User login
Screen all patients for cannabis use before surgery: Guideline
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
FROM REGIONAL ANETHESIA AND MEDICINE
FDA considers regulating CBD products
The products can have drug-like effects on the body and contain CBD (cannabidiol) and THC (tetrahydrocannabinol). Both CBD and THC can be derived from hemp, which was legalized by Congress in 2018.
“Given what we know about the safety of CBD so far, it raises concerns for FDA about whether these existing regulatory pathways for food and dietary supplements are appropriate for this substance,” FDA Principal Deputy Commissioner Janet Woodcock, MD, told The Wall Street Journal.
A 2021 FDA report valued the CBD market at $4.6 billion and projected it to quadruple by 2026. The only FDA-approved CBD product is an oil called Epidiolex, which can be prescribed for the seizure-associated disease epilepsy. Research on CBD to treat other diseases is ongoing.
Food, beverage, and beauty products containing CBD are sold in stores and online in many forms, including oils, vaporized liquids, and oil-based capsules, but “research supporting the drug’s benefits is still limited,” the Mayo Clinic said.
Recently, investigations have found that many CBD products also contain THC, which can be derived from legal hemp in a form that is referred to as Delta 8 and produces a psychoactive high. The CDC warned in 2022 that people “mistook” THC products for CBD products, which are often sold at the same stores, and experienced “adverse events.”
The Centers for Disease Control and Prevention and FDA warn that much is unknown about CBD and delta-8 products. The CDC says known CBD risks include liver damage; interference with other drugs you are taking, which may lead to injury or serious side effects; drowsiness or sleepiness; diarrhea or changes in appetite; changes in mood, such as crankiness; potential negative effects on fetuses during pregnancy or on babies during breastfeeding; or unintentional poisoning of children when mistaking THC products for CBD products or due to containing other ingredients such as THC or pesticides.
“I don’t think that we can have the perfect be the enemy of the good when we’re looking at such a vast market that is so available and utilized,” Norman Birenbaum, a senior FDA adviser who is working on the regulatory issue, told the Journal. “You’ve got a widely unregulated market.”
A version of this article first appeared on WebMD.com.
The products can have drug-like effects on the body and contain CBD (cannabidiol) and THC (tetrahydrocannabinol). Both CBD and THC can be derived from hemp, which was legalized by Congress in 2018.
“Given what we know about the safety of CBD so far, it raises concerns for FDA about whether these existing regulatory pathways for food and dietary supplements are appropriate for this substance,” FDA Principal Deputy Commissioner Janet Woodcock, MD, told The Wall Street Journal.
A 2021 FDA report valued the CBD market at $4.6 billion and projected it to quadruple by 2026. The only FDA-approved CBD product is an oil called Epidiolex, which can be prescribed for the seizure-associated disease epilepsy. Research on CBD to treat other diseases is ongoing.
Food, beverage, and beauty products containing CBD are sold in stores and online in many forms, including oils, vaporized liquids, and oil-based capsules, but “research supporting the drug’s benefits is still limited,” the Mayo Clinic said.
Recently, investigations have found that many CBD products also contain THC, which can be derived from legal hemp in a form that is referred to as Delta 8 and produces a psychoactive high. The CDC warned in 2022 that people “mistook” THC products for CBD products, which are often sold at the same stores, and experienced “adverse events.”
The Centers for Disease Control and Prevention and FDA warn that much is unknown about CBD and delta-8 products. The CDC says known CBD risks include liver damage; interference with other drugs you are taking, which may lead to injury or serious side effects; drowsiness or sleepiness; diarrhea or changes in appetite; changes in mood, such as crankiness; potential negative effects on fetuses during pregnancy or on babies during breastfeeding; or unintentional poisoning of children when mistaking THC products for CBD products or due to containing other ingredients such as THC or pesticides.
“I don’t think that we can have the perfect be the enemy of the good when we’re looking at such a vast market that is so available and utilized,” Norman Birenbaum, a senior FDA adviser who is working on the regulatory issue, told the Journal. “You’ve got a widely unregulated market.”
A version of this article first appeared on WebMD.com.
The products can have drug-like effects on the body and contain CBD (cannabidiol) and THC (tetrahydrocannabinol). Both CBD and THC can be derived from hemp, which was legalized by Congress in 2018.
“Given what we know about the safety of CBD so far, it raises concerns for FDA about whether these existing regulatory pathways for food and dietary supplements are appropriate for this substance,” FDA Principal Deputy Commissioner Janet Woodcock, MD, told The Wall Street Journal.
A 2021 FDA report valued the CBD market at $4.6 billion and projected it to quadruple by 2026. The only FDA-approved CBD product is an oil called Epidiolex, which can be prescribed for the seizure-associated disease epilepsy. Research on CBD to treat other diseases is ongoing.
Food, beverage, and beauty products containing CBD are sold in stores and online in many forms, including oils, vaporized liquids, and oil-based capsules, but “research supporting the drug’s benefits is still limited,” the Mayo Clinic said.
Recently, investigations have found that many CBD products also contain THC, which can be derived from legal hemp in a form that is referred to as Delta 8 and produces a psychoactive high. The CDC warned in 2022 that people “mistook” THC products for CBD products, which are often sold at the same stores, and experienced “adverse events.”
The Centers for Disease Control and Prevention and FDA warn that much is unknown about CBD and delta-8 products. The CDC says known CBD risks include liver damage; interference with other drugs you are taking, which may lead to injury or serious side effects; drowsiness or sleepiness; diarrhea or changes in appetite; changes in mood, such as crankiness; potential negative effects on fetuses during pregnancy or on babies during breastfeeding; or unintentional poisoning of children when mistaking THC products for CBD products or due to containing other ingredients such as THC or pesticides.
“I don’t think that we can have the perfect be the enemy of the good when we’re looking at such a vast market that is so available and utilized,” Norman Birenbaum, a senior FDA adviser who is working on the regulatory issue, told the Journal. “You’ve got a widely unregulated market.”
A version of this article first appeared on WebMD.com.
Problematic alcohol use on the rise among physicians?
However, good data on exactly how common this is and on salient risk factors are lacking.
In a systematic literature review, investigators found the prevalence of self-reported problematic alcohol use varied widely, but could affect up to one third of physicians.
However, all studies were survey-based and self-reported, and definitions of problematic alcohol use were mixed, with inconsistent reporting on differences across sex, age, physician specialty, and career stage.
“Key epidemiologic information of the prevalence of problematic alcohol use in physicians and associated risk factors are unknown, hampering the ability to identify high-risk individuals for targeted interventions,” Manish Sood, MD, University of Ottawa, and colleagues wrote.
The findings were published online in JAMA Network Open.
Serious concern
The researchers noted that physicians are at a higher risk for burnout and mental health conditions, including depression and anxiety, than the general population, which could contribute to problematic drinking.
Problematic drinking among physicians poses a “serious concern” to their health and ability to provide care, the investigators wrote. Understanding the extent and characteristics of the issue is important to guide interventions.
To better characterize problematic drinking among physicians, the investigators reviewed 31 studies from 2006 to 2020 involving 51,680 residents, fellows, or staff physicians in 17 countries.
In the studies, problematic alcohol use was measured by a validated tool: the Alcohol Use Disorders Identification Test, AUDIT Version C (AUDIT-C), or the Cut down, Annoyed, Guilty, and Eye-opener (CAGE) questionnaire.
“Problematic alcohol use” included hazardous, potentially hazardous, risky, at-risk, harmful, problematic, or heavy drinking or alcohol use, as well as alcohol misuse, alcohol dependence, and alcohol use more than low-risk guidelines and alcohol use disorder.
Results showed problematic alcohol use “varied widely” regardless of measurement method used. The rate was 0%-34% with AUDIT, 9%-35% with AUDIT-C, and 4%-22% with CAGE.
The data also showed an increase in reported problematic alcohol use over time, rising from 16.3% between 2006 and 2010 to 26.8% between 2017 and 2020.
