User login
Transitioning patients with opioid use disorder from methadone to buprenorphine
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
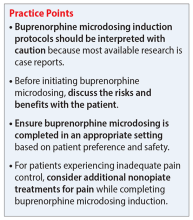
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
Buprenorphine linked with lower risk for neonatal harms than methadone
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Your patients are rotting their teeth with vaping
Primary care physicians, and especially pediatricians, should consider telling their patients about the long-term oral health problems associated with vaping.
A new study found that patients who use vapes were at a higher risk of developing tooth decay and periodontal disease.
Vapes were introduced to the U.S. market in 2006 as an alternative to conventional cigarettes and have become widely popular among youth. According to a 2022 survey from the U.S. Centers for Disease Control and Prevention, 2.55 million middle and high school students in this country reported using the devices in the previous 30 days.
The new study, published in the Journal of the American Dental Association, expands on an initial case series published in 2020 of patients who reported use of vapes and who had severe dental decay. Karina Irusa, BDS, assistant professor of comprehensive care at Tufts University, Boston, and lead author of the case series, wanted to investigate whether her initial findings would apply to a large population of vape users.
For the new study, Dr. Irusa and colleagues collected data on 13,216 patients aged 16-40 who attended Tufts dental clinics between 2019 and 2021. All patients had received a diagnosis of tooth decay, had a tooth decay risk assessment on record, and had answered “yes” or “no” to use of vapes in a health history questionnaire.
Patients had records on file of varying types of dental lesions, cavities filled within the previous 3 years, heavy plaque on teeth, inadequate brushing and flushing, and a self-report of recreational drug use and frequent snacking. If patients had these factors on their file, they were at high risk of developing decay that leads to cavities.
The study found that 79% of patients who responded “yes” to being a current user of vapes were at high risk for dental decay, compared with 60% of those who did not report using the devices.
Materials in the vaping liquids further cause an inflammatory response that disrupts an individual’s internal microbiome, according to numerous studies.
“All the ingredients of vaping are surely a recipe for overgrowth of cavities causing bacteria,” said Jennifer Genuardi, MD, an internist and pediatrician at federally qualified community health center Urban Health Plan, in New York, who was not involved in the study.
Dr. Irusa said information on patient’s vaping habits should be included in routine dental and medical history questionnaires as part of their overall electronic health record.
“Decay in its severe form not only affects one’s ability to eat but affects facial aesthetics and self-esteem as well,” Dr. Irusa said.
Dr. Genuardi called the findings unsurprising.
“We are learning daily more and more about the dangers of vaping,” Dr. Genuardi said. “There’s a focus of today’s research on the effect of actions on our microbiome and the subsequent effects on our health.”
Dr. Genuardi also said many of her teenage patients do not enjoy dental visits or having cavities filled, which could serve as a useful deterrent to vaping for a demographic that has been targeted with marketing from vape manufacturers.
“Cavity formation and the experience of having cavities filled is an experience teens can identify with, so this to me seems like perhaps an even more effective angle to try to curb this unhealthy behavior of vaping,” Dr. Genuardi said.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Primary care physicians, and especially pediatricians, should consider telling their patients about the long-term oral health problems associated with vaping.
A new study found that patients who use vapes were at a higher risk of developing tooth decay and periodontal disease.
Vapes were introduced to the U.S. market in 2006 as an alternative to conventional cigarettes and have become widely popular among youth. According to a 2022 survey from the U.S. Centers for Disease Control and Prevention, 2.55 million middle and high school students in this country reported using the devices in the previous 30 days.
The new study, published in the Journal of the American Dental Association, expands on an initial case series published in 2020 of patients who reported use of vapes and who had severe dental decay. Karina Irusa, BDS, assistant professor of comprehensive care at Tufts University, Boston, and lead author of the case series, wanted to investigate whether her initial findings would apply to a large population of vape users.
For the new study, Dr. Irusa and colleagues collected data on 13,216 patients aged 16-40 who attended Tufts dental clinics between 2019 and 2021. All patients had received a diagnosis of tooth decay, had a tooth decay risk assessment on record, and had answered “yes” or “no” to use of vapes in a health history questionnaire.
Patients had records on file of varying types of dental lesions, cavities filled within the previous 3 years, heavy plaque on teeth, inadequate brushing and flushing, and a self-report of recreational drug use and frequent snacking. If patients had these factors on their file, they were at high risk of developing decay that leads to cavities.
The study found that 79% of patients who responded “yes” to being a current user of vapes were at high risk for dental decay, compared with 60% of those who did not report using the devices.
Materials in the vaping liquids further cause an inflammatory response that disrupts an individual’s internal microbiome, according to numerous studies.
“All the ingredients of vaping are surely a recipe for overgrowth of cavities causing bacteria,” said Jennifer Genuardi, MD, an internist and pediatrician at federally qualified community health center Urban Health Plan, in New York, who was not involved in the study.
Dr. Irusa said information on patient’s vaping habits should be included in routine dental and medical history questionnaires as part of their overall electronic health record.
“Decay in its severe form not only affects one’s ability to eat but affects facial aesthetics and self-esteem as well,” Dr. Irusa said.
Dr. Genuardi called the findings unsurprising.
“We are learning daily more and more about the dangers of vaping,” Dr. Genuardi said. “There’s a focus of today’s research on the effect of actions on our microbiome and the subsequent effects on our health.”
Dr. Genuardi also said many of her teenage patients do not enjoy dental visits or having cavities filled, which could serve as a useful deterrent to vaping for a demographic that has been targeted with marketing from vape manufacturers.
“Cavity formation and the experience of having cavities filled is an experience teens can identify with, so this to me seems like perhaps an even more effective angle to try to curb this unhealthy behavior of vaping,” Dr. Genuardi said.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Primary care physicians, and especially pediatricians, should consider telling their patients about the long-term oral health problems associated with vaping.
A new study found that patients who use vapes were at a higher risk of developing tooth decay and periodontal disease.
Vapes were introduced to the U.S. market in 2006 as an alternative to conventional cigarettes and have become widely popular among youth. According to a 2022 survey from the U.S. Centers for Disease Control and Prevention, 2.55 million middle and high school students in this country reported using the devices in the previous 30 days.
The new study, published in the Journal of the American Dental Association, expands on an initial case series published in 2020 of patients who reported use of vapes and who had severe dental decay. Karina Irusa, BDS, assistant professor of comprehensive care at Tufts University, Boston, and lead author of the case series, wanted to investigate whether her initial findings would apply to a large population of vape users.
For the new study, Dr. Irusa and colleagues collected data on 13,216 patients aged 16-40 who attended Tufts dental clinics between 2019 and 2021. All patients had received a diagnosis of tooth decay, had a tooth decay risk assessment on record, and had answered “yes” or “no” to use of vapes in a health history questionnaire.
Patients had records on file of varying types of dental lesions, cavities filled within the previous 3 years, heavy plaque on teeth, inadequate brushing and flushing, and a self-report of recreational drug use and frequent snacking. If patients had these factors on their file, they were at high risk of developing decay that leads to cavities.
The study found that 79% of patients who responded “yes” to being a current user of vapes were at high risk for dental decay, compared with 60% of those who did not report using the devices.
Materials in the vaping liquids further cause an inflammatory response that disrupts an individual’s internal microbiome, according to numerous studies.
“All the ingredients of vaping are surely a recipe for overgrowth of cavities causing bacteria,” said Jennifer Genuardi, MD, an internist and pediatrician at federally qualified community health center Urban Health Plan, in New York, who was not involved in the study.
Dr. Irusa said information on patient’s vaping habits should be included in routine dental and medical history questionnaires as part of their overall electronic health record.
“Decay in its severe form not only affects one’s ability to eat but affects facial aesthetics and self-esteem as well,” Dr. Irusa said.
Dr. Genuardi called the findings unsurprising.
“We are learning daily more and more about the dangers of vaping,” Dr. Genuardi said. “There’s a focus of today’s research on the effect of actions on our microbiome and the subsequent effects on our health.”
Dr. Genuardi also said many of her teenage patients do not enjoy dental visits or having cavities filled, which could serve as a useful deterrent to vaping for a demographic that has been targeted with marketing from vape manufacturers.
“Cavity formation and the experience of having cavities filled is an experience teens can identify with, so this to me seems like perhaps an even more effective angle to try to curb this unhealthy behavior of vaping,” Dr. Genuardi said.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN DENTAL ASSOCIATION
Highly processed foods ‘as addictive’ as tobacco
according to a new U.S. study that proposes a set of criteria to assess the addictive potential of some foods.
The research suggests that health care professionals are taking steps toward framing food addiction as a clinical entity in its own right; it currently lacks validated treatment protocols and recognition as a clinical diagnosis.
Meanwhile, other data, reported by researchers at the 2022 Diabetes Professional Care conference in London also add support to the clinical recognition of food addiction.
Clinical psychologist Jen Unwin, PhD, from Southport, England, showed that a 3-month online program of low-carbohydrate diet together with psychoeducational support significantly reduced food addiction symptoms among a varied group of individuals, not all of whom were overweight or had obesity.
Dr. Unwin said her new data represent the first wide-scale clinical audit of its kind, other than a prior report of three patients with food addiction who were successfully treated with a ketogenic diet.
“Food addiction explains so much of what we see in clinical practice, where intelligent people understand what we tell them about the physiology associated with a low-carb diet, and they follow it for a while, but then they relapse,” said Dr. Unwin, explaining the difficulties faced by around 20% of her patients who are considered to have food addiction.
Meanwhile, the authors of the U.S. study, led by Ashley N. Gearhardt, PhD, a psychologist from the University of Michigan, Ann Arbor, wrote that the ability of highly processed foods (HPFs) “to rapidly deliver high doses of refined carbohydrates and/or fat appear key to their addictive potential. Thus, we conclude that HPFs can be considered addictive substances based on scientifically established criteria.”
They asserted that the contribution to preventable deaths by a diet dominated by highly processed foods is comparable with that of tobacco products, and as such, like Dr. Unwin, the authors sought clinical recognition and a more formalized protocol to manage food addiction.
“Understanding whether addiction contributes to HPF intake may lead to new treatments, as preliminary research finds that behavioral and pharmacological interventions that target addictive mechanisms may reduce compulsive HPF intake,” they stated.
