User login
ACR, AAD, AAO, RDS issue joint statement on safe use of hydroxychloroquine
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
FROM ARTHRITIS & rHEUMATOLOGY
Consider home subcutaneous immune globulin for refractory dermatomyositis
Home-based subcutaneous immune globulin therapy is a promising alternative to intravenous immune globulin therapy for patients with refractory dermatomyositis or polymyositis, Anna Postolova, MD, MPH, declared at the 2021 Rheumatology Winter Clinical Symposium.
“This is really exciting. I think in the years to come we may see a change to having our patients be able to do immune globulin therapy at home,” said Dr. Postolova, a rheumatologist and allergist/immunologist at Stanford (Calif.) Health Care.
“The technology is there. I think our patients might feel more comfortable getting immune globulin at home,” she said. “I would love to switch more patients from IVIg to SCIg [subcutaneous immune globulin] in my practice.”
A few caveats: SCIg remains off label for treatment of dermatomyositis (DM) or polymyositis (PM). Its approved indication is as replacement therapy in patients with primary or secondary immunodeficiency diseases. IVIg is approved for this indication, but is also approved for DM/PM refractory to high-dose corticosteroids and immunosuppressants. Yet SCIg is clearly effective for these autoimmune inflammatory diseases, albeit to date the supporting evidence comes chiefly from observational studies and anecdotal experience.
“I don’t know if insurers will cover it, but they should because it’s obviously a lot cheaper to do it at home,” she noted.
SCIg advantages
SCIg offers compelling advantages over IVIg in addition to its substantially lower cost. These include far fewer systemic side effects, shorter infusion time, greater bioavailability, and better quality of life. Patients self-administer SCIg at home, avoiding the inconvenience of IVIg therapy, which entails travel time for once-monthly hospitalization or long hours spent in an infusion center, she explained.
French investigators recently documented a previously unappreciated further advantage of home-based SCIg. They convened a focus group of patients with DM or PM experienced with both IVIg and home SCIg and determined that participants uniformly preferred home SCIg. The patients cited a new and welcome feeling of autonomy and control.
“All patients with experience of IVIg and SCIg expressed a clear preference for SCIg, which was described to be easy, less disruptive for daily life, well tolerated, and less time-consuming. Preference was mainly related to a restoration of autonomy. Home-based self-administration reinforced the feeling of independence,” according to the investigators.
Available products
Six preparations of SCIg are commercially available. Most are in 10% concentration, as are all IVIg products. However, a 20% formulation of SCIg known as Hizentra allows for a smaller infusion volume and quicker completion of a treatment session. And one SCIg product, HyQvia, uses recombinant human hyaluronidase-facilitated 10% immune globulin, allowing home infusion of large volumes of sustained-release immune globulin on a once-monthly basis.
The relatively recent introduction of home SCIg for treatment of autoimmune inflammatory diseases, including DM, PM, and chronic inflammatory demyelinating polyneuropathy, has been pioneered mainly by European investigators. The treatment is often given by programmable mechanical pump once weekly. Italian investigators have reported efficacy in DM using 0.2 g/kg per week, which is about half the monthly total dose of IVIg employed. The infusion rate is 10-40 mL/hour, with a volume of around 35 mL per injection site.
Alternatively, SCIg can be delivered by rapid push infusions of smaller volumes with a syringe two or three times per week; that’s the regimen that was used at 2 g/kg over the course of a month by patients in the French focus group study, who didn’t mind the more frequent dosing.
“As they have had severe long-lasting symptoms, SCIg was perceived as a curative rather than a preventive therapy,” according to the French investigators.
More than 40% of patients experience adverse reactions to IVIg. These often involve headaches, nausea, back or abdominal pain, arthralgias, and/or difficulty breathing. Thromboembolic events and acute renal failure occur occasionally. For this reason, many physicians give a prophylactic dose of corticosteroids an hour before a patient’s first dose of IVIg. These systemic side effects are so rare with SCIg that Dr. Postolova has never pretreated with steroids, even though the main reason she resorts to the home therapy is a patient’s track record of poor tolerance of IVIg. The lower abdomen and thigh are the most commonly used subcutaneous infusion sites. Mild local infusion site reactions are fairly common.
Formulating IVIg and SCIg is a complex process that entails plasma procurement and pooling, fractionation, and purification. It takes 10,000-60,000 plasma donations to make one lot of IVIg. Donations are accepted only from repeated donors. Samples are held for 6 months and tested for infectious agents. However, efforts are underway to develop bioengineered recombinant immune globulin products that don’t require donated plasma. These products are being designed to capture and enhance the most important mechanisms of benefit of plasma-derived immunoglobulins using Fc fragments that target key receptors, rather than relying on full-length immune globulin. The goal is enhanced efficacy at much lower doses than with IVIg or SCIg.
Dr. Postolova reported having no financial conflicts regarding her presentation.
Home-based subcutaneous immune globulin therapy is a promising alternative to intravenous immune globulin therapy for patients with refractory dermatomyositis or polymyositis, Anna Postolova, MD, MPH, declared at the 2021 Rheumatology Winter Clinical Symposium.
“This is really exciting. I think in the years to come we may see a change to having our patients be able to do immune globulin therapy at home,” said Dr. Postolova, a rheumatologist and allergist/immunologist at Stanford (Calif.) Health Care.
“The technology is there. I think our patients might feel more comfortable getting immune globulin at home,” she said. “I would love to switch more patients from IVIg to SCIg [subcutaneous immune globulin] in my practice.”
A few caveats: SCIg remains off label for treatment of dermatomyositis (DM) or polymyositis (PM). Its approved indication is as replacement therapy in patients with primary or secondary immunodeficiency diseases. IVIg is approved for this indication, but is also approved for DM/PM refractory to high-dose corticosteroids and immunosuppressants. Yet SCIg is clearly effective for these autoimmune inflammatory diseases, albeit to date the supporting evidence comes chiefly from observational studies and anecdotal experience.
“I don’t know if insurers will cover it, but they should because it’s obviously a lot cheaper to do it at home,” she noted.
SCIg advantages
SCIg offers compelling advantages over IVIg in addition to its substantially lower cost. These include far fewer systemic side effects, shorter infusion time, greater bioavailability, and better quality of life. Patients self-administer SCIg at home, avoiding the inconvenience of IVIg therapy, which entails travel time for once-monthly hospitalization or long hours spent in an infusion center, she explained.
French investigators recently documented a previously unappreciated further advantage of home-based SCIg. They convened a focus group of patients with DM or PM experienced with both IVIg and home SCIg and determined that participants uniformly preferred home SCIg. The patients cited a new and welcome feeling of autonomy and control.
“All patients with experience of IVIg and SCIg expressed a clear preference for SCIg, which was described to be easy, less disruptive for daily life, well tolerated, and less time-consuming. Preference was mainly related to a restoration of autonomy. Home-based self-administration reinforced the feeling of independence,” according to the investigators.
Available products
Six preparations of SCIg are commercially available. Most are in 10% concentration, as are all IVIg products. However, a 20% formulation of SCIg known as Hizentra allows for a smaller infusion volume and quicker completion of a treatment session. And one SCIg product, HyQvia, uses recombinant human hyaluronidase-facilitated 10% immune globulin, allowing home infusion of large volumes of sustained-release immune globulin on a once-monthly basis.
The relatively recent introduction of home SCIg for treatment of autoimmune inflammatory diseases, including DM, PM, and chronic inflammatory demyelinating polyneuropathy, has been pioneered mainly by European investigators. The treatment is often given by programmable mechanical pump once weekly. Italian investigators have reported efficacy in DM using 0.2 g/kg per week, which is about half the monthly total dose of IVIg employed. The infusion rate is 10-40 mL/hour, with a volume of around 35 mL per injection site.
Alternatively, SCIg can be delivered by rapid push infusions of smaller volumes with a syringe two or three times per week; that’s the regimen that was used at 2 g/kg over the course of a month by patients in the French focus group study, who didn’t mind the more frequent dosing.
“As they have had severe long-lasting symptoms, SCIg was perceived as a curative rather than a preventive therapy,” according to the French investigators.
More than 40% of patients experience adverse reactions to IVIg. These often involve headaches, nausea, back or abdominal pain, arthralgias, and/or difficulty breathing. Thromboembolic events and acute renal failure occur occasionally. For this reason, many physicians give a prophylactic dose of corticosteroids an hour before a patient’s first dose of IVIg. These systemic side effects are so rare with SCIg that Dr. Postolova has never pretreated with steroids, even though the main reason she resorts to the home therapy is a patient’s track record of poor tolerance of IVIg. The lower abdomen and thigh are the most commonly used subcutaneous infusion sites. Mild local infusion site reactions are fairly common.
Formulating IVIg and SCIg is a complex process that entails plasma procurement and pooling, fractionation, and purification. It takes 10,000-60,000 plasma donations to make one lot of IVIg. Donations are accepted only from repeated donors. Samples are held for 6 months and tested for infectious agents. However, efforts are underway to develop bioengineered recombinant immune globulin products that don’t require donated plasma. These products are being designed to capture and enhance the most important mechanisms of benefit of plasma-derived immunoglobulins using Fc fragments that target key receptors, rather than relying on full-length immune globulin. The goal is enhanced efficacy at much lower doses than with IVIg or SCIg.
Dr. Postolova reported having no financial conflicts regarding her presentation.
Home-based subcutaneous immune globulin therapy is a promising alternative to intravenous immune globulin therapy for patients with refractory dermatomyositis or polymyositis, Anna Postolova, MD, MPH, declared at the 2021 Rheumatology Winter Clinical Symposium.
“This is really exciting. I think in the years to come we may see a change to having our patients be able to do immune globulin therapy at home,” said Dr. Postolova, a rheumatologist and allergist/immunologist at Stanford (Calif.) Health Care.
“The technology is there. I think our patients might feel more comfortable getting immune globulin at home,” she said. “I would love to switch more patients from IVIg to SCIg [subcutaneous immune globulin] in my practice.”
