User login
50.6 million tobacco users are not a homogeneous group
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.
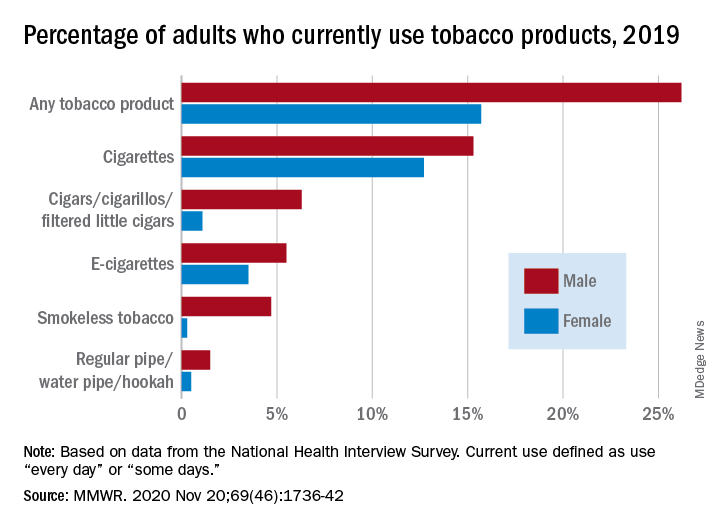
with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
FROM MMWR
Can mental health teams de-escalate crises in NYC?
“Defund the police”: It’s a slogan, or perhaps a battle cry, that has emerged from the Black Lives Matter movement as a response to race-related police brutality and concerns that people of color are profiled, targeted, arrested, charged, manhandled, and killed by law enforcement in a disproportionate and unjust manner. It crosses into our realm as psychiatrists as mental health emergency calls are handled by the police and not by mental health professionals. The result is sometimes tragic: As many as half of police shootings involve people with psychiatric disorders, and the hope is that many of the police shootings could be avoided if crises were handed by mental health clinicians instead of, or in cooperation with, the police.
At best, police officers receive a week of specialized, crisis intervention training about how to approach those with psychiatric disorders; most officers receive no training. This leaves psychiatry as the only field where medical crises are routinely handled by the police – it is demeaning and embarrassing for some of our patients and dangerous for others. The reality remains, however, that there are times when psychiatric disorders result in violent behavior, and patients being taken for involuntary treatment often resist transport, so either way there is risk, both to the patient and to anyone who responds to a call for assistance.
Early this month, the office of New York City Mayor Bill de Blasio announced that a major change would be made in how mental health calls to 911 are handled in two “high-need” areas. The mayor’s website states:
“Beginning in February 2021, new Mental Health Teams will use their physical and mental health expertise, and experience in crisis response to de-escalate emergency situations, will help reduce the number of times police will need to respond to 911 mental health calls in these precincts. These teams will have the expertise to respond to a range of behavioral health problems, such as suicide attempts, substance misuse, and serious mental illness, as well as physical health problems, which can be exacerbated by or mask mental health problems. NYC Health + Hospitals will train and provide ongoing technical assistance and support. In selecting team members for this program, FDNY will prioritize professionals with significant experience with mental health crises.”
The press release goes on to say that, in situations where there is a weapon or reason to believe there is a risk of violence, the police will be dispatched along with the new mental health team.
“This is the first time in our history that health professionals will be the default responders to mental health emergencies,” New York City First Lady Chirlane McCray said as she announced the new program. “Treating mental health crises as mental health challenges and not public safety ones is the modern and more appropriate approach.”
New York City is not the first city to employ this model. In the United States, the CAHOOTS (Crisis Assistance Helping Out on the Streets) program in Eugene, Ore., has been run by the White Bird Clinic since 1989 as part of a community policing initiative. Last year, the team responded to 24,000 calls and police backup was required on only 150 of those responses. The CAHOOTS website states:
“The CAHOOTS model has been in the spotlight recently as our nation struggles to reimagine public safety. The program mobilizes two-person teams consisting of a medic (a nurse, paramedic, or EMT) and a crisis worker who has substantial training and experience in the mental health field. The CAHOOTS teams deal with a wide range of mental health-related crises, including conflict resolution, welfare checks, substance abuse, suicide threats, and more, relying on trauma-informed de-escalation and harm reduction techniques. CAHOOTS staff are not law enforcement officers and do not carry weapons; their training and experience are the tools they use to ensure a non-violent resolution of crisis situations. They also handle non-emergent medical issues, avoiding costly ambulance transport and emergency room treatment.”
Other cities in the United States are also looking at implementing programs where mental health teams, and not the police, respond to emergency calls. Last year, Oakland, Calif.’s city council invested $40,000 in research to assess how they could best implement a program like the one in Eugene. They hope to begin the Mobile Assistance Community Responders of Oakland (MACROS) next year. Sigal Samuel writes in a Vox article, “The goal is to launch the pilot next year with funding from the city budget, and although supporters are not yet sure what its size and duration will be, they’re hopeful it’ll make a big difference to Oakland’s overpoliced community of people without homes. They were among those who first called for a non-policing approach.”
The model is not unique to the United States. In 2005, Stockholm started a program with a psychiatric ambulance – equipped with comfortable seating rather than a stretcher – to respond to mental health emergencies. The ambulance responds to 130 calls a month. It is staffed with a driver and two psychiatric nurses, and for half of the calls, the police also come. While the Swedish program was not about removing resources from the police, it has relieved the police of the responsibility for many psychiatric emergencies.
The New York City program will be modeled after the CAHOOTS initiative in Eugene. It differs from the mobile crisis response services in many other cities because CAHOOTS is hooked directly into the 911 emergency services system. Its website notes that the program has saved money:
“The cost savings are considerable. The CAHOOTS program budget is about $2.1 million annually, while the combined annual budgets for the Eugene and Springfield police departments are $90 million. In 2017, the CAHOOTS teams answered 17% of the Eugene Police Department’s overall call volume. The program saves the city of Eugene an estimated $8.5 million in public safety spending annually.”
Some worry there is an unpredictable aspect to calls for psychiatric emergencies, and the potential for mental health professions to be injured or killed. Annette Hanson, MD, a forensic psychiatrist at University of Maryland, Baltimore, voiced her concerns, “While multidisciplinary teams are useful, there have been rare cases of violence against responding mental health providers. People with serious mental illness are rarely violent but their dangerousness is unpredictable and cannot be predicted by case screening.”
Daniel Felts is a mental health crisis counselor who has worked at CAHOOTS for the past 4* years. He has responded to about 8,000 calls, and called for police backup only three times to request an immediate "Code 3 cover" when someone's safety has been in danger. Mr. Felts calls the police about once a month for concerns that do not require an immediate response for safety.* “Over the last 4 years, I am only aware of three instances when a team member’s safety was compromised because of a client’s violent behavior. No employee has been seriously physically harmed. In 30 years, with hundreds of thousands (millions?) of calls responded to, no CAHOOTS worker has ever been killed, shot, or stabbed in the line of duty,” Mr. Felts noted.
Emergency calls are screened. “It is not uncommon for CAHOOTS to be dispatched to ‘stage’ for calls involving active disputes or acutely suicidal individuals where means are present. “Staging” entails us parking roughly a mile away while police make first contact and advise whether it is safe for CAHOOTS to engage.”
Mr. Felts went on to discuss the program’s relationship with the community. “ and how we operate. Having operated in Eugene for 30 years, our service is well understood to be one that does not kill, harm, or violate personal boundaries or liberties.”
Would a program like the ones in Stockholm or in Eugene work in other places? Eugene is a city with a population of 172,000 with a low crime rate. Whether a program implemented in one city can be mimicked in another very different city is not clear.
Paul Appelbaum, MD, a forensic psychiatrist at Columbia University, New York, is optimistic about New York City’s forthcoming program.
“The proposed pilot project in NYC is a real step forward. Work that we’ve done looking at fatal encounters involving the police found that roughly 25% of all deaths at the hands of the police are of people with mental illness. In many of those cases, police were initially called to bring people who were clearly troubled for psychiatric evaluation, but as the situation escalated, the police turned to their weapons to control it, which led to a fatal outcome. Taking police out of the picture whenever possible in favor of trained mental health personnel is clearly a better approach. It will be important for the city to collect good outcome data to enable independent evaluation of the pilot project – not something that political entities are inclined toward, but a critical element in assessing the effectiveness of this approach.”
There are questions that remain about the new program. Mayor de Blasio’s office has not released information about which areas of the city are being chosen for the new program, how much the program will cost, or what the funding source will be. If it can be implemented safely and effectively, it has the potential to provide more sensitive care to patients in crisis, and to save lives.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
*Correction, 11/27/2020: An earlier version of this article misstated the number of years Daniel Felts has worked at CAHOOTS.
“Defund the police”: It’s a slogan, or perhaps a battle cry, that has emerged from the Black Lives Matter movement as a response to race-related police brutality and concerns that people of color are profiled, targeted, arrested, charged, manhandled, and killed by law enforcement in a disproportionate and unjust manner. It crosses into our realm as psychiatrists as mental health emergency calls are handled by the police and not by mental health professionals. The result is sometimes tragic: As many as half of police shootings involve people with psychiatric disorders, and the hope is that many of the police shootings could be avoided if crises were handed by mental health clinicians instead of, or in cooperation with, the police.
At best, police officers receive a week of specialized, crisis intervention training about how to approach those with psychiatric disorders; most officers receive no training. This leaves psychiatry as the only field where medical crises are routinely handled by the police – it is demeaning and embarrassing for some of our patients and dangerous for others. The reality remains, however, that there are times when psychiatric disorders result in violent behavior, and patients being taken for involuntary treatment often resist transport, so either way there is risk, both to the patient and to anyone who responds to a call for assistance.
Early this month, the office of New York City Mayor Bill de Blasio announced that a major change would be made in how mental health calls to 911 are handled in two “high-need” areas. The mayor’s website states:
“Beginning in February 2021, new Mental Health Teams will use their physical and mental health expertise, and experience in crisis response to de-escalate emergency situations, will help reduce the number of times police will need to respond to 911 mental health calls in these precincts. These teams will have the expertise to respond to a range of behavioral health problems, such as suicide attempts, substance misuse, and serious mental illness, as well as physical health problems, which can be exacerbated by or mask mental health problems. NYC Health + Hospitals will train and provide ongoing technical assistance and support. In selecting team members for this program, FDNY will prioritize professionals with significant experience with mental health crises.”
The press release goes on to say that, in situations where there is a weapon or reason to believe there is a risk of violence, the police will be dispatched along with the new mental health team.
“This is the first time in our history that health professionals will be the default responders to mental health emergencies,” New York City First Lady Chirlane McCray said as she announced the new program. “Treating mental health crises as mental health challenges and not public safety ones is the modern and more appropriate approach.”
New York City is not the first city to employ this model. In the United States, the CAHOOTS (Crisis Assistance Helping Out on the Streets) program in Eugene, Ore., has been run by the White Bird Clinic since 1989 as part of a community policing initiative. Last year, the team responded to 24,000 calls and police backup was required on only 150 of those responses. The CAHOOTS website states:
“The CAHOOTS model has been in the spotlight recently as our nation struggles to reimagine public safety. The program mobilizes two-person teams consisting of a medic (a nurse, paramedic, or EMT) and a crisis worker who has substantial training and experience in the mental health field. The CAHOOTS teams deal with a wide range of mental health-related crises, including conflict resolution, welfare checks, substance abuse, suicide threats, and more, relying on trauma-informed de-escalation and harm reduction techniques. CAHOOTS staff are not law enforcement officers and do not carry weapons; their training and experience are the tools they use to ensure a non-violent resolution of crisis situations. They also handle non-emergent medical issues, avoiding costly ambulance transport and emergency room treatment.”
