User login
When does a bicarb drip make sense?
A 70-year-old woman is admitted to the intensive care unit with a pH of 7.1, an acute kidney injury (AKI), and ketonuria. She is volume depleted and her history is consistent with starvation ketosis. This LOL truly is in NAD (that’s little old lady in no acute distress, for those who haven’t read The House of God). She is clinically stable and seemingly unperturbed by the flurry of activity surrounding her admission.
Your resident is concerned by the severity of the acidosis and suggests starting an intravenous bicarbonate drip. The fellow is adamantly against it. He’s been taught that intravenous bicarbonate increases the serum pH but paradoxically causes intracellular acidosis. As the attending you elect to observe fellow autonomy – no bicarb is given. Because any debate on rounds is a “teachable moment,” you decide to review the evidence and physiology behind infusing bicarbonate.
What do the data reveal?
An excellent review published in CHEST in 2000 covers the physiologic effects of bicarbonate, specifically related to lactic acidosis, which our patient didn’t have. Aside from that difference, the review validates the fellow’s opinion. In short, It is unlikely to provoke hemodynamic or respiratory compromise outside the setting of shock or hypercapnia. Intravenous bicarbonate can lead to intracellular acidosis, hypercapnia, hypocalcemia, and a reduction in oxygen delivery via the Bohr effect. The authors concluded that because the benefits are unproven and the negative effects are real, intravenous bicarbonate should not be used to correct a metabolic acidosis.
The CHEST review hardly settles the issue, though. A survey published a few years later found a majority of intensivists and nephrologists used intravenous bicarbonate to treat metabolic acidosis while the Surviving Sepsis Campaign Guidelines for the Management of Sepsis and Septic Shock published in 2017 recommended against bicarbonate for acidosis. It wasn’t until 2018 that we reached the holy grail: a randomized controlled trial.
The BICAR-ICU study randomly assigned patients with a pH of 7.20 or less, PCO2 of 45 mm Hg or less, and sodium bicarbonate concentration of 20 mmol/L or less to receive no bicarbonate versus a sodium bicarbonate drip to maintain a pH greater than 7.30. There’s additional nuance to the trial design and even more detail in the results. To summarize, there was signal for an improvement in renal outcomes across all patients, and those with AKI saw a mortality benefit. Post–BICAR-ICU iterations of the Surviving Sepsis Campaign Guidelines have incorporated these findings by recommending intravenous bicarbonate for patients with sepsis who have AKI and a pH of 7.20 or less.
That’s not to say BICAR-ICU has settled the issue. Although it’s far and away the best we have, there were fewer than 400 total patients in their intention-to-treat analysis. It was open label, with lots of crossover. The primary outcome was negative for the entire population, with only a subgroup (albeit a prespecified one) showing benefit. Finally, the results weren’t stratified by etiology for the metabolic acidosis. There was also evidence of alkalosis and hypocalcemia in the treatment group.
Last but not least in terms of importance, in most cases when bicarbonate is being considered, wouldn’t some form of renal replacement therapy (RRT) be preferred? This point was raised by nephrologists and intensivists when we covered BICAR-ICU in a journal club at my former program. It’s also mentioned in an accompanying editorial. RRT timing is controversial, and a detailed discussion is outside the scope of this piece and beyond the limits of my current knowledge base. But I do know that the A in the A-E-I-O-U acute indications for dialysis pneumonic stands for acidosis.
Our patient had AKI, a pH of 7.20 or less, and a pCO2 well under 45 mm Hg. Does BICAR-ICU support the resident’s inclination to start a drip? Sort of. The majority of patients enrolled in BICAR-ICU were in shock or were recovering from cardiac arrest, so it’s not clear the results can be generalized to our LOL with starvation ketosis. Extrapolating from studies of diabetic ketoacidosis (DKA) seems more appropriate, and here the data are poor but equivocal. Reviews are generally negative but don’t rule out the use of intravenous bicarbonate in certain patients with DKA.
Key takeaways
Our patient survived a 24-hour ICU stay with neither cardiopulmonary decompensation nor a need for RRT. Not sure how she did out of the ICU; presumably she was discharged soon after transfer. As is always the case with anecdotal medicine, the absence of a control prevents assessment of the counterfactual. Is it possible she may have done “better” with intravenous bicarbonate? Seems unlikely to me, though I doubt there would have been demonstrable adverse effects. Perhaps next time the fellow can observe resident autonomy?
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University of the Health Sciences, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
A 70-year-old woman is admitted to the intensive care unit with a pH of 7.1, an acute kidney injury (AKI), and ketonuria. She is volume depleted and her history is consistent with starvation ketosis. This LOL truly is in NAD (that’s little old lady in no acute distress, for those who haven’t read The House of God). She is clinically stable and seemingly unperturbed by the flurry of activity surrounding her admission.
Your resident is concerned by the severity of the acidosis and suggests starting an intravenous bicarbonate drip. The fellow is adamantly against it. He’s been taught that intravenous bicarbonate increases the serum pH but paradoxically causes intracellular acidosis. As the attending you elect to observe fellow autonomy – no bicarb is given. Because any debate on rounds is a “teachable moment,” you decide to review the evidence and physiology behind infusing bicarbonate.
What do the data reveal?
An excellent review published in CHEST in 2000 covers the physiologic effects of bicarbonate, specifically related to lactic acidosis, which our patient didn’t have. Aside from that difference, the review validates the fellow’s opinion. In short, It is unlikely to provoke hemodynamic or respiratory compromise outside the setting of shock or hypercapnia. Intravenous bicarbonate can lead to intracellular acidosis, hypercapnia, hypocalcemia, and a reduction in oxygen delivery via the Bohr effect. The authors concluded that because the benefits are unproven and the negative effects are real, intravenous bicarbonate should not be used to correct a metabolic acidosis.
The CHEST review hardly settles the issue, though. A survey published a few years later found a majority of intensivists and nephrologists used intravenous bicarbonate to treat metabolic acidosis while the Surviving Sepsis Campaign Guidelines for the Management of Sepsis and Septic Shock published in 2017 recommended against bicarbonate for acidosis. It wasn’t until 2018 that we reached the holy grail: a randomized controlled trial.
The BICAR-ICU study randomly assigned patients with a pH of 7.20 or less, PCO2 of 45 mm Hg or less, and sodium bicarbonate concentration of 20 mmol/L or less to receive no bicarbonate versus a sodium bicarbonate drip to maintain a pH greater than 7.30. There’s additional nuance to the trial design and even more detail in the results. To summarize, there was signal for an improvement in renal outcomes across all patients, and those with AKI saw a mortality benefit. Post–BICAR-ICU iterations of the Surviving Sepsis Campaign Guidelines have incorporated these findings by recommending intravenous bicarbonate for patients with sepsis who have AKI and a pH of 7.20 or less.
That’s not to say BICAR-ICU has settled the issue. Although it’s far and away the best we have, there were fewer than 400 total patients in their intention-to-treat analysis. It was open label, with lots of crossover. The primary outcome was negative for the entire population, with only a subgroup (albeit a prespecified one) showing benefit. Finally, the results weren’t stratified by etiology for the metabolic acidosis. There was also evidence of alkalosis and hypocalcemia in the treatment group.
Last but not least in terms of importance, in most cases when bicarbonate is being considered, wouldn’t some form of renal replacement therapy (RRT) be preferred? This point was raised by nephrologists and intensivists when we covered BICAR-ICU in a journal club at my former program. It’s also mentioned in an accompanying editorial. RRT timing is controversial, and a detailed discussion is outside the scope of this piece and beyond the limits of my current knowledge base. But I do know that the A in the A-E-I-O-U acute indications for dialysis pneumonic stands for acidosis.
Our patient had AKI, a pH of 7.20 or less, and a pCO2 well under 45 mm Hg. Does BICAR-ICU support the resident’s inclination to start a drip? Sort of. The majority of patients enrolled in BICAR-ICU were in shock or were recovering from cardiac arrest, so it’s not clear the results can be generalized to our LOL with starvation ketosis. Extrapolating from studies of diabetic ketoacidosis (DKA) seems more appropriate, and here the data are poor but equivocal. Reviews are generally negative but don’t rule out the use of intravenous bicarbonate in certain patients with DKA.
Key takeaways
Our patient survived a 24-hour ICU stay with neither cardiopulmonary decompensation nor a need for RRT. Not sure how she did out of the ICU; presumably she was discharged soon after transfer. As is always the case with anecdotal medicine, the absence of a control prevents assessment of the counterfactual. Is it possible she may have done “better” with intravenous bicarbonate? Seems unlikely to me, though I doubt there would have been demonstrable adverse effects. Perhaps next time the fellow can observe resident autonomy?
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University of the Health Sciences, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
A 70-year-old woman is admitted to the intensive care unit with a pH of 7.1, an acute kidney injury (AKI), and ketonuria. She is volume depleted and her history is consistent with starvation ketosis. This LOL truly is in NAD (that’s little old lady in no acute distress, for those who haven’t read The House of God). She is clinically stable and seemingly unperturbed by the flurry of activity surrounding her admission.
Your resident is concerned by the severity of the acidosis and suggests starting an intravenous bicarbonate drip. The fellow is adamantly against it. He’s been taught that intravenous bicarbonate increases the serum pH but paradoxically causes intracellular acidosis. As the attending you elect to observe fellow autonomy – no bicarb is given. Because any debate on rounds is a “teachable moment,” you decide to review the evidence and physiology behind infusing bicarbonate.
What do the data reveal?
An excellent review published in CHEST in 2000 covers the physiologic effects of bicarbonate, specifically related to lactic acidosis, which our patient didn’t have. Aside from that difference, the review validates the fellow’s opinion. In short, It is unlikely to provoke hemodynamic or respiratory compromise outside the setting of shock or hypercapnia. Intravenous bicarbonate can lead to intracellular acidosis, hypercapnia, hypocalcemia, and a reduction in oxygen delivery via the Bohr effect. The authors concluded that because the benefits are unproven and the negative effects are real, intravenous bicarbonate should not be used to correct a metabolic acidosis.
The CHEST review hardly settles the issue, though. A survey published a few years later found a majority of intensivists and nephrologists used intravenous bicarbonate to treat metabolic acidosis while the Surviving Sepsis Campaign Guidelines for the Management of Sepsis and Septic Shock published in 2017 recommended against bicarbonate for acidosis. It wasn’t until 2018 that we reached the holy grail: a randomized controlled trial.
The BICAR-ICU study randomly assigned patients with a pH of 7.20 or less, PCO2 of 45 mm Hg or less, and sodium bicarbonate concentration of 20 mmol/L or less to receive no bicarbonate versus a sodium bicarbonate drip to maintain a pH greater than 7.30. There’s additional nuance to the trial design and even more detail in the results. To summarize, there was signal for an improvement in renal outcomes across all patients, and those with AKI saw a mortality benefit. Post–BICAR-ICU iterations of the Surviving Sepsis Campaign Guidelines have incorporated these findings by recommending intravenous bicarbonate for patients with sepsis who have AKI and a pH of 7.20 or less.
That’s not to say BICAR-ICU has settled the issue. Although it’s far and away the best we have, there were fewer than 400 total patients in their intention-to-treat analysis. It was open label, with lots of crossover. The primary outcome was negative for the entire population, with only a subgroup (albeit a prespecified one) showing benefit. Finally, the results weren’t stratified by etiology for the metabolic acidosis. There was also evidence of alkalosis and hypocalcemia in the treatment group.
Last but not least in terms of importance, in most cases when bicarbonate is being considered, wouldn’t some form of renal replacement therapy (RRT) be preferred? This point was raised by nephrologists and intensivists when we covered BICAR-ICU in a journal club at my former program. It’s also mentioned in an accompanying editorial. RRT timing is controversial, and a detailed discussion is outside the scope of this piece and beyond the limits of my current knowledge base. But I do know that the A in the A-E-I-O-U acute indications for dialysis pneumonic stands for acidosis.
Our patient had AKI, a pH of 7.20 or less, and a pCO2 well under 45 mm Hg. Does BICAR-ICU support the resident’s inclination to start a drip? Sort of. The majority of patients enrolled in BICAR-ICU were in shock or were recovering from cardiac arrest, so it’s not clear the results can be generalized to our LOL with starvation ketosis. Extrapolating from studies of diabetic ketoacidosis (DKA) seems more appropriate, and here the data are poor but equivocal. Reviews are generally negative but don’t rule out the use of intravenous bicarbonate in certain patients with DKA.
Key takeaways
Our patient survived a 24-hour ICU stay with neither cardiopulmonary decompensation nor a need for RRT. Not sure how she did out of the ICU; presumably she was discharged soon after transfer. As is always the case with anecdotal medicine, the absence of a control prevents assessment of the counterfactual. Is it possible she may have done “better” with intravenous bicarbonate? Seems unlikely to me, though I doubt there would have been demonstrable adverse effects. Perhaps next time the fellow can observe resident autonomy?
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University of the Health Sciences, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
The magic of music
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
‘Decapitated’ boy saved by surgery team
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: I am joined today by Dr. Ohad Einav. He’s a staff surgeon in orthopedics at Hadassah Medical Center in Jerusalem. He’s with me to talk about an absolutely incredible surgical case, something that is terrifying to most non–orthopedic surgeons and I imagine is fairly scary for spine surgeons like him as well. But what we don’t have is information about how this works from a medical perspective. So, first of all, Dr. Einav, thank you for taking time to speak with me today.
Ohad Einav, MD: Thank you for having me.
Dr. Wilson: Can you tell us about Suleiman Hassan and what happened to him before he came into your care?
Dr. Einav: Hassan is a 12-year-old child who was riding his bicycle on the West Bank, about 40 minutes from here. Unfortunately, he was involved in a motor vehicle accident and he suffered injuries to his abdomen and cervical spine. He was transported to our service by helicopter from the scene of the accident.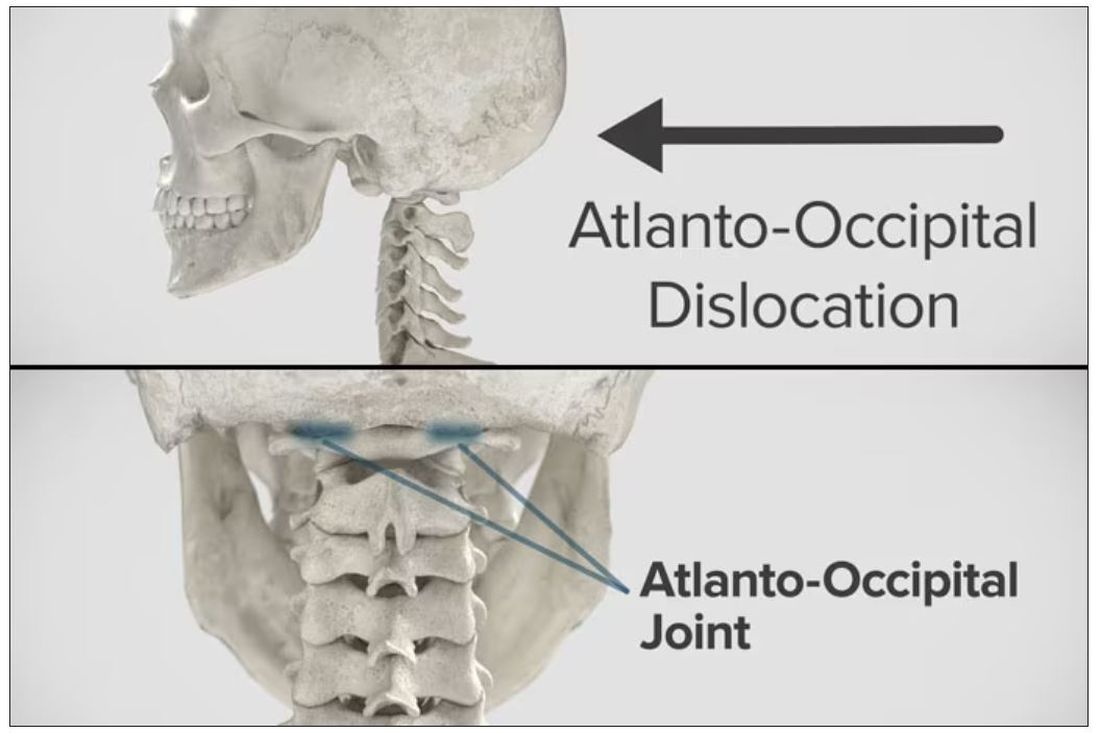
Dr. Wilson: “Injury to the cervical spine” might be something of an understatement. He had what’s called atlanto-occipital dislocation, colloquially often referred to as internal decapitation. Can you tell us what that means? It sounds terrifying.
Dr. Einav: It’s an injury to the ligaments between the occiput and the upper cervical spine, with or without bony fracture. The atlanto-occipital joint is formed by the superior articular facet of the atlas and the occipital condyle, stabilized by an articular capsule between the head and neck, and is supported by various ligaments around it that stabilize the joint and allow joint movements, including flexion, extension, and some rotation in the lower levels.
Dr. Wilson: This joint has several degrees of freedom, which means it needs a lot of support. With this type of injury, where essentially you have severing of the ligaments, is it usually survivable? How dangerous is this?
Dr. Einav: The mortality rate is 50%-60%, depending on the primary impact, the injury, transportation later on, and then the surgery and surgical management.
Dr. Wilson: Tell us a bit about this patient’s status when he came to your medical center. I assume he was in bad shape.
Dr. Einav: Hassan arrived at our medical center with a Glasgow Coma Scale score of 15. He was fully conscious. He was hemodynamically stable except for a bad laceration on his abdomen. He had a Philadelphia collar around his neck. He was transported by chopper because the paramedics suspected that he had a cervical spine injury and decided to bring him to a Level 1 trauma center.
He was monitored and we treated him according to the ATLS [advanced trauma life support] protocol. He didn’t have any gross sensory deficits, but he was a little confused about the whole situation and the accident. Therefore, we could do a general examination but we couldn’t rely on that regarding any sensory deficit that he may or may not have. We decided as a team that it would be better to slow down and control the situation. We decided not to operate on him immediately. We basically stabilized him and made sure that he didn’t have any traumatic internal organ damage. Later on we took him to the OR and performed surgery.
Dr. Wilson: It’s amazing that he had intact motor function, considering the extent of his injury. The spinal cord was spared somewhat during the injury. There must have been a moment when you realized that this kid, who was conscious and could move all four extremities, had a very severe neck injury. Was that due to a CT scan or physical exam? And what was your feeling when you saw that he had atlanto-occipital dislocation?
Dr. Einav: As a surgeon, you have a gut feeling in regard to the general examination of the patient. But I never rely on gut feelings. On the CT, I understood exactly what he had, what we needed to do, and the time frame.
Dr. Wilson: You’ve done these types of surgeries before, right? Obviously, no one has done a lot of them because this isn’t very common. But you knew what to do. Did you have a plan? Where does your experience come into play in a situation like this?
Dr. Einav: I graduated from the spine program of Toronto University, where I did a fellowship in trauma of the spine and complex spine surgery. I had very good teachers, and during my fellowship I treated a few cases in older patients that were similar but not the same. Therefore, I knew exactly what needed to be done.