A few caveats: SCIg remains off label for treatment of dermatomyositis (DM) or polymyositis (PM). Its approved indication is as replacement therapy in patients with primary or secondary immunodeficiency diseases. IVIg is approved for this indication, but is also approved for DM/PM refractory to high-dose corticosteroids and immunosuppressants. Yet SCIg is clearly effective for these autoimmune inflammatory diseases, albeit to date the supporting evidence comes chiefly from observational studies and anecdotal experience.
“I don’t know if insurers will cover it, but they should because it’s obviously a lot cheaper to do it at home,” she noted.
SCIg advantages
SCIg offers compelling advantages over IVIg in addition to its substantially lower cost. These include far fewer systemic side effects, shorter infusion time, greater bioavailability, and better quality of life. Patients self-administer SCIg at home, avoiding the inconvenience of IVIg therapy, which entails travel time for once-monthly hospitalization or long hours spent in an infusion center, she explained.
French investigators recently documented a previously unappreciated further advantage of home-based SCIg. They convened a focus group of patients with DM or PM experienced with both IVIg and home SCIg and determined that participants uniformly preferred home SCIg. The patients cited a new and welcome feeling of autonomy and control.
“All patients with experience of IVIg and SCIg expressed a clear preference for SCIg, which was described to be easy, less disruptive for daily life, well tolerated, and less time-consuming. Preference was mainly related to a restoration of autonomy. Home-based self-administration reinforced the feeling of independence,” according to the investigators.
Available products
Six preparations of SCIg are commercially available. Most are in 10% concentration, as are all IVIg products. However, a 20% formulation of SCIg known as Hizentra allows for a smaller infusion volume and quicker completion of a treatment session. And one SCIg product, HyQvia, uses recombinant human hyaluronidase-facilitated 10% immune globulin, allowing home infusion of large volumes of sustained-release immune globulin on a once-monthly basis.
The relatively recent introduction of home SCIg for treatment of autoimmune inflammatory diseases, including DM, PM, and chronic inflammatory demyelinating polyneuropathy, has been pioneered mainly by European investigators. The treatment is often given by programmable mechanical pump once weekly. Italian investigators have reported efficacy in DM using 0.2 g/kg per week, which is about half the monthly total dose of IVIg employed. The infusion rate is 10-40 mL/hour, with a volume of around 35 mL per injection site.
Alternatively, SCIg can be delivered by rapid push infusions of smaller volumes with a syringe two or three times per week; that’s the regimen that was used at 2 g/kg over the course of a month by patients in the French focus group study, who didn’t mind the more frequent dosing.
“As they have had severe long-lasting symptoms, SCIg was perceived as a curative rather than a preventive therapy,” according to the French investigators.
More than 40% of patients experience adverse reactions to IVIg. These often involve headaches, nausea, back or abdominal pain, arthralgias, and/or difficulty breathing. Thromboembolic events and acute renal failure occur occasionally. For this reason, many physicians give a prophylactic dose of corticosteroids an hour before a patient’s first dose of IVIg. These systemic side effects are so rare with SCIg that Dr. Postolova has never pretreated with steroids, even though the main reason she resorts to the home therapy is a patient’s track record of poor tolerance of IVIg. The lower abdomen and thigh are the most commonly used subcutaneous infusion sites. Mild local infusion site reactions are fairly common.
Formulating IVIg and SCIg is a complex process that entails plasma procurement and pooling, fractionation, and purification. It takes 10,000-60,000 plasma donations to make one lot of IVIg. Donations are accepted only from repeated donors. Samples are held for 6 months and tested for infectious agents. However, efforts are underway to develop bioengineered recombinant immune globulin products that don’t require donated plasma. These products are being designed to capture and enhance the most important mechanisms of benefit of plasma-derived immunoglobulins using Fc fragments that target key receptors, rather than relying on full-length immune globulin. The goal is enhanced efficacy at much lower doses than with IVIg or SCIg.
Dr. Postolova reported having no financial conflicts regarding her presentation.
FROM RWCS 2021
Checkpoint inhibitors’ ‘big picture’ safety shown with preexisting autoimmune diseases
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
FROM ANNALS OF INTERNAL MEDICINE
Meta-analysis finds much less lupus than expected
The prevalence of systemic lupus erythematosus (SLE) appears to be much lower than previously believed and may pose “a potential risk to research funding for the disease,” according to results of a meta-analysis involving a network of population-based registries.
“When we started this study, a widely cited lupus statistic was that approximately 1.5 million Americans were affected. Our meta-analysis found the actual prevalence to be slightly more than 200,000: a number that approaches the [Food and Drug Administration’s] definition of a rare disease,” Emily Somers, PhD, ScM, senior author and associate professor of rheumatology and environmental health sciences at the University of Michigan, Ann Arbor, said in a written statement.
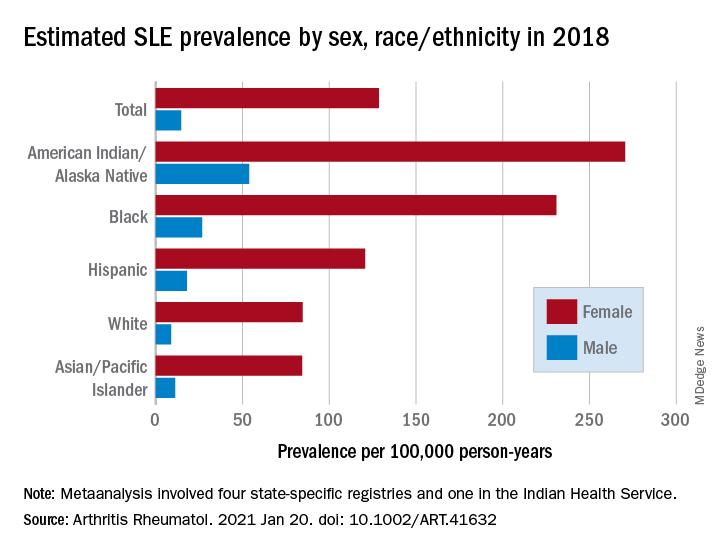
Their estimates, published online in Arthritis & Rheumatology, put the overall SLE prevalence in the United States at 72.8 per 100,000 person-years in 2018, with nearly nine times more females affected (128.7 cases per 100,000) than males (14.6 per 100,000). Race and ethnicity also play a role, as prevalence was highest among American Indian/Alaska Native and Black females, with Hispanic females lower but still higher than White and Asian/Pacific Islander females, Peter M. Izmirly, MD, MSc, of New York University, the lead author, and associates said.
SLE prevalence was distributed similarly in men, although there was a greater relative margin between American Indians/Alaska Natives (53.8 cases per 100,000 person-years) and Blacks (26.7 per 100,000), and Asians/Pacific Islanders were higher than Whites (11.2 vs. 8.9), the investigators reported.
The meta-analysis leveraged data from the Centers for Disease Control and Prevention’s national lupus registries, which include four state-specific SLE registries and a fifth in the Indian Health Service. All cases of SLE occurred in 2002-2009, and the data were age adjusted to the 2000 U.S. population and separately extrapolated to the 2018 U.S. Census population, they explained.
The analysis was funded by cooperative agreements between the New York City Department of Health and Mental Hygiene and New York University, and the CDC and National Institute of Health.
The prevalence of systemic lupus erythematosus (SLE) appears to be much lower than previously believed and may pose “a potential risk to research funding for the disease,” according to results of a meta-analysis involving a network of population-based registries.

“When we started this study, a widely cited lupus statistic was that approximately 1.5 million Americans were affected. Our meta-analysis found the actual prevalence to be slightly more than 200,000: a number that approaches the [Food and Drug Administration’s] definition of a rare disease,” Emily Somers, PhD, ScM, senior author and associate professor of rheumatology and environmental health sciences at the University of Michigan, Ann Arbor, said in a written statement.
Their estimates, published online in Arthritis & Rheumatology, put the overall SLE prevalence in the United States at 72.8 per 100,000 person-years in 2018, with nearly nine times more females affected (128.7 cases per 100,000) than males (14.6 per 100,000). Race and ethnicity also play a role, as prevalence was highest among American Indian/Alaska Native and Black females, with Hispanic females lower but still higher than White and Asian/Pacific Islander females, Peter M. Izmirly, MD, MSc, of New York University, the lead author, and associates said.
SLE prevalence was distributed similarly in men, although there was a greater relative margin between American Indians/Alaska Natives (53.8 cases per 100,000 person-years) and Blacks (26.7 per 100,000), and Asians/Pacific Islanders were higher than Whites (11.2 vs. 8.9), the investigators reported.
The meta-analysis leveraged data from the Centers for Disease Control and Prevention’s national lupus registries, which include four state-specific SLE registries and a fifth in the Indian Health Service. All cases of SLE occurred in 2002-2009, and the data were age adjusted to the 2000 U.S. population and separately extrapolated to the 2018 U.S. Census population, they explained.
The analysis was funded by cooperative agreements between the New York City Department of Health and Mental Hygiene and New York University, and the CDC and National Institute of Health.
The prevalence of systemic lupus erythematosus (SLE) appears to be much lower than previously believed and may pose “a potential risk to research funding for the disease,” according to results of a meta-analysis involving a network of population-based registries.

“When we started this study, a widely cited lupus statistic was that approximately 1.5 million Americans were affected. Our meta-analysis found the actual prevalence to be slightly more than 200,000: a number that approaches the [Food and Drug Administration’s] definition of a rare disease,” Emily Somers, PhD, ScM, senior author and associate professor of rheumatology and environmental health sciences at the University of Michigan, Ann Arbor, said in a written statement.
Their estimates, published online in Arthritis & Rheumatology, put the overall SLE prevalence in the United States at 72.8 per 100,000 person-years in 2018, with nearly nine times more females affected (128.7 cases per 100,000) than males (14.6 per 100,000). Race and ethnicity also play a role, as prevalence was highest among American Indian/Alaska Native and Black females, with Hispanic females lower but still higher than White and Asian/Pacific Islander females, Peter M. Izmirly, MD, MSc, of New York University, the lead author, and associates said.
