User login
Psychedelic experience and “oneiroid” state
David Nichols, PhD, a leading expert in the field of psychedelic research and the founding president of the Heffter Research Institute, often answers the question, “What is a psychedelic?” by saying, “The scientific definition I always use is that they are substances that produce changes in thought, mood, and affect, which only occur during dreaming or religious exaltation.”1,2 However, this definition does not account for the experiences of naturally occurring psychoses, such as those seen in schizophrenia.
The phenomenology of psychotic experiences between drug-induced and naturally occurring psychoses differs, and the use of psychedelics does not necessarily replicate or simulate the symptoms of schizophrenia. For example, drug induced hallucinations are often described as more intense and vivid, while those associated with schizophrenia are described as more persistent and distressing.3
An altered state of consciousness is a hallmark of a psychedelic trip, but not a characteristic of schizophrenia. Patients with schizophrenia who have used psychedelics typically report that their experiences under the influence of these drugs are distinct from their usual experiences with the condition. Additionally, according to many experts, the underlying neurobiological mechanisms are different, with psychedelics affecting serotonin receptors and schizophrenia thought to be linked more to dopamine dysregulation, among other factors.
However, there are some instances of naturally occurring psychoses that are difficult to distinguish from the experiences reported by those who take psychedelics. This article explores the similarities between psychedelic experiences induced by serotonergic psychedelics, 5-HT2A agonists, such as LSD, psilocybin, and mescaline, and a rare psychiatric disorder known as the “oneiroid state.”
Origins and definitions
The term “oneiroia” is derived from the Greek words for “sleep” and “similar,” referring to the dreamlike character of the condition. It is widely acknowledged that psychedelics can temporarily induce dreamlike states,4 but this article focuses specifically on the naturally occurring, endogenous dreamlike state.
Oneiroid state was first described by German psychiatrist Wilhelm Mayer-Gross in the early 20th century and was once well-known among European psychiatrists. Some classified it as a part of schizophrenia, others saw it as an unusual manifestation of affective disorder, and still others considered it an atypical psychosis. Nevertheless, this phenomenon has received limited attention in American psychiatric journals.
The key characteristic of this condition is a distinctive state of consciousness marked by vivid and florid hallucinations, and a succession of constantly shifting dreamlike or surrealistic visuals and imagery often similar to mystical or cosmic experiences. Self-awareness and orientation in time and place are often disturbed, and delusions are experienced within this altered state.5
To gain a better understanding of the oneiroid state, it may be helpful to turn to European or other schools of psychiatry with a history of studying this phenomenon, as American psychiatry has limited knowledge in this area.
As described in the “Handbook of Psychiatry” by Russian psychiatrist A.V. Snezhnevsky, published in 1983, oneiroid state, also known as oneiroid syndrome, is a dreamlike and imaginative state characterized by a bizarre combination of reality and vivid phantasmagoric imaginations.6 In this state, individuals are completely detached from their surroundings and experience a change in self-awareness, often displaying either a lack of movement or senseless excitement.
Patients often experience the oneiroid state as active participants, as if they are in a movie theater, not only watching the story, but also being part of it, reacting to it with either “external immobility” or senseless excitement, completely detached from their surroundings. This is similar to the portrayal of the emotions of the characters in Steven Spielberg’s film “Ready Player One.”
The entry in Dr. Snezhnevsky’s “Handbook” states: “Some patients in Oneiroid State experience travels to other worlds, such as interacting with inhabitants on Mars, collecting gems on the moon, exploring invisible cities, participating in conspiracies and insurrections, fighting with pirates, chasing The Flying Dutchman, wandering through ancient Rome, and even visiting heaven or hell. At times, the patient’s imagination reaches a state of mystical contemplation.”6
In contrast to delirium, which never impairs self-awareness, oneiroid state is marked by drastic changes in the sense of self. The memory of the subjective experience during the oneiroia is much more vivid and consistent than in delirium. Patients who have experienced the oneiroid state often have complete recall of their experience, as if they have just woken up from a dream.
It was commonly thought that oneiroid state was part of the group of functional psychoses, rather than organic psychoses, and was not considered to be a result of mind-altering psychedelics. It was not considered a manifestation of epilepsy either. Oneiroid state could last for weeks or even months, making it unlikely to be related to an epileptic seizure. Furthermore, EEG results did not show any seizurelike activity during the state.
An excellent case study on oneiroid state was published in Israel in 2000. The authors described two patients who experienced the oneiroid state for several days or even weeks.7 One of the patients reported that during the illness, he experienced himself aboard a spaceship as a cosmonaut, heading for a different universe. On another occasion, the patient perceived himself as a person living 2,000 years ago and being guilty of Christ’s death.
The second patient reported that everything around her appeared “like in the movies,” and she saw others as characters from comic strips. Both patients alternated between catatonic excitement and sluggishness and would sometimes come back to reality for a few minutes to respond to questions. Physical exams, laboratory tests, neurological tests, and a brain scan were all normal in both cases.
Conclusions
As mentioned earlier, oneiroid state is not widely discussed in American psychiatric journals and is now considered a rare condition globally, despite being a common occurrence in the past century. A diagnosis similar to oneiroid state, known as bouffée délirante, is still in use in some French-speaking countries, with a noticeable decline in frequency.2,8 The widespread acceptance of international classification systems such as the ICD-10, which does not recognize the diagnosis, is likely one of the reasons for this decline.
However, the decrease in the prevalence of the oneiroid state is not unique, as other forms of mental illness, such as the catatonic subtype of schizophrenia, are also becoming less prevalent. The cause of this decline is uncertain. Could changes in the way mental disorders affect 5HT2A receptors be a contributing factor?
In conclusion, as the field of psychedelic research experiences a resurgence, this little-known manifestation of mental illness, oneiroid state, may be worth reexamining.
With the expected approval and regulation of psilocybin and MDMA by the FDA, and classical psychedelics widely regarded as nonaddictive and safer than other recreational drugs, , rather than as a cause for concern.
Dr. Khetsuriani is a supervising psychiatrist at the Bronx Psychiatric Center in Bronx, N.Y., and has a private psychiatric practice located in Manhattan, N.Y.
References
1. Nichols DE. Keynote address, 39th Telluride Mushroom Festival, Aug. 15-18, 2019. https://www.youtube.com/watch?v=RlDCM5JQzRk.
2. Nichols DE. Psychedelics. Pharmacol Rev. 2016 Apr;68(2):264-355. doi: 10.1124/pr.115.011478.
3. Leptourgos P et al. Hallucinations Under Psychedelics and in the Schizophrenia Spectrum: An Interdisciplinary and Multiscale Comparison. Schizophr Bull. 2020 Dec 1;46(6):1396-1408. doi: 10.1093/schbul/sbaa117.
4. Kraehenmann R. Dreams and Psychedelics: Neurophenomenological Comparison and Therapeutic Implications. Curr Neuropharmacol. 2017;15(7):1032-42. doi: 10.2174/1573413713666170619092629.
5. Henri EY et al. “Acute Delusional Psychosis” in Hirsch SR and Shepherd M, eds. Themes and variations in European psychiatry: An anthology. Charlottesville: University Press of Virginia, 1974.
6. Snezhnevsky A, ed. Handbook of Psychiatry. Moscow: Meditsina, 1983.
7. Kaptsan A et al. Oneiroid syndrome: A concept of use for Western psychiatry. Isr J Psychiatry Relat Sci. 2000;37(4):278-85.
8. https://en.wikipedia.org/wiki/Bouff%C3%A9e_d%C3%A9lirante.
David Nichols, PhD, a leading expert in the field of psychedelic research and the founding president of the Heffter Research Institute, often answers the question, “What is a psychedelic?” by saying, “The scientific definition I always use is that they are substances that produce changes in thought, mood, and affect, which only occur during dreaming or religious exaltation.”1,2 However, this definition does not account for the experiences of naturally occurring psychoses, such as those seen in schizophrenia.
The phenomenology of psychotic experiences between drug-induced and naturally occurring psychoses differs, and the use of psychedelics does not necessarily replicate or simulate the symptoms of schizophrenia. For example, drug induced hallucinations are often described as more intense and vivid, while those associated with schizophrenia are described as more persistent and distressing.3
An altered state of consciousness is a hallmark of a psychedelic trip, but not a characteristic of schizophrenia. Patients with schizophrenia who have used psychedelics typically report that their experiences under the influence of these drugs are distinct from their usual experiences with the condition. Additionally, according to many experts, the underlying neurobiological mechanisms are different, with psychedelics affecting serotonin receptors and schizophrenia thought to be linked more to dopamine dysregulation, among other factors.
However, there are some instances of naturally occurring psychoses that are difficult to distinguish from the experiences reported by those who take psychedelics. This article explores the similarities between psychedelic experiences induced by serotonergic psychedelics, 5-HT2A agonists, such as LSD, psilocybin, and mescaline, and a rare psychiatric disorder known as the “oneiroid state.”
Origins and definitions
The term “oneiroia” is derived from the Greek words for “sleep” and “similar,” referring to the dreamlike character of the condition. It is widely acknowledged that psychedelics can temporarily induce dreamlike states,4 but this article focuses specifically on the naturally occurring, endogenous dreamlike state.
