User login
Tense Bullae With Widespread Erosions
The Diagnosis: Linear IgA Bullous Dermatosis
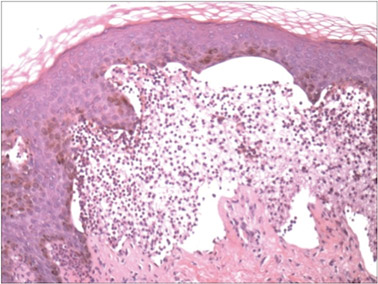
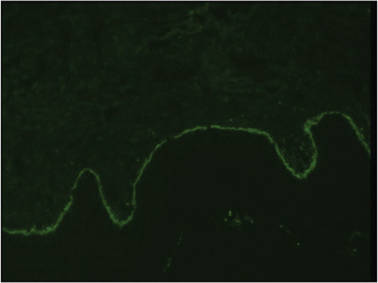
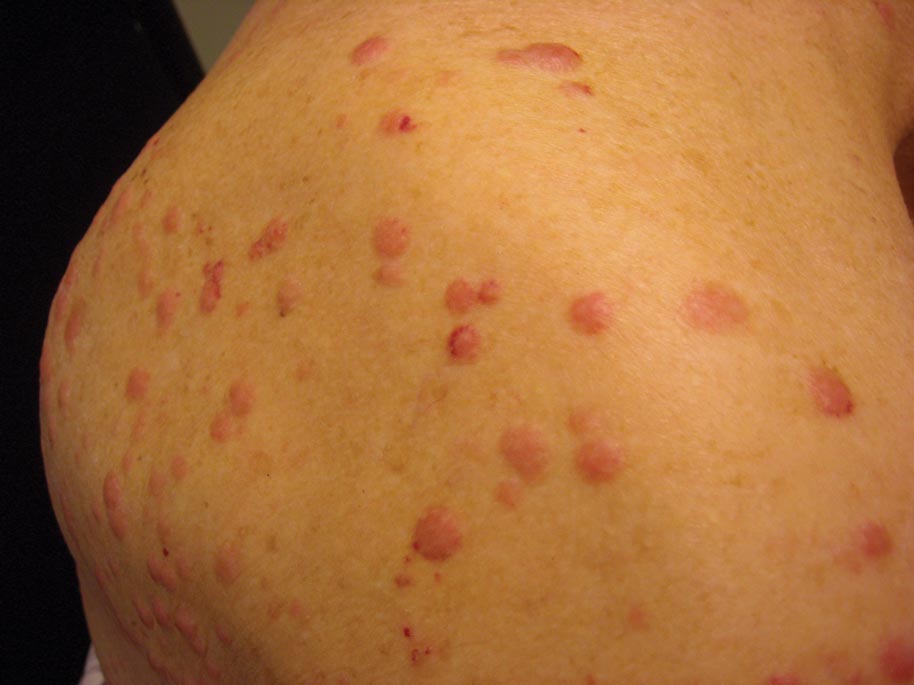
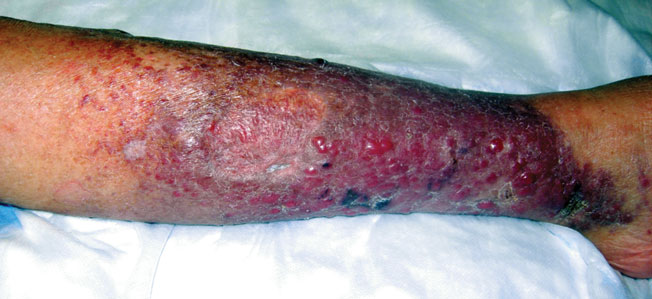
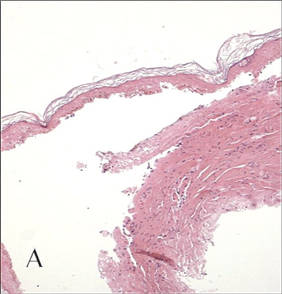
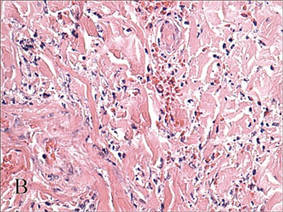
A biopsy specimen from an intact vesicle was obtained. Histologic findings showed a basket weave stratum corneum suggestive of an acute process. There was subepidermal separation with an inflammatory infiltrate of neutrophils (Figure 1). Direct immunofluorescence yielded a pattern of IgA deposition along the dermoepidermal junction (Figure 2). A diagnosis of linear IgA bullous dermatosis (LABD) was made. The patient was started on 100 mg daily of dapsone. The dose was subsequently increased to 175 mg twice daily, resulting in complete clearance. He became dermatologically disease free after 10 months and the dapsone was successfully tapered.
|
Linear IgA bullous dermatosis is an autoimmune subepidermal blistering disease with linear IgA deposits found along the basement membrane of the skin. There are 3 major categories of LABD: drug induced, systemic disorder related, and idiopathic.1 Patients with LABD present with a pruritic vesicobullous eruption that tends to favor the trunk, proximal extremities, and acral regions of the body. Mucous membrane lesions are present in less than 50% of patients.2 Linear IgA bullous dermatosis may resemble bullous pemphigoid, erythema multiforme, dermatitis herpetiformis, or toxic epidermal necrolysis. The gold standard for diagnosis is immunofluorescence staining that shows linear IgA deposition along the skin’s basement membrane.1 Prognosis for LABD is variable; there is risk for persistence and scarring.2 The drug-induced form of LABD is associated with clearance with the removal of the inciting agent.1
There are several autoimmune disorders that have been described in association with human immunodeficiency virus (HIV).3 Autoimmune bullous dermatoses, while described, are very uncommon in the setting of HIV infection. Previously reported cases include bullous pemphigoid, epidermolysis bullosa acquisita, pemphigus herpetiformis, pemphigus vegetans, pemphigus vulgaris, and cicatricial pemphigoid.4-12 The presentation of LABD in an HIV-positive patient is extremely rare.
There are 3 proposed mechanisms by which HIV and autoimmune bullous dermatoses coexist: unregulated B-cell activation, loss of T-suppressor cell regulation, and molecular mimicry. In patients with HIV, infected macrophages increase production of IL-1 and IL-6, causing nonspecific stimulation of B cells. Further production of tumor necrosis factor and other lymphotoxins may kill CD8+ T-suppressor cells, which further reduces B-cell regulation and production of nonspecific antibodies. Unregulated B-cell activation could lead to proliferation of antiself-specific B cells and autoantibodies. Additionally, various autoantibodies may arise due to mimicry between HIV antigens and human proteins. Some of the antibodies produced may be cytotoxic antilymphocyte antibodies that further disrupt B-cell regulation.13,14
Zandman-Goddard and Shoenfeld14 proposed a staging system of autoimmune disease and HIV with respect to CD4 count and viral load. Stage I is clinical latency of HIV, with a high CD4 count (>500 cells/mm3) and high viral load, which correlates with an acute infection of HIV and an intact immune system. Autoimmune disease can be seen in this stage. Stage II is cellular response, a quiescent period without overt manifestations of AIDS. The CD4 count is declining (200–499 cells/mm3), indicating immunosuppression, and the viral count is high. Autoimmune disease can occur and typically includes immune complex–mediated disease and vasculitis. Stage III is immune deficiency. The CD4 count is low (<200 cells/mm3), viral load is high, and AIDS develops. Autoimmune disease is not seen during this stage. Stage IV is the period of immune restoration following the advent of highly active antiretroviral therapy. There is a high CD4 count (>500 cells/mm3) and low viral load. There is a resurgence of autoimmune disease in this stage. Autoimmune disease can occur with an immune system capable of B- and T-cell interactions and a normal CD4 count. Autoimmunity is possible in stages I, II, and IV.14 Our patient developed bullous disease in stage II.
Although uncommon, autoimmune disease is possible in the setting of immune deficiency. The presence of autoimmune disease in a patient with HIV can only be seen during certain stages of infection. Knowledge of the possible scenarios of autoimmune disease can assist the clinician with monitoring status of the HIV infection or immune reconstitution.
1. Bouldin MB, Clowers-Webb HE, Davis JL, et al. Naproxen-associated linear IgA bullous dermatosis: case report and review. Mayo Clin Proc. 2000;75:967-970.
2. Nousari HC, Kimyai-Asadi A, Caeiro JP, et al. Clinical, demographic, and immunohistologic features of vancomycin-induced linear IgA bullous disease of the skin: report of 2 cases and review of the literature. Medicine. 1999;78:1-8.
3. Gala S, Fulcher DA. How HIV leads to autoimmune disorders. Med J Aust. 1996;164:224-226.
4. Lateef A, Packles MR, White SM, et al. Pemphigus vegetans in association with human immunodeficiency virus. Int J Dermatol. 1999;38:778-781.
5. Levy PM, Balavoine JF, Salomon D, et al. Ritodrine-responsive bullous pemphigoing in a patient with AIDS-related complex. Br J Dermatol. 1986;114:635-636.
6. Bull RH, Fallowfield ME, Marsden RA. Autoimmune blistering diseases associated with HIV infection. Clin Exp Dermatol. 1994;19:47-50.
7. Chou K, Kauh YC, Jacoby RA, et al. Autoimmune bullous disease in a patient with HIV infection. J Am Acad Dermatol. 1991;24:1022-1023.
8. Mahé A, Flageul B, Prost C, et al. Pemphigus vegetans in an HIV-1-infected man. Clin Exp Dermatol. 1994;19:447.
9. Capizzi R, Marasca G, De Luca A, et al. Pemphigus vulgaris in a human-immunodeficiency-virus-infected patient. Dermatology. 1998;197:97-98.
10. Splaver A, Silos S, Lowell B, et al. Case report: pemphigus vulgaris in a patient infected with HIV. AIDS Patient Care STDS. 2000;14:295-296.
11. Hodgson TA, Fidler SJ, Speight PM, et al. Oral pemphigus vulgaris associated with HIV infection. J Am Acad Dermatol. 2003;49:313-315.
12. Demathé A, Arede LT, Miyahara GI. Mucous membrane pemphigoid in HIV patient: a case report. Cases J. 2008;1:345.
13. Etzioni A. Immune deficiency and autoimmunity. Autoimmun Rev. 2003;2:364-369.
14. Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
The Diagnosis: Linear IgA Bullous Dermatosis
A biopsy specimen from an intact vesicle was obtained. Histologic findings showed a basket weave stratum corneum suggestive of an acute process. There was subepidermal separation with an inflammatory infiltrate of neutrophils (Figure 1). Direct immunofluorescence yielded a pattern of IgA deposition along the dermoepidermal junction (Figure 2). A diagnosis of linear IgA bullous dermatosis (LABD) was made. The patient was started on 100 mg daily of dapsone. The dose was subsequently increased to 175 mg twice daily, resulting in complete clearance. He became dermatologically disease free after 10 months and the dapsone was successfully tapered.
|
Linear IgA bullous dermatosis is an autoimmune subepidermal blistering disease with linear IgA deposits found along the basement membrane of the skin. There are 3 major categories of LABD: drug induced, systemic disorder related, and idiopathic.1 Patients with LABD present with a pruritic vesicobullous eruption that tends to favor the trunk, proximal extremities, and acral regions of the body. Mucous membrane lesions are present in less than 50% of patients.2 Linear IgA bullous dermatosis may resemble bullous pemphigoid, erythema multiforme, dermatitis herpetiformis, or toxic epidermal necrolysis. The gold standard for diagnosis is immunofluorescence staining that shows linear IgA deposition along the skin’s basement membrane.1 Prognosis for LABD is variable; there is risk for persistence and scarring.2 The drug-induced form of LABD is associated with clearance with the removal of the inciting agent.1
There are several autoimmune disorders that have been described in association with human immunodeficiency virus (HIV).3 Autoimmune bullous dermatoses, while described, are very uncommon in the setting of HIV infection. Previously reported cases include bullous pemphigoid, epidermolysis bullosa acquisita, pemphigus herpetiformis, pemphigus vegetans, pemphigus vulgaris, and cicatricial pemphigoid.4-12 The presentation of LABD in an HIV-positive patient is extremely rare.
There are 3 proposed mechanisms by which HIV and autoimmune bullous dermatoses coexist: unregulated B-cell activation, loss of T-suppressor cell regulation, and molecular mimicry. In patients with HIV, infected macrophages increase production of IL-1 and IL-6, causing nonspecific stimulation of B cells. Further production of tumor necrosis factor and other lymphotoxins may kill CD8+ T-suppressor cells, which further reduces B-cell regulation and production of nonspecific antibodies. Unregulated B-cell activation could lead to proliferation of antiself-specific B cells and autoantibodies. Additionally, various autoantibodies may arise due to mimicry between HIV antigens and human proteins. Some of the antibodies produced may be cytotoxic antilymphocyte antibodies that further disrupt B-cell regulation.13,14
Zandman-Goddard and Shoenfeld14 proposed a staging system of autoimmune disease and HIV with respect to CD4 count and viral load. Stage I is clinical latency of HIV, with a high CD4 count (>500 cells/mm3) and high viral load, which correlates with an acute infection of HIV and an intact immune system. Autoimmune disease can be seen in this stage. Stage II is cellular response, a quiescent period without overt manifestations of AIDS. The CD4 count is declining (200–499 cells/mm3), indicating immunosuppression, and the viral count is high. Autoimmune disease can occur and typically includes immune complex–mediated disease and vasculitis. Stage III is immune deficiency. The CD4 count is low (<200 cells/mm3), viral load is high, and AIDS develops. Autoimmune disease is not seen during this stage. Stage IV is the period of immune restoration following the advent of highly active antiretroviral therapy. There is a high CD4 count (>500 cells/mm3) and low viral load. There is a resurgence of autoimmune disease in this stage. Autoimmune disease can occur with an immune system capable of B- and T-cell interactions and a normal CD4 count. Autoimmunity is possible in stages I, II, and IV.14 Our patient developed bullous disease in stage II.
Although uncommon, autoimmune disease is possible in the setting of immune deficiency. The presence of autoimmune disease in a patient with HIV can only be seen during certain stages of infection. Knowledge of the possible scenarios of autoimmune disease can assist the clinician with monitoring status of the HIV infection or immune reconstitution.
The Diagnosis: Linear IgA Bullous Dermatosis
A biopsy specimen from an intact vesicle was obtained. Histologic findings showed a basket weave stratum corneum suggestive of an acute process. There was subepidermal separation with an inflammatory infiltrate of neutrophils (Figure 1). Direct immunofluorescence yielded a pattern of IgA deposition along the dermoepidermal junction (Figure 2). A diagnosis of linear IgA bullous dermatosis (LABD) was made. The patient was started on 100 mg daily of dapsone. The dose was subsequently increased to 175 mg twice daily, resulting in complete clearance. He became dermatologically disease free after 10 months and the dapsone was successfully tapered.
|
Linear IgA bullous dermatosis is an autoimmune subepidermal blistering disease with linear IgA deposits found along the basement membrane of the skin. There are 3 major categories of LABD: drug induced, systemic disorder related, and idiopathic.1 Patients with LABD present with a pruritic vesicobullous eruption that tends to favor the trunk, proximal extremities, and acral regions of the body. Mucous membrane lesions are present in less than 50% of patients.2 Linear IgA bullous dermatosis may resemble bullous pemphigoid, erythema multiforme, dermatitis herpetiformis, or toxic epidermal necrolysis. The gold standard for diagnosis is immunofluorescence staining that shows linear IgA deposition along the skin’s basement membrane.1 Prognosis for LABD is variable; there is risk for persistence and scarring.2 The drug-induced form of LABD is associated with clearance with the removal of the inciting agent.1
There are several autoimmune disorders that have been described in association with human immunodeficiency virus (HIV).3 Autoimmune bullous dermatoses, while described, are very uncommon in the setting of HIV infection. Previously reported cases include bullous pemphigoid, epidermolysis bullosa acquisita, pemphigus herpetiformis, pemphigus vegetans, pemphigus vulgaris, and cicatricial pemphigoid.4-12 The presentation of LABD in an HIV-positive patient is extremely rare.
There are 3 proposed mechanisms by which HIV and autoimmune bullous dermatoses coexist: unregulated B-cell activation, loss of T-suppressor cell regulation, and molecular mimicry. In patients with HIV, infected macrophages increase production of IL-1 and IL-6, causing nonspecific stimulation of B cells. Further production of tumor necrosis factor and other lymphotoxins may kill CD8+ T-suppressor cells, which further reduces B-cell regulation and production of nonspecific antibodies. Unregulated B-cell activation could lead to proliferation of antiself-specific B cells and autoantibodies. Additionally, various autoantibodies may arise due to mimicry between HIV antigens and human proteins. Some of the antibodies produced may be cytotoxic antilymphocyte antibodies that further disrupt B-cell regulation.13,14
Zandman-Goddard and Shoenfeld14 proposed a staging system of autoimmune disease and HIV with respect to CD4 count and viral load. Stage I is clinical latency of HIV, with a high CD4 count (>500 cells/mm3) and high viral load, which correlates with an acute infection of HIV and an intact immune system. Autoimmune disease can be seen in this stage. Stage II is cellular response, a quiescent period without overt manifestations of AIDS. The CD4 count is declining (200–499 cells/mm3), indicating immunosuppression, and the viral count is high. Autoimmune disease can occur and typically includes immune complex–mediated disease and vasculitis. Stage III is immune deficiency. The CD4 count is low (<200 cells/mm3), viral load is high, and AIDS develops. Autoimmune disease is not seen during this stage. Stage IV is the period of immune restoration following the advent of highly active antiretroviral therapy. There is a high CD4 count (>500 cells/mm3) and low viral load. There is a resurgence of autoimmune disease in this stage. Autoimmune disease can occur with an immune system capable of B- and T-cell interactions and a normal CD4 count. Autoimmunity is possible in stages I, II, and IV.14 Our patient developed bullous disease in stage II.
