User login
Melanoma incidence highest in Oregon, lowest in Texas in 2015
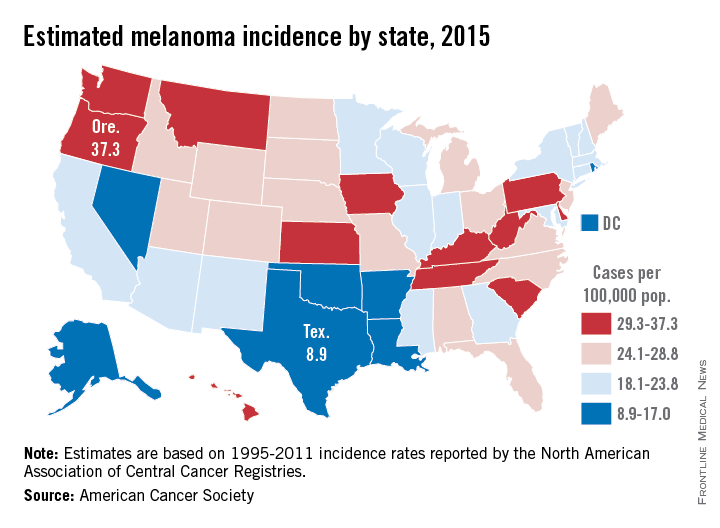
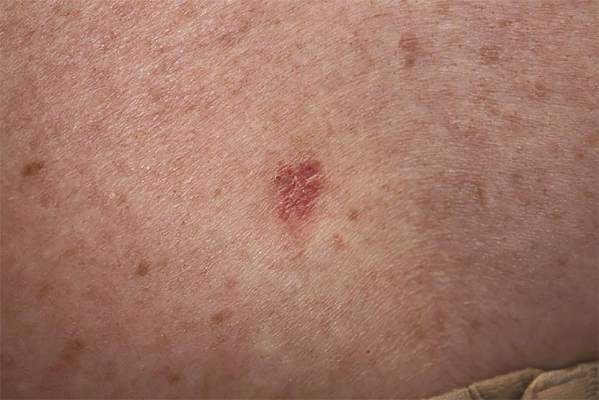
Projections for the number of new melanoma cases in 2015 give Texas the lowest estimated incidence and Oregon the highest, according to a report from the American Cancer Society.
Texas is expected to have 2,410 new cases of melanoma this year, for an incidence of 8.9 cases per 100,000 people. Oregon’s projected 1,480 melanoma cases for 2015 results in an incidence of 37.3 cases per 100,000 population. (The ACS projected the number of new cases, so incidences here are calculated via recent Census Bureau population estimates.)
After Texas, Louisiana should have the lowest melanoma rate at 11.6 per 100,000, followed by Arkansas and the District of Columbia, both at 12.1 per 100,000. On the upper end of the scale, Oregon will likely be followed by Washington, where the incidence for 2015 is expected to be 34.8 per 100,000, the ACS data show.
Using incidences (1995-2011) reported by the North American Association of Central Cancer Registries, the ACS projected that 73,870 new cases of melanoma will be diagnosed in the United States this year – meaning an overall incidence of 23.2 per 100,000, based on the same Census Bureau figures.
The ACS estimated that there will be 9,940 deaths from melanoma in 2015 – an incidence of 3.1 per 100,000 population – as well as 3,400 deaths from other forms of skin cancer, not including basal cell and squamous cell carcinomas.
Projections for the number of new melanoma cases in 2015 give Texas the lowest estimated incidence and Oregon the highest, according to a report from the American Cancer Society.
Texas is expected to have 2,410 new cases of melanoma this year, for an incidence of 8.9 cases per 100,000 people. Oregon’s projected 1,480 melanoma cases for 2015 results in an incidence of 37.3 cases per 100,000 population. (The ACS projected the number of new cases, so incidences here are calculated via recent Census Bureau population estimates.)
After Texas, Louisiana should have the lowest melanoma rate at 11.6 per 100,000, followed by Arkansas and the District of Columbia, both at 12.1 per 100,000. On the upper end of the scale, Oregon will likely be followed by Washington, where the incidence for 2015 is expected to be 34.8 per 100,000, the ACS data show.
Using incidences (1995-2011) reported by the North American Association of Central Cancer Registries, the ACS projected that 73,870 new cases of melanoma will be diagnosed in the United States this year – meaning an overall incidence of 23.2 per 100,000, based on the same Census Bureau figures.
The ACS estimated that there will be 9,940 deaths from melanoma in 2015 – an incidence of 3.1 per 100,000 population – as well as 3,400 deaths from other forms of skin cancer, not including basal cell and squamous cell carcinomas.

Projections for the number of new melanoma cases in 2015 give Texas the lowest estimated incidence and Oregon the highest, according to a report from the American Cancer Society.
Texas is expected to have 2,410 new cases of melanoma this year, for an incidence of 8.9 cases per 100,000 people. Oregon’s projected 1,480 melanoma cases for 2015 results in an incidence of 37.3 cases per 100,000 population. (The ACS projected the number of new cases, so incidences here are calculated via recent Census Bureau population estimates.)
After Texas, Louisiana should have the lowest melanoma rate at 11.6 per 100,000, followed by Arkansas and the District of Columbia, both at 12.1 per 100,000. On the upper end of the scale, Oregon will likely be followed by Washington, where the incidence for 2015 is expected to be 34.8 per 100,000, the ACS data show.
Using incidences (1995-2011) reported by the North American Association of Central Cancer Registries, the ACS projected that 73,870 new cases of melanoma will be diagnosed in the United States this year – meaning an overall incidence of 23.2 per 100,000, based on the same Census Bureau figures.
The ACS estimated that there will be 9,940 deaths from melanoma in 2015 – an incidence of 3.1 per 100,000 population – as well as 3,400 deaths from other forms of skin cancer, not including basal cell and squamous cell carcinomas.

