User login
Putting an end to chronic opioid prescriptions
Thanks to Dr. Linn et al for “Tips and tools for safe opioid prescribing” (J Fam Pract. 2020;69:280-292), which addressed an important topic: the risks of, and poor evidence for, chronic opioids in noncancer pain.
Pain management is challenging, and it is easy to prescribe opioids from a desire to help. However, we must translate the evidence of chronic opioids’ poor benefit and real harms into practice. No studies show a long-term benefit of opioids for chronic noncancer pain, but they do demonstrate abundant findings of harm. As a family medicine community, we should be practicing at the highest level of evidence and addressing legacy opioid prescribing for chronic noncancer pain.
Increasing opioid doses for pain only offers short-term benefits and can result in rapid tolerance and withdrawal. We should not be starting people on opioids for knee and back pain. We do not need more ways to initiate opioids or tables on how to dose long-acting opioids—drugs that increase mortality.1 Let’s stop using poorly validated tools like DIRE to ignore the evidence against opioids (validated with 61 retrospective chart reviews; 81% sensitivity, 76% specificity for predicting efficacy of opioids).2,3
A 2018 randomized controlled trial of 240 patients with back, knee, or hip osteoarthritis found opioids were not superior to nonopioid medication for pain-related function at 12 months and had more adverse effects.4 A 2015 systematic review concluded there was insufficient evidence of long-term benefits of opioids but a dose-dependent risk of serious harm.5 Just 1 year of taking low-dose opioids can increase the risk of opioid use disorder by 0.7%, compared with 0.004% with no opioids.5
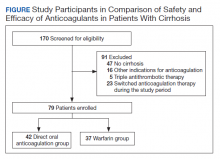
Practical approaches exist. Excellent examples of modern pain care have been developed by the Department of Veterans Affairs/Department of Defense, the Department of Health and Human Services, and state-level initiatives such as the Oregon Pain Guidance.6-8 All use a similar clinical algorithm (FIGURE). If pain is poorly controlled, a slow medically supervised tapering of opioids is indicated.
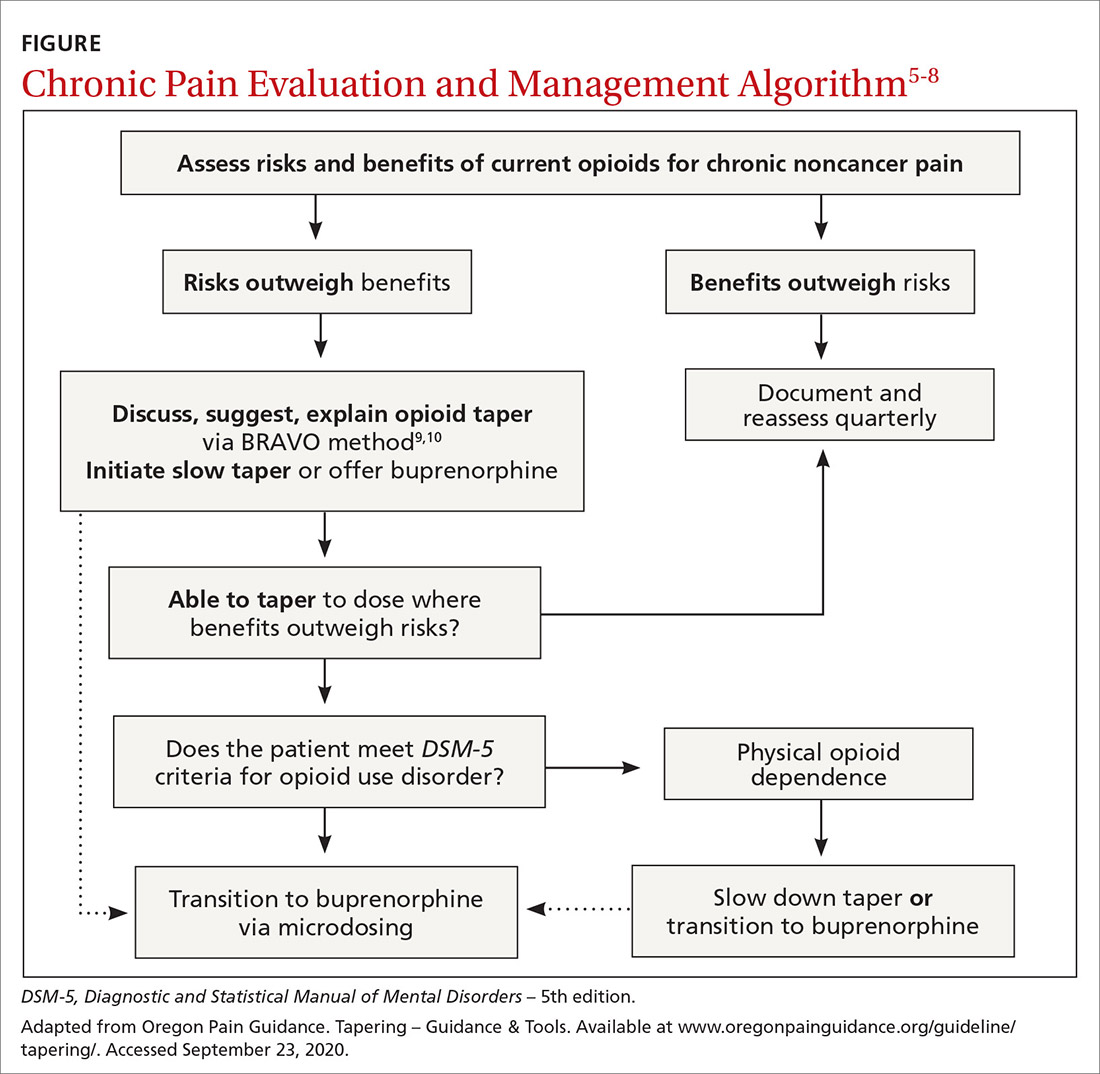
It can be challenging to raise the subject of opioid tapering with patients; I use Stanford’s BRAVO method to guide these conversations.9,10 At my facility, we are tapering about 50 legacy opioid patients, and most are surprised to find that their pain is the same or better with reduced to no opioids, with fewer adverse effects. Many are happier on sublingual buprenorphine, a safer opioid analgesic.11 The algorithm shown in the FIGURE and the BRAVO method should be more widely used within our specialty for a safe and patient-centered approach to chronic pain.
Above all, let the patient know that you are with them on this journey to safe pain management. Start the conversation: “I’ve been thinking a lot about your chronic pain and how best to help you with it. Our understanding of what opioids do for pain has changed, and I worry they’re causing more harm than good now. This is a scary thing to talk about, but I’ll be with you every step of the way.”
Matt Perez, MD
Neighborcare Health
Seattle
1. Ray WA, Chung CP, Murray KT, et al. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315:2415-23.
2. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
3. Brennan MJ. Letter to the editor. J Pain. 2007;8:185.
4. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018;319:872-882.
5. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
6. Oldfield BJ, Edens EL, Agnoli A, et al. Multimodal treatment options, including rotating to buprenorphine, within a multidisciplinary pain clinic for patients on risky opioid regimens: a quality improvement study. Pain Med. 2018;19(suppl 1):S38–S45.
7. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of long-term opioid analgesics. US Department of Health of Human Services Web site. www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf. October 2019. Accessed September 29, 2020.
8. Pain treatment guidelines. Oregon Pain Guidance Web site. www.oregonpainguidance.org/pain-treatment-guidelines/. Accessed September 29, 2020.
9. Tapering – BRAVO – a collaborative approach clinical update March 2020. Oregon Pain Guidance Web site. www.oregonpainguidance.org/guideline/tapering/. Accessed September 29, 2020.
10. How to taper patients off of chronic opioid therapy. Stanford Center for Continuing Medical Education Web site. https://stanford.cloud-cme.com/default.aspx?P=0&EID=20909. Accessed September 29, 2020.
11. Chou R, Ballantyne J, Lembke A, et al. Rethinking opioid dose tapering, prescription opioid dependence, and indications for buprenorphine. Ann Intern Med. 2019;171:427-429.
Thanks to Dr. Linn et al for “Tips and tools for safe opioid prescribing” (J Fam Pract. 2020;69:280-292), which addressed an important topic: the risks of, and poor evidence for, chronic opioids in noncancer pain.
Pain management is challenging, and it is easy to prescribe opioids from a desire to help. However, we must translate the evidence of chronic opioids’ poor benefit and real harms into practice. No studies show a long-term benefit of opioids for chronic noncancer pain, but they do demonstrate abundant findings of harm. As a family medicine community, we should be practicing at the highest level of evidence and addressing legacy opioid prescribing for chronic noncancer pain.
Increasing opioid doses for pain only offers short-term benefits and can result in rapid tolerance and withdrawal. We should not be starting people on opioids for knee and back pain. We do not need more ways to initiate opioids or tables on how to dose long-acting opioids—drugs that increase mortality.1 Let’s stop using poorly validated tools like DIRE to ignore the evidence against opioids (validated with 61 retrospective chart reviews; 81% sensitivity, 76% specificity for predicting efficacy of opioids).2,3
A 2018 randomized controlled trial of 240 patients with back, knee, or hip osteoarthritis found opioids were not superior to nonopioid medication for pain-related function at 12 months and had more adverse effects.4 A 2015 systematic review concluded there was insufficient evidence of long-term benefits of opioids but a dose-dependent risk of serious harm.5 Just 1 year of taking low-dose opioids can increase the risk of opioid use disorder by 0.7%, compared with 0.004% with no opioids.5
Practical approaches exist. Excellent examples of modern pain care have been developed by the Department of Veterans Affairs/Department of Defense, the Department of Health and Human Services, and state-level initiatives such as the Oregon Pain Guidance.6-8 All use a similar clinical algorithm (FIGURE). If pain is poorly controlled, a slow medically supervised tapering of opioids is indicated.

It can be challenging to raise the subject of opioid tapering with patients; I use Stanford’s BRAVO method to guide these conversations.9,10 At my facility, we are tapering about 50 legacy opioid patients, and most are surprised to find that their pain is the same or better with reduced to no opioids, with fewer adverse effects. Many are happier on sublingual buprenorphine, a safer opioid analgesic.11 The algorithm shown in the FIGURE and the BRAVO method should be more widely used within our specialty for a safe and patient-centered approach to chronic pain.
Above all, let the patient know that you are with them on this journey to safe pain management. Start the conversation: “I’ve been thinking a lot about your chronic pain and how best to help you with it. Our understanding of what opioids do for pain has changed, and I worry they’re causing more harm than good now. This is a scary thing to talk about, but I’ll be with you every step of the way.”
Matt Perez, MD
Neighborcare Health
Seattle
Thanks to Dr. Linn et al for “Tips and tools for safe opioid prescribing” (J Fam Pract. 2020;69:280-292), which addressed an important topic: the risks of, and poor evidence for, chronic opioids in noncancer pain.
Pain management is challenging, and it is easy to prescribe opioids from a desire to help. However, we must translate the evidence of chronic opioids’ poor benefit and real harms into practice. No studies show a long-term benefit of opioids for chronic noncancer pain, but they do demonstrate abundant findings of harm. As a family medicine community, we should be practicing at the highest level of evidence and addressing legacy opioid prescribing for chronic noncancer pain.
Increasing opioid doses for pain only offers short-term benefits and can result in rapid tolerance and withdrawal. We should not be starting people on opioids for knee and back pain. We do not need more ways to initiate opioids or tables on how to dose long-acting opioids—drugs that increase mortality.1 Let’s stop using poorly validated tools like DIRE to ignore the evidence against opioids (validated with 61 retrospective chart reviews; 81% sensitivity, 76% specificity for predicting efficacy of opioids).2,3
A 2018 randomized controlled trial of 240 patients with back, knee, or hip osteoarthritis found opioids were not superior to nonopioid medication for pain-related function at 12 months and had more adverse effects.4 A 2015 systematic review concluded there was insufficient evidence of long-term benefits of opioids but a dose-dependent risk of serious harm.5 Just 1 year of taking low-dose opioids can increase the risk of opioid use disorder by 0.7%, compared with 0.004% with no opioids.5
Practical approaches exist. Excellent examples of modern pain care have been developed by the Department of Veterans Affairs/Department of Defense, the Department of Health and Human Services, and state-level initiatives such as the Oregon Pain Guidance.6-8 All use a similar clinical algorithm (FIGURE). If pain is poorly controlled, a slow medically supervised tapering of opioids is indicated.

It can be challenging to raise the subject of opioid tapering with patients; I use Stanford’s BRAVO method to guide these conversations.9,10 At my facility, we are tapering about 50 legacy opioid patients, and most are surprised to find that their pain is the same or better with reduced to no opioids, with fewer adverse effects. Many are happier on sublingual buprenorphine, a safer opioid analgesic.11 The algorithm shown in the FIGURE and the BRAVO method should be more widely used within our specialty for a safe and patient-centered approach to chronic pain.
Above all, let the patient know that you are with them on this journey to safe pain management. Start the conversation: “I’ve been thinking a lot about your chronic pain and how best to help you with it. Our understanding of what opioids do for pain has changed, and I worry they’re causing more harm than good now. This is a scary thing to talk about, but I’ll be with you every step of the way.”
Matt Perez, MD
Neighborcare Health
Seattle
1. Ray WA, Chung CP, Murray KT, et al. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315:2415-23.
2. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
3. Brennan MJ. Letter to the editor. J Pain. 2007;8:185.
4. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018;319:872-882.
5. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
6. Oldfield BJ, Edens EL, Agnoli A, et al. Multimodal treatment options, including rotating to buprenorphine, within a multidisciplinary pain clinic for patients on risky opioid regimens: a quality improvement study. Pain Med. 2018;19(suppl 1):S38–S45.
7. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of long-term opioid analgesics. US Department of Health of Human Services Web site. www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf. October 2019. Accessed September 29, 2020.
8. Pain treatment guidelines. Oregon Pain Guidance Web site. www.oregonpainguidance.org/pain-treatment-guidelines/. Accessed September 29, 2020.
9. Tapering – BRAVO – a collaborative approach clinical update March 2020. Oregon Pain Guidance Web site. www.oregonpainguidance.org/guideline/tapering/. Accessed September 29, 2020.
10. How to taper patients off of chronic opioid therapy. Stanford Center for Continuing Medical Education Web site. https://stanford.cloud-cme.com/default.aspx?P=0&EID=20909. Accessed September 29, 2020.
11. Chou R, Ballantyne J, Lembke A, et al. Rethinking opioid dose tapering, prescription opioid dependence, and indications for buprenorphine. Ann Intern Med. 2019;171:427-429.
1. Ray WA, Chung CP, Murray KT, et al. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315:2415-23.
2. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
3. Brennan MJ. Letter to the editor. J Pain. 2007;8:185.
4. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018;319:872-882.
5. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
6. Oldfield BJ, Edens EL, Agnoli A, et al. Multimodal treatment options, including rotating to buprenorphine, within a multidisciplinary pain clinic for patients on risky opioid regimens: a quality improvement study. Pain Med. 2018;19(suppl 1):S38–S45.
7. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of long-term opioid analgesics. US Department of Health of Human Services Web site. www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf. October 2019. Accessed September 29, 2020.
8. Pain treatment guidelines. Oregon Pain Guidance Web site. www.oregonpainguidance.org/pain-treatment-guidelines/. Accessed September 29, 2020.
9. Tapering – BRAVO – a collaborative approach clinical update March 2020. Oregon Pain Guidance Web site. www.oregonpainguidance.org/guideline/tapering/. Accessed September 29, 2020.
10. How to taper patients off of chronic opioid therapy. Stanford Center for Continuing Medical Education Web site. https://stanford.cloud-cme.com/default.aspx?P=0&EID=20909. Accessed September 29, 2020.
11. Chou R, Ballantyne J, Lembke A, et al. Rethinking opioid dose tapering, prescription opioid dependence, and indications for buprenorphine. Ann Intern Med. 2019;171:427-429.
Is your patient’s cannabis use problematic?
CASE
Jessica F is a new 23-year-old patient at your clinic who is seeing you to discuss her severe anxiety. She also has asthma and reports during your exploration of her family history that her father has been diagnosed with schizophrenia. She has been using 3 cartridges of cannabis vape daily to help “calm her mind” but has never tried other psychotropic medications and has never been referred to a psychiatrist.
How would you proceed with this patient?
