User login
The Importance of Service Learning in Dermatology Residency: An Actionable Approach to Improve Resident Education and Skin Health Equity
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):
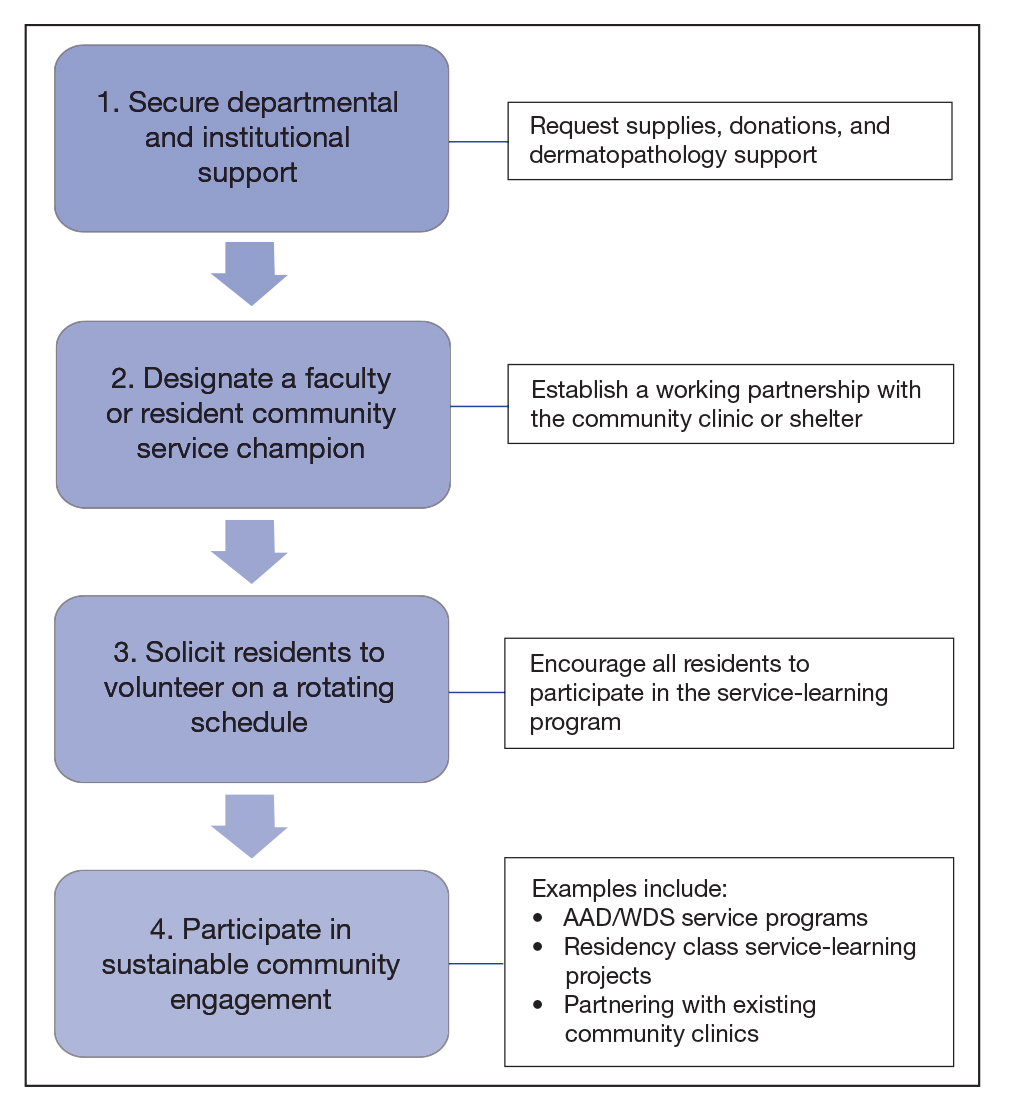
• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):

• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):

• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
Practice Points
- In 2019, nearly 30 million Americans did not have health insurance. Dermatologists in the United States should be cognizant of the challenges faced by underserved patients when accessing dermatologic care.
- Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive learning experience, meet societal needs, and fulfill Accreditation Council for Graduate Medical Education requirements.
- Actionable methods to increase service learning in dermatology residency training include volunteering in community service programs offered by national dermatology organizations, implementing service-learning projects, and partnering with free and federally funded community practices.
- Dermatology residents who participate in service learning will help increase access to equitable dermatologic care and experience practicing in settings with limited resources.
Bonds and Bridges: The Role of Social Capital in Building a More Diverse Dermatology Workforce
As our specialty seeks to address its lack of racial diversity, many dermatologists have answered recent calls to action.1,2 As we work toward dismantling systemic issues that have created pervasive inequality in our residency application review and interview processes, consideration also should be given to psychosocial issues that underrepresented-in-medicine (UIM) students face before their applications come to our attention. In this article, we explore how potential differences in the social capital of UIM and other disadvantaged dermatology residency applicants contribute to persistent homogeneity among dermatology training programs and the workforce.
The Theory of Capital
The concepts of economic, social, and cultural capital originate from the writings of social theorist Pierre Bourdieu.3 All 3 forms of capital are interconnected, and they relate to each other in ways that often facilitate social division and inequality. Economic capital denotes an individual’s economic resources or wealth, while cultural capital refers to the knowledge, behaviors, and skills that demonstrate his/her economic class (eg, communication style, table manners).3 Social capital refers to an individual’s interpersonal connections in personal and professional settings and can be subdivided into 3 categories: bonds, bridges, and linkages.4,5 Herein, we will focus on bonds and bridges.
It has been suggested that bonds are important for “getting by,” while bridges are critical for “getting ahead.”5 Bonds refer to close relationships within a community of people with shared characteristics, such as racial/ethnic identity and culture, access to information, and resources (eg, family, friends). These bonds provide trust, safety, and financial and emotional support; however, they are considered to be inward-looking and can promote exclusion and homogeneity.5
On the other hand, bridges refer to social relationships that extend outward beyond one’s close circle of family and friends to other people with shared interests and goals who may have different social or cultural identities (eg, professional colleagues). These bridges are considered to be outward-looking and provide many benefits to individuals and society. They link diverse individuals, which tends to increase tolerance and disrupt stereotypes, and they facilitate the sharing of ideas, information, and innovation. Additionally, bridges between individuals from different networks facilitate access to increased resources and opportunities for all parties.5
The 3 forms of capital are inextricably linked. For example, with economic capital, a child’s family can purchase access to a prestigious private high school, where he/she will gain valuable social capital through bridges with other students and their families. At this school, the child also will accumulate cultural capital that increases his/her sense of belonging in these circles. Subsequently, both the social and cultural capital accumulated at this private high school can be exchanged for economic capital via social networks, skills, values, and behaviors that facilitate entry into higher education and professional training. As such, these 3 forms of capital work together to continue social/class divisions, hierarchies, and ultimately inequality.
Impact of Social Capital in Pursuing a Medical Career
For medical students whose bonds (ie, close family, friends) include physicians or other health care professionals, the journey to studying medicine and entering their chosen specialty will be facilitated by financial security, valuable “inside information” about the application process, study skills, and even clinical guidance. Additionally, these students will have access to professional networks for mentorship, shadowing experiences, and other potential advantages. Furthermore, social capital is associated with higher self-esteem,6 which likely improves academic performance and wards off imposter syndrome in these students.
For medical students from lower socioeconomic status backgrounds or those whose inner circles do not include physicians or other health care professionals, accumulating the social and cultural capital needed to successfully navigate a medical career is more difficult. Although they may receive support and encouragement from family and friends, they will not have access to the same valuable information and connections that facilitate success; rather, they will have a further distance to travel, and this distance should be acknowledged in the residency application review process.
Acquiring Social Capital as a UIM Student
Despite the benefits of social and cultural capital, acquiring them takes a toll. For those UIM students who start life from a disadvantaged place, the accumulation of social capital does not come easily; rather, it demands effort and time that has the potential to detract from a student’s focus on the academic demands of medical education.7 Programs that attempt to improve disadvantaged students’ access to credible information, role models, and mentors can help lift some of the burden from the individual student’s shoulders. For example, studies have demonstrated the benefits of harnessing technology to enhance mentorship programs that increase social capital of disadvantaged populations.8-11 This approach already is in progress, bolstered by advances made in digital communications during the coronavirus disease 2019 pandemic.12 Student-led networking groups that connect remotely have been shown to build social capital bonds and bridges that facilitate collaborative learning, relationship building, and information sharing.8-11 There are existing online UIM student networks that individual dermatologists, institutions, and national organizations can partner with to facilitate the construction of bridges between these UIM student groups and dermatologists who can provide accurate, high-yield information and professional networking; however, one limitation of this suggestion is the disparate access to technology in the UIM community.
Final Thoughts
It is important to note that assumptions should not be made about the level of economic, social, or cultural capital an individual possesses based on his/her race or ethnicity. Instead, mentors should attempt to be available to a diverse pool of students; take the time to get to know these students; and then provide the types of mentorship, information, exposure, and networking that each individual student needs. Another approach is to make a concerted effort to ensure that all students receive the same amount and quality of information about medical education and our specialty regardless of their level of economic, cultural, or social capital. Moreover, beyond the promotion of diversity through increasing numbers of UIM applicants, we should seek to reshape our specialty into a space that does not require students to subdue their existing diverse forms of capital but rather to bring these different perspectives and lived experiences to the table.13
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:7.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Bourdieu P. The forms of capital. In: Richardson J, ed. Handbook of Theory and Research for the Sociology of Education. Westport, CT: Greenwood; 1986:241-258.
- Granovetter MS. The strength of weak ties. Am J Sociol. 1973;78:1360-1380.
- Putnam RD. Bowling alone: America’s declining social capital. J Democracy. 1995;6:65-78.
- Han S. Longitudinal association between social capital and self-esteem: a matter of context. Psychiatry Research. 2015;226:340-346.
- Kirschling JM. Building social capital: leading and leveraging constituencies outside the college. J Nurs Educ. 2004;43:517-519.
- Radlick RL, Svedberg P, Nygren JM, et al. Digitally enhanced mentoring for immigrant youth social capital: protocol for a mixed methods pilot study and a randomized controlled trial [published online March 17, 2020]. JMIR Research Protocols. doi:10.2196/16472.
- Koh LC, Walker R, Wollersheim D, et al. I think someone is walking with me: the use of mobile phone for social capital development among women in four refugee communities. Int J Migration Health Social Care. 2018;14:411-424.
- Hartley A, Kassam AA. Social networking for learning in higher education: capitalising on social capital. ResearchGate website.https://www.researchgate.net/publication/311097860_Social_Networking_for_Learning_in_Higher_Education_Capitalising_on_Social_Capital. Published November 2016. Accessed October 19, 2020.
- Zalon ML. Using technology to build community in professional associations. J Contin Educ Nurs. 2008;39:235-240.
- Stewart CR, Chernoff KA, Wildman HF, et al. Recommendations for medical student preparedness and equity for dermatology residency applications during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E225-E226.
- Brosnan C, Southgate E, Outram S, et al. Experiences of medical students who are first in family to attend university. Med Educ. 2016;50:842-851.
As our specialty seeks to address its lack of racial diversity, many dermatologists have answered recent calls to action.1,2 As we work toward dismantling systemic issues that have created pervasive inequality in our residency application review and interview processes, consideration also should be given to psychosocial issues that underrepresented-in-medicine (UIM) students face before their applications come to our attention. In this article, we explore how potential differences in the social capital of UIM and other disadvantaged dermatology residency applicants contribute to persistent homogeneity among dermatology training programs and the workforce.
The Theory of Capital
The concepts of economic, social, and cultural capital originate from the writings of social theorist Pierre Bourdieu.3 All 3 forms of capital are interconnected, and they relate to each other in ways that often facilitate social division and inequality. Economic capital denotes an individual’s economic resources or wealth, while cultural capital refers to the knowledge, behaviors, and skills that demonstrate his/her economic class (eg, communication style, table manners).3 Social capital refers to an individual’s interpersonal connections in personal and professional settings and can be subdivided into 3 categories: bonds, bridges, and linkages.4,5 Herein, we will focus on bonds and bridges.
It has been suggested that bonds are important for “getting by,” while bridges are critical for “getting ahead.”5 Bonds refer to close relationships within a community of people with shared characteristics, such as racial/ethnic identity and culture, access to information, and resources (eg, family, friends). These bonds provide trust, safety, and financial and emotional support; however, they are considered to be inward-looking and can promote exclusion and homogeneity.5
On the other hand, bridges refer to social relationships that extend outward beyond one’s close circle of family and friends to other people with shared interests and goals who may have different social or cultural identities (eg, professional colleagues). These bridges are considered to be outward-looking and provide many benefits to individuals and society. They link diverse individuals, which tends to increase tolerance and disrupt stereotypes, and they facilitate the sharing of ideas, information, and innovation. Additionally, bridges between individuals from different networks facilitate access to increased resources and opportunities for all parties.5
The 3 forms of capital are inextricably linked. For example, with economic capital, a child’s family can purchase access to a prestigious private high school, where he/she will gain valuable social capital through bridges with other students and their families. At this school, the child also will accumulate cultural capital that increases his/her sense of belonging in these circles. Subsequently, both the social and cultural capital accumulated at this private high school can be exchanged for economic capital via social networks, skills, values, and behaviors that facilitate entry into higher education and professional training. As such, these 3 forms of capital work together to continue social/class divisions, hierarchies, and ultimately inequality.
Impact of Social Capital in Pursuing a Medical Career
For medical students whose bonds (ie, close family, friends) include physicians or other health care professionals, the journey to studying medicine and entering their chosen specialty will be facilitated by financial security, valuable “inside information” about the application process, study skills, and even clinical guidance. Additionally, these students will have access to professional networks for mentorship, shadowing experiences, and other potential advantages. Furthermore, social capital is associated with higher self-esteem,6 which likely improves academic performance and wards off imposter syndrome in these students.
For medical students from lower socioeconomic status backgrounds or those whose inner circles do not include physicians or other health care professionals, accumulating the social and cultural capital needed to successfully navigate a medical career is more difficult. Although they may receive support and encouragement from family and friends, they will not have access to the same valuable information and connections that facilitate success; rather, they will have a further distance to travel, and this distance should be acknowledged in the residency application review process.
Acquiring Social Capital as a UIM Student
Despite the benefits of social and cultural capital, acquiring them takes a toll. For those UIM students who start life from a disadvantaged place, the accumulation of social capital does not come easily; rather, it demands effort and time that has the potential to detract from a student’s focus on the academic demands of medical education.7 Programs that attempt to improve disadvantaged students’ access to credible information, role models, and mentors can help lift some of the burden from the individual student’s shoulders. For example, studies have demonstrated the benefits of harnessing technology to enhance mentorship programs that increase social capital of disadvantaged populations.8-11 This approach already is in progress, bolstered by advances made in digital communications during the coronavirus disease 2019 pandemic.12 Student-led networking groups that connect remotely have been shown to build social capital bonds and bridges that facilitate collaborative learning, relationship building, and information sharing.8-11 There are existing online UIM student networks that individual dermatologists, institutions, and national organizations can partner with to facilitate the construction of bridges between these UIM student groups and dermatologists who can provide accurate, high-yield information and professional networking; however, one limitation of this suggestion is the disparate access to technology in the UIM community.
Final Thoughts
It is important to note that assumptions should not be made about the level of economic, social, or cultural capital an individual possesses based on his/her race or ethnicity. Instead, mentors should attempt to be available to a diverse pool of students; take the time to get to know these students; and then provide the types of mentorship, information, exposure, and networking that each individual student needs. Another approach is to make a concerted effort to ensure that all students receive the same amount and quality of information about medical education and our specialty regardless of their level of economic, cultural, or social capital. Moreover, beyond the promotion of diversity through increasing numbers of UIM applicants, we should seek to reshape our specialty into a space that does not require students to subdue their existing diverse forms of capital but rather to bring these different perspectives and lived experiences to the table.13
As our specialty seeks to address its lack of racial diversity, many dermatologists have answered recent calls to action.1,2 As we work toward dismantling systemic issues that have created pervasive inequality in our residency application review and interview processes, consideration also should be given to psychosocial issues that underrepresented-in-medicine (UIM) students face before their applications come to our attention. In this article, we explore how potential differences in the social capital of UIM and other disadvantaged dermatology residency applicants contribute to persistent homogeneity among dermatology training programs and the workforce.
The Theory of Capital
The concepts of economic, social, and cultural capital originate from the writings of social theorist Pierre Bourdieu.3 All 3 forms of capital are interconnected, and they relate to each other in ways that often facilitate social division and inequality. Economic capital denotes an individual’s economic resources or wealth, while cultural capital refers to the knowledge, behaviors, and skills that demonstrate his/her economic class (eg, communication style, table manners).3 Social capital refers to an individual’s interpersonal connections in personal and professional settings and can be subdivided into 3 categories: bonds, bridges, and linkages.4,5 Herein, we will focus on bonds and bridges.
It has been suggested that bonds are important for “getting by,” while bridges are critical for “getting ahead.”5 Bonds refer to close relationships within a community of people with shared characteristics, such as racial/ethnic identity and culture, access to information, and resources (eg, family, friends). These bonds provide trust, safety, and financial and emotional support; however, they are considered to be inward-looking and can promote exclusion and homogeneity.5
On the other hand, bridges refer to social relationships that extend outward beyond one’s close circle of family and friends to other people with shared interests and goals who may have different social or cultural identities (eg, professional colleagues). These bridges are considered to be outward-looking and provide many benefits to individuals and society. They link diverse individuals, which tends to increase tolerance and disrupt stereotypes, and they facilitate the sharing of ideas, information, and innovation. Additionally, bridges between individuals from different networks facilitate access to increased resources and opportunities for all parties.5
The 3 forms of capital are inextricably linked. For example, with economic capital, a child’s family can purchase access to a prestigious private high school, where he/she will gain valuable social capital through bridges with other students and their families. At this school, the child also will accumulate cultural capital that increases his/her sense of belonging in these circles. Subsequently, both the social and cultural capital accumulated at this private high school can be exchanged for economic capital via social networks, skills, values, and behaviors that facilitate entry into higher education and professional training. As such, these 3 forms of capital work together to continue social/class divisions, hierarchies, and ultimately inequality.
Impact of Social Capital in Pursuing a Medical Career
For medical students whose bonds (ie, close family, friends) include physicians or other health care professionals, the journey to studying medicine and entering their chosen specialty will be facilitated by financial security, valuable “inside information” about the application process, study skills, and even clinical guidance. Additionally, these students will have access to professional networks for mentorship, shadowing experiences, and other potential advantages. Furthermore, social capital is associated with higher self-esteem,6 which likely improves academic performance and wards off imposter syndrome in these students.
For medical students from lower socioeconomic status backgrounds or those whose inner circles do not include physicians or other health care professionals, accumulating the social and cultural capital needed to successfully navigate a medical career is more difficult. Although they may receive support and encouragement from family and friends, they will not have access to the same valuable information and connections that facilitate success; rather, they will have a further distance to travel, and this distance should be acknowledged in the residency application review process.
Acquiring Social Capital as a UIM Student
Despite the benefits of social and cultural capital, acquiring them takes a toll. For those UIM students who start life from a disadvantaged place, the accumulation of social capital does not come easily; rather, it demands effort and time that has the potential to detract from a student’s focus on the academic demands of medical education.7 Programs that attempt to improve disadvantaged students’ access to credible information, role models, and mentors can help lift some of the burden from the individual student’s shoulders. For example, studies have demonstrated the benefits of harnessing technology to enhance mentorship programs that increase social capital of disadvantaged populations.8-11 This approach already is in progress, bolstered by advances made in digital communications during the coronavirus disease 2019 pandemic.12 Student-led networking groups that connect remotely have been shown to build social capital bonds and bridges that facilitate collaborative learning, relationship building, and information sharing.8-11 There are existing online UIM student networks that individual dermatologists, institutions, and national organizations can partner with to facilitate the construction of bridges between these UIM student groups and dermatologists who can provide accurate, high-yield information and professional networking; however, one limitation of this suggestion is the disparate access to technology in the UIM community.
Final Thoughts
It is important to note that assumptions should not be made about the level of economic, social, or cultural capital an individual possesses based on his/her race or ethnicity. Instead, mentors should attempt to be available to a diverse pool of students; take the time to get to know these students; and then provide the types of mentorship, information, exposure, and networking that each individual student needs. Another approach is to make a concerted effort to ensure that all students receive the same amount and quality of information about medical education and our specialty regardless of their level of economic, cultural, or social capital. Moreover, beyond the promotion of diversity through increasing numbers of UIM applicants, we should seek to reshape our specialty into a space that does not require students to subdue their existing diverse forms of capital but rather to bring these different perspectives and lived experiences to the table.13
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:7.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Bourdieu P. The forms of capital. In: Richardson J, ed. Handbook of Theory and Research for the Sociology of Education. Westport, CT: Greenwood; 1986:241-258.
- Granovetter MS. The strength of weak ties. Am J Sociol. 1973;78:1360-1380.
- Putnam RD. Bowling alone: America’s declining social capital. J Democracy. 1995;6:65-78.
- Han S. Longitudinal association between social capital and self-esteem: a matter of context. Psychiatry Research. 2015;226:340-346.
- Kirschling JM. Building social capital: leading and leveraging constituencies outside the college. J Nurs Educ. 2004;43:517-519.
- Radlick RL, Svedberg P, Nygren JM, et al. Digitally enhanced mentoring for immigrant youth social capital: protocol for a mixed methods pilot study and a randomized controlled trial [published online March 17, 2020]. JMIR Research Protocols. doi:10.2196/16472.
- Koh LC, Walker R, Wollersheim D, et al. I think someone is walking with me: the use of mobile phone for social capital development among women in four refugee communities. Int J Migration Health Social Care. 2018;14:411-424.
- Hartley A, Kassam AA. Social networking for learning in higher education: capitalising on social capital. ResearchGate website.https://www.researchgate.net/publication/311097860_Social_Networking_for_Learning_in_Higher_Education_Capitalising_on_Social_Capital. Published November 2016. Accessed October 19, 2020.
- Zalon ML. Using technology to build community in professional associations. J Contin Educ Nurs. 2008;39:235-240.
- Stewart CR, Chernoff KA, Wildman HF, et al. Recommendations for medical student preparedness and equity for dermatology residency applications during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E225-E226.
- Brosnan C, Southgate E, Outram S, et al. Experiences of medical students who are first in family to attend university. Med Educ. 2016;50:842-851.
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:7.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Bourdieu P. The forms of capital. In: Richardson J, ed. Handbook of Theory and Research for the Sociology of Education. Westport, CT: Greenwood; 1986:241-258.
- Granovetter MS. The strength of weak ties. Am J Sociol. 1973;78:1360-1380.
- Putnam RD. Bowling alone: America’s declining social capital. J Democracy. 1995;6:65-78.
- Han S. Longitudinal association between social capital and self-esteem: a matter of context. Psychiatry Research. 2015;226:340-346.
- Kirschling JM. Building social capital: leading and leveraging constituencies outside the college. J Nurs Educ. 2004;43:517-519.
- Radlick RL, Svedberg P, Nygren JM, et al. Digitally enhanced mentoring for immigrant youth social capital: protocol for a mixed methods pilot study and a randomized controlled trial [published online March 17, 2020]. JMIR Research Protocols. doi:10.2196/16472.