The study led by Dr. Gearhardt was published in the journal Addiction, and the study led by Unwin was also recently published in Frontiers in Psychiatry.
Addiction criteria similar to tobacco
HPFs can be associated with an eating phenotype “that reflects the hallmarks of addiction,” said Dr. Gearhardt and coauthors; typically, loss of control over intake, intense cravings, inability to cut down, and continued use despite negative consequences.
Acknowledging the lack of a single addictive agent, they explain that food addiction reflects mechanisms implicated in other addictive disorders such as smoking.
As such, in their study, Dr. Gearhardt and colleagues proposed a set of scientifically based criteria for the evaluation of whether certain foods are addictive. “Specifically, we propose the primary criteria used to resolve one of the last major controversies over whether a substance, tobacco products, was addictive.”
They consider certain foods according to the primary criteria that have stood the test of time after being proposed in 1988 by the U.S. Surgeon General to establish the addictive potential of tobacco: they trigger compulsive use, they have psychoactive effects, and they are reinforcing.
They have updated these criteria to include the ability to trigger urges and cravings, and added that “both these products [tobacco and HPFs] are legal, easily accessible, inexpensive, lack an intoxication syndrome, and are major causes of preventable death.”
For example, with compulsive use, tobacco meets this criterion because evidence suggests that most smokers would like to quit but are unable to do so.
Likewise, wrote Dr. Gearhardt and colleagues, even “in the face of significant diet-related health consequences (e.g., diabetes and cardiovascular disease), the majority of patients are unable to adhere to medically recommended dietary plans that require a reduction in HPF intake.”
Reinforcement, through tobacco use, is demonstrated by its ‘being sufficiently rewarding to maintain self-administration” because of its ability to deliver nicotine, they said, quoting the Surgeon General’s report, and likewise, with food addiction, “both adults and children will self-administer HPFs (e.g., potato chips, candy, and cookies) even when satiated.”
Online group food addiction intervention study
Dr. Unwin and coauthors want people with food addiction to be able to access a validated treatment protocol. Their study aimed to evaluate an online group intervention across multiple sites in the United States, Canada, and the United Kingdom, involving an abstinent, low-carbohydrate diet and biopsychosocial education focused on addiction and recovery in people self-identifying as having food addiction.
“Lots of people with food addiction go to GPs who don’t clinically recognize this, or if they attend addiction services and psychiatry, then they tend to only specialize in drugs, alcohol, and gambling. Eating disorder services are linked but their programs mostly don’t work for a food addict,” Dr. Unwin remarked in an interview.
“We feel running groups, as well as training professionals to run groups, is the best way to manage food addiction,” she said, reflecting on the scale of the problem, with around 10% of adults in the U.K. general population considered to have food addiction. In Dr. Unwin’s study, some people had type 2 diabetes and some overweight/obesity, but she added that some participants were underweight or of normal weight.
Initially, the 103 participants received weekly group (8-24 people) sessions for 10-14 weeks, and then monthly maintenance comprising follow-up that involved coaching participants on how to cope with relapse and get back on track.
Food addiction symptoms were assessed pre- and post program using the modified Yale Food Addiction Scale (mYFAS) 2.0; ICD-10 symptoms of food-related substance use disorder (CRAVED); and mental health well-being measured using the short version of the Warwick Edinburgh Mental Wellbeing scale and body weight.
“The program eliminates processed foods with a personalized, abstinence food plan that involves education around mechanisms involved,” said Dr. Unwin, who explained that processed foods deliver a dopamine high, and in response to this, the brain lowers the number of dopamine receptors to effectively counteract the increase in dopamine. This drop in dopamine receptors explains the depression often associated with food addiction.
Dr. Unwin reported that food addiction symptoms were significantly reduced, with the mYFAS dropping by 1.52, the CRAVED score by 1.53, and body weight by 2.34 kg (5.2 lb). Mental health, as measured by the Warwick Edinburgh Mental Wellbeing scale, improved by 2.37 points.
“We were very interested in mental health and well-being because it impacts so much across our lives, and we saw significant improvements here, but we were less interested in weight because food addicts come in all shapes and sizes with some people underweight,” said Dr. Unwin. “Food addiction symptoms were significantly improved in the group, but we now need to look at the longer-term outcomes.”
Dr. Unwin runs a low-carbohydrate program for type 2 diabetes with her husband David Unwin, MD, who is a GP in Southport, England. She said that they ask patients if they think they have food addiction, and most say they do.
“I always try to explain to patients about the dopamine high, and how this starts the craving which makes people wonder when and where they can find the next sugar hit. Just thinking about the next chocolate bar gets the dopamine running for many people, and the more they tread this path then the worse it gets because the dopamine receptors keep reducing.”
Lorraine Avery, RN, a diabetes nurse specialist for Solent NHS Trust, who attended the DPC conference, welcomed Dr. Unwin’s presentation.
“My concern as a diabetes nurse specialist is that I’m unsure all our patients recognize their food addiction, and there are often more drivers to eating than just the food in front of them,” she said in an interview. “I think there’s an emotional element, too. These people are often ‘yo-yo’ dieters, and they join lots of expert companies to help them lose weight, but these companies want them to regain and re-join their programs,” she said.
“I think there is something about helping patients recognize they have a food addiction and they need to consider that other approaches might be helpful.”
Dr. Unwin reported no relevant financial relationships; some other authors have fee-paying clients with food addiction. Dr. Gearhardt and Ms. Avery reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new U.S. study that proposes a set of criteria to assess the addictive potential of some foods.
The research suggests that health care professionals are taking steps toward framing food addiction as a clinical entity in its own right; it currently lacks validated treatment protocols and recognition as a clinical diagnosis.
Meanwhile, other data, reported by researchers at the 2022 Diabetes Professional Care conference in London also add support to the clinical recognition of food addiction.
Clinical psychologist Jen Unwin, PhD, from Southport, England, showed that a 3-month online program of low-carbohydrate diet together with psychoeducational support significantly reduced food addiction symptoms among a varied group of individuals, not all of whom were overweight or had obesity.
Dr. Unwin said her new data represent the first wide-scale clinical audit of its kind, other than a prior report of three patients with food addiction who were successfully treated with a ketogenic diet.
“Food addiction explains so much of what we see in clinical practice, where intelligent people understand what we tell them about the physiology associated with a low-carb diet, and they follow it for a while, but then they relapse,” said Dr. Unwin, explaining the difficulties faced by around 20% of her patients who are considered to have food addiction.
Meanwhile, the authors of the U.S. study, led by Ashley N. Gearhardt, PhD, a psychologist from the University of Michigan, Ann Arbor, wrote that the ability of highly processed foods (HPFs) “to rapidly deliver high doses of refined carbohydrates and/or fat appear key to their addictive potential. Thus, we conclude that HPFs can be considered addictive substances based on scientifically established criteria.”
They asserted that the contribution to preventable deaths by a diet dominated by highly processed foods is comparable with that of tobacco products, and as such, like Dr. Unwin, the authors sought clinical recognition and a more formalized protocol to manage food addiction.
“Understanding whether addiction contributes to HPF intake may lead to new treatments, as preliminary research finds that behavioral and pharmacological interventions that target addictive mechanisms may reduce compulsive HPF intake,” they stated.
The study led by Dr. Gearhardt was published in the journal Addiction, and the study led by Unwin was also recently published in Frontiers in Psychiatry.
Addiction criteria similar to tobacco
HPFs can be associated with an eating phenotype “that reflects the hallmarks of addiction,” said Dr. Gearhardt and coauthors; typically, loss of control over intake, intense cravings, inability to cut down, and continued use despite negative consequences.
Acknowledging the lack of a single addictive agent, they explain that food addiction reflects mechanisms implicated in other addictive disorders such as smoking.
As such, in their study, Dr. Gearhardt and colleagues proposed a set of scientifically based criteria for the evaluation of whether certain foods are addictive. “Specifically, we propose the primary criteria used to resolve one of the last major controversies over whether a substance, tobacco products, was addictive.”
They consider certain foods according to the primary criteria that have stood the test of time after being proposed in 1988 by the U.S. Surgeon General to establish the addictive potential of tobacco: they trigger compulsive use, they have psychoactive effects, and they are reinforcing.
They have updated these criteria to include the ability to trigger urges and cravings, and added that “both these products [tobacco and HPFs] are legal, easily accessible, inexpensive, lack an intoxication syndrome, and are major causes of preventable death.”
For example, with compulsive use, tobacco meets this criterion because evidence suggests that most smokers would like to quit but are unable to do so.
Likewise, wrote Dr. Gearhardt and colleagues, even “in the face of significant diet-related health consequences (e.g., diabetes and cardiovascular disease), the majority of patients are unable to adhere to medically recommended dietary plans that require a reduction in HPF intake.”
Reinforcement, through tobacco use, is demonstrated by its ‘being sufficiently rewarding to maintain self-administration” because of its ability to deliver nicotine, they said, quoting the Surgeon General’s report, and likewise, with food addiction, “both adults and children will self-administer HPFs (e.g., potato chips, candy, and cookies) even when satiated.”
Online group food addiction intervention study
Dr. Unwin and coauthors want people with food addiction to be able to access a validated treatment protocol. Their study aimed to evaluate an online group intervention across multiple sites in the United States, Canada, and the United Kingdom, involving an abstinent, low-carbohydrate diet and biopsychosocial education focused on addiction and recovery in people self-identifying as having food addiction.
“Lots of people with food addiction go to GPs who don’t clinically recognize this, or if they attend addiction services and psychiatry, then they tend to only specialize in drugs, alcohol, and gambling. Eating disorder services are linked but their programs mostly don’t work for a food addict,” Dr. Unwin remarked in an interview.
“We feel running groups, as well as training professionals to run groups, is the best way to manage food addiction,” she said, reflecting on the scale of the problem, with around 10% of adults in the U.K. general population considered to have food addiction. In Dr. Unwin’s study, some people had type 2 diabetes and some overweight/obesity, but she added that some participants were underweight or of normal weight.
Initially, the 103 participants received weekly group (8-24 people) sessions for 10-14 weeks, and then monthly maintenance comprising follow-up that involved coaching participants on how to cope with relapse and get back on track.