Despite emerging evidence of the harmful effects of cannabis consumption, public perception of harm has steadily declined over the past 10 years.1,2 More adults are using cannabis than before and using it more frequently. Among primary care patients who consume cannabis recreationally, about half report less than monthly consumption; 15% use it weekly, and 20% daily.3 The potency of cannabis products has also increased. In the past 2 decades, the average tetrahydrocannabinol (THC) content of recreational cannabis rose from 3% to 19%, and high-THC content delivery modalities such as vaporizer pens (“vapes”) were introduced.4,5
Health hazards of cannabis use include gastrointestinal dysfunction (eg, cannabinoid hyperemesis syndrome), acute psychosis or exacerbation of an existing mood, anxiety, or psychotic disorder, and cardiovascular sequelae such as myocardial infarction or dysrhythmia.6 Potential long-term effects include neurocognitive impairment among adolescents who use cannabis,7-9 worse outcomes in anxiety and mood disorders,10 schizophrenia,11 cardiovascular sequelae,12 chronic bronchitis,13 negative impact on reproductive function,14 and poor birth outcomes.15-17
Hidden in plain sight. Many patients who use cannabis report that their primary care physicians are unaware of their cannabis consumption.18 Inadequate screening for cannabis can be attributed to time constraints, inconsistent definitions for problematic or risky cannabis use, and lack of guidance.19,20 This article offers a more inclusive definition of “problematic cannabis use,” presents an up-to-date framework for evaluating it in the outpatient setting, and outlines potential interventions.

Your patient doesn’t meetthe DSM criteria, but …
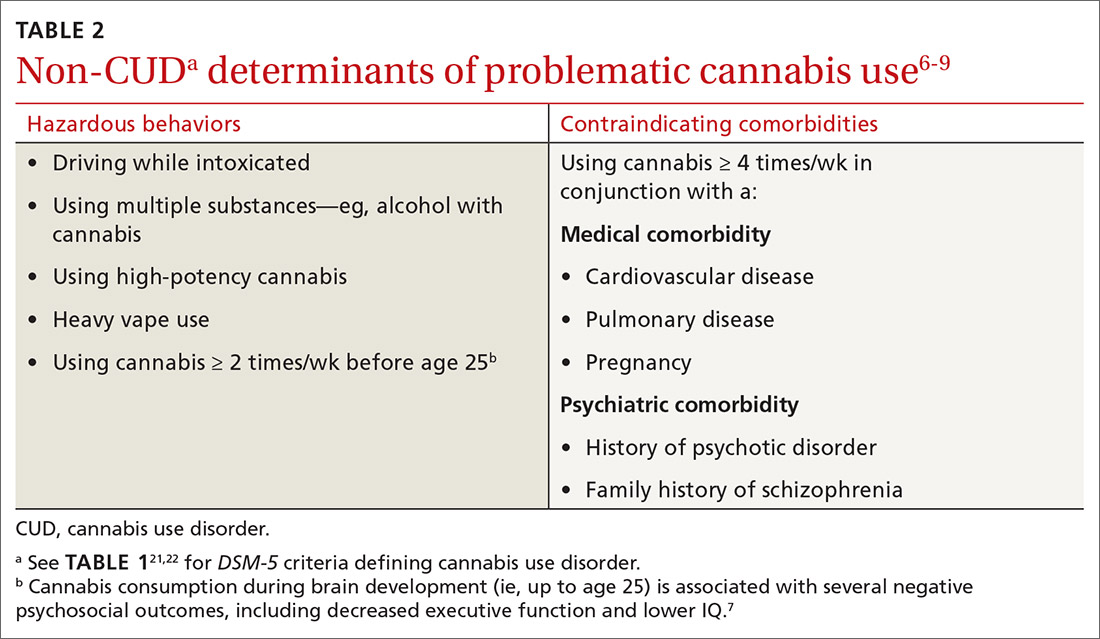
Although it is important to identify cannabis use disorder (CUD) as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; TABLE 121,22), consider also the immediate and long-term consequences of cannabis use for individuals who do not meet criteria for CUD. “Problematic cannabis use,” as we define it, may also involve (a) high-risk behaviors or (b) contraindicating medical or psychiatric comorbidities (TABLE 26-9).
CASE
The patient in our case exhibited
Continue to: Guidelines for screening and evaluation
Guidelines for screening and evaluation
All primary care patients should be screened for problematic cannabis use, but especially teenagers, young adults, pregnant women, and patients with a mental health or substance use history. A variation of the single question used to screen for alcohol use disorder can be applied to cannabis use.23 We recommend asking the initial question, “Over the past month, how many days a week on average have you used cannabis and products that contain THC?” Although some guidelines emphasize frequency of cannabis use when identifying problematic consumption,24,25 duration of behavior and content of THC are also important indicators.19 Inquire about cannabis consumption over 1 month to differentiate sporadic use from longstanding persistent use.
Explore what types of cannabis the patient is ingesting and whether the patient uses cannabis heavily (4 or more times a week on average). Also determine the method of ingestion (eg, eating, vaping, smoking), THC-content (%, if known), and estimated weight of daily cannabis use in grams (TABLE 326). Although patients may not always be able to provide accurate answers, you can gain a sense of the quantity and forms of cannabis a patient is ingesting to inform future conversations on risk and harm reduction.27

Assess a patient’s risk for harm
Cannabis use has the potential to cause immediate harm (linked to a single event of problematic cannabis use) and long-term harm (linked to a recurring pattern of problematic consumption). Cannabis can be especially harmful for patients with the following medical comorbidities or psychosocial factors, and should be avoided.
Cardiovascular disease. Cannabis is associated with an elevated risk for acute coronary syndrome and cardiovascular disease.28 Long-term cannabis use is linked to increased frequency of anginal events, development of cardiac arrhythmias, peripheral arteritis, coronary vasospasms, and problems with platelet aggregation.29,30 Strongly caution against cannabis use with patients who have a history of cardiovascular disease, orthostatic hypotension, tachyarrhythmia, or hypertension.
Pulmonary disease. Patients with pulmonary disease such as asthma may find cannabis helpful as a short-term bronchodilator.31 However, for patients with underlying pulmonary disease who also smoke cigarettes, strongly discourage the smoking of cannabis or hashish, as that may worsen asthma symptoms,32 increase risk of chronic bronchitis,33 and increase cough, sputum production, and wheezing.31 There is currently insufficient evidence to suggest a positive association between cannabis use and the development of chronic obstructive pulmonary disease.34
Continue to: Family history of psychotic disorders
Family history of psychotic disorders. Cannabis is associated with a dose-dependent risk of schizophrenia, which is especially pronounced in patients with a family history of schizophrenia.35 Among patients with a history of psychosis, heavy cannabis use has been associated with increased hospitalizations, increased positive symptoms, and more frequent relapses.36-38
Pregnancy, current or planned. Some women turn to cannabis during pregnancy due to its antiemetic properties. However, perinatal exposure to cannabis is associated with significant risk to the offspring. Maternal cannabis use during the first and second trimesters of pregnancy is associated with decreased performance of the child on measures of function at 3 years of age.39 In addition, cannabis consumption during pregnancy is linked to increased frequency of childhood behavioral issues, inattention, hyperactivity, and impulsivity.40 Peripartum cannabis exposure can affect birth outcomes and is correlated with lower birth weight, incidence of preterm labor, and neonatal intensive care unit admission.15-17,41 Of note, the THC concentration in breast milk peaks at 1 hour after the nursing mother inhales cannabis and typically dissipates after 4 hours.42
Age < 25 years. Chronic heavy use of cannabis in those younger than 25 is associated with higher likelihood of developing CUD, lower IQ,9 lower level of educational attainment, lower income,43 and decreased executive function.8
Substance use disorder history. Recreational cannabis use can hinder recovery from other substance use disorders.44
Consider these 5 interventions
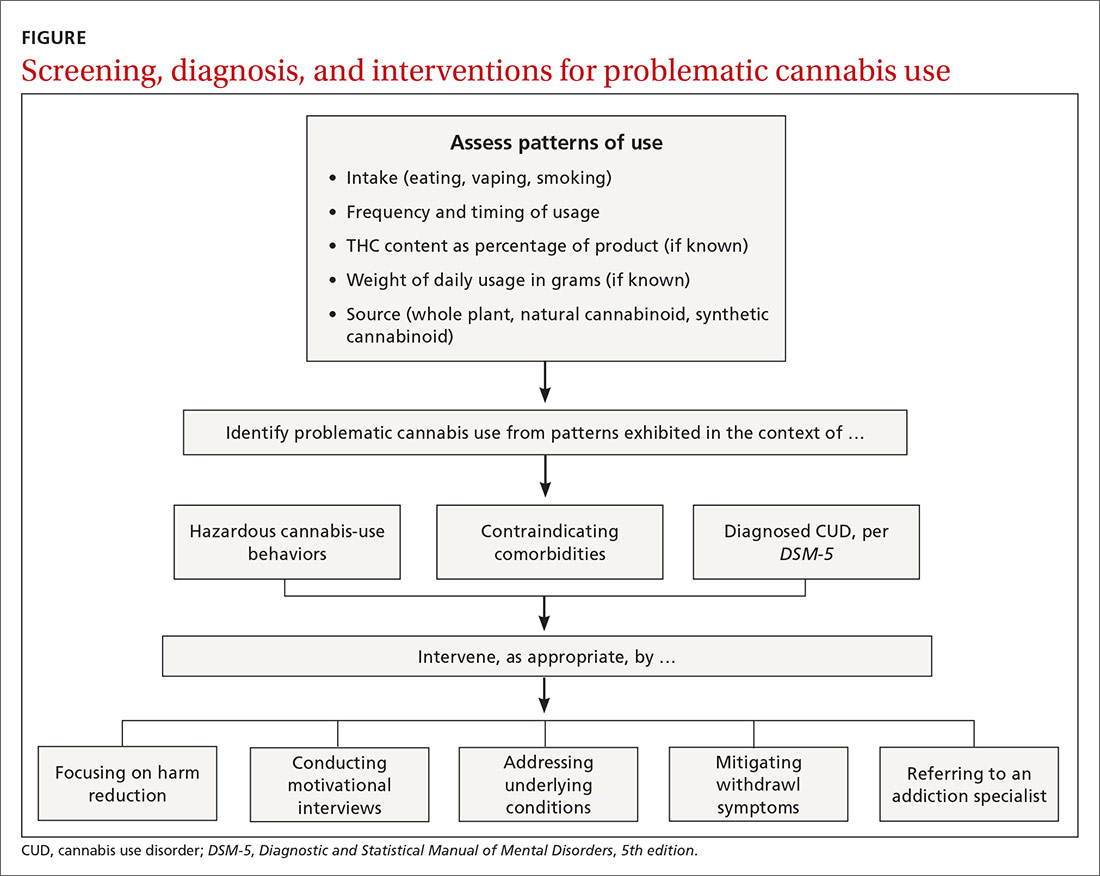
Physicians can address problematic cannabis use with a 5-pronged approach: (1) harm reduction, (2) motivational interviewing, (3) addressing underlying conditions, (4) mitigating withdrawal symptoms, and (5) referring to an addiction specialist (FIGURE).
Continue to: Harm reduction
Harm reduction
Harm reduction applies to all individuals who use cannabis but especially to problematic cannabis users. Ask users to abstain from cannabis for limited periods of time to see how such abstinence affects other areas of their life. While abstinence is a goal, be prepared to perform non-abstinence-based interventions. The goal of harm reduction is to encourage behaviors that minimize health risks to which cannabis users are exposed. Encourage patients to:
Abstain from driving while intoxicated. Cannabis use while driving slows reaction time,45 impairs road tracking (driving with correct road position),46 increases weaving,47 and causes a loss of anticipatory reactions learned in driving practice.48 Risk of crashing is significantly increased with elevated levels of THC, and driving within 1 hour of cannabis ingestion nearly doubles the risk of a crash.49-51
Abstain from vaping THC-containing products. The Centers for Disease Control and Prevention recommends that patients minimize the use of THC-containing e-cigarette or vaping products in light of the thousands of reports in the United States of product-associated lung injury, which in some cases have led to death.52
Clarify serving sizes and recognize delayed effects. Inexperienced cannabis users often are confused by recommended serving sizes for edible cannabis products. A typical cannabis-infused brownie may contain 100 mg of THC when the recommended serving size typically is 10 mg. THC content is included on the label of cannabis edibles purchased in state-regulated stores; these products are tested regularly in laboratories designated by the state.
Due to the delayed onset of THC’s effect, there have been numerous cases of patients taking a higher-than-intended dose of edible cannabis that caused acute intoxication and psychomedical sequelae leading to emergency hospital visits and, in some cases, death.6,53 Individuals should start at a low dose and gradually work up to a higher dose as tolerated. Patients naïve to cannabis should be especially cautious when ingesting edible products.
Continue to: Abstain from cannabis with high THC content
Abstain from cannabis with high THC content. High-potency cannabis (> 10% THC) is associated with earlier onset of first-episode psychosis.54,55
Motivational interviewing
Motivational interviewing (MI) is a psychosocial approach that emphasizes a patient’s self-efficacy and an interviewer’s positive feedback to collaboratively address substance use.56 MI can be performed in short, discrete sessions. Such interventions can reduce the average number of days of cannabis use. One large-scale Cochrane review found that cognitive behavioral therapy (CBT), motivational enhancement therapy, or the 2 therapies combined most consistently reduced the frequency of cannabis use reported by patients at early follow-up.57
Address underlying conditions
Some patients use cannabis to self-medicate for pain, insomnia, nausea, and anxiety. Identify these conditions and address them with first-line pharmacologic or psychotherapeutic interventions when possible. This is especially important for conditions in which long-term cannabis use may adversely impact outcomes, such as in posttraumatic stress disorder, anxiety, and mood disorders.58-60 Little evidence exists for the use of cannabis as treatment of any primary psychiatric disorder.61,62 Family physicians who are uncomfortable treating a specific underlying condition can consult specialists in pain management, sleep medicine, psychiatry, and neurology.
Mitigate withdrawal symptoms
Discontinuation of cannabis use may lead to withdrawal symptoms such as waxing and waning irritability, restlessness, sweating, aggression, anxiety, depressed mood, sleep disturbance, or changes in appetite.63,64 These symptoms typically emerge within the first couple days of abstinence and can last up to 28 days.63,64 Although the US Food and Drug Administration has not approved any medications for CUD treatment, and there are no established protocols for detoxification, there is evidence that CBT or medications such as gabapentin or zolpidem can reduce the intensity of withdrawal symptoms.65,66
Refer to an addiction specialist
Consider referring patients with problematic cannabis use to an addiction specialist with expertise in psychopharmacologic and psychotherapeutic approaches to managing substance use.
Continue to: CASE
CASE
You renew Ms. F’s asthma medications, discuss her cannabis use, start her on a selective serotonin reuptake inhibitor, and refer her to an outpatient psychiatrist. Over the next few weeks, you and the outpatient psychiatrist employ brief motivational interviewing around cannabis use, and you provide psychoeducation around potential harms of use when driving and in light of the patient’s asthma.
The patient’s anxiety symptoms decrease with up-titration of the SSRI by the outpatient psychiatrist and with enrollment in individual CBT. She is slowly able to taper off cannabis vaping with continued motivational interviewing and encouragement, despite withdrawal-induced anxiety and sleep disturbance.
CORRESPONDENCE
Michael Hsu, MD, Brigham & Women’s Hospital, 75 Francis Street, Boston, MA 02215; [email protected].
1. Sarvet AL, Wall MM, Keyes KM, et al. Recent rapid decrease in adolescents’ perception that marijuana is harmful, but no concurrent increase in use. Drug Alcohol Depend. 2018;186:68-74.
2. Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002-14: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954-964.
3. Lapham GT, Lee AK, Caldeiro RM, et al. Frequency of cannabis use among primary care patients in Washington state. J Am Board Fam Med. 2017;30:795‐805.
4. Chandra S, Radwan MM, Majumdar CG, et al. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5-15.
5. Sevigny EL, Pacula RL, Heaton P. The effects of medical marijuana laws on potency. Int J Drug Policy. 2014;25:308-319.
6. Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med. 2019;170:531-537.
7. Scott JC, Slomiak ST, Jones JD, et al. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585-595.
8. Gruber SA, Sagar KA, Dahlgren MK, et al. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012;26:496-506.
9. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657-E2664.
10. Mammen G, Rueda S, Roerecke M, et al. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79:17r11839.
11. Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry. 2016;79:549-556.
12. Singh A, Saluja S, Kumar A, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7:45-59.
13. Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219-2227.
14. Bari M, Battista N, Pirazzi V, et al. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed). 2011;16:498-516.
15. Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215-219.
16. Saurel-Cubizolles M-J, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121:971-977.
17. Prunet C, Delnord M, Saurel-Cubizolles M-J, et al. Risk factors of preterm birth in France in 2010 and changes since 1995: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46:19-28.
18. Kondrad EC, Reed AJ, Simpson MJ, et al. Lack of communication about medical marijuana use between doctors and their patients. J Am Board Fam Med. 2018;31:805-808.
19. Casajuana C, López-Pelayo H, Balcells MM, et al. Definitions of risky and problematic cannabis use: a systematic review. Subst Use Misuse. 2016;51:1760-1770.
20. Norberg MM, Gates P, Dillon P, et al. Screening and managing cannabis use: comparing GP’s and nurses’ knowledge, beliefs, and behavior. Subst Abuse Treat Prev Policy. 2012;7:31.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: APA Publishing; 2013:509-516.
22. Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72:1235-1242.
23. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
24. Fischer B, Jones W, Shuper P, et al. 12-month follow-up of an exploratory ‘brief intervention’ for high-frequency cannabis users among Canadian university students. Subst Abuse Treat Prev Policy. 2012;7:15.
25. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Physician. 2014;60:801-808.
26. Smart R, Caulkins JP, Kilmer B, et al. Variation in cannabis potency & prices in a newly-legal market: evidence from 30 million cannabis sales in Washington State. Addiction. 2017;112:2167-2177.
27. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708-1709.
28. Richards JR, Bing ML, Moulin AK, et al. Cannabis use and acute coronary syndrome. Clin Toxicol (Phila). 2019;57:831-841.
29. Subramaniam VN, Menezes AR, DeSchutter A, et al. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116:146-153.
30. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S-63S.
31. Tetrault JM, Crothers K, Moore BA, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221-228.
32. Bramness JG, von Soest T. A longitudinal study of cannabis use increasing the use of asthma medication in young Norwegian adults. BMC Pulm Med. 2019;19:52.
33. Moore BA, Augustson EM, Moser RP, et al. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med. 2005;20:33-37.
34. Tashkin DP. Does marijuana pose risks for chronic airflow obstruction? Ann Am Thorac Soc. 2015;12:235-236.
35. McGuire PK, Jones P, Harvey I, et al. Morbid risk of schizophrenia for relatives of patients with cannabis-associated psychosis. Schizophr Res. 1995;15:277-281.
36. Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry. 2008;7:68-71.
37. Gerlach J, Koret B, Gereš N, et al. Clinical challenges in patients with first episode psychosis and cannabis use: mini-review and a case study. Psychiatr Danub. 2019;31(suppl 2):162-170.
38. Patel R, Wilson R, Jackson R, et al. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open. 2016;6:e009888.
39. Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16:169-175.
40. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
41. Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322:145-152.
42. Baker T, Datta P, Rewers-Felkins K, et al. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol. 2018;131:783-788.
43. Thompson K, Leadbeater B, Ames M, et al. Associations between marijuana use trajectories and educational and occupational success in young adulthood. Prev Sci. 2019;20:257-269.
44. Yuan M, Kanellopoulos T, Kotbi N. Cannabis use and psychiatric illness in the context of medical marijuana legalization: a clinical perspective. Gen Hosp Psychiatry. 2019;61:82-83.
45. Ronen A, Gershon P, Drobiner H, et al. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev. 2008;40:926-934.
46. Robbe H. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum Psychopharmacol Clin Exp. 1998;13(suppl 2):S70-S78.
47. Lenné MG, Dietze PM, Triggs TJ, et al. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid Anal Prev. 2010;42:859-866.
48. Anderson BM, Rizzo M, Block RI, et al. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42:19-30.
49. Laumon B, Gadegbeku B, Martin J-L, Biecheler M-B. Cannabis intoxication and fatal road crashes in France: population based case-control study. BMJ. 2005;331:1371.
50. Asbridge M, Poulin C, Donato A. Motor vehicle collision risk and driving under the influence of cannabis: evidence from adolescents in Atlantic Canada. Accid Anal Prev. 2005;37:1025-1034.
51. Mann RE, Adlaf E, Zhao J, et al. Cannabis use and self-reported collisions in a representative sample of adult drivers. J Safety Res. 2007;38:669-674.
52. Taylor J, Wiens T, Peterson J, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement—Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1096-1100.
53. Hancock-Allen JB, Barker L, VanDyke M, et al. Notes from the field: death following ingestion of an edible marijuana product—Colorado, March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:771-772.
54. Murray RM, Quigley H, Quattrone D, et al. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry. 2016;15:195-204.
55. Di Forti MD, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509-1517.
56. Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21:835-842.
57. Gates PJ, Sabioni P, Copeland J, et al. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;(5):CD005336.
58. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76:1174-1180.
59. Cougle JR, Bonn-Miller MO, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25:554-558.
60. Johnson MJ, Pierce JD, Mavandadi S, et al. Mental health symptom severity in cannabis using and non-using veterans with probable PTSD. J Affect Disord. 2016;190:439-442.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77:1050-1064.
62. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:995-1010.
63. Bonnet U, Preuss U. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9-37.
64. Vandrey R, Smith MT, McCann UD, et al. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117:38-44.
65. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
66. Weinstein A, Miller H, Tal E, et al. Treatment of cannabis withdrawal syndrome using cognitive-behavioral therapy and relapse prevention for cannabis dependence. J Groups Addict Recover. 2010;5:240-263.
CASE
Jessica F is a new 23-year-old patient at your clinic who is seeing you to discuss her severe anxiety. She also has asthma and reports during your exploration of her family history that her father has been diagnosed with schizophrenia. She has been using 3 cartridges of cannabis vape daily to help “calm her mind” but has never tried other psychotropic medications and has never been referred to a psychiatrist.
How would you proceed with this patient?
Despite emerging evidence of the harmful effects of cannabis consumption, public perception of harm has steadily declined over the past 10 years.1,2 More adults are using cannabis than before and using it more frequently. Among primary care patients who consume cannabis recreationally, about half report less than monthly consumption; 15% use it weekly, and 20% daily.3 The potency of cannabis products has also increased. In the past 2 decades, the average tetrahydrocannabinol (THC) content of recreational cannabis rose from 3% to 19%, and high-THC content delivery modalities such as vaporizer pens (“vapes”) were introduced.4,5
Health hazards of cannabis use include gastrointestinal dysfunction (eg, cannabinoid hyperemesis syndrome), acute psychosis or exacerbation of an existing mood, anxiety, or psychotic disorder, and cardiovascular sequelae such as myocardial infarction or dysrhythmia.6 Potential long-term effects include neurocognitive impairment among adolescents who use cannabis,7-9 worse outcomes in anxiety and mood disorders,10 schizophrenia,11 cardiovascular sequelae,12 chronic bronchitis,13 negative impact on reproductive function,14 and poor birth outcomes.15-17
Hidden in plain sight. Many patients who use cannabis report that their primary care physicians are unaware of their cannabis consumption.18 Inadequate screening for cannabis can be attributed to time constraints, inconsistent definitions for problematic or risky cannabis use, and lack of guidance.19,20 This article offers a more inclusive definition of “problematic cannabis use,” presents an up-to-date framework for evaluating it in the outpatient setting, and outlines potential interventions.

Your patient doesn’t meetthe DSM criteria, but …
Although it is important to identify cannabis use disorder (CUD) as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; TABLE 121,22), consider also the immediate and long-term consequences of cannabis use for individuals who do not meet criteria for CUD. “Problematic cannabis use,” as we define it, may also involve (a) high-risk behaviors or (b) contraindicating medical or psychiatric comorbidities (TABLE 26-9).

CASE
The patient in our case exhibited
Continue to: Guidelines for screening and evaluation
Guidelines for screening and evaluation
All primary care patients should be screened for problematic cannabis use, but especially teenagers, young adults, pregnant women, and patients with a mental health or substance use history. A variation of the single question used to screen for alcohol use disorder can be applied to cannabis use.23 We recommend asking the initial question, “Over the past month, how many days a week on average have you used cannabis and products that contain THC?” Although some guidelines emphasize frequency of cannabis use when identifying problematic consumption,24,25 duration of behavior and content of THC are also important indicators.19 Inquire about cannabis consumption over 1 month to differentiate sporadic use from longstanding persistent use.
Explore what types of cannabis the patient is ingesting and whether the patient uses cannabis heavily (4 or more times a week on average). Also determine the method of ingestion (eg, eating, vaping, smoking), THC-content (%, if known), and estimated weight of daily cannabis use in grams (TABLE 326). Although patients may not always be able to provide accurate answers, you can gain a sense of the quantity and forms of cannabis a patient is ingesting to inform future conversations on risk and harm reduction.27

Assess a patient’s risk for harm
Cannabis use has the potential to cause immediate harm (linked to a single event of problematic cannabis use) and long-term harm (linked to a recurring pattern of problematic consumption). Cannabis can be especially harmful for patients with the following medical comorbidities or psychosocial factors, and should be avoided.
Cardiovascular disease. Cannabis is associated with an elevated risk for acute coronary syndrome and cardiovascular disease.28 Long-term cannabis use is linked to increased frequency of anginal events, development of cardiac arrhythmias, peripheral arteritis, coronary vasospasms, and problems with platelet aggregation.29,30 Strongly caution against cannabis use with patients who have a history of cardiovascular disease, orthostatic hypotension, tachyarrhythmia, or hypertension.
Pulmonary disease. Patients with pulmonary disease such as asthma may find cannabis helpful as a short-term bronchodilator.31 However, for patients with underlying pulmonary disease who also smoke cigarettes, strongly discourage the smoking of cannabis or hashish, as that may worsen asthma symptoms,32 increase risk of chronic bronchitis,33 and increase cough, sputum production, and wheezing.31 There is currently insufficient evidence to suggest a positive association between cannabis use and the development of chronic obstructive pulmonary disease.34
Continue to: Family history of psychotic disorders
Family history of psychotic disorders. Cannabis is associated with a dose-dependent risk of schizophrenia, which is especially pronounced in patients with a family history of schizophrenia.35 Among patients with a history of psychosis, heavy cannabis use has been associated with increased hospitalizations, increased positive symptoms, and more frequent relapses.36-38
Pregnancy, current or planned. Some women turn to cannabis during pregnancy due to its antiemetic properties. However, perinatal exposure to cannabis is associated with significant risk to the offspring. Maternal cannabis use during the first and second trimesters of pregnancy is associated with decreased performance of the child on measures of function at 3 years of age.39 In addition, cannabis consumption during pregnancy is linked to increased frequency of childhood behavioral issues, inattention, hyperactivity, and impulsivity.40 Peripartum cannabis exposure can affect birth outcomes and is correlated with lower birth weight, incidence of preterm labor, and neonatal intensive care unit admission.15-17,41 Of note, the THC concentration in breast milk peaks at 1 hour after the nursing mother inhales cannabis and typically dissipates after 4 hours.42
Age < 25 years. Chronic heavy use of cannabis in those younger than 25 is associated with higher likelihood of developing CUD, lower IQ,9 lower level of educational attainment, lower income,43 and decreased executive function.8
Substance use disorder history. Recreational cannabis use can hinder recovery from other substance use disorders.44
Consider these 5 interventions
Physicians can address problematic cannabis use with a 5-pronged approach: (1) harm reduction, (2) motivational interviewing, (3) addressing underlying conditions, (4) mitigating withdrawal symptoms, and (5) referring to an addiction specialist (FIGURE).

Continue to: Harm reduction
Harm reduction
Harm reduction applies to all individuals who use cannabis but especially to problematic cannabis users. Ask users to abstain from cannabis for limited periods of time to see how such abstinence affects other areas of their life. While abstinence is a goal, be prepared to perform non-abstinence-based interventions. The goal of harm reduction is to encourage behaviors that minimize health risks to which cannabis users are exposed. Encourage patients to:
Abstain from driving while intoxicated. Cannabis use while driving slows reaction time,45 impairs road tracking (driving with correct road position),46 increases weaving,47 and causes a loss of anticipatory reactions learned in driving practice.48 Risk of crashing is significantly increased with elevated levels of THC, and driving within 1 hour of cannabis ingestion nearly doubles the risk of a crash.49-51
Abstain from vaping THC-containing products. The Centers for Disease Control and Prevention recommends that patients minimize the use of THC-containing e-cigarette or vaping products in light of the thousands of reports in the United States of product-associated lung injury, which in some cases have led to death.52
Clarify serving sizes and recognize delayed effects. Inexperienced cannabis users often are confused by recommended serving sizes for edible cannabis products. A typical cannabis-infused brownie may contain 100 mg of THC when the recommended serving size typically is 10 mg. THC content is included on the label of cannabis edibles purchased in state-regulated stores; these products are tested regularly in laboratories designated by the state.
Due to the delayed onset of THC’s effect, there have been numerous cases of patients taking a higher-than-intended dose of edible cannabis that caused acute intoxication and psychomedical sequelae leading to emergency hospital visits and, in some cases, death.6,53 Individuals should start at a low dose and gradually work up to a higher dose as tolerated. Patients naïve to cannabis should be especially cautious when ingesting edible products.
Continue to: Abstain from cannabis with high THC content
Abstain from cannabis with high THC content. High-potency cannabis (> 10% THC) is associated with earlier onset of first-episode psychosis.54,55
Motivational interviewing
Motivational interviewing (MI) is a psychosocial approach that emphasizes a patient’s self-efficacy and an interviewer’s positive feedback to collaboratively address substance use.56 MI can be performed in short, discrete sessions. Such interventions can reduce the average number of days of cannabis use. One large-scale Cochrane review found that cognitive behavioral therapy (CBT), motivational enhancement therapy, or the 2 therapies combined most consistently reduced the frequency of cannabis use reported by patients at early follow-up.57
Address underlying conditions
Some patients use cannabis to self-medicate for pain, insomnia, nausea, and anxiety. Identify these conditions and address them with first-line pharmacologic or psychotherapeutic interventions when possible. This is especially important for conditions in which long-term cannabis use may adversely impact outcomes, such as in posttraumatic stress disorder, anxiety, and mood disorders.58-60 Little evidence exists for the use of cannabis as treatment of any primary psychiatric disorder.61,62 Family physicians who are uncomfortable treating a specific underlying condition can consult specialists in pain management, sleep medicine, psychiatry, and neurology.
Mitigate withdrawal symptoms
Discontinuation of cannabis use may lead to withdrawal symptoms such as waxing and waning irritability, restlessness, sweating, aggression, anxiety, depressed mood, sleep disturbance, or changes in appetite.63,64 These symptoms typically emerge within the first couple days of abstinence and can last up to 28 days.63,64 Although the US Food and Drug Administration has not approved any medications for CUD treatment, and there are no established protocols for detoxification, there is evidence that CBT or medications such as gabapentin or zolpidem can reduce the intensity of withdrawal symptoms.65,66
Refer to an addiction specialist
Consider referring patients with problematic cannabis use to an addiction specialist with expertise in psychopharmacologic and psychotherapeutic approaches to managing substance use.
Continue to: CASE
CASE
You renew Ms. F’s asthma medications, discuss her cannabis use, start her on a selective serotonin reuptake inhibitor, and refer her to an outpatient psychiatrist. Over the next few weeks, you and the outpatient psychiatrist employ brief motivational interviewing around cannabis use, and you provide psychoeducation around potential harms of use when driving and in light of the patient’s asthma.
The patient’s anxiety symptoms decrease with up-titration of the SSRI by the outpatient psychiatrist and with enrollment in individual CBT. She is slowly able to taper off cannabis vaping with continued motivational interviewing and encouragement, despite withdrawal-induced anxiety and sleep disturbance.
CORRESPONDENCE
Michael Hsu, MD, Brigham & Women’s Hospital, 75 Francis Street, Boston, MA 02215; [email protected].
CASE
Jessica F is a new 23-year-old patient at your clinic who is seeing you to discuss her severe anxiety. She also has asthma and reports during your exploration of her family history that her father has been diagnosed with schizophrenia. She has been using 3 cartridges of cannabis vape daily to help “calm her mind” but has never tried other psychotropic medications and has never been referred to a psychiatrist.
How would you proceed with this patient?
Despite emerging evidence of the harmful effects of cannabis consumption, public perception of harm has steadily declined over the past 10 years.1,2 More adults are using cannabis than before and using it more frequently. Among primary care patients who consume cannabis recreationally, about half report less than monthly consumption; 15% use it weekly, and 20% daily.3 The potency of cannabis products has also increased. In the past 2 decades, the average tetrahydrocannabinol (THC) content of recreational cannabis rose from 3% to 19%, and high-THC content delivery modalities such as vaporizer pens (“vapes”) were introduced.4,5
Health hazards of cannabis use include gastrointestinal dysfunction (eg, cannabinoid hyperemesis syndrome), acute psychosis or exacerbation of an existing mood, anxiety, or psychotic disorder, and cardiovascular sequelae such as myocardial infarction or dysrhythmia.6 Potential long-term effects include neurocognitive impairment among adolescents who use cannabis,7-9 worse outcomes in anxiety and mood disorders,10 schizophrenia,11 cardiovascular sequelae,12 chronic bronchitis,13 negative impact on reproductive function,14 and poor birth outcomes.15-17
Hidden in plain sight. Many patients who use cannabis report that their primary care physicians are unaware of their cannabis consumption.18 Inadequate screening for cannabis can be attributed to time constraints, inconsistent definitions for problematic or risky cannabis use, and lack of guidance.19,20 This article offers a more inclusive definition of “problematic cannabis use,” presents an up-to-date framework for evaluating it in the outpatient setting, and outlines potential interventions.

Your patient doesn’t meetthe DSM criteria, but …
Although it is important to identify cannabis use disorder (CUD) as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; TABLE 121,22), consider also the immediate and long-term consequences of cannabis use for individuals who do not meet criteria for CUD. “Problematic cannabis use,” as we define it, may also involve (a) high-risk behaviors or (b) contraindicating medical or psychiatric comorbidities (TABLE 26-9).

