User login
FFR-guided PCI falls short vs. surgery in multivessel disease: FAME 3
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”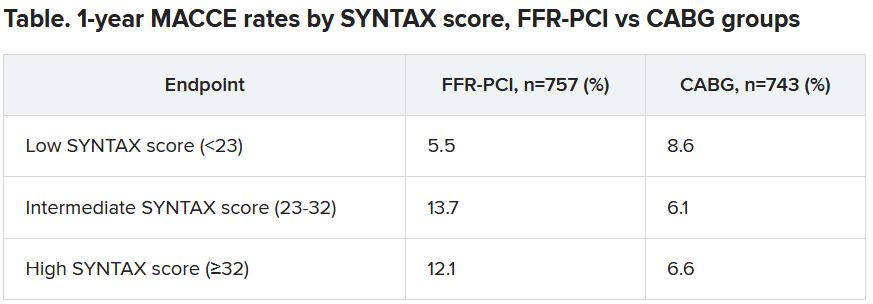
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
SUGAR trial finds superior stent for those with diabetes and CAD
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Superiority shown on TLF endpoint
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021
COVID-19 has brought more complex, longer office visits
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
AHA dietary guidance cites structural challenges to heart-healthy patterns
In a new scientific statement on diet and lifestyle recommendations, the American Heart Association is highlighting, for the first time, structural challenges that impede the adoption of heart-healthy dietary patterns.
This is in addition to stressing aspects of diet that improve cardiovascular health and reduce cardiovascular risk, with an emphasis on dietary patterns and food-based guidance beyond naming individual foods or nutrients.
The 2021 Dietary Guidance to Improve Cardiovascular Health scientific statement, developed under Alice H. Lichtenstein, DSc, chair of the AHA writing group, provides 10 evidence-based guidance recommendations to promote cardiometabolic health.
“The way to make heart-healthy choices every day,” said Dr. Lichtenstein, of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University in Boston, in a statement, “is to step back, look at the environment in which you eat, whether it be at home, at work, during social interaction, and then identify what the best choices are. And if there are no good choices, then think about how you can modify your environment so that there are good choices.”
The statement, published in Circulation, underscores growing evidence that nutrition-related chronic diseases have maternal-nutritional origins, and that prevention of pediatric obesity is a key to preserving and prolonging ideal cardiovascular health.
The features are as follows:
- Adjust energy intake and expenditure to achieve and maintain a healthy body weight. To counter the shift toward higher energy intake and more sedentary lifestyles over the past 3 decades, the statement recommends at least 150 minutes of moderate physical activity per week, adjusted for individual’s age, activity level, sex, and size.
- Eat plenty of fruits and vegetables; choose a wide variety. Observational and intervention studies document that dietary patterns rich in varied fruits and vegetables, with the exception of white potatoes, are linked to a lower risk of cardiovascular disease (CVD). Also, whole fruits and vegetables, which more readily provide fiber and satiety, are preferred over juices.
- Choose whole grain foods and products made mostly with whole grains rather than refined grains. Evidence from observational, interventional, and clinical studies confirm the benefits of frequent consumption of whole grains over infrequent consumption or over refined grains in terms of CVD risk, coronary heart disease (CHD), stroke, metabolic syndrome, cardiometabolic risk factors, laxation, and gut microbiota.
- Choose healthy sources of protein, mostly from plants (legumes and nuts).
- Higher intake of legumes, which are rich in protein and fiber, is associated with lower CVD risk, while higher nut intake is associated with lower risks of CVD, CHD, and stroke mortality/incidence. Replacing animal-source foods with plant-source whole foods, beyond health benefits, lowers the diet’s carbon footprint. Meat alternatives are often ultraprocessed and evidence on their short- and long-term health effects is limited. Unsaturated fats are preferred, as are lean, nonprocessed meats.
- Use liquid plant oils rather than tropical oils (coconut, palm, and palm kernel), animal fats (butter and lard), and partially hydrogenated fats. Saturated and trans fats (animal and dairy fats, and partially hydrogenated fat) should be replaced with nontropical liquid plant oils. Evidence supports cardiovascular benefits of dietary unsaturated fats, especially polyunsaturated fats primarily from plant oils (e.g. soybean, corn, safflower and sunflower oils, walnuts, and flax seeds).
- Choose minimally processed foods instead of ultraprocessed foods. Because of their proven association with adverse health outcomes, including overweight and obesity, cardiometabolic disorders (type 2 diabetes, CVD), and all-cause mortality, the consumption of many ultraprocessed foods is of concern. Ultraprocessed foods include artificial colors and flavors and preservatives that promote shelf stability, preserve texture, and increase palatability. A general principle is to emphasize unprocessed or minimally processed foods.
- Minimize intake of beverages and foods with added sugars. Added sugars (commonly glucose, dextrose, sucrose, corn syrup, honey, maple syrup, and concentrated fruit juice) are tied to elevated risk for type 2 diabetes, high cholesterol, and excess body weight. Findings from meta-analyses on body weight and metabolic outcomes for replacing added sugars with low-energy sweeteners are mixed, and the possibility of reverse causality has been raised.
- Choose and prepare foods with little or no salt. In general, the effects of sodium reduction on blood pressure tend to be higher in Black people, middle-aged and older people, and those with hypertension. In the United States, the main combined sources of sodium intake are processed foods, those prepared outside the home, packaged foods, and restaurant foods. Potassium-enriched salts are a promising alternative.
- If you don’t drink alcohol, don’t start; if you choose to drink, limit intake.
- While relationships between alcohol intake and cardiovascular outcomes are complex, the 2020 Dietary Guidelines Advisory Committee recently concluded that those who do drink should consume no more than one drink per day and should not drink alcohol in binges; the 2020 Dietary Guidelines for Americans continues to recommend no more than one drink per day for women and two drinks per day for men.
- Adhere to the guidance regardless in all settings. Food-based dietary guidance applies to all foods and beverages, regardless of where prepared, procured, and consumed. Policies should be enacted that encourage healthier default options (for example, whole grains, minimized sodium and sugar content).
Recognizing impediments
The AHA/ASA scientific statement closes with the declaration: “Creating an environment that facilitates, rather than impedes, adherence to heart-healthy dietary patterns among all individuals is a public health imperative.” It points to the National Institutes of Health’s 2020-2030 Strategic Plan for National Institutes of Health Nutrition Research, which focuses on precision nutrition as a means “to determine the impact on health of not only what individuals eat, but also of why, when, and how they eat throughout the life course.”
Ultimately, precision nutrition may provide personalized diets for CVD prevention. But the “food environment,” often conditioned by “rampant nutrition misinformation” through local, state, and federal practices and policies, may impede the adoption of heart-healthy dietary patterns. Factors such as targeted food marketing (for example, of processed food and beverages in minority neighborhoods), structural racism, neighborhood segregation, unhealthy built environments, and food insecurity create environments in which unhealthy foods are the default option.”
These factors compound adverse dietary and health effects, and underscore a need to “directly combat nutrition misinformation among the public and health care professionals.” They also explain why, despite widespread knowledge of heart-healthy dietary pattern components, little progress has been made in achieving dietary goals in the United States.
Dr. Lichtenstein’s office, in response to a request regarding AHA advocacy and consumer programs, provided the following links: Voices for Healthy Kids initiative site and choosing healthier processed foods and one on fresh, frozen, and canned fruits and vegetables.
Dr. Lichtenstein had no disclosures. Disclosures for the writing group members are included in the statement.
In a new scientific statement on diet and lifestyle recommendations, the American Heart Association is highlighting, for the first time, structural challenges that impede the adoption of heart-healthy dietary patterns.
