User login
The lives of drug users are more important than stopping drug use
One quiet afternoon at a mobile outreach clinic, where I had been working on the West Side of Chicago, a young man without a home to go to, and clothes he kept as clean as he could, came to get a refill of buprenorphine. The drug, which works on the same opioid receptors as heroin, was helping him feel normal. It was also probably helping to keep him alive, as a study found that taking it after an overdose was associated with a one-third reduction in all-cause mortality.
He was still using drugs, but now only a few days a week instead of multiple times a day. He had put on some weight and looked visibly healthier.
I gave him his prescription and thanked him for coming back. As he got up to leave, he turned to our outreach team and said, “Thank you for being here and caring about us. Because a lot of people don’t. They don’t care if we live or die.”
But a lot of people do care and are still failing him and others who use drugs. When I first started treating addictions, I was taught to cut people like him off treatment. We could give patients a medication, but they had to follow the rules, first and foremost to stop using drugs. Keep using, even if you were using less and your health was improving, and I would have to dismiss you from the practice. This was the kind of “tough love” that many doctors have been taught, and are, in many cases, still being taught today. Even though we know that this approach does not work.
For too long, doctors, nurses, caregivers, and the broader American public have favored abstinence only treatment, criminalization, and prohibition. The proof that this approach does not work is in the spectacular overdose crisis we are experiencing in this country, as CDC data documents. While we continue to blame drugs like fentanyl and methamphetamine (and thirty years ago, crack and heroin), we fail to see how our approach contributes to these overdose deaths.
For instance, treating with buprenorphine or methadone was associated with reductions in overdose and serious opioid-related acute care use compared with detox alone. But only one in three centers offer these medications, the gold standard of care. We continue to imprison people who use drugs, even though we have known for 15 years that the risk of overdose is exponentially higher in the first few weeks after people leave prison.
Patients who use opioids safely for decades are also arbitrarily being forced off their prescriptions because too many clinicians equate opioid use with opioid addiction, despite the fact that opioid tapering was associated with increased rates of overdose. And prohibition has led to a change in the drug supply that is now dominated by methamphetamine and fentanyl, substances far more deadly than the ones we demonized and seized decades ago.
We have tried and failed to rid the country of many drugs. We never will. Human beings will seek mind-altering substances, from caffeine to alcohol to hallucinogens. But we can stop the grim massacre of people who use drugs. We have the tools. What we lack is moral clarity.
In lecture after lecture of physicians and medical students, I hear the refrain that patients are not often “ready” for treatment. There’s nothing that doctors can do, they say, if the patient doesn’t want help. Yet they do not examine why that may be. Are we offering the help that they need? Time and again I have seen that if we meet people where they are, we can help virtually anyone.
Tools for fighting the opioid crisis
The reason our policies have failed is because we have not confronted a simple truth: We must care more about saving and improving the lives of people who use drugs than stopping drug use. With that framework, the approach is clear and multifactorial. First, we must make methadone treatment less draconian. Methadone, like buprenorphine, has been associated with a large reduction in all-cause mortality for people who have a history of overdose.
In this country, to access it, however, you must go to a clinic daily for the first 90 days of treatment and jump through hoops that often make it impossible to have a job and accomplish other goals. Other countries have safely moved methadone to primary care offices, and so should we. The other main drug for opioid addiction, buprenorphine, requires a special license to prescribe, even though it is far safer than other opioids that any physician can prescribe. This requirement has been weakened, but it should be removed entirely.
Moreover, the DEA conducts regular audits of buprenorphine prescribers in an effort to prevent diversion, discouraging doctors from prescribing it. This despite the fact that it is almost impossible to overdose on buprenorphine alone, and a study suggests that diversion of buprenorphine is associated with a lower overdose risk in a community by making the medication available to more people who can benefit.
Treatment is not the only way we can help people using drugs. Naloxone, an overdose rescue drug, should be available in every first aid kit and free at pharmacies without a prescription. Clean needles and pipes for people who use can help prevent infections, potentially mitigating the severity of outbreaks. Overdose prevention sites, where people can safely use, should be opened across the country.
We need accessible drug testing so people do not accidentally overdose and so they can know what they are using. We should stop sending people to jail for drug use when we know that it is too often tantamount to a death sentence, and offer effective medical treatment to anyone who is incarcerated.
All these interventions remain controversial within medicine and in the larger culture. If our metric, however, is lives saved and harm avoided, these are sure-fire approaches.
Right now, I am focused on clinical care and changing the culture of medicine, where we have opportunities to help but too often do harm instead. The impact of a shift in mentality would be huge, because we would realize there is no one we cannot help, only millions of people we do not listen to. But this is a national crisis and requires a national response. Until we are clear that our goal should and must be to stem the mounting deaths and harms above all else, we will continue to fail.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures.
One quiet afternoon at a mobile outreach clinic, where I had been working on the West Side of Chicago, a young man without a home to go to, and clothes he kept as clean as he could, came to get a refill of buprenorphine. The drug, which works on the same opioid receptors as heroin, was helping him feel normal. It was also probably helping to keep him alive, as a study found that taking it after an overdose was associated with a one-third reduction in all-cause mortality.
He was still using drugs, but now only a few days a week instead of multiple times a day. He had put on some weight and looked visibly healthier.
I gave him his prescription and thanked him for coming back. As he got up to leave, he turned to our outreach team and said, “Thank you for being here and caring about us. Because a lot of people don’t. They don’t care if we live or die.”
But a lot of people do care and are still failing him and others who use drugs. When I first started treating addictions, I was taught to cut people like him off treatment. We could give patients a medication, but they had to follow the rules, first and foremost to stop using drugs. Keep using, even if you were using less and your health was improving, and I would have to dismiss you from the practice. This was the kind of “tough love” that many doctors have been taught, and are, in many cases, still being taught today. Even though we know that this approach does not work.
For too long, doctors, nurses, caregivers, and the broader American public have favored abstinence only treatment, criminalization, and prohibition. The proof that this approach does not work is in the spectacular overdose crisis we are experiencing in this country, as CDC data documents. While we continue to blame drugs like fentanyl and methamphetamine (and thirty years ago, crack and heroin), we fail to see how our approach contributes to these overdose deaths.
For instance, treating with buprenorphine or methadone was associated with reductions in overdose and serious opioid-related acute care use compared with detox alone. But only one in three centers offer these medications, the gold standard of care. We continue to imprison people who use drugs, even though we have known for 15 years that the risk of overdose is exponentially higher in the first few weeks after people leave prison.
Patients who use opioids safely for decades are also arbitrarily being forced off their prescriptions because too many clinicians equate opioid use with opioid addiction, despite the fact that opioid tapering was associated with increased rates of overdose. And prohibition has led to a change in the drug supply that is now dominated by methamphetamine and fentanyl, substances far more deadly than the ones we demonized and seized decades ago.
We have tried and failed to rid the country of many drugs. We never will. Human beings will seek mind-altering substances, from caffeine to alcohol to hallucinogens. But we can stop the grim massacre of people who use drugs. We have the tools. What we lack is moral clarity.
In lecture after lecture of physicians and medical students, I hear the refrain that patients are not often “ready” for treatment. There’s nothing that doctors can do, they say, if the patient doesn’t want help. Yet they do not examine why that may be. Are we offering the help that they need? Time and again I have seen that if we meet people where they are, we can help virtually anyone.
Tools for fighting the opioid crisis
The reason our policies have failed is because we have not confronted a simple truth: We must care more about saving and improving the lives of people who use drugs than stopping drug use. With that framework, the approach is clear and multifactorial. First, we must make methadone treatment less draconian. Methadone, like buprenorphine, has been associated with a large reduction in all-cause mortality for people who have a history of overdose.
In this country, to access it, however, you must go to a clinic daily for the first 90 days of treatment and jump through hoops that often make it impossible to have a job and accomplish other goals. Other countries have safely moved methadone to primary care offices, and so should we. The other main drug for opioid addiction, buprenorphine, requires a special license to prescribe, even though it is far safer than other opioids that any physician can prescribe. This requirement has been weakened, but it should be removed entirely.
Moreover, the DEA conducts regular audits of buprenorphine prescribers in an effort to prevent diversion, discouraging doctors from prescribing it. This despite the fact that it is almost impossible to overdose on buprenorphine alone, and a study suggests that diversion of buprenorphine is associated with a lower overdose risk in a community by making the medication available to more people who can benefit.
Treatment is not the only way we can help people using drugs. Naloxone, an overdose rescue drug, should be available in every first aid kit and free at pharmacies without a prescription. Clean needles and pipes for people who use can help prevent infections, potentially mitigating the severity of outbreaks. Overdose prevention sites, where people can safely use, should be opened across the country.
We need accessible drug testing so people do not accidentally overdose and so they can know what they are using. We should stop sending people to jail for drug use when we know that it is too often tantamount to a death sentence, and offer effective medical treatment to anyone who is incarcerated.
All these interventions remain controversial within medicine and in the larger culture. If our metric, however, is lives saved and harm avoided, these are sure-fire approaches.
Right now, I am focused on clinical care and changing the culture of medicine, where we have opportunities to help but too often do harm instead. The impact of a shift in mentality would be huge, because we would realize there is no one we cannot help, only millions of people we do not listen to. But this is a national crisis and requires a national response. Until we are clear that our goal should and must be to stem the mounting deaths and harms above all else, we will continue to fail.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures.
One quiet afternoon at a mobile outreach clinic, where I had been working on the West Side of Chicago, a young man without a home to go to, and clothes he kept as clean as he could, came to get a refill of buprenorphine. The drug, which works on the same opioid receptors as heroin, was helping him feel normal. It was also probably helping to keep him alive, as a study found that taking it after an overdose was associated with a one-third reduction in all-cause mortality.
He was still using drugs, but now only a few days a week instead of multiple times a day. He had put on some weight and looked visibly healthier.
I gave him his prescription and thanked him for coming back. As he got up to leave, he turned to our outreach team and said, “Thank you for being here and caring about us. Because a lot of people don’t. They don’t care if we live or die.”
But a lot of people do care and are still failing him and others who use drugs. When I first started treating addictions, I was taught to cut people like him off treatment. We could give patients a medication, but they had to follow the rules, first and foremost to stop using drugs. Keep using, even if you were using less and your health was improving, and I would have to dismiss you from the practice. This was the kind of “tough love” that many doctors have been taught, and are, in many cases, still being taught today. Even though we know that this approach does not work.
For too long, doctors, nurses, caregivers, and the broader American public have favored abstinence only treatment, criminalization, and prohibition. The proof that this approach does not work is in the spectacular overdose crisis we are experiencing in this country, as CDC data documents. While we continue to blame drugs like fentanyl and methamphetamine (and thirty years ago, crack and heroin), we fail to see how our approach contributes to these overdose deaths.
For instance, treating with buprenorphine or methadone was associated with reductions in overdose and serious opioid-related acute care use compared with detox alone. But only one in three centers offer these medications, the gold standard of care. We continue to imprison people who use drugs, even though we have known for 15 years that the risk of overdose is exponentially higher in the first few weeks after people leave prison.
Patients who use opioids safely for decades are also arbitrarily being forced off their prescriptions because too many clinicians equate opioid use with opioid addiction, despite the fact that opioid tapering was associated with increased rates of overdose. And prohibition has led to a change in the drug supply that is now dominated by methamphetamine and fentanyl, substances far more deadly than the ones we demonized and seized decades ago.
We have tried and failed to rid the country of many drugs. We never will. Human beings will seek mind-altering substances, from caffeine to alcohol to hallucinogens. But we can stop the grim massacre of people who use drugs. We have the tools. What we lack is moral clarity.
In lecture after lecture of physicians and medical students, I hear the refrain that patients are not often “ready” for treatment. There’s nothing that doctors can do, they say, if the patient doesn’t want help. Yet they do not examine why that may be. Are we offering the help that they need? Time and again I have seen that if we meet people where they are, we can help virtually anyone.
Tools for fighting the opioid crisis
The reason our policies have failed is because we have not confronted a simple truth: We must care more about saving and improving the lives of people who use drugs than stopping drug use. With that framework, the approach is clear and multifactorial. First, we must make methadone treatment less draconian. Methadone, like buprenorphine, has been associated with a large reduction in all-cause mortality for people who have a history of overdose.
In this country, to access it, however, you must go to a clinic daily for the first 90 days of treatment and jump through hoops that often make it impossible to have a job and accomplish other goals. Other countries have safely moved methadone to primary care offices, and so should we. The other main drug for opioid addiction, buprenorphine, requires a special license to prescribe, even though it is far safer than other opioids that any physician can prescribe. This requirement has been weakened, but it should be removed entirely.
Moreover, the DEA conducts regular audits of buprenorphine prescribers in an effort to prevent diversion, discouraging doctors from prescribing it. This despite the fact that it is almost impossible to overdose on buprenorphine alone, and a study suggests that diversion of buprenorphine is associated with a lower overdose risk in a community by making the medication available to more people who can benefit.
Treatment is not the only way we can help people using drugs. Naloxone, an overdose rescue drug, should be available in every first aid kit and free at pharmacies without a prescription. Clean needles and pipes for people who use can help prevent infections, potentially mitigating the severity of outbreaks. Overdose prevention sites, where people can safely use, should be opened across the country.
We need accessible drug testing so people do not accidentally overdose and so they can know what they are using. We should stop sending people to jail for drug use when we know that it is too often tantamount to a death sentence, and offer effective medical treatment to anyone who is incarcerated.
All these interventions remain controversial within medicine and in the larger culture. If our metric, however, is lives saved and harm avoided, these are sure-fire approaches.
Right now, I am focused on clinical care and changing the culture of medicine, where we have opportunities to help but too often do harm instead. The impact of a shift in mentality would be huge, because we would realize there is no one we cannot help, only millions of people we do not listen to. But this is a national crisis and requires a national response. Until we are clear that our goal should and must be to stem the mounting deaths and harms above all else, we will continue to fail.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures.
