User login
Nail Psoriasis Tips
What does your patient need to know at the first visit?
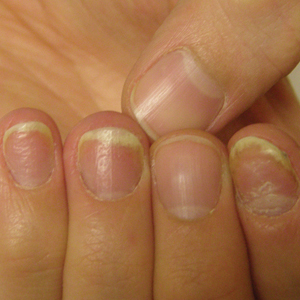
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.
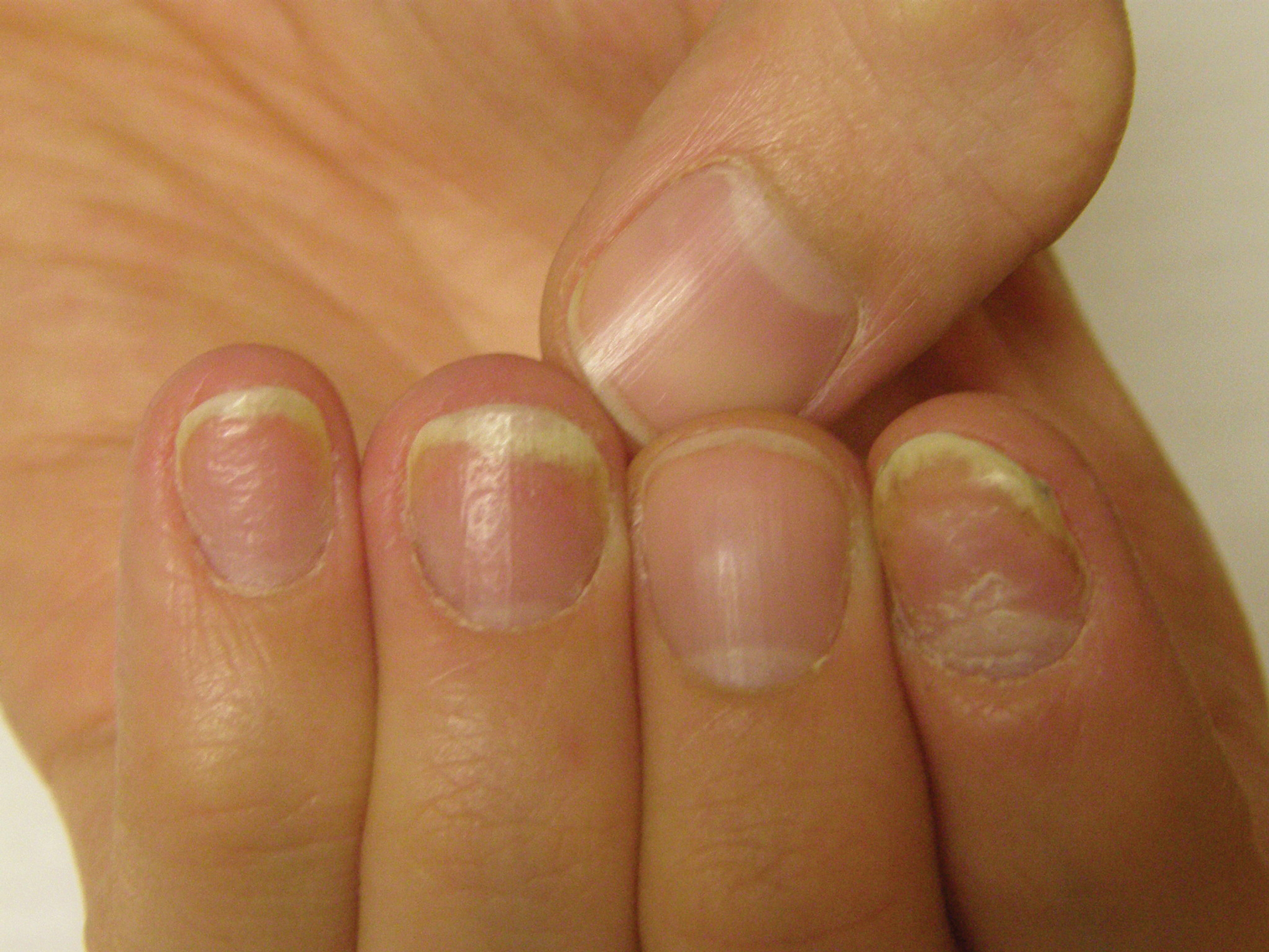
The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.

The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.

The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
Wound Closure Tips
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
Clinical Pearl: Mohs Cantaloupe Analogy for the Dermatology Resident
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
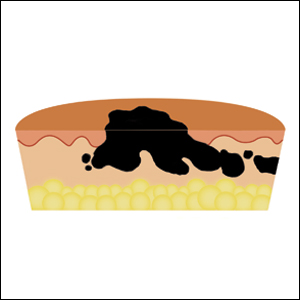
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).

