User login
Implementation of a VHA Virtual Oncology Training Pilot Program for Clinical Pharmacists
Purpose/Background
Oncology clinical pharmacist practitioners (CPP) play a critical role in optimizing drug therapy, managing side effects, and ensuring medication adherence. As a specialized clinical area, specific training is needed to ensure quality of care. Oncology pharmacy training programs are commercially available but pose a financial burden and are not specific to the Veterans Health Administration (VHA). A comprehensive, virtual Oncology Bootcamp series was implemented to upskill new oncology pharmacists (or pharmacists seeking to further their understanding of oncology practice), with didactic materials and clinical tools to enhance and standardize quality care delivery.
Methods
This program was comprised of an online platform of 23 one hour-long continuing education accredited sessions, delivered by leading subject matter experts. Pharmacists from two Veteran Integrated Service Networks (VISNs) were invited for the first year of the bootcamp. The curriculum encompassed fundamentals of oncology practice, patient care assessment, chemotherapy protocol review, practice management, and supportive care. Participants also received in-depth training on managing various cancer types, including but not limited to prostate, lung, gastrointestinal and hematologic malignancies. VHA specific information, including utilization of Oncology Clinical Pathways to promote standardized care was included where applicable. The interactive nature of the virtual sessions provided opportunities for real-time discussion and immediate feedback. To measure the impact of this program, a pre and post program evaluation of participants was conducted.
Results
Over the course of the program, more than 40 pharmacists across two VISNs participated in the bootcamp series. Results of the program evaluation showed an increase in self-reported comfort and skill levels in all criteria that were assessed (oncology pharmacotherapy, solid tumor malignancies, hematologic malignancies and oral anti-cancer therapy management). Additionally, 85% of respondents stated the series met their overall goals and over 90% of respondents stated they were either satisfied or very satisfied with the content, speakers and organization of the course.
Implications/Significance
This initiative has established the viability and significance of a highly accessible, VHA pathway specific and Veteran centric platform for oncology pharmacy professional development. Future directions for the program include a broader nationwide audience, increased content coverage and self-paced learning options.
Purpose/Background
Oncology clinical pharmacist practitioners (CPP) play a critical role in optimizing drug therapy, managing side effects, and ensuring medication adherence. As a specialized clinical area, specific training is needed to ensure quality of care. Oncology pharmacy training programs are commercially available but pose a financial burden and are not specific to the Veterans Health Administration (VHA). A comprehensive, virtual Oncology Bootcamp series was implemented to upskill new oncology pharmacists (or pharmacists seeking to further their understanding of oncology practice), with didactic materials and clinical tools to enhance and standardize quality care delivery.
Methods
This program was comprised of an online platform of 23 one hour-long continuing education accredited sessions, delivered by leading subject matter experts. Pharmacists from two Veteran Integrated Service Networks (VISNs) were invited for the first year of the bootcamp. The curriculum encompassed fundamentals of oncology practice, patient care assessment, chemotherapy protocol review, practice management, and supportive care. Participants also received in-depth training on managing various cancer types, including but not limited to prostate, lung, gastrointestinal and hematologic malignancies. VHA specific information, including utilization of Oncology Clinical Pathways to promote standardized care was included where applicable. The interactive nature of the virtual sessions provided opportunities for real-time discussion and immediate feedback. To measure the impact of this program, a pre and post program evaluation of participants was conducted.
Results
Over the course of the program, more than 40 pharmacists across two VISNs participated in the bootcamp series. Results of the program evaluation showed an increase in self-reported comfort and skill levels in all criteria that were assessed (oncology pharmacotherapy, solid tumor malignancies, hematologic malignancies and oral anti-cancer therapy management). Additionally, 85% of respondents stated the series met their overall goals and over 90% of respondents stated they were either satisfied or very satisfied with the content, speakers and organization of the course.
Implications/Significance
This initiative has established the viability and significance of a highly accessible, VHA pathway specific and Veteran centric platform for oncology pharmacy professional development. Future directions for the program include a broader nationwide audience, increased content coverage and self-paced learning options.
Purpose/Background
Oncology clinical pharmacist practitioners (CPP) play a critical role in optimizing drug therapy, managing side effects, and ensuring medication adherence. As a specialized clinical area, specific training is needed to ensure quality of care. Oncology pharmacy training programs are commercially available but pose a financial burden and are not specific to the Veterans Health Administration (VHA). A comprehensive, virtual Oncology Bootcamp series was implemented to upskill new oncology pharmacists (or pharmacists seeking to further their understanding of oncology practice), with didactic materials and clinical tools to enhance and standardize quality care delivery.
Methods
This program was comprised of an online platform of 23 one hour-long continuing education accredited sessions, delivered by leading subject matter experts. Pharmacists from two Veteran Integrated Service Networks (VISNs) were invited for the first year of the bootcamp. The curriculum encompassed fundamentals of oncology practice, patient care assessment, chemotherapy protocol review, practice management, and supportive care. Participants also received in-depth training on managing various cancer types, including but not limited to prostate, lung, gastrointestinal and hematologic malignancies. VHA specific information, including utilization of Oncology Clinical Pathways to promote standardized care was included where applicable. The interactive nature of the virtual sessions provided opportunities for real-time discussion and immediate feedback. To measure the impact of this program, a pre and post program evaluation of participants was conducted.
Results
Over the course of the program, more than 40 pharmacists across two VISNs participated in the bootcamp series. Results of the program evaluation showed an increase in self-reported comfort and skill levels in all criteria that were assessed (oncology pharmacotherapy, solid tumor malignancies, hematologic malignancies and oral anti-cancer therapy management). Additionally, 85% of respondents stated the series met their overall goals and over 90% of respondents stated they were either satisfied or very satisfied with the content, speakers and organization of the course.
Implications/Significance
This initiative has established the viability and significance of a highly accessible, VHA pathway specific and Veteran centric platform for oncology pharmacy professional development. Future directions for the program include a broader nationwide audience, increased content coverage and self-paced learning options.
Virtual Reality: An Innovative Approach to Cancer Distress Management
Objective
To assess the impact of virtual reality on the distress and pain levels of oncology patients in a VA outpatient infusion clinic.
Background
It is known that distress in cancer care leads to several problems including decreased survival, decreased treatment adherence, and inability to make treatment decisions. Virtual reality (VR) has proven to be beneficial to Veterans suffering from stress, anxiety, and other mental health ailments. This VA Oncology Infusion clinic is assessing the impact of VR on its Veterans’ distress and pain levels.
Methods
The pilot phase will last from 3/5/25- 9/5/25. Prior to each VR session, Veterans are administered an NCCN cancer distress screening tool and a numerical pain assessment. Post-VR session, Veterans are reassessed for distress and pain. The veterans are asked the following questions after each session: 1) Would you recommend VR to other veterans? and 2) Was the VR headset easy to use? Each VR session is approximately 10-15 minutes long, and the Veterans choose to engage in mindfulness activities, breathing exercises, or view scenery of their choice.
Results
Preliminary results indicate receptiveness and positive experiences amongst Veterans. 66% of Veterans who have used the VR headset have demonstrated a decrease in Cancer Distress by at least 2 points after a 10–15-minute VR session. 92% of Veterans that have used the VR headset report that it is easy to use and that they would recommend it to other Veterans.
Feasibility
The VA has created the Extended Reality Network (XR) to support the implementation of VR at the local site level. Resources and training are widely available to ensure program success.
Sustainability and Impact
A clearly developed standard of work and protocol that is tailored to the local site’s workflow, including a VR champion is needed to ensure sustainability. Preliminary data shows that veterans are engaged and responding positively to this innovative approach to cancer distress management, as evidenced by decreased distress levels and anxiety.
Objective
To assess the impact of virtual reality on the distress and pain levels of oncology patients in a VA outpatient infusion clinic.
Background
It is known that distress in cancer care leads to several problems including decreased survival, decreased treatment adherence, and inability to make treatment decisions. Virtual reality (VR) has proven to be beneficial to Veterans suffering from stress, anxiety, and other mental health ailments. This VA Oncology Infusion clinic is assessing the impact of VR on its Veterans’ distress and pain levels.
Methods
The pilot phase will last from 3/5/25- 9/5/25. Prior to each VR session, Veterans are administered an NCCN cancer distress screening tool and a numerical pain assessment. Post-VR session, Veterans are reassessed for distress and pain. The veterans are asked the following questions after each session: 1) Would you recommend VR to other veterans? and 2) Was the VR headset easy to use? Each VR session is approximately 10-15 minutes long, and the Veterans choose to engage in mindfulness activities, breathing exercises, or view scenery of their choice.
Results
Preliminary results indicate receptiveness and positive experiences amongst Veterans. 66% of Veterans who have used the VR headset have demonstrated a decrease in Cancer Distress by at least 2 points after a 10–15-minute VR session. 92% of Veterans that have used the VR headset report that it is easy to use and that they would recommend it to other Veterans.
Feasibility
The VA has created the Extended Reality Network (XR) to support the implementation of VR at the local site level. Resources and training are widely available to ensure program success.
Sustainability and Impact
A clearly developed standard of work and protocol that is tailored to the local site’s workflow, including a VR champion is needed to ensure sustainability. Preliminary data shows that veterans are engaged and responding positively to this innovative approach to cancer distress management, as evidenced by decreased distress levels and anxiety.
Objective
To assess the impact of virtual reality on the distress and pain levels of oncology patients in a VA outpatient infusion clinic.
Background
It is known that distress in cancer care leads to several problems including decreased survival, decreased treatment adherence, and inability to make treatment decisions. Virtual reality (VR) has proven to be beneficial to Veterans suffering from stress, anxiety, and other mental health ailments. This VA Oncology Infusion clinic is assessing the impact of VR on its Veterans’ distress and pain levels.
Methods
The pilot phase will last from 3/5/25- 9/5/25. Prior to each VR session, Veterans are administered an NCCN cancer distress screening tool and a numerical pain assessment. Post-VR session, Veterans are reassessed for distress and pain. The veterans are asked the following questions after each session: 1) Would you recommend VR to other veterans? and 2) Was the VR headset easy to use? Each VR session is approximately 10-15 minutes long, and the Veterans choose to engage in mindfulness activities, breathing exercises, or view scenery of their choice.
Results
Preliminary results indicate receptiveness and positive experiences amongst Veterans. 66% of Veterans who have used the VR headset have demonstrated a decrease in Cancer Distress by at least 2 points after a 10–15-minute VR session. 92% of Veterans that have used the VR headset report that it is easy to use and that they would recommend it to other Veterans.
Feasibility
The VA has created the Extended Reality Network (XR) to support the implementation of VR at the local site level. Resources and training are widely available to ensure program success.
Sustainability and Impact
A clearly developed standard of work and protocol that is tailored to the local site’s workflow, including a VR champion is needed to ensure sustainability. Preliminary data shows that veterans are engaged and responding positively to this innovative approach to cancer distress management, as evidenced by decreased distress levels and anxiety.
Enhancing Veteran Access to Cutting-Edge Treatments: Launching a T Cell Engager Therapy Administration Program
Background
The rise in the number of T-cell engager therapies highlights their importance in modern cancer treatment paradigms. Having recognized the need for, and complexities of, administering these innovative medications to our patients, our team assessed our institution’s capability to provide these therapies to our patients. We identified that our facility was wellequipped for implementation of T-cell engager therapy due to inpatient administration capabilities, an outpatient infusion center, on-hand supportive care medications (tocilizumab), and access to higher levels of care. Key players included medical oncologists, pharmacists, inpatient and infusion nurses, staff physicians, critical care practitioners, and care coordinators.
Clinical Practice Initiative
Barriers identified: education, toxicity concerns, formulary management, and logistics. To overcome these obstacles, comprehensive plans for procurement, hospital admission, monitoring, and training were developed as a facility-specific standard operating procedure (SOP). All available Tcell engager therapies were presented to the formulary committee and received local approval. Physician and pharmacist champions were registered for the associated risk evaluation and mitigation strategies (REMS) programs. Recorded webinars were done to provide education on REMS requirements, medication logistics, and adverse event management.
An admission plan was formulated to outline admission criteria, medication administration, and safety logistics. Order sets created by pharmacists, encompassed pre, post, and as needed medications for cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome. To facilitate safe discharge and meet REMS criteria, patients received wallet cards, dexamethasone and acetaminophen PRNs with detailed instructions for use, and direction for seeking emergency care with consideration of local tocilizumab availability.
Conclusions
Our SOP has enabled administration of six T-cell engager therapies for six diseases. The primary limitation for some of these agents is the need for inpatient monitoring at initiation, which may not be available at smaller centers. Facilities that lack these capabilities could utilize community care or partner with a neighboring Veterans Affairs medical center for initial administration, then transition back for continued treatment. Facilities that lack inpatient oncology nursing could administer the drug in the infusion center followed by admission for monitoring and toxicity management. Our implementation plan serves as a scalable model for improving veteran access to novel therapies.
Background
The rise in the number of T-cell engager therapies highlights their importance in modern cancer treatment paradigms. Having recognized the need for, and complexities of, administering these innovative medications to our patients, our team assessed our institution’s capability to provide these therapies to our patients. We identified that our facility was wellequipped for implementation of T-cell engager therapy due to inpatient administration capabilities, an outpatient infusion center, on-hand supportive care medications (tocilizumab), and access to higher levels of care. Key players included medical oncologists, pharmacists, inpatient and infusion nurses, staff physicians, critical care practitioners, and care coordinators.
Clinical Practice Initiative
Barriers identified: education, toxicity concerns, formulary management, and logistics. To overcome these obstacles, comprehensive plans for procurement, hospital admission, monitoring, and training were developed as a facility-specific standard operating procedure (SOP). All available Tcell engager therapies were presented to the formulary committee and received local approval. Physician and pharmacist champions were registered for the associated risk evaluation and mitigation strategies (REMS) programs. Recorded webinars were done to provide education on REMS requirements, medication logistics, and adverse event management.
An admission plan was formulated to outline admission criteria, medication administration, and safety logistics. Order sets created by pharmacists, encompassed pre, post, and as needed medications for cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome. To facilitate safe discharge and meet REMS criteria, patients received wallet cards, dexamethasone and acetaminophen PRNs with detailed instructions for use, and direction for seeking emergency care with consideration of local tocilizumab availability.
Conclusions
Our SOP has enabled administration of six T-cell engager therapies for six diseases. The primary limitation for some of these agents is the need for inpatient monitoring at initiation, which may not be available at smaller centers. Facilities that lack these capabilities could utilize community care or partner with a neighboring Veterans Affairs medical center for initial administration, then transition back for continued treatment. Facilities that lack inpatient oncology nursing could administer the drug in the infusion center followed by admission for monitoring and toxicity management. Our implementation plan serves as a scalable model for improving veteran access to novel therapies.
Background
The rise in the number of T-cell engager therapies highlights their importance in modern cancer treatment paradigms. Having recognized the need for, and complexities of, administering these innovative medications to our patients, our team assessed our institution’s capability to provide these therapies to our patients. We identified that our facility was wellequipped for implementation of T-cell engager therapy due to inpatient administration capabilities, an outpatient infusion center, on-hand supportive care medications (tocilizumab), and access to higher levels of care. Key players included medical oncologists, pharmacists, inpatient and infusion nurses, staff physicians, critical care practitioners, and care coordinators.
Clinical Practice Initiative
Barriers identified: education, toxicity concerns, formulary management, and logistics. To overcome these obstacles, comprehensive plans for procurement, hospital admission, monitoring, and training were developed as a facility-specific standard operating procedure (SOP). All available Tcell engager therapies were presented to the formulary committee and received local approval. Physician and pharmacist champions were registered for the associated risk evaluation and mitigation strategies (REMS) programs. Recorded webinars were done to provide education on REMS requirements, medication logistics, and adverse event management.
An admission plan was formulated to outline admission criteria, medication administration, and safety logistics. Order sets created by pharmacists, encompassed pre, post, and as needed medications for cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome. To facilitate safe discharge and meet REMS criteria, patients received wallet cards, dexamethasone and acetaminophen PRNs with detailed instructions for use, and direction for seeking emergency care with consideration of local tocilizumab availability.
Conclusions
Our SOP has enabled administration of six T-cell engager therapies for six diseases. The primary limitation for some of these agents is the need for inpatient monitoring at initiation, which may not be available at smaller centers. Facilities that lack these capabilities could utilize community care or partner with a neighboring Veterans Affairs medical center for initial administration, then transition back for continued treatment. Facilities that lack inpatient oncology nursing could administer the drug in the infusion center followed by admission for monitoring and toxicity management. Our implementation plan serves as a scalable model for improving veteran access to novel therapies.
Centralized Psychosocial Distress Screening Led by RN Care Coordinator
Background
Unmet psychosocial health needs negatively impact cancer care and outcomes. The American College of Surgeons’ Commission on Cancer (CoC) accreditation requirements include Psychosocial Distress Screening (PDS) for all newly diagnosed patients. To enhance cancer care and meet CoC standards, the Tibor Rubin Veterans Affairs Medical Center (TRVAMC) developed and implemented a closed-loop, centralized PDS pathway.
Objectives
Develop processes/methods to: (1) identify all newly diagnosed cancer patients; (2) track initiation of first course of treatment; (3) offer and complete PDS at initiation of first course of treatment; and (4) ensure placement of appropriate referrals.
Methods
All staff members were trained in PDS and competency completed. A standard operating procedure (SOP) was created to identify patients meeting criteria for PDS. Newly diagnosed patients were identified from cancer registry lists, tumor boards, radiology and pathology reports. Patients were placed on a tracking tool by the nurse care coordinator (NCC) and monitored to facilitate timely workup and initiation of treatment. Nurses in the cancer program offered and completed PDS and placed all necessary referrals (to > 11 services). Patients were removed from the tracker only after confirmation of PDS and referrals.
Results
Prior to implementation of PDS, no patients received comprehensive screening and referrals. After implementation, data were collected over a 2 year period. In 2023 and 2024, 277/565 (49%) and 256/526 (48.7%) newly diagnosed patients were eligible for PDS, respectively. All eligible patients were offered PDS (100%). Of patients who underwent PDS, 37% scored their distress at a level of 4/10 or higher, underscoring the severity of distress and unmet need. Referrals to various services were indicated and made in 43.8% patients, most frequently to Social Work, Primary Care or Psychology/Mental Health. More recently, nurses in the Infusion Clinic and Radiation Oncology were trained in and also started conducting PDS on patients coming for treatment.
Conclusions
Implementation of comprehensive and timely PDS resulted in early identification and interventions to address diverse facets of distress that are known to interfere with quality of life, compliance with cancer treatments and outcomes. The program also met the CoC standard for accreditation of TRVAMC in 2024.
Background
Unmet psychosocial health needs negatively impact cancer care and outcomes. The American College of Surgeons’ Commission on Cancer (CoC) accreditation requirements include Psychosocial Distress Screening (PDS) for all newly diagnosed patients. To enhance cancer care and meet CoC standards, the Tibor Rubin Veterans Affairs Medical Center (TRVAMC) developed and implemented a closed-loop, centralized PDS pathway.
Objectives
Develop processes/methods to: (1) identify all newly diagnosed cancer patients; (2) track initiation of first course of treatment; (3) offer and complete PDS at initiation of first course of treatment; and (4) ensure placement of appropriate referrals.
Methods
All staff members were trained in PDS and competency completed. A standard operating procedure (SOP) was created to identify patients meeting criteria for PDS. Newly diagnosed patients were identified from cancer registry lists, tumor boards, radiology and pathology reports. Patients were placed on a tracking tool by the nurse care coordinator (NCC) and monitored to facilitate timely workup and initiation of treatment. Nurses in the cancer program offered and completed PDS and placed all necessary referrals (to > 11 services). Patients were removed from the tracker only after confirmation of PDS and referrals.
Results
Prior to implementation of PDS, no patients received comprehensive screening and referrals. After implementation, data were collected over a 2 year period. In 2023 and 2024, 277/565 (49%) and 256/526 (48.7%) newly diagnosed patients were eligible for PDS, respectively. All eligible patients were offered PDS (100%). Of patients who underwent PDS, 37% scored their distress at a level of 4/10 or higher, underscoring the severity of distress and unmet need. Referrals to various services were indicated and made in 43.8% patients, most frequently to Social Work, Primary Care or Psychology/Mental Health. More recently, nurses in the Infusion Clinic and Radiation Oncology were trained in and also started conducting PDS on patients coming for treatment.
Conclusions
Implementation of comprehensive and timely PDS resulted in early identification and interventions to address diverse facets of distress that are known to interfere with quality of life, compliance with cancer treatments and outcomes. The program also met the CoC standard for accreditation of TRVAMC in 2024.
Background
Unmet psychosocial health needs negatively impact cancer care and outcomes. The American College of Surgeons’ Commission on Cancer (CoC) accreditation requirements include Psychosocial Distress Screening (PDS) for all newly diagnosed patients. To enhance cancer care and meet CoC standards, the Tibor Rubin Veterans Affairs Medical Center (TRVAMC) developed and implemented a closed-loop, centralized PDS pathway.
Objectives
Develop processes/methods to: (1) identify all newly diagnosed cancer patients; (2) track initiation of first course of treatment; (3) offer and complete PDS at initiation of first course of treatment; and (4) ensure placement of appropriate referrals.
Methods
All staff members were trained in PDS and competency completed. A standard operating procedure (SOP) was created to identify patients meeting criteria for PDS. Newly diagnosed patients were identified from cancer registry lists, tumor boards, radiology and pathology reports. Patients were placed on a tracking tool by the nurse care coordinator (NCC) and monitored to facilitate timely workup and initiation of treatment. Nurses in the cancer program offered and completed PDS and placed all necessary referrals (to > 11 services). Patients were removed from the tracker only after confirmation of PDS and referrals.
Results
Prior to implementation of PDS, no patients received comprehensive screening and referrals. After implementation, data were collected over a 2 year period. In 2023 and 2024, 277/565 (49%) and 256/526 (48.7%) newly diagnosed patients were eligible for PDS, respectively. All eligible patients were offered PDS (100%). Of patients who underwent PDS, 37% scored their distress at a level of 4/10 or higher, underscoring the severity of distress and unmet need. Referrals to various services were indicated and made in 43.8% patients, most frequently to Social Work, Primary Care or Psychology/Mental Health. More recently, nurses in the Infusion Clinic and Radiation Oncology were trained in and also started conducting PDS on patients coming for treatment.
Conclusions
Implementation of comprehensive and timely PDS resulted in early identification and interventions to address diverse facets of distress that are known to interfere with quality of life, compliance with cancer treatments and outcomes. The program also met the CoC standard for accreditation of TRVAMC in 2024.
Access to Germline Genetic Testing through Clinical Pathways in Veterans With Prostate Cancer
Background
Germline genetic testing (GGT) is essential in prostate cancer care, informing clinical decisions. The Veterans Affairs National Oncology Program (VA NOP) recommends GGT for patients with specific risk factors in non-metastatic prostate cancer and all patients with metastatic disease. Understanding GGT access helps evaluate care quality and guide improvements. Since 2021, VA NOP has implemented pathway health factor (HF) templates to standardize cancer care documentation, including GGT status, enabling data extraction from the Corporate Data Warehouse (CDW) rather than requiring manual review of clinical notes. This work aims to evaluate Veterans’ access to GGT in prostate cancer care by leveraging pathway HF templates, and to assess the feasibility of using structured electronic health record (EHR) data to monitor adherence to GGT recommendations.
Methods
Process delivery diagrams (PDDs) were used to map data flow from prostate cancer clinical pathways to the VA CDW. We identified and categorized HFs related to prostate cancer GGT through the computerized patient record system (CPRS). Descriptive statistics were used to summarize access, ordering, and consent rates.
Results
We identified 5,744 Veterans with at least one prostate cancer GGT-relevant HF entered between 02/01/2021 and 12/31/2024. Of these, 5,125 (89.2%) had access to GGT, with 4,569 (89.2%) consenting to or having GGT ordered, while 556 (10.8%) declined testing. Among the 619 (10.8%) Veterans without GGT access, providers reported plans to discuss GGT in the future for 528 (85.3%) patients, while 91 (14.7%) were off pathway.
Conclusions
NOP-developed HF templates enabled extraction of GGT information from structured EHR data, eliminating manual extraction from clinical notes. We observed high GGT utilization among Veterans with pathway-entered HFs. However, low overall HF utilization may introduce selection bias. Future work includes developing a Natural Language Processing pipeline using large language models to automatically extract GGT information from clinical notes, with HF data serving as ground truth.
Background
Germline genetic testing (GGT) is essential in prostate cancer care, informing clinical decisions. The Veterans Affairs National Oncology Program (VA NOP) recommends GGT for patients with specific risk factors in non-metastatic prostate cancer and all patients with metastatic disease. Understanding GGT access helps evaluate care quality and guide improvements. Since 2021, VA NOP has implemented pathway health factor (HF) templates to standardize cancer care documentation, including GGT status, enabling data extraction from the Corporate Data Warehouse (CDW) rather than requiring manual review of clinical notes. This work aims to evaluate Veterans’ access to GGT in prostate cancer care by leveraging pathway HF templates, and to assess the feasibility of using structured electronic health record (EHR) data to monitor adherence to GGT recommendations.
Methods
Process delivery diagrams (PDDs) were used to map data flow from prostate cancer clinical pathways to the VA CDW. We identified and categorized HFs related to prostate cancer GGT through the computerized patient record system (CPRS). Descriptive statistics were used to summarize access, ordering, and consent rates.
Results
We identified 5,744 Veterans with at least one prostate cancer GGT-relevant HF entered between 02/01/2021 and 12/31/2024. Of these, 5,125 (89.2%) had access to GGT, with 4,569 (89.2%) consenting to or having GGT ordered, while 556 (10.8%) declined testing. Among the 619 (10.8%) Veterans without GGT access, providers reported plans to discuss GGT in the future for 528 (85.3%) patients, while 91 (14.7%) were off pathway.
Conclusions
NOP-developed HF templates enabled extraction of GGT information from structured EHR data, eliminating manual extraction from clinical notes. We observed high GGT utilization among Veterans with pathway-entered HFs. However, low overall HF utilization may introduce selection bias. Future work includes developing a Natural Language Processing pipeline using large language models to automatically extract GGT information from clinical notes, with HF data serving as ground truth.
Background
Germline genetic testing (GGT) is essential in prostate cancer care, informing clinical decisions. The Veterans Affairs National Oncology Program (VA NOP) recommends GGT for patients with specific risk factors in non-metastatic prostate cancer and all patients with metastatic disease. Understanding GGT access helps evaluate care quality and guide improvements. Since 2021, VA NOP has implemented pathway health factor (HF) templates to standardize cancer care documentation, including GGT status, enabling data extraction from the Corporate Data Warehouse (CDW) rather than requiring manual review of clinical notes. This work aims to evaluate Veterans’ access to GGT in prostate cancer care by leveraging pathway HF templates, and to assess the feasibility of using structured electronic health record (EHR) data to monitor adherence to GGT recommendations.
Methods
Process delivery diagrams (PDDs) were used to map data flow from prostate cancer clinical pathways to the VA CDW. We identified and categorized HFs related to prostate cancer GGT through the computerized patient record system (CPRS). Descriptive statistics were used to summarize access, ordering, and consent rates.
Results
We identified 5,744 Veterans with at least one prostate cancer GGT-relevant HF entered between 02/01/2021 and 12/31/2024. Of these, 5,125 (89.2%) had access to GGT, with 4,569 (89.2%) consenting to or having GGT ordered, while 556 (10.8%) declined testing. Among the 619 (10.8%) Veterans without GGT access, providers reported plans to discuss GGT in the future for 528 (85.3%) patients, while 91 (14.7%) were off pathway.
Conclusions
NOP-developed HF templates enabled extraction of GGT information from structured EHR data, eliminating manual extraction from clinical notes. We observed high GGT utilization among Veterans with pathway-entered HFs. However, low overall HF utilization may introduce selection bias. Future work includes developing a Natural Language Processing pipeline using large language models to automatically extract GGT information from clinical notes, with HF data serving as ground truth.
VA Ann Arbor Immunotherapy Stewardship Program
Purpose
To compare vial utilization and spending between fixed and weight-based dosing of pembrolizumab in Veterans. Promote and assess pembrolizumab extended interval dosing.
Background
FDA approved pembrolizumab label change from weight-based to fixed dosing without evidence of fixed-dosing’s superiority. Retrospective studies demonstrate equivalent outcomes for 2 mg/kg every 3 weeks (Q3W), 200 mg Q3W, 4 mg/kg every 6 weeks (Q6W), and 400 mg Q6W.
Methods
In July 2024 VAAAHS (VA Ann Arbor Healthcare System) initiated an immunotherapy stewardship quality improvement program to deprescribe unnecessary pembrolizumab units and promote extended-interval dosing. Specific interventions included order template modification and targeted outreach to key stakeholders.
Data Analysis
All pembrolizumab doses administered at VAAAHS between July 1, 2024 (launch) and March 31, 2025 (data cutoff) were extracted from EHR. Drug utilization, spending, and healthcare contact hours averted were compared to a fixed-dosing counterfactual.
Results
Sixty-three Veterans received 286 total pembrolizumab doses, of which 107 (37.4%) were Q6W and 179 (62.6%) were Q3W. In total, 741 vials were utilized, against expectation of 786 (5.7% reduction), reflecting approximately $182,000 in savings (annualized, $243,000) and 86.5% of the theoretical maximum savings were captured. Q6W’s share of all doses rose from 27.3% in July 2024 to 53.8% in March 2025. Amongst monotherapy, Q6W’s share rose from 60.0% in July 2024 to 86.7% in March 2025. Q6W adoption saved 381 Veteran-healthcare contact hours, not including travel time.
Conclusions
Stewardship efforts reduced unnecessary pembrolizumab utilization and spending while saving Veterans and VAAAHS providers’ time. Continued provider reinforcement, preparation for Oracle/ Cerner implementation, VISN expansion, refinement of pembrolizumab dose-banding, and development of dose bands for other immunotherapies are underway.
Significance
National implementation would improve Veteran convenience and quality of life, enable reductions in drug and resource costs, and enhance clinic throughput.
Purpose
To compare vial utilization and spending between fixed and weight-based dosing of pembrolizumab in Veterans. Promote and assess pembrolizumab extended interval dosing.
Background
FDA approved pembrolizumab label change from weight-based to fixed dosing without evidence of fixed-dosing’s superiority. Retrospective studies demonstrate equivalent outcomes for 2 mg/kg every 3 weeks (Q3W), 200 mg Q3W, 4 mg/kg every 6 weeks (Q6W), and 400 mg Q6W.
Methods
In July 2024 VAAAHS (VA Ann Arbor Healthcare System) initiated an immunotherapy stewardship quality improvement program to deprescribe unnecessary pembrolizumab units and promote extended-interval dosing. Specific interventions included order template modification and targeted outreach to key stakeholders.
Data Analysis
All pembrolizumab doses administered at VAAAHS between July 1, 2024 (launch) and March 31, 2025 (data cutoff) were extracted from EHR. Drug utilization, spending, and healthcare contact hours averted were compared to a fixed-dosing counterfactual.
Results
Sixty-three Veterans received 286 total pembrolizumab doses, of which 107 (37.4%) were Q6W and 179 (62.6%) were Q3W. In total, 741 vials were utilized, against expectation of 786 (5.7% reduction), reflecting approximately $182,000 in savings (annualized, $243,000) and 86.5% of the theoretical maximum savings were captured. Q6W’s share of all doses rose from 27.3% in July 2024 to 53.8% in March 2025. Amongst monotherapy, Q6W’s share rose from 60.0% in July 2024 to 86.7% in March 2025. Q6W adoption saved 381 Veteran-healthcare contact hours, not including travel time.
Conclusions
Stewardship efforts reduced unnecessary pembrolizumab utilization and spending while saving Veterans and VAAAHS providers’ time. Continued provider reinforcement, preparation for Oracle/ Cerner implementation, VISN expansion, refinement of pembrolizumab dose-banding, and development of dose bands for other immunotherapies are underway.
Significance
National implementation would improve Veteran convenience and quality of life, enable reductions in drug and resource costs, and enhance clinic throughput.
Purpose
To compare vial utilization and spending between fixed and weight-based dosing of pembrolizumab in Veterans. Promote and assess pembrolizumab extended interval dosing.
Background
FDA approved pembrolizumab label change from weight-based to fixed dosing without evidence of fixed-dosing’s superiority. Retrospective studies demonstrate equivalent outcomes for 2 mg/kg every 3 weeks (Q3W), 200 mg Q3W, 4 mg/kg every 6 weeks (Q6W), and 400 mg Q6W.
Methods
In July 2024 VAAAHS (VA Ann Arbor Healthcare System) initiated an immunotherapy stewardship quality improvement program to deprescribe unnecessary pembrolizumab units and promote extended-interval dosing. Specific interventions included order template modification and targeted outreach to key stakeholders.
Data Analysis
All pembrolizumab doses administered at VAAAHS between July 1, 2024 (launch) and March 31, 2025 (data cutoff) were extracted from EHR. Drug utilization, spending, and healthcare contact hours averted were compared to a fixed-dosing counterfactual.
Results
Sixty-three Veterans received 286 total pembrolizumab doses, of which 107 (37.4%) were Q6W and 179 (62.6%) were Q3W. In total, 741 vials were utilized, against expectation of 786 (5.7% reduction), reflecting approximately $182,000 in savings (annualized, $243,000) and 86.5% of the theoretical maximum savings were captured. Q6W’s share of all doses rose from 27.3% in July 2024 to 53.8% in March 2025. Amongst monotherapy, Q6W’s share rose from 60.0% in July 2024 to 86.7% in March 2025. Q6W adoption saved 381 Veteran-healthcare contact hours, not including travel time.
Conclusions
Stewardship efforts reduced unnecessary pembrolizumab utilization and spending while saving Veterans and VAAAHS providers’ time. Continued provider reinforcement, preparation for Oracle/ Cerner implementation, VISN expansion, refinement of pembrolizumab dose-banding, and development of dose bands for other immunotherapies are underway.
Significance
National implementation would improve Veteran convenience and quality of life, enable reductions in drug and resource costs, and enhance clinic throughput.
From Screening to Support: Enhancing Cancer Care Through eScreener Technology
Background
Addressing cancer-related distress is a critical component of comprehensive oncology care. In alignment with the National Comprehensive Cancer Network (NCCN) guidelines, which advocate for routine distress screening as a standard of care, our institution aimed to enhance a previously underutilized paper-based screening process by implementing a more efficient and accessible solution.
Objective
To improve screening rates and streamline the identification of psychosocial needs of Veterans who have cancer.
Population
This initiative was conducted in an outpatient Hematology/Oncology clinic at a Midwest Federal Healthcare Center.
Methods
The Plan-Do-Study-Act (PDSA) quality improvement model was used to guide the implementation of the electronic screener. The eScreener was integrated into routine clinical workflow and staff received training to facilitate implementation. Veterans self-identified their needs through the screener, which included a range of practical, family/social, physical, religious or emotional concerns. Clinical staff then review the responses, assessed the identified needs, and entered appropriate referrals into the electronic health record. A dedicated certified nursing assistant (CNA) was incorporated into the workflow to support implementation efforts. As part of their role, the CNA was tasked with ensuring that all Veterans completed the distress screener either electronically or on paper during their visit
Results
Between January 2025 and March 2025, a total of 180 distress screens were completed using the newly implement method. During the same period in the previous year, only 60 screens were completed, representing a 200% increase. The new process enabled timely referrals based on identified needs, resulting in 39 referrals to physicians, 32 to psychologists, 10 to social work, 7 to dieticians, 6 to nurses, and 1 to pastoral care. These outcomes reflect a significant improvement in both accessibility and patient engagement.
Conclusions
The implementation of an electronic cancer distress screener, along with a dedicated staff member resulted in a substantial increase in screening completion rates and multidisciplinary referrals. These preliminary finds suggest that digital tools can significantly enhance psychosocial assessment, improve coordination, and support the delivery of timely, patient-centered oncology care.
Background
Addressing cancer-related distress is a critical component of comprehensive oncology care. In alignment with the National Comprehensive Cancer Network (NCCN) guidelines, which advocate for routine distress screening as a standard of care, our institution aimed to enhance a previously underutilized paper-based screening process by implementing a more efficient and accessible solution.
Objective
To improve screening rates and streamline the identification of psychosocial needs of Veterans who have cancer.
Population
This initiative was conducted in an outpatient Hematology/Oncology clinic at a Midwest Federal Healthcare Center.
Methods
The Plan-Do-Study-Act (PDSA) quality improvement model was used to guide the implementation of the electronic screener. The eScreener was integrated into routine clinical workflow and staff received training to facilitate implementation. Veterans self-identified their needs through the screener, which included a range of practical, family/social, physical, religious or emotional concerns. Clinical staff then review the responses, assessed the identified needs, and entered appropriate referrals into the electronic health record. A dedicated certified nursing assistant (CNA) was incorporated into the workflow to support implementation efforts. As part of their role, the CNA was tasked with ensuring that all Veterans completed the distress screener either electronically or on paper during their visit
Results
Between January 2025 and March 2025, a total of 180 distress screens were completed using the newly implement method. During the same period in the previous year, only 60 screens were completed, representing a 200% increase. The new process enabled timely referrals based on identified needs, resulting in 39 referrals to physicians, 32 to psychologists, 10 to social work, 7 to dieticians, 6 to nurses, and 1 to pastoral care. These outcomes reflect a significant improvement in both accessibility and patient engagement.
Conclusions
The implementation of an electronic cancer distress screener, along with a dedicated staff member resulted in a substantial increase in screening completion rates and multidisciplinary referrals. These preliminary finds suggest that digital tools can significantly enhance psychosocial assessment, improve coordination, and support the delivery of timely, patient-centered oncology care.
Background
Addressing cancer-related distress is a critical component of comprehensive oncology care. In alignment with the National Comprehensive Cancer Network (NCCN) guidelines, which advocate for routine distress screening as a standard of care, our institution aimed to enhance a previously underutilized paper-based screening process by implementing a more efficient and accessible solution.
Objective
To improve screening rates and streamline the identification of psychosocial needs of Veterans who have cancer.
Population
This initiative was conducted in an outpatient Hematology/Oncology clinic at a Midwest Federal Healthcare Center.
Methods
The Plan-Do-Study-Act (PDSA) quality improvement model was used to guide the implementation of the electronic screener. The eScreener was integrated into routine clinical workflow and staff received training to facilitate implementation. Veterans self-identified their needs through the screener, which included a range of practical, family/social, physical, religious or emotional concerns. Clinical staff then review the responses, assessed the identified needs, and entered appropriate referrals into the electronic health record. A dedicated certified nursing assistant (CNA) was incorporated into the workflow to support implementation efforts. As part of their role, the CNA was tasked with ensuring that all Veterans completed the distress screener either electronically or on paper during their visit
Results
Between January 2025 and March 2025, a total of 180 distress screens were completed using the newly implement method. During the same period in the previous year, only 60 screens were completed, representing a 200% increase. The new process enabled timely referrals based on identified needs, resulting in 39 referrals to physicians, 32 to psychologists, 10 to social work, 7 to dieticians, 6 to nurses, and 1 to pastoral care. These outcomes reflect a significant improvement in both accessibility and patient engagement.
Conclusions
The implementation of an electronic cancer distress screener, along with a dedicated staff member resulted in a substantial increase in screening completion rates and multidisciplinary referrals. These preliminary finds suggest that digital tools can significantly enhance psychosocial assessment, improve coordination, and support the delivery of timely, patient-centered oncology care.
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
Quality improvement (QI) initiatives within the US Department of Veterans Affairs (VA) play an important role in enhancing health care for veterans.1,2 While effective QI programs are often developed, veterans benefit only if they receive care at sites where the program is offered.3 It is estimated only 1% to 5% of patients receive benefit from evidence-based programs, limiting the opportunity for widespread impact.4,5
The Chronic Obstructive Pulmonary Disease (COPD) Coordinated Access to Reduce Exacerbations (CARE) Academy is a national training program designed to promote the adoption of a COPD primary care service.6 The Academy was created and iteratively refined by VA staff to include both clinical training emphasizing COPD management and program implementation strategies. Training programs such as COPD CARE are commonly described as a method to support adoption of health care services, but there is no consensus on a universal approach to training design.
This article describes COPD CARE training and implementation strategies (Table). The Academy began as a training program at 1 VA medical center (VAMC) and has expanded to 49 diverse VAMCs. The Academy illustrates how implementation strategies can be leveraged to develop pragmatic and impactful training. Highlights from the Academy's 9-year history are outlined in this article.