Other cities in the United States are also looking at implementing programs where mental health teams, and not the police, respond to emergency calls. Last year, Oakland, Calif.’s city council invested $40,000 in research to assess how they could best implement a program like the one in Eugene. They hope to begin the Mobile Assistance Community Responders of Oakland (MACROS) next year. Sigal Samuel writes in a Vox article, “The goal is to launch the pilot next year with funding from the city budget, and although supporters are not yet sure what its size and duration will be, they’re hopeful it’ll make a big difference to Oakland’s overpoliced community of people without homes. They were among those who first called for a non-policing approach.”
The model is not unique to the United States. In 2005, Stockholm started a program with a psychiatric ambulance – equipped with comfortable seating rather than a stretcher – to respond to mental health emergencies. The ambulance responds to 130 calls a month. It is staffed with a driver and two psychiatric nurses, and for half of the calls, the police also come. While the Swedish program was not about removing resources from the police, it has relieved the police of the responsibility for many psychiatric emergencies.
The New York City program will be modeled after the CAHOOTS initiative in Eugene. It differs from the mobile crisis response services in many other cities because CAHOOTS is hooked directly into the 911 emergency services system. Its website notes that the program has saved money:
“The cost savings are considerable. The CAHOOTS program budget is about $2.1 million annually, while the combined annual budgets for the Eugene and Springfield police departments are $90 million. In 2017, the CAHOOTS teams answered 17% of the Eugene Police Department’s overall call volume. The program saves the city of Eugene an estimated $8.5 million in public safety spending annually.”
Some worry there is an unpredictable aspect to calls for psychiatric emergencies, and the potential for mental health professions to be injured or killed. Annette Hanson, MD, a forensic psychiatrist at University of Maryland, Baltimore, voiced her concerns, “While multidisciplinary teams are useful, there have been rare cases of violence against responding mental health providers. People with serious mental illness are rarely violent but their dangerousness is unpredictable and cannot be predicted by case screening.”
Daniel Felts is a mental health crisis counselor who has worked at CAHOOTS for the past 4* years. He has responded to about 8,000 calls, and called for police backup only three times to request an immediate "Code 3 cover" when someone's safety has been in danger. Mr. Felts calls the police about once a month for concerns that do not require an immediate response for safety.* “Over the last 4 years, I am only aware of three instances when a team member’s safety was compromised because of a client’s violent behavior. No employee has been seriously physically harmed. In 30 years, with hundreds of thousands (millions?) of calls responded to, no CAHOOTS worker has ever been killed, shot, or stabbed in the line of duty,” Mr. Felts noted.
Emergency calls are screened. “It is not uncommon for CAHOOTS to be dispatched to ‘stage’ for calls involving active disputes or acutely suicidal individuals where means are present. “Staging” entails us parking roughly a mile away while police make first contact and advise whether it is safe for CAHOOTS to engage.”
Mr. Felts went on to discuss the program’s relationship with the community. “ and how we operate. Having operated in Eugene for 30 years, our service is well understood to be one that does not kill, harm, or violate personal boundaries or liberties.”
Would a program like the ones in Stockholm or in Eugene work in other places? Eugene is a city with a population of 172,000 with a low crime rate. Whether a program implemented in one city can be mimicked in another very different city is not clear.
Paul Appelbaum, MD, a forensic psychiatrist at Columbia University, New York, is optimistic about New York City’s forthcoming program.
“The proposed pilot project in NYC is a real step forward. Work that we’ve done looking at fatal encounters involving the police found that roughly 25% of all deaths at the hands of the police are of people with mental illness. In many of those cases, police were initially called to bring people who were clearly troubled for psychiatric evaluation, but as the situation escalated, the police turned to their weapons to control it, which led to a fatal outcome. Taking police out of the picture whenever possible in favor of trained mental health personnel is clearly a better approach. It will be important for the city to collect good outcome data to enable independent evaluation of the pilot project – not something that political entities are inclined toward, but a critical element in assessing the effectiveness of this approach.”
There are questions that remain about the new program. Mayor de Blasio’s office has not released information about which areas of the city are being chosen for the new program, how much the program will cost, or what the funding source will be. If it can be implemented safely and effectively, it has the potential to provide more sensitive care to patients in crisis, and to save lives.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
*Correction, 11/27/2020: An earlier version of this article misstated the number of years Daniel Felts has worked at CAHOOTS.
“Defund the police”: It’s a slogan, or perhaps a battle cry, that has emerged from the Black Lives Matter movement as a response to race-related police brutality and concerns that people of color are profiled, targeted, arrested, charged, manhandled, and killed by law enforcement in a disproportionate and unjust manner. It crosses into our realm as psychiatrists as mental health emergency calls are handled by the police and not by mental health professionals. The result is sometimes tragic: As many as half of police shootings involve people with psychiatric disorders, and the hope is that many of the police shootings could be avoided if crises were handed by mental health clinicians instead of, or in cooperation with, the police.
At best, police officers receive a week of specialized, crisis intervention training about how to approach those with psychiatric disorders; most officers receive no training. This leaves psychiatry as the only field where medical crises are routinely handled by the police – it is demeaning and embarrassing for some of our patients and dangerous for others. The reality remains, however, that there are times when psychiatric disorders result in violent behavior, and patients being taken for involuntary treatment often resist transport, so either way there is risk, both to the patient and to anyone who responds to a call for assistance.
Early this month, the office of New York City Mayor Bill de Blasio announced that a major change would be made in how mental health calls to 911 are handled in two “high-need” areas. The mayor’s website states:
“Beginning in February 2021, new Mental Health Teams will use their physical and mental health expertise, and experience in crisis response to de-escalate emergency situations, will help reduce the number of times police will need to respond to 911 mental health calls in these precincts. These teams will have the expertise to respond to a range of behavioral health problems, such as suicide attempts, substance misuse, and serious mental illness, as well as physical health problems, which can be exacerbated by or mask mental health problems. NYC Health + Hospitals will train and provide ongoing technical assistance and support. In selecting team members for this program, FDNY will prioritize professionals with significant experience with mental health crises.”
The press release goes on to say that, in situations where there is a weapon or reason to believe there is a risk of violence, the police will be dispatched along with the new mental health team.
“This is the first time in our history that health professionals will be the default responders to mental health emergencies,” New York City First Lady Chirlane McCray said as she announced the new program. “Treating mental health crises as mental health challenges and not public safety ones is the modern and more appropriate approach.”
New York City is not the first city to employ this model. In the United States, the CAHOOTS (Crisis Assistance Helping Out on the Streets) program in Eugene, Ore., has been run by the White Bird Clinic since 1989 as part of a community policing initiative. Last year, the team responded to 24,000 calls and police backup was required on only 150 of those responses. The CAHOOTS website states:
“The CAHOOTS model has been in the spotlight recently as our nation struggles to reimagine public safety. The program mobilizes two-person teams consisting of a medic (a nurse, paramedic, or EMT) and a crisis worker who has substantial training and experience in the mental health field. The CAHOOTS teams deal with a wide range of mental health-related crises, including conflict resolution, welfare checks, substance abuse, suicide threats, and more, relying on trauma-informed de-escalation and harm reduction techniques. CAHOOTS staff are not law enforcement officers and do not carry weapons; their training and experience are the tools they use to ensure a non-violent resolution of crisis situations. They also handle non-emergent medical issues, avoiding costly ambulance transport and emergency room treatment.”
Other cities in the United States are also looking at implementing programs where mental health teams, and not the police, respond to emergency calls. Last year, Oakland, Calif.’s city council invested $40,000 in research to assess how they could best implement a program like the one in Eugene. They hope to begin the Mobile Assistance Community Responders of Oakland (MACROS) next year. Sigal Samuel writes in a Vox article, “The goal is to launch the pilot next year with funding from the city budget, and although supporters are not yet sure what its size and duration will be, they’re hopeful it’ll make a big difference to Oakland’s overpoliced community of people without homes. They were among those who first called for a non-policing approach.”
The model is not unique to the United States. In 2005, Stockholm started a program with a psychiatric ambulance – equipped with comfortable seating rather than a stretcher – to respond to mental health emergencies. The ambulance responds to 130 calls a month. It is staffed with a driver and two psychiatric nurses, and for half of the calls, the police also come. While the Swedish program was not about removing resources from the police, it has relieved the police of the responsibility for many psychiatric emergencies.
The New York City program will be modeled after the CAHOOTS initiative in Eugene. It differs from the mobile crisis response services in many other cities because CAHOOTS is hooked directly into the 911 emergency services system. Its website notes that the program has saved money:
“The cost savings are considerable. The CAHOOTS program budget is about $2.1 million annually, while the combined annual budgets for the Eugene and Springfield police departments are $90 million. In 2017, the CAHOOTS teams answered 17% of the Eugene Police Department’s overall call volume. The program saves the city of Eugene an estimated $8.5 million in public safety spending annually.”
Some worry there is an unpredictable aspect to calls for psychiatric emergencies, and the potential for mental health professions to be injured or killed. Annette Hanson, MD, a forensic psychiatrist at University of Maryland, Baltimore, voiced her concerns, “While multidisciplinary teams are useful, there have been rare cases of violence against responding mental health providers. People with serious mental illness are rarely violent but their dangerousness is unpredictable and cannot be predicted by case screening.”
Daniel Felts is a mental health crisis counselor who has worked at CAHOOTS for the past 4* years. He has responded to about 8,000 calls, and called for police backup only three times to request an immediate "Code 3 cover" when someone's safety has been in danger. Mr. Felts calls the police about once a month for concerns that do not require an immediate response for safety.* “Over the last 4 years, I am only aware of three instances when a team member’s safety was compromised because of a client’s violent behavior. No employee has been seriously physically harmed. In 30 years, with hundreds of thousands (millions?) of calls responded to, no CAHOOTS worker has ever been killed, shot, or stabbed in the line of duty,” Mr. Felts noted.
Emergency calls are screened. “It is not uncommon for CAHOOTS to be dispatched to ‘stage’ for calls involving active disputes or acutely suicidal individuals where means are present. “Staging” entails us parking roughly a mile away while police make first contact and advise whether it is safe for CAHOOTS to engage.”
Mr. Felts went on to discuss the program’s relationship with the community. “ and how we operate. Having operated in Eugene for 30 years, our service is well understood to be one that does not kill, harm, or violate personal boundaries or liberties.”
Would a program like the ones in Stockholm or in Eugene work in other places? Eugene is a city with a population of 172,000 with a low crime rate. Whether a program implemented in one city can be mimicked in another very different city is not clear.
Paul Appelbaum, MD, a forensic psychiatrist at Columbia University, New York, is optimistic about New York City’s forthcoming program.
“The proposed pilot project in NYC is a real step forward. Work that we’ve done looking at fatal encounters involving the police found that roughly 25% of all deaths at the hands of the police are of people with mental illness. In many of those cases, police were initially called to bring people who were clearly troubled for psychiatric evaluation, but as the situation escalated, the police turned to their weapons to control it, which led to a fatal outcome. Taking police out of the picture whenever possible in favor of trained mental health personnel is clearly a better approach. It will be important for the city to collect good outcome data to enable independent evaluation of the pilot project – not something that political entities are inclined toward, but a critical element in assessing the effectiveness of this approach.”
There are questions that remain about the new program. Mayor de Blasio’s office has not released information about which areas of the city are being chosen for the new program, how much the program will cost, or what the funding source will be. If it can be implemented safely and effectively, it has the potential to provide more sensitive care to patients in crisis, and to save lives.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
*Correction, 11/27/2020: An earlier version of this article misstated the number of years Daniel Felts has worked at CAHOOTS.
Excited delirium: Is it time to change the status quo?