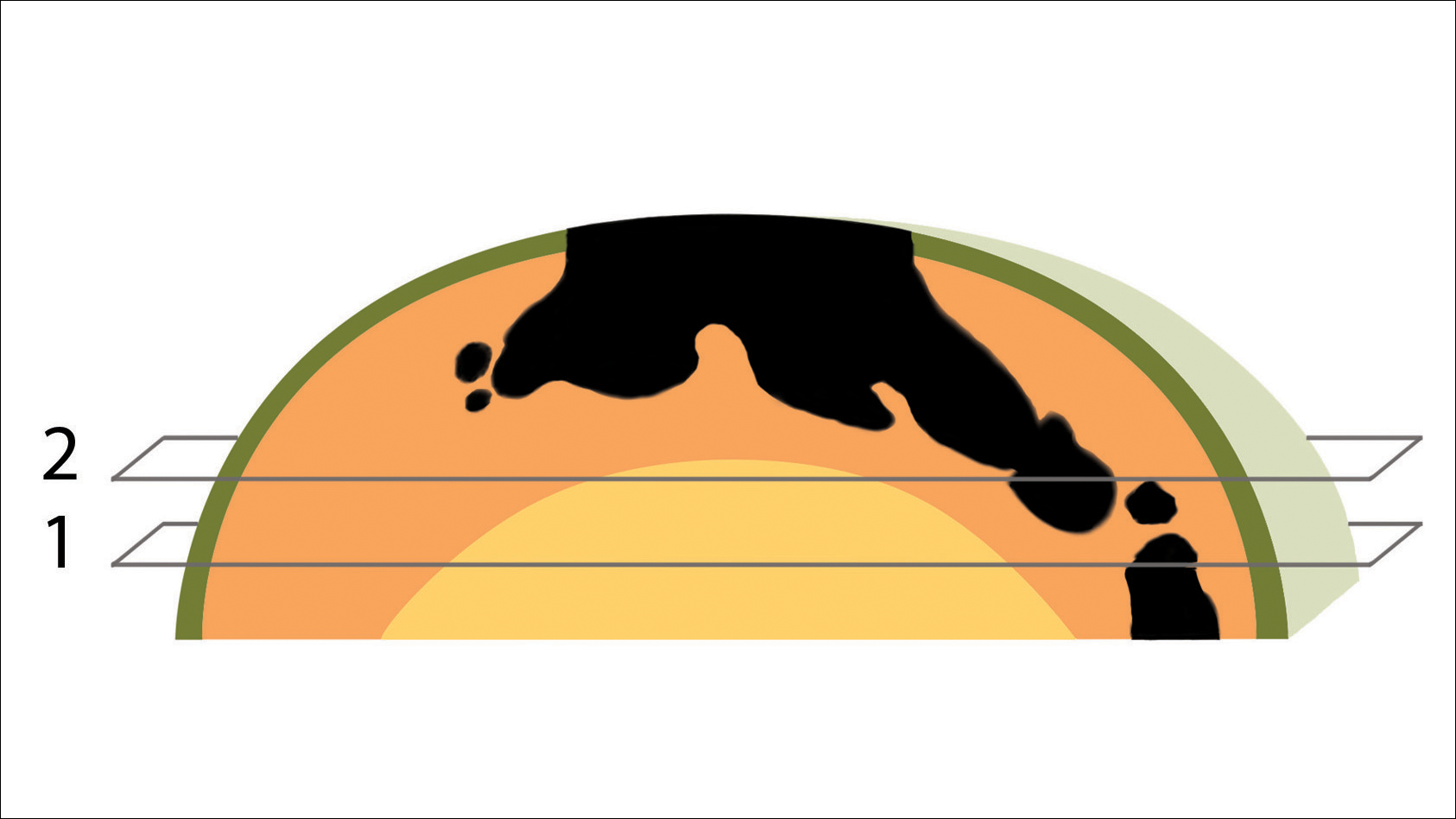
In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.
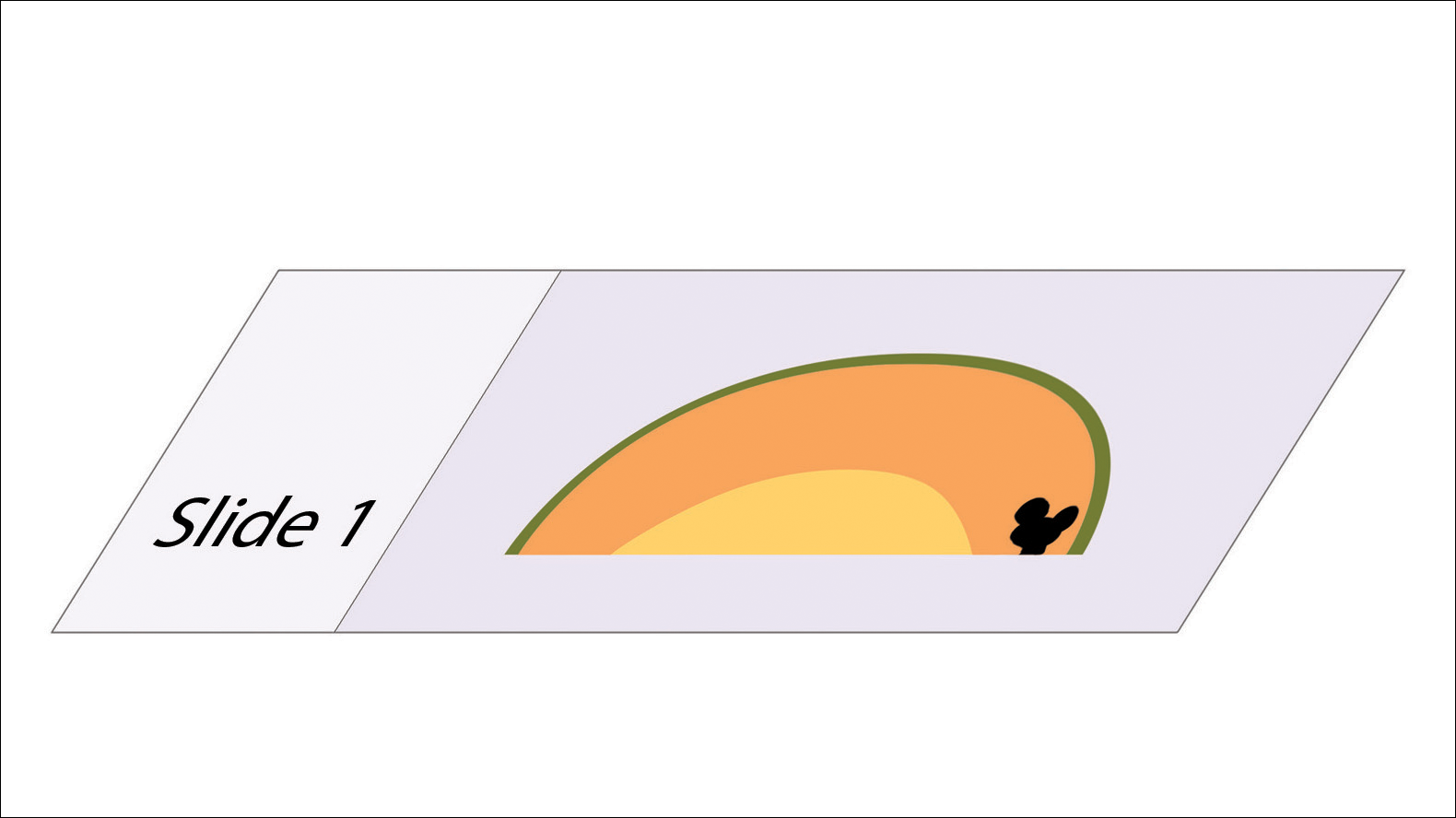
Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
Biologics and Systemic Therapies for Psoriasis: Treat the Patient, Not the Disease
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
Onychomycosis Diagnosis and Long-term Treatment
What does your patient need to know at the first visit?