Dr. Wilson: For those of us who aren’t surgeons, take us into the OR with you. This is obviously an incredibly delicate procedure. You are high up in the spinal cord at the base of the brain. The slightest mistake could have devastating consequences. What are the key elements of this procedure? What can go wrong here? What is the number-one thing you have to look out for when you’re trying to fix an internal decapitation?
Dr. Einav: The key element in surgeries of the cervical spine – trauma and complex spine surgery – is planning. I never go to the OR without knowing what I’m going to do. I have a few plans – plan A, plan B, plan C – in case something fails. So, I definitely know what the next step will be. I always think about the surgery a few hours before, if I have time to prepare.
The second thing that is very important is teamwork. The team needs to be coordinated. Everybody needs to know what their job is. With these types of injuries, it’s not the time for rookies. If you are new, please stand back and let the more experienced people do that job. I’m talking about surgeons, nurses, anesthesiologists – everyone.
Another important thing in planning is choosing the right hardware. For example, in this case we had a problem because most of the hardware is designed for adults, and we had to improvise because there isn’t a lot of hardware on the market for the pediatric population. The adult plates and screws are too big, so we had to improvise.
Dr. Wilson: Tell us more about that. How do you improvise spinal hardware for a 12-year-old?
Dr. Einav: In this case, I chose to use hardware from one of the companies that works with us.
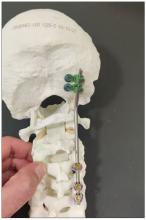
You can see in this model the area of the injury, and the area that we worked on. To perform the surgery, I had to use some plates and rods from a different company. This company’s (NuVasive) hardware has a small attachment to the skull, which was helpful for affixing the skull to the cervical spine, instead of using a big plate that would sit at the base of the skull and would not be very good for him. Most of the hardware is made for adults and not for kids.
Dr. Wilson: Will that hardware preserve the motor function of his neck? Will he be able to turn his head and extend and flex it?
Dr. Einav: The injury leads to instability and destruction of both articulations between the head and neck. Therefore, those articulations won’t be able to function the same way in the future. There is a decrease of something like 50% of the flexion and extension of Hassan’s cervical spine. Therefore, I decided that in this case there would be no chance of saving Hassan’s motor function unless we performed a fusion between the head and the neck, and therefore I decided that this would be the best procedure with the best survival rate. So, in the future, he will have some diminished flexion, extension, and rotation of his head.
Dr. Wilson: How long did his surgery take?
Dr. Einav: To be honest, I don’t remember. But I can tell you that it took us time. It was very challenging to coordinate with everyone. The most problematic part of the surgery to perform is what we call “flip-over.”
The anesthesiologist intubated the patient when he was supine, and later on, we flipped him prone to operate on the spine. This maneuver can actually lead to injury by itself, and injury at this level is fatal. So, we took our time and got Hassan into the OR. The anesthesiologist did a great job with the GlideScope – inserting the endotracheal tube. Later on, we neuromonitored him. Basically, we connected Hassan’s peripheral nerves to a computer and monitored his motor function. Gently we flipped him over, and after that we saw a little change in his motor function, so we had to modify his position so we could preserve his motor function. We then started the procedure, which took a few hours. I don’t know exactly how many.
Dr. Wilson: That just speaks to how delicate this is for everything from the intubation, where typically you’re manipulating the head, to the repositioning. Clearly this requires a lot of teamwork.
What happened after the operation? How is he doing?
Dr. Einav: After the operation, Hassan had a great recovery. He’s doing well. He doesn’t have any motor or sensory deficits. He’s able to ambulate without any aid. He had no signs of infection, which can happen after a car accident, neither from his abdominal wound nor from the occipital cervical surgery. He feels well. We saw him in the clinic. We removed his collar. We monitored him at the clinic. He looked amazing.
Dr. Wilson: That’s incredible. Are there long-term risks for him that you need to be looking out for?
Dr. Einav: Yes, and that’s the reason that we are monitoring him post surgery. While he was in the hospital, we monitored his motor and sensory functions, as well as his wound healing. Later on, in the clinic, for a few weeks after surgery we monitored for any failure of the hardware and bone graft. We check for healing of the bone graft and bone substitutes we put in to heal those bones.
Dr. Wilson: He will grow, right? He’s only 12, so he still has some years of growth in him. Is he going to need more surgery or any kind of hardware upgrade?
Dr. Einav: I hope not. In my surgeries, I never rely on the hardware for long durations. If I decide to do, for example, fusion, I rely on the hardware for a certain amount of time. And then I plan that the biology will do the work. If I plan for fusion, I put bone grafts in the preferred area for a fusion. Then if the hardware fails, I wouldn’t need to take out the hardware, and there would be no change in the condition of the patient.
Dr. Wilson: What an incredible story. It’s clear that you and your team kept your cool despite a very high-acuity situation with a ton of risk. What a tremendous outcome that this boy is not only alive but fully functional. So, congratulations to you and your team. That was very strong work.
Dr. Einav: Thank you very much. I would like to thank our team. We have to remember that the surgeon is not standing alone in the war. Hassan’s story is a success story of a very big group of people from various backgrounds and religions. They work day and night to help people and save lives. To the paramedics, the physiologists, the traumatologists, the pediatricians, the nurses, the physiotherapists, and obviously the surgeons, a big thank you. His story is our success story.
Dr. Wilson: It’s inspiring to see so many people come together to do what we all are here for, which is to fight against suffering, disease, and death. Thank you for keeping up that fight. And thank you for joining me here.
Dr. Einav: Thank you very much.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: I am joined today by Dr. Ohad Einav. He’s a staff surgeon in orthopedics at Hadassah Medical Center in Jerusalem. He’s with me to talk about an absolutely incredible surgical case, something that is terrifying to most non–orthopedic surgeons and I imagine is fairly scary for spine surgeons like him as well. But what we don’t have is information about how this works from a medical perspective. So, first of all, Dr. Einav, thank you for taking time to speak with me today.
Ohad Einav, MD: Thank you for having me.
Dr. Wilson: Can you tell us about Suleiman Hassan and what happened to him before he came into your care?
Dr. Einav: Hassan is a 12-year-old child who was riding his bicycle on the West Bank, about 40 minutes from here. Unfortunately, he was involved in a motor vehicle accident and he suffered injuries to his abdomen and cervical spine. He was transported to our service by helicopter from the scene of the accident.
Dr. Wilson: “Injury to the cervical spine” might be something of an understatement. He had what’s called atlanto-occipital dislocation, colloquially often referred to as internal decapitation. Can you tell us what that means? It sounds terrifying.
Dr. Einav: It’s an injury to the ligaments between the occiput and the upper cervical spine, with or without bony fracture. The atlanto-occipital joint is formed by the superior articular facet of the atlas and the occipital condyle, stabilized by an articular capsule between the head and neck, and is supported by various ligaments around it that stabilize the joint and allow joint movements, including flexion, extension, and some rotation in the lower levels.
Dr. Wilson: This joint has several degrees of freedom, which means it needs a lot of support. With this type of injury, where essentially you have severing of the ligaments, is it usually survivable? How dangerous is this?
Dr. Einav: The mortality rate is 50%-60%, depending on the primary impact, the injury, transportation later on, and then the surgery and surgical management.
Dr. Wilson: Tell us a bit about this patient’s status when he came to your medical center. I assume he was in bad shape.
Dr. Einav: Hassan arrived at our medical center with a Glasgow Coma Scale score of 15. He was fully conscious. He was hemodynamically stable except for a bad laceration on his abdomen. He had a Philadelphia collar around his neck. He was transported by chopper because the paramedics suspected that he had a cervical spine injury and decided to bring him to a Level 1 trauma center.
He was monitored and we treated him according to the ATLS [advanced trauma life support] protocol. He didn’t have any gross sensory deficits, but he was a little confused about the whole situation and the accident. Therefore, we could do a general examination but we couldn’t rely on that regarding any sensory deficit that he may or may not have. We decided as a team that it would be better to slow down and control the situation. We decided not to operate on him immediately. We basically stabilized him and made sure that he didn’t have any traumatic internal organ damage. Later on we took him to the OR and performed surgery.
Dr. Wilson: It’s amazing that he had intact motor function, considering the extent of his injury. The spinal cord was spared somewhat during the injury. There must have been a moment when you realized that this kid, who was conscious and could move all four extremities, had a very severe neck injury. Was that due to a CT scan or physical exam? And what was your feeling when you saw that he had atlanto-occipital dislocation?
Dr. Einav: As a surgeon, you have a gut feeling in regard to the general examination of the patient. But I never rely on gut feelings. On the CT, I understood exactly what he had, what we needed to do, and the time frame.
Dr. Wilson: You’ve done these types of surgeries before, right? Obviously, no one has done a lot of them because this isn’t very common. But you knew what to do. Did you have a plan? Where does your experience come into play in a situation like this?
Dr. Einav: I graduated from the spine program of Toronto University, where I did a fellowship in trauma of the spine and complex spine surgery. I had very good teachers, and during my fellowship I treated a few cases in older patients that were similar but not the same. Therefore, I knew exactly what needed to be done.
Dr. Wilson: For those of us who aren’t surgeons, take us into the OR with you. This is obviously an incredibly delicate procedure. You are high up in the spinal cord at the base of the brain. The slightest mistake could have devastating consequences. What are the key elements of this procedure? What can go wrong here? What is the number-one thing you have to look out for when you’re trying to fix an internal decapitation?
Dr. Einav: The key element in surgeries of the cervical spine – trauma and complex spine surgery – is planning. I never go to the OR without knowing what I’m going to do. I have a few plans – plan A, plan B, plan C – in case something fails. So, I definitely know what the next step will be. I always think about the surgery a few hours before, if I have time to prepare.
The second thing that is very important is teamwork. The team needs to be coordinated. Everybody needs to know what their job is. With these types of injuries, it’s not the time for rookies. If you are new, please stand back and let the more experienced people do that job. I’m talking about surgeons, nurses, anesthesiologists – everyone.
Another important thing in planning is choosing the right hardware. For example, in this case we had a problem because most of the hardware is designed for adults, and we had to improvise because there isn’t a lot of hardware on the market for the pediatric population. The adult plates and screws are too big, so we had to improvise.
Dr. Wilson: Tell us more about that. How do you improvise spinal hardware for a 12-year-old?
Dr. Einav: In this case, I chose to use hardware from one of the companies that works with us.
You can see in this model the area of the injury, and the area that we worked on. To perform the surgery, I had to use some plates and rods from a different company. This company’s (NuVasive) hardware has a small attachment to the skull, which was helpful for affixing the skull to the cervical spine, instead of using a big plate that would sit at the base of the skull and would not be very good for him. Most of the hardware is made for adults and not for kids.
Dr. Wilson: Will that hardware preserve the motor function of his neck? Will he be able to turn his head and extend and flex it?
Dr. Einav: The injury leads to instability and destruction of both articulations between the head and neck. Therefore, those articulations won’t be able to function the same way in the future. There is a decrease of something like 50% of the flexion and extension of Hassan’s cervical spine. Therefore, I decided that in this case there would be no chance of saving Hassan’s motor function unless we performed a fusion between the head and the neck, and therefore I decided that this would be the best procedure with the best survival rate. So, in the future, he will have some diminished flexion, extension, and rotation of his head.
Dr. Wilson: How long did his surgery take?
Dr. Einav: To be honest, I don’t remember. But I can tell you that it took us time. It was very challenging to coordinate with everyone. The most problematic part of the surgery to perform is what we call “flip-over.”
The anesthesiologist intubated the patient when he was supine, and later on, we flipped him prone to operate on the spine. This maneuver can actually lead to injury by itself, and injury at this level is fatal. So, we took our time and got Hassan into the OR. The anesthesiologist did a great job with the GlideScope – inserting the endotracheal tube. Later on, we neuromonitored him. Basically, we connected Hassan’s peripheral nerves to a computer and monitored his motor function. Gently we flipped him over, and after that we saw a little change in his motor function, so we had to modify his position so we could preserve his motor function. We then started the procedure, which took a few hours. I don’t know exactly how many.
Dr. Wilson: That just speaks to how delicate this is for everything from the intubation, where typically you’re manipulating the head, to the repositioning. Clearly this requires a lot of teamwork.
What happened after the operation? How is he doing?
Dr. Einav: After the operation, Hassan had a great recovery. He’s doing well. He doesn’t have any motor or sensory deficits. He’s able to ambulate without any aid. He had no signs of infection, which can happen after a car accident, neither from his abdominal wound nor from the occipital cervical surgery. He feels well. We saw him in the clinic. We removed his collar. We monitored him at the clinic. He looked amazing.
Dr. Wilson: That’s incredible. Are there long-term risks for him that you need to be looking out for?
Dr. Einav: Yes, and that’s the reason that we are monitoring him post surgery. While he was in the hospital, we monitored his motor and sensory functions, as well as his wound healing. Later on, in the clinic, for a few weeks after surgery we monitored for any failure of the hardware and bone graft. We check for healing of the bone graft and bone substitutes we put in to heal those bones.
Dr. Wilson: He will grow, right? He’s only 12, so he still has some years of growth in him. Is he going to need more surgery or any kind of hardware upgrade?
Dr. Einav: I hope not. In my surgeries, I never rely on the hardware for long durations. If I decide to do, for example, fusion, I rely on the hardware for a certain amount of time. And then I plan that the biology will do the work. If I plan for fusion, I put bone grafts in the preferred area for a fusion. Then if the hardware fails, I wouldn’t need to take out the hardware, and there would be no change in the condition of the patient.
Dr. Wilson: What an incredible story. It’s clear that you and your team kept your cool despite a very high-acuity situation with a ton of risk. What a tremendous outcome that this boy is not only alive but fully functional. So, congratulations to you and your team. That was very strong work.
Dr. Einav: Thank you very much. I would like to thank our team. We have to remember that the surgeon is not standing alone in the war. Hassan’s story is a success story of a very big group of people from various backgrounds and religions. They work day and night to help people and save lives. To the paramedics, the physiologists, the traumatologists, the pediatricians, the nurses, the physiotherapists, and obviously the surgeons, a big thank you. His story is our success story.
Dr. Wilson: It’s inspiring to see so many people come together to do what we all are here for, which is to fight against suffering, disease, and death. Thank you for keeping up that fight. And thank you for joining me here.
Dr. Einav: Thank you very much.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: I am joined today by Dr. Ohad Einav. He’s a staff surgeon in orthopedics at Hadassah Medical Center in Jerusalem. He’s with me to talk about an absolutely incredible surgical case, something that is terrifying to most non–orthopedic surgeons and I imagine is fairly scary for spine surgeons like him as well. But what we don’t have is information about how this works from a medical perspective. So, first of all, Dr. Einav, thank you for taking time to speak with me today.
Ohad Einav, MD: Thank you for having me.
Dr. Wilson: Can you tell us about Suleiman Hassan and what happened to him before he came into your care?
Dr. Einav: Hassan is a 12-year-old child who was riding his bicycle on the West Bank, about 40 minutes from here. Unfortunately, he was involved in a motor vehicle accident and he suffered injuries to his abdomen and cervical spine. He was transported to our service by helicopter from the scene of the accident.
Dr. Wilson: “Injury to the cervical spine” might be something of an understatement. He had what’s called atlanto-occipital dislocation, colloquially often referred to as internal decapitation. Can you tell us what that means? It sounds terrifying.
Dr. Einav: It’s an injury to the ligaments between the occiput and the upper cervical spine, with or without bony fracture. The atlanto-occipital joint is formed by the superior articular facet of the atlas and the occipital condyle, stabilized by an articular capsule between the head and neck, and is supported by various ligaments around it that stabilize the joint and allow joint movements, including flexion, extension, and some rotation in the lower levels.
Dr. Wilson: This joint has several degrees of freedom, which means it needs a lot of support. With this type of injury, where essentially you have severing of the ligaments, is it usually survivable? How dangerous is this?
Dr. Einav: The mortality rate is 50%-60%, depending on the primary impact, the injury, transportation later on, and then the surgery and surgical management.
Dr. Wilson: Tell us a bit about this patient’s status when he came to your medical center. I assume he was in bad shape.
Dr. Einav: Hassan arrived at our medical center with a Glasgow Coma Scale score of 15. He was fully conscious. He was hemodynamically stable except for a bad laceration on his abdomen. He had a Philadelphia collar around his neck. He was transported by chopper because the paramedics suspected that he had a cervical spine injury and decided to bring him to a Level 1 trauma center.
He was monitored and we treated him according to the ATLS [advanced trauma life support] protocol. He didn’t have any gross sensory deficits, but he was a little confused about the whole situation and the accident. Therefore, we could do a general examination but we couldn’t rely on that regarding any sensory deficit that he may or may not have. We decided as a team that it would be better to slow down and control the situation. We decided not to operate on him immediately. We basically stabilized him and made sure that he didn’t have any traumatic internal organ damage. Later on we took him to the OR and performed surgery.
Dr. Wilson: It’s amazing that he had intact motor function, considering the extent of his injury. The spinal cord was spared somewhat during the injury. There must have been a moment when you realized that this kid, who was conscious and could move all four extremities, had a very severe neck injury. Was that due to a CT scan or physical exam? And what was your feeling when you saw that he had atlanto-occipital dislocation?
Dr. Einav: As a surgeon, you have a gut feeling in regard to the general examination of the patient. But I never rely on gut feelings. On the CT, I understood exactly what he had, what we needed to do, and the time frame.
Dr. Wilson: You’ve done these types of surgeries before, right? Obviously, no one has done a lot of them because this isn’t very common. But you knew what to do. Did you have a plan? Where does your experience come into play in a situation like this?
Dr. Einav: I graduated from the spine program of Toronto University, where I did a fellowship in trauma of the spine and complex spine surgery. I had very good teachers, and during my fellowship I treated a few cases in older patients that were similar but not the same. Therefore, I knew exactly what needed to be done.
Dr. Wilson: For those of us who aren’t surgeons, take us into the OR with you. This is obviously an incredibly delicate procedure. You are high up in the spinal cord at the base of the brain. The slightest mistake could have devastating consequences. What are the key elements of this procedure? What can go wrong here? What is the number-one thing you have to look out for when you’re trying to fix an internal decapitation?
Dr. Einav: The key element in surgeries of the cervical spine – trauma and complex spine surgery – is planning. I never go to the OR without knowing what I’m going to do. I have a few plans – plan A, plan B, plan C – in case something fails. So, I definitely know what the next step will be. I always think about the surgery a few hours before, if I have time to prepare.
The second thing that is very important is teamwork. The team needs to be coordinated. Everybody needs to know what their job is. With these types of injuries, it’s not the time for rookies. If you are new, please stand back and let the more experienced people do that job. I’m talking about surgeons, nurses, anesthesiologists – everyone.
Another important thing in planning is choosing the right hardware. For example, in this case we had a problem because most of the hardware is designed for adults, and we had to improvise because there isn’t a lot of hardware on the market for the pediatric population. The adult plates and screws are too big, so we had to improvise.
Dr. Wilson: Tell us more about that. How do you improvise spinal hardware for a 12-year-old?
Dr. Einav: In this case, I chose to use hardware from one of the companies that works with us.
