User login
Physical fitness tied to lower risk of Alzheimer’s disease
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
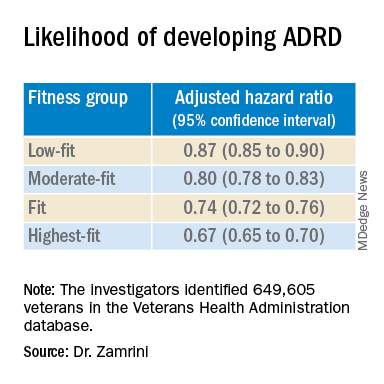
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.
The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.

The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.

The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
Some reproductive factors linked with risk of dementia
Certain reproductive factors are associated with greater or lower risk of dementia, according to researchers who conducted a large population-based study with UK Biobank data.
Jessica Gong, a PhD candidate at the George Institute for Global Health at University of New South Wales in Australia, and coauthors found a greater dementia risk in women with early and late menarche, women who were younger when they first gave birth, and those who had had a hysterectomy, especially those who had a hysterectomy without concomitant oophorectomy or with a previous oophorectomy.
After controlling for key confounders, the researchers found lower risk of all-cause dementia if women had ever been pregnant, ever had an abortion, had a longer reproductive span, or had later menopause.
Use of oral contraceptive pills was associated with a lower dementia risk, they found.
In this study, there was no evidence that hormone therapy (HT) was associated with dementia risk (hazard ratio, 0.99, 95% confidence interval [0.90-1.09], P =.0828).
The analysis, published online April 5 in PLOS Medicine, comprised 273,240 women and 228,957 men without prevalent dementia.
The authors noted that dementia rates are increasing. Globally, 50 million people live with dementia, and the number is expected to triple by 2050, according to Alzheimer’s Disease International.
“Our study identified certain reproductive factors related to shorter exposure to endogenous estrogen were associated with increased risk of dementia, highlighting the susceptibility in dementia risk pertaining to women,” Ms. Gong told this publication.
Risk comparison of men and women
Men were included in this study to compare the association between number of children fathered and the risk of all-cause dementia, with the association in their female counterparts.
The U-shaped associations between the number of children and dementia risk were similar for both sexes, suggesting that the risk difference in women may not be associated with factors associated with childbearing
“It may be more related to social and behavioral factors in parenthood, rather than biological factors involved in childbearing,” Ms. Gong said.
Compared with those with two children, for those without children, the multiple adjusted HR (95% CI) was 1.18 (1.04, 1.33) (P = .027) for women and 1.10 (0.98-1.23) P = .164) for men.
For those with four or more children, the HR was 1.14 (0.98, 1.33) (P = .132) for women and 1.26 (1.10-1.45) (P = .003) for men.
Rachel Buckley, PhD, assistant professor of neurology with a dual appointment at Brigham and Women’s and Massachusetts General hospitals in Boston, told this publication she found the comparison of dementia risk with number of children in men and women “fascinating.”
She said the argument usually is that if women have had more births, then they have had more estrogen through their body because women get a huge injection of hormones in pregnancy.
“The idea is that the more pregnancies you have the more protected you are. But this study put that on its head, because if men and women are showing increased [dementia] risk in the number of children they have, it suggests there must be something about having the children – not necessarily the circulating hormones – that might be having an impact,” Dr. Buckley said.
“I had never thought to compare the number of children in men. I do find that very interesting,” she said.
As for the lack of a link between HT and dementia risk, in this study she said, she wouldn’t shut the door on that discussion just yet.
She noted the long history of controversy in the field about whether there is a protective factor against dementia for estrogen or whether exposure to estrogen leads to increased risk.
Before the landmark Women’s Health Initiative (WHI) study in the 1990s, she pointed out, there was evidence in many observational studies that women who had longer exposure to estrogen – whether that was earlier age at first period and later age at menopause combined or women had taken hormone therapy at some point, had less risk for dementia.
Dr. Buckley said that in a secondary outcome of WHI, however, “there was increased risk for progression to dementia in women who were taking hormone therapy which essentially flipped the field on its ahead because until that point everybody thought that estrogen was a protective factor.”
She said although this study found no association with dementia, she still thinks HT has a role to play and that it may just need to be better tailored to individuals.
“If you think about it, we have our tailored cocktail of hormones in our body and who’s to say that my hormones are going to be the same as yours? Why should you and I be put on the same hormone therapy and assume that will give us the same outcome? I think we could do a lot better with customization and calibration of hormones to aid in women’s health.”
Lifetime approach to dementia
Ms. Gong says future dementia risk-reduction strategies should consider sex-specific risk, and consider the reproductive events that took place in women’s lifespans as well as their entire hormone history when assessing dementia risk, to ensure that the strategies are sex sensitive.
Dr. Buckley agrees: “I don’t think we should ever think about dementia in terms of 65 onwards. We know this disease is insidious and it starts very, very early.”
Regarding limitations, the authors noted that it was a retrospective study that included self-reported measures of reproductive factors, which may be inherently subject to recall bias.
A coauthor does consultant work for Amgen, Freeline, and Kirin outside the submitted work. There were no other relevant financial disclosures.
Certain reproductive factors are associated with greater or lower risk of dementia, according to researchers who conducted a large population-based study with UK Biobank data.
Jessica Gong, a PhD candidate at the George Institute for Global Health at University of New South Wales in Australia, and coauthors found a greater dementia risk in women with early and late menarche, women who were younger when they first gave birth, and those who had had a hysterectomy, especially those who had a hysterectomy without concomitant oophorectomy or with a previous oophorectomy.
After controlling for key confounders, the researchers found lower risk of all-cause dementia if women had ever been pregnant, ever had an abortion, had a longer reproductive span, or had later menopause.
Use of oral contraceptive pills was associated with a lower dementia risk, they found.
In this study, there was no evidence that hormone therapy (HT) was associated with dementia risk (hazard ratio, 0.99, 95% confidence interval [0.90-1.09], P =.0828).
The analysis, published online April 5 in PLOS Medicine, comprised 273,240 women and 228,957 men without prevalent dementia.
The authors noted that dementia rates are increasing. Globally, 50 million people live with dementia, and the number is expected to triple by 2050, according to Alzheimer’s Disease International.
“Our study identified certain reproductive factors related to shorter exposure to endogenous estrogen were associated with increased risk of dementia, highlighting the susceptibility in dementia risk pertaining to women,” Ms. Gong told this publication.
Risk comparison of men and women
Men were included in this study to compare the association between number of children fathered and the risk of all-cause dementia, with the association in their female counterparts.
The U-shaped associations between the number of children and dementia risk were similar for both sexes, suggesting that the risk difference in women may not be associated with factors associated with childbearing
“It may be more related to social and behavioral factors in parenthood, rather than biological factors involved in childbearing,” Ms. Gong said.
Compared with those with two children, for those without children, the multiple adjusted HR (95% CI) was 1.18 (1.04, 1.33) (P = .027) for women and 1.10 (0.98-1.23) P = .164) for men.
For those with four or more children, the HR was 1.14 (0.98, 1.33) (P = .132) for women and 1.26 (1.10-1.45) (P = .003) for men.
Rachel Buckley, PhD, assistant professor of neurology with a dual appointment at Brigham and Women’s and Massachusetts General hospitals in Boston, told this publication she found the comparison of dementia risk with number of children in men and women “fascinating.”
She said the argument usually is that if women have had more births, then they have had more estrogen through their body because women get a huge injection of hormones in pregnancy.
“The idea is that the more pregnancies you have the more protected you are. But this study put that on its head, because if men and women are showing increased [dementia] risk in the number of children they have, it suggests there must be something about having the children – not necessarily the circulating hormones – that might be having an impact,” Dr. Buckley said.
“I had never thought to compare the number of children in men. I do find that very interesting,” she said.
As for the lack of a link between HT and dementia risk, in this study she said, she wouldn’t shut the door on that discussion just yet.
She noted the long history of controversy in the field about whether there is a protective factor against dementia for estrogen or whether exposure to estrogen leads to increased risk.
Before the landmark Women’s Health Initiative (WHI) study in the 1990s, she pointed out, there was evidence in many observational studies that women who had longer exposure to estrogen – whether that was earlier age at first period and later age at menopause combined or women had taken hormone therapy at some point, had less risk for dementia.
Dr. Buckley said that in a secondary outcome of WHI, however, “there was increased risk for progression to dementia in women who were taking hormone therapy which essentially flipped the field on its ahead because until that point everybody thought that estrogen was a protective factor.”
She said although this study found no association with dementia, she still thinks HT has a role to play and that it may just need to be better tailored to individuals.
“If you think about it, we have our tailored cocktail of hormones in our body and who’s to say that my hormones are going to be the same as yours? Why should you and I be put on the same hormone therapy and assume that will give us the same outcome? I think we could do a lot better with customization and calibration of hormones to aid in women’s health.”
Lifetime approach to dementia
Ms. Gong says future dementia risk-reduction strategies should consider sex-specific risk, and consider the reproductive events that took place in women’s lifespans as well as their entire hormone history when assessing dementia risk, to ensure that the strategies are sex sensitive.
Dr. Buckley agrees: “I don’t think we should ever think about dementia in terms of 65 onwards. We know this disease is insidious and it starts very, very early.”
Regarding limitations, the authors noted that it was a retrospective study that included self-reported measures of reproductive factors, which may be inherently subject to recall bias.
A coauthor does consultant work for Amgen, Freeline, and Kirin outside the submitted work. There were no other relevant financial disclosures.
Certain reproductive factors are associated with greater or lower risk of dementia, according to researchers who conducted a large population-based study with UK Biobank data.
Jessica Gong, a PhD candidate at the George Institute for Global Health at University of New South Wales in Australia, and coauthors found a greater dementia risk in women with early and late menarche, women who were younger when they first gave birth, and those who had had a hysterectomy, especially those who had a hysterectomy without concomitant oophorectomy or with a previous oophorectomy.
After controlling for key confounders, the researchers found lower risk of all-cause dementia if women had ever been pregnant, ever had an abortion, had a longer reproductive span, or had later menopause.
Use of oral contraceptive pills was associated with a lower dementia risk, they found.
In this study, there was no evidence that hormone therapy (HT) was associated with dementia risk (hazard ratio, 0.99, 95% confidence interval [0.90-1.09], P =.0828).
The analysis, published online April 5 in PLOS Medicine, comprised 273,240 women and 228,957 men without prevalent dementia.
The authors noted that dementia rates are increasing. Globally, 50 million people live with dementia, and the number is expected to triple by 2050, according to Alzheimer’s Disease International.
“Our study identified certain reproductive factors related to shorter exposure to endogenous estrogen were associated with increased risk of dementia, highlighting the susceptibility in dementia risk pertaining to women,” Ms. Gong told this publication.
Risk comparison of men and women
Men were included in this study to compare the association between number of children fathered and the risk of all-cause dementia, with the association in their female counterparts.
The U-shaped associations between the number of children and dementia risk were similar for both sexes, suggesting that the risk difference in women may not be associated with factors associated with childbearing
“It may be more related to social and behavioral factors in parenthood, rather than biological factors involved in childbearing,” Ms. Gong said.
Compared with those with two children, for those without children, the multiple adjusted HR (95% CI) was 1.18 (1.04, 1.33) (P = .027) for women and 1.10 (0.98-1.23) P = .164) for men.
For those with four or more children, the HR was 1.14 (0.98, 1.33) (P = .132) for women and 1.26 (1.10-1.45) (P = .003) for men.
Rachel Buckley, PhD, assistant professor of neurology with a dual appointment at Brigham and Women’s and Massachusetts General hospitals in Boston, told this publication she found the comparison of dementia risk with number of children in men and women “fascinating.”
She said the argument usually is that if women have had more births, then they have had more estrogen through their body because women get a huge injection of hormones in pregnancy.
“The idea is that the more pregnancies you have the more protected you are. But this study put that on its head, because if men and women are showing increased [dementia] risk in the number of children they have, it suggests there must be something about having the children – not necessarily the circulating hormones – that might be having an impact,” Dr. Buckley said.
“I had never thought to compare the number of children in men. I do find that very interesting,” she said.
As for the lack of a link between HT and dementia risk, in this study she said, she wouldn’t shut the door on that discussion just yet.
She noted the long history of controversy in the field about whether there is a protective factor against dementia for estrogen or whether exposure to estrogen leads to increased risk.
Before the landmark Women’s Health Initiative (WHI) study in the 1990s, she pointed out, there was evidence in many observational studies that women who had longer exposure to estrogen – whether that was earlier age at first period and later age at menopause combined or women had taken hormone therapy at some point, had less risk for dementia.
Dr. Buckley said that in a secondary outcome of WHI, however, “there was increased risk for progression to dementia in women who were taking hormone therapy which essentially flipped the field on its ahead because until that point everybody thought that estrogen was a protective factor.”
She said although this study found no association with dementia, she still thinks HT has a role to play and that it may just need to be better tailored to individuals.
“If you think about it, we have our tailored cocktail of hormones in our body and who’s to say that my hormones are going to be the same as yours? Why should you and I be put on the same hormone therapy and assume that will give us the same outcome? I think we could do a lot better with customization and calibration of hormones to aid in women’s health.”
Lifetime approach to dementia
Ms. Gong says future dementia risk-reduction strategies should consider sex-specific risk, and consider the reproductive events that took place in women’s lifespans as well as their entire hormone history when assessing dementia risk, to ensure that the strategies are sex sensitive.