Risk factors for onychomycosis include prior trauma, history of tinea pedis, sports activities, frequenting gyms and pools, hyperhidrosis, advancing age, diabetes mellitus, immunosuppression, smoking, and family history of onychomycosis. Toenails are involved more frequently than fingernails, and typical physical examination findings are distal and lateral nail plate onycholysis with subungual hyperkeratosis. In more severe cases, there may be nail plate thickening, crumbling, yellowing, and involvement of the nail matrix.
Because other nail conditions may resemble onychomycosis, it is imperative to confirm the diagnosis using histopathology, direct microscopy, fungal culture, and/or polymerase chain reaction on nail plate clippings or subungual debris.
What are your go-to treatments? What are the side effects?
After laboratory confirmation, assess the patient for the severity of the infection based on the surface area of nail plate affected, nail plate thickness, involvement of the nail matrix, and number of nails affected. United States Food and Drug Administration-approved oral and topical antifungals are used first line for the treatment of onychomycosis. Devices such as lasers are approved by the US Food and Drug Administration for temporary cosmetic improvement in the appearance of the nail without eradicating the fungus.
Oral antifungals such as terbinafine, itraconazole, and fluconazole (off label) are indicated for patients with severe disease. Patients with mild to moderate disease may benefit from oral or topical antifungals such as efinaconazole, tavaborole, or ciclopirox.
I recommend terbinafine to many of my patients due to its high complete and mycological cure rates, short list of drug-drug interactions, and low incidence of side effects. Adverse reactions are uncommon, with the most common being gastrointestinal upset. While liver injury has been reported, it is exceedingly rare. Itraconazole has many important drug interactions and is contraindicated in patients with congestive heart failure. With topical antifungals, side effects are uncommon, but dermatitis, ingrown nails, and vesicles may occur.
How do you keep patients compliant with treatment?
Patients on a 3-month course of daily oral terbinafine or itraconazole for toenail onychomycosis are typically highly compliant. Compliance for patients on oral fluconazole (off label) is generally more challenging because it is dosed weekly until the nail grows out (1-1.5 years for toenails). To circumvent missed fluconazole doses, I recommend that the patient schedule quarterly visits with me and also to set a cell phone alarm as a weekly reminder to take the medication.
Because topical medications are prescribed for the toenails for a year-long course (with avoidance of nail polish during this period), I prescribe topical antifungals only to highly motivated patients. In addition, because topical antifungals are retained in the nail plate for at least several days after a month-long application, I tell my patients that if they have a big event to attend that they can take a vacation from the topical antifungal, get a pedicure, and then resume treatment after the event.
What do you do if they refuse treatment?
In 2018, we have many options to treat onychomycosis effectively, and therapy is individualized based on the patient's severity of disease, infecting organism(s), comorbidities, concomitant medications, and preferences. If the patient's fungal nail infection is asymptomatic and not aesthetically bothersome, he/she may opt for observation rather than treatment. If the decision is observation, I recommend use of a topical antifungal on the feet and web spaces to prevent worsening of onychomycosis.
Suggested Readings
Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31:1111-1118.
Lipner SR, Scher RK. Long-standing onychodystrophy in a young woman. JAMA. 2016;316:1915-1916.
Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
Lipner SR, Scher RK. Onychomycosis: current and investigational therapies. Cutis. 2014;94:E21-E24.
What does your patient need to know at the first visit?
Risk factors for onychomycosis include prior trauma, history of tinea pedis, sports activities, frequenting gyms and pools, hyperhidrosis, advancing age, diabetes mellitus, immunosuppression, smoking, and family history of onychomycosis. Toenails are involved more frequently than fingernails, and typical physical examination findings are distal and lateral nail plate onycholysis with subungual hyperkeratosis. In more severe cases, there may be nail plate thickening, crumbling, yellowing, and involvement of the nail matrix.
Because other nail conditions may resemble onychomycosis, it is imperative to confirm the diagnosis using histopathology, direct microscopy, fungal culture, and/or polymerase chain reaction on nail plate clippings or subungual debris.
