User login
Lasmiditan is associated with driving impairment
Philadelphia – according to research presented at the annual meeting of the American Headache Society. This impairment coincides with the time of peak drug concentration and is resolved by 8 hours after dosing.
Lasmiditan, a 5-HT1F receptor agonist, is under regulatory review as an acute treatment of migraine in adults. In two phase 3 trials, a significant proportion of participants who received the treatment were pain-free at 2 hours and free of their most bothersome symptom at 2 hours, compared with controls. The most common treatment-emergent adverse events (dizziness, paresthesia, somnolence, fatigue, and hypoaesthesia) reflected the drug’s penetration of the CNS. Because CNS-related adverse events could affect a patient’s ability to drive, the effects of lasmiditan on simulated driving were studied, consistent with regulatory guidance.
Two studies in healthy participants
Eric Pearlman, MD, PhD, senior medical director for neuroscience, U.S. Medical Affairs, at Eli Lilly, and colleagues conducted two simulated driving studies with crossover designs. In the first study, the researchers randomized 90 healthy subjects (mean age, 34.9 years) to 50 mg, 100 mg, or 200 mg of lasmiditan; 1 mg of alprazolam (an active control); or placebo. After appropriate washout periods, participants received each treatment in random order. At 1.5 hours after each treatment, participants underwent a driving assessment using a driving simulator.
In the second study, Dr. Pearlman and colleagues randomized 68 healthy subjects (mean age, 32.8 years) to 100 mg or 200 mg of lasmiditan, 50 mg of diphenhydramine (an active control), or placebo. Participants underwent driving assessments at 8, 12, and 24 hours after baseline. Participants randomized to lasmiditan received treatment at baseline, but participants randomized to diphenhydramine received treatment 2 hours before each driving assessment. The diphenhydramine arm was intended to test the sensitivity of the driving assessment to impairment, as was the alprazolam arm in the first study.
During the driving assessments, participants were asked to maintain a speed of 100 km/h and to stay in the middle of the lane. The simulated road included gentle curves and hills, and oncoming traffic appeared occasionally. Participants also completed a secondary attention task, mimicking real-world conditions. Each assessment took about 1 hour. The primary endpoint was the standard deviation of lateral position (SDLP), which is known colloquially as weaving. The investigators defined noninferiority to placebo as an SDLP of 4.4 cm or less.
Participants reported that they could drive safely
In the first study, Dr. Pearlman and colleagues reported a dose-related increase in SDLP at 1.5 hours post treatment. All three doses of lasmiditan were inferior to placebo, in terms of their effect on SDLP. In the second study, both doses of lasmiditan were noninferior to placebo at 8 hours, 12 hours, and 24 hours. In both studies, the positive control confirmed the assay’s sensitivity to driving impairment. Secondary endpoints in the second study did not indicate an association between lasmiditan and clinically meaningful driving impairment at 8, 12, and 24 hours after dosing.
Single oral doses of lasmiditan were absorbed rapidly. The median time to peak concentration was approximately 2 hours, and the mean elimination half-life was about 4.25 hours. Exposure to lasmiditan was approximately dose proportional.
Before each driving assessment, investigators asked participants, “Do you feel safe to drive?” Depending on the dose, 55%-80% of participants responded affirmatively, but the majority of participants had clinically meaningful changes in SDLP. “This [result] is consistent with the FDA guidance in the literature that subject perception of safety to drive is faulty and supports the need for formal driving assessments,” said Dr. Pearlman.
Dizziness, somnolence, and headache were the most common adverse events in the study, and this result was similar to those of the phase 3 trials. Most of the adverse events were of mild to moderate severity.
Questions for further study
Among the questions that future research could address is whether the lasmiditan-related effects seen in these studies in healthy subjects are similar to those in patients with migraine when lasmiditan is taken to treat a migraine attack, said Dr. Pearlman. Another open question is whether migraine has ictal and interictal effects on driving performance.
Eli Lilly, which has developed lasmiditan, sponsored the studies. Dr. Pearlman and several of coinvestigators are employees of the company.
SOURCE: Pearlman E et al. AHS 2019, Abstract IOR06.
Philadelphia – according to research presented at the annual meeting of the American Headache Society. This impairment coincides with the time of peak drug concentration and is resolved by 8 hours after dosing.
Lasmiditan, a 5-HT1F receptor agonist, is under regulatory review as an acute treatment of migraine in adults. In two phase 3 trials, a significant proportion of participants who received the treatment were pain-free at 2 hours and free of their most bothersome symptom at 2 hours, compared with controls. The most common treatment-emergent adverse events (dizziness, paresthesia, somnolence, fatigue, and hypoaesthesia) reflected the drug’s penetration of the CNS. Because CNS-related adverse events could affect a patient’s ability to drive, the effects of lasmiditan on simulated driving were studied, consistent with regulatory guidance.
Two studies in healthy participants
Eric Pearlman, MD, PhD, senior medical director for neuroscience, U.S. Medical Affairs, at Eli Lilly, and colleagues conducted two simulated driving studies with crossover designs. In the first study, the researchers randomized 90 healthy subjects (mean age, 34.9 years) to 50 mg, 100 mg, or 200 mg of lasmiditan; 1 mg of alprazolam (an active control); or placebo. After appropriate washout periods, participants received each treatment in random order. At 1.5 hours after each treatment, participants underwent a driving assessment using a driving simulator.
In the second study, Dr. Pearlman and colleagues randomized 68 healthy subjects (mean age, 32.8 years) to 100 mg or 200 mg of lasmiditan, 50 mg of diphenhydramine (an active control), or placebo. Participants underwent driving assessments at 8, 12, and 24 hours after baseline. Participants randomized to lasmiditan received treatment at baseline, but participants randomized to diphenhydramine received treatment 2 hours before each driving assessment. The diphenhydramine arm was intended to test the sensitivity of the driving assessment to impairment, as was the alprazolam arm in the first study.
During the driving assessments, participants were asked to maintain a speed of 100 km/h and to stay in the middle of the lane. The simulated road included gentle curves and hills, and oncoming traffic appeared occasionally. Participants also completed a secondary attention task, mimicking real-world conditions. Each assessment took about 1 hour. The primary endpoint was the standard deviation of lateral position (SDLP), which is known colloquially as weaving. The investigators defined noninferiority to placebo as an SDLP of 4.4 cm or less.
Participants reported that they could drive safely
In the first study, Dr. Pearlman and colleagues reported a dose-related increase in SDLP at 1.5 hours post treatment. All three doses of lasmiditan were inferior to placebo, in terms of their effect on SDLP. In the second study, both doses of lasmiditan were noninferior to placebo at 8 hours, 12 hours, and 24 hours. In both studies, the positive control confirmed the assay’s sensitivity to driving impairment. Secondary endpoints in the second study did not indicate an association between lasmiditan and clinically meaningful driving impairment at 8, 12, and 24 hours after dosing.
Single oral doses of lasmiditan were absorbed rapidly. The median time to peak concentration was approximately 2 hours, and the mean elimination half-life was about 4.25 hours. Exposure to lasmiditan was approximately dose proportional.
Before each driving assessment, investigators asked participants, “Do you feel safe to drive?” Depending on the dose, 55%-80% of participants responded affirmatively, but the majority of participants had clinically meaningful changes in SDLP. “This [result] is consistent with the FDA guidance in the literature that subject perception of safety to drive is faulty and supports the need for formal driving assessments,” said Dr. Pearlman.
Dizziness, somnolence, and headache were the most common adverse events in the study, and this result was similar to those of the phase 3 trials. Most of the adverse events were of mild to moderate severity.
Questions for further study
Among the questions that future research could address is whether the lasmiditan-related effects seen in these studies in healthy subjects are similar to those in patients with migraine when lasmiditan is taken to treat a migraine attack, said Dr. Pearlman. Another open question is whether migraine has ictal and interictal effects on driving performance.
Eli Lilly, which has developed lasmiditan, sponsored the studies. Dr. Pearlman and several of coinvestigators are employees of the company.
SOURCE: Pearlman E et al. AHS 2019, Abstract IOR06.
Philadelphia – according to research presented at the annual meeting of the American Headache Society. This impairment coincides with the time of peak drug concentration and is resolved by 8 hours after dosing.
Lasmiditan, a 5-HT1F receptor agonist, is under regulatory review as an acute treatment of migraine in adults. In two phase 3 trials, a significant proportion of participants who received the treatment were pain-free at 2 hours and free of their most bothersome symptom at 2 hours, compared with controls. The most common treatment-emergent adverse events (dizziness, paresthesia, somnolence, fatigue, and hypoaesthesia) reflected the drug’s penetration of the CNS. Because CNS-related adverse events could affect a patient’s ability to drive, the effects of lasmiditan on simulated driving were studied, consistent with regulatory guidance.
Two studies in healthy participants
Eric Pearlman, MD, PhD, senior medical director for neuroscience, U.S. Medical Affairs, at Eli Lilly, and colleagues conducted two simulated driving studies with crossover designs. In the first study, the researchers randomized 90 healthy subjects (mean age, 34.9 years) to 50 mg, 100 mg, or 200 mg of lasmiditan; 1 mg of alprazolam (an active control); or placebo. After appropriate washout periods, participants received each treatment in random order. At 1.5 hours after each treatment, participants underwent a driving assessment using a driving simulator.
In the second study, Dr. Pearlman and colleagues randomized 68 healthy subjects (mean age, 32.8 years) to 100 mg or 200 mg of lasmiditan, 50 mg of diphenhydramine (an active control), or placebo. Participants underwent driving assessments at 8, 12, and 24 hours after baseline. Participants randomized to lasmiditan received treatment at baseline, but participants randomized to diphenhydramine received treatment 2 hours before each driving assessment. The diphenhydramine arm was intended to test the sensitivity of the driving assessment to impairment, as was the alprazolam arm in the first study.
During the driving assessments, participants were asked to maintain a speed of 100 km/h and to stay in the middle of the lane. The simulated road included gentle curves and hills, and oncoming traffic appeared occasionally. Participants also completed a secondary attention task, mimicking real-world conditions. Each assessment took about 1 hour. The primary endpoint was the standard deviation of lateral position (SDLP), which is known colloquially as weaving. The investigators defined noninferiority to placebo as an SDLP of 4.4 cm or less.
Participants reported that they could drive safely
In the first study, Dr. Pearlman and colleagues reported a dose-related increase in SDLP at 1.5 hours post treatment. All three doses of lasmiditan were inferior to placebo, in terms of their effect on SDLP. In the second study, both doses of lasmiditan were noninferior to placebo at 8 hours, 12 hours, and 24 hours. In both studies, the positive control confirmed the assay’s sensitivity to driving impairment. Secondary endpoints in the second study did not indicate an association between lasmiditan and clinically meaningful driving impairment at 8, 12, and 24 hours after dosing.
Single oral doses of lasmiditan were absorbed rapidly. The median time to peak concentration was approximately 2 hours, and the mean elimination half-life was about 4.25 hours. Exposure to lasmiditan was approximately dose proportional.
Before each driving assessment, investigators asked participants, “Do you feel safe to drive?” Depending on the dose, 55%-80% of participants responded affirmatively, but the majority of participants had clinically meaningful changes in SDLP. “This [result] is consistent with the FDA guidance in the literature that subject perception of safety to drive is faulty and supports the need for formal driving assessments,” said Dr. Pearlman.
