User login
Improving Diagnostic Accuracy in Skin of Color Using an Educational Module
Dermatologic disparities disproportionately affect patients with skin of color (SOC). Two studies assessing the diagnostic accuracy of medical students have shown disparities in diagnosing common skin conditions presenting in darker skin compared to lighter skin at early stages of training.1,2 This knowledge gap could be attributed to the underrepresentation of SOC in dermatologic textbooks, journals, and educational curricula.3-6 It is important for dermatologists as well as physicians in other specialties and ancillary health care workers involved in treating or triaging dermatologic diseases to recognize common skin conditions presenting in SOC. We sought to evaluate the effectiveness of a focused educational module for improving diagnostic accuracy and confidence in treating SOC among interprofessional health care providers.
Methods
Interprofessional health care providers—medical students, residents/fellows, attending physicians, advanced practice providers (APPs), and nurses practicing across various medical specialties—at The University of Texas at Austin Dell Medical School and Ascension Medical Group (both in Austin, Texas) were invited to participate in an institutional review board–exempt study involving a virtual SOC educational module from February through May 2021. The 1-hour module involved a pretest, a 15-minute lecture, an immediate posttest, and a 3-month posttest. All tests included the same 40 multiple-choice questions of 20 dermatologic conditions portrayed in lighter and darker skin types from VisualDx.com, and participants were asked to identify the condition in each photograph. Questions appeared one at a time in a randomized order, and answers could not be changed once submitted.
For analysis, the dermatologic conditions were categorized into 4 groups: cancerous, infectious, inflammatory, and SOC-associated conditions. Cancerous conditions included basal cell carcinoma, squamous cell carcinoma, and melanoma. Infectious conditions included herpes zoster, tinea corporis, tinea versicolor, staphylococcal scalded skin syndrome, and verruca vulgaris. Inflammatory conditions included acne, atopic dermatitis, pityriasis rosea, psoriasis, seborrheic dermatitis, contact dermatitis, lichen planus, and urticaria. Skin of color–associated conditions included hidradenitis suppurativa, acanthosis nigricans, keloid, and melasma. Two questions utilizing a 5-point Likert scale assessing confidence in diagnosing light and dark skin also were included.
The pre-recorded 15-minute video lecture was given by 2 dermatology residents (P.L.K. and C.P.), and the learning objectives covered morphologic differences in lighter skin and darker skin, comparisons of common dermatologic diseases in lighter skin and darker skin, diseases more commonly affecting patients with SOC, and treatment considerations for conditions affecting skin and hair in patients with SOC. Photographs from the diagnostic accuracy assessment were not reused in the lecture. Detailed explanations on morphology, diagnostic pearls, and treatment options for all conditions tested were provided to participants upon completion of the 3-month posttest.
Statistical Analysis—Test scores were compared between conditions shown in lighter and darker skin types and from the pretest to the immediate posttest and 3-month posttest. Multiple linear regression was used to assess for intervention effects on lighter and darker skin scores controlling for provider type and specialty. All tests were 2-sided with significance at P<.05. Analyses were conducted using Stata 17.
Results
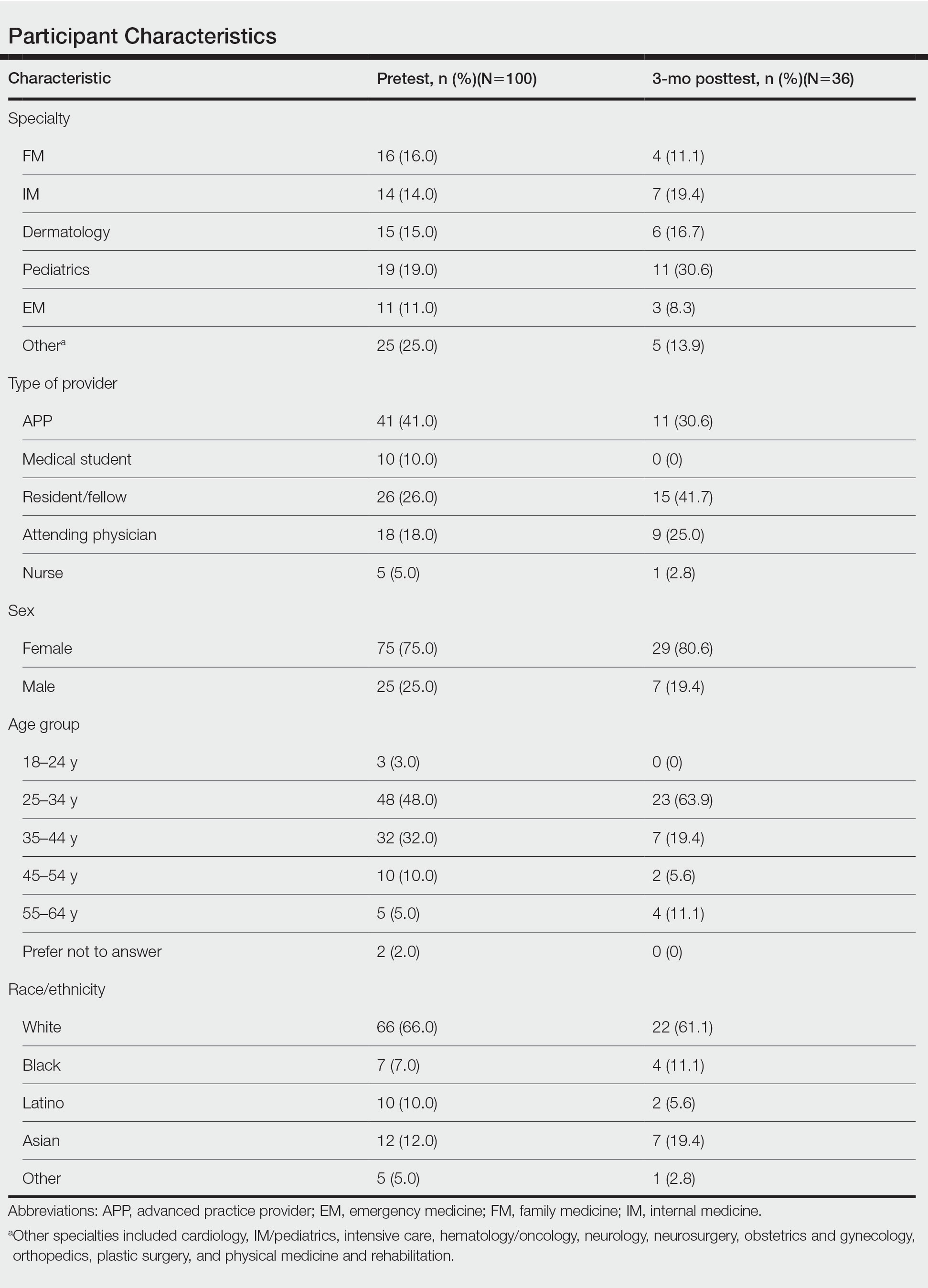
One hundred participants completed the pretest and immediate posttest, 36 of whom also completed the 3-month posttest (Table). There was no significant difference in baseline characteristics between the pretest and 3-month posttest groups.
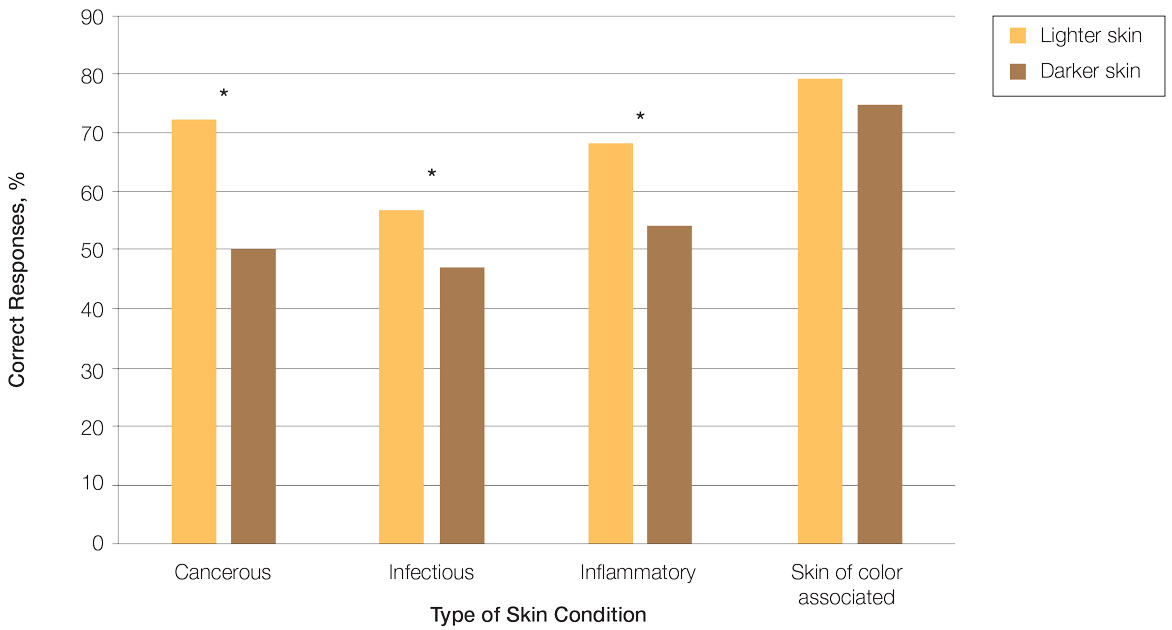
Test scores were correlated with provider type and specialty but not age, sex, or race/ethnicity. Specializing in dermatology and being a resident or attending physician were independently associated with higher test scores. Mean pretest diagnostic accuracy and confidence scores were higher for skin conditions shown in lighter skin compared with those shown in darker skin (13.6 vs 11.3 and 2.7 vs 1.9, respectively; both P<.001). Pretest diagnostic accuracy was significantly higher for skin conditions shown in lighter skin compared with darker skin for cancerous, inflammatory, and infectious conditions (72% vs 50%, 68% vs 55%, and 57% vs 47%, respectively; P<.001 for all)(Figure 1). Skin of color–associated conditions were not associated with significantly different scores for lighter skin compared with darker skin (79% vs 75%; P=.059).
Controlling for provider type and specialty, significantly improved diagnostic accuracy was seen in immediate posttest scores compared with pretest scores for conditions shown in both lighter and darker skin types (lighter: 15.2 vs 13.6; darker: 13.3 vs 11.3; both P<.001)(Figure 2). The immediate posttest demonstrated higher mean diagnostic accuracy and confidence scores for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 15.2 vs 13.3; confidence: 3.0 vs 2.6; both P<.001), but the disparity between scores was less than in the pretest.
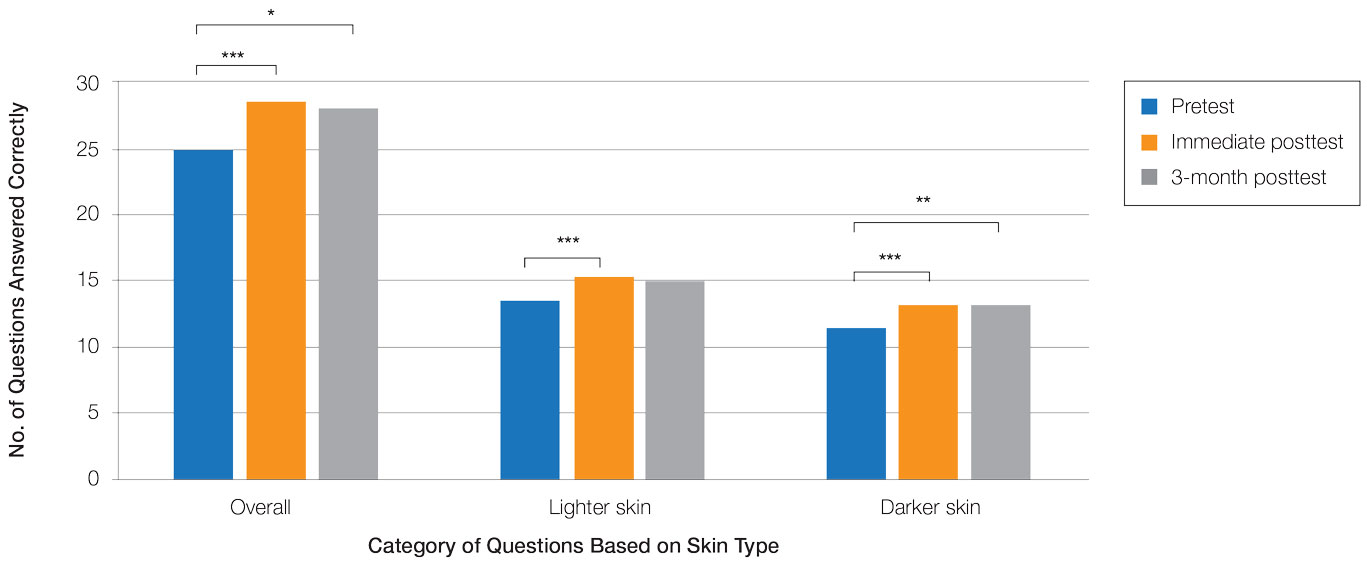
Following the 3-month posttest, improvement in diagnostic accuracy was noted among both lighter and darker skin types compared with the pretest, but the difference remained significant only for conditions shown in darker skin (mean scores, 11.3 vs 13.3; P<.01). Similarly, confidence in diagnosing conditions in both lighter and darker skin improved following the immediate posttest (mean scores, 2.7 vs 3.0 and 1.9 vs 2.6; both P<.001), and this improvement remained significant for only darker skin following the 3-month posttest (mean scores, 1.9 vs 2.3; P<.001). Despite these improvements, diagnostic accuracy and confidence remained higher for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 14.7 vs 13.3; P<.01; confidence: 2.8 vs 2.3; P<.001), though the disparity between scores was again less than in the pretest.
Comment
Our study showed that there are diagnostic disparities between lighter and darker skin types among interprofessional health care providers. Education on SOC should extend to interprofessional health care providers and other medical specialties involved in treating or triaging dermatologic diseases. A focused educational module may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in SOC. Differences in diagnostic accuracy between conditions shown in lighter and darker skin types were noted for the disease categories of infectious, cancerous, and inflammatory conditions, with the exception of conditions more frequently seen in patients with SOC. Learning resources for SOC-associated conditions are more likely to have greater representation of images depicting darker skin types.7 Future educational interventions may need to focus on dermatologic conditions that are not preferentially seen in patients with SOC. In our study, the pretest scores for conditions shown in darker skin were lowest among infectious and cancerous conditions. For infections, certain morphologic clues such as erythema are important for diagnosis but may be more subtle or difficult to discern in darker skin. It also is possible that providers may be less likely to suspect skin cancer in patients with SOC given that the morphologic presentation and/or anatomic site of involvement for skin cancers in SOC differs from those in lighter skin. Future educational interventions targeting disparities in diagnostic accuracy should focus on conditions that are not specifically associated with SOC.
Limitations of our study included the small number of participants, the study population came from a single institution, and a possible selection bias for providers interested in dermatology.
Conclusion
Disparities exist among interprofessional health care providers when treating conditions in patients with lighter skin compared to darker skin. An educational module for health care providers may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in patients with SOC.
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958. doi:10.1016/j.jaad.2019.12.078
- Mamo A, Szeto MD, Rietcheck H, et al. Evaluating medical student assessment of common dermatologic conditions across Fitzpatrick phototypes and skin of color. J Am Acad Dermatol. 2022;87:167-169. doi:10.1016/j.jaad.2021.06.868
- Guda VA, Paek SY. Skin of color representation in commonly utilized medical student dermatology resources. J Drugs Dermatol. 2021;20:799. doi:10.36849/JDD.5726
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of color and diversity and inclusion content of dermatologic published literature: an analysis and call to action. Int J Womens Dermatol. 2021;7:391-397. doi:10.1016/j.ijwd.2021.04.001
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Gupta R, Ibraheim MK, Dao H Jr, et al. Assessing dermatology resident confidence in caring for patients with skin of color. Clin Dermatol. 2021;39:873-878. doi:10.1016/j.clindermatol.2021.08.019
- Chang MJ, Lipner SR. Analysis of skin color on the American Academy of Dermatology public education website. J Drugs Dermatol. 2020;19:1236-1237. doi:10.36849/JDD.2020.5545
Dermatologic disparities disproportionately affect patients with skin of color (SOC). Two studies assessing the diagnostic accuracy of medical students have shown disparities in diagnosing common skin conditions presenting in darker skin compared to lighter skin at early stages of training.1,2 This knowledge gap could be attributed to the underrepresentation of SOC in dermatologic textbooks, journals, and educational curricula.3-6 It is important for dermatologists as well as physicians in other specialties and ancillary health care workers involved in treating or triaging dermatologic diseases to recognize common skin conditions presenting in SOC. We sought to evaluate the effectiveness of a focused educational module for improving diagnostic accuracy and confidence in treating SOC among interprofessional health care providers.
Methods
Interprofessional health care providers—medical students, residents/fellows, attending physicians, advanced practice providers (APPs), and nurses practicing across various medical specialties—at The University of Texas at Austin Dell Medical School and Ascension Medical Group (both in Austin, Texas) were invited to participate in an institutional review board–exempt study involving a virtual SOC educational module from February through May 2021. The 1-hour module involved a pretest, a 15-minute lecture, an immediate posttest, and a 3-month posttest. All tests included the same 40 multiple-choice questions of 20 dermatologic conditions portrayed in lighter and darker skin types from VisualDx.com, and participants were asked to identify the condition in each photograph. Questions appeared one at a time in a randomized order, and answers could not be changed once submitted.
For analysis, the dermatologic conditions were categorized into 4 groups: cancerous, infectious, inflammatory, and SOC-associated conditions. Cancerous conditions included basal cell carcinoma, squamous cell carcinoma, and melanoma. Infectious conditions included herpes zoster, tinea corporis, tinea versicolor, staphylococcal scalded skin syndrome, and verruca vulgaris. Inflammatory conditions included acne, atopic dermatitis, pityriasis rosea, psoriasis, seborrheic dermatitis, contact dermatitis, lichen planus, and urticaria. Skin of color–associated conditions included hidradenitis suppurativa, acanthosis nigricans, keloid, and melasma. Two questions utilizing a 5-point Likert scale assessing confidence in diagnosing light and dark skin also were included.
The pre-recorded 15-minute video lecture was given by 2 dermatology residents (P.L.K. and C.P.), and the learning objectives covered morphologic differences in lighter skin and darker skin, comparisons of common dermatologic diseases in lighter skin and darker skin, diseases more commonly affecting patients with SOC, and treatment considerations for conditions affecting skin and hair in patients with SOC. Photographs from the diagnostic accuracy assessment were not reused in the lecture. Detailed explanations on morphology, diagnostic pearls, and treatment options for all conditions tested were provided to participants upon completion of the 3-month posttest.
Statistical Analysis—Test scores were compared between conditions shown in lighter and darker skin types and from the pretest to the immediate posttest and 3-month posttest. Multiple linear regression was used to assess for intervention effects on lighter and darker skin scores controlling for provider type and specialty. All tests were 2-sided with significance at P<.05. Analyses were conducted using Stata 17.
Results
One hundred participants completed the pretest and immediate posttest, 36 of whom also completed the 3-month posttest (Table). There was no significant difference in baseline characteristics between the pretest and 3-month posttest groups.

Test scores were correlated with provider type and specialty but not age, sex, or race/ethnicity. Specializing in dermatology and being a resident or attending physician were independently associated with higher test scores. Mean pretest diagnostic accuracy and confidence scores were higher for skin conditions shown in lighter skin compared with those shown in darker skin (13.6 vs 11.3 and 2.7 vs 1.9, respectively; both P<.001). Pretest diagnostic accuracy was significantly higher for skin conditions shown in lighter skin compared with darker skin for cancerous, inflammatory, and infectious conditions (72% vs 50%, 68% vs 55%, and 57% vs 47%, respectively; P<.001 for all)(Figure 1). Skin of color–associated conditions were not associated with significantly different scores for lighter skin compared with darker skin (79% vs 75%; P=.059).

Controlling for provider type and specialty, significantly improved diagnostic accuracy was seen in immediate posttest scores compared with pretest scores for conditions shown in both lighter and darker skin types (lighter: 15.2 vs 13.6; darker: 13.3 vs 11.3; both P<.001)(Figure 2). The immediate posttest demonstrated higher mean diagnostic accuracy and confidence scores for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 15.2 vs 13.3; confidence: 3.0 vs 2.6; both P<.001), but the disparity between scores was less than in the pretest.