CASE
The patient in our case exhibited
Continue to: Guidelines for screening and evaluation
Guidelines for screening and evaluation
All primary care patients should be screened for problematic cannabis use, but especially teenagers, young adults, pregnant women, and patients with a mental health or substance use history. A variation of the single question used to screen for alcohol use disorder can be applied to cannabis use.23 We recommend asking the initial question, “Over the past month, how many days a week on average have you used cannabis and products that contain THC?” Although some guidelines emphasize frequency of cannabis use when identifying problematic consumption,24,25 duration of behavior and content of THC are also important indicators.19 Inquire about cannabis consumption over 1 month to differentiate sporadic use from longstanding persistent use.
Explore what types of cannabis the patient is ingesting and whether the patient uses cannabis heavily (4 or more times a week on average). Also determine the method of ingestion (eg, eating, vaping, smoking), THC-content (%, if known), and estimated weight of daily cannabis use in grams (TABLE 326). Although patients may not always be able to provide accurate answers, you can gain a sense of the quantity and forms of cannabis a patient is ingesting to inform future conversations on risk and harm reduction.27

Assess a patient’s risk for harm
Cannabis use has the potential to cause immediate harm (linked to a single event of problematic cannabis use) and long-term harm (linked to a recurring pattern of problematic consumption). Cannabis can be especially harmful for patients with the following medical comorbidities or psychosocial factors, and should be avoided.
Cardiovascular disease. Cannabis is associated with an elevated risk for acute coronary syndrome and cardiovascular disease.28 Long-term cannabis use is linked to increased frequency of anginal events, development of cardiac arrhythmias, peripheral arteritis, coronary vasospasms, and problems with platelet aggregation.29,30 Strongly caution against cannabis use with patients who have a history of cardiovascular disease, orthostatic hypotension, tachyarrhythmia, or hypertension.
Pulmonary disease. Patients with pulmonary disease such as asthma may find cannabis helpful as a short-term bronchodilator.31 However, for patients with underlying pulmonary disease who also smoke cigarettes, strongly discourage the smoking of cannabis or hashish, as that may worsen asthma symptoms,32 increase risk of chronic bronchitis,33 and increase cough, sputum production, and wheezing.31 There is currently insufficient evidence to suggest a positive association between cannabis use and the development of chronic obstructive pulmonary disease.34
Continue to: Family history of psychotic disorders
Family history of psychotic disorders. Cannabis is associated with a dose-dependent risk of schizophrenia, which is especially pronounced in patients with a family history of schizophrenia.35 Among patients with a history of psychosis, heavy cannabis use has been associated with increased hospitalizations, increased positive symptoms, and more frequent relapses.36-38
Pregnancy, current or planned. Some women turn to cannabis during pregnancy due to its antiemetic properties. However, perinatal exposure to cannabis is associated with significant risk to the offspring. Maternal cannabis use during the first and second trimesters of pregnancy is associated with decreased performance of the child on measures of function at 3 years of age.39 In addition, cannabis consumption during pregnancy is linked to increased frequency of childhood behavioral issues, inattention, hyperactivity, and impulsivity.40 Peripartum cannabis exposure can affect birth outcomes and is correlated with lower birth weight, incidence of preterm labor, and neonatal intensive care unit admission.15-17,41 Of note, the THC concentration in breast milk peaks at 1 hour after the nursing mother inhales cannabis and typically dissipates after 4 hours.42
Age < 25 years. Chronic heavy use of cannabis in those younger than 25 is associated with higher likelihood of developing CUD, lower IQ,9 lower level of educational attainment, lower income,43 and decreased executive function.8
Substance use disorder history. Recreational cannabis use can hinder recovery from other substance use disorders.44
Consider these 5 interventions
Physicians can address problematic cannabis use with a 5-pronged approach: (1) harm reduction, (2) motivational interviewing, (3) addressing underlying conditions, (4) mitigating withdrawal symptoms, and (5) referring to an addiction specialist (FIGURE).

Continue to: Harm reduction
Harm reduction
Harm reduction applies to all individuals who use cannabis but especially to problematic cannabis users. Ask users to abstain from cannabis for limited periods of time to see how such abstinence affects other areas of their life. While abstinence is a goal, be prepared to perform non-abstinence-based interventions. The goal of harm reduction is to encourage behaviors that minimize health risks to which cannabis users are exposed. Encourage patients to:
Abstain from driving while intoxicated. Cannabis use while driving slows reaction time,45 impairs road tracking (driving with correct road position),46 increases weaving,47 and causes a loss of anticipatory reactions learned in driving practice.48 Risk of crashing is significantly increased with elevated levels of THC, and driving within 1 hour of cannabis ingestion nearly doubles the risk of a crash.49-51
Abstain from vaping THC-containing products. The Centers for Disease Control and Prevention recommends that patients minimize the use of THC-containing e-cigarette or vaping products in light of the thousands of reports in the United States of product-associated lung injury, which in some cases have led to death.52
Clarify serving sizes and recognize delayed effects. Inexperienced cannabis users often are confused by recommended serving sizes for edible cannabis products. A typical cannabis-infused brownie may contain 100 mg of THC when the recommended serving size typically is 10 mg. THC content is included on the label of cannabis edibles purchased in state-regulated stores; these products are tested regularly in laboratories designated by the state.
Due to the delayed onset of THC’s effect, there have been numerous cases of patients taking a higher-than-intended dose of edible cannabis that caused acute intoxication and psychomedical sequelae leading to emergency hospital visits and, in some cases, death.6,53 Individuals should start at a low dose and gradually work up to a higher dose as tolerated. Patients naïve to cannabis should be especially cautious when ingesting edible products.
Continue to: Abstain from cannabis with high THC content
Abstain from cannabis with high THC content. High-potency cannabis (> 10% THC) is associated with earlier onset of first-episode psychosis.54,55
Motivational interviewing
Motivational interviewing (MI) is a psychosocial approach that emphasizes a patient’s self-efficacy and an interviewer’s positive feedback to collaboratively address substance use.56 MI can be performed in short, discrete sessions. Such interventions can reduce the average number of days of cannabis use. One large-scale Cochrane review found that cognitive behavioral therapy (CBT), motivational enhancement therapy, or the 2 therapies combined most consistently reduced the frequency of cannabis use reported by patients at early follow-up.57
Address underlying conditions
Some patients use cannabis to self-medicate for pain, insomnia, nausea, and anxiety. Identify these conditions and address them with first-line pharmacologic or psychotherapeutic interventions when possible. This is especially important for conditions in which long-term cannabis use may adversely impact outcomes, such as in posttraumatic stress disorder, anxiety, and mood disorders.58-60 Little evidence exists for the use of cannabis as treatment of any primary psychiatric disorder.61,62 Family physicians who are uncomfortable treating a specific underlying condition can consult specialists in pain management, sleep medicine, psychiatry, and neurology.
Mitigate withdrawal symptoms
Discontinuation of cannabis use may lead to withdrawal symptoms such as waxing and waning irritability, restlessness, sweating, aggression, anxiety, depressed mood, sleep disturbance, or changes in appetite.63,64 These symptoms typically emerge within the first couple days of abstinence and can last up to 28 days.63,64 Although the US Food and Drug Administration has not approved any medications for CUD treatment, and there are no established protocols for detoxification, there is evidence that CBT or medications such as gabapentin or zolpidem can reduce the intensity of withdrawal symptoms.65,66
Refer to an addiction specialist
Consider referring patients with problematic cannabis use to an addiction specialist with expertise in psychopharmacologic and psychotherapeutic approaches to managing substance use.
Continue to: CASE
CASE
You renew Ms. F’s asthma medications, discuss her cannabis use, start her on a selective serotonin reuptake inhibitor, and refer her to an outpatient psychiatrist. Over the next few weeks, you and the outpatient psychiatrist employ brief motivational interviewing around cannabis use, and you provide psychoeducation around potential harms of use when driving and in light of the patient’s asthma.
The patient’s anxiety symptoms decrease with up-titration of the SSRI by the outpatient psychiatrist and with enrollment in individual CBT. She is slowly able to taper off cannabis vaping with continued motivational interviewing and encouragement, despite withdrawal-induced anxiety and sleep disturbance.
CORRESPONDENCE
Michael Hsu, MD, Brigham & Women’s Hospital, 75 Francis Street, Boston, MA 02215; [email protected].
1. Sarvet AL, Wall MM, Keyes KM, et al. Recent rapid decrease in adolescents’ perception that marijuana is harmful, but no concurrent increase in use. Drug Alcohol Depend. 2018;186:68-74.
2. Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002-14: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954-964.
3. Lapham GT, Lee AK, Caldeiro RM, et al. Frequency of cannabis use among primary care patients in Washington state. J Am Board Fam Med. 2017;30:795‐805.
4. Chandra S, Radwan MM, Majumdar CG, et al. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5-15.
5. Sevigny EL, Pacula RL, Heaton P. The effects of medical marijuana laws on potency. Int J Drug Policy. 2014;25:308-319.
6. Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med. 2019;170:531-537.
7. Scott JC, Slomiak ST, Jones JD, et al. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585-595.
8. Gruber SA, Sagar KA, Dahlgren MK, et al. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012;26:496-506.
9. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657-E2664.
10. Mammen G, Rueda S, Roerecke M, et al. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79:17r11839.
11. Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry. 2016;79:549-556.
12. Singh A, Saluja S, Kumar A, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7:45-59.
13. Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219-2227.
14. Bari M, Battista N, Pirazzi V, et al. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed). 2011;16:498-516.
15. Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215-219.
16. Saurel-Cubizolles M-J, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121:971-977.
17. Prunet C, Delnord M, Saurel-Cubizolles M-J, et al. Risk factors of preterm birth in France in 2010 and changes since 1995: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46:19-28.
18. Kondrad EC, Reed AJ, Simpson MJ, et al. Lack of communication about medical marijuana use between doctors and their patients. J Am Board Fam Med. 2018;31:805-808.
19. Casajuana C, López-Pelayo H, Balcells MM, et al. Definitions of risky and problematic cannabis use: a systematic review. Subst Use Misuse. 2016;51:1760-1770.
20. Norberg MM, Gates P, Dillon P, et al. Screening and managing cannabis use: comparing GP’s and nurses’ knowledge, beliefs, and behavior. Subst Abuse Treat Prev Policy. 2012;7:31.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: APA Publishing; 2013:509-516.
22. Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72:1235-1242.
23. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
24. Fischer B, Jones W, Shuper P, et al. 12-month follow-up of an exploratory ‘brief intervention’ for high-frequency cannabis users among Canadian university students. Subst Abuse Treat Prev Policy. 2012;7:15.
25. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Physician. 2014;60:801-808.
26. Smart R, Caulkins JP, Kilmer B, et al. Variation in cannabis potency & prices in a newly-legal market: evidence from 30 million cannabis sales in Washington State. Addiction. 2017;112:2167-2177.
27. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708-1709.
28. Richards JR, Bing ML, Moulin AK, et al. Cannabis use and acute coronary syndrome. Clin Toxicol (Phila). 2019;57:831-841.
29. Subramaniam VN, Menezes AR, DeSchutter A, et al. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116:146-153.
30. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S-63S.
31. Tetrault JM, Crothers K, Moore BA, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221-228.
32. Bramness JG, von Soest T. A longitudinal study of cannabis use increasing the use of asthma medication in young Norwegian adults. BMC Pulm Med. 2019;19:52.
33. Moore BA, Augustson EM, Moser RP, et al. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med. 2005;20:33-37.
34. Tashkin DP. Does marijuana pose risks for chronic airflow obstruction? Ann Am Thorac Soc. 2015;12:235-236.
35. McGuire PK, Jones P, Harvey I, et al. Morbid risk of schizophrenia for relatives of patients with cannabis-associated psychosis. Schizophr Res. 1995;15:277-281.
36. Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry. 2008;7:68-71.
37. Gerlach J, Koret B, Gereš N, et al. Clinical challenges in patients with first episode psychosis and cannabis use: mini-review and a case study. Psychiatr Danub. 2019;31(suppl 2):162-170.
38. Patel R, Wilson R, Jackson R, et al. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open. 2016;6:e009888.
39. Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16:169-175.
40. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
41. Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322:145-152.
42. Baker T, Datta P, Rewers-Felkins K, et al. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol. 2018;131:783-788.
43. Thompson K, Leadbeater B, Ames M, et al. Associations between marijuana use trajectories and educational and occupational success in young adulthood. Prev Sci. 2019;20:257-269.
44. Yuan M, Kanellopoulos T, Kotbi N. Cannabis use and psychiatric illness in the context of medical marijuana legalization: a clinical perspective. Gen Hosp Psychiatry. 2019;61:82-83.
45. Ronen A, Gershon P, Drobiner H, et al. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev. 2008;40:926-934.
46. Robbe H. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum Psychopharmacol Clin Exp. 1998;13(suppl 2):S70-S78.
47. Lenné MG, Dietze PM, Triggs TJ, et al. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid Anal Prev. 2010;42:859-866.
48. Anderson BM, Rizzo M, Block RI, et al. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42:19-30.
49. Laumon B, Gadegbeku B, Martin J-L, Biecheler M-B. Cannabis intoxication and fatal road crashes in France: population based case-control study. BMJ. 2005;331:1371.
50. Asbridge M, Poulin C, Donato A. Motor vehicle collision risk and driving under the influence of cannabis: evidence from adolescents in Atlantic Canada. Accid Anal Prev. 2005;37:1025-1034.
51. Mann RE, Adlaf E, Zhao J, et al. Cannabis use and self-reported collisions in a representative sample of adult drivers. J Safety Res. 2007;38:669-674.
52. Taylor J, Wiens T, Peterson J, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement—Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1096-1100.
53. Hancock-Allen JB, Barker L, VanDyke M, et al. Notes from the field: death following ingestion of an edible marijuana product—Colorado, March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:771-772.
54. Murray RM, Quigley H, Quattrone D, et al. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry. 2016;15:195-204.
55. Di Forti MD, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509-1517.
56. Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21:835-842.
57. Gates PJ, Sabioni P, Copeland J, et al. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;(5):CD005336.
58. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76:1174-1180.
59. Cougle JR, Bonn-Miller MO, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25:554-558.
60. Johnson MJ, Pierce JD, Mavandadi S, et al. Mental health symptom severity in cannabis using and non-using veterans with probable PTSD. J Affect Disord. 2016;190:439-442.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77:1050-1064.
62. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:995-1010.
63. Bonnet U, Preuss U. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9-37.
64. Vandrey R, Smith MT, McCann UD, et al. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117:38-44.
65. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
66. Weinstein A, Miller H, Tal E, et al. Treatment of cannabis withdrawal syndrome using cognitive-behavioral therapy and relapse prevention for cannabis dependence. J Groups Addict Recover. 2010;5:240-263.
1. Sarvet AL, Wall MM, Keyes KM, et al. Recent rapid decrease in adolescents’ perception that marijuana is harmful, but no concurrent increase in use. Drug Alcohol Depend. 2018;186:68-74.
2. Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002-14: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954-964.
3. Lapham GT, Lee AK, Caldeiro RM, et al. Frequency of cannabis use among primary care patients in Washington state. J Am Board Fam Med. 2017;30:795‐805.
4. Chandra S, Radwan MM, Majumdar CG, et al. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5-15.
5. Sevigny EL, Pacula RL, Heaton P. The effects of medical marijuana laws on potency. Int J Drug Policy. 2014;25:308-319.
6. Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med. 2019;170:531-537.
7. Scott JC, Slomiak ST, Jones JD, et al. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585-595.
8. Gruber SA, Sagar KA, Dahlgren MK, et al. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012;26:496-506.
9. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657-E2664.
10. Mammen G, Rueda S, Roerecke M, et al. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79:17r11839.
11. Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry. 2016;79:549-556.
12. Singh A, Saluja S, Kumar A, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7:45-59.
13. Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219-2227.
14. Bari M, Battista N, Pirazzi V, et al. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed). 2011;16:498-516.
15. Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215-219.
16. Saurel-Cubizolles M-J, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121:971-977.
17. Prunet C, Delnord M, Saurel-Cubizolles M-J, et al. Risk factors of preterm birth in France in 2010 and changes since 1995: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46:19-28.
18. Kondrad EC, Reed AJ, Simpson MJ, et al. Lack of communication about medical marijuana use between doctors and their patients. J Am Board Fam Med. 2018;31:805-808.
19. Casajuana C, López-Pelayo H, Balcells MM, et al. Definitions of risky and problematic cannabis use: a systematic review. Subst Use Misuse. 2016;51:1760-1770.
20. Norberg MM, Gates P, Dillon P, et al. Screening and managing cannabis use: comparing GP’s and nurses’ knowledge, beliefs, and behavior. Subst Abuse Treat Prev Policy. 2012;7:31.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: APA Publishing; 2013:509-516.
22. Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72:1235-1242.
23. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
24. Fischer B, Jones W, Shuper P, et al. 12-month follow-up of an exploratory ‘brief intervention’ for high-frequency cannabis users among Canadian university students. Subst Abuse Treat Prev Policy. 2012;7:15.
25. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Physician. 2014;60:801-808.