SLE prevalence was distributed similarly in men, although there was a greater relative margin between American Indians/Alaska Natives (53.8 cases per 100,000 person-years) and Blacks (26.7 per 100,000), and Asians/Pacific Islanders were higher than Whites (11.2 vs. 8.9), the investigators reported.
The meta-analysis leveraged data from the Centers for Disease Control and Prevention’s national lupus registries, which include four state-specific SLE registries and a fifth in the Indian Health Service. All cases of SLE occurred in 2002-2009, and the data were age adjusted to the 2000 U.S. population and separately extrapolated to the 2018 U.S. Census population, they explained.
The analysis was funded by cooperative agreements between the New York City Department of Health and Mental Hygiene and New York University, and the CDC and National Institute of Health.
FROM ARTHRITIS & RHEUMATOLOGY
Severe renal arteriosclerosis may indicate cardiovascular risk in lupus nephritis
Severe renal arteriosclerosis was associated with a ninefold increased risk of atherosclerotic cardiovascular disease in patients with lupus nephritis, based on data from an observational study of 189 individuals.
Atherosclerotic cardiovascular disease (ASCVD) has traditionally been thought to be a late complication of systemic lupus erythematosus (SLE), but this has been challenged in recent population-based studies of patients with SLE and lupus nephritis (LN) that indicated an early and increased risk of ASCVD at the time of diagnosis. However, it is unclear which early risk factors may predispose patients to ASCVD, Shivani Garg, MD, of the University of Wisconsin, Madison, and colleagues wrote in a study published in Arthritis Care & Research.
In patients with IgA nephropathy and renal transplantation, previous studies have shown that severe renal arteriosclerosis (r-ASCL) based on kidney biopsies at the time of diagnosis predicts ASCVD, but “a few studies including LN biopsies failed to report a similar association between the presence of severe r-ASCL and ASCVD occurrence,” possibly because of underreporting of r-ASCL. Dr. Garg and colleagues also noted the problem of underreporting of r-ASCL in their own previous study of its prevalence in LN patients at the time of diagnosis.
To get a more detailed view of how r-ASCL may be linked to early occurrence of ASCVD in LN patients, Dr. Garg and coauthors identified 189 consecutive patients with incident LN who underwent diagnostic biopsies between 1994 and 2017. The median age of the patients was 25 years, 78% were women, and 73% were white. The researchers developed a composite score for r-ASCL severity based on reported and overread biopsies.
Overall, 31% of the patients had any reported r-ASCL, and 7% had moderate-severe r-ASCL. After incorporating systematically reexamined r-ASCL grades, the prevalence of any and moderate-severe r-ASCL increased to 39% and 12%, respectively.
Based on their composite of reported and overread r-ASCL grade, severe r-ASCL in diagnostic LN biopsies was associated with a ninefold increased risk of ASCVD.
The researchers identified 22 incident ASCVD events over an 11-year follow-up for an overall 12% incidence of ASCVD in LN. ASCVD was defined as ischemic heart disease (including myocardial infarction, coronary artery revascularization, abnormal stress test, abnormal angiogram, and events documented by a cardiologist); stroke and transient ischemic attack (TIA); and peripheral vascular disease. Incident ASCVD was defined as the first ASCVD event between 1 and 10 years after LN diagnosis.
The most common ASCVD events were stroke or TIA (12 patients), events related to ischemic heart disease (7 patients), and events related to peripheral vascular disease (3 patients).
Lack of statin use
The researchers also hypothesized that the presence of gaps in statin use among eligible LN patients would be present in their study population. “Among the 20 patients with incident ASCVD events after LN diagnosis in our cohort, none was on statin therapy at the time of LN diagnosis,” the researchers said, noting that current guidelines from the American College of Rheumatology and the European League Against Rheumatism (now known as the European Alliance of Associations for Rheumatology) recommend initiating statin therapy at the time of LN diagnosis in all patients who have hyperlipidemia and chronic kidney disease (CKD) stage ≥3. “Further, 11 patients (55%) met high-risk criteria (hyperlipidemia and CKD stage ≥3) to implement statin therapy at the time of LN diagnosis, yet only one patient (9%) was initiated on statin therapy.” In addition, patients with stage 3 or higher CKD were more likely to develop ASCVD than patients without stage 3 or higher CKD, they said.
The study findings were limited by several factors including the majority white study population, the ability to overread only 25% of the biopsies, and the lack of data on the potential role of chronic lesions in ASCVD, the researchers noted. However, the results were strengthened by the use of a validated LN cohort, and the data provide “the basis to establish severe composite r-ASCL as a predictor of ASCVD events using a larger sample size in different cohorts,” they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Severe renal arteriosclerosis was associated with a ninefold increased risk of atherosclerotic cardiovascular disease in patients with lupus nephritis, based on data from an observational study of 189 individuals.
Atherosclerotic cardiovascular disease (ASCVD) has traditionally been thought to be a late complication of systemic lupus erythematosus (SLE), but this has been challenged in recent population-based studies of patients with SLE and lupus nephritis (LN) that indicated an early and increased risk of ASCVD at the time of diagnosis. However, it is unclear which early risk factors may predispose patients to ASCVD, Shivani Garg, MD, of the University of Wisconsin, Madison, and colleagues wrote in a study published in Arthritis Care & Research.
In patients with IgA nephropathy and renal transplantation, previous studies have shown that severe renal arteriosclerosis (r-ASCL) based on kidney biopsies at the time of diagnosis predicts ASCVD, but “a few studies including LN biopsies failed to report a similar association between the presence of severe r-ASCL and ASCVD occurrence,” possibly because of underreporting of r-ASCL. Dr. Garg and colleagues also noted the problem of underreporting of r-ASCL in their own previous study of its prevalence in LN patients at the time of diagnosis.
To get a more detailed view of how r-ASCL may be linked to early occurrence of ASCVD in LN patients, Dr. Garg and coauthors identified 189 consecutive patients with incident LN who underwent diagnostic biopsies between 1994 and 2017. The median age of the patients was 25 years, 78% were women, and 73% were white. The researchers developed a composite score for r-ASCL severity based on reported and overread biopsies.
Overall, 31% of the patients had any reported r-ASCL, and 7% had moderate-severe r-ASCL. After incorporating systematically reexamined r-ASCL grades, the prevalence of any and moderate-severe r-ASCL increased to 39% and 12%, respectively.
Based on their composite of reported and overread r-ASCL grade, severe r-ASCL in diagnostic LN biopsies was associated with a ninefold increased risk of ASCVD.
The researchers identified 22 incident ASCVD events over an 11-year follow-up for an overall 12% incidence of ASCVD in LN. ASCVD was defined as ischemic heart disease (including myocardial infarction, coronary artery revascularization, abnormal stress test, abnormal angiogram, and events documented by a cardiologist); stroke and transient ischemic attack (TIA); and peripheral vascular disease. Incident ASCVD was defined as the first ASCVD event between 1 and 10 years after LN diagnosis.
The most common ASCVD events were stroke or TIA (12 patients), events related to ischemic heart disease (7 patients), and events related to peripheral vascular disease (3 patients).
Lack of statin use
The researchers also hypothesized that the presence of gaps in statin use among eligible LN patients would be present in their study population. “Among the 20 patients with incident ASCVD events after LN diagnosis in our cohort, none was on statin therapy at the time of LN diagnosis,” the researchers said, noting that current guidelines from the American College of Rheumatology and the European League Against Rheumatism (now known as the European Alliance of Associations for Rheumatology) recommend initiating statin therapy at the time of LN diagnosis in all patients who have hyperlipidemia and chronic kidney disease (CKD) stage ≥3. “Further, 11 patients (55%) met high-risk criteria (hyperlipidemia and CKD stage ≥3) to implement statin therapy at the time of LN diagnosis, yet only one patient (9%) was initiated on statin therapy.” In addition, patients with stage 3 or higher CKD were more likely to develop ASCVD than patients without stage 3 or higher CKD, they said.
The study findings were limited by several factors including the majority white study population, the ability to overread only 25% of the biopsies, and the lack of data on the potential role of chronic lesions in ASCVD, the researchers noted. However, the results were strengthened by the use of a validated LN cohort, and the data provide “the basis to establish severe composite r-ASCL as a predictor of ASCVD events using a larger sample size in different cohorts,” they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Severe renal arteriosclerosis was associated with a ninefold increased risk of atherosclerotic cardiovascular disease in patients with lupus nephritis, based on data from an observational study of 189 individuals.
Atherosclerotic cardiovascular disease (ASCVD) has traditionally been thought to be a late complication of systemic lupus erythematosus (SLE), but this has been challenged in recent population-based studies of patients with SLE and lupus nephritis (LN) that indicated an early and increased risk of ASCVD at the time of diagnosis. However, it is unclear which early risk factors may predispose patients to ASCVD, Shivani Garg, MD, of the University of Wisconsin, Madison, and colleagues wrote in a study published in Arthritis Care & Research.
In patients with IgA nephropathy and renal transplantation, previous studies have shown that severe renal arteriosclerosis (r-ASCL) based on kidney biopsies at the time of diagnosis predicts ASCVD, but “a few studies including LN biopsies failed to report a similar association between the presence of severe r-ASCL and ASCVD occurrence,” possibly because of underreporting of r-ASCL. Dr. Garg and colleagues also noted the problem of underreporting of r-ASCL in their own previous study of its prevalence in LN patients at the time of diagnosis.
To get a more detailed view of how r-ASCL may be linked to early occurrence of ASCVD in LN patients, Dr. Garg and coauthors identified 189 consecutive patients with incident LN who underwent diagnostic biopsies between 1994 and 2017. The median age of the patients was 25 years, 78% were women, and 73% were white. The researchers developed a composite score for r-ASCL severity based on reported and overread biopsies.
Overall, 31% of the patients had any reported r-ASCL, and 7% had moderate-severe r-ASCL. After incorporating systematically reexamined r-ASCL grades, the prevalence of any and moderate-severe r-ASCL increased to 39% and 12%, respectively.