This is in addition to stressing aspects of diet that improve cardiovascular health and reduce cardiovascular risk, with an emphasis on dietary patterns and food-based guidance beyond naming individual foods or nutrients.
The 2021 Dietary Guidance to Improve Cardiovascular Health scientific statement, developed under Alice H. Lichtenstein, DSc, chair of the AHA writing group, provides 10 evidence-based guidance recommendations to promote cardiometabolic health.
“The way to make heart-healthy choices every day,” said Dr. Lichtenstein, of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University in Boston, in a statement, “is to step back, look at the environment in which you eat, whether it be at home, at work, during social interaction, and then identify what the best choices are. And if there are no good choices, then think about how you can modify your environment so that there are good choices.”
The statement, published in Circulation, underscores growing evidence that nutrition-related chronic diseases have maternal-nutritional origins, and that prevention of pediatric obesity is a key to preserving and prolonging ideal cardiovascular health.
The features are as follows:
- Adjust energy intake and expenditure to achieve and maintain a healthy body weight. To counter the shift toward higher energy intake and more sedentary lifestyles over the past 3 decades, the statement recommends at least 150 minutes of moderate physical activity per week, adjusted for individual’s age, activity level, sex, and size.
- Eat plenty of fruits and vegetables; choose a wide variety. Observational and intervention studies document that dietary patterns rich in varied fruits and vegetables, with the exception of white potatoes, are linked to a lower risk of cardiovascular disease (CVD). Also, whole fruits and vegetables, which more readily provide fiber and satiety, are preferred over juices.
- Choose whole grain foods and products made mostly with whole grains rather than refined grains. Evidence from observational, interventional, and clinical studies confirm the benefits of frequent consumption of whole grains over infrequent consumption or over refined grains in terms of CVD risk, coronary heart disease (CHD), stroke, metabolic syndrome, cardiometabolic risk factors, laxation, and gut microbiota.
- Choose healthy sources of protein, mostly from plants (legumes and nuts).
- Higher intake of legumes, which are rich in protein and fiber, is associated with lower CVD risk, while higher nut intake is associated with lower risks of CVD, CHD, and stroke mortality/incidence. Replacing animal-source foods with plant-source whole foods, beyond health benefits, lowers the diet’s carbon footprint. Meat alternatives are often ultraprocessed and evidence on their short- and long-term health effects is limited. Unsaturated fats are preferred, as are lean, nonprocessed meats.
- Use liquid plant oils rather than tropical oils (coconut, palm, and palm kernel), animal fats (butter and lard), and partially hydrogenated fats. Saturated and trans fats (animal and dairy fats, and partially hydrogenated fat) should be replaced with nontropical liquid plant oils. Evidence supports cardiovascular benefits of dietary unsaturated fats, especially polyunsaturated fats primarily from plant oils (e.g. soybean, corn, safflower and sunflower oils, walnuts, and flax seeds).
- Choose minimally processed foods instead of ultraprocessed foods. Because of their proven association with adverse health outcomes, including overweight and obesity, cardiometabolic disorders (type 2 diabetes, CVD), and all-cause mortality, the consumption of many ultraprocessed foods is of concern. Ultraprocessed foods include artificial colors and flavors and preservatives that promote shelf stability, preserve texture, and increase palatability. A general principle is to emphasize unprocessed or minimally processed foods.
- Minimize intake of beverages and foods with added sugars. Added sugars (commonly glucose, dextrose, sucrose, corn syrup, honey, maple syrup, and concentrated fruit juice) are tied to elevated risk for type 2 diabetes, high cholesterol, and excess body weight. Findings from meta-analyses on body weight and metabolic outcomes for replacing added sugars with low-energy sweeteners are mixed, and the possibility of reverse causality has been raised.
- Choose and prepare foods with little or no salt. In general, the effects of sodium reduction on blood pressure tend to be higher in Black people, middle-aged and older people, and those with hypertension. In the United States, the main combined sources of sodium intake are processed foods, those prepared outside the home, packaged foods, and restaurant foods. Potassium-enriched salts are a promising alternative.
- If you don’t drink alcohol, don’t start; if you choose to drink, limit intake.
- While relationships between alcohol intake and cardiovascular outcomes are complex, the 2020 Dietary Guidelines Advisory Committee recently concluded that those who do drink should consume no more than one drink per day and should not drink alcohol in binges; the 2020 Dietary Guidelines for Americans continues to recommend no more than one drink per day for women and two drinks per day for men.
- Adhere to the guidance regardless in all settings. Food-based dietary guidance applies to all foods and beverages, regardless of where prepared, procured, and consumed. Policies should be enacted that encourage healthier default options (for example, whole grains, minimized sodium and sugar content).
Recognizing impediments
The AHA/ASA scientific statement closes with the declaration: “Creating an environment that facilitates, rather than impedes, adherence to heart-healthy dietary patterns among all individuals is a public health imperative.” It points to the National Institutes of Health’s 2020-2030 Strategic Plan for National Institutes of Health Nutrition Research, which focuses on precision nutrition as a means “to determine the impact on health of not only what individuals eat, but also of why, when, and how they eat throughout the life course.”
Ultimately, precision nutrition may provide personalized diets for CVD prevention. But the “food environment,” often conditioned by “rampant nutrition misinformation” through local, state, and federal practices and policies, may impede the adoption of heart-healthy dietary patterns. Factors such as targeted food marketing (for example, of processed food and beverages in minority neighborhoods), structural racism, neighborhood segregation, unhealthy built environments, and food insecurity create environments in which unhealthy foods are the default option.”
These factors compound adverse dietary and health effects, and underscore a need to “directly combat nutrition misinformation among the public and health care professionals.” They also explain why, despite widespread knowledge of heart-healthy dietary pattern components, little progress has been made in achieving dietary goals in the United States.
Dr. Lichtenstein’s office, in response to a request regarding AHA advocacy and consumer programs, provided the following links: Voices for Healthy Kids initiative site and choosing healthier processed foods and one on fresh, frozen, and canned fruits and vegetables.
Dr. Lichtenstein had no disclosures. Disclosures for the writing group members are included in the statement.
In a new scientific statement on diet and lifestyle recommendations, the American Heart Association is highlighting, for the first time, structural challenges that impede the adoption of heart-healthy dietary patterns.
This is in addition to stressing aspects of diet that improve cardiovascular health and reduce cardiovascular risk, with an emphasis on dietary patterns and food-based guidance beyond naming individual foods or nutrients.
The 2021 Dietary Guidance to Improve Cardiovascular Health scientific statement, developed under Alice H. Lichtenstein, DSc, chair of the AHA writing group, provides 10 evidence-based guidance recommendations to promote cardiometabolic health.
“The way to make heart-healthy choices every day,” said Dr. Lichtenstein, of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University in Boston, in a statement, “is to step back, look at the environment in which you eat, whether it be at home, at work, during social interaction, and then identify what the best choices are. And if there are no good choices, then think about how you can modify your environment so that there are good choices.”
The statement, published in Circulation, underscores growing evidence that nutrition-related chronic diseases have maternal-nutritional origins, and that prevention of pediatric obesity is a key to preserving and prolonging ideal cardiovascular health.
The features are as follows:
- Adjust energy intake and expenditure to achieve and maintain a healthy body weight. To counter the shift toward higher energy intake and more sedentary lifestyles over the past 3 decades, the statement recommends at least 150 minutes of moderate physical activity per week, adjusted for individual’s age, activity level, sex, and size.