True prevalence unknown
“It remains unknown whether this increase is indeed accurate or whether it is due to increased transparency by physicians in self-reporting problematic alcohol use because of a changing culture of medicine,” the investigators wrote.
The data suggest that problematic alcohol use is more common in male than female physicians; but no firm conclusions can be drawn from the data on how problematic alcohol use varies based on physician age, sex, specialty, and career stage, the researchers noted.
True prevalence of problematic alcohol use among physicians remains unknown – and identifying this type of behavior is difficult, they pointed out.
They added that physicians with problematic use may be “high functioning,” making identifying potential impairment a challenge. Also, societal stigma and fear of reprisal from professional colleges for reporting or seeking care for problematic alcohol use may encourage physicians with alcohol problems to keep their problems hidden.
The researchers noted that future population-based studies with longitudinal designs or using health administrative data could help identify the prevalence of and salient risk factors for problematic alcohol use in physicians.
The study was supported by the Canadian Medical Association. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
However, good data on exactly how common this is and on salient risk factors are lacking.
In a systematic literature review, investigators found the prevalence of self-reported problematic alcohol use varied widely, but could affect up to one third of physicians.
However, all studies were survey-based and self-reported, and definitions of problematic alcohol use were mixed, with inconsistent reporting on differences across sex, age, physician specialty, and career stage.
“Key epidemiologic information of the prevalence of problematic alcohol use in physicians and associated risk factors are unknown, hampering the ability to identify high-risk individuals for targeted interventions,” Manish Sood, MD, University of Ottawa, and colleagues wrote.
The findings were published online in JAMA Network Open.
Serious concern
The researchers noted that physicians are at a higher risk for burnout and mental health conditions, including depression and anxiety, than the general population, which could contribute to problematic drinking.
Problematic drinking among physicians poses a “serious concern” to their health and ability to provide care, the investigators wrote. Understanding the extent and characteristics of the issue is important to guide interventions.
To better characterize problematic drinking among physicians, the investigators reviewed 31 studies from 2006 to 2020 involving 51,680 residents, fellows, or staff physicians in 17 countries.
In the studies, problematic alcohol use was measured by a validated tool: the Alcohol Use Disorders Identification Test, AUDIT Version C (AUDIT-C), or the Cut down, Annoyed, Guilty, and Eye-opener (CAGE) questionnaire.
“Problematic alcohol use” included hazardous, potentially hazardous, risky, at-risk, harmful, problematic, or heavy drinking or alcohol use, as well as alcohol misuse, alcohol dependence, and alcohol use more than low-risk guidelines and alcohol use disorder.
Results showed problematic alcohol use “varied widely” regardless of measurement method used. The rate was 0%-34% with AUDIT, 9%-35% with AUDIT-C, and 4%-22% with CAGE.
The data also showed an increase in reported problematic alcohol use over time, rising from 16.3% between 2006 and 2010 to 26.8% between 2017 and 2020.
True prevalence unknown
“It remains unknown whether this increase is indeed accurate or whether it is due to increased transparency by physicians in self-reporting problematic alcohol use because of a changing culture of medicine,” the investigators wrote.
The data suggest that problematic alcohol use is more common in male than female physicians; but no firm conclusions can be drawn from the data on how problematic alcohol use varies based on physician age, sex, specialty, and career stage, the researchers noted.
True prevalence of problematic alcohol use among physicians remains unknown – and identifying this type of behavior is difficult, they pointed out.
They added that physicians with problematic use may be “high functioning,” making identifying potential impairment a challenge. Also, societal stigma and fear of reprisal from professional colleges for reporting or seeking care for problematic alcohol use may encourage physicians with alcohol problems to keep their problems hidden.
The researchers noted that future population-based studies with longitudinal designs or using health administrative data could help identify the prevalence of and salient risk factors for problematic alcohol use in physicians.
The study was supported by the Canadian Medical Association. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
However, good data on exactly how common this is and on salient risk factors are lacking.
In a systematic literature review, investigators found the prevalence of self-reported problematic alcohol use varied widely, but could affect up to one third of physicians.
However, all studies were survey-based and self-reported, and definitions of problematic alcohol use were mixed, with inconsistent reporting on differences across sex, age, physician specialty, and career stage.
“Key epidemiologic information of the prevalence of problematic alcohol use in physicians and associated risk factors are unknown, hampering the ability to identify high-risk individuals for targeted interventions,” Manish Sood, MD, University of Ottawa, and colleagues wrote.
The findings were published online in JAMA Network Open.
Serious concern
The researchers noted that physicians are at a higher risk for burnout and mental health conditions, including depression and anxiety, than the general population, which could contribute to problematic drinking.
Problematic drinking among physicians poses a “serious concern” to their health and ability to provide care, the investigators wrote. Understanding the extent and characteristics of the issue is important to guide interventions.
To better characterize problematic drinking among physicians, the investigators reviewed 31 studies from 2006 to 2020 involving 51,680 residents, fellows, or staff physicians in 17 countries.
In the studies, problematic alcohol use was measured by a validated tool: the Alcohol Use Disorders Identification Test, AUDIT Version C (AUDIT-C), or the Cut down, Annoyed, Guilty, and Eye-opener (CAGE) questionnaire.
“Problematic alcohol use” included hazardous, potentially hazardous, risky, at-risk, harmful, problematic, or heavy drinking or alcohol use, as well as alcohol misuse, alcohol dependence, and alcohol use more than low-risk guidelines and alcohol use disorder.
Results showed problematic alcohol use “varied widely” regardless of measurement method used. The rate was 0%-34% with AUDIT, 9%-35% with AUDIT-C, and 4%-22% with CAGE.
The data also showed an increase in reported problematic alcohol use over time, rising from 16.3% between 2006 and 2010 to 26.8% between 2017 and 2020.
True prevalence unknown
“It remains unknown whether this increase is indeed accurate or whether it is due to increased transparency by physicians in self-reporting problematic alcohol use because of a changing culture of medicine,” the investigators wrote.
The data suggest that problematic alcohol use is more common in male than female physicians; but no firm conclusions can be drawn from the data on how problematic alcohol use varies based on physician age, sex, specialty, and career stage, the researchers noted.
True prevalence of problematic alcohol use among physicians remains unknown – and identifying this type of behavior is difficult, they pointed out.
They added that physicians with problematic use may be “high functioning,” making identifying potential impairment a challenge. Also, societal stigma and fear of reprisal from professional colleges for reporting or seeking care for problematic alcohol use may encourage physicians with alcohol problems to keep their problems hidden.
The researchers noted that future population-based studies with longitudinal designs or using health administrative data could help identify the prevalence of and salient risk factors for problematic alcohol use in physicians.
The study was supported by the Canadian Medical Association. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Behavioral treatment tied to lower medical, pharmacy costs
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Let people take illegal drugs under medical supervision?
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m the director of the division of medical ethics at New York University.
One is up in Washington Heights in Manhattan; the other, I believe, is over in Harlem.
These two centers will supervise people taking drugs. They have available all of the anti-overdose medications, such as Narcan. If you overdose, they will help you and try to counsel you to get off drugs, but they don’t insist that you do so. You can go there, even if you’re an addict, and continue to take drugs under supervision. This is called a risk-reduction strategy.
Some people note that there are over 100 centers like this worldwide. They’re in Canada, Switzerland, and many other countries, and they seem to work. “Working” means more people seem to come off drugs slowly – not huge numbers, but some – than if you don’t do something like this, and death rates from overdose go way down.
By the way, having these centers in place has other benefits. They save money because when someone overdoses out in the community, you have to pay all the costs of the ambulances and emergency rooms, and there are risks to the first responders due to fentanyl or other things. There are fewer syringes littering parks and public places where people shoot up. You have everything controlled when they come into a center, so that’s less burden on the community.
It turns out that you have less crime because people just aren’t out there harming or robbing other people to get money to get their next fix. The drugs are provided for them. Crime rates in neighborhoods around the world where these centers operate seem to dip. There are many positives.