COPD CARE
One in 4 veterans have a COPD diagnosis, and the 5-year mortality rate following a COPD flare is ≥ 50%.7,8 In 2015, a pharmacy resident designed and piloted COPD CARE, a program that used evidence-based practice to optimize management of the disease.9,10
The COPD CARE program is delivered by interprofessional team members. It includes a postacute care call completed 48 hours postdischarge, a wellness visit (face-to-face or virtual) 1 month postdischarge, and a follow-up visit scheduled 2 months postdischarge. Clinical pharmacist practitioners (CPPs) prescribe and collaborate with the COPD CARE health care team. Evidence-based practices embedded within COPD CARE include treatment optimization, symptom evaluation, severity staging, vaccination promotion, referrals, tobacco treatment, and comorbidity management.11-16 The initial COPD CARE pilot demonstrated promising results; patients received timely care and high rates of COPD best practices.11
Academy Design and Implementation
Initial COPD CARE training was tailored to the culture, context, and workflow of the William S. Middleton Memorial Veteran’s Hospital in Madison, Wisconsin. Further service expansion required integration of implementation strategies that enable learners to apply and adapt content to fit different processes, staffing, and patient needs.
Formal Implementation Blueprint
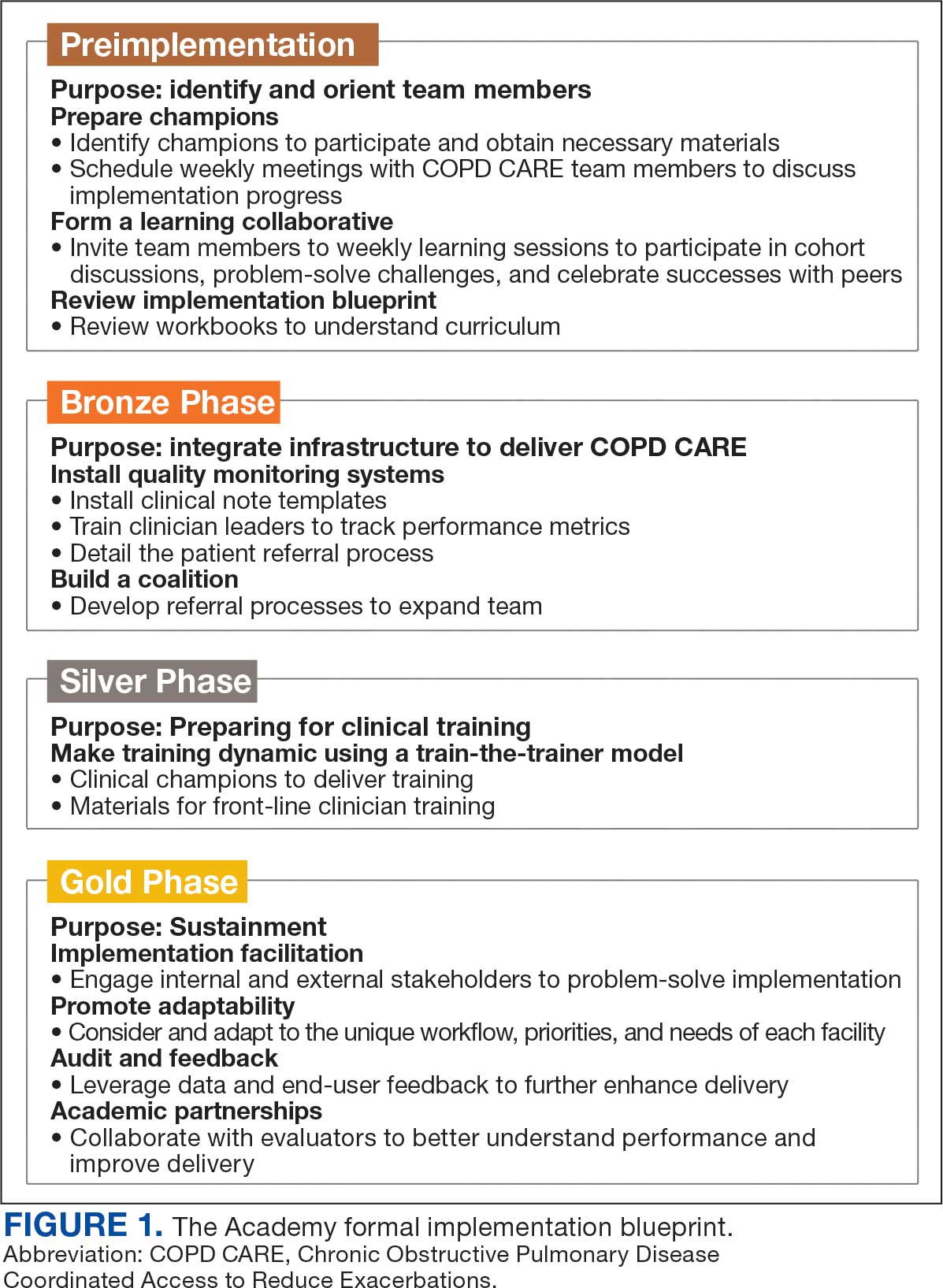
A key aspect of the Academy is the integration of a formal implementation blueprint that includes training goals, scope, and key milestones to guide implementation. The Academy blueprint includes 4 phased training workbooks: (1) preimplementation support from local stakeholders; (2) integration of COPD CARE operational infrastructure into workflows; (3) preparing clinical champions; and (4) leading clinical training (Figure 1). Five weekly 1-hour synchronous virtual discussions are used for learning the workbook content that include learning objectives and opportunities to strategize how to overcome implementation barriers.
Promoting and Facilitating Implementation
As clinicians apply content from the Academy to install informatics tools, coordinate clinical training, and build relationships across service lines, implementation barriers may occur. A learning collaborative allows peer-mentorship and shared problem solving. The Academy learning collaborative includes attendees across multiple VAMCs, allowing for diverse perspectives and cross-site learning. Within the field of dissemination and implementation science, this process of shared problem-solving to support individuals is referred to as implementation facilitation.17 Academy facilitators with prior experience provide a unique perspective and external facilitation from outside local VAMCs. Academy learners form local teams to engage in shared decision-making when applying Academy content. Following Academy completion, learning collaboratives continue to meet monthly to share clinical insights and operational updates.
Local Champions Promote Adaptability
One or more local champions were identified at each VAMC who were focused on the implementation of clinical training content and operational implementation of Academy content.18 Champions have helped develop adaptations of Academy content, such as integrating telehealth nursing within the COPD CARE referral process, which have become new best practices. Champions attend Academy sessions, which provide an opportunity to share adaptations to meet local needs.19
Using a Train-The-Trainer Model
Clinical training was designed to be dynamic and included video modeling, such as recorded examples of CPPs conducting COPD CARE visits and video clips highlighting clinical content. Each learner received a clinical workbook summarizing the content. The champion shares discussion questions to relate training content to the local clinical practice setting. The combination of live training, with videos of clinic visits and case-based discussion was intended to address differing learning styles. Clinical training was delivered using a train-the-trainer model led by the local champion, which allows clinicians with expertise to tailor their training. The use of a train-the-trainer model was intended to promote local buy-in and was often completed by frontline clinicians.
Informatics note templates provide clinicians with information needed to deliver training content during clinic visits. Direct hyperlinks to symptomatic scoring tools, resources to promote evidence-based medication optimization, and patient education resources were embedded within the electronic health record note templates. Direct links to consults for COPD referrals services discussed during clinical training were also included to promote ease of care coordination and awareness of referral opportunities. The integration of clinical training with informatics note template support was intentional to directly relate clinical training to clinical care delivery.
Audit and Feedback
To inform COPD CARE practice, the Academy included informatics infrastructure that allowed for timely local quality monitoring. Electronic health record note templates with embedded data fields track COPD CARE service implementation, including timely completion of patient visits, completion of patient medication reviews, appropriate testing, symptom assessment, and interventions made. Champions can organize template installation and integrate templates into COPD CARE clinical training. Data are included on a COPD CARE implementation dashboard.
An audit and feedback process is allows for the review of performance metrics and development of action plans.20,21 Data reports from note templates are described during the Academy, along with resources to help teams enhance delivery of their program based on performance metrics.
Building a Coalition
Within VA primary care, clinical care delivery is optimized through a team-based coalition of clinicians using the patient aligned care team (PACT) framework. The VA patient-centered team-based care delivery model, patient facilitates coordination of patient referrals, including patient review, scheduling, and completion of patient visits.22
Partnerships with VA Pharmacy Benefits Manager, VA Diffusion of Excellence, VA Quality Enhancement Research Initiative, VA Office of Pulmonary Medicine, and the VA Office of Rural Health have facilitated COPD CARE successes. Collaborations with VA Centers of Innovation helped benchmark the Academy’s impact. An academic partnership with the University of Wisconsin-Madison was established in 2017 and has provided evaluation expertise and leadership as the Academy has been iteratively developed, and revised.
Preliminary Metrics
COPD CARE has delivered > 2000 visits. CPPs have delivered COPD care, with a mean 9.4 of 10 best practices per patient visit. Improvements in veteran COPD symptoms have also been observed following COPD CARE patient visits.
DISCUSSION
The COPD CARE Academy was developed to promote rapid scale-up of a complex, team-based COPD service delivered during veteran care transitions. The implementation blueprint for the Academy is multifaceted and integrates both clinical-focused and implementation-focused infrastructure to apply training content.23 A randomized control trial evaluating the efficacy of training modalities found a need to expand implementation blueprints beyond clinical training alone, as training by itself may not be sufficient to change behavior.24 VA staff designed the Academy using clinical- and implementation-focused content within its implementation blueprint. Key components included leveraging clinical champions, using a train-the-trainer approach, and incorporating facilitation strategies to overcome adoption barriers.
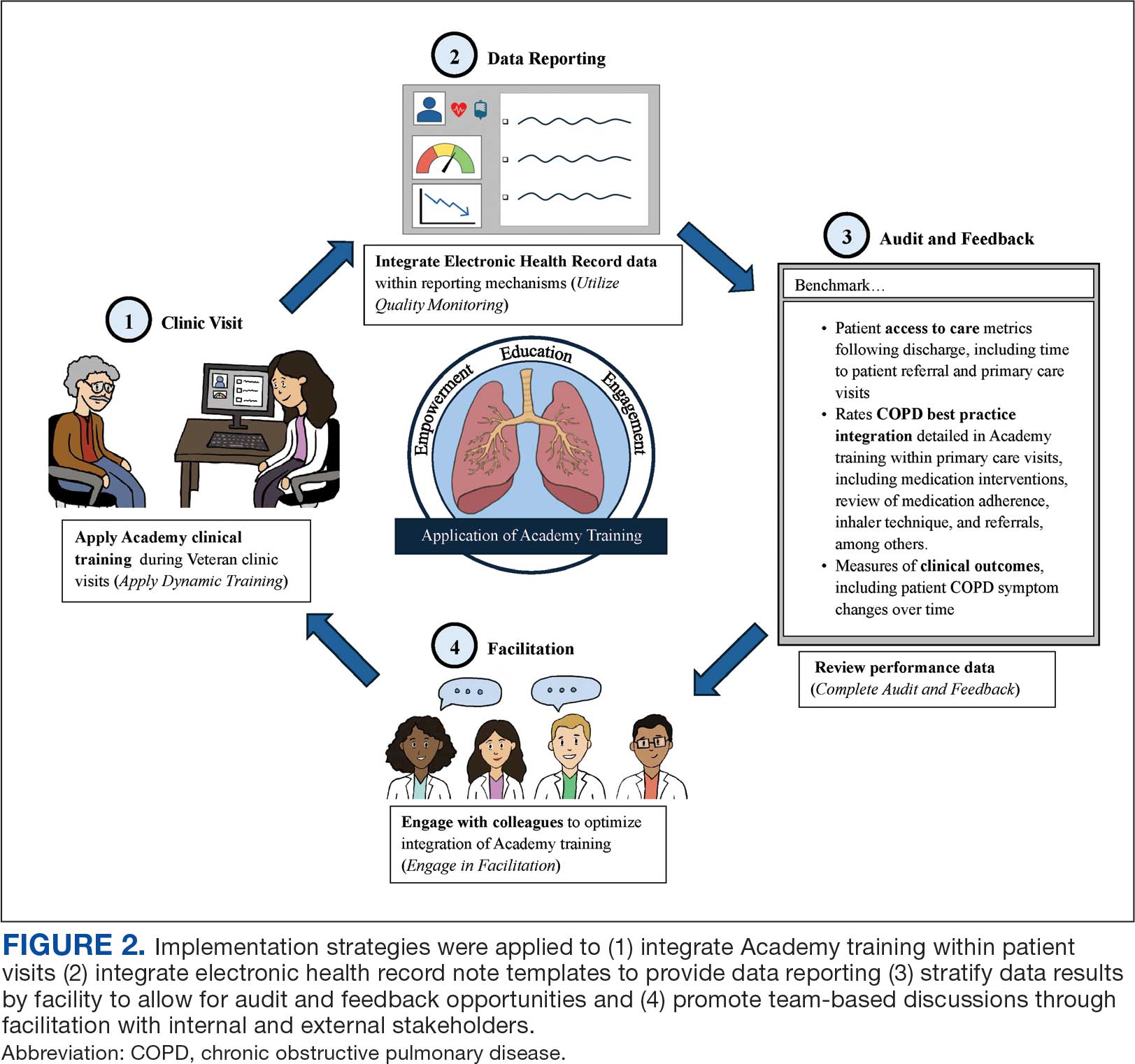
Lewis et al emphasize matching implementation strategies to barriers within VA staff who identify care coordination as a key challenge.23 The informatics infrastructure developed for Academy learners, including standardized note templates, video modeling examples of clinic visits, and data capture for audit and feedback, was designed to complement clinical training and standardize service workflows (Figure 2). There are opportunities to explore how to optimize technology in the Academy.
While Academy clinical training specifically focuses on COPD management, many implementation strategies can be considered to promote care delivery services for other chronic conditions. The Academy blueprint and implementation infrastructure, are strategies that may be considered within and outside the federal health care system. The opportunity for adaptations to Academy training enables clinical champions to promote tailored content to the needs of each unique VAMC. The translation of Academy implementation strategies for new chronic conditions will similarly require adaptations at each VAMC to promote adoption of content.
CONCLUSIONS
COPD CARE Academy is an example of the collaborative spirit within VA, and the opportunity for further advancement of health care programs. The VA is a national leader in Learning Health Systems implementation, in which “science, informatics, incentives and culture are aligned for continuous improvement and innovation.”25,26 There are many opportunities for VA staff to learn from one another to form partnerships between leaders, clinicians, and scientists to optimize health care delivery and further the VA’s work as a learning health system.
- Robinson CH, Thompto AJ, Lima EN, Damschroder LJ. Continuous quality improvement at the frontline: one interdisciplinary clinical team's four-year journey after completing a virtual learning program. Learn Health Syst. 2022;6(4):e10345. doi:10.1002/lrh2.10345
- US Department of Veterans Affairs. Continuous quality improvement (CQI) for clinical teams: a systematic review of reviews. Accessed July 24, 2025. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4151
- Dondanville KA, Fina BA, Straud CL, et al. Launching a competency-based training program in evidence-based treatments for PTSD: supporting veteran-serving mental health providers in Texas. Community Ment Health J. 2021;57(5):910-919. doi:10.1007/S10597-020-00676-7
- Abildso CG, Zizzi SJ, Reger-Nash B. Evaluating an insurance- sponsored weight management program with the RE-AIM model, West Virginia, 2004-2008. Prev Chronic Dis. 2010;7(3):A46.
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National institutes of health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274- 1281. doi:10.2105/AJPH.2012.300755
- Portillo EC, Maurer MA, Kettner JT, et al. Applying RE-AIM to examine the impact of an implementation facilitation package to scale up a program for veterans with chronic obstructive pulmonary disease. Implement Sci Commun. 2023;4(1):143. doi:10.1186/S43058-023-00520-5
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748- 1755. doi:10.1378/chest.06-3018
- Anderson E, Wiener RS, Resnick K, Elwy AR, Rinne ST. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/AJMC.2020.42394
- 2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease - GOLD. Accessed July 24, 2025. https://goldcopd.org/2024-gold-report/
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-e69. doi:10.1164/rccm.202003-0625ST
- Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
- Portillo EC, Gruber S, Lehmann M, et al. Application of the replicating effective programs framework to design a COPD training program. J Am Pharm Assoc. 2021;61(2):e129-e135. doi:10.1016/J.JAPH.2020.10.023
- Portillo EC, Lehmann MR, Hagen TL, et al. Integration of the patient-centered medical home to deliver a care bundle for chronic obstructive pulmonary disease management. J Am Pharm Assoc. 2023;63(1):212-219. doi:10.1016/j.japh.2022.10.003
- Portillo E, Lehmann M, Hagen T, et al. Evaluation of an implementation package to deliver the COPD CARE service. BMJ Open Qual. 2023;12(1). doi:10.1136/BMJOQ-2022-002074
- Portillo E, Lehmann M, Maurer M, et al. Barriers to implementing a pharmacist-led COPD care bundle in rural settings: A qualitative evaluation. 2025 (under review).
- Population Health Management. American Hospital Association. Accessed July 24, 2025. https://www.aha.org/center/population-health-management
- Ritchie MJ, Dollar KM, Miller CK, et al. Using implementation facilitation to improve healthcare: implementation facilitation training manual. Accessed July 11, 2024. https:// www.queri.research.va.gov/tools/Facilitation-Manual.pdf
- Morena AL, Gaias LM, Larkin C. Understanding the role of clinical champions and their impact on clinician behavior change: the need for causal pathway mechanisms. Front Health Serv. 2022;2:896885. doi:10.3389/FRHS.2022.896885
- Ayele RA, Rabin BA, McCreight M, Battaglia C. Editorial: understanding, assessing, and guiding adaptations in public health and health systems interventions: current and future directions. Front Public Health. 2023;11:1228437. doi:10.3389/fpubh.2023.1228437
- Jamtvedt G, Flottorp S, Ivers N. Audit and feedback as a quality strategy. In: Improving Healthcare Services. World Health Organization; 2019. Accessed July 24, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549284/
- Snider MDH, Boyd MR, Walker MR, Powell BJ, Lewis CC. Using audit and feedback to guide tailored implementations of measurement-based care in community mental health: a multiple case study. Implement Sci Commun. 2023;4(1):94. doi:10.1186/s43058-023-00474-8
- Patient Aligned Care Team (PACT) – Patient Care Services. US Department of Veterans Affairs. Accessed July 24, 2025. https://www.patientcare.va.gov/primarycare/PACT.asp
- Lewis CC, Scott K, Marriott BR. A methodology for generating a tailored implementation blueprint: an exemplar from a youth residential setting. Implementat Sci. 2018;13(1):68. doi:10.1186/s13012-018-0761-6
- Beidas RS, Edmunds JM, Marcus SC, Kendall PC. Training and consultation to promote implementation of an empirically supported treatment: a randomized trial. Psychiatr Serv. 2012;63(7):660-665. doi:10.1176/appi.ps.201100401
- Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. doi:10.1002/LRH2.10333
- Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: implications from a qualitative analysis of the scientific literature. Learn Health Syst. 2021;6(2):e10287. doi:10.1002/LRH2.10287
Quality improvement (QI) initiatives within the US Department of Veterans Affairs (VA) play an important role in enhancing health care for veterans.1,2 While effective QI programs are often developed, veterans benefit only if they receive care at sites where the program is offered.3 It is estimated only 1% to 5% of patients receive benefit from evidence-based programs, limiting the opportunity for widespread impact.4,5
The Chronic Obstructive Pulmonary Disease (COPD) Coordinated Access to Reduce Exacerbations (CARE) Academy is a national training program designed to promote the adoption of a COPD primary care service.6 The Academy was created and iteratively refined by VA staff to include both clinical training emphasizing COPD management and program implementation strategies. Training programs such as COPD CARE are commonly described as a method to support adoption of health care services, but there is no consensus on a universal approach to training design.
This article describes COPD CARE training and implementation strategies (Table). The Academy began as a training program at 1 VA medical center (VAMC) and has expanded to 49 diverse VAMCs. The Academy illustrates how implementation strategies can be leveraged to develop pragmatic and impactful training. Highlights from the Academy's 9-year history are outlined in this article.