Medications for Opioid Use Disorder Program in a VA Emergency Department
Opioid use disorder (OUD) is a public health crisis significantly affecting veterans. A substantial increase in veterans diagnosed with OUD has occurred, nearly tripling from 25,031 in 2003 to 69,142 in 2017
For patients with active OUD, medications for opioid use disorder (MOUD) reduce the risk of overdose and all-cause mortality.3 In 2009, the US Department of Veterans Affairs (VA) and Department of Defense (DoD) published clinical practice guidelines for substance use disorders that strongly recommended MOUD with either buprenorphine or methadone as a first-line treatment. In 2015 updated guidelines encouraged buprenorphine initiation in primary care settings.4,5 This was followed by an academic detailing campaign designed to encourage VA clinicians to initiate MOUD.1 Despite this institutional support, MOUD remains underutilized within the VA, with widely variable rates of prescribing among VA sites.1
Efforts to further expand MOUD cultivated interest in administering buprenorphine in VA emergency departments (EDs). Patients with OUD often use the ED for same-day care, providing opportunities to initiate buprenorphine in the ED 24 hours, 7 days per week. This has been especially true during the COVID-19 pandemic during which reliable access to usual recovery services has been disrupted and EDs have served as a safety net.6
Buprenorphine’s safety profile and prolonged effect duration make it superior to other MOUD options for ED administration. As a partial opioid agonist, buprenorphine is unlikely to cause significant sedation or respiratory depression compared with full agonists like methadone. This is known as the ceiling effect. Additionally, at higher doses, buprenorphine’s effects can last for about 3 days, potentially obviating the need for repeat dosing. D’Onofrio and colleagues seminal 2015 paper conceptually proved the feasibility and value of initiating buprenorphine in the ED; patients who received ED initiation therapy were more likely to be engaged in addiction treatment 30 days after their visit and have reduced rates of illicit opioid drug use.7 Such ED harm-reduction strategies are increasingly recognized as essential, given that 1 in 20 patients treated for a nonfatal opioid overdose in an ED will die within 1 year of their visit, many within 2 days.8 Finally, a significant barrier faced by physicians wanting to administer or prescribe buprenorphine for patients with OUD has been the special licensing required by the Drug Enforcement Administration Drug Addiction Treatment Act of 2000, also known as an X-waiver. A notable exception to this X-waiver requirement is the 72-hour rule, which allows nonwaivered practitioners to administer (but not prescribe for home use) buprenorphine to a patient to relieve acute withdrawal symptoms for up to 72 hours while arranging for specialist referral.Under the 72-hour rule, ED clinicians have a unique opportunity to treat patients experiencing acute withdrawal symptoms and bridge them to specialty care, without the burden of an X-waiver requirement.
The VA Greater Los Angeles Healthcare System (VAGLAHS), therefore, developed and implemented a program to administer buprenorphine in the ED to bridge patients with OUD to an appointment with substance use disorder (SUD) services. We describe our development, implementation and evaluation of this program protocol as a model for other VA EDs. This project was determined to be quality improvement (nonresearch) by the VAGLAHS Institutional Review Board.
ED MOUD Program
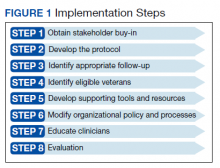
We engaged in a 2-month (January-March 2019) preimplementation process during which we (1) obtained stakeholder buy-in; (2) developed a protocol and supporting resources and tools; (3) worked with stakeholders to enact local organizational policy and process modifications; and (4) educated practitioners.
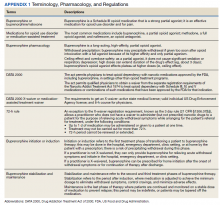
Appendix 1 provides an overview of MOUD terminology, pharmacology, and regulations. We developed an 8-step program implementation plan for the ED MOUD program (Figure 1).
Obtaining Stakeholder Buy-in
Two ED physician champions (MC, JH) organized all activities. Champions obtained stakeholder buy-in from clinical and administrative leaders as well as from frontline personnel in OUD specialty care, ED, and pharmacy services. ED social workers and clerks who schedule post-ED appointments also were engaged. These stakeholders emphasized the importance of fitting the developed protocol into the existing ED workflows as well as minimizing additional resources required to initiate and maintain the program.
We ascertained that in fiscal year 2018, VAGLAHS had 156 ED visits with International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes related to OUD for 108 unique patients. Based on these data and in consultation with OUD specialty care, we determined that the potential number of referrals to the SUD clinic would be manageable with existing resources. Additionally, there was consensus that most opioid withdrawal patients could be treated in the urgent care portion of our ED since these patients generally do not require special monitoring. This consideration was important for obtaining ED stakeholder buy-in and for planning protocol logistics.
Developing the Protocol
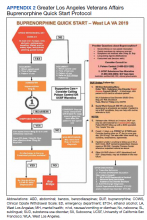
We customized resources created by CalBridge Behavioral Health Navigator Program (CA Bridge), formerly called ED Bridge, a program of the Public Health Institute in Oakland, California, funded through California Department of Health Care Services. CA Bridge offers technical assistance and support for hospitals as well as guidance and tools for establishing processes for EDs providing buprenorphine prescriptions for the management of acute opioid withdrawal and serving as a bridge to follow-up care in SUD clinics.9 We also reviewed protocols described by D’Onofrio and colleagues. With iterative input from stakeholders, we created a protocol concretely delineating each process and corresponding responsible party with the overall aim of removing potential barriers to MOUD initiation and follow-up (Appendix 2).
Identifying Appropriate Follow-up
To operationalize protocol implementation, we built on VA’s Emergency Department Rapid Access Clinic (ED-RAC) process, a mechanism for scheduling appointments for post-ED specialty follow-up care. This process facilitated veterans’ access to urgent specialty care follow-up after ED visits by scheduling appointments prior to ED discharge.10 For the ED MOUD program, we adapted the ED-RAC process to schedule appointments in SUD clinic prior to ED discharge. These appointments allowed patients to be seen by an SUD clinician within 72 hours of ED discharge. This step was critical to working within the 72-hour rule without relying on X-waiver licensing of ED clinicians. Alternatively, as was previous practice, per patient preference, patients were also referred to non-VA residential rehabilitation services if the facility had capacity and patients met criteria for admission.
Identification of Eligible Veterans
Target patients were those primarily presenting with a request for treatment of opioid dependence or withdrawal. Patients were not actively screened for OUD. Clinicians diagnosed and assessed for OUD as per their usual practice. Patients with OUD who presented to the ED for other reasons were assessed, at clinician discretion, for their interest in receiving MOUD. If patients presented in moderate-to-severe withdrawal (eg, Clinical Opiate Withdrawal Scale [COWS] ≥ 8), buprenorphine was initiated in the ED. These patients were subsequently referred to either the local SUD clinic or to a residential treatment center. Patients presenting with a COWS score < 8 were referred to the outpatient SUD clinic or residential treatment centers without initiating buprenorphine from the ED. The SUD clinic or residential treatment centers could offer buprenorphine or other MOUD options. From the ED, prescribing buprenorphine for patients to self-initiate at home was not available as this required an X-waivered prescriber, which were limited during the program time frame.
Support Tools and Resources
To facilitate ED clinicians using the protocol, we worked with a programmer experienced with the Computerized Patient Record System, the VA electronic health record (EHR), to create electronic order menu sets that directed clinicians to the protocol and educational materials (Appendix 3). These menus are readily accessible and embedded into the ED clinician workflow. The menus highlight key elements of the protocol, including indications for initiation, contraindications, recommended dosing with quick orders, and how to obtain follow-up for the patient. Links also are provided to the protocol and patient discharge handouts, including the CA Bridge website.
Organizational Policy and Processes
Before implementing the developed protocol, we worked with stakeholders to modify organizational policies and processes. Our pharmacy agreed to stock buprenorphine in the ED to make it readily available. EHR restrictions that historically prohibited ordering buprenorphine for ED administration by nonwaivered clinicians were modified. Additionally, our chief of staff, pharmacy, and credentialing department agreed that physicians did not need to apply for additional delineated privileges.
Clinician Education
The final preparation step was educating clinicians and other protocol users. The VAGLAHS SUD chief presented a lecture and answered questions about MOUD to core ED faculty about the rising prevalence of OUD and use of buprenorphine as a recommended treatment.
Evaluation
To assess adherence to the developed protocol, we conducted a retrospective health record review of all ED visits March 1 to October 25, 2019, in which the patient had OUD and may have qualified for MOUD. To do this, we identified (1) ED visits with an OUD ICD-10 code as a primary or secondary diagnoses; (2) ED referrals to outpatient SUD treatment; and/or (3) ED visits in which buprenorphine was given or prescribed. We included the latter 2 criteria as application of ICD-10 codes for OUD care was inconsistent. Visits were excluded if patients did not have OUD, had OUD in remission, were already maintained on a stable MOUD regimen and no longer using illicit drugs or craving additional opioids, or were presenting solely for a refill or administration of a missed dose. Patients who relapsed were categorized as unstable. Visits were excluded if the patient was admitted to the hospital or left against medical advice. Patients on MOUD who had relapsed or requested a change in MOUD treatment were included. For all included visits, 2 ED physicians (MC, JH) reviewed the ED clinician and nursing notes, pharmacy and referral records, diagnostic codes, and veteran demographics.
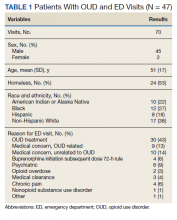
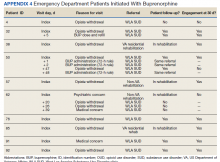
In the evaluation, there were 130 visits with 92 unique veterans meeting inclusion criteria. The final sample included 70 visits with 47 unique veterans (Table 1). Of note, 24 (53%) patients self-identified as homeless or were engaged with VA housing services. Twelve veterans had multiple ED visits (7 patients with 2 visits; 5 patients with ≥ 3 visits). In 30 (43%) visits the veteran’s primary reason for seeking ED care was to obtain treatment for opioid withdrawal or receive MOUD. Type of opiate used was specified in 58% of visits; of these, 69% indicated heroin use and 17% prescription medications. Buprenorphine was initiated in the ED in 18 (26%) visits for 10 veterans. Appendix 4 outlines the clinical course and follow-up after these visits. Some veterans returned to the ED for buprenorphine redosing per the 72-hour rule. SUD clinic appointments were provided in 11 visits, and direct transfer to an inpatient rehabilitation center was arranged in 4 visits. In 42 (60%) visits, across 32 unique veterans, buprenorphine was not given in the ED, but patients were referred for SUD treat
A majority of veterans who received buprenorphine and a referral for an SUD appointment went to their initial SUD follow-up appointment and had ongoing engagement in addiction care 30 days after their index ED visit. Among veterans who did not receive buprenorphine but were referred for SUD treatment, about half went to their SUD appointments and about 1 in 5 had ongoing engagement in addiction care at 30 days after the index ED visit. Of note, 2 patients who received referrals died within 1 year of their index ED visit. The cause of death for one patient was an overdose; the other was unspecified.
DISCUSSION
We developed the ED MOUD program as a bridge to SUD specialty care. Our 8 implementation steps can serve as a model for implementing programs at other VA EDs. We demonstrated feasibility, high follow-up rates, and high retention in treatment.
Patients who received ED buprenorphine initiation were more likely to follow up and had higher rates of ongoing engagement at 30 days than did those who received only a clinic referral. In a similar Canadian study, buprenorphine was initiated in the ED, and patients followed up as a walk-in for addiction services; however, only 54% of patients presented to this initial follow-up.11 Our higher initial follow-up rate may be due to our ability to directly schedule clinic appointments. Our 70% 30-day follow-up rate is comparable, but slightly lower than the 2015 D’Onofrio and colleagues study in which 78% of patients remained engaged at 30 days.7 A possible reason is that in the D’Onofrio and colleagues study, all study physicians obtained X-waiver training and were able to prescribe buprenorphine after ED initiation or for self-initiation at home. X-waiver training was not required of our clinicians, and none of our patients were offered a prescription for self-initiation.
Our program demonstrates that it is feasible to develop a protocol without X-waiver licensing. This program provides a supportive framework for the use of MOUD and allows nonspecialists to gain experience and confidence in using buprenorphine. Any clinician could administer buprenorphine in the ED, and patients could be bridged at later ED visits until follow-up with a specialist. Of note, only a small percentage of the total visits for buprenorphine initiation required multiple daily visits for buprenorphine. Appointments with the specialist were assured to fall within a 72-hour window.
Our program has some limitations. First, the number of patients who were candidates for our ED MOUD program was small. In our 7-month review, only 47 patients were identified as potential candidates for MOUD treatment across 70 visits, and only 10 were initiated in the ED. Second, all patients were not actively screened for OUD. There was potential for missing eligible veterans as inclusion criteria relied on clinicians both recognizing OUD and manually entering a correct diagnostic code. We attempted to mitigate this by also reviewing all ED referrals to the SUD clinic and all patients who received buprenorphine in the ED. In addition, we do not have data on preimplementation rates of follow-up for comparison.
Future Directions
More than half of our patients did not receive ED buprenorphine initiation because they were not in moderate or severe withdrawal (COWS ≥ 8) similar to 57% of patients cited in the D’Onofrio and colleagues study.7 Teaching veterans how to start buprenorphine at home could greatly expand enrollment. However, this requires a prescription from an X-waiver licensed clinician. In 2021, the US Department of Health and Human Services removed the 8-hour training requirement for obtaining an X-waiver.12 However, clinicians are still required to apply for licensing. Eliminating the X-waiver requirement, as proposed by D’Onofrio and colleagues in a 2021 editorial, would have allowed all clinicians to offer home initiation.13
Previous studies suggest that despite the ability to provide a prescription, clinicians may be reluctant to offer home initiation.14–17 In a national VA 2019 survey, many emergency medicine physicians believe that SUD care is not in their scope of practice, as Dieujuste and colleagues described in Federal Practitioner.14 Although it is likely some attitudes have changed with the increased visibility of ED MOUD programs, there is still much work to be done to change perceptions.
Another area for improvement is screening for OUD in the ED to better reveal MOUD candidates. Missed opportunities (neither referral nor treatment offered) occurred in 21% of our visits. D’Onofrio and colleagues identified 66% of patients by screening all ED patients.7 Although universal screening for SUD in routine health care settings has been recommended, 2021 VA guidelines state that there is insufficient evidence to recommend universal screening.18-20 There are also limited data on the best screening tool for OUD in the ED.21 Further research on how to effectively and efficiently identify OUD patients in the ED is needed.
Conclusions
With minimal resource allocation, we started the program to offer MOUD with buprenorphine for patients with OUD at a VA ED and provided addiction treatment follow-up. This program, the first of its kind within VA, can be modeled and expanded to other VA facilities. Given increasing numbers of fatal opioid overdose, and significant adverse impacts of the COVID-19 pandemic on the OUD crisis, developing local and national strategies to treat OUD is essential. Future steps include improved screening and expanding capacity to offer home initiation by increasing the number of X-waiver ED clinicians.6
Acknowledgments
Thank you to Jeffrey Balsam, PharmD, BCPS, Veterans Affairs Greater Los Angeles Clinical Applications Coordinator for his contributions in creating a Computerized Patient Record System opioid use disorder screening tool. Thank you to Gracielle Tan, MD, Veterans Affairs Greater Los Angeles Health Science Specialist for her administrative assistance in manuscript preparation.
1. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: historical perspective, lessons learned, and next steps. Subst Abuse. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
2. Bohnert ASB, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs health system. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. Ma J, Bao Y-P, Wang R-J, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1968-1983. doi:10.1038/s41380-018-0094-5
4. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 2.0. US Department of Veterans Affairs; 2009.
5. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 3.0. US Department of Veterans Affairs. 2015. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
6. Hulsey J, Mellis A, Kelly B. COVID-19 pandemic impact on patients, families and individuals in recovery from substance use disorder. Accessed July 7, 2021. https://www.addictionpolicy.org/covid19-report
7. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opiod dependence. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
8. Weiner SG, Baker O, Bernson D, Schuur JD. One-year mortality of patients after emergency department treatment for non-fatal opioid overdose. Ann Emerg Med. 2020;75(1):13-17. doi:10.1016/j.annemergmed.2019.04.020
9. CA Bridge. Updated 2021. Accessed July 1, 2022. https://cabridge.org
10. Penney L, Miake-Lye I, Lewis D, et al. Proceedings from the 11th annual conference on the science of dissemination and implementation: S72 spreading VA’s emergency department-rapid access clinics (ED-RAC) intervention: key factors for success. Implementation Sci. 2019;14(suppl 1). doi:10.1186/s13012-019-0878-2
11. Hu T, Snider-Alder M, Nijmeh L, Pyle A. Buprenorphine/naloxone induction in a Canadian emergency department with rapid access to community-based addictions providers. CJEM. 2019;21(4):492-498. doi:10.1017/cem.2019.24
12. US Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Federal Register. Accessed July 1, 2022. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
13. D’Onofrio G, Melnick ER, Hawk KF. Improve access to care for opioid use disorder: a call to eliminate the x-waiver requirement now. Ann Emerg Med. 2021;78(2):220-222. doi:10.1016/j.annemergmed.2021.03.023
14. Dieujuste N, Johnson-Koenke R, Celedon M, et al. Provider perceptions of opioid safety measures in VHA emergency department and urgent care centers. Fed Pract. 2021;38(9):412-419. doi:10.12788/fp.0179
15. Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and faciliatators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw Open. 2020;3(5):e204561. doi:10.1001/jamanetworkopen.2020.4561
16. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. doi:10.1016/j.ajem.2019.02.025
17. Srivastava A, Kahan M, Leece P, McAndrew A. Buprenorphine unobserved “home” induction: a survey of Ontario’s addiction physicians. Addic Sci Clin Pract. 2019;14(1):18. doi:10.1186/s13722-019-0146-4
18. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 4.0. US Department of Veterans Affairs. 2021. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPG.pdf
19. Patnode CD, Perdue LA, Rushkin M, et al. Screening for unhealthy drug use updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(22):2310-2338. doi:10.1001/jama.2019.21381
20. Coles S, Vosooney A. Evidence lacking to support universal unhealthy drug use screening. Am Fam Physician. 2021;103(2):72-73.
21. Sahota PK, Sharstry S, Mukamel DB, et al. Screening emergency department patients for opioid drug use: a qualitative systematic review. Addict Behav. 2018;85:139-146. doi:10.1016/j.addbeh.2018.05.022
Opioid use disorder (OUD) is a public health crisis significantly affecting veterans. A substantial increase in veterans diagnosed with OUD has occurred, nearly tripling from 25,031 in 2003 to 69,142 in 2017
For patients with active OUD, medications for opioid use disorder (MOUD) reduce the risk of overdose and all-cause mortality.3 In 2009, the US Department of Veterans Affairs (VA) and Department of Defense (DoD) published clinical practice guidelines for substance use disorders that strongly recommended MOUD with either buprenorphine or methadone as a first-line treatment. In 2015 updated guidelines encouraged buprenorphine initiation in primary care settings.4,5 This was followed by an academic detailing campaign designed to encourage VA clinicians to initiate MOUD.1 Despite this institutional support, MOUD remains underutilized within the VA, with widely variable rates of prescribing among VA sites.1
Efforts to further expand MOUD cultivated interest in administering buprenorphine in VA emergency departments (EDs). Patients with OUD often use the ED for same-day care, providing opportunities to initiate buprenorphine in the ED 24 hours, 7 days per week. This has been especially true during the COVID-19 pandemic during which reliable access to usual recovery services has been disrupted and EDs have served as a safety net.6
Buprenorphine’s safety profile and prolonged effect duration make it superior to other MOUD options for ED administration. As a partial opioid agonist, buprenorphine is unlikely to cause significant sedation or respiratory depression compared with full agonists like methadone. This is known as the ceiling effect. Additionally, at higher doses, buprenorphine’s effects can last for about 3 days, potentially obviating the need for repeat dosing. D’Onofrio and colleagues seminal 2015 paper conceptually proved the feasibility and value of initiating buprenorphine in the ED; patients who received ED initiation therapy were more likely to be engaged in addiction treatment 30 days after their visit and have reduced rates of illicit opioid drug use.7 Such ED harm-reduction strategies are increasingly recognized as essential, given that 1 in 20 patients treated for a nonfatal opioid overdose in an ED will die within 1 year of their visit, many within 2 days.8 Finally, a significant barrier faced by physicians wanting to administer or prescribe buprenorphine for patients with OUD has been the special licensing required by the Drug Enforcement Administration Drug Addiction Treatment Act of 2000, also known as an X-waiver. A notable exception to this X-waiver requirement is the 72-hour rule, which allows nonwaivered practitioners to administer (but not prescribe for home use) buprenorphine to a patient to relieve acute withdrawal symptoms for up to 72 hours while arranging for specialist referral.Under the 72-hour rule, ED clinicians have a unique opportunity to treat patients experiencing acute withdrawal symptoms and bridge them to specialty care, without the burden of an X-waiver requirement.
The VA Greater Los Angeles Healthcare System (VAGLAHS), therefore, developed and implemented a program to administer buprenorphine in the ED to bridge patients with OUD to an appointment with substance use disorder (SUD) services. We describe our development, implementation and evaluation of this program protocol as a model for other VA EDs. This project was determined to be quality improvement (nonresearch) by the VAGLAHS Institutional Review Board.
ED MOUD Program
We engaged in a 2-month (January-March 2019) preimplementation process during which we (1) obtained stakeholder buy-in; (2) developed a protocol and supporting resources and tools; (3) worked with stakeholders to enact local organizational policy and process modifications; and (4) educated practitioners.
Appendix 1 provides an overview of MOUD terminology, pharmacology, and regulations. We developed an 8-step program implementation plan for the ED MOUD program (Figure 1).
Obtaining Stakeholder Buy-in
Two ED physician champions (MC, JH) organized all activities. Champions obtained stakeholder buy-in from clinical and administrative leaders as well as from frontline personnel in OUD specialty care, ED, and pharmacy services. ED social workers and clerks who schedule post-ED appointments also were engaged. These stakeholders emphasized the importance of fitting the developed protocol into the existing ED workflows as well as minimizing additional resources required to initiate and maintain the program.
We ascertained that in fiscal year 2018, VAGLAHS had 156 ED visits with International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes related to OUD for 108 unique patients. Based on these data and in consultation with OUD specialty care, we determined that the potential number of referrals to the SUD clinic would be manageable with existing resources. Additionally, there was consensus that most opioid withdrawal patients could be treated in the urgent care portion of our ED since these patients generally do not require special monitoring. This consideration was important for obtaining ED stakeholder buy-in and for planning protocol logistics.
Developing the Protocol
We customized resources created by CalBridge Behavioral Health Navigator Program (CA Bridge), formerly called ED Bridge, a program of the Public Health Institute in Oakland, California, funded through California Department of Health Care Services. CA Bridge offers technical assistance and support for hospitals as well as guidance and tools for establishing processes for EDs providing buprenorphine prescriptions for the management of acute opioid withdrawal and serving as a bridge to follow-up care in SUD clinics.9 We also reviewed protocols described by D’Onofrio and colleagues. With iterative input from stakeholders, we created a protocol concretely delineating each process and corresponding responsible party with the overall aim of removing potential barriers to MOUD initiation and follow-up (Appendix 2).
Identifying Appropriate Follow-up
To operationalize protocol implementation, we built on VA’s Emergency Department Rapid Access Clinic (ED-RAC) process, a mechanism for scheduling appointments for post-ED specialty follow-up care. This process facilitated veterans’ access to urgent specialty care follow-up after ED visits by scheduling appointments prior to ED discharge.10 For the ED MOUD program, we adapted the ED-RAC process to schedule appointments in SUD clinic prior to ED discharge. These appointments allowed patients to be seen by an SUD clinician within 72 hours of ED discharge. This step was critical to working within the 72-hour rule without relying on X-waiver licensing of ED clinicians. Alternatively, as was previous practice, per patient preference, patients were also referred to non-VA residential rehabilitation services if the facility had capacity and patients met criteria for admission.
Identification of Eligible Veterans
Target patients were those primarily presenting with a request for treatment of opioid dependence or withdrawal. Patients were not actively screened for OUD. Clinicians diagnosed and assessed for OUD as per their usual practice. Patients with OUD who presented to the ED for other reasons were assessed, at clinician discretion, for their interest in receiving MOUD. If patients presented in moderate-to-severe withdrawal (eg, Clinical Opiate Withdrawal Scale [COWS] ≥ 8), buprenorphine was initiated in the ED. These patients were subsequently referred to either the local SUD clinic or to a residential treatment center. Patients presenting with a COWS score < 8 were referred to the outpatient SUD clinic or residential treatment centers without initiating buprenorphine from the ED. The SUD clinic or residential treatment centers could offer buprenorphine or other MOUD options. From the ED, prescribing buprenorphine for patients to self-initiate at home was not available as this required an X-waivered prescriber, which were limited during the program time frame.
Support Tools and Resources
To facilitate ED clinicians using the protocol, we worked with a programmer experienced with the Computerized Patient Record System, the VA electronic health record (EHR), to create electronic order menu sets that directed clinicians to the protocol and educational materials (Appendix 3). These menus are readily accessible and embedded into the ED clinician workflow. The menus highlight key elements of the protocol, including indications for initiation, contraindications, recommended dosing with quick orders, and how to obtain follow-up for the patient. Links also are provided to the protocol and patient discharge handouts, including the CA Bridge website.
Organizational Policy and Processes
Before implementing the developed protocol, we worked with stakeholders to modify organizational policies and processes. Our pharmacy agreed to stock buprenorphine in the ED to make it readily available. EHR restrictions that historically prohibited ordering buprenorphine for ED administration by nonwaivered clinicians were modified. Additionally, our chief of staff, pharmacy, and credentialing department agreed that physicians did not need to apply for additional delineated privileges.
Clinician Education
The final preparation step was educating clinicians and other protocol users. The VAGLAHS SUD chief presented a lecture and answered questions about MOUD to core ED faculty about the rising prevalence of OUD and use of buprenorphine as a recommended treatment.
Evaluation
To assess adherence to the developed protocol, we conducted a retrospective health record review of all ED visits March 1 to October 25, 2019, in which the patient had OUD and may have qualified for MOUD. To do this, we identified (1) ED visits with an OUD ICD-10 code as a primary or secondary diagnoses; (2) ED referrals to outpatient SUD treatment; and/or (3) ED visits in which buprenorphine was given or prescribed. We included the latter 2 criteria as application of ICD-10 codes for OUD care was inconsistent. Visits were excluded if patients did not have OUD, had OUD in remission, were already maintained on a stable MOUD regimen and no longer using illicit drugs or craving additional opioids, or were presenting solely for a refill or administration of a missed dose. Patients who relapsed were categorized as unstable. Visits were excluded if the patient was admitted to the hospital or left against medical advice. Patients on MOUD who had relapsed or requested a change in MOUD treatment were included. For all included visits, 2 ED physicians (MC, JH) reviewed the ED clinician and nursing notes, pharmacy and referral records, diagnostic codes, and veteran demographics.
In the evaluation, there were 130 visits with 92 unique veterans meeting inclusion criteria. The final sample included 70 visits with 47 unique veterans (Table 1). Of note, 24 (53%) patients self-identified as homeless or were engaged with VA housing services. Twelve veterans had multiple ED visits (7 patients with 2 visits; 5 patients with ≥ 3 visits). In 30 (43%) visits the veteran’s primary reason for seeking ED care was to obtain treatment for opioid withdrawal or receive MOUD. Type of opiate used was specified in 58% of visits; of these, 69% indicated heroin use and 17% prescription medications. Buprenorphine was initiated in the ED in 18 (26%) visits for 10 veterans. Appendix 4 outlines the clinical course and follow-up after these visits. Some veterans returned to the ED for buprenorphine redosing per the 72-hour rule. SUD clinic appointments were provided in 11 visits, and direct transfer to an inpatient rehabilitation center was arranged in 4 visits. In 42 (60%) visits, across 32 unique veterans, buprenorphine was not given in the ED, but patients were referred for SUD treat
A majority of veterans who received buprenorphine and a referral for an SUD appointment went to their initial SUD follow-up appointment and had ongoing engagement in addiction care 30 days after their index ED visit. Among veterans who did not receive buprenorphine but were referred for SUD treatment, about half went to their SUD appointments and about 1 in 5 had ongoing engagement in addiction care at 30 days after the index ED visit. Of note, 2 patients who received referrals died within 1 year of their index ED visit. The cause of death for one patient was an overdose; the other was unspecified.
DISCUSSION
We developed the ED MOUD program as a bridge to SUD specialty care. Our 8 implementation steps can serve as a model for implementing programs at other VA EDs. We demonstrated feasibility, high follow-up rates, and high retention in treatment.
Patients who received ED buprenorphine initiation were more likely to follow up and had higher rates of ongoing engagement at 30 days than did those who received only a clinic referral. In a similar Canadian study, buprenorphine was initiated in the ED, and patients followed up as a walk-in for addiction services; however, only 54% of patients presented to this initial follow-up.11 Our higher initial follow-up rate may be due to our ability to directly schedule clinic appointments. Our 70% 30-day follow-up rate is comparable, but slightly lower than the 2015 D’Onofrio and colleagues study in which 78% of patients remained engaged at 30 days.7 A possible reason is that in the D’Onofrio and colleagues study, all study physicians obtained X-waiver training and were able to prescribe buprenorphine after ED initiation or for self-initiation at home. X-waiver training was not required of our clinicians, and none of our patients were offered a prescription for self-initiation.
Our program demonstrates that it is feasible to develop a protocol without X-waiver licensing. This program provides a supportive framework for the use of MOUD and allows nonspecialists to gain experience and confidence in using buprenorphine. Any clinician could administer buprenorphine in the ED, and patients could be bridged at later ED visits until follow-up with a specialist. Of note, only a small percentage of the total visits for buprenorphine initiation required multiple daily visits for buprenorphine. Appointments with the specialist were assured to fall within a 72-hour window.
Our program has some limitations. First, the number of patients who were candidates for our ED MOUD program was small. In our 7-month review, only 47 patients were identified as potential candidates for MOUD treatment across 70 visits, and only 10 were initiated in the ED. Second, all patients were not actively screened for OUD. There was potential for missing eligible veterans as inclusion criteria relied on clinicians both recognizing OUD and manually entering a correct diagnostic code. We attempted to mitigate this by also reviewing all ED referrals to the SUD clinic and all patients who received buprenorphine in the ED. In addition, we do not have data on preimplementation rates of follow-up for comparison.