Following the 3-month posttest, improvement in diagnostic accuracy was noted among both lighter and darker skin types compared with the pretest, but the difference remained significant only for conditions shown in darker skin (mean scores, 11.3 vs 13.3; P<.01). Similarly, confidence in diagnosing conditions in both lighter and darker skin improved following the immediate posttest (mean scores, 2.7 vs 3.0 and 1.9 vs 2.6; both P<.001), and this improvement remained significant for only darker skin following the 3-month posttest (mean scores, 1.9 vs 2.3; P<.001). Despite these improvements, diagnostic accuracy and confidence remained higher for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 14.7 vs 13.3; P<.01; confidence: 2.8 vs 2.3; P<.001), though the disparity between scores was again less than in the pretest.
Comment
Our study showed that there are diagnostic disparities between lighter and darker skin types among interprofessional health care providers. Education on SOC should extend to interprofessional health care providers and other medical specialties involved in treating or triaging dermatologic diseases. A focused educational module may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in SOC. Differences in diagnostic accuracy between conditions shown in lighter and darker skin types were noted for the disease categories of infectious, cancerous, and inflammatory conditions, with the exception of conditions more frequently seen in patients with SOC. Learning resources for SOC-associated conditions are more likely to have greater representation of images depicting darker skin types.7 Future educational interventions may need to focus on dermatologic conditions that are not preferentially seen in patients with SOC. In our study, the pretest scores for conditions shown in darker skin were lowest among infectious and cancerous conditions. For infections, certain morphologic clues such as erythema are important for diagnosis but may be more subtle or difficult to discern in darker skin. It also is possible that providers may be less likely to suspect skin cancer in patients with SOC given that the morphologic presentation and/or anatomic site of involvement for skin cancers in SOC differs from those in lighter skin. Future educational interventions targeting disparities in diagnostic accuracy should focus on conditions that are not specifically associated with SOC.
Limitations of our study included the small number of participants, the study population came from a single institution, and a possible selection bias for providers interested in dermatology.
Conclusion
Disparities exist among interprofessional health care providers when treating conditions in patients with lighter skin compared to darker skin. An educational module for health care providers may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in patients with SOC.
Dermatologic disparities disproportionately affect patients with skin of color (SOC). Two studies assessing the diagnostic accuracy of medical students have shown disparities in diagnosing common skin conditions presenting in darker skin compared to lighter skin at early stages of training.1,2 This knowledge gap could be attributed to the underrepresentation of SOC in dermatologic textbooks, journals, and educational curricula.3-6 It is important for dermatologists as well as physicians in other specialties and ancillary health care workers involved in treating or triaging dermatologic diseases to recognize common skin conditions presenting in SOC. We sought to evaluate the effectiveness of a focused educational module for improving diagnostic accuracy and confidence in treating SOC among interprofessional health care providers.
Methods
Interprofessional health care providers—medical students, residents/fellows, attending physicians, advanced practice providers (APPs), and nurses practicing across various medical specialties—at The University of Texas at Austin Dell Medical School and Ascension Medical Group (both in Austin, Texas) were invited to participate in an institutional review board–exempt study involving a virtual SOC educational module from February through May 2021. The 1-hour module involved a pretest, a 15-minute lecture, an immediate posttest, and a 3-month posttest. All tests included the same 40 multiple-choice questions of 20 dermatologic conditions portrayed in lighter and darker skin types from VisualDx.com, and participants were asked to identify the condition in each photograph. Questions appeared one at a time in a randomized order, and answers could not be changed once submitted.
For analysis, the dermatologic conditions were categorized into 4 groups: cancerous, infectious, inflammatory, and SOC-associated conditions. Cancerous conditions included basal cell carcinoma, squamous cell carcinoma, and melanoma. Infectious conditions included herpes zoster, tinea corporis, tinea versicolor, staphylococcal scalded skin syndrome, and verruca vulgaris. Inflammatory conditions included acne, atopic dermatitis, pityriasis rosea, psoriasis, seborrheic dermatitis, contact dermatitis, lichen planus, and urticaria. Skin of color–associated conditions included hidradenitis suppurativa, acanthosis nigricans, keloid, and melasma. Two questions utilizing a 5-point Likert scale assessing confidence in diagnosing light and dark skin also were included.
The pre-recorded 15-minute video lecture was given by 2 dermatology residents (P.L.K. and C.P.), and the learning objectives covered morphologic differences in lighter skin and darker skin, comparisons of common dermatologic diseases in lighter skin and darker skin, diseases more commonly affecting patients with SOC, and treatment considerations for conditions affecting skin and hair in patients with SOC. Photographs from the diagnostic accuracy assessment were not reused in the lecture. Detailed explanations on morphology, diagnostic pearls, and treatment options for all conditions tested were provided to participants upon completion of the 3-month posttest.
Statistical Analysis—Test scores were compared between conditions shown in lighter and darker skin types and from the pretest to the immediate posttest and 3-month posttest. Multiple linear regression was used to assess for intervention effects on lighter and darker skin scores controlling for provider type and specialty. All tests were 2-sided with significance at P<.05. Analyses were conducted using Stata 17.
Results
One hundred participants completed the pretest and immediate posttest, 36 of whom also completed the 3-month posttest (Table). There was no significant difference in baseline characteristics between the pretest and 3-month posttest groups.

Test scores were correlated with provider type and specialty but not age, sex, or race/ethnicity. Specializing in dermatology and being a resident or attending physician were independently associated with higher test scores. Mean pretest diagnostic accuracy and confidence scores were higher for skin conditions shown in lighter skin compared with those shown in darker skin (13.6 vs 11.3 and 2.7 vs 1.9, respectively; both P<.001). Pretest diagnostic accuracy was significantly higher for skin conditions shown in lighter skin compared with darker skin for cancerous, inflammatory, and infectious conditions (72% vs 50%, 68% vs 55%, and 57% vs 47%, respectively; P<.001 for all)(Figure 1). Skin of color–associated conditions were not associated with significantly different scores for lighter skin compared with darker skin (79% vs 75%; P=.059).

Controlling for provider type and specialty, significantly improved diagnostic accuracy was seen in immediate posttest scores compared with pretest scores for conditions shown in both lighter and darker skin types (lighter: 15.2 vs 13.6; darker: 13.3 vs 11.3; both P<.001)(Figure 2). The immediate posttest demonstrated higher mean diagnostic accuracy and confidence scores for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 15.2 vs 13.3; confidence: 3.0 vs 2.6; both P<.001), but the disparity between scores was less than in the pretest.

Following the 3-month posttest, improvement in diagnostic accuracy was noted among both lighter and darker skin types compared with the pretest, but the difference remained significant only for conditions shown in darker skin (mean scores, 11.3 vs 13.3; P<.01). Similarly, confidence in diagnosing conditions in both lighter and darker skin improved following the immediate posttest (mean scores, 2.7 vs 3.0 and 1.9 vs 2.6; both P<.001), and this improvement remained significant for only darker skin following the 3-month posttest (mean scores, 1.9 vs 2.3; P<.001). Despite these improvements, diagnostic accuracy and confidence remained higher for skin conditions shown in lighter skin compared with darker skin (diagnostic accuracy: 14.7 vs 13.3; P<.01; confidence: 2.8 vs 2.3; P<.001), though the disparity between scores was again less than in the pretest.
Comment
Our study showed that there are diagnostic disparities between lighter and darker skin types among interprofessional health care providers. Education on SOC should extend to interprofessional health care providers and other medical specialties involved in treating or triaging dermatologic diseases. A focused educational module may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in SOC. Differences in diagnostic accuracy between conditions shown in lighter and darker skin types were noted for the disease categories of infectious, cancerous, and inflammatory conditions, with the exception of conditions more frequently seen in patients with SOC. Learning resources for SOC-associated conditions are more likely to have greater representation of images depicting darker skin types.7 Future educational interventions may need to focus on dermatologic conditions that are not preferentially seen in patients with SOC. In our study, the pretest scores for conditions shown in darker skin were lowest among infectious and cancerous conditions. For infections, certain morphologic clues such as erythema are important for diagnosis but may be more subtle or difficult to discern in darker skin. It also is possible that providers may be less likely to suspect skin cancer in patients with SOC given that the morphologic presentation and/or anatomic site of involvement for skin cancers in SOC differs from those in lighter skin. Future educational interventions targeting disparities in diagnostic accuracy should focus on conditions that are not specifically associated with SOC.
Limitations of our study included the small number of participants, the study population came from a single institution, and a possible selection bias for providers interested in dermatology.
Conclusion
Disparities exist among interprofessional health care providers when treating conditions in patients with lighter skin compared to darker skin. An educational module for health care providers may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in patients with SOC.
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958. doi:10.1016/j.jaad.2019.12.078
- Mamo A, Szeto MD, Rietcheck H, et al. Evaluating medical student assessment of common dermatologic conditions across Fitzpatrick phototypes and skin of color. J Am Acad Dermatol. 2022;87:167-169. doi:10.1016/j.jaad.2021.06.868
- Guda VA, Paek SY. Skin of color representation in commonly utilized medical student dermatology resources. J Drugs Dermatol. 2021;20:799. doi:10.36849/JDD.5726
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of color and diversity and inclusion content of dermatologic published literature: an analysis and call to action. Int J Womens Dermatol. 2021;7:391-397. doi:10.1016/j.ijwd.2021.04.001
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Gupta R, Ibraheim MK, Dao H Jr, et al. Assessing dermatology resident confidence in caring for patients with skin of color. Clin Dermatol. 2021;39:873-878. doi:10.1016/j.clindermatol.2021.08.019
- Chang MJ, Lipner SR. Analysis of skin color on the American Academy of Dermatology public education website. J Drugs Dermatol. 2020;19:1236-1237. doi:10.36849/JDD.2020.5545
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958. doi:10.1016/j.jaad.2019.12.078
- Mamo A, Szeto MD, Rietcheck H, et al. Evaluating medical student assessment of common dermatologic conditions across Fitzpatrick phototypes and skin of color. J Am Acad Dermatol. 2022;87:167-169. doi:10.1016/j.jaad.2021.06.868
- Guda VA, Paek SY. Skin of color representation in commonly utilized medical student dermatology resources. J Drugs Dermatol. 2021;20:799. doi:10.36849/JDD.5726
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of color and diversity and inclusion content of dermatologic published literature: an analysis and call to action. Int J Womens Dermatol. 2021;7:391-397. doi:10.1016/j.ijwd.2021.04.001
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Gupta R, Ibraheim MK, Dao H Jr, et al. Assessing dermatology resident confidence in caring for patients with skin of color. Clin Dermatol. 2021;39:873-878. doi:10.1016/j.clindermatol.2021.08.019
- Chang MJ, Lipner SR. Analysis of skin color on the American Academy of Dermatology public education website. J Drugs Dermatol. 2020;19:1236-1237. doi:10.36849/JDD.2020.5545
Practice Points
- Disparities exist among interprofessional health care providers when diagnosing conditions in patients with lighter and darker skin, specifically for infectious, cancerous, or inflammatory conditions vs conditions that are preferentially seen in patients with skin of color (SOC).
- A focused educational module for health care providers may provide long-term improvements in diagnostic accuracy and confidence for conditions presenting in patients with SOC.
Treatment of Frontal Fibrosing Alopecia in Black Patients: A Systematic Review
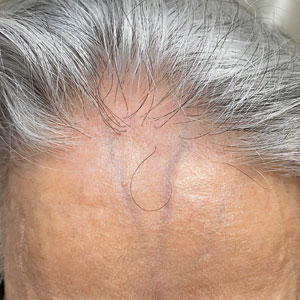
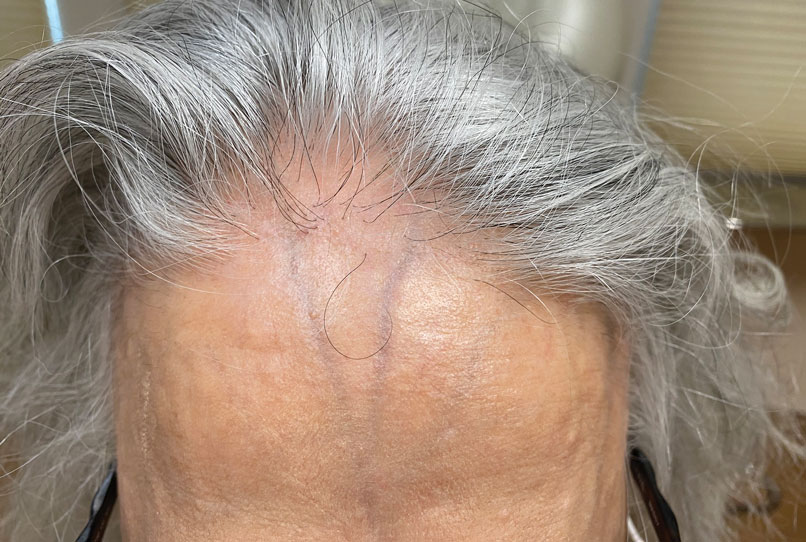
Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
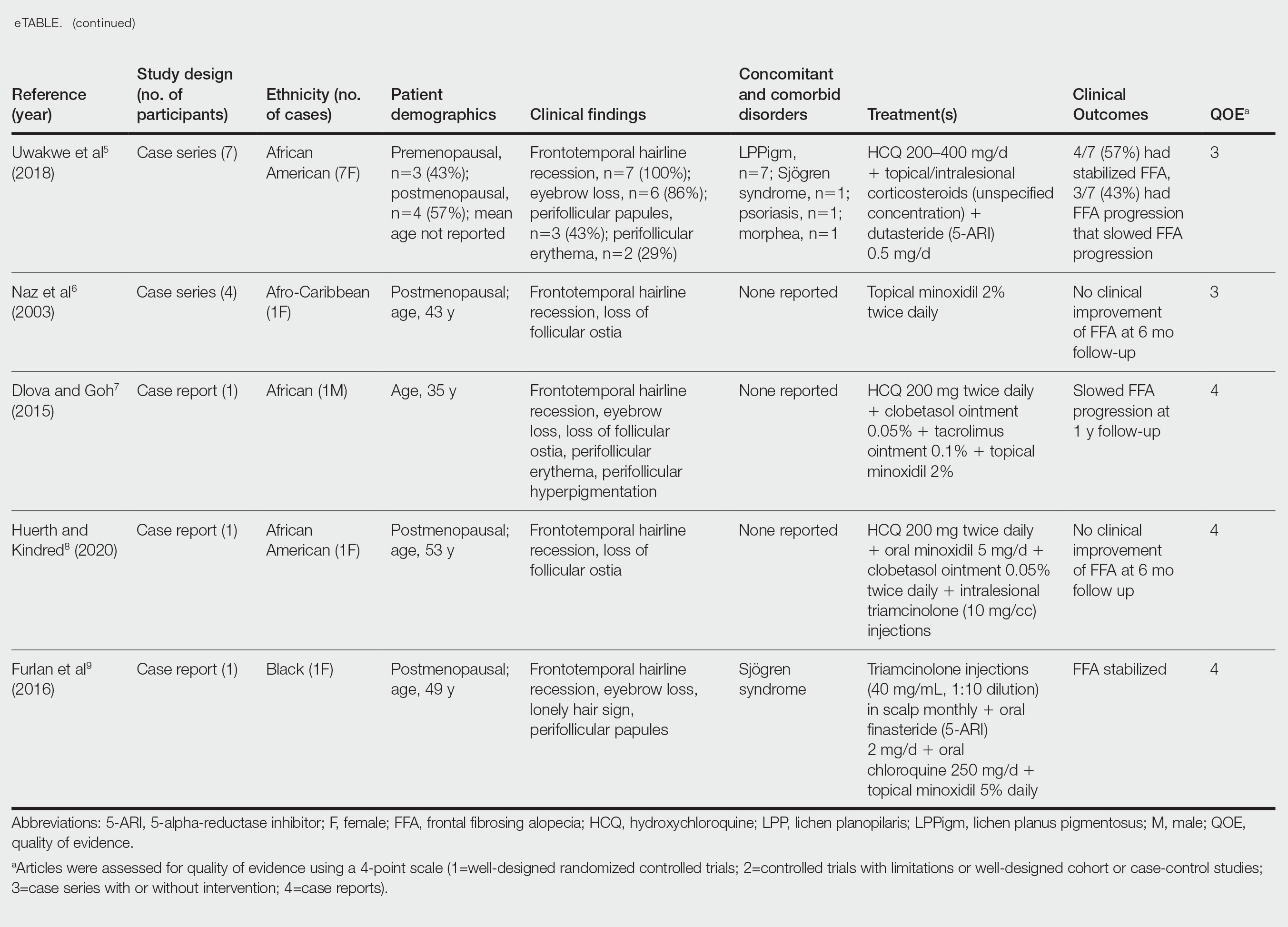
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
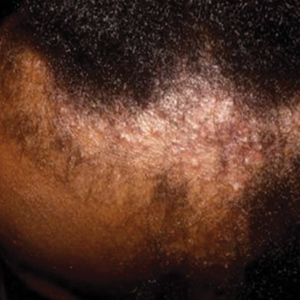
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.
The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
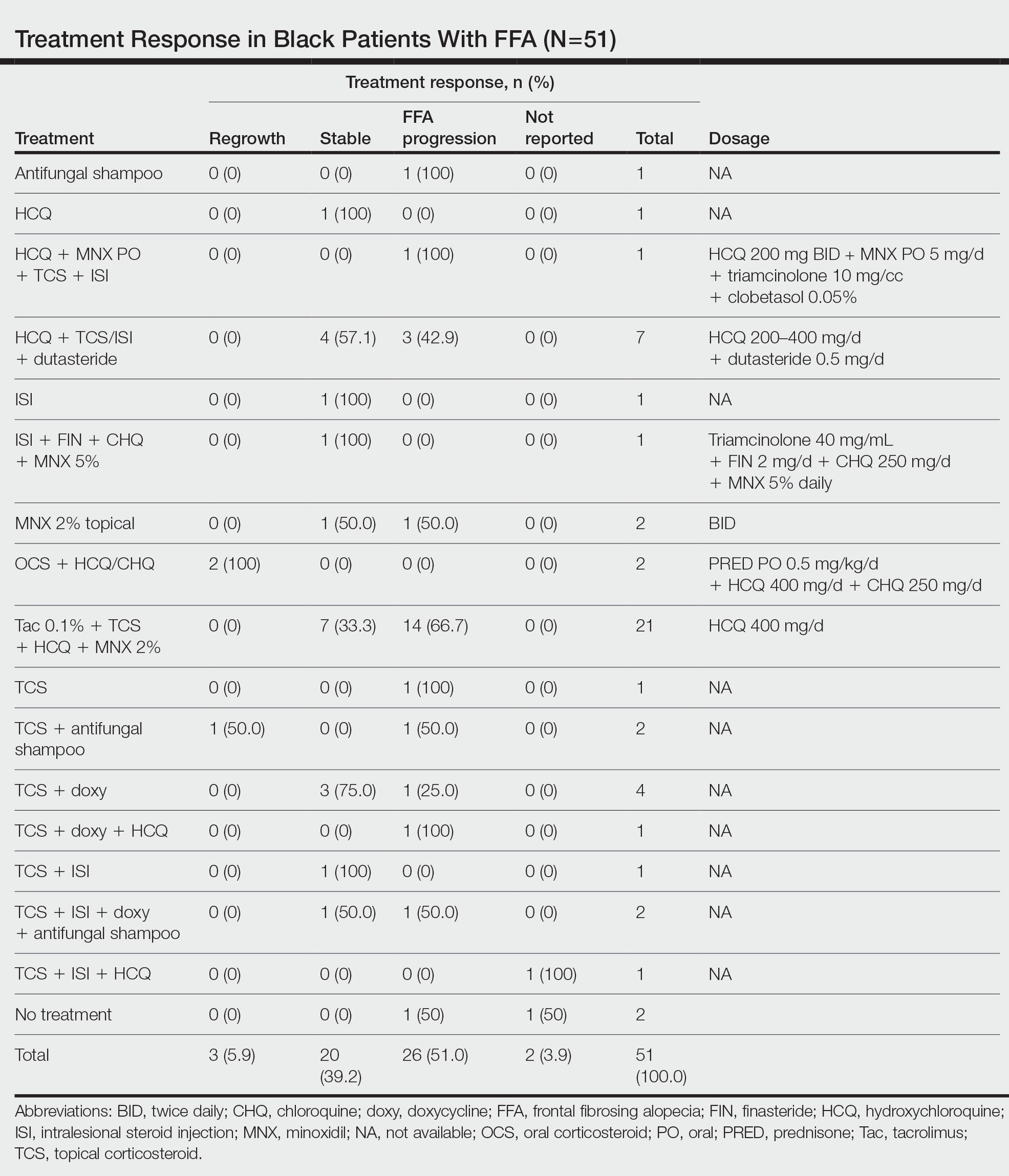
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.
Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.

- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.

The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.

Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.


Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.

The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.

Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.


- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
Practice Points
- Treatment of frontal fibrosing alopecia (FFA) is challenging, and there are no evidence-based treatment guidelines available. Patients with skin of color (SOC) may have varying responses to treatment modalities.
- Special consideration should be taken when treating FFA in patients with SOC.
- Histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
The CROWNing Event on Hair Loss in Women of Color: A Framework for Advocacy and Community Engagement (FACE) Survey Analysis
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.
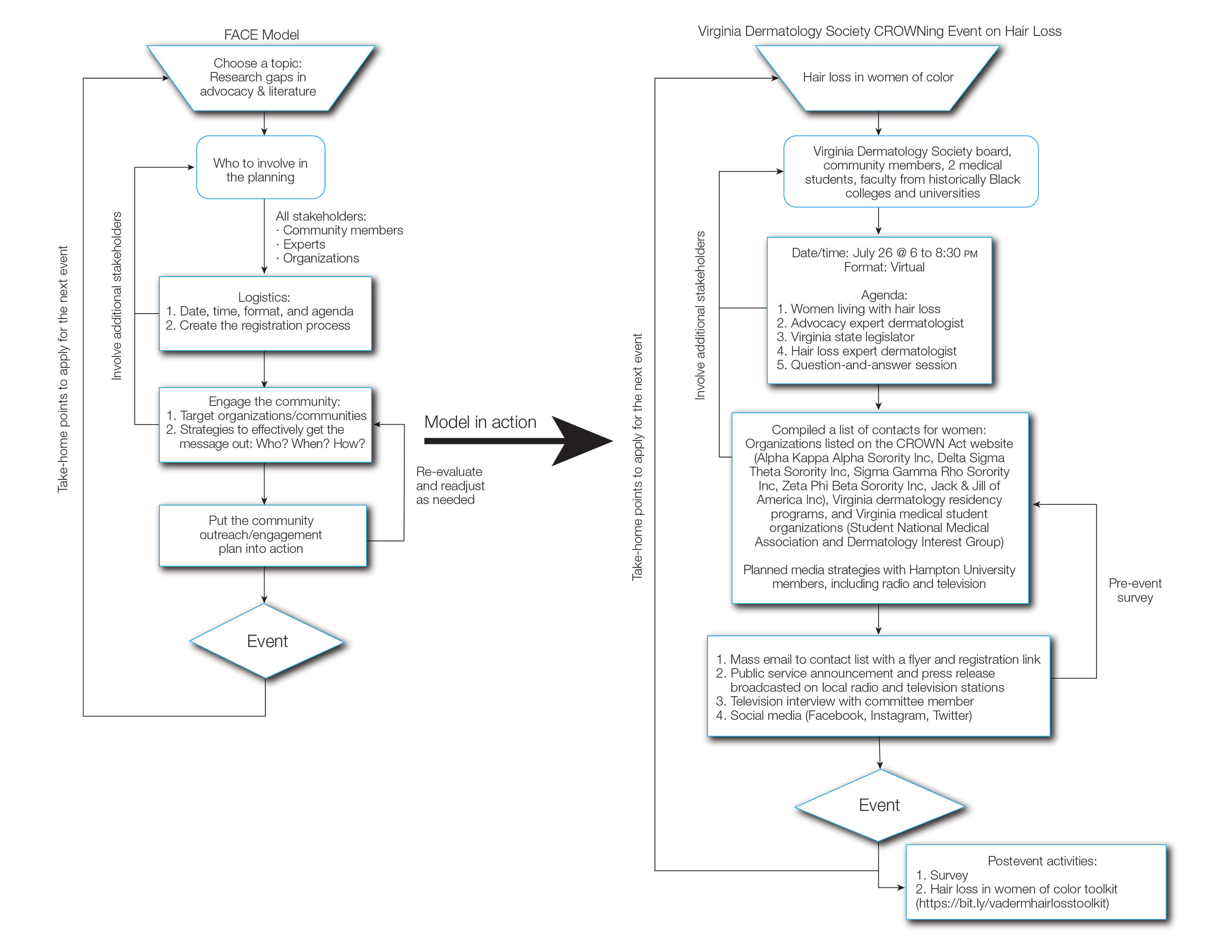
We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.
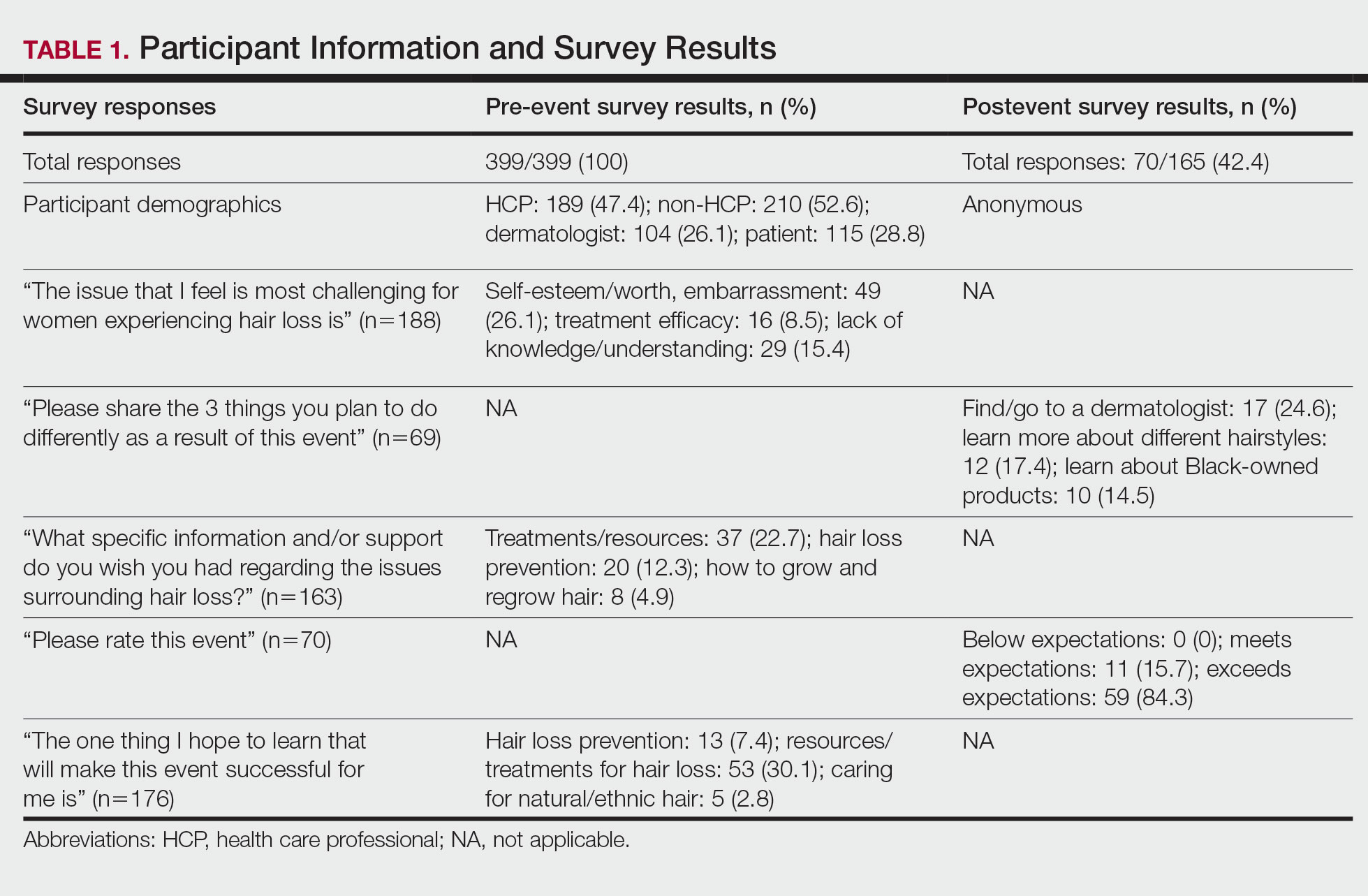
Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.
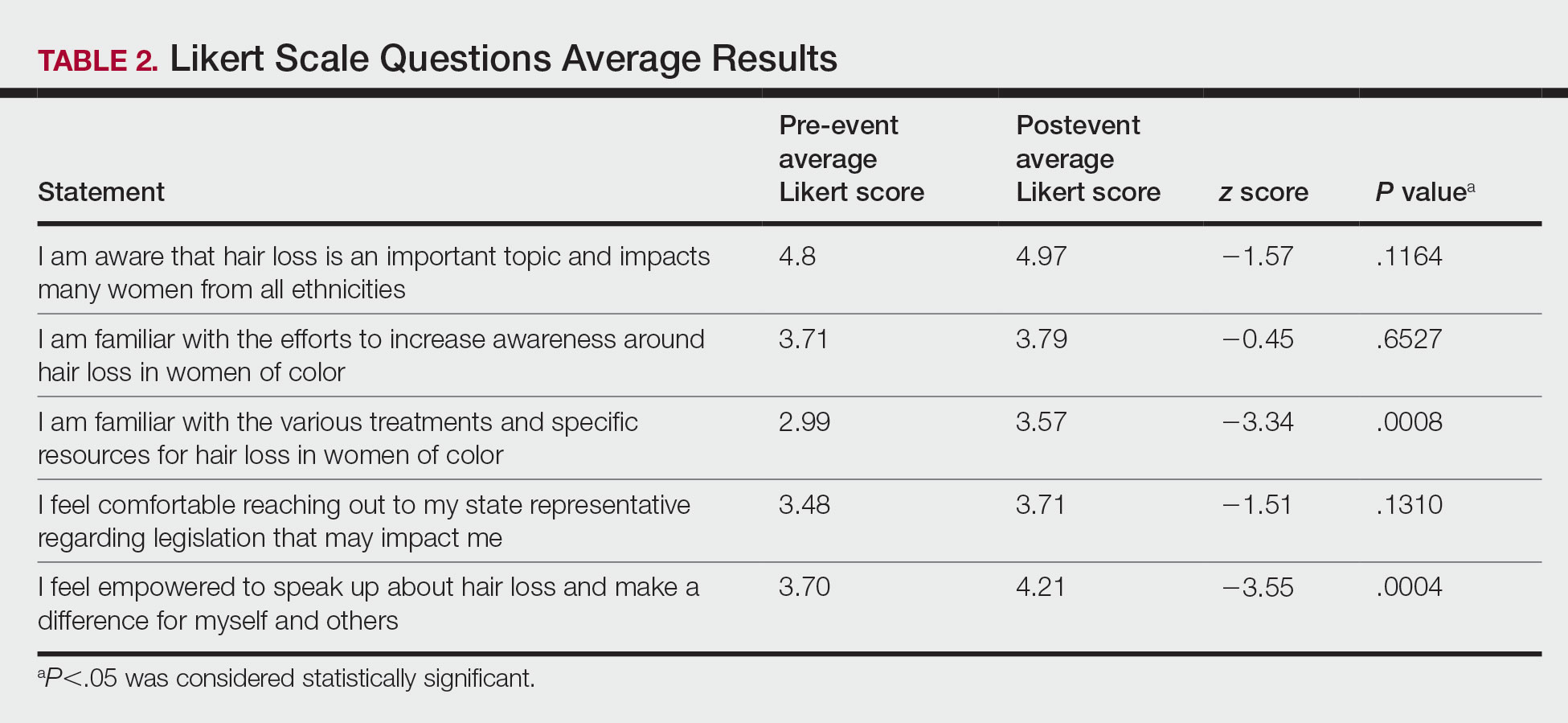
Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Practice Points
- Hair loss is associated with low self-esteem in women with skin of color; therefore, it is important to both acknowledge the social and psychological impacts of hair loss in this population and provide educational resources and community events that address patient concerns.
- There is a deficit of dermatology advocacy efforts that address conditions affecting patients with skin of color. Highlighting this disparity is the first step to catalyzing change.
- Dermatologists are responsible for advocating for women with skin of color and for addressing the social issues that impact their quality of life.
- The Framework for Advocacy and Community Efforts (FACE) model is a template for others to use when planning community engagement and advocacy efforts.
Perceptions of Community Service in Dermatology Residency Training Programs: A Survey-Based Study of Program Directors, Residents, and Recent Dermatology Residency Graduates
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.
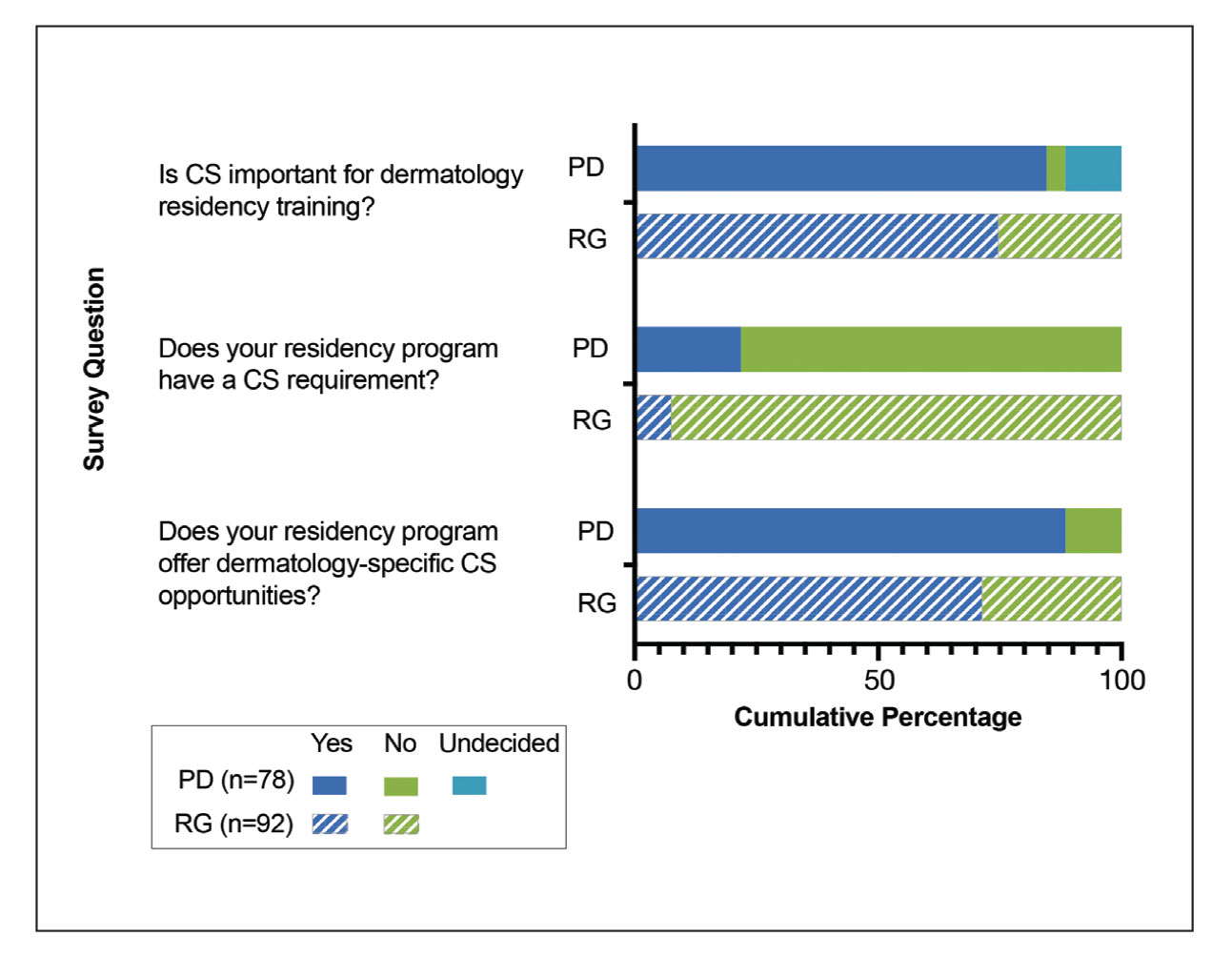
Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).
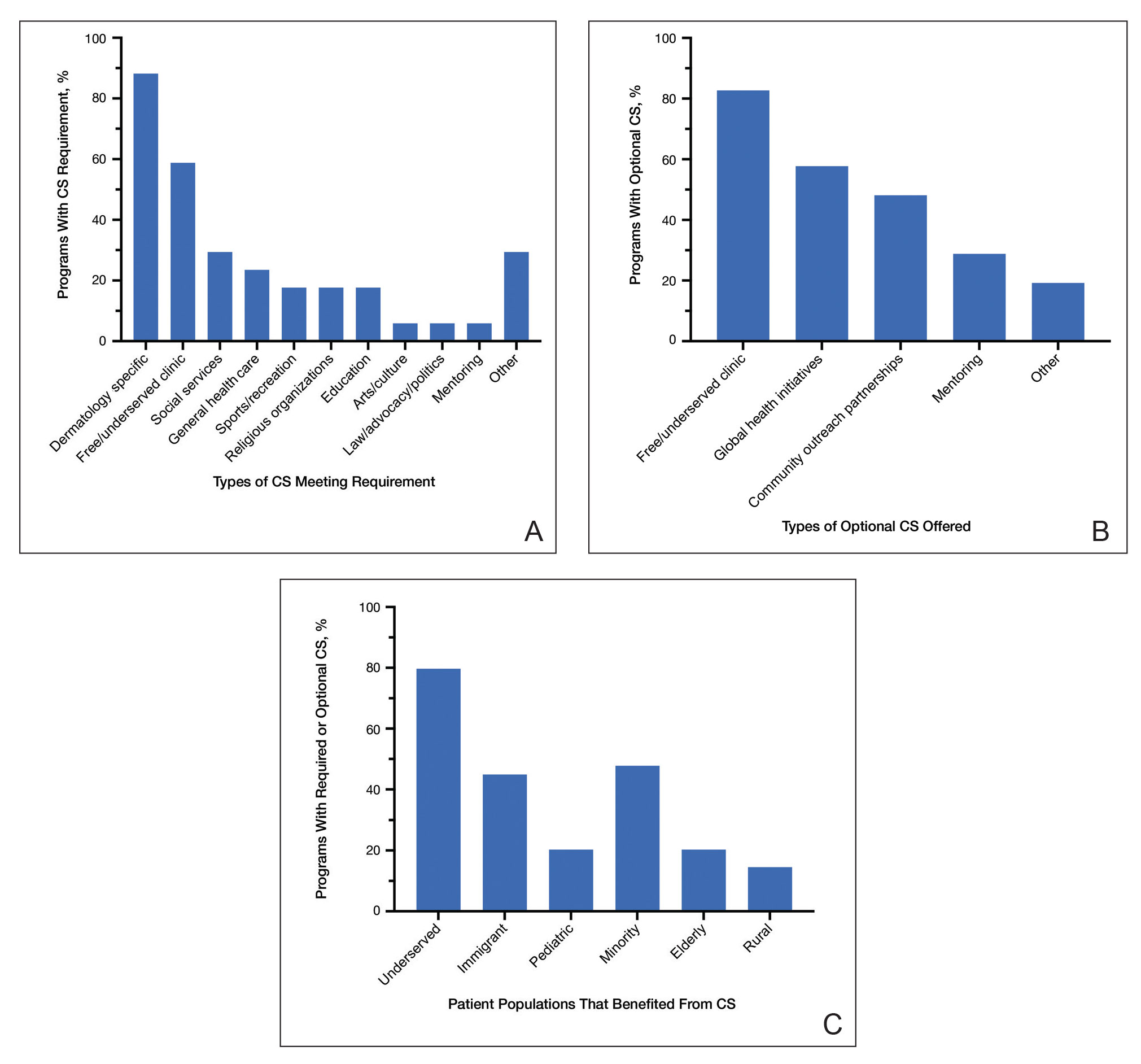
Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.
Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.

Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).

Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.

Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.

Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).

Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.

Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Practice Points
- Participation of dermatology residents in service-learning experiences increases awareness of health disparities and social factors impacting dermatologic care and promotes a lifelong commitment to serving vulnerable populations.
- Integrating service learning into the dermatology residency program curriculum enhances trainees’ cultural sensitivity and encourages the prioritization of skin health equity.
- Service learning will help bridge the gap in access to dermatologic care for patients in medically marginalized communities.
Tinted Sunscreens: Consumer Preferences Based on Light, Medium, and Dark Skin Tones
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.

Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.
Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.

Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.

Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.

Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.

Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
Practice Points
- Visible light has been shown to increase tyrosinase activity and induce immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.
- The formulation of sunscreens with iron oxides and pigmentary titanium dioxide are a safe and effective way to protect against high-energy visible light, especially when combined with zinc oxide.
- Physicians should be aware of sunscreen characteristics that patients like and dislike to tailor recommendations that are appropriate for each individual to enhance adherence.
- Cosmetic elegance and tone compatibility are the most important criteria for individuals seeking tinted sunscreens.
The Leaky Pipeline: A Narrative Review of Diversity in Dermatology
With a majority-minority population expected in the United States by 2044, improving diversity and cultural competency in the dermatology workforce is now more important than ever. A more diverse workforce increases the cultural competence of all providers, provides greater opportunities for mentorship and sponsorship of underrepresented minority (URM) trainees, establishes a more inclusive environment for learners, and enhances the knowledge and productivity of the workforce.1-3 Additionally, it is imperative to address clinical care disparities seen in minority patients in dermatology, including treatment of skin cancer, psoriasis, acne, atopic dermatitis, and other diseases.4-7
Despite the attention that has been devoted to improving diversity in medicine,8-10 dermatology remains one of the least diverse specialties, prompting additional calls to action within the field.11 Why does the lack of diversity still exist in dermatology, and what is the path to correcting this problem? In this article, we review the evidence of diversity barriers at different stages of medical education training that may impede academic advancement for minority learners pursuing careers in dermatology.
Undergraduate Medical Education
The term leaky pipeline refers to the progressive decline in the number of URMs along a given career path, including in dermatology. The Association of American Medical Colleges defines URMs as racial/ethnic populations that are “underrepresented in the medical profession relative to their numbers in the general population.”9 The first leak in the pipeline is that URMs are not applying to medical school. From 2002 and 2017, rates of both application and matriculation to medical school were lower by 30% to 70% in URM groups compared to White students, including Hispanic, Black, and American Indian/Alaska Native students.12,13 The decision not to apply to medical school was greater in URM undergraduate students irrespective of scholastic ability as measured by SAT scores.14
A striking statistic is that the number of Black men matriculating into medical school in 2014 was less than it was in 1978 despite the increase in the number of US medical schools and efforts to recruit more diverse student populations. The Association of American Medical Colleges identified potential reasons for this decline, including poor early education, lack of mentorship, negative perceptions of Black men due to racial stereotypes, and lack of financial and academic resources to support the application process.8,13,15-17 Implicit racial bias by admission committees also may play a role.
Medical School Matriculation and Applying to Dermatology Residency
There is greater representation of URM students in medical school than in dermatology residency, which means URM students are either not applying to dermatology programs or they are not matching into the specialty. In the Electronic Residency Application Service’s 2016-2017 application cycle (N=776), there were 76 (9.8%) URM dermatology residency applicants.18 In 2018, there was a notable decline in representation of Black students among residency applicants (4.9%) to matched residents (3.7%), and there were only 133 (9.3%) URM dermatology residents in total (PGY2-PGY4 classes).19 The lack of exposure to medical subspecialties and the recommendation by medical schools for URM medical students to pursue careers in primary care have been cited as reasons that these students may not apply to residency programs in specialty care.20,21 The presence of an Accreditation Council for Graduate Medical Education dermatology residency program, fellowships, and dermatology interest groups at their medical schools correlated with higher proportions of URM students applying to dermatology programs.20
Underrepresented minority students face critical challenges during medical school, including receiving lower grades in both standardized and school-designated assessments and clerkship grades.21,22 A 2019 National Board of Medical Examiners study found that Hispanic and Black test takers scored 12.1 and 16.6 points lower than White men, respectively, on the
A recent cross-sectional study showed that lack of equitable resources, lack of support, financial constrictions, and lack of group identity were 4 barriers to URM students matching into dermatology.26 Dermatology is a competitive specialty with the highest median Electronic Residency Application Service applications submitted per US applicant (n=90)27 and an approximate total cost per US applicant of $10,781.28,29 Disadvantaged URM applicants noted relying on loans while non-URM applicants cited family financial support as being beneficial.26 In addition, an increasing number of applicants take gap years for research, which pose additional costs for finances and resources. In contrast, mentorship and participation in pipeline/enrichment programs were factors associated with URM students matching into dermatology.26
Dermatology Residency and the Transition to Advanced Dermatology Fellowships
Similar to the transition from medical school into dermatology residency, URM dermatology residents are either not applying to fellowships or are not getting in. In the 2018-2019 academic year, there were no Black, Hispanic, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native Mohs micrographic surgery and dermatologic oncology fellows.19 Similarly, there were no Black, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native dermatopathology fellows. There were 4 (6%) Hispanic dermatopathology fellows.19
There also is marked underrepresentation of minority groups—and minimal growth over time—in the dermatology procedural subspecialty. Whereas the percentage of female Mohs surgeons increased considerably from 1985 to 2005 (12.7% to 40.9%, respectively), the percentage of URM Mohs surgeons remained steady from 4.2% to 4.6%, respectively, and remained at 4.5% in 2014.30
There are no available data on the race/ethnicity of fellowship applicants, as these demographic data for the application process have not been consistently or traditionally collected. The reasons why there are so few URM dermatology fellows is not known; whether this is due to a lack of mentorship or whether other factors lead to residents not applying for advanced training needs further study. Financial factors related to prolonged training, which include lower salaries and delayed loan repayment, may present barriers to applying to fellowships.
Lack of URM Academic Faculty in Dermatology
At the academic faculty level, URM representation continues to worsen. Lett et al31 found that there is declining racial and ethnic representation in clinical academic medicine relative to US census data for 16 US medical specialties, including dermatology, with growing underrepresentation of Black and Hispanic faculty at the associate professor and full professor levels and underrepresentation in all faculty ranks. From 1970 to 2018, URM faculty in dermatology only increased from 4.8% to 7.4%, respectively. Non-URM female and male faculty members increased by 13.8 and 10.8 faculty members per year, respectively, while URM female and male faculty members increased by 1.2 and 0.8 faculty members per year, respectively.32
Underrepresentation of minorities seen in dermatology faculty may result from clinical demands, minority taxation (defined as the extensive service requirements uniquely experienced by URM faculty to disproportionately serve as representatives on academic committees and to mentor URM students), and barriers to academic promotion, which are challenges uniquely encountered by URMs in academic dermatology.33 Increased clinical demand may result from the fact that URM physicians are more likely to care for underserved populations, those of lower socioeconomic status, non-English–speaking patients, those on Medicaid, and those who are uninsured, which may impact renumeration. Minority tax experienced by URM faculty includes mentoring URM medical students, providing cultural expertise to departments and institutions, and participating in community service projects and outreach programs. Specifically, many institutional committees require the participation of a URM member, resulting in URM faculty members experiencing higher committee service burden. Many, if not all, of these responsibilities often are not compensated through salary or academic promotion.
A Call to Action
There are several steps that can be taken to create a pathway to dermatology that is inclusive, flexible, and supportive of URMs.
• Increase early exposure to dermatology in medical school. Early exposure and mentorship opportunities are associated with higher rates of students pursuing specialty field careers.34 Increased early opportunities allow for URM students to consider and explore a career in dermatology; receive mentorship; and ensure that dermatology, including topics related to skin of color (SOC), is incorporated into their learning. The American Academy of Dermatology has contributed to these efforts by its presence at every national meeting of the Student National Medical Association and Latino Medical Student Association, as well as its involvement with Nth Dimensions, which offers various educational opportunities for URM medical students.
• Implement equitable grading and holistic review processes in medical school. Racial/ethnic differences in clinical grading and standardized test scores in medical school demonstrate why holistic review of dermatology residency applicants is needed and why other metrics such as USMLE scores and AΩA status should be de-emphasized or eliminated when evaluating candidates. To support equity, many medical schools have eliminated honors grading, and some schools have eliminated AΩA distinction.
• Increase diversity of dermatology residents and residency programs. Implicit bias training for a medical school admissions committee has been shown to increase diversity in medical school enrollment.35 Whether implicit bias training and other diversity training may benefit dermatology residency selection must be examined, including study of unintended consequences, such as reduced diversity, increased microaggressions toward minority colleagues, and the illusion of fairness.36-39 Increasing representation is not sufficient—creating inclusive residency training environments is a critical parallel aim. Prioritizing diversity in dermatology residency recruitment is imperative. Creating dermatology residency positions specifically for URM residents may be an important option, as done at the University of Pennsylvania (Philadelphia, Pennsylvania) and Duke University (Durham, North Carolina).
• Create effective programs for URM mentorship. Due to the competitive nature of dermatology residency, the need for mentors in dermatology is critically vital for URM medical students, especially those without a home dermatology program at their medical school. Further development of formal mentorship and pipeline programs is essential at both the local and national levels. Some national examples of these initiatives include diversity mentorship programs offered by the American Academy of Dermatology, Skin of Color Society, Women’s Dermatologic Society, and Student National Medical Association. Many institutional programs also offer invaluable opportunities, such as the summer research fellowship at the University of California, San Francisco (UCSF); visiting clerkship grants for URMs at the University of Pennsylvania (Philadelphia, Pennsylvania) and Johns Hopkins University (Baltimore, Maryland); and integrated programs, such as the Visiting Elective Scholarship Program at UCSF, which provides funding and faculty mentorship for URM students completing an away rotation at UCSF.
• Establish longitudinal skin-of-color curricula and increased opportunities for research. More robust SOC training may lead to an increasingly diverse workforce. It is important that medical student and dermatology resident and fellow education include training on SOC to ensure high-quality care to diverse patient populations, which also may enhance the knowledge of trainees, encourage clinical and research interest in this field, and reduce health care disparities. Increasing research opportunities and offering formalized longitudinal training in SOC as well as incorporating more diverse images in medical school education may foster greater interest in this field at a time when trainees are establishing their career interests. At present, there is considerable room for improvement. Nijhawan et al40 surveyed 63 dermatology chief residents and 41 program directors and found only 14.3% and 14.6%, respectively, reported having an expert who conducts clinic specializing in SOC. Only 52.4% and 65.9% reported having didactic sessions or lectures focused on SOC diseases, and 30.2% and 12.2% reported having a dedicated rotation for residents to gain experience in SOC.40 A more recent study showed that when faculty were asked to incorporate more SOC content into lectures, the most commonly identified barrier to implementation was a lack of SOC images.41 Additionally, there remains a paucity of published research on this topic, with SOC articles representing only 2.7% of the literature.42 These numbers demonstrate the continued need for a more inclusive and comprehensive curriculum in dermatology residency programs and more robust funding for SOC research.
• Recruit and support URM faculty. Increasing diversity in dermatology residency programs likely will increase the number of potential URMs pursuing additional fellowship training and academic dermatology with active career mentorship and support. In addition, promoting faculty retention by combatting the progressive loss of URMs at all faculty levels is paramount. Mentorship for URM physicians has been shown to play a key role in the decision to pursue academic medicine as well as academic productivity and job satisfaction.43,44 The visibility, cultural competency, clinical work, academic productivity, and mentorship efforts that URM faculty provide are essential to enhancing patient care, teaching diverse groups of learners, and recruiting more diverse trainees. Protected time to participate in professional development opportunities has been shown to improve recruitment and retention of URM faculty and offer additional opportunities for junior faculty to find mentors.35,36 Incentivizing clinical care of underserved populations also may augment financial stability for URM physicians who choose to care for these patients. Finally, diversity work and community service should be legitimized and count toward faculty promotion.
Conclusion
There are numerous factors that contribute to the leaky pipeline in dermatology (eFigure). Many challenges that are unique to the URM population disadvantage these students from entering medical school, applying to dermatology residency, matching into dermatology fellowships, pursuing and staying in faculty positions, and achieving faculty advancement into leadership positions. With each progressive step along this trajectory, there is less minority representation. All dermatologists, regardless of race/ethnicity, need to play an active role and must prioritize diversity, equity, and inclusion efforts at all levels of education and training for the betterment of the specialty.
- Dixon G, Kind T, Wright J, et al. Factors that influence the choice of academic pediatrics by underrepresented minorities. Pediatrics. 2019;144:E20182759. doi:10.1542/peds.2018-2759
- Yehia BR, Cronholm PF, Wilson N, et al. Mentorship and pursuit of academic medicine careers: a mixed methods study of residents from diverse backgrounds. BMC Med Educ. 2014:14:2-26. doi:10.1186/1472-6920-14-26
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145. doi:10.1001/jama.300.10.1135
- Hsu DY, Gordon K, Silverberg JI. The patient burden of psoriasis in the United States. J Am Acad Dermatol. 2016;75:33-41. doi:10.1016/j.jaad.2016.03.048
- Silverberg JI. Racial and ethnic disparities in atopic dermatitis. Curr Dermatol Rep. 2015;4:44-48.
- Buster KJ, Sevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Smedley BD, Stith AY, Colburn L, et al. The Right Thing To Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions. National Academies Press; 2001.
- Association of American Medical Colleges. Minorities in medical education: fact and figures 2019. Accessed December 9, 2021. https://www.aamc.org/datareports/workforce/report/diversity-medicine-facts-and-figures-2019
- Liaison Committee on Medical Education (LCME) standards on diversity. University of South Florida Health website. Accessed December 9, 2021. https://health.usf.edu/~/media/Files/Medicine/MD%20Program/Diversity/LCMEStandardsonDiversity1.ashx?la=en
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500. doi:10.1001/jamadermatol.2017.0296
- Lett LA, Murdock HM, Orji W, et al. Trends in racial/ethnic representation among US medical students. JAMA Netw Open. 2019;2:e1910490. doi:10.1001/jamanetworkopen.2019.10490
- Association of American Medical Colleges. Altering the course: Black males in medicine. Published 2015. Accessed December 8, 2021. https://store.aamc.org/downloadable/download/sample/sample_id/84/
- Barr DA, Gonzalez ME, Wanat SF. The leaky pipeline: factors associated with early decline in interest in premedical studies among underrepresented minority undergraduate students. Acad Med. 2008;83:5:503-511. doi:10.1097/ACM.0b013e31816bda16
- Flores RL. The rising gap between rich and poor: a look at the persistence of educational disparities in the United States and why we should worry. Cogent Soc Sci. 2017;3:1323698.
- Jackson D. Why am I behind? an examination of low income and minority students’ preparedness for college. McNair Sch J. 2012;13:121-138.
- Rothstein R. The racial achievement gap, segregated schools, andsegregated neighborhoods: a constitutional insult. Race Soc Probl. 2015;7:21-30.
- Association of American Medical Colleges. Residency Applicants From US MD Granting Medical Schools to ACGME-Accredited Programs by Specialty and Race/Ethnicity. Association of American Medical Colleges; 2017.
- Brotherton SE, Etzel SL. Graduate medical education, 2018-2019. JAMA. 2019;322:996-1016. doi:10.1001/jama.2019.10155
- Barnes LA, Bae GH, Nambudiri V. Sex and racial/ethnic diversity of US medical students and their exposure to dermatology programs. JAMA Dermatol. 2019;155:490-491. doi:10.1001/jamadermatol.2018.5025
- Soliman YS, Rzepecki AK, Guzman AK. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Low D, Pollack SW, Liao Z, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance and United States medical licensing examination scores. Acad Med. 2019;94;364-370. doi:10.1097/ACM.0000000000002366
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the alpha omega honor society. JAMA Intern Med. 2017;177:659-665. doi:10.1001/jamainternmed.2016.9623
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey [published online March 17, 2014]. Dermatol Res Pract. doi:10.1155/2014/692760
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Results of the 2019 NRMP applicant survey by preferred specialty and applicant type. National Resident Matching Program website. Published July 2019. Accessed December 8, 2021. https://www.nrmp.org/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Polacco MA, Lally J, Walls A, et al. Digging into debt: the financial burden associated with the otolaryngology match. Otolaryngol Head Neck Surg. 2017;12:1091-1096. doi:10.1177/0194599816686538
- Feng H, Feng PW, Geronemus RG. Diversity in the US Mohs micrographic surgery workforce. Dermatol Surg. 2020:46:1451-1455. doi:10.1097/DSS.0000000000002080
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS ONE. 2018;13:e0207274. doi:10.1371/journal.pone.020727432. Xierali IM, Nivet MA, Pandya AG. US Dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970-2018. JAMA Dermatol. 2020;156:280-287. doi:10.1001/jamadermatol.2019.4297
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Intl J Womens Dermatol. 2020;6:57-60. doi:10.1016/j.ijwd.2019.09.009
- Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86:2335-2338. doi:10.2106/00004623-200410000-00031
- Capers Q, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369. doi:10.1097/ACM.0000000000001388
- Dobbin F, Kalev A. Why diversity programs fail. Harvard Business Rev. 2016;52-60. Accessed December 8, 2021. https://hbr.org/2016/07/why-diversity-programs-fail
- Kalev A, Dobbin F, Kelly E. Best practices or best guesses? assessing the efficacy of corporate affirmative action and diversity policies. Am Sociol Rev. 2006;71:589-617.
- Sanchez JI, Medkik N. The effects of diversity awareness training on differential treatment. Group Organ Manag. 2004;29:517-536.
- Kaiser CR, Major B, Jurcevic I, et al. Presumed fair: ironic effects of organizational diversity structures. J Pers Soc Psychol. 2013;104:504-519. doi:10.1037/a0030838
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-617.
- Jia JL, Gordon JS, Lester JC, et al. Integrating skin of color and sexual and gender minority content into dermatology residency curricula: a prospective program initiative [published online April 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.018
- Amuzie AU, Lia JL, Taylor SC, et al. Skin of color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Beech BM, Calles-Escandon J, Hairston KC, et al. Mentoring programs for underrepresented minority faculty in academic medical center: a systematic review of the literature. Acad Med. 2013;88:541-549. doi:10.1097/ACM.0b013e31828589e3
- Daley S, Wingard DL, Reznik V. Improving the retention of underrepresented minority faculty in academic medicine. J Natl Med Assoc. 2006;98:1435-1440. doi:10.1016/s0027-9684(15)31449-8
- Association of American Medical Colleges. US medical school faculty by sex, race/ethnicity, rank, and department, 2019. Published December 31, 2019. Accessed December 20, 2021. https://www.aamc.org/media/8476/download?attachment
With a majority-minority population expected in the United States by 2044, improving diversity and cultural competency in the dermatology workforce is now more important than ever. A more diverse workforce increases the cultural competence of all providers, provides greater opportunities for mentorship and sponsorship of underrepresented minority (URM) trainees, establishes a more inclusive environment for learners, and enhances the knowledge and productivity of the workforce.1-3 Additionally, it is imperative to address clinical care disparities seen in minority patients in dermatology, including treatment of skin cancer, psoriasis, acne, atopic dermatitis, and other diseases.4-7
Despite the attention that has been devoted to improving diversity in medicine,8-10 dermatology remains one of the least diverse specialties, prompting additional calls to action within the field.11 Why does the lack of diversity still exist in dermatology, and what is the path to correcting this problem? In this article, we review the evidence of diversity barriers at different stages of medical education training that may impede academic advancement for minority learners pursuing careers in dermatology.
Undergraduate Medical Education
The term leaky pipeline refers to the progressive decline in the number of URMs along a given career path, including in dermatology. The Association of American Medical Colleges defines URMs as racial/ethnic populations that are “underrepresented in the medical profession relative to their numbers in the general population.”9 The first leak in the pipeline is that URMs are not applying to medical school. From 2002 and 2017, rates of both application and matriculation to medical school were lower by 30% to 70% in URM groups compared to White students, including Hispanic, Black, and American Indian/Alaska Native students.12,13 The decision not to apply to medical school was greater in URM undergraduate students irrespective of scholastic ability as measured by SAT scores.14
A striking statistic is that the number of Black men matriculating into medical school in 2014 was less than it was in 1978 despite the increase in the number of US medical schools and efforts to recruit more diverse student populations. The Association of American Medical Colleges identified potential reasons for this decline, including poor early education, lack of mentorship, negative perceptions of Black men due to racial stereotypes, and lack of financial and academic resources to support the application process.8,13,15-17 Implicit racial bias by admission committees also may play a role.
Medical School Matriculation and Applying to Dermatology Residency
There is greater representation of URM students in medical school than in dermatology residency, which means URM students are either not applying to dermatology programs or they are not matching into the specialty. In the Electronic Residency Application Service’s 2016-2017 application cycle (N=776), there were 76 (9.8%) URM dermatology residency applicants.18 In 2018, there was a notable decline in representation of Black students among residency applicants (4.9%) to matched residents (3.7%), and there were only 133 (9.3%) URM dermatology residents in total (PGY2-PGY4 classes).19 The lack of exposure to medical subspecialties and the recommendation by medical schools for URM medical students to pursue careers in primary care have been cited as reasons that these students may not apply to residency programs in specialty care.20,21 The presence of an Accreditation Council for Graduate Medical Education dermatology residency program, fellowships, and dermatology interest groups at their medical schools correlated with higher proportions of URM students applying to dermatology programs.20
Underrepresented minority students face critical challenges during medical school, including receiving lower grades in both standardized and school-designated assessments and clerkship grades.21,22 A 2019 National Board of Medical Examiners study found that Hispanic and Black test takers scored 12.1 and 16.6 points lower than White men, respectively, on the
A recent cross-sectional study showed that lack of equitable resources, lack of support, financial constrictions, and lack of group identity were 4 barriers to URM students matching into dermatology.26 Dermatology is a competitive specialty with the highest median Electronic Residency Application Service applications submitted per US applicant (n=90)27 and an approximate total cost per US applicant of $10,781.28,29 Disadvantaged URM applicants noted relying on loans while non-URM applicants cited family financial support as being beneficial.26 In addition, an increasing number of applicants take gap years for research, which pose additional costs for finances and resources. In contrast, mentorship and participation in pipeline/enrichment programs were factors associated with URM students matching into dermatology.26
Dermatology Residency and the Transition to Advanced Dermatology Fellowships
Similar to the transition from medical school into dermatology residency, URM dermatology residents are either not applying to fellowships or are not getting in. In the 2018-2019 academic year, there were no Black, Hispanic, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native Mohs micrographic surgery and dermatologic oncology fellows.19 Similarly, there were no Black, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native dermatopathology fellows. There were 4 (6%) Hispanic dermatopathology fellows.19
There also is marked underrepresentation of minority groups—and minimal growth over time—in the dermatology procedural subspecialty. Whereas the percentage of female Mohs surgeons increased considerably from 1985 to 2005 (12.7% to 40.9%, respectively), the percentage of URM Mohs surgeons remained steady from 4.2% to 4.6%, respectively, and remained at 4.5% in 2014.30
There are no available data on the race/ethnicity of fellowship applicants, as these demographic data for the application process have not been consistently or traditionally collected. The reasons why there are so few URM dermatology fellows is not known; whether this is due to a lack of mentorship or whether other factors lead to residents not applying for advanced training needs further study. Financial factors related to prolonged training, which include lower salaries and delayed loan repayment, may present barriers to applying to fellowships.
Lack of URM Academic Faculty in Dermatology
At the academic faculty level, URM representation continues to worsen. Lett et al31 found that there is declining racial and ethnic representation in clinical academic medicine relative to US census data for 16 US medical specialties, including dermatology, with growing underrepresentation of Black and Hispanic faculty at the associate professor and full professor levels and underrepresentation in all faculty ranks. From 1970 to 2018, URM faculty in dermatology only increased from 4.8% to 7.4%, respectively. Non-URM female and male faculty members increased by 13.8 and 10.8 faculty members per year, respectively, while URM female and male faculty members increased by 1.2 and 0.8 faculty members per year, respectively.32
Underrepresentation of minorities seen in dermatology faculty may result from clinical demands, minority taxation (defined as the extensive service requirements uniquely experienced by URM faculty to disproportionately serve as representatives on academic committees and to mentor URM students), and barriers to academic promotion, which are challenges uniquely encountered by URMs in academic dermatology.33 Increased clinical demand may result from the fact that URM physicians are more likely to care for underserved populations, those of lower socioeconomic status, non-English–speaking patients, those on Medicaid, and those who are uninsured, which may impact renumeration. Minority tax experienced by URM faculty includes mentoring URM medical students, providing cultural expertise to departments and institutions, and participating in community service projects and outreach programs. Specifically, many institutional committees require the participation of a URM member, resulting in URM faculty members experiencing higher committee service burden. Many, if not all, of these responsibilities often are not compensated through salary or academic promotion.
A Call to Action
There are several steps that can be taken to create a pathway to dermatology that is inclusive, flexible, and supportive of URMs.
• Increase early exposure to dermatology in medical school. Early exposure and mentorship opportunities are associated with higher rates of students pursuing specialty field careers.34 Increased early opportunities allow for URM students to consider and explore a career in dermatology; receive mentorship; and ensure that dermatology, including topics related to skin of color (SOC), is incorporated into their learning. The American Academy of Dermatology has contributed to these efforts by its presence at every national meeting of the Student National Medical Association and Latino Medical Student Association, as well as its involvement with Nth Dimensions, which offers various educational opportunities for URM medical students.
• Implement equitable grading and holistic review processes in medical school. Racial/ethnic differences in clinical grading and standardized test scores in medical school demonstrate why holistic review of dermatology residency applicants is needed and why other metrics such as USMLE scores and AΩA status should be de-emphasized or eliminated when evaluating candidates. To support equity, many medical schools have eliminated honors grading, and some schools have eliminated AΩA distinction.
• Increase diversity of dermatology residents and residency programs. Implicit bias training for a medical school admissions committee has been shown to increase diversity in medical school enrollment.35 Whether implicit bias training and other diversity training may benefit dermatology residency selection must be examined, including study of unintended consequences, such as reduced diversity, increased microaggressions toward minority colleagues, and the illusion of fairness.36-39 Increasing representation is not sufficient—creating inclusive residency training environments is a critical parallel aim. Prioritizing diversity in dermatology residency recruitment is imperative. Creating dermatology residency positions specifically for URM residents may be an important option, as done at the University of Pennsylvania (Philadelphia, Pennsylvania) and Duke University (Durham, North Carolina).
• Create effective programs for URM mentorship. Due to the competitive nature of dermatology residency, the need for mentors in dermatology is critically vital for URM medical students, especially those without a home dermatology program at their medical school. Further development of formal mentorship and pipeline programs is essential at both the local and national levels. Some national examples of these initiatives include diversity mentorship programs offered by the American Academy of Dermatology, Skin of Color Society, Women’s Dermatologic Society, and Student National Medical Association. Many institutional programs also offer invaluable opportunities, such as the summer research fellowship at the University of California, San Francisco (UCSF); visiting clerkship grants for URMs at the University of Pennsylvania (Philadelphia, Pennsylvania) and Johns Hopkins University (Baltimore, Maryland); and integrated programs, such as the Visiting Elective Scholarship Program at UCSF, which provides funding and faculty mentorship for URM students completing an away rotation at UCSF.
• Establish longitudinal skin-of-color curricula and increased opportunities for research. More robust SOC training may lead to an increasingly diverse workforce. It is important that medical student and dermatology resident and fellow education include training on SOC to ensure high-quality care to diverse patient populations, which also may enhance the knowledge of trainees, encourage clinical and research interest in this field, and reduce health care disparities. Increasing research opportunities and offering formalized longitudinal training in SOC as well as incorporating more diverse images in medical school education may foster greater interest in this field at a time when trainees are establishing their career interests. At present, there is considerable room for improvement. Nijhawan et al40 surveyed 63 dermatology chief residents and 41 program directors and found only 14.3% and 14.6%, respectively, reported having an expert who conducts clinic specializing in SOC. Only 52.4% and 65.9% reported having didactic sessions or lectures focused on SOC diseases, and 30.2% and 12.2% reported having a dedicated rotation for residents to gain experience in SOC.40 A more recent study showed that when faculty were asked to incorporate more SOC content into lectures, the most commonly identified barrier to implementation was a lack of SOC images.41 Additionally, there remains a paucity of published research on this topic, with SOC articles representing only 2.7% of the literature.42 These numbers demonstrate the continued need for a more inclusive and comprehensive curriculum in dermatology residency programs and more robust funding for SOC research.
• Recruit and support URM faculty. Increasing diversity in dermatology residency programs likely will increase the number of potential URMs pursuing additional fellowship training and academic dermatology with active career mentorship and support. In addition, promoting faculty retention by combatting the progressive loss of URMs at all faculty levels is paramount. Mentorship for URM physicians has been shown to play a key role in the decision to pursue academic medicine as well as academic productivity and job satisfaction.43,44 The visibility, cultural competency, clinical work, academic productivity, and mentorship efforts that URM faculty provide are essential to enhancing patient care, teaching diverse groups of learners, and recruiting more diverse trainees. Protected time to participate in professional development opportunities has been shown to improve recruitment and retention of URM faculty and offer additional opportunities for junior faculty to find mentors.35,36 Incentivizing clinical care of underserved populations also may augment financial stability for URM physicians who choose to care for these patients. Finally, diversity work and community service should be legitimized and count toward faculty promotion.
Conclusion
There are numerous factors that contribute to the leaky pipeline in dermatology (eFigure). Many challenges that are unique to the URM population disadvantage these students from entering medical school, applying to dermatology residency, matching into dermatology fellowships, pursuing and staying in faculty positions, and achieving faculty advancement into leadership positions. With each progressive step along this trajectory, there is less minority representation. All dermatologists, regardless of race/ethnicity, need to play an active role and must prioritize diversity, equity, and inclusion efforts at all levels of education and training for the betterment of the specialty.