26. Smart R, Caulkins JP, Kilmer B, et al. Variation in cannabis potency & prices in a newly-legal market: evidence from 30 million cannabis sales in Washington State. Addiction. 2017;112:2167-2177.
27. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708-1709.
28. Richards JR, Bing ML, Moulin AK, et al. Cannabis use and acute coronary syndrome. Clin Toxicol (Phila). 2019;57:831-841.
29. Subramaniam VN, Menezes AR, DeSchutter A, et al. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116:146-153.
30. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S-63S.
31. Tetrault JM, Crothers K, Moore BA, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221-228.
32. Bramness JG, von Soest T. A longitudinal study of cannabis use increasing the use of asthma medication in young Norwegian adults. BMC Pulm Med. 2019;19:52.
33. Moore BA, Augustson EM, Moser RP, et al. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med. 2005;20:33-37.
34. Tashkin DP. Does marijuana pose risks for chronic airflow obstruction? Ann Am Thorac Soc. 2015;12:235-236.
35. McGuire PK, Jones P, Harvey I, et al. Morbid risk of schizophrenia for relatives of patients with cannabis-associated psychosis. Schizophr Res. 1995;15:277-281.
36. Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry. 2008;7:68-71.
37. Gerlach J, Koret B, Gereš N, et al. Clinical challenges in patients with first episode psychosis and cannabis use: mini-review and a case study. Psychiatr Danub. 2019;31(suppl 2):162-170.
38. Patel R, Wilson R, Jackson R, et al. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open. 2016;6:e009888.
39. Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16:169-175.
40. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
41. Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322:145-152.
42. Baker T, Datta P, Rewers-Felkins K, et al. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol. 2018;131:783-788.
43. Thompson K, Leadbeater B, Ames M, et al. Associations between marijuana use trajectories and educational and occupational success in young adulthood. Prev Sci. 2019;20:257-269.
44. Yuan M, Kanellopoulos T, Kotbi N. Cannabis use and psychiatric illness in the context of medical marijuana legalization: a clinical perspective. Gen Hosp Psychiatry. 2019;61:82-83.
45. Ronen A, Gershon P, Drobiner H, et al. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev. 2008;40:926-934.
46. Robbe H. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum Psychopharmacol Clin Exp. 1998;13(suppl 2):S70-S78.
47. Lenné MG, Dietze PM, Triggs TJ, et al. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid Anal Prev. 2010;42:859-866.
48. Anderson BM, Rizzo M, Block RI, et al. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42:19-30.
49. Laumon B, Gadegbeku B, Martin J-L, Biecheler M-B. Cannabis intoxication and fatal road crashes in France: population based case-control study. BMJ. 2005;331:1371.
50. Asbridge M, Poulin C, Donato A. Motor vehicle collision risk and driving under the influence of cannabis: evidence from adolescents in Atlantic Canada. Accid Anal Prev. 2005;37:1025-1034.
51. Mann RE, Adlaf E, Zhao J, et al. Cannabis use and self-reported collisions in a representative sample of adult drivers. J Safety Res. 2007;38:669-674.
52. Taylor J, Wiens T, Peterson J, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement—Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1096-1100.
53. Hancock-Allen JB, Barker L, VanDyke M, et al. Notes from the field: death following ingestion of an edible marijuana product—Colorado, March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:771-772.
54. Murray RM, Quigley H, Quattrone D, et al. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry. 2016;15:195-204.
55. Di Forti MD, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509-1517.
56. Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21:835-842.
57. Gates PJ, Sabioni P, Copeland J, et al. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;(5):CD005336.
58. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76:1174-1180.
59. Cougle JR, Bonn-Miller MO, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25:554-558.
60. Johnson MJ, Pierce JD, Mavandadi S, et al. Mental health symptom severity in cannabis using and non-using veterans with probable PTSD. J Affect Disord. 2016;190:439-442.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77:1050-1064.
62. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:995-1010.
63. Bonnet U, Preuss U. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9-37.
64. Vandrey R, Smith MT, McCann UD, et al. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117:38-44.
65. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
66. Weinstein A, Miller H, Tal E, et al. Treatment of cannabis withdrawal syndrome using cognitive-behavioral therapy and relapse prevention for cannabis dependence. J Groups Addict Recover. 2010;5:240-263.
PRACTICE RECOMMENDATIONS
› Address underlying conditions for which patients use recreational cannabis to manage symptoms. B
› Consider discrete, in-office sessions of motivational interviewing and referral for cognitive behavioral therapy for patients with problematic cannabis use. B
› Provide counseling around harm reduction for all patients—especially those with problematic cannabis use. C
› Consider referral to an addiction specialist for patients with cannabis use disorder or other problematic cannabis use. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Alcohol problems linked to legal performance-enhancement products
Adolescent alcohol use among boys was prospectively associated with use of legal performance-enhancing substances in young adulthood, based on prospective cohort data from more than 12,000 individuals, wrote Kyle T. Ganson, PhD, MSW, of the University of Toronto, and colleagues.
In addition, legal use of performance-enhancing substances (PES) among young men was associated with increased risk of alcohol use problems.
Although previous studies have shown a range of adverse effects associated with the use of anabolic-androgenic steroid derivatives (defined as illegal PES), the possible adverse effects of legal PES (defined in this report as protein powders, creatine monohydrate, dehydroepiandrostenedione, and amino acids) have not been well studied, the researchers wrote.
In a study published in Pediatrics, the researchers reviewed data from 12,133 young adults aged 18-26 years who were part of the National Longitudinal Study of Adolescent to Adult Health from 1994 to 2008.
Overall, 16% of young men and 1% of young women reported using legal PES in the past year. Among men, legal PES use was prospectively associated with increased risk of a range of alcohol-related problem behaviors including binge drinking (adjusted odds ratio, 1.35), injurious and risky behaviors (aOR, 1.78), legal problems (aOR, 1.52), reduced activities and socializing (aOR, 1.91), and problems with emotional or physical health (aOR, 1.44).
Legal PES use among young adult women was associated with an increased risk of emotional or physical health problems (aOR, 3.00).
Adolescent impact
Between adolescence and young adulthood (an average of 7 years’ follow-up), alcohol use was prospectively associated with legal PES use in young men (OR, 1.39), but neither cigarette smoking nor marijuana use in adolescence was associated with later use of legal PES. Among young women, no type of adolescent substance use was prospectively associated with later use of legal PES.
“To date, legal PES have not been largely considered as part of the spectrum of substances used among adolescents, have not been subject to the same regulatory scrutiny as other substances known to be linked to subsequent substance use and are freely available over the counter to adolescents,” Dr. Ganson and associates noted.
“Clearly, the robust reciprocal temporal relationship between substance use and legal PES suggests that each may serve as a gateway for the other,” they wrote.
The study findings were limited by several factors including the inability to identify outcomes associated with variable PES components, incomplete data collection on several drinking-related risk behaviors, and inability to analyze prospective use of illegal or other substances associated with use of legal PES, the researchers wrote.
However, “these results provide further evidence in support of the gateway theory and prospective health risk behaviors associated with legal PES and substance use,” they wrote.
The data may inform policy on the additional regulation of legal PES use in minors. In the meantime, “it is important for medical providers and clinicians to assess problematic alcohol use and drinking-related risk behaviors among young adult men who have previously used legal PES,” Dr. Ganson and associates concluded.
Challenges to clinicians
An important point to recognize is that PES is a misleading term, Steven Cuff, MD, of the Ohio State University, Columbus, and Michele LaBotz, MD, of Tufts University, Boston, wrote in an accompanying editorial. “Most legal supplements marketed for athletic performance enhancement are ineffective at increasing muscle mass or athletic performance beyond what can be achieved through appropriate nutrition and training,” they emphasized. The current study findings suggest that “legal PES should be integrated into the gateway hypothesis regarding patterns and progression of substance use through adolescence and early adulthood,” and support discouragement of any PES use among adolescents and young adults.
Even legal PES can be dangerous because of the lack of oversight of dietary supplements by the Food and Drug Administration. “There is widespread evidence that many over-the-counter dietary supplements lack stated ingredients, contain unlabeled ingredients (including potential allergens), or are contaminated with impurities or illegal or dangerous substances, such as steroids and stimulants,” the editorialists emphasized.
In addition, the association found in the study between muscle dysphoria and both PES use and substance use disorders, notably alcohol-related morbidity, highlights the need for a proactive approach by pediatricians to minimize the risk, they noted.
“For pediatricians uncomfortable with initiating discussions on PES use with their patients, an American Academy of Pediatrics–supported role-play simulation is available,” they concluded.
The study is important because “PES use is ubiquitous among adolescents and young adults,” Dr. LaBotz said in an interview. “Although it is widely believed that PES use serves as a likely ‘gateway’ to use of anabolic steroids and other substances, this is one of the very few studies that explores this relationship. Their findings that alcohol use appears to correlate with subsequent use of PES, and that PES use appears to correlate with future alcohol-related issues, suggest that this is not a simple linear progression of problematic behavior.”
Dr. LaBotz added that she was not surprised by the study findings, and emphasized that pediatric health care providers should be aware of the association between PES and alcohol use. “PES screening should be incorporated into screening done for alcohol and other substance use. This appears to be particularly true for athletes and other subpopulations who are at higher risk for problematic alcohol use.”
She said much of PES use is driven by the desire by young men for a muscular appearance, but more research is needed on young women. “In the past, this was a goal primarily associated with males, but females have become increasingly interested in achieving muscularity as well, which suggests an increasing risk of PES use among females as compared to earlier reports. We need updated data on patterns, prevalence and consequences of PES use in females.”
In addition, “although preparticipation physical examination forms include screening questions for PES use among athletes, further information is needed on how to incorporate PES into substance use screening that is performed in a general pediatric population, such as including athletes and nonathletes,” Dr. LaBotz said.
The study was supported by the National Institutes of Health and by grants to one of the coauthors from the Pediatric Scientist Development Program funded by the American Academy of Pediatrics and the American Pediatric Society, as well as the American Heart Association Career Development Award. The researchers had no financial conflicts to disclose. Dr. Cuff and Dr. LaBotz had no financial conflicts to disclose.
SOURCE: Ganson KT et al. Pediatrics. 2020 Sep. doi: 10.1542/peds.2020-0409.
Adolescent alcohol use among boys was prospectively associated with use of legal performance-enhancing substances in young adulthood, based on prospective cohort data from more than 12,000 individuals, wrote Kyle T. Ganson, PhD, MSW, of the University of Toronto, and colleagues.
In addition, legal use of performance-enhancing substances (PES) among young men was associated with increased risk of alcohol use problems.
Although previous studies have shown a range of adverse effects associated with the use of anabolic-androgenic steroid derivatives (defined as illegal PES), the possible adverse effects of legal PES (defined in this report as protein powders, creatine monohydrate, dehydroepiandrostenedione, and amino acids) have not been well studied, the researchers wrote.
In a study published in Pediatrics, the researchers reviewed data from 12,133 young adults aged 18-26 years who were part of the National Longitudinal Study of Adolescent to Adult Health from 1994 to 2008.
Overall, 16% of young men and 1% of young women reported using legal PES in the past year. Among men, legal PES use was prospectively associated with increased risk of a range of alcohol-related problem behaviors including binge drinking (adjusted odds ratio, 1.35), injurious and risky behaviors (aOR, 1.78), legal problems (aOR, 1.52), reduced activities and socializing (aOR, 1.91), and problems with emotional or physical health (aOR, 1.44).
Legal PES use among young adult women was associated with an increased risk of emotional or physical health problems (aOR, 3.00).
Adolescent impact
Between adolescence and young adulthood (an average of 7 years’ follow-up), alcohol use was prospectively associated with legal PES use in young men (OR, 1.39), but neither cigarette smoking nor marijuana use in adolescence was associated with later use of legal PES. Among young women, no type of adolescent substance use was prospectively associated with later use of legal PES.
“To date, legal PES have not been largely considered as part of the spectrum of substances used among adolescents, have not been subject to the same regulatory scrutiny as other substances known to be linked to subsequent substance use and are freely available over the counter to adolescents,” Dr. Ganson and associates noted.
“Clearly, the robust reciprocal temporal relationship between substance use and legal PES suggests that each may serve as a gateway for the other,” they wrote.
The study findings were limited by several factors including the inability to identify outcomes associated with variable PES components, incomplete data collection on several drinking-related risk behaviors, and inability to analyze prospective use of illegal or other substances associated with use of legal PES, the researchers wrote.
However, “these results provide further evidence in support of the gateway theory and prospective health risk behaviors associated with legal PES and substance use,” they wrote.
The data may inform policy on the additional regulation of legal PES use in minors. In the meantime, “it is important for medical providers and clinicians to assess problematic alcohol use and drinking-related risk behaviors among young adult men who have previously used legal PES,” Dr. Ganson and associates concluded.
Challenges to clinicians
An important point to recognize is that PES is a misleading term, Steven Cuff, MD, of the Ohio State University, Columbus, and Michele LaBotz, MD, of Tufts University, Boston, wrote in an accompanying editorial. “Most legal supplements marketed for athletic performance enhancement are ineffective at increasing muscle mass or athletic performance beyond what can be achieved through appropriate nutrition and training,” they emphasized. The current study findings suggest that “legal PES should be integrated into the gateway hypothesis regarding patterns and progression of substance use through adolescence and early adulthood,” and support discouragement of any PES use among adolescents and young adults.
Even legal PES can be dangerous because of the lack of oversight of dietary supplements by the Food and Drug Administration. “There is widespread evidence that many over-the-counter dietary supplements lack stated ingredients, contain unlabeled ingredients (including potential allergens), or are contaminated with impurities or illegal or dangerous substances, such as steroids and stimulants,” the editorialists emphasized.
In addition, the association found in the study between muscle dysphoria and both PES use and substance use disorders, notably alcohol-related morbidity, highlights the need for a proactive approach by pediatricians to minimize the risk, they noted.
“For pediatricians uncomfortable with initiating discussions on PES use with their patients, an American Academy of Pediatrics–supported role-play simulation is available,” they concluded.
The study is important because “PES use is ubiquitous among adolescents and young adults,” Dr. LaBotz said in an interview. “Although it is widely believed that PES use serves as a likely ‘gateway’ to use of anabolic steroids and other substances, this is one of the very few studies that explores this relationship. Their findings that alcohol use appears to correlate with subsequent use of PES, and that PES use appears to correlate with future alcohol-related issues, suggest that this is not a simple linear progression of problematic behavior.”
Dr. LaBotz added that she was not surprised by the study findings, and emphasized that pediatric health care providers should be aware of the association between PES and alcohol use. “PES screening should be incorporated into screening done for alcohol and other substance use. This appears to be particularly true for athletes and other subpopulations who are at higher risk for problematic alcohol use.”
She said much of PES use is driven by the desire by young men for a muscular appearance, but more research is needed on young women. “In the past, this was a goal primarily associated with males, but females have become increasingly interested in achieving muscularity as well, which suggests an increasing risk of PES use among females as compared to earlier reports. We need updated data on patterns, prevalence and consequences of PES use in females.”
In addition, “although preparticipation physical examination forms include screening questions for PES use among athletes, further information is needed on how to incorporate PES into substance use screening that is performed in a general pediatric population, such as including athletes and nonathletes,” Dr. LaBotz said.
The study was supported by the National Institutes of Health and by grants to one of the coauthors from the Pediatric Scientist Development Program funded by the American Academy of Pediatrics and the American Pediatric Society, as well as the American Heart Association Career Development Award. The researchers had no financial conflicts to disclose. Dr. Cuff and Dr. LaBotz had no financial conflicts to disclose.
SOURCE: Ganson KT et al. Pediatrics. 2020 Sep. doi: 10.1542/peds.2020-0409.
Adolescent alcohol use among boys was prospectively associated with use of legal performance-enhancing substances in young adulthood, based on prospective cohort data from more than 12,000 individuals, wrote Kyle T. Ganson, PhD, MSW, of the University of Toronto, and colleagues.
In addition, legal use of performance-enhancing substances (PES) among young men was associated with increased risk of alcohol use problems.
Although previous studies have shown a range of adverse effects associated with the use of anabolic-androgenic steroid derivatives (defined as illegal PES), the possible adverse effects of legal PES (defined in this report as protein powders, creatine monohydrate, dehydroepiandrostenedione, and amino acids) have not been well studied, the researchers wrote.
In a study published in Pediatrics, the researchers reviewed data from 12,133 young adults aged 18-26 years who were part of the National Longitudinal Study of Adolescent to Adult Health from 1994 to 2008.
Overall, 16% of young men and 1% of young women reported using legal PES in the past year. Among men, legal PES use was prospectively associated with increased risk of a range of alcohol-related problem behaviors including binge drinking (adjusted odds ratio, 1.35), injurious and risky behaviors (aOR, 1.78), legal problems (aOR, 1.52), reduced activities and socializing (aOR, 1.91), and problems with emotional or physical health (aOR, 1.44).
Legal PES use among young adult women was associated with an increased risk of emotional or physical health problems (aOR, 3.00).