What are your go-to treatments? What are the side effects?
After laboratory confirmation, assess the patient for the severity of the infection based on the surface area of nail plate affected, nail plate thickness, involvement of the nail matrix, and number of nails affected. United States Food and Drug Administration-approved oral and topical antifungals are used first line for the treatment of onychomycosis. Devices such as lasers are approved by the US Food and Drug Administration for temporary cosmetic improvement in the appearance of the nail without eradicating the fungus.
Oral antifungals such as terbinafine, itraconazole, and fluconazole (off label) are indicated for patients with severe disease. Patients with mild to moderate disease may benefit from oral or topical antifungals such as efinaconazole, tavaborole, or ciclopirox.
I recommend terbinafine to many of my patients due to its high complete and mycological cure rates, short list of drug-drug interactions, and low incidence of side effects. Adverse reactions are uncommon, with the most common being gastrointestinal upset. While liver injury has been reported, it is exceedingly rare. Itraconazole has many important drug interactions and is contraindicated in patients with congestive heart failure. With topical antifungals, side effects are uncommon, but dermatitis, ingrown nails, and vesicles may occur.
How do you keep patients compliant with treatment?
Patients on a 3-month course of daily oral terbinafine or itraconazole for toenail onychomycosis are typically highly compliant. Compliance for patients on oral fluconazole (off label) is generally more challenging because it is dosed weekly until the nail grows out (1-1.5 years for toenails). To circumvent missed fluconazole doses, I recommend that the patient schedule quarterly visits with me and also to set a cell phone alarm as a weekly reminder to take the medication.
Because topical medications are prescribed for the toenails for a year-long course (with avoidance of nail polish during this period), I prescribe topical antifungals only to highly motivated patients. In addition, because topical antifungals are retained in the nail plate for at least several days after a month-long application, I tell my patients that if they have a big event to attend that they can take a vacation from the topical antifungal, get a pedicure, and then resume treatment after the event.
What do you do if they refuse treatment?
In 2018, we have many options to treat onychomycosis effectively, and therapy is individualized based on the patient's severity of disease, infecting organism(s), comorbidities, concomitant medications, and preferences. If the patient's fungal nail infection is asymptomatic and not aesthetically bothersome, he/she may opt for observation rather than treatment. If the decision is observation, I recommend use of a topical antifungal on the feet and web spaces to prevent worsening of onychomycosis.
Suggested Readings
Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31:1111-1118.
Lipner SR, Scher RK. Long-standing onychodystrophy in a young woman. JAMA. 2016;316:1915-1916.
Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
Lipner SR, Scher RK. Onychomycosis: current and investigational therapies. Cutis. 2014;94:E21-E24.
What does your patient need to know at the first visit?
Risk factors for onychomycosis include prior trauma, history of tinea pedis, sports activities, frequenting gyms and pools, hyperhidrosis, advancing age, diabetes mellitus, immunosuppression, smoking, and family history of onychomycosis. Toenails are involved more frequently than fingernails, and typical physical examination findings are distal and lateral nail plate onycholysis with subungual hyperkeratosis. In more severe cases, there may be nail plate thickening, crumbling, yellowing, and involvement of the nail matrix.
Because other nail conditions may resemble onychomycosis, it is imperative to confirm the diagnosis using histopathology, direct microscopy, fungal culture, and/or polymerase chain reaction on nail plate clippings or subungual debris.
What are your go-to treatments? What are the side effects?
After laboratory confirmation, assess the patient for the severity of the infection based on the surface area of nail plate affected, nail plate thickness, involvement of the nail matrix, and number of nails affected. United States Food and Drug Administration-approved oral and topical antifungals are used first line for the treatment of onychomycosis. Devices such as lasers are approved by the US Food and Drug Administration for temporary cosmetic improvement in the appearance of the nail without eradicating the fungus.
Oral antifungals such as terbinafine, itraconazole, and fluconazole (off label) are indicated for patients with severe disease. Patients with mild to moderate disease may benefit from oral or topical antifungals such as efinaconazole, tavaborole, or ciclopirox.
I recommend terbinafine to many of my patients due to its high complete and mycological cure rates, short list of drug-drug interactions, and low incidence of side effects. Adverse reactions are uncommon, with the most common being gastrointestinal upset. While liver injury has been reported, it is exceedingly rare. Itraconazole has many important drug interactions and is contraindicated in patients with congestive heart failure. With topical antifungals, side effects are uncommon, but dermatitis, ingrown nails, and vesicles may occur.
How do you keep patients compliant with treatment?
Patients on a 3-month course of daily oral terbinafine or itraconazole for toenail onychomycosis are typically highly compliant. Compliance for patients on oral fluconazole (off label) is generally more challenging because it is dosed weekly until the nail grows out (1-1.5 years for toenails). To circumvent missed fluconazole doses, I recommend that the patient schedule quarterly visits with me and also to set a cell phone alarm as a weekly reminder to take the medication.
Because topical medications are prescribed for the toenails for a year-long course (with avoidance of nail polish during this period), I prescribe topical antifungals only to highly motivated patients. In addition, because topical antifungals are retained in the nail plate for at least several days after a month-long application, I tell my patients that if they have a big event to attend that they can take a vacation from the topical antifungal, get a pedicure, and then resume treatment after the event.
What do you do if they refuse treatment?
