User login
Tender Nodule on the Umbilicus
Tender Nodule on the Umbilicus
THE DIAGNOSIS: Villar Nodule
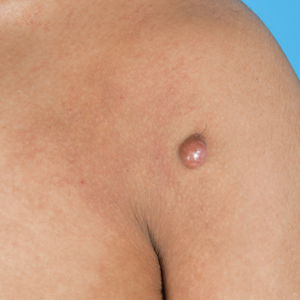
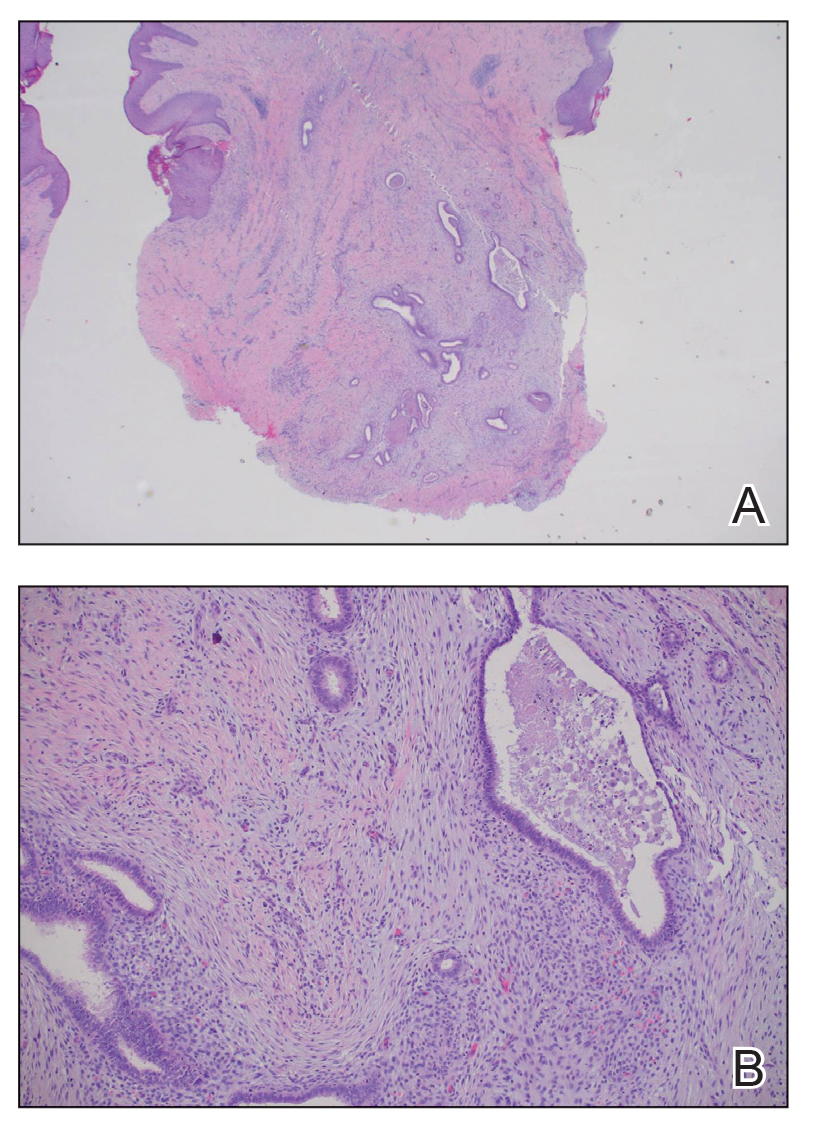
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.
Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
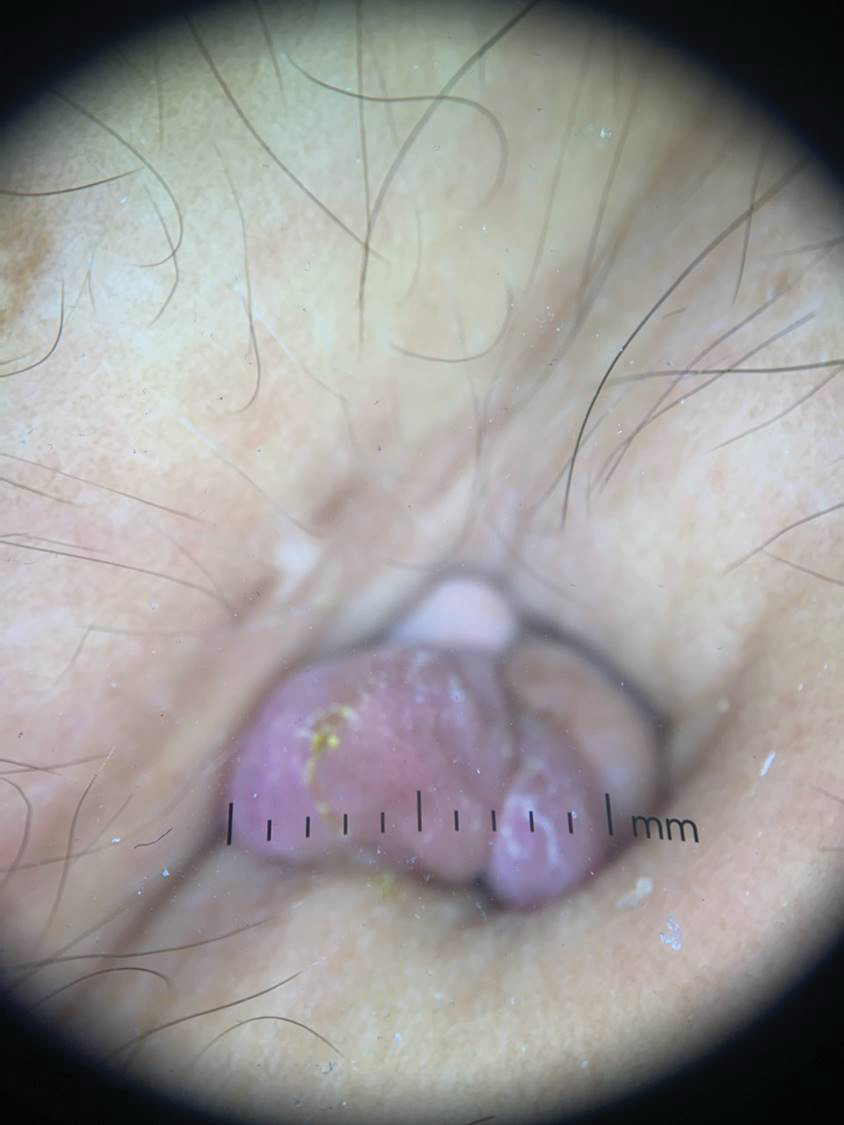
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.
Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
THE DIAGNOSIS: Villar Nodule
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.

Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.

Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
THE DIAGNOSIS: Villar Nodule
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.

Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.

Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
Tender Nodule on the Umbilicus
Tender Nodule on the Umbilicus
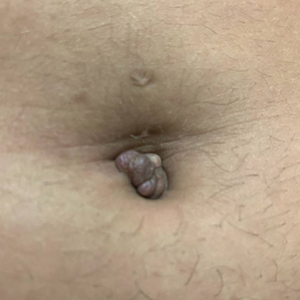
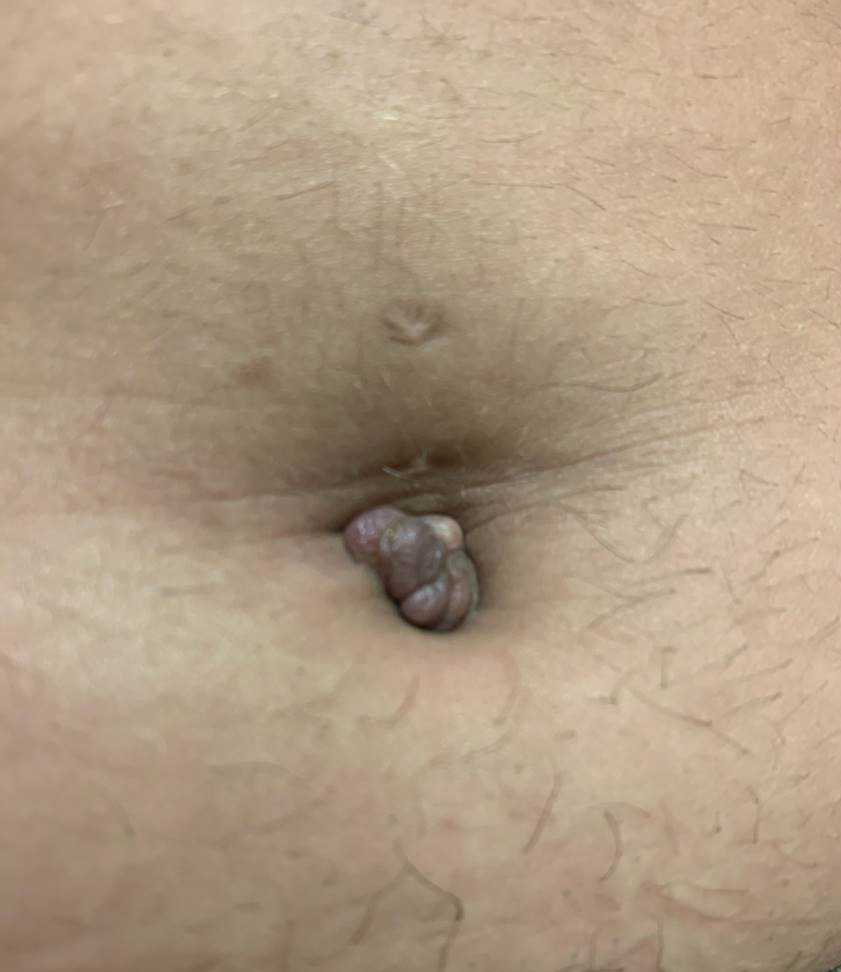
A 25-year-old woman was referred to the dermatology clinic by her primary care provider for evaluation of a tender nodule on the inferior umbilicus of 2 years' duration at the site of a preexisting keloid scar. The patient reported that the lesion caused occasional pain and tenderness. A few weeks prior to the current presentation, a dark-red bloody discharge developed at the superior aspect of the lesion that subsequently crusted over. The patient denied any use of oral contraceptives or history of abdominal surgery.
The original keloid scar had been treated successfully by an outside physician with intralesional steroid injections, and the patient was interested in a similar procedure for the current nodule. She also had a history of a hyperpigmented hypertrophic scar on the superior periumbilical area from a previous piercing that had resolved several years prior to presentation.
Physical examination of the lesion revealed a 1.2-cm, soft, tender, violaceous nodule with scant yellow crust along the superior surface of the umbilicus. There was no palpable abdominal wall defect, and the nodule was not reducible into the abdominal cavity. An interval history revealed bleeding of the lesion during the patient's menstrual cycle with persistent pain and tenderness. A punch biopsy was performed.
Diffuse Pruritic Keratotic Papules
Diffuse Pruritic Keratotic Papules
THE DIAGNOSIS: Reactive Perforating Collagenosis
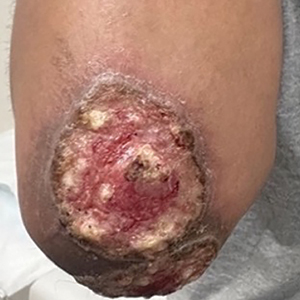
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).
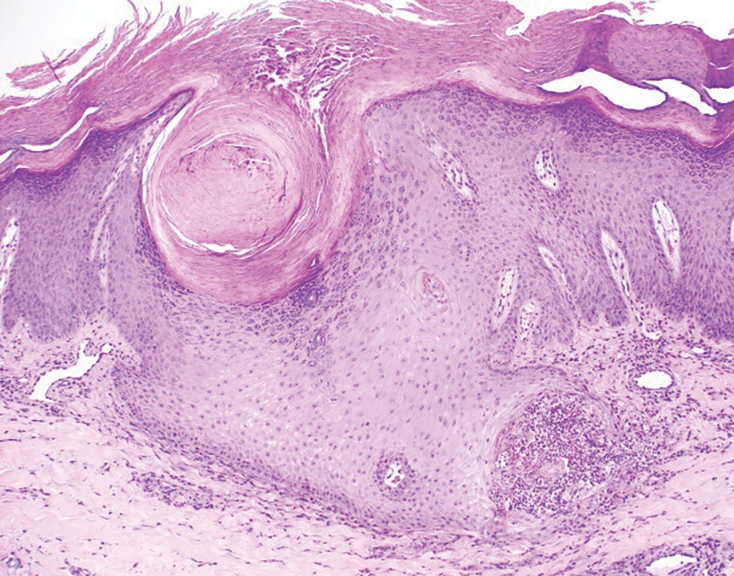
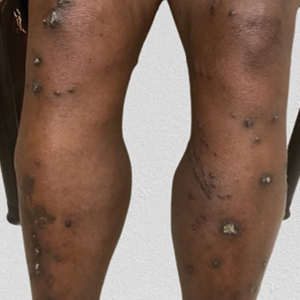
Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).

Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).

Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
Diffuse Pruritic Keratotic Papules
Diffuse Pruritic Keratotic Papules
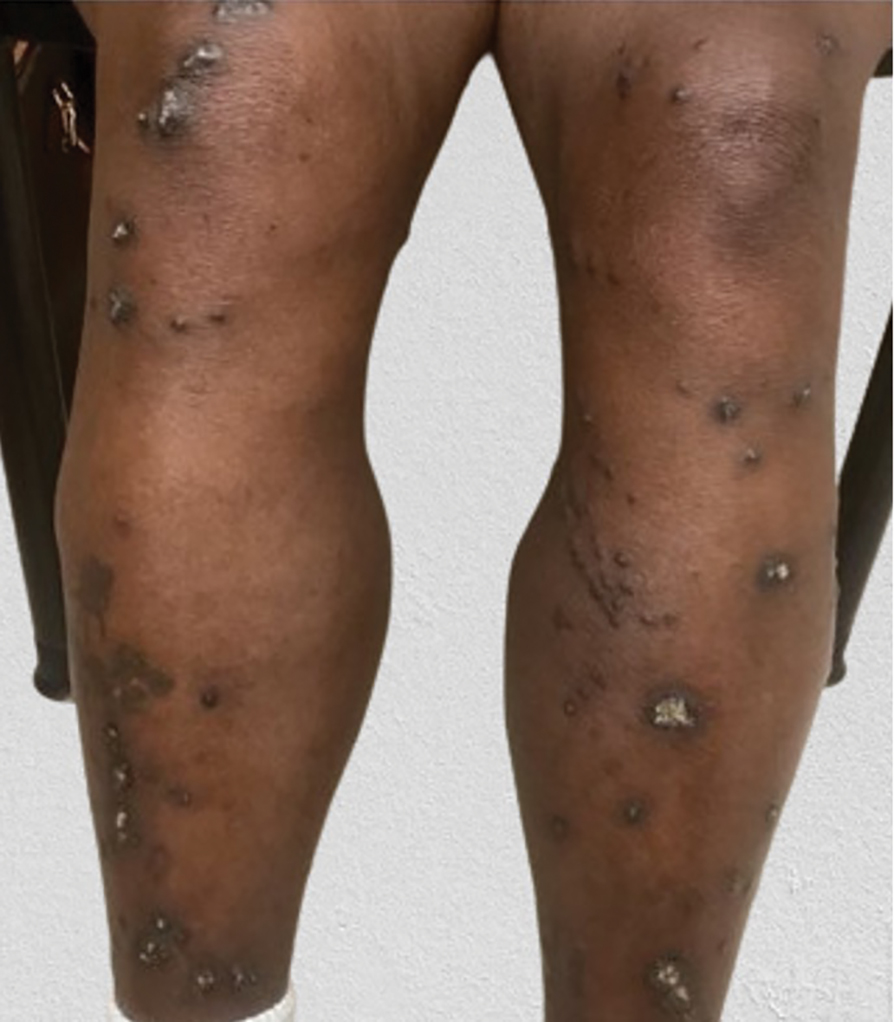
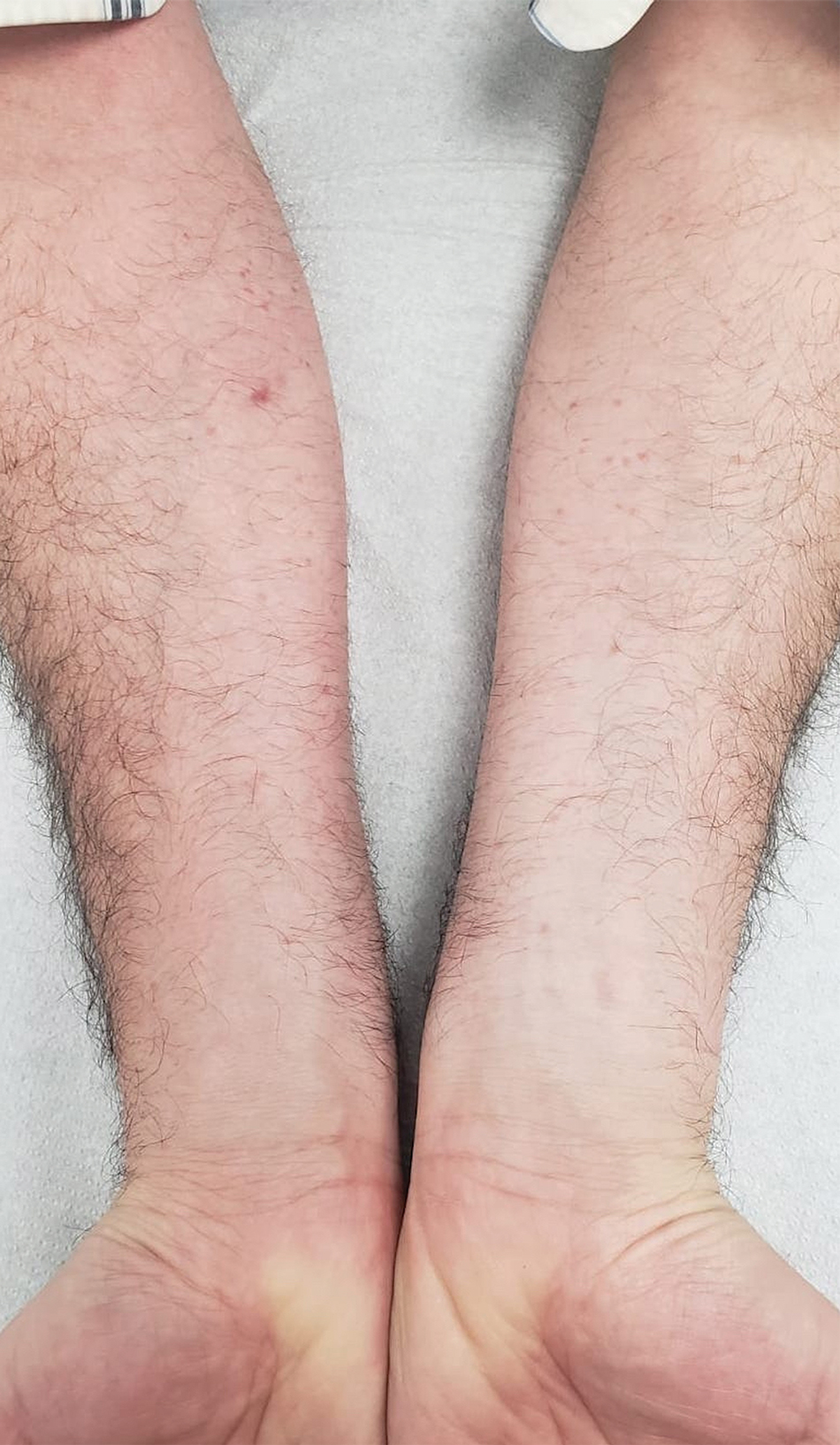
A 65-year-old woman presented to dermatology with an intensely pruritic rash on the arms, legs, neck, and face of several months’ duration. The patient reported scratching the lesions but denied any recent trauma to the affected areas. She previously had been evaluated by her primary care provider, who prescribed cephalexin with no improvement. Her medical history was remarkable for chronic renal failure on dialysis, diabetes, hypertension, and congestive heart failure. Physical examination of the skin revealed hard white cutaneous nodules distributed on the proximal posterior upper arms, bilateral proximal pretibial regions, right elbow, and left knee. Two shave biopsies from the right elbow and left knee were obtained for histopathology.
Painful Edematous Labial Erosions
Painful Edematous Labial Erosions
THE DIAGNOSIS: Kaposi Varicelliform Eruption
Genital erosions tested positive for herpes simplex virus (HSV) 2 via PCR, confirming a Kaposi varicelliform eruption (KVE) in a patient with mycosis fungoides. The medical team began antiviral therapy with intravenous (IV) acyclovir; however, susceptibility testing during the hospital admission confirmed acyclovir resistance, requiring a transition to cidofovir cream 1% and IV foscarnet.1 Subsequent concerns by the care team about chemical burns, dysuria, and renal impairment led to discontinuation of both the cidofovir and foscarnet, considerably narrowing the treatment options.1 The patient’s condition was complicated by polymicrobial bacteremia. Additionally, worsening acidosis and acute kidney injury required initiation of continuous renal replacement therapy.1 Considering these conditions, the patient was enrolled in a promising clinical trial for pritelivir, a novel antiviral medication; however, due to the development of oliguria and the progression of renal failure, this course of treatment had to be discontinued. Faced with potential viral encephalitis, the infectious disease team concluded that, despite previous adverse reactions, resumption of IV foscarnet treatment would present more benefits than risks, given the patient’s critical situation.1
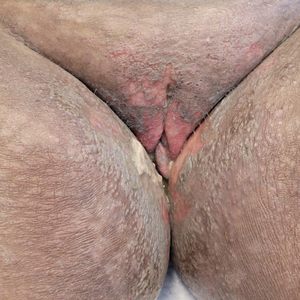
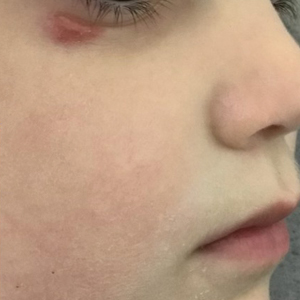
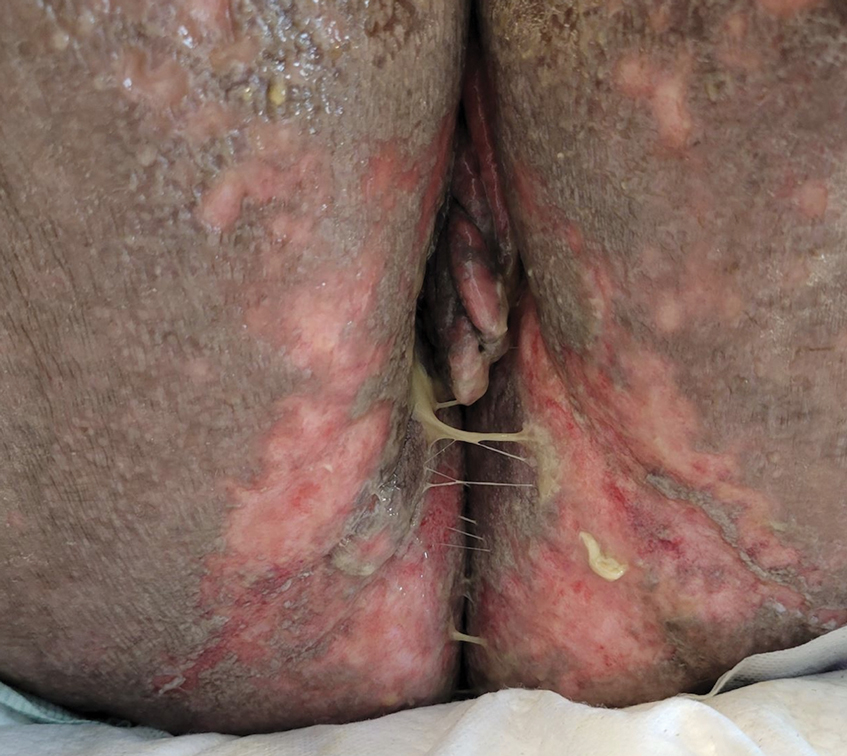
Mycosis fungoides (MF) is a slowly progressive cutaneous T-cell lymphoma of CD4+ cells that primarily affects the skin. Clinically, it often is characterized by pruritic scaly patches or plaques with sharply demarcated borders, the enduring nature of which consistently poses a therapeutic challenge due to their noted resistance to preliminary lines of treatment. Presently, potential cures are limited to allogeneic stem cell transplantation and unilesional radiotherapy for advanced MF; however, no treatment has been found to notably improve survival rates.1 Mycosis fungoides can result in various complications including diffuse spread of a skin infection caused by HSV, known as KVE.1 Kaposi varicelliform eruption usually manifests clinically with painful skin vesicles that often are accompanied by systemic signs such as fever and malaise. The vesicles rapidly progress into pustules or erosions, predominantly affecting regions such as the head, neck, groin, and upper torso (Figure 1).2 Kaposi varicelliform eruption is considered a dermatologic emergency due to its potential to precipitate serious complications such as life-threatening secondary bacterial infection, HSV viremia, and multiorgan involvement; it also carries the risk of instigating ocular complications, such as keratitis, conjunctivitis, blepharitis, uveitis, and potential vision loss.2
Kaposi varicelliform eruption usually is diagnosed through clinical examination supported by polymerase chain reaction, viral culture, histopathology, HSV serology, and Tzanck smear.2 The differential diagnosis includes varicella, atypical varicella, herpes genitalis, herpes zoster, allergic or irritant contact dermatitis, or MF, which may result in painful skin ulcers.2-4 If an HSV superinfection is suspected, a polymerase chain reaction test ideally should be conducted within the first 72 hours of symptom onset.2 Herpes simplex virus infection may be reinforced by histologic features such as intraepidermal blistering, acantholysis, keratinocyte ballooning degeneration, and multinuclear giant cells with intranuclear inclusions. Given its severe nature, immediate empiric antiviral treatment for KVE is essential, even while awaiting confirmatory tests. The recommended treatment protocol involves acyclovir (400 mg orally 3 times daily or 10 mg/kg IV) or valacyclovir (500 mg orally twice daily), continued until KVE resolves.2
Herpes genitalis caused by HSV-2 is estimated to affect approximately 45 million adults in the United States.2 First-line treatment for HSV-2 includes acyclovir and its derivatives, which are viral nucleoside analogs that inhibit viral DNA polymerases.5,6 However, over the past 2 decades, increasing HSV resistance to acyclovir and its derivatives has been noted among immunocompromised patients.5,6 Second-line agents, such as IV foscarnet and cidofovir, require close laboratory monitoring for nephrotoxicity and are contraindicated in those with renal insufficiency, thus limiting their use.5 To combat acyclovir resistance, novel antivirals such as pritelivir are being developed. Pritelivir targets the HSV helicase-primase complex and has been shown to outperform acyclovir in in-vitro animal models.7 Due to its unique mechanism of action (Figure 2), pritelivir is effective against acyclovir-resistant HSV strains, and clinical trials suggest its serum half-life may allow for daily dosing. A phase 2 study showed pritelivir reduced viral shedding days, sped up genital lesion healing in adults infected with HSV-2, and exhibited a good safety profile.7 Our patient participated in ongoing open-label trials of pritelivir that aimed to assess its efficacy and safety in immunocompromised patients. Given the limited alternative treatments for acyclovir-resistant HSV-2, clinicians need to stay updated on antiviral agents under development.
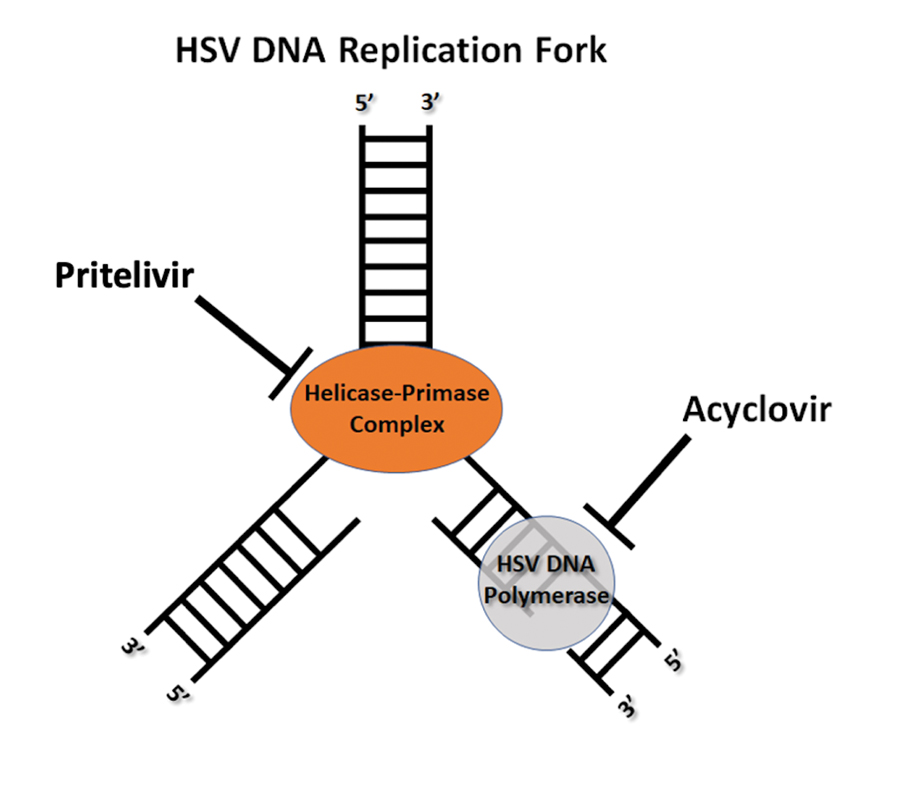
- García-Díaz N, Piris MÁ, Ortiz-Romero PL, et al. Mycosis fungoides and Sézary syndrome: an integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers. 2021;13:1931. doi:10.3390/cancers13081931
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Shin D, Lee MS, Kim DY, et al. Increased large unstained cells value in varicella patients: a valuable parameter to aid rapid diagnosis of varicella infection. J Dermatol. 2015;42:795-799. doi:10.1111
- Joshi A, Sah SP, Agrawal S. Kaposi’s varicelliform eruption or atypical chickenpox in a normal individual. Australas J Dermatol. 2000;41:126-127.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Fleming DT, Leone P, Esposito D, et al. Herpes virus type 2 infection and genital symptoms in primary care patients. Sex Transm Dis. 2006;33:416-421. doi:10.1097/01.olq.0000200578.86276.0b
- Poole CL, James SH. Antiviral therapies for herpesviruses: current agents and new directions. Clin Ther. 2018;40:1282-1298. doi:10.1016 /j.clinthera.2018.07.006.
THE DIAGNOSIS: Kaposi Varicelliform Eruption
Genital erosions tested positive for herpes simplex virus (HSV) 2 via PCR, confirming a Kaposi varicelliform eruption (KVE) in a patient with mycosis fungoides. The medical team began antiviral therapy with intravenous (IV) acyclovir; however, susceptibility testing during the hospital admission confirmed acyclovir resistance, requiring a transition to cidofovir cream 1% and IV foscarnet.1 Subsequent concerns by the care team about chemical burns, dysuria, and renal impairment led to discontinuation of both the cidofovir and foscarnet, considerably narrowing the treatment options.1 The patient’s condition was complicated by polymicrobial bacteremia. Additionally, worsening acidosis and acute kidney injury required initiation of continuous renal replacement therapy.1 Considering these conditions, the patient was enrolled in a promising clinical trial for pritelivir, a novel antiviral medication; however, due to the development of oliguria and the progression of renal failure, this course of treatment had to be discontinued. Faced with potential viral encephalitis, the infectious disease team concluded that, despite previous adverse reactions, resumption of IV foscarnet treatment would present more benefits than risks, given the patient’s critical situation.1
Mycosis fungoides (MF) is a slowly progressive cutaneous T-cell lymphoma of CD4+ cells that primarily affects the skin. Clinically, it often is characterized by pruritic scaly patches or plaques with sharply demarcated borders, the enduring nature of which consistently poses a therapeutic challenge due to their noted resistance to preliminary lines of treatment. Presently, potential cures are limited to allogeneic stem cell transplantation and unilesional radiotherapy for advanced MF; however, no treatment has been found to notably improve survival rates.1 Mycosis fungoides can result in various complications including diffuse spread of a skin infection caused by HSV, known as KVE.1 Kaposi varicelliform eruption usually manifests clinically with painful skin vesicles that often are accompanied by systemic signs such as fever and malaise. The vesicles rapidly progress into pustules or erosions, predominantly affecting regions such as the head, neck, groin, and upper torso (Figure 1).2 Kaposi varicelliform eruption is considered a dermatologic emergency due to its potential to precipitate serious complications such as life-threatening secondary bacterial infection, HSV viremia, and multiorgan involvement; it also carries the risk of instigating ocular complications, such as keratitis, conjunctivitis, blepharitis, uveitis, and potential vision loss.2