Oneiroid state was first described by German psychiatrist Wilhelm Mayer-Gross in the early 20th century and was once well-known among European psychiatrists. Some classified it as a part of schizophrenia, others saw it as an unusual manifestation of affective disorder, and still others considered it an atypical psychosis. Nevertheless, this phenomenon has received limited attention in American psychiatric journals.
The key characteristic of this condition is a distinctive state of consciousness marked by vivid and florid hallucinations, and a succession of constantly shifting dreamlike or surrealistic visuals and imagery often similar to mystical or cosmic experiences. Self-awareness and orientation in time and place are often disturbed, and delusions are experienced within this altered state.5
To gain a better understanding of the oneiroid state, it may be helpful to turn to European or other schools of psychiatry with a history of studying this phenomenon, as American psychiatry has limited knowledge in this area.
As described in the “Handbook of Psychiatry” by Russian psychiatrist A.V. Snezhnevsky, published in 1983, oneiroid state, also known as oneiroid syndrome, is a dreamlike and imaginative state characterized by a bizarre combination of reality and vivid phantasmagoric imaginations.6 In this state, individuals are completely detached from their surroundings and experience a change in self-awareness, often displaying either a lack of movement or senseless excitement.
Patients often experience the oneiroid state as active participants, as if they are in a movie theater, not only watching the story, but also being part of it, reacting to it with either “external immobility” or senseless excitement, completely detached from their surroundings. This is similar to the portrayal of the emotions of the characters in Steven Spielberg’s film “Ready Player One.”
The entry in Dr. Snezhnevsky’s “Handbook” states: “Some patients in Oneiroid State experience travels to other worlds, such as interacting with inhabitants on Mars, collecting gems on the moon, exploring invisible cities, participating in conspiracies and insurrections, fighting with pirates, chasing The Flying Dutchman, wandering through ancient Rome, and even visiting heaven or hell. At times, the patient’s imagination reaches a state of mystical contemplation.”6
In contrast to delirium, which never impairs self-awareness, oneiroid state is marked by drastic changes in the sense of self. The memory of the subjective experience during the oneiroia is much more vivid and consistent than in delirium. Patients who have experienced the oneiroid state often have complete recall of their experience, as if they have just woken up from a dream.
It was commonly thought that oneiroid state was part of the group of functional psychoses, rather than organic psychoses, and was not considered to be a result of mind-altering psychedelics. It was not considered a manifestation of epilepsy either. Oneiroid state could last for weeks or even months, making it unlikely to be related to an epileptic seizure. Furthermore, EEG results did not show any seizurelike activity during the state.
An excellent case study on oneiroid state was published in Israel in 2000. The authors described two patients who experienced the oneiroid state for several days or even weeks.7 One of the patients reported that during the illness, he experienced himself aboard a spaceship as a cosmonaut, heading for a different universe. On another occasion, the patient perceived himself as a person living 2,000 years ago and being guilty of Christ’s death.
The second patient reported that everything around her appeared “like in the movies,” and she saw others as characters from comic strips. Both patients alternated between catatonic excitement and sluggishness and would sometimes come back to reality for a few minutes to respond to questions. Physical exams, laboratory tests, neurological tests, and a brain scan were all normal in both cases.
Conclusions
As mentioned earlier, oneiroid state is not widely discussed in American psychiatric journals and is now considered a rare condition globally, despite being a common occurrence in the past century. A diagnosis similar to oneiroid state, known as bouffée délirante, is still in use in some French-speaking countries, with a noticeable decline in frequency.2,8 The widespread acceptance of international classification systems such as the ICD-10, which does not recognize the diagnosis, is likely one of the reasons for this decline.
However, the decrease in the prevalence of the oneiroid state is not unique, as other forms of mental illness, such as the catatonic subtype of schizophrenia, are also becoming less prevalent. The cause of this decline is uncertain. Could changes in the way mental disorders affect 5HT2A receptors be a contributing factor?
In conclusion, as the field of psychedelic research experiences a resurgence, this little-known manifestation of mental illness, oneiroid state, may be worth reexamining.
With the expected approval and regulation of psilocybin and MDMA by the FDA, and classical psychedelics widely regarded as nonaddictive and safer than other recreational drugs, , rather than as a cause for concern.
Dr. Khetsuriani is a supervising psychiatrist at the Bronx Psychiatric Center in Bronx, N.Y., and has a private psychiatric practice located in Manhattan, N.Y.
References
1. Nichols DE. Keynote address, 39th Telluride Mushroom Festival, Aug. 15-18, 2019. https://www.youtube.com/watch?v=RlDCM5JQzRk.
2. Nichols DE. Psychedelics. Pharmacol Rev. 2016 Apr;68(2):264-355. doi: 10.1124/pr.115.011478.
3. Leptourgos P et al. Hallucinations Under Psychedelics and in the Schizophrenia Spectrum: An Interdisciplinary and Multiscale Comparison. Schizophr Bull. 2020 Dec 1;46(6):1396-1408. doi: 10.1093/schbul/sbaa117.
4. Kraehenmann R. Dreams and Psychedelics: Neurophenomenological Comparison and Therapeutic Implications. Curr Neuropharmacol. 2017;15(7):1032-42. doi: 10.2174/1573413713666170619092629.
5. Henri EY et al. “Acute Delusional Psychosis” in Hirsch SR and Shepherd M, eds. Themes and variations in European psychiatry: An anthology. Charlottesville: University Press of Virginia, 1974.
6. Snezhnevsky A, ed. Handbook of Psychiatry. Moscow: Meditsina, 1983.
7. Kaptsan A et al. Oneiroid syndrome: A concept of use for Western psychiatry. Isr J Psychiatry Relat Sci. 2000;37(4):278-85.
8. https://en.wikipedia.org/wiki/Bouff%C3%A9e_d%C3%A9lirante.
David Nichols, PhD, a leading expert in the field of psychedelic research and the founding president of the Heffter Research Institute, often answers the question, “What is a psychedelic?” by saying, “The scientific definition I always use is that they are substances that produce changes in thought, mood, and affect, which only occur during dreaming or religious exaltation.”1,2 However, this definition does not account for the experiences of naturally occurring psychoses, such as those seen in schizophrenia.
The phenomenology of psychotic experiences between drug-induced and naturally occurring psychoses differs, and the use of psychedelics does not necessarily replicate or simulate the symptoms of schizophrenia. For example, drug induced hallucinations are often described as more intense and vivid, while those associated with schizophrenia are described as more persistent and distressing.3
An altered state of consciousness is a hallmark of a psychedelic trip, but not a characteristic of schizophrenia. Patients with schizophrenia who have used psychedelics typically report that their experiences under the influence of these drugs are distinct from their usual experiences with the condition. Additionally, according to many experts, the underlying neurobiological mechanisms are different, with psychedelics affecting serotonin receptors and schizophrenia thought to be linked more to dopamine dysregulation, among other factors.
However, there are some instances of naturally occurring psychoses that are difficult to distinguish from the experiences reported by those who take psychedelics. This article explores the similarities between psychedelic experiences induced by serotonergic psychedelics, 5-HT2A agonists, such as LSD, psilocybin, and mescaline, and a rare psychiatric disorder known as the “oneiroid state.”
Origins and definitions
The term “oneiroia” is derived from the Greek words for “sleep” and “similar,” referring to the dreamlike character of the condition. It is widely acknowledged that psychedelics can temporarily induce dreamlike states,4 but this article focuses specifically on the naturally occurring, endogenous dreamlike state.
Oneiroid state was first described by German psychiatrist Wilhelm Mayer-Gross in the early 20th century and was once well-known among European psychiatrists. Some classified it as a part of schizophrenia, others saw it as an unusual manifestation of affective disorder, and still others considered it an atypical psychosis. Nevertheless, this phenomenon has received limited attention in American psychiatric journals.
The key characteristic of this condition is a distinctive state of consciousness marked by vivid and florid hallucinations, and a succession of constantly shifting dreamlike or surrealistic visuals and imagery often similar to mystical or cosmic experiences. Self-awareness and orientation in time and place are often disturbed, and delusions are experienced within this altered state.5
To gain a better understanding of the oneiroid state, it may be helpful to turn to European or other schools of psychiatry with a history of studying this phenomenon, as American psychiatry has limited knowledge in this area.
As described in the “Handbook of Psychiatry” by Russian psychiatrist A.V. Snezhnevsky, published in 1983, oneiroid state, also known as oneiroid syndrome, is a dreamlike and imaginative state characterized by a bizarre combination of reality and vivid phantasmagoric imaginations.6 In this state, individuals are completely detached from their surroundings and experience a change in self-awareness, often displaying either a lack of movement or senseless excitement.
Patients often experience the oneiroid state as active participants, as if they are in a movie theater, not only watching the story, but also being part of it, reacting to it with either “external immobility” or senseless excitement, completely detached from their surroundings. This is similar to the portrayal of the emotions of the characters in Steven Spielberg’s film “Ready Player One.”
The entry in Dr. Snezhnevsky’s “Handbook” states: “Some patients in Oneiroid State experience travels to other worlds, such as interacting with inhabitants on Mars, collecting gems on the moon, exploring invisible cities, participating in conspiracies and insurrections, fighting with pirates, chasing The Flying Dutchman, wandering through ancient Rome, and even visiting heaven or hell. At times, the patient’s imagination reaches a state of mystical contemplation.”6
In contrast to delirium, which never impairs self-awareness, oneiroid state is marked by drastic changes in the sense of self. The memory of the subjective experience during the oneiroia is much more vivid and consistent than in delirium. Patients who have experienced the oneiroid state often have complete recall of their experience, as if they have just woken up from a dream.