COPD CARE
One in 4 veterans have a COPD diagnosis, and the 5-year mortality rate following a COPD flare is ≥ 50%.7,8 In 2015, a pharmacy resident designed and piloted COPD CARE, a program that used evidence-based practice to optimize management of the disease.9,10
The COPD CARE program is delivered by interprofessional team members. It includes a postacute care call completed 48 hours postdischarge, a wellness visit (face-to-face or virtual) 1 month postdischarge, and a follow-up visit scheduled 2 months postdischarge. Clinical pharmacist practitioners (CPPs) prescribe and collaborate with the COPD CARE health care team. Evidence-based practices embedded within COPD CARE include treatment optimization, symptom evaluation, severity staging, vaccination promotion, referrals, tobacco treatment, and comorbidity management.11-16 The initial COPD CARE pilot demonstrated promising results; patients received timely care and high rates of COPD best practices.11
Academy Design and Implementation
Initial COPD CARE training was tailored to the culture, context, and workflow of the William S. Middleton Memorial Veteran’s Hospital in Madison, Wisconsin. Further service expansion required integration of implementation strategies that enable learners to apply and adapt content to fit different processes, staffing, and patient needs.
Formal Implementation Blueprint
A key aspect of the Academy is the integration of a formal implementation blueprint that includes training goals, scope, and key milestones to guide implementation. The Academy blueprint includes 4 phased training workbooks: (1) preimplementation support from local stakeholders; (2) integration of COPD CARE operational infrastructure into workflows; (3) preparing clinical champions; and (4) leading clinical training (Figure 1). Five weekly 1-hour synchronous virtual discussions are used for learning the workbook content that include learning objectives and opportunities to strategize how to overcome implementation barriers.

Promoting and Facilitating Implementation
As clinicians apply content from the Academy to install informatics tools, coordinate clinical training, and build relationships across service lines, implementation barriers may occur. A learning collaborative allows peer-mentorship and shared problem solving. The Academy learning collaborative includes attendees across multiple VAMCs, allowing for diverse perspectives and cross-site learning. Within the field of dissemination and implementation science, this process of shared problem-solving to support individuals is referred to as implementation facilitation.17 Academy facilitators with prior experience provide a unique perspective and external facilitation from outside local VAMCs. Academy learners form local teams to engage in shared decision-making when applying Academy content. Following Academy completion, learning collaboratives continue to meet monthly to share clinical insights and operational updates.
Local Champions Promote Adaptability
One or more local champions were identified at each VAMC who were focused on the implementation of clinical training content and operational implementation of Academy content.18 Champions have helped develop adaptations of Academy content, such as integrating telehealth nursing within the COPD CARE referral process, which have become new best practices. Champions attend Academy sessions, which provide an opportunity to share adaptations to meet local needs.19
Using a Train-The-Trainer Model
Clinical training was designed to be dynamic and included video modeling, such as recorded examples of CPPs conducting COPD CARE visits and video clips highlighting clinical content. Each learner received a clinical workbook summarizing the content. The champion shares discussion questions to relate training content to the local clinical practice setting. The combination of live training, with videos of clinic visits and case-based discussion was intended to address differing learning styles. Clinical training was delivered using a train-the-trainer model led by the local champion, which allows clinicians with expertise to tailor their training. The use of a train-the-trainer model was intended to promote local buy-in and was often completed by frontline clinicians.
Informatics note templates provide clinicians with information needed to deliver training content during clinic visits. Direct hyperlinks to symptomatic scoring tools, resources to promote evidence-based medication optimization, and patient education resources were embedded within the electronic health record note templates. Direct links to consults for COPD referrals services discussed during clinical training were also included to promote ease of care coordination and awareness of referral opportunities. The integration of clinical training with informatics note template support was intentional to directly relate clinical training to clinical care delivery.
Audit and Feedback
To inform COPD CARE practice, the Academy included informatics infrastructure that allowed for timely local quality monitoring. Electronic health record note templates with embedded data fields track COPD CARE service implementation, including timely completion of patient visits, completion of patient medication reviews, appropriate testing, symptom assessment, and interventions made. Champions can organize template installation and integrate templates into COPD CARE clinical training. Data are included on a COPD CARE implementation dashboard.
An audit and feedback process is allows for the review of performance metrics and development of action plans.20,21 Data reports from note templates are described during the Academy, along with resources to help teams enhance delivery of their program based on performance metrics.
Building a Coalition
Within VA primary care, clinical care delivery is optimized through a team-based coalition of clinicians using the patient aligned care team (PACT) framework. The VA patient-centered team-based care delivery model, patient facilitates coordination of patient referrals, including patient review, scheduling, and completion of patient visits.22
Partnerships with VA Pharmacy Benefits Manager, VA Diffusion of Excellence, VA Quality Enhancement Research Initiative, VA Office of Pulmonary Medicine, and the VA Office of Rural Health have facilitated COPD CARE successes. Collaborations with VA Centers of Innovation helped benchmark the Academy’s impact. An academic partnership with the University of Wisconsin-Madison was established in 2017 and has provided evaluation expertise and leadership as the Academy has been iteratively developed, and revised.
Preliminary Metrics
COPD CARE has delivered > 2000 visits. CPPs have delivered COPD care, with a mean 9.4 of 10 best practices per patient visit. Improvements in veteran COPD symptoms have also been observed following COPD CARE patient visits.
DISCUSSION
The COPD CARE Academy was developed to promote rapid scale-up of a complex, team-based COPD service delivered during veteran care transitions. The implementation blueprint for the Academy is multifaceted and integrates both clinical-focused and implementation-focused infrastructure to apply training content.23 A randomized control trial evaluating the efficacy of training modalities found a need to expand implementation blueprints beyond clinical training alone, as training by itself may not be sufficient to change behavior.24 VA staff designed the Academy using clinical- and implementation-focused content within its implementation blueprint. Key components included leveraging clinical champions, using a train-the-trainer approach, and incorporating facilitation strategies to overcome adoption barriers.
Lewis et al emphasize matching implementation strategies to barriers within VA staff who identify care coordination as a key challenge.23 The informatics infrastructure developed for Academy learners, including standardized note templates, video modeling examples of clinic visits, and data capture for audit and feedback, was designed to complement clinical training and standardize service workflows (Figure 2). There are opportunities to explore how to optimize technology in the Academy.