Overall survival plateaus at 3 years for ipilimumab-treated melanoma patients
Among patients with advanced melanoma who were treated with ipilimumab, about 20%-26% survived to 3 years, and these patients are likely to have a good long-term outcome, according to a pooled analysis of survival data published online Feb. 9 in the Journal of Clinical Oncology.
Investigators pooled data from ten prospective (including two phase III trials) and two retrospective studies with a total of 1,257 previously treated and 604 treatment-naive patients. At least 3 years after receiving ipilimumab, 254 patients were still alive, with a median follow up for this subset of 69 months. Around year 3, the Kaplan-Meier overall survival (OS) curve began to plateau and extended to 9.9 years for the longest survival follow-up.
“These results suggest that the majority of patients who reached this milestone time point had a low risk of death thereafter,” wrote Dr. Dirk Schadendorf and his associates (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]).
Compared with patients who were previously treated, treatment-naive patients had a higher median overall survival (13.5 months [95% confidence interval, 11.9-15.4] vs. 10.7 months [9.6-11.4]) and higher 3-year-survival rates (26% [21%-30%] vs. 20% [18%-23%]). No definitive conclusion could be drawn from this observation, however, since nonrandomized subsets were used for this analysis. Subset analysis by dose showed similar median OS and 3-year survival rates for ipilimumab 3 mg/kg, 10 mg/kg, and other dosing regimens.
The researchers expanded the study to include overall survival (OS) data from 2,985 patients enrolled in a U.S. multicenter, open-label, expanded-access treatment protocol (EAP). This group included patients with poorer prognostic factors, some of whom were ineligible for clinical trials. The expanded group showed a lower median OS of 9.5 months and 3 year–survival rate of 21%, with the familiar OS curve plateau around 3 years that extended up to 10 years in some patients.
While this analysis only examined overall survival rates, individual ipilimumab studies that tracked patient responses to the drug have shown that some proportion of long-term survivors did not achieve a response. Identifying the specific disease characteristics of the long-term survivors will require further study.
“Considering the historic median OS of approximately 8-10 months and a 5-year survival rate of approximately 10% in advanced melanoma, the results presented herein are encouraging for patients diagnosed with this aggressive disease,” the authors wrote.
Dr. Schadendorf and his associates demonstrate a plateau in the survival curve of ipilimumab-treated patients beginning at about 3 years and representing about 21% of the treatment group. The curve suggests that those who survive to 3 years are highly likely to have a good long-term outcome, which provides a strong motivating factor in the decision to consider ipilimumab treatment. While pooled data adds information far beyond individual trials, a major drawback lies in the loss of control data necessary to isolate the added benefit of the study drug.
An indirect comparison using historic control series, in this case a large cohort documented in the American Joint Committee on Cancer (AJCC) Melanoma Staging Database, can substitute for missing control data in the pooled analysis. Reviewing data for stage IIIc and IV patients, the overall survival Kaplan-Meier curves in this population also show a plateau, but much later than that reported for ipilimumab, at beyond 8 years.
The AJCC melanoma classification gives survival rates at 3, 5, and 10 years of 19%, 13%, and 9%, respectively. Comparison with ipilimumab data suggests that survival at 3 years is similar, but thereafter improves with ipilimumab by 10% over other treatments that were available at the time. This difference is similar to the percentage of patients who achieved objective responses with ipilimumab. Although assessing response rate and progression-free survival in patients treated with ipilimumab presents challenges, the long-term benefits of ipilimumab could be better ascertained if information on the number of patients in the 21% plateau who were disease free or stably maintaining response had been collected.
Evaluation of long-term benefits of ipilimumab should consider toxicities and costs, as it is one of the most costly systemic therapies used for cancer treatment. The phase III trial using the drug at 3 mg/kg demonstrated that the large majority of patients had no serious adverse effects. If older patients and those with advanced disease are candidates, then the 10%-15% of grade 3 or 4 adverse events may translate to hospitalization and added expense, putting health regulatory systems in the position to deny widespread use of the agent despite proven benefit.
As the first agent to benefit overall survival of patients with advanced melanoma, ipilimumab may pave the way to broader improvements in a larger proportion of patients by combining with targeted therapies, such as BRAF and MEK inhibitors, and other new immunotherapies, such as anti-PD-1 antibodies.
Dr. Antoni Ribas is an oncologist with the Jonsson Comprehensive Cancer Center, Los Angles, and Dr. Keith T. Flaherty is an oncologist with Massachusetts General Hospital Cancer Center, Boston. These remarks were part of an editorial accompanying the report (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]). Dr. Ribas has an advisory role with Merck, Amgen, Novartis, GlaxoSmithKline, and Genentech/Roche. Dr. Flaherty has an advisory role with GlaxoSmithKline, Genentech/Roche, Novartis, and Merck.
Dr. Schadendorf and his associates demonstrate a plateau in the survival curve of ipilimumab-treated patients beginning at about 3 years and representing about 21% of the treatment group. The curve suggests that those who survive to 3 years are highly likely to have a good long-term outcome, which provides a strong motivating factor in the decision to consider ipilimumab treatment. While pooled data adds information far beyond individual trials, a major drawback lies in the loss of control data necessary to isolate the added benefit of the study drug.
An indirect comparison using historic control series, in this case a large cohort documented in the American Joint Committee on Cancer (AJCC) Melanoma Staging Database, can substitute for missing control data in the pooled analysis. Reviewing data for stage IIIc and IV patients, the overall survival Kaplan-Meier curves in this population also show a plateau, but much later than that reported for ipilimumab, at beyond 8 years.
The AJCC melanoma classification gives survival rates at 3, 5, and 10 years of 19%, 13%, and 9%, respectively. Comparison with ipilimumab data suggests that survival at 3 years is similar, but thereafter improves with ipilimumab by 10% over other treatments that were available at the time. This difference is similar to the percentage of patients who achieved objective responses with ipilimumab. Although assessing response rate and progression-free survival in patients treated with ipilimumab presents challenges, the long-term benefits of ipilimumab could be better ascertained if information on the number of patients in the 21% plateau who were disease free or stably maintaining response had been collected.
Evaluation of long-term benefits of ipilimumab should consider toxicities and costs, as it is one of the most costly systemic therapies used for cancer treatment. The phase III trial using the drug at 3 mg/kg demonstrated that the large majority of patients had no serious adverse effects. If older patients and those with advanced disease are candidates, then the 10%-15% of grade 3 or 4 adverse events may translate to hospitalization and added expense, putting health regulatory systems in the position to deny widespread use of the agent despite proven benefit.
As the first agent to benefit overall survival of patients with advanced melanoma, ipilimumab may pave the way to broader improvements in a larger proportion of patients by combining with targeted therapies, such as BRAF and MEK inhibitors, and other new immunotherapies, such as anti-PD-1 antibodies.
Dr. Antoni Ribas is an oncologist with the Jonsson Comprehensive Cancer Center, Los Angles, and Dr. Keith T. Flaherty is an oncologist with Massachusetts General Hospital Cancer Center, Boston. These remarks were part of an editorial accompanying the report (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]). Dr. Ribas has an advisory role with Merck, Amgen, Novartis, GlaxoSmithKline, and Genentech/Roche. Dr. Flaherty has an advisory role with GlaxoSmithKline, Genentech/Roche, Novartis, and Merck.
Dr. Schadendorf and his associates demonstrate a plateau in the survival curve of ipilimumab-treated patients beginning at about 3 years and representing about 21% of the treatment group. The curve suggests that those who survive to 3 years are highly likely to have a good long-term outcome, which provides a strong motivating factor in the decision to consider ipilimumab treatment. While pooled data adds information far beyond individual trials, a major drawback lies in the loss of control data necessary to isolate the added benefit of the study drug.
An indirect comparison using historic control series, in this case a large cohort documented in the American Joint Committee on Cancer (AJCC) Melanoma Staging Database, can substitute for missing control data in the pooled analysis. Reviewing data for stage IIIc and IV patients, the overall survival Kaplan-Meier curves in this population also show a plateau, but much later than that reported for ipilimumab, at beyond 8 years.
The AJCC melanoma classification gives survival rates at 3, 5, and 10 years of 19%, 13%, and 9%, respectively. Comparison with ipilimumab data suggests that survival at 3 years is similar, but thereafter improves with ipilimumab by 10% over other treatments that were available at the time. This difference is similar to the percentage of patients who achieved objective responses with ipilimumab. Although assessing response rate and progression-free survival in patients treated with ipilimumab presents challenges, the long-term benefits of ipilimumab could be better ascertained if information on the number of patients in the 21% plateau who were disease free or stably maintaining response had been collected.
Evaluation of long-term benefits of ipilimumab should consider toxicities and costs, as it is one of the most costly systemic therapies used for cancer treatment. The phase III trial using the drug at 3 mg/kg demonstrated that the large majority of patients had no serious adverse effects. If older patients and those with advanced disease are candidates, then the 10%-15% of grade 3 or 4 adverse events may translate to hospitalization and added expense, putting health regulatory systems in the position to deny widespread use of the agent despite proven benefit.