Based on their composite of reported and overread r-ASCL grade, severe r-ASCL in diagnostic LN biopsies was associated with a ninefold increased risk of ASCVD.
The researchers identified 22 incident ASCVD events over an 11-year follow-up for an overall 12% incidence of ASCVD in LN. ASCVD was defined as ischemic heart disease (including myocardial infarction, coronary artery revascularization, abnormal stress test, abnormal angiogram, and events documented by a cardiologist); stroke and transient ischemic attack (TIA); and peripheral vascular disease. Incident ASCVD was defined as the first ASCVD event between 1 and 10 years after LN diagnosis.
The most common ASCVD events were stroke or TIA (12 patients), events related to ischemic heart disease (7 patients), and events related to peripheral vascular disease (3 patients).
Lack of statin use
The researchers also hypothesized that the presence of gaps in statin use among eligible LN patients would be present in their study population. “Among the 20 patients with incident ASCVD events after LN diagnosis in our cohort, none was on statin therapy at the time of LN diagnosis,” the researchers said, noting that current guidelines from the American College of Rheumatology and the European League Against Rheumatism (now known as the European Alliance of Associations for Rheumatology) recommend initiating statin therapy at the time of LN diagnosis in all patients who have hyperlipidemia and chronic kidney disease (CKD) stage ≥3. “Further, 11 patients (55%) met high-risk criteria (hyperlipidemia and CKD stage ≥3) to implement statin therapy at the time of LN diagnosis, yet only one patient (9%) was initiated on statin therapy.” In addition, patients with stage 3 or higher CKD were more likely to develop ASCVD than patients without stage 3 or higher CKD, they said.
The study findings were limited by several factors including the majority white study population, the ability to overread only 25% of the biopsies, and the lack of data on the potential role of chronic lesions in ASCVD, the researchers noted. However, the results were strengthened by the use of a validated LN cohort, and the data provide “the basis to establish severe composite r-ASCL as a predictor of ASCVD events using a larger sample size in different cohorts,” they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM ARTHRITIS CARE & RESEARCH
High hydroxychloroquine blood level may lower thrombosis risk in lupus
Maintaining an average hydroxychloroquine whole blood level above 1,068 ng/mL significantly reduced the risk of thrombosis in adults with systemic lupus erythematosus, based on data from 739 patients.
Hydroxychloroquine (HCQ) is a common treatment for systemic lupus erythematosus (SLE); studies suggest that it may protect against thrombosis, but the optimal dosing for this purpose remains unknown, wrote Michelle Petri, MD, of Johns Hopkins University, Baltimore, and colleagues. In a study published in Arthritis & Rheumatology, the researchers examined data on HCQ levels from 739 adults with SLE who were part of the Hopkins Lupus Cohort, a longitudinal study of outcomes in SLE patients. Of these, 38 (5.1%) developed thrombosis during 2,330 person-years of follow-up.
Overall, the average HCQ blood level was significantly lower in patients who experienced thrombosis, compared to those who did not (720 ng/mL vs. 935 ng/mL; P = .025). “Prescribed hydroxychloroquine doses did not predict hydroxychloroquine blood levels,” the researchers noted.
In addition, Dr. Petri and associates found a dose-response relationship in which the thrombosis rate declined approximately 13% for every 200-ng/mL increase in the mean HCQ blood level measurement and for the most recent HCQ blood level measurement after controlling for factors that included age, ethnicity, lupus anticoagulant, low C3, and hypertension.
In a multivariate analysis, thrombotic events decreased by 69% in patients with mean HCQ blood levels greater than 1,068 ng/mL, compared to those with average HCQ blood levels less than 648 ng/mL.
The average age of the patients at the time HCQ measurements began was 43 years, 93% were female, and 46% were White. Patients visited a clinic every 3 months, and HCQ levels were determined by liquid chromatography-tandem mass spectrometry.
“Between-person and within-person correlation coefficients were used to measure the strength of the linear association between HCQ blood levels and commonly prescribed HCQ doses from 4.5 to 6.5 mg/kg,” the researchers said.
Higher doses of HCQ have been associated with increased risk for retinopathy, and current guidelines recommend using less than 5 mg/kg of ideal body weight, the researchers said. “Importantly, there was no correlation between the prescribed dose and the hydroxychloroquine blood level over the range (4.5 to 6.5 mg/kg) used in clinical practice, highlighting the need for personalized hydroxychloroquine drug level-guided therapy and dose adjustment,” they emphasized.
The study findings were limited by several factors, including the observational design and potential confounding from variables not included in the model, as well as the small sample size, single site, and single rheumatologist involved in the study, the researchers noted.
The results suggest that aiming for a blood HCQ level of 1,068 ng/mL can be done safely to help prevent thrombosis in patients with SLE, the researchers said. “Routine clinical integration of hydroxychloroquine blood level measurement offers an opportunity for personalized drug dosing and risk management beyond rigid empirical dosing recommendations in patients with SLE,” they concluded.
The study was supported in part by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no relevant financial conflicts to disclose.
SOURCE: Petri M et al. Arthritis Rheumatol. 2021 Jan 6. doi: 10.1002/ART.41621.
Maintaining an average hydroxychloroquine whole blood level above 1,068 ng/mL significantly reduced the risk of thrombosis in adults with systemic lupus erythematosus, based on data from 739 patients.
Hydroxychloroquine (HCQ) is a common treatment for systemic lupus erythematosus (SLE); studies suggest that it may protect against thrombosis, but the optimal dosing for this purpose remains unknown, wrote Michelle Petri, MD, of Johns Hopkins University, Baltimore, and colleagues. In a study published in Arthritis & Rheumatology, the researchers examined data on HCQ levels from 739 adults with SLE who were part of the Hopkins Lupus Cohort, a longitudinal study of outcomes in SLE patients. Of these, 38 (5.1%) developed thrombosis during 2,330 person-years of follow-up.
Overall, the average HCQ blood level was significantly lower in patients who experienced thrombosis, compared to those who did not (720 ng/mL vs. 935 ng/mL; P = .025). “Prescribed hydroxychloroquine doses did not predict hydroxychloroquine blood levels,” the researchers noted.
In addition, Dr. Petri and associates found a dose-response relationship in which the thrombosis rate declined approximately 13% for every 200-ng/mL increase in the mean HCQ blood level measurement and for the most recent HCQ blood level measurement after controlling for factors that included age, ethnicity, lupus anticoagulant, low C3, and hypertension.
In a multivariate analysis, thrombotic events decreased by 69% in patients with mean HCQ blood levels greater than 1,068 ng/mL, compared to those with average HCQ blood levels less than 648 ng/mL.
The average age of the patients at the time HCQ measurements began was 43 years, 93% were female, and 46% were White. Patients visited a clinic every 3 months, and HCQ levels were determined by liquid chromatography-tandem mass spectrometry.
“Between-person and within-person correlation coefficients were used to measure the strength of the linear association between HCQ blood levels and commonly prescribed HCQ doses from 4.5 to 6.5 mg/kg,” the researchers said.
Higher doses of HCQ have been associated with increased risk for retinopathy, and current guidelines recommend using less than 5 mg/kg of ideal body weight, the researchers said. “Importantly, there was no correlation between the prescribed dose and the hydroxychloroquine blood level over the range (4.5 to 6.5 mg/kg) used in clinical practice, highlighting the need for personalized hydroxychloroquine drug level-guided therapy and dose adjustment,” they emphasized.
The study findings were limited by several factors, including the observational design and potential confounding from variables not included in the model, as well as the small sample size, single site, and single rheumatologist involved in the study, the researchers noted.
The results suggest that aiming for a blood HCQ level of 1,068 ng/mL can be done safely to help prevent thrombosis in patients with SLE, the researchers said. “Routine clinical integration of hydroxychloroquine blood level measurement offers an opportunity for personalized drug dosing and risk management beyond rigid empirical dosing recommendations in patients with SLE,” they concluded.
The study was supported in part by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no relevant financial conflicts to disclose.
SOURCE: Petri M et al. Arthritis Rheumatol. 2021 Jan 6. doi: 10.1002/ART.41621.
Maintaining an average hydroxychloroquine whole blood level above 1,068 ng/mL significantly reduced the risk of thrombosis in adults with systemic lupus erythematosus, based on data from 739 patients.
Hydroxychloroquine (HCQ) is a common treatment for systemic lupus erythematosus (SLE); studies suggest that it may protect against thrombosis, but the optimal dosing for this purpose remains unknown, wrote Michelle Petri, MD, of Johns Hopkins University, Baltimore, and colleagues. In a study published in Arthritis & Rheumatology, the researchers examined data on HCQ levels from 739 adults with SLE who were part of the Hopkins Lupus Cohort, a longitudinal study of outcomes in SLE patients. Of these, 38 (5.1%) developed thrombosis during 2,330 person-years of follow-up.
Overall, the average HCQ blood level was significantly lower in patients who experienced thrombosis, compared to those who did not (720 ng/mL vs. 935 ng/mL; P = .025). “Prescribed hydroxychloroquine doses did not predict hydroxychloroquine blood levels,” the researchers noted.
In addition, Dr. Petri and associates found a dose-response relationship in which the thrombosis rate declined approximately 13% for every 200-ng/mL increase in the mean HCQ blood level measurement and for the most recent HCQ blood level measurement after controlling for factors that included age, ethnicity, lupus anticoagulant, low C3, and hypertension.
In a multivariate analysis, thrombotic events decreased by 69% in patients with mean HCQ blood levels greater than 1,068 ng/mL, compared to those with average HCQ blood levels less than 648 ng/mL.
The average age of the patients at the time HCQ measurements began was 43 years, 93% were female, and 46% were White. Patients visited a clinic every 3 months, and HCQ levels were determined by liquid chromatography-tandem mass spectrometry.
“Between-person and within-person correlation coefficients were used to measure the strength of the linear association between HCQ blood levels and commonly prescribed HCQ doses from 4.5 to 6.5 mg/kg,” the researchers said.