While Academy clinical training specifically focuses on COPD management, many implementation strategies can be considered to promote care delivery services for other chronic conditions. The Academy blueprint and implementation infrastructure, are strategies that may be considered within and outside the federal health care system. The opportunity for adaptations to Academy training enables clinical champions to promote tailored content to the needs of each unique VAMC. The translation of Academy implementation strategies for new chronic conditions will similarly require adaptations at each VAMC to promote adoption of content.
CONCLUSIONS
COPD CARE Academy is an example of the collaborative spirit within VA, and the opportunity for further advancement of health care programs. The VA is a national leader in Learning Health Systems implementation, in which “science, informatics, incentives and culture are aligned for continuous improvement and innovation.”25,26 There are many opportunities for VA staff to learn from one another to form partnerships between leaders, clinicians, and scientists to optimize health care delivery and further the VA’s work as a learning health system.
Quality improvement (QI) initiatives within the US Department of Veterans Affairs (VA) play an important role in enhancing health care for veterans.1,2 While effective QI programs are often developed, veterans benefit only if they receive care at sites where the program is offered.3 It is estimated only 1% to 5% of patients receive benefit from evidence-based programs, limiting the opportunity for widespread impact.4,5
The Chronic Obstructive Pulmonary Disease (COPD) Coordinated Access to Reduce Exacerbations (CARE) Academy is a national training program designed to promote the adoption of a COPD primary care service.6 The Academy was created and iteratively refined by VA staff to include both clinical training emphasizing COPD management and program implementation strategies. Training programs such as COPD CARE are commonly described as a method to support adoption of health care services, but there is no consensus on a universal approach to training design.
This article describes COPD CARE training and implementation strategies (Table). The Academy began as a training program at 1 VA medical center (VAMC) and has expanded to 49 diverse VAMCs. The Academy illustrates how implementation strategies can be leveraged to develop pragmatic and impactful training. Highlights from the Academy's 9-year history are outlined in this article.

COPD CARE
One in 4 veterans have a COPD diagnosis, and the 5-year mortality rate following a COPD flare is ≥ 50%.7,8 In 2015, a pharmacy resident designed and piloted COPD CARE, a program that used evidence-based practice to optimize management of the disease.9,10
The COPD CARE program is delivered by interprofessional team members. It includes a postacute care call completed 48 hours postdischarge, a wellness visit (face-to-face or virtual) 1 month postdischarge, and a follow-up visit scheduled 2 months postdischarge. Clinical pharmacist practitioners (CPPs) prescribe and collaborate with the COPD CARE health care team. Evidence-based practices embedded within COPD CARE include treatment optimization, symptom evaluation, severity staging, vaccination promotion, referrals, tobacco treatment, and comorbidity management.11-16 The initial COPD CARE pilot demonstrated promising results; patients received timely care and high rates of COPD best practices.11
Academy Design and Implementation
Initial COPD CARE training was tailored to the culture, context, and workflow of the William S. Middleton Memorial Veteran’s Hospital in Madison, Wisconsin. Further service expansion required integration of implementation strategies that enable learners to apply and adapt content to fit different processes, staffing, and patient needs.
Formal Implementation Blueprint
A key aspect of the Academy is the integration of a formal implementation blueprint that includes training goals, scope, and key milestones to guide implementation. The Academy blueprint includes 4 phased training workbooks: (1) preimplementation support from local stakeholders; (2) integration of COPD CARE operational infrastructure into workflows; (3) preparing clinical champions; and (4) leading clinical training (Figure 1). Five weekly 1-hour synchronous virtual discussions are used for learning the workbook content that include learning objectives and opportunities to strategize how to overcome implementation barriers.

Promoting and Facilitating Implementation
As clinicians apply content from the Academy to install informatics tools, coordinate clinical training, and build relationships across service lines, implementation barriers may occur. A learning collaborative allows peer-mentorship and shared problem solving. The Academy learning collaborative includes attendees across multiple VAMCs, allowing for diverse perspectives and cross-site learning. Within the field of dissemination and implementation science, this process of shared problem-solving to support individuals is referred to as implementation facilitation.17 Academy facilitators with prior experience provide a unique perspective and external facilitation from outside local VAMCs. Academy learners form local teams to engage in shared decision-making when applying Academy content. Following Academy completion, learning collaboratives continue to meet monthly to share clinical insights and operational updates.
Local Champions Promote Adaptability
One or more local champions were identified at each VAMC who were focused on the implementation of clinical training content and operational implementation of Academy content.18 Champions have helped develop adaptations of Academy content, such as integrating telehealth nursing within the COPD CARE referral process, which have become new best practices. Champions attend Academy sessions, which provide an opportunity to share adaptations to meet local needs.19
Using a Train-The-Trainer Model
Clinical training was designed to be dynamic and included video modeling, such as recorded examples of CPPs conducting COPD CARE visits and video clips highlighting clinical content. Each learner received a clinical workbook summarizing the content. The champion shares discussion questions to relate training content to the local clinical practice setting. The combination of live training, with videos of clinic visits and case-based discussion was intended to address differing learning styles. Clinical training was delivered using a train-the-trainer model led by the local champion, which allows clinicians with expertise to tailor their training. The use of a train-the-trainer model was intended to promote local buy-in and was often completed by frontline clinicians.
Informatics note templates provide clinicians with information needed to deliver training content during clinic visits. Direct hyperlinks to symptomatic scoring tools, resources to promote evidence-based medication optimization, and patient education resources were embedded within the electronic health record note templates. Direct links to consults for COPD referrals services discussed during clinical training were also included to promote ease of care coordination and awareness of referral opportunities. The integration of clinical training with informatics note template support was intentional to directly relate clinical training to clinical care delivery.
Audit and Feedback
To inform COPD CARE practice, the Academy included informatics infrastructure that allowed for timely local quality monitoring. Electronic health record note templates with embedded data fields track COPD CARE service implementation, including timely completion of patient visits, completion of patient medication reviews, appropriate testing, symptom assessment, and interventions made. Champions can organize template installation and integrate templates into COPD CARE clinical training. Data are included on a COPD CARE implementation dashboard.
An audit and feedback process is allows for the review of performance metrics and development of action plans.20,21 Data reports from note templates are described during the Academy, along with resources to help teams enhance delivery of their program based on performance metrics.
Building a Coalition
Within VA primary care, clinical care delivery is optimized through a team-based coalition of clinicians using the patient aligned care team (PACT) framework. The VA patient-centered team-based care delivery model, patient facilitates coordination of patient referrals, including patient review, scheduling, and completion of patient visits.22
Partnerships with VA Pharmacy Benefits Manager, VA Diffusion of Excellence, VA Quality Enhancement Research Initiative, VA Office of Pulmonary Medicine, and the VA Office of Rural Health have facilitated COPD CARE successes. Collaborations with VA Centers of Innovation helped benchmark the Academy’s impact. An academic partnership with the University of Wisconsin-Madison was established in 2017 and has provided evaluation expertise and leadership as the Academy has been iteratively developed, and revised.
Preliminary Metrics
COPD CARE has delivered > 2000 visits. CPPs have delivered COPD care, with a mean 9.4 of 10 best practices per patient visit. Improvements in veteran COPD symptoms have also been observed following COPD CARE patient visits.
DISCUSSION
The COPD CARE Academy was developed to promote rapid scale-up of a complex, team-based COPD service delivered during veteran care transitions. The implementation blueprint for the Academy is multifaceted and integrates both clinical-focused and implementation-focused infrastructure to apply training content.23 A randomized control trial evaluating the efficacy of training modalities found a need to expand implementation blueprints beyond clinical training alone, as training by itself may not be sufficient to change behavior.24 VA staff designed the Academy using clinical- and implementation-focused content within its implementation blueprint. Key components included leveraging clinical champions, using a train-the-trainer approach, and incorporating facilitation strategies to overcome adoption barriers.
Lewis et al emphasize matching implementation strategies to barriers within VA staff who identify care coordination as a key challenge.23 The informatics infrastructure developed for Academy learners, including standardized note templates, video modeling examples of clinic visits, and data capture for audit and feedback, was designed to complement clinical training and standardize service workflows (Figure 2). There are opportunities to explore how to optimize technology in the Academy.

While Academy clinical training specifically focuses on COPD management, many implementation strategies can be considered to promote care delivery services for other chronic conditions. The Academy blueprint and implementation infrastructure, are strategies that may be considered within and outside the federal health care system. The opportunity for adaptations to Academy training enables clinical champions to promote tailored content to the needs of each unique VAMC. The translation of Academy implementation strategies for new chronic conditions will similarly require adaptations at each VAMC to promote adoption of content.
CONCLUSIONS
COPD CARE Academy is an example of the collaborative spirit within VA, and the opportunity for further advancement of health care programs. The VA is a national leader in Learning Health Systems implementation, in which “science, informatics, incentives and culture are aligned for continuous improvement and innovation.”25,26 There are many opportunities for VA staff to learn from one another to form partnerships between leaders, clinicians, and scientists to optimize health care delivery and further the VA’s work as a learning health system.
- Robinson CH, Thompto AJ, Lima EN, Damschroder LJ. Continuous quality improvement at the frontline: one interdisciplinary clinical team's four-year journey after completing a virtual learning program. Learn Health Syst. 2022;6(4):e10345. doi:10.1002/lrh2.10345
- US Department of Veterans Affairs. Continuous quality improvement (CQI) for clinical teams: a systematic review of reviews. Accessed July 24, 2025. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4151
- Dondanville KA, Fina BA, Straud CL, et al. Launching a competency-based training program in evidence-based treatments for PTSD: supporting veteran-serving mental health providers in Texas. Community Ment Health J. 2021;57(5):910-919. doi:10.1007/S10597-020-00676-7
- Abildso CG, Zizzi SJ, Reger-Nash B. Evaluating an insurance- sponsored weight management program with the RE-AIM model, West Virginia, 2004-2008. Prev Chronic Dis. 2010;7(3):A46.
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National institutes of health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274- 1281. doi:10.2105/AJPH.2012.300755
- Portillo EC, Maurer MA, Kettner JT, et al. Applying RE-AIM to examine the impact of an implementation facilitation package to scale up a program for veterans with chronic obstructive pulmonary disease. Implement Sci Commun. 2023;4(1):143. doi:10.1186/S43058-023-00520-5
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748- 1755. doi:10.1378/chest.06-3018
- Anderson E, Wiener RS, Resnick K, Elwy AR, Rinne ST. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/AJMC.2020.42394
- 2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease - GOLD. Accessed July 24, 2025. https://goldcopd.org/2024-gold-report/
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-e69. doi:10.1164/rccm.202003-0625ST
- Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
- Portillo EC, Gruber S, Lehmann M, et al. Application of the replicating effective programs framework to design a COPD training program. J Am Pharm Assoc. 2021;61(2):e129-e135. doi:10.1016/J.JAPH.2020.10.023
- Portillo EC, Lehmann MR, Hagen TL, et al. Integration of the patient-centered medical home to deliver a care bundle for chronic obstructive pulmonary disease management. J Am Pharm Assoc. 2023;63(1):212-219. doi:10.1016/j.japh.2022.10.003
- Portillo E, Lehmann M, Hagen T, et al. Evaluation of an implementation package to deliver the COPD CARE service. BMJ Open Qual. 2023;12(1). doi:10.1136/BMJOQ-2022-002074
- Portillo E, Lehmann M, Maurer M, et al. Barriers to implementing a pharmacist-led COPD care bundle in rural settings: A qualitative evaluation. 2025 (under review).
- Population Health Management. American Hospital Association. Accessed July 24, 2025. https://www.aha.org/center/population-health-management
- Ritchie MJ, Dollar KM, Miller CK, et al. Using implementation facilitation to improve healthcare: implementation facilitation training manual. Accessed July 11, 2024. https:// www.queri.research.va.gov/tools/Facilitation-Manual.pdf
- Morena AL, Gaias LM, Larkin C. Understanding the role of clinical champions and their impact on clinician behavior change: the need for causal pathway mechanisms. Front Health Serv. 2022;2:896885. doi:10.3389/FRHS.2022.896885
- Ayele RA, Rabin BA, McCreight M, Battaglia C. Editorial: understanding, assessing, and guiding adaptations in public health and health systems interventions: current and future directions. Front Public Health. 2023;11:1228437. doi:10.3389/fpubh.2023.1228437
- Jamtvedt G, Flottorp S, Ivers N. Audit and feedback as a quality strategy. In: Improving Healthcare Services. World Health Organization; 2019. Accessed July 24, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549284/
- Snider MDH, Boyd MR, Walker MR, Powell BJ, Lewis CC. Using audit and feedback to guide tailored implementations of measurement-based care in community mental health: a multiple case study. Implement Sci Commun. 2023;4(1):94. doi:10.1186/s43058-023-00474-8
- Patient Aligned Care Team (PACT) – Patient Care Services. US Department of Veterans Affairs. Accessed July 24, 2025. https://www.patientcare.va.gov/primarycare/PACT.asp
- Lewis CC, Scott K, Marriott BR. A methodology for generating a tailored implementation blueprint: an exemplar from a youth residential setting. Implementat Sci. 2018;13(1):68. doi:10.1186/s13012-018-0761-6
- Beidas RS, Edmunds JM, Marcus SC, Kendall PC. Training and consultation to promote implementation of an empirically supported treatment: a randomized trial. Psychiatr Serv. 2012;63(7):660-665. doi:10.1176/appi.ps.201100401
- Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. doi:10.1002/LRH2.10333
- Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: implications from a qualitative analysis of the scientific literature. Learn Health Syst. 2021;6(2):e10287. doi:10.1002/LRH2.10287
- Robinson CH, Thompto AJ, Lima EN, Damschroder LJ. Continuous quality improvement at the frontline: one interdisciplinary clinical team's four-year journey after completing a virtual learning program. Learn Health Syst. 2022;6(4):e10345. doi:10.1002/lrh2.10345
- US Department of Veterans Affairs. Continuous quality improvement (CQI) for clinical teams: a systematic review of reviews. Accessed July 24, 2025. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4151
- Dondanville KA, Fina BA, Straud CL, et al. Launching a competency-based training program in evidence-based treatments for PTSD: supporting veteran-serving mental health providers in Texas. Community Ment Health J. 2021;57(5):910-919. doi:10.1007/S10597-020-00676-7
- Abildso CG, Zizzi SJ, Reger-Nash B. Evaluating an insurance- sponsored weight management program with the RE-AIM model, West Virginia, 2004-2008. Prev Chronic Dis. 2010;7(3):A46.
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National institutes of health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274- 1281. doi:10.2105/AJPH.2012.300755
- Portillo EC, Maurer MA, Kettner JT, et al. Applying RE-AIM to examine the impact of an implementation facilitation package to scale up a program for veterans with chronic obstructive pulmonary disease. Implement Sci Commun. 2023;4(1):143. doi:10.1186/S43058-023-00520-5
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748- 1755. doi:10.1378/chest.06-3018
- Anderson E, Wiener RS, Resnick K, Elwy AR, Rinne ST. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/AJMC.2020.42394
- 2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease - GOLD. Accessed July 24, 2025. https://goldcopd.org/2024-gold-report/
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-e69. doi:10.1164/rccm.202003-0625ST
- Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
- Portillo EC, Gruber S, Lehmann M, et al. Application of the replicating effective programs framework to design a COPD training program. J Am Pharm Assoc. 2021;61(2):e129-e135. doi:10.1016/J.JAPH.2020.10.023
- Portillo EC, Lehmann MR, Hagen TL, et al. Integration of the patient-centered medical home to deliver a care bundle for chronic obstructive pulmonary disease management. J Am Pharm Assoc. 2023;63(1):212-219. doi:10.1016/j.japh.2022.10.003
- Portillo E, Lehmann M, Hagen T, et al. Evaluation of an implementation package to deliver the COPD CARE service. BMJ Open Qual. 2023;12(1). doi:10.1136/BMJOQ-2022-002074
- Portillo E, Lehmann M, Maurer M, et al. Barriers to implementing a pharmacist-led COPD care bundle in rural settings: A qualitative evaluation. 2025 (under review).
- Population Health Management. American Hospital Association. Accessed July 24, 2025. https://www.aha.org/center/population-health-management
- Ritchie MJ, Dollar KM, Miller CK, et al. Using implementation facilitation to improve healthcare: implementation facilitation training manual. Accessed July 11, 2024. https:// www.queri.research.va.gov/tools/Facilitation-Manual.pdf
- Morena AL, Gaias LM, Larkin C. Understanding the role of clinical champions and their impact on clinician behavior change: the need for causal pathway mechanisms. Front Health Serv. 2022;2:896885. doi:10.3389/FRHS.2022.896885
- Ayele RA, Rabin BA, McCreight M, Battaglia C. Editorial: understanding, assessing, and guiding adaptations in public health and health systems interventions: current and future directions. Front Public Health. 2023;11:1228437. doi:10.3389/fpubh.2023.1228437
- Jamtvedt G, Flottorp S, Ivers N. Audit and feedback as a quality strategy. In: Improving Healthcare Services. World Health Organization; 2019. Accessed July 24, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549284/
- Snider MDH, Boyd MR, Walker MR, Powell BJ, Lewis CC. Using audit and feedback to guide tailored implementations of measurement-based care in community mental health: a multiple case study. Implement Sci Commun. 2023;4(1):94. doi:10.1186/s43058-023-00474-8
- Patient Aligned Care Team (PACT) – Patient Care Services. US Department of Veterans Affairs. Accessed July 24, 2025. https://www.patientcare.va.gov/primarycare/PACT.asp
- Lewis CC, Scott K, Marriott BR. A methodology for generating a tailored implementation blueprint: an exemplar from a youth residential setting. Implementat Sci. 2018;13(1):68. doi:10.1186/s13012-018-0761-6
- Beidas RS, Edmunds JM, Marcus SC, Kendall PC. Training and consultation to promote implementation of an empirically supported treatment: a randomized trial. Psychiatr Serv. 2012;63(7):660-665. doi:10.1176/appi.ps.201100401
- Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. doi:10.1002/LRH2.10333
- Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: implications from a qualitative analysis of the scientific literature. Learn Health Syst. 2021;6(2):e10287. doi:10.1002/LRH2.10287
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
Evaluation of Subcutaneous Contraception for Patient Self-Administration at North Florida/South Georgia Veterans Health System
Evaluation of Subcutaneous Contraception for Patient Self-Administration at North Florida/South Georgia Veterans Health System
Medroxyprogesterone acetate is an injectable medication indicated for contraception and management of endometriosis-associated pain in females of reproductive age.1 Medroxyprogesterone inhibits gonadotropin secretion, which prevents follicular maturation and ovulation. This leads to endometrial thinning and a contraceptive effect. Adverse drug reactions (ADRs), such as weight gain, menstrual bleeding irregularities, and bone loss appear to be dose- and time-related. Two formulations of medroxyprogesterone acetate are available: 150 mg depot medroxyprogesterone acetate intramuscular (DMPA-IM) and 104 mg DMPA subcutaneous (DMPA-SC).2 Originally, medroxyprogesterone acetate injections required administration by a health care worker. While the current labeling for DMPA-SC still indicates a requirement for administration by a health care worker, data show that the medication can be safe and effective when self-administered.3
Self-Administered Contraception
The 2019 World Health Organization (WHO) guideline on self-care interventions recommends making self-administered injectable contraception available to individuals of reproductive age.3 The WHO recommendation is based on evidence from the Depo Self-Administration Study, which included 401 patients randomized 1:1 to receive self-administered or clinic-administered DMPA-SC. This study concluded that self-administration improved continuation of contraception.4
The North Florida/South Georgia Veterans Health System (NFSGVHS) is the largest US Department of Veterans Affairs (VA) health care system, serving > 22,000 female veterans. All primary care practitioners (PCP) have been trained in women’s health (WH).
The WH patient-aligned care team (PACT) clinical pharmacy practitioner (CPP) proposed using DMPA-SC for outpatient self-administration to increase access, improve patient satisfaction, and reduce burden on patients and nurses for administration appointments. The Pharmacy and Therapeutics Committee (P&T), WH Medical Director, and Chief of Gynecology approved the proposal. DMPA-SC was added to the ordering menu with order sets. The order set included instructions that outlined the 12-week dosing interval, instructions to contact the prescriber if the injection was > 2 weeks overdue (aligning with dosing recommendations for administration every 12 to 14 weeks), and an optional order for a home pregnancy test if necessary. These instructions were designed to ensure proper self-administration of the medication and timely follow-up care.
The gynecology and PACT health care practitioners (HCPs), including physicians, pharmacists, nurses, and medical assistants, received DMPA-SC education, which consisted of a review of medication, ADRs, contraindications, and administration. An NFSGVHS procedure was developed to ensure patients received self-administration education. DMPA-SC prescriptions were mailed to patients with scheduled nursing appointments. The patient would then bring DMPA-SC to the nursing appointment where they received administration instruction and completed the first injection under nurse supervision to ensure appropriate technique. Patients were offered supplementary educational documents and a calendar to keep track of injection days. The patients were responsible for ordering refills and administering subsequent injections at home. Once all stakeholders received education and order sets were in place, prescribers and nurses could begin offering the option for initiation of self-administered DMPA-SC to patients. All conversions or new prescriptions were initiated by prescribers as a part of usual care.
Medication Use Evaluation
A medication use evaluation was conducted about 1 year after the rollout to assess use, adherence, and impact of DMPA-SC for patient-self administration as a new contraceptive option for NFSGVHS patients.
A retrospective chart review was conducted for patients dispensed DMPA-SC from June 1, 2022, to July 1, 2023. Baseline body mass index (BMI), recorded prior to initiation of DMPA-SC, was compared with the most recent BMI on record at the completion of the study to evaluate weight change. Nursing visit attendance for the first injection was also assessed. Adherence was evaluated by reviewing the date of the initial DMPA-SC prescription, the date of the patient's first nursing visit, and subsequent refill patterns. A 2-week margin of error was established to account for the flexibility within the recommended dosing interval and delays in postal service delivery.
Forty patients were initiated on DMPA-SC for patient self-administration. The mean age of patients was 37.2 years. All 40 patients were female. Twenty-two patients (55%) identified as Black, 17 (43%) as White, and 1 (3%) as Asian. The majority (90%) of patients were non-Hispanic. The mean baseline BMI was 30 and BMI after DMPA-SC initiation was 30.4.
Twenty-eight (70%) patients had a nursing appointment, adhering to the NFSGVHS protocol. Five patients (13%) discontinued use and switched to DMPA-IM administered by an HCP and 4 (10%) discontinued use following an ADR (hives, mood changes, bruising, and menometrorrhagia). Of the 31 patients who continued therapy, 25 (81%) were refilling appropriately (Table).

