User login
Sleep aids and dementia: Studies find both risks and benefits
LOS ANGELES – While a large number of older adults take prescription and nonprescription medications to help them sleep, the effect of these medications on dementia risk is unclear, with most researchers advocating a cautious and conservative approach to prescribing.
Research is increasingly revealing a bidirectional relationship between sleep and dementia. Poor sleep – especially from insomnia, sleep deprivation, or obstructive sleep apnea – is known to increase dementia risk. Dementias, meanwhile, are associated with serious circadian rhythm disturbances, leading to nighttime sleep loss and increasing the likelihood of institutionalization.
At the Alzheimer’s Association International Conference, researchers presented findings assessing the links between sleep medication use and dementia and also what agents or approaches might safely improve sleep in people with sleep disorders who are at risk for dementia or who have been diagnosed with dementia.
Sex- and race-based differences in risk
Yue Leng, PhD, of the University of California, San Francisco, reported a link between frequent sleep medication use and later dementia – but only in white adults. Dr. Leng presented findings from the National Institutes of Health–funded Health, Aging, and Body Composition Study, which recruited 3,068 subjects aged 70-79 and followed them for 15 years. At baseline, 2.7% of African Americans and 7.7% of whites in the study reported taking sleep medications “often” or “almost always.”
Dr. Leng and her colleagues found that white subjects who reported taking sleep aids five or more times a month at baseline had a nearly 80% higher risk of developing dementia during the course of the study (hazard ratio, 1.79; 95% confidence interval, 1.21-2.66), compared with people who reported never taking sleep aids or taking them less frequently.
The researchers saw no between-sex differences for this finding, and adjusted for a variety of genetic and lifestyle confounders. Importantly, no significant increase in dementia risk was seen for black subjects, who made up more than one-third of the cohort.
Dr. Leng told the conference that the researchers could not explain why black participants did not see similarly increased dementia risk. Also, she noted, researchers did not have information on the specific sleep medications people used: benzodiazepines, antihistamines, antidepressants, or other types of drugs. Nonetheless, she told the conference, the findings ratified the cautious approach many dementia experts are already stressing.
“Do we really need to prescribe so many sleep meds to older adults who are already at risk for cognitive impairment?” Dr. Leng said, adding: “I am a big advocate of behavioral sleep interventions.” People with clinical sleep problems “should be referred to sleep centers” for a fuller assessment before medication is prescribed, she said.
Findings from another cohort study, meanwhile, suggest that there could be sex-related differences in how sleep aids affect dementia risk. Investigators at Utah State University in Logan used data from some 3,656 older adults in the Cache County Study on Memory and Aging, an NIH-backed cohort study of white adults in Utah without dementia at baseline who were followed for 12 years.
The investigators, led by doctoral student Elizabeth Vernon, found that men reporting use of sleep medication saw more than threefold higher risk of developing Alzheimer’s disease than did men who did not use sleep aids (HR, 3.604; P = .0001).
Women who did not report having sleep disturbance but used sleep-inducing medications were at nearly fourfold greater risk for developing Alzheimer’s disease (HR, 3.916; P = .0001). Women who self-reported sleep disturbances at baseline, meanwhile, saw a reduction in Alzheimer’s risk of about one-third associated with the use of sleep medications.
Ms. Vernon told the conference that, despite the finding of risk reduction for this particular group of women, caution in prescribing sleep aids was warranted.
Common sleep drugs linked to cognitive aging
Chris Fox, MD, a researcher at the University of East Anglia in Norwich, England, and his colleagues demonstrated in 2018 that long-term exposure to anticholinergic drugs, a class that includes some antidepressants and antihistamines used to promote sleep, was associated with a higher risk of dementia, while use of benzodiazepines, a class of sedatives used commonly in older people as sleep aids, was not. (Whether benzodiazepine exposure relates to dementia remains controversial.)
At AAIC 2019, Dr. Fox presented findings from a study of 337 brains in a U.K. brain bank, of which 17% and 21% came from users of benzodiazepines and anticholinergic drugs, whose usage history was well documented. Dr. Fox and his colleagues found that, while neither anticholinergic nor benzodiazepine exposure was associated with brain pathology specific to that seen in Alzheimer’s disease, both classes of drugs were associated with “slight signals in neuronal loss” in one brain region, the nucleus basalis of Meynert. Dr. Fox described the drugs as causing “an increase in cognitive aging” which could bear on Alzheimer’s risk without being directly causative.
Newer sleep drugs may help Alzheimer’s patients
Scientists working for drug manufacturers presented findings on agents to counter the circadian rhythm disturbances seen in people with Alzheimer’s disease. Margaret Moline, PhD, of Eisai in Woodcliff Lake, N.J., showed some results from a phase 2, dose-ranging, placebo-controlled study of the experimental agent lemborexant in 62 subjects aged 60-90 with mild to moderate Alzheimer’s disease and sleep disturbances. (Lemborexant, an orexin receptor agonist that acts to regulate wakefulness, is being investigated in a broad range of sleep disorders.) Patients were randomized to one of four doses of lemborexant or placebo and wore a device for sleep monitoring. Nighttime activity indicating arousal was significantly lower for people in two dosage arms, 5 mg and 10 mg, compared with placebo, and treatment groups saw trends toward less sleep fragmentation and higher total sleep time, Dr. Moline told the conference.
Suvorexant (Belsomra), the only orexin receptor antagonist currently licensed as a sleep aid, is also being tested in people with Alzheimer’s disease. At AAIC 2019, Joseph Herring, MD, PhD, of Merck in Kenilworth, N.J., presented results from a placebo-controlled trial of 277 patients with Alzheimer’s disease and insomnia, and reported that treatment with 10 or 20 mg of suvorexant over 4 weeks was associated with about an extra half hour of total nightly sleep, with a 73-minute mean increase from baseline, compared with 45 minutes for patients receiving placebo (95% CI, 11-45; P less than .005).
Trazodone linked to slower cognitive decline
An inexpensive antidepressant used in low doses as a sleep aid, including in people with Alzheimer’s disease, was associated with a delay in cognitive decline in older adults, according to results from a retrospective study. Elissaios Karageorgiou, MD, PhD, of the University of California, San Francisco, and the Neurological Institute of Athens presented results derived from two cohorts: patients enrolled at the UCSF Memory and Aging Center and women enrolled in the Study for Osteoporotic Fractures (SOF) in Women. The investigators were able to identify trazodone users in the studies (with two or more contiguous study visits reporting trazodone use) and match them with control patients from the same cohorts who did not use trazodone.
Trazodone was studied because previous research suggests it increases total sleep time in patients with Alzheimer’s disease without affecting next-day cognitive performance.
Trazodone-using patients in the UCSF cohort (n = 25) saw significantly less decline in Mini Mental State Exam (MMSE) scores over 4 years, compared with nonusers (0.27 vs. 0.70 points per year; P = .023), an effect that remained statistically significant even after adjusting for sedative and stimulant use and the expected progression of Alzheimer’s disease pathology. Importantly, the slower decline was seen only among subjects with sleep complaints at baseline and especially those whose sleep improved over time, suggesting that the cognitive benefit was mediated by improved sleep.
In the SOF cohort of 46 trazodone users matched with 148 nonusers, no significant protective or negative effect related to long-term trazodone use was found using the MMSE or the Trails Making Test. In this analysis, however, baseline and longitudinal sleep quality was not captured in the group-matching process, neither was the use of other medications. The patient group was slightly older, and all patients were women.
Dr. Karageorgiou said in an interview that the link between improved sleep, trazodone, and cognition needs to be validated in prospective intervention studies. Trazodone, he said, appears to work best in people with a specific type of insomnia characterized by cortical and behavioral hyperarousal, and its cognitive effect appears limited to people whose sleep improves with treatment. “You’re not going to see long-term cognitive benefits if it’s not improving your sleep,” Dr. Karageorgiou said. “So, whether trazodone improves sleep or not in a patient after a few months can be an early indicator for the clinician to continue using it or suspend it, because it is unlikely to help their cognition otherwise.”
He stressed that physicians need to be broadly focused on improving sleep to help patients with, or at risk for, dementia by consolidating their sleep rhythms.
“Trazodone is not the magic bullet, and I don’t think we will ever have a magic bullet,” Dr. Karageorgiou said. “Because when our brain degenerates, it’s not just one chemical, or one system, it’s many. And our body changes as well. The important thing is to help the patient consolidate their rhythms, whether through light therapy, daily exercise, cognitive behavioral therapy for insomnia, or other evidence-based interventions and their combination. The same applies for a person with dementia as for the rest of us.”
None of the investigators outside of the industry-sponsored studies had relevant disclosures.
LOS ANGELES – While a large number of older adults take prescription and nonprescription medications to help them sleep, the effect of these medications on dementia risk is unclear, with most researchers advocating a cautious and conservative approach to prescribing.
Research is increasingly revealing a bidirectional relationship between sleep and dementia. Poor sleep – especially from insomnia, sleep deprivation, or obstructive sleep apnea – is known to increase dementia risk. Dementias, meanwhile, are associated with serious circadian rhythm disturbances, leading to nighttime sleep loss and increasing the likelihood of institutionalization.
At the Alzheimer’s Association International Conference, researchers presented findings assessing the links between sleep medication use and dementia and also what agents or approaches might safely improve sleep in people with sleep disorders who are at risk for dementia or who have been diagnosed with dementia.
Sex- and race-based differences in risk
Yue Leng, PhD, of the University of California, San Francisco, reported a link between frequent sleep medication use and later dementia – but only in white adults. Dr. Leng presented findings from the National Institutes of Health–funded Health, Aging, and Body Composition Study, which recruited 3,068 subjects aged 70-79 and followed them for 15 years. At baseline, 2.7% of African Americans and 7.7% of whites in the study reported taking sleep medications “often” or “almost always.”
Dr. Leng and her colleagues found that white subjects who reported taking sleep aids five or more times a month at baseline had a nearly 80% higher risk of developing dementia during the course of the study (hazard ratio, 1.79; 95% confidence interval, 1.21-2.66), compared with people who reported never taking sleep aids or taking them less frequently.
The researchers saw no between-sex differences for this finding, and adjusted for a variety of genetic and lifestyle confounders. Importantly, no significant increase in dementia risk was seen for black subjects, who made up more than one-third of the cohort.
Dr. Leng told the conference that the researchers could not explain why black participants did not see similarly increased dementia risk. Also, she noted, researchers did not have information on the specific sleep medications people used: benzodiazepines, antihistamines, antidepressants, or other types of drugs. Nonetheless, she told the conference, the findings ratified the cautious approach many dementia experts are already stressing.
“Do we really need to prescribe so many sleep meds to older adults who are already at risk for cognitive impairment?” Dr. Leng said, adding: “I am a big advocate of behavioral sleep interventions.” People with clinical sleep problems “should be referred to sleep centers” for a fuller assessment before medication is prescribed, she said.
Findings from another cohort study, meanwhile, suggest that there could be sex-related differences in how sleep aids affect dementia risk. Investigators at Utah State University in Logan used data from some 3,656 older adults in the Cache County Study on Memory and Aging, an NIH-backed cohort study of white adults in Utah without dementia at baseline who were followed for 12 years.
The investigators, led by doctoral student Elizabeth Vernon, found that men reporting use of sleep medication saw more than threefold higher risk of developing Alzheimer’s disease than did men who did not use sleep aids (HR, 3.604; P = .0001).
Women who did not report having sleep disturbance but used sleep-inducing medications were at nearly fourfold greater risk for developing Alzheimer’s disease (HR, 3.916; P = .0001). Women who self-reported sleep disturbances at baseline, meanwhile, saw a reduction in Alzheimer’s risk of about one-third associated with the use of sleep medications.
Ms. Vernon told the conference that, despite the finding of risk reduction for this particular group of women, caution in prescribing sleep aids was warranted.
Common sleep drugs linked to cognitive aging
Chris Fox, MD, a researcher at the University of East Anglia in Norwich, England, and his colleagues demonstrated in 2018 that long-term exposure to anticholinergic drugs, a class that includes some antidepressants and antihistamines used to promote sleep, was associated with a higher risk of dementia, while use of benzodiazepines, a class of sedatives used commonly in older people as sleep aids, was not. (Whether benzodiazepine exposure relates to dementia remains controversial.)
At AAIC 2019, Dr. Fox presented findings from a study of 337 brains in a U.K. brain bank, of which 17% and 21% came from users of benzodiazepines and anticholinergic drugs, whose usage history was well documented. Dr. Fox and his colleagues found that, while neither anticholinergic nor benzodiazepine exposure was associated with brain pathology specific to that seen in Alzheimer’s disease, both classes of drugs were associated with “slight signals in neuronal loss” in one brain region, the nucleus basalis of Meynert. Dr. Fox described the drugs as causing “an increase in cognitive aging” which could bear on Alzheimer’s risk without being directly causative.