Future Directions
More than half of our patients did not receive ED buprenorphine initiation because they were not in moderate or severe withdrawal (COWS ≥ 8) similar to 57% of patients cited in the D’Onofrio and colleagues study.7 Teaching veterans how to start buprenorphine at home could greatly expand enrollment. However, this requires a prescription from an X-waiver licensed clinician. In 2021, the US Department of Health and Human Services removed the 8-hour training requirement for obtaining an X-waiver.12 However, clinicians are still required to apply for licensing. Eliminating the X-waiver requirement, as proposed by D’Onofrio and colleagues in a 2021 editorial, would have allowed all clinicians to offer home initiation.13
Previous studies suggest that despite the ability to provide a prescription, clinicians may be reluctant to offer home initiation.14–17 In a national VA 2019 survey, many emergency medicine physicians believe that SUD care is not in their scope of practice, as Dieujuste and colleagues described in Federal Practitioner.14 Although it is likely some attitudes have changed with the increased visibility of ED MOUD programs, there is still much work to be done to change perceptions.
Another area for improvement is screening for OUD in the ED to better reveal MOUD candidates. Missed opportunities (neither referral nor treatment offered) occurred in 21% of our visits. D’Onofrio and colleagues identified 66% of patients by screening all ED patients.7 Although universal screening for SUD in routine health care settings has been recommended, 2021 VA guidelines state that there is insufficient evidence to recommend universal screening.18-20 There are also limited data on the best screening tool for OUD in the ED.21 Further research on how to effectively and efficiently identify OUD patients in the ED is needed.
Conclusions
With minimal resource allocation, we started the program to offer MOUD with buprenorphine for patients with OUD at a VA ED and provided addiction treatment follow-up. This program, the first of its kind within VA, can be modeled and expanded to other VA facilities. Given increasing numbers of fatal opioid overdose, and significant adverse impacts of the COVID-19 pandemic on the OUD crisis, developing local and national strategies to treat OUD is essential. Future steps include improved screening and expanding capacity to offer home initiation by increasing the number of X-waiver ED clinicians.6
Acknowledgments
Thank you to Jeffrey Balsam, PharmD, BCPS, Veterans Affairs Greater Los Angeles Clinical Applications Coordinator for his contributions in creating a Computerized Patient Record System opioid use disorder screening tool. Thank you to Gracielle Tan, MD, Veterans Affairs Greater Los Angeles Health Science Specialist for her administrative assistance in manuscript preparation.
Opioid use disorder (OUD) is a public health crisis significantly affecting veterans. A substantial increase in veterans diagnosed with OUD has occurred, nearly tripling from 25,031 in 2003 to 69,142 in 2017
For patients with active OUD, medications for opioid use disorder (MOUD) reduce the risk of overdose and all-cause mortality.3 In 2009, the US Department of Veterans Affairs (VA) and Department of Defense (DoD) published clinical practice guidelines for substance use disorders that strongly recommended MOUD with either buprenorphine or methadone as a first-line treatment. In 2015 updated guidelines encouraged buprenorphine initiation in primary care settings.4,5 This was followed by an academic detailing campaign designed to encourage VA clinicians to initiate MOUD.1 Despite this institutional support, MOUD remains underutilized within the VA, with widely variable rates of prescribing among VA sites.1
Efforts to further expand MOUD cultivated interest in administering buprenorphine in VA emergency departments (EDs). Patients with OUD often use the ED for same-day care, providing opportunities to initiate buprenorphine in the ED 24 hours, 7 days per week. This has been especially true during the COVID-19 pandemic during which reliable access to usual recovery services has been disrupted and EDs have served as a safety net.6
Buprenorphine’s safety profile and prolonged effect duration make it superior to other MOUD options for ED administration. As a partial opioid agonist, buprenorphine is unlikely to cause significant sedation or respiratory depression compared with full agonists like methadone. This is known as the ceiling effect. Additionally, at higher doses, buprenorphine’s effects can last for about 3 days, potentially obviating the need for repeat dosing. D’Onofrio and colleagues seminal 2015 paper conceptually proved the feasibility and value of initiating buprenorphine in the ED; patients who received ED initiation therapy were more likely to be engaged in addiction treatment 30 days after their visit and have reduced rates of illicit opioid drug use.7 Such ED harm-reduction strategies are increasingly recognized as essential, given that 1 in 20 patients treated for a nonfatal opioid overdose in an ED will die within 1 year of their visit, many within 2 days.8 Finally, a significant barrier faced by physicians wanting to administer or prescribe buprenorphine for patients with OUD has been the special licensing required by the Drug Enforcement Administration Drug Addiction Treatment Act of 2000, also known as an X-waiver. A notable exception to this X-waiver requirement is the 72-hour rule, which allows nonwaivered practitioners to administer (but not prescribe for home use) buprenorphine to a patient to relieve acute withdrawal symptoms for up to 72 hours while arranging for specialist referral.Under the 72-hour rule, ED clinicians have a unique opportunity to treat patients experiencing acute withdrawal symptoms and bridge them to specialty care, without the burden of an X-waiver requirement.
The VA Greater Los Angeles Healthcare System (VAGLAHS), therefore, developed and implemented a program to administer buprenorphine in the ED to bridge patients with OUD to an appointment with substance use disorder (SUD) services. We describe our development, implementation and evaluation of this program protocol as a model for other VA EDs. This project was determined to be quality improvement (nonresearch) by the VAGLAHS Institutional Review Board.
ED MOUD Program
We engaged in a 2-month (January-March 2019) preimplementation process during which we (1) obtained stakeholder buy-in; (2) developed a protocol and supporting resources and tools; (3) worked with stakeholders to enact local organizational policy and process modifications; and (4) educated practitioners.
Appendix 1 provides an overview of MOUD terminology, pharmacology, and regulations. We developed an 8-step program implementation plan for the ED MOUD program (Figure 1).
Obtaining Stakeholder Buy-in
Two ED physician champions (MC, JH) organized all activities. Champions obtained stakeholder buy-in from clinical and administrative leaders as well as from frontline personnel in OUD specialty care, ED, and pharmacy services. ED social workers and clerks who schedule post-ED appointments also were engaged. These stakeholders emphasized the importance of fitting the developed protocol into the existing ED workflows as well as minimizing additional resources required to initiate and maintain the program.
We ascertained that in fiscal year 2018, VAGLAHS had 156 ED visits with International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes related to OUD for 108 unique patients. Based on these data and in consultation with OUD specialty care, we determined that the potential number of referrals to the SUD clinic would be manageable with existing resources. Additionally, there was consensus that most opioid withdrawal patients could be treated in the urgent care portion of our ED since these patients generally do not require special monitoring. This consideration was important for obtaining ED stakeholder buy-in and for planning protocol logistics.
Developing the Protocol
We customized resources created by CalBridge Behavioral Health Navigator Program (CA Bridge), formerly called ED Bridge, a program of the Public Health Institute in Oakland, California, funded through California Department of Health Care Services. CA Bridge offers technical assistance and support for hospitals as well as guidance and tools for establishing processes for EDs providing buprenorphine prescriptions for the management of acute opioid withdrawal and serving as a bridge to follow-up care in SUD clinics.9 We also reviewed protocols described by D’Onofrio and colleagues. With iterative input from stakeholders, we created a protocol concretely delineating each process and corresponding responsible party with the overall aim of removing potential barriers to MOUD initiation and follow-up (Appendix 2).
Identifying Appropriate Follow-up
To operationalize protocol implementation, we built on VA’s Emergency Department Rapid Access Clinic (ED-RAC) process, a mechanism for scheduling appointments for post-ED specialty follow-up care. This process facilitated veterans’ access to urgent specialty care follow-up after ED visits by scheduling appointments prior to ED discharge.10 For the ED MOUD program, we adapted the ED-RAC process to schedule appointments in SUD clinic prior to ED discharge. These appointments allowed patients to be seen by an SUD clinician within 72 hours of ED discharge. This step was critical to working within the 72-hour rule without relying on X-waiver licensing of ED clinicians. Alternatively, as was previous practice, per patient preference, patients were also referred to non-VA residential rehabilitation services if the facility had capacity and patients met criteria for admission.
Identification of Eligible Veterans
Target patients were those primarily presenting with a request for treatment of opioid dependence or withdrawal. Patients were not actively screened for OUD. Clinicians diagnosed and assessed for OUD as per their usual practice. Patients with OUD who presented to the ED for other reasons were assessed, at clinician discretion, for their interest in receiving MOUD. If patients presented in moderate-to-severe withdrawal (eg, Clinical Opiate Withdrawal Scale [COWS] ≥ 8), buprenorphine was initiated in the ED. These patients were subsequently referred to either the local SUD clinic or to a residential treatment center. Patients presenting with a COWS score < 8 were referred to the outpatient SUD clinic or residential treatment centers without initiating buprenorphine from the ED. The SUD clinic or residential treatment centers could offer buprenorphine or other MOUD options. From the ED, prescribing buprenorphine for patients to self-initiate at home was not available as this required an X-waivered prescriber, which were limited during the program time frame.
Support Tools and Resources
To facilitate ED clinicians using the protocol, we worked with a programmer experienced with the Computerized Patient Record System, the VA electronic health record (EHR), to create electronic order menu sets that directed clinicians to the protocol and educational materials (Appendix 3). These menus are readily accessible and embedded into the ED clinician workflow. The menus highlight key elements of the protocol, including indications for initiation, contraindications, recommended dosing with quick orders, and how to obtain follow-up for the patient. Links also are provided to the protocol and patient discharge handouts, including the CA Bridge website.
Organizational Policy and Processes
Before implementing the developed protocol, we worked with stakeholders to modify organizational policies and processes. Our pharmacy agreed to stock buprenorphine in the ED to make it readily available. EHR restrictions that historically prohibited ordering buprenorphine for ED administration by nonwaivered clinicians were modified. Additionally, our chief of staff, pharmacy, and credentialing department agreed that physicians did not need to apply for additional delineated privileges.
Clinician Education
The final preparation step was educating clinicians and other protocol users. The VAGLAHS SUD chief presented a lecture and answered questions about MOUD to core ED faculty about the rising prevalence of OUD and use of buprenorphine as a recommended treatment.
Evaluation
To assess adherence to the developed protocol, we conducted a retrospective health record review of all ED visits March 1 to October 25, 2019, in which the patient had OUD and may have qualified for MOUD. To do this, we identified (1) ED visits with an OUD ICD-10 code as a primary or secondary diagnoses; (2) ED referrals to outpatient SUD treatment; and/or (3) ED visits in which buprenorphine was given or prescribed. We included the latter 2 criteria as application of ICD-10 codes for OUD care was inconsistent. Visits were excluded if patients did not have OUD, had OUD in remission, were already maintained on a stable MOUD regimen and no longer using illicit drugs or craving additional opioids, or were presenting solely for a refill or administration of a missed dose. Patients who relapsed were categorized as unstable. Visits were excluded if the patient was admitted to the hospital or left against medical advice. Patients on MOUD who had relapsed or requested a change in MOUD treatment were included. For all included visits, 2 ED physicians (MC, JH) reviewed the ED clinician and nursing notes, pharmacy and referral records, diagnostic codes, and veteran demographics.
In the evaluation, there were 130 visits with 92 unique veterans meeting inclusion criteria. The final sample included 70 visits with 47 unique veterans (Table 1). Of note, 24 (53%) patients self-identified as homeless or were engaged with VA housing services. Twelve veterans had multiple ED visits (7 patients with 2 visits; 5 patients with ≥ 3 visits). In 30 (43%) visits the veteran’s primary reason for seeking ED care was to obtain treatment for opioid withdrawal or receive MOUD. Type of opiate used was specified in 58% of visits; of these, 69% indicated heroin use and 17% prescription medications. Buprenorphine was initiated in the ED in 18 (26%) visits for 10 veterans. Appendix 4 outlines the clinical course and follow-up after these visits. Some veterans returned to the ED for buprenorphine redosing per the 72-hour rule. SUD clinic appointments were provided in 11 visits, and direct transfer to an inpatient rehabilitation center was arranged in 4 visits. In 42 (60%) visits, across 32 unique veterans, buprenorphine was not given in the ED, but patients were referred for SUD treat
A majority of veterans who received buprenorphine and a referral for an SUD appointment went to their initial SUD follow-up appointment and had ongoing engagement in addiction care 30 days after their index ED visit. Among veterans who did not receive buprenorphine but were referred for SUD treatment, about half went to their SUD appointments and about 1 in 5 had ongoing engagement in addiction care at 30 days after the index ED visit. Of note, 2 patients who received referrals died within 1 year of their index ED visit. The cause of death for one patient was an overdose; the other was unspecified.
DISCUSSION
We developed the ED MOUD program as a bridge to SUD specialty care. Our 8 implementation steps can serve as a model for implementing programs at other VA EDs. We demonstrated feasibility, high follow-up rates, and high retention in treatment.
Patients who received ED buprenorphine initiation were more likely to follow up and had higher rates of ongoing engagement at 30 days than did those who received only a clinic referral. In a similar Canadian study, buprenorphine was initiated in the ED, and patients followed up as a walk-in for addiction services; however, only 54% of patients presented to this initial follow-up.11 Our higher initial follow-up rate may be due to our ability to directly schedule clinic appointments. Our 70% 30-day follow-up rate is comparable, but slightly lower than the 2015 D’Onofrio and colleagues study in which 78% of patients remained engaged at 30 days.7 A possible reason is that in the D’Onofrio and colleagues study, all study physicians obtained X-waiver training and were able to prescribe buprenorphine after ED initiation or for self-initiation at home. X-waiver training was not required of our clinicians, and none of our patients were offered a prescription for self-initiation.
Our program demonstrates that it is feasible to develop a protocol without X-waiver licensing. This program provides a supportive framework for the use of MOUD and allows nonspecialists to gain experience and confidence in using buprenorphine. Any clinician could administer buprenorphine in the ED, and patients could be bridged at later ED visits until follow-up with a specialist. Of note, only a small percentage of the total visits for buprenorphine initiation required multiple daily visits for buprenorphine. Appointments with the specialist were assured to fall within a 72-hour window.
Our program has some limitations. First, the number of patients who were candidates for our ED MOUD program was small. In our 7-month review, only 47 patients were identified as potential candidates for MOUD treatment across 70 visits, and only 10 were initiated in the ED. Second, all patients were not actively screened for OUD. There was potential for missing eligible veterans as inclusion criteria relied on clinicians both recognizing OUD and manually entering a correct diagnostic code. We attempted to mitigate this by also reviewing all ED referrals to the SUD clinic and all patients who received buprenorphine in the ED. In addition, we do not have data on preimplementation rates of follow-up for comparison.
Future Directions
More than half of our patients did not receive ED buprenorphine initiation because they were not in moderate or severe withdrawal (COWS ≥ 8) similar to 57% of patients cited in the D’Onofrio and colleagues study.7 Teaching veterans how to start buprenorphine at home could greatly expand enrollment. However, this requires a prescription from an X-waiver licensed clinician. In 2021, the US Department of Health and Human Services removed the 8-hour training requirement for obtaining an X-waiver.12 However, clinicians are still required to apply for licensing. Eliminating the X-waiver requirement, as proposed by D’Onofrio and colleagues in a 2021 editorial, would have allowed all clinicians to offer home initiation.13
Previous studies suggest that despite the ability to provide a prescription, clinicians may be reluctant to offer home initiation.14–17 In a national VA 2019 survey, many emergency medicine physicians believe that SUD care is not in their scope of practice, as Dieujuste and colleagues described in Federal Practitioner.14 Although it is likely some attitudes have changed with the increased visibility of ED MOUD programs, there is still much work to be done to change perceptions.