Although uncommon, autoimmune disease is possible in the setting of immune deficiency. The presence of autoimmune disease in a patient with HIV can only be seen during certain stages of infection. Knowledge of the possible scenarios of autoimmune disease can assist the clinician with monitoring status of the HIV infection or immune reconstitution.
1. Bouldin MB, Clowers-Webb HE, Davis JL, et al. Naproxen-associated linear IgA bullous dermatosis: case report and review. Mayo Clin Proc. 2000;75:967-970.
2. Nousari HC, Kimyai-Asadi A, Caeiro JP, et al. Clinical, demographic, and immunohistologic features of vancomycin-induced linear IgA bullous disease of the skin: report of 2 cases and review of the literature. Medicine. 1999;78:1-8.
3. Gala S, Fulcher DA. How HIV leads to autoimmune disorders. Med J Aust. 1996;164:224-226.
4. Lateef A, Packles MR, White SM, et al. Pemphigus vegetans in association with human immunodeficiency virus. Int J Dermatol. 1999;38:778-781.
5. Levy PM, Balavoine JF, Salomon D, et al. Ritodrine-responsive bullous pemphigoing in a patient with AIDS-related complex. Br J Dermatol. 1986;114:635-636.
6. Bull RH, Fallowfield ME, Marsden RA. Autoimmune blistering diseases associated with HIV infection. Clin Exp Dermatol. 1994;19:47-50.
7. Chou K, Kauh YC, Jacoby RA, et al. Autoimmune bullous disease in a patient with HIV infection. J Am Acad Dermatol. 1991;24:1022-1023.
8. Mahé A, Flageul B, Prost C, et al. Pemphigus vegetans in an HIV-1-infected man. Clin Exp Dermatol. 1994;19:447.
9. Capizzi R, Marasca G, De Luca A, et al. Pemphigus vulgaris in a human-immunodeficiency-virus-infected patient. Dermatology. 1998;197:97-98.
10. Splaver A, Silos S, Lowell B, et al. Case report: pemphigus vulgaris in a patient infected with HIV. AIDS Patient Care STDS. 2000;14:295-296.
11. Hodgson TA, Fidler SJ, Speight PM, et al. Oral pemphigus vulgaris associated with HIV infection. J Am Acad Dermatol. 2003;49:313-315.
12. Demathé A, Arede LT, Miyahara GI. Mucous membrane pemphigoid in HIV patient: a case report. Cases J. 2008;1:345.
13. Etzioni A. Immune deficiency and autoimmunity. Autoimmun Rev. 2003;2:364-369.
14. Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
1. Bouldin MB, Clowers-Webb HE, Davis JL, et al. Naproxen-associated linear IgA bullous dermatosis: case report and review. Mayo Clin Proc. 2000;75:967-970.
2. Nousari HC, Kimyai-Asadi A, Caeiro JP, et al. Clinical, demographic, and immunohistologic features of vancomycin-induced linear IgA bullous disease of the skin: report of 2 cases and review of the literature. Medicine. 1999;78:1-8.
3. Gala S, Fulcher DA. How HIV leads to autoimmune disorders. Med J Aust. 1996;164:224-226.
4. Lateef A, Packles MR, White SM, et al. Pemphigus vegetans in association with human immunodeficiency virus. Int J Dermatol. 1999;38:778-781.
5. Levy PM, Balavoine JF, Salomon D, et al. Ritodrine-responsive bullous pemphigoing in a patient with AIDS-related complex. Br J Dermatol. 1986;114:635-636.
6. Bull RH, Fallowfield ME, Marsden RA. Autoimmune blistering diseases associated with HIV infection. Clin Exp Dermatol. 1994;19:47-50.
7. Chou K, Kauh YC, Jacoby RA, et al. Autoimmune bullous disease in a patient with HIV infection. J Am Acad Dermatol. 1991;24:1022-1023.
8. Mahé A, Flageul B, Prost C, et al. Pemphigus vegetans in an HIV-1-infected man. Clin Exp Dermatol. 1994;19:447.
9. Capizzi R, Marasca G, De Luca A, et al. Pemphigus vulgaris in a human-immunodeficiency-virus-infected patient. Dermatology. 1998;197:97-98.
10. Splaver A, Silos S, Lowell B, et al. Case report: pemphigus vulgaris in a patient infected with HIV. AIDS Patient Care STDS. 2000;14:295-296.
11. Hodgson TA, Fidler SJ, Speight PM, et al. Oral pemphigus vulgaris associated with HIV infection. J Am Acad Dermatol. 2003;49:313-315.
12. Demathé A, Arede LT, Miyahara GI. Mucous membrane pemphigoid in HIV patient: a case report. Cases J. 2008;1:345.
13. Etzioni A. Immune deficiency and autoimmunity. Autoimmun Rev. 2003;2:364-369.
14. Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
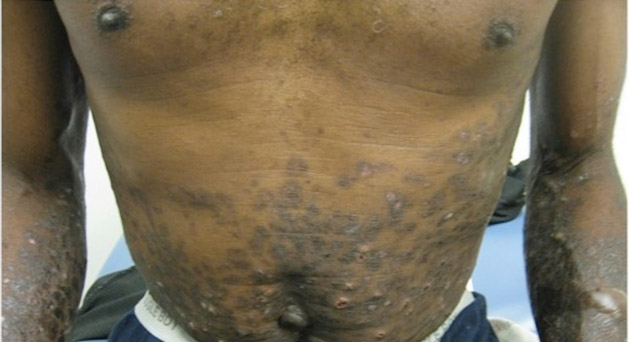
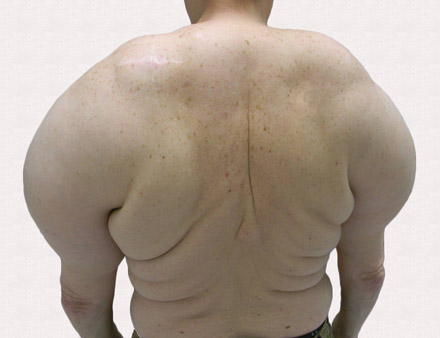
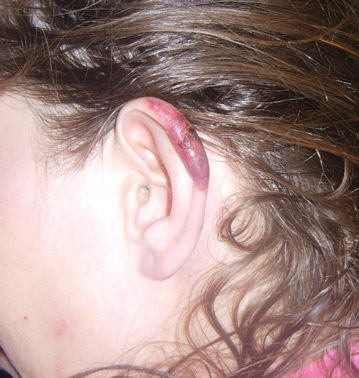
A 50-year-old black man presented with a new-onset widespread pruritic bullous eruption 7 months after being diagnosed with human immunodeficiency virus. The CD4 lymphocyte count was 421 cells/mm3 and viral load was 7818 copies/mL. Results of a viral culture were negative for herpes simplex virus. Dermatologic examination revealed numerous intact tense bullae as well as scattered erosions on the trunk and extremities. Postinflammatory hyperpigmentation was prominent, with some areas of hypopigmentation and depigmentation.
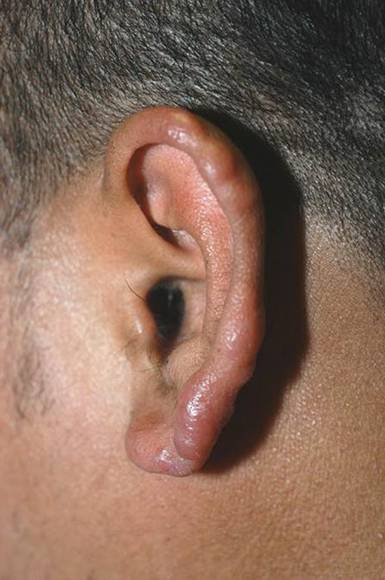
Lobular-Appearing Nodule on the Scalp
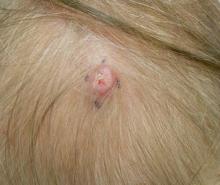
The Diagnosis: Dermal Cylindroma
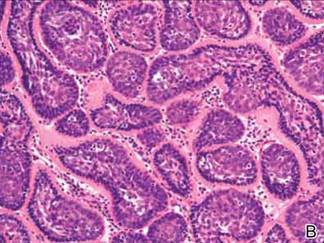
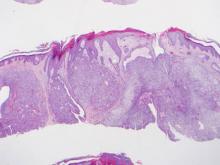
Microsopic evaluation of a tangential biopsy revealed findings of a dermal process consisting of well-circumscribed islands of pale and darker blue cells with little cytoplasm outlined by a hyaline basement membrane (Figure). These cellular islands were arranged in a jigsawlike configuration. These findings were thought to be consistent with a diagnosis of cylindroma.
|
Cylindromas are benign appendageal neoplasms with a somewhat controversial histogenesis. Munger and colleagues1 investigated the pattern of acid mucopolysaccharide secretion by these tumors in association with prosecretory vacuoles in proximity to the Golgi apparatus, which led to their impression that cylindromas most resemble eccrine rather than apocrine sweat glands. Other researchers, however, have concluded that cylindromas are of apocrine derivation.2
Clinically, cylindromas appear most often in 2 settings: isolated or as a manifestation of one of several inherited familial syndromes. One such syndrome is familial cylindromatosis, a rare autosomal-dominant disorder in which affected individuals develop multiple cylindromas, usually on the head and neck. The merging of multiple lesions gives rise to the often-employed term turban tumor.3 This syndrome has been linked to mutations in the cylindromatosis gene, CYLD.4 Brooke-Spiegler syndrome also has been associated with the development of multiple cylindromas. Similar to familial cylindromatosis, it is inherited in an autosomal-dominant fashion. Brooke-Spiegler syndrome is typified by the appearance of multiple cylindromas, trichoepitheliomas, and less commonly spiradenomas. Mutations in the CYLD gene also have been linked to Brooke-Spiegler syndrome in some cases.5
Although considered a benign entity, in rare cases cylindromas have shown evidence of malignant transformation to cylindrocarcinoma. This more aggressive tumor may occur in the setting of isolated cylindromas or more commonly in individuals with numerous lesions, as with both familial cylindromatosis and Brooke-Spiegler syndrome. These lesions may appear to grow rapidly, ulcerate, or bleed, traits that are not associated with their benign counterparts.
Diagnosis of cylindromas rests on histopathologic confirmation, which demonstrates well-defined dermal islands of epithelial cells comprised of dark- and pale-staining nuclei. These tumor islands are surrounded by a hyaline basement membrane and often take on the appearance of a jigsaw puzzle. Cylindrocarcinomas exhibit greater cellular pleomorphism and higher mitotic rates.
Dermal cylindromas require no further treatment but can be electively excised, while treatment of cylindrocarcinoma with excision is curative.6 Definitive excision was offered to our patient, but she declined treatment.
1. Munger BL, Graham JH, Helwig EB. Ultrastructure and histochemical characteristics of dermal eccrine cylindroma (turban tumor). J Invest Dermatol. 1962;39:577-595.
2. Tellechea O, Reis JP, Ilheu O, et al. Dermal cylindroma. an immunohistochemical study of thirteen cases. Am J Dermatopathol. 1995;17:260-265.
3. Biggs PJ, Wooster R, Ford D, et al. Familial cylindromatosis (turban tumour syndrome) gene localised to chromosome 16q12-q13: evidence for its role as a tumour suppressor gene. Nat Genet. 1995;11:441-443.
4. Bignell GR, Warren W, Seal S, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160-165.
5. Bowen S, Gill M, Lee DA, et al. Mutations in the CYLD gene in Brooke-Spiegler syndrome, familial cylindromatosis, and multiple familial trichoepithelioma: lack of genotype-phenotype correlation. J Invest Dermatol. 2005;124:919-920.
6. Gerretsen AL, van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
The Diagnosis: Dermal Cylindroma
Microsopic evaluation of a tangential biopsy revealed findings of a dermal process consisting of well-circumscribed islands of pale and darker blue cells with little cytoplasm outlined by a hyaline basement membrane (Figure). These cellular islands were arranged in a jigsawlike configuration. These findings were thought to be consistent with a diagnosis of cylindroma.
|
Cylindromas are benign appendageal neoplasms with a somewhat controversial histogenesis. Munger and colleagues1 investigated the pattern of acid mucopolysaccharide secretion by these tumors in association with prosecretory vacuoles in proximity to the Golgi apparatus, which led to their impression that cylindromas most resemble eccrine rather than apocrine sweat glands. Other researchers, however, have concluded that cylindromas are of apocrine derivation.2
Clinically, cylindromas appear most often in 2 settings: isolated or as a manifestation of one of several inherited familial syndromes. One such syndrome is familial cylindromatosis, a rare autosomal-dominant disorder in which affected individuals develop multiple cylindromas, usually on the head and neck. The merging of multiple lesions gives rise to the often-employed term turban tumor.3 This syndrome has been linked to mutations in the cylindromatosis gene, CYLD.4 Brooke-Spiegler syndrome also has been associated with the development of multiple cylindromas. Similar to familial cylindromatosis, it is inherited in an autosomal-dominant fashion. Brooke-Spiegler syndrome is typified by the appearance of multiple cylindromas, trichoepitheliomas, and less commonly spiradenomas. Mutations in the CYLD gene also have been linked to Brooke-Spiegler syndrome in some cases.5
Although considered a benign entity, in rare cases cylindromas have shown evidence of malignant transformation to cylindrocarcinoma. This more aggressive tumor may occur in the setting of isolated cylindromas or more commonly in individuals with numerous lesions, as with both familial cylindromatosis and Brooke-Spiegler syndrome. These lesions may appear to grow rapidly, ulcerate, or bleed, traits that are not associated with their benign counterparts.
Diagnosis of cylindromas rests on histopathologic confirmation, which demonstrates well-defined dermal islands of epithelial cells comprised of dark- and pale-staining nuclei. These tumor islands are surrounded by a hyaline basement membrane and often take on the appearance of a jigsaw puzzle. Cylindrocarcinomas exhibit greater cellular pleomorphism and higher mitotic rates.
Dermal cylindromas require no further treatment but can be electively excised, while treatment of cylindrocarcinoma with excision is curative.6 Definitive excision was offered to our patient, but she declined treatment.
The Diagnosis: Dermal Cylindroma
Microsopic evaluation of a tangential biopsy revealed findings of a dermal process consisting of well-circumscribed islands of pale and darker blue cells with little cytoplasm outlined by a hyaline basement membrane (Figure). These cellular islands were arranged in a jigsawlike configuration. These findings were thought to be consistent with a diagnosis of cylindroma.
|
Cylindromas are benign appendageal neoplasms with a somewhat controversial histogenesis. Munger and colleagues1 investigated the pattern of acid mucopolysaccharide secretion by these tumors in association with prosecretory vacuoles in proximity to the Golgi apparatus, which led to their impression that cylindromas most resemble eccrine rather than apocrine sweat glands. Other researchers, however, have concluded that cylindromas are of apocrine derivation.2
Clinically, cylindromas appear most often in 2 settings: isolated or as a manifestation of one of several inherited familial syndromes. One such syndrome is familial cylindromatosis, a rare autosomal-dominant disorder in which affected individuals develop multiple cylindromas, usually on the head and neck. The merging of multiple lesions gives rise to the often-employed term turban tumor.3 This syndrome has been linked to mutations in the cylindromatosis gene, CYLD.4 Brooke-Spiegler syndrome also has been associated with the development of multiple cylindromas. Similar to familial cylindromatosis, it is inherited in an autosomal-dominant fashion. Brooke-Spiegler syndrome is typified by the appearance of multiple cylindromas, trichoepitheliomas, and less commonly spiradenomas. Mutations in the CYLD gene also have been linked to Brooke-Spiegler syndrome in some cases.5
Although considered a benign entity, in rare cases cylindromas have shown evidence of malignant transformation to cylindrocarcinoma. This more aggressive tumor may occur in the setting of isolated cylindromas or more commonly in individuals with numerous lesions, as with both familial cylindromatosis and Brooke-Spiegler syndrome. These lesions may appear to grow rapidly, ulcerate, or bleed, traits that are not associated with their benign counterparts.
Diagnosis of cylindromas rests on histopathologic confirmation, which demonstrates well-defined dermal islands of epithelial cells comprised of dark- and pale-staining nuclei. These tumor islands are surrounded by a hyaline basement membrane and often take on the appearance of a jigsaw puzzle. Cylindrocarcinomas exhibit greater cellular pleomorphism and higher mitotic rates.
Dermal cylindromas require no further treatment but can be electively excised, while treatment of cylindrocarcinoma with excision is curative.6 Definitive excision was offered to our patient, but she declined treatment.
1. Munger BL, Graham JH, Helwig EB. Ultrastructure and histochemical characteristics of dermal eccrine cylindroma (turban tumor). J Invest Dermatol. 1962;39:577-595.
2. Tellechea O, Reis JP, Ilheu O, et al. Dermal cylindroma. an immunohistochemical study of thirteen cases. Am J Dermatopathol. 1995;17:260-265.
3. Biggs PJ, Wooster R, Ford D, et al. Familial cylindromatosis (turban tumour syndrome) gene localised to chromosome 16q12-q13: evidence for its role as a tumour suppressor gene. Nat Genet. 1995;11:441-443.
4. Bignell GR, Warren W, Seal S, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160-165.
5. Bowen S, Gill M, Lee DA, et al. Mutations in the CYLD gene in Brooke-Spiegler syndrome, familial cylindromatosis, and multiple familial trichoepithelioma: lack of genotype-phenotype correlation. J Invest Dermatol. 2005;124:919-920.