Newer sleep drugs may help Alzheimer’s patients
Scientists working for drug manufacturers presented findings on agents to counter the circadian rhythm disturbances seen in people with Alzheimer’s disease. Margaret Moline, PhD, of Eisai in Woodcliff Lake, N.J., showed some results from a phase 2, dose-ranging, placebo-controlled study of the experimental agent lemborexant in 62 subjects aged 60-90 with mild to moderate Alzheimer’s disease and sleep disturbances. (Lemborexant, an orexin receptor agonist that acts to regulate wakefulness, is being investigated in a broad range of sleep disorders.) Patients were randomized to one of four doses of lemborexant or placebo and wore a device for sleep monitoring. Nighttime activity indicating arousal was significantly lower for people in two dosage arms, 5 mg and 10 mg, compared with placebo, and treatment groups saw trends toward less sleep fragmentation and higher total sleep time, Dr. Moline told the conference.
Suvorexant (Belsomra), the only orexin receptor antagonist currently licensed as a sleep aid, is also being tested in people with Alzheimer’s disease. At AAIC 2019, Joseph Herring, MD, PhD, of Merck in Kenilworth, N.J., presented results from a placebo-controlled trial of 277 patients with Alzheimer’s disease and insomnia, and reported that treatment with 10 or 20 mg of suvorexant over 4 weeks was associated with about an extra half hour of total nightly sleep, with a 73-minute mean increase from baseline, compared with 45 minutes for patients receiving placebo (95% CI, 11-45; P less than .005).
Trazodone linked to slower cognitive decline
An inexpensive antidepressant used in low doses as a sleep aid, including in people with Alzheimer’s disease, was associated with a delay in cognitive decline in older adults, according to results from a retrospective study. Elissaios Karageorgiou, MD, PhD, of the University of California, San Francisco, and the Neurological Institute of Athens presented results derived from two cohorts: patients enrolled at the UCSF Memory and Aging Center and women enrolled in the Study for Osteoporotic Fractures (SOF) in Women. The investigators were able to identify trazodone users in the studies (with two or more contiguous study visits reporting trazodone use) and match them with control patients from the same cohorts who did not use trazodone.
Trazodone was studied because previous research suggests it increases total sleep time in patients with Alzheimer’s disease without affecting next-day cognitive performance.
Trazodone-using patients in the UCSF cohort (n = 25) saw significantly less decline in Mini Mental State Exam (MMSE) scores over 4 years, compared with nonusers (0.27 vs. 0.70 points per year; P = .023), an effect that remained statistically significant even after adjusting for sedative and stimulant use and the expected progression of Alzheimer’s disease pathology. Importantly, the slower decline was seen only among subjects with sleep complaints at baseline and especially those whose sleep improved over time, suggesting that the cognitive benefit was mediated by improved sleep.
In the SOF cohort of 46 trazodone users matched with 148 nonusers, no significant protective or negative effect related to long-term trazodone use was found using the MMSE or the Trails Making Test. In this analysis, however, baseline and longitudinal sleep quality was not captured in the group-matching process, neither was the use of other medications. The patient group was slightly older, and all patients were women.
Dr. Karageorgiou said in an interview that the link between improved sleep, trazodone, and cognition needs to be validated in prospective intervention studies. Trazodone, he said, appears to work best in people with a specific type of insomnia characterized by cortical and behavioral hyperarousal, and its cognitive effect appears limited to people whose sleep improves with treatment. “You’re not going to see long-term cognitive benefits if it’s not improving your sleep,” Dr. Karageorgiou said. “So, whether trazodone improves sleep or not in a patient after a few months can be an early indicator for the clinician to continue using it or suspend it, because it is unlikely to help their cognition otherwise.”
He stressed that physicians need to be broadly focused on improving sleep to help patients with, or at risk for, dementia by consolidating their sleep rhythms.
“Trazodone is not the magic bullet, and I don’t think we will ever have a magic bullet,” Dr. Karageorgiou said. “Because when our brain degenerates, it’s not just one chemical, or one system, it’s many. And our body changes as well. The important thing is to help the patient consolidate their rhythms, whether through light therapy, daily exercise, cognitive behavioral therapy for insomnia, or other evidence-based interventions and their combination. The same applies for a person with dementia as for the rest of us.”
None of the investigators outside of the industry-sponsored studies had relevant disclosures.
LOS ANGELES – While a large number of older adults take prescription and nonprescription medications to help them sleep, the effect of these medications on dementia risk is unclear, with most researchers advocating a cautious and conservative approach to prescribing.
Research is increasingly revealing a bidirectional relationship between sleep and dementia. Poor sleep – especially from insomnia, sleep deprivation, or obstructive sleep apnea – is known to increase dementia risk. Dementias, meanwhile, are associated with serious circadian rhythm disturbances, leading to nighttime sleep loss and increasing the likelihood of institutionalization.
At the Alzheimer’s Association International Conference, researchers presented findings assessing the links between sleep medication use and dementia and also what agents or approaches might safely improve sleep in people with sleep disorders who are at risk for dementia or who have been diagnosed with dementia.
Sex- and race-based differences in risk
Yue Leng, PhD, of the University of California, San Francisco, reported a link between frequent sleep medication use and later dementia – but only in white adults. Dr. Leng presented findings from the National Institutes of Health–funded Health, Aging, and Body Composition Study, which recruited 3,068 subjects aged 70-79 and followed them for 15 years. At baseline, 2.7% of African Americans and 7.7% of whites in the study reported taking sleep medications “often” or “almost always.”
Dr. Leng and her colleagues found that white subjects who reported taking sleep aids five or more times a month at baseline had a nearly 80% higher risk of developing dementia during the course of the study (hazard ratio, 1.79; 95% confidence interval, 1.21-2.66), compared with people who reported never taking sleep aids or taking them less frequently.
The researchers saw no between-sex differences for this finding, and adjusted for a variety of genetic and lifestyle confounders. Importantly, no significant increase in dementia risk was seen for black subjects, who made up more than one-third of the cohort.
Dr. Leng told the conference that the researchers could not explain why black participants did not see similarly increased dementia risk. Also, she noted, researchers did not have information on the specific sleep medications people used: benzodiazepines, antihistamines, antidepressants, or other types of drugs. Nonetheless, she told the conference, the findings ratified the cautious approach many dementia experts are already stressing.
“Do we really need to prescribe so many sleep meds to older adults who are already at risk for cognitive impairment?” Dr. Leng said, adding: “I am a big advocate of behavioral sleep interventions.” People with clinical sleep problems “should be referred to sleep centers” for a fuller assessment before medication is prescribed, she said.
Findings from another cohort study, meanwhile, suggest that there could be sex-related differences in how sleep aids affect dementia risk. Investigators at Utah State University in Logan used data from some 3,656 older adults in the Cache County Study on Memory and Aging, an NIH-backed cohort study of white adults in Utah without dementia at baseline who were followed for 12 years.
The investigators, led by doctoral student Elizabeth Vernon, found that men reporting use of sleep medication saw more than threefold higher risk of developing Alzheimer’s disease than did men who did not use sleep aids (HR, 3.604; P = .0001).
Women who did not report having sleep disturbance but used sleep-inducing medications were at nearly fourfold greater risk for developing Alzheimer’s disease (HR, 3.916; P = .0001). Women who self-reported sleep disturbances at baseline, meanwhile, saw a reduction in Alzheimer’s risk of about one-third associated with the use of sleep medications.
Ms. Vernon told the conference that, despite the finding of risk reduction for this particular group of women, caution in prescribing sleep aids was warranted.
Common sleep drugs linked to cognitive aging
Chris Fox, MD, a researcher at the University of East Anglia in Norwich, England, and his colleagues demonstrated in 2018 that long-term exposure to anticholinergic drugs, a class that includes some antidepressants and antihistamines used to promote sleep, was associated with a higher risk of dementia, while use of benzodiazepines, a class of sedatives used commonly in older people as sleep aids, was not. (Whether benzodiazepine exposure relates to dementia remains controversial.)
At AAIC 2019, Dr. Fox presented findings from a study of 337 brains in a U.K. brain bank, of which 17% and 21% came from users of benzodiazepines and anticholinergic drugs, whose usage history was well documented. Dr. Fox and his colleagues found that, while neither anticholinergic nor benzodiazepine exposure was associated with brain pathology specific to that seen in Alzheimer’s disease, both classes of drugs were associated with “slight signals in neuronal loss” in one brain region, the nucleus basalis of Meynert. Dr. Fox described the drugs as causing “an increase in cognitive aging” which could bear on Alzheimer’s risk without being directly causative.
Newer sleep drugs may help Alzheimer’s patients
Scientists working for drug manufacturers presented findings on agents to counter the circadian rhythm disturbances seen in people with Alzheimer’s disease. Margaret Moline, PhD, of Eisai in Woodcliff Lake, N.J., showed some results from a phase 2, dose-ranging, placebo-controlled study of the experimental agent lemborexant in 62 subjects aged 60-90 with mild to moderate Alzheimer’s disease and sleep disturbances. (Lemborexant, an orexin receptor agonist that acts to regulate wakefulness, is being investigated in a broad range of sleep disorders.) Patients were randomized to one of four doses of lemborexant or placebo and wore a device for sleep monitoring. Nighttime activity indicating arousal was significantly lower for people in two dosage arms, 5 mg and 10 mg, compared with placebo, and treatment groups saw trends toward less sleep fragmentation and higher total sleep time, Dr. Moline told the conference.
Suvorexant (Belsomra), the only orexin receptor antagonist currently licensed as a sleep aid, is also being tested in people with Alzheimer’s disease. At AAIC 2019, Joseph Herring, MD, PhD, of Merck in Kenilworth, N.J., presented results from a placebo-controlled trial of 277 patients with Alzheimer’s disease and insomnia, and reported that treatment with 10 or 20 mg of suvorexant over 4 weeks was associated with about an extra half hour of total nightly sleep, with a 73-minute mean increase from baseline, compared with 45 minutes for patients receiving placebo (95% CI, 11-45; P less than .005).
Trazodone linked to slower cognitive decline
An inexpensive antidepressant used in low doses as a sleep aid, including in people with Alzheimer’s disease, was associated with a delay in cognitive decline in older adults, according to results from a retrospective study. Elissaios Karageorgiou, MD, PhD, of the University of California, San Francisco, and the Neurological Institute of Athens presented results derived from two cohorts: patients enrolled at the UCSF Memory and Aging Center and women enrolled in the Study for Osteoporotic Fractures (SOF) in Women. The investigators were able to identify trazodone users in the studies (with two or more contiguous study visits reporting trazodone use) and match them with control patients from the same cohorts who did not use trazodone.
Trazodone was studied because previous research suggests it increases total sleep time in patients with Alzheimer’s disease without affecting next-day cognitive performance.
Trazodone-using patients in the UCSF cohort (n = 25) saw significantly less decline in Mini Mental State Exam (MMSE) scores over 4 years, compared with nonusers (0.27 vs. 0.70 points per year; P = .023), an effect that remained statistically significant even after adjusting for sedative and stimulant use and the expected progression of Alzheimer’s disease pathology. Importantly, the slower decline was seen only among subjects with sleep complaints at baseline and especially those whose sleep improved over time, suggesting that the cognitive benefit was mediated by improved sleep.
In the SOF cohort of 46 trazodone users matched with 148 nonusers, no significant protective or negative effect related to long-term trazodone use was found using the MMSE or the Trails Making Test. In this analysis, however, baseline and longitudinal sleep quality was not captured in the group-matching process, neither was the use of other medications. The patient group was slightly older, and all patients were women.
Dr. Karageorgiou said in an interview that the link between improved sleep, trazodone, and cognition needs to be validated in prospective intervention studies. Trazodone, he said, appears to work best in people with a specific type of insomnia characterized by cortical and behavioral hyperarousal, and its cognitive effect appears limited to people whose sleep improves with treatment. “You’re not going to see long-term cognitive benefits if it’s not improving your sleep,” Dr. Karageorgiou said. “So, whether trazodone improves sleep or not in a patient after a few months can be an early indicator for the clinician to continue using it or suspend it, because it is unlikely to help their cognition otherwise.”
He stressed that physicians need to be broadly focused on improving sleep to help patients with, or at risk for, dementia by consolidating their sleep rhythms.
“Trazodone is not the magic bullet, and I don’t think we will ever have a magic bullet,” Dr. Karageorgiou said. “Because when our brain degenerates, it’s not just one chemical, or one system, it’s many. And our body changes as well. The important thing is to help the patient consolidate their rhythms, whether through light therapy, daily exercise, cognitive behavioral therapy for insomnia, or other evidence-based interventions and their combination. The same applies for a person with dementia as for the rest of us.”
None of the investigators outside of the industry-sponsored studies had relevant disclosures.