Six patients with unidentified reasons for nonadherence were contacted to determine if there were unmet contraceptive needs. This subgroup included patients with an active prescription for DMPA-SC that did not meet refill expectations. Nonadherence was mostly due to forgetfulness, however 1 patient was unable to refill her DMPA-SC in a timely manner due to an outside hospital admission and another was unreachable. These conversations were documented in the electronic health record (EHR) and all patients requesting follow-up, reinitiation of therapy, or alternative regimens, the appropriate parties were notified to coordinate care.
Discussion
The uptake in DMPA-SC prescribing suggests prescribers and patients have embraced self-administration as an option for contraception. Most patients were appropriately scheduled for nursing appointments to reinforce education and ensure appropriate self-injection technique, as outlined in the NFSGVHS procedure.
The need to improve adherence to NFSGVHS procedure was identified because not all patients had scheduled nursing appointments. This is concerning because some patients may have started self-injecting DMPA-SC without proper education, which could lead to improper injection technique and diminished effectiveness. Nursing appointments ensure appropriate self-injection techniques and reinforce the importance of refilling every 12 weeks for proper effectiveness. Nonadherence to contraceptive therapy may result in unintended pregnancy, although no pregnancies were reported by patients in this study. Pharmacist involvement in DMPA-SC initiation and follow-up monitoring may help ensure adherence to local procedure for initiation and improve patient adherence.
There is limited evidence comparing weight gain related to DMPA-SC vs DMPA-IM. However, in a small, 2-year, randomized study, weight changes were considered comparable for both cohorts with a mean increase of 3.5 kg in the DMPA-IM group vs 3.4 kg in the DMPA-SC group.5 While our analysis did not formally evaluate weight changes, BMI data were collected to evaluate for evidence of weight change. The duration of therapy varied per patient and may not have been long enough to see comparable weight changes.
Strengths of this project include the use of the PACT multidisciplinary approach in primary care including physicians, pharmacists, and nurses. The NFSGVHS EHR is comprehensive, and data including appointments and pharmacy refill information was readily available for collection and evaluation. Limitations included inconsistent documentation in the patient’s EHR which made collection of some data difficult.
Cost Estimates
NFSGVHS had 231 patients prescribed DMPA-IM at the time of DMPA-SC rollout and 40 patients initiated DMPA-SC therapy in the first year. There are possible cost savings associated with the use of DMPA-SC compared to DMPA-IM. Although DMPA-IM costs about $120 annually and DMPA-SC costs about $252 annually, this does not account for indirect costs such as supplies, overhead cost, nursing visits, and patient travel.6 Additionally, allowing patients to self-administer the DMPA-SC injection at home provides nurses time to care for other patients.
Moving forward, the PACT and gynecology teams will receive instruction on the importance of adhering to NFSGVHS procedures to ensure new patients prescribed DMPA-SC receive education and present for nursing appointments to ensure appropriate self-injection.
DMPA has historically been administered in the clinic setting by an HCP; therefore, the prescriber was available to assess adherence to therapy based on patient’s attendance to scheduled clinic appointments. Some prescribers may feel apprehensive about shifting the onus of medication adherence to the patient when prescribing DMPA-SC. However, this model is comparable to any other prescription form of birth control, such as combined hormonal contraceptive pills, where the prescriber expects the patient to take the medication as prescribed and refill their prescriptions in a timely manner to avoid gaps in therapy. The findings of this project suggest the majority of patients who were prescribed self-administered DMPA-SC for contraception were adherent to therapy. The utility of self-administration of DMPA-SC for other labeled or off-label indications was not evaluated; however, it is possible that patients who are motivated to self-administer the medication (regardless of indication) would also demonstrate similar adherence rates.
Conclusions
The majority of patients who started DMPA-SC tolerated the medication well and continued to refill therapy within the recommended time period. Patient self-administration of DMPA-SC can enhance access by removing barriers to administration, increase patient autonomy and contraceptive continuation rates. Overall, the increase in DMPA-SC prescriptions suggests that patients and HCPs support the option for DMPA-SC self-administration at NFSGVHS.
- Depo-SubQ Provera. Package insert. Pharmacia & Upjohn Co; 2019.
- Kaunitz AM. Depot medroxyprogesterone acetate. UpToDate. Updated June 12, 2025. Accessed July 11, 2025. https://www.uptodate.com/contents/depot-medroxyprogesterone-acetate-dmpa-formulations-patient-selection-and-drug-administration
- World Health Organization. WHO guideline on self-care interventions for health and well-being, 2022 revision. World Health Organization. 2022. Accessed July 17, 2025. https://iris.who.int/bitstream/handle/10665/357828/9789240052192-eng.pdf
- Kohn JE, Simons HR, Della Badia L, et al. Increased 1-year continuation of DMPA among women randomized to self-administration: results from a randomized controlled trial at Planned Parenthood. Contraception. 2018;97(3):198-204. doi:10.1016/j.contraception.2017.11.009
- Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80(1):7-17. doi:10.1016/j.contraception.2009.02.005
- UpToDate, Lexidrug. Medroxyprogesterone acetate. Accessed July 16, 2025. https://online.lexi.com
Medroxyprogesterone acetate is an injectable medication indicated for contraception and management of endometriosis-associated pain in females of reproductive age.1 Medroxyprogesterone inhibits gonadotropin secretion, which prevents follicular maturation and ovulation. This leads to endometrial thinning and a contraceptive effect. Adverse drug reactions (ADRs), such as weight gain, menstrual bleeding irregularities, and bone loss appear to be dose- and time-related. Two formulations of medroxyprogesterone acetate are available: 150 mg depot medroxyprogesterone acetate intramuscular (DMPA-IM) and 104 mg DMPA subcutaneous (DMPA-SC).2 Originally, medroxyprogesterone acetate injections required administration by a health care worker. While the current labeling for DMPA-SC still indicates a requirement for administration by a health care worker, data show that the medication can be safe and effective when self-administered.3
Self-Administered Contraception
The 2019 World Health Organization (WHO) guideline on self-care interventions recommends making self-administered injectable contraception available to individuals of reproductive age.3 The WHO recommendation is based on evidence from the Depo Self-Administration Study, which included 401 patients randomized 1:1 to receive self-administered or clinic-administered DMPA-SC. This study concluded that self-administration improved continuation of contraception.4
The North Florida/South Georgia Veterans Health System (NFSGVHS) is the largest US Department of Veterans Affairs (VA) health care system, serving > 22,000 female veterans. All primary care practitioners (PCP) have been trained in women’s health (WH).
The WH patient-aligned care team (PACT) clinical pharmacy practitioner (CPP) proposed using DMPA-SC for outpatient self-administration to increase access, improve patient satisfaction, and reduce burden on patients and nurses for administration appointments. The Pharmacy and Therapeutics Committee (P&T), WH Medical Director, and Chief of Gynecology approved the proposal. DMPA-SC was added to the ordering menu with order sets. The order set included instructions that outlined the 12-week dosing interval, instructions to contact the prescriber if the injection was > 2 weeks overdue (aligning with dosing recommendations for administration every 12 to 14 weeks), and an optional order for a home pregnancy test if necessary. These instructions were designed to ensure proper self-administration of the medication and timely follow-up care.
The gynecology and PACT health care practitioners (HCPs), including physicians, pharmacists, nurses, and medical assistants, received DMPA-SC education, which consisted of a review of medication, ADRs, contraindications, and administration. An NFSGVHS procedure was developed to ensure patients received self-administration education. DMPA-SC prescriptions were mailed to patients with scheduled nursing appointments. The patient would then bring DMPA-SC to the nursing appointment where they received administration instruction and completed the first injection under nurse supervision to ensure appropriate technique. Patients were offered supplementary educational documents and a calendar to keep track of injection days. The patients were responsible for ordering refills and administering subsequent injections at home. Once all stakeholders received education and order sets were in place, prescribers and nurses could begin offering the option for initiation of self-administered DMPA-SC to patients. All conversions or new prescriptions were initiated by prescribers as a part of usual care.
Medication Use Evaluation
A medication use evaluation was conducted about 1 year after the rollout to assess use, adherence, and impact of DMPA-SC for patient-self administration as a new contraceptive option for NFSGVHS patients.
A retrospective chart review was conducted for patients dispensed DMPA-SC from June 1, 2022, to July 1, 2023. Baseline body mass index (BMI), recorded prior to initiation of DMPA-SC, was compared with the most recent BMI on record at the completion of the study to evaluate weight change. Nursing visit attendance for the first injection was also assessed. Adherence was evaluated by reviewing the date of the initial DMPA-SC prescription, the date of the patient's first nursing visit, and subsequent refill patterns. A 2-week margin of error was established to account for the flexibility within the recommended dosing interval and delays in postal service delivery.
Forty patients were initiated on DMPA-SC for patient self-administration. The mean age of patients was 37.2 years. All 40 patients were female. Twenty-two patients (55%) identified as Black, 17 (43%) as White, and 1 (3%) as Asian. The majority (90%) of patients were non-Hispanic. The mean baseline BMI was 30 and BMI after DMPA-SC initiation was 30.4.
Twenty-eight (70%) patients had a nursing appointment, adhering to the NFSGVHS protocol. Five patients (13%) discontinued use and switched to DMPA-IM administered by an HCP and 4 (10%) discontinued use following an ADR (hives, mood changes, bruising, and menometrorrhagia). Of the 31 patients who continued therapy, 25 (81%) were refilling appropriately (Table).

Six patients with unidentified reasons for nonadherence were contacted to determine if there were unmet contraceptive needs. This subgroup included patients with an active prescription for DMPA-SC that did not meet refill expectations. Nonadherence was mostly due to forgetfulness, however 1 patient was unable to refill her DMPA-SC in a timely manner due to an outside hospital admission and another was unreachable. These conversations were documented in the electronic health record (EHR) and all patients requesting follow-up, reinitiation of therapy, or alternative regimens, the appropriate parties were notified to coordinate care.
Discussion
The uptake in DMPA-SC prescribing suggests prescribers and patients have embraced self-administration as an option for contraception. Most patients were appropriately scheduled for nursing appointments to reinforce education and ensure appropriate self-injection technique, as outlined in the NFSGVHS procedure.
The need to improve adherence to NFSGVHS procedure was identified because not all patients had scheduled nursing appointments. This is concerning because some patients may have started self-injecting DMPA-SC without proper education, which could lead to improper injection technique and diminished effectiveness. Nursing appointments ensure appropriate self-injection techniques and reinforce the importance of refilling every 12 weeks for proper effectiveness. Nonadherence to contraceptive therapy may result in unintended pregnancy, although no pregnancies were reported by patients in this study. Pharmacist involvement in DMPA-SC initiation and follow-up monitoring may help ensure adherence to local procedure for initiation and improve patient adherence.
There is limited evidence comparing weight gain related to DMPA-SC vs DMPA-IM. However, in a small, 2-year, randomized study, weight changes were considered comparable for both cohorts with a mean increase of 3.5 kg in the DMPA-IM group vs 3.4 kg in the DMPA-SC group.5 While our analysis did not formally evaluate weight changes, BMI data were collected to evaluate for evidence of weight change. The duration of therapy varied per patient and may not have been long enough to see comparable weight changes.
Strengths of this project include the use of the PACT multidisciplinary approach in primary care including physicians, pharmacists, and nurses. The NFSGVHS EHR is comprehensive, and data including appointments and pharmacy refill information was readily available for collection and evaluation. Limitations included inconsistent documentation in the patient’s EHR which made collection of some data difficult.
Cost Estimates
NFSGVHS had 231 patients prescribed DMPA-IM at the time of DMPA-SC rollout and 40 patients initiated DMPA-SC therapy in the first year. There are possible cost savings associated with the use of DMPA-SC compared to DMPA-IM. Although DMPA-IM costs about $120 annually and DMPA-SC costs about $252 annually, this does not account for indirect costs such as supplies, overhead cost, nursing visits, and patient travel.6 Additionally, allowing patients to self-administer the DMPA-SC injection at home provides nurses time to care for other patients.
Moving forward, the PACT and gynecology teams will receive instruction on the importance of adhering to NFSGVHS procedures to ensure new patients prescribed DMPA-SC receive education and present for nursing appointments to ensure appropriate self-injection.
DMPA has historically been administered in the clinic setting by an HCP; therefore, the prescriber was available to assess adherence to therapy based on patient’s attendance to scheduled clinic appointments. Some prescribers may feel apprehensive about shifting the onus of medication adherence to the patient when prescribing DMPA-SC. However, this model is comparable to any other prescription form of birth control, such as combined hormonal contraceptive pills, where the prescriber expects the patient to take the medication as prescribed and refill their prescriptions in a timely manner to avoid gaps in therapy. The findings of this project suggest the majority of patients who were prescribed self-administered DMPA-SC for contraception were adherent to therapy. The utility of self-administration of DMPA-SC for other labeled or off-label indications was not evaluated; however, it is possible that patients who are motivated to self-administer the medication (regardless of indication) would also demonstrate similar adherence rates.
Conclusions
The majority of patients who started DMPA-SC tolerated the medication well and continued to refill therapy within the recommended time period. Patient self-administration of DMPA-SC can enhance access by removing barriers to administration, increase patient autonomy and contraceptive continuation rates. Overall, the increase in DMPA-SC prescriptions suggests that patients and HCPs support the option for DMPA-SC self-administration at NFSGVHS.
Medroxyprogesterone acetate is an injectable medication indicated for contraception and management of endometriosis-associated pain in females of reproductive age.1 Medroxyprogesterone inhibits gonadotropin secretion, which prevents follicular maturation and ovulation. This leads to endometrial thinning and a contraceptive effect. Adverse drug reactions (ADRs), such as weight gain, menstrual bleeding irregularities, and bone loss appear to be dose- and time-related. Two formulations of medroxyprogesterone acetate are available: 150 mg depot medroxyprogesterone acetate intramuscular (DMPA-IM) and 104 mg DMPA subcutaneous (DMPA-SC).2 Originally, medroxyprogesterone acetate injections required administration by a health care worker. While the current labeling for DMPA-SC still indicates a requirement for administration by a health care worker, data show that the medication can be safe and effective when self-administered.3
Self-Administered Contraception
The 2019 World Health Organization (WHO) guideline on self-care interventions recommends making self-administered injectable contraception available to individuals of reproductive age.3 The WHO recommendation is based on evidence from the Depo Self-Administration Study, which included 401 patients randomized 1:1 to receive self-administered or clinic-administered DMPA-SC. This study concluded that self-administration improved continuation of contraception.4
The North Florida/South Georgia Veterans Health System (NFSGVHS) is the largest US Department of Veterans Affairs (VA) health care system, serving > 22,000 female veterans. All primary care practitioners (PCP) have been trained in women’s health (WH).
The WH patient-aligned care team (PACT) clinical pharmacy practitioner (CPP) proposed using DMPA-SC for outpatient self-administration to increase access, improve patient satisfaction, and reduce burden on patients and nurses for administration appointments. The Pharmacy and Therapeutics Committee (P&T), WH Medical Director, and Chief of Gynecology approved the proposal. DMPA-SC was added to the ordering menu with order sets. The order set included instructions that outlined the 12-week dosing interval, instructions to contact the prescriber if the injection was > 2 weeks overdue (aligning with dosing recommendations for administration every 12 to 14 weeks), and an optional order for a home pregnancy test if necessary. These instructions were designed to ensure proper self-administration of the medication and timely follow-up care.
The gynecology and PACT health care practitioners (HCPs), including physicians, pharmacists, nurses, and medical assistants, received DMPA-SC education, which consisted of a review of medication, ADRs, contraindications, and administration. An NFSGVHS procedure was developed to ensure patients received self-administration education. DMPA-SC prescriptions were mailed to patients with scheduled nursing appointments. The patient would then bring DMPA-SC to the nursing appointment where they received administration instruction and completed the first injection under nurse supervision to ensure appropriate technique. Patients were offered supplementary educational documents and a calendar to keep track of injection days. The patients were responsible for ordering refills and administering subsequent injections at home. Once all stakeholders received education and order sets were in place, prescribers and nurses could begin offering the option for initiation of self-administered DMPA-SC to patients. All conversions or new prescriptions were initiated by prescribers as a part of usual care.
Medication Use Evaluation
A medication use evaluation was conducted about 1 year after the rollout to assess use, adherence, and impact of DMPA-SC for patient-self administration as a new contraceptive option for NFSGVHS patients.
A retrospective chart review was conducted for patients dispensed DMPA-SC from June 1, 2022, to July 1, 2023. Baseline body mass index (BMI), recorded prior to initiation of DMPA-SC, was compared with the most recent BMI on record at the completion of the study to evaluate weight change. Nursing visit attendance for the first injection was also assessed. Adherence was evaluated by reviewing the date of the initial DMPA-SC prescription, the date of the patient's first nursing visit, and subsequent refill patterns. A 2-week margin of error was established to account for the flexibility within the recommended dosing interval and delays in postal service delivery.
Forty patients were initiated on DMPA-SC for patient self-administration. The mean age of patients was 37.2 years. All 40 patients were female. Twenty-two patients (55%) identified as Black, 17 (43%) as White, and 1 (3%) as Asian. The majority (90%) of patients were non-Hispanic. The mean baseline BMI was 30 and BMI after DMPA-SC initiation was 30.4.
Twenty-eight (70%) patients had a nursing appointment, adhering to the NFSGVHS protocol. Five patients (13%) discontinued use and switched to DMPA-IM administered by an HCP and 4 (10%) discontinued use following an ADR (hives, mood changes, bruising, and menometrorrhagia). Of the 31 patients who continued therapy, 25 (81%) were refilling appropriately (Table).