There are also some negatives. People say it shouldn’t be the job of the state to keep people addicted. It’s just not the right role. Everything should be aimed at getting people off drugs, maybe including criminal penalties if that’s what it takes to get them to stop using.
My own view is that hasn’t worked. Implementing tough prison sentences in trying to fight the war on drugs just doesn’t seem to work. We had 100,000 deaths last year from drug overdoses. That number has been climbing. We all know that we’ve got a terrible epidemic of deaths due to drug overdose.
It seems to me that these centers that are involved in risk reduction are a better option for now, until we figure out some interventions that can cut the desire or the drive to use drugs, or antidotes that are effective for months or years, to prevent people from getting high no matter what drugs they take.
I’m going to come out and say that I think the New York experiment has worked. I think it has saved upward of 600 lives, they estimate, in the past year that would have been overdoses. I think costwise, it’s effective. [Reductions in] related damages and injuries from syringes being scattered around, and robbery, and so forth, are all to the good. There are even a few people coming off drugs due to counseling, which is a better outcome than we get when they’re just out in the streets.
I think other cities want to try this. I know Philadelphia does. I know New York wants to expand its program. The federal government isn’t sure, but I think the time has come to try an expansion. I think we’ve got something that – although far from perfect and I wish we had other tools – may be the best we’ve got. In the war on drugs, little victories ought to be reinforced.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m the director of the division of medical ethics at New York University.
One is up in Washington Heights in Manhattan; the other, I believe, is over in Harlem.
These two centers will supervise people taking drugs. They have available all of the anti-overdose medications, such as Narcan. If you overdose, they will help you and try to counsel you to get off drugs, but they don’t insist that you do so. You can go there, even if you’re an addict, and continue to take drugs under supervision. This is called a risk-reduction strategy.
Some people note that there are over 100 centers like this worldwide. They’re in Canada, Switzerland, and many other countries, and they seem to work. “Working” means more people seem to come off drugs slowly – not huge numbers, but some – than if you don’t do something like this, and death rates from overdose go way down.
By the way, having these centers in place has other benefits. They save money because when someone overdoses out in the community, you have to pay all the costs of the ambulances and emergency rooms, and there are risks to the first responders due to fentanyl or other things. There are fewer syringes littering parks and public places where people shoot up. You have everything controlled when they come into a center, so that’s less burden on the community.
It turns out that you have less crime because people just aren’t out there harming or robbing other people to get money to get their next fix. The drugs are provided for them. Crime rates in neighborhoods around the world where these centers operate seem to dip. There are many positives.
There are also some negatives. People say it shouldn’t be the job of the state to keep people addicted. It’s just not the right role. Everything should be aimed at getting people off drugs, maybe including criminal penalties if that’s what it takes to get them to stop using.
My own view is that hasn’t worked. Implementing tough prison sentences in trying to fight the war on drugs just doesn’t seem to work. We had 100,000 deaths last year from drug overdoses. That number has been climbing. We all know that we’ve got a terrible epidemic of deaths due to drug overdose.
It seems to me that these centers that are involved in risk reduction are a better option for now, until we figure out some interventions that can cut the desire or the drive to use drugs, or antidotes that are effective for months or years, to prevent people from getting high no matter what drugs they take.
I’m going to come out and say that I think the New York experiment has worked. I think it has saved upward of 600 lives, they estimate, in the past year that would have been overdoses. I think costwise, it’s effective. [Reductions in] related damages and injuries from syringes being scattered around, and robbery, and so forth, are all to the good. There are even a few people coming off drugs due to counseling, which is a better outcome than we get when they’re just out in the streets.
I think other cities want to try this. I know Philadelphia does. I know New York wants to expand its program. The federal government isn’t sure, but I think the time has come to try an expansion. I think we’ve got something that – although far from perfect and I wish we had other tools – may be the best we’ve got. In the war on drugs, little victories ought to be reinforced.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m the director of the division of medical ethics at New York University.
One is up in Washington Heights in Manhattan; the other, I believe, is over in Harlem.
These two centers will supervise people taking drugs. They have available all of the anti-overdose medications, such as Narcan. If you overdose, they will help you and try to counsel you to get off drugs, but they don’t insist that you do so. You can go there, even if you’re an addict, and continue to take drugs under supervision. This is called a risk-reduction strategy.
Some people note that there are over 100 centers like this worldwide. They’re in Canada, Switzerland, and many other countries, and they seem to work. “Working” means more people seem to come off drugs slowly – not huge numbers, but some – than if you don’t do something like this, and death rates from overdose go way down.
By the way, having these centers in place has other benefits. They save money because when someone overdoses out in the community, you have to pay all the costs of the ambulances and emergency rooms, and there are risks to the first responders due to fentanyl or other things. There are fewer syringes littering parks and public places where people shoot up. You have everything controlled when they come into a center, so that’s less burden on the community.
It turns out that you have less crime because people just aren’t out there harming or robbing other people to get money to get their next fix. The drugs are provided for them. Crime rates in neighborhoods around the world where these centers operate seem to dip. There are many positives.
There are also some negatives. People say it shouldn’t be the job of the state to keep people addicted. It’s just not the right role. Everything should be aimed at getting people off drugs, maybe including criminal penalties if that’s what it takes to get them to stop using.
My own view is that hasn’t worked. Implementing tough prison sentences in trying to fight the war on drugs just doesn’t seem to work. We had 100,000 deaths last year from drug overdoses. That number has been climbing. We all know that we’ve got a terrible epidemic of deaths due to drug overdose.
It seems to me that these centers that are involved in risk reduction are a better option for now, until we figure out some interventions that can cut the desire or the drive to use drugs, or antidotes that are effective for months or years, to prevent people from getting high no matter what drugs they take.
I’m going to come out and say that I think the New York experiment has worked. I think it has saved upward of 600 lives, they estimate, in the past year that would have been overdoses. I think costwise, it’s effective. [Reductions in] related damages and injuries from syringes being scattered around, and robbery, and so forth, are all to the good. There are even a few people coming off drugs due to counseling, which is a better outcome than we get when they’re just out in the streets.
I think other cities want to try this. I know Philadelphia does. I know New York wants to expand its program. The federal government isn’t sure, but I think the time has come to try an expansion. I think we’ve got something that – although far from perfect and I wish we had other tools – may be the best we’ve got. In the war on drugs, little victories ought to be reinforced.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
Poison centers fielding more calls about teen cannabis use
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
‘Meth’ heart failure on the rise, often more severe
a literature review indicates.
MethHF is associated with increased severity for HF, longer inpatient stay, and more readmissions, compared with non-MethHF, the data show.
Clinicians “need to consider methamphetamine as a potential etiology for heart failure and include a substance use history when evaluating patients. Treating methamphetamine use disorder improves heart failure outcomes,” first author Veena Manja, MD, PhD, with Stanford (Calif.) University, said in an interview.
The study was published online in the journal Heart.
Poor outcomes, ‘staggering’ costs
This “thoughtful” review is “important and necessary,” Jonathan Davis, MD, director of the heart failure program, Zuckerberg San Francisco General Hospital, wrote in an editorial in the journal.
Dr. Davis noted that patients with Meth HF are at increased risk for poor outcomes and death and the health care costs related to MethHF are “staggering.”
As an example, inpatient data for California show annual charges related to MethHF rose by 840% from 2008 to 2018, from $41.5 million to $390.2 million, compared with 82% for all HF, which rose from $3.5 billion to $6.8 billion.
Illicit use of methamphetamine – also known as “crystal meth,” “ice,” and “speed” – has been linked to hypertension, MI, stroke, aortic dissection, and sudden death. But until now, there was no comprehensive systematic review of published studies on MethHF.
“Our goal was to compile current knowledge on the topic, increase awareness of this condition and identify areas for future research,” Dr. Manja said.
The researchers reviewed 21 observational studies, mostly from the United States (14 from California), between 1997 and 2020. The mean age of adults with MethHF ranged in age from 35 to 60 and more than half were male (57%).
Illicit methamphetamine was inhaled, injected, swallowed, smoked, and snorted. The reported frequency ranged from daily to every other week, and the total monthly dose ranged from 0.35 g to 24.5 g.