REPORTING FROM AAIC 2019
CDC finds that too little naloxone is dispensed
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Benzodiazepines, hypnotics don’t increase Alzheimer’s pathology
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
REPORTING FROM AAIC 2019
A Novel Pharmaceutical Care Model for High-Risk Patients
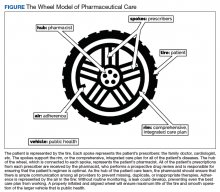
Nonadherence is a significant problem that has a negative impact on both patients and public health. Patients with multiple diseases often have complicated medication regimens, which can be difficult for them to manage. Unfortunately, nonadherence in these high-risk patients can have drastic consequences, including disease progression, hospitalization, and death, resulting in billions of dollars in unnecessary costs nationwide.1,2 The Wheel Model of Pharmaceutical Care (Figure) is a novel care model developed at the Gallup Indian Medical Center (GIMC) in New Mexico to address these problems by positioning pharmacy as a proactive service. The Wheel Model of Pharmaceutical Care was designed to improve adherence and patient outcomes and to encourage communication among the patient, pharmacists, prescribers, and other health care team members.
Pharmacists are central to managing patients’ medication therapies and coordinating communication among the health care providers (HCPs).1,3 Medication therapy management (MTM), a required component of Medicare Part D plans, helps ensure appropriate drug use and reduce the risk of adverse events.3 Since pharmacists receive prescriptions from all of the patient’s HCPs, patients may see pharmacists more often than they see any other HCP. GIMC is currently piloting a new clinic, the Medication Optimization, Synchronization, and Adherence Improvement Clinic (MOSAIC), that was created to implement the Wheel Model of Pharmaceutical Care. MOSAIC aims to provide proactive pharmacy services and continuous MTM to high-risk patients and will enable the effectiveness of this new pharmaceutical care model to be assessed.
Methods
Studies have identified certain populations who are at an increased risk for nonadherence: the elderly, patients with complex or extensive medication regimens, patients with multiple chronic medical conditions, substance misusers, certain ethnicities, patients of lower socioeconomic status, patients with limited literacy, and the homeless.2,4 Federal regulations require that Medicare Part D plans target beneficiaries who meet specific criteria for MTM programs. Under these rules, plans must target beneficiaries with ≥ 3 chronic diseases and ≥ 8 chronic medications, although plans also may include patients with fewer medications and diseases.3 Although the Wheel Model of Pharmaceutical Care is postulated to be an accurate model for the ideal care of all patients, initial implementation should be targeted toward populations who are likely to benefit the most from intervention. For these reasons, elderly Native American patients who have ≥ 2 chronic diseases and who take ≥ 5 chronic medications were targeted for initial enrollment in MOSAIC at GIMC.
Overview
In MOSAIC, pharmacists act as the hub of the pharmaceutical care wheel. Pharmacists work to ensure optimization of the patient’s comprehensive, integrated care plan—the rim of the wheel. As a part of this optimization process, MOSAIC pharmacists facilitate synchronization of the patient’s prescriptions to a monthly or quarterly target fill date. The patient’s current medication therapy is organized, and pharmacists track which medications are due to be filled instead of depending on the patient to request each prescription refill. This process effectively changes pharmacy from a requested service to a provided service.
Pharmacists also monitor the air in the tire to promote adherence. This is accomplished by providing efficient monthly or quarterly telephone or in-person consultations, which helps the patient better understand his or her comprehensive, integrated care plan. MOSAIC eliminates the possibility of nonadherence due to running out of refills. Specialized packaging, such as pill boxes or blister packs, can also improve adherence for certain patients.
MOSAIC ensures that pharmacists stay connected with the spokes, which represent a patient’s numerous prescribers, and close communication loops. Pharmacists can make prescribers aware of potential gaps or overlaps in treatment and assist them in the optimization and development of the patient’s comprehensive, integrated care plan. Pharmacists also make sure that the patient’s medication profile is current and accurate in the electronic health record (EHR). Any pertinent information discovered during MOSAIC encounters, such as abnormal laboratory results or changes in medications or disease, is documented in an EHR note. The patient’s prescribers are made aware of this information by tagging them as additional signers to the note in the EHR.
Keeping patients—the tires—healthy will ensure smooth operation of the vehicle and have a positive impact on public health. MOSAIC is expected to not only improve individual patient outcomes, but also decrease health care costs for patients and society due to nonadherence, suboptimal regimens, stockpiled home medications, and preventable hospital admissions.
Traditionally, pharmacy has been a requested service: A patient requests each of their prescriptions to be refilled, and the pharmacy fills the prescription. Ideally, pharmacy must become a provided service, with pharmacists keeping track of when a patient’s medications are due to be filled and actively looking for medication therapy optimization opportunities. This is accomplished by synchronizing the patient’s medications to the same monthly or quarterly fill date; screening for any potentially inappropriate medications, including high-risk medications in elderly patients, duplications, and omissions; verifying any medication changes with the patient each fill; and then providing all needed medications to the patient at a scheduled time.
To facilitate this process, custom software was developed for MOSAIC. In addition, a collaborative practice agreement (CPA) was drafted that allowed MOSAIC pharmacists to make certain medication therapy optimizations on behalf of the patient’s primary care provider. As part of this CPA, pharmacists also may order and act on certain laboratory tests, which helps to monitor disease progression, ensure safe medication use, and meet Government Performance and Results Act (GPRA) measures. As a novel model of pharmaceutical care, the effects of this approach are not yet known; however, research suggests that increased communication among HCPs and patient-centered approaches to care are beneficial to patient outcomes, adherence, and public health.1,5
Investigated Outcomes
As patients continue to enroll in MOSAIC, the effectiveness of the clinic will be evaluated. Specifically, quality of life, patient and HCP satisfaction with the program, adherence metrics, hospitalization rates, and all-cause mortality will be assessed for patients enrolled in MOSAIC as well as similar patients who are not enrolled in MOSAIC. Also, pharmacists will log all recommended medication therapy interventions so that the optimization component of MOSAIC may be quantified. GPRA measures and the financial implications of the interventions made by MOSAIC will also be evaluated.
Discussion
There are a number of factors, such as MTM services and interprofessional care teams, that research has shown to independently improve patient outcomes, adherence, or public health. By synthesizing these factors, a completely new approach—the Wheel Model of Pharmaceutical Care—was developed. This model presents a radical departure from traditional, requested-service practices and posits pharmacy as a provided service instead. Although the ideas of MTM and interprofessional care teams are not new, there has never been a practical way to truly integrate community pharmacists into the patient care team or to ensure adequate communication among all of the patient’s HCPs. The Wheel Model of Pharmaceutical Care includes public health as one of its core components and provides a framework for pharmacies to meaningfully impact health outcomes for patients.
The Wheel Model of Pharmaceutical Care was designed to minimize the likelihood of nonadherence. Despite this, patients might willfully choose to be nonadherent, forget to take their medications, or neglect to pick up their medications. Additionally, in health care systems where patients must pay for their medications, prescription drug costs might be a barrier to adherence.
When nonadherence is suspected, the Wheel Model of Pharmaceutical Care directs pharmacists in MOSAIC to take action. First, the underlying cause of the nonadherence must be determined. For example, if a patient is nonadherent because of an adverse drug reaction, a therapy change may be indicated. If a patient is nonadherent due to apathy toward their health or therapy, the patient may benefit from education about their condition and treatment options; thus, the patient can make shared, informed decisions and feel more actively involved with his or her health. If a patients is nonadherent due to forgetfulness, adherence packaging dispense methods should be considered as an alternative to traditional vials. Depending on the services offered by a given pharmacy, adherence packaging options may include blister packs, pill boxes, or strips prepared by robotic dispensing systems. The use of medication reminders, whether in the form of a smartphone application or a simple alarm clock, should be discussed with the patient. If the patient does not pick up their medications on time, a pharmacist can contact the patient to determine why the medications were not picked up and to assess any nonadherence. In this case, mail order pharmacy services, if available, should be offered to patients as a more convenient option.
The medication regimen optimization component of MOSAIC helps reduce the workload of primary care providers and allows pharmacists to act autonomously based on clinical judgment, within the scope of the CPA. This can prevent delays in care caused by no refills remaining on a prescription. The laboratory monitoring component allows pharmacists to track diseases and take action if necessary, which should have a favorable impact on GPRA measures. Medication optimizations can reduce wasted resources by identifying cost-saving formulary alternatives, potentially inappropriate medications, and suboptimal doses.
Since many Indian Health Service beneficiaries do not have private insurance and therefore do not generate third-party reimbursements for services and care provided by GIMC, keeping patients healthy and out of the hospital is a top priority. As more patients are enrolled in MOSAIC, the program is expected to have a favorable impact on pharmacy workload and workflow as well. Prescriptions are anticipated and filled in advance, which decreases the amount of patients calling and presenting to the pharmacy for same-day refill requests. Scheduling when MOSAIC patients’ medications are to be filled and dispensed creates a predictable workload that allows the pharmacy staff to be managed more efficiently.
Conclusion
Adherence is the responsibility of the patient, but the Wheel Model of Pharmaceutical Care aims to provide pharmacists with a framework to monitor and encourage adherence in their patients. By taking this patient-centered approach, MOSAIC is expected to improve outcomes and decrease hospitalizations for high-risk patients who simply need a little extra help with their medications.
1. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
2. Vlasnik JJ, Aliotta SL, DeLor B. Medication adherence: factors influencing compliance with prescribed medication plans. Case Manager. 2005;16(2):47-51.
3. Drug utilization management, quality assurance, and medication therapy management programs (MTMPs). Fed Regist. 2012;77(71):2207-22175. To be codified at 42 CFR § 423.153.
4. Thiruchselvam T, Naglie G, Moineddin R, et al. Risk factors for medication nonadherence in older adults with cognitive impairment who live alone. Int J Geriatr Psychiatry. 2012;27(12):1275-1282.
5. Liddy C, Blazkho V, Mill K. Challenges of self-management when living with multiple chronic conditions: systematic review of the qualitative literature. Can Fam Physician. 2014;60(12):1123-1133.
Nonadherence is a significant problem that has a negative impact on both patients and public health. Patients with multiple diseases often have complicated medication regimens, which can be difficult for them to manage. Unfortunately, nonadherence in these high-risk patients can have drastic consequences, including disease progression, hospitalization, and death, resulting in billions of dollars in unnecessary costs nationwide.1,2 The Wheel Model of Pharmaceutical Care (Figure) is a novel care model developed at the Gallup Indian Medical Center (GIMC) in New Mexico to address these problems by positioning pharmacy as a proactive service. The Wheel Model of Pharmaceutical Care was designed to improve adherence and patient outcomes and to encourage communication among the patient, pharmacists, prescribers, and other health care team members.
Pharmacists are central to managing patients’ medication therapies and coordinating communication among the health care providers (HCPs).1,3 Medication therapy management (MTM), a required component of Medicare Part D plans, helps ensure appropriate drug use and reduce the risk of adverse events.3 Since pharmacists receive prescriptions from all of the patient’s HCPs, patients may see pharmacists more often than they see any other HCP. GIMC is currently piloting a new clinic, the Medication Optimization, Synchronization, and Adherence Improvement Clinic (MOSAIC), that was created to implement the Wheel Model of Pharmaceutical Care. MOSAIC aims to provide proactive pharmacy services and continuous MTM to high-risk patients and will enable the effectiveness of this new pharmaceutical care model to be assessed.
Methods
Studies have identified certain populations who are at an increased risk for nonadherence: the elderly, patients with complex or extensive medication regimens, patients with multiple chronic medical conditions, substance misusers, certain ethnicities, patients of lower socioeconomic status, patients with limited literacy, and the homeless.2,4 Federal regulations require that Medicare Part D plans target beneficiaries who meet specific criteria for MTM programs. Under these rules, plans must target beneficiaries with ≥ 3 chronic diseases and ≥ 8 chronic medications, although plans also may include patients with fewer medications and diseases.3 Although the Wheel Model of Pharmaceutical Care is postulated to be an accurate model for the ideal care of all patients, initial implementation should be targeted toward populations who are likely to benefit the most from intervention. For these reasons, elderly Native American patients who have ≥ 2 chronic diseases and who take ≥ 5 chronic medications were targeted for initial enrollment in MOSAIC at GIMC.
Overview
In MOSAIC, pharmacists act as the hub of the pharmaceutical care wheel. Pharmacists work to ensure optimization of the patient’s comprehensive, integrated care plan—the rim of the wheel. As a part of this optimization process, MOSAIC pharmacists facilitate synchronization of the patient’s prescriptions to a monthly or quarterly target fill date. The patient’s current medication therapy is organized, and pharmacists track which medications are due to be filled instead of depending on the patient to request each prescription refill. This process effectively changes pharmacy from a requested service to a provided service.
Pharmacists also monitor the air in the tire to promote adherence. This is accomplished by providing efficient monthly or quarterly telephone or in-person consultations, which helps the patient better understand his or her comprehensive, integrated care plan. MOSAIC eliminates the possibility of nonadherence due to running out of refills. Specialized packaging, such as pill boxes or blister packs, can also improve adherence for certain patients.