Higher doses of HCQ have been associated with increased risk for retinopathy, and current guidelines recommend using less than 5 mg/kg of ideal body weight, the researchers said. “Importantly, there was no correlation between the prescribed dose and the hydroxychloroquine blood level over the range (4.5 to 6.5 mg/kg) used in clinical practice, highlighting the need for personalized hydroxychloroquine drug level-guided therapy and dose adjustment,” they emphasized.
The study findings were limited by several factors, including the observational design and potential confounding from variables not included in the model, as well as the small sample size, single site, and single rheumatologist involved in the study, the researchers noted.
The results suggest that aiming for a blood HCQ level of 1,068 ng/mL can be done safely to help prevent thrombosis in patients with SLE, the researchers said. “Routine clinical integration of hydroxychloroquine blood level measurement offers an opportunity for personalized drug dosing and risk management beyond rigid empirical dosing recommendations in patients with SLE,” they concluded.
The study was supported in part by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no relevant financial conflicts to disclose.
SOURCE: Petri M et al. Arthritis Rheumatol. 2021 Jan 6. doi: 10.1002/ART.41621.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Higher blood levels of hydroxychloroquine (HCQ) were protective against thrombosis in adults with systemic lupus erythematosus (SLE).
Major finding: The average HCQ in SLE patients who developed thrombosis was 720 ng/mL, compared to 935 ng/mL in those without thrombosis (P = .025).
Study details: The data come from an observational study of 739 adults with SLE; 5.1% developed thrombosis during the study period.
Disclosures: The study was supported in part by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no relevant financial conflicts to disclose.
Source: Petri M et al. Arthritis Rheumatol. 2021 Jan 6. doi: 10.1002/ART.41621.
Swedish registry study finds atopic dermatitis significantly associated with autoimmune diseases
in a case control study derived from Swedish national health care registry data.
Atopic dermatitis (AD) is known to be associated with other atopic conditions, and there is increasing evidence it is associated with some nonatopic conditions, including some cancers, cardiovascular disease, and neuropsychiatric disorders, according to Lina U. Ivert, MD, of the dermatology and venereology unit at the Karolinska Institutet, Stockholm, and coauthors. There are also some data indicating that autoimmune diseases, particularly those involving the skin and gastrointestinal tract, are more common in people with AD.
The aim of their study, published in the British Journal of Dermatology, was to investigate a wide spectrum of autoimmune diseases for associations with AD in a large-scale, population-based study using Swedish registers. Findings could lead to better monitoring of comorbidities and deeper understanding of disease burden and AD pathophysiology, they noted.
Large-scale study
With data from the Swedish Board of Health and Welfare’s National Patient Register on inpatient diagnoses since 1964 and specialist outpatient visits since 2001, the investigators included all patients aged 15 years and older with AD diagnoses (104,832) and matched them with controls from the general population (1,022,435). The authors noted that the large number of people included in the analysis allowed for robust estimates, and underscored that 80% of the AD patients included had received their diagnosis in a dermatology department, which reduces the risk of misclassification.
Association with autoimmune disease
The investigators found an association between AD and autoimmune disease, with an adjusted odds ratio) of 1.97 (95% confidence interval, 1.93-2.01). The association was present with several organ systems, particularly the skin and gastrointestinal tract, and with connective tissue diseases. The strongest associations with autoimmune skin diseases were found for dermatitis herpetiformis (aOR, 9.76; 95% CI, 8.10-11.8), alopecia areata (aOR, 5.11; 95% CI, 4.75-5.49), and chronic urticaria (aOR, 4.82; 95% CI, 4.48-5.19).
AD was associated with gastrointestinal diseases, including celiac disease (aOR, 1.96; 95% CI, 1.84-2.09), Crohn disease (aOR 1.83; CI, 1.71-1.96), and ulcerative colitis (aOR 1.58; 95% CI, 1.49-1.68).
Connective tissue diseases significantly associated with AD included systemic lupus erythematosus (aOR, 1.65; 95% CI, 1.42-1.90), ankylosing spondylitis (aOR, 1.46; 95% CI, 1.29-1.66), and RA (aOR, 1.44; 95% CI,1.34-1.54]). Hematologic or hepatic autoimmune disease associations with AD were not observed.
Stronger association with multiple diseases
The association between AD and two or more autoimmune diseases was significantly stronger than the association between AD and having one autoimmune disease. For example, the OR for AD among people with three to five autoimmune diseases was 3.33 (95% CI, 2.86-3.87), and was stronger in men (OR, 3.96; 95% CI, 2.92-5.37) than in women (OR, 3.14; 95% CI, 2.63-3.74).
Sex differences
In the study overall, the association with AD and autoimmune diseases was stronger in men (aOR, 2.18; 95% CI, 2.10-2.25), compared with women (aOR, 1.89; 95% CI, 1.85-1.93), but this “sex difference was only statistically significant between AD and RA and between AD and Celiac disease,” they noted.
Associations between AD and dermatomyositis, systemic scleroderma, systemic lupus erythematosus, Hashimoto’s disease, Graves disease, multiple sclerosis, and polymyalgia rheumatica were found only in women. Dr. Ivert and coauthors observed that “women are in general more likely to develop autoimmune diseases, and 80% of patients with autoimmune diseases are women.”
Provocative questions
Commenting on the findings, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said, “At a high level, it is important for clinicians to recognize that atopic dermatitis is a systemic immune-mediated disease. AD is associated with higher rates of comorbid autoimmune disease, similar to psoriasis and other chronic inflammatory skin diseases.”
“At this point, there is nothing immediately actionable about these results,” noted Dr. Silverberg, who was not an author of this study. “That said, in my mind, they raise some provocative questions: What is the difference between AD in adults who do versus those who do not get comorbid autoimmune disease? Does AD then present differently? Does it respond to the same therapies? These will have to be the subject of future research.”
The study was funded by the Swedish Asthma and Allergy Association Research Foundation, Hudfonden (the Welander-Finsen Foundation), and the Swedish Society for Dermatology and Venereology. The authors disclosed no conflicts of interest.
SOURCE: Ivert LU et al. Br J Dermatol. 2020 Oct 22. doi: 10.1111/bjd.19624.
in a case control study derived from Swedish national health care registry data.
Atopic dermatitis (AD) is known to be associated with other atopic conditions, and there is increasing evidence it is associated with some nonatopic conditions, including some cancers, cardiovascular disease, and neuropsychiatric disorders, according to Lina U. Ivert, MD, of the dermatology and venereology unit at the Karolinska Institutet, Stockholm, and coauthors. There are also some data indicating that autoimmune diseases, particularly those involving the skin and gastrointestinal tract, are more common in people with AD.
The aim of their study, published in the British Journal of Dermatology, was to investigate a wide spectrum of autoimmune diseases for associations with AD in a large-scale, population-based study using Swedish registers. Findings could lead to better monitoring of comorbidities and deeper understanding of disease burden and AD pathophysiology, they noted.
Large-scale study
With data from the Swedish Board of Health and Welfare’s National Patient Register on inpatient diagnoses since 1964 and specialist outpatient visits since 2001, the investigators included all patients aged 15 years and older with AD diagnoses (104,832) and matched them with controls from the general population (1,022,435). The authors noted that the large number of people included in the analysis allowed for robust estimates, and underscored that 80% of the AD patients included had received their diagnosis in a dermatology department, which reduces the risk of misclassification.
Association with autoimmune disease
The investigators found an association between AD and autoimmune disease, with an adjusted odds ratio) of 1.97 (95% confidence interval, 1.93-2.01). The association was present with several organ systems, particularly the skin and gastrointestinal tract, and with connective tissue diseases. The strongest associations with autoimmune skin diseases were found for dermatitis herpetiformis (aOR, 9.76; 95% CI, 8.10-11.8), alopecia areata (aOR, 5.11; 95% CI, 4.75-5.49), and chronic urticaria (aOR, 4.82; 95% CI, 4.48-5.19).
AD was associated with gastrointestinal diseases, including celiac disease (aOR, 1.96; 95% CI, 1.84-2.09), Crohn disease (aOR 1.83; CI, 1.71-1.96), and ulcerative colitis (aOR 1.58; 95% CI, 1.49-1.68).
Connective tissue diseases significantly associated with AD included systemic lupus erythematosus (aOR, 1.65; 95% CI, 1.42-1.90), ankylosing spondylitis (aOR, 1.46; 95% CI, 1.29-1.66), and RA (aOR, 1.44; 95% CI,1.34-1.54]). Hematologic or hepatic autoimmune disease associations with AD were not observed.
Stronger association with multiple diseases
The association between AD and two or more autoimmune diseases was significantly stronger than the association between AD and having one autoimmune disease. For example, the OR for AD among people with three to five autoimmune diseases was 3.33 (95% CI, 2.86-3.87), and was stronger in men (OR, 3.96; 95% CI, 2.92-5.37) than in women (OR, 3.14; 95% CI, 2.63-3.74).
Sex differences
In the study overall, the association with AD and autoimmune diseases was stronger in men (aOR, 2.18; 95% CI, 2.10-2.25), compared with women (aOR, 1.89; 95% CI, 1.85-1.93), but this “sex difference was only statistically significant between AD and RA and between AD and Celiac disease,” they noted.
Associations between AD and dermatomyositis, systemic scleroderma, systemic lupus erythematosus, Hashimoto’s disease, Graves disease, multiple sclerosis, and polymyalgia rheumatica were found only in women. Dr. Ivert and coauthors observed that “women are in general more likely to develop autoimmune diseases, and 80% of patients with autoimmune diseases are women.”
Provocative questions
Commenting on the findings, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said, “At a high level, it is important for clinicians to recognize that atopic dermatitis is a systemic immune-mediated disease. AD is associated with higher rates of comorbid autoimmune disease, similar to psoriasis and other chronic inflammatory skin diseases.”
“At this point, there is nothing immediately actionable about these results,” noted Dr. Silverberg, who was not an author of this study. “That said, in my mind, they raise some provocative questions: What is the difference between AD in adults who do versus those who do not get comorbid autoimmune disease? Does AD then present differently? Does it respond to the same therapies? These will have to be the subject of future research.”
