 (29%) | 6 (40%) | 0.58 |
| Dominant shoulder | 14 (58%) | 8 (53%) | 0.81 |
| Primary Diagnosis | |||
| Osteoarthritis | 22 (92%) | 15 (100%) | |
| Rheumatoid arthritis | 2 (8%) | 0 (0%) |
Fifteen patients underwent LTO, and 24 underwent ST. One patient underwent a tenotomy of the right shoulder and LTO of the left shoulder. Three LTOs were performed by the surgeon who primarily performed ST, owing to potential benefits of LTO. He eventually returned to his preferred technique of ST because of surgeon preference. Three ST procedures were completed by the surgeon who typically performed LTO at the start of the series prior to establishing LTO as his preferred technique. There was no significant difference between the study populations in terms of age, follow-up, male-to-female ratio, hand dominance, and primary diagnosis of osteoarthritis vs rheumatoid arthritis.
Continue to: There was no significant difference...
RADIOGRAPHIC DATA
There was no significant difference in preoperative HAD between the LTO and ST groups (9.5 ± 2.4 mm vs 10.9 ± 2.7 mm, P = .11). The immediate postoperative HAD was statistically significant between the LTO and ST groups (11.9 ± 3.7 mm vs 15.9 ± 4.5 mm, P = .005). There was as statistically significant difference noted in the final follow-up films between the LTO and ST groups (11.8 ± 3.2 mm vs 14.5 ± 3.9 mm, P = .025) (Table 2).
Table 2. Radiographic Data | |||||
Humeral Acromial Distance | |||||
| LTO | ST | P-Value | ||
Preoperative, mm | 9.5 | [2.4] | 10.9 | [2.7] | 0.11 |
Postoperative, mm | 11.9 | [3.7] | 15.9 | [4.5] | 0.005 |
Final follow-up, mm | 11.8 | [3.2] | 14.5 | [3.9] | 0.025 |
Subsidence | |||||
| LTO | ST | P-Value | ||
Subsidence, mm | 2.8 | [3.1] | 2.5 | [3.1] | 0.72 |
Subluxation Index | |||||
| LTO | ST | P-Value | ||
Preoperative, % | 0.55 | [0.06] | 0.54 | [0.07] | 0.45 |
Postoperative, % | 0.55 | [0.09] | 0.48 | [0.05] | 0.015 |
Lucent Lines | |||||
| LTO | ST | P-Value | ||
Lines >2 mm, % | 0.00 | 0.08 | 0.51 | ||
Abbreviations: LTO, lesser tuberosity osteotomy; ST, subscapularis tenotomy.
There were no statistically significant differences found in subsidence between LTO and ST groups at final follow-up (2.8 mm ± 3.1 mm vs 2.5 mm ± 3.1 mm, P = .72) (Table 2). No statistically significant difference was noted in the subluxation index between the LTO and ST groups (0.55% ± .06% vs 0.54% ± 0.07%, P = .45), but there was a statistically significant difference noted postoperatively between the LTO and ST groups (0.55% ± 0.09% vs .48% ± 0.05%, P = .015) (Table 2).
Two stems were noted to have lucent lines >2 mm, both within the ST cohort. Each had 1 stem zone >2 mm, 1 in zone 7, and 1 in zone 4. No statistically significant difference was identified between the LTO and ST groups (0/15 vs 2/24, P = .51) (Table 2).
FUNCTIONAL OUTCOMES
Study patients were evaluated using functional outcome scores, including the Constant, WOOS, and DASH scores (Table 3).
| Table 3. Functional Data | |||||
| LTO | ST | P-Value | |||
| WOOS index | 93.3 | [5.3] | 81.5 | [20.8] | 0.013 |
| DASH score | 8.4 | [6.6] | 13.8 | [4.9] | 0.13 |
| Constant score | 83.3 | [9.1] | 81.8 | [10.1] | 0.64 |
Abbreviations: DASH, disabilities of the arm, shoulder and hand; WOOS, Western Ontario Osteoarthritis of the Shoulder.
No statistically significant differences were noted in the DASH scores (8.4 ± 6.6 vs 13.8 ± 4.9, P = .13) or Constant scores (83.3 ± 9.1 vs 81.8 ± 10.1, P = .64) between the LTO and ST cohorts. There was a statistically significant difference between the WOOS scores (93.3 ± 5.3 vs 81.5 ± 20.8, P = .013). Because separate radiographic reviews were done by 3 independent personnel at 3 different times, it was important to ensure agreement among the reviewers. This was compared using the intraclass correlation coefficients. In the statistical analysis completed, the intraclass coefficients showed the 3 reviewers agreed with each other throughout the radiographic analysis (Table 4).
| Table 4. Testing Agreement: ICC | ||||
| ICC | CI, 2.5% | CI, 97.5% | ||
| HAD | Preoperative | 0.4451 | 0.2202 | 0.6443 |
| Postoperative | 0.6997 | 0.4836 | 0.834 | |
| Final follow-up | 0.5575 | 0.3592 | 0.7218 | |
| Subsidence | 0.6863 | 0.5349 | 0.807 | |
| SI | Preoperative | 0.3087 | 0.1061 | 0.5213 |
| Final follow-up | 0.5364 | 0.299 | 0.7186 |
Abbreviations: CI, confidence interval; HAD, humeral acromial distance; ICC, intraclass correlation coefficient; SI, subluxation index.
DISCUSSION
At final follow-up, we identified no statistically significant difference between the LTO and ST patients in subsidence, lucent lines >2 mm, or functional outcomes (Constant and DASH scores) in patients who underwent TSA with the same proximal collar press-fit humeral stem. In regard to the functional outcome scores, although the WOOS score was statistically significant (P = .013) between the LTO and ST cohorts, we do not feel that this is clinically relevant because it does not reach the minimal clinically important difference threshold of 15 points.8
A statistically significant difference was noted in postoperative subluxation index but was not clinically relevant, because the values between the LTO and ST groups (0.55 vs 0.48) still showed a centered humeral head. Gerber and colleagues3 discussed using a value of 0.65 as a measure of posterior humeral head subluxation, whereas Walch and colleagues12 defined posterior humeral head subluxation as a value >0.55. On the basis of these numbers, the values obtained in this study demonstrated that the postoperative values were still centered on the glenoid, and therefore were not clinically significant.3,12
Continue to: In regard to HAD, there...
In regard to HAD, there was a statistically significant difference noted postoperatively (P = .005) and at final follow-up (P = .025) between the LTO and ST cohorts. Saupe and colleagues13 demonstrated that a HAD <7 mm was considered abnormal and reflected subacromial space narrowing. The values noted in the LTO and ST patients on postoperative and final follow-up radiographs were statistically significant (Table 2), but not clinically relevant because both were >7 mm. A potential source for the variation in HAD may be due to X-ray position and angle.
Studies have shown a concern regarding the integrity of the subscapularis after tenotomy or peel used in TSA with abnormal subscapularis function.14,15 Miller and colleagues15 reported 41 patients, nearly two-thirds, of whom described subscapularis dysfunction. Those authors’ response to the poor clinical outcomes was to remove a fleck of bone with the tendon to achieve “bone-to-bone” healing.14 Gerber and colleagues16 reported on a series of patients using LTO and repair in TSA with 75% and 89% intact subscapularis function on clinical testing.16 Studies by Qureshi and colleagues17 and Scalise and colleagues18 showed similar results after LTO. Biomechanical studies have shown mixed results. Ponce and colleagues19 showed biomechanically superior results for LTO in comparison to the various repair techniques for ST. In another study, Giuseffi and colleagues20 showed no difference in LTO vs ST during biomechanical testing. In response to the increased concern regarding subscapularis integrity, Caplan and colleagues21 reported on 45 arthroplasties in 43 patients with improved postoperative testing with intact subscapularis testing in 90% to 100% of patients. A level 1 randomized control trial conducted by Lapner and colleagues22 did not demonstrate any clear clinical advantage of LTO vs ST. Controversy still exists regarding which is the preferred technique for TSA.
Sanchez-Sotelo and colleagues4 evaluated uncemented humeral components in 72 patients who underwent TSA. They found a humeral component was at risk for loosening if a radiolucent line ≥2 mm was present in at least 3 radiographic zones. They also evaluated tilt or subsidence by measurement and whether the components were observed to have changed. Their measured values correlated with their observed values. That study provided a benchmark for evaluation of loosening and subsidence used during this study.4 Although radiographic follow-up is limited in this study, we feel that any potential subsidence secondary to use of the LTO technique would be radiographically apparent at 1 year. There were 16 patients without adequate radiographic follow-up included in the study. However, we feel that this was not a large concern, because the study was adequately powered with the patients available to determine a difference based on subsidence.
CONCLUSION
We found no difference in subsidence, lucent lines >2 mm, posterior subluxation, and the Constant and DASH functional outcome scores when we compared TSA performed by a LTO with an ST technique with proximal collar press-fit humeral stem. These data cannot be extrapolated to metaphyseal fit stems, which may exhibit different settling characteristics in the setting of the LTO technique.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Lesser tuberosity osteotomy (LTO) and subscapularis tenotomy (ST) are used for takedown of the subscapularis during shoulder arthroplasty. LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis. However, humeral stem subsidence and loosening may be greater when osteotomy is performed, which may compromise functional outcomes. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique.
During the surgical approach for total shoulder arthroplasty (TSA), the subscapularis is taken down for adequate exposure to the glenohumeral joint. Various methods are available for taking down the subscapularis, including lesser tuberosity osteotomy (LTO) and a subscapularis tenotomy (ST). LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis secondary to bone-to-bone healing. One concern, however, is that humeral stem subsidence may be greater when an osteotomy is performed owing to compromise of metaphyseal cortical bone, which may compromise functional outcomes. The humeral stem design may also influence subsidence when metaphyseal bone proximally is compromised. This is a concern in both metaphyseal and diaphyseal fitting stems. Metaphyseal collars on diaphyseal fitting stems rely on adequate bone stock in the metaphysis to provide the additional support needed. Also, posterior subluxation remains a challenge in shoulder arthroplasty. The integrity of the subscapularis is important in prevention of posterior subluxation.1 To our knowledge, no study to date has directly compared differences in humeral stem subsidence, loosening, or posterior subluxation between LTO and ST techniques with any humeral stem design. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique. We also hypothesize that no difference in posterior subluxation exists between LTO and ST techniques.
MATERIALS AND METHODS
INCLUSION CRITERIA
Consecutive patients with a minimum of 12 months of radiographic follow-up were selected from 2007 to 2010 after TSA was performed by 1 of the senior authors (Dr. Miller and Dr. Voloshin). Study patients underwent primary TSA for primary osteoarthritis or rheumatoid arthritis.
EXCLUSION CRITERIA
Patients were excluded if they underwent TSA for posttraumatic glenohumeral arthritis, hemiarthroplasty, or osteonecrosis. Patients were also excluded if a rotator cuff tear was discovered intraoperatively or if they had a history of a rotator cuff repair. Additional exclusion criteria included postoperative trauma to the operative shoulder, postoperative infection, extensive documentation of chronic pain, and underlying neurologic disorder (eg, Parkinson disease, dystonia). Patients with a history of diabetes mellitus were not excluded.
SURGICAL TECHNIQUE
All patients underwent TSA via a deltopectoral approach in a modified beach chair position. Biceps tendons were tenodesed at the level of the pectoralis major. All patients received the same proximal collar press-fit implant (Bigliani-Flatow; Zimmer Biomet). These stems provide rotational stability in the metaphyseal segment via fins, vertical stability with the proximal collar, and distal fixation via an interference fit. All parts of the procedure were performed in similar fashion with the exception of ST vs LTO (Figures 1A-1D).
Continue to: LTO was performed as the primary...
LESSER TUBEROSITY OSTEOTOMY
LTO was performed as the primary or preferred technique of 1 surgeon. After completion of the biceps tenodesis, the lesser tuberosity is reflected off with the subscapularis intact using an osteotome. After placement of the press-fit humeral stem, the LTO is repaired using No. 5 Ethibond Excel sutures (Ethicon) passed through previously created bone tunnels in the greater tuberosity. These sutures are tied over metal buttons over the lateral cortex of the greater tuberosity. Last, the lateral corner of the rotator interval is repaired using a single No. 2 FiberWire (Arthrex).2
SUBSCAPULARIS TENOTOMY
ST is the preferred surgical technique of the second surgeon. After a biceps tenodesis, the subscapularis tendon is released from the lesser tuberosity at the margin of the bicipital groove. Through careful dissection, a single flap including the underlying capsule is created and reflected medially to the level of the coracoid. After placement of the press-fit humeral stem and humeral head, the subscapularis is repaired back in place through previous bone tunnels and with a No. 5 Ethibond Excel suture under the appropriate tension. Then, the lateral corner of the rotator interval is closed using a single No. 2 Ethibond Excel suture in a figure-of-eight fashion.2
RADIOGRAPHIC ANALYSIS
The primary variables analyzed were subsidence and loosening. Additional variables, including humeral-acromial distance (HAD) and subluxation index, were also analyzed to assess for any additional impact caused by subsidence or loosening.3 All radiographic measurements were taken from the Grashey (true anteroposterior) view, except subluxation index, which was calculated using the axillary view. All radiographic measurements were completed by 3 independent reviewers. All radiographs were completed in a consistent manner according to postoperative protocols.
HAD was measured preoperatively, immediately postoperatively, and at final follow-up at a minimum of 1 year. The HAD was measured from the lowest point on the acromion to the humerus using a perpendicular line (Figure 2).
Subsidence of the prosthesis was calculated by determining the difference between immediate postoperative heights of the prosthesis in comparison to the value of the final follow-up films. To calculate the height, 2 lines were drawn, 1 line was drawn perpendicular to the top of the prosthetic head and 1 perpendicular to the top of the greater tuberosity (Figure 3).
Continue to: Posterior subluxation is indicated...
Posterior subluxation is indicated by a value >65%, a centered head is between 35% and 65%, and anterior subluxation is indicated by a value <35% (Figure 4).3
The humeral stems were evaluated for loosening by assessing for lucency on final radiographic follow-up films. These were evaluated in a zonal fashion as demonstrated by Sanchez-Sotelo and colleagues4 and in Figure 5.
FUNCTIONAL OUTCOME EVALUATION
Before clinical evaluation, each study patient completed the Western Ontario Osteoarthritis of the Shoulder (WOOS) index; the Disabilities of the Hand, Arm and Shoulder (DASH) questionnaire, and the pain and function sections of the Constant score. The functional outcomes scores were captured postoperatively from October to November 2011. The WOOS is a validated outcome measure specific to osteoarthritis of the shoulder and has been used in prior studies evaluating outcomes of TSA.5-7 Previous studies have determined that the minimal clinically important difference for the WOOS score is 15 on a normalized 0 to 100 scale (100 being the best). The DASH score is a validated outcome measure for disorders of the upper extremity but is not specific to osteoarthritis of the shoulder.8 The Constant score is a validated outcome measure for a number of shoulder disorders, including TSA.9,10
STATISTICAL ANALYSIS
Statistical analyses were completed by a trained biostatistician. A power analysis was calculated using the noninferiority test to determine if adequate data had been obtained for this study. This was calculated by using previously accepted data demonstrating a statistically significant difference for subsidence and HAD. The data from these studies were used to make assumptions regarding accepted standard deviations and noninferiority margins, as calculated from the mean values of the 2 groups analyzed in each respective study.4,11 This analysis demonstrated power of 0.97 and 0.85 for the subsidence and HAD, respectively, given the current sample sizes. Intraclass coefficients were calculated to evaluate the measurements obtained during the radiographic analysis to determine the interrater agreement. Two samples’ t tests were calculated for the variables analyzed, along with P values and means.
RESULTS
DEMOGRAPHICS
A total of 51 consecutive patients were retrospectively selected for analysis. Of these, 16 patients were excluded from the study because they had <9 months of radiographic follow-up and were unavailable for further follow-up evaluation. Of the remaining 35 patients available for analysis, 4 patients had bilateral TSA, providing 39 shoulders for evaluation. Demographic characteristics of the study cohort are reported in Table 1.
| Table 1. Demographic Characteristics | |||
| Tenotomy (n = 24) | Osteotomy (n = 15) | P-value | |
| Age | 68.2 [7.4] | 70.2 [7.1] | 0.46 |
| Follow-up | 20.6 [11.5] | 18.5 [6.25] | 0.94 |
| Females | 7 (29%) | 6 (40%) | 0.58 |
| Dominant shoulder | 14 (58%) | 8 (53%) | 0.81 |
| Primary Diagnosis | |||
| Osteoarthritis | 22 (92%) | 15 (100%) | |
| Rheumatoid arthritis | 2 (8%) | 0 (0%) |
Fifteen patients underwent LTO, and 24 underwent ST. One patient underwent a tenotomy of the right shoulder and LTO of the left shoulder. Three LTOs were performed by the surgeon who primarily performed ST, owing to potential benefits of LTO. He eventually returned to his preferred technique of ST because of surgeon preference. Three ST procedures were completed by the surgeon who typically performed LTO at the start of the series prior to establishing LTO as his preferred technique. There was no significant difference between the study populations in terms of age, follow-up, male-to-female ratio, hand dominance, and primary diagnosis of osteoarthritis vs rheumatoid arthritis.
Continue to: There was no significant difference...
RADIOGRAPHIC DATA
There was no significant difference in preoperative HAD between the LTO and ST groups (9.5 ± 2.4 mm vs 10.9 ± 2.7 mm, P = .11). The immediate postoperative HAD was statistically significant between the LTO and ST groups (11.9 ± 3.7 mm vs 15.9 ± 4.5 mm, P = .005). There was as statistically significant difference noted in the final follow-up films between the LTO and ST groups (11.8 ± 3.2 mm vs 14.5 ± 3.9 mm, P = .025) (Table 2).
Table 2. Radiographic Data | |||||
Humeral Acromial Distance | |||||
| LTO | ST | P-Value | ||
Preoperative, mm | 9.5 | [2.4] | 10.9 | [2.7] | 0.11 |
Postoperative, mm | 11.9 | [3.7] | 15.9 | [4.5] | 0.005 |
Final follow-up, mm | 11.8 | [3.2] | 14.5 | [3.9] | 0.025 |
Subsidence | |||||
| LTO | ST | P-Value | ||
Subsidence, mm | 2.8 | [3.1] | 2.5 | [3.1] | 0.72 |
Subluxation Index | |||||
| LTO | ST | P-Value | ||
Preoperative, % | 0.55 | [0.06] | 0.54 | [0.07] | 0.45 |
Postoperative, % | 0.55 | [0.09] | 0.48 | [0.05] | 0.015 |
Lucent Lines | |||||
| LTO | ST | P-Value | ||
Lines >2 mm, % | 0.00 | 0.08 | 0.51 | ||
Abbreviations: LTO, lesser tuberosity osteotomy; ST, subscapularis tenotomy.
There were no statistically significant differences found in subsidence between LTO and ST groups at final follow-up (2.8 mm ± 3.1 mm vs 2.5 mm ± 3.1 mm, P = .72) (Table 2). No statistically significant difference was noted in the subluxation index between the LTO and ST groups (0.55% ± .06% vs 0.54% ± 0.07%, P = .45), but there was a statistically significant difference noted postoperatively between the LTO and ST groups (0.55% ± 0.09% vs .48% ± 0.05%, P = .015) (Table 2).
Two stems were noted to have lucent lines >2 mm, both within the ST cohort. Each had 1 stem zone >2 mm, 1 in zone 7, and 1 in zone 4. No statistically significant difference was identified between the LTO and ST groups (0/15 vs 2/24, P = .51) (Table 2).
FUNCTIONAL OUTCOMES
Study patients were evaluated using functional outcome scores, including the Constant, WOOS, and DASH scores (Table 3).
| Table 3. Functional Data | |||||
| LTO | ST | P-Value | |||
| WOOS index | 93.3 | [5.3] | 81.5 | [20.8] | 0.013 |
| DASH score | 8.4 | [6.6] | 13.8 | [4.9] | 0.13 |
| Constant score | 83.3 | [9.1] | 81.8 | [10.1] | 0.64 |
Abbreviations: DASH, disabilities of the arm, shoulder and hand; WOOS, Western Ontario Osteoarthritis of the Shoulder.
No statistically significant differences were noted in the DASH scores (8.4 ± 6.6 vs 13.8 ± 4.9, P = .13) or Constant scores (83.3 ± 9.1 vs 81.8 ± 10.1, P = .64) between the LTO and ST cohorts. There was a statistically significant difference between the WOOS scores (93.3 ± 5.3 vs 81.5 ± 20.8, P = .013). Because separate radiographic reviews were done by 3 independent personnel at 3 different times, it was important to ensure agreement among the reviewers. This was compared using the intraclass correlation coefficients. In the statistical analysis completed, the intraclass coefficients showed the 3 reviewers agreed with each other throughout the radiographic analysis (Table 4).
| Table 4. Testing Agreement: ICC | ||||
| ICC | CI, 2.5% | CI, 97.5% | ||
| HAD | Preoperative | 0.4451 | 0.2202 | 0.6443 |
| Postoperative | 0.6997 | 0.4836 | 0.834 | |
| Final follow-up | 0.5575 | 0.3592 | 0.7218 | |
| Subsidence | 0.6863 | 0.5349 | 0.807 | |
| SI | Preoperative | 0.3087 | 0.1061 | 0.5213 |
| Final follow-up | 0.5364 | 0.299 | 0.7186 |
Abbreviations: CI, confidence interval; HAD, humeral acromial distance; ICC, intraclass correlation coefficient; SI, subluxation index.
DISCUSSION
At final follow-up, we identified no statistically significant difference between the LTO and ST patients in subsidence, lucent lines >2 mm, or functional outcomes (Constant and DASH scores) in patients who underwent TSA with the same proximal collar press-fit humeral stem. In regard to the functional outcome scores, although the WOOS score was statistically significant (P = .013) between the LTO and ST cohorts, we do not feel that this is clinically relevant because it does not reach the minimal clinically important difference threshold of 15 points.8
A statistically significant difference was noted in postoperative subluxation index but was not clinically relevant, because the values between the LTO and ST groups (0.55 vs 0.48) still showed a centered humeral head. Gerber and colleagues3 discussed using a value of 0.65 as a measure of posterior humeral head subluxation, whereas Walch and colleagues12 defined posterior humeral head subluxation as a value >0.55. On the basis of these numbers, the values obtained in this study demonstrated that the postoperative values were still centered on the glenoid, and therefore were not clinically significant.3,12
Continue to: In regard to HAD, there...
In regard to HAD, there was a statistically significant difference noted postoperatively (P = .005) and at final follow-up (P = .025) between the LTO and ST cohorts. Saupe and colleagues13 demonstrated that a HAD <7 mm was considered abnormal and reflected subacromial space narrowing. The values noted in the LTO and ST patients on postoperative and final follow-up radiographs were statistically significant (Table 2), but not clinically relevant because both were >7 mm. A potential source for the variation in HAD may be due to X-ray position and angle.
Studies have shown a concern regarding the integrity of the subscapularis after tenotomy or peel used in TSA with abnormal subscapularis function.14,15 Miller and colleagues15 reported 41 patients, nearly two-thirds, of whom described subscapularis dysfunction. Those authors’ response to the poor clinical outcomes was to remove a fleck of bone with the tendon to achieve “bone-to-bone” healing.14 Gerber and colleagues16 reported on a series of patients using LTO and repair in TSA with 75% and 89% intact subscapularis function on clinical testing.16 Studies by Qureshi and colleagues17 and Scalise and colleagues18 showed similar results after LTO. Biomechanical studies have shown mixed results. Ponce and colleagues19 showed biomechanically superior results for LTO in comparison to the various repair techniques for ST. In another study, Giuseffi and colleagues20 showed no difference in LTO vs ST during biomechanical testing. In response to the increased concern regarding subscapularis integrity, Caplan and colleagues21 reported on 45 arthroplasties in 43 patients with improved postoperative testing with intact subscapularis testing in 90% to 100% of patients. A level 1 randomized control trial conducted by Lapner and colleagues22 did not demonstrate any clear clinical advantage of LTO vs ST. Controversy still exists regarding which is the preferred technique for TSA.
Sanchez-Sotelo and colleagues4 evaluated uncemented humeral components in 72 patients who underwent TSA. They found a humeral component was at risk for loosening if a radiolucent line ≥2 mm was present in at least 3 radiographic zones. They also evaluated tilt or subsidence by measurement and whether the components were observed to have changed. Their measured values correlated with their observed values. That study provided a benchmark for evaluation of loosening and subsidence used during this study.4 Although radiographic follow-up is limited in this study, we feel that any potential subsidence secondary to use of the LTO technique would be radiographically apparent at 1 year. There were 16 patients without adequate radiographic follow-up included in the study. However, we feel that this was not a large concern, because the study was adequately powered with the patients available to determine a difference based on subsidence.
CONCLUSION
We found no difference in subsidence, lucent lines >2 mm, posterior subluxation, and the Constant and DASH functional outcome scores when we compared TSA performed by a LTO with an ST technique with proximal collar press-fit humeral stem. These data cannot be extrapolated to metaphyseal fit stems, which may exhibit different settling characteristics in the setting of the LTO technique.
This paper will be judged for the Resident Writer’s Award.
1. Blasier R, Soslowsky L, Malicky D, Palmer M. Posterior glenohumeral subluxation: Active and passive stabilization in a biomechanical model. J Bone Joint Surg Am. 1997;79-A(3):433-440.
2. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317. doi:10.1016/j.jse.2013.12.009.
3. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510. doi:10.1016/j.jse.2009.03.003.
4. Sanchez-Sotelo J, Wright TW, O'Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplasty. 2001;16(2):180-187.
5. Litchfield RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthrtitis of the shoulder: A prospective, randomized, double-blind clinical trial-A JOINTs Canada Project. J Shoulder Elbow Surg. 2013;20(4):529-536. doi:10.1016/j.jse.2011.01.041.
6. Lo IK, Griffin S, Kirkley A. The development of a disease specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778. doi:10.1053/joca.2001.0474
7. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality of life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185. doi:10.2106/JBJS.D.02198
8. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602-608. doi:10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
9. Constant CR, Gerber C, Emery RJ, Sojbjerg JO, Gohlke F, Boileau P. A review of the constant score: Modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17(2):355-361. doi:10.1016/j.jse.2007.06.022.
10. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
11. Mayerhoefer ME, Breitenseher MJ, Wurnig C, Roposch A. Shoulder impingement: Relationship of clinical symptoms and imaging criteria. Clin J Sport Med. 2009;19(2):83-89. doi:10.1097/JSM.0b013e318198e2e3.
12. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasy. 1999;14(6):756-760.
13. Saupe N, Pfirmann CW, Schmid MR, et al. Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006;187(2):376-382. doi:10.2214/AJR.05.0435.
14. Jackson J, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090. doi:10.1016/j.jse.2010.04.001.
15. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34. doi:10.1067/mse.2003.128195.
16. Gerber C, Yian EH, Pfirrmann AW, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745. doi:10.2106/JBJS.D.02788.
17. Qureshi S, Hsiao A, Klug RA, Lee E, Braman J, Flatow EL. Subscapularis function after total shoulder replacement: results with lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2008;17(1): 68-72. doi:10.1016/j.jse.2007.04.018.
18. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634. doi:10.2106/JBJS.G.01461.
19. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87 Suppl 2:1-8.
20. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095. doi:10.1016/j.jse.2011.07.008.
21. Caplan JL, Whitfield W, Nevasier RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196. doi:10.1016/j.jse.2008.10.019.
22. Lapner PLC, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of LTO to subscapularis peel in shoulder arthroplasty. J Bone Joint Surg Am. 2012;94(24):2239-2246. doi:10.2106/JBJS.K.01365.
1. Blasier R, Soslowsky L, Malicky D, Palmer M. Posterior glenohumeral subluxation: Active and passive stabilization in a biomechanical model. J Bone Joint Surg Am. 1997;79-A(3):433-440.
2. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317. doi:10.1016/j.jse.2013.12.009.
3. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510. doi:10.1016/j.jse.2009.03.003.
4. Sanchez-Sotelo J, Wright TW, O'Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplasty. 2001;16(2):180-187.
5. Litchfield RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthrtitis of the shoulder: A prospective, randomized, double-blind clinical trial-A JOINTs Canada Project. J Shoulder Elbow Surg. 2013;20(4):529-536. doi:10.1016/j.jse.2011.01.041.
6. Lo IK, Griffin S, Kirkley A. The development of a disease specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778. doi:10.1053/joca.2001.0474
7. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality of life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185. doi:10.2106/JBJS.D.02198
8. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602-608. doi:10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
9. Constant CR, Gerber C, Emery RJ, Sojbjerg JO, Gohlke F, Boileau P. A review of the constant score: Modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17(2):355-361. doi:10.1016/j.jse.2007.06.022.
10. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
11. Mayerhoefer ME, Breitenseher MJ, Wurnig C, Roposch A. Shoulder impingement: Relationship of clinical symptoms and imaging criteria. Clin J Sport Med. 2009;19(2):83-89. doi:10.1097/JSM.0b013e318198e2e3.
12. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasy. 1999;14(6):756-760.
13. Saupe N, Pfirmann CW, Schmid MR, et al. Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006;187(2):376-382. doi:10.2214/AJR.05.0435.
14. Jackson J, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090. doi:10.1016/j.jse.2010.04.001.
15. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34. doi:10.1067/mse.2003.128195.
16. Gerber C, Yian EH, Pfirrmann AW, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745. doi:10.2106/JBJS.D.02788.
17. Qureshi S, Hsiao A, Klug RA, Lee E, Braman J, Flatow EL. Subscapularis function after total shoulder replacement: results with lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2008;17(1): 68-72. doi:10.1016/j.jse.2007.04.018.
18. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634. doi:10.2106/JBJS.G.01461.
19. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87 Suppl 2:1-8.
20. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095. doi:10.1016/j.jse.2011.07.008.
21. Caplan JL, Whitfield W, Nevasier RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196. doi:10.1016/j.jse.2008.10.019.
22. Lapner PLC, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of LTO to subscapularis peel in shoulder arthroplasty. J Bone Joint Surg Am. 2012;94(24):2239-2246. doi:10.2106/JBJS.K.01365.
TAKE-HOME POINTS
- LTO and ST remain viable options for takedown of the subscapularis.
- No difference exists in subsidence, lucent lines, and posterior subluxation on radiographic evaluation between LTO and ST.
- No clinically significant difference exists between outcome scores of patients with either technique.
- HAD was statistically significant but not clinically relevant between the 2 techniques.
- Results from the study do not apply to metaphyseal fitting stems, only diaphyseal fitting stems.
Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination (FULL)
From 1982 to 2011, the melanoma incidence rate doubled in the US.1 In 2018, an estimated 87,290 cases of melanoma in situ and 91,270 cases of invasive melanoma will be diagnosed in the US, and 9,320 deaths will be attributable to melanoma.2 Early detection of melanoma is critically important to reduce melanoma-related mortality, with 5-year survival rates as high as 97% at stage 1A vs a 20% 5-year survival when there is distant metastasis.2,3 Melanoma is particularly relevant for medical providers working with veterans because melanoma disproportionately affects service members with an incidence rate ratio of 1.62 (95% confidence interval [CI], 1.40-1.86) compared with that of the general population.4
Biopsy is the definitive diagnostic tool for melanoma. Histologic analysis differentiates melanoma from seborrheic keratoses, pigmented nevi, dermatofibromas, and other pigmented lesions that can resemble melanoma on clinical examination. However, biopsy must be used judiciously as unnecessary biopsies contribute to health care costs and leave scars, which can have psychosocial implications. With benign nevi outnumbering melanoma about 2 million to 1, biopsy is indicated once a threshold of suspicion is obtained.5
Dermoscopic Tool
Dermoscopy is a microscopy-based tool to improve noninvasive diagnostic discrimination of skin lesions based on color and structure analysis. Coloration provides an indication of the composition of elements present in the skin with keratin appearing yellow, blood appearing red, and collagen appearing white. Coloration also suggests pigment depth as melanin appears black when located in the stratum corneum, brown when located deeper in the epidermis, and blue when located in the dermis.6 Finally, characteristic histopathologic alterations in the dermoepidermal junction, rete ridges, pigment-containing cells, and/or melanocyte granules that occur in melanoma are recognizable with dermoscopy.6
In 2001, Bafounta and colleagues performed the first meta-analysis on the efficacy of dermoscopy compared with that of clinical evaluation, finding that dermoscopy performed specifically by dermatology-trained clinicians improved the accuracy of identifying melanoma from an odds ratio of 16 (95% CI, 9-31) with naked eye examination to 76 (95% CI, 25-223) with dermoscopy.7
More recently, Terushkin and colleagues demonstrated that diagnosis specificity improves when a general dermatologist is trained in dermoscopic pattern recognition. Naked eye examination produced a benign to malignant ratio (BMR) of 18.4:1, indicating that about 18 of 19 biopsies considered suspicious for melanoma ultimately yielded benign melanocytic lesions. Although the BMR for the general dermatologist experienced an increase after dermoscopy training, the ratio eventually decreased to 7.9:1.8
Dermoscopic Analysis
Some of the common patterns recognized in melanoma are demonstrated in Figures 1 and 2. Figure 1 is a close-up of a patient’s upper back showing a solitary asymmetric melanocytic lesion containing multiple red, brown, black, and blue hues.
Pattern analysis, the dermoscopic interpretation method preferred by pigmented lesion specialists, requires simultaneously assessing numerous lesion patterns that vary depending on body site.10 Alternative dermoscopic algorithms that focus on the most common features of melanoma have been developed to aid practitioners with the interpretation of dermoscopy findings: the 7-point checklist, the Menzies method, the ABCD rule, and the CASH algorithm (Tables 2, 3, 4, and 5).
Argenziano and colleagues developed the 7-point checklist in 1998. Two points are assigned to the lesion for each of the major criteria and 1 point for each minor criteria.
The Menzies method was developed by Menzies and colleagues in 1996. To be classified as melanoma, the pigmented lesion must show an absence of pattern symmetry and color uniformity while simultaneously exhibiting at least one of the following: blue-white veil, multiple brown dots, pseudopods, radial streaming, scarlike depigmentation, peripheral block dots/globules, 5 to 6 colors, multiple blue/gray dots, and a broadened network.12
The ABCD rule is a more technical dermoscopic evaluation algorithm developed in 1994 by Stolz and colleagues. This method yields a numeric value called the total dermoscopic score (TDS) based on Asymmetry, Border pigment pattern, Color variation, and 5 Different structural components.
Henning and colleagues developed the CASH algorithm in 2006 with the intention of assisting less experienced dermoscopy users with lesion evaluation.14 This algorithm tallies points for Color, Architectural disorder, Symmetry, and Homogeneity. One point is attributed to a lesion for each light brown, dark brown, black, red, white, and/or blue region present. Architectural disorder is assigned a point value between 0 and 2, with 0 indicating the absence of or minimal architectural disorder, 1 indicating moderate disorder, and 2 indicating marked disorder. Symmetry is assigned a point value between 0 and 2, with 0 points assigned to a lesion that exhibits biaxial symmetry, 1 point assigned to a lesion that exhibits monoaxial symmetry, and 2 points assigned to a lesion that exhibits biaxial asymmetry. Finally, 1 point is attributed to a lesion for evidence of each of the following: atypical network, dots/globules, streaks/pseudopods, blue-white veil, regression structures, blotches > 10% of the overall lesion size, and polymorphous blood vessels. The lesion in Figure 2 scores 16 points out of the maximum total CASH score of 17. Any lesion scoring 8 or more is suggestive of malignant melanoma.14
Finally, the TADA method was developed by Rogers and colleagues in 2016.15 This method uses sequential questions to evaluate lesions. First, “Does the lesion exhibit clear dermoscopic evidence of an angioma, dermatofibroma, or seborrheic keratosis?” If “yes,” then no additional dermoscopic evaluation is necessary, and it is recommended to monitor the lesion. If the answer to the first question is “no,” then ask, “Does the lesion exhibit architectural disorder?” The presence of architectural disorder is based on an overall impression of the lesion, which includes symmetry with regard to structures and colors. Any lesion deemed to exhibit architectural disorder should be biopsied. If the lesion has no architectural disorder, the third question is, “Does the lesion contain any starburst patterns, blue-black or gray coloration, shiny white structures, negative networks, ulcers or erosions, and/or vessels?” If “yes,” then the lesion should be biopsied. Since the lesion in Figure 2 exhibits marked architectural disorder in terms of symmetry and color, analysis of the lesion with the TADA method would yield a recommendation for biopsy.15
Dermoscopy in Practice
A. Bernard Ackerman, MD, a key figure in the modern era of dermatopathology, wrote an editorial for the Journal of the American Academy of Dermatology in 1985 titled “No one should die of malignant melanoma.” The editorial highlighted that the visual changes associated with melanoma often manifest years prior to malignant invasion and advocated that all physicians should have competence in melanoma detection, specifically mentioning the importance of training primary care physicians (PCPs), dermatologists, and pathologists in this regard.16 This sentiment is equally as true now as it was in 1985.
Naked eye examination paired with an evaluation of patient risk factors for melanoma, including fair skin types, significant sun exposure history, history of sunburn, geographic location, and personal and family history of melanoma, are the foundation of melanoma detection efforts.17 Studies suggest that the triage skills of PCPs could be improved in regard to the evaluation of pigmented lesions. Primary care residents, for instance, did not accurately diagnose 40% of malignant melanoma cases.18,19 Additionally, a meta-analysis demonstrated that PCP accuracy when diagnosing malignant melanoma ranged between 49% and 80%, significantly less than the 85% to 89% exhibited by practicing dermatologists.19 Dermoscopy could be incorporated as an element of the skin examination to enhance lesion discrimination among PCPs, as it has proven use in dermatologic practice.
Dermoscopy is not readily used by PCPs. A survey study of 705 family practitioners in the US performed by Morris and colleagues demonstrated that only 8.3% of participants currently use a dermatoscope to evaluate pigmented lesions.20 The most common barriers to dermoscopy use cited by PCPs in the US include the cost of the dermatoscope, the time required to acquire proficiency, and the lack of financial reimbursement.16 True utilization of dermoscopy among PCPs is higher than this figure suggests due to the number of PCPs who access dermoscopic evaluations via teledermatology. All 21 Veterans Integrated Services Networks of the Veterans Health Administration (VHA) system, for instance, participate in teledermatology and jointly employ more than 1,150 trained telehealth clinical technicians who created a collective 107,000 teledermatology encounters with and without dermoscopy for evaluation by dermatologists in the most recent fiscal year(Martin Weinstock, written communication, October 2017). Nonetheless, it is necessary to determine the contribution that wider utilization of dermoscopy among PCPs would have on melanoma surveillance.
Studies show that dermoscopic algorithms improve the sensitivity while slightly decreasing the specificity of PCPs to detect melanoma compared with that of the naked eye examination. Dolianitis and colleagues demonstrated that a baseline sensitivity of 60.9% for melanoma detection improved to 85.4% with the 7-point checklist, 85.4% with Menzies method, and 77.5% with the ABCD rule, while the baseline specificity of 85.4% moderated to 73.0%, 77.7%, and 80.4%, respectively, among 61 medical practitioners after studying dermoscopy techniques from 2 CDs.21 Westerhoff and colleagues performed a randomized controlled trial with 74 PCPs to determine the effect of a minimal intervention on melanoma diagnostic accuracy. The intervention consisted of providing participants in the experimental group with an atlas of microscopic features common to melanoma to be read at the participants’ leisure, a 1-hour presentation on microscopy, and a 25-questionpractice quiz. Participants randomized to the intervention group improved their diagnostic accuracy from 57.8% to 75.9% with the use of dermoscopy. This group also experiencedimproved accuracy in its clinical diagnosis of melanoma from 54.6% to 62.7%.22
Argenziano and colleagues demonstrated similar results after PCPs attended a 4-hour workshop on dermoscopy. The 73 PCPs in this study evaluated 2,522 lesions randomized to naked eye examination or dermoscopy. The BMR, calculated from the data provided, improved from 12.6:1 to 10.5:1, respectively, when dermoscopy was incorporated into lesion analysis, while the sensitivity increased from 54.1% to 79.2% and the negative predictive value increased from 95.8% to 98.1%. It is important to note that the BMR and negative predictive value improved in tandem, indicating that PCPs were more discriminatory with their referrals for evaluation by dermatology while capturing a greater percentage of melanomas.23
These studies are not without limitations that preclude broad generalizations. For example, Dolianitis and colleagues and Westerhoff and colleagues provided participants with dermoscopic images of the lesions to be evaluated instead of requiring personal use of a dermatoscope, whereas the study by Argenziano and colleagues incorporated only 6 histopathologically proven malignant melanomas into each of the naked eye examination and the experimental dermoscopy groups.21-23 Yet these studies suggest that broader use of dermoscopy among PCPs could improve the accuracy of melanoma detection given clinically relevant training.
Several additional studies identify positive correlations associated with dermoscopy use among PCPs. A recent survey of 425 French general practitioners found that 8% of the study participants acknowledged owning a dermatoscope. Dermatoscope owners spent a statistically significant longer time analyzing each pigmented skin lesions, exhibited greater confidence in their analysis of pigmented lesions, and issued fewer overall referrals to dermatologists.24
Similarly, Rosendahl and colleagues evaluated the number needed to treat (NNT) (equivalent to the BMR) among 193 Australian PCPs and found that the NNT was inversely correlated to the frequency with which the physicians used dermoscopy. However, it was difficult to determine the definitive cause of the reduced NNT in this study because a similar effect was observed when NNT was evaluated based on general practitioner subspecialization.25 Again, despite limitations, these studies suggest that increased dermoscopy use among PCPs could reduce the morbidity of lifelong scarring as well as the short-term anxiety associated with a possible melanoma diagnosis.
Limitations
Even in the hands of a trained dermatologist, dermoscopy has limitations. Featureless melanoma is a term applied to melanoma lesions bereft of classical findings on both naked eye examination and dermoscopy. Menzies, a dermatologic pioneer in dermoscopy, acknowledged this limitation in 1996 while showing that 8% of melanomas evaded dermoscopic detection. He proceeded to discuss the importance of clinical history in melanoma detection because all of the featureless melanomas exhibited recent changes in size, shape, and/or color.26 More recently, sequential dermoscopy (mole mapping) imaging has been implemented to successfully identify these lesions.27 Thus, dermoscopy cannot replace dermatologists trained in the art of visual assessment with honed clinical diagnostic acumen. Rather, dermoscopy is a tool to enhance the assessment of clinically suspicious lesions and aid diagnostic discrimination of uncertain pigmented lesions.
Conclusion
Primary care physicians are on the frontline of medicine and often the first to have the opportunity to detect the presence of melanoma. Notably, 52.2% of the 884.7 million medical office visits performed annually in the US are with PCPs.28 Despite the benefits, dermoscopy is not uniformly used by dermatologists in the US. Of dermatologists practicing for more than 20 years, 76.2% use dermoscopy compared with 97.8% of dermatologists in practice for less than 5 years. This illustrates an increased use in tandem with dermatology residencies integrating dermoscopy training as a component of the curriculum, showing the importance of incorporating dermoscopy into medical school and residency training for PCPs..29-31 Guidelines regarding dermoscopy training and dermoscopic evaluation algorithms should be established, routinely taught in medical education, and actively incorporated into training curriculum for PCPs in order to improve patient care and realize the potential health care savings associated with the early diagnosis and treatment of melanoma. Dermoscopic-teledermatology consultations present a viable opportunity within the VHA to expedite access to care for veterans and simultaneously offer evaluative feedback on lesions to referring PCPs, as skilled, dermoscopy-trained dermatologists render the diagnoses. Given the devastating mortality rate of melanoma, continued multidisciplinary education on identifying melanoma is of the utmost importance for patient care. Widespread implementation of dermoscopy and dermoscopic-teledermatology consultations could save lives and slow the ever-increasing economic burden associated with melanoma treatment, costing $1.467 billion in 2016.32
1. Guy GP Jr, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC. Vital signs: melanoma incidence and mortality trends and projections-United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591-596.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
3. American Cancer Society. Cancer facts & figures 2017. Atlanta: American Cancer Society; 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed April 19, 2018.
4. Lea CS, Efird JT, Toland AE, Lewis DR, Phillips CJ. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179(3):247-253.
5. Thomas L, Puig S. Dermoscopy, digital dermoscopy and other diagnostic tools in the early detection of melanoma and follow-up of high-risk skin cancer patients. Acta Derm Venereol. 2017;97(218):14-21.
6. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88(7):441-450.
7. Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137(10):1343-1350.
8. Terushkin V, Warycha M, Levy M, Kopf AW, Cohen DE, Polsky D. Analysis of the benign to malignant ratio of lesions biopsied by a general dermatologist before and after the adoption of dermoscopy. Arch Dermatol. 2010;146(3):343-344.
9. Wolner ZJ, Yélamos O, Liopyris K, Rogers T, Marchetti MA, Marghoob AA. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35(4):417-437.
10. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148(5):981-984.
11. Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134(12):1563-1570.
12. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
13. Nachbar F, Stolz W, Merkle T, et al. The ABCD rule of dermatoscopy. High prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol. 1994;30(4):551-559.
14. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56(1):45-52.
15. Rogers T, Marino M, Dusza SW, Bajaj S, Marchetti MA, Marghoob A. Triage amalgamated dermoscopic algorithm (TADA) for skin cancer screening. Dermatol Pract Concept. 2017;7(2):39-46.
16. Ackerman AB. No one should die of malignant melanoma. J Am Acad Dermatol. 1985;12(1):115-116.
17. Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II: sun exposure. Eur J Cancer. 2005;41(1):45-60.
18. Gerbert B, Maurer T, Berger T, et al. Primary care physicians as gatekeepers in managed care. Primary care physicians’ and dermatologists’ skills at secondary prevention of skin cancer. Arch Dermatol. 1996;132(9):1030-1038.
19. Corbo MD, Wismer J. Agreement between dermatologists and primary care practitioners in the diagnosis of malignant melanoma: review of the literature. J Cutan Med Surg. 2012;16(5):306-310.
20. Morris JB, Alfonso SV, Hernandez N, Fernández MI. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7(4):63-70.
21. Dolianitis C, Kelly J, Wolfe R, Simpson P. Comparative performance of 4 dermoscopic algorithms by nonexperts for the diagnosis of melanocytic lesions. Arch Dermatol. 2005;141(8):1008-1014.
22. Westerhoff K, Mccarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143(5):1016-1020.
23. Argenziano G, Puig S, Zalaudek I, et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J Clin Oncol. 2006;24(12):1877-1882.
24. Chappuis P, Duru G, Marchal O, Girier P, Dalle S, Thomas L. Dermoscopy, a useful tool for general practitioners in melanoma screening: a nationwide survey. Br J Dermatol. 2016;175(4):744-750.
25. Rosendahl C, Williams G, Eley D, et al. The impact of subspecialization and dermatoscopy use on accuracy of melanoma diagnosis among primary care doctors in Australia. J Am Acad Dermatol. 2012;67(5):846-852.
26. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
27. Kittler H, Guitera P, Riedl E, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch Dermatol. 2006;142(9):1113-1119.
28. Centers for Disease Control and Prevention. Ambulatory care use and physician office visits. https://www.cdc.gov/nchs/fastats/physician-visits.htm. Updated May 3, 2017. Accessed April 10, 2018.
29. Murzaku EC, Hayan S, Rao BK. Methods and rates of dermoscopy usage: a cross-sectional survey of US dermatologists stratified by years in practice. J Am Acad Dermatol. 2014;71(2):393-395.
30. Nehal KS, Oliveria SA, Marghoob AA, et al. Use of and beliefs about dermoscopy in the management of patients with pigmented lesions: a survey of dermatology residency programmes in the United States. Melanoma Res. 2002;12(6):601-605.
31. Wu TP, Newlove T, Smith L, Vuong CH, Stein JA, Polsky D. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68(6):1000-1005.
32. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958-972
From 1982 to 2011, the melanoma incidence rate doubled in the US.1 In 2018, an estimated 87,290 cases of melanoma in situ and 91,270 cases of invasive melanoma will be diagnosed in the US, and 9,320 deaths will be attributable to melanoma.2 Early detection of melanoma is critically important to reduce melanoma-related mortality, with 5-year survival rates as high as 97% at stage 1A vs a 20% 5-year survival when there is distant metastasis.2,3 Melanoma is particularly relevant for medical providers working with veterans because melanoma disproportionately affects service members with an incidence rate ratio of 1.62 (95% confidence interval [CI], 1.40-1.86) compared with that of the general population.4
Biopsy is the definitive diagnostic tool for melanoma. Histologic analysis differentiates melanoma from seborrheic keratoses, pigmented nevi, dermatofibromas, and other pigmented lesions that can resemble melanoma on clinical examination. However, biopsy must be used judiciously as unnecessary biopsies contribute to health care costs and leave scars, which can have psychosocial implications. With benign nevi outnumbering melanoma about 2 million to 1, biopsy is indicated once a threshold of suspicion is obtained.5
Dermoscopic Tool
Dermoscopy is a microscopy-based tool to improve noninvasive diagnostic discrimination of skin lesions based on color and structure analysis. Coloration provides an indication of the composition of elements present in the skin with keratin appearing yellow, blood appearing red, and collagen appearing white. Coloration also suggests pigment depth as melanin appears black when located in the stratum corneum, brown when located deeper in the epidermis, and blue when located in the dermis.6 Finally, characteristic histopathologic alterations in the dermoepidermal junction, rete ridges, pigment-containing cells, and/or melanocyte granules that occur in melanoma are recognizable with dermoscopy.6
In 2001, Bafounta and colleagues performed the first meta-analysis on the efficacy of dermoscopy compared with that of clinical evaluation, finding that dermoscopy performed specifically by dermatology-trained clinicians improved the accuracy of identifying melanoma from an odds ratio of 16 (95% CI, 9-31) with naked eye examination to 76 (95% CI, 25-223) with dermoscopy.7
More recently, Terushkin and colleagues demonstrated that diagnosis specificity improves when a general dermatologist is trained in dermoscopic pattern recognition. Naked eye examination produced a benign to malignant ratio (BMR) of 18.4:1, indicating that about 18 of 19 biopsies considered suspicious for melanoma ultimately yielded benign melanocytic lesions. Although the BMR for the general dermatologist experienced an increase after dermoscopy training, the ratio eventually decreased to 7.9:1.8
Dermoscopic Analysis
Some of the common patterns recognized in melanoma are demonstrated in Figures 1 and 2. Figure 1 is a close-up of a patient’s upper back showing a solitary asymmetric melanocytic lesion containing multiple red, brown, black, and blue hues.
Pattern analysis, the dermoscopic interpretation method preferred by pigmented lesion specialists, requires simultaneously assessing numerous lesion patterns that vary depending on body site.10 Alternative dermoscopic algorithms that focus on the most common features of melanoma have been developed to aid practitioners with the interpretation of dermoscopy findings: the 7-point checklist, the Menzies method, the ABCD rule, and the CASH algorithm (Tables 2, 3, 4, and 5).
Argenziano and colleagues developed the 7-point checklist in 1998. Two points are assigned to the lesion for each of the major criteria and 1 point for each minor criteria.
The Menzies method was developed by Menzies and colleagues in 1996. To be classified as melanoma, the pigmented lesion must show an absence of pattern symmetry and color uniformity while simultaneously exhibiting at least one of the following: blue-white veil, multiple brown dots, pseudopods, radial streaming, scarlike depigmentation, peripheral block dots/globules, 5 to 6 colors, multiple blue/gray dots, and a broadened network.12
The ABCD rule is a more technical dermoscopic evaluation algorithm developed in 1994 by Stolz and colleagues. This method yields a numeric value called the total dermoscopic score (TDS) based on Asymmetry, Border pigment pattern, Color variation, and 5 Different structural components.
Henning and colleagues developed the CASH algorithm in 2006 with the intention of assisting less experienced dermoscopy users with lesion evaluation.14 This algorithm tallies points for Color, Architectural disorder, Symmetry, and Homogeneity. One point is attributed to a lesion for each light brown, dark brown, black, red, white, and/or blue region present. Architectural disorder is assigned a point value between 0 and 2, with 0 indicating the absence of or minimal architectural disorder, 1 indicating moderate disorder, and 2 indicating marked disorder. Symmetry is assigned a point value between 0 and 2, with 0 points assigned to a lesion that exhibits biaxial symmetry, 1 point assigned to a lesion that exhibits monoaxial symmetry, and 2 points assigned to a lesion that exhibits biaxial asymmetry. Finally, 1 point is attributed to a lesion for evidence of each of the following: atypical network, dots/globules, streaks/pseudopods, blue-white veil, regression structures, blotches > 10% of the overall lesion size, and polymorphous blood vessels. The lesion in Figure 2 scores 16 points out of the maximum total CASH score of 17. Any lesion scoring 8 or more is suggestive of malignant melanoma.14
Finally, the TADA method was developed by Rogers and colleagues in 2016.15 This method uses sequential questions to evaluate lesions. First, “Does the lesion exhibit clear dermoscopic evidence of an angioma, dermatofibroma, or seborrheic keratosis?” If “yes,” then no additional dermoscopic evaluation is necessary, and it is recommended to monitor the lesion. If the answer to the first question is “no,” then ask, “Does the lesion exhibit architectural disorder?” The presence of architectural disorder is based on an overall impression of the lesion, which includes symmetry with regard to structures and colors. Any lesion deemed to exhibit architectural disorder should be biopsied. If the lesion has no architectural disorder, the third question is, “Does the lesion contain any starburst patterns, blue-black or gray coloration, shiny white structures, negative networks, ulcers or erosions, and/or vessels?” If “yes,” then the lesion should be biopsied. Since the lesion in Figure 2 exhibits marked architectural disorder in terms of symmetry and color, analysis of the lesion with the TADA method would yield a recommendation for biopsy.15
Dermoscopy in Practice
A. Bernard Ackerman, MD, a key figure in the modern era of dermatopathology, wrote an editorial for the Journal of the American Academy of Dermatology in 1985 titled “No one should die of malignant melanoma.” The editorial highlighted that the visual changes associated with melanoma often manifest years prior to malignant invasion and advocated that all physicians should have competence in melanoma detection, specifically mentioning the importance of training primary care physicians (PCPs), dermatologists, and pathologists in this regard.16 This sentiment is equally as true now as it was in 1985.
Naked eye examination paired with an evaluation of patient risk factors for melanoma, including fair skin types, significant sun exposure history, history of sunburn, geographic location, and personal and family history of melanoma, are the foundation of melanoma detection efforts.17 Studies suggest that the triage skills of PCPs could be improved in regard to the evaluation of pigmented lesions. Primary care residents, for instance, did not accurately diagnose 40% of malignant melanoma cases.18,19 Additionally, a meta-analysis demonstrated that PCP accuracy when diagnosing malignant melanoma ranged between 49% and 80%, significantly less than the 85% to 89% exhibited by practicing dermatologists.19 Dermoscopy could be incorporated as an element of the skin examination to enhance lesion discrimination among PCPs, as it has proven use in dermatologic practice.
Dermoscopy is not readily used by PCPs. A survey study of 705 family practitioners in the US performed by Morris and colleagues demonstrated that only 8.3% of participants currently use a dermatoscope to evaluate pigmented lesions.20 The most common barriers to dermoscopy use cited by PCPs in the US include the cost of the dermatoscope, the time required to acquire proficiency, and the lack of financial reimbursement.16 True utilization of dermoscopy among PCPs is higher than this figure suggests due to the number of PCPs who access dermoscopic evaluations via teledermatology. All 21 Veterans Integrated Services Networks of the Veterans Health Administration (VHA) system, for instance, participate in teledermatology and jointly employ more than 1,150 trained telehealth clinical technicians who created a collective 107,000 teledermatology encounters with and without dermoscopy for evaluation by dermatologists in the most recent fiscal year(Martin Weinstock, written communication, October 2017). Nonetheless, it is necessary to determine the contribution that wider utilization of dermoscopy among PCPs would have on melanoma surveillance.
Studies show that dermoscopic algorithms improve the sensitivity while slightly decreasing the specificity of PCPs to detect melanoma compared with that of the naked eye examination. Dolianitis and colleagues demonstrated that a baseline sensitivity of 60.9% for melanoma detection improved to 85.4% with the 7-point checklist, 85.4% with Menzies method, and 77.5% with the ABCD rule, while the baseline specificity of 85.4% moderated to 73.0%, 77.7%, and 80.4%, respectively, among 61 medical practitioners after studying dermoscopy techniques from 2 CDs.21 Westerhoff and colleagues performed a randomized controlled trial with 74 PCPs to determine the effect of a minimal intervention on melanoma diagnostic accuracy. The intervention consisted of providing participants in the experimental group with an atlas of microscopic features common to melanoma to be read at the participants’ leisure, a 1-hour presentation on microscopy, and a 25-questionpractice quiz. Participants randomized to the intervention group improved their diagnostic accuracy from 57.8% to 75.9% with the use of dermoscopy. This group also experiencedimproved accuracy in its clinical diagnosis of melanoma from 54.6% to 62.7%.22
Argenziano and colleagues demonstrated similar results after PCPs attended a 4-hour workshop on dermoscopy. The 73 PCPs in this study evaluated 2,522 lesions randomized to naked eye examination or dermoscopy. The BMR, calculated from the data provided, improved from 12.6:1 to 10.5:1, respectively, when dermoscopy was incorporated into lesion analysis, while the sensitivity increased from 54.1% to 79.2% and the negative predictive value increased from 95.8% to 98.1%. It is important to note that the BMR and negative predictive value improved in tandem, indicating that PCPs were more discriminatory with their referrals for evaluation by dermatology while capturing a greater percentage of melanomas.23
These studies are not without limitations that preclude broad generalizations. For example, Dolianitis and colleagues and Westerhoff and colleagues provided participants with dermoscopic images of the lesions to be evaluated instead of requiring personal use of a dermatoscope, whereas the study by Argenziano and colleagues incorporated only 6 histopathologically proven malignant melanomas into each of the naked eye examination and the experimental dermoscopy groups.21-23 Yet these studies suggest that broader use of dermoscopy among PCPs could improve the accuracy of melanoma detection given clinically relevant training.
Several additional studies identify positive correlations associated with dermoscopy use among PCPs. A recent survey of 425 French general practitioners found that 8% of the study participants acknowledged owning a dermatoscope. Dermatoscope owners spent a statistically significant longer time analyzing each pigmented skin lesions, exhibited greater confidence in their analysis of pigmented lesions, and issued fewer overall referrals to dermatologists.24
Similarly, Rosendahl and colleagues evaluated the number needed to treat (NNT) (equivalent to the BMR) among 193 Australian PCPs and found that the NNT was inversely correlated to the frequency with which the physicians used dermoscopy. However, it was difficult to determine the definitive cause of the reduced NNT in this study because a similar effect was observed when NNT was evaluated based on general practitioner subspecialization.25 Again, despite limitations, these studies suggest that increased dermoscopy use among PCPs could reduce the morbidity of lifelong scarring as well as the short-term anxiety associated with a possible melanoma diagnosis.