The average duration of meth use before HF diagnosis was 5 years. However, 18% of users developed HF within 1 year of starting to use illicit methamphetamine. In some cases, HF was diagnosed after a single use.
The researchers also note that MethHF with preserved left ventricular ejection fraction, seen in up to 44% of cases, is a distinct entity that may progress to reduced LVEF with continued use.
MethHF is also associated with a greater likelihood of other substance abuse, PTSD, depression, and other heart and kidney disease.
Factors associated with improved MethHF outcomes include female sex, meth abstinence, and adherence to guideline-directed HF therapy.
Improvement in MethHF outcomes is possible even if abstinence is not consistent, a finding that lends support to harm reduction principles of “meeting patients where they are instead of insisting on complete abstinence,” the researchers said.
Large gaps in knowledge
They were unable to combine the results into a meta-analysis because of heterogeneity in study design, population, comparator, and outcome assessment. Also, the overall risk of bias is moderate because of the presence of confounders, selection bias and poor matching, and the overall certainty in the evidence is very low,.
No study evaluated the incidence or prevalence of HF among methamphetamine users and inconsistent history taking and testing in patients with HF impeded accurate MethHF prevalence assessment.
Several studies, however, document an increasing incidence of MethHF, particularly over the past decade.
One study from California reported a 585% increase in MethHF hospital admissions between 2008 and 2018. An analysis of the National Inpatient Survey found a 12-fold increase in annual MethHF hospitalizations between 2002 and 2014.
“The results of this systematic review highlight large gaps in our knowledge” of MethHF, Dr. Manja said in an interview.
“We need to understand the epidemiology, prevalence, factors that confer susceptibility to cardiovascular outcomes, and need research into treatment targeted toward this disease,” Dr. Manja added. “We should consider options to integrate substance use treatment in HF/cardiology/primary care clinics and design a multidisciplinary patient-centered approach.”
Dr. Davis agreed. This work “highlights that the standard of care academically and clinically must be a broad team across the care spectrum to simultaneously address methamphetamine use, heart failure, and social determinants of health.”
This research had no specific funding. Dr. Manja and Dr. Davis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
a literature review indicates.
MethHF is associated with increased severity for HF, longer inpatient stay, and more readmissions, compared with non-MethHF, the data show.
Clinicians “need to consider methamphetamine as a potential etiology for heart failure and include a substance use history when evaluating patients. Treating methamphetamine use disorder improves heart failure outcomes,” first author Veena Manja, MD, PhD, with Stanford (Calif.) University, said in an interview.
The study was published online in the journal Heart.
Poor outcomes, ‘staggering’ costs
This “thoughtful” review is “important and necessary,” Jonathan Davis, MD, director of the heart failure program, Zuckerberg San Francisco General Hospital, wrote in an editorial in the journal.
Dr. Davis noted that patients with Meth HF are at increased risk for poor outcomes and death and the health care costs related to MethHF are “staggering.”
As an example, inpatient data for California show annual charges related to MethHF rose by 840% from 2008 to 2018, from $41.5 million to $390.2 million, compared with 82% for all HF, which rose from $3.5 billion to $6.8 billion.
Illicit use of methamphetamine – also known as “crystal meth,” “ice,” and “speed” – has been linked to hypertension, MI, stroke, aortic dissection, and sudden death. But until now, there was no comprehensive systematic review of published studies on MethHF.
“Our goal was to compile current knowledge on the topic, increase awareness of this condition and identify areas for future research,” Dr. Manja said.
The researchers reviewed 21 observational studies, mostly from the United States (14 from California), between 1997 and 2020. The mean age of adults with MethHF ranged in age from 35 to 60 and more than half were male (57%).
Illicit methamphetamine was inhaled, injected, swallowed, smoked, and snorted. The reported frequency ranged from daily to every other week, and the total monthly dose ranged from 0.35 g to 24.5 g.
The average duration of meth use before HF diagnosis was 5 years. However, 18% of users developed HF within 1 year of starting to use illicit methamphetamine. In some cases, HF was diagnosed after a single use.
The researchers also note that MethHF with preserved left ventricular ejection fraction, seen in up to 44% of cases, is a distinct entity that may progress to reduced LVEF with continued use.
MethHF is also associated with a greater likelihood of other substance abuse, PTSD, depression, and other heart and kidney disease.
Factors associated with improved MethHF outcomes include female sex, meth abstinence, and adherence to guideline-directed HF therapy.
Improvement in MethHF outcomes is possible even if abstinence is not consistent, a finding that lends support to harm reduction principles of “meeting patients where they are instead of insisting on complete abstinence,” the researchers said.
Large gaps in knowledge
They were unable to combine the results into a meta-analysis because of heterogeneity in study design, population, comparator, and outcome assessment. Also, the overall risk of bias is moderate because of the presence of confounders, selection bias and poor matching, and the overall certainty in the evidence is very low,.
No study evaluated the incidence or prevalence of HF among methamphetamine users and inconsistent history taking and testing in patients with HF impeded accurate MethHF prevalence assessment.
Several studies, however, document an increasing incidence of MethHF, particularly over the past decade.
One study from California reported a 585% increase in MethHF hospital admissions between 2008 and 2018. An analysis of the National Inpatient Survey found a 12-fold increase in annual MethHF hospitalizations between 2002 and 2014.
“The results of this systematic review highlight large gaps in our knowledge” of MethHF, Dr. Manja said in an interview.
“We need to understand the epidemiology, prevalence, factors that confer susceptibility to cardiovascular outcomes, and need research into treatment targeted toward this disease,” Dr. Manja added. “We should consider options to integrate substance use treatment in HF/cardiology/primary care clinics and design a multidisciplinary patient-centered approach.”
Dr. Davis agreed. This work “highlights that the standard of care academically and clinically must be a broad team across the care spectrum to simultaneously address methamphetamine use, heart failure, and social determinants of health.”
This research had no specific funding. Dr. Manja and Dr. Davis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
a literature review indicates.
MethHF is associated with increased severity for HF, longer inpatient stay, and more readmissions, compared with non-MethHF, the data show.
Clinicians “need to consider methamphetamine as a potential etiology for heart failure and include a substance use history when evaluating patients. Treating methamphetamine use disorder improves heart failure outcomes,” first author Veena Manja, MD, PhD, with Stanford (Calif.) University, said in an interview.
The study was published online in the journal Heart.
Poor outcomes, ‘staggering’ costs
This “thoughtful” review is “important and necessary,” Jonathan Davis, MD, director of the heart failure program, Zuckerberg San Francisco General Hospital, wrote in an editorial in the journal.
Dr. Davis noted that patients with Meth HF are at increased risk for poor outcomes and death and the health care costs related to MethHF are “staggering.”
As an example, inpatient data for California show annual charges related to MethHF rose by 840% from 2008 to 2018, from $41.5 million to $390.2 million, compared with 82% for all HF, which rose from $3.5 billion to $6.8 billion.
Illicit use of methamphetamine – also known as “crystal meth,” “ice,” and “speed” – has been linked to hypertension, MI, stroke, aortic dissection, and sudden death. But until now, there was no comprehensive systematic review of published studies on MethHF.
“Our goal was to compile current knowledge on the topic, increase awareness of this condition and identify areas for future research,” Dr. Manja said.
The researchers reviewed 21 observational studies, mostly from the United States (14 from California), between 1997 and 2020. The mean age of adults with MethHF ranged in age from 35 to 60 and more than half were male (57%).
Illicit methamphetamine was inhaled, injected, swallowed, smoked, and snorted. The reported frequency ranged from daily to every other week, and the total monthly dose ranged from 0.35 g to 24.5 g.
The average duration of meth use before HF diagnosis was 5 years. However, 18% of users developed HF within 1 year of starting to use illicit methamphetamine. In some cases, HF was diagnosed after a single use.
The researchers also note that MethHF with preserved left ventricular ejection fraction, seen in up to 44% of cases, is a distinct entity that may progress to reduced LVEF with continued use.