Adolescent impact
Between adolescence and young adulthood (an average of 7 years’ follow-up), alcohol use was prospectively associated with legal PES use in young men (OR, 1.39), but neither cigarette smoking nor marijuana use in adolescence was associated with later use of legal PES. Among young women, no type of adolescent substance use was prospectively associated with later use of legal PES.
“To date, legal PES have not been largely considered as part of the spectrum of substances used among adolescents, have not been subject to the same regulatory scrutiny as other substances known to be linked to subsequent substance use and are freely available over the counter to adolescents,” Dr. Ganson and associates noted.
“Clearly, the robust reciprocal temporal relationship between substance use and legal PES suggests that each may serve as a gateway for the other,” they wrote.
The study findings were limited by several factors including the inability to identify outcomes associated with variable PES components, incomplete data collection on several drinking-related risk behaviors, and inability to analyze prospective use of illegal or other substances associated with use of legal PES, the researchers wrote.
However, “these results provide further evidence in support of the gateway theory and prospective health risk behaviors associated with legal PES and substance use,” they wrote.
The data may inform policy on the additional regulation of legal PES use in minors. In the meantime, “it is important for medical providers and clinicians to assess problematic alcohol use and drinking-related risk behaviors among young adult men who have previously used legal PES,” Dr. Ganson and associates concluded.
Challenges to clinicians
An important point to recognize is that PES is a misleading term, Steven Cuff, MD, of the Ohio State University, Columbus, and Michele LaBotz, MD, of Tufts University, Boston, wrote in an accompanying editorial. “Most legal supplements marketed for athletic performance enhancement are ineffective at increasing muscle mass or athletic performance beyond what can be achieved through appropriate nutrition and training,” they emphasized. The current study findings suggest that “legal PES should be integrated into the gateway hypothesis regarding patterns and progression of substance use through adolescence and early adulthood,” and support discouragement of any PES use among adolescents and young adults.
Even legal PES can be dangerous because of the lack of oversight of dietary supplements by the Food and Drug Administration. “There is widespread evidence that many over-the-counter dietary supplements lack stated ingredients, contain unlabeled ingredients (including potential allergens), or are contaminated with impurities or illegal or dangerous substances, such as steroids and stimulants,” the editorialists emphasized.
In addition, the association found in the study between muscle dysphoria and both PES use and substance use disorders, notably alcohol-related morbidity, highlights the need for a proactive approach by pediatricians to minimize the risk, they noted.
“For pediatricians uncomfortable with initiating discussions on PES use with their patients, an American Academy of Pediatrics–supported role-play simulation is available,” they concluded.
The study is important because “PES use is ubiquitous among adolescents and young adults,” Dr. LaBotz said in an interview. “Although it is widely believed that PES use serves as a likely ‘gateway’ to use of anabolic steroids and other substances, this is one of the very few studies that explores this relationship. Their findings that alcohol use appears to correlate with subsequent use of PES, and that PES use appears to correlate with future alcohol-related issues, suggest that this is not a simple linear progression of problematic behavior.”
Dr. LaBotz added that she was not surprised by the study findings, and emphasized that pediatric health care providers should be aware of the association between PES and alcohol use. “PES screening should be incorporated into screening done for alcohol and other substance use. This appears to be particularly true for athletes and other subpopulations who are at higher risk for problematic alcohol use.”
She said much of PES use is driven by the desire by young men for a muscular appearance, but more research is needed on young women. “In the past, this was a goal primarily associated with males, but females have become increasingly interested in achieving muscularity as well, which suggests an increasing risk of PES use among females as compared to earlier reports. We need updated data on patterns, prevalence and consequences of PES use in females.”
In addition, “although preparticipation physical examination forms include screening questions for PES use among athletes, further information is needed on how to incorporate PES into substance use screening that is performed in a general pediatric population, such as including athletes and nonathletes,” Dr. LaBotz said.
The study was supported by the National Institutes of Health and by grants to one of the coauthors from the Pediatric Scientist Development Program funded by the American Academy of Pediatrics and the American Pediatric Society, as well as the American Heart Association Career Development Award. The researchers had no financial conflicts to disclose. Dr. Cuff and Dr. LaBotz had no financial conflicts to disclose.
SOURCE: Ganson KT et al. Pediatrics. 2020 Sep. doi: 10.1542/peds.2020-0409.
FROM PEDIATRICS
Retrospective Review on the Safety and Efficacy of Direct Oral Anticoagulants Compared With Warfarin in Patients With Cirrhosis
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
The Other Pandemic: Addiction
May 20 of this plague year, Reuters reported the death of a 32-year-old Florida nurse who had worked tirelessly to treat patients with COVID-19.1 The presumption is that, like so many selfless health care providers (HCPs), this nurse was exposed to and then sadly succumbed to the virus. That presumption would be wrong: COVID-19 did not take his young life. The other pandemic—addiction— did. Bereaved friends and family reported that the nurse had been in recovery from opioid use disorder (OUD) before the onslaught of the public health crisis. The chronicle of his relapse is instructive for the devastating effect COVID-19 has had on persons struggling with addiction, even those like the nurse who was in sustained remission from OUD with a bright future.
Many of the themes are familiar to HCPs and have been the subject of prior columns in this COVID-19 series. The nurse experienced acute stress symptoms, such as nightmares from the repeated crises of sick and dying patients in the intensive care unit where he worked.2 Like so many other HCPs, while he was desperately trying to save others, he also worried about having sufficient access to appropriate personal protective equipment (PPE).
Most relevant to this column, the caregiver was unable to access his primary source of support for his sobriety—attendance at 12-step meetings. Social distancing, which is one of the only proven means we have of reducing transmission of the virus, has had unintended consequences. Although many have found virtual connections rewarding, this nurse needed the curtailed face-to-face contact. The courage that had led him to volunteer for hazardous duty unwontedly resulted in his estrangement: Friends feared that he would expose them to the virus, and he worried that he would expose his family to danger. As in the 1918 flu pandemic, the humans we depend on for reality testing and companionship have been cruelly transformed into potential vectors of the virus.3
Isolation is the worst of all possible counselors as the great Spanish philosopher of alienation Miguel de Unamuno has argued. The deceptive promise of a rapid deliverance from anxiety and pain that substances of abuse proffer apparently led the nurse back to opioids. The virtue of being clean permitted the dirty drug to take advantage of the nurses’ reduced physiologic tolerance to opioids. It is suspected but not confirmed that he fatally overdosed alone in his car.
This Florida nurse is an especially tragic example of a terrible phenomenon being repeated all over the country. And the epidemic of substance use disorders (SUDs) related to COVID-19 is not confined to the US; there are similar reports from other afflicted nations, making addiction truly the other pandemic.4 The Centers for Disease Control and Prevention reported that 13.3% of American adults have started or increased their substance use as a means of managing the negative emotions associated with the pandemic.5 Also from March to May 2020, researchers in Baltimore found a 17.6% increase in suspected overdoses in counties advising social distancing and/or mandating stay at home orders.5
These data reinforce a well-known maxim in the addiction community that “addiction is a disease of isolation.”6-8 The burden of the lockdown falls harder on many of the patients we treat in the federal health care system whose other mental and physical health conditions, including chronic pain, depression, and posttraumatic stress disorder already placed them at elevated risk of SUDs.9 Director of the National Institute of Drug Abuse Nora Volkow, MD, recently traced the well-known arc from isolation to increased use of drugs and alcohol.10 Isolation is stressful and amplifies negative thoughts, dysphoria, and fearful emotions, which are recognized triggers for the use of substances of abuse. The usually available means of coping with craving, and in many cases withdrawal, such as prescribed medications, visits to therapists, participation in support groups are either not available or much more difficult to access.10 Nor are those without a current or even historical SUD immune to the psychosocial pressures of the pandemic: Isolation also constitutes a risk for the development of de novo addiction particularly among already marginalized groups, such as the elderly and disabled.
The federal government has initiated several important measures to reduce the adverse impact of isolation on persons with SUDs. The Drug Enforcement Administration is exempting qualified practitioners of medication-assisted treatment from the in-person evaluation that is usually required for the prescription of controlled substances, including buprenorphine. This exemption applies to both established patient prescriptions for buprenorphine and new buprenorphine patient prescriptions.11 These and other administrative contingencies at the federal government level can assist persons with OUD to continue to receive medicationassisted treatment.
As individual clinicians in federal practice, we alone cannot engineer such major policy accommodations in response to COVID-19, yet we can still make a difference in the lives of our patients. We can focus a few minutes of our telehealth interactions on checking in with patients who have a history or a current SUD. We can remember to use evidence-based screens for these patients and those with other risk factors to detect drug or alcohol use before it becomes a disorder. And we can identify and refer not only patients but also our beleaguered colleagues who feel alone at sea—to the many lifelines our agencies have cast into what other commentators have referred to as a Perfect Storm of COVID-19 and the opioid crisis (Table).12
1. Borter G. A nurse struggled with COVID-19 trauma. He was found dead in his car. Reuters. May 20, 2020. https:// www.reuters.com/article/us-health-coronavirus-nurse -death-insigh/a-nurse-struggled-with-covid-19-trauma-he -was-found-dead-in-his-car-idUSKBN22W1JD Accessed September 15, 2020.
2. Geppert CMA. The duty to care and its exceptions in a pandemic. Fed Pract. 2020;37(5):210-211.
3. Kim NY. How the 1918 pandemic frayed social bonds. The Atlantic. March 31, 2020. https://www.theatlantic.com /family/archive/2020/03/coronavirus-loneliness-and-mistrust -1918-flu-pandemic-quarantine/609163. Accessed September 18, 2020.
4. Jemberie WB, Stewart Williams J, Eriksson M, et al. Substance use disorders and COVID-19: multi-faceted problems which require multi-pronged solutions. Front Psychiatry. 2020;11:714. Published 2020 Jul 21. doi:10.3389/fpsyt.2020.00714
5. Alter A, Yeager C. COVID-19 impact on US national overdose crises. http://www.odmap.org/Content/docs/news/2020 /ODMAP-Report-June-2020.pdf. Published May 2020. Accessed September 18, 2020.
6. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. Published 2020 Aug 14. doi:10.15585/mmwr.mm6932a1
7. Grinspoon P. A tale of two epidemics: when COVID-19 and opioid addiction collide. https://www.health.harvard.edu /blog/a-tale-of-two-epidemics-when-covid-19-and-opioid -addiction-collide-2020042019569. Published April 20, 2020. Accessed September 16, 2020
8. Bebinger M. Addiction is “a disease of isolation”—so pandemic puts recovery at risk. https://khn.org/news/addiction -is-a-disease-of-isolation-so-pandemic-puts-recovery-at-risk. Published March 30, 2020. Accessed September 23, 2020.
9. National Institute of Drug Abuse. Substance abuse and military life. DrugFacts. https://www.drugabuse.gov/publications /drugfacts/substance-use-military-life. Published October 2019. Accessed September 16, 2020.
10. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62. doi:10.7326/M20-1212
11. Substance Abuse and Mental Health Administration. FAQS: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency. https:// www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing -and-dispensing.pdf. Updated April 21, 2020. Accessed September 22, 2020.
12. Spagnolo PA, Montemitro C, Leggio L. New challenges in addiction medicine: COVID-19 infection in patients with alcohol and substance usedisorders-the perfect storm. Am J Psychiatry. 2020;177(9):805-807. doi:10.1176/appi. ajp.2020.20040417
May 20 of this plague year, Reuters reported the death of a 32-year-old Florida nurse who had worked tirelessly to treat patients with COVID-19.1 The presumption is that, like so many selfless health care providers (HCPs), this nurse was exposed to and then sadly succumbed to the virus. That presumption would be wrong: COVID-19 did not take his young life. The other pandemic—addiction— did. Bereaved friends and family reported that the nurse had been in recovery from opioid use disorder (OUD) before the onslaught of the public health crisis. The chronicle of his relapse is instructive for the devastating effect COVID-19 has had on persons struggling with addiction, even those like the nurse who was in sustained remission from OUD with a bright future.
Many of the themes are familiar to HCPs and have been the subject of prior columns in this COVID-19 series. The nurse experienced acute stress symptoms, such as nightmares from the repeated crises of sick and dying patients in the intensive care unit where he worked.2 Like so many other HCPs, while he was desperately trying to save others, he also worried about having sufficient access to appropriate personal protective equipment (PPE).
Most relevant to this column, the caregiver was unable to access his primary source of support for his sobriety—attendance at 12-step meetings. Social distancing, which is one of the only proven means we have of reducing transmission of the virus, has had unintended consequences. Although many have found virtual connections rewarding, this nurse needed the curtailed face-to-face contact. The courage that had led him to volunteer for hazardous duty unwontedly resulted in his estrangement: Friends feared that he would expose them to the virus, and he worried that he would expose his family to danger. As in the 1918 flu pandemic, the humans we depend on for reality testing and companionship have been cruelly transformed into potential vectors of the virus.3
Isolation is the worst of all possible counselors as the great Spanish philosopher of alienation Miguel de Unamuno has argued. The deceptive promise of a rapid deliverance from anxiety and pain that substances of abuse proffer apparently led the nurse back to opioids. The virtue of being clean permitted the dirty drug to take advantage of the nurses’ reduced physiologic tolerance to opioids. It is suspected but not confirmed that he fatally overdosed alone in his car.
This Florida nurse is an especially tragic example of a terrible phenomenon being repeated all over the country. And the epidemic of substance use disorders (SUDs) related to COVID-19 is not confined to the US; there are similar reports from other afflicted nations, making addiction truly the other pandemic.4 The Centers for Disease Control and Prevention reported that 13.3% of American adults have started or increased their substance use as a means of managing the negative emotions associated with the pandemic.5 Also from March to May 2020, researchers in Baltimore found a 17.6% increase in suspected overdoses in counties advising social distancing and/or mandating stay at home orders.5
These data reinforce a well-known maxim in the addiction community that “addiction is a disease of isolation.”6-8 The burden of the lockdown falls harder on many of the patients we treat in the federal health care system whose other mental and physical health conditions, including chronic pain, depression, and posttraumatic stress disorder already placed them at elevated risk of SUDs.9 Director of the National Institute of Drug Abuse Nora Volkow, MD, recently traced the well-known arc from isolation to increased use of drugs and alcohol.10 Isolation is stressful and amplifies negative thoughts, dysphoria, and fearful emotions, which are recognized triggers for the use of substances of abuse. The usually available means of coping with craving, and in many cases withdrawal, such as prescribed medications, visits to therapists, participation in support groups are either not available or much more difficult to access.10 Nor are those without a current or even historical SUD immune to the psychosocial pressures of the pandemic: Isolation also constitutes a risk for the development of de novo addiction particularly among already marginalized groups, such as the elderly and disabled.
The federal government has initiated several important measures to reduce the adverse impact of isolation on persons with SUDs. The Drug Enforcement Administration is exempting qualified practitioners of medication-assisted treatment from the in-person evaluation that is usually required for the prescription of controlled substances, including buprenorphine. This exemption applies to both established patient prescriptions for buprenorphine and new buprenorphine patient prescriptions.11 These and other administrative contingencies at the federal government level can assist persons with OUD to continue to receive medicationassisted treatment.
As individual clinicians in federal practice, we alone cannot engineer such major policy accommodations in response to COVID-19, yet we can still make a difference in the lives of our patients. We can focus a few minutes of our telehealth interactions on checking in with patients who have a history or a current SUD. We can remember to use evidence-based screens for these patients and those with other risk factors to detect drug or alcohol use before it becomes a disorder. And we can identify and refer not only patients but also our beleaguered colleagues who feel alone at sea—to the many lifelines our agencies have cast into what other commentators have referred to as a Perfect Storm of COVID-19 and the opioid crisis (Table).12
May 20 of this plague year, Reuters reported the death of a 32-year-old Florida nurse who had worked tirelessly to treat patients with COVID-19.1 The presumption is that, like so many selfless health care providers (HCPs), this nurse was exposed to and then sadly succumbed to the virus. That presumption would be wrong: COVID-19 did not take his young life. The other pandemic—addiction— did. Bereaved friends and family reported that the nurse had been in recovery from opioid use disorder (OUD) before the onslaught of the public health crisis. The chronicle of his relapse is instructive for the devastating effect COVID-19 has had on persons struggling with addiction, even those like the nurse who was in sustained remission from OUD with a bright future.
Many of the themes are familiar to HCPs and have been the subject of prior columns in this COVID-19 series. The nurse experienced acute stress symptoms, such as nightmares from the repeated crises of sick and dying patients in the intensive care unit where he worked.2 Like so many other HCPs, while he was desperately trying to save others, he also worried about having sufficient access to appropriate personal protective equipment (PPE).