The study was funded by the Swedish Asthma and Allergy Association Research Foundation, Hudfonden (the Welander-Finsen Foundation), and the Swedish Society for Dermatology and Venereology. The authors disclosed no conflicts of interest.
SOURCE: Ivert LU et al. Br J Dermatol. 2020 Oct 22. doi: 10.1111/bjd.19624.
in a case control study derived from Swedish national health care registry data.
Atopic dermatitis (AD) is known to be associated with other atopic conditions, and there is increasing evidence it is associated with some nonatopic conditions, including some cancers, cardiovascular disease, and neuropsychiatric disorders, according to Lina U. Ivert, MD, of the dermatology and venereology unit at the Karolinska Institutet, Stockholm, and coauthors. There are also some data indicating that autoimmune diseases, particularly those involving the skin and gastrointestinal tract, are more common in people with AD.
The aim of their study, published in the British Journal of Dermatology, was to investigate a wide spectrum of autoimmune diseases for associations with AD in a large-scale, population-based study using Swedish registers. Findings could lead to better monitoring of comorbidities and deeper understanding of disease burden and AD pathophysiology, they noted.
Large-scale study
With data from the Swedish Board of Health and Welfare’s National Patient Register on inpatient diagnoses since 1964 and specialist outpatient visits since 2001, the investigators included all patients aged 15 years and older with AD diagnoses (104,832) and matched them with controls from the general population (1,022,435). The authors noted that the large number of people included in the analysis allowed for robust estimates, and underscored that 80% of the AD patients included had received their diagnosis in a dermatology department, which reduces the risk of misclassification.
Association with autoimmune disease
The investigators found an association between AD and autoimmune disease, with an adjusted odds ratio) of 1.97 (95% confidence interval, 1.93-2.01). The association was present with several organ systems, particularly the skin and gastrointestinal tract, and with connective tissue diseases. The strongest associations with autoimmune skin diseases were found for dermatitis herpetiformis (aOR, 9.76; 95% CI, 8.10-11.8), alopecia areata (aOR, 5.11; 95% CI, 4.75-5.49), and chronic urticaria (aOR, 4.82; 95% CI, 4.48-5.19).
AD was associated with gastrointestinal diseases, including celiac disease (aOR, 1.96; 95% CI, 1.84-2.09), Crohn disease (aOR 1.83; CI, 1.71-1.96), and ulcerative colitis (aOR 1.58; 95% CI, 1.49-1.68).
Connective tissue diseases significantly associated with AD included systemic lupus erythematosus (aOR, 1.65; 95% CI, 1.42-1.90), ankylosing spondylitis (aOR, 1.46; 95% CI, 1.29-1.66), and RA (aOR, 1.44; 95% CI,1.34-1.54]). Hematologic or hepatic autoimmune disease associations with AD were not observed.
Stronger association with multiple diseases
The association between AD and two or more autoimmune diseases was significantly stronger than the association between AD and having one autoimmune disease. For example, the OR for AD among people with three to five autoimmune diseases was 3.33 (95% CI, 2.86-3.87), and was stronger in men (OR, 3.96; 95% CI, 2.92-5.37) than in women (OR, 3.14; 95% CI, 2.63-3.74).
Sex differences
In the study overall, the association with AD and autoimmune diseases was stronger in men (aOR, 2.18; 95% CI, 2.10-2.25), compared with women (aOR, 1.89; 95% CI, 1.85-1.93), but this “sex difference was only statistically significant between AD and RA and between AD and Celiac disease,” they noted.
Associations between AD and dermatomyositis, systemic scleroderma, systemic lupus erythematosus, Hashimoto’s disease, Graves disease, multiple sclerosis, and polymyalgia rheumatica were found only in women. Dr. Ivert and coauthors observed that “women are in general more likely to develop autoimmune diseases, and 80% of patients with autoimmune diseases are women.”
Provocative questions
Commenting on the findings, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said, “At a high level, it is important for clinicians to recognize that atopic dermatitis is a systemic immune-mediated disease. AD is associated with higher rates of comorbid autoimmune disease, similar to psoriasis and other chronic inflammatory skin diseases.”
“At this point, there is nothing immediately actionable about these results,” noted Dr. Silverberg, who was not an author of this study. “That said, in my mind, they raise some provocative questions: What is the difference between AD in adults who do versus those who do not get comorbid autoimmune disease? Does AD then present differently? Does it respond to the same therapies? These will have to be the subject of future research.”
The study was funded by the Swedish Asthma and Allergy Association Research Foundation, Hudfonden (the Welander-Finsen Foundation), and the Swedish Society for Dermatology and Venereology. The authors disclosed no conflicts of interest.
SOURCE: Ivert LU et al. Br J Dermatol. 2020 Oct 22. doi: 10.1111/bjd.19624.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Slow taper off antimalarial is best to avoid lupus flare during remission
Slowly tapering off – or remaining on – antimalarial medications can help prevent disease flare in patients with systemic lupus erythematosus (SLE) who’ve achieved clinical remission for at least a year, according to a new study that was presented at the virtual annual meeting of the American College of Rheumatology.
“Except in the setting of toxicity, cessation of antimalarial medication in patients with disease quiescence is feasible using a slow taper,” lead author Danaë Papachristos, MBBS, said during an oral abstract presentation at the online meeting. Dr. Papachristos conducted the research while a clinical and research fellow at the University of Toronto’s lupus clinic, but is now a consultant rheumatologist at the Wesley Hospital in Brisbane, Queensland, Australia.
To investigate flare in patients with SLE who were on or recently off antimalarial medications (AMs), the researchers identified 1,573 potential participants from a long-term observational cohort study at the university’s lupus clinic. From that larger group, 88 cases – patients who achieved clinical remission for at least a year and stopped taking AMs – were matched to at least one control – patients who also achieved remission and continued on medication. Most cases were also matched to a second control, bringing the total number to 173. All patients had at least 2 years of follow-up.
Flare was defined as any increase in the SLEDAI-2K score, with major flare defined as an increase of 4 or more. Patients in the case group were roughly 44 years old, compared with an average age of 46 in the control group. Both groups were almost entirely female and largely white. Reasons for withdrawal in the case group included self-cessation, disease quiescence, and retinal, mucocutaneous, or cardiac toxicities. Twenty participants in the case group reported AM toxicity, compared with four controls.
Dr. Papachristos noted in her presentation that the toxicity disparity was expected, “because controls are those who continue their medication, and most patients who have toxicity will stop their medication.”
Disease flare occurred in 61.4% of cases, compared with 45.1% of controls (P = .002), with the most common types being cutaneous and musculoskeletal flares. After multivariate analysis, the risk of flare more than doubled for those who ceased AMs (odds ratio, 2.26; 95% confidence interval, 1.24-4.11; P = .008). More than half of the cases (n = 46) restarted AMs after withdrawal, which was largely due to disease flare. Of the patients who restarted due to flare, 88% either recaptured control or improved, and the remaining 12% had further flares.
Of the 88 patients in the case group, 51 abruptly withdrew AMs while 37 tapered off. Patients who tapered had fewer flares (45.9%), compared with patients who withdrew abruptly (72.6%). After multivariate analysis, the risk of flare more than tripled for the abrupt withdrawal group (OR, 3.42; 95% CI, 1.26-9.26; P = .016). Fewer patients who tapered later restarted AMs, compared with the abrupt withdrawal group (37.8% vs. 62.7%; P = .02).
When asked about other differences in medications between the two groups, Dr. Papachristos answered: “We didn’t look into that specifically. We did look at those patients who were on prednisone and on any immunosuppression, although we didn’t look at specific therapies. Those variables were adjusted for in the analysis, and it didn’t make any difference if patients were on immunosuppression or prednisone at the point of index date.
“But we would like to look into the different forms of immunosuppression,” she added, “just to see if that made any difference.”
Withdrawing hydroxychloroquine in older patients
Older patients with SLE who discontinue their use of hydroxychloroquine (HCQ) are also not at increased risk of disease flare, according to a retrospective chart review from rheumatologists Ruth Fernandez-Ruiz, MD, and Peter M. Izmirly, MD, of New York University (Arthritis Res Ther. 2020;22:191. doi: 10.1186/s13075-020-02282-0).
“We wanted to focus on older patients who may have a lower risk of flaring and a higher risk of side effects from the drug,” Dr. Fernandez-Ruiz said in an interview.
The doctors embarked on the study after noticing eye and heart toxicities in certain older patients. They matched 26 lupus patients who had been on HCQ for at least 5 years before discontinuing the drug with 32 control patients who remained on HCQ, ultimately finding that withdrawal had no effect on their risk of lupus flares within a year.
“After starting a drug, the second question most people ask, after ‘What are the side effects?’ is ‘How long do I have to be on this?’ ” Dr. Izmirly said in an interview. “These patients are having side effects associated with long-term HCQ use. And we were noticing that, after you stop the drug, despite what you’re taught, they weren’t flaring.”
Only five patients from each group – 19.2% of the withdrawal group and 15.6% of the continuation group – experienced a flare (OR, 1.28; 95% CI, 0.31-5.30; P = .73). Most of the flares were cutaneous and musculoskeletal in nature, and no severe flares occurred in either group.
“On each side, the overall flare rate was not that high, and they were all relatively mild,” Dr. Izmirly said.
The two doctors acknowledged their study’s smaller sample size, compared with the study by Papachristos and colleagues, along with the advanced age of their patient population, which limits the generalizability of their findings. “We selected patients who had a very low disease activity to begin with, and who were older,” Dr. Fernandez-Ruiz noted.
That said, they reinforced the scarcity of existing research on this subset of lupus patients, one that will only continue to grow.
“Older [patients with] lupus,” Dr. Izmirly said, are “an understudied demographic.”
One of the authors of the study presented at ACR 2020 acknowledged receiving research support and consulting fees from various pharmaceutical companies. The HCQ study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; its authors declared no conflicts of interest.
SOURCE: Papachristos D et al. Arthritis Rheumatol. 2020;72(suppl 10). Abstract 0983.