Dr. Buckley agrees: “I don’t think we should ever think about dementia in terms of 65 onwards. We know this disease is insidious and it starts very, very early.”
Regarding limitations, the authors noted that it was a retrospective study that included self-reported measures of reproductive factors, which may be inherently subject to recall bias.
A coauthor does consultant work for Amgen, Freeline, and Kirin outside the submitted work. There were no other relevant financial disclosures.
FROM PLOS MEDICINE
Experimental drug may boost executive function in patients with Alzheimer’s disease
Patients performed better in cognitive testing after just 2 weeks, especially in areas of executive functioning. Clinicians involved in the study also reported improvements in patients’ ability to complete daily activities, especially in complex tasks such as using a computer, carrying out household chores, and managing their medications.
“It’s pretty incredible to see improvement over the course of a week to a week and a half,” said study investigator Aaron Koenig, MD, vice president of Early Clinical Development at Sage Therapeutics in Cambridge, Mass. “Not only are we seeing objective improvement, we’re also seeing a subjective benefit.”
The drug, SAGE-718, is also under study for MCI in patients with Huntington’s disease, the drug’s primary indication, and Parkinson’s disease.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Improved executive function
SAGE-718 is in a new class of drugs called positive allosteric modulator of N-methyl-D-aspartate (NMDA) receptors, which are thought to improve neuroplasticity.
For the phase 2a open-label LUMINARY trial, researchers enrolled 26 patients ages 50-80 years with Alzheimer’s disease who had MCI. Patients completed a battery of cognitive tests at the study outset, again at the end of treatment, and again after 28 days.
Participants received SAGE-718 daily for 2 weeks and were followed for another 2 weeks.
The study’s primary outcome was safety. Seven patients (26.9%) reported mild or moderate treatment-emergent adverse events (AEs), and there were no serious AEs or deaths.
However, after 14 days, researchers also noted improvements from baseline on multiple tests of executive functioning, learning, and memory. And at 28 days, participants demonstrated significantly better Montreal Cognitive Assessment scores, compared with baseline (+2.3 points; P < .05), suggesting improvement in global cognition.
“We know that in Alzheimer’s and other neurodegenerative conditions there is a change in cognition, the ability to think, the ability to do things,” Dr. Koenig said. “What we’ve seen with SAGE-718 to date, all the way back to our phase 1 studies, is a cognitively beneficial effect, but more specifically an improvement in executive functioning.”
Intentional small study design
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement for the Alzheimer’s Association, said that because of the small study size, short follow-up, and limited data available in the conference abstract, “we cannot speculate about the efficacy of this investigational therapy.”
“Bigger picture, the real-world clinical meaningfulness of research results that are generated in highly controlled circumstances is an important question that is being discussed right now throughout the Alzheimer’s field,” he added.
However, Dr. Koenig countered that the small study design was intentional. “Over the course of a year, we can get to the answer in different patient populations rather than running these rather large and arduous trials that may pan out to not be positive,” he said.
“The purpose here is to say, directionally, are we seeing improvement that warrants further investigation? If you don’t see an effect in a small number of patients, if you don’t see that effect rather quickly, and if you don’t see an effect that should translate into something meaningful, we at SAGE believe that you may not have a drug there,” he added.
Sage Therapeutics plans to launch a phase 2b placebo-controlled trial later this year to study SAGE-718 in more Alzheimer’s patients over a longer period of time.
The study was funded by SAGE Therapeutics. Dr. Koenig is an employee of SAGE and reports no other conflicts. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients performed better in cognitive testing after just 2 weeks, especially in areas of executive functioning. Clinicians involved in the study also reported improvements in patients’ ability to complete daily activities, especially in complex tasks such as using a computer, carrying out household chores, and managing their medications.
“It’s pretty incredible to see improvement over the course of a week to a week and a half,” said study investigator Aaron Koenig, MD, vice president of Early Clinical Development at Sage Therapeutics in Cambridge, Mass. “Not only are we seeing objective improvement, we’re also seeing a subjective benefit.”
The drug, SAGE-718, is also under study for MCI in patients with Huntington’s disease, the drug’s primary indication, and Parkinson’s disease.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Improved executive function
SAGE-718 is in a new class of drugs called positive allosteric modulator of N-methyl-D-aspartate (NMDA) receptors, which are thought to improve neuroplasticity.
For the phase 2a open-label LUMINARY trial, researchers enrolled 26 patients ages 50-80 years with Alzheimer’s disease who had MCI. Patients completed a battery of cognitive tests at the study outset, again at the end of treatment, and again after 28 days.
Participants received SAGE-718 daily for 2 weeks and were followed for another 2 weeks.
The study’s primary outcome was safety. Seven patients (26.9%) reported mild or moderate treatment-emergent adverse events (AEs), and there were no serious AEs or deaths.
However, after 14 days, researchers also noted improvements from baseline on multiple tests of executive functioning, learning, and memory. And at 28 days, participants demonstrated significantly better Montreal Cognitive Assessment scores, compared with baseline (+2.3 points; P < .05), suggesting improvement in global cognition.
“We know that in Alzheimer’s and other neurodegenerative conditions there is a change in cognition, the ability to think, the ability to do things,” Dr. Koenig said. “What we’ve seen with SAGE-718 to date, all the way back to our phase 1 studies, is a cognitively beneficial effect, but more specifically an improvement in executive functioning.”
Intentional small study design
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement for the Alzheimer’s Association, said that because of the small study size, short follow-up, and limited data available in the conference abstract, “we cannot speculate about the efficacy of this investigational therapy.”
“Bigger picture, the real-world clinical meaningfulness of research results that are generated in highly controlled circumstances is an important question that is being discussed right now throughout the Alzheimer’s field,” he added.
However, Dr. Koenig countered that the small study design was intentional. “Over the course of a year, we can get to the answer in different patient populations rather than running these rather large and arduous trials that may pan out to not be positive,” he said.
“The purpose here is to say, directionally, are we seeing improvement that warrants further investigation? If you don’t see an effect in a small number of patients, if you don’t see that effect rather quickly, and if you don’t see an effect that should translate into something meaningful, we at SAGE believe that you may not have a drug there,” he added.
Sage Therapeutics plans to launch a phase 2b placebo-controlled trial later this year to study SAGE-718 in more Alzheimer’s patients over a longer period of time.
The study was funded by SAGE Therapeutics. Dr. Koenig is an employee of SAGE and reports no other conflicts. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients performed better in cognitive testing after just 2 weeks, especially in areas of executive functioning. Clinicians involved in the study also reported improvements in patients’ ability to complete daily activities, especially in complex tasks such as using a computer, carrying out household chores, and managing their medications.
“It’s pretty incredible to see improvement over the course of a week to a week and a half,” said study investigator Aaron Koenig, MD, vice president of Early Clinical Development at Sage Therapeutics in Cambridge, Mass. “Not only are we seeing objective improvement, we’re also seeing a subjective benefit.”
The drug, SAGE-718, is also under study for MCI in patients with Huntington’s disease, the drug’s primary indication, and Parkinson’s disease.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Improved executive function
SAGE-718 is in a new class of drugs called positive allosteric modulator of N-methyl-D-aspartate (NMDA) receptors, which are thought to improve neuroplasticity.
For the phase 2a open-label LUMINARY trial, researchers enrolled 26 patients ages 50-80 years with Alzheimer’s disease who had MCI. Patients completed a battery of cognitive tests at the study outset, again at the end of treatment, and again after 28 days.
Participants received SAGE-718 daily for 2 weeks and were followed for another 2 weeks.
The study’s primary outcome was safety. Seven patients (26.9%) reported mild or moderate treatment-emergent adverse events (AEs), and there were no serious AEs or deaths.
However, after 14 days, researchers also noted improvements from baseline on multiple tests of executive functioning, learning, and memory. And at 28 days, participants demonstrated significantly better Montreal Cognitive Assessment scores, compared with baseline (+2.3 points; P < .05), suggesting improvement in global cognition.
“We know that in Alzheimer’s and other neurodegenerative conditions there is a change in cognition, the ability to think, the ability to do things,” Dr. Koenig said. “What we’ve seen with SAGE-718 to date, all the way back to our phase 1 studies, is a cognitively beneficial effect, but more specifically an improvement in executive functioning.”
Intentional small study design
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement for the Alzheimer’s Association, said that because of the small study size, short follow-up, and limited data available in the conference abstract, “we cannot speculate about the efficacy of this investigational therapy.”
“Bigger picture, the real-world clinical meaningfulness of research results that are generated in highly controlled circumstances is an important question that is being discussed right now throughout the Alzheimer’s field,” he added.
However, Dr. Koenig countered that the small study design was intentional. “Over the course of a year, we can get to the answer in different patient populations rather than running these rather large and arduous trials that may pan out to not be positive,” he said.
“The purpose here is to say, directionally, are we seeing improvement that warrants further investigation? If you don’t see an effect in a small number of patients, if you don’t see that effect rather quickly, and if you don’t see an effect that should translate into something meaningful, we at SAGE believe that you may not have a drug there,” he added.
Sage Therapeutics plans to launch a phase 2b placebo-controlled trial later this year to study SAGE-718 in more Alzheimer’s patients over a longer period of time.
The study was funded by SAGE Therapeutics. Dr. Koenig is an employee of SAGE and reports no other conflicts. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
More evidence that COVID ‘brain fog’ is biologically based
Researchers found elevated levels of CSF immune activation and immunovascular markers in individuals with cognitive postacute sequelae of SARS-CoV-2 infection (PASC). Patients whose cognitive symptoms developed during the acute phase of COVID-19 had the highest levels of brain inflammation.
The findings add to a growing body of evidence that suggests the condition often referred to as “brain fog” has a neurologic basis, said lead author Joanna Hellmuth, MD, MHS, assistant professor of neurology at the University of California, San Francisco Weill Institute of Neurosciences and the UCSF Memory and Aging Center.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Inflammatory response
There are no effective diagnostic tests or treatments for cognitive PASC, which prompted the investigators to study inflammation in patients with the condition. Initial findings were reported earlier in 2022, which showed abnormalities in the CSF in 77% of patients with cognitive impairment. Patients without cognitive impairments had normal CSF.
Extending that work in this new study, researchers studied patients from the Long-term Impact of Infection With Novel Coronavirus (LIINC) study with confirmed SARS-CoV-2 infection who were not hospitalized. They conducted 2-hour neurocognitive interviews and identified 23 people with new, persistent cognitive symptoms (cognitive PASC) and 10 with no cognitive symptoms who served as controls.
All participants underwent additional neurologic examination and neuropsychological testing, and half agreed to a lumbar puncture to allow researchers to collect CSF samples. The CSF was collected a median of 10.2 months after initial COVID symptoms began.
Participants with cognitive PASC had higher median levels of CSF acute phase reactants C-reactive protein (0.007 mg/L vs. 0.000 mg/L; P =.004) and serum amyloid A (0.001 mg/L vs. 0.000 mg/L; P = .001), compared with COVID controls.
The PASC group also had elevated levels of CSF immune activation markers interferon gamma–inducible protein (IP-10), interleukin-8, and immunovascular markers vascular endothelial growth factor-C and VEGFR-1, although the differences with the control group were not statistically significant.
The timing of the onset of cognitive problems was also associated with higher levels of immune activation and immunovascular markers. Patients with brain fog that developed during the acute phase of COVID-19 had higher levels of CSF VEGF-C, compared with patients whose cognitive symptoms developed more than a month after initial COVID symptoms (173 pg/mL vs. 99 pg/mL; P = .048) and COVID controls (79 pg/mL; P = .048).
Acute onset cognitive PASC participants had higher CSF levels of IP-10 (P = .030), IL-8 (P = .048), placental growth factor (P = .030) and intercellular adhesion molecule-1 (P = .045), compared with COVID controls.
Researchers believe these new findings could mean that intrathecal immune activation and endothelial activation/dysfunction may contribute to cognitive PASC and that the mechanisms involved may be different in patients with acute cognitive PASC versus those with delayed onset.
“Our data suggests that perhaps in these people with more acute cognitive changes they don’t have the return to homeostasis,” Dr. Hellmuth said, while patients with delayed onset cognitive PASC had levels more in line with COVID patients who had no cognitive issues.
Moving the needle forward
Commenting on the findings, William Schaffner, MD, professor of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said that, while the study doesn’t rule out a possible psychological basis for cognitive PASC, it adds more weight to the biological argument.
“When you have nonspecific symptoms for which specific tests are unavailable,” Dr. Schaffner explained, “there is a natural question that always comes up: Is this principally a biologically induced phenomenon or psychological? This moves the needle substantially in the direction of a biological phenomenon.”
Another important element to the study, Dr. Schaffner said, is that the patients involved had mild COVID.
“Not every patient with long COVID symptoms had been hospitalized with severe disease,” he said. “There are inflammatory phenomenon in various organ systems such that even if the inflammatory response in the lung was not severe enough to get you into the hospital, there were inflammatory responses in other organ systems that could persist once the acute infection resolved.”
Although the small size of the study is a limitation, Dr. Schaffner said that shouldn’t minimize the importance of these findings.
“That it’s small doesn’t diminish its value,” he said. “The next step forward might be to try to associate the markers more specifically with COVID. The more precise we can be, the more convincing the story will become.”