Kaposi varicelliform eruption usually is diagnosed through clinical examination supported by polymerase chain reaction, viral culture, histopathology, HSV serology, and Tzanck smear.2 The differential diagnosis includes varicella, atypical varicella, herpes genitalis, herpes zoster, allergic or irritant contact dermatitis, or MF, which may result in painful skin ulcers.2-4 If an HSV superinfection is suspected, a polymerase chain reaction test ideally should be conducted within the first 72 hours of symptom onset.2 Herpes simplex virus infection may be reinforced by histologic features such as intraepidermal blistering, acantholysis, keratinocyte ballooning degeneration, and multinuclear giant cells with intranuclear inclusions. Given its severe nature, immediate empiric antiviral treatment for KVE is essential, even while awaiting confirmatory tests. The recommended treatment protocol involves acyclovir (400 mg orally 3 times daily or 10 mg/kg IV) or valacyclovir (500 mg orally twice daily), continued until KVE resolves.2
Herpes genitalis caused by HSV-2 is estimated to affect approximately 45 million adults in the United States.2 First-line treatment for HSV-2 includes acyclovir and its derivatives, which are viral nucleoside analogs that inhibit viral DNA polymerases.5,6 However, over the past 2 decades, increasing HSV resistance to acyclovir and its derivatives has been noted among immunocompromised patients.5,6 Second-line agents, such as IV foscarnet and cidofovir, require close laboratory monitoring for nephrotoxicity and are contraindicated in those with renal insufficiency, thus limiting their use.5 To combat acyclovir resistance, novel antivirals such as pritelivir are being developed. Pritelivir targets the HSV helicase-primase complex and has been shown to outperform acyclovir in in-vitro animal models.7 Due to its unique mechanism of action (Figure 2), pritelivir is effective against acyclovir-resistant HSV strains, and clinical trials suggest its serum half-life may allow for daily dosing. A phase 2 study showed pritelivir reduced viral shedding days, sped up genital lesion healing in adults infected with HSV-2, and exhibited a good safety profile.7 Our patient participated in ongoing open-label trials of pritelivir that aimed to assess its efficacy and safety in immunocompromised patients. Given the limited alternative treatments for acyclovir-resistant HSV-2, clinicians need to stay updated on antiviral agents under development.

THE DIAGNOSIS: Kaposi Varicelliform Eruption
Genital erosions tested positive for herpes simplex virus (HSV) 2 via PCR, confirming a Kaposi varicelliform eruption (KVE) in a patient with mycosis fungoides. The medical team began antiviral therapy with intravenous (IV) acyclovir; however, susceptibility testing during the hospital admission confirmed acyclovir resistance, requiring a transition to cidofovir cream 1% and IV foscarnet.1 Subsequent concerns by the care team about chemical burns, dysuria, and renal impairment led to discontinuation of both the cidofovir and foscarnet, considerably narrowing the treatment options.1 The patient’s condition was complicated by polymicrobial bacteremia. Additionally, worsening acidosis and acute kidney injury required initiation of continuous renal replacement therapy.1 Considering these conditions, the patient was enrolled in a promising clinical trial for pritelivir, a novel antiviral medication; however, due to the development of oliguria and the progression of renal failure, this course of treatment had to be discontinued. Faced with potential viral encephalitis, the infectious disease team concluded that, despite previous adverse reactions, resumption of IV foscarnet treatment would present more benefits than risks, given the patient’s critical situation.1
Mycosis fungoides (MF) is a slowly progressive cutaneous T-cell lymphoma of CD4+ cells that primarily affects the skin. Clinically, it often is characterized by pruritic scaly patches or plaques with sharply demarcated borders, the enduring nature of which consistently poses a therapeutic challenge due to their noted resistance to preliminary lines of treatment. Presently, potential cures are limited to allogeneic stem cell transplantation and unilesional radiotherapy for advanced MF; however, no treatment has been found to notably improve survival rates.1 Mycosis fungoides can result in various complications including diffuse spread of a skin infection caused by HSV, known as KVE.1 Kaposi varicelliform eruption usually manifests clinically with painful skin vesicles that often are accompanied by systemic signs such as fever and malaise. The vesicles rapidly progress into pustules or erosions, predominantly affecting regions such as the head, neck, groin, and upper torso (Figure 1).2 Kaposi varicelliform eruption is considered a dermatologic emergency due to its potential to precipitate serious complications such as life-threatening secondary bacterial infection, HSV viremia, and multiorgan involvement; it also carries the risk of instigating ocular complications, such as keratitis, conjunctivitis, blepharitis, uveitis, and potential vision loss.2

Kaposi varicelliform eruption usually is diagnosed through clinical examination supported by polymerase chain reaction, viral culture, histopathology, HSV serology, and Tzanck smear.2 The differential diagnosis includes varicella, atypical varicella, herpes genitalis, herpes zoster, allergic or irritant contact dermatitis, or MF, which may result in painful skin ulcers.2-4 If an HSV superinfection is suspected, a polymerase chain reaction test ideally should be conducted within the first 72 hours of symptom onset.2 Herpes simplex virus infection may be reinforced by histologic features such as intraepidermal blistering, acantholysis, keratinocyte ballooning degeneration, and multinuclear giant cells with intranuclear inclusions. Given its severe nature, immediate empiric antiviral treatment for KVE is essential, even while awaiting confirmatory tests. The recommended treatment protocol involves acyclovir (400 mg orally 3 times daily or 10 mg/kg IV) or valacyclovir (500 mg orally twice daily), continued until KVE resolves.2
Herpes genitalis caused by HSV-2 is estimated to affect approximately 45 million adults in the United States.2 First-line treatment for HSV-2 includes acyclovir and its derivatives, which are viral nucleoside analogs that inhibit viral DNA polymerases.5,6 However, over the past 2 decades, increasing HSV resistance to acyclovir and its derivatives has been noted among immunocompromised patients.5,6 Second-line agents, such as IV foscarnet and cidofovir, require close laboratory monitoring for nephrotoxicity and are contraindicated in those with renal insufficiency, thus limiting their use.5 To combat acyclovir resistance, novel antivirals such as pritelivir are being developed. Pritelivir targets the HSV helicase-primase complex and has been shown to outperform acyclovir in in-vitro animal models.7 Due to its unique mechanism of action (Figure 2), pritelivir is effective against acyclovir-resistant HSV strains, and clinical trials suggest its serum half-life may allow for daily dosing. A phase 2 study showed pritelivir reduced viral shedding days, sped up genital lesion healing in adults infected with HSV-2, and exhibited a good safety profile.7 Our patient participated in ongoing open-label trials of pritelivir that aimed to assess its efficacy and safety in immunocompromised patients. Given the limited alternative treatments for acyclovir-resistant HSV-2, clinicians need to stay updated on antiviral agents under development.