MethHF is also associated with a greater likelihood of other substance abuse, PTSD, depression, and other heart and kidney disease.
Factors associated with improved MethHF outcomes include female sex, meth abstinence, and adherence to guideline-directed HF therapy.
Improvement in MethHF outcomes is possible even if abstinence is not consistent, a finding that lends support to harm reduction principles of “meeting patients where they are instead of insisting on complete abstinence,” the researchers said.
Large gaps in knowledge
They were unable to combine the results into a meta-analysis because of heterogeneity in study design, population, comparator, and outcome assessment. Also, the overall risk of bias is moderate because of the presence of confounders, selection bias and poor matching, and the overall certainty in the evidence is very low,.
No study evaluated the incidence or prevalence of HF among methamphetamine users and inconsistent history taking and testing in patients with HF impeded accurate MethHF prevalence assessment.
Several studies, however, document an increasing incidence of MethHF, particularly over the past decade.
One study from California reported a 585% increase in MethHF hospital admissions between 2008 and 2018. An analysis of the National Inpatient Survey found a 12-fold increase in annual MethHF hospitalizations between 2002 and 2014.
“The results of this systematic review highlight large gaps in our knowledge” of MethHF, Dr. Manja said in an interview.
“We need to understand the epidemiology, prevalence, factors that confer susceptibility to cardiovascular outcomes, and need research into treatment targeted toward this disease,” Dr. Manja added. “We should consider options to integrate substance use treatment in HF/cardiology/primary care clinics and design a multidisciplinary patient-centered approach.”
Dr. Davis agreed. This work “highlights that the standard of care academically and clinically must be a broad team across the care spectrum to simultaneously address methamphetamine use, heart failure, and social determinants of health.”
This research had no specific funding. Dr. Manja and Dr. Davis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM HEART
Overdose deaths up 81% in pregnant, postpartum women
Drug overdose deaths in pregnant and postpartum women rose by about 81% from 2017 to 2020, researchers report in a JAMA research letter published online Dec. 6.
Pregnancy-associated overdose deaths were highest in 2020 as the COVID pandemic began, according to the researchers, Emilie Bruzelius, MPH, and Silvia S. Martins, MD, PHD, with the department of epidemiology, Columbia University School of Public Health in New York.
The deaths were identified using International Statistical Classification of Diseases and Related Health Problems (ICD-10) pregnancy-related codes and death certificate pregnancy checkbox status.
The checkbox, part of all states’ death certificates, asks whether the person was pregnant at the time of death, within 42 days of death (early postpartum) or within 43-365 days of death (late postpartum).
Sharp increase at start of COVID pandemic
The authors note that pregnancy-related overdose deaths have been climbing from 2007 to 2019, but increased sharply in 2020.
“Pregnant and postpartum persons are known to face barriers to accessing drug treatment and harm-reduction services, which when compounded by pandemic-associated stressors, health care shutdowns, and increasingly volatile unregulated drug supply may have increased fatal overdose risk,” the authors write.
Of the 7,642 pregnancy-related deaths in the study period, 1,249 were overdose-related, leading to a cumulative overdose death rate of 8.35 per 100,000. From 2017 to 2020, pregnancy-related overdose deaths rose from 6.56 to 11.85 per 100,000. That translates to an absolute change rate of 5.30 per 100,000 and a relative increase of 81%.
The trend mirrors a pattern in people of reproductive age overall, the authors write.
Overdose mortality among reproductive age women similarly increased from 14.37 to 19.76 per 100,000 (absolute change rate, 5.39 [95% confidence interval, 4.94-5.85] per 100,000; relative increase of 38%).
Fentanyl deaths increase
The researchers found large increases in deaths involving fentanyl and other synthetics and psychostimulants (methamphetamine and cocaine, for example).
Pregnancy-associated overdose deaths involving benzodiazepines, heroin, and prescription opioids, however, were mostly stable from 2017 to 2020.
Numbers of late postpartum overdose deaths were notable in the paper.
In that group, there were 3.95 deaths per 100,000, compared with those pregnant at the time of death (2.99 per 100,000 or those identified as early postpartum (1.39 per 100 000).
Davida Schiff, MD, director of the Perinatal and Family-based Substance Use Disorder Care Massachusetts General Hospital substance use disorders initiative in Boston, told this publication it’s important to realize from this study that late postpartum period is the highest-risk period and also the time “when many states that have not expanded Medicaid cut off insurance needed to access life-saving health care services.”“Pregnancy is an important touch point of increased health care access, yet pregnant and parenting people face unique social and legal consequences from their substance use,” Dr. Schiff said.
She added, “I’m left wondering how many of the deaths reported could have been avoided if fear of a punitive response when engaging with our health care system had not prevented them from seeking out the care they needed.”
Dr. Schiff said the study highlights the importance of the pregnancy-checkbox addition to death records to better characterize pregnancy-associated deaths that previously were likely undercounted.
The authors and Dr. Schiff declare no relevant financial relationships.
Drug overdose deaths in pregnant and postpartum women rose by about 81% from 2017 to 2020, researchers report in a JAMA research letter published online Dec. 6.
Pregnancy-associated overdose deaths were highest in 2020 as the COVID pandemic began, according to the researchers, Emilie Bruzelius, MPH, and Silvia S. Martins, MD, PHD, with the department of epidemiology, Columbia University School of Public Health in New York.
The deaths were identified using International Statistical Classification of Diseases and Related Health Problems (ICD-10) pregnancy-related codes and death certificate pregnancy checkbox status.
The checkbox, part of all states’ death certificates, asks whether the person was pregnant at the time of death, within 42 days of death (early postpartum) or within 43-365 days of death (late postpartum).
Sharp increase at start of COVID pandemic
The authors note that pregnancy-related overdose deaths have been climbing from 2007 to 2019, but increased sharply in 2020.
“Pregnant and postpartum persons are known to face barriers to accessing drug treatment and harm-reduction services, which when compounded by pandemic-associated stressors, health care shutdowns, and increasingly volatile unregulated drug supply may have increased fatal overdose risk,” the authors write.
Of the 7,642 pregnancy-related deaths in the study period, 1,249 were overdose-related, leading to a cumulative overdose death rate of 8.35 per 100,000. From 2017 to 2020, pregnancy-related overdose deaths rose from 6.56 to 11.85 per 100,000. That translates to an absolute change rate of 5.30 per 100,000 and a relative increase of 81%.
The trend mirrors a pattern in people of reproductive age overall, the authors write.
Overdose mortality among reproductive age women similarly increased from 14.37 to 19.76 per 100,000 (absolute change rate, 5.39 [95% confidence interval, 4.94-5.85] per 100,000; relative increase of 38%).
Fentanyl deaths increase
The researchers found large increases in deaths involving fentanyl and other synthetics and psychostimulants (methamphetamine and cocaine, for example).
Pregnancy-associated overdose deaths involving benzodiazepines, heroin, and prescription opioids, however, were mostly stable from 2017 to 2020.
Numbers of late postpartum overdose deaths were notable in the paper.
In that group, there were 3.95 deaths per 100,000, compared with those pregnant at the time of death (2.99 per 100,000 or those identified as early postpartum (1.39 per 100 000).
Davida Schiff, MD, director of the Perinatal and Family-based Substance Use Disorder Care Massachusetts General Hospital substance use disorders initiative in Boston, told this publication it’s important to realize from this study that late postpartum period is the highest-risk period and also the time “when many states that have not expanded Medicaid cut off insurance needed to access life-saving health care services.”“Pregnancy is an important touch point of increased health care access, yet pregnant and parenting people face unique social and legal consequences from their substance use,” Dr. Schiff said.
She added, “I’m left wondering how many of the deaths reported could have been avoided if fear of a punitive response when engaging with our health care system had not prevented them from seeking out the care they needed.”
Dr. Schiff said the study highlights the importance of the pregnancy-checkbox addition to death records to better characterize pregnancy-associated deaths that previously were likely undercounted.