- Koh LC, Walker R, Wollersheim D, et al. I think someone is walking with me: the use of mobile phone for social capital development among women in four refugee communities. Int J Migration Health Social Care. 2018;14:411-424.
- Hartley A, Kassam AA. Social networking for learning in higher education: capitalising on social capital. ResearchGate website.https://www.researchgate.net/publication/311097860_Social_Networking_for_Learning_in_Higher_Education_Capitalising_on_Social_Capital. Published November 2016. Accessed October 19, 2020.
- Zalon ML. Using technology to build community in professional associations. J Contin Educ Nurs. 2008;39:235-240.
- Stewart CR, Chernoff KA, Wildman HF, et al. Recommendations for medical student preparedness and equity for dermatology residency applications during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E225-E226.
- Brosnan C, Southgate E, Outram S, et al. Experiences of medical students who are first in family to attend university. Med Educ. 2016;50:842-851.
Practice Points
- Achieving diversity in the field of dermatology will require a concerted effort to equalize access to mentorship, information, exposure, and networking for students of all backgrounds.
- Valuing diverse forms of capital in applicants ultimately will strengthen the dermatology workforce through inclusion of various lived experiences and perspectives.
How to Obtain a Dermatology Residency: A Guide Targeted to Underrepresented in Medicine Medical Students
There has been increasing attention focused on the lack of diversity within dermatology academic and residency programs.1-6 Several factors have been identified as contributing to this narrow pipeline of qualified applicants, including lack of mentorship, delayed exposure to the field, implicit bias, and lack of an overall holistic review of applications with overemphasis on board scores.1,5 In an effort to provide guidance to underrepresented in medicine (UIM) students who are interested in dermatology, the Skin of Color Society (SOCS) has created a detailed, step-by-step guide on how to obtain a position in a dermatology residency program,7 which was modeled after a similar resource created by the American Academy of Orthopaedic Surgeons.8 Here, we highlight the main SOCS recommendations to help guide medical students through a systematic approach to becoming successful applicants for dermatology residency.
Start Early
Competitive fields such as dermatology require intentional efforts starting at the beginning of medical school. Regardless of what specialty is right for you, begin by constructing a well-rounded application for residency immediately. Start by shadowing dermatologists and attending Grand Rounds held in your institution’s dermatology department to ensure that this field is right for you. Students are encouraged to meet with academic advisors and upperclassmen to seek guidance on gaining early exposure to dermatology at their home institutions (or nearby programs) during their first year. As a platform for learning about community-based dermatology activities, join your school’s Dermatology Interest Group, keeping in mind that an executive position in such a group can help foster relationships with faculty and residents of the dermatology department. A long-term commitment to community service also contributes to your depth as an applicant. Getting involved early helps students uncover health disparities in medicine and allows time to formulate ideas to implement change. Forming a well-rounded application mandates maintaining good academic standing, and students should prioritize mastering the curriculum, excelling in clinical rotations, and studying for the US Medical Licensing Examination (USMLE).
Choose a Mentor
The summer between your first and second years of medical school is an opportune time to explore research opportunities. Students successfully complete research by taking ownership of a project, efficiently meeting deadlines, maintaining contact with research mentors by quickly responding to emails, and producing quality work. Research outside of dermatology also is valued. Research mentors often provide future letters of recommendation, so commit to doing an outstanding job. For those finding it difficult to locate a mentor, consider searching the American Academy of Dermatology (AAD)(https://www.aad.org/mentorship/) or SOCS (https://skinofcolorsociety.org/) websites. The AAD has an established Diversity Mentorship Program (https://www.aad.org/member/career/volunteer/diversity-mentorship) that provides members with direct guidance from dermatologists for 4 weeks. Students use this time to conduct research, learn more about the specialty, and foster a relationship with their mentor. Students can apply any year of medical school; however, the typical awardee usually is a third-year or fourth-year student. The AAD may provide a stipend to help offset expenses.
Prepare for Boards
Second year is a continuation of the agenda set forth in first year, now with the focus shifting toward board preparation and excelling in clinical core didactics and rotations. According to data from the 2018 National Resident Matching Program,9 the mean USMLE Step 1 score for US allopathic senior medical students who matched into dermatology was 249 compared to 241 who did not match into dermatology. However, the mean score is just that—a mean—and people have matched with lower scores. Do not be intimidated by this number; instead, be driven to commit the time and resources to master the content and do your personal best on the USMLE Step examinations. Given the shift in some programs for earlier clinical exposure and postponement of boards until the third year, the recommendations in this timeline can be catered to fit a medical student’s specific situation.
Build Your Application
The third year of medical school is a busy year. Prepare for third-year clinical rotations by speaking with upperclassmen and clinical preceptors as you progress through your rotations. Evaluations and recommendations are weighed heavily by residency program directors, as this information is used to ascertain your clinical abilities. Seek feedback from your preceptors early and often with a sincere attempt to integrate suggested improvements. Schedule a dermatology rotation at your home institution after completing the core rotations. Although they are not required, applicants may complete away rotations early in their fourth year; the application period for visiting student learning opportunities typically opens April 1 of the third year, if not earlier. Free resources are available to help prepare for your dermatology rotations. Start by reviewing the Basic Dermatology Curriculum on the AAD website (https://www.aad.org/member/education/residents/bdc). Make contributions to
Interviewing for Residency
During your fourth year of medical school, you will be completing dermatology rotations, submitting your applications through the Electronic Residency Application Service, and interviewing with residency programs. When deciding which programs to apply to, consider referencing the American Medical Association Residency and Fellowship Database (https://freida.ama-assn.org/Freida/#/). Also keep in mind that, depending on your competitiveness, you should expect to receive 1 interview for every 10 programs you apply to, thus the application process can be quite costly. It is highly encouraged that you ask for letters of recommendation prior to August 15 and that you submit your applications by September 15. Complete mock interviews with a mentor and research commonly asked questions. Prior to your interview day, you want to spend time researching the program, browsing faculty publications, and reviewing your application. Dress in a comfortable suit, shoes, and minimal accessories; arrive early knowing that your interview begins even before you meet your interviewer, so treat everyone you meet with respect. Refrain from speaking to anyone in a casual way and have questions prepared to ask each interviewer. After your interviews, be sure to write thank you notes or emails if a program does not specifically discourage postinterview communication. Continuous efforts will improve your success in obtaining a dermatology residency position.
Final Thoughts
Recent articles have underscored and emphasized the importance of diversity in our field, with a call to action to find meaningful and overdue solutions.2,6 We acknowledge the important role that mentors play in providing timely, honest, and encouraging guidance to UIM students interested in careers in dermatology. We hope to provide readily available and detailed guidance to these students on how they can present themselves as excellent and qualified applicants through this summary and other platforms.
Acknowledgment
The authors would like to thank the members of the SOCS Diversity Task Force for their assistance in creating the original guide.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Taylor SC. Meeting the unique dermatologic needs of black patients [published online August 21, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1963.
- Skin of Color Society. How to obtain a position in a dermatology residency program. https://skinofcolorsociety.org/wp-content/uploads/2019/10/How-to-Obtain-a-Position-in-a-Dermatology-Residency-Program-10-08-2019.pdf. Accessed June 24, 2020.
- American Academy of Orthopaedic Surgeons. How to obtain an orthopedic residency by the American Academy of Orthopaedic Surgeons. https://www.aaos.org/globalassets/about/diversity/how-to-obtain-an-orthopaedic-residency.pdf. Accessed June 24, 2020.
- Results and Data—2018 Main Residency Match. Washington, DC: National Resident Matching Program; 2018. Published April 2018. Accessed June 24, 2020.
There has been increasing attention focused on the lack of diversity within dermatology academic and residency programs.1-6 Several factors have been identified as contributing to this narrow pipeline of qualified applicants, including lack of mentorship, delayed exposure to the field, implicit bias, and lack of an overall holistic review of applications with overemphasis on board scores.1,5 In an effort to provide guidance to underrepresented in medicine (UIM) students who are interested in dermatology, the Skin of Color Society (SOCS) has created a detailed, step-by-step guide on how to obtain a position in a dermatology residency program,7 which was modeled after a similar resource created by the American Academy of Orthopaedic Surgeons.8 Here, we highlight the main SOCS recommendations to help guide medical students through a systematic approach to becoming successful applicants for dermatology residency.
Start Early
Competitive fields such as dermatology require intentional efforts starting at the beginning of medical school. Regardless of what specialty is right for you, begin by constructing a well-rounded application for residency immediately. Start by shadowing dermatologists and attending Grand Rounds held in your institution’s dermatology department to ensure that this field is right for you. Students are encouraged to meet with academic advisors and upperclassmen to seek guidance on gaining early exposure to dermatology at their home institutions (or nearby programs) during their first year. As a platform for learning about community-based dermatology activities, join your school’s Dermatology Interest Group, keeping in mind that an executive position in such a group can help foster relationships with faculty and residents of the dermatology department. A long-term commitment to community service also contributes to your depth as an applicant. Getting involved early helps students uncover health disparities in medicine and allows time to formulate ideas to implement change. Forming a well-rounded application mandates maintaining good academic standing, and students should prioritize mastering the curriculum, excelling in clinical rotations, and studying for the US Medical Licensing Examination (USMLE).
Choose a Mentor
The summer between your first and second years of medical school is an opportune time to explore research opportunities. Students successfully complete research by taking ownership of a project, efficiently meeting deadlines, maintaining contact with research mentors by quickly responding to emails, and producing quality work. Research outside of dermatology also is valued. Research mentors often provide future letters of recommendation, so commit to doing an outstanding job. For those finding it difficult to locate a mentor, consider searching the American Academy of Dermatology (AAD)(https://www.aad.org/mentorship/) or SOCS (https://skinofcolorsociety.org/) websites. The AAD has an established Diversity Mentorship Program (https://www.aad.org/member/career/volunteer/diversity-mentorship) that provides members with direct guidance from dermatologists for 4 weeks. Students use this time to conduct research, learn more about the specialty, and foster a relationship with their mentor. Students can apply any year of medical school; however, the typical awardee usually is a third-year or fourth-year student. The AAD may provide a stipend to help offset expenses.
Prepare for Boards
Second year is a continuation of the agenda set forth in first year, now with the focus shifting toward board preparation and excelling in clinical core didactics and rotations. According to data from the 2018 National Resident Matching Program,9 the mean USMLE Step 1 score for US allopathic senior medical students who matched into dermatology was 249 compared to 241 who did not match into dermatology. However, the mean score is just that—a mean—and people have matched with lower scores. Do not be intimidated by this number; instead, be driven to commit the time and resources to master the content and do your personal best on the USMLE Step examinations. Given the shift in some programs for earlier clinical exposure and postponement of boards until the third year, the recommendations in this timeline can be catered to fit a medical student’s specific situation.
Build Your Application
The third year of medical school is a busy year. Prepare for third-year clinical rotations by speaking with upperclassmen and clinical preceptors as you progress through your rotations. Evaluations and recommendations are weighed heavily by residency program directors, as this information is used to ascertain your clinical abilities. Seek feedback from your preceptors early and often with a sincere attempt to integrate suggested improvements. Schedule a dermatology rotation at your home institution after completing the core rotations. Although they are not required, applicants may complete away rotations early in their fourth year; the application period for visiting student learning opportunities typically opens April 1 of the third year, if not earlier. Free resources are available to help prepare for your dermatology rotations. Start by reviewing the Basic Dermatology Curriculum on the AAD website (https://www.aad.org/member/education/residents/bdc). Make contributions to
Interviewing for Residency
During your fourth year of medical school, you will be completing dermatology rotations, submitting your applications through the Electronic Residency Application Service, and interviewing with residency programs. When deciding which programs to apply to, consider referencing the American Medical Association Residency and Fellowship Database (https://freida.ama-assn.org/Freida/#/). Also keep in mind that, depending on your competitiveness, you should expect to receive 1 interview for every 10 programs you apply to, thus the application process can be quite costly. It is highly encouraged that you ask for letters of recommendation prior to August 15 and that you submit your applications by September 15. Complete mock interviews with a mentor and research commonly asked questions. Prior to your interview day, you want to spend time researching the program, browsing faculty publications, and reviewing your application. Dress in a comfortable suit, shoes, and minimal accessories; arrive early knowing that your interview begins even before you meet your interviewer, so treat everyone you meet with respect. Refrain from speaking to anyone in a casual way and have questions prepared to ask each interviewer. After your interviews, be sure to write thank you notes or emails if a program does not specifically discourage postinterview communication. Continuous efforts will improve your success in obtaining a dermatology residency position.
Final Thoughts
Recent articles have underscored and emphasized the importance of diversity in our field, with a call to action to find meaningful and overdue solutions.2,6 We acknowledge the important role that mentors play in providing timely, honest, and encouraging guidance to UIM students interested in careers in dermatology. We hope to provide readily available and detailed guidance to these students on how they can present themselves as excellent and qualified applicants through this summary and other platforms.
Acknowledgment
The authors would like to thank the members of the SOCS Diversity Task Force for their assistance in creating the original guide.
There has been increasing attention focused on the lack of diversity within dermatology academic and residency programs.1-6 Several factors have been identified as contributing to this narrow pipeline of qualified applicants, including lack of mentorship, delayed exposure to the field, implicit bias, and lack of an overall holistic review of applications with overemphasis on board scores.1,5 In an effort to provide guidance to underrepresented in medicine (UIM) students who are interested in dermatology, the Skin of Color Society (SOCS) has created a detailed, step-by-step guide on how to obtain a position in a dermatology residency program,7 which was modeled after a similar resource created by the American Academy of Orthopaedic Surgeons.8 Here, we highlight the main SOCS recommendations to help guide medical students through a systematic approach to becoming successful applicants for dermatology residency.
Start Early
Competitive fields such as dermatology require intentional efforts starting at the beginning of medical school. Regardless of what specialty is right for you, begin by constructing a well-rounded application for residency immediately. Start by shadowing dermatologists and attending Grand Rounds held in your institution’s dermatology department to ensure that this field is right for you. Students are encouraged to meet with academic advisors and upperclassmen to seek guidance on gaining early exposure to dermatology at their home institutions (or nearby programs) during their first year. As a platform for learning about community-based dermatology activities, join your school’s Dermatology Interest Group, keeping in mind that an executive position in such a group can help foster relationships with faculty and residents of the dermatology department. A long-term commitment to community service also contributes to your depth as an applicant. Getting involved early helps students uncover health disparities in medicine and allows time to formulate ideas to implement change. Forming a well-rounded application mandates maintaining good academic standing, and students should prioritize mastering the curriculum, excelling in clinical rotations, and studying for the US Medical Licensing Examination (USMLE).
Choose a Mentor
The summer between your first and second years of medical school is an opportune time to explore research opportunities. Students successfully complete research by taking ownership of a project, efficiently meeting deadlines, maintaining contact with research mentors by quickly responding to emails, and producing quality work. Research outside of dermatology also is valued. Research mentors often provide future letters of recommendation, so commit to doing an outstanding job. For those finding it difficult to locate a mentor, consider searching the American Academy of Dermatology (AAD)(https://www.aad.org/mentorship/) or SOCS (https://skinofcolorsociety.org/) websites. The AAD has an established Diversity Mentorship Program (https://www.aad.org/member/career/volunteer/diversity-mentorship) that provides members with direct guidance from dermatologists for 4 weeks. Students use this time to conduct research, learn more about the specialty, and foster a relationship with their mentor. Students can apply any year of medical school; however, the typical awardee usually is a third-year or fourth-year student. The AAD may provide a stipend to help offset expenses.
Prepare for Boards
Second year is a continuation of the agenda set forth in first year, now with the focus shifting toward board preparation and excelling in clinical core didactics and rotations. According to data from the 2018 National Resident Matching Program,9 the mean USMLE Step 1 score for US allopathic senior medical students who matched into dermatology was 249 compared to 241 who did not match into dermatology. However, the mean score is just that—a mean—and people have matched with lower scores. Do not be intimidated by this number; instead, be driven to commit the time and resources to master the content and do your personal best on the USMLE Step examinations. Given the shift in some programs for earlier clinical exposure and postponement of boards until the third year, the recommendations in this timeline can be catered to fit a medical student’s specific situation.
Build Your Application
The third year of medical school is a busy year. Prepare for third-year clinical rotations by speaking with upperclassmen and clinical preceptors as you progress through your rotations. Evaluations and recommendations are weighed heavily by residency program directors, as this information is used to ascertain your clinical abilities. Seek feedback from your preceptors early and often with a sincere attempt to integrate suggested improvements. Schedule a dermatology rotation at your home institution after completing the core rotations. Although they are not required, applicants may complete away rotations early in their fourth year; the application period for visiting student learning opportunities typically opens April 1 of the third year, if not earlier. Free resources are available to help prepare for your dermatology rotations. Start by reviewing the Basic Dermatology Curriculum on the AAD website (https://www.aad.org/member/education/residents/bdc). Make contributions to
Interviewing for Residency
During your fourth year of medical school, you will be completing dermatology rotations, submitting your applications through the Electronic Residency Application Service, and interviewing with residency programs. When deciding which programs to apply to, consider referencing the American Medical Association Residency and Fellowship Database (https://freida.ama-assn.org/Freida/#/). Also keep in mind that, depending on your competitiveness, you should expect to receive 1 interview for every 10 programs you apply to, thus the application process can be quite costly. It is highly encouraged that you ask for letters of recommendation prior to August 15 and that you submit your applications by September 15. Complete mock interviews with a mentor and research commonly asked questions. Prior to your interview day, you want to spend time researching the program, browsing faculty publications, and reviewing your application. Dress in a comfortable suit, shoes, and minimal accessories; arrive early knowing that your interview begins even before you meet your interviewer, so treat everyone you meet with respect. Refrain from speaking to anyone in a casual way and have questions prepared to ask each interviewer. After your interviews, be sure to write thank you notes or emails if a program does not specifically discourage postinterview communication. Continuous efforts will improve your success in obtaining a dermatology residency position.
Final Thoughts
Recent articles have underscored and emphasized the importance of diversity in our field, with a call to action to find meaningful and overdue solutions.2,6 We acknowledge the important role that mentors play in providing timely, honest, and encouraging guidance to UIM students interested in careers in dermatology. We hope to provide readily available and detailed guidance to these students on how they can present themselves as excellent and qualified applicants through this summary and other platforms.
Acknowledgment
The authors would like to thank the members of the SOCS Diversity Task Force for their assistance in creating the original guide.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Taylor SC. Meeting the unique dermatologic needs of black patients [published online August 21, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1963.
- Skin of Color Society. How to obtain a position in a dermatology residency program. https://skinofcolorsociety.org/wp-content/uploads/2019/10/How-to-Obtain-a-Position-in-a-Dermatology-Residency-Program-10-08-2019.pdf. Accessed June 24, 2020.
- American Academy of Orthopaedic Surgeons. How to obtain an orthopedic residency by the American Academy of Orthopaedic Surgeons. https://www.aaos.org/globalassets/about/diversity/how-to-obtain-an-orthopaedic-residency.pdf. Accessed June 24, 2020.
- Results and Data—2018 Main Residency Match. Washington, DC: National Resident Matching Program; 2018. Published April 2018. Accessed June 24, 2020.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Taylor SC. Meeting the unique dermatologic needs of black patients [published online August 21, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1963.
- Skin of Color Society. How to obtain a position in a dermatology residency program. https://skinofcolorsociety.org/wp-content/uploads/2019/10/How-to-Obtain-a-Position-in-a-Dermatology-Residency-Program-10-08-2019.pdf. Accessed June 24, 2020.
- American Academy of Orthopaedic Surgeons. How to obtain an orthopedic residency by the American Academy of Orthopaedic Surgeons. https://www.aaos.org/globalassets/about/diversity/how-to-obtain-an-orthopaedic-residency.pdf. Accessed June 24, 2020.
- Results and Data—2018 Main Residency Match. Washington, DC: National Resident Matching Program; 2018. Published April 2018. Accessed June 24, 2020.
Practice Points
- Students interested in dermatology are encouraged to seek mentorship, strive for their academic best, and maintain their unique personal interests that make them a well-rounded applicant.
- Increasing diversity in dermatology requires initiative from students as well as dermatologists who are willing to mentor and sponsor.
Hair Care Products Used by Women of African Descent: Review of Ingredients
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.
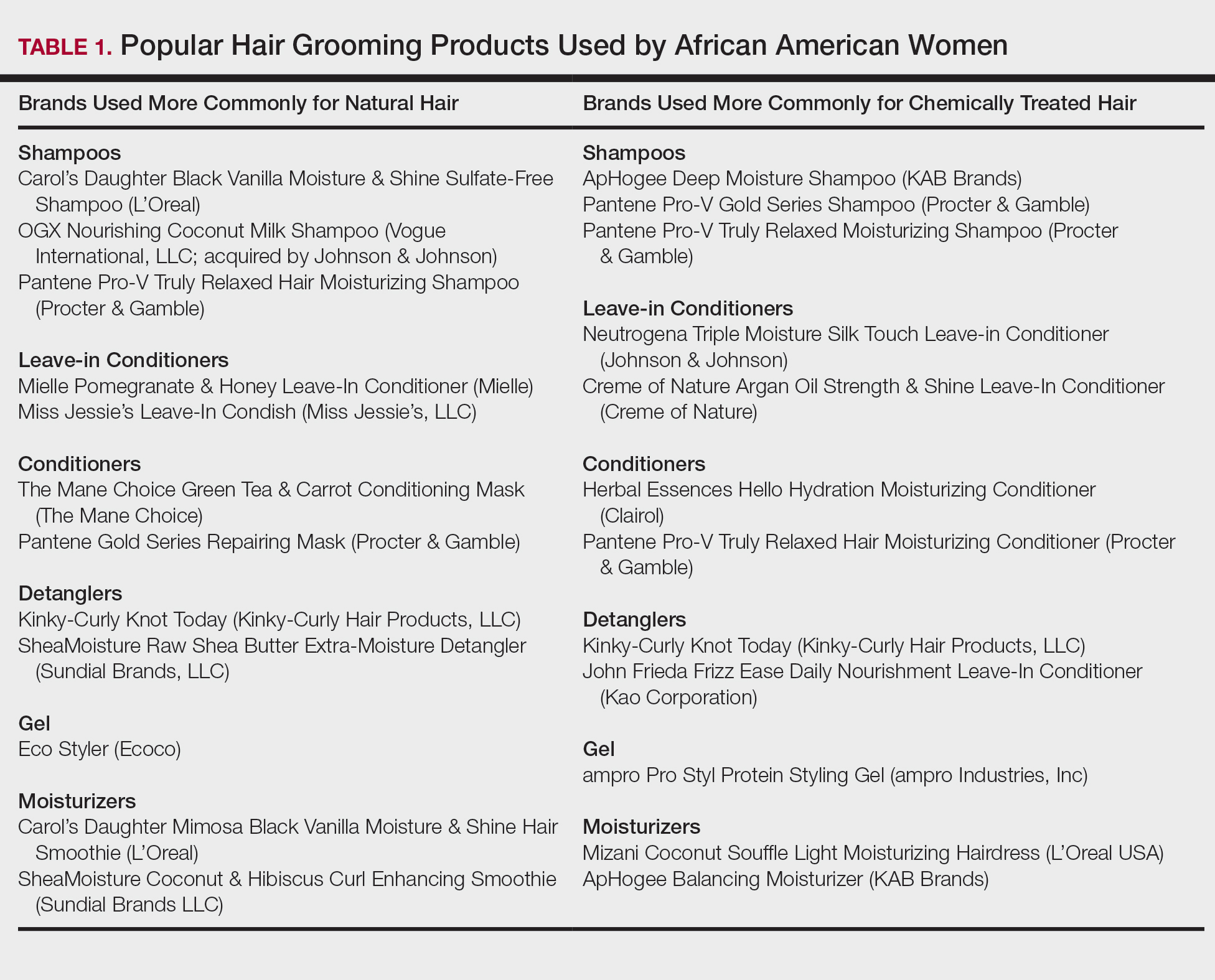
Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.

Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.

Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
Practice Points
- Dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent patient interactions and treatment.
- Common ingredients in popular hair care products used by African Americans include sulfates, cationic surfactants and polymers, silicone, oils, and parabens.
Racial Limitations of Fitzpatrick Skin Type
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20
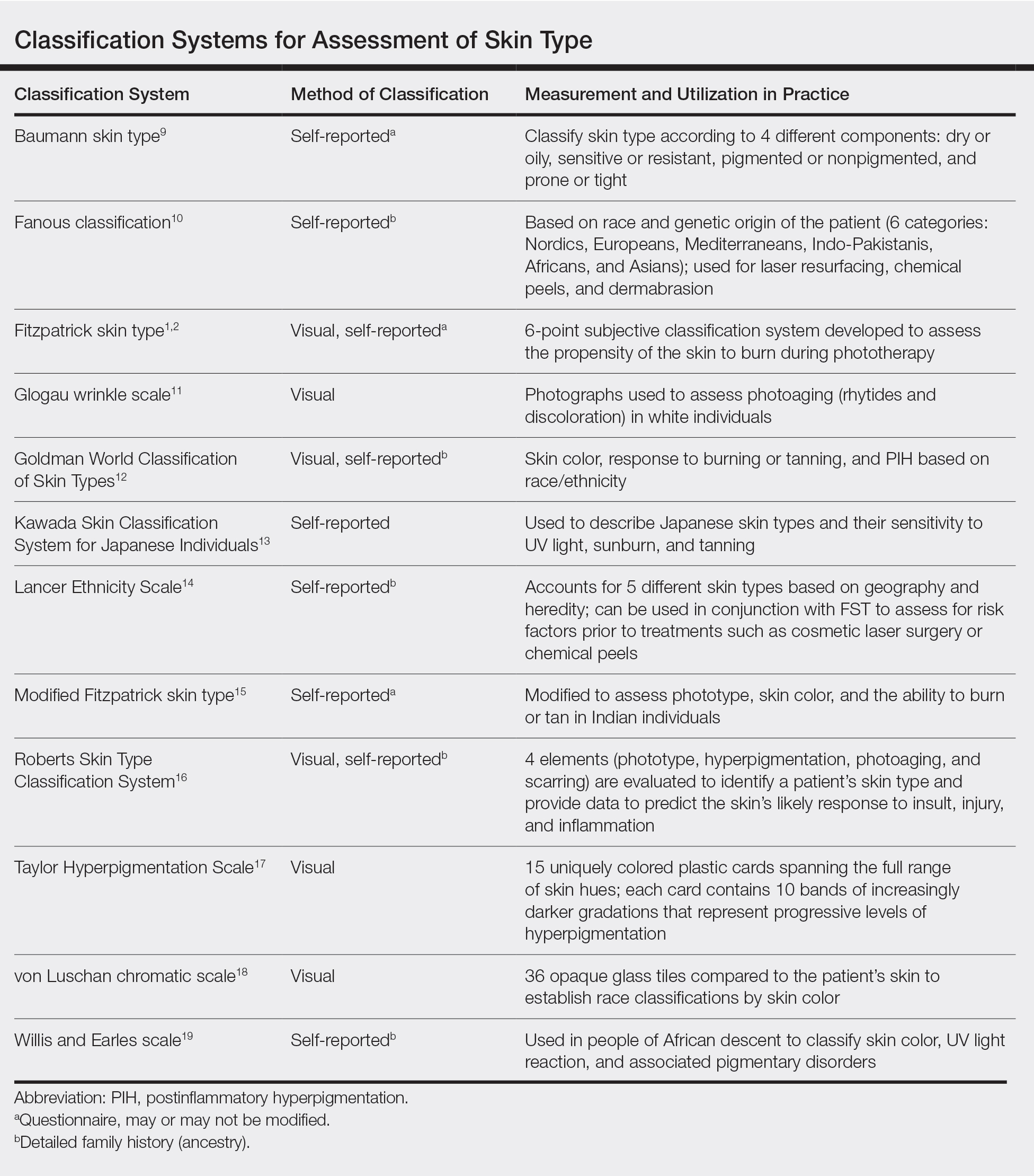
Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20

Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20

Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Practice Points
- Medical providers should be cognizant of conflating race and ethnicity with Fitzpatrick skin type (FST).
- Misuse of FST may occur more frequently among physicians who do not identify as having skin of color.
- Although alternative skin type classification systems have been proposed, more clinically relevant methods for describing skin of color need to be developed.
Atopic Dermatitis in Adolescents With Skin of Color
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).

Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6
The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).

Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6

The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).

Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6

The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
Practice Points
- Atopic dermatitis (AD) can be a lifelong issue that affects academic and occupational performance, with higher rates of absenteeism seen in black patients.
- The FLG loss-of-function mutation seems to play a smaller role in black patients, and other genes may be involved in skin barrier dysfunction, which could be why there is a higher rate of skin of color patients with treatment-resistant AD.
- Diagnosing AD in skin of color patients can pose a particular challenge, and severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin tones.
- There are several areas of opportunity for further research to better treat AD in this patient population and improve
quality of life.
Establishing the Diagnosis of Rosacea in Skin of Color Patients
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.

Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.

Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.

Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.

Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.

Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.

Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
Practice Points
- The clinical signs of rosacea may be similar in all skin types; however, dermatologists must have a high clinical index of suspicion for rosacea in patients with skin of color (SOC).
- Dermatologists should consider a wide differential diagnosis when presented with an SOC patient with facial erythema and/or papules and pustules.
Dermoscopic Patterns of Acral Melanocytic Lesions in Skin of Color
Acral lentiginous melanoma (ALM) is a rare subtype of melanoma that occurs on the palms, soles, and nail apparatus. Unlike more common types of melanoma, ALM occurs on sun-protected areas of the skin and has distinct clinical, histologic, and genetic features. Acral lentiginous melanoma accounts for a larger proportion of melanomas in individuals with skin of color and has a worse prognosis and recurrence rate than other forms of melanoma.
Population Trends in Skin of Color
Much of the literature on malignant melanoma historically has involved non-Hispanic white patients, but the incidence in lighter-skinned populations has been increasing steadily over the last few decades.1 Although ALM can occur in any race, it disproportionately affects skin of color populations; ALM accounts for only 0.8% to 1% of all melanomas in white populations, but it constitutes 4% to 58% of melanomas in ethnic populations and is the most common melanoma subtype among black Americans.2-5 Acral lentiginous melanoma also is associated with a worse prognosis compared to other subtypes, which may indicate a more aggressive biological nature6 but also may point toward socioeconomic and cultural barriers (eg, low income or education levels, lack of insurance, lower health literacy), leading to disparities in access to care and diagnosis at advanced stages.5
Similarly, the distribution of acral melanocytic nevi appears to demonstrate an association with ethnicity and skin pigmentation. Although skin of color patients have fewer nevi than non-Hispanic whites, the proportion of acral melanocytic nevi tends to be greater.6,7 Given its grim prognosis, accurately differentiating ALM from acral nevi is of utmost importance.
Diagnostic Challenges of Acral Lesions
Due to the unique nature of the surfaces of acral sites, melanocytic lesions on the palms, soles, and nail apparatus present many diagnostic challenges. It can be difficult to distinguish acral melanoma from benign lesions using the naked eye alone. Volar surfaces are characterized by the presence of dermatoglyphics, and pigment deposition along ridges and furrows create particular dermoscopic patterns exclusive to these sites.8 Thus, dermoscopy can be useful on acral surfaces, but the dermoscopic features are different from those on the rest of the body and must be learned separately.
In addition, nearly half of patients are unaware of their acral lesions.6 Acral surfaces may not always be examined by clinicians during total-body skin examinations, leading to further possibility of overlooking a lesion. Obtaining biopsies on glabrous skin or nails also is challenging because they can be more painful and hemostasis can be more difficult, especially in the nail. Acral melanomas also may be amelanotic, including those at subungual sites.
Dermoscopic Patterns of Acral Volar Skin
Dermoscopy is a useful noninvasive tool for distinguishing between benign and malignant acral melanocytic lesions, and its efficacy in improving diagnostic accuracy and decreasing unnecessary biopsies is well-established in the literature.13,14 Acral dermoscopy allows for visualization of pigment along the dermatoglyphics that constitute the characteristic dermoscopic patterns.
Acral Lentiginous Melanoma
The hallmark dermoscopic pattern and most important finding of ALM is the parallel ridge pattern, characterized by parallel linear pigmentation along the ridges of dermatoglyphics. In the early phases of malignancy, the pattern appears light brown and involves most of the lesion; as the tumor develops, increasing melanin production results in focal areas of the parallel ridge pattern with darker bands.15,16 The sensitivity and specificity of a parallel ridge pattern for diagnosing early ALM has been shown to be 86% and 99%, respectively.15,16
A pattern of irregular diffuse pigmentation also can be observed in more advanced ALM. Dermoscopy may reveal a structureless pattern (ie, lack of identifiable structures or patterns) in a background of tan-black coloration due to more exuberant melanocyte proliferation along the epidermis.15 Sensitivity and specificity of this dermoscopic finding for invasive lesions is high at 94% and 97%, respectively.16,17 Interestingly, once ALM lesions have advanced even further, conventional melanoma-associated structures (ie, blue-white veil, polymorphous blood vessels, ulceration, irregular dots/globules or streaks) or atypical forms of typically benign acral dermoscopic patterns may be observed.15
Per a 3-step diagnostic algorithm created by Koga and Saida,18 a suspected acral lesion should first be evaluated for a parallel ridge pattern to determine the need for biopsy, as it is seen in approximately two-thirds of ALMs.19 If no parallel ridge pattern is observed, the lesion should then be checked for any of the typical dermoscopic patterns seen in benign acral nevi (eg, parallel furrow, latticelike, or fibrillar patterns).18 The maximum diameter should be measured only if the lesion does not exhibit any of the typical dermoscopic patterns. If the lesion’s diameter is greater than 7 mm in diameter, it should be biopsied; if the diameter is less than 7 mm, it should have regular clinical and dermoscopic follow-up.18
In 2015, Lallas et al20 developed the BRAAFF checklist, a scoring system of 6 variables: blotches, ridge pattern, asymmetry of structures, asymmetry of colors, parallel furrow pattern, and fibrillar pattern. The checklist also was shown to substantially improve diagnostic accuracy of dermoscopy for ALM, with sensitivity and specificity at 93.1% and 86.7%, respectively.20
Acquired Acral Nevi
Three classic dermoscopic patterns are associated with acquired acral nevi: parallel furrow pattern, latticelike pattern, and fibrillar pattern.15,21 Approximately three-quarters of all acquired acral nevi exhibit one of these patterns, roughly half exhibiting parallel furrow with tan-brown bandlike pigmentation along dermatoglyphic grooves.16,17
Latticelike patterns also are characterized by brown parallel lines along the sulci of dermatoglyphics but additionally have multiple intersecting lines. Thus, this pattern can be considered a variant of the parallel furrow pattern.15 The crisscross markings can be predominantly found in the plantar arch.22 This dermoscopic pattern comprises 15% to 25% of all acral nevi.21
Fibrillar pattern accounts for 10% to 20% of all acral melanocytic nevi.21 Dermoscopically, these lesions demonstrate parallel filamentous streaks that cross dermatoglyphics obliquely. The fibrillar pattern is predominantly found on weight-bearing areas of the sole,22 which likely is explained by pressure causing slanting of melanin columns in the horny layer.23 The fibrillar pattern has been shown to be the benign acral dermoscopic pattern that is most commonly misdiagnosed, with higher reported rates of biopsy.24
Acral Congenital Melanocytic Nevi
Congenital melanocytic nevi (CMN) present at birth or appear during the first few weeks of life. Congenital melanocytic nevi can vary widely in size, shape, and color, and they are occasionally biopsied in cases of larger diameter or dermoscopic atypia to differentiate from melanoma.25 Congenital melanocytic nevi also can occur on acral volar surfaces. Possible dermoscopic patterns include parallel furrow or fibrillar patterns as well as a crista dotted pattern, defined as evenly spaced dots/globules on the ridges near the openings of eccrine ducts.26 A more commonly observed dermoscopic pattern in acral CMN is a combination of the crista dotted and parallel furrow patterns, known as the peas-in-a-pod pattern. Changes in the clinical appearance and dermoscopic features of an acral CMN are possible over time; some lesions also may fade with age.26
Final Thoughts
Acral lentiginous melanoma is a rare but potentially aggressive melanoma subtype that accounts for a larger proportion of melanomas in patients with skin of color than in white patients. Dermoscopy of acral volar skin provides invaluable diagnostic information and allows for better management of acral melanocytic lesions. Dermoscopic patterns such as the parallel ridge, parallel furrow, latticelike, fibrillar, and peas-in-a-pod patterns are unique to acral sites and can be used to differentiate between ALMs, acquired nevi, or CMNs.
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136:1161-1171.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19:42.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691.
- Madankumar R, Gumaste PV, Martires K, et al. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74:724.e1-730.e1.
- Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch Dermatol. 2010;146:1085-1094.
- Thomas L, Phan A, Pralong P, et al. Special locations dermoscopy: facial, acral, and nail. Dermatol Clin. 2013;31:615-624.
- Gong HZ, Zheng HY, Li J. Amelanotic melanoma [published online January 21, 2019]. Melanoma Res. doi:10.1097/CMR.0000000000000571.
- Ise M, Yasuda F, Konohana I, et al. Acral melanoma with hyperkeratosis mimicking a pigmented wart. Dermatol Pract Concept. 2013;3:37-39.
- Serarslan G, Akçaly CM, Atik E. Acral lentiginous melanoma misdiagnosed as tinea pedis: a case report. Int J Dermatol. 2004;43:37-38.
- Gumaste P, Penn L, Cohen N, et al. Acral lentiginous melanoma of the foot misdiagnosed as a traumatic ulcer. a cautionary case. J Am Podiatr Med Assoc. 2015;105:189-194.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-689.
- Carli P, de Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997-2001. Br J Dermatol. 2004;150:687-692.
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34.
- Ishihara Y, Saida T, Miyazaki A, et al. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol. 2006;28:21-27.
- Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238.
- Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch Dermatol. 2011;147:741-743.
- Lallas A, Sgouros D, Zalaudek I, et al. Palmar and plantar melanomas differ for sex prevalence and tumor thickness but not for dermoscopic patterns. Melanoma Res. 2014;24:83-87.
- Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173:1041-1049.
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426.
- Miyazaki A, Saida T, Koga H, et al. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J Am Acad Dermatol. 2005;53:230-236.
- Watanabe S, Sawada M, Ishizaki S, et al. Comparison of dermatoscopic images of acral lentiginous melanoma and acral melanocytic nevus occurring on body weight-bearing areas. Dermatol Pract Concept. 2014;4:47-50.
- Costello CM, Ghanavatian S, Temkit M, et al. Educational and practice gaps in the management of volar melanocytic lesions. J Eur Acad Dermatol Venereol. 2018;32:1450-1455.
- Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? part I. clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012;67:495.e1-495.e17; quiz 512-514.
- Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch Dermatol. 2011;147:809-813.
Acral lentiginous melanoma (ALM) is a rare subtype of melanoma that occurs on the palms, soles, and nail apparatus. Unlike more common types of melanoma, ALM occurs on sun-protected areas of the skin and has distinct clinical, histologic, and genetic features. Acral lentiginous melanoma accounts for a larger proportion of melanomas in individuals with skin of color and has a worse prognosis and recurrence rate than other forms of melanoma.
Population Trends in Skin of Color
Much of the literature on malignant melanoma historically has involved non-Hispanic white patients, but the incidence in lighter-skinned populations has been increasing steadily over the last few decades.1 Although ALM can occur in any race, it disproportionately affects skin of color populations; ALM accounts for only 0.8% to 1% of all melanomas in white populations, but it constitutes 4% to 58% of melanomas in ethnic populations and is the most common melanoma subtype among black Americans.2-5 Acral lentiginous melanoma also is associated with a worse prognosis compared to other subtypes, which may indicate a more aggressive biological nature6 but also may point toward socioeconomic and cultural barriers (eg, low income or education levels, lack of insurance, lower health literacy), leading to disparities in access to care and diagnosis at advanced stages.5
Similarly, the distribution of acral melanocytic nevi appears to demonstrate an association with ethnicity and skin pigmentation. Although skin of color patients have fewer nevi than non-Hispanic whites, the proportion of acral melanocytic nevi tends to be greater.6,7 Given its grim prognosis, accurately differentiating ALM from acral nevi is of utmost importance.
Diagnostic Challenges of Acral Lesions
Due to the unique nature of the surfaces of acral sites, melanocytic lesions on the palms, soles, and nail apparatus present many diagnostic challenges. It can be difficult to distinguish acral melanoma from benign lesions using the naked eye alone. Volar surfaces are characterized by the presence of dermatoglyphics, and pigment deposition along ridges and furrows create particular dermoscopic patterns exclusive to these sites.8 Thus, dermoscopy can be useful on acral surfaces, but the dermoscopic features are different from those on the rest of the body and must be learned separately.
In addition, nearly half of patients are unaware of their acral lesions.6 Acral surfaces may not always be examined by clinicians during total-body skin examinations, leading to further possibility of overlooking a lesion. Obtaining biopsies on glabrous skin or nails also is challenging because they can be more painful and hemostasis can be more difficult, especially in the nail. Acral melanomas also may be amelanotic, including those at subungual sites.
Dermoscopic Patterns of Acral Volar Skin
Dermoscopy is a useful noninvasive tool for distinguishing between benign and malignant acral melanocytic lesions, and its efficacy in improving diagnostic accuracy and decreasing unnecessary biopsies is well-established in the literature.13,14 Acral dermoscopy allows for visualization of pigment along the dermatoglyphics that constitute the characteristic dermoscopic patterns.
Acral Lentiginous Melanoma
The hallmark dermoscopic pattern and most important finding of ALM is the parallel ridge pattern, characterized by parallel linear pigmentation along the ridges of dermatoglyphics. In the early phases of malignancy, the pattern appears light brown and involves most of the lesion; as the tumor develops, increasing melanin production results in focal areas of the parallel ridge pattern with darker bands.15,16 The sensitivity and specificity of a parallel ridge pattern for diagnosing early ALM has been shown to be 86% and 99%, respectively.15,16
A pattern of irregular diffuse pigmentation also can be observed in more advanced ALM. Dermoscopy may reveal a structureless pattern (ie, lack of identifiable structures or patterns) in a background of tan-black coloration due to more exuberant melanocyte proliferation along the epidermis.15 Sensitivity and specificity of this dermoscopic finding for invasive lesions is high at 94% and 97%, respectively.16,17 Interestingly, once ALM lesions have advanced even further, conventional melanoma-associated structures (ie, blue-white veil, polymorphous blood vessels, ulceration, irregular dots/globules or streaks) or atypical forms of typically benign acral dermoscopic patterns may be observed.15
Per a 3-step diagnostic algorithm created by Koga and Saida,18 a suspected acral lesion should first be evaluated for a parallel ridge pattern to determine the need for biopsy, as it is seen in approximately two-thirds of ALMs.19 If no parallel ridge pattern is observed, the lesion should then be checked for any of the typical dermoscopic patterns seen in benign acral nevi (eg, parallel furrow, latticelike, or fibrillar patterns).18 The maximum diameter should be measured only if the lesion does not exhibit any of the typical dermoscopic patterns. If the lesion’s diameter is greater than 7 mm in diameter, it should be biopsied; if the diameter is less than 7 mm, it should have regular clinical and dermoscopic follow-up.18
In 2015, Lallas et al20 developed the BRAAFF checklist, a scoring system of 6 variables: blotches, ridge pattern, asymmetry of structures, asymmetry of colors, parallel furrow pattern, and fibrillar pattern. The checklist also was shown to substantially improve diagnostic accuracy of dermoscopy for ALM, with sensitivity and specificity at 93.1% and 86.7%, respectively.20
Acquired Acral Nevi
Three classic dermoscopic patterns are associated with acquired acral nevi: parallel furrow pattern, latticelike pattern, and fibrillar pattern.15,21 Approximately three-quarters of all acquired acral nevi exhibit one of these patterns, roughly half exhibiting parallel furrow with tan-brown bandlike pigmentation along dermatoglyphic grooves.16,17
Latticelike patterns also are characterized by brown parallel lines along the sulci of dermatoglyphics but additionally have multiple intersecting lines. Thus, this pattern can be considered a variant of the parallel furrow pattern.15 The crisscross markings can be predominantly found in the plantar arch.22 This dermoscopic pattern comprises 15% to 25% of all acral nevi.21
