User login
One-month delay in cancer treatment linked to increase in mortality
In light of the treatment delays resulting from the pandemic, Canadian and U.K. researchers carried out a review and analysis of relevant studies published between January 2000 and April 2020.
Included studies examined data on surgical interventions, systemic therapy, or radiotherapy for seven forms of cancer – bladder, breast, colon, rectum, lung, cervix, and head and neck. Delays were measured from diagnosis to the first treatment or from the completion of one treatment to the start of the next.
The search identified 34 suitable studies for 17 indications, with data from more than 1.2 million patients. The analysis identified a significant association between delay and increased mortality for 13 of the 17 indications (P < .05).
For surgery, there was a 6%-8% increase in the risk of death for every 4-week treatment delay. Estimates for systemic treatment varied (hazard ratio range, 1.01-1.28). Four-week delays in radiotherapy were for radical radiotherapy for head and neck cancer (HR, 1.09; 95% confidence interval, 1.05-1.14), adjuvant radiotherapy after breast-conserving surgery (HR, 0.98; 95% CI, 0.88-1.09), and cervical cancer adjuvant radiotherapy (HR, 1.23; 95% CI, 1.00-1.50).
Delays of up to 8 and 12 weeks further increased mortality. An 8-week delay in breast cancer surgery was linked to a 17% increased mortality, and a 12-week delay would increase mortality by 26%.
A surgical delay of 12 weeks for patients with breast cancer continuing for 1 year – which is likely to be the case as the pandemic continues – would lead to 1,400 excess deaths in the United Kingdom.
The authors said the results of this study could be used to guide policy making on the organization of cancer services, particularly as the pandemic continues and further delays are expected.
This article originally appeared on Univadis, part of the Medscape Professional Network.
In light of the treatment delays resulting from the pandemic, Canadian and U.K. researchers carried out a review and analysis of relevant studies published between January 2000 and April 2020.
Included studies examined data on surgical interventions, systemic therapy, or radiotherapy for seven forms of cancer – bladder, breast, colon, rectum, lung, cervix, and head and neck. Delays were measured from diagnosis to the first treatment or from the completion of one treatment to the start of the next.
The search identified 34 suitable studies for 17 indications, with data from more than 1.2 million patients. The analysis identified a significant association between delay and increased mortality for 13 of the 17 indications (P < .05).
For surgery, there was a 6%-8% increase in the risk of death for every 4-week treatment delay. Estimates for systemic treatment varied (hazard ratio range, 1.01-1.28). Four-week delays in radiotherapy were for radical radiotherapy for head and neck cancer (HR, 1.09; 95% confidence interval, 1.05-1.14), adjuvant radiotherapy after breast-conserving surgery (HR, 0.98; 95% CI, 0.88-1.09), and cervical cancer adjuvant radiotherapy (HR, 1.23; 95% CI, 1.00-1.50).
Delays of up to 8 and 12 weeks further increased mortality. An 8-week delay in breast cancer surgery was linked to a 17% increased mortality, and a 12-week delay would increase mortality by 26%.
A surgical delay of 12 weeks for patients with breast cancer continuing for 1 year – which is likely to be the case as the pandemic continues – would lead to 1,400 excess deaths in the United Kingdom.
The authors said the results of this study could be used to guide policy making on the organization of cancer services, particularly as the pandemic continues and further delays are expected.
This article originally appeared on Univadis, part of the Medscape Professional Network.
In light of the treatment delays resulting from the pandemic, Canadian and U.K. researchers carried out a review and analysis of relevant studies published between January 2000 and April 2020.
Included studies examined data on surgical interventions, systemic therapy, or radiotherapy for seven forms of cancer – bladder, breast, colon, rectum, lung, cervix, and head and neck. Delays were measured from diagnosis to the first treatment or from the completion of one treatment to the start of the next.
The search identified 34 suitable studies for 17 indications, with data from more than 1.2 million patients. The analysis identified a significant association between delay and increased mortality for 13 of the 17 indications (P < .05).
For surgery, there was a 6%-8% increase in the risk of death for every 4-week treatment delay. Estimates for systemic treatment varied (hazard ratio range, 1.01-1.28). Four-week delays in radiotherapy were for radical radiotherapy for head and neck cancer (HR, 1.09; 95% confidence interval, 1.05-1.14), adjuvant radiotherapy after breast-conserving surgery (HR, 0.98; 95% CI, 0.88-1.09), and cervical cancer adjuvant radiotherapy (HR, 1.23; 95% CI, 1.00-1.50).
Delays of up to 8 and 12 weeks further increased mortality. An 8-week delay in breast cancer surgery was linked to a 17% increased mortality, and a 12-week delay would increase mortality by 26%.
A surgical delay of 12 weeks for patients with breast cancer continuing for 1 year – which is likely to be the case as the pandemic continues – would lead to 1,400 excess deaths in the United Kingdom.
The authors said the results of this study could be used to guide policy making on the organization of cancer services, particularly as the pandemic continues and further delays are expected.
This article originally appeared on Univadis, part of the Medscape Professional Network.
New estimates for breast cancer risk with HRT
The study was published online on October 28 in The BMJ.
“The study confirms increased risk of breast cancer in patients taking HRT but shows that the magnitude of risk depends on a number of factors,” first author Yana Vinogradova, PhD, said in an interview. Dr. Vinogradova is a medical statistician at the University of Nottingham (England).
The study also suggests the risk may be lower than was estimated in a large meta-analysis of 24 trials that was published in 2019 in The Lancet. In that study, researchers suggested the risk for breast cancer with HRT was higher and persisted longer than had been thought.
This conclusion from the meta-analysis was widely reported in the lay press and led to the UK Medicine and Healthcare Products Regulatory Agency issuing a safety alert for HRT regarding breast cancer. Experts in the field questioned the alert and said it caused undue anxiety. The European Medicines Agency also issued a safety alert because of the study.
This new study was begun before publication of the meta-analysis. Although the results are broadly similar in suggesting increased risk for breast cancer with HRT use, findings from the new study suggest the risk is lower than had been estimated in the meta-analysis and that the risk diminishes more rapidly after stopping HRT than was suggested by the meta-analysis.
“The publicity surrounding publication of the meta-analysis highlighted unexpectedly high risks and led to a heightened level of concern in some quarters,” Dr. Vinogradova commented. “Our study, based on general population data, has not confirmed any such findings. In general, it showed lower levels of risk and clarified the variability of magnitude within them.”
Dr. Vinogradova said the discrepancy could be related to the fact that the studies were designed differently. The meta-analysis relied on results from 24 studies that were conducted around the world at different periods and included women of different ages and backgrounds. The studies in the meta-analysis used different methods, including questionnaires that relied on women’s memories and therefore could have been biased, she said.
In contrast, the new study analyzed EMR data collected prospectively by general practices in the United Kingdom. The data came from the QResearch and from the Clinical Practice Research Datalink (CPRD) databases, the two largest primary care databases in the United Kingdom, which were linked to hospital, mortality, and cancer registries.
Because this study used a “consistent design” and “consistent data sources,” these new results “are likely to be more accurate and reliable for assessing risks among HRT users,” Dr. Vinogradova commented.
This study used an observational design, so it cannot prove that HRT causes breast cancer. These results may better represent women in the general U.K. population, compared with the earlier meta-analysis, she added.
Commenting on the new study, Michael Jones, PhD, senior staff scientist in genetics and epidemiology at the Institute of Cancer Research, London, also emphasized that it was large and its data came from general practitioner medical records, “so the strong statistical associations are unlikely to be due to chance.
“The results of this study generally confirm what has been seen before and is well established – that the use of combined estrogen plus progestogen HRT is associated with increased risk of breast cancer, and this risk increases with duration of use. But reassuringly, after stopping HRT, the raised risk of breast cancer mostly returns to that seen in nonusers of HRT,” he said.
“It’s important to note that no one study should be considered in isolation,” he added. “Even though some risks were found to be slightly smaller than those reported in another meta-analysis of the worldwide epidemiological evidence recently published in 2019, women considering use of HRT should still follow advice given to them by their [general practitioners].”
Study details
In the study, researchers evaluated all types of HRT commonly prescribed in the United Kingdom over the past 20 years, including topical estrogen, vaginal pessaries, and creams. They grouped HRT use by recent (within the past 5 years) and past (5 or more years ago) and HRT duration as short term (less than 5 years) and long term (5 years or longer). Results were adjusted for a range of factors that could affect breast cancer risk, including lifestyle, smoking, alcohol consumption, other medical conditions, family history, and use of other prescribed drugs.
The analysis included 98,611 women aged 50-79 years who were first diagnosed with breast cancer between 1998 and 2019. These women were matched by age and general practice to 457,498 women who were not diagnosed with breast cancer over these years. HRT use was reported in 34% (33,703) of women with breast cancer and in 31% (134,391) of women without breast cancer.
Overall, the risk for breast cancer was increased with use of most HRT drugs (adjusted odds ratio, 1.21; 95% confidence, 1.19-1.23), compared with not using HRT drugs. The highest risk was tied to combined estrogen/progestogen HRT (adjusted OR, 1.26; 95% CI, 1.24-1.29). The lowest risk was tied to estrogen-only HRT (adjusted OR, 1.06; 95% CI, 1.03-1.10). Estrogen cream and vaginal estrogen were not associated with increased breast cancer risk.
In general, breast cancer risk was higher among recent HRT users and those receiving long-term therapy. HRT-associated breast cancer risk increased with age and declined after discontinuing treatment. Therapy of less than 1 year was not associated with increased breast cancer risk.
Women who had recently been receiving long-term combined estrogen/progestogen HRT had a 79% increased risk for breast cancer (adjusted OR, 1.79; 95% CI, 1.73-1.85), compared with never-users. Among recent long-term users of combined HRT, breast cancer risk was highest for norethisterone (adjusted OR, 1.88; 95% CI, 1.79-1.99) and lowest for dydrogesterone (adjusted OR, 1.24; 95% CI, 1.03-1.48). Women who had recently been receiving long-term estrogen-only HRT had a 15% increased risk for breast cancer compared to never-users (adjusted OR, 1.15; 95% CI, 1.09-1.21).
Among women who discontinued HRT 5 or more years ago, risk for breast cancer was no longer increased for long-term estrogen-only therapy and short-term estrogen/progestogen therapy. However, breast cancer risk remained elevated 5 years after discontinuing long-term estrogen/progestogen (adjusted OR, 1.16; 95% CI, 1.11-1.21).
HRT-associated risk for breast cancer increased with age across all durations of therapy.
Compared with never-use, recent long-term estrogen-only therapy was associated with zero extra breast cancer cases per 10,000 women-years among women aged 50-59 years and eight extra cases per 10,000 women-years among women aged 70-79.
Recent long-term estrogen/progestogen use was associated with 15 extra breast cancer cases among women aged 50-59 and 36 extra cases among women aged 70-79 per 10,000 women-years.
Past long-term estrogen/progestogen use was associated with zero extra breast cancer cases among women aged 50-59 and eight extra cases among women aged 70-79 per 10,000 women-years.
Summarizing, Dr. Vinogradova said the increased risk for breast cancer with HRT appears to be “relatively small, particularly for younger women and for any women who use HRT only for a restricted period.”
Decisions about whether to use HRT and which type to use should depend on symptom severity, patient factors, and suitability of other treatment options, she commented.
“Particularly for those women who our study has shown to be most at risk, these decisions should be made through discussions between the patient and her doctor,” she concluded. “We hope that the new and more detailed information provided by our study will facilitate such prescribing decisions.”
The study was partially funded by the School for Primary Care Research of the National Institute for Health Research, by Cancer Research UK, and by the Cancer Research UK Oxford Center. Dr. Vinogradova has disclosed no relevant financial relationships. Senior author Julia Hippisley-Cox is an unpaid director of QResearch and was a paid director of ClinRisk until 2019. The other authors have disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
The study was published online on October 28 in The BMJ.
“The study confirms increased risk of breast cancer in patients taking HRT but shows that the magnitude of risk depends on a number of factors,” first author Yana Vinogradova, PhD, said in an interview. Dr. Vinogradova is a medical statistician at the University of Nottingham (England).
The study also suggests the risk may be lower than was estimated in a large meta-analysis of 24 trials that was published in 2019 in The Lancet. In that study, researchers suggested the risk for breast cancer with HRT was higher and persisted longer than had been thought.
This conclusion from the meta-analysis was widely reported in the lay press and led to the UK Medicine and Healthcare Products Regulatory Agency issuing a safety alert for HRT regarding breast cancer. Experts in the field questioned the alert and said it caused undue anxiety. The European Medicines Agency also issued a safety alert because of the study.
This new study was begun before publication of the meta-analysis. Although the results are broadly similar in suggesting increased risk for breast cancer with HRT use, findings from the new study suggest the risk is lower than had been estimated in the meta-analysis and that the risk diminishes more rapidly after stopping HRT than was suggested by the meta-analysis.
“The publicity surrounding publication of the meta-analysis highlighted unexpectedly high risks and led to a heightened level of concern in some quarters,” Dr. Vinogradova commented. “Our study, based on general population data, has not confirmed any such findings. In general, it showed lower levels of risk and clarified the variability of magnitude within them.”
Dr. Vinogradova said the discrepancy could be related to the fact that the studies were designed differently. The meta-analysis relied on results from 24 studies that were conducted around the world at different periods and included women of different ages and backgrounds. The studies in the meta-analysis used different methods, including questionnaires that relied on women’s memories and therefore could have been biased, she said.
In contrast, the new study analyzed EMR data collected prospectively by general practices in the United Kingdom. The data came from the QResearch and from the Clinical Practice Research Datalink (CPRD) databases, the two largest primary care databases in the United Kingdom, which were linked to hospital, mortality, and cancer registries.
Because this study used a “consistent design” and “consistent data sources,” these new results “are likely to be more accurate and reliable for assessing risks among HRT users,” Dr. Vinogradova commented.
This study used an observational design, so it cannot prove that HRT causes breast cancer. These results may better represent women in the general U.K. population, compared with the earlier meta-analysis, she added.
Commenting on the new study, Michael Jones, PhD, senior staff scientist in genetics and epidemiology at the Institute of Cancer Research, London, also emphasized that it was large and its data came from general practitioner medical records, “so the strong statistical associations are unlikely to be due to chance.
“The results of this study generally confirm what has been seen before and is well established – that the use of combined estrogen plus progestogen HRT is associated with increased risk of breast cancer, and this risk increases with duration of use. But reassuringly, after stopping HRT, the raised risk of breast cancer mostly returns to that seen in nonusers of HRT,” he said.
“It’s important to note that no one study should be considered in isolation,” he added. “Even though some risks were found to be slightly smaller than those reported in another meta-analysis of the worldwide epidemiological evidence recently published in 2019, women considering use of HRT should still follow advice given to them by their [general practitioners].”
Study details
In the study, researchers evaluated all types of HRT commonly prescribed in the United Kingdom over the past 20 years, including topical estrogen, vaginal pessaries, and creams. They grouped HRT use by recent (within the past 5 years) and past (5 or more years ago) and HRT duration as short term (less than 5 years) and long term (5 years or longer). Results were adjusted for a range of factors that could affect breast cancer risk, including lifestyle, smoking, alcohol consumption, other medical conditions, family history, and use of other prescribed drugs.
The analysis included 98,611 women aged 50-79 years who were first diagnosed with breast cancer between 1998 and 2019. These women were matched by age and general practice to 457,498 women who were not diagnosed with breast cancer over these years. HRT use was reported in 34% (33,703) of women with breast cancer and in 31% (134,391) of women without breast cancer.
Overall, the risk for breast cancer was increased with use of most HRT drugs (adjusted odds ratio, 1.21; 95% confidence, 1.19-1.23), compared with not using HRT drugs. The highest risk was tied to combined estrogen/progestogen HRT (adjusted OR, 1.26; 95% CI, 1.24-1.29). The lowest risk was tied to estrogen-only HRT (adjusted OR, 1.06; 95% CI, 1.03-1.10). Estrogen cream and vaginal estrogen were not associated with increased breast cancer risk.
In general, breast cancer risk was higher among recent HRT users and those receiving long-term therapy. HRT-associated breast cancer risk increased with age and declined after discontinuing treatment. Therapy of less than 1 year was not associated with increased breast cancer risk.
Women who had recently been receiving long-term combined estrogen/progestogen HRT had a 79% increased risk for breast cancer (adjusted OR, 1.79; 95% CI, 1.73-1.85), compared with never-users. Among recent long-term users of combined HRT, breast cancer risk was highest for norethisterone (adjusted OR, 1.88; 95% CI, 1.79-1.99) and lowest for dydrogesterone (adjusted OR, 1.24; 95% CI, 1.03-1.48). Women who had recently been receiving long-term estrogen-only HRT had a 15% increased risk for breast cancer compared to never-users (adjusted OR, 1.15; 95% CI, 1.09-1.21).
Among women who discontinued HRT 5 or more years ago, risk for breast cancer was no longer increased for long-term estrogen-only therapy and short-term estrogen/progestogen therapy. However, breast cancer risk remained elevated 5 years after discontinuing long-term estrogen/progestogen (adjusted OR, 1.16; 95% CI, 1.11-1.21).
HRT-associated risk for breast cancer increased with age across all durations of therapy.
Compared with never-use, recent long-term estrogen-only therapy was associated with zero extra breast cancer cases per 10,000 women-years among women aged 50-59 years and eight extra cases per 10,000 women-years among women aged 70-79.
Recent long-term estrogen/progestogen use was associated with 15 extra breast cancer cases among women aged 50-59 and 36 extra cases among women aged 70-79 per 10,000 women-years.
Past long-term estrogen/progestogen use was associated with zero extra breast cancer cases among women aged 50-59 and eight extra cases among women aged 70-79 per 10,000 women-years.
Summarizing, Dr. Vinogradova said the increased risk for breast cancer with HRT appears to be “relatively small, particularly for younger women and for any women who use HRT only for a restricted period.”
Decisions about whether to use HRT and which type to use should depend on symptom severity, patient factors, and suitability of other treatment options, she commented.
“Particularly for those women who our study has shown to be most at risk, these decisions should be made through discussions between the patient and her doctor,” she concluded. “We hope that the new and more detailed information provided by our study will facilitate such prescribing decisions.”
The study was partially funded by the School for Primary Care Research of the National Institute for Health Research, by Cancer Research UK, and by the Cancer Research UK Oxford Center. Dr. Vinogradova has disclosed no relevant financial relationships. Senior author Julia Hippisley-Cox is an unpaid director of QResearch and was a paid director of ClinRisk until 2019. The other authors have disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
The study was published online on October 28 in The BMJ.
“The study confirms increased risk of breast cancer in patients taking HRT but shows that the magnitude of risk depends on a number of factors,” first author Yana Vinogradova, PhD, said in an interview. Dr. Vinogradova is a medical statistician at the University of Nottingham (England).
The study also suggests the risk may be lower than was estimated in a large meta-analysis of 24 trials that was published in 2019 in The Lancet. In that study, researchers suggested the risk for breast cancer with HRT was higher and persisted longer than had been thought.
This conclusion from the meta-analysis was widely reported in the lay press and led to the UK Medicine and Healthcare Products Regulatory Agency issuing a safety alert for HRT regarding breast cancer. Experts in the field questioned the alert and said it caused undue anxiety. The European Medicines Agency also issued a safety alert because of the study.
This new study was begun before publication of the meta-analysis. Although the results are broadly similar in suggesting increased risk for breast cancer with HRT use, findings from the new study suggest the risk is lower than had been estimated in the meta-analysis and that the risk diminishes more rapidly after stopping HRT than was suggested by the meta-analysis.
“The publicity surrounding publication of the meta-analysis highlighted unexpectedly high risks and led to a heightened level of concern in some quarters,” Dr. Vinogradova commented. “Our study, based on general population data, has not confirmed any such findings. In general, it showed lower levels of risk and clarified the variability of magnitude within them.”
Dr. Vinogradova said the discrepancy could be related to the fact that the studies were designed differently. The meta-analysis relied on results from 24 studies that were conducted around the world at different periods and included women of different ages and backgrounds. The studies in the meta-analysis used different methods, including questionnaires that relied on women’s memories and therefore could have been biased, she said.
In contrast, the new study analyzed EMR data collected prospectively by general practices in the United Kingdom. The data came from the QResearch and from the Clinical Practice Research Datalink (CPRD) databases, the two largest primary care databases in the United Kingdom, which were linked to hospital, mortality, and cancer registries.
Because this study used a “consistent design” and “consistent data sources,” these new results “are likely to be more accurate and reliable for assessing risks among HRT users,” Dr. Vinogradova commented.
This study used an observational design, so it cannot prove that HRT causes breast cancer. These results may better represent women in the general U.K. population, compared with the earlier meta-analysis, she added.
Commenting on the new study, Michael Jones, PhD, senior staff scientist in genetics and epidemiology at the Institute of Cancer Research, London, also emphasized that it was large and its data came from general practitioner medical records, “so the strong statistical associations are unlikely to be due to chance.
“The results of this study generally confirm what has been seen before and is well established – that the use of combined estrogen plus progestogen HRT is associated with increased risk of breast cancer, and this risk increases with duration of use. But reassuringly, after stopping HRT, the raised risk of breast cancer mostly returns to that seen in nonusers of HRT,” he said.
“It’s important to note that no one study should be considered in isolation,” he added. “Even though some risks were found to be slightly smaller than those reported in another meta-analysis of the worldwide epidemiological evidence recently published in 2019, women considering use of HRT should still follow advice given to them by their [general practitioners].”