The authors and Dr. Schiff declare no relevant financial relationships.
Drug overdose deaths in pregnant and postpartum women rose by about 81% from 2017 to 2020, researchers report in a JAMA research letter published online Dec. 6.
Pregnancy-associated overdose deaths were highest in 2020 as the COVID pandemic began, according to the researchers, Emilie Bruzelius, MPH, and Silvia S. Martins, MD, PHD, with the department of epidemiology, Columbia University School of Public Health in New York.
The deaths were identified using International Statistical Classification of Diseases and Related Health Problems (ICD-10) pregnancy-related codes and death certificate pregnancy checkbox status.
The checkbox, part of all states’ death certificates, asks whether the person was pregnant at the time of death, within 42 days of death (early postpartum) or within 43-365 days of death (late postpartum).
Sharp increase at start of COVID pandemic
The authors note that pregnancy-related overdose deaths have been climbing from 2007 to 2019, but increased sharply in 2020.
“Pregnant and postpartum persons are known to face barriers to accessing drug treatment and harm-reduction services, which when compounded by pandemic-associated stressors, health care shutdowns, and increasingly volatile unregulated drug supply may have increased fatal overdose risk,” the authors write.
Of the 7,642 pregnancy-related deaths in the study period, 1,249 were overdose-related, leading to a cumulative overdose death rate of 8.35 per 100,000. From 2017 to 2020, pregnancy-related overdose deaths rose from 6.56 to 11.85 per 100,000. That translates to an absolute change rate of 5.30 per 100,000 and a relative increase of 81%.
The trend mirrors a pattern in people of reproductive age overall, the authors write.
Overdose mortality among reproductive age women similarly increased from 14.37 to 19.76 per 100,000 (absolute change rate, 5.39 [95% confidence interval, 4.94-5.85] per 100,000; relative increase of 38%).
Fentanyl deaths increase
The researchers found large increases in deaths involving fentanyl and other synthetics and psychostimulants (methamphetamine and cocaine, for example).
Pregnancy-associated overdose deaths involving benzodiazepines, heroin, and prescription opioids, however, were mostly stable from 2017 to 2020.
Numbers of late postpartum overdose deaths were notable in the paper.
In that group, there were 3.95 deaths per 100,000, compared with those pregnant at the time of death (2.99 per 100,000 or those identified as early postpartum (1.39 per 100 000).
Davida Schiff, MD, director of the Perinatal and Family-based Substance Use Disorder Care Massachusetts General Hospital substance use disorders initiative in Boston, told this publication it’s important to realize from this study that late postpartum period is the highest-risk period and also the time “when many states that have not expanded Medicaid cut off insurance needed to access life-saving health care services.”“Pregnancy is an important touch point of increased health care access, yet pregnant and parenting people face unique social and legal consequences from their substance use,” Dr. Schiff said.
She added, “I’m left wondering how many of the deaths reported could have been avoided if fear of a punitive response when engaging with our health care system had not prevented them from seeking out the care they needed.”
Dr. Schiff said the study highlights the importance of the pregnancy-checkbox addition to death records to better characterize pregnancy-associated deaths that previously were likely undercounted.
The authors and Dr. Schiff declare no relevant financial relationships.
FROM JAMA
Higher potency of fentanyl affects addiction treatment, screening
As fentanyl-related overdose deaths continue to increase, clinicians should take note of important differences that set the drug apart from the other drugs of misuse – and the troubling reality that fentanyl now contaminates most of them.
“It would be fair to tell patients, if you’re buying any illicit drugs – pills, powder, liquid, whatever it is, you’ve got to assume it’s either contaminated with or replaced by fentanyl,” said Edwin Salsitz, MD, an associate clinical professor at the Icahn School of Medicine at Mount Sinai, New York, during a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In many if not most cases, he noted, patients become addicted to fentanyl unknowingly. They assume they are ingesting oxycodone, cocaine, or another drug, and have no realization that they are even exposed to fentanyl until they test positive for it – or overdose.
Meanwhile, the high potency of fentanyl can overcome the opioid blockade of addiction treatment therapies – methadone and buprenorphine – that take away the high that users get from less potent drugs such as heroin.
“Fentanyl is overcoming this blockade that methadone and buprenorphine used to provide,” Dr. Salsitz said. “With fentanyl having such a higher potency, patients are saying ‘no, I still feel the fentanyl effects,’ and they continue feeling it even with 200 milligrams of methadone or 24 milligrams of buprenorphine.”
‘Wooden chest syndrome’
Among the lesser-known dangers of fentanyl is the possibility that some overdose deaths may occur as the result of a syndrome previously reported as a rare complication following the medical use of fentanyl in critically ill patients – fentanyl-induced chest-wall rigidity, or “wooden chest syndrome,” Dr. Salsitz explained.
In such cases, the muscles of respiration become rigid and paralyzed, causing suffocation within a matter of minutes – too soon to benefit from the overdose rescue medication naloxone.
In one recent study published in Clinical Toxicology , nearly half of fentanyl overdose deaths were found to have occurred even before the body had a chance to produce norfentanyl, a metabolite of fentanyl that takes only about 2-3 minutes to appear in the system, suggesting the deaths occurred rapidly.
In the study of 48 fentanyl deaths, no appreciable concentrations of norfentanyl could be detected in 20 of the 48 overdose deaths (42%), and concentrations were less than 1 ng/mL in 25 cases (52%).
“The lack of any measurable norfentanyl in half of our cases suggests a very rapid death, consistent with acute chest rigidity,” the authors reported.
“In several cases fentanyl concentrations were strikingly high (22 ng/mL and 20 ng/mL) with no norfentanyl detected,” they said.
Dr. Salsitz noted that the syndrome is not well known among the addiction treatment community.
“This is different than the usual respiratory opioid overdose where there’s a gradual decrease in the breathing rate and a gradual decrease in how much air is going in and out of the lungs,” Dr. Salsitz explained.
“With those cases, some may survive for an hour or longer, allowing time for someone to administer naloxone or to get the patient to the emergency room,” he said. “But with this, breathing stops and people can die within minutes.
“I think that this is one of the reasons that fentanyl deaths keep going up despite more and more naloxone availability out there,” he said.
Clearance may take longer
In toxicology testing for fentanyl, clinicians should also note the important difference between fentanyl and other opioids – that fentanyl, because of its high lipophilicity, may be detected in urine toxicology testing up to 3 weeks after last use. This is much longer than the 2- to 4-day clearance observed with other opioids, possibly causing patients to continue to test positive for the drug weeks after cessation.
This effect was observed in one recent study of 12 opioid use disorder patients in a residential treatment program who had previously been exposed to daily fentanyl.
The study showed the mean amount of time of fentanyl clearance was 2 weeks, with a range of 4-26 days after last use.
The authors pointed out that the findings “might explain recent reports of difficulty in buprenorphine inductions for persons who use fentanyl, and point to a need to better understand the pharmacokinetics of fentanyl in the context of opioid withdrawal in persons who regularly use fentanyl.”
Though the study was small, Dr. Salsitz said “that’s not a stumbling block to the important finding that, with regular use of fentanyl, the drug may stay in the urine for a long time.”
Dr. Salsitz noted that similar observations have been made at his center, with clinicians logically assuming that patients were still somehow getting fentanyl.
“When we initially found this in patients, we thought that they were using on the unit, perhaps that they brought in the fentanyl, because otherwise how could it stay in the urine that long,” he noted. “But fentanyl appears to be more lipophilic and gets into the fat; it’s then excreted very slowly and then stays in the urine.”
Dr. Salsitz said most practitioners think of fentanyl as a short-acting drug, so “it’s important to realize that people may continue to test positive and it should be thought of as a long-acting opioid.”
Opiate screening tests don’t work
Dr. Salsitz warned of another misconception in fentanyl testing – the common mistake of assuming that fentanyl should show up in a test for opiates – when in fact fentanyl is not, technically, an opiate.
“The word opiate only refers to morphine, codeine, heroin and sometimes hydrocodone,” he explained. “Other opioids are classified as semisynthetic, such as oxycodone, or synthetics, such as fentanyl and methadone, buprenorphine.”