Limitations
Even in the hands of a trained dermatologist, dermoscopy has limitations. Featureless melanoma is a term applied to melanoma lesions bereft of classical findings on both naked eye examination and dermoscopy. Menzies, a dermatologic pioneer in dermoscopy, acknowledged this limitation in 1996 while showing that 8% of melanomas evaded dermoscopic detection. He proceeded to discuss the importance of clinical history in melanoma detection because all of the featureless melanomas exhibited recent changes in size, shape, and/or color.26 More recently, sequential dermoscopy (mole mapping) imaging has been implemented to successfully identify these lesions.27 Thus, dermoscopy cannot replace dermatologists trained in the art of visual assessment with honed clinical diagnostic acumen. Rather, dermoscopy is a tool to enhance the assessment of clinically suspicious lesions and aid diagnostic discrimination of uncertain pigmented lesions.
Conclusion
Primary care physicians are on the frontline of medicine and often the first to have the opportunity to detect the presence of melanoma. Notably, 52.2% of the 884.7 million medical office visits performed annually in the US are with PCPs.28 Despite the benefits, dermoscopy is not uniformly used by dermatologists in the US. Of dermatologists practicing for more than 20 years, 76.2% use dermoscopy compared with 97.8% of dermatologists in practice for less than 5 years. This illustrates an increased use in tandem with dermatology residencies integrating dermoscopy training as a component of the curriculum, showing the importance of incorporating dermoscopy into medical school and residency training for PCPs..29-31 Guidelines regarding dermoscopy training and dermoscopic evaluation algorithms should be established, routinely taught in medical education, and actively incorporated into training curriculum for PCPs in order to improve patient care and realize the potential health care savings associated with the early diagnosis and treatment of melanoma. Dermoscopic-teledermatology consultations present a viable opportunity within the VHA to expedite access to care for veterans and simultaneously offer evaluative feedback on lesions to referring PCPs, as skilled, dermoscopy-trained dermatologists render the diagnoses. Given the devastating mortality rate of melanoma, continued multidisciplinary education on identifying melanoma is of the utmost importance for patient care. Widespread implementation of dermoscopy and dermoscopic-teledermatology consultations could save lives and slow the ever-increasing economic burden associated with melanoma treatment, costing $1.467 billion in 2016.32
From 1982 to 2011, the melanoma incidence rate doubled in the US.1 In 2018, an estimated 87,290 cases of melanoma in situ and 91,270 cases of invasive melanoma will be diagnosed in the US, and 9,320 deaths will be attributable to melanoma.2 Early detection of melanoma is critically important to reduce melanoma-related mortality, with 5-year survival rates as high as 97% at stage 1A vs a 20% 5-year survival when there is distant metastasis.2,3 Melanoma is particularly relevant for medical providers working with veterans because melanoma disproportionately affects service members with an incidence rate ratio of 1.62 (95% confidence interval [CI], 1.40-1.86) compared with that of the general population.4
Biopsy is the definitive diagnostic tool for melanoma. Histologic analysis differentiates melanoma from seborrheic keratoses, pigmented nevi, dermatofibromas, and other pigmented lesions that can resemble melanoma on clinical examination. However, biopsy must be used judiciously as unnecessary biopsies contribute to health care costs and leave scars, which can have psychosocial implications. With benign nevi outnumbering melanoma about 2 million to 1, biopsy is indicated once a threshold of suspicion is obtained.5
Dermoscopic Tool
Dermoscopy is a microscopy-based tool to improve noninvasive diagnostic discrimination of skin lesions based on color and structure analysis. Coloration provides an indication of the composition of elements present in the skin with keratin appearing yellow, blood appearing red, and collagen appearing white. Coloration also suggests pigment depth as melanin appears black when located in the stratum corneum, brown when located deeper in the epidermis, and blue when located in the dermis.6 Finally, characteristic histopathologic alterations in the dermoepidermal junction, rete ridges, pigment-containing cells, and/or melanocyte granules that occur in melanoma are recognizable with dermoscopy.6
In 2001, Bafounta and colleagues performed the first meta-analysis on the efficacy of dermoscopy compared with that of clinical evaluation, finding that dermoscopy performed specifically by dermatology-trained clinicians improved the accuracy of identifying melanoma from an odds ratio of 16 (95% CI, 9-31) with naked eye examination to 76 (95% CI, 25-223) with dermoscopy.7
More recently, Terushkin and colleagues demonstrated that diagnosis specificity improves when a general dermatologist is trained in dermoscopic pattern recognition. Naked eye examination produced a benign to malignant ratio (BMR) of 18.4:1, indicating that about 18 of 19 biopsies considered suspicious for melanoma ultimately yielded benign melanocytic lesions. Although the BMR for the general dermatologist experienced an increase after dermoscopy training, the ratio eventually decreased to 7.9:1.8
Dermoscopic Analysis
Some of the common patterns recognized in melanoma are demonstrated in Figures 1 and 2. Figure 1 is a close-up of a patient’s upper back showing a solitary asymmetric melanocytic lesion containing multiple red, brown, black, and blue hues.
Pattern analysis, the dermoscopic interpretation method preferred by pigmented lesion specialists, requires simultaneously assessing numerous lesion patterns that vary depending on body site.10 Alternative dermoscopic algorithms that focus on the most common features of melanoma have been developed to aid practitioners with the interpretation of dermoscopy findings: the 7-point checklist, the Menzies method, the ABCD rule, and the CASH algorithm (Tables 2, 3, 4, and 5).
Argenziano and colleagues developed the 7-point checklist in 1998. Two points are assigned to the lesion for each of the major criteria and 1 point for each minor criteria.
The Menzies method was developed by Menzies and colleagues in 1996. To be classified as melanoma, the pigmented lesion must show an absence of pattern symmetry and color uniformity while simultaneously exhibiting at least one of the following: blue-white veil, multiple brown dots, pseudopods, radial streaming, scarlike depigmentation, peripheral block dots/globules, 5 to 6 colors, multiple blue/gray dots, and a broadened network.12
The ABCD rule is a more technical dermoscopic evaluation algorithm developed in 1994 by Stolz and colleagues. This method yields a numeric value called the total dermoscopic score (TDS) based on Asymmetry, Border pigment pattern, Color variation, and 5 Different structural components.
Henning and colleagues developed the CASH algorithm in 2006 with the intention of assisting less experienced dermoscopy users with lesion evaluation.14 This algorithm tallies points for Color, Architectural disorder, Symmetry, and Homogeneity. One point is attributed to a lesion for each light brown, dark brown, black, red, white, and/or blue region present. Architectural disorder is assigned a point value between 0 and 2, with 0 indicating the absence of or minimal architectural disorder, 1 indicating moderate disorder, and 2 indicating marked disorder. Symmetry is assigned a point value between 0 and 2, with 0 points assigned to a lesion that exhibits biaxial symmetry, 1 point assigned to a lesion that exhibits monoaxial symmetry, and 2 points assigned to a lesion that exhibits biaxial asymmetry. Finally, 1 point is attributed to a lesion for evidence of each of the following: atypical network, dots/globules, streaks/pseudopods, blue-white veil, regression structures, blotches > 10% of the overall lesion size, and polymorphous blood vessels. The lesion in Figure 2 scores 16 points out of the maximum total CASH score of 17. Any lesion scoring 8 or more is suggestive of malignant melanoma.14
Finally, the TADA method was developed by Rogers and colleagues in 2016.15 This method uses sequential questions to evaluate lesions. First, “Does the lesion exhibit clear dermoscopic evidence of an angioma, dermatofibroma, or seborrheic keratosis?” If “yes,” then no additional dermoscopic evaluation is necessary, and it is recommended to monitor the lesion. If the answer to the first question is “no,” then ask, “Does the lesion exhibit architectural disorder?” The presence of architectural disorder is based on an overall impression of the lesion, which includes symmetry with regard to structures and colors. Any lesion deemed to exhibit architectural disorder should be biopsied. If the lesion has no architectural disorder, the third question is, “Does the lesion contain any starburst patterns, blue-black or gray coloration, shiny white structures, negative networks, ulcers or erosions, and/or vessels?” If “yes,” then the lesion should be biopsied. Since the lesion in Figure 2 exhibits marked architectural disorder in terms of symmetry and color, analysis of the lesion with the TADA method would yield a recommendation for biopsy.15
Dermoscopy in Practice
A. Bernard Ackerman, MD, a key figure in the modern era of dermatopathology, wrote an editorial for the Journal of the American Academy of Dermatology in 1985 titled “No one should die of malignant melanoma.” The editorial highlighted that the visual changes associated with melanoma often manifest years prior to malignant invasion and advocated that all physicians should have competence in melanoma detection, specifically mentioning the importance of training primary care physicians (PCPs), dermatologists, and pathologists in this regard.16 This sentiment is equally as true now as it was in 1985.
Naked eye examination paired with an evaluation of patient risk factors for melanoma, including fair skin types, significant sun exposure history, history of sunburn, geographic location, and personal and family history of melanoma, are the foundation of melanoma detection efforts.17 Studies suggest that the triage skills of PCPs could be improved in regard to the evaluation of pigmented lesions. Primary care residents, for instance, did not accurately diagnose 40% of malignant melanoma cases.18,19 Additionally, a meta-analysis demonstrated that PCP accuracy when diagnosing malignant melanoma ranged between 49% and 80%, significantly less than the 85% to 89% exhibited by practicing dermatologists.19 Dermoscopy could be incorporated as an element of the skin examination to enhance lesion discrimination among PCPs, as it has proven use in dermatologic practice.
Dermoscopy is not readily used by PCPs. A survey study of 705 family practitioners in the US performed by Morris and colleagues demonstrated that only 8.3% of participants currently use a dermatoscope to evaluate pigmented lesions.20 The most common barriers to dermoscopy use cited by PCPs in the US include the cost of the dermatoscope, the time required to acquire proficiency, and the lack of financial reimbursement.16 True utilization of dermoscopy among PCPs is higher than this figure suggests due to the number of PCPs who access dermoscopic evaluations via teledermatology. All 21 Veterans Integrated Services Networks of the Veterans Health Administration (VHA) system, for instance, participate in teledermatology and jointly employ more than 1,150 trained telehealth clinical technicians who created a collective 107,000 teledermatology encounters with and without dermoscopy for evaluation by dermatologists in the most recent fiscal year(Martin Weinstock, written communication, October 2017). Nonetheless, it is necessary to determine the contribution that wider utilization of dermoscopy among PCPs would have on melanoma surveillance.
Studies show that dermoscopic algorithms improve the sensitivity while slightly decreasing the specificity of PCPs to detect melanoma compared with that of the naked eye examination. Dolianitis and colleagues demonstrated that a baseline sensitivity of 60.9% for melanoma detection improved to 85.4% with the 7-point checklist, 85.4% with Menzies method, and 77.5% with the ABCD rule, while the baseline specificity of 85.4% moderated to 73.0%, 77.7%, and 80.4%, respectively, among 61 medical practitioners after studying dermoscopy techniques from 2 CDs.21 Westerhoff and colleagues performed a randomized controlled trial with 74 PCPs to determine the effect of a minimal intervention on melanoma diagnostic accuracy. The intervention consisted of providing participants in the experimental group with an atlas of microscopic features common to melanoma to be read at the participants’ leisure, a 1-hour presentation on microscopy, and a 25-questionpractice quiz. Participants randomized to the intervention group improved their diagnostic accuracy from 57.8% to 75.9% with the use of dermoscopy. This group also experiencedimproved accuracy in its clinical diagnosis of melanoma from 54.6% to 62.7%.22
Argenziano and colleagues demonstrated similar results after PCPs attended a 4-hour workshop on dermoscopy. The 73 PCPs in this study evaluated 2,522 lesions randomized to naked eye examination or dermoscopy. The BMR, calculated from the data provided, improved from 12.6:1 to 10.5:1, respectively, when dermoscopy was incorporated into lesion analysis, while the sensitivity increased from 54.1% to 79.2% and the negative predictive value increased from 95.8% to 98.1%. It is important to note that the BMR and negative predictive value improved in tandem, indicating that PCPs were more discriminatory with their referrals for evaluation by dermatology while capturing a greater percentage of melanomas.23
These studies are not without limitations that preclude broad generalizations. For example, Dolianitis and colleagues and Westerhoff and colleagues provided participants with dermoscopic images of the lesions to be evaluated instead of requiring personal use of a dermatoscope, whereas the study by Argenziano and colleagues incorporated only 6 histopathologically proven malignant melanomas into each of the naked eye examination and the experimental dermoscopy groups.21-23 Yet these studies suggest that broader use of dermoscopy among PCPs could improve the accuracy of melanoma detection given clinically relevant training.
Several additional studies identify positive correlations associated with dermoscopy use among PCPs. A recent survey of 425 French general practitioners found that 8% of the study participants acknowledged owning a dermatoscope. Dermatoscope owners spent a statistically significant longer time analyzing each pigmented skin lesions, exhibited greater confidence in their analysis of pigmented lesions, and issued fewer overall referrals to dermatologists.24
Similarly, Rosendahl and colleagues evaluated the number needed to treat (NNT) (equivalent to the BMR) among 193 Australian PCPs and found that the NNT was inversely correlated to the frequency with which the physicians used dermoscopy. However, it was difficult to determine the definitive cause of the reduced NNT in this study because a similar effect was observed when NNT was evaluated based on general practitioner subspecialization.25 Again, despite limitations, these studies suggest that increased dermoscopy use among PCPs could reduce the morbidity of lifelong scarring as well as the short-term anxiety associated with a possible melanoma diagnosis.
Limitations
Even in the hands of a trained dermatologist, dermoscopy has limitations. Featureless melanoma is a term applied to melanoma lesions bereft of classical findings on both naked eye examination and dermoscopy. Menzies, a dermatologic pioneer in dermoscopy, acknowledged this limitation in 1996 while showing that 8% of melanomas evaded dermoscopic detection. He proceeded to discuss the importance of clinical history in melanoma detection because all of the featureless melanomas exhibited recent changes in size, shape, and/or color.26 More recently, sequential dermoscopy (mole mapping) imaging has been implemented to successfully identify these lesions.27 Thus, dermoscopy cannot replace dermatologists trained in the art of visual assessment with honed clinical diagnostic acumen. Rather, dermoscopy is a tool to enhance the assessment of clinically suspicious lesions and aid diagnostic discrimination of uncertain pigmented lesions.
Conclusion
Primary care physicians are on the frontline of medicine and often the first to have the opportunity to detect the presence of melanoma. Notably, 52.2% of the 884.7 million medical office visits performed annually in the US are with PCPs.28 Despite the benefits, dermoscopy is not uniformly used by dermatologists in the US. Of dermatologists practicing for more than 20 years, 76.2% use dermoscopy compared with 97.8% of dermatologists in practice for less than 5 years. This illustrates an increased use in tandem with dermatology residencies integrating dermoscopy training as a component of the curriculum, showing the importance of incorporating dermoscopy into medical school and residency training for PCPs..29-31 Guidelines regarding dermoscopy training and dermoscopic evaluation algorithms should be established, routinely taught in medical education, and actively incorporated into training curriculum for PCPs in order to improve patient care and realize the potential health care savings associated with the early diagnosis and treatment of melanoma. Dermoscopic-teledermatology consultations present a viable opportunity within the VHA to expedite access to care for veterans and simultaneously offer evaluative feedback on lesions to referring PCPs, as skilled, dermoscopy-trained dermatologists render the diagnoses. Given the devastating mortality rate of melanoma, continued multidisciplinary education on identifying melanoma is of the utmost importance for patient care. Widespread implementation of dermoscopy and dermoscopic-teledermatology consultations could save lives and slow the ever-increasing economic burden associated with melanoma treatment, costing $1.467 billion in 2016.32
1. Guy GP Jr, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC. Vital signs: melanoma incidence and mortality trends and projections-United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591-596.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
3. American Cancer Society. Cancer facts & figures 2017. Atlanta: American Cancer Society; 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed April 19, 2018.
4. Lea CS, Efird JT, Toland AE, Lewis DR, Phillips CJ. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179(3):247-253.
5. Thomas L, Puig S. Dermoscopy, digital dermoscopy and other diagnostic tools in the early detection of melanoma and follow-up of high-risk skin cancer patients. Acta Derm Venereol. 2017;97(218):14-21.
6. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88(7):441-450.
7. Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137(10):1343-1350.
8. Terushkin V, Warycha M, Levy M, Kopf AW, Cohen DE, Polsky D. Analysis of the benign to malignant ratio of lesions biopsied by a general dermatologist before and after the adoption of dermoscopy. Arch Dermatol. 2010;146(3):343-344.
9. Wolner ZJ, Yélamos O, Liopyris K, Rogers T, Marchetti MA, Marghoob AA. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35(4):417-437.
10. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148(5):981-984.
11. Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134(12):1563-1570.
12. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
13. Nachbar F, Stolz W, Merkle T, et al. The ABCD rule of dermatoscopy. High prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol. 1994;30(4):551-559.
14. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56(1):45-52.
15. Rogers T, Marino M, Dusza SW, Bajaj S, Marchetti MA, Marghoob A. Triage amalgamated dermoscopic algorithm (TADA) for skin cancer screening. Dermatol Pract Concept. 2017;7(2):39-46.
16. Ackerman AB. No one should die of malignant melanoma. J Am Acad Dermatol. 1985;12(1):115-116.
17. Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II: sun exposure. Eur J Cancer. 2005;41(1):45-60.
18. Gerbert B, Maurer T, Berger T, et al. Primary care physicians as gatekeepers in managed care. Primary care physicians’ and dermatologists’ skills at secondary prevention of skin cancer. Arch Dermatol. 1996;132(9):1030-1038.
19. Corbo MD, Wismer J. Agreement between dermatologists and primary care practitioners in the diagnosis of malignant melanoma: review of the literature. J Cutan Med Surg. 2012;16(5):306-310.
20. Morris JB, Alfonso SV, Hernandez N, Fernández MI. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7(4):63-70.
21. Dolianitis C, Kelly J, Wolfe R, Simpson P. Comparative performance of 4 dermoscopic algorithms by nonexperts for the diagnosis of melanocytic lesions. Arch Dermatol. 2005;141(8):1008-1014.
22. Westerhoff K, Mccarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143(5):1016-1020.
23. Argenziano G, Puig S, Zalaudek I, et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J Clin Oncol. 2006;24(12):1877-1882.
24. Chappuis P, Duru G, Marchal O, Girier P, Dalle S, Thomas L. Dermoscopy, a useful tool for general practitioners in melanoma screening: a nationwide survey. Br J Dermatol. 2016;175(4):744-750.
25. Rosendahl C, Williams G, Eley D, et al. The impact of subspecialization and dermatoscopy use on accuracy of melanoma diagnosis among primary care doctors in Australia. J Am Acad Dermatol. 2012;67(5):846-852.
26. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
27. Kittler H, Guitera P, Riedl E, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch Dermatol. 2006;142(9):1113-1119.
28. Centers for Disease Control and Prevention. Ambulatory care use and physician office visits. https://www.cdc.gov/nchs/fastats/physician-visits.htm. Updated May 3, 2017. Accessed April 10, 2018.
29. Murzaku EC, Hayan S, Rao BK. Methods and rates of dermoscopy usage: a cross-sectional survey of US dermatologists stratified by years in practice. J Am Acad Dermatol. 2014;71(2):393-395.
30. Nehal KS, Oliveria SA, Marghoob AA, et al. Use of and beliefs about dermoscopy in the management of patients with pigmented lesions: a survey of dermatology residency programmes in the United States. Melanoma Res. 2002;12(6):601-605.
31. Wu TP, Newlove T, Smith L, Vuong CH, Stein JA, Polsky D. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68(6):1000-1005.
32. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958-972
1. Guy GP Jr, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC. Vital signs: melanoma incidence and mortality trends and projections-United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591-596.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
3. American Cancer Society. Cancer facts & figures 2017. Atlanta: American Cancer Society; 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed April 19, 2018.
4. Lea CS, Efird JT, Toland AE, Lewis DR, Phillips CJ. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179(3):247-253.
5. Thomas L, Puig S. Dermoscopy, digital dermoscopy and other diagnostic tools in the early detection of melanoma and follow-up of high-risk skin cancer patients. Acta Derm Venereol. 2017;97(218):14-21.
6. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88(7):441-450.
7. Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137(10):1343-1350.
8. Terushkin V, Warycha M, Levy M, Kopf AW, Cohen DE, Polsky D. Analysis of the benign to malignant ratio of lesions biopsied by a general dermatologist before and after the adoption of dermoscopy. Arch Dermatol. 2010;146(3):343-344.
9. Wolner ZJ, Yélamos O, Liopyris K, Rogers T, Marchetti MA, Marghoob AA. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35(4):417-437.
10. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148(5):981-984.
11. Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134(12):1563-1570.
12. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
13. Nachbar F, Stolz W, Merkle T, et al. The ABCD rule of dermatoscopy. High prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol. 1994;30(4):551-559.
14. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56(1):45-52.
15. Rogers T, Marino M, Dusza SW, Bajaj S, Marchetti MA, Marghoob A. Triage amalgamated dermoscopic algorithm (TADA) for skin cancer screening. Dermatol Pract Concept. 2017;7(2):39-46.
16. Ackerman AB. No one should die of malignant melanoma. J Am Acad Dermatol. 1985;12(1):115-116.
17. Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II: sun exposure. Eur J Cancer. 2005;41(1):45-60.
18. Gerbert B, Maurer T, Berger T, et al. Primary care physicians as gatekeepers in managed care. Primary care physicians’ and dermatologists’ skills at secondary prevention of skin cancer. Arch Dermatol. 1996;132(9):1030-1038.
19. Corbo MD, Wismer J. Agreement between dermatologists and primary care practitioners in the diagnosis of malignant melanoma: review of the literature. J Cutan Med Surg. 2012;16(5):306-310.
20. Morris JB, Alfonso SV, Hernandez N, Fernández MI. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7(4):63-70.
21. Dolianitis C, Kelly J, Wolfe R, Simpson P. Comparative performance of 4 dermoscopic algorithms by nonexperts for the diagnosis of melanocytic lesions. Arch Dermatol. 2005;141(8):1008-1014.
22. Westerhoff K, Mccarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143(5):1016-1020.
23. Argenziano G, Puig S, Zalaudek I, et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J Clin Oncol. 2006;24(12):1877-1882.
24. Chappuis P, Duru G, Marchal O, Girier P, Dalle S, Thomas L. Dermoscopy, a useful tool for general practitioners in melanoma screening: a nationwide survey. Br J Dermatol. 2016;175(4):744-750.
25. Rosendahl C, Williams G, Eley D, et al. The impact of subspecialization and dermatoscopy use on accuracy of melanoma diagnosis among primary care doctors in Australia. J Am Acad Dermatol. 2012;67(5):846-852.
26. Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178-1182.
27. Kittler H, Guitera P, Riedl E, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch Dermatol. 2006;142(9):1113-1119.
28. Centers for Disease Control and Prevention. Ambulatory care use and physician office visits. https://www.cdc.gov/nchs/fastats/physician-visits.htm. Updated May 3, 2017. Accessed April 10, 2018.
29. Murzaku EC, Hayan S, Rao BK. Methods and rates of dermoscopy usage: a cross-sectional survey of US dermatologists stratified by years in practice. J Am Acad Dermatol. 2014;71(2):393-395.
30. Nehal KS, Oliveria SA, Marghoob AA, et al. Use of and beliefs about dermoscopy in the management of patients with pigmented lesions: a survey of dermatology residency programmes in the United States. Melanoma Res. 2002;12(6):601-605.
31. Wu TP, Newlove T, Smith L, Vuong CH, Stein JA, Polsky D. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68(6):1000-1005.
32. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958-972
Blood Loss Reduction with Tranexamic Acid and a Bipolar Sealer in Direct Anterior Total Hip Arthroplasty
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
TAKE-HOME POINTS
- TXA reduces blood loss and transfusion requirements in THA.
- The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation.
- The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements.
- The addition of a bipolar sealer did not offer an advantage to transfusion requirements in anterior THA.
- TXA should be used routinely in THA.
Nonoperative Treatment of Closed Extra-Articular Distal Humeral Shaft Fractures in Adults: A Comparison of Functional Bracing and Above-Elbow Casting
ABSTRACT
Diaphyseal fractures of the distal humerus have a high rate of union when treated with a functional brace or an above-elbow cast (AEC). This study compares alignment of the humerus and motion of the elbow after functional brace or AEC treatment.
One-hundred and five consecutive patients with a closed, extra-articular fracture of the distal humeral diaphysis were identified in the orthopedic trauma databases of 3 hospitals between 2003 and 2012. Seventy-five patients with a follow-up of at least 6 months or with radiographic and clinical evidence of fracture union were included (51 treated with functional bracing and 24 treated with an AEC).
All of the fractures healed. The average arc of elbow flexion was 130° ± 9° in braced patients vs 127° ± 12° in casted patients. Four patients (8%) in the bracing group and 4 (17%) in the casting group lost >20° of elbow motion. The average varus angulation on radiographs was 17° ± 8° in braced and 13° ± 8° in casted patients, while the average posterior angulation was 9° ± 6° vs 7° ± 7°, respectively.
Closed extra-articular distal diaphyseal humerus fractures heal with both bracing and casting and there are no differences in average elbow motion or radiographic alignment.
Nonoperative treatment of closed fractures of the humeral shaft (AO/OTA [Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association] type 12) with a functional brace or above-elbow cast (AEC) is associated with a high union rate, good motion, and good function. Advocates of casting believe that a brace cannot control fracture alignment as well as a cast that allows for immobilization and molding. Advocates of brace treatment are concerned that immobilization in a cast will cause elbow stiffness.1-11
Continue to: In our differing institutions...
In our differing institutions, there are advocates of each type of treatment, providing the opportunity for a comparison. This retrospective study compares brace and cast treatment. The working hypothesis was that there is no difference in elbow motion 6 months or more after fracture. We also compared radiographic alignment after union.
MATERIALS AND METHODS
Between 2003 and 2012, consecutive adult patients treated for a nonpathological fracture of the diaphysis of the distal humerus at the orthopedic trauma service of 3 level 1 academic trauma centers were identified from prospectively collected trauma injury databases. Patients with vascular injury, ipsilateral upper extremity fracture, and periprosthetic fractures were excluded. The attending orthopedic surgeon chose the treatment method and evaluated the range of motion (ROM) of the elbow and radiographic union at the final ambulatory visit. We included patients followed to clinical and radiographic union with a minimum of 6 months of follow-up. We also included patients with <6 months’ follow-up who demonstrated union and had elbow ROM within 10° of the uninjured arm.
We identified 105 consecutive adult patients with a closed nonpathological extra-articular distal humeral shaft fracture (fracture of the distal humeral shaft with an AO/OTA type-12.A, 12.B, or 12.C pattern) treated with an AEC or a brace in our databases.12 Two patients in the brace group chose surgery to improve alignment within 3 weeks of injury and were excluded from the analysis. Twenty-eight patients had inadequate follow-up.
A total of 75 patients were included in the study. At the first and second institutions, 51 patients were treated with functional bracing with an average follow-up of 7 months. At the third institution, 24 patients were treated with an AEC with an average follow-up of 4 months. Seventeen out of 24 patients in the long arm casting group and 19 out of 51 patients in the bracing group, who were included since they had <6 months of follow-up, demonstrated union and had elbow ROM within 10° of the uninjured arm. Differing methods of closed immobilization were the result of differing treatment algorithms at each institution.
The patients who were treated with a functional brace averaged 34 years of age (range, 18-90 years) and included 27 men and 24 women. The brace was removed at an average of 11.5 weeks (range, 8-18 weeks) after initial injury. Six patients had an injury-associated radial nerve palsy, all of which fully recovered within an average of 4 months (range, 0.5-7 months). Sixteen patients were injured due to a fall from standing height, 2 due to a fall from a greater height than standing, 16 in a motor-vehicle accident, 15 during a sport activity, and 2 were not specifically documented.
Continue to: Four patients had concomitant...
Four patients had concomitant injuries: one patient had a mid-shaft humeral fracture on the contralateral arm; a second had an ankle fracture; a third had an ankle fracture, acetabular fracture, a rib fracture, and pneumothorax; and the fourth had 2 rib fractures.
The patients who were treated with an AEC had an average age of 32 years (range,18-82 years) and included 14 men and 10 women. The cast was removed at an average of 4.2 weeks (range, 3-7 weeks) after the initial injury. Two patients had an injury-associated radial nerve palsy, both of which fully recovered. Five patients were injured due to a fall from standing height, 1 due to a fall from a height greater than standing, 7 during a motor-vehicle accident, 5 during a sport activity, and 6 were not documented. Two patients sustained concomitant injuries: one patient sustained a tibia-fibula fracture, and another patient sustained facial trauma.
The 2 groups were comparable in age and gender, as well as the injury mechanism (Table).