Prior to George Floyd’s death, Officer Thomas Lane reportedly said, “I am worried about excited delirium or whatever” to his colleague, Officer Derek Chauvin.1 For those of us who frequently work with law enforcement and in correctional facilities, “excited delirium” is a common refrain. It would be too facile to dismiss the concept as an attempt by police officers to inappropriately use medically sounding jargon to justify violence. “Excited delirium” is a reminder of the complex situations faced by police officers and the need for better medical training, as well as the attention of research on this commonly used label.
Many law enforcement facilities, in particular jails that receive inmates directly from the community, will have large posters educating staff on the “signs of excited delirium.” The concept is not covered in residency training programs, or many of the leading textbooks of psychiatry. Yet, it has become common parlance in law enforcement. Officers in training receive education programs on excited delirium, although those are rarely conducted by clinicians.
In our practice and experience, “excited delirium” has been used by law enforcement officers to describe mood lability from the stress of arrest, acute agitation from stimulant or phencyclidine intoxication, actual delirium from a medical comorbidity, sociopathic aggression for the purpose of violence, and incoherence from psychosis, along with simply describing a person not following direction from a police officer.
Our differential diagnosis when informed that someone was described by a nonclinician as having so-called excited delirium is wider than the Diagnostic and Statistical Manual (DSM). In addition, the term comes at a cost. Its use has been implicated in police-related deaths and brutality.2 There is also concern of its disproportionate application to Black people.3,4
Nonetheless, the term “excited delirium” can sometimes accurately describe critical medical situations. We particularly remember a case of altered mental status from serotonin syndrome, a case of delirium tremens from alcohol withdrawal, and a case of life-threatening dehydration in the context of stimulant intoxication. Each of those cases was appropriately recognized as problematic by perceptive and caring police officers. It is important for police officers to recognize these life-threatening conditions, and they need the language to do so. Having a common label that can be used across professional fields and law enforcement departments to express medical concern in the context of aggressive behavior has value. The question is: can psychiatry help law enforcement describe situations more accurately?
As physicians, it would be overly simple to point out the limited understanding of medical information by police and correctional officers. Naming many behaviors poses significant challenges for psychiatrists and nonclinicians. Examples include the use of the word “agitation” to describe mild restlessness, “delusional” for uncooperative, and “irritable” for opinionated. We must also be cognizant of the infinite demands placed on police officers and that labels must be available to them to express complex situations without being forced to use medical diagnosis and terminology for which they do not have the license or expertise. It is possible that “excited delirium” serves an important role; the problem may not be as much “excited delirium,” the term itself, as the diversion of its use to justify poor policing.
It must be acknowledged that debates, concerns, poor nomenclature, confusing labels, and different interpretations of diagnoses and symptoms are not unusual things in psychiatry, even among professionals. In the 1970s, the famous American and British study of diagnostic criteria, showed that psychiatrists used the diagnosis of schizophrenia to describe vastly different patients.5 The findings of the study were a significant cause of the paradigm shift of the DSM in its 3rd edition. More recently, the DSM-5 field trials suggested that the field of psychiatry continues to struggle with this problem.6 Nonetheless, each edition of the DSM presents a new opportunity to discuss, refine, and improve our ability to communicate while emphasizing the importance of improving our common language.
Emergency physicians face delirious patients brought to them from the community on a regular basis. As such, it makes sense that they have been at the forefront of this issue and the American College of Emergency Physicians has recognized excited delirium as a condition since 2009.7 The emergency physician literature points out that death from excited delirium also happens in hospitals and is not a unique consequence of law enforcement. There is no accepted definition. Reported symptoms include agitation, bizarre behavior, tirelessness, unusual strength, pain tolerance, noncompliance, attraction to reflective surfaces, stupor, fear, panic, hyperthermia, inappropriate clothing, tachycardia, tachypnea, diaphoresis, seizure, and mydriasis. Etiology is suspected to be from catecholaminergic endogenous stress-related catecholamines and exogenous catecholaminergic drugs. In particular is the importance of dopamine through the use of stimulants, specifically cocaine. The literature makes some reference to management, including recommendations aimed at keeping patients on one of their sides, using de-escalation techniques, and performing evaluation in quiet rooms.
We certainly condone and commend efforts to understand and define this condition in the medical literature. The indiscriminate use of “excited delirium” to represent all sorts of behaviors by nonmedical personnel warrants intelligent, relevant, and researched commentary by physicians. There are several potentially appropriate ways forward. First, psychiatry may decide that excited delirium is not a useful diagnosis in the clinical setting and does not belong in the DSM. That distinction in itself would be potentially useful to law enforcement officers, who might welcome the opportunity to create their own nomenclature and classification. Second, psychiatry may decide that excited delirium is not a useful diagnosis in the clinical setting but warrants a definition nonetheless, akin to the ways homelessness and extreme poverty are defined in the DSM; this definition could take into account the wide use of the term by nonclinicians. Third, psychiatry may decide that excited delirium warrants a clinical diagnosis that warrants a distinction and clarification from the current delirium diagnosis with the hyperactive specifier.
At this time, the status quo doesn’t protect or help clinicians in their respective fields of work. “Excited delirium” is routinely used by law enforcement officers without clear meaning. Experts have difficulty pointing out the poor or ill-intended use of the term without a precise or accepted definition to rely on. Some of the proposed criteria, such as “unusual strength,” have unclear scientific legitimacy. Some, such as agitation or bizarre behavior, often have different meanings to nonphysicians. Some, such as poor clothing, may facilitate discrimination. The current state allows some professionals to hide their limited attempts at de-escalation by describing the person of interest as having excited delirium. On the other hand, the current state also prevents well-intended officers from using proper terminology that is understood by others as describing a concerning behavior reliably.
We wonder whether excited delirium is an important facet of the current dilemma of reconsidering the role of law enforcement in society. Frequent use of “excited delirium” by police officers is itself a testament to their desire to have assistance or delegation of certain duties to other social services, such as health care. In some ways, police officers face a difficult position: Admission that a behavior may be attributable to excited delirium should warrant a medical evaluation and, thus, render the person of interest a patient rather than a suspect. As such, this person interacting with police officers should be treated as someone in need of medical care, which makes many interventions – including neck compression – seemingly inappropriate. The frequent use of “excited delirium” suggests that law enforcement is ill-equipped in handling many situations and that an attempt to diversify the composition and funding of emergency response might be warranted. Psychiatry should be at the forefront of this research and effort.
References
1. State of Minnesota v. Derek Michael Chauvin (4th Judicial District, 2020 May 29).
2. J Forensic Leg Med. 2008 May 15(4):227-30.
3. “Excited delirium: Rare and deadly syndrome or a condition to excuse deaths by police?” Florida Today. 2020 Jan 20.
4. J Forensic Sci. 1997 Jan;42(1):25-31.
5. Arch Gen Psychiatry. 1971;25(2):123-30.
6. Am J Psychiatry. 2013 Jan;170(1):59-70.
7. White Paper Report on Excited Delirium Syndrome. ACEP Excited Delirium Task Force. 2009 Sep 10.
Dr. Amendolara is a first-year psychiatry resident at University of California, San Diego. He spent years advocating for survivors of rape and domestic violence at the Crime Victims Treatment Center in New York and conducted public health research at Lourdes Center for Public Health in Camden, N.J. Dr. Amendolara has no disclosures. Dr. Malik is a first-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures. Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology, and correctional mental health. He holds teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures. Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Prior to George Floyd’s death, Officer Thomas Lane reportedly said, “I am worried about excited delirium or whatever” to his colleague, Officer Derek Chauvin.1 For those of us who frequently work with law enforcement and in correctional facilities, “excited delirium” is a common refrain. It would be too facile to dismiss the concept as an attempt by police officers to inappropriately use medically sounding jargon to justify violence. “Excited delirium” is a reminder of the complex situations faced by police officers and the need for better medical training, as well as the attention of research on this commonly used label.
Many law enforcement facilities, in particular jails that receive inmates directly from the community, will have large posters educating staff on the “signs of excited delirium.” The concept is not covered in residency training programs, or many of the leading textbooks of psychiatry. Yet, it has become common parlance in law enforcement. Officers in training receive education programs on excited delirium, although those are rarely conducted by clinicians.
In our practice and experience, “excited delirium” has been used by law enforcement officers to describe mood lability from the stress of arrest, acute agitation from stimulant or phencyclidine intoxication, actual delirium from a medical comorbidity, sociopathic aggression for the purpose of violence, and incoherence from psychosis, along with simply describing a person not following direction from a police officer.
Our differential diagnosis when informed that someone was described by a nonclinician as having so-called excited delirium is wider than the Diagnostic and Statistical Manual (DSM). In addition, the term comes at a cost. Its use has been implicated in police-related deaths and brutality.2 There is also concern of its disproportionate application to Black people.3,4
Nonetheless, the term “excited delirium” can sometimes accurately describe critical medical situations. We particularly remember a case of altered mental status from serotonin syndrome, a case of delirium tremens from alcohol withdrawal, and a case of life-threatening dehydration in the context of stimulant intoxication. Each of those cases was appropriately recognized as problematic by perceptive and caring police officers. It is important for police officers to recognize these life-threatening conditions, and they need the language to do so. Having a common label that can be used across professional fields and law enforcement departments to express medical concern in the context of aggressive behavior has value. The question is: can psychiatry help law enforcement describe situations more accurately?
As physicians, it would be overly simple to point out the limited understanding of medical information by police and correctional officers. Naming many behaviors poses significant challenges for psychiatrists and nonclinicians. Examples include the use of the word “agitation” to describe mild restlessness, “delusional” for uncooperative, and “irritable” for opinionated. We must also be cognizant of the infinite demands placed on police officers and that labels must be available to them to express complex situations without being forced to use medical diagnosis and terminology for which they do not have the license or expertise. It is possible that “excited delirium” serves an important role; the problem may not be as much “excited delirium,” the term itself, as the diversion of its use to justify poor policing.
It must be acknowledged that debates, concerns, poor nomenclature, confusing labels, and different interpretations of diagnoses and symptoms are not unusual things in psychiatry, even among professionals. In the 1970s, the famous American and British study of diagnostic criteria, showed that psychiatrists used the diagnosis of schizophrenia to describe vastly different patients.5 The findings of the study were a significant cause of the paradigm shift of the DSM in its 3rd edition. More recently, the DSM-5 field trials suggested that the field of psychiatry continues to struggle with this problem.6 Nonetheless, each edition of the DSM presents a new opportunity to discuss, refine, and improve our ability to communicate while emphasizing the importance of improving our common language.
Emergency physicians face delirious patients brought to them from the community on a regular basis. As such, it makes sense that they have been at the forefront of this issue and the American College of Emergency Physicians has recognized excited delirium as a condition since 2009.7 The emergency physician literature points out that death from excited delirium also happens in hospitals and is not a unique consequence of law enforcement. There is no accepted definition. Reported symptoms include agitation, bizarre behavior, tirelessness, unusual strength, pain tolerance, noncompliance, attraction to reflective surfaces, stupor, fear, panic, hyperthermia, inappropriate clothing, tachycardia, tachypnea, diaphoresis, seizure, and mydriasis. Etiology is suspected to be from catecholaminergic endogenous stress-related catecholamines and exogenous catecholaminergic drugs. In particular is the importance of dopamine through the use of stimulants, specifically cocaine. The literature makes some reference to management, including recommendations aimed at keeping patients on one of their sides, using de-escalation techniques, and performing evaluation in quiet rooms.
We certainly condone and commend efforts to understand and define this condition in the medical literature. The indiscriminate use of “excited delirium” to represent all sorts of behaviors by nonmedical personnel warrants intelligent, relevant, and researched commentary by physicians. There are several potentially appropriate ways forward. First, psychiatry may decide that excited delirium is not a useful diagnosis in the clinical setting and does not belong in the DSM. That distinction in itself would be potentially useful to law enforcement officers, who might welcome the opportunity to create their own nomenclature and classification. Second, psychiatry may decide that excited delirium is not a useful diagnosis in the clinical setting but warrants a definition nonetheless, akin to the ways homelessness and extreme poverty are defined in the DSM; this definition could take into account the wide use of the term by nonclinicians. Third, psychiatry may decide that excited delirium warrants a clinical diagnosis that warrants a distinction and clarification from the current delirium diagnosis with the hyperactive specifier.