- García-Díaz N, Piris MÁ, Ortiz-Romero PL, et al. Mycosis fungoides and Sézary syndrome: an integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers. 2021;13:1931. doi:10.3390/cancers13081931
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Shin D, Lee MS, Kim DY, et al. Increased large unstained cells value in varicella patients: a valuable parameter to aid rapid diagnosis of varicella infection. J Dermatol. 2015;42:795-799. doi:10.1111
- Joshi A, Sah SP, Agrawal S. Kaposi’s varicelliform eruption or atypical chickenpox in a normal individual. Australas J Dermatol. 2000;41:126-127.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Fleming DT, Leone P, Esposito D, et al. Herpes virus type 2 infection and genital symptoms in primary care patients. Sex Transm Dis. 2006;33:416-421. doi:10.1097/01.olq.0000200578.86276.0b
- Poole CL, James SH. Antiviral therapies for herpesviruses: current agents and new directions. Clin Ther. 2018;40:1282-1298. doi:10.1016 /j.clinthera.2018.07.006.
- García-Díaz N, Piris MÁ, Ortiz-Romero PL, et al. Mycosis fungoides and Sézary syndrome: an integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers. 2021;13:1931. doi:10.3390/cancers13081931
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Shin D, Lee MS, Kim DY, et al. Increased large unstained cells value in varicella patients: a valuable parameter to aid rapid diagnosis of varicella infection. J Dermatol. 2015;42:795-799. doi:10.1111
- Joshi A, Sah SP, Agrawal S. Kaposi’s varicelliform eruption or atypical chickenpox in a normal individual. Australas J Dermatol. 2000;41:126-127.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Fleming DT, Leone P, Esposito D, et al. Herpes virus type 2 infection and genital symptoms in primary care patients. Sex Transm Dis. 2006;33:416-421. doi:10.1097/01.olq.0000200578.86276.0b
- Poole CL, James SH. Antiviral therapies for herpesviruses: current agents and new directions. Clin Ther. 2018;40:1282-1298. doi:10.1016 /j.clinthera.2018.07.006.
Painful Edematous Labial Erosions
Painful Edematous Labial Erosions
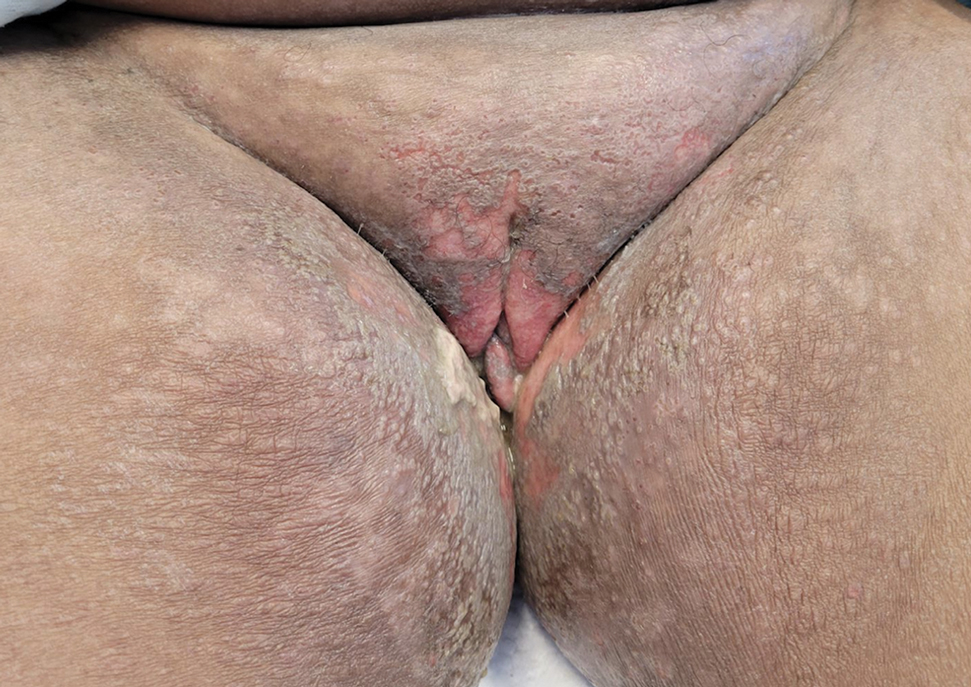
A 40-year-old woman presented to the emergency department with painful, well-defined, edematous labial erosions of several weeks’ duration. The patient reported a medical history of stage IIIA (T4N0M0B0) mycosis fungoides. She had been hospitalized 2 months prior for sepsis that was attributed to a polymicrobial bacteremia involving Acinetobacter baumannii and Staphylococcus epidermidis. During that admission, a polymerase chain reaction test conducted on a skin swab from a lesion on the labia majora confirmed the presence of herpes simplex virus type 2. At the current presentation, physical examination by dermatology also revealed discrete, coalescing, erythematous erosions on the buttocks, groin, and proximal thighs with a punched-out appearance. These areas also exhibited skin sloughing and were covered with a gray-brown exudate. No other mucosal surfaces were involved.
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
THE DIAGNOSIS: Impetigo Herpetiformis
Histopathology revealed epidermal acanthosis and spongiosis with overlying parakeratosis associated with subcorneal and intracorneal neutrophils, papillary dermal edema, and dermal mixed inflammation with neutrophils and eosinophils. Both direct immunofluorescence and periodic acid–Schiff studies were negative. Blood and pustule cultures were sterile and the skin flora were normal. Based on these findings, a diagnosis of impetigo herpetiformis (IH) was made. The condition improved with systemic and topical steroids, supportive care, and an intravenous infusion of infliximab 5 mg/kg. At 3 weeks’ follow-up, the patient demonstrated near-complete resolution and later delivered successfully at 40 weeks’ gestation without complications.
Impetigo herpetiformis, also known as pustular psoriasis of pregnancy, is an exceedingly rare gestational dermatosis that typically manifests in the third trimester and can be life-threatening for both the mother and fetus. The term was first used in 1872 to describe 5 pregnant women with extensive acute pustular eruptions, all in unstable condition; 4 (80%)of the cases resulted in maternal death, and all resulted in fetal death.1 Impetigo herpetiformis is characterized by pruritic and painful erythematous patches studded at the periphery with subcorneal pustules. Eruptions usually occur in the flexural areas and spread centrifugally, with extension of the lesions peripherally as the center erodes and crusts. Sparing of the face, palms, and soles is expected, and mucosal involvement is rare. Generalized involvement and exfoliation may occur in extreme cases.2 While IH typically manifests during the third trimester, it may occur any time throughout pregnancy or immediately postpartum.3 A few cases have been reported in the puerperium.2 Common symptoms include fever, chills, malaise, anorexia, nausea, vomiting, diarrhea, and arthralgias. Less common complications include hypoalbuminemia and severe hypocalcemia leading to tetany, seizures, and delirium.2,3 While maternal mortality is uncommon, fetal mortality often is a more pressing risk and is attributed to placental insufficiency.3,4 For this reason, early delivery commonly is considered in severe cases.
Whether IH is a separate entity or a variant of pustular psoriasis remains heavily debated. Although the histopathology of IH is identical to pustular psoriasis, the lack of a personal and family history of psoriasis, symptom resolution with delivery, and possible recurrence during successive pregnancies help differentiate IH from generalized pustular psoriasis.2,5 Earlier onset, diffuse involvement, faster progression, and recurrence in subsequent pregnancies all have been linked to a worse prognosis.4
The differential diagnosis for IH includes acute generalized exanthematous pustulosis, pemphigoid gestationis, dermatitis herpetiformis, and subcorneal pustular dermatosis. Acute generalized exanthematous pustulosis is an uncommon severe cutaneous drug reaction characterized by the sudden onset of numerous sterile pustules on erythematous skin within 48 hours of exposure. The most common offending medications include pristinamycin and beta-lactam antibiotics. A high fever, neutrophilic leukocytosis, and hypocalcemia often accompany acute generalized exanthematous pustulosis.6 Prompt diagnosis and withdrawal of the offending drug as well as supportive care and symptomatic treatment are crucial for disease management, as systemic symptoms and even organ involvement may occur.6
Pemphigoid gestationis, also known as gestational pemphigoid or herpes gestationis, is a rare autoimmune blistering disorder that primarily affects pregnant women. It typically manifests in the second or third trimester or shortly after delivery. Clinically, it manifests as an intensely pruritic polymorphic eruption of urticarial papules and plaques accompanied by vesicles and bullae and often is distributed on the abdomen and extends to other body regions. Although the exact etiology is unknown, pemphigoid gestationis is caused by autoantibodies targeting the BP180 and BP230 hemidesmosomal proteins.7 Treatment usually involves systemic corticosteroids and may require additional immunosuppressive therapy. In most cases, patients see resolution within 6 months of delivery.7
Dermatitis herpetiformis is a chronic autoimmune blistering skin disorder characterized by intensely pruritic, grouped vesicles and papules, often distributed symmetrically on extensor surfaces such as the elbows, knees, buttocks, and back. It is closely associated with celiac disease and is triggered by gluten ingestion in genetically predisposed individuals with human leukocyte antigen DQ2 and DQ8 haplotypes. Dermatitis herpetiformis is caused by deposition of IgA antibodies that target tissue transglutaminase 3 at the dermal papillae, leading to inflammation and blister formation. 8 Treatment typically involves a gluten-free diet and medications such as dapsone to alleviate symptoms and prevent recurrence.
Subcorneal pustular dermatosis, also known as Sneddon-Wilkinson disease, is a rare chronic relapsing pustular dermatosis characterized by sterile superficial pustules arranged in annular or circinate patterns on erythematous plaques. It predominantly affects middleaged women and often is associated with underlying conditions such as IgA gammopathy or monoclonal gammopathy of undetermined significance. The pathogenesis remains unclear, but immune dysregulation is thought to play a role. Some authors still question whether subcorneal pustular dermatosis is a distinct entity from pustular psoriasis.4,5,12 Dapsone is the preferred first-line treatment, with adjunct therapies including topical or systemic corticosteroids, other immunosuppressive agents, tumor necrosis factor inhibitors, and UV light therapy.9
Definitive management of IH is achieved through early delivery; however, systemic corticosteroids often are used in varying doses to control the disease and to extend the pregnancy period closer to term or until delivery is considered viable. Additional therapies that can be considered include infliximab, cyclosporine, and topical corticosteroids, in conjunction with fluid and electrolyte maintenance.2,4,10 If symptoms persist despite supportive care and pharmacologic intervention, induction of labor or termination of pregnancy may be indicated. In nonbreastfeeding postpartum mothers with persistent disease, therapies commonly used in generalized pustular psoriasis may be given.11
- Hebra F. Ueber einzelne wahrend Schwangerschaft, des wacherbette unde bei uterinal. Krankheiten der Frauen zu beobachtende Hautkrankheiten. Wien Med Wochenschr. 1872;48:1197-1202.
- Fouda UM, Fouda RM, Ammar HM, et al. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J. 2009;2:9338. doi:10.1186/1757-1626-2-9338
- Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1-22. doi:10.1067/mjd.2001.114595
- Liu J, Ali K, Lou H, et al. First-trimester impetigo herpetiformis leads to stillbirth: a case report. Dermatol Ther (Heidelb). 2022;12:1271-1279. doi:10.1007/s13555-022-00735-9
- Lotem M, Katzenelson V, Rotem A, et al. Impetigo herpetiformis: a variant of pustular psoriasis or a separate entity? J Am Acad Dermatol. 1989;20:338-41. doi:10.1016/s0190-9622(89)70042-6
- Stadler PC, Oschmann A, Kerl-French K, et al. Acute generalized exanthematous pustulosis: clinical characteristics, pathogenesis, and management. Dermatology. 2023;239:328-333. doi:10.1159/000529218
- Abdelhafez MMA, Ahmed KAM, Daud MNBM, et al. Pemphigoid gestationis and adverse pregnancy outcomes: a literature review. J Gynecol Obstet Hum Reprod. 2022;51:102370. doi:10.1016 /j.jogoh.2022.102370
- Reunala T, Hervonen K, Salmi T. Dermatitis herpetiformis: an update on diagnosis and management. Am J Clin Dermatol. 2021;22:329-338. doi:10.1007/s40257-020-00584-2
- Watts PJ, Khachemoune A. Subcorneal pustular dermatosis: a review of 30 years of progress. Am J Clin Dermatol. 2016;17:653-671. doi:10.1007 /s40257-016-0202-8
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/j.jaad.2011.01.032
- Bukhari IA. Impetigo herpetiformis in a primigravida: successful treatment with etanercept. J Drugs Dermatol. 2004;3:449-451.
- Chang SE, Kim HH, Choi JH, et al. Impetigo herpetiformis followed by generalized pustular psoriasis: more evidence of same disease entity. Int J Dermatol. 2003;42(9):754-755.
THE DIAGNOSIS: Impetigo Herpetiformis
Histopathology revealed epidermal acanthosis and spongiosis with overlying parakeratosis associated with subcorneal and intracorneal neutrophils, papillary dermal edema, and dermal mixed inflammation with neutrophils and eosinophils. Both direct immunofluorescence and periodic acid–Schiff studies were negative. Blood and pustule cultures were sterile and the skin flora were normal. Based on these findings, a diagnosis of impetigo herpetiformis (IH) was made. The condition improved with systemic and topical steroids, supportive care, and an intravenous infusion of infliximab 5 mg/kg. At 3 weeks’ follow-up, the patient demonstrated near-complete resolution and later delivered successfully at 40 weeks’ gestation without complications.
Impetigo herpetiformis, also known as pustular psoriasis of pregnancy, is an exceedingly rare gestational dermatosis that typically manifests in the third trimester and can be life-threatening for both the mother and fetus. The term was first used in 1872 to describe 5 pregnant women with extensive acute pustular eruptions, all in unstable condition; 4 (80%)of the cases resulted in maternal death, and all resulted in fetal death.1 Impetigo herpetiformis is characterized by pruritic and painful erythematous patches studded at the periphery with subcorneal pustules. Eruptions usually occur in the flexural areas and spread centrifugally, with extension of the lesions peripherally as the center erodes and crusts. Sparing of the face, palms, and soles is expected, and mucosal involvement is rare. Generalized involvement and exfoliation may occur in extreme cases.2 While IH typically manifests during the third trimester, it may occur any time throughout pregnancy or immediately postpartum.3 A few cases have been reported in the puerperium.2 Common symptoms include fever, chills, malaise, anorexia, nausea, vomiting, diarrhea, and arthralgias. Less common complications include hypoalbuminemia and severe hypocalcemia leading to tetany, seizures, and delirium.2,3 While maternal mortality is uncommon, fetal mortality often is a more pressing risk and is attributed to placental insufficiency.3,4 For this reason, early delivery commonly is considered in severe cases.
Whether IH is a separate entity or a variant of pustular psoriasis remains heavily debated. Although the histopathology of IH is identical to pustular psoriasis, the lack of a personal and family history of psoriasis, symptom resolution with delivery, and possible recurrence during successive pregnancies help differentiate IH from generalized pustular psoriasis.2,5 Earlier onset, diffuse involvement, faster progression, and recurrence in subsequent pregnancies all have been linked to a worse prognosis.4
The differential diagnosis for IH includes acute generalized exanthematous pustulosis, pemphigoid gestationis, dermatitis herpetiformis, and subcorneal pustular dermatosis. Acute generalized exanthematous pustulosis is an uncommon severe cutaneous drug reaction characterized by the sudden onset of numerous sterile pustules on erythematous skin within 48 hours of exposure. The most common offending medications include pristinamycin and beta-lactam antibiotics. A high fever, neutrophilic leukocytosis, and hypocalcemia often accompany acute generalized exanthematous pustulosis.6 Prompt diagnosis and withdrawal of the offending drug as well as supportive care and symptomatic treatment are crucial for disease management, as systemic symptoms and even organ involvement may occur.6
Pemphigoid gestationis, also known as gestational pemphigoid or herpes gestationis, is a rare autoimmune blistering disorder that primarily affects pregnant women. It typically manifests in the second or third trimester or shortly after delivery. Clinically, it manifests as an intensely pruritic polymorphic eruption of urticarial papules and plaques accompanied by vesicles and bullae and often is distributed on the abdomen and extends to other body regions. Although the exact etiology is unknown, pemphigoid gestationis is caused by autoantibodies targeting the BP180 and BP230 hemidesmosomal proteins.7 Treatment usually involves systemic corticosteroids and may require additional immunosuppressive therapy. In most cases, patients see resolution within 6 months of delivery.7
Dermatitis herpetiformis is a chronic autoimmune blistering skin disorder characterized by intensely pruritic, grouped vesicles and papules, often distributed symmetrically on extensor surfaces such as the elbows, knees, buttocks, and back. It is closely associated with celiac disease and is triggered by gluten ingestion in genetically predisposed individuals with human leukocyte antigen DQ2 and DQ8 haplotypes. Dermatitis herpetiformis is caused by deposition of IgA antibodies that target tissue transglutaminase 3 at the dermal papillae, leading to inflammation and blister formation. 8 Treatment typically involves a gluten-free diet and medications such as dapsone to alleviate symptoms and prevent recurrence.
Subcorneal pustular dermatosis, also known as Sneddon-Wilkinson disease, is a rare chronic relapsing pustular dermatosis characterized by sterile superficial pustules arranged in annular or circinate patterns on erythematous plaques. It predominantly affects middleaged women and often is associated with underlying conditions such as IgA gammopathy or monoclonal gammopathy of undetermined significance. The pathogenesis remains unclear, but immune dysregulation is thought to play a role. Some authors still question whether subcorneal pustular dermatosis is a distinct entity from pustular psoriasis.4,5,12 Dapsone is the preferred first-line treatment, with adjunct therapies including topical or systemic corticosteroids, other immunosuppressive agents, tumor necrosis factor inhibitors, and UV light therapy.9
Definitive management of IH is achieved through early delivery; however, systemic corticosteroids often are used in varying doses to control the disease and to extend the pregnancy period closer to term or until delivery is considered viable. Additional therapies that can be considered include infliximab, cyclosporine, and topical corticosteroids, in conjunction with fluid and electrolyte maintenance.2,4,10 If symptoms persist despite supportive care and pharmacologic intervention, induction of labor or termination of pregnancy may be indicated. In nonbreastfeeding postpartum mothers with persistent disease, therapies commonly used in generalized pustular psoriasis may be given.11
THE DIAGNOSIS: Impetigo Herpetiformis
Histopathology revealed epidermal acanthosis and spongiosis with overlying parakeratosis associated with subcorneal and intracorneal neutrophils, papillary dermal edema, and dermal mixed inflammation with neutrophils and eosinophils. Both direct immunofluorescence and periodic acid–Schiff studies were negative. Blood and pustule cultures were sterile and the skin flora were normal. Based on these findings, a diagnosis of impetigo herpetiformis (IH) was made. The condition improved with systemic and topical steroids, supportive care, and an intravenous infusion of infliximab 5 mg/kg. At 3 weeks’ follow-up, the patient demonstrated near-complete resolution and later delivered successfully at 40 weeks’ gestation without complications.
Impetigo herpetiformis, also known as pustular psoriasis of pregnancy, is an exceedingly rare gestational dermatosis that typically manifests in the third trimester and can be life-threatening for both the mother and fetus. The term was first used in 1872 to describe 5 pregnant women with extensive acute pustular eruptions, all in unstable condition; 4 (80%)of the cases resulted in maternal death, and all resulted in fetal death.1 Impetigo herpetiformis is characterized by pruritic and painful erythematous patches studded at the periphery with subcorneal pustules. Eruptions usually occur in the flexural areas and spread centrifugally, with extension of the lesions peripherally as the center erodes and crusts. Sparing of the face, palms, and soles is expected, and mucosal involvement is rare. Generalized involvement and exfoliation may occur in extreme cases.2 While IH typically manifests during the third trimester, it may occur any time throughout pregnancy or immediately postpartum.3 A few cases have been reported in the puerperium.2 Common symptoms include fever, chills, malaise, anorexia, nausea, vomiting, diarrhea, and arthralgias. Less common complications include hypoalbuminemia and severe hypocalcemia leading to tetany, seizures, and delirium.2,3 While maternal mortality is uncommon, fetal mortality often is a more pressing risk and is attributed to placental insufficiency.3,4 For this reason, early delivery commonly is considered in severe cases.
Whether IH is a separate entity or a variant of pustular psoriasis remains heavily debated. Although the histopathology of IH is identical to pustular psoriasis, the lack of a personal and family history of psoriasis, symptom resolution with delivery, and possible recurrence during successive pregnancies help differentiate IH from generalized pustular psoriasis.2,5 Earlier onset, diffuse involvement, faster progression, and recurrence in subsequent pregnancies all have been linked to a worse prognosis.4
The differential diagnosis for IH includes acute generalized exanthematous pustulosis, pemphigoid gestationis, dermatitis herpetiformis, and subcorneal pustular dermatosis. Acute generalized exanthematous pustulosis is an uncommon severe cutaneous drug reaction characterized by the sudden onset of numerous sterile pustules on erythematous skin within 48 hours of exposure. The most common offending medications include pristinamycin and beta-lactam antibiotics. A high fever, neutrophilic leukocytosis, and hypocalcemia often accompany acute generalized exanthematous pustulosis.6 Prompt diagnosis and withdrawal of the offending drug as well as supportive care and symptomatic treatment are crucial for disease management, as systemic symptoms and even organ involvement may occur.6
Pemphigoid gestationis, also known as gestational pemphigoid or herpes gestationis, is a rare autoimmune blistering disorder that primarily affects pregnant women. It typically manifests in the second or third trimester or shortly after delivery. Clinically, it manifests as an intensely pruritic polymorphic eruption of urticarial papules and plaques accompanied by vesicles and bullae and often is distributed on the abdomen and extends to other body regions. Although the exact etiology is unknown, pemphigoid gestationis is caused by autoantibodies targeting the BP180 and BP230 hemidesmosomal proteins.7 Treatment usually involves systemic corticosteroids and may require additional immunosuppressive therapy. In most cases, patients see resolution within 6 months of delivery.7
Dermatitis herpetiformis is a chronic autoimmune blistering skin disorder characterized by intensely pruritic, grouped vesicles and papules, often distributed symmetrically on extensor surfaces such as the elbows, knees, buttocks, and back. It is closely associated with celiac disease and is triggered by gluten ingestion in genetically predisposed individuals with human leukocyte antigen DQ2 and DQ8 haplotypes. Dermatitis herpetiformis is caused by deposition of IgA antibodies that target tissue transglutaminase 3 at the dermal papillae, leading to inflammation and blister formation. 8 Treatment typically involves a gluten-free diet and medications such as dapsone to alleviate symptoms and prevent recurrence.
Subcorneal pustular dermatosis, also known as Sneddon-Wilkinson disease, is a rare chronic relapsing pustular dermatosis characterized by sterile superficial pustules arranged in annular or circinate patterns on erythematous plaques. It predominantly affects middleaged women and often is associated with underlying conditions such as IgA gammopathy or monoclonal gammopathy of undetermined significance. The pathogenesis remains unclear, but immune dysregulation is thought to play a role. Some authors still question whether subcorneal pustular dermatosis is a distinct entity from pustular psoriasis.4,5,12 Dapsone is the preferred first-line treatment, with adjunct therapies including topical or systemic corticosteroids, other immunosuppressive agents, tumor necrosis factor inhibitors, and UV light therapy.9
Definitive management of IH is achieved through early delivery; however, systemic corticosteroids often are used in varying doses to control the disease and to extend the pregnancy period closer to term or until delivery is considered viable. Additional therapies that can be considered include infliximab, cyclosporine, and topical corticosteroids, in conjunction with fluid and electrolyte maintenance.2,4,10 If symptoms persist despite supportive care and pharmacologic intervention, induction of labor or termination of pregnancy may be indicated. In nonbreastfeeding postpartum mothers with persistent disease, therapies commonly used in generalized pustular psoriasis may be given.11
- Hebra F. Ueber einzelne wahrend Schwangerschaft, des wacherbette unde bei uterinal. Krankheiten der Frauen zu beobachtende Hautkrankheiten. Wien Med Wochenschr. 1872;48:1197-1202.
- Fouda UM, Fouda RM, Ammar HM, et al. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J. 2009;2:9338. doi:10.1186/1757-1626-2-9338
- Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1-22. doi:10.1067/mjd.2001.114595
- Liu J, Ali K, Lou H, et al. First-trimester impetigo herpetiformis leads to stillbirth: a case report. Dermatol Ther (Heidelb). 2022;12:1271-1279. doi:10.1007/s13555-022-00735-9
- Lotem M, Katzenelson V, Rotem A, et al. Impetigo herpetiformis: a variant of pustular psoriasis or a separate entity? J Am Acad Dermatol. 1989;20:338-41. doi:10.1016/s0190-9622(89)70042-6
- Stadler PC, Oschmann A, Kerl-French K, et al. Acute generalized exanthematous pustulosis: clinical characteristics, pathogenesis, and management. Dermatology. 2023;239:328-333. doi:10.1159/000529218
- Abdelhafez MMA, Ahmed KAM, Daud MNBM, et al. Pemphigoid gestationis and adverse pregnancy outcomes: a literature review. J Gynecol Obstet Hum Reprod. 2022;51:102370. doi:10.1016 /j.jogoh.2022.102370
- Reunala T, Hervonen K, Salmi T. Dermatitis herpetiformis: an update on diagnosis and management. Am J Clin Dermatol. 2021;22:329-338. doi:10.1007/s40257-020-00584-2
- Watts PJ, Khachemoune A. Subcorneal pustular dermatosis: a review of 30 years of progress. Am J Clin Dermatol. 2016;17:653-671. doi:10.1007 /s40257-016-0202-8
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/j.jaad.2011.01.032
- Bukhari IA. Impetigo herpetiformis in a primigravida: successful treatment with etanercept. J Drugs Dermatol. 2004;3:449-451.
- Chang SE, Kim HH, Choi JH, et al. Impetigo herpetiformis followed by generalized pustular psoriasis: more evidence of same disease entity. Int J Dermatol. 2003;42(9):754-755.
- Hebra F. Ueber einzelne wahrend Schwangerschaft, des wacherbette unde bei uterinal. Krankheiten der Frauen zu beobachtende Hautkrankheiten. Wien Med Wochenschr. 1872;48:1197-1202.
- Fouda UM, Fouda RM, Ammar HM, et al. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J. 2009;2:9338. doi:10.1186/1757-1626-2-9338
- Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1-22. doi:10.1067/mjd.2001.114595
- Liu J, Ali K, Lou H, et al. First-trimester impetigo herpetiformis leads to stillbirth: a case report. Dermatol Ther (Heidelb). 2022;12:1271-1279. doi:10.1007/s13555-022-00735-9
- Lotem M, Katzenelson V, Rotem A, et al. Impetigo herpetiformis: a variant of pustular psoriasis or a separate entity? J Am Acad Dermatol. 1989;20:338-41. doi:10.1016/s0190-9622(89)70042-6
- Stadler PC, Oschmann A, Kerl-French K, et al. Acute generalized exanthematous pustulosis: clinical characteristics, pathogenesis, and management. Dermatology. 2023;239:328-333. doi:10.1159/000529218
- Abdelhafez MMA, Ahmed KAM, Daud MNBM, et al. Pemphigoid gestationis and adverse pregnancy outcomes: a literature review. J Gynecol Obstet Hum Reprod. 2022;51:102370. doi:10.1016 /j.jogoh.2022.102370
- Reunala T, Hervonen K, Salmi T. Dermatitis herpetiformis: an update on diagnosis and management. Am J Clin Dermatol. 2021;22:329-338. doi:10.1007/s40257-020-00584-2
- Watts PJ, Khachemoune A. Subcorneal pustular dermatosis: a review of 30 years of progress. Am J Clin Dermatol. 2016;17:653-671. doi:10.1007 /s40257-016-0202-8
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/j.jaad.2011.01.032
- Bukhari IA. Impetigo herpetiformis in a primigravida: successful treatment with etanercept. J Drugs Dermatol. 2004;3:449-451.
- Chang SE, Kim HH, Choi JH, et al. Impetigo herpetiformis followed by generalized pustular psoriasis: more evidence of same disease entity. Int J Dermatol. 2003;42(9):754-755.
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
A 17-year-old girl was admitted to the hospital at 19 weeks' gestation for a widespread eruption of erythematous plaques with pustules covering more than 60% of the body and signs of sepsis. The rash initially appeared as a few small spots on the upper chest and under the breasts 5 weeks prior to hospital admission with subsequent spread to the abdomen and groin. At admission, the patient had a mild fever and tachycardia. She reported a history of eczema, herpes simplex virus, and intertrigo. Physical examination performed by dermatology revealed generalized erythematous plaques with pustule-studded margins and overlying scale involving the neck, torso, arms, and legs favoring the flexural areas. There was no involvement of the face, eyes, oral mucosa, palms, soles, or nails. Laboratory testing revealed hypoalbuminemia (2.4 g/dL [reference range, 3.5-5.5 g/dL]) and elevated inflammatory markers, including leukocytosis (15.83×103μL [reference range, 4.50- 11.00×103/μL]), absolute neutrophil count (12.87×103/μL [reference range, 1.50-8.00×103/μL]), and erythrocyte sedimentation rate (124 mm/h [reference range, 0-20 mm/h]). A culture from an abdominal pustule grew 1 colony of taphylococcus epidermidis, a suspected contaminant. A biopsy from a lesion on the right chest was performed.
Painless Nodule on the Lower Eyelid
Painless Nodule on the Lower Eyelid
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).
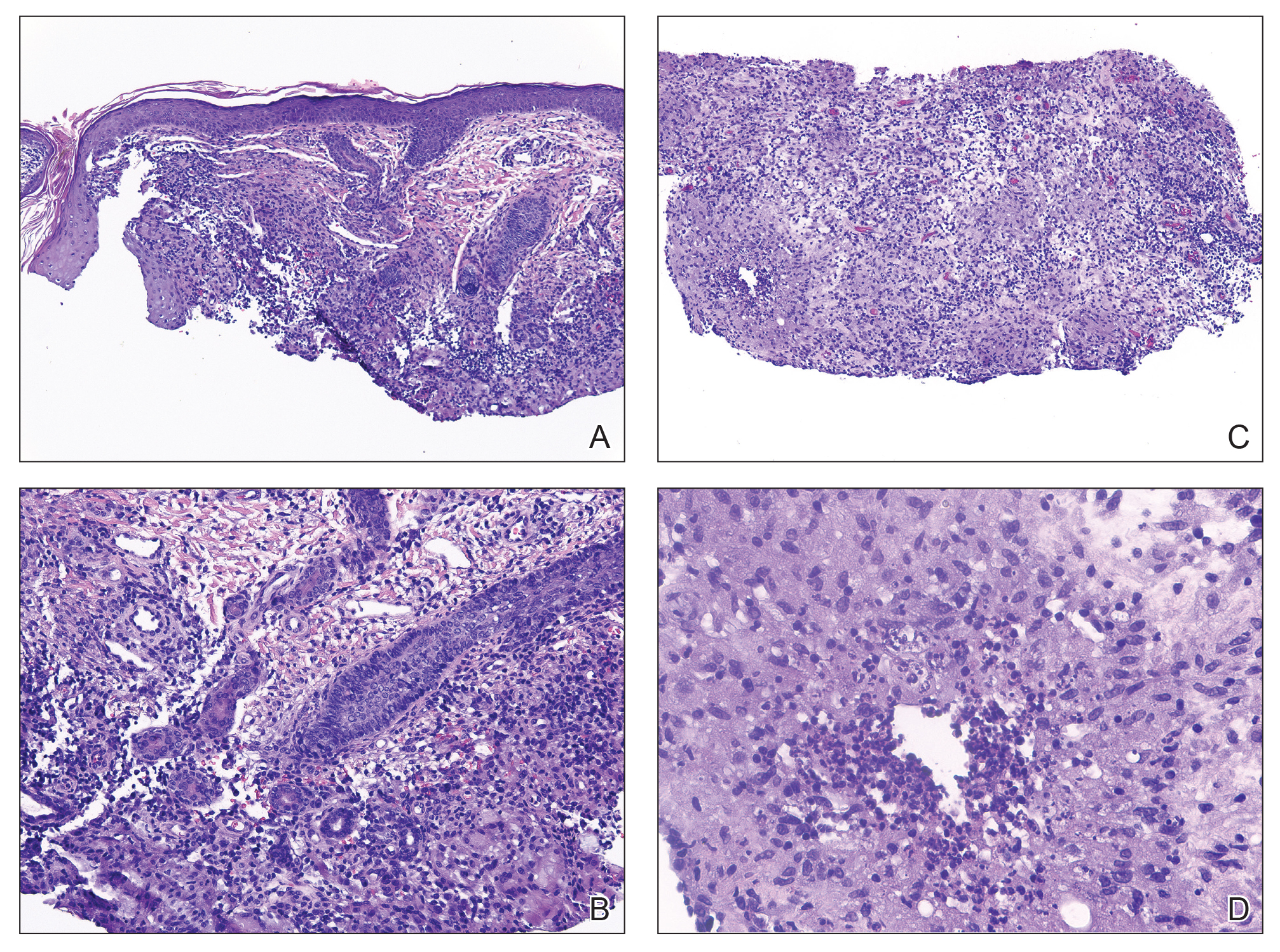
First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).