It was commonly thought that oneiroid state was part of the group of functional psychoses, rather than organic psychoses, and was not considered to be a result of mind-altering psychedelics. It was not considered a manifestation of epilepsy either. Oneiroid state could last for weeks or even months, making it unlikely to be related to an epileptic seizure. Furthermore, EEG results did not show any seizurelike activity during the state.
An excellent case study on oneiroid state was published in Israel in 2000. The authors described two patients who experienced the oneiroid state for several days or even weeks.7 One of the patients reported that during the illness, he experienced himself aboard a spaceship as a cosmonaut, heading for a different universe. On another occasion, the patient perceived himself as a person living 2,000 years ago and being guilty of Christ’s death.
The second patient reported that everything around her appeared “like in the movies,” and she saw others as characters from comic strips. Both patients alternated between catatonic excitement and sluggishness and would sometimes come back to reality for a few minutes to respond to questions. Physical exams, laboratory tests, neurological tests, and a brain scan were all normal in both cases.
Conclusions
As mentioned earlier, oneiroid state is not widely discussed in American psychiatric journals and is now considered a rare condition globally, despite being a common occurrence in the past century. A diagnosis similar to oneiroid state, known as bouffée délirante, is still in use in some French-speaking countries, with a noticeable decline in frequency.2,8 The widespread acceptance of international classification systems such as the ICD-10, which does not recognize the diagnosis, is likely one of the reasons for this decline.
However, the decrease in the prevalence of the oneiroid state is not unique, as other forms of mental illness, such as the catatonic subtype of schizophrenia, are also becoming less prevalent. The cause of this decline is uncertain. Could changes in the way mental disorders affect 5HT2A receptors be a contributing factor?
In conclusion, as the field of psychedelic research experiences a resurgence, this little-known manifestation of mental illness, oneiroid state, may be worth reexamining.
With the expected approval and regulation of psilocybin and MDMA by the FDA, and classical psychedelics widely regarded as nonaddictive and safer than other recreational drugs, , rather than as a cause for concern.
Dr. Khetsuriani is a supervising psychiatrist at the Bronx Psychiatric Center in Bronx, N.Y., and has a private psychiatric practice located in Manhattan, N.Y.
References
1. Nichols DE. Keynote address, 39th Telluride Mushroom Festival, Aug. 15-18, 2019. https://www.youtube.com/watch?v=RlDCM5JQzRk.
2. Nichols DE. Psychedelics. Pharmacol Rev. 2016 Apr;68(2):264-355. doi: 10.1124/pr.115.011478.
3. Leptourgos P et al. Hallucinations Under Psychedelics and in the Schizophrenia Spectrum: An Interdisciplinary and Multiscale Comparison. Schizophr Bull. 2020 Dec 1;46(6):1396-1408. doi: 10.1093/schbul/sbaa117.
4. Kraehenmann R. Dreams and Psychedelics: Neurophenomenological Comparison and Therapeutic Implications. Curr Neuropharmacol. 2017;15(7):1032-42. doi: 10.2174/1573413713666170619092629.
5. Henri EY et al. “Acute Delusional Psychosis” in Hirsch SR and Shepherd M, eds. Themes and variations in European psychiatry: An anthology. Charlottesville: University Press of Virginia, 1974.
6. Snezhnevsky A, ed. Handbook of Psychiatry. Moscow: Meditsina, 1983.
7. Kaptsan A et al. Oneiroid syndrome: A concept of use for Western psychiatry. Isr J Psychiatry Relat Sci. 2000;37(4):278-85.
8. https://en.wikipedia.org/wiki/Bouff%C3%A9e_d%C3%A9lirante.
Bad blood: Could brain bleeds be contagious?
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.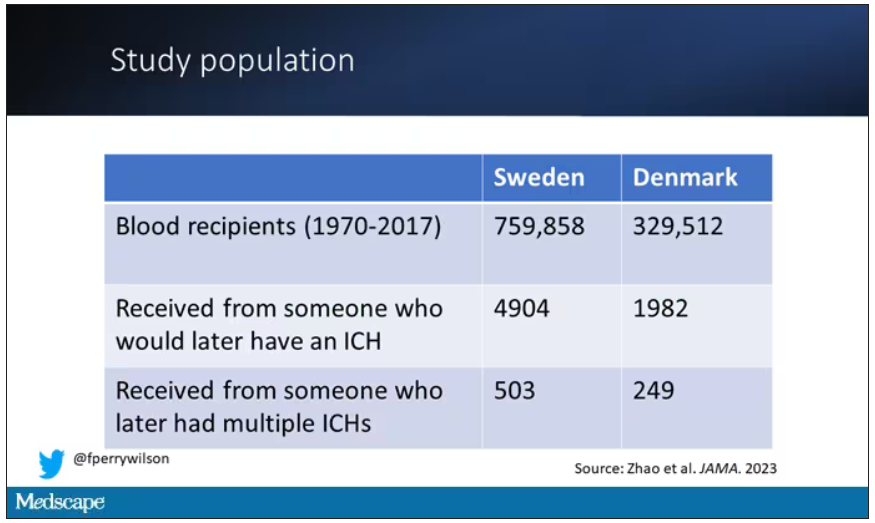
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.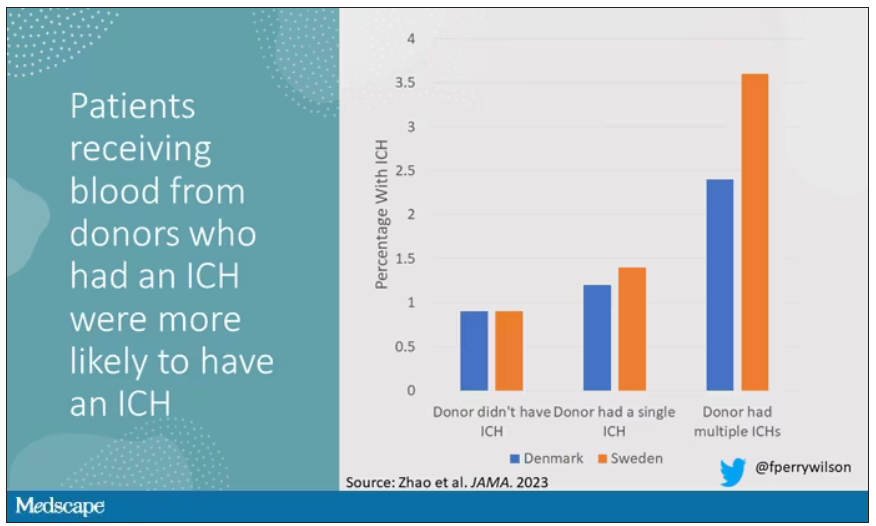
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.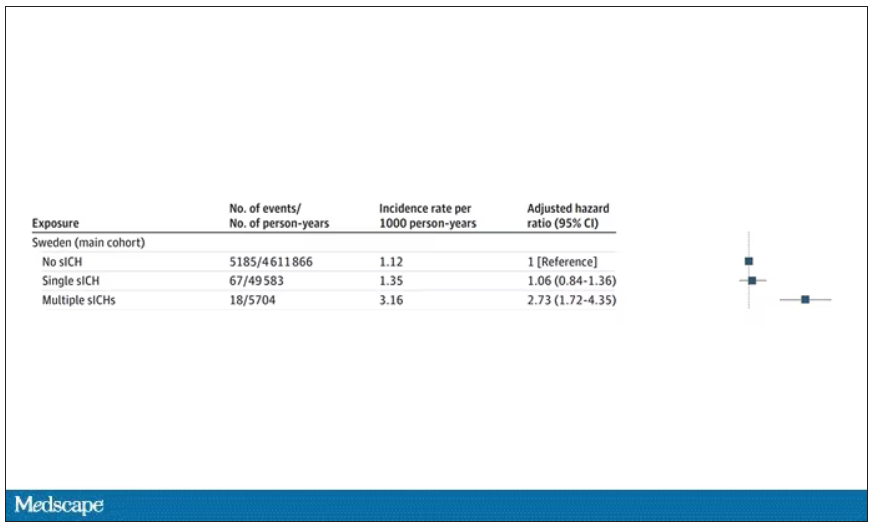
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Disenfranchised grief: What it looks like, where it goes
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
Universal anxiety screening recommendation is a good start
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Should people who play sports pay higher medical insurance premiums?
This transcript has been edited for clarity.
If you’re anywhere near Seattle, anywhere near Florida, or anywhere where it might be not oppressively hot outside but encouraging some people who might want to go out and get a little exercise, you’ve undoubtedly seen or heard of pickleball.
This took off, I think, out of Bainbridge Island, Wash. It was meant as a gentlemanly game where people didn’t exert themselves too much. The joke is you could play it while holding a drink in one hand. It’s gotten more popular and more competitive. It’s kind of a miniature version of tennis, with a smaller court, a plastic ball, and a wooden paddle. The ball can go back and forth rapidly, but you’re always playing doubles and it doesn’t take as much energy, exertion, and, if you will, fitness as a game like singles tennis.