At this time, the status quo doesn’t protect or help clinicians in their respective fields of work. “Excited delirium” is routinely used by law enforcement officers without clear meaning. Experts have difficulty pointing out the poor or ill-intended use of the term without a precise or accepted definition to rely on. Some of the proposed criteria, such as “unusual strength,” have unclear scientific legitimacy. Some, such as agitation or bizarre behavior, often have different meanings to nonphysicians. Some, such as poor clothing, may facilitate discrimination. The current state allows some professionals to hide their limited attempts at de-escalation by describing the person of interest as having excited delirium. On the other hand, the current state also prevents well-intended officers from using proper terminology that is understood by others as describing a concerning behavior reliably.
We wonder whether excited delirium is an important facet of the current dilemma of reconsidering the role of law enforcement in society. Frequent use of “excited delirium” by police officers is itself a testament to their desire to have assistance or delegation of certain duties to other social services, such as health care. In some ways, police officers face a difficult position: Admission that a behavior may be attributable to excited delirium should warrant a medical evaluation and, thus, render the person of interest a patient rather than a suspect. As such, this person interacting with police officers should be treated as someone in need of medical care, which makes many interventions – including neck compression – seemingly inappropriate. The frequent use of “excited delirium” suggests that law enforcement is ill-equipped in handling many situations and that an attempt to diversify the composition and funding of emergency response might be warranted. Psychiatry should be at the forefront of this research and effort.
References
1. State of Minnesota v. Derek Michael Chauvin (4th Judicial District, 2020 May 29).
2. J Forensic Leg Med. 2008 May 15(4):227-30.
3. “Excited delirium: Rare and deadly syndrome or a condition to excuse deaths by police?” Florida Today. 2020 Jan 20.
4. J Forensic Sci. 1997 Jan;42(1):25-31.
5. Arch Gen Psychiatry. 1971;25(2):123-30.
6. Am J Psychiatry. 2013 Jan;170(1):59-70.
7. White Paper Report on Excited Delirium Syndrome. ACEP Excited Delirium Task Force. 2009 Sep 10.
Dr. Amendolara is a first-year psychiatry resident at University of California, San Diego. He spent years advocating for survivors of rape and domestic violence at the Crime Victims Treatment Center in New York and conducted public health research at Lourdes Center for Public Health in Camden, N.J. Dr. Amendolara has no disclosures. Dr. Malik is a first-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures. Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology, and correctional mental health. He holds teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures. Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Prior to George Floyd’s death, Officer Thomas Lane reportedly said, “I am worried about excited delirium or whatever” to his colleague, Officer Derek Chauvin.1 For those of us who frequently work with law enforcement and in correctional facilities, “excited delirium” is a common refrain. It would be too facile to dismiss the concept as an attempt by police officers to inappropriately use medically sounding jargon to justify violence. “Excited delirium” is a reminder of the complex situations faced by police officers and the need for better medical training, as well as the attention of research on this commonly used label.
Many law enforcement facilities, in particular jails that receive inmates directly from the community, will have large posters educating staff on the “signs of excited delirium.” The concept is not covered in residency training programs, or many of the leading textbooks of psychiatry. Yet, it has become common parlance in law enforcement. Officers in training receive education programs on excited delirium, although those are rarely conducted by clinicians.
In our practice and experience, “excited delirium” has been used by law enforcement officers to describe mood lability from the stress of arrest, acute agitation from stimulant or phencyclidine intoxication, actual delirium from a medical comorbidity, sociopathic aggression for the purpose of violence, and incoherence from psychosis, along with simply describing a person not following direction from a police officer.
Our differential diagnosis when informed that someone was described by a nonclinician as having so-called excited delirium is wider than the Diagnostic and Statistical Manual (DSM). In addition, the term comes at a cost. Its use has been implicated in police-related deaths and brutality.2 There is also concern of its disproportionate application to Black people.3,4
Nonetheless, the term “excited delirium” can sometimes accurately describe critical medical situations. We particularly remember a case of altered mental status from serotonin syndrome, a case of delirium tremens from alcohol withdrawal, and a case of life-threatening dehydration in the context of stimulant intoxication. Each of those cases was appropriately recognized as problematic by perceptive and caring police officers. It is important for police officers to recognize these life-threatening conditions, and they need the language to do so. Having a common label that can be used across professional fields and law enforcement departments to express medical concern in the context of aggressive behavior has value. The question is: can psychiatry help law enforcement describe situations more accurately?
As physicians, it would be overly simple to point out the limited understanding of medical information by police and correctional officers. Naming many behaviors poses significant challenges for psychiatrists and nonclinicians. Examples include the use of the word “agitation” to describe mild restlessness, “delusional” for uncooperative, and “irritable” for opinionated. We must also be cognizant of the infinite demands placed on police officers and that labels must be available to them to express complex situations without being forced to use medical diagnosis and terminology for which they do not have the license or expertise. It is possible that “excited delirium” serves an important role; the problem may not be as much “excited delirium,” the term itself, as the diversion of its use to justify poor policing.
It must be acknowledged that debates, concerns, poor nomenclature, confusing labels, and different interpretations of diagnoses and symptoms are not unusual things in psychiatry, even among professionals. In the 1970s, the famous American and British study of diagnostic criteria, showed that psychiatrists used the diagnosis of schizophrenia to describe vastly different patients.5 The findings of the study were a significant cause of the paradigm shift of the DSM in its 3rd edition. More recently, the DSM-5 field trials suggested that the field of psychiatry continues to struggle with this problem.6 Nonetheless, each edition of the DSM presents a new opportunity to discuss, refine, and improve our ability to communicate while emphasizing the importance of improving our common language.
Emergency physicians face delirious patients brought to them from the community on a regular basis. As such, it makes sense that they have been at the forefront of this issue and the American College of Emergency Physicians has recognized excited delirium as a condition since 2009.7 The emergency physician literature points out that death from excited delirium also happens in hospitals and is not a unique consequence of law enforcement. There is no accepted definition. Reported symptoms include agitation, bizarre behavior, tirelessness, unusual strength, pain tolerance, noncompliance, attraction to reflective surfaces, stupor, fear, panic, hyperthermia, inappropriate clothing, tachycardia, tachypnea, diaphoresis, seizure, and mydriasis. Etiology is suspected to be from catecholaminergic endogenous stress-related catecholamines and exogenous catecholaminergic drugs. In particular is the importance of dopamine through the use of stimulants, specifically cocaine. The literature makes some reference to management, including recommendations aimed at keeping patients on one of their sides, using de-escalation techniques, and performing evaluation in quiet rooms.
We certainly condone and commend efforts to understand and define this condition in the medical literature. The indiscriminate use of “excited delirium” to represent all sorts of behaviors by nonmedical personnel warrants intelligent, relevant, and researched commentary by physicians. There are several potentially appropriate ways forward. First, psychiatry may decide that excited delirium is not a useful diagnosis in the clinical setting and does not belong in the DSM. That distinction in itself would be potentially useful to law enforcement officers, who might welcome the opportunity to create their own nomenclature and classification. Second, psychiatry may decide that excited delirium is not a useful diagnosis in the clinical setting but warrants a definition nonetheless, akin to the ways homelessness and extreme poverty are defined in the DSM; this definition could take into account the wide use of the term by nonclinicians. Third, psychiatry may decide that excited delirium warrants a clinical diagnosis that warrants a distinction and clarification from the current delirium diagnosis with the hyperactive specifier.
At this time, the status quo doesn’t protect or help clinicians in their respective fields of work. “Excited delirium” is routinely used by law enforcement officers without clear meaning. Experts have difficulty pointing out the poor or ill-intended use of the term without a precise or accepted definition to rely on. Some of the proposed criteria, such as “unusual strength,” have unclear scientific legitimacy. Some, such as agitation or bizarre behavior, often have different meanings to nonphysicians. Some, such as poor clothing, may facilitate discrimination. The current state allows some professionals to hide their limited attempts at de-escalation by describing the person of interest as having excited delirium. On the other hand, the current state also prevents well-intended officers from using proper terminology that is understood by others as describing a concerning behavior reliably.
We wonder whether excited delirium is an important facet of the current dilemma of reconsidering the role of law enforcement in society. Frequent use of “excited delirium” by police officers is itself a testament to their desire to have assistance or delegation of certain duties to other social services, such as health care. In some ways, police officers face a difficult position: Admission that a behavior may be attributable to excited delirium should warrant a medical evaluation and, thus, render the person of interest a patient rather than a suspect. As such, this person interacting with police officers should be treated as someone in need of medical care, which makes many interventions – including neck compression – seemingly inappropriate. The frequent use of “excited delirium” suggests that law enforcement is ill-equipped in handling many situations and that an attempt to diversify the composition and funding of emergency response might be warranted. Psychiatry should be at the forefront of this research and effort.
References
1. State of Minnesota v. Derek Michael Chauvin (4th Judicial District, 2020 May 29).
2. J Forensic Leg Med. 2008 May 15(4):227-30.
3. “Excited delirium: Rare and deadly syndrome or a condition to excuse deaths by police?” Florida Today. 2020 Jan 20.
4. J Forensic Sci. 1997 Jan;42(1):25-31.
5. Arch Gen Psychiatry. 1971;25(2):123-30.
6. Am J Psychiatry. 2013 Jan;170(1):59-70.
7. White Paper Report on Excited Delirium Syndrome. ACEP Excited Delirium Task Force. 2009 Sep 10.
Dr. Amendolara is a first-year psychiatry resident at University of California, San Diego. He spent years advocating for survivors of rape and domestic violence at the Crime Victims Treatment Center in New York and conducted public health research at Lourdes Center for Public Health in Camden, N.J. Dr. Amendolara has no disclosures. Dr. Malik is a first-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures. Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology, and correctional mental health. He holds teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures. Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Siblings of patients with bipolar disorder at increased risk
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
FROM ECNP 2020
Dripping, dabbing, and bongs: Can’t tell the players without a scorecard
E-cigarettes may be synonymous with vaping to most physicians, but there are other ways for patients to inhale nicotine or tetrahydrocannabinol-containing aerosols, according to investigators at the Cleveland Clinic. 
Humberto Choi, MD, and associates wrote in the Annals of the American Thoracic Society.
These “alternate aerosol inhalation methods” have been poorly described thus far, so little is known about their scope of use and potential health impact, they noted.
Dripping involves an e-cigarette modified to expose the heating coil. The e-cigarette liquid is dripped directly onto the hot coil, which produces immediate aerosolization and results in a thicker cloud.
Dripping “may expose users to higher levels of nicotine compared to e-cigarette inhalation” and lead to “increased release of volatile aldehydes as a result of the higher heating potential of direct atomizer exposure,” the investigators suggested.
Water pipes, or bongs, produce both smoke and vapor, although an electronic vaporizer can be attached to create a “vape bong.” About 21% of daily cannabis users report using a bong, but tobacco inhalation is less common. Cases of severe pulmonary infections have been associated with bong use, along with a couple of tuberculosis clusters, Dr. Choi and associates said.
Dabbing uses butane-extracted, concentrated cannabis oil inhaled through a modified water pipe or bong or a smaller device called a “dab pen.” A small amount, or “dab,” of the product is placed on the “nail,” which replaces the bowl of the water pipe, heated with a blowtorch, and inhaled through the pipe, the researchers explained.
The prevalence of dabbing is unknown, but “the most recent Monitoring the Future survey of high school seniors shows that 11.9% of students have used a marijuana vaporizer at some point in their life,” they said.