The study was funded by the National Institutes of Health. Dr. Hellmuth received grant support from the National Institutes of Health/National Institute of Mental Health supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found elevated levels of CSF immune activation and immunovascular markers in individuals with cognitive postacute sequelae of SARS-CoV-2 infection (PASC). Patients whose cognitive symptoms developed during the acute phase of COVID-19 had the highest levels of brain inflammation.
The findings add to a growing body of evidence that suggests the condition often referred to as “brain fog” has a neurologic basis, said lead author Joanna Hellmuth, MD, MHS, assistant professor of neurology at the University of California, San Francisco Weill Institute of Neurosciences and the UCSF Memory and Aging Center.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Inflammatory response
There are no effective diagnostic tests or treatments for cognitive PASC, which prompted the investigators to study inflammation in patients with the condition. Initial findings were reported earlier in 2022, which showed abnormalities in the CSF in 77% of patients with cognitive impairment. Patients without cognitive impairments had normal CSF.
Extending that work in this new study, researchers studied patients from the Long-term Impact of Infection With Novel Coronavirus (LIINC) study with confirmed SARS-CoV-2 infection who were not hospitalized. They conducted 2-hour neurocognitive interviews and identified 23 people with new, persistent cognitive symptoms (cognitive PASC) and 10 with no cognitive symptoms who served as controls.
All participants underwent additional neurologic examination and neuropsychological testing, and half agreed to a lumbar puncture to allow researchers to collect CSF samples. The CSF was collected a median of 10.2 months after initial COVID symptoms began.
Participants with cognitive PASC had higher median levels of CSF acute phase reactants C-reactive protein (0.007 mg/L vs. 0.000 mg/L; P =.004) and serum amyloid A (0.001 mg/L vs. 0.000 mg/L; P = .001), compared with COVID controls.
The PASC group also had elevated levels of CSF immune activation markers interferon gamma–inducible protein (IP-10), interleukin-8, and immunovascular markers vascular endothelial growth factor-C and VEGFR-1, although the differences with the control group were not statistically significant.
The timing of the onset of cognitive problems was also associated with higher levels of immune activation and immunovascular markers. Patients with brain fog that developed during the acute phase of COVID-19 had higher levels of CSF VEGF-C, compared with patients whose cognitive symptoms developed more than a month after initial COVID symptoms (173 pg/mL vs. 99 pg/mL; P = .048) and COVID controls (79 pg/mL; P = .048).
Acute onset cognitive PASC participants had higher CSF levels of IP-10 (P = .030), IL-8 (P = .048), placental growth factor (P = .030) and intercellular adhesion molecule-1 (P = .045), compared with COVID controls.
Researchers believe these new findings could mean that intrathecal immune activation and endothelial activation/dysfunction may contribute to cognitive PASC and that the mechanisms involved may be different in patients with acute cognitive PASC versus those with delayed onset.
“Our data suggests that perhaps in these people with more acute cognitive changes they don’t have the return to homeostasis,” Dr. Hellmuth said, while patients with delayed onset cognitive PASC had levels more in line with COVID patients who had no cognitive issues.
Moving the needle forward
Commenting on the findings, William Schaffner, MD, professor of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said that, while the study doesn’t rule out a possible psychological basis for cognitive PASC, it adds more weight to the biological argument.
“When you have nonspecific symptoms for which specific tests are unavailable,” Dr. Schaffner explained, “there is a natural question that always comes up: Is this principally a biologically induced phenomenon or psychological? This moves the needle substantially in the direction of a biological phenomenon.”
Another important element to the study, Dr. Schaffner said, is that the patients involved had mild COVID.
“Not every patient with long COVID symptoms had been hospitalized with severe disease,” he said. “There are inflammatory phenomenon in various organ systems such that even if the inflammatory response in the lung was not severe enough to get you into the hospital, there were inflammatory responses in other organ systems that could persist once the acute infection resolved.”
Although the small size of the study is a limitation, Dr. Schaffner said that shouldn’t minimize the importance of these findings.
“That it’s small doesn’t diminish its value,” he said. “The next step forward might be to try to associate the markers more specifically with COVID. The more precise we can be, the more convincing the story will become.”
The study was funded by the National Institutes of Health. Dr. Hellmuth received grant support from the National Institutes of Health/National Institute of Mental Health supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found elevated levels of CSF immune activation and immunovascular markers in individuals with cognitive postacute sequelae of SARS-CoV-2 infection (PASC). Patients whose cognitive symptoms developed during the acute phase of COVID-19 had the highest levels of brain inflammation.
The findings add to a growing body of evidence that suggests the condition often referred to as “brain fog” has a neurologic basis, said lead author Joanna Hellmuth, MD, MHS, assistant professor of neurology at the University of California, San Francisco Weill Institute of Neurosciences and the UCSF Memory and Aging Center.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Inflammatory response
There are no effective diagnostic tests or treatments for cognitive PASC, which prompted the investigators to study inflammation in patients with the condition. Initial findings were reported earlier in 2022, which showed abnormalities in the CSF in 77% of patients with cognitive impairment. Patients without cognitive impairments had normal CSF.
Extending that work in this new study, researchers studied patients from the Long-term Impact of Infection With Novel Coronavirus (LIINC) study with confirmed SARS-CoV-2 infection who were not hospitalized. They conducted 2-hour neurocognitive interviews and identified 23 people with new, persistent cognitive symptoms (cognitive PASC) and 10 with no cognitive symptoms who served as controls.
All participants underwent additional neurologic examination and neuropsychological testing, and half agreed to a lumbar puncture to allow researchers to collect CSF samples. The CSF was collected a median of 10.2 months after initial COVID symptoms began.
Participants with cognitive PASC had higher median levels of CSF acute phase reactants C-reactive protein (0.007 mg/L vs. 0.000 mg/L; P =.004) and serum amyloid A (0.001 mg/L vs. 0.000 mg/L; P = .001), compared with COVID controls.
The PASC group also had elevated levels of CSF immune activation markers interferon gamma–inducible protein (IP-10), interleukin-8, and immunovascular markers vascular endothelial growth factor-C and VEGFR-1, although the differences with the control group were not statistically significant.
The timing of the onset of cognitive problems was also associated with higher levels of immune activation and immunovascular markers. Patients with brain fog that developed during the acute phase of COVID-19 had higher levels of CSF VEGF-C, compared with patients whose cognitive symptoms developed more than a month after initial COVID symptoms (173 pg/mL vs. 99 pg/mL; P = .048) and COVID controls (79 pg/mL; P = .048).
Acute onset cognitive PASC participants had higher CSF levels of IP-10 (P = .030), IL-8 (P = .048), placental growth factor (P = .030) and intercellular adhesion molecule-1 (P = .045), compared with COVID controls.
Researchers believe these new findings could mean that intrathecal immune activation and endothelial activation/dysfunction may contribute to cognitive PASC and that the mechanisms involved may be different in patients with acute cognitive PASC versus those with delayed onset.
“Our data suggests that perhaps in these people with more acute cognitive changes they don’t have the return to homeostasis,” Dr. Hellmuth said, while patients with delayed onset cognitive PASC had levels more in line with COVID patients who had no cognitive issues.
Moving the needle forward
Commenting on the findings, William Schaffner, MD, professor of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said that, while the study doesn’t rule out a possible psychological basis for cognitive PASC, it adds more weight to the biological argument.
“When you have nonspecific symptoms for which specific tests are unavailable,” Dr. Schaffner explained, “there is a natural question that always comes up: Is this principally a biologically induced phenomenon or psychological? This moves the needle substantially in the direction of a biological phenomenon.”
Another important element to the study, Dr. Schaffner said, is that the patients involved had mild COVID.
“Not every patient with long COVID symptoms had been hospitalized with severe disease,” he said. “There are inflammatory phenomenon in various organ systems such that even if the inflammatory response in the lung was not severe enough to get you into the hospital, there were inflammatory responses in other organ systems that could persist once the acute infection resolved.”
Although the small size of the study is a limitation, Dr. Schaffner said that shouldn’t minimize the importance of these findings.
“That it’s small doesn’t diminish its value,” he said. “The next step forward might be to try to associate the markers more specifically with COVID. The more precise we can be, the more convincing the story will become.”
The study was funded by the National Institutes of Health. Dr. Hellmuth received grant support from the National Institutes of Health/National Institute of Mental Health supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
Does evidence support benefits of omega-3 fatty acids?
Dietary supplements that contain omega-3 fatty acids have been widely consumed for years. Researchers have been investigating the benefits of such preparations for cardiovascular, neurologic, and psychological conditions. A recently published study on omega-3 fatty acids and depression inspired neurologist Hans-Christoph Diener, MD, PhD, of the Institute for Epidemiology at the University Duisburg-Essen (Germany), to examine scientific publications concerning omega-3 fatty acids or fish-oil capsules in more detail.
Prevention of depression
Dr. Diener told the story of how he stumbled upon an interesting article in JAMA in December 2021. It was about a placebo-controlled study that investigated whether omega-3 fatty acids can prevent incident depression.
As the study authors reported, treatment with omega-3 preparations in adults aged 50 years or older without clinically relevant symptoms of depression at study initiation was associated with a small but statistically significant increase in the risk for depression or clinically relevant symptoms of depression. There was no difference in mood scale value, however, over a median follow-up of 5.3 years. According to the study authors, these results did not support the administration of omega-3 preparations for the prevention of depression.
This study was, as Dr. Diener said, somewhat negative, but it did arouse his interest in questions such as what biological effects omega-3 fatty acids have and what is known “about this topic with regard to neurology,” he said. When reviewing the literature, he noticed that there “were association studies, i.e., studies that describe that the intake of omega-3 fatty acids may possibly be associated with a lower risk of certain diseases.”
Beginning with the Inuit
It all started “with observations of the Inuit [population] in Greenland and Alaska after World War II, because it was remarked upon that these people ate a lot of fish and seal meat and had a very low incidence of cardiovascular diseases.” Over the years, a large number of association studies have been published, which may have encouraged the assumption that omega-3 fatty acids have positive health effects on various conditions, such as cardiovascular diseases, hyperlipidemia, type 2 diabetes, various malignancies, cognitive impairments, Alzheimer’s disease, depression and anxiety disorders, heart failure, slipped disks, ADHD, symptoms of menopause, rheumatoid arthritis, asthma, periodontitis, epilepsy, chemotherapy tolerance, premenstrual syndrome, and nonalcoholic fatty liver disease.
Dr. Diener believes that the problem is that these are association studies. But association does not mean that there is a causal relationship.
Disappointing study results
On the contrary, the results from the randomized placebo-controlled studies are truly frustrating, according to the neurologist. A meta-analysis of the use of omega-3 fatty acids in cardiovascular diseases included 86 studies with over 162,000 patients. According to Dr. Diener, it did not reveal any benefit for overall and cardiovascular mortality, nor any benefit for the reduction of myocardial infarction and stroke.
The results did indicate a trend, however, for reduced mortality in coronary heart disease. Even so, the number needed to treat for this was 334, which means that 334 people would have to take omega-3 fatty acids for years to prevent one fatal cardiac event.
Aside from this study, Dr. Diener found six studies on Alzheimer’s disease and three studies on dementia with patient populations between 600 and 800. In these studies, too, a positive effect of omega-3 fatty acids could not be identified. Then he discovered another 31 placebo-controlled studies of omega-3 fatty acids for the treatment or prevention of depression and anxiety disorder. Despite including 50,000 patients, these studies also did not show any positive effect.
“I see a significant discrepancy between the promotion of omega-3 fatty acids, whether it’s on television, in the ‘yellow’ [journalism] press, or in advertisements, and the actual scientific evidence,” said Dr. Diener. “At least from a neurological perspective, there is no evidence that omega-3 fatty acids have any benefit. This is true for strokes, dementia, Alzheimer’s disease, depression, and anxiety disorders.”
Potential adverse effects
Omega-3 fatty acids also have potentially adverse effects. The VITAL Rhythm study recently provided evidence that, depending on the dose, preparations with omega-3 fatty acids may increase the risk for atrial fibrillation. As the authors wrote, the results do not support taking omega-3 fatty acids to prevent atrial fibrillation.
In 2019, the global market for omega-3 fatty acids reached a value of $4.1 billion. This value is expected to double by 2025, according to a comment by Gregory Curfman, MD, deputy editor of JAMA and lecturer in health care policy at Harvard Medical School, Boston.
As Dr. Curfman wrote, this impressive amount of expenditure shows how beloved these products are and how strongly many people believe that omega-3 fatty acids are beneficial for their health. It is therefore important to know the potential risks of such preparations. One such example for this would be the risk for atrial fibrillation.
According to Dr. Curfman, in the last 2 years, four randomized clinical studies have provided data on the risk for atrial fibrillation associated with omega-3 fatty acids. In the STRENGTH study, 13,078 high-risk patients with cardiovascular diseases were randomly assigned to one of two groups. The subjects received either a high dose (4 g/day) of a combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) or corn oil. After a median of 42 months, there was no significant difference between the two groups in the primary composite cardiovascular endpoint, but more frequent atrial fibrillation in the omega-3 fatty acid group, compared with the corn oil group (2.2% vs. 1.3%; hazard ratio, 1.69; 95% confidence interval, 1.29-2.21; P < .001).