The upside is it’s gotten many people outdoors getting some exercise and socializing. That’s all to the good. But a recent study suggested that there are about $500 million worth of injuries coming into the health care system associated with pickleball. There have been leg sprains, broken bones, people getting hit in the eye, hamstring pulls, and many other problems. I’ve been told that many of the spectators who show up for pickleball matches are there with a cast or have some kind of a wrap on because they were injured.
Well, many people have argued in the past about what we are going to do about health care costs. Some suggest if you voluntarily incur health care damage, you ought to pay for that yourself and you ought to have a big copay.
If you decide you’re going to do cross-country skiing or downhill skiing and you injure yourself, you chose to do it, so you pay. If you’re not going to maintain your weight, you’re going to smoke, or you’re going to ride around without a helmet, that’s your choice. You ought to pay.
I think the pickleball example is really a good challenge to these views. You obviously want people to go out and get some exercise. Here, we’re talking about a population that’s a little older and oftentimes doesn’t get out there as much as doctors would like to get the exercise that’s still important that they need, and yet it does incur injuries and problems.
My suggestion would be to make the game a little safer. Let’s try to encourage people to warm up more before they get out there and jump out of the car and engage in their pickleball battles. Goggles might be important to prevent the eye injuries in a game that’s played up close. Maybe we want to make sure that people look out for one another out there. If they think they’re getting dehydrated or tired, they should say, “Let’s sit down.”
I’m not willing to put a tax or a copay on the pickleball players of America. I know they choose to do it. It’s got an upside and benefits, as many things like skiing and other behaviors that have some risk do, but I think we want to be encouraging, not discouraging, of it.
I don’t like a society where anybody who tries to do something that takes risk winds up bearing extra cost for doing that. I understand that that gets people irritated when it comes to dangerous, hyper-risky behavior like smoking and not wearing a motorcycle helmet. I think the way to engage is not to call out the sinner or to try and punish those who are trying to do things that bring them enjoyment, reward, or in some of these cases, physical fitness, but to try to make things safer and try to gradually improve and get rid of the risk side to capture the full benefit side.
I’m not sure I’ve come up with all the best ways to make pickleball safer, but I think that’s where our thinking in health care should go. My view is to get out there and play pickleball. If you do pull your hamstring, raise my insurance premium a little bit. I’ll help to pay for it. Better you get some enjoyment and some exercise.
I get the downside, but come on, folks, we ought to be, as a community, somewhat supportive of the fun and recreation that our fellow citizens engage in.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center. He disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); and as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re anywhere near Seattle, anywhere near Florida, or anywhere where it might be not oppressively hot outside but encouraging some people who might want to go out and get a little exercise, you’ve undoubtedly seen or heard of pickleball.
This took off, I think, out of Bainbridge Island, Wash. It was meant as a gentlemanly game where people didn’t exert themselves too much. The joke is you could play it while holding a drink in one hand. It’s gotten more popular and more competitive. It’s kind of a miniature version of tennis, with a smaller court, a plastic ball, and a wooden paddle. The ball can go back and forth rapidly, but you’re always playing doubles and it doesn’t take as much energy, exertion, and, if you will, fitness as a game like singles tennis.
The upside is it’s gotten many people outdoors getting some exercise and socializing. That’s all to the good. But a recent study suggested that there are about $500 million worth of injuries coming into the health care system associated with pickleball. There have been leg sprains, broken bones, people getting hit in the eye, hamstring pulls, and many other problems. I’ve been told that many of the spectators who show up for pickleball matches are there with a cast or have some kind of a wrap on because they were injured.
Well, many people have argued in the past about what we are going to do about health care costs. Some suggest if you voluntarily incur health care damage, you ought to pay for that yourself and you ought to have a big copay.
If you decide you’re going to do cross-country skiing or downhill skiing and you injure yourself, you chose to do it, so you pay. If you’re not going to maintain your weight, you’re going to smoke, or you’re going to ride around without a helmet, that’s your choice. You ought to pay.
I think the pickleball example is really a good challenge to these views. You obviously want people to go out and get some exercise. Here, we’re talking about a population that’s a little older and oftentimes doesn’t get out there as much as doctors would like to get the exercise that’s still important that they need, and yet it does incur injuries and problems.
My suggestion would be to make the game a little safer. Let’s try to encourage people to warm up more before they get out there and jump out of the car and engage in their pickleball battles. Goggles might be important to prevent the eye injuries in a game that’s played up close. Maybe we want to make sure that people look out for one another out there. If they think they’re getting dehydrated or tired, they should say, “Let’s sit down.”
I’m not willing to put a tax or a copay on the pickleball players of America. I know they choose to do it. It’s got an upside and benefits, as many things like skiing and other behaviors that have some risk do, but I think we want to be encouraging, not discouraging, of it.
I don’t like a society where anybody who tries to do something that takes risk winds up bearing extra cost for doing that. I understand that that gets people irritated when it comes to dangerous, hyper-risky behavior like smoking and not wearing a motorcycle helmet. I think the way to engage is not to call out the sinner or to try and punish those who are trying to do things that bring them enjoyment, reward, or in some of these cases, physical fitness, but to try to make things safer and try to gradually improve and get rid of the risk side to capture the full benefit side.
I’m not sure I’ve come up with all the best ways to make pickleball safer, but I think that’s where our thinking in health care should go. My view is to get out there and play pickleball. If you do pull your hamstring, raise my insurance premium a little bit. I’ll help to pay for it. Better you get some enjoyment and some exercise.
I get the downside, but come on, folks, we ought to be, as a community, somewhat supportive of the fun and recreation that our fellow citizens engage in.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center. He disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); and as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re anywhere near Seattle, anywhere near Florida, or anywhere where it might be not oppressively hot outside but encouraging some people who might want to go out and get a little exercise, you’ve undoubtedly seen or heard of pickleball.
This took off, I think, out of Bainbridge Island, Wash. It was meant as a gentlemanly game where people didn’t exert themselves too much. The joke is you could play it while holding a drink in one hand. It’s gotten more popular and more competitive. It’s kind of a miniature version of tennis, with a smaller court, a plastic ball, and a wooden paddle. The ball can go back and forth rapidly, but you’re always playing doubles and it doesn’t take as much energy, exertion, and, if you will, fitness as a game like singles tennis.
The upside is it’s gotten many people outdoors getting some exercise and socializing. That’s all to the good. But a recent study suggested that there are about $500 million worth of injuries coming into the health care system associated with pickleball. There have been leg sprains, broken bones, people getting hit in the eye, hamstring pulls, and many other problems. I’ve been told that many of the spectators who show up for pickleball matches are there with a cast or have some kind of a wrap on because they were injured.
Well, many people have argued in the past about what we are going to do about health care costs. Some suggest if you voluntarily incur health care damage, you ought to pay for that yourself and you ought to have a big copay.
If you decide you’re going to do cross-country skiing or downhill skiing and you injure yourself, you chose to do it, so you pay. If you’re not going to maintain your weight, you’re going to smoke, or you’re going to ride around without a helmet, that’s your choice. You ought to pay.
I think the pickleball example is really a good challenge to these views. You obviously want people to go out and get some exercise. Here, we’re talking about a population that’s a little older and oftentimes doesn’t get out there as much as doctors would like to get the exercise that’s still important that they need, and yet it does incur injuries and problems.
My suggestion would be to make the game a little safer. Let’s try to encourage people to warm up more before they get out there and jump out of the car and engage in their pickleball battles. Goggles might be important to prevent the eye injuries in a game that’s played up close. Maybe we want to make sure that people look out for one another out there. If they think they’re getting dehydrated or tired, they should say, “Let’s sit down.”
I’m not willing to put a tax or a copay on the pickleball players of America. I know they choose to do it. It’s got an upside and benefits, as many things like skiing and other behaviors that have some risk do, but I think we want to be encouraging, not discouraging, of it.
I don’t like a society where anybody who tries to do something that takes risk winds up bearing extra cost for doing that. I understand that that gets people irritated when it comes to dangerous, hyper-risky behavior like smoking and not wearing a motorcycle helmet. I think the way to engage is not to call out the sinner or to try and punish those who are trying to do things that bring them enjoyment, reward, or in some of these cases, physical fitness, but to try to make things safer and try to gradually improve and get rid of the risk side to capture the full benefit side.
I’m not sure I’ve come up with all the best ways to make pickleball safer, but I think that’s where our thinking in health care should go. My view is to get out there and play pickleball. If you do pull your hamstring, raise my insurance premium a little bit. I’ll help to pay for it. Better you get some enjoyment and some exercise.
I get the downside, but come on, folks, we ought to be, as a community, somewhat supportive of the fun and recreation that our fellow citizens engage in.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center. He disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); and as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
Barbie has an anxiety disorder
And it’s a great time to be a therapist
The Barbie movie is generating a lot of feelings, ranging from praise to vitriol. However one feels about the movie, let’s all pause and reflect for a moment on the fact that the number-one grossing film of 2023 is about our childhood doll trying to treat her anxiety disorder.
“Life imitates art more than art imitates life.” So said Oscar Wilde in 1889.
When my adult daughter, a childhood Barbie enthusiast, asked me to see the film, we put on pink and went. Twice. Little did I know that it would stir up so many thoughts and feelings. The one I want to share is how blessed I feel at this moment in time to be a mental health care provider! No longer is mental health something to be whispered about at the water cooler; instead, even Barbie is suffering. And with all the controversy in the press about the movie, no one seems at all surprised by this storyline.