Fibrillar pattern accounts for 10% to 20% of all acral melanocytic nevi.21 Dermoscopically, these lesions demonstrate parallel filamentous streaks that cross dermatoglyphics obliquely. The fibrillar pattern is predominantly found on weight-bearing areas of the sole,22 which likely is explained by pressure causing slanting of melanin columns in the horny layer.23 The fibrillar pattern has been shown to be the benign acral dermoscopic pattern that is most commonly misdiagnosed, with higher reported rates of biopsy.24
Acral Congenital Melanocytic Nevi
Congenital melanocytic nevi (CMN) present at birth or appear during the first few weeks of life. Congenital melanocytic nevi can vary widely in size, shape, and color, and they are occasionally biopsied in cases of larger diameter or dermoscopic atypia to differentiate from melanoma.25 Congenital melanocytic nevi also can occur on acral volar surfaces. Possible dermoscopic patterns include parallel furrow or fibrillar patterns as well as a crista dotted pattern, defined as evenly spaced dots/globules on the ridges near the openings of eccrine ducts.26 A more commonly observed dermoscopic pattern in acral CMN is a combination of the crista dotted and parallel furrow patterns, known as the peas-in-a-pod pattern. Changes in the clinical appearance and dermoscopic features of an acral CMN are possible over time; some lesions also may fade with age.26
Final Thoughts
Acral lentiginous melanoma is a rare but potentially aggressive melanoma subtype that accounts for a larger proportion of melanomas in patients with skin of color than in white patients. Dermoscopy of acral volar skin provides invaluable diagnostic information and allows for better management of acral melanocytic lesions. Dermoscopic patterns such as the parallel ridge, parallel furrow, latticelike, fibrillar, and peas-in-a-pod patterns are unique to acral sites and can be used to differentiate between ALMs, acquired nevi, or CMNs.
Acral lentiginous melanoma (ALM) is a rare subtype of melanoma that occurs on the palms, soles, and nail apparatus. Unlike more common types of melanoma, ALM occurs on sun-protected areas of the skin and has distinct clinical, histologic, and genetic features. Acral lentiginous melanoma accounts for a larger proportion of melanomas in individuals with skin of color and has a worse prognosis and recurrence rate than other forms of melanoma.
Population Trends in Skin of Color
Much of the literature on malignant melanoma historically has involved non-Hispanic white patients, but the incidence in lighter-skinned populations has been increasing steadily over the last few decades.1 Although ALM can occur in any race, it disproportionately affects skin of color populations; ALM accounts for only 0.8% to 1% of all melanomas in white populations, but it constitutes 4% to 58% of melanomas in ethnic populations and is the most common melanoma subtype among black Americans.2-5 Acral lentiginous melanoma also is associated with a worse prognosis compared to other subtypes, which may indicate a more aggressive biological nature6 but also may point toward socioeconomic and cultural barriers (eg, low income or education levels, lack of insurance, lower health literacy), leading to disparities in access to care and diagnosis at advanced stages.5
Similarly, the distribution of acral melanocytic nevi appears to demonstrate an association with ethnicity and skin pigmentation. Although skin of color patients have fewer nevi than non-Hispanic whites, the proportion of acral melanocytic nevi tends to be greater.6,7 Given its grim prognosis, accurately differentiating ALM from acral nevi is of utmost importance.
Diagnostic Challenges of Acral Lesions
Due to the unique nature of the surfaces of acral sites, melanocytic lesions on the palms, soles, and nail apparatus present many diagnostic challenges. It can be difficult to distinguish acral melanoma from benign lesions using the naked eye alone. Volar surfaces are characterized by the presence of dermatoglyphics, and pigment deposition along ridges and furrows create particular dermoscopic patterns exclusive to these sites.8 Thus, dermoscopy can be useful on acral surfaces, but the dermoscopic features are different from those on the rest of the body and must be learned separately.
In addition, nearly half of patients are unaware of their acral lesions.6 Acral surfaces may not always be examined by clinicians during total-body skin examinations, leading to further possibility of overlooking a lesion. Obtaining biopsies on glabrous skin or nails also is challenging because they can be more painful and hemostasis can be more difficult, especially in the nail. Acral melanomas also may be amelanotic, including those at subungual sites.
Dermoscopic Patterns of Acral Volar Skin
Dermoscopy is a useful noninvasive tool for distinguishing between benign and malignant acral melanocytic lesions, and its efficacy in improving diagnostic accuracy and decreasing unnecessary biopsies is well-established in the literature.13,14 Acral dermoscopy allows for visualization of pigment along the dermatoglyphics that constitute the characteristic dermoscopic patterns.
Acral Lentiginous Melanoma
The hallmark dermoscopic pattern and most important finding of ALM is the parallel ridge pattern, characterized by parallel linear pigmentation along the ridges of dermatoglyphics. In the early phases of malignancy, the pattern appears light brown and involves most of the lesion; as the tumor develops, increasing melanin production results in focal areas of the parallel ridge pattern with darker bands.15,16 The sensitivity and specificity of a parallel ridge pattern for diagnosing early ALM has been shown to be 86% and 99%, respectively.15,16
A pattern of irregular diffuse pigmentation also can be observed in more advanced ALM. Dermoscopy may reveal a structureless pattern (ie, lack of identifiable structures or patterns) in a background of tan-black coloration due to more exuberant melanocyte proliferation along the epidermis.15 Sensitivity and specificity of this dermoscopic finding for invasive lesions is high at 94% and 97%, respectively.16,17 Interestingly, once ALM lesions have advanced even further, conventional melanoma-associated structures (ie, blue-white veil, polymorphous blood vessels, ulceration, irregular dots/globules or streaks) or atypical forms of typically benign acral dermoscopic patterns may be observed.15
Per a 3-step diagnostic algorithm created by Koga and Saida,18 a suspected acral lesion should first be evaluated for a parallel ridge pattern to determine the need for biopsy, as it is seen in approximately two-thirds of ALMs.19 If no parallel ridge pattern is observed, the lesion should then be checked for any of the typical dermoscopic patterns seen in benign acral nevi (eg, parallel furrow, latticelike, or fibrillar patterns).18 The maximum diameter should be measured only if the lesion does not exhibit any of the typical dermoscopic patterns. If the lesion’s diameter is greater than 7 mm in diameter, it should be biopsied; if the diameter is less than 7 mm, it should have regular clinical and dermoscopic follow-up.18
In 2015, Lallas et al20 developed the BRAAFF checklist, a scoring system of 6 variables: blotches, ridge pattern, asymmetry of structures, asymmetry of colors, parallel furrow pattern, and fibrillar pattern. The checklist also was shown to substantially improve diagnostic accuracy of dermoscopy for ALM, with sensitivity and specificity at 93.1% and 86.7%, respectively.20
Acquired Acral Nevi
Three classic dermoscopic patterns are associated with acquired acral nevi: parallel furrow pattern, latticelike pattern, and fibrillar pattern.15,21 Approximately three-quarters of all acquired acral nevi exhibit one of these patterns, roughly half exhibiting parallel furrow with tan-brown bandlike pigmentation along dermatoglyphic grooves.16,17
Latticelike patterns also are characterized by brown parallel lines along the sulci of dermatoglyphics but additionally have multiple intersecting lines. Thus, this pattern can be considered a variant of the parallel furrow pattern.15 The crisscross markings can be predominantly found in the plantar arch.22 This dermoscopic pattern comprises 15% to 25% of all acral nevi.21
Fibrillar pattern accounts for 10% to 20% of all acral melanocytic nevi.21 Dermoscopically, these lesions demonstrate parallel filamentous streaks that cross dermatoglyphics obliquely. The fibrillar pattern is predominantly found on weight-bearing areas of the sole,22 which likely is explained by pressure causing slanting of melanin columns in the horny layer.23 The fibrillar pattern has been shown to be the benign acral dermoscopic pattern that is most commonly misdiagnosed, with higher reported rates of biopsy.24
Acral Congenital Melanocytic Nevi
Congenital melanocytic nevi (CMN) present at birth or appear during the first few weeks of life. Congenital melanocytic nevi can vary widely in size, shape, and color, and they are occasionally biopsied in cases of larger diameter or dermoscopic atypia to differentiate from melanoma.25 Congenital melanocytic nevi also can occur on acral volar surfaces. Possible dermoscopic patterns include parallel furrow or fibrillar patterns as well as a crista dotted pattern, defined as evenly spaced dots/globules on the ridges near the openings of eccrine ducts.26 A more commonly observed dermoscopic pattern in acral CMN is a combination of the crista dotted and parallel furrow patterns, known as the peas-in-a-pod pattern. Changes in the clinical appearance and dermoscopic features of an acral CMN are possible over time; some lesions also may fade with age.26
Final Thoughts
Acral lentiginous melanoma is a rare but potentially aggressive melanoma subtype that accounts for a larger proportion of melanomas in patients with skin of color than in white patients. Dermoscopy of acral volar skin provides invaluable diagnostic information and allows for better management of acral melanocytic lesions. Dermoscopic patterns such as the parallel ridge, parallel furrow, latticelike, fibrillar, and peas-in-a-pod patterns are unique to acral sites and can be used to differentiate between ALMs, acquired nevi, or CMNs.
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136:1161-1171.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19:42.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691.
- Madankumar R, Gumaste PV, Martires K, et al. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74:724.e1-730.e1.
- Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch Dermatol. 2010;146:1085-1094.
- Thomas L, Phan A, Pralong P, et al. Special locations dermoscopy: facial, acral, and nail. Dermatol Clin. 2013;31:615-624.
- Gong HZ, Zheng HY, Li J. Amelanotic melanoma [published online January 21, 2019]. Melanoma Res. doi:10.1097/CMR.0000000000000571.
- Ise M, Yasuda F, Konohana I, et al. Acral melanoma with hyperkeratosis mimicking a pigmented wart. Dermatol Pract Concept. 2013;3:37-39.
- Serarslan G, Akçaly CM, Atik E. Acral lentiginous melanoma misdiagnosed as tinea pedis: a case report. Int J Dermatol. 2004;43:37-38.
- Gumaste P, Penn L, Cohen N, et al. Acral lentiginous melanoma of the foot misdiagnosed as a traumatic ulcer. a cautionary case. J Am Podiatr Med Assoc. 2015;105:189-194.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-689.
- Carli P, de Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997-2001. Br J Dermatol. 2004;150:687-692.
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34.
- Ishihara Y, Saida T, Miyazaki A, et al. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol. 2006;28:21-27.
- Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238.
- Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch Dermatol. 2011;147:741-743.
- Lallas A, Sgouros D, Zalaudek I, et al. Palmar and plantar melanomas differ for sex prevalence and tumor thickness but not for dermoscopic patterns. Melanoma Res. 2014;24:83-87.
- Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173:1041-1049.
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426.
- Miyazaki A, Saida T, Koga H, et al. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J Am Acad Dermatol. 2005;53:230-236.
- Watanabe S, Sawada M, Ishizaki S, et al. Comparison of dermatoscopic images of acral lentiginous melanoma and acral melanocytic nevus occurring on body weight-bearing areas. Dermatol Pract Concept. 2014;4:47-50.
- Costello CM, Ghanavatian S, Temkit M, et al. Educational and practice gaps in the management of volar melanocytic lesions. J Eur Acad Dermatol Venereol. 2018;32:1450-1455.
- Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? part I. clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012;67:495.e1-495.e17; quiz 512-514.
- Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch Dermatol. 2011;147:809-813.
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136:1161-1171.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19:42.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691.
- Madankumar R, Gumaste PV, Martires K, et al. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74:724.e1-730.e1.
- Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch Dermatol. 2010;146:1085-1094.
- Thomas L, Phan A, Pralong P, et al. Special locations dermoscopy: facial, acral, and nail. Dermatol Clin. 2013;31:615-624.
- Gong HZ, Zheng HY, Li J. Amelanotic melanoma [published online January 21, 2019]. Melanoma Res. doi:10.1097/CMR.0000000000000571.
- Ise M, Yasuda F, Konohana I, et al. Acral melanoma with hyperkeratosis mimicking a pigmented wart. Dermatol Pract Concept. 2013;3:37-39.
- Serarslan G, Akçaly CM, Atik E. Acral lentiginous melanoma misdiagnosed as tinea pedis: a case report. Int J Dermatol. 2004;43:37-38.
- Gumaste P, Penn L, Cohen N, et al. Acral lentiginous melanoma of the foot misdiagnosed as a traumatic ulcer. a cautionary case. J Am Podiatr Med Assoc. 2015;105:189-194.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-689.
- Carli P, de Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997-2001. Br J Dermatol. 2004;150:687-692.
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34.
- Ishihara Y, Saida T, Miyazaki A, et al. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol. 2006;28:21-27.
- Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238.
- Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch Dermatol. 2011;147:741-743.
- Lallas A, Sgouros D, Zalaudek I, et al. Palmar and plantar melanomas differ for sex prevalence and tumor thickness but not for dermoscopic patterns. Melanoma Res. 2014;24:83-87.
- Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173:1041-1049.
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426.
- Miyazaki A, Saida T, Koga H, et al. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J Am Acad Dermatol. 2005;53:230-236.
- Watanabe S, Sawada M, Ishizaki S, et al. Comparison of dermatoscopic images of acral lentiginous melanoma and acral melanocytic nevus occurring on body weight-bearing areas. Dermatol Pract Concept. 2014;4:47-50.
- Costello CM, Ghanavatian S, Temkit M, et al. Educational and practice gaps in the management of volar melanocytic lesions. J Eur Acad Dermatol Venereol. 2018;32:1450-1455.
- Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? part I. clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012;67:495.e1-495.e17; quiz 512-514.
- Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch Dermatol. 2011;147:809-813.
Practice Points
- Dermatologists should be familiar with common dermoscopic patterns seen at acral sites in patients with skin of color as well as the most up-to-date diagnostic algorithms.
- Acral lentiginous melanoma should be strongly suspected if dermoscopy reveals a parallel ridge pattern or if dermoscopy of volar skin reveals a lack of typical dermoscopic patterns in lesions with a diameter greater than 7 mm.
Managing Postinflammatory Hyperpigmentation in Pediatric Patients With Skin of Color
Postnflammatory hyperpigmentation (PIH) is an acquired hypermelanosis that can occur in children and adults following an inflammatory cutaneous disease or trauma. Postinflammatory hyperpigmentation may last for months to even years. Although PIH may occur in all skin types, it is more common and presents with greater severity and intensity in individuals with skin of color. By the year 2050, 1 in 3 US residents is projected to be Hispanic.1 It is projected that by 2044, non-Hispanic white individuals (all ages) will make up less than 50% of the US population.2 Currently, the majority of the US residents younger than 18 years are minorities. The majority minority population in the United States already exists in those younger than 18 years and is predicted to occur in the adult population by 2044.2
Effective treatment options and management strategies for PIH in adults with skin of color have been described in the literature.3 Due to a paucity of research, the approach to management of PIH in children with skin of color has been based on clinical experience and lessons learned from adult patients. This article focuses on management of PIH in pediatric patients with skin of color, which includes black/African American, African-Caribbean, Hispanic, Asian, Pacific Islander, and American Indian individuals.
Underlying Inflammatory Dermatoses Resulting in PIH
There are numerous conditions that may result in PIH, including but not limited to atopic dermatitis (AD), acne, arthropod bites, and injuries to the skin. Postinflammatory hyperpigmentation may have more of a psychological impact than the inciting disease or injury itself. The most important step in the approach to managing PIH is treating the underlying inflammatory condition that caused the pigmentation.