MOSAIC ensures that pharmacists stay connected with the spokes, which represent a patient’s numerous prescribers, and close communication loops. Pharmacists can make prescribers aware of potential gaps or overlaps in treatment and assist them in the optimization and development of the patient’s comprehensive, integrated care plan. Pharmacists also make sure that the patient’s medication profile is current and accurate in the electronic health record (EHR). Any pertinent information discovered during MOSAIC encounters, such as abnormal laboratory results or changes in medications or disease, is documented in an EHR note. The patient’s prescribers are made aware of this information by tagging them as additional signers to the note in the EHR.
Keeping patients—the tires—healthy will ensure smooth operation of the vehicle and have a positive impact on public health. MOSAIC is expected to not only improve individual patient outcomes, but also decrease health care costs for patients and society due to nonadherence, suboptimal regimens, stockpiled home medications, and preventable hospital admissions.
Traditionally, pharmacy has been a requested service: A patient requests each of their prescriptions to be refilled, and the pharmacy fills the prescription. Ideally, pharmacy must become a provided service, with pharmacists keeping track of when a patient’s medications are due to be filled and actively looking for medication therapy optimization opportunities. This is accomplished by synchronizing the patient’s medications to the same monthly or quarterly fill date; screening for any potentially inappropriate medications, including high-risk medications in elderly patients, duplications, and omissions; verifying any medication changes with the patient each fill; and then providing all needed medications to the patient at a scheduled time.
To facilitate this process, custom software was developed for MOSAIC. In addition, a collaborative practice agreement (CPA) was drafted that allowed MOSAIC pharmacists to make certain medication therapy optimizations on behalf of the patient’s primary care provider. As part of this CPA, pharmacists also may order and act on certain laboratory tests, which helps to monitor disease progression, ensure safe medication use, and meet Government Performance and Results Act (GPRA) measures. As a novel model of pharmaceutical care, the effects of this approach are not yet known; however, research suggests that increased communication among HCPs and patient-centered approaches to care are beneficial to patient outcomes, adherence, and public health.1,5
Investigated Outcomes
As patients continue to enroll in MOSAIC, the effectiveness of the clinic will be evaluated. Specifically, quality of life, patient and HCP satisfaction with the program, adherence metrics, hospitalization rates, and all-cause mortality will be assessed for patients enrolled in MOSAIC as well as similar patients who are not enrolled in MOSAIC. Also, pharmacists will log all recommended medication therapy interventions so that the optimization component of MOSAIC may be quantified. GPRA measures and the financial implications of the interventions made by MOSAIC will also be evaluated.
Discussion
There are a number of factors, such as MTM services and interprofessional care teams, that research has shown to independently improve patient outcomes, adherence, or public health. By synthesizing these factors, a completely new approach—the Wheel Model of Pharmaceutical Care—was developed. This model presents a radical departure from traditional, requested-service practices and posits pharmacy as a provided service instead. Although the ideas of MTM and interprofessional care teams are not new, there has never been a practical way to truly integrate community pharmacists into the patient care team or to ensure adequate communication among all of the patient’s HCPs. The Wheel Model of Pharmaceutical Care includes public health as one of its core components and provides a framework for pharmacies to meaningfully impact health outcomes for patients.
The Wheel Model of Pharmaceutical Care was designed to minimize the likelihood of nonadherence. Despite this, patients might willfully choose to be nonadherent, forget to take their medications, or neglect to pick up their medications. Additionally, in health care systems where patients must pay for their medications, prescription drug costs might be a barrier to adherence.
When nonadherence is suspected, the Wheel Model of Pharmaceutical Care directs pharmacists in MOSAIC to take action. First, the underlying cause of the nonadherence must be determined. For example, if a patient is nonadherent because of an adverse drug reaction, a therapy change may be indicated. If a patient is nonadherent due to apathy toward their health or therapy, the patient may benefit from education about their condition and treatment options; thus, the patient can make shared, informed decisions and feel more actively involved with his or her health. If a patients is nonadherent due to forgetfulness, adherence packaging dispense methods should be considered as an alternative to traditional vials. Depending on the services offered by a given pharmacy, adherence packaging options may include blister packs, pill boxes, or strips prepared by robotic dispensing systems. The use of medication reminders, whether in the form of a smartphone application or a simple alarm clock, should be discussed with the patient. If the patient does not pick up their medications on time, a pharmacist can contact the patient to determine why the medications were not picked up and to assess any nonadherence. In this case, mail order pharmacy services, if available, should be offered to patients as a more convenient option.
The medication regimen optimization component of MOSAIC helps reduce the workload of primary care providers and allows pharmacists to act autonomously based on clinical judgment, within the scope of the CPA. This can prevent delays in care caused by no refills remaining on a prescription. The laboratory monitoring component allows pharmacists to track diseases and take action if necessary, which should have a favorable impact on GPRA measures. Medication optimizations can reduce wasted resources by identifying cost-saving formulary alternatives, potentially inappropriate medications, and suboptimal doses.
Since many Indian Health Service beneficiaries do not have private insurance and therefore do not generate third-party reimbursements for services and care provided by GIMC, keeping patients healthy and out of the hospital is a top priority. As more patients are enrolled in MOSAIC, the program is expected to have a favorable impact on pharmacy workload and workflow as well. Prescriptions are anticipated and filled in advance, which decreases the amount of patients calling and presenting to the pharmacy for same-day refill requests. Scheduling when MOSAIC patients’ medications are to be filled and dispensed creates a predictable workload that allows the pharmacy staff to be managed more efficiently.
Conclusion
Adherence is the responsibility of the patient, but the Wheel Model of Pharmaceutical Care aims to provide pharmacists with a framework to monitor and encourage adherence in their patients. By taking this patient-centered approach, MOSAIC is expected to improve outcomes and decrease hospitalizations for high-risk patients who simply need a little extra help with their medications.
Nonadherence is a significant problem that has a negative impact on both patients and public health. Patients with multiple diseases often have complicated medication regimens, which can be difficult for them to manage. Unfortunately, nonadherence in these high-risk patients can have drastic consequences, including disease progression, hospitalization, and death, resulting in billions of dollars in unnecessary costs nationwide.1,2 The Wheel Model of Pharmaceutical Care (Figure) is a novel care model developed at the Gallup Indian Medical Center (GIMC) in New Mexico to address these problems by positioning pharmacy as a proactive service. The Wheel Model of Pharmaceutical Care was designed to improve adherence and patient outcomes and to encourage communication among the patient, pharmacists, prescribers, and other health care team members.
Pharmacists are central to managing patients’ medication therapies and coordinating communication among the health care providers (HCPs).1,3 Medication therapy management (MTM), a required component of Medicare Part D plans, helps ensure appropriate drug use and reduce the risk of adverse events.3 Since pharmacists receive prescriptions from all of the patient’s HCPs, patients may see pharmacists more often than they see any other HCP. GIMC is currently piloting a new clinic, the Medication Optimization, Synchronization, and Adherence Improvement Clinic (MOSAIC), that was created to implement the Wheel Model of Pharmaceutical Care. MOSAIC aims to provide proactive pharmacy services and continuous MTM to high-risk patients and will enable the effectiveness of this new pharmaceutical care model to be assessed.
Methods
Studies have identified certain populations who are at an increased risk for nonadherence: the elderly, patients with complex or extensive medication regimens, patients with multiple chronic medical conditions, substance misusers, certain ethnicities, patients of lower socioeconomic status, patients with limited literacy, and the homeless.2,4 Federal regulations require that Medicare Part D plans target beneficiaries who meet specific criteria for MTM programs. Under these rules, plans must target beneficiaries with ≥ 3 chronic diseases and ≥ 8 chronic medications, although plans also may include patients with fewer medications and diseases.3 Although the Wheel Model of Pharmaceutical Care is postulated to be an accurate model for the ideal care of all patients, initial implementation should be targeted toward populations who are likely to benefit the most from intervention. For these reasons, elderly Native American patients who have ≥ 2 chronic diseases and who take ≥ 5 chronic medications were targeted for initial enrollment in MOSAIC at GIMC.
Overview
In MOSAIC, pharmacists act as the hub of the pharmaceutical care wheel. Pharmacists work to ensure optimization of the patient’s comprehensive, integrated care plan—the rim of the wheel. As a part of this optimization process, MOSAIC pharmacists facilitate synchronization of the patient’s prescriptions to a monthly or quarterly target fill date. The patient’s current medication therapy is organized, and pharmacists track which medications are due to be filled instead of depending on the patient to request each prescription refill. This process effectively changes pharmacy from a requested service to a provided service.
Pharmacists also monitor the air in the tire to promote adherence. This is accomplished by providing efficient monthly or quarterly telephone or in-person consultations, which helps the patient better understand his or her comprehensive, integrated care plan. MOSAIC eliminates the possibility of nonadherence due to running out of refills. Specialized packaging, such as pill boxes or blister packs, can also improve adherence for certain patients.
MOSAIC ensures that pharmacists stay connected with the spokes, which represent a patient’s numerous prescribers, and close communication loops. Pharmacists can make prescribers aware of potential gaps or overlaps in treatment and assist them in the optimization and development of the patient’s comprehensive, integrated care plan. Pharmacists also make sure that the patient’s medication profile is current and accurate in the electronic health record (EHR). Any pertinent information discovered during MOSAIC encounters, such as abnormal laboratory results or changes in medications or disease, is documented in an EHR note. The patient’s prescribers are made aware of this information by tagging them as additional signers to the note in the EHR.
Keeping patients—the tires—healthy will ensure smooth operation of the vehicle and have a positive impact on public health. MOSAIC is expected to not only improve individual patient outcomes, but also decrease health care costs for patients and society due to nonadherence, suboptimal regimens, stockpiled home medications, and preventable hospital admissions.
Traditionally, pharmacy has been a requested service: A patient requests each of their prescriptions to be refilled, and the pharmacy fills the prescription. Ideally, pharmacy must become a provided service, with pharmacists keeping track of when a patient’s medications are due to be filled and actively looking for medication therapy optimization opportunities. This is accomplished by synchronizing the patient’s medications to the same monthly or quarterly fill date; screening for any potentially inappropriate medications, including high-risk medications in elderly patients, duplications, and omissions; verifying any medication changes with the patient each fill; and then providing all needed medications to the patient at a scheduled time.
To facilitate this process, custom software was developed for MOSAIC. In addition, a collaborative practice agreement (CPA) was drafted that allowed MOSAIC pharmacists to make certain medication therapy optimizations on behalf of the patient’s primary care provider. As part of this CPA, pharmacists also may order and act on certain laboratory tests, which helps to monitor disease progression, ensure safe medication use, and meet Government Performance and Results Act (GPRA) measures. As a novel model of pharmaceutical care, the effects of this approach are not yet known; however, research suggests that increased communication among HCPs and patient-centered approaches to care are beneficial to patient outcomes, adherence, and public health.1,5
Investigated Outcomes
As patients continue to enroll in MOSAIC, the effectiveness of the clinic will be evaluated. Specifically, quality of life, patient and HCP satisfaction with the program, adherence metrics, hospitalization rates, and all-cause mortality will be assessed for patients enrolled in MOSAIC as well as similar patients who are not enrolled in MOSAIC. Also, pharmacists will log all recommended medication therapy interventions so that the optimization component of MOSAIC may be quantified. GPRA measures and the financial implications of the interventions made by MOSAIC will also be evaluated.
Discussion
There are a number of factors, such as MTM services and interprofessional care teams, that research has shown to independently improve patient outcomes, adherence, or public health. By synthesizing these factors, a completely new approach—the Wheel Model of Pharmaceutical Care—was developed. This model presents a radical departure from traditional, requested-service practices and posits pharmacy as a provided service instead. Although the ideas of MTM and interprofessional care teams are not new, there has never been a practical way to truly integrate community pharmacists into the patient care team or to ensure adequate communication among all of the patient’s HCPs. The Wheel Model of Pharmaceutical Care includes public health as one of its core components and provides a framework for pharmacies to meaningfully impact health outcomes for patients.
The Wheel Model of Pharmaceutical Care was designed to minimize the likelihood of nonadherence. Despite this, patients might willfully choose to be nonadherent, forget to take their medications, or neglect to pick up their medications. Additionally, in health care systems where patients must pay for their medications, prescription drug costs might be a barrier to adherence.
When nonadherence is suspected, the Wheel Model of Pharmaceutical Care directs pharmacists in MOSAIC to take action. First, the underlying cause of the nonadherence must be determined. For example, if a patient is nonadherent because of an adverse drug reaction, a therapy change may be indicated. If a patient is nonadherent due to apathy toward their health or therapy, the patient may benefit from education about their condition and treatment options; thus, the patient can make shared, informed decisions and feel more actively involved with his or her health. If a patients is nonadherent due to forgetfulness, adherence packaging dispense methods should be considered as an alternative to traditional vials. Depending on the services offered by a given pharmacy, adherence packaging options may include blister packs, pill boxes, or strips prepared by robotic dispensing systems. The use of medication reminders, whether in the form of a smartphone application or a simple alarm clock, should be discussed with the patient. If the patient does not pick up their medications on time, a pharmacist can contact the patient to determine why the medications were not picked up and to assess any nonadherence. In this case, mail order pharmacy services, if available, should be offered to patients as a more convenient option.