In the REDUCE-IT study, 8179 subjects were randomly assigned to a high dose (4 g/day, as in STRENGTH) of an omega-3 fatty acid preparation consisting of a purified EPA (icosapent ethyl) or mineral oil. After a median observation period of 4.9 years, icosapent ethyl was associated with a relative reduction of the primary composite cardiovascular endpoint by 25%, compared with mineral oil. As in the STRENGTH study, this study found that the risk for atrial fibrillation associated with omega-3 fatty acids, compared with mineral oil, was significantly higher (5.3% vs. 3.9%; P = .003).
In a third study (OMEMI), as Dr. Curfman reported, 1027 elderly patients who had recently had a myocardial infarction were randomly assigned to receive either a median dose of 1.8 g/day of omega-3 fatty acids (a combination of EPA and DHA) or corn oil. After 2 years, there was no significant difference between the two groups in primary composite cardiovascular endpoints, but 7.2% of the patients taking omega-3 fatty acids developed atrial fibrillation. In the corn oil group, this proportion was 4% (HR, 1.84; 95% CI, 0.98-3.45; P = .06).
The data from the four studies together indicate a potential dose-dependent risk for atrial fibrillation associated with omega-3 fatty acids, according to Dr. Curfman. At a dose of 4.0 g/day, there is a highly significant risk increase (almost double). With a median dose of 1.8 g/day, the risk increase (HR, 1.84) did not reach statistical significance. At a daily standard dose of 840 mg/day, an increase in risk could not be determined.
Dr. Curfman’s recommendation is that patients who take, or want to take, preparations with omega-3 fatty acids be informed of the potential development of arrhythmia at higher dosages. These patients also should undergo cardiological monitoring.
A version of this article first appeared on Medscape.com.
Dietary supplements that contain omega-3 fatty acids have been widely consumed for years. Researchers have been investigating the benefits of such preparations for cardiovascular, neurologic, and psychological conditions. A recently published study on omega-3 fatty acids and depression inspired neurologist Hans-Christoph Diener, MD, PhD, of the Institute for Epidemiology at the University Duisburg-Essen (Germany), to examine scientific publications concerning omega-3 fatty acids or fish-oil capsules in more detail.
Prevention of depression
Dr. Diener told the story of how he stumbled upon an interesting article in JAMA in December 2021. It was about a placebo-controlled study that investigated whether omega-3 fatty acids can prevent incident depression.
As the study authors reported, treatment with omega-3 preparations in adults aged 50 years or older without clinically relevant symptoms of depression at study initiation was associated with a small but statistically significant increase in the risk for depression or clinically relevant symptoms of depression. There was no difference in mood scale value, however, over a median follow-up of 5.3 years. According to the study authors, these results did not support the administration of omega-3 preparations for the prevention of depression.
This study was, as Dr. Diener said, somewhat negative, but it did arouse his interest in questions such as what biological effects omega-3 fatty acids have and what is known “about this topic with regard to neurology,” he said. When reviewing the literature, he noticed that there “were association studies, i.e., studies that describe that the intake of omega-3 fatty acids may possibly be associated with a lower risk of certain diseases.”
Beginning with the Inuit
It all started “with observations of the Inuit [population] in Greenland and Alaska after World War II, because it was remarked upon that these people ate a lot of fish and seal meat and had a very low incidence of cardiovascular diseases.” Over the years, a large number of association studies have been published, which may have encouraged the assumption that omega-3 fatty acids have positive health effects on various conditions, such as cardiovascular diseases, hyperlipidemia, type 2 diabetes, various malignancies, cognitive impairments, Alzheimer’s disease, depression and anxiety disorders, heart failure, slipped disks, ADHD, symptoms of menopause, rheumatoid arthritis, asthma, periodontitis, epilepsy, chemotherapy tolerance, premenstrual syndrome, and nonalcoholic fatty liver disease.
Dr. Diener believes that the problem is that these are association studies. But association does not mean that there is a causal relationship.
Disappointing study results
On the contrary, the results from the randomized placebo-controlled studies are truly frustrating, according to the neurologist. A meta-analysis of the use of omega-3 fatty acids in cardiovascular diseases included 86 studies with over 162,000 patients. According to Dr. Diener, it did not reveal any benefit for overall and cardiovascular mortality, nor any benefit for the reduction of myocardial infarction and stroke.
The results did indicate a trend, however, for reduced mortality in coronary heart disease. Even so, the number needed to treat for this was 334, which means that 334 people would have to take omega-3 fatty acids for years to prevent one fatal cardiac event.
Aside from this study, Dr. Diener found six studies on Alzheimer’s disease and three studies on dementia with patient populations between 600 and 800. In these studies, too, a positive effect of omega-3 fatty acids could not be identified. Then he discovered another 31 placebo-controlled studies of omega-3 fatty acids for the treatment or prevention of depression and anxiety disorder. Despite including 50,000 patients, these studies also did not show any positive effect.
“I see a significant discrepancy between the promotion of omega-3 fatty acids, whether it’s on television, in the ‘yellow’ [journalism] press, or in advertisements, and the actual scientific evidence,” said Dr. Diener. “At least from a neurological perspective, there is no evidence that omega-3 fatty acids have any benefit. This is true for strokes, dementia, Alzheimer’s disease, depression, and anxiety disorders.”
Potential adverse effects
Omega-3 fatty acids also have potentially adverse effects. The VITAL Rhythm study recently provided evidence that, depending on the dose, preparations with omega-3 fatty acids may increase the risk for atrial fibrillation. As the authors wrote, the results do not support taking omega-3 fatty acids to prevent atrial fibrillation.
In 2019, the global market for omega-3 fatty acids reached a value of $4.1 billion. This value is expected to double by 2025, according to a comment by Gregory Curfman, MD, deputy editor of JAMA and lecturer in health care policy at Harvard Medical School, Boston.
As Dr. Curfman wrote, this impressive amount of expenditure shows how beloved these products are and how strongly many people believe that omega-3 fatty acids are beneficial for their health. It is therefore important to know the potential risks of such preparations. One such example for this would be the risk for atrial fibrillation.
According to Dr. Curfman, in the last 2 years, four randomized clinical studies have provided data on the risk for atrial fibrillation associated with omega-3 fatty acids. In the STRENGTH study, 13,078 high-risk patients with cardiovascular diseases were randomly assigned to one of two groups. The subjects received either a high dose (4 g/day) of a combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) or corn oil. After a median of 42 months, there was no significant difference between the two groups in the primary composite cardiovascular endpoint, but more frequent atrial fibrillation in the omega-3 fatty acid group, compared with the corn oil group (2.2% vs. 1.3%; hazard ratio, 1.69; 95% confidence interval, 1.29-2.21; P < .001).
In the REDUCE-IT study, 8179 subjects were randomly assigned to a high dose (4 g/day, as in STRENGTH) of an omega-3 fatty acid preparation consisting of a purified EPA (icosapent ethyl) or mineral oil. After a median observation period of 4.9 years, icosapent ethyl was associated with a relative reduction of the primary composite cardiovascular endpoint by 25%, compared with mineral oil. As in the STRENGTH study, this study found that the risk for atrial fibrillation associated with omega-3 fatty acids, compared with mineral oil, was significantly higher (5.3% vs. 3.9%; P = .003).
In a third study (OMEMI), as Dr. Curfman reported, 1027 elderly patients who had recently had a myocardial infarction were randomly assigned to receive either a median dose of 1.8 g/day of omega-3 fatty acids (a combination of EPA and DHA) or corn oil. After 2 years, there was no significant difference between the two groups in primary composite cardiovascular endpoints, but 7.2% of the patients taking omega-3 fatty acids developed atrial fibrillation. In the corn oil group, this proportion was 4% (HR, 1.84; 95% CI, 0.98-3.45; P = .06).
The data from the four studies together indicate a potential dose-dependent risk for atrial fibrillation associated with omega-3 fatty acids, according to Dr. Curfman. At a dose of 4.0 g/day, there is a highly significant risk increase (almost double). With a median dose of 1.8 g/day, the risk increase (HR, 1.84) did not reach statistical significance. At a daily standard dose of 840 mg/day, an increase in risk could not be determined.
Dr. Curfman’s recommendation is that patients who take, or want to take, preparations with omega-3 fatty acids be informed of the potential development of arrhythmia at higher dosages. These patients also should undergo cardiological monitoring.
A version of this article first appeared on Medscape.com.
Dietary supplements that contain omega-3 fatty acids have been widely consumed for years. Researchers have been investigating the benefits of such preparations for cardiovascular, neurologic, and psychological conditions. A recently published study on omega-3 fatty acids and depression inspired neurologist Hans-Christoph Diener, MD, PhD, of the Institute for Epidemiology at the University Duisburg-Essen (Germany), to examine scientific publications concerning omega-3 fatty acids or fish-oil capsules in more detail.
Prevention of depression
Dr. Diener told the story of how he stumbled upon an interesting article in JAMA in December 2021. It was about a placebo-controlled study that investigated whether omega-3 fatty acids can prevent incident depression.
As the study authors reported, treatment with omega-3 preparations in adults aged 50 years or older without clinically relevant symptoms of depression at study initiation was associated with a small but statistically significant increase in the risk for depression or clinically relevant symptoms of depression. There was no difference in mood scale value, however, over a median follow-up of 5.3 years. According to the study authors, these results did not support the administration of omega-3 preparations for the prevention of depression.
This study was, as Dr. Diener said, somewhat negative, but it did arouse his interest in questions such as what biological effects omega-3 fatty acids have and what is known “about this topic with regard to neurology,” he said. When reviewing the literature, he noticed that there “were association studies, i.e., studies that describe that the intake of omega-3 fatty acids may possibly be associated with a lower risk of certain diseases.”
Beginning with the Inuit
It all started “with observations of the Inuit [population] in Greenland and Alaska after World War II, because it was remarked upon that these people ate a lot of fish and seal meat and had a very low incidence of cardiovascular diseases.” Over the years, a large number of association studies have been published, which may have encouraged the assumption that omega-3 fatty acids have positive health effects on various conditions, such as cardiovascular diseases, hyperlipidemia, type 2 diabetes, various malignancies, cognitive impairments, Alzheimer’s disease, depression and anxiety disorders, heart failure, slipped disks, ADHD, symptoms of menopause, rheumatoid arthritis, asthma, periodontitis, epilepsy, chemotherapy tolerance, premenstrual syndrome, and nonalcoholic fatty liver disease.
Dr. Diener believes that the problem is that these are association studies. But association does not mean that there is a causal relationship.
Disappointing study results
On the contrary, the results from the randomized placebo-controlled studies are truly frustrating, according to the neurologist. A meta-analysis of the use of omega-3 fatty acids in cardiovascular diseases included 86 studies with over 162,000 patients. According to Dr. Diener, it did not reveal any benefit for overall and cardiovascular mortality, nor any benefit for the reduction of myocardial infarction and stroke.
The results did indicate a trend, however, for reduced mortality in coronary heart disease. Even so, the number needed to treat for this was 334, which means that 334 people would have to take omega-3 fatty acids for years to prevent one fatal cardiac event.
Aside from this study, Dr. Diener found six studies on Alzheimer’s disease and three studies on dementia with patient populations between 600 and 800. In these studies, too, a positive effect of omega-3 fatty acids could not be identified. Then he discovered another 31 placebo-controlled studies of omega-3 fatty acids for the treatment or prevention of depression and anxiety disorder. Despite including 50,000 patients, these studies also did not show any positive effect.
“I see a significant discrepancy between the promotion of omega-3 fatty acids, whether it’s on television, in the ‘yellow’ [journalism] press, or in advertisements, and the actual scientific evidence,” said Dr. Diener. “At least from a neurological perspective, there is no evidence that omega-3 fatty acids have any benefit. This is true for strokes, dementia, Alzheimer’s disease, depression, and anxiety disorders.”
Potential adverse effects
Omega-3 fatty acids also have potentially adverse effects. The VITAL Rhythm study recently provided evidence that, depending on the dose, preparations with omega-3 fatty acids may increase the risk for atrial fibrillation. As the authors wrote, the results do not support taking omega-3 fatty acids to prevent atrial fibrillation.
In 2019, the global market for omega-3 fatty acids reached a value of $4.1 billion. This value is expected to double by 2025, according to a comment by Gregory Curfman, MD, deputy editor of JAMA and lecturer in health care policy at Harvard Medical School, Boston.
As Dr. Curfman wrote, this impressive amount of expenditure shows how beloved these products are and how strongly many people believe that omega-3 fatty acids are beneficial for their health. It is therefore important to know the potential risks of such preparations. One such example for this would be the risk for atrial fibrillation.
According to Dr. Curfman, in the last 2 years, four randomized clinical studies have provided data on the risk for atrial fibrillation associated with omega-3 fatty acids. In the STRENGTH study, 13,078 high-risk patients with cardiovascular diseases were randomly assigned to one of two groups. The subjects received either a high dose (4 g/day) of a combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) or corn oil. After a median of 42 months, there was no significant difference between the two groups in the primary composite cardiovascular endpoint, but more frequent atrial fibrillation in the omega-3 fatty acid group, compared with the corn oil group (2.2% vs. 1.3%; hazard ratio, 1.69; 95% confidence interval, 1.29-2.21; P < .001).
In the REDUCE-IT study, 8179 subjects were randomly assigned to a high dose (4 g/day, as in STRENGTH) of an omega-3 fatty acid preparation consisting of a purified EPA (icosapent ethyl) or mineral oil. After a median observation period of 4.9 years, icosapent ethyl was associated with a relative reduction of the primary composite cardiovascular endpoint by 25%, compared with mineral oil. As in the STRENGTH study, this study found that the risk for atrial fibrillation associated with omega-3 fatty acids, compared with mineral oil, was significantly higher (5.3% vs. 3.9%; P = .003).