With a majority-minority population expected in the United States by 2044, improving diversity and cultural competency in the dermatology workforce is now more important than ever. A more diverse workforce increases the cultural competence of all providers, provides greater opportunities for mentorship and sponsorship of underrepresented minority (URM) trainees, establishes a more inclusive environment for learners, and enhances the knowledge and productivity of the workforce.1-3 Additionally, it is imperative to address clinical care disparities seen in minority patients in dermatology, including treatment of skin cancer, psoriasis, acne, atopic dermatitis, and other diseases.4-7
Despite the attention that has been devoted to improving diversity in medicine,8-10 dermatology remains one of the least diverse specialties, prompting additional calls to action within the field.11 Why does the lack of diversity still exist in dermatology, and what is the path to correcting this problem? In this article, we review the evidence of diversity barriers at different stages of medical education training that may impede academic advancement for minority learners pursuing careers in dermatology.
Undergraduate Medical Education
The term leaky pipeline refers to the progressive decline in the number of URMs along a given career path, including in dermatology. The Association of American Medical Colleges defines URMs as racial/ethnic populations that are “underrepresented in the medical profession relative to their numbers in the general population.”9 The first leak in the pipeline is that URMs are not applying to medical school. From 2002 and 2017, rates of both application and matriculation to medical school were lower by 30% to 70% in URM groups compared to White students, including Hispanic, Black, and American Indian/Alaska Native students.12,13 The decision not to apply to medical school was greater in URM undergraduate students irrespective of scholastic ability as measured by SAT scores.14
A striking statistic is that the number of Black men matriculating into medical school in 2014 was less than it was in 1978 despite the increase in the number of US medical schools and efforts to recruit more diverse student populations. The Association of American Medical Colleges identified potential reasons for this decline, including poor early education, lack of mentorship, negative perceptions of Black men due to racial stereotypes, and lack of financial and academic resources to support the application process.8,13,15-17 Implicit racial bias by admission committees also may play a role.
Medical School Matriculation and Applying to Dermatology Residency
There is greater representation of URM students in medical school than in dermatology residency, which means URM students are either not applying to dermatology programs or they are not matching into the specialty. In the Electronic Residency Application Service’s 2016-2017 application cycle (N=776), there were 76 (9.8%) URM dermatology residency applicants.18 In 2018, there was a notable decline in representation of Black students among residency applicants (4.9%) to matched residents (3.7%), and there were only 133 (9.3%) URM dermatology residents in total (PGY2-PGY4 classes).19 The lack of exposure to medical subspecialties and the recommendation by medical schools for URM medical students to pursue careers in primary care have been cited as reasons that these students may not apply to residency programs in specialty care.20,21 The presence of an Accreditation Council for Graduate Medical Education dermatology residency program, fellowships, and dermatology interest groups at their medical schools correlated with higher proportions of URM students applying to dermatology programs.20
Underrepresented minority students face critical challenges during medical school, including receiving lower grades in both standardized and school-designated assessments and clerkship grades.21,22 A 2019 National Board of Medical Examiners study found that Hispanic and Black test takers scored 12.1 and 16.6 points lower than White men, respectively, on the
A recent cross-sectional study showed that lack of equitable resources, lack of support, financial constrictions, and lack of group identity were 4 barriers to URM students matching into dermatology.26 Dermatology is a competitive specialty with the highest median Electronic Residency Application Service applications submitted per US applicant (n=90)27 and an approximate total cost per US applicant of $10,781.28,29 Disadvantaged URM applicants noted relying on loans while non-URM applicants cited family financial support as being beneficial.26 In addition, an increasing number of applicants take gap years for research, which pose additional costs for finances and resources. In contrast, mentorship and participation in pipeline/enrichment programs were factors associated with URM students matching into dermatology.26
Dermatology Residency and the Transition to Advanced Dermatology Fellowships
Similar to the transition from medical school into dermatology residency, URM dermatology residents are either not applying to fellowships or are not getting in. In the 2018-2019 academic year, there were no Black, Hispanic, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native Mohs micrographic surgery and dermatologic oncology fellows.19 Similarly, there were no Black, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native dermatopathology fellows. There were 4 (6%) Hispanic dermatopathology fellows.19
There also is marked underrepresentation of minority groups—and minimal growth over time—in the dermatology procedural subspecialty. Whereas the percentage of female Mohs surgeons increased considerably from 1985 to 2005 (12.7% to 40.9%, respectively), the percentage of URM Mohs surgeons remained steady from 4.2% to 4.6%, respectively, and remained at 4.5% in 2014.30
There are no available data on the race/ethnicity of fellowship applicants, as these demographic data for the application process have not been consistently or traditionally collected. The reasons why there are so few URM dermatology fellows is not known; whether this is due to a lack of mentorship or whether other factors lead to residents not applying for advanced training needs further study. Financial factors related to prolonged training, which include lower salaries and delayed loan repayment, may present barriers to applying to fellowships.
Lack of URM Academic Faculty in Dermatology
At the academic faculty level, URM representation continues to worsen. Lett et al31 found that there is declining racial and ethnic representation in clinical academic medicine relative to US census data for 16 US medical specialties, including dermatology, with growing underrepresentation of Black and Hispanic faculty at the associate professor and full professor levels and underrepresentation in all faculty ranks. From 1970 to 2018, URM faculty in dermatology only increased from 4.8% to 7.4%, respectively. Non-URM female and male faculty members increased by 13.8 and 10.8 faculty members per year, respectively, while URM female and male faculty members increased by 1.2 and 0.8 faculty members per year, respectively.32
Underrepresentation of minorities seen in dermatology faculty may result from clinical demands, minority taxation (defined as the extensive service requirements uniquely experienced by URM faculty to disproportionately serve as representatives on academic committees and to mentor URM students), and barriers to academic promotion, which are challenges uniquely encountered by URMs in academic dermatology.33 Increased clinical demand may result from the fact that URM physicians are more likely to care for underserved populations, those of lower socioeconomic status, non-English–speaking patients, those on Medicaid, and those who are uninsured, which may impact renumeration. Minority tax experienced by URM faculty includes mentoring URM medical students, providing cultural expertise to departments and institutions, and participating in community service projects and outreach programs. Specifically, many institutional committees require the participation of a URM member, resulting in URM faculty members experiencing higher committee service burden. Many, if not all, of these responsibilities often are not compensated through salary or academic promotion.
A Call to Action
There are several steps that can be taken to create a pathway to dermatology that is inclusive, flexible, and supportive of URMs.
• Increase early exposure to dermatology in medical school. Early exposure and mentorship opportunities are associated with higher rates of students pursuing specialty field careers.34 Increased early opportunities allow for URM students to consider and explore a career in dermatology; receive mentorship; and ensure that dermatology, including topics related to skin of color (SOC), is incorporated into their learning. The American Academy of Dermatology has contributed to these efforts by its presence at every national meeting of the Student National Medical Association and Latino Medical Student Association, as well as its involvement with Nth Dimensions, which offers various educational opportunities for URM medical students.
• Implement equitable grading and holistic review processes in medical school. Racial/ethnic differences in clinical grading and standardized test scores in medical school demonstrate why holistic review of dermatology residency applicants is needed and why other metrics such as USMLE scores and AΩA status should be de-emphasized or eliminated when evaluating candidates. To support equity, many medical schools have eliminated honors grading, and some schools have eliminated AΩA distinction.
• Increase diversity of dermatology residents and residency programs. Implicit bias training for a medical school admissions committee has been shown to increase diversity in medical school enrollment.35 Whether implicit bias training and other diversity training may benefit dermatology residency selection must be examined, including study of unintended consequences, such as reduced diversity, increased microaggressions toward minority colleagues, and the illusion of fairness.36-39 Increasing representation is not sufficient—creating inclusive residency training environments is a critical parallel aim. Prioritizing diversity in dermatology residency recruitment is imperative. Creating dermatology residency positions specifically for URM residents may be an important option, as done at the University of Pennsylvania (Philadelphia, Pennsylvania) and Duke University (Durham, North Carolina).
• Create effective programs for URM mentorship. Due to the competitive nature of dermatology residency, the need for mentors in dermatology is critically vital for URM medical students, especially those without a home dermatology program at their medical school. Further development of formal mentorship and pipeline programs is essential at both the local and national levels. Some national examples of these initiatives include diversity mentorship programs offered by the American Academy of Dermatology, Skin of Color Society, Women’s Dermatologic Society, and Student National Medical Association. Many institutional programs also offer invaluable opportunities, such as the summer research fellowship at the University of California, San Francisco (UCSF); visiting clerkship grants for URMs at the University of Pennsylvania (Philadelphia, Pennsylvania) and Johns Hopkins University (Baltimore, Maryland); and integrated programs, such as the Visiting Elective Scholarship Program at UCSF, which provides funding and faculty mentorship for URM students completing an away rotation at UCSF.
• Establish longitudinal skin-of-color curricula and increased opportunities for research. More robust SOC training may lead to an increasingly diverse workforce. It is important that medical student and dermatology resident and fellow education include training on SOC to ensure high-quality care to diverse patient populations, which also may enhance the knowledge of trainees, encourage clinical and research interest in this field, and reduce health care disparities. Increasing research opportunities and offering formalized longitudinal training in SOC as well as incorporating more diverse images in medical school education may foster greater interest in this field at a time when trainees are establishing their career interests. At present, there is considerable room for improvement. Nijhawan et al40 surveyed 63 dermatology chief residents and 41 program directors and found only 14.3% and 14.6%, respectively, reported having an expert who conducts clinic specializing in SOC. Only 52.4% and 65.9% reported having didactic sessions or lectures focused on SOC diseases, and 30.2% and 12.2% reported having a dedicated rotation for residents to gain experience in SOC.40 A more recent study showed that when faculty were asked to incorporate more SOC content into lectures, the most commonly identified barrier to implementation was a lack of SOC images.41 Additionally, there remains a paucity of published research on this topic, with SOC articles representing only 2.7% of the literature.42 These numbers demonstrate the continued need for a more inclusive and comprehensive curriculum in dermatology residency programs and more robust funding for SOC research.
• Recruit and support URM faculty. Increasing diversity in dermatology residency programs likely will increase the number of potential URMs pursuing additional fellowship training and academic dermatology with active career mentorship and support. In addition, promoting faculty retention by combatting the progressive loss of URMs at all faculty levels is paramount. Mentorship for URM physicians has been shown to play a key role in the decision to pursue academic medicine as well as academic productivity and job satisfaction.43,44 The visibility, cultural competency, clinical work, academic productivity, and mentorship efforts that URM faculty provide are essential to enhancing patient care, teaching diverse groups of learners, and recruiting more diverse trainees. Protected time to participate in professional development opportunities has been shown to improve recruitment and retention of URM faculty and offer additional opportunities for junior faculty to find mentors.35,36 Incentivizing clinical care of underserved populations also may augment financial stability for URM physicians who choose to care for these patients. Finally, diversity work and community service should be legitimized and count toward faculty promotion.
Conclusion
There are numerous factors that contribute to the leaky pipeline in dermatology (eFigure). Many challenges that are unique to the URM population disadvantage these students from entering medical school, applying to dermatology residency, matching into dermatology fellowships, pursuing and staying in faculty positions, and achieving faculty advancement into leadership positions. With each progressive step along this trajectory, there is less minority representation. All dermatologists, regardless of race/ethnicity, need to play an active role and must prioritize diversity, equity, and inclusion efforts at all levels of education and training for the betterment of the specialty.