Food addiction symptoms were assessed pre- and post program using the modified Yale Food Addiction Scale (mYFAS) 2.0; ICD-10 symptoms of food-related substance use disorder (CRAVED); and mental health well-being measured using the short version of the Warwick Edinburgh Mental Wellbeing scale and body weight.
“The program eliminates processed foods with a personalized, abstinence food plan that involves education around mechanisms involved,” said Dr. Unwin, who explained that processed foods deliver a dopamine high, and in response to this, the brain lowers the number of dopamine receptors to effectively counteract the increase in dopamine. This drop in dopamine receptors explains the depression often associated with food addiction.
Dr. Unwin reported that food addiction symptoms were significantly reduced, with the mYFAS dropping by 1.52, the CRAVED score by 1.53, and body weight by 2.34 kg (5.2 lb). Mental health, as measured by the Warwick Edinburgh Mental Wellbeing scale, improved by 2.37 points.
“We were very interested in mental health and well-being because it impacts so much across our lives, and we saw significant improvements here, but we were less interested in weight because food addicts come in all shapes and sizes with some people underweight,” said Dr. Unwin. “Food addiction symptoms were significantly improved in the group, but we now need to look at the longer-term outcomes.”
Dr. Unwin runs a low-carbohydrate program for type 2 diabetes with her husband David Unwin, MD, who is a GP in Southport, England. She said that they ask patients if they think they have food addiction, and most say they do.
“I always try to explain to patients about the dopamine high, and how this starts the craving which makes people wonder when and where they can find the next sugar hit. Just thinking about the next chocolate bar gets the dopamine running for many people, and the more they tread this path then the worse it gets because the dopamine receptors keep reducing.”
Lorraine Avery, RN, a diabetes nurse specialist for Solent NHS Trust, who attended the DPC conference, welcomed Dr. Unwin’s presentation.
“My concern as a diabetes nurse specialist is that I’m unsure all our patients recognize their food addiction, and there are often more drivers to eating than just the food in front of them,” she said in an interview. “I think there’s an emotional element, too. These people are often ‘yo-yo’ dieters, and they join lots of expert companies to help them lose weight, but these companies want them to regain and re-join their programs,” she said.
“I think there is something about helping patients recognize they have a food addiction and they need to consider that other approaches might be helpful.”
Dr. Unwin reported no relevant financial relationships; some other authors have fee-paying clients with food addiction. Dr. Gearhardt and Ms. Avery reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new U.S. study that proposes a set of criteria to assess the addictive potential of some foods.
The research suggests that health care professionals are taking steps toward framing food addiction as a clinical entity in its own right; it currently lacks validated treatment protocols and recognition as a clinical diagnosis.
Meanwhile, other data, reported by researchers at the 2022 Diabetes Professional Care conference in London also add support to the clinical recognition of food addiction.
Clinical psychologist Jen Unwin, PhD, from Southport, England, showed that a 3-month online program of low-carbohydrate diet together with psychoeducational support significantly reduced food addiction symptoms among a varied group of individuals, not all of whom were overweight or had obesity.
Dr. Unwin said her new data represent the first wide-scale clinical audit of its kind, other than a prior report of three patients with food addiction who were successfully treated with a ketogenic diet.
“Food addiction explains so much of what we see in clinical practice, where intelligent people understand what we tell them about the physiology associated with a low-carb diet, and they follow it for a while, but then they relapse,” said Dr. Unwin, explaining the difficulties faced by around 20% of her patients who are considered to have food addiction.
Meanwhile, the authors of the U.S. study, led by Ashley N. Gearhardt, PhD, a psychologist from the University of Michigan, Ann Arbor, wrote that the ability of highly processed foods (HPFs) “to rapidly deliver high doses of refined carbohydrates and/or fat appear key to their addictive potential. Thus, we conclude that HPFs can be considered addictive substances based on scientifically established criteria.”
They asserted that the contribution to preventable deaths by a diet dominated by highly processed foods is comparable with that of tobacco products, and as such, like Dr. Unwin, the authors sought clinical recognition and a more formalized protocol to manage food addiction.
“Understanding whether addiction contributes to HPF intake may lead to new treatments, as preliminary research finds that behavioral and pharmacological interventions that target addictive mechanisms may reduce compulsive HPF intake,” they stated.
The study led by Dr. Gearhardt was published in the journal Addiction, and the study led by Unwin was also recently published in Frontiers in Psychiatry.
Addiction criteria similar to tobacco
HPFs can be associated with an eating phenotype “that reflects the hallmarks of addiction,” said Dr. Gearhardt and coauthors; typically, loss of control over intake, intense cravings, inability to cut down, and continued use despite negative consequences.
Acknowledging the lack of a single addictive agent, they explain that food addiction reflects mechanisms implicated in other addictive disorders such as smoking.
As such, in their study, Dr. Gearhardt and colleagues proposed a set of scientifically based criteria for the evaluation of whether certain foods are addictive. “Specifically, we propose the primary criteria used to resolve one of the last major controversies over whether a substance, tobacco products, was addictive.”
They consider certain foods according to the primary criteria that have stood the test of time after being proposed in 1988 by the U.S. Surgeon General to establish the addictive potential of tobacco: they trigger compulsive use, they have psychoactive effects, and they are reinforcing.
They have updated these criteria to include the ability to trigger urges and cravings, and added that “both these products [tobacco and HPFs] are legal, easily accessible, inexpensive, lack an intoxication syndrome, and are major causes of preventable death.”
For example, with compulsive use, tobacco meets this criterion because evidence suggests that most smokers would like to quit but are unable to do so.
Likewise, wrote Dr. Gearhardt and colleagues, even “in the face of significant diet-related health consequences (e.g., diabetes and cardiovascular disease), the majority of patients are unable to adhere to medically recommended dietary plans that require a reduction in HPF intake.”
Reinforcement, through tobacco use, is demonstrated by its ‘being sufficiently rewarding to maintain self-administration” because of its ability to deliver nicotine, they said, quoting the Surgeon General’s report, and likewise, with food addiction, “both adults and children will self-administer HPFs (e.g., potato chips, candy, and cookies) even when satiated.”
Online group food addiction intervention study
Dr. Unwin and coauthors want people with food addiction to be able to access a validated treatment protocol. Their study aimed to evaluate an online group intervention across multiple sites in the United States, Canada, and the United Kingdom, involving an abstinent, low-carbohydrate diet and biopsychosocial education focused on addiction and recovery in people self-identifying as having food addiction.
“Lots of people with food addiction go to GPs who don’t clinically recognize this, or if they attend addiction services and psychiatry, then they tend to only specialize in drugs, alcohol, and gambling. Eating disorder services are linked but their programs mostly don’t work for a food addict,” Dr. Unwin remarked in an interview.
“We feel running groups, as well as training professionals to run groups, is the best way to manage food addiction,” she said, reflecting on the scale of the problem, with around 10% of adults in the U.K. general population considered to have food addiction. In Dr. Unwin’s study, some people had type 2 diabetes and some overweight/obesity, but she added that some participants were underweight or of normal weight.
Initially, the 103 participants received weekly group (8-24 people) sessions for 10-14 weeks, and then monthly maintenance comprising follow-up that involved coaching participants on how to cope with relapse and get back on track.
Food addiction symptoms were assessed pre- and post program using the modified Yale Food Addiction Scale (mYFAS) 2.0; ICD-10 symptoms of food-related substance use disorder (CRAVED); and mental health well-being measured using the short version of the Warwick Edinburgh Mental Wellbeing scale and body weight.
“The program eliminates processed foods with a personalized, abstinence food plan that involves education around mechanisms involved,” said Dr. Unwin, who explained that processed foods deliver a dopamine high, and in response to this, the brain lowers the number of dopamine receptors to effectively counteract the increase in dopamine. This drop in dopamine receptors explains the depression often associated with food addiction.
Dr. Unwin reported that food addiction symptoms were significantly reduced, with the mYFAS dropping by 1.52, the CRAVED score by 1.53, and body weight by 2.34 kg (5.2 lb). Mental health, as measured by the Warwick Edinburgh Mental Wellbeing scale, improved by 2.37 points.
“We were very interested in mental health and well-being because it impacts so much across our lives, and we saw significant improvements here, but we were less interested in weight because food addicts come in all shapes and sizes with some people underweight,” said Dr. Unwin. “Food addiction symptoms were significantly improved in the group, but we now need to look at the longer-term outcomes.”
Dr. Unwin runs a low-carbohydrate program for type 2 diabetes with her husband David Unwin, MD, who is a GP in Southport, England. She said that they ask patients if they think they have food addiction, and most say they do.
“I always try to explain to patients about the dopamine high, and how this starts the craving which makes people wonder when and where they can find the next sugar hit. Just thinking about the next chocolate bar gets the dopamine running for many people, and the more they tread this path then the worse it gets because the dopamine receptors keep reducing.”
Lorraine Avery, RN, a diabetes nurse specialist for Solent NHS Trust, who attended the DPC conference, welcomed Dr. Unwin’s presentation.
“My concern as a diabetes nurse specialist is that I’m unsure all our patients recognize their food addiction, and there are often more drivers to eating than just the food in front of them,” she said in an interview. “I think there’s an emotional element, too. These people are often ‘yo-yo’ dieters, and they join lots of expert companies to help them lose weight, but these companies want them to regain and re-join their programs,” she said.
“I think there is something about helping patients recognize they have a food addiction and they need to consider that other approaches might be helpful.”
Dr. Unwin reported no relevant financial relationships; some other authors have fee-paying clients with food addiction. Dr. Gearhardt and Ms. Avery reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fentanyl vaccine a potential ‘game changer’ for opioid crisis
Texas-based researchers have developed a vaccine that blocks the euphoric effects of fentanyl, a potent synthetic opioid that is increasingly involved in opioid overdose deaths in the United States.
In studies in male and female mice, the vaccine generated significant and long-lasting levels of anti-fentanyl antibodies that were highly effective at reducing the antinociceptive, behavioral, and physiological effects of the drug.