I was raised by two child psychiatrists and have been practicing as an adult psychiatrist since 1991. The start of the pandemic was the most difficult time of my career, as almost every patient was struggling simultaneously, as was I. Three long years later, we are gradually emerging from our shared trauma. How ironic, now with the opportunity to go back to work, I have elected to maintain the majority of my practice online from home. It seems that most patients and providers prefer this mode of treatment, with a full 90 percent of practitioners saying they are using a hybrid model.
As mental health professionals, we know that anywhere from 3% to 49% of those experiencing trauma will develop posttraumatic stress disorder (PTSD), and we have been trained to treat them.
But what happens when an entire global population is exposed simultaneously to trauma? Historians and social scientists refer to such events by many different names, such as: Singularity, Black Swan Event, and Tipping Point. These events are incredibly rare, and afterwards everything is different. These global traumas always lead to massive change.
I think we are at that tipping point. This is the singularity. This is our Black Swan Event. Within a 3-year span, we have experienced the following:
- A global traumatic event (COVID-19).
- A sudden and seemingly permanent shift from office to remote video meetings mostly from home.
- Upending of traditional fundamentals of the stock market as the game literally stopped in January 2021.
- Rapid and widespread availability of Artificial Intelligence.
- The first generation to be fully raised on the Internet and social media (Gen Z) is now entering the workforce.
- Ongoing war in Ukraine.
That’s already an overwhelming list, and I could go on, but let’s get back to Barbie’s anxiety disorder.
The awareness about and acceptance of mental health issues has never been higher. The access to treatment never greater. There are now more online therapy options than ever. Treatment options have dramatically expanded in recent years, from Transcranial Magnetic Stimulation (TMS) to ketamine centers and psychedelics, as well as more mainstream options such as dialectical behavior therapy (DBT), cognitive behavioral therapy (CBT), selective serotonin reuptake inhibitors (SSRIs), and so many more.
What is particularly unique about this moment is the direct access to care. Self-help books abound with many making it to the New York Times bestseller list. YouTube is loaded with fantastic content on overcoming many mental health issues, although one should be careful with selecting reliable sources. Apps like HeadSpace and Calm are being downloaded by millions of people around the globe. Investors provided a record-breaking $1.5 billion to mental health startups in 2020 alone.
For most practitioners, our phones have been ringing off the hook since 2020. Applications to psychology, psychiatric residency, social work, and counseling degree programs are on the rise, with workforce shortages expected to continue for decades. Psychological expertise has been embraced by businesses especially for DEI (diversity, equity, and inclusion). Mental health experts are the most asked-for experts through media request services. Elite athletes are talking openly about bringing us on their teams.
In this unique moment, when everything seems set to transform into something else, it is time for mental health professionals to exert some agency and influence over where mental health will go from here. I think the next frontier for mental health specialists is to figure out how to speak collectively and help guide society.
Neil Howe, in his sweeping book “The Fourth Turning is Here,” says we have another 10 years in this “Millennial Crisis” phase. He calls this our “winter,” and it remains to be seen how we will emerge from our current challenges. I think we can make a difference.
If the Barbie movie is indeed a canary in the coal mine, I see positive trends ahead as we move past some of the societal and structural issues facing us, and work together to create a more open and egalitarian society. We must find creative solutions that will solve truly massive problems threatening our well-being and perhaps even our existence.
I am so grateful to be able to continue to practice and share my thoughts with you here from my home office, and I hope you can take a break and see this movie, which is not only entertaining but also thought- and emotion-provoking.
Dr. Ritvo has almost 30 years’ experience in psychiatry and is currently practicing telemedicine. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She has no conflicts of interest.
And it’s a great time to be a therapist
And it’s a great time to be a therapist
The Barbie movie is generating a lot of feelings, ranging from praise to vitriol. However one feels about the movie, let’s all pause and reflect for a moment on the fact that the number-one grossing film of 2023 is about our childhood doll trying to treat her anxiety disorder.
“Life imitates art more than art imitates life.” So said Oscar Wilde in 1889.
When my adult daughter, a childhood Barbie enthusiast, asked me to see the film, we put on pink and went. Twice. Little did I know that it would stir up so many thoughts and feelings. The one I want to share is how blessed I feel at this moment in time to be a mental health care provider! No longer is mental health something to be whispered about at the water cooler; instead, even Barbie is suffering. And with all the controversy in the press about the movie, no one seems at all surprised by this storyline.
I was raised by two child psychiatrists and have been practicing as an adult psychiatrist since 1991. The start of the pandemic was the most difficult time of my career, as almost every patient was struggling simultaneously, as was I. Three long years later, we are gradually emerging from our shared trauma. How ironic, now with the opportunity to go back to work, I have elected to maintain the majority of my practice online from home. It seems that most patients and providers prefer this mode of treatment, with a full 90 percent of practitioners saying they are using a hybrid model.
As mental health professionals, we know that anywhere from 3% to 49% of those experiencing trauma will develop posttraumatic stress disorder (PTSD), and we have been trained to treat them.
But what happens when an entire global population is exposed simultaneously to trauma? Historians and social scientists refer to such events by many different names, such as: Singularity, Black Swan Event, and Tipping Point. These events are incredibly rare, and afterwards everything is different. These global traumas always lead to massive change.
I think we are at that tipping point. This is the singularity. This is our Black Swan Event. Within a 3-year span, we have experienced the following:
- A global traumatic event (COVID-19).
- A sudden and seemingly permanent shift from office to remote video meetings mostly from home.
- Upending of traditional fundamentals of the stock market as the game literally stopped in January 2021.
- Rapid and widespread availability of Artificial Intelligence.
- The first generation to be fully raised on the Internet and social media (Gen Z) is now entering the workforce.
- Ongoing war in Ukraine.
That’s already an overwhelming list, and I could go on, but let’s get back to Barbie’s anxiety disorder.
The awareness about and acceptance of mental health issues has never been higher. The access to treatment never greater. There are now more online therapy options than ever. Treatment options have dramatically expanded in recent years, from Transcranial Magnetic Stimulation (TMS) to ketamine centers and psychedelics, as well as more mainstream options such as dialectical behavior therapy (DBT), cognitive behavioral therapy (CBT), selective serotonin reuptake inhibitors (SSRIs), and so many more.
What is particularly unique about this moment is the direct access to care. Self-help books abound with many making it to the New York Times bestseller list. YouTube is loaded with fantastic content on overcoming many mental health issues, although one should be careful with selecting reliable sources. Apps like HeadSpace and Calm are being downloaded by millions of people around the globe. Investors provided a record-breaking $1.5 billion to mental health startups in 2020 alone.
For most practitioners, our phones have been ringing off the hook since 2020. Applications to psychology, psychiatric residency, social work, and counseling degree programs are on the rise, with workforce shortages expected to continue for decades. Psychological expertise has been embraced by businesses especially for DEI (diversity, equity, and inclusion). Mental health experts are the most asked-for experts through media request services. Elite athletes are talking openly about bringing us on their teams.
In this unique moment, when everything seems set to transform into something else, it is time for mental health professionals to exert some agency and influence over where mental health will go from here. I think the next frontier for mental health specialists is to figure out how to speak collectively and help guide society.
Neil Howe, in his sweeping book “The Fourth Turning is Here,” says we have another 10 years in this “Millennial Crisis” phase. He calls this our “winter,” and it remains to be seen how we will emerge from our current challenges. I think we can make a difference.
If the Barbie movie is indeed a canary in the coal mine, I see positive trends ahead as we move past some of the societal and structural issues facing us, and work together to create a more open and egalitarian society. We must find creative solutions that will solve truly massive problems threatening our well-being and perhaps even our existence.
I am so grateful to be able to continue to practice and share my thoughts with you here from my home office, and I hope you can take a break and see this movie, which is not only entertaining but also thought- and emotion-provoking.
Dr. Ritvo has almost 30 years’ experience in psychiatry and is currently practicing telemedicine. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She has no conflicts of interest.
The Barbie movie is generating a lot of feelings, ranging from praise to vitriol. However one feels about the movie, let’s all pause and reflect for a moment on the fact that the number-one grossing film of 2023 is about our childhood doll trying to treat her anxiety disorder.
“Life imitates art more than art imitates life.” So said Oscar Wilde in 1889.
When my adult daughter, a childhood Barbie enthusiast, asked me to see the film, we put on pink and went. Twice. Little did I know that it would stir up so many thoughts and feelings. The one I want to share is how blessed I feel at this moment in time to be a mental health care provider! No longer is mental health something to be whispered about at the water cooler; instead, even Barbie is suffering. And with all the controversy in the press about the movie, no one seems at all surprised by this storyline.
I was raised by two child psychiatrists and have been practicing as an adult psychiatrist since 1991. The start of the pandemic was the most difficult time of my career, as almost every patient was struggling simultaneously, as was I. Three long years later, we are gradually emerging from our shared trauma. How ironic, now with the opportunity to go back to work, I have elected to maintain the majority of my practice online from home. It seems that most patients and providers prefer this mode of treatment, with a full 90 percent of practitioners saying they are using a hybrid model.
As mental health professionals, we know that anywhere from 3% to 49% of those experiencing trauma will develop posttraumatic stress disorder (PTSD), and we have been trained to treat them.