- Eat plenty of fruits and vegetables; choose a wide variety. Observational and intervention studies document that dietary patterns rich in varied fruits and vegetables, with the exception of white potatoes, are linked to a lower risk of cardiovascular disease (CVD). Also, whole fruits and vegetables, which more readily provide fiber and satiety, are preferred over juices.
- Choose whole grain foods and products made mostly with whole grains rather than refined grains. Evidence from observational, interventional, and clinical studies confirm the benefits of frequent consumption of whole grains over infrequent consumption or over refined grains in terms of CVD risk, coronary heart disease (CHD), stroke, metabolic syndrome, cardiometabolic risk factors, laxation, and gut microbiota.
- Choose healthy sources of protein, mostly from plants (legumes and nuts).
- Higher intake of legumes, which are rich in protein and fiber, is associated with lower CVD risk, while higher nut intake is associated with lower risks of CVD, CHD, and stroke mortality/incidence. Replacing animal-source foods with plant-source whole foods, beyond health benefits, lowers the diet’s carbon footprint. Meat alternatives are often ultraprocessed and evidence on their short- and long-term health effects is limited. Unsaturated fats are preferred, as are lean, nonprocessed meats.
- Use liquid plant oils rather than tropical oils (coconut, palm, and palm kernel), animal fats (butter and lard), and partially hydrogenated fats. Saturated and trans fats (animal and dairy fats, and partially hydrogenated fat) should be replaced with nontropical liquid plant oils. Evidence supports cardiovascular benefits of dietary unsaturated fats, especially polyunsaturated fats primarily from plant oils (e.g. soybean, corn, safflower and sunflower oils, walnuts, and flax seeds).
- Choose minimally processed foods instead of ultraprocessed foods. Because of their proven association with adverse health outcomes, including overweight and obesity, cardiometabolic disorders (type 2 diabetes, CVD), and all-cause mortality, the consumption of many ultraprocessed foods is of concern. Ultraprocessed foods include artificial colors and flavors and preservatives that promote shelf stability, preserve texture, and increase palatability. A general principle is to emphasize unprocessed or minimally processed foods.
- Minimize intake of beverages and foods with added sugars. Added sugars (commonly glucose, dextrose, sucrose, corn syrup, honey, maple syrup, and concentrated fruit juice) are tied to elevated risk for type 2 diabetes, high cholesterol, and excess body weight. Findings from meta-analyses on body weight and metabolic outcomes for replacing added sugars with low-energy sweeteners are mixed, and the possibility of reverse causality has been raised.
- Choose and prepare foods with little or no salt. In general, the effects of sodium reduction on blood pressure tend to be higher in Black people, middle-aged and older people, and those with hypertension. In the United States, the main combined sources of sodium intake are processed foods, those prepared outside the home, packaged foods, and restaurant foods. Potassium-enriched salts are a promising alternative.
- If you don’t drink alcohol, don’t start; if you choose to drink, limit intake.
- While relationships between alcohol intake and cardiovascular outcomes are complex, the 2020 Dietary Guidelines Advisory Committee recently concluded that those who do drink should consume no more than one drink per day and should not drink alcohol in binges; the 2020 Dietary Guidelines for Americans continues to recommend no more than one drink per day for women and two drinks per day for men.
- Adhere to the guidance regardless in all settings. Food-based dietary guidance applies to all foods and beverages, regardless of where prepared, procured, and consumed. Policies should be enacted that encourage healthier default options (for example, whole grains, minimized sodium and sugar content).
Recognizing impediments
The AHA/ASA scientific statement closes with the declaration: “Creating an environment that facilitates, rather than impedes, adherence to heart-healthy dietary patterns among all individuals is a public health imperative.” It points to the National Institutes of Health’s 2020-2030 Strategic Plan for National Institutes of Health Nutrition Research, which focuses on precision nutrition as a means “to determine the impact on health of not only what individuals eat, but also of why, when, and how they eat throughout the life course.”
Ultimately, precision nutrition may provide personalized diets for CVD prevention. But the “food environment,” often conditioned by “rampant nutrition misinformation” through local, state, and federal practices and policies, may impede the adoption of heart-healthy dietary patterns. Factors such as targeted food marketing (for example, of processed food and beverages in minority neighborhoods), structural racism, neighborhood segregation, unhealthy built environments, and food insecurity create environments in which unhealthy foods are the default option.”
These factors compound adverse dietary and health effects, and underscore a need to “directly combat nutrition misinformation among the public and health care professionals.” They also explain why, despite widespread knowledge of heart-healthy dietary pattern components, little progress has been made in achieving dietary goals in the United States.
Dr. Lichtenstein’s office, in response to a request regarding AHA advocacy and consumer programs, provided the following links: Voices for Healthy Kids initiative site and choosing healthier processed foods and one on fresh, frozen, and canned fruits and vegetables.
Dr. Lichtenstein had no disclosures. Disclosures for the writing group members are included in the statement.
FROM CIRCULATION
Nondiabetes hospitalization is wrong time to up diabetes meds
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
FROM JAMA NETWORK OPEN
Social determinants of health may drive CVD risk in Black Americans
Investigators analyzed 20 years of data on over 50,500 U.S. adults drawn from the National Health and Nutrition Examination Surveys (NHANES) and found that, in the overall population, body mass index and hemoglobin A1c were significantly increased between 1999 and 2018, while serum total cholesterol and cigarette smoking were significantly decreased. Mean systolic blood pressure decreased between 1999 and 2010, but then increased after 2010.
The mean age- and sex-adjusted estimated 10-year risk for atherosclerotic cardiovascular disease (ASCVD) was consistently higher in Black participants vs. White participants, but the difference was attenuated after further adjusting for education, income, home ownership, employment, health insurance, and access to health care.
“These findings are helpful to guide the development of national public health policies for targeted interventions aimed at eliminating health disparities,” Jiang He, MD, PhD, Joseph S. Copes Chair and professor of epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
“Interventions on social determinants of cardiovascular health should be tested in rigorous designed intervention trials,” said Dr. He, director of the Tulane University Translational Science Institute.
The study was published online Oct. 5 in JAMA.
‘Flattened’ CVD mortality?
Recent data show that the CVD mortality rate flattened, while the total number of cardiovascular deaths increased in the U.S. general population from 2010 to 2018, “but the reasons for this deceleration in the decline of CVD mortality are not entirely understood,” Dr. He said.
Moreover, “racial and ethnic differences in CVD mortality persist in the U.S. general population [but] the secular trends of cardiovascular risk factors among U.S. subpopulations with various racial and ethnic backgrounds and socioeconomic status are [also] not well understood,” he added. The effects of social determinants of health, such as education, income, home ownership, employment, health insurance, and access to health care on racial/ethnic differences in CVD risk, “are not well documented.”
To investigate these questions, the researchers drew on data from NHANES, a series of cross-sectional surveys in nationally representative samples of the U.S. population aged 20 years and older. The surveys are conducted in 2-year cycles and include data from 10 cycles conducted from 1999-2000 to 2017-2018 (n = 50,571, mean age 49.0-51.8 years; 48.2%-51.3% female).
Every 2 years, participants provided sociodemographic information, including age, race/ethnicity, sex, education, income, employment, housing, health insurance, and access to health care, as well as medical history and medication use. They underwent a physical examination that included weight and height, blood pressure, lipid levels, plasma glucose, and hemoglobin A1c.
Social determinants of health
Between 1999-2000 and 2017-2018, age- and sex-adjusted mean BMI and hemoglobin A1c increased, while mean serum total cholesterol and prevalence of smoking decreased (all P < .001).