Slowly tapering off – or remaining on – antimalarial medications can help prevent disease flare in patients with systemic lupus erythematosus (SLE) who’ve achieved clinical remission for at least a year, according to a new study that was presented at the virtual annual meeting of the American College of Rheumatology.
“Except in the setting of toxicity, cessation of antimalarial medication in patients with disease quiescence is feasible using a slow taper,” lead author Danaë Papachristos, MBBS, said during an oral abstract presentation at the online meeting. Dr. Papachristos conducted the research while a clinical and research fellow at the University of Toronto’s lupus clinic, but is now a consultant rheumatologist at the Wesley Hospital in Brisbane, Queensland, Australia.
To investigate flare in patients with SLE who were on or recently off antimalarial medications (AMs), the researchers identified 1,573 potential participants from a long-term observational cohort study at the university’s lupus clinic. From that larger group, 88 cases – patients who achieved clinical remission for at least a year and stopped taking AMs – were matched to at least one control – patients who also achieved remission and continued on medication. Most cases were also matched to a second control, bringing the total number to 173. All patients had at least 2 years of follow-up.
Flare was defined as any increase in the SLEDAI-2K score, with major flare defined as an increase of 4 or more. Patients in the case group were roughly 44 years old, compared with an average age of 46 in the control group. Both groups were almost entirely female and largely white. Reasons for withdrawal in the case group included self-cessation, disease quiescence, and retinal, mucocutaneous, or cardiac toxicities. Twenty participants in the case group reported AM toxicity, compared with four controls.
Dr. Papachristos noted in her presentation that the toxicity disparity was expected, “because controls are those who continue their medication, and most patients who have toxicity will stop their medication.”
Disease flare occurred in 61.4% of cases, compared with 45.1% of controls (P = .002), with the most common types being cutaneous and musculoskeletal flares. After multivariate analysis, the risk of flare more than doubled for those who ceased AMs (odds ratio, 2.26; 95% confidence interval, 1.24-4.11; P = .008). More than half of the cases (n = 46) restarted AMs after withdrawal, which was largely due to disease flare. Of the patients who restarted due to flare, 88% either recaptured control or improved, and the remaining 12% had further flares.
Of the 88 patients in the case group, 51 abruptly withdrew AMs while 37 tapered off. Patients who tapered had fewer flares (45.9%), compared with patients who withdrew abruptly (72.6%). After multivariate analysis, the risk of flare more than tripled for the abrupt withdrawal group (OR, 3.42; 95% CI, 1.26-9.26; P = .016). Fewer patients who tapered later restarted AMs, compared with the abrupt withdrawal group (37.8% vs. 62.7%; P = .02).
When asked about other differences in medications between the two groups, Dr. Papachristos answered: “We didn’t look into that specifically. We did look at those patients who were on prednisone and on any immunosuppression, although we didn’t look at specific therapies. Those variables were adjusted for in the analysis, and it didn’t make any difference if patients were on immunosuppression or prednisone at the point of index date.
“But we would like to look into the different forms of immunosuppression,” she added, “just to see if that made any difference.”
Withdrawing hydroxychloroquine in older patients
Older patients with SLE who discontinue their use of hydroxychloroquine (HCQ) are also not at increased risk of disease flare, according to a retrospective chart review from rheumatologists Ruth Fernandez-Ruiz, MD, and Peter M. Izmirly, MD, of New York University (Arthritis Res Ther. 2020;22:191. doi: 10.1186/s13075-020-02282-0).
“We wanted to focus on older patients who may have a lower risk of flaring and a higher risk of side effects from the drug,” Dr. Fernandez-Ruiz said in an interview.
The doctors embarked on the study after noticing eye and heart toxicities in certain older patients. They matched 26 lupus patients who had been on HCQ for at least 5 years before discontinuing the drug with 32 control patients who remained on HCQ, ultimately finding that withdrawal had no effect on their risk of lupus flares within a year.
“After starting a drug, the second question most people ask, after ‘What are the side effects?’ is ‘How long do I have to be on this?’ ” Dr. Izmirly said in an interview. “These patients are having side effects associated with long-term HCQ use. And we were noticing that, after you stop the drug, despite what you’re taught, they weren’t flaring.”
Only five patients from each group – 19.2% of the withdrawal group and 15.6% of the continuation group – experienced a flare (OR, 1.28; 95% CI, 0.31-5.30; P = .73). Most of the flares were cutaneous and musculoskeletal in nature, and no severe flares occurred in either group.
“On each side, the overall flare rate was not that high, and they were all relatively mild,” Dr. Izmirly said.
The two doctors acknowledged their study’s smaller sample size, compared with the study by Papachristos and colleagues, along with the advanced age of their patient population, which limits the generalizability of their findings. “We selected patients who had a very low disease activity to begin with, and who were older,” Dr. Fernandez-Ruiz noted.
That said, they reinforced the scarcity of existing research on this subset of lupus patients, one that will only continue to grow.
“Older [patients with] lupus,” Dr. Izmirly said, are “an understudied demographic.”
One of the authors of the study presented at ACR 2020 acknowledged receiving research support and consulting fees from various pharmaceutical companies. The HCQ study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; its authors declared no conflicts of interest.
SOURCE: Papachristos D et al. Arthritis Rheumatol. 2020;72(suppl 10). Abstract 0983.
Slowly tapering off – or remaining on – antimalarial medications can help prevent disease flare in patients with systemic lupus erythematosus (SLE) who’ve achieved clinical remission for at least a year, according to a new study that was presented at the virtual annual meeting of the American College of Rheumatology.
“Except in the setting of toxicity, cessation of antimalarial medication in patients with disease quiescence is feasible using a slow taper,” lead author Danaë Papachristos, MBBS, said during an oral abstract presentation at the online meeting. Dr. Papachristos conducted the research while a clinical and research fellow at the University of Toronto’s lupus clinic, but is now a consultant rheumatologist at the Wesley Hospital in Brisbane, Queensland, Australia.
To investigate flare in patients with SLE who were on or recently off antimalarial medications (AMs), the researchers identified 1,573 potential participants from a long-term observational cohort study at the university’s lupus clinic. From that larger group, 88 cases – patients who achieved clinical remission for at least a year and stopped taking AMs – were matched to at least one control – patients who also achieved remission and continued on medication. Most cases were also matched to a second control, bringing the total number to 173. All patients had at least 2 years of follow-up.
Flare was defined as any increase in the SLEDAI-2K score, with major flare defined as an increase of 4 or more. Patients in the case group were roughly 44 years old, compared with an average age of 46 in the control group. Both groups were almost entirely female and largely white. Reasons for withdrawal in the case group included self-cessation, disease quiescence, and retinal, mucocutaneous, or cardiac toxicities. Twenty participants in the case group reported AM toxicity, compared with four controls.
Dr. Papachristos noted in her presentation that the toxicity disparity was expected, “because controls are those who continue their medication, and most patients who have toxicity will stop their medication.”
Disease flare occurred in 61.4% of cases, compared with 45.1% of controls (P = .002), with the most common types being cutaneous and musculoskeletal flares. After multivariate analysis, the risk of flare more than doubled for those who ceased AMs (odds ratio, 2.26; 95% confidence interval, 1.24-4.11; P = .008). More than half of the cases (n = 46) restarted AMs after withdrawal, which was largely due to disease flare. Of the patients who restarted due to flare, 88% either recaptured control or improved, and the remaining 12% had further flares.
Of the 88 patients in the case group, 51 abruptly withdrew AMs while 37 tapered off. Patients who tapered had fewer flares (45.9%), compared with patients who withdrew abruptly (72.6%). After multivariate analysis, the risk of flare more than tripled for the abrupt withdrawal group (OR, 3.42; 95% CI, 1.26-9.26; P = .016). Fewer patients who tapered later restarted AMs, compared with the abrupt withdrawal group (37.8% vs. 62.7%; P = .02).
When asked about other differences in medications between the two groups, Dr. Papachristos answered: “We didn’t look into that specifically. We did look at those patients who were on prednisone and on any immunosuppression, although we didn’t look at specific therapies. Those variables were adjusted for in the analysis, and it didn’t make any difference if patients were on immunosuppression or prednisone at the point of index date.
“But we would like to look into the different forms of immunosuppression,” she added, “just to see if that made any difference.”
Withdrawing hydroxychloroquine in older patients
Older patients with SLE who discontinue their use of hydroxychloroquine (HCQ) are also not at increased risk of disease flare, according to a retrospective chart review from rheumatologists Ruth Fernandez-Ruiz, MD, and Peter M. Izmirly, MD, of New York University (Arthritis Res Ther. 2020;22:191. doi: 10.1186/s13075-020-02282-0).
“We wanted to focus on older patients who may have a lower risk of flaring and a higher risk of side effects from the drug,” Dr. Fernandez-Ruiz said in an interview.
The doctors embarked on the study after noticing eye and heart toxicities in certain older patients. They matched 26 lupus patients who had been on HCQ for at least 5 years before discontinuing the drug with 32 control patients who remained on HCQ, ultimately finding that withdrawal had no effect on their risk of lupus flares within a year.
“After starting a drug, the second question most people ask, after ‘What are the side effects?’ is ‘How long do I have to be on this?’ ” Dr. Izmirly said in an interview. “These patients are having side effects associated with long-term HCQ use. And we were noticing that, after you stop the drug, despite what you’re taught, they weren’t flaring.”
Only five patients from each group – 19.2% of the withdrawal group and 15.6% of the continuation group – experienced a flare (OR, 1.28; 95% CI, 0.31-5.30; P = .73). Most of the flares were cutaneous and musculoskeletal in nature, and no severe flares occurred in either group.
“On each side, the overall flare rate was not that high, and they were all relatively mild,” Dr. Izmirly said.
The two doctors acknowledged their study’s smaller sample size, compared with the study by Papachristos and colleagues, along with the advanced age of their patient population, which limits the generalizability of their findings. “We selected patients who had a very low disease activity to begin with, and who were older,” Dr. Fernandez-Ruiz noted.