You can see in this model the area of the injury, and the area that we worked on. To perform the surgery, I had to use some plates and rods from a different company. This company’s (NuVasive) hardware has a small attachment to the skull, which was helpful for affixing the skull to the cervical spine, instead of using a big plate that would sit at the base of the skull and would not be very good for him. Most of the hardware is made for adults and not for kids.
Dr. Wilson: Will that hardware preserve the motor function of his neck? Will he be able to turn his head and extend and flex it?
Dr. Einav: The injury leads to instability and destruction of both articulations between the head and neck. Therefore, those articulations won’t be able to function the same way in the future. There is a decrease of something like 50% of the flexion and extension of Hassan’s cervical spine. Therefore, I decided that in this case there would be no chance of saving Hassan’s motor function unless we performed a fusion between the head and the neck, and therefore I decided that this would be the best procedure with the best survival rate. So, in the future, he will have some diminished flexion, extension, and rotation of his head.
Dr. Wilson: How long did his surgery take?
Dr. Einav: To be honest, I don’t remember. But I can tell you that it took us time. It was very challenging to coordinate with everyone. The most problematic part of the surgery to perform is what we call “flip-over.”
The anesthesiologist intubated the patient when he was supine, and later on, we flipped him prone to operate on the spine. This maneuver can actually lead to injury by itself, and injury at this level is fatal. So, we took our time and got Hassan into the OR. The anesthesiologist did a great job with the GlideScope – inserting the endotracheal tube. Later on, we neuromonitored him. Basically, we connected Hassan’s peripheral nerves to a computer and monitored his motor function. Gently we flipped him over, and after that we saw a little change in his motor function, so we had to modify his position so we could preserve his motor function. We then started the procedure, which took a few hours. I don’t know exactly how many.
Dr. Wilson: That just speaks to how delicate this is for everything from the intubation, where typically you’re manipulating the head, to the repositioning. Clearly this requires a lot of teamwork.
What happened after the operation? How is he doing?
Dr. Einav: After the operation, Hassan had a great recovery. He’s doing well. He doesn’t have any motor or sensory deficits. He’s able to ambulate without any aid. He had no signs of infection, which can happen after a car accident, neither from his abdominal wound nor from the occipital cervical surgery. He feels well. We saw him in the clinic. We removed his collar. We monitored him at the clinic. He looked amazing.
Dr. Wilson: That’s incredible. Are there long-term risks for him that you need to be looking out for?
Dr. Einav: Yes, and that’s the reason that we are monitoring him post surgery. While he was in the hospital, we monitored his motor and sensory functions, as well as his wound healing. Later on, in the clinic, for a few weeks after surgery we monitored for any failure of the hardware and bone graft. We check for healing of the bone graft and bone substitutes we put in to heal those bones.
Dr. Wilson: He will grow, right? He’s only 12, so he still has some years of growth in him. Is he going to need more surgery or any kind of hardware upgrade?
Dr. Einav: I hope not. In my surgeries, I never rely on the hardware for long durations. If I decide to do, for example, fusion, I rely on the hardware for a certain amount of time. And then I plan that the biology will do the work. If I plan for fusion, I put bone grafts in the preferred area for a fusion. Then if the hardware fails, I wouldn’t need to take out the hardware, and there would be no change in the condition of the patient.
Dr. Wilson: What an incredible story. It’s clear that you and your team kept your cool despite a very high-acuity situation with a ton of risk. What a tremendous outcome that this boy is not only alive but fully functional. So, congratulations to you and your team. That was very strong work.
Dr. Einav: Thank you very much. I would like to thank our team. We have to remember that the surgeon is not standing alone in the war. Hassan’s story is a success story of a very big group of people from various backgrounds and religions. They work day and night to help people and save lives. To the paramedics, the physiologists, the traumatologists, the pediatricians, the nurses, the physiotherapists, and obviously the surgeons, a big thank you. His story is our success story.
Dr. Wilson: It’s inspiring to see so many people come together to do what we all are here for, which is to fight against suffering, disease, and death. Thank you for keeping up that fight. And thank you for joining me here.
Dr. Einav: Thank you very much.
A version of this article first appeared on Medscape.com.
Targeted warnings
I was probably about 9 or 10 and I am assuming it was early winter when my mother took me aside and said in her usual quiet tone, “Willy, don’t ever stick your tongue on a metal pipe when it is cold outside.”
Putting my tongue on a frozen pipe was something that had never occurred to me even in my wildest preadolescent dreams. My mother’s caution only served to pique my interest and provide me with one more tempting scenario to consider.
Recently, a prank has gone viral on TikTok that shows an adult, usually the parent, cracking (not smashing) an egg on the child’s head and then emptying the egg contents into a bowl. Unlike the tongue-pipe disaster, it is hard to imagine how this stunt can be dangerous as long as the child is old enough to be walking around. But, at least one pediatrician has warned that there is a risk to the child from contracting salmonella.
There may be a few young children who are frightened by having an egg cracked on their head, but I can’t imagine that it would leave any lasting emotional scars. Given the minuscule theoretical risk of infection and the fact that the videos have accumulated more than 670 million views, this is another example of when we “experts” should keep a low profile and let the virus fade into Internet oblivion.
There is, however, a difference between harmless foolishness and stupidity, and one wonders when and in what manner we pediatricians should become involved. For example, in a recent study published in the journal Pediatrics, the investigators searched through a national emergency department database and found that
There were two peaks of distribution, one at less than 1 year of age and another at age 4. The older children were more often injured playing on furniture, most often bunk beds. The younger children were more likely to have been injured by being lifted or tossed in the air. No deaths were reported.
Is this a phenomenon that demands a response by pediatricians? Do we have time to ask every family if they have a ceiling fan? Should we be handing out brochures to every family? To whom should we target our message? This is a situation that seems to sort easily into two categories. One that involves stupidity and a second that is ignorance that may respond to education.
Tossing young children in the air is fun for the tosser and the child. I am sure there are a few children every year who slip out of the grasp of an adult and are injured. I have never seen a child brought in with this history. But it must happen. The result is likely to trigger a very tricky child protective investigation. But tossing a child underneath a ceiling fan is just plain stupid. I’m not sure our intervention is going to prevent it from happening. Bunk beds and ceiling fans are a different story. Posters in our offices and warnings and labels at the point of purchase of both fans and bunk beds makes some sense.
And while we are sticking labels on furniture, we should take a hard look at couches. Researchers have recently found that the accumulation of sedentary time in childhood can lead to early evidence of heart damage, which may portend heart disease in adulthood. Instead of those tags under the cushions, we need a big blaze orange sticker in prominent view that warns of the danger of becoming a couch potato.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I was probably about 9 or 10 and I am assuming it was early winter when my mother took me aside and said in her usual quiet tone, “Willy, don’t ever stick your tongue on a metal pipe when it is cold outside.”
Putting my tongue on a frozen pipe was something that had never occurred to me even in my wildest preadolescent dreams. My mother’s caution only served to pique my interest and provide me with one more tempting scenario to consider.
Recently, a prank has gone viral on TikTok that shows an adult, usually the parent, cracking (not smashing) an egg on the child’s head and then emptying the egg contents into a bowl. Unlike the tongue-pipe disaster, it is hard to imagine how this stunt can be dangerous as long as the child is old enough to be walking around. But, at least one pediatrician has warned that there is a risk to the child from contracting salmonella.
There may be a few young children who are frightened by having an egg cracked on their head, but I can’t imagine that it would leave any lasting emotional scars. Given the minuscule theoretical risk of infection and the fact that the videos have accumulated more than 670 million views, this is another example of when we “experts” should keep a low profile and let the virus fade into Internet oblivion.
There is, however, a difference between harmless foolishness and stupidity, and one wonders when and in what manner we pediatricians should become involved. For example, in a recent study published in the journal Pediatrics, the investigators searched through a national emergency department database and found that
There were two peaks of distribution, one at less than 1 year of age and another at age 4. The older children were more often injured playing on furniture, most often bunk beds. The younger children were more likely to have been injured by being lifted or tossed in the air. No deaths were reported.
Is this a phenomenon that demands a response by pediatricians? Do we have time to ask every family if they have a ceiling fan? Should we be handing out brochures to every family? To whom should we target our message? This is a situation that seems to sort easily into two categories. One that involves stupidity and a second that is ignorance that may respond to education.
Tossing young children in the air is fun for the tosser and the child. I am sure there are a few children every year who slip out of the grasp of an adult and are injured. I have never seen a child brought in with this history. But it must happen. The result is likely to trigger a very tricky child protective investigation. But tossing a child underneath a ceiling fan is just plain stupid. I’m not sure our intervention is going to prevent it from happening. Bunk beds and ceiling fans are a different story. Posters in our offices and warnings and labels at the point of purchase of both fans and bunk beds makes some sense.
And while we are sticking labels on furniture, we should take a hard look at couches. Researchers have recently found that the accumulation of sedentary time in childhood can lead to early evidence of heart damage, which may portend heart disease in adulthood. Instead of those tags under the cushions, we need a big blaze orange sticker in prominent view that warns of the danger of becoming a couch potato.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I was probably about 9 or 10 and I am assuming it was early winter when my mother took me aside and said in her usual quiet tone, “Willy, don’t ever stick your tongue on a metal pipe when it is cold outside.”
Putting my tongue on a frozen pipe was something that had never occurred to me even in my wildest preadolescent dreams. My mother’s caution only served to pique my interest and provide me with one more tempting scenario to consider.
Recently, a prank has gone viral on TikTok that shows an adult, usually the parent, cracking (not smashing) an egg on the child’s head and then emptying the egg contents into a bowl. Unlike the tongue-pipe disaster, it is hard to imagine how this stunt can be dangerous as long as the child is old enough to be walking around. But, at least one pediatrician has warned that there is a risk to the child from contracting salmonella.
There may be a few young children who are frightened by having an egg cracked on their head, but I can’t imagine that it would leave any lasting emotional scars. Given the minuscule theoretical risk of infection and the fact that the videos have accumulated more than 670 million views, this is another example of when we “experts” should keep a low profile and let the virus fade into Internet oblivion.
There is, however, a difference between harmless foolishness and stupidity, and one wonders when and in what manner we pediatricians should become involved. For example, in a recent study published in the journal Pediatrics, the investigators searched through a national emergency department database and found that
There were two peaks of distribution, one at less than 1 year of age and another at age 4. The older children were more often injured playing on furniture, most often bunk beds. The younger children were more likely to have been injured by being lifted or tossed in the air. No deaths were reported.
Is this a phenomenon that demands a response by pediatricians? Do we have time to ask every family if they have a ceiling fan? Should we be handing out brochures to every family? To whom should we target our message? This is a situation that seems to sort easily into two categories. One that involves stupidity and a second that is ignorance that may respond to education.
Tossing young children in the air is fun for the tosser and the child. I am sure there are a few children every year who slip out of the grasp of an adult and are injured. I have never seen a child brought in with this history. But it must happen. The result is likely to trigger a very tricky child protective investigation. But tossing a child underneath a ceiling fan is just plain stupid. I’m not sure our intervention is going to prevent it from happening. Bunk beds and ceiling fans are a different story. Posters in our offices and warnings and labels at the point of purchase of both fans and bunk beds makes some sense.
And while we are sticking labels on furniture, we should take a hard look at couches. Researchers have recently found that the accumulation of sedentary time in childhood can lead to early evidence of heart damage, which may portend heart disease in adulthood. Instead of those tags under the cushions, we need a big blaze orange sticker in prominent view that warns of the danger of becoming a couch potato.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Mammography breast density reporting: What it means for clinicians
This transcript has been edited for clarity.
Today, I’m going to talk about the 2023 Food and Drug Administration regulation that requires breast density to be reported on all mammogram results nationwide, and for that report to go to both clinicians and patients. Previously this was the rule in some states, but not in others. This is important because 40%-50% of women have dense breasts. I’m going to discuss what that means for you, and for our patients.
First
Breast density describes the appearance of the breast on mammography. Appearance varies on the basis of breast tissue composition, with fibroglandular tissue being more dense than fatty tissue. Breast density is important because it relates to both the risk for cancer and the ability of mammography to detect cancer.
Breast density is defined and classified according to the American College of Radiology’s BI-RADS four-category scale. Categories 1 and 2 refer to breast tissue that is not dense, accounting for about 50% of the population. Categories 3 and 4 describe heterogeneously dense and extremely dense breast tissue, which occur in approximately 40% and 50% of women, respectively. When speaking about dense breast tissue readings on mammography, we are referring to categories 3 and 4.
Women with dense breast tissue have an increased risk of developing breast cancer and are less likely to have early breast cancer detected on mammography.
Let’s go over the details by category:
For women in categories 1 and 2 (considered not dense breast tissue), the sensitivity of mammography for detecting early breast cancer is 80%-90%. In categories 3 and 4, the sensitivity of mammography drops to 60%-70%.
Compared with women with average breast density, the risk of developing breast cancer is 20% higher in women with BI-RADS category 3 breasts, and more than twice as high (relative risk, 2.1) in those with BI-RADS category 4 breasts. Thus, the risk of developing breast cancer is higher, but the sensitivity of the test is lower.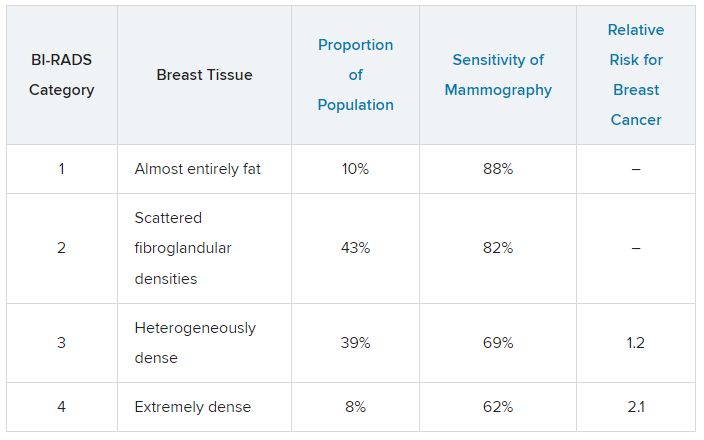
The clinical question is, what should we do about this? For women who have a normal mammogram with dense breasts, should follow-up testing be done, and if so, what test? The main follow-up testing options are either ultrasound or MRI, usually ultrasound. Additional testing will detect additional cancers that were not picked up on the initial mammogram and will also lead to additional biopsies for false-positive tests from the additional testing.
An American College of Gynecology and Obstetrics practice advisory nicely summarizes the evidence and clarifies that this decision is made in the context of a lack of published evidence demonstrating improved outcomes, specifically no reduction in breast cancer mortality, with supplemental testing. The official ACOG stance is that they “do not recommend routine use of alternative or adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.”
This is an area where it is important to understand the data. We are all going to be getting test results back that indicate level of breast density, and those test results will also be sent to our patients, so we are going to be asked about this by interested patients. Should this be something that we talk to patients about, utilizing shared decision-making to decide about whether follow-up testing is necessary in women with dense breasts? That is something each clinician will need to decide, and knowing the data is a critically important step in that decision.
Neil Skolnik, MD, is a professor, department of family medicine, at Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director, department of family medicine, Abington (Pennsylvania) Jefferson Health.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Today, I’m going to talk about the 2023 Food and Drug Administration regulation that requires breast density to be reported on all mammogram results nationwide, and for that report to go to both clinicians and patients. Previously this was the rule in some states, but not in others. This is important because 40%-50% of women have dense breasts. I’m going to discuss what that means for you, and for our patients.
First
Breast density describes the appearance of the breast on mammography. Appearance varies on the basis of breast tissue composition, with fibroglandular tissue being more dense than fatty tissue. Breast density is important because it relates to both the risk for cancer and the ability of mammography to detect cancer.
Breast density is defined and classified according to the American College of Radiology’s BI-RADS four-category scale. Categories 1 and 2 refer to breast tissue that is not dense, accounting for about 50% of the population. Categories 3 and 4 describe heterogeneously dense and extremely dense breast tissue, which occur in approximately 40% and 50% of women, respectively. When speaking about dense breast tissue readings on mammography, we are referring to categories 3 and 4.
Women with dense breast tissue have an increased risk of developing breast cancer and are less likely to have early breast cancer detected on mammography.
Let’s go over the details by category:
For women in categories 1 and 2 (considered not dense breast tissue), the sensitivity of mammography for detecting early breast cancer is 80%-90%. In categories 3 and 4, the sensitivity of mammography drops to 60%-70%.
Compared with women with average breast density, the risk of developing breast cancer is 20% higher in women with BI-RADS category 3 breasts, and more than twice as high (relative risk, 2.1) in those with BI-RADS category 4 breasts. Thus, the risk of developing breast cancer is higher, but the sensitivity of the test is lower.
The clinical question is, what should we do about this? For women who have a normal mammogram with dense breasts, should follow-up testing be done, and if so, what test? The main follow-up testing options are either ultrasound or MRI, usually ultrasound. Additional testing will detect additional cancers that were not picked up on the initial mammogram and will also lead to additional biopsies for false-positive tests from the additional testing.
An American College of Gynecology and Obstetrics practice advisory nicely summarizes the evidence and clarifies that this decision is made in the context of a lack of published evidence demonstrating improved outcomes, specifically no reduction in breast cancer mortality, with supplemental testing. The official ACOG stance is that they “do not recommend routine use of alternative or adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.”
This is an area where it is important to understand the data. We are all going to be getting test results back that indicate level of breast density, and those test results will also be sent to our patients, so we are going to be asked about this by interested patients. Should this be something that we talk to patients about, utilizing shared decision-making to decide about whether follow-up testing is necessary in women with dense breasts? That is something each clinician will need to decide, and knowing the data is a critically important step in that decision.
Neil Skolnik, MD, is a professor, department of family medicine, at Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director, department of family medicine, Abington (Pennsylvania) Jefferson Health.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Today, I’m going to talk about the 2023 Food and Drug Administration regulation that requires breast density to be reported on all mammogram results nationwide, and for that report to go to both clinicians and patients. Previously this was the rule in some states, but not in others. This is important because 40%-50% of women have dense breasts. I’m going to discuss what that means for you, and for our patients.
First
Breast density describes the appearance of the breast on mammography. Appearance varies on the basis of breast tissue composition, with fibroglandular tissue being more dense than fatty tissue. Breast density is important because it relates to both the risk for cancer and the ability of mammography to detect cancer.
Breast density is defined and classified according to the American College of Radiology’s BI-RADS four-category scale. Categories 1 and 2 refer to breast tissue that is not dense, accounting for about 50% of the population. Categories 3 and 4 describe heterogeneously dense and extremely dense breast tissue, which occur in approximately 40% and 50% of women, respectively. When speaking about dense breast tissue readings on mammography, we are referring to categories 3 and 4.
Women with dense breast tissue have an increased risk of developing breast cancer and are less likely to have early breast cancer detected on mammography.
Let’s go over the details by category:
For women in categories 1 and 2 (considered not dense breast tissue), the sensitivity of mammography for detecting early breast cancer is 80%-90%. In categories 3 and 4, the sensitivity of mammography drops to 60%-70%.
Compared with women with average breast density, the risk of developing breast cancer is 20% higher in women with BI-RADS category 3 breasts, and more than twice as high (relative risk, 2.1) in those with BI-RADS category 4 breasts. Thus, the risk of developing breast cancer is higher, but the sensitivity of the test is lower.