Table. Patient Demographics and Outcome Data
| Functional Bracing (n = 51) | Long Arm Casting (n = 24) | Significance (P < .05) |
Sex |
|
|
|
Male | 27 (54%) | 14 (58%) |
|
Female | 24 (46%) | 10 (42%) |
|
Average age (y) | 34 (range, 18-90) | 32 (range, 18-82) |
|
Mechanism of injury |
|
|
|
Standing height | 16 (31%) | 5 (20%) |
|
Greater height | 2 (4%) | 1 (4%) |
|
Motor vehicle collision | 16 (31%) | 7 (29%) |
|
Sports activity | 15 (29 %) | 5 (21%) |
|
Other | 2 (4%) | 6 (25%) |
|
Follow-up (months) | 7 (range, 2-25) | 4 (range, 2-15) |
|
Elbow range of motion (degrees) | 130 ± 9.4 | 127 ± 11.9 | P = .26 |
Varus/valgus angulation (degrees) | 17 ± 7.8 varus | 13 ± 8.4 varus | P = .11 |
Anterior/posterior angulation (degrees) | 9 ± 6.2 posterior | 7 ± 7.5 posterior | P = .54 |
FUNCTIONAL BRACING TECHNIQUE
Upon presentation after injury, patients were immobilized in a coaptation splint (Figure 1A). Within 10 days, the arm was placed in a pre-manufactured polyethylene functional brace (Corflex) and the arm was supported with a simple sling. Patients were allowed to use the hand for light tasks and move the elbow, but most patients were not capable of active elbow flexion exercises until early healing was established 4 to 6 weeks after injury. Shoulder motion was discouraged until radiographic union. Patients started active, self-assisted elbow and shoulder stretching exercises, and weaned from the brace once radiographic union was confirmed between 6 and 10 weeks after injury (Figures 1B, 1C).
ABOVE-ELBOW CASE
Patients were also initially immobilized in a coaptation splint upon initial presentation. Within 7 days, an above-elbow fiberglass cast with neutral forearm rotation and 90° of elbow flexion was applied with a supracondylar mold, followed by radiographic imaging (Figure 2A). With the fractured arm dependent, a valgus mold was applied as the material hardened in order to align the fracture site and limit varus angulation.
Continue to: There were no shoulder...
There were no shoulder ROM restrictions. Casts were removed, skin checked, and replaced every week for 4 to 6 weeks. Casts were removed when callus was noted on radiographs. After cast removal, physician-taught active and active-assisted elbow stretching exercises were given to patients to be performed on a daily basis at home. Patients were followed until clinical and radiographic union and elbow ROM to within 10° of the injured arm (Figures 2B, 2C).
STATISTICAL ANALYSIS
Alignment of the humerus (including varus-valgus alignment and apex anterior-posterior alignment) was measured on anteroposterior and lateral radiographs as the angle between lines bisecting the humeral diaphysis proximal and distal to the fracture. The normality of the data was tested using the Kolmogorov-Smirnov test. To statistically compare continuous variables with a normal distribution, t-tests were used; otherwise the Wilcoxon t-test was applied. The Pearson’s Chi-Square test was used to statistically compare dichotomous variables, except when expected cell frequency was <5, in which case the Fisher exact test was used. The level of significance was set at P < .05.
RESULTS
RANGE OF MOTION AND RADIOGRAPHIC ALIGNMENT
The average range of elbow motion was 130° ± 9° after brace treatment and 127° ± 12° after cast treatment (P = .26). Four patients (8%) treated with a brace and 3 (12%) treated with a cast lost >20° of elbow motion.
All the fractures healed. The average varus angulation on the anteroposterior radiograph was 17° (range, 2°-26°) in braced patients and 13 (range, 5°-31°) in casted patients (P = .11). The average posterior angulation on the lateral radiograph was 9° (range, 0°-28°) in braced patients vs 7° (range, 2°-33°) in casted patients (P = .54).
Continue to: Two weeks after initiating brace...
COMPLICATIONS
Two weeks after initiating brace treatment, an obese patient suffered a rash with desquamation that necessitated discontinuation of the brace. However, the skin and fracture ultimately healed with a coaptation splint and sling support without additional complications. In the casting cohort, 2 patients returned to the emergency department after AEC placement because of swelling of the hand and pain in the cast. Both casts were removed and reapplied.
DISCUSSION
Fractures of the distal third of the humeral diaphysis heal without surgery. Fracture angulation and elbow stiffness are the concerns that lead to variations in nonoperative treatment.1-3 Advocates of casting believe they can get better alignment without losing elbow motion, and advocates of bracing feel that the brace is less cumbersome.1-3,5-8 We compared these treatments retrospectively and found them comparable.
This study should be considered in light of its limitations. Many patients were lost to follow-up in our urban trauma centers. We do not know if these patients did better, worse, or the same as the patients we were able to evaluate, but our opinion is that patients having problems were more likely to return. The evaluation time was relatively short, but motion can only improve in the longer-term. Two patients that were initially braced chose surgery, probably because either they or their surgeon were nervous about the radiographic appearance of the fracture. In our opinion, continued nonoperative treatment of these patients would not affect the findings.
Cast treatment of distal diaphyseal humerus fractures does not cause permanent elbow stiffness. This is confirmed by our results; as casted patients did not lose final ROM compared to the bracing cohort. These injuries are extra-articular and casted patients are transitioned to bracing once humeri have significant union demonstrated by the arm moving as a unit. To our knowledge, there is no other study that has evaluated casting for these fractures, but it may be that evidence of permanent stiffness with nonoperative treatment of distal metaphyseal fractures of the humerus [AO/OTA type 13] is misapplied to distal humeral shaft fractures [AO/OTA type 12].3,9,10,12 For brace treatment, Sarmiento and colleagues9 showed no significant elbow stiffness in a consecutive cohort of 69 patients, while Jawa and colleagues5 showed no increased elbow stiffness compared to plate fixation. Given the accumulated data,3,5,6,8,13 advocates of operative treatment for distal third diaphyseal humerus fractures12 can no longer site elbow stiffness as a disadvantage of nonoperative treatment, whether with cast or brace.
As shown in this study, patients that choose nonoperative treatment can expect their fracture to heal with an average of approximately 15° of varus angulation, as well as 2 others evaluating brace treatment.5,9 Some will heal with as much as 30° of varus angulation.5,9 The arm may look a little different, particularly in thin patients, but there is no evidence that this angulation affects function. The risks, discomforts, and inconveniences of surgery can be balanced with the ability of surgery to improve alignment and allow elbow motion a few weeks earlier. The aesthetics of the scar after surgery may not be better than the deformity after nonoperative treatment. Patients should be involved in these decisions.
Continue to: No cost comparison...
No cost comparison was done between these 2 treatment modalities. However, both casting and bracing offer substantially lower costs comparted to surgical treatment with high efficacy and less risk for the patient. In some billing environments, closed treatments of fractures are captured as “surgical interventions” with global periods included in the reimbursement. Both casting and bracing are relatively inexpensive with materials that are readily accessible in nearly any general or subspecialty orthopedic practice.
There is a passive implication that operative treatment of distal third diaphyseal humerus fractures affords better results and union for patients in the discussed literature. Our results demonstrate that the distal diaphyseal humerus has a natural anatomic and biologic propensity to heal with closed immobilization. Patients should be made aware that while operative treatments exist for this fracture pattern, nonoperative treatment modalities have proven to be efficacious using a variety of immobilization methods. Thus, patients that prefer nonoperative treatment of a distal third diaphyseal humerus fracture can choose between a cast or a brace with confidence of the efficacy of the nonoperative treatment.
1. McKee MD. Fractures of the shaft of the humerus. In: Bucholz R, Heckman JD, Court-Brown C, eds. Rockwood and Green’s Fractures in Adults. 6th ed. Philadelphia: Lippencott Williams & Wilkins; 2006:1117-1159.
2. Schemitsch E, Bhandari M, Talbot M. Fractures of the humeral shaft. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma. 4th ed. Philadelphia: Saunders-Elsevier Company; 2009:1593-1622.
3. Walker M, Palumbo B, Badman B, Brooks J, Van Gelderen J, Mighell M. Humeral shaft fractures: a review. J Shoulder Elbow Surg. 2011;20(5):833-844. doi:10.1016/j.jse.2010.11.030.
4. Balfour GW, Mooney V, Ashby ME. Diaphyseal fractures of the humerus treated with a ready-made fracture brace. J Bone Joint Surg Am. 1982;64(1):11-13. doi:10.2106/00004623-198264010-00002.
5. Jawa A, McCarty P, Doornberg J, Harris M, Ring D. Extra-articular distal-third diaphyseal fractures of the humerus. A comparison of functional bracing and plate fixation. J Bone Joint Surg Am. 2006;88(11):2343-2347. doi:10.2106/JBJS.F.00334.
6. Pehlivan O. Functional treatment of the distal third humeral shaft fractures. Arch Orthop Trauma Surg. 2002;122(7):390-395. doi:10.1007/s00402-002-0403-x.
7. Ring D, Chin K, Taghinia AH, Jupiter JB. Nonunion after functional brace treatment of diaphyseal humerus fractures. J Trauma. 2007;62(5):1157-1158. doi:10.1097/01.ta.0000222719.52619.2c.
8. Sarmiento A, Horowitch A, Aboulafia A, Vangsness CT Jr. Functional bracing for comminuted extra-articular fractures of the distal third of the humerus. J Bone Joint Surg Br. 1990;72(4):283-287.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
10. Toivanen JA, Nieminen J, Laine HJ, Honkonen SE, Jarvinen MJ. Functional treatment of closed humeral shaft fractures. Int Orthop. 2005;29(1):10-13. doi:10.1007/s00264-004-0612-8.
11. Wallny T, Westermann K, Sagebiel C, Reimer M, Wagner UA. Functional treatment of humeral shaft fractures: indications and results. J Orthop Trauma. 1997;11(4):283-287.
12. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 Suppl):S1-S133.
13. Paris H, Tropiano P, Clouet D'orval B, Chaudet H, Poitout DG. Fractures of the shaft of the humerus: systematic plate fixation. Anatomic and functional results in 156 cases and a review of the literature. Rev Chir Orthop Reparatrice Appar Mot. 2000;86(4):346-359.
ABSTRACT
Diaphyseal fractures of the distal humerus have a high rate of union when treated with a functional brace or an above-elbow cast (AEC). This study compares alignment of the humerus and motion of the elbow after functional brace or AEC treatment.
One-hundred and five consecutive patients with a closed, extra-articular fracture of the distal humeral diaphysis were identified in the orthopedic trauma databases of 3 hospitals between 2003 and 2012. Seventy-five patients with a follow-up of at least 6 months or with radiographic and clinical evidence of fracture union were included (51 treated with functional bracing and 24 treated with an AEC).
All of the fractures healed. The average arc of elbow flexion was 130° ± 9° in braced patients vs 127° ± 12° in casted patients. Four patients (8%) in the bracing group and 4 (17%) in the casting group lost >20° of elbow motion. The average varus angulation on radiographs was 17° ± 8° in braced and 13° ± 8° in casted patients, while the average posterior angulation was 9° ± 6° vs 7° ± 7°, respectively.
Closed extra-articular distal diaphyseal humerus fractures heal with both bracing and casting and there are no differences in average elbow motion or radiographic alignment.
Nonoperative treatment of closed fractures of the humeral shaft (AO/OTA [Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association] type 12) with a functional brace or above-elbow cast (AEC) is associated with a high union rate, good motion, and good function. Advocates of casting believe that a brace cannot control fracture alignment as well as a cast that allows for immobilization and molding. Advocates of brace treatment are concerned that immobilization in a cast will cause elbow stiffness.1-11
Continue to: In our differing institutions...
In our differing institutions, there are advocates of each type of treatment, providing the opportunity for a comparison. This retrospective study compares brace and cast treatment. The working hypothesis was that there is no difference in elbow motion 6 months or more after fracture. We also compared radiographic alignment after union.
MATERIALS AND METHODS
Between 2003 and 2012, consecutive adult patients treated for a nonpathological fracture of the diaphysis of the distal humerus at the orthopedic trauma service of 3 level 1 academic trauma centers were identified from prospectively collected trauma injury databases. Patients with vascular injury, ipsilateral upper extremity fracture, and periprosthetic fractures were excluded. The attending orthopedic surgeon chose the treatment method and evaluated the range of motion (ROM) of the elbow and radiographic union at the final ambulatory visit. We included patients followed to clinical and radiographic union with a minimum of 6 months of follow-up. We also included patients with <6 months’ follow-up who demonstrated union and had elbow ROM within 10° of the uninjured arm.
We identified 105 consecutive adult patients with a closed nonpathological extra-articular distal humeral shaft fracture (fracture of the distal humeral shaft with an AO/OTA type-12.A, 12.B, or 12.C pattern) treated with an AEC or a brace in our databases.12 Two patients in the brace group chose surgery to improve alignment within 3 weeks of injury and were excluded from the analysis. Twenty-eight patients had inadequate follow-up.
A total of 75 patients were included in the study. At the first and second institutions, 51 patients were treated with functional bracing with an average follow-up of 7 months. At the third institution, 24 patients were treated with an AEC with an average follow-up of 4 months. Seventeen out of 24 patients in the long arm casting group and 19 out of 51 patients in the bracing group, who were included since they had <6 months of follow-up, demonstrated union and had elbow ROM within 10° of the uninjured arm. Differing methods of closed immobilization were the result of differing treatment algorithms at each institution.
The patients who were treated with a functional brace averaged 34 years of age (range, 18-90 years) and included 27 men and 24 women. The brace was removed at an average of 11.5 weeks (range, 8-18 weeks) after initial injury. Six patients had an injury-associated radial nerve palsy, all of which fully recovered within an average of 4 months (range, 0.5-7 months). Sixteen patients were injured due to a fall from standing height, 2 due to a fall from a greater height than standing, 16 in a motor-vehicle accident, 15 during a sport activity, and 2 were not specifically documented.
Continue to: Four patients had concomitant...
Four patients had concomitant injuries: one patient had a mid-shaft humeral fracture on the contralateral arm; a second had an ankle fracture; a third had an ankle fracture, acetabular fracture, a rib fracture, and pneumothorax; and the fourth had 2 rib fractures.
The patients who were treated with an AEC had an average age of 32 years (range,18-82 years) and included 14 men and 10 women. The cast was removed at an average of 4.2 weeks (range, 3-7 weeks) after the initial injury. Two patients had an injury-associated radial nerve palsy, both of which fully recovered. Five patients were injured due to a fall from standing height, 1 due to a fall from a height greater than standing, 7 during a motor-vehicle accident, 5 during a sport activity, and 6 were not documented. Two patients sustained concomitant injuries: one patient sustained a tibia-fibula fracture, and another patient sustained facial trauma.
The 2 groups were comparable in age and gender, as well as the injury mechanism (Table).
Table. Patient Demographics and Outcome Data
| Functional Bracing (n = 51) | Long Arm Casting (n = 24) | Significance (P < .05) |
Sex |
|
|
|
Male | 27 (54%) | 14 (58%) |
|
Female | 24 (46%) | 10 (42%) |
|
Average age (y) | 34 (range, 18-90) | 32 (range, 18-82) |
|
Mechanism of injury |
|
|
|
Standing height | 16 (31%) | 5 (20%) |
|
Greater height | 2 (4%) | 1 (4%) |
|
Motor vehicle collision | 16 (31%) | 7 (29%) |
|
Sports activity | 15 (29 %) | 5 (21%) |
|
Other | 2 (4%) | 6 (25%) |
|
Follow-up (months) | 7 (range, 2-25) | 4 (range, 2-15) |
|
Elbow range of motion (degrees) | 130 ± 9.4 | 127 ± 11.9 | P = .26 |
Varus/valgus angulation (degrees) | 17 ± 7.8 varus | 13 ± 8.4 varus | P = .11 |
Anterior/posterior angulation (degrees) | 9 ± 6.2 posterior | 7 ± 7.5 posterior | P = .54 |
FUNCTIONAL BRACING TECHNIQUE
Upon presentation after injury, patients were immobilized in a coaptation splint (Figure 1A). Within 10 days, the arm was placed in a pre-manufactured polyethylene functional brace (Corflex) and the arm was supported with a simple sling. Patients were allowed to use the hand for light tasks and move the elbow, but most patients were not capable of active elbow flexion exercises until early healing was established 4 to 6 weeks after injury. Shoulder motion was discouraged until radiographic union. Patients started active, self-assisted elbow and shoulder stretching exercises, and weaned from the brace once radiographic union was confirmed between 6 and 10 weeks after injury (Figures 1B, 1C).
ABOVE-ELBOW CASE
Patients were also initially immobilized in a coaptation splint upon initial presentation. Within 7 days, an above-elbow fiberglass cast with neutral forearm rotation and 90° of elbow flexion was applied with a supracondylar mold, followed by radiographic imaging (Figure 2A). With the fractured arm dependent, a valgus mold was applied as the material hardened in order to align the fracture site and limit varus angulation.
Continue to: There were no shoulder...
There were no shoulder ROM restrictions. Casts were removed, skin checked, and replaced every week for 4 to 6 weeks. Casts were removed when callus was noted on radiographs. After cast removal, physician-taught active and active-assisted elbow stretching exercises were given to patients to be performed on a daily basis at home. Patients were followed until clinical and radiographic union and elbow ROM to within 10° of the injured arm (Figures 2B, 2C).
STATISTICAL ANALYSIS
Alignment of the humerus (including varus-valgus alignment and apex anterior-posterior alignment) was measured on anteroposterior and lateral radiographs as the angle between lines bisecting the humeral diaphysis proximal and distal to the fracture. The normality of the data was tested using the Kolmogorov-Smirnov test. To statistically compare continuous variables with a normal distribution, t-tests were used; otherwise the Wilcoxon t-test was applied. The Pearson’s Chi-Square test was used to statistically compare dichotomous variables, except when expected cell frequency was <5, in which case the Fisher exact test was used. The level of significance was set at P < .05.
RESULTS
RANGE OF MOTION AND RADIOGRAPHIC ALIGNMENT
The average range of elbow motion was 130° ± 9° after brace treatment and 127° ± 12° after cast treatment (P = .26). Four patients (8%) treated with a brace and 3 (12%) treated with a cast lost >20° of elbow motion.
All the fractures healed. The average varus angulation on the anteroposterior radiograph was 17° (range, 2°-26°) in braced patients and 13 (range, 5°-31°) in casted patients (P = .11). The average posterior angulation on the lateral radiograph was 9° (range, 0°-28°) in braced patients vs 7° (range, 2°-33°) in casted patients (P = .54).
Continue to: Two weeks after initiating brace...
COMPLICATIONS
Two weeks after initiating brace treatment, an obese patient suffered a rash with desquamation that necessitated discontinuation of the brace. However, the skin and fracture ultimately healed with a coaptation splint and sling support without additional complications. In the casting cohort, 2 patients returned to the emergency department after AEC placement because of swelling of the hand and pain in the cast. Both casts were removed and reapplied.
DISCUSSION
Fractures of the distal third of the humeral diaphysis heal without surgery. Fracture angulation and elbow stiffness are the concerns that lead to variations in nonoperative treatment.1-3 Advocates of casting believe they can get better alignment without losing elbow motion, and advocates of bracing feel that the brace is less cumbersome.1-3,5-8 We compared these treatments retrospectively and found them comparable.
This study should be considered in light of its limitations. Many patients were lost to follow-up in our urban trauma centers. We do not know if these patients did better, worse, or the same as the patients we were able to evaluate, but our opinion is that patients having problems were more likely to return. The evaluation time was relatively short, but motion can only improve in the longer-term. Two patients that were initially braced chose surgery, probably because either they or their surgeon were nervous about the radiographic appearance of the fracture. In our opinion, continued nonoperative treatment of these patients would not affect the findings.
Cast treatment of distal diaphyseal humerus fractures does not cause permanent elbow stiffness. This is confirmed by our results; as casted patients did not lose final ROM compared to the bracing cohort. These injuries are extra-articular and casted patients are transitioned to bracing once humeri have significant union demonstrated by the arm moving as a unit. To our knowledge, there is no other study that has evaluated casting for these fractures, but it may be that evidence of permanent stiffness with nonoperative treatment of distal metaphyseal fractures of the humerus [AO/OTA type 13] is misapplied to distal humeral shaft fractures [AO/OTA type 12].3,9,10,12 For brace treatment, Sarmiento and colleagues9 showed no significant elbow stiffness in a consecutive cohort of 69 patients, while Jawa and colleagues5 showed no increased elbow stiffness compared to plate fixation. Given the accumulated data,3,5,6,8,13 advocates of operative treatment for distal third diaphyseal humerus fractures12 can no longer site elbow stiffness as a disadvantage of nonoperative treatment, whether with cast or brace.
As shown in this study, patients that choose nonoperative treatment can expect their fracture to heal with an average of approximately 15° of varus angulation, as well as 2 others evaluating brace treatment.5,9 Some will heal with as much as 30° of varus angulation.5,9 The arm may look a little different, particularly in thin patients, but there is no evidence that this angulation affects function. The risks, discomforts, and inconveniences of surgery can be balanced with the ability of surgery to improve alignment and allow elbow motion a few weeks earlier. The aesthetics of the scar after surgery may not be better than the deformity after nonoperative treatment. Patients should be involved in these decisions.
Continue to: No cost comparison...
No cost comparison was done between these 2 treatment modalities. However, both casting and bracing offer substantially lower costs comparted to surgical treatment with high efficacy and less risk for the patient. In some billing environments, closed treatments of fractures are captured as “surgical interventions” with global periods included in the reimbursement. Both casting and bracing are relatively inexpensive with materials that are readily accessible in nearly any general or subspecialty orthopedic practice.
There is a passive implication that operative treatment of distal third diaphyseal humerus fractures affords better results and union for patients in the discussed literature. Our results demonstrate that the distal diaphyseal humerus has a natural anatomic and biologic propensity to heal with closed immobilization. Patients should be made aware that while operative treatments exist for this fracture pattern, nonoperative treatment modalities have proven to be efficacious using a variety of immobilization methods. Thus, patients that prefer nonoperative treatment of a distal third diaphyseal humerus fracture can choose between a cast or a brace with confidence of the efficacy of the nonoperative treatment.
ABSTRACT
Diaphyseal fractures of the distal humerus have a high rate of union when treated with a functional brace or an above-elbow cast (AEC). This study compares alignment of the humerus and motion of the elbow after functional brace or AEC treatment.
One-hundred and five consecutive patients with a closed, extra-articular fracture of the distal humeral diaphysis were identified in the orthopedic trauma databases of 3 hospitals between 2003 and 2012. Seventy-five patients with a follow-up of at least 6 months or with radiographic and clinical evidence of fracture union were included (51 treated with functional bracing and 24 treated with an AEC).
All of the fractures healed. The average arc of elbow flexion was 130° ± 9° in braced patients vs 127° ± 12° in casted patients. Four patients (8%) in the bracing group and 4 (17%) in the casting group lost >20° of elbow motion. The average varus angulation on radiographs was 17° ± 8° in braced and 13° ± 8° in casted patients, while the average posterior angulation was 9° ± 6° vs 7° ± 7°, respectively.
Closed extra-articular distal diaphyseal humerus fractures heal with both bracing and casting and there are no differences in average elbow motion or radiographic alignment.
Nonoperative treatment of closed fractures of the humeral shaft (AO/OTA [Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association] type 12) with a functional brace or above-elbow cast (AEC) is associated with a high union rate, good motion, and good function. Advocates of casting believe that a brace cannot control fracture alignment as well as a cast that allows for immobilization and molding. Advocates of brace treatment are concerned that immobilization in a cast will cause elbow stiffness.1-11
Continue to: In our differing institutions...
In our differing institutions, there are advocates of each type of treatment, providing the opportunity for a comparison. This retrospective study compares brace and cast treatment. The working hypothesis was that there is no difference in elbow motion 6 months or more after fracture. We also compared radiographic alignment after union.
MATERIALS AND METHODS
Between 2003 and 2012, consecutive adult patients treated for a nonpathological fracture of the diaphysis of the distal humerus at the orthopedic trauma service of 3 level 1 academic trauma centers were identified from prospectively collected trauma injury databases. Patients with vascular injury, ipsilateral upper extremity fracture, and periprosthetic fractures were excluded. The attending orthopedic surgeon chose the treatment method and evaluated the range of motion (ROM) of the elbow and radiographic union at the final ambulatory visit. We included patients followed to clinical and radiographic union with a minimum of 6 months of follow-up. We also included patients with <6 months’ follow-up who demonstrated union and had elbow ROM within 10° of the uninjured arm.
We identified 105 consecutive adult patients with a closed nonpathological extra-articular distal humeral shaft fracture (fracture of the distal humeral shaft with an AO/OTA type-12.A, 12.B, or 12.C pattern) treated with an AEC or a brace in our databases.12 Two patients in the brace group chose surgery to improve alignment within 3 weeks of injury and were excluded from the analysis. Twenty-eight patients had inadequate follow-up.
A total of 75 patients were included in the study. At the first and second institutions, 51 patients were treated with functional bracing with an average follow-up of 7 months. At the third institution, 24 patients were treated with an AEC with an average follow-up of 4 months. Seventeen out of 24 patients in the long arm casting group and 19 out of 51 patients in the bracing group, who were included since they had <6 months of follow-up, demonstrated union and had elbow ROM within 10° of the uninjured arm. Differing methods of closed immobilization were the result of differing treatment algorithms at each institution.
The patients who were treated with a functional brace averaged 34 years of age (range, 18-90 years) and included 27 men and 24 women. The brace was removed at an average of 11.5 weeks (range, 8-18 weeks) after initial injury. Six patients had an injury-associated radial nerve palsy, all of which fully recovered within an average of 4 months (range, 0.5-7 months). Sixteen patients were injured due to a fall from standing height, 2 due to a fall from a greater height than standing, 16 in a motor-vehicle accident, 15 during a sport activity, and 2 were not specifically documented.
Continue to: Four patients had concomitant...
Four patients had concomitant injuries: one patient had a mid-shaft humeral fracture on the contralateral arm; a second had an ankle fracture; a third had an ankle fracture, acetabular fracture, a rib fracture, and pneumothorax; and the fourth had 2 rib fractures.
The patients who were treated with an AEC had an average age of 32 years (range,18-82 years) and included 14 men and 10 women. The cast was removed at an average of 4.2 weeks (range, 3-7 weeks) after the initial injury. Two patients had an injury-associated radial nerve palsy, both of which fully recovered. Five patients were injured due to a fall from standing height, 1 due to a fall from a height greater than standing, 7 during a motor-vehicle accident, 5 during a sport activity, and 6 were not documented. Two patients sustained concomitant injuries: one patient sustained a tibia-fibula fracture, and another patient sustained facial trauma.
The 2 groups were comparable in age and gender, as well as the injury mechanism (Table).
Table. Patient Demographics and Outcome Data
| Functional Bracing (n = 51) | Long Arm Casting (n = 24) | Significance (P < .05) |
Sex |
|
|
|
Male | 27 (54%) | 14 (58%) |
|
Female | 24 (46%) | 10 (42%) |
|
Average age (y) | 34 (range, 18-90) | 32 (range, 18-82) |
|
Mechanism of injury |
|
|
|
Standing height | 16 (31%) | 5 (20%) |
|
Greater height | 2 (4%) | 1 (4%) |
|
Motor vehicle collision | 16 (31%) | 7 (29%) |
|
Sports activity | 15 (29 %) | 5 (21%) |
|
Other | 2 (4%) | 6 (25%) |
|
Follow-up (months) | 7 (range, 2-25) | 4 (range, 2-15) |
|
Elbow range of motion (degrees) | 130 ± 9.4 | 127 ± 11.9 | P = .26 |
Varus/valgus angulation (degrees) | 17 ± 7.8 varus | 13 ± 8.4 varus | P = .11 |
Anterior/posterior angulation (degrees) | 9 ± 6.2 posterior | 7 ± 7.5 posterior | P = .54 |
FUNCTIONAL BRACING TECHNIQUE
Upon presentation after injury, patients were immobilized in a coaptation splint (Figure 1A). Within 10 days, the arm was placed in a pre-manufactured polyethylene functional brace (Corflex) and the arm was supported with a simple sling. Patients were allowed to use the hand for light tasks and move the elbow, but most patients were not capable of active elbow flexion exercises until early healing was established 4 to 6 weeks after injury. Shoulder motion was discouraged until radiographic union. Patients started active, self-assisted elbow and shoulder stretching exercises, and weaned from the brace once radiographic union was confirmed between 6 and 10 weeks after injury (Figures 1B, 1C).
ABOVE-ELBOW CASE
Patients were also initially immobilized in a coaptation splint upon initial presentation. Within 7 days, an above-elbow fiberglass cast with neutral forearm rotation and 90° of elbow flexion was applied with a supracondylar mold, followed by radiographic imaging (Figure 2A). With the fractured arm dependent, a valgus mold was applied as the material hardened in order to align the fracture site and limit varus angulation.
Continue to: There were no shoulder...
There were no shoulder ROM restrictions. Casts were removed, skin checked, and replaced every week for 4 to 6 weeks. Casts were removed when callus was noted on radiographs. After cast removal, physician-taught active and active-assisted elbow stretching exercises were given to patients to be performed on a daily basis at home. Patients were followed until clinical and radiographic union and elbow ROM to within 10° of the injured arm (Figures 2B, 2C).
STATISTICAL ANALYSIS
Alignment of the humerus (including varus-valgus alignment and apex anterior-posterior alignment) was measured on anteroposterior and lateral radiographs as the angle between lines bisecting the humeral diaphysis proximal and distal to the fracture. The normality of the data was tested using the Kolmogorov-Smirnov test. To statistically compare continuous variables with a normal distribution, t-tests were used; otherwise the Wilcoxon t-test was applied. The Pearson’s Chi-Square test was used to statistically compare dichotomous variables, except when expected cell frequency was <5, in which case the Fisher exact test was used. The level of significance was set at P < .05.
RESULTS
RANGE OF MOTION AND RADIOGRAPHIC ALIGNMENT
The average range of elbow motion was 130° ± 9° after brace treatment and 127° ± 12° after cast treatment (P = .26). Four patients (8%) treated with a brace and 3 (12%) treated with a cast lost >20° of elbow motion.
All the fractures healed. The average varus angulation on the anteroposterior radiograph was 17° (range, 2°-26°) in braced patients and 13 (range, 5°-31°) in casted patients (P = .11). The average posterior angulation on the lateral radiograph was 9° (range, 0°-28°) in braced patients vs 7° (range, 2°-33°) in casted patients (P = .54).