6. Gerretsen AL, van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
1. Munger BL, Graham JH, Helwig EB. Ultrastructure and histochemical characteristics of dermal eccrine cylindroma (turban tumor). J Invest Dermatol. 1962;39:577-595.
2. Tellechea O, Reis JP, Ilheu O, et al. Dermal cylindroma. an immunohistochemical study of thirteen cases. Am J Dermatopathol. 1995;17:260-265.
3. Biggs PJ, Wooster R, Ford D, et al. Familial cylindromatosis (turban tumour syndrome) gene localised to chromosome 16q12-q13: evidence for its role as a tumour suppressor gene. Nat Genet. 1995;11:441-443.
4. Bignell GR, Warren W, Seal S, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160-165.
5. Bowen S, Gill M, Lee DA, et al. Mutations in the CYLD gene in Brooke-Spiegler syndrome, familial cylindromatosis, and multiple familial trichoepithelioma: lack of genotype-phenotype correlation. J Invest Dermatol. 2005;124:919-920.
6. Gerretsen AL, van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
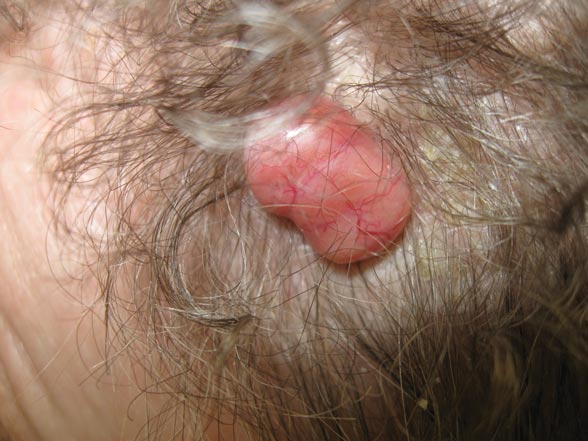
A 79-year-old woman presented with a lesion on the left side of the scalp of several years’ duration that had slowly increased in size. Despite its growth, the lesion remained asymptomatic. Physical examination revealed an exophytic, lobular-appearing nodule on the left side of the temporoparietal scalp, measuring 1.5 cm in size.
Lesions With a Distinct Fingerprint Presentation
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
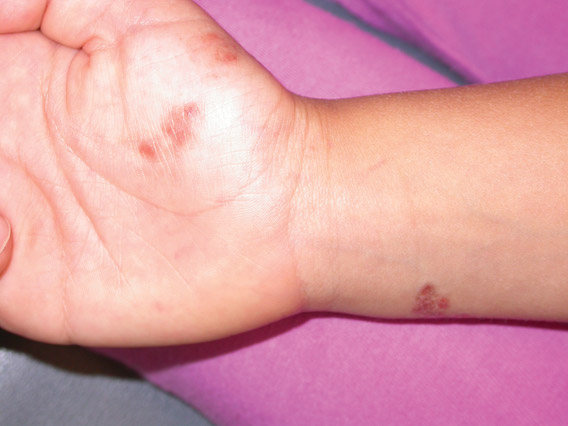
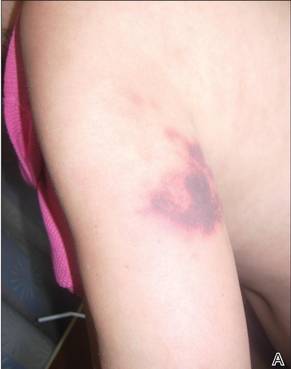
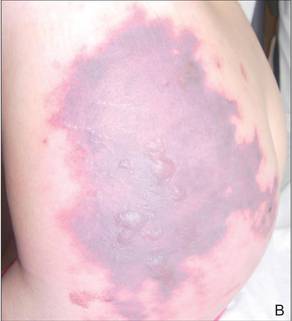
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.

A 17-year-old adolescent girl presented with scattered brown macules over the dorsal aspect of the hands bilaterally and a brown patch in the shape of a hand on the right upper arm of 3 weeks’ duration.
What Is Your Diagnosis? Lepromatous Leprosy
The Diagnosis: Lepromatous Leprosy
Histopathologic examination of a punch biopsy specimen (Figures 1 and 2) disclosed a grenz zone and a diffuse infiltrative process beneath a normal-appearing epidermis. Higher-power examination revealed areas containing macrophages (Virchow cells) with cloudy regions devoid of nuclei (globi). Fite stain demonstrated numerous intracytoplasmic acid-fast bacilli (Figure 3). Laboratory test results for rapid plasma reagin and human immunodeficiency virus were negative, and a complete blood cell count was normal.



On further questioning the patient revealed he was an immigrant from Micronesia, and he described decreased sensation and numbness in the lesions that had been present from onset. Physical examination was consistent with this history and revealed hypoesthesia of the lesions, particularly over the central aspect of the depigmented macules. Based on the clinical examination and histopathologic findings, a diagnosis of lepromatous leprosy was made.
Therapy with rifampin, clofazimine, and dapsone was initiated. Unfortunately, compliance was poor, and at clinic follow-up 10 months later the patient demonstrated formation of new indurated lesions as well as mild eyelid swelling and edema of the hands thought to be consistent with erythema nodosum leprosum. Prednisone was then initiated and the dose of clofazimine was increased from 50 mg daily to 100 mg daily with excellent clinical response.
Mycobacterium leprae is a small, slightly curved rod that is an acid-fast, obligate, intracellular organism. It remains endemic in Brazil and Southeast Asia but may present outside of these areas secondary to immigration.1
Hallmarks of the disease are anesthetic skin or mucous membrane lesions with thickened peripheral nerves.2 It grows best at 27°C to 33°C, thereby affecting cooler areas of the human body such as earlobes, knees, and distal extremities.3 It is most likely spread by aerosolized respiratory droplets and less commonly by direct contact. There have been reports suggesting transmission via armadillos.4
Genetic susceptibility influences the development of leprosy, while HLA type influences the immune response and hence the type of leprosy.5 Ridley and Jopling6 devised a classification system based on the immunologic response to M leprae. Highly reactive hosts with a vigorous cell-mediated response to M leprae develop tuberculoid leprosy and exhibit few skin lesions containing rare organisms. In contrast, anergic hosts develop lepromatous leprosy, characterized by multiple skin lesions, abundant organisms, and diffuse disease. Borderline tuberculoid, borderline, and borderline lepromatous make up the middle of the spectrum.6-9 Skin lesions can present with poorly defined, hypopigmented macules of indeterminate leprosy on one end and diffuse skin involvement of lepromatous leprosy on the opposite end. Diffuse involvement includes facial skin thickening, classic leonine facies, loss of eyebrows and eyelashes, anesthetic lesions, and anhidrosis.
Erythema nodosum leprosum occurs with chronic infection from M leprae, most commonly lepromatous leprosy. Immune complex deposition results in vasculitis and inflammatory foci. This phenomenon is thought to be secondary to high antigen load released by dying mycobacteria, causing secretion of tumor necrosis factor a from macrophages.1 Erythema nodosum leprosum demonstrates rapid onset of tender erythematous plaques or nodules, most commonly on the face and extensor surfaces of the extremities, with fever, malaise, iritis, arthralgia, and orchitis. Clofazimine therapy probably decreases the occurence.1 Treatment includes systemic corticosteroids and/or thalidomide.
- Moschella S, Ooi W. Update on leprosy in immigrants in the United States: status in the year 2000. Clin Infect Dis. 2001;32:930-937.
- Abraham S, Job C, Joseph G, et al. Epidemiological significance of first skin lesion in leprosy. Int J Lep Other Mycobact Dis. 1998;66:131-139.
- Shepard C. The experimental disease that follows the injection of human bacilli into footpads of mice. J Exp Med. 1960;112:445-454.
- Leprosy: global target attained. Wkly Epidemiol Rec. 2001;20:155-156.
- World Health Organization. Global leprosy situation, 2005. Wkly Epidemiol Rec. 2005;80:289-295.
- Ridley DS, Jopling WH. A classification of leprosy for research purposes. Lepr Rev. 1962;33:119-128.
- Lane J. Borderline tuberculoid leprosy in a woman from the state of Georgia with armadillo exposure. J Am Acad Dermatol. 2006;55:714-716.
- Fitness J, Tosh K, Hill AV. Genetics of susceptibility to leprosy. Genes Immun. 2002;3:441-453.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
The Diagnosis: Lepromatous Leprosy
Histopathologic examination of a punch biopsy specimen (Figures 1 and 2) disclosed a grenz zone and a diffuse infiltrative process beneath a normal-appearing epidermis. Higher-power examination revealed areas containing macrophages (Virchow cells) with cloudy regions devoid of nuclei (globi). Fite stain demonstrated numerous intracytoplasmic acid-fast bacilli (Figure 3). Laboratory test results for rapid plasma reagin and human immunodeficiency virus were negative, and a complete blood cell count was normal.



On further questioning the patient revealed he was an immigrant from Micronesia, and he described decreased sensation and numbness in the lesions that had been present from onset. Physical examination was consistent with this history and revealed hypoesthesia of the lesions, particularly over the central aspect of the depigmented macules. Based on the clinical examination and histopathologic findings, a diagnosis of lepromatous leprosy was made.
Therapy with rifampin, clofazimine, and dapsone was initiated. Unfortunately, compliance was poor, and at clinic follow-up 10 months later the patient demonstrated formation of new indurated lesions as well as mild eyelid swelling and edema of the hands thought to be consistent with erythema nodosum leprosum. Prednisone was then initiated and the dose of clofazimine was increased from 50 mg daily to 100 mg daily with excellent clinical response.
Mycobacterium leprae is a small, slightly curved rod that is an acid-fast, obligate, intracellular organism. It remains endemic in Brazil and Southeast Asia but may present outside of these areas secondary to immigration.1
Hallmarks of the disease are anesthetic skin or mucous membrane lesions with thickened peripheral nerves.2 It grows best at 27°C to 33°C, thereby affecting cooler areas of the human body such as earlobes, knees, and distal extremities.3 It is most likely spread by aerosolized respiratory droplets and less commonly by direct contact. There have been reports suggesting transmission via armadillos.4
Genetic susceptibility influences the development of leprosy, while HLA type influences the immune response and hence the type of leprosy.5 Ridley and Jopling6 devised a classification system based on the immunologic response to M leprae. Highly reactive hosts with a vigorous cell-mediated response to M leprae develop tuberculoid leprosy and exhibit few skin lesions containing rare organisms. In contrast, anergic hosts develop lepromatous leprosy, characterized by multiple skin lesions, abundant organisms, and diffuse disease. Borderline tuberculoid, borderline, and borderline lepromatous make up the middle of the spectrum.6-9 Skin lesions can present with poorly defined, hypopigmented macules of indeterminate leprosy on one end and diffuse skin involvement of lepromatous leprosy on the opposite end. Diffuse involvement includes facial skin thickening, classic leonine facies, loss of eyebrows and eyelashes, anesthetic lesions, and anhidrosis.
Erythema nodosum leprosum occurs with chronic infection from M leprae, most commonly lepromatous leprosy. Immune complex deposition results in vasculitis and inflammatory foci. This phenomenon is thought to be secondary to high antigen load released by dying mycobacteria, causing secretion of tumor necrosis factor a from macrophages.1 Erythema nodosum leprosum demonstrates rapid onset of tender erythematous plaques or nodules, most commonly on the face and extensor surfaces of the extremities, with fever, malaise, iritis, arthralgia, and orchitis. Clofazimine therapy probably decreases the occurence.1 Treatment includes systemic corticosteroids and/or thalidomide.
The Diagnosis: Lepromatous Leprosy
Histopathologic examination of a punch biopsy specimen (Figures 1 and 2) disclosed a grenz zone and a diffuse infiltrative process beneath a normal-appearing epidermis. Higher-power examination revealed areas containing macrophages (Virchow cells) with cloudy regions devoid of nuclei (globi). Fite stain demonstrated numerous intracytoplasmic acid-fast bacilli (Figure 3). Laboratory test results for rapid plasma reagin and human immunodeficiency virus were negative, and a complete blood cell count was normal.



On further questioning the patient revealed he was an immigrant from Micronesia, and he described decreased sensation and numbness in the lesions that had been present from onset. Physical examination was consistent with this history and revealed hypoesthesia of the lesions, particularly over the central aspect of the depigmented macules. Based on the clinical examination and histopathologic findings, a diagnosis of lepromatous leprosy was made.
Therapy with rifampin, clofazimine, and dapsone was initiated. Unfortunately, compliance was poor, and at clinic follow-up 10 months later the patient demonstrated formation of new indurated lesions as well as mild eyelid swelling and edema of the hands thought to be consistent with erythema nodosum leprosum. Prednisone was then initiated and the dose of clofazimine was increased from 50 mg daily to 100 mg daily with excellent clinical response.
Mycobacterium leprae is a small, slightly curved rod that is an acid-fast, obligate, intracellular organism. It remains endemic in Brazil and Southeast Asia but may present outside of these areas secondary to immigration.1
Hallmarks of the disease are anesthetic skin or mucous membrane lesions with thickened peripheral nerves.2 It grows best at 27°C to 33°C, thereby affecting cooler areas of the human body such as earlobes, knees, and distal extremities.3 It is most likely spread by aerosolized respiratory droplets and less commonly by direct contact. There have been reports suggesting transmission via armadillos.4
Genetic susceptibility influences the development of leprosy, while HLA type influences the immune response and hence the type of leprosy.5 Ridley and Jopling6 devised a classification system based on the immunologic response to M leprae. Highly reactive hosts with a vigorous cell-mediated response to M leprae develop tuberculoid leprosy and exhibit few skin lesions containing rare organisms. In contrast, anergic hosts develop lepromatous leprosy, characterized by multiple skin lesions, abundant organisms, and diffuse disease. Borderline tuberculoid, borderline, and borderline lepromatous make up the middle of the spectrum.6-9 Skin lesions can present with poorly defined, hypopigmented macules of indeterminate leprosy on one end and diffuse skin involvement of lepromatous leprosy on the opposite end. Diffuse involvement includes facial skin thickening, classic leonine facies, loss of eyebrows and eyelashes, anesthetic lesions, and anhidrosis.
Erythema nodosum leprosum occurs with chronic infection from M leprae, most commonly lepromatous leprosy. Immune complex deposition results in vasculitis and inflammatory foci. This phenomenon is thought to be secondary to high antigen load released by dying mycobacteria, causing secretion of tumor necrosis factor a from macrophages.1 Erythema nodosum leprosum demonstrates rapid onset of tender erythematous plaques or nodules, most commonly on the face and extensor surfaces of the extremities, with fever, malaise, iritis, arthralgia, and orchitis. Clofazimine therapy probably decreases the occurence.1 Treatment includes systemic corticosteroids and/or thalidomide.
- Moschella S, Ooi W. Update on leprosy in immigrants in the United States: status in the year 2000. Clin Infect Dis. 2001;32:930-937.
- Abraham S, Job C, Joseph G, et al. Epidemiological significance of first skin lesion in leprosy. Int J Lep Other Mycobact Dis. 1998;66:131-139.
- Shepard C. The experimental disease that follows the injection of human bacilli into footpads of mice. J Exp Med. 1960;112:445-454.
- Leprosy: global target attained. Wkly Epidemiol Rec. 2001;20:155-156.
- World Health Organization. Global leprosy situation, 2005. Wkly Epidemiol Rec. 2005;80:289-295.
- Ridley DS, Jopling WH. A classification of leprosy for research purposes. Lepr Rev. 1962;33:119-128.
- Lane J. Borderline tuberculoid leprosy in a woman from the state of Georgia with armadillo exposure. J Am Acad Dermatol. 2006;55:714-716.
- Fitness J, Tosh K, Hill AV. Genetics of susceptibility to leprosy. Genes Immun. 2002;3:441-453.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
- Moschella S, Ooi W. Update on leprosy in immigrants in the United States: status in the year 2000. Clin Infect Dis. 2001;32:930-937.
- Abraham S, Job C, Joseph G, et al. Epidemiological significance of first skin lesion in leprosy. Int J Lep Other Mycobact Dis. 1998;66:131-139.
- Shepard C. The experimental disease that follows the injection of human bacilli into footpads of mice. J Exp Med. 1960;112:445-454.
- Leprosy: global target attained. Wkly Epidemiol Rec. 2001;20:155-156.
- World Health Organization. Global leprosy situation, 2005. Wkly Epidemiol Rec. 2005;80:289-295.
- Ridley DS, Jopling WH. A classification of leprosy for research purposes. Lepr Rev. 1962;33:119-128.
- Lane J. Borderline tuberculoid leprosy in a woman from the state of Georgia with armadillo exposure. J Am Acad Dermatol. 2006;55:714-716.
- Fitness J, Tosh K, Hill AV. Genetics of susceptibility to leprosy. Genes Immun. 2002;3:441-453.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
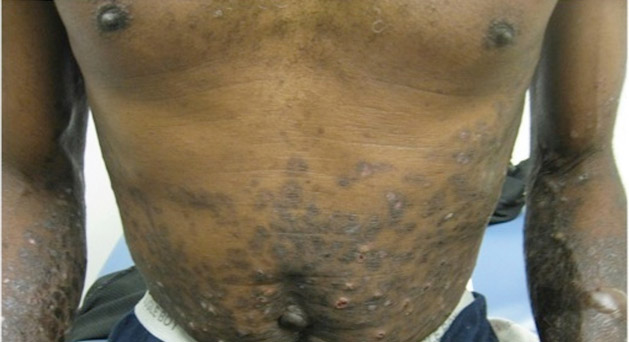
A 37-year-old man presented with pruritic lesions over the arms, legs, face, and back of 4 months’ duration that had been refractory to topical steroid treatment. He reported a 15-lb weight loss that he attributed to recent intranasal cocaine use. His medical history revealed obesity. There was no known history of sexually transmitted diseases, human immunodeficiency virus infection, tuberculosis, diabetes mellitus, or intravenous drug use. Physical examination revealed small nodules over the pinnae, plaques on the forehead, and large plaques with depigmented macules of variable sizes over the extremities and back. Some lesions on the extremities were violaceous in appearance, while others on the upper extremities had raised borders.