That said, they reinforced the scarcity of existing research on this subset of lupus patients, one that will only continue to grow.
“Older [patients with] lupus,” Dr. Izmirly said, are “an understudied demographic.”
One of the authors of the study presented at ACR 2020 acknowledged receiving research support and consulting fees from various pharmaceutical companies. The HCQ study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; its authors declared no conflicts of interest.
SOURCE: Papachristos D et al. Arthritis Rheumatol. 2020;72(suppl 10). Abstract 0983.
FROM ACR 2020
Methotrexate users need tuberculosis tests in high-TB areas
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
Transcutaneous VNS on the ear shows positive effects in lupus pilot trial
during a brief pilot trial.
“Our study population included individuals with significant pain and exemplifies the unmet need for adequate control of pain and fatigue in SLE. Importantly, this was a double-blind, sham-controlled study and neither the subject nor assessor was aware of a subject’s intervention. Objective outcomes, that is, tender and swollen joint counts, were also significantly reduced in subjects receiving taVNS, compared with those receiving [sham stimulation]. The stimulation was well tolerated with no adverse events attributed to the intervention, and, clinical benefits continued after taVNS was stopped,” first author Cynthia Aranow, MD, and her colleagues at the Feinstein Institutes for Medical Research in Manhasset, N.Y., wrote in Annals of the Rheumatic Diseases.
Stimulation of the vagus nerve can be achieved through the ear via its auricular branch, which innervates the cymba concha in the outer ear. Past pilot studies of implanted VNS devices lasting 6 weeks to 6 months in patients with Crohn’s disease or rheumatoid arthritis have shown improvements in measures of disease activity as well as objective markers of inflammation, and a more recent trial testing a transcutaneous devices’s effect in patients with Sjögren’s syndrome found significant reductions in fatigue over a 26-day period, the investigators noted.
In the taVNS device study, the researchers recruited 18 patients with SLE who had musculoskeletal pain rated as 4 or higher on a 10-cm visual analog scale and randomized them in a 2:1 ratio to receive taVNS once per day for 5 minutes for 4 consecutive days versus sham stimulation. Patients were allowed to be on stable doses of disease-modifying antirheumatic drugs, biologics, and/or prednisone ≤ 10 mg/day, with no change of dose within 28 days prior to baseline. The study excluded patients who used tobacco or an anticholinergic medication and those with a diagnosis of fibromyalgia.
The 12 patients who received actual taVNS had a significantly greater reduction in their pain, compared with 6 sham-treated patients (–5.00 vs. 0.10; P = .049), with 10 of 12 and 1 of 6 having a clinical response (a reduction of at least 1.58 on a 10-cm visual analog scale from baseline to day 5). Stimulation-treated patients also reported significantly greater reductions in fatigue, with 10 of 12 achieving a meaningful reduction, defined as a 4-point improvement on the Functional Assessment of Chronic Illness Therapy Fatigue Subscale; none of the sham-treated patients experienced meaningful improvement of fatigue. The patients who received taVNS had resolution of all swollen and tender joints, compared with 5.3% of tender and 9.1% of swollen joints in sham-treated patients. Ex vivo lipopolysaccharide stimulation of whole-blood samples from taVNS-treated patients, however, showed no reductions of inflammatory mediators or chemokines in tests on day 5 and day 12.
The investigators reported that there were no adverse events attributed to taVNS, including no reports of headache, lightheadedness, tinnitus, ear irritation, or changes to the external skin of the outer ear.
The study was supported by a grant from the John and Marcia Goldman Foundation. One author reported a financial relationship with Set Point Medical and My String, and three authors reported having a provisional patent application titled “Auricular stimulation device, system and methods of use.”
SOURCE: Aranow C et al. Ann Rheum Dis. 2020 Nov 3. doi: 10.1136/annrheumdis-2020-217872.
during a brief pilot trial.
“Our study population included individuals with significant pain and exemplifies the unmet need for adequate control of pain and fatigue in SLE. Importantly, this was a double-blind, sham-controlled study and neither the subject nor assessor was aware of a subject’s intervention. Objective outcomes, that is, tender and swollen joint counts, were also significantly reduced in subjects receiving taVNS, compared with those receiving [sham stimulation]. The stimulation was well tolerated with no adverse events attributed to the intervention, and, clinical benefits continued after taVNS was stopped,” first author Cynthia Aranow, MD, and her colleagues at the Feinstein Institutes for Medical Research in Manhasset, N.Y., wrote in Annals of the Rheumatic Diseases.
Stimulation of the vagus nerve can be achieved through the ear via its auricular branch, which innervates the cymba concha in the outer ear. Past pilot studies of implanted VNS devices lasting 6 weeks to 6 months in patients with Crohn’s disease or rheumatoid arthritis have shown improvements in measures of disease activity as well as objective markers of inflammation, and a more recent trial testing a transcutaneous devices’s effect in patients with Sjögren’s syndrome found significant reductions in fatigue over a 26-day period, the investigators noted.
In the taVNS device study, the researchers recruited 18 patients with SLE who had musculoskeletal pain rated as 4 or higher on a 10-cm visual analog scale and randomized them in a 2:1 ratio to receive taVNS once per day for 5 minutes for 4 consecutive days versus sham stimulation. Patients were allowed to be on stable doses of disease-modifying antirheumatic drugs, biologics, and/or prednisone ≤ 10 mg/day, with no change of dose within 28 days prior to baseline. The study excluded patients who used tobacco or an anticholinergic medication and those with a diagnosis of fibromyalgia.
The 12 patients who received actual taVNS had a significantly greater reduction in their pain, compared with 6 sham-treated patients (–5.00 vs. 0.10; P = .049), with 10 of 12 and 1 of 6 having a clinical response (a reduction of at least 1.58 on a 10-cm visual analog scale from baseline to day 5). Stimulation-treated patients also reported significantly greater reductions in fatigue, with 10 of 12 achieving a meaningful reduction, defined as a 4-point improvement on the Functional Assessment of Chronic Illness Therapy Fatigue Subscale; none of the sham-treated patients experienced meaningful improvement of fatigue. The patients who received taVNS had resolution of all swollen and tender joints, compared with 5.3% of tender and 9.1% of swollen joints in sham-treated patients. Ex vivo lipopolysaccharide stimulation of whole-blood samples from taVNS-treated patients, however, showed no reductions of inflammatory mediators or chemokines in tests on day 5 and day 12.
The investigators reported that there were no adverse events attributed to taVNS, including no reports of headache, lightheadedness, tinnitus, ear irritation, or changes to the external skin of the outer ear.
The study was supported by a grant from the John and Marcia Goldman Foundation. One author reported a financial relationship with Set Point Medical and My String, and three authors reported having a provisional patent application titled “Auricular stimulation device, system and methods of use.”
SOURCE: Aranow C et al. Ann Rheum Dis. 2020 Nov 3. doi: 10.1136/annrheumdis-2020-217872.
during a brief pilot trial.
“Our study population included individuals with significant pain and exemplifies the unmet need for adequate control of pain and fatigue in SLE. Importantly, this was a double-blind, sham-controlled study and neither the subject nor assessor was aware of a subject’s intervention. Objective outcomes, that is, tender and swollen joint counts, were also significantly reduced in subjects receiving taVNS, compared with those receiving [sham stimulation]. The stimulation was well tolerated with no adverse events attributed to the intervention, and, clinical benefits continued after taVNS was stopped,” first author Cynthia Aranow, MD, and her colleagues at the Feinstein Institutes for Medical Research in Manhasset, N.Y., wrote in Annals of the Rheumatic Diseases.
Stimulation of the vagus nerve can be achieved through the ear via its auricular branch, which innervates the cymba concha in the outer ear. Past pilot studies of implanted VNS devices lasting 6 weeks to 6 months in patients with Crohn’s disease or rheumatoid arthritis have shown improvements in measures of disease activity as well as objective markers of inflammation, and a more recent trial testing a transcutaneous devices’s effect in patients with Sjögren’s syndrome found significant reductions in fatigue over a 26-day period, the investigators noted.
In the taVNS device study, the researchers recruited 18 patients with SLE who had musculoskeletal pain rated as 4 or higher on a 10-cm visual analog scale and randomized them in a 2:1 ratio to receive taVNS once per day for 5 minutes for 4 consecutive days versus sham stimulation. Patients were allowed to be on stable doses of disease-modifying antirheumatic drugs, biologics, and/or prednisone ≤ 10 mg/day, with no change of dose within 28 days prior to baseline. The study excluded patients who used tobacco or an anticholinergic medication and those with a diagnosis of fibromyalgia.
The 12 patients who received actual taVNS had a significantly greater reduction in their pain, compared with 6 sham-treated patients (–5.00 vs. 0.10; P = .049), with 10 of 12 and 1 of 6 having a clinical response (a reduction of at least 1.58 on a 10-cm visual analog scale from baseline to day 5). Stimulation-treated patients also reported significantly greater reductions in fatigue, with 10 of 12 achieving a meaningful reduction, defined as a 4-point improvement on the Functional Assessment of Chronic Illness Therapy Fatigue Subscale; none of the sham-treated patients experienced meaningful improvement of fatigue. The patients who received taVNS had resolution of all swollen and tender joints, compared with 5.3% of tender and 9.1% of swollen joints in sham-treated patients. Ex vivo lipopolysaccharide stimulation of whole-blood samples from taVNS-treated patients, however, showed no reductions of inflammatory mediators or chemokines in tests on day 5 and day 12.
The investigators reported that there were no adverse events attributed to taVNS, including no reports of headache, lightheadedness, tinnitus, ear irritation, or changes to the external skin of the outer ear.
The study was supported by a grant from the John and Marcia Goldman Foundation. One author reported a financial relationship with Set Point Medical and My String, and three authors reported having a provisional patent application titled “Auricular stimulation device, system and methods of use.”
SOURCE: Aranow C et al. Ann Rheum Dis. 2020 Nov 3. doi: 10.1136/annrheumdis-2020-217872.
FROM ANNALS OF THE RHEUMATIC DISEASES