But what happens when an entire global population is exposed simultaneously to trauma? Historians and social scientists refer to such events by many different names, such as: Singularity, Black Swan Event, and Tipping Point. These events are incredibly rare, and afterwards everything is different. These global traumas always lead to massive change.
I think we are at that tipping point. This is the singularity. This is our Black Swan Event. Within a 3-year span, we have experienced the following:
- A global traumatic event (COVID-19).
- A sudden and seemingly permanent shift from office to remote video meetings mostly from home.
- Upending of traditional fundamentals of the stock market as the game literally stopped in January 2021.
- Rapid and widespread availability of Artificial Intelligence.
- The first generation to be fully raised on the Internet and social media (Gen Z) is now entering the workforce.
- Ongoing war in Ukraine.
That’s already an overwhelming list, and I could go on, but let’s get back to Barbie’s anxiety disorder.
The awareness about and acceptance of mental health issues has never been higher. The access to treatment never greater. There are now more online therapy options than ever. Treatment options have dramatically expanded in recent years, from Transcranial Magnetic Stimulation (TMS) to ketamine centers and psychedelics, as well as more mainstream options such as dialectical behavior therapy (DBT), cognitive behavioral therapy (CBT), selective serotonin reuptake inhibitors (SSRIs), and so many more.
What is particularly unique about this moment is the direct access to care. Self-help books abound with many making it to the New York Times bestseller list. YouTube is loaded with fantastic content on overcoming many mental health issues, although one should be careful with selecting reliable sources. Apps like HeadSpace and Calm are being downloaded by millions of people around the globe. Investors provided a record-breaking $1.5 billion to mental health startups in 2020 alone.
For most practitioners, our phones have been ringing off the hook since 2020. Applications to psychology, psychiatric residency, social work, and counseling degree programs are on the rise, with workforce shortages expected to continue for decades. Psychological expertise has been embraced by businesses especially for DEI (diversity, equity, and inclusion). Mental health experts are the most asked-for experts through media request services. Elite athletes are talking openly about bringing us on their teams.
In this unique moment, when everything seems set to transform into something else, it is time for mental health professionals to exert some agency and influence over where mental health will go from here. I think the next frontier for mental health specialists is to figure out how to speak collectively and help guide society.
Neil Howe, in his sweeping book “The Fourth Turning is Here,” says we have another 10 years in this “Millennial Crisis” phase. He calls this our “winter,” and it remains to be seen how we will emerge from our current challenges. I think we can make a difference.