Six patients with unidentified reasons for nonadherence were contacted to determine if there were unmet contraceptive needs. This subgroup included patients with an active prescription for DMPA-SC that did not meet refill expectations. Nonadherence was mostly due to forgetfulness, however 1 patient was unable to refill her DMPA-SC in a timely manner due to an outside hospital admission and another was unreachable. These conversations were documented in the electronic health record (EHR) and all patients requesting follow-up, reinitiation of therapy, or alternative regimens, the appropriate parties were notified to coordinate care.
Discussion
The uptake in DMPA-SC prescribing suggests prescribers and patients have embraced self-administration as an option for contraception. Most patients were appropriately scheduled for nursing appointments to reinforce education and ensure appropriate self-injection technique, as outlined in the NFSGVHS procedure.
The need to improve adherence to NFSGVHS procedure was identified because not all patients had scheduled nursing appointments. This is concerning because some patients may have started self-injecting DMPA-SC without proper education, which could lead to improper injection technique and diminished effectiveness. Nursing appointments ensure appropriate self-injection techniques and reinforce the importance of refilling every 12 weeks for proper effectiveness. Nonadherence to contraceptive therapy may result in unintended pregnancy, although no pregnancies were reported by patients in this study. Pharmacist involvement in DMPA-SC initiation and follow-up monitoring may help ensure adherence to local procedure for initiation and improve patient adherence.
There is limited evidence comparing weight gain related to DMPA-SC vs DMPA-IM. However, in a small, 2-year, randomized study, weight changes were considered comparable for both cohorts with a mean increase of 3.5 kg in the DMPA-IM group vs 3.4 kg in the DMPA-SC group.5 While our analysis did not formally evaluate weight changes, BMI data were collected to evaluate for evidence of weight change. The duration of therapy varied per patient and may not have been long enough to see comparable weight changes.
Strengths of this project include the use of the PACT multidisciplinary approach in primary care including physicians, pharmacists, and nurses. The NFSGVHS EHR is comprehensive, and data including appointments and pharmacy refill information was readily available for collection and evaluation. Limitations included inconsistent documentation in the patient’s EHR which made collection of some data difficult.
Cost Estimates
NFSGVHS had 231 patients prescribed DMPA-IM at the time of DMPA-SC rollout and 40 patients initiated DMPA-SC therapy in the first year. There are possible cost savings associated with the use of DMPA-SC compared to DMPA-IM. Although DMPA-IM costs about $120 annually and DMPA-SC costs about $252 annually, this does not account for indirect costs such as supplies, overhead cost, nursing visits, and patient travel.6 Additionally, allowing patients to self-administer the DMPA-SC injection at home provides nurses time to care for other patients.
Moving forward, the PACT and gynecology teams will receive instruction on the importance of adhering to NFSGVHS procedures to ensure new patients prescribed DMPA-SC receive education and present for nursing appointments to ensure appropriate self-injection.
DMPA has historically been administered in the clinic setting by an HCP; therefore, the prescriber was available to assess adherence to therapy based on patient’s attendance to scheduled clinic appointments. Some prescribers may feel apprehensive about shifting the onus of medication adherence to the patient when prescribing DMPA-SC. However, this model is comparable to any other prescription form of birth control, such as combined hormonal contraceptive pills, where the prescriber expects the patient to take the medication as prescribed and refill their prescriptions in a timely manner to avoid gaps in therapy. The findings of this project suggest the majority of patients who were prescribed self-administered DMPA-SC for contraception were adherent to therapy. The utility of self-administration of DMPA-SC for other labeled or off-label indications was not evaluated; however, it is possible that patients who are motivated to self-administer the medication (regardless of indication) would also demonstrate similar adherence rates.
Conclusions
The majority of patients who started DMPA-SC tolerated the medication well and continued to refill therapy within the recommended time period. Patient self-administration of DMPA-SC can enhance access by removing barriers to administration, increase patient autonomy and contraceptive continuation rates. Overall, the increase in DMPA-SC prescriptions suggests that patients and HCPs support the option for DMPA-SC self-administration at NFSGVHS.
- Depo-SubQ Provera. Package insert. Pharmacia & Upjohn Co; 2019.
- Kaunitz AM. Depot medroxyprogesterone acetate. UpToDate. Updated June 12, 2025. Accessed July 11, 2025. https://www.uptodate.com/contents/depot-medroxyprogesterone-acetate-dmpa-formulations-patient-selection-and-drug-administration
- World Health Organization. WHO guideline on self-care interventions for health and well-being, 2022 revision. World Health Organization. 2022. Accessed July 17, 2025. https://iris.who.int/bitstream/handle/10665/357828/9789240052192-eng.pdf
- Kohn JE, Simons HR, Della Badia L, et al. Increased 1-year continuation of DMPA among women randomized to self-administration: results from a randomized controlled trial at Planned Parenthood. Contraception. 2018;97(3):198-204. doi:10.1016/j.contraception.2017.11.009
- Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80(1):7-17. doi:10.1016/j.contraception.2009.02.005
- UpToDate, Lexidrug. Medroxyprogesterone acetate. Accessed July 16, 2025. https://online.lexi.com
- Depo-SubQ Provera. Package insert. Pharmacia & Upjohn Co; 2019.
- Kaunitz AM. Depot medroxyprogesterone acetate. UpToDate. Updated June 12, 2025. Accessed July 11, 2025. https://www.uptodate.com/contents/depot-medroxyprogesterone-acetate-dmpa-formulations-patient-selection-and-drug-administration
- World Health Organization. WHO guideline on self-care interventions for health and well-being, 2022 revision. World Health Organization. 2022. Accessed July 17, 2025. https://iris.who.int/bitstream/handle/10665/357828/9789240052192-eng.pdf
- Kohn JE, Simons HR, Della Badia L, et al. Increased 1-year continuation of DMPA among women randomized to self-administration: results from a randomized controlled trial at Planned Parenthood. Contraception. 2018;97(3):198-204. doi:10.1016/j.contraception.2017.11.009
- Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80(1):7-17. doi:10.1016/j.contraception.2009.02.005
- UpToDate, Lexidrug. Medroxyprogesterone acetate. Accessed July 16, 2025. https://online.lexi.com
Evaluation of Subcutaneous Contraception for Patient Self-Administration at North Florida/South Georgia Veterans Health System
Evaluation of Subcutaneous Contraception for Patient Self-Administration at North Florida/South Georgia Veterans Health System
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project
Penicillin allergy is common in the United States. About 9.0% to 13.8% of patients have a diagnosed penicillin allergy documented in their electronic health record. The annual incidence rates is 1.1% in males and 1.4% in females.1,2
Penicillin hypersensitivity likely wanes over time. A 1981 study found that 93% of patients who experienced an allergic reaction to penicillin had a positive skin test 7 to 12 months postreaction, but only 22% still had a positive test after 10 years.3 Confirmed type 1 hypersensitivity penicillin allergies, as demonstrated by positive skin prick testing, also are decreasing over time.4 Furthermore, many patients’ reactions may have been misdiagnosed as a penicillin allergy. Upon actual confirmatory testing of penicillin allergy, only 8.5% to 13.8% of patients believed to have a penicillin allergy were positive on skin prick testing of penicillin products.5,6 A 2024 US study found that 11% of individuals with a history of a penicillin reaction tested positive on skin testing.7
The positive predictive value of penicillin allergy skin testing is poorly defined due to the ethical dilemma of orally challenging a patient who demonstrates skin test reactivity. Due to its high negative predictive value (NPV), skin prick combined with intradermal testing has been the gold-standard test in cases of clinical concern.6 Patients with positive skin testing are assumed to be truly positive, and therefore penicillin allergic, even though false-positive results to penicillin skin testing are known to occur.8
Misdiagnosis of penicillin allergy carries substantial clinical and economic consequences. A 2011 study suggested a statistically significant 1.8% increased absolute risk of mortality and 5.5% increased absolute risk of intensive care unit admission for those labeled with penicillin allergy and admitted for an infection.9 Another study found a 14% increase in mortality associated with the diagnosis of penicillin allergy.10 In a 2014 case-control study, penicillin allergy also was associated with a 23.4% greater risk of Clostridioides difficile, 14.1% more methicillin-resistant Staphylococcus aureus, and 30.1% more vancomycin-resistant enterococci infections.11Direct cost savings during an inpatient admission for infection were as much as $609 per patient with additional indirect cost savings of up to $4254 per admission.12 When viewed from the perspective of a health care system, these costs quickly accumulate, negatively impacting the fiscal stability of our patients and placing additional financial strain on an over-burdened system.
If 10% of US patients have penicillin allergy labels, then about 33 million patients might be eligible for delabeling. There are only 6309 board-certified allergists actively practicing in the US, which could amount to about 5231 potential penicillin challenges per allergist, not even including the 3.3 million new patients per year (assuming a 1% incidence).13 Clarifying each patient’s tolerance of penicillin products will clearly require nonallergist cooperation.
The 2022 drug allergy practice parameter update recommends several consensus-based statements (CBSs) to directly address penicillin allergy.14 This guideline recommends proactive efforts to delabel patients with a reported penicillin allergy (CBS 4); advise against testing in cases where the history is inconsistent with a true allergic reaction, though a challenge may be offered (CBS 5); skin testing for those with a history of anaphylaxis or a recent reaction (CBS 6); advise against multiple-day penicillin challenges (CBS 7); advise against skin testing for pediatric patients with benign cutaneous reactions (CBS 8); and recommends direct oral challenge for adults with distant or benign cutaneous reactions (CBS 9). These recommendations create a potentially high demand for delabeling with allergy specialists. One potential solution is to perform direct oral challenges in primary care, emergency departments, and urgent care clinics.
Evidence supporting the safety of direct oral penicillin challenges in low-risk patients was initially noted in the allergy community, but now evidence for their use in primary care clinics is growing—including in children.15 In a military-specific population, an amoxicillin challenge of Marine recruits with suspected penicillin allergy revealed that only 1.5% of those challenged acutely reacted and should be considered allergic to penicillin.16 Historically, in order to refute the diagnosis of penicillin allergy, an allergist would order penicillin skin prick testing. If the test was negative, an allergist would proceed to intradermal testing and if negative again (NPV of 97.9%), proceed to a graded oral challenge.6 However, this process is not fully reproducible in most clinics because the minor determinants mixture used in skin testing is not commercially available.17 Additionally, the full skin testing procedure requires specialized training, is more time-consuming, causes more discomfort, lacks US Food and Drug Administration approval for children, and has a higher cost ($220 per test for each patient as of 2016).18 As such, the movement toward direct oral challenges is progressing. Nonetheless, the best method for primary care or emergency department clinicians to determine who the appropriate patients are for this procedure has not been fully established. Risk tools have been created in the past to help delineate low-risk patients who would be appropriate for direct oral amoxicillin challenges, but these were not widely replicated or validated.19 The PEN-FAST standardized risk score was first published in 2020 and has since been validated in different groups with additional safety data. This scoring system ranges from 0 to 5 points, assigning 2 points for a penicillin reaction within the past five (F) years, 2 points for angioedema/anaphylaxis (A) or a severe (S) cutaneous reaction, and 1 point if treatment (T) was required for the reaction. A score < 3 is considered low-risk and safe for direct oral challenge, although most of the safety data are in patients with a score of 0 or 1.20 The PEN-FAST guided direct oral challenge with an NPV of 96.3% has now been prospectively shown to be noninferior to standard skin prick test/intradermal test/graded challenge for low-risk patients with a PEN-FAST score < 3.21 The PEN-FAST validating study was conducted predominantly with an Australian population of adult White women, but now it also has been validated in children aged > 12 years, as well as in European and North American cohorts.22-24
Air Force Delabeling Program
This article describes a method for proactively, safely, and efficiently delabeling penicillin allergic patients at an Air Force clinic. This quality improvement (QI) report provides a successful model for penicillin allergy delabeling, illustrates lessons learned, and suggests next steps toward improving patient options for an invaluable antibiotic class.
The first step was to proactively delabel penicillin allergy from a population of active duty service members and their dependents. Electronic health record (EHR) allergy search functions are a helpful tool in finding patients with allergy labels. The Kadena Medical Clinic, in Okinawa, Japan, uses the Military Health System GENESIS EHR, which includes a discern reporting portal with a patient allergy search that creates a patient-specific medication allergy report. To compile the most complete database of patients with a penicillin allergy, all 15 potential allergy search options for “penicillin” were selected, as were 4 relevant options for amoxicillin (including options with clavulanate). Including so many options for specific penicillin medication allergies helps add specificity to the diagnosis in the EHR but can make aggregation of data more difficult. The
The complete compiled list was manually reviewed for high-risk patients with severe cutaneous adverse reactions (SCARs) of any age. Patients with pregnancy, unsuitable medical histories (ie, severe asthma), or taking β-blockers were excluded. Patients remaining on the list were contacted by telephone and offered appointments during a single week that was dedicated to penicillin allergy delabeling. Allergists in the Air Force are assigned to a region where they offer allergy services at clinics without a regular allergist. The allergist for the region traveled to the QI site for a 1-week campaign at an estimated cost of $4600. When the patients were contacted, they were briefly informed of the goal of the penicillin delabeling campaign, and if interested, they were scheduled for 1 of 50 available appointments that week. Patients were contacted with enough lead time to stop oral antihistamines (OAH) for ≥ 7 days before the appointment.
Patients were given an intake questionnaire and interviewed about their penicillin allergy history. This questionnaire inquired about the nature of the allergy, mental and physical health impacts of the allergy label, PEN-FAST scoring questions, and posttest attitude toward delabeling, if applicable. Patients with a PEN-FAST score < 3 were offered direct, graded oral challenge or the standard skin prick, followed by intradermal, followed by graded oral challenge protocol. Patients with PEN-FAST scores of ≥ 3 were offered skin testing prior to oral challenge protocol. Patients could decline further testing. If patients wished to proceed, they were asked to complete a written informed consent document.
Oral challenges followed a 10%/90% protocol, beginning with 50 mg of liquid amoxicillin followed by 450 mg after 15 minutes, as long as the patient remained asymptomatic. Challenge forms are available in the eAppendix . After receiving the 450-mg amoxicillin dose, the patient remained in the clinic for 60 minutes before a final clinical evaluation. If the patient remained asymptomatic after this period, the penicillin or amoxicillin allergy was marked as resolved in the EHR. The patients were given contact information for the clinic for follow-up if a delayed reaction was noted and they wished the medication allergy to be re-entered. An EHR encounter note was created for each patient detailing the allergy testing and delabeling.
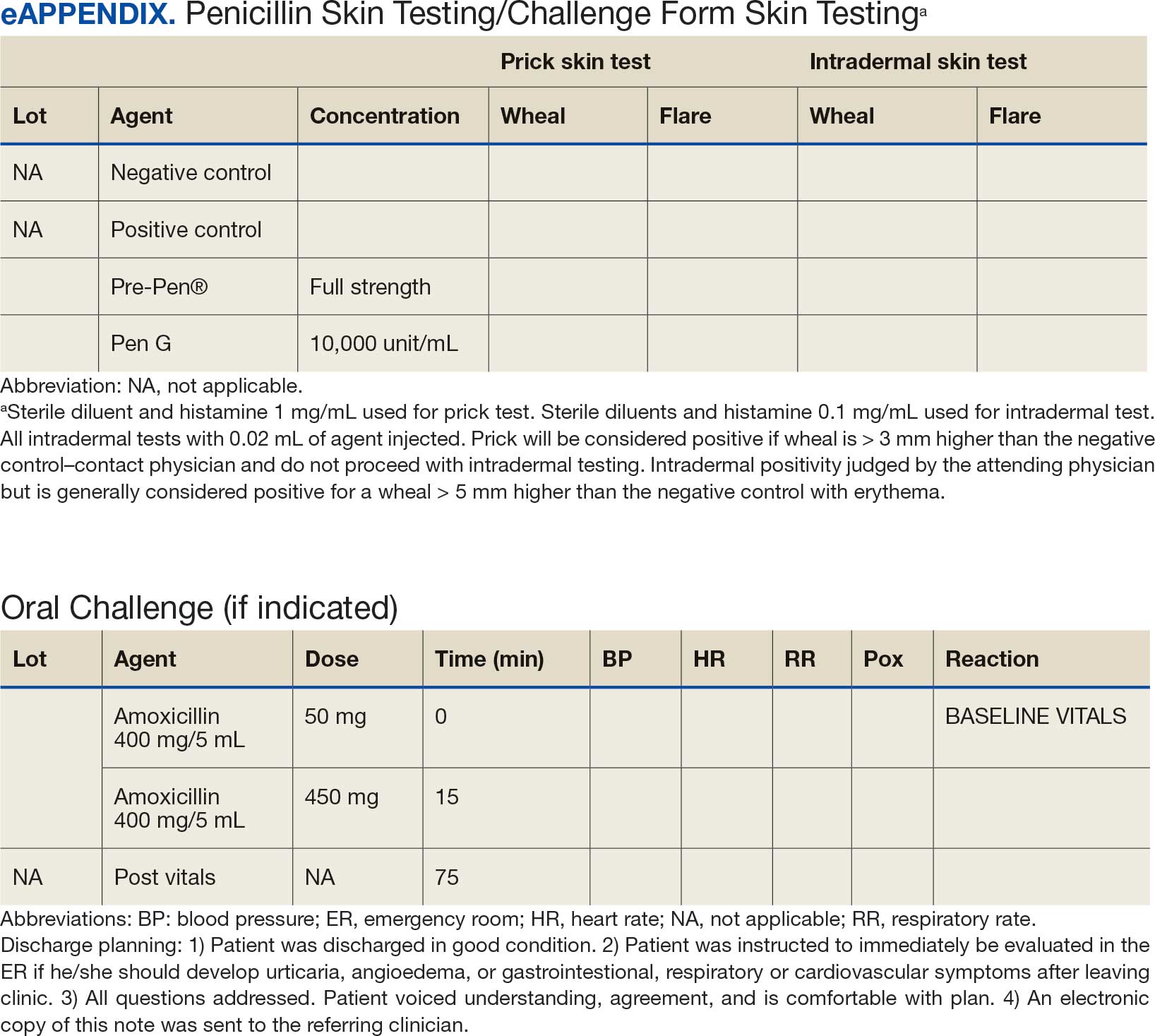
This campaign was conducted at a basic life support-only facility by a single clinician without medical technician support. An allergic reaction medication kit was available and contained OAHs, intramuscular antihistamines, intramuscular epinephrine, intramuscular corticosteroids, and short-acting β-agonists for nebulization. The facility also had an urgent care room (staffed by primary care practitioners [PCPs]) that could help establish intravenous access and administer fluids if necessary and had previously established plans for emergency patient transport to a higher level of care, if necessary.
Program Outcomes
A list of 65 patients that included both active-duty service members and dependents with penicillin or amoxicillin allergy was created. This list was reviewed by an allergist to identify high-risk individuals, which required about 90 minutes. Two patients (3%) were excluded; 1 had a history of SCAR to penicillin and 1 had a complex medical history requiring continued OAH use. Sixty-three patients were contacted via telephone, and 29 patients (46%) scheduled an appointment. One patient (2%) was identified as penicillin-tolerant during the booking process, and the penicillin allergy was removed without testing (Figure 1).
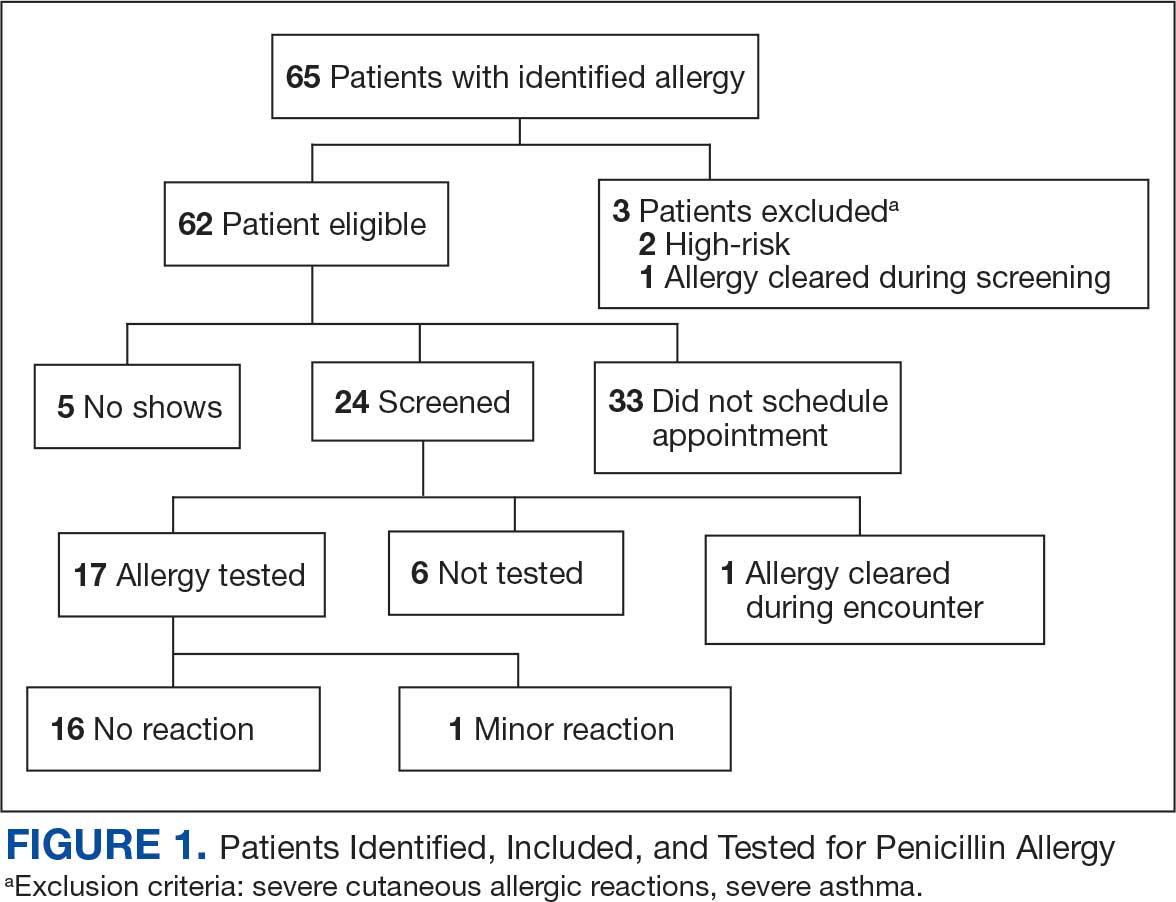
Of the 29 scheduled patients, 5 patients (17%) failed to present for care. Of the potential appointments set aside for the program, only 42% were used. One patient (4%) who was seen in clinic was delabeled based on history alone as they had previously successfully tolerated a course of amoxicillin. Four patients (17%) declined further testing with a PEN-FAST score > 2 due to a clear history of acute immunoglobulin (Ig) E-mediated reaction to a penicillin product within the past year. One patient (4%) was unable to be tested due to ongoing OAH use and 1 patient (4%) declined further penicillin testing after the discussion about risks, benefits, and alternatives to the procedures offered.
Of the 24 patients who arrived for a clinic appointment, 17 (71%) underwent penicillin allergy delabeling testing: 14 (82%) underwent direct challenge, and 3 (18%) underwent the skin testing before oral amoxicillin challenge procedure. Of the 17 who were tested, 16 (94%) tolerated a total dose of 500 mg of oral amoxicillin within the 1-hour observation period. One tested patient (6%) in the direct oral challenge group experienced an adverse reaction that was described as dull headache and hand tremor after the 50-mg dose; although it self-resolved within 15 minutes, this prompted the patient to discontinue the challenge. This adverse reaction was determined to be very unlikely IgE-mediated. None of the 3 patients who underwent the skin testing before oral challenge protocol experienced an adverse drug reaction (ADR). None of the 17 patients who received any oral amoxicillin required follow-up or reported a delayed cutaneous ADR to the challenge. No OAHs or epinephrine were used for any of the challenges.
Data collected from patient questionnaires displayed perceived health impacts of a penicillin allergy on the patient population. Patients reported a variety of ADRs to previous administration of penicillin products: 17 (71%) reported urticaria, 2 (8%) reported anaphylaxis, and 3 (13%) were unable to recall the reaction (Figure 2). Nine patients (38%) felt their initial reaction was distressing. Fifteen patients (88%) felt relief following negative testing (Table).
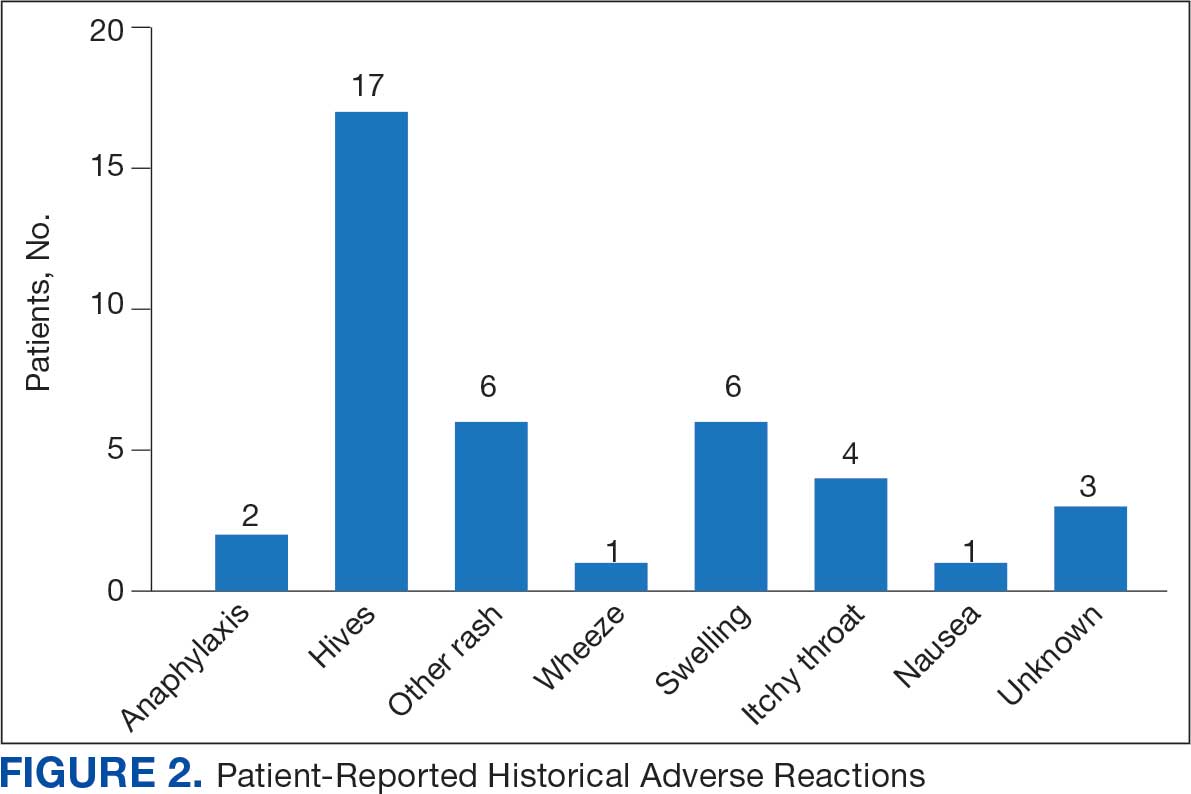
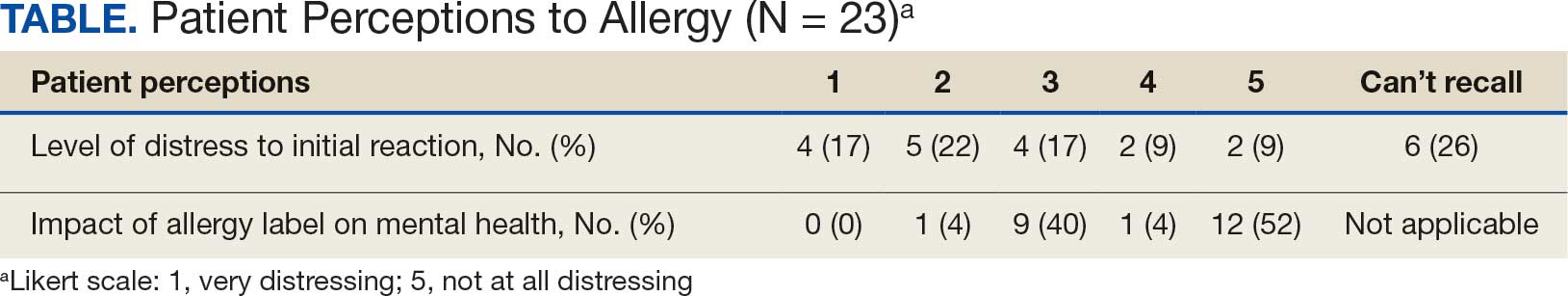
Discussion
To our knowledge, this was the first documented proactive penicillin delabeling QI project in a military clinic treating both active-duty service members and their dependents, modeled on the 2022 drug allergy guidelines.14 Several interesting lessons were learned that may improve future similar QI projects. Only 46% of patients identified as having penicillin allergy presented for evaluation, leaving 42% of available appointments unused. Without prior data on anticipated participation rates, these data provide a crude benchmark for utilization rates, which can inform future resource planning. While attempts were made to contact each patient, additional efforts to publicize the penicillin allergy delabeling campaign would have been useful to improve efficiency.
In addition, when patients with a PEN-FAST score of < 3 were educated about the risks and benefits of each procedure and offered the direct oral graded challenge and skin testing prior to oral challenge, 82% preferred the direct challenge. None of the patients who experienced a penicillin ADR in the past year wished to undergo skin testing or oral challenge, though each was educated on penicillin allergy and the possibility of testing in the future, making each encounter beneficial. Of the 17 patients tested, 16 (94%) tolerated oral amoxicillin and 1 (6%) experienced a mild, self-resolving ADR that was very unlikely of an IgE-mediated origin. Additionally, while plans and preparations for ADRs to the challenges were available, none were required. Patient questionnaires demonstrated the heterogeneity of previous ADRs and their attitude toward their allergy diagnosis. The positive impact of delabeling on patient well-being noted by 88% of patients reinforced the benefit of the effort.
This project was limited by a relatively small sample size, which may not have been large enough to detect ADRs, especially IgE-mediated allergic reactions. Herein lies the importance of having clinicians equipped to treat allergic ADRs to conduct penicillin challenges in the primary care setting. It is prudent to ensure not only proper training of physicians performing these challenges, but also appropriate equipment, medication, and response personnel. Medications that are useful include epinephrine, OAHs, albuterol, steroids, and intravenous fluids.
Having a response area and plan are essential to ensure appropriate care in the rare instance of allergic ADRs progressing to anaphylaxis. In rare cases, emergency medical services may be required and having a plan with appropriate response and transport time is essential to patient safety. This may not be practical in more rural or smaller practices. In those scenarios, it may be helpful to partner with a larger practice to send patients for delabeling or to use clinical space in closer proximity to emergency services. Perhaps an ideal setting might be urgent or emergent care centers due to high acuity resources and frequent prescription of amoxicillin antibiotics; however, this may be complicated by concurrent infections raising the incidence of delayed benign eruptions with amoxicillin ingestion and complicating the patient’s allergy records. Further training of urgent and emergent care practitioners would be helpful for proper patient education regarding antibiotic-associated reactions.
Full testing integration into other primary care clinics may be limited due to the specialized training required for complete skin testing. Nevertheless, as shown in this project, most patients may be delabeled based on a PEN-FAST evaluation followed by oral challenge alone. Incorporation in other QI projects could involve continuing medical education to train staff physicians on PEN-FAST, teaching primary care residents during training, and site visits by allergists to train local physicians on testing. This project involved training 2 PCPs to conduct skin and oral challenge testing using PEN-FAST to guide clinical decision-making with an allergist available for consultation if needed. Future projects might model a similar approach or perhaps focus on training more physicians on oral challenges alone to reach a high percentage of the target population.
Conclusions
This project demonstrates a safe, efficient, and cost-effective model for penicillin allergy delabeling in clinics without regular access to allergy services. The use of PEN-FAST allows a quick and simple method to screen patients with penicillin allergy to meet the goals of the 2022 CBSs, but data are still accumulating to validate this method of screening across populations. This project demonstrates additional support for the use of PEN-FAST, while illustrating appropriate education regarding oral testing technique and its limitations.
Using an EHR report limited the patients in the testing pool and subsequent sample size. This suggests that a primary care identification-driven enrollment in testing may offer even more benefit both in allergy detection and education of testing benefits. Oral challenges are more cost effective, shorter in duration, and have fewer training requirements when compared with antecedent skin testing, making them an ideal option for PCPs in a clinic setting. Trained PCPs may opt to offer periodic appointments for delabeling, or offer days dedicated to delabeling as many patients as possible. Penicillin delabeling is an urgent and expansive charge; this study offers a replicable model for executing this important task.
- Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7787. doi:10.1016/j.amjmed.2009.01.034
- Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68(3):171-180. doi:10.1016/0091-6749(81)90180-9
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13(2):12-18. doi:10.7812/TPP/08-073
- Fox SJ, Park MA. Penicillin skin testing is a safe and effective tool for evaluating penicillin allergy in the pediatric population. J Allergy Clin Immunol Pract. 2014;2(4):439-444. doi:10.1016/j.jaip.2014.04.013
- Solensky R, Jacobs J, Lester M, et al. Penicillin Allergy Evaluation: A Prospective, Multicenter, Open-Label Evaluation of a Comprehensive Penicillin Skin Test Kit. J Allergy Clin Immunol Pract. 2019;7(6):1876-1885.e3. doi:10.1016/j.jaip.2019.02.040 7.
- Gonzalez-Estrada A, Park MA, Accarino JJO, et al. Predicting penicillin allergy: A United States multicenter retrospective study. J Allergy Clin Immunol Pract. 2024;12(5):1181-1191.e10. doi:10.1016/j.jaip.2024.01.010
- Stüwe HT, Geissler W, Paap A, Cromwell O. The presence of latex can induce false-positive skin tests in subjects tested with penicillin determinants. Allergy. 1997;52(12):1243. doi:10.1111/j.1398-9995.1997.tb00975.x
- Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011;31(8):742-747. doi:10.1592/phco.31.8.742
- Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med. 2019;34(9):1685-1687. doi:10.1007/s11606-019-04991-y
- Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
- Mattingly TJ II, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6(5):1649-1654.e4. doi:10.1016/j.jaip.2017.12.033
- Diplomate Statistics. American Board of Allergy and Immunology website. Published February, 18 2021. Accessed July 28, 2025. https://www.abai.org/statistics_diplomates.asp
- Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333-1393. doi:10.1016/j.jaci.2022.08.028
- Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170:e160033. doi:10.1001/jamapediatrics.2016.0033
- Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188–99. doi:10.1001/jama.2018.19283
- Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6(3):1019-1027.e2. doi:10.1016/j.jaip.2017.08.006
- Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
- Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745-752. doi:10.1001/jamainternmed.2020.0403
- Copaescu AM, Vogrin S, James F, et al. Efficacy of a clinical decision rule to enable direct oral challenge in patients with low-risk penicillin allergy: the PALACE randomized clinical trial. JAMA Intern Med. 2023;183(9):944-952. doi:10.1001/jamainternmed.2023.2986
- Copaescu AM, Vogrin S, Shand G, et al. Validation of the PEN-FAST score in a pediatric population. JAMA Netw Open. 2022;5(9):e2233703. doi:10.1001/jamanetworkopen.2022.33703
- Piotin A, Godet J, Trubiano JA, et al. Predictive factors of amoxicillin immediate hypersensitivity and validation of PEN-FAST clinical decision rule. Ann Allergy Asthma Immunol. 2022;128(1):27-32. doi:10.1016/j.anai.2021.07.005
- Su C, Belmont A, Liao J, et al. Evaluating the PEN-FAST clinical decision-making tool to enhance penicillin allergy delabeling. JAMA Intern Med. 2023;183(8):883-885. doi:10.1001/jamainternmed.2023.1572
Penicillin allergy is common in the United States. About 9.0% to 13.8% of patients have a diagnosed penicillin allergy documented in their electronic health record. The annual incidence rates is 1.1% in males and 1.4% in females.1,2
Penicillin hypersensitivity likely wanes over time. A 1981 study found that 93% of patients who experienced an allergic reaction to penicillin had a positive skin test 7 to 12 months postreaction, but only 22% still had a positive test after 10 years.3 Confirmed type 1 hypersensitivity penicillin allergies, as demonstrated by positive skin prick testing, also are decreasing over time.4 Furthermore, many patients’ reactions may have been misdiagnosed as a penicillin allergy. Upon actual confirmatory testing of penicillin allergy, only 8.5% to 13.8% of patients believed to have a penicillin allergy were positive on skin prick testing of penicillin products.5,6 A 2024 US study found that 11% of individuals with a history of a penicillin reaction tested positive on skin testing.7
The positive predictive value of penicillin allergy skin testing is poorly defined due to the ethical dilemma of orally challenging a patient who demonstrates skin test reactivity. Due to its high negative predictive value (NPV), skin prick combined with intradermal testing has been the gold-standard test in cases of clinical concern.6 Patients with positive skin testing are assumed to be truly positive, and therefore penicillin allergic, even though false-positive results to penicillin skin testing are known to occur.8
Misdiagnosis of penicillin allergy carries substantial clinical and economic consequences. A 2011 study suggested a statistically significant 1.8% increased absolute risk of mortality and 5.5% increased absolute risk of intensive care unit admission for those labeled with penicillin allergy and admitted for an infection.9 Another study found a 14% increase in mortality associated with the diagnosis of penicillin allergy.10 In a 2014 case-control study, penicillin allergy also was associated with a 23.4% greater risk of Clostridioides difficile, 14.1% more methicillin-resistant Staphylococcus aureus, and 30.1% more vancomycin-resistant enterococci infections.11Direct cost savings during an inpatient admission for infection were as much as $609 per patient with additional indirect cost savings of up to $4254 per admission.12 When viewed from the perspective of a health care system, these costs quickly accumulate, negatively impacting the fiscal stability of our patients and placing additional financial strain on an over-burdened system.
If 10% of US patients have penicillin allergy labels, then about 33 million patients might be eligible for delabeling. There are only 6309 board-certified allergists actively practicing in the US, which could amount to about 5231 potential penicillin challenges per allergist, not even including the 3.3 million new patients per year (assuming a 1% incidence).13 Clarifying each patient’s tolerance of penicillin products will clearly require nonallergist cooperation.
The 2022 drug allergy practice parameter update recommends several consensus-based statements (CBSs) to directly address penicillin allergy.14 This guideline recommends proactive efforts to delabel patients with a reported penicillin allergy (CBS 4); advise against testing in cases where the history is inconsistent with a true allergic reaction, though a challenge may be offered (CBS 5); skin testing for those with a history of anaphylaxis or a recent reaction (CBS 6); advise against multiple-day penicillin challenges (CBS 7); advise against skin testing for pediatric patients with benign cutaneous reactions (CBS 8); and recommends direct oral challenge for adults with distant or benign cutaneous reactions (CBS 9). These recommendations create a potentially high demand for delabeling with allergy specialists. One potential solution is to perform direct oral challenges in primary care, emergency departments, and urgent care clinics.
Evidence supporting the safety of direct oral penicillin challenges in low-risk patients was initially noted in the allergy community, but now evidence for their use in primary care clinics is growing—including in children.15 In a military-specific population, an amoxicillin challenge of Marine recruits with suspected penicillin allergy revealed that only 1.5% of those challenged acutely reacted and should be considered allergic to penicillin.16 Historically, in order to refute the diagnosis of penicillin allergy, an allergist would order penicillin skin prick testing. If the test was negative, an allergist would proceed to intradermal testing and if negative again (NPV of 97.9%), proceed to a graded oral challenge.6 However, this process is not fully reproducible in most clinics because the minor determinants mixture used in skin testing is not commercially available.17 Additionally, the full skin testing procedure requires specialized training, is more time-consuming, causes more discomfort, lacks US Food and Drug Administration approval for children, and has a higher cost ($220 per test for each patient as of 2016).18 As such, the movement toward direct oral challenges is progressing. Nonetheless, the best method for primary care or emergency department clinicians to determine who the appropriate patients are for this procedure has not been fully established. Risk tools have been created in the past to help delineate low-risk patients who would be appropriate for direct oral amoxicillin challenges, but these were not widely replicated or validated.19 The PEN-FAST standardized risk score was first published in 2020 and has since been validated in different groups with additional safety data. This scoring system ranges from 0 to 5 points, assigning 2 points for a penicillin reaction within the past five (F) years, 2 points for angioedema/anaphylaxis (A) or a severe (S) cutaneous reaction, and 1 point if treatment (T) was required for the reaction. A score < 3 is considered low-risk and safe for direct oral challenge, although most of the safety data are in patients with a score of 0 or 1.20 The PEN-FAST guided direct oral challenge with an NPV of 96.3% has now been prospectively shown to be noninferior to standard skin prick test/intradermal test/graded challenge for low-risk patients with a PEN-FAST score < 3.21 The PEN-FAST validating study was conducted predominantly with an Australian population of adult White women, but now it also has been validated in children aged > 12 years, as well as in European and North American cohorts.22-24
Air Force Delabeling Program
This article describes a method for proactively, safely, and efficiently delabeling penicillin allergic patients at an Air Force clinic. This quality improvement (QI) report provides a successful model for penicillin allergy delabeling, illustrates lessons learned, and suggests next steps toward improving patient options for an invaluable antibiotic class.
The first step was to proactively delabel penicillin allergy from a population of active duty service members and their dependents. Electronic health record (EHR) allergy search functions are a helpful tool in finding patients with allergy labels. The Kadena Medical Clinic, in Okinawa, Japan, uses the Military Health System GENESIS EHR, which includes a discern reporting portal with a patient allergy search that creates a patient-specific medication allergy report. To compile the most complete database of patients with a penicillin allergy, all 15 potential allergy search options for “penicillin” were selected, as were 4 relevant options for amoxicillin (including options with clavulanate). Including so many options for specific penicillin medication allergies helps add specificity to the diagnosis in the EHR but can make aggregation of data more difficult. The
The complete compiled list was manually reviewed for high-risk patients with severe cutaneous adverse reactions (SCARs) of any age. Patients with pregnancy, unsuitable medical histories (ie, severe asthma), or taking β-blockers were excluded. Patients remaining on the list were contacted by telephone and offered appointments during a single week that was dedicated to penicillin allergy delabeling. Allergists in the Air Force are assigned to a region where they offer allergy services at clinics without a regular allergist. The allergist for the region traveled to the QI site for a 1-week campaign at an estimated cost of $4600. When the patients were contacted, they were briefly informed of the goal of the penicillin delabeling campaign, and if interested, they were scheduled for 1 of 50 available appointments that week. Patients were contacted with enough lead time to stop oral antihistamines (OAH) for ≥ 7 days before the appointment.
Patients were given an intake questionnaire and interviewed about their penicillin allergy history. This questionnaire inquired about the nature of the allergy, mental and physical health impacts of the allergy label, PEN-FAST scoring questions, and posttest attitude toward delabeling, if applicable. Patients with a PEN-FAST score < 3 were offered direct, graded oral challenge or the standard skin prick, followed by intradermal, followed by graded oral challenge protocol. Patients with PEN-FAST scores of ≥ 3 were offered skin testing prior to oral challenge protocol. Patients could decline further testing. If patients wished to proceed, they were asked to complete a written informed consent document.
Oral challenges followed a 10%/90% protocol, beginning with 50 mg of liquid amoxicillin followed by 450 mg after 15 minutes, as long as the patient remained asymptomatic. Challenge forms are available in the eAppendix . After receiving the 450-mg amoxicillin dose, the patient remained in the clinic for 60 minutes before a final clinical evaluation. If the patient remained asymptomatic after this period, the penicillin or amoxicillin allergy was marked as resolved in the EHR. The patients were given contact information for the clinic for follow-up if a delayed reaction was noted and they wished the medication allergy to be re-entered. An EHR encounter note was created for each patient detailing the allergy testing and delabeling.

