User login
FDA approves third trastuzumab biosimilar
The Food and Drug Administration .
Ontruzant (trastuzumab-dttb), marketed by Samsung Bioepis, is the third approved biosimilar to Genentech’s Herceptin in the United States.
Patients should be selected for treatment with Ontruzant using an FDA-approved companion diagnostic for a trastuzumab product.
The prescribing information for the newly approved biosimilar includes a boxed warning highlighting the risk of cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity associated with the drug.
Ontruzant was shown to be equivalent to Herceptin in a phase 3 study (Eur J Cancer. 2018 Apr;93:19-27).
The trial included 875 patients with HER2-positive early breast cancer. Patients were randomized to receive Ontruzant or Herceptin for eight cycles concurrently with chemotherapy. The chemotherapy consisted of four cycles of docetaxel followed by four cycles of 5-fluorouracil/epirubicin/cyclophosphamide.
The patients then underwent surgery, which was followed by 10 cycles of Ontruzant (n=380) or Herceptin (n=384).
The median follow-up was 437 days in the Ontruzant arm and 438 days in the Herceptin arm. Safety and efficacy results were similar between the treatment arms.
Treatment-emergent adverse events occurred in 97.5% of patients in the Ontruzant arm and 96.1% of those in the Herceptin arm.
The 12-month event-free survival rate was 93.7% in the Ontruzant arm and 93.4% in the Herceptin arm.
The Food and Drug Administration .
Ontruzant (trastuzumab-dttb), marketed by Samsung Bioepis, is the third approved biosimilar to Genentech’s Herceptin in the United States.
Patients should be selected for treatment with Ontruzant using an FDA-approved companion diagnostic for a trastuzumab product.
The prescribing information for the newly approved biosimilar includes a boxed warning highlighting the risk of cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity associated with the drug.
Ontruzant was shown to be equivalent to Herceptin in a phase 3 study (Eur J Cancer. 2018 Apr;93:19-27).
The trial included 875 patients with HER2-positive early breast cancer. Patients were randomized to receive Ontruzant or Herceptin for eight cycles concurrently with chemotherapy. The chemotherapy consisted of four cycles of docetaxel followed by four cycles of 5-fluorouracil/epirubicin/cyclophosphamide.
The patients then underwent surgery, which was followed by 10 cycles of Ontruzant (n=380) or Herceptin (n=384).
The median follow-up was 437 days in the Ontruzant arm and 438 days in the Herceptin arm. Safety and efficacy results were similar between the treatment arms.
Treatment-emergent adverse events occurred in 97.5% of patients in the Ontruzant arm and 96.1% of those in the Herceptin arm.
The 12-month event-free survival rate was 93.7% in the Ontruzant arm and 93.4% in the Herceptin arm.
The Food and Drug Administration .
Ontruzant (trastuzumab-dttb), marketed by Samsung Bioepis, is the third approved biosimilar to Genentech’s Herceptin in the United States.
Patients should be selected for treatment with Ontruzant using an FDA-approved companion diagnostic for a trastuzumab product.
The prescribing information for the newly approved biosimilar includes a boxed warning highlighting the risk of cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity associated with the drug.
Ontruzant was shown to be equivalent to Herceptin in a phase 3 study (Eur J Cancer. 2018 Apr;93:19-27).
The trial included 875 patients with HER2-positive early breast cancer. Patients were randomized to receive Ontruzant or Herceptin for eight cycles concurrently with chemotherapy. The chemotherapy consisted of four cycles of docetaxel followed by four cycles of 5-fluorouracil/epirubicin/cyclophosphamide.
The patients then underwent surgery, which was followed by 10 cycles of Ontruzant (n=380) or Herceptin (n=384).
The median follow-up was 437 days in the Ontruzant arm and 438 days in the Herceptin arm. Safety and efficacy results were similar between the treatment arms.
Treatment-emergent adverse events occurred in 97.5% of patients in the Ontruzant arm and 96.1% of those in the Herceptin arm.
The 12-month event-free survival rate was 93.7% in the Ontruzant arm and 93.4% in the Herceptin arm.
High postpartum breast cancer metastasis risk may persist for a decade
Increased risk of metastasis associated with postpartum breast cancer (PPBC) in women 45 years or younger may persist for 10 years after childbirth, a finding that may give reason to extend the 5-year window currently defining PPBC.
Analysis of more than 700 patients showed that risk of metastasis was approximately twofold higher for a decade after childbirth, with risks about 3.5- to fivefold higher in women diagnosed with stage I or II disease, reported lead author Erica Goddard, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues. Regardless of parity status, patients diagnosed with stage III disease had poor outcomes.
“The high risk for metastasis is independent of poor prognostic indicators, including biological subtype, stage, age, or year of diagnosis,” the investigators wrote in JAMA Network Open. “Yet, PPBC is an underrecognized subset of breast cancer, and few studies address the associated high risk for metastasis.”
The cohort study involved 701 women 45 years or younger who were diagnosed with breast cancer between 1981 and 2014. Early cases were retrospective, until the study switched to a prospective method in 2004. The investigators analyzed rates of distant metastasis and looked for associations with tumor cell proliferation, lymphovascular invasion, lymph node involvement, and other clinical attributes. Distant metastasis was defined by spread beyond the ipsilateral breast or local draining lymph node, as detected by physical exam, imaging, and/or pathological testing. The investigators also stained available tumor samples for Ki67 positivity, which is used for prognostic purposes, and to distinguish between ER-positive luminal A versus ER-positive luminal B disease.
Compared with nulliparous patients, women under 45 who were diagnosed with PPBC within 5 years of childbirth were 2.13 times as likely to develop metastasis (P = .009). This risk persisted for 5 more years. Women diagnosed within 5-10 years of childbirth showed a similar hazard ratio, of 2.23 (P = .006). After 10 years, the hazard ratio dropped to 1.6, but this value was statistically insignificant (P = .13). Patients identified with stage I or II disease had more dramatic risk profiles, with hazard ratios of 3.5 and 5.2, for diagnoses up to 5 years postpartum, and diagnoses 5-10 years postpartum, respectively. These findings suggest that, for some patients, the 5- to 10-year window may be the riskiest time for metastasis, and, incidentally, one that has historically been excluded from the definition of PPBC.
In addition, patients diagnosed with estrogen receptor–positive breast cancer within 10 years of childbirth had outcomes similar to those of nulliparous women with estrogen receptor–negative breast cancer, and postpartum women with estrogen receptor–negative breast cancer had worse outcomes than did nulliparous women with the same subtype. Furthermore, PPBC was associated with higher rates of lymph node involvement and lymphovascular invasion. Collectively, these findings suggest that PPBC is generally more aggressive than nulliparous breast cancer. In contrast, Ki67 positivity, identifying the luminal B subtype, was associated with worse outcome regardless of parity status, but this finding was statistically insignificant.
“[T]hese data suggest that stages I and II breast cancer in patients with PPBC diagnosed within 10 years of parturition may be underestimated in their risk for metastasis, as parity status is not currently factored into clinical decision-making algorithms, such as the National Comprehensive Cancer Network guidelines,” the investigators concluded. “In sum, we suggest that poor-prognostic PPBC is an increasing problem that merits more dedicated research.”
The study was funded by the National Cancer Institute, the National Institutes of Health, the U.S. Department of Defense, and other organizations. Dr. Goddard reported funding from the NCI and NIH. Dr. Mori reported financial support from the Department of Defense.
SOURCE: Goddard et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.
Increased risk of metastasis associated with postpartum breast cancer (PPBC) in women 45 years or younger may persist for 10 years after childbirth, a finding that may give reason to extend the 5-year window currently defining PPBC.
Analysis of more than 700 patients showed that risk of metastasis was approximately twofold higher for a decade after childbirth, with risks about 3.5- to fivefold higher in women diagnosed with stage I or II disease, reported lead author Erica Goddard, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues. Regardless of parity status, patients diagnosed with stage III disease had poor outcomes.
“The high risk for metastasis is independent of poor prognostic indicators, including biological subtype, stage, age, or year of diagnosis,” the investigators wrote in JAMA Network Open. “Yet, PPBC is an underrecognized subset of breast cancer, and few studies address the associated high risk for metastasis.”
The cohort study involved 701 women 45 years or younger who were diagnosed with breast cancer between 1981 and 2014. Early cases were retrospective, until the study switched to a prospective method in 2004. The investigators analyzed rates of distant metastasis and looked for associations with tumor cell proliferation, lymphovascular invasion, lymph node involvement, and other clinical attributes. Distant metastasis was defined by spread beyond the ipsilateral breast or local draining lymph node, as detected by physical exam, imaging, and/or pathological testing. The investigators also stained available tumor samples for Ki67 positivity, which is used for prognostic purposes, and to distinguish between ER-positive luminal A versus ER-positive luminal B disease.
Compared with nulliparous patients, women under 45 who were diagnosed with PPBC within 5 years of childbirth were 2.13 times as likely to develop metastasis (P = .009). This risk persisted for 5 more years. Women diagnosed within 5-10 years of childbirth showed a similar hazard ratio, of 2.23 (P = .006). After 10 years, the hazard ratio dropped to 1.6, but this value was statistically insignificant (P = .13). Patients identified with stage I or II disease had more dramatic risk profiles, with hazard ratios of 3.5 and 5.2, for diagnoses up to 5 years postpartum, and diagnoses 5-10 years postpartum, respectively. These findings suggest that, for some patients, the 5- to 10-year window may be the riskiest time for metastasis, and, incidentally, one that has historically been excluded from the definition of PPBC.
In addition, patients diagnosed with estrogen receptor–positive breast cancer within 10 years of childbirth had outcomes similar to those of nulliparous women with estrogen receptor–negative breast cancer, and postpartum women with estrogen receptor–negative breast cancer had worse outcomes than did nulliparous women with the same subtype. Furthermore, PPBC was associated with higher rates of lymph node involvement and lymphovascular invasion. Collectively, these findings suggest that PPBC is generally more aggressive than nulliparous breast cancer. In contrast, Ki67 positivity, identifying the luminal B subtype, was associated with worse outcome regardless of parity status, but this finding was statistically insignificant.
“[T]hese data suggest that stages I and II breast cancer in patients with PPBC diagnosed within 10 years of parturition may be underestimated in their risk for metastasis, as parity status is not currently factored into clinical decision-making algorithms, such as the National Comprehensive Cancer Network guidelines,” the investigators concluded. “In sum, we suggest that poor-prognostic PPBC is an increasing problem that merits more dedicated research.”
The study was funded by the National Cancer Institute, the National Institutes of Health, the U.S. Department of Defense, and other organizations. Dr. Goddard reported funding from the NCI and NIH. Dr. Mori reported financial support from the Department of Defense.
SOURCE: Goddard et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.
Increased risk of metastasis associated with postpartum breast cancer (PPBC) in women 45 years or younger may persist for 10 years after childbirth, a finding that may give reason to extend the 5-year window currently defining PPBC.
Analysis of more than 700 patients showed that risk of metastasis was approximately twofold higher for a decade after childbirth, with risks about 3.5- to fivefold higher in women diagnosed with stage I or II disease, reported lead author Erica Goddard, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues. Regardless of parity status, patients diagnosed with stage III disease had poor outcomes.
“The high risk for metastasis is independent of poor prognostic indicators, including biological subtype, stage, age, or year of diagnosis,” the investigators wrote in JAMA Network Open. “Yet, PPBC is an underrecognized subset of breast cancer, and few studies address the associated high risk for metastasis.”
The cohort study involved 701 women 45 years or younger who were diagnosed with breast cancer between 1981 and 2014. Early cases were retrospective, until the study switched to a prospective method in 2004. The investigators analyzed rates of distant metastasis and looked for associations with tumor cell proliferation, lymphovascular invasion, lymph node involvement, and other clinical attributes. Distant metastasis was defined by spread beyond the ipsilateral breast or local draining lymph node, as detected by physical exam, imaging, and/or pathological testing. The investigators also stained available tumor samples for Ki67 positivity, which is used for prognostic purposes, and to distinguish between ER-positive luminal A versus ER-positive luminal B disease.
Compared with nulliparous patients, women under 45 who were diagnosed with PPBC within 5 years of childbirth were 2.13 times as likely to develop metastasis (P = .009). This risk persisted for 5 more years. Women diagnosed within 5-10 years of childbirth showed a similar hazard ratio, of 2.23 (P = .006). After 10 years, the hazard ratio dropped to 1.6, but this value was statistically insignificant (P = .13). Patients identified with stage I or II disease had more dramatic risk profiles, with hazard ratios of 3.5 and 5.2, for diagnoses up to 5 years postpartum, and diagnoses 5-10 years postpartum, respectively. These findings suggest that, for some patients, the 5- to 10-year window may be the riskiest time for metastasis, and, incidentally, one that has historically been excluded from the definition of PPBC.
In addition, patients diagnosed with estrogen receptor–positive breast cancer within 10 years of childbirth had outcomes similar to those of nulliparous women with estrogen receptor–negative breast cancer, and postpartum women with estrogen receptor–negative breast cancer had worse outcomes than did nulliparous women with the same subtype. Furthermore, PPBC was associated with higher rates of lymph node involvement and lymphovascular invasion. Collectively, these findings suggest that PPBC is generally more aggressive than nulliparous breast cancer. In contrast, Ki67 positivity, identifying the luminal B subtype, was associated with worse outcome regardless of parity status, but this finding was statistically insignificant.
“[T]hese data suggest that stages I and II breast cancer in patients with PPBC diagnosed within 10 years of parturition may be underestimated in their risk for metastasis, as parity status is not currently factored into clinical decision-making algorithms, such as the National Comprehensive Cancer Network guidelines,” the investigators concluded. “In sum, we suggest that poor-prognostic PPBC is an increasing problem that merits more dedicated research.”
The study was funded by the National Cancer Institute, the National Institutes of Health, the U.S. Department of Defense, and other organizations. Dr. Goddard reported funding from the NCI and NIH. Dr. Mori reported financial support from the Department of Defense.
SOURCE: Goddard et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.
FROM JAMA NETWORK OPEN
Key clinical point: Increased risk of metastasis associated with postpartum breast cancer in women 45 years or younger may persist for 10 years after childbirth, instead of 5 years, as previously reported.
Major finding: Compared with nulliparous breast cancer patients, women 45 years or younger diagnosed with breast cancer within 5-10 years of childbirth were 2.23 times as likely to develop metastasis.
Study details: A retrospective and prospective cohort study involving 701 women with stage I, II, or III breast cancer who were 45 years or younger at time of diagnosis.
Disclosures: The study was funded by the National Cancer Institute, the National Institutes of Health, the U.S. Department of Defense, and other organizations. Dr. Goddard reported funding from the NCI and NIH. Dr. Mori reported financial support from the Department of Defense.
Source: Goddard et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.6997.
Progress in cancer research slowed by broken system
NEW YORK – Conflicts of interest, whether reported or unreported, are just one component of a broken system that has misaligned incentives with meaningful advances in breast cancer research, agreed a panel of experts who participated in an evening program sponsored by SHARE, a breast cancer patient advocacy group.
“The issue of disclosures, while it is important, is not really the key issue. The key issue for me is about the amazing amount of money that is changing hands,” said Fran Visco, president of the National Breast Cancer Coalition, Washington. She contended not enough money in breast cancer research is being applied to save women’s lives rather than driving profits for institutions, physicians, and the pharmaceutical industry.
The other two participants in this panel, called Conflicts of Interest in Research and Treatment: What Breast Cancer Patients Should Know, largely agreed. In turn, they each explained that major sums of money in play for physicians and institutions collaborating with industry are earmarked for generating income much more than improving outcomes.
“Most of where this money is going is for these little tiny improvements and there is no association between the outcome increment that you get and what you pay for that outcome increment,” said Robert Cook-Deegan, MD, a bioethicist and a professor at the School for Innovation in Society, Arizona State University, Tempe.
Leaving aside whether physician-scientists working at nonprofit research institutions can avoid conflicts of interest when money is funneled to them from start-up investment opportunities, pharmaceutical board memberships, and direct compensation deals for consultancies and other services, Dr. Cook-Deegan was referring to the bigger problem of misaligned incentives. Large financial rewards are available for any drug with market potential instead of being limited to those that save lives, he said.
Tracing the current system to the Bayh-Dole Act in 1980, which permitted researchers to claim patents for intellectual property developed with federal funding, Dr. Cook-Deegan described a system of minnows, fish, and sharks. Researchers, who are the minnows, seek potential new therapeutics to be developed by for-profit startups or other commercial entities, which are the fish. If the drug continues to show promise, it brings investors, who are the sharks.
Once the new therapy reaches the sharks, the excitement about a new and more effective treatment has been overshadowed by the excitement about profits, which are essential to pay for the large investment required for the clinical development that brings new drugs to market. If the drug is marketable, it is considered a success even when the clinical benefits barely register.
“There have been about 20 new [cancer] drugs approved by the [Food and Drug Administration] in the last 2 years. The increased median disease-free survival is usually about 3 months,” said Otis W. Brawley, MD, who recently joined the Kimmel Cancer Center at Johns Hopkins University after resigning his position as chief medical officer at the American Cancer Society (ACS).
Dr. Brawley joined the others in declaring: “The system is broken.” The flow of money is not the only incentive for researchers to participate in clinical development programs focused on minor advances rather than blockbuster discoveries. Unrestricted research grants are now much harder to obtain, according to Dr. Brawley, making collaboration with industry essential not just for research funding but career advancement. Research at major cancer centers is still being conducted at the highest level, but there are unavoidable interdependent relationships for nonprofit institutions and for-profit enterprises.
“The doc who prescribes the drug to the average patient probably does not have this conflict of interest,” he said. “However, they are influenced by people who have these conflicts.”
The SHARE panel on conflicts of interest was convened after a series of articles co-reported by ProPublica and the New York Times described the failure of the chief medical officer at Memorial Sloan Kettering Cancer Center, José Baselga, MD, to disclose his financial conflicts of interest in publications, such as the New England Journal of Medicine, where they are required. Dr. Baselga resigned his position.
However, the failure to disclose financial relationships with industry was a minor topic during the SHARE panel. Robert Bazell, PhD, an adjunct professor in the division of molecular, cellular, and developmental biology at Yale University, New Haven, Conn., moderated the panel. He said in his opening remarks that the issues “go much deeper.”
While the articles did not reveal “anything illegal, it opened a lot of peoples’ eyes as to how much money there was in the system,” said Dr. Bazell, who was for many years the chief science and health correspondent for NBC News.
Specifically, it has heightened concern about whether profit motives are subverting the goals of science, according to Dr. Bazell. “This money bomb has fallen on a lot of science but it has particularly fallen on oncology,” he said.
Not everyone, including the bioethicist, agreed that profits by themselves are the problem. Big rewards may be a reasonable price for discoveries that save lives, but all agreed that the system does not necessarily reserve big rewards for life-saving advances.
“The focus isn’t really on drugs that will save lives, its just on more drugs,” said Ms. Visco, echoing the incentive system described by Dr. Cook-Deegan. She believes the current system handsomely rewards scientists and physicians for developing drugs with little or no significant clinical benefit. When a drug shows a progression-free survival benefit relative to a previous standard, even of modest statistical or clinical significance, it will be prescribed and profits will be generated.
Early in her tenure at the National Breast Cancer Coalition, Ms. Visco, who was a practicing lawyer prior to taking the helm of the coalition, considered fund raising for research the primary goal. She hoped that the coalition could help the breast cancer research community identify and fund priorities, but her perspective has changed.
“I used to think that we should be collaborators but now I am coming to the conclusion that we should be in charge. Educated, trained patient advocates with a constituency should be in charge, because our only agenda is to save lives and end breast cancer,” Ms. Visco said. She no longer believes in simply increasing funding even at research centers such as the National Cancer Institute without a fundamental reorganization of priorities.
Many leaders in medicine are aware of the problem, according to Dr. Brawley, who cited a recent statement by the American Society of Clinical Oncology that expressed concern about the plethora of cancer trials showing very small gains. He did agree with the others, however, that incentives are now misaligned.
“We are designing clinical trials not to look for big gains,” Dr. Brawley agreed. But he also cautioned that the system is complex. One reason that drugs offering modest or little gain over a previous standard are highly marketable is that patients themselves demand them. Direct-to-consumer marketing supports drugs with little or even nothing to offer.
“I see patients who want drugs that they should not get,” said Dr. Brawley, who indicated that clinicians are under pressure to offer something to desperate patients even when options are expensive and not shown to provide any change in outcome.
Overall, in the shake-up at Memorial Sloan Kettering, it was the failure to disclose significant financial relationships rather than the financial relationships themselves that represented an important breach in ethics, according to Dr. Brawley, who indicated that researcher relationships with industry are not inherently wrong. In an article published in November 2018, the New York Times reported that Dr. Brawley left his post at the ACS because of “his dismay” over some partnerships the ACS had formed with industry, but Dr. Brawley would not confirm or deny this report.
Relative to conflicts of interest at major research institutions, Dr. Cook-Deegan was more circumspect about whether disclosure is enough. Although he agreed with the premise that close collaboration with industry might be clinically valuable, which one investigator at Memorial Sloan Kettering claimed when speaking with the New York Times, he questioned whether there is a line over which the relationship is too intertwined.
“Do you really need to serve on the board? Do you really need the types of financial ties that have the potential to influence clinical decisions?” he asked.
Although Dr. Cook-Deegan agreed with the others that he does not know exactly how best to fix the system, he believes a fix may be coming.
“I think we are at an inflection point,” he said. “The symptoms of a system that has been running off the rails for a while are getting severe enough that we are starting to pay attention,” he said. One sign of movement is that both political parties have “introduced bills to address pricing, which is directly related to the problems we are talking about.”
NEW YORK – Conflicts of interest, whether reported or unreported, are just one component of a broken system that has misaligned incentives with meaningful advances in breast cancer research, agreed a panel of experts who participated in an evening program sponsored by SHARE, a breast cancer patient advocacy group.
“The issue of disclosures, while it is important, is not really the key issue. The key issue for me is about the amazing amount of money that is changing hands,” said Fran Visco, president of the National Breast Cancer Coalition, Washington. She contended not enough money in breast cancer research is being applied to save women’s lives rather than driving profits for institutions, physicians, and the pharmaceutical industry.
The other two participants in this panel, called Conflicts of Interest in Research and Treatment: What Breast Cancer Patients Should Know, largely agreed. In turn, they each explained that major sums of money in play for physicians and institutions collaborating with industry are earmarked for generating income much more than improving outcomes.
“Most of where this money is going is for these little tiny improvements and there is no association between the outcome increment that you get and what you pay for that outcome increment,” said Robert Cook-Deegan, MD, a bioethicist and a professor at the School for Innovation in Society, Arizona State University, Tempe.
Leaving aside whether physician-scientists working at nonprofit research institutions can avoid conflicts of interest when money is funneled to them from start-up investment opportunities, pharmaceutical board memberships, and direct compensation deals for consultancies and other services, Dr. Cook-Deegan was referring to the bigger problem of misaligned incentives. Large financial rewards are available for any drug with market potential instead of being limited to those that save lives, he said.
Tracing the current system to the Bayh-Dole Act in 1980, which permitted researchers to claim patents for intellectual property developed with federal funding, Dr. Cook-Deegan described a system of minnows, fish, and sharks. Researchers, who are the minnows, seek potential new therapeutics to be developed by for-profit startups or other commercial entities, which are the fish. If the drug continues to show promise, it brings investors, who are the sharks.
Once the new therapy reaches the sharks, the excitement about a new and more effective treatment has been overshadowed by the excitement about profits, which are essential to pay for the large investment required for the clinical development that brings new drugs to market. If the drug is marketable, it is considered a success even when the clinical benefits barely register.
“There have been about 20 new [cancer] drugs approved by the [Food and Drug Administration] in the last 2 years. The increased median disease-free survival is usually about 3 months,” said Otis W. Brawley, MD, who recently joined the Kimmel Cancer Center at Johns Hopkins University after resigning his position as chief medical officer at the American Cancer Society (ACS).
Dr. Brawley joined the others in declaring: “The system is broken.” The flow of money is not the only incentive for researchers to participate in clinical development programs focused on minor advances rather than blockbuster discoveries. Unrestricted research grants are now much harder to obtain, according to Dr. Brawley, making collaboration with industry essential not just for research funding but career advancement. Research at major cancer centers is still being conducted at the highest level, but there are unavoidable interdependent relationships for nonprofit institutions and for-profit enterprises.
“The doc who prescribes the drug to the average patient probably does not have this conflict of interest,” he said. “However, they are influenced by people who have these conflicts.”
The SHARE panel on conflicts of interest was convened after a series of articles co-reported by ProPublica and the New York Times described the failure of the chief medical officer at Memorial Sloan Kettering Cancer Center, José Baselga, MD, to disclose his financial conflicts of interest in publications, such as the New England Journal of Medicine, where they are required. Dr. Baselga resigned his position.
However, the failure to disclose financial relationships with industry was a minor topic during the SHARE panel. Robert Bazell, PhD, an adjunct professor in the division of molecular, cellular, and developmental biology at Yale University, New Haven, Conn., moderated the panel. He said in his opening remarks that the issues “go much deeper.”
While the articles did not reveal “anything illegal, it opened a lot of peoples’ eyes as to how much money there was in the system,” said Dr. Bazell, who was for many years the chief science and health correspondent for NBC News.
Specifically, it has heightened concern about whether profit motives are subverting the goals of science, according to Dr. Bazell. “This money bomb has fallen on a lot of science but it has particularly fallen on oncology,” he said.
Not everyone, including the bioethicist, agreed that profits by themselves are the problem. Big rewards may be a reasonable price for discoveries that save lives, but all agreed that the system does not necessarily reserve big rewards for life-saving advances.
“The focus isn’t really on drugs that will save lives, its just on more drugs,” said Ms. Visco, echoing the incentive system described by Dr. Cook-Deegan. She believes the current system handsomely rewards scientists and physicians for developing drugs with little or no significant clinical benefit. When a drug shows a progression-free survival benefit relative to a previous standard, even of modest statistical or clinical significance, it will be prescribed and profits will be generated.
Early in her tenure at the National Breast Cancer Coalition, Ms. Visco, who was a practicing lawyer prior to taking the helm of the coalition, considered fund raising for research the primary goal. She hoped that the coalition could help the breast cancer research community identify and fund priorities, but her perspective has changed.
“I used to think that we should be collaborators but now I am coming to the conclusion that we should be in charge. Educated, trained patient advocates with a constituency should be in charge, because our only agenda is to save lives and end breast cancer,” Ms. Visco said. She no longer believes in simply increasing funding even at research centers such as the National Cancer Institute without a fundamental reorganization of priorities.
Many leaders in medicine are aware of the problem, according to Dr. Brawley, who cited a recent statement by the American Society of Clinical Oncology that expressed concern about the plethora of cancer trials showing very small gains. He did agree with the others, however, that incentives are now misaligned.
“We are designing clinical trials not to look for big gains,” Dr. Brawley agreed. But he also cautioned that the system is complex. One reason that drugs offering modest or little gain over a previous standard are highly marketable is that patients themselves demand them. Direct-to-consumer marketing supports drugs with little or even nothing to offer.
“I see patients who want drugs that they should not get,” said Dr. Brawley, who indicated that clinicians are under pressure to offer something to desperate patients even when options are expensive and not shown to provide any change in outcome.
Overall, in the shake-up at Memorial Sloan Kettering, it was the failure to disclose significant financial relationships rather than the financial relationships themselves that represented an important breach in ethics, according to Dr. Brawley, who indicated that researcher relationships with industry are not inherently wrong. In an article published in November 2018, the New York Times reported that Dr. Brawley left his post at the ACS because of “his dismay” over some partnerships the ACS had formed with industry, but Dr. Brawley would not confirm or deny this report.
Relative to conflicts of interest at major research institutions, Dr. Cook-Deegan was more circumspect about whether disclosure is enough. Although he agreed with the premise that close collaboration with industry might be clinically valuable, which one investigator at Memorial Sloan Kettering claimed when speaking with the New York Times, he questioned whether there is a line over which the relationship is too intertwined.
“Do you really need to serve on the board? Do you really need the types of financial ties that have the potential to influence clinical decisions?” he asked.
Although Dr. Cook-Deegan agreed with the others that he does not know exactly how best to fix the system, he believes a fix may be coming.
“I think we are at an inflection point,” he said. “The symptoms of a system that has been running off the rails for a while are getting severe enough that we are starting to pay attention,” he said. One sign of movement is that both political parties have “introduced bills to address pricing, which is directly related to the problems we are talking about.”
NEW YORK – Conflicts of interest, whether reported or unreported, are just one component of a broken system that has misaligned incentives with meaningful advances in breast cancer research, agreed a panel of experts who participated in an evening program sponsored by SHARE, a breast cancer patient advocacy group.
“The issue of disclosures, while it is important, is not really the key issue. The key issue for me is about the amazing amount of money that is changing hands,” said Fran Visco, president of the National Breast Cancer Coalition, Washington. She contended not enough money in breast cancer research is being applied to save women’s lives rather than driving profits for institutions, physicians, and the pharmaceutical industry.
The other two participants in this panel, called Conflicts of Interest in Research and Treatment: What Breast Cancer Patients Should Know, largely agreed. In turn, they each explained that major sums of money in play for physicians and institutions collaborating with industry are earmarked for generating income much more than improving outcomes.
“Most of where this money is going is for these little tiny improvements and there is no association between the outcome increment that you get and what you pay for that outcome increment,” said Robert Cook-Deegan, MD, a bioethicist and a professor at the School for Innovation in Society, Arizona State University, Tempe.
Leaving aside whether physician-scientists working at nonprofit research institutions can avoid conflicts of interest when money is funneled to them from start-up investment opportunities, pharmaceutical board memberships, and direct compensation deals for consultancies and other services, Dr. Cook-Deegan was referring to the bigger problem of misaligned incentives. Large financial rewards are available for any drug with market potential instead of being limited to those that save lives, he said.
Tracing the current system to the Bayh-Dole Act in 1980, which permitted researchers to claim patents for intellectual property developed with federal funding, Dr. Cook-Deegan described a system of minnows, fish, and sharks. Researchers, who are the minnows, seek potential new therapeutics to be developed by for-profit startups or other commercial entities, which are the fish. If the drug continues to show promise, it brings investors, who are the sharks.
Once the new therapy reaches the sharks, the excitement about a new and more effective treatment has been overshadowed by the excitement about profits, which are essential to pay for the large investment required for the clinical development that brings new drugs to market. If the drug is marketable, it is considered a success even when the clinical benefits barely register.
“There have been about 20 new [cancer] drugs approved by the [Food and Drug Administration] in the last 2 years. The increased median disease-free survival is usually about 3 months,” said Otis W. Brawley, MD, who recently joined the Kimmel Cancer Center at Johns Hopkins University after resigning his position as chief medical officer at the American Cancer Society (ACS).
Dr. Brawley joined the others in declaring: “The system is broken.” The flow of money is not the only incentive for researchers to participate in clinical development programs focused on minor advances rather than blockbuster discoveries. Unrestricted research grants are now much harder to obtain, according to Dr. Brawley, making collaboration with industry essential not just for research funding but career advancement. Research at major cancer centers is still being conducted at the highest level, but there are unavoidable interdependent relationships for nonprofit institutions and for-profit enterprises.
“The doc who prescribes the drug to the average patient probably does not have this conflict of interest,” he said. “However, they are influenced by people who have these conflicts.”
The SHARE panel on conflicts of interest was convened after a series of articles co-reported by ProPublica and the New York Times described the failure of the chief medical officer at Memorial Sloan Kettering Cancer Center, José Baselga, MD, to disclose his financial conflicts of interest in publications, such as the New England Journal of Medicine, where they are required. Dr. Baselga resigned his position.
However, the failure to disclose financial relationships with industry was a minor topic during the SHARE panel. Robert Bazell, PhD, an adjunct professor in the division of molecular, cellular, and developmental biology at Yale University, New Haven, Conn., moderated the panel. He said in his opening remarks that the issues “go much deeper.”
While the articles did not reveal “anything illegal, it opened a lot of peoples’ eyes as to how much money there was in the system,” said Dr. Bazell, who was for many years the chief science and health correspondent for NBC News.
Specifically, it has heightened concern about whether profit motives are subverting the goals of science, according to Dr. Bazell. “This money bomb has fallen on a lot of science but it has particularly fallen on oncology,” he said.
Not everyone, including the bioethicist, agreed that profits by themselves are the problem. Big rewards may be a reasonable price for discoveries that save lives, but all agreed that the system does not necessarily reserve big rewards for life-saving advances.
“The focus isn’t really on drugs that will save lives, its just on more drugs,” said Ms. Visco, echoing the incentive system described by Dr. Cook-Deegan. She believes the current system handsomely rewards scientists and physicians for developing drugs with little or no significant clinical benefit. When a drug shows a progression-free survival benefit relative to a previous standard, even of modest statistical or clinical significance, it will be prescribed and profits will be generated.
Early in her tenure at the National Breast Cancer Coalition, Ms. Visco, who was a practicing lawyer prior to taking the helm of the coalition, considered fund raising for research the primary goal. She hoped that the coalition could help the breast cancer research community identify and fund priorities, but her perspective has changed.
“I used to think that we should be collaborators but now I am coming to the conclusion that we should be in charge. Educated, trained patient advocates with a constituency should be in charge, because our only agenda is to save lives and end breast cancer,” Ms. Visco said. She no longer believes in simply increasing funding even at research centers such as the National Cancer Institute without a fundamental reorganization of priorities.
Many leaders in medicine are aware of the problem, according to Dr. Brawley, who cited a recent statement by the American Society of Clinical Oncology that expressed concern about the plethora of cancer trials showing very small gains. He did agree with the others, however, that incentives are now misaligned.
“We are designing clinical trials not to look for big gains,” Dr. Brawley agreed. But he also cautioned that the system is complex. One reason that drugs offering modest or little gain over a previous standard are highly marketable is that patients themselves demand them. Direct-to-consumer marketing supports drugs with little or even nothing to offer.
“I see patients who want drugs that they should not get,” said Dr. Brawley, who indicated that clinicians are under pressure to offer something to desperate patients even when options are expensive and not shown to provide any change in outcome.
Overall, in the shake-up at Memorial Sloan Kettering, it was the failure to disclose significant financial relationships rather than the financial relationships themselves that represented an important breach in ethics, according to Dr. Brawley, who indicated that researcher relationships with industry are not inherently wrong. In an article published in November 2018, the New York Times reported that Dr. Brawley left his post at the ACS because of “his dismay” over some partnerships the ACS had formed with industry, but Dr. Brawley would not confirm or deny this report.
Relative to conflicts of interest at major research institutions, Dr. Cook-Deegan was more circumspect about whether disclosure is enough. Although he agreed with the premise that close collaboration with industry might be clinically valuable, which one investigator at Memorial Sloan Kettering claimed when speaking with the New York Times, he questioned whether there is a line over which the relationship is too intertwined.
“Do you really need to serve on the board? Do you really need the types of financial ties that have the potential to influence clinical decisions?” he asked.
Although Dr. Cook-Deegan agreed with the others that he does not know exactly how best to fix the system, he believes a fix may be coming.
“I think we are at an inflection point,” he said. “The symptoms of a system that has been running off the rails for a while are getting severe enough that we are starting to pay attention,” he said. One sign of movement is that both political parties have “introduced bills to address pricing, which is directly related to the problems we are talking about.”
Soy didn’t up all-cause mortality in breast cancer survivors
A cohort of Chinese women who are breast cancer survivors had no increased mortality from soy intake, according to a new study.
The work adds to the existing body of evidence that women with breast cancer, or risk for breast cancer, don’t need to modify their soy intake to mitigate risk, said the study’s first author, Suzanne C. Ho, PhD.
Speaking at the annual meeting of the North American Menopause Society, Dr. Ho noted that the combination of increasing breast cancer incidence and improved outcome has resulted in larger numbers of breast cancer survivors in Hong Kong, where she is professor emerita at the Chinese University of Hong Kong.
The prospective, ongoing study examines the association between soy intake pre- and postdiagnosis and total mortality for Chinese women who are breast cancer survivors. Dr. Ho said that she and her colleagues hypothesized that they would not see higher mortality among women who had higher soy intake – and this was the case.
Of 1,497 breast cancer survivors drawn from two facilities in Hong Kong, those who consumed higher quantities of dietary soy did not have increased risk of all-cause mortality, compared with those in the lowest tertile of soy consumption.
There are theoretical underpinnings for thinking that soy could be a player in cancer risk, but the biochemistry and epidemiology behind the studies are complicated. Estrogen plays a role in human breast cancer, and many modern breast cancer treatments actually dampen endogenous estrogens.
However, epidemiologic data have shown that consumption of soy-based foods – which contain phytoestrogens, primarily in the form of isoflavones – is inversely associated with developing breast cancer.
This is all part of why soy-based foods have been thought of as a mixed bag with regard to breast cancer: Soy isoflavones are, said Dr. Ho, “Natural estrogen receptor modulators that possess both estrogenlike and antiestrogenic properties.”
Other chemicals contained in soy may fight cancer, with effects that are antioxidative and strengthen immune response. Soy constituents also inhibit DNA topoisomerase I and II, proteases, tyrosine kinases, and inositol phosphate, effects that can slow tumor growth. Still, one soy isoflavone, genistein, actually can promote growth of estrogen-dependent tumors in rats, said Dr. Ho
Dr. Ho and her colleagues enrolled Hong Kong residents for the study of mortality among breast cancer survivors. Participants were included if they were Chinese, female, aged 24-77 years, and had their first primary breast cancer histologically confirmed within 12 months of entering the study. Cancer had to be graded below stage III.
Using a 109-item validated food questionnaire, investigators gathered information about participants’ soy intake and general diet for the year prior to breast cancer diagnosis. Other patient characteristics, relevant prognostic information from medical records, and anthropometric data were collected at baseline, and repeated at 18, 36, and 60 months.
The primary outcome measure – all-cause mortality during the follow-up period – was tracked for a mean 50.9 months, with a 78% retention rate for study participants, said Dr. Ho. In total, 96 patients died during follow-up, making up 5.9% of the premenopausal and 7% of the postmenopausal participants.
Statistical analysis corrected for potential confounders, including patient and disease characteristics and treatment modalities, as well as overall energy consumption.
Patients were evenly divided into tertiles of soy isoflavone intake, with cutpoints of 3.77 mg/1,000 kcal and 10.05 mg/1,000 kcal for the lower limit of the two higher tertiles. For the highest tertile, though, mean isoflavone intake was actually 20.87 mg/1,000 kcal.
Patient, disease, and treatment characteristics did not differ significantly among the tertiles.
An adjusted statistical analysis looked at pre- and postmenopausal women separately by tertile of soy isoflavone consumption, setting the hazard ratio for all-cause mortality at 1.00 for women in the lowest tertile of soy consumption.
For premenopausal women in the middle tertile, the HR was 0.45 (95% confidence interval, 0.20-1.00), and 0.86 for those in the highest tertile (95% CI, 0.43-1.72); 782 participants, in all, were premenopausal.
For the 715 postmenopausal women, the HR for those in the middle tertile of soy consumption was 0.94 (95% CI, 0.43-2.05), and 1.11 in the highest (95% CI, 0.54-2.29).
Taking all pre- and postmenopausal participants together, those in the middle tertile of soy isoflavone intake had an all-cause mortality HR of 0.63 (95% CI, 0.37-1.09). For the highest tertile of the full cohort, the HR was 0.95 (95% CI, 0.58-1.55).
Confidence intervals were wide in these findings, but Dr. Ho noted that “moderate soy food intake might be associated with better survival.”
“Prediagnosis soy intake did not increase the risk of all-cause mortality in breast cancer survivors,” said Dr. Ho, findings she called “consistent with the literature that soy consumption does not adversely effect breast cancer survival.”
The study is ongoing, she explained, and “longer follow-up will provide further evidence on the effect of pre- and postdiagnosis soy intake on breast cancer outcomes.”
The study had a homogeneous population of southern Chinese women, with fairly good retention and robust statistical adjustment for confounders. However, it wasn’t possible to assess bioavailability of isoflavones and their metabolites, which can vary according to individual microbiota. Also, researchers did not track whether patients used traditional Chinese medicine.
The World Cancer Research Fund International supported the study. Dr. Ho reported no conflicts of interest.
SOURCE: Ho S et al. NAMS 2018, Abstract S-23.
A cohort of Chinese women who are breast cancer survivors had no increased mortality from soy intake, according to a new study.
The work adds to the existing body of evidence that women with breast cancer, or risk for breast cancer, don’t need to modify their soy intake to mitigate risk, said the study’s first author, Suzanne C. Ho, PhD.
Speaking at the annual meeting of the North American Menopause Society, Dr. Ho noted that the combination of increasing breast cancer incidence and improved outcome has resulted in larger numbers of breast cancer survivors in Hong Kong, where she is professor emerita at the Chinese University of Hong Kong.
The prospective, ongoing study examines the association between soy intake pre- and postdiagnosis and total mortality for Chinese women who are breast cancer survivors. Dr. Ho said that she and her colleagues hypothesized that they would not see higher mortality among women who had higher soy intake – and this was the case.
Of 1,497 breast cancer survivors drawn from two facilities in Hong Kong, those who consumed higher quantities of dietary soy did not have increased risk of all-cause mortality, compared with those in the lowest tertile of soy consumption.
There are theoretical underpinnings for thinking that soy could be a player in cancer risk, but the biochemistry and epidemiology behind the studies are complicated. Estrogen plays a role in human breast cancer, and many modern breast cancer treatments actually dampen endogenous estrogens.
However, epidemiologic data have shown that consumption of soy-based foods – which contain phytoestrogens, primarily in the form of isoflavones – is inversely associated with developing breast cancer.
This is all part of why soy-based foods have been thought of as a mixed bag with regard to breast cancer: Soy isoflavones are, said Dr. Ho, “Natural estrogen receptor modulators that possess both estrogenlike and antiestrogenic properties.”
Other chemicals contained in soy may fight cancer, with effects that are antioxidative and strengthen immune response. Soy constituents also inhibit DNA topoisomerase I and II, proteases, tyrosine kinases, and inositol phosphate, effects that can slow tumor growth. Still, one soy isoflavone, genistein, actually can promote growth of estrogen-dependent tumors in rats, said Dr. Ho
Dr. Ho and her colleagues enrolled Hong Kong residents for the study of mortality among breast cancer survivors. Participants were included if they were Chinese, female, aged 24-77 years, and had their first primary breast cancer histologically confirmed within 12 months of entering the study. Cancer had to be graded below stage III.
Using a 109-item validated food questionnaire, investigators gathered information about participants’ soy intake and general diet for the year prior to breast cancer diagnosis. Other patient characteristics, relevant prognostic information from medical records, and anthropometric data were collected at baseline, and repeated at 18, 36, and 60 months.
The primary outcome measure – all-cause mortality during the follow-up period – was tracked for a mean 50.9 months, with a 78% retention rate for study participants, said Dr. Ho. In total, 96 patients died during follow-up, making up 5.9% of the premenopausal and 7% of the postmenopausal participants.
Statistical analysis corrected for potential confounders, including patient and disease characteristics and treatment modalities, as well as overall energy consumption.
Patients were evenly divided into tertiles of soy isoflavone intake, with cutpoints of 3.77 mg/1,000 kcal and 10.05 mg/1,000 kcal for the lower limit of the two higher tertiles. For the highest tertile, though, mean isoflavone intake was actually 20.87 mg/1,000 kcal.
Patient, disease, and treatment characteristics did not differ significantly among the tertiles.
An adjusted statistical analysis looked at pre- and postmenopausal women separately by tertile of soy isoflavone consumption, setting the hazard ratio for all-cause mortality at 1.00 for women in the lowest tertile of soy consumption.
For premenopausal women in the middle tertile, the HR was 0.45 (95% confidence interval, 0.20-1.00), and 0.86 for those in the highest tertile (95% CI, 0.43-1.72); 782 participants, in all, were premenopausal.
For the 715 postmenopausal women, the HR for those in the middle tertile of soy consumption was 0.94 (95% CI, 0.43-2.05), and 1.11 in the highest (95% CI, 0.54-2.29).
Taking all pre- and postmenopausal participants together, those in the middle tertile of soy isoflavone intake had an all-cause mortality HR of 0.63 (95% CI, 0.37-1.09). For the highest tertile of the full cohort, the HR was 0.95 (95% CI, 0.58-1.55).
Confidence intervals were wide in these findings, but Dr. Ho noted that “moderate soy food intake might be associated with better survival.”
“Prediagnosis soy intake did not increase the risk of all-cause mortality in breast cancer survivors,” said Dr. Ho, findings she called “consistent with the literature that soy consumption does not adversely effect breast cancer survival.”
The study is ongoing, she explained, and “longer follow-up will provide further evidence on the effect of pre- and postdiagnosis soy intake on breast cancer outcomes.”
The study had a homogeneous population of southern Chinese women, with fairly good retention and robust statistical adjustment for confounders. However, it wasn’t possible to assess bioavailability of isoflavones and their metabolites, which can vary according to individual microbiota. Also, researchers did not track whether patients used traditional Chinese medicine.
The World Cancer Research Fund International supported the study. Dr. Ho reported no conflicts of interest.
SOURCE: Ho S et al. NAMS 2018, Abstract S-23.
A cohort of Chinese women who are breast cancer survivors had no increased mortality from soy intake, according to a new study.
The work adds to the existing body of evidence that women with breast cancer, or risk for breast cancer, don’t need to modify their soy intake to mitigate risk, said the study’s first author, Suzanne C. Ho, PhD.
Speaking at the annual meeting of the North American Menopause Society, Dr. Ho noted that the combination of increasing breast cancer incidence and improved outcome has resulted in larger numbers of breast cancer survivors in Hong Kong, where she is professor emerita at the Chinese University of Hong Kong.
The prospective, ongoing study examines the association between soy intake pre- and postdiagnosis and total mortality for Chinese women who are breast cancer survivors. Dr. Ho said that she and her colleagues hypothesized that they would not see higher mortality among women who had higher soy intake – and this was the case.
Of 1,497 breast cancer survivors drawn from two facilities in Hong Kong, those who consumed higher quantities of dietary soy did not have increased risk of all-cause mortality, compared with those in the lowest tertile of soy consumption.
There are theoretical underpinnings for thinking that soy could be a player in cancer risk, but the biochemistry and epidemiology behind the studies are complicated. Estrogen plays a role in human breast cancer, and many modern breast cancer treatments actually dampen endogenous estrogens.
However, epidemiologic data have shown that consumption of soy-based foods – which contain phytoestrogens, primarily in the form of isoflavones – is inversely associated with developing breast cancer.
This is all part of why soy-based foods have been thought of as a mixed bag with regard to breast cancer: Soy isoflavones are, said Dr. Ho, “Natural estrogen receptor modulators that possess both estrogenlike and antiestrogenic properties.”
Other chemicals contained in soy may fight cancer, with effects that are antioxidative and strengthen immune response. Soy constituents also inhibit DNA topoisomerase I and II, proteases, tyrosine kinases, and inositol phosphate, effects that can slow tumor growth. Still, one soy isoflavone, genistein, actually can promote growth of estrogen-dependent tumors in rats, said Dr. Ho
Dr. Ho and her colleagues enrolled Hong Kong residents for the study of mortality among breast cancer survivors. Participants were included if they were Chinese, female, aged 24-77 years, and had their first primary breast cancer histologically confirmed within 12 months of entering the study. Cancer had to be graded below stage III.
Using a 109-item validated food questionnaire, investigators gathered information about participants’ soy intake and general diet for the year prior to breast cancer diagnosis. Other patient characteristics, relevant prognostic information from medical records, and anthropometric data were collected at baseline, and repeated at 18, 36, and 60 months.
The primary outcome measure – all-cause mortality during the follow-up period – was tracked for a mean 50.9 months, with a 78% retention rate for study participants, said Dr. Ho. In total, 96 patients died during follow-up, making up 5.9% of the premenopausal and 7% of the postmenopausal participants.
Statistical analysis corrected for potential confounders, including patient and disease characteristics and treatment modalities, as well as overall energy consumption.
Patients were evenly divided into tertiles of soy isoflavone intake, with cutpoints of 3.77 mg/1,000 kcal and 10.05 mg/1,000 kcal for the lower limit of the two higher tertiles. For the highest tertile, though, mean isoflavone intake was actually 20.87 mg/1,000 kcal.
Patient, disease, and treatment characteristics did not differ significantly among the tertiles.
An adjusted statistical analysis looked at pre- and postmenopausal women separately by tertile of soy isoflavone consumption, setting the hazard ratio for all-cause mortality at 1.00 for women in the lowest tertile of soy consumption.
For premenopausal women in the middle tertile, the HR was 0.45 (95% confidence interval, 0.20-1.00), and 0.86 for those in the highest tertile (95% CI, 0.43-1.72); 782 participants, in all, were premenopausal.
For the 715 postmenopausal women, the HR for those in the middle tertile of soy consumption was 0.94 (95% CI, 0.43-2.05), and 1.11 in the highest (95% CI, 0.54-2.29).
Taking all pre- and postmenopausal participants together, those in the middle tertile of soy isoflavone intake had an all-cause mortality HR of 0.63 (95% CI, 0.37-1.09). For the highest tertile of the full cohort, the HR was 0.95 (95% CI, 0.58-1.55).
Confidence intervals were wide in these findings, but Dr. Ho noted that “moderate soy food intake might be associated with better survival.”
“Prediagnosis soy intake did not increase the risk of all-cause mortality in breast cancer survivors,” said Dr. Ho, findings she called “consistent with the literature that soy consumption does not adversely effect breast cancer survival.”
The study is ongoing, she explained, and “longer follow-up will provide further evidence on the effect of pre- and postdiagnosis soy intake on breast cancer outcomes.”
The study had a homogeneous population of southern Chinese women, with fairly good retention and robust statistical adjustment for confounders. However, it wasn’t possible to assess bioavailability of isoflavones and their metabolites, which can vary according to individual microbiota. Also, researchers did not track whether patients used traditional Chinese medicine.
The World Cancer Research Fund International supported the study. Dr. Ho reported no conflicts of interest.
SOURCE: Ho S et al. NAMS 2018, Abstract S-23.
REPORTING FROM NAMS 2018
Key clinical point: Soy consumption did not increase mortality risk in breast cancer survivors.
Major finding: The hazard ratios for all-cause mortality were 0.63 and 0.95 for the two highest tertiles of soy consumption.
Study details: An ongoing prospective cohort study of 1,497 female breast cancer survivors in Hong Kong.
Disclosures: The World Cancer Research Fund International supported the study. Dr. Ho reported no conflicts of interest.
Source: Ho S et al. NAMS 2018, Abstract S-23.
Daily News Special: SABCS
Stories include: uUing low-dose tamoxifen, the latest findings from the KATHERINE trial, results of a meta-analysis of neoadjuvant chemotherapy, and capecitabine in early stage triple negative breast cancer.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Stories include: uUing low-dose tamoxifen, the latest findings from the KATHERINE trial, results of a meta-analysis of neoadjuvant chemotherapy, and capecitabine in early stage triple negative breast cancer.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Stories include: uUing low-dose tamoxifen, the latest findings from the KATHERINE trial, results of a meta-analysis of neoadjuvant chemotherapy, and capecitabine in early stage triple negative breast cancer.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Neoadjuvant degarelix more effective than triptorelin for ovarian suppression
Degarelix, the gonadotropin-releasing hormone (GnRH) antagonist approved for prostate cancer, was more effective than a GnRH agonist in achieving ovarian function suppression in women with breast cancer, results of a randomized trial show.
Ovarian function suppression was achieved more rapidly and maintained more effectively with degarelix, compared with triptorelin, in the premenopausal women who were receiving letrozole for neoadjuvant endocrine therapy, investigators said.
Adverse events including hot flashes and injection site reactions were reported more often with degarelix versus the GnRH agonist in this randomized, phase 2 trial of 51 subjects.
Additional research is needed to determine whether degarelix results in superior disease control versus the current standard of care, reported Silvia Dellapasqua, MD, of the European Institute of Oncology IRCCS in Milan, Italy, and coinvestigators.
“The study is hypothesis-generating, and supports later studies to assess whether maintenance of ovarian function suppression with degarelix translates into a better clinical outcome and is worth a trade-off of increased rate of some adverse events,” the researchers wrote. The report is in the Journal of Clinical Oncology.
Patients were randomly assigned to receive degarelix plus letrozole or triptorelin plus letrozole for six 28-day cycles. Degarelix was administered subcutaneously on day 1 of each cycle, while triptorelin was administered intramuscularly on day 1 of each cycle, and oral letrozole was to be taken daily. Surgery was performed a few weeks after the last injection.
All patients achieved optimal ovarian function suppression by the end of the first cycle. However, that endpoint was achieved significantly faster among patients in the degarelix arm, at a median of 3 days, versus a median of 14 days for the GnRH agonist, the investigators reported.
The optimal ovarian function suppression was seen three times faster with degarelix (hazard ratio, 3.05; 95% confidence interval, 1.65-5.65; P less than 001), they added.
One hundred percent of patients receiving degarelix and letrozole maintained optimal ovarian function suppression throughout the study, while about 15% of patients assigned to triptorelin had suboptimal suppression after that first cycle.
The group of patients receiving degarelix had a higher rate of node-negative disease at surgery, and a higher rate of breast-conserving surgery compared with the triptorelin group, the investigators said.
There were two grade 3 adverse events, hypertension and anemia, which both occurred in the triptorelin group, and no grade 4 adverse events. The most common adverse events reported were hot flashes, occurring in 80.0% and 69.2% of the degarelix and triptorelin groups, respectively; arthralgias in 32.0% and 53.8%; insomnia in 24.0% and 11.5%; injection site reactions in 24.0% and 0%; and nausea in 16.0% and 3.8%.
The study was supported by Ferring, and by the International Breast Cancer Study Group via Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research, Cancer Research Switzerland, Oncosuisse, Swiss Cancer League, and the Foundation for Clinical Cancer Research of Eastern Switzerland. The authors reported disclosures related to Ferring, Novartis, Ipsen, DVAX, Roche, Genentech, Pfizer, Celgene, and Merck, among others.
SOURCE: Dellapasqua S et al. J Clin Oncol. 2018 Dec 27. doi: 10.1200/JCO.18.00296.
Degarelix, the gonadotropin-releasing hormone (GnRH) antagonist approved for prostate cancer, was more effective than a GnRH agonist in achieving ovarian function suppression in women with breast cancer, results of a randomized trial show.
Ovarian function suppression was achieved more rapidly and maintained more effectively with degarelix, compared with triptorelin, in the premenopausal women who were receiving letrozole for neoadjuvant endocrine therapy, investigators said.
Adverse events including hot flashes and injection site reactions were reported more often with degarelix versus the GnRH agonist in this randomized, phase 2 trial of 51 subjects.
Additional research is needed to determine whether degarelix results in superior disease control versus the current standard of care, reported Silvia Dellapasqua, MD, of the European Institute of Oncology IRCCS in Milan, Italy, and coinvestigators.
“The study is hypothesis-generating, and supports later studies to assess whether maintenance of ovarian function suppression with degarelix translates into a better clinical outcome and is worth a trade-off of increased rate of some adverse events,” the researchers wrote. The report is in the Journal of Clinical Oncology.
Patients were randomly assigned to receive degarelix plus letrozole or triptorelin plus letrozole for six 28-day cycles. Degarelix was administered subcutaneously on day 1 of each cycle, while triptorelin was administered intramuscularly on day 1 of each cycle, and oral letrozole was to be taken daily. Surgery was performed a few weeks after the last injection.
All patients achieved optimal ovarian function suppression by the end of the first cycle. However, that endpoint was achieved significantly faster among patients in the degarelix arm, at a median of 3 days, versus a median of 14 days for the GnRH agonist, the investigators reported.
The optimal ovarian function suppression was seen three times faster with degarelix (hazard ratio, 3.05; 95% confidence interval, 1.65-5.65; P less than 001), they added.
One hundred percent of patients receiving degarelix and letrozole maintained optimal ovarian function suppression throughout the study, while about 15% of patients assigned to triptorelin had suboptimal suppression after that first cycle.
The group of patients receiving degarelix had a higher rate of node-negative disease at surgery, and a higher rate of breast-conserving surgery compared with the triptorelin group, the investigators said.
There were two grade 3 adverse events, hypertension and anemia, which both occurred in the triptorelin group, and no grade 4 adverse events. The most common adverse events reported were hot flashes, occurring in 80.0% and 69.2% of the degarelix and triptorelin groups, respectively; arthralgias in 32.0% and 53.8%; insomnia in 24.0% and 11.5%; injection site reactions in 24.0% and 0%; and nausea in 16.0% and 3.8%.
The study was supported by Ferring, and by the International Breast Cancer Study Group via Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research, Cancer Research Switzerland, Oncosuisse, Swiss Cancer League, and the Foundation for Clinical Cancer Research of Eastern Switzerland. The authors reported disclosures related to Ferring, Novartis, Ipsen, DVAX, Roche, Genentech, Pfizer, Celgene, and Merck, among others.
SOURCE: Dellapasqua S et al. J Clin Oncol. 2018 Dec 27. doi: 10.1200/JCO.18.00296.
Degarelix, the gonadotropin-releasing hormone (GnRH) antagonist approved for prostate cancer, was more effective than a GnRH agonist in achieving ovarian function suppression in women with breast cancer, results of a randomized trial show.
Ovarian function suppression was achieved more rapidly and maintained more effectively with degarelix, compared with triptorelin, in the premenopausal women who were receiving letrozole for neoadjuvant endocrine therapy, investigators said.
Adverse events including hot flashes and injection site reactions were reported more often with degarelix versus the GnRH agonist in this randomized, phase 2 trial of 51 subjects.
Additional research is needed to determine whether degarelix results in superior disease control versus the current standard of care, reported Silvia Dellapasqua, MD, of the European Institute of Oncology IRCCS in Milan, Italy, and coinvestigators.
“The study is hypothesis-generating, and supports later studies to assess whether maintenance of ovarian function suppression with degarelix translates into a better clinical outcome and is worth a trade-off of increased rate of some adverse events,” the researchers wrote. The report is in the Journal of Clinical Oncology.
Patients were randomly assigned to receive degarelix plus letrozole or triptorelin plus letrozole for six 28-day cycles. Degarelix was administered subcutaneously on day 1 of each cycle, while triptorelin was administered intramuscularly on day 1 of each cycle, and oral letrozole was to be taken daily. Surgery was performed a few weeks after the last injection.
All patients achieved optimal ovarian function suppression by the end of the first cycle. However, that endpoint was achieved significantly faster among patients in the degarelix arm, at a median of 3 days, versus a median of 14 days for the GnRH agonist, the investigators reported.
The optimal ovarian function suppression was seen three times faster with degarelix (hazard ratio, 3.05; 95% confidence interval, 1.65-5.65; P less than 001), they added.
One hundred percent of patients receiving degarelix and letrozole maintained optimal ovarian function suppression throughout the study, while about 15% of patients assigned to triptorelin had suboptimal suppression after that first cycle.
The group of patients receiving degarelix had a higher rate of node-negative disease at surgery, and a higher rate of breast-conserving surgery compared with the triptorelin group, the investigators said.
There were two grade 3 adverse events, hypertension and anemia, which both occurred in the triptorelin group, and no grade 4 adverse events. The most common adverse events reported were hot flashes, occurring in 80.0% and 69.2% of the degarelix and triptorelin groups, respectively; arthralgias in 32.0% and 53.8%; insomnia in 24.0% and 11.5%; injection site reactions in 24.0% and 0%; and nausea in 16.0% and 3.8%.
The study was supported by Ferring, and by the International Breast Cancer Study Group via Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research, Cancer Research Switzerland, Oncosuisse, Swiss Cancer League, and the Foundation for Clinical Cancer Research of Eastern Switzerland. The authors reported disclosures related to Ferring, Novartis, Ipsen, DVAX, Roche, Genentech, Pfizer, Celgene, and Merck, among others.
SOURCE: Dellapasqua S et al. J Clin Oncol. 2018 Dec 27. doi: 10.1200/JCO.18.00296.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Degarelix, the gonadotropin-releasing hormone (GnRH) antagonist approved for prostate cancer, was more effective than the GnRH agonist triptorelin in achieving ovarian function suppression.
Major finding: Ovarian function suppression occurred three times faster with degarelix (hazard ratio, 3.05; 95% confidence interval, 1.65-5.65; P less than .001) and in contrast to the triptorelin group, none had suboptimal suppression on subsequent cycles.
Study details: A randomized phase 2 trial including 51 premenopausal women receiving letrozole for locally advanced, endocrine-responsive breast cancer.
Disclosures: The study was supported in part by Ferring. Authors reported disclosures related to Ferring, Novartis, Ipsen, DVAX, Roche, Genentech, Pfizer, Celgene, and Merck, among others.
Source: Dellapasqua S et al. J Clin Oncol. 2018 Dec 27. doi: 10.1200/JCO.18.00296.
Managing menopausal vasomotor and genitourinary symptoms after breast cancer
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.
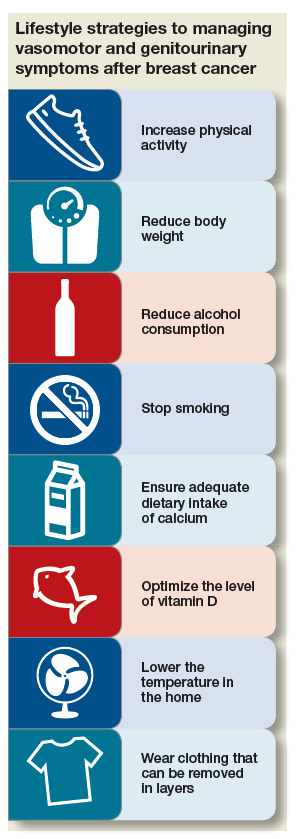
The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.

The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.

The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
Actionable mutations more likely in women with both breast and uterine cancer
Women with both breast and uterine cancer are more likely to carry genetic mutations than women with either breast or uterine cancer alone, according to results of a retrospective analysis of test results.
The majority of the mutations identified were actionable, suggesting that women with breast and uterine cancer should be offered expanded genetic testing, authors of the analysis wrote in Gynecologic Oncology.
“Expanded testing for patients with breast and uterine cancer can help guide management by identifying more patients who may benefit from additional surveillance, along with at-risk family members,” said Kelly Fulk, an oncology genetic specialist at Ambry Genetics, and her coauthors.
The analysis by Ms. Fulk and her colleagues at St. Thomas Health, Nashville, and the University of California, Irvine, was based on a cohort of 52,000 women who had undergone multigene panel testing.
That cohort included 1,650 women with both breast and uterine cancer, of whom about 70% were white, and more than 94% were older than age 50 years at the time of testing. Their median age at first diagnosis was 56 years for breast cancer and 58 years for uterine cancer.
A total of 231 women with breast and uterine cancer, or 14.0%, carried at least one pathogenic mutation or likely pathogenic variant, Ms. Fulk and her colleagues reported. By comparison, mutations were seen in 9.3% of women with breast cancer only (P less than .001), 11.5% of women with uterine cancer only (P = 4.63 x 10–3), and 6.8% of women with no personal cancer history (P less than .001).
Women with both breast and uterine cancer more often had mutations in ATM, BARD1, BRCA2, MSH2, MSH6, PALB2, PMS2, and PTEN, when compared with those women with no personal cancer history, according to the report by Ms. Fulk and her coauthors.
When compared with women with just breast cancer, the women with both breast and uterine cancer more often had mutations in BRCA1, MLH1, MSH2, MSH6, PMS2 and PTEN, they added, noting that women with both cancers were twice as likely to have a BRCA1 mutation (odds ratio, 2.01; 95% confidence interval, 1.08-3.39, P = .016).
While this study had limitations, including its retrospective nature and use of genetic tests with varying numbers of genes analyzed, authors said the results nonetheless support expanded testing in women with both breast and uterine cancers to help guide therapy and cancer surveillance.
“Mutations associated with hereditary breast and ovarian cancer, Lynch syndrome, Cowden syndrome, and Li-Fraumeni syndrome have clear guidelines regarding management and surveillance for other cancers, which can benefit patients and their at-risk family members,” the investigators said.
Ms. Fulk and five coauthors reported that they are paid employees of Ambry Genetics. No other disclosures were provided.
SOURCE: Fulk K et al. Gynecol Oncol. 2019 Jan 3. doi: 10.1016/j.ygyno.2018.12.021.
Women with both breast and uterine cancer are more likely to carry genetic mutations than women with either breast or uterine cancer alone, according to results of a retrospective analysis of test results.
The majority of the mutations identified were actionable, suggesting that women with breast and uterine cancer should be offered expanded genetic testing, authors of the analysis wrote in Gynecologic Oncology.
“Expanded testing for patients with breast and uterine cancer can help guide management by identifying more patients who may benefit from additional surveillance, along with at-risk family members,” said Kelly Fulk, an oncology genetic specialist at Ambry Genetics, and her coauthors.
The analysis by Ms. Fulk and her colleagues at St. Thomas Health, Nashville, and the University of California, Irvine, was based on a cohort of 52,000 women who had undergone multigene panel testing.
That cohort included 1,650 women with both breast and uterine cancer, of whom about 70% were white, and more than 94% were older than age 50 years at the time of testing. Their median age at first diagnosis was 56 years for breast cancer and 58 years for uterine cancer.
A total of 231 women with breast and uterine cancer, or 14.0%, carried at least one pathogenic mutation or likely pathogenic variant, Ms. Fulk and her colleagues reported. By comparison, mutations were seen in 9.3% of women with breast cancer only (P less than .001), 11.5% of women with uterine cancer only (P = 4.63 x 10–3), and 6.8% of women with no personal cancer history (P less than .001).
Women with both breast and uterine cancer more often had mutations in ATM, BARD1, BRCA2, MSH2, MSH6, PALB2, PMS2, and PTEN, when compared with those women with no personal cancer history, according to the report by Ms. Fulk and her coauthors.
When compared with women with just breast cancer, the women with both breast and uterine cancer more often had mutations in BRCA1, MLH1, MSH2, MSH6, PMS2 and PTEN, they added, noting that women with both cancers were twice as likely to have a BRCA1 mutation (odds ratio, 2.01; 95% confidence interval, 1.08-3.39, P = .016).
While this study had limitations, including its retrospective nature and use of genetic tests with varying numbers of genes analyzed, authors said the results nonetheless support expanded testing in women with both breast and uterine cancers to help guide therapy and cancer surveillance.
“Mutations associated with hereditary breast and ovarian cancer, Lynch syndrome, Cowden syndrome, and Li-Fraumeni syndrome have clear guidelines regarding management and surveillance for other cancers, which can benefit patients and their at-risk family members,” the investigators said.
Ms. Fulk and five coauthors reported that they are paid employees of Ambry Genetics. No other disclosures were provided.
SOURCE: Fulk K et al. Gynecol Oncol. 2019 Jan 3. doi: 10.1016/j.ygyno.2018.12.021.
Women with both breast and uterine cancer are more likely to carry genetic mutations than women with either breast or uterine cancer alone, according to results of a retrospective analysis of test results.
The majority of the mutations identified were actionable, suggesting that women with breast and uterine cancer should be offered expanded genetic testing, authors of the analysis wrote in Gynecologic Oncology.
“Expanded testing for patients with breast and uterine cancer can help guide management by identifying more patients who may benefit from additional surveillance, along with at-risk family members,” said Kelly Fulk, an oncology genetic specialist at Ambry Genetics, and her coauthors.
The analysis by Ms. Fulk and her colleagues at St. Thomas Health, Nashville, and the University of California, Irvine, was based on a cohort of 52,000 women who had undergone multigene panel testing.
That cohort included 1,650 women with both breast and uterine cancer, of whom about 70% were white, and more than 94% were older than age 50 years at the time of testing. Their median age at first diagnosis was 56 years for breast cancer and 58 years for uterine cancer.
A total of 231 women with breast and uterine cancer, or 14.0%, carried at least one pathogenic mutation or likely pathogenic variant, Ms. Fulk and her colleagues reported. By comparison, mutations were seen in 9.3% of women with breast cancer only (P less than .001), 11.5% of women with uterine cancer only (P = 4.63 x 10–3), and 6.8% of women with no personal cancer history (P less than .001).
Women with both breast and uterine cancer more often had mutations in ATM, BARD1, BRCA2, MSH2, MSH6, PALB2, PMS2, and PTEN, when compared with those women with no personal cancer history, according to the report by Ms. Fulk and her coauthors.
When compared with women with just breast cancer, the women with both breast and uterine cancer more often had mutations in BRCA1, MLH1, MSH2, MSH6, PMS2 and PTEN, they added, noting that women with both cancers were twice as likely to have a BRCA1 mutation (odds ratio, 2.01; 95% confidence interval, 1.08-3.39, P = .016).
While this study had limitations, including its retrospective nature and use of genetic tests with varying numbers of genes analyzed, authors said the results nonetheless support expanded testing in women with both breast and uterine cancers to help guide therapy and cancer surveillance.
“Mutations associated with hereditary breast and ovarian cancer, Lynch syndrome, Cowden syndrome, and Li-Fraumeni syndrome have clear guidelines regarding management and surveillance for other cancers, which can benefit patients and their at-risk family members,” the investigators said.
Ms. Fulk and five coauthors reported that they are paid employees of Ambry Genetics. No other disclosures were provided.
SOURCE: Fulk K et al. Gynecol Oncol. 2019 Jan 3. doi: 10.1016/j.ygyno.2018.12.021.
FROM GYNECOLOGIC ONCOLOGY
Key clinical point: Women with both breast and uterine cancer are more likely to carry actionable mutations than do women with breast or uterine cancer alone.
Major finding: At least one actionable mutation was seen in 14% of women with breast and uterine cancer, compared with 9.3% of women with breast cancer only, 11.5% of women with uterine cancer only, and 6.8% of women with no personal cancer history.
Study details: A retrospective analysis of a cohort of nearly 52,000 patients who underwent multigene panel testing.
Disclosures: Ms. Fulk and five coauthors reported that they are paid employees of Ambry Genetics.
Source: Fulk K et al. Gynecol Oncol. 2019 Jan 3. doi: 10.1016/j.ygyno.2018.12.021.
Poor-prognosis cancers linked to highest suicide risk in first year
Suicide risk significantly increases within the first year of a cancer diagnosis, with risk varying by type of cancer, according to investigators who conducted a retrospective analysis representing nearly 4.7 million patients.
Risk of suicide in that first year after diagnosis was especially high in pancreatic and lung cancers, while by contrast, breast and prostate cancer did not increase suicide risk, reported the researchers, led by Hesham Hamoda, MD, MPH, of Boston Children’s Hospital/Harvard Medical School, and Ahmad Alfaar, MBBCh, MSc, of Charité–Universitätsmedizin Berlin.
That variation in suicide risk by cancer type suggests that prognosis and 5-year relative survival play a role in increasing suicide rates, according to Dr. Hamoda, Dr. Alfaar, and their coauthors.
“After the diagnosis, it is important that health care providers be vigilant in screening for suicide and ensuring that patients have access to social and emotional support,” they wrote in a report published in Cancer. Their analysis was based on 4,671,989 patients with a diagnosis of cancer in the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2014. Out of 1,005,825 of those patients who died within the first year of diagnosis, the cause of death was suicide for 1,585, or 0.16%.
Overall, the risk of suicide increased significantly among cancer patients versus the general population, with an observed-to-expected (O/E) ratio of 2.51 per 10,000 person-years, the investigators found. The risk was highest in the first 6 months, with an O/E mortality of 3.13 versus 1.8 in the latter 6 months.
The highest ratios were seen for pancreatic cancer, with an O/E ratio of 8.01, and lung cancer, with a ratio of 6.05, the researchers found in further analysis.
Significant increases in suicide risk were also seen for colorectal cancer (2.08) and melanoma (1.45), though rates were not significantly different versus the general population for breast (1.23) and prostate (0.99), according to the reported data.
Suicide risk was relatively high for any cancer with distant metastases (5.63), though still significantly higher at 1.65 in persons with localized/regional disease, the data show.
The increased suicide risk persisted more than 1 year after the cancer diagnosis, though not to the degree observed within that first year, they added.
Most patients with suicide as a cause of death were white (90.2%) and male (87%). Nearly 60% were between the ages of 65 and 84 at the time of suicide.
Social support plays an integral role in suicide prevention among cancer patients, the researchers noted.
Previous studies suggest that support programs may decrease suicide risk by making patients better aware of their prognosis, receptive to decreased social stigma, or less likely to have stress related to cost of care, they said.
“Discussing the quality of life after diagnosis, the effectiveness of therapy, and the prognosis of the disease and maintaining a trusting relationship with health care professionals all decrease the likelihood of suicide immediately after a diagnosis of cancer,” they said.
Dr. Hamoda, Dr. Alfaar, and their coauthors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service (Dr. Alfaar).
SOURCE: Saad AM, et al. Cancer 2019 Jan 7. doi: 10.1002/cncr.31876.
Suicide risk significantly increases within the first year of a cancer diagnosis, with risk varying by type of cancer, according to investigators who conducted a retrospective analysis representing nearly 4.7 million patients.
Risk of suicide in that first year after diagnosis was especially high in pancreatic and lung cancers, while by contrast, breast and prostate cancer did not increase suicide risk, reported the researchers, led by Hesham Hamoda, MD, MPH, of Boston Children’s Hospital/Harvard Medical School, and Ahmad Alfaar, MBBCh, MSc, of Charité–Universitätsmedizin Berlin.
That variation in suicide risk by cancer type suggests that prognosis and 5-year relative survival play a role in increasing suicide rates, according to Dr. Hamoda, Dr. Alfaar, and their coauthors.
“After the diagnosis, it is important that health care providers be vigilant in screening for suicide and ensuring that patients have access to social and emotional support,” they wrote in a report published in Cancer. Their analysis was based on 4,671,989 patients with a diagnosis of cancer in the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2014. Out of 1,005,825 of those patients who died within the first year of diagnosis, the cause of death was suicide for 1,585, or 0.16%.
Overall, the risk of suicide increased significantly among cancer patients versus the general population, with an observed-to-expected (O/E) ratio of 2.51 per 10,000 person-years, the investigators found. The risk was highest in the first 6 months, with an O/E mortality of 3.13 versus 1.8 in the latter 6 months.
The highest ratios were seen for pancreatic cancer, with an O/E ratio of 8.01, and lung cancer, with a ratio of 6.05, the researchers found in further analysis.
Significant increases in suicide risk were also seen for colorectal cancer (2.08) and melanoma (1.45), though rates were not significantly different versus the general population for breast (1.23) and prostate (0.99), according to the reported data.
Suicide risk was relatively high for any cancer with distant metastases (5.63), though still significantly higher at 1.65 in persons with localized/regional disease, the data show.
The increased suicide risk persisted more than 1 year after the cancer diagnosis, though not to the degree observed within that first year, they added.
Most patients with suicide as a cause of death were white (90.2%) and male (87%). Nearly 60% were between the ages of 65 and 84 at the time of suicide.
Social support plays an integral role in suicide prevention among cancer patients, the researchers noted.
Previous studies suggest that support programs may decrease suicide risk by making patients better aware of their prognosis, receptive to decreased social stigma, or less likely to have stress related to cost of care, they said.
“Discussing the quality of life after diagnosis, the effectiveness of therapy, and the prognosis of the disease and maintaining a trusting relationship with health care professionals all decrease the likelihood of suicide immediately after a diagnosis of cancer,” they said.
Dr. Hamoda, Dr. Alfaar, and their coauthors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service (Dr. Alfaar).
SOURCE: Saad AM, et al. Cancer 2019 Jan 7. doi: 10.1002/cncr.31876.
Suicide risk significantly increases within the first year of a cancer diagnosis, with risk varying by type of cancer, according to investigators who conducted a retrospective analysis representing nearly 4.7 million patients.
Risk of suicide in that first year after diagnosis was especially high in pancreatic and lung cancers, while by contrast, breast and prostate cancer did not increase suicide risk, reported the researchers, led by Hesham Hamoda, MD, MPH, of Boston Children’s Hospital/Harvard Medical School, and Ahmad Alfaar, MBBCh, MSc, of Charité–Universitätsmedizin Berlin.
That variation in suicide risk by cancer type suggests that prognosis and 5-year relative survival play a role in increasing suicide rates, according to Dr. Hamoda, Dr. Alfaar, and their coauthors.
“After the diagnosis, it is important that health care providers be vigilant in screening for suicide and ensuring that patients have access to social and emotional support,” they wrote in a report published in Cancer. Their analysis was based on 4,671,989 patients with a diagnosis of cancer in the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2014. Out of 1,005,825 of those patients who died within the first year of diagnosis, the cause of death was suicide for 1,585, or 0.16%.
Overall, the risk of suicide increased significantly among cancer patients versus the general population, with an observed-to-expected (O/E) ratio of 2.51 per 10,000 person-years, the investigators found. The risk was highest in the first 6 months, with an O/E mortality of 3.13 versus 1.8 in the latter 6 months.
The highest ratios were seen for pancreatic cancer, with an O/E ratio of 8.01, and lung cancer, with a ratio of 6.05, the researchers found in further analysis.
Significant increases in suicide risk were also seen for colorectal cancer (2.08) and melanoma (1.45), though rates were not significantly different versus the general population for breast (1.23) and prostate (0.99), according to the reported data.
Suicide risk was relatively high for any cancer with distant metastases (5.63), though still significantly higher at 1.65 in persons with localized/regional disease, the data show.
The increased suicide risk persisted more than 1 year after the cancer diagnosis, though not to the degree observed within that first year, they added.
Most patients with suicide as a cause of death were white (90.2%) and male (87%). Nearly 60% were between the ages of 65 and 84 at the time of suicide.
Social support plays an integral role in suicide prevention among cancer patients, the researchers noted.
Previous studies suggest that support programs may decrease suicide risk by making patients better aware of their prognosis, receptive to decreased social stigma, or less likely to have stress related to cost of care, they said.
“Discussing the quality of life after diagnosis, the effectiveness of therapy, and the prognosis of the disease and maintaining a trusting relationship with health care professionals all decrease the likelihood of suicide immediately after a diagnosis of cancer,” they said.
Dr. Hamoda, Dr. Alfaar, and their coauthors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service (Dr. Alfaar).
SOURCE: Saad AM, et al. Cancer 2019 Jan 7. doi: 10.1002/cncr.31876.
FROM CANCER
Key clinical point: A cancer diagnosis significantly increases risk of suicide in comparison to the general population, particularly for poorer-prognosis cancers.
Major finding: The observed-to-expected mortality ratio was substantially higher for pancreatic cancer (8.01), and lung cancer (6.05), but not significantly increased for breast (1.23) and prostate (0.99).
Study details: A retrospective population-based study of 4,671,989 cancer patients.
Disclosures: The authors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service.
Source: Saad AM et al. Cancer. 2019 Jan 7. doi: 10.1002/cncr.31876.
Young women opt for mastectomy even when neoadjuvant chemo works well
SAN ANTONIO – Response to neoadjuvant chemotherapy has little if any influence on the choice of surgery among young women with early-stage breast cancer, suggests a multicenter, prospective cohort study reported at the San Antonio Breast Cancer Symposium.
Randomized, controlled trials have found high levels of mastectomy among patients who are eligible for breast-conserving surgery, according to first author Hee Jeong Kim, MD, PhD, a visiting scholar at the Dana-Farber Cancer Institute, Boston, and an associate professor in the division of breast in the department of surgery at the University of Ulsan, Seoul, South Korea.
“Young women are more likely to present with large tumors and particularly benefit from a neoadjuvant systemic approach,” she noted. “Recent data suggest that response rates, including pathological complete response, are higher in women younger than 40 than in older women, but little is known about how response to neoadjuvant chemotherapy influences surgical decisions in young women.”
The investigators studied 315 women aged 40 years or younger at diagnosis of unilateral stage I-III breast cancer who received neoadjuvant chemotherapy. Results showed that the chemotherapy doubled the proportion who were eligible for breast-conserving surgery, but 41% of all women eligible after neoadjuvant chemotherapy opted to undergo mastectomy, and the value was essentially the same (42%) among the subset who achieved a complete clinical response. The leading reason given in the medical record for this choice was personal preference in the absence of any known high-risk predisposition.
“Surgical decisions among young women with breast cancer appear to be driven by factors beyond the extent of disease and response to neoadjuvant chemotherapy,” she commented. “We should focus our efforts to optimize surgical decisions in these patients.”
The study complements another study undertaken in the same cohort, also reported at the symposium, that assessed longer-term quality of life according to which surgery women chose; this quality-of-life study found poorer measures after mastectomy.
Drivers and explanatory factors
Session moderator Fatima Cardoso, MD, director of the Breast Unit at the Champalimaud Clinical Center in Lisbon, asked, “Do you think this is really the patient preference, or is this more the surgeon’s preference that is passed on to the patient? Because there is now data showing that breast conservation with radiation is better, even in terms of survival, than mastectomy.”
“Patient preference includes a variety of things. Maybe it is a real patient preference [driven by] fear of recurrence or their peace of mind, but another important factor is maybe the doctor, especially the surgeon. That’s why we should be aware of surgical overtreatment, especially in these young early breast cancer patients,” Dr. Kim replied. “But the good news from this study is that neoadjuvant chemotherapy can give options to the patients, they can choose mastectomy. I think that it’s totally different when the patient has no option other than mastectomy versus the patient can choose mastectomy.”
Two main groups of patients in the United States are being given neoadjuvant chemotherapy, noted session attendee Steven E. Vogl, MD, an oncologist at Montefiore Medical Center, New York. One group has large tumors, and the goal is to shrink the tumor; the other group is planning to have unilateral or bilateral mastectomy with some type of reconstruction by a plastic surgeon.
“The medical oncologist, having decided the [latter] patient needs chemotherapy, chooses to give the chemotherapy preoperatively, so it’s not delayed by 3-5 months for the wounds to heal,” he elaborated. “How many of your patients were in the second category?”
The study did not tease out that population, Dr. Kim replied.
Study details
The women studied were participants in the Young Women’s Breast Cancer Study (YWS). Some 67% had a clinical complete response (no palpable tumor in the breast) to their neoadjuvant chemotherapy, and 32% had a pathological complete response (no tumor in the breast, with or without ductal carcinoma in situ [DCIS], and no tumor deposit exceeding 0.2 mm in the lymph nodes).
Before neoadjuvant chemotherapy, 26% of the women overall were eligible for breast-conserving surgery, but after neoadjuvant chemotherapy, 42% were eligible, Dr. Kim reported.
However, in the entire cohort, breast-conserving surgery was the initial surgery in just 25% of women and the final (definitive) surgery in just 23%.
Among patients eligible for breast conservation after neoadjuvant chemotherapy, 41% chose mastectomy instead as their initial surgery. Response to the chemotherapy seemingly did not influence this choice given that 42% of the subset with a clinical complete response still chose mastectomy. Furthermore, among those eligible for breast conservation who underwent mastectomy, 35% had a pathologic complete response to the chemotherapy.
Of all patients eligible for breast-conserving surgery who opted for mastectomy (and usually a bilateral procedure), the most common reason for choosing this more extensive surgery was personal preference, documented in 53% of cases, followed by presence of a BRCA or p53 mutation or a strong family history, documented in 40%. Reasons were similar among the breast conservation–eligible women who had a clinical complete response and/or ultimately a pathological complete response but chose mastectomy.
The study did not analyze disease factors that may have influenced choice of surgery, such as multicentricity or presence of DCIS, acknowledged Dr. Kim, who disclosed that she had no relevant conflicts of interest.
In an exploratory analysis, use of neoadjuvant chemotherapy increased over time among YWS participants, from 23% among those with diagnosis in 2006-2007 to 44% among those with diagnosis in 2014-2015. There were concurrent improvements in the proportions who achieved a clinical complete response (from 64% to 77%) and a pathological complete response (from 23% to 34%). Yet the proportion undergoing breast-conserving surgery as their initial surgery fell slightly, from 21% to 19%, during the same period.
SOURCE: Kim HJ et al. SABCS 2018, Abstract GS6-01,
SAN ANTONIO – Response to neoadjuvant chemotherapy has little if any influence on the choice of surgery among young women with early-stage breast cancer, suggests a multicenter, prospective cohort study reported at the San Antonio Breast Cancer Symposium.
Randomized, controlled trials have found high levels of mastectomy among patients who are eligible for breast-conserving surgery, according to first author Hee Jeong Kim, MD, PhD, a visiting scholar at the Dana-Farber Cancer Institute, Boston, and an associate professor in the division of breast in the department of surgery at the University of Ulsan, Seoul, South Korea.
“Young women are more likely to present with large tumors and particularly benefit from a neoadjuvant systemic approach,” she noted. “Recent data suggest that response rates, including pathological complete response, are higher in women younger than 40 than in older women, but little is known about how response to neoadjuvant chemotherapy influences surgical decisions in young women.”
The investigators studied 315 women aged 40 years or younger at diagnosis of unilateral stage I-III breast cancer who received neoadjuvant chemotherapy. Results showed that the chemotherapy doubled the proportion who were eligible for breast-conserving surgery, but 41% of all women eligible after neoadjuvant chemotherapy opted to undergo mastectomy, and the value was essentially the same (42%) among the subset who achieved a complete clinical response. The leading reason given in the medical record for this choice was personal preference in the absence of any known high-risk predisposition.
“Surgical decisions among young women with breast cancer appear to be driven by factors beyond the extent of disease and response to neoadjuvant chemotherapy,” she commented. “We should focus our efforts to optimize surgical decisions in these patients.”
The study complements another study undertaken in the same cohort, also reported at the symposium, that assessed longer-term quality of life according to which surgery women chose; this quality-of-life study found poorer measures after mastectomy.
Drivers and explanatory factors
Session moderator Fatima Cardoso, MD, director of the Breast Unit at the Champalimaud Clinical Center in Lisbon, asked, “Do you think this is really the patient preference, or is this more the surgeon’s preference that is passed on to the patient? Because there is now data showing that breast conservation with radiation is better, even in terms of survival, than mastectomy.”
“Patient preference includes a variety of things. Maybe it is a real patient preference [driven by] fear of recurrence or their peace of mind, but another important factor is maybe the doctor, especially the surgeon. That’s why we should be aware of surgical overtreatment, especially in these young early breast cancer patients,” Dr. Kim replied. “But the good news from this study is that neoadjuvant chemotherapy can give options to the patients, they can choose mastectomy. I think that it’s totally different when the patient has no option other than mastectomy versus the patient can choose mastectomy.”
Two main groups of patients in the United States are being given neoadjuvant chemotherapy, noted session attendee Steven E. Vogl, MD, an oncologist at Montefiore Medical Center, New York. One group has large tumors, and the goal is to shrink the tumor; the other group is planning to have unilateral or bilateral mastectomy with some type of reconstruction by a plastic surgeon.
“The medical oncologist, having decided the [latter] patient needs chemotherapy, chooses to give the chemotherapy preoperatively, so it’s not delayed by 3-5 months for the wounds to heal,” he elaborated. “How many of your patients were in the second category?”
The study did not tease out that population, Dr. Kim replied.
Study details
The women studied were participants in the Young Women’s Breast Cancer Study (YWS). Some 67% had a clinical complete response (no palpable tumor in the breast) to their neoadjuvant chemotherapy, and 32% had a pathological complete response (no tumor in the breast, with or without ductal carcinoma in situ [DCIS], and no tumor deposit exceeding 0.2 mm in the lymph nodes).
Before neoadjuvant chemotherapy, 26% of the women overall were eligible for breast-conserving surgery, but after neoadjuvant chemotherapy, 42% were eligible, Dr. Kim reported.
However, in the entire cohort, breast-conserving surgery was the initial surgery in just 25% of women and the final (definitive) surgery in just 23%.
Among patients eligible for breast conservation after neoadjuvant chemotherapy, 41% chose mastectomy instead as their initial surgery. Response to the chemotherapy seemingly did not influence this choice given that 42% of the subset with a clinical complete response still chose mastectomy. Furthermore, among those eligible for breast conservation who underwent mastectomy, 35% had a pathologic complete response to the chemotherapy.
Of all patients eligible for breast-conserving surgery who opted for mastectomy (and usually a bilateral procedure), the most common reason for choosing this more extensive surgery was personal preference, documented in 53% of cases, followed by presence of a BRCA or p53 mutation or a strong family history, documented in 40%. Reasons were similar among the breast conservation–eligible women who had a clinical complete response and/or ultimately a pathological complete response but chose mastectomy.
The study did not analyze disease factors that may have influenced choice of surgery, such as multicentricity or presence of DCIS, acknowledged Dr. Kim, who disclosed that she had no relevant conflicts of interest.
In an exploratory analysis, use of neoadjuvant chemotherapy increased over time among YWS participants, from 23% among those with diagnosis in 2006-2007 to 44% among those with diagnosis in 2014-2015. There were concurrent improvements in the proportions who achieved a clinical complete response (from 64% to 77%) and a pathological complete response (from 23% to 34%). Yet the proportion undergoing breast-conserving surgery as their initial surgery fell slightly, from 21% to 19%, during the same period.
SOURCE: Kim HJ et al. SABCS 2018, Abstract GS6-01,
SAN ANTONIO – Response to neoadjuvant chemotherapy has little if any influence on the choice of surgery among young women with early-stage breast cancer, suggests a multicenter, prospective cohort study reported at the San Antonio Breast Cancer Symposium.
Randomized, controlled trials have found high levels of mastectomy among patients who are eligible for breast-conserving surgery, according to first author Hee Jeong Kim, MD, PhD, a visiting scholar at the Dana-Farber Cancer Institute, Boston, and an associate professor in the division of breast in the department of surgery at the University of Ulsan, Seoul, South Korea.
“Young women are more likely to present with large tumors and particularly benefit from a neoadjuvant systemic approach,” she noted. “Recent data suggest that response rates, including pathological complete response, are higher in women younger than 40 than in older women, but little is known about how response to neoadjuvant chemotherapy influences surgical decisions in young women.”
The investigators studied 315 women aged 40 years or younger at diagnosis of unilateral stage I-III breast cancer who received neoadjuvant chemotherapy. Results showed that the chemotherapy doubled the proportion who were eligible for breast-conserving surgery, but 41% of all women eligible after neoadjuvant chemotherapy opted to undergo mastectomy, and the value was essentially the same (42%) among the subset who achieved a complete clinical response. The leading reason given in the medical record for this choice was personal preference in the absence of any known high-risk predisposition.
“Surgical decisions among young women with breast cancer appear to be driven by factors beyond the extent of disease and response to neoadjuvant chemotherapy,” she commented. “We should focus our efforts to optimize surgical decisions in these patients.”
The study complements another study undertaken in the same cohort, also reported at the symposium, that assessed longer-term quality of life according to which surgery women chose; this quality-of-life study found poorer measures after mastectomy.
Drivers and explanatory factors
Session moderator Fatima Cardoso, MD, director of the Breast Unit at the Champalimaud Clinical Center in Lisbon, asked, “Do you think this is really the patient preference, or is this more the surgeon’s preference that is passed on to the patient? Because there is now data showing that breast conservation with radiation is better, even in terms of survival, than mastectomy.”
“Patient preference includes a variety of things. Maybe it is a real patient preference [driven by] fear of recurrence or their peace of mind, but another important factor is maybe the doctor, especially the surgeon. That’s why we should be aware of surgical overtreatment, especially in these young early breast cancer patients,” Dr. Kim replied. “But the good news from this study is that neoadjuvant chemotherapy can give options to the patients, they can choose mastectomy. I think that it’s totally different when the patient has no option other than mastectomy versus the patient can choose mastectomy.”
Two main groups of patients in the United States are being given neoadjuvant chemotherapy, noted session attendee Steven E. Vogl, MD, an oncologist at Montefiore Medical Center, New York. One group has large tumors, and the goal is to shrink the tumor; the other group is planning to have unilateral or bilateral mastectomy with some type of reconstruction by a plastic surgeon.
“The medical oncologist, having decided the [latter] patient needs chemotherapy, chooses to give the chemotherapy preoperatively, so it’s not delayed by 3-5 months for the wounds to heal,” he elaborated. “How many of your patients were in the second category?”
The study did not tease out that population, Dr. Kim replied.
Study details
The women studied were participants in the Young Women’s Breast Cancer Study (YWS). Some 67% had a clinical complete response (no palpable tumor in the breast) to their neoadjuvant chemotherapy, and 32% had a pathological complete response (no tumor in the breast, with or without ductal carcinoma in situ [DCIS], and no tumor deposit exceeding 0.2 mm in the lymph nodes).
Before neoadjuvant chemotherapy, 26% of the women overall were eligible for breast-conserving surgery, but after neoadjuvant chemotherapy, 42% were eligible, Dr. Kim reported.
However, in the entire cohort, breast-conserving surgery was the initial surgery in just 25% of women and the final (definitive) surgery in just 23%.
Among patients eligible for breast conservation after neoadjuvant chemotherapy, 41% chose mastectomy instead as their initial surgery. Response to the chemotherapy seemingly did not influence this choice given that 42% of the subset with a clinical complete response still chose mastectomy. Furthermore, among those eligible for breast conservation who underwent mastectomy, 35% had a pathologic complete response to the chemotherapy.
Of all patients eligible for breast-conserving surgery who opted for mastectomy (and usually a bilateral procedure), the most common reason for choosing this more extensive surgery was personal preference, documented in 53% of cases, followed by presence of a BRCA or p53 mutation or a strong family history, documented in 40%. Reasons were similar among the breast conservation–eligible women who had a clinical complete response and/or ultimately a pathological complete response but chose mastectomy.
The study did not analyze disease factors that may have influenced choice of surgery, such as multicentricity or presence of DCIS, acknowledged Dr. Kim, who disclosed that she had no relevant conflicts of interest.
In an exploratory analysis, use of neoadjuvant chemotherapy increased over time among YWS participants, from 23% among those with diagnosis in 2006-2007 to 44% among those with diagnosis in 2014-2015. There were concurrent improvements in the proportions who achieved a clinical complete response (from 64% to 77%) and a pathological complete response (from 23% to 34%). Yet the proportion undergoing breast-conserving surgery as their initial surgery fell slightly, from 21% to 19%, during the same period.
SOURCE: Kim HJ et al. SABCS 2018, Abstract GS6-01,
REPORTING FROM SABCS 2018
Key clinical point: Response to neoadjuvant chemotherapy does not alter choice of surgery among young breast cancer patients.
Major finding: Neoadjuvant chemotherapy increased the proportion eligible for breast-conserving surgery from 26% to 42%, but about 40% of those eligible chose mastectomy regardless of chemotherapy response, mainly because of personal preference.
Study details: A multicenter, prospective cohort study of 315 women aged 40 years or younger at diagnosis of early-stage breast cancer who received neoadjuvant chemotherapy (Young Women’s Breast Cancer Study).
Disclosures: Dr. Kim disclosed that she had no relevant conflicts of interest.
Source: Kim HJ et al. SABCS 2018, Abstract GS6-01.