Most relevant to this column, the caregiver was unable to access his primary source of support for his sobriety—attendance at 12-step meetings. Social distancing, which is one of the only proven means we have of reducing transmission of the virus, has had unintended consequences. Although many have found virtual connections rewarding, this nurse needed the curtailed face-to-face contact. The courage that had led him to volunteer for hazardous duty unwontedly resulted in his estrangement: Friends feared that he would expose them to the virus, and he worried that he would expose his family to danger. As in the 1918 flu pandemic, the humans we depend on for reality testing and companionship have been cruelly transformed into potential vectors of the virus.3
Isolation is the worst of all possible counselors as the great Spanish philosopher of alienation Miguel de Unamuno has argued. The deceptive promise of a rapid deliverance from anxiety and pain that substances of abuse proffer apparently led the nurse back to opioids. The virtue of being clean permitted the dirty drug to take advantage of the nurses’ reduced physiologic tolerance to opioids. It is suspected but not confirmed that he fatally overdosed alone in his car.
This Florida nurse is an especially tragic example of a terrible phenomenon being repeated all over the country. And the epidemic of substance use disorders (SUDs) related to COVID-19 is not confined to the US; there are similar reports from other afflicted nations, making addiction truly the other pandemic.4 The Centers for Disease Control and Prevention reported that 13.3% of American adults have started or increased their substance use as a means of managing the negative emotions associated with the pandemic.5 Also from March to May 2020, researchers in Baltimore found a 17.6% increase in suspected overdoses in counties advising social distancing and/or mandating stay at home orders.5
These data reinforce a well-known maxim in the addiction community that “addiction is a disease of isolation.”6-8 The burden of the lockdown falls harder on many of the patients we treat in the federal health care system whose other mental and physical health conditions, including chronic pain, depression, and posttraumatic stress disorder already placed them at elevated risk of SUDs.9 Director of the National Institute of Drug Abuse Nora Volkow, MD, recently traced the well-known arc from isolation to increased use of drugs and alcohol.10 Isolation is stressful and amplifies negative thoughts, dysphoria, and fearful emotions, which are recognized triggers for the use of substances of abuse. The usually available means of coping with craving, and in many cases withdrawal, such as prescribed medications, visits to therapists, participation in support groups are either not available or much more difficult to access.10 Nor are those without a current or even historical SUD immune to the psychosocial pressures of the pandemic: Isolation also constitutes a risk for the development of de novo addiction particularly among already marginalized groups, such as the elderly and disabled.
The federal government has initiated several important measures to reduce the adverse impact of isolation on persons with SUDs. The Drug Enforcement Administration is exempting qualified practitioners of medication-assisted treatment from the in-person evaluation that is usually required for the prescription of controlled substances, including buprenorphine. This exemption applies to both established patient prescriptions for buprenorphine and new buprenorphine patient prescriptions.11 These and other administrative contingencies at the federal government level can assist persons with OUD to continue to receive medicationassisted treatment.
As individual clinicians in federal practice, we alone cannot engineer such major policy accommodations in response to COVID-19, yet we can still make a difference in the lives of our patients. We can focus a few minutes of our telehealth interactions on checking in with patients who have a history or a current SUD. We can remember to use evidence-based screens for these patients and those with other risk factors to detect drug or alcohol use before it becomes a disorder. And we can identify and refer not only patients but also our beleaguered colleagues who feel alone at sea—to the many lifelines our agencies have cast into what other commentators have referred to as a Perfect Storm of COVID-19 and the opioid crisis (Table).12
1. Borter G. A nurse struggled with COVID-19 trauma. He was found dead in his car. Reuters. May 20, 2020. https:// www.reuters.com/article/us-health-coronavirus-nurse -death-insigh/a-nurse-struggled-with-covid-19-trauma-he -was-found-dead-in-his-car-idUSKBN22W1JD Accessed September 15, 2020.
2. Geppert CMA. The duty to care and its exceptions in a pandemic. Fed Pract. 2020;37(5):210-211.
3. Kim NY. How the 1918 pandemic frayed social bonds. The Atlantic. March 31, 2020. https://www.theatlantic.com /family/archive/2020/03/coronavirus-loneliness-and-mistrust -1918-flu-pandemic-quarantine/609163. Accessed September 18, 2020.
4. Jemberie WB, Stewart Williams J, Eriksson M, et al. Substance use disorders and COVID-19: multi-faceted problems which require multi-pronged solutions. Front Psychiatry. 2020;11:714. Published 2020 Jul 21. doi:10.3389/fpsyt.2020.00714
5. Alter A, Yeager C. COVID-19 impact on US national overdose crises. http://www.odmap.org/Content/docs/news/2020 /ODMAP-Report-June-2020.pdf. Published May 2020. Accessed September 18, 2020.
6. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. Published 2020 Aug 14. doi:10.15585/mmwr.mm6932a1
7. Grinspoon P. A tale of two epidemics: when COVID-19 and opioid addiction collide. https://www.health.harvard.edu /blog/a-tale-of-two-epidemics-when-covid-19-and-opioid -addiction-collide-2020042019569. Published April 20, 2020. Accessed September 16, 2020
8. Bebinger M. Addiction is “a disease of isolation”—so pandemic puts recovery at risk. https://khn.org/news/addiction -is-a-disease-of-isolation-so-pandemic-puts-recovery-at-risk. Published March 30, 2020. Accessed September 23, 2020.
9. National Institute of Drug Abuse. Substance abuse and military life. DrugFacts. https://www.drugabuse.gov/publications /drugfacts/substance-use-military-life. Published October 2019. Accessed September 16, 2020.
10. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62. doi:10.7326/M20-1212
11. Substance Abuse and Mental Health Administration. FAQS: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency. https:// www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing -and-dispensing.pdf. Updated April 21, 2020. Accessed September 22, 2020.
12. Spagnolo PA, Montemitro C, Leggio L. New challenges in addiction medicine: COVID-19 infection in patients with alcohol and substance usedisorders-the perfect storm. Am J Psychiatry. 2020;177(9):805-807. doi:10.1176/appi. ajp.2020.20040417
1. Borter G. A nurse struggled with COVID-19 trauma. He was found dead in his car. Reuters. May 20, 2020. https:// www.reuters.com/article/us-health-coronavirus-nurse -death-insigh/a-nurse-struggled-with-covid-19-trauma-he -was-found-dead-in-his-car-idUSKBN22W1JD Accessed September 15, 2020.
2. Geppert CMA. The duty to care and its exceptions in a pandemic. Fed Pract. 2020;37(5):210-211.
3. Kim NY. How the 1918 pandemic frayed social bonds. The Atlantic. March 31, 2020. https://www.theatlantic.com /family/archive/2020/03/coronavirus-loneliness-and-mistrust -1918-flu-pandemic-quarantine/609163. Accessed September 18, 2020.
4. Jemberie WB, Stewart Williams J, Eriksson M, et al. Substance use disorders and COVID-19: multi-faceted problems which require multi-pronged solutions. Front Psychiatry. 2020;11:714. Published 2020 Jul 21. doi:10.3389/fpsyt.2020.00714
5. Alter A, Yeager C. COVID-19 impact on US national overdose crises. http://www.odmap.org/Content/docs/news/2020 /ODMAP-Report-June-2020.pdf. Published May 2020. Accessed September 18, 2020.
6. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. Published 2020 Aug 14. doi:10.15585/mmwr.mm6932a1
7. Grinspoon P. A tale of two epidemics: when COVID-19 and opioid addiction collide. https://www.health.harvard.edu /blog/a-tale-of-two-epidemics-when-covid-19-and-opioid -addiction-collide-2020042019569. Published April 20, 2020. Accessed September 16, 2020
8. Bebinger M. Addiction is “a disease of isolation”—so pandemic puts recovery at risk. https://khn.org/news/addiction -is-a-disease-of-isolation-so-pandemic-puts-recovery-at-risk. Published March 30, 2020. Accessed September 23, 2020.
9. National Institute of Drug Abuse. Substance abuse and military life. DrugFacts. https://www.drugabuse.gov/publications /drugfacts/substance-use-military-life. Published October 2019. Accessed September 16, 2020.
10. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62. doi:10.7326/M20-1212
11. Substance Abuse and Mental Health Administration. FAQS: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency. https:// www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing -and-dispensing.pdf. Updated April 21, 2020. Accessed September 22, 2020.
12. Spagnolo PA, Montemitro C, Leggio L. New challenges in addiction medicine: COVID-19 infection in patients with alcohol and substance usedisorders-the perfect storm. Am J Psychiatry. 2020;177(9):805-807. doi:10.1176/appi. ajp.2020.20040417
Substance use tied to increased COVID-19 risk
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Children’s opioid harms vary by race, location
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.
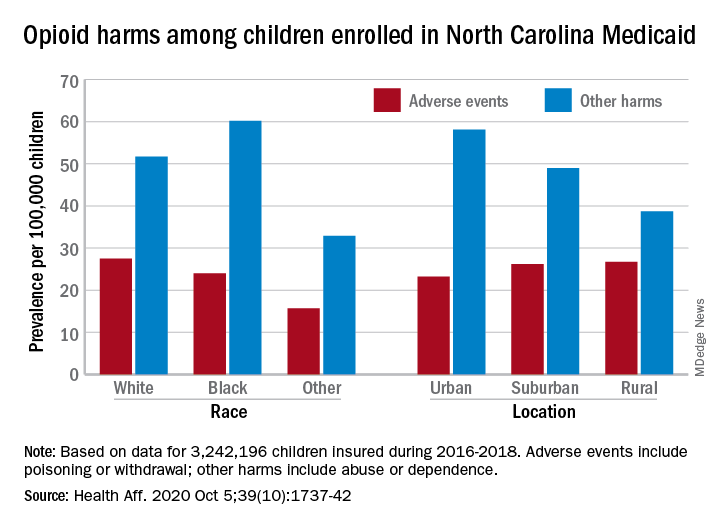
Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.

Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.

Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
FROM HEALTH AFFAIRS
Use of e-cigarettes may be linked to sleep deprivation
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.
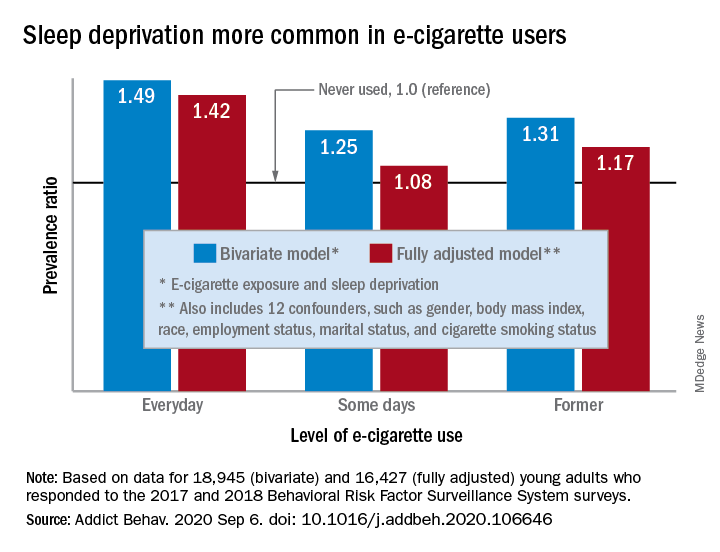
“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
FROM ADDICTIVE BEHAVIORS
‘Overwhelming evidence’ FDA’s opioid approval process is shoddy
Despite the ongoing epidemic of misuse, overuse, and diversion of opioids, the Food and Drug Administration has set a low bar for approval of these medications over the past 20 years, new research suggests.
The study results also show that the FDA did not require manufacturers to collect safety data on tolerance, withdrawal, overdose, misuse, and diversion in any rigorous fashion.
In addition, during the study period, 17 of the 39 new drug applications (NDAs) (only one was an innovator product, known as a new molecular entity) for chronic pain were approved with an “enriched enrollment randomized withdrawal” (EERW) trial design. Such a design, in this case, allowed manufacturers to exclude 32%-43% of the initially enrolled patients from the double-blind treatment phase.
“The question for regulators, policy makers, and others is: How did we get to a point where these approvals took place based on trials that were by design unlikely to yield some of the most important information about safety and efficacy that patients and clinicians would care about?” study investigator G. Caleb Alexander, MD, Johns Hopkins University, Baltimore, said in an interview.
The study was published online Sept. 29 in the Annals of Internal Medicine.
‘Cooking the books’
Little is known about the evidence required by the FDA for new approvals of opioid analgesics.
To characterize the quality of safety and efficacy data in NDAs for opioid analgesics approved by the FDA between 1997 and 2018, the investigators conducted the cross-sectional analysis using data from ClinicalTrials.gov, FDA reviews, and peer-reviewed publications regarding phase 3 pivotal trials.
The investigators examined the key characteristics of each NDA, including the number, size, and duration of pivotal trials, trial control groups, use of EERW, and systematically measured safety outcomes.
Results showed that most of the 48 NDAs evaluated were for new dosage forms (52.1%) or new formulations (18.8%). Only one (2.1%) was for a new molecular entity.
Of 39 NDAs approved for the treatment of chronic pain, only 21 products were supported by at least one pivotal trial. The mean duration of these 28 trials was 84 days, and they enrolled a median of 299 patients.
Results showed that, for 17 of the 39 opioids approved for chronic pain, pivotal trials had an EERW design. For the latest period – 2012-2018 – trials of all eight of the approved opioids used the EERW method.
This EERW design allows the manufacturer to assess efficacy “among a subset of patients most likely to respond and least likely to have adverse effects, reducing generalizability to real-world settings,” the investigators noted.
They called on the FDA to stop relying on this type of trial to assess opioid efficacy.
In an August 2020 article, Andrew Kolodny, MD, pointed out the pitfalls of the EERW approach. In such a study, all participants are made physiologically dependent on the opioid in a 4- to 6-week open-label phase. Only those who tolerate the drug and find it helpful are included in the randomized study. Dr. Kolodny is codirector of opioid policy research at Brandeis University, Waltham, Mass.
“Critics of EERW have correctly described this methodology as ‘cooking the books,’ ” Dr. Kolodny writes.
He noted that the agency’s decision to rely on EERW trials for opioids was “based on discussions at private meetings between FDA officials and pharmaceutical company executives hosted by an organization called Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.” The 2013 meetings were reported in an article published in the Washington Post.
Little sign of change
Among NDAs for chronic pain, the investigators found that eight (20.5%) included pooled safety reviews that reported systematic assessment of diversion. Seven (17.9%) reported systematic measurement of nonmedical use, and 15 (38.5%) assessed incident tolerance.
The study revealed that eight of nine products that were approved for acute pain were supported by at least one pivotal trial. The median duration of these 19 trials was 1 day, and they enrolled a median of 329 patients.
The investigators noted that the findings “underscore the evidence gaps that have limited clinicians’ and patients’ understanding and appreciation of the inherent risks of prescription opioid analgesics.”
Dr. Alexander, who has been an FDA advisory committee chairman and currently serves as a consultant to plaintiffs who are suing opioid manufacturers in federal multidistrict litigation, said the study “is a story about missed opportunities to improve the safety and to improve the regulatory review of these products.”
Coinvestigator Peter Lurie, MD, who was an official at the FDA from 2009 to 2017, said that “there’s not a lot of signs that things are changing” at the agency.
The study shows that the FDA has “accepted what the companies have been presenting,” said Dr. Lurie, who is president of the Center for Science in the Public Interest.
The FDA “absolutely has the authority” to require manufacturers to undertake more rigorous trials, but agency culture keeps it from making such demands, especially if doing so means a new applicant might have to conduct trials that weren’t previously required, Dr. Lurie said in an interview.
“FDA is pretty rigorous about trying to establish a level playing field. That’s a virtuous thing, but it becomes problematic when that prevents change,” said Dr. Lurie.
The most recent FDA guidance to manufacturers, issued in 2019, does not provide advice on criteria for endpoints, study duration, or which populations are most likely to benefit from opioid treatment. The agency also does not require drug manufacturers to formally collect data on safety, tolerance, overdose symptoms, or constipation.
The guidance does suggest that the agency would likely take into account public health considerations when evaluating opioids, such as the risk to the overall population for overdose and diversion.
‘Overwhelming evidence’
Dr. Kolodny said that, as far as he is aware, “this is the first scientific publication in a peer-reviewed journal demonstrating clearly the problems with FDA’s opioid approval process.”
The article offers “overwhelming evidence that they are improperly approving the most dangerous medications – medications that killed more people than any other medication on the market,” added Dr. Kolodny, who is also president of Physicians for Responsible Opioid Prescribing.
Asked to respond to the study findings, FDA spokesperson Charles Kohler said the agency “does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
A version of this article originally appeared on Medscape.com.
Despite the ongoing epidemic of misuse, overuse, and diversion of opioids, the Food and Drug Administration has set a low bar for approval of these medications over the past 20 years, new research suggests.
The study results also show that the FDA did not require manufacturers to collect safety data on tolerance, withdrawal, overdose, misuse, and diversion in any rigorous fashion.
In addition, during the study period, 17 of the 39 new drug applications (NDAs) (only one was an innovator product, known as a new molecular entity) for chronic pain were approved with an “enriched enrollment randomized withdrawal” (EERW) trial design. Such a design, in this case, allowed manufacturers to exclude 32%-43% of the initially enrolled patients from the double-blind treatment phase.