Dizziness, somnolence, and headache were the most common adverse events in the study, and this result was similar to those of the phase 3 trials. Most of the adverse events were of mild to moderate severity.
Questions for further study
Among the questions that future research could address is whether the lasmiditan-related effects seen in these studies in healthy subjects are similar to those in patients with migraine when lasmiditan is taken to treat a migraine attack, said Dr. Pearlman. Another open question is whether migraine has ictal and interictal effects on driving performance.
Eli Lilly, which has developed lasmiditan, sponsored the studies. Dr. Pearlman and several of coinvestigators are employees of the company.
SOURCE: Pearlman E et al. AHS 2019, Abstract IOR06.
REPORTING FROM AHS 2019
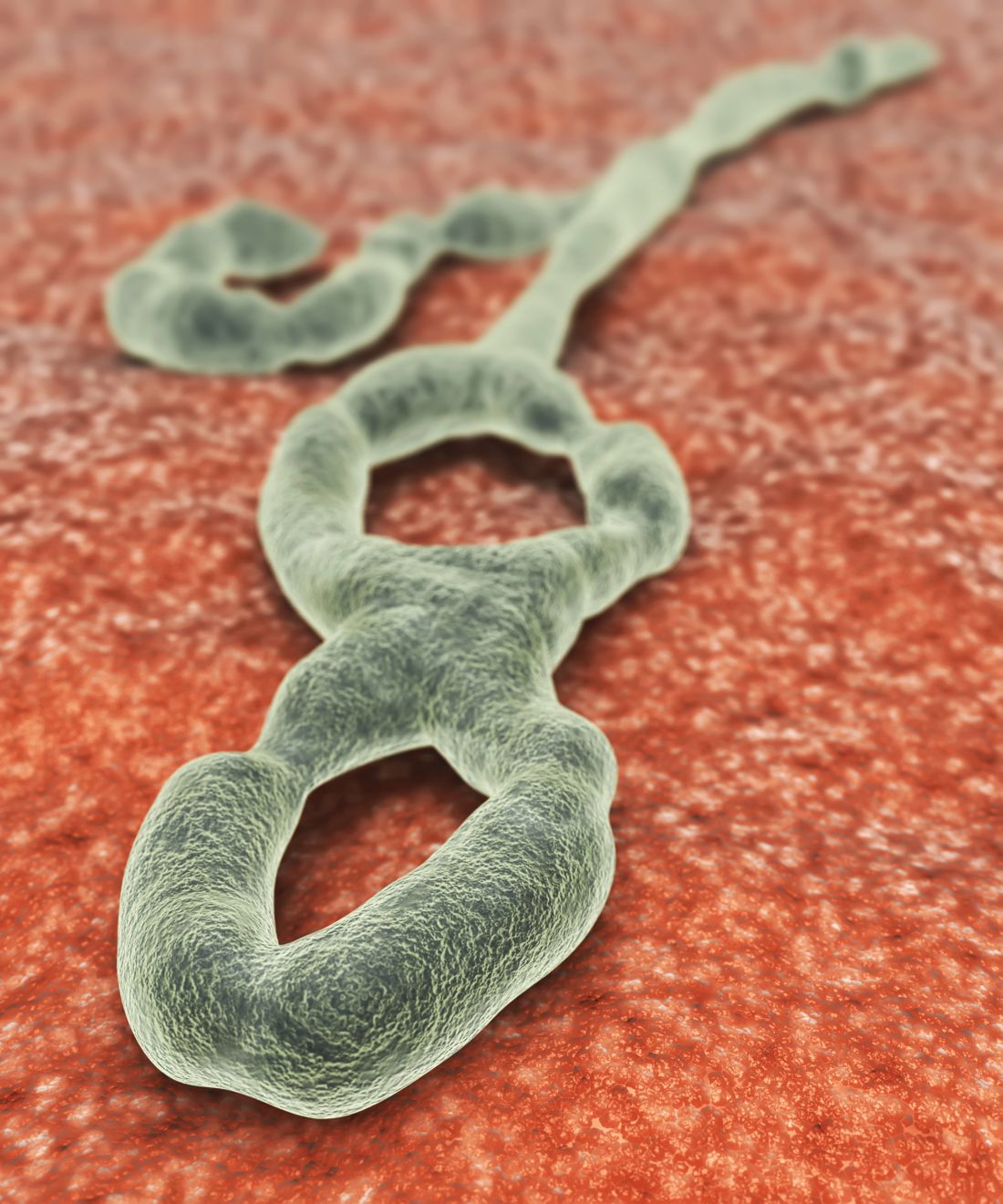
Favorable Ebola results lead to drug trial termination, new focus
An investigational agent known as REGN-EB3 has met an early stopping criterion in the protocol of an Ebola therapeutics trial, according to a National Institutes of Health media advisory.
Preliminary results in 499 study participants showed that individuals receiving either of two treatments, REGN-EB3 or mAb114, had a greater chance of survival, compared with participants in the other two study arms.
The randomized, controlled Pamoja Tulinde Maisha (PALM) study, which began Nov. 20, 2018, was designed to evaluate four investigational agents (ZMapp, remdesivir, mAb114, and REGN-EB3) for the treatment of patients with Ebola virus disease in the Democratic Republic of the Congo (DRC) as part of the emergency response to an ongoing outbreak in the North Kivu and Ituri provinces.
As of Aug. 9, 2019, the trial had enrolled 681 patients at four Ebola treatment centers in live outbreak regions of the DRC, with the goal of enrolling 725 patients in total.
The trial investigators and study cosponsors accepted the recommendation for early termination, and staff at the trial sites in the DRC were promptly informed, according to the media advisory. Additional patient randomizations in the now-revised trial will be limited to treatment either with REGN-EB3 or mAb114. Patients randomized to the ZMapp or remdesivir arms in the last 10 days of the original trial will be given the option, at the discretion of their treating physician, to receive either of the two more effective treatments, according to the NIH.
“While the final analysis of the data can occur only after all the data are generated and collected (likely late September/early October 2019), the DSMB [Data and Safety Monitoring Board] and the study leadership felt the preliminary analysis of the existing data was compelling enough to recommend and implement these changes in the trial immediately. The complete results will be submitted for publication in the peer-reviewed medical literature as soon as possible,” the NIH stated.
The study is cosponsored and funded by the NIH, carried out by an international research consortium coordinated by the World Health Organization, and supported by four pharmaceutical companies (MappBio, Gilead, Regeneron, and Ridgeback Biotherapeutics).
An investigational agent known as REGN-EB3 has met an early stopping criterion in the protocol of an Ebola therapeutics trial, according to a National Institutes of Health media advisory.
Preliminary results in 499 study participants showed that individuals receiving either of two treatments, REGN-EB3 or mAb114, had a greater chance of survival, compared with participants in the other two study arms.
The randomized, controlled Pamoja Tulinde Maisha (PALM) study, which began Nov. 20, 2018, was designed to evaluate four investigational agents (ZMapp, remdesivir, mAb114, and REGN-EB3) for the treatment of patients with Ebola virus disease in the Democratic Republic of the Congo (DRC) as part of the emergency response to an ongoing outbreak in the North Kivu and Ituri provinces.
As of Aug. 9, 2019, the trial had enrolled 681 patients at four Ebola treatment centers in live outbreak regions of the DRC, with the goal of enrolling 725 patients in total.
The trial investigators and study cosponsors accepted the recommendation for early termination, and staff at the trial sites in the DRC were promptly informed, according to the media advisory. Additional patient randomizations in the now-revised trial will be limited to treatment either with REGN-EB3 or mAb114. Patients randomized to the ZMapp or remdesivir arms in the last 10 days of the original trial will be given the option, at the discretion of their treating physician, to receive either of the two more effective treatments, according to the NIH.
“While the final analysis of the data can occur only after all the data are generated and collected (likely late September/early October 2019), the DSMB [Data and Safety Monitoring Board] and the study leadership felt the preliminary analysis of the existing data was compelling enough to recommend and implement these changes in the trial immediately. The complete results will be submitted for publication in the peer-reviewed medical literature as soon as possible,” the NIH stated.
The study is cosponsored and funded by the NIH, carried out by an international research consortium coordinated by the World Health Organization, and supported by four pharmaceutical companies (MappBio, Gilead, Regeneron, and Ridgeback Biotherapeutics).
An investigational agent known as REGN-EB3 has met an early stopping criterion in the protocol of an Ebola therapeutics trial, according to a National Institutes of Health media advisory.
Preliminary results in 499 study participants showed that individuals receiving either of two treatments, REGN-EB3 or mAb114, had a greater chance of survival, compared with participants in the other two study arms.
The randomized, controlled Pamoja Tulinde Maisha (PALM) study, which began Nov. 20, 2018, was designed to evaluate four investigational agents (ZMapp, remdesivir, mAb114, and REGN-EB3) for the treatment of patients with Ebola virus disease in the Democratic Republic of the Congo (DRC) as part of the emergency response to an ongoing outbreak in the North Kivu and Ituri provinces.
As of Aug. 9, 2019, the trial had enrolled 681 patients at four Ebola treatment centers in live outbreak regions of the DRC, with the goal of enrolling 725 patients in total.
The trial investigators and study cosponsors accepted the recommendation for early termination, and staff at the trial sites in the DRC were promptly informed, according to the media advisory. Additional patient randomizations in the now-revised trial will be limited to treatment either with REGN-EB3 or mAb114. Patients randomized to the ZMapp or remdesivir arms in the last 10 days of the original trial will be given the option, at the discretion of their treating physician, to receive either of the two more effective treatments, according to the NIH.
“While the final analysis of the data can occur only after all the data are generated and collected (likely late September/early October 2019), the DSMB [Data and Safety Monitoring Board] and the study leadership felt the preliminary analysis of the existing data was compelling enough to recommend and implement these changes in the trial immediately. The complete results will be submitted for publication in the peer-reviewed medical literature as soon as possible,” the NIH stated.
The study is cosponsored and funded by the NIH, carried out by an international research consortium coordinated by the World Health Organization, and supported by four pharmaceutical companies (MappBio, Gilead, Regeneron, and Ridgeback Biotherapeutics).
Ibrutinib/rituximab effective, safe as frontline treatment for older patients with MCL
LUGANO, SWITZERLAND – The chemotherapy-free combination of ibrutinib (Imbruvica) and rituximab is highly effective as frontline therapy for older, transplant-ineligible patients with nonblastoid mantle cell lymphoma, according to investigators.
In a phase 2 study of patients with a median age of 71 years, 38 of 41 patients (93%) had an objective response, and the regimen was both safe and easy to administer, reported Preetesh Jain, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
“The adverse event profile was generally favorable, with specific monitoring recommended for patients with cardiovascular comorbidities and a history of atrial fibrillation,” he said at the International Conference on Malignant Lymphoma.
The investigators enrolled 48 patients aged 65 years and older with previously untreated mantle cell lymphoma (MCL), of whom 41 were evaluable for the primary endpoints of overall response rate (ORR) and safety. The patients had good performance status and normal organ function, with the largest tumor size less than 10 cm. Patients with atrial fibrillation could participate, if the fibrillation was controlled. Patients with Ki-67 protein levels of 50% or greater and blastoid/pleomorphic histology were excluded.
Patients were treated with ibrutinib 560 mg orally daily for each 28-day cycle, with therapy continued until disease progression, or until therapy was stopped for any other reason. Patients also received intravenous rituximab 375 mg/m2 on days 1, 8, 15 and 22, plus or minus one day for cycle 1, on day 1 of cycles 3-8, and on day 1 of every other cycle for up to 2 years.