The medication regimen optimization component of MOSAIC helps reduce the workload of primary care providers and allows pharmacists to act autonomously based on clinical judgment, within the scope of the CPA. This can prevent delays in care caused by no refills remaining on a prescription. The laboratory monitoring component allows pharmacists to track diseases and take action if necessary, which should have a favorable impact on GPRA measures. Medication optimizations can reduce wasted resources by identifying cost-saving formulary alternatives, potentially inappropriate medications, and suboptimal doses.
Since many Indian Health Service beneficiaries do not have private insurance and therefore do not generate third-party reimbursements for services and care provided by GIMC, keeping patients healthy and out of the hospital is a top priority. As more patients are enrolled in MOSAIC, the program is expected to have a favorable impact on pharmacy workload and workflow as well. Prescriptions are anticipated and filled in advance, which decreases the amount of patients calling and presenting to the pharmacy for same-day refill requests. Scheduling when MOSAIC patients’ medications are to be filled and dispensed creates a predictable workload that allows the pharmacy staff to be managed more efficiently.
Conclusion
Adherence is the responsibility of the patient, but the Wheel Model of Pharmaceutical Care aims to provide pharmacists with a framework to monitor and encourage adherence in their patients. By taking this patient-centered approach, MOSAIC is expected to improve outcomes and decrease hospitalizations for high-risk patients who simply need a little extra help with their medications.
1. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
2. Vlasnik JJ, Aliotta SL, DeLor B. Medication adherence: factors influencing compliance with prescribed medication plans. Case Manager. 2005;16(2):47-51.
3. Drug utilization management, quality assurance, and medication therapy management programs (MTMPs). Fed Regist. 2012;77(71):2207-22175. To be codified at 42 CFR § 423.153.
4. Thiruchselvam T, Naglie G, Moineddin R, et al. Risk factors for medication nonadherence in older adults with cognitive impairment who live alone. Int J Geriatr Psychiatry. 2012;27(12):1275-1282.
5. Liddy C, Blazkho V, Mill K. Challenges of self-management when living with multiple chronic conditions: systematic review of the qualitative literature. Can Fam Physician. 2014;60(12):1123-1133.
1. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
2. Vlasnik JJ, Aliotta SL, DeLor B. Medication adherence: factors influencing compliance with prescribed medication plans. Case Manager. 2005;16(2):47-51.
3. Drug utilization management, quality assurance, and medication therapy management programs (MTMPs). Fed Regist. 2012;77(71):2207-22175. To be codified at 42 CFR § 423.153.
4. Thiruchselvam T, Naglie G, Moineddin R, et al. Risk factors for medication nonadherence in older adults with cognitive impairment who live alone. Int J Geriatr Psychiatry. 2012;27(12):1275-1282.
5. Liddy C, Blazkho V, Mill K. Challenges of self-management when living with multiple chronic conditions: systematic review of the qualitative literature. Can Fam Physician. 2014;60(12):1123-1133.
Older patients who stop statins may be increasing their cardiovascular risk
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
FROM THE EUROPEAN HEART JOURNAL
FDA approves darolutamide for nonmetastatic CRPC
The Food and Drug Administration has approved darolutamide for nonmetastatic, castration-resistant prostate cancer.
The approval was based on improved metastasis-free survival (MFS) in the randomized ARAMIS trial of 1,509 patients with nonmetastatic, castration-resistant prostate cancer.
Median MFS was 40.4 months (95% confidence interval, 34.3 months to not reached) for patients treated with darolutamide, compared with 18.4 months (95% CI, 15.5-22.3 months) for those receiving placebo (hazard ratio, 0.41; 95% CI, 0.34-0.50; P less than .0001), according to the FDA.
MFS is defined as the time from randomization to first evidence of distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first.
In ARAMIS, patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n = 955) or matching placebo (n = 554). All patients received a gonadotropin-releasing hormone analog concurrently or had a previous bilateral orchiectomy. Twelve patients with previous seizure histories were treated on the darolutamide arm.
Overall survival data is not yet mature, the FDA said.
The most common adverse reactions in patients who received darolutamide were fatigue, extremity pain, and rash. Ischemic heart disease (4.3%) and heart failure (2.1%) were more common on the darolutamide arm, while seizure incidence was similar in the two arms (0.2%).
The recommended darolutamide dose is 600 mg (two 300-mg tablets) administered orally twice daily with food. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy, the FDA said.
Darolutamide is marketed as Nubeqa by Bayer HealthCare Pharmaceuticals.
The Food and Drug Administration has approved darolutamide for nonmetastatic, castration-resistant prostate cancer.
The approval was based on improved metastasis-free survival (MFS) in the randomized ARAMIS trial of 1,509 patients with nonmetastatic, castration-resistant prostate cancer.
Median MFS was 40.4 months (95% confidence interval, 34.3 months to not reached) for patients treated with darolutamide, compared with 18.4 months (95% CI, 15.5-22.3 months) for those receiving placebo (hazard ratio, 0.41; 95% CI, 0.34-0.50; P less than .0001), according to the FDA.
MFS is defined as the time from randomization to first evidence of distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first.
In ARAMIS, patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n = 955) or matching placebo (n = 554). All patients received a gonadotropin-releasing hormone analog concurrently or had a previous bilateral orchiectomy. Twelve patients with previous seizure histories were treated on the darolutamide arm.
Overall survival data is not yet mature, the FDA said.
The most common adverse reactions in patients who received darolutamide were fatigue, extremity pain, and rash. Ischemic heart disease (4.3%) and heart failure (2.1%) were more common on the darolutamide arm, while seizure incidence was similar in the two arms (0.2%).
The recommended darolutamide dose is 600 mg (two 300-mg tablets) administered orally twice daily with food. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy, the FDA said.
Darolutamide is marketed as Nubeqa by Bayer HealthCare Pharmaceuticals.
The Food and Drug Administration has approved darolutamide for nonmetastatic, castration-resistant prostate cancer.
The approval was based on improved metastasis-free survival (MFS) in the randomized ARAMIS trial of 1,509 patients with nonmetastatic, castration-resistant prostate cancer.
Median MFS was 40.4 months (95% confidence interval, 34.3 months to not reached) for patients treated with darolutamide, compared with 18.4 months (95% CI, 15.5-22.3 months) for those receiving placebo (hazard ratio, 0.41; 95% CI, 0.34-0.50; P less than .0001), according to the FDA.
MFS is defined as the time from randomization to first evidence of distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first.
In ARAMIS, patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n = 955) or matching placebo (n = 554). All patients received a gonadotropin-releasing hormone analog concurrently or had a previous bilateral orchiectomy. Twelve patients with previous seizure histories were treated on the darolutamide arm.
Overall survival data is not yet mature, the FDA said.
The most common adverse reactions in patients who received darolutamide were fatigue, extremity pain, and rash. Ischemic heart disease (4.3%) and heart failure (2.1%) were more common on the darolutamide arm, while seizure incidence was similar in the two arms (0.2%).
The recommended darolutamide dose is 600 mg (two 300-mg tablets) administered orally twice daily with food. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy, the FDA said.
Darolutamide is marketed as Nubeqa by Bayer HealthCare Pharmaceuticals.
Clopidogrel matches aspirin for reducing risk of colorectal cancer
Clopidogrel appears to reduce the risk of colorectal cancer (CRC) as much as low-dose aspirin, based on a case-control study involving more than 15,000 cases.
Source: American Gastroenterological Association
Risk of CRC was reduced by 20%-30% when clopidogrel was given alone or in combination with aspirin, reported lead author Antonio Rodríguez-Miguel of Príncipe de Asturias University Hospital in Madrid and colleagues. This finding adds support to the hypothesis that low-dose aspirin is chemoprotective primarily because of its antiplatelet properties, they noted.
“The mechanism of action of low-dose aspirin to explain its protective effect is subject to debate,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Although aspirin is a nonsteroidal anti-inflammatory drug (NSAID) and these drugs are known to prevent CRC through the inhibition of cyclooxygenase (COX)-2 in epithelial and stromal cells in the large bowel, at low doses (75-300 mg/d) aspirin has only transient effects on this isozyme, while permanently inactivating platelet COX-1 and suppressing thromboxane A2 production. The apparent lack of dose-dependence of the chemoprotective effect of aspirin, as well as the potential role of locally activated platelets in upregulating COX-2 expression in adjacent nucleated cells of the intestinal mucosa, have led [to] the postulation that low-dose aspirin could exert its chemoprotective effect via its antiplatelet action.”
Although previous studies have explored the chemoprotective potential of other antiplatelet agents, such as clopidogrel, the resultant body of evidence remains small. In 2017, for example, Avi Leader, MD, and colleagues reported that the chemoprotective effect of dual-antiplatelet therapy (DAPT) with clopidogrel and aspirin was superior to aspirin monotherapy, based on an additional 8% risk reduction. The present study aimed to build on such findings with evaluation of a Mediterranean cohort, which could reduce confounding lifestyle factors, owing to a lower rate of cardiovascular morbidity than other populations.
The nested, case-control study involved 15,491 cases of CRC and 60,000 controls who were randomly selected and frequency matched by sex, age, and year of indexing. Data were drawn from Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP), a Spanish medical record database with more than 7 million patients. Records of patients involved in the present study were screened for prescription of three antiplatelet agents: low-dose aspirin, clopidogrel, and triflusal. Additional categorization identified current users, recent users, past users, and nonusers. The effects of clopidogrel and aspirin were evaluated separately, as monotherapies, and together, as DAPT.
Demographically, the mean age of the entire study population was 68.6 years, with a slight male predominance (59%). Median follow-up was similar between cases and controls, at approximately 3 years, ranging from about 1.5 to 6 years. Cases showed higher rates of gout, alcohol abuse, acute digestive diseases, and peripheral artery disease, whereas controls were more likely to have histories involving stroke, acute myocardial infarction, chronic digestive diseases, and constipation.
Controls were more likely to be current aspirin users than patients diagnosed with CRC (12.8% vs. 12.2%), giving an associated adjusted odds ratio (AOR) of 0.83. Risk reduction became statistically apparent after 180 days of aspirin usage, with an AOR of 0.79, and more prominent in the 1- to 3-year range, with an AOR of 0.73. This chemoprotective effect faded rapidly with discontinuation.
Current clopidogrel usage led to a comparable level of risk reduction, with an AOR of 0.80. It wasn’t until a year of continuous clopidogrel monotherapy that risk reduction became statistically significant, with an AOR of 0.65, which dropped to 0.57 between years 1 and 3.
Turning to a matched comparison of aspirin or clopidogrel monotherapy versus DAPT, the investigators found similar rates of chemoprotection. Current aspirin usage of any duration offered an adjusted risk reduction of 17%, compared with 25% for clopidogrel, and 29% for DAPT. Beyond 1 year of continuous and current usage, the superiority of DAPT was called into question, as clopidogrel monotherapy offered the greatest risk reduction, at 37%, compared with 22% for aspirin, and 22% for DAPT. Risk analyses involving triflusal lacked statistical significance.
“The results of the present study are compatible with a chemoprotective effect of clopidogrel against CRC, equivalent in magnitude to the one observed for low-dose aspirin,” the investigators wrote. “This finding indirectly supports the hypothesis that the chemoprotective effect of low-dose aspirin is mediated mostly through the permanent inactivation of platelet COX-1.”
The investigators pointed out that the chemoprotective effects of antiplatelet therapy begin to appear early in treatment, independently from lifestyle factors, but risk reduction depends on current usage. Although short-term usage of either aspirin or clopidogrel was associated with an increased risk of CRC, the investigators suggested that this was more likely a perceived risk rather than an actual one. “In our view, this observation could be explained in part by a detection bias, owing to an increased risk of GI bleeding induced by antiplatelet agents that could lead to a greater number of colonoscopies, and, as a result, an early cancer diagnosis,” they wrote.
The study was funded by the Fundación Instituto Teófilo Hernando. Dr. García-Rodríguez disclosed a relationship with CEIFE, which has received funding from Bayer and AstraZeneca.
SOURCE: Rodríguez-Miguel et al. Clin Gastrenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.012.