Besides the fire risks involved in creating the material needed for dabbing – use of heating plates, ovens, and devices for removing butane vapors – inhalation of residual butane vapors could lead to vomiting, cardiac arrhythmias, acute encephalopathy, and respiratory depression, Dr. Choi and associates said.
Nicotine dependence is also a concern, as is the possibility of withdrawal symptoms. “Patients presenting with prolonged and severe vomiting, psychotic symptoms, or other acute neuropsychiatric symptoms should raise the suspicion of [tetrahydrocannabinol]-containing products especially synthetic cannabinoids,” they wrote.
SOURCE: Choi H et al. Ann Am Thorac Soc. 2020 Oct 14. doi: 10.1513/AnnalsATS.202005-511CME.
E-cigarettes may be synonymous with vaping to most physicians, but there are other ways for patients to inhale nicotine or tetrahydrocannabinol-containing aerosols, according to investigators at the Cleveland Clinic. 
Humberto Choi, MD, and associates wrote in the Annals of the American Thoracic Society.
These “alternate aerosol inhalation methods” have been poorly described thus far, so little is known about their scope of use and potential health impact, they noted.
Dripping involves an e-cigarette modified to expose the heating coil. The e-cigarette liquid is dripped directly onto the hot coil, which produces immediate aerosolization and results in a thicker cloud.
Dripping “may expose users to higher levels of nicotine compared to e-cigarette inhalation” and lead to “increased release of volatile aldehydes as a result of the higher heating potential of direct atomizer exposure,” the investigators suggested.
Water pipes, or bongs, produce both smoke and vapor, although an electronic vaporizer can be attached to create a “vape bong.” About 21% of daily cannabis users report using a bong, but tobacco inhalation is less common. Cases of severe pulmonary infections have been associated with bong use, along with a couple of tuberculosis clusters, Dr. Choi and associates said.
Dabbing uses butane-extracted, concentrated cannabis oil inhaled through a modified water pipe or bong or a smaller device called a “dab pen.” A small amount, or “dab,” of the product is placed on the “nail,” which replaces the bowl of the water pipe, heated with a blowtorch, and inhaled through the pipe, the researchers explained.
The prevalence of dabbing is unknown, but “the most recent Monitoring the Future survey of high school seniors shows that 11.9% of students have used a marijuana vaporizer at some point in their life,” they said.
Besides the fire risks involved in creating the material needed for dabbing – use of heating plates, ovens, and devices for removing butane vapors – inhalation of residual butane vapors could lead to vomiting, cardiac arrhythmias, acute encephalopathy, and respiratory depression, Dr. Choi and associates said.
Nicotine dependence is also a concern, as is the possibility of withdrawal symptoms. “Patients presenting with prolonged and severe vomiting, psychotic symptoms, or other acute neuropsychiatric symptoms should raise the suspicion of [tetrahydrocannabinol]-containing products especially synthetic cannabinoids,” they wrote.
SOURCE: Choi H et al. Ann Am Thorac Soc. 2020 Oct 14. doi: 10.1513/AnnalsATS.202005-511CME.
E-cigarettes may be synonymous with vaping to most physicians, but there are other ways for patients to inhale nicotine or tetrahydrocannabinol-containing aerosols, according to investigators at the Cleveland Clinic. 
Humberto Choi, MD, and associates wrote in the Annals of the American Thoracic Society.
These “alternate aerosol inhalation methods” have been poorly described thus far, so little is known about their scope of use and potential health impact, they noted.
Dripping involves an e-cigarette modified to expose the heating coil. The e-cigarette liquid is dripped directly onto the hot coil, which produces immediate aerosolization and results in a thicker cloud.
Dripping “may expose users to higher levels of nicotine compared to e-cigarette inhalation” and lead to “increased release of volatile aldehydes as a result of the higher heating potential of direct atomizer exposure,” the investigators suggested.
Water pipes, or bongs, produce both smoke and vapor, although an electronic vaporizer can be attached to create a “vape bong.” About 21% of daily cannabis users report using a bong, but tobacco inhalation is less common. Cases of severe pulmonary infections have been associated with bong use, along with a couple of tuberculosis clusters, Dr. Choi and associates said.
Dabbing uses butane-extracted, concentrated cannabis oil inhaled through a modified water pipe or bong or a smaller device called a “dab pen.” A small amount, or “dab,” of the product is placed on the “nail,” which replaces the bowl of the water pipe, heated with a blowtorch, and inhaled through the pipe, the researchers explained.
The prevalence of dabbing is unknown, but “the most recent Monitoring the Future survey of high school seniors shows that 11.9% of students have used a marijuana vaporizer at some point in their life,” they said.
Besides the fire risks involved in creating the material needed for dabbing – use of heating plates, ovens, and devices for removing butane vapors – inhalation of residual butane vapors could lead to vomiting, cardiac arrhythmias, acute encephalopathy, and respiratory depression, Dr. Choi and associates said.
Nicotine dependence is also a concern, as is the possibility of withdrawal symptoms. “Patients presenting with prolonged and severe vomiting, psychotic symptoms, or other acute neuropsychiatric symptoms should raise the suspicion of [tetrahydrocannabinol]-containing products especially synthetic cannabinoids,” they wrote.
SOURCE: Choi H et al. Ann Am Thorac Soc. 2020 Oct 14. doi: 10.1513/AnnalsATS.202005-511CME.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Cirrhosis, Child-Pugh score predict ERCP complications
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
FROM ACG 2020
How mental health care would look under a Trump vs. Biden administration
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
Addressing adolescent substance use requires establishing consistent procedures
according to Lucien Gonzalez, MD, assistant professor of psychiatry and behavioral sciences at the University of Minnesota, Minneapolis.
In a presentation at the annual meeting of the American Academy of Pediatrics, held virtually this year, Dr. Gonzalez discussed some of the common challenges pediatricians face in appropriately screening, diagnosing, and managing or referring youth when it comes to substance use.
Substance use screening
One of these included picking the right assessment tool and frequency for screening patients for substance use. A number of validated tools are out there, including the Screening to Brief Intervention (S2BI) and CRAFFT Screening Tool for Adolescent Substance Abuse. Regardless of which screening tool providers choose, “the important thing is to use a tool that is validated in the pediatric population and ideally has frequency results in it,” Dr. Gonzalez said.
In terms of frequency, screening young people at least once a year is fairly standard, but it may be necessary to screen adolescents more often or to screen them at acute visits.
“As many of you who work with adolescents know, you can’t always rely on the yearly well child visit because after a certain age, you start to see drop-off,” Dr. Gonzalez said. “They often aren’t coming for well child visits, and they often are then only showing up for acute visits.”
That means doctors need to think about how their clinics operate, how often they see their teen patients, and other factors – including how much can happen in a single year of adolescence – to ensure that screening captures these patients at least once a year, but more if that works within the practice.
Screening vs. diagnosis
Dr. Gonzalez also addressed the difference between screening and diagnosis, a very familiar distinction to physicians in other areas of medicine but often a source of confusion in the area of substance use.
“Screening is the presumptive identification of unrecognized disease in apparently healthy people who don’t have symptoms, using assessments that can be used rapidly,” Dr. Gonzalez said. “When we move into the diagnostic realm, these are people who present with symptoms or they have positive results on our screening test prompting further investigation.”
Sonia Khan, MD, a pediatrician and the medical director of the substance use disorder counseling program in the department of health and human services in Fremont, Calif., who heard the talk, particularly appreciated this point about screening versus diagnosis.
“As soon as you get a hint that there’s a problem with the kid, you’re no longer screening. You’re doing diagnostic investigation,” Dr. Khan, also the human relations commissioner for the city of Fremont, Calif., said in an interview. “Screening is about the kids you don’t know about. It seems like a small point to make a big deal out of, but it’s not.”
Sometimes a screening tool can serve as an introductory interview guide when beginning a clinical investigation with a patient who already shows symptoms, but that doesn’t mean it’s a screen.
Dr. Gonzalez emphasized the importance of not prescreening.
“A prescreener looks at a kid and decides whether or not they need to be screened,” Dr. Gonzalez said. “We have research that demonstrates that that doesn’t work. Physicians are not good at determining this by eyeballing it, and it’s fraught with bias. Universal screening with a validated screening tool is what works.”
Again, the idea of confronting one’s own personal biases and how they could interfere with screening really resonated with Dr. Khan.
“When it comes to the prescreening, if you’re only screening the ones you [think you] need to screen, you’re introducing bias into your screening,” she said. “It’s usually judgmental. It’s important to focus on really getting the bias out of what you’re doing because it’s a field fraught with bias and expectations.”
Brief interventions
Another area of confusion for many providers is what qualifies as a brief intervention and how to deliver it. The brief intervention needs to focus on increasing the patient’s knowledge, insights, and awareness when it comes to their own substance use and how it affects others. It should also support motivation in the patient to make behavioral changes. “It is always given in a nonjudgmental, supportive manner,” Dr. Gonzalez said.
Though motivational interviewing is often discussed as though it’s a brief intervention, it is actually the mechanism for delivering the intervention – not the intervention itself.
Dr. Gonzalez highly recommended that providers seek motivational interviewing training if they haven’t already. He went on to caution attendees about behavior goals in interventions: They should be the patient’s change goals, not the provider’s, and the provider is there to facilitate the teen’s clarification of those goals.
“It’s very important to use those listening skills that we have and honor their decision-making and listen to their language in establishing their own goals,” he said. It’s also important to keep cultural relevance and respect in mind when delivering the intervention. He shared a chart showing the dominant and nondominant groups along various demographic cultural influences, including age, disability status, faith, race/ethnicity, indigenous heritage, socioeconomic status, national origin, gender and sexuality.
For example, the dominant age groups are the young and middle-aged while the nondominant are children and elderly. The dominant faith in the United States is Christian or secular, and the dominant sexuality is heterosexual; the corresponding nondominant groups would be non-Christian and nonheterosexual. It’s important for providers to consider the child’s needs within that entire behavioral context to understand where they’re coming from.
“Have you ever characterized a kid’s situation with regard to substance use and diagnoses based on certain characteristics?” Dr. Gonzalez asked attendees. “We like to think that we don’t, but research on diagnostic disparities indicates otherwise.”
A way to help avoid this is to know who you are in the room and who you’re with in terms of dominant and nondominant groups. “Oftentimes a kid’s cultural make-up holds a big part of the answer to what they need,” Dr. Gonzalez said. He provided the example of a patient who was witnessing domestic violence in the home. A key part to helping him meet his goal of reducing cannabis and alcohol use was understanding his relationship with his dad, his response to trauma, and his depression, all within his cultural and religious background.
Preserving the medical home
Finally, when it comes to referrals, consider what are you referring a patient for and whom are you referring them to because not all programs and all clinicians are created equal. Create, build, and maintain relationships with as many behavioral health clinicians and practices as you can, he advised.
Further, it’s important to preserve the medical home, though that can require extra effort, particularly with children who have seen a lot of providers. Each physician will need to develop their own strategy for how to do this. Sometimes kids feel passed around and there’s poor communication within programs, leaving kids and their families feeling unwelcome at your practice.
“No child is a hot potato,” he said. Because they may feel like they’re being bounced around among different providers, programs, emergency departments, facilities, and such, it’s important to convey strongly that you want to continue to care for them.
“Whether we’ve been part of that or not, we become part of that,” Dr. Gonzalez said. “They may think that you don’t want to see them again. You want to keep them, and you might have to continue giving repeated messages. Sometimes we need to be very overt and repeat ourselves and say no, ‘I really, really, really want you to come back. This is your home and I want you to come back.’ ”
Dr. Gonzalez and Dr. Khan have no disclosures.
according to Lucien Gonzalez, MD, assistant professor of psychiatry and behavioral sciences at the University of Minnesota, Minneapolis.