Another area for improvement is screening for OUD in the ED to better reveal MOUD candidates. Missed opportunities (neither referral nor treatment offered) occurred in 21% of our visits. D’Onofrio and colleagues identified 66% of patients by screening all ED patients.7 Although universal screening for SUD in routine health care settings has been recommended, 2021 VA guidelines state that there is insufficient evidence to recommend universal screening.18-20 There are also limited data on the best screening tool for OUD in the ED.21 Further research on how to effectively and efficiently identify OUD patients in the ED is needed.
Conclusions
With minimal resource allocation, we started the program to offer MOUD with buprenorphine for patients with OUD at a VA ED and provided addiction treatment follow-up. This program, the first of its kind within VA, can be modeled and expanded to other VA facilities. Given increasing numbers of fatal opioid overdose, and significant adverse impacts of the COVID-19 pandemic on the OUD crisis, developing local and national strategies to treat OUD is essential. Future steps include improved screening and expanding capacity to offer home initiation by increasing the number of X-waiver ED clinicians.6
Acknowledgments
Thank you to Jeffrey Balsam, PharmD, BCPS, Veterans Affairs Greater Los Angeles Clinical Applications Coordinator for his contributions in creating a Computerized Patient Record System opioid use disorder screening tool. Thank you to Gracielle Tan, MD, Veterans Affairs Greater Los Angeles Health Science Specialist for her administrative assistance in manuscript preparation.
1. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: historical perspective, lessons learned, and next steps. Subst Abuse. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
2. Bohnert ASB, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs health system. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. Ma J, Bao Y-P, Wang R-J, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1968-1983. doi:10.1038/s41380-018-0094-5
4. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 2.0. US Department of Veterans Affairs; 2009.
5. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 3.0. US Department of Veterans Affairs. 2015. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
6. Hulsey J, Mellis A, Kelly B. COVID-19 pandemic impact on patients, families and individuals in recovery from substance use disorder. Accessed July 7, 2021. https://www.addictionpolicy.org/covid19-report
7. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opiod dependence. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
8. Weiner SG, Baker O, Bernson D, Schuur JD. One-year mortality of patients after emergency department treatment for non-fatal opioid overdose. Ann Emerg Med. 2020;75(1):13-17. doi:10.1016/j.annemergmed.2019.04.020
9. CA Bridge. Updated 2021. Accessed July 1, 2022. https://cabridge.org
10. Penney L, Miake-Lye I, Lewis D, et al. Proceedings from the 11th annual conference on the science of dissemination and implementation: S72 spreading VA’s emergency department-rapid access clinics (ED-RAC) intervention: key factors for success. Implementation Sci. 2019;14(suppl 1). doi:10.1186/s13012-019-0878-2
11. Hu T, Snider-Alder M, Nijmeh L, Pyle A. Buprenorphine/naloxone induction in a Canadian emergency department with rapid access to community-based addictions providers. CJEM. 2019;21(4):492-498. doi:10.1017/cem.2019.24
12. US Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Federal Register. Accessed July 1, 2022. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
13. D’Onofrio G, Melnick ER, Hawk KF. Improve access to care for opioid use disorder: a call to eliminate the x-waiver requirement now. Ann Emerg Med. 2021;78(2):220-222. doi:10.1016/j.annemergmed.2021.03.023
14. Dieujuste N, Johnson-Koenke R, Celedon M, et al. Provider perceptions of opioid safety measures in VHA emergency department and urgent care centers. Fed Pract. 2021;38(9):412-419. doi:10.12788/fp.0179
15. Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and faciliatators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw Open. 2020;3(5):e204561. doi:10.1001/jamanetworkopen.2020.4561
16. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. doi:10.1016/j.ajem.2019.02.025
17. Srivastava A, Kahan M, Leece P, McAndrew A. Buprenorphine unobserved “home” induction: a survey of Ontario’s addiction physicians. Addic Sci Clin Pract. 2019;14(1):18. doi:10.1186/s13722-019-0146-4
18. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 4.0. US Department of Veterans Affairs. 2021. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPG.pdf
19. Patnode CD, Perdue LA, Rushkin M, et al. Screening for unhealthy drug use updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(22):2310-2338. doi:10.1001/jama.2019.21381
20. Coles S, Vosooney A. Evidence lacking to support universal unhealthy drug use screening. Am Fam Physician. 2021;103(2):72-73.
21. Sahota PK, Sharstry S, Mukamel DB, et al. Screening emergency department patients for opioid drug use: a qualitative systematic review. Addict Behav. 2018;85:139-146. doi:10.1016/j.addbeh.2018.05.022
1. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: historical perspective, lessons learned, and next steps. Subst Abuse. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
2. Bohnert ASB, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs health system. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. Ma J, Bao Y-P, Wang R-J, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1968-1983. doi:10.1038/s41380-018-0094-5
4. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 2.0. US Department of Veterans Affairs; 2009.
5. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 3.0. US Department of Veterans Affairs. 2015. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
6. Hulsey J, Mellis A, Kelly B. COVID-19 pandemic impact on patients, families and individuals in recovery from substance use disorder. Accessed July 7, 2021. https://www.addictionpolicy.org/covid19-report
7. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opiod dependence. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
8. Weiner SG, Baker O, Bernson D, Schuur JD. One-year mortality of patients after emergency department treatment for non-fatal opioid overdose. Ann Emerg Med. 2020;75(1):13-17. doi:10.1016/j.annemergmed.2019.04.020
9. CA Bridge. Updated 2021. Accessed July 1, 2022. https://cabridge.org
10. Penney L, Miake-Lye I, Lewis D, et al. Proceedings from the 11th annual conference on the science of dissemination and implementation: S72 spreading VA’s emergency department-rapid access clinics (ED-RAC) intervention: key factors for success. Implementation Sci. 2019;14(suppl 1). doi:10.1186/s13012-019-0878-2
11. Hu T, Snider-Alder M, Nijmeh L, Pyle A. Buprenorphine/naloxone induction in a Canadian emergency department with rapid access to community-based addictions providers. CJEM. 2019;21(4):492-498. doi:10.1017/cem.2019.24
12. US Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Federal Register. Accessed July 1, 2022. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
13. D’Onofrio G, Melnick ER, Hawk KF. Improve access to care for opioid use disorder: a call to eliminate the x-waiver requirement now. Ann Emerg Med. 2021;78(2):220-222. doi:10.1016/j.annemergmed.2021.03.023
14. Dieujuste N, Johnson-Koenke R, Celedon M, et al. Provider perceptions of opioid safety measures in VHA emergency department and urgent care centers. Fed Pract. 2021;38(9):412-419. doi:10.12788/fp.0179
15. Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and faciliatators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw Open. 2020;3(5):e204561. doi:10.1001/jamanetworkopen.2020.4561
16. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. doi:10.1016/j.ajem.2019.02.025
17. Srivastava A, Kahan M, Leece P, McAndrew A. Buprenorphine unobserved “home” induction: a survey of Ontario’s addiction physicians. Addic Sci Clin Pract. 2019;14(1):18. doi:10.1186/s13722-019-0146-4
18. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 4.0. US Department of Veterans Affairs. 2021. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPG.pdf
19. Patnode CD, Perdue LA, Rushkin M, et al. Screening for unhealthy drug use updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(22):2310-2338. doi:10.1001/jama.2019.21381
20. Coles S, Vosooney A. Evidence lacking to support universal unhealthy drug use screening. Am Fam Physician. 2021;103(2):72-73.
21. Sahota PK, Sharstry S, Mukamel DB, et al. Screening emergency department patients for opioid drug use: a qualitative systematic review. Addict Behav. 2018;85:139-146. doi:10.1016/j.addbeh.2018.05.022
‘Disturbing’ lack of follow-up care after psychiatric crises
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PSYCHIATRIC SERVICES
The truth about the ‘happy hormone’: Why we shouldn’t mess with dopamine
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Clinical psychoeconomics: Accounting for money matters in psychiatric assessment and treatment
Despite money’s central role in our psychic lives, many trainees – and some seasoned practitioners – skirt around financial issues. Some clinicians confess that inquiring about patients’ finances feels “too personal.” They fear that asking about money could suggest that the clinician is primarily concerned with getting paid. Some clinicians feel that looking into patients’ finances might be unprofessional, outside one’s scope of practice. But it is not.
Trainees often receive little guidance concerning money matters in patients’ lives and treatments, considerations we have labeled clinical psychoeconomics. Considerable evidence suggests that financial concerns often provoke emotional distress and dysfunctional behaviors, and directly influence patient’s health care decisions. Financial issues also influence how clinicians view and react to patients.
We have recently reviewed (and illustrated through case vignettes) how money matters might impact psychiatric assessment, case formulation, treatment planning, and ongoing psychiatric treatments including psychotherapies.1 Consider how money affects people’s lives: Money helps people meet multiple practical, psychological, and social needs by enabling them to obtain food, clothing, shelter, other material goods, services, discretionary time, and opportunities. And money strongly influences relationships. Regardless of poverty or wealth, thoughts and behaviors connected to acquiring, possessing, and disposing of money, and feelings accompanying these processes such as greed, neediness, envy, pride, shame, guilt, and self-satisfaction often underly intrapsychic and interpersonal conflicts.
Individuals constantly engage in numerous simultaneous conscious, preconscious, and unconscious neuro-economic trade-offs that determine goals, efforts, and timing. Many are financially influenced. Money influences how virtually all patients seek, receive, and sustain their mental health care including psychotherapy.
Money problems can be associated with insecurity, impotence, feeling unloved, and lack of freedom or subjugation. Individuals may resent how they’re forced to acquire money, and feel shamed or morally injured by their jobs, financial dependence on other family members, public assistance, or their questionable ways of obtaining money.
Impoverished individuals may face choosing between food, housing, medications, and medical care. Domestically abused individuals may reluctantly remain with their abusers, risking physical harm or death rather than face destitution. Some families tolerate severely disabled individuals at home because they rely on their disability checks and caregiver payments. Suicides may turn on how individuals forecast financial repercussions affecting their families. Desires to avoid debt may lead to treatment avoidance.
Individuals with enough money to get by face daily financially related choices involving competing needs, desires, values, and loyalties. They may experience conflicts concerning spending on necessities vs. indulgences or spending on oneself vs. significant others.
Whereas some wealthy individuals may assume unwarranted airs of superiority and entitlement, others may feel guilty about wealth, or fearful that others like them only for their money. Individuals on the receiving end of wealth may feel emotionally and behaviorally manipulated by their benefactors.
Assessment
Assessments should consider how financial matters have shaped patients’ early psychological development as well their current lives. How do patients’ emotions, thoughts, and behaviors reflect money matters? What money-related pathologies are evident? What aspects of the patient’s “financial world” seem modifiable?
Financial questions should be posed colloquially. Screeners include: “Where do you live?”, “Who’s in the home?”, “How do you (all) manage financially?”, “What do you all do for a living?”, “How do you make ends meet?”, and “What financial problems are you facing?” Clinicians can quickly learn about patients’ financial self-sufficiencies, individuals for whom they bear financial responsibility, and others they rely on for support, for example, relatives. If patients avoid answering such questions forthrightly, particularly when financial arrangements are “complicated,” clinicians will want to revisit these issues later after establishing a firmer alliance but continue to wonder about the meaning of the patient’s reluctance.
When explicit, patients, families, and couples are fully aware of the conflicts but have difficulty resolving financial disputes. When conflicts are implicit, money problems may be unacknowledged, avoided, denied, or minimized. Conflicts concerning money are often transmitted trans-generationally.
Psychopathological conditions unequivocally linked to money include compulsive shopping, gambling disorders, miserly hoarding, impulse buying, and spending sprees during hypomanic and manic states. Mounting debts may create progressively insurmountable sources of distress. Money can be weaponized to sadistically create enticement, envy, or deprivation. Some monetarily antisocial individuals compromise interpersonal relationships as well as treatments. Individuals with alcohol/substance use disorders may spend so much on substances that little is left for necessities. Financially needy individuals may engage in morally questionable behaviors they might otherwise shun.
Case formulation and treatment planning
Incorporating money matters into case formulations entails demonstrating how financial concerns influenced maladaptive development and distort current attitudes, perceptions, and behaviors.
Concurrently, clinicians should acknowledge patients’ reality-based fiscal decisions, appreciating cultural and family value differences concerning how money should be acquired and spent. Since money often determines frequency and duration of treatment visits, clinicians are ethically obligated to discuss with patients what they might expect from different medications and psychotherapies, and their comparative costs.
Money matters’ impact on psychotherapies
Money matters often affect transference and countertransference reactions. Some reactions stem from how patients and clinicians compare their own financial situations with those of the other.
To help identify and ameliorate money-related countertransference responses, clinicians can reflect on questions such as: “How comfortable are you with people who are much poorer or richer than you are?” “How comfortable are you with impoverished individuals or with multimillionaires or their children?” And “why?” For trainees, all these reactions should be discussed in supervision.
Conclusions
To summarize, four clinical psychoeconomic issues should be routinely assessed and factored into psychiatric case formulations and treatment plans: how financial issues 1) have impacted patients’ psychological development; 2) impact patients’ current lives; 3) are likely to impact access, type, intensity, and duration of treatment visits; and 4) might provoke money-related transference and countertransference concerns.
In advising patients about treatment options, clinicians should discuss each treatment’s relative effectiveness and estimated costs of care. Patients’ decisions will likely be heavily influenced by financial considerations.
Dr. Yager is based in the department of psychiatry, University of Colorado at Denver, Aurora. Dr. Kay is based in the department of psychiatry, Wright State University, Dayton, Ohio. No external funds were received for this project, and the authors have no conflicts to disclose.
Reference
1. Yager J and Kay J. Money matters in psychiatric assessment, case formulation, treatment planning, and ongoing psychotherapy: Clinical psychoeconomics. J Nerv Ment Dis. 2022 Jun 10. doi: 10.1097/NMD.0000000000001552.
Despite money’s central role in our psychic lives, many trainees – and some seasoned practitioners – skirt around financial issues. Some clinicians confess that inquiring about patients’ finances feels “too personal.” They fear that asking about money could suggest that the clinician is primarily concerned with getting paid. Some clinicians feel that looking into patients’ finances might be unprofessional, outside one’s scope of practice. But it is not.
Trainees often receive little guidance concerning money matters in patients’ lives and treatments, considerations we have labeled clinical psychoeconomics. Considerable evidence suggests that financial concerns often provoke emotional distress and dysfunctional behaviors, and directly influence patient’s health care decisions. Financial issues also influence how clinicians view and react to patients.