In 2018, we have many options to treat onychomycosis effectively, and therapy is individualized based on the patient's severity of disease, infecting organism(s), comorbidities, concomitant medications, and preferences. If the patient's fungal nail infection is asymptomatic and not aesthetically bothersome, he/she may opt for observation rather than treatment. If the decision is observation, I recommend use of a topical antifungal on the feet and web spaces to prevent worsening of onychomycosis.
Suggested Readings
Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31:1111-1118.
Lipner SR, Scher RK. Long-standing onychodystrophy in a young woman. JAMA. 2016;316:1915-1916.
Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
Lipner SR, Scher RK. Onychomycosis: current and investigational therapies. Cutis. 2014;94:E21-E24.
Pearls in Dermatology: 2017
The Pearls in Dermatology collection consists of our popular pearls from the year in one convenient file. Topics include:
- Nail psoriasis and psoriasis on the hands and feet
- Genital wart treatment
- Isotretinoin for acne
- Cosmeceuticals for rosacea
- Surgical technique with the flexible scalpel blade
Editor’s Commentary provided by Vincent A. DeLeo, MD, Editor-in-Chief, Cutis.
Save this collection, print it, and/or share it with your colleagues. We hope this comprehensive collection will positively impact how you manage patients.

The Pearls in Dermatology collection consists of our popular pearls from the year in one convenient file. Topics include:
- Nail psoriasis and psoriasis on the hands and feet
- Genital wart treatment
- Isotretinoin for acne
- Cosmeceuticals for rosacea
- Surgical technique with the flexible scalpel blade
Editor’s Commentary provided by Vincent A. DeLeo, MD, Editor-in-Chief, Cutis.
Save this collection, print it, and/or share it with your colleagues. We hope this comprehensive collection will positively impact how you manage patients.

The Pearls in Dermatology collection consists of our popular pearls from the year in one convenient file. Topics include:
- Nail psoriasis and psoriasis on the hands and feet
- Genital wart treatment
- Isotretinoin for acne
- Cosmeceuticals for rosacea
- Surgical technique with the flexible scalpel blade
Editor’s Commentary provided by Vincent A. DeLeo, MD, Editor-in-Chief, Cutis.
Save this collection, print it, and/or share it with your colleagues. We hope this comprehensive collection will positively impact how you manage patients.

Topical 5-Fluorouracil Made Easy?
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
Clinical Pearl: A Simple and Effective Technique for Improving Surgical Closures for the Early-Learning Resident
Practice Gap
For first-year dermatology residents, dermatologic surgeries can present many challenges. Although approximation of wound edges following excision may be intuitive for the experienced surgeon, an early trainee may need some guidance. Infusion of anesthetics can distort the normal skin field or it may be difficult for the patient to remain in the same position for the required period of time; for example, an elderly patient who requires an excision on the posterior aspect of the neck may be unable to assume the same position for the full duration of the procedure. We offer a simple and effective technique for early-learning dermatology residents to improve surgical closures.
The Technique
We propose drawing straight lines using a sterile marking pen perpendicular to the fusiform plane laid out for any simple, intermediate, or complex linear closure (Figure 1). These lines can then be used as scaffolding for the surgical closure (Figure 2). We recommend drawing the lines at the time of initial planning when the site of excision is in the normal anatomic position.
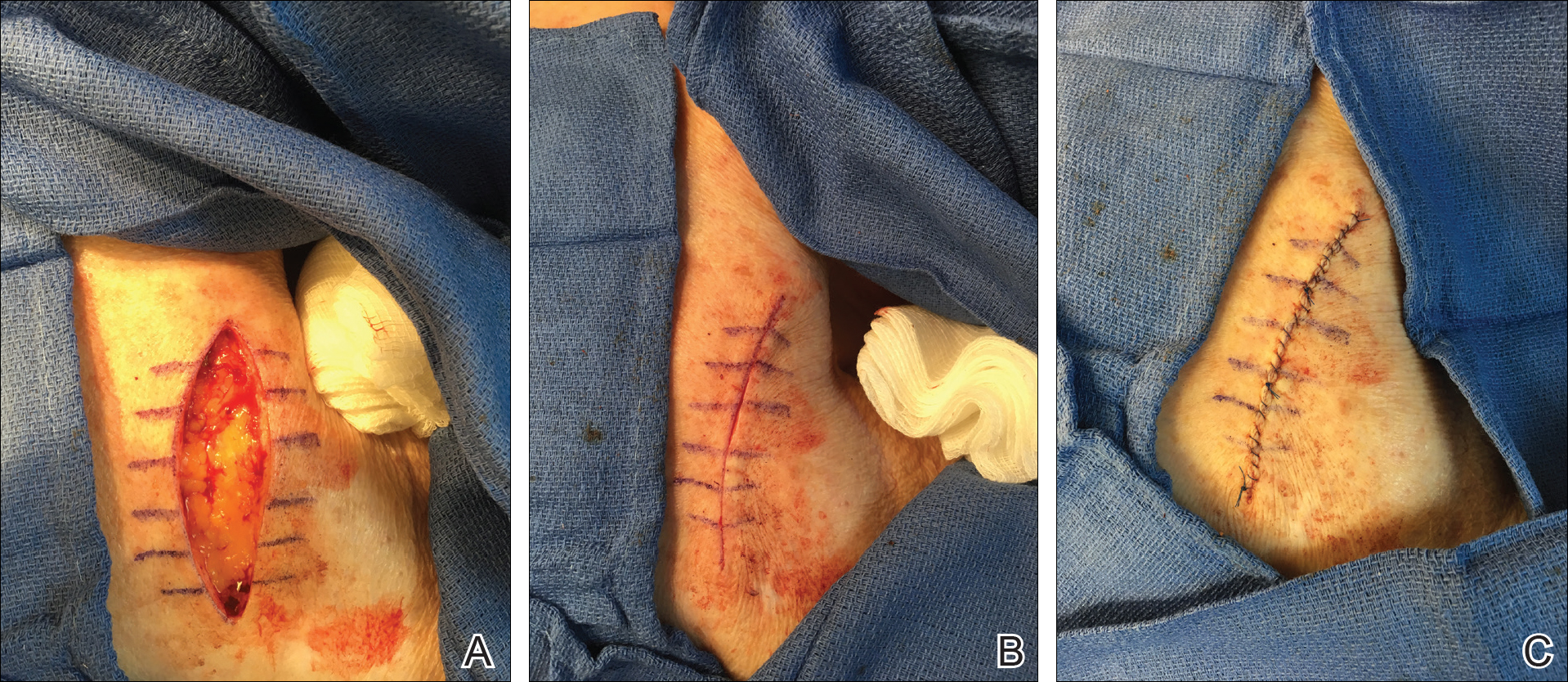
Practice Implications
By creating a sketch with perpendicular lines, approximation of skin edges and surgical closures may become easier for the learning resident. Patients also can rest more comfortably during the procedure, and the overall cosmesis, healing, and outcome of the procedure may improve. The addition of a sterile marking pen to the surgical tray may aide in highlighting faded pen markings for easier visualization after cleansing of the surgical site.