In a presentation at the annual meeting of the American Academy of Pediatrics, held virtually this year, Dr. Gonzalez discussed some of the common challenges pediatricians face in appropriately screening, diagnosing, and managing or referring youth when it comes to substance use.
Substance use screening
One of these included picking the right assessment tool and frequency for screening patients for substance use. A number of validated tools are out there, including the Screening to Brief Intervention (S2BI) and CRAFFT Screening Tool for Adolescent Substance Abuse. Regardless of which screening tool providers choose, “the important thing is to use a tool that is validated in the pediatric population and ideally has frequency results in it,” Dr. Gonzalez said.
In terms of frequency, screening young people at least once a year is fairly standard, but it may be necessary to screen adolescents more often or to screen them at acute visits.
“As many of you who work with adolescents know, you can’t always rely on the yearly well child visit because after a certain age, you start to see drop-off,” Dr. Gonzalez said. “They often aren’t coming for well child visits, and they often are then only showing up for acute visits.”
That means doctors need to think about how their clinics operate, how often they see their teen patients, and other factors – including how much can happen in a single year of adolescence – to ensure that screening captures these patients at least once a year, but more if that works within the practice.
Screening vs. diagnosis
Dr. Gonzalez also addressed the difference between screening and diagnosis, a very familiar distinction to physicians in other areas of medicine but often a source of confusion in the area of substance use.
“Screening is the presumptive identification of unrecognized disease in apparently healthy people who don’t have symptoms, using assessments that can be used rapidly,” Dr. Gonzalez said. “When we move into the diagnostic realm, these are people who present with symptoms or they have positive results on our screening test prompting further investigation.”
Sonia Khan, MD, a pediatrician and the medical director of the substance use disorder counseling program in the department of health and human services in Fremont, Calif., who heard the talk, particularly appreciated this point about screening versus diagnosis.
“As soon as you get a hint that there’s a problem with the kid, you’re no longer screening. You’re doing diagnostic investigation,” Dr. Khan, also the human relations commissioner for the city of Fremont, Calif., said in an interview. “Screening is about the kids you don’t know about. It seems like a small point to make a big deal out of, but it’s not.”
Sometimes a screening tool can serve as an introductory interview guide when beginning a clinical investigation with a patient who already shows symptoms, but that doesn’t mean it’s a screen.
Dr. Gonzalez emphasized the importance of not prescreening.
“A prescreener looks at a kid and decides whether or not they need to be screened,” Dr. Gonzalez said. “We have research that demonstrates that that doesn’t work. Physicians are not good at determining this by eyeballing it, and it’s fraught with bias. Universal screening with a validated screening tool is what works.”
Again, the idea of confronting one’s own personal biases and how they could interfere with screening really resonated with Dr. Khan.
“When it comes to the prescreening, if you’re only screening the ones you [think you] need to screen, you’re introducing bias into your screening,” she said. “It’s usually judgmental. It’s important to focus on really getting the bias out of what you’re doing because it’s a field fraught with bias and expectations.”
Brief interventions
Another area of confusion for many providers is what qualifies as a brief intervention and how to deliver it. The brief intervention needs to focus on increasing the patient’s knowledge, insights, and awareness when it comes to their own substance use and how it affects others. It should also support motivation in the patient to make behavioral changes. “It is always given in a nonjudgmental, supportive manner,” Dr. Gonzalez said.
Though motivational interviewing is often discussed as though it’s a brief intervention, it is actually the mechanism for delivering the intervention – not the intervention itself.
Dr. Gonzalez highly recommended that providers seek motivational interviewing training if they haven’t already. He went on to caution attendees about behavior goals in interventions: They should be the patient’s change goals, not the provider’s, and the provider is there to facilitate the teen’s clarification of those goals.
“It’s very important to use those listening skills that we have and honor their decision-making and listen to their language in establishing their own goals,” he said. It’s also important to keep cultural relevance and respect in mind when delivering the intervention. He shared a chart showing the dominant and nondominant groups along various demographic cultural influences, including age, disability status, faith, race/ethnicity, indigenous heritage, socioeconomic status, national origin, gender and sexuality.
For example, the dominant age groups are the young and middle-aged while the nondominant are children and elderly. The dominant faith in the United States is Christian or secular, and the dominant sexuality is heterosexual; the corresponding nondominant groups would be non-Christian and nonheterosexual. It’s important for providers to consider the child’s needs within that entire behavioral context to understand where they’re coming from.
“Have you ever characterized a kid’s situation with regard to substance use and diagnoses based on certain characteristics?” Dr. Gonzalez asked attendees. “We like to think that we don’t, but research on diagnostic disparities indicates otherwise.”
A way to help avoid this is to know who you are in the room and who you’re with in terms of dominant and nondominant groups. “Oftentimes a kid’s cultural make-up holds a big part of the answer to what they need,” Dr. Gonzalez said. He provided the example of a patient who was witnessing domestic violence in the home. A key part to helping him meet his goal of reducing cannabis and alcohol use was understanding his relationship with his dad, his response to trauma, and his depression, all within his cultural and religious background.
Preserving the medical home
Finally, when it comes to referrals, consider what are you referring a patient for and whom are you referring them to because not all programs and all clinicians are created equal. Create, build, and maintain relationships with as many behavioral health clinicians and practices as you can, he advised.
Further, it’s important to preserve the medical home, though that can require extra effort, particularly with children who have seen a lot of providers. Each physician will need to develop their own strategy for how to do this. Sometimes kids feel passed around and there’s poor communication within programs, leaving kids and their families feeling unwelcome at your practice.
“No child is a hot potato,” he said. Because they may feel like they’re being bounced around among different providers, programs, emergency departments, facilities, and such, it’s important to convey strongly that you want to continue to care for them.
“Whether we’ve been part of that or not, we become part of that,” Dr. Gonzalez said. “They may think that you don’t want to see them again. You want to keep them, and you might have to continue giving repeated messages. Sometimes we need to be very overt and repeat ourselves and say no, ‘I really, really, really want you to come back. This is your home and I want you to come back.’ ”
Dr. Gonzalez and Dr. Khan have no disclosures.
according to Lucien Gonzalez, MD, assistant professor of psychiatry and behavioral sciences at the University of Minnesota, Minneapolis.
In a presentation at the annual meeting of the American Academy of Pediatrics, held virtually this year, Dr. Gonzalez discussed some of the common challenges pediatricians face in appropriately screening, diagnosing, and managing or referring youth when it comes to substance use.
Substance use screening
One of these included picking the right assessment tool and frequency for screening patients for substance use. A number of validated tools are out there, including the Screening to Brief Intervention (S2BI) and CRAFFT Screening Tool for Adolescent Substance Abuse. Regardless of which screening tool providers choose, “the important thing is to use a tool that is validated in the pediatric population and ideally has frequency results in it,” Dr. Gonzalez said.
In terms of frequency, screening young people at least once a year is fairly standard, but it may be necessary to screen adolescents more often or to screen them at acute visits.
“As many of you who work with adolescents know, you can’t always rely on the yearly well child visit because after a certain age, you start to see drop-off,” Dr. Gonzalez said. “They often aren’t coming for well child visits, and they often are then only showing up for acute visits.”
That means doctors need to think about how their clinics operate, how often they see their teen patients, and other factors – including how much can happen in a single year of adolescence – to ensure that screening captures these patients at least once a year, but more if that works within the practice.
Screening vs. diagnosis
Dr. Gonzalez also addressed the difference between screening and diagnosis, a very familiar distinction to physicians in other areas of medicine but often a source of confusion in the area of substance use.
“Screening is the presumptive identification of unrecognized disease in apparently healthy people who don’t have symptoms, using assessments that can be used rapidly,” Dr. Gonzalez said. “When we move into the diagnostic realm, these are people who present with symptoms or they have positive results on our screening test prompting further investigation.”
Sonia Khan, MD, a pediatrician and the medical director of the substance use disorder counseling program in the department of health and human services in Fremont, Calif., who heard the talk, particularly appreciated this point about screening versus diagnosis.
“As soon as you get a hint that there’s a problem with the kid, you’re no longer screening. You’re doing diagnostic investigation,” Dr. Khan, also the human relations commissioner for the city of Fremont, Calif., said in an interview. “Screening is about the kids you don’t know about. It seems like a small point to make a big deal out of, but it’s not.”
Sometimes a screening tool can serve as an introductory interview guide when beginning a clinical investigation with a patient who already shows symptoms, but that doesn’t mean it’s a screen.
Dr. Gonzalez emphasized the importance of not prescreening.
“A prescreener looks at a kid and decides whether or not they need to be screened,” Dr. Gonzalez said. “We have research that demonstrates that that doesn’t work. Physicians are not good at determining this by eyeballing it, and it’s fraught with bias. Universal screening with a validated screening tool is what works.”
Again, the idea of confronting one’s own personal biases and how they could interfere with screening really resonated with Dr. Khan.
“When it comes to the prescreening, if you’re only screening the ones you [think you] need to screen, you’re introducing bias into your screening,” she said. “It’s usually judgmental. It’s important to focus on really getting the bias out of what you’re doing because it’s a field fraught with bias and expectations.”
Brief interventions
Another area of confusion for many providers is what qualifies as a brief intervention and how to deliver it. The brief intervention needs to focus on increasing the patient’s knowledge, insights, and awareness when it comes to their own substance use and how it affects others. It should also support motivation in the patient to make behavioral changes. “It is always given in a nonjudgmental, supportive manner,” Dr. Gonzalez said.
Though motivational interviewing is often discussed as though it’s a brief intervention, it is actually the mechanism for delivering the intervention – not the intervention itself.
Dr. Gonzalez highly recommended that providers seek motivational interviewing training if they haven’t already. He went on to caution attendees about behavior goals in interventions: They should be the patient’s change goals, not the provider’s, and the provider is there to facilitate the teen’s clarification of those goals.
“It’s very important to use those listening skills that we have and honor their decision-making and listen to their language in establishing their own goals,” he said. It’s also important to keep cultural relevance and respect in mind when delivering the intervention. He shared a chart showing the dominant and nondominant groups along various demographic cultural influences, including age, disability status, faith, race/ethnicity, indigenous heritage, socioeconomic status, national origin, gender and sexuality.
For example, the dominant age groups are the young and middle-aged while the nondominant are children and elderly. The dominant faith in the United States is Christian or secular, and the dominant sexuality is heterosexual; the corresponding nondominant groups would be non-Christian and nonheterosexual. It’s important for providers to consider the child’s needs within that entire behavioral context to understand where they’re coming from.
“Have you ever characterized a kid’s situation with regard to substance use and diagnoses based on certain characteristics?” Dr. Gonzalez asked attendees. “We like to think that we don’t, but research on diagnostic disparities indicates otherwise.”
A way to help avoid this is to know who you are in the room and who you’re with in terms of dominant and nondominant groups. “Oftentimes a kid’s cultural make-up holds a big part of the answer to what they need,” Dr. Gonzalez said. He provided the example of a patient who was witnessing domestic violence in the home. A key part to helping him meet his goal of reducing cannabis and alcohol use was understanding his relationship with his dad, his response to trauma, and his depression, all within his cultural and religious background.
Preserving the medical home
Finally, when it comes to referrals, consider what are you referring a patient for and whom are you referring them to because not all programs and all clinicians are created equal. Create, build, and maintain relationships with as many behavioral health clinicians and practices as you can, he advised.
Further, it’s important to preserve the medical home, though that can require extra effort, particularly with children who have seen a lot of providers. Each physician will need to develop their own strategy for how to do this. Sometimes kids feel passed around and there’s poor communication within programs, leaving kids and their families feeling unwelcome at your practice.
“No child is a hot potato,” he said. Because they may feel like they’re being bounced around among different providers, programs, emergency departments, facilities, and such, it’s important to convey strongly that you want to continue to care for them.