Of the 41 patients evaluable for response, 26 (64%) had a complete response (CR) and 12 (29%) had a partial response. Three additional patients had stable disease, for an objective response rate of 93%.
Of 34 patients with PET scans, all had negative scans. Of 37 patients evaluable for minimal residual disease (MRD) by flow cytometry, 21 (58%) were MRD negative.
Patients with low or intermediate Mantle Cell Lymphoma International Prognostic Index (MIPI) scores had a higher ORR (100% vs. 89% for patients with high MIPI scores), and patients with low Ki-67 levels had a higher response rate than that of patients with KI-67 of 30% or greater (80% vs. 87%).
Neither median 3-year progression-free survival nor median 3-year overall survival have been reached, with respective 3-year rates of 87% and 95%.
Four patients experienced disease progression at 4, 10, 13 and 33 months of treatment. Three of these patients had disease that had transformed to blastoid/pleomorphic variant, two had Ki-67 of 30% or greater, one had mutations in TP53, and one had FAT1 and SF3B1 mutations.
Two patients died after ibrutinib therapy, one who had discontinued therapy because of bleeding, and the other who died on treatment at 13 months from transformed disease. Both of these patients had high Ki-67 levels.
Grade 3 or 4 hematological adverse events were neutropenia in four patients, and thrombocytopenia in two patients. There were no cases of grade 3 or 4 anemia.
Grade 3 or 4 nonhematological adverse events were fatigue, myalgia, and atrial fibrillation in seven patients each, diarrhea in six patients, and petechiae/bleeding in three patients.
Patients will continue to be followed for late adverse events, secondary cancers, and relapses, and further studies on clonal evolution, mutation profiling, and MRD are ongoing and will be reported at a later date, Dr. Jain said.
The National Cancer Institute supported the study. Dr. Jain reported having no financial disclosures.
SOURCE: Jain P et al. 15-ICML. Abstract 011.
LUGANO, SWITZERLAND – The chemotherapy-free combination of ibrutinib (Imbruvica) and rituximab is highly effective as frontline therapy for older, transplant-ineligible patients with nonblastoid mantle cell lymphoma, according to investigators.
In a phase 2 study of patients with a median age of 71 years, 38 of 41 patients (93%) had an objective response, and the regimen was both safe and easy to administer, reported Preetesh Jain, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
“The adverse event profile was generally favorable, with specific monitoring recommended for patients with cardiovascular comorbidities and a history of atrial fibrillation,” he said at the International Conference on Malignant Lymphoma.
The investigators enrolled 48 patients aged 65 years and older with previously untreated mantle cell lymphoma (MCL), of whom 41 were evaluable for the primary endpoints of overall response rate (ORR) and safety. The patients had good performance status and normal organ function, with the largest tumor size less than 10 cm. Patients with atrial fibrillation could participate, if the fibrillation was controlled. Patients with Ki-67 protein levels of 50% or greater and blastoid/pleomorphic histology were excluded.
Patients were treated with ibrutinib 560 mg orally daily for each 28-day cycle, with therapy continued until disease progression, or until therapy was stopped for any other reason. Patients also received intravenous rituximab 375 mg/m2 on days 1, 8, 15 and 22, plus or minus one day for cycle 1, on day 1 of cycles 3-8, and on day 1 of every other cycle for up to 2 years.
Of the 41 patients evaluable for response, 26 (64%) had a complete response (CR) and 12 (29%) had a partial response. Three additional patients had stable disease, for an objective response rate of 93%.
Of 34 patients with PET scans, all had negative scans. Of 37 patients evaluable for minimal residual disease (MRD) by flow cytometry, 21 (58%) were MRD negative.
Patients with low or intermediate Mantle Cell Lymphoma International Prognostic Index (MIPI) scores had a higher ORR (100% vs. 89% for patients with high MIPI scores), and patients with low Ki-67 levels had a higher response rate than that of patients with KI-67 of 30% or greater (80% vs. 87%).
Neither median 3-year progression-free survival nor median 3-year overall survival have been reached, with respective 3-year rates of 87% and 95%.
Four patients experienced disease progression at 4, 10, 13 and 33 months of treatment. Three of these patients had disease that had transformed to blastoid/pleomorphic variant, two had Ki-67 of 30% or greater, one had mutations in TP53, and one had FAT1 and SF3B1 mutations.
Two patients died after ibrutinib therapy, one who had discontinued therapy because of bleeding, and the other who died on treatment at 13 months from transformed disease. Both of these patients had high Ki-67 levels.
Grade 3 or 4 hematological adverse events were neutropenia in four patients, and thrombocytopenia in two patients. There were no cases of grade 3 or 4 anemia.
Grade 3 or 4 nonhematological adverse events were fatigue, myalgia, and atrial fibrillation in seven patients each, diarrhea in six patients, and petechiae/bleeding in three patients.
Patients will continue to be followed for late adverse events, secondary cancers, and relapses, and further studies on clonal evolution, mutation profiling, and MRD are ongoing and will be reported at a later date, Dr. Jain said.
The National Cancer Institute supported the study. Dr. Jain reported having no financial disclosures.
SOURCE: Jain P et al. 15-ICML. Abstract 011.
LUGANO, SWITZERLAND – The chemotherapy-free combination of ibrutinib (Imbruvica) and rituximab is highly effective as frontline therapy for older, transplant-ineligible patients with nonblastoid mantle cell lymphoma, according to investigators.
In a phase 2 study of patients with a median age of 71 years, 38 of 41 patients (93%) had an objective response, and the regimen was both safe and easy to administer, reported Preetesh Jain, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
“The adverse event profile was generally favorable, with specific monitoring recommended for patients with cardiovascular comorbidities and a history of atrial fibrillation,” he said at the International Conference on Malignant Lymphoma.
The investigators enrolled 48 patients aged 65 years and older with previously untreated mantle cell lymphoma (MCL), of whom 41 were evaluable for the primary endpoints of overall response rate (ORR) and safety. The patients had good performance status and normal organ function, with the largest tumor size less than 10 cm. Patients with atrial fibrillation could participate, if the fibrillation was controlled. Patients with Ki-67 protein levels of 50% or greater and blastoid/pleomorphic histology were excluded.
Patients were treated with ibrutinib 560 mg orally daily for each 28-day cycle, with therapy continued until disease progression, or until therapy was stopped for any other reason. Patients also received intravenous rituximab 375 mg/m2 on days 1, 8, 15 and 22, plus or minus one day for cycle 1, on day 1 of cycles 3-8, and on day 1 of every other cycle for up to 2 years.
Of the 41 patients evaluable for response, 26 (64%) had a complete response (CR) and 12 (29%) had a partial response. Three additional patients had stable disease, for an objective response rate of 93%.
Of 34 patients with PET scans, all had negative scans. Of 37 patients evaluable for minimal residual disease (MRD) by flow cytometry, 21 (58%) were MRD negative.
Patients with low or intermediate Mantle Cell Lymphoma International Prognostic Index (MIPI) scores had a higher ORR (100% vs. 89% for patients with high MIPI scores), and patients with low Ki-67 levels had a higher response rate than that of patients with KI-67 of 30% or greater (80% vs. 87%).
Neither median 3-year progression-free survival nor median 3-year overall survival have been reached, with respective 3-year rates of 87% and 95%.
Four patients experienced disease progression at 4, 10, 13 and 33 months of treatment. Three of these patients had disease that had transformed to blastoid/pleomorphic variant, two had Ki-67 of 30% or greater, one had mutations in TP53, and one had FAT1 and SF3B1 mutations.
Two patients died after ibrutinib therapy, one who had discontinued therapy because of bleeding, and the other who died on treatment at 13 months from transformed disease. Both of these patients had high Ki-67 levels.
Grade 3 or 4 hematological adverse events were neutropenia in four patients, and thrombocytopenia in two patients. There were no cases of grade 3 or 4 anemia.
Grade 3 or 4 nonhematological adverse events were fatigue, myalgia, and atrial fibrillation in seven patients each, diarrhea in six patients, and petechiae/bleeding in three patients.
Patients will continue to be followed for late adverse events, secondary cancers, and relapses, and further studies on clonal evolution, mutation profiling, and MRD are ongoing and will be reported at a later date, Dr. Jain said.
The National Cancer Institute supported the study. Dr. Jain reported having no financial disclosures.
SOURCE: Jain P et al. 15-ICML. Abstract 011.
REPORTING FROM 15-ICML
Immune-related toxicities, hospitalization common with checkpoint inhibitor therapy
, according to a retrospective cohort study.
In addition, the majority of the immune-related toxicities were high-grade events of grade 3 or higher (65%), necessitated multidisciplinary care (91%), and eventually improved or resolved (65%). The results highlight potential risk factors for hospitalizations due to immune-related toxicities in oncology patients.
“[We aimed to] characterize the spectrum of toxicities, management, and outcomes of hospitalizations for immune-related adverse events,” wrote Aanika Balaji, BS, of Johns Hopkins University, Baltimore, and colleagues. The findings were reported in the Journal of Oncology Practice.
The researchers studied 443 patients admitted to solid tumor oncology service at an oncology center over a period of 6-months. Of these, 100 patients had at any point received checkpoint inhibitor therapy.
The proportion of hospital admissions for patients with confirmed immune-related toxicities and associations between hospitalizations due to immune-related toxicity and patient characteristics were assessed by the team. Nearly half of the patients admitted with immune-related toxicities had thoracic or head and neck cancers.
In the analysis, patients treated with combination checkpoint inhibitor therapy (odds ratio, 6.8; 95% confidence interval, 2.0-23.2), in addition to those aged 65 years and over (OR, 5.4; 95% CI, 1.6-17.8), were more likely to be hospitalized for immune-related toxicities.
Overall, 5% of all hospitalizations were the result of immune-related toxicities. Furthermore, 87% of patients discontinued checkpoint inhibitor therapy post discharge.
“We found that the most common immune-related adverse events warranting hospital admission were pneumonitis (26%) and colitis (17%),” they wrote.
The researchers acknowledged two key limitations of the study were the small sample size and lack of generalizability in community settings. Future studies that include patients from community oncology settings could improve the generalizability of the results.
“These data indicate potential risk factors for immune-related adverse event hospitalization and are likely to indicate future service needs” they concluded.
Financial support was provided by Jarushka Naidoo. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Compugen, Genentech, GlaxoSmithKline, Exelixis, MedImmune, and several others.
SOURCE: Balaji A et al. J Oncol Pract. 2019 Aug 6. doi: 10.1200/JOP.18.00703.
, according to a retrospective cohort study.
In addition, the majority of the immune-related toxicities were high-grade events of grade 3 or higher (65%), necessitated multidisciplinary care (91%), and eventually improved or resolved (65%). The results highlight potential risk factors for hospitalizations due to immune-related toxicities in oncology patients.
“[We aimed to] characterize the spectrum of toxicities, management, and outcomes of hospitalizations for immune-related adverse events,” wrote Aanika Balaji, BS, of Johns Hopkins University, Baltimore, and colleagues. The findings were reported in the Journal of Oncology Practice.
The researchers studied 443 patients admitted to solid tumor oncology service at an oncology center over a period of 6-months. Of these, 100 patients had at any point received checkpoint inhibitor therapy.