“Thus, the individual will not feel the euphoric effects and can ‘get back on the wagon’ to sobriety,” lead investigator Colin Haile, MD, PhD, with University of Houston and founding member of the UH Drug Discovery Institute, said in a news release. The study was published online in the journal Pharmaceutics.
“The anti-fentanyl antibodies were specific to fentanyl and a fentanyl derivative and did not cross-react with other opioids, such as morphine. That means a vaccinated person would still be able to be treated for pain relief with other opioids,” said Dr. Haile.
The vaccine did not cause any adverse effects in the immunized mice. The research team plans to start manufacturing clinical-grade vaccine in the coming months with clinical trials in humans planned soon.
If proven safe and effective in clinical testing, the vaccine could have major implications for the nation’s opioid epidemic by becoming a relapse prevention agent for people trying to quit using opioids, the researchers note.
The United States in 2021 recorded more than 107,000 drug overdose deaths – a record high, according to federal health officials – and fentanyl was involved in most of these deaths.
Senior author Therese Kosten, PhD, director of the UH Developmental, Cognitive & Behavioral Neuroscience program, calls the new fentanyl vaccine a potential “game changer.”
“Fentanyl use and overdose is a particular treatment challenge that is not adequately addressed with current medications because of its pharmacodynamics, and managing acute overdose with the short-acting naloxone [Narcan] is not appropriately effective as multiple doses of naloxone are often needed to reverse fentanyl’s fatal effects,” said Dr. Kosten.
Funding for the study was provided by the Department of Defense through the Alcohol and Substance Abuse Disorders Program managed by RTI International’s Pharmacotherapies for Alcohol and Substance Use Disorders Alliance, which has funded Dr. Haile’s lab for several years to develop the anti-fentanyl vaccine. The authors have no relevant conflicts of interest. A provisional patent has been submitted by the University of Houston on behalf of four of the investigators containing technology related to the fentanyl vaccine.
A version of this article first appeared on Medscape.com.
Texas-based researchers have developed a vaccine that blocks the euphoric effects of fentanyl, a potent synthetic opioid that is increasingly involved in opioid overdose deaths in the United States.
In studies in male and female mice, the vaccine generated significant and long-lasting levels of anti-fentanyl antibodies that were highly effective at reducing the antinociceptive, behavioral, and physiological effects of the drug.
“Thus, the individual will not feel the euphoric effects and can ‘get back on the wagon’ to sobriety,” lead investigator Colin Haile, MD, PhD, with University of Houston and founding member of the UH Drug Discovery Institute, said in a news release. The study was published online in the journal Pharmaceutics.
“The anti-fentanyl antibodies were specific to fentanyl and a fentanyl derivative and did not cross-react with other opioids, such as morphine. That means a vaccinated person would still be able to be treated for pain relief with other opioids,” said Dr. Haile.
The vaccine did not cause any adverse effects in the immunized mice. The research team plans to start manufacturing clinical-grade vaccine in the coming months with clinical trials in humans planned soon.
If proven safe and effective in clinical testing, the vaccine could have major implications for the nation’s opioid epidemic by becoming a relapse prevention agent for people trying to quit using opioids, the researchers note.
The United States in 2021 recorded more than 107,000 drug overdose deaths – a record high, according to federal health officials – and fentanyl was involved in most of these deaths.
Senior author Therese Kosten, PhD, director of the UH Developmental, Cognitive & Behavioral Neuroscience program, calls the new fentanyl vaccine a potential “game changer.”
“Fentanyl use and overdose is a particular treatment challenge that is not adequately addressed with current medications because of its pharmacodynamics, and managing acute overdose with the short-acting naloxone [Narcan] is not appropriately effective as multiple doses of naloxone are often needed to reverse fentanyl’s fatal effects,” said Dr. Kosten.
Funding for the study was provided by the Department of Defense through the Alcohol and Substance Abuse Disorders Program managed by RTI International’s Pharmacotherapies for Alcohol and Substance Use Disorders Alliance, which has funded Dr. Haile’s lab for several years to develop the anti-fentanyl vaccine. The authors have no relevant conflicts of interest. A provisional patent has been submitted by the University of Houston on behalf of four of the investigators containing technology related to the fentanyl vaccine.
A version of this article first appeared on Medscape.com.
Texas-based researchers have developed a vaccine that blocks the euphoric effects of fentanyl, a potent synthetic opioid that is increasingly involved in opioid overdose deaths in the United States.
In studies in male and female mice, the vaccine generated significant and long-lasting levels of anti-fentanyl antibodies that were highly effective at reducing the antinociceptive, behavioral, and physiological effects of the drug.
“Thus, the individual will not feel the euphoric effects and can ‘get back on the wagon’ to sobriety,” lead investigator Colin Haile, MD, PhD, with University of Houston and founding member of the UH Drug Discovery Institute, said in a news release. The study was published online in the journal Pharmaceutics.
“The anti-fentanyl antibodies were specific to fentanyl and a fentanyl derivative and did not cross-react with other opioids, such as morphine. That means a vaccinated person would still be able to be treated for pain relief with other opioids,” said Dr. Haile.
The vaccine did not cause any adverse effects in the immunized mice. The research team plans to start manufacturing clinical-grade vaccine in the coming months with clinical trials in humans planned soon.
If proven safe and effective in clinical testing, the vaccine could have major implications for the nation’s opioid epidemic by becoming a relapse prevention agent for people trying to quit using opioids, the researchers note.
The United States in 2021 recorded more than 107,000 drug overdose deaths – a record high, according to federal health officials – and fentanyl was involved in most of these deaths.
Senior author Therese Kosten, PhD, director of the UH Developmental, Cognitive & Behavioral Neuroscience program, calls the new fentanyl vaccine a potential “game changer.”
“Fentanyl use and overdose is a particular treatment challenge that is not adequately addressed with current medications because of its pharmacodynamics, and managing acute overdose with the short-acting naloxone [Narcan] is not appropriately effective as multiple doses of naloxone are often needed to reverse fentanyl’s fatal effects,” said Dr. Kosten.
Funding for the study was provided by the Department of Defense through the Alcohol and Substance Abuse Disorders Program managed by RTI International’s Pharmacotherapies for Alcohol and Substance Use Disorders Alliance, which has funded Dr. Haile’s lab for several years to develop the anti-fentanyl vaccine. The authors have no relevant conflicts of interest. A provisional patent has been submitted by the University of Houston on behalf of four of the investigators containing technology related to the fentanyl vaccine.
A version of this article first appeared on Medscape.com.
FROM PHARMACEUTICS
Is opioid abuse leading to pediatric head trauma?
As a physician in the heart of the opioid epidemic, Pavirthra R. Ellison, MD, has watched for years as her patients have lost parents to overdoses. More than 1,400 adults in West Virginia, where she practices, died of opioid abuse in 2021 alone, government statistics show.
The grim toll made Ellison wonder: What was happening to children in the state? The answer, according to a new study, is not reassuring.
Ellison and her colleagues have found a troubling link between a surge in critical head and neck injuries among youth in West Virginia and a spike in positive tests for opioids and benzodiazepines among children who arrive at emergency departments in the state. They don’t think the pattern is a coincidence.
“What we found was really kind of scary,” said Dr. Ellison, a professor of anesthesiology and pediatrics at West Virginia University, Morgantown. “Children in this region often get exposure to these drugs early on.”
A region in crisis
According to a 2020 report from the Department of Health & Human Services, about 9.9 million Americans abused prescription opioids in 2018. That same year, almost 47,000 died following an overdose of the painkillers. In 2017, Appalachian counties experienced a death rate from opioid overdoses that was 72% higher than that of the rest of the country.
Dr. Ellison and associates who presented their findings recently at the 2022 annual meeting of the American Society of Anesthesiologists, examined rates of pediatric trauma injuries, injury severity, and results of drug screenings throughout West Virginia between 2009 and 2019.
The study included 4,538 children and adolescents younger than 18 years who had been treated for head and neck trauma. The youth were divided into two groups: 3,356 who were treated from 2009 to 2016, and 1,182 who were treated between 2017 and 2019.
The incidence of critical head injuries increased from 3.7% in the period 2009-2016 to 7.2% in the period 2017-2019 (P = .007). The incidence of serious neck injuries increased from 12.2% to 27.1% (P = .007) during that period, according to the researchers. The number of days that these patients spent on ventilators more than doubled, from 3.1 to 6.3 (P < .001), they reported.
At the same time, the rate of positive urine drug tests rose sharply, from 0.8% to 1.8% (P < .001) for benzodiazepines and from 1% to 4.9% for opioids (P < .001).
Drug testing of children hospitalized for trauma rose more than threefold, from 6.9% to 23.2% (P < .001). Dr. Ellison’s group was unable to match positive drug screens with patients who came in with injuries.
Dr. Ellison said her research “warrants further evaluation of current policies and protocols targeting substance use in children and adolescents.” To that end, her team is planning to conduct a prospective study in mid 2023 to further illuminate the trends.
“I hope early next year we can put together a group of physicians, pediatric general surgeons, neurosurgeons, and anesthesiologists,” she said. “I want to look at what we can do to reduce the severity of injury.”
She also wants to reach the population that these findings directly affect.
“The next step that we are currently working on is community awareness of the issue,” Dr. Ellison said. “Our trauma institute is partnering with middle school and high school kids to create material to raise awareness.”
Rural Appalachia faces several other endemic problems that affect the health and well-being of children and families, including limited access to health care, poverty, and minimal community support, according to Dr. Ellison. Children and teens in the region who live with parents who abuse opioids are more likely to experience family conflict, mental health challenges, legal troubles, and negative health effects, including physical trauma.
A call to action
Toufic Jildeh, MD, assistant professor of orthopedics, Michigan State University Health Care, East Lansing, who has studied ways to reduce opioid use among surgery patients, called the new findings “alarming.”
After reviewing the study, Dr. Jildeh said that in his opinion, the results support standardized drug testing of children, particularly in the context of severe trauma.
Bruce Bassi, MD, an addiction psychiatrist and owner of TelepsychHealth, a private, online psychiatric practice, agreed. “The main take-home message is that drug screening should be the standard of care for pediatric patients in this region, because it changes the management of those individuals,” Dr. Bassi said.