Age- and sex-adjusted 10-year atherosclerotic cardiovascular disease (ASCVD) risk decreased from 7.6% (6.9%-8.2%) in 1999-2000 to 6.5% (6.1%-6.8%) in 2011-2012, with no significant changes thereafter.
When the researchers looked at specific racial and ethnic groups, they found that age- and sex-adjusted BMI, systolic BP, and hemoglobin A1c were “consistently higher” in non-Hispanic Black participants compared with non-Hispanic White participants, but total cholesterol was lower (all P < .001).
Participants with at least a college education or high family income had “consistently lower levels” of cardiovascular risk factors. And although the mean age- and sex-adjusted 10-year risk for ASCVD was significantly higher in non-Hispanic Black vs. non-Hispanic White participants (difference, 1.4% [1.0%-1.7%] in 1999-2008 and 2.0% [1.7%-2.4%] in 2009-2018), the difference was attenuated (by –0.3% in 1999-2008 and 0.7% in 2009-2018) after the researchers further adjusted for education, income, home ownership, employment, health insurance, and access to health care.
The differences in cardiovascular risk factors between Black and White participants “may have been moderated by social determinants of health,” the authors noted.
Provide appropriate education
Commenting on the study in an interview, Mary Ann McLaughlin, MD, MPH, associate professor of medicine, cardiology, Icahn School of Medicine at Mount Sinai, New York, pointed out that two important cardiovascular risk factors associated with being overweight – hypertension and diabetes – remained higher in the Black population compared with the White population in this analysis.
“Physicians and health care systems should provide appropriate education and resources regarding risk factor modification regarding diet, exercise, and blood pressure control,” advised Dr. McLaughlin, who was not involved with the study.
“Importantly, smoking rates and cholesterol levels are lower in the Black population, compared to the White population, when adjusted for many important socioeconomic factors,” she pointed out.
Dr. McLaughlin added that other “important social determinants of health, such as neighborhood and access to healthy food, were not measured and should be addressed by physicians when optimizing cardiovascular risk.”
The research reported in this publication was supported by the National Heart, Lung, and Blood Institute and by the National Institute of General Medical Sciences. One of the researchers, Joshua D. Bundy, PhD, was supported by a grant from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. He and the other coauthors and Dr. McLaughlin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed 20 years of data on over 50,500 U.S. adults drawn from the National Health and Nutrition Examination Surveys (NHANES) and found that, in the overall population, body mass index and hemoglobin A1c were significantly increased between 1999 and 2018, while serum total cholesterol and cigarette smoking were significantly decreased. Mean systolic blood pressure decreased between 1999 and 2010, but then increased after 2010.
The mean age- and sex-adjusted estimated 10-year risk for atherosclerotic cardiovascular disease (ASCVD) was consistently higher in Black participants vs. White participants, but the difference was attenuated after further adjusting for education, income, home ownership, employment, health insurance, and access to health care.
“These findings are helpful to guide the development of national public health policies for targeted interventions aimed at eliminating health disparities,” Jiang He, MD, PhD, Joseph S. Copes Chair and professor of epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
“Interventions on social determinants of cardiovascular health should be tested in rigorous designed intervention trials,” said Dr. He, director of the Tulane University Translational Science Institute.
The study was published online Oct. 5 in JAMA.
‘Flattened’ CVD mortality?
Recent data show that the CVD mortality rate flattened, while the total number of cardiovascular deaths increased in the U.S. general population from 2010 to 2018, “but the reasons for this deceleration in the decline of CVD mortality are not entirely understood,” Dr. He said.
Moreover, “racial and ethnic differences in CVD mortality persist in the U.S. general population [but] the secular trends of cardiovascular risk factors among U.S. subpopulations with various racial and ethnic backgrounds and socioeconomic status are [also] not well understood,” he added. The effects of social determinants of health, such as education, income, home ownership, employment, health insurance, and access to health care on racial/ethnic differences in CVD risk, “are not well documented.”
To investigate these questions, the researchers drew on data from NHANES, a series of cross-sectional surveys in nationally representative samples of the U.S. population aged 20 years and older. The surveys are conducted in 2-year cycles and include data from 10 cycles conducted from 1999-2000 to 2017-2018 (n = 50,571, mean age 49.0-51.8 years; 48.2%-51.3% female).
Every 2 years, participants provided sociodemographic information, including age, race/ethnicity, sex, education, income, employment, housing, health insurance, and access to health care, as well as medical history and medication use. They underwent a physical examination that included weight and height, blood pressure, lipid levels, plasma glucose, and hemoglobin A1c.
Social determinants of health
Between 1999-2000 and 2017-2018, age- and sex-adjusted mean BMI and hemoglobin A1c increased, while mean serum total cholesterol and prevalence of smoking decreased (all P < .001).
Age- and sex-adjusted 10-year atherosclerotic cardiovascular disease (ASCVD) risk decreased from 7.6% (6.9%-8.2%) in 1999-2000 to 6.5% (6.1%-6.8%) in 2011-2012, with no significant changes thereafter.
When the researchers looked at specific racial and ethnic groups, they found that age- and sex-adjusted BMI, systolic BP, and hemoglobin A1c were “consistently higher” in non-Hispanic Black participants compared with non-Hispanic White participants, but total cholesterol was lower (all P < .001).
Participants with at least a college education or high family income had “consistently lower levels” of cardiovascular risk factors. And although the mean age- and sex-adjusted 10-year risk for ASCVD was significantly higher in non-Hispanic Black vs. non-Hispanic White participants (difference, 1.4% [1.0%-1.7%] in 1999-2008 and 2.0% [1.7%-2.4%] in 2009-2018), the difference was attenuated (by –0.3% in 1999-2008 and 0.7% in 2009-2018) after the researchers further adjusted for education, income, home ownership, employment, health insurance, and access to health care.
The differences in cardiovascular risk factors between Black and White participants “may have been moderated by social determinants of health,” the authors noted.
Provide appropriate education
Commenting on the study in an interview, Mary Ann McLaughlin, MD, MPH, associate professor of medicine, cardiology, Icahn School of Medicine at Mount Sinai, New York, pointed out that two important cardiovascular risk factors associated with being overweight – hypertension and diabetes – remained higher in the Black population compared with the White population in this analysis.
“Physicians and health care systems should provide appropriate education and resources regarding risk factor modification regarding diet, exercise, and blood pressure control,” advised Dr. McLaughlin, who was not involved with the study.
“Importantly, smoking rates and cholesterol levels are lower in the Black population, compared to the White population, when adjusted for many important socioeconomic factors,” she pointed out.
Dr. McLaughlin added that other “important social determinants of health, such as neighborhood and access to healthy food, were not measured and should be addressed by physicians when optimizing cardiovascular risk.”
The research reported in this publication was supported by the National Heart, Lung, and Blood Institute and by the National Institute of General Medical Sciences. One of the researchers, Joshua D. Bundy, PhD, was supported by a grant from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. He and the other coauthors and Dr. McLaughlin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed 20 years of data on over 50,500 U.S. adults drawn from the National Health and Nutrition Examination Surveys (NHANES) and found that, in the overall population, body mass index and hemoglobin A1c were significantly increased between 1999 and 2018, while serum total cholesterol and cigarette smoking were significantly decreased. Mean systolic blood pressure decreased between 1999 and 2010, but then increased after 2010.
The mean age- and sex-adjusted estimated 10-year risk for atherosclerotic cardiovascular disease (ASCVD) was consistently higher in Black participants vs. White participants, but the difference was attenuated after further adjusting for education, income, home ownership, employment, health insurance, and access to health care.