The proportion of hospital admissions for patients with confirmed immune-related toxicities and associations between hospitalizations due to immune-related toxicity and patient characteristics were assessed by the team. Nearly half of the patients admitted with immune-related toxicities had thoracic or head and neck cancers.
In the analysis, patients treated with combination checkpoint inhibitor therapy (odds ratio, 6.8; 95% confidence interval, 2.0-23.2), in addition to those aged 65 years and over (OR, 5.4; 95% CI, 1.6-17.8), were more likely to be hospitalized for immune-related toxicities.
Overall, 5% of all hospitalizations were the result of immune-related toxicities. Furthermore, 87% of patients discontinued checkpoint inhibitor therapy post discharge.
“We found that the most common immune-related adverse events warranting hospital admission were pneumonitis (26%) and colitis (17%),” they wrote.
The researchers acknowledged two key limitations of the study were the small sample size and lack of generalizability in community settings. Future studies that include patients from community oncology settings could improve the generalizability of the results.
“These data indicate potential risk factors for immune-related adverse event hospitalization and are likely to indicate future service needs” they concluded.
Financial support was provided by Jarushka Naidoo. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Compugen, Genentech, GlaxoSmithKline, Exelixis, MedImmune, and several others.
SOURCE: Balaji A et al. J Oncol Pract. 2019 Aug 6. doi: 10.1200/JOP.18.00703.
, according to a retrospective cohort study.
In addition, the majority of the immune-related toxicities were high-grade events of grade 3 or higher (65%), necessitated multidisciplinary care (91%), and eventually improved or resolved (65%). The results highlight potential risk factors for hospitalizations due to immune-related toxicities in oncology patients.
“[We aimed to] characterize the spectrum of toxicities, management, and outcomes of hospitalizations for immune-related adverse events,” wrote Aanika Balaji, BS, of Johns Hopkins University, Baltimore, and colleagues. The findings were reported in the Journal of Oncology Practice.
The researchers studied 443 patients admitted to solid tumor oncology service at an oncology center over a period of 6-months. Of these, 100 patients had at any point received checkpoint inhibitor therapy.
The proportion of hospital admissions for patients with confirmed immune-related toxicities and associations between hospitalizations due to immune-related toxicity and patient characteristics were assessed by the team. Nearly half of the patients admitted with immune-related toxicities had thoracic or head and neck cancers.
In the analysis, patients treated with combination checkpoint inhibitor therapy (odds ratio, 6.8; 95% confidence interval, 2.0-23.2), in addition to those aged 65 years and over (OR, 5.4; 95% CI, 1.6-17.8), were more likely to be hospitalized for immune-related toxicities.
Overall, 5% of all hospitalizations were the result of immune-related toxicities. Furthermore, 87% of patients discontinued checkpoint inhibitor therapy post discharge.
“We found that the most common immune-related adverse events warranting hospital admission were pneumonitis (26%) and colitis (17%),” they wrote.
The researchers acknowledged two key limitations of the study were the small sample size and lack of generalizability in community settings. Future studies that include patients from community oncology settings could improve the generalizability of the results.
“These data indicate potential risk factors for immune-related adverse event hospitalization and are likely to indicate future service needs” they concluded.
Financial support was provided by Jarushka Naidoo. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Compugen, Genentech, GlaxoSmithKline, Exelixis, MedImmune, and several others.
SOURCE: Balaji A et al. J Oncol Pract. 2019 Aug 6. doi: 10.1200/JOP.18.00703.
FROM JOURNAL OF ONCOLOGY PRACTICE
Oral semaglutide monotherapy delivers HbA1c improvements in type 2 diabetes
Oral semaglutide monotherapy was superior to placebo for improving glycated hemoglobin (HbA1c) levels at all doses tested in adults with type 2 diabetes who had been previously insufficiently managed with diet and exercise, according to findings from a global, randomized trial.
The drug also showed dose-dependent weight loss, with a statistically significant effect on body weight, compared with placebo, at higher doses.
To date, the glucagon-like peptide–1 receptor agonist has been available as weekly subcutaneous shots for patients with type 2 diabetes, and in that form they have been shown to be effective in reducing HbA1c, inducing weight loss, and lowering the risk of cardiovascular events in patients with cardiovascular disease or those who are at high risk for it, wrote Vanita R. Aroda, MD, of Brigham and Women’s Hospital, Boston, and colleagues. The report is in Diabetes Care.
The novel oral semaglutide tablet is designed to enhance medication absorption, and the pharmacokinetics and dosage were established in phase 2 studies, they noted.
In the phase 3 Peptide Innovation for Early Diabetes Treatment 1 (PIONEER 1) study, Dr. Aroda and colleagues randomized 703 adults with type 2 diabetes to receive either 3 mg, 7 mg, or 14 mg of oral semaglutide daily, or placebo. The average age of the patients was 55 years, about half were women, and the average baseline HbA1c was 8.0% (64 mmol/mol). The primary endpoint was change in HbA1c level from baseline to week 26, and the secondary endpoint was change in body weight over the same period.
After 26 weeks of once-daily treatment, patients in semaglutide group showed significant reductions in HbA1c from baseline with all three doses: –0.6% (3 mg), –0.9% (7 mg), and –1.1% (14 mg), with P less than .001 for all, based on an intention-to-treat analysis. Similar results occurred using an on-treatment analysis, with differences of –0.7%, –1.2%, and –1.4%, respectively, for the three doses.
In addition, patients in all dose groups achieved the secondary endpoint of reduction in body weight, compared with placebo, from baseline to 26 weeks based on both types of analyses. “Significantly more patients achieved body weight loss of at least 5% with oral semaglutide at 7 mg and 14 mg, compared with placebo,” Dr. Aroda and colleagues wrote (intention-to-treat: –0.1 for 3 mg daily [P = .87], –0.9 for 7 mg [P = .09], –2.3 for 14 mg [P less than .001]; and on-treatment: –0.2 for 3 mg [P = .71], –1.0 for 7 mg [P = .01], –2.6 for 14 mg [P less than .001]).
The overall incidence of adverse events and serious adverse events was similar in the treatment and placebo groups, with the most frequent being nausea and diarrhea. No deaths occurred among patients on the medication.
The findings were limited by several factors, including a patient population that had a relatively short duration of diabetes (mean, 3.5 years) and that the oral semaglutide was used as first-line monotherapy, without first using metformin, the researchers noted. However, oral semaglutide “achieved clinically meaningful and superior glucose lowering,” compared with placebo, at all three doses, they wrote.
“Ongoing additional studies in the PIONEER program will further define the effect when used in combination with other glucose-lowering therapies and in other populations of interest, such as those with high cardiovascular risk or renal impairment,” they emphasized
Novo Nordisk funded the study. The lead author disclosed relationships with Novo Nordisk, and several coauthors disclosed relationships with or employment by the company.
SOURCE: Aroda VR et al. Diabetes Care. 2019 Jul. doi: 10.2337/dc19-0749.
Oral semaglutide monotherapy was superior to placebo for improving glycated hemoglobin (HbA1c) levels at all doses tested in adults with type 2 diabetes who had been previously insufficiently managed with diet and exercise, according to findings from a global, randomized trial.
The drug also showed dose-dependent weight loss, with a statistically significant effect on body weight, compared with placebo, at higher doses.
To date, the glucagon-like peptide–1 receptor agonist has been available as weekly subcutaneous shots for patients with type 2 diabetes, and in that form they have been shown to be effective in reducing HbA1c, inducing weight loss, and lowering the risk of cardiovascular events in patients with cardiovascular disease or those who are at high risk for it, wrote Vanita R. Aroda, MD, of Brigham and Women’s Hospital, Boston, and colleagues. The report is in Diabetes Care.
The novel oral semaglutide tablet is designed to enhance medication absorption, and the pharmacokinetics and dosage were established in phase 2 studies, they noted.
In the phase 3 Peptide Innovation for Early Diabetes Treatment 1 (PIONEER 1) study, Dr. Aroda and colleagues randomized 703 adults with type 2 diabetes to receive either 3 mg, 7 mg, or 14 mg of oral semaglutide daily, or placebo. The average age of the patients was 55 years, about half were women, and the average baseline HbA1c was 8.0% (64 mmol/mol). The primary endpoint was change in HbA1c level from baseline to week 26, and the secondary endpoint was change in body weight over the same period.
After 26 weeks of once-daily treatment, patients in semaglutide group showed significant reductions in HbA1c from baseline with all three doses: –0.6% (3 mg), –0.9% (7 mg), and –1.1% (14 mg), with P less than .001 for all, based on an intention-to-treat analysis. Similar results occurred using an on-treatment analysis, with differences of –0.7%, –1.2%, and –1.4%, respectively, for the three doses.
In addition, patients in all dose groups achieved the secondary endpoint of reduction in body weight, compared with placebo, from baseline to 26 weeks based on both types of analyses. “Significantly more patients achieved body weight loss of at least 5% with oral semaglutide at 7 mg and 14 mg, compared with placebo,” Dr. Aroda and colleagues wrote (intention-to-treat: –0.1 for 3 mg daily [P = .87], –0.9 for 7 mg [P = .09], –2.3 for 14 mg [P less than .001]; and on-treatment: –0.2 for 3 mg [P = .71], –1.0 for 7 mg [P = .01], –2.6 for 14 mg [P less than .001]).
The overall incidence of adverse events and serious adverse events was similar in the treatment and placebo groups, with the most frequent being nausea and diarrhea. No deaths occurred among patients on the medication.
The findings were limited by several factors, including a patient population that had a relatively short duration of diabetes (mean, 3.5 years) and that the oral semaglutide was used as first-line monotherapy, without first using metformin, the researchers noted. However, oral semaglutide “achieved clinically meaningful and superior glucose lowering,” compared with placebo, at all three doses, they wrote.
“Ongoing additional studies in the PIONEER program will further define the effect when used in combination with other glucose-lowering therapies and in other populations of interest, such as those with high cardiovascular risk or renal impairment,” they emphasized
Novo Nordisk funded the study. The lead author disclosed relationships with Novo Nordisk, and several coauthors disclosed relationships with or employment by the company.
SOURCE: Aroda VR et al. Diabetes Care. 2019 Jul. doi: 10.2337/dc19-0749.
Oral semaglutide monotherapy was superior to placebo for improving glycated hemoglobin (HbA1c) levels at all doses tested in adults with type 2 diabetes who had been previously insufficiently managed with diet and exercise, according to findings from a global, randomized trial.
The drug also showed dose-dependent weight loss, with a statistically significant effect on body weight, compared with placebo, at higher doses.
To date, the glucagon-like peptide–1 receptor agonist has been available as weekly subcutaneous shots for patients with type 2 diabetes, and in that form they have been shown to be effective in reducing HbA1c, inducing weight loss, and lowering the risk of cardiovascular events in patients with cardiovascular disease or those who are at high risk for it, wrote Vanita R. Aroda, MD, of Brigham and Women’s Hospital, Boston, and colleagues. The report is in Diabetes Care.
The novel oral semaglutide tablet is designed to enhance medication absorption, and the pharmacokinetics and dosage were established in phase 2 studies, they noted.
In the phase 3 Peptide Innovation for Early Diabetes Treatment 1 (PIONEER 1) study, Dr. Aroda and colleagues randomized 703 adults with type 2 diabetes to receive either 3 mg, 7 mg, or 14 mg of oral semaglutide daily, or placebo. The average age of the patients was 55 years, about half were women, and the average baseline HbA1c was 8.0% (64 mmol/mol). The primary endpoint was change in HbA1c level from baseline to week 26, and the secondary endpoint was change in body weight over the same period.