But identifying these patients is just the first step. “We should continue to educate and raise awareness, not only in the health care system,” Dr. Bassi said. “We also need to let parents know that the possibility of children obtaining access to medications is high.”
The study was independently supported. Dr. Ellison and Dr. Jildeh reported no relevant financial relationships. Dr. Bassi owns a private psychiatry practice called Telepsychhealth but has no other relevant financial relationships.
A version of this article first appeared on Medscape.com.
As a physician in the heart of the opioid epidemic, Pavirthra R. Ellison, MD, has watched for years as her patients have lost parents to overdoses. More than 1,400 adults in West Virginia, where she practices, died of opioid abuse in 2021 alone, government statistics show.
The grim toll made Ellison wonder: What was happening to children in the state? The answer, according to a new study, is not reassuring.
Ellison and her colleagues have found a troubling link between a surge in critical head and neck injuries among youth in West Virginia and a spike in positive tests for opioids and benzodiazepines among children who arrive at emergency departments in the state. They don’t think the pattern is a coincidence.
“What we found was really kind of scary,” said Dr. Ellison, a professor of anesthesiology and pediatrics at West Virginia University, Morgantown. “Children in this region often get exposure to these drugs early on.”
A region in crisis
According to a 2020 report from the Department of Health & Human Services, about 9.9 million Americans abused prescription opioids in 2018. That same year, almost 47,000 died following an overdose of the painkillers. In 2017, Appalachian counties experienced a death rate from opioid overdoses that was 72% higher than that of the rest of the country.
Dr. Ellison and associates who presented their findings recently at the 2022 annual meeting of the American Society of Anesthesiologists, examined rates of pediatric trauma injuries, injury severity, and results of drug screenings throughout West Virginia between 2009 and 2019.
The study included 4,538 children and adolescents younger than 18 years who had been treated for head and neck trauma. The youth were divided into two groups: 3,356 who were treated from 2009 to 2016, and 1,182 who were treated between 2017 and 2019.
The incidence of critical head injuries increased from 3.7% in the period 2009-2016 to 7.2% in the period 2017-2019 (P = .007). The incidence of serious neck injuries increased from 12.2% to 27.1% (P = .007) during that period, according to the researchers. The number of days that these patients spent on ventilators more than doubled, from 3.1 to 6.3 (P < .001), they reported.
At the same time, the rate of positive urine drug tests rose sharply, from 0.8% to 1.8% (P < .001) for benzodiazepines and from 1% to 4.9% for opioids (P < .001).
Drug testing of children hospitalized for trauma rose more than threefold, from 6.9% to 23.2% (P < .001). Dr. Ellison’s group was unable to match positive drug screens with patients who came in with injuries.
Dr. Ellison said her research “warrants further evaluation of current policies and protocols targeting substance use in children and adolescents.” To that end, her team is planning to conduct a prospective study in mid 2023 to further illuminate the trends.
“I hope early next year we can put together a group of physicians, pediatric general surgeons, neurosurgeons, and anesthesiologists,” she said. “I want to look at what we can do to reduce the severity of injury.”
She also wants to reach the population that these findings directly affect.
“The next step that we are currently working on is community awareness of the issue,” Dr. Ellison said. “Our trauma institute is partnering with middle school and high school kids to create material to raise awareness.”
Rural Appalachia faces several other endemic problems that affect the health and well-being of children and families, including limited access to health care, poverty, and minimal community support, according to Dr. Ellison. Children and teens in the region who live with parents who abuse opioids are more likely to experience family conflict, mental health challenges, legal troubles, and negative health effects, including physical trauma.
A call to action
Toufic Jildeh, MD, assistant professor of orthopedics, Michigan State University Health Care, East Lansing, who has studied ways to reduce opioid use among surgery patients, called the new findings “alarming.”
After reviewing the study, Dr. Jildeh said that in his opinion, the results support standardized drug testing of children, particularly in the context of severe trauma.
Bruce Bassi, MD, an addiction psychiatrist and owner of TelepsychHealth, a private, online psychiatric practice, agreed. “The main take-home message is that drug screening should be the standard of care for pediatric patients in this region, because it changes the management of those individuals,” Dr. Bassi said.
But identifying these patients is just the first step. “We should continue to educate and raise awareness, not only in the health care system,” Dr. Bassi said. “We also need to let parents know that the possibility of children obtaining access to medications is high.”
The study was independently supported. Dr. Ellison and Dr. Jildeh reported no relevant financial relationships. Dr. Bassi owns a private psychiatry practice called Telepsychhealth but has no other relevant financial relationships.
A version of this article first appeared on Medscape.com.
As a physician in the heart of the opioid epidemic, Pavirthra R. Ellison, MD, has watched for years as her patients have lost parents to overdoses. More than 1,400 adults in West Virginia, where she practices, died of opioid abuse in 2021 alone, government statistics show.
The grim toll made Ellison wonder: What was happening to children in the state? The answer, according to a new study, is not reassuring.
Ellison and her colleagues have found a troubling link between a surge in critical head and neck injuries among youth in West Virginia and a spike in positive tests for opioids and benzodiazepines among children who arrive at emergency departments in the state. They don’t think the pattern is a coincidence.
“What we found was really kind of scary,” said Dr. Ellison, a professor of anesthesiology and pediatrics at West Virginia University, Morgantown. “Children in this region often get exposure to these drugs early on.”
A region in crisis
According to a 2020 report from the Department of Health & Human Services, about 9.9 million Americans abused prescription opioids in 2018. That same year, almost 47,000 died following an overdose of the painkillers. In 2017, Appalachian counties experienced a death rate from opioid overdoses that was 72% higher than that of the rest of the country.
Dr. Ellison and associates who presented their findings recently at the 2022 annual meeting of the American Society of Anesthesiologists, examined rates of pediatric trauma injuries, injury severity, and results of drug screenings throughout West Virginia between 2009 and 2019.
The study included 4,538 children and adolescents younger than 18 years who had been treated for head and neck trauma. The youth were divided into two groups: 3,356 who were treated from 2009 to 2016, and 1,182 who were treated between 2017 and 2019.
The incidence of critical head injuries increased from 3.7% in the period 2009-2016 to 7.2% in the period 2017-2019 (P = .007). The incidence of serious neck injuries increased from 12.2% to 27.1% (P = .007) during that period, according to the researchers. The number of days that these patients spent on ventilators more than doubled, from 3.1 to 6.3 (P < .001), they reported.
At the same time, the rate of positive urine drug tests rose sharply, from 0.8% to 1.8% (P < .001) for benzodiazepines and from 1% to 4.9% for opioids (P < .001).
Drug testing of children hospitalized for trauma rose more than threefold, from 6.9% to 23.2% (P < .001). Dr. Ellison’s group was unable to match positive drug screens with patients who came in with injuries.
Dr. Ellison said her research “warrants further evaluation of current policies and protocols targeting substance use in children and adolescents.” To that end, her team is planning to conduct a prospective study in mid 2023 to further illuminate the trends.
“I hope early next year we can put together a group of physicians, pediatric general surgeons, neurosurgeons, and anesthesiologists,” she said. “I want to look at what we can do to reduce the severity of injury.”
She also wants to reach the population that these findings directly affect.
“The next step that we are currently working on is community awareness of the issue,” Dr. Ellison said. “Our trauma institute is partnering with middle school and high school kids to create material to raise awareness.”
Rural Appalachia faces several other endemic problems that affect the health and well-being of children and families, including limited access to health care, poverty, and minimal community support, according to Dr. Ellison. Children and teens in the region who live with parents who abuse opioids are more likely to experience family conflict, mental health challenges, legal troubles, and negative health effects, including physical trauma.
A call to action
Toufic Jildeh, MD, assistant professor of orthopedics, Michigan State University Health Care, East Lansing, who has studied ways to reduce opioid use among surgery patients, called the new findings “alarming.”
After reviewing the study, Dr. Jildeh said that in his opinion, the results support standardized drug testing of children, particularly in the context of severe trauma.
Bruce Bassi, MD, an addiction psychiatrist and owner of TelepsychHealth, a private, online psychiatric practice, agreed. “The main take-home message is that drug screening should be the standard of care for pediatric patients in this region, because it changes the management of those individuals,” Dr. Bassi said.
But identifying these patients is just the first step. “We should continue to educate and raise awareness, not only in the health care system,” Dr. Bassi said. “We also need to let parents know that the possibility of children obtaining access to medications is high.”
The study was independently supported. Dr. Ellison and Dr. Jildeh reported no relevant financial relationships. Dr. Bassi owns a private psychiatry practice called Telepsychhealth but has no other relevant financial relationships.
A version of this article first appeared on Medscape.com.
Numbers of adolescents who vape within 5 minutes of waking jumps
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
FROM JAMA NETWORK OPEN
Nicotine blocks estrogen production in women’s brains
VIENNA – The production of estrogen in the thalamus appears to be curtailed by just one dose of nicotine, equivalent to that in a cigarette, reveals a whole brain analysis of healthy women in the first study of its kind.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
The researchers performed both MRI and positron emission tomography (PET) scans in 10 healthy women using a tracer that binds to aromatase, also known as estrogen synthase.
They found that, following an intranasal spray delivering 1 mg of nicotine, there was a significant reduction in estrogen synthase in both the right and left thalamus.
“For the first time, we can see that nicotine works to shut down the estrogen production mechanism in the brains of women,” said lead researcher Erika Comasco, PhD, department of neuroscience, Uppsala University, Sweden, in a release.
“We were surprised to see that this effect could be seen even with a single dose of nicotine, equivalent to just one cigarette, showing how powerful the effects of smoking are on a woman’s brain.”
Emphasizing the preliminary nature of the study and the need for a larger sample, she added: “We’re still not sure what the behavioral or cognitive outcomes are, only that nicotine acts on this area of the brain.
“However, we note that the affected brain system is a target for addictive drugs, such as nicotine.”
Previous research has revealed that women are less successful at quitting smoking than men, and appear to be more resistant to nicotine replacement therapy, and experience more relapses.
There is evidence to suggest that there is a complex interaction between sex and steroid hormones and the reward effect of nicotine, modulated by the dopaminergic system.
Moreover, women who smoke enter menopause earlier than nonsmokers, and have lower plasma estrogen levels, Dr. Camasco told this news organization.