“These findings are helpful to guide the development of national public health policies for targeted interventions aimed at eliminating health disparities,” Jiang He, MD, PhD, Joseph S. Copes Chair and professor of epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
“Interventions on social determinants of cardiovascular health should be tested in rigorous designed intervention trials,” said Dr. He, director of the Tulane University Translational Science Institute.
The study was published online Oct. 5 in JAMA.
‘Flattened’ CVD mortality?
Recent data show that the CVD mortality rate flattened, while the total number of cardiovascular deaths increased in the U.S. general population from 2010 to 2018, “but the reasons for this deceleration in the decline of CVD mortality are not entirely understood,” Dr. He said.
Moreover, “racial and ethnic differences in CVD mortality persist in the U.S. general population [but] the secular trends of cardiovascular risk factors among U.S. subpopulations with various racial and ethnic backgrounds and socioeconomic status are [also] not well understood,” he added. The effects of social determinants of health, such as education, income, home ownership, employment, health insurance, and access to health care on racial/ethnic differences in CVD risk, “are not well documented.”
To investigate these questions, the researchers drew on data from NHANES, a series of cross-sectional surveys in nationally representative samples of the U.S. population aged 20 years and older. The surveys are conducted in 2-year cycles and include data from 10 cycles conducted from 1999-2000 to 2017-2018 (n = 50,571, mean age 49.0-51.8 years; 48.2%-51.3% female).
Every 2 years, participants provided sociodemographic information, including age, race/ethnicity, sex, education, income, employment, housing, health insurance, and access to health care, as well as medical history and medication use. They underwent a physical examination that included weight and height, blood pressure, lipid levels, plasma glucose, and hemoglobin A1c.
Social determinants of health
Between 1999-2000 and 2017-2018, age- and sex-adjusted mean BMI and hemoglobin A1c increased, while mean serum total cholesterol and prevalence of smoking decreased (all P < .001).
Age- and sex-adjusted 10-year atherosclerotic cardiovascular disease (ASCVD) risk decreased from 7.6% (6.9%-8.2%) in 1999-2000 to 6.5% (6.1%-6.8%) in 2011-2012, with no significant changes thereafter.
When the researchers looked at specific racial and ethnic groups, they found that age- and sex-adjusted BMI, systolic BP, and hemoglobin A1c were “consistently higher” in non-Hispanic Black participants compared with non-Hispanic White participants, but total cholesterol was lower (all P < .001).
Participants with at least a college education or high family income had “consistently lower levels” of cardiovascular risk factors. And although the mean age- and sex-adjusted 10-year risk for ASCVD was significantly higher in non-Hispanic Black vs. non-Hispanic White participants (difference, 1.4% [1.0%-1.7%] in 1999-2008 and 2.0% [1.7%-2.4%] in 2009-2018), the difference was attenuated (by –0.3% in 1999-2008 and 0.7% in 2009-2018) after the researchers further adjusted for education, income, home ownership, employment, health insurance, and access to health care.
The differences in cardiovascular risk factors between Black and White participants “may have been moderated by social determinants of health,” the authors noted.
Provide appropriate education
Commenting on the study in an interview, Mary Ann McLaughlin, MD, MPH, associate professor of medicine, cardiology, Icahn School of Medicine at Mount Sinai, New York, pointed out that two important cardiovascular risk factors associated with being overweight – hypertension and diabetes – remained higher in the Black population compared with the White population in this analysis.
“Physicians and health care systems should provide appropriate education and resources regarding risk factor modification regarding diet, exercise, and blood pressure control,” advised Dr. McLaughlin, who was not involved with the study.
“Importantly, smoking rates and cholesterol levels are lower in the Black population, compared to the White population, when adjusted for many important socioeconomic factors,” she pointed out.
Dr. McLaughlin added that other “important social determinants of health, such as neighborhood and access to healthy food, were not measured and should be addressed by physicians when optimizing cardiovascular risk.”
The research reported in this publication was supported by the National Heart, Lung, and Blood Institute and by the National Institute of General Medical Sciences. One of the researchers, Joshua D. Bundy, PhD, was supported by a grant from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. He and the other coauthors and Dr. McLaughlin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study points to ideal age for CAC testing in young adults
New risk equations can help determine the need for a first coronary artery calcium (CAC) scan in young adults to identify those most at risk for premature atherosclerosis, researchers say.
“To our knowledge this is the first time to derive a clinical risk equation for the initial conversion from CAC 0, which can be used actually to guide the timing of CAC testing in young adults,” Omar Dzaye, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, said in an interview.
CAC is an independent predictor of adverse atherosclerotic cardiovascular disease (ASCVD), but routine screening is not recommended in low-risk groups. U.S. guidelines say CAC testing may be considered (class IIa) for risk stratification in adults 40 to 75 years at intermediate risk (estimated 10-year ASCVD risk 7.5% to 20%) when the decision to start preventive therapies is unclear.
The new sex-specific risk equations were derived from 22,346 adults 30 to 50 years of age who underwent CAC testing between 1991 and 2010 for ASCVD risk prediction at four high-volume centers in the CAC Consortium. The average age was 43.5 years, 25% were women, and 12.3% were non-White.
The participants were free of clinical ASCVD or CV symptoms at the time of scanning but had underlying traditional ASCVD risk factors (dyslipidemia in 49.6%, hypertension in 20.0%, active smokers 11.0%, and diabetes in 4.0%), an intermediate 10-year ASCVD risk (2.6%), and/or a significant family history of CHD (49.3%).
As reported in the Journal of the American College of Cardiology, 92.7% of participants had a low 10-year ASCVD risk below 5%, but 34.4% had CAC scores above 0 (median, 20 Agatston units).
Assuming a 25% testing yield (number needed to scan equals four to detect one CAC score above 0), the optimal age for a first scan in young men without risk factors was 42.3 years, and for women it was 57.6 years.
Young adults with one or more risk factors, however, would convert to CAC above 0 at least 3.3 years earlier on average. Diabetes had the strongest influence on the probability of conversion, with men and women predicted to develop incident CAC a respective 5.5 years and 7.3 years earlier on average.
The findings build on previous observations by the team showing that diabetes confers a 40% reduction in the so-called “warranty period” of a CAC score of 0, Dr. Dzaye noted. The National Lipid Association 2020 statement on CAC scoring also suggests it’s reasonable to obtain a CAC scan in people with diabetes aged 30 to 39 years.
“The predicted utility of CAC for ASCVD outcomes is similar in type 1 and type 2 diabetes; however, individuals with type 1 diabetes may actually develop CAC as young as 17 years of age,” he said. “Therefore, definitely, CAC studies in this population are required.”
In contrast, hypertension, dyslipidemia, active smoking, and a family history of CHD were individually associated with the development of CAC 3.3 to 4.3 years earlier. In general, the time to premature CAC was longer for women than for men with a given risk-factor profile.
The predicted age for a first CAC was 37.5 years for men and 48.9 years for women with an intermediate risk-factor profile (for example, smoking plus hypertension) and 33.8 years and 44.7 years, respectively, for those with a high-risk profile (for example, diabetes plus dyslipidemia).
Asked whether the risk equations can be used to guide CAC scanning in clinical practice, Dr. Dzaye said, “we very much believe that this can be used because for the process we published the internal validation, and we also did an external validation that is not published at the moment in [the] MESA [trial].”
He pointed out that study participants did not have a second CAC scan for true modeling of longitudinal CAC and do not represent the general population but, rather, a general cardiology referral population enriched with ASCVD risk factors. Future studies are needed that incorporate a more diverse population, multiple CAC scans, and genetic risk factors.