After 26 weeks of once-daily treatment, patients in semaglutide group showed significant reductions in HbA1c from baseline with all three doses: –0.6% (3 mg), –0.9% (7 mg), and –1.1% (14 mg), with P less than .001 for all, based on an intention-to-treat analysis. Similar results occurred using an on-treatment analysis, with differences of –0.7%, –1.2%, and –1.4%, respectively, for the three doses.
In addition, patients in all dose groups achieved the secondary endpoint of reduction in body weight, compared with placebo, from baseline to 26 weeks based on both types of analyses. “Significantly more patients achieved body weight loss of at least 5% with oral semaglutide at 7 mg and 14 mg, compared with placebo,” Dr. Aroda and colleagues wrote (intention-to-treat: –0.1 for 3 mg daily [P = .87], –0.9 for 7 mg [P = .09], –2.3 for 14 mg [P less than .001]; and on-treatment: –0.2 for 3 mg [P = .71], –1.0 for 7 mg [P = .01], –2.6 for 14 mg [P less than .001]).
The overall incidence of adverse events and serious adverse events was similar in the treatment and placebo groups, with the most frequent being nausea and diarrhea. No deaths occurred among patients on the medication.
The findings were limited by several factors, including a patient population that had a relatively short duration of diabetes (mean, 3.5 years) and that the oral semaglutide was used as first-line monotherapy, without first using metformin, the researchers noted. However, oral semaglutide “achieved clinically meaningful and superior glucose lowering,” compared with placebo, at all three doses, they wrote.
“Ongoing additional studies in the PIONEER program will further define the effect when used in combination with other glucose-lowering therapies and in other populations of interest, such as those with high cardiovascular risk or renal impairment,” they emphasized
Novo Nordisk funded the study. The lead author disclosed relationships with Novo Nordisk, and several coauthors disclosed relationships with or employment by the company.
SOURCE: Aroda VR et al. Diabetes Care. 2019 Jul. doi: 10.2337/dc19-0749.
FROM DIABETES CARE
Beyond sunscreen: Skin cancer preventive agents finding a role
NEW YORK – (KCs) that deserves to be considered for selective use in at-risk patients, according to an update at the American Academy of Dermatology summer meeting.
In providing her perspective on the available options, Rebecca Hartman, MD, MPH, director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, emphasized that the therapies are not interchangeable but deserve to be used selectively according to their relative protection and relative risks.
Of oral agents, she characterized two, nicotinamide and acitretin, as “clinic-ready.” Acitretin is “an oldie but goodie,” but there is an important issue of tolerability. In the published studies, 15%-39% of patients withdrew because of adverse events, according to Dr. Hartman, which suggests the need for a motivated patient.
In addition, acitretin can be esterified into etretinate, a teratogen that can persist as long as 3 years after the drug is discontinued, making this drug contraindicated in women of childbearing potential, she noted.
However, most patients in need of prophylaxis for KCs are older, so teratogenicity is not an issue. In her practice, she offers acitretin to patients who are developing three or more KCs per year, as well as in situations of extensive skin damage in which a course of acitretin might provide some degree of clearing.
“When you are faced with the potential of a large number of biopsies, you could start acitretin to see if lesions can be reduced,” Dr. Hartman said .
Prevention of KCs became somewhat more attractive as a routine practice following publication of a phase 3 trial with nicotinamide. In this study, nicotinamide, an over-the-counter water-soluble form of vitamin B3, was associated with significantly reduced nonmelanoma skin cancers, including KCs and actinic keratoses, relative to placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26). Importantly, there was no greater risk of adverse events relative to placebo.
When assessed individually, the relative reduction in squamous cell carcinomas (SCCs; P = .05) and basal cell carcinomas (P = .12) fell short of statistical significance, but there was a highly significant 13% reduction in actinic keratoses after 12 months (P less than .001). An increase in SCCs was observed after therapy was stopped, which led Dr. Hartman to conclude that nicotinamide must be used on a “use-it-or-lose-it” basis. However, she does routinely offer this option.
“When do I recommend nicotinamide? Any patient with multiple actinic keratoses who wants to get ahead of the game and wants something that is relative safe,” Dr. Hartman explained. She uses the same dosing employed in the study, which was 500 mg twice daily.
There are other options for chemoprevention of KCs, but they are less attractive.
For example, capecitabine is effective, but tolerability is an even greater issue with this agent than it is for acitretin. According to Dr. Hartman, “we use this therapy very rarely and only in select cases.” As an alternative to the 14 days on and 7 days off schedule used for treatment of cancer, capecitabine is sometimes better tolerated in a 7 day on and 7 day off schedule, she said.
Topical 5-fluorouracil with or without calcipotriol is another chemoprevention option for those who can tolerate a skin reaction that lasts several days, Dr. Hartman said. She cited one study that associated this therapy with a nearly 80% reduction in face and scalp SCC.
Ultimately, she offers 5-fluorouracil with or without calcipotriol to “patients who want an evidence-based chemoprevention,” but she indicated that patients must be motivated to endure the adverse effects.
Many remain unaware of the array of options for chemoprevention of KCs, but Dr. Hartman emphasized that this is an area of active research with new options expected.
“I am really excited about the future direction of chemoprevention in skin cancer,” said Dr. Hartman, citing ongoing work to develop vitamin A, polypodium leucotomas extract, and human papillomavirus vaccine as options.
“If we can stop skin cancer in the first place, avoiding the morbidity and mortality of treatment, we will also hopefully save costs as well,” she commented. So far, essentially all of the strategies for chemoprevention, other than sunscreen, involve KCs, which leaves a large unmet need for better ways to prevent melanoma. However, Dr. Hartman noted that KCs represent the most common type of cancer of any type.
Just days after Dr. Hartman spoke at the meeting, a prospective study of vitamin A that found an inverse association between vitamin A intake and cutaneous SCC risk was, in fact, published in JAMA Dermatology (2019 Jul 31. doi: 10.1001/jamadermatol.2019.1937).
Dr. Hartman reported no financial relationships relevant to her presentation.
NEW YORK – (KCs) that deserves to be considered for selective use in at-risk patients, according to an update at the American Academy of Dermatology summer meeting.
In providing her perspective on the available options, Rebecca Hartman, MD, MPH, director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, emphasized that the therapies are not interchangeable but deserve to be used selectively according to their relative protection and relative risks.
Of oral agents, she characterized two, nicotinamide and acitretin, as “clinic-ready.” Acitretin is “an oldie but goodie,” but there is an important issue of tolerability. In the published studies, 15%-39% of patients withdrew because of adverse events, according to Dr. Hartman, which suggests the need for a motivated patient.
In addition, acitretin can be esterified into etretinate, a teratogen that can persist as long as 3 years after the drug is discontinued, making this drug contraindicated in women of childbearing potential, she noted.
However, most patients in need of prophylaxis for KCs are older, so teratogenicity is not an issue. In her practice, she offers acitretin to patients who are developing three or more KCs per year, as well as in situations of extensive skin damage in which a course of acitretin might provide some degree of clearing.
“When you are faced with the potential of a large number of biopsies, you could start acitretin to see if lesions can be reduced,” Dr. Hartman said .
Prevention of KCs became somewhat more attractive as a routine practice following publication of a phase 3 trial with nicotinamide. In this study, nicotinamide, an over-the-counter water-soluble form of vitamin B3, was associated with significantly reduced nonmelanoma skin cancers, including KCs and actinic keratoses, relative to placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26). Importantly, there was no greater risk of adverse events relative to placebo.
When assessed individually, the relative reduction in squamous cell carcinomas (SCCs; P = .05) and basal cell carcinomas (P = .12) fell short of statistical significance, but there was a highly significant 13% reduction in actinic keratoses after 12 months (P less than .001). An increase in SCCs was observed after therapy was stopped, which led Dr. Hartman to conclude that nicotinamide must be used on a “use-it-or-lose-it” basis. However, she does routinely offer this option.
“When do I recommend nicotinamide? Any patient with multiple actinic keratoses who wants to get ahead of the game and wants something that is relative safe,” Dr. Hartman explained. She uses the same dosing employed in the study, which was 500 mg twice daily.
There are other options for chemoprevention of KCs, but they are less attractive.
For example, capecitabine is effective, but tolerability is an even greater issue with this agent than it is for acitretin. According to Dr. Hartman, “we use this therapy very rarely and only in select cases.” As an alternative to the 14 days on and 7 days off schedule used for treatment of cancer, capecitabine is sometimes better tolerated in a 7 day on and 7 day off schedule, she said.
Topical 5-fluorouracil with or without calcipotriol is another chemoprevention option for those who can tolerate a skin reaction that lasts several days, Dr. Hartman said. She cited one study that associated this therapy with a nearly 80% reduction in face and scalp SCC.
Ultimately, she offers 5-fluorouracil with or without calcipotriol to “patients who want an evidence-based chemoprevention,” but she indicated that patients must be motivated to endure the adverse effects.
Many remain unaware of the array of options for chemoprevention of KCs, but Dr. Hartman emphasized that this is an area of active research with new options expected.
“I am really excited about the future direction of chemoprevention in skin cancer,” said Dr. Hartman, citing ongoing work to develop vitamin A, polypodium leucotomas extract, and human papillomavirus vaccine as options.
“If we can stop skin cancer in the first place, avoiding the morbidity and mortality of treatment, we will also hopefully save costs as well,” she commented. So far, essentially all of the strategies for chemoprevention, other than sunscreen, involve KCs, which leaves a large unmet need for better ways to prevent melanoma. However, Dr. Hartman noted that KCs represent the most common type of cancer of any type.
Just days after Dr. Hartman spoke at the meeting, a prospective study of vitamin A that found an inverse association between vitamin A intake and cutaneous SCC risk was, in fact, published in JAMA Dermatology (2019 Jul 31. doi: 10.1001/jamadermatol.2019.1937).
Dr. Hartman reported no financial relationships relevant to her presentation.
NEW YORK – (KCs) that deserves to be considered for selective use in at-risk patients, according to an update at the American Academy of Dermatology summer meeting.
In providing her perspective on the available options, Rebecca Hartman, MD, MPH, director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, emphasized that the therapies are not interchangeable but deserve to be used selectively according to their relative protection and relative risks.
Of oral agents, she characterized two, nicotinamide and acitretin, as “clinic-ready.” Acitretin is “an oldie but goodie,” but there is an important issue of tolerability. In the published studies, 15%-39% of patients withdrew because of adverse events, according to Dr. Hartman, which suggests the need for a motivated patient.
In addition, acitretin can be esterified into etretinate, a teratogen that can persist as long as 3 years after the drug is discontinued, making this drug contraindicated in women of childbearing potential, she noted.
However, most patients in need of prophylaxis for KCs are older, so teratogenicity is not an issue. In her practice, she offers acitretin to patients who are developing three or more KCs per year, as well as in situations of extensive skin damage in which a course of acitretin might provide some degree of clearing.
“When you are faced with the potential of a large number of biopsies, you could start acitretin to see if lesions can be reduced,” Dr. Hartman said .