Practice Gap
For first-year dermatology residents, dermatologic surgeries can present many challenges. Although approximation of wound edges following excision may be intuitive for the experienced surgeon, an early trainee may need some guidance. Infusion of anesthetics can distort the normal skin field or it may be difficult for the patient to remain in the same position for the required period of time; for example, an elderly patient who requires an excision on the posterior aspect of the neck may be unable to assume the same position for the full duration of the procedure. We offer a simple and effective technique for early-learning dermatology residents to improve surgical closures.
The Technique
We propose drawing straight lines using a sterile marking pen perpendicular to the fusiform plane laid out for any simple, intermediate, or complex linear closure (Figure 1). These lines can then be used as scaffolding for the surgical closure (Figure 2). We recommend drawing the lines at the time of initial planning when the site of excision is in the normal anatomic position.


Practice Implications
By creating a sketch with perpendicular lines, approximation of skin edges and surgical closures may become easier for the learning resident. Patients also can rest more comfortably during the procedure, and the overall cosmesis, healing, and outcome of the procedure may improve. The addition of a sterile marking pen to the surgical tray may aide in highlighting faded pen markings for easier visualization after cleansing of the surgical site.
Practice Gap
For first-year dermatology residents, dermatologic surgeries can present many challenges. Although approximation of wound edges following excision may be intuitive for the experienced surgeon, an early trainee may need some guidance. Infusion of anesthetics can distort the normal skin field or it may be difficult for the patient to remain in the same position for the required period of time; for example, an elderly patient who requires an excision on the posterior aspect of the neck may be unable to assume the same position for the full duration of the procedure. We offer a simple and effective technique for early-learning dermatology residents to improve surgical closures.
The Technique
We propose drawing straight lines using a sterile marking pen perpendicular to the fusiform plane laid out for any simple, intermediate, or complex linear closure (Figure 1). These lines can then be used as scaffolding for the surgical closure (Figure 2). We recommend drawing the lines at the time of initial planning when the site of excision is in the normal anatomic position.


Practice Implications
By creating a sketch with perpendicular lines, approximation of skin edges and surgical closures may become easier for the learning resident. Patients also can rest more comfortably during the procedure, and the overall cosmesis, healing, and outcome of the procedure may improve. The addition of a sterile marking pen to the surgical tray may aide in highlighting faded pen markings for easier visualization after cleansing of the surgical site.
Recognizing and Preventing Arbovirus Infections
What do patients need to know about arboviruses?
Dengue is the most common arbovirus worldwide, with more than 300 million individuals infected each year, most of them asymptomatic carriers. It is the most common febrile illness in travelers returning from Southeast Asia, South America, and the Caribbean. Dengue symptoms typically begin 3 to 12 days after exposure and may include fever; headache; conjunctivitis; and a biphasic rash that begins with blanching macular erythema, which patients may mistake for sunburn, followed by a morbilliform to petechial rash with islands of sparing (white islands in a sea of red). Severe dengue (formerly known as dengue hemorrhagic fever) may present with dramatic skin and mucosal hemorrhage including purpura, hemorrhagic bullae, and bleeding from orifices and injection sites, with associated thrombocytopenia and hypotension (dengue shock syndrome). Patients with onset of such symptoms need to go to the emergency department for inpatient management.
Individuals living in the United States should be particularly aware of West Nile virus, with infections reported in all US states in 2017, except Alaska and Hawaii thus far. Transmitted by the bite of the Culex mosquito, most infections are symptomatic; however, up to 20% of patients may present with nonspecific symptoms such as mild febrile or flulike symptoms, nonspecific morbilliform rash, and headaches, and up to 1% of patients may develop encephalitis or meningitis, with approximately 10% mortality rates.
What are your go-to treatments?
Arboviral infections generally are self-limited and there are no specific treatments available. Supportive care, including fluid resuscitation, and analgesia (if needed for joint, muscle, or bone pain) are the mainstays of management. Diagnoses generally are confirmed via viral polymerase chain reaction from serum (<7 days), IgM enzyme-linked immunosorbent assay (>4 days), or IgG serologies for later presentations.