Dr. Comasco explained that “besides its role in reproductive function and sexual behavior, estrogen has an impact on the brain wherever there are receptors, which is basically regions that are related to emotional regulation, cognitive function, and so on.”
Estrogen, she continued, has two main mechanisms of action, via dopaminergic and serotonergic signaling. However, levels of the hormone cannot be measured directly in the brain.
The researchers therefore turned to estrogen synthase, which regulates the synthesis of estrogen, and is highly expressed in the limbic system, a brain region associated with addiction.
Moreover, estrogen synthase levels can be measured in vivo, and previous animal studies have indicated that nicotine inhibits estrogen synthase.
To investigate its impact in humans, the researchers performed structural MRI and two 11C-cetrozole PET scans in 10 healthy women.
The assessments were performed before and after the nasal administration of 1 mg of nicotine, the dose contained in one cigarette, via two sprays of a nasal spray each containing 0.5 mg of nicotine.
A whole brain analysis was then used to determine changes in nondisplaceable binding potential of 11C-cetrozole to estrogen synthase between the two scans to indicate the availability of the enzyme at the two time points.
The results showed that, at baseline, high availability of estrogen synthase was observed in the thalamus, hypothalamus, and amygdala, with the highest levels in the right and left thalamus.
However, nicotine exposure was associated with a significant reduction in estrogen binding bilaterally in the thalamus when averaged across the participants (P < .01).
Region-of-interest analysis using within-individual voxel-wise comparison confirmed reduced estrogen synthase levels in both the right and left thalamus (P < .05), as well as in the subthalamic area.
Next, Dr. Comasco would like to test the impact of nicotine on estrogen synthase in men.
While men have lower levels of estrogen then women, “the reaction will take place anyway,” she said, although the “impact would be different.”
She would also like to look at the behavioral effects of reductions in estrogen synthase, and look at the effect of nicotine from a functional point of view.
Wim van den Brink, MD, PhD, professor of psychiatry and addiction at the Academic Medical Center, University of Amsterdam, commented that this is an “important first finding.”
“Smoking has many adverse effects in men and in women, but this particular effect of nicotine on the reduction of estrogen production in women was not known before,” he added in the release.
However, he underlined that tobacco addition is a “complex disorder” and it is “unlikely that this specific effect of nicotine on the thalamus explains all the observed differences in the development, treatment, and outcomes between male and female smokers.”
“It is still a long way from a nicotine-induced reduction in estrogen production to a reduced risk of nicotine addiction and negative effects of treatment and relapse in female cigarette smokers, but this work merits further investigation,” Dr. van den Brink said.
The study was funded by the Science for Life Laboratory/Uppsala University.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
VIENNA – The production of estrogen in the thalamus appears to be curtailed by just one dose of nicotine, equivalent to that in a cigarette, reveals a whole brain analysis of healthy women in the first study of its kind.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
The researchers performed both MRI and positron emission tomography (PET) scans in 10 healthy women using a tracer that binds to aromatase, also known as estrogen synthase.
They found that, following an intranasal spray delivering 1 mg of nicotine, there was a significant reduction in estrogen synthase in both the right and left thalamus.
“For the first time, we can see that nicotine works to shut down the estrogen production mechanism in the brains of women,” said lead researcher Erika Comasco, PhD, department of neuroscience, Uppsala University, Sweden, in a release.
“We were surprised to see that this effect could be seen even with a single dose of nicotine, equivalent to just one cigarette, showing how powerful the effects of smoking are on a woman’s brain.”
Emphasizing the preliminary nature of the study and the need for a larger sample, she added: “We’re still not sure what the behavioral or cognitive outcomes are, only that nicotine acts on this area of the brain.
“However, we note that the affected brain system is a target for addictive drugs, such as nicotine.”
Previous research has revealed that women are less successful at quitting smoking than men, and appear to be more resistant to nicotine replacement therapy, and experience more relapses.
There is evidence to suggest that there is a complex interaction between sex and steroid hormones and the reward effect of nicotine, modulated by the dopaminergic system.
Moreover, women who smoke enter menopause earlier than nonsmokers, and have lower plasma estrogen levels, Dr. Camasco told this news organization.
Dr. Comasco explained that “besides its role in reproductive function and sexual behavior, estrogen has an impact on the brain wherever there are receptors, which is basically regions that are related to emotional regulation, cognitive function, and so on.”
Estrogen, she continued, has two main mechanisms of action, via dopaminergic and serotonergic signaling. However, levels of the hormone cannot be measured directly in the brain.
The researchers therefore turned to estrogen synthase, which regulates the synthesis of estrogen, and is highly expressed in the limbic system, a brain region associated with addiction.
Moreover, estrogen synthase levels can be measured in vivo, and previous animal studies have indicated that nicotine inhibits estrogen synthase.
To investigate its impact in humans, the researchers performed structural MRI and two 11C-cetrozole PET scans in 10 healthy women.
The assessments were performed before and after the nasal administration of 1 mg of nicotine, the dose contained in one cigarette, via two sprays of a nasal spray each containing 0.5 mg of nicotine.
A whole brain analysis was then used to determine changes in nondisplaceable binding potential of 11C-cetrozole to estrogen synthase between the two scans to indicate the availability of the enzyme at the two time points.
The results showed that, at baseline, high availability of estrogen synthase was observed in the thalamus, hypothalamus, and amygdala, with the highest levels in the right and left thalamus.
However, nicotine exposure was associated with a significant reduction in estrogen binding bilaterally in the thalamus when averaged across the participants (P < .01).
Region-of-interest analysis using within-individual voxel-wise comparison confirmed reduced estrogen synthase levels in both the right and left thalamus (P < .05), as well as in the subthalamic area.
Next, Dr. Comasco would like to test the impact of nicotine on estrogen synthase in men.
While men have lower levels of estrogen then women, “the reaction will take place anyway,” she said, although the “impact would be different.”
She would also like to look at the behavioral effects of reductions in estrogen synthase, and look at the effect of nicotine from a functional point of view.
Wim van den Brink, MD, PhD, professor of psychiatry and addiction at the Academic Medical Center, University of Amsterdam, commented that this is an “important first finding.”
“Smoking has many adverse effects in men and in women, but this particular effect of nicotine on the reduction of estrogen production in women was not known before,” he added in the release.
However, he underlined that tobacco addition is a “complex disorder” and it is “unlikely that this specific effect of nicotine on the thalamus explains all the observed differences in the development, treatment, and outcomes between male and female smokers.”
“It is still a long way from a nicotine-induced reduction in estrogen production to a reduced risk of nicotine addiction and negative effects of treatment and relapse in female cigarette smokers, but this work merits further investigation,” Dr. van den Brink said.
The study was funded by the Science for Life Laboratory/Uppsala University.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
VIENNA – The production of estrogen in the thalamus appears to be curtailed by just one dose of nicotine, equivalent to that in a cigarette, reveals a whole brain analysis of healthy women in the first study of its kind.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
The researchers performed both MRI and positron emission tomography (PET) scans in 10 healthy women using a tracer that binds to aromatase, also known as estrogen synthase.
They found that, following an intranasal spray delivering 1 mg of nicotine, there was a significant reduction in estrogen synthase in both the right and left thalamus.
“For the first time, we can see that nicotine works to shut down the estrogen production mechanism in the brains of women,” said lead researcher Erika Comasco, PhD, department of neuroscience, Uppsala University, Sweden, in a release.
“We were surprised to see that this effect could be seen even with a single dose of nicotine, equivalent to just one cigarette, showing how powerful the effects of smoking are on a woman’s brain.”
Emphasizing the preliminary nature of the study and the need for a larger sample, she added: “We’re still not sure what the behavioral or cognitive outcomes are, only that nicotine acts on this area of the brain.
“However, we note that the affected brain system is a target for addictive drugs, such as nicotine.”
Previous research has revealed that women are less successful at quitting smoking than men, and appear to be more resistant to nicotine replacement therapy, and experience more relapses.
There is evidence to suggest that there is a complex interaction between sex and steroid hormones and the reward effect of nicotine, modulated by the dopaminergic system.
Moreover, women who smoke enter menopause earlier than nonsmokers, and have lower plasma estrogen levels, Dr. Camasco told this news organization.
Dr. Comasco explained that “besides its role in reproductive function and sexual behavior, estrogen has an impact on the brain wherever there are receptors, which is basically regions that are related to emotional regulation, cognitive function, and so on.”
Estrogen, she continued, has two main mechanisms of action, via dopaminergic and serotonergic signaling. However, levels of the hormone cannot be measured directly in the brain.
The researchers therefore turned to estrogen synthase, which regulates the synthesis of estrogen, and is highly expressed in the limbic system, a brain region associated with addiction.
Moreover, estrogen synthase levels can be measured in vivo, and previous animal studies have indicated that nicotine inhibits estrogen synthase.
To investigate its impact in humans, the researchers performed structural MRI and two 11C-cetrozole PET scans in 10 healthy women.
The assessments were performed before and after the nasal administration of 1 mg of nicotine, the dose contained in one cigarette, via two sprays of a nasal spray each containing 0.5 mg of nicotine.
A whole brain analysis was then used to determine changes in nondisplaceable binding potential of 11C-cetrozole to estrogen synthase between the two scans to indicate the availability of the enzyme at the two time points.
The results showed that, at baseline, high availability of estrogen synthase was observed in the thalamus, hypothalamus, and amygdala, with the highest levels in the right and left thalamus.
However, nicotine exposure was associated with a significant reduction in estrogen binding bilaterally in the thalamus when averaged across the participants (P < .01).
Region-of-interest analysis using within-individual voxel-wise comparison confirmed reduced estrogen synthase levels in both the right and left thalamus (P < .05), as well as in the subthalamic area.
Next, Dr. Comasco would like to test the impact of nicotine on estrogen synthase in men.
While men have lower levels of estrogen then women, “the reaction will take place anyway,” she said, although the “impact would be different.”
She would also like to look at the behavioral effects of reductions in estrogen synthase, and look at the effect of nicotine from a functional point of view.
Wim van den Brink, MD, PhD, professor of psychiatry and addiction at the Academic Medical Center, University of Amsterdam, commented that this is an “important first finding.”