Parents/guardians may report a chief concern of dark spots, manchas (stains), blemishes, or stains on the skin, often with no mention of a coexisting inflammatory dermatosis. Parents/guardians of children with skin of color often have personally experienced PIH and may be determined to shield their children from similar angst associated with the condition. Although physicians may see just another pediatric patient with PIH, the child’s parents/guardians may see a condition that will be readily perceptible during major life events, such as the child’s prom or even his/her wedding day. Promptly diagnosing and instituting early treatment of inflammatory conditions associated with PIH may accelerate resolution and prevent worsening of the pigmentation.3
Select inflammatory dermatoses that are common in children with skin of color and may lead to PIH are highlighted below. Although this list is not comprehensive, the approach and management strategies should prompt creation of plans that keep PIH in mind when treating primary inflammatory skin diseases.
Atopic Dermatitis
Atopic dermatitis may induce PIH or hypopigmentation of the skin in children with skin of color. Developing a plan for AD flare prevention, as well as management of mild, moderate, and severe AD flares, is imperative in pediatric patients. Prevention plans should include gentle skin care, twice-daily application of emollients to the full body, and reduction of Staphylococcus aureus loads on the skin. The treatment action plan for mild to moderate flares may include topical corticosteroids, immunomodulators, and nonsteroidal agents. Treatment options for severe AD or patients who were unsuccessfully treated with other therapies may include phototherapy, biologics, and methotrexate, among others.4 Creating action plans for AD flares is a vital step in the prevention of PIH in patients with skin of color. Additionally, PIH should not be considered a sign of AD treatment failure.
Acne
Acne is a common skin disorder seen in patients with skin of color.5 A prospective observational study found that 34.3% of 683 children aged 9 to 14 years in a pediatric ambulatory clinic had acne.6 The number of preadolescents with acne is growing. Most cases are not associated with underlying endocrinopathy.7 With the growing population of children with skin of color in the United States along with the increasing childhood acne rate and subsequent inherent risk for hyperpigmentation, early acne interventions should be considered in pediatric acne patients with skin of color to reduce the impact of PIH in those at risk.
In a survey study of 313 adult acne patients with skin of color, 37.2% reported the presence of dark marks lasting 4 months or longer.5 Regardless of the severity of the acne, treatment should be initiated as tolerated in those with PIH. Adolescent acne patients with skin of color may develop PIH that is more severe and longer lasting than the acne itself.
The foundation for treatment of acne in adolescent skin of color patients is the same as those without skin of color, including topical retinoids, topical antibiotics, oral antibiotics, and isotretinoin when needed. Topical tretinoin, adapalene, azelaic acid, and tazarotene not only treat acne but also are a valuable part of the treatment armamentarium for PIH. Several studies in adults with skin of color have demonstrated improvement of PIH from the use of topical retinoids alone.8-10 Despite wanting to treat the acne aggressively, special guidance should be given to prevent retinoid dermatitis, which may lead to PIH.10 Demonstrating the application of the topical acne medications, discussing how to avoid potential side effects, and giving permission to skip applications, if needed, may empower families to make adjustments between visits to limit irritation that might prompt further PIH. Incorporating α-hydroxy acid–based cleansers, α-hydroxy acid–based chemical peels, or salicylic acid chemical peels may be warranted in the setting of intense PIH. Selecting treatments that not only help the inflammatory disease leading to the PIH but also can help improve the pigmentation are preferred; however, the risks and benefits have to be weighed because many treatments that work well for PIH also may cause irritation, leading to new or worsening PIH.
Arthropod Bites
Arthropod bites cause inflamed pruritic papules and nodules, and the resulting PIH in those with darker skin types may be quite dramatic. Parents/guardians should be instructed to have a low-potency topical corticosteroid on hand to use on bites for a few days when they appear, which will not only help with the inflammation associated with the bite but also will help decrease pruritus and subsequently skin injury from scratching. In homes with pets, checking animals routinely for fleas and other infestations is helpful. In the setting of repeated arthropod bites in the spring and summer, applying bug repellant with 10% to 30% DEET (N,N-diethyl-meta-toluamide) on the child’s clothing and exposed body areas before playing outside or in the morning before school or camp may prevent some bites. There are DEET alternatives, such as picaridin, that may be used. Product instructions should be followed when using insect repellants in the pediatric population.11
PIH Management Strategies
Gentle Skin Care Routine
There are misconceptions that areas of hyperpigmentation on the skin are caused by dirt and that scrubbing the skin harder may help to lighten the affected areas. Parents/guardians may report that the child’s skin looks dirty or, in the setting of acne, view dirt as the cause of the skin condition, which may prompt the patient to scrub the skin and the friction further worsens the PIH. Use of daily gentle cleansers and moisturizers is advised to keep the skin moisturized and free of further potential irritation and dryness that may prompt scratching or flares of the underlying condition.
Photoprotection
During the treatment course for PIH, using sun protection is helpful to prevent further darkening of the PIH areas. Sun protection may be in the form of broad-spectrum sunscreen, hats, or sun-protective clothing. Patients should be encouraged to apply sunscreen daily and to reapply every 2 hours and after water-based activities.12 For pediatric and adolescent populations, practicing sun-protective behaviors before school or outdoor activities also should be advised, as many families only think about sun protection in the setting of sunny vacation activities. Research has demonstrated that individuals with skin of color may not realize that they can be affected by skin cancer,13 thus they may not have any experience selecting, applying, or regularly using sunscreens. Products that do not leave a white hue on the skin are suggested for adolescents who may be sensitive about their appearance following sunscreen application.
Skin Lightening Treatments
Although the most important therapy for PIH is to treat the underlying inflammatory conditions, some parents/guardians may desire additional options due to the extent of involvement of the PIH, its psychological impact on the child, or adverse effect on the child’s quality of life.14 In adolescents, incorporating an α-hydroxy acid–based cleanser, glycolic acid chemical peels, salicylic acid chemical peels, and topical cosmeceuticals may be warranted in the setting of intense PIH and acne. However, irritation may lead to further dyspigmentation.
Topical ammonium lactate 12% is lactic acid neutralized with ammonium hydroxide that is formulated as a lotion or a cream. It is used to hydrate dry skin and may decrease corneocyte cohesion.15 Topical ammonium lactate also has been used anecdotally for PIH on the body during periods of watchful waiting.
Topical hydroquinone, the gold standard for treating hyperpigmentation,3,16 is not approved in children, but some parents/guardians elect to utilize hydroquinone off label to accelerate the clearing of distressing PIH in adolescents. Careful consideration including a discussion of potential risks and alternatives (eg, watchful waiting) should be highlighted.
In the setting of chronic inflammatory conditions that recur and remit, potentially irritating topical treatments should be used only during periods when symptoms of inflammation such as itching or erythema are absent.
Conclusion
Despite the best management efforts, PIH in some patients with skin of color may be present for months to years. In the pediatric skin of color population, treatment of the underlying inflammatory condition, gentle skin care, use of photoprotection, and time may be all that is needed for PIH resolution. With their parent/guardians’ consent, adolescents distressed by PIH may decide to pursue more aggressive, potentially irritating treatments. Above all, the most important management in the setting of PIH is to treat the underlying inflammatory condition causing the PIH and set reasonable expectations. For challenging cases, pediatric dermatologists with special expertise in treating pediatric and adolescent patients with skin of color may be consulted.
- Broughton A. Minorities expected to be majority in 2050. CNN. August 13, 2008. Accessed January 2, 2019.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. Published March 2015. Accessed January 23, 2019.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49-S57.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Napolitano M, Ruggiero G, Monfrecola G, et al. Acne prevalence in 9 to 14-year-old patients attending pediatric ambulatory clinics in Italy. Int J Dermatol. 2018;57:1320-1323.
- Mancini AJ, Baldwin HE, Eichenfield LF. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg 2011;30:2-5.
- Lowe NJ, Rizk D, Grimes P, et al. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin Ther. 1998;20:945-959.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoid acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- American Academy of Pediatrics. Choosing an insect repellent for your child. Healthy Children website. Updated July 18, 2018. Accessed January 8, 2019.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:312-317.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Downie J. Help prevent and reverse post-inflammatory hyperpigmentation. Pract Dermatol Pediatr. May/June 2011:12-14. Accessed January 18, 2019.
- Ammonium lactate lotion 12% [package insert]. Bronx, New York: Perrigo New York, Inc; 2006.
- Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77-85.
Postnflammatory hyperpigmentation (PIH) is an acquired hypermelanosis that can occur in children and adults following an inflammatory cutaneous disease or trauma. Postinflammatory hyperpigmentation may last for months to even years. Although PIH may occur in all skin types, it is more common and presents with greater severity and intensity in individuals with skin of color. By the year 2050, 1 in 3 US residents is projected to be Hispanic.1 It is projected that by 2044, non-Hispanic white individuals (all ages) will make up less than 50% of the US population.2 Currently, the majority of the US residents younger than 18 years are minorities. The majority minority population in the United States already exists in those younger than 18 years and is predicted to occur in the adult population by 2044.2
Effective treatment options and management strategies for PIH in adults with skin of color have been described in the literature.3 Due to a paucity of research, the approach to management of PIH in children with skin of color has been based on clinical experience and lessons learned from adult patients. This article focuses on management of PIH in pediatric patients with skin of color, which includes black/African American, African-Caribbean, Hispanic, Asian, Pacific Islander, and American Indian individuals.
Underlying Inflammatory Dermatoses Resulting in PIH
There are numerous conditions that may result in PIH, including but not limited to atopic dermatitis (AD), acne, arthropod bites, and injuries to the skin. Postinflammatory hyperpigmentation may have more of a psychological impact than the inciting disease or injury itself. The most important step in the approach to managing PIH is treating the underlying inflammatory condition that caused the pigmentation.
Parents/guardians may report a chief concern of dark spots, manchas (stains), blemishes, or stains on the skin, often with no mention of a coexisting inflammatory dermatosis. Parents/guardians of children with skin of color often have personally experienced PIH and may be determined to shield their children from similar angst associated with the condition. Although physicians may see just another pediatric patient with PIH, the child’s parents/guardians may see a condition that will be readily perceptible during major life events, such as the child’s prom or even his/her wedding day. Promptly diagnosing and instituting early treatment of inflammatory conditions associated with PIH may accelerate resolution and prevent worsening of the pigmentation.3
Select inflammatory dermatoses that are common in children with skin of color and may lead to PIH are highlighted below. Although this list is not comprehensive, the approach and management strategies should prompt creation of plans that keep PIH in mind when treating primary inflammatory skin diseases.
Atopic Dermatitis
Atopic dermatitis may induce PIH or hypopigmentation of the skin in children with skin of color. Developing a plan for AD flare prevention, as well as management of mild, moderate, and severe AD flares, is imperative in pediatric patients. Prevention plans should include gentle skin care, twice-daily application of emollients to the full body, and reduction of Staphylococcus aureus loads on the skin. The treatment action plan for mild to moderate flares may include topical corticosteroids, immunomodulators, and nonsteroidal agents. Treatment options for severe AD or patients who were unsuccessfully treated with other therapies may include phototherapy, biologics, and methotrexate, among others.4 Creating action plans for AD flares is a vital step in the prevention of PIH in patients with skin of color. Additionally, PIH should not be considered a sign of AD treatment failure.
Acne
Acne is a common skin disorder seen in patients with skin of color.5 A prospective observational study found that 34.3% of 683 children aged 9 to 14 years in a pediatric ambulatory clinic had acne.6 The number of preadolescents with acne is growing. Most cases are not associated with underlying endocrinopathy.7 With the growing population of children with skin of color in the United States along with the increasing childhood acne rate and subsequent inherent risk for hyperpigmentation, early acne interventions should be considered in pediatric acne patients with skin of color to reduce the impact of PIH in those at risk.
In a survey study of 313 adult acne patients with skin of color, 37.2% reported the presence of dark marks lasting 4 months or longer.5 Regardless of the severity of the acne, treatment should be initiated as tolerated in those with PIH. Adolescent acne patients with skin of color may develop PIH that is more severe and longer lasting than the acne itself.
The foundation for treatment of acne in adolescent skin of color patients is the same as those without skin of color, including topical retinoids, topical antibiotics, oral antibiotics, and isotretinoin when needed. Topical tretinoin, adapalene, azelaic acid, and tazarotene not only treat acne but also are a valuable part of the treatment armamentarium for PIH. Several studies in adults with skin of color have demonstrated improvement of PIH from the use of topical retinoids alone.8-10 Despite wanting to treat the acne aggressively, special guidance should be given to prevent retinoid dermatitis, which may lead to PIH.10 Demonstrating the application of the topical acne medications, discussing how to avoid potential side effects, and giving permission to skip applications, if needed, may empower families to make adjustments between visits to limit irritation that might prompt further PIH. Incorporating α-hydroxy acid–based cleansers, α-hydroxy acid–based chemical peels, or salicylic acid chemical peels may be warranted in the setting of intense PIH. Selecting treatments that not only help the inflammatory disease leading to the PIH but also can help improve the pigmentation are preferred; however, the risks and benefits have to be weighed because many treatments that work well for PIH also may cause irritation, leading to new or worsening PIH.
Arthropod Bites
Arthropod bites cause inflamed pruritic papules and nodules, and the resulting PIH in those with darker skin types may be quite dramatic. Parents/guardians should be instructed to have a low-potency topical corticosteroid on hand to use on bites for a few days when they appear, which will not only help with the inflammation associated with the bite but also will help decrease pruritus and subsequently skin injury from scratching. In homes with pets, checking animals routinely for fleas and other infestations is helpful. In the setting of repeated arthropod bites in the spring and summer, applying bug repellant with 10% to 30% DEET (N,N-diethyl-meta-toluamide) on the child’s clothing and exposed body areas before playing outside or in the morning before school or camp may prevent some bites. There are DEET alternatives, such as picaridin, that may be used. Product instructions should be followed when using insect repellants in the pediatric population.11
PIH Management Strategies
Gentle Skin Care Routine
There are misconceptions that areas of hyperpigmentation on the skin are caused by dirt and that scrubbing the skin harder may help to lighten the affected areas. Parents/guardians may report that the child’s skin looks dirty or, in the setting of acne, view dirt as the cause of the skin condition, which may prompt the patient to scrub the skin and the friction further worsens the PIH. Use of daily gentle cleansers and moisturizers is advised to keep the skin moisturized and free of further potential irritation and dryness that may prompt scratching or flares of the underlying condition.