We have recently reviewed (and illustrated through case vignettes) how money matters might impact psychiatric assessment, case formulation, treatment planning, and ongoing psychiatric treatments including psychotherapies.1 Consider how money affects people’s lives: Money helps people meet multiple practical, psychological, and social needs by enabling them to obtain food, clothing, shelter, other material goods, services, discretionary time, and opportunities. And money strongly influences relationships. Regardless of poverty or wealth, thoughts and behaviors connected to acquiring, possessing, and disposing of money, and feelings accompanying these processes such as greed, neediness, envy, pride, shame, guilt, and self-satisfaction often underly intrapsychic and interpersonal conflicts.
Individuals constantly engage in numerous simultaneous conscious, preconscious, and unconscious neuro-economic trade-offs that determine goals, efforts, and timing. Many are financially influenced. Money influences how virtually all patients seek, receive, and sustain their mental health care including psychotherapy.
Money problems can be associated with insecurity, impotence, feeling unloved, and lack of freedom or subjugation. Individuals may resent how they’re forced to acquire money, and feel shamed or morally injured by their jobs, financial dependence on other family members, public assistance, or their questionable ways of obtaining money.
Impoverished individuals may face choosing between food, housing, medications, and medical care. Domestically abused individuals may reluctantly remain with their abusers, risking physical harm or death rather than face destitution. Some families tolerate severely disabled individuals at home because they rely on their disability checks and caregiver payments. Suicides may turn on how individuals forecast financial repercussions affecting their families. Desires to avoid debt may lead to treatment avoidance.
Individuals with enough money to get by face daily financially related choices involving competing needs, desires, values, and loyalties. They may experience conflicts concerning spending on necessities vs. indulgences or spending on oneself vs. significant others.
Whereas some wealthy individuals may assume unwarranted airs of superiority and entitlement, others may feel guilty about wealth, or fearful that others like them only for their money. Individuals on the receiving end of wealth may feel emotionally and behaviorally manipulated by their benefactors.
Assessment
Assessments should consider how financial matters have shaped patients’ early psychological development as well their current lives. How do patients’ emotions, thoughts, and behaviors reflect money matters? What money-related pathologies are evident? What aspects of the patient’s “financial world” seem modifiable?
Financial questions should be posed colloquially. Screeners include: “Where do you live?”, “Who’s in the home?”, “How do you (all) manage financially?”, “What do you all do for a living?”, “How do you make ends meet?”, and “What financial problems are you facing?” Clinicians can quickly learn about patients’ financial self-sufficiencies, individuals for whom they bear financial responsibility, and others they rely on for support, for example, relatives. If patients avoid answering such questions forthrightly, particularly when financial arrangements are “complicated,” clinicians will want to revisit these issues later after establishing a firmer alliance but continue to wonder about the meaning of the patient’s reluctance.
When explicit, patients, families, and couples are fully aware of the conflicts but have difficulty resolving financial disputes. When conflicts are implicit, money problems may be unacknowledged, avoided, denied, or minimized. Conflicts concerning money are often transmitted trans-generationally.
Psychopathological conditions unequivocally linked to money include compulsive shopping, gambling disorders, miserly hoarding, impulse buying, and spending sprees during hypomanic and manic states. Mounting debts may create progressively insurmountable sources of distress. Money can be weaponized to sadistically create enticement, envy, or deprivation. Some monetarily antisocial individuals compromise interpersonal relationships as well as treatments. Individuals with alcohol/substance use disorders may spend so much on substances that little is left for necessities. Financially needy individuals may engage in morally questionable behaviors they might otherwise shun.
Case formulation and treatment planning
Incorporating money matters into case formulations entails demonstrating how financial concerns influenced maladaptive development and distort current attitudes, perceptions, and behaviors.
Concurrently, clinicians should acknowledge patients’ reality-based fiscal decisions, appreciating cultural and family value differences concerning how money should be acquired and spent. Since money often determines frequency and duration of treatment visits, clinicians are ethically obligated to discuss with patients what they might expect from different medications and psychotherapies, and their comparative costs.
Money matters’ impact on psychotherapies
Money matters often affect transference and countertransference reactions. Some reactions stem from how patients and clinicians compare their own financial situations with those of the other.
To help identify and ameliorate money-related countertransference responses, clinicians can reflect on questions such as: “How comfortable are you with people who are much poorer or richer than you are?” “How comfortable are you with impoverished individuals or with multimillionaires or their children?” And “why?” For trainees, all these reactions should be discussed in supervision.
Conclusions
To summarize, four clinical psychoeconomic issues should be routinely assessed and factored into psychiatric case formulations and treatment plans: how financial issues 1) have impacted patients’ psychological development; 2) impact patients’ current lives; 3) are likely to impact access, type, intensity, and duration of treatment visits; and 4) might provoke money-related transference and countertransference concerns.
In advising patients about treatment options, clinicians should discuss each treatment’s relative effectiveness and estimated costs of care. Patients’ decisions will likely be heavily influenced by financial considerations.
Dr. Yager is based in the department of psychiatry, University of Colorado at Denver, Aurora. Dr. Kay is based in the department of psychiatry, Wright State University, Dayton, Ohio. No external funds were received for this project, and the authors have no conflicts to disclose.
Reference
1. Yager J and Kay J. Money matters in psychiatric assessment, case formulation, treatment planning, and ongoing psychotherapy: Clinical psychoeconomics. J Nerv Ment Dis. 2022 Jun 10. doi: 10.1097/NMD.0000000000001552.
Despite money’s central role in our psychic lives, many trainees – and some seasoned practitioners – skirt around financial issues. Some clinicians confess that inquiring about patients’ finances feels “too personal.” They fear that asking about money could suggest that the clinician is primarily concerned with getting paid. Some clinicians feel that looking into patients’ finances might be unprofessional, outside one’s scope of practice. But it is not.
Trainees often receive little guidance concerning money matters in patients’ lives and treatments, considerations we have labeled clinical psychoeconomics. Considerable evidence suggests that financial concerns often provoke emotional distress and dysfunctional behaviors, and directly influence patient’s health care decisions. Financial issues also influence how clinicians view and react to patients.
We have recently reviewed (and illustrated through case vignettes) how money matters might impact psychiatric assessment, case formulation, treatment planning, and ongoing psychiatric treatments including psychotherapies.1 Consider how money affects people’s lives: Money helps people meet multiple practical, psychological, and social needs by enabling them to obtain food, clothing, shelter, other material goods, services, discretionary time, and opportunities. And money strongly influences relationships. Regardless of poverty or wealth, thoughts and behaviors connected to acquiring, possessing, and disposing of money, and feelings accompanying these processes such as greed, neediness, envy, pride, shame, guilt, and self-satisfaction often underly intrapsychic and interpersonal conflicts.
Individuals constantly engage in numerous simultaneous conscious, preconscious, and unconscious neuro-economic trade-offs that determine goals, efforts, and timing. Many are financially influenced. Money influences how virtually all patients seek, receive, and sustain their mental health care including psychotherapy.
Money problems can be associated with insecurity, impotence, feeling unloved, and lack of freedom or subjugation. Individuals may resent how they’re forced to acquire money, and feel shamed or morally injured by their jobs, financial dependence on other family members, public assistance, or their questionable ways of obtaining money.
Impoverished individuals may face choosing between food, housing, medications, and medical care. Domestically abused individuals may reluctantly remain with their abusers, risking physical harm or death rather than face destitution. Some families tolerate severely disabled individuals at home because they rely on their disability checks and caregiver payments. Suicides may turn on how individuals forecast financial repercussions affecting their families. Desires to avoid debt may lead to treatment avoidance.
Individuals with enough money to get by face daily financially related choices involving competing needs, desires, values, and loyalties. They may experience conflicts concerning spending on necessities vs. indulgences or spending on oneself vs. significant others.
Whereas some wealthy individuals may assume unwarranted airs of superiority and entitlement, others may feel guilty about wealth, or fearful that others like them only for their money. Individuals on the receiving end of wealth may feel emotionally and behaviorally manipulated by their benefactors.
Assessment
Assessments should consider how financial matters have shaped patients’ early psychological development as well their current lives. How do patients’ emotions, thoughts, and behaviors reflect money matters? What money-related pathologies are evident? What aspects of the patient’s “financial world” seem modifiable?
Financial questions should be posed colloquially. Screeners include: “Where do you live?”, “Who’s in the home?”, “How do you (all) manage financially?”, “What do you all do for a living?”, “How do you make ends meet?”, and “What financial problems are you facing?” Clinicians can quickly learn about patients’ financial self-sufficiencies, individuals for whom they bear financial responsibility, and others they rely on for support, for example, relatives. If patients avoid answering such questions forthrightly, particularly when financial arrangements are “complicated,” clinicians will want to revisit these issues later after establishing a firmer alliance but continue to wonder about the meaning of the patient’s reluctance.
When explicit, patients, families, and couples are fully aware of the conflicts but have difficulty resolving financial disputes. When conflicts are implicit, money problems may be unacknowledged, avoided, denied, or minimized. Conflicts concerning money are often transmitted trans-generationally.
Psychopathological conditions unequivocally linked to money include compulsive shopping, gambling disorders, miserly hoarding, impulse buying, and spending sprees during hypomanic and manic states. Mounting debts may create progressively insurmountable sources of distress. Money can be weaponized to sadistically create enticement, envy, or deprivation. Some monetarily antisocial individuals compromise interpersonal relationships as well as treatments. Individuals with alcohol/substance use disorders may spend so much on substances that little is left for necessities. Financially needy individuals may engage in morally questionable behaviors they might otherwise shun.
Case formulation and treatment planning
Incorporating money matters into case formulations entails demonstrating how financial concerns influenced maladaptive development and distort current attitudes, perceptions, and behaviors.
Concurrently, clinicians should acknowledge patients’ reality-based fiscal decisions, appreciating cultural and family value differences concerning how money should be acquired and spent. Since money often determines frequency and duration of treatment visits, clinicians are ethically obligated to discuss with patients what they might expect from different medications and psychotherapies, and their comparative costs.
Money matters’ impact on psychotherapies
Money matters often affect transference and countertransference reactions. Some reactions stem from how patients and clinicians compare their own financial situations with those of the other.
To help identify and ameliorate money-related countertransference responses, clinicians can reflect on questions such as: “How comfortable are you with people who are much poorer or richer than you are?” “How comfortable are you with impoverished individuals or with multimillionaires or their children?” And “why?” For trainees, all these reactions should be discussed in supervision.
Conclusions
To summarize, four clinical psychoeconomic issues should be routinely assessed and factored into psychiatric case formulations and treatment plans: how financial issues 1) have impacted patients’ psychological development; 2) impact patients’ current lives; 3) are likely to impact access, type, intensity, and duration of treatment visits; and 4) might provoke money-related transference and countertransference concerns.
In advising patients about treatment options, clinicians should discuss each treatment’s relative effectiveness and estimated costs of care. Patients’ decisions will likely be heavily influenced by financial considerations.
Dr. Yager is based in the department of psychiatry, University of Colorado at Denver, Aurora. Dr. Kay is based in the department of psychiatry, Wright State University, Dayton, Ohio. No external funds were received for this project, and the authors have no conflicts to disclose.
Reference
1. Yager J and Kay J. Money matters in psychiatric assessment, case formulation, treatment planning, and ongoing psychotherapy: Clinical psychoeconomics. J Nerv Ment Dis. 2022 Jun 10. doi: 10.1097/NMD.0000000000001552.
Long-acting naltrexone effective in alcohol use disorder
according to findings presented at the annual meeting of the American College of Emergency Physicians.
The results show the feasibility of such a program and underscore the importance of the ED in combating AUD, said the researchers, from the University of California, San Francisco.
“According to the National Institute on Alcohol Abuse and Alcoholism, 18% of ED visits had alcohol as a contributing factor – the volume of alcohol-related ED visits has been climbing every year, and it is a significant public health problem,” said Maria Raven, MD, MPH, professor of emergency medicine at UCSF. “Right now, we do very little for people who come to the ED with AUD, so it is a missed opportunity to intervene, especially given the volume of visits we see and that our patient population is one that often has significant barriers to accessing outpatient treatment.”
The findings come from a 12-week, prospective, single-arm study of ED patients who were actively drinking adults with known or suspected AUD and who had positive scores on a screening test. Of 179 patients who were approached, 32 agreed to enroll; the enrollment yield was 18%. Participants were given monthly extended-release naltrexone and case management services.
Of the 32 participants, 25 completed all their study visits and 22 (69%) continued taking naltrexone after the 12 weeks.
The researchers said the results surprised them. The average daily alcohol consumption at baseline was 7.6 drinks a day, and it fell by 7.5 drinks a day – in other words, to almost no consumption.
“The median alcohol consumption when measured over the last 2 weeks of the study was zero,” Dr. Raven said. “This doesn’t mean everyone was at zero, but this was the median and reflects that many participants stopped drinking altogether. We were pleasantly surprised by this. I don’t know that we thought so many people who participated would actually fully abstain.”
On the Kemp Quality of Life Scale – with scores from 1 to 7, with 1 being “life is very distressing,” 4 being “life is so-so,” and 7 being “life is great” – the average baseline score was 3.6. That score rose by 1.2 points by the study’s end.
Dr. Raven said she hoped more would enroll but that “a number of people actually did not want the injection or were not ready to think about stopping.” Still, the 18% enrollment is “a major improvement,” considering that no attempt was made to initiate treatment with naltrexone prior to the study. Oral naltrexone, rather than the injection, could be offered to improve participation, but oral naltrexone has to be taken daily.
She said a larger study is planned at UCSF and that other institutions are interested in starting a similar program.
“When someone is in the ED for an AUD-related issue, it can serve as a turning point for them in some cases,” she said.
Erik S. Anderson, MD, associate research director at Oakland, Calif.–based Alameda Health System, who has studied naltrexone in the ED, said the findings dovetail with what his team has found at his center. He added that psychosocial support is important as well and that his team has found that navigation services are the most important factor in connecting patients with follow-up care – even more so than providing medications.
“In my mind, this is a situation where we have treatment options and approaches that work, and it’s really about implementing these services in a novel care setting,” he said. “ED patients are at higher risk of complications for AUD simply because they are in the ED in the first place – initiating AUD treatment in this setting is the right thing to do.”
Dr. Raven and Dr. Anderson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to findings presented at the annual meeting of the American College of Emergency Physicians.
The results show the feasibility of such a program and underscore the importance of the ED in combating AUD, said the researchers, from the University of California, San Francisco.
“According to the National Institute on Alcohol Abuse and Alcoholism, 18% of ED visits had alcohol as a contributing factor – the volume of alcohol-related ED visits has been climbing every year, and it is a significant public health problem,” said Maria Raven, MD, MPH, professor of emergency medicine at UCSF. “Right now, we do very little for people who come to the ED with AUD, so it is a missed opportunity to intervene, especially given the volume of visits we see and that our patient population is one that often has significant barriers to accessing outpatient treatment.”