“This is helpful from a descriptive, epidemiologic point of view and helps us understand the approximate prevalence of coronary calcium greater than 0 in younger men and women, but I’m not convinced that it will or should change clinical practice,” cardiologist Philip Greenland, MD, a professor of preventive medicine and professor of medicine at Northwestern University in Chicago, said in an interview.
Dr. Greenland, who coauthored a review on CAC testing earlier this month, said CAC is the strongest tool we have to improve risk prediction beyond standard risk scores but does involve radiation exposure and some added costs. CAC testing is especially useful as a tiebreaker in older intermediate-risk patients who may be on the fence about starting primary prevention medications but could fall short among “younger, low-risk patients where, as they show here, the proportion of people who have a positive test is well below half.”
“So that means you’re going to have a very large number of people who are CAC 0, which is what we would expect in relatively younger people, but I wouldn’t be happy to try to explain that to a patient: ‘We’re not seeing coronary atherosclerosis right now, but we still want to treat your risk factors.’ That’s kind of a dissonant message,” Dr. Greenland said.
An accompanying editorial suggests “the study has filled an important clinical gap, providing highly actionable data that could help guide clinical decision making for ASCVD prevention.”
Nevertheless, Tasneem Naqvi, MD, Mayo Clinic, Scottsdale, Arizona, and Tamar Polonsky, MD, University of Chicago, question the generalizability of the results and point out that CAC screening at the authors’ recommended ages “could still miss a substantial number of young women with incident MI.”
Exposure to ionizing radiation with CAC is lower than that used in screening mammography for breast cancer but, they agree, should be considered, particularly in young women.
“Alternatively, ultrasonography avoids radiation altogether and can detect plaque earlier than the development of CAC,” write Dr. Naqvi and Dr. Polonsky. Further, the 2019 European Society of Cardiology guidelines for CV risk give ultrasound assessment of carotid artery and femoral plaque a class IIa recommendation and CAC a class IIb recommendation.
Commenting for this news organization, Roger Blumenthal, MD, director of the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, said the class IIb recommendation “never really made any sense because the data with coronary calcium is so much stronger than it is with carotid ultrasound.”
“Sometimes smart scientists and researchers differ, but in my strong opinion, the European Society of Cardiology in 2019 did not give it the right classification, while the group I was part of, the American Heart Association/American College of Cardiology [2019 guideline], got it right and emphasized that this is the most cost-effective and useful way to improve risk assessment.”
Dr. Blumenthal, who was not part of the study, noted that U.S. guidelines say CAC measurement is not intended as a screening test for everyone but may be used selectively as a decision aid.
“This study adds to the information about how to use that type of testing. So, I personally think it will be a highly referenced article in the next set of guidelines that the American Heart Association, American College of Cardiology, and other organizations have.”
The study was supported in part by a research grant from the National Institutes of Health National Heart, Lung, and Blood Institute. Dr. Dzaye, Dr. Blumenthal, Dr. Naqvi, and Dr. Polonsky report having no relevant financial relationships.
A version of this article appeared on Medscape.com.
New risk equations can help determine the need for a first coronary artery calcium (CAC) scan in young adults to identify those most at risk for premature atherosclerosis, researchers say.
“To our knowledge this is the first time to derive a clinical risk equation for the initial conversion from CAC 0, which can be used actually to guide the timing of CAC testing in young adults,” Omar Dzaye, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, said in an interview.
CAC is an independent predictor of adverse atherosclerotic cardiovascular disease (ASCVD), but routine screening is not recommended in low-risk groups. U.S. guidelines say CAC testing may be considered (class IIa) for risk stratification in adults 40 to 75 years at intermediate risk (estimated 10-year ASCVD risk 7.5% to 20%) when the decision to start preventive therapies is unclear.
The new sex-specific risk equations were derived from 22,346 adults 30 to 50 years of age who underwent CAC testing between 1991 and 2010 for ASCVD risk prediction at four high-volume centers in the CAC Consortium. The average age was 43.5 years, 25% were women, and 12.3% were non-White.
The participants were free of clinical ASCVD or CV symptoms at the time of scanning but had underlying traditional ASCVD risk factors (dyslipidemia in 49.6%, hypertension in 20.0%, active smokers 11.0%, and diabetes in 4.0%), an intermediate 10-year ASCVD risk (2.6%), and/or a significant family history of CHD (49.3%).
As reported in the Journal of the American College of Cardiology, 92.7% of participants had a low 10-year ASCVD risk below 5%, but 34.4% had CAC scores above 0 (median, 20 Agatston units).
Assuming a 25% testing yield (number needed to scan equals four to detect one CAC score above 0), the optimal age for a first scan in young men without risk factors was 42.3 years, and for women it was 57.6 years.
Young adults with one or more risk factors, however, would convert to CAC above 0 at least 3.3 years earlier on average. Diabetes had the strongest influence on the probability of conversion, with men and women predicted to develop incident CAC a respective 5.5 years and 7.3 years earlier on average.
The findings build on previous observations by the team showing that diabetes confers a 40% reduction in the so-called “warranty period” of a CAC score of 0, Dr. Dzaye noted. The National Lipid Association 2020 statement on CAC scoring also suggests it’s reasonable to obtain a CAC scan in people with diabetes aged 30 to 39 years.
“The predicted utility of CAC for ASCVD outcomes is similar in type 1 and type 2 diabetes; however, individuals with type 1 diabetes may actually develop CAC as young as 17 years of age,” he said. “Therefore, definitely, CAC studies in this population are required.”
In contrast, hypertension, dyslipidemia, active smoking, and a family history of CHD were individually associated with the development of CAC 3.3 to 4.3 years earlier. In general, the time to premature CAC was longer for women than for men with a given risk-factor profile.
The predicted age for a first CAC was 37.5 years for men and 48.9 years for women with an intermediate risk-factor profile (for example, smoking plus hypertension) and 33.8 years and 44.7 years, respectively, for those with a high-risk profile (for example, diabetes plus dyslipidemia).
Asked whether the risk equations can be used to guide CAC scanning in clinical practice, Dr. Dzaye said, “we very much believe that this can be used because for the process we published the internal validation, and we also did an external validation that is not published at the moment in [the] MESA [trial].”
He pointed out that study participants did not have a second CAC scan for true modeling of longitudinal CAC and do not represent the general population but, rather, a general cardiology referral population enriched with ASCVD risk factors. Future studies are needed that incorporate a more diverse population, multiple CAC scans, and genetic risk factors.
“This is helpful from a descriptive, epidemiologic point of view and helps us understand the approximate prevalence of coronary calcium greater than 0 in younger men and women, but I’m not convinced that it will or should change clinical practice,” cardiologist Philip Greenland, MD, a professor of preventive medicine and professor of medicine at Northwestern University in Chicago, said in an interview.
Dr. Greenland, who coauthored a review on CAC testing earlier this month, said CAC is the strongest tool we have to improve risk prediction beyond standard risk scores but does involve radiation exposure and some added costs. CAC testing is especially useful as a tiebreaker in older intermediate-risk patients who may be on the fence about starting primary prevention medications but could fall short among “younger, low-risk patients where, as they show here, the proportion of people who have a positive test is well below half.”
“So that means you’re going to have a very large number of people who are CAC 0, which is what we would expect in relatively younger people, but I wouldn’t be happy to try to explain that to a patient: ‘We’re not seeing coronary atherosclerosis right now, but we still want to treat your risk factors.’ That’s kind of a dissonant message,” Dr. Greenland said.
An accompanying editorial suggests “the study has filled an important clinical gap, providing highly actionable data that could help guide clinical decision making for ASCVD prevention.”