The clinical question is, what should we do about this? For women who have a normal mammogram with dense breasts, should follow-up testing be done, and if so, what test? The main follow-up testing options are either ultrasound or MRI, usually ultrasound. Additional testing will detect additional cancers that were not picked up on the initial mammogram and will also lead to additional biopsies for false-positive tests from the additional testing.
An American College of Gynecology and Obstetrics practice advisory nicely summarizes the evidence and clarifies that this decision is made in the context of a lack of published evidence demonstrating improved outcomes, specifically no reduction in breast cancer mortality, with supplemental testing. The official ACOG stance is that they “do not recommend routine use of alternative or adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.”
This is an area where it is important to understand the data. We are all going to be getting test results back that indicate level of breast density, and those test results will also be sent to our patients, so we are going to be asked about this by interested patients. Should this be something that we talk to patients about, utilizing shared decision-making to decide about whether follow-up testing is necessary in women with dense breasts? That is something each clinician will need to decide, and knowing the data is a critically important step in that decision.
Neil Skolnik, MD, is a professor, department of family medicine, at Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director, department of family medicine, Abington (Pennsylvania) Jefferson Health.
A version of this article first appeared on Medscape.com.
Coping with burnout and repetitive injuries
Dear colleagues,
We are all part of one of the most exciting and varied fields of medicine and hope to have long and productive careers. In this month’s AGA Perspectives we explore two different impediments to longevity as a gastroenterologist: work-related disability and burnout.
Physician burnout has reached almost epidemic levels in medicine and is best approached in a multimodal manner, incorporating both institutional and individual changes. Dr. Sumeet Tewani discusses ways in which groups and institutions can foster physician wellness to reduce burnout. In particular, he will explore how flexibility in work schedules, among other initiatives, can improve workplace morale. In an accompanying perspective, Dr. Anna Lipowska and Dr. Amandeep Shergill explore how to incorporate ergonomics in endoscopy to prevent injury. Endoscopic practice, with its repetitive tasks and physical demands, can predispose to injury at all levels of training and experience. Ergonomics is thus a critical topic that is unfortunately covered too little, if at all, in our endoscopy training.
We hope these essays will help your medical practice and welcome your thoughts on these important issues at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Fostering physician wellness to prevent burnout
BY SUMEET TEWANI, MD
Gastroenterology can be a challenging field, both professionally and personally, as it requires providers to have high clinical knowledge, expertise, and emotional intelligence. Burnout is a state of emotional, physical, and mental exhaustion, depersonalization, and a reduced sense of personal accomplishment, caused by prolonged or excessive stress. Burnout can be a serious and progressive chronic condition that negatively impacts the provider and patient experience, with serious consequences on the provider’s health, job performance, patient satisfaction, and personal relationships.
Adopting strategies to combat burnout are of vital importance to promoting provider wellness, a healthy work environment, and positive interactions with colleagues, staff, and patients. This also positively impacts providers’ out-of-work experiences and relationships. on an institutional level and on an individual level.
Multiple factors affecting burnout have been identified, including individual factors, work volume, professional risk and responsibility, resources, and relationships with colleagues and patients. Practicing gastroenterology frequently requires long and irregular work hours, heavy workloads, large panels of complex patients, invasive procedures, and high amounts of stress. Additional stressors may include an inefficient work environment, inadequate support, and loss of value and meaning in work. Nearly 50% of physicians meet criteria for burnout, citing such reasons as excessive bureaucratic tasks, lack of control, flexibility and autonomy, lack of peer respect, increasing computerization of practice, and lack of respect from patients.
Preventing burnout
When coping with burnout, many providers choose positive mechanisms such as exercise, listening to music, meditation, and talking with family and friends. Others become more isolated, eat junk food or binge eat, or turn to drug or alcohol abuse. Our primary approach to preventing burnout at an individual level is to ensure providers have access to self-care techniques such as stress management and mindfulness, and to encourage wellness with regular exercise and healthy habits. Promoting a culture of work-life balance requires providing adequate time for personal activities and hobbies, rest, relaxation, and spending time with family and friends. Allowing providers to personally shape their career paths aligns their personal and professional goals, leading to greater satisfaction. To this end, providers may become involved in clinical research, medical education, and clinical and administrative committees through the practice, local medical school and hospitals, and local and national societies. We provide ample vacation time and CME opportunities for our providers. Vacation time is flexible and can be taken in half-day or full-day increments, or on an hourly basis for personal time as necessary. This allows for enhanced flexibility with scheduling time off from work.
On an institutional level, leadership plays a prime role in creating a healthy work environment. Having good leaders influences the well-being and satisfaction of everyone within the organization. Leaders can have a positive impact by aligning values and work culture, using incentives in a productive manner, and promoting strategies to reduce burnout. Involving physician partners in leadership on a rotating basis allows them to better understand the roles of the leaders in the organization and empowers them to have a voice in changing policies to reduce administrative burdens and foster wellness.
We promote the concept of working together as a team for the success of the practice. All partners have an equal say in the management of the group. We eliminated the stress of competition within the group, equalizing pay across all physician partners, while maintaining equal exposure to work and equal time off from work. This levels the playing field between physicians who have varied interests and expertise, so that everyone is working towards the success of the practice and not individual compensation. To that end, our providers do not have individual offices, but work out of a “bullpen” with an open concept where we have individual workspaces and interact with each other continuously throughout the day. This promotes cohesion and teamwork between the providers for all our patients and promotes professional relationships and peer support. Efforts to promote workplace morale include access to a fully stocked deli and a newly installed espresso machine.
The concept of teamwork
The concept of teamwork also needs to pervade through the entire organization. To manage the demands of a busy workday, we have directly trained advanced practice nurses in the clinic and inpatient settings, allowing our physicians to increase throughput and procedures while maintaining a high level of patient care, satisfaction, and efficiency. Providers report excessive administrative tasks and frustration with the electronic health record as major factors contributing to burnout. Delegating tasks to staff, commensurate with their training and scope of practice, alleviates some of this burden. Each of the providers in our practice has a triage nurse who functions in a key capacity to ensure the appropriate clinical and administrative tasks are complete. Medical scribes, medical assistants, nurses, and physician assistants can be utilized for data entry and other tasks. We have developed templates within the electronic health record that can be standardized across the practice. Promoting teamwork with staff also means respecting the staff and understanding their needs. A highly functioning health care team can provide comprehensive care proactively and efficiently, with improved professional satisfaction.
In summary, I identify several ways to promote physician wellness. Every GI practice should strive to implement local approaches to prevent physician burnout and help maintain a happy and productive workforce.
Dr. Tewani is a gastroenterologist with Rockford Gastroenterology Associates in Rockford, Ill. He has no relevant disclosures.
References
1. Koval ML. Medscape gastroenterologist lifestyle, happiness & burnout report 2023: Contentment amid stress. Medscape. 24 Feb 2023.
2. Ong J et al. The prevalence of burnout, risk factors, and job-related stressors in gastroenterologists: A systematic review. J Gastroenterol Hepatol. 2021 Sep;36(9):2338-48.
3. Anderson J et al. Strategies to combat physician burnout in gastroenterology. Am J Gastroenterol. 2017 Sep;112(9):1356-9.
4. Keswani R et al. Burnout in gastroenterologists and how to prevent it. Gastroenterology. 2014 Jul;147(1):11-4.
The hazards of endoscopy: Ergonomics guide the way
BY ANNA LIPOWSKA, MD, AND AMANDEEP SHERGILL, MD, MS
Preventing disability and promoting a long and successful endoscopic career involves proactive measures to support well-being, and ergonomics plays a key role. Ergonomics is the science of fitting a job to the worker, with a primary goal of working smarter and safer. When hazards are identified, mitigation measures, guided by a hierarchy of controls, must be implemented that improve the fit of the tool, task and job to the worker in order to reduce the risk of endoscopy-related injury (ERI). As more women enter the field and as the overall GI physician population ages, ensuring that endoscopy is designed to be safely performed within the capacity of a diverse group of workers will be critical to creating an inclusive and equitable work environment.
Ergonomic education is foundational: Awareness of ERI risk factors allows endoscopists to identify hazards and advocate for effective control solutions. Ergonomic education materials are available through all of the major GI societies. A road map for implementing an endoscopy ergonomics program has been previously published and provides guidance on risk assessment and mitigation measures.
Respect pain
Overuse injuries occur when the physical demands of a job are greater than tissue tolerances, leading to cumulative microtrauma. The first sign of microtraumatic injury is discomfort and pain. Studies have demonstrated that an estimated three-quarters of gastroenterologists experience ERI, with 20% requiring time off work and 12% requiring surgery. Gastroenterology trainees are also at risk, with 20% of surveyed fellows endorsing overuse injury, some even requiring work-related leaves of absence. In the early stages of ERI, aching and tiredness occur during the work shift only. In the intermediate stage, aching and tiredness occur early in the work shift and persist at night, and may be associated with a reduced capacity for repetitive work. In the late stages, aching, fatigue and weakness persist at rest and may be associated with inability to sleep and to perform light duties. Pain is an important signal indicating mitigation measures are required to control exposures.
Utilize the hierarchy of controls
The responsibility for adoption of ergonomically friendly practices does not lie solely with the physician; both institutional and industry-level support are key to its success. The hierarchy of controls defines which actions will best mitigate exposures to hazards in the workplace, highlighting that modifications to personal practice have the smallest impact. Current endoscope design does not accommodate the full range of hand strengths and sizes and contributes to ERI.
Advancements at the industry level by eliminating or substituting hazards, or designing engineering controls to reduce exposure, will be most effective at preventing distal upper extremity ERI. The next most effective controls are at the institutional level, with endoscopy units ensuring access to engineering controls and implementing effective administrative controls. For example, institutional support and investment in adjustable workstations is imperative to accommodate a range of anthropometric dimensions of the population. Support for ergonomic education, scheduling changes and a culture where safety is a priority can help reduce exposure to hazards and injury risk.
Adjust the endoscopy suite to achieve a comfortable position before every procedure
Neutral body posture is our position of greatest comfort and maximum strength, and any deviation from neutral posture decreases the amount of force the muscles can produce and causes the muscles to fatigue sooner. The most important factor affecting the endoscopists’ overall posture is the monitor position and height. Monitors must be adjustable. Place the monitor directly in front of you, with the center of the screen 15-25 degrees below eye height for a neutral neck position and resting eye position. Procedure bed height should be adjusted 0-10 cm below elbow height to allow for neutral elbow postures and relaxed shoulders. Antifatigue mats and shoes with supportive insoles can reduce fatigue. Two-piece lead aprons distribute a portion of the static load to the hips and decrease back strain. Incorporate a preprocedure ergonomic time-out, to assess proper room set up, body mechanics, equipment and team preparedness.
Give yourself a break
Breaks should be built into the endoscopy schedule, especially for a full day of endoscopy. At a minimum, incorporate microbreaks during procedures, which have been found to alleviate pain and improve performance. Exercises and stretching can be incorporated between cases, including routines designed specifically for endoscopists.
Getting older isn’t for the weak
Currently, 50% of our gastroenterologists are over 55 years old. The aging process leads to a distinct muscle mass and strength loss. Women are already at a disadvantage because, on average, they have less muscle mass than men in all age groups. Muscle starts to deteriorate when we reach our 30s, and after age 40, we lose on average 8% of our muscle mass every decade which accelerates at an even faster rate after age 60. Both resistance and aerobic exercise can be very useful to counteract sarcopenia and maintain strength. Given the physical demands of endoscopy, exercise can help safeguard career longevity and maintain overall wellness. Multiple resources are available to tailor a program that fits your time, budget and needs.
Optimize all of your workstations
Prolonged computer use and desk work is also a significant part of a gastroenterologist’s profession. If using a sitting desk, chair height should allow for 90-degree flexion at the hips and knees and for feet to rest flatly on the floor. The chair should also provide adequate back support for a relaxed upright position. Similar to endoscopy, place the monitor directly in front with the center of the screen slightly below eye level. For mouse and keyboard placement, aim to have the elbows at or slightly below 90 degrees and one’s wrists and fingers in neutral position.
Endoscopy can be hazardous to the endoscopists’ health. Incorporating ergonomic principles creates a safer and more efficient work environment. At the individual level, ergonomically optimized postures during endoscopy as well as during computer-related tasks, room set up, inclusion of microbreaks, and protective exercises can help decrease the risk of repetitive strain injury and prevent disability. Importantly, change at the industry and institutional level has the greatest potential for positive impact. Adoption of ergonomic practices promotes career longevity and ensures that gastroenterologists can continue successful and long careers and provide quality care to their patients without compromising their own health.
Dr. Lipowska is an assistant professor in the division of gastroenterology and hepatology, University of Illinois at Chicago. She disclosed no conflicts. Dr. Shergill is chief of gastroenterology for the San Francisco VA Health Care System. She disclosed consulting work for Boston Scientific and Neptune Medical, honoraria for visiting professorship with Intuitive Surgical, and a research gift from Pentax.
References
Shergill AK. Top tips for implementing an endoscopy ergonomics program. Gastrointest Endosc. 2023 Feb;97(2):361-4.
Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
Austin K et al. Musculoskeletal injuries are commonly reported among gastroenterology trainees: Results of a national survey. Dig Dis Sci. 2019;64(6):1439-47.
Lipowska A et al. Ergonomics in the unit: Modeling the environment around the endoscopist. Tech Innov Gastrointest Endosc. 2021;23(3):256-62.
Park A et al. Intraoperative “Micro Breaks” with a targeted stretching enhance surgeon physical function and mental focus: A multicenter cohort study. Ann Surg. 2017;265(2):340-6.
Shergill A et al. Ergonomic endoscopy: An oxymoron or realistic goal? Gastrointest Endosc. 2019;90(6):966-70.
Dear colleagues,
We are all part of one of the most exciting and varied fields of medicine and hope to have long and productive careers. In this month’s AGA Perspectives we explore two different impediments to longevity as a gastroenterologist: work-related disability and burnout.
Physician burnout has reached almost epidemic levels in medicine and is best approached in a multimodal manner, incorporating both institutional and individual changes. Dr. Sumeet Tewani discusses ways in which groups and institutions can foster physician wellness to reduce burnout. In particular, he will explore how flexibility in work schedules, among other initiatives, can improve workplace morale. In an accompanying perspective, Dr. Anna Lipowska and Dr. Amandeep Shergill explore how to incorporate ergonomics in endoscopy to prevent injury. Endoscopic practice, with its repetitive tasks and physical demands, can predispose to injury at all levels of training and experience. Ergonomics is thus a critical topic that is unfortunately covered too little, if at all, in our endoscopy training.
We hope these essays will help your medical practice and welcome your thoughts on these important issues at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Fostering physician wellness to prevent burnout
BY SUMEET TEWANI, MD
Gastroenterology can be a challenging field, both professionally and personally, as it requires providers to have high clinical knowledge, expertise, and emotional intelligence. Burnout is a state of emotional, physical, and mental exhaustion, depersonalization, and a reduced sense of personal accomplishment, caused by prolonged or excessive stress. Burnout can be a serious and progressive chronic condition that negatively impacts the provider and patient experience, with serious consequences on the provider’s health, job performance, patient satisfaction, and personal relationships.
Adopting strategies to combat burnout are of vital importance to promoting provider wellness, a healthy work environment, and positive interactions with colleagues, staff, and patients. This also positively impacts providers’ out-of-work experiences and relationships. on an institutional level and on an individual level.
Multiple factors affecting burnout have been identified, including individual factors, work volume, professional risk and responsibility, resources, and relationships with colleagues and patients. Practicing gastroenterology frequently requires long and irregular work hours, heavy workloads, large panels of complex patients, invasive procedures, and high amounts of stress. Additional stressors may include an inefficient work environment, inadequate support, and loss of value and meaning in work. Nearly 50% of physicians meet criteria for burnout, citing such reasons as excessive bureaucratic tasks, lack of control, flexibility and autonomy, lack of peer respect, increasing computerization of practice, and lack of respect from patients.
Preventing burnout
When coping with burnout, many providers choose positive mechanisms such as exercise, listening to music, meditation, and talking with family and friends. Others become more isolated, eat junk food or binge eat, or turn to drug or alcohol abuse. Our primary approach to preventing burnout at an individual level is to ensure providers have access to self-care techniques such as stress management and mindfulness, and to encourage wellness with regular exercise and healthy habits. Promoting a culture of work-life balance requires providing adequate time for personal activities and hobbies, rest, relaxation, and spending time with family and friends. Allowing providers to personally shape their career paths aligns their personal and professional goals, leading to greater satisfaction. To this end, providers may become involved in clinical research, medical education, and clinical and administrative committees through the practice, local medical school and hospitals, and local and national societies. We provide ample vacation time and CME opportunities for our providers. Vacation time is flexible and can be taken in half-day or full-day increments, or on an hourly basis for personal time as necessary. This allows for enhanced flexibility with scheduling time off from work.
On an institutional level, leadership plays a prime role in creating a healthy work environment. Having good leaders influences the well-being and satisfaction of everyone within the organization. Leaders can have a positive impact by aligning values and work culture, using incentives in a productive manner, and promoting strategies to reduce burnout. Involving physician partners in leadership on a rotating basis allows them to better understand the roles of the leaders in the organization and empowers them to have a voice in changing policies to reduce administrative burdens and foster wellness.
We promote the concept of working together as a team for the success of the practice. All partners have an equal say in the management of the group. We eliminated the stress of competition within the group, equalizing pay across all physician partners, while maintaining equal exposure to work and equal time off from work. This levels the playing field between physicians who have varied interests and expertise, so that everyone is working towards the success of the practice and not individual compensation. To that end, our providers do not have individual offices, but work out of a “bullpen” with an open concept where we have individual workspaces and interact with each other continuously throughout the day. This promotes cohesion and teamwork between the providers for all our patients and promotes professional relationships and peer support. Efforts to promote workplace morale include access to a fully stocked deli and a newly installed espresso machine.
The concept of teamwork
The concept of teamwork also needs to pervade through the entire organization. To manage the demands of a busy workday, we have directly trained advanced practice nurses in the clinic and inpatient settings, allowing our physicians to increase throughput and procedures while maintaining a high level of patient care, satisfaction, and efficiency. Providers report excessive administrative tasks and frustration with the electronic health record as major factors contributing to burnout. Delegating tasks to staff, commensurate with their training and scope of practice, alleviates some of this burden. Each of the providers in our practice has a triage nurse who functions in a key capacity to ensure the appropriate clinical and administrative tasks are complete. Medical scribes, medical assistants, nurses, and physician assistants can be utilized for data entry and other tasks. We have developed templates within the electronic health record that can be standardized across the practice. Promoting teamwork with staff also means respecting the staff and understanding their needs. A highly functioning health care team can provide comprehensive care proactively and efficiently, with improved professional satisfaction.
In summary, I identify several ways to promote physician wellness. Every GI practice should strive to implement local approaches to prevent physician burnout and help maintain a happy and productive workforce.
Dr. Tewani is a gastroenterologist with Rockford Gastroenterology Associates in Rockford, Ill. He has no relevant disclosures.