The findings come from a 12-week, prospective, single-arm study of ED patients who were actively drinking adults with known or suspected AUD and who had positive scores on a screening test. Of 179 patients who were approached, 32 agreed to enroll; the enrollment yield was 18%. Participants were given monthly extended-release naltrexone and case management services.
Of the 32 participants, 25 completed all their study visits and 22 (69%) continued taking naltrexone after the 12 weeks.
The researchers said the results surprised them. The average daily alcohol consumption at baseline was 7.6 drinks a day, and it fell by 7.5 drinks a day – in other words, to almost no consumption.
“The median alcohol consumption when measured over the last 2 weeks of the study was zero,” Dr. Raven said. “This doesn’t mean everyone was at zero, but this was the median and reflects that many participants stopped drinking altogether. We were pleasantly surprised by this. I don’t know that we thought so many people who participated would actually fully abstain.”
On the Kemp Quality of Life Scale – with scores from 1 to 7, with 1 being “life is very distressing,” 4 being “life is so-so,” and 7 being “life is great” – the average baseline score was 3.6. That score rose by 1.2 points by the study’s end.
Dr. Raven said she hoped more would enroll but that “a number of people actually did not want the injection or were not ready to think about stopping.” Still, the 18% enrollment is “a major improvement,” considering that no attempt was made to initiate treatment with naltrexone prior to the study. Oral naltrexone, rather than the injection, could be offered to improve participation, but oral naltrexone has to be taken daily.
She said a larger study is planned at UCSF and that other institutions are interested in starting a similar program.
“When someone is in the ED for an AUD-related issue, it can serve as a turning point for them in some cases,” she said.
Erik S. Anderson, MD, associate research director at Oakland, Calif.–based Alameda Health System, who has studied naltrexone in the ED, said the findings dovetail with what his team has found at his center. He added that psychosocial support is important as well and that his team has found that navigation services are the most important factor in connecting patients with follow-up care – even more so than providing medications.
“In my mind, this is a situation where we have treatment options and approaches that work, and it’s really about implementing these services in a novel care setting,” he said. “ED patients are at higher risk of complications for AUD simply because they are in the ED in the first place – initiating AUD treatment in this setting is the right thing to do.”
Dr. Raven and Dr. Anderson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to findings presented at the annual meeting of the American College of Emergency Physicians.
The results show the feasibility of such a program and underscore the importance of the ED in combating AUD, said the researchers, from the University of California, San Francisco.
“According to the National Institute on Alcohol Abuse and Alcoholism, 18% of ED visits had alcohol as a contributing factor – the volume of alcohol-related ED visits has been climbing every year, and it is a significant public health problem,” said Maria Raven, MD, MPH, professor of emergency medicine at UCSF. “Right now, we do very little for people who come to the ED with AUD, so it is a missed opportunity to intervene, especially given the volume of visits we see and that our patient population is one that often has significant barriers to accessing outpatient treatment.”
The findings come from a 12-week, prospective, single-arm study of ED patients who were actively drinking adults with known or suspected AUD and who had positive scores on a screening test. Of 179 patients who were approached, 32 agreed to enroll; the enrollment yield was 18%. Participants were given monthly extended-release naltrexone and case management services.
Of the 32 participants, 25 completed all their study visits and 22 (69%) continued taking naltrexone after the 12 weeks.
The researchers said the results surprised them. The average daily alcohol consumption at baseline was 7.6 drinks a day, and it fell by 7.5 drinks a day – in other words, to almost no consumption.
“The median alcohol consumption when measured over the last 2 weeks of the study was zero,” Dr. Raven said. “This doesn’t mean everyone was at zero, but this was the median and reflects that many participants stopped drinking altogether. We were pleasantly surprised by this. I don’t know that we thought so many people who participated would actually fully abstain.”
On the Kemp Quality of Life Scale – with scores from 1 to 7, with 1 being “life is very distressing,” 4 being “life is so-so,” and 7 being “life is great” – the average baseline score was 3.6. That score rose by 1.2 points by the study’s end.
Dr. Raven said she hoped more would enroll but that “a number of people actually did not want the injection or were not ready to think about stopping.” Still, the 18% enrollment is “a major improvement,” considering that no attempt was made to initiate treatment with naltrexone prior to the study. Oral naltrexone, rather than the injection, could be offered to improve participation, but oral naltrexone has to be taken daily.
She said a larger study is planned at UCSF and that other institutions are interested in starting a similar program.
“When someone is in the ED for an AUD-related issue, it can serve as a turning point for them in some cases,” she said.
Erik S. Anderson, MD, associate research director at Oakland, Calif.–based Alameda Health System, who has studied naltrexone in the ED, said the findings dovetail with what his team has found at his center. He added that psychosocial support is important as well and that his team has found that navigation services are the most important factor in connecting patients with follow-up care – even more so than providing medications.
“In my mind, this is a situation where we have treatment options and approaches that work, and it’s really about implementing these services in a novel care setting,” he said. “ED patients are at higher risk of complications for AUD simply because they are in the ED in the first place – initiating AUD treatment in this setting is the right thing to do.”
Dr. Raven and Dr. Anderson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
More on varenicline
Murray et al have written a timely, thoughtful, and useful article (“Smoking cessation: Varenicline and the risk of neuropsychiatric adverse events,”
Just a few caveats regarding Murray et al’s excellent summary:
• The article did not address that nicotine is consumed in multiple ways, such as vaping, snuff, chewing tobacco, and hookah
• The safety of varenicline appears fair when psychiatric illness is well controlled but can be problematic (and even severely detrimental) when mental illness is not well controlled. This should not be glossed over, especially since it was the reason for the original black-box warning (for risks including behavioral impulsivity, suicidality, severe insomnia, and nightmares) that was removed in 2016
• Patients with severe mental illness may not fully understand the risks, benefits, and priorities of the treatment intervention. The importance of psychiatric and internal medicine in addition to pharmacy follow-up is critical and needs to be documented.
Varenicline has been contextualized in its current role as a first-line treatment for smoking cessation. By bypassing a sizeable population of patients who have unstable psychiatric illness (especially bipolar I disorder), the path has been opened for risky “off-label” varenicline prescribing to this population by internists, who should be very cautious and prudent about prescribing for such patients. This alone is probably a good reason to reinstate the black-box warning.
Interestingly, one review found that only 1 of 11 patients receiving varenicline stopped smoking.1 Not dramatically beneficial for a first-line treatment! Decreasing smoking occurs as well and is more robust with combinational use with bupropion, nicotine replacement therapy, and cognitive-behavioral therapy.
If we are focusing on patients with unstable mental illness—who are seen primarily by psychiatrists—adherence, urgency of intervention, and context regarding acute safety for this population must be seen as top priorities.
So-called “second-line” treatment options must also be considered. Sandiego et al3 make excellent points regarding the role of alpha-adrenergic agonists such as guanfacine, which have been shown to be helpful in smoking cessation. They work by decreasing cortical dopamine release and their calming effects on the noradrenergic system, which may decrease smoking precipitated by stress. For the particularly challenging subpopulation of unstable smokers, the combination of varenicline plus guanfacine ER may turn out to be a game-changer.
Varenicline has not proven itself to be useful in patients who are severely mentally ill, and due to its low success rate, expectations should remain tempered, pragmatically realistic, and safety-based.4,5 The bottom line is that in an unstable psychiatrically ill patient, interventions other than varenicline should be first-line.
1. Crawford P, Cieslak D. Varenicline for smoking cessation. Am Fam Physician. 2017;96(5).
2. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824.
3. Sandiego CM, Matuskey D, Lavery M, et al. The effect of treatment with guanfacine, an alpha2 adrenergic agonist, on dopaminergic tone in tobacco smokers: an [11C]FLB457 PET study. Neuropsychopharmacology. 2018;43(5):1052-1058.
4. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120.
5. Tofler IR. Varenicline for smoking cessation in the bipolar patient. J Clin Psychiatry. 2015;76(5):625.
Murray et al have written a timely, thoughtful, and useful article (“Smoking cessation: Varenicline and the risk of neuropsychiatric adverse events,”
Just a few caveats regarding Murray et al’s excellent summary:
• The article did not address that nicotine is consumed in multiple ways, such as vaping, snuff, chewing tobacco, and hookah
• The safety of varenicline appears fair when psychiatric illness is well controlled but can be problematic (and even severely detrimental) when mental illness is not well controlled. This should not be glossed over, especially since it was the reason for the original black-box warning (for risks including behavioral impulsivity, suicidality, severe insomnia, and nightmares) that was removed in 2016
• Patients with severe mental illness may not fully understand the risks, benefits, and priorities of the treatment intervention. The importance of psychiatric and internal medicine in addition to pharmacy follow-up is critical and needs to be documented.
Varenicline has been contextualized in its current role as a first-line treatment for smoking cessation. By bypassing a sizeable population of patients who have unstable psychiatric illness (especially bipolar I disorder), the path has been opened for risky “off-label” varenicline prescribing to this population by internists, who should be very cautious and prudent about prescribing for such patients. This alone is probably a good reason to reinstate the black-box warning.
Interestingly, one review found that only 1 of 11 patients receiving varenicline stopped smoking.1 Not dramatically beneficial for a first-line treatment! Decreasing smoking occurs as well and is more robust with combinational use with bupropion, nicotine replacement therapy, and cognitive-behavioral therapy.
If we are focusing on patients with unstable mental illness—who are seen primarily by psychiatrists—adherence, urgency of intervention, and context regarding acute safety for this population must be seen as top priorities.
So-called “second-line” treatment options must also be considered. Sandiego et al3 make excellent points regarding the role of alpha-adrenergic agonists such as guanfacine, which have been shown to be helpful in smoking cessation. They work by decreasing cortical dopamine release and their calming effects on the noradrenergic system, which may decrease smoking precipitated by stress. For the particularly challenging subpopulation of unstable smokers, the combination of varenicline plus guanfacine ER may turn out to be a game-changer.
Varenicline has not proven itself to be useful in patients who are severely mentally ill, and due to its low success rate, expectations should remain tempered, pragmatically realistic, and safety-based.4,5 The bottom line is that in an unstable psychiatrically ill patient, interventions other than varenicline should be first-line.
Murray et al have written a timely, thoughtful, and useful article (“Smoking cessation: Varenicline and the risk of neuropsychiatric adverse events,”
Just a few caveats regarding Murray et al’s excellent summary:
• The article did not address that nicotine is consumed in multiple ways, such as vaping, snuff, chewing tobacco, and hookah
• The safety of varenicline appears fair when psychiatric illness is well controlled but can be problematic (and even severely detrimental) when mental illness is not well controlled. This should not be glossed over, especially since it was the reason for the original black-box warning (for risks including behavioral impulsivity, suicidality, severe insomnia, and nightmares) that was removed in 2016
• Patients with severe mental illness may not fully understand the risks, benefits, and priorities of the treatment intervention. The importance of psychiatric and internal medicine in addition to pharmacy follow-up is critical and needs to be documented.
Varenicline has been contextualized in its current role as a first-line treatment for smoking cessation. By bypassing a sizeable population of patients who have unstable psychiatric illness (especially bipolar I disorder), the path has been opened for risky “off-label” varenicline prescribing to this population by internists, who should be very cautious and prudent about prescribing for such patients. This alone is probably a good reason to reinstate the black-box warning.
Interestingly, one review found that only 1 of 11 patients receiving varenicline stopped smoking.1 Not dramatically beneficial for a first-line treatment! Decreasing smoking occurs as well and is more robust with combinational use with bupropion, nicotine replacement therapy, and cognitive-behavioral therapy.
If we are focusing on patients with unstable mental illness—who are seen primarily by psychiatrists—adherence, urgency of intervention, and context regarding acute safety for this population must be seen as top priorities.
So-called “second-line” treatment options must also be considered. Sandiego et al3 make excellent points regarding the role of alpha-adrenergic agonists such as guanfacine, which have been shown to be helpful in smoking cessation. They work by decreasing cortical dopamine release and their calming effects on the noradrenergic system, which may decrease smoking precipitated by stress. For the particularly challenging subpopulation of unstable smokers, the combination of varenicline plus guanfacine ER may turn out to be a game-changer.
Varenicline has not proven itself to be useful in patients who are severely mentally ill, and due to its low success rate, expectations should remain tempered, pragmatically realistic, and safety-based.4,5 The bottom line is that in an unstable psychiatrically ill patient, interventions other than varenicline should be first-line.
1. Crawford P, Cieslak D. Varenicline for smoking cessation. Am Fam Physician. 2017;96(5).
2. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824.
3. Sandiego CM, Matuskey D, Lavery M, et al. The effect of treatment with guanfacine, an alpha2 adrenergic agonist, on dopaminergic tone in tobacco smokers: an [11C]FLB457 PET study. Neuropsychopharmacology. 2018;43(5):1052-1058.
4. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120.
5. Tofler IR. Varenicline for smoking cessation in the bipolar patient. J Clin Psychiatry. 2015;76(5):625.
1. Crawford P, Cieslak D. Varenicline for smoking cessation. Am Fam Physician. 2017;96(5).
2. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824.
3. Sandiego CM, Matuskey D, Lavery M, et al. The effect of treatment with guanfacine, an alpha2 adrenergic agonist, on dopaminergic tone in tobacco smokers: an [11C]FLB457 PET study. Neuropsychopharmacology. 2018;43(5):1052-1058.
4. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120.
5. Tofler IR. Varenicline for smoking cessation in the bipolar patient. J Clin Psychiatry. 2015;76(5):625.
Postop analgesia in Saudi Arabia and the United States: A resident’s perspective
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
Safer opioid supply program in Canada helps those who face overdose risks
An analysis indicates that the program is associated with a reduction in emergency department visits, hospitalizations, and overall health care costs. In addition, there were no opioid-related deaths among participants who were at high risk of overdose.
“Not only did hospital engagements decline immediately after starting SOS programs, but also the risk of overdose did not change, and there were no opioid-related deaths in the 1-year follow-up,” study author Tara Gomes, PhD, an assistant professor of health policy, management, and evaluation at the University of Toronto and a scientist at the Li Ka Shing Knowledge Institute of St. Michael’s Hospital in Toronto, said in an interview.
Dr. Gomes is the lead principal investigator of the Ontario Drug Policy Research Network, a collaboration between researchers and drug policy decision-makers in the province.
“These changes were not seen in a group of similar individuals who lived in the same city – so were exposed to the same illicit drug supply – but who were not part of this program, helping to reinforce that these changes are specific to SOS participation,” she said.
The study was published in the Canadian Medical Association Journal.
Hospital admissions declined
More than 29,000 opioid-related toxicity deaths occurred in Canada between 2016 and 2021, often as a result of high levels of fentanyl in the drug supply, according to the investigators. In response, SOS programs have been launched in several provinces, including the first formal SOS program at the London (Ont.) InterCommunity Health Centre. As part of the program, clients are prescribed pharmaceutical opioids as an alternative to the fentanyl-adulterated drug supply and are given health and social supports.