The role of aspirin in reducing the risk of colorectal cancer is well established, although the mechanisms of actions are not entirely clear. One possible mechanism is through inhibition of the cyclooxygenase-1 (COX-1) pathway. The authors investigated the role of aspirin but also clopidogrel, another antiplatelet drug that works through inhibition of the COX-1 pathway in reducing the risk of CRC in a case-control study from Spain. CRC cases were randomly matched with cancer-free controls, and the use of aspirin and clopidogrel as a risk factor for CRC was studied. Not surprisingly, aspirin use was associated with reduced risk of CRC by 17%, However, what’s new is that the use of clopidogrel was associated with reduced risk of CRC by 20% also but use of dual therapy (aspirin plus clopidogrel) did not confer additional benefit. The results did not differ by patient age or sex. The caveat is that history of CRC screening or colonoscopy was not known for cases or controls, and many other confounders, such as diet, exercise, and other lifestyle and medication history that may account for the differences could not be easily teased apart. If confirmed by others, these data suggest an additional beneficial effect of antiplatelet agent clopidogrel in reducing risk of CRC, if taken for more than 1 year. The study opens the door to exploring mechanisms by which antiplatelet agents may reduce risk of CRC, and the potential role of other antiplatelet agents in reducing risk of CRC.
Aasma Shaukat, MD, MPH, GI section chief Minneapolis VAMC and professor of medicine, University of Minnesota, Minneapolis. She has no conflicts of interest.
The role of aspirin in reducing the risk of colorectal cancer is well established, although the mechanisms of actions are not entirely clear. One possible mechanism is through inhibition of the cyclooxygenase-1 (COX-1) pathway. The authors investigated the role of aspirin but also clopidogrel, another antiplatelet drug that works through inhibition of the COX-1 pathway in reducing the risk of CRC in a case-control study from Spain. CRC cases were randomly matched with cancer-free controls, and the use of aspirin and clopidogrel as a risk factor for CRC was studied. Not surprisingly, aspirin use was associated with reduced risk of CRC by 17%, However, what’s new is that the use of clopidogrel was associated with reduced risk of CRC by 20% also but use of dual therapy (aspirin plus clopidogrel) did not confer additional benefit. The results did not differ by patient age or sex. The caveat is that history of CRC screening or colonoscopy was not known for cases or controls, and many other confounders, such as diet, exercise, and other lifestyle and medication history that may account for the differences could not be easily teased apart. If confirmed by others, these data suggest an additional beneficial effect of antiplatelet agent clopidogrel in reducing risk of CRC, if taken for more than 1 year. The study opens the door to exploring mechanisms by which antiplatelet agents may reduce risk of CRC, and the potential role of other antiplatelet agents in reducing risk of CRC.
Aasma Shaukat, MD, MPH, GI section chief Minneapolis VAMC and professor of medicine, University of Minnesota, Minneapolis. She has no conflicts of interest.
The role of aspirin in reducing the risk of colorectal cancer is well established, although the mechanisms of actions are not entirely clear. One possible mechanism is through inhibition of the cyclooxygenase-1 (COX-1) pathway. The authors investigated the role of aspirin but also clopidogrel, another antiplatelet drug that works through inhibition of the COX-1 pathway in reducing the risk of CRC in a case-control study from Spain. CRC cases were randomly matched with cancer-free controls, and the use of aspirin and clopidogrel as a risk factor for CRC was studied. Not surprisingly, aspirin use was associated with reduced risk of CRC by 17%, However, what’s new is that the use of clopidogrel was associated with reduced risk of CRC by 20% also but use of dual therapy (aspirin plus clopidogrel) did not confer additional benefit. The results did not differ by patient age or sex. The caveat is that history of CRC screening or colonoscopy was not known for cases or controls, and many other confounders, such as diet, exercise, and other lifestyle and medication history that may account for the differences could not be easily teased apart. If confirmed by others, these data suggest an additional beneficial effect of antiplatelet agent clopidogrel in reducing risk of CRC, if taken for more than 1 year. The study opens the door to exploring mechanisms by which antiplatelet agents may reduce risk of CRC, and the potential role of other antiplatelet agents in reducing risk of CRC.
Aasma Shaukat, MD, MPH, GI section chief Minneapolis VAMC and professor of medicine, University of Minnesota, Minneapolis. She has no conflicts of interest.
Clopidogrel appears to reduce the risk of colorectal cancer (CRC) as much as low-dose aspirin, based on a case-control study involving more than 15,000 cases.
Source: American Gastroenterological Association
Risk of CRC was reduced by 20%-30% when clopidogrel was given alone or in combination with aspirin, reported lead author Antonio Rodríguez-Miguel of Príncipe de Asturias University Hospital in Madrid and colleagues. This finding adds support to the hypothesis that low-dose aspirin is chemoprotective primarily because of its antiplatelet properties, they noted.
“The mechanism of action of low-dose aspirin to explain its protective effect is subject to debate,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Although aspirin is a nonsteroidal anti-inflammatory drug (NSAID) and these drugs are known to prevent CRC through the inhibition of cyclooxygenase (COX)-2 in epithelial and stromal cells in the large bowel, at low doses (75-300 mg/d) aspirin has only transient effects on this isozyme, while permanently inactivating platelet COX-1 and suppressing thromboxane A2 production. The apparent lack of dose-dependence of the chemoprotective effect of aspirin, as well as the potential role of locally activated platelets in upregulating COX-2 expression in adjacent nucleated cells of the intestinal mucosa, have led [to] the postulation that low-dose aspirin could exert its chemoprotective effect via its antiplatelet action.”
Although previous studies have explored the chemoprotective potential of other antiplatelet agents, such as clopidogrel, the resultant body of evidence remains small. In 2017, for example, Avi Leader, MD, and colleagues reported that the chemoprotective effect of dual-antiplatelet therapy (DAPT) with clopidogrel and aspirin was superior to aspirin monotherapy, based on an additional 8% risk reduction. The present study aimed to build on such findings with evaluation of a Mediterranean cohort, which could reduce confounding lifestyle factors, owing to a lower rate of cardiovascular morbidity than other populations.
The nested, case-control study involved 15,491 cases of CRC and 60,000 controls who were randomly selected and frequency matched by sex, age, and year of indexing. Data were drawn from Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP), a Spanish medical record database with more than 7 million patients. Records of patients involved in the present study were screened for prescription of three antiplatelet agents: low-dose aspirin, clopidogrel, and triflusal. Additional categorization identified current users, recent users, past users, and nonusers. The effects of clopidogrel and aspirin were evaluated separately, as monotherapies, and together, as DAPT.
Demographically, the mean age of the entire study population was 68.6 years, with a slight male predominance (59%). Median follow-up was similar between cases and controls, at approximately 3 years, ranging from about 1.5 to 6 years. Cases showed higher rates of gout, alcohol abuse, acute digestive diseases, and peripheral artery disease, whereas controls were more likely to have histories involving stroke, acute myocardial infarction, chronic digestive diseases, and constipation.
Controls were more likely to be current aspirin users than patients diagnosed with CRC (12.8% vs. 12.2%), giving an associated adjusted odds ratio (AOR) of 0.83. Risk reduction became statistically apparent after 180 days of aspirin usage, with an AOR of 0.79, and more prominent in the 1- to 3-year range, with an AOR of 0.73. This chemoprotective effect faded rapidly with discontinuation.
Current clopidogrel usage led to a comparable level of risk reduction, with an AOR of 0.80. It wasn’t until a year of continuous clopidogrel monotherapy that risk reduction became statistically significant, with an AOR of 0.65, which dropped to 0.57 between years 1 and 3.
Turning to a matched comparison of aspirin or clopidogrel monotherapy versus DAPT, the investigators found similar rates of chemoprotection. Current aspirin usage of any duration offered an adjusted risk reduction of 17%, compared with 25% for clopidogrel, and 29% for DAPT. Beyond 1 year of continuous and current usage, the superiority of DAPT was called into question, as clopidogrel monotherapy offered the greatest risk reduction, at 37%, compared with 22% for aspirin, and 22% for DAPT. Risk analyses involving triflusal lacked statistical significance.
“The results of the present study are compatible with a chemoprotective effect of clopidogrel against CRC, equivalent in magnitude to the one observed for low-dose aspirin,” the investigators wrote. “This finding indirectly supports the hypothesis that the chemoprotective effect of low-dose aspirin is mediated mostly through the permanent inactivation of platelet COX-1.”
The investigators pointed out that the chemoprotective effects of antiplatelet therapy begin to appear early in treatment, independently from lifestyle factors, but risk reduction depends on current usage. Although short-term usage of either aspirin or clopidogrel was associated with an increased risk of CRC, the investigators suggested that this was more likely a perceived risk rather than an actual one. “In our view, this observation could be explained in part by a detection bias, owing to an increased risk of GI bleeding induced by antiplatelet agents that could lead to a greater number of colonoscopies, and, as a result, an early cancer diagnosis,” they wrote.
The study was funded by the Fundación Instituto Teófilo Hernando. Dr. García-Rodríguez disclosed a relationship with CEIFE, which has received funding from Bayer and AstraZeneca.
SOURCE: Rodríguez-Miguel et al. Clin Gastrenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.012.
Clopidogrel appears to reduce the risk of colorectal cancer (CRC) as much as low-dose aspirin, based on a case-control study involving more than 15,000 cases.
Source: American Gastroenterological Association
Risk of CRC was reduced by 20%-30% when clopidogrel was given alone or in combination with aspirin, reported lead author Antonio Rodríguez-Miguel of Príncipe de Asturias University Hospital in Madrid and colleagues. This finding adds support to the hypothesis that low-dose aspirin is chemoprotective primarily because of its antiplatelet properties, they noted.
“The mechanism of action of low-dose aspirin to explain its protective effect is subject to debate,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Although aspirin is a nonsteroidal anti-inflammatory drug (NSAID) and these drugs are known to prevent CRC through the inhibition of cyclooxygenase (COX)-2 in epithelial and stromal cells in the large bowel, at low doses (75-300 mg/d) aspirin has only transient effects on this isozyme, while permanently inactivating platelet COX-1 and suppressing thromboxane A2 production. The apparent lack of dose-dependence of the chemoprotective effect of aspirin, as well as the potential role of locally activated platelets in upregulating COX-2 expression in adjacent nucleated cells of the intestinal mucosa, have led [to] the postulation that low-dose aspirin could exert its chemoprotective effect via its antiplatelet action.”
Although previous studies have explored the chemoprotective potential of other antiplatelet agents, such as clopidogrel, the resultant body of evidence remains small. In 2017, for example, Avi Leader, MD, and colleagues reported that the chemoprotective effect of dual-antiplatelet therapy (DAPT) with clopidogrel and aspirin was superior to aspirin monotherapy, based on an additional 8% risk reduction. The present study aimed to build on such findings with evaluation of a Mediterranean cohort, which could reduce confounding lifestyle factors, owing to a lower rate of cardiovascular morbidity than other populations.
The nested, case-control study involved 15,491 cases of CRC and 60,000 controls who were randomly selected and frequency matched by sex, age, and year of indexing. Data were drawn from Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP), a Spanish medical record database with more than 7 million patients. Records of patients involved in the present study were screened for prescription of three antiplatelet agents: low-dose aspirin, clopidogrel, and triflusal. Additional categorization identified current users, recent users, past users, and nonusers. The effects of clopidogrel and aspirin were evaluated separately, as monotherapies, and together, as DAPT.
Demographically, the mean age of the entire study population was 68.6 years, with a slight male predominance (59%). Median follow-up was similar between cases and controls, at approximately 3 years, ranging from about 1.5 to 6 years. Cases showed higher rates of gout, alcohol abuse, acute digestive diseases, and peripheral artery disease, whereas controls were more likely to have histories involving stroke, acute myocardial infarction, chronic digestive diseases, and constipation.
Controls were more likely to be current aspirin users than patients diagnosed with CRC (12.8% vs. 12.2%), giving an associated adjusted odds ratio (AOR) of 0.83. Risk reduction became statistically apparent after 180 days of aspirin usage, with an AOR of 0.79, and more prominent in the 1- to 3-year range, with an AOR of 0.73. This chemoprotective effect faded rapidly with discontinuation.
Current clopidogrel usage led to a comparable level of risk reduction, with an AOR of 0.80. It wasn’t until a year of continuous clopidogrel monotherapy that risk reduction became statistically significant, with an AOR of 0.65, which dropped to 0.57 between years 1 and 3.
Turning to a matched comparison of aspirin or clopidogrel monotherapy versus DAPT, the investigators found similar rates of chemoprotection. Current aspirin usage of any duration offered an adjusted risk reduction of 17%, compared with 25% for clopidogrel, and 29% for DAPT. Beyond 1 year of continuous and current usage, the superiority of DAPT was called into question, as clopidogrel monotherapy offered the greatest risk reduction, at 37%, compared with 22% for aspirin, and 22% for DAPT. Risk analyses involving triflusal lacked statistical significance.
“The results of the present study are compatible with a chemoprotective effect of clopidogrel against CRC, equivalent in magnitude to the one observed for low-dose aspirin,” the investigators wrote. “This finding indirectly supports the hypothesis that the chemoprotective effect of low-dose aspirin is mediated mostly through the permanent inactivation of platelet COX-1.”
The investigators pointed out that the chemoprotective effects of antiplatelet therapy begin to appear early in treatment, independently from lifestyle factors, but risk reduction depends on current usage. Although short-term usage of either aspirin or clopidogrel was associated with an increased risk of CRC, the investigators suggested that this was more likely a perceived risk rather than an actual one. “In our view, this observation could be explained in part by a detection bias, owing to an increased risk of GI bleeding induced by antiplatelet agents that could lead to a greater number of colonoscopies, and, as a result, an early cancer diagnosis,” they wrote.