As the first agent to benefit overall survival of patients with advanced melanoma, ipilimumab may pave the way to broader improvements in a larger proportion of patients by combining with targeted therapies, such as BRAF and MEK inhibitors, and other new immunotherapies, such as anti-PD-1 antibodies.
Dr. Antoni Ribas is an oncologist with the Jonsson Comprehensive Cancer Center, Los Angles, and Dr. Keith T. Flaherty is an oncologist with Massachusetts General Hospital Cancer Center, Boston. These remarks were part of an editorial accompanying the report (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]). Dr. Ribas has an advisory role with Merck, Amgen, Novartis, GlaxoSmithKline, and Genentech/Roche. Dr. Flaherty has an advisory role with GlaxoSmithKline, Genentech/Roche, Novartis, and Merck.
Among patients with advanced melanoma who were treated with ipilimumab, about 20%-26% survived to 3 years, and these patients are likely to have a good long-term outcome, according to a pooled analysis of survival data published online Feb. 9 in the Journal of Clinical Oncology.
Investigators pooled data from ten prospective (including two phase III trials) and two retrospective studies with a total of 1,257 previously treated and 604 treatment-naive patients. At least 3 years after receiving ipilimumab, 254 patients were still alive, with a median follow up for this subset of 69 months. Around year 3, the Kaplan-Meier overall survival (OS) curve began to plateau and extended to 9.9 years for the longest survival follow-up.
“These results suggest that the majority of patients who reached this milestone time point had a low risk of death thereafter,” wrote Dr. Dirk Schadendorf and his associates (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]).
Compared with patients who were previously treated, treatment-naive patients had a higher median overall survival (13.5 months [95% confidence interval, 11.9-15.4] vs. 10.7 months [9.6-11.4]) and higher 3-year-survival rates (26% [21%-30%] vs. 20% [18%-23%]). No definitive conclusion could be drawn from this observation, however, since nonrandomized subsets were used for this analysis. Subset analysis by dose showed similar median OS and 3-year survival rates for ipilimumab 3 mg/kg, 10 mg/kg, and other dosing regimens.
The researchers expanded the study to include overall survival (OS) data from 2,985 patients enrolled in a U.S. multicenter, open-label, expanded-access treatment protocol (EAP). This group included patients with poorer prognostic factors, some of whom were ineligible for clinical trials. The expanded group showed a lower median OS of 9.5 months and 3 year–survival rate of 21%, with the familiar OS curve plateau around 3 years that extended up to 10 years in some patients.
While this analysis only examined overall survival rates, individual ipilimumab studies that tracked patient responses to the drug have shown that some proportion of long-term survivors did not achieve a response. Identifying the specific disease characteristics of the long-term survivors will require further study.
“Considering the historic median OS of approximately 8-10 months and a 5-year survival rate of approximately 10% in advanced melanoma, the results presented herein are encouraging for patients diagnosed with this aggressive disease,” the authors wrote.
Among patients with advanced melanoma who were treated with ipilimumab, about 20%-26% survived to 3 years, and these patients are likely to have a good long-term outcome, according to a pooled analysis of survival data published online Feb. 9 in the Journal of Clinical Oncology.
Investigators pooled data from ten prospective (including two phase III trials) and two retrospective studies with a total of 1,257 previously treated and 604 treatment-naive patients. At least 3 years after receiving ipilimumab, 254 patients were still alive, with a median follow up for this subset of 69 months. Around year 3, the Kaplan-Meier overall survival (OS) curve began to plateau and extended to 9.9 years for the longest survival follow-up.
“These results suggest that the majority of patients who reached this milestone time point had a low risk of death thereafter,” wrote Dr. Dirk Schadendorf and his associates (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.56.2736]).
Compared with patients who were previously treated, treatment-naive patients had a higher median overall survival (13.5 months [95% confidence interval, 11.9-15.4] vs. 10.7 months [9.6-11.4]) and higher 3-year-survival rates (26% [21%-30%] vs. 20% [18%-23%]). No definitive conclusion could be drawn from this observation, however, since nonrandomized subsets were used for this analysis. Subset analysis by dose showed similar median OS and 3-year survival rates for ipilimumab 3 mg/kg, 10 mg/kg, and other dosing regimens.
The researchers expanded the study to include overall survival (OS) data from 2,985 patients enrolled in a U.S. multicenter, open-label, expanded-access treatment protocol (EAP). This group included patients with poorer prognostic factors, some of whom were ineligible for clinical trials. The expanded group showed a lower median OS of 9.5 months and 3 year–survival rate of 21%, with the familiar OS curve plateau around 3 years that extended up to 10 years in some patients.
While this analysis only examined overall survival rates, individual ipilimumab studies that tracked patient responses to the drug have shown that some proportion of long-term survivors did not achieve a response. Identifying the specific disease characteristics of the long-term survivors will require further study.
“Considering the historic median OS of approximately 8-10 months and a 5-year survival rate of approximately 10% in advanced melanoma, the results presented herein are encouraging for patients diagnosed with this aggressive disease,” the authors wrote.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Ipilimumab-treated advanced melanoma patients alive at 3 years tend to have good long-term outcomes.
Major finding: Around year 3, the Kaplan-Meier OS curve began to plateau and extended to 9.9 years for the longest survival follow-up.
Data source: Pooled overall survival data from 12 studies including 1,861 ipilimumab-treated patients with advanced melanoma.
Disclosures: Dr. Schadendorf disclosed that he is a consultant for Bristol-Myers Squibb. Bristol-Myers Squibb sponsored this study.
Xerosis is significant risk during targeted anticancer treatments
Patients receiving targeted anticancer treatments are at a significant risk of developing xerosis, or abnormal dryness, according to Dr. Johannah Valentine and her associates.
In a systematic review and meta-analysis of clinical trials involving 58 targeted agents, nearly 18% of all patients developed xerosis, with 1% of patients developing high-grade xerosis. The incidence may be affected by age, concomitant medications, comorbidities, and underlying malignancies or skin conditions, and reporting may vary among physicians and institutions, the researchers said.
Patients should be counseled and treated early for this symptom to prevent suboptimal dosing and quality-of-life impairment, the investigators recommended.
Read the full article at the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2014.12.010).
Patients receiving targeted anticancer treatments are at a significant risk of developing xerosis, or abnormal dryness, according to Dr. Johannah Valentine and her associates.
In a systematic review and meta-analysis of clinical trials involving 58 targeted agents, nearly 18% of all patients developed xerosis, with 1% of patients developing high-grade xerosis. The incidence may be affected by age, concomitant medications, comorbidities, and underlying malignancies or skin conditions, and reporting may vary among physicians and institutions, the researchers said.
Patients should be counseled and treated early for this symptom to prevent suboptimal dosing and quality-of-life impairment, the investigators recommended.
Read the full article at the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2014.12.010).
Patients receiving targeted anticancer treatments are at a significant risk of developing xerosis, or abnormal dryness, according to Dr. Johannah Valentine and her associates.
In a systematic review and meta-analysis of clinical trials involving 58 targeted agents, nearly 18% of all patients developed xerosis, with 1% of patients developing high-grade xerosis. The incidence may be affected by age, concomitant medications, comorbidities, and underlying malignancies or skin conditions, and reporting may vary among physicians and institutions, the researchers said.
Patients should be counseled and treated early for this symptom to prevent suboptimal dosing and quality-of-life impairment, the investigators recommended.
Read the full article at the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2014.12.010).
Patient-led teledermoscopy appears feasible and effective
Patient-administered teledermoscopy using an iPhone-based mobile dermatoscope attachment and app is an effective and feasible method for short-term monitoring of clinically atypical nevi, with the added benefit of improving patient and physician convenience, based on data from a pilot study of 29 patients.
Researchers found a high level of diagnostic concordance (0.87) between dermatoscope images taken and assessed by an office-based dermatologist and those taken by the patient – albeit in the clinic setting – using the mobile dermatoscope and assessed by a teledermatologist.
All but one of the 29 patients with clinically atypical nevi who completed the study were able to acquire evaluable baseline and follow-up images, the researchers noted. In addition, most of the patients reported that the device was easy to use and that it saved them a trip to the doctor’s office. The study findings were published online Jan. 28 in JAMA Dermatology (doi:10.1001/jamadermatol.2014.3837).
“Under our modality of care, patients needing short-term monitoring will have an established relationship with their dermatologists, who will be the ones identifying concerning lesions that need to be monitored and the ones who evaluate the lesions via teledermoscopy and communicate treatment options directly with the patients,” wrote Xinyuan Wu of Memorial Sloan Kettering Cancer Center, New York, and colleagues.
The authors of an accompanying editorial wrote that recommendations for screening and follow-up for melanoma placed considerable burdens on patients, physicians, and the health care system, and that the patient-led mobile teledermoscopy described in the study was one of a number of options being considered to reduce that burden.
“The study by Wu and colleagues in this issue adds significantly to the discussion on whether regular follow-up visits with clinicians could be replaced by patient self-monitoring with remote feedback by a teledermatologist,” wrote Monika Janda, Ph.D., of the Queensland University of Technology in Brisbane, Australia, and colleagues.
One editorial author reported shares and consultancies with e-derm-consult GmbH and MoleMap, but there were no other conflicts of interest declared.