Photoprotection
During the treatment course for PIH, using sun protection is helpful to prevent further darkening of the PIH areas. Sun protection may be in the form of broad-spectrum sunscreen, hats, or sun-protective clothing. Patients should be encouraged to apply sunscreen daily and to reapply every 2 hours and after water-based activities.12 For pediatric and adolescent populations, practicing sun-protective behaviors before school or outdoor activities also should be advised, as many families only think about sun protection in the setting of sunny vacation activities. Research has demonstrated that individuals with skin of color may not realize that they can be affected by skin cancer,13 thus they may not have any experience selecting, applying, or regularly using sunscreens. Products that do not leave a white hue on the skin are suggested for adolescents who may be sensitive about their appearance following sunscreen application.
Skin Lightening Treatments
Although the most important therapy for PIH is to treat the underlying inflammatory conditions, some parents/guardians may desire additional options due to the extent of involvement of the PIH, its psychological impact on the child, or adverse effect on the child’s quality of life.14 In adolescents, incorporating an α-hydroxy acid–based cleanser, glycolic acid chemical peels, salicylic acid chemical peels, and topical cosmeceuticals may be warranted in the setting of intense PIH and acne. However, irritation may lead to further dyspigmentation.
Topical ammonium lactate 12% is lactic acid neutralized with ammonium hydroxide that is formulated as a lotion or a cream. It is used to hydrate dry skin and may decrease corneocyte cohesion.15 Topical ammonium lactate also has been used anecdotally for PIH on the body during periods of watchful waiting.
Topical hydroquinone, the gold standard for treating hyperpigmentation,3,16 is not approved in children, but some parents/guardians elect to utilize hydroquinone off label to accelerate the clearing of distressing PIH in adolescents. Careful consideration including a discussion of potential risks and alternatives (eg, watchful waiting) should be highlighted.
In the setting of chronic inflammatory conditions that recur and remit, potentially irritating topical treatments should be used only during periods when symptoms of inflammation such as itching or erythema are absent.
Conclusion
Despite the best management efforts, PIH in some patients with skin of color may be present for months to years. In the pediatric skin of color population, treatment of the underlying inflammatory condition, gentle skin care, use of photoprotection, and time may be all that is needed for PIH resolution. With their parent/guardians’ consent, adolescents distressed by PIH may decide to pursue more aggressive, potentially irritating treatments. Above all, the most important management in the setting of PIH is to treat the underlying inflammatory condition causing the PIH and set reasonable expectations. For challenging cases, pediatric dermatologists with special expertise in treating pediatric and adolescent patients with skin of color may be consulted.
Postnflammatory hyperpigmentation (PIH) is an acquired hypermelanosis that can occur in children and adults following an inflammatory cutaneous disease or trauma. Postinflammatory hyperpigmentation may last for months to even years. Although PIH may occur in all skin types, it is more common and presents with greater severity and intensity in individuals with skin of color. By the year 2050, 1 in 3 US residents is projected to be Hispanic.1 It is projected that by 2044, non-Hispanic white individuals (all ages) will make up less than 50% of the US population.2 Currently, the majority of the US residents younger than 18 years are minorities. The majority minority population in the United States already exists in those younger than 18 years and is predicted to occur in the adult population by 2044.2
Effective treatment options and management strategies for PIH in adults with skin of color have been described in the literature.3 Due to a paucity of research, the approach to management of PIH in children with skin of color has been based on clinical experience and lessons learned from adult patients. This article focuses on management of PIH in pediatric patients with skin of color, which includes black/African American, African-Caribbean, Hispanic, Asian, Pacific Islander, and American Indian individuals.
Underlying Inflammatory Dermatoses Resulting in PIH
There are numerous conditions that may result in PIH, including but not limited to atopic dermatitis (AD), acne, arthropod bites, and injuries to the skin. Postinflammatory hyperpigmentation may have more of a psychological impact than the inciting disease or injury itself. The most important step in the approach to managing PIH is treating the underlying inflammatory condition that caused the pigmentation.
Parents/guardians may report a chief concern of dark spots, manchas (stains), blemishes, or stains on the skin, often with no mention of a coexisting inflammatory dermatosis. Parents/guardians of children with skin of color often have personally experienced PIH and may be determined to shield their children from similar angst associated with the condition. Although physicians may see just another pediatric patient with PIH, the child’s parents/guardians may see a condition that will be readily perceptible during major life events, such as the child’s prom or even his/her wedding day. Promptly diagnosing and instituting early treatment of inflammatory conditions associated with PIH may accelerate resolution and prevent worsening of the pigmentation.3
Select inflammatory dermatoses that are common in children with skin of color and may lead to PIH are highlighted below. Although this list is not comprehensive, the approach and management strategies should prompt creation of plans that keep PIH in mind when treating primary inflammatory skin diseases.
Atopic Dermatitis
Atopic dermatitis may induce PIH or hypopigmentation of the skin in children with skin of color. Developing a plan for AD flare prevention, as well as management of mild, moderate, and severe AD flares, is imperative in pediatric patients. Prevention plans should include gentle skin care, twice-daily application of emollients to the full body, and reduction of Staphylococcus aureus loads on the skin. The treatment action plan for mild to moderate flares may include topical corticosteroids, immunomodulators, and nonsteroidal agents. Treatment options for severe AD or patients who were unsuccessfully treated with other therapies may include phototherapy, biologics, and methotrexate, among others.4 Creating action plans for AD flares is a vital step in the prevention of PIH in patients with skin of color. Additionally, PIH should not be considered a sign of AD treatment failure.
Acne
Acne is a common skin disorder seen in patients with skin of color.5 A prospective observational study found that 34.3% of 683 children aged 9 to 14 years in a pediatric ambulatory clinic had acne.6 The number of preadolescents with acne is growing. Most cases are not associated with underlying endocrinopathy.7 With the growing population of children with skin of color in the United States along with the increasing childhood acne rate and subsequent inherent risk for hyperpigmentation, early acne interventions should be considered in pediatric acne patients with skin of color to reduce the impact of PIH in those at risk.
In a survey study of 313 adult acne patients with skin of color, 37.2% reported the presence of dark marks lasting 4 months or longer.5 Regardless of the severity of the acne, treatment should be initiated as tolerated in those with PIH. Adolescent acne patients with skin of color may develop PIH that is more severe and longer lasting than the acne itself.
The foundation for treatment of acne in adolescent skin of color patients is the same as those without skin of color, including topical retinoids, topical antibiotics, oral antibiotics, and isotretinoin when needed. Topical tretinoin, adapalene, azelaic acid, and tazarotene not only treat acne but also are a valuable part of the treatment armamentarium for PIH. Several studies in adults with skin of color have demonstrated improvement of PIH from the use of topical retinoids alone.8-10 Despite wanting to treat the acne aggressively, special guidance should be given to prevent retinoid dermatitis, which may lead to PIH.10 Demonstrating the application of the topical acne medications, discussing how to avoid potential side effects, and giving permission to skip applications, if needed, may empower families to make adjustments between visits to limit irritation that might prompt further PIH. Incorporating α-hydroxy acid–based cleansers, α-hydroxy acid–based chemical peels, or salicylic acid chemical peels may be warranted in the setting of intense PIH. Selecting treatments that not only help the inflammatory disease leading to the PIH but also can help improve the pigmentation are preferred; however, the risks and benefits have to be weighed because many treatments that work well for PIH also may cause irritation, leading to new or worsening PIH.
Arthropod Bites
Arthropod bites cause inflamed pruritic papules and nodules, and the resulting PIH in those with darker skin types may be quite dramatic. Parents/guardians should be instructed to have a low-potency topical corticosteroid on hand to use on bites for a few days when they appear, which will not only help with the inflammation associated with the bite but also will help decrease pruritus and subsequently skin injury from scratching. In homes with pets, checking animals routinely for fleas and other infestations is helpful. In the setting of repeated arthropod bites in the spring and summer, applying bug repellant with 10% to 30% DEET (N,N-diethyl-meta-toluamide) on the child’s clothing and exposed body areas before playing outside or in the morning before school or camp may prevent some bites. There are DEET alternatives, such as picaridin, that may be used. Product instructions should be followed when using insect repellants in the pediatric population.11
PIH Management Strategies
Gentle Skin Care Routine
There are misconceptions that areas of hyperpigmentation on the skin are caused by dirt and that scrubbing the skin harder may help to lighten the affected areas. Parents/guardians may report that the child’s skin looks dirty or, in the setting of acne, view dirt as the cause of the skin condition, which may prompt the patient to scrub the skin and the friction further worsens the PIH. Use of daily gentle cleansers and moisturizers is advised to keep the skin moisturized and free of further potential irritation and dryness that may prompt scratching or flares of the underlying condition.
Photoprotection
During the treatment course for PIH, using sun protection is helpful to prevent further darkening of the PIH areas. Sun protection may be in the form of broad-spectrum sunscreen, hats, or sun-protective clothing. Patients should be encouraged to apply sunscreen daily and to reapply every 2 hours and after water-based activities.12 For pediatric and adolescent populations, practicing sun-protective behaviors before school or outdoor activities also should be advised, as many families only think about sun protection in the setting of sunny vacation activities. Research has demonstrated that individuals with skin of color may not realize that they can be affected by skin cancer,13 thus they may not have any experience selecting, applying, or regularly using sunscreens. Products that do not leave a white hue on the skin are suggested for adolescents who may be sensitive about their appearance following sunscreen application.
Skin Lightening Treatments
Although the most important therapy for PIH is to treat the underlying inflammatory conditions, some parents/guardians may desire additional options due to the extent of involvement of the PIH, its psychological impact on the child, or adverse effect on the child’s quality of life.14 In adolescents, incorporating an α-hydroxy acid–based cleanser, glycolic acid chemical peels, salicylic acid chemical peels, and topical cosmeceuticals may be warranted in the setting of intense PIH and acne. However, irritation may lead to further dyspigmentation.
Topical ammonium lactate 12% is lactic acid neutralized with ammonium hydroxide that is formulated as a lotion or a cream. It is used to hydrate dry skin and may decrease corneocyte cohesion.15 Topical ammonium lactate also has been used anecdotally for PIH on the body during periods of watchful waiting.
Topical hydroquinone, the gold standard for treating hyperpigmentation,3,16 is not approved in children, but some parents/guardians elect to utilize hydroquinone off label to accelerate the clearing of distressing PIH in adolescents. Careful consideration including a discussion of potential risks and alternatives (eg, watchful waiting) should be highlighted.
In the setting of chronic inflammatory conditions that recur and remit, potentially irritating topical treatments should be used only during periods when symptoms of inflammation such as itching or erythema are absent.
Conclusion
Despite the best management efforts, PIH in some patients with skin of color may be present for months to years. In the pediatric skin of color population, treatment of the underlying inflammatory condition, gentle skin care, use of photoprotection, and time may be all that is needed for PIH resolution. With their parent/guardians’ consent, adolescents distressed by PIH may decide to pursue more aggressive, potentially irritating treatments. Above all, the most important management in the setting of PIH is to treat the underlying inflammatory condition causing the PIH and set reasonable expectations. For challenging cases, pediatric dermatologists with special expertise in treating pediatric and adolescent patients with skin of color may be consulted.
- Broughton A. Minorities expected to be majority in 2050. CNN. August 13, 2008. Accessed January 2, 2019.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. Published March 2015. Accessed January 23, 2019.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49-S57.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Napolitano M, Ruggiero G, Monfrecola G, et al. Acne prevalence in 9 to 14-year-old patients attending pediatric ambulatory clinics in Italy. Int J Dermatol. 2018;57:1320-1323.
- Mancini AJ, Baldwin HE, Eichenfield LF. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg 2011;30:2-5.
- Lowe NJ, Rizk D, Grimes P, et al. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin Ther. 1998;20:945-959.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoid acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- American Academy of Pediatrics. Choosing an insect repellent for your child. Healthy Children website. Updated July 18, 2018. Accessed January 8, 2019.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:312-317.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Downie J. Help prevent and reverse post-inflammatory hyperpigmentation. Pract Dermatol Pediatr. May/June 2011:12-14. Accessed January 18, 2019.
- Ammonium lactate lotion 12% [package insert]. Bronx, New York: Perrigo New York, Inc; 2006.
- Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77-85.
- Broughton A. Minorities expected to be majority in 2050. CNN. August 13, 2008. Accessed January 2, 2019.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. Published March 2015. Accessed January 23, 2019.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49-S57.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Napolitano M, Ruggiero G, Monfrecola G, et al. Acne prevalence in 9 to 14-year-old patients attending pediatric ambulatory clinics in Italy. Int J Dermatol. 2018;57:1320-1323.
- Mancini AJ, Baldwin HE, Eichenfield LF. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg 2011;30:2-5.
- Lowe NJ, Rizk D, Grimes P, et al. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin Ther. 1998;20:945-959.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoid acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- American Academy of Pediatrics. Choosing an insect repellent for your child. Healthy Children website. Updated July 18, 2018. Accessed January 8, 2019.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:312-317.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Downie J. Help prevent and reverse post-inflammatory hyperpigmentation. Pract Dermatol Pediatr. May/June 2011:12-14. Accessed January 18, 2019.
- Ammonium lactate lotion 12% [package insert]. Bronx, New York: Perrigo New York, Inc; 2006.
- Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77-85.
Practice Points
- The US population of children with skin of color is growing rapidly.
- Treating the underlying inflammatory dermatosis is the most important step in managing postinflammatory hyperpigmentation (PIH); however, many pediatric PIH patients and their parents/guardians presenting with a chief concern of pigmentary changes are unaware of the associated inflammatory condition.
- When appropriate, choose treatments for the underlying inflammatory condition that can simultaneously improve any existing PIH. Gentle skin care, avoidance of rubbing and scrubbing the skin, and photoprotection are essential to halt worsening of PIH.
- Patients’ parents/guardians may consent to more aggressive PIH treatment in select cases (eg, emotional distress in adolescents).