The study was funded by the Fundación Instituto Teófilo Hernando. Dr. García-Rodríguez disclosed a relationship with CEIFE, which has received funding from Bayer and AstraZeneca.
SOURCE: Rodríguez-Miguel et al. Clin Gastrenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.012.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Clopidogrel usage appears to reduce the risk of colorectal cancer as much as low-dose aspirin.
Major finding: Current clopidogrel usage was associated with a 20% reduced risk of colorectal cancer (adjusted odds ratio, 0.8).
Study details: A nested case-control study involving 15,491 cases of colorectal cancer and 60,000 controls.
Disclosures: The study was funded by the Fundación Instituto Teófilo Hernando. Dr. García-Rodríguez disclosed a relationship with CEIFE, which has received funding from Bayer and AstraZeneca.
Source: Rodríguez-Miguel A et al. Clin Gastrenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.012.
No increase in UTI risk with SGLT-2 inhibitors
Sodium-glucose cotransporter 2 inhibitors do not appear to increase the risk of urinary tract infections, compared with other antidiabetic medications, new research has found.
In a paper published in Annals of Internal Medicine, researchers reported the outcomes of a population-based, propensity-matched cohort study in which the analyzed data from 235,730 individuals newly prescribed sodium-glucose cotransporter 2 (SGLT-2) inhibitors, dipeptidyl peptidase–4 (DPP-4) inhibitors, and glucagonlike peptide–1 receptor (GLP-1) agonists.
In the first cohort, comparing SGLT-2 inhibitors with DDP-4 inhibitors, there were no significant differences between the two groups in the incidence rate of severe urinary tract infections (UTIs) (adjusted hazard ratio, 0.98; 95% confidence interval, 0.68-1.41).
In the second cohort, which compared patients taking GLP-1 receptor agonists with those taking SGLT-2 inhibitors, researchers saw a slightly lower incidence of severe UTIs among individuals on SGLT-2 inhibitors (HR, 0.72; 95% CI, 0.53-0.99; P = .04).
The analysis also failed to find any evidence that SGLT-2 inhibitors were associated with an increase in the risk of hospitalization or outpatient treatment for a UTI.
Chintan V. Dave, PharmD – now at Rutgers University, New Brunswick, N.J. – and colleagues from Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote that the findings have significant clinical implications.
“Patients who may be good candidates to receive SGLT-2 inhibitors for diabetes control but who have a history of recurrent UTIs may be precluded from being prescribed these agents; because UTIs are highly prevalent in patients with diabetes, this could exclude a substantial number of patients from receiving an entire class of medications that has been shown to decrease risk for major cardiovascular events and death,” they wrote.
The researchers stressed that “other factors beyond risk for UTI events should be considered in decisions about whether to prescribe SGLT-2 therapy for patients with diabetes.”
The authors did note that their study was subject to the usual limitations of observational studies, such as susceptibility to confounding, and was also limited to people with health insurance.
An accompanying editorial by Kristian B. Filion, PhD, and Oriana H. Yu, MD, of McGill University, Montreal, commented that the data provided reassuring, real-world evidence on the potential safety issue of UTI risk with SGLT-2 inhibitors.
However, they also stressed that the study excluded individuals at high risk or with a history of UTIs, and that these were subgroups who required further investigation.
The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
SOURCE: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.
Sodium-glucose cotransporter 2 inhibitors do not appear to increase the risk of urinary tract infections, compared with other antidiabetic medications, new research has found.
In a paper published in Annals of Internal Medicine, researchers reported the outcomes of a population-based, propensity-matched cohort study in which the analyzed data from 235,730 individuals newly prescribed sodium-glucose cotransporter 2 (SGLT-2) inhibitors, dipeptidyl peptidase–4 (DPP-4) inhibitors, and glucagonlike peptide–1 receptor (GLP-1) agonists.
In the first cohort, comparing SGLT-2 inhibitors with DDP-4 inhibitors, there were no significant differences between the two groups in the incidence rate of severe urinary tract infections (UTIs) (adjusted hazard ratio, 0.98; 95% confidence interval, 0.68-1.41).
In the second cohort, which compared patients taking GLP-1 receptor agonists with those taking SGLT-2 inhibitors, researchers saw a slightly lower incidence of severe UTIs among individuals on SGLT-2 inhibitors (HR, 0.72; 95% CI, 0.53-0.99; P = .04).
The analysis also failed to find any evidence that SGLT-2 inhibitors were associated with an increase in the risk of hospitalization or outpatient treatment for a UTI.
Chintan V. Dave, PharmD – now at Rutgers University, New Brunswick, N.J. – and colleagues from Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote that the findings have significant clinical implications.
“Patients who may be good candidates to receive SGLT-2 inhibitors for diabetes control but who have a history of recurrent UTIs may be precluded from being prescribed these agents; because UTIs are highly prevalent in patients with diabetes, this could exclude a substantial number of patients from receiving an entire class of medications that has been shown to decrease risk for major cardiovascular events and death,” they wrote.
The researchers stressed that “other factors beyond risk for UTI events should be considered in decisions about whether to prescribe SGLT-2 therapy for patients with diabetes.”
The authors did note that their study was subject to the usual limitations of observational studies, such as susceptibility to confounding, and was also limited to people with health insurance.
An accompanying editorial by Kristian B. Filion, PhD, and Oriana H. Yu, MD, of McGill University, Montreal, commented that the data provided reassuring, real-world evidence on the potential safety issue of UTI risk with SGLT-2 inhibitors.
However, they also stressed that the study excluded individuals at high risk or with a history of UTIs, and that these were subgroups who required further investigation.
The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
SOURCE: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.
Sodium-glucose cotransporter 2 inhibitors do not appear to increase the risk of urinary tract infections, compared with other antidiabetic medications, new research has found.
In a paper published in Annals of Internal Medicine, researchers reported the outcomes of a population-based, propensity-matched cohort study in which the analyzed data from 235,730 individuals newly prescribed sodium-glucose cotransporter 2 (SGLT-2) inhibitors, dipeptidyl peptidase–4 (DPP-4) inhibitors, and glucagonlike peptide–1 receptor (GLP-1) agonists.
In the first cohort, comparing SGLT-2 inhibitors with DDP-4 inhibitors, there were no significant differences between the two groups in the incidence rate of severe urinary tract infections (UTIs) (adjusted hazard ratio, 0.98; 95% confidence interval, 0.68-1.41).
In the second cohort, which compared patients taking GLP-1 receptor agonists with those taking SGLT-2 inhibitors, researchers saw a slightly lower incidence of severe UTIs among individuals on SGLT-2 inhibitors (HR, 0.72; 95% CI, 0.53-0.99; P = .04).
The analysis also failed to find any evidence that SGLT-2 inhibitors were associated with an increase in the risk of hospitalization or outpatient treatment for a UTI.
Chintan V. Dave, PharmD – now at Rutgers University, New Brunswick, N.J. – and colleagues from Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote that the findings have significant clinical implications.
“Patients who may be good candidates to receive SGLT-2 inhibitors for diabetes control but who have a history of recurrent UTIs may be precluded from being prescribed these agents; because UTIs are highly prevalent in patients with diabetes, this could exclude a substantial number of patients from receiving an entire class of medications that has been shown to decrease risk for major cardiovascular events and death,” they wrote.
The researchers stressed that “other factors beyond risk for UTI events should be considered in decisions about whether to prescribe SGLT-2 therapy for patients with diabetes.”
The authors did note that their study was subject to the usual limitations of observational studies, such as susceptibility to confounding, and was also limited to people with health insurance.
An accompanying editorial by Kristian B. Filion, PhD, and Oriana H. Yu, MD, of McGill University, Montreal, commented that the data provided reassuring, real-world evidence on the potential safety issue of UTI risk with SGLT-2 inhibitors.
However, they also stressed that the study excluded individuals at high risk or with a history of UTIs, and that these were subgroups who required further investigation.
The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
SOURCE: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Sodium-glucose cotransporter 2 inhibitors are not associated with a greater risk of urinary tract infections, compared with dipeptidyl peptidase-4 inhibitors or glucagonlike peptide–1 receptor agonists.
Major finding: The incidence of severe urinary tract infections is similar among patients taking sodium-glucose cotransporter 2 inhibitors, dipeptidyl peptidase–4, inhibitors or glucagonlike peptide–1 receptor agonists.
Study details: A population-based, propensity-matched cohort study in 235,730 individuals with type 2 diabetes.
Disclosures: The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
Source: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.
Real-world data for immunotherapy-treated NSCLC found robust
Real-world outcome data from patients with advanced non–small cell lung cancer (NSCLC) treated with immunotherapy are robust enough to use for regulatory and payer decisions, suggests an analysis of six data sets and more than 13,000 patients.
“Study of routinely collected health care data is increasingly important for various stakeholders who are interested in better understanding particular patient populations, evaluating drug safety in the postmarketing setting, measuring health care use and clinical outcomes, performing comparative effectiveness research, and optimizing drug pricing models,” noted lead investigator Mark Stewart, PhD, Friends of Cancer Research, Washington, and coinvestigators. “However, before [real-world data] finds widespread use as an adjunct to – or in unique settings, an alternative for – [randomized clinical trials], the validity of readily extractable clinical outcomes measures – real-world endpoints – must be established.”
The investigators undertook a retrospective cohort study using administrative claims and electronic health records for patients with advanced NSCLC treated with inhibitors of programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1) between January 2011 and October 2017 in real-world settings. They analyzed six data sets having 269 to 6,924 patients each (13,639 patients total).
Results reported in JCO Clinical Cancer Informatics showed that the real-world intermediate endpoints of time to treatment discontinuation and time to next treatment were moderately to highly correlated with real-world overall survival (Spearman’s rank correlation coefficient, 0.36 to 0.89, with most values 0.60 or higher).
In real-world settings, the 1-year rate of overall survival after starting immunotherapy ranged from 40% to 57%. Real-world data and trial data were similar with respect to median overall survival (8.6-13.5 months vs. 9.2-12.7 months).
The data sources used for the study have been extensively used for research and are curated on an ongoing basis to ensure the data are accurate and as complete as possible, Dr. Stewart and coinvestigators noted.
“These findings demonstrate that real-world endpoints are generally consistent with each other and with outcomes observed in randomized clinical trials, which substantiates the potential validity of real-world data to support regulatory and payer decision-making,” they maintained. “Differences observed likely reflect true differences between real-world and protocol-driven practices.
“Additional studies are needed to further support the use of [real-world evidence] and inform the development of regulatory guidance,” the investigators concluded. “Standardizing definitions for real-world endpoints and determining appropriate analytic methodologies for [real-world data] will be critical for broader adoption of real-world studies and will provide greater confidence in associated findings. As more refined and standardized approaches are developed that incorporate deep clinical and bioinformatics expertise, the greater the utility of [real-world data] will be for detecting even small, but important, differences in treatment effects.”
Dr. Stewart disclosed no conflicts of interest. The study was supported in part by the National Cancer Institute and the Patient Centered Outcomes Research Institute.
SOURCE: Stewart M et al. JCO Clin Cancer Inform. 2019 July 23. doi: 10.1200/CCI.18.00155.
Real-world outcome data from patients with advanced non–small cell lung cancer (NSCLC) treated with immunotherapy are robust enough to use for regulatory and payer decisions, suggests an analysis of six data sets and more than 13,000 patients.
“Study of routinely collected health care data is increasingly important for various stakeholders who are interested in better understanding particular patient populations, evaluating drug safety in the postmarketing setting, measuring health care use and clinical outcomes, performing comparative effectiveness research, and optimizing drug pricing models,” noted lead investigator Mark Stewart, PhD, Friends of Cancer Research, Washington, and coinvestigators. “However, before [real-world data] finds widespread use as an adjunct to – or in unique settings, an alternative for – [randomized clinical trials], the validity of readily extractable clinical outcomes measures – real-world endpoints – must be established.”
The investigators undertook a retrospective cohort study using administrative claims and electronic health records for patients with advanced NSCLC treated with inhibitors of programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1) between January 2011 and October 2017 in real-world settings. They analyzed six data sets having 269 to 6,924 patients each (13,639 patients total).
Results reported in JCO Clinical Cancer Informatics showed that the real-world intermediate endpoints of time to treatment discontinuation and time to next treatment were moderately to highly correlated with real-world overall survival (Spearman’s rank correlation coefficient, 0.36 to 0.89, with most values 0.60 or higher).
In real-world settings, the 1-year rate of overall survival after starting immunotherapy ranged from 40% to 57%. Real-world data and trial data were similar with respect to median overall survival (8.6-13.5 months vs. 9.2-12.7 months).
The data sources used for the study have been extensively used for research and are curated on an ongoing basis to ensure the data are accurate and as complete as possible, Dr. Stewart and coinvestigators noted.