Patient-administered teledermoscopy using an iPhone-based mobile dermatoscope attachment and app is an effective and feasible method for short-term monitoring of clinically atypical nevi, with the added benefit of improving patient and physician convenience, based on data from a pilot study of 29 patients.
Researchers found a high level of diagnostic concordance (0.87) between dermatoscope images taken and assessed by an office-based dermatologist and those taken by the patient – albeit in the clinic setting – using the mobile dermatoscope and assessed by a teledermatologist.
All but one of the 29 patients with clinically atypical nevi who completed the study were able to acquire evaluable baseline and follow-up images, the researchers noted. In addition, most of the patients reported that the device was easy to use and that it saved them a trip to the doctor’s office. The study findings were published online Jan. 28 in JAMA Dermatology (doi:10.1001/jamadermatol.2014.3837).
“Under our modality of care, patients needing short-term monitoring will have an established relationship with their dermatologists, who will be the ones identifying concerning lesions that need to be monitored and the ones who evaluate the lesions via teledermoscopy and communicate treatment options directly with the patients,” wrote Xinyuan Wu of Memorial Sloan Kettering Cancer Center, New York, and colleagues.
The authors of an accompanying editorial wrote that recommendations for screening and follow-up for melanoma placed considerable burdens on patients, physicians, and the health care system, and that the patient-led mobile teledermoscopy described in the study was one of a number of options being considered to reduce that burden.
“The study by Wu and colleagues in this issue adds significantly to the discussion on whether regular follow-up visits with clinicians could be replaced by patient self-monitoring with remote feedback by a teledermatologist,” wrote Monika Janda, Ph.D., of the Queensland University of Technology in Brisbane, Australia, and colleagues.
One editorial author reported shares and consultancies with e-derm-consult GmbH and MoleMap, but there were no other conflicts of interest declared.
Patient-administered teledermoscopy using an iPhone-based mobile dermatoscope attachment and app is an effective and feasible method for short-term monitoring of clinically atypical nevi, with the added benefit of improving patient and physician convenience, based on data from a pilot study of 29 patients.
Researchers found a high level of diagnostic concordance (0.87) between dermatoscope images taken and assessed by an office-based dermatologist and those taken by the patient – albeit in the clinic setting – using the mobile dermatoscope and assessed by a teledermatologist.
All but one of the 29 patients with clinically atypical nevi who completed the study were able to acquire evaluable baseline and follow-up images, the researchers noted. In addition, most of the patients reported that the device was easy to use and that it saved them a trip to the doctor’s office. The study findings were published online Jan. 28 in JAMA Dermatology (doi:10.1001/jamadermatol.2014.3837).
“Under our modality of care, patients needing short-term monitoring will have an established relationship with their dermatologists, who will be the ones identifying concerning lesions that need to be monitored and the ones who evaluate the lesions via teledermoscopy and communicate treatment options directly with the patients,” wrote Xinyuan Wu of Memorial Sloan Kettering Cancer Center, New York, and colleagues.
The authors of an accompanying editorial wrote that recommendations for screening and follow-up for melanoma placed considerable burdens on patients, physicians, and the health care system, and that the patient-led mobile teledermoscopy described in the study was one of a number of options being considered to reduce that burden.
“The study by Wu and colleagues in this issue adds significantly to the discussion on whether regular follow-up visits with clinicians could be replaced by patient self-monitoring with remote feedback by a teledermatologist,” wrote Monika Janda, Ph.D., of the Queensland University of Technology in Brisbane, Australia, and colleagues.
One editorial author reported shares and consultancies with e-derm-consult GmbH and MoleMap, but there were no other conflicts of interest declared.
FROM JAMA DERMATOLOGY
Key clinical point: Patient-administered teledermoscopy using an iPhone-based mobile dermatoscope attachment and app is an effective and feasible method for short-term monitoring of clinically atypical nevi.
Major finding: Researchers found a high level of diagnostic concordance (0.87) between dermatoscope images taken and assessed by the office-based dermatologist and those taken by the patient using an iPhone.
Data source:A prospective cohort study in 34 patients – 29 of whom completed follow-up – with clinically atypical nevi.
Disclosures: One editorial author reported shares and consultancies with e-derm-consult GmbH and MoleMap. No other conflicts of interest were declared.
Teledermoscopy referrals surpass paper for managing skin cancer patients
Smartphone teledermoscopy referrals were faster and allowed for more efficient management of patients with skin cancer, compared with paper referrals, according to Dr. Alexander Börve of the University of Gothenburg, Sweden, and his associates.
The waiting time was significantly shorter using teledermoscopy for patients with various melanomas and carcinomas when surgical treatment was necessary. “Triage decisions were also more reliable with teledermoscopy, and over 40% of the teledermoscopy patients could potentially have avoided face-to-face visits,” the researchers noted (Acta. Derm. Venereol. 2015;95:186-90).
Less than 1% of teledermoscopy referrals were excluded because of poor image quality, they said.
Read the full article at Acta Dermato-Venereologica (doi:10.2340/00015555-1906).
Smartphone teledermoscopy referrals were faster and allowed for more efficient management of patients with skin cancer, compared with paper referrals, according to Dr. Alexander Börve of the University of Gothenburg, Sweden, and his associates.
The waiting time was significantly shorter using teledermoscopy for patients with various melanomas and carcinomas when surgical treatment was necessary. “Triage decisions were also more reliable with teledermoscopy, and over 40% of the teledermoscopy patients could potentially have avoided face-to-face visits,” the researchers noted (Acta. Derm. Venereol. 2015;95:186-90).
Less than 1% of teledermoscopy referrals were excluded because of poor image quality, they said.
Read the full article at Acta Dermato-Venereologica (doi:10.2340/00015555-1906).
Smartphone teledermoscopy referrals were faster and allowed for more efficient management of patients with skin cancer, compared with paper referrals, according to Dr. Alexander Börve of the University of Gothenburg, Sweden, and his associates.
The waiting time was significantly shorter using teledermoscopy for patients with various melanomas and carcinomas when surgical treatment was necessary. “Triage decisions were also more reliable with teledermoscopy, and over 40% of the teledermoscopy patients could potentially have avoided face-to-face visits,” the researchers noted (Acta. Derm. Venereol. 2015;95:186-90).
Less than 1% of teledermoscopy referrals were excluded because of poor image quality, they said.
Read the full article at Acta Dermato-Venereologica (doi:10.2340/00015555-1906).
Handheld device illuminates possible routes of melanoma metastases
Investigators using a handheld dermoscopy device that allows visualization of colors, structures, and patterns in skin lesions not evident to the naked eye were able to visualize nonblanching blue and red lines in a branched pattern in two patients with in-transit cutaneous melanoma metastases.
Dr. Michael A. Marchetti and his associates at Memorial Sloan Kettering Cancer Center, New York, reported the “intriguing” visualization of dissemination for cutaneous melanoma metastases in a letter to JAMA Dermatology.
In-transit cutaneous melanoma metastases are those located more than 2 cm from the primary melanoma, but not beyond the regional nodal basin.
The first patient had wide local excision of a primary cutaneous melanoma on the forehead, and a year later, received localized irradiation for satellite skin metastases. A year after that, skin examination revealed six blue macules on the scalp more than 2 cm from the excision scar. Dermoscopy revealed nonblanching bluish lines in a branched pattern. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal lymphatics, Dr. Marchetti and his associates reported (JAMA Dermatology 2015;103-5)
The second patient had a history of multiple primary melanomas, the most recent being one on the chest treated with wide local excision. At a follow-up visit 5 years later, skin examination revealed eight blue-gray macules on the chest, all more than 2 cm from the excision scar. Dermoscopy revealed nonblanching, red-bluish, fuzzy, branching lines. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal blood vessels, the investigators wrote.
Typical dermoscopic features of cutaneous melanoma metastases include peripheral gray spots, atypical vessels, and a blue nevus-like pattern. The histopathologic findings in these two cases suggest that the dermoscopic color differences correspond to unique microanatomic routes of melanoma dissemination, with blue and red-blue lines corresponding to lymphatic and hematogenous dissemination of tumors, respectively, they said.
“While the factors driving lymphatic vs. hematogenous in-transit dissemination of melanoma remain unknown, as do any differences in their biologic significance, our finding is an intriguing clinical/dermoscopic/histopathologic observation,” the investigators concluded.
On Twitter @nikolaideslaura
Investigators using a handheld dermoscopy device that allows visualization of colors, structures, and patterns in skin lesions not evident to the naked eye were able to visualize nonblanching blue and red lines in a branched pattern in two patients with in-transit cutaneous melanoma metastases.
Dr. Michael A. Marchetti and his associates at Memorial Sloan Kettering Cancer Center, New York, reported the “intriguing” visualization of dissemination for cutaneous melanoma metastases in a letter to JAMA Dermatology.
In-transit cutaneous melanoma metastases are those located more than 2 cm from the primary melanoma, but not beyond the regional nodal basin.
The first patient had wide local excision of a primary cutaneous melanoma on the forehead, and a year later, received localized irradiation for satellite skin metastases. A year after that, skin examination revealed six blue macules on the scalp more than 2 cm from the excision scar. Dermoscopy revealed nonblanching bluish lines in a branched pattern. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal lymphatics, Dr. Marchetti and his associates reported (JAMA Dermatology 2015;103-5)