Prevention of KCs became somewhat more attractive as a routine practice following publication of a phase 3 trial with nicotinamide. In this study, nicotinamide, an over-the-counter water-soluble form of vitamin B3, was associated with significantly reduced nonmelanoma skin cancers, including KCs and actinic keratoses, relative to placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26). Importantly, there was no greater risk of adverse events relative to placebo.
When assessed individually, the relative reduction in squamous cell carcinomas (SCCs; P = .05) and basal cell carcinomas (P = .12) fell short of statistical significance, but there was a highly significant 13% reduction in actinic keratoses after 12 months (P less than .001). An increase in SCCs was observed after therapy was stopped, which led Dr. Hartman to conclude that nicotinamide must be used on a “use-it-or-lose-it” basis. However, she does routinely offer this option.
“When do I recommend nicotinamide? Any patient with multiple actinic keratoses who wants to get ahead of the game and wants something that is relative safe,” Dr. Hartman explained. She uses the same dosing employed in the study, which was 500 mg twice daily.
There are other options for chemoprevention of KCs, but they are less attractive.
For example, capecitabine is effective, but tolerability is an even greater issue with this agent than it is for acitretin. According to Dr. Hartman, “we use this therapy very rarely and only in select cases.” As an alternative to the 14 days on and 7 days off schedule used for treatment of cancer, capecitabine is sometimes better tolerated in a 7 day on and 7 day off schedule, she said.
Topical 5-fluorouracil with or without calcipotriol is another chemoprevention option for those who can tolerate a skin reaction that lasts several days, Dr. Hartman said. She cited one study that associated this therapy with a nearly 80% reduction in face and scalp SCC.
Ultimately, she offers 5-fluorouracil with or without calcipotriol to “patients who want an evidence-based chemoprevention,” but she indicated that patients must be motivated to endure the adverse effects.
Many remain unaware of the array of options for chemoprevention of KCs, but Dr. Hartman emphasized that this is an area of active research with new options expected.
“I am really excited about the future direction of chemoprevention in skin cancer,” said Dr. Hartman, citing ongoing work to develop vitamin A, polypodium leucotomas extract, and human papillomavirus vaccine as options.
“If we can stop skin cancer in the first place, avoiding the morbidity and mortality of treatment, we will also hopefully save costs as well,” she commented. So far, essentially all of the strategies for chemoprevention, other than sunscreen, involve KCs, which leaves a large unmet need for better ways to prevent melanoma. However, Dr. Hartman noted that KCs represent the most common type of cancer of any type.
Just days after Dr. Hartman spoke at the meeting, a prospective study of vitamin A that found an inverse association between vitamin A intake and cutaneous SCC risk was, in fact, published in JAMA Dermatology (2019 Jul 31. doi: 10.1001/jamadermatol.2019.1937).
Dr. Hartman reported no financial relationships relevant to her presentation.
EXPERT ANALYSIS FROM SUMMER AAD 2019
FDA panel backs Descovy as HIV PrEP for men and transgender women who have sex with men
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
FROM AN FDA ADVISORY COMMITTEE MEETING
Vitamin D supplementation may improve ulcerative colitis
Vitamin D supplementation may lead to significant improvements in ulcerative colitis (UC), based on a placebo-controlled trial involving 60 patients with active disease.
Those who achieved vitamin D levels greater than 40 ng/mL were most likely to benefit, reported lead author Rizwan Ahamed Z, MD, of the Postgraduate Institute of Medical Education and Research in Chandigarh, India, and colleagues. They noted that the findings contribute much-needed clinical data to a largely theoretical subject area.
“[T]he discovery of vitamin D receptors on lymphocytes, monocytes, and dendritic cells initiated various studies which have highlighted the role of vitamin D in regulating gut mucosal immunity and gut barrier,” the investigators wrote in Journal of Clinical Gastroenterology. “In experimental interleukin (IL)-10 knockout mice models, vitamin D deficiency was found to result in severe colitis, progressive wasting, and high mortality. However, vitamin D supplementation not only prevented but also ameliorated symptoms of colitis in the mice model.”
Human studies have revealed similar associations between vitamin D supplementation and inflammatory bowel disease, such as a study by Jørgensen and colleagues that found a lower risk of relapse in Crohn’s disease, and another by Sharifi and colleagues that showed injectable vitamin D could reduce erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in patients with UC. Still, the investigators suggested that more clinical data are needed, particularly for outcomes after vitamin D therapy. In addition to providing such data, the present trial was also the first of its kind to test oral nano vitamin D3, which may have better bioavailability than conventional supplements.
The investigators initially recruited 110 patients with active UC who had an ulcerative colitis disease activity index (UCDAI) of at least 3. After screening, 50 patients were excluded because they had vitamin D levels greater than 40 ng/mL, were already taking a vitamin D supplement, had severe UC requiring hospitalization, or exhibited severe systemic illness. The remaining 60 patients were randomized in a 1:1 ratio to receive either 60,000 IU nano vitamin D3 once daily for 8 days, or placebo. Disease parameters, which were measured at baseline and then again at 4 weeks, included UCDAI, ESR, CRP, and fecal calprotectin. The primary outcome was response, defined as a UCDAI reduction of at least 3 points. Secondary outcome measures included stool frequency, stool consistency, and remission (UCDAI less than 3); in addition, the investigators evaluated histologic, endoscopic, fecal, and serum inflammatory markers.
The majority of patients in the study were men (60%), with a mean age of 36 years. Most patients had moderate UC (73.3%), while smaller proportions had severe (18%) or mild (8%) disease. All patients were taking a 5-aminosalicylic acid oral compound and some (16.6%) were also taking azathioprine. At baseline, the mean vitamin D level was 14 ng/mL. Most patients (70%) were diagnosed with vitamin D deficiency, based on measurements below 20 ng/mL. The remaining patients were diagnosed with insufficiency (13%; 20-30 ng/mL) or suboptimal levels (17%; 30-40 ng/mL).
From baseline to 4-week follow-up, median vitamin D level in the supplement group increased from 15.4 to 40.83 ng/mL, compared with a much smaller increase in the placebo group, from 13.45 to 18.85 ng/mL. Compared with the placebo group, significantly more patients given nano vitamin D3 achieved a UCDAI 3-point reduction (53% vs 13%; P = .001); this translated to a Pearson correlation coefficient (rho) of –0.713, between vitamin D level and UCDAI. Similar, albeit less strong, inverse relationships were detected between vitamin D level and CRP (rho = −0.603) and calprotectin (rho = −0.368).
Benefits observed in the supplement group also extended to stool frequency, stool consistency, and histologic measures. Those who achieved a vitamin D level greater than 40 ng/mL were 4 times more likely to have a UCDAI 3-point reduction than those who did not meet the same criteria (80% vs 20%; P = .038). Independent predictors of response included baseline histologic activity (odds ratio, 1.92), and to a greater extent, vitamin D supplementation (OR, 9.17). No patients achieved remission, which the investigators attributed to the relatively short study duration.
Minor, self-limiting side effects occurred in 13.3% and 10% of patients given the vitamin D supplement and placebo, respectively.
“[T]he present study showed significant improvement in all inflammatory parameters of the disease including clinical, endoscopic, histopathologic, and serum and fecal markers of inflammation, all of which paralleled each other in showing [the benefit of] oral nano vitamin D supplementation,” the investigators concluded. They advised that larger, longer-term studies are needed before the findings can be generalized to all patients with active UC.
The investigators disclosed no external funding or conflicts of interest.
SOURCE: Ahamed R et al. J Clin Gastroenterol. 2019 Jul 24. doi: 10.1097/MCG.0000000000001233.
Vitamin D supplementation may lead to significant improvements in ulcerative colitis (UC), based on a placebo-controlled trial involving 60 patients with active disease.
Those who achieved vitamin D levels greater than 40 ng/mL were most likely to benefit, reported lead author Rizwan Ahamed Z, MD, of the Postgraduate Institute of Medical Education and Research in Chandigarh, India, and colleagues. They noted that the findings contribute much-needed clinical data to a largely theoretical subject area.
“[T]he discovery of vitamin D receptors on lymphocytes, monocytes, and dendritic cells initiated various studies which have highlighted the role of vitamin D in regulating gut mucosal immunity and gut barrier,” the investigators wrote in Journal of Clinical Gastroenterology. “In experimental interleukin (IL)-10 knockout mice models, vitamin D deficiency was found to result in severe colitis, progressive wasting, and high mortality. However, vitamin D supplementation not only prevented but also ameliorated symptoms of colitis in the mice model.”
Human studies have revealed similar associations between vitamin D supplementation and inflammatory bowel disease, such as a study by Jørgensen and colleagues that found a lower risk of relapse in Crohn’s disease, and another by Sharifi and colleagues that showed injectable vitamin D could reduce erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in patients with UC. Still, the investigators suggested that more clinical data are needed, particularly for outcomes after vitamin D therapy. In addition to providing such data, the present trial was also the first of its kind to test oral nano vitamin D3, which may have better bioavailability than conventional supplements.
The investigators initially recruited 110 patients with active UC who had an ulcerative colitis disease activity index (UCDAI) of at least 3. After screening, 50 patients were excluded because they had vitamin D levels greater than 40 ng/mL, were already taking a vitamin D supplement, had severe UC requiring hospitalization, or exhibited severe systemic illness. The remaining 60 patients were randomized in a 1:1 ratio to receive either 60,000 IU nano vitamin D3 once daily for 8 days, or placebo. Disease parameters, which were measured at baseline and then again at 4 weeks, included UCDAI, ESR, CRP, and fecal calprotectin. The primary outcome was response, defined as a UCDAI reduction of at least 3 points. Secondary outcome measures included stool frequency, stool consistency, and remission (UCDAI less than 3); in addition, the investigators evaluated histologic, endoscopic, fecal, and serum inflammatory markers.
The majority of patients in the study were men (60%), with a mean age of 36 years. Most patients had moderate UC (73.3%), while smaller proportions had severe (18%) or mild (8%) disease. All patients were taking a 5-aminosalicylic acid oral compound and some (16.6%) were also taking azathioprine. At baseline, the mean vitamin D level was 14 ng/mL. Most patients (70%) were diagnosed with vitamin D deficiency, based on measurements below 20 ng/mL. The remaining patients were diagnosed with insufficiency (13%; 20-30 ng/mL) or suboptimal levels (17%; 30-40 ng/mL).
From baseline to 4-week follow-up, median vitamin D level in the supplement group increased from 15.4 to 40.83 ng/mL, compared with a much smaller increase in the placebo group, from 13.45 to 18.85 ng/mL. Compared with the placebo group, significantly more patients given nano vitamin D3 achieved a UCDAI 3-point reduction (53% vs 13%; P = .001); this translated to a Pearson correlation coefficient (rho) of –0.713, between vitamin D level and UCDAI. Similar, albeit less strong, inverse relationships were detected between vitamin D level and CRP (rho = −0.603) and calprotectin (rho = −0.368).
Benefits observed in the supplement group also extended to stool frequency, stool consistency, and histologic measures. Those who achieved a vitamin D level greater than 40 ng/mL were 4 times more likely to have a UCDAI 3-point reduction than those who did not meet the same criteria (80% vs 20%; P = .038). Independent predictors of response included baseline histologic activity (odds ratio, 1.92), and to a greater extent, vitamin D supplementation (OR, 9.17). No patients achieved remission, which the investigators attributed to the relatively short study duration.
Minor, self-limiting side effects occurred in 13.3% and 10% of patients given the vitamin D supplement and placebo, respectively.