- Dixon G, Kind T, Wright J, et al. Factors that influence the choice of academic pediatrics by underrepresented minorities. Pediatrics. 2019;144:E20182759. doi:10.1542/peds.2018-2759
- Yehia BR, Cronholm PF, Wilson N, et al. Mentorship and pursuit of academic medicine careers: a mixed methods study of residents from diverse backgrounds. BMC Med Educ. 2014:14:2-26. doi:10.1186/1472-6920-14-26
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145. doi:10.1001/jama.300.10.1135
- Hsu DY, Gordon K, Silverberg JI. The patient burden of psoriasis in the United States. J Am Acad Dermatol. 2016;75:33-41. doi:10.1016/j.jaad.2016.03.048
- Silverberg JI. Racial and ethnic disparities in atopic dermatitis. Curr Dermatol Rep. 2015;4:44-48.
- Buster KJ, Sevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Smedley BD, Stith AY, Colburn L, et al. The Right Thing To Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions. National Academies Press; 2001.
- Association of American Medical Colleges. Minorities in medical education: fact and figures 2019. Accessed December 9, 2021. https://www.aamc.org/datareports/workforce/report/diversity-medicine-facts-and-figures-2019
- Liaison Committee on Medical Education (LCME) standards on diversity. University of South Florida Health website. Accessed December 9, 2021. https://health.usf.edu/~/media/Files/Medicine/MD%20Program/Diversity/LCMEStandardsonDiversity1.ashx?la=en
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500. doi:10.1001/jamadermatol.2017.0296
- Lett LA, Murdock HM, Orji W, et al. Trends in racial/ethnic representation among US medical students. JAMA Netw Open. 2019;2:e1910490. doi:10.1001/jamanetworkopen.2019.10490
- Association of American Medical Colleges. Altering the course: Black males in medicine. Published 2015. Accessed December 8, 2021. https://store.aamc.org/downloadable/download/sample/sample_id/84/
- Barr DA, Gonzalez ME, Wanat SF. The leaky pipeline: factors associated with early decline in interest in premedical studies among underrepresented minority undergraduate students. Acad Med. 2008;83:5:503-511. doi:10.1097/ACM.0b013e31816bda16
- Flores RL. The rising gap between rich and poor: a look at the persistence of educational disparities in the United States and why we should worry. Cogent Soc Sci. 2017;3:1323698.
- Jackson D. Why am I behind? an examination of low income and minority students’ preparedness for college. McNair Sch J. 2012;13:121-138.
- Rothstein R. The racial achievement gap, segregated schools, andsegregated neighborhoods: a constitutional insult. Race Soc Probl. 2015;7:21-30.
- Association of American Medical Colleges. Residency Applicants From US MD Granting Medical Schools to ACGME-Accredited Programs by Specialty and Race/Ethnicity. Association of American Medical Colleges; 2017.
- Brotherton SE, Etzel SL. Graduate medical education, 2018-2019. JAMA. 2019;322:996-1016. doi:10.1001/jama.2019.10155
- Barnes LA, Bae GH, Nambudiri V. Sex and racial/ethnic diversity of US medical students and their exposure to dermatology programs. JAMA Dermatol. 2019;155:490-491. doi:10.1001/jamadermatol.2018.5025
- Soliman YS, Rzepecki AK, Guzman AK. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Low D, Pollack SW, Liao Z, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance and United States medical licensing examination scores. Acad Med. 2019;94;364-370. doi:10.1097/ACM.0000000000002366
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the alpha omega honor society. JAMA Intern Med. 2017;177:659-665. doi:10.1001/jamainternmed.2016.9623
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey [published online March 17, 2014]. Dermatol Res Pract. doi:10.1155/2014/692760
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Results of the 2019 NRMP applicant survey by preferred specialty and applicant type. National Resident Matching Program website. Published July 2019. Accessed December 8, 2021. https://www.nrmp.org/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Polacco MA, Lally J, Walls A, et al. Digging into debt: the financial burden associated with the otolaryngology match. Otolaryngol Head Neck Surg. 2017;12:1091-1096. doi:10.1177/0194599816686538
- Feng H, Feng PW, Geronemus RG. Diversity in the US Mohs micrographic surgery workforce. Dermatol Surg. 2020:46:1451-1455. doi:10.1097/DSS.0000000000002080
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS ONE. 2018;13:e0207274. doi:10.1371/journal.pone.020727432. Xierali IM, Nivet MA, Pandya AG. US Dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970-2018. JAMA Dermatol. 2020;156:280-287. doi:10.1001/jamadermatol.2019.4297
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Intl J Womens Dermatol. 2020;6:57-60. doi:10.1016/j.ijwd.2019.09.009
- Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86:2335-2338. doi:10.2106/00004623-200410000-00031
- Capers Q, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369. doi:10.1097/ACM.0000000000001388
- Dobbin F, Kalev A. Why diversity programs fail. Harvard Business Rev. 2016;52-60. Accessed December 8, 2021. https://hbr.org/2016/07/why-diversity-programs-fail
- Kalev A, Dobbin F, Kelly E. Best practices or best guesses? assessing the efficacy of corporate affirmative action and diversity policies. Am Sociol Rev. 2006;71:589-617.
- Sanchez JI, Medkik N. The effects of diversity awareness training on differential treatment. Group Organ Manag. 2004;29:517-536.
- Kaiser CR, Major B, Jurcevic I, et al. Presumed fair: ironic effects of organizational diversity structures. J Pers Soc Psychol. 2013;104:504-519. doi:10.1037/a0030838
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-617.
- Jia JL, Gordon JS, Lester JC, et al. Integrating skin of color and sexual and gender minority content into dermatology residency curricula: a prospective program initiative [published online April 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.018
- Amuzie AU, Lia JL, Taylor SC, et al. Skin of color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Beech BM, Calles-Escandon J, Hairston KC, et al. Mentoring programs for underrepresented minority faculty in academic medical center: a systematic review of the literature. Acad Med. 2013;88:541-549. doi:10.1097/ACM.0b013e31828589e3
- Daley S, Wingard DL, Reznik V. Improving the retention of underrepresented minority faculty in academic medicine. J Natl Med Assoc. 2006;98:1435-1440. doi:10.1016/s0027-9684(15)31449-8
- Association of American Medical Colleges. US medical school faculty by sex, race/ethnicity, rank, and department, 2019. Published December 31, 2019. Accessed December 20, 2021. https://www.aamc.org/media/8476/download?attachment
- Dixon G, Kind T, Wright J, et al. Factors that influence the choice of academic pediatrics by underrepresented minorities. Pediatrics. 2019;144:E20182759. doi:10.1542/peds.2018-2759
- Yehia BR, Cronholm PF, Wilson N, et al. Mentorship and pursuit of academic medicine careers: a mixed methods study of residents from diverse backgrounds. BMC Med Educ. 2014:14:2-26. doi:10.1186/1472-6920-14-26
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145. doi:10.1001/jama.300.10.1135
- Hsu DY, Gordon K, Silverberg JI. The patient burden of psoriasis in the United States. J Am Acad Dermatol. 2016;75:33-41. doi:10.1016/j.jaad.2016.03.048
- Silverberg JI. Racial and ethnic disparities in atopic dermatitis. Curr Dermatol Rep. 2015;4:44-48.
- Buster KJ, Sevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Smedley BD, Stith AY, Colburn L, et al. The Right Thing To Do, The Smart Thing to Do: Enhancing Diversity in the Health Professions. National Academies Press; 2001.
- Association of American Medical Colleges. Minorities in medical education: fact and figures 2019. Accessed December 9, 2021. https://www.aamc.org/datareports/workforce/report/diversity-medicine-facts-and-figures-2019
- Liaison Committee on Medical Education (LCME) standards on diversity. University of South Florida Health website. Accessed December 9, 2021. https://health.usf.edu/~/media/Files/Medicine/MD%20Program/Diversity/LCMEStandardsonDiversity1.ashx?la=en
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500. doi:10.1001/jamadermatol.2017.0296
- Lett LA, Murdock HM, Orji W, et al. Trends in racial/ethnic representation among US medical students. JAMA Netw Open. 2019;2:e1910490. doi:10.1001/jamanetworkopen.2019.10490
- Association of American Medical Colleges. Altering the course: Black males in medicine. Published 2015. Accessed December 8, 2021. https://store.aamc.org/downloadable/download/sample/sample_id/84/
- Barr DA, Gonzalez ME, Wanat SF. The leaky pipeline: factors associated with early decline in interest in premedical studies among underrepresented minority undergraduate students. Acad Med. 2008;83:5:503-511. doi:10.1097/ACM.0b013e31816bda16
- Flores RL. The rising gap between rich and poor: a look at the persistence of educational disparities in the United States and why we should worry. Cogent Soc Sci. 2017;3:1323698.
- Jackson D. Why am I behind? an examination of low income and minority students’ preparedness for college. McNair Sch J. 2012;13:121-138.
- Rothstein R. The racial achievement gap, segregated schools, andsegregated neighborhoods: a constitutional insult. Race Soc Probl. 2015;7:21-30.
- Association of American Medical Colleges. Residency Applicants From US MD Granting Medical Schools to ACGME-Accredited Programs by Specialty and Race/Ethnicity. Association of American Medical Colleges; 2017.
- Brotherton SE, Etzel SL. Graduate medical education, 2018-2019. JAMA. 2019;322:996-1016. doi:10.1001/jama.2019.10155
- Barnes LA, Bae GH, Nambudiri V. Sex and racial/ethnic diversity of US medical students and their exposure to dermatology programs. JAMA Dermatol. 2019;155:490-491. doi:10.1001/jamadermatol.2018.5025
- Soliman YS, Rzepecki AK, Guzman AK. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Low D, Pollack SW, Liao Z, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance and United States medical licensing examination scores. Acad Med. 2019;94;364-370. doi:10.1097/ACM.0000000000002366
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the alpha omega honor society. JAMA Intern Med. 2017;177:659-665. doi:10.1001/jamainternmed.2016.9623
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey [published online March 17, 2014]. Dermatol Res Pract. doi:10.1155/2014/692760
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Results of the 2019 NRMP applicant survey by preferred specialty and applicant type. National Resident Matching Program website. Published July 2019. Accessed December 8, 2021. https://www.nrmp.org/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Polacco MA, Lally J, Walls A, et al. Digging into debt: the financial burden associated with the otolaryngology match. Otolaryngol Head Neck Surg. 2017;12:1091-1096. doi:10.1177/0194599816686538
- Feng H, Feng PW, Geronemus RG. Diversity in the US Mohs micrographic surgery workforce. Dermatol Surg. 2020:46:1451-1455. doi:10.1097/DSS.0000000000002080
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS ONE. 2018;13:e0207274. doi:10.1371/journal.pone.020727432. Xierali IM, Nivet MA, Pandya AG. US Dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970-2018. JAMA Dermatol. 2020;156:280-287. doi:10.1001/jamadermatol.2019.4297
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Intl J Womens Dermatol. 2020;6:57-60. doi:10.1016/j.ijwd.2019.09.009
- Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86:2335-2338. doi:10.2106/00004623-200410000-00031
- Capers Q, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369. doi:10.1097/ACM.0000000000001388
- Dobbin F, Kalev A. Why diversity programs fail. Harvard Business Rev. 2016;52-60. Accessed December 8, 2021. https://hbr.org/2016/07/why-diversity-programs-fail
- Kalev A, Dobbin F, Kelly E. Best practices or best guesses? assessing the efficacy of corporate affirmative action and diversity policies. Am Sociol Rev. 2006;71:589-617.
- Sanchez JI, Medkik N. The effects of diversity awareness training on differential treatment. Group Organ Manag. 2004;29:517-536.
- Kaiser CR, Major B, Jurcevic I, et al. Presumed fair: ironic effects of organizational diversity structures. J Pers Soc Psychol. 2013;104:504-519. doi:10.1037/a0030838
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-617.
- Jia JL, Gordon JS, Lester JC, et al. Integrating skin of color and sexual and gender minority content into dermatology residency curricula: a prospective program initiative [published online April 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.018
- Amuzie AU, Lia JL, Taylor SC, et al. Skin of color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Beech BM, Calles-Escandon J, Hairston KC, et al. Mentoring programs for underrepresented minority faculty in academic medical center: a systematic review of the literature. Acad Med. 2013;88:541-549. doi:10.1097/ACM.0b013e31828589e3
- Daley S, Wingard DL, Reznik V. Improving the retention of underrepresented minority faculty in academic medicine. J Natl Med Assoc. 2006;98:1435-1440. doi:10.1016/s0027-9684(15)31449-8
- Association of American Medical Colleges. US medical school faculty by sex, race/ethnicity, rank, and department, 2019. Published December 31, 2019. Accessed December 20, 2021. https://www.aamc.org/media/8476/download?attachment
Practice Points
- Dermatology remains the second least diverse specialty in medicine, which has important implications for the workforce and clinical excellence of the specialty.
- Barriers presenting at different stages of medical education and training result in the loss of underrepresented minority (URM) learners pursuing or advancing careers in dermatology.
- Understanding these barriers is the first step to creating and implementing important structural changes to the way we mentor, teach, and support URM students in the specialty.
Skin of Color in Preclinical Medical Education: A Cross-Institutional Comparison and A Call to Action
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.
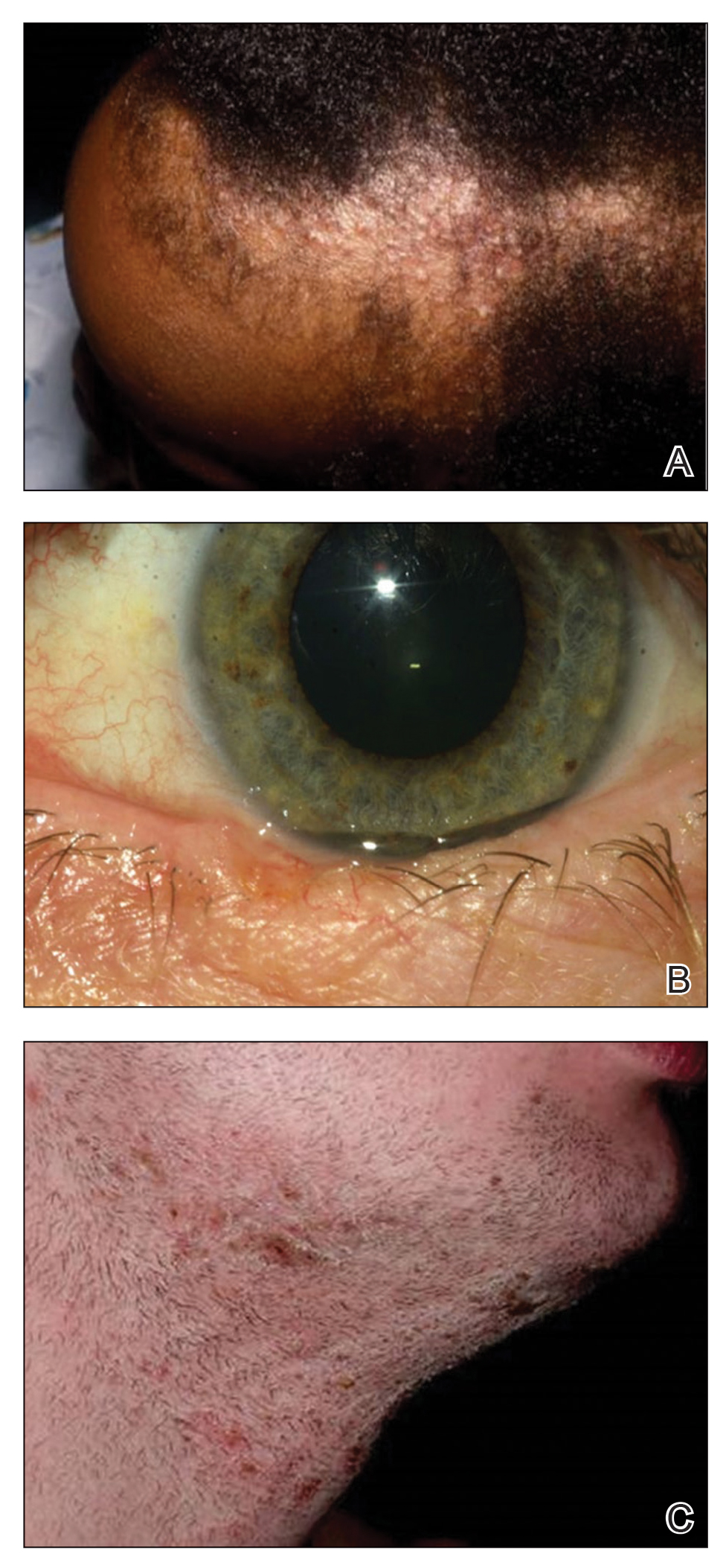
We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.
Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).
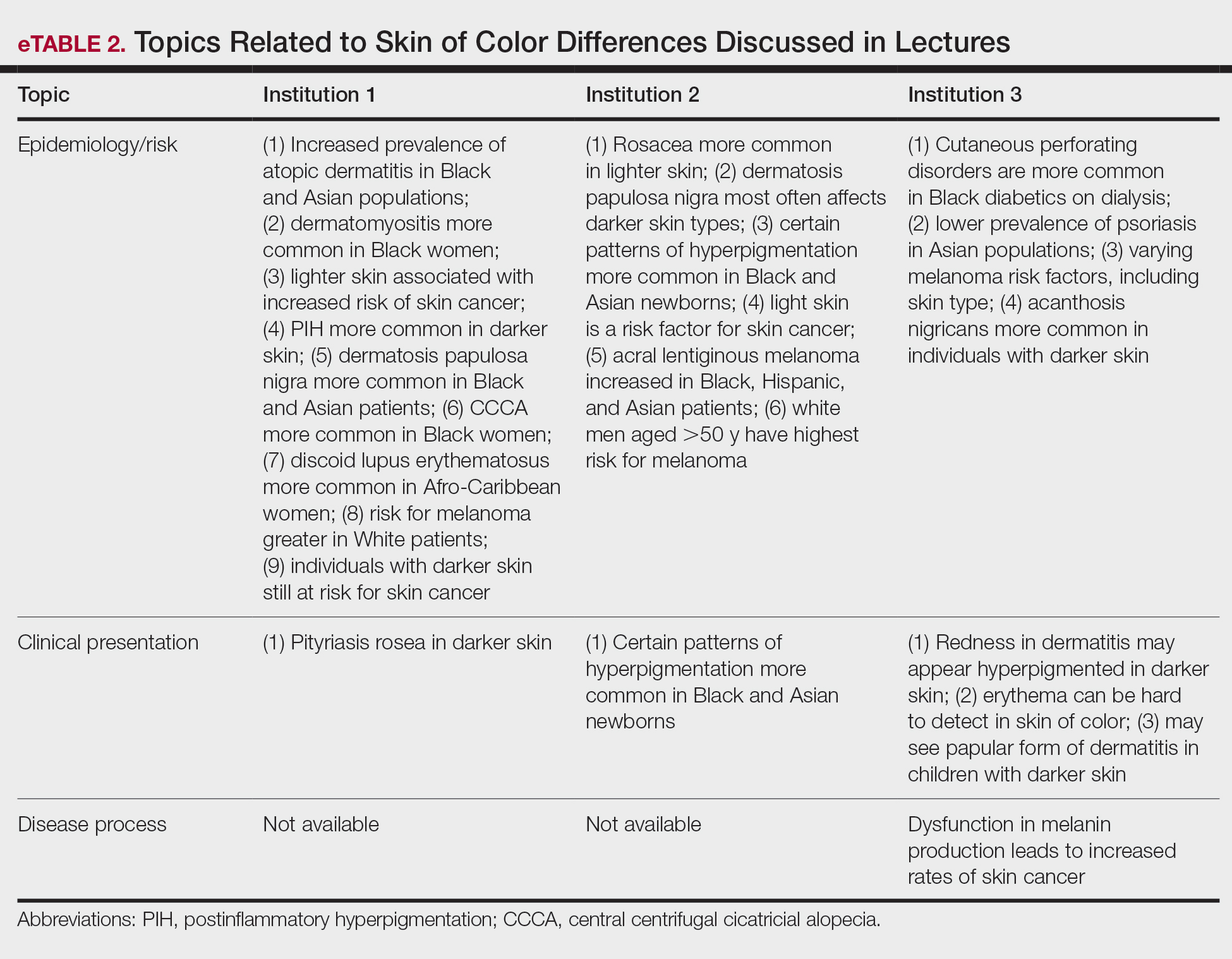
Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.

We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.

Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).

Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.

We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.

Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).

Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
Practice Points
- The United States rapidly is becoming a country in which the majority of citizens will have skin of color.
- Our study results strongly suggest that skin of color may be seriously underrepresented in medical education and can guide modifications to preclinical dermatology medical education to develop a more comprehensive and inclusive curriculum.
- Efforts should be made to increase images and discussion of skin of color in preclinical didactics.
Counseling About Traction Alopecia: A "Compliment, Discuss, and Suggest" Method
Traction alopecia (TA)--one of the most common types of hair loss in Black women (although not exclusive to Black women)--is reversible when early corrective measures are taken; if chronic tension continues, however, permanent scarring alopecia ensues. Dermatologists can prevent worsening of this distressing hair loss. Due to a dearth of training among dermatologists in conditions occurring in patients with tightly coiled hair, it is imperative to add practical methods to the body of dermatology literature, with the goal of enhancing cultural humility.
Hairstyling among Black women often is a lengthy process and often results in relationship bonding with the hair care giver, in turn imparting hair care traditions to the next generation. Therefore, a well-received discussion about TA prevention not only has an impact on the patient but potentially on a multigenerational family of women and friends. We present a memory aid for discussing TA, with a focus on cultural humility and patient-centered communication.
Factors contributing to the risk of TA are hairstyles and hair care practices commonly used in Black individuals, including braids, locs, weaves, wigs, and chemical straightening.1 These styles often are worn to increase hair manageability or as a creative expression of beauty.
Discussing TA can be distressing for physicians and patients, especially in the setting of hair texture discordance. In a study that surveyed Black patients' perception of their dermatologic care both in and outside of a skin of color clinic, 71% of respondents (12/17) said that they prefer a race-concordant dermatologist. Some respondents reported that non-skin of color clinic dermatologists examined their hair with the end of a pencil or not at all; patients interpreted these interactions as disrespectful and racially insensitive.2 Another study found that only 30.2% (19/63) of dermatology chief residents and 12.2% (5/41) of program directors reported a specific rotation during which residents gained experience treating skin of color patients.3
Due to a paucity of training in diagnosing and treating patients with tightly coiled hair who experience hair loss, some physicians might feel uncomfortable caring for patients who have tightly coiled hair. Although many Black patients prefer to see a race-concordant dermatologist because of their perceived cultural competence and shared experience, there is a paucity of Black dermatologists to see all patients who have tightly coiled hair.4 Therefore, all dermatologists should become skilled and comfortable discussing and treating TA in patients with all hair types.
METHOD FOR COUNSELING
The following scenarios are a guide to begin closing the competency gap in counseling about TA, using a "compliment, discuss, and suggest" method.
Scenario 1
A Black woman presents with a concern of "thinning edges" (a popular term on social media for TA). A hair-discordant dermatologist tells her, first, that she has TA caused by wearing tight hairstyles and, second, that the treatment is to stop wearing tight braids and weaves and to discontinue chemical relaxers. The dermatologist then leaves the room.
The Patient's Perspective
It is not uncommon for the patient to have feelings of frustration about how they will style their hair, especially if they are unfamiliar with caring for their hair in its natural state.5 Also, they might have feelings of dismay that the loving childhood hair care giver, often their mother or grandmother, unintentionally harmed them with a tight style. They also might feel betrayed by their hairstylist, who might not have encouraged them to see a dermatologist, or who continued to oblige their request for a high-risk hairstyle. The patient might feel uncomfortable communicating the dermatologist's new recommendations to their hair care team, who also are part of her emotional support system. The patient also might think that the hair-discordant dermatologist has no idea what they "go through" with their hair.
"Compliment, Discuss, and Suggest" Counseling
Traction alopecia is caused by tight hairstyles that often hurt when they are put in as tight braids, weaves, and ponytails.6 Risk increases if tight styles are applied to chemically straightened hair.1 Braids, sew-in weaves, and wigs with adhesive sometimes are referred to as protective styles. However, these styles can still lead to TA due to excessive tension.
- Compliment: "Your hair looks great. I know that you get many compliments."
- Discuss: "However, some of the styles might be increasing your risk for hair loss. Our goal is to preserve as many of your follicles as possible."
- Suggest: "Let's start by loosening the hairstyle if it is painful when being applied. Pain means inflammation, which can lead to scarring of hair follicles and worsening of hair loss."
Using pronouns such as we, us, and our is intentional. Doing so signals that the dermatologist is a partner with the patient in the treatment of TA. Starting with a simple initial recommendation gives the patient time to process the common thoughts highlighted in The Patient's Perspective section.6
Scenario 2
A Black child (we'll call her "Janet") is accompanied by her mother for follow-up of mild atopic dermatitis on the body and scalp. When the dermatologist examines the patient's scalp, they note that she has the fringe sign--retained short hairs along the frontal hairline--that is consistent with TA. Janet's hair is adorned with 2 tight ponytails in the front with colorful decorative balls on ponytail ties, barrettes, and 6 cornrow braids in the back with plastic beads on the ends. The dermatologist counsels about the atopic dermatitis and leaves the room.
"Compliment, Discuss, and Suggest" Counseling
The use of tight decorative balls on ponytail ties and numerous plastic beads increases the amount of tension and weight on the hair, which may lead to a higher risk for developing traction alopecia.6 It is quite common for children of African descent to wear hair adornments. Proper counseling regarding their use and possible implications is essential.
- Compliment: "You're doing a great job controlling the atopic dermatitis, which can cause Janet's scalp to be dry. Also, her hair is beautiful--it looks like you spent a lot of time on her hair. And Janet, I like the color of your barrettes."
- Discuss: "Mom, I just noticed that a few areas look tight. Let's look together." (The dermatologist points out areas where the scalp is tented upward due to traction, follicular pustules or papules, or the frontal fringe sign.) "I'm on a mission to #savetheedges because we want Janet to grow up with full edges." (Again, loss of "edges" refers to TA.)
- Suggest: "When you do Janet's hair, it's OK if every hair is not in place. In fact, making styles look and feel 1 or 2 weeks old will lessen tension on the scalp. Remove Janet's hair ties to release tension when she is at home and while she's sleeping, if possible. Every minute that the hair is loose really does help."6
The Parent's Perspective
All parents take pride in their children. In some Black communities, mothers are judged by how well they manage and style their children's hair. Some people might even suggest that parents of children with nonstyled, tightly coiled hair are not fit parents. Anthropologist Sylvia Boone, PhD, found that among the Mende tribe in Sierra Leone, "unkempt, 'neglected,' or 'messy' hair implied that a woman either had loose morals or was insane."7
Braids are commonly worn by people of African heritage for a variety of reasons, including ease of manageability, to decrease daily hairstyling time, and as an expression of creativity. Intricate neat hairstyles, despite the risk of pain and TA, are perceived as a sign that the child is cared for and loved.6
FINAL THOUGHTS
Patient-centered communication is associated with the patient trusting the physician, which is especially important in race-discordant physician-patient relationships. A study found that patient-physician race discordance led to shorter visits, a lower rating of patient affect, and less shared decision-making.8 Moreover, in a study of primary care clinicians, implicit bias was found to affect communication patterns and social interactions, impacting patient outcomes. Downstream effects of racial bias resulted in less speaking, smiling, and social comments when interacting with Black patients.9
These findings highlight the need to address interpersonal barriers to effective communication in race-discordant patient-physician dyads. A history of segregated neighborhoods and schools might contribute to structural barriers, resulting in lack of familiarity with cultural norms outside one's culture, which might globally perpetuate poor communication and patient outcomes.
The "compliment, discuss, and suggest" method might lead to more positive physician-patient encounters by having the dermatologist focus on empathetically understanding the patient's perspective.10 Effective communication, understanding cultural hair care practices, and a thorough scalp examination are paramount for patients with tightly coiled hair.11 Early intervention in TA is crucial and involves partnering with patients and parents to amend high-risk hairstyling routines with cultural humility.
Traction alopecia (TA)--one of the most common types of hair loss in Black women (although not exclusive to Black women)--is reversible when early corrective measures are taken; if chronic tension continues, however, permanent scarring alopecia ensues. Dermatologists can prevent worsening of this distressing hair loss. Due to a dearth of training among dermatologists in conditions occurring in patients with tightly coiled hair, it is imperative to add practical methods to the body of dermatology literature, with the goal of enhancing cultural humility.
Hairstyling among Black women often is a lengthy process and often results in relationship bonding with the hair care giver, in turn imparting hair care traditions to the next generation. Therefore, a well-received discussion about TA prevention not only has an impact on the patient but potentially on a multigenerational family of women and friends. We present a memory aid for discussing TA, with a focus on cultural humility and patient-centered communication.
Factors contributing to the risk of TA are hairstyles and hair care practices commonly used in Black individuals, including braids, locs, weaves, wigs, and chemical straightening.1 These styles often are worn to increase hair manageability or as a creative expression of beauty.
Discussing TA can be distressing for physicians and patients, especially in the setting of hair texture discordance. In a study that surveyed Black patients' perception of their dermatologic care both in and outside of a skin of color clinic, 71% of respondents (12/17) said that they prefer a race-concordant dermatologist. Some respondents reported that non-skin of color clinic dermatologists examined their hair with the end of a pencil or not at all; patients interpreted these interactions as disrespectful and racially insensitive.2 Another study found that only 30.2% (19/63) of dermatology chief residents and 12.2% (5/41) of program directors reported a specific rotation during which residents gained experience treating skin of color patients.3
Due to a paucity of training in diagnosing and treating patients with tightly coiled hair who experience hair loss, some physicians might feel uncomfortable caring for patients who have tightly coiled hair. Although many Black patients prefer to see a race-concordant dermatologist because of their perceived cultural competence and shared experience, there is a paucity of Black dermatologists to see all patients who have tightly coiled hair.4 Therefore, all dermatologists should become skilled and comfortable discussing and treating TA in patients with all hair types.
METHOD FOR COUNSELING
The following scenarios are a guide to begin closing the competency gap in counseling about TA, using a "compliment, discuss, and suggest" method.
Scenario 1
A Black woman presents with a concern of "thinning edges" (a popular term on social media for TA). A hair-discordant dermatologist tells her, first, that she has TA caused by wearing tight hairstyles and, second, that the treatment is to stop wearing tight braids and weaves and to discontinue chemical relaxers. The dermatologist then leaves the room.
The Patient's Perspective
It is not uncommon for the patient to have feelings of frustration about how they will style their hair, especially if they are unfamiliar with caring for their hair in its natural state.5 Also, they might have feelings of dismay that the loving childhood hair care giver, often their mother or grandmother, unintentionally harmed them with a tight style. They also might feel betrayed by their hairstylist, who might not have encouraged them to see a dermatologist, or who continued to oblige their request for a high-risk hairstyle. The patient might feel uncomfortable communicating the dermatologist's new recommendations to their hair care team, who also are part of her emotional support system. The patient also might think that the hair-discordant dermatologist has no idea what they "go through" with their hair.
"Compliment, Discuss, and Suggest" Counseling
Traction alopecia is caused by tight hairstyles that often hurt when they are put in as tight braids, weaves, and ponytails.6 Risk increases if tight styles are applied to chemically straightened hair.1 Braids, sew-in weaves, and wigs with adhesive sometimes are referred to as protective styles. However, these styles can still lead to TA due to excessive tension.
- Compliment: "Your hair looks great. I know that you get many compliments."
- Discuss: "However, some of the styles might be increasing your risk for hair loss. Our goal is to preserve as many of your follicles as possible."
- Suggest: "Let's start by loosening the hairstyle if it is painful when being applied. Pain means inflammation, which can lead to scarring of hair follicles and worsening of hair loss."
Using pronouns such as we, us, and our is intentional. Doing so signals that the dermatologist is a partner with the patient in the treatment of TA. Starting with a simple initial recommendation gives the patient time to process the common thoughts highlighted in The Patient's Perspective section.6
Scenario 2
A Black child (we'll call her "Janet") is accompanied by her mother for follow-up of mild atopic dermatitis on the body and scalp. When the dermatologist examines the patient's scalp, they note that she has the fringe sign--retained short hairs along the frontal hairline--that is consistent with TA. Janet's hair is adorned with 2 tight ponytails in the front with colorful decorative balls on ponytail ties, barrettes, and 6 cornrow braids in the back with plastic beads on the ends. The dermatologist counsels about the atopic dermatitis and leaves the room.
"Compliment, Discuss, and Suggest" Counseling
The use of tight decorative balls on ponytail ties and numerous plastic beads increases the amount of tension and weight on the hair, which may lead to a higher risk for developing traction alopecia.6 It is quite common for children of African descent to wear hair adornments. Proper counseling regarding their use and possible implications is essential.
- Compliment: "You're doing a great job controlling the atopic dermatitis, which can cause Janet's scalp to be dry. Also, her hair is beautiful--it looks like you spent a lot of time on her hair. And Janet, I like the color of your barrettes."
- Discuss: "Mom, I just noticed that a few areas look tight. Let's look together." (The dermatologist points out areas where the scalp is tented upward due to traction, follicular pustules or papules, or the frontal fringe sign.) "I'm on a mission to #savetheedges because we want Janet to grow up with full edges." (Again, loss of "edges" refers to TA.)
- Suggest: "When you do Janet's hair, it's OK if every hair is not in place. In fact, making styles look and feel 1 or 2 weeks old will lessen tension on the scalp. Remove Janet's hair ties to release tension when she is at home and while she's sleeping, if possible. Every minute that the hair is loose really does help."6
The Parent's Perspective
All parents take pride in their children. In some Black communities, mothers are judged by how well they manage and style their children's hair. Some people might even suggest that parents of children with nonstyled, tightly coiled hair are not fit parents. Anthropologist Sylvia Boone, PhD, found that among the Mende tribe in Sierra Leone, "unkempt, 'neglected,' or 'messy' hair implied that a woman either had loose morals or was insane."7
Braids are commonly worn by people of African heritage for a variety of reasons, including ease of manageability, to decrease daily hairstyling time, and as an expression of creativity. Intricate neat hairstyles, despite the risk of pain and TA, are perceived as a sign that the child is cared for and loved.6
FINAL THOUGHTS
Patient-centered communication is associated with the patient trusting the physician, which is especially important in race-discordant physician-patient relationships. A study found that patient-physician race discordance led to shorter visits, a lower rating of patient affect, and less shared decision-making.8 Moreover, in a study of primary care clinicians, implicit bias was found to affect communication patterns and social interactions, impacting patient outcomes. Downstream effects of racial bias resulted in less speaking, smiling, and social comments when interacting with Black patients.9
These findings highlight the need to address interpersonal barriers to effective communication in race-discordant patient-physician dyads. A history of segregated neighborhoods and schools might contribute to structural barriers, resulting in lack of familiarity with cultural norms outside one's culture, which might globally perpetuate poor communication and patient outcomes.
The "compliment, discuss, and suggest" method might lead to more positive physician-patient encounters by having the dermatologist focus on empathetically understanding the patient's perspective.10 Effective communication, understanding cultural hair care practices, and a thorough scalp examination are paramount for patients with tightly coiled hair.11 Early intervention in TA is crucial and involves partnering with patients and parents to amend high-risk hairstyling routines with cultural humility.
Traction alopecia (TA)--one of the most common types of hair loss in Black women (although not exclusive to Black women)--is reversible when early corrective measures are taken; if chronic tension continues, however, permanent scarring alopecia ensues. Dermatologists can prevent worsening of this distressing hair loss. Due to a dearth of training among dermatologists in conditions occurring in patients with tightly coiled hair, it is imperative to add practical methods to the body of dermatology literature, with the goal of enhancing cultural humility.
Hairstyling among Black women often is a lengthy process and often results in relationship bonding with the hair care giver, in turn imparting hair care traditions to the next generation. Therefore, a well-received discussion about TA prevention not only has an impact on the patient but potentially on a multigenerational family of women and friends. We present a memory aid for discussing TA, with a focus on cultural humility and patient-centered communication.
Factors contributing to the risk of TA are hairstyles and hair care practices commonly used in Black individuals, including braids, locs, weaves, wigs, and chemical straightening.1 These styles often are worn to increase hair manageability or as a creative expression of beauty.
Discussing TA can be distressing for physicians and patients, especially in the setting of hair texture discordance. In a study that surveyed Black patients' perception of their dermatologic care both in and outside of a skin of color clinic, 71% of respondents (12/17) said that they prefer a race-concordant dermatologist. Some respondents reported that non-skin of color clinic dermatologists examined their hair with the end of a pencil or not at all; patients interpreted these interactions as disrespectful and racially insensitive.2 Another study found that only 30.2% (19/63) of dermatology chief residents and 12.2% (5/41) of program directors reported a specific rotation during which residents gained experience treating skin of color patients.3
Due to a paucity of training in diagnosing and treating patients with tightly coiled hair who experience hair loss, some physicians might feel uncomfortable caring for patients who have tightly coiled hair. Although many Black patients prefer to see a race-concordant dermatologist because of their perceived cultural competence and shared experience, there is a paucity of Black dermatologists to see all patients who have tightly coiled hair.4 Therefore, all dermatologists should become skilled and comfortable discussing and treating TA in patients with all hair types.
METHOD FOR COUNSELING
The following scenarios are a guide to begin closing the competency gap in counseling about TA, using a "compliment, discuss, and suggest" method.
Scenario 1
A Black woman presents with a concern of "thinning edges" (a popular term on social media for TA). A hair-discordant dermatologist tells her, first, that she has TA caused by wearing tight hairstyles and, second, that the treatment is to stop wearing tight braids and weaves and to discontinue chemical relaxers. The dermatologist then leaves the room.
The Patient's Perspective
It is not uncommon for the patient to have feelings of frustration about how they will style their hair, especially if they are unfamiliar with caring for their hair in its natural state.5 Also, they might have feelings of dismay that the loving childhood hair care giver, often their mother or grandmother, unintentionally harmed them with a tight style. They also might feel betrayed by their hairstylist, who might not have encouraged them to see a dermatologist, or who continued to oblige their request for a high-risk hairstyle. The patient might feel uncomfortable communicating the dermatologist's new recommendations to their hair care team, who also are part of her emotional support system. The patient also might think that the hair-discordant dermatologist has no idea what they "go through" with their hair.
"Compliment, Discuss, and Suggest" Counseling
Traction alopecia is caused by tight hairstyles that often hurt when they are put in as tight braids, weaves, and ponytails.6 Risk increases if tight styles are applied to chemically straightened hair.1 Braids, sew-in weaves, and wigs with adhesive sometimes are referred to as protective styles. However, these styles can still lead to TA due to excessive tension.
- Compliment: "Your hair looks great. I know that you get many compliments."
- Discuss: "However, some of the styles might be increasing your risk for hair loss. Our goal is to preserve as many of your follicles as possible."
- Suggest: "Let's start by loosening the hairstyle if it is painful when being applied. Pain means inflammation, which can lead to scarring of hair follicles and worsening of hair loss."
Using pronouns such as we, us, and our is intentional. Doing so signals that the dermatologist is a partner with the patient in the treatment of TA. Starting with a simple initial recommendation gives the patient time to process the common thoughts highlighted in The Patient's Perspective section.6
Scenario 2
A Black child (we'll call her "Janet") is accompanied by her mother for follow-up of mild atopic dermatitis on the body and scalp. When the dermatologist examines the patient's scalp, they note that she has the fringe sign--retained short hairs along the frontal hairline--that is consistent with TA. Janet's hair is adorned with 2 tight ponytails in the front with colorful decorative balls on ponytail ties, barrettes, and 6 cornrow braids in the back with plastic beads on the ends. The dermatologist counsels about the atopic dermatitis and leaves the room.
"Compliment, Discuss, and Suggest" Counseling
The use of tight decorative balls on ponytail ties and numerous plastic beads increases the amount of tension and weight on the hair, which may lead to a higher risk for developing traction alopecia.6 It is quite common for children of African descent to wear hair adornments. Proper counseling regarding their use and possible implications is essential.
- Compliment: "You're doing a great job controlling the atopic dermatitis, which can cause Janet's scalp to be dry. Also, her hair is beautiful--it looks like you spent a lot of time on her hair. And Janet, I like the color of your barrettes."
- Discuss: "Mom, I just noticed that a few areas look tight. Let's look together." (The dermatologist points out areas where the scalp is tented upward due to traction, follicular pustules or papules, or the frontal fringe sign.) "I'm on a mission to #savetheedges because we want Janet to grow up with full edges." (Again, loss of "edges" refers to TA.)
- Suggest: "When you do Janet's hair, it's OK if every hair is not in place. In fact, making styles look and feel 1 or 2 weeks old will lessen tension on the scalp. Remove Janet's hair ties to release tension when she is at home and while she's sleeping, if possible. Every minute that the hair is loose really does help."6
The Parent's Perspective
All parents take pride in their children. In some Black communities, mothers are judged by how well they manage and style their children's hair. Some people might even suggest that parents of children with nonstyled, tightly coiled hair are not fit parents. Anthropologist Sylvia Boone, PhD, found that among the Mende tribe in Sierra Leone, "unkempt, 'neglected,' or 'messy' hair implied that a woman either had loose morals or was insane."7
Braids are commonly worn by people of African heritage for a variety of reasons, including ease of manageability, to decrease daily hairstyling time, and as an expression of creativity. Intricate neat hairstyles, despite the risk of pain and TA, are perceived as a sign that the child is cared for and loved.6
FINAL THOUGHTS
Patient-centered communication is associated with the patient trusting the physician, which is especially important in race-discordant physician-patient relationships. A study found that patient-physician race discordance led to shorter visits, a lower rating of patient affect, and less shared decision-making.8 Moreover, in a study of primary care clinicians, implicit bias was found to affect communication patterns and social interactions, impacting patient outcomes. Downstream effects of racial bias resulted in less speaking, smiling, and social comments when interacting with Black patients.9
These findings highlight the need to address interpersonal barriers to effective communication in race-discordant patient-physician dyads. A history of segregated neighborhoods and schools might contribute to structural barriers, resulting in lack of familiarity with cultural norms outside one's culture, which might globally perpetuate poor communication and patient outcomes.
The "compliment, discuss, and suggest" method might lead to more positive physician-patient encounters by having the dermatologist focus on empathetically understanding the patient's perspective.10 Effective communication, understanding cultural hair care practices, and a thorough scalp examination are paramount for patients with tightly coiled hair.11 Early intervention in TA is crucial and involves partnering with patients and parents to amend high-risk hairstyling routines with cultural humility.
Practice Points
- When communicating with patients regarding traction alopecia (TA), it is crucial to display cultural humility and empathy.
- Understanding the patient’s hair care goals and perspective allows dermatologists to take a more individualized approach to counseling about TA.
- The “compliment, discuss, and suggest” method is an empathetic and culturally sensitive method for discussing TA with patients.
Atopic Dermatitis
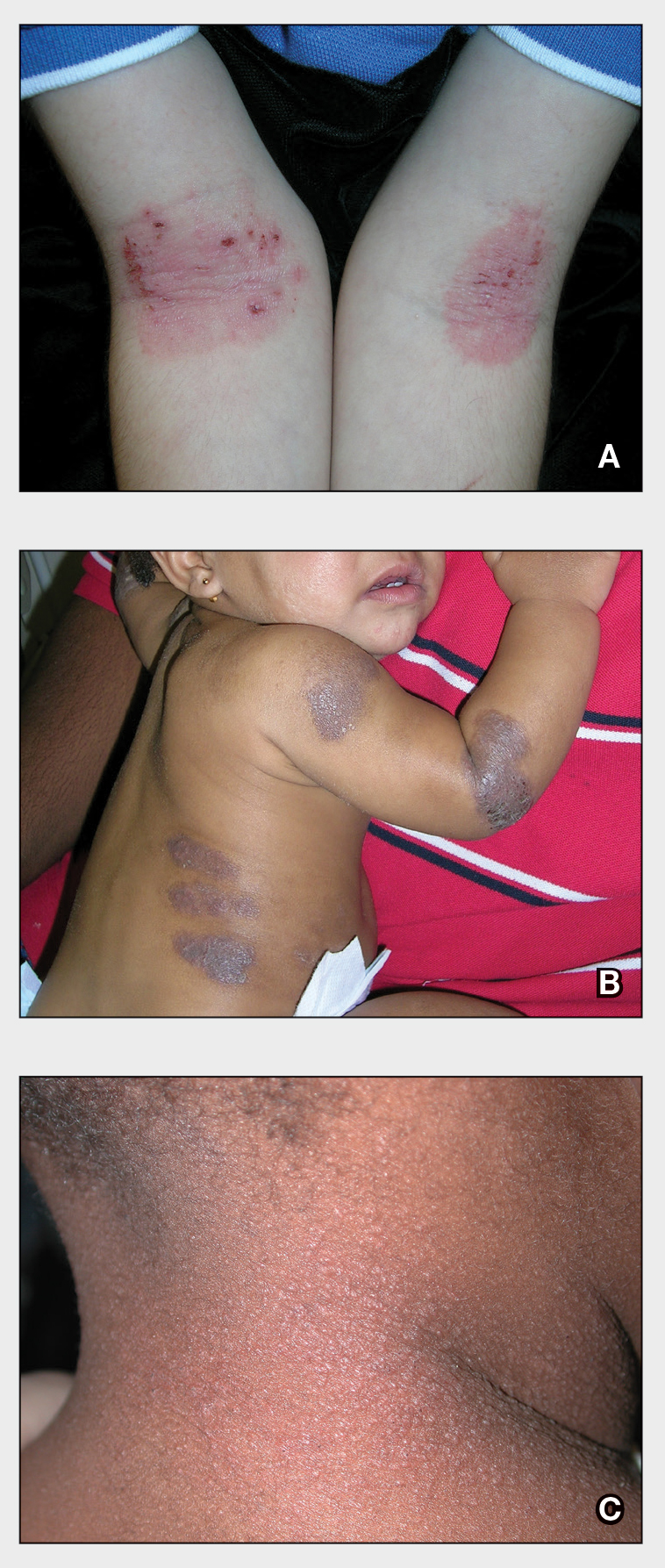
The Comparison
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-ofpocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016 /j.anai.2019.05.014
- Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016 /j.jid.2018.10.029
- Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432

The Comparison
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-ofpocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4