“These findings demonstrate that real-world endpoints are generally consistent with each other and with outcomes observed in randomized clinical trials, which substantiates the potential validity of real-world data to support regulatory and payer decision-making,” they maintained. “Differences observed likely reflect true differences between real-world and protocol-driven practices.
“Additional studies are needed to further support the use of [real-world evidence] and inform the development of regulatory guidance,” the investigators concluded. “Standardizing definitions for real-world endpoints and determining appropriate analytic methodologies for [real-world data] will be critical for broader adoption of real-world studies and will provide greater confidence in associated findings. As more refined and standardized approaches are developed that incorporate deep clinical and bioinformatics expertise, the greater the utility of [real-world data] will be for detecting even small, but important, differences in treatment effects.”
Dr. Stewart disclosed no conflicts of interest. The study was supported in part by the National Cancer Institute and the Patient Centered Outcomes Research Institute.
SOURCE: Stewart M et al. JCO Clin Cancer Inform. 2019 July 23. doi: 10.1200/CCI.18.00155.
Real-world outcome data from patients with advanced non–small cell lung cancer (NSCLC) treated with immunotherapy are robust enough to use for regulatory and payer decisions, suggests an analysis of six data sets and more than 13,000 patients.
“Study of routinely collected health care data is increasingly important for various stakeholders who are interested in better understanding particular patient populations, evaluating drug safety in the postmarketing setting, measuring health care use and clinical outcomes, performing comparative effectiveness research, and optimizing drug pricing models,” noted lead investigator Mark Stewart, PhD, Friends of Cancer Research, Washington, and coinvestigators. “However, before [real-world data] finds widespread use as an adjunct to – or in unique settings, an alternative for – [randomized clinical trials], the validity of readily extractable clinical outcomes measures – real-world endpoints – must be established.”
The investigators undertook a retrospective cohort study using administrative claims and electronic health records for patients with advanced NSCLC treated with inhibitors of programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1) between January 2011 and October 2017 in real-world settings. They analyzed six data sets having 269 to 6,924 patients each (13,639 patients total).
Results reported in JCO Clinical Cancer Informatics showed that the real-world intermediate endpoints of time to treatment discontinuation and time to next treatment were moderately to highly correlated with real-world overall survival (Spearman’s rank correlation coefficient, 0.36 to 0.89, with most values 0.60 or higher).
In real-world settings, the 1-year rate of overall survival after starting immunotherapy ranged from 40% to 57%. Real-world data and trial data were similar with respect to median overall survival (8.6-13.5 months vs. 9.2-12.7 months).
The data sources used for the study have been extensively used for research and are curated on an ongoing basis to ensure the data are accurate and as complete as possible, Dr. Stewart and coinvestigators noted.
“These findings demonstrate that real-world endpoints are generally consistent with each other and with outcomes observed in randomized clinical trials, which substantiates the potential validity of real-world data to support regulatory and payer decision-making,” they maintained. “Differences observed likely reflect true differences between real-world and protocol-driven practices.
“Additional studies are needed to further support the use of [real-world evidence] and inform the development of regulatory guidance,” the investigators concluded. “Standardizing definitions for real-world endpoints and determining appropriate analytic methodologies for [real-world data] will be critical for broader adoption of real-world studies and will provide greater confidence in associated findings. As more refined and standardized approaches are developed that incorporate deep clinical and bioinformatics expertise, the greater the utility of [real-world data] will be for detecting even small, but important, differences in treatment effects.”
Dr. Stewart disclosed no conflicts of interest. The study was supported in part by the National Cancer Institute and the Patient Centered Outcomes Research Institute.
SOURCE: Stewart M et al. JCO Clin Cancer Inform. 2019 July 23. doi: 10.1200/CCI.18.00155.
FROM JCO CLINICAL CANCER INFORMATICS
FDA advisors recommend nintedanib for SSc interstitial lung disease
The Food and Drug Administration Arthritis Advisory Committee recommended approval of nintedanib for the treatment of interstitial lung disease in patients with systemic sclerosis by a 10-7 vote on July 25, 2019. If the FDA acts in accord with the panel’s recommendation, it would make nintedanib (Ofev) the first drug to receive marketing approval for this indication.
Nintedanib has had FDA approval for treating idiopathic pulmonary fibrosis since 2014, and the manufacturer, Boehringer Ingelheim, designed the current pivotal trial with 576 patients to broaden the indication to patients with a different but similar fibrotic lung disease, interstitial lung disease (ILD), that is a common and eventually lethal complication of systemic sclerosis. The results of the pivotal study, the SENSCIS (Safety and Efficacy of Nintedanib in Systemic Sclerosis) trial, recently appeared in print and showed that patients randomized to receive 150 mg of nintedanib orally twice daily had an average 41-mL cut in the rate of loss of forced vital capacity (FVC) during 52 weeks on treatment, compared with those randomized to placebo. This was a 44% relative reduction in rate of FVC loss that was statistically significant for the study’s primary endpoint (N Engl J Med. 2019 June 27;380[26]:2518-28).
Votes in favor of FDA approval for many on the panel seemed to stem from a combination of the fact that nintedanib met the pivotal trial’s primary endpoint; which had been developed in consultation with the FDA, as well as the absence of any new safety signals when compared with prior experience using the drug; the lack of any treatment specifically recognized as beneficial to systemic sclerosis patients who develop the terminal complication of ILD; and the challenge of running a second trial in an orphan disease with an estimated U.S. prevalence of no more than 100,000 patients. Several committee members who voted in favor of nintedanib’s approval also voiced concern that the case in favor of its benefit/risk balance was not open and shut.
“I have a fair amount of apprehension,” admitted the committee’s chair, Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School, Boston. “I support the needs of patients, but we don’t want to give them false hope. We need to be able to say who will benefit, and the single study [SENSCIS] results don’t tell us how to use the drug. I want to understand which patient subgroups benefit.” He suggested that the FDA mandate further data collection through postmarketing studies.
Comments from panel members who voted against recommending approval generally focused on what was generally agreed to be a very modest treatment effect with a 41-mL average difference in FVC decline that has marginal clinical meaningfulness. Although the SENSCIS results met the study’s primary endpoint it was neutral for all prespecified secondary endpoints, including a measure of quality of life, although many on the panel agreed that a good measure of quality of life in the target patient population is lacking. Some sensitivity analyses run by FDA staffers also failed to confirm the primary result. Fewer questions arose about safety, although some panelists expressed concern about gastrointestinal effects, especially diarrhea, that seemed to link with treatment, as well as a signal for an increased incidence of pneumonia among patients on nintedanib. The data also showed a possible signal of reduced efficacy among patients who also received treatment with the immunosuppressive agent mycophenolate mofetil, often used off label to treat systemic sclerosis patients with ILD. However, a statistician involved in the discussion warned against overinterpreting this or other subgroup analyses.
Dr. Solomon has received research support from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, Janssen, and Pfizer.
The Food and Drug Administration Arthritis Advisory Committee recommended approval of nintedanib for the treatment of interstitial lung disease in patients with systemic sclerosis by a 10-7 vote on July 25, 2019. If the FDA acts in accord with the panel’s recommendation, it would make nintedanib (Ofev) the first drug to receive marketing approval for this indication.
Nintedanib has had FDA approval for treating idiopathic pulmonary fibrosis since 2014, and the manufacturer, Boehringer Ingelheim, designed the current pivotal trial with 576 patients to broaden the indication to patients with a different but similar fibrotic lung disease, interstitial lung disease (ILD), that is a common and eventually lethal complication of systemic sclerosis. The results of the pivotal study, the SENSCIS (Safety and Efficacy of Nintedanib in Systemic Sclerosis) trial, recently appeared in print and showed that patients randomized to receive 150 mg of nintedanib orally twice daily had an average 41-mL cut in the rate of loss of forced vital capacity (FVC) during 52 weeks on treatment, compared with those randomized to placebo. This was a 44% relative reduction in rate of FVC loss that was statistically significant for the study’s primary endpoint (N Engl J Med. 2019 June 27;380[26]:2518-28).
Votes in favor of FDA approval for many on the panel seemed to stem from a combination of the fact that nintedanib met the pivotal trial’s primary endpoint; which had been developed in consultation with the FDA, as well as the absence of any new safety signals when compared with prior experience using the drug; the lack of any treatment specifically recognized as beneficial to systemic sclerosis patients who develop the terminal complication of ILD; and the challenge of running a second trial in an orphan disease with an estimated U.S. prevalence of no more than 100,000 patients. Several committee members who voted in favor of nintedanib’s approval also voiced concern that the case in favor of its benefit/risk balance was not open and shut.
“I have a fair amount of apprehension,” admitted the committee’s chair, Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School, Boston. “I support the needs of patients, but we don’t want to give them false hope. We need to be able to say who will benefit, and the single study [SENSCIS] results don’t tell us how to use the drug. I want to understand which patient subgroups benefit.” He suggested that the FDA mandate further data collection through postmarketing studies.
Comments from panel members who voted against recommending approval generally focused on what was generally agreed to be a very modest treatment effect with a 41-mL average difference in FVC decline that has marginal clinical meaningfulness. Although the SENSCIS results met the study’s primary endpoint it was neutral for all prespecified secondary endpoints, including a measure of quality of life, although many on the panel agreed that a good measure of quality of life in the target patient population is lacking. Some sensitivity analyses run by FDA staffers also failed to confirm the primary result. Fewer questions arose about safety, although some panelists expressed concern about gastrointestinal effects, especially diarrhea, that seemed to link with treatment, as well as a signal for an increased incidence of pneumonia among patients on nintedanib. The data also showed a possible signal of reduced efficacy among patients who also received treatment with the immunosuppressive agent mycophenolate mofetil, often used off label to treat systemic sclerosis patients with ILD. However, a statistician involved in the discussion warned against overinterpreting this or other subgroup analyses.
Dr. Solomon has received research support from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, Janssen, and Pfizer.
The Food and Drug Administration Arthritis Advisory Committee recommended approval of nintedanib for the treatment of interstitial lung disease in patients with systemic sclerosis by a 10-7 vote on July 25, 2019. If the FDA acts in accord with the panel’s recommendation, it would make nintedanib (Ofev) the first drug to receive marketing approval for this indication.
Nintedanib has had FDA approval for treating idiopathic pulmonary fibrosis since 2014, and the manufacturer, Boehringer Ingelheim, designed the current pivotal trial with 576 patients to broaden the indication to patients with a different but similar fibrotic lung disease, interstitial lung disease (ILD), that is a common and eventually lethal complication of systemic sclerosis. The results of the pivotal study, the SENSCIS (Safety and Efficacy of Nintedanib in Systemic Sclerosis) trial, recently appeared in print and showed that patients randomized to receive 150 mg of nintedanib orally twice daily had an average 41-mL cut in the rate of loss of forced vital capacity (FVC) during 52 weeks on treatment, compared with those randomized to placebo. This was a 44% relative reduction in rate of FVC loss that was statistically significant for the study’s primary endpoint (N Engl J Med. 2019 June 27;380[26]:2518-28).
Votes in favor of FDA approval for many on the panel seemed to stem from a combination of the fact that nintedanib met the pivotal trial’s primary endpoint; which had been developed in consultation with the FDA, as well as the absence of any new safety signals when compared with prior experience using the drug; the lack of any treatment specifically recognized as beneficial to systemic sclerosis patients who develop the terminal complication of ILD; and the challenge of running a second trial in an orphan disease with an estimated U.S. prevalence of no more than 100,000 patients. Several committee members who voted in favor of nintedanib’s approval also voiced concern that the case in favor of its benefit/risk balance was not open and shut.
“I have a fair amount of apprehension,” admitted the committee’s chair, Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School, Boston. “I support the needs of patients, but we don’t want to give them false hope. We need to be able to say who will benefit, and the single study [SENSCIS] results don’t tell us how to use the drug. I want to understand which patient subgroups benefit.” He suggested that the FDA mandate further data collection through postmarketing studies.
Comments from panel members who voted against recommending approval generally focused on what was generally agreed to be a very modest treatment effect with a 41-mL average difference in FVC decline that has marginal clinical meaningfulness. Although the SENSCIS results met the study’s primary endpoint it was neutral for all prespecified secondary endpoints, including a measure of quality of life, although many on the panel agreed that a good measure of quality of life in the target patient population is lacking. Some sensitivity analyses run by FDA staffers also failed to confirm the primary result. Fewer questions arose about safety, although some panelists expressed concern about gastrointestinal effects, especially diarrhea, that seemed to link with treatment, as well as a signal for an increased incidence of pneumonia among patients on nintedanib. The data also showed a possible signal of reduced efficacy among patients who also received treatment with the immunosuppressive agent mycophenolate mofetil, often used off label to treat systemic sclerosis patients with ILD. However, a statistician involved in the discussion warned against overinterpreting this or other subgroup analyses.
Dr. Solomon has received research support from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, Janssen, and Pfizer.