References
1. Koval ML. Medscape gastroenterologist lifestyle, happiness & burnout report 2023: Contentment amid stress. Medscape. 24 Feb 2023.
2. Ong J et al. The prevalence of burnout, risk factors, and job-related stressors in gastroenterologists: A systematic review. J Gastroenterol Hepatol. 2021 Sep;36(9):2338-48.
3. Anderson J et al. Strategies to combat physician burnout in gastroenterology. Am J Gastroenterol. 2017 Sep;112(9):1356-9.
4. Keswani R et al. Burnout in gastroenterologists and how to prevent it. Gastroenterology. 2014 Jul;147(1):11-4.
The hazards of endoscopy: Ergonomics guide the way
BY ANNA LIPOWSKA, MD, AND AMANDEEP SHERGILL, MD, MS
Preventing disability and promoting a long and successful endoscopic career involves proactive measures to support well-being, and ergonomics plays a key role. Ergonomics is the science of fitting a job to the worker, with a primary goal of working smarter and safer. When hazards are identified, mitigation measures, guided by a hierarchy of controls, must be implemented that improve the fit of the tool, task and job to the worker in order to reduce the risk of endoscopy-related injury (ERI). As more women enter the field and as the overall GI physician population ages, ensuring that endoscopy is designed to be safely performed within the capacity of a diverse group of workers will be critical to creating an inclusive and equitable work environment.
Ergonomic education is foundational: Awareness of ERI risk factors allows endoscopists to identify hazards and advocate for effective control solutions. Ergonomic education materials are available through all of the major GI societies. A road map for implementing an endoscopy ergonomics program has been previously published and provides guidance on risk assessment and mitigation measures.
Respect pain
Overuse injuries occur when the physical demands of a job are greater than tissue tolerances, leading to cumulative microtrauma. The first sign of microtraumatic injury is discomfort and pain. Studies have demonstrated that an estimated three-quarters of gastroenterologists experience ERI, with 20% requiring time off work and 12% requiring surgery. Gastroenterology trainees are also at risk, with 20% of surveyed fellows endorsing overuse injury, some even requiring work-related leaves of absence. In the early stages of ERI, aching and tiredness occur during the work shift only. In the intermediate stage, aching and tiredness occur early in the work shift and persist at night, and may be associated with a reduced capacity for repetitive work. In the late stages, aching, fatigue and weakness persist at rest and may be associated with inability to sleep and to perform light duties. Pain is an important signal indicating mitigation measures are required to control exposures.
Utilize the hierarchy of controls
The responsibility for adoption of ergonomically friendly practices does not lie solely with the physician; both institutional and industry-level support are key to its success. The hierarchy of controls defines which actions will best mitigate exposures to hazards in the workplace, highlighting that modifications to personal practice have the smallest impact. Current endoscope design does not accommodate the full range of hand strengths and sizes and contributes to ERI.
Advancements at the industry level by eliminating or substituting hazards, or designing engineering controls to reduce exposure, will be most effective at preventing distal upper extremity ERI. The next most effective controls are at the institutional level, with endoscopy units ensuring access to engineering controls and implementing effective administrative controls. For example, institutional support and investment in adjustable workstations is imperative to accommodate a range of anthropometric dimensions of the population. Support for ergonomic education, scheduling changes and a culture where safety is a priority can help reduce exposure to hazards and injury risk.
Adjust the endoscopy suite to achieve a comfortable position before every procedure
Neutral body posture is our position of greatest comfort and maximum strength, and any deviation from neutral posture decreases the amount of force the muscles can produce and causes the muscles to fatigue sooner. The most important factor affecting the endoscopists’ overall posture is the monitor position and height. Monitors must be adjustable. Place the monitor directly in front of you, with the center of the screen 15-25 degrees below eye height for a neutral neck position and resting eye position. Procedure bed height should be adjusted 0-10 cm below elbow height to allow for neutral elbow postures and relaxed shoulders. Antifatigue mats and shoes with supportive insoles can reduce fatigue. Two-piece lead aprons distribute a portion of the static load to the hips and decrease back strain. Incorporate a preprocedure ergonomic time-out, to assess proper room set up, body mechanics, equipment and team preparedness.
Give yourself a break
Breaks should be built into the endoscopy schedule, especially for a full day of endoscopy. At a minimum, incorporate microbreaks during procedures, which have been found to alleviate pain and improve performance. Exercises and stretching can be incorporated between cases, including routines designed specifically for endoscopists.
Getting older isn’t for the weak
Currently, 50% of our gastroenterologists are over 55 years old. The aging process leads to a distinct muscle mass and strength loss. Women are already at a disadvantage because, on average, they have less muscle mass than men in all age groups. Muscle starts to deteriorate when we reach our 30s, and after age 40, we lose on average 8% of our muscle mass every decade which accelerates at an even faster rate after age 60. Both resistance and aerobic exercise can be very useful to counteract sarcopenia and maintain strength. Given the physical demands of endoscopy, exercise can help safeguard career longevity and maintain overall wellness. Multiple resources are available to tailor a program that fits your time, budget and needs.
Optimize all of your workstations
Prolonged computer use and desk work is also a significant part of a gastroenterologist’s profession. If using a sitting desk, chair height should allow for 90-degree flexion at the hips and knees and for feet to rest flatly on the floor. The chair should also provide adequate back support for a relaxed upright position. Similar to endoscopy, place the monitor directly in front with the center of the screen slightly below eye level. For mouse and keyboard placement, aim to have the elbows at or slightly below 90 degrees and one’s wrists and fingers in neutral position.
Endoscopy can be hazardous to the endoscopists’ health. Incorporating ergonomic principles creates a safer and more efficient work environment. At the individual level, ergonomically optimized postures during endoscopy as well as during computer-related tasks, room set up, inclusion of microbreaks, and protective exercises can help decrease the risk of repetitive strain injury and prevent disability. Importantly, change at the industry and institutional level has the greatest potential for positive impact. Adoption of ergonomic practices promotes career longevity and ensures that gastroenterologists can continue successful and long careers and provide quality care to their patients without compromising their own health.
Dr. Lipowska is an assistant professor in the division of gastroenterology and hepatology, University of Illinois at Chicago. She disclosed no conflicts. Dr. Shergill is chief of gastroenterology for the San Francisco VA Health Care System. She disclosed consulting work for Boston Scientific and Neptune Medical, honoraria for visiting professorship with Intuitive Surgical, and a research gift from Pentax.
References
Shergill AK. Top tips for implementing an endoscopy ergonomics program. Gastrointest Endosc. 2023 Feb;97(2):361-4.
Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
Austin K et al. Musculoskeletal injuries are commonly reported among gastroenterology trainees: Results of a national survey. Dig Dis Sci. 2019;64(6):1439-47.
Lipowska A et al. Ergonomics in the unit: Modeling the environment around the endoscopist. Tech Innov Gastrointest Endosc. 2021;23(3):256-62.
Park A et al. Intraoperative “Micro Breaks” with a targeted stretching enhance surgeon physical function and mental focus: A multicenter cohort study. Ann Surg. 2017;265(2):340-6.
Shergill A et al. Ergonomic endoscopy: An oxymoron or realistic goal? Gastrointest Endosc. 2019;90(6):966-70.
Dear colleagues,
We are all part of one of the most exciting and varied fields of medicine and hope to have long and productive careers. In this month’s AGA Perspectives we explore two different impediments to longevity as a gastroenterologist: work-related disability and burnout.
Physician burnout has reached almost epidemic levels in medicine and is best approached in a multimodal manner, incorporating both institutional and individual changes. Dr. Sumeet Tewani discusses ways in which groups and institutions can foster physician wellness to reduce burnout. In particular, he will explore how flexibility in work schedules, among other initiatives, can improve workplace morale. In an accompanying perspective, Dr. Anna Lipowska and Dr. Amandeep Shergill explore how to incorporate ergonomics in endoscopy to prevent injury. Endoscopic practice, with its repetitive tasks and physical demands, can predispose to injury at all levels of training and experience. Ergonomics is thus a critical topic that is unfortunately covered too little, if at all, in our endoscopy training.
We hope these essays will help your medical practice and welcome your thoughts on these important issues at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Fostering physician wellness to prevent burnout
BY SUMEET TEWANI, MD
Gastroenterology can be a challenging field, both professionally and personally, as it requires providers to have high clinical knowledge, expertise, and emotional intelligence. Burnout is a state of emotional, physical, and mental exhaustion, depersonalization, and a reduced sense of personal accomplishment, caused by prolonged or excessive stress. Burnout can be a serious and progressive chronic condition that negatively impacts the provider and patient experience, with serious consequences on the provider’s health, job performance, patient satisfaction, and personal relationships.
Adopting strategies to combat burnout are of vital importance to promoting provider wellness, a healthy work environment, and positive interactions with colleagues, staff, and patients. This also positively impacts providers’ out-of-work experiences and relationships. on an institutional level and on an individual level.
Multiple factors affecting burnout have been identified, including individual factors, work volume, professional risk and responsibility, resources, and relationships with colleagues and patients. Practicing gastroenterology frequently requires long and irregular work hours, heavy workloads, large panels of complex patients, invasive procedures, and high amounts of stress. Additional stressors may include an inefficient work environment, inadequate support, and loss of value and meaning in work. Nearly 50% of physicians meet criteria for burnout, citing such reasons as excessive bureaucratic tasks, lack of control, flexibility and autonomy, lack of peer respect, increasing computerization of practice, and lack of respect from patients.
Preventing burnout
When coping with burnout, many providers choose positive mechanisms such as exercise, listening to music, meditation, and talking with family and friends. Others become more isolated, eat junk food or binge eat, or turn to drug or alcohol abuse. Our primary approach to preventing burnout at an individual level is to ensure providers have access to self-care techniques such as stress management and mindfulness, and to encourage wellness with regular exercise and healthy habits. Promoting a culture of work-life balance requires providing adequate time for personal activities and hobbies, rest, relaxation, and spending time with family and friends. Allowing providers to personally shape their career paths aligns their personal and professional goals, leading to greater satisfaction. To this end, providers may become involved in clinical research, medical education, and clinical and administrative committees through the practice, local medical school and hospitals, and local and national societies. We provide ample vacation time and CME opportunities for our providers. Vacation time is flexible and can be taken in half-day or full-day increments, or on an hourly basis for personal time as necessary. This allows for enhanced flexibility with scheduling time off from work.
On an institutional level, leadership plays a prime role in creating a healthy work environment. Having good leaders influences the well-being and satisfaction of everyone within the organization. Leaders can have a positive impact by aligning values and work culture, using incentives in a productive manner, and promoting strategies to reduce burnout. Involving physician partners in leadership on a rotating basis allows them to better understand the roles of the leaders in the organization and empowers them to have a voice in changing policies to reduce administrative burdens and foster wellness.
We promote the concept of working together as a team for the success of the practice. All partners have an equal say in the management of the group. We eliminated the stress of competition within the group, equalizing pay across all physician partners, while maintaining equal exposure to work and equal time off from work. This levels the playing field between physicians who have varied interests and expertise, so that everyone is working towards the success of the practice and not individual compensation. To that end, our providers do not have individual offices, but work out of a “bullpen” with an open concept where we have individual workspaces and interact with each other continuously throughout the day. This promotes cohesion and teamwork between the providers for all our patients and promotes professional relationships and peer support. Efforts to promote workplace morale include access to a fully stocked deli and a newly installed espresso machine.
The concept of teamwork
The concept of teamwork also needs to pervade through the entire organization. To manage the demands of a busy workday, we have directly trained advanced practice nurses in the clinic and inpatient settings, allowing our physicians to increase throughput and procedures while maintaining a high level of patient care, satisfaction, and efficiency. Providers report excessive administrative tasks and frustration with the electronic health record as major factors contributing to burnout. Delegating tasks to staff, commensurate with their training and scope of practice, alleviates some of this burden. Each of the providers in our practice has a triage nurse who functions in a key capacity to ensure the appropriate clinical and administrative tasks are complete. Medical scribes, medical assistants, nurses, and physician assistants can be utilized for data entry and other tasks. We have developed templates within the electronic health record that can be standardized across the practice. Promoting teamwork with staff also means respecting the staff and understanding their needs. A highly functioning health care team can provide comprehensive care proactively and efficiently, with improved professional satisfaction.
In summary, I identify several ways to promote physician wellness. Every GI practice should strive to implement local approaches to prevent physician burnout and help maintain a happy and productive workforce.
Dr. Tewani is a gastroenterologist with Rockford Gastroenterology Associates in Rockford, Ill. He has no relevant disclosures.
References
1. Koval ML. Medscape gastroenterologist lifestyle, happiness & burnout report 2023: Contentment amid stress. Medscape. 24 Feb 2023.
2. Ong J et al. The prevalence of burnout, risk factors, and job-related stressors in gastroenterologists: A systematic review. J Gastroenterol Hepatol. 2021 Sep;36(9):2338-48.
3. Anderson J et al. Strategies to combat physician burnout in gastroenterology. Am J Gastroenterol. 2017 Sep;112(9):1356-9.
4. Keswani R et al. Burnout in gastroenterologists and how to prevent it. Gastroenterology. 2014 Jul;147(1):11-4.
The hazards of endoscopy: Ergonomics guide the way
BY ANNA LIPOWSKA, MD, AND AMANDEEP SHERGILL, MD, MS
Preventing disability and promoting a long and successful endoscopic career involves proactive measures to support well-being, and ergonomics plays a key role. Ergonomics is the science of fitting a job to the worker, with a primary goal of working smarter and safer. When hazards are identified, mitigation measures, guided by a hierarchy of controls, must be implemented that improve the fit of the tool, task and job to the worker in order to reduce the risk of endoscopy-related injury (ERI). As more women enter the field and as the overall GI physician population ages, ensuring that endoscopy is designed to be safely performed within the capacity of a diverse group of workers will be critical to creating an inclusive and equitable work environment.
Ergonomic education is foundational: Awareness of ERI risk factors allows endoscopists to identify hazards and advocate for effective control solutions. Ergonomic education materials are available through all of the major GI societies. A road map for implementing an endoscopy ergonomics program has been previously published and provides guidance on risk assessment and mitigation measures.
Respect pain
Overuse injuries occur when the physical demands of a job are greater than tissue tolerances, leading to cumulative microtrauma. The first sign of microtraumatic injury is discomfort and pain. Studies have demonstrated that an estimated three-quarters of gastroenterologists experience ERI, with 20% requiring time off work and 12% requiring surgery. Gastroenterology trainees are also at risk, with 20% of surveyed fellows endorsing overuse injury, some even requiring work-related leaves of absence. In the early stages of ERI, aching and tiredness occur during the work shift only. In the intermediate stage, aching and tiredness occur early in the work shift and persist at night, and may be associated with a reduced capacity for repetitive work. In the late stages, aching, fatigue and weakness persist at rest and may be associated with inability to sleep and to perform light duties. Pain is an important signal indicating mitigation measures are required to control exposures.
Utilize the hierarchy of controls
The responsibility for adoption of ergonomically friendly practices does not lie solely with the physician; both institutional and industry-level support are key to its success. The hierarchy of controls defines which actions will best mitigate exposures to hazards in the workplace, highlighting that modifications to personal practice have the smallest impact. Current endoscope design does not accommodate the full range of hand strengths and sizes and contributes to ERI.
Advancements at the industry level by eliminating or substituting hazards, or designing engineering controls to reduce exposure, will be most effective at preventing distal upper extremity ERI. The next most effective controls are at the institutional level, with endoscopy units ensuring access to engineering controls and implementing effective administrative controls. For example, institutional support and investment in adjustable workstations is imperative to accommodate a range of anthropometric dimensions of the population. Support for ergonomic education, scheduling changes and a culture where safety is a priority can help reduce exposure to hazards and injury risk.
Adjust the endoscopy suite to achieve a comfortable position before every procedure
Neutral body posture is our position of greatest comfort and maximum strength, and any deviation from neutral posture decreases the amount of force the muscles can produce and causes the muscles to fatigue sooner. The most important factor affecting the endoscopists’ overall posture is the monitor position and height. Monitors must be adjustable. Place the monitor directly in front of you, with the center of the screen 15-25 degrees below eye height for a neutral neck position and resting eye position. Procedure bed height should be adjusted 0-10 cm below elbow height to allow for neutral elbow postures and relaxed shoulders. Antifatigue mats and shoes with supportive insoles can reduce fatigue. Two-piece lead aprons distribute a portion of the static load to the hips and decrease back strain. Incorporate a preprocedure ergonomic time-out, to assess proper room set up, body mechanics, equipment and team preparedness.
Give yourself a break
Breaks should be built into the endoscopy schedule, especially for a full day of endoscopy. At a minimum, incorporate microbreaks during procedures, which have been found to alleviate pain and improve performance. Exercises and stretching can be incorporated between cases, including routines designed specifically for endoscopists.
Getting older isn’t for the weak
Currently, 50% of our gastroenterologists are over 55 years old. The aging process leads to a distinct muscle mass and strength loss. Women are already at a disadvantage because, on average, they have less muscle mass than men in all age groups. Muscle starts to deteriorate when we reach our 30s, and after age 40, we lose on average 8% of our muscle mass every decade which accelerates at an even faster rate after age 60. Both resistance and aerobic exercise can be very useful to counteract sarcopenia and maintain strength. Given the physical demands of endoscopy, exercise can help safeguard career longevity and maintain overall wellness. Multiple resources are available to tailor a program that fits your time, budget and needs.
Optimize all of your workstations
Prolonged computer use and desk work is also a significant part of a gastroenterologist’s profession. If using a sitting desk, chair height should allow for 90-degree flexion at the hips and knees and for feet to rest flatly on the floor. The chair should also provide adequate back support for a relaxed upright position. Similar to endoscopy, place the monitor directly in front with the center of the screen slightly below eye level. For mouse and keyboard placement, aim to have the elbows at or slightly below 90 degrees and one’s wrists and fingers in neutral position.
Endoscopy can be hazardous to the endoscopists’ health. Incorporating ergonomic principles creates a safer and more efficient work environment. At the individual level, ergonomically optimized postures during endoscopy as well as during computer-related tasks, room set up, inclusion of microbreaks, and protective exercises can help decrease the risk of repetitive strain injury and prevent disability. Importantly, change at the industry and institutional level has the greatest potential for positive impact. Adoption of ergonomic practices promotes career longevity and ensures that gastroenterologists can continue successful and long careers and provide quality care to their patients without compromising their own health.
Dr. Lipowska is an assistant professor in the division of gastroenterology and hepatology, University of Illinois at Chicago. She disclosed no conflicts. Dr. Shergill is chief of gastroenterology for the San Francisco VA Health Care System. She disclosed consulting work for Boston Scientific and Neptune Medical, honoraria for visiting professorship with Intuitive Surgical, and a research gift from Pentax.
References
Shergill AK. Top tips for implementing an endoscopy ergonomics program. Gastrointest Endosc. 2023 Feb;97(2):361-4.
Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
Austin K et al. Musculoskeletal injuries are commonly reported among gastroenterology trainees: Results of a national survey. Dig Dis Sci. 2019;64(6):1439-47.
Lipowska A et al. Ergonomics in the unit: Modeling the environment around the endoscopist. Tech Innov Gastrointest Endosc. 2021;23(3):256-62.