First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).

First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
Painless Nodule on the Lower Eyelid
Painless Nodule on the Lower Eyelid
A 4-year-old girl presented to the dermatology clinic with a painless, red to golden-yellowish nodule on the right lower eyelid of 4 months’ duration. The patient had no history of skin disease and was otherwise healthy. Physical examination revealed a single 1-cm, soft, erythematous and yellowish plaque on the right lower eyelid that was subtly fluctuant on palpation. She had no associated systemic symptoms or lymphadenopathy. A punch biopsy of the lesion was performed.
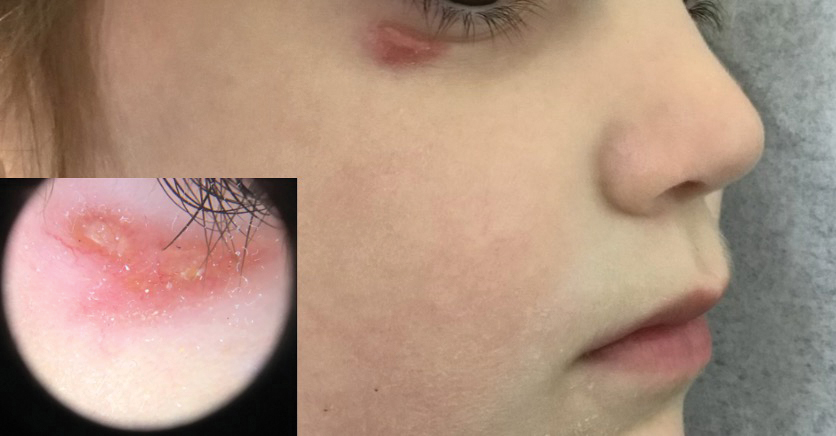
Reddish Nodule on the Left Shoulder
Reddish Nodule on the Left Shoulder
THE DIAGNOSIS: Atypical Fibroxanthoma
Given the appearance of the nodule and the absence of features of a keloid scar, a soft-tissue or adnexal tumor was suspected. Histology revealed a thin epidermis with loss of rete ridges and a Grenz zone. There was a nodular uncircumscribed dermal proliferation of spindle cells forming interweaving fascicles with elongated ovoid nuclei and prominent nucleoli (Figure). There was moderate cellular and nuclear atypia, and no necrosis was observed. The spindle cells stained positive for CD10 and negative for AE1/AE3, cytokeratin 5/6, S100, melanoma triple marker, Factor XIII 1, ERG, CD31, CD34, desmin, and smooth muscle actin; ERG, CD31, CD34, and SMA highlighted small vessels within the tumor. The histologic diagnosis was an atypical spindle cell tumor favoring atypical fibroxanthoma (AFX). The excisional biopsy margins were clear.
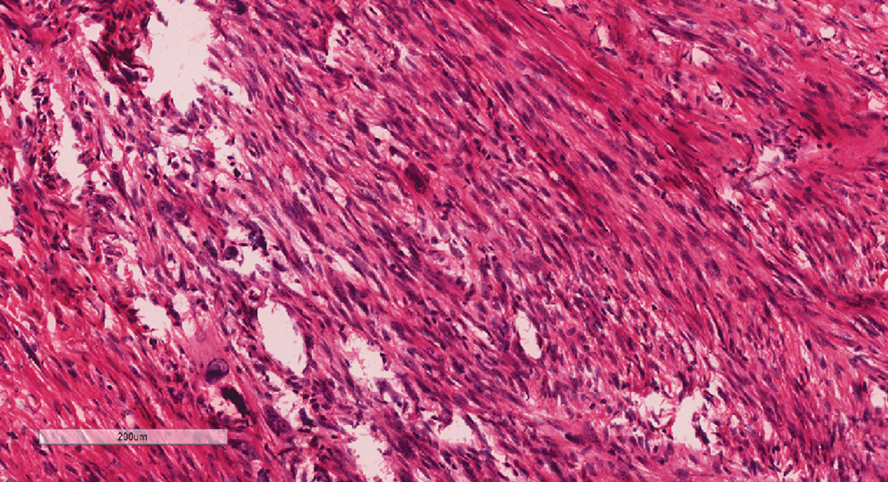
The patient was referred to surgical oncology to consider re-excision of margins after the diagnosis was made. A chest radiograph was clear, and magnetic resonance imaging showed mild skin thickening and image enhancement at the left shoulder—possibly a postsurgical change—with no nodularity suggesting a residual or recurrent tumor. Surgical oncology determined that the patient did not require further excision and placed him on regular follow-up every 2 to 3 months for the next 2 years.
uncertain origin that is considered to be on a spectrum with the more aggressive pleomorphic dermal sarcoma (PDS); it can be distinguished from PDS by histologic features such as nerve or vessel invasion.1 Both entities share oncogenes (eg, tumor protein 53 gene mutations) and are histologically and immunohistochemically similar. Atypical fibroxanthoma largely is viewed as an intermediate-risk tumor that is locally aggressive but rarely metastasizes, with a reported local recurrence rate of 5% to 11% and metastasis risk of 1% to 2%. Conversely, PDS is a more aggressive diagnosis with a high risk for local recurrence and metastasis (7%-69% and 4%-20%, respectively).1
Atypical fibroxanthomas may mimic other entities, both clinically and histologically. It commonly manifests as a flesh-colored to erythematous, sometimes ulcerated nodule on sun-exposed skin in elderly patients, leading to a broad range of clinical differential diagnoses, including other primary cutaneous malignancies (eg, squamous cell carcinoma, amelanotic melanoma), cutaneous sarcomas (eg, dermatofibrosarcoma protuberans), adnexal and other tumors (eg, pleomorphic fibroma, pilomatricoma), cutaneous metastases, and even keloid scars. As the differentials can look clinically similar, a skin biopsy may be necessary to confirm the diagnosis.
Histologically, AFX tends to show an undifferentiated pleomorphic spindle cell morphology. Notably, histology can be highly variable, with other reported histologic patterns including keloidlike, pleomorphic, epithelioid, rhabdoid, clear-cell, foamy cell, granular cell, bizarre cell, pseudoangiomatous, inflammatory, and osteoclast-rich patterns.2 Thus, the histologic differential diagnosis also is broad, and AFX primarily is a diagnosis of exclusion without specific immunohistochemical markers that serve to exclude other diagnoses. For example, AFX tends to stain positive for CD10 and CD68, though these are not specific markers for AFX. Furthermore, although certain histologic markers may commonly be more positive in AFX than PDS (eg, CD74 stains positive in 20% of AFXs and only 1% of PDSs), this is not reliable enough to be diagnostic.3 As such, AFX is distinguished from PDS primarily by histologic features such as subcutaneous tissue invasion, vascular or perineural invasion, necrosis, or local invasion/ metastases.1 Given the rarity of both tumors, no established management guidelines exist, although excision (wide local excision or Mohs micrographic surgery) usually is recommended, with some authors suggesting margins of 1 cm for AFX and 2 cm to 3 cm for PDS.1
This atypical case of AFX arising in non–sun-exposed skin in a young man raises questions about whether unknown genetic factors or possibly prior immunosuppression could have contributed to the development of the tumor. A thorough history and physical examination can provide valuable clues for biopsy, including ongoing growth, absence of known prior trauma or acne at the site, and clinical appearance, such as the reddish, solitary, dome-shaped lesion in our patient.
- Ørholt M, Abebe K, Rasmussen LE, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: local recurrence and metastasis in a nationwide population-based cohort of 1118 patients. J Am Acad Dermatol. 2023;89:1177-1184. doi:10.1016/j.jaad.2023.08.050
- Agaimy A. The many faces of atypical fibroxanthoma. Semin Diagn Pathol. 2023;40:306-312. doi:10.1053/j.semdp.2023.06.001
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier Health Sciences; 2021.
THE DIAGNOSIS: Atypical Fibroxanthoma
Given the appearance of the nodule and the absence of features of a keloid scar, a soft-tissue or adnexal tumor was suspected. Histology revealed a thin epidermis with loss of rete ridges and a Grenz zone. There was a nodular uncircumscribed dermal proliferation of spindle cells forming interweaving fascicles with elongated ovoid nuclei and prominent nucleoli (Figure). There was moderate cellular and nuclear atypia, and no necrosis was observed. The spindle cells stained positive for CD10 and negative for AE1/AE3, cytokeratin 5/6, S100, melanoma triple marker, Factor XIII 1, ERG, CD31, CD34, desmin, and smooth muscle actin; ERG, CD31, CD34, and SMA highlighted small vessels within the tumor. The histologic diagnosis was an atypical spindle cell tumor favoring atypical fibroxanthoma (AFX). The excisional biopsy margins were clear.

The patient was referred to surgical oncology to consider re-excision of margins after the diagnosis was made. A chest radiograph was clear, and magnetic resonance imaging showed mild skin thickening and image enhancement at the left shoulder—possibly a postsurgical change—with no nodularity suggesting a residual or recurrent tumor. Surgical oncology determined that the patient did not require further excision and placed him on regular follow-up every 2 to 3 months for the next 2 years.
uncertain origin that is considered to be on a spectrum with the more aggressive pleomorphic dermal sarcoma (PDS); it can be distinguished from PDS by histologic features such as nerve or vessel invasion.1 Both entities share oncogenes (eg, tumor protein 53 gene mutations) and are histologically and immunohistochemically similar. Atypical fibroxanthoma largely is viewed as an intermediate-risk tumor that is locally aggressive but rarely metastasizes, with a reported local recurrence rate of 5% to 11% and metastasis risk of 1% to 2%. Conversely, PDS is a more aggressive diagnosis with a high risk for local recurrence and metastasis (7%-69% and 4%-20%, respectively).1
Atypical fibroxanthomas may mimic other entities, both clinically and histologically. It commonly manifests as a flesh-colored to erythematous, sometimes ulcerated nodule on sun-exposed skin in elderly patients, leading to a broad range of clinical differential diagnoses, including other primary cutaneous malignancies (eg, squamous cell carcinoma, amelanotic melanoma), cutaneous sarcomas (eg, dermatofibrosarcoma protuberans), adnexal and other tumors (eg, pleomorphic fibroma, pilomatricoma), cutaneous metastases, and even keloid scars. As the differentials can look clinically similar, a skin biopsy may be necessary to confirm the diagnosis.
Histologically, AFX tends to show an undifferentiated pleomorphic spindle cell morphology. Notably, histology can be highly variable, with other reported histologic patterns including keloidlike, pleomorphic, epithelioid, rhabdoid, clear-cell, foamy cell, granular cell, bizarre cell, pseudoangiomatous, inflammatory, and osteoclast-rich patterns.2 Thus, the histologic differential diagnosis also is broad, and AFX primarily is a diagnosis of exclusion without specific immunohistochemical markers that serve to exclude other diagnoses. For example, AFX tends to stain positive for CD10 and CD68, though these are not specific markers for AFX. Furthermore, although certain histologic markers may commonly be more positive in AFX than PDS (eg, CD74 stains positive in 20% of AFXs and only 1% of PDSs), this is not reliable enough to be diagnostic.3 As such, AFX is distinguished from PDS primarily by histologic features such as subcutaneous tissue invasion, vascular or perineural invasion, necrosis, or local invasion/ metastases.1 Given the rarity of both tumors, no established management guidelines exist, although excision (wide local excision or Mohs micrographic surgery) usually is recommended, with some authors suggesting margins of 1 cm for AFX and 2 cm to 3 cm for PDS.1
This atypical case of AFX arising in non–sun-exposed skin in a young man raises questions about whether unknown genetic factors or possibly prior immunosuppression could have contributed to the development of the tumor. A thorough history and physical examination can provide valuable clues for biopsy, including ongoing growth, absence of known prior trauma or acne at the site, and clinical appearance, such as the reddish, solitary, dome-shaped lesion in our patient.
THE DIAGNOSIS: Atypical Fibroxanthoma
Given the appearance of the nodule and the absence of features of a keloid scar, a soft-tissue or adnexal tumor was suspected. Histology revealed a thin epidermis with loss of rete ridges and a Grenz zone. There was a nodular uncircumscribed dermal proliferation of spindle cells forming interweaving fascicles with elongated ovoid nuclei and prominent nucleoli (Figure). There was moderate cellular and nuclear atypia, and no necrosis was observed. The spindle cells stained positive for CD10 and negative for AE1/AE3, cytokeratin 5/6, S100, melanoma triple marker, Factor XIII 1, ERG, CD31, CD34, desmin, and smooth muscle actin; ERG, CD31, CD34, and SMA highlighted small vessels within the tumor. The histologic diagnosis was an atypical spindle cell tumor favoring atypical fibroxanthoma (AFX). The excisional biopsy margins were clear.

The patient was referred to surgical oncology to consider re-excision of margins after the diagnosis was made. A chest radiograph was clear, and magnetic resonance imaging showed mild skin thickening and image enhancement at the left shoulder—possibly a postsurgical change—with no nodularity suggesting a residual or recurrent tumor. Surgical oncology determined that the patient did not require further excision and placed him on regular follow-up every 2 to 3 months for the next 2 years.
uncertain origin that is considered to be on a spectrum with the more aggressive pleomorphic dermal sarcoma (PDS); it can be distinguished from PDS by histologic features such as nerve or vessel invasion.1 Both entities share oncogenes (eg, tumor protein 53 gene mutations) and are histologically and immunohistochemically similar. Atypical fibroxanthoma largely is viewed as an intermediate-risk tumor that is locally aggressive but rarely metastasizes, with a reported local recurrence rate of 5% to 11% and metastasis risk of 1% to 2%. Conversely, PDS is a more aggressive diagnosis with a high risk for local recurrence and metastasis (7%-69% and 4%-20%, respectively).1
Atypical fibroxanthomas may mimic other entities, both clinically and histologically. It commonly manifests as a flesh-colored to erythematous, sometimes ulcerated nodule on sun-exposed skin in elderly patients, leading to a broad range of clinical differential diagnoses, including other primary cutaneous malignancies (eg, squamous cell carcinoma, amelanotic melanoma), cutaneous sarcomas (eg, dermatofibrosarcoma protuberans), adnexal and other tumors (eg, pleomorphic fibroma, pilomatricoma), cutaneous metastases, and even keloid scars. As the differentials can look clinically similar, a skin biopsy may be necessary to confirm the diagnosis.
Histologically, AFX tends to show an undifferentiated pleomorphic spindle cell morphology. Notably, histology can be highly variable, with other reported histologic patterns including keloidlike, pleomorphic, epithelioid, rhabdoid, clear-cell, foamy cell, granular cell, bizarre cell, pseudoangiomatous, inflammatory, and osteoclast-rich patterns.2 Thus, the histologic differential diagnosis also is broad, and AFX primarily is a diagnosis of exclusion without specific immunohistochemical markers that serve to exclude other diagnoses. For example, AFX tends to stain positive for CD10 and CD68, though these are not specific markers for AFX. Furthermore, although certain histologic markers may commonly be more positive in AFX than PDS (eg, CD74 stains positive in 20% of AFXs and only 1% of PDSs), this is not reliable enough to be diagnostic.3 As such, AFX is distinguished from PDS primarily by histologic features such as subcutaneous tissue invasion, vascular or perineural invasion, necrosis, or local invasion/ metastases.1 Given the rarity of both tumors, no established management guidelines exist, although excision (wide local excision or Mohs micrographic surgery) usually is recommended, with some authors suggesting margins of 1 cm for AFX and 2 cm to 3 cm for PDS.1
This atypical case of AFX arising in non–sun-exposed skin in a young man raises questions about whether unknown genetic factors or possibly prior immunosuppression could have contributed to the development of the tumor. A thorough history and physical examination can provide valuable clues for biopsy, including ongoing growth, absence of known prior trauma or acne at the site, and clinical appearance, such as the reddish, solitary, dome-shaped lesion in our patient.
- Ørholt M, Abebe K, Rasmussen LE, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: local recurrence and metastasis in a nationwide population-based cohort of 1118 patients. J Am Acad Dermatol. 2023;89:1177-1184. doi:10.1016/j.jaad.2023.08.050
- Agaimy A. The many faces of atypical fibroxanthoma. Semin Diagn Pathol. 2023;40:306-312. doi:10.1053/j.semdp.2023.06.001
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier Health Sciences; 2021.
- Ørholt M, Abebe K, Rasmussen LE, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: local recurrence and metastasis in a nationwide population-based cohort of 1118 patients. J Am Acad Dermatol. 2023;89:1177-1184. doi:10.1016/j.jaad.2023.08.050
- Agaimy A. The many faces of atypical fibroxanthoma. Semin Diagn Pathol. 2023;40:306-312. doi:10.1053/j.semdp.2023.06.001
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier Health Sciences; 2021.
Reddish Nodule on the Left Shoulder
Reddish Nodule on the Left Shoulder
A 20-year-old man presented to the dermatology clinic for evaluation of a slow-growing nodule on the left shoulder of 1 year’s duration. The patient reported a history of eczema since childhood, which had been treated by an external physician with cyclosporine and methotrexate; however, exact treatment records were unavailable as the patient had been treated at another institution. The eczema had been well controlled over the past year on topical steroids alone. The nodule was asymptomatic, and the patient denied any history of trauma or acne at the affected site. He also denied any family history of similar nodules or other notable skin findings. Physical examination revealed a well circumscribed, 15×12-mm, firm, flesh-colored to reddish nodule on the left shoulder with a slightly whitish center. An excisional biopsy was performed.
Pedunculated Pink Papule on the Nose
THE DIAGNOSIS: Pedunculated Lipofibroma
Histopathology confirmed a pedunculated/polypoid lesion with intradermal lobules of adipocytes/mature adipose tissue admixed with connective tissue bundles and vascular ectasias. Overlying epidermal acanthosis with slight papillomatosis and hyperkeratosis was present (Figure 1). Masson trichrome staining highlighted admixed collagen bundles (Figure 2). Verhoeff–van Gieson staining showed marked reduction in elastic fibers (Figure 3). Immunostaining was negative for smooth muscle actin and desmin. A diagnosis of pedunculated lipofibroma on the nose was made based on both clinical and histopathologic findings.
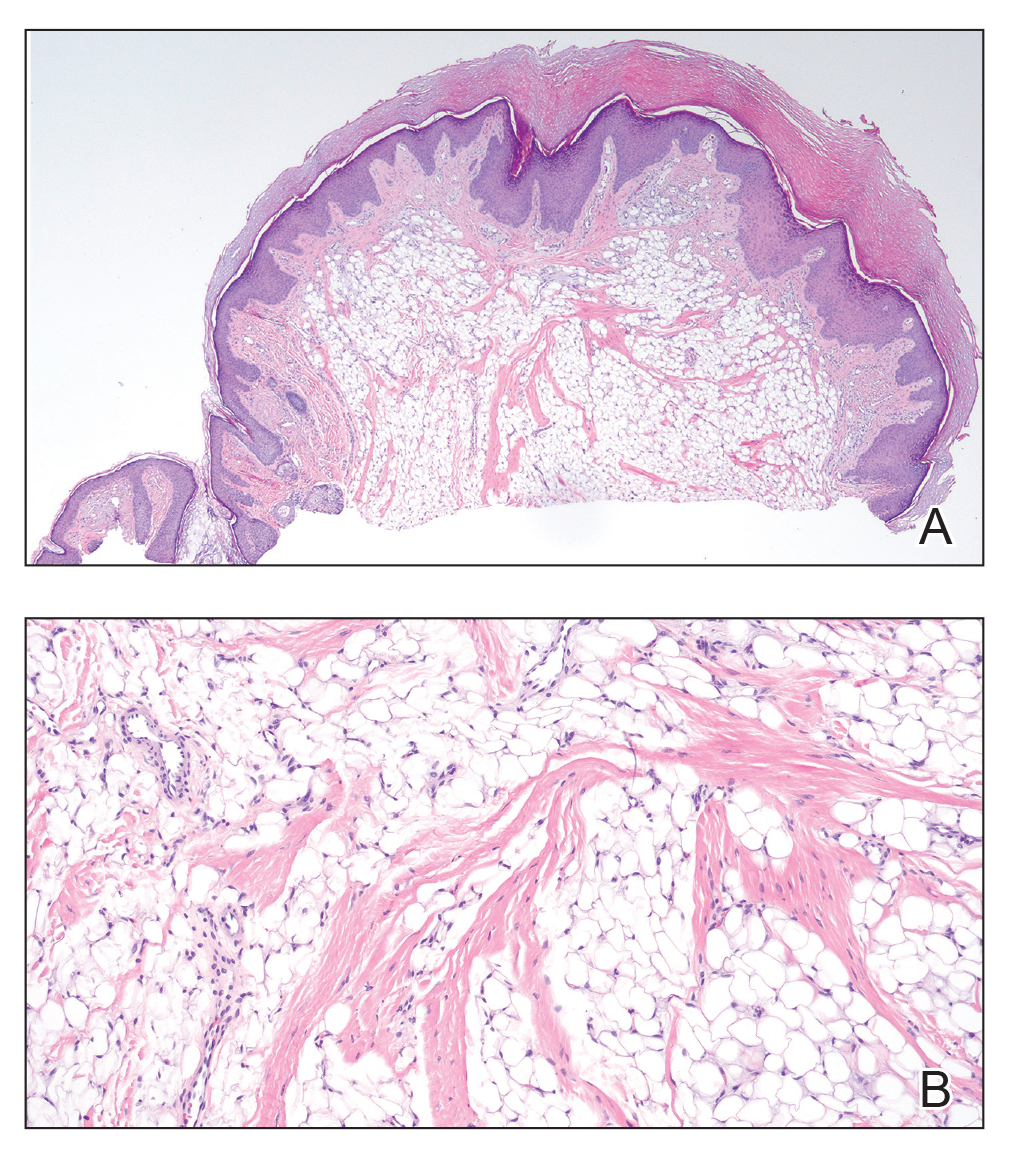
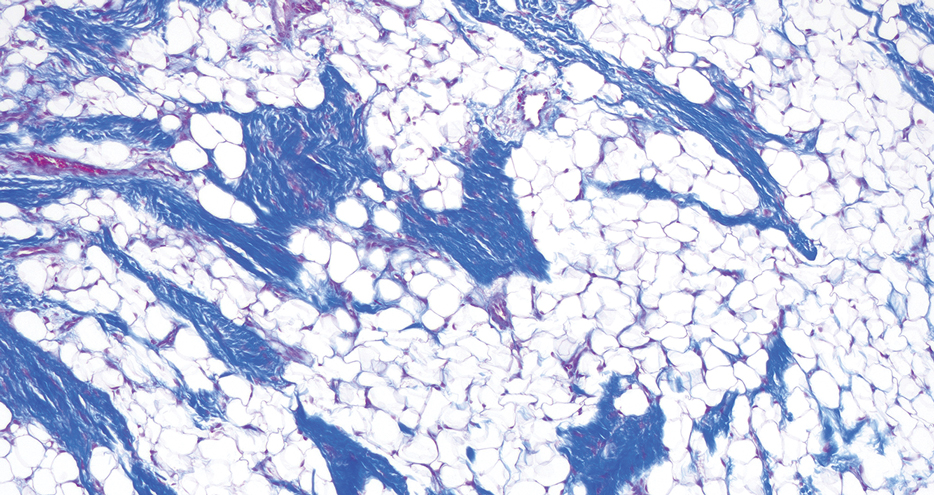
Pedunculated lipofibroma (or solitary lipofibroma) is the solitary form of nevus lipomatosus cutaneous superficialis (NLCS).7 First described by Hoffmann and Zurhelle1 in 1921, NLCS is an uncommon benign hamartomatous cutaneous lesion/connective tissue nevus that also has a classic multiple form.1-13 The etiology of NLCS remains unclear, but several theories have been proposed to explain its pathogenesis, including deposition of adipocytes secondary to degenerative changes in dermal connective tissue, focal/local heterotopic development of adipose tissue, and derivation from differentiating lipoblasts (preadipose tissue) originating from precursor vascular or perivascular cells.2-13
Pedunculated lipofibroma usually develops during the third to sixth decades of life and manifests as a single cutaneous lesion with a smooth surface, often on a non–pelvic girdle location.7-13 No particular predilection sites are noted, with lesions reported on the arm, axilla, back, upper thigh, knee, and sole.5,12 There are rare reports of this type of NLCS on the ear, scalp, forehead, or eyelid.7-11
In the classic form of NLCS, multiple cutaneous lesions are present at birth or develop within the first 2 to 3 decades of life.2-6 Lesions consist of soft, nontender, pedunculated, flesh-colored or yellowish papules and nodules with a verrucoid or cerebriform surface that may later coalesce to form plaques.2-6 Predilection sites include the pelvic girdle, buttocks, sacral and coccygeal regions, and upper posterior thighs, with a linear or zosteriform pattern of distribution.2-6 Rarely, the classic form can arise in elderly patients and/or at an atypical anatomic location (eg, clitoris,3 shoulder,5 thorax,5 abdomen5) and can demonstrate extension of lesions across the midline.4 Rare cases of classic NLCS on the scalp2 and face3-6 have been reported, including lesions localized to the nose3 and chin4 and others extending from the right mandible to the neck5 and right lower lip to the submandibular/posteriorateral cervical region.6 In some cases, lesions clinically resemble plane xanthoma4 and localized scleroderma.6
Adotama et al13 proposed a set of clinical features to differentiate classic NLCS, pedunculated lipofibroma (solitary NLCS), and fibroepithelial polyp with adipocytes (distinguished by their furrowed surface, hyperpigmentation, and anatomic predilection for the neck and axilla). Lesions are asymptomatic in both forms of NLCS.2-13 Family history or predominant sex involvement have not been reported in either clinical type.2-13 Reported associations with NLCS include a number of endocrinologic conditions including diabetes.7 Other coexisting skin findings can include café-au-lait macules, leukodermic (white) spots, overlying hypertrichosis, comedolike alterations, angiokeratoma, hemangioma, and folliculosebaceous cystic hamartoma.4 None of these were evident in our patient.
Lesions from both types of NLCS are indistinguishable on histopathology, characterized by the presence of a central core of ectopic mature adipocytes in the papillary/reticular dermis.2-13 Additional light microscopic features (some seen in our case) have been described, including thickened collagen bundles, reduction of elastic fibers, increased numbers of fibroblasts and/or mast cells, increased (small-vessel) vascularity, focal mucin deposition/myxoid degeneration, a mild perivascular lymphocytic infiltrate, attenuation of adnexal structures, and abnormalities of the epidermis (eg, surface ulceration).2-13
Prior to biopsy, the differential diagnosis in our patient included angiofibroma, pyogenic granuloma, and basal cell carcinoma given the exophytic, pink, papular appearance of the lesion; however, the histopathologic differential diagnosis included angiofibroma, angiomyolipoma, lymphangioma, nevus sebaceus, and spindle cell lipoma (SCL). In angiofibroma, a dermal proliferation of stellate fibroblasts, dilated blood vessels, and collagenous stroma are seen. Cutaneous angiomyolipoma demonstrates smooth muscle bundles in addition to thickened blood vessels and variable proportions of mature adipocytes. Lymphangioma is characterized by dilated lymph channels lined by flat endothelial cells. Nevus sebaceus shows superficial immature and abnormally formed pilosebaceous units, with epidermal papillomatosis.
Rare cases of SCL on the nose have been described.14 Similar to pedunculated lipofibroma, reported examples demonstrate mature univacuolar adipocytes with thick collagen fibers and bland uniform spindle cells. Unlike the lesion seen in our patient, nasal SCL may be clinically mobile and typically is localized to the subcutaneous tissue, although dermal tumors also occur.14 Variably reported histopathologic findings in nasal SCL include circumscription/encapsulation, spindle cells arranged in short fascicles with nuclear palisading, a myxoid/mucinous interstitial matrix, and/or multinucleated giant cells—all light microscopic features that were not identified in our case; however, variable proportions of adipocytic, fibrous, and myxoid components among reported examples of SCL on the nose14 can make distinction from pedunculated lipofibroma difficult, as both are benign lipomatous tumor variants.
Clinically, pedunculated lipofibroma may be confused with more common benign cutaneous lesions and must be distinguished from other fibrolipomatous lesions on the nose. Specifically, the differential diagnosis includes benign cutaneous papillomas such as acrochordon, angiofibroma, melanocytic nevi, neurofibroma, nevus sebaceus, lymphangioma, and eccrine poroma.7-13 These all can be readily excluded on histopathology. Pedunculated lipofibroma on the nose, as in our patient, must be distinguished from fibrolipoma15 and dendritic myxofibrolipoma.16 Fibrolipoma is a subcutaneous proliferation of mature adipose tissue and fibrous tissue and comprises 1.6% of all facial lipomas reported worldwide.15 Dendritic myxofibrolipoma is a recently described benign soft-tissue tumor characterized by an admixture of mature adipose tissue, spindle and stellate cells, and an abundant myxoid stroma with prominent collagenization.16
Treatment of pedunculated lipofibroma on the nose is not indicated except for cosmetic reasons, in which case simple surgical excision would be considered satisfactory. Following biopsy, no further treatment was pursued in our patient.
- Hoffmann E, Zurhelle E. Uber einen naevus lipomatodes cutaneous superficialis der linken Glutaalgegend. Arch Derm Syph. 1921;130:327-333.
- Chanoki M, Isukos S, Suzuki S, et al. Nevus lipomatosus cutaneus superficialis of the scalp. Cutis. 1989;43:143-144.
- Sáez Rodríguez M, Rodríguez-Martin M, Carnerero A, et al. Naevus lipomatosus cutaneous superficialis on the nose. J Eur Acad Dermatol Venereol. 2005;19:751-752.
- Hassab-El-Naby HMM, Rageh MA. Adult-onset nevus lipomatosus cutaneous superficialis mimicking plane xanthoma. J Clin Aesthet Dermatol. 2022;15:10-11.
- Park HJ, Park CJ, Yi JY, et al. Nevus lipomatosus superficialis on the face. Int J Dermatol. 1997;36:435-437.
- Ioannidou DJ, Stefanidou MP, Panayiotides JG, et al. Nevus lipomatosus cutaneous superficialis (Hoffman-Zurhelle) with localized scleroderma like appearance. Int J Dermatol. 2001;40:54-57.
- Nogita T, Wong TY, Hidano A, et al. Pedunculated lipofibroma. a clinicopathologic study of thirty-two cases supporting a simplified nomenclature. J Am Acad Dermatol. 1994;31(2 pt 1):235-240.
- Sawada Y. Solitary nevus lipomatosus superficialis on the forehead. Ann Plast Surg. 1986;16:356-358.
- Knoth W. Uber Naevus lipomatosus cutaneus superficialis Hoffmann-Zurhelle und uber Naevus naevocellularis partim lipomatodes. Dermatologica. 1962;125:161.
- Weitzner S. Solitary naevus lipomatosus cutaneus superficialis of scalp. Arch Dermatol. 1968;97:540-542.
- Kaw P, Carlson A, Meyer DR. Nevus lipomatosus (pedunculated lipofibroma) of the eyelid. Ophthalmic Plast Reconstr Surg. 2005;21:74-76.
- Vano-Galvan S, Moreno C, Vano-Galvan E, et al. Solitary naevus lipomatosus cutaneous superficialis on the sole. Eur J Dermatol. 2008;18:353-354.
- Adotama P, Hutson SD, Rieder EA, et al. Revisiting solitary pedunculated lipofibromas. Am J Clin Pathol. 2021;156:954-957.
- Kubin ME, Lantto U, Lindgren O, et al. A rare, recurrent spindle cell lipoma of the nose. Acta Derm Venereol. 2021;101:adv00571.
- Jung SN, Shin JW, Kwon H, et al. Fibrolipoma of the tip of the nose. J Craniofac Surg. 2009;20:555-556.
- Han XC, Zheng LQ, Shang XL. Dendritic fibromyxolipoma on the nasal tip in an old patient. Int J Clin Exp Pathol. 2014;7:7064-7067.
THE DIAGNOSIS: Pedunculated Lipofibroma
Histopathology confirmed a pedunculated/polypoid lesion with intradermal lobules of adipocytes/mature adipose tissue admixed with connective tissue bundles and vascular ectasias. Overlying epidermal acanthosis with slight papillomatosis and hyperkeratosis was present (Figure 1). Masson trichrome staining highlighted admixed collagen bundles (Figure 2). Verhoeff–van Gieson staining showed marked reduction in elastic fibers (Figure 3). Immunostaining was negative for smooth muscle actin and desmin. A diagnosis of pedunculated lipofibroma on the nose was made based on both clinical and histopathologic findings.