This campaign was conducted at a basic life support-only facility by a single clinician without medical technician support. An allergic reaction medication kit was available and contained OAHs, intramuscular antihistamines, intramuscular epinephrine, intramuscular corticosteroids, and short-acting β-agonists for nebulization. The facility also had an urgent care room (staffed by primary care practitioners [PCPs]) that could help establish intravenous access and administer fluids if necessary and had previously established plans for emergency patient transport to a higher level of care, if necessary.
Program Outcomes
A list of 65 patients that included both active-duty service members and dependents with penicillin or amoxicillin allergy was created. This list was reviewed by an allergist to identify high-risk individuals, which required about 90 minutes. Two patients (3%) were excluded; 1 had a history of SCAR to penicillin and 1 had a complex medical history requiring continued OAH use. Sixty-three patients were contacted via telephone, and 29 patients (46%) scheduled an appointment. One patient (2%) was identified as penicillin-tolerant during the booking process, and the penicillin allergy was removed without testing (Figure 1).

Of the 29 scheduled patients, 5 patients (17%) failed to present for care. Of the potential appointments set aside for the program, only 42% were used. One patient (4%) who was seen in clinic was delabeled based on history alone as they had previously successfully tolerated a course of amoxicillin. Four patients (17%) declined further testing with a PEN-FAST score > 2 due to a clear history of acute immunoglobulin (Ig) E-mediated reaction to a penicillin product within the past year. One patient (4%) was unable to be tested due to ongoing OAH use and 1 patient (4%) declined further penicillin testing after the discussion about risks, benefits, and alternatives to the procedures offered.
Of the 24 patients who arrived for a clinic appointment, 17 (71%) underwent penicillin allergy delabeling testing: 14 (82%) underwent direct challenge, and 3 (18%) underwent the skin testing before oral amoxicillin challenge procedure. Of the 17 who were tested, 16 (94%) tolerated a total dose of 500 mg of oral amoxicillin within the 1-hour observation period. One tested patient (6%) in the direct oral challenge group experienced an adverse reaction that was described as dull headache and hand tremor after the 50-mg dose; although it self-resolved within 15 minutes, this prompted the patient to discontinue the challenge. This adverse reaction was determined to be very unlikely IgE-mediated. None of the 3 patients who underwent the skin testing before oral challenge protocol experienced an adverse drug reaction (ADR). None of the 17 patients who received any oral amoxicillin required follow-up or reported a delayed cutaneous ADR to the challenge. No OAHs or epinephrine were used for any of the challenges.
Data collected from patient questionnaires displayed perceived health impacts of a penicillin allergy on the patient population. Patients reported a variety of ADRs to previous administration of penicillin products: 17 (71%) reported urticaria, 2 (8%) reported anaphylaxis, and 3 (13%) were unable to recall the reaction (Figure 2). Nine patients (38%) felt their initial reaction was distressing. Fifteen patients (88%) felt relief following negative testing (Table).