Dr. Gomes and colleagues conducted an interrupted time series analysis of residents in London, Ont., who had received a diagnosis of opioid use disorder and had had a health care encounter related to the diagnosis between January 2016 and March 2019. They followed 82 participants who entered the SOS program, as well as a comparison group of 303 people who were matched on the basis of demographic and clinical characteristics but who did not participate in the program.
The research team focused on the population’s numbers of emergency department visits, hospital admissions, infection rates, and health care costs. They used autoregressive integrated moving average models to evaluate the effect of starting the SOS program and to compare the population’s outcome rates in the year before and after entering the program.
For participants who entered the program, the rate of emergency department visits declined by about 14 visits per 100 people. In addition, hospital admissions declined by about 5 admissions per 100 people. Health care costs that weren’t related to primary care or outpatient medications declined by about $922 per person. The rate of hospital admission for infections remained about the same; the investigators observed a decline of about 1.6 infections per 100 people.
In the year after entry into the program, emergency department visits, hospital admissions, infection-related admissions, and total health care costs declined significantly among SOS clients, compared with the year before.
Conversely, there were no significant changes in any of the measured outcomes among the 303 people who didn’t participate in the program.
Medication costs increased
DR. Gomes and colleagues noted that the findings provide preliminary evidence that SOS programs can play a role in the harm-reduction options available to those who are at high risk of drug poisoning and overdose. At the same time, many questions remain.
For instance, although total health care costs declined among those enrolled in the program, the medication-related costs increased. About 34% of participants had HIV, 69.5% had hepatitis C virus infection, and 28% had infectious complications in the year before entering the program. This finding may indicate that the participants had serious medical complications resulting from their drug use and were able to seek health care services.
“We interpret that to be a positive finding, because of the very high prevalence of HIV and hepatitis C in the SOS clients. Treatments for HIV and hepatitis C are lifesaving but expensive,” said Dr. Gomes. “Therefore, these higher medication costs are likely reflective of improved access to treatments for these infections, which can greatly improve people’s health and quality of life but also save the health care system money over the longer term.”
DR. Gomes and colleagues are now beginning to evaluate other SOS programs across Ontario. They hope to better understand the various approaches that are available and determine which models can best support people who face high risks because of drug use.
A limited solution?
Commenting on the study, Andrew Ivsins, PhD, a postdoctoral fellow in social medicine at the University of British Columbia in Vancouver and a research scientist at the British Columbia Centre on Substance Abuse, said, “This is an important study and one of the first to show how safe supply can help by building connections to the health care system that didn’t exist previously.”
Dr. Ivsins, who wasn’t involved with this study, has researched safe supply programs around Vancouver. He and colleagues found that among participants in these programs, the use of illicit street-purchased drugs decreased, which led to improved health and wellness.
“Safe supply is fundamentally, at the most basic level, a response to the highly toxic drug supply and out-of-control poisoning crisis in North America,” he said. “It’s a contentious issue, but it makes so much sense that, if what’s killing people is highly toxic drugs, we need to find a way to provide an option that doesn’t kill them.”
“Up to now, safer supply has mostly been used to reduce harms, including mortality and morbidity, in persons using illicit opioids. But if we really want to lower the risk linked to heavy contamination of the unregulated drug supply, safer supply programs will have to be extended to all substances potentially sold illegally,” Marie-Eve Goyer, MD, an assistant professor of family medicine at the University of Montreal, said in an interview.
Dr. Goyer, who wasn’t involved with this study, has conducted research about substance replacement therapy in Quebec. She found that many provinces are now reporting on new potent designer benzodiazepines that are being used or that are contaminating fentanyl, which calls for a broader approach to address the drug overdose crisis.
“Let’s realize that safer supply prescription is a very medicalized (and limited) solution to an epidemic that is made of stigma, criminalization, and repressive public policies,” she said. “Without true changes in the law, we will continue to see our people dying every day.”
The study was funded by grants from the Ontario Ministry of Health and the Canadian Institutes of Health Research. Dr. Gomes has received grants to support the research of both groups, and other authors have received support or fees related to the London InterCommunity Health Centre. Dr. Ivsins and Dr. Goyer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis indicates that the program is associated with a reduction in emergency department visits, hospitalizations, and overall health care costs. In addition, there were no opioid-related deaths among participants who were at high risk of overdose.
“Not only did hospital engagements decline immediately after starting SOS programs, but also the risk of overdose did not change, and there were no opioid-related deaths in the 1-year follow-up,” study author Tara Gomes, PhD, an assistant professor of health policy, management, and evaluation at the University of Toronto and a scientist at the Li Ka Shing Knowledge Institute of St. Michael’s Hospital in Toronto, said in an interview.
Dr. Gomes is the lead principal investigator of the Ontario Drug Policy Research Network, a collaboration between researchers and drug policy decision-makers in the province.
“These changes were not seen in a group of similar individuals who lived in the same city – so were exposed to the same illicit drug supply – but who were not part of this program, helping to reinforce that these changes are specific to SOS participation,” she said.
The study was published in the Canadian Medical Association Journal.
Hospital admissions declined
More than 29,000 opioid-related toxicity deaths occurred in Canada between 2016 and 2021, often as a result of high levels of fentanyl in the drug supply, according to the investigators. In response, SOS programs have been launched in several provinces, including the first formal SOS program at the London (Ont.) InterCommunity Health Centre. As part of the program, clients are prescribed pharmaceutical opioids as an alternative to the fentanyl-adulterated drug supply and are given health and social supports.
Dr. Gomes and colleagues conducted an interrupted time series analysis of residents in London, Ont., who had received a diagnosis of opioid use disorder and had had a health care encounter related to the diagnosis between January 2016 and March 2019. They followed 82 participants who entered the SOS program, as well as a comparison group of 303 people who were matched on the basis of demographic and clinical characteristics but who did not participate in the program.
The research team focused on the population’s numbers of emergency department visits, hospital admissions, infection rates, and health care costs. They used autoregressive integrated moving average models to evaluate the effect of starting the SOS program and to compare the population’s outcome rates in the year before and after entering the program.
For participants who entered the program, the rate of emergency department visits declined by about 14 visits per 100 people. In addition, hospital admissions declined by about 5 admissions per 100 people. Health care costs that weren’t related to primary care or outpatient medications declined by about $922 per person. The rate of hospital admission for infections remained about the same; the investigators observed a decline of about 1.6 infections per 100 people.
In the year after entry into the program, emergency department visits, hospital admissions, infection-related admissions, and total health care costs declined significantly among SOS clients, compared with the year before.
Conversely, there were no significant changes in any of the measured outcomes among the 303 people who didn’t participate in the program.
Medication costs increased
DR. Gomes and colleagues noted that the findings provide preliminary evidence that SOS programs can play a role in the harm-reduction options available to those who are at high risk of drug poisoning and overdose. At the same time, many questions remain.
For instance, although total health care costs declined among those enrolled in the program, the medication-related costs increased. About 34% of participants had HIV, 69.5% had hepatitis C virus infection, and 28% had infectious complications in the year before entering the program. This finding may indicate that the participants had serious medical complications resulting from their drug use and were able to seek health care services.
“We interpret that to be a positive finding, because of the very high prevalence of HIV and hepatitis C in the SOS clients. Treatments for HIV and hepatitis C are lifesaving but expensive,” said Dr. Gomes. “Therefore, these higher medication costs are likely reflective of improved access to treatments for these infections, which can greatly improve people’s health and quality of life but also save the health care system money over the longer term.”
DR. Gomes and colleagues are now beginning to evaluate other SOS programs across Ontario. They hope to better understand the various approaches that are available and determine which models can best support people who face high risks because of drug use.
A limited solution?
Commenting on the study, Andrew Ivsins, PhD, a postdoctoral fellow in social medicine at the University of British Columbia in Vancouver and a research scientist at the British Columbia Centre on Substance Abuse, said, “This is an important study and one of the first to show how safe supply can help by building connections to the health care system that didn’t exist previously.”
Dr. Ivsins, who wasn’t involved with this study, has researched safe supply programs around Vancouver. He and colleagues found that among participants in these programs, the use of illicit street-purchased drugs decreased, which led to improved health and wellness.
“Safe supply is fundamentally, at the most basic level, a response to the highly toxic drug supply and out-of-control poisoning crisis in North America,” he said. “It’s a contentious issue, but it makes so much sense that, if what’s killing people is highly toxic drugs, we need to find a way to provide an option that doesn’t kill them.”
“Up to now, safer supply has mostly been used to reduce harms, including mortality and morbidity, in persons using illicit opioids. But if we really want to lower the risk linked to heavy contamination of the unregulated drug supply, safer supply programs will have to be extended to all substances potentially sold illegally,” Marie-Eve Goyer, MD, an assistant professor of family medicine at the University of Montreal, said in an interview.
Dr. Goyer, who wasn’t involved with this study, has conducted research about substance replacement therapy in Quebec. She found that many provinces are now reporting on new potent designer benzodiazepines that are being used or that are contaminating fentanyl, which calls for a broader approach to address the drug overdose crisis.
“Let’s realize that safer supply prescription is a very medicalized (and limited) solution to an epidemic that is made of stigma, criminalization, and repressive public policies,” she said. “Without true changes in the law, we will continue to see our people dying every day.”
The study was funded by grants from the Ontario Ministry of Health and the Canadian Institutes of Health Research. Dr. Gomes has received grants to support the research of both groups, and other authors have received support or fees related to the London InterCommunity Health Centre. Dr. Ivsins and Dr. Goyer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis indicates that the program is associated with a reduction in emergency department visits, hospitalizations, and overall health care costs. In addition, there were no opioid-related deaths among participants who were at high risk of overdose.
“Not only did hospital engagements decline immediately after starting SOS programs, but also the risk of overdose did not change, and there were no opioid-related deaths in the 1-year follow-up,” study author Tara Gomes, PhD, an assistant professor of health policy, management, and evaluation at the University of Toronto and a scientist at the Li Ka Shing Knowledge Institute of St. Michael’s Hospital in Toronto, said in an interview.
Dr. Gomes is the lead principal investigator of the Ontario Drug Policy Research Network, a collaboration between researchers and drug policy decision-makers in the province.
“These changes were not seen in a group of similar individuals who lived in the same city – so were exposed to the same illicit drug supply – but who were not part of this program, helping to reinforce that these changes are specific to SOS participation,” she said.
The study was published in the Canadian Medical Association Journal.
Hospital admissions declined
More than 29,000 opioid-related toxicity deaths occurred in Canada between 2016 and 2021, often as a result of high levels of fentanyl in the drug supply, according to the investigators. In response, SOS programs have been launched in several provinces, including the first formal SOS program at the London (Ont.) InterCommunity Health Centre. As part of the program, clients are prescribed pharmaceutical opioids as an alternative to the fentanyl-adulterated drug supply and are given health and social supports.
Dr. Gomes and colleagues conducted an interrupted time series analysis of residents in London, Ont., who had received a diagnosis of opioid use disorder and had had a health care encounter related to the diagnosis between January 2016 and March 2019. They followed 82 participants who entered the SOS program, as well as a comparison group of 303 people who were matched on the basis of demographic and clinical characteristics but who did not participate in the program.
The research team focused on the population’s numbers of emergency department visits, hospital admissions, infection rates, and health care costs. They used autoregressive integrated moving average models to evaluate the effect of starting the SOS program and to compare the population’s outcome rates in the year before and after entering the program.
For participants who entered the program, the rate of emergency department visits declined by about 14 visits per 100 people. In addition, hospital admissions declined by about 5 admissions per 100 people. Health care costs that weren’t related to primary care or outpatient medications declined by about $922 per person. The rate of hospital admission for infections remained about the same; the investigators observed a decline of about 1.6 infections per 100 people.
In the year after entry into the program, emergency department visits, hospital admissions, infection-related admissions, and total health care costs declined significantly among SOS clients, compared with the year before.
Conversely, there were no significant changes in any of the measured outcomes among the 303 people who didn’t participate in the program.
Medication costs increased
DR. Gomes and colleagues noted that the findings provide preliminary evidence that SOS programs can play a role in the harm-reduction options available to those who are at high risk of drug poisoning and overdose. At the same time, many questions remain.
For instance, although total health care costs declined among those enrolled in the program, the medication-related costs increased. About 34% of participants had HIV, 69.5% had hepatitis C virus infection, and 28% had infectious complications in the year before entering the program. This finding may indicate that the participants had serious medical complications resulting from their drug use and were able to seek health care services.
“We interpret that to be a positive finding, because of the very high prevalence of HIV and hepatitis C in the SOS clients. Treatments for HIV and hepatitis C are lifesaving but expensive,” said Dr. Gomes. “Therefore, these higher medication costs are likely reflective of improved access to treatments for these infections, which can greatly improve people’s health and quality of life but also save the health care system money over the longer term.”
DR. Gomes and colleagues are now beginning to evaluate other SOS programs across Ontario. They hope to better understand the various approaches that are available and determine which models can best support people who face high risks because of drug use.
A limited solution?
Commenting on the study, Andrew Ivsins, PhD, a postdoctoral fellow in social medicine at the University of British Columbia in Vancouver and a research scientist at the British Columbia Centre on Substance Abuse, said, “This is an important study and one of the first to show how safe supply can help by building connections to the health care system that didn’t exist previously.”
Dr. Ivsins, who wasn’t involved with this study, has researched safe supply programs around Vancouver. He and colleagues found that among participants in these programs, the use of illicit street-purchased drugs decreased, which led to improved health and wellness.
“Safe supply is fundamentally, at the most basic level, a response to the highly toxic drug supply and out-of-control poisoning crisis in North America,” he said. “It’s a contentious issue, but it makes so much sense that, if what’s killing people is highly toxic drugs, we need to find a way to provide an option that doesn’t kill them.”
“Up to now, safer supply has mostly been used to reduce harms, including mortality and morbidity, in persons using illicit opioids. But if we really want to lower the risk linked to heavy contamination of the unregulated drug supply, safer supply programs will have to be extended to all substances potentially sold illegally,” Marie-Eve Goyer, MD, an assistant professor of family medicine at the University of Montreal, said in an interview.
Dr. Goyer, who wasn’t involved with this study, has conducted research about substance replacement therapy in Quebec. She found that many provinces are now reporting on new potent designer benzodiazepines that are being used or that are contaminating fentanyl, which calls for a broader approach to address the drug overdose crisis.
“Let’s realize that safer supply prescription is a very medicalized (and limited) solution to an epidemic that is made of stigma, criminalization, and repressive public policies,” she said. “Without true changes in the law, we will continue to see our people dying every day.”
The study was funded by grants from the Ontario Ministry of Health and the Canadian Institutes of Health Research. Dr. Gomes has received grants to support the research of both groups, and other authors have received support or fees related to the London InterCommunity Health Centre. Dr. Ivsins and Dr. Goyer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANADIAN MEDICAL ASSOCIATION JOURNAL
Could a vaccine (and more) fix the fentanyl crisis?
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.