If the Barbie movie is indeed a canary in the coal mine, I see positive trends ahead as we move past some of the societal and structural issues facing us, and work together to create a more open and egalitarian society. We must find creative solutions that will solve truly massive problems threatening our well-being and perhaps even our existence.
I am so grateful to be able to continue to practice and share my thoughts with you here from my home office, and I hope you can take a break and see this movie, which is not only entertaining but also thought- and emotion-provoking.
Dr. Ritvo has almost 30 years’ experience in psychiatry and is currently practicing telemedicine. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She has no conflicts of interest.
The new normal in body temperature
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.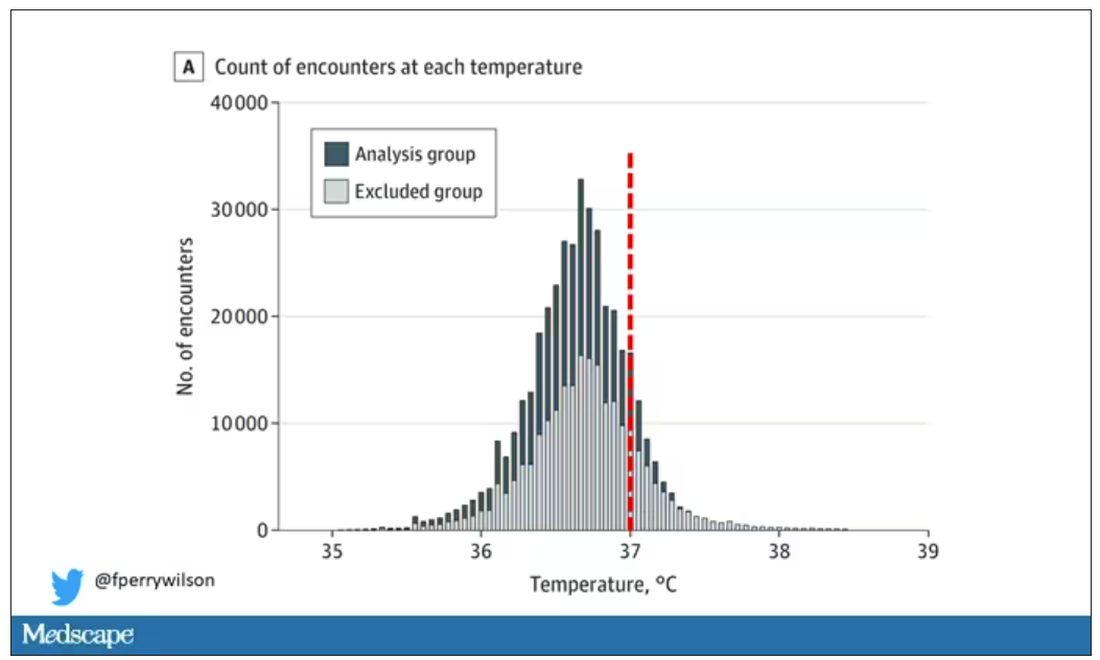
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.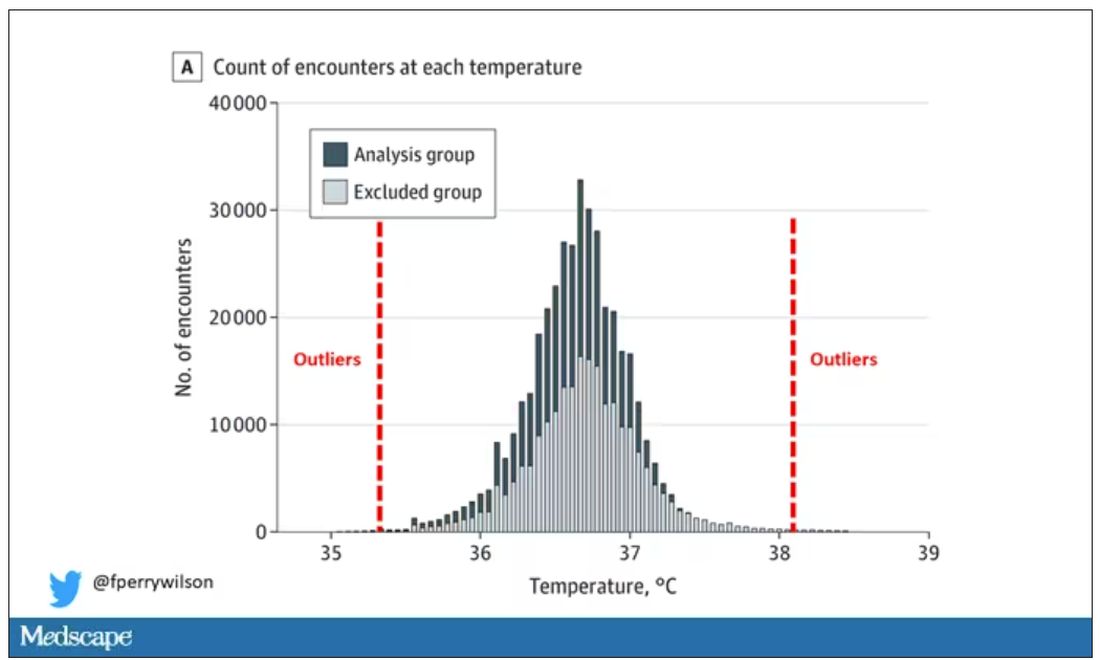
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.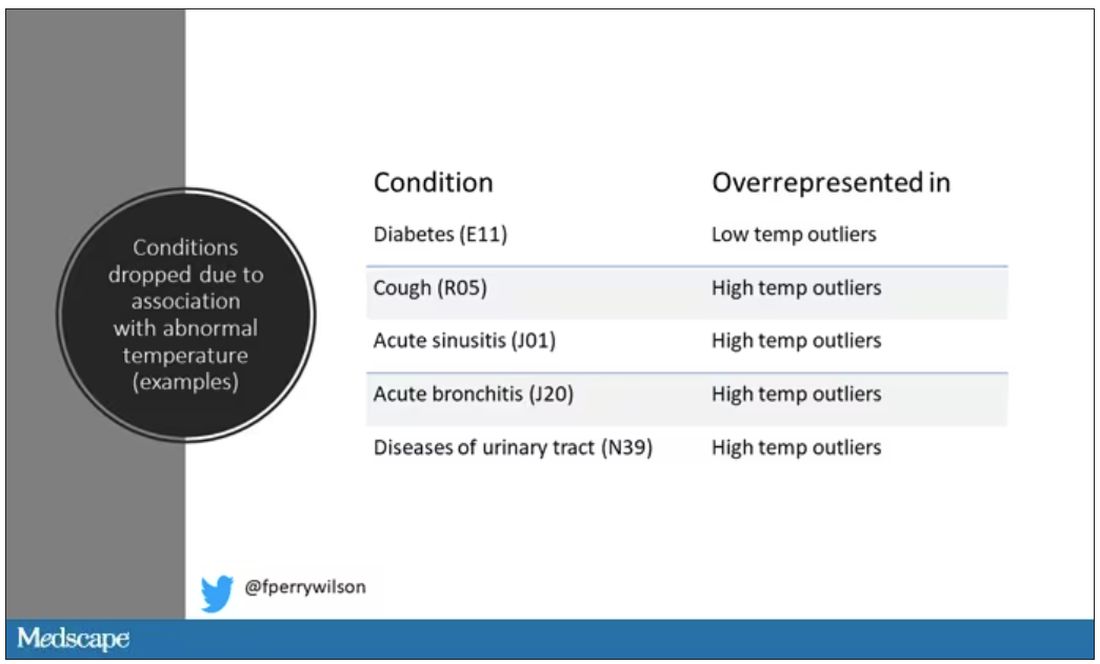
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.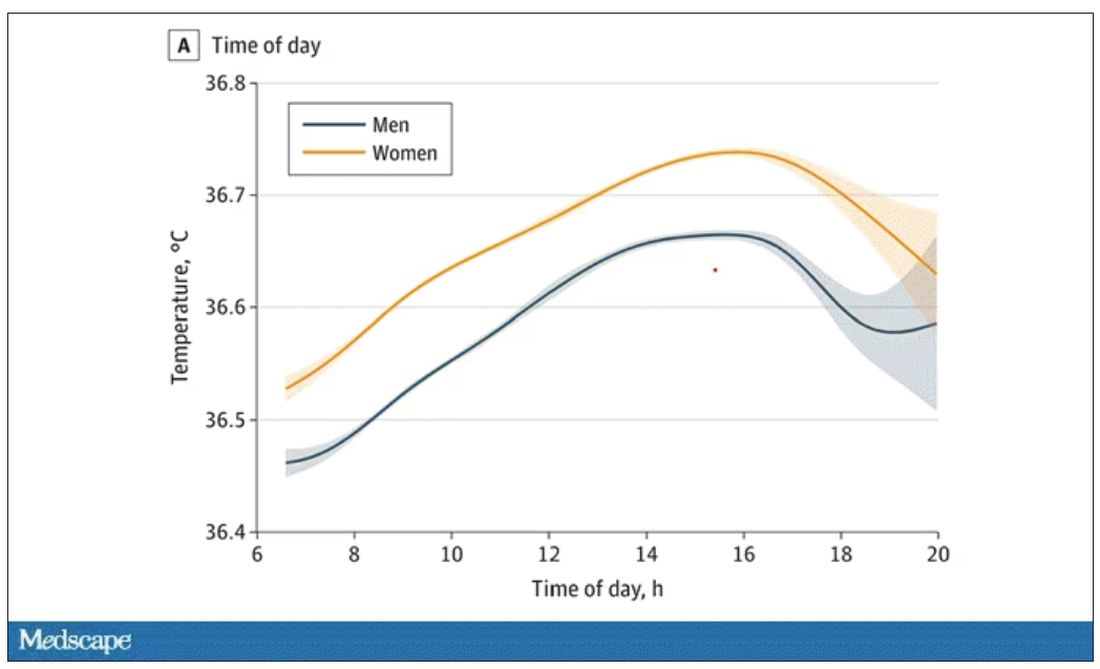
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.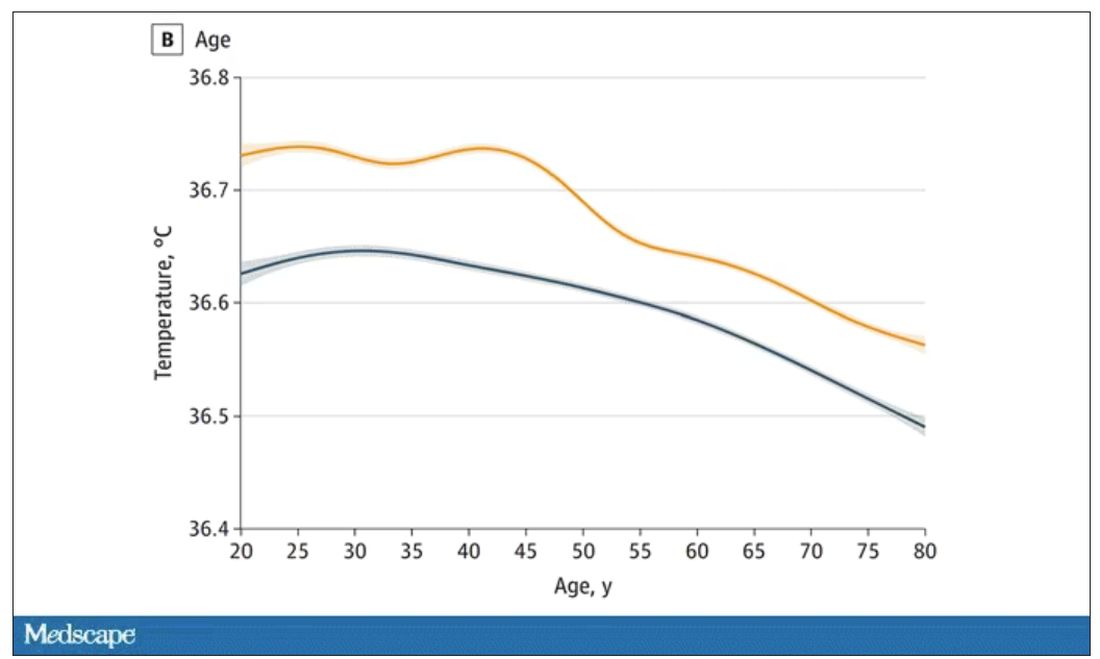
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.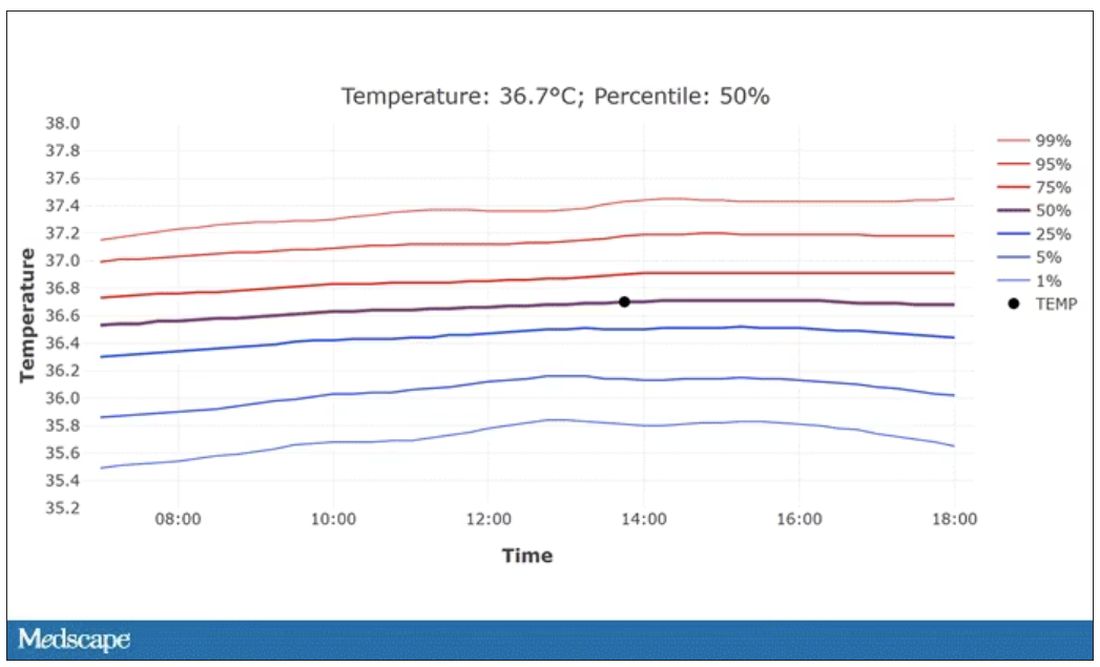
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
The cult of the suicide risk assessment
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
IQ and concussion recovery
Pediatric concussion is one of those rare phenomena in which we may be witnessing its emergence and clarification in a generation. When I was serving as the game doctor for our local high school football team in the 1970s, I and many other physicians had a very simplistic view of concussion. If the patient never lost conscious and had a reasonably intact short-term memory, we didn’t seriously entertain concussion as a diagnosis. “What’s the score and who is the president?” Were my favorite screening questions.
Obviously, we were underdiagnosing and mismanaging concussion. In part thanks to some high-profile athletes who suffered multiple concussions and eventually chronic traumatic encephalopathy (CTE) physicians began to realize that they should be looking more closely at children who sustained a head injury. The diagnostic criteria were expanded to include any injury that even temporarily effected brain function.
With the new appreciation for the risk of multiple concussions, the focus broadened to include the question of when is it safe for the athlete to return to competition. What signs or symptoms can the patient offer us so we can be sure his or her brain is sufficiently recovered? Here we stepped off into a deep abyss of ignorance. Fortunately, it became obvious fairly quickly that imaging studies weren’t going to help us, as they were invariably normal or at least didn’t tell us anything that wasn’t obvious on a physical exam.