Discussion
To our knowledge, this was the first documented proactive penicillin delabeling QI project in a military clinic treating both active-duty service members and their dependents, modeled on the 2022 drug allergy guidelines.14 Several interesting lessons were learned that may improve future similar QI projects. Only 46% of patients identified as having penicillin allergy presented for evaluation, leaving 42% of available appointments unused. Without prior data on anticipated participation rates, these data provide a crude benchmark for utilization rates, which can inform future resource planning. While attempts were made to contact each patient, additional efforts to publicize the penicillin allergy delabeling campaign would have been useful to improve efficiency.
In addition, when patients with a PEN-FAST score of < 3 were educated about the risks and benefits of each procedure and offered the direct oral graded challenge and skin testing prior to oral challenge, 82% preferred the direct challenge. None of the patients who experienced a penicillin ADR in the past year wished to undergo skin testing or oral challenge, though each was educated on penicillin allergy and the possibility of testing in the future, making each encounter beneficial. Of the 17 patients tested, 16 (94%) tolerated oral amoxicillin and 1 (6%) experienced a mild, self-resolving ADR that was very unlikely of an IgE-mediated origin. Additionally, while plans and preparations for ADRs to the challenges were available, none were required. Patient questionnaires demonstrated the heterogeneity of previous ADRs and their attitude toward their allergy diagnosis. The positive impact of delabeling on patient well-being noted by 88% of patients reinforced the benefit of the effort.
This project was limited by a relatively small sample size, which may not have been large enough to detect ADRs, especially IgE-mediated allergic reactions. Herein lies the importance of having clinicians equipped to treat allergic ADRs to conduct penicillin challenges in the primary care setting. It is prudent to ensure not only proper training of physicians performing these challenges, but also appropriate equipment, medication, and response personnel. Medications that are useful include epinephrine, OAHs, albuterol, steroids, and intravenous fluids.
Having a response area and plan are essential to ensure appropriate care in the rare instance of allergic ADRs progressing to anaphylaxis. In rare cases, emergency medical services may be required and having a plan with appropriate response and transport time is essential to patient safety. This may not be practical in more rural or smaller practices. In those scenarios, it may be helpful to partner with a larger practice to send patients for delabeling or to use clinical space in closer proximity to emergency services. Perhaps an ideal setting might be urgent or emergent care centers due to high acuity resources and frequent prescription of amoxicillin antibiotics; however, this may be complicated by concurrent infections raising the incidence of delayed benign eruptions with amoxicillin ingestion and complicating the patient’s allergy records. Further training of urgent and emergent care practitioners would be helpful for proper patient education regarding antibiotic-associated reactions.
Full testing integration into other primary care clinics may be limited due to the specialized training required for complete skin testing. Nevertheless, as shown in this project, most patients may be delabeled based on a PEN-FAST evaluation followed by oral challenge alone. Incorporation in other QI projects could involve continuing medical education to train staff physicians on PEN-FAST, teaching primary care residents during training, and site visits by allergists to train local physicians on testing. This project involved training 2 PCPs to conduct skin and oral challenge testing using PEN-FAST to guide clinical decision-making with an allergist available for consultation if needed. Future projects might model a similar approach or perhaps focus on training more physicians on oral challenges alone to reach a high percentage of the target population.
Conclusions
This project demonstrates a safe, efficient, and cost-effective model for penicillin allergy delabeling in clinics without regular access to allergy services. The use of PEN-FAST allows a quick and simple method to screen patients with penicillin allergy to meet the goals of the 2022 CBSs, but data are still accumulating to validate this method of screening across populations. This project demonstrates additional support for the use of PEN-FAST, while illustrating appropriate education regarding oral testing technique and its limitations.
Using an EHR report limited the patients in the testing pool and subsequent sample size. This suggests that a primary care identification-driven enrollment in testing may offer even more benefit both in allergy detection and education of testing benefits. Oral challenges are more cost effective, shorter in duration, and have fewer training requirements when compared with antecedent skin testing, making them an ideal option for PCPs in a clinic setting. Trained PCPs may opt to offer periodic appointments for delabeling, or offer days dedicated to delabeling as many patients as possible. Penicillin delabeling is an urgent and expansive charge; this study offers a replicable model for executing this important task.
Penicillin allergy is common in the United States. About 9.0% to 13.8% of patients have a diagnosed penicillin allergy documented in their electronic health record. The annual incidence rates is 1.1% in males and 1.4% in females.1,2
Penicillin hypersensitivity likely wanes over time. A 1981 study found that 93% of patients who experienced an allergic reaction to penicillin had a positive skin test 7 to 12 months postreaction, but only 22% still had a positive test after 10 years.3 Confirmed type 1 hypersensitivity penicillin allergies, as demonstrated by positive skin prick testing, also are decreasing over time.4 Furthermore, many patients’ reactions may have been misdiagnosed as a penicillin allergy. Upon actual confirmatory testing of penicillin allergy, only 8.5% to 13.8% of patients believed to have a penicillin allergy were positive on skin prick testing of penicillin products.5,6 A 2024 US study found that 11% of individuals with a history of a penicillin reaction tested positive on skin testing.7
The positive predictive value of penicillin allergy skin testing is poorly defined due to the ethical dilemma of orally challenging a patient who demonstrates skin test reactivity. Due to its high negative predictive value (NPV), skin prick combined with intradermal testing has been the gold-standard test in cases of clinical concern.6 Patients with positive skin testing are assumed to be truly positive, and therefore penicillin allergic, even though false-positive results to penicillin skin testing are known to occur.8
Misdiagnosis of penicillin allergy carries substantial clinical and economic consequences. A 2011 study suggested a statistically significant 1.8% increased absolute risk of mortality and 5.5% increased absolute risk of intensive care unit admission for those labeled with penicillin allergy and admitted for an infection.9 Another study found a 14% increase in mortality associated with the diagnosis of penicillin allergy.10 In a 2014 case-control study, penicillin allergy also was associated with a 23.4% greater risk of Clostridioides difficile, 14.1% more methicillin-resistant Staphylococcus aureus, and 30.1% more vancomycin-resistant enterococci infections.11Direct cost savings during an inpatient admission for infection were as much as $609 per patient with additional indirect cost savings of up to $4254 per admission.12 When viewed from the perspective of a health care system, these costs quickly accumulate, negatively impacting the fiscal stability of our patients and placing additional financial strain on an over-burdened system.
If 10% of US patients have penicillin allergy labels, then about 33 million patients might be eligible for delabeling. There are only 6309 board-certified allergists actively practicing in the US, which could amount to about 5231 potential penicillin challenges per allergist, not even including the 3.3 million new patients per year (assuming a 1% incidence).13 Clarifying each patient’s tolerance of penicillin products will clearly require nonallergist cooperation.
The 2022 drug allergy practice parameter update recommends several consensus-based statements (CBSs) to directly address penicillin allergy.14 This guideline recommends proactive efforts to delabel patients with a reported penicillin allergy (CBS 4); advise against testing in cases where the history is inconsistent with a true allergic reaction, though a challenge may be offered (CBS 5); skin testing for those with a history of anaphylaxis or a recent reaction (CBS 6); advise against multiple-day penicillin challenges (CBS 7); advise against skin testing for pediatric patients with benign cutaneous reactions (CBS 8); and recommends direct oral challenge for adults with distant or benign cutaneous reactions (CBS 9). These recommendations create a potentially high demand for delabeling with allergy specialists. One potential solution is to perform direct oral challenges in primary care, emergency departments, and urgent care clinics.
Evidence supporting the safety of direct oral penicillin challenges in low-risk patients was initially noted in the allergy community, but now evidence for their use in primary care clinics is growing—including in children.15 In a military-specific population, an amoxicillin challenge of Marine recruits with suspected penicillin allergy revealed that only 1.5% of those challenged acutely reacted and should be considered allergic to penicillin.16 Historically, in order to refute the diagnosis of penicillin allergy, an allergist would order penicillin skin prick testing. If the test was negative, an allergist would proceed to intradermal testing and if negative again (NPV of 97.9%), proceed to a graded oral challenge.6 However, this process is not fully reproducible in most clinics because the minor determinants mixture used in skin testing is not commercially available.17 Additionally, the full skin testing procedure requires specialized training, is more time-consuming, causes more discomfort, lacks US Food and Drug Administration approval for children, and has a higher cost ($220 per test for each patient as of 2016).18 As such, the movement toward direct oral challenges is progressing. Nonetheless, the best method for primary care or emergency department clinicians to determine who the appropriate patients are for this procedure has not been fully established. Risk tools have been created in the past to help delineate low-risk patients who would be appropriate for direct oral amoxicillin challenges, but these were not widely replicated or validated.19 The PEN-FAST standardized risk score was first published in 2020 and has since been validated in different groups with additional safety data. This scoring system ranges from 0 to 5 points, assigning 2 points for a penicillin reaction within the past five (F) years, 2 points for angioedema/anaphylaxis (A) or a severe (S) cutaneous reaction, and 1 point if treatment (T) was required for the reaction. A score < 3 is considered low-risk and safe for direct oral challenge, although most of the safety data are in patients with a score of 0 or 1.20 The PEN-FAST guided direct oral challenge with an NPV of 96.3% has now been prospectively shown to be noninferior to standard skin prick test/intradermal test/graded challenge for low-risk patients with a PEN-FAST score < 3.21 The PEN-FAST validating study was conducted predominantly with an Australian population of adult White women, but now it also has been validated in children aged > 12 years, as well as in European and North American cohorts.22-24
Air Force Delabeling Program
This article describes a method for proactively, safely, and efficiently delabeling penicillin allergic patients at an Air Force clinic. This quality improvement (QI) report provides a successful model for penicillin allergy delabeling, illustrates lessons learned, and suggests next steps toward improving patient options for an invaluable antibiotic class.
The first step was to proactively delabel penicillin allergy from a population of active duty service members and their dependents. Electronic health record (EHR) allergy search functions are a helpful tool in finding patients with allergy labels. The Kadena Medical Clinic, in Okinawa, Japan, uses the Military Health System GENESIS EHR, which includes a discern reporting portal with a patient allergy search that creates a patient-specific medication allergy report. To compile the most complete database of patients with a penicillin allergy, all 15 potential allergy search options for “penicillin” were selected, as were 4 relevant options for amoxicillin (including options with clavulanate). Including so many options for specific penicillin medication allergies helps add specificity to the diagnosis in the EHR but can make aggregation of data more difficult. The
The complete compiled list was manually reviewed for high-risk patients with severe cutaneous adverse reactions (SCARs) of any age. Patients with pregnancy, unsuitable medical histories (ie, severe asthma), or taking β-blockers were excluded. Patients remaining on the list were contacted by telephone and offered appointments during a single week that was dedicated to penicillin allergy delabeling. Allergists in the Air Force are assigned to a region where they offer allergy services at clinics without a regular allergist. The allergist for the region traveled to the QI site for a 1-week campaign at an estimated cost of $4600. When the patients were contacted, they were briefly informed of the goal of the penicillin delabeling campaign, and if interested, they were scheduled for 1 of 50 available appointments that week. Patients were contacted with enough lead time to stop oral antihistamines (OAH) for ≥ 7 days before the appointment.
Patients were given an intake questionnaire and interviewed about their penicillin allergy history. This questionnaire inquired about the nature of the allergy, mental and physical health impacts of the allergy label, PEN-FAST scoring questions, and posttest attitude toward delabeling, if applicable. Patients with a PEN-FAST score < 3 were offered direct, graded oral challenge or the standard skin prick, followed by intradermal, followed by graded oral challenge protocol. Patients with PEN-FAST scores of ≥ 3 were offered skin testing prior to oral challenge protocol. Patients could decline further testing. If patients wished to proceed, they were asked to complete a written informed consent document.
Oral challenges followed a 10%/90% protocol, beginning with 50 mg of liquid amoxicillin followed by 450 mg after 15 minutes, as long as the patient remained asymptomatic. Challenge forms are available in the eAppendix . After receiving the 450-mg amoxicillin dose, the patient remained in the clinic for 60 minutes before a final clinical evaluation. If the patient remained asymptomatic after this period, the penicillin or amoxicillin allergy was marked as resolved in the EHR. The patients were given contact information for the clinic for follow-up if a delayed reaction was noted and they wished the medication allergy to be re-entered. An EHR encounter note was created for each patient detailing the allergy testing and delabeling.

This campaign was conducted at a basic life support-only facility by a single clinician without medical technician support. An allergic reaction medication kit was available and contained OAHs, intramuscular antihistamines, intramuscular epinephrine, intramuscular corticosteroids, and short-acting β-agonists for nebulization. The facility also had an urgent care room (staffed by primary care practitioners [PCPs]) that could help establish intravenous access and administer fluids if necessary and had previously established plans for emergency patient transport to a higher level of care, if necessary.
Program Outcomes
A list of 65 patients that included both active-duty service members and dependents with penicillin or amoxicillin allergy was created. This list was reviewed by an allergist to identify high-risk individuals, which required about 90 minutes. Two patients (3%) were excluded; 1 had a history of SCAR to penicillin and 1 had a complex medical history requiring continued OAH use. Sixty-three patients were contacted via telephone, and 29 patients (46%) scheduled an appointment. One patient (2%) was identified as penicillin-tolerant during the booking process, and the penicillin allergy was removed without testing (Figure 1).

Of the 29 scheduled patients, 5 patients (17%) failed to present for care. Of the potential appointments set aside for the program, only 42% were used. One patient (4%) who was seen in clinic was delabeled based on history alone as they had previously successfully tolerated a course of amoxicillin. Four patients (17%) declined further testing with a PEN-FAST score > 2 due to a clear history of acute immunoglobulin (Ig) E-mediated reaction to a penicillin product within the past year. One patient (4%) was unable to be tested due to ongoing OAH use and 1 patient (4%) declined further penicillin testing after the discussion about risks, benefits, and alternatives to the procedures offered.
Of the 24 patients who arrived for a clinic appointment, 17 (71%) underwent penicillin allergy delabeling testing: 14 (82%) underwent direct challenge, and 3 (18%) underwent the skin testing before oral amoxicillin challenge procedure. Of the 17 who were tested, 16 (94%) tolerated a total dose of 500 mg of oral amoxicillin within the 1-hour observation period. One tested patient (6%) in the direct oral challenge group experienced an adverse reaction that was described as dull headache and hand tremor after the 50-mg dose; although it self-resolved within 15 minutes, this prompted the patient to discontinue the challenge. This adverse reaction was determined to be very unlikely IgE-mediated. None of the 3 patients who underwent the skin testing before oral challenge protocol experienced an adverse drug reaction (ADR). None of the 17 patients who received any oral amoxicillin required follow-up or reported a delayed cutaneous ADR to the challenge. No OAHs or epinephrine were used for any of the challenges.
Data collected from patient questionnaires displayed perceived health impacts of a penicillin allergy on the patient population. Patients reported a variety of ADRs to previous administration of penicillin products: 17 (71%) reported urticaria, 2 (8%) reported anaphylaxis, and 3 (13%) were unable to recall the reaction (Figure 2). Nine patients (38%) felt their initial reaction was distressing. Fifteen patients (88%) felt relief following negative testing (Table).


Discussion
To our knowledge, this was the first documented proactive penicillin delabeling QI project in a military clinic treating both active-duty service members and their dependents, modeled on the 2022 drug allergy guidelines.14 Several interesting lessons were learned that may improve future similar QI projects. Only 46% of patients identified as having penicillin allergy presented for evaluation, leaving 42% of available appointments unused. Without prior data on anticipated participation rates, these data provide a crude benchmark for utilization rates, which can inform future resource planning. While attempts were made to contact each patient, additional efforts to publicize the penicillin allergy delabeling campaign would have been useful to improve efficiency.
In addition, when patients with a PEN-FAST score of < 3 were educated about the risks and benefits of each procedure and offered the direct oral graded challenge and skin testing prior to oral challenge, 82% preferred the direct challenge. None of the patients who experienced a penicillin ADR in the past year wished to undergo skin testing or oral challenge, though each was educated on penicillin allergy and the possibility of testing in the future, making each encounter beneficial. Of the 17 patients tested, 16 (94%) tolerated oral amoxicillin and 1 (6%) experienced a mild, self-resolving ADR that was very unlikely of an IgE-mediated origin. Additionally, while plans and preparations for ADRs to the challenges were available, none were required. Patient questionnaires demonstrated the heterogeneity of previous ADRs and their attitude toward their allergy diagnosis. The positive impact of delabeling on patient well-being noted by 88% of patients reinforced the benefit of the effort.
This project was limited by a relatively small sample size, which may not have been large enough to detect ADRs, especially IgE-mediated allergic reactions. Herein lies the importance of having clinicians equipped to treat allergic ADRs to conduct penicillin challenges in the primary care setting. It is prudent to ensure not only proper training of physicians performing these challenges, but also appropriate equipment, medication, and response personnel. Medications that are useful include epinephrine, OAHs, albuterol, steroids, and intravenous fluids.
Having a response area and plan are essential to ensure appropriate care in the rare instance of allergic ADRs progressing to anaphylaxis. In rare cases, emergency medical services may be required and having a plan with appropriate response and transport time is essential to patient safety. This may not be practical in more rural or smaller practices. In those scenarios, it may be helpful to partner with a larger practice to send patients for delabeling or to use clinical space in closer proximity to emergency services. Perhaps an ideal setting might be urgent or emergent care centers due to high acuity resources and frequent prescription of amoxicillin antibiotics; however, this may be complicated by concurrent infections raising the incidence of delayed benign eruptions with amoxicillin ingestion and complicating the patient’s allergy records. Further training of urgent and emergent care practitioners would be helpful for proper patient education regarding antibiotic-associated reactions.
Full testing integration into other primary care clinics may be limited due to the specialized training required for complete skin testing. Nevertheless, as shown in this project, most patients may be delabeled based on a PEN-FAST evaluation followed by oral challenge alone. Incorporation in other QI projects could involve continuing medical education to train staff physicians on PEN-FAST, teaching primary care residents during training, and site visits by allergists to train local physicians on testing. This project involved training 2 PCPs to conduct skin and oral challenge testing using PEN-FAST to guide clinical decision-making with an allergist available for consultation if needed. Future projects might model a similar approach or perhaps focus on training more physicians on oral challenges alone to reach a high percentage of the target population.
Conclusions
This project demonstrates a safe, efficient, and cost-effective model for penicillin allergy delabeling in clinics without regular access to allergy services. The use of PEN-FAST allows a quick and simple method to screen patients with penicillin allergy to meet the goals of the 2022 CBSs, but data are still accumulating to validate this method of screening across populations. This project demonstrates additional support for the use of PEN-FAST, while illustrating appropriate education regarding oral testing technique and its limitations.
Using an EHR report limited the patients in the testing pool and subsequent sample size. This suggests that a primary care identification-driven enrollment in testing may offer even more benefit both in allergy detection and education of testing benefits. Oral challenges are more cost effective, shorter in duration, and have fewer training requirements when compared with antecedent skin testing, making them an ideal option for PCPs in a clinic setting. Trained PCPs may opt to offer periodic appointments for delabeling, or offer days dedicated to delabeling as many patients as possible. Penicillin delabeling is an urgent and expansive charge; this study offers a replicable model for executing this important task.
- Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7787. doi:10.1016/j.amjmed.2009.01.034
- Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68(3):171-180. doi:10.1016/0091-6749(81)90180-9
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13(2):12-18. doi:10.7812/TPP/08-073
- Fox SJ, Park MA. Penicillin skin testing is a safe and effective tool for evaluating penicillin allergy in the pediatric population. J Allergy Clin Immunol Pract. 2014;2(4):439-444. doi:10.1016/j.jaip.2014.04.013
- Solensky R, Jacobs J, Lester M, et al. Penicillin Allergy Evaluation: A Prospective, Multicenter, Open-Label Evaluation of a Comprehensive Penicillin Skin Test Kit. J Allergy Clin Immunol Pract. 2019;7(6):1876-1885.e3. doi:10.1016/j.jaip.2019.02.040 7.
- Gonzalez-Estrada A, Park MA, Accarino JJO, et al. Predicting penicillin allergy: A United States multicenter retrospective study. J Allergy Clin Immunol Pract. 2024;12(5):1181-1191.e10. doi:10.1016/j.jaip.2024.01.010
- Stüwe HT, Geissler W, Paap A, Cromwell O. The presence of latex can induce false-positive skin tests in subjects tested with penicillin determinants. Allergy. 1997;52(12):1243. doi:10.1111/j.1398-9995.1997.tb00975.x
- Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011;31(8):742-747. doi:10.1592/phco.31.8.742
- Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med. 2019;34(9):1685-1687. doi:10.1007/s11606-019-04991-y
- Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
- Mattingly TJ II, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6(5):1649-1654.e4. doi:10.1016/j.jaip.2017.12.033
- Diplomate Statistics. American Board of Allergy and Immunology website. Published February, 18 2021. Accessed July 28, 2025. https://www.abai.org/statistics_diplomates.asp
- Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333-1393. doi:10.1016/j.jaci.2022.08.028
- Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170:e160033. doi:10.1001/jamapediatrics.2016.0033
- Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188–99. doi:10.1001/jama.2018.19283
- Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6(3):1019-1027.e2. doi:10.1016/j.jaip.2017.08.006
- Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
- Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745-752. doi:10.1001/jamainternmed.2020.0403
- Copaescu AM, Vogrin S, James F, et al. Efficacy of a clinical decision rule to enable direct oral challenge in patients with low-risk penicillin allergy: the PALACE randomized clinical trial. JAMA Intern Med. 2023;183(9):944-952. doi:10.1001/jamainternmed.2023.2986
- Copaescu AM, Vogrin S, Shand G, et al. Validation of the PEN-FAST score in a pediatric population. JAMA Netw Open. 2022;5(9):e2233703. doi:10.1001/jamanetworkopen.2022.33703
- Piotin A, Godet J, Trubiano JA, et al. Predictive factors of amoxicillin immediate hypersensitivity and validation of PEN-FAST clinical decision rule. Ann Allergy Asthma Immunol. 2022;128(1):27-32. doi:10.1016/j.anai.2021.07.005
- Su C, Belmont A, Liao J, et al. Evaluating the PEN-FAST clinical decision-making tool to enhance penicillin allergy delabeling. JAMA Intern Med. 2023;183(8):883-885. doi:10.1001/jamainternmed.2023.1572
- Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7787. doi:10.1016/j.amjmed.2009.01.034
- Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68(3):171-180. doi:10.1016/0091-6749(81)90180-9
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13(2):12-18. doi:10.7812/TPP/08-073
- Fox SJ, Park MA. Penicillin skin testing is a safe and effective tool for evaluating penicillin allergy in the pediatric population. J Allergy Clin Immunol Pract. 2014;2(4):439-444. doi:10.1016/j.jaip.2014.04.013
- Solensky R, Jacobs J, Lester M, et al. Penicillin Allergy Evaluation: A Prospective, Multicenter, Open-Label Evaluation of a Comprehensive Penicillin Skin Test Kit. J Allergy Clin Immunol Pract. 2019;7(6):1876-1885.e3. doi:10.1016/j.jaip.2019.02.040 7.
- Gonzalez-Estrada A, Park MA, Accarino JJO, et al. Predicting penicillin allergy: A United States multicenter retrospective study. J Allergy Clin Immunol Pract. 2024;12(5):1181-1191.e10. doi:10.1016/j.jaip.2024.01.010
- Stüwe HT, Geissler W, Paap A, Cromwell O. The presence of latex can induce false-positive skin tests in subjects tested with penicillin determinants. Allergy. 1997;52(12):1243. doi:10.1111/j.1398-9995.1997.tb00975.x
- Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011;31(8):742-747. doi:10.1592/phco.31.8.742
- Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med. 2019;34(9):1685-1687. doi:10.1007/s11606-019-04991-y
- Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
- Mattingly TJ II, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6(5):1649-1654.e4. doi:10.1016/j.jaip.2017.12.033
- Diplomate Statistics. American Board of Allergy and Immunology website. Published February, 18 2021. Accessed July 28, 2025. https://www.abai.org/statistics_diplomates.asp
- Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333-1393. doi:10.1016/j.jaci.2022.08.028
- Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170:e160033. doi:10.1001/jamapediatrics.2016.0033
- Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188–99. doi:10.1001/jama.2018.19283
- Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6(3):1019-1027.e2. doi:10.1016/j.jaip.2017.08.006
- Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
- Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745-752. doi:10.1001/jamainternmed.2020.0403
- Copaescu AM, Vogrin S, James F, et al. Efficacy of a clinical decision rule to enable direct oral challenge in patients with low-risk penicillin allergy: the PALACE randomized clinical trial. JAMA Intern Med. 2023;183(9):944-952. doi:10.1001/jamainternmed.2023.2986
- Copaescu AM, Vogrin S, Shand G, et al. Validation of the PEN-FAST score in a pediatric population. JAMA Netw Open. 2022;5(9):e2233703. doi:10.1001/jamanetworkopen.2022.33703
- Piotin A, Godet J, Trubiano JA, et al. Predictive factors of amoxicillin immediate hypersensitivity and validation of PEN-FAST clinical decision rule. Ann Allergy Asthma Immunol. 2022;128(1):27-32. doi:10.1016/j.anai.2021.07.005
- Su C, Belmont A, Liao J, et al. Evaluating the PEN-FAST clinical decision-making tool to enhance penicillin allergy delabeling. JAMA Intern Med. 2023;183(8):883-885. doi:10.1001/jamainternmed.2023.1572
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project