The second patient had a history of multiple primary melanomas, the most recent being one on the chest treated with wide local excision. At a follow-up visit 5 years later, skin examination revealed eight blue-gray macules on the chest, all more than 2 cm from the excision scar. Dermoscopy revealed nonblanching, red-bluish, fuzzy, branching lines. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal blood vessels, the investigators wrote.
Typical dermoscopic features of cutaneous melanoma metastases include peripheral gray spots, atypical vessels, and a blue nevus-like pattern. The histopathologic findings in these two cases suggest that the dermoscopic color differences correspond to unique microanatomic routes of melanoma dissemination, with blue and red-blue lines corresponding to lymphatic and hematogenous dissemination of tumors, respectively, they said.
“While the factors driving lymphatic vs. hematogenous in-transit dissemination of melanoma remain unknown, as do any differences in their biologic significance, our finding is an intriguing clinical/dermoscopic/histopathologic observation,” the investigators concluded.
On Twitter @nikolaideslaura
Investigators using a handheld dermoscopy device that allows visualization of colors, structures, and patterns in skin lesions not evident to the naked eye were able to visualize nonblanching blue and red lines in a branched pattern in two patients with in-transit cutaneous melanoma metastases.
Dr. Michael A. Marchetti and his associates at Memorial Sloan Kettering Cancer Center, New York, reported the “intriguing” visualization of dissemination for cutaneous melanoma metastases in a letter to JAMA Dermatology.
In-transit cutaneous melanoma metastases are those located more than 2 cm from the primary melanoma, but not beyond the regional nodal basin.
The first patient had wide local excision of a primary cutaneous melanoma on the forehead, and a year later, received localized irradiation for satellite skin metastases. A year after that, skin examination revealed six blue macules on the scalp more than 2 cm from the excision scar. Dermoscopy revealed nonblanching bluish lines in a branched pattern. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal lymphatics, Dr. Marchetti and his associates reported (JAMA Dermatology 2015;103-5)
The second patient had a history of multiple primary melanomas, the most recent being one on the chest treated with wide local excision. At a follow-up visit 5 years later, skin examination revealed eight blue-gray macules on the chest, all more than 2 cm from the excision scar. Dermoscopy revealed nonblanching, red-bluish, fuzzy, branching lines. Histopathologic examination of a skin biopsy confirmed in-transit metastatic melanoma with atypical melanocytes present in superficial dermal blood vessels, the investigators wrote.
Typical dermoscopic features of cutaneous melanoma metastases include peripheral gray spots, atypical vessels, and a blue nevus-like pattern. The histopathologic findings in these two cases suggest that the dermoscopic color differences correspond to unique microanatomic routes of melanoma dissemination, with blue and red-blue lines corresponding to lymphatic and hematogenous dissemination of tumors, respectively, they said.
“While the factors driving lymphatic vs. hematogenous in-transit dissemination of melanoma remain unknown, as do any differences in their biologic significance, our finding is an intriguing clinical/dermoscopic/histopathologic observation,” the investigators concluded.
On Twitter @nikolaideslaura
FROM JAMA DERMATOLOGY
MEK inhibitors can induce skin eruptions with distinctive duskiness
Case reports of unusual drug hypersensitivity to MEK inhibitors, involving skin eruptions with distinctive central duskiness, have been described online in JAMA Dermatology.
Three patients who were receiving different MEK inhibitors (selumetinib, cobimetinib, and trametinib) developed grade 2 or 3 eruptions, all associated with unique duskiness, reported Dr. Urvi Patel and associates at Washington University, St. Louis.
A 60-year-old man with pancreatic cancer who was receiving selumetinib as part of a clinical trial presented with a grade 2 generalized eruption and pruritus 12 days after initiating therapy. He had diffuse targetoid patches with central duskiness. Selumetinib and other study drugs were withheld, the patient was given topical corticosteroid treatment, and the eruption completely resolved after 4 weeks. The patient did not restart the study drugs because of an elevated alkaline phosphatase level and fatigue.
A woman in her 40s who was receiving cobimetinib and other medication for metastatic melanoma developed grade 2 coalescing urticarial patches with surrounding duskiness on day 28 of treatment. Histopathologic examination showed a superficial perivascular lymphocytic infiltrate with rare eosinophils. After treatment was halted for 7 days and a regimen of oral prednisone was started, cobimetinib therapy was reinstituted at a lower dose. There was no recurrence of the eruption 1 year after cobimetinib therapy was restarted, Dr. Patel and associates reported (JAMA Dermatol. 2015 Jan. 14 [doi:10.1001/jamadermatol.2014.3207]).
The third patient, a woman in her 50s with metastatic melanoma, developed a grade 3 eruption 7 weeks into trametinib treatment together with another drug. The worsening urticarial patches and plaques had surrounding diffuse duskiness. After trametinib treatment was withheld for a week, and a regimen of oral prednisone was begun, trametinib therapy was restarted and the eruption did not return.
“As shown in our patients, successful treatment of this MEK inhibitor–associated cutaneous eruption can include a drug holiday and oral corticosteroid therapy, with reinstitution of the drug at a lower dose without recurrence,” Dr. Patel and his associates wrote.
MEK inhibitors target the mitogen-activated protein kinase pathway. Trametinib has been approved for treating advanced melanoma, and more than a dozen other MEK inhibitors are in clinical trials (including selumetinib and cobimetinib) for treatment of melanoma and other solid-organ malignant neoplasms, including pancreatic, hepatocellular, colorectal, and non–small cell lung cancer, the authors noted.
On Twitter @nikolaideslaura
Case reports of unusual drug hypersensitivity to MEK inhibitors, involving skin eruptions with distinctive central duskiness, have been described online in JAMA Dermatology.
Three patients who were receiving different MEK inhibitors (selumetinib, cobimetinib, and trametinib) developed grade 2 or 3 eruptions, all associated with unique duskiness, reported Dr. Urvi Patel and associates at Washington University, St. Louis.
A 60-year-old man with pancreatic cancer who was receiving selumetinib as part of a clinical trial presented with a grade 2 generalized eruption and pruritus 12 days after initiating therapy. He had diffuse targetoid patches with central duskiness. Selumetinib and other study drugs were withheld, the patient was given topical corticosteroid treatment, and the eruption completely resolved after 4 weeks. The patient did not restart the study drugs because of an elevated alkaline phosphatase level and fatigue.
A woman in her 40s who was receiving cobimetinib and other medication for metastatic melanoma developed grade 2 coalescing urticarial patches with surrounding duskiness on day 28 of treatment. Histopathologic examination showed a superficial perivascular lymphocytic infiltrate with rare eosinophils. After treatment was halted for 7 days and a regimen of oral prednisone was started, cobimetinib therapy was reinstituted at a lower dose. There was no recurrence of the eruption 1 year after cobimetinib therapy was restarted, Dr. Patel and associates reported (JAMA Dermatol. 2015 Jan. 14 [doi:10.1001/jamadermatol.2014.3207]).
The third patient, a woman in her 50s with metastatic melanoma, developed a grade 3 eruption 7 weeks into trametinib treatment together with another drug. The worsening urticarial patches and plaques had surrounding diffuse duskiness. After trametinib treatment was withheld for a week, and a regimen of oral prednisone was begun, trametinib therapy was restarted and the eruption did not return.
“As shown in our patients, successful treatment of this MEK inhibitor–associated cutaneous eruption can include a drug holiday and oral corticosteroid therapy, with reinstitution of the drug at a lower dose without recurrence,” Dr. Patel and his associates wrote.
MEK inhibitors target the mitogen-activated protein kinase pathway. Trametinib has been approved for treating advanced melanoma, and more than a dozen other MEK inhibitors are in clinical trials (including selumetinib and cobimetinib) for treatment of melanoma and other solid-organ malignant neoplasms, including pancreatic, hepatocellular, colorectal, and non–small cell lung cancer, the authors noted.
On Twitter @nikolaideslaura
Case reports of unusual drug hypersensitivity to MEK inhibitors, involving skin eruptions with distinctive central duskiness, have been described online in JAMA Dermatology.
Three patients who were receiving different MEK inhibitors (selumetinib, cobimetinib, and trametinib) developed grade 2 or 3 eruptions, all associated with unique duskiness, reported Dr. Urvi Patel and associates at Washington University, St. Louis.
A 60-year-old man with pancreatic cancer who was receiving selumetinib as part of a clinical trial presented with a grade 2 generalized eruption and pruritus 12 days after initiating therapy. He had diffuse targetoid patches with central duskiness. Selumetinib and other study drugs were withheld, the patient was given topical corticosteroid treatment, and the eruption completely resolved after 4 weeks. The patient did not restart the study drugs because of an elevated alkaline phosphatase level and fatigue.
A woman in her 40s who was receiving cobimetinib and other medication for metastatic melanoma developed grade 2 coalescing urticarial patches with surrounding duskiness on day 28 of treatment. Histopathologic examination showed a superficial perivascular lymphocytic infiltrate with rare eosinophils. After treatment was halted for 7 days and a regimen of oral prednisone was started, cobimetinib therapy was reinstituted at a lower dose. There was no recurrence of the eruption 1 year after cobimetinib therapy was restarted, Dr. Patel and associates reported (JAMA Dermatol. 2015 Jan. 14 [doi:10.1001/jamadermatol.2014.3207]).
The third patient, a woman in her 50s with metastatic melanoma, developed a grade 3 eruption 7 weeks into trametinib treatment together with another drug. The worsening urticarial patches and plaques had surrounding diffuse duskiness. After trametinib treatment was withheld for a week, and a regimen of oral prednisone was begun, trametinib therapy was restarted and the eruption did not return.
“As shown in our patients, successful treatment of this MEK inhibitor–associated cutaneous eruption can include a drug holiday and oral corticosteroid therapy, with reinstitution of the drug at a lower dose without recurrence,” Dr. Patel and his associates wrote.