Multiple Firm Pink Papules and Nodules
The Diagnosis: Myeloid Leukemia Cutis
Leukemia cutis represents the infiltration of leukemic cells into the skin. It has been described in the setting of both myeloid and lymphoid leukemia. In the setting of acute myeloid leukemia, it has been reported to occur in 2% to 13% of patients overall,1,2 but it may occur in 31% of patients with the acute myelomonocytic or acute monocytic leukemia subtypes.3 Leukemia cutis is less common, with chronic myeloid leukemia occurring in 2.7% of patients in one study.4 In another study, 65% of patients with myeloid leukemia cutis had an acute myeloid leukemia.5
Myeloid leukemia cutis has been reported in patients aged 22 days to 90 years, with a median age of 62 years. There is a male predominance (1.4:1 ratio).5,6 The diagnosis of leukemia cutis is made concurrently with the diagnosis of leukemia in approximately 30% of cases, subsequent to the diagnosis of leukemia in approximately 60% of cases, and prior to the diagnosis of leukemia in approximately 10% of cases.5
Clinically, myeloid leukemia cutis presents as an asymptomatic solitary lesion in 23% of cases or as multiple lesions in 77% of cases. Lesions consist of pink to red to violaceous papules, nodules, and macules that are occasionally purpuric and involve any cutaneous surface.5
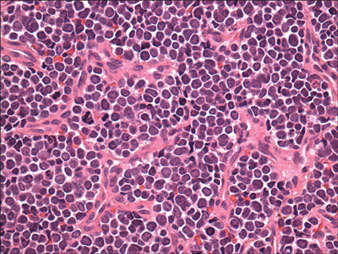
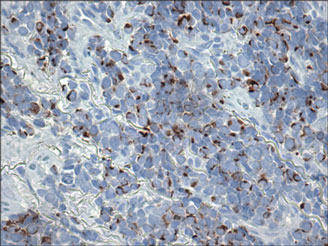
Histologically, the epidermis is unremarkable. Beneath a grenz zone within the dermis and usually extending into the subcutis there is a diffuse or nodular proliferation of neoplastic cells, often with perivascular and periadnexal accentuation and sometimes single filing of cells between collagen bundles (Figure 1). The cells are immature myeloid cells with irregular nuclear contours that may be indented or reniform (Figure 2). Nuclei contain finely dispersed chromatin with variably prominent nucleoli.5,6 Immunohistochemically, CD68 is positive in approximately 97% of cases, myeloperoxidase in 62%, and lysozyme in 85%. CD168, CD14, CD4, CD33, CD117, CD34, CD56, CD123, and CD303 are variably positive. CD3 and CD20, markers of lymphoid leukemia, are negative.5-8
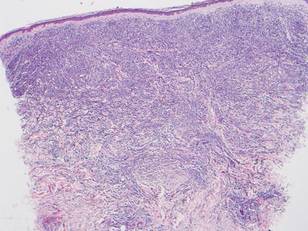
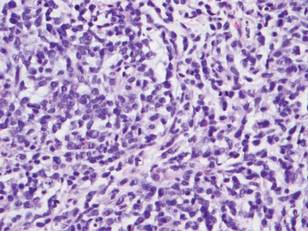
Leukemia cutis in the setting of a myeloid leukemia portends a grave prognosis. In a series of 18 patients, 16 had additional extramedullary leukemia, including meningeal leukemia in 6 patients.2 Most patients with myeloid leukemia cutis die within an average of 1 to 8 months of diagnosis.9
- Boggs DR, Wintrobe MM, Cartwright GE. The acute leukemias. analysis of 322 cases and review of the literature. Medicine (Baltimore). 1962;41:163-225.
- Baer MR, Barcos M, Farrell H, et al. Acute myelogenous leukemia with leukemia cutis. eighteen cases seen between 1969 and 1986. Cancer. 1989;63:2192-2200.
- Straus DJ, Mertelsmann R, Koziner B, et al. The acute monocytic leukemias: multidisciplinary studies in 45 patients. Medicine (Baltimore). 1980;59:409-425.
- Rosenthal S, Canellos GP, DeVita VT Jr, et al. Characteristics of blast crisis in chronic granulocytic leukemia. Blood. 1977;49:705-714.
- Bénet C, Gomez A, Aguilar C, et al. Histologic and immunohistologic characterization of skin localization of myeloid disorders: a study of 173 cases. Am J Clin Pathol. 2011;135:278-290.
- Cronin DM, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009;132:101-110.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Su WP, Buechner SA, Li CY. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
The Diagnosis: Myeloid Leukemia Cutis
Leukemia cutis represents the infiltration of leukemic cells into the skin. It has been described in the setting of both myeloid and lymphoid leukemia. In the setting of acute myeloid leukemia, it has been reported to occur in 2% to 13% of patients overall,1,2 but it may occur in 31% of patients with the acute myelomonocytic or acute monocytic leukemia subtypes.3 Leukemia cutis is less common, with chronic myeloid leukemia occurring in 2.7% of patients in one study.4 In another study, 65% of patients with myeloid leukemia cutis had an acute myeloid leukemia.5
Myeloid leukemia cutis has been reported in patients aged 22 days to 90 years, with a median age of 62 years. There is a male predominance (1.4:1 ratio).5,6 The diagnosis of leukemia cutis is made concurrently with the diagnosis of leukemia in approximately 30% of cases, subsequent to the diagnosis of leukemia in approximately 60% of cases, and prior to the diagnosis of leukemia in approximately 10% of cases.5
Clinically, myeloid leukemia cutis presents as an asymptomatic solitary lesion in 23% of cases or as multiple lesions in 77% of cases. Lesions consist of pink to red to violaceous papules, nodules, and macules that are occasionally purpuric and involve any cutaneous surface.5
Histologically, the epidermis is unremarkable. Beneath a grenz zone within the dermis and usually extending into the subcutis there is a diffuse or nodular proliferation of neoplastic cells, often with perivascular and periadnexal accentuation and sometimes single filing of cells between collagen bundles (Figure 1). The cells are immature myeloid cells with irregular nuclear contours that may be indented or reniform (Figure 2). Nuclei contain finely dispersed chromatin with variably prominent nucleoli.5,6 Immunohistochemically, CD68 is positive in approximately 97% of cases, myeloperoxidase in 62%, and lysozyme in 85%. CD168, CD14, CD4, CD33, CD117, CD34, CD56, CD123, and CD303 are variably positive. CD3 and CD20, markers of lymphoid leukemia, are negative.5-8


Leukemia cutis in the setting of a myeloid leukemia portends a grave prognosis. In a series of 18 patients, 16 had additional extramedullary leukemia, including meningeal leukemia in 6 patients.2 Most patients with myeloid leukemia cutis die within an average of 1 to 8 months of diagnosis.9
The Diagnosis: Myeloid Leukemia Cutis
Leukemia cutis represents the infiltration of leukemic cells into the skin. It has been described in the setting of both myeloid and lymphoid leukemia. In the setting of acute myeloid leukemia, it has been reported to occur in 2% to 13% of patients overall,1,2 but it may occur in 31% of patients with the acute myelomonocytic or acute monocytic leukemia subtypes.3 Leukemia cutis is less common, with chronic myeloid leukemia occurring in 2.7% of patients in one study.4 In another study, 65% of patients with myeloid leukemia cutis had an acute myeloid leukemia.5
Myeloid leukemia cutis has been reported in patients aged 22 days to 90 years, with a median age of 62 years. There is a male predominance (1.4:1 ratio).5,6 The diagnosis of leukemia cutis is made concurrently with the diagnosis of leukemia in approximately 30% of cases, subsequent to the diagnosis of leukemia in approximately 60% of cases, and prior to the diagnosis of leukemia in approximately 10% of cases.5
Clinically, myeloid leukemia cutis presents as an asymptomatic solitary lesion in 23% of cases or as multiple lesions in 77% of cases. Lesions consist of pink to red to violaceous papules, nodules, and macules that are occasionally purpuric and involve any cutaneous surface.5
Histologically, the epidermis is unremarkable. Beneath a grenz zone within the dermis and usually extending into the subcutis there is a diffuse or nodular proliferation of neoplastic cells, often with perivascular and periadnexal accentuation and sometimes single filing of cells between collagen bundles (Figure 1). The cells are immature myeloid cells with irregular nuclear contours that may be indented or reniform (Figure 2). Nuclei contain finely dispersed chromatin with variably prominent nucleoli.5,6 Immunohistochemically, CD68 is positive in approximately 97% of cases, myeloperoxidase in 62%, and lysozyme in 85%. CD168, CD14, CD4, CD33, CD117, CD34, CD56, CD123, and CD303 are variably positive. CD3 and CD20, markers of lymphoid leukemia, are negative.5-8


Leukemia cutis in the setting of a myeloid leukemia portends a grave prognosis. In a series of 18 patients, 16 had additional extramedullary leukemia, including meningeal leukemia in 6 patients.2 Most patients with myeloid leukemia cutis die within an average of 1 to 8 months of diagnosis.9
- Boggs DR, Wintrobe MM, Cartwright GE. The acute leukemias. analysis of 322 cases and review of the literature. Medicine (Baltimore). 1962;41:163-225.
- Baer MR, Barcos M, Farrell H, et al. Acute myelogenous leukemia with leukemia cutis. eighteen cases seen between 1969 and 1986. Cancer. 1989;63:2192-2200.
- Straus DJ, Mertelsmann R, Koziner B, et al. The acute monocytic leukemias: multidisciplinary studies in 45 patients. Medicine (Baltimore). 1980;59:409-425.
- Rosenthal S, Canellos GP, DeVita VT Jr, et al. Characteristics of blast crisis in chronic granulocytic leukemia. Blood. 1977;49:705-714.
- Bénet C, Gomez A, Aguilar C, et al. Histologic and immunohistologic characterization of skin localization of myeloid disorders: a study of 173 cases. Am J Clin Pathol. 2011;135:278-290.
- Cronin DM, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009;132:101-110.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Su WP, Buechner SA, Li CY. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
- Boggs DR, Wintrobe MM, Cartwright GE. The acute leukemias. analysis of 322 cases and review of the literature. Medicine (Baltimore). 1962;41:163-225.
- Baer MR, Barcos M, Farrell H, et al. Acute myelogenous leukemia with leukemia cutis. eighteen cases seen between 1969 and 1986. Cancer. 1989;63:2192-2200.
- Straus DJ, Mertelsmann R, Koziner B, et al. The acute monocytic leukemias: multidisciplinary studies in 45 patients. Medicine (Baltimore). 1980;59:409-425.
- Rosenthal S, Canellos GP, DeVita VT Jr, et al. Characteristics of blast crisis in chronic granulocytic leukemia. Blood. 1977;49:705-714.
- Bénet C, Gomez A, Aguilar C, et al. Histologic and immunohistologic characterization of skin localization of myeloid disorders: a study of 173 cases. Am J Clin Pathol. 2011;135:278-290.
- Cronin DM, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009;132:101-110.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Su WP, Buechner SA, Li CY. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
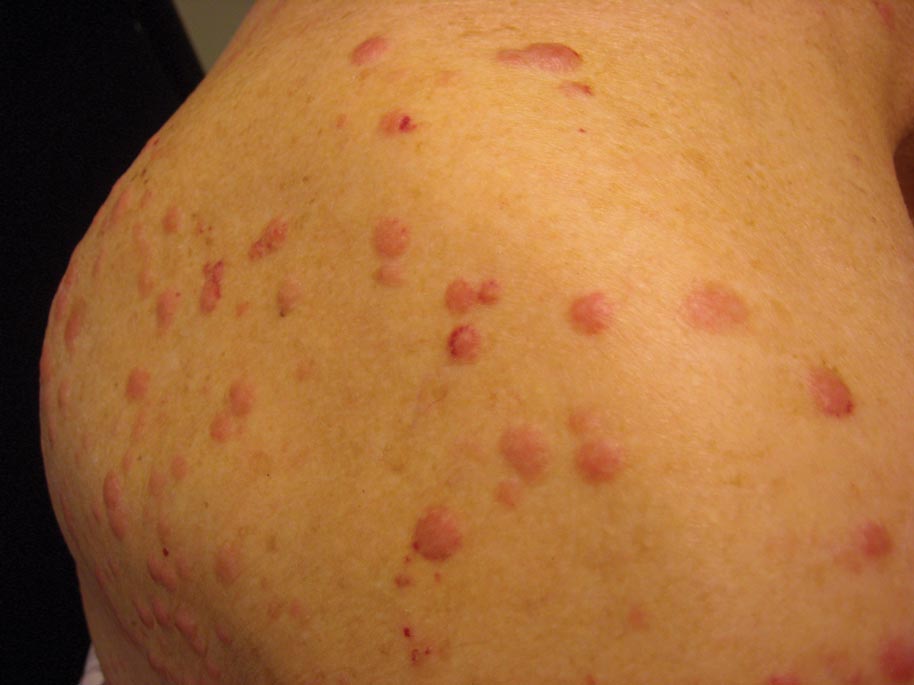
A 91-year-old man presented with numerous, scattered, asymptomatic, 3- to 9-mm, smooth, firm, pink papules and nodules involving the neck, trunk, and arms and legs of 1 week’s duration.
Erythematous Nodular Plaque Encircling the Lower Leg
The Diagnosis: Merkel Cell Carcinoma
Histopathology showed small round cells (Figure 1) that stained positive for cytokeratin 20 in a paranuclear dotlike pattern (Figure 2). The tumor stained negative for lymphoma (CD45) marker. Fluorodeoxyglucose positron emission tomography showed focally increased activity at cutaneous sites corresponding to the nodules, but lymph nodes and visceral sites did not show areas of increased metabolic activity. She underwent an above-knee amputation. She was started on a chemotherapy regimen of etoposide and carboplatin given that the pathology of the excised limb demonstrated vascular and lymphatic invasion by the tumor cells in the proximal skin margin. After 4 months she presented with gangrenous changes of the amputated limb and evidence of metastasis to the region of the skin flap. Similar tumors presented on the ipsilateral hip. Given her general poor condition and aggressive nature of the tumor, the patient decided to pursue hospice care 6 months after her diagnosis of Merkel cell carcinoma (MCC).
Figure 1. The tumor was composed of sheets of cells with a high nuclear-cytoplasmic ratio and fine chromatin without nucleoli (H&E, original magnification ×40). |
Figure 2. Immunohistochemical staining for cytokera-tin 20 showed positivity in a paranuclear dotlike pattern (original magnification ×40). |
Merkel cell carcinoma usually presents as firm, red to purple papules on sun-exposed skin in older patients with light skin. Factors strongly associated with the development of MCC are age (>65 years), lighter skin types, history of extensive sun exposure, and chronic immune suppression (eg, kidney or heart transplantation, human immunodeficiency virus).1 The rate of MCC has increased 3-fold between 1986 and 2001; the rate of MCC was 0.15 cases per 100,000 individuals in 1986, climbing up to 0.44 cases per 100,000 individuals in 2001.2 Our patient had been on immunosuppressants—prednisone, cyclosporine, and sirolimus—for nearly a decade following kidney transplants, which had been discontinued 2 years prior to presentation.
Heath et al3 defined an acronym AEIOU (asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin) for MCC features derived from 195 patients. They advised that a biopsy is warranted if the patient presents with more than 3 of these features.3
The 1991 MCC staging system was revised in 1999 and 2005 based on experience at Memorial Sloan Kettering Cancer Center (New York, New York).4 In 2010 the American Joint Committee on Cancer staging was introduced for MCC, which follows other skin malignancies.5 Using this TNM staging system for primary tumors, regional lymph nodes, and distant metastasis, our patient at the time of presentation was stage IIB (T3N0M0), with tumor size greater than 5 cm, nodes negative by clinical examination, and no distant metastasis. In a span of 3 months, she had metastasis to the skin, subcutaneous tissue, and distant lymph nodes, which resulted in classification as stage IV, proving the aggressive nature of the tumor.
The newly discovered Merkel cell polyomavirus (MCPyV) is found integrating into the Merkel cell genome. Merkel cell polyomavirus is present in 80% of cancers and is expressed in a clonal pattern, while 90% of MCC patients are seropositive for the same. Unlike antibodies to MCPyV VP1 protein, antibodies to the T antigen for MCPyV track disease burden and may be a useful biomarker for MCC in the future.6
A study of 251 patients in 1970-2002 showed that pathologic nodal staging identifies a group of patients with excellent long-term survival.1 Our patient preferred to undergo positron emission tomography rather than a sentinel lymph node biopsy prior to surgery. Also, after margin-negative excision and pathologic nodal staging, local and nodal recurrence rates were low. Adjuvant chemotherapy for stage III patients showed a trend (P=.08) to decreased survival compared with stage II patients who did not receive chemotherapy.7 A multidisciplinary approach to treatment including surgery, radiation,8 and chemotherapy needs to be created for each individual patient. Merkel cell carcinoma is the cause of death in 35% of patients within 3 years of diagnosis.9
Merkel cell carcinoma is a rare orphan tumor with rapidly increasing incidence in an era of immunosuppression. It has a grave prognosis, as demonstrated in our case, if not detected early. People at increased risk for MCC must have regular skin checks. Unfortunately, our patient was a nursing home resident and had not had a skin check for 2 years prior to presentation.
1. Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
2. Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
3. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
4. Edge S, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. New York, NY: Springer; 2010.
5. Assouline A, Tai P, Joseph K, et al. Merkel cell carcinoma of skin-current controversies and recommendations.Rare Tumors. 2011;4:e23.
6. Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to Merkel cell polyomavirus T-antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70:8388-8397.
7. Poulsen M, Rischin D, Walpole E, et al; Trans-Tasman Radiation Oncology Group. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study—TROG 96:07. J Clin Oncol. 2003;21:4371-4376.
8. Lewis KG, Weinstock MA, Weaver AL, et al. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142:693-700.
9. Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
The Diagnosis: Merkel Cell Carcinoma
Histopathology showed small round cells (Figure 1) that stained positive for cytokeratin 20 in a paranuclear dotlike pattern (Figure 2). The tumor stained negative for lymphoma (CD45) marker. Fluorodeoxyglucose positron emission tomography showed focally increased activity at cutaneous sites corresponding to the nodules, but lymph nodes and visceral sites did not show areas of increased metabolic activity. She underwent an above-knee amputation. She was started on a chemotherapy regimen of etoposide and carboplatin given that the pathology of the excised limb demonstrated vascular and lymphatic invasion by the tumor cells in the proximal skin margin. After 4 months she presented with gangrenous changes of the amputated limb and evidence of metastasis to the region of the skin flap. Similar tumors presented on the ipsilateral hip. Given her general poor condition and aggressive nature of the tumor, the patient decided to pursue hospice care 6 months after her diagnosis of Merkel cell carcinoma (MCC).
Figure 1. The tumor was composed of sheets of cells with a high nuclear-cytoplasmic ratio and fine chromatin without nucleoli (H&E, original magnification ×40). |
Figure 2. Immunohistochemical staining for cytokera-tin 20 showed positivity in a paranuclear dotlike pattern (original magnification ×40). |
Merkel cell carcinoma usually presents as firm, red to purple papules on sun-exposed skin in older patients with light skin. Factors strongly associated with the development of MCC are age (>65 years), lighter skin types, history of extensive sun exposure, and chronic immune suppression (eg, kidney or heart transplantation, human immunodeficiency virus).1 The rate of MCC has increased 3-fold between 1986 and 2001; the rate of MCC was 0.15 cases per 100,000 individuals in 1986, climbing up to 0.44 cases per 100,000 individuals in 2001.2 Our patient had been on immunosuppressants—prednisone, cyclosporine, and sirolimus—for nearly a decade following kidney transplants, which had been discontinued 2 years prior to presentation.
Heath et al3 defined an acronym AEIOU (asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin) for MCC features derived from 195 patients. They advised that a biopsy is warranted if the patient presents with more than 3 of these features.3
The 1991 MCC staging system was revised in 1999 and 2005 based on experience at Memorial Sloan Kettering Cancer Center (New York, New York).4 In 2010 the American Joint Committee on Cancer staging was introduced for MCC, which follows other skin malignancies.5 Using this TNM staging system for primary tumors, regional lymph nodes, and distant metastasis, our patient at the time of presentation was stage IIB (T3N0M0), with tumor size greater than 5 cm, nodes negative by clinical examination, and no distant metastasis. In a span of 3 months, she had metastasis to the skin, subcutaneous tissue, and distant lymph nodes, which resulted in classification as stage IV, proving the aggressive nature of the tumor.
The newly discovered Merkel cell polyomavirus (MCPyV) is found integrating into the Merkel cell genome. Merkel cell polyomavirus is present in 80% of cancers and is expressed in a clonal pattern, while 90% of MCC patients are seropositive for the same. Unlike antibodies to MCPyV VP1 protein, antibodies to the T antigen for MCPyV track disease burden and may be a useful biomarker for MCC in the future.6
A study of 251 patients in 1970-2002 showed that pathologic nodal staging identifies a group of patients with excellent long-term survival.1 Our patient preferred to undergo positron emission tomography rather than a sentinel lymph node biopsy prior to surgery. Also, after margin-negative excision and pathologic nodal staging, local and nodal recurrence rates were low. Adjuvant chemotherapy for stage III patients showed a trend (P=.08) to decreased survival compared with stage II patients who did not receive chemotherapy.7 A multidisciplinary approach to treatment including surgery, radiation,8 and chemotherapy needs to be created for each individual patient. Merkel cell carcinoma is the cause of death in 35% of patients within 3 years of diagnosis.9
Merkel cell carcinoma is a rare orphan tumor with rapidly increasing incidence in an era of immunosuppression. It has a grave prognosis, as demonstrated in our case, if not detected early. People at increased risk for MCC must have regular skin checks. Unfortunately, our patient was a nursing home resident and had not had a skin check for 2 years prior to presentation.
The Diagnosis: Merkel Cell Carcinoma
Histopathology showed small round cells (Figure 1) that stained positive for cytokeratin 20 in a paranuclear dotlike pattern (Figure 2). The tumor stained negative for lymphoma (CD45) marker. Fluorodeoxyglucose positron emission tomography showed focally increased activity at cutaneous sites corresponding to the nodules, but lymph nodes and visceral sites did not show areas of increased metabolic activity. She underwent an above-knee amputation. She was started on a chemotherapy regimen of etoposide and carboplatin given that the pathology of the excised limb demonstrated vascular and lymphatic invasion by the tumor cells in the proximal skin margin. After 4 months she presented with gangrenous changes of the amputated limb and evidence of metastasis to the region of the skin flap. Similar tumors presented on the ipsilateral hip. Given her general poor condition and aggressive nature of the tumor, the patient decided to pursue hospice care 6 months after her diagnosis of Merkel cell carcinoma (MCC).
Figure 1. The tumor was composed of sheets of cells with a high nuclear-cytoplasmic ratio and fine chromatin without nucleoli (H&E, original magnification ×40). |
Figure 2. Immunohistochemical staining for cytokera-tin 20 showed positivity in a paranuclear dotlike pattern (original magnification ×40). |
Merkel cell carcinoma usually presents as firm, red to purple papules on sun-exposed skin in older patients with light skin. Factors strongly associated with the development of MCC are age (>65 years), lighter skin types, history of extensive sun exposure, and chronic immune suppression (eg, kidney or heart transplantation, human immunodeficiency virus).1 The rate of MCC has increased 3-fold between 1986 and 2001; the rate of MCC was 0.15 cases per 100,000 individuals in 1986, climbing up to 0.44 cases per 100,000 individuals in 2001.2 Our patient had been on immunosuppressants—prednisone, cyclosporine, and sirolimus—for nearly a decade following kidney transplants, which had been discontinued 2 years prior to presentation.
Heath et al3 defined an acronym AEIOU (asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin) for MCC features derived from 195 patients. They advised that a biopsy is warranted if the patient presents with more than 3 of these features.3
The 1991 MCC staging system was revised in 1999 and 2005 based on experience at Memorial Sloan Kettering Cancer Center (New York, New York).4 In 2010 the American Joint Committee on Cancer staging was introduced for MCC, which follows other skin malignancies.5 Using this TNM staging system for primary tumors, regional lymph nodes, and distant metastasis, our patient at the time of presentation was stage IIB (T3N0M0), with tumor size greater than 5 cm, nodes negative by clinical examination, and no distant metastasis. In a span of 3 months, she had metastasis to the skin, subcutaneous tissue, and distant lymph nodes, which resulted in classification as stage IV, proving the aggressive nature of the tumor.
The newly discovered Merkel cell polyomavirus (MCPyV) is found integrating into the Merkel cell genome. Merkel cell polyomavirus is present in 80% of cancers and is expressed in a clonal pattern, while 90% of MCC patients are seropositive for the same. Unlike antibodies to MCPyV VP1 protein, antibodies to the T antigen for MCPyV track disease burden and may be a useful biomarker for MCC in the future.6
A study of 251 patients in 1970-2002 showed that pathologic nodal staging identifies a group of patients with excellent long-term survival.1 Our patient preferred to undergo positron emission tomography rather than a sentinel lymph node biopsy prior to surgery. Also, after margin-negative excision and pathologic nodal staging, local and nodal recurrence rates were low. Adjuvant chemotherapy for stage III patients showed a trend (P=.08) to decreased survival compared with stage II patients who did not receive chemotherapy.7 A multidisciplinary approach to treatment including surgery, radiation,8 and chemotherapy needs to be created for each individual patient. Merkel cell carcinoma is the cause of death in 35% of patients within 3 years of diagnosis.9
Merkel cell carcinoma is a rare orphan tumor with rapidly increasing incidence in an era of immunosuppression. It has a grave prognosis, as demonstrated in our case, if not detected early. People at increased risk for MCC must have regular skin checks. Unfortunately, our patient was a nursing home resident and had not had a skin check for 2 years prior to presentation.
1. Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
2. Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
3. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
4. Edge S, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. New York, NY: Springer; 2010.
5. Assouline A, Tai P, Joseph K, et al. Merkel cell carcinoma of skin-current controversies and recommendations.Rare Tumors. 2011;4:e23.
6. Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to Merkel cell polyomavirus T-antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70:8388-8397.
7. Poulsen M, Rischin D, Walpole E, et al; Trans-Tasman Radiation Oncology Group. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study—TROG 96:07. J Clin Oncol. 2003;21:4371-4376.
8. Lewis KG, Weinstock MA, Weaver AL, et al. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142:693-700.
9. Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
1. Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
2. Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
3. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
4. Edge S, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. New York, NY: Springer; 2010.
5. Assouline A, Tai P, Joseph K, et al. Merkel cell carcinoma of skin-current controversies and recommendations.Rare Tumors. 2011;4:e23.
6. Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to Merkel cell polyomavirus T-antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70:8388-8397.
7. Poulsen M, Rischin D, Walpole E, et al; Trans-Tasman Radiation Oncology Group. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study—TROG 96:07. J Clin Oncol. 2003;21:4371-4376.
8. Lewis KG, Weinstock MA, Weaver AL, et al. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142:693-700.
9. Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
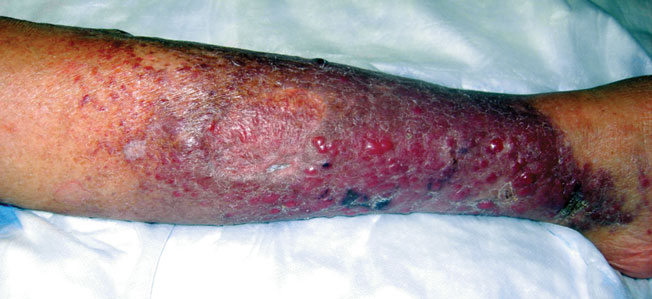
A 66-year-old woman presented with red to violaceous, rapidly growing nodules on the skin. Her medical history was remarkable for diabetes mellitus, hypertension, dyslipidemia, and renal failure. She had 2 rejected kidney transplants and was on hemodialysis at the time of presentation. She noticed asymptomatic nodules present on the left lower leg that progressively coalesced, finally encroaching the whole girth of the limb, spreading from the foot to the knee in a short duration of 3 months. The regional lymph nodes were not clinically palpable.
Erythematous Plaques on the Hand and Wrist
The Diagnosis: Lichen Aureus
Lichen aureus (LA) is classified as a pigmented purpuric dermatosis (PPD), a collection of conditions that are characterized by petechiae, pigmentation, and occasionally telangiectasia without a causative underlying disorder. As in our case, the lesions of LA are usually asymptomatic. They appear as circumscribed areas of discrete or confluent macules and papules that can range in color from gold or copper to purple (Figure 1). The lesions typically occur unilaterally on the lower extremities but can occur on all body regions. The etiology is unknown, but explanations such as venous insufficiency,1 contact allergens,2 and drugs3,4 have been proposed. Unlike other PPDs, LA tends to occur abruptly and then either stabilizes or progresses slowly over years. Studies have reported resolution in 2 to 7 years.5 The average age of onset is in the 20s and 30s, with pediatric cases accounting for only 17%.6 Pediatric cases are more likely to be self-limited and occur in uncommon sites such as the trunk and arms.7
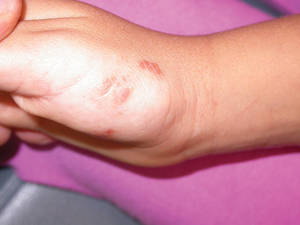
Lichen aureus is characterized histopathologically by a dense, bandlike, dermal inflammatory infiltrate (Figure 2). Additionally, there is variable exocytosis of lymphocytes and marked accumulation of siderotic macrophages (Figure 3). These qualities in the proper clinical setting help differentiate LA from other PPDs that share findings of capillaritis, hemosiderin deposition, and erythrocyte extravasation near dermal vessels. An iron stain assists in the diagnosis of LA (Figure 3), as it differentiates the disease from other lichenoid conditions such as lichen planus. Zaballos et al8 also demonstrated a role for dermoscopy to clinically differentiate LA from other similar-appearing lesions such as lichen planus.
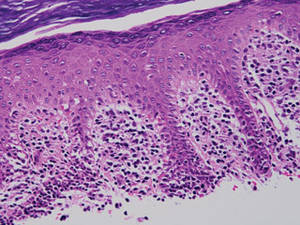
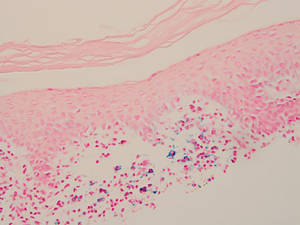
The lesions of LA are benign. Because the predominantly T-cell infiltrate is monoclonal in approximately 50% of cases,2,9-11 authors have suggested the possibility of progression to cutaneous T-cell lymphoma.9,12 Guitart and Magro13 classified LA as a T-cell lymphoid dyscrasia with potential for progression. Despite these reports, the general consensus is that LA is a benign, self-limiting condition. The benign nature of LA is supported by Fink-Puches et al2 who followed 23 patients for a mean 102.1 months and did not observe a single case of progression to malignancy.
There have been many treatment regimens attempted for patients with LA. Topical corticosteroids have not been found to be beneficial14; however, there have been isolated cases reporting its efficacy in children.7,15 Other medications that have been effective in small trials include psoralen plus UVA,16 topical pimecrolimus,5 calcium dobesilate,17 and combination therapy with pentoxifylline and prostacyclin.18 Despite some reported benefit, the use of potent immunomodulating medications is not indicated due to the benign nature of the disease. Alternative supplements including oral bioflavonoids and ascorbic acid have also been explored with modest benefit.19
1. Reinhardt L, Wilkin JK, Tausend R. Vascular abnormalities in lichen aureus. J Am Acad Dermatol. 1983;8:417-420.
2. Fink-Puches R, Wolf P, Kerl H, et al. Lichen aureus: clinicopathologic features, natural history, and relationship to mycosis fungoides. Arch Dermatol. 2008;144:1169-1173.
3. Nishioka K, Katayama I, Masuzawa M, et al. Drug-induced chronic pigmented purpura. J Dermatol. 1989;16:220-222.
4. Yazdi AS, Mayser P, Sander CA. Lichen aureus with clonal T cells in a child possibly induced by regular consumption of an energy drink. J Cutan Pathol. 2008;35:960-962.
5. Bohm M, Bonsmann G, Luger TA. Resolution of lichen aureus in a 10-year-old child after topical pimecrolimus. Br J Dermatol. 2004;151:519-520.
6. Gelmetti C, Cerri D, Grimalt R. Lichen aureus in childhood. Pediatr Dermatol. 1991;8:280-283.
7. Kim MJ, Kim BY, Park KC, et al. A case of childhood lichen aureus. Ann Dermatol. 2009;21:393-395.
8. Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
9. Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
10. Magro CM, Schaefer JT, Crowson AN, et al. Pigmented purpuric dermatosis: classification by phenotypic and molecular profiles. Am J Clin Pathol. 2007;128:218-229.
11. Crowson AN, Magro CM, Zahorchak R. Atypical pigmentary purpura: a clinical, histopathologic, and genotypic study. Hum Pathol. 1999;30:1004-1012.
12. Barnhill RL, Braverman IM. Progression of pigmented purpura-like eruptions to mycosis fungoides: report of three cases. J Am Acad Dermatol. 1988;19(1, pt 1):25-31.
13. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
14. Graham RM, English JS, Emmerson RW. Lichen aureus—a study of twelve cases. Clin Exp Dermatol. 1984;9:393-401.
15. Fujita H, Iguchi M, Ikari Y, et al. Lichen aureus on the back in a 6-year-old girl. J Dermatol. 2007;34:148-149.
16. Ling TC, Goulden V, Goodfield MJ. PUVA therapy in lichen aureus. J Am Acad Dermatol. 2001;45:145-146.
17. Agrawal SK, Gandhi V, Bhattacharya SN. Calcium dobesilate (Cd) in pigmented purpuric dermatosis (PPD): a pilot evaluation. J Dermatol. 2004;31:98-103.
18. Lee HW, Lee DK, Chang SE, et al. Segmental lichen aureus: combination therapy with pentoxifylline and prostacyclin. J Eur Acad Dermatol Venereol. 2006;20:1378-1380.
19. Reinhold U, Seiter S, Ugurel S, et al. Treatment of progressive pigmented purpura with oral bioflavonoids and ascorbic acid: an open pilot study in 3 patients. J Am Acad Dermatol. 1999;4(2, pt 1):207-208.