In a third study (OMEMI), as Dr. Curfman reported, 1027 elderly patients who had recently had a myocardial infarction were randomly assigned to receive either a median dose of 1.8 g/day of omega-3 fatty acids (a combination of EPA and DHA) or corn oil. After 2 years, there was no significant difference between the two groups in primary composite cardiovascular endpoints, but 7.2% of the patients taking omega-3 fatty acids developed atrial fibrillation. In the corn oil group, this proportion was 4% (HR, 1.84; 95% CI, 0.98-3.45; P = .06).
The data from the four studies together indicate a potential dose-dependent risk for atrial fibrillation associated with omega-3 fatty acids, according to Dr. Curfman. At a dose of 4.0 g/day, there is a highly significant risk increase (almost double). With a median dose of 1.8 g/day, the risk increase (HR, 1.84) did not reach statistical significance. At a daily standard dose of 840 mg/day, an increase in risk could not be determined.
Dr. Curfman’s recommendation is that patients who take, or want to take, preparations with omega-3 fatty acids be informed of the potential development of arrhythmia at higher dosages. These patients also should undergo cardiological monitoring.
A version of this article first appeared on Medscape.com.
Psychotropic med use tied to ‘striking’ post-COVID dementia risk
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN MEDICINE
Neuropsychiatric outcomes similar for hospitalized COVID-19 patients and non–COVID-19 patients
Hospitalized COVID-19 survivors showed greater cognitive impairment 6 months later, compared with patients hospitalized for other causes, but the overall disease burden was similar, based on data from 85 adults with COVID-19.
Previous studies have shown that cognitive and neuropsychiatric symptoms can occur from 2-6 months after COVID-19 recovery, and such symptoms are known to be associated with hospitalization for other severe medical conditions, Vardan Nersesjan, MD, of Copenhagen University Hospital, and colleagues wrote.
However, it remains unknown if COVID-19 is associated with a unique pattern of cognitive and mental impairment compared with other similarly severe medical conditions, they said.
In a study published in JAMA Psychiatry (2022 Mar 23. doi: 10.1001/jamapsychiatry.2022.0284), the researchers identified 85 adult COVID-19 survivors and 61 controls with non-COVID medical conditions who were treated and released between July 2020 and July 2021. The COVID-19 patients and controls were matched for age, sex, and ICU status. Cognitive impairment was assessed using the Mini-International Neuropsychiatric Interview, the Montreal Cognitive Assessment (MoCA), neurologic examination, and a semistructured interview to determine subjective symptoms.
The primary outcomes were the total scores on the MoCA and any new-onset psychiatric diagnoses. Secondary outcomes included specific psychiatric diagnoses such as depression, neurologic examination findings, and self-reported neuropsychiatric and cognitive symptoms. The mean age of the COVID-19 patients was 56.8 years, and 42% were women.
At 6 months’ follow-up, cognitive status was significantly lower in COVID-19 survivors, compared with controls, based on total geometric mean MoCA scores (26.7 vs. 27.5, P = .01). However, cognitive status improved significantly from 19.2 at hospital discharge to 26.1 at 6 months in 15 of the COVID-19 patients (P = .004), the researchers noted.
New-onset psychiatric diagnoses occurred in 16 COVID-19 patients and 12 of the controls (19% vs. 20%); this difference was not significant.
Secondary outcomes were not significantly different at 6 months between the groups, with the exception of anosmia, which was significantly more common in the COVID-19 patients; however, the significance disappeared in adjusted analysis, the researchers said.
The study findings were limited by several factors including the inability to prove causality because of the case-control feature and by the inability to detect small differences in neuropsychiatric outcomes, the researchers noted.
However, the results were strengthened by the use of a prospectively matched control group with similar disease severity admitted to the same hospital in the same time frame. Although the overall burden of neuropsychiatric and neurologic symptoms and diagnoses appeared similar in COVID-19 patients and those with other medical conditions, more research in larger populations is needed to determine smaller differences in neuropsychiatric profiles, the researchers noted.
Study fills research gap
The study is important at this time because, although prolonged neuropsychiatric and cognitive symptoms have been reported after COVID-19, the field lacked prospective case-control studies with well-matched controls to investigate whether these outcomes differed from those seen in other critical illnesses that had also required hospitalization, corresponding author Michael E. Benros, MD, of the Mental Health Center, Copenhagen, said in an interview.
“I was surprised that there was a significant worse cognitive functioning among COVID-19 patients 6 months after symptom onset also when compared to this well-matched control group that had been hospitalized for non–COVID-19 illness, although the absolute difference between the groups in cognition score were small,” said Dr. Benros. “Another notable finding is the large improvement in cognitive functioning from discharge to follow-up,” he added on behalf of himself and fellow corresponding author Daniel Kondziella, MD.
The study results show that cognitive function affected by COVID-19 and critical illness as observed at discharge showed a substantial improvement at 6 months after symptom onset, said Dr. Benros. “However, the cognitive function was significantly worse among severely ill COVID-19 patients 6 months after symptom onset when compared to a matched control group of individuals hospitalized for non–COVID-19 illness, although this difference in cognitive function was rather small in absolute numbers, and smaller than what had been suggested by other studies that lacked control groups. Strikingly, neuropsychiatric disorders were similar across the two groups, which was also the case when looking at neuropsychiatric symptoms.
“Larger prospective case-control studies of neuropsychiatric and cognitive functioning after COVID-19, compared with matched controls are still needed to detect smaller differences, and more detailed cognitive domains, and with longer follow-up time, which we are currently conducting,” Dr. Benros said.
Controlled studies will help planning
“Lingering neuropsychiatric complications are common after COVID-19, but only controlled studies can tell us whether these complications are specific to COVID-19, rather than a general effect of having been medically ill,” Alasdair G. Rooney, MRCPsych MD PhD, of the University of Edinburgh, said in an interview. “The answer matters ultimately because COVID-19 is a new disease; societies and health care services need to be able to plan for its specific consequences.”
The health status of the control group is important as well. “Most previous studies had compared COVID-19 survivors against healthy controls or patients from a historical database. This new study compared COVID-19 survivors against those hospitalized for other medical causes over the same period,” Dr. Rooney said. “This is a more stringent test of whether COVID-19 has specific neurocognitive and neuropsychiatric consequences.
“The study found that new-onset neuropsychiatric diagnoses and symptoms were no more likely to occur after COVID-19 than after similarly severe medical illnesses,” Dr. Rooney said. “This negative finding runs counter to some earlier studies and may surprise some.” The findings need to be replicated in larger samples, but the current study shows the importance of prospectively recruiting active controls.
“In a subgroup analysis, some patients showed good improvement in cognitive scores between discharge and follow-up. While unsurprising, this is encouraging and suggests that the early postdischarge months are an important time for neurocognitive recovery,” Dr. Rooney noted.
“The findings suggest that COVID-19 may impair attention more selectively than other medical causes of hospitalization. COVID-19 survivors may also be at higher risk of significant overall cognitive impairment than survivors of similarly severe medical illnesses, after a similar duration,” said Dr. Rooney. “If the results are replicated by other prospective studies, they would suggest that there is something about COVID-19 that causes clinically significant neurocognitive difficulties in a minority of survivors.
“Larger well-controlled studies are required, with longer follow-up and more detailed neurocognitive testing,” as the duration of impairment and scope for further recovery are not known, Dr. Rooney added. Also unknown is whether COVID-19 affects attention permanently, or whether recovery is simply slower after COVID-19 compared to other medical illnesses.
“Knowing who is at the greatest risk of severe cognitive impairment after COVID-19 is important and likely to allow tailoring of more effective shielding strategies,” said Dr. Rooney. “This study was conducted before the widespread availability of vaccines for COVID-19. Long-term neuropsychiatric outcomes in vaccinated patients remain largely unknown. Arguably, these are now more important to understand, as future COVID-19 waves will occur mainly among vaccinated individuals.”
The study was supported by the Lundbeck Foundation and the Novo Nordisk Foundation. Lead author Dr. Nersesjan had no financial conflicts to disclose. Dr. Benros reported grants from Lundbeck Foundation and Novo Nordisk Foundation during the conduct of the study. Dr. Rooney had no financial conflicts to disclose.
This article was updated 3/25/22.
Hospitalized COVID-19 survivors showed greater cognitive impairment 6 months later, compared with patients hospitalized for other causes, but the overall disease burden was similar, based on data from 85 adults with COVID-19.
Previous studies have shown that cognitive and neuropsychiatric symptoms can occur from 2-6 months after COVID-19 recovery, and such symptoms are known to be associated with hospitalization for other severe medical conditions, Vardan Nersesjan, MD, of Copenhagen University Hospital, and colleagues wrote.
However, it remains unknown if COVID-19 is associated with a unique pattern of cognitive and mental impairment compared with other similarly severe medical conditions, they said.
In a study published in JAMA Psychiatry (2022 Mar 23. doi: 10.1001/jamapsychiatry.2022.0284), the researchers identified 85 adult COVID-19 survivors and 61 controls with non-COVID medical conditions who were treated and released between July 2020 and July 2021. The COVID-19 patients and controls were matched for age, sex, and ICU status. Cognitive impairment was assessed using the Mini-International Neuropsychiatric Interview, the Montreal Cognitive Assessment (MoCA), neurologic examination, and a semistructured interview to determine subjective symptoms.
The primary outcomes were the total scores on the MoCA and any new-onset psychiatric diagnoses. Secondary outcomes included specific psychiatric diagnoses such as depression, neurologic examination findings, and self-reported neuropsychiatric and cognitive symptoms. The mean age of the COVID-19 patients was 56.8 years, and 42% were women.
At 6 months’ follow-up, cognitive status was significantly lower in COVID-19 survivors, compared with controls, based on total geometric mean MoCA scores (26.7 vs. 27.5, P = .01). However, cognitive status improved significantly from 19.2 at hospital discharge to 26.1 at 6 months in 15 of the COVID-19 patients (P = .004), the researchers noted.
New-onset psychiatric diagnoses occurred in 16 COVID-19 patients and 12 of the controls (19% vs. 20%); this difference was not significant.
Secondary outcomes were not significantly different at 6 months between the groups, with the exception of anosmia, which was significantly more common in the COVID-19 patients; however, the significance disappeared in adjusted analysis, the researchers said.
The study findings were limited by several factors including the inability to prove causality because of the case-control feature and by the inability to detect small differences in neuropsychiatric outcomes, the researchers noted.
However, the results were strengthened by the use of a prospectively matched control group with similar disease severity admitted to the same hospital in the same time frame. Although the overall burden of neuropsychiatric and neurologic symptoms and diagnoses appeared similar in COVID-19 patients and those with other medical conditions, more research in larger populations is needed to determine smaller differences in neuropsychiatric profiles, the researchers noted.
Study fills research gap
The study is important at this time because, although prolonged neuropsychiatric and cognitive symptoms have been reported after COVID-19, the field lacked prospective case-control studies with well-matched controls to investigate whether these outcomes differed from those seen in other critical illnesses that had also required hospitalization, corresponding author Michael E. Benros, MD, of the Mental Health Center, Copenhagen, said in an interview.
“I was surprised that there was a significant worse cognitive functioning among COVID-19 patients 6 months after symptom onset also when compared to this well-matched control group that had been hospitalized for non–COVID-19 illness, although the absolute difference between the groups in cognition score were small,” said Dr. Benros. “Another notable finding is the large improvement in cognitive functioning from discharge to follow-up,” he added on behalf of himself and fellow corresponding author Daniel Kondziella, MD.
The study results show that cognitive function affected by COVID-19 and critical illness as observed at discharge showed a substantial improvement at 6 months after symptom onset, said Dr. Benros. “However, the cognitive function was significantly worse among severely ill COVID-19 patients 6 months after symptom onset when compared to a matched control group of individuals hospitalized for non–COVID-19 illness, although this difference in cognitive function was rather small in absolute numbers, and smaller than what had been suggested by other studies that lacked control groups. Strikingly, neuropsychiatric disorders were similar across the two groups, which was also the case when looking at neuropsychiatric symptoms.
“Larger prospective case-control studies of neuropsychiatric and cognitive functioning after COVID-19, compared with matched controls are still needed to detect smaller differences, and more detailed cognitive domains, and with longer follow-up time, which we are currently conducting,” Dr. Benros said.
Controlled studies will help planning
“Lingering neuropsychiatric complications are common after COVID-19, but only controlled studies can tell us whether these complications are specific to COVID-19, rather than a general effect of having been medically ill,” Alasdair G. Rooney, MRCPsych MD PhD, of the University of Edinburgh, said in an interview. “The answer matters ultimately because COVID-19 is a new disease; societies and health care services need to be able to plan for its specific consequences.”
The health status of the control group is important as well. “Most previous studies had compared COVID-19 survivors against healthy controls or patients from a historical database. This new study compared COVID-19 survivors against those hospitalized for other medical causes over the same period,” Dr. Rooney said. “This is a more stringent test of whether COVID-19 has specific neurocognitive and neuropsychiatric consequences.