Park A et al. Intraoperative “Micro Breaks” with a targeted stretching enhance surgeon physical function and mental focus: A multicenter cohort study. Ann Surg. 2017;265(2):340-6.
Shergill A et al. Ergonomic endoscopy: An oxymoron or realistic goal? Gastrointest Endosc. 2019;90(6):966-70.
Really? Cancer screening doesn’t save lives?
This transcript from Impact Factor has been edited for clarity.
If you are my age or older, and like me, you are something of a rule follower, then you’re getting screened for various cancers.
Colonoscopies, mammograms, cervical cancer screening, chest CTs for people with a significant smoking history. The tests are done and usually, but not always, they are negative. And if positive, usually, but not always, follow-up tests are negative, and if they aren’t and a new cancer is diagnosed you tell yourself, Well, at least we caught it early. Isn’t it good that I’m a rule follower? My life was just saved.
But it turns out, proving that cancer screening actually saves lives is quite difficult. Is it possible that all this screening is for nothing?
The benefits, risks, or perhaps futility of cancer screening is in the news this week because of this article, appearing in JAMA Internal Medicine.
It’s a meta-analysis of very specific randomized trials of cancer screening modalities and concludes that, with the exception of sigmoidoscopy for colon cancer screening, none of them meaningfully change life expectancy.
Now – a bit of inside baseball here – I almost never choose to discuss meta-analyses on Impact Factor. It’s hard enough to dig deep into the methodology of a single study, but with a meta-analysis, you’re sort of obligated to review all the included studies, and, what’s worse, the studies that were not included but might bear on the central question.
In this case, though, the topic is important enough to think about a bit more, and the conclusions have large enough implications for public health that we should question them a bit.
First, let’s run down the study as presented.
The authors searched for randomized trials of cancer screening modalities. This is important, and I think appropriate. They wanted studies that took some people and assigned them to screening, and some people to no screening – avoiding the confounding that would come from observational data (rule followers like me tend to live longer owing to a variety of healthful behaviors, not just cancer screening).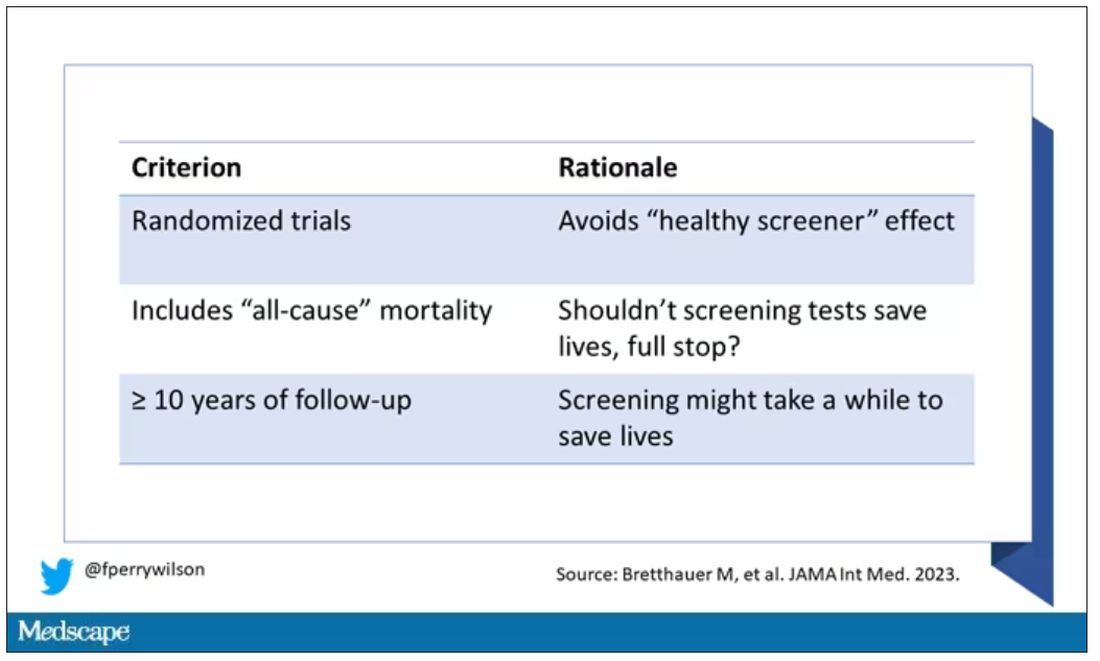
They didn’t stop at just randomized trials, though. They wanted trials that reported on all-cause, not cancer-specific, mortality. We’ll dig into the distinction in a sec. Finally, they wanted trials with at least 10 years of follow-up time.
These are pretty strict criteria – and after applying that filter, we are left with a grand total of 18 studies to analyze. Most were in the colon cancer space; only two studies met criteria for mammography screening.
Right off the bat, this raises concerns to me. In the universe of high-quality studies of cancer screening modalities, this is just the tip of the iceberg. And the results of meta-analyses are always dependent on the included studies – definitionally.
The results as presented are compelling.
(Side note: Averages are tricky here. It’s not like everyone who gets screened gets 110 extra days. Most people get nothing, and some people – those whose colon cancer was detected early – get a bunch of extra days.)
And a thing about meta-analysis: Meeting the criteria to be included in a meta-analysis does not necessarily mean the study was a good one. For example, one of the two mammography screening studies included is this one, from Miller and colleagues.
On the surface, it looks good – a large randomized trial of mammography screening in Canada, with long-term follow-up including all-cause mortality. Showing, by the way, no effect of screening on either breast cancer–specific or all-cause mortality.
But that study came under a lot of criticism owing to allegations that randomization was broken and women with palpable breast masses were preferentially put into the mammography group, making those outcomes worse.
The authors of the current meta-analysis don’t mention this. Indeed, they state that they don’t perform any assessments of the quality of the included studies.
But I don’t want to criticize all the included studies. Let’s think bigger picture.
Randomized trials of screening for cancers like colon, breast, and lung cancer in smokers have generally shown that those randomized to screening had lower target-cancer–specific mortality. Across all the randomized mammography studies, for example, women randomized to mammography were about 20% less likely to die of breast cancer than were those who were randomized to not be screened – particularly among those above age 50.
But it’s true that all-cause mortality, on the whole, has not differed statistically between those randomized to mammography vs. no mammography. What’s the deal?
Well, the authors of the meta-analysis engage in some zero-sum thinking here. They say that if it is true that screening tests reduce cancer-specific deaths, but all-cause mortality is not different, screening tests must increase mortality due to other causes. How? They cite colonic perforation during colonoscopy as an example of a harm that could lead to earlier death, which makes some sense. For mammogram and other less invasive screening modalities, they suggest that the stress and anxiety associated with screening might increase the risk for death – this is a bit harder for me to defend.
The thing is, statistics really isn’t a zero-sum game. It’s a question of signal vs. noise. Take breast cancer, for example. Without screening, about 3.2% of women in this country would die of breast cancer. With screening, 2.8% would die (that’s a 20% reduction on the relative scale). The truth is, most women don’t die of breast cancer. Most people don’t die of colon cancer. Even most smokers don’t die of lung cancer. Most people die of heart disease. And then cancer – but there are a lot of cancers out there, and only a handful have decent screening tests.
In other words, the screening tests are unlikely to help most people because most people will not die of the particular type of cancer being screened for. But it will help some small number of those people being screened a lot, potentially saving their lives. If we knew who those people were in advance, it would be great, but then I suppose we wouldn’t need the screening test in the first place.
It’s not fair, then, to say that mammography increases non–breast cancer causes of death. In reality, it’s just that the impact of mammography on all-cause mortality is washed out by the random noise inherent to studying a sample of individuals rather than the entire population.
I’m reminded of that old story about the girl on the beach after a storm, throwing beached starfish back into the water. Someone comes by and says, “Why are you doing that? There are millions of starfish here – it doesn’t matter if you throw a few back.” And she says, “It matters for this one.”
There are other issues with aggregating data like these and concluding that there is no effect on all-cause mortality. For one, it assumes the people randomized to no screening never got screening. Most of these studies lasted 5-10 years, some with longer follow-up, but many people in the no-screening arm may have been screened as recommendations have changed. That would tend to bias the results against screening because the so-called control group, well, isn’t.
It also fails to acknowledge the reality that screening for disease can be thought of as a package deal. Instead of asking whether screening for breast cancer, and colon cancer, and lung cancer individually saves lives, the real relevant question is whether a policy of screening for cancer in general saves lives. And that hasn’t been studied very broadly, except in one trial looking at screening for four cancers. That study is in this meta-analysis and, interestingly, seems to suggest that the policy does extend life – by 123 days. Again, be careful how you think about that average.
I don’t want to be an absolutist here. Whether these screening tests are a good idea or not is actually a moving target. As treatment for cancer gets better, detecting cancer early may not be as important. As new screening modalities emerge, older ones may not be preferable any longer. Better testing, genetic or otherwise, might allow us to tailor screening more narrowly than the population-based approach we have now.
But I worry that a meta-analysis like this, which concludes that screening doesn’t help on the basis of a handful of studies – without acknowledgment of the signal-to-noise problem, without accounting for screening in the control group, without acknowledging that screening should be thought of as a package – will lead some people to make the decision to forgo screening. for, say, 49 out of 50 of them, that may be fine. But for 1 out of 50 or so, well, it matters for that one.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript from Impact Factor has been edited for clarity.
If you are my age or older, and like me, you are something of a rule follower, then you’re getting screened for various cancers.
Colonoscopies, mammograms, cervical cancer screening, chest CTs for people with a significant smoking history. The tests are done and usually, but not always, they are negative. And if positive, usually, but not always, follow-up tests are negative, and if they aren’t and a new cancer is diagnosed you tell yourself, Well, at least we caught it early. Isn’t it good that I’m a rule follower? My life was just saved.
But it turns out, proving that cancer screening actually saves lives is quite difficult. Is it possible that all this screening is for nothing?
The benefits, risks, or perhaps futility of cancer screening is in the news this week because of this article, appearing in JAMA Internal Medicine.
It’s a meta-analysis of very specific randomized trials of cancer screening modalities and concludes that, with the exception of sigmoidoscopy for colon cancer screening, none of them meaningfully change life expectancy.
Now – a bit of inside baseball here – I almost never choose to discuss meta-analyses on Impact Factor. It’s hard enough to dig deep into the methodology of a single study, but with a meta-analysis, you’re sort of obligated to review all the included studies, and, what’s worse, the studies that were not included but might bear on the central question.
In this case, though, the topic is important enough to think about a bit more, and the conclusions have large enough implications for public health that we should question them a bit.
First, let’s run down the study as presented.
The authors searched for randomized trials of cancer screening modalities. This is important, and I think appropriate. They wanted studies that took some people and assigned them to screening, and some people to no screening – avoiding the confounding that would come from observational data (rule followers like me tend to live longer owing to a variety of healthful behaviors, not just cancer screening).
They didn’t stop at just randomized trials, though. They wanted trials that reported on all-cause, not cancer-specific, mortality. We’ll dig into the distinction in a sec. Finally, they wanted trials with at least 10 years of follow-up time.
These are pretty strict criteria – and after applying that filter, we are left with a grand total of 18 studies to analyze. Most were in the colon cancer space; only two studies met criteria for mammography screening.
Right off the bat, this raises concerns to me. In the universe of high-quality studies of cancer screening modalities, this is just the tip of the iceberg. And the results of meta-analyses are always dependent on the included studies – definitionally.
The results as presented are compelling.
(Side note: Averages are tricky here. It’s not like everyone who gets screened gets 110 extra days. Most people get nothing, and some people – those whose colon cancer was detected early – get a bunch of extra days.)
And a thing about meta-analysis: Meeting the criteria to be included in a meta-analysis does not necessarily mean the study was a good one. For example, one of the two mammography screening studies included is this one, from Miller and colleagues.
On the surface, it looks good – a large randomized trial of mammography screening in Canada, with long-term follow-up including all-cause mortality. Showing, by the way, no effect of screening on either breast cancer–specific or all-cause mortality.
But that study came under a lot of criticism owing to allegations that randomization was broken and women with palpable breast masses were preferentially put into the mammography group, making those outcomes worse.
The authors of the current meta-analysis don’t mention this. Indeed, they state that they don’t perform any assessments of the quality of the included studies.
But I don’t want to criticize all the included studies. Let’s think bigger picture.
Randomized trials of screening for cancers like colon, breast, and lung cancer in smokers have generally shown that those randomized to screening had lower target-cancer–specific mortality. Across all the randomized mammography studies, for example, women randomized to mammography were about 20% less likely to die of breast cancer than were those who were randomized to not be screened – particularly among those above age 50.
But it’s true that all-cause mortality, on the whole, has not differed statistically between those randomized to mammography vs. no mammography. What’s the deal?
Well, the authors of the meta-analysis engage in some zero-sum thinking here. They say that if it is true that screening tests reduce cancer-specific deaths, but all-cause mortality is not different, screening tests must increase mortality due to other causes. How? They cite colonic perforation during colonoscopy as an example of a harm that could lead to earlier death, which makes some sense. For mammogram and other less invasive screening modalities, they suggest that the stress and anxiety associated with screening might increase the risk for death – this is a bit harder for me to defend.
The thing is, statistics really isn’t a zero-sum game. It’s a question of signal vs. noise. Take breast cancer, for example. Without screening, about 3.2% of women in this country would die of breast cancer. With screening, 2.8% would die (that’s a 20% reduction on the relative scale). The truth is, most women don’t die of breast cancer. Most people don’t die of colon cancer. Even most smokers don’t die of lung cancer. Most people die of heart disease. And then cancer – but there are a lot of cancers out there, and only a handful have decent screening tests.
In other words, the screening tests are unlikely to help most people because most people will not die of the particular type of cancer being screened for. But it will help some small number of those people being screened a lot, potentially saving their lives. If we knew who those people were in advance, it would be great, but then I suppose we wouldn’t need the screening test in the first place.
It’s not fair, then, to say that mammography increases non–breast cancer causes of death. In reality, it’s just that the impact of mammography on all-cause mortality is washed out by the random noise inherent to studying a sample of individuals rather than the entire population.
I’m reminded of that old story about the girl on the beach after a storm, throwing beached starfish back into the water. Someone comes by and says, “Why are you doing that? There are millions of starfish here – it doesn’t matter if you throw a few back.” And she says, “It matters for this one.”
There are other issues with aggregating data like these and concluding that there is no effect on all-cause mortality. For one, it assumes the people randomized to no screening never got screening. Most of these studies lasted 5-10 years, some with longer follow-up, but many people in the no-screening arm may have been screened as recommendations have changed. That would tend to bias the results against screening because the so-called control group, well, isn’t.
It also fails to acknowledge the reality that screening for disease can be thought of as a package deal. Instead of asking whether screening for breast cancer, and colon cancer, and lung cancer individually saves lives, the real relevant question is whether a policy of screening for cancer in general saves lives. And that hasn’t been studied very broadly, except in one trial looking at screening for four cancers. That study is in this meta-analysis and, interestingly, seems to suggest that the policy does extend life – by 123 days. Again, be careful how you think about that average.
I don’t want to be an absolutist here. Whether these screening tests are a good idea or not is actually a moving target. As treatment for cancer gets better, detecting cancer early may not be as important. As new screening modalities emerge, older ones may not be preferable any longer. Better testing, genetic or otherwise, might allow us to tailor screening more narrowly than the population-based approach we have now.
But I worry that a meta-analysis like this, which concludes that screening doesn’t help on the basis of a handful of studies – without acknowledgment of the signal-to-noise problem, without accounting for screening in the control group, without acknowledging that screening should be thought of as a package – will lead some people to make the decision to forgo screening. for, say, 49 out of 50 of them, that may be fine. But for 1 out of 50 or so, well, it matters for that one.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript from Impact Factor has been edited for clarity.
If you are my age or older, and like me, you are something of a rule follower, then you’re getting screened for various cancers.
Colonoscopies, mammograms, cervical cancer screening, chest CTs for people with a significant smoking history. The tests are done and usually, but not always, they are negative. And if positive, usually, but not always, follow-up tests are negative, and if they aren’t and a new cancer is diagnosed you tell yourself, Well, at least we caught it early. Isn’t it good that I’m a rule follower? My life was just saved.
But it turns out, proving that cancer screening actually saves lives is quite difficult. Is it possible that all this screening is for nothing?
The benefits, risks, or perhaps futility of cancer screening is in the news this week because of this article, appearing in JAMA Internal Medicine.
It’s a meta-analysis of very specific randomized trials of cancer screening modalities and concludes that, with the exception of sigmoidoscopy for colon cancer screening, none of them meaningfully change life expectancy.
Now – a bit of inside baseball here – I almost never choose to discuss meta-analyses on Impact Factor. It’s hard enough to dig deep into the methodology of a single study, but with a meta-analysis, you’re sort of obligated to review all the included studies, and, what’s worse, the studies that were not included but might bear on the central question.
In this case, though, the topic is important enough to think about a bit more, and the conclusions have large enough implications for public health that we should question them a bit.
First, let’s run down the study as presented.
The authors searched for randomized trials of cancer screening modalities. This is important, and I think appropriate. They wanted studies that took some people and assigned them to screening, and some people to no screening – avoiding the confounding that would come from observational data (rule followers like me tend to live longer owing to a variety of healthful behaviors, not just cancer screening).
They didn’t stop at just randomized trials, though. They wanted trials that reported on all-cause, not cancer-specific, mortality. We’ll dig into the distinction in a sec. Finally, they wanted trials with at least 10 years of follow-up time.
These are pretty strict criteria – and after applying that filter, we are left with a grand total of 18 studies to analyze. Most were in the colon cancer space; only two studies met criteria for mammography screening.
Right off the bat, this raises concerns to me. In the universe of high-quality studies of cancer screening modalities, this is just the tip of the iceberg. And the results of meta-analyses are always dependent on the included studies – definitionally.
The results as presented are compelling.
(Side note: Averages are tricky here. It’s not like everyone who gets screened gets 110 extra days. Most people get nothing, and some people – those whose colon cancer was detected early – get a bunch of extra days.)
And a thing about meta-analysis: Meeting the criteria to be included in a meta-analysis does not necessarily mean the study was a good one. For example, one of the two mammography screening studies included is this one, from Miller and colleagues.
On the surface, it looks good – a large randomized trial of mammography screening in Canada, with long-term follow-up including all-cause mortality. Showing, by the way, no effect of screening on either breast cancer–specific or all-cause mortality.
But that study came under a lot of criticism owing to allegations that randomization was broken and women with palpable breast masses were preferentially put into the mammography group, making those outcomes worse.
The authors of the current meta-analysis don’t mention this. Indeed, they state that they don’t perform any assessments of the quality of the included studies.
But I don’t want to criticize all the included studies. Let’s think bigger picture.
Randomized trials of screening for cancers like colon, breast, and lung cancer in smokers have generally shown that those randomized to screening had lower target-cancer–specific mortality. Across all the randomized mammography studies, for example, women randomized to mammography were about 20% less likely to die of breast cancer than were those who were randomized to not be screened – particularly among those above age 50.