“The question for regulators, policy makers, and others is: How did we get to a point where these approvals took place based on trials that were by design unlikely to yield some of the most important information about safety and efficacy that patients and clinicians would care about?” study investigator G. Caleb Alexander, MD, Johns Hopkins University, Baltimore, said in an interview.
The study was published online Sept. 29 in the Annals of Internal Medicine.
‘Cooking the books’
Little is known about the evidence required by the FDA for new approvals of opioid analgesics.
To characterize the quality of safety and efficacy data in NDAs for opioid analgesics approved by the FDA between 1997 and 2018, the investigators conducted the cross-sectional analysis using data from ClinicalTrials.gov, FDA reviews, and peer-reviewed publications regarding phase 3 pivotal trials.
The investigators examined the key characteristics of each NDA, including the number, size, and duration of pivotal trials, trial control groups, use of EERW, and systematically measured safety outcomes.
Results showed that most of the 48 NDAs evaluated were for new dosage forms (52.1%) or new formulations (18.8%). Only one (2.1%) was for a new molecular entity.
Of 39 NDAs approved for the treatment of chronic pain, only 21 products were supported by at least one pivotal trial. The mean duration of these 28 trials was 84 days, and they enrolled a median of 299 patients.
Results showed that, for 17 of the 39 opioids approved for chronic pain, pivotal trials had an EERW design. For the latest period – 2012-2018 – trials of all eight of the approved opioids used the EERW method.
This EERW design allows the manufacturer to assess efficacy “among a subset of patients most likely to respond and least likely to have adverse effects, reducing generalizability to real-world settings,” the investigators noted.
They called on the FDA to stop relying on this type of trial to assess opioid efficacy.
In an August 2020 article, Andrew Kolodny, MD, pointed out the pitfalls of the EERW approach. In such a study, all participants are made physiologically dependent on the opioid in a 4- to 6-week open-label phase. Only those who tolerate the drug and find it helpful are included in the randomized study. Dr. Kolodny is codirector of opioid policy research at Brandeis University, Waltham, Mass.
“Critics of EERW have correctly described this methodology as ‘cooking the books,’ ” Dr. Kolodny writes.
He noted that the agency’s decision to rely on EERW trials for opioids was “based on discussions at private meetings between FDA officials and pharmaceutical company executives hosted by an organization called Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.” The 2013 meetings were reported in an article published in the Washington Post.
Little sign of change
Among NDAs for chronic pain, the investigators found that eight (20.5%) included pooled safety reviews that reported systematic assessment of diversion. Seven (17.9%) reported systematic measurement of nonmedical use, and 15 (38.5%) assessed incident tolerance.
The study revealed that eight of nine products that were approved for acute pain were supported by at least one pivotal trial. The median duration of these 19 trials was 1 day, and they enrolled a median of 329 patients.
The investigators noted that the findings “underscore the evidence gaps that have limited clinicians’ and patients’ understanding and appreciation of the inherent risks of prescription opioid analgesics.”
Dr. Alexander, who has been an FDA advisory committee chairman and currently serves as a consultant to plaintiffs who are suing opioid manufacturers in federal multidistrict litigation, said the study “is a story about missed opportunities to improve the safety and to improve the regulatory review of these products.”
Coinvestigator Peter Lurie, MD, who was an official at the FDA from 2009 to 2017, said that “there’s not a lot of signs that things are changing” at the agency.
The study shows that the FDA has “accepted what the companies have been presenting,” said Dr. Lurie, who is president of the Center for Science in the Public Interest.
The FDA “absolutely has the authority” to require manufacturers to undertake more rigorous trials, but agency culture keeps it from making such demands, especially if doing so means a new applicant might have to conduct trials that weren’t previously required, Dr. Lurie said in an interview.
“FDA is pretty rigorous about trying to establish a level playing field. That’s a virtuous thing, but it becomes problematic when that prevents change,” said Dr. Lurie.
The most recent FDA guidance to manufacturers, issued in 2019, does not provide advice on criteria for endpoints, study duration, or which populations are most likely to benefit from opioid treatment. The agency also does not require drug manufacturers to formally collect data on safety, tolerance, overdose symptoms, or constipation.
The guidance does suggest that the agency would likely take into account public health considerations when evaluating opioids, such as the risk to the overall population for overdose and diversion.
‘Overwhelming evidence’
Dr. Kolodny said that, as far as he is aware, “this is the first scientific publication in a peer-reviewed journal demonstrating clearly the problems with FDA’s opioid approval process.”
The article offers “overwhelming evidence that they are improperly approving the most dangerous medications – medications that killed more people than any other medication on the market,” added Dr. Kolodny, who is also president of Physicians for Responsible Opioid Prescribing.
Asked to respond to the study findings, FDA spokesperson Charles Kohler said the agency “does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
A version of this article originally appeared on Medscape.com.
Despite the ongoing epidemic of misuse, overuse, and diversion of opioids, the Food and Drug Administration has set a low bar for approval of these medications over the past 20 years, new research suggests.
The study results also show that the FDA did not require manufacturers to collect safety data on tolerance, withdrawal, overdose, misuse, and diversion in any rigorous fashion.
In addition, during the study period, 17 of the 39 new drug applications (NDAs) (only one was an innovator product, known as a new molecular entity) for chronic pain were approved with an “enriched enrollment randomized withdrawal” (EERW) trial design. Such a design, in this case, allowed manufacturers to exclude 32%-43% of the initially enrolled patients from the double-blind treatment phase.
“The question for regulators, policy makers, and others is: How did we get to a point where these approvals took place based on trials that were by design unlikely to yield some of the most important information about safety and efficacy that patients and clinicians would care about?” study investigator G. Caleb Alexander, MD, Johns Hopkins University, Baltimore, said in an interview.
The study was published online Sept. 29 in the Annals of Internal Medicine.
‘Cooking the books’
Little is known about the evidence required by the FDA for new approvals of opioid analgesics.
To characterize the quality of safety and efficacy data in NDAs for opioid analgesics approved by the FDA between 1997 and 2018, the investigators conducted the cross-sectional analysis using data from ClinicalTrials.gov, FDA reviews, and peer-reviewed publications regarding phase 3 pivotal trials.
The investigators examined the key characteristics of each NDA, including the number, size, and duration of pivotal trials, trial control groups, use of EERW, and systematically measured safety outcomes.
Results showed that most of the 48 NDAs evaluated were for new dosage forms (52.1%) or new formulations (18.8%). Only one (2.1%) was for a new molecular entity.
Of 39 NDAs approved for the treatment of chronic pain, only 21 products were supported by at least one pivotal trial. The mean duration of these 28 trials was 84 days, and they enrolled a median of 299 patients.
Results showed that, for 17 of the 39 opioids approved for chronic pain, pivotal trials had an EERW design. For the latest period – 2012-2018 – trials of all eight of the approved opioids used the EERW method.
This EERW design allows the manufacturer to assess efficacy “among a subset of patients most likely to respond and least likely to have adverse effects, reducing generalizability to real-world settings,” the investigators noted.
They called on the FDA to stop relying on this type of trial to assess opioid efficacy.
In an August 2020 article, Andrew Kolodny, MD, pointed out the pitfalls of the EERW approach. In such a study, all participants are made physiologically dependent on the opioid in a 4- to 6-week open-label phase. Only those who tolerate the drug and find it helpful are included in the randomized study. Dr. Kolodny is codirector of opioid policy research at Brandeis University, Waltham, Mass.
“Critics of EERW have correctly described this methodology as ‘cooking the books,’ ” Dr. Kolodny writes.
He noted that the agency’s decision to rely on EERW trials for opioids was “based on discussions at private meetings between FDA officials and pharmaceutical company executives hosted by an organization called Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.” The 2013 meetings were reported in an article published in the Washington Post.
Little sign of change
Among NDAs for chronic pain, the investigators found that eight (20.5%) included pooled safety reviews that reported systematic assessment of diversion. Seven (17.9%) reported systematic measurement of nonmedical use, and 15 (38.5%) assessed incident tolerance.
The study revealed that eight of nine products that were approved for acute pain were supported by at least one pivotal trial. The median duration of these 19 trials was 1 day, and they enrolled a median of 329 patients.
The investigators noted that the findings “underscore the evidence gaps that have limited clinicians’ and patients’ understanding and appreciation of the inherent risks of prescription opioid analgesics.”
Dr. Alexander, who has been an FDA advisory committee chairman and currently serves as a consultant to plaintiffs who are suing opioid manufacturers in federal multidistrict litigation, said the study “is a story about missed opportunities to improve the safety and to improve the regulatory review of these products.”
Coinvestigator Peter Lurie, MD, who was an official at the FDA from 2009 to 2017, said that “there’s not a lot of signs that things are changing” at the agency.
The study shows that the FDA has “accepted what the companies have been presenting,” said Dr. Lurie, who is president of the Center for Science in the Public Interest.
The FDA “absolutely has the authority” to require manufacturers to undertake more rigorous trials, but agency culture keeps it from making such demands, especially if doing so means a new applicant might have to conduct trials that weren’t previously required, Dr. Lurie said in an interview.
“FDA is pretty rigorous about trying to establish a level playing field. That’s a virtuous thing, but it becomes problematic when that prevents change,” said Dr. Lurie.
The most recent FDA guidance to manufacturers, issued in 2019, does not provide advice on criteria for endpoints, study duration, or which populations are most likely to benefit from opioid treatment. The agency also does not require drug manufacturers to formally collect data on safety, tolerance, overdose symptoms, or constipation.
The guidance does suggest that the agency would likely take into account public health considerations when evaluating opioids, such as the risk to the overall population for overdose and diversion.
‘Overwhelming evidence’
Dr. Kolodny said that, as far as he is aware, “this is the first scientific publication in a peer-reviewed journal demonstrating clearly the problems with FDA’s opioid approval process.”
The article offers “overwhelming evidence that they are improperly approving the most dangerous medications – medications that killed more people than any other medication on the market,” added Dr. Kolodny, who is also president of Physicians for Responsible Opioid Prescribing.
Asked to respond to the study findings, FDA spokesperson Charles Kohler said the agency “does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
A version of this article originally appeared on Medscape.com.
Treating patients during COVID-19: What I observed
I am a psychiatrist at a community mental health center located close to a large city. I want to report on our experience treating 100 consecutive, non-duplicative patients during the coronavirus disease 2019 (COVID-19) pandemic. Most of these patients had medical assistance or Medicare. Fifty-one were white, 46 were black, and 3 were Asian; 50 were men, and their ages ranged from 16 to 83 (mean: 54; median: 56). Using each patient as his/her own control (pre- and post–COVID-19), here I report 6 observations I made while treating these patients.
1. Telehealth worked for most patients. Of the 100 patients, 18 were seen in-person. Of the 18 seen in person, 14 received long-acting IM injections, and 2 patients presented with urgent matters that I felt required in-person evaluations. One patient needed to fill out several forms and provide consents, and 1 patient with chronic illness was treated at the clinic because he mistakenly arrived in person for his appointment.
The remaining 82 patients had telehealth sessions. Only 9 patients said they were able to use video conferencing, so the remaining 73 patients were treated by phone. These patients were mostly poor and/or older and had no access to smartphones or computers. This is especially important because the current emergency telehealth rules allow phone-only sessions, while regular telehealth rules do not. Our clinic strongly advocates for the extension of emergency telehealth rules. I have e-mailed many elected officials about this, but I have received few replies and no substantive responses. Our clinic also needs to help our patients obtain increased audiovisual capabilities.
2. Female patients fared better in their treatment than males.
3. Older patients did better than younger patients. Older patients’ experiences of living through past crises were helpful because they were able to compare how they persevered in the past with the current pandemic.
4. White patients showed more improvements compared with black patients. White patients generally had greater access to resources and support.
5. Patients with psychotic diagnoses/symptoms improved more than those with neurotic/anxiety/depressive diagnoses or symptoms. Most of our patients with psychotic diagnoses were already in a supportive, structured living environment, so the new “COVID-19 world” may be less disruptive for them. Additionally, it was more difficult for our patients to get substances of abuse because they had less mobility and access during the pandemic.
Continue to: Support
6. Support, especially from family but also institutional support, trumped other factors. The more support and structure our patients had, the better they did.
My observations may not be generalizable because I am reporting on a relatively small population size, most patients were older, and most were established patients who were likely more stable. I plan to follow up with these patients to see how the new COVID-19 world continues to affect them, and us.
Daniel D. Storch, MD
Key Point Health Services
Catonsville, Maryland
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
I am a psychiatrist at a community mental health center located close to a large city. I want to report on our experience treating 100 consecutive, non-duplicative patients during the coronavirus disease 2019 (COVID-19) pandemic. Most of these patients had medical assistance or Medicare. Fifty-one were white, 46 were black, and 3 were Asian; 50 were men, and their ages ranged from 16 to 83 (mean: 54; median: 56). Using each patient as his/her own control (pre- and post–COVID-19), here I report 6 observations I made while treating these patients.
1. Telehealth worked for most patients. Of the 100 patients, 18 were seen in-person. Of the 18 seen in person, 14 received long-acting IM injections, and 2 patients presented with urgent matters that I felt required in-person evaluations. One patient needed to fill out several forms and provide consents, and 1 patient with chronic illness was treated at the clinic because he mistakenly arrived in person for his appointment.
The remaining 82 patients had telehealth sessions. Only 9 patients said they were able to use video conferencing, so the remaining 73 patients were treated by phone. These patients were mostly poor and/or older and had no access to smartphones or computers. This is especially important because the current emergency telehealth rules allow phone-only sessions, while regular telehealth rules do not. Our clinic strongly advocates for the extension of emergency telehealth rules. I have e-mailed many elected officials about this, but I have received few replies and no substantive responses. Our clinic also needs to help our patients obtain increased audiovisual capabilities.
2. Female patients fared better in their treatment than males.
3. Older patients did better than younger patients. Older patients’ experiences of living through past crises were helpful because they were able to compare how they persevered in the past with the current pandemic.
4. White patients showed more improvements compared with black patients. White patients generally had greater access to resources and support.
5. Patients with psychotic diagnoses/symptoms improved more than those with neurotic/anxiety/depressive diagnoses or symptoms. Most of our patients with psychotic diagnoses were already in a supportive, structured living environment, so the new “COVID-19 world” may be less disruptive for them. Additionally, it was more difficult for our patients to get substances of abuse because they had less mobility and access during the pandemic.
Continue to: Support
6. Support, especially from family but also institutional support, trumped other factors. The more support and structure our patients had, the better they did.
My observations may not be generalizable because I am reporting on a relatively small population size, most patients were older, and most were established patients who were likely more stable. I plan to follow up with these patients to see how the new COVID-19 world continues to affect them, and us.
Daniel D. Storch, MD
Key Point Health Services
Catonsville, Maryland
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
I am a psychiatrist at a community mental health center located close to a large city. I want to report on our experience treating 100 consecutive, non-duplicative patients during the coronavirus disease 2019 (COVID-19) pandemic. Most of these patients had medical assistance or Medicare. Fifty-one were white, 46 were black, and 3 were Asian; 50 were men, and their ages ranged from 16 to 83 (mean: 54; median: 56). Using each patient as his/her own control (pre- and post–COVID-19), here I report 6 observations I made while treating these patients.
1. Telehealth worked for most patients. Of the 100 patients, 18 were seen in-person. Of the 18 seen in person, 14 received long-acting IM injections, and 2 patients presented with urgent matters that I felt required in-person evaluations. One patient needed to fill out several forms and provide consents, and 1 patient with chronic illness was treated at the clinic because he mistakenly arrived in person for his appointment.
The remaining 82 patients had telehealth sessions. Only 9 patients said they were able to use video conferencing, so the remaining 73 patients were treated by phone. These patients were mostly poor and/or older and had no access to smartphones or computers. This is especially important because the current emergency telehealth rules allow phone-only sessions, while regular telehealth rules do not. Our clinic strongly advocates for the extension of emergency telehealth rules. I have e-mailed many elected officials about this, but I have received few replies and no substantive responses. Our clinic also needs to help our patients obtain increased audiovisual capabilities.
2. Female patients fared better in their treatment than males.
3. Older patients did better than younger patients. Older patients’ experiences of living through past crises were helpful because they were able to compare how they persevered in the past with the current pandemic.
4. White patients showed more improvements compared with black patients. White patients generally had greater access to resources and support.
5. Patients with psychotic diagnoses/symptoms improved more than those with neurotic/anxiety/depressive diagnoses or symptoms. Most of our patients with psychotic diagnoses were already in a supportive, structured living environment, so the new “COVID-19 world” may be less disruptive for them. Additionally, it was more difficult for our patients to get substances of abuse because they had less mobility and access during the pandemic.
Continue to: Support
6. Support, especially from family but also institutional support, trumped other factors. The more support and structure our patients had, the better they did.
My observations may not be generalizable because I am reporting on a relatively small population size, most patients were older, and most were established patients who were likely more stable. I plan to follow up with these patients to see how the new COVID-19 world continues to affect them, and us.
Daniel D. Storch, MD
Key Point Health Services
Catonsville, Maryland
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.