The Comparison
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-ofpocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016 /j.anai.2019.05.014
- Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016 /j.jid.2018.10.029
- Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016 /j.anai.2019.05.014
- Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016 /j.jid.2018.10.029
- Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
Microaggressions in Medicine
As manifestations of overt racism and macroaggressions have gained increased visibility, there is a need for discussion of another expression of racism: microaggressions. Although racism classically is viewed as blatant structural, attitudinal, and behavioral prejudice, experts pose that the face of racism has evolved into a more covert insidious form. This form of racism was originally coined racial microaggressions by psychiatrist Chester M. Pierce, MD, 50 years ago.1,2 Since that time, microaggressions have further expanded to describe “brief and commonplace daily verbal, behavioral, and environmental indignities, whether intentional or unintentional, that communicate hostile, derogatory, or negative racial, gender, sexual-orientation, and religious slights and insults to the target person or group.” 3 This article aims to define and depict examples of microaggressions in medicine, discuss the resulting harmful effects, and offer strategies to minimize and counter these negative ramifications.
What are microaggressions?
Microaggressions are behaviors that stem from implicit bias and occur at an interpersonal level. Implicit bias refers to unconscious stereotypes, assumptions, and beliefs held about an individual’s identity. One of the earliest microaggressions—invisibility—was characterized by Ralph Ellison in his novel Invisible Man. Ellison states, “I am invisible, understand, simply because people refuse to see me . . . When they approach me they see only my surroundings, themselves, or figments of their imagination—indeed, everything and anything except me.”4 This concept of invisibility is a primary microaggression faced by people of color.
In medicine, microaggressions and implicit bias may be encountered throughout medical training and clinical practice in interactions with colleagues, superiors, patients, and patients’ families.5,6 Examples of microaggressions in medicine include demeaning comments, nonverbal disrespect, generalizations of social identity, assumption of nonphysician status, role- or credential-questioning behavior, explicit epithets, rejection of care, questioning or inquiries of ethnic/racial origin, and sexual harassment.7
An example of microaggressions in medicine was fully displayed when physician Tamika Cross described her experience of being turned away from helping an unresponsive passenger during a flight emergency.
[T]he flight attendant yells “call overhead for a physician on board.” I raised my hand to grab her attention. She said to me “oh no sweetie put [your] hand down, we are looking for actual physicians or nurses or some type of medical personnel, we don’t have time to talk to you” . . . Another “seasoned” white male approaches the row and says he is a physician as well. She says to me “thanks for your help but he can help us, and he has his credentials.”8
What are the effects of microaggressions?
Although microaggressions may be unconscious and unintentional by the offender, the negative ramifications are notable. Recent studies report that women and underrepresented minority (URM) medical students, residents, and physicians experience microaggressions and implicit bias at a higher prevalence and frequency compared with their male and non-URM counterparts.7,9 Repetitive microaggressions are harmful to the health and safety of women and URM medical students, residents, physicians, other providers, and patients. The Table provides example scenarios of microaggressions in medicine categorized according to Berk.10
Microaggressions negatively impact physical, mental, and emotional well-being. Current data support that medical students and residents who experience microaggressions are more likely to report associated symptoms of burnout, depression, and suicidal thoughts.11,12 Subjection to persistent bias can lead to minority status stress and racial battle fatigue, creating feelings of invisibility, isolation, exclusion, and loneliness for those impacted.13,14
In the book Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine, Damon Tweedy, MD, reflects on race in medicine. Tweedy notes his experience as a medical student when a professor mistakenly assumed he was a maintenance worker in the classroom. Tweedy describes how he internalized the exchange and, despite his success throughout the course of his medical training, combatted feelings of anxiety, self-doubt, and implied inferiority.15
Although microaggressions are harmful to one’s health, they also undermine the learning and teaching experience for students, residents, and faculty, and they detract from the larger goal of providing care for patients.11 Frequent devaluing and questioning of an individual’s contributions, qualifications, and credentials based on identity can lower productivity and problem-solving abilities. These behaviors cultivate an unwelcome and hostile work/learning environment that is stressful and polarizing for the recipient.
Despite the heavy burden of microaggressions, most students, residents, and faculty physicians do not report incidents to their institutions and feel that training, resources, and policies to respond to bias adequately are lacking.7 As a result of implicit bias and microaggressions, women and URM medical students and providers are unable to focus solely on the practice of medicine. They are tasked with the additional burden of shouldering the emotional and cognitive complexities that microaggressions produce.16
What are strategies to reduce microaggressions in medicine?
To minimize the harmful effects of microaggressions, intervention strategies must be implemented that reduce the likelihood of the occurrence of microaggressions and challenge the stereotypes that undergird implicit bias. These strategies include cultivating allies, followed by demanding structural accountability. Allies are members of the majority group who collectively collaborate with members of the nonmajority group to effect change through the promotion of diversity, equity, and inclusion efforts.17 Cultivating allies involves building a network of collaboration among these groups and emphasizes education. Education is critical for allies to address microaggressions at the interpersonal level. This process of education involves personal reflection and self-awareness in exploring one’s biases, fears, and assumptions. Integral to this step is broadening one’s acceptance of different cultures, racial/ethnic groups, and identities. There must be a willingness to engage in difficult or uncomfortable conversations and a readiness to actively listen to concerns rather than perpetuating further harm through avoidance and dismissive or defensive behavior.18
Demanding structural accountability facilitates deconstruction of bias and microaggression at the larger systemic level. This strategy involves implicit bias and antiracism training, development of retention plans, and identification of mentors for women and URM providers and students. Implicit bias and microaggression training and policies should be incorporated into medical education and resident curriculums. Similarly, educational resources and training must be made available to practicing physicians, faculty, and other providers through their institutions and places of employment. Equipping students and providers with the tools needed when microaggressions are witnessed or experienced demonstrates systemic-level accountability and communicates the importance of the issue. Furthermore, the development of retention plans and identification of mentors provide a support system and foster a culture of inclusion where recipients of microaggressions feel protected and valued. Increased feelings of inclusivity and belonging help bridge the gap created through microaggressions and implicit bias.
Final Thoughts
Despite an often covert nature, the detrimental effects of microaggressions are tangible and far reaching. As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice. It is our obligation to undertake the challenge of “making the ‘invisible’ visible” as we confront microaggressions and implicit bias to promote a safer and more inclusive medical community and workforce.19
- Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154:868-872. doi:10.1001/jamasurg.2019.1648
- Williams MT. Microaggressions: clarification, evidence, and impact. Perspect Psychol Sci. 2020;15:3-26. doi:10.1177/1745691619827499
- Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Wiley; 2010.
- Ellison R. Invisible Man. Random House; 1952.
- Molina MF, Landry AI, Chary AN, et al. Addressing the elephant in the room: microaggressions in medicine. Ann Emerg Med. 2020;76:387-391. doi:10.1016/j.annemergmed.2020.04.009
- Overland MK, Zumsteg JM, Lindo EG, et al. Microaggressions in clinical training and practice. PM R. 2019;11:1004-1012. doi:10.1002/pmrj.12229
- de Bourmont SS, Burra A, Nouri SS, et al. Resident physician experiences with and responses to biased patients. JAMA Netw Open. 2020;3:e2021769. doi:10.1001/jamanetworkopen.2020.21769
- TK Cross Facebook page. October 9, 2016. Accessed April 19, 2021. https://www.facebook.com/tamika.cross.52/posts/658443077654049
- Periyakoil VS, Chaudron L, Hill EV, et al. Common types of gender-based microaggressions in medicine. Acad Med. 2020;95:450-457. doi:10.1097/ACM.0000000000003057
- Berk RA. Microaggressions trilogy: part 1. why do microaggressions matter? J Fac Dev. 2017;31:63-73.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2020:S0027-9684(20)30428-4. doi:10.1016/j.jnma.2020.11.009
- Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381:1741-1752. doi:10.1056/NEJMsa1903759
- Acholonu RG, Oyeku SO. Addressing microaggressions in the health care workforce-a path toward achieving equity and inclusion. JAMA Netw Open. 2020;3:E2021770. doi:10.1001/jamanetworkopen.2020.21770
- O’Keefe VM, Wingate LR, Cole AB, et al. Seemingly harmless racial communications are not so harmless: racial microaggressions lead to suicidal ideation by way of depression symptoms. Suicide Life Threat Behav. 2015;45:567-576. doi:10.1111/sltb.12150
- Tweedy D. Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine. Picador; 2016.
- Osseo-Asare A, Balasuriya L, Huot SJ, et al. Minority resident physicians’ views on the role of race/ethnicity in their training experiences in the workplace. JAMA Netw Open. 2018;1:E182723. doi: 10.1001/jamanetworkopen.2018.2723
- Melaku TM, Beeman A, Smith DG, et al. Be a better ally. Harvard Business Review. Published November-December 2020. Accessed April 23, 2021. https://hbr.org/2020/11/be-a-better-ally
- Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62:271-286. doi:10.1037/0003-066X.62.4.271
- Sue DW. Whiteness and ethnocentric monoculturalism: making the “invisible” visible. Am Psychol. 2004;59:761-769. doi:10.1037/0003-066X.59.8.761
As manifestations of overt racism and macroaggressions have gained increased visibility, there is a need for discussion of another expression of racism: microaggressions. Although racism classically is viewed as blatant structural, attitudinal, and behavioral prejudice, experts pose that the face of racism has evolved into a more covert insidious form. This form of racism was originally coined racial microaggressions by psychiatrist Chester M. Pierce, MD, 50 years ago.1,2 Since that time, microaggressions have further expanded to describe “brief and commonplace daily verbal, behavioral, and environmental indignities, whether intentional or unintentional, that communicate hostile, derogatory, or negative racial, gender, sexual-orientation, and religious slights and insults to the target person or group.” 3 This article aims to define and depict examples of microaggressions in medicine, discuss the resulting harmful effects, and offer strategies to minimize and counter these negative ramifications.
What are microaggressions?
Microaggressions are behaviors that stem from implicit bias and occur at an interpersonal level. Implicit bias refers to unconscious stereotypes, assumptions, and beliefs held about an individual’s identity. One of the earliest microaggressions—invisibility—was characterized by Ralph Ellison in his novel Invisible Man. Ellison states, “I am invisible, understand, simply because people refuse to see me . . . When they approach me they see only my surroundings, themselves, or figments of their imagination—indeed, everything and anything except me.”4 This concept of invisibility is a primary microaggression faced by people of color.
In medicine, microaggressions and implicit bias may be encountered throughout medical training and clinical practice in interactions with colleagues, superiors, patients, and patients’ families.5,6 Examples of microaggressions in medicine include demeaning comments, nonverbal disrespect, generalizations of social identity, assumption of nonphysician status, role- or credential-questioning behavior, explicit epithets, rejection of care, questioning or inquiries of ethnic/racial origin, and sexual harassment.7
An example of microaggressions in medicine was fully displayed when physician Tamika Cross described her experience of being turned away from helping an unresponsive passenger during a flight emergency.
[T]he flight attendant yells “call overhead for a physician on board.” I raised my hand to grab her attention. She said to me “oh no sweetie put [your] hand down, we are looking for actual physicians or nurses or some type of medical personnel, we don’t have time to talk to you” . . . Another “seasoned” white male approaches the row and says he is a physician as well. She says to me “thanks for your help but he can help us, and he has his credentials.”8
What are the effects of microaggressions?
Although microaggressions may be unconscious and unintentional by the offender, the negative ramifications are notable. Recent studies report that women and underrepresented minority (URM) medical students, residents, and physicians experience microaggressions and implicit bias at a higher prevalence and frequency compared with their male and non-URM counterparts.7,9 Repetitive microaggressions are harmful to the health and safety of women and URM medical students, residents, physicians, other providers, and patients. The Table provides example scenarios of microaggressions in medicine categorized according to Berk.10

Microaggressions negatively impact physical, mental, and emotional well-being. Current data support that medical students and residents who experience microaggressions are more likely to report associated symptoms of burnout, depression, and suicidal thoughts.11,12 Subjection to persistent bias can lead to minority status stress and racial battle fatigue, creating feelings of invisibility, isolation, exclusion, and loneliness for those impacted.13,14
In the book Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine, Damon Tweedy, MD, reflects on race in medicine. Tweedy notes his experience as a medical student when a professor mistakenly assumed he was a maintenance worker in the classroom. Tweedy describes how he internalized the exchange and, despite his success throughout the course of his medical training, combatted feelings of anxiety, self-doubt, and implied inferiority.15
Although microaggressions are harmful to one’s health, they also undermine the learning and teaching experience for students, residents, and faculty, and they detract from the larger goal of providing care for patients.11 Frequent devaluing and questioning of an individual’s contributions, qualifications, and credentials based on identity can lower productivity and problem-solving abilities. These behaviors cultivate an unwelcome and hostile work/learning environment that is stressful and polarizing for the recipient.
Despite the heavy burden of microaggressions, most students, residents, and faculty physicians do not report incidents to their institutions and feel that training, resources, and policies to respond to bias adequately are lacking.7 As a result of implicit bias and microaggressions, women and URM medical students and providers are unable to focus solely on the practice of medicine. They are tasked with the additional burden of shouldering the emotional and cognitive complexities that microaggressions produce.16
What are strategies to reduce microaggressions in medicine?
To minimize the harmful effects of microaggressions, intervention strategies must be implemented that reduce the likelihood of the occurrence of microaggressions and challenge the stereotypes that undergird implicit bias. These strategies include cultivating allies, followed by demanding structural accountability. Allies are members of the majority group who collectively collaborate with members of the nonmajority group to effect change through the promotion of diversity, equity, and inclusion efforts.17 Cultivating allies involves building a network of collaboration among these groups and emphasizes education. Education is critical for allies to address microaggressions at the interpersonal level. This process of education involves personal reflection and self-awareness in exploring one’s biases, fears, and assumptions. Integral to this step is broadening one’s acceptance of different cultures, racial/ethnic groups, and identities. There must be a willingness to engage in difficult or uncomfortable conversations and a readiness to actively listen to concerns rather than perpetuating further harm through avoidance and dismissive or defensive behavior.18
Demanding structural accountability facilitates deconstruction of bias and microaggression at the larger systemic level. This strategy involves implicit bias and antiracism training, development of retention plans, and identification of mentors for women and URM providers and students. Implicit bias and microaggression training and policies should be incorporated into medical education and resident curriculums. Similarly, educational resources and training must be made available to practicing physicians, faculty, and other providers through their institutions and places of employment. Equipping students and providers with the tools needed when microaggressions are witnessed or experienced demonstrates systemic-level accountability and communicates the importance of the issue. Furthermore, the development of retention plans and identification of mentors provide a support system and foster a culture of inclusion where recipients of microaggressions feel protected and valued. Increased feelings of inclusivity and belonging help bridge the gap created through microaggressions and implicit bias.
Final Thoughts
Despite an often covert nature, the detrimental effects of microaggressions are tangible and far reaching. As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice. It is our obligation to undertake the challenge of “making the ‘invisible’ visible” as we confront microaggressions and implicit bias to promote a safer and more inclusive medical community and workforce.19
As manifestations of overt racism and macroaggressions have gained increased visibility, there is a need for discussion of another expression of racism: microaggressions. Although racism classically is viewed as blatant structural, attitudinal, and behavioral prejudice, experts pose that the face of racism has evolved into a more covert insidious form. This form of racism was originally coined racial microaggressions by psychiatrist Chester M. Pierce, MD, 50 years ago.1,2 Since that time, microaggressions have further expanded to describe “brief and commonplace daily verbal, behavioral, and environmental indignities, whether intentional or unintentional, that communicate hostile, derogatory, or negative racial, gender, sexual-orientation, and religious slights and insults to the target person or group.” 3 This article aims to define and depict examples of microaggressions in medicine, discuss the resulting harmful effects, and offer strategies to minimize and counter these negative ramifications.
What are microaggressions?
Microaggressions are behaviors that stem from implicit bias and occur at an interpersonal level. Implicit bias refers to unconscious stereotypes, assumptions, and beliefs held about an individual’s identity. One of the earliest microaggressions—invisibility—was characterized by Ralph Ellison in his novel Invisible Man. Ellison states, “I am invisible, understand, simply because people refuse to see me . . . When they approach me they see only my surroundings, themselves, or figments of their imagination—indeed, everything and anything except me.”4 This concept of invisibility is a primary microaggression faced by people of color.
In medicine, microaggressions and implicit bias may be encountered throughout medical training and clinical practice in interactions with colleagues, superiors, patients, and patients’ families.5,6 Examples of microaggressions in medicine include demeaning comments, nonverbal disrespect, generalizations of social identity, assumption of nonphysician status, role- or credential-questioning behavior, explicit epithets, rejection of care, questioning or inquiries of ethnic/racial origin, and sexual harassment.7
An example of microaggressions in medicine was fully displayed when physician Tamika Cross described her experience of being turned away from helping an unresponsive passenger during a flight emergency.
[T]he flight attendant yells “call overhead for a physician on board.” I raised my hand to grab her attention. She said to me “oh no sweetie put [your] hand down, we are looking for actual physicians or nurses or some type of medical personnel, we don’t have time to talk to you” . . . Another “seasoned” white male approaches the row and says he is a physician as well. She says to me “thanks for your help but he can help us, and he has his credentials.”8
What are the effects of microaggressions?
Although microaggressions may be unconscious and unintentional by the offender, the negative ramifications are notable. Recent studies report that women and underrepresented minority (URM) medical students, residents, and physicians experience microaggressions and implicit bias at a higher prevalence and frequency compared with their male and non-URM counterparts.7,9 Repetitive microaggressions are harmful to the health and safety of women and URM medical students, residents, physicians, other providers, and patients. The Table provides example scenarios of microaggressions in medicine categorized according to Berk.10

Microaggressions negatively impact physical, mental, and emotional well-being. Current data support that medical students and residents who experience microaggressions are more likely to report associated symptoms of burnout, depression, and suicidal thoughts.11,12 Subjection to persistent bias can lead to minority status stress and racial battle fatigue, creating feelings of invisibility, isolation, exclusion, and loneliness for those impacted.13,14
In the book Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine, Damon Tweedy, MD, reflects on race in medicine. Tweedy notes his experience as a medical student when a professor mistakenly assumed he was a maintenance worker in the classroom. Tweedy describes how he internalized the exchange and, despite his success throughout the course of his medical training, combatted feelings of anxiety, self-doubt, and implied inferiority.15
Although microaggressions are harmful to one’s health, they also undermine the learning and teaching experience for students, residents, and faculty, and they detract from the larger goal of providing care for patients.11 Frequent devaluing and questioning of an individual’s contributions, qualifications, and credentials based on identity can lower productivity and problem-solving abilities. These behaviors cultivate an unwelcome and hostile work/learning environment that is stressful and polarizing for the recipient.
Despite the heavy burden of microaggressions, most students, residents, and faculty physicians do not report incidents to their institutions and feel that training, resources, and policies to respond to bias adequately are lacking.7 As a result of implicit bias and microaggressions, women and URM medical students and providers are unable to focus solely on the practice of medicine. They are tasked with the additional burden of shouldering the emotional and cognitive complexities that microaggressions produce.16
What are strategies to reduce microaggressions in medicine?
To minimize the harmful effects of microaggressions, intervention strategies must be implemented that reduce the likelihood of the occurrence of microaggressions and challenge the stereotypes that undergird implicit bias. These strategies include cultivating allies, followed by demanding structural accountability. Allies are members of the majority group who collectively collaborate with members of the nonmajority group to effect change through the promotion of diversity, equity, and inclusion efforts.17 Cultivating allies involves building a network of collaboration among these groups and emphasizes education. Education is critical for allies to address microaggressions at the interpersonal level. This process of education involves personal reflection and self-awareness in exploring one’s biases, fears, and assumptions. Integral to this step is broadening one’s acceptance of different cultures, racial/ethnic groups, and identities. There must be a willingness to engage in difficult or uncomfortable conversations and a readiness to actively listen to concerns rather than perpetuating further harm through avoidance and dismissive or defensive behavior.18
Demanding structural accountability facilitates deconstruction of bias and microaggression at the larger systemic level. This strategy involves implicit bias and antiracism training, development of retention plans, and identification of mentors for women and URM providers and students. Implicit bias and microaggression training and policies should be incorporated into medical education and resident curriculums. Similarly, educational resources and training must be made available to practicing physicians, faculty, and other providers through their institutions and places of employment. Equipping students and providers with the tools needed when microaggressions are witnessed or experienced demonstrates systemic-level accountability and communicates the importance of the issue. Furthermore, the development of retention plans and identification of mentors provide a support system and foster a culture of inclusion where recipients of microaggressions feel protected and valued. Increased feelings of inclusivity and belonging help bridge the gap created through microaggressions and implicit bias.
Final Thoughts
Despite an often covert nature, the detrimental effects of microaggressions are tangible and far reaching. As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice. It is our obligation to undertake the challenge of “making the ‘invisible’ visible” as we confront microaggressions and implicit bias to promote a safer and more inclusive medical community and workforce.19
- Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154:868-872. doi:10.1001/jamasurg.2019.1648
- Williams MT. Microaggressions: clarification, evidence, and impact. Perspect Psychol Sci. 2020;15:3-26. doi:10.1177/1745691619827499
- Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Wiley; 2010.
- Ellison R. Invisible Man. Random House; 1952.
- Molina MF, Landry AI, Chary AN, et al. Addressing the elephant in the room: microaggressions in medicine. Ann Emerg Med. 2020;76:387-391. doi:10.1016/j.annemergmed.2020.04.009
- Overland MK, Zumsteg JM, Lindo EG, et al. Microaggressions in clinical training and practice. PM R. 2019;11:1004-1012. doi:10.1002/pmrj.12229
- de Bourmont SS, Burra A, Nouri SS, et al. Resident physician experiences with and responses to biased patients. JAMA Netw Open. 2020;3:e2021769. doi:10.1001/jamanetworkopen.2020.21769
- TK Cross Facebook page. October 9, 2016. Accessed April 19, 2021. https://www.facebook.com/tamika.cross.52/posts/658443077654049
- Periyakoil VS, Chaudron L, Hill EV, et al. Common types of gender-based microaggressions in medicine. Acad Med. 2020;95:450-457. doi:10.1097/ACM.0000000000003057
- Berk RA. Microaggressions trilogy: part 1. why do microaggressions matter? J Fac Dev. 2017;31:63-73.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2020:S0027-9684(20)30428-4. doi:10.1016/j.jnma.2020.11.009
- Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381:1741-1752. doi:10.1056/NEJMsa1903759
- Acholonu RG, Oyeku SO. Addressing microaggressions in the health care workforce-a path toward achieving equity and inclusion. JAMA Netw Open. 2020;3:E2021770. doi:10.1001/jamanetworkopen.2020.21770
- O’Keefe VM, Wingate LR, Cole AB, et al. Seemingly harmless racial communications are not so harmless: racial microaggressions lead to suicidal ideation by way of depression symptoms. Suicide Life Threat Behav. 2015;45:567-576. doi:10.1111/sltb.12150
- Tweedy D. Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine. Picador; 2016.
- Osseo-Asare A, Balasuriya L, Huot SJ, et al. Minority resident physicians’ views on the role of race/ethnicity in their training experiences in the workplace. JAMA Netw Open. 2018;1:E182723. doi: 10.1001/jamanetworkopen.2018.2723
- Melaku TM, Beeman A, Smith DG, et al. Be a better ally. Harvard Business Review. Published November-December 2020. Accessed April 23, 2021. https://hbr.org/2020/11/be-a-better-ally
- Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62:271-286. doi:10.1037/0003-066X.62.4.271
- Sue DW. Whiteness and ethnocentric monoculturalism: making the “invisible” visible. Am Psychol. 2004;59:761-769. doi:10.1037/0003-066X.59.8.761
- Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154:868-872. doi:10.1001/jamasurg.2019.1648
- Williams MT. Microaggressions: clarification, evidence, and impact. Perspect Psychol Sci. 2020;15:3-26. doi:10.1177/1745691619827499
- Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Wiley; 2010.
- Ellison R. Invisible Man. Random House; 1952.
- Molina MF, Landry AI, Chary AN, et al. Addressing the elephant in the room: microaggressions in medicine. Ann Emerg Med. 2020;76:387-391. doi:10.1016/j.annemergmed.2020.04.009
- Overland MK, Zumsteg JM, Lindo EG, et al. Microaggressions in clinical training and practice. PM R. 2019;11:1004-1012. doi:10.1002/pmrj.12229
- de Bourmont SS, Burra A, Nouri SS, et al. Resident physician experiences with and responses to biased patients. JAMA Netw Open. 2020;3:e2021769. doi:10.1001/jamanetworkopen.2020.21769
- TK Cross Facebook page. October 9, 2016. Accessed April 19, 2021. https://www.facebook.com/tamika.cross.52/posts/658443077654049
- Periyakoil VS, Chaudron L, Hill EV, et al. Common types of gender-based microaggressions in medicine. Acad Med. 2020;95:450-457. doi:10.1097/ACM.0000000000003057
- Berk RA. Microaggressions trilogy: part 1. why do microaggressions matter? J Fac Dev. 2017;31:63-73.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2020:S0027-9684(20)30428-4. doi:10.1016/j.jnma.2020.11.009
- Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381:1741-1752. doi:10.1056/NEJMsa1903759
- Acholonu RG, Oyeku SO. Addressing microaggressions in the health care workforce-a path toward achieving equity and inclusion. JAMA Netw Open. 2020;3:E2021770. doi:10.1001/jamanetworkopen.2020.21770
- O’Keefe VM, Wingate LR, Cole AB, et al. Seemingly harmless racial communications are not so harmless: racial microaggressions lead to suicidal ideation by way of depression symptoms. Suicide Life Threat Behav. 2015;45:567-576. doi:10.1111/sltb.12150
- Tweedy D. Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine. Picador; 2016.
- Osseo-Asare A, Balasuriya L, Huot SJ, et al. Minority resident physicians’ views on the role of race/ethnicity in their training experiences in the workplace. JAMA Netw Open. 2018;1:E182723. doi: 10.1001/jamanetworkopen.2018.2723
- Melaku TM, Beeman A, Smith DG, et al. Be a better ally. Harvard Business Review. Published November-December 2020. Accessed April 23, 2021. https://hbr.org/2020/11/be-a-better-ally
- Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62:271-286. doi:10.1037/0003-066X.62.4.271
- Sue DW. Whiteness and ethnocentric monoculturalism: making the “invisible” visible. Am Psychol. 2004;59:761-769. doi:10.1037/0003-066X.59.8.761
Practice Points
- As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice.
- Intervention strategies must be implemented to reduce the likelihood of the occurrence of microaggressions in medicine and challenge the stereotypes that undergird implicit bias.
- It is important to promote collaboration in diversity, equity, and inclusion efforts to demonstrate support for women and underrepresented minority medical students, residents, physicians, providers, and patients.