MEK inhibitors target the mitogen-activated protein kinase pathway. Trametinib has been approved for treating advanced melanoma, and more than a dozen other MEK inhibitors are in clinical trials (including selumetinib and cobimetinib) for treatment of melanoma and other solid-organ malignant neoplasms, including pancreatic, hepatocellular, colorectal, and non–small cell lung cancer, the authors noted.
On Twitter @nikolaideslaura
FROM JAMA DERMATOLOGY
Key clinical point: This MEK inhibitor–associated cutaneous eruption can be treated with a drug holiday and oral corticosteroid treatment, restarting the drug at a lower dose without recurrence.
Major finding: Three patients who were receiving different MEK inhibitors (selumetinib, cobimetinib, and trametinib) developed grade 2 or 3 eruptions, all associated with unique duskiness.
Data source: Three case studies of patients receiving different MEK inhibitors.
Disclosures: Dr. Lynn Cornelius has received a research grant from Genentech and is a clinical subinvestigator for GlaxoSmithKline. Dr. Milan J. Anadkat has received honoraria as a speaker and/or consultant from AstraZeneca, Bristol-Myers Squibb, Eisai, ImClone, and Therakos. No other disclosures were reported.
RNA sequencing characterized high-risk squamous cell carcinomas
SAN DIEGO – Cutaneous squamous cell carcinomas from organ transplant recipients had a more aggressive molecular profile than did tumor samples from immunocompetent patients, according to an RNA sequencing study presented at the annual meeting of the American Society for Dermatologic Surgery.
Specimens from organ transplant recipients showed greater induction of biologic pathways related to cancer signaling, fibrosis, and extracellular matrix remodeling, said Dr. Cameron Chesnut, a dermatologist in private practice in Spokane, Wash., who carried out the research while he was a dermatologic surgery resident at the University of California, Los Angeles.
Furthermore, the TP53 tumor suppressor gene was inhibited at least five times more in samples from organ transplant recipients, compared with those from immunocompetent patients, Dr. Chesnut said in an interview.
Squamous cell carcinoma (SCC) is the most common cancer to occur after organ transplantation, Dr. Chesnut and his associates noted. The malignancy is 65-250 times more common, is more than 4 times more likely to metastasize, and has a mortality rate of 5% compared with a rate of less than 1% in immunocompetent patients, based on data published online in the journal F1000 Prime Reports, they said.
To characterize these high-risk SCCs and compare them with lower-risk SCCs, the researchers performed RNA sequencing of three normal skin samples and SCC specimens from 15 patients – 7 organ transplant recipients and 8 otherwise healthy individuals. The researchers used an Illumina GAIIx RNA Seq instrument to generate RNA sequencing libraries of the specimens. They also used the web-based Ingenuity Pathway Analysis technique to identify the major biological pathways regulated within the tumors.
In all, 690 highly expressed genes were induced at least fivefold in SCCs from organ transplant recipients compared with those from otherwise healthy patients. These genes encoded pathways related to fibrosis, extracellular remodeling, the cell cycle, and tumor signaling, the investigators said. The COX-2 pathway for prostaglandin synthesis also was induced fivefold or more in the high-risk SCCs compared with those from immunocompetent patients, Dr. Chesnut added.
The researchers also identified 1,290 highly expressed genes that were inhibited at least fivefold in SCCs from organ transplant recipients compared with specimens from immunocompetent patients. The most strongly inhibited pathways were related to sterol biosynthesis and epithelial differentiation, followed by nucleotide excision repair, interleukin-6 and IL-17, and apoptosis, they said.
Based on these findings, novel therapeutics might someday be able to target specific biologic pathways that are highly induced in SCCs from organ transplant recipients, Dr. Chesnut said. “It’s hard to say what the most likely candidates are,” but based on the study findings, “regulating inflammation may be a target,” he added. Dr. Chesnut and his associates reported no external funding sources or conflicts of interest.
SAN DIEGO – Cutaneous squamous cell carcinomas from organ transplant recipients had a more aggressive molecular profile than did tumor samples from immunocompetent patients, according to an RNA sequencing study presented at the annual meeting of the American Society for Dermatologic Surgery.
Specimens from organ transplant recipients showed greater induction of biologic pathways related to cancer signaling, fibrosis, and extracellular matrix remodeling, said Dr. Cameron Chesnut, a dermatologist in private practice in Spokane, Wash., who carried out the research while he was a dermatologic surgery resident at the University of California, Los Angeles.
Furthermore, the TP53 tumor suppressor gene was inhibited at least five times more in samples from organ transplant recipients, compared with those from immunocompetent patients, Dr. Chesnut said in an interview.
Squamous cell carcinoma (SCC) is the most common cancer to occur after organ transplantation, Dr. Chesnut and his associates noted. The malignancy is 65-250 times more common, is more than 4 times more likely to metastasize, and has a mortality rate of 5% compared with a rate of less than 1% in immunocompetent patients, based on data published online in the journal F1000 Prime Reports, they said.
To characterize these high-risk SCCs and compare them with lower-risk SCCs, the researchers performed RNA sequencing of three normal skin samples and SCC specimens from 15 patients – 7 organ transplant recipients and 8 otherwise healthy individuals. The researchers used an Illumina GAIIx RNA Seq instrument to generate RNA sequencing libraries of the specimens. They also used the web-based Ingenuity Pathway Analysis technique to identify the major biological pathways regulated within the tumors.
In all, 690 highly expressed genes were induced at least fivefold in SCCs from organ transplant recipients compared with those from otherwise healthy patients. These genes encoded pathways related to fibrosis, extracellular remodeling, the cell cycle, and tumor signaling, the investigators said. The COX-2 pathway for prostaglandin synthesis also was induced fivefold or more in the high-risk SCCs compared with those from immunocompetent patients, Dr. Chesnut added.
The researchers also identified 1,290 highly expressed genes that were inhibited at least fivefold in SCCs from organ transplant recipients compared with specimens from immunocompetent patients. The most strongly inhibited pathways were related to sterol biosynthesis and epithelial differentiation, followed by nucleotide excision repair, interleukin-6 and IL-17, and apoptosis, they said.
Based on these findings, novel therapeutics might someday be able to target specific biologic pathways that are highly induced in SCCs from organ transplant recipients, Dr. Chesnut said. “It’s hard to say what the most likely candidates are,” but based on the study findings, “regulating inflammation may be a target,” he added. Dr. Chesnut and his associates reported no external funding sources or conflicts of interest.
SAN DIEGO – Cutaneous squamous cell carcinomas from organ transplant recipients had a more aggressive molecular profile than did tumor samples from immunocompetent patients, according to an RNA sequencing study presented at the annual meeting of the American Society for Dermatologic Surgery.
Specimens from organ transplant recipients showed greater induction of biologic pathways related to cancer signaling, fibrosis, and extracellular matrix remodeling, said Dr. Cameron Chesnut, a dermatologist in private practice in Spokane, Wash., who carried out the research while he was a dermatologic surgery resident at the University of California, Los Angeles.
Furthermore, the TP53 tumor suppressor gene was inhibited at least five times more in samples from organ transplant recipients, compared with those from immunocompetent patients, Dr. Chesnut said in an interview.
Squamous cell carcinoma (SCC) is the most common cancer to occur after organ transplantation, Dr. Chesnut and his associates noted. The malignancy is 65-250 times more common, is more than 4 times more likely to metastasize, and has a mortality rate of 5% compared with a rate of less than 1% in immunocompetent patients, based on data published online in the journal F1000 Prime Reports, they said.
To characterize these high-risk SCCs and compare them with lower-risk SCCs, the researchers performed RNA sequencing of three normal skin samples and SCC specimens from 15 patients – 7 organ transplant recipients and 8 otherwise healthy individuals. The researchers used an Illumina GAIIx RNA Seq instrument to generate RNA sequencing libraries of the specimens. They also used the web-based Ingenuity Pathway Analysis technique to identify the major biological pathways regulated within the tumors.
In all, 690 highly expressed genes were induced at least fivefold in SCCs from organ transplant recipients compared with those from otherwise healthy patients. These genes encoded pathways related to fibrosis, extracellular remodeling, the cell cycle, and tumor signaling, the investigators said. The COX-2 pathway for prostaglandin synthesis also was induced fivefold or more in the high-risk SCCs compared with those from immunocompetent patients, Dr. Chesnut added.
The researchers also identified 1,290 highly expressed genes that were inhibited at least fivefold in SCCs from organ transplant recipients compared with specimens from immunocompetent patients. The most strongly inhibited pathways were related to sterol biosynthesis and epithelial differentiation, followed by nucleotide excision repair, interleukin-6 and IL-17, and apoptosis, they said.
Based on these findings, novel therapeutics might someday be able to target specific biologic pathways that are highly induced in SCCs from organ transplant recipients, Dr. Chesnut said. “It’s hard to say what the most likely candidates are,” but based on the study findings, “regulating inflammation may be a target,” he added. Dr. Chesnut and his associates reported no external funding sources or conflicts of interest.
Key clinical point: Squamous cell carcinomas from organ transplant recipients showed a more aggressive molecular profile than did those from immunocompetent individuals.
Major finding: The high-risk tumors showed greater induction of biologic pathways related to cancer signaling, fibrosis, and extracellular matrix remodeling, and inhibition of the tp53 tumor suppressor gene.
Data source: RNA sequencing of 15 squamous cell carcinomas, including seven from organ transplant recipients.
Disclosures: The investigators reported no external funding sources or conflicts of interest.
Reduced Degree of Irritation During a Second Cycle of Ingenol Mebutate Gel 0.015% for the Treatment of Actinic Keratosis
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.
|