“In order to detect the synthetics, you must have a separate strip for each one of those drugs. They will not show up positive on a screen for opiates,” he noted.
The belief that fentanyl and other synthetic and semisynthetic opioids will show positive on an opiate screen is a common misconception, he said. “The misunderstanding in toxicology interpretation is a problem for many practitioners, [but] it’s essential to understand because otherwise false assumptions about the patient will be considered.”
Another important testing misreading can occur with the antidepressant drug trazodone, which Dr. Salsitz cautioned may falsely test as positive for fentanyl on immunoassays.
“Trazodone is very commonly used in addiction treatment centers, but it can give a false positive on the fentanyl immunoassay and we’ve had a number of those cases,” he said.
Dr. Salsitz had no disclosures to report.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
As fentanyl-related overdose deaths continue to increase, clinicians should take note of important differences that set the drug apart from the other drugs of misuse – and the troubling reality that fentanyl now contaminates most of them.
“It would be fair to tell patients, if you’re buying any illicit drugs – pills, powder, liquid, whatever it is, you’ve got to assume it’s either contaminated with or replaced by fentanyl,” said Edwin Salsitz, MD, an associate clinical professor at the Icahn School of Medicine at Mount Sinai, New York, during a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In many if not most cases, he noted, patients become addicted to fentanyl unknowingly. They assume they are ingesting oxycodone, cocaine, or another drug, and have no realization that they are even exposed to fentanyl until they test positive for it – or overdose.
Meanwhile, the high potency of fentanyl can overcome the opioid blockade of addiction treatment therapies – methadone and buprenorphine – that take away the high that users get from less potent drugs such as heroin.
“Fentanyl is overcoming this blockade that methadone and buprenorphine used to provide,” Dr. Salsitz said. “With fentanyl having such a higher potency, patients are saying ‘no, I still feel the fentanyl effects,’ and they continue feeling it even with 200 milligrams of methadone or 24 milligrams of buprenorphine.”
‘Wooden chest syndrome’
Among the lesser-known dangers of fentanyl is the possibility that some overdose deaths may occur as the result of a syndrome previously reported as a rare complication following the medical use of fentanyl in critically ill patients – fentanyl-induced chest-wall rigidity, or “wooden chest syndrome,” Dr. Salsitz explained.
In such cases, the muscles of respiration become rigid and paralyzed, causing suffocation within a matter of minutes – too soon to benefit from the overdose rescue medication naloxone.
In one recent study published in Clinical Toxicology , nearly half of fentanyl overdose deaths were found to have occurred even before the body had a chance to produce norfentanyl, a metabolite of fentanyl that takes only about 2-3 minutes to appear in the system, suggesting the deaths occurred rapidly.
In the study of 48 fentanyl deaths, no appreciable concentrations of norfentanyl could be detected in 20 of the 48 overdose deaths (42%), and concentrations were less than 1 ng/mL in 25 cases (52%).
“The lack of any measurable norfentanyl in half of our cases suggests a very rapid death, consistent with acute chest rigidity,” the authors reported.
“In several cases fentanyl concentrations were strikingly high (22 ng/mL and 20 ng/mL) with no norfentanyl detected,” they said.
Dr. Salsitz noted that the syndrome is not well known among the addiction treatment community.
“This is different than the usual respiratory opioid overdose where there’s a gradual decrease in the breathing rate and a gradual decrease in how much air is going in and out of the lungs,” Dr. Salsitz explained.
“With those cases, some may survive for an hour or longer, allowing time for someone to administer naloxone or to get the patient to the emergency room,” he said. “But with this, breathing stops and people can die within minutes.
“I think that this is one of the reasons that fentanyl deaths keep going up despite more and more naloxone availability out there,” he said.
Clearance may take longer
In toxicology testing for fentanyl, clinicians should also note the important difference between fentanyl and other opioids – that fentanyl, because of its high lipophilicity, may be detected in urine toxicology testing up to 3 weeks after last use. This is much longer than the 2- to 4-day clearance observed with other opioids, possibly causing patients to continue to test positive for the drug weeks after cessation.
This effect was observed in one recent study of 12 opioid use disorder patients in a residential treatment program who had previously been exposed to daily fentanyl.
The study showed the mean amount of time of fentanyl clearance was 2 weeks, with a range of 4-26 days after last use.
The authors pointed out that the findings “might explain recent reports of difficulty in buprenorphine inductions for persons who use fentanyl, and point to a need to better understand the pharmacokinetics of fentanyl in the context of opioid withdrawal in persons who regularly use fentanyl.”
Though the study was small, Dr. Salsitz said “that’s not a stumbling block to the important finding that, with regular use of fentanyl, the drug may stay in the urine for a long time.”
Dr. Salsitz noted that similar observations have been made at his center, with clinicians logically assuming that patients were still somehow getting fentanyl.
“When we initially found this in patients, we thought that they were using on the unit, perhaps that they brought in the fentanyl, because otherwise how could it stay in the urine that long,” he noted. “But fentanyl appears to be more lipophilic and gets into the fat; it’s then excreted very slowly and then stays in the urine.”
Dr. Salsitz said most practitioners think of fentanyl as a short-acting drug, so “it’s important to realize that people may continue to test positive and it should be thought of as a long-acting opioid.”
Opiate screening tests don’t work
Dr. Salsitz warned of another misconception in fentanyl testing – the common mistake of assuming that fentanyl should show up in a test for opiates – when in fact fentanyl is not, technically, an opiate.
“The word opiate only refers to morphine, codeine, heroin and sometimes hydrocodone,” he explained. “Other opioids are classified as semisynthetic, such as oxycodone, or synthetics, such as fentanyl and methadone, buprenorphine.”
“In order to detect the synthetics, you must have a separate strip for each one of those drugs. They will not show up positive on a screen for opiates,” he noted.
The belief that fentanyl and other synthetic and semisynthetic opioids will show positive on an opiate screen is a common misconception, he said. “The misunderstanding in toxicology interpretation is a problem for many practitioners, [but] it’s essential to understand because otherwise false assumptions about the patient will be considered.”
Another important testing misreading can occur with the antidepressant drug trazodone, which Dr. Salsitz cautioned may falsely test as positive for fentanyl on immunoassays.
“Trazodone is very commonly used in addiction treatment centers, but it can give a false positive on the fentanyl immunoassay and we’ve had a number of those cases,” he said.
Dr. Salsitz had no disclosures to report.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
As fentanyl-related overdose deaths continue to increase, clinicians should take note of important differences that set the drug apart from the other drugs of misuse – and the troubling reality that fentanyl now contaminates most of them.
“It would be fair to tell patients, if you’re buying any illicit drugs – pills, powder, liquid, whatever it is, you’ve got to assume it’s either contaminated with or replaced by fentanyl,” said Edwin Salsitz, MD, an associate clinical professor at the Icahn School of Medicine at Mount Sinai, New York, during a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In many if not most cases, he noted, patients become addicted to fentanyl unknowingly. They assume they are ingesting oxycodone, cocaine, or another drug, and have no realization that they are even exposed to fentanyl until they test positive for it – or overdose.
Meanwhile, the high potency of fentanyl can overcome the opioid blockade of addiction treatment therapies – methadone and buprenorphine – that take away the high that users get from less potent drugs such as heroin.
“Fentanyl is overcoming this blockade that methadone and buprenorphine used to provide,” Dr. Salsitz said. “With fentanyl having such a higher potency, patients are saying ‘no, I still feel the fentanyl effects,’ and they continue feeling it even with 200 milligrams of methadone or 24 milligrams of buprenorphine.”
‘Wooden chest syndrome’
Among the lesser-known dangers of fentanyl is the possibility that some overdose deaths may occur as the result of a syndrome previously reported as a rare complication following the medical use of fentanyl in critically ill patients – fentanyl-induced chest-wall rigidity, or “wooden chest syndrome,” Dr. Salsitz explained.