“The study found that new-onset neuropsychiatric diagnoses and symptoms were no more likely to occur after COVID-19 than after similarly severe medical illnesses,” Dr. Rooney said. “This negative finding runs counter to some earlier studies and may surprise some.” The findings need to be replicated in larger samples, but the current study shows the importance of prospectively recruiting active controls.
“In a subgroup analysis, some patients showed good improvement in cognitive scores between discharge and follow-up. While unsurprising, this is encouraging and suggests that the early postdischarge months are an important time for neurocognitive recovery,” Dr. Rooney noted.
“The findings suggest that COVID-19 may impair attention more selectively than other medical causes of hospitalization. COVID-19 survivors may also be at higher risk of significant overall cognitive impairment than survivors of similarly severe medical illnesses, after a similar duration,” said Dr. Rooney. “If the results are replicated by other prospective studies, they would suggest that there is something about COVID-19 that causes clinically significant neurocognitive difficulties in a minority of survivors.
“Larger well-controlled studies are required, with longer follow-up and more detailed neurocognitive testing,” as the duration of impairment and scope for further recovery are not known, Dr. Rooney added. Also unknown is whether COVID-19 affects attention permanently, or whether recovery is simply slower after COVID-19 compared to other medical illnesses.
“Knowing who is at the greatest risk of severe cognitive impairment after COVID-19 is important and likely to allow tailoring of more effective shielding strategies,” said Dr. Rooney. “This study was conducted before the widespread availability of vaccines for COVID-19. Long-term neuropsychiatric outcomes in vaccinated patients remain largely unknown. Arguably, these are now more important to understand, as future COVID-19 waves will occur mainly among vaccinated individuals.”
The study was supported by the Lundbeck Foundation and the Novo Nordisk Foundation. Lead author Dr. Nersesjan had no financial conflicts to disclose. Dr. Benros reported grants from Lundbeck Foundation and Novo Nordisk Foundation during the conduct of the study. Dr. Rooney had no financial conflicts to disclose.
This article was updated 3/25/22.
Hospitalized COVID-19 survivors showed greater cognitive impairment 6 months later, compared with patients hospitalized for other causes, but the overall disease burden was similar, based on data from 85 adults with COVID-19.
Previous studies have shown that cognitive and neuropsychiatric symptoms can occur from 2-6 months after COVID-19 recovery, and such symptoms are known to be associated with hospitalization for other severe medical conditions, Vardan Nersesjan, MD, of Copenhagen University Hospital, and colleagues wrote.
However, it remains unknown if COVID-19 is associated with a unique pattern of cognitive and mental impairment compared with other similarly severe medical conditions, they said.
In a study published in JAMA Psychiatry (2022 Mar 23. doi: 10.1001/jamapsychiatry.2022.0284), the researchers identified 85 adult COVID-19 survivors and 61 controls with non-COVID medical conditions who were treated and released between July 2020 and July 2021. The COVID-19 patients and controls were matched for age, sex, and ICU status. Cognitive impairment was assessed using the Mini-International Neuropsychiatric Interview, the Montreal Cognitive Assessment (MoCA), neurologic examination, and a semistructured interview to determine subjective symptoms.
The primary outcomes were the total scores on the MoCA and any new-onset psychiatric diagnoses. Secondary outcomes included specific psychiatric diagnoses such as depression, neurologic examination findings, and self-reported neuropsychiatric and cognitive symptoms. The mean age of the COVID-19 patients was 56.8 years, and 42% were women.
At 6 months’ follow-up, cognitive status was significantly lower in COVID-19 survivors, compared with controls, based on total geometric mean MoCA scores (26.7 vs. 27.5, P = .01). However, cognitive status improved significantly from 19.2 at hospital discharge to 26.1 at 6 months in 15 of the COVID-19 patients (P = .004), the researchers noted.
New-onset psychiatric diagnoses occurred in 16 COVID-19 patients and 12 of the controls (19% vs. 20%); this difference was not significant.
Secondary outcomes were not significantly different at 6 months between the groups, with the exception of anosmia, which was significantly more common in the COVID-19 patients; however, the significance disappeared in adjusted analysis, the researchers said.
The study findings were limited by several factors including the inability to prove causality because of the case-control feature and by the inability to detect small differences in neuropsychiatric outcomes, the researchers noted.
However, the results were strengthened by the use of a prospectively matched control group with similar disease severity admitted to the same hospital in the same time frame. Although the overall burden of neuropsychiatric and neurologic symptoms and diagnoses appeared similar in COVID-19 patients and those with other medical conditions, more research in larger populations is needed to determine smaller differences in neuropsychiatric profiles, the researchers noted.
Study fills research gap
The study is important at this time because, although prolonged neuropsychiatric and cognitive symptoms have been reported after COVID-19, the field lacked prospective case-control studies with well-matched controls to investigate whether these outcomes differed from those seen in other critical illnesses that had also required hospitalization, corresponding author Michael E. Benros, MD, of the Mental Health Center, Copenhagen, said in an interview.
“I was surprised that there was a significant worse cognitive functioning among COVID-19 patients 6 months after symptom onset also when compared to this well-matched control group that had been hospitalized for non–COVID-19 illness, although the absolute difference between the groups in cognition score were small,” said Dr. Benros. “Another notable finding is the large improvement in cognitive functioning from discharge to follow-up,” he added on behalf of himself and fellow corresponding author Daniel Kondziella, MD.
The study results show that cognitive function affected by COVID-19 and critical illness as observed at discharge showed a substantial improvement at 6 months after symptom onset, said Dr. Benros. “However, the cognitive function was significantly worse among severely ill COVID-19 patients 6 months after symptom onset when compared to a matched control group of individuals hospitalized for non–COVID-19 illness, although this difference in cognitive function was rather small in absolute numbers, and smaller than what had been suggested by other studies that lacked control groups. Strikingly, neuropsychiatric disorders were similar across the two groups, which was also the case when looking at neuropsychiatric symptoms.
“Larger prospective case-control studies of neuropsychiatric and cognitive functioning after COVID-19, compared with matched controls are still needed to detect smaller differences, and more detailed cognitive domains, and with longer follow-up time, which we are currently conducting,” Dr. Benros said.
Controlled studies will help planning
“Lingering neuropsychiatric complications are common after COVID-19, but only controlled studies can tell us whether these complications are specific to COVID-19, rather than a general effect of having been medically ill,” Alasdair G. Rooney, MRCPsych MD PhD, of the University of Edinburgh, said in an interview. “The answer matters ultimately because COVID-19 is a new disease; societies and health care services need to be able to plan for its specific consequences.”
The health status of the control group is important as well. “Most previous studies had compared COVID-19 survivors against healthy controls or patients from a historical database. This new study compared COVID-19 survivors against those hospitalized for other medical causes over the same period,” Dr. Rooney said. “This is a more stringent test of whether COVID-19 has specific neurocognitive and neuropsychiatric consequences.
“The study found that new-onset neuropsychiatric diagnoses and symptoms were no more likely to occur after COVID-19 than after similarly severe medical illnesses,” Dr. Rooney said. “This negative finding runs counter to some earlier studies and may surprise some.” The findings need to be replicated in larger samples, but the current study shows the importance of prospectively recruiting active controls.
“In a subgroup analysis, some patients showed good improvement in cognitive scores between discharge and follow-up. While unsurprising, this is encouraging and suggests that the early postdischarge months are an important time for neurocognitive recovery,” Dr. Rooney noted.
“The findings suggest that COVID-19 may impair attention more selectively than other medical causes of hospitalization. COVID-19 survivors may also be at higher risk of significant overall cognitive impairment than survivors of similarly severe medical illnesses, after a similar duration,” said Dr. Rooney. “If the results are replicated by other prospective studies, they would suggest that there is something about COVID-19 that causes clinically significant neurocognitive difficulties in a minority of survivors.
“Larger well-controlled studies are required, with longer follow-up and more detailed neurocognitive testing,” as the duration of impairment and scope for further recovery are not known, Dr. Rooney added. Also unknown is whether COVID-19 affects attention permanently, or whether recovery is simply slower after COVID-19 compared to other medical illnesses.
“Knowing who is at the greatest risk of severe cognitive impairment after COVID-19 is important and likely to allow tailoring of more effective shielding strategies,” said Dr. Rooney. “This study was conducted before the widespread availability of vaccines for COVID-19. Long-term neuropsychiatric outcomes in vaccinated patients remain largely unknown. Arguably, these are now more important to understand, as future COVID-19 waves will occur mainly among vaccinated individuals.”
The study was supported by the Lundbeck Foundation and the Novo Nordisk Foundation. Lead author Dr. Nersesjan had no financial conflicts to disclose. Dr. Benros reported grants from Lundbeck Foundation and Novo Nordisk Foundation during the conduct of the study. Dr. Rooney had no financial conflicts to disclose.
This article was updated 3/25/22.
FROM JAMA PSYCHIATRY
Aducanumab and ARIA: Does the FDA’s prescribing label put patients at risk?
Specifically, the drug’s label calls for three MRI brain scans before, and during, the titration period. The problem is the trial data used for the drug’s approval by the U.S. Food and Drug Administration included five MRIs to screen for ARIA.
“We recommend proceeding as per the clinical trials,” said Meghan Riddle, MD, associate director, Memory and Aging program, Butler Hospital, and assistant professor of psychiatry and human behavior, Brown University, Providence, R.I.
Dr. Riddle shared her team’s clinical experience with aducanumab, as well as information on four ARIA cases from their clinic, during a presentation at the American Association for Geriatric Psychiatry (AAGP) 2022 Annual Meeting.
Significant safety risk?
As previously reported by this news organization, the FDA granted accelerated approval of aducanumab for AD last year.
ARIA is the most common risk associated with aducanumab and has two types: ARIA-E (with edema) and ARIA-H (with hemosiderin). These can co-occur, particularly in areas of high amyloid burden, Dr. Riddle noted during her presentation.
ARIA is often detected incidentally via MRI. Patients are usually asymptomatic, but when they do have symptoms, headache, dizziness, and vision changes are the most common complaints. However, these are generally mild, said Dr. Riddle.
Nevertheless, in some cases, there can be severe sequelae, including severe edema or bleeding and seizures, she added.
A major risk factor for ARIA is apolipoprotein 4 (APOE ε4) status. Carriers are twice as likely to develop ARIA as non-carriers.
“If you’re heterozygote for APOE ε4, you have about a 40% chance of developing ARIA, and if you’re homozygote, you have about a 66% chance of developing ARIA,” Dr. Riddle said.
Given the high rate of ARIA in APOE ε4 carriers, the team from Butler Hospital recommends APOE testing prior to treatment with aducanumab.
The risk for developing ARIA is highest within the year of dose titration, Dr. Riddle noted. The current FDA label recommends obtaining a recent brain MRI, within 1 year, and then scans before the 7th and 12th infusions. However, the protocol during the clinical trials of aducanumab included MRI at baseline and prior to the 5th, 7th, 9th, and 12th infusions.
Dr. Riddle’s group has opted to continue the research protocol with new patients. “There’s concern that the decreased MRI monitoring based on the current FDA label may pose a significant safety risk, particularly among those who we know are already at a higher risk of developing ARIA,” she said.
Dr. Riddle also shared how her team selects aducanumab candidates. They need to have mild cognitive impairment (MCI), a mini-mental state examination (MMSE) score of 24 to 30, and a recent MRI to review for eligibility and APOE testing.
The most common reason for treatment exclusion is advanced disease and comorbidity, such as stroke.
Once approved for treatment, patients receive monthly infusions titrated over 6 months – 1 mg/kg for 2 months, 3 mg/kg for 2 months, 6 mg/kg for 2 months, then 10 mg/kg.
Patients are monitored to ensure safety and tolerability and regular review of MRI findings. In addition, patients and their families receive ongoing education about the drug.
Dr. Riddle and her team permanently discontinue the aducanumab if patients develop microhemorrhage, more than one area of superficial siderosis, more than 10 microhemorrhages, more than two episodes of ARIA, or severe symptoms of ARIA.
Four cases
Of the 11 patients who were candidates for aducanumab treatment, four developed ARIA. All are APOE ε4 carriers, with two homozygotes and two heterozygotes. All had severe radiographic ARIA-E, with one developing ARIA-H.
“Importantly, they were all initially asymptomatic and the ARIA was just picked up on their regular surveillance MRI,” said Dr. Riddle. She added that the drug was discontinued in all four cases.
Three of the ARIA cases were detected prior to the 5th scan, which is “concerning,” said Dr. Riddle. “Based on the current FDA label of safety monitoring, they don’t recommend doing that MRI. So [clinicians] would have dosed through that ARIA, which could put someone at much greater risk of developing severe symptoms.”
In addition, 14 patients at the center are receiving treatment with aducanumab. However, at this point they have not yet received their first MRI screen.
Dr. Riddle noted that when patients are told they are not candidates for treatment, or when treatment is discontinued, they are upset. However, she added, there is also a substantial level of understanding.
“We have a very layered discussion that includes the simple fact that we still aren’t sure if this is going to provide any clinical benefit, that this decision [to approve the drug] was accelerated, and that data are still being gathered,” Dr. Riddle added.
Dr. Riddle noted that the risk of ARIA is highest during the dose titration period: “There’s a signal that once you get to the 10 mg/kg dose, that plateaus.”
None of the patients at her center have reached that 12-month treatment mark. “The current plan is to do the MRI at 12 months then to give serial MRIs but less frequently, and whether that’s at 6 months or annually is yet to be determined.”