“Smoking has many adverse effects in men and in women, but this particular effect of nicotine on the reduction of estrogen production in women was not known before,” he added in the release.
However, he underlined that tobacco addition is a “complex disorder” and it is “unlikely that this specific effect of nicotine on the thalamus explains all the observed differences in the development, treatment, and outcomes between male and female smokers.”
“It is still a long way from a nicotine-induced reduction in estrogen production to a reduced risk of nicotine addiction and negative effects of treatment and relapse in female cigarette smokers, but this work merits further investigation,” Dr. van den Brink said.
The study was funded by the Science for Life Laboratory/Uppsala University.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
AT ECNP 2022
What’s the best age to stop smoking? Study offers clue
Researchers also quantified the benefit of quitting for those older than 35. The added risk of death associated with smoking was reduced by 90% for those who quit before age 45 and 66% for those who quit at ages 45 to 64.
“The distal nature of the health consequences for young smokers is a challenge for professionals trying to motivate quitting in younger age groups. Without a proximal goal, it is tempting for smokers to abandon a quit attempt with cognitions such as ‘I don’t really need to do it just now,’ ” John P. Pierce, PhD, director for Population Sciences at UC-San Diego’s Moores Cancer Center, wrote in a commentary.
Current smokers were twice as likely to die from any cause during the study, compared with the group researchers called “never smokers,” who were defined as smoking fewer than 100 lifetime cigarettes.
Published in JAMA Network Open, the study involved 551,388 U.S. participants using information collected by the CDC from 1997 to 2018. Researchers collected data for specific causes of death of participants through the end of 2019.
The results echo past findings but also established whether demographic factors such as a smoker’s race and gender impact the benefits of quitting. (In many areas of health research, a person’s race or gender is associated with varying risks.)
The researchers found that the benefits of quitting smoking in reducing risk of death are comparable across demographic groups.
“Among former smokers in each racial and ethnic group, whether male or female, quitting was associated with reductions of approximately 80% of the excess mortality associated with continued smoking,” the authors stated. “These associations were generally consistent for deaths from cancer, cardiovascular disease, and lower respiratory disease.”
The findings are also important for guiding stop-smoking efforts because while smoking nationwide has decreased, the reduction has varied across demographic groups.
“Monitoring the association of smoking with mortality by race, ethnicity, and sex is critical to understanding how the U.S. tobacco epidemic continues to evolve over time and who is most affected by the changes,” the authors stated. “Despite continued decreases in U.S. smoking prevalence in recent decades, progress has not been equal across demographic groups. Recent progress in raising the quit ratio among U.S. ever-smokers overall has been modest, and the quit ratio has been consistently lower among Black and Hispanic ever-smokers than among non-Hispanic White ever-smokers.”
A version of this article first appeared on WebMD.com.
This article was updated 10/27/22.
Researchers also quantified the benefit of quitting for those older than 35. The added risk of death associated with smoking was reduced by 90% for those who quit before age 45 and 66% for those who quit at ages 45 to 64.
“The distal nature of the health consequences for young smokers is a challenge for professionals trying to motivate quitting in younger age groups. Without a proximal goal, it is tempting for smokers to abandon a quit attempt with cognitions such as ‘I don’t really need to do it just now,’ ” John P. Pierce, PhD, director for Population Sciences at UC-San Diego’s Moores Cancer Center, wrote in a commentary.
Current smokers were twice as likely to die from any cause during the study, compared with the group researchers called “never smokers,” who were defined as smoking fewer than 100 lifetime cigarettes.
Published in JAMA Network Open, the study involved 551,388 U.S. participants using information collected by the CDC from 1997 to 2018. Researchers collected data for specific causes of death of participants through the end of 2019.
The results echo past findings but also established whether demographic factors such as a smoker’s race and gender impact the benefits of quitting. (In many areas of health research, a person’s race or gender is associated with varying risks.)
The researchers found that the benefits of quitting smoking in reducing risk of death are comparable across demographic groups.
“Among former smokers in each racial and ethnic group, whether male or female, quitting was associated with reductions of approximately 80% of the excess mortality associated with continued smoking,” the authors stated. “These associations were generally consistent for deaths from cancer, cardiovascular disease, and lower respiratory disease.”
The findings are also important for guiding stop-smoking efforts because while smoking nationwide has decreased, the reduction has varied across demographic groups.
“Monitoring the association of smoking with mortality by race, ethnicity, and sex is critical to understanding how the U.S. tobacco epidemic continues to evolve over time and who is most affected by the changes,” the authors stated. “Despite continued decreases in U.S. smoking prevalence in recent decades, progress has not been equal across demographic groups. Recent progress in raising the quit ratio among U.S. ever-smokers overall has been modest, and the quit ratio has been consistently lower among Black and Hispanic ever-smokers than among non-Hispanic White ever-smokers.”
A version of this article first appeared on WebMD.com.
This article was updated 10/27/22.
Researchers also quantified the benefit of quitting for those older than 35. The added risk of death associated with smoking was reduced by 90% for those who quit before age 45 and 66% for those who quit at ages 45 to 64.
“The distal nature of the health consequences for young smokers is a challenge for professionals trying to motivate quitting in younger age groups. Without a proximal goal, it is tempting for smokers to abandon a quit attempt with cognitions such as ‘I don’t really need to do it just now,’ ” John P. Pierce, PhD, director for Population Sciences at UC-San Diego’s Moores Cancer Center, wrote in a commentary.
Current smokers were twice as likely to die from any cause during the study, compared with the group researchers called “never smokers,” who were defined as smoking fewer than 100 lifetime cigarettes.
Published in JAMA Network Open, the study involved 551,388 U.S. participants using information collected by the CDC from 1997 to 2018. Researchers collected data for specific causes of death of participants through the end of 2019.
The results echo past findings but also established whether demographic factors such as a smoker’s race and gender impact the benefits of quitting. (In many areas of health research, a person’s race or gender is associated with varying risks.)
The researchers found that the benefits of quitting smoking in reducing risk of death are comparable across demographic groups.
“Among former smokers in each racial and ethnic group, whether male or female, quitting was associated with reductions of approximately 80% of the excess mortality associated with continued smoking,” the authors stated. “These associations were generally consistent for deaths from cancer, cardiovascular disease, and lower respiratory disease.”
The findings are also important for guiding stop-smoking efforts because while smoking nationwide has decreased, the reduction has varied across demographic groups.
“Monitoring the association of smoking with mortality by race, ethnicity, and sex is critical to understanding how the U.S. tobacco epidemic continues to evolve over time and who is most affected by the changes,” the authors stated. “Despite continued decreases in U.S. smoking prevalence in recent decades, progress has not been equal across demographic groups. Recent progress in raising the quit ratio among U.S. ever-smokers overall has been modest, and the quit ratio has been consistently lower among Black and Hispanic ever-smokers than among non-Hispanic White ever-smokers.”
A version of this article first appeared on WebMD.com.
This article was updated 10/27/22.
FROM JAMA NETWORK OPEN
Four commonly abused drugs linked with atrial fibrillation
Cocaine, methamphetamine, opioids, and cannabis may independently increase risk of atrial fibrillation (AFib), based on data from almost 24 million people.
While more work is needed to uncover causal links, physicians should be aware that these commonly abused substances could be driving new cases of AFib, reported investigators from the University of California, San Francisco.
“Though alcohol and tobacco smoking have each been associated with a heightened risk of [AFib], relationships between other drug use and [AFib] are poorly understood,” they wrote in European Heart Journal.
Some previous studies have ventured into this terrain, but most focused on fatal arrhythmias, or offered anecdotal evidence. This knowledge gap is particularly concerning for cannabis, the researchers noted, as medical and recreational use are on the rise.
The present analysis included data from 23.5 million adults in California who received care through a hospital, emergency department, or outpatient surgery center during 2005-2015. Based on ICD-9 diagnostic codes, 132,834 of these patients used cannabis, 98,271 used methamphetamines, 48,701 used cocaine, and 10,032 used opiates. Inclusion required lack of AFib at baseline.
Reliance on ICD-9 codes makes the data “quite specific,” but lacking sensitivity, according to principal author Gregory M. Marcus, MD, cardiologist and professor of medicine at UCSF.
“If they were designated as using these drugs, that is very likely true,” Dr. Marcus said in an interview. “But certainly, the absence of any mention of use of these drugs does not exclude the possibility that some people were still using them. That would not create spurious false-positive relationships; if anything, it attenuates existing relationships.”
In other words, using ICD-9 codes reduced the power to detect an association between each drug and AFib, meaning any relationship needed to be sufficiently strong enough to generate a significant result.
At the end of the decade-long study period, 998,747 patients (4.2%) had developed incident AFib. After adjusting for potential confounders and mediators, all four drugs showed significant, independent associations with AFib. Methamphetamines presented the greatest risk (hazard ratio, 1.86%), followed by opiates (HR, 1.74), cocaine (HR, 1.61), and cannabis (HR, 1.35).
“Our findings provide the first evidence utilizing a longitudinal cohort to demonstrate that cannabis use predicts the future onset of AFib,” Dr. Marcus and colleagues wrote.
Dose-response relationships were not detected for any of the substances; however, usage levels were also derived from ICD-9 codes, which may have been insufficient for this purpose, according to the investigators.
Causal mechanisms deserve a closer look
Causal links between AFib and each of the drugs remain unclear. Citing prior research, Dr. Marcus and colleagues explained how methamphetamines are capable of “significant cardiac electrical remodeling,” while cocaine may cause sodium channel dysregulation, and opioids can render atrial myocytes more susceptible to oxidative damage. Although cannabis has previously been linked with hospitalization for arrhythmia, a pharmacologic driver of this phenomenon remains largely unexamined.
“We don’t know for sure precisely what the constituents are that are responsible for our findings,” Dr. Marcus said. “It’s possible that there are some effects that are much more generic, such as inhaling a burned substance. There is good evidence that if you inhale pretty much any sort of particulate matter, that increases inflammation in the body. Inflammation is known to be a trigger for atrial fibrillation.”
Alternatively, all four drugs – whether stimulants or depressants – cause “quite dramatic and often rapid effects on the autonomic nervous system,” Dr. Marcus said, noting that these rapid swings are a known trigger for AFib.