Nevertheless, Tasneem Naqvi, MD, Mayo Clinic, Scottsdale, Arizona, and Tamar Polonsky, MD, University of Chicago, question the generalizability of the results and point out that CAC screening at the authors’ recommended ages “could still miss a substantial number of young women with incident MI.”
Exposure to ionizing radiation with CAC is lower than that used in screening mammography for breast cancer but, they agree, should be considered, particularly in young women.
“Alternatively, ultrasonography avoids radiation altogether and can detect plaque earlier than the development of CAC,” write Dr. Naqvi and Dr. Polonsky. Further, the 2019 European Society of Cardiology guidelines for CV risk give ultrasound assessment of carotid artery and femoral plaque a class IIa recommendation and CAC a class IIb recommendation.
Commenting for this news organization, Roger Blumenthal, MD, director of the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, said the class IIb recommendation “never really made any sense because the data with coronary calcium is so much stronger than it is with carotid ultrasound.”
“Sometimes smart scientists and researchers differ, but in my strong opinion, the European Society of Cardiology in 2019 did not give it the right classification, while the group I was part of, the American Heart Association/American College of Cardiology [2019 guideline], got it right and emphasized that this is the most cost-effective and useful way to improve risk assessment.”
Dr. Blumenthal, who was not part of the study, noted that U.S. guidelines say CAC measurement is not intended as a screening test for everyone but may be used selectively as a decision aid.
“This study adds to the information about how to use that type of testing. So, I personally think it will be a highly referenced article in the next set of guidelines that the American Heart Association, American College of Cardiology, and other organizations have.”
The study was supported in part by a research grant from the National Institutes of Health National Heart, Lung, and Blood Institute. Dr. Dzaye, Dr. Blumenthal, Dr. Naqvi, and Dr. Polonsky report having no relevant financial relationships.
A version of this article appeared on Medscape.com.
New risk equations can help determine the need for a first coronary artery calcium (CAC) scan in young adults to identify those most at risk for premature atherosclerosis, researchers say.
“To our knowledge this is the first time to derive a clinical risk equation for the initial conversion from CAC 0, which can be used actually to guide the timing of CAC testing in young adults,” Omar Dzaye, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, said in an interview.
CAC is an independent predictor of adverse atherosclerotic cardiovascular disease (ASCVD), but routine screening is not recommended in low-risk groups. U.S. guidelines say CAC testing may be considered (class IIa) for risk stratification in adults 40 to 75 years at intermediate risk (estimated 10-year ASCVD risk 7.5% to 20%) when the decision to start preventive therapies is unclear.
The new sex-specific risk equations were derived from 22,346 adults 30 to 50 years of age who underwent CAC testing between 1991 and 2010 for ASCVD risk prediction at four high-volume centers in the CAC Consortium. The average age was 43.5 years, 25% were women, and 12.3% were non-White.
The participants were free of clinical ASCVD or CV symptoms at the time of scanning but had underlying traditional ASCVD risk factors (dyslipidemia in 49.6%, hypertension in 20.0%, active smokers 11.0%, and diabetes in 4.0%), an intermediate 10-year ASCVD risk (2.6%), and/or a significant family history of CHD (49.3%).
As reported in the Journal of the American College of Cardiology, 92.7% of participants had a low 10-year ASCVD risk below 5%, but 34.4% had CAC scores above 0 (median, 20 Agatston units).
Assuming a 25% testing yield (number needed to scan equals four to detect one CAC score above 0), the optimal age for a first scan in young men without risk factors was 42.3 years, and for women it was 57.6 years.
Young adults with one or more risk factors, however, would convert to CAC above 0 at least 3.3 years earlier on average. Diabetes had the strongest influence on the probability of conversion, with men and women predicted to develop incident CAC a respective 5.5 years and 7.3 years earlier on average.
The findings build on previous observations by the team showing that diabetes confers a 40% reduction in the so-called “warranty period” of a CAC score of 0, Dr. Dzaye noted. The National Lipid Association 2020 statement on CAC scoring also suggests it’s reasonable to obtain a CAC scan in people with diabetes aged 30 to 39 years.
“The predicted utility of CAC for ASCVD outcomes is similar in type 1 and type 2 diabetes; however, individuals with type 1 diabetes may actually develop CAC as young as 17 years of age,” he said. “Therefore, definitely, CAC studies in this population are required.”
In contrast, hypertension, dyslipidemia, active smoking, and a family history of CHD were individually associated with the development of CAC 3.3 to 4.3 years earlier. In general, the time to premature CAC was longer for women than for men with a given risk-factor profile.
The predicted age for a first CAC was 37.5 years for men and 48.9 years for women with an intermediate risk-factor profile (for example, smoking plus hypertension) and 33.8 years and 44.7 years, respectively, for those with a high-risk profile (for example, diabetes plus dyslipidemia).
Asked whether the risk equations can be used to guide CAC scanning in clinical practice, Dr. Dzaye said, “we very much believe that this can be used because for the process we published the internal validation, and we also did an external validation that is not published at the moment in [the] MESA [trial].”
He pointed out that study participants did not have a second CAC scan for true modeling of longitudinal CAC and do not represent the general population but, rather, a general cardiology referral population enriched with ASCVD risk factors. Future studies are needed that incorporate a more diverse population, multiple CAC scans, and genetic risk factors.
“This is helpful from a descriptive, epidemiologic point of view and helps us understand the approximate prevalence of coronary calcium greater than 0 in younger men and women, but I’m not convinced that it will or should change clinical practice,” cardiologist Philip Greenland, MD, a professor of preventive medicine and professor of medicine at Northwestern University in Chicago, said in an interview.
Dr. Greenland, who coauthored a review on CAC testing earlier this month, said CAC is the strongest tool we have to improve risk prediction beyond standard risk scores but does involve radiation exposure and some added costs. CAC testing is especially useful as a tiebreaker in older intermediate-risk patients who may be on the fence about starting primary prevention medications but could fall short among “younger, low-risk patients where, as they show here, the proportion of people who have a positive test is well below half.”
“So that means you’re going to have a very large number of people who are CAC 0, which is what we would expect in relatively younger people, but I wouldn’t be happy to try to explain that to a patient: ‘We’re not seeing coronary atherosclerosis right now, but we still want to treat your risk factors.’ That’s kind of a dissonant message,” Dr. Greenland said.
An accompanying editorial suggests “the study has filled an important clinical gap, providing highly actionable data that could help guide clinical decision making for ASCVD prevention.”
Nevertheless, Tasneem Naqvi, MD, Mayo Clinic, Scottsdale, Arizona, and Tamar Polonsky, MD, University of Chicago, question the generalizability of the results and point out that CAC screening at the authors’ recommended ages “could still miss a substantial number of young women with incident MI.”
Exposure to ionizing radiation with CAC is lower than that used in screening mammography for breast cancer but, they agree, should be considered, particularly in young women.
“Alternatively, ultrasonography avoids radiation altogether and can detect plaque earlier than the development of CAC,” write Dr. Naqvi and Dr. Polonsky. Further, the 2019 European Society of Cardiology guidelines for CV risk give ultrasound assessment of carotid artery and femoral plaque a class IIa recommendation and CAC a class IIb recommendation.
Commenting for this news organization, Roger Blumenthal, MD, director of the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, said the class IIb recommendation “never really made any sense because the data with coronary calcium is so much stronger than it is with carotid ultrasound.”
“Sometimes smart scientists and researchers differ, but in my strong opinion, the European Society of Cardiology in 2019 did not give it the right classification, while the group I was part of, the American Heart Association/American College of Cardiology [2019 guideline], got it right and emphasized that this is the most cost-effective and useful way to improve risk assessment.”