The Diagnosis: Lichen Aureus
Lichen aureus (LA) is classified as a pigmented purpuric dermatosis (PPD), a collection of conditions that are characterized by petechiae, pigmentation, and occasionally telangiectasia without a causative underlying disorder. As in our case, the lesions of LA are usually asymptomatic. They appear as circumscribed areas of discrete or confluent macules and papules that can range in color from gold or copper to purple (Figure 1). The lesions typically occur unilaterally on the lower extremities but can occur on all body regions. The etiology is unknown, but explanations such as venous insufficiency,1 contact allergens,2 and drugs3,4 have been proposed. Unlike other PPDs, LA tends to occur abruptly and then either stabilizes or progresses slowly over years. Studies have reported resolution in 2 to 7 years.5 The average age of onset is in the 20s and 30s, with pediatric cases accounting for only 17%.6 Pediatric cases are more likely to be self-limited and occur in uncommon sites such as the trunk and arms.7

Lichen aureus is characterized histopathologically by a dense, bandlike, dermal inflammatory infiltrate (Figure 2). Additionally, there is variable exocytosis of lymphocytes and marked accumulation of siderotic macrophages (Figure 3). These qualities in the proper clinical setting help differentiate LA from other PPDs that share findings of capillaritis, hemosiderin deposition, and erythrocyte extravasation near dermal vessels. An iron stain assists in the diagnosis of LA (Figure 3), as it differentiates the disease from other lichenoid conditions such as lichen planus. Zaballos et al8 also demonstrated a role for dermoscopy to clinically differentiate LA from other similar-appearing lesions such as lichen planus.


The lesions of LA are benign. Because the predominantly T-cell infiltrate is monoclonal in approximately 50% of cases,2,9-11 authors have suggested the possibility of progression to cutaneous T-cell lymphoma.9,12 Guitart and Magro13 classified LA as a T-cell lymphoid dyscrasia with potential for progression. Despite these reports, the general consensus is that LA is a benign, self-limiting condition. The benign nature of LA is supported by Fink-Puches et al2 who followed 23 patients for a mean 102.1 months and did not observe a single case of progression to malignancy.
There have been many treatment regimens attempted for patients with LA. Topical corticosteroids have not been found to be beneficial14; however, there have been isolated cases reporting its efficacy in children.7,15 Other medications that have been effective in small trials include psoralen plus UVA,16 topical pimecrolimus,5 calcium dobesilate,17 and combination therapy with pentoxifylline and prostacyclin.18 Despite some reported benefit, the use of potent immunomodulating medications is not indicated due to the benign nature of the disease. Alternative supplements including oral bioflavonoids and ascorbic acid have also been explored with modest benefit.19
The Diagnosis: Lichen Aureus
Lichen aureus (LA) is classified as a pigmented purpuric dermatosis (PPD), a collection of conditions that are characterized by petechiae, pigmentation, and occasionally telangiectasia without a causative underlying disorder. As in our case, the lesions of LA are usually asymptomatic. They appear as circumscribed areas of discrete or confluent macules and papules that can range in color from gold or copper to purple (Figure 1). The lesions typically occur unilaterally on the lower extremities but can occur on all body regions. The etiology is unknown, but explanations such as venous insufficiency,1 contact allergens,2 and drugs3,4 have been proposed. Unlike other PPDs, LA tends to occur abruptly and then either stabilizes or progresses slowly over years. Studies have reported resolution in 2 to 7 years.5 The average age of onset is in the 20s and 30s, with pediatric cases accounting for only 17%.6 Pediatric cases are more likely to be self-limited and occur in uncommon sites such as the trunk and arms.7

Lichen aureus is characterized histopathologically by a dense, bandlike, dermal inflammatory infiltrate (Figure 2). Additionally, there is variable exocytosis of lymphocytes and marked accumulation of siderotic macrophages (Figure 3). These qualities in the proper clinical setting help differentiate LA from other PPDs that share findings of capillaritis, hemosiderin deposition, and erythrocyte extravasation near dermal vessels. An iron stain assists in the diagnosis of LA (Figure 3), as it differentiates the disease from other lichenoid conditions such as lichen planus. Zaballos et al8 also demonstrated a role for dermoscopy to clinically differentiate LA from other similar-appearing lesions such as lichen planus.


The lesions of LA are benign. Because the predominantly T-cell infiltrate is monoclonal in approximately 50% of cases,2,9-11 authors have suggested the possibility of progression to cutaneous T-cell lymphoma.9,12 Guitart and Magro13 classified LA as a T-cell lymphoid dyscrasia with potential for progression. Despite these reports, the general consensus is that LA is a benign, self-limiting condition. The benign nature of LA is supported by Fink-Puches et al2 who followed 23 patients for a mean 102.1 months and did not observe a single case of progression to malignancy.
There have been many treatment regimens attempted for patients with LA. Topical corticosteroids have not been found to be beneficial14; however, there have been isolated cases reporting its efficacy in children.7,15 Other medications that have been effective in small trials include psoralen plus UVA,16 topical pimecrolimus,5 calcium dobesilate,17 and combination therapy with pentoxifylline and prostacyclin.18 Despite some reported benefit, the use of potent immunomodulating medications is not indicated due to the benign nature of the disease. Alternative supplements including oral bioflavonoids and ascorbic acid have also been explored with modest benefit.19
1. Reinhardt L, Wilkin JK, Tausend R. Vascular abnormalities in lichen aureus. J Am Acad Dermatol. 1983;8:417-420.
2. Fink-Puches R, Wolf P, Kerl H, et al. Lichen aureus: clinicopathologic features, natural history, and relationship to mycosis fungoides. Arch Dermatol. 2008;144:1169-1173.
3. Nishioka K, Katayama I, Masuzawa M, et al. Drug-induced chronic pigmented purpura. J Dermatol. 1989;16:220-222.
4. Yazdi AS, Mayser P, Sander CA. Lichen aureus with clonal T cells in a child possibly induced by regular consumption of an energy drink. J Cutan Pathol. 2008;35:960-962.
5. Bohm M, Bonsmann G, Luger TA. Resolution of lichen aureus in a 10-year-old child after topical pimecrolimus. Br J Dermatol. 2004;151:519-520.
6. Gelmetti C, Cerri D, Grimalt R. Lichen aureus in childhood. Pediatr Dermatol. 1991;8:280-283.
7. Kim MJ, Kim BY, Park KC, et al. A case of childhood lichen aureus. Ann Dermatol. 2009;21:393-395.
8. Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
9. Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
10. Magro CM, Schaefer JT, Crowson AN, et al. Pigmented purpuric dermatosis: classification by phenotypic and molecular profiles. Am J Clin Pathol. 2007;128:218-229.
11. Crowson AN, Magro CM, Zahorchak R. Atypical pigmentary purpura: a clinical, histopathologic, and genotypic study. Hum Pathol. 1999;30:1004-1012.
12. Barnhill RL, Braverman IM. Progression of pigmented purpura-like eruptions to mycosis fungoides: report of three cases. J Am Acad Dermatol. 1988;19(1, pt 1):25-31.
13. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
14. Graham RM, English JS, Emmerson RW. Lichen aureus—a study of twelve cases. Clin Exp Dermatol. 1984;9:393-401.
15. Fujita H, Iguchi M, Ikari Y, et al. Lichen aureus on the back in a 6-year-old girl. J Dermatol. 2007;34:148-149.
16. Ling TC, Goulden V, Goodfield MJ. PUVA therapy in lichen aureus. J Am Acad Dermatol. 2001;45:145-146.
17. Agrawal SK, Gandhi V, Bhattacharya SN. Calcium dobesilate (Cd) in pigmented purpuric dermatosis (PPD): a pilot evaluation. J Dermatol. 2004;31:98-103.
18. Lee HW, Lee DK, Chang SE, et al. Segmental lichen aureus: combination therapy with pentoxifylline and prostacyclin. J Eur Acad Dermatol Venereol. 2006;20:1378-1380.
19. Reinhold U, Seiter S, Ugurel S, et al. Treatment of progressive pigmented purpura with oral bioflavonoids and ascorbic acid: an open pilot study in 3 patients. J Am Acad Dermatol. 1999;4(2, pt 1):207-208.
1. Reinhardt L, Wilkin JK, Tausend R. Vascular abnormalities in lichen aureus. J Am Acad Dermatol. 1983;8:417-420.
2. Fink-Puches R, Wolf P, Kerl H, et al. Lichen aureus: clinicopathologic features, natural history, and relationship to mycosis fungoides. Arch Dermatol. 2008;144:1169-1173.
3. Nishioka K, Katayama I, Masuzawa M, et al. Drug-induced chronic pigmented purpura. J Dermatol. 1989;16:220-222.
4. Yazdi AS, Mayser P, Sander CA. Lichen aureus with clonal T cells in a child possibly induced by regular consumption of an energy drink. J Cutan Pathol. 2008;35:960-962.
5. Bohm M, Bonsmann G, Luger TA. Resolution of lichen aureus in a 10-year-old child after topical pimecrolimus. Br J Dermatol. 2004;151:519-520.
6. Gelmetti C, Cerri D, Grimalt R. Lichen aureus in childhood. Pediatr Dermatol. 1991;8:280-283.
7. Kim MJ, Kim BY, Park KC, et al. A case of childhood lichen aureus. Ann Dermatol. 2009;21:393-395.
8. Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
9. Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
10. Magro CM, Schaefer JT, Crowson AN, et al. Pigmented purpuric dermatosis: classification by phenotypic and molecular profiles. Am J Clin Pathol. 2007;128:218-229.
11. Crowson AN, Magro CM, Zahorchak R. Atypical pigmentary purpura: a clinical, histopathologic, and genotypic study. Hum Pathol. 1999;30:1004-1012.
12. Barnhill RL, Braverman IM. Progression of pigmented purpura-like eruptions to mycosis fungoides: report of three cases. J Am Acad Dermatol. 1988;19(1, pt 1):25-31.
13. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
14. Graham RM, English JS, Emmerson RW. Lichen aureus—a study of twelve cases. Clin Exp Dermatol. 1984;9:393-401.
15. Fujita H, Iguchi M, Ikari Y, et al. Lichen aureus on the back in a 6-year-old girl. J Dermatol. 2007;34:148-149.
16. Ling TC, Goulden V, Goodfield MJ. PUVA therapy in lichen aureus. J Am Acad Dermatol. 2001;45:145-146.
17. Agrawal SK, Gandhi V, Bhattacharya SN. Calcium dobesilate (Cd) in pigmented purpuric dermatosis (PPD): a pilot evaluation. J Dermatol. 2004;31:98-103.
18. Lee HW, Lee DK, Chang SE, et al. Segmental lichen aureus: combination therapy with pentoxifylline and prostacyclin. J Eur Acad Dermatol Venereol. 2006;20:1378-1380.
19. Reinhold U, Seiter S, Ugurel S, et al. Treatment of progressive pigmented purpura with oral bioflavonoids and ascorbic acid: an open pilot study in 3 patients. J Am Acad Dermatol. 1999;4(2, pt 1):207-208.
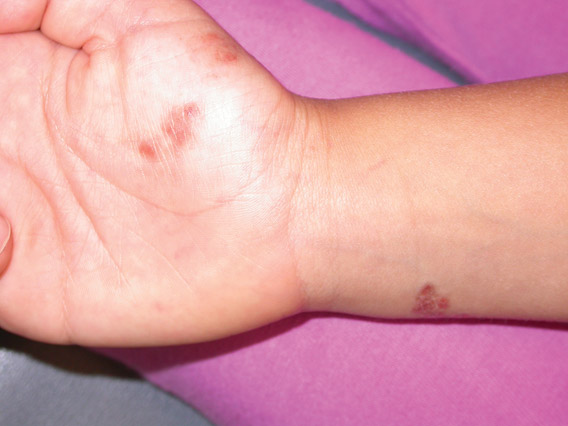
What Is Your Diagnosis? Clear Cell Hidradenoma
A 70-year-old woman presented to our dermatology clinic with an enlarging lesion on the left anterior aspect of the scalp of 4 years’ duration. She had a history of breast carcinoma in the left breast with positive lymph nodes 2 years prior. Physical examination revealed a 2.5-cm pink, pearly, exophytic plaque on the left anterior aspect of the scalp. The lesion was removed with clear margins by excisional surgery.
The Diagnosis: Clear Cell Hidradenoma
Clear cell hidradenoma (CCH) is a variant of nodular hidradenoma that may contain varying quantities of solid and cystic components and comprises approximately one-third of hidradenomas.1 Clear cell hidradenomas are slow-growing and fairly uncommon adnexal neoplasms derived from either eccrine sweat glands or apocrine glands. Some researchers have regarded hidradenomas as apocrine tumors due to evidence of apocrine decapitation secretion, whereas others note the lack of apocrine and ultrastructural features of immature eccrine glands.2 Clear cell hidradenomas typically develop between the fourth and eighth decades of life, usually peaking during the sixth decade.3 Clear cell hidradenomas usually range in size from 5 to 30 mm and frequently present on the scalp, head, chest, and abdomen; rarely, CCHs present on the joint spaces of the shoulders and knees.3-5 This neoplasm is more common in women than men3 and generally has a flesh-colored, erythematous, red-brown or blue appearance with a tendency to ulcerate and exude a serous discharge (Figure 1).5 The clinical differential diagnosis includes metastatic cancer (eg, renal cell carcinoma, keratoacanthoma, trichoblastoma, trichilemmoma) or other benign adnexal neoplasms.
Histopathologic examination of a CCH generally reveals an unencapsulated and circumscribed neoplasm in the mid or upper dermis with occasional extensions into the subcutaneous fat (Figure 2). The tumor typically presents with 2 types of cells: (1) round, fusiform, or polygonal cells with vesicular nuclei and eosinophilic cytoplasm, and (2) cells with clear cytoplasm and basophilic, often eccentrically located nuclei.6 Ducts are scattered within the neoplasm and are lined by a layer of cuboidal cells that can be highlighted on carcinoembryonic antigen and epithelial membrane antigen immunostaining.6 The tumor cells themselves are highlighted on cytokeratin AE1/AE3 staining.
Malignant transformation rarely is associated with CCH, with de novo clear cell hidradenocarcinoma being more common. Only approximately 6.7% of CCHs have been shown to be malignant, and the malignant tumors feature nuclear atypia, abnormal mitotic figures, necrosis, and infiltration.1,7 Although CCH is a benign adnexal neoplasm, it has a high recurrence rate (approximately 10%) following excision.7 The treatment of choice is complete surgical excision, though Mohs micrographic surgery is advocated, as it promotes thorough examination of the tumor margin to ensure complete tumor removal.8 Our case illustrates the importance of a broad differential diagnosis when treating patients with CCH as well as keeping in mind nonmalignant lesions are far more common than malignant lesions.
1. Volmar K, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
2. Goh SG, Carr R, Dayrit JF, et al. Mucinous hidradenoma: a report of three cases. J Cutan Pathol. 2007;34:497-502.
3. Gonul M, Cakmak SK, Gul U, et al. A skin tumor in a young girl. diagnosis: clear cell hidradenoma. Indian J Dermatol Venereol Leprol. 2010;76:445-446.
4. Singhal V, Sharma SC, Anil J, et al. Giant benign nodular hidradenoma of the shoulder: a rare tumor of orthopedic practice. Int J Shoulder Surg. 2010;4:93-96.
5. Yu G, Goodloe S Jr, D’Angelis CA, et al. Giant clear cell hidradenoma of the knee. J Cutan Pathol. 2010;37:E37-E41.
6. McKee PH, Calonje E, Granter SR. Tumors of the sweat glands. In: McKee PH, Calonje E, Granter SR. Pathology of the Skin With Clinical Correlations. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2005:1632-1635.
7. Ozawa T, Fujiwara M, Nose K, et al. Clear-cell hidradenoma of the forearm in a young boy. Pediatr Dermatol. 2005;22:450-452.
8. Yavel R, Hinshaw M, Rao V, et al. Hidradenoma and a hidradenocarcinoma of the scalp managed by Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatol Surg. 2009;35:273-281.
A 70-year-old woman presented to our dermatology clinic with an enlarging lesion on the left anterior aspect of the scalp of 4 years’ duration. She had a history of breast carcinoma in the left breast with positive lymph nodes 2 years prior. Physical examination revealed a 2.5-cm pink, pearly, exophytic plaque on the left anterior aspect of the scalp. The lesion was removed with clear margins by excisional surgery.
The Diagnosis: Clear Cell Hidradenoma
Clear cell hidradenoma (CCH) is a variant of nodular hidradenoma that may contain varying quantities of solid and cystic components and comprises approximately one-third of hidradenomas.1 Clear cell hidradenomas are slow-growing and fairly uncommon adnexal neoplasms derived from either eccrine sweat glands or apocrine glands. Some researchers have regarded hidradenomas as apocrine tumors due to evidence of apocrine decapitation secretion, whereas others note the lack of apocrine and ultrastructural features of immature eccrine glands.2 Clear cell hidradenomas typically develop between the fourth and eighth decades of life, usually peaking during the sixth decade.3 Clear cell hidradenomas usually range in size from 5 to 30 mm and frequently present on the scalp, head, chest, and abdomen; rarely, CCHs present on the joint spaces of the shoulders and knees.3-5 This neoplasm is more common in women than men3 and generally has a flesh-colored, erythematous, red-brown or blue appearance with a tendency to ulcerate and exude a serous discharge (Figure 1).5 The clinical differential diagnosis includes metastatic cancer (eg, renal cell carcinoma, keratoacanthoma, trichoblastoma, trichilemmoma) or other benign adnexal neoplasms.
Histopathologic examination of a CCH generally reveals an unencapsulated and circumscribed neoplasm in the mid or upper dermis with occasional extensions into the subcutaneous fat (Figure 2). The tumor typically presents with 2 types of cells: (1) round, fusiform, or polygonal cells with vesicular nuclei and eosinophilic cytoplasm, and (2) cells with clear cytoplasm and basophilic, often eccentrically located nuclei.6 Ducts are scattered within the neoplasm and are lined by a layer of cuboidal cells that can be highlighted on carcinoembryonic antigen and epithelial membrane antigen immunostaining.6 The tumor cells themselves are highlighted on cytokeratin AE1/AE3 staining.