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
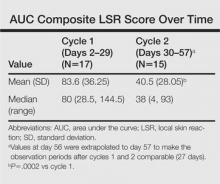
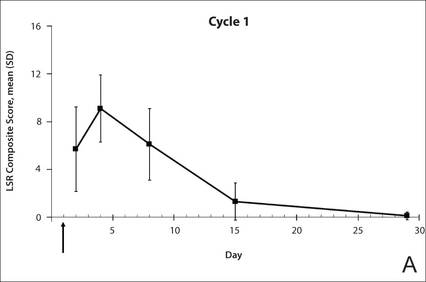
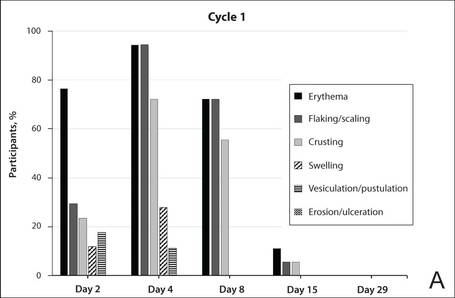
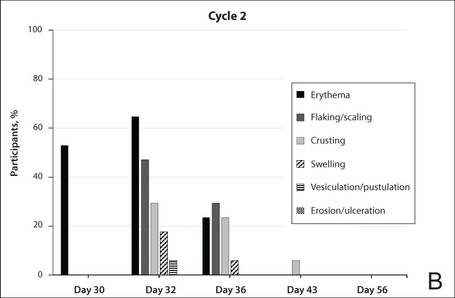
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.
|
|
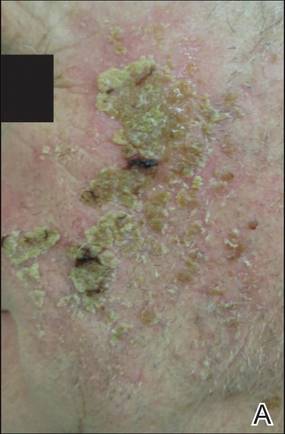
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes
|