In such cases, the muscles of respiration become rigid and paralyzed, causing suffocation within a matter of minutes – too soon to benefit from the overdose rescue medication naloxone.
In one recent study published in Clinical Toxicology , nearly half of fentanyl overdose deaths were found to have occurred even before the body had a chance to produce norfentanyl, a metabolite of fentanyl that takes only about 2-3 minutes to appear in the system, suggesting the deaths occurred rapidly.
In the study of 48 fentanyl deaths, no appreciable concentrations of norfentanyl could be detected in 20 of the 48 overdose deaths (42%), and concentrations were less than 1 ng/mL in 25 cases (52%).
“The lack of any measurable norfentanyl in half of our cases suggests a very rapid death, consistent with acute chest rigidity,” the authors reported.
“In several cases fentanyl concentrations were strikingly high (22 ng/mL and 20 ng/mL) with no norfentanyl detected,” they said.
Dr. Salsitz noted that the syndrome is not well known among the addiction treatment community.
“This is different than the usual respiratory opioid overdose where there’s a gradual decrease in the breathing rate and a gradual decrease in how much air is going in and out of the lungs,” Dr. Salsitz explained.
“With those cases, some may survive for an hour or longer, allowing time for someone to administer naloxone or to get the patient to the emergency room,” he said. “But with this, breathing stops and people can die within minutes.
“I think that this is one of the reasons that fentanyl deaths keep going up despite more and more naloxone availability out there,” he said.
Clearance may take longer
In toxicology testing for fentanyl, clinicians should also note the important difference between fentanyl and other opioids – that fentanyl, because of its high lipophilicity, may be detected in urine toxicology testing up to 3 weeks after last use. This is much longer than the 2- to 4-day clearance observed with other opioids, possibly causing patients to continue to test positive for the drug weeks after cessation.
This effect was observed in one recent study of 12 opioid use disorder patients in a residential treatment program who had previously been exposed to daily fentanyl.
The study showed the mean amount of time of fentanyl clearance was 2 weeks, with a range of 4-26 days after last use.
The authors pointed out that the findings “might explain recent reports of difficulty in buprenorphine inductions for persons who use fentanyl, and point to a need to better understand the pharmacokinetics of fentanyl in the context of opioid withdrawal in persons who regularly use fentanyl.”
Though the study was small, Dr. Salsitz said “that’s not a stumbling block to the important finding that, with regular use of fentanyl, the drug may stay in the urine for a long time.”
Dr. Salsitz noted that similar observations have been made at his center, with clinicians logically assuming that patients were still somehow getting fentanyl.
“When we initially found this in patients, we thought that they were using on the unit, perhaps that they brought in the fentanyl, because otherwise how could it stay in the urine that long,” he noted. “But fentanyl appears to be more lipophilic and gets into the fat; it’s then excreted very slowly and then stays in the urine.”
Dr. Salsitz said most practitioners think of fentanyl as a short-acting drug, so “it’s important to realize that people may continue to test positive and it should be thought of as a long-acting opioid.”
Opiate screening tests don’t work
Dr. Salsitz warned of another misconception in fentanyl testing – the common mistake of assuming that fentanyl should show up in a test for opiates – when in fact fentanyl is not, technically, an opiate.
“The word opiate only refers to morphine, codeine, heroin and sometimes hydrocodone,” he explained. “Other opioids are classified as semisynthetic, such as oxycodone, or synthetics, such as fentanyl and methadone, buprenorphine.”
“In order to detect the synthetics, you must have a separate strip for each one of those drugs. They will not show up positive on a screen for opiates,” he noted.
The belief that fentanyl and other synthetic and semisynthetic opioids will show positive on an opiate screen is a common misconception, he said. “The misunderstanding in toxicology interpretation is a problem for many practitioners, [but] it’s essential to understand because otherwise false assumptions about the patient will be considered.”
Another important testing misreading can occur with the antidepressant drug trazodone, which Dr. Salsitz cautioned may falsely test as positive for fentanyl on immunoassays.
“Trazodone is very commonly used in addiction treatment centers, but it can give a false positive on the fentanyl immunoassay and we’ve had a number of those cases,” he said.
Dr. Salsitz had no disclosures to report.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
Psychedelics for treating psychiatric disorders: Are they safe?
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40
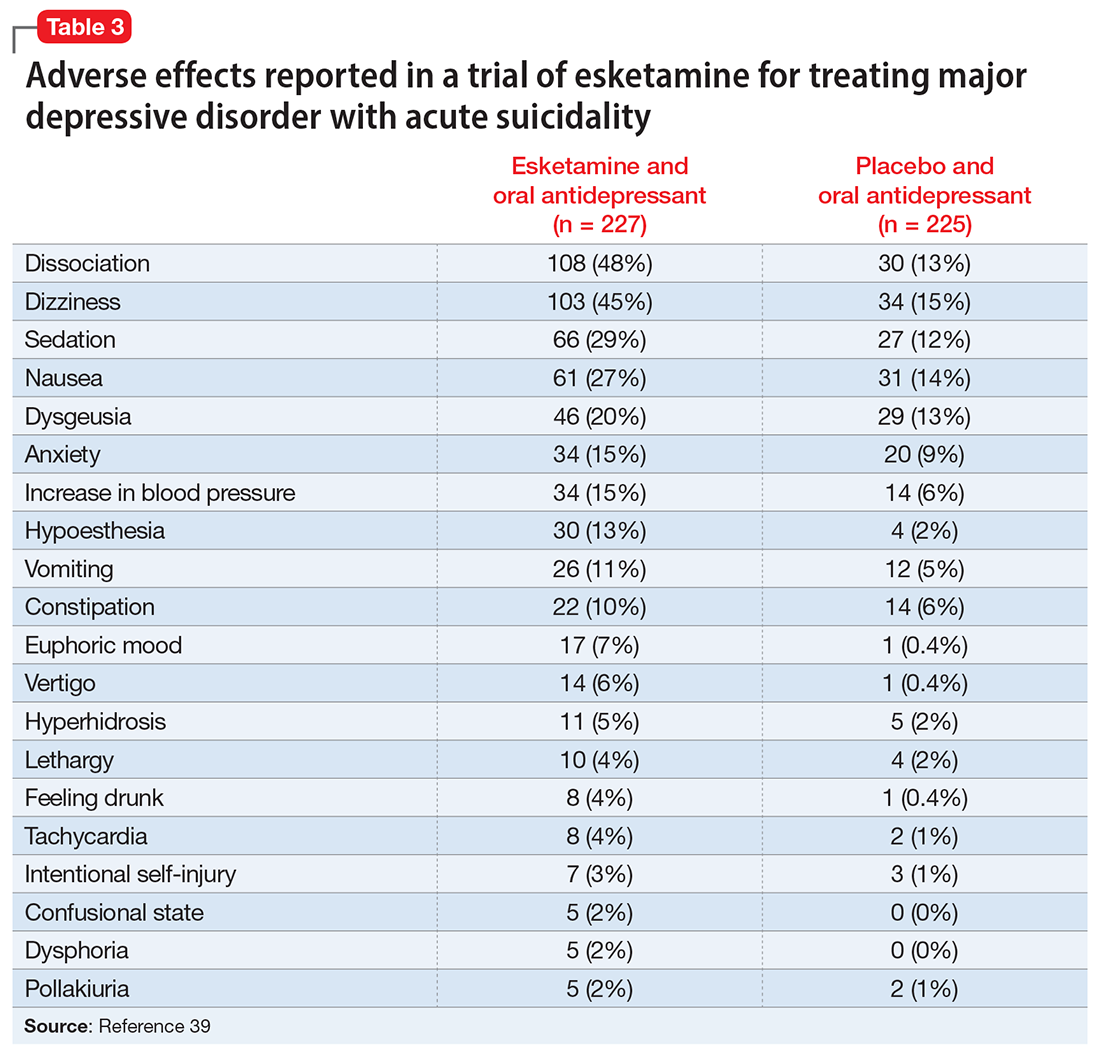
Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40

Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40

Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.