Study details
In the study, researchers evaluated all types of HRT commonly prescribed in the United Kingdom over the past 20 years, including topical estrogen, vaginal pessaries, and creams. They grouped HRT use by recent (within the past 5 years) and past (5 or more years ago) and HRT duration as short term (less than 5 years) and long term (5 years or longer). Results were adjusted for a range of factors that could affect breast cancer risk, including lifestyle, smoking, alcohol consumption, other medical conditions, family history, and use of other prescribed drugs.
The analysis included 98,611 women aged 50-79 years who were first diagnosed with breast cancer between 1998 and 2019. These women were matched by age and general practice to 457,498 women who were not diagnosed with breast cancer over these years. HRT use was reported in 34% (33,703) of women with breast cancer and in 31% (134,391) of women without breast cancer.
Overall, the risk for breast cancer was increased with use of most HRT drugs (adjusted odds ratio, 1.21; 95% confidence, 1.19-1.23), compared with not using HRT drugs. The highest risk was tied to combined estrogen/progestogen HRT (adjusted OR, 1.26; 95% CI, 1.24-1.29). The lowest risk was tied to estrogen-only HRT (adjusted OR, 1.06; 95% CI, 1.03-1.10). Estrogen cream and vaginal estrogen were not associated with increased breast cancer risk.
In general, breast cancer risk was higher among recent HRT users and those receiving long-term therapy. HRT-associated breast cancer risk increased with age and declined after discontinuing treatment. Therapy of less than 1 year was not associated with increased breast cancer risk.
Women who had recently been receiving long-term combined estrogen/progestogen HRT had a 79% increased risk for breast cancer (adjusted OR, 1.79; 95% CI, 1.73-1.85), compared with never-users. Among recent long-term users of combined HRT, breast cancer risk was highest for norethisterone (adjusted OR, 1.88; 95% CI, 1.79-1.99) and lowest for dydrogesterone (adjusted OR, 1.24; 95% CI, 1.03-1.48). Women who had recently been receiving long-term estrogen-only HRT had a 15% increased risk for breast cancer compared to never-users (adjusted OR, 1.15; 95% CI, 1.09-1.21).
Among women who discontinued HRT 5 or more years ago, risk for breast cancer was no longer increased for long-term estrogen-only therapy and short-term estrogen/progestogen therapy. However, breast cancer risk remained elevated 5 years after discontinuing long-term estrogen/progestogen (adjusted OR, 1.16; 95% CI, 1.11-1.21).
HRT-associated risk for breast cancer increased with age across all durations of therapy.
Compared with never-use, recent long-term estrogen-only therapy was associated with zero extra breast cancer cases per 10,000 women-years among women aged 50-59 years and eight extra cases per 10,000 women-years among women aged 70-79.
Recent long-term estrogen/progestogen use was associated with 15 extra breast cancer cases among women aged 50-59 and 36 extra cases among women aged 70-79 per 10,000 women-years.
Past long-term estrogen/progestogen use was associated with zero extra breast cancer cases among women aged 50-59 and eight extra cases among women aged 70-79 per 10,000 women-years.
Summarizing, Dr. Vinogradova said the increased risk for breast cancer with HRT appears to be “relatively small, particularly for younger women and for any women who use HRT only for a restricted period.”
Decisions about whether to use HRT and which type to use should depend on symptom severity, patient factors, and suitability of other treatment options, she commented.
“Particularly for those women who our study has shown to be most at risk, these decisions should be made through discussions between the patient and her doctor,” she concluded. “We hope that the new and more detailed information provided by our study will facilitate such prescribing decisions.”
The study was partially funded by the School for Primary Care Research of the National Institute for Health Research, by Cancer Research UK, and by the Cancer Research UK Oxford Center. Dr. Vinogradova has disclosed no relevant financial relationships. Senior author Julia Hippisley-Cox is an unpaid director of QResearch and was a paid director of ClinRisk until 2019. The other authors have disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
SRS instead of WBRT for patients with multiple brain metastases
Stereotactic radiosurgery (SRS) should replace whole-brain radiotherapy (WBRT) as the new standard of care for patients with four or more brain metastases, say researchers who report results from a randomized trial conducted in patients with four to 15 brain metastases
“SRS was associated with reduced risk of neurocognitive deterioration compared to WBRT, as demonstrated by a constellation of neurocognitive tests, individually or by composite scores,” said lead author Jing Li, MD, PhD, associate professor of radiation oncology and codirector of the Brain Metastasis Clinic at the University of Texas MD Anderson Cancer Center, Houston.
She was speaking at the American Society for Radiation Oncology (ASTRO) 2020 Annual Meeting, which was held online this year because of the COVID pandemic.
“The results from this phase 3 randomized trial strongly support the use of SRS in patients with four to 15 brain metastases to better preserve cognitive function and to minimize interruption of systemic therapy, without compromising overall survival,” said Li.
SRS is already the standard of care for patients with one to three brain metastases. Two previous phase 3 randomized trials showed that SRS was better at preserving cognitive function without compromising overall survival in comparison to WBRT.
However, there has been some controversy over the use of SRS for patients with multiple brain metastases, commented study discussant Sue S. Yom, MD, PhD, a professor in the Departments of Radiation Oncology and Otolaryngology–Head and Neck Surgery, University of California, San Francisco.
This study has shown, “in a practice-changing manner, that giving SRS can improve the quality of life of patients with metastatic disease,” she said.
Up to 30% of cancer patients develop brain metastases. Historically, these have been associated with poor overall survival, in the range of 1 to 4 months.
Reduces cognitive decline
The new trial involved 72 patients with four to 15 untreated, nonmelanoma brain metastases (up to 20 lesions were allowed at the time of treatment); the median number of brain metastases was eight. Most (83%) of the trial participants were White, nearly half were aged 60 years or older, and 58% were women.
Patients were randomly assigned to receive either SRS (15–24 Gy per Radiation Therapy Oncology Group protocol 9005) or WBRT (30 Gy in 10 fractions). On the basis of previous research, 62% of patients in the WBRT arm were also given memantine, a dementia drug that can help preserve cognitive function.
All participants completed neurocognitive testing, including testing of learning, memory, attention span, executive function, verbal fluency, processing speed, and motor dexterity, at enrollment and longitudinally.
The primary endpoints were Hopkins Verbal Learning Test – Revised Total Recall (HVLT-R TR) score and local control at 4 months. Secondary endpoints included overall survival, distant brain failure, toxicity, and time to initiation of systemic therapy.
In the primary endpoint analysis, at 4 months, the HVLT-R TR standardized z-score increased by +0.21 (standard error [SE], 0.27) for patients who received SRS, but it declined by –0.74 (SE, 0.36) for WBRT-treated patients (P = .041). On the basis of Clinical Trial Battery Composite score, neurocognitive function of patients in the SRS arm improved on average +0.23 (SE, 0.14) but declined an average –0.73 (SE, 0.35) in the WBRT arm (P = .008).
Li pointed out that there was also a “clinically meaningful and statistically significant benefit” with SRS at 1 month (P = .033) and 6 months (P = .012).
A total of 69 patients (35 for SRS and 34 for WBRT) were evaluable for overall survival, which was similar between the groups (SRS median, 7.8 months; WBRT median, 8.9 months; P = .59). Treatment with SRS resulted in better local control rates (95% at 4 months with SRS and 86.7% with WBRT; P = .09), but the median time to distant brain failure was shorter (10.5 months for WBRT and 6.3 months for SRS; P = .37).
In her discussion of the study, Yom noted that overall survival time was similar in the two arms and that, numerically, it may have even been a little longer in the SRS group. “While it is true that they had more relapses in untreated portions of the brain, they lived as long or longer than those who received WBRT and had better cognitive function,” she noted
Yom also noted that of particular importance was the finding that SRS was associated with shorter interruptions of systemic therapy (time to systemic therapy: SRS, 1.7 weeks; WBRT, 4.1 weeks; P = .001). Patients with metastatic disease usually have cancer in locations other than the brain. They may be receiving some type of systemic therapy, which is interrupted with WBRT, Li commented.
Toxicities of grade 3 or higher were observed in four patients in the WBRT arm and two in the SRS arm. Radiographic evidence of radiation necrosis, a side effect associated with SRS, was observed in 17% patients in the SRS arm of the trial (4% of all treated lesions).
The trial was halted early owing to the publication of another phase 3 trial (NRG Oncology CC 001), which provided level 1 evidence for replacing standard WBRT with hippocampal-avoidance WBRT. Despite the early trial termination, Li concluded that these results “strongly support the use of SRS in patients with four to 15 brain metastases to better preserve cognitive function and to minimize interruption of systemic therapy, without compromising overall survival.”
Li has received research funding from BMS and Medtronic and honorarium from Novocure and Monteris.
This article first appeared on Medscape.com.
Stereotactic radiosurgery (SRS) should replace whole-brain radiotherapy (WBRT) as the new standard of care for patients with four or more brain metastases, say researchers who report results from a randomized trial conducted in patients with four to 15 brain metastases
“SRS was associated with reduced risk of neurocognitive deterioration compared to WBRT, as demonstrated by a constellation of neurocognitive tests, individually or by composite scores,” said lead author Jing Li, MD, PhD, associate professor of radiation oncology and codirector of the Brain Metastasis Clinic at the University of Texas MD Anderson Cancer Center, Houston.
She was speaking at the American Society for Radiation Oncology (ASTRO) 2020 Annual Meeting, which was held online this year because of the COVID pandemic.
“The results from this phase 3 randomized trial strongly support the use of SRS in patients with four to 15 brain metastases to better preserve cognitive function and to minimize interruption of systemic therapy, without compromising overall survival,” said Li.
SRS is already the standard of care for patients with one to three brain metastases. Two previous phase 3 randomized trials showed that SRS was better at preserving cognitive function without compromising overall survival in comparison to WBRT.
However, there has been some controversy over the use of SRS for patients with multiple brain metastases, commented study discussant Sue S. Yom, MD, PhD, a professor in the Departments of Radiation Oncology and Otolaryngology–Head and Neck Surgery, University of California, San Francisco.
This study has shown, “in a practice-changing manner, that giving SRS can improve the quality of life of patients with metastatic disease,” she said.
Up to 30% of cancer patients develop brain metastases. Historically, these have been associated with poor overall survival, in the range of 1 to 4 months.
Reduces cognitive decline
The new trial involved 72 patients with four to 15 untreated, nonmelanoma brain metastases (up to 20 lesions were allowed at the time of treatment); the median number of brain metastases was eight. Most (83%) of the trial participants were White, nearly half were aged 60 years or older, and 58% were women.
Patients were randomly assigned to receive either SRS (15–24 Gy per Radiation Therapy Oncology Group protocol 9005) or WBRT (30 Gy in 10 fractions). On the basis of previous research, 62% of patients in the WBRT arm were also given memantine, a dementia drug that can help preserve cognitive function.
All participants completed neurocognitive testing, including testing of learning, memory, attention span, executive function, verbal fluency, processing speed, and motor dexterity, at enrollment and longitudinally.
The primary endpoints were Hopkins Verbal Learning Test – Revised Total Recall (HVLT-R TR) score and local control at 4 months. Secondary endpoints included overall survival, distant brain failure, toxicity, and time to initiation of systemic therapy.
In the primary endpoint analysis, at 4 months, the HVLT-R TR standardized z-score increased by +0.21 (standard error [SE], 0.27) for patients who received SRS, but it declined by –0.74 (SE, 0.36) for WBRT-treated patients (P = .041). On the basis of Clinical Trial Battery Composite score, neurocognitive function of patients in the SRS arm improved on average +0.23 (SE, 0.14) but declined an average –0.73 (SE, 0.35) in the WBRT arm (P = .008).
Li pointed out that there was also a “clinically meaningful and statistically significant benefit” with SRS at 1 month (P = .033) and 6 months (P = .012).
A total of 69 patients (35 for SRS and 34 for WBRT) were evaluable for overall survival, which was similar between the groups (SRS median, 7.8 months; WBRT median, 8.9 months; P = .59). Treatment with SRS resulted in better local control rates (95% at 4 months with SRS and 86.7% with WBRT; P = .09), but the median time to distant brain failure was shorter (10.5 months for WBRT and 6.3 months for SRS; P = .37).
In her discussion of the study, Yom noted that overall survival time was similar in the two arms and that, numerically, it may have even been a little longer in the SRS group. “While it is true that they had more relapses in untreated portions of the brain, they lived as long or longer than those who received WBRT and had better cognitive function,” she noted
Yom also noted that of particular importance was the finding that SRS was associated with shorter interruptions of systemic therapy (time to systemic therapy: SRS, 1.7 weeks; WBRT, 4.1 weeks; P = .001). Patients with metastatic disease usually have cancer in locations other than the brain. They may be receiving some type of systemic therapy, which is interrupted with WBRT, Li commented.
Toxicities of grade 3 or higher were observed in four patients in the WBRT arm and two in the SRS arm. Radiographic evidence of radiation necrosis, a side effect associated with SRS, was observed in 17% patients in the SRS arm of the trial (4% of all treated lesions).
The trial was halted early owing to the publication of another phase 3 trial (NRG Oncology CC 001), which provided level 1 evidence for replacing standard WBRT with hippocampal-avoidance WBRT. Despite the early trial termination, Li concluded that these results “strongly support the use of SRS in patients with four to 15 brain metastases to better preserve cognitive function and to minimize interruption of systemic therapy, without compromising overall survival.”
Li has received research funding from BMS and Medtronic and honorarium from Novocure and Monteris.
This article first appeared on Medscape.com.
Stereotactic radiosurgery (SRS) should replace whole-brain radiotherapy (WBRT) as the new standard of care for patients with four or more brain metastases, say researchers who report results from a randomized trial conducted in patients with four to 15 brain metastases
“SRS was associated with reduced risk of neurocognitive deterioration compared to WBRT, as demonstrated by a constellation of neurocognitive tests, individually or by composite scores,” said lead author Jing Li, MD, PhD, associate professor of radiation oncology and codirector of the Brain Metastasis Clinic at the University of Texas MD Anderson Cancer Center, Houston.
She was speaking at the American Society for Radiation Oncology (ASTRO) 2020 Annual Meeting, which was held online this year because of the COVID pandemic.
“The results from this phase 3 randomized trial strongly support the use of SRS in patients with four to 15 brain metastases to better preserve cognitive function and to minimize interruption of systemic therapy, without compromising overall survival,” said Li.
SRS is already the standard of care for patients with one to three brain metastases. Two previous phase 3 randomized trials showed that SRS was better at preserving cognitive function without compromising overall survival in comparison to WBRT.
However, there has been some controversy over the use of SRS for patients with multiple brain metastases, commented study discussant Sue S. Yom, MD, PhD, a professor in the Departments of Radiation Oncology and Otolaryngology–Head and Neck Surgery, University of California, San Francisco.
This study has shown, “in a practice-changing manner, that giving SRS can improve the quality of life of patients with metastatic disease,” she said.
Up to 30% of cancer patients develop brain metastases. Historically, these have been associated with poor overall survival, in the range of 1 to 4 months.
Reduces cognitive decline
The new trial involved 72 patients with four to 15 untreated, nonmelanoma brain metastases (up to 20 lesions were allowed at the time of treatment); the median number of brain metastases was eight. Most (83%) of the trial participants were White, nearly half were aged 60 years or older, and 58% were women.
Patients were randomly assigned to receive either SRS (15–24 Gy per Radiation Therapy Oncology Group protocol 9005) or WBRT (30 Gy in 10 fractions). On the basis of previous research, 62% of patients in the WBRT arm were also given memantine, a dementia drug that can help preserve cognitive function.
All participants completed neurocognitive testing, including testing of learning, memory, attention span, executive function, verbal fluency, processing speed, and motor dexterity, at enrollment and longitudinally.
The primary endpoints were Hopkins Verbal Learning Test – Revised Total Recall (HVLT-R TR) score and local control at 4 months. Secondary endpoints included overall survival, distant brain failure, toxicity, and time to initiation of systemic therapy.
In the primary endpoint analysis, at 4 months, the HVLT-R TR standardized z-score increased by +0.21 (standard error [SE], 0.27) for patients who received SRS, but it declined by –0.74 (SE, 0.36) for WBRT-treated patients (P = .041). On the basis of Clinical Trial Battery Composite score, neurocognitive function of patients in the SRS arm improved on average +0.23 (SE, 0.14) but declined an average –0.73 (SE, 0.35) in the WBRT arm (P = .008).
Li pointed out that there was also a “clinically meaningful and statistically significant benefit” with SRS at 1 month (P = .033) and 6 months (P = .012).
A total of 69 patients (35 for SRS and 34 for WBRT) were evaluable for overall survival, which was similar between the groups (SRS median, 7.8 months; WBRT median, 8.9 months; P = .59). Treatment with SRS resulted in better local control rates (95% at 4 months with SRS and 86.7% with WBRT; P = .09), but the median time to distant brain failure was shorter (10.5 months for WBRT and 6.3 months for SRS; P = .37).
In her discussion of the study, Yom noted that overall survival time was similar in the two arms and that, numerically, it may have even been a little longer in the SRS group. “While it is true that they had more relapses in untreated portions of the brain, they lived as long or longer than those who received WBRT and had better cognitive function,” she noted
Yom also noted that of particular importance was the finding that SRS was associated with shorter interruptions of systemic therapy (time to systemic therapy: SRS, 1.7 weeks; WBRT, 4.1 weeks; P = .001). Patients with metastatic disease usually have cancer in locations other than the brain. They may be receiving some type of systemic therapy, which is interrupted with WBRT, Li commented.
Toxicities of grade 3 or higher were observed in four patients in the WBRT arm and two in the SRS arm. Radiographic evidence of radiation necrosis, a side effect associated with SRS, was observed in 17% patients in the SRS arm of the trial (4% of all treated lesions).
The trial was halted early owing to the publication of another phase 3 trial (NRG Oncology CC 001), which provided level 1 evidence for replacing standard WBRT with hippocampal-avoidance WBRT. Despite the early trial termination, Li concluded that these results “strongly support the use of SRS in patients with four to 15 brain metastases to better preserve cognitive function and to minimize interruption of systemic therapy, without compromising overall survival.”
Li has received research funding from BMS and Medtronic and honorarium from Novocure and Monteris.
This article first appeared on Medscape.com.
Cancer therapy affects sexual health in most patients
Sexual dysfunction is a common treatment-related problem observed across numerous cancer diagnoses, and a new survey finds that 87% of cancer survivors have had such problems.
However, most of them also reported that their oncologist had not formally discussed the topic, and female patients were particularly unlikely to be asked about sexual dysfunction.
“The main takeaway from our study is that sexual side effects following treatment are very common,” said lead author James Taylor, MD, MPH, chief resident in radiation oncology at the Sidney Kimmel Cancer Center at Thomas Jefferson University in Philadelphia, Pennsylvania.
“Nearly 9 in 10 patients reported some change after cancer treatment that negatively affected their sexual health,” he said.
Taylor was speaking at the American Society for Radiation Oncology (ASTRO) Annual Meeting, held virtually this year because of the pandemic.
“Negative effects on sexual health after cancer treatment are unfortunately very common,” he said. “This is not just patients treated with radiation but this includes chemotherapy, hormonal therapy, surgery, and other treatment modalities.”
Potential issues include physical complications such as erectile dysfunction with prostate cancer treatment or vaginal dryness with gynecological cancer treatment. One recent study found that one-third of men who had undergone treatment for prostate cancer reported that a subsequent lack of sexual function has had the greatest impact on their quality of life. Another study reported that nearly all patients with breast cancer taking endocrine therapy experience a high degree of sexual dysfunction, including vulvovaginal dryness and severe dyspareunia.
Not discussed, not warned
Taylor and colleagues developed a questionnaire with input from radiation oncologists, medical oncologists, and surgeons, which consisted of more than 25 questions and was specifically targeted at cancer survivors.
A total of 405 adults completed the electronic survey about their experiences with sexual side effects after cancer treatment (391 responses were eligible for analysis). Most of the respondents were women (81%), and the most common cancer types were breast (67%), prostate (16%), and endometrial (6%). Treatments included chemotherapy (78%), radiation therapy (54%), and hormone therapy (47%).
“The questionnaires were distributed at Thomas Jefferson and throughout social media,” said Taylor. “The responses from social media are important because it shows a broad representation of patients who are treated in multiple clinics across the United States.”
Most of the survivors who responded (n = 337, 87%) stated cancer treatment had impacted sexual function or desire, with 53.8% reporting body image distortion, 73.4% with dyspareunia, and 42.3% unable to achieve orgasm.
Only about one-quarter (27.9%) said they had been formally asked about their sexual health by their clinician.
“Only about 40% said that they have been preemptively warned that their sexual health may be affected by treatment,” said Taylor.
Women were far less likely to be asked about their sexual health by their provider. The survey showed that male respondents were twice as likely to say they had been asked about sexual health and counseled about the potential toxicity (53% vs 22%; P < .001), and a substantially higher percentage of men reported receiving a formal assessment tool such as a survey (32% vs 5%; P = .001) compared with female respondents.
Taylor noted that the survey demonstrated several things. “One is that sexual toxicity is exceedingly common, and number two, it identified a gender disparity,” he said. “But number 3, and I think that this is an important aspect of our study, is that the majority of respondents felt that they would like a standard questionnaire to initiate and guide a discussion on sexual health with their provider.”
The reason that aspect is very important, he emphasized, is that “we know metrics and questionnaires already exist, so this gives us an actionable intervention that we can distribute and help mitigate some of these disparities.”
Importance of being holistic
The results of the survey “highlight the importance of being holistic in our approach to patient survivorship,” commented Karen Winkfield, MD, PhD, associate professor of radiation oncology at Wake Forest University, Winston-Salem, North Carolina, and executive director of the Meharry-Vanderbilt Alliance, Nashville, Tennessee.
“We need to ask patients about all parts of their well-being, including sexual health,” Winkfield said. “Body dysmorphism can impact anyone, but especially patients who have had surgery or radiation,” she said, while chemotherapy can impact energy and libido and have other toxicities that impact sexual health.
“I encourage all oncologists to ask patients about their sexual health, and a standardized form that can be used across all sites will make this much easier,” Winkfield commented. “We owe it to our patients to treat them holistically.”
The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Sexual dysfunction is a common treatment-related problem observed across numerous cancer diagnoses, and a new survey finds that 87% of cancer survivors have had such problems.
However, most of them also reported that their oncologist had not formally discussed the topic, and female patients were particularly unlikely to be asked about sexual dysfunction.
“The main takeaway from our study is that sexual side effects following treatment are very common,” said lead author James Taylor, MD, MPH, chief resident in radiation oncology at the Sidney Kimmel Cancer Center at Thomas Jefferson University in Philadelphia, Pennsylvania.
“Nearly 9 in 10 patients reported some change after cancer treatment that negatively affected their sexual health,” he said.
Taylor was speaking at the American Society for Radiation Oncology (ASTRO) Annual Meeting, held virtually this year because of the pandemic.
“Negative effects on sexual health after cancer treatment are unfortunately very common,” he said. “This is not just patients treated with radiation but this includes chemotherapy, hormonal therapy, surgery, and other treatment modalities.”
Potential issues include physical complications such as erectile dysfunction with prostate cancer treatment or vaginal dryness with gynecological cancer treatment. One recent study found that one-third of men who had undergone treatment for prostate cancer reported that a subsequent lack of sexual function has had the greatest impact on their quality of life. Another study reported that nearly all patients with breast cancer taking endocrine therapy experience a high degree of sexual dysfunction, including vulvovaginal dryness and severe dyspareunia.
Not discussed, not warned
Taylor and colleagues developed a questionnaire with input from radiation oncologists, medical oncologists, and surgeons, which consisted of more than 25 questions and was specifically targeted at cancer survivors.
A total of 405 adults completed the electronic survey about their experiences with sexual side effects after cancer treatment (391 responses were eligible for analysis). Most of the respondents were women (81%), and the most common cancer types were breast (67%), prostate (16%), and endometrial (6%). Treatments included chemotherapy (78%), radiation therapy (54%), and hormone therapy (47%).
“The questionnaires were distributed at Thomas Jefferson and throughout social media,” said Taylor. “The responses from social media are important because it shows a broad representation of patients who are treated in multiple clinics across the United States.”
Most of the survivors who responded (n = 337, 87%) stated cancer treatment had impacted sexual function or desire, with 53.8% reporting body image distortion, 73.4% with dyspareunia, and 42.3% unable to achieve orgasm.
Only about one-quarter (27.9%) said they had been formally asked about their sexual health by their clinician.
“Only about 40% said that they have been preemptively warned that their sexual health may be affected by treatment,” said Taylor.
Women were far less likely to be asked about their sexual health by their provider. The survey showed that male respondents were twice as likely to say they had been asked about sexual health and counseled about the potential toxicity (53% vs 22%; P < .001), and a substantially higher percentage of men reported receiving a formal assessment tool such as a survey (32% vs 5%; P = .001) compared with female respondents.
Taylor noted that the survey demonstrated several things. “One is that sexual toxicity is exceedingly common, and number two, it identified a gender disparity,” he said. “But number 3, and I think that this is an important aspect of our study, is that the majority of respondents felt that they would like a standard questionnaire to initiate and guide a discussion on sexual health with their provider.”
The reason that aspect is very important, he emphasized, is that “we know metrics and questionnaires already exist, so this gives us an actionable intervention that we can distribute and help mitigate some of these disparities.”
Importance of being holistic
The results of the survey “highlight the importance of being holistic in our approach to patient survivorship,” commented Karen Winkfield, MD, PhD, associate professor of radiation oncology at Wake Forest University, Winston-Salem, North Carolina, and executive director of the Meharry-Vanderbilt Alliance, Nashville, Tennessee.
“We need to ask patients about all parts of their well-being, including sexual health,” Winkfield said. “Body dysmorphism can impact anyone, but especially patients who have had surgery or radiation,” she said, while chemotherapy can impact energy and libido and have other toxicities that impact sexual health.
“I encourage all oncologists to ask patients about their sexual health, and a standardized form that can be used across all sites will make this much easier,” Winkfield commented. “We owe it to our patients to treat them holistically.”
The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Sexual dysfunction is a common treatment-related problem observed across numerous cancer diagnoses, and a new survey finds that 87% of cancer survivors have had such problems.
However, most of them also reported that their oncologist had not formally discussed the topic, and female patients were particularly unlikely to be asked about sexual dysfunction.
“The main takeaway from our study is that sexual side effects following treatment are very common,” said lead author James Taylor, MD, MPH, chief resident in radiation oncology at the Sidney Kimmel Cancer Center at Thomas Jefferson University in Philadelphia, Pennsylvania.
“Nearly 9 in 10 patients reported some change after cancer treatment that negatively affected their sexual health,” he said.
Taylor was speaking at the American Society for Radiation Oncology (ASTRO) Annual Meeting, held virtually this year because of the pandemic.
“Negative effects on sexual health after cancer treatment are unfortunately very common,” he said. “This is not just patients treated with radiation but this includes chemotherapy, hormonal therapy, surgery, and other treatment modalities.”
Potential issues include physical complications such as erectile dysfunction with prostate cancer treatment or vaginal dryness with gynecological cancer treatment. One recent study found that one-third of men who had undergone treatment for prostate cancer reported that a subsequent lack of sexual function has had the greatest impact on their quality of life. Another study reported that nearly all patients with breast cancer taking endocrine therapy experience a high degree of sexual dysfunction, including vulvovaginal dryness and severe dyspareunia.
Not discussed, not warned
Taylor and colleagues developed a questionnaire with input from radiation oncologists, medical oncologists, and surgeons, which consisted of more than 25 questions and was specifically targeted at cancer survivors.
A total of 405 adults completed the electronic survey about their experiences with sexual side effects after cancer treatment (391 responses were eligible for analysis). Most of the respondents were women (81%), and the most common cancer types were breast (67%), prostate (16%), and endometrial (6%). Treatments included chemotherapy (78%), radiation therapy (54%), and hormone therapy (47%).
“The questionnaires were distributed at Thomas Jefferson and throughout social media,” said Taylor. “The responses from social media are important because it shows a broad representation of patients who are treated in multiple clinics across the United States.”
Most of the survivors who responded (n = 337, 87%) stated cancer treatment had impacted sexual function or desire, with 53.8% reporting body image distortion, 73.4% with dyspareunia, and 42.3% unable to achieve orgasm.
Only about one-quarter (27.9%) said they had been formally asked about their sexual health by their clinician.
“Only about 40% said that they have been preemptively warned that their sexual health may be affected by treatment,” said Taylor.
Women were far less likely to be asked about their sexual health by their provider. The survey showed that male respondents were twice as likely to say they had been asked about sexual health and counseled about the potential toxicity (53% vs 22%; P < .001), and a substantially higher percentage of men reported receiving a formal assessment tool such as a survey (32% vs 5%; P = .001) compared with female respondents.
Taylor noted that the survey demonstrated several things. “One is that sexual toxicity is exceedingly common, and number two, it identified a gender disparity,” he said. “But number 3, and I think that this is an important aspect of our study, is that the majority of respondents felt that they would like a standard questionnaire to initiate and guide a discussion on sexual health with their provider.”
The reason that aspect is very important, he emphasized, is that “we know metrics and questionnaires already exist, so this gives us an actionable intervention that we can distribute and help mitigate some of these disparities.”
Importance of being holistic
The results of the survey “highlight the importance of being holistic in our approach to patient survivorship,” commented Karen Winkfield, MD, PhD, associate professor of radiation oncology at Wake Forest University, Winston-Salem, North Carolina, and executive director of the Meharry-Vanderbilt Alliance, Nashville, Tennessee.
“We need to ask patients about all parts of their well-being, including sexual health,” Winkfield said. “Body dysmorphism can impact anyone, but especially patients who have had surgery or radiation,” she said, while chemotherapy can impact energy and libido and have other toxicities that impact sexual health.
“I encourage all oncologists to ask patients about their sexual health, and a standardized form that can be used across all sites will make this much easier,” Winkfield commented. “We owe it to our patients to treat them holistically.”
The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
‘Tour de force’ study reveals therapeutic targets in 38% of cancer patients
The effort is the National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) trial. For this study, researchers performed next-generation sequencing on tumor biopsy specimens to identify therapeutically actionable molecular alterations in patients with “underexplored” cancer types.
The trial included 5,954 patients with cancers that had progressed on standard treatments or rare cancers for which there is no standard treatment. If actionable alterations were found in these patients, they could receive new drugs in development that showed promise in other clinical trials or drugs that were approved by the Food and Drug Administration to treat at least one cancer type.
Data newly reported in the Journal of Clinical Oncology showed that 37.6% of patients had alterations that could be matched to targeted drugs, and 17.8% of patients were assigned to targeted treatment. Multiple actionable tumor mutations were seen in 11.9% of specimens, and resistance-conferring mutations were seen in 71.3% of specimens.
“The bottom line from this report is that next-generation sequencing is an efficient way to identify both approved and promising investigational therapies. For this reason, it should be considered standard of care for patients with advanced cancers,” said study chair Keith T. Flaherty, MD, director of the Henri and Belinda Termeer Center for Targeted Therapy at Massachusetts General Hospital Cancer Center in Boston.
“This study sets the benchmark for the ‘actionability’ of next-generation sequencing,” Dr. Flaherty added. “We expect this number [of actionable alterations] will continue to rise steadily as further advances are made in the development of therapies that target some of the genetic alterations for which we could not offer treatment options in NCI-MATCH.”
Relapsed/refractory vs. primary tumors
The NCI-MATCH researchers focused on the most commonly found genetic alterations and performed biopsies at study entry to provide the most accurate picture of the genetic landscape of relapsed/refractory cancer patients. That makes this cohort distinct from The Cancer Genome Atlas (TCGA), a database of patients with mostly untreated primary tumors, and other published cohorts that include genetic analysis of primary tumors and biopsies from the time of initial metastatic recurrence.
The researchers compared the tumor gene makeup of NCI-MATCH and TCGA patients with seven cancer types – breast, bile duct, cervix, colorectal, lung, pancreas, and prostate.
“Perhaps the biggest surprise was the relatively minimal change in the genetic alterations found in these relapsed/refractory patients, compared to primary tumors,” Dr. Flaherty said. “These findings suggest that it is very reasonable to perform next-generation sequencing at the time of initial metastatic cancer diagnosis and to rely on those findings for the purposes of considering FDA-approved therapies and clinical trial participation.”
Multiple alterations and resistance
The complex genetics of cancers has led researchers to explore combinations of targeted and other therapies to address multiple defects at the same time.
“Not surprisingly, the most common collision of multiple genetic alterations within the same tumor was in the commonly altered tumor suppressor genes: TP53, APC, and PTEN,” Dr. Flaherty said.
“An increasing body of evidence supports a role for loss-of-function alterations in these genes to confer resistance to many targeted therapies,” he added. “While we don’t have targeted therapies yet established to directly counter this form of therapeutic resistance, we hypothesize that various types of combination therapy may be able to indirectly undercut resistance and enhance the benefit of many targeted therapies.”
The NCI-MATCH researchers will continue to mine this large dataset to better understand the many small, genetically defined cancer subpopulations.
“We will continue to report the outcome of the individual treatment subprotocols, and combining this genetic analysis with those outcomes will likely inform the next clinical trials,” Dr. Flaherty said.
Actionable mutations make a difference
Precision oncology experts agree that NCI-MATCH results are impressive and add a fuller appreciation that actionable mutations make a clinical difference.
“This is a powerful, extremely well-designed study, a tour de force of collaborative science,” said Stephen Gruber, MD, PhD, director of the Center for Precision Medicine at City of Hope National Medical Center in Duarte, Calif.
“The future holds even more promise,” he added. “Our ability to interrogate the genomic landscape of cancer is improving rapidly. Tumor testing helps get the right drug to the right tumor faster than a guidelines-based approach from historical data of combination chemotherapy. This is a likely game changer for the way oncologists will practice in the future, especially as we learn more results of subset trials. The NCI-MATCH researchers have taken a laser-focused look at the current data, but we now know we can look far more comprehensively at genomic profiles of tumors.”
From the viewpoint of the practicing oncologist, co-occurring resistance mutations make a difference in defining what combinations are likely and, more importantly, less likely to be effective. “When we see two mutations and one is likely to confer resistance, we can make a choice to avoid a drug that is not likely to work,” Dr. Gruber said.
“The NCI-MATCH trial allows both approved and investigational agents, which expands the possibility of matching patients to newer agents. This is especially relevant if there are no FDA-approved drugs yet for some molecular aberrations,” said Lillian L. Siu, MD, a senior medical oncologist at the Princess Margaret Cancer Centre in Toronto. “This trial enables such evaluations under the auspice of a clinical trial to provide important knowledge.”
Both experts agree that in-depth biological interrogations of cancer will move the field of precision oncology forward. Dr. Gruber said that “studies have not yet fully addressed the power of germline genetic testing of DNA. Inherited susceptibility will drive therapeutic choices – for example, PARP inhibitors that access homologous recombination deficiency for breast, ovarian, and prostate cancer. We will learn more about treatment choices for those cancers.”
Dr. Siu added: “I truly believe that liquid biopsies [circulating tumor DNA] will help us perform target-drug matching in a less invasive way. We need to explore beyond the genome to look at the transcriptome, proteome, epigenome, and immunome, among others. It is likely that multiomic predictors are going to be able to identify more therapeutic options compared to single genomic predictors.”
Dr. Flaherty noted that all tumor samples from patients assigned to treatment are being subjected to whole-exome sequencing to further the discovery of the genetic features of responsive and nonresponsive tumors.
NCI-MATCH was funded by the National Cancer Institute. Dr. Flaherty disclosed relationships with Clovis Oncology, Loxo, X4 Pharma, and many other companies. His coauthors disclosed many conflicts as well. Dr. Gruber is cofounder of Brogent International. Dr. Siu disclosed relationships with Agios, Treadwell Therapeutics, Merck, Pfizer, and many other companies.
SOURCE: Flaherty KT et al. J Clin Oncol. 2020 Oct 13. doi: 10.1200/JCO.19.03010.
The effort is the National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) trial. For this study, researchers performed next-generation sequencing on tumor biopsy specimens to identify therapeutically actionable molecular alterations in patients with “underexplored” cancer types.
The trial included 5,954 patients with cancers that had progressed on standard treatments or rare cancers for which there is no standard treatment. If actionable alterations were found in these patients, they could receive new drugs in development that showed promise in other clinical trials or drugs that were approved by the Food and Drug Administration to treat at least one cancer type.
Data newly reported in the Journal of Clinical Oncology showed that 37.6% of patients had alterations that could be matched to targeted drugs, and 17.8% of patients were assigned to targeted treatment. Multiple actionable tumor mutations were seen in 11.9% of specimens, and resistance-conferring mutations were seen in 71.3% of specimens.
“The bottom line from this report is that next-generation sequencing is an efficient way to identify both approved and promising investigational therapies. For this reason, it should be considered standard of care for patients with advanced cancers,” said study chair Keith T. Flaherty, MD, director of the Henri and Belinda Termeer Center for Targeted Therapy at Massachusetts General Hospital Cancer Center in Boston.
“This study sets the benchmark for the ‘actionability’ of next-generation sequencing,” Dr. Flaherty added. “We expect this number [of actionable alterations] will continue to rise steadily as further advances are made in the development of therapies that target some of the genetic alterations for which we could not offer treatment options in NCI-MATCH.”
Relapsed/refractory vs. primary tumors
The NCI-MATCH researchers focused on the most commonly found genetic alterations and performed biopsies at study entry to provide the most accurate picture of the genetic landscape of relapsed/refractory cancer patients. That makes this cohort distinct from The Cancer Genome Atlas (TCGA), a database of patients with mostly untreated primary tumors, and other published cohorts that include genetic analysis of primary tumors and biopsies from the time of initial metastatic recurrence.
The researchers compared the tumor gene makeup of NCI-MATCH and TCGA patients with seven cancer types – breast, bile duct, cervix, colorectal, lung, pancreas, and prostate.
“Perhaps the biggest surprise was the relatively minimal change in the genetic alterations found in these relapsed/refractory patients, compared to primary tumors,” Dr. Flaherty said. “These findings suggest that it is very reasonable to perform next-generation sequencing at the time of initial metastatic cancer diagnosis and to rely on those findings for the purposes of considering FDA-approved therapies and clinical trial participation.”
Multiple alterations and resistance
The complex genetics of cancers has led researchers to explore combinations of targeted and other therapies to address multiple defects at the same time.
“Not surprisingly, the most common collision of multiple genetic alterations within the same tumor was in the commonly altered tumor suppressor genes: TP53, APC, and PTEN,” Dr. Flaherty said.
“An increasing body of evidence supports a role for loss-of-function alterations in these genes to confer resistance to many targeted therapies,” he added. “While we don’t have targeted therapies yet established to directly counter this form of therapeutic resistance, we hypothesize that various types of combination therapy may be able to indirectly undercut resistance and enhance the benefit of many targeted therapies.”
The NCI-MATCH researchers will continue to mine this large dataset to better understand the many small, genetically defined cancer subpopulations.
“We will continue to report the outcome of the individual treatment subprotocols, and combining this genetic analysis with those outcomes will likely inform the next clinical trials,” Dr. Flaherty said.
Actionable mutations make a difference
Precision oncology experts agree that NCI-MATCH results are impressive and add a fuller appreciation that actionable mutations make a clinical difference.
“This is a powerful, extremely well-designed study, a tour de force of collaborative science,” said Stephen Gruber, MD, PhD, director of the Center for Precision Medicine at City of Hope National Medical Center in Duarte, Calif.
“The future holds even more promise,” he added. “Our ability to interrogate the genomic landscape of cancer is improving rapidly. Tumor testing helps get the right drug to the right tumor faster than a guidelines-based approach from historical data of combination chemotherapy. This is a likely game changer for the way oncologists will practice in the future, especially as we learn more results of subset trials. The NCI-MATCH researchers have taken a laser-focused look at the current data, but we now know we can look far more comprehensively at genomic profiles of tumors.”
From the viewpoint of the practicing oncologist, co-occurring resistance mutations make a difference in defining what combinations are likely and, more importantly, less likely to be effective. “When we see two mutations and one is likely to confer resistance, we can make a choice to avoid a drug that is not likely to work,” Dr. Gruber said.
“The NCI-MATCH trial allows both approved and investigational agents, which expands the possibility of matching patients to newer agents. This is especially relevant if there are no FDA-approved drugs yet for some molecular aberrations,” said Lillian L. Siu, MD, a senior medical oncologist at the Princess Margaret Cancer Centre in Toronto. “This trial enables such evaluations under the auspice of a clinical trial to provide important knowledge.”
Both experts agree that in-depth biological interrogations of cancer will move the field of precision oncology forward. Dr. Gruber said that “studies have not yet fully addressed the power of germline genetic testing of DNA. Inherited susceptibility will drive therapeutic choices – for example, PARP inhibitors that access homologous recombination deficiency for breast, ovarian, and prostate cancer. We will learn more about treatment choices for those cancers.”
Dr. Siu added: “I truly believe that liquid biopsies [circulating tumor DNA] will help us perform target-drug matching in a less invasive way. We need to explore beyond the genome to look at the transcriptome, proteome, epigenome, and immunome, among others. It is likely that multiomic predictors are going to be able to identify more therapeutic options compared to single genomic predictors.”
Dr. Flaherty noted that all tumor samples from patients assigned to treatment are being subjected to whole-exome sequencing to further the discovery of the genetic features of responsive and nonresponsive tumors.
NCI-MATCH was funded by the National Cancer Institute. Dr. Flaherty disclosed relationships with Clovis Oncology, Loxo, X4 Pharma, and many other companies. His coauthors disclosed many conflicts as well. Dr. Gruber is cofounder of Brogent International. Dr. Siu disclosed relationships with Agios, Treadwell Therapeutics, Merck, Pfizer, and many other companies.
SOURCE: Flaherty KT et al. J Clin Oncol. 2020 Oct 13. doi: 10.1200/JCO.19.03010.
The effort is the National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) trial. For this study, researchers performed next-generation sequencing on tumor biopsy specimens to identify therapeutically actionable molecular alterations in patients with “underexplored” cancer types.
The trial included 5,954 patients with cancers that had progressed on standard treatments or rare cancers for which there is no standard treatment. If actionable alterations were found in these patients, they could receive new drugs in development that showed promise in other clinical trials or drugs that were approved by the Food and Drug Administration to treat at least one cancer type.
Data newly reported in the Journal of Clinical Oncology showed that 37.6% of patients had alterations that could be matched to targeted drugs, and 17.8% of patients were assigned to targeted treatment. Multiple actionable tumor mutations were seen in 11.9% of specimens, and resistance-conferring mutations were seen in 71.3% of specimens.
“The bottom line from this report is that next-generation sequencing is an efficient way to identify both approved and promising investigational therapies. For this reason, it should be considered standard of care for patients with advanced cancers,” said study chair Keith T. Flaherty, MD, director of the Henri and Belinda Termeer Center for Targeted Therapy at Massachusetts General Hospital Cancer Center in Boston.
“This study sets the benchmark for the ‘actionability’ of next-generation sequencing,” Dr. Flaherty added. “We expect this number [of actionable alterations] will continue to rise steadily as further advances are made in the development of therapies that target some of the genetic alterations for which we could not offer treatment options in NCI-MATCH.”
Relapsed/refractory vs. primary tumors
The NCI-MATCH researchers focused on the most commonly found genetic alterations and performed biopsies at study entry to provide the most accurate picture of the genetic landscape of relapsed/refractory cancer patients. That makes this cohort distinct from The Cancer Genome Atlas (TCGA), a database of patients with mostly untreated primary tumors, and other published cohorts that include genetic analysis of primary tumors and biopsies from the time of initial metastatic recurrence.
The researchers compared the tumor gene makeup of NCI-MATCH and TCGA patients with seven cancer types – breast, bile duct, cervix, colorectal, lung, pancreas, and prostate.
“Perhaps the biggest surprise was the relatively minimal change in the genetic alterations found in these relapsed/refractory patients, compared to primary tumors,” Dr. Flaherty said. “These findings suggest that it is very reasonable to perform next-generation sequencing at the time of initial metastatic cancer diagnosis and to rely on those findings for the purposes of considering FDA-approved therapies and clinical trial participation.”
Multiple alterations and resistance
The complex genetics of cancers has led researchers to explore combinations of targeted and other therapies to address multiple defects at the same time.
“Not surprisingly, the most common collision of multiple genetic alterations within the same tumor was in the commonly altered tumor suppressor genes: TP53, APC, and PTEN,” Dr. Flaherty said.
“An increasing body of evidence supports a role for loss-of-function alterations in these genes to confer resistance to many targeted therapies,” he added. “While we don’t have targeted therapies yet established to directly counter this form of therapeutic resistance, we hypothesize that various types of combination therapy may be able to indirectly undercut resistance and enhance the benefit of many targeted therapies.”
The NCI-MATCH researchers will continue to mine this large dataset to better understand the many small, genetically defined cancer subpopulations.
“We will continue to report the outcome of the individual treatment subprotocols, and combining this genetic analysis with those outcomes will likely inform the next clinical trials,” Dr. Flaherty said.
Actionable mutations make a difference
Precision oncology experts agree that NCI-MATCH results are impressive and add a fuller appreciation that actionable mutations make a clinical difference.
“This is a powerful, extremely well-designed study, a tour de force of collaborative science,” said Stephen Gruber, MD, PhD, director of the Center for Precision Medicine at City of Hope National Medical Center in Duarte, Calif.
“The future holds even more promise,” he added. “Our ability to interrogate the genomic landscape of cancer is improving rapidly. Tumor testing helps get the right drug to the right tumor faster than a guidelines-based approach from historical data of combination chemotherapy. This is a likely game changer for the way oncologists will practice in the future, especially as we learn more results of subset trials. The NCI-MATCH researchers have taken a laser-focused look at the current data, but we now know we can look far more comprehensively at genomic profiles of tumors.”
From the viewpoint of the practicing oncologist, co-occurring resistance mutations make a difference in defining what combinations are likely and, more importantly, less likely to be effective. “When we see two mutations and one is likely to confer resistance, we can make a choice to avoid a drug that is not likely to work,” Dr. Gruber said.
“The NCI-MATCH trial allows both approved and investigational agents, which expands the possibility of matching patients to newer agents. This is especially relevant if there are no FDA-approved drugs yet for some molecular aberrations,” said Lillian L. Siu, MD, a senior medical oncologist at the Princess Margaret Cancer Centre in Toronto. “This trial enables such evaluations under the auspice of a clinical trial to provide important knowledge.”
Both experts agree that in-depth biological interrogations of cancer will move the field of precision oncology forward. Dr. Gruber said that “studies have not yet fully addressed the power of germline genetic testing of DNA. Inherited susceptibility will drive therapeutic choices – for example, PARP inhibitors that access homologous recombination deficiency for breast, ovarian, and prostate cancer. We will learn more about treatment choices for those cancers.”
Dr. Siu added: “I truly believe that liquid biopsies [circulating tumor DNA] will help us perform target-drug matching in a less invasive way. We need to explore beyond the genome to look at the transcriptome, proteome, epigenome, and immunome, among others. It is likely that multiomic predictors are going to be able to identify more therapeutic options compared to single genomic predictors.”
Dr. Flaherty noted that all tumor samples from patients assigned to treatment are being subjected to whole-exome sequencing to further the discovery of the genetic features of responsive and nonresponsive tumors.
NCI-MATCH was funded by the National Cancer Institute. Dr. Flaherty disclosed relationships with Clovis Oncology, Loxo, X4 Pharma, and many other companies. His coauthors disclosed many conflicts as well. Dr. Gruber is cofounder of Brogent International. Dr. Siu disclosed relationships with Agios, Treadwell Therapeutics, Merck, Pfizer, and many other companies.
SOURCE: Flaherty KT et al. J Clin Oncol. 2020 Oct 13. doi: 10.1200/JCO.19.03010.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Lower BP and better tumor control with drug combo?
It’s not ready for the clinic, but new research suggests that angiotensin receptor II blockers (ARBs) widely used to treat hypertension may improve responses to cancer immunotherapy agents targeted against the programmed death-1/ligand-1 (PD-1/PD-L1) pathway.
That conclusion comes from an observational study of 597 patients with more than 3 dozen different cancer types treated in clinical trials at the US National Institutes of Health. Investigators found that both objective response rates and 3-year overall survival (OS) rates were significantly higher for patients treated with a PD-1/PD-L1 inhibitor who were on ARBs, compared with patients who weren’t taking the antihypertensive agents.
An association was also seen between higher ORR and OS rates for patients taking ACE inhibitors, but it was not statistically significant, reported Julius Strauss, MD, from the Center for Cancer Research at the National Cancer Institute in Bethesda, Md.
All study patients received PD-1/PD-L1 inhibitors, and the ORR for patients treated with ARBs was 33.8%, compared with 19.5% for those treated with ACE inhibitors, and 17% for those who took neither drug. The respective complete response (CR) rates were 11.3%, 3.7%, and 3.1%.
Strauss discussed the data during an online briefing prior to his presentation of the findings during the 32nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, which is taking place virtually.
Several early studies have suggested that angiotensin II, in addition to its effect on blood pressure, can also affect cancer growth by leading to downstream production of two proteins: vascular endothelial growth factor (VEGF) and transforming growth factor–beta (TGF-beta), he explained.
“Both of these [proteins] have been linked to cancer growth and cancer resistance to immune system attack,” Strauss observed.
He also discussed the mechanics of possible effects. Angiotensin II increases VEGF and TGF-beta through binding to the AT1 receptor, but has the opposite effect when it binds to the AT2 receptor, resulting in a decrease in both of the growth factors, he added.
ACE inhibitors prevent the conversion of angiotensin I to angiotensin II, with the result being that the drugs indirectly block both the AT1 and AT2 receptors.
In contrast, ARBs block only the AT1 receptor and leave the AT2 counter-regulatory receptor alone, said Strauss.
More data, including on overall survival
Strauss and colleagues examined whether ACE inhibitors and/or ARBs could have an effect on the response to PD-1/PD-L1 immune checkpoint inhibitors delivered with or without other immunotherapies, such as anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) checkpoint inhibitors, or targeted agents such as tyrosine kinase inhibitors (TKIs).
They pooled data on 597 patients receiving PD-1/PD-L1 inhibitors in clinical trials for various cancers, including 71 receiving concomitant ARBs, 82 receiving an ACE inhibitor, and 444 who were not receiving either class of antihypertensives.
The above-mentioned improvement in ORR with ARBs compared with patients not receiving the drug was statistically significant (P = .001), as was the improvement in CR rates (P = .002). In contrast, neither ORR nor CR were significantly better with patients on ACE inhibitors compared with patients not taking these drugs.
In multiple regression analysis controlling for age, gender, body mass index (BMI), tumor type, and additional therapies given, the superior ORR and CR rates with ARBs remained (P = .039 and .002, respectively), while there continued to be no significant additional benefit with ACE inhibitors.
The median overall survival was 35.2 months for patients on ARBs, 26.2 months for those on ACE inhibitors, and 18.8 months for patients on neither drug. The respective 3-year OS rates were 48.1%, 37.2%, and 31.5%, with the difference between the ARB and no-drug groups being significant (P = .0078).
In regression analysis controlling for the factors mentioned before, the OS advantage with ARBs but not ACE inhibitors remained significant (P = .006 for ARBs, and .078 for ACE inhibitors).
Strauss emphasized that further study is needed to determine if AT1 blockade can improve outcomes when combined anti-PD-1/PD-L1-based therapy.
It might be reasonable for patients who are taking ACE inhibitors to control blood pressure and are also receiving immunotherapy with a PD-1/PD-L1 inhibitor to be switched to an ARB if it is deemed safe and if further research bears it out, said Strauss in response to a question from Medscape Medical News.
Hypothesis-generating study
Meeting cochair Emiliano Calvo, MD, PhD, from Hospital de Madrid Norte Sanchinarro in Madrid, who attended the media briefing but was not involved in the study, commented that hypothesis-generating research using drugs already on the market for other indications adds important value to cancer therapy.
James Gulley, MD, PhD, from the Center for Cancer Research at the NCI, also a meeting cochair, agreed with Calvo.
“Thinking about utilizing the data that already exists to really get hypothesis-generating questions, it also opens up the possibility for real-world data, real-world evidence from these big datasets from [electronic medical records] that we could really interrogate and understand what we might see and get these hypothesis-generating findings that we could then prospectively evaluate,” Gulley said.
The research was funded by the National Cancer Institute. Strauss and Gulley are National Cancer Institute employees. Calvo disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
It’s not ready for the clinic, but new research suggests that angiotensin receptor II blockers (ARBs) widely used to treat hypertension may improve responses to cancer immunotherapy agents targeted against the programmed death-1/ligand-1 (PD-1/PD-L1) pathway.
That conclusion comes from an observational study of 597 patients with more than 3 dozen different cancer types treated in clinical trials at the US National Institutes of Health. Investigators found that both objective response rates and 3-year overall survival (OS) rates were significantly higher for patients treated with a PD-1/PD-L1 inhibitor who were on ARBs, compared with patients who weren’t taking the antihypertensive agents.
An association was also seen between higher ORR and OS rates for patients taking ACE inhibitors, but it was not statistically significant, reported Julius Strauss, MD, from the Center for Cancer Research at the National Cancer Institute in Bethesda, Md.
All study patients received PD-1/PD-L1 inhibitors, and the ORR for patients treated with ARBs was 33.8%, compared with 19.5% for those treated with ACE inhibitors, and 17% for those who took neither drug. The respective complete response (CR) rates were 11.3%, 3.7%, and 3.1%.
Strauss discussed the data during an online briefing prior to his presentation of the findings during the 32nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, which is taking place virtually.
Several early studies have suggested that angiotensin II, in addition to its effect on blood pressure, can also affect cancer growth by leading to downstream production of two proteins: vascular endothelial growth factor (VEGF) and transforming growth factor–beta (TGF-beta), he explained.
“Both of these [proteins] have been linked to cancer growth and cancer resistance to immune system attack,” Strauss observed.
He also discussed the mechanics of possible effects. Angiotensin II increases VEGF and TGF-beta through binding to the AT1 receptor, but has the opposite effect when it binds to the AT2 receptor, resulting in a decrease in both of the growth factors, he added.
ACE inhibitors prevent the conversion of angiotensin I to angiotensin II, with the result being that the drugs indirectly block both the AT1 and AT2 receptors.
In contrast, ARBs block only the AT1 receptor and leave the AT2 counter-regulatory receptor alone, said Strauss.
More data, including on overall survival
Strauss and colleagues examined whether ACE inhibitors and/or ARBs could have an effect on the response to PD-1/PD-L1 immune checkpoint inhibitors delivered with or without other immunotherapies, such as anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) checkpoint inhibitors, or targeted agents such as tyrosine kinase inhibitors (TKIs).
They pooled data on 597 patients receiving PD-1/PD-L1 inhibitors in clinical trials for various cancers, including 71 receiving concomitant ARBs, 82 receiving an ACE inhibitor, and 444 who were not receiving either class of antihypertensives.
The above-mentioned improvement in ORR with ARBs compared with patients not receiving the drug was statistically significant (P = .001), as was the improvement in CR rates (P = .002). In contrast, neither ORR nor CR were significantly better with patients on ACE inhibitors compared with patients not taking these drugs.
In multiple regression analysis controlling for age, gender, body mass index (BMI), tumor type, and additional therapies given, the superior ORR and CR rates with ARBs remained (P = .039 and .002, respectively), while there continued to be no significant additional benefit with ACE inhibitors.
The median overall survival was 35.2 months for patients on ARBs, 26.2 months for those on ACE inhibitors, and 18.8 months for patients on neither drug. The respective 3-year OS rates were 48.1%, 37.2%, and 31.5%, with the difference between the ARB and no-drug groups being significant (P = .0078).
In regression analysis controlling for the factors mentioned before, the OS advantage with ARBs but not ACE inhibitors remained significant (P = .006 for ARBs, and .078 for ACE inhibitors).
Strauss emphasized that further study is needed to determine if AT1 blockade can improve outcomes when combined anti-PD-1/PD-L1-based therapy.
It might be reasonable for patients who are taking ACE inhibitors to control blood pressure and are also receiving immunotherapy with a PD-1/PD-L1 inhibitor to be switched to an ARB if it is deemed safe and if further research bears it out, said Strauss in response to a question from Medscape Medical News.
Hypothesis-generating study
Meeting cochair Emiliano Calvo, MD, PhD, from Hospital de Madrid Norte Sanchinarro in Madrid, who attended the media briefing but was not involved in the study, commented that hypothesis-generating research using drugs already on the market for other indications adds important value to cancer therapy.
James Gulley, MD, PhD, from the Center for Cancer Research at the NCI, also a meeting cochair, agreed with Calvo.
“Thinking about utilizing the data that already exists to really get hypothesis-generating questions, it also opens up the possibility for real-world data, real-world evidence from these big datasets from [electronic medical records] that we could really interrogate and understand what we might see and get these hypothesis-generating findings that we could then prospectively evaluate,” Gulley said.
The research was funded by the National Cancer Institute. Strauss and Gulley are National Cancer Institute employees. Calvo disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
It’s not ready for the clinic, but new research suggests that angiotensin receptor II blockers (ARBs) widely used to treat hypertension may improve responses to cancer immunotherapy agents targeted against the programmed death-1/ligand-1 (PD-1/PD-L1) pathway.
That conclusion comes from an observational study of 597 patients with more than 3 dozen different cancer types treated in clinical trials at the US National Institutes of Health. Investigators found that both objective response rates and 3-year overall survival (OS) rates were significantly higher for patients treated with a PD-1/PD-L1 inhibitor who were on ARBs, compared with patients who weren’t taking the antihypertensive agents.
An association was also seen between higher ORR and OS rates for patients taking ACE inhibitors, but it was not statistically significant, reported Julius Strauss, MD, from the Center for Cancer Research at the National Cancer Institute in Bethesda, Md.
All study patients received PD-1/PD-L1 inhibitors, and the ORR for patients treated with ARBs was 33.8%, compared with 19.5% for those treated with ACE inhibitors, and 17% for those who took neither drug. The respective complete response (CR) rates were 11.3%, 3.7%, and 3.1%.
Strauss discussed the data during an online briefing prior to his presentation of the findings during the 32nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, which is taking place virtually.
Several early studies have suggested that angiotensin II, in addition to its effect on blood pressure, can also affect cancer growth by leading to downstream production of two proteins: vascular endothelial growth factor (VEGF) and transforming growth factor–beta (TGF-beta), he explained.
“Both of these [proteins] have been linked to cancer growth and cancer resistance to immune system attack,” Strauss observed.
He also discussed the mechanics of possible effects. Angiotensin II increases VEGF and TGF-beta through binding to the AT1 receptor, but has the opposite effect when it binds to the AT2 receptor, resulting in a decrease in both of the growth factors, he added.
ACE inhibitors prevent the conversion of angiotensin I to angiotensin II, with the result being that the drugs indirectly block both the AT1 and AT2 receptors.
In contrast, ARBs block only the AT1 receptor and leave the AT2 counter-regulatory receptor alone, said Strauss.
More data, including on overall survival
Strauss and colleagues examined whether ACE inhibitors and/or ARBs could have an effect on the response to PD-1/PD-L1 immune checkpoint inhibitors delivered with or without other immunotherapies, such as anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) checkpoint inhibitors, or targeted agents such as tyrosine kinase inhibitors (TKIs).
They pooled data on 597 patients receiving PD-1/PD-L1 inhibitors in clinical trials for various cancers, including 71 receiving concomitant ARBs, 82 receiving an ACE inhibitor, and 444 who were not receiving either class of antihypertensives.
The above-mentioned improvement in ORR with ARBs compared with patients not receiving the drug was statistically significant (P = .001), as was the improvement in CR rates (P = .002). In contrast, neither ORR nor CR were significantly better with patients on ACE inhibitors compared with patients not taking these drugs.
In multiple regression analysis controlling for age, gender, body mass index (BMI), tumor type, and additional therapies given, the superior ORR and CR rates with ARBs remained (P = .039 and .002, respectively), while there continued to be no significant additional benefit with ACE inhibitors.
The median overall survival was 35.2 months for patients on ARBs, 26.2 months for those on ACE inhibitors, and 18.8 months for patients on neither drug. The respective 3-year OS rates were 48.1%, 37.2%, and 31.5%, with the difference between the ARB and no-drug groups being significant (P = .0078).
In regression analysis controlling for the factors mentioned before, the OS advantage with ARBs but not ACE inhibitors remained significant (P = .006 for ARBs, and .078 for ACE inhibitors).
Strauss emphasized that further study is needed to determine if AT1 blockade can improve outcomes when combined anti-PD-1/PD-L1-based therapy.
It might be reasonable for patients who are taking ACE inhibitors to control blood pressure and are also receiving immunotherapy with a PD-1/PD-L1 inhibitor to be switched to an ARB if it is deemed safe and if further research bears it out, said Strauss in response to a question from Medscape Medical News.
Hypothesis-generating study
Meeting cochair Emiliano Calvo, MD, PhD, from Hospital de Madrid Norte Sanchinarro in Madrid, who attended the media briefing but was not involved in the study, commented that hypothesis-generating research using drugs already on the market for other indications adds important value to cancer therapy.
James Gulley, MD, PhD, from the Center for Cancer Research at the NCI, also a meeting cochair, agreed with Calvo.
“Thinking about utilizing the data that already exists to really get hypothesis-generating questions, it also opens up the possibility for real-world data, real-world evidence from these big datasets from [electronic medical records] that we could really interrogate and understand what we might see and get these hypothesis-generating findings that we could then prospectively evaluate,” Gulley said.
The research was funded by the National Cancer Institute. Strauss and Gulley are National Cancer Institute employees. Calvo disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Thermography plus software shows efficacy for breast cancer screening
Sensitivity and area under the curve (AUC) analyses of thermography that is combined with diagnostic software demonstrate “the efficacy of the tool for breast cancer screening,” concludes an observational, comparative study from India published online Oct. 1 in JCO Global Oncology, a publication of the American Society of Clinical Oncology.
Siva Teja Kakileti of Niramai Health Analytix, Koramangala, Bangalore, India, and colleagues said that the product, Thermalytix, is potentially a good fit for low- and middle-income countries because it is portable and provides automated quantitative analysis of thermal images – and thus can be conducted by technicians with “minimal training.”
Conventional thermography involves manual interpretation of complex thermal images, which “often results in erroneous results owing to subjectivity,” said the study authors.
That manual interpretation of thermal images might involve looking at 200 color shades, which is “high cognitive overload for the thermographer,” explained Mr. Kakileti in an interview.
However, an American mammography expert who was approached for comment dismissed thermography – even with the new twist of software-aided diagnostic scoring by Thermalytix – as wholly inappropriate for the detection of early breast cancer, owing to inherent limitations.
“Thermal imaging of any type has no value in finding early breast cancer,” Daniel Kopans, MD, of Harvard University and Massachusetts General Hospital, both in Boston, said in an interview. He said that thermal imaging only detects heat on the skin and perhaps a few millimeters beneath the skin and thus misses deeper cancers, the heat from which is carried away by the vascular system.
The new study included 470 women who presented for breast screening at two centers in Bangalore, India. A total of 238 women had symptoms such as breast lump, nipple discharge, skin changes, or breast pain; the remaining 232 women were asymptomatic.
All participants underwent a Thermalytix test and one or more standard-of-care tests for breast cancer screening (such as mammography, ultrasonography, biopsy, fine-needle aspiration, or elastography). A total of 78 women, or 16.6% of the group overall, were diagnosed with a malignancy. For the overall group of 470 women, Thermalytix had a sensitivity of 91.02% (symptomatic, 89.85%; asymptomatic,100%) and a specificity of 82.39% (symptomatic, 69.04%; asymptomatic, 92.41%) in detection of breast malignancy. Thermalytix showed an overall AUC of 0.90, with an AUC of 0.82 for symptomatic and 0.98 for asymptomatic women.
The study authors characterized both the sensitivity and AUC as “high.”
The results from the study, which the authors characterized as preliminary, encouraged the study sponsor, Niramai, to start planning a large-scale, multicountry trial.
But Dr. Kopans, who serves as a consultant to DART, which produces digital breast tomosynthesis units in China, suggested that this research will be fruitless. “Thermal imaging seems to raise its head every few years since it is passive, but it does not work and is a waste of money,” Dr. Kopans reiterated.
“Its use can be dangerous by dissuading women from being screened with mammography, which has been proven to save lives,” he stressed.
Thermalytix compared with mammography
Investigators also compared screening results in the subset of 242 women who underwent both Thermalytix and mammography. Results showed that Thermalytix had a higher sensitivity than did mammography (91.23% vs. 85.96%), but mammography had a higher specificity than Thermalytix did (94.05% vs. 68.65%).
In the asymptomatic group who underwent both tests (n = 95), four cancers were detected, and Thermalytix demonstrated superior sensitivity than mammography (100% vs. 50%), Mr. Kakileti and colleagues state.
Thermalytix evaluates vascularity variations too
In the subset of 228 women who did not undergo mammography (owing to dense breasts, younger age, or other reasons), Thermalytix detected tumors in all but 3 of 21 patients who went on to be diagnosed with breast cancer. The authors state that, because their artificial intelligence–based analysis uses vascularity, as well as temperature variations on the skin, to complement hot-spot detection, it is able to detect small lesions.
In the current study, 24 malignant tumors were less than 2 cm in diameter, and Thermalytix was able to identify 17 of the tumors as positive, for a 71% sensitivity rate for T1 tumors. This compared with a 68% sensitivity rate for mammography for detecting the same T1 tumors. Thermalytix also showed promising results in women younger than 40 years, for whom screening mammography is not usually recommended. The automated test picked up all 11 tumors eventually diagnosed in this younger cohort.
“Thermalytix is a portable, noninvasive, radiation-free test that has shown promising results in this preliminary study,” the investigators wrote, “[and] it can be an affordable and scalable method of screening in remote areas,” they added.
“We believe that Thermalytix ... is poised to be a promising modality for breast cancer screening,” Mr. Kakileti and colleagues summarized.
The FDA warns about thermography in place of mammography
The US Food and Drug Administration fairly recently warned against the use of thermography as an alternative to mammography for breast cancer screening or diagnosis, noting that it has received reports that facilities where thermography is offered often provide false information about the technology that can mislead patients into believing that it is either an alternative to or a better option than mammography.
Dr. Kopans says that other groups have invested in thermography research. “The Israelis spent millions working on a similar approach that didn’t work,” he commented.
The new software from Thermalytix, which is derived from artificial intelligence, is a “gimmick,” says the Boston radiologist. “If the basic information is not there, a computer cannot find it,” he stated, referring to what he believes are deeper-tissue tumors that are inaccessible to heat-detecting technology.
Mr. Kakileti is an employee of Nirami Health Analytix and owns stock and has filed patents with the company. Other investigators are also employed by the same company or receive research and other funding or have patents filed by the company as well. Dr. Kopans serves as a consultant to DART, which produces digital breast tomosynthesis units in China.
A version of this article originally appeared on Medscape.com.
Sensitivity and area under the curve (AUC) analyses of thermography that is combined with diagnostic software demonstrate “the efficacy of the tool for breast cancer screening,” concludes an observational, comparative study from India published online Oct. 1 in JCO Global Oncology, a publication of the American Society of Clinical Oncology.
Siva Teja Kakileti of Niramai Health Analytix, Koramangala, Bangalore, India, and colleagues said that the product, Thermalytix, is potentially a good fit for low- and middle-income countries because it is portable and provides automated quantitative analysis of thermal images – and thus can be conducted by technicians with “minimal training.”
Conventional thermography involves manual interpretation of complex thermal images, which “often results in erroneous results owing to subjectivity,” said the study authors.
That manual interpretation of thermal images might involve looking at 200 color shades, which is “high cognitive overload for the thermographer,” explained Mr. Kakileti in an interview.
However, an American mammography expert who was approached for comment dismissed thermography – even with the new twist of software-aided diagnostic scoring by Thermalytix – as wholly inappropriate for the detection of early breast cancer, owing to inherent limitations.
“Thermal imaging of any type has no value in finding early breast cancer,” Daniel Kopans, MD, of Harvard University and Massachusetts General Hospital, both in Boston, said in an interview. He said that thermal imaging only detects heat on the skin and perhaps a few millimeters beneath the skin and thus misses deeper cancers, the heat from which is carried away by the vascular system.
The new study included 470 women who presented for breast screening at two centers in Bangalore, India. A total of 238 women had symptoms such as breast lump, nipple discharge, skin changes, or breast pain; the remaining 232 women were asymptomatic.
All participants underwent a Thermalytix test and one or more standard-of-care tests for breast cancer screening (such as mammography, ultrasonography, biopsy, fine-needle aspiration, or elastography). A total of 78 women, or 16.6% of the group overall, were diagnosed with a malignancy. For the overall group of 470 women, Thermalytix had a sensitivity of 91.02% (symptomatic, 89.85%; asymptomatic,100%) and a specificity of 82.39% (symptomatic, 69.04%; asymptomatic, 92.41%) in detection of breast malignancy. Thermalytix showed an overall AUC of 0.90, with an AUC of 0.82 for symptomatic and 0.98 for asymptomatic women.
The study authors characterized both the sensitivity and AUC as “high.”
The results from the study, which the authors characterized as preliminary, encouraged the study sponsor, Niramai, to start planning a large-scale, multicountry trial.
But Dr. Kopans, who serves as a consultant to DART, which produces digital breast tomosynthesis units in China, suggested that this research will be fruitless. “Thermal imaging seems to raise its head every few years since it is passive, but it does not work and is a waste of money,” Dr. Kopans reiterated.
“Its use can be dangerous by dissuading women from being screened with mammography, which has been proven to save lives,” he stressed.
Thermalytix compared with mammography
Investigators also compared screening results in the subset of 242 women who underwent both Thermalytix and mammography. Results showed that Thermalytix had a higher sensitivity than did mammography (91.23% vs. 85.96%), but mammography had a higher specificity than Thermalytix did (94.05% vs. 68.65%).
In the asymptomatic group who underwent both tests (n = 95), four cancers were detected, and Thermalytix demonstrated superior sensitivity than mammography (100% vs. 50%), Mr. Kakileti and colleagues state.
Thermalytix evaluates vascularity variations too
In the subset of 228 women who did not undergo mammography (owing to dense breasts, younger age, or other reasons), Thermalytix detected tumors in all but 3 of 21 patients who went on to be diagnosed with breast cancer. The authors state that, because their artificial intelligence–based analysis uses vascularity, as well as temperature variations on the skin, to complement hot-spot detection, it is able to detect small lesions.
In the current study, 24 malignant tumors were less than 2 cm in diameter, and Thermalytix was able to identify 17 of the tumors as positive, for a 71% sensitivity rate for T1 tumors. This compared with a 68% sensitivity rate for mammography for detecting the same T1 tumors. Thermalytix also showed promising results in women younger than 40 years, for whom screening mammography is not usually recommended. The automated test picked up all 11 tumors eventually diagnosed in this younger cohort.
“Thermalytix is a portable, noninvasive, radiation-free test that has shown promising results in this preliminary study,” the investigators wrote, “[and] it can be an affordable and scalable method of screening in remote areas,” they added.
“We believe that Thermalytix ... is poised to be a promising modality for breast cancer screening,” Mr. Kakileti and colleagues summarized.
The FDA warns about thermography in place of mammography
The US Food and Drug Administration fairly recently warned against the use of thermography as an alternative to mammography for breast cancer screening or diagnosis, noting that it has received reports that facilities where thermography is offered often provide false information about the technology that can mislead patients into believing that it is either an alternative to or a better option than mammography.
Dr. Kopans says that other groups have invested in thermography research. “The Israelis spent millions working on a similar approach that didn’t work,” he commented.
The new software from Thermalytix, which is derived from artificial intelligence, is a “gimmick,” says the Boston radiologist. “If the basic information is not there, a computer cannot find it,” he stated, referring to what he believes are deeper-tissue tumors that are inaccessible to heat-detecting technology.
Mr. Kakileti is an employee of Nirami Health Analytix and owns stock and has filed patents with the company. Other investigators are also employed by the same company or receive research and other funding or have patents filed by the company as well. Dr. Kopans serves as a consultant to DART, which produces digital breast tomosynthesis units in China.
A version of this article originally appeared on Medscape.com.
Sensitivity and area under the curve (AUC) analyses of thermography that is combined with diagnostic software demonstrate “the efficacy of the tool for breast cancer screening,” concludes an observational, comparative study from India published online Oct. 1 in JCO Global Oncology, a publication of the American Society of Clinical Oncology.
Siva Teja Kakileti of Niramai Health Analytix, Koramangala, Bangalore, India, and colleagues said that the product, Thermalytix, is potentially a good fit for low- and middle-income countries because it is portable and provides automated quantitative analysis of thermal images – and thus can be conducted by technicians with “minimal training.”
Conventional thermography involves manual interpretation of complex thermal images, which “often results in erroneous results owing to subjectivity,” said the study authors.
That manual interpretation of thermal images might involve looking at 200 color shades, which is “high cognitive overload for the thermographer,” explained Mr. Kakileti in an interview.
However, an American mammography expert who was approached for comment dismissed thermography – even with the new twist of software-aided diagnostic scoring by Thermalytix – as wholly inappropriate for the detection of early breast cancer, owing to inherent limitations.
“Thermal imaging of any type has no value in finding early breast cancer,” Daniel Kopans, MD, of Harvard University and Massachusetts General Hospital, both in Boston, said in an interview. He said that thermal imaging only detects heat on the skin and perhaps a few millimeters beneath the skin and thus misses deeper cancers, the heat from which is carried away by the vascular system.
The new study included 470 women who presented for breast screening at two centers in Bangalore, India. A total of 238 women had symptoms such as breast lump, nipple discharge, skin changes, or breast pain; the remaining 232 women were asymptomatic.
All participants underwent a Thermalytix test and one or more standard-of-care tests for breast cancer screening (such as mammography, ultrasonography, biopsy, fine-needle aspiration, or elastography). A total of 78 women, or 16.6% of the group overall, were diagnosed with a malignancy. For the overall group of 470 women, Thermalytix had a sensitivity of 91.02% (symptomatic, 89.85%; asymptomatic,100%) and a specificity of 82.39% (symptomatic, 69.04%; asymptomatic, 92.41%) in detection of breast malignancy. Thermalytix showed an overall AUC of 0.90, with an AUC of 0.82 for symptomatic and 0.98 for asymptomatic women.
The study authors characterized both the sensitivity and AUC as “high.”
The results from the study, which the authors characterized as preliminary, encouraged the study sponsor, Niramai, to start planning a large-scale, multicountry trial.
But Dr. Kopans, who serves as a consultant to DART, which produces digital breast tomosynthesis units in China, suggested that this research will be fruitless. “Thermal imaging seems to raise its head every few years since it is passive, but it does not work and is a waste of money,” Dr. Kopans reiterated.
“Its use can be dangerous by dissuading women from being screened with mammography, which has been proven to save lives,” he stressed.
Thermalytix compared with mammography
Investigators also compared screening results in the subset of 242 women who underwent both Thermalytix and mammography. Results showed that Thermalytix had a higher sensitivity than did mammography (91.23% vs. 85.96%), but mammography had a higher specificity than Thermalytix did (94.05% vs. 68.65%).
In the asymptomatic group who underwent both tests (n = 95), four cancers were detected, and Thermalytix demonstrated superior sensitivity than mammography (100% vs. 50%), Mr. Kakileti and colleagues state.
Thermalytix evaluates vascularity variations too
In the subset of 228 women who did not undergo mammography (owing to dense breasts, younger age, or other reasons), Thermalytix detected tumors in all but 3 of 21 patients who went on to be diagnosed with breast cancer. The authors state that, because their artificial intelligence–based analysis uses vascularity, as well as temperature variations on the skin, to complement hot-spot detection, it is able to detect small lesions.
In the current study, 24 malignant tumors were less than 2 cm in diameter, and Thermalytix was able to identify 17 of the tumors as positive, for a 71% sensitivity rate for T1 tumors. This compared with a 68% sensitivity rate for mammography for detecting the same T1 tumors. Thermalytix also showed promising results in women younger than 40 years, for whom screening mammography is not usually recommended. The automated test picked up all 11 tumors eventually diagnosed in this younger cohort.
“Thermalytix is a portable, noninvasive, radiation-free test that has shown promising results in this preliminary study,” the investigators wrote, “[and] it can be an affordable and scalable method of screening in remote areas,” they added.
“We believe that Thermalytix ... is poised to be a promising modality for breast cancer screening,” Mr. Kakileti and colleagues summarized.
The FDA warns about thermography in place of mammography
The US Food and Drug Administration fairly recently warned against the use of thermography as an alternative to mammography for breast cancer screening or diagnosis, noting that it has received reports that facilities where thermography is offered often provide false information about the technology that can mislead patients into believing that it is either an alternative to or a better option than mammography.
Dr. Kopans says that other groups have invested in thermography research. “The Israelis spent millions working on a similar approach that didn’t work,” he commented.
The new software from Thermalytix, which is derived from artificial intelligence, is a “gimmick,” says the Boston radiologist. “If the basic information is not there, a computer cannot find it,” he stated, referring to what he believes are deeper-tissue tumors that are inaccessible to heat-detecting technology.
Mr. Kakileti is an employee of Nirami Health Analytix and owns stock and has filed patents with the company. Other investigators are also employed by the same company or receive research and other funding or have patents filed by the company as well. Dr. Kopans serves as a consultant to DART, which produces digital breast tomosynthesis units in China.
A version of this article originally appeared on Medscape.com.
Are oncologists ready to confront a second wave of COVID-19?
Canceled appointments, postponed surgeries, and delayed cancer diagnoses – all are a recipe for exhaustion for oncologists around the world, struggling to reach and treat their patients during the pandemic. Physicians and their teams felt the pain as COVID-19 took its initial march around the globe.
“We saw the distress of people with cancer who could no longer get to anyone on the phone. Their medical visit was usually canceled. Their radiotherapy session was postponed or modified, and chemotherapy postponed,” says Axel Kahn, MD, chairman of the board of directors of La Ligue Nationale Contre le Cancer (National League Against Cancer). “In the vast majority of cases, cancer treatment can be postponed or readjusted, without affecting the patient’s chances of survival, but there has been a lot of anxiety because the patients do not know that.”
The stay-at-home factor was one that played out across many months during the first wave.
“I believe that the ‘stay-home’ message that we transmitted was rigorously followed by patients who should have come to the emergency room much earlier and who, therefore, were admitted with a much more deteriorated general condition than in non-COVID-19 times,” says Benjamín Domingo Arrué, MD, from the department of medical oncology at Hospital Universitari i Politècnic La Fe in Valencia, Spain.
And in Brazil, some of the impact from the initial hit of COVID-19 on oncology is only now being felt, according to Laura Testa, MD, head of breast medical oncology, Instituto do Câncer do Estado de São Paulo.
“We are starting to see a lot of cancer cases that didn’t show up at the beginning of the pandemic, but now they are arriving to us already in advanced stages,” she said. “These patients need hospital care. If the situation worsens and goes back to what we saw at the peak of the curve, I fear the public system won’t be able to treat properly the oncology patients that need hospital care and the patients with cancer who also have COVID-19.”
But even as health care worker fatigue and concerns linger, oncologists say that what they have learned in the last 6 months has helped them prepare as COVID-19 cases increase and a second global wave kicks up.
Lessons from the first wave
In the United States, COVID-19 hit different regions at different times and to different degrees. One of the areas hit first was Seattle.
“We jumped on top of this, we were evidence based, we put things in place very, very quickly,” said Julie Gralow, MD, professor at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
“We did a really good job keeping COVID out of our cancer centers,” Dr. Gralow said. “We learned how to be super safe, and to keep symptomatic people out of the building, and to limit the extra people they could bring with them. It’s all about the number of contacts you have.”
The story was different, though, for oncologists in several other countries, and sometimes it varied immensely within each nation.
“We treated fewer patients with cancer during the first wave,” says Dirk Arnold, MD, medical director of the Asklepios Tumor Center Hamburg (Germany), in an interview. “In part, this was because staff were quarantined and because we had a completely different infrastructure in all of the hospitals. But also fewer patients with cancer came to the clinic at all. A lot of resources were directed toward COVID-19.”
In Spain, telemedicine helped keep up with visits, but other areas felt the effect of COVID-19 patient loads.
“At least in the oncology department of our center, we have practically maintained 100% of visits, mostly by telephone,” says Dr. Arrué, “but the reality is that our country has not yet been prepared for telemedicine.”
Laura Mezquita, MD, of the department of medical oncology at Hospital Clinic de Barcelona, describes a more dramatic situation: “We have seen how some of our patients, especially with metastatic disease, have been dismissed for intensive care and life-support treatments, as well as specific treatments against COVID-19 (tocilizumab, remdesivir, etc.) due to the general health collapse of the former wave,” she said. She adds that specific oncologic populations, such as those with thoracic tumors, have been more affected.
Distress among oncologists
Many oncologists are still feeling stressed and fatigued after the first wave, just as a second string of outbreaks is on its way.
A survey presented at last month’s ESMO 2020 Congress found that, in July-August, moral distress was reported by one-third of the oncologists who responded, and more than half reported a feeling of exhaustion.
“The tiredness and team exhaustion is noticeable,” said Dr. Arnold. “We recently had a task force discussion about what will happen when we have a second wave and how the department and our services will adapt. It was clear that those who were at the very front in the first wave had only a limited desire to do that again in the second wave.”
Another concern: COVID-19’s effect on staffing levels.
“We have a population of young caregivers who are affected by the COVID-19 disease with an absenteeism rate that is quite unprecedented,” said Sophie Beaupère, general delegate of Unicancer since January.
She said that, in general, the absenteeism rate in the cancer centers averages 5%-6%, depending on the year. But that rate is now skyrocketing.
Stop-start cycle for surgery
As caregivers quarantined around the world, more than 10% of patients with cancer had treatment canceled or delayed during the first wave of the pandemic, according to another survey from ESMO, involving 109 oncologists from 18 countries.
Difficulties were reported for surgeries by 34% of the centers, but also difficulties with delivering chemotherapy (22% of centers), radiotherapy (13.7%), and therapy with checkpoint inhibitors (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Stopping surgery is a real concern in France, noted Dr. Kahn, the National League Against Cancer chair. He says that in regions that were badly hit by COVID-19, “it was not possible to have access to the operating room for people who absolutely needed surgery; for example, patients with lung cancer that was still operable. Most of the recovery rooms were mobilized for resuscitation.”
There may be some solutions, suggested Thierry Breton, director general of the National Institute of Cancer in France. “We are getting prepared, with the health ministry, for a possible increase in hospital tension, which would lead to a situation where we would have to reschedule operations. Nationally, regionally, and locally, we are seeing how we can resume and prioritize surgeries that have not been done.”
Delays in cancer diagnosis
While COVID-19 affected treatment, many oncologists say the major impact of the first wave was a delay in diagnosing cancer. Some of this was a result of the suspension of cancer screening programs, but there was also fear among the general public about visiting clinics and hospitals during a pandemic.
“We didn’t do so well with cancer during the first wave here in the U.K.,” said Karol Sikora, PhD, MBBChir, professor of cancer medicine and founding dean at the University of Buckingham Medical School, London. “Cancer diagnostic pathways virtually stalled partly because patients didn’t seek help, but getting scans and biopsies was also very difficult. Even patients referred urgently under the ‘2-weeks-wait’ rule were turned down.”
In France, “the delay in diagnosis is indisputable,” said Dr. Kahn. “About 50% of the cancer diagnoses one would expect during this period were missed.”
“I am worried that there remains a major traffic jam that has not been caught up with, and, in the meantime, the health crisis is worsening,” he added.
In Seattle, Dr. Gralow said the first COVID-19 wave had little impact on treatment for breast cancer, but it was in screening for breast cancer “where things really got messed up.”
“Even though we’ve been fully ramped up again,” she said, concerns remain. To ensure that screening mammography is maintained, “we have spaced out the visits to keep our waiting rooms less populated, with a longer time between using the machine so we can clean it. To do this, we have extended operating hours and are now opening on Saturday.
“So we’re actually at 100% of our capacity, but I’m really nervous, though, that a lot of people put off their screening mammogram and aren’t going to come in and get it.
“Not only did people get the message to stay home and not do nonessential things, but I think a lot of people lost their health insurance when they lost their jobs,” she said, and without health insurance, they are not covered for cancer screening.
Looking ahead, with a plan
Many oncologists agree that access to care can and must be improved – and there were some positive moves.
“Some regimens changed during the first months of the pandemic, and I don’t see them going back to the way they were anytime soon,” said Dr. Testa. “The changes/adaptations that were made to minimize the chance of SARS-CoV-2 infection are still in place and will go on for a while. In this context, telemedicine helped a lot. The pandemic forced the stakeholders to step up and put it in place in March. And now it’s here to stay.”
The experience gained in the last several months has driven preparation for the next wave.
“We are not going to see the disorganization that we saw during the first wave,” said Florence Joly, MD, PhD, head of medical oncology at the Centre François Baclesse in Caen, France. “The difference between now and earlier this year is that COVID diagnostic tests are available. That was one of the problems in the first wave. We had no way to diagnose.”
On the East Coast of the United States, medical oncologist Charu Aggarwal, MD, MPH, is also optimistic: “I think we’re at a place where we can manage.”
“I believe if there was going to be a new wave of COVID-19 cases we would be: better psychologically prepared and better organized,” said Dr. Aggarwal, assistant professor of medicine in the hematology-oncology division at the University of Pennsylvania, Philadelphia. “We already have experience with all of the tools, we have telemedicine available, we have screening protocols available, we have testing, we are already universally masking, everyone’s hand-washing, so I do think that means we would be okay.”
Dr. Arnold agreed that “we are much better prepared than for the first wave, but … we have immense tasks in the area of patient management, the digitization of patient care, the clear allocation of resources when there is a second or third wave. In many areas of preparation, I believe, unfortunately, we are not as well positioned as we had actually hoped.”
The first wave of COVID hit cancer services in the United Kingdom particularly hard: One modeling study suggested that delays in cancer referrals will lead to thousands of additional deaths and tens of thousands of life-years lost.
“Cancer services are working at near normal levels now, but they are still fragile and could be severely compromised again if the NHS [National Health Service] gets flooded by COVID patients,” said Dr. Sikora.
The second wave may be different. “Although the number of infections has increased, the hospitalizations have only risen a little. Let’s see what happens,” he said in an interview. Since then, however, infections have continued to rise, and there has been an increase in hospitalizations. New social distancing measures in the United Kingdom were put into place on Oct. 12, with the aim of protecting the NHS from overload.
Dr. Arrué describes it this way: “The reality is that the ‘second wave’ has left behind the initial grief and shock that both patients and health professionals experienced when faced with something that, until now, we had only seen in the movies.” The second wave has led to new restrictions – including a partial lockdown since the beginning of October.
Dr. Aggarwal says her department recently had a conference with Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, about the impact of COVID-19 on oncology.
“I asked him what advice he’d give oncologists, and he said to go back to as much screening as you were doing previously as quickly as possible. That’s what must be relayed to our oncologists in the community – and also to primary care physicians – because they are often the ones who are ordering and championing the screening efforts.”
This article was originated by Aude Lecrubier, Medscape French edition, and developed by Zosia Chustecka, Medscape Oncology. With additional reporting by Kate Johnson, freelance medical journalist, Claudia Gottschling for Medscape Germany, Leoleli Schwartz for Medscape em português, Tim Locke for Medscape United Kingdom, and Carla Nieto Martínez, freelance medical journalist for Medscape Spanish edition.
This article first appeared on Medscape.com.
Canceled appointments, postponed surgeries, and delayed cancer diagnoses – all are a recipe for exhaustion for oncologists around the world, struggling to reach and treat their patients during the pandemic. Physicians and their teams felt the pain as COVID-19 took its initial march around the globe.
“We saw the distress of people with cancer who could no longer get to anyone on the phone. Their medical visit was usually canceled. Their radiotherapy session was postponed or modified, and chemotherapy postponed,” says Axel Kahn, MD, chairman of the board of directors of La Ligue Nationale Contre le Cancer (National League Against Cancer). “In the vast majority of cases, cancer treatment can be postponed or readjusted, without affecting the patient’s chances of survival, but there has been a lot of anxiety because the patients do not know that.”
The stay-at-home factor was one that played out across many months during the first wave.
“I believe that the ‘stay-home’ message that we transmitted was rigorously followed by patients who should have come to the emergency room much earlier and who, therefore, were admitted with a much more deteriorated general condition than in non-COVID-19 times,” says Benjamín Domingo Arrué, MD, from the department of medical oncology at Hospital Universitari i Politècnic La Fe in Valencia, Spain.
And in Brazil, some of the impact from the initial hit of COVID-19 on oncology is only now being felt, according to Laura Testa, MD, head of breast medical oncology, Instituto do Câncer do Estado de São Paulo.
“We are starting to see a lot of cancer cases that didn’t show up at the beginning of the pandemic, but now they are arriving to us already in advanced stages,” she said. “These patients need hospital care. If the situation worsens and goes back to what we saw at the peak of the curve, I fear the public system won’t be able to treat properly the oncology patients that need hospital care and the patients with cancer who also have COVID-19.”
But even as health care worker fatigue and concerns linger, oncologists say that what they have learned in the last 6 months has helped them prepare as COVID-19 cases increase and a second global wave kicks up.
Lessons from the first wave
In the United States, COVID-19 hit different regions at different times and to different degrees. One of the areas hit first was Seattle.
“We jumped on top of this, we were evidence based, we put things in place very, very quickly,” said Julie Gralow, MD, professor at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
“We did a really good job keeping COVID out of our cancer centers,” Dr. Gralow said. “We learned how to be super safe, and to keep symptomatic people out of the building, and to limit the extra people they could bring with them. It’s all about the number of contacts you have.”
The story was different, though, for oncologists in several other countries, and sometimes it varied immensely within each nation.
“We treated fewer patients with cancer during the first wave,” says Dirk Arnold, MD, medical director of the Asklepios Tumor Center Hamburg (Germany), in an interview. “In part, this was because staff were quarantined and because we had a completely different infrastructure in all of the hospitals. But also fewer patients with cancer came to the clinic at all. A lot of resources were directed toward COVID-19.”
In Spain, telemedicine helped keep up with visits, but other areas felt the effect of COVID-19 patient loads.
“At least in the oncology department of our center, we have practically maintained 100% of visits, mostly by telephone,” says Dr. Arrué, “but the reality is that our country has not yet been prepared for telemedicine.”
Laura Mezquita, MD, of the department of medical oncology at Hospital Clinic de Barcelona, describes a more dramatic situation: “We have seen how some of our patients, especially with metastatic disease, have been dismissed for intensive care and life-support treatments, as well as specific treatments against COVID-19 (tocilizumab, remdesivir, etc.) due to the general health collapse of the former wave,” she said. She adds that specific oncologic populations, such as those with thoracic tumors, have been more affected.
Distress among oncologists
Many oncologists are still feeling stressed and fatigued after the first wave, just as a second string of outbreaks is on its way.
A survey presented at last month’s ESMO 2020 Congress found that, in July-August, moral distress was reported by one-third of the oncologists who responded, and more than half reported a feeling of exhaustion.
“The tiredness and team exhaustion is noticeable,” said Dr. Arnold. “We recently had a task force discussion about what will happen when we have a second wave and how the department and our services will adapt. It was clear that those who were at the very front in the first wave had only a limited desire to do that again in the second wave.”
Another concern: COVID-19’s effect on staffing levels.
“We have a population of young caregivers who are affected by the COVID-19 disease with an absenteeism rate that is quite unprecedented,” said Sophie Beaupère, general delegate of Unicancer since January.
She said that, in general, the absenteeism rate in the cancer centers averages 5%-6%, depending on the year. But that rate is now skyrocketing.
Stop-start cycle for surgery
As caregivers quarantined around the world, more than 10% of patients with cancer had treatment canceled or delayed during the first wave of the pandemic, according to another survey from ESMO, involving 109 oncologists from 18 countries.
Difficulties were reported for surgeries by 34% of the centers, but also difficulties with delivering chemotherapy (22% of centers), radiotherapy (13.7%), and therapy with checkpoint inhibitors (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Stopping surgery is a real concern in France, noted Dr. Kahn, the National League Against Cancer chair. He says that in regions that were badly hit by COVID-19, “it was not possible to have access to the operating room for people who absolutely needed surgery; for example, patients with lung cancer that was still operable. Most of the recovery rooms were mobilized for resuscitation.”
There may be some solutions, suggested Thierry Breton, director general of the National Institute of Cancer in France. “We are getting prepared, with the health ministry, for a possible increase in hospital tension, which would lead to a situation where we would have to reschedule operations. Nationally, regionally, and locally, we are seeing how we can resume and prioritize surgeries that have not been done.”
Delays in cancer diagnosis
While COVID-19 affected treatment, many oncologists say the major impact of the first wave was a delay in diagnosing cancer. Some of this was a result of the suspension of cancer screening programs, but there was also fear among the general public about visiting clinics and hospitals during a pandemic.
“We didn’t do so well with cancer during the first wave here in the U.K.,” said Karol Sikora, PhD, MBBChir, professor of cancer medicine and founding dean at the University of Buckingham Medical School, London. “Cancer diagnostic pathways virtually stalled partly because patients didn’t seek help, but getting scans and biopsies was also very difficult. Even patients referred urgently under the ‘2-weeks-wait’ rule were turned down.”
In France, “the delay in diagnosis is indisputable,” said Dr. Kahn. “About 50% of the cancer diagnoses one would expect during this period were missed.”
“I am worried that there remains a major traffic jam that has not been caught up with, and, in the meantime, the health crisis is worsening,” he added.
In Seattle, Dr. Gralow said the first COVID-19 wave had little impact on treatment for breast cancer, but it was in screening for breast cancer “where things really got messed up.”
“Even though we’ve been fully ramped up again,” she said, concerns remain. To ensure that screening mammography is maintained, “we have spaced out the visits to keep our waiting rooms less populated, with a longer time between using the machine so we can clean it. To do this, we have extended operating hours and are now opening on Saturday.
“So we’re actually at 100% of our capacity, but I’m really nervous, though, that a lot of people put off their screening mammogram and aren’t going to come in and get it.
“Not only did people get the message to stay home and not do nonessential things, but I think a lot of people lost their health insurance when they lost their jobs,” she said, and without health insurance, they are not covered for cancer screening.
Looking ahead, with a plan
Many oncologists agree that access to care can and must be improved – and there were some positive moves.
“Some regimens changed during the first months of the pandemic, and I don’t see them going back to the way they were anytime soon,” said Dr. Testa. “The changes/adaptations that were made to minimize the chance of SARS-CoV-2 infection are still in place and will go on for a while. In this context, telemedicine helped a lot. The pandemic forced the stakeholders to step up and put it in place in March. And now it’s here to stay.”
The experience gained in the last several months has driven preparation for the next wave.
“We are not going to see the disorganization that we saw during the first wave,” said Florence Joly, MD, PhD, head of medical oncology at the Centre François Baclesse in Caen, France. “The difference between now and earlier this year is that COVID diagnostic tests are available. That was one of the problems in the first wave. We had no way to diagnose.”
On the East Coast of the United States, medical oncologist Charu Aggarwal, MD, MPH, is also optimistic: “I think we’re at a place where we can manage.”
“I believe if there was going to be a new wave of COVID-19 cases we would be: better psychologically prepared and better organized,” said Dr. Aggarwal, assistant professor of medicine in the hematology-oncology division at the University of Pennsylvania, Philadelphia. “We already have experience with all of the tools, we have telemedicine available, we have screening protocols available, we have testing, we are already universally masking, everyone’s hand-washing, so I do think that means we would be okay.”
Dr. Arnold agreed that “we are much better prepared than for the first wave, but … we have immense tasks in the area of patient management, the digitization of patient care, the clear allocation of resources when there is a second or third wave. In many areas of preparation, I believe, unfortunately, we are not as well positioned as we had actually hoped.”
The first wave of COVID hit cancer services in the United Kingdom particularly hard: One modeling study suggested that delays in cancer referrals will lead to thousands of additional deaths and tens of thousands of life-years lost.
“Cancer services are working at near normal levels now, but they are still fragile and could be severely compromised again if the NHS [National Health Service] gets flooded by COVID patients,” said Dr. Sikora.
The second wave may be different. “Although the number of infections has increased, the hospitalizations have only risen a little. Let’s see what happens,” he said in an interview. Since then, however, infections have continued to rise, and there has been an increase in hospitalizations. New social distancing measures in the United Kingdom were put into place on Oct. 12, with the aim of protecting the NHS from overload.
Dr. Arrué describes it this way: “The reality is that the ‘second wave’ has left behind the initial grief and shock that both patients and health professionals experienced when faced with something that, until now, we had only seen in the movies.” The second wave has led to new restrictions – including a partial lockdown since the beginning of October.
Dr. Aggarwal says her department recently had a conference with Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, about the impact of COVID-19 on oncology.
“I asked him what advice he’d give oncologists, and he said to go back to as much screening as you were doing previously as quickly as possible. That’s what must be relayed to our oncologists in the community – and also to primary care physicians – because they are often the ones who are ordering and championing the screening efforts.”
This article was originated by Aude Lecrubier, Medscape French edition, and developed by Zosia Chustecka, Medscape Oncology. With additional reporting by Kate Johnson, freelance medical journalist, Claudia Gottschling for Medscape Germany, Leoleli Schwartz for Medscape em português, Tim Locke for Medscape United Kingdom, and Carla Nieto Martínez, freelance medical journalist for Medscape Spanish edition.
This article first appeared on Medscape.com.
Canceled appointments, postponed surgeries, and delayed cancer diagnoses – all are a recipe for exhaustion for oncologists around the world, struggling to reach and treat their patients during the pandemic. Physicians and their teams felt the pain as COVID-19 took its initial march around the globe.
“We saw the distress of people with cancer who could no longer get to anyone on the phone. Their medical visit was usually canceled. Their radiotherapy session was postponed or modified, and chemotherapy postponed,” says Axel Kahn, MD, chairman of the board of directors of La Ligue Nationale Contre le Cancer (National League Against Cancer). “In the vast majority of cases, cancer treatment can be postponed or readjusted, without affecting the patient’s chances of survival, but there has been a lot of anxiety because the patients do not know that.”
The stay-at-home factor was one that played out across many months during the first wave.
“I believe that the ‘stay-home’ message that we transmitted was rigorously followed by patients who should have come to the emergency room much earlier and who, therefore, were admitted with a much more deteriorated general condition than in non-COVID-19 times,” says Benjamín Domingo Arrué, MD, from the department of medical oncology at Hospital Universitari i Politècnic La Fe in Valencia, Spain.
And in Brazil, some of the impact from the initial hit of COVID-19 on oncology is only now being felt, according to Laura Testa, MD, head of breast medical oncology, Instituto do Câncer do Estado de São Paulo.
“We are starting to see a lot of cancer cases that didn’t show up at the beginning of the pandemic, but now they are arriving to us already in advanced stages,” she said. “These patients need hospital care. If the situation worsens and goes back to what we saw at the peak of the curve, I fear the public system won’t be able to treat properly the oncology patients that need hospital care and the patients with cancer who also have COVID-19.”
But even as health care worker fatigue and concerns linger, oncologists say that what they have learned in the last 6 months has helped them prepare as COVID-19 cases increase and a second global wave kicks up.
Lessons from the first wave
In the United States, COVID-19 hit different regions at different times and to different degrees. One of the areas hit first was Seattle.
“We jumped on top of this, we were evidence based, we put things in place very, very quickly,” said Julie Gralow, MD, professor at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
“We did a really good job keeping COVID out of our cancer centers,” Dr. Gralow said. “We learned how to be super safe, and to keep symptomatic people out of the building, and to limit the extra people they could bring with them. It’s all about the number of contacts you have.”
The story was different, though, for oncologists in several other countries, and sometimes it varied immensely within each nation.
“We treated fewer patients with cancer during the first wave,” says Dirk Arnold, MD, medical director of the Asklepios Tumor Center Hamburg (Germany), in an interview. “In part, this was because staff were quarantined and because we had a completely different infrastructure in all of the hospitals. But also fewer patients with cancer came to the clinic at all. A lot of resources were directed toward COVID-19.”
In Spain, telemedicine helped keep up with visits, but other areas felt the effect of COVID-19 patient loads.
“At least in the oncology department of our center, we have practically maintained 100% of visits, mostly by telephone,” says Dr. Arrué, “but the reality is that our country has not yet been prepared for telemedicine.”
Laura Mezquita, MD, of the department of medical oncology at Hospital Clinic de Barcelona, describes a more dramatic situation: “We have seen how some of our patients, especially with metastatic disease, have been dismissed for intensive care and life-support treatments, as well as specific treatments against COVID-19 (tocilizumab, remdesivir, etc.) due to the general health collapse of the former wave,” she said. She adds that specific oncologic populations, such as those with thoracic tumors, have been more affected.
Distress among oncologists
Many oncologists are still feeling stressed and fatigued after the first wave, just as a second string of outbreaks is on its way.
A survey presented at last month’s ESMO 2020 Congress found that, in July-August, moral distress was reported by one-third of the oncologists who responded, and more than half reported a feeling of exhaustion.
“The tiredness and team exhaustion is noticeable,” said Dr. Arnold. “We recently had a task force discussion about what will happen when we have a second wave and how the department and our services will adapt. It was clear that those who were at the very front in the first wave had only a limited desire to do that again in the second wave.”
Another concern: COVID-19’s effect on staffing levels.
“We have a population of young caregivers who are affected by the COVID-19 disease with an absenteeism rate that is quite unprecedented,” said Sophie Beaupère, general delegate of Unicancer since January.
She said that, in general, the absenteeism rate in the cancer centers averages 5%-6%, depending on the year. But that rate is now skyrocketing.
Stop-start cycle for surgery
As caregivers quarantined around the world, more than 10% of patients with cancer had treatment canceled or delayed during the first wave of the pandemic, according to another survey from ESMO, involving 109 oncologists from 18 countries.
Difficulties were reported for surgeries by 34% of the centers, but also difficulties with delivering chemotherapy (22% of centers), radiotherapy (13.7%), and therapy with checkpoint inhibitors (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Stopping surgery is a real concern in France, noted Dr. Kahn, the National League Against Cancer chair. He says that in regions that were badly hit by COVID-19, “it was not possible to have access to the operating room for people who absolutely needed surgery; for example, patients with lung cancer that was still operable. Most of the recovery rooms were mobilized for resuscitation.”
There may be some solutions, suggested Thierry Breton, director general of the National Institute of Cancer in France. “We are getting prepared, with the health ministry, for a possible increase in hospital tension, which would lead to a situation where we would have to reschedule operations. Nationally, regionally, and locally, we are seeing how we can resume and prioritize surgeries that have not been done.”
Delays in cancer diagnosis
While COVID-19 affected treatment, many oncologists say the major impact of the first wave was a delay in diagnosing cancer. Some of this was a result of the suspension of cancer screening programs, but there was also fear among the general public about visiting clinics and hospitals during a pandemic.
“We didn’t do so well with cancer during the first wave here in the U.K.,” said Karol Sikora, PhD, MBBChir, professor of cancer medicine and founding dean at the University of Buckingham Medical School, London. “Cancer diagnostic pathways virtually stalled partly because patients didn’t seek help, but getting scans and biopsies was also very difficult. Even patients referred urgently under the ‘2-weeks-wait’ rule were turned down.”
In France, “the delay in diagnosis is indisputable,” said Dr. Kahn. “About 50% of the cancer diagnoses one would expect during this period were missed.”
“I am worried that there remains a major traffic jam that has not been caught up with, and, in the meantime, the health crisis is worsening,” he added.
In Seattle, Dr. Gralow said the first COVID-19 wave had little impact on treatment for breast cancer, but it was in screening for breast cancer “where things really got messed up.”
“Even though we’ve been fully ramped up again,” she said, concerns remain. To ensure that screening mammography is maintained, “we have spaced out the visits to keep our waiting rooms less populated, with a longer time between using the machine so we can clean it. To do this, we have extended operating hours and are now opening on Saturday.
“So we’re actually at 100% of our capacity, but I’m really nervous, though, that a lot of people put off their screening mammogram and aren’t going to come in and get it.
“Not only did people get the message to stay home and not do nonessential things, but I think a lot of people lost their health insurance when they lost their jobs,” she said, and without health insurance, they are not covered for cancer screening.
Looking ahead, with a plan
Many oncologists agree that access to care can and must be improved – and there were some positive moves.
“Some regimens changed during the first months of the pandemic, and I don’t see them going back to the way they were anytime soon,” said Dr. Testa. “The changes/adaptations that were made to minimize the chance of SARS-CoV-2 infection are still in place and will go on for a while. In this context, telemedicine helped a lot. The pandemic forced the stakeholders to step up and put it in place in March. And now it’s here to stay.”
The experience gained in the last several months has driven preparation for the next wave.
“We are not going to see the disorganization that we saw during the first wave,” said Florence Joly, MD, PhD, head of medical oncology at the Centre François Baclesse in Caen, France. “The difference between now and earlier this year is that COVID diagnostic tests are available. That was one of the problems in the first wave. We had no way to diagnose.”
On the East Coast of the United States, medical oncologist Charu Aggarwal, MD, MPH, is also optimistic: “I think we’re at a place where we can manage.”
“I believe if there was going to be a new wave of COVID-19 cases we would be: better psychologically prepared and better organized,” said Dr. Aggarwal, assistant professor of medicine in the hematology-oncology division at the University of Pennsylvania, Philadelphia. “We already have experience with all of the tools, we have telemedicine available, we have screening protocols available, we have testing, we are already universally masking, everyone’s hand-washing, so I do think that means we would be okay.”
Dr. Arnold agreed that “we are much better prepared than for the first wave, but … we have immense tasks in the area of patient management, the digitization of patient care, the clear allocation of resources when there is a second or third wave. In many areas of preparation, I believe, unfortunately, we are not as well positioned as we had actually hoped.”
The first wave of COVID hit cancer services in the United Kingdom particularly hard: One modeling study suggested that delays in cancer referrals will lead to thousands of additional deaths and tens of thousands of life-years lost.
“Cancer services are working at near normal levels now, but they are still fragile and could be severely compromised again if the NHS [National Health Service] gets flooded by COVID patients,” said Dr. Sikora.
The second wave may be different. “Although the number of infections has increased, the hospitalizations have only risen a little. Let’s see what happens,” he said in an interview. Since then, however, infections have continued to rise, and there has been an increase in hospitalizations. New social distancing measures in the United Kingdom were put into place on Oct. 12, with the aim of protecting the NHS from overload.
Dr. Arrué describes it this way: “The reality is that the ‘second wave’ has left behind the initial grief and shock that both patients and health professionals experienced when faced with something that, until now, we had only seen in the movies.” The second wave has led to new restrictions – including a partial lockdown since the beginning of October.
Dr. Aggarwal says her department recently had a conference with Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, about the impact of COVID-19 on oncology.
“I asked him what advice he’d give oncologists, and he said to go back to as much screening as you were doing previously as quickly as possible. That’s what must be relayed to our oncologists in the community – and also to primary care physicians – because they are often the ones who are ordering and championing the screening efforts.”
This article was originated by Aude Lecrubier, Medscape French edition, and developed by Zosia Chustecka, Medscape Oncology. With additional reporting by Kate Johnson, freelance medical journalist, Claudia Gottschling for Medscape Germany, Leoleli Schwartz for Medscape em português, Tim Locke for Medscape United Kingdom, and Carla Nieto Martínez, freelance medical journalist for Medscape Spanish edition.
This article first appeared on Medscape.com.
It’s not time to abandon routine screening mammography in average-risk women in their 40s
In the 1970s and early 1980s, population-based screening mammography was studied in numerous randomized control trials (RCTs), with the primary outcome of reduced breast cancer mortality. Although technology and the sensitivity of mammography in the 1980s was somewhat rudimentary compared with current screening, a meta-analysis of these RCTs demonstrated a clear mortality benefit for screening mammography.1 As a result, widespread population-based mammography was introduced in the mid-1980s in the United States and has become a standard for breast cancer screening.
Since that time, few RCTs of screening mammography versus observation have been conducted because of the ethical challenges of entering women into such studies as well as the difficulty and expense of long-term follow-up to measure the effect of screening on breast cancer mortality. Without ongoing RCTs of mammography, retrospective, observational, and computer simulation trials of the efficacy and harms of screening mammography have been conducted using proxy measures of mortality (such as stage at diagnosis), and some have questioned the overall benefit of screening mammography.2,3
To further complicate this controversy, some national guidelines have recommended against routinely recommending screening mammography for women aged 40 to 49 based on concerns that the harms (callbacks, benign breast biopsies, overdiagnosis) exceed the potential benefits (earlier diagnosis, possible decrease in needed treatments, reduced breast cancer mortality).4 This has resulted in a confusing morass of national recommendations with uncertainty regarding the question of whether to routinely offer screening mammography for women in their 40s at average risk for breast cancer.4-6
Recently, to address this question Duffy and colleagues conducted a large RCT of women in their 40s to evaluate the long-term effect of mammography on breast cancer mortality.7 Here, I review the study in depth and offer some guidance to clinicians and women struggling with screening decisions.
Breast cancer mortality significantly lower in the screening group
The RCT, known as the UK Age trial, was conducted in England, Wales, and Scotland and enrolled 160,921 women from 1990 through 1997.7 Women were randomly assigned in a 2:1 ratio to observation or annual screening mammogram beginning at age 39–41 until age 48. (In the United Kingdom, all women are screened starting at age 50.) Study enrollees were followed for a median of 22.8 years, and the primary outcome was breast cancer mortality.
The study results showed a 25% relative risk (RR) reduction in breast cancer mortality at 10 years of follow-up in the mammography group compared with the unscreened women (83 breast cancer deaths in the mammography group vs 219 in the observation group [RR, 0.75; 95% confidence interval (CI), 0.58–0.97; P = .029]). Based on the prevalence of breast cancer in women in their 40s, this 25% relative risk reduction translates into approximately 1 less death per 1,000 women who undergo routine screening in their 40s.
While there was no additional significant mortality reduction beyond 10 years of follow-up, as noted mammography is offered routinely starting at age 50 to all women in the United Kingdom. The authors concluded that “reducing the lower age limit for screening from 50 to 40 years [of age] could potentially reduce breast cancer mortality.”
Was overdiagnosis a concern? Another finding in this trial was related to overdiagnosis of breast cancer in the screened group. Overdiagnosis refers to mammographic-only diagnosis (that is, no clinical findings) of nonaggressive breast cancer, which would remain indolent and not harm the patient. The study results demonstrated essentially no overdiagnosis in women screened at age 40 compared with the unscreened group.
Continue to: Large trial, long follow-up are key strengths...
Large trial, long follow-up are key strengths
The UK Age trial’s primary strength is its study design: a large population-based RCT that included diverse participants with the critical study outcome for cancer screening (mortality). The study’s long-term follow-up is another key strength, since breast cancer mortality typically occurs 7 to 10 years after diagnosis. In addition, results were available for 99.9% of the women enrolled in the trial (that is, only 0.1% of women were lost to follow-up). Interestingly, the demonstrated mortality reduction with screening mammography for women in their 40s validates the mortality benefit demonstrated in other large RCTs of women in their 40s.1
Another strong point is that the study addresses the issue of whether screening women in their 40s results in overdiagnosis compared with women who start screening in their 50s. Further, this study validates a prior observational study that mammographic findings of nonprogressive cancers do not disappear, so nonaggressive cancers that present on mammography in women in their 40s still would be detected when women start screening in their 50s.8
Study limitations should be noted
The study has several limitations. For example, significant improvements have been made in breast cancer treatments that may mitigate against the positive impact of screening mammography. The impact of changed breast cancer management over the past 20 years could not be addressed with this study’s design since women would have been treated in the 1990s. In addition, substantial improvements have occurred in breast cancer screening standards (2 views vs the single view used in the study) and technology since the 1990s. Current mammography includes nearly uniform use of either digital mammography (DM) or digital breast tomosynthesis (DBT), both of which improve breast cancer detection for women in their 40s compared with the older film-screen technology. In addition, DBT reduces false-positive results by approximately 40%, resulting in fewer callbacks and biopsies. While improved cancer detection and reduced false-positive results are seen with DM and DBT, whether these technology improvements result in improved breast cancer mortality has not yet been sufficiently studied.
Perhaps the most important limitation in this study is that the women did not undergo routine risk assessment before trial entry to assure that they all were at “average risk.” As a result, both high- and average-risk women would have been included in this population-based trial. Without risk stratification, it remains uncertain whether the reduction in breast cancer mortality disproportionately exists within a high-risk subgroup (such as breast cancer gene mutation carriers).
Finally, the cost efficacy of routine screening mammography for women in their 40s was not evaluated in this study.
The UK Age trial in perspective
The good news is that there is the clear evidence that breast cancer mortality rates (deaths per 100,000) have decreased by about 40% over the past 50 years, likely due to improvements in breast cancer treatment and routine screening mammography.9 Breast cancer mortality reduction is particularly important because breast cancer remains the most common cancer and is the second leading cause of cancer death in women in the United States. In the past decade, considerable debate has arisen arguing whether this reduction in breast cancer mortality is due to improved treatments, routine screening mammography, or both. Authors of a retrospective trial in Australia, recently reviewed in OBG Management, suggested that the majority of improvement is due to improvements in treatment.3,10 However, as the authors pointed out, due to the trial’s retrospective design, causality only can be inferred. The current UK Age trial does add to the numerous prospective trials demonstrating mortality benefit for mammography in women in their 40s.11
What remains a challenge for clinicians, and for women struggling with the mammography question, is the absence of risk assessment in these long-term RCT trials as well as in the large retrospective database studies. Without risk stratification, these studies treated all the study population as “average risk.” Because breast cancer risk assessment is sporadically performed in clinical practice and there are no published RCTs of screening mammography in risk-assessed “average risk” women in their 40s, it remains uncertain whether the women benefiting from screening in their 40s are in a high-risk group or whether women of average risk in this age group also are benefiting from routine screening mammography.
Continue to: What’s next: Incorporate routine risk assessment into clinical practice...
What’s next: Incorporate routine risk assessment into clinical practice
It is not time to abandon screening mammography for all women in their 40s. Rather, routine risk assessment should be performed using one of many available validated or widely tested tools, a recommendation supported by the American College of Obstetricians and Gynecologists, the National Comprehensive Cancer Network, and the US Preventive Services Task Force.5,6,12
Ideally, these tools can be incorporated into an electronic health record and prepopulated using already available patient data (such as age, reproductive risk factors, current medications, breast density if available, and family history). Prepopulating available data into breast cancer risk calculators would allow clinicians to spend time on counseling women regarding breast cancer risk and appropriate screening methods. The TABLE provides a summary of useful breast cancer risk calculators and includes comments about their utility and significant limitations and benefits. In addition to breast cancer risk, the more comprehensive risk calculators (Tyrer-Cuzick and BOADICEA) allow calculation of ovarian cancer risk and gene mutation risk.
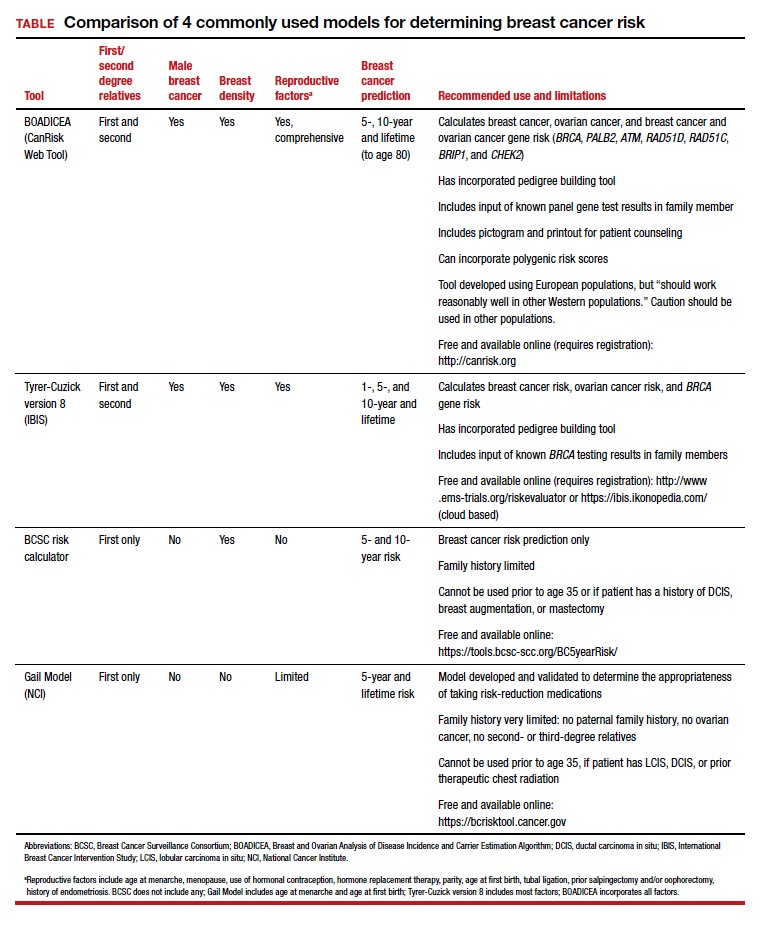
Routinely performing breast cancer risk assessment can guide discussions of screening mammography and can provide data for conducting a more individualized discussion on cancer genetic counseling and testing, risk reduction methods in high-risk women, and possible use of intensive breast cancer screening tools in identified high-risk women.
Ultimately, debating the question of whether all women should have routine breast cancer screening in their 40s should be passé. Ideally, all women should undergo breast cancer risk assessment in their 20s. Risk assessment results can then be used to guide the discussion of multiple potential interventions for women in their 40s (or earlier if appropriate), including routine screening mammography, cancer genetic counseling and testing in appropriate individuals, and intervention for women who are identified at high risk.
Absent breast cancer risk assessment, screening mammography still should be offered to women in their 40s, and the decision to proceed should be based on a discussion of risks, benefits, and the value the patient places on these factors.●
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244-255.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249-e.
- Nelson HD, Cantor A, Humphrey L, et al. A systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence syntheses No. 124. AHRQ Publication No. 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- ACOG Committee on Practice Bulletins–Gynecology. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Arleo EK, Monticciolo DL, Monsees B, et al. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14:863-867.
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451.
- Kaunitz AM. How effective is screening mammography for preventing breast cancer mortality? OBG Manag. 2020;32(8):17,49.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:652-665.
In the 1970s and early 1980s, population-based screening mammography was studied in numerous randomized control trials (RCTs), with the primary outcome of reduced breast cancer mortality. Although technology and the sensitivity of mammography in the 1980s was somewhat rudimentary compared with current screening, a meta-analysis of these RCTs demonstrated a clear mortality benefit for screening mammography.1 As a result, widespread population-based mammography was introduced in the mid-1980s in the United States and has become a standard for breast cancer screening.
Since that time, few RCTs of screening mammography versus observation have been conducted because of the ethical challenges of entering women into such studies as well as the difficulty and expense of long-term follow-up to measure the effect of screening on breast cancer mortality. Without ongoing RCTs of mammography, retrospective, observational, and computer simulation trials of the efficacy and harms of screening mammography have been conducted using proxy measures of mortality (such as stage at diagnosis), and some have questioned the overall benefit of screening mammography.2,3
To further complicate this controversy, some national guidelines have recommended against routinely recommending screening mammography for women aged 40 to 49 based on concerns that the harms (callbacks, benign breast biopsies, overdiagnosis) exceed the potential benefits (earlier diagnosis, possible decrease in needed treatments, reduced breast cancer mortality).4 This has resulted in a confusing morass of national recommendations with uncertainty regarding the question of whether to routinely offer screening mammography for women in their 40s at average risk for breast cancer.4-6
Recently, to address this question Duffy and colleagues conducted a large RCT of women in their 40s to evaluate the long-term effect of mammography on breast cancer mortality.7 Here, I review the study in depth and offer some guidance to clinicians and women struggling with screening decisions.
Breast cancer mortality significantly lower in the screening group
The RCT, known as the UK Age trial, was conducted in England, Wales, and Scotland and enrolled 160,921 women from 1990 through 1997.7 Women were randomly assigned in a 2:1 ratio to observation or annual screening mammogram beginning at age 39–41 until age 48. (In the United Kingdom, all women are screened starting at age 50.) Study enrollees were followed for a median of 22.8 years, and the primary outcome was breast cancer mortality.
The study results showed a 25% relative risk (RR) reduction in breast cancer mortality at 10 years of follow-up in the mammography group compared with the unscreened women (83 breast cancer deaths in the mammography group vs 219 in the observation group [RR, 0.75; 95% confidence interval (CI), 0.58–0.97; P = .029]). Based on the prevalence of breast cancer in women in their 40s, this 25% relative risk reduction translates into approximately 1 less death per 1,000 women who undergo routine screening in their 40s.
While there was no additional significant mortality reduction beyond 10 years of follow-up, as noted mammography is offered routinely starting at age 50 to all women in the United Kingdom. The authors concluded that “reducing the lower age limit for screening from 50 to 40 years [of age] could potentially reduce breast cancer mortality.”
Was overdiagnosis a concern? Another finding in this trial was related to overdiagnosis of breast cancer in the screened group. Overdiagnosis refers to mammographic-only diagnosis (that is, no clinical findings) of nonaggressive breast cancer, which would remain indolent and not harm the patient. The study results demonstrated essentially no overdiagnosis in women screened at age 40 compared with the unscreened group.
Continue to: Large trial, long follow-up are key strengths...
Large trial, long follow-up are key strengths
The UK Age trial’s primary strength is its study design: a large population-based RCT that included diverse participants with the critical study outcome for cancer screening (mortality). The study’s long-term follow-up is another key strength, since breast cancer mortality typically occurs 7 to 10 years after diagnosis. In addition, results were available for 99.9% of the women enrolled in the trial (that is, only 0.1% of women were lost to follow-up). Interestingly, the demonstrated mortality reduction with screening mammography for women in their 40s validates the mortality benefit demonstrated in other large RCTs of women in their 40s.1
Another strong point is that the study addresses the issue of whether screening women in their 40s results in overdiagnosis compared with women who start screening in their 50s. Further, this study validates a prior observational study that mammographic findings of nonprogressive cancers do not disappear, so nonaggressive cancers that present on mammography in women in their 40s still would be detected when women start screening in their 50s.8
Study limitations should be noted
The study has several limitations. For example, significant improvements have been made in breast cancer treatments that may mitigate against the positive impact of screening mammography. The impact of changed breast cancer management over the past 20 years could not be addressed with this study’s design since women would have been treated in the 1990s. In addition, substantial improvements have occurred in breast cancer screening standards (2 views vs the single view used in the study) and technology since the 1990s. Current mammography includes nearly uniform use of either digital mammography (DM) or digital breast tomosynthesis (DBT), both of which improve breast cancer detection for women in their 40s compared with the older film-screen technology. In addition, DBT reduces false-positive results by approximately 40%, resulting in fewer callbacks and biopsies. While improved cancer detection and reduced false-positive results are seen with DM and DBT, whether these technology improvements result in improved breast cancer mortality has not yet been sufficiently studied.
Perhaps the most important limitation in this study is that the women did not undergo routine risk assessment before trial entry to assure that they all were at “average risk.” As a result, both high- and average-risk women would have been included in this population-based trial. Without risk stratification, it remains uncertain whether the reduction in breast cancer mortality disproportionately exists within a high-risk subgroup (such as breast cancer gene mutation carriers).
Finally, the cost efficacy of routine screening mammography for women in their 40s was not evaluated in this study.
The UK Age trial in perspective
The good news is that there is the clear evidence that breast cancer mortality rates (deaths per 100,000) have decreased by about 40% over the past 50 years, likely due to improvements in breast cancer treatment and routine screening mammography.9 Breast cancer mortality reduction is particularly important because breast cancer remains the most common cancer and is the second leading cause of cancer death in women in the United States. In the past decade, considerable debate has arisen arguing whether this reduction in breast cancer mortality is due to improved treatments, routine screening mammography, or both. Authors of a retrospective trial in Australia, recently reviewed in OBG Management, suggested that the majority of improvement is due to improvements in treatment.3,10 However, as the authors pointed out, due to the trial’s retrospective design, causality only can be inferred. The current UK Age trial does add to the numerous prospective trials demonstrating mortality benefit for mammography in women in their 40s.11
What remains a challenge for clinicians, and for women struggling with the mammography question, is the absence of risk assessment in these long-term RCT trials as well as in the large retrospective database studies. Without risk stratification, these studies treated all the study population as “average risk.” Because breast cancer risk assessment is sporadically performed in clinical practice and there are no published RCTs of screening mammography in risk-assessed “average risk” women in their 40s, it remains uncertain whether the women benefiting from screening in their 40s are in a high-risk group or whether women of average risk in this age group also are benefiting from routine screening mammography.
Continue to: What’s next: Incorporate routine risk assessment into clinical practice...
What’s next: Incorporate routine risk assessment into clinical practice
It is not time to abandon screening mammography for all women in their 40s. Rather, routine risk assessment should be performed using one of many available validated or widely tested tools, a recommendation supported by the American College of Obstetricians and Gynecologists, the National Comprehensive Cancer Network, and the US Preventive Services Task Force.5,6,12
Ideally, these tools can be incorporated into an electronic health record and prepopulated using already available patient data (such as age, reproductive risk factors, current medications, breast density if available, and family history). Prepopulating available data into breast cancer risk calculators would allow clinicians to spend time on counseling women regarding breast cancer risk and appropriate screening methods. The TABLE provides a summary of useful breast cancer risk calculators and includes comments about their utility and significant limitations and benefits. In addition to breast cancer risk, the more comprehensive risk calculators (Tyrer-Cuzick and BOADICEA) allow calculation of ovarian cancer risk and gene mutation risk.

Routinely performing breast cancer risk assessment can guide discussions of screening mammography and can provide data for conducting a more individualized discussion on cancer genetic counseling and testing, risk reduction methods in high-risk women, and possible use of intensive breast cancer screening tools in identified high-risk women.
Ultimately, debating the question of whether all women should have routine breast cancer screening in their 40s should be passé. Ideally, all women should undergo breast cancer risk assessment in their 20s. Risk assessment results can then be used to guide the discussion of multiple potential interventions for women in their 40s (or earlier if appropriate), including routine screening mammography, cancer genetic counseling and testing in appropriate individuals, and intervention for women who are identified at high risk.
Absent breast cancer risk assessment, screening mammography still should be offered to women in their 40s, and the decision to proceed should be based on a discussion of risks, benefits, and the value the patient places on these factors.●
In the 1970s and early 1980s, population-based screening mammography was studied in numerous randomized control trials (RCTs), with the primary outcome of reduced breast cancer mortality. Although technology and the sensitivity of mammography in the 1980s was somewhat rudimentary compared with current screening, a meta-analysis of these RCTs demonstrated a clear mortality benefit for screening mammography.1 As a result, widespread population-based mammography was introduced in the mid-1980s in the United States and has become a standard for breast cancer screening.
Since that time, few RCTs of screening mammography versus observation have been conducted because of the ethical challenges of entering women into such studies as well as the difficulty and expense of long-term follow-up to measure the effect of screening on breast cancer mortality. Without ongoing RCTs of mammography, retrospective, observational, and computer simulation trials of the efficacy and harms of screening mammography have been conducted using proxy measures of mortality (such as stage at diagnosis), and some have questioned the overall benefit of screening mammography.2,3
To further complicate this controversy, some national guidelines have recommended against routinely recommending screening mammography for women aged 40 to 49 based on concerns that the harms (callbacks, benign breast biopsies, overdiagnosis) exceed the potential benefits (earlier diagnosis, possible decrease in needed treatments, reduced breast cancer mortality).4 This has resulted in a confusing morass of national recommendations with uncertainty regarding the question of whether to routinely offer screening mammography for women in their 40s at average risk for breast cancer.4-6
Recently, to address this question Duffy and colleagues conducted a large RCT of women in their 40s to evaluate the long-term effect of mammography on breast cancer mortality.7 Here, I review the study in depth and offer some guidance to clinicians and women struggling with screening decisions.
Breast cancer mortality significantly lower in the screening group
The RCT, known as the UK Age trial, was conducted in England, Wales, and Scotland and enrolled 160,921 women from 1990 through 1997.7 Women were randomly assigned in a 2:1 ratio to observation or annual screening mammogram beginning at age 39–41 until age 48. (In the United Kingdom, all women are screened starting at age 50.) Study enrollees were followed for a median of 22.8 years, and the primary outcome was breast cancer mortality.
The study results showed a 25% relative risk (RR) reduction in breast cancer mortality at 10 years of follow-up in the mammography group compared with the unscreened women (83 breast cancer deaths in the mammography group vs 219 in the observation group [RR, 0.75; 95% confidence interval (CI), 0.58–0.97; P = .029]). Based on the prevalence of breast cancer in women in their 40s, this 25% relative risk reduction translates into approximately 1 less death per 1,000 women who undergo routine screening in their 40s.
While there was no additional significant mortality reduction beyond 10 years of follow-up, as noted mammography is offered routinely starting at age 50 to all women in the United Kingdom. The authors concluded that “reducing the lower age limit for screening from 50 to 40 years [of age] could potentially reduce breast cancer mortality.”
Was overdiagnosis a concern? Another finding in this trial was related to overdiagnosis of breast cancer in the screened group. Overdiagnosis refers to mammographic-only diagnosis (that is, no clinical findings) of nonaggressive breast cancer, which would remain indolent and not harm the patient. The study results demonstrated essentially no overdiagnosis in women screened at age 40 compared with the unscreened group.
Continue to: Large trial, long follow-up are key strengths...
Large trial, long follow-up are key strengths
The UK Age trial’s primary strength is its study design: a large population-based RCT that included diverse participants with the critical study outcome for cancer screening (mortality). The study’s long-term follow-up is another key strength, since breast cancer mortality typically occurs 7 to 10 years after diagnosis. In addition, results were available for 99.9% of the women enrolled in the trial (that is, only 0.1% of women were lost to follow-up). Interestingly, the demonstrated mortality reduction with screening mammography for women in their 40s validates the mortality benefit demonstrated in other large RCTs of women in their 40s.1
Another strong point is that the study addresses the issue of whether screening women in their 40s results in overdiagnosis compared with women who start screening in their 50s. Further, this study validates a prior observational study that mammographic findings of nonprogressive cancers do not disappear, so nonaggressive cancers that present on mammography in women in their 40s still would be detected when women start screening in their 50s.8
Study limitations should be noted
The study has several limitations. For example, significant improvements have been made in breast cancer treatments that may mitigate against the positive impact of screening mammography. The impact of changed breast cancer management over the past 20 years could not be addressed with this study’s design since women would have been treated in the 1990s. In addition, substantial improvements have occurred in breast cancer screening standards (2 views vs the single view used in the study) and technology since the 1990s. Current mammography includes nearly uniform use of either digital mammography (DM) or digital breast tomosynthesis (DBT), both of which improve breast cancer detection for women in their 40s compared with the older film-screen technology. In addition, DBT reduces false-positive results by approximately 40%, resulting in fewer callbacks and biopsies. While improved cancer detection and reduced false-positive results are seen with DM and DBT, whether these technology improvements result in improved breast cancer mortality has not yet been sufficiently studied.
Perhaps the most important limitation in this study is that the women did not undergo routine risk assessment before trial entry to assure that they all were at “average risk.” As a result, both high- and average-risk women would have been included in this population-based trial. Without risk stratification, it remains uncertain whether the reduction in breast cancer mortality disproportionately exists within a high-risk subgroup (such as breast cancer gene mutation carriers).
Finally, the cost efficacy of routine screening mammography for women in their 40s was not evaluated in this study.
The UK Age trial in perspective
The good news is that there is the clear evidence that breast cancer mortality rates (deaths per 100,000) have decreased by about 40% over the past 50 years, likely due to improvements in breast cancer treatment and routine screening mammography.9 Breast cancer mortality reduction is particularly important because breast cancer remains the most common cancer and is the second leading cause of cancer death in women in the United States. In the past decade, considerable debate has arisen arguing whether this reduction in breast cancer mortality is due to improved treatments, routine screening mammography, or both. Authors of a retrospective trial in Australia, recently reviewed in OBG Management, suggested that the majority of improvement is due to improvements in treatment.3,10 However, as the authors pointed out, due to the trial’s retrospective design, causality only can be inferred. The current UK Age trial does add to the numerous prospective trials demonstrating mortality benefit for mammography in women in their 40s.11
What remains a challenge for clinicians, and for women struggling with the mammography question, is the absence of risk assessment in these long-term RCT trials as well as in the large retrospective database studies. Without risk stratification, these studies treated all the study population as “average risk.” Because breast cancer risk assessment is sporadically performed in clinical practice and there are no published RCTs of screening mammography in risk-assessed “average risk” women in their 40s, it remains uncertain whether the women benefiting from screening in their 40s are in a high-risk group or whether women of average risk in this age group also are benefiting from routine screening mammography.
Continue to: What’s next: Incorporate routine risk assessment into clinical practice...
What’s next: Incorporate routine risk assessment into clinical practice
It is not time to abandon screening mammography for all women in their 40s. Rather, routine risk assessment should be performed using one of many available validated or widely tested tools, a recommendation supported by the American College of Obstetricians and Gynecologists, the National Comprehensive Cancer Network, and the US Preventive Services Task Force.5,6,12
Ideally, these tools can be incorporated into an electronic health record and prepopulated using already available patient data (such as age, reproductive risk factors, current medications, breast density if available, and family history). Prepopulating available data into breast cancer risk calculators would allow clinicians to spend time on counseling women regarding breast cancer risk and appropriate screening methods. The TABLE provides a summary of useful breast cancer risk calculators and includes comments about their utility and significant limitations and benefits. In addition to breast cancer risk, the more comprehensive risk calculators (Tyrer-Cuzick and BOADICEA) allow calculation of ovarian cancer risk and gene mutation risk.

Routinely performing breast cancer risk assessment can guide discussions of screening mammography and can provide data for conducting a more individualized discussion on cancer genetic counseling and testing, risk reduction methods in high-risk women, and possible use of intensive breast cancer screening tools in identified high-risk women.
Ultimately, debating the question of whether all women should have routine breast cancer screening in their 40s should be passé. Ideally, all women should undergo breast cancer risk assessment in their 20s. Risk assessment results can then be used to guide the discussion of multiple potential interventions for women in their 40s (or earlier if appropriate), including routine screening mammography, cancer genetic counseling and testing in appropriate individuals, and intervention for women who are identified at high risk.
Absent breast cancer risk assessment, screening mammography still should be offered to women in their 40s, and the decision to proceed should be based on a discussion of risks, benefits, and the value the patient places on these factors.●
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244-255.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249-e.
- Nelson HD, Cantor A, Humphrey L, et al. A systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence syntheses No. 124. AHRQ Publication No. 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- ACOG Committee on Practice Bulletins–Gynecology. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Arleo EK, Monticciolo DL, Monsees B, et al. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14:863-867.
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451.
- Kaunitz AM. How effective is screening mammography for preventing breast cancer mortality? OBG Manag. 2020;32(8):17,49.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244-255.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249-e.
- Nelson HD, Cantor A, Humphrey L, et al. A systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence syntheses No. 124. AHRQ Publication No. 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- ACOG Committee on Practice Bulletins–Gynecology. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Arleo EK, Monticciolo DL, Monsees B, et al. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14:863-867.
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451.
- Kaunitz AM. How effective is screening mammography for preventing breast cancer mortality? OBG Manag. 2020;32(8):17,49.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:652-665.
Pathologic CR in HER2+ breast cancer predicts long-term survival
In fact, for the majority of women, pCR appears to be a marker of cure.
The trial was conducted among 455 women with HER2-positive breast cancer tumors measuring at least 2 cm who were randomized to neoadjuvant trastuzumab, lapatinib, or both drugs in combination, each together with paclitaxel, followed by more chemotherapy and more of the same targeted therapy after surgery.
Relative to trastuzumab alone, trastuzumab plus lapatinib improved rates of pCR, as shown by data published in The Lancet in 2012. However, the dual therapy did not significantly prolong event-free or overall survival, according to data published in The Lancet Oncology in 2014. Findings were similar in an update at a median follow-up of 6.7 years, published in the European Journal of Cancer in 2019.
Study investigator Paolo Nuciforo, MD, PhD, of the Vall d’Hebron Institute of Oncology in Barcelona, reported the trial’s final results, now at a median follow-up of 9.7 years, at the 12th European Breast Cancer Conference.
There were no significant differences in 9-year outcomes by specific HER2-targeted therapy. However, in a landmark analysis among women who were event free and still on follow-up 30 weeks after randomization, those achieving pCR with any of the therapies were 52% less likely to experience events and 63% less likely to die. Benefit was greatest in the subset of patients with hormone receptor–negative disease.
“The long-term follow-up confirms that, independent of the treatment regimen that we use – in this case, the dual blockade was with lapatinib, but similar results can be expected with other dual blockade – the pCR is a very robust surrogate biomarker of long-term survival,” Dr. Nuciforo commented in a press conference, noting that dual trastuzumab and pertuzumab has emerged as the standard of care.
“If we really pay attention to the curve, it’s maybe interesting to see that, after year 6, we actually don’t see any events in the pCR population. So this means that these patients are almost cured. We cannot say the word ‘cure’ in cancer, but it’s very reassuring to see the long-term survival analysis support the use of pCR as an endpoint,” he elaborated.
“Our results support the design of future trial concepts in HER2-positive early breast cancer which use pCR as an early efficacy readout of long-term benefit to escalate or deescalate therapy, particularly for hormone receptor–negative tumors,” Dr. Nuciforo concluded.
Support for current practice
“The study lends support for the current practice of risk-stratifying by pCR as well as making treatment decisions regarding T-DM1 [trastuzumab emtansine], and there hasn’t been a big change between 5-year and 9-year outcomes,” Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill Lineberger Comprehensive Cancer Center, commented in an interview.
The lack of late events in the group with pCR technically meets the definition of cure, Dr. Carey said. “I think it speaks to the relatively early relapse risk in HER2-positive breast cancer and the impact of anti-HER2 therapy that carries forward. In general, these are findings similar to long-term findings of other trials and I suspect will be the same for any regimen.”
Although the analysis of dual lapatinib-trastuzumab therapy was underpowered, the trends seen align with favorable results in the adjuvant APHINITY trial (which combined trastuzumab with pertuzumab) and the neoadjuvant CALGB 40601 trial (which combined trastuzumab with lapatinib), according to Dr. Carey. “There has been a trend in every other study [of dual therapy] performed, so this is consistent.”
Study details
NeoALTTO is noteworthy for having the longest follow-up among all neoadjuvant studies of dual HER2 blockade in early breast cancer, Dr. Nuciforo said.
He reported no significant difference in survival between the treatment arms at 9 years.
The 9-year rate of event-free survival was 69% with lapatinib-trastuzumab, 63% with lapatinib alone, and 65% with trastuzumab alone. The corresponding 9-year rates of overall survival were 80%, 77%, and 76%, respectively.
However, there were significant differences in event-free and overall survival among women who achieved pCR and those who did not.
“pCR was achieved for almost twice as many patients treated with dual HER2 blockade, compared with patients in the single-agent arms,” Dr. Nuciforo pointed out. The pCR rate was 51.3% with lapatinib-trastuzumab, 24.7% with lapatinib alone, and 29.5% with trastuzumab alone.
Relative to peers who did not achieve pCR, women who did had better 9-year event-free survival (77% vs. 61%; adjusted hazard ratio, 0.48; P = .0008). The benefit was stronger in hormone receptor–negative disease (HR, 0.43; P = .002) than in hormone receptor–positive disease (HR, 0.60; P = .15).
The pattern was similar for overall survival at 9 years – 88% in those who achieved a pCR and 72% in those who did not (adjusted HR, 0.37; P = .0004). Again, greater benefit was seen in hormone receptor–negative disease (HR, 0.33; P = .002) than in hormone receptor–positive disease (HR, 0.44; P = .09).
“Biomarker-driven approaches may improve selection of those patients who are more likely to respond to anti-HER2 therapies,” Dr. Nuciforo proposed.
From 6 years onward, there were no additional fatal adverse events or nonfatal serious adverse events recorded, and no additional primary cardiac endpoints were recorded.
The study was funded by Novartis. Dr. Nuciforo and Dr. Carey disclosed no conflicts of interest.
SOURCE: Nuciforo P et al. EBCC-12 Virtual Conference, Abstract 23.
In fact, for the majority of women, pCR appears to be a marker of cure.
The trial was conducted among 455 women with HER2-positive breast cancer tumors measuring at least 2 cm who were randomized to neoadjuvant trastuzumab, lapatinib, or both drugs in combination, each together with paclitaxel, followed by more chemotherapy and more of the same targeted therapy after surgery.
Relative to trastuzumab alone, trastuzumab plus lapatinib improved rates of pCR, as shown by data published in The Lancet in 2012. However, the dual therapy did not significantly prolong event-free or overall survival, according to data published in The Lancet Oncology in 2014. Findings were similar in an update at a median follow-up of 6.7 years, published in the European Journal of Cancer in 2019.
Study investigator Paolo Nuciforo, MD, PhD, of the Vall d’Hebron Institute of Oncology in Barcelona, reported the trial’s final results, now at a median follow-up of 9.7 years, at the 12th European Breast Cancer Conference.
There were no significant differences in 9-year outcomes by specific HER2-targeted therapy. However, in a landmark analysis among women who were event free and still on follow-up 30 weeks after randomization, those achieving pCR with any of the therapies were 52% less likely to experience events and 63% less likely to die. Benefit was greatest in the subset of patients with hormone receptor–negative disease.
“The long-term follow-up confirms that, independent of the treatment regimen that we use – in this case, the dual blockade was with lapatinib, but similar results can be expected with other dual blockade – the pCR is a very robust surrogate biomarker of long-term survival,” Dr. Nuciforo commented in a press conference, noting that dual trastuzumab and pertuzumab has emerged as the standard of care.
“If we really pay attention to the curve, it’s maybe interesting to see that, after year 6, we actually don’t see any events in the pCR population. So this means that these patients are almost cured. We cannot say the word ‘cure’ in cancer, but it’s very reassuring to see the long-term survival analysis support the use of pCR as an endpoint,” he elaborated.
“Our results support the design of future trial concepts in HER2-positive early breast cancer which use pCR as an early efficacy readout of long-term benefit to escalate or deescalate therapy, particularly for hormone receptor–negative tumors,” Dr. Nuciforo concluded.
Support for current practice
“The study lends support for the current practice of risk-stratifying by pCR as well as making treatment decisions regarding T-DM1 [trastuzumab emtansine], and there hasn’t been a big change between 5-year and 9-year outcomes,” Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill Lineberger Comprehensive Cancer Center, commented in an interview.
The lack of late events in the group with pCR technically meets the definition of cure, Dr. Carey said. “I think it speaks to the relatively early relapse risk in HER2-positive breast cancer and the impact of anti-HER2 therapy that carries forward. In general, these are findings similar to long-term findings of other trials and I suspect will be the same for any regimen.”
Although the analysis of dual lapatinib-trastuzumab therapy was underpowered, the trends seen align with favorable results in the adjuvant APHINITY trial (which combined trastuzumab with pertuzumab) and the neoadjuvant CALGB 40601 trial (which combined trastuzumab with lapatinib), according to Dr. Carey. “There has been a trend in every other study [of dual therapy] performed, so this is consistent.”
Study details
NeoALTTO is noteworthy for having the longest follow-up among all neoadjuvant studies of dual HER2 blockade in early breast cancer, Dr. Nuciforo said.
He reported no significant difference in survival between the treatment arms at 9 years.
The 9-year rate of event-free survival was 69% with lapatinib-trastuzumab, 63% with lapatinib alone, and 65% with trastuzumab alone. The corresponding 9-year rates of overall survival were 80%, 77%, and 76%, respectively.
However, there were significant differences in event-free and overall survival among women who achieved pCR and those who did not.
“pCR was achieved for almost twice as many patients treated with dual HER2 blockade, compared with patients in the single-agent arms,” Dr. Nuciforo pointed out. The pCR rate was 51.3% with lapatinib-trastuzumab, 24.7% with lapatinib alone, and 29.5% with trastuzumab alone.
Relative to peers who did not achieve pCR, women who did had better 9-year event-free survival (77% vs. 61%; adjusted hazard ratio, 0.48; P = .0008). The benefit was stronger in hormone receptor–negative disease (HR, 0.43; P = .002) than in hormone receptor–positive disease (HR, 0.60; P = .15).
The pattern was similar for overall survival at 9 years – 88% in those who achieved a pCR and 72% in those who did not (adjusted HR, 0.37; P = .0004). Again, greater benefit was seen in hormone receptor–negative disease (HR, 0.33; P = .002) than in hormone receptor–positive disease (HR, 0.44; P = .09).
“Biomarker-driven approaches may improve selection of those patients who are more likely to respond to anti-HER2 therapies,” Dr. Nuciforo proposed.
From 6 years onward, there were no additional fatal adverse events or nonfatal serious adverse events recorded, and no additional primary cardiac endpoints were recorded.
The study was funded by Novartis. Dr. Nuciforo and Dr. Carey disclosed no conflicts of interest.
SOURCE: Nuciforo P et al. EBCC-12 Virtual Conference, Abstract 23.
In fact, for the majority of women, pCR appears to be a marker of cure.
The trial was conducted among 455 women with HER2-positive breast cancer tumors measuring at least 2 cm who were randomized to neoadjuvant trastuzumab, lapatinib, or both drugs in combination, each together with paclitaxel, followed by more chemotherapy and more of the same targeted therapy after surgery.
Relative to trastuzumab alone, trastuzumab plus lapatinib improved rates of pCR, as shown by data published in The Lancet in 2012. However, the dual therapy did not significantly prolong event-free or overall survival, according to data published in The Lancet Oncology in 2014. Findings were similar in an update at a median follow-up of 6.7 years, published in the European Journal of Cancer in 2019.
Study investigator Paolo Nuciforo, MD, PhD, of the Vall d’Hebron Institute of Oncology in Barcelona, reported the trial’s final results, now at a median follow-up of 9.7 years, at the 12th European Breast Cancer Conference.
There were no significant differences in 9-year outcomes by specific HER2-targeted therapy. However, in a landmark analysis among women who were event free and still on follow-up 30 weeks after randomization, those achieving pCR with any of the therapies were 52% less likely to experience events and 63% less likely to die. Benefit was greatest in the subset of patients with hormone receptor–negative disease.
“The long-term follow-up confirms that, independent of the treatment regimen that we use – in this case, the dual blockade was with lapatinib, but similar results can be expected with other dual blockade – the pCR is a very robust surrogate biomarker of long-term survival,” Dr. Nuciforo commented in a press conference, noting that dual trastuzumab and pertuzumab has emerged as the standard of care.
“If we really pay attention to the curve, it’s maybe interesting to see that, after year 6, we actually don’t see any events in the pCR population. So this means that these patients are almost cured. We cannot say the word ‘cure’ in cancer, but it’s very reassuring to see the long-term survival analysis support the use of pCR as an endpoint,” he elaborated.
“Our results support the design of future trial concepts in HER2-positive early breast cancer which use pCR as an early efficacy readout of long-term benefit to escalate or deescalate therapy, particularly for hormone receptor–negative tumors,” Dr. Nuciforo concluded.
Support for current practice
“The study lends support for the current practice of risk-stratifying by pCR as well as making treatment decisions regarding T-DM1 [trastuzumab emtansine], and there hasn’t been a big change between 5-year and 9-year outcomes,” Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill Lineberger Comprehensive Cancer Center, commented in an interview.
The lack of late events in the group with pCR technically meets the definition of cure, Dr. Carey said. “I think it speaks to the relatively early relapse risk in HER2-positive breast cancer and the impact of anti-HER2 therapy that carries forward. In general, these are findings similar to long-term findings of other trials and I suspect will be the same for any regimen.”
Although the analysis of dual lapatinib-trastuzumab therapy was underpowered, the trends seen align with favorable results in the adjuvant APHINITY trial (which combined trastuzumab with pertuzumab) and the neoadjuvant CALGB 40601 trial (which combined trastuzumab with lapatinib), according to Dr. Carey. “There has been a trend in every other study [of dual therapy] performed, so this is consistent.”
Study details
NeoALTTO is noteworthy for having the longest follow-up among all neoadjuvant studies of dual HER2 blockade in early breast cancer, Dr. Nuciforo said.
He reported no significant difference in survival between the treatment arms at 9 years.
The 9-year rate of event-free survival was 69% with lapatinib-trastuzumab, 63% with lapatinib alone, and 65% with trastuzumab alone. The corresponding 9-year rates of overall survival were 80%, 77%, and 76%, respectively.
However, there were significant differences in event-free and overall survival among women who achieved pCR and those who did not.
“pCR was achieved for almost twice as many patients treated with dual HER2 blockade, compared with patients in the single-agent arms,” Dr. Nuciforo pointed out. The pCR rate was 51.3% with lapatinib-trastuzumab, 24.7% with lapatinib alone, and 29.5% with trastuzumab alone.
Relative to peers who did not achieve pCR, women who did had better 9-year event-free survival (77% vs. 61%; adjusted hazard ratio, 0.48; P = .0008). The benefit was stronger in hormone receptor–negative disease (HR, 0.43; P = .002) than in hormone receptor–positive disease (HR, 0.60; P = .15).
The pattern was similar for overall survival at 9 years – 88% in those who achieved a pCR and 72% in those who did not (adjusted HR, 0.37; P = .0004). Again, greater benefit was seen in hormone receptor–negative disease (HR, 0.33; P = .002) than in hormone receptor–positive disease (HR, 0.44; P = .09).
“Biomarker-driven approaches may improve selection of those patients who are more likely to respond to anti-HER2 therapies,” Dr. Nuciforo proposed.
From 6 years onward, there were no additional fatal adverse events or nonfatal serious adverse events recorded, and no additional primary cardiac endpoints were recorded.
The study was funded by Novartis. Dr. Nuciforo and Dr. Carey disclosed no conflicts of interest.
SOURCE: Nuciforo P et al. EBCC-12 Virtual Conference, Abstract 23.
FROM EBCC-12 VIRTUAL CONFERENCE