Brian Olshansky, MD, emeritus professor of internal medicine-cardiovascular medicine at the University of Iowa, Iowa City, suggested that nonpharmacologic factors are likely also playing a role.
“All these drugs have slightly different mechanisms of action, so there’s not one mechanism that would explain why all of them would cause atrial fibrillation,” Dr. Olshansky said in an interview. “That does suggest that there’s something else going on, besides just the drug itself. It would be potentially concerning if we were to lay the blame totally on these drugs.”
Dr. Olshansky, who recently coauthored a review of stimulant drugs and arrhythmias, suggested that lifestyle, comorbidities, and drug impurities may have added to the risk of AF.
“[The investigators] did try to correct for that kind of stuff, but it’s very hard to correct for a lot of the issues that may be ongoing with individuals who partake in these drugs,” Dr. Olshansky said in an interview. “They may not be a healthy lot, in general.”
Still, considering previous data linking drugs of abuse with arrhythmias, he said the detected risks were “intriguing,” and deserved a closer look.
“It’s a nice groundbreaking study, with regard to the fact that they showed unique relationships that we don’t completely understand,” Dr. Olshansky said. “It opens up a new opportunity for further investigation.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Olshansky disclosed no relevant competing interests.
Cocaine, methamphetamine, opioids, and cannabis may independently increase risk of atrial fibrillation (AFib), based on data from almost 24 million people.
While more work is needed to uncover causal links, physicians should be aware that these commonly abused substances could be driving new cases of AFib, reported investigators from the University of California, San Francisco.
“Though alcohol and tobacco smoking have each been associated with a heightened risk of [AFib], relationships between other drug use and [AFib] are poorly understood,” they wrote in European Heart Journal.
Some previous studies have ventured into this terrain, but most focused on fatal arrhythmias, or offered anecdotal evidence. This knowledge gap is particularly concerning for cannabis, the researchers noted, as medical and recreational use are on the rise.
The present analysis included data from 23.5 million adults in California who received care through a hospital, emergency department, or outpatient surgery center during 2005-2015. Based on ICD-9 diagnostic codes, 132,834 of these patients used cannabis, 98,271 used methamphetamines, 48,701 used cocaine, and 10,032 used opiates. Inclusion required lack of AFib at baseline.
Reliance on ICD-9 codes makes the data “quite specific,” but lacking sensitivity, according to principal author Gregory M. Marcus, MD, cardiologist and professor of medicine at UCSF.
“If they were designated as using these drugs, that is very likely true,” Dr. Marcus said in an interview. “But certainly, the absence of any mention of use of these drugs does not exclude the possibility that some people were still using them. That would not create spurious false-positive relationships; if anything, it attenuates existing relationships.”
In other words, using ICD-9 codes reduced the power to detect an association between each drug and AFib, meaning any relationship needed to be sufficiently strong enough to generate a significant result.
At the end of the decade-long study period, 998,747 patients (4.2%) had developed incident AFib. After adjusting for potential confounders and mediators, all four drugs showed significant, independent associations with AFib. Methamphetamines presented the greatest risk (hazard ratio, 1.86%), followed by opiates (HR, 1.74), cocaine (HR, 1.61), and cannabis (HR, 1.35).
“Our findings provide the first evidence utilizing a longitudinal cohort to demonstrate that cannabis use predicts the future onset of AFib,” Dr. Marcus and colleagues wrote.
Dose-response relationships were not detected for any of the substances; however, usage levels were also derived from ICD-9 codes, which may have been insufficient for this purpose, according to the investigators.
Causal mechanisms deserve a closer look
Causal links between AFib and each of the drugs remain unclear. Citing prior research, Dr. Marcus and colleagues explained how methamphetamines are capable of “significant cardiac electrical remodeling,” while cocaine may cause sodium channel dysregulation, and opioids can render atrial myocytes more susceptible to oxidative damage. Although cannabis has previously been linked with hospitalization for arrhythmia, a pharmacologic driver of this phenomenon remains largely unexamined.
“We don’t know for sure precisely what the constituents are that are responsible for our findings,” Dr. Marcus said. “It’s possible that there are some effects that are much more generic, such as inhaling a burned substance. There is good evidence that if you inhale pretty much any sort of particulate matter, that increases inflammation in the body. Inflammation is known to be a trigger for atrial fibrillation.”
Alternatively, all four drugs – whether stimulants or depressants – cause “quite dramatic and often rapid effects on the autonomic nervous system,” Dr. Marcus said, noting that these rapid swings are a known trigger for AFib.
Brian Olshansky, MD, emeritus professor of internal medicine-cardiovascular medicine at the University of Iowa, Iowa City, suggested that nonpharmacologic factors are likely also playing a role.
“All these drugs have slightly different mechanisms of action, so there’s not one mechanism that would explain why all of them would cause atrial fibrillation,” Dr. Olshansky said in an interview. “That does suggest that there’s something else going on, besides just the drug itself. It would be potentially concerning if we were to lay the blame totally on these drugs.”
Dr. Olshansky, who recently coauthored a review of stimulant drugs and arrhythmias, suggested that lifestyle, comorbidities, and drug impurities may have added to the risk of AF.
“[The investigators] did try to correct for that kind of stuff, but it’s very hard to correct for a lot of the issues that may be ongoing with individuals who partake in these drugs,” Dr. Olshansky said in an interview. “They may not be a healthy lot, in general.”
Still, considering previous data linking drugs of abuse with arrhythmias, he said the detected risks were “intriguing,” and deserved a closer look.
“It’s a nice groundbreaking study, with regard to the fact that they showed unique relationships that we don’t completely understand,” Dr. Olshansky said. “It opens up a new opportunity for further investigation.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Olshansky disclosed no relevant competing interests.
Cocaine, methamphetamine, opioids, and cannabis may independently increase risk of atrial fibrillation (AFib), based on data from almost 24 million people.
While more work is needed to uncover causal links, physicians should be aware that these commonly abused substances could be driving new cases of AFib, reported investigators from the University of California, San Francisco.
“Though alcohol and tobacco smoking have each been associated with a heightened risk of [AFib], relationships between other drug use and [AFib] are poorly understood,” they wrote in European Heart Journal.
Some previous studies have ventured into this terrain, but most focused on fatal arrhythmias, or offered anecdotal evidence. This knowledge gap is particularly concerning for cannabis, the researchers noted, as medical and recreational use are on the rise.
The present analysis included data from 23.5 million adults in California who received care through a hospital, emergency department, or outpatient surgery center during 2005-2015. Based on ICD-9 diagnostic codes, 132,834 of these patients used cannabis, 98,271 used methamphetamines, 48,701 used cocaine, and 10,032 used opiates. Inclusion required lack of AFib at baseline.
Reliance on ICD-9 codes makes the data “quite specific,” but lacking sensitivity, according to principal author Gregory M. Marcus, MD, cardiologist and professor of medicine at UCSF.
“If they were designated as using these drugs, that is very likely true,” Dr. Marcus said in an interview. “But certainly, the absence of any mention of use of these drugs does not exclude the possibility that some people were still using them. That would not create spurious false-positive relationships; if anything, it attenuates existing relationships.”
In other words, using ICD-9 codes reduced the power to detect an association between each drug and AFib, meaning any relationship needed to be sufficiently strong enough to generate a significant result.
At the end of the decade-long study period, 998,747 patients (4.2%) had developed incident AFib. After adjusting for potential confounders and mediators, all four drugs showed significant, independent associations with AFib. Methamphetamines presented the greatest risk (hazard ratio, 1.86%), followed by opiates (HR, 1.74), cocaine (HR, 1.61), and cannabis (HR, 1.35).
“Our findings provide the first evidence utilizing a longitudinal cohort to demonstrate that cannabis use predicts the future onset of AFib,” Dr. Marcus and colleagues wrote.
Dose-response relationships were not detected for any of the substances; however, usage levels were also derived from ICD-9 codes, which may have been insufficient for this purpose, according to the investigators.
Causal mechanisms deserve a closer look
Causal links between AFib and each of the drugs remain unclear. Citing prior research, Dr. Marcus and colleagues explained how methamphetamines are capable of “significant cardiac electrical remodeling,” while cocaine may cause sodium channel dysregulation, and opioids can render atrial myocytes more susceptible to oxidative damage. Although cannabis has previously been linked with hospitalization for arrhythmia, a pharmacologic driver of this phenomenon remains largely unexamined.
“We don’t know for sure precisely what the constituents are that are responsible for our findings,” Dr. Marcus said. “It’s possible that there are some effects that are much more generic, such as inhaling a burned substance. There is good evidence that if you inhale pretty much any sort of particulate matter, that increases inflammation in the body. Inflammation is known to be a trigger for atrial fibrillation.”
Alternatively, all four drugs – whether stimulants or depressants – cause “quite dramatic and often rapid effects on the autonomic nervous system,” Dr. Marcus said, noting that these rapid swings are a known trigger for AFib.
Brian Olshansky, MD, emeritus professor of internal medicine-cardiovascular medicine at the University of Iowa, Iowa City, suggested that nonpharmacologic factors are likely also playing a role.
“All these drugs have slightly different mechanisms of action, so there’s not one mechanism that would explain why all of them would cause atrial fibrillation,” Dr. Olshansky said in an interview. “That does suggest that there’s something else going on, besides just the drug itself. It would be potentially concerning if we were to lay the blame totally on these drugs.”
Dr. Olshansky, who recently coauthored a review of stimulant drugs and arrhythmias, suggested that lifestyle, comorbidities, and drug impurities may have added to the risk of AF.
“[The investigators] did try to correct for that kind of stuff, but it’s very hard to correct for a lot of the issues that may be ongoing with individuals who partake in these drugs,” Dr. Olshansky said in an interview. “They may not be a healthy lot, in general.”
Still, considering previous data linking drugs of abuse with arrhythmias, he said the detected risks were “intriguing,” and deserved a closer look.
“It’s a nice groundbreaking study, with regard to the fact that they showed unique relationships that we don’t completely understand,” Dr. Olshansky said. “It opens up a new opportunity for further investigation.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Olshansky disclosed no relevant competing interests.
FROM EUROPEAN HEART JOURNAL