“[T]he present study showed significant improvement in all inflammatory parameters of the disease including clinical, endoscopic, histopathologic, and serum and fecal markers of inflammation, all of which paralleled each other in showing [the benefit of] oral nano vitamin D supplementation,” the investigators concluded. They advised that larger, longer-term studies are needed before the findings can be generalized to all patients with active UC.
The investigators disclosed no external funding or conflicts of interest.
SOURCE: Ahamed R et al. J Clin Gastroenterol. 2019 Jul 24. doi: 10.1097/MCG.0000000000001233.
Vitamin D supplementation may lead to significant improvements in ulcerative colitis (UC), based on a placebo-controlled trial involving 60 patients with active disease.
Those who achieved vitamin D levels greater than 40 ng/mL were most likely to benefit, reported lead author Rizwan Ahamed Z, MD, of the Postgraduate Institute of Medical Education and Research in Chandigarh, India, and colleagues. They noted that the findings contribute much-needed clinical data to a largely theoretical subject area.
“[T]he discovery of vitamin D receptors on lymphocytes, monocytes, and dendritic cells initiated various studies which have highlighted the role of vitamin D in regulating gut mucosal immunity and gut barrier,” the investigators wrote in Journal of Clinical Gastroenterology. “In experimental interleukin (IL)-10 knockout mice models, vitamin D deficiency was found to result in severe colitis, progressive wasting, and high mortality. However, vitamin D supplementation not only prevented but also ameliorated symptoms of colitis in the mice model.”
Human studies have revealed similar associations between vitamin D supplementation and inflammatory bowel disease, such as a study by Jørgensen and colleagues that found a lower risk of relapse in Crohn’s disease, and another by Sharifi and colleagues that showed injectable vitamin D could reduce erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in patients with UC. Still, the investigators suggested that more clinical data are needed, particularly for outcomes after vitamin D therapy. In addition to providing such data, the present trial was also the first of its kind to test oral nano vitamin D3, which may have better bioavailability than conventional supplements.
The investigators initially recruited 110 patients with active UC who had an ulcerative colitis disease activity index (UCDAI) of at least 3. After screening, 50 patients were excluded because they had vitamin D levels greater than 40 ng/mL, were already taking a vitamin D supplement, had severe UC requiring hospitalization, or exhibited severe systemic illness. The remaining 60 patients were randomized in a 1:1 ratio to receive either 60,000 IU nano vitamin D3 once daily for 8 days, or placebo. Disease parameters, which were measured at baseline and then again at 4 weeks, included UCDAI, ESR, CRP, and fecal calprotectin. The primary outcome was response, defined as a UCDAI reduction of at least 3 points. Secondary outcome measures included stool frequency, stool consistency, and remission (UCDAI less than 3); in addition, the investigators evaluated histologic, endoscopic, fecal, and serum inflammatory markers.
The majority of patients in the study were men (60%), with a mean age of 36 years. Most patients had moderate UC (73.3%), while smaller proportions had severe (18%) or mild (8%) disease. All patients were taking a 5-aminosalicylic acid oral compound and some (16.6%) were also taking azathioprine. At baseline, the mean vitamin D level was 14 ng/mL. Most patients (70%) were diagnosed with vitamin D deficiency, based on measurements below 20 ng/mL. The remaining patients were diagnosed with insufficiency (13%; 20-30 ng/mL) or suboptimal levels (17%; 30-40 ng/mL).
From baseline to 4-week follow-up, median vitamin D level in the supplement group increased from 15.4 to 40.83 ng/mL, compared with a much smaller increase in the placebo group, from 13.45 to 18.85 ng/mL. Compared with the placebo group, significantly more patients given nano vitamin D3 achieved a UCDAI 3-point reduction (53% vs 13%; P = .001); this translated to a Pearson correlation coefficient (rho) of –0.713, between vitamin D level and UCDAI. Similar, albeit less strong, inverse relationships were detected between vitamin D level and CRP (rho = −0.603) and calprotectin (rho = −0.368).
Benefits observed in the supplement group also extended to stool frequency, stool consistency, and histologic measures. Those who achieved a vitamin D level greater than 40 ng/mL were 4 times more likely to have a UCDAI 3-point reduction than those who did not meet the same criteria (80% vs 20%; P = .038). Independent predictors of response included baseline histologic activity (odds ratio, 1.92), and to a greater extent, vitamin D supplementation (OR, 9.17). No patients achieved remission, which the investigators attributed to the relatively short study duration.
Minor, self-limiting side effects occurred in 13.3% and 10% of patients given the vitamin D supplement and placebo, respectively.
“[T]he present study showed significant improvement in all inflammatory parameters of the disease including clinical, endoscopic, histopathologic, and serum and fecal markers of inflammation, all of which paralleled each other in showing [the benefit of] oral nano vitamin D supplementation,” the investigators concluded. They advised that larger, longer-term studies are needed before the findings can be generalized to all patients with active UC.
The investigators disclosed no external funding or conflicts of interest.
SOURCE: Ahamed R et al. J Clin Gastroenterol. 2019 Jul 24. doi: 10.1097/MCG.0000000000001233.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Bevacizumab or pemetrexed, but not both, efficacious for NSCLC maintenance
Single-agent therapy with either bevacizumab or pemetrexed is efficacious as maintenance therapy for patients with advanced nonsquamous non–small cell lung cancer (NSCLC), but the combination of the two agents offers no survival benefit and is more toxic, results of the randomized ECOG-ACRIN 5508 study show.
For patients with no disease progression after four cycles of induction chemotherapy who were assigned to one of three maintenance therapy strategies, there were no differences in overall survival between those randomized to monotherapy with pemetrexed or bevacizumab or to a combination of the two agents, although progression-free survival (PFS) was better with the combination, reported Suresh S. Ramalingam, MD, from the Winship Cancer Institute of Emory University, Atlanta, and colleagues.
The incidence of grade 3 or greater adverse events was also significantly higher with the combination, compared with bevacizumab monotherapy.
Even in the age of immune checkpoint inhibitor therapy in the front line, “[i]t is clear that maintenance therapy will remain an integral part of the treatment approach to advanced nonsquamous NSCLC. The results of ECOG-ACRIN 5508 support the use of either pemetrexed or bevacizumab as a single agent in this setting,” the investigators wrote in the Journal of Clinical Oncology.
Although the combination of bevacizumab and pemetrexed was associated with a significant improvement in PFS in a randomized trial, the three maintenance strategies have never before been directly compared, Dr. Ramalingam and associates noted.
In ECOG-ACRIN 5508, 1,516 patients with advanced nonsquamous NSCLC who had not received prior systemic therapy were given standard induction chemotherapy, consisting of carboplatin to an area under the curve of 6, paclitaxel 200 mg/m2, and bevacizumab 15 mg/kg for up to four cycles.
Patients without progression after four cycles (874) were randomly assigned to maintenance therapy with bevacizumab at 15 mg/kg , pemetrexed 500 mg/m2, or a combination of the two agents.
For the primary endpoint of overall survival, with bevacizumab serving as the control group for comparison, the investigators found that, at a median follow-up of 50.6 months, median survival was 15.9 months with pemetrexed, compared with 14.4 months with bevacizumab, a difference that was not statistically significant. Median survival with the combination was 16.4 months and was also not significantly different from bevacizumab.
Median PFS was 4.2 months and 5.1 months for the pemetrexed and bevacizumab groups, respectively, with no significant difference. In contrast, median was 7.5 months with the combination, which was significantly better than controls, with a hazard ratio of 0.67 (P less than .001).
Patients received a median of six maintenance therapy cycles for each of the single agents, and a median of eight for the combinations. The incidence of grade 3-4 toxicity was 29% with bevacizumab, 37% with pemetrexed, and 51% with the combination. The combination was associated with a significantly greater incidence of toxicities, compared with bevacizumab (P less than .001).
The study was supported by grants from the National Cancer Institute. Dr. Ramalingam reported a consulting/advisory role for bevacizumab maker Genentech/Roche and others. Multiple coauthors reported similar disclosures.
SOURCE: Ramalingam SS et al. J Clin Oncol. 2019 Jul 30. doi: 10.1200/JCO.19.01006.
Single-agent therapy with either bevacizumab or pemetrexed is efficacious as maintenance therapy for patients with advanced nonsquamous non–small cell lung cancer (NSCLC), but the combination of the two agents offers no survival benefit and is more toxic, results of the randomized ECOG-ACRIN 5508 study show.
For patients with no disease progression after four cycles of induction chemotherapy who were assigned to one of three maintenance therapy strategies, there were no differences in overall survival between those randomized to monotherapy with pemetrexed or bevacizumab or to a combination of the two agents, although progression-free survival (PFS) was better with the combination, reported Suresh S. Ramalingam, MD, from the Winship Cancer Institute of Emory University, Atlanta, and colleagues.
The incidence of grade 3 or greater adverse events was also significantly higher with the combination, compared with bevacizumab monotherapy.
Even in the age of immune checkpoint inhibitor therapy in the front line, “[i]t is clear that maintenance therapy will remain an integral part of the treatment approach to advanced nonsquamous NSCLC. The results of ECOG-ACRIN 5508 support the use of either pemetrexed or bevacizumab as a single agent in this setting,” the investigators wrote in the Journal of Clinical Oncology.
Although the combination of bevacizumab and pemetrexed was associated with a significant improvement in PFS in a randomized trial, the three maintenance strategies have never before been directly compared, Dr. Ramalingam and associates noted.
In ECOG-ACRIN 5508, 1,516 patients with advanced nonsquamous NSCLC who had not received prior systemic therapy were given standard induction chemotherapy, consisting of carboplatin to an area under the curve of 6, paclitaxel 200 mg/m2, and bevacizumab 15 mg/kg for up to four cycles.
Patients without progression after four cycles (874) were randomly assigned to maintenance therapy with bevacizumab at 15 mg/kg , pemetrexed 500 mg/m2, or a combination of the two agents.
For the primary endpoint of overall survival, with bevacizumab serving as the control group for comparison, the investigators found that, at a median follow-up of 50.6 months, median survival was 15.9 months with pemetrexed, compared with 14.4 months with bevacizumab, a difference that was not statistically significant. Median survival with the combination was 16.4 months and was also not significantly different from bevacizumab.
Median PFS was 4.2 months and 5.1 months for the pemetrexed and bevacizumab groups, respectively, with no significant difference. In contrast, median was 7.5 months with the combination, which was significantly better than controls, with a hazard ratio of 0.67 (P less than .001).
Patients received a median of six maintenance therapy cycles for each of the single agents, and a median of eight for the combinations. The incidence of grade 3-4 toxicity was 29% with bevacizumab, 37% with pemetrexed, and 51% with the combination. The combination was associated with a significantly greater incidence of toxicities, compared with bevacizumab (P less than .001).
The study was supported by grants from the National Cancer Institute. Dr. Ramalingam reported a consulting/advisory role for bevacizumab maker Genentech/Roche and others. Multiple coauthors reported similar disclosures.
SOURCE: Ramalingam SS et al. J Clin Oncol. 2019 Jul 30. doi: 10.1200/JCO.19.01006.
Single-agent therapy with either bevacizumab or pemetrexed is efficacious as maintenance therapy for patients with advanced nonsquamous non–small cell lung cancer (NSCLC), but the combination of the two agents offers no survival benefit and is more toxic, results of the randomized ECOG-ACRIN 5508 study show.