|
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.

|

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.

|

|
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes

|

|
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.

|

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.

|

|
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes

|

|
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
Practice Points
- Reapplication of ingenol mebutate gel 0.015% to the same treatment area on the face or scalp produced a less intense inflammatory reaction than the first treatment course.
- Ingenol mebutate may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over 2 treatment cycles.
- Almost all patients were either clear or almost clear of actinic keratosis lesions by 4 weeks following the second application of ingenol mebutate.
Ionizing radiation linked to BCC
SAN DIEGO – Patients treated with ionizing radiation were 2.65 to 3.78 times more likely to develop basal cell carcinoma than were controls, and exposure at younger ages or relatively high doses further increased that risk, according to a pooled analysis presented at the annual meeting of the American Society for Dermatologic Surgery.
“Concomitant exposure to ultraviolet radiation may potentiate this effect,” said Dr. Min Deng, a dermatology resident at the University of Chicago. “With newer treatment protocols and improved shielding, it would be interesting to see if the effect of ionizing radiation has changed, or whether we as dermatologists should more actively screen this at-risk population.”
Basal cell carcinoma (BCC) is the most common skin cancer worldwide, but relatively few dermatology papers have assessed the effects of ionizing radiation on the incidence of BCC, said Dr. Deng and coauthor Dr. Diana Bolotin, also of the University of Chicago.
To better understand the link, the researchers searched PubMed for controlled studies on the topic by using the terms “radiation,” “risk,” and “basal cell carcinoma.” They excluded case reports, animal studies, studies published in languages other than English, and trials of radiation as a treatment of BCC, they said. They also excluded studies of atomic bomb survivors, because exposure was uncontrolled and methods to estimate exposure in this group have changed over time, they noted.
In all, six studies published between 1991 and 2012 met the inclusion criteria, and all six showed a statistically significant relative risk or odds of BCC with ionizing radiation exposure, the investigators reported. Three analyses calculated the relative risk of BCC in patients who received radiation for tinea capitis or who underwent total-body irradiation before hematopoietic cell transplantation, they said. Two studies evaluated the odds of BCC in patients with a past history of radiation exposure, and one study assessed time to subsequent BCCs in patients with a history of BCC.
For the three studies that calculated relative risk, the researchers calculated a pooled RR of BCC after ionizing radiation treatment of 2.65 (95% confidence interval, 1.22-5.72) compared with controls. The study of total-body irradiation (TBI) did not report or control for the primary disease for which patients were treated, “which may have confounded the results,” they said. When they excluded that study from their calculation, the overall RR rose to 3.78 (95% CI, 2.62 to 5.44).
Notably, in the two studies of patients with tinea capitis, the relative risk of BCC fell by 12% to 16% for every additional year of age at which patients received radiation treatment, the researchers said. Similarly, risk of BCC dropped by 10.9% for every 1-year increase in age at total-body irradiation prior to cell transplantation.
The combined odds ratio for the next two studies was 4.28 (1.45-12.63). “Likewise, both studies found an elevated odds ratio with younger age at radiation exposure,” the researchers added. One study that stratified patients by type of medical condition detected a “markedly elevated” 8.7 odds of BCC after radiation treatment for acne (2.0 to 38.0), they noted.
The sixth study was a nested case-control analysis that found a statistically significant increase in the odds of BCC starting at a 1-Gy dose of ionizing radiation, and rising linearly up to doses of 35-63.3 Gy. “The risk for developing multiple BCCs also appears to be elevated in patients with a history of radiation therapy,” the researchers said. Patients who had been exposed to ionizing ratio were 2.3 times more likely to develop new BCCs compared with unexposed patients (1.7 to 3.1), they said.
The investigators reported no funding sources or conflicts of interest.
SAN DIEGO – Patients treated with ionizing radiation were 2.65 to 3.78 times more likely to develop basal cell carcinoma than were controls, and exposure at younger ages or relatively high doses further increased that risk, according to a pooled analysis presented at the annual meeting of the American Society for Dermatologic Surgery.
“Concomitant exposure to ultraviolet radiation may potentiate this effect,” said Dr. Min Deng, a dermatology resident at the University of Chicago. “With newer treatment protocols and improved shielding, it would be interesting to see if the effect of ionizing radiation has changed, or whether we as dermatologists should more actively screen this at-risk population.”
Basal cell carcinoma (BCC) is the most common skin cancer worldwide, but relatively few dermatology papers have assessed the effects of ionizing radiation on the incidence of BCC, said Dr. Deng and coauthor Dr. Diana Bolotin, also of the University of Chicago.
To better understand the link, the researchers searched PubMed for controlled studies on the topic by using the terms “radiation,” “risk,” and “basal cell carcinoma.” They excluded case reports, animal studies, studies published in languages other than English, and trials of radiation as a treatment of BCC, they said. They also excluded studies of atomic bomb survivors, because exposure was uncontrolled and methods to estimate exposure in this group have changed over time, they noted.
In all, six studies published between 1991 and 2012 met the inclusion criteria, and all six showed a statistically significant relative risk or odds of BCC with ionizing radiation exposure, the investigators reported. Three analyses calculated the relative risk of BCC in patients who received radiation for tinea capitis or who underwent total-body irradiation before hematopoietic cell transplantation, they said. Two studies evaluated the odds of BCC in patients with a past history of radiation exposure, and one study assessed time to subsequent BCCs in patients with a history of BCC.
For the three studies that calculated relative risk, the researchers calculated a pooled RR of BCC after ionizing radiation treatment of 2.65 (95% confidence interval, 1.22-5.72) compared with controls. The study of total-body irradiation (TBI) did not report or control for the primary disease for which patients were treated, “which may have confounded the results,” they said. When they excluded that study from their calculation, the overall RR rose to 3.78 (95% CI, 2.62 to 5.44).
Notably, in the two studies of patients with tinea capitis, the relative risk of BCC fell by 12% to 16% for every additional year of age at which patients received radiation treatment, the researchers said. Similarly, risk of BCC dropped by 10.9% for every 1-year increase in age at total-body irradiation prior to cell transplantation.
The combined odds ratio for the next two studies was 4.28 (1.45-12.63). “Likewise, both studies found an elevated odds ratio with younger age at radiation exposure,” the researchers added. One study that stratified patients by type of medical condition detected a “markedly elevated” 8.7 odds of BCC after radiation treatment for acne (2.0 to 38.0), they noted.
The sixth study was a nested case-control analysis that found a statistically significant increase in the odds of BCC starting at a 1-Gy dose of ionizing radiation, and rising linearly up to doses of 35-63.3 Gy. “The risk for developing multiple BCCs also appears to be elevated in patients with a history of radiation therapy,” the researchers said. Patients who had been exposed to ionizing ratio were 2.3 times more likely to develop new BCCs compared with unexposed patients (1.7 to 3.1), they said.
The investigators reported no funding sources or conflicts of interest.
SAN DIEGO – Patients treated with ionizing radiation were 2.65 to 3.78 times more likely to develop basal cell carcinoma than were controls, and exposure at younger ages or relatively high doses further increased that risk, according to a pooled analysis presented at the annual meeting of the American Society for Dermatologic Surgery.
“Concomitant exposure to ultraviolet radiation may potentiate this effect,” said Dr. Min Deng, a dermatology resident at the University of Chicago. “With newer treatment protocols and improved shielding, it would be interesting to see if the effect of ionizing radiation has changed, or whether we as dermatologists should more actively screen this at-risk population.”
Basal cell carcinoma (BCC) is the most common skin cancer worldwide, but relatively few dermatology papers have assessed the effects of ionizing radiation on the incidence of BCC, said Dr. Deng and coauthor Dr. Diana Bolotin, also of the University of Chicago.
To better understand the link, the researchers searched PubMed for controlled studies on the topic by using the terms “radiation,” “risk,” and “basal cell carcinoma.” They excluded case reports, animal studies, studies published in languages other than English, and trials of radiation as a treatment of BCC, they said. They also excluded studies of atomic bomb survivors, because exposure was uncontrolled and methods to estimate exposure in this group have changed over time, they noted.
In all, six studies published between 1991 and 2012 met the inclusion criteria, and all six showed a statistically significant relative risk or odds of BCC with ionizing radiation exposure, the investigators reported. Three analyses calculated the relative risk of BCC in patients who received radiation for tinea capitis or who underwent total-body irradiation before hematopoietic cell transplantation, they said. Two studies evaluated the odds of BCC in patients with a past history of radiation exposure, and one study assessed time to subsequent BCCs in patients with a history of BCC.
For the three studies that calculated relative risk, the researchers calculated a pooled RR of BCC after ionizing radiation treatment of 2.65 (95% confidence interval, 1.22-5.72) compared with controls. The study of total-body irradiation (TBI) did not report or control for the primary disease for which patients were treated, “which may have confounded the results,” they said. When they excluded that study from their calculation, the overall RR rose to 3.78 (95% CI, 2.62 to 5.44).
Notably, in the two studies of patients with tinea capitis, the relative risk of BCC fell by 12% to 16% for every additional year of age at which patients received radiation treatment, the researchers said. Similarly, risk of BCC dropped by 10.9% for every 1-year increase in age at total-body irradiation prior to cell transplantation.
The combined odds ratio for the next two studies was 4.28 (1.45-12.63). “Likewise, both studies found an elevated odds ratio with younger age at radiation exposure,” the researchers added. One study that stratified patients by type of medical condition detected a “markedly elevated” 8.7 odds of BCC after radiation treatment for acne (2.0 to 38.0), they noted.
The sixth study was a nested case-control analysis that found a statistically significant increase in the odds of BCC starting at a 1-Gy dose of ionizing radiation, and rising linearly up to doses of 35-63.3 Gy. “The risk for developing multiple BCCs also appears to be elevated in patients with a history of radiation therapy,” the researchers said. Patients who had been exposed to ionizing ratio were 2.3 times more likely to develop new BCCs compared with unexposed patients (1.7 to 3.1), they said.
The investigators reported no funding sources or conflicts of interest.
Key clinical point: Ionizing radiation therapy significantly increased risk of basal cell carcinoma, especially when patients were younger or were treated at relatively high doses.
Major finding: The pooled relative risk of BCC after ionizing radiation treatment was 2.65 (95% confidence interval, 1.22-5.72) compared with controls.
Data source: Pooled analysis of six studies of ionizing radiation exposure and risk or odds of basal cell carcinoma.
Disclosures: The researchers reported no funding sources or conflicts of interest.