Patients should avoid the use of nonsteroidal anti-inflammatory drugs if there is a possibility of dengue virus infection, as they may potentiate the risk for hemorrhagic complications in patients with severe dengue. Instead, acetaminophen is recommended for analgesia and antipyretic purposes, if needed.
How do you recommend patients prevent infection while traveling?
Primary prevention of infection and secondary prevention of transmission are important. Although mosquito bed netting is helpful in preventing some mosquito-borne viruses, many arboviruses (ie, dengue, Zika, chikun-gunya) are transmitted by primarily daytime-biting Aedes mosquitoes. In an endemic area, travelers should try to stay within air-conditioned buildings with intact window and door screens. When outdoors, wear long sleeves and pants and use Environmental Protection Agency-registered mosquito repellents. Conventional repellents include the following:
- DEET: concentrations 10% to 30% are safe for children 2 months and older and pregnant women; concentrations around 10% are effective for periods of approximately 2 hours; as the concentration of DEET increases, the duration of protection increases.
- Picaridin: concentrations of 5% to 20%; effective for 4 to 8 hours depending on the concentration; most effective concentration is 20%; is not effective against ticks.
Biopesticide repellents include the following:
- IR3535 (ethyl butylacetylaminopropionate): concentration 10% to 30% has been used as an insect repellent in Europe for 20 years with no substantial adverse effects.
- 2-undecanone: a natural compound from leaves and stems of the wild tomato plant; is a biopesticide product less toxic than conventional pesticides.
- Oil of lemon eucalyptus (OLE) or PMD (the synthesized version of OLE): concentration 30%; "pure" oil of lemon eucalyptus (essential oil not formulated as a repellent) is not recommended.
- Natural oils (eg, soybean, lemongrass, citronella, cedar, peppermint, lavender, geranium) are exempted from Environmental Protection Agency registration; duration of effectiveness is estimated between 30 minutes and 2 hours.
Products that combine sunscreen and repellent are not recommended because sunscreen may need to be reapplied, increasing the toxicity of the repellent. Use separate products, applying sunscreen first and then applying the repellent.
Permethrin-treated clothing may provide an additional measure of protection, though in some endemic areas, resistance has been reported.
Because sexual transmission has been reported for Zika virus (between both male and female partners), any known or possibly infected persons should use condoms. Durations of abstinence or protected sex recommendations vary by situation and more detailed recommendations can be found from the Centers for Disease Control and Prevention. Pregnant women and women trying to get pregnant should take preventive measures with respect to Zika due to the possibility of the virus causing severe birth defects (congenital Zika syndrome) including microcephaly, joint deformities, ocular damage, and hypertonia.
What do patients need to know about arboviruses?
Dengue is the most common arbovirus worldwide, with more than 300 million individuals infected each year, most of them asymptomatic carriers. It is the most common febrile illness in travelers returning from Southeast Asia, South America, and the Caribbean. Dengue symptoms typically begin 3 to 12 days after exposure and may include fever; headache; conjunctivitis; and a biphasic rash that begins with blanching macular erythema, which patients may mistake for sunburn, followed by a morbilliform to petechial rash with islands of sparing (white islands in a sea of red). Severe dengue (formerly known as dengue hemorrhagic fever) may present with dramatic skin and mucosal hemorrhage including purpura, hemorrhagic bullae, and bleeding from orifices and injection sites, with associated thrombocytopenia and hypotension (dengue shock syndrome). Patients with onset of such symptoms need to go to the emergency department for inpatient management.
Individuals living in the United States should be particularly aware of West Nile virus, with infections reported in all US states in 2017, except Alaska and Hawaii thus far. Transmitted by the bite of the Culex mosquito, most infections are symptomatic; however, up to 20% of patients may present with nonspecific symptoms such as mild febrile or flulike symptoms, nonspecific morbilliform rash, and headaches, and up to 1% of patients may develop encephalitis or meningitis, with approximately 10% mortality rates.
What are your go-to treatments?
Arboviral infections generally are self-limited and there are no specific treatments available. Supportive care, including fluid resuscitation, and analgesia (if needed for joint, muscle, or bone pain) are the mainstays of management. Diagnoses generally are confirmed via viral polymerase chain reaction from serum (<7 days), IgM enzyme-linked immunosorbent assay (>4 days), or IgG serologies for later presentations.
Patients should avoid the use of nonsteroidal anti-inflammatory drugs if there is a possibility of dengue virus infection, as they may potentiate the risk for hemorrhagic complications in patients with severe dengue. Instead, acetaminophen is recommended for analgesia and antipyretic purposes, if needed.
How do you recommend patients prevent infection while traveling?
Primary prevention of infection and secondary prevention of transmission are important. Although mosquito bed netting is helpful in preventing some mosquito-borne viruses, many arboviruses (ie, dengue, Zika, chikun-gunya) are transmitted by primarily daytime-biting Aedes mosquitoes. In an endemic area, travelers should try to stay within air-conditioned buildings with intact window and door screens. When outdoors, wear long sleeves and pants and use Environmental Protection Agency-registered mosquito repellents. Conventional repellents include the following:
- DEET: concentrations 10% to 30% are safe for children 2 months and older and pregnant women; concentrations around 10% are effective for periods of approximately 2 hours; as the concentration of DEET increases, the duration of protection increases.
- Picaridin: concentrations of 5% to 20%; effective for 4 to 8 hours depending on the concentration; most effective concentration is 20%; is not effective against ticks.
Biopesticide repellents include the following:
- IR3535 (ethyl butylacetylaminopropionate): concentration 10% to 30% has been used as an insect repellent in Europe for 20 years with no substantial adverse effects.
- 2-undecanone: a natural compound from leaves and stems of the wild tomato plant; is a biopesticide product less toxic than conventional pesticides.