Continue to: Two weeks after initiating brace...
COMPLICATIONS
Two weeks after initiating brace treatment, an obese patient suffered a rash with desquamation that necessitated discontinuation of the brace. However, the skin and fracture ultimately healed with a coaptation splint and sling support without additional complications. In the casting cohort, 2 patients returned to the emergency department after AEC placement because of swelling of the hand and pain in the cast. Both casts were removed and reapplied.
DISCUSSION
Fractures of the distal third of the humeral diaphysis heal without surgery. Fracture angulation and elbow stiffness are the concerns that lead to variations in nonoperative treatment.1-3 Advocates of casting believe they can get better alignment without losing elbow motion, and advocates of bracing feel that the brace is less cumbersome.1-3,5-8 We compared these treatments retrospectively and found them comparable.
This study should be considered in light of its limitations. Many patients were lost to follow-up in our urban trauma centers. We do not know if these patients did better, worse, or the same as the patients we were able to evaluate, but our opinion is that patients having problems were more likely to return. The evaluation time was relatively short, but motion can only improve in the longer-term. Two patients that were initially braced chose surgery, probably because either they or their surgeon were nervous about the radiographic appearance of the fracture. In our opinion, continued nonoperative treatment of these patients would not affect the findings.
Cast treatment of distal diaphyseal humerus fractures does not cause permanent elbow stiffness. This is confirmed by our results; as casted patients did not lose final ROM compared to the bracing cohort. These injuries are extra-articular and casted patients are transitioned to bracing once humeri have significant union demonstrated by the arm moving as a unit. To our knowledge, there is no other study that has evaluated casting for these fractures, but it may be that evidence of permanent stiffness with nonoperative treatment of distal metaphyseal fractures of the humerus [AO/OTA type 13] is misapplied to distal humeral shaft fractures [AO/OTA type 12].3,9,10,12 For brace treatment, Sarmiento and colleagues9 showed no significant elbow stiffness in a consecutive cohort of 69 patients, while Jawa and colleagues5 showed no increased elbow stiffness compared to plate fixation. Given the accumulated data,3,5,6,8,13 advocates of operative treatment for distal third diaphyseal humerus fractures12 can no longer site elbow stiffness as a disadvantage of nonoperative treatment, whether with cast or brace.
As shown in this study, patients that choose nonoperative treatment can expect their fracture to heal with an average of approximately 15° of varus angulation, as well as 2 others evaluating brace treatment.5,9 Some will heal with as much as 30° of varus angulation.5,9 The arm may look a little different, particularly in thin patients, but there is no evidence that this angulation affects function. The risks, discomforts, and inconveniences of surgery can be balanced with the ability of surgery to improve alignment and allow elbow motion a few weeks earlier. The aesthetics of the scar after surgery may not be better than the deformity after nonoperative treatment. Patients should be involved in these decisions.
Continue to: No cost comparison...
No cost comparison was done between these 2 treatment modalities. However, both casting and bracing offer substantially lower costs comparted to surgical treatment with high efficacy and less risk for the patient. In some billing environments, closed treatments of fractures are captured as “surgical interventions” with global periods included in the reimbursement. Both casting and bracing are relatively inexpensive with materials that are readily accessible in nearly any general or subspecialty orthopedic practice.
There is a passive implication that operative treatment of distal third diaphyseal humerus fractures affords better results and union for patients in the discussed literature. Our results demonstrate that the distal diaphyseal humerus has a natural anatomic and biologic propensity to heal with closed immobilization. Patients should be made aware that while operative treatments exist for this fracture pattern, nonoperative treatment modalities have proven to be efficacious using a variety of immobilization methods. Thus, patients that prefer nonoperative treatment of a distal third diaphyseal humerus fracture can choose between a cast or a brace with confidence of the efficacy of the nonoperative treatment.
1. McKee MD. Fractures of the shaft of the humerus. In: Bucholz R, Heckman JD, Court-Brown C, eds. Rockwood and Green’s Fractures in Adults. 6th ed. Philadelphia: Lippencott Williams & Wilkins; 2006:1117-1159.
2. Schemitsch E, Bhandari M, Talbot M. Fractures of the humeral shaft. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma. 4th ed. Philadelphia: Saunders-Elsevier Company; 2009:1593-1622.
3. Walker M, Palumbo B, Badman B, Brooks J, Van Gelderen J, Mighell M. Humeral shaft fractures: a review. J Shoulder Elbow Surg. 2011;20(5):833-844. doi:10.1016/j.jse.2010.11.030.
4. Balfour GW, Mooney V, Ashby ME. Diaphyseal fractures of the humerus treated with a ready-made fracture brace. J Bone Joint Surg Am. 1982;64(1):11-13. doi:10.2106/00004623-198264010-00002.
5. Jawa A, McCarty P, Doornberg J, Harris M, Ring D. Extra-articular distal-third diaphyseal fractures of the humerus. A comparison of functional bracing and plate fixation. J Bone Joint Surg Am. 2006;88(11):2343-2347. doi:10.2106/JBJS.F.00334.
6. Pehlivan O. Functional treatment of the distal third humeral shaft fractures. Arch Orthop Trauma Surg. 2002;122(7):390-395. doi:10.1007/s00402-002-0403-x.
7. Ring D, Chin K, Taghinia AH, Jupiter JB. Nonunion after functional brace treatment of diaphyseal humerus fractures. J Trauma. 2007;62(5):1157-1158. doi:10.1097/01.ta.0000222719.52619.2c.
8. Sarmiento A, Horowitch A, Aboulafia A, Vangsness CT Jr. Functional bracing for comminuted extra-articular fractures of the distal third of the humerus. J Bone Joint Surg Br. 1990;72(4):283-287.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
10. Toivanen JA, Nieminen J, Laine HJ, Honkonen SE, Jarvinen MJ. Functional treatment of closed humeral shaft fractures. Int Orthop. 2005;29(1):10-13. doi:10.1007/s00264-004-0612-8.
11. Wallny T, Westermann K, Sagebiel C, Reimer M, Wagner UA. Functional treatment of humeral shaft fractures: indications and results. J Orthop Trauma. 1997;11(4):283-287.
12. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 Suppl):S1-S133.
13. Paris H, Tropiano P, Clouet D'orval B, Chaudet H, Poitout DG. Fractures of the shaft of the humerus: systematic plate fixation. Anatomic and functional results in 156 cases and a review of the literature. Rev Chir Orthop Reparatrice Appar Mot. 2000;86(4):346-359.
1. McKee MD. Fractures of the shaft of the humerus. In: Bucholz R, Heckman JD, Court-Brown C, eds. Rockwood and Green’s Fractures in Adults. 6th ed. Philadelphia: Lippencott Williams & Wilkins; 2006:1117-1159.
2. Schemitsch E, Bhandari M, Talbot M. Fractures of the humeral shaft. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma. 4th ed. Philadelphia: Saunders-Elsevier Company; 2009:1593-1622.
3. Walker M, Palumbo B, Badman B, Brooks J, Van Gelderen J, Mighell M. Humeral shaft fractures: a review. J Shoulder Elbow Surg. 2011;20(5):833-844. doi:10.1016/j.jse.2010.11.030.
4. Balfour GW, Mooney V, Ashby ME. Diaphyseal fractures of the humerus treated with a ready-made fracture brace. J Bone Joint Surg Am. 1982;64(1):11-13. doi:10.2106/00004623-198264010-00002.
5. Jawa A, McCarty P, Doornberg J, Harris M, Ring D. Extra-articular distal-third diaphyseal fractures of the humerus. A comparison of functional bracing and plate fixation. J Bone Joint Surg Am. 2006;88(11):2343-2347. doi:10.2106/JBJS.F.00334.
6. Pehlivan O. Functional treatment of the distal third humeral shaft fractures. Arch Orthop Trauma Surg. 2002;122(7):390-395. doi:10.1007/s00402-002-0403-x.
7. Ring D, Chin K, Taghinia AH, Jupiter JB. Nonunion after functional brace treatment of diaphyseal humerus fractures. J Trauma. 2007;62(5):1157-1158. doi:10.1097/01.ta.0000222719.52619.2c.
8. Sarmiento A, Horowitch A, Aboulafia A, Vangsness CT Jr. Functional bracing for comminuted extra-articular fractures of the distal third of the humerus. J Bone Joint Surg Br. 1990;72(4):283-287.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
10. Toivanen JA, Nieminen J, Laine HJ, Honkonen SE, Jarvinen MJ. Functional treatment of closed humeral shaft fractures. Int Orthop. 2005;29(1):10-13. doi:10.1007/s00264-004-0612-8.
11. Wallny T, Westermann K, Sagebiel C, Reimer M, Wagner UA. Functional treatment of humeral shaft fractures: indications and results. J Orthop Trauma. 1997;11(4):283-287.
12. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 Suppl):S1-S133.
13. Paris H, Tropiano P, Clouet D'orval B, Chaudet H, Poitout DG. Fractures of the shaft of the humerus: systematic plate fixation. Anatomic and functional results in 156 cases and a review of the literature. Rev Chir Orthop Reparatrice Appar Mot. 2000;86(4):346-359.
TAKE-HOME POINTS
- Closed extra-articular distal diaphyseal humerus fractures heal predictably with both bracing and casting.
- There are no differences in average elbow motion between bracing and casting of these fractures.
- There are no differences in radiographic alignment between bracing and casting of these fractures.
- The distal diaphyseal humerus has a natural anatomic and biologic propensity to heal with closed immobilization.
- Patients preferring nonoperative treatment can choose between a cast or a brace with confidence of the efficacy of either treatment.
The Effect of Immunonutrition on Veterans Undergoing Major Surgery for Gastrointestinal Cancer (FULL)
Immunonutrition involves the use of omega-3 fatty acids, glutamine, arginine, and/or nucleotides individually or in combination at therapeutic levels to specifically modulate the immune system against altering inflammatory and metabolic pathways.1 Current literature supports the routine use of immune-enhancing formulas (containing both arginine and fish oil) in surgical patients.2-4 Although most of the literature favors the use of immunonutrition in surgical patients, some studies reported no benefit over standard oral nutrition supplementation.5
Background
Most studies evaluating the effect of immunonutrition for those undergoing elective surgery have been conducted in surgical oncology patients.6-12 Advanced cancers and older age can lead to cancer cachexia and sarcopenia, respectively. These conditions increase a patient’s surgical morbidity and mortality risk likely because of the negative effects on lean body mass, nutrient intake, and inflammatory and metabolic profile.13 However, early detection of some cancers through routine screening might lead to earlier surgical intervention that minimizes these negative tumor effects on the patient. Immunonutrition provided to well-nourished and malnourished patients has shown benefits, which supports the premise that a combination of immunonutrients included in immune-enhancing diets might have a beneficial pharmacotherapeutic effect beyond that of providing energy, protein, vitamins, and minerals for nutritional support.7,14
There are a lack of data regarding whether there is a window of opportunity for improved outcomes. Is the greatest need for immunonutrients during the peak of the injury, which might be immediately after surgery, or is it before the procedure? Arginine is a conditionally essential amino acid that has been shown to have a beneficial effect on the immune system by enhancing T-lymphocyte response when supplemented in surgical patients. When the arginase 1 (ARG 1) enzyme in myeloid cells is expressed during the inflammatory response to injury, accelerated use of arginine can deplete endogenous arginine, making it conditionally essential.
If adequate arginine cannot be synthesized or an exogenous source is not provided, T-cell dysfunction and decreased nitric oxide production leads to immune and vascular dysfunction, respectively.15,16 Providing arginine and omega-3 fatty acids might have a synergistic effect by shifting to an anti-inflammatory prostaglandin profile that has been shown to decrease ARG 1 expression while providing an exogenous source of arginine.17 Postsurgical inflammation might be caused in part by pro-inflammatory mediators and the anti-inflammatory properties of omega-3 fatty acids might offset this if cell membranes are loaded preoperatively.18 Therefore, preoperative immunonutrition might allow tissues to recover from planned surgical trauma. Bouwens and colleagues demonstrated that intake of eicosapentaenoic acid/docosahexaenoic acid over 26 weeks can alter the gene expression profiles of immune cells to a more anti-inflammatory status.19 However, Senkal and colleagues recommended that 3 to 7 days preoperatively is adequate to positively alter the lipid profile of tissues.20
Oncology patients preparing for surgery often are exposed to the physiologic stress of radiation and chemotherapy as neoadjuvant treatment to surgery. Oncology treatment and the adverse nutritional effects of treatment increase risk for arginine deficiency, such as poor nutrition intake, increased requirements, decreased production. Braga and colleagues demonstrated improved gut microprofusion and gut oxygenation intraoperatively, an effect that continued for up to 5 days after surgery.21 Waitzberg conducted a systematic review of randomized clinical trials evaluating immunonutrition in preoperative, postoperative, and perioperative periods. The results showed that the greatest improvements in postoperative infections and length of stay occurred in patients receiving preoperative 0.5 to 1 L/d of an immune nutrition product containing supplemental omega-3 fatty acids, arginine, and nucleotides for 5 to 7 days.22
It is unclear which population of surgical patients benefit the most from immunonutrition. Some results in the literature favor use in malnourished patients.18,23 However, other studies also have found benefit in well-nourished patients.7,14,21
Veterans who seek medical care at the Department of Veteran Affairs (VA) have higher rates of cancer, obesity, and diabetes mellitus, which complicate surgical outcomes.24 In addition to comorbidities, veterans who seek medical care at the VA are more likely to have been deployed overseas and have more physical and mental health disorders compared with that of nonveteran patients or veterans who do not use the VA. Because of higher comorbidities, unique deployment history, and mental health disorders, all of which may impact quality of life concerns, veterans are clinically more complex, which makes comparisons with the private sector difficult. The VA has the advantage of providing comprehensive care to veterans in all settings, including preparation for surgery and postsurgical follow-up with an interdisciplinary team.
The objective of this study was to compare surgical outcomes in veterans who receive preoperative supplementation using an immune-modulating formula with veterans who received a standard oral supplement. Although practice guidelines have been developed from studies in US nonveteran populations, there are no high- quality randomized studies of veterans.
This study design also would allow the VA to gauge cost-effectiveness of immunonutrition before implementing new protocols. There is convincing data supporting significant economic benefit; however, more cost-benefit studies are needed to fully assess.18,25-27 Immunonutrition products are more expensive than are standard nutrition supplements, but overall cost of care when immunonutrition products are used could be lower because of reduction of complications and hospital resources.
Methods
From November 2011 to January 2016, the authors conducted a single-center, prospective, randomized parallel-group study in veterans undergoing elective gastrointestinal oncologic surgery. Inclusion criteria included planned esophageal, gastric, pancreatic, colorectal, or liver resections in veterans with histologically documented neoplasm of the gastrointestinal tract. Patients were excluded if they were admitted to the intensive care unit (ICU) before surgery, were receiving steroids or other immunosuppressive medications, had a recent hospital admission for pulmonary, cardiac, or renal disease, or were exhibiting signs or symptoms of infection or sepsis, including elevated white blood cells (WBC) > 10,000/mL or a temperature > 37.7° C.
The study was approved by the research and development committee and the institutional review board at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida. The clinicaltrials.gov identifier for the study was NCT01471743.
Nutrition Formula
Subjects were randomized into 2 oral supplement groups: immunonutrition group (ING) patients received immunonutrition, and standard nutrition group (SNG) received a standard formula (Table 1).
Study Procedures
All veterans with planned gastrointestinal surgeries were evaluated in the JAHVH general surgery clinic. Veterans meeting the inclusion criteria were invited to participate in the study, and informed consent was obtained. A research randomizer program assigned subjects to the groups to reach equal 1:1 randomization. Enrolled participants were provided their randomized supplement (unblinded) in the general surgery clinic and instructed on the amount of supplement to consume and date to begin taking the supplement. Participants were instructed to continue with their normal diet in addition to the supplement. No additional nutrition education was provided. Participants were asked to keep track of their daily supplement intake. Patients in both groups also used preoperative bowel preparations when indicated.
At the time of enrollment, presurgical comorbidities, anthropometric data, and nutrition status parameters were obtained. Postoperatively, study personnel interviewed each patient about formula consumption and tolerance. Thirty days postoperatively, patient demographics, surgical characteristics (eg, surgery, operative time, blood loss), nutrition risk screening (NRS 2002) score, diet/enteral orders, days spent NPO, days in the hospital or in the ICU, and complications (eg, wound infection, abscess, sepsis, pneumonia, urinary tract infection, intestinal fistula, ileus, or anastomotic leakage) were collected from the electronic health record.
Statistical Analysis
The primary outcome measure was overall postoperative complication rate and postoperative infection rate. Based on reviews of similar studies available at the time of protocol development, it was assumed that a postoperative infection rate of 38% in the SNG and 15% in the ING would indicate treatment efficacy. A sample size of 54 patients in each group would provide a Type I error level α = .05 and a power of 80%. A total of 108 patients enrolled in the study. Chi-square analysis was used to determine this primary outcome measure.
Secondary outcomes (mean number of complications, hospital days, NPO (nothing by mouth) days, and ICU days) were evaluated with Mann Whitney U test because of violation of assumptions for the t test. All P values were 2-tailed and statistical significance was accepted at P < .05 with clinical significance accepted at P < .10. Analysis for intention to treat (ITT) and per protocol are provided for outcome measures. For the ITT analysis, multiple imputation (last observation carried forward) was used. Sensitivity analysis found that the data were missing at random. SPSS software version 21.0 (Chicago, IL) was used for statistical analysis.
Results
During the study period, 137 patients were assessed for eligibility (Figure).
The sample was predominately white and male, which is consistent with the veteran population. There were no statistical differences for baseline patient or surgical characteristics between the groups (Table 2).
There was a significant difference (P = .09) in the surgical procedures completed. There was only 1 pancreatic surgery completed in the ING and 9 pancreatic surgeries completed in the SNG.
Primary Outcomes
The overall rate of complications differed between the groups (Table 3).
Given the large number of colorectal procedures, a separate per-protocol analysis included 37 patients from ING and 36 patients in the SNG (Table 4).
Secondary Outcomes
The ITT analysis found that overall number of hospital days was slightly higher in the ING compared with that of the SNG, 9.4 vs 9.3 days, respectively. In the per-protocol analysis there were 1.3 fewer hospital days for those who received immunonutrition (P = .059). No significant differences were found between the groups in the number of days spent in the ICU or number of days NPO (Table 3). Death within 30 days postoperative was twice as high for those in the SNG vs ING, with no deaths in the per-protocol analysis for those in the ING.
The colorectal analysis found 8.5 hospital days for ING patients vs 10.0 days for SNG patients, (P = .08). There were no deaths in the ING and 1 death in the SNG for colorectal procedure patients.
Discussion
Surgery is traumatic to healthy patients with or without cancer. Patients with cancer who receive surgical intervention might be at an even higher risk for complications because of altered metabolic pathways, nutritional deficiencies, and depressed immune function.13 Meta-analyses of immunonutrition studies conducted over the past 2 decades have come to different conclusions regarding the benefit of immunonutrition in the elective gastrointestinal cancer surgery population.3,5,18 Although practice guidelines from the American Society of Parenteral and Enteral Nutrition and the European Society of Parenteral and Enteral Nutrition recommend routine use of immune-modulating formulas in surgical oncology patients, there is still some debate about the optimal timing, dose, individual formula constituents, and populations that will benefit.2,25 Earlier studies evaluating the economics of immunonutrition have shown significant cost savings related to reduction in length of stay and decrease in infectious complications even after accounting for the extra cost of the formula.26,27 More recent economic analyses confirmed these cost savings showing a savings of about $1,000 to $2,500 per patient with higher savings when immunonutrition was given preoperatively.28,29
For practitioners treating veterans with cancer, good stewardship of federal dollars and optimal outcomes are important considerations before implementing new therapies. Therefore, JAHVH set out to evaluate whether standard oral nutrition supplementation would be as effective as the higher cost immunonutrition supplementation in cancer patients receiving elective surgical procedures.
Rates of Complications
In this study, favorable effects of immunonutrition were found on total postoperative complications and number of hospital days. The total number of patients who experienced complications was 39% lower in the ING than it was in SNG in the ITT analysis and 37% lower in the colorectal per-protocol analysis. These rates are similar to the 48% lower rate Braga and colleagues found in their study in patients with colorectal cancer who received 5 days of preoperative immunonutrition.21 Because more than half of the patients in this study had colorectal cancer, the group is comparable to the Braga and colleagues study population. The overall supplement adherence rate was 86%, which was slightly lower than the 90% adherence rate that Braga and colleagues found. Lower consumption rates might have been a factor in not achieving a greater therapeutic benefit for infectious complications. Some studies suggest a therapeutic goal intake of greater than two-thirds of the prescribed amount.10,30 In the present study, 70.4% of the ING and 83% of the SNG met that recommended therapeutic goal, which is more than Hübner colleagues reported in their study (53% of the ING and 60% in the SNG meeting therapeutic intake goal).
Okamoto and colleagues also reported a much lower complication rate in gastric cancer patients who received immunonutrition (13.3%) compared with that of those receiving an isoenergetic formula (40%).11 The group receiving immunonutrition in the Okamoto and colleagues study had 4 times fewer infectious complications than did the standard group (P = .039), and a contributing reason might be that they supplemented for 7 days preoperatively. Similar to the current study’s results, Giger-Pabst and colleagues and Hübner and colleagues did not find any significant difference in infectious complications.10,30 Important notes of comparison include a low adherence rate in the study conducted by Hübner and colleagues and the lower dose of immunonutrition used by Giger-Pabst andcolleagues who used 3 days of preoperative supplementation, which may not be long enough to promote the tissue benefits of immunonutrition.
Although, the current study did not find any statistically significant difference in infectious complications, the SNG experienced 1.8 times more infections than did the ING, which indicates that immunonutrition support may be clinically beneficial. Based on previous literature and the results of this study, the authors speculate that at least 5 days of intake of the study immunonutrition formula could positively affect outcomes.
The authors suspect that the added arginine and fish oil in the immunonutrition product act synergistically as therapeutic ingredients to shift toward a preoperative anti-inflammatory prostaglandin environment while providing exogenous arginine to possibly prevent or correct a conditionally essential need for arginine that would promote adequate nitric oxide production. Another crucial factor is that the a priori power analysis was looking at a 38% complication rate in the SNG and only 15% complication rate in the ING, which generated a sample size of 108 participants. The post hoc power analysis indicates that this study is underpowered based on the complication rates, which could be a reason for insignificant infectious complications.
The benefits of immunonutrients are still being studied. Future studies in a controlled surgical setting could determine whether immunonutrition has a clinical outcome effect on operative time and surgical blood loss. A challenge for the investigators was to decide whether the difference in operative time and blood loss was a surgical characteristic or a clinical outcome. The positive impact of immunonutrients on tissue perfusion and cell integrity have been shown in other studies to reduce tissue inflammation and alter gene expression, which could affect how tissues respond to surgical insults.10,11 Because JAHVH is a teaching institution and multiple surgeons are involved with the patients, this question will continue to be unresolved. Future research may want to consider controlling for variability in surgical technique and perioperative protocols to evaluate this as a clinical outcome.
Limitations
Several limitations of this trial need to be addressed. Although the design of the study was a randomized controlled trial, it was an unblinded, single-center study with a small sample size. Surgeons were not aware of which supplement each subject had received; however, researchers took no measures to ensure the surgeons were blinded. To minimize bias, 2 investigators evaluated the records for complication rates to confirm consistency, and any discrepancies were resolved by a third investigator. Although adherence was evaluated, it was patient-reported, and lab testing was not conducted to ensure that tissues were loaded with therapeutic amounts of immunonutrients or to determine baseline levels of nutrient intake, which could show a nutrient response curve.
The use of other nutritional supplements, such as vitamins, probiotics, or additional fatty acids were not monitored, and the study formulas differed in protein and fiber content, which could have impacted the overall nutrient intake and affected the primary outcomes. Another limitation includes the variety of surgeons used over the period of the study. At a teaching institution, it is not feasible to limit the number of surgeons performing surgery.
Additionally, the study period was 5 years, and there have been changes in fasting times, medications, and bowel preparation over the course of that period, which could not be accounted for. Postoperative immunonutrition was not provided in this study based on the limited evidence available when the protocol was initiated. However, since that time, evidence supports and encourages postoperative therapy and might have proven beneficial to the patients. Data were not collected on the need for additional surgery within the study period, which could significantly impact outcomes.
Future studies would benefit from a longer postoperative monitoring period because this study looked only at the 30-day postoperative period. Last, randomization did not account for equal allocation of surgical procedures, and a higher number of pancreatic surgeries in the SNG could account for the higher complication rate found in that group. Although the colorectal analysis is underpowered, the results continue to show beneficial results with the use of immunonutrition.
Conclusion
The primary purpose of this research was to determine whether the veteran population would benefit from an immunonutrition preoperative protocol as recommended by several practice guidelines. The results of the initial analysis and the colorectal analysis were presented to the hospital interdisciplinary nutrition committee who voted that a preoperative immunonutrition protocol will be implemented at JAHVH because of the high comorbidity rate experienced by veterans.
1. Grimble RF. Immunonutrition. Curr Opin Gastroenterol. 2005;21(2):216-222.
2. McClave SA, Martindale RG, Vanek VW, et al; A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2009;33(3):277-316.
3. Marimuthu K, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann Surg. 2012;255(6):1060-1068.
4. Bharadwaj S, Trivax B, Tandon P, Alkam B, Hanouneh I, Steiger E. Should perioperative immunonutrition for elective surgery be the current standard of care? Gastroenterol Rep (Oxford). 2016;4(2):87-95.
5. Hegazi RA, Hustead DS, Evans DC. Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg. 2014;219(5):1078-1087.
6. Xu J, Zhong Y, Jing D, Wu Z. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg. 2006;30(7):1284-1289.
7. Horie H, Okada M, Kojima M, Nagai H. Favorable effects of preoperative enteral immunonutrition on a surgical site infection in patients with colorectal cancer without malnutrition. Surg Today. 2006;36(12):1063-1068.
8. Fujitani K, Tsujinaka T, Fujita J, et al; Osaka Gastrointestinal Cancer Chemotherapy Study Group. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg. 2012;99(5):621-629.
9. Braga M, Gianotti L, Nespoli L, Radaelli G, Di Carlo V. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg. 2002;137(2):174-180.
10. Giger-Pabst U, Lange J, Maurer C, et al. Short-term preoperative supplementation of an immunoenriched diet does not improve clinical outcome in well-nourished patients undergoing abdominal cancer surgery. Nutrition. 2013;29(5):724-729.
11. Okamoto Y, Okano K, Izuishi K, Usuki H, Wakabayashi H, Suzuki Y. Attenuation of the systemic inflammatory response and infectious complications after gastrectomy with preoperative oral arginine and omega-3 fatty acids supplemented immunonutrition. World J Surg. 2009;33(9):1815-1821.
12. Yildiz SY, Yazicoiog˘lu MB, Tiryaki Ç, Çiftçi A, Boyaciog˘lu Z. The effect of enteral immunonutrition in upper gastrointestinal surgery for cancer: a prospective study. Turk J Med Sci. 2016;46(2):393-400.
13. Peterson SJ, Mozer M. Differentiating sarcopenia and cachexia among patients with cancer. Nutr Clin Pract. 2017;32(1):30-39.
14. Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122(7):1763-1770.
15. Daly JM, Reynolds J, Thom A, et al. Immune and metabolic effects of arginine in the surgical patient. Ann Surg. 1988;208(4):512-523.
16. Aida T, Furukawa K, Suzuki D, et al. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreato-duodenectomy. Surgery. 2014;155(1):124-133.
17. Bansal V, Syres KM, Makarenkova V, et al. Interactions between fatty acids and arginine metabolism: implications for the design of immune-enhancing diets. JPEN J Parenter Enteral Nutr. 2005;29(1 suppl):S75-S80.
18. Osland E, Hossain MB, Khan S, Memon MA. Effect of timing of pharmaconutrition (immunonutrition) administration on outcomes of elective surgery for gastrointestinal malignancies: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. 2014;38(1):53-69.
19. Bouwens M, van de Rest O, Dellschaft N, et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90(2):415-424.
20. Senkal M, Haaker R, Linseisen J, Wolfram G, Homann HH, Stehle P. Preoperative oral supplementation with long-chain omega-3 fatty acids beneficially alters phospholipid fatty acid patterns in liver, gut mucosa, and tumor tissue. JPEN J Parenter Enteral Nutr. 2005;29(4):236-240.
21. Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132(5):805-814.
22. Waitzberg DL, Saito H, Plank LD, et al. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006;30(8):1592-1604.
23. Klek S, Sierzega M, Szybinski P, et al. The immunomodulating enteral nutrition in malnourished surgical patients—a prospective, randomized, double-blind clinical trial. Clin Nutr. 2011;30(3):282-288.
24. Farmer CM, Hosek SD, Adamson DM. Balancing demand and supply for veteran’s health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
25. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11-48.
26. Mauskopf JA, Candrilli SD, Chevrou-Séverac H, Ochoa JB. Immunonutrition for patients undergoing elective surgery for gastrointestinal cancer: Impact on hospital costs. World J Surg Oncol. 2012;10:136.
27. Senkal M, Mumme A, Eickhoff U, et al. Early postoperative enteral immunonutrition: clinical outcome and cost-comparison analysis in surgical patients. Crit Care Med. 1997;25(9):1489-1496.
28. Chevrou-Séverac H, Pinget C, Cerantola Y, Demartines N, Wasserfallen JB, Schäfer M. Cost-effectiveness analysis of immune-modulating nutritional support for gastrointestinal cancer patients. Clin Nutr. 2014;33(4):649-654.