“We’re kind of writing these protocols as information evolves,” Dr. Riddle said.
The Memory and Aging Program receives grants from NIH-ADNI, Alzheimer’s Association, Fain Family Foundation, Joukowsky Family Foundation, Winter Family, Rhode Island Foundation, Goodman Family Foundation, and Global Alzheimer Platform Foundation; and clinical trials include: Lilly, Biogen, Genentech, Avid, Roche, Eisai, and Novartis. Dr. Riddle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Specifically, the drug’s label calls for three MRI brain scans before, and during, the titration period. The problem is the trial data used for the drug’s approval by the U.S. Food and Drug Administration included five MRIs to screen for ARIA.
“We recommend proceeding as per the clinical trials,” said Meghan Riddle, MD, associate director, Memory and Aging program, Butler Hospital, and assistant professor of psychiatry and human behavior, Brown University, Providence, R.I.
Dr. Riddle shared her team’s clinical experience with aducanumab, as well as information on four ARIA cases from their clinic, during a presentation at the American Association for Geriatric Psychiatry (AAGP) 2022 Annual Meeting.
Significant safety risk?
As previously reported by this news organization, the FDA granted accelerated approval of aducanumab for AD last year.
ARIA is the most common risk associated with aducanumab and has two types: ARIA-E (with edema) and ARIA-H (with hemosiderin). These can co-occur, particularly in areas of high amyloid burden, Dr. Riddle noted during her presentation.
ARIA is often detected incidentally via MRI. Patients are usually asymptomatic, but when they do have symptoms, headache, dizziness, and vision changes are the most common complaints. However, these are generally mild, said Dr. Riddle.
Nevertheless, in some cases, there can be severe sequelae, including severe edema or bleeding and seizures, she added.
A major risk factor for ARIA is apolipoprotein 4 (APOE ε4) status. Carriers are twice as likely to develop ARIA as non-carriers.
“If you’re heterozygote for APOE ε4, you have about a 40% chance of developing ARIA, and if you’re homozygote, you have about a 66% chance of developing ARIA,” Dr. Riddle said.
Given the high rate of ARIA in APOE ε4 carriers, the team from Butler Hospital recommends APOE testing prior to treatment with aducanumab.
The risk for developing ARIA is highest within the year of dose titration, Dr. Riddle noted. The current FDA label recommends obtaining a recent brain MRI, within 1 year, and then scans before the 7th and 12th infusions. However, the protocol during the clinical trials of aducanumab included MRI at baseline and prior to the 5th, 7th, 9th, and 12th infusions.
Dr. Riddle’s group has opted to continue the research protocol with new patients. “There’s concern that the decreased MRI monitoring based on the current FDA label may pose a significant safety risk, particularly among those who we know are already at a higher risk of developing ARIA,” she said.
Dr. Riddle also shared how her team selects aducanumab candidates. They need to have mild cognitive impairment (MCI), a mini-mental state examination (MMSE) score of 24 to 30, and a recent MRI to review for eligibility and APOE testing.
The most common reason for treatment exclusion is advanced disease and comorbidity, such as stroke.
Once approved for treatment, patients receive monthly infusions titrated over 6 months – 1 mg/kg for 2 months, 3 mg/kg for 2 months, 6 mg/kg for 2 months, then 10 mg/kg.
Patients are monitored to ensure safety and tolerability and regular review of MRI findings. In addition, patients and their families receive ongoing education about the drug.
Dr. Riddle and her team permanently discontinue the aducanumab if patients develop microhemorrhage, more than one area of superficial siderosis, more than 10 microhemorrhages, more than two episodes of ARIA, or severe symptoms of ARIA.
Four cases
Of the 11 patients who were candidates for aducanumab treatment, four developed ARIA. All are APOE ε4 carriers, with two homozygotes and two heterozygotes. All had severe radiographic ARIA-E, with one developing ARIA-H.
“Importantly, they were all initially asymptomatic and the ARIA was just picked up on their regular surveillance MRI,” said Dr. Riddle. She added that the drug was discontinued in all four cases.
Three of the ARIA cases were detected prior to the 5th scan, which is “concerning,” said Dr. Riddle. “Based on the current FDA label of safety monitoring, they don’t recommend doing that MRI. So [clinicians] would have dosed through that ARIA, which could put someone at much greater risk of developing severe symptoms.”
In addition, 14 patients at the center are receiving treatment with aducanumab. However, at this point they have not yet received their first MRI screen.
Dr. Riddle noted that when patients are told they are not candidates for treatment, or when treatment is discontinued, they are upset. However, she added, there is also a substantial level of understanding.
“We have a very layered discussion that includes the simple fact that we still aren’t sure if this is going to provide any clinical benefit, that this decision [to approve the drug] was accelerated, and that data are still being gathered,” Dr. Riddle added.
Dr. Riddle noted that the risk of ARIA is highest during the dose titration period: “There’s a signal that once you get to the 10 mg/kg dose, that plateaus.”
None of the patients at her center have reached that 12-month treatment mark. “The current plan is to do the MRI at 12 months then to give serial MRIs but less frequently, and whether that’s at 6 months or annually is yet to be determined.”
“We’re kind of writing these protocols as information evolves,” Dr. Riddle said.
The Memory and Aging Program receives grants from NIH-ADNI, Alzheimer’s Association, Fain Family Foundation, Joukowsky Family Foundation, Winter Family, Rhode Island Foundation, Goodman Family Foundation, and Global Alzheimer Platform Foundation; and clinical trials include: Lilly, Biogen, Genentech, Avid, Roche, Eisai, and Novartis. Dr. Riddle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Specifically, the drug’s label calls for three MRI brain scans before, and during, the titration period. The problem is the trial data used for the drug’s approval by the U.S. Food and Drug Administration included five MRIs to screen for ARIA.
“We recommend proceeding as per the clinical trials,” said Meghan Riddle, MD, associate director, Memory and Aging program, Butler Hospital, and assistant professor of psychiatry and human behavior, Brown University, Providence, R.I.
Dr. Riddle shared her team’s clinical experience with aducanumab, as well as information on four ARIA cases from their clinic, during a presentation at the American Association for Geriatric Psychiatry (AAGP) 2022 Annual Meeting.
Significant safety risk?
As previously reported by this news organization, the FDA granted accelerated approval of aducanumab for AD last year.
ARIA is the most common risk associated with aducanumab and has two types: ARIA-E (with edema) and ARIA-H (with hemosiderin). These can co-occur, particularly in areas of high amyloid burden, Dr. Riddle noted during her presentation.
ARIA is often detected incidentally via MRI. Patients are usually asymptomatic, but when they do have symptoms, headache, dizziness, and vision changes are the most common complaints. However, these are generally mild, said Dr. Riddle.
Nevertheless, in some cases, there can be severe sequelae, including severe edema or bleeding and seizures, she added.
A major risk factor for ARIA is apolipoprotein 4 (APOE ε4) status. Carriers are twice as likely to develop ARIA as non-carriers.
“If you’re heterozygote for APOE ε4, you have about a 40% chance of developing ARIA, and if you’re homozygote, you have about a 66% chance of developing ARIA,” Dr. Riddle said.
Given the high rate of ARIA in APOE ε4 carriers, the team from Butler Hospital recommends APOE testing prior to treatment with aducanumab.
The risk for developing ARIA is highest within the year of dose titration, Dr. Riddle noted. The current FDA label recommends obtaining a recent brain MRI, within 1 year, and then scans before the 7th and 12th infusions. However, the protocol during the clinical trials of aducanumab included MRI at baseline and prior to the 5th, 7th, 9th, and 12th infusions.
Dr. Riddle’s group has opted to continue the research protocol with new patients. “There’s concern that the decreased MRI monitoring based on the current FDA label may pose a significant safety risk, particularly among those who we know are already at a higher risk of developing ARIA,” she said.
Dr. Riddle also shared how her team selects aducanumab candidates. They need to have mild cognitive impairment (MCI), a mini-mental state examination (MMSE) score of 24 to 30, and a recent MRI to review for eligibility and APOE testing.
The most common reason for treatment exclusion is advanced disease and comorbidity, such as stroke.
Once approved for treatment, patients receive monthly infusions titrated over 6 months – 1 mg/kg for 2 months, 3 mg/kg for 2 months, 6 mg/kg for 2 months, then 10 mg/kg.
Patients are monitored to ensure safety and tolerability and regular review of MRI findings. In addition, patients and their families receive ongoing education about the drug.
Dr. Riddle and her team permanently discontinue the aducanumab if patients develop microhemorrhage, more than one area of superficial siderosis, more than 10 microhemorrhages, more than two episodes of ARIA, or severe symptoms of ARIA.
Four cases
Of the 11 patients who were candidates for aducanumab treatment, four developed ARIA. All are APOE ε4 carriers, with two homozygotes and two heterozygotes. All had severe radiographic ARIA-E, with one developing ARIA-H.
“Importantly, they were all initially asymptomatic and the ARIA was just picked up on their regular surveillance MRI,” said Dr. Riddle. She added that the drug was discontinued in all four cases.
Three of the ARIA cases were detected prior to the 5th scan, which is “concerning,” said Dr. Riddle. “Based on the current FDA label of safety monitoring, they don’t recommend doing that MRI. So [clinicians] would have dosed through that ARIA, which could put someone at much greater risk of developing severe symptoms.”
In addition, 14 patients at the center are receiving treatment with aducanumab. However, at this point they have not yet received their first MRI screen.
Dr. Riddle noted that when patients are told they are not candidates for treatment, or when treatment is discontinued, they are upset. However, she added, there is also a substantial level of understanding.
“We have a very layered discussion that includes the simple fact that we still aren’t sure if this is going to provide any clinical benefit, that this decision [to approve the drug] was accelerated, and that data are still being gathered,” Dr. Riddle added.
Dr. Riddle noted that the risk of ARIA is highest during the dose titration period: “There’s a signal that once you get to the 10 mg/kg dose, that plateaus.”
None of the patients at her center have reached that 12-month treatment mark. “The current plan is to do the MRI at 12 months then to give serial MRIs but less frequently, and whether that’s at 6 months or annually is yet to be determined.”
“We’re kind of writing these protocols as information evolves,” Dr. Riddle said.
The Memory and Aging Program receives grants from NIH-ADNI, Alzheimer’s Association, Fain Family Foundation, Joukowsky Family Foundation, Winter Family, Rhode Island Foundation, Goodman Family Foundation, and Global Alzheimer Platform Foundation; and clinical trials include: Lilly, Biogen, Genentech, Avid, Roche, Eisai, and Novartis. Dr. Riddle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAGP 2022
Restoring ‘sixth sense’ may reduce falls in Alzheimer’s
(AD), new research confirms.
Falls are twice as common in patients with AD versu older individuals without the disorder and significantly increase the likelihood of institutionalization.
However, researchers recorded fewer falls in patients with a better functioning vestibular system, which detects head movements and plays a critical role in spatial orientation, posture, gait, and balance.
The results suggest that improving vestibular function with currently available therapies may prevent falls, something the researchers will investigate in a new clinical trial launching next month.
“One of the most dangerous and impactful symptoms in terms of function in patients with Alzheimer’s disease is their increased predisposition to falls,” study investigator Yuri Agrawal, MD, department of otolaryngology–head and neck surgery, Johns Hopkins University School of Medicine, Baltimore, said in an interview. “Alzheimer’s is the sixth leading cause of death in the U.S., and some people actually say that that high mortality rate is because of their predisposition to falls and the injuries that occur.”
The study was published online Feb. 14 in the Journal of Alzheimer’s Disease.
The ‘sixth hidden sense’
The vestibular system consists of three semicircular canals, which detect rotational head movement, and two otolith organs called the utricle and the saccule, which sense linear head movements and the orientation of the head with respect to gravity.
“We call the vestibular system the sixth hidden sense because it’s not a conscious perception like taste or smell,” Dr. Agrawal said. “It’s constantly providing input to our brain about where we are in space.”
Dr. Agrawal and colleagues previously reported that vestibular loss is twice as common in Alzheimer’s patients as in cognitively unimpaired age-matched controls. Now, they wanted to know if this sensory loss was associated with an increased risk for falls in this population.
The study included 48 patients age greater than or equal to 60 years with mild-to-moderate AD between 2018 and 2020. They also included an age-matched control group of healthy controls with no cognitive impairment.
Researchers assessed vestibular function at baseline by measuring semicircular canal and saccular function. One test required participants to wear goggles and complete a series of tests with their eyes open and closed while researchers recorded their eye movement with video-oculography. They also measured participants’ balance using the Berg Balance Scale.
Relative to matched controls, AD patients exhibited increased lateral instability when their eyes were open (P = .033) and closed (P = .042). Studies suggest that lateral stability declines more quickly with age and that instability with eyes closed is the single biggest predictor of incident falls in community-dwelling adults.
To determine if poor vestibular function increased fall risk in patients with AD, researchers followed the cohort for up to 2 years.
“We found that patients with vestibular loss at baseline were 50% more likely to fall, adjusting for other factors that could contribute to that,” Dr. Agrawal said.
Specifically, better semicircular canal function was significantly associated with lower likelihood of falls, even after adjusting for confounders (adjusted hazard ratio, 0.65; P = .009).
Can therapy help?
Commenting on the findings, James Burke, MD, PhD, professor of neurology at Duke University Medical Center, Durham, N.C., said that the finding that impaired vestibular function is associated with increased falls “significantly advances our understanding of the topic” and suggests that treating vestibular dysfunction could reduce falls in Alzheimer’s patients.