- Oil of lemon eucalyptus (OLE) or PMD (the synthesized version of OLE): concentration 30%; "pure" oil of lemon eucalyptus (essential oil not formulated as a repellent) is not recommended.
- Natural oils (eg, soybean, lemongrass, citronella, cedar, peppermint, lavender, geranium) are exempted from Environmental Protection Agency registration; duration of effectiveness is estimated between 30 minutes and 2 hours.
Products that combine sunscreen and repellent are not recommended because sunscreen may need to be reapplied, increasing the toxicity of the repellent. Use separate products, applying sunscreen first and then applying the repellent.
Permethrin-treated clothing may provide an additional measure of protection, though in some endemic areas, resistance has been reported.
Because sexual transmission has been reported for Zika virus (between both male and female partners), any known or possibly infected persons should use condoms. Durations of abstinence or protected sex recommendations vary by situation and more detailed recommendations can be found from the Centers for Disease Control and Prevention. Pregnant women and women trying to get pregnant should take preventive measures with respect to Zika due to the possibility of the virus causing severe birth defects (congenital Zika syndrome) including microcephaly, joint deformities, ocular damage, and hypertonia.
What do patients need to know about arboviruses?
Dengue is the most common arbovirus worldwide, with more than 300 million individuals infected each year, most of them asymptomatic carriers. It is the most common febrile illness in travelers returning from Southeast Asia, South America, and the Caribbean. Dengue symptoms typically begin 3 to 12 days after exposure and may include fever; headache; conjunctivitis; and a biphasic rash that begins with blanching macular erythema, which patients may mistake for sunburn, followed by a morbilliform to petechial rash with islands of sparing (white islands in a sea of red). Severe dengue (formerly known as dengue hemorrhagic fever) may present with dramatic skin and mucosal hemorrhage including purpura, hemorrhagic bullae, and bleeding from orifices and injection sites, with associated thrombocytopenia and hypotension (dengue shock syndrome). Patients with onset of such symptoms need to go to the emergency department for inpatient management.
Individuals living in the United States should be particularly aware of West Nile virus, with infections reported in all US states in 2017, except Alaska and Hawaii thus far. Transmitted by the bite of the Culex mosquito, most infections are symptomatic; however, up to 20% of patients may present with nonspecific symptoms such as mild febrile or flulike symptoms, nonspecific morbilliform rash, and headaches, and up to 1% of patients may develop encephalitis or meningitis, with approximately 10% mortality rates.
What are your go-to treatments?
Arboviral infections generally are self-limited and there are no specific treatments available. Supportive care, including fluid resuscitation, and analgesia (if needed for joint, muscle, or bone pain) are the mainstays of management. Diagnoses generally are confirmed via viral polymerase chain reaction from serum (<7 days), IgM enzyme-linked immunosorbent assay (>4 days), or IgG serologies for later presentations.
Patients should avoid the use of nonsteroidal anti-inflammatory drugs if there is a possibility of dengue virus infection, as they may potentiate the risk for hemorrhagic complications in patients with severe dengue. Instead, acetaminophen is recommended for analgesia and antipyretic purposes, if needed.
How do you recommend patients prevent infection while traveling?
Primary prevention of infection and secondary prevention of transmission are important. Although mosquito bed netting is helpful in preventing some mosquito-borne viruses, many arboviruses (ie, dengue, Zika, chikun-gunya) are transmitted by primarily daytime-biting Aedes mosquitoes. In an endemic area, travelers should try to stay within air-conditioned buildings with intact window and door screens. When outdoors, wear long sleeves and pants and use Environmental Protection Agency-registered mosquito repellents. Conventional repellents include the following:
- DEET: concentrations 10% to 30% are safe for children 2 months and older and pregnant women; concentrations around 10% are effective for periods of approximately 2 hours; as the concentration of DEET increases, the duration of protection increases.
- Picaridin: concentrations of 5% to 20%; effective for 4 to 8 hours depending on the concentration; most effective concentration is 20%; is not effective against ticks.
Biopesticide repellents include the following:
- IR3535 (ethyl butylacetylaminopropionate): concentration 10% to 30% has been used as an insect repellent in Europe for 20 years with no substantial adverse effects.
- 2-undecanone: a natural compound from leaves and stems of the wild tomato plant; is a biopesticide product less toxic than conventional pesticides.
- Oil of lemon eucalyptus (OLE) or PMD (the synthesized version of OLE): concentration 30%; "pure" oil of lemon eucalyptus (essential oil not formulated as a repellent) is not recommended.
- Natural oils (eg, soybean, lemongrass, citronella, cedar, peppermint, lavender, geranium) are exempted from Environmental Protection Agency registration; duration of effectiveness is estimated between 30 minutes and 2 hours.
Products that combine sunscreen and repellent are not recommended because sunscreen may need to be reapplied, increasing the toxicity of the repellent. Use separate products, applying sunscreen first and then applying the repellent.
Permethrin-treated clothing may provide an additional measure of protection, though in some endemic areas, resistance has been reported.
Because sexual transmission has been reported for Zika virus (between both male and female partners), any known or possibly infected persons should use condoms. Durations of abstinence or protected sex recommendations vary by situation and more detailed recommendations can be found from the Centers for Disease Control and Prevention. Pregnant women and women trying to get pregnant should take preventive measures with respect to Zika due to the possibility of the virus causing severe birth defects (congenital Zika syndrome) including microcephaly, joint deformities, ocular damage, and hypertonia.
Incorporating New Atopic Dermatitis Medications in Your Practice
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.