29. Strickland A, Brogan A, Krauss J, Martindale R, Cresci G. Is the use of specialized nutritional formulations a cost-effective strategy? A national database evaluation. JPEN J Parenter Enteral Nutr. 2005;29(1 suppl):S81-S91.
30. Hübner M, Cerantola Y, Grass F, Bertrand PC, Schäfer M, Demartines N. Preoperative immunonutrition in patients at nutritional risk: results of a double-blinded randomized clinical trial. Eur J Clin Nutr. 2012;66(7):850-855.
Immunonutrition involves the use of omega-3 fatty acids, glutamine, arginine, and/or nucleotides individually or in combination at therapeutic levels to specifically modulate the immune system against altering inflammatory and metabolic pathways.1 Current literature supports the routine use of immune-enhancing formulas (containing both arginine and fish oil) in surgical patients.2-4 Although most of the literature favors the use of immunonutrition in surgical patients, some studies reported no benefit over standard oral nutrition supplementation.5
Background
Most studies evaluating the effect of immunonutrition for those undergoing elective surgery have been conducted in surgical oncology patients.6-12 Advanced cancers and older age can lead to cancer cachexia and sarcopenia, respectively. These conditions increase a patient’s surgical morbidity and mortality risk likely because of the negative effects on lean body mass, nutrient intake, and inflammatory and metabolic profile.13 However, early detection of some cancers through routine screening might lead to earlier surgical intervention that minimizes these negative tumor effects on the patient. Immunonutrition provided to well-nourished and malnourished patients has shown benefits, which supports the premise that a combination of immunonutrients included in immune-enhancing diets might have a beneficial pharmacotherapeutic effect beyond that of providing energy, protein, vitamins, and minerals for nutritional support.7,14
There are a lack of data regarding whether there is a window of opportunity for improved outcomes. Is the greatest need for immunonutrients during the peak of the injury, which might be immediately after surgery, or is it before the procedure? Arginine is a conditionally essential amino acid that has been shown to have a beneficial effect on the immune system by enhancing T-lymphocyte response when supplemented in surgical patients. When the arginase 1 (ARG 1) enzyme in myeloid cells is expressed during the inflammatory response to injury, accelerated use of arginine can deplete endogenous arginine, making it conditionally essential.
If adequate arginine cannot be synthesized or an exogenous source is not provided, T-cell dysfunction and decreased nitric oxide production leads to immune and vascular dysfunction, respectively.15,16 Providing arginine and omega-3 fatty acids might have a synergistic effect by shifting to an anti-inflammatory prostaglandin profile that has been shown to decrease ARG 1 expression while providing an exogenous source of arginine.17 Postsurgical inflammation might be caused in part by pro-inflammatory mediators and the anti-inflammatory properties of omega-3 fatty acids might offset this if cell membranes are loaded preoperatively.18 Therefore, preoperative immunonutrition might allow tissues to recover from planned surgical trauma. Bouwens and colleagues demonstrated that intake of eicosapentaenoic acid/docosahexaenoic acid over 26 weeks can alter the gene expression profiles of immune cells to a more anti-inflammatory status.19 However, Senkal and colleagues recommended that 3 to 7 days preoperatively is adequate to positively alter the lipid profile of tissues.20
Oncology patients preparing for surgery often are exposed to the physiologic stress of radiation and chemotherapy as neoadjuvant treatment to surgery. Oncology treatment and the adverse nutritional effects of treatment increase risk for arginine deficiency, such as poor nutrition intake, increased requirements, decreased production. Braga and colleagues demonstrated improved gut microprofusion and gut oxygenation intraoperatively, an effect that continued for up to 5 days after surgery.21 Waitzberg conducted a systematic review of randomized clinical trials evaluating immunonutrition in preoperative, postoperative, and perioperative periods. The results showed that the greatest improvements in postoperative infections and length of stay occurred in patients receiving preoperative 0.5 to 1 L/d of an immune nutrition product containing supplemental omega-3 fatty acids, arginine, and nucleotides for 5 to 7 days.22
It is unclear which population of surgical patients benefit the most from immunonutrition. Some results in the literature favor use in malnourished patients.18,23 However, other studies also have found benefit in well-nourished patients.7,14,21
Veterans who seek medical care at the Department of Veteran Affairs (VA) have higher rates of cancer, obesity, and diabetes mellitus, which complicate surgical outcomes.24 In addition to comorbidities, veterans who seek medical care at the VA are more likely to have been deployed overseas and have more physical and mental health disorders compared with that of nonveteran patients or veterans who do not use the VA. Because of higher comorbidities, unique deployment history, and mental health disorders, all of which may impact quality of life concerns, veterans are clinically more complex, which makes comparisons with the private sector difficult. The VA has the advantage of providing comprehensive care to veterans in all settings, including preparation for surgery and postsurgical follow-up with an interdisciplinary team.
The objective of this study was to compare surgical outcomes in veterans who receive preoperative supplementation using an immune-modulating formula with veterans who received a standard oral supplement. Although practice guidelines have been developed from studies in US nonveteran populations, there are no high- quality randomized studies of veterans.
This study design also would allow the VA to gauge cost-effectiveness of immunonutrition before implementing new protocols. There is convincing data supporting significant economic benefit; however, more cost-benefit studies are needed to fully assess.18,25-27 Immunonutrition products are more expensive than are standard nutrition supplements, but overall cost of care when immunonutrition products are used could be lower because of reduction of complications and hospital resources.
Methods
From November 2011 to January 2016, the authors conducted a single-center, prospective, randomized parallel-group study in veterans undergoing elective gastrointestinal oncologic surgery. Inclusion criteria included planned esophageal, gastric, pancreatic, colorectal, or liver resections in veterans with histologically documented neoplasm of the gastrointestinal tract. Patients were excluded if they were admitted to the intensive care unit (ICU) before surgery, were receiving steroids or other immunosuppressive medications, had a recent hospital admission for pulmonary, cardiac, or renal disease, or were exhibiting signs or symptoms of infection or sepsis, including elevated white blood cells (WBC) > 10,000/mL or a temperature > 37.7° C.
The study was approved by the research and development committee and the institutional review board at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida. The clinicaltrials.gov identifier for the study was NCT01471743.
Nutrition Formula
Subjects were randomized into 2 oral supplement groups: immunonutrition group (ING) patients received immunonutrition, and standard nutrition group (SNG) received a standard formula (Table 1).
Study Procedures
All veterans with planned gastrointestinal surgeries were evaluated in the JAHVH general surgery clinic. Veterans meeting the inclusion criteria were invited to participate in the study, and informed consent was obtained. A research randomizer program assigned subjects to the groups to reach equal 1:1 randomization. Enrolled participants were provided their randomized supplement (unblinded) in the general surgery clinic and instructed on the amount of supplement to consume and date to begin taking the supplement. Participants were instructed to continue with their normal diet in addition to the supplement. No additional nutrition education was provided. Participants were asked to keep track of their daily supplement intake. Patients in both groups also used preoperative bowel preparations when indicated.
At the time of enrollment, presurgical comorbidities, anthropometric data, and nutrition status parameters were obtained. Postoperatively, study personnel interviewed each patient about formula consumption and tolerance. Thirty days postoperatively, patient demographics, surgical characteristics (eg, surgery, operative time, blood loss), nutrition risk screening (NRS 2002) score, diet/enteral orders, days spent NPO, days in the hospital or in the ICU, and complications (eg, wound infection, abscess, sepsis, pneumonia, urinary tract infection, intestinal fistula, ileus, or anastomotic leakage) were collected from the electronic health record.
Statistical Analysis
The primary outcome measure was overall postoperative complication rate and postoperative infection rate. Based on reviews of similar studies available at the time of protocol development, it was assumed that a postoperative infection rate of 38% in the SNG and 15% in the ING would indicate treatment efficacy. A sample size of 54 patients in each group would provide a Type I error level α = .05 and a power of 80%. A total of 108 patients enrolled in the study. Chi-square analysis was used to determine this primary outcome measure.
Secondary outcomes (mean number of complications, hospital days, NPO (nothing by mouth) days, and ICU days) were evaluated with Mann Whitney U test because of violation of assumptions for the t test. All P values were 2-tailed and statistical significance was accepted at P < .05 with clinical significance accepted at P < .10. Analysis for intention to treat (ITT) and per protocol are provided for outcome measures. For the ITT analysis, multiple imputation (last observation carried forward) was used. Sensitivity analysis found that the data were missing at random. SPSS software version 21.0 (Chicago, IL) was used for statistical analysis.
Results
During the study period, 137 patients were assessed for eligibility (Figure).
The sample was predominately white and male, which is consistent with the veteran population. There were no statistical differences for baseline patient or surgical characteristics between the groups (Table 2).
There was a significant difference (P = .09) in the surgical procedures completed. There was only 1 pancreatic surgery completed in the ING and 9 pancreatic surgeries completed in the SNG.
Primary Outcomes
The overall rate of complications differed between the groups (Table 3).
Given the large number of colorectal procedures, a separate per-protocol analysis included 37 patients from ING and 36 patients in the SNG (Table 4).
Secondary Outcomes
The ITT analysis found that overall number of hospital days was slightly higher in the ING compared with that of the SNG, 9.4 vs 9.3 days, respectively. In the per-protocol analysis there were 1.3 fewer hospital days for those who received immunonutrition (P = .059). No significant differences were found between the groups in the number of days spent in the ICU or number of days NPO (Table 3). Death within 30 days postoperative was twice as high for those in the SNG vs ING, with no deaths in the per-protocol analysis for those in the ING.
The colorectal analysis found 8.5 hospital days for ING patients vs 10.0 days for SNG patients, (P = .08). There were no deaths in the ING and 1 death in the SNG for colorectal procedure patients.
Discussion
Surgery is traumatic to healthy patients with or without cancer. Patients with cancer who receive surgical intervention might be at an even higher risk for complications because of altered metabolic pathways, nutritional deficiencies, and depressed immune function.13 Meta-analyses of immunonutrition studies conducted over the past 2 decades have come to different conclusions regarding the benefit of immunonutrition in the elective gastrointestinal cancer surgery population.3,5,18 Although practice guidelines from the American Society of Parenteral and Enteral Nutrition and the European Society of Parenteral and Enteral Nutrition recommend routine use of immune-modulating formulas in surgical oncology patients, there is still some debate about the optimal timing, dose, individual formula constituents, and populations that will benefit.2,25 Earlier studies evaluating the economics of immunonutrition have shown significant cost savings related to reduction in length of stay and decrease in infectious complications even after accounting for the extra cost of the formula.26,27 More recent economic analyses confirmed these cost savings showing a savings of about $1,000 to $2,500 per patient with higher savings when immunonutrition was given preoperatively.28,29
For practitioners treating veterans with cancer, good stewardship of federal dollars and optimal outcomes are important considerations before implementing new therapies. Therefore, JAHVH set out to evaluate whether standard oral nutrition supplementation would be as effective as the higher cost immunonutrition supplementation in cancer patients receiving elective surgical procedures.
Rates of Complications
In this study, favorable effects of immunonutrition were found on total postoperative complications and number of hospital days. The total number of patients who experienced complications was 39% lower in the ING than it was in SNG in the ITT analysis and 37% lower in the colorectal per-protocol analysis. These rates are similar to the 48% lower rate Braga and colleagues found in their study in patients with colorectal cancer who received 5 days of preoperative immunonutrition.21 Because more than half of the patients in this study had colorectal cancer, the group is comparable to the Braga and colleagues study population. The overall supplement adherence rate was 86%, which was slightly lower than the 90% adherence rate that Braga and colleagues found. Lower consumption rates might have been a factor in not achieving a greater therapeutic benefit for infectious complications. Some studies suggest a therapeutic goal intake of greater than two-thirds of the prescribed amount.10,30 In the present study, 70.4% of the ING and 83% of the SNG met that recommended therapeutic goal, which is more than Hübner colleagues reported in their study (53% of the ING and 60% in the SNG meeting therapeutic intake goal).
Okamoto and colleagues also reported a much lower complication rate in gastric cancer patients who received immunonutrition (13.3%) compared with that of those receiving an isoenergetic formula (40%).11 The group receiving immunonutrition in the Okamoto and colleagues study had 4 times fewer infectious complications than did the standard group (P = .039), and a contributing reason might be that they supplemented for 7 days preoperatively. Similar to the current study’s results, Giger-Pabst and colleagues and Hübner and colleagues did not find any significant difference in infectious complications.10,30 Important notes of comparison include a low adherence rate in the study conducted by Hübner and colleagues and the lower dose of immunonutrition used by Giger-Pabst andcolleagues who used 3 days of preoperative supplementation, which may not be long enough to promote the tissue benefits of immunonutrition.
Although, the current study did not find any statistically significant difference in infectious complications, the SNG experienced 1.8 times more infections than did the ING, which indicates that immunonutrition support may be clinically beneficial. Based on previous literature and the results of this study, the authors speculate that at least 5 days of intake of the study immunonutrition formula could positively affect outcomes.
The authors suspect that the added arginine and fish oil in the immunonutrition product act synergistically as therapeutic ingredients to shift toward a preoperative anti-inflammatory prostaglandin environment while providing exogenous arginine to possibly prevent or correct a conditionally essential need for arginine that would promote adequate nitric oxide production. Another crucial factor is that the a priori power analysis was looking at a 38% complication rate in the SNG and only 15% complication rate in the ING, which generated a sample size of 108 participants. The post hoc power analysis indicates that this study is underpowered based on the complication rates, which could be a reason for insignificant infectious complications.
The benefits of immunonutrients are still being studied. Future studies in a controlled surgical setting could determine whether immunonutrition has a clinical outcome effect on operative time and surgical blood loss. A challenge for the investigators was to decide whether the difference in operative time and blood loss was a surgical characteristic or a clinical outcome. The positive impact of immunonutrients on tissue perfusion and cell integrity have been shown in other studies to reduce tissue inflammation and alter gene expression, which could affect how tissues respond to surgical insults.10,11 Because JAHVH is a teaching institution and multiple surgeons are involved with the patients, this question will continue to be unresolved. Future research may want to consider controlling for variability in surgical technique and perioperative protocols to evaluate this as a clinical outcome.
Limitations
Several limitations of this trial need to be addressed. Although the design of the study was a randomized controlled trial, it was an unblinded, single-center study with a small sample size. Surgeons were not aware of which supplement each subject had received; however, researchers took no measures to ensure the surgeons were blinded. To minimize bias, 2 investigators evaluated the records for complication rates to confirm consistency, and any discrepancies were resolved by a third investigator. Although adherence was evaluated, it was patient-reported, and lab testing was not conducted to ensure that tissues were loaded with therapeutic amounts of immunonutrients or to determine baseline levels of nutrient intake, which could show a nutrient response curve.
The use of other nutritional supplements, such as vitamins, probiotics, or additional fatty acids were not monitored, and the study formulas differed in protein and fiber content, which could have impacted the overall nutrient intake and affected the primary outcomes. Another limitation includes the variety of surgeons used over the period of the study. At a teaching institution, it is not feasible to limit the number of surgeons performing surgery.
Additionally, the study period was 5 years, and there have been changes in fasting times, medications, and bowel preparation over the course of that period, which could not be accounted for. Postoperative immunonutrition was not provided in this study based on the limited evidence available when the protocol was initiated. However, since that time, evidence supports and encourages postoperative therapy and might have proven beneficial to the patients. Data were not collected on the need for additional surgery within the study period, which could significantly impact outcomes.
Future studies would benefit from a longer postoperative monitoring period because this study looked only at the 30-day postoperative period. Last, randomization did not account for equal allocation of surgical procedures, and a higher number of pancreatic surgeries in the SNG could account for the higher complication rate found in that group. Although the colorectal analysis is underpowered, the results continue to show beneficial results with the use of immunonutrition.
Conclusion
The primary purpose of this research was to determine whether the veteran population would benefit from an immunonutrition preoperative protocol as recommended by several practice guidelines. The results of the initial analysis and the colorectal analysis were presented to the hospital interdisciplinary nutrition committee who voted that a preoperative immunonutrition protocol will be implemented at JAHVH because of the high comorbidity rate experienced by veterans.
Immunonutrition involves the use of omega-3 fatty acids, glutamine, arginine, and/or nucleotides individually or in combination at therapeutic levels to specifically modulate the immune system against altering inflammatory and metabolic pathways.1 Current literature supports the routine use of immune-enhancing formulas (containing both arginine and fish oil) in surgical patients.2-4 Although most of the literature favors the use of immunonutrition in surgical patients, some studies reported no benefit over standard oral nutrition supplementation.5
Background
Most studies evaluating the effect of immunonutrition for those undergoing elective surgery have been conducted in surgical oncology patients.6-12 Advanced cancers and older age can lead to cancer cachexia and sarcopenia, respectively. These conditions increase a patient’s surgical morbidity and mortality risk likely because of the negative effects on lean body mass, nutrient intake, and inflammatory and metabolic profile.13 However, early detection of some cancers through routine screening might lead to earlier surgical intervention that minimizes these negative tumor effects on the patient. Immunonutrition provided to well-nourished and malnourished patients has shown benefits, which supports the premise that a combination of immunonutrients included in immune-enhancing diets might have a beneficial pharmacotherapeutic effect beyond that of providing energy, protein, vitamins, and minerals for nutritional support.7,14
There are a lack of data regarding whether there is a window of opportunity for improved outcomes. Is the greatest need for immunonutrients during the peak of the injury, which might be immediately after surgery, or is it before the procedure? Arginine is a conditionally essential amino acid that has been shown to have a beneficial effect on the immune system by enhancing T-lymphocyte response when supplemented in surgical patients. When the arginase 1 (ARG 1) enzyme in myeloid cells is expressed during the inflammatory response to injury, accelerated use of arginine can deplete endogenous arginine, making it conditionally essential.
If adequate arginine cannot be synthesized or an exogenous source is not provided, T-cell dysfunction and decreased nitric oxide production leads to immune and vascular dysfunction, respectively.15,16 Providing arginine and omega-3 fatty acids might have a synergistic effect by shifting to an anti-inflammatory prostaglandin profile that has been shown to decrease ARG 1 expression while providing an exogenous source of arginine.17 Postsurgical inflammation might be caused in part by pro-inflammatory mediators and the anti-inflammatory properties of omega-3 fatty acids might offset this if cell membranes are loaded preoperatively.18 Therefore, preoperative immunonutrition might allow tissues to recover from planned surgical trauma. Bouwens and colleagues demonstrated that intake of eicosapentaenoic acid/docosahexaenoic acid over 26 weeks can alter the gene expression profiles of immune cells to a more anti-inflammatory status.19 However, Senkal and colleagues recommended that 3 to 7 days preoperatively is adequate to positively alter the lipid profile of tissues.20
Oncology patients preparing for surgery often are exposed to the physiologic stress of radiation and chemotherapy as neoadjuvant treatment to surgery. Oncology treatment and the adverse nutritional effects of treatment increase risk for arginine deficiency, such as poor nutrition intake, increased requirements, decreased production. Braga and colleagues demonstrated improved gut microprofusion and gut oxygenation intraoperatively, an effect that continued for up to 5 days after surgery.21 Waitzberg conducted a systematic review of randomized clinical trials evaluating immunonutrition in preoperative, postoperative, and perioperative periods. The results showed that the greatest improvements in postoperative infections and length of stay occurred in patients receiving preoperative 0.5 to 1 L/d of an immune nutrition product containing supplemental omega-3 fatty acids, arginine, and nucleotides for 5 to 7 days.22
It is unclear which population of surgical patients benefit the most from immunonutrition. Some results in the literature favor use in malnourished patients.18,23 However, other studies also have found benefit in well-nourished patients.7,14,21
Veterans who seek medical care at the Department of Veteran Affairs (VA) have higher rates of cancer, obesity, and diabetes mellitus, which complicate surgical outcomes.24 In addition to comorbidities, veterans who seek medical care at the VA are more likely to have been deployed overseas and have more physical and mental health disorders compared with that of nonveteran patients or veterans who do not use the VA. Because of higher comorbidities, unique deployment history, and mental health disorders, all of which may impact quality of life concerns, veterans are clinically more complex, which makes comparisons with the private sector difficult. The VA has the advantage of providing comprehensive care to veterans in all settings, including preparation for surgery and postsurgical follow-up with an interdisciplinary team.
The objective of this study was to compare surgical outcomes in veterans who receive preoperative supplementation using an immune-modulating formula with veterans who received a standard oral supplement. Although practice guidelines have been developed from studies in US nonveteran populations, there are no high- quality randomized studies of veterans.
This study design also would allow the VA to gauge cost-effectiveness of immunonutrition before implementing new protocols. There is convincing data supporting significant economic benefit; however, more cost-benefit studies are needed to fully assess.18,25-27 Immunonutrition products are more expensive than are standard nutrition supplements, but overall cost of care when immunonutrition products are used could be lower because of reduction of complications and hospital resources.
Methods
From November 2011 to January 2016, the authors conducted a single-center, prospective, randomized parallel-group study in veterans undergoing elective gastrointestinal oncologic surgery. Inclusion criteria included planned esophageal, gastric, pancreatic, colorectal, or liver resections in veterans with histologically documented neoplasm of the gastrointestinal tract. Patients were excluded if they were admitted to the intensive care unit (ICU) before surgery, were receiving steroids or other immunosuppressive medications, had a recent hospital admission for pulmonary, cardiac, or renal disease, or were exhibiting signs or symptoms of infection or sepsis, including elevated white blood cells (WBC) > 10,000/mL or a temperature > 37.7° C.
The study was approved by the research and development committee and the institutional review board at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida. The clinicaltrials.gov identifier for the study was NCT01471743.
Nutrition Formula
Subjects were randomized into 2 oral supplement groups: immunonutrition group (ING) patients received immunonutrition, and standard nutrition group (SNG) received a standard formula (Table 1).
Study Procedures
All veterans with planned gastrointestinal surgeries were evaluated in the JAHVH general surgery clinic. Veterans meeting the inclusion criteria were invited to participate in the study, and informed consent was obtained. A research randomizer program assigned subjects to the groups to reach equal 1:1 randomization. Enrolled participants were provided their randomized supplement (unblinded) in the general surgery clinic and instructed on the amount of supplement to consume and date to begin taking the supplement. Participants were instructed to continue with their normal diet in addition to the supplement. No additional nutrition education was provided. Participants were asked to keep track of their daily supplement intake. Patients in both groups also used preoperative bowel preparations when indicated.
At the time of enrollment, presurgical comorbidities, anthropometric data, and nutrition status parameters were obtained. Postoperatively, study personnel interviewed each patient about formula consumption and tolerance. Thirty days postoperatively, patient demographics, surgical characteristics (eg, surgery, operative time, blood loss), nutrition risk screening (NRS 2002) score, diet/enteral orders, days spent NPO, days in the hospital or in the ICU, and complications (eg, wound infection, abscess, sepsis, pneumonia, urinary tract infection, intestinal fistula, ileus, or anastomotic leakage) were collected from the electronic health record.
Statistical Analysis
The primary outcome measure was overall postoperative complication rate and postoperative infection rate. Based on reviews of similar studies available at the time of protocol development, it was assumed that a postoperative infection rate of 38% in the SNG and 15% in the ING would indicate treatment efficacy. A sample size of 54 patients in each group would provide a Type I error level α = .05 and a power of 80%. A total of 108 patients enrolled in the study. Chi-square analysis was used to determine this primary outcome measure.
Secondary outcomes (mean number of complications, hospital days, NPO (nothing by mouth) days, and ICU days) were evaluated with Mann Whitney U test because of violation of assumptions for the t test. All P values were 2-tailed and statistical significance was accepted at P < .05 with clinical significance accepted at P < .10. Analysis for intention to treat (ITT) and per protocol are provided for outcome measures. For the ITT analysis, multiple imputation (last observation carried forward) was used. Sensitivity analysis found that the data were missing at random. SPSS software version 21.0 (Chicago, IL) was used for statistical analysis.
Results
During the study period, 137 patients were assessed for eligibility (Figure).
The sample was predominately white and male, which is consistent with the veteran population. There were no statistical differences for baseline patient or surgical characteristics between the groups (Table 2).
There was a significant difference (P = .09) in the surgical procedures completed. There was only 1 pancreatic surgery completed in the ING and 9 pancreatic surgeries completed in the SNG.
Primary Outcomes
The overall rate of complications differed between the groups (Table 3).
Given the large number of colorectal procedures, a separate per-protocol analysis included 37 patients from ING and 36 patients in the SNG (Table 4).
Secondary Outcomes
The ITT analysis found that overall number of hospital days was slightly higher in the ING compared with that of the SNG, 9.4 vs 9.3 days, respectively. In the per-protocol analysis there were 1.3 fewer hospital days for those who received immunonutrition (P = .059). No significant differences were found between the groups in the number of days spent in the ICU or number of days NPO (Table 3). Death within 30 days postoperative was twice as high for those in the SNG vs ING, with no deaths in the per-protocol analysis for those in the ING.
The colorectal analysis found 8.5 hospital days for ING patients vs 10.0 days for SNG patients, (P = .08). There were no deaths in the ING and 1 death in the SNG for colorectal procedure patients.
Discussion
Surgery is traumatic to healthy patients with or without cancer. Patients with cancer who receive surgical intervention might be at an even higher risk for complications because of altered metabolic pathways, nutritional deficiencies, and depressed immune function.13 Meta-analyses of immunonutrition studies conducted over the past 2 decades have come to different conclusions regarding the benefit of immunonutrition in the elective gastrointestinal cancer surgery population.3,5,18 Although practice guidelines from the American Society of Parenteral and Enteral Nutrition and the European Society of Parenteral and Enteral Nutrition recommend routine use of immune-modulating formulas in surgical oncology patients, there is still some debate about the optimal timing, dose, individual formula constituents, and populations that will benefit.2,25 Earlier studies evaluating the economics of immunonutrition have shown significant cost savings related to reduction in length of stay and decrease in infectious complications even after accounting for the extra cost of the formula.26,27 More recent economic analyses confirmed these cost savings showing a savings of about $1,000 to $2,500 per patient with higher savings when immunonutrition was given preoperatively.28,29
For practitioners treating veterans with cancer, good stewardship of federal dollars and optimal outcomes are important considerations before implementing new therapies. Therefore, JAHVH set out to evaluate whether standard oral nutrition supplementation would be as effective as the higher cost immunonutrition supplementation in cancer patients receiving elective surgical procedures.
Rates of Complications
In this study, favorable effects of immunonutrition were found on total postoperative complications and number of hospital days. The total number of patients who experienced complications was 39% lower in the ING than it was in SNG in the ITT analysis and 37% lower in the colorectal per-protocol analysis. These rates are similar to the 48% lower rate Braga and colleagues found in their study in patients with colorectal cancer who received 5 days of preoperative immunonutrition.21 Because more than half of the patients in this study had colorectal cancer, the group is comparable to the Braga and colleagues study population. The overall supplement adherence rate was 86%, which was slightly lower than the 90% adherence rate that Braga and colleagues found. Lower consumption rates might have been a factor in not achieving a greater therapeutic benefit for infectious complications. Some studies suggest a therapeutic goal intake of greater than two-thirds of the prescribed amount.10,30 In the present study, 70.4% of the ING and 83% of the SNG met that recommended therapeutic goal, which is more than Hübner colleagues reported in their study (53% of the ING and 60% in the SNG meeting therapeutic intake goal).
Okamoto and colleagues also reported a much lower complication rate in gastric cancer patients who received immunonutrition (13.3%) compared with that of those receiving an isoenergetic formula (40%).11 The group receiving immunonutrition in the Okamoto and colleagues study had 4 times fewer infectious complications than did the standard group (P = .039), and a contributing reason might be that they supplemented for 7 days preoperatively. Similar to the current study’s results, Giger-Pabst and colleagues and Hübner and colleagues did not find any significant difference in infectious complications.10,30 Important notes of comparison include a low adherence rate in the study conducted by Hübner and colleagues and the lower dose of immunonutrition used by Giger-Pabst andcolleagues who used 3 days of preoperative supplementation, which may not be long enough to promote the tissue benefits of immunonutrition.
Although, the current study did not find any statistically significant difference in infectious complications, the SNG experienced 1.8 times more infections than did the ING, which indicates that immunonutrition support may be clinically beneficial. Based on previous literature and the results of this study, the authors speculate that at least 5 days of intake of the study immunonutrition formula could positively affect outcomes.
The authors suspect that the added arginine and fish oil in the immunonutrition product act synergistically as therapeutic ingredients to shift toward a preoperative anti-inflammatory prostaglandin environment while providing exogenous arginine to possibly prevent or correct a conditionally essential need for arginine that would promote adequate nitric oxide production. Another crucial factor is that the a priori power analysis was looking at a 38% complication rate in the SNG and only 15% complication rate in the ING, which generated a sample size of 108 participants. The post hoc power analysis indicates that this study is underpowered based on the complication rates, which could be a reason for insignificant infectious complications.
The benefits of immunonutrients are still being studied. Future studies in a controlled surgical setting could determine whether immunonutrition has a clinical outcome effect on operative time and surgical blood loss. A challenge for the investigators was to decide whether the difference in operative time and blood loss was a surgical characteristic or a clinical outcome. The positive impact of immunonutrients on tissue perfusion and cell integrity have been shown in other studies to reduce tissue inflammation and alter gene expression, which could affect how tissues respond to surgical insults.10,11 Because JAHVH is a teaching institution and multiple surgeons are involved with the patients, this question will continue to be unresolved. Future research may want to consider controlling for variability in surgical technique and perioperative protocols to evaluate this as a clinical outcome.