But it’s true that all-cause mortality, on the whole, has not differed statistically between those randomized to mammography vs. no mammography. What’s the deal?
Well, the authors of the meta-analysis engage in some zero-sum thinking here. They say that if it is true that screening tests reduce cancer-specific deaths, but all-cause mortality is not different, screening tests must increase mortality due to other causes. How? They cite colonic perforation during colonoscopy as an example of a harm that could lead to earlier death, which makes some sense. For mammogram and other less invasive screening modalities, they suggest that the stress and anxiety associated with screening might increase the risk for death – this is a bit harder for me to defend.
The thing is, statistics really isn’t a zero-sum game. It’s a question of signal vs. noise. Take breast cancer, for example. Without screening, about 3.2% of women in this country would die of breast cancer. With screening, 2.8% would die (that’s a 20% reduction on the relative scale). The truth is, most women don’t die of breast cancer. Most people don’t die of colon cancer. Even most smokers don’t die of lung cancer. Most people die of heart disease. And then cancer – but there are a lot of cancers out there, and only a handful have decent screening tests.
In other words, the screening tests are unlikely to help most people because most people will not die of the particular type of cancer being screened for. But it will help some small number of those people being screened a lot, potentially saving their lives. If we knew who those people were in advance, it would be great, but then I suppose we wouldn’t need the screening test in the first place.
It’s not fair, then, to say that mammography increases non–breast cancer causes of death. In reality, it’s just that the impact of mammography on all-cause mortality is washed out by the random noise inherent to studying a sample of individuals rather than the entire population.
I’m reminded of that old story about the girl on the beach after a storm, throwing beached starfish back into the water. Someone comes by and says, “Why are you doing that? There are millions of starfish here – it doesn’t matter if you throw a few back.” And she says, “It matters for this one.”
There are other issues with aggregating data like these and concluding that there is no effect on all-cause mortality. For one, it assumes the people randomized to no screening never got screening. Most of these studies lasted 5-10 years, some with longer follow-up, but many people in the no-screening arm may have been screened as recommendations have changed. That would tend to bias the results against screening because the so-called control group, well, isn’t.
It also fails to acknowledge the reality that screening for disease can be thought of as a package deal. Instead of asking whether screening for breast cancer, and colon cancer, and lung cancer individually saves lives, the real relevant question is whether a policy of screening for cancer in general saves lives. And that hasn’t been studied very broadly, except in one trial looking at screening for four cancers. That study is in this meta-analysis and, interestingly, seems to suggest that the policy does extend life – by 123 days. Again, be careful how you think about that average.
I don’t want to be an absolutist here. Whether these screening tests are a good idea or not is actually a moving target. As treatment for cancer gets better, detecting cancer early may not be as important. As new screening modalities emerge, older ones may not be preferable any longer. Better testing, genetic or otherwise, might allow us to tailor screening more narrowly than the population-based approach we have now.
But I worry that a meta-analysis like this, which concludes that screening doesn’t help on the basis of a handful of studies – without acknowledgment of the signal-to-noise problem, without accounting for screening in the control group, without acknowledging that screening should be thought of as a package – will lead some people to make the decision to forgo screening. for, say, 49 out of 50 of them, that may be fine. But for 1 out of 50 or so, well, it matters for that one.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s not an assembly line
A lot of businesses benefit from being in private equity funds.
Health care isn’t one of them, and a recent report found that
This really shouldn’t surprise anyone. Such funds may offer glittering phrases like “improved technology” and “greater efficiency” but the bottom line is that they’re run by – and for – the shareholders. The majority of them aren’t going to be medical people or realize that you can’t run a medical practice like it’s a clothing retailer or electronic car manufacturer.
I’m not saying medicine isn’t a business – it is. I depend on my little practice to support three families, so keeping it in the black is important. But it also needs to run well to do that. Measures to increase revenue, like cutting my staff down (there are only two of them) or overbooking patients would seriously impact me effectively doing my part, which is playing doctor.
You can predict pretty accurately how long it will take to put a motor and bumper assembly on a specific model of car, but you can’t do that in medicine because people aren’t standardized. Even if you control variables such as same sex, age, and diagnosis, personalities vary widely, as do treatment decisions, questions they’ll have, and the “oh, another thing” factor.
That doesn’t happen at a bottling plant.
In the business model of health care, you’re hoping revenue will pay overhead and a reasonable salary for everyone. But when you add a private equity firm in, the shareholders also expect to be paid. Which means either revenue has to go up significantly, or costs have to be cut (layoffs, short staffing, reduced benefits, etc.), or a combination of both.
Regardless of which option is chosen, it isn’t good for the medical staff or the patients. Increasing the number of people seen in a given amount of time per doctor may be good for the shareholders, but it’s not good for the doctor or the person being cared for. Think of Lucy and Ethyl at the chocolate factory.
Even in an auto factory, if you speed up the rate of cars going through the assembly line, sooner or later mistakes will be made. Humans can’t keep up, and even robots will make errors if things aren’t aligned correctly, or are a few seconds ahead or behind the program. This is why they (hopefully) have quality control, to try and catch those things before they’re on the road.
Of course, cars are more easily fixable. When the mistake is found you repair or replace the part. You can’t do that as easily in people, and when serious mistakes happen it’s the doctor who’s held at fault – not the shareholders who pressured him or her to see patients faster and with less support.
Unfortunately, this is the way the current trend is going. The more people who are involved in the practice of medicine, in person or behind the scenes, the smaller each slice of the pie gets.
That’s not good for the patient, who’s the person at the center of it all and the reason why we’re here.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A lot of businesses benefit from being in private equity funds.
Health care isn’t one of them, and a recent report found that
This really shouldn’t surprise anyone. Such funds may offer glittering phrases like “improved technology” and “greater efficiency” but the bottom line is that they’re run by – and for – the shareholders. The majority of them aren’t going to be medical people or realize that you can’t run a medical practice like it’s a clothing retailer or electronic car manufacturer.
I’m not saying medicine isn’t a business – it is. I depend on my little practice to support three families, so keeping it in the black is important. But it also needs to run well to do that. Measures to increase revenue, like cutting my staff down (there are only two of them) or overbooking patients would seriously impact me effectively doing my part, which is playing doctor.
You can predict pretty accurately how long it will take to put a motor and bumper assembly on a specific model of car, but you can’t do that in medicine because people aren’t standardized. Even if you control variables such as same sex, age, and diagnosis, personalities vary widely, as do treatment decisions, questions they’ll have, and the “oh, another thing” factor.
That doesn’t happen at a bottling plant.
In the business model of health care, you’re hoping revenue will pay overhead and a reasonable salary for everyone. But when you add a private equity firm in, the shareholders also expect to be paid. Which means either revenue has to go up significantly, or costs have to be cut (layoffs, short staffing, reduced benefits, etc.), or a combination of both.
Regardless of which option is chosen, it isn’t good for the medical staff or the patients. Increasing the number of people seen in a given amount of time per doctor may be good for the shareholders, but it’s not good for the doctor or the person being cared for. Think of Lucy and Ethyl at the chocolate factory.
Even in an auto factory, if you speed up the rate of cars going through the assembly line, sooner or later mistakes will be made. Humans can’t keep up, and even robots will make errors if things aren’t aligned correctly, or are a few seconds ahead or behind the program. This is why they (hopefully) have quality control, to try and catch those things before they’re on the road.
Of course, cars are more easily fixable. When the mistake is found you repair or replace the part. You can’t do that as easily in people, and when serious mistakes happen it’s the doctor who’s held at fault – not the shareholders who pressured him or her to see patients faster and with less support.
Unfortunately, this is the way the current trend is going. The more people who are involved in the practice of medicine, in person or behind the scenes, the smaller each slice of the pie gets.
That’s not good for the patient, who’s the person at the center of it all and the reason why we’re here.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A lot of businesses benefit from being in private equity funds.
Health care isn’t one of them, and a recent report found that
This really shouldn’t surprise anyone. Such funds may offer glittering phrases like “improved technology” and “greater efficiency” but the bottom line is that they’re run by – and for – the shareholders. The majority of them aren’t going to be medical people or realize that you can’t run a medical practice like it’s a clothing retailer or electronic car manufacturer.
I’m not saying medicine isn’t a business – it is. I depend on my little practice to support three families, so keeping it in the black is important. But it also needs to run well to do that. Measures to increase revenue, like cutting my staff down (there are only two of them) or overbooking patients would seriously impact me effectively doing my part, which is playing doctor.
You can predict pretty accurately how long it will take to put a motor and bumper assembly on a specific model of car, but you can’t do that in medicine because people aren’t standardized. Even if you control variables such as same sex, age, and diagnosis, personalities vary widely, as do treatment decisions, questions they’ll have, and the “oh, another thing” factor.
That doesn’t happen at a bottling plant.
In the business model of health care, you’re hoping revenue will pay overhead and a reasonable salary for everyone. But when you add a private equity firm in, the shareholders also expect to be paid. Which means either revenue has to go up significantly, or costs have to be cut (layoffs, short staffing, reduced benefits, etc.), or a combination of both.
Regardless of which option is chosen, it isn’t good for the medical staff or the patients. Increasing the number of people seen in a given amount of time per doctor may be good for the shareholders, but it’s not good for the doctor or the person being cared for. Think of Lucy and Ethyl at the chocolate factory.
Even in an auto factory, if you speed up the rate of cars going through the assembly line, sooner or later mistakes will be made. Humans can’t keep up, and even robots will make errors if things aren’t aligned correctly, or are a few seconds ahead or behind the program. This is why they (hopefully) have quality control, to try and catch those things before they’re on the road.
Of course, cars are more easily fixable. When the mistake is found you repair or replace the part. You can’t do that as easily in people, and when serious mistakes happen it’s the doctor who’s held at fault – not the shareholders who pressured him or her to see patients faster and with less support.
Unfortunately, this is the way the current trend is going. The more people who are involved in the practice of medicine, in person or behind the scenes, the smaller each slice of the pie gets.
That’s not good for the patient, who’s the person at the center of it all and the reason why we’re here.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A nurse’s view: Blood test for severe preeclampsia will save lives
There is amazing news for the world of obstetrics and for all pregnant women. Severe preeclampsia is a critical obstetrical condition that can have serious outcomes for a mother and baby. It can lead to eclampsia, an obstetrical emergency, which often results in death of the mother and/or baby.
Based on research published in the Journal of the American Heart Association, the incidence of new‐onset hypertensive disorders of pregnancy (gestational hypertension and preeclampsia/eclampsia) have nearly doubled in the United States from 2007 to 2019. And they continue to climb.
According to the Preeclampsia Foundation, 5%-8% of all pregnancies in the United States will result in preeclampsia. Black women are at a 60% higher risk than white women, and according to various sources, other risk groups include those who became pregnant via in vitro fertilization, mothers of multiples (twins and triplets), women with gestational diabetes, women over age 35, women with chronic hypertension, obesity, polycystic ovary syndrome, sickle cell disease, rheumatoid arthritis, lupus, migraines, antiphospholipid syndrome, previous pregnancy with preeclampsia, family history, and scleroderma.
Screening and treatment
Preeclampsia is a multiorgan disease of pregnancy, and can be mild, but may quickly progress to severe, which can be life-threatening for mother and baby. It was previously referred to as toxemia or the high blood pressure disease of pregnancy. It primarily involves the cardiovascular, neurologic and renal systems, and the liver. Patients typically present with elevated blood pressures, but other symptoms may include headache, swelling of hands and feet, blurry/double vision or seeing spots, nausea/vomiting, and epigastric pain. It is diagnosed with elevated blood pressures, blood work, and protein in the urine.
Early screening for preeclampsia is done in the first trimester. Presently, a combination of prenatal blood work, blood pressure monitoring, and recognition of high-risk groups is used to determine a treatment plan going forward. The American Congress of Obstetricians and Gynecologists recommends women that fall into this group for potentially developing preeclampsia take daily aspirin as a preventative measure.
In its milder form, a pregnant woman can be observed as an outpatient – monitored with antepartum testing, lab work, and patient education to report significant symptoms as listed above. Teaching patients about fetal kick counts to monitor their baby’s movements is equally important. Women with mild preeclampsia usually can safely deliver at term, being induced between 37-39 weeks’ gestation.
On the other hand, if mild preeclampsia progresses to severe preeclampsia, delivery may be preterm for the safety of mother and baby. Severe preeclampsia can lead to maternal organ damage, seizures, and even death of mother and/or baby.
About 20% of women with severe preeclampsia will develop HELLP (Hemolysis, Elevated Liver enzymes, and Low Platelets) syndrome, a life-threatening disease that often warrants immediate delivery. According to the National Library of Medicine, the mortality rate of women with HELLP syndrome is up to 24% and the perinatal death rate is up as high as 37%. These serious conditions can cause ineffective maternal clotting, liver rupture, placental abruption, and postpartum hemorrhage. It is most prevalent in the third trimester but can occur within 48 hours of delivery.
The only cure for preeclampsia in any form is delivery.
Patients with severe preeclampsia are hospitalized until delivery – sometimes a few days to a couple of weeks. Mother and baby are closely watched for further progression, including signs of organ damage in the mother and changes to the well-being of the baby. If the mother’s health is severely compromised, then the baby will be compromised as well. A preterm delivery may be necessary.
Impact of the new test
The National Institute of Health states that preterm babies born from preeclamptic mothers can suffer many health problems including cerebral palsy, deafness, blindness, epilepsy, and a host of other respiratory, cardiovascular, and endocrine issues. But the biggest issue is preterm birth, defined as birth before 37 weeks gestation. Being born preterm can require a long stay in the intensive care nursery.
This is where the first-of-its-kind prognostic blood test comes into play. The test’s ability to predict severe preeclampsia within 2 weeks can help save lives. The test can offer health care providers the ability to administer steroids for fetal lung maturity before delivery and be more prepared to care for what could be a very compromised newborn.
The blood test, which is recommended between 23-35 weeks gestation, involves analyzing a ratio between two proteins from the placenta, sFlt1 and PIGF. The higher the ratio, the higher the risk that severe preeclampsia will develop. Results can be available within 30 minutes, which is critical when contemplating treatment.
An example of the use of this ratio is illustrated with chronic hypertension in pregnancy, which is defined as elevated blood pressure before 20 weeks or even before conception. Since chronic hypertension can be a primary precursor to preeclampsia, patients with this condition are at higher risk. The FDA-approved blood test would be helpful in determining the plan of care; that is, delivery versus hospitalization versus monitor as an outpatient.
With a positive test result, a pregnant woman can be immediately hospitalized where she can get the care she and baby need as they await delivery. Since health care providers already know the high-risk groups, surveillance can begin early, utilizing this blood test to predict the progression to severe preeclampsia. Conversely, if the test is negative, a treatment plan can be made as an outpatient and the pregnancy continues.
Not all hospitals are equipped to care for premature babies. If delivery is not imminent, providers can use this blood test to identify those that should be transferred to a tertiary center for observation and monitoring. Mother and baby would then not be separated after birth.
We really don’t know who will develop severe preeclampsia and who won’t. This new blood test will be a critical tool as pregnant patients go through their second and third trimesters. It will be especially pivotal for these women, but important for all pregnant women in reducing maternal and fetal mortality and morbidity.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
There is amazing news for the world of obstetrics and for all pregnant women. Severe preeclampsia is a critical obstetrical condition that can have serious outcomes for a mother and baby. It can lead to eclampsia, an obstetrical emergency, which often results in death of the mother and/or baby.
Based on research published in the Journal of the American Heart Association, the incidence of new‐onset hypertensive disorders of pregnancy (gestational hypertension and preeclampsia/eclampsia) have nearly doubled in the United States from 2007 to 2019. And they continue to climb.
According to the Preeclampsia Foundation, 5%-8% of all pregnancies in the United States will result in preeclampsia. Black women are at a 60% higher risk than white women, and according to various sources, other risk groups include those who became pregnant via in vitro fertilization, mothers of multiples (twins and triplets), women with gestational diabetes, women over age 35, women with chronic hypertension, obesity, polycystic ovary syndrome, sickle cell disease, rheumatoid arthritis, lupus, migraines, antiphospholipid syndrome, previous pregnancy with preeclampsia, family history, and scleroderma.
Screening and treatment
Preeclampsia is a multiorgan disease of pregnancy, and can be mild, but may quickly progress to severe, which can be life-threatening for mother and baby. It was previously referred to as toxemia or the high blood pressure disease of pregnancy. It primarily involves the cardiovascular, neurologic and renal systems, and the liver. Patients typically present with elevated blood pressures, but other symptoms may include headache, swelling of hands and feet, blurry/double vision or seeing spots, nausea/vomiting, and epigastric pain. It is diagnosed with elevated blood pressures, blood work, and protein in the urine.
Early screening for preeclampsia is done in the first trimester. Presently, a combination of prenatal blood work, blood pressure monitoring, and recognition of high-risk groups is used to determine a treatment plan going forward. The American Congress of Obstetricians and Gynecologists recommends women that fall into this group for potentially developing preeclampsia take daily aspirin as a preventative measure.
In its milder form, a pregnant woman can be observed as an outpatient – monitored with antepartum testing, lab work, and patient education to report significant symptoms as listed above. Teaching patients about fetal kick counts to monitor their baby’s movements is equally important. Women with mild preeclampsia usually can safely deliver at term, being induced between 37-39 weeks’ gestation.
On the other hand, if mild preeclampsia progresses to severe preeclampsia, delivery may be preterm for the safety of mother and baby. Severe preeclampsia can lead to maternal organ damage, seizures, and even death of mother and/or baby.
About 20% of women with severe preeclampsia will develop HELLP (Hemolysis, Elevated Liver enzymes, and Low Platelets) syndrome, a life-threatening disease that often warrants immediate delivery. According to the National Library of Medicine, the mortality rate of women with HELLP syndrome is up to 24% and the perinatal death rate is up as high as 37%. These serious conditions can cause ineffective maternal clotting, liver rupture, placental abruption, and postpartum hemorrhage. It is most prevalent in the third trimester but can occur within 48 hours of delivery.
The only cure for preeclampsia in any form is delivery.
Patients with severe preeclampsia are hospitalized until delivery – sometimes a few days to a couple of weeks. Mother and baby are closely watched for further progression, including signs of organ damage in the mother and changes to the well-being of the baby. If the mother’s health is severely compromised, then the baby will be compromised as well. A preterm delivery may be necessary.
Impact of the new test
The National Institute of Health states that preterm babies born from preeclamptic mothers can suffer many health problems including cerebral palsy, deafness, blindness, epilepsy, and a host of other respiratory, cardiovascular, and endocrine issues. But the biggest issue is preterm birth, defined as birth before 37 weeks gestation. Being born preterm can require a long stay in the intensive care nursery.
This is where the first-of-its-kind prognostic blood test comes into play. The test’s ability to predict severe preeclampsia within 2 weeks can help save lives. The test can offer health care providers the ability to administer steroids for fetal lung maturity before delivery and be more prepared to care for what could be a very compromised newborn.
The blood test, which is recommended between 23-35 weeks gestation, involves analyzing a ratio between two proteins from the placenta, sFlt1 and PIGF. The higher the ratio, the higher the risk that severe preeclampsia will develop. Results can be available within 30 minutes, which is critical when contemplating treatment.