If the patient had a headache, complained of dizziness, or manifested amnesia, monitoring the patient was fairly straightforward. But, in the absence of symptoms and no obvious way to determine the pace of recovery of an organ we couldn’t visualize, clinicians were pulling criteria and time tables out of thin air. Guessing that the concussed brain was in some ways like a torn muscle or overstretched tendon, “brain rest” was often suggested. So no TV, no reading, and certainly none of the cerebral challenging activity of school. Fortunately, we don’t hear much about the notion of brain rest anymore and there is at least one study that suggests that patients kept home from school recover more slowly.
But . Sometimes they describe headache or dizziness but often they complain of a vague mental unwellness. “Brain fog,” a term that has emerged in the wake of the COVID pandemic, might be an apt descriptor. Management of these slow recoverers has been a challenge.
However, two recent articles in the journal Pediatrics may provide some clarity and offer guidance in their management. In a study coming from the psychology department at Georgia State University, researchers reported that they have been able to find “no evidence of clinical meaningful differences in IQ after pediatric concussion.” In their words there is “strong evidence against reduced intelligence in the first few weeks to month after pediatric concussion.”
While their findings may simply toss the IQ onto the pile of worthless measures of healing, a companion commentary by Talin Babikian, PhD, a psychologist at the Semel Institute for Neuroscience and Human Behavior at UCLA, provides a more nuanced interpretation. He writes that if we are looking for an explanation when a patient’s recovery is taking longer than we might expect we need to look beyond some structural damage. Maybe the patient has a previously undiagnosed premorbid condition effecting his or her intellectual, cognitive, or learning abilities. Could the stall in improvement be the result of other symptoms? Here fatigue and sleep deprivation may be the culprits. Could some underlying emotional factor such as anxiety or depression be the problem? For example, I have seen patients whose fear of re-injury has prevented their return to full function. And, finally, the patient may be avoiding a “nonpreferred or challenging situation” unrelated to the injury.
In other words, the concussion may simply be the most obvious rip in a fabric that was already frayed and under stress. This kind of broad holistic (a word I usually like to avoid) thinking may be what is lacking as we struggle to understand other mysterious and chronic conditions such as Lyme disease and chronic fatigue syndrome.
While these two papers help provide some clarity in the management of pediatric concussion, what they fail to address is the bigger question of the relationship between head injury and CTE. The answers to that conundrum are enshrouded in a mix of politics and publicity that I doubt will clear in the near future.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Pediatric concussion is one of those rare phenomena in which we may be witnessing its emergence and clarification in a generation. When I was serving as the game doctor for our local high school football team in the 1970s, I and many other physicians had a very simplistic view of concussion. If the patient never lost conscious and had a reasonably intact short-term memory, we didn’t seriously entertain concussion as a diagnosis. “What’s the score and who is the president?” Were my favorite screening questions.
Obviously, we were underdiagnosing and mismanaging concussion. In part thanks to some high-profile athletes who suffered multiple concussions and eventually chronic traumatic encephalopathy (CTE) physicians began to realize that they should be looking more closely at children who sustained a head injury. The diagnostic criteria were expanded to include any injury that even temporarily effected brain function.
With the new appreciation for the risk of multiple concussions, the focus broadened to include the question of when is it safe for the athlete to return to competition. What signs or symptoms can the patient offer us so we can be sure his or her brain is sufficiently recovered? Here we stepped off into a deep abyss of ignorance. Fortunately, it became obvious fairly quickly that imaging studies weren’t going to help us, as they were invariably normal or at least didn’t tell us anything that wasn’t obvious on a physical exam.
If the patient had a headache, complained of dizziness, or manifested amnesia, monitoring the patient was fairly straightforward. But, in the absence of symptoms and no obvious way to determine the pace of recovery of an organ we couldn’t visualize, clinicians were pulling criteria and time tables out of thin air. Guessing that the concussed brain was in some ways like a torn muscle or overstretched tendon, “brain rest” was often suggested. So no TV, no reading, and certainly none of the cerebral challenging activity of school. Fortunately, we don’t hear much about the notion of brain rest anymore and there is at least one study that suggests that patients kept home from school recover more slowly.
But . Sometimes they describe headache or dizziness but often they complain of a vague mental unwellness. “Brain fog,” a term that has emerged in the wake of the COVID pandemic, might be an apt descriptor. Management of these slow recoverers has been a challenge.
However, two recent articles in the journal Pediatrics may provide some clarity and offer guidance in their management. In a study coming from the psychology department at Georgia State University, researchers reported that they have been able to find “no evidence of clinical meaningful differences in IQ after pediatric concussion.” In their words there is “strong evidence against reduced intelligence in the first few weeks to month after pediatric concussion.”
While their findings may simply toss the IQ onto the pile of worthless measures of healing, a companion commentary by Talin Babikian, PhD, a psychologist at the Semel Institute for Neuroscience and Human Behavior at UCLA, provides a more nuanced interpretation. He writes that if we are looking for an explanation when a patient’s recovery is taking longer than we might expect we need to look beyond some structural damage. Maybe the patient has a previously undiagnosed premorbid condition effecting his or her intellectual, cognitive, or learning abilities. Could the stall in improvement be the result of other symptoms? Here fatigue and sleep deprivation may be the culprits. Could some underlying emotional factor such as anxiety or depression be the problem? For example, I have seen patients whose fear of re-injury has prevented their return to full function. And, finally, the patient may be avoiding a “nonpreferred or challenging situation” unrelated to the injury.
In other words, the concussion may simply be the most obvious rip in a fabric that was already frayed and under stress. This kind of broad holistic (a word I usually like to avoid) thinking may be what is lacking as we struggle to understand other mysterious and chronic conditions such as Lyme disease and chronic fatigue syndrome.
While these two papers help provide some clarity in the management of pediatric concussion, what they fail to address is the bigger question of the relationship between head injury and CTE. The answers to that conundrum are enshrouded in a mix of politics and publicity that I doubt will clear in the near future.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Pediatric concussion is one of those rare phenomena in which we may be witnessing its emergence and clarification in a generation. When I was serving as the game doctor for our local high school football team in the 1970s, I and many other physicians had a very simplistic view of concussion. If the patient never lost conscious and had a reasonably intact short-term memory, we didn’t seriously entertain concussion as a diagnosis. “What’s the score and who is the president?” Were my favorite screening questions.
Obviously, we were underdiagnosing and mismanaging concussion. In part thanks to some high-profile athletes who suffered multiple concussions and eventually chronic traumatic encephalopathy (CTE) physicians began to realize that they should be looking more closely at children who sustained a head injury. The diagnostic criteria were expanded to include any injury that even temporarily effected brain function.
With the new appreciation for the risk of multiple concussions, the focus broadened to include the question of when is it safe for the athlete to return to competition. What signs or symptoms can the patient offer us so we can be sure his or her brain is sufficiently recovered? Here we stepped off into a deep abyss of ignorance. Fortunately, it became obvious fairly quickly that imaging studies weren’t going to help us, as they were invariably normal or at least didn’t tell us anything that wasn’t obvious on a physical exam.
If the patient had a headache, complained of dizziness, or manifested amnesia, monitoring the patient was fairly straightforward. But, in the absence of symptoms and no obvious way to determine the pace of recovery of an organ we couldn’t visualize, clinicians were pulling criteria and time tables out of thin air. Guessing that the concussed brain was in some ways like a torn muscle or overstretched tendon, “brain rest” was often suggested. So no TV, no reading, and certainly none of the cerebral challenging activity of school. Fortunately, we don’t hear much about the notion of brain rest anymore and there is at least one study that suggests that patients kept home from school recover more slowly.
But . Sometimes they describe headache or dizziness but often they complain of a vague mental unwellness. “Brain fog,” a term that has emerged in the wake of the COVID pandemic, might be an apt descriptor. Management of these slow recoverers has been a challenge.
However, two recent articles in the journal Pediatrics may provide some clarity and offer guidance in their management. In a study coming from the psychology department at Georgia State University, researchers reported that they have been able to find “no evidence of clinical meaningful differences in IQ after pediatric concussion.” In their words there is “strong evidence against reduced intelligence in the first few weeks to month after pediatric concussion.”
While their findings may simply toss the IQ onto the pile of worthless measures of healing, a companion commentary by Talin Babikian, PhD, a psychologist at the Semel Institute for Neuroscience and Human Behavior at UCLA, provides a more nuanced interpretation. He writes that if we are looking for an explanation when a patient’s recovery is taking longer than we might expect we need to look beyond some structural damage. Maybe the patient has a previously undiagnosed premorbid condition effecting his or her intellectual, cognitive, or learning abilities. Could the stall in improvement be the result of other symptoms? Here fatigue and sleep deprivation may be the culprits. Could some underlying emotional factor such as anxiety or depression be the problem? For example, I have seen patients whose fear of re-injury has prevented their return to full function. And, finally, the patient may be avoiding a “nonpreferred or challenging situation” unrelated to the injury.
In other words, the concussion may simply be the most obvious rip in a fabric that was already frayed and under stress. This kind of broad holistic (a word I usually like to avoid) thinking may be what is lacking as we struggle to understand other mysterious and chronic conditions such as Lyme disease and chronic fatigue syndrome.
While these two papers help provide some clarity in the management of pediatric concussion, what they fail to address is the bigger question of the relationship between head injury and CTE. The answers to that conundrum are enshrouded in a mix of politics and publicity that I doubt will clear in the near future.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Can a decrease in dopamine lead to binge eating?
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.