Limitations
Several limitations of this trial need to be addressed. Although the design of the study was a randomized controlled trial, it was an unblinded, single-center study with a small sample size. Surgeons were not aware of which supplement each subject had received; however, researchers took no measures to ensure the surgeons were blinded. To minimize bias, 2 investigators evaluated the records for complication rates to confirm consistency, and any discrepancies were resolved by a third investigator. Although adherence was evaluated, it was patient-reported, and lab testing was not conducted to ensure that tissues were loaded with therapeutic amounts of immunonutrients or to determine baseline levels of nutrient intake, which could show a nutrient response curve.
The use of other nutritional supplements, such as vitamins, probiotics, or additional fatty acids were not monitored, and the study formulas differed in protein and fiber content, which could have impacted the overall nutrient intake and affected the primary outcomes. Another limitation includes the variety of surgeons used over the period of the study. At a teaching institution, it is not feasible to limit the number of surgeons performing surgery.
Additionally, the study period was 5 years, and there have been changes in fasting times, medications, and bowel preparation over the course of that period, which could not be accounted for. Postoperative immunonutrition was not provided in this study based on the limited evidence available when the protocol was initiated. However, since that time, evidence supports and encourages postoperative therapy and might have proven beneficial to the patients. Data were not collected on the need for additional surgery within the study period, which could significantly impact outcomes.
Future studies would benefit from a longer postoperative monitoring period because this study looked only at the 30-day postoperative period. Last, randomization did not account for equal allocation of surgical procedures, and a higher number of pancreatic surgeries in the SNG could account for the higher complication rate found in that group. Although the colorectal analysis is underpowered, the results continue to show beneficial results with the use of immunonutrition.
Conclusion
The primary purpose of this research was to determine whether the veteran population would benefit from an immunonutrition preoperative protocol as recommended by several practice guidelines. The results of the initial analysis and the colorectal analysis were presented to the hospital interdisciplinary nutrition committee who voted that a preoperative immunonutrition protocol will be implemented at JAHVH because of the high comorbidity rate experienced by veterans.
1. Grimble RF. Immunonutrition. Curr Opin Gastroenterol. 2005;21(2):216-222.
2. McClave SA, Martindale RG, Vanek VW, et al; A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2009;33(3):277-316.
3. Marimuthu K, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann Surg. 2012;255(6):1060-1068.
4. Bharadwaj S, Trivax B, Tandon P, Alkam B, Hanouneh I, Steiger E. Should perioperative immunonutrition for elective surgery be the current standard of care? Gastroenterol Rep (Oxford). 2016;4(2):87-95.
5. Hegazi RA, Hustead DS, Evans DC. Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg. 2014;219(5):1078-1087.
6. Xu J, Zhong Y, Jing D, Wu Z. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg. 2006;30(7):1284-1289.
7. Horie H, Okada M, Kojima M, Nagai H. Favorable effects of preoperative enteral immunonutrition on a surgical site infection in patients with colorectal cancer without malnutrition. Surg Today. 2006;36(12):1063-1068.
8. Fujitani K, Tsujinaka T, Fujita J, et al; Osaka Gastrointestinal Cancer Chemotherapy Study Group. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg. 2012;99(5):621-629.
9. Braga M, Gianotti L, Nespoli L, Radaelli G, Di Carlo V. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg. 2002;137(2):174-180.
10. Giger-Pabst U, Lange J, Maurer C, et al. Short-term preoperative supplementation of an immunoenriched diet does not improve clinical outcome in well-nourished patients undergoing abdominal cancer surgery. Nutrition. 2013;29(5):724-729.
11. Okamoto Y, Okano K, Izuishi K, Usuki H, Wakabayashi H, Suzuki Y. Attenuation of the systemic inflammatory response and infectious complications after gastrectomy with preoperative oral arginine and omega-3 fatty acids supplemented immunonutrition. World J Surg. 2009;33(9):1815-1821.
12. Yildiz SY, Yazicoiog˘lu MB, Tiryaki Ç, Çiftçi A, Boyaciog˘lu Z. The effect of enteral immunonutrition in upper gastrointestinal surgery for cancer: a prospective study. Turk J Med Sci. 2016;46(2):393-400.
13. Peterson SJ, Mozer M. Differentiating sarcopenia and cachexia among patients with cancer. Nutr Clin Pract. 2017;32(1):30-39.
14. Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122(7):1763-1770.
15. Daly JM, Reynolds J, Thom A, et al. Immune and metabolic effects of arginine in the surgical patient. Ann Surg. 1988;208(4):512-523.
16. Aida T, Furukawa K, Suzuki D, et al. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreato-duodenectomy. Surgery. 2014;155(1):124-133.
17. Bansal V, Syres KM, Makarenkova V, et al. Interactions between fatty acids and arginine metabolism: implications for the design of immune-enhancing diets. JPEN J Parenter Enteral Nutr. 2005;29(1 suppl):S75-S80.
18. Osland E, Hossain MB, Khan S, Memon MA. Effect of timing of pharmaconutrition (immunonutrition) administration on outcomes of elective surgery for gastrointestinal malignancies: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. 2014;38(1):53-69.
19. Bouwens M, van de Rest O, Dellschaft N, et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90(2):415-424.
20. Senkal M, Haaker R, Linseisen J, Wolfram G, Homann HH, Stehle P. Preoperative oral supplementation with long-chain omega-3 fatty acids beneficially alters phospholipid fatty acid patterns in liver, gut mucosa, and tumor tissue. JPEN J Parenter Enteral Nutr. 2005;29(4):236-240.
21. Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132(5):805-814.
22. Waitzberg DL, Saito H, Plank LD, et al. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006;30(8):1592-1604.
23. Klek S, Sierzega M, Szybinski P, et al. The immunomodulating enteral nutrition in malnourished surgical patients—a prospective, randomized, double-blind clinical trial. Clin Nutr. 2011;30(3):282-288.
24. Farmer CM, Hosek SD, Adamson DM. Balancing demand and supply for veteran’s health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
25. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11-48.
26. Mauskopf JA, Candrilli SD, Chevrou-Séverac H, Ochoa JB. Immunonutrition for patients undergoing elective surgery for gastrointestinal cancer: Impact on hospital costs. World J Surg Oncol. 2012;10:136.
27. Senkal M, Mumme A, Eickhoff U, et al. Early postoperative enteral immunonutrition: clinical outcome and cost-comparison analysis in surgical patients. Crit Care Med. 1997;25(9):1489-1496.
28. Chevrou-Séverac H, Pinget C, Cerantola Y, Demartines N, Wasserfallen JB, Schäfer M. Cost-effectiveness analysis of immune-modulating nutritional support for gastrointestinal cancer patients. Clin Nutr. 2014;33(4):649-654.
29. Strickland A, Brogan A, Krauss J, Martindale R, Cresci G. Is the use of specialized nutritional formulations a cost-effective strategy? A national database evaluation. JPEN J Parenter Enteral Nutr. 2005;29(1 suppl):S81-S91.
30. Hübner M, Cerantola Y, Grass F, Bertrand PC, Schäfer M, Demartines N. Preoperative immunonutrition in patients at nutritional risk: results of a double-blinded randomized clinical trial. Eur J Clin Nutr. 2012;66(7):850-855.
1. Grimble RF. Immunonutrition. Curr Opin Gastroenterol. 2005;21(2):216-222.
2. McClave SA, Martindale RG, Vanek VW, et al; A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2009;33(3):277-316.
3. Marimuthu K, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann Surg. 2012;255(6):1060-1068.
4. Bharadwaj S, Trivax B, Tandon P, Alkam B, Hanouneh I, Steiger E. Should perioperative immunonutrition for elective surgery be the current standard of care? Gastroenterol Rep (Oxford). 2016;4(2):87-95.
5. Hegazi RA, Hustead DS, Evans DC. Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg. 2014;219(5):1078-1087.
6. Xu J, Zhong Y, Jing D, Wu Z. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg. 2006;30(7):1284-1289.
7. Horie H, Okada M, Kojima M, Nagai H. Favorable effects of preoperative enteral immunonutrition on a surgical site infection in patients with colorectal cancer without malnutrition. Surg Today. 2006;36(12):1063-1068.
8. Fujitani K, Tsujinaka T, Fujita J, et al; Osaka Gastrointestinal Cancer Chemotherapy Study Group. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg. 2012;99(5):621-629.
9. Braga M, Gianotti L, Nespoli L, Radaelli G, Di Carlo V. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg. 2002;137(2):174-180.
10. Giger-Pabst U, Lange J, Maurer C, et al. Short-term preoperative supplementation of an immunoenriched diet does not improve clinical outcome in well-nourished patients undergoing abdominal cancer surgery. Nutrition. 2013;29(5):724-729.
11. Okamoto Y, Okano K, Izuishi K, Usuki H, Wakabayashi H, Suzuki Y. Attenuation of the systemic inflammatory response and infectious complications after gastrectomy with preoperative oral arginine and omega-3 fatty acids supplemented immunonutrition. World J Surg. 2009;33(9):1815-1821.
12. Yildiz SY, Yazicoiog˘lu MB, Tiryaki Ç, Çiftçi A, Boyaciog˘lu Z. The effect of enteral immunonutrition in upper gastrointestinal surgery for cancer: a prospective study. Turk J Med Sci. 2016;46(2):393-400.
13. Peterson SJ, Mozer M. Differentiating sarcopenia and cachexia among patients with cancer. Nutr Clin Pract. 2017;32(1):30-39.
14. Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122(7):1763-1770.
15. Daly JM, Reynolds J, Thom A, et al. Immune and metabolic effects of arginine in the surgical patient. Ann Surg. 1988;208(4):512-523.
16. Aida T, Furukawa K, Suzuki D, et al. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreato-duodenectomy. Surgery. 2014;155(1):124-133.
17. Bansal V, Syres KM, Makarenkova V, et al. Interactions between fatty acids and arginine metabolism: implications for the design of immune-enhancing diets. JPEN J Parenter Enteral Nutr. 2005;29(1 suppl):S75-S80.
18. Osland E, Hossain MB, Khan S, Memon MA. Effect of timing of pharmaconutrition (immunonutrition) administration on outcomes of elective surgery for gastrointestinal malignancies: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. 2014;38(1):53-69.
19. Bouwens M, van de Rest O, Dellschaft N, et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90(2):415-424.
20. Senkal M, Haaker R, Linseisen J, Wolfram G, Homann HH, Stehle P. Preoperative oral supplementation with long-chain omega-3 fatty acids beneficially alters phospholipid fatty acid patterns in liver, gut mucosa, and tumor tissue. JPEN J Parenter Enteral Nutr. 2005;29(4):236-240.
21. Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132(5):805-814.
22. Waitzberg DL, Saito H, Plank LD, et al. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006;30(8):1592-1604.
23. Klek S, Sierzega M, Szybinski P, et al. The immunomodulating enteral nutrition in malnourished surgical patients—a prospective, randomized, double-blind clinical trial. Clin Nutr. 2011;30(3):282-288.
24. Farmer CM, Hosek SD, Adamson DM. Balancing demand and supply for veteran’s health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
25. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11-48.
26. Mauskopf JA, Candrilli SD, Chevrou-Séverac H, Ochoa JB. Immunonutrition for patients undergoing elective surgery for gastrointestinal cancer: Impact on hospital costs. World J Surg Oncol. 2012;10:136.
27. Senkal M, Mumme A, Eickhoff U, et al. Early postoperative enteral immunonutrition: clinical outcome and cost-comparison analysis in surgical patients. Crit Care Med. 1997;25(9):1489-1496.
28. Chevrou-Séverac H, Pinget C, Cerantola Y, Demartines N, Wasserfallen JB, Schäfer M. Cost-effectiveness analysis of immune-modulating nutritional support for gastrointestinal cancer patients. Clin Nutr. 2014;33(4):649-654.
29. Strickland A, Brogan A, Krauss J, Martindale R, Cresci G. Is the use of specialized nutritional formulations a cost-effective strategy? A national database evaluation. JPEN J Parenter Enteral Nutr. 2005;29(1 suppl):S81-S91.
30. Hübner M, Cerantola Y, Grass F, Bertrand PC, Schäfer M, Demartines N. Preoperative immunonutrition in patients at nutritional risk: results of a double-blinded randomized clinical trial. Eur J Clin Nutr. 2012;66(7):850-855.
The Effects of Ranolazine on Hemoglobin A1c in a Veteran Population
Diabetes mellitus (DM) is a risk factor for cardiovascular disease(CVD).1-4 Death rates from heart disease are 2- to 4-times higher among adults with DM compared with those of adults without DM. In the US, it is estimated that 21.1 million adults have diagnosed DM, 8.1 million adults have undiagnosed DM, and 80.8 million adults have prediabetes.3 The American Heart Association has identified an untreated fasting blood glucose level < 100 mg/dL as a component of ideal cardiovascular health.3
Although the use of antidiabetic agents has been shown to reduce the risks of microvascular complications among patients with DM, a cardiovascular benefit has not been consistently demonstrated with all available agents, and some used in the treatment of DM are associated with cardiovascular harm.5 In addition, some cardiovascular medications may contribute to the development of DM or may mask the symptoms of hypoglycemia.6 Given the risk for CVD among patients with DM, a medication with utility in both DM and CVD could be beneficial.
Evidence for Use of Ranolazine
Ranolazine is indicated for the treatment of chronic angina.7 In clinical trials, ranolazine also was found to decrease hemoglobin A1c (HbA1c).8-15 The possible mechanisms for lowering HbA1c with ranolazine include preservation of pancreatic β-cells and an increase in glucose-dependent insulin secretion.6 The most common adverse effects associated with ranolazine include dizziness, headache, constipation, and nausea.7
Ranolazine has been shown to be efficacious and safe in the reduction of angina symptoms among patients with and without DM.8-12 In addition to improving symptoms of angina, studies have demonstrated a reduction in HbA1c among patients taking ranolazine.9,13-15 In an open-label extension of the Combination Assessment of Ranolazine in Stable Angina (CARISA) trial, ranolazine 750 mg twice daily and 1,000 mg twice daily led to a greater reduction in HbA1c when each was compared with placebo (-0.48% HbA1c, P = .008; and -0.70% HbA1c, P = .001, respectively).9
Among the 5,576 patients enrolled in the Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes—Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) trial with a baseline HbA1c, ranolazine significantly reduced HbA1c at 4 months when compared with placebo among patients with and without DM.13 In addition, patients with DM who were treated with ranolazine were more likely to achieve a HbA1c < 7% at 4 months when compared with placebo (59% vs 49%; P < .001). Ranolazine was not found to increase the incidence of hypoglycemia.
A subgroup analysis of MERLIN-TIMI 36 evaluated the effects of ranolazine compared with those of placebo on fasting plasma glucose and HbA1c in patients with moderate DM (HbA1c ≥ 6% but < 8%, fasting plasma glucose < 250 mg/dL) and severe DM (A1c ≥ 8%, fasting plasma glucose 150-400 mg/dL).14 A significant reduction in HbA1c with ranolazine in addition to standard of care antidiabetes treatment was observed among both groups. The placebo-corrected decrease in HbA1c in the moderate DM group was 0.28% (95% confidence interval [CI] -0.55 to 0; P = .045) and in the severe DM group was 0.59% (95% CI -0.99 to -0.20; P < .001).
In a trial designed to evaluate change in HbA1c in patients taking ranolazine 1,000 mg twice daily compared with that of placebo, ranolazine led to a greater decrease in HbA1c compared with that of placebo (placebo corrected change in HbA1c -0.56%, P = .001).15 In addition, a higher percentage of patients achieved HbA1c < 7% at 24 weeks in the ranolazine group compared with that of placebo (41.2% vs 25.7%; P = .001). No patient experienced severe hypoglycemia or had documented hypoglycemia in this study.
These trials suggest that ranolazine, in addition to decreasing anginal events, is potentially beneficial in achieving better control of DM. However, more studies are needed to determine this benefit. In addition, no trials have examined the 500-mg twice daily dose of ranolazine in HbA1c reduction.
The purpose of this study was to evaluate the change in HbA1c among veterans with DM after the initiation of ranolazine. The study compared the percentage of veterans achieving HbA1c < 7% or < 8% after initiation of ranolazine with the baseline, to determine whether there is a dose-related change in HbA1c among different ranolazine regimens and to determine whether there is a change in the incidence of hypoglycemia after the initiation of ranolazine.
Methods
This was a multicenter, retrospective study. The institutional review board and research and development committee for 3 Veterans Affairs medical centers (VAMCs) approved this study and waived informed consent. Additionally, this study was approved for access to national patient information through the Corporate Data Warehouse (CDW).
Subjects were eligible for inclusion in this study if they were aged ≥ 18 years, had a diagnosis of type 2 DM, and received their first prescription of ranolazine at a VAMC from January 1, 2008 through March 31, 2015. Exclusion criteria included subjects with no baseline HbA1c (defined as the HbA1c result closest to the ranolazine initiation date and within 90 days before to 14 days after ranolazine initiation), no follow-up HbA1c (defined as the first HbA1c result within 60 to 180 days after the ranolazine initiation date), any change to their DM medication regimen during the follow-up period, or who discontinued ranolazine prior to collection of the follow-up HbA1c.
Data were collected from the electronic health record (EHR) for each subject from 6 months prior to the ranolazine initiation date through 6 months after the ranolazine initiation date. The ranolazine initiation date was defined as the date ranolazine was picked up in person at a VAMC pharmacy or 7 days after the date filled for medications sent by mail.
The primary endpoint of this study was the change in HbA1c after ranolazine initiation. The secondary endpoint was the percentage of study subjects achieving HbA1c < 7% and < 8% before and after the initiation of ranolazine.
To achieve 80% power to detect a change in HbA1c of 0.4%, a sample size of 52 patients was required. For the primary endpoint, a paired t test was used to test for statistical significance. The McNemar test was used to evaluate for a significant change in subjects achieving an HbA1c < 7% and HbA1c < 8% with the initiation of ranolazine.
Results
A total of 523 patients were evaluated for study inclusion, of which 66 patients were included (Figure). The most common reasons for exclusion included no HbA1c at baseline and changes to the DM medication regimen during follow-up.
Ranolazine at any dose was associated with a change in HbA1c of -0.3% (P < .001).
A dose of 500 mg ranolazine twice daily also was associated with a significant decrease in HbA1c by 0.3% (P = .001). A significant increase in veterans achieving HbA1c < 7% after ranolazine initiation was observed (42.3% before ranolazine initiation vs 73.1% after ranolazine initiation; P = .001), and a nonsignificant increase in veterans achieving HbA1c < 8% was observed (82.7% before ranolazine initiation vs 90.4% after ranolazine initiation, P = .37).
At a dose of 1,000 mg twice daily, a 0.4% decrease in HbA1c was observed. However, this result was not found to be statistically significant (P = .09), and the study was underpowered to detect a significant change in HbA1c at this dose.
Hypoglycemia was not reported in a majority of study patient progress notes; thus, it was not evaluated further.
Discussion
In this study of a veteran population, ranolazine was associated with an HbA1c decrease of 0.3%. This change is less than that observed in previous studies, which may be related to a lower baseline HbA1c for the patients in this study. In addition, a greater percentage of veterans achieved an HbA1c < 7% after initiation of ranolazine compared with that of the baseline.
To the authors’ knowledge, this is the first study evaluating ranolazine and HbA1c in a veteran population. It also is the first study to demonstrate an association between HbA1c lowering and ranolazine at a dose of 500 mg twice daily. These results suggest that in patients with chronic angina and type 2 DM, ranolazine could potentially play a dual role in therapy.
Limitations
The authors recognize several limitations in this study. Given its observational design, it cannot be definitively concluded that the decrease in HbA1c was due to the initiation of ranolazine. While excluding patients with changes to their antidiabetic medication regimen was done in an effort to minimize confounding factors, it is possible that other factors, such as lifestyle, also could explain changes in HbA1c. It is possible that changes to the DM medication regimen were made but not documented in the EHR. In addition, information on hypoglycemia was not readily available; thus, the safety of ranolazine among patients with DM could not be evaluated fully. Finally, the patient population characteristics may limit external validity.
Conclusion
In this observational study, ranolazine was associated with a statistically significant decrease in HbA1c among veterans with DM, which supports previously published literature.9, 13-15 However, no randomized controlled trials have been performed specifically studying the impact of ranolazine on HbA1c among patient with DM. Ideally, future prospective, randomized placebo-controlled studies will take place to further evaluate the association between ranolazine use and HbA1c lowering.
1. Kannel WB, McGee DL. Diabetes and cardiovascular disease—the Framingham study. JAMA. 1979;241(19): 2035-2038.
2. Selvin E, Coresh J, Golden SH, Boland LL, Brancati FL, Steffes MW; Atherosclerosis risk in communities study. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28(8):1965-1973.
3. Writing Group Members, Mozaffarian D, Benjamion EJ, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360.
4. Conaway DG, O’Keefe JH, Reid KJ, Spertus J. Frequency of undiagnosed diabetes mellitus in patients with acute coronary syndrome. Am J Cardiol. 2005;96(3):363-365.
5. Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs—insights from the rosiglitazone experience. N Engl J Med. 2013;369(14):1285-1287.
6. Ning Y, Zhen W, Fu Z, et al. Ranolazine increases β-cell survival and improves glucose homeostasis in low-dose streptozotocin-induced diabetes in mice. J Pharmacol Exp Ther. 2011;337(1):50-58.
7. Ranexa [package insert]. Foster City, CA: Gilead Sciences Inc; 2016.
8. Chaitman BR, Pepine CJ, Parker JO, et al; Combination Assessment of Ranolazine In Stable Angina (CARISA) Investigators. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291(3):309-316.
9. Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J. 2006;27(1):42-48.
10. Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al; MERLIN-TIMI 36 Trial Investigators. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297(16):1775-1783.
11. Kosiborod M, Arnold SV, Spertus JA, et al. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina). J Am Coll Cardiol. 2013;61(20):2038-2045.
12. Arnold SV, McGuire DK, Spertus JA, et al. Effectiveness of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina according to baseline hemoglobin A1c. Am Heart J. 2014;168(4):457-465.e2.
13. Morrow DA, Scirica BM, Chaitman BR, et al; MERLIN-TIMI 36 Trial Investigators. Evaluation of the glycometabolic effects of ranolazine patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation. 2009;119(15):2032-2039.
14. Chisholm JW, Goldfine AB, Dhalla AK, et al. Effect of ranolazine on A1c and glucose levels in hyperglycemic patients with non-ST elevation acute coronary syndrome. Diabetes Care. 2010;33(6):1163-1168.
15. Eckel RH, Henry RR, Yue P, et al. Effect of ranolazine monotherapy on glycemic control in subjects with type 2 diabetes. Diabetes Care. 2015;38(7):1189-1196.
Diabetes mellitus (DM) is a risk factor for cardiovascular disease(CVD).1-4 Death rates from heart disease are 2- to 4-times higher among adults with DM compared with those of adults without DM. In the US, it is estimated that 21.1 million adults have diagnosed DM, 8.1 million adults have undiagnosed DM, and 80.8 million adults have prediabetes.3 The American Heart Association has identified an untreated fasting blood glucose level < 100 mg/dL as a component of ideal cardiovascular health.3
Although the use of antidiabetic agents has been shown to reduce the risks of microvascular complications among patients with DM, a cardiovascular benefit has not been consistently demonstrated with all available agents, and some used in the treatment of DM are associated with cardiovascular harm.5 In addition, some cardiovascular medications may contribute to the development of DM or may mask the symptoms of hypoglycemia.6 Given the risk for CVD among patients with DM, a medication with utility in both DM and CVD could be beneficial.
Evidence for Use of Ranolazine
Ranolazine is indicated for the treatment of chronic angina.7 In clinical trials, ranolazine also was found to decrease hemoglobin A1c (HbA1c).8-15 The possible mechanisms for lowering HbA1c with ranolazine include preservation of pancreatic β-cells and an increase in glucose-dependent insulin secretion.6 The most common adverse effects associated with ranolazine include dizziness, headache, constipation, and nausea.7
Ranolazine has been shown to be efficacious and safe in the reduction of angina symptoms among patients with and without DM.8-12 In addition to improving symptoms of angina, studies have demonstrated a reduction in HbA1c among patients taking ranolazine.9,13-15 In an open-label extension of the Combination Assessment of Ranolazine in Stable Angina (CARISA) trial, ranolazine 750 mg twice daily and 1,000 mg twice daily led to a greater reduction in HbA1c when each was compared with placebo (-0.48% HbA1c, P = .008; and -0.70% HbA1c, P = .001, respectively).9
Among the 5,576 patients enrolled in the Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes—Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) trial with a baseline HbA1c, ranolazine significantly reduced HbA1c at 4 months when compared with placebo among patients with and without DM.13 In addition, patients with DM who were treated with ranolazine were more likely to achieve a HbA1c < 7% at 4 months when compared with placebo (59% vs 49%; P < .001). Ranolazine was not found to increase the incidence of hypoglycemia.
A subgroup analysis of MERLIN-TIMI 36 evaluated the effects of ranolazine compared with those of placebo on fasting plasma glucose and HbA1c in patients with moderate DM (HbA1c ≥ 6% but < 8%, fasting plasma glucose < 250 mg/dL) and severe DM (A1c ≥ 8%, fasting plasma glucose 150-400 mg/dL).14 A significant reduction in HbA1c with ranolazine in addition to standard of care antidiabetes treatment was observed among both groups. The placebo-corrected decrease in HbA1c in the moderate DM group was 0.28% (95% confidence interval [CI] -0.55 to 0; P = .045) and in the severe DM group was 0.59% (95% CI -0.99 to -0.20; P < .001).
In a trial designed to evaluate change in HbA1c in patients taking ranolazine 1,000 mg twice daily compared with that of placebo, ranolazine led to a greater decrease in HbA1c compared with that of placebo (placebo corrected change in HbA1c -0.56%, P = .001).15 In addition, a higher percentage of patients achieved HbA1c < 7% at 24 weeks in the ranolazine group compared with that of placebo (41.2% vs 25.7%; P = .001). No patient experienced severe hypoglycemia or had documented hypoglycemia in this study.
These trials suggest that ranolazine, in addition to decreasing anginal events, is potentially beneficial in achieving better control of DM. However, more studies are needed to determine this benefit. In addition, no trials have examined the 500-mg twice daily dose of ranolazine in HbA1c reduction.
The purpose of this study was to evaluate the change in HbA1c among veterans with DM after the initiation of ranolazine. The study compared the percentage of veterans achieving HbA1c < 7% or < 8% after initiation of ranolazine with the baseline, to determine whether there is a dose-related change in HbA1c among different ranolazine regimens and to determine whether there is a change in the incidence of hypoglycemia after the initiation of ranolazine.
Methods
This was a multicenter, retrospective study. The institutional review board and research and development committee for 3 Veterans Affairs medical centers (VAMCs) approved this study and waived informed consent. Additionally, this study was approved for access to national patient information through the Corporate Data Warehouse (CDW).
Subjects were eligible for inclusion in this study if they were aged ≥ 18 years, had a diagnosis of type 2 DM, and received their first prescription of ranolazine at a VAMC from January 1, 2008 through March 31, 2015. Exclusion criteria included subjects with no baseline HbA1c (defined as the HbA1c result closest to the ranolazine initiation date and within 90 days before to 14 days after ranolazine initiation), no follow-up HbA1c (defined as the first HbA1c result within 60 to 180 days after the ranolazine initiation date), any change to their DM medication regimen during the follow-up period, or who discontinued ranolazine prior to collection of the follow-up HbA1c.
Data were collected from the electronic health record (EHR) for each subject from 6 months prior to the ranolazine initiation date through 6 months after the ranolazine initiation date. The ranolazine initiation date was defined as the date ranolazine was picked up in person at a VAMC pharmacy or 7 days after the date filled for medications sent by mail.
The primary endpoint of this study was the change in HbA1c after ranolazine initiation. The secondary endpoint was the percentage of study subjects achieving HbA1c < 7% and < 8% before and after the initiation of ranolazine.
To achieve 80% power to detect a change in HbA1c of 0.4%, a sample size of 52 patients was required. For the primary endpoint, a paired t test was used to test for statistical significance. The McNemar test was used to evaluate for a significant change in subjects achieving an HbA1c < 7% and HbA1c < 8% with the initiation of ranolazine.
Results
A total of 523 patients were evaluated for study inclusion, of which 66 patients were included (Figure). The most common reasons for exclusion included no HbA1c at baseline and changes to the DM medication regimen during follow-up.
Ranolazine at any dose was associated with a change in HbA1c of -0.3% (P < .001).
A dose of 500 mg ranolazine twice daily also was associated with a significant decrease in HbA1c by 0.3% (P = .001). A significant increase in veterans achieving HbA1c < 7% after ranolazine initiation was observed (42.3% before ranolazine initiation vs 73.1% after ranolazine initiation; P = .001), and a nonsignificant increase in veterans achieving HbA1c < 8% was observed (82.7% before ranolazine initiation vs 90.4% after ranolazine initiation, P = .37).
At a dose of 1,000 mg twice daily, a 0.4% decrease in HbA1c was observed. However, this result was not found to be statistically significant (P = .09), and the study was underpowered to detect a significant change in HbA1c at this dose.
Hypoglycemia was not reported in a majority of study patient progress notes; thus, it was not evaluated further.
Discussion
In this study of a veteran population, ranolazine was associated with an HbA1c decrease of 0.3%. This change is less than that observed in previous studies, which may be related to a lower baseline HbA1c for the patients in this study. In addition, a greater percentage of veterans achieved an HbA1c < 7% after initiation of ranolazine compared with that of the baseline.
To the authors’ knowledge, this is the first study evaluating ranolazine and HbA1c in a veteran population. It also is the first study to demonstrate an association between HbA1c lowering and ranolazine at a dose of 500 mg twice daily. These results suggest that in patients with chronic angina and type 2 DM, ranolazine could potentially play a dual role in therapy.