“Whether we’ve been part of that or not, we become part of that,” Dr. Gonzalez said. “They may think that you don’t want to see them again. You want to keep them, and you might have to continue giving repeated messages. Sometimes we need to be very overt and repeat ourselves and say no, ‘I really, really, really want you to come back. This is your home and I want you to come back.’ ”
Dr. Gonzalez and Dr. Khan have no disclosures.
FROM AAP 2020
National lung cancer screening guidelines may miss younger African American individuals at high risk
in a recent retrospective study, the lead author reported at the annual meeting of the American College of Chest Physicians.
The finding highlights a health disparity issue that may be addressed through an update of those guidelines that is in the works, said Carol Velez Martinez, MD, a third-year internal medicine resident at Louisiana State University Health Sciences Center in Shreveport, La.
About one-third of the lung cancer patients in the retrospective cohort study were diagnosed before the age of 55 years, which means they would not have been recommended for screening with low-dose computed tomography (LDCT) based on the 2013 lung cancer guidelines from the United States Preventive Services Task Force (USPSTF), said Dr. Velez Martinez.
By contrast, 12.5% of screening-ineligible patients would have been counted as LDCT eligible based on guidelines from the National Comprehensive Cancer Network (NCCN), Dr. Velez Martinez and coauthors found in their analysis.
In a draft recommendation statement posted July 7, the USPSTF said they would now recommend that screening at age 50 years, rather than 55, and that the pack-years of smoking history that would make an individual eligible for screening would be dropped from 30 pack-years to 20, changes that task force members said would be more inclusive of African Americans and women.
Dr. Velez Martinez said she is looking forward to a formal recommendation from USPSTF soon: “I’m hoping that’s where they’re heading,” she said in an interview. “When I’m in practice as a resident, I actually bring it up to my patients, and if I have to call the insurance I don’t have a problem – but I still have to call them because they’re still going by the prior guidelines.”
“I think there are going to be a lot of other health disparities,” Dr. Revelo said in an interview. “[Dr. Velez Martinez’s] study was limited by the fact that she cared mostly for Caucasians and also African Americans, but maybe no Latinos or Hispanics that I’m sure would also be affected if we were looking to that in a bigger or national study.”
The 2013 USPSTF guidelines were based on benefits observed in the National Lung Screening Trial (NLST), which indicated a 20% relative risk reduction in death from lung cancer; however, the generalizability of the study beyond White males has been questioned, said Dr. Velez Martinez in a presentation at the CHEST annual meeting.
About 90% of NSLT participants were White and 59% were male, according to results published in 2011.
Other studies have shown that African Americans are more likely to get lung cancer than Whites, despite comparable smoking rates between the races, and that African American men are more likely to die from lung cancer than White men, Dr. Velez Martinez said. Many African Americans live below the poverty line, which means they have limited resources for insurance and health providers, and they also participate less often in clinical trials, she added.
In their retrospective observational cohort study, Dr. Velez Martinez and coinvestigators reviewed 1,500 medical records of patients with newly diagnosed stage 1-4 lung cancers from the LSU Health Science Center Shreveport between 2011 and 2015.
They found that 33% of those lung cancer patients were diagnosed before the age of 55 years, meaning they did not meet the 2013 USPSTF screening guidelines, which recommend annual LDCT in adults aged 55-80 years with a 30 pack-year smoking history who currently smoke or have quit within the past 15 years.
Next, they sought to classify those screening-ineligible patients based on NCCN guidelines, which recommend LDCT in patients 50 years of age or older with at least a 20 pack-year smoking history and a 6-year risk of lung cancer of at least 1.3% based on the Tammemagi lung cancer risk calculator. The Tammemagi calculator considers factors such as age, education, body mass index, prior lung disease, familial cancer history, race and ethnicity, and smoking history.
After applying the risk stratification, the investigators found that 12.5% of these patients would have been categorized as high risk and therefore recommended for LDCT, and of that group, more than 65% were African American, Dr. Velez Martinez reported.
Dr. Revelo, who chaired the CHEST session where the findings were reported, said that shared decision-making will still be as important regardless of any changes to lung screening guidelines given the recognized potential harms of LDCT screening, such as false positives, radiation exposure, and psychological distress.
“I think we will continue to have a very personal conversation and make important decisions focused on what the patient wants,” he said.
Authors reported no disclosures.
in a recent retrospective study, the lead author reported at the annual meeting of the American College of Chest Physicians.
The finding highlights a health disparity issue that may be addressed through an update of those guidelines that is in the works, said Carol Velez Martinez, MD, a third-year internal medicine resident at Louisiana State University Health Sciences Center in Shreveport, La.
About one-third of the lung cancer patients in the retrospective cohort study were diagnosed before the age of 55 years, which means they would not have been recommended for screening with low-dose computed tomography (LDCT) based on the 2013 lung cancer guidelines from the United States Preventive Services Task Force (USPSTF), said Dr. Velez Martinez.
By contrast, 12.5% of screening-ineligible patients would have been counted as LDCT eligible based on guidelines from the National Comprehensive Cancer Network (NCCN), Dr. Velez Martinez and coauthors found in their analysis.
In a draft recommendation statement posted July 7, the USPSTF said they would now recommend that screening at age 50 years, rather than 55, and that the pack-years of smoking history that would make an individual eligible for screening would be dropped from 30 pack-years to 20, changes that task force members said would be more inclusive of African Americans and women.
Dr. Velez Martinez said she is looking forward to a formal recommendation from USPSTF soon: “I’m hoping that’s where they’re heading,” she said in an interview. “When I’m in practice as a resident, I actually bring it up to my patients, and if I have to call the insurance I don’t have a problem – but I still have to call them because they’re still going by the prior guidelines.”
“I think there are going to be a lot of other health disparities,” Dr. Revelo said in an interview. “[Dr. Velez Martinez’s] study was limited by the fact that she cared mostly for Caucasians and also African Americans, but maybe no Latinos or Hispanics that I’m sure would also be affected if we were looking to that in a bigger or national study.”
The 2013 USPSTF guidelines were based on benefits observed in the National Lung Screening Trial (NLST), which indicated a 20% relative risk reduction in death from lung cancer; however, the generalizability of the study beyond White males has been questioned, said Dr. Velez Martinez in a presentation at the CHEST annual meeting.
About 90% of NSLT participants were White and 59% were male, according to results published in 2011.
Other studies have shown that African Americans are more likely to get lung cancer than Whites, despite comparable smoking rates between the races, and that African American men are more likely to die from lung cancer than White men, Dr. Velez Martinez said. Many African Americans live below the poverty line, which means they have limited resources for insurance and health providers, and they also participate less often in clinical trials, she added.
In their retrospective observational cohort study, Dr. Velez Martinez and coinvestigators reviewed 1,500 medical records of patients with newly diagnosed stage 1-4 lung cancers from the LSU Health Science Center Shreveport between 2011 and 2015.
They found that 33% of those lung cancer patients were diagnosed before the age of 55 years, meaning they did not meet the 2013 USPSTF screening guidelines, which recommend annual LDCT in adults aged 55-80 years with a 30 pack-year smoking history who currently smoke or have quit within the past 15 years.
Next, they sought to classify those screening-ineligible patients based on NCCN guidelines, which recommend LDCT in patients 50 years of age or older with at least a 20 pack-year smoking history and a 6-year risk of lung cancer of at least 1.3% based on the Tammemagi lung cancer risk calculator. The Tammemagi calculator considers factors such as age, education, body mass index, prior lung disease, familial cancer history, race and ethnicity, and smoking history.
After applying the risk stratification, the investigators found that 12.5% of these patients would have been categorized as high risk and therefore recommended for LDCT, and of that group, more than 65% were African American, Dr. Velez Martinez reported.
Dr. Revelo, who chaired the CHEST session where the findings were reported, said that shared decision-making will still be as important regardless of any changes to lung screening guidelines given the recognized potential harms of LDCT screening, such as false positives, radiation exposure, and psychological distress.
“I think we will continue to have a very personal conversation and make important decisions focused on what the patient wants,” he said.
Authors reported no disclosures.
in a recent retrospective study, the lead author reported at the annual meeting of the American College of Chest Physicians.
The finding highlights a health disparity issue that may be addressed through an update of those guidelines that is in the works, said Carol Velez Martinez, MD, a third-year internal medicine resident at Louisiana State University Health Sciences Center in Shreveport, La.
About one-third of the lung cancer patients in the retrospective cohort study were diagnosed before the age of 55 years, which means they would not have been recommended for screening with low-dose computed tomography (LDCT) based on the 2013 lung cancer guidelines from the United States Preventive Services Task Force (USPSTF), said Dr. Velez Martinez.
By contrast, 12.5% of screening-ineligible patients would have been counted as LDCT eligible based on guidelines from the National Comprehensive Cancer Network (NCCN), Dr. Velez Martinez and coauthors found in their analysis.
In a draft recommendation statement posted July 7, the USPSTF said they would now recommend that screening at age 50 years, rather than 55, and that the pack-years of smoking history that would make an individual eligible for screening would be dropped from 30 pack-years to 20, changes that task force members said would be more inclusive of African Americans and women.
Dr. Velez Martinez said she is looking forward to a formal recommendation from USPSTF soon: “I’m hoping that’s where they’re heading,” she said in an interview. “When I’m in practice as a resident, I actually bring it up to my patients, and if I have to call the insurance I don’t have a problem – but I still have to call them because they’re still going by the prior guidelines.”
“I think there are going to be a lot of other health disparities,” Dr. Revelo said in an interview. “[Dr. Velez Martinez’s] study was limited by the fact that she cared mostly for Caucasians and also African Americans, but maybe no Latinos or Hispanics that I’m sure would also be affected if we were looking to that in a bigger or national study.”
The 2013 USPSTF guidelines were based on benefits observed in the National Lung Screening Trial (NLST), which indicated a 20% relative risk reduction in death from lung cancer; however, the generalizability of the study beyond White males has been questioned, said Dr. Velez Martinez in a presentation at the CHEST annual meeting.
About 90% of NSLT participants were White and 59% were male, according to results published in 2011.
Other studies have shown that African Americans are more likely to get lung cancer than Whites, despite comparable smoking rates between the races, and that African American men are more likely to die from lung cancer than White men, Dr. Velez Martinez said. Many African Americans live below the poverty line, which means they have limited resources for insurance and health providers, and they also participate less often in clinical trials, she added.
In their retrospective observational cohort study, Dr. Velez Martinez and coinvestigators reviewed 1,500 medical records of patients with newly diagnosed stage 1-4 lung cancers from the LSU Health Science Center Shreveport between 2011 and 2015.
They found that 33% of those lung cancer patients were diagnosed before the age of 55 years, meaning they did not meet the 2013 USPSTF screening guidelines, which recommend annual LDCT in adults aged 55-80 years with a 30 pack-year smoking history who currently smoke or have quit within the past 15 years.
Next, they sought to classify those screening-ineligible patients based on NCCN guidelines, which recommend LDCT in patients 50 years of age or older with at least a 20 pack-year smoking history and a 6-year risk of lung cancer of at least 1.3% based on the Tammemagi lung cancer risk calculator. The Tammemagi calculator considers factors such as age, education, body mass index, prior lung disease, familial cancer history, race and ethnicity, and smoking history.
After applying the risk stratification, the investigators found that 12.5% of these patients would have been categorized as high risk and therefore recommended for LDCT, and of that group, more than 65% were African American, Dr. Velez Martinez reported.
Dr. Revelo, who chaired the CHEST session where the findings were reported, said that shared decision-making will still be as important regardless of any changes to lung screening guidelines given the recognized potential harms of LDCT screening, such as false positives, radiation exposure, and psychological distress.
“I think we will continue to have a very personal conversation and make important decisions focused on what the patient wants,” he said.