“Screening patients with Alzheimer’s disease for impaired vestibular function could lead to identification of individuals at high risk of falls and target those who would benefit from vestibular therapy,” he said.
Vestibular rehabilitation therapy is often used to treat a number of disorders related to vestibular function loss. There are also studies underway to measure the efficacy of a vestibular implant that works much like a cochlear implant.
While evaluation of vestibular function is currently not routinely included in AD care, studies such as these suggest it may be time to consider adding it to the standard of care, Jennifer Coto, PhD, assistant professor of otolaryngology at the University of Miami Miller School of Medicine, said in an interview.
“Best practice guidelines for management of Alzheimer’s patients should be revised to include routine vestibular evaluation and support from a multidisciplinary team that may address other crucial areas of functioning, particularly psychological functioning, sleep, and independence,” she said.
“Future research also needs to evaluate the effectiveness of vestibular therapy in patients with Alzheimer’s and the benefits of early identification and intervention for preventing recurrent falls.”
Dr. Agrawal is leading a 5-year, $3.5 million National Institute on Aging study that seeks to do just that. Enrollment in the study begins next month. Patients will complete an initial in-person screening, but the remainder of the study will be conducted virtually.
Therapies will be noninvasive, nonpharmaceutical, and performed in participants’ homes. If the therapy is successful at reducing falls, Dr. Agrawal said the virtual design would significantly broaden its potential patient reach.
The study was funded by the National Institute on Aging. Study authors’ disclosures are reported in the original article. Dr. Coto and Dr. Burke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AD), new research confirms.
Falls are twice as common in patients with AD versu older individuals without the disorder and significantly increase the likelihood of institutionalization.
However, researchers recorded fewer falls in patients with a better functioning vestibular system, which detects head movements and plays a critical role in spatial orientation, posture, gait, and balance.
The results suggest that improving vestibular function with currently available therapies may prevent falls, something the researchers will investigate in a new clinical trial launching next month.
“One of the most dangerous and impactful symptoms in terms of function in patients with Alzheimer’s disease is their increased predisposition to falls,” study investigator Yuri Agrawal, MD, department of otolaryngology–head and neck surgery, Johns Hopkins University School of Medicine, Baltimore, said in an interview. “Alzheimer’s is the sixth leading cause of death in the U.S., and some people actually say that that high mortality rate is because of their predisposition to falls and the injuries that occur.”
The study was published online Feb. 14 in the Journal of Alzheimer’s Disease.
The ‘sixth hidden sense’
The vestibular system consists of three semicircular canals, which detect rotational head movement, and two otolith organs called the utricle and the saccule, which sense linear head movements and the orientation of the head with respect to gravity.
“We call the vestibular system the sixth hidden sense because it’s not a conscious perception like taste or smell,” Dr. Agrawal said. “It’s constantly providing input to our brain about where we are in space.”
Dr. Agrawal and colleagues previously reported that vestibular loss is twice as common in Alzheimer’s patients as in cognitively unimpaired age-matched controls. Now, they wanted to know if this sensory loss was associated with an increased risk for falls in this population.
The study included 48 patients age greater than or equal to 60 years with mild-to-moderate AD between 2018 and 2020. They also included an age-matched control group of healthy controls with no cognitive impairment.
Researchers assessed vestibular function at baseline by measuring semicircular canal and saccular function. One test required participants to wear goggles and complete a series of tests with their eyes open and closed while researchers recorded their eye movement with video-oculography. They also measured participants’ balance using the Berg Balance Scale.
Relative to matched controls, AD patients exhibited increased lateral instability when their eyes were open (P = .033) and closed (P = .042). Studies suggest that lateral stability declines more quickly with age and that instability with eyes closed is the single biggest predictor of incident falls in community-dwelling adults.
To determine if poor vestibular function increased fall risk in patients with AD, researchers followed the cohort for up to 2 years.
“We found that patients with vestibular loss at baseline were 50% more likely to fall, adjusting for other factors that could contribute to that,” Dr. Agrawal said.
Specifically, better semicircular canal function was significantly associated with lower likelihood of falls, even after adjusting for confounders (adjusted hazard ratio, 0.65; P = .009).
Can therapy help?
Commenting on the findings, James Burke, MD, PhD, professor of neurology at Duke University Medical Center, Durham, N.C., said that the finding that impaired vestibular function is associated with increased falls “significantly advances our understanding of the topic” and suggests that treating vestibular dysfunction could reduce falls in Alzheimer’s patients.
“Screening patients with Alzheimer’s disease for impaired vestibular function could lead to identification of individuals at high risk of falls and target those who would benefit from vestibular therapy,” he said.
Vestibular rehabilitation therapy is often used to treat a number of disorders related to vestibular function loss. There are also studies underway to measure the efficacy of a vestibular implant that works much like a cochlear implant.
While evaluation of vestibular function is currently not routinely included in AD care, studies such as these suggest it may be time to consider adding it to the standard of care, Jennifer Coto, PhD, assistant professor of otolaryngology at the University of Miami Miller School of Medicine, said in an interview.
“Best practice guidelines for management of Alzheimer’s patients should be revised to include routine vestibular evaluation and support from a multidisciplinary team that may address other crucial areas of functioning, particularly psychological functioning, sleep, and independence,” she said.
“Future research also needs to evaluate the effectiveness of vestibular therapy in patients with Alzheimer’s and the benefits of early identification and intervention for preventing recurrent falls.”
Dr. Agrawal is leading a 5-year, $3.5 million National Institute on Aging study that seeks to do just that. Enrollment in the study begins next month. Patients will complete an initial in-person screening, but the remainder of the study will be conducted virtually.
Therapies will be noninvasive, nonpharmaceutical, and performed in participants’ homes. If the therapy is successful at reducing falls, Dr. Agrawal said the virtual design would significantly broaden its potential patient reach.
The study was funded by the National Institute on Aging. Study authors’ disclosures are reported in the original article. Dr. Coto and Dr. Burke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AD), new research confirms.
Falls are twice as common in patients with AD versu older individuals without the disorder and significantly increase the likelihood of institutionalization.
However, researchers recorded fewer falls in patients with a better functioning vestibular system, which detects head movements and plays a critical role in spatial orientation, posture, gait, and balance.
The results suggest that improving vestibular function with currently available therapies may prevent falls, something the researchers will investigate in a new clinical trial launching next month.
“One of the most dangerous and impactful symptoms in terms of function in patients with Alzheimer’s disease is their increased predisposition to falls,” study investigator Yuri Agrawal, MD, department of otolaryngology–head and neck surgery, Johns Hopkins University School of Medicine, Baltimore, said in an interview. “Alzheimer’s is the sixth leading cause of death in the U.S., and some people actually say that that high mortality rate is because of their predisposition to falls and the injuries that occur.”
The study was published online Feb. 14 in the Journal of Alzheimer’s Disease.
The ‘sixth hidden sense’
The vestibular system consists of three semicircular canals, which detect rotational head movement, and two otolith organs called the utricle and the saccule, which sense linear head movements and the orientation of the head with respect to gravity.
“We call the vestibular system the sixth hidden sense because it’s not a conscious perception like taste or smell,” Dr. Agrawal said. “It’s constantly providing input to our brain about where we are in space.”
Dr. Agrawal and colleagues previously reported that vestibular loss is twice as common in Alzheimer’s patients as in cognitively unimpaired age-matched controls. Now, they wanted to know if this sensory loss was associated with an increased risk for falls in this population.
The study included 48 patients age greater than or equal to 60 years with mild-to-moderate AD between 2018 and 2020. They also included an age-matched control group of healthy controls with no cognitive impairment.
Researchers assessed vestibular function at baseline by measuring semicircular canal and saccular function. One test required participants to wear goggles and complete a series of tests with their eyes open and closed while researchers recorded their eye movement with video-oculography. They also measured participants’ balance using the Berg Balance Scale.
Relative to matched controls, AD patients exhibited increased lateral instability when their eyes were open (P = .033) and closed (P = .042). Studies suggest that lateral stability declines more quickly with age and that instability with eyes closed is the single biggest predictor of incident falls in community-dwelling adults.
To determine if poor vestibular function increased fall risk in patients with AD, researchers followed the cohort for up to 2 years.
“We found that patients with vestibular loss at baseline were 50% more likely to fall, adjusting for other factors that could contribute to that,” Dr. Agrawal said.
Specifically, better semicircular canal function was significantly associated with lower likelihood of falls, even after adjusting for confounders (adjusted hazard ratio, 0.65; P = .009).
Can therapy help?
Commenting on the findings, James Burke, MD, PhD, professor of neurology at Duke University Medical Center, Durham, N.C., said that the finding that impaired vestibular function is associated with increased falls “significantly advances our understanding of the topic” and suggests that treating vestibular dysfunction could reduce falls in Alzheimer’s patients.
“Screening patients with Alzheimer’s disease for impaired vestibular function could lead to identification of individuals at high risk of falls and target those who would benefit from vestibular therapy,” he said.
Vestibular rehabilitation therapy is often used to treat a number of disorders related to vestibular function loss. There are also studies underway to measure the efficacy of a vestibular implant that works much like a cochlear implant.
While evaluation of vestibular function is currently not routinely included in AD care, studies such as these suggest it may be time to consider adding it to the standard of care, Jennifer Coto, PhD, assistant professor of otolaryngology at the University of Miami Miller School of Medicine, said in an interview.
“Best practice guidelines for management of Alzheimer’s patients should be revised to include routine vestibular evaluation and support from a multidisciplinary team that may address other crucial areas of functioning, particularly psychological functioning, sleep, and independence,” she said.
“Future research also needs to evaluate the effectiveness of vestibular therapy in patients with Alzheimer’s and the benefits of early identification and intervention for preventing recurrent falls.”
Dr. Agrawal is leading a 5-year, $3.5 million National Institute on Aging study that seeks to do just that. Enrollment in the study begins next month. Patients will complete an initial in-person screening, but the remainder of the study will be conducted virtually.
Therapies will be noninvasive, nonpharmaceutical, and performed in participants’ homes. If the therapy is successful at reducing falls, Dr. Agrawal said the virtual design would significantly broaden its potential patient reach.
The study was funded by the National Institute on Aging. Study authors’ disclosures are reported in the original article. Dr. Coto and Dr. Burke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
FDA clears once-weekly transdermal patch for Alzheimer’s
Adlarity is the first and only once-weekly patch to continuously deliver consistent doses of the acetylcholinesterase inhibitor through the skin, bypassing the digestive system and resulting in low likelihood of gastrointestinal side effects associated with oral donepezil, the company said in a press release.
Each patch delivers either 5 mg or 10 mg of donepezil daily for 7 days. After that, it is removed and a new patch is applied.
“The availability of a once-weekly patch formulation of donepezil has the potential to substantially benefit patients, caregivers, and health care providers,” Pierre Tariot, MD, director of the Banner Alzheimer’s Institute, Phoenix, said in the release.
“It offers effective, well-tolerated, and stable dosing for 7 days for patients who cannot take daily oral donepezil reliably because of impaired memory. It can also offer benefits for those patients who have diminished ability to swallow or have GI side effects associated with ingestion of oral donepezil,” Dr. Tariot added.
The FDA approved Adlarity through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
The company expects the donepezil transdermal patch to be available in early Fall 2022.
A version of this article first appeared on Medscape.com.
Adlarity is the first and only once-weekly patch to continuously deliver consistent doses of the acetylcholinesterase inhibitor through the skin, bypassing the digestive system and resulting in low likelihood of gastrointestinal side effects associated with oral donepezil, the company said in a press release.
Each patch delivers either 5 mg or 10 mg of donepezil daily for 7 days. After that, it is removed and a new patch is applied.
“The availability of a once-weekly patch formulation of donepezil has the potential to substantially benefit patients, caregivers, and health care providers,” Pierre Tariot, MD, director of the Banner Alzheimer’s Institute, Phoenix, said in the release.
“It offers effective, well-tolerated, and stable dosing for 7 days for patients who cannot take daily oral donepezil reliably because of impaired memory. It can also offer benefits for those patients who have diminished ability to swallow or have GI side effects associated with ingestion of oral donepezil,” Dr. Tariot added.
The FDA approved Adlarity through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
The company expects the donepezil transdermal patch to be available in early Fall 2022.
A version of this article first appeared on Medscape.com.
Adlarity is the first and only once-weekly patch to continuously deliver consistent doses of the acetylcholinesterase inhibitor through the skin, bypassing the digestive system and resulting in low likelihood of gastrointestinal side effects associated with oral donepezil, the company said in a press release.
Each patch delivers either 5 mg or 10 mg of donepezil daily for 7 days. After that, it is removed and a new patch is applied.
“The availability of a once-weekly patch formulation of donepezil has the potential to substantially benefit patients, caregivers, and health care providers,” Pierre Tariot, MD, director of the Banner Alzheimer’s Institute, Phoenix, said in the release.
“It offers effective, well-tolerated, and stable dosing for 7 days for patients who cannot take daily oral donepezil reliably because of impaired memory. It can also offer benefits for those patients who have diminished ability to swallow or have GI side effects associated with ingestion of oral donepezil,” Dr. Tariot added.
The FDA approved Adlarity through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
The company expects the donepezil transdermal patch to be available in early Fall 2022.
A version of this article first appeared on Medscape.com.