For patients with no disease progression after four cycles of induction chemotherapy who were assigned to one of three maintenance therapy strategies, there were no differences in overall survival between those randomized to monotherapy with pemetrexed or bevacizumab or to a combination of the two agents, although progression-free survival (PFS) was better with the combination, reported Suresh S. Ramalingam, MD, from the Winship Cancer Institute of Emory University, Atlanta, and colleagues.
The incidence of grade 3 or greater adverse events was also significantly higher with the combination, compared with bevacizumab monotherapy.
Even in the age of immune checkpoint inhibitor therapy in the front line, “[i]t is clear that maintenance therapy will remain an integral part of the treatment approach to advanced nonsquamous NSCLC. The results of ECOG-ACRIN 5508 support the use of either pemetrexed or bevacizumab as a single agent in this setting,” the investigators wrote in the Journal of Clinical Oncology.
Although the combination of bevacizumab and pemetrexed was associated with a significant improvement in PFS in a randomized trial, the three maintenance strategies have never before been directly compared, Dr. Ramalingam and associates noted.
In ECOG-ACRIN 5508, 1,516 patients with advanced nonsquamous NSCLC who had not received prior systemic therapy were given standard induction chemotherapy, consisting of carboplatin to an area under the curve of 6, paclitaxel 200 mg/m2, and bevacizumab 15 mg/kg for up to four cycles.
Patients without progression after four cycles (874) were randomly assigned to maintenance therapy with bevacizumab at 15 mg/kg , pemetrexed 500 mg/m2, or a combination of the two agents.
For the primary endpoint of overall survival, with bevacizumab serving as the control group for comparison, the investigators found that, at a median follow-up of 50.6 months, median survival was 15.9 months with pemetrexed, compared with 14.4 months with bevacizumab, a difference that was not statistically significant. Median survival with the combination was 16.4 months and was also not significantly different from bevacizumab.
Median PFS was 4.2 months and 5.1 months for the pemetrexed and bevacizumab groups, respectively, with no significant difference. In contrast, median was 7.5 months with the combination, which was significantly better than controls, with a hazard ratio of 0.67 (P less than .001).
Patients received a median of six maintenance therapy cycles for each of the single agents, and a median of eight for the combinations. The incidence of grade 3-4 toxicity was 29% with bevacizumab, 37% with pemetrexed, and 51% with the combination. The combination was associated with a significantly greater incidence of toxicities, compared with bevacizumab (P less than .001).
The study was supported by grants from the National Cancer Institute. Dr. Ramalingam reported a consulting/advisory role for bevacizumab maker Genentech/Roche and others. Multiple coauthors reported similar disclosures.
SOURCE: Ramalingam SS et al. J Clin Oncol. 2019 Jul 30. doi: 10.1200/JCO.19.01006.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
NSAIDs a significant mediator of cardiovascular risk in osteoarthritis
Writing in Arthritis & Rheumatology, researchers reported the outcomes of a longitudinal, population-based cohort study of 7,743 individuals with osteoarthritis patients and 23,229 age- and sex-matched controls without osteoarthritis.
“The prevailing hypothesis in the OA to CVD relationship has been that OA patients frequently take NSAIDs to control their pain and inflammation and that this may lead to them developing CVD,” wrote Mohammad Atiquzzaman, a PhD student at the University of British Columbia, Vancouver, and his coauthors. However they commented that no studies had so far examined this directly in patients with osteoarthritis.
Overall, people with osteoarthritis had a significant 23% higher risk of cardiovascular disease, compared with controls, after adjustment for factors such body mass index, hypertension, diabetes, hyperlipidemia, and socioeconomic status. They also had a 42% higher risk of congestive heart failure, 17% higher risk of ischemic heart disease, and 14% higher risk of stroke.
NSAID use was five times more common among people with osteoarthritis, and NSAIDs alone were associated with a greater than fourfold higher risk of cardiovascular disease, after adjusting for osteoarthritis and other potential confounders.
When the authors performed modeling to break down the effect of osteoarthritis on CVD risk into the direct effect of osteoarthritis itself and the indirect effect mediated by NSAID use, they concluded that 41% of the total effect of osteoarthritis on cardiovascular risk was mediated by NSAIDs. The effect of NSAIDs was particularly pronounced for stroke, in which cases they estimated that the drugs contributed to 64% of the increased in risk, and in ischemic heart disease, in which they contributed to 56% of the increased risk.
Subgroup analysis suggested that conventional NSAIDs were responsible for around 29% of the total increased risk of cardiovascular disease, while selective COX-2 inhibitors, or coxibs, such as celecoxib, lumiracoxib, rofecoxib, and valdecoxib mediated around 21%. For ischemic heart disease, conventional NSAIDs explained around 45% of the increased risk, while selective coxibs explained around 32% of the risk. Similarly, with congestive heart failure and stroke, the proportion of risk mediated by NSAIDs was higher for conventional NSAIDs, compared with coxibs.
The authors noted that while a number of previous studies have found osteoarthritis is an independent risk factor for cardiovascular disease, theirs was the first study to specifically examine the role that NSAIDs play in that increased risk.
However, they noted that their information on NSAID use was gleaned from prescription claims data, which did not include information on over-the-counter NSAID use. Their analysis was also unable to include information on family history of cardiovascular disease, smoking, and physical activity, which are important cardiovascular disease risk factors. They did observe that the rates of obesity were higher among the osteoarthritis group when compared with controls (29% vs. 20%), and hypertension and COPD were also more common among individuals with osteoarthritis.
There was no outside funding for the study, and the authors had no conflicts of interest to declare.
SOURCE: Atiquzzaman M et al. Arthritis Rheumatol. 2019 Aug 6. doi: 10.1002/art.41027
Writing in Arthritis & Rheumatology, researchers reported the outcomes of a longitudinal, population-based cohort study of 7,743 individuals with osteoarthritis patients and 23,229 age- and sex-matched controls without osteoarthritis.
“The prevailing hypothesis in the OA to CVD relationship has been that OA patients frequently take NSAIDs to control their pain and inflammation and that this may lead to them developing CVD,” wrote Mohammad Atiquzzaman, a PhD student at the University of British Columbia, Vancouver, and his coauthors. However they commented that no studies had so far examined this directly in patients with osteoarthritis.
Overall, people with osteoarthritis had a significant 23% higher risk of cardiovascular disease, compared with controls, after adjustment for factors such body mass index, hypertension, diabetes, hyperlipidemia, and socioeconomic status. They also had a 42% higher risk of congestive heart failure, 17% higher risk of ischemic heart disease, and 14% higher risk of stroke.
NSAID use was five times more common among people with osteoarthritis, and NSAIDs alone were associated with a greater than fourfold higher risk of cardiovascular disease, after adjusting for osteoarthritis and other potential confounders.
When the authors performed modeling to break down the effect of osteoarthritis on CVD risk into the direct effect of osteoarthritis itself and the indirect effect mediated by NSAID use, they concluded that 41% of the total effect of osteoarthritis on cardiovascular risk was mediated by NSAIDs. The effect of NSAIDs was particularly pronounced for stroke, in which cases they estimated that the drugs contributed to 64% of the increased in risk, and in ischemic heart disease, in which they contributed to 56% of the increased risk.
Subgroup analysis suggested that conventional NSAIDs were responsible for around 29% of the total increased risk of cardiovascular disease, while selective COX-2 inhibitors, or coxibs, such as celecoxib, lumiracoxib, rofecoxib, and valdecoxib mediated around 21%. For ischemic heart disease, conventional NSAIDs explained around 45% of the increased risk, while selective coxibs explained around 32% of the risk. Similarly, with congestive heart failure and stroke, the proportion of risk mediated by NSAIDs was higher for conventional NSAIDs, compared with coxibs.
The authors noted that while a number of previous studies have found osteoarthritis is an independent risk factor for cardiovascular disease, theirs was the first study to specifically examine the role that NSAIDs play in that increased risk.
However, they noted that their information on NSAID use was gleaned from prescription claims data, which did not include information on over-the-counter NSAID use. Their analysis was also unable to include information on family history of cardiovascular disease, smoking, and physical activity, which are important cardiovascular disease risk factors. They did observe that the rates of obesity were higher among the osteoarthritis group when compared with controls (29% vs. 20%), and hypertension and COPD were also more common among individuals with osteoarthritis.
There was no outside funding for the study, and the authors had no conflicts of interest to declare.
SOURCE: Atiquzzaman M et al. Arthritis Rheumatol. 2019 Aug 6. doi: 10.1002/art.41027
Writing in Arthritis & Rheumatology, researchers reported the outcomes of a longitudinal, population-based cohort study of 7,743 individuals with osteoarthritis patients and 23,229 age- and sex-matched controls without osteoarthritis.
“The prevailing hypothesis in the OA to CVD relationship has been that OA patients frequently take NSAIDs to control their pain and inflammation and that this may lead to them developing CVD,” wrote Mohammad Atiquzzaman, a PhD student at the University of British Columbia, Vancouver, and his coauthors. However they commented that no studies had so far examined this directly in patients with osteoarthritis.
Overall, people with osteoarthritis had a significant 23% higher risk of cardiovascular disease, compared with controls, after adjustment for factors such body mass index, hypertension, diabetes, hyperlipidemia, and socioeconomic status. They also had a 42% higher risk of congestive heart failure, 17% higher risk of ischemic heart disease, and 14% higher risk of stroke.
NSAID use was five times more common among people with osteoarthritis, and NSAIDs alone were associated with a greater than fourfold higher risk of cardiovascular disease, after adjusting for osteoarthritis and other potential confounders.
When the authors performed modeling to break down the effect of osteoarthritis on CVD risk into the direct effect of osteoarthritis itself and the indirect effect mediated by NSAID use, they concluded that 41% of the total effect of osteoarthritis on cardiovascular risk was mediated by NSAIDs. The effect of NSAIDs was particularly pronounced for stroke, in which cases they estimated that the drugs contributed to 64% of the increased in risk, and in ischemic heart disease, in which they contributed to 56% of the increased risk.
Subgroup analysis suggested that conventional NSAIDs were responsible for around 29% of the total increased risk of cardiovascular disease, while selective COX-2 inhibitors, or coxibs, such as celecoxib, lumiracoxib, rofecoxib, and valdecoxib mediated around 21%. For ischemic heart disease, conventional NSAIDs explained around 45% of the increased risk, while selective coxibs explained around 32% of the risk. Similarly, with congestive heart failure and stroke, the proportion of risk mediated by NSAIDs was higher for conventional NSAIDs, compared with coxibs.
The authors noted that while a number of previous studies have found osteoarthritis is an independent risk factor for cardiovascular disease, theirs was the first study to specifically examine the role that NSAIDs play in that increased risk.
However, they noted that their information on NSAID use was gleaned from prescription claims data, which did not include information on over-the-counter NSAID use. Their analysis was also unable to include information on family history of cardiovascular disease, smoking, and physical activity, which are important cardiovascular disease risk factors. They did observe that the rates of obesity were higher among the osteoarthritis group when compared with controls (29% vs. 20%), and hypertension and COPD were also more common among individuals with osteoarthritis.
There was no outside funding for the study, and the authors had no conflicts of interest to declare.
SOURCE: Atiquzzaman M et al. Arthritis Rheumatol. 2019 Aug 6. doi: 10.1002/art.41027
FROM ARTHRITIS & RHEUMATOLOGY