Authors reported no disclosures.
FROM CHEST 2020
Tobacco-free homes yield more tobacco-free youth
Tsu-Suan Wu and Benjamin W. Chaffee, DDS, PhD, of the University of California, San Francisco, advised in their study in Pediatrics.
Previous studies have shown that children who grow up in a nonsmoking household are less likely to begin smoking themselves, and active parental engagement in interventions shows promise overall in protecting children from drug, alcohol, and illicit drug use. Households with rigid rules against smoking offer a deterrent for children who might otherwise be tempted, the researchers noted.
Other studies have shown that while youth smoking is on the decline, use of noncigarette products is increasing sharply. The inconspicuous appearance and attractive scents these delivery devices afford make it easier to conceal them from parents.
In the current study, using data from the Population Assessment of Tobacco and Health (PATH) Study involving 23,170 parents and youth ages 9 and up, Mr. Wu and Dr. Chaffee sought to assess to what extent parents had knowledge or suspicions of tobacco use and also to evaluate the association between youth initiating tobacco use and the establishment of household rules and engaging in regular conversation about tobacco.
Study results revealed in three of the four groups evaluated that youth were most likely to engage in using several different types of tobacco (polytobacco) products; in the fourth group, e-cigarette use was most common. Among polytobacco users, fully 77%-80% reported cigarette usage.
Parental knowledge and actions
Overall, Mr. Wu and Dr. Chaffee “identified substantial lapses in parents’ awareness of their children’s tobacco use.” Parents were most likely to register awareness when their children smoked cigarettes; half as many parents were aware or suspected use when noncigarette products were used.
Parents who had heightened awareness about possible tobacco usage tended to be the child’s mother, had completed lower levels of education, parented children who were older, male and non-Hispanic, and lived with a tobacco user.
Noteworthy was the growing percentage of parents who report awareness or suspicions of cigarette usage – approximately 70% – compared with previous study findings – about 40%. The researchers speculated that this increase could be directly tied to growing social concern regarding youth smoking. Unfortunately, parents will continue to be challenged to keep up with constantly changing e-cigarette designs in maintaining their awareness, Mr. Wu and Dr. Chaffee noted.
Establishing strict household rules was found to be more effective than just talking with youth about usage, which half of the youth reported their parents did. At all time points, the risk of tobacco initiation was 20%-26% lower for children who lived in a house with strict household rules forbidding any tobacco use by anyone. The researchers observed that success with the household rules method was best achieved with children at younger ages.
The study did not measure the quality or frequency of antitobacco conversations but it should not be concluded definitively that all parental communication is unhelpful, the researchers cautioned.
To their knowledge, this study is the first to analyze the effects of household antitobacco strategies on discouraging initiation the use of tobacco and other smoking products as well as assessing parental awareness surrounding tobacco usage among youth.
What to tell parents
In a separate interview, Kelly Curran, MD, MA, assistant professor of pediatrics at the University of Oklahoma, Oklahoma City, commented on the explosive growth of e-cigarette use in the last 7 years.
What makes e-cigs so difficult to detect is that they “can resemble common objects such as flash drives or pens, and as a result, can often be hidden or overlooked by parents,” noted Dr. Curran.
The most important message for parents from this study is that they have the potential to have a large impact in the prevention of tobacco initiation, she said. “This effort requires parents to ‘walk the walk’ instead of just ‘talking the talk.”
As the study revealed, simply talking to teens about not using tobacco products doesn’t decrease use, but “creating strict household rules around no tobacco use for all visitors and inhabitants has a significant impact in decreasing youth tobacco initiation – by nearly 25%,” she added. “When counseling patients and families about tobacco prevention, clinicians should encourage them to create a tobacco-free home.”
The study was funded by a National Institutes of Health grant and the Delta Dental Community Care Foundation. The authors have no relevant financial disclosures. Dr. Curran, who is a member of the Pediatric News editorial advisory board, said she had no relevant financial disclosures.
SOURCE: Wu T-S and Chaffee BW. Pediatrics 2020 October. doi: 10.1542/peds.2019-4034.
Tsu-Suan Wu and Benjamin W. Chaffee, DDS, PhD, of the University of California, San Francisco, advised in their study in Pediatrics.
Previous studies have shown that children who grow up in a nonsmoking household are less likely to begin smoking themselves, and active parental engagement in interventions shows promise overall in protecting children from drug, alcohol, and illicit drug use. Households with rigid rules against smoking offer a deterrent for children who might otherwise be tempted, the researchers noted.
Other studies have shown that while youth smoking is on the decline, use of noncigarette products is increasing sharply. The inconspicuous appearance and attractive scents these delivery devices afford make it easier to conceal them from parents.
In the current study, using data from the Population Assessment of Tobacco and Health (PATH) Study involving 23,170 parents and youth ages 9 and up, Mr. Wu and Dr. Chaffee sought to assess to what extent parents had knowledge or suspicions of tobacco use and also to evaluate the association between youth initiating tobacco use and the establishment of household rules and engaging in regular conversation about tobacco.
Study results revealed in three of the four groups evaluated that youth were most likely to engage in using several different types of tobacco (polytobacco) products; in the fourth group, e-cigarette use was most common. Among polytobacco users, fully 77%-80% reported cigarette usage.
Parental knowledge and actions
Overall, Mr. Wu and Dr. Chaffee “identified substantial lapses in parents’ awareness of their children’s tobacco use.” Parents were most likely to register awareness when their children smoked cigarettes; half as many parents were aware or suspected use when noncigarette products were used.
Parents who had heightened awareness about possible tobacco usage tended to be the child’s mother, had completed lower levels of education, parented children who were older, male and non-Hispanic, and lived with a tobacco user.
Noteworthy was the growing percentage of parents who report awareness or suspicions of cigarette usage – approximately 70% – compared with previous study findings – about 40%. The researchers speculated that this increase could be directly tied to growing social concern regarding youth smoking. Unfortunately, parents will continue to be challenged to keep up with constantly changing e-cigarette designs in maintaining their awareness, Mr. Wu and Dr. Chaffee noted.
Establishing strict household rules was found to be more effective than just talking with youth about usage, which half of the youth reported their parents did. At all time points, the risk of tobacco initiation was 20%-26% lower for children who lived in a house with strict household rules forbidding any tobacco use by anyone. The researchers observed that success with the household rules method was best achieved with children at younger ages.
The study did not measure the quality or frequency of antitobacco conversations but it should not be concluded definitively that all parental communication is unhelpful, the researchers cautioned.
To their knowledge, this study is the first to analyze the effects of household antitobacco strategies on discouraging initiation the use of tobacco and other smoking products as well as assessing parental awareness surrounding tobacco usage among youth.
What to tell parents
In a separate interview, Kelly Curran, MD, MA, assistant professor of pediatrics at the University of Oklahoma, Oklahoma City, commented on the explosive growth of e-cigarette use in the last 7 years.
What makes e-cigs so difficult to detect is that they “can resemble common objects such as flash drives or pens, and as a result, can often be hidden or overlooked by parents,” noted Dr. Curran.
The most important message for parents from this study is that they have the potential to have a large impact in the prevention of tobacco initiation, she said. “This effort requires parents to ‘walk the walk’ instead of just ‘talking the talk.”
As the study revealed, simply talking to teens about not using tobacco products doesn’t decrease use, but “creating strict household rules around no tobacco use for all visitors and inhabitants has a significant impact in decreasing youth tobacco initiation – by nearly 25%,” she added. “When counseling patients and families about tobacco prevention, clinicians should encourage them to create a tobacco-free home.”
The study was funded by a National Institutes of Health grant and the Delta Dental Community Care Foundation. The authors have no relevant financial disclosures. Dr. Curran, who is a member of the Pediatric News editorial advisory board, said she had no relevant financial disclosures.
SOURCE: Wu T-S and Chaffee BW. Pediatrics 2020 October. doi: 10.1542/peds.2019-4034.
Tsu-Suan Wu and Benjamin W. Chaffee, DDS, PhD, of the University of California, San Francisco, advised in their study in Pediatrics.
Previous studies have shown that children who grow up in a nonsmoking household are less likely to begin smoking themselves, and active parental engagement in interventions shows promise overall in protecting children from drug, alcohol, and illicit drug use. Households with rigid rules against smoking offer a deterrent for children who might otherwise be tempted, the researchers noted.
Other studies have shown that while youth smoking is on the decline, use of noncigarette products is increasing sharply. The inconspicuous appearance and attractive scents these delivery devices afford make it easier to conceal them from parents.
In the current study, using data from the Population Assessment of Tobacco and Health (PATH) Study involving 23,170 parents and youth ages 9 and up, Mr. Wu and Dr. Chaffee sought to assess to what extent parents had knowledge or suspicions of tobacco use and also to evaluate the association between youth initiating tobacco use and the establishment of household rules and engaging in regular conversation about tobacco.
Study results revealed in three of the four groups evaluated that youth were most likely to engage in using several different types of tobacco (polytobacco) products; in the fourth group, e-cigarette use was most common. Among polytobacco users, fully 77%-80% reported cigarette usage.
Parental knowledge and actions
Overall, Mr. Wu and Dr. Chaffee “identified substantial lapses in parents’ awareness of their children’s tobacco use.” Parents were most likely to register awareness when their children smoked cigarettes; half as many parents were aware or suspected use when noncigarette products were used.
Parents who had heightened awareness about possible tobacco usage tended to be the child’s mother, had completed lower levels of education, parented children who were older, male and non-Hispanic, and lived with a tobacco user.
Noteworthy was the growing percentage of parents who report awareness or suspicions of cigarette usage – approximately 70% – compared with previous study findings – about 40%. The researchers speculated that this increase could be directly tied to growing social concern regarding youth smoking. Unfortunately, parents will continue to be challenged to keep up with constantly changing e-cigarette designs in maintaining their awareness, Mr. Wu and Dr. Chaffee noted.
Establishing strict household rules was found to be more effective than just talking with youth about usage, which half of the youth reported their parents did. At all time points, the risk of tobacco initiation was 20%-26% lower for children who lived in a house with strict household rules forbidding any tobacco use by anyone. The researchers observed that success with the household rules method was best achieved with children at younger ages.
The study did not measure the quality or frequency of antitobacco conversations but it should not be concluded definitively that all parental communication is unhelpful, the researchers cautioned.
To their knowledge, this study is the first to analyze the effects of household antitobacco strategies on discouraging initiation the use of tobacco and other smoking products as well as assessing parental awareness surrounding tobacco usage among youth.
What to tell parents
In a separate interview, Kelly Curran, MD, MA, assistant professor of pediatrics at the University of Oklahoma, Oklahoma City, commented on the explosive growth of e-cigarette use in the last 7 years.
What makes e-cigs so difficult to detect is that they “can resemble common objects such as flash drives or pens, and as a result, can often be hidden or overlooked by parents,” noted Dr. Curran.
The most important message for parents from this study is that they have the potential to have a large impact in the prevention of tobacco initiation, she said. “This effort requires parents to ‘walk the walk’ instead of just ‘talking the talk.”
As the study revealed, simply talking to teens about not using tobacco products doesn’t decrease use, but “creating strict household rules around no tobacco use for all visitors and inhabitants has a significant impact in decreasing youth tobacco initiation – by nearly 25%,” she added. “When counseling patients and families about tobacco prevention, clinicians should encourage them to create a tobacco-free home.”
The study was funded by a National Institutes of Health grant and the Delta Dental Community Care Foundation. The authors have no relevant financial disclosures. Dr. Curran, who is a member of the Pediatric News editorial advisory board, said she had no relevant financial disclosures.
SOURCE: Wu T-S and Chaffee BW. Pediatrics 2020 October. doi: 10.1542/peds.2019-4034.
FROM PEDIATRICS