Malignant transformation rarely is associated with CCH, with de novo clear cell hidradenocarcinoma being more common. Only approximately 6.7% of CCHs have been shown to be malignant, and the malignant tumors feature nuclear atypia, abnormal mitotic figures, necrosis, and infiltration.1,7 Although CCH is a benign adnexal neoplasm, it has a high recurrence rate (approximately 10%) following excision.7 The treatment of choice is complete surgical excision, though Mohs micrographic surgery is advocated, as it promotes thorough examination of the tumor margin to ensure complete tumor removal.8 Our case illustrates the importance of a broad differential diagnosis when treating patients with CCH as well as keeping in mind nonmalignant lesions are far more common than malignant lesions.
A 70-year-old woman presented to our dermatology clinic with an enlarging lesion on the left anterior aspect of the scalp of 4 years’ duration. She had a history of breast carcinoma in the left breast with positive lymph nodes 2 years prior. Physical examination revealed a 2.5-cm pink, pearly, exophytic plaque on the left anterior aspect of the scalp. The lesion was removed with clear margins by excisional surgery.
The Diagnosis: Clear Cell Hidradenoma
Clear cell hidradenoma (CCH) is a variant of nodular hidradenoma that may contain varying quantities of solid and cystic components and comprises approximately one-third of hidradenomas.1 Clear cell hidradenomas are slow-growing and fairly uncommon adnexal neoplasms derived from either eccrine sweat glands or apocrine glands. Some researchers have regarded hidradenomas as apocrine tumors due to evidence of apocrine decapitation secretion, whereas others note the lack of apocrine and ultrastructural features of immature eccrine glands.2 Clear cell hidradenomas typically develop between the fourth and eighth decades of life, usually peaking during the sixth decade.3 Clear cell hidradenomas usually range in size from 5 to 30 mm and frequently present on the scalp, head, chest, and abdomen; rarely, CCHs present on the joint spaces of the shoulders and knees.3-5 This neoplasm is more common in women than men3 and generally has a flesh-colored, erythematous, red-brown or blue appearance with a tendency to ulcerate and exude a serous discharge (Figure 1).5 The clinical differential diagnosis includes metastatic cancer (eg, renal cell carcinoma, keratoacanthoma, trichoblastoma, trichilemmoma) or other benign adnexal neoplasms.
Histopathologic examination of a CCH generally reveals an unencapsulated and circumscribed neoplasm in the mid or upper dermis with occasional extensions into the subcutaneous fat (Figure 2). The tumor typically presents with 2 types of cells: (1) round, fusiform, or polygonal cells with vesicular nuclei and eosinophilic cytoplasm, and (2) cells with clear cytoplasm and basophilic, often eccentrically located nuclei.6 Ducts are scattered within the neoplasm and are lined by a layer of cuboidal cells that can be highlighted on carcinoembryonic antigen and epithelial membrane antigen immunostaining.6 The tumor cells themselves are highlighted on cytokeratin AE1/AE3 staining.
Malignant transformation rarely is associated with CCH, with de novo clear cell hidradenocarcinoma being more common. Only approximately 6.7% of CCHs have been shown to be malignant, and the malignant tumors feature nuclear atypia, abnormal mitotic figures, necrosis, and infiltration.1,7 Although CCH is a benign adnexal neoplasm, it has a high recurrence rate (approximately 10%) following excision.7 The treatment of choice is complete surgical excision, though Mohs micrographic surgery is advocated, as it promotes thorough examination of the tumor margin to ensure complete tumor removal.8 Our case illustrates the importance of a broad differential diagnosis when treating patients with CCH as well as keeping in mind nonmalignant lesions are far more common than malignant lesions.
1. Volmar K, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
2. Goh SG, Carr R, Dayrit JF, et al. Mucinous hidradenoma: a report of three cases. J Cutan Pathol. 2007;34:497-502.
3. Gonul M, Cakmak SK, Gul U, et al. A skin tumor in a young girl. diagnosis: clear cell hidradenoma. Indian J Dermatol Venereol Leprol. 2010;76:445-446.
4. Singhal V, Sharma SC, Anil J, et al. Giant benign nodular hidradenoma of the shoulder: a rare tumor of orthopedic practice. Int J Shoulder Surg. 2010;4:93-96.
5. Yu G, Goodloe S Jr, D’Angelis CA, et al. Giant clear cell hidradenoma of the knee. J Cutan Pathol. 2010;37:E37-E41.
6. McKee PH, Calonje E, Granter SR. Tumors of the sweat glands. In: McKee PH, Calonje E, Granter SR. Pathology of the Skin With Clinical Correlations. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2005:1632-1635.
7. Ozawa T, Fujiwara M, Nose K, et al. Clear-cell hidradenoma of the forearm in a young boy. Pediatr Dermatol. 2005;22:450-452.
8. Yavel R, Hinshaw M, Rao V, et al. Hidradenoma and a hidradenocarcinoma of the scalp managed by Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatol Surg. 2009;35:273-281.
1. Volmar K, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
2. Goh SG, Carr R, Dayrit JF, et al. Mucinous hidradenoma: a report of three cases. J Cutan Pathol. 2007;34:497-502.
3. Gonul M, Cakmak SK, Gul U, et al. A skin tumor in a young girl. diagnosis: clear cell hidradenoma. Indian J Dermatol Venereol Leprol. 2010;76:445-446.
4. Singhal V, Sharma SC, Anil J, et al. Giant benign nodular hidradenoma of the shoulder: a rare tumor of orthopedic practice. Int J Shoulder Surg. 2010;4:93-96.
5. Yu G, Goodloe S Jr, D’Angelis CA, et al. Giant clear cell hidradenoma of the knee. J Cutan Pathol. 2010;37:E37-E41.
6. McKee PH, Calonje E, Granter SR. Tumors of the sweat glands. In: McKee PH, Calonje E, Granter SR. Pathology of the Skin With Clinical Correlations. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2005:1632-1635.
7. Ozawa T, Fujiwara M, Nose K, et al. Clear-cell hidradenoma of the forearm in a young boy. Pediatr Dermatol. 2005;22:450-452.
8. Yavel R, Hinshaw M, Rao V, et al. Hidradenoma and a hidradenocarcinoma of the scalp managed by Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatol Surg. 2009;35:273-281.
Large Subcutaneous Masses
The Diagnosis: Madelung Disease (Benign Symmetric Lipomatosis)
A 56-year-old man presented for evaluation of massive subcutaneous nodules the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs (Figures 1 and 2) that developed over the last 12 to 18 months and continued to enlarge. In addition, he was beginning to develop symptoms of neuropathy of the bilateral hands. The patient had a long-standing history of alcohol abuse. Biopsies performed by the patient’s primary care physician revealed benign adipose tissue. He was referred to the dermatology clinic and subsequently diagnosed with Madelung disease.
|
|
Madelung disease, also known as benign symmetric lipomatosis and Launois-Bensaude syndrome, is characterized by multiple large masses of nonencapsulated adipose tissue. These masses are symmetric and most prominent on the head, neck, trunk, and proximal extremities. Classically, a pseudoathletic appearance is described. Madelung disease most frequently affects men aged 30 to 60 years. In more than 90% of cases, it is associated with alcoholism.
In general, the masses of adipose tissue are asymptomatic. However, airway compression and dysphagia requiring surgical intervention has been reported in the otolaryngology literature.1 In addition, neuropathy develops in 84% of cases.2 Nerve biopsies from patients with Madelung disease have revealed a pattern of axonopathy that is distinct from alcohol-induced neuropathy.3 This neuropathy can involve sensory and motor nerves, with the most prominent findings being muscle weakness, tendon areflexia, interosseous muscle atrophy, vibratory sensation loss, and hypoesthesia. Furthermore, dysfunction of the autonomic nervous system can lead to segmental hyperhidrosis, gustatory sweating, and abnormal autonomic cardiovascular reflexes.2 Many patients who develop neuropathy will eventually become incapacitated.
The etiology of Madelung disease is not fully understood. There are several theories on the pathogenesis of this disease, most describing metabolic disturbances induced by alcohol. Specifically, studies have revealed chronic alcohol use causes numerous deletions in mitochondrial DNA.4,5 The mitochondrial DNA damage may explain both the resistance of the lipomatous masses to lipolysis and the nerve-related changes. Comparisons between human immunodeficiency virus/highly active antiretroviral therapy–associated lipodystrophy and Madelung disease lend credence to the metabolic disturbance theory and may help clarify the specific mechanisms involved.6
There have been no cases of spontaneous resolution, even in patients who stop consuming alcohol. For this reason, most patients are referred to a surgeon. Many surgeons prefer open excision for debulking large lipomatous masses, but this technique typically requires general anesthesia. For those in whom this treatment is not an appropriate option, liposuction can be considered with tumescent anesthesia.7 A combination of these surgical modalities also is an option.
Madelung disease is a distinctive disorder typically affecting chronic alcoholics. Recognition of this clinical entity is important, as severe neuropathy and airway compromise may ensue. Although surgical excision is an attractive option for cosmesis and airway compromise, the associated neuropathy can be extremely difficult to treat and can be quite debilitating.
1. Palacios E, Neitzschman HR, Nguyen J. Madelung disease: multiple symmetric lipomatosis. Ear Nose Throat J. 2014;93:94-96.
2. Enzi G, Angelini C, Negrin P, et al. Sensory, motor, and autonomic neuropathy in patients with multiple symmetric lipomatosis. Medicine (Baltimore). 1985;64:388-393.
3. Pollock M, Nicholson GI, Nukada H, et al. Neuropathy in multiple symmetric lipomatosis. Madelung’s disease. Brain. 1988;111:1157-1171.
4. Klopstock T, Naumann M, Schalke B, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology. 1994;44:862-866.
5. Mansouri A, Fromenty B, Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96-102.
6. Urso R, Gentile M. Are ‘buffalo hump’ syndrome, Madelung's disease and multiple symmetrical lipomatosis variants of the same dysmetabolism? AIDS. 2001;15:290-291.
7. Grassegger A, Häussler R, Schmalzl F. Tumescent liposuction in a patient with Launois-Bensaude syndrome and severe hepatopathy. Dermatol Surg. 2007;33:982-985.
The Diagnosis: Madelung Disease (Benign Symmetric Lipomatosis)
A 56-year-old man presented for evaluation of massive subcutaneous nodules the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs (Figures 1 and 2) that developed over the last 12 to 18 months and continued to enlarge. In addition, he was beginning to develop symptoms of neuropathy of the bilateral hands. The patient had a long-standing history of alcohol abuse. Biopsies performed by the patient’s primary care physician revealed benign adipose tissue. He was referred to the dermatology clinic and subsequently diagnosed with Madelung disease.

|

|
Madelung disease, also known as benign symmetric lipomatosis and Launois-Bensaude syndrome, is characterized by multiple large masses of nonencapsulated adipose tissue. These masses are symmetric and most prominent on the head, neck, trunk, and proximal extremities. Classically, a pseudoathletic appearance is described. Madelung disease most frequently affects men aged 30 to 60 years. In more than 90% of cases, it is associated with alcoholism.
In general, the masses of adipose tissue are asymptomatic. However, airway compression and dysphagia requiring surgical intervention has been reported in the otolaryngology literature.1 In addition, neuropathy develops in 84% of cases.2 Nerve biopsies from patients with Madelung disease have revealed a pattern of axonopathy that is distinct from alcohol-induced neuropathy.3 This neuropathy can involve sensory and motor nerves, with the most prominent findings being muscle weakness, tendon areflexia, interosseous muscle atrophy, vibratory sensation loss, and hypoesthesia. Furthermore, dysfunction of the autonomic nervous system can lead to segmental hyperhidrosis, gustatory sweating, and abnormal autonomic cardiovascular reflexes.2 Many patients who develop neuropathy will eventually become incapacitated.
The etiology of Madelung disease is not fully understood. There are several theories on the pathogenesis of this disease, most describing metabolic disturbances induced by alcohol. Specifically, studies have revealed chronic alcohol use causes numerous deletions in mitochondrial DNA.4,5 The mitochondrial DNA damage may explain both the resistance of the lipomatous masses to lipolysis and the nerve-related changes. Comparisons between human immunodeficiency virus/highly active antiretroviral therapy–associated lipodystrophy and Madelung disease lend credence to the metabolic disturbance theory and may help clarify the specific mechanisms involved.6
There have been no cases of spontaneous resolution, even in patients who stop consuming alcohol. For this reason, most patients are referred to a surgeon. Many surgeons prefer open excision for debulking large lipomatous masses, but this technique typically requires general anesthesia. For those in whom this treatment is not an appropriate option, liposuction can be considered with tumescent anesthesia.7 A combination of these surgical modalities also is an option.
Madelung disease is a distinctive disorder typically affecting chronic alcoholics. Recognition of this clinical entity is important, as severe neuropathy and airway compromise may ensue. Although surgical excision is an attractive option for cosmesis and airway compromise, the associated neuropathy can be extremely difficult to treat and can be quite debilitating.
The Diagnosis: Madelung Disease (Benign Symmetric Lipomatosis)
A 56-year-old man presented for evaluation of massive subcutaneous nodules the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs (Figures 1 and 2) that developed over the last 12 to 18 months and continued to enlarge. In addition, he was beginning to develop symptoms of neuropathy of the bilateral hands. The patient had a long-standing history of alcohol abuse. Biopsies performed by the patient’s primary care physician revealed benign adipose tissue. He was referred to the dermatology clinic and subsequently diagnosed with Madelung disease.

|

|
Madelung disease, also known as benign symmetric lipomatosis and Launois-Bensaude syndrome, is characterized by multiple large masses of nonencapsulated adipose tissue. These masses are symmetric and most prominent on the head, neck, trunk, and proximal extremities. Classically, a pseudoathletic appearance is described. Madelung disease most frequently affects men aged 30 to 60 years. In more than 90% of cases, it is associated with alcoholism.
In general, the masses of adipose tissue are asymptomatic. However, airway compression and dysphagia requiring surgical intervention has been reported in the otolaryngology literature.1 In addition, neuropathy develops in 84% of cases.2 Nerve biopsies from patients with Madelung disease have revealed a pattern of axonopathy that is distinct from alcohol-induced neuropathy.3 This neuropathy can involve sensory and motor nerves, with the most prominent findings being muscle weakness, tendon areflexia, interosseous muscle atrophy, vibratory sensation loss, and hypoesthesia. Furthermore, dysfunction of the autonomic nervous system can lead to segmental hyperhidrosis, gustatory sweating, and abnormal autonomic cardiovascular reflexes.2 Many patients who develop neuropathy will eventually become incapacitated.
The etiology of Madelung disease is not fully understood. There are several theories on the pathogenesis of this disease, most describing metabolic disturbances induced by alcohol. Specifically, studies have revealed chronic alcohol use causes numerous deletions in mitochondrial DNA.4,5 The mitochondrial DNA damage may explain both the resistance of the lipomatous masses to lipolysis and the nerve-related changes. Comparisons between human immunodeficiency virus/highly active antiretroviral therapy–associated lipodystrophy and Madelung disease lend credence to the metabolic disturbance theory and may help clarify the specific mechanisms involved.6
There have been no cases of spontaneous resolution, even in patients who stop consuming alcohol. For this reason, most patients are referred to a surgeon. Many surgeons prefer open excision for debulking large lipomatous masses, but this technique typically requires general anesthesia. For those in whom this treatment is not an appropriate option, liposuction can be considered with tumescent anesthesia.7 A combination of these surgical modalities also is an option.
Madelung disease is a distinctive disorder typically affecting chronic alcoholics. Recognition of this clinical entity is important, as severe neuropathy and airway compromise may ensue. Although surgical excision is an attractive option for cosmesis and airway compromise, the associated neuropathy can be extremely difficult to treat and can be quite debilitating.
1. Palacios E, Neitzschman HR, Nguyen J. Madelung disease: multiple symmetric lipomatosis. Ear Nose Throat J. 2014;93:94-96.
2. Enzi G, Angelini C, Negrin P, et al. Sensory, motor, and autonomic neuropathy in patients with multiple symmetric lipomatosis. Medicine (Baltimore). 1985;64:388-393.
3. Pollock M, Nicholson GI, Nukada H, et al. Neuropathy in multiple symmetric lipomatosis. Madelung’s disease. Brain. 1988;111:1157-1171.
4. Klopstock T, Naumann M, Schalke B, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology. 1994;44:862-866.
5. Mansouri A, Fromenty B, Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96-102.
6. Urso R, Gentile M. Are ‘buffalo hump’ syndrome, Madelung's disease and multiple symmetrical lipomatosis variants of the same dysmetabolism? AIDS. 2001;15:290-291.
7. Grassegger A, Häussler R, Schmalzl F. Tumescent liposuction in a patient with Launois-Bensaude syndrome and severe hepatopathy. Dermatol Surg. 2007;33:982-985.
1. Palacios E, Neitzschman HR, Nguyen J. Madelung disease: multiple symmetric lipomatosis. Ear Nose Throat J. 2014;93:94-96.
2. Enzi G, Angelini C, Negrin P, et al. Sensory, motor, and autonomic neuropathy in patients with multiple symmetric lipomatosis. Medicine (Baltimore). 1985;64:388-393.
3. Pollock M, Nicholson GI, Nukada H, et al. Neuropathy in multiple symmetric lipomatosis. Madelung’s disease. Brain. 1988;111:1157-1171.