An example of the use of this ratio is illustrated with chronic hypertension in pregnancy, which is defined as elevated blood pressure before 20 weeks or even before conception. Since chronic hypertension can be a primary precursor to preeclampsia, patients with this condition are at higher risk. The FDA-approved blood test would be helpful in determining the plan of care; that is, delivery versus hospitalization versus monitor as an outpatient.
With a positive test result, a pregnant woman can be immediately hospitalized where she can get the care she and baby need as they await delivery. Since health care providers already know the high-risk groups, surveillance can begin early, utilizing this blood test to predict the progression to severe preeclampsia. Conversely, if the test is negative, a treatment plan can be made as an outpatient and the pregnancy continues.
Not all hospitals are equipped to care for premature babies. If delivery is not imminent, providers can use this blood test to identify those that should be transferred to a tertiary center for observation and monitoring. Mother and baby would then not be separated after birth.
We really don’t know who will develop severe preeclampsia and who won’t. This new blood test will be a critical tool as pregnant patients go through their second and third trimesters. It will be especially pivotal for these women, but important for all pregnant women in reducing maternal and fetal mortality and morbidity.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
There is amazing news for the world of obstetrics and for all pregnant women. Severe preeclampsia is a critical obstetrical condition that can have serious outcomes for a mother and baby. It can lead to eclampsia, an obstetrical emergency, which often results in death of the mother and/or baby.
Based on research published in the Journal of the American Heart Association, the incidence of new‐onset hypertensive disorders of pregnancy (gestational hypertension and preeclampsia/eclampsia) have nearly doubled in the United States from 2007 to 2019. And they continue to climb.
According to the Preeclampsia Foundation, 5%-8% of all pregnancies in the United States will result in preeclampsia. Black women are at a 60% higher risk than white women, and according to various sources, other risk groups include those who became pregnant via in vitro fertilization, mothers of multiples (twins and triplets), women with gestational diabetes, women over age 35, women with chronic hypertension, obesity, polycystic ovary syndrome, sickle cell disease, rheumatoid arthritis, lupus, migraines, antiphospholipid syndrome, previous pregnancy with preeclampsia, family history, and scleroderma.
Screening and treatment
Preeclampsia is a multiorgan disease of pregnancy, and can be mild, but may quickly progress to severe, which can be life-threatening for mother and baby. It was previously referred to as toxemia or the high blood pressure disease of pregnancy. It primarily involves the cardiovascular, neurologic and renal systems, and the liver. Patients typically present with elevated blood pressures, but other symptoms may include headache, swelling of hands and feet, blurry/double vision or seeing spots, nausea/vomiting, and epigastric pain. It is diagnosed with elevated blood pressures, blood work, and protein in the urine.
Early screening for preeclampsia is done in the first trimester. Presently, a combination of prenatal blood work, blood pressure monitoring, and recognition of high-risk groups is used to determine a treatment plan going forward. The American Congress of Obstetricians and Gynecologists recommends women that fall into this group for potentially developing preeclampsia take daily aspirin as a preventative measure.
In its milder form, a pregnant woman can be observed as an outpatient – monitored with antepartum testing, lab work, and patient education to report significant symptoms as listed above. Teaching patients about fetal kick counts to monitor their baby’s movements is equally important. Women with mild preeclampsia usually can safely deliver at term, being induced between 37-39 weeks’ gestation.
On the other hand, if mild preeclampsia progresses to severe preeclampsia, delivery may be preterm for the safety of mother and baby. Severe preeclampsia can lead to maternal organ damage, seizures, and even death of mother and/or baby.
About 20% of women with severe preeclampsia will develop HELLP (Hemolysis, Elevated Liver enzymes, and Low Platelets) syndrome, a life-threatening disease that often warrants immediate delivery. According to the National Library of Medicine, the mortality rate of women with HELLP syndrome is up to 24% and the perinatal death rate is up as high as 37%. These serious conditions can cause ineffective maternal clotting, liver rupture, placental abruption, and postpartum hemorrhage. It is most prevalent in the third trimester but can occur within 48 hours of delivery.
The only cure for preeclampsia in any form is delivery.
Patients with severe preeclampsia are hospitalized until delivery – sometimes a few days to a couple of weeks. Mother and baby are closely watched for further progression, including signs of organ damage in the mother and changes to the well-being of the baby. If the mother’s health is severely compromised, then the baby will be compromised as well. A preterm delivery may be necessary.
Impact of the new test
The National Institute of Health states that preterm babies born from preeclamptic mothers can suffer many health problems including cerebral palsy, deafness, blindness, epilepsy, and a host of other respiratory, cardiovascular, and endocrine issues. But the biggest issue is preterm birth, defined as birth before 37 weeks gestation. Being born preterm can require a long stay in the intensive care nursery.
This is where the first-of-its-kind prognostic blood test comes into play. The test’s ability to predict severe preeclampsia within 2 weeks can help save lives. The test can offer health care providers the ability to administer steroids for fetal lung maturity before delivery and be more prepared to care for what could be a very compromised newborn.
The blood test, which is recommended between 23-35 weeks gestation, involves analyzing a ratio between two proteins from the placenta, sFlt1 and PIGF. The higher the ratio, the higher the risk that severe preeclampsia will develop. Results can be available within 30 minutes, which is critical when contemplating treatment.
An example of the use of this ratio is illustrated with chronic hypertension in pregnancy, which is defined as elevated blood pressure before 20 weeks or even before conception. Since chronic hypertension can be a primary precursor to preeclampsia, patients with this condition are at higher risk. The FDA-approved blood test would be helpful in determining the plan of care; that is, delivery versus hospitalization versus monitor as an outpatient.
With a positive test result, a pregnant woman can be immediately hospitalized where she can get the care she and baby need as they await delivery. Since health care providers already know the high-risk groups, surveillance can begin early, utilizing this blood test to predict the progression to severe preeclampsia. Conversely, if the test is negative, a treatment plan can be made as an outpatient and the pregnancy continues.
Not all hospitals are equipped to care for premature babies. If delivery is not imminent, providers can use this blood test to identify those that should be transferred to a tertiary center for observation and monitoring. Mother and baby would then not be separated after birth.
We really don’t know who will develop severe preeclampsia and who won’t. This new blood test will be a critical tool as pregnant patients go through their second and third trimesters. It will be especially pivotal for these women, but important for all pregnant women in reducing maternal and fetal mortality and morbidity.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
Answering the protein question when prescribing plant-based diets
Science supports the use of a whole food, predominantly plant-based dietary pattern for optimal health, including reduced risk for chronic disease, and best practice in treatment of leading chronic disease.
We’ve all heard it, and it’s understandable. Patients know that protein is essential for their health and strength, and animal foods have developed a reputation for being the premier protein sources that humans should prioritize through diet. But widespread misconceptions about human needs for protein have inaccurately equated animal food as the best and only sources of protein, augmented by fad diets and modern food marketing. All of this leads to confusion about how much protein people should actually consume and the quality of protein found in plant foods, making many patients reluctant to fully embrace a whole food, predominately plant-based diet.
To ensure that patients have all the facts when making dietary decisions, clinicians need to be prepared to respond to concerns about protein adequacy and quality with evidence-based information. A good starting point for these conversations is to assess how much protein patients are already consuming. A review of the 2015-2016 National Health and Nutrition Examination Survey found that women normally consume an average of 69 g and men an average of 97 g of protein daily.
As a general point of reference, the recommended dietary allowance for protein is about 0.8 g/kg of bodyweight (or 0.36 g/lb), which equates to about 52 g of protein per day for a 145-lb woman and 65 g for a 180-lb man. But for many patients, it may be best to get a more precise recommendation based upon age, gender and physical activity level by using a handy Department of Agriculture tool for health care professionals to calculate daily protein and other nutrient needs. Patients can also use one of countless apps to track their protein and other nutrient intake. By using the tool and a tracking app, both clinician and patients can be fully informed whether protein needs are being met.
The recommended daily allowances for protein are easily met by consuming a variety of whole plant foods, including a variety of minimally processed vegetables, fruits, whole grains, legumes, nuts, and seeds. One cup of cooked red lentils or black beans, for example, contains between 15 g and 18 g of protein. A quarter cup of almonds contains about 7 g of protein and one cup of cooked oats has 5 g.
What about those amino acids?
An area of contention around plant food protein is “complete versus incomplete protein,” terms used to describe whether a protein contains all nine essential amino acids that our bodies require from a single source. Animal food sources usually contain all the essential amino acids, whereas plant sources of protein may contain varying amounts of these amino acids or may even be missing some.
This leads to a misconception that someone adopting a diet of predominately plant food may have to stack or combine specific plant foods in a meal to ensure their protein intake includes an appropriate proportion of amino acids. But the process of protein breakdown turnover solves this problem. The body continuously breaks down protein and recombines it with amino acids stored in tissue for use when needed. Once absorbed by the small intestine, it doesn’t matter whether the protein or amino acids came from the same meal. As long as a person is eating a variety of plant-based protein sources, they will consume adequate amounts of all essential amino acids.
This is true even for athletes, older adults and pregnant women. It is also the position of the Academy of Nutrition and Dietetics that a whole-food, predominately plant-based eating pattern is appropriate for athletes and “all stages of the life cycle, including pregnancy, lactation, infancy, childhood, adolescence, older adulthood.”
The plant-based diet
For examples of healthy plant-based eating plans, The American College of Lifestyle Medicine offers a complimentary guide for a whole food, predominantly plant-based diet that demonstrates how easily the recommended dietary allowance of protein is satisfied. A breakfast of rolled oats, a lunch of bean burritos, and a dinner of mashed potatoes, with chickpeas with a couple snacks throughout the day, adds up to 71 g of protein. Other plant-based meal plans top 100 g or 90 g, with all meal plans meeting or surpassing recommended allowances.
Along with the protein, plant food delivers other beneficial nutrients and dietary components like fiber, antioxidants, anti-inflammatory properties, various vitamins and nutrients, and phytochemicals and vitamin D, without the saturated fats and sodium in meat. But U.S. adults get approximately two-thirds of their protein from animal sources, which lack fiber and have higher levels of saturated fats or sodium that can raise cholesterol and increase the risks for heart disease and stroke.
For clinicians, ACLM published a 10-part series of research white papers on the benefits of a whole food, plant-predominant dietary lifestyle and offers a catalogue of food as medicine continuing medical education and continuing education courses.
Patients hunger for knowledge about health-promoting nutrition but may have difficulty sorting myths from evidence-based facts. Each healthcare professional has an important and powerful opportunity to steer patients in a healthier direction through their diet.
Dr. Collings is director of lifestyle medicine, Silicon Valley Medical Development; President, American College of Lifestyle Medicine, Mountain View, Calif. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Science supports the use of a whole food, predominantly plant-based dietary pattern for optimal health, including reduced risk for chronic disease, and best practice in treatment of leading chronic disease.
We’ve all heard it, and it’s understandable. Patients know that protein is essential for their health and strength, and animal foods have developed a reputation for being the premier protein sources that humans should prioritize through diet. But widespread misconceptions about human needs for protein have inaccurately equated animal food as the best and only sources of protein, augmented by fad diets and modern food marketing. All of this leads to confusion about how much protein people should actually consume and the quality of protein found in plant foods, making many patients reluctant to fully embrace a whole food, predominately plant-based diet.
To ensure that patients have all the facts when making dietary decisions, clinicians need to be prepared to respond to concerns about protein adequacy and quality with evidence-based information. A good starting point for these conversations is to assess how much protein patients are already consuming. A review of the 2015-2016 National Health and Nutrition Examination Survey found that women normally consume an average of 69 g and men an average of 97 g of protein daily.
As a general point of reference, the recommended dietary allowance for protein is about 0.8 g/kg of bodyweight (or 0.36 g/lb), which equates to about 52 g of protein per day for a 145-lb woman and 65 g for a 180-lb man. But for many patients, it may be best to get a more precise recommendation based upon age, gender and physical activity level by using a handy Department of Agriculture tool for health care professionals to calculate daily protein and other nutrient needs. Patients can also use one of countless apps to track their protein and other nutrient intake. By using the tool and a tracking app, both clinician and patients can be fully informed whether protein needs are being met.
The recommended daily allowances for protein are easily met by consuming a variety of whole plant foods, including a variety of minimally processed vegetables, fruits, whole grains, legumes, nuts, and seeds. One cup of cooked red lentils or black beans, for example, contains between 15 g and 18 g of protein. A quarter cup of almonds contains about 7 g of protein and one cup of cooked oats has 5 g.
What about those amino acids?
An area of contention around plant food protein is “complete versus incomplete protein,” terms used to describe whether a protein contains all nine essential amino acids that our bodies require from a single source. Animal food sources usually contain all the essential amino acids, whereas plant sources of protein may contain varying amounts of these amino acids or may even be missing some.
This leads to a misconception that someone adopting a diet of predominately plant food may have to stack or combine specific plant foods in a meal to ensure their protein intake includes an appropriate proportion of amino acids. But the process of protein breakdown turnover solves this problem. The body continuously breaks down protein and recombines it with amino acids stored in tissue for use when needed. Once absorbed by the small intestine, it doesn’t matter whether the protein or amino acids came from the same meal. As long as a person is eating a variety of plant-based protein sources, they will consume adequate amounts of all essential amino acids.
This is true even for athletes, older adults and pregnant women. It is also the position of the Academy of Nutrition and Dietetics that a whole-food, predominately plant-based eating pattern is appropriate for athletes and “all stages of the life cycle, including pregnancy, lactation, infancy, childhood, adolescence, older adulthood.”
The plant-based diet
For examples of healthy plant-based eating plans, The American College of Lifestyle Medicine offers a complimentary guide for a whole food, predominantly plant-based diet that demonstrates how easily the recommended dietary allowance of protein is satisfied. A breakfast of rolled oats, a lunch of bean burritos, and a dinner of mashed potatoes, with chickpeas with a couple snacks throughout the day, adds up to 71 g of protein. Other plant-based meal plans top 100 g or 90 g, with all meal plans meeting or surpassing recommended allowances.
Along with the protein, plant food delivers other beneficial nutrients and dietary components like fiber, antioxidants, anti-inflammatory properties, various vitamins and nutrients, and phytochemicals and vitamin D, without the saturated fats and sodium in meat. But U.S. adults get approximately two-thirds of their protein from animal sources, which lack fiber and have higher levels of saturated fats or sodium that can raise cholesterol and increase the risks for heart disease and stroke.
For clinicians, ACLM published a 10-part series of research white papers on the benefits of a whole food, plant-predominant dietary lifestyle and offers a catalogue of food as medicine continuing medical education and continuing education courses.
Patients hunger for knowledge about health-promoting nutrition but may have difficulty sorting myths from evidence-based facts. Each healthcare professional has an important and powerful opportunity to steer patients in a healthier direction through their diet.
Dr. Collings is director of lifestyle medicine, Silicon Valley Medical Development; President, American College of Lifestyle Medicine, Mountain View, Calif. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Science supports the use of a whole food, predominantly plant-based dietary pattern for optimal health, including reduced risk for chronic disease, and best practice in treatment of leading chronic disease.
We’ve all heard it, and it’s understandable. Patients know that protein is essential for their health and strength, and animal foods have developed a reputation for being the premier protein sources that humans should prioritize through diet. But widespread misconceptions about human needs for protein have inaccurately equated animal food as the best and only sources of protein, augmented by fad diets and modern food marketing. All of this leads to confusion about how much protein people should actually consume and the quality of protein found in plant foods, making many patients reluctant to fully embrace a whole food, predominately plant-based diet.
To ensure that patients have all the facts when making dietary decisions, clinicians need to be prepared to respond to concerns about protein adequacy and quality with evidence-based information. A good starting point for these conversations is to assess how much protein patients are already consuming. A review of the 2015-2016 National Health and Nutrition Examination Survey found that women normally consume an average of 69 g and men an average of 97 g of protein daily.
As a general point of reference, the recommended dietary allowance for protein is about 0.8 g/kg of bodyweight (or 0.36 g/lb), which equates to about 52 g of protein per day for a 145-lb woman and 65 g for a 180-lb man. But for many patients, it may be best to get a more precise recommendation based upon age, gender and physical activity level by using a handy Department of Agriculture tool for health care professionals to calculate daily protein and other nutrient needs. Patients can also use one of countless apps to track their protein and other nutrient intake. By using the tool and a tracking app, both clinician and patients can be fully informed whether protein needs are being met.
The recommended daily allowances for protein are easily met by consuming a variety of whole plant foods, including a variety of minimally processed vegetables, fruits, whole grains, legumes, nuts, and seeds. One cup of cooked red lentils or black beans, for example, contains between 15 g and 18 g of protein. A quarter cup of almonds contains about 7 g of protein and one cup of cooked oats has 5 g.
What about those amino acids?
An area of contention around plant food protein is “complete versus incomplete protein,” terms used to describe whether a protein contains all nine essential amino acids that our bodies require from a single source. Animal food sources usually contain all the essential amino acids, whereas plant sources of protein may contain varying amounts of these amino acids or may even be missing some.
This leads to a misconception that someone adopting a diet of predominately plant food may have to stack or combine specific plant foods in a meal to ensure their protein intake includes an appropriate proportion of amino acids. But the process of protein breakdown turnover solves this problem. The body continuously breaks down protein and recombines it with amino acids stored in tissue for use when needed. Once absorbed by the small intestine, it doesn’t matter whether the protein or amino acids came from the same meal. As long as a person is eating a variety of plant-based protein sources, they will consume adequate amounts of all essential amino acids.
This is true even for athletes, older adults and pregnant women. It is also the position of the Academy of Nutrition and Dietetics that a whole-food, predominately plant-based eating pattern is appropriate for athletes and “all stages of the life cycle, including pregnancy, lactation, infancy, childhood, adolescence, older adulthood.”
The plant-based diet
For examples of healthy plant-based eating plans, The American College of Lifestyle Medicine offers a complimentary guide for a whole food, predominantly plant-based diet that demonstrates how easily the recommended dietary allowance of protein is satisfied. A breakfast of rolled oats, a lunch of bean burritos, and a dinner of mashed potatoes, with chickpeas with a couple snacks throughout the day, adds up to 71 g of protein. Other plant-based meal plans top 100 g or 90 g, with all meal plans meeting or surpassing recommended allowances.
Along with the protein, plant food delivers other beneficial nutrients and dietary components like fiber, antioxidants, anti-inflammatory properties, various vitamins and nutrients, and phytochemicals and vitamin D, without the saturated fats and sodium in meat. But U.S. adults get approximately two-thirds of their protein from animal sources, which lack fiber and have higher levels of saturated fats or sodium that can raise cholesterol and increase the risks for heart disease and stroke.
For clinicians, ACLM published a 10-part series of research white papers on the benefits of a whole food, plant-predominant dietary lifestyle and offers a catalogue of food as medicine continuing medical education and continuing education courses.
Patients hunger for knowledge about health-promoting nutrition but may have difficulty sorting myths from evidence-based facts. Each healthcare professional has an important and powerful opportunity to steer patients in a healthier direction through their diet.
Dr. Collings is director of lifestyle medicine, Silicon Valley Medical Development; President, American College of Lifestyle Medicine, Mountain View, Calif. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.