Limitations
The authors recognize several limitations in this study. Given its observational design, it cannot be definitively concluded that the decrease in HbA1c was due to the initiation of ranolazine. While excluding patients with changes to their antidiabetic medication regimen was done in an effort to minimize confounding factors, it is possible that other factors, such as lifestyle, also could explain changes in HbA1c. It is possible that changes to the DM medication regimen were made but not documented in the EHR. In addition, information on hypoglycemia was not readily available; thus, the safety of ranolazine among patients with DM could not be evaluated fully. Finally, the patient population characteristics may limit external validity.
Conclusion
In this observational study, ranolazine was associated with a statistically significant decrease in HbA1c among veterans with DM, which supports previously published literature.9, 13-15 However, no randomized controlled trials have been performed specifically studying the impact of ranolazine on HbA1c among patient with DM. Ideally, future prospective, randomized placebo-controlled studies will take place to further evaluate the association between ranolazine use and HbA1c lowering.
Diabetes mellitus (DM) is a risk factor for cardiovascular disease(CVD).1-4 Death rates from heart disease are 2- to 4-times higher among adults with DM compared with those of adults without DM. In the US, it is estimated that 21.1 million adults have diagnosed DM, 8.1 million adults have undiagnosed DM, and 80.8 million adults have prediabetes.3 The American Heart Association has identified an untreated fasting blood glucose level < 100 mg/dL as a component of ideal cardiovascular health.3
Although the use of antidiabetic agents has been shown to reduce the risks of microvascular complications among patients with DM, a cardiovascular benefit has not been consistently demonstrated with all available agents, and some used in the treatment of DM are associated with cardiovascular harm.5 In addition, some cardiovascular medications may contribute to the development of DM or may mask the symptoms of hypoglycemia.6 Given the risk for CVD among patients with DM, a medication with utility in both DM and CVD could be beneficial.
Evidence for Use of Ranolazine
Ranolazine is indicated for the treatment of chronic angina.7 In clinical trials, ranolazine also was found to decrease hemoglobin A1c (HbA1c).8-15 The possible mechanisms for lowering HbA1c with ranolazine include preservation of pancreatic β-cells and an increase in glucose-dependent insulin secretion.6 The most common adverse effects associated with ranolazine include dizziness, headache, constipation, and nausea.7
Ranolazine has been shown to be efficacious and safe in the reduction of angina symptoms among patients with and without DM.8-12 In addition to improving symptoms of angina, studies have demonstrated a reduction in HbA1c among patients taking ranolazine.9,13-15 In an open-label extension of the Combination Assessment of Ranolazine in Stable Angina (CARISA) trial, ranolazine 750 mg twice daily and 1,000 mg twice daily led to a greater reduction in HbA1c when each was compared with placebo (-0.48% HbA1c, P = .008; and -0.70% HbA1c, P = .001, respectively).9
Among the 5,576 patients enrolled in the Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes—Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) trial with a baseline HbA1c, ranolazine significantly reduced HbA1c at 4 months when compared with placebo among patients with and without DM.13 In addition, patients with DM who were treated with ranolazine were more likely to achieve a HbA1c < 7% at 4 months when compared with placebo (59% vs 49%; P < .001). Ranolazine was not found to increase the incidence of hypoglycemia.
A subgroup analysis of MERLIN-TIMI 36 evaluated the effects of ranolazine compared with those of placebo on fasting plasma glucose and HbA1c in patients with moderate DM (HbA1c ≥ 6% but < 8%, fasting plasma glucose < 250 mg/dL) and severe DM (A1c ≥ 8%, fasting plasma glucose 150-400 mg/dL).14 A significant reduction in HbA1c with ranolazine in addition to standard of care antidiabetes treatment was observed among both groups. The placebo-corrected decrease in HbA1c in the moderate DM group was 0.28% (95% confidence interval [CI] -0.55 to 0; P = .045) and in the severe DM group was 0.59% (95% CI -0.99 to -0.20; P < .001).
In a trial designed to evaluate change in HbA1c in patients taking ranolazine 1,000 mg twice daily compared with that of placebo, ranolazine led to a greater decrease in HbA1c compared with that of placebo (placebo corrected change in HbA1c -0.56%, P = .001).15 In addition, a higher percentage of patients achieved HbA1c < 7% at 24 weeks in the ranolazine group compared with that of placebo (41.2% vs 25.7%; P = .001). No patient experienced severe hypoglycemia or had documented hypoglycemia in this study.
These trials suggest that ranolazine, in addition to decreasing anginal events, is potentially beneficial in achieving better control of DM. However, more studies are needed to determine this benefit. In addition, no trials have examined the 500-mg twice daily dose of ranolazine in HbA1c reduction.
The purpose of this study was to evaluate the change in HbA1c among veterans with DM after the initiation of ranolazine. The study compared the percentage of veterans achieving HbA1c < 7% or < 8% after initiation of ranolazine with the baseline, to determine whether there is a dose-related change in HbA1c among different ranolazine regimens and to determine whether there is a change in the incidence of hypoglycemia after the initiation of ranolazine.
Methods
This was a multicenter, retrospective study. The institutional review board and research and development committee for 3 Veterans Affairs medical centers (VAMCs) approved this study and waived informed consent. Additionally, this study was approved for access to national patient information through the Corporate Data Warehouse (CDW).
Subjects were eligible for inclusion in this study if they were aged ≥ 18 years, had a diagnosis of type 2 DM, and received their first prescription of ranolazine at a VAMC from January 1, 2008 through March 31, 2015. Exclusion criteria included subjects with no baseline HbA1c (defined as the HbA1c result closest to the ranolazine initiation date and within 90 days before to 14 days after ranolazine initiation), no follow-up HbA1c (defined as the first HbA1c result within 60 to 180 days after the ranolazine initiation date), any change to their DM medication regimen during the follow-up period, or who discontinued ranolazine prior to collection of the follow-up HbA1c.
Data were collected from the electronic health record (EHR) for each subject from 6 months prior to the ranolazine initiation date through 6 months after the ranolazine initiation date. The ranolazine initiation date was defined as the date ranolazine was picked up in person at a VAMC pharmacy or 7 days after the date filled for medications sent by mail.
The primary endpoint of this study was the change in HbA1c after ranolazine initiation. The secondary endpoint was the percentage of study subjects achieving HbA1c < 7% and < 8% before and after the initiation of ranolazine.
To achieve 80% power to detect a change in HbA1c of 0.4%, a sample size of 52 patients was required. For the primary endpoint, a paired t test was used to test for statistical significance. The McNemar test was used to evaluate for a significant change in subjects achieving an HbA1c < 7% and HbA1c < 8% with the initiation of ranolazine.
Results
A total of 523 patients were evaluated for study inclusion, of which 66 patients were included (Figure). The most common reasons for exclusion included no HbA1c at baseline and changes to the DM medication regimen during follow-up.
Ranolazine at any dose was associated with a change in HbA1c of -0.3% (P < .001).
A dose of 500 mg ranolazine twice daily also was associated with a significant decrease in HbA1c by 0.3% (P = .001). A significant increase in veterans achieving HbA1c < 7% after ranolazine initiation was observed (42.3% before ranolazine initiation vs 73.1% after ranolazine initiation; P = .001), and a nonsignificant increase in veterans achieving HbA1c < 8% was observed (82.7% before ranolazine initiation vs 90.4% after ranolazine initiation, P = .37).
At a dose of 1,000 mg twice daily, a 0.4% decrease in HbA1c was observed. However, this result was not found to be statistically significant (P = .09), and the study was underpowered to detect a significant change in HbA1c at this dose.
Hypoglycemia was not reported in a majority of study patient progress notes; thus, it was not evaluated further.
Discussion
In this study of a veteran population, ranolazine was associated with an HbA1c decrease of 0.3%. This change is less than that observed in previous studies, which may be related to a lower baseline HbA1c for the patients in this study. In addition, a greater percentage of veterans achieved an HbA1c < 7% after initiation of ranolazine compared with that of the baseline.
To the authors’ knowledge, this is the first study evaluating ranolazine and HbA1c in a veteran population. It also is the first study to demonstrate an association between HbA1c lowering and ranolazine at a dose of 500 mg twice daily. These results suggest that in patients with chronic angina and type 2 DM, ranolazine could potentially play a dual role in therapy.
Limitations
The authors recognize several limitations in this study. Given its observational design, it cannot be definitively concluded that the decrease in HbA1c was due to the initiation of ranolazine. While excluding patients with changes to their antidiabetic medication regimen was done in an effort to minimize confounding factors, it is possible that other factors, such as lifestyle, also could explain changes in HbA1c. It is possible that changes to the DM medication regimen were made but not documented in the EHR. In addition, information on hypoglycemia was not readily available; thus, the safety of ranolazine among patients with DM could not be evaluated fully. Finally, the patient population characteristics may limit external validity.
Conclusion
In this observational study, ranolazine was associated with a statistically significant decrease in HbA1c among veterans with DM, which supports previously published literature.9, 13-15 However, no randomized controlled trials have been performed specifically studying the impact of ranolazine on HbA1c among patient with DM. Ideally, future prospective, randomized placebo-controlled studies will take place to further evaluate the association between ranolazine use and HbA1c lowering.
1. Kannel WB, McGee DL. Diabetes and cardiovascular disease—the Framingham study. JAMA. 1979;241(19): 2035-2038.
2. Selvin E, Coresh J, Golden SH, Boland LL, Brancati FL, Steffes MW; Atherosclerosis risk in communities study. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28(8):1965-1973.
3. Writing Group Members, Mozaffarian D, Benjamion EJ, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360.
4. Conaway DG, O’Keefe JH, Reid KJ, Spertus J. Frequency of undiagnosed diabetes mellitus in patients with acute coronary syndrome. Am J Cardiol. 2005;96(3):363-365.
5. Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs—insights from the rosiglitazone experience. N Engl J Med. 2013;369(14):1285-1287.
6. Ning Y, Zhen W, Fu Z, et al. Ranolazine increases β-cell survival and improves glucose homeostasis in low-dose streptozotocin-induced diabetes in mice. J Pharmacol Exp Ther. 2011;337(1):50-58.
7. Ranexa [package insert]. Foster City, CA: Gilead Sciences Inc; 2016.
8. Chaitman BR, Pepine CJ, Parker JO, et al; Combination Assessment of Ranolazine In Stable Angina (CARISA) Investigators. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291(3):309-316.
9. Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J. 2006;27(1):42-48.
10. Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al; MERLIN-TIMI 36 Trial Investigators. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297(16):1775-1783.
11. Kosiborod M, Arnold SV, Spertus JA, et al. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina). J Am Coll Cardiol. 2013;61(20):2038-2045.
12. Arnold SV, McGuire DK, Spertus JA, et al. Effectiveness of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina according to baseline hemoglobin A1c. Am Heart J. 2014;168(4):457-465.e2.
13. Morrow DA, Scirica BM, Chaitman BR, et al; MERLIN-TIMI 36 Trial Investigators. Evaluation of the glycometabolic effects of ranolazine patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation. 2009;119(15):2032-2039.
14. Chisholm JW, Goldfine AB, Dhalla AK, et al. Effect of ranolazine on A1c and glucose levels in hyperglycemic patients with non-ST elevation acute coronary syndrome. Diabetes Care. 2010;33(6):1163-1168.
15. Eckel RH, Henry RR, Yue P, et al. Effect of ranolazine monotherapy on glycemic control in subjects with type 2 diabetes. Diabetes Care. 2015;38(7):1189-1196.
1. Kannel WB, McGee DL. Diabetes and cardiovascular disease—the Framingham study. JAMA. 1979;241(19): 2035-2038.
2. Selvin E, Coresh J, Golden SH, Boland LL, Brancati FL, Steffes MW; Atherosclerosis risk in communities study. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28(8):1965-1973.
3. Writing Group Members, Mozaffarian D, Benjamion EJ, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360.
4. Conaway DG, O’Keefe JH, Reid KJ, Spertus J. Frequency of undiagnosed diabetes mellitus in patients with acute coronary syndrome. Am J Cardiol. 2005;96(3):363-365.
5. Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs—insights from the rosiglitazone experience. N Engl J Med. 2013;369(14):1285-1287.
6. Ning Y, Zhen W, Fu Z, et al. Ranolazine increases β-cell survival and improves glucose homeostasis in low-dose streptozotocin-induced diabetes in mice. J Pharmacol Exp Ther. 2011;337(1):50-58.
7. Ranexa [package insert]. Foster City, CA: Gilead Sciences Inc; 2016.
8. Chaitman BR, Pepine CJ, Parker JO, et al; Combination Assessment of Ranolazine In Stable Angina (CARISA) Investigators. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291(3):309-316.
9. Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J. 2006;27(1):42-48.
10. Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al; MERLIN-TIMI 36 Trial Investigators. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297(16):1775-1783.
11. Kosiborod M, Arnold SV, Spertus JA, et al. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina). J Am Coll Cardiol. 2013;61(20):2038-2045.
12. Arnold SV, McGuire DK, Spertus JA, et al. Effectiveness of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina according to baseline hemoglobin A1c. Am Heart J. 2014;168(4):457-465.e2.
13. Morrow DA, Scirica BM, Chaitman BR, et al; MERLIN-TIMI 36 Trial Investigators. Evaluation of the glycometabolic effects of ranolazine patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation. 2009;119(15):2032-2039.
14. Chisholm JW, Goldfine AB, Dhalla AK, et al. Effect of ranolazine on A1c and glucose levels in hyperglycemic patients with non-ST elevation acute coronary syndrome. Diabetes Care. 2010;33(6):1163-1168.
15. Eckel RH, Henry RR, Yue P, et al. Effect of ranolazine monotherapy on glycemic control in subjects with type 2 diabetes. Diabetes Care. 2015;38(7):1189-1196.
Lung Cancer Screening: Translating Research Into Practice (FULL)
According to the National Lung Screening Trial (NLST), use of low-dose computed tomography (LDCT) reduced lung cancer deaths by 20%. That finding led the U.S. Preventive Services Task Force (USPSTF) to recommend screening for high-risk individuals (current and former heavy smokers). And that, in turn, led to hospitals nationwide setting up lung cancer screening programs—a number that rose “dramatically,” according to the National Cancer Institute (NCI), after the Centers for Medicare and Medicaid Services decided to cover LDCT screening for high-risk Medicare beneficiaries.
But how does the screening recommendation pan out in real life? Primary care physicians and pulmonologists alike were concerned about the workability of putting LDCT into real-life practice. And not without basis: Published experience with implementation of lung cancer screening (LCS) is limited, say VHA researchers. Their 3-year demonstration project bears out the concerns, they add.
When they designed the study, the researchers wanted to see how feasible LCS would be in terms of resources and effort, whether patients would take part, what their clinical experience might be, and what type of findings the screenings might produce. The researchers found that establishing and sustaining a screening program requires “significant clinical effort for as-yet uncertain patient benefit.” They also found “wide variation” in both processes and patient experiences among the 8 VA hospitals in the study. Moreover, they found that, although most patients had findings that required follow-up, few had early-stage lung cancers.
Of the 2,106 screened patients in a JAMA Internal Medicine study, 1,257 had nodules. More than half of those required tracking, and 2% required further evaluation, but the findings were not cancer. Just 1.5% had lung cancer. Scans of 857 patients (40.7%) also revealed a variety of incidental findings, such as emphysema, other pulmonary abnormalities, and coronary artery calcification.
The researchers say that implementing a comprehensive program that followed recommendations was “challenging and complex,” requiring new tools and processes for staff as well as for dedicated patient coordination. As an example, they say creating electronic tools to capture the necessary clinical data in real time that met the needs of the screening coordinators proved to be difficult, “even with the VHA’s highly regarded electronic medical record.”
Also, finding out who actually had a smoking history of > 30 pack-years of smoking per the USPSTF recommendation was not easy. Lead investigator Linda Kinsinger, MD, MPH, points out, “People who smoke don’t track that sort of thing as closely as you think they would, and they don’t smoke at the same level for years and years.”
The researchers estimate that nearly 900,000 veterans meet the initial screening criteria for age, smoking history, and medical history, but they caution that accurately identifying the patients and discussing risks and benefits will take “significant effort” for primary care teams. Even if that number were reduced by 16% to account for longer medical contraindications, the number of veterans who might be candidates for annual LCS would be “substantial.” And based on the researchers’ experience, a bit more than half the candidates will agree to be screened.
Although screening programs are a complex endeavor, the researchers say, the results of their study can help the VHA plan for broader implementation of comprehensive screening programs.
“What [the VHA] is reporting is the initial experience for almost everybody,” said Lynn Tanoue, MD, director of the Lung Screening and Nodule Program at Yale Cancer Center in New Haven, Connecticut, in an interview with the NCI. “Until people really started doing lung cancer screening and began to understand the challenges of doing it properly, you couldn’t have known what it was going to be like.” But she adds, “The data from NLST were very clear. We should accept that there is benefit and choose the right population to screen.”
However, although the LDCT screening can find signs of early lung cancer, a biopsy is often necessary. Researchers from Boston University suggest an effective, much less invasive approach: analyzing gene expression in nasal cell samples.
The researchers collected and analyzed nasal cell samples from 505 current and former smokers for gene expression. They found 535 genes that were expressed differently between patients who were diagnosed with lung cancer and those whose lesions were benign.
Comparing those data with data from bronchial airway samples from the same patients, the researchers found changes that were similar between the nose and lung samples of patients with lung cancer, suggesting that smoking might cause similar genetic changes throughout the entire airway.
The researchers used the 30 most prominent changes to create a biomarker panel and tested it in 130 other patients. Compared with a clinical risk factor model that considered age, smoking status, and other factors, the biomarker panel was better at predicting lung cancer. Combining the 2 models further improved detection. “We find that nasal gene expression contains information about the presence of cancer that is independent of standard clinical risk factors,” said one of the co-lead investigators.
Lung cancer screening is still experiencing “growing pains,” the NCI says. And the need for screening is both acute and chronic: A 2015 study found that only 4% of people who meet the criteria for screening actually undergo screening.
These studies not only open avenues for better screening and diagnosis, but also highlight the need for better patient education.
Click here to read the digital edition.
Kinsinger LS, Anderson C, Kim J, et al. JAMA Intern Med. 2017;177(3):399-406.
National Cancer Institute. https://www.cancer.gov/types/lung/research/nlst. Updated September 8, 2014. Accessed April 25, 2017.
National Institutes of Health. https://www.nih.gov/news-events/nih-research-matters/noninvasive-strategies-lung-cancer-testing. Published March 7, 2017. Accessed April
25, 2017.
AEGIS Study Team. J. Natl Cancer Inst. 2017;109(7).
National Cancer Institute. https://www.cancer.gov/news-events/cancer-currents-blog/2017/lung-cancer-screening-challenges. Published February 27, 2017. Accessed April
25, 2017.
According to the National Lung Screening Trial (NLST), use of low-dose computed tomography (LDCT) reduced lung cancer deaths by 20%. That finding led the U.S. Preventive Services Task Force (USPSTF) to recommend screening for high-risk individuals (current and former heavy smokers). And that, in turn, led to hospitals nationwide setting up lung cancer screening programs—a number that rose “dramatically,” according to the National Cancer Institute (NCI), after the Centers for Medicare and Medicaid Services decided to cover LDCT screening for high-risk Medicare beneficiaries.
But how does the screening recommendation pan out in real life? Primary care physicians and pulmonologists alike were concerned about the workability of putting LDCT into real-life practice. And not without basis: Published experience with implementation of lung cancer screening (LCS) is limited, say VHA researchers. Their 3-year demonstration project bears out the concerns, they add.
When they designed the study, the researchers wanted to see how feasible LCS would be in terms of resources and effort, whether patients would take part, what their clinical experience might be, and what type of findings the screenings might produce. The researchers found that establishing and sustaining a screening program requires “significant clinical effort for as-yet uncertain patient benefit.” They also found “wide variation” in both processes and patient experiences among the 8 VA hospitals in the study. Moreover, they found that, although most patients had findings that required follow-up, few had early-stage lung cancers.
Of the 2,106 screened patients in a JAMA Internal Medicine study, 1,257 had nodules. More than half of those required tracking, and 2% required further evaluation, but the findings were not cancer. Just 1.5% had lung cancer. Scans of 857 patients (40.7%) also revealed a variety of incidental findings, such as emphysema, other pulmonary abnormalities, and coronary artery calcification.
The researchers say that implementing a comprehensive program that followed recommendations was “challenging and complex,” requiring new tools and processes for staff as well as for dedicated patient coordination. As an example, they say creating electronic tools to capture the necessary clinical data in real time that met the needs of the screening coordinators proved to be difficult, “even with the VHA’s highly regarded electronic medical record.”
Also, finding out who actually had a smoking history of > 30 pack-years of smoking per the USPSTF recommendation was not easy. Lead investigator Linda Kinsinger, MD, MPH, points out, “People who smoke don’t track that sort of thing as closely as you think they would, and they don’t smoke at the same level for years and years.”
The researchers estimate that nearly 900,000 veterans meet the initial screening criteria for age, smoking history, and medical history, but they caution that accurately identifying the patients and discussing risks and benefits will take “significant effort” for primary care teams. Even if that number were reduced by 16% to account for longer medical contraindications, the number of veterans who might be candidates for annual LCS would be “substantial.” And based on the researchers’ experience, a bit more than half the candidates will agree to be screened.
Although screening programs are a complex endeavor, the researchers say, the results of their study can help the VHA plan for broader implementation of comprehensive screening programs.
“What [the VHA] is reporting is the initial experience for almost everybody,” said Lynn Tanoue, MD, director of the Lung Screening and Nodule Program at Yale Cancer Center in New Haven, Connecticut, in an interview with the NCI. “Until people really started doing lung cancer screening and began to understand the challenges of doing it properly, you couldn’t have known what it was going to be like.” But she adds, “The data from NLST were very clear. We should accept that there is benefit and choose the right population to screen.”
However, although the LDCT screening can find signs of early lung cancer, a biopsy is often necessary. Researchers from Boston University suggest an effective, much less invasive approach: analyzing gene expression in nasal cell samples.
The researchers collected and analyzed nasal cell samples from 505 current and former smokers for gene expression. They found 535 genes that were expressed differently between patients who were diagnosed with lung cancer and those whose lesions were benign.
Comparing those data with data from bronchial airway samples from the same patients, the researchers found changes that were similar between the nose and lung samples of patients with lung cancer, suggesting that smoking might cause similar genetic changes throughout the entire airway.
The researchers used the 30 most prominent changes to create a biomarker panel and tested it in 130 other patients. Compared with a clinical risk factor model that considered age, smoking status, and other factors, the biomarker panel was better at predicting lung cancer. Combining the 2 models further improved detection. “We find that nasal gene expression contains information about the presence of cancer that is independent of standard clinical risk factors,” said one of the co-lead investigators.
Lung cancer screening is still experiencing “growing pains,” the NCI says. And the need for screening is both acute and chronic: A 2015 study found that only 4% of people who meet the criteria for screening actually undergo screening.
These studies not only open avenues for better screening and diagnosis, but also highlight the need for better patient education.
Click here to read the digital edition.
According to the National Lung Screening Trial (NLST), use of low-dose computed tomography (LDCT) reduced lung cancer deaths by 20%. That finding led the U.S. Preventive Services Task Force (USPSTF) to recommend screening for high-risk individuals (current and former heavy smokers). And that, in turn, led to hospitals nationwide setting up lung cancer screening programs—a number that rose “dramatically,” according to the National Cancer Institute (NCI), after the Centers for Medicare and Medicaid Services decided to cover LDCT screening for high-risk Medicare beneficiaries.
But how does the screening recommendation pan out in real life? Primary care physicians and pulmonologists alike were concerned about the workability of putting LDCT into real-life practice. And not without basis: Published experience with implementation of lung cancer screening (LCS) is limited, say VHA researchers. Their 3-year demonstration project bears out the concerns, they add.
When they designed the study, the researchers wanted to see how feasible LCS would be in terms of resources and effort, whether patients would take part, what their clinical experience might be, and what type of findings the screenings might produce. The researchers found that establishing and sustaining a screening program requires “significant clinical effort for as-yet uncertain patient benefit.” They also found “wide variation” in both processes and patient experiences among the 8 VA hospitals in the study. Moreover, they found that, although most patients had findings that required follow-up, few had early-stage lung cancers.
Of the 2,106 screened patients in a JAMA Internal Medicine study, 1,257 had nodules. More than half of those required tracking, and 2% required further evaluation, but the findings were not cancer. Just 1.5% had lung cancer. Scans of 857 patients (40.7%) also revealed a variety of incidental findings, such as emphysema, other pulmonary abnormalities, and coronary artery calcification.
The researchers say that implementing a comprehensive program that followed recommendations was “challenging and complex,” requiring new tools and processes for staff as well as for dedicated patient coordination. As an example, they say creating electronic tools to capture the necessary clinical data in real time that met the needs of the screening coordinators proved to be difficult, “even with the VHA’s highly regarded electronic medical record.”
Also, finding out who actually had a smoking history of > 30 pack-years of smoking per the USPSTF recommendation was not easy. Lead investigator Linda Kinsinger, MD, MPH, points out, “People who smoke don’t track that sort of thing as closely as you think they would, and they don’t smoke at the same level for years and years.”
The researchers estimate that nearly 900,000 veterans meet the initial screening criteria for age, smoking history, and medical history, but they caution that accurately identifying the patients and discussing risks and benefits will take “significant effort” for primary care teams. Even if that number were reduced by 16% to account for longer medical contraindications, the number of veterans who might be candidates for annual LCS would be “substantial.” And based on the researchers’ experience, a bit more than half the candidates will agree to be screened.
Although screening programs are a complex endeavor, the researchers say, the results of their study can help the VHA plan for broader implementation of comprehensive screening programs.
“What [the VHA] is reporting is the initial experience for almost everybody,” said Lynn Tanoue, MD, director of the Lung Screening and Nodule Program at Yale Cancer Center in New Haven, Connecticut, in an interview with the NCI. “Until people really started doing lung cancer screening and began to understand the challenges of doing it properly, you couldn’t have known what it was going to be like.” But she adds, “The data from NLST were very clear. We should accept that there is benefit and choose the right population to screen.”
However, although the LDCT screening can find signs of early lung cancer, a biopsy is often necessary. Researchers from Boston University suggest an effective, much less invasive approach: analyzing gene expression in nasal cell samples.
The researchers collected and analyzed nasal cell samples from 505 current and former smokers for gene expression. They found 535 genes that were expressed differently between patients who were diagnosed with lung cancer and those whose lesions were benign.
Comparing those data with data from bronchial airway samples from the same patients, the researchers found changes that were similar between the nose and lung samples of patients with lung cancer, suggesting that smoking might cause similar genetic changes throughout the entire airway.
The researchers used the 30 most prominent changes to create a biomarker panel and tested it in 130 other patients. Compared with a clinical risk factor model that considered age, smoking status, and other factors, the biomarker panel was better at predicting lung cancer. Combining the 2 models further improved detection. “We find that nasal gene expression contains information about the presence of cancer that is independent of standard clinical risk factors,” said one of the co-lead investigators.
Lung cancer screening is still experiencing “growing pains,” the NCI says. And the need for screening is both acute and chronic: A 2015 study found that only 4% of people who meet the criteria for screening actually undergo screening.
These studies not only open avenues for better screening and diagnosis, but also highlight the need for better patient education.
Click here to read the digital edition.
Kinsinger LS, Anderson C, Kim J, et al. JAMA Intern Med. 2017;177(3):399-406.
National Cancer Institute. https://www.cancer.gov/types/lung/research/nlst. Updated September 8, 2014. Accessed April 25, 2017.
National Institutes of Health. https://www.nih.gov/news-events/nih-research-matters/noninvasive-strategies-lung-cancer-testing. Published March 7, 2017. Accessed April
25, 2017.
AEGIS Study Team. J. Natl Cancer Inst. 2017;109(7).
National Cancer Institute. https://www.cancer.gov/news-events/cancer-currents-blog/2017/lung-cancer-screening-challenges. Published February 27, 2017. Accessed April
25, 2017.
Kinsinger LS, Anderson C, Kim J, et al. JAMA Intern Med. 2017;177(3):399-406.
National Cancer Institute. https://www.cancer.gov/types/lung/research/nlst. Updated September 8, 2014. Accessed April 25, 2017.
National Institutes of Health. https://www.nih.gov/news-events/nih-research-matters/noninvasive-strategies-lung-cancer-testing. Published March 7, 2017. Accessed April
25, 2017.
AEGIS Study Team. J. Natl Cancer Inst. 2017;109(7).
National Cancer Institute. https://www.cancer.gov/news-events/cancer-currents-blog/2017/lung-cancer-screening-challenges. Published February 27, 2017. Accessed April
25, 2017.
Enhanced Melanoma Diagnosis With Multispectral Digital Skin Lesion Analysis
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods
Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.
Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods
Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.
Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods
Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.
Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
Practice Points
- Multispectral digital skin lesion analysis (MSDSLA) can be a valuable tool in the evaluation of pigmented skin lesions (PSLs).
- MSDSLA may help to better identify high-risk PSLs and improve cost of care.
Mohs Micrographic Surgery for Digital Melanoma and Nonmelanoma Skin Cancers
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.
Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.
Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.
Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.

Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.
Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.

Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
Practice Points
- Melanoma and nonmelanoma skin cancers of the digits traditionally have been treated with wide local surgical excision and even amputation.
- Conservative tissue sparing techniques such as Mohs micrographic surgery can be used to treat digital skin cancers with high cure rates and improved functional and cosmetic results.