4. Klopstock T, Naumann M, Schalke B, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology. 1994;44:862-866.
5. Mansouri A, Fromenty B, Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96-102.
6. Urso R, Gentile M. Are ‘buffalo hump’ syndrome, Madelung's disease and multiple symmetrical lipomatosis variants of the same dysmetabolism? AIDS. 2001;15:290-291.
7. Grassegger A, Häussler R, Schmalzl F. Tumescent liposuction in a patient with Launois-Bensaude syndrome and severe hepatopathy. Dermatol Surg. 2007;33:982-985.
A 56-year-old man presented for evaluation of massive subcutaneous nodules on the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs that developed over the last 12 to 18 months and continued to enlarge. His medical history was remarkable for alcoholism, hyperlipidemia, and hypertension.
Cutaneous Manifestations of Cocaine Use
The Diagnosis: Levamisole-Induced Cutaneous Vasculopathy
In our patient, tender stellate purpura and occasional bullae were present on the ears, arms and legs, groin, and buttocks (Figure 1). Histopathologic examination revealed subepidermal detachment, perivascular neutrophilic infiltrate, and red blood cell extravasation, consistent with early leukocyctoclastic vasculitis (Figure 2).
|
Levamisole-induced vasculopathy is a condition related primarily to cocaine use. Levamisole is an immunomodulatory agent, historically used as a disease-modifying antirheumatic drug for rheumatoid arthritis and as adjuvant chemotherapy for various types of cancer. However, levamisole for human use was banned from US and Canadian markets in 1999 and 2003, respectively, due to increased risk for agranulocytosis, retiform purpura, and epilepsy.1 Currently, veterinarians use levamisole as an anthelminthic agent to deworm house and farm animals. In Europe, pediatric nephrologists use it as a steroid-sparing agent in children with steroid-dependent nephritic syndrome.
Over the last decade, levamisole has increasingly been used as a cocaine adulterant or bulking agent. This contaminant closely resembles cocaine physically and is theorized to prolong or attenuate cocaine’s “high.” Approximately 69% of cocaine sampled by the US Drug Enforcement Administration is adulterated with levamisole.2 Similarly, levamisole-contaminated cocaine also has been found in Europe, Australia, and other parts of the world. Potential complications include vasculitis, thromboembolism, neutropenia, and agranulocytosis.3
Levamisole-induced vasculopathy appears to affect cocaine users of all ages, ethnicities, and genders. Cocaine can be smoked, snorted, or injected. In nearly all reported cases, patients characteristically present with hemorrhagic bullae of the bilateral ear helix, cheeks, or nasal tip. Any body site can be affected with retiform purpura or necrotic bullae. Along with skin lesions, arthralgia is commonly reported, as are constitutional symptoms (eg, fever, night sweats, weight loss, malaise)4; oral mucosal involvement also has been reported.5 Laboratory investigation can reveal neutropenia, positive antineutrophil cytoplasmic antibodies (ANCAs) in the perinuclear or cytoplasmic pattern, positive proteinase 3, and negative or mildly elevated antimyeloperoxidase.3-5 Acute renal injury and pulmonary hemorrhage are other potentially serious copmlications.4 Antihuman neutrophil elastase antibody testing can help distinguish levamisole-induced vasculopathy from other forms of immune-mediated vasculitis and will be negative in immune-mediated vasculitides such as Churg-Strauss syndrome (allergic granulomatosis), Wegener granulomatosis (granulomatosis with polyangiitis), and polyarteritis nodosa.6 On histology, microvascular thrombosis or leukocytoclastic vasculitis can both, or individually, be seen. Epidermal necrosis, dermal hemorrhage, and endothelial hyperplasia have all been noted in skin biopsied from necrotic bullae.
|
Levamisole’s short half-life (approximately 5–6 hours) makes it difficult to detect on routine blood draws. An astute physician suspecting this diagnosis on initial presentation can ask for levamisole detection on urine toxicology screening.7 Urine samples also can be sent for testing with gas chromatography–mass spectrometry, though this test may only be available at major research centers.8
Differential diagnosis of levamisole toxicity includes different types of vasculitides such as cryoglobulinemia (positive serum IgM and IgG cryoglobulins; possible hepatitis C infection), Wegener granulomatosis (cytoplasmic ANCA positive; associated with upper and lower respiratory tract inflammation, glomerulonephritis), Churg-Strauss syndrome (perinuclear ANCA positive; associated with asthma and eosinophilia), and polyarteritis nodosa (medium vessel involvement only; associated with livedo reticularis, subcutaneous nodules, ulcers).9 Necrotic lesions also may raise the possibility of warfarin necrosis, heparin necrosis, or cholesterol emboli. Cholesterol embolism most frequently presents with small vessel vasculitis and necrosis of distal extremities such as the toes. With large areas of skin involvement and bullae, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis also should be considered.9
Definitive treatment of this condition requires complete and immediate cessation of cocaine use. Levamisole also has been found as a contaminant in heroin.1 Thus, it may be prudent to recommend heroin avoidance to the patient to prevent recurrences. Management of acute levamisole-induced vasculopathy is primarily symptomatic. Some patients with severe neutropenia at risk for infection have been treated with granulocyte colony-stimulating factor, while others have only required pain control, usually with nonsteroidal anti-inflammatory drugs.10 Oral prednisone and colchicine also have been used with reported success.5
Given the increasing incidence of levamisole toxicity and public health implications, clinicians should be aware of this association and the classic clinical and laboratory findings.
1. Aberastury MN, Silva WH, Vaccarezza MM, et al. Epilepsia partialis continua associated with levamisole. Pediatr Neurol. 2011;44:385-388.
2. Nationwide public health alert issued concerning life-threatening risk posed by cocaine laced with veterinary anti-parasite drug [press release]. Rockville, MD: Substance Abuse and Mental Health Services Administration; September 21, 2009. http://beta.samhsa.gov/newsroom/press-announcements/200909211245. Accessed October 9, 2014.
3. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
4. McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasm antibody-associated disease. Clin J Am Soc Nephrol. 2011;6:2799-2805.
5. Poon SH, Baliog CR Jr, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leucopenia. Semin Arthritis Rheum. 2011;41:434-444.
6. Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
7. Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
8. Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2001;35:545-550.
9. Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
10. Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
The Diagnosis: Levamisole-Induced Cutaneous Vasculopathy
In our patient, tender stellate purpura and occasional bullae were present on the ears, arms and legs, groin, and buttocks (Figure 1). Histopathologic examination revealed subepidermal detachment, perivascular neutrophilic infiltrate, and red blood cell extravasation, consistent with early leukocyctoclastic vasculitis (Figure 2).
|
Levamisole-induced vasculopathy is a condition related primarily to cocaine use. Levamisole is an immunomodulatory agent, historically used as a disease-modifying antirheumatic drug for rheumatoid arthritis and as adjuvant chemotherapy for various types of cancer. However, levamisole for human use was banned from US and Canadian markets in 1999 and 2003, respectively, due to increased risk for agranulocytosis, retiform purpura, and epilepsy.1 Currently, veterinarians use levamisole as an anthelminthic agent to deworm house and farm animals. In Europe, pediatric nephrologists use it as a steroid-sparing agent in children with steroid-dependent nephritic syndrome.
Over the last decade, levamisole has increasingly been used as a cocaine adulterant or bulking agent. This contaminant closely resembles cocaine physically and is theorized to prolong or attenuate cocaine’s “high.” Approximately 69% of cocaine sampled by the US Drug Enforcement Administration is adulterated with levamisole.2 Similarly, levamisole-contaminated cocaine also has been found in Europe, Australia, and other parts of the world. Potential complications include vasculitis, thromboembolism, neutropenia, and agranulocytosis.3
Levamisole-induced vasculopathy appears to affect cocaine users of all ages, ethnicities, and genders. Cocaine can be smoked, snorted, or injected. In nearly all reported cases, patients characteristically present with hemorrhagic bullae of the bilateral ear helix, cheeks, or nasal tip. Any body site can be affected with retiform purpura or necrotic bullae. Along with skin lesions, arthralgia is commonly reported, as are constitutional symptoms (eg, fever, night sweats, weight loss, malaise)4; oral mucosal involvement also has been reported.5 Laboratory investigation can reveal neutropenia, positive antineutrophil cytoplasmic antibodies (ANCAs) in the perinuclear or cytoplasmic pattern, positive proteinase 3, and negative or mildly elevated antimyeloperoxidase.3-5 Acute renal injury and pulmonary hemorrhage are other potentially serious copmlications.4 Antihuman neutrophil elastase antibody testing can help distinguish levamisole-induced vasculopathy from other forms of immune-mediated vasculitis and will be negative in immune-mediated vasculitides such as Churg-Strauss syndrome (allergic granulomatosis), Wegener granulomatosis (granulomatosis with polyangiitis), and polyarteritis nodosa.6 On histology, microvascular thrombosis or leukocytoclastic vasculitis can both, or individually, be seen. Epidermal necrosis, dermal hemorrhage, and endothelial hyperplasia have all been noted in skin biopsied from necrotic bullae.
|
Levamisole’s short half-life (approximately 5–6 hours) makes it difficult to detect on routine blood draws. An astute physician suspecting this diagnosis on initial presentation can ask for levamisole detection on urine toxicology screening.7 Urine samples also can be sent for testing with gas chromatography–mass spectrometry, though this test may only be available at major research centers.8
Differential diagnosis of levamisole toxicity includes different types of vasculitides such as cryoglobulinemia (positive serum IgM and IgG cryoglobulins; possible hepatitis C infection), Wegener granulomatosis (cytoplasmic ANCA positive; associated with upper and lower respiratory tract inflammation, glomerulonephritis), Churg-Strauss syndrome (perinuclear ANCA positive; associated with asthma and eosinophilia), and polyarteritis nodosa (medium vessel involvement only; associated with livedo reticularis, subcutaneous nodules, ulcers).9 Necrotic lesions also may raise the possibility of warfarin necrosis, heparin necrosis, or cholesterol emboli. Cholesterol embolism most frequently presents with small vessel vasculitis and necrosis of distal extremities such as the toes. With large areas of skin involvement and bullae, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis also should be considered.9
Definitive treatment of this condition requires complete and immediate cessation of cocaine use. Levamisole also has been found as a contaminant in heroin.1 Thus, it may be prudent to recommend heroin avoidance to the patient to prevent recurrences. Management of acute levamisole-induced vasculopathy is primarily symptomatic. Some patients with severe neutropenia at risk for infection have been treated with granulocyte colony-stimulating factor, while others have only required pain control, usually with nonsteroidal anti-inflammatory drugs.10 Oral prednisone and colchicine also have been used with reported success.5
Given the increasing incidence of levamisole toxicity and public health implications, clinicians should be aware of this association and the classic clinical and laboratory findings.
The Diagnosis: Levamisole-Induced Cutaneous Vasculopathy
In our patient, tender stellate purpura and occasional bullae were present on the ears, arms and legs, groin, and buttocks (Figure 1). Histopathologic examination revealed subepidermal detachment, perivascular neutrophilic infiltrate, and red blood cell extravasation, consistent with early leukocyctoclastic vasculitis (Figure 2).
|
Levamisole-induced vasculopathy is a condition related primarily to cocaine use. Levamisole is an immunomodulatory agent, historically used as a disease-modifying antirheumatic drug for rheumatoid arthritis and as adjuvant chemotherapy for various types of cancer. However, levamisole for human use was banned from US and Canadian markets in 1999 and 2003, respectively, due to increased risk for agranulocytosis, retiform purpura, and epilepsy.1 Currently, veterinarians use levamisole as an anthelminthic agent to deworm house and farm animals. In Europe, pediatric nephrologists use it as a steroid-sparing agent in children with steroid-dependent nephritic syndrome.
Over the last decade, levamisole has increasingly been used as a cocaine adulterant or bulking agent. This contaminant closely resembles cocaine physically and is theorized to prolong or attenuate cocaine’s “high.” Approximately 69% of cocaine sampled by the US Drug Enforcement Administration is adulterated with levamisole.2 Similarly, levamisole-contaminated cocaine also has been found in Europe, Australia, and other parts of the world. Potential complications include vasculitis, thromboembolism, neutropenia, and agranulocytosis.3
Levamisole-induced vasculopathy appears to affect cocaine users of all ages, ethnicities, and genders. Cocaine can be smoked, snorted, or injected. In nearly all reported cases, patients characteristically present with hemorrhagic bullae of the bilateral ear helix, cheeks, or nasal tip. Any body site can be affected with retiform purpura or necrotic bullae. Along with skin lesions, arthralgia is commonly reported, as are constitutional symptoms (eg, fever, night sweats, weight loss, malaise)4; oral mucosal involvement also has been reported.5 Laboratory investigation can reveal neutropenia, positive antineutrophil cytoplasmic antibodies (ANCAs) in the perinuclear or cytoplasmic pattern, positive proteinase 3, and negative or mildly elevated antimyeloperoxidase.3-5 Acute renal injury and pulmonary hemorrhage are other potentially serious copmlications.4 Antihuman neutrophil elastase antibody testing can help distinguish levamisole-induced vasculopathy from other forms of immune-mediated vasculitis and will be negative in immune-mediated vasculitides such as Churg-Strauss syndrome (allergic granulomatosis), Wegener granulomatosis (granulomatosis with polyangiitis), and polyarteritis nodosa.6 On histology, microvascular thrombosis or leukocytoclastic vasculitis can both, or individually, be seen. Epidermal necrosis, dermal hemorrhage, and endothelial hyperplasia have all been noted in skin biopsied from necrotic bullae.
|
Levamisole’s short half-life (approximately 5–6 hours) makes it difficult to detect on routine blood draws. An astute physician suspecting this diagnosis on initial presentation can ask for levamisole detection on urine toxicology screening.7 Urine samples also can be sent for testing with gas chromatography–mass spectrometry, though this test may only be available at major research centers.8
Differential diagnosis of levamisole toxicity includes different types of vasculitides such as cryoglobulinemia (positive serum IgM and IgG cryoglobulins; possible hepatitis C infection), Wegener granulomatosis (cytoplasmic ANCA positive; associated with upper and lower respiratory tract inflammation, glomerulonephritis), Churg-Strauss syndrome (perinuclear ANCA positive; associated with asthma and eosinophilia), and polyarteritis nodosa (medium vessel involvement only; associated with livedo reticularis, subcutaneous nodules, ulcers).9 Necrotic lesions also may raise the possibility of warfarin necrosis, heparin necrosis, or cholesterol emboli. Cholesterol embolism most frequently presents with small vessel vasculitis and necrosis of distal extremities such as the toes. With large areas of skin involvement and bullae, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis also should be considered.9
Definitive treatment of this condition requires complete and immediate cessation of cocaine use. Levamisole also has been found as a contaminant in heroin.1 Thus, it may be prudent to recommend heroin avoidance to the patient to prevent recurrences. Management of acute levamisole-induced vasculopathy is primarily symptomatic. Some patients with severe neutropenia at risk for infection have been treated with granulocyte colony-stimulating factor, while others have only required pain control, usually with nonsteroidal anti-inflammatory drugs.10 Oral prednisone and colchicine also have been used with reported success.5
Given the increasing incidence of levamisole toxicity and public health implications, clinicians should be aware of this association and the classic clinical and laboratory findings.
1. Aberastury MN, Silva WH, Vaccarezza MM, et al. Epilepsia partialis continua associated with levamisole. Pediatr Neurol. 2011;44:385-388.
2. Nationwide public health alert issued concerning life-threatening risk posed by cocaine laced with veterinary anti-parasite drug [press release]. Rockville, MD: Substance Abuse and Mental Health Services Administration; September 21, 2009. http://beta.samhsa.gov/newsroom/press-announcements/200909211245. Accessed October 9, 2014.
3. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
4. McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasm antibody-associated disease. Clin J Am Soc Nephrol. 2011;6:2799-2805.
5. Poon SH, Baliog CR Jr, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leucopenia. Semin Arthritis Rheum. 2011;41:434-444.
6. Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
7. Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
8. Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2001;35:545-550.
9. Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
10. Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
1. Aberastury MN, Silva WH, Vaccarezza MM, et al. Epilepsia partialis continua associated with levamisole. Pediatr Neurol. 2011;44:385-388.
2. Nationwide public health alert issued concerning life-threatening risk posed by cocaine laced with veterinary anti-parasite drug [press release]. Rockville, MD: Substance Abuse and Mental Health Services Administration; September 21, 2009. http://beta.samhsa.gov/newsroom/press-announcements/200909211245. Accessed October 9, 2014.
3. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
4. McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasm antibody-associated disease. Clin J Am Soc Nephrol. 2011;6:2799-2805.
5. Poon SH, Baliog CR Jr, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leucopenia. Semin Arthritis Rheum. 2011;41:434-444.
6. Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
7. Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
8. Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2001;35:545-550.
9. Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
10. Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
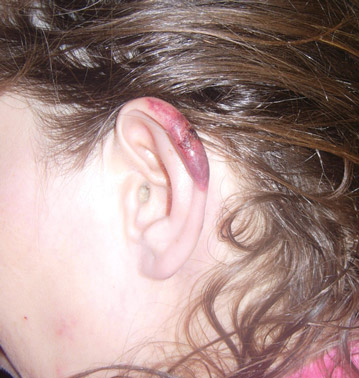
A 43-year-old woman presented to the emergency department with painful skin lesions of 1 day’s duration. Physical examination revealed tender stellate purpura and occasional bullae on the ears, arms and legs, groin, and buttocks. Laboratory results revealed neutropenia and positive lupus anticoagulant; antineutrophil cytoplasmic antibody, antinuclear antibody, and double-stranded DNA antibodies were all negative. Urine toxicology was positive for cocaine and opioids. An incisional biopsy of the left arm was performed.