Pedunculated lipofibroma (or solitary lipofibroma) is the solitary form of nevus lipomatosus cutaneous superficialis (NLCS).7 First described by Hoffmann and Zurhelle1 in 1921, NLCS is an uncommon benign hamartomatous cutaneous lesion/connective tissue nevus that also has a classic multiple form.1-13 The etiology of NLCS remains unclear, but several theories have been proposed to explain its pathogenesis, including deposition of adipocytes secondary to degenerative changes in dermal connective tissue, focal/local heterotopic development of adipose tissue, and derivation from differentiating lipoblasts (preadipose tissue) originating from precursor vascular or perivascular cells.2-13
Pedunculated lipofibroma usually develops during the third to sixth decades of life and manifests as a single cutaneous lesion with a smooth surface, often on a non–pelvic girdle location.7-13 No particular predilection sites are noted, with lesions reported on the arm, axilla, back, upper thigh, knee, and sole.5,12 There are rare reports of this type of NLCS on the ear, scalp, forehead, or eyelid.7-11
In the classic form of NLCS, multiple cutaneous lesions are present at birth or develop within the first 2 to 3 decades of life.2-6 Lesions consist of soft, nontender, pedunculated, flesh-colored or yellowish papules and nodules with a verrucoid or cerebriform surface that may later coalesce to form plaques.2-6 Predilection sites include the pelvic girdle, buttocks, sacral and coccygeal regions, and upper posterior thighs, with a linear or zosteriform pattern of distribution.2-6 Rarely, the classic form can arise in elderly patients and/or at an atypical anatomic location (eg, clitoris,3 shoulder,5 thorax,5 abdomen5) and can demonstrate extension of lesions across the midline.4 Rare cases of classic NLCS on the scalp2 and face3-6 have been reported, including lesions localized to the nose3 and chin4 and others extending from the right mandible to the neck5 and right lower lip to the submandibular/posteriorateral cervical region.6 In some cases, lesions clinically resemble plane xanthoma4 and localized scleroderma.6
Adotama et al13 proposed a set of clinical features to differentiate classic NLCS, pedunculated lipofibroma (solitary NLCS), and fibroepithelial polyp with adipocytes (distinguished by their furrowed surface, hyperpigmentation, and anatomic predilection for the neck and axilla). Lesions are asymptomatic in both forms of NLCS.2-13 Family history or predominant sex involvement have not been reported in either clinical type.2-13 Reported associations with NLCS include a number of endocrinologic conditions including diabetes.7 Other coexisting skin findings can include café-au-lait macules, leukodermic (white) spots, overlying hypertrichosis, comedolike alterations, angiokeratoma, hemangioma, and folliculosebaceous cystic hamartoma.4 None of these were evident in our patient.
Lesions from both types of NLCS are indistinguishable on histopathology, characterized by the presence of a central core of ectopic mature adipocytes in the papillary/reticular dermis.2-13 Additional light microscopic features (some seen in our case) have been described, including thickened collagen bundles, reduction of elastic fibers, increased numbers of fibroblasts and/or mast cells, increased (small-vessel) vascularity, focal mucin deposition/myxoid degeneration, a mild perivascular lymphocytic infiltrate, attenuation of adnexal structures, and abnormalities of the epidermis (eg, surface ulceration).2-13
Prior to biopsy, the differential diagnosis in our patient included angiofibroma, pyogenic granuloma, and basal cell carcinoma given the exophytic, pink, papular appearance of the lesion; however, the histopathologic differential diagnosis included angiofibroma, angiomyolipoma, lymphangioma, nevus sebaceus, and spindle cell lipoma (SCL). In angiofibroma, a dermal proliferation of stellate fibroblasts, dilated blood vessels, and collagenous stroma are seen. Cutaneous angiomyolipoma demonstrates smooth muscle bundles in addition to thickened blood vessels and variable proportions of mature adipocytes. Lymphangioma is characterized by dilated lymph channels lined by flat endothelial cells. Nevus sebaceus shows superficial immature and abnormally formed pilosebaceous units, with epidermal papillomatosis.
Rare cases of SCL on the nose have been described.14 Similar to pedunculated lipofibroma, reported examples demonstrate mature univacuolar adipocytes with thick collagen fibers and bland uniform spindle cells. Unlike the lesion seen in our patient, nasal SCL may be clinically mobile and typically is localized to the subcutaneous tissue, although dermal tumors also occur.14 Variably reported histopathologic findings in nasal SCL include circumscription/encapsulation, spindle cells arranged in short fascicles with nuclear palisading, a myxoid/mucinous interstitial matrix, and/or multinucleated giant cells—all light microscopic features that were not identified in our case; however, variable proportions of adipocytic, fibrous, and myxoid components among reported examples of SCL on the nose14 can make distinction from pedunculated lipofibroma difficult, as both are benign lipomatous tumor variants.
Clinically, pedunculated lipofibroma may be confused with more common benign cutaneous lesions and must be distinguished from other fibrolipomatous lesions on the nose. Specifically, the differential diagnosis includes benign cutaneous papillomas such as acrochordon, angiofibroma, melanocytic nevi, neurofibroma, nevus sebaceus, lymphangioma, and eccrine poroma.7-13 These all can be readily excluded on histopathology. Pedunculated lipofibroma on the nose, as in our patient, must be distinguished from fibrolipoma15 and dendritic myxofibrolipoma.16 Fibrolipoma is a subcutaneous proliferation of mature adipose tissue and fibrous tissue and comprises 1.6% of all facial lipomas reported worldwide.15 Dendritic myxofibrolipoma is a recently described benign soft-tissue tumor characterized by an admixture of mature adipose tissue, spindle and stellate cells, and an abundant myxoid stroma with prominent collagenization.16
Treatment of pedunculated lipofibroma on the nose is not indicated except for cosmetic reasons, in which case simple surgical excision would be considered satisfactory. Following biopsy, no further treatment was pursued in our patient.
THE DIAGNOSIS: Pedunculated Lipofibroma
Histopathology confirmed a pedunculated/polypoid lesion with intradermal lobules of adipocytes/mature adipose tissue admixed with connective tissue bundles and vascular ectasias. Overlying epidermal acanthosis with slight papillomatosis and hyperkeratosis was present (Figure 1). Masson trichrome staining highlighted admixed collagen bundles (Figure 2). Verhoeff–van Gieson staining showed marked reduction in elastic fibers (Figure 3). Immunostaining was negative for smooth muscle actin and desmin. A diagnosis of pedunculated lipofibroma on the nose was made based on both clinical and histopathologic findings.



Pedunculated lipofibroma (or solitary lipofibroma) is the solitary form of nevus lipomatosus cutaneous superficialis (NLCS).7 First described by Hoffmann and Zurhelle1 in 1921, NLCS is an uncommon benign hamartomatous cutaneous lesion/connective tissue nevus that also has a classic multiple form.1-13 The etiology of NLCS remains unclear, but several theories have been proposed to explain its pathogenesis, including deposition of adipocytes secondary to degenerative changes in dermal connective tissue, focal/local heterotopic development of adipose tissue, and derivation from differentiating lipoblasts (preadipose tissue) originating from precursor vascular or perivascular cells.2-13
Pedunculated lipofibroma usually develops during the third to sixth decades of life and manifests as a single cutaneous lesion with a smooth surface, often on a non–pelvic girdle location.7-13 No particular predilection sites are noted, with lesions reported on the arm, axilla, back, upper thigh, knee, and sole.5,12 There are rare reports of this type of NLCS on the ear, scalp, forehead, or eyelid.7-11
In the classic form of NLCS, multiple cutaneous lesions are present at birth or develop within the first 2 to 3 decades of life.2-6 Lesions consist of soft, nontender, pedunculated, flesh-colored or yellowish papules and nodules with a verrucoid or cerebriform surface that may later coalesce to form plaques.2-6 Predilection sites include the pelvic girdle, buttocks, sacral and coccygeal regions, and upper posterior thighs, with a linear or zosteriform pattern of distribution.2-6 Rarely, the classic form can arise in elderly patients and/or at an atypical anatomic location (eg, clitoris,3 shoulder,5 thorax,5 abdomen5) and can demonstrate extension of lesions across the midline.4 Rare cases of classic NLCS on the scalp2 and face3-6 have been reported, including lesions localized to the nose3 and chin4 and others extending from the right mandible to the neck5 and right lower lip to the submandibular/posteriorateral cervical region.6 In some cases, lesions clinically resemble plane xanthoma4 and localized scleroderma.6
Adotama et al13 proposed a set of clinical features to differentiate classic NLCS, pedunculated lipofibroma (solitary NLCS), and fibroepithelial polyp with adipocytes (distinguished by their furrowed surface, hyperpigmentation, and anatomic predilection for the neck and axilla). Lesions are asymptomatic in both forms of NLCS.2-13 Family history or predominant sex involvement have not been reported in either clinical type.2-13 Reported associations with NLCS include a number of endocrinologic conditions including diabetes.7 Other coexisting skin findings can include café-au-lait macules, leukodermic (white) spots, overlying hypertrichosis, comedolike alterations, angiokeratoma, hemangioma, and folliculosebaceous cystic hamartoma.4 None of these were evident in our patient.
Lesions from both types of NLCS are indistinguishable on histopathology, characterized by the presence of a central core of ectopic mature adipocytes in the papillary/reticular dermis.2-13 Additional light microscopic features (some seen in our case) have been described, including thickened collagen bundles, reduction of elastic fibers, increased numbers of fibroblasts and/or mast cells, increased (small-vessel) vascularity, focal mucin deposition/myxoid degeneration, a mild perivascular lymphocytic infiltrate, attenuation of adnexal structures, and abnormalities of the epidermis (eg, surface ulceration).2-13
Prior to biopsy, the differential diagnosis in our patient included angiofibroma, pyogenic granuloma, and basal cell carcinoma given the exophytic, pink, papular appearance of the lesion; however, the histopathologic differential diagnosis included angiofibroma, angiomyolipoma, lymphangioma, nevus sebaceus, and spindle cell lipoma (SCL). In angiofibroma, a dermal proliferation of stellate fibroblasts, dilated blood vessels, and collagenous stroma are seen. Cutaneous angiomyolipoma demonstrates smooth muscle bundles in addition to thickened blood vessels and variable proportions of mature adipocytes. Lymphangioma is characterized by dilated lymph channels lined by flat endothelial cells. Nevus sebaceus shows superficial immature and abnormally formed pilosebaceous units, with epidermal papillomatosis.
Rare cases of SCL on the nose have been described.14 Similar to pedunculated lipofibroma, reported examples demonstrate mature univacuolar adipocytes with thick collagen fibers and bland uniform spindle cells. Unlike the lesion seen in our patient, nasal SCL may be clinically mobile and typically is localized to the subcutaneous tissue, although dermal tumors also occur.14 Variably reported histopathologic findings in nasal SCL include circumscription/encapsulation, spindle cells arranged in short fascicles with nuclear palisading, a myxoid/mucinous interstitial matrix, and/or multinucleated giant cells—all light microscopic features that were not identified in our case; however, variable proportions of adipocytic, fibrous, and myxoid components among reported examples of SCL on the nose14 can make distinction from pedunculated lipofibroma difficult, as both are benign lipomatous tumor variants.
Clinically, pedunculated lipofibroma may be confused with more common benign cutaneous lesions and must be distinguished from other fibrolipomatous lesions on the nose. Specifically, the differential diagnosis includes benign cutaneous papillomas such as acrochordon, angiofibroma, melanocytic nevi, neurofibroma, nevus sebaceus, lymphangioma, and eccrine poroma.7-13 These all can be readily excluded on histopathology. Pedunculated lipofibroma on the nose, as in our patient, must be distinguished from fibrolipoma15 and dendritic myxofibrolipoma.16 Fibrolipoma is a subcutaneous proliferation of mature adipose tissue and fibrous tissue and comprises 1.6% of all facial lipomas reported worldwide.15 Dendritic myxofibrolipoma is a recently described benign soft-tissue tumor characterized by an admixture of mature adipose tissue, spindle and stellate cells, and an abundant myxoid stroma with prominent collagenization.16
Treatment of pedunculated lipofibroma on the nose is not indicated except for cosmetic reasons, in which case simple surgical excision would be considered satisfactory. Following biopsy, no further treatment was pursued in our patient.
- Hoffmann E, Zurhelle E. Uber einen naevus lipomatodes cutaneous superficialis der linken Glutaalgegend. Arch Derm Syph. 1921;130:327-333.
- Chanoki M, Isukos S, Suzuki S, et al. Nevus lipomatosus cutaneus superficialis of the scalp. Cutis. 1989;43:143-144.
- Sáez Rodríguez M, Rodríguez-Martin M, Carnerero A, et al. Naevus lipomatosus cutaneous superficialis on the nose. J Eur Acad Dermatol Venereol. 2005;19:751-752.
- Hassab-El-Naby HMM, Rageh MA. Adult-onset nevus lipomatosus cutaneous superficialis mimicking plane xanthoma. J Clin Aesthet Dermatol. 2022;15:10-11.
- Park HJ, Park CJ, Yi JY, et al. Nevus lipomatosus superficialis on the face. Int J Dermatol. 1997;36:435-437.
- Ioannidou DJ, Stefanidou MP, Panayiotides JG, et al. Nevus lipomatosus cutaneous superficialis (Hoffman-Zurhelle) with localized scleroderma like appearance. Int J Dermatol. 2001;40:54-57.
- Nogita T, Wong TY, Hidano A, et al. Pedunculated lipofibroma. a clinicopathologic study of thirty-two cases supporting a simplified nomenclature. J Am Acad Dermatol. 1994;31(2 pt 1):235-240.
- Sawada Y. Solitary nevus lipomatosus superficialis on the forehead. Ann Plast Surg. 1986;16:356-358.
- Knoth W. Uber Naevus lipomatosus cutaneus superficialis Hoffmann-Zurhelle und uber Naevus naevocellularis partim lipomatodes. Dermatologica. 1962;125:161.
- Weitzner S. Solitary naevus lipomatosus cutaneus superficialis of scalp. Arch Dermatol. 1968;97:540-542.
- Kaw P, Carlson A, Meyer DR. Nevus lipomatosus (pedunculated lipofibroma) of the eyelid. Ophthalmic Plast Reconstr Surg. 2005;21:74-76.
- Vano-Galvan S, Moreno C, Vano-Galvan E, et al. Solitary naevus lipomatosus cutaneous superficialis on the sole. Eur J Dermatol. 2008;18:353-354.
- Adotama P, Hutson SD, Rieder EA, et al. Revisiting solitary pedunculated lipofibromas. Am J Clin Pathol. 2021;156:954-957.
- Kubin ME, Lantto U, Lindgren O, et al. A rare, recurrent spindle cell lipoma of the nose. Acta Derm Venereol. 2021;101:adv00571.
- Jung SN, Shin JW, Kwon H, et al. Fibrolipoma of the tip of the nose. J Craniofac Surg. 2009;20:555-556.
- Han XC, Zheng LQ, Shang XL. Dendritic fibromyxolipoma on the nasal tip in an old patient. Int J Clin Exp Pathol. 2014;7:7064-7067.
- Hoffmann E, Zurhelle E. Uber einen naevus lipomatodes cutaneous superficialis der linken Glutaalgegend. Arch Derm Syph. 1921;130:327-333.
- Chanoki M, Isukos S, Suzuki S, et al. Nevus lipomatosus cutaneus superficialis of the scalp. Cutis. 1989;43:143-144.
- Sáez Rodríguez M, Rodríguez-Martin M, Carnerero A, et al. Naevus lipomatosus cutaneous superficialis on the nose. J Eur Acad Dermatol Venereol. 2005;19:751-752.
- Hassab-El-Naby HMM, Rageh MA. Adult-onset nevus lipomatosus cutaneous superficialis mimicking plane xanthoma. J Clin Aesthet Dermatol. 2022;15:10-11.
- Park HJ, Park CJ, Yi JY, et al. Nevus lipomatosus superficialis on the face. Int J Dermatol. 1997;36:435-437.
- Ioannidou DJ, Stefanidou MP, Panayiotides JG, et al. Nevus lipomatosus cutaneous superficialis (Hoffman-Zurhelle) with localized scleroderma like appearance. Int J Dermatol. 2001;40:54-57.
- Nogita T, Wong TY, Hidano A, et al. Pedunculated lipofibroma. a clinicopathologic study of thirty-two cases supporting a simplified nomenclature. J Am Acad Dermatol. 1994;31(2 pt 1):235-240.
- Sawada Y. Solitary nevus lipomatosus superficialis on the forehead. Ann Plast Surg. 1986;16:356-358.
- Knoth W. Uber Naevus lipomatosus cutaneus superficialis Hoffmann-Zurhelle und uber Naevus naevocellularis partim lipomatodes. Dermatologica. 1962;125:161.
- Weitzner S. Solitary naevus lipomatosus cutaneus superficialis of scalp. Arch Dermatol. 1968;97:540-542.
- Kaw P, Carlson A, Meyer DR. Nevus lipomatosus (pedunculated lipofibroma) of the eyelid. Ophthalmic Plast Reconstr Surg. 2005;21:74-76.
- Vano-Galvan S, Moreno C, Vano-Galvan E, et al. Solitary naevus lipomatosus cutaneous superficialis on the sole. Eur J Dermatol. 2008;18:353-354.
- Adotama P, Hutson SD, Rieder EA, et al. Revisiting solitary pedunculated lipofibromas. Am J Clin Pathol. 2021;156:954-957.
- Kubin ME, Lantto U, Lindgren O, et al. A rare, recurrent spindle cell lipoma of the nose. Acta Derm Venereol. 2021;101:adv00571.
- Jung SN, Shin JW, Kwon H, et al. Fibrolipoma of the tip of the nose. J Craniofac Surg. 2009;20:555-556.
- Han XC, Zheng LQ, Shang XL. Dendritic fibromyxolipoma on the nasal tip in an old patient. Int J Clin Exp Pathol. 2014;7:7064-7067.
A 60-year-old woman presented to the dermatology department with a 6-mm, firm, pink, nonulcerated, nonmobile papule on the right nasal side wall of 1 year’s duration. It had grown slowly and was asymptomatic with no tenderness or bleeding. No other skin lesions were noted on physical examination, and her medical history was otherwise unremarkable. A shave biopsy was performed.
Erythematous Rash on the Face and Neck
The Diagnosis: Allergic Contact Dermatitis
In our patient, the erythematous pruritic rash on the face and neck, the lack of systemic symptoms, and her history of atopic dermatitis suggested a diagnosis of allergic contact dermatitis (ACD). She underwent patch testing with standard, fragrance, and cosmetic panels in addition to 6 of her personal care products. Her first patch test, which was read on day 2, showed a positive reaction to isopropyl myristate (IPM), a penetration enhancer used in cosmetics, topical medications (eg, tretinoin), and cosmeceuticals. The reading on day 5 showed a 2+ reaction to IPM, which was found in several of her personal care products, including her shampoo, leave-in conditioner, and eczema-calming cream. Isopropyl myristate is used in these products because of its ability to enhance their penetration into the skin and also can be found in commercially used products such as hand sanitizers. The patient was given information on this allergen and how to identify and avoid triggers. At follow-up, the ACD had resolved with avoidance of IPM.
Contact dermatitis is an inflammatory skin condition that is triggered by contact with a specific causative agent. There are 2 types of contact dermatitis: irritant and allergic; the irritant type is more common (approximately 80% of cases worldwide).1 Allergic contact dermatitis is a type IV (delayed-type) hypersensitivity reaction; common causative agents include shampoos, moisturizers, makeup, certain metals (eg, nickel), fragrances, latex, and certain plants (eg, poison ivy).2 In cases of ACD, a new reaction can develop from exposure to a product that the patient has used for years. It manifests clinically as erythema, pruritus, scaling, and vesicle formation.1 Certain populations, such as those with atopic dermatitis, are more prone to developing ACD due to a breakdown of the skin barrier, frequent use of topical products, and immune dysregulation.1,2 Patch testing performed by dermatologists and allergists is the gold standard for diagnosing ACD.1,3
Annually, allergists, dermatologists, and primary care physicians see thousands of cases of contact dermatitis.1 Early recognition and appropriate treatment can help reduce the severity and duration of symptoms and improve patient outcomes. The main treatment for ACD is identification of the causative agent followed by patient education on how to identify and avoid triggers.2 Once patch testing has been completed, patients can be given access to the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) to help them identify and avoid products that contain triggering allergens.
Topical corticosteroids are the first-line pharmacologic treatments for atopic dermatitis.4 When our patient presented with the facial rash, her atopic dermatitis had been well controlled with both dupilumab and topical triamcinolone. The lack of response to previously successful therapies in a new area of involvement made a flare of atopic dermatitis less likely. For flares of ACD after exposure, topical corticosteroids and topical calcineurin inhibitors can help. If needed due to severity, oral corticosteroids also can be used.1
Dermatomyositis is an inflammatory myopathy that has several skin manifestations, including a heliotrope rash and poikiloderma.5 While our patient’s rash covered the periorbital area, she did not have other classic skin findings of dermatomyositis, such as nail-fold capillary changes or poikiloderma in a shawl or holster distribution.6 She also lacked signs of systemic involvement including myositis and elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and creatine kinase levels.5
Erythematotelangiectatic rosacea is characterized by telangiectasias and transient flushing and erythema on the central face.5 Rosacea typically is triggered by temperature changes, alcohol consumption, sun exposure, spicy foods, and stress5 and would be expected to involve the nose, which was not observed in our patient. The fixed nature of our patient’s patches and the absence of telangiectasias also argued against this diagnosis.
The classic cutaneous finding of systemic lupus erythematosus is a malar rash, which appears as erythematous patches or thin plaques across the bridge of the nose and over the cheeks, sparing the nasolabial folds.5 Systemic lupus erythematosus is associated with laboratory abnormalities, such as positive antinuclear antibodies and elevated CRP and ESR levels.5 Our patient had notable sparing of the nose, negative antinuclear antibodies, and normal CRP and ESR levels, making systemic lupus erythematosus unlikely. Systemic lupus erythematosus also can manifest with photosensitivity,7 and involvement of the submental skin in our patient argued against a photosensitive eruption.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76. doi:10.1016/j.mcna.2019.08.012
- Fonacier LS, Sher JM. Allergic contact dermatitis. Ann Allergy Asthma Immunol. 2014;113:9-12. doi:10.1016/j.anai.2014.03.018
- Uyesugi BA, Sheehan MP. Patch testing pearls. Clin Rev Allergy Immunol. 2019;56:110-118. doi:10.1007/s12016-018-8715-y
- Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2018;14(suppl 2):52. doi:10.1186/s13223-018-0281-6
- Naji S. Malar rash. StatPearls. Updated September 4, 2023. Accessed June 30, 2025. https://www.statpearls.com/point-of-care/24661
- Muro Y, Sugiura K, Akiyama M. Cutaneous manifestations in dermatomyositis: key clinical and serological features—a comprehensive review. Clin Rev Allergy Immunol. 2016;51:293-302. doi:10.1007 /s12016-015-8496-5
- Hannon CW, McCourt C, Lima HC, et al. Interventions for cutaneous disease in systemic lupus erythematosus. Cochrane Database Syst Rev. 2021;3(3):CD007478. doi:10.1002/14651858.CD007478.pub2
The Diagnosis: Allergic Contact Dermatitis
In our patient, the erythematous pruritic rash on the face and neck, the lack of systemic symptoms, and her history of atopic dermatitis suggested a diagnosis of allergic contact dermatitis (ACD). She underwent patch testing with standard, fragrance, and cosmetic panels in addition to 6 of her personal care products. Her first patch test, which was read on day 2, showed a positive reaction to isopropyl myristate (IPM), a penetration enhancer used in cosmetics, topical medications (eg, tretinoin), and cosmeceuticals. The reading on day 5 showed a 2+ reaction to IPM, which was found in several of her personal care products, including her shampoo, leave-in conditioner, and eczema-calming cream. Isopropyl myristate is used in these products because of its ability to enhance their penetration into the skin and also can be found in commercially used products such as hand sanitizers. The patient was given information on this allergen and how to identify and avoid triggers. At follow-up, the ACD had resolved with avoidance of IPM.
Contact dermatitis is an inflammatory skin condition that is triggered by contact with a specific causative agent. There are 2 types of contact dermatitis: irritant and allergic; the irritant type is more common (approximately 80% of cases worldwide).1 Allergic contact dermatitis is a type IV (delayed-type) hypersensitivity reaction; common causative agents include shampoos, moisturizers, makeup, certain metals (eg, nickel), fragrances, latex, and certain plants (eg, poison ivy).2 In cases of ACD, a new reaction can develop from exposure to a product that the patient has used for years. It manifests clinically as erythema, pruritus, scaling, and vesicle formation.1 Certain populations, such as those with atopic dermatitis, are more prone to developing ACD due to a breakdown of the skin barrier, frequent use of topical products, and immune dysregulation.1,2 Patch testing performed by dermatologists and allergists is the gold standard for diagnosing ACD.1,3
Annually, allergists, dermatologists, and primary care physicians see thousands of cases of contact dermatitis.1 Early recognition and appropriate treatment can help reduce the severity and duration of symptoms and improve patient outcomes. The main treatment for ACD is identification of the causative agent followed by patient education on how to identify and avoid triggers.2 Once patch testing has been completed, patients can be given access to the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) to help them identify and avoid products that contain triggering allergens.
Topical corticosteroids are the first-line pharmacologic treatments for atopic dermatitis.4 When our patient presented with the facial rash, her atopic dermatitis had been well controlled with both dupilumab and topical triamcinolone. The lack of response to previously successful therapies in a new area of involvement made a flare of atopic dermatitis less likely. For flares of ACD after exposure, topical corticosteroids and topical calcineurin inhibitors can help. If needed due to severity, oral corticosteroids also can be used.1
Dermatomyositis is an inflammatory myopathy that has several skin manifestations, including a heliotrope rash and poikiloderma.5 While our patient’s rash covered the periorbital area, she did not have other classic skin findings of dermatomyositis, such as nail-fold capillary changes or poikiloderma in a shawl or holster distribution.6 She also lacked signs of systemic involvement including myositis and elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and creatine kinase levels.5
Erythematotelangiectatic rosacea is characterized by telangiectasias and transient flushing and erythema on the central face.5 Rosacea typically is triggered by temperature changes, alcohol consumption, sun exposure, spicy foods, and stress5 and would be expected to involve the nose, which was not observed in our patient. The fixed nature of our patient’s patches and the absence of telangiectasias also argued against this diagnosis.
The classic cutaneous finding of systemic lupus erythematosus is a malar rash, which appears as erythematous patches or thin plaques across the bridge of the nose and over the cheeks, sparing the nasolabial folds.5 Systemic lupus erythematosus is associated with laboratory abnormalities, such as positive antinuclear antibodies and elevated CRP and ESR levels.5 Our patient had notable sparing of the nose, negative antinuclear antibodies, and normal CRP and ESR levels, making systemic lupus erythematosus unlikely. Systemic lupus erythematosus also can manifest with photosensitivity,7 and involvement of the submental skin in our patient argued against a photosensitive eruption.
The Diagnosis: Allergic Contact Dermatitis
In our patient, the erythematous pruritic rash on the face and neck, the lack of systemic symptoms, and her history of atopic dermatitis suggested a diagnosis of allergic contact dermatitis (ACD). She underwent patch testing with standard, fragrance, and cosmetic panels in addition to 6 of her personal care products. Her first patch test, which was read on day 2, showed a positive reaction to isopropyl myristate (IPM), a penetration enhancer used in cosmetics, topical medications (eg, tretinoin), and cosmeceuticals. The reading on day 5 showed a 2+ reaction to IPM, which was found in several of her personal care products, including her shampoo, leave-in conditioner, and eczema-calming cream. Isopropyl myristate is used in these products because of its ability to enhance their penetration into the skin and also can be found in commercially used products such as hand sanitizers. The patient was given information on this allergen and how to identify and avoid triggers. At follow-up, the ACD had resolved with avoidance of IPM.
Contact dermatitis is an inflammatory skin condition that is triggered by contact with a specific causative agent. There are 2 types of contact dermatitis: irritant and allergic; the irritant type is more common (approximately 80% of cases worldwide).1 Allergic contact dermatitis is a type IV (delayed-type) hypersensitivity reaction; common causative agents include shampoos, moisturizers, makeup, certain metals (eg, nickel), fragrances, latex, and certain plants (eg, poison ivy).2 In cases of ACD, a new reaction can develop from exposure to a product that the patient has used for years. It manifests clinically as erythema, pruritus, scaling, and vesicle formation.1 Certain populations, such as those with atopic dermatitis, are more prone to developing ACD due to a breakdown of the skin barrier, frequent use of topical products, and immune dysregulation.1,2 Patch testing performed by dermatologists and allergists is the gold standard for diagnosing ACD.1,3
Annually, allergists, dermatologists, and primary care physicians see thousands of cases of contact dermatitis.1 Early recognition and appropriate treatment can help reduce the severity and duration of symptoms and improve patient outcomes. The main treatment for ACD is identification of the causative agent followed by patient education on how to identify and avoid triggers.2 Once patch testing has been completed, patients can be given access to the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) to help them identify and avoid products that contain triggering allergens.
Topical corticosteroids are the first-line pharmacologic treatments for atopic dermatitis.4 When our patient presented with the facial rash, her atopic dermatitis had been well controlled with both dupilumab and topical triamcinolone. The lack of response to previously successful therapies in a new area of involvement made a flare of atopic dermatitis less likely. For flares of ACD after exposure, topical corticosteroids and topical calcineurin inhibitors can help. If needed due to severity, oral corticosteroids also can be used.1
Dermatomyositis is an inflammatory myopathy that has several skin manifestations, including a heliotrope rash and poikiloderma.5 While our patient’s rash covered the periorbital area, she did not have other classic skin findings of dermatomyositis, such as nail-fold capillary changes or poikiloderma in a shawl or holster distribution.6 She also lacked signs of systemic involvement including myositis and elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and creatine kinase levels.5
Erythematotelangiectatic rosacea is characterized by telangiectasias and transient flushing and erythema on the central face.5 Rosacea typically is triggered by temperature changes, alcohol consumption, sun exposure, spicy foods, and stress5 and would be expected to involve the nose, which was not observed in our patient. The fixed nature of our patient’s patches and the absence of telangiectasias also argued against this diagnosis.
The classic cutaneous finding of systemic lupus erythematosus is a malar rash, which appears as erythematous patches or thin plaques across the bridge of the nose and over the cheeks, sparing the nasolabial folds.5 Systemic lupus erythematosus is associated with laboratory abnormalities, such as positive antinuclear antibodies and elevated CRP and ESR levels.5 Our patient had notable sparing of the nose, negative antinuclear antibodies, and normal CRP and ESR levels, making systemic lupus erythematosus unlikely. Systemic lupus erythematosus also can manifest with photosensitivity,7 and involvement of the submental skin in our patient argued against a photosensitive eruption.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76. doi:10.1016/j.mcna.2019.08.012
- Fonacier LS, Sher JM. Allergic contact dermatitis. Ann Allergy Asthma Immunol. 2014;113:9-12. doi:10.1016/j.anai.2014.03.018
- Uyesugi BA, Sheehan MP. Patch testing pearls. Clin Rev Allergy Immunol. 2019;56:110-118. doi:10.1007/s12016-018-8715-y
- Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2018;14(suppl 2):52. doi:10.1186/s13223-018-0281-6
- Naji S. Malar rash. StatPearls. Updated September 4, 2023. Accessed June 30, 2025. https://www.statpearls.com/point-of-care/24661
- Muro Y, Sugiura K, Akiyama M. Cutaneous manifestations in dermatomyositis: key clinical and serological features—a comprehensive review. Clin Rev Allergy Immunol. 2016;51:293-302. doi:10.1007 /s12016-015-8496-5
- Hannon CW, McCourt C, Lima HC, et al. Interventions for cutaneous disease in systemic lupus erythematosus. Cochrane Database Syst Rev. 2021;3(3):CD007478. doi:10.1002/14651858.CD007478.pub2
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76. doi:10.1016/j.mcna.2019.08.012
- Fonacier LS, Sher JM. Allergic contact dermatitis. Ann Allergy Asthma Immunol. 2014;113:9-12. doi:10.1016/j.anai.2014.03.018
- Uyesugi BA, Sheehan MP. Patch testing pearls. Clin Rev Allergy Immunol. 2019;56:110-118. doi:10.1007/s12016-018-8715-y
- Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2018;14(suppl 2):52. doi:10.1186/s13223-018-0281-6
- Naji S. Malar rash. StatPearls. Updated September 4, 2023. Accessed June 30, 2025. https://www.statpearls.com/point-of-care/24661
- Muro Y, Sugiura K, Akiyama M. Cutaneous manifestations in dermatomyositis: key clinical and serological features—a comprehensive review. Clin Rev Allergy Immunol. 2016;51:293-302. doi:10.1007 /s12016-015-8496-5
- Hannon CW, McCourt C, Lima HC, et al. Interventions for cutaneous disease in systemic lupus erythematosus. Cochrane Database Syst Rev. 2021;3(3):CD007478. doi:10.1002/14651858.CD007478.pub2
A 23-year-old woman with atopic dermatitis and seasonal allergic rhinitis presented to the dermatology department with an erythematous pruritic rash of 1 year’s duration involving the forehead, periorbital and submental skin, and neck. The patient’s atopic dermatitis was stable and had been well controlled with dupilumab and topical triamcinolone as needed for flares. The patient denied any other symptoms including fever, fatigue, and muscle weakness. Physical examination of the hands and nails revealed no abnormalities. She was treated with topical triamcinolone acetonide 0.1% without improvement. Short-term prednisone tapers fully resolved the rash, but it recurred within 5 days after discontinuation of prednisone. Results of testing for rheumatoid factor, antinuclear antibodies, complete blood count, comprehensive metabolic panel, C-reactive protein, erythrocyte sedimentation rate, and antistreptolysin O antibodies were unremarkable.
Multiple Fungating Plaques on the Face, Arms, and Legs
Multiple Fungating Plaques on the Face, Arms, and Legs
THE DIAGNOSIS: Mpox
Histologic examination demonstrated dense aggregates of necrotic cellular debris composed of karyorrhectic nuclear fragments intermixed with neutrophils, lymphocytes, and histiocytes. Eosinophilic intracytoplasmic inclusions also were observed (Figure 1). The bacterial, fungal, and mycobacterial histologic special stains and cultures were negative. Three weeks after the initial visit with dermatology, the patient was admitted to the hospital for worsening symptoms of fever, chills, and painful erythema surrounding the skin lesions. Serology and viral workup revealed a positive mpox polymerase chain reaction test, suggesting a diagnosis of mpox. Following the Centers for Disease Control and Prevention protocol, the patient was started on oral tecovirimat 200 mg twice daily for 3 weeks and intravenous infusions of cidofovir 345 mg once weekly for 2 weeks. After treatment was initiated, the skin lesions showed rapid improvement (Figure 2), and he was discharged from the hospital after finishing the second dose of cidofovir. Four months after the initial dermatology consultation, the lesions had resolved completely with residual scarring. At that time, the patient had full movement of the right eye.
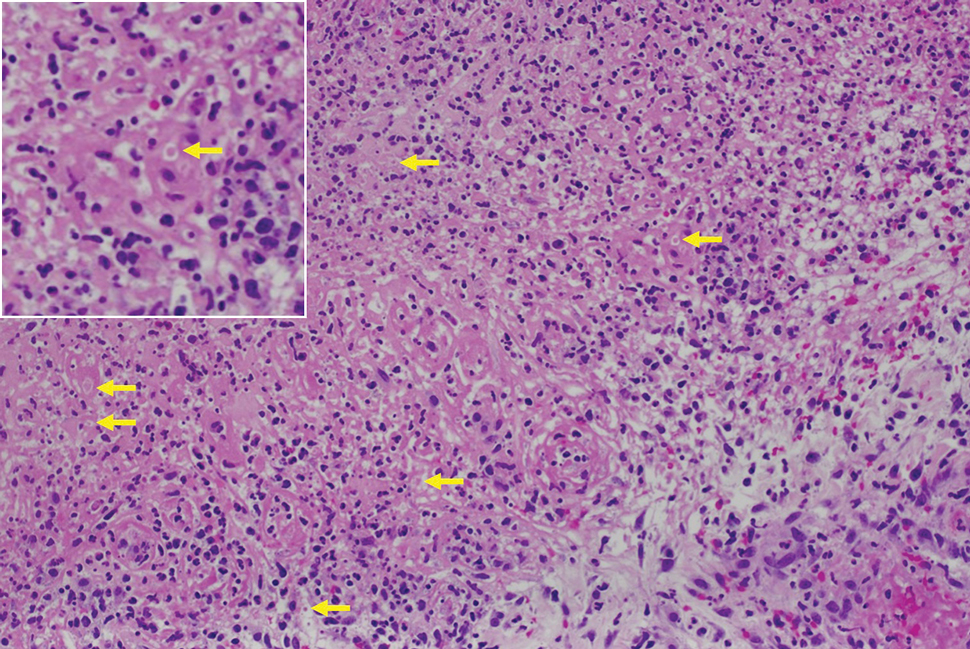
shows higher digital magnification of eosinophilic inclusions observed throughout the biopsy specimen (original magnification ×400).
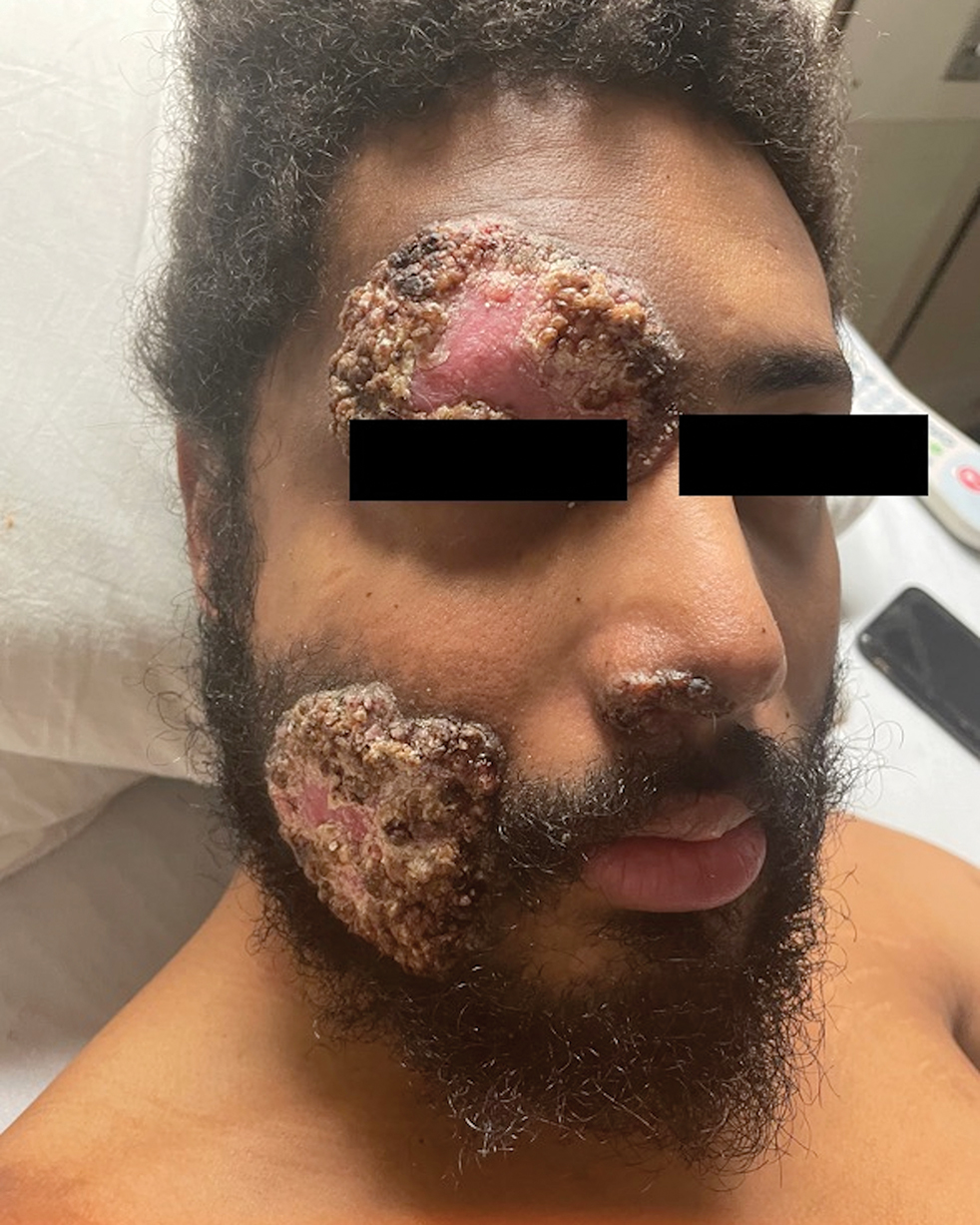
Mpox virus is a member of the Poxviridae family of zoonotic viruses, which are transmitted from animals to humans. The mpox virus is brick-shaped (rectangular) and has a genome of linear double-stranded DNA encoding 180 proteins.1 Primates and rodents are the typical host reservoirs for viral circulation of mpox.2 Animal-to-human transmission occurs through direct contact with mucous membranes, bodily fluids, or tissues of an infected animal. Human-to-human transmission occurs through direct contact with infected mucous membranes, bodily fluids, respiratory droplets, and contaminated fomites.2
Symptoms typically occur within 1 week of exposure to the mpox virus. Prodromal symptoms of fever, sore throat, body aches, and headaches last for 3 days.1 Many patients experience a facial rash that spreads to the arms and legs over a period of 2 to 4 weeks. The rash initially manifests as small papules that progress to painful pustules and vesicles measuring 0.5 to 1.0 cm in diameter.3 The mpox virus is transmitted through these skin lesions until they crust over and re-epithelialize.1 The case fatality rate for mpox infection remains low (0.18%).4
Mpox outbreaks mainly were limited to central and western Africa prior to 2022. From May 17, 2022, through October 6, 2022, 26,384 cases of mpox were reported in the United States.5 During this outbreak, immunocompromised patients diagnosed with HIV and men who have sex with men were disproportionately affected.5
Due to the similarities between the smallpox virus and other orthopoxviruses, certain smallpox vaccines have been indicated for pre-exposure prophylaxis.6 The efficacy of prophylactic vaccination is believed to stem from the production of neutralizing antibodies that are cross-protective against other orthopoxviruses, including mpox.7 The 2 vaccines approved in the United States for mpox prophylaxis are JYNNEOS and ACAM2000, which are both live attenuated vaccines. Pre-exposure prophylaxis is indicated for patients at risk for severe disease, including men who have sex with men, individuals diagnosed with HIV or other immunosuppressive disorders, and individuals with recent diagnoses of one or more sexually transmitted diseases.8
Most mpox cases resolve within 2 to 4 weeks and only require supportive care (eg, nonsteroidal anti-inflammatory drugs, topical steroids, topical anesthetics) to treat pain.8 For patients at risk for severe disease, antiviral medications are warranted. Tecovirimat, brincidofovir, and cidofovir are antiviral medications used to treat smallpox that are thought to be effective against mpox.8,9 Tecovirimat and cidofovir have been shown to be effective against mpox in animal trials, but randomized or nonrandomized trials have not been performed in humans.9-11 Tecovirimat currently is available for the treatment of severe mpox in patients who meet the Centers for Disease Control and Prevention’s Investigational New Drug protocol; for these patients, a 200-mg course is administered orally or intravenously every 12 hours for 2 weeks.8
- Lu J, Xing H, Wang C, et al. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther. 2023;8:458. doi:10.1038/s41392-023-01675-
- Lim CK, Roberts J, Moso M, et al. Mpox diagnostics: review of current and emerging technologies. J Med Virol. 2023;95:e28429. doi:10.1002/jmv.28429
- Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi:10.3390/tropicalmed1010008
- World Health Organization. Mpox (monkeypox) World Health Organization. Published April 18, 2023. Accessed May 28, 2025. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. 2022:1449-1456. https://www.cdc.gov/mmwr/volumes/71/wr/mm7145a4.htm?s_cid=mm7145a4_w
- Rizk JG, Lippi G, Henry BM, et al. Prevention and treatment of monkeypox. Drugs. 2022;82:957-963. doi:10.1007/s40265-022-01742-y
- Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740-747. doi:10.1038 /nm1261
- Centers for Disease Control and Prevention. Mpox treatment information for healthcare professionals. Updated June 18, 2024. Accessed May 28, 2025. https://www.cdc.gov/mpox/hcp/clinical-care/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html
- Mitja O, Ogoina D, Titanji BK, et al. Monkeypox. Lancet. 2023;401:60-74. doi:10.1016/S0140-6736(22)02075-X
- Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620-2625. doi:10.1128/aac.00021-09
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44-53. doi:10.1056 /nejmoa1705688
THE DIAGNOSIS: Mpox
Histologic examination demonstrated dense aggregates of necrotic cellular debris composed of karyorrhectic nuclear fragments intermixed with neutrophils, lymphocytes, and histiocytes. Eosinophilic intracytoplasmic inclusions also were observed (Figure 1). The bacterial, fungal, and mycobacterial histologic special stains and cultures were negative. Three weeks after the initial visit with dermatology, the patient was admitted to the hospital for worsening symptoms of fever, chills, and painful erythema surrounding the skin lesions. Serology and viral workup revealed a positive mpox polymerase chain reaction test, suggesting a diagnosis of mpox. Following the Centers for Disease Control and Prevention protocol, the patient was started on oral tecovirimat 200 mg twice daily for 3 weeks and intravenous infusions of cidofovir 345 mg once weekly for 2 weeks. After treatment was initiated, the skin lesions showed rapid improvement (Figure 2), and he was discharged from the hospital after finishing the second dose of cidofovir. Four months after the initial dermatology consultation, the lesions had resolved completely with residual scarring. At that time, the patient had full movement of the right eye.

shows higher digital magnification of eosinophilic inclusions observed throughout the biopsy specimen (original magnification ×400).

Mpox virus is a member of the Poxviridae family of zoonotic viruses, which are transmitted from animals to humans. The mpox virus is brick-shaped (rectangular) and has a genome of linear double-stranded DNA encoding 180 proteins.1 Primates and rodents are the typical host reservoirs for viral circulation of mpox.2 Animal-to-human transmission occurs through direct contact with mucous membranes, bodily fluids, or tissues of an infected animal. Human-to-human transmission occurs through direct contact with infected mucous membranes, bodily fluids, respiratory droplets, and contaminated fomites.2
Symptoms typically occur within 1 week of exposure to the mpox virus. Prodromal symptoms of fever, sore throat, body aches, and headaches last for 3 days.1 Many patients experience a facial rash that spreads to the arms and legs over a period of 2 to 4 weeks. The rash initially manifests as small papules that progress to painful pustules and vesicles measuring 0.5 to 1.0 cm in diameter.3 The mpox virus is transmitted through these skin lesions until they crust over and re-epithelialize.1 The case fatality rate for mpox infection remains low (0.18%).4
Mpox outbreaks mainly were limited to central and western Africa prior to 2022. From May 17, 2022, through October 6, 2022, 26,384 cases of mpox were reported in the United States.5 During this outbreak, immunocompromised patients diagnosed with HIV and men who have sex with men were disproportionately affected.5
Due to the similarities between the smallpox virus and other orthopoxviruses, certain smallpox vaccines have been indicated for pre-exposure prophylaxis.6 The efficacy of prophylactic vaccination is believed to stem from the production of neutralizing antibodies that are cross-protective against other orthopoxviruses, including mpox.7 The 2 vaccines approved in the United States for mpox prophylaxis are JYNNEOS and ACAM2000, which are both live attenuated vaccines. Pre-exposure prophylaxis is indicated for patients at risk for severe disease, including men who have sex with men, individuals diagnosed with HIV or other immunosuppressive disorders, and individuals with recent diagnoses of one or more sexually transmitted diseases.8
Most mpox cases resolve within 2 to 4 weeks and only require supportive care (eg, nonsteroidal anti-inflammatory drugs, topical steroids, topical anesthetics) to treat pain.8 For patients at risk for severe disease, antiviral medications are warranted. Tecovirimat, brincidofovir, and cidofovir are antiviral medications used to treat smallpox that are thought to be effective against mpox.8,9 Tecovirimat and cidofovir have been shown to be effective against mpox in animal trials, but randomized or nonrandomized trials have not been performed in humans.9-11 Tecovirimat currently is available for the treatment of severe mpox in patients who meet the Centers for Disease Control and Prevention’s Investigational New Drug protocol; for these patients, a 200-mg course is administered orally or intravenously every 12 hours for 2 weeks.8
THE DIAGNOSIS: Mpox
Histologic examination demonstrated dense aggregates of necrotic cellular debris composed of karyorrhectic nuclear fragments intermixed with neutrophils, lymphocytes, and histiocytes. Eosinophilic intracytoplasmic inclusions also were observed (Figure 1). The bacterial, fungal, and mycobacterial histologic special stains and cultures were negative. Three weeks after the initial visit with dermatology, the patient was admitted to the hospital for worsening symptoms of fever, chills, and painful erythema surrounding the skin lesions. Serology and viral workup revealed a positive mpox polymerase chain reaction test, suggesting a diagnosis of mpox. Following the Centers for Disease Control and Prevention protocol, the patient was started on oral tecovirimat 200 mg twice daily for 3 weeks and intravenous infusions of cidofovir 345 mg once weekly for 2 weeks. After treatment was initiated, the skin lesions showed rapid improvement (Figure 2), and he was discharged from the hospital after finishing the second dose of cidofovir. Four months after the initial dermatology consultation, the lesions had resolved completely with residual scarring. At that time, the patient had full movement of the right eye.

shows higher digital magnification of eosinophilic inclusions observed throughout the biopsy specimen (original magnification ×400).

Mpox virus is a member of the Poxviridae family of zoonotic viruses, which are transmitted from animals to humans. The mpox virus is brick-shaped (rectangular) and has a genome of linear double-stranded DNA encoding 180 proteins.1 Primates and rodents are the typical host reservoirs for viral circulation of mpox.2 Animal-to-human transmission occurs through direct contact with mucous membranes, bodily fluids, or tissues of an infected animal. Human-to-human transmission occurs through direct contact with infected mucous membranes, bodily fluids, respiratory droplets, and contaminated fomites.2
Symptoms typically occur within 1 week of exposure to the mpox virus. Prodromal symptoms of fever, sore throat, body aches, and headaches last for 3 days.1 Many patients experience a facial rash that spreads to the arms and legs over a period of 2 to 4 weeks. The rash initially manifests as small papules that progress to painful pustules and vesicles measuring 0.5 to 1.0 cm in diameter.3 The mpox virus is transmitted through these skin lesions until they crust over and re-epithelialize.1 The case fatality rate for mpox infection remains low (0.18%).4
Mpox outbreaks mainly were limited to central and western Africa prior to 2022. From May 17, 2022, through October 6, 2022, 26,384 cases of mpox were reported in the United States.5 During this outbreak, immunocompromised patients diagnosed with HIV and men who have sex with men were disproportionately affected.5
Due to the similarities between the smallpox virus and other orthopoxviruses, certain smallpox vaccines have been indicated for pre-exposure prophylaxis.6 The efficacy of prophylactic vaccination is believed to stem from the production of neutralizing antibodies that are cross-protective against other orthopoxviruses, including mpox.7 The 2 vaccines approved in the United States for mpox prophylaxis are JYNNEOS and ACAM2000, which are both live attenuated vaccines. Pre-exposure prophylaxis is indicated for patients at risk for severe disease, including men who have sex with men, individuals diagnosed with HIV or other immunosuppressive disorders, and individuals with recent diagnoses of one or more sexually transmitted diseases.8
Most mpox cases resolve within 2 to 4 weeks and only require supportive care (eg, nonsteroidal anti-inflammatory drugs, topical steroids, topical anesthetics) to treat pain.8 For patients at risk for severe disease, antiviral medications are warranted. Tecovirimat, brincidofovir, and cidofovir are antiviral medications used to treat smallpox that are thought to be effective against mpox.8,9 Tecovirimat and cidofovir have been shown to be effective against mpox in animal trials, but randomized or nonrandomized trials have not been performed in humans.9-11 Tecovirimat currently is available for the treatment of severe mpox in patients who meet the Centers for Disease Control and Prevention’s Investigational New Drug protocol; for these patients, a 200-mg course is administered orally or intravenously every 12 hours for 2 weeks.8
- Lu J, Xing H, Wang C, et al. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther. 2023;8:458. doi:10.1038/s41392-023-01675-
- Lim CK, Roberts J, Moso M, et al. Mpox diagnostics: review of current and emerging technologies. J Med Virol. 2023;95:e28429. doi:10.1002/jmv.28429
- Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi:10.3390/tropicalmed1010008
- World Health Organization. Mpox (monkeypox) World Health Organization. Published April 18, 2023. Accessed May 28, 2025. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. 2022:1449-1456. https://www.cdc.gov/mmwr/volumes/71/wr/mm7145a4.htm?s_cid=mm7145a4_w
- Rizk JG, Lippi G, Henry BM, et al. Prevention and treatment of monkeypox. Drugs. 2022;82:957-963. doi:10.1007/s40265-022-01742-y
- Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740-747. doi:10.1038 /nm1261
- Centers for Disease Control and Prevention. Mpox treatment information for healthcare professionals. Updated June 18, 2024. Accessed May 28, 2025. https://www.cdc.gov/mpox/hcp/clinical-care/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html
- Mitja O, Ogoina D, Titanji BK, et al. Monkeypox. Lancet. 2023;401:60-74. doi:10.1016/S0140-6736(22)02075-X
- Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620-2625. doi:10.1128/aac.00021-09
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44-53. doi:10.1056 /nejmoa1705688
- Lu J, Xing H, Wang C, et al. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther. 2023;8:458. doi:10.1038/s41392-023-01675-
- Lim CK, Roberts J, Moso M, et al. Mpox diagnostics: review of current and emerging technologies. J Med Virol. 2023;95:e28429. doi:10.1002/jmv.28429
- Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi:10.3390/tropicalmed1010008
- World Health Organization. Mpox (monkeypox) World Health Organization. Published April 18, 2023. Accessed May 28, 2025. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. 2022:1449-1456. https://www.cdc.gov/mmwr/volumes/71/wr/mm7145a4.htm?s_cid=mm7145a4_w
- Rizk JG, Lippi G, Henry BM, et al. Prevention and treatment of monkeypox. Drugs. 2022;82:957-963. doi:10.1007/s40265-022-01742-y
- Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740-747. doi:10.1038 /nm1261
- Centers for Disease Control and Prevention. Mpox treatment information for healthcare professionals. Updated June 18, 2024. Accessed May 28, 2025. https://www.cdc.gov/mpox/hcp/clinical-care/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html
- Mitja O, Ogoina D, Titanji BK, et al. Monkeypox. Lancet. 2023;401:60-74. doi:10.1016/S0140-6736(22)02075-X
- Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620-2625. doi:10.1128/aac.00021-09
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44-53. doi:10.1056 /nejmoa1705688
Multiple Fungating Plaques on the Face, Arms, and Legs
Multiple Fungating Plaques on the Face, Arms, and Legs
A 27-year-old man presented to his primary care physician after he was struck in the head by a tree branch while working outside. The next day, ulcerating lesions emerged on the right supraorbital ridge, along with subjective fevers, chills, fatigue, and shortness of breath. The patient reported a history of unprotected sexual intercourse with a male partner who was HIV positive. His medical history included syphilis status posttreatment with a course of 5 penicillin injections, hepatitis C, and HIV diagnosed one month prior to presentation (CD4 count, 169 cells/mm3 [reference range, 500-1500 cells/mm3]). A punch biopsy performed by the primary care physician revealed suppurative granulomatous inflammation, and the patient was prescribed antibiotics with mild improvement. He then was referred to dermatology for further evaluation of the ulcerating lesions.
Three months after the initial trauma, the patient presented to the dermatology clinic for evaluation of multiple large fungating plaques affecting multiple sites on the face (top), arms (bottom), and legs. Physical examination revealed large circinate verrucous plaques involving the right supraorbital ridge and eyelid. The patient was unable to fully open the right eye. Similar plaques also were observed on the right malar cheek, arms, and feet. Four 5-mm punch biopsies from lesions on the right elbow and left ankle were obtained with fungal and bacterial cultures.
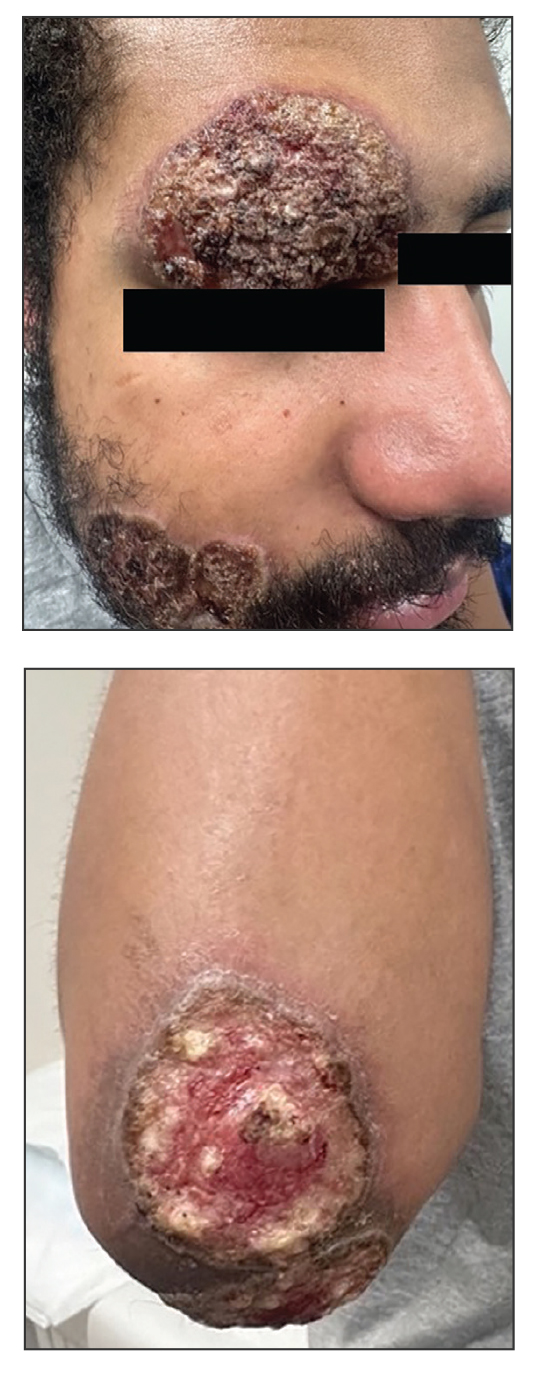
Eruptive Erythematous Papules on the Forearms
Eruptive Erythematous Papules on the Forearms
THE DIAGNOSIS: Acral Eruptive Syringoma
Syringomas are small, benign, often asymptomatic eccrine tumors that originate in the intraepidermal portion of eccrine sweat ducts.1 Clinically, they present as multiple symmetric white-to-yellow or discrete flesh-colored papules measuring 1 to 3 mm in diameter, often located on the face (most commonly on the eyelids), with a greater prevalence in middle-aged women. Occasionally, they manifest in other locations such as the cheeks, chest, axillae, abdomen, and groin.2
In 1987, Friedman and Butler3 developed a classification system categorizing syringomas into 4 clinical subtypes: familial syringoma, localized syringoma, Down syndrome–related syringoma, and generalized syringoma. The fourth subtype includes the variant of eruptive syringoma,3 a rare clinical manifestation that often develops before or during puberty with several flesh-colored or lightly pigmented papules on the neck, anterior chest, upper abdomen, axillae, periumbilical region, and/or genital region.1,4,5 The etiology of eruptive syringomas is unclear, although it has been linked to abnormal proliferation of sweat glands due to an underlying local inflammatory process.6
Acral distribution of syringomas is a rare variant that can manifest as part of generalized eruptive syringoma with consequent involvement of the arms and other areas.5,7 There are limited case reports on eruptive syringomas with predominant acral distribution.8 Compared to classic syringomas, the acral variant is associated with an older age of onset as well as a similar prevalence between men and women.9 Acral eruptive syringoma (AES) usually is isolated to the distal arms and legs. The most commonly affected region is the anterior surface of the forearms, although involvement of the dorsal hands, wrists, and feet also has been reported.10-16
The first known case of AES, which was reported in 1977, described eruptive syringomas on the dorsal hands of a healthy 31-year-old man.17 Several cases have been reported since then, mostly in patients aged 30 to 60 years, with predominant involvement of the dorsal hands and forearms.18-24 A review of Embase as well as PubMed articles indexed for MEDLINE using the search terms syringoma OR eccrine ductal tumor and eruptive OR acral OR arms OR forearms OR extremities identified 19 reported cases of AES between 1977 and 2023. For the reported AES cases, the mean (SD) age at diagnosis was 45.1 years (15.96 years), with patient ages ranging from 19 to 76 years. Notably, most cases occurred in individuals aged between 30 and 60 years, which deviates from the typical age of onset of localized syringomas, commonly seen during puberty or early adulthood.
Currently, AES is categorized within the clinical presentation of eruptive syringoma. Nevertheless, some authors have proposed classifying it as a distinct fifth clinical group due to specific features that distinguish it from generalized eruptive syringoma.9 This reclassification has considerable implications for the differential diagnosis, particularly because exclusive acral involvement poses a substantial diagnostic challenge and often requires histologic confirmation.
As shown in the Figure, histopathologic examination revealed tubular structures in the upper dermis with characteristic comma-shaped extensions. Some of these structures were lined with cuboidal cells and contained eosinophilic material within the lumen. There was no involvement of the epidermis or deeper dermis. The histologic features were consistent with syringoma, which is distinguished by its predominant involvement of the upper dermis and the presence of enlarged, dilated eccrine ducts, as observed in our case.
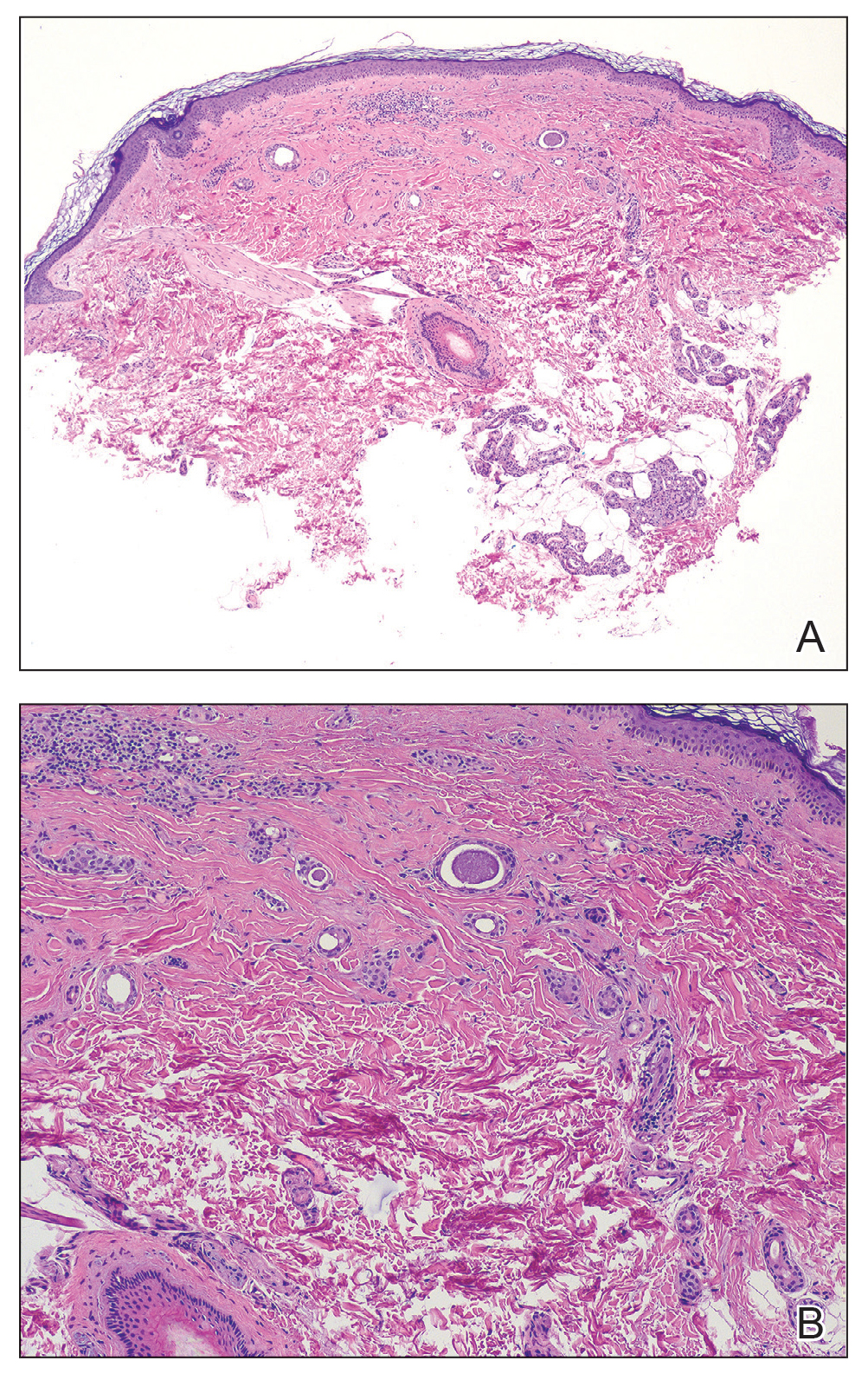
Treatment of syringomas often is challenging due to the high rate of recurrence and the risk for postinflammatory hyperpigmentation. Since the condition is benign, treatment typically is pursued for aesthetic reasons. Various therapeutic approaches have been reported, each with diverse response rates. The most common method involves surgical intervention, either with electrodesiccation or CO2 laser—both of which have shown satisfactory resolution of lesions without recurrence at 1-year follow-up, with no major scarring reported.25,26 Alternatively, topical management with retinoids daily over a 4-month period leads to flattening of the tumors with no further appearance of new lesions.27 Despite the availability of numerous management options, establishing a first-line treatment remains controversial due to the high risk for recurrence and the variability in the number and location of lesions among individual patients. In our case, given the benign nature of syringomas, the asymptomatic nature of the lesions, the involvement of noncritical aesthetic areas, and the limited response to noninvasive therapeutic options, the patient was informed of the diagnosis, and no further pharmacologic or surgical intervention was pursued.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.E9. doi:10.1016 /j.jaad.2015.12.006
- Resende C, Araújo C, Santos R, et al. Late-onset of eruptive syringomas: a diagnostic challenge. An Bras Dermatol. 2015;90(3 suppl 1):239-241. doi:10.1590/abd1806-4841.20153899
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Avhad G, Ghuge P, Jerajani HR. Generalized eruptive syringoma. Indian J Dermatol. 2015;60:214. doi:10.4103/0019-5154.152586
- Ning WV, Bashey S, Cole C, et al. Multiple eruptive syringomas on the penis. Cutis. 2019;103:E15-E16.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Jamalipour M, Heidarpour M, Rajabi P. Generalized eruptive syringomas. Indian J Dermatol. 2009;54:65-67. doi:10.4103/0019-5154.48992
- Mohaghegh F, Amiri A, Fatemi Naeini F, et al. Acral eruptive syringoma: an unusual presentation with misdiagnosis. Case Rep Dermatol Med. 2020;2020:5416285. doi:10.1155/2020/5416285
- Valdivielso-Ramos M, de la Cueva P, Gimeno M, et al. Acral syringomas. Actas Dermosifiliogr. 2010;101:458-460.
- Patel K, Lundgren AD, Ahmed AM, et al. Disseminated syringomas of the upper extremities in a young woman. Cureus. 2018;10:E3619. doi:10.7759/cureus.3619
- Balci DD, Atik E, Altintas S. Coexistence of acral syringomas and multiple trichoepitheliomas on the face. J Cutan Med Surg. 2009;13:169-171. doi:10.2310/7750.2008.08011
- Martín-García RF, Muñoz CM. Acral syringomas presenting as a photosensitive papular eruption. Cutis. 2006;77:33-36.
- Varas-Meis E, Prada-García C, Samaniego-González E, et al. Acral syringomas associated with hematological neoplasm. Indian J Dermatol Venereol Leprol. 2017;83:136. doi:10.4103/0378-6323.192961
- Berbis P, Fabre JF, Jancovici E, et al. Late-onset syringomas of the upper extremities associated with a carcinoid tumor. Arch Dermatol. 1989;125:848-849.
- Metze D, Jurecka W, Gebhart W. Disseminated syringomas of the upper extremities. case history and immunohistochemical and ultrastructural study. Dermatologica. 1990;180:228-235. doi:10.1159/000248036
- Gómez-de Castro C, Vivanco Allende B, García-García B. Multiple acral syringomas. siringomas acrales múltiples. Actas Dermosifiliogr (Engl Ed). 2018;109:834-836. doi:10.1016/j.ad.2017.10.014
- Hughes PS, Apisarnthanarax P. Acral syringoma. Arch Dermatol. 1977;113:1435-1436.
- Asai Y, Ishii M, Hamada T. Acral syringoma: electron microscopic studies on its origin. Acta Derm Venereol. 1982;62:64-68.
- van den Broek H, Lundquist CD. Syringomas of the upper extremities with onset in the sixth decade. J Am Acad Dermatol. 1982,6:534-536. doi:10.1016/S0190-9622(82)80368-X
- Garcia C, Krunic AL, Grichnik J, et al. Multiple acral syringomata with uniform involvement of the hands and feet. Cutis. 1997;59:213-214, 216.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Iglesias Sancho M, Serra Llobet J, Salleras Redonnet M, et al. Siringomas disem- inados de inicio acral, aparecidos en la octava década. Actas Dermosifiliofr. 1999;90:253-257.
- Muniesa C, Fortuño Y, Moreno A, et al. Papules on the dorsum of the fingers. Actas Dermosifiliogr. 2008;99:812-813. doi:10.1016 /S1578-2190(08)70371-8
- Koh MJ. Multiple acral syringomas involving the hands. Clin Exp Dermatol. 2009;34:E438. doi:10.1111/j.1365-2230.2009.03462.x
- Karam P, Benedetto AV. Syringomas: new approach to an old technique. Int J Dermatol. 1996;35:219-220. doi:10.1111/j.1365-4362 .1996.tb01647.x
- Wang JI, Roenigk HH. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139. doi:10.1046/j.1524-4725.1999.08111.x
- Gómez MI, Pérez B, Azaña JM, et al. Eruptive syringoma: treatment with topical tretinoin. Dermatology. 2009;189:105-106. doi:10.1159/000246803
THE DIAGNOSIS: Acral Eruptive Syringoma
Syringomas are small, benign, often asymptomatic eccrine tumors that originate in the intraepidermal portion of eccrine sweat ducts.1 Clinically, they present as multiple symmetric white-to-yellow or discrete flesh-colored papules measuring 1 to 3 mm in diameter, often located on the face (most commonly on the eyelids), with a greater prevalence in middle-aged women. Occasionally, they manifest in other locations such as the cheeks, chest, axillae, abdomen, and groin.2
In 1987, Friedman and Butler3 developed a classification system categorizing syringomas into 4 clinical subtypes: familial syringoma, localized syringoma, Down syndrome–related syringoma, and generalized syringoma. The fourth subtype includes the variant of eruptive syringoma,3 a rare clinical manifestation that often develops before or during puberty with several flesh-colored or lightly pigmented papules on the neck, anterior chest, upper abdomen, axillae, periumbilical region, and/or genital region.1,4,5 The etiology of eruptive syringomas is unclear, although it has been linked to abnormal proliferation of sweat glands due to an underlying local inflammatory process.6
Acral distribution of syringomas is a rare variant that can manifest as part of generalized eruptive syringoma with consequent involvement of the arms and other areas.5,7 There are limited case reports on eruptive syringomas with predominant acral distribution.8 Compared to classic syringomas, the acral variant is associated with an older age of onset as well as a similar prevalence between men and women.9 Acral eruptive syringoma (AES) usually is isolated to the distal arms and legs. The most commonly affected region is the anterior surface of the forearms, although involvement of the dorsal hands, wrists, and feet also has been reported.10-16
The first known case of AES, which was reported in 1977, described eruptive syringomas on the dorsal hands of a healthy 31-year-old man.17 Several cases have been reported since then, mostly in patients aged 30 to 60 years, with predominant involvement of the dorsal hands and forearms.18-24 A review of Embase as well as PubMed articles indexed for MEDLINE using the search terms syringoma OR eccrine ductal tumor and eruptive OR acral OR arms OR forearms OR extremities identified 19 reported cases of AES between 1977 and 2023. For the reported AES cases, the mean (SD) age at diagnosis was 45.1 years (15.96 years), with patient ages ranging from 19 to 76 years. Notably, most cases occurred in individuals aged between 30 and 60 years, which deviates from the typical age of onset of localized syringomas, commonly seen during puberty or early adulthood.
Currently, AES is categorized within the clinical presentation of eruptive syringoma. Nevertheless, some authors have proposed classifying it as a distinct fifth clinical group due to specific features that distinguish it from generalized eruptive syringoma.9 This reclassification has considerable implications for the differential diagnosis, particularly because exclusive acral involvement poses a substantial diagnostic challenge and often requires histologic confirmation.
As shown in the Figure, histopathologic examination revealed tubular structures in the upper dermis with characteristic comma-shaped extensions. Some of these structures were lined with cuboidal cells and contained eosinophilic material within the lumen. There was no involvement of the epidermis or deeper dermis. The histologic features were consistent with syringoma, which is distinguished by its predominant involvement of the upper dermis and the presence of enlarged, dilated eccrine ducts, as observed in our case.

Treatment of syringomas often is challenging due to the high rate of recurrence and the risk for postinflammatory hyperpigmentation. Since the condition is benign, treatment typically is pursued for aesthetic reasons. Various therapeutic approaches have been reported, each with diverse response rates. The most common method involves surgical intervention, either with electrodesiccation or CO2 laser—both of which have shown satisfactory resolution of lesions without recurrence at 1-year follow-up, with no major scarring reported.25,26 Alternatively, topical management with retinoids daily over a 4-month period leads to flattening of the tumors with no further appearance of new lesions.27 Despite the availability of numerous management options, establishing a first-line treatment remains controversial due to the high risk for recurrence and the variability in the number and location of lesions among individual patients. In our case, given the benign nature of syringomas, the asymptomatic nature of the lesions, the involvement of noncritical aesthetic areas, and the limited response to noninvasive therapeutic options, the patient was informed of the diagnosis, and no further pharmacologic or surgical intervention was pursued.
THE DIAGNOSIS: Acral Eruptive Syringoma
Syringomas are small, benign, often asymptomatic eccrine tumors that originate in the intraepidermal portion of eccrine sweat ducts.1 Clinically, they present as multiple symmetric white-to-yellow or discrete flesh-colored papules measuring 1 to 3 mm in diameter, often located on the face (most commonly on the eyelids), with a greater prevalence in middle-aged women. Occasionally, they manifest in other locations such as the cheeks, chest, axillae, abdomen, and groin.2
In 1987, Friedman and Butler3 developed a classification system categorizing syringomas into 4 clinical subtypes: familial syringoma, localized syringoma, Down syndrome–related syringoma, and generalized syringoma. The fourth subtype includes the variant of eruptive syringoma,3 a rare clinical manifestation that often develops before or during puberty with several flesh-colored or lightly pigmented papules on the neck, anterior chest, upper abdomen, axillae, periumbilical region, and/or genital region.1,4,5 The etiology of eruptive syringomas is unclear, although it has been linked to abnormal proliferation of sweat glands due to an underlying local inflammatory process.6
Acral distribution of syringomas is a rare variant that can manifest as part of generalized eruptive syringoma with consequent involvement of the arms and other areas.5,7 There are limited case reports on eruptive syringomas with predominant acral distribution.8 Compared to classic syringomas, the acral variant is associated with an older age of onset as well as a similar prevalence between men and women.9 Acral eruptive syringoma (AES) usually is isolated to the distal arms and legs. The most commonly affected region is the anterior surface of the forearms, although involvement of the dorsal hands, wrists, and feet also has been reported.10-16
The first known case of AES, which was reported in 1977, described eruptive syringomas on the dorsal hands of a healthy 31-year-old man.17 Several cases have been reported since then, mostly in patients aged 30 to 60 years, with predominant involvement of the dorsal hands and forearms.18-24 A review of Embase as well as PubMed articles indexed for MEDLINE using the search terms syringoma OR eccrine ductal tumor and eruptive OR acral OR arms OR forearms OR extremities identified 19 reported cases of AES between 1977 and 2023. For the reported AES cases, the mean (SD) age at diagnosis was 45.1 years (15.96 years), with patient ages ranging from 19 to 76 years. Notably, most cases occurred in individuals aged between 30 and 60 years, which deviates from the typical age of onset of localized syringomas, commonly seen during puberty or early adulthood.
Currently, AES is categorized within the clinical presentation of eruptive syringoma. Nevertheless, some authors have proposed classifying it as a distinct fifth clinical group due to specific features that distinguish it from generalized eruptive syringoma.9 This reclassification has considerable implications for the differential diagnosis, particularly because exclusive acral involvement poses a substantial diagnostic challenge and often requires histologic confirmation.
As shown in the Figure, histopathologic examination revealed tubular structures in the upper dermis with characteristic comma-shaped extensions. Some of these structures were lined with cuboidal cells and contained eosinophilic material within the lumen. There was no involvement of the epidermis or deeper dermis. The histologic features were consistent with syringoma, which is distinguished by its predominant involvement of the upper dermis and the presence of enlarged, dilated eccrine ducts, as observed in our case.

Treatment of syringomas often is challenging due to the high rate of recurrence and the risk for postinflammatory hyperpigmentation. Since the condition is benign, treatment typically is pursued for aesthetic reasons. Various therapeutic approaches have been reported, each with diverse response rates. The most common method involves surgical intervention, either with electrodesiccation or CO2 laser—both of which have shown satisfactory resolution of lesions without recurrence at 1-year follow-up, with no major scarring reported.25,26 Alternatively, topical management with retinoids daily over a 4-month period leads to flattening of the tumors with no further appearance of new lesions.27 Despite the availability of numerous management options, establishing a first-line treatment remains controversial due to the high risk for recurrence and the variability in the number and location of lesions among individual patients. In our case, given the benign nature of syringomas, the asymptomatic nature of the lesions, the involvement of noncritical aesthetic areas, and the limited response to noninvasive therapeutic options, the patient was informed of the diagnosis, and no further pharmacologic or surgical intervention was pursued.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.E9. doi:10.1016 /j.jaad.2015.12.006
- Resende C, Araújo C, Santos R, et al. Late-onset of eruptive syringomas: a diagnostic challenge. An Bras Dermatol. 2015;90(3 suppl 1):239-241. doi:10.1590/abd1806-4841.20153899
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Avhad G, Ghuge P, Jerajani HR. Generalized eruptive syringoma. Indian J Dermatol. 2015;60:214. doi:10.4103/0019-5154.152586
- Ning WV, Bashey S, Cole C, et al. Multiple eruptive syringomas on the penis. Cutis. 2019;103:E15-E16.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Jamalipour M, Heidarpour M, Rajabi P. Generalized eruptive syringomas. Indian J Dermatol. 2009;54:65-67. doi:10.4103/0019-5154.48992
- Mohaghegh F, Amiri A, Fatemi Naeini F, et al. Acral eruptive syringoma: an unusual presentation with misdiagnosis. Case Rep Dermatol Med. 2020;2020:5416285. doi:10.1155/2020/5416285
- Valdivielso-Ramos M, de la Cueva P, Gimeno M, et al. Acral syringomas. Actas Dermosifiliogr. 2010;101:458-460.
- Patel K, Lundgren AD, Ahmed AM, et al. Disseminated syringomas of the upper extremities in a young woman. Cureus. 2018;10:E3619. doi:10.7759/cureus.3619
- Balci DD, Atik E, Altintas S. Coexistence of acral syringomas and multiple trichoepitheliomas on the face. J Cutan Med Surg. 2009;13:169-171. doi:10.2310/7750.2008.08011
- Martín-García RF, Muñoz CM. Acral syringomas presenting as a photosensitive papular eruption. Cutis. 2006;77:33-36.
- Varas-Meis E, Prada-García C, Samaniego-González E, et al. Acral syringomas associated with hematological neoplasm. Indian J Dermatol Venereol Leprol. 2017;83:136. doi:10.4103/0378-6323.192961
- Berbis P, Fabre JF, Jancovici E, et al. Late-onset syringomas of the upper extremities associated with a carcinoid tumor. Arch Dermatol. 1989;125:848-849.
- Metze D, Jurecka W, Gebhart W. Disseminated syringomas of the upper extremities. case history and immunohistochemical and ultrastructural study. Dermatologica. 1990;180:228-235. doi:10.1159/000248036
- Gómez-de Castro C, Vivanco Allende B, García-García B. Multiple acral syringomas. siringomas acrales múltiples. Actas Dermosifiliogr (Engl Ed). 2018;109:834-836. doi:10.1016/j.ad.2017.10.014
- Hughes PS, Apisarnthanarax P. Acral syringoma. Arch Dermatol. 1977;113:1435-1436.
- Asai Y, Ishii M, Hamada T. Acral syringoma: electron microscopic studies on its origin. Acta Derm Venereol. 1982;62:64-68.
- van den Broek H, Lundquist CD. Syringomas of the upper extremities with onset in the sixth decade. J Am Acad Dermatol. 1982,6:534-536. doi:10.1016/S0190-9622(82)80368-X
- Garcia C, Krunic AL, Grichnik J, et al. Multiple acral syringomata with uniform involvement of the hands and feet. Cutis. 1997;59:213-214, 216.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Iglesias Sancho M, Serra Llobet J, Salleras Redonnet M, et al. Siringomas disem- inados de inicio acral, aparecidos en la octava década. Actas Dermosifiliofr. 1999;90:253-257.
- Muniesa C, Fortuño Y, Moreno A, et al. Papules on the dorsum of the fingers. Actas Dermosifiliogr. 2008;99:812-813. doi:10.1016 /S1578-2190(08)70371-8
- Koh MJ. Multiple acral syringomas involving the hands. Clin Exp Dermatol. 2009;34:E438. doi:10.1111/j.1365-2230.2009.03462.x
- Karam P, Benedetto AV. Syringomas: new approach to an old technique. Int J Dermatol. 1996;35:219-220. doi:10.1111/j.1365-4362 .1996.tb01647.x
- Wang JI, Roenigk HH. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139. doi:10.1046/j.1524-4725.1999.08111.x
- Gómez MI, Pérez B, Azaña JM, et al. Eruptive syringoma: treatment with topical tretinoin. Dermatology. 2009;189:105-106. doi:10.1159/000246803
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.E9. doi:10.1016 /j.jaad.2015.12.006
- Resende C, Araújo C, Santos R, et al. Late-onset of eruptive syringomas: a diagnostic challenge. An Bras Dermatol. 2015;90(3 suppl 1):239-241. doi:10.1590/abd1806-4841.20153899
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Avhad G, Ghuge P, Jerajani HR. Generalized eruptive syringoma. Indian J Dermatol. 2015;60:214. doi:10.4103/0019-5154.152586
- Ning WV, Bashey S, Cole C, et al. Multiple eruptive syringomas on the penis. Cutis. 2019;103:E15-E16.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Jamalipour M, Heidarpour M, Rajabi P. Generalized eruptive syringomas. Indian J Dermatol. 2009;54:65-67. doi:10.4103/0019-5154.48992
- Mohaghegh F, Amiri A, Fatemi Naeini F, et al. Acral eruptive syringoma: an unusual presentation with misdiagnosis. Case Rep Dermatol Med. 2020;2020:5416285. doi:10.1155/2020/5416285
- Valdivielso-Ramos M, de la Cueva P, Gimeno M, et al. Acral syringomas. Actas Dermosifiliogr. 2010;101:458-460.
- Patel K, Lundgren AD, Ahmed AM, et al. Disseminated syringomas of the upper extremities in a young woman. Cureus. 2018;10:E3619. doi:10.7759/cureus.3619
- Balci DD, Atik E, Altintas S. Coexistence of acral syringomas and multiple trichoepitheliomas on the face. J Cutan Med Surg. 2009;13:169-171. doi:10.2310/7750.2008.08011
- Martín-García RF, Muñoz CM. Acral syringomas presenting as a photosensitive papular eruption. Cutis. 2006;77:33-36.
- Varas-Meis E, Prada-García C, Samaniego-González E, et al. Acral syringomas associated with hematological neoplasm. Indian J Dermatol Venereol Leprol. 2017;83:136. doi:10.4103/0378-6323.192961
- Berbis P, Fabre JF, Jancovici E, et al. Late-onset syringomas of the upper extremities associated with a carcinoid tumor. Arch Dermatol. 1989;125:848-849.
- Metze D, Jurecka W, Gebhart W. Disseminated syringomas of the upper extremities. case history and immunohistochemical and ultrastructural study. Dermatologica. 1990;180:228-235. doi:10.1159/000248036
- Gómez-de Castro C, Vivanco Allende B, García-García B. Multiple acral syringomas. siringomas acrales múltiples. Actas Dermosifiliogr (Engl Ed). 2018;109:834-836. doi:10.1016/j.ad.2017.10.014
- Hughes PS, Apisarnthanarax P. Acral syringoma. Arch Dermatol. 1977;113:1435-1436.
- Asai Y, Ishii M, Hamada T. Acral syringoma: electron microscopic studies on its origin. Acta Derm Venereol. 1982;62:64-68.
- van den Broek H, Lundquist CD. Syringomas of the upper extremities with onset in the sixth decade. J Am Acad Dermatol. 1982,6:534-536. doi:10.1016/S0190-9622(82)80368-X
- Garcia C, Krunic AL, Grichnik J, et al. Multiple acral syringomata with uniform involvement of the hands and feet. Cutis. 1997;59:213-214, 216.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Iglesias Sancho M, Serra Llobet J, Salleras Redonnet M, et al. Siringomas disem- inados de inicio acral, aparecidos en la octava década. Actas Dermosifiliofr. 1999;90:253-257.
- Muniesa C, Fortuño Y, Moreno A, et al. Papules on the dorsum of the fingers. Actas Dermosifiliogr. 2008;99:812-813. doi:10.1016 /S1578-2190(08)70371-8
- Koh MJ. Multiple acral syringomas involving the hands. Clin Exp Dermatol. 2009;34:E438. doi:10.1111/j.1365-2230.2009.03462.x
- Karam P, Benedetto AV. Syringomas: new approach to an old technique. Int J Dermatol. 1996;35:219-220. doi:10.1111/j.1365-4362 .1996.tb01647.x
- Wang JI, Roenigk HH. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139. doi:10.1046/j.1524-4725.1999.08111.x
- Gómez MI, Pérez B, Azaña JM, et al. Eruptive syringoma: treatment with topical tretinoin. Dermatology. 2009;189:105-106. doi:10.1159/000246803
Eruptive Erythematous Papules on the Forearms
Eruptive Erythematous Papules on the Forearms
A 44-year-old man presented to the dermatology department with multiple eruptive, nonconfluent, erythematous papules on the anterior forearms of 2 years’ duration. The patient’s medical history was notable for right-sided testicular cancer diagnosed in childhood and 3 excised basal cell carcinomas, the most recent of which was concurrent with the present case. The patient denied any recent pruritus, exposure to irritants, or use of over-the-counter medications. Physical examination was remarkable for numerous monomorphic, symmetric, nonconfluent, flesh-colored to slightly pigmented papules on the dorsal aspect of the forearms. No involvement of the fingers or lower extremities was observed. Two punch biopsies of representative lesions on the right and left forearms were taken. Histopathologic examination revealed eccrine ductal proliferations lined by cuboidal cells embedded within bundles of sclerotic collagen.