Dr. Blumenthal, who was not part of the study, noted that U.S. guidelines say CAC measurement is not intended as a screening test for everyone but may be used selectively as a decision aid.
“This study adds to the information about how to use that type of testing. So, I personally think it will be a highly referenced article in the next set of guidelines that the American Heart Association, American College of Cardiology, and other organizations have.”
The study was supported in part by a research grant from the National Institutes of Health National Heart, Lung, and Blood Institute. Dr. Dzaye, Dr. Blumenthal, Dr. Naqvi, and Dr. Polonsky report having no relevant financial relationships.
A version of this article appeared on Medscape.com.
New FDA guidance aims to cut sodium in processed foods
The Food and Drug Administration has issued voluntary, short-term sodium reduction targets for food manufacturers, chain restaurants, and food service operators for processed, packaged, and prepared foods, with an eye toward reducing diet-related conditions such as heart disease and obesity.
The new targets seek to decrease average sodium intake from approximately 3,400 mg/day to 3,000 mg/day, about a 12% reduction, over the next 2.5 years, acting FDA Commissioner Janet Woodcock, MD, and Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition, said in joint statement.
Although this reduction keeps the average intake above the recommended limit of 2,300 mg/day for individuals 14 years and older as per the Dietary Guidelines for Americans, “we know that even these modest reductions made slowly over the next few years will substantially decrease diet-related diseases,” they added.
The FDA first proposed recommendations for reducing sodium content in draft guidance released in 2016.
Since, then a number of companies in the food industry have already made changes to sodium content in their products, “which is encouraging, but additional support across all types of foods to help consumers meet recommended sodium limits is needed,” Dr. Woodcock and Dr. Mayne said.
They emphasized that the new guidance represents short-term goals that the food industry should work to meet as soon as possible to help optimize public health.
“We will continue our discussions with the food industry as we monitor the sodium content of the food supply to evaluate progress. In the future, we plan to issue revised, subsequent targets to further lower the sodium content incrementally and continue to help reduce sodium intake,” Dr. Woodcock and Dr. Mayne said.
AHA: A good first step that does not go far enough
In a statement, the American Heart Association said the new targets will play “a critical role in helping people across the country achieve healthier levels of sodium and improved well-being overall. These targets will be an important driver to reduce sodium consumption, which can have significant health benefits and lead to lower medical costs.”
“Lowering sodium levels in the food supply would reduce risk of hypertension, heart disease, stroke, heart attack, and death in addition to saving billions of dollars in health care costs over the next decade,” the AHA said.
But the AHA also said lowering sodium intake to 3,000 mg/day is not enough.
“Lowering sodium further to 2,300 mg could prevent an estimated 450,000 cases of cardiovascular disease, gain 2 million quality-adjusted life-years, and save approximately $40 billion in health care costs over a 20-year period,” the AHA said.
The AHA is urging the FDA to “follow [this] action with additional targets to further lower the amount of sodium in the food supply and help people in America attain an appropriate sodium intake.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued voluntary, short-term sodium reduction targets for food manufacturers, chain restaurants, and food service operators for processed, packaged, and prepared foods, with an eye toward reducing diet-related conditions such as heart disease and obesity.
The new targets seek to decrease average sodium intake from approximately 3,400 mg/day to 3,000 mg/day, about a 12% reduction, over the next 2.5 years, acting FDA Commissioner Janet Woodcock, MD, and Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition, said in joint statement.
Although this reduction keeps the average intake above the recommended limit of 2,300 mg/day for individuals 14 years and older as per the Dietary Guidelines for Americans, “we know that even these modest reductions made slowly over the next few years will substantially decrease diet-related diseases,” they added.
The FDA first proposed recommendations for reducing sodium content in draft guidance released in 2016.
Since, then a number of companies in the food industry have already made changes to sodium content in their products, “which is encouraging, but additional support across all types of foods to help consumers meet recommended sodium limits is needed,” Dr. Woodcock and Dr. Mayne said.
They emphasized that the new guidance represents short-term goals that the food industry should work to meet as soon as possible to help optimize public health.
“We will continue our discussions with the food industry as we monitor the sodium content of the food supply to evaluate progress. In the future, we plan to issue revised, subsequent targets to further lower the sodium content incrementally and continue to help reduce sodium intake,” Dr. Woodcock and Dr. Mayne said.
AHA: A good first step that does not go far enough
In a statement, the American Heart Association said the new targets will play “a critical role in helping people across the country achieve healthier levels of sodium and improved well-being overall. These targets will be an important driver to reduce sodium consumption, which can have significant health benefits and lead to lower medical costs.”
“Lowering sodium levels in the food supply would reduce risk of hypertension, heart disease, stroke, heart attack, and death in addition to saving billions of dollars in health care costs over the next decade,” the AHA said.
But the AHA also said lowering sodium intake to 3,000 mg/day is not enough.
“Lowering sodium further to 2,300 mg could prevent an estimated 450,000 cases of cardiovascular disease, gain 2 million quality-adjusted life-years, and save approximately $40 billion in health care costs over a 20-year period,” the AHA said.
The AHA is urging the FDA to “follow [this] action with additional targets to further lower the amount of sodium in the food supply and help people in America attain an appropriate sodium intake.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued voluntary, short-term sodium reduction targets for food manufacturers, chain restaurants, and food service operators for processed, packaged, and prepared foods, with an eye toward reducing diet-related conditions such as heart disease and obesity.
The new targets seek to decrease average sodium intake from approximately 3,400 mg/day to 3,000 mg/day, about a 12% reduction, over the next 2.5 years, acting FDA Commissioner Janet Woodcock, MD, and Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition, said in joint statement.
Although this reduction keeps the average intake above the recommended limit of 2,300 mg/day for individuals 14 years and older as per the Dietary Guidelines for Americans, “we know that even these modest reductions made slowly over the next few years will substantially decrease diet-related diseases,” they added.
The FDA first proposed recommendations for reducing sodium content in draft guidance released in 2016.
Since, then a number of companies in the food industry have already made changes to sodium content in their products, “which is encouraging, but additional support across all types of foods to help consumers meet recommended sodium limits is needed,” Dr. Woodcock and Dr. Mayne said.
They emphasized that the new guidance represents short-term goals that the food industry should work to meet as soon as possible to help optimize public health.
“We will continue our discussions with the food industry as we monitor the sodium content of the food supply to evaluate progress. In the future, we plan to issue revised, subsequent targets to further lower the sodium content incrementally and continue to help reduce sodium intake,” Dr. Woodcock and Dr. Mayne said.
AHA: A good first step that does not go far enough
In a statement, the American Heart Association said the new targets will play “a critical role in helping people across the country achieve healthier levels of sodium and improved well-being overall. These targets will be an important driver to reduce sodium consumption, which can have significant health benefits and lead to lower medical costs.”
“Lowering sodium levels in the food supply would reduce risk of hypertension, heart disease, stroke, heart attack, and death in addition to saving billions of dollars in health care costs over the next decade,” the AHA said.
But the AHA also said lowering sodium intake to 3,000 mg/day is not enough.
“Lowering sodium further to 2,300 mg could prevent an estimated 450,000 cases of cardiovascular disease, gain 2 million quality-adjusted life-years, and save approximately $40 billion in health care costs over a 20-year period,” the AHA said.
The AHA is urging the FDA to “follow [this] action with additional targets to further lower the amount of sodium in the food supply and help people in America attain an appropriate sodium intake.”
A version of this article first appeared on Medscape.com.
USPSTF rules out aspirin for over 60s in primary CVD prevention
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
High-dose